Abstract
Background
There are limited data on trends in nationwide cardiac electrophysiology (EP) procedures in the United States before and during the global COVID-19 pandemic.
Objective
We aimed to understand contemporary EP procedural trends and how the COVID-19 pandemic impacted them.
Methods
Trends were obtained from publicly reported Centers for Medicare and Medicaid Services data from 2013 to 2020 (latest available). Rates of catheter-based EP procedures (EP studies and ablations) and cardiac implantable electronic device (CIED) procedures were analyzed. All procedural rates were calculated per 100,000 Medicare beneficiaries (year specific). Procedure physician subspecialty was also reported.
Results
From 2013 to 2019, annual rate of all cardiac EP procedures increased from 817.91 to 1089.68 per 100,000 beneficiaries. Catheter-based EP procedures increased from 323.73 to 675.01, while CIED rates decreased from 494.18 to 414.67. While all ablation procedures increased over time, relative proportion of ablation procedures being pulmonary vein isolation (PVI) increased (9.9% of ablations in 2013, to 18.2% in 2019). In 2020, rates of both catheter-based EP procedures and CIED procedures decreased; however, PVI share of ablation continued to increase in 2020 comprising 25.2% of ablation procedures.
Conclusion
Rates of EP procedures have increased among Medicare beneficiaries, with catheter-based procedures now eclipsing CIEDs. Additionally, a greater proportion of catheter-based EP procedures are PVI, but they still represent a minority of all ablations. In 2020, rates of EP procedures were attenuated, yet the proportion of PVI ablations increased to over one-fourth of ablation procedures. These data have important implications for the EP workforce.
Keywords: Electrophysiology, Procedure trends, Medicare, Ablation, Implantable devices
Key Findings.
-
▪
Rates of all cardiac electrophysiology (EP) procedures in Medicare have increased from 2013 to 2019, primarily driven by catheter-based EP procedures.
-
▪
The frequency of catheter-based EP procedures surpassed that of cardiac implantable electronic device procedures in 2016.
-
▪
The proportion of catheter-based EP procedures being pulmonary vein isolation for atrial fibrillation continued to increase from 2013 to 2019.
-
▪
During the COVID-19 pandemic, overall rates of catheter-based EP procedures and cardiac implantable electronic device procedures decreased from 2019 to 2020; however, rates pulmonary vein isolation and ventricular tachycardia ablation continued to increase.
Introduction
As the U.S. population ages, the rate of age-related and acquired cardiac disease is likely to rise. The projected increase in prevalence of cardiac arrhythmia, particularly atrial fibrillation (AF), has been well documented and is expected to increase both dependent on and independent of other cardiac disease.1 Additionally, as therapies improve for cardiac comorbidities, such as coronary artery disease and heart failure, additional survivors of these conditions require interventions for arrhythmia. Last, contemporary and emerging evidence supporting earlier arrhythmia interventions has the potential to increase utilization of electrophysiology (EP) procedures.2,3 Despite these circumstances, there remain little data on the trends in rates of EP procedures in the United States and the clinicians performing them.
Currently available data on broad EP procedure volumes are either outdated or from outside the United States.4, 5, 6, 7 Furthermore, there are few data on contemporary characteristics including the impact of the global COVID-19 pandemic on procedural rates. The overall aim of the present study was to measure the trends in EP procedures in contemporary U.S. practice. Specifically, the objectives were to (1) measure overall trends in rates of catheter-based EP procedures (EP study [EPS] and ablation and cardiac implantable device [CIED] procedures), (2) measure the relative rates of specific ablations (ie, pulmonary vein isolation [PVI]) for AF compared with ablation for supraventricular tachycardia [SVT] or ventricular tachycardia [VT]), and (3) understand the impact of the global COVID-19 pandemic on nationwide EP procedures in 2020.
Methods
We used publicly available Centers for Medicare and Medicaid Services (CMS) data to measure trends in EP procedures, which included the years 2013 to 2020 (the latest available), consistent with other, similar analyses.8 The primary data source was annual versions of the Medicare Physician and Other Practitioners by Provider and Service dataset. These datasets describe service volumes, payments, and submitted charges organized by clinician (via National Provider Identifier [NPI]), Healthcare Common Procedure Coding System code (standardized Current Procedural Terminology codes), and place of service. For each year, these datasets were filtered for only procedures of interest (see the following section), by unique clinicians (irrespective of place of service). While some procedure codes may allow multiple instances in the same day for a single beneficiary, we limited our analysis to procedures per beneficiary per day. Physician operator characteristics are in the same dataset and derived from NPI registrant information.
