Dear editor, recently in this journal Shamez Ladhani presented the need of verified, objective evidence of significant benefit without potential or proven harms for the implementation of COVID-19 mitigation strategies among children.1 Besides face masking, regular use of SARS-CoV-2 rapid antigen detection tests (RDT) has been established as infection control strategy in nurseries and schools.(2) Despite this, a large-scale, real-life analysis of RDT performance among children and adolescents considering COVID-19 vaccination status and SARS-CoV-2 virus variants of concern (VOC) is still missing.3, 4, 5, 6, 7
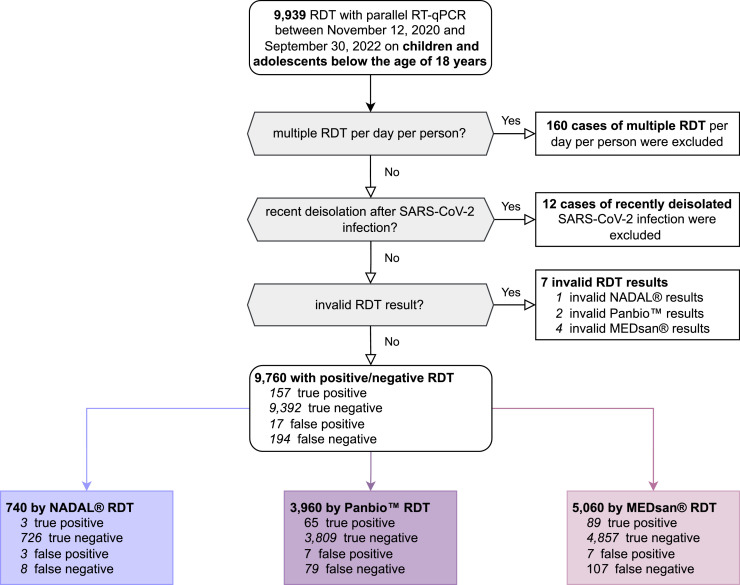
From the 12th of November 2020 to the 30th of September 2022, the RDT performance was evaluated prospectively in comparison to quantitative reverse transcription polymerase chain reaction (RT-qPCR) with oropharyngeal sampling as screening test strategy for all hospitalised children and adolescents under the age of 18 in a tertiary care hospital in Bavaria/Germany. 9760 RDT/RT-qPCR tandems on 7472 individuals (median age: 5 years) with equal gender composition were enrolled. Three different RDT were used (NADAL®, PANBIO™, and MEDsan®; Fig. 1 ). As this study follows two former RDT performance assessments as paediatric follow-up, details on the study protocol, VOC assessment, and RT-qPCR are described earlier.7 , 8 A logistic lasso regression analysis identified factors being associated with the RDT result. Using a tenfold cross-validation procedure for model parameters estimation, the model with the lowest mean squared error (MSE) of ∼0.89 was chosen.
Fig. 1.
Enrolment of antigen rapid diagnostic test (RDT) results.
RDT: Antigen rapid diagnostic test.
RT-qPCR: Quantitative reverse transcription polymerase chain reaction.
351 of 9760 enrolled samples tested RT-qPCR positive, the overall RDT sensitivity was 44.7% (157/351, 95%CI: 39.6–50.0%), specificity 99.8% (9392/9409, 95%CI: 99.7–99.9%).
In the logistic lasso regression analysis, the factors viral load, and Omicron VOC infection showed associations influencing the RDT result. The viral load level significantly increased the odds of having a positive RDT (p < 0.0001) while the independent negative influence of Omicron VOC on the RDT result was not significant (p = 0.12).
RT-qPCR detected a median viral load of 1.8 × 106 (IQR: 2.7 × 104–3.8 × 107) RNA copies per ml in SARS-CoV-2 positive children and adolescents. Significantly higher median viral loads were obtained for RDT positive samples (median: 4.0 × 107) than for RDT negative samples (median: 4.6 × 104, p < 0.0001, Mann-Whitney U test). RDT sensitivity increased significantly by viral load. Considering the viral load threshold of 106 SARS-CoV-2 RNA copies per ml, suggested as SARS-CoV-2 infectivity threshold,9 RDT sensitivity was estimated 71.0% (95%CI: 64.1–77.1%).
