Abstract
Background
A third dose of the BNT162b2 SARS-CoV-2 vaccine leads to a significant increase in antibody levels, however, concerns regarding the long-term persistence of this response exist. We assessed the humoral response for one year following vaccination.
Methods
A prospective study among immunocompetent healthcare workers (HCW) who received three doses of BNT162b2. anti-spike antibody titers were measured at six predefined timepoints, from before the second vaccine dose, and up to one year afterwards, which is 4–6 months after the third dose. HCW with a history of SARS-CoV-2 infection were excluded.
Results
Seventy-six HCW had all the six serological measurements. Antibody titers significantly increased shortly following the third vaccine dose, and while declining, remained higher from all previous measurements for up to six months.
Conclusions
A third dose of BNT162b2 leads to a profound humoral response, which remains significantly higher than previous measurements, even after 6 months.
Keywords: COVID-19, SARS-CoV-2, Pfizer-BioNTech vaccine, Vaccination, Humoral response
1. Introduction
1.1. Background
Widespread provision of effective SARS-CoV-2 vaccines, such as BNT162b2 (Pfizer-BioNTech), have led to reduced transmission rates and a significant decrease of severe COVID-19 [1]. The combination of waning immunity over time and the emergence of highly transmissible variants of concern, have led to breakthrough infections in vaccinated individuals [2]. A third vaccine dose improves the short-term immune response and provided protection from infection [3], [4], yet the long-term persistence of immune protection is unknown. Concerns regarding further decline of immunity, concomitant with a surge in COVID-19 cases caused by the highly infectious B.1.1529 (Omicron) variant worldwide, have led to the authorization of a fourth vaccine dose by the Israeli Ministry of Health, initially for high-risk and elderly individuals and healthcare workers (HCW). We assessed titers of anti-spike immunoglobulin G (IgG anti-S) in vaccinated HCW for one year, including early (1–2 months) and later (4–6 months) after the third dose.
2. Methods
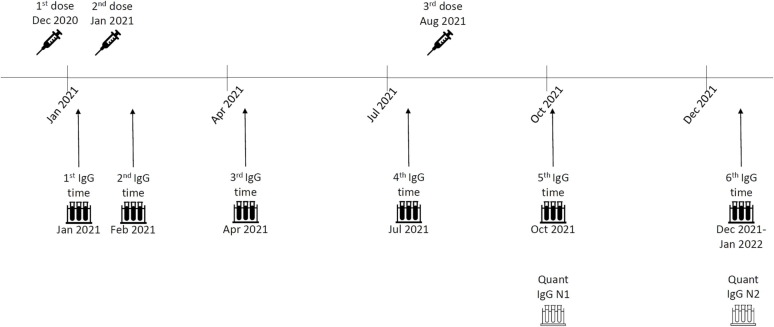
This is a single-center prospective study which enrolled vaccinated HCW from Barzilai Medical Center, at Ashkelon, Israel. HCW volunteered to participate in a serological survey which included measurements of anti-spike (anti-S) and anti-nucleocapsid (anti-N) antibodies at predetermined time points: 0–5 days before-, and 1, 3, 6, 9, and 12 months after the second vaccine dose, which is 4–6 months after the third dose (Fig. 1 ). Vaccination with BNT162b2 began in December 2020, the second dose was administered 21-days later as recommended, and the third dose during July-August 2021. HCW who had a history of solid or hematological malignancy, or who were otherwise immunocompromised were excluded from participation. Since we wanted to assess only the response to vaccination, HCW with evidence of past SARS-CoV-2 infection, either a positive RT-PCR swab or positive anti-N antibodies, were also excluded.
Fig. 1.
Study timeline First vaccine doses were administered to HCW in December 2020. Second doses were administered 21-days later, in January 2021, and the third doses during August 2021. IgG anti-S measurements began 0–5 days prior to the second dose (time 1), and then one month (time 2), three months (time 3), six months (time 4), nine months (time 5), and 12 months (time 6) after the second dose. Measurements of Quant anti-S were done at time points 5 and 6, after we identified very high IgG anti-S levels which were higher than the assay's upper limit following the third dose.
At all the time points, IgG anti-S were measured using Liaison chemiluminescent immunoassay kit (DiaSorin, Saluggia, Italy), and anti-N Abs were measured using Abbott SARS-CoV-2 IgG nucleocapsid protein assay (Abbott, Abbott Park, IL) on an Architect analyzer to identify asymptomatic SARS-CoV-2 infection. The upper detection limit of the Liaison assay is 400 AU/ml, yet all participants had level > 400 AU/ml at the 5th time point and some at the 6th time point (both after the third dose). To overcome this, we have performed serum dilution according to the manufacturer instructions. No measurements > 400 AU/ml were measured before the third vaccine dose. We additionally performed measurements of anti-S Abs using Abbott AdviseDx SARS-CoV-2 IgG II Quant assay (Abbott, Abbott Park, IL), which can differentiate higher antibody levels, for the time points after the third vaccine dose (time points 5 and 6, Quant anti-S). Antibody levels between the two assays are not interchangeable.
Regarding anti-N Abs, index values of 0.8 or higher were considered positive, as previously suggested [5], [6].