Data collection of procedure trends
All EPS and ablation procedures were identified by the following Healthcare Common Procedure Coding System codes: 93613, 93618, 93619, 93620, 93621, 93622, 93623, 93624, 93650, 93651, 93653, 93654, 93655. 93656, and 93657. Non-AF EPS and ablation procedures were identified with the previous codes, excluding 93656 and 93657. Catheter ablation for AF (PVI) was identified using code 93656, a code unique to that procedure starting in 2013. Code 93657 was not used because it is primarily an add-on code for AF ablation that is not billed independently from 93656. Catheter ablation for SVT was identified by code 93653 and for VT by code 93654. Procedures related to CIEDs were identified by the following codes: 33206, 33207, 33208, 33212, 33213, 33214, 33215, 33216, 33217, 33218, 33220, 33221, 33222, 33223, 33224, 33225, 33226, 33227, 33228, 33229, 33230, 33231, 33240, and 33249. Implantable loop recorder procedures were excluded, as these are often performed in an office setting, by nonelectrophysiologists.
Numbers and rates of procedures were measured for each enrollment year. Rates of procedures were adjusted for 100,000 beneficiaries enrolled in “classic” Medicare (Medicare A and B) for that particular year, as reported by public CMS data enrollment data. Additionally, physician subspecialties of physician operators are described by year. Physician specialty was stratified by cardiac EP, other cardiology (general, interventional, heart failure), cardiothoracic surgery, other surgery, or internal medicine (IM) physician (no further specialty reported). Physician location is available; however, it was found to be unreliable depending on recency of the update for an individual physician.
Statistical methods
Data are expressed as number and percentage or proportion, where appropriate. All data cleaning and analyses were performed using R (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria) and RStudio (Version 2021.09.0 Build 351; RStudio, Boston, MA), with packages specifically geared to such analyses.9 As this was an observational analysis of publicly available data and did not involve patients, Institutional Review Board approval was not required. The research reported here adhered to Declaration of Helsinki.
Results
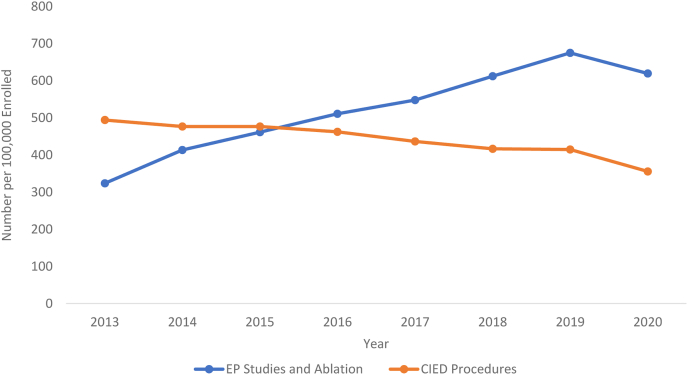
From 2013 to 2019, overall EP procedures, including EPS and ablation and CIED procedures, increased from 817.91 to 1089.68 procedures per 100,000 beneficiaries. This was primarily driven by an increase in EPS and ablation from 323.73 to 675.01 procedures per 100,000 beneficiaries, compared with a decrease in rates of CIED procedures from 494.18 to 414.67 procedures per 100,000 beneficiaries. In 2020, rates of both EPS and ablation and CIED procedures decreased from 675.02 to 619.43 per 100,000 beneficiaries and 414.67 to 355.82 per 100,000 beneficiaries, respectively. These data are shown in Figure 1.
Figure 1.
Trend in number of electrophysiology (EP) studies and ablations vs cardiac implantable electronic device (CIED) implantations per 100,000 Medicare beneficiaries in the years 2013–2020.