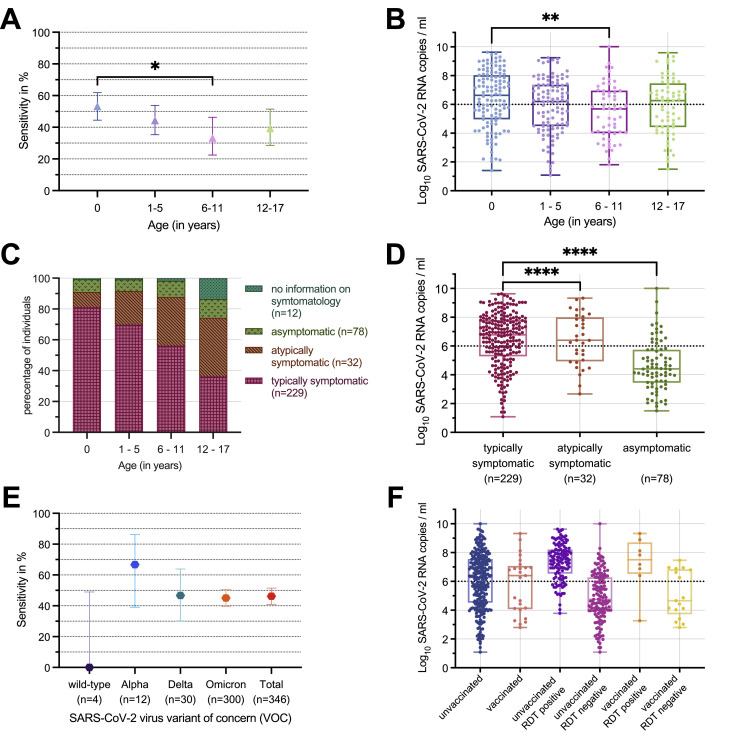
The median viral load was 2.6 × 104 (IQR: 2.8 × 103–5.5 × 105) SARS-CoV-2 RNA copies per ml among 78 asymptomatic, 2.5 × 106 (IQR: 8.6 × 104–9.7 × 107) among 32 atypically symptomatic (e.g. seizures, diarrhoea), and 6.1 × 106 (IQR: 1.9 × 105–6.4 × 107) among 229 typically COVID-19 symptomatic children. RDT sensitivity was significantly reduced in asymptomatic (20.5%) compared to the symptomatic children (52.9%; p = 0.0022, Fisher's exact test, Fig. 2 D).
Fig. 2.
RDT performance in comparison to RT-qPCR stratified by age categories, VOC, and COVID-19 symptomatology.
Fig. 2 A–C: RDT sensitivity (n = 351), logarithmised viral load (n = 348), and symptomatology (n = 351) in SARS-CoV-2 RNA copies/ml stratified by age categories (first year of life, 1 to 5 years, 6 to 11 years, 12 to 17 years). D portrays the logarithmised viral load in SARS-CoV-2 RNA copies/ml, separated by RDT and COVID-19 symptomatology, n = 339. The viral load of specimen of typically COVID-19 symptomatic children and adolescents exceeded statistically significant the viral load of atypically symptomatic or asymptomatic individuals. E included 346 specimens with either molecularly confirmed or epidemiologically assigned VOC (in case of no molecular VOC diagnostics or, if available, known VOC of the infection source, VOC was assigned based on the VOC corresponding to at least 90% of the German COVID-19 cases at RDT performance).F: Viral load in 306 specimens with known COVID-19 vaccination status stratified by vaccination status only and by vaccination status and RDT result.
The viral load threshold of 106 SARS-CoV-2 RNA copies/ml, suggested as infectivity threshold, is added as horizontal dotted line to B,D,F.9
n: Number of enrolled RDT per group.
RDT: Antigen rapid diagnostic test.
RT-qPCR: Quantitative reverse transcription polymerase chain reaction.
*p < 0.05.
⁎⁎p < 0.01.
⁎⁎⁎⁎p < 0.0001.
RDT sensitivity ranged from 52.3% (65/122, 95%CI: 44.5–61.9%) for children in the first year of life, to 44.3% (47/106, 95%CI: 35.3–54.0%) for children aged 1 to 5 years, 33.3% (19/57, 95%CI: 22.49–46.28%) aged 6 to 11 years and 39.4% (26/66, 95%CI: 28.5–51.5%) aged 12 to 17 years (Fig. 2A)in line with differing median viral loads (Fig. 2B). Children between 6 and 11 years showed a significantly reduced RDT sensitivity (p = 0.016, Fisher's exact test, Fig. 2A) and viral load (p = 0.0033, Mann-Whitney U test, Fig. 2B) compared to children in the first year of life going in line with a lower rate of typically symptomatic children (Fig. 2C).
Sensitivity decreased from the Alpha VOC (66.7%, 8/12, 95%CI: 39.1–86.2%) over the Delta VOC (46.7%, 14/30, 95%CI: 30.2–63.9%), to the Omicron VOC (45.0%, 135/300, 95%CI: 39.5–50.7%). Differences in VOC specific sensitivity were not significant (pairwise comparisons using Fisher's exact test, all p > 0.08, Fig. 2E).
In 7990 of 9760 (81.9%) enrolled RDT, information on COVID-19 vaccination status was available: 6977 RDT (87.3%) were performed on unvaccinated, 1013 (12.7%) on children and adolescents with at least one dose of COVID-19 vaccine. Among unvaccinated, RDT sensitivity was 44.8% (126/281, 95%CI: 39.1–50.7%), specificity 99.8% (6681/6696, 95%CI: 99.6–99.9%). Among vaccinated, sensitivity was 32.0% (8/25, 95%CI: 17.2–51.6%), specificity 100.00% (988/988, 95%CI: 99.6–100.0%). Differences in sensitivity (p = 0.29) and specificity (p = 0.24, both Fisher's exact test) were not significant. Viral load did not significantly differ comparing COVID-19 unvaccinated with vaccinated (p = 0.20, Mann-Whitney U test, Fig. 2F).
Compared to previous data, the presented sensitivtiy scores are at the lower end. However, they were obtained in a study which resulted in more case numbers in a real-life point-of-care setting.3, 4, 5 Reliability of RDT performance clearly depended on specimens’ viral load which is influenced by age and days since symptom onset. The differences in the age-stratified viral load levels may be explained by the different proportions of COVID-19 symptomatology. As typically symptomatic infants in the first year of life may enlist the hospitals’ medical care early and large-scale, the school children aged 6 to 11 years may be detected coincidentally in the COVID-19 screening having differing non-infectious reasons for medical consultation. No significantly reduced viral load was observed in Omicron VOC(7) being potentially explained by the dominating proportion of Omicron VOC in this study. RDT sensitivity did not correlate with immunisation status which has been reported in a preprint analysis as factor impairing RDT sensitivity.6
The study is limited in several aspects: Data collection in the real-life and point-of-care setting led to differing distributions and proportional use of the three RDT across the pediatric departments and over the study period. The direct comparability between the manufacturers’ is limited. The potential influence and inhomogeneity in sampling, especially considering the preanalytical challenges in children, in test execution, and in interpretation is probable. Molecularly based VOC determination was only performed from January 2021 to January 2022.10
For children and adolescents, the indication, as well as advantages and disadvantages for RDT usage is comparable to the one for adults.7 , 8 Due to the low sensitivity in asymptomatic individuals, the usefulness of RDT seems limited in large-scale SARS-CoV-2 screening programs. This intrahospital assessed data on RDT reliability should also be considered in terms of RDT screening usage, including its self-testing option for children and adolescents as COVID-19 management strategy in the context of schools and nurseries.
Data access, responsibility, and analysis
Dr Krone and Ms Wagenhäuser had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing statement
Individual participant data that underlie the results reported in this article after deidentification is available on request immediately following publication ending 5 years following article publication to researchers who provide a methodological sound proposal to achieve aims in the approved proposal. Proposals should be directed to krone_m@ukw.de.
Information on previous presentation of the information
1034 RDT results on children and adolescents have already been included in an age independent RDT performance assessment in a cohort of 5068 RDT with data collection from the 12th of November 2020 to the 28th of February 2021 8 as wells as the sequel up to the 30th of January 2022 including 35479 specimen within 5623 on children and adolescents.7
Ethics committee approval
The Ethics committee of the University of Wuerzburg considered the study protocol and waived the need to formally apply for ethical clearance due to the study design (File 20221018 01).