Trends in ablation, 2013 to 2019
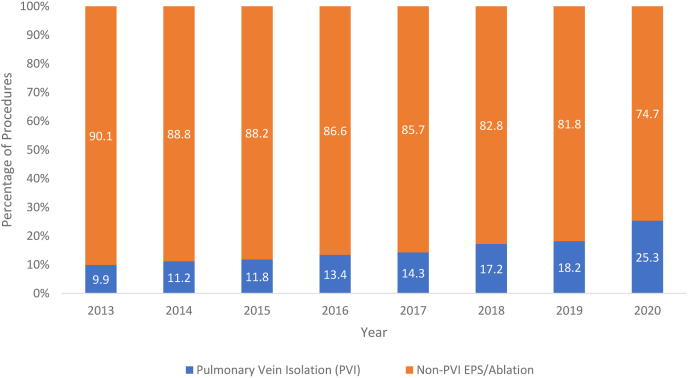
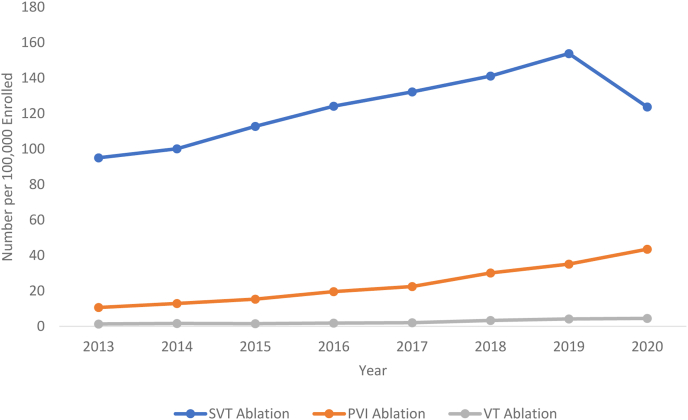
Specific ablation procedural trends (a subgroup of the overall EPS and ablation data) include PVI, SVT, and VT ablation. The overall ablation procedural trends increased from 106.98 to 193.08 procedures per 100,000 beneficiaries. Rates of PVI increased from 10.62 to 35.12 procedures per 100,000 beneficiaries from 2013 to 2019. As a proportion of all ablation codes, rates of PVI increased from 9.92% in 2013 to 18.19% in 2019. The contribution of PVI procedure rates to overall ablation procedure rates is shown in Figure 2. Rates of SVT and VT ablation procedures also showed an increase from 2013 to 2019. SVT ablation increased from 95.02 to 153.80 procedures per 100,000 beneficiaries. VT ablation showed the greatest proportional increase, from 1.34 to 4.15 procedures per 100,000 beneficiaries. Rates of all SVT, PVI, and VT procedures are further depicted in Figure 3.
Figure 2.
Trend in percentage of pulmonary vein isolation (PVI) procedures vs non-PVI procedures for all electrophysiology studies (EPSs) or ablations in the years 2013–2020.
Figure 3.
Trends in number of supraventricular tachycardia (SVT), pulmonary vein isolation (PVI), and ventricular tachycardia (VT) ablation procedures per 100,000 Medicare beneficiaries in the years 2013–2020.
Trends in number of CIED procedures, 2013 to 2019
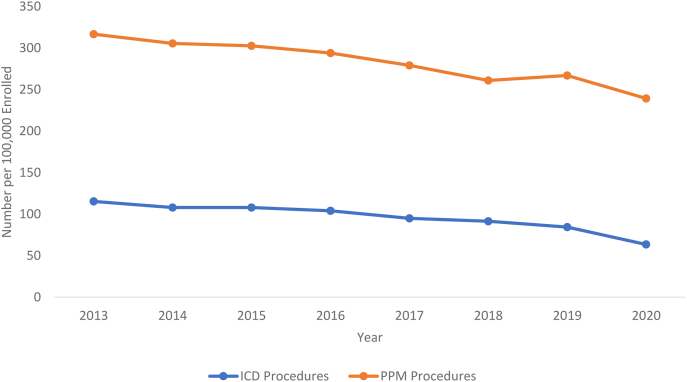
CIED procedural rates are shown in Figure 4. Pacemaker-associated procedure rates decreased from 316.67 to 267.01 per 100,000 beneficiaries from 2013 to 2019. Implantable cardioverter-defibrillator (ICD)–related procedures decreased from 115.31 to 84.47 per 100,000 beneficiaries from 2013 to 2019.
Figure 4.
Trend in number of implantable cardioverter-defibrillators (ICDs) vs permanent pacemakers (PPMs) per 100,000 Medicare beneficiaries in the years 2013–2020. These trends of PPM and ICD do not sum into the cardiac implantable electronic device procedural group, as there are other procedures within this group (ie, nonspecific lead changes).
Trends in operator subspecialty performing CIED, 2013 to 2019
From 2013 to 2019, there was an increase in proportion of EP physicians performing permanent pacemaker (PPM) procedures from 29.2% to 50.8%, decrease in cardiothoracic (CT) surgery physicians from 4.5% to 2.0%, decrease in non-EP cardiology physicians from 60.4% to 42.3%, decrease in IM physicians from 2.7% to 2.5%, and decrease in non-CT surgery from 2.5% to 0.9%. Among ICD procedures, there was an increase in EP physicians from 46.1% to 69.3%, decrease in CT surgery physicians from 1.3% to 0.6%, decrease in non-EP cardiology physicians from 49.4% to 26.1%, and a decrease in IM physicians from 2.8% to 1.7%. These data are described in Table 1.
Table 1.
Physician subspecialty performing PPM and ICD implantations in the years 2013 to 2020
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|---|---|
| Subspecialty of operator performing PPM implantations | ||||||||
| Cardiac electrophysiology | 28.9 | 32.7 | 37.5 | 42.9 | 45.9 | 48.9 | 50.6 | 53.8 |
| Cardiothoracic surgery | 4.6 | 3.7 | 3.3 | 2.7 | 2.4 | 2.4 | 2.0 | 1.8 |
| Other cardiology | 60.6 | 58.5 | 53.9 | 49.8 | 47.1 | 44.2 | 42.4 | 39.5 |
| Internal medicine | 2.7 | 2.5 | 2.5 | 1.9 | 2.1 | 2.1 | 2.4 | 2.1 |
| Other surgery | 2.6 | 1.9 | 2.0 | 1.7 | 1.5 | 1.0 | 0.9 | 0.9 |
| Subspeciality of operator performing ICD implantations | ||||||||
| Cardiac electrophysiology | 46.1 | 49.7 | 55.9 | 60.2 | 63.7 | 66.5 | 69.3 | 70.4 |
| Cardiothoracic surgery | 1.3 | 1.6 | 1.2 | 0.7 | 0.9 | 0.5 | 0.6 | 0.4 |
| Other cardiology | 49.4 | 45.7 | 39.9 | 36.0 | 31.8 | 29.5 | 26.1 | 24.5 |
| Internal medicine | 2.8 | 2.5 | 2.3 | 1.0 | 2.1 | 1.7 | 2.2 | 1.9 |
| Other surgery | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 |
Values are %.
ICD = implantable cardioverter-defibrillator; PPM = permanent pacemaker.
Procedure trends in 2020
In 2020, the number of unique physicians performing EP procedures decreased (from 2019) by roughly 10% (from 1776 to 1661 performing ablation, from 3299 to 3026 performing PPM implantation, from 1607 to 1269 performing ICD implantation). In 2020, rates of EPS and ablation codes decreased from 675.02 to 619.43 per 100,000 beneficiaries, and CIED procedures decreased from 414.67 to 355.82 per 100,000 beneficiaries.
With respect to any ablation, procedures decreased from 193.08 to 171.64 per 100,000 beneficiaries. SVT ablation rates decreased, while both PVI and VT ablation increased. SVT ablation decreased from 153.80 to 123.68 per 100,000 beneficiaries, PVI ablation increased from 35.12 to 43.48 per 100,000 beneficiaries, and VT ablation increased from 4.16 to 4.47 per 100,000 beneficiaries. Trends of SVT, PVI, and VT ablation procedures are shown in Figure 3. The proportion of PVI to all ablation procedures increased from 18.2% to 25.3%, and this is shown in Figure 2.
With respect to CIED procedures specifically, ICD and PPM procedures from 2019 to 2020 both decreased. ICD implantation decreased from 84.47 to 63.46, and PPM implantation decreased from 267.01 to 239.28 per 100,000 beneficiaries. This is shown in Figure 4.
While the overall number of physicians performing EP procedures decreased, the percentage of EP physicians performing these procedures did continue to increase in 2020. The percentage of EP physicians performing PPM implantation increased from 50.6% to 53.6%, and EP physicians performing ICD implantation increased from 69.3% to 70.4% (Table 1).
Discussion
Our study provides important epidemiologic data about national trends in the performance of EP procedures among older Americans and the specific effect of the global COVID-19 pandemic on those trends. We found that the overall rate of EP procedures increased from 2013 to 2019; EPSs and ablations rose dramatically, while CIED procedures did not. Further, VT ablation, the least common procedure performed in general, demonstrated proportionally the greatest growth, a nearly 3-fold increase over the study period. While overall EP procedures decreased in 2020 in the setting of the COVID-19 pandemic, the rate of both PVI and VT ablation continued to increase. Ablation of AF (PVI) in 2020 compromised over one-fourth of all ablation procedures. These data have important implications for the treatment of patients with arrhythmia disorders moving forward, specifically related to workforce development, deployment of new technology, and areas for additional research.