Declaration of Competing Interest
Manuel Krone receives honoraria from Abbott outside the submitted work. None of the other authors has any conflicts of interests to declare.
Acknowledgments
Funding statement
This study was funded by the Federal Ministry for Education and Science (BMBF) via a grant provided to the University Hospital of Wuerzburg by the Network University Medicine on COVID-19 (B-FAST, grant-No. 01KX2021), by the Bavarian Staten Ministry of Health and Care via Bay-VOC as well as by the Free State of Bavaria with COVID-research funds provided to the University of Wuerzburg, Germany.
Nils Petri is supported by the German Research Foundation (DFG) funded scholarship UNION CVD.
Role of the funding source
This study was initiated by the investigators. The sponsoring institutions had no function in study design, data collection, analysis, and interpretation of data as well as in writing of the manuscript. All authors had unlimited access to all data. Dr Krone, Ms Wagenhäuser, Prof Liese, and Dr Andres had the final responsibility for the decision to submit for publication.
Acknowledgments
We thank all hospital staff for conducting RDT testing and documenting test results and all laboratory staff in the virological diagnostic laboratory for performing RT-qPCR testing. We thank accounting department from medical controlling for SAP support.
References
- 1.Ladhani S.N. Face masking for children - time to reconsider. J Infect. 2022;85(6):623–624. doi: 10.1016/j.jinf.2022.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster J., Streng A., Rudolph P., Rücker V., Wallstabe J., Timme S., et al. Feasibility of SARS-CoV-2 surveillance testing among children and childcare workers at German day care centers: a nonrandomized controlled trial. JAMA Netw Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.42057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbonell-Sahuquillo S., Lázaro-Carreño M.I., Camacho J., Barrés-Fernández A., Albert E., Torres I., et al. Evaluation of a rapid antigen detection test (Panbio™ COVID-19 Ag rapid test device) as a point-of-care diagnostic tool for COVID-19 in a pediatric emergency department. J Med Virol. 2021;93(12):6803–6807. doi: 10.1002/jmv.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González-Donapetry P., García-Clemente P., Bloise I., García-Sánchez C., MÁ S.C., Romero M.P., et al. Think of the children: evaluation of SARS-CoV-2 rapid antigen test in pediatric population. Pediatr Infect Dis J. 2021;40(5):385–388. doi: 10.1097/INF.0000000000003101. [DOI] [PubMed] [Google Scholar]
- 5.L’Huillier A.G., Lacour M., Sadiku D., Gadiri M.A., Siebenthal L.D., Schibler M., et al. Diagnostic accuracy of SARS-CoV-2 rapid antigen detection testing in symptomatic and asymptomatic children in the clinical setting. J Clin Microbiol. 2021;59(9):e0099121. doi: 10.1128/JCM.00991-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meiners L., Horn J., Mühlemann B., Schmidt M.L., Walper F., Menzel P., et al. SARS-CoV-2 rapid antigen test sensitivity and viral load in freshly symptomatic hospital employees, December 2020 to February 2022. SSRN Preprint: 10.2139/ssrn.4099425. [DOI] [PubMed]
- 7.I. Wagenhäuser, K. Knies, D. Hofmann, V. Rauschenberger, M. Eisenmann, J. Reusch, et al., Virus variant specific clinical performance of SARS-CoV-2 rapid antigen tests in point-of-care use, November 2020 to January 2022, Clin Microbiol Infect, 2022. Article in Press. [DOI] [PMC free article] [PubMed]
- 8.I. Wagenhäuser, K. Knies, V. Rauschenberger, M. Eisenmann, M. McDonogh, N. Petri, et al., Clinical performance evaluation of SARS-CoV-2 rapid antigen testing in point of care usage in comparison to RT-qPCR, EBioMedicine, 69, 2021,103455. [DOI] [PMC free article] [PubMed]
- 9.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 10.Robert Koch-Institut (RKI): Anzahl und Anteile von VOC und VOI in Deutschland [Available from: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/VOC_VOI_Tabelle.html] (Accessed 1 October 2022).