After the adjustment for CMS enrollment, we observed a growth in catheter ablation procedures, specifically a 3-fold increase in VT ablation and a doubling of AF ablation rates. This growth is likely attributable to the combination of increasing disease burden, procedural availability and efficiency, training programs that provide more exposure to and training in complex ablation of both atrial and ventricular arrhythmias, and evidence supporting invasive management earlier.3,10, 11, 12 As treatments for other structural heart diseases improve, including those for heart failure and coronary artery disease, the need for arrhythmia management has increased (including both AF and VT) as people live longer with coexistent cardiovascular disease. Additionally, over the period studied, several randomized clinical trials of catheter ablation for AF and VT demonstrated favorable results for the use of these procedures.10, 11, 12, 13, 14, 15 Last, there has become heightened emphasis on quality-of-life outcomes, which have been demonstrated to be better in general with invasive therapy compared with medical therapy.16
Overall, CIED procedures have declined, including pacemakers and ICDs. The first likely reason is that neither the indications for permanent pacing nor the rates of degenerative conduction disease have changed significantly. Next, battery longevity and automated pacing output algorithms (eg, ‘capture management’) result in less procedures for CIED generator replacements. Additionally, these trends may be in part driven by an attenuation in deployment of primary prevention ICDs. Sudden death rates decline with more sophisticated medical therapy for heart failure,17 and major studies such as the Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality have suggested older patients may be unlikely to benefit.18 However, even with a decrease in CIED procedures, the overall trend in EP procedures has still increased.
Changes in clinician specialty may be more difficult to interpret. For example, the dramatic increase in proportion of electrophysiologists performing these procedures may in part be due to simply to physicians updating their subspecialty classification in the NPI system (from, for example, IM or general cardiology). However, this is unlikely to account for the drop in surgical specialists performing these procedures, as clinicians are unlikely to shift between surgical and medical specialties. These data suggest that surgeons are less and less likely to be performing CIED procedures in favor of electrophysiologists. Several factors may be contributing to this trend, including the increasing complexity of CIED implantation and management, more novel devices being developed, consistent data that support improved outcomes with implantation by electrophysiologists compared with others,19 and potentially financial pressures.
From 2019 to 2020, we found a decrease in the overall trend of both EPS and ablation and CIED procedures coinciding with the COVID-19 pandemic—by nearly 10%. This reflects the reality of healthcare during this time, with a shift in emphasis from routine care to the need for addressing a public health emergency. Our data provide a much broader, wider, and long-term view than the primary, previous report of EP procedures from a single U.S. city during the pandemic.20 Here, we capture the nationwide effect and annual outlook. While overall procedures declined, ablation of AF and VT still showed increases. This is likely explained by several factors: VT ablation is less likely to be elective and is more likely to be an urgent or emergent procedure that was often still performed during this time; additionally, AF has been shown to have a significant effect on quality of life, comparable to acute myocardial infarction21—when given the choice of delaying or proceeding, patients with AF may have been more willing to engage with the healthcare system during a pandemic, in order to improve their quality of life with ablation, compared with, for example, patients with SVT (which showed a marked decline during this period). Last, financial pressures on stressed healthcare institutions may have provided significant motivation to minimize lost elective procedural volume. In point of fact, financial pressures are an increasingly important consideration with respect to these data, as recently enacted and proposed cuts to reimbursement for catheter ablation procedures may attenuate this growth and development despite mounting evidence of safety and effectiveness.
Limitations
These data include only procedures performed within Medicare and do not include those paid for by other payers; private payer rates may vary. Any records that are derived from 10 or fewer beneficiaries are excluded from the Medicare Provider Utilization and Payment Data (to protect beneficiary privacy) and thus are not included in these counts. Operators who perform <10 procedures annually for a given individual procedure code were also excluded from the Medicare Provider Utilization and Payment Data; therefore, procedures that are performed less frequently, including ablation of VT, could be underrepresented in the study. Additionally, our data cannot exclude the concomitant performance of multiple different procedures at once. However, this is more common for non-AF catheter-based EPS and ablation procedures (eg, EP study and ablation for supraventricular tachycardia) and thus would artificially inflate the frequency of non-PVI ablations, suggesting that the true proportion of catheter-based procedures that are PVI may be even higher than that reported herein. Last, physician subspecialty is self-reported and may not be updated regularly.
Conclusion
Rates of catheter-based EP procedures have grown dramatically from 2013 to 2019, with a modest decline in CIED procedures. In comparison with 2019, 2020 rates of both catheter-based EP procedures and CIED procedures decreased in the setting of the COVID-19 pandemic. However, while there continues to be a robust demand and use of catheter ablation treatment for both AF and VT, AF ablation now comprises over one-quarter of all ablation procedures among Medicare beneficiaries.
Acknowledgments
Funding Sources
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL143156 (to BAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The following relationships exist related to this presentation: Benjamin A. Steinberg reports research support from AHA/PCORI, Abbott, Sanofi, Cardiva, and AltaThera; and consulting to Sanofi, InCarda, Pfizer, MileStone, and AltaThera. All other authors did not report any relevant disclosures.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Patient consent was not applicable, as this was an observational analysis of publicly available data.
Ethics Statement
Institutional review board approval was not required, as this was an observational analysis of publicly available data and did not involve patients. The research reported here adhered to the Helsinki declaration.
References
- 1.Go A.S., Hylek E.M., Phillips K.A., et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P., Camm A.J., Goette A., et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 3.Andrade J.G., Deyell M.W., Macle L., et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2022 Nov 7 doi: 10.1056/NEJMoa2212540. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Faryan M., Buchta P., Kowalski O., et al. Temporal trends in the availability and efficacy of catheter ablation for atrial fibrillation and atrial flutter in a highly populated urban area. Kardiol Pol. 2020;78:537–544. doi: 10.33963/KP.15275. [DOI] [PubMed] [Google Scholar]
- 5.Foo F.S., Stiles M.K., Lee M., et al. Ten year trends in cardiac implantable electronic devices in New Zealand: a national data linkage study (ANZACS-QI 51) Intern Med J. 2022;52:614–622. doi: 10.1111/imj.15103. [DOI] [PubMed] [Google Scholar]
- 6.Kneeland P.P., Fang M.C. Trends in catheter ablation for atrial fibrillation in the United States. J Hosp Med. 2009;4:E1–E5. doi: 10.1002/jhm.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J.H., Lee S.R., Choi E.K., et al. Temporal trends of cardiac implantable electronic device implantations: a nationwide population-based study. Korean Circ J. 2019;49:841–852. doi: 10.4070/kcj.2018.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell S.J., Simpson T., Atkinson T., Pellegrini C.N., Nazer B. Temporal and geographical trends in women operators of electrophysiology procedures in the United States. Heart Rhythm. 2022;19:807–811. doi: 10.1016/j.hrthm.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 9.tableone: create 'Table 1' to describe baseline characteristics (R package) https://cran.r-project.org/web/packages/tableone/tableone.pdf Version 0.9.22018. Available at:
- 10.Kuck K.H., Schaumann A., Eckardt L., et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 11.Cosedis Nielsen J., Johannessen A., Raatikainen P., et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 12.Sapp J.L., Wells G.A., Parkash R., et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614. [DOI] [PubMed] [Google Scholar]
- 13.Prasitlumkum N., Navaravong L., Desai A., et al. Impact of early ventricular tachycardia ablation in patients with an implantable cardioverter defibrillator: an updated systematic review and meta-analysis of randomized control trials. Heart Rhythm. 2022;19:2054–2061. doi: 10.1016/j.hrthm.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Tung R., Vaseghi M., Frankel D.S., et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12:1997–2007. doi: 10.1016/j.hrthm.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade J.G., Wells G.A., Deyell M.W., et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 16.Mark D.B., Anstrom K.J., Sheng S., et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg B.A., Mulpuru S.K., Fang J.C., Gersh B.J. Sudden death mechanisms in nonischemic cardiomyopathies: insights gleaned from clinical implantable cardioverter-defibrillator trials. Heart Rhythm. 2017;14:1839–1848. doi: 10.1016/j.hrthm.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Kober L., Thune J.J., Nielsen J.C., et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 19.Chui P.W., Wang Y., Ranasinghe I., et al. Association of physician specialty with long-term implantable cardioverter-defibrillator complication and reoperations rates. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pothineni N.V.K., Santangeli P. Electrophysiology and interventional cardiology procedure volumes during the coronavirus disease 2019 pandemic. Card Electrophysiol Clin. 2022;14:105–110. doi: 10.1016/j.ccep.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorian P., Jung W., Newman D., et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]