We compared geometric means of anti-S antibodies at different time points for both the Liaison (IgG anti-S) and the Abbott (Quant anti-S) assays. We assessed the association between antibody levels and baseline participants characteristics including age, gender, birthplace, overweight BMI > 25, self-reported smoking status, self-reported use of chronic medications, and whether the profession included direct contact with patients (physicians, nurses, physiotherapists etc.) or not (administrators, IT workers, housekeepers, laboratory workers etc.). We used ANOVA for comparing Abs levels between categorical variables and Chi square or Fisher's Exact test when appropriated for comparing proportions. Statistical significance was set on p ≤ 0.05. Data analysis was performed using SPSS 25.0 (SPSS, Chicago, IL).
The study was approved by the Ethics Committee and Institutional Review Board of Barzilai Medical Center (no. BRZ-0009–21). The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants signed an informed consent form prior to study entry.
Results are reported according to STROBE guidelines.
3. Results
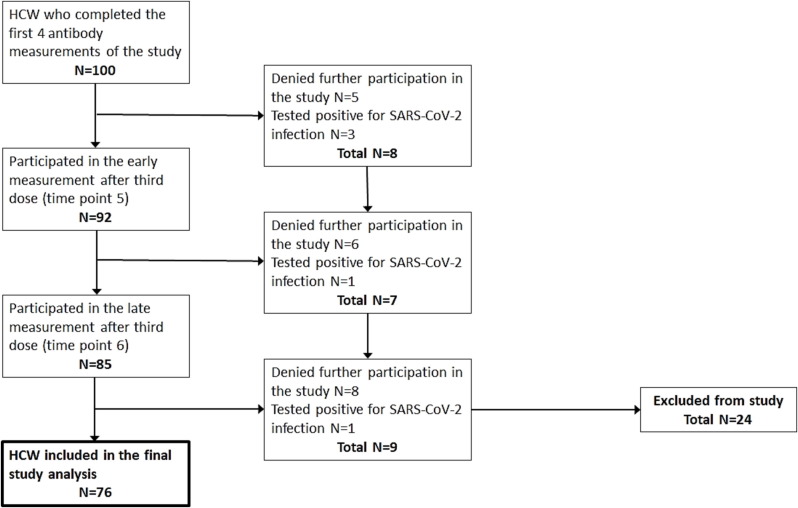
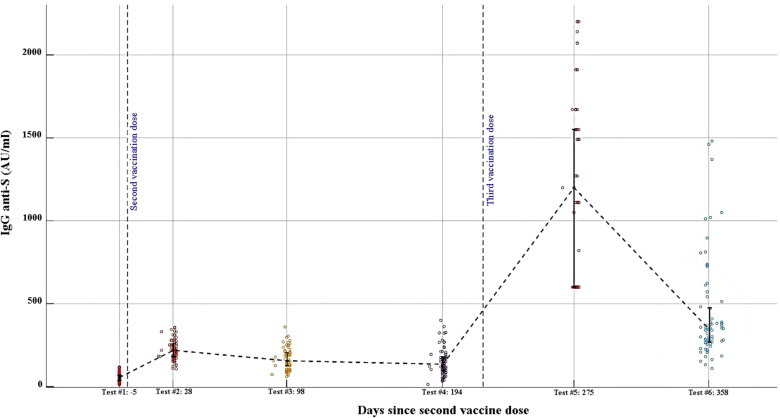
Of 100 HCW who completed the first 4 measurements and were invited to continue the study, 19 denied further participation and 5 were excluded because they were diagnosed with SARS-CoV-2 infection. Thus, a total of Seventy-six HCW had antibody titers measurements from all six time points and were included in the study (Fig. 2 ). Of participants 79 % were women, and median age was 51 (Table 1 ). IgG anti-S titers significantly increased after the third dose, from a median of 135 AU/ml to 1,405 AU/ml (>400 AU/ml in all cases, p < 0.001). The last measurements were taken a median of 359 days after the second dose and 148 after the third. IgG anti-S levels at the last measurement were 432 AU/ml, significantly higher than all measurements before the third dose (p < 0.001) yet lower than the previous measurement (p < 0.001, Fig. 3 ). Levels of Quant anti-S also significantly declined from a median of 16,224 AU/ml (interquartile range 17,129, mean 20,526 ± 11,139 AU/ml) at the early measurement after the third dose to 5,320 AU/ml (interquartile range 5,624, mean 7,073 ± 6,182 AU/ml) at the late measurements (p=<0.0001). HCW who have direct contact with patients had lower Quant anti-S levels at the early measurement than those without patient contact (medians 14,959 AU/ml vs 20,640 AU/ml, respectively, p = 0.03). There were no other associations between antibody levels and baseline characteristics.
Fig. 2.
Study participants flow chart After exclusion of 5 HCW who were diagnosed with SARS-CoV-2 infection and 19 HCW who denied further participation, the number of participants in the final analysis of the study was 76.
Table 1.
Background characteristics of the study population (N = 76).
| Variable | n (%) or Mean ± SD |
|---|---|
|
Sex Male Female |
16 (21.1) 60 (78.9) |
| Age [years] | 49.7 ± 10.4 |
|
Place of Birth Israel America/Europe Asia/Africa |
15 (19.7) 26 (34.2) 35 (46.1) |
|
Chronic use of medications* Yes No |
37 (55.2) 30 (44.8) |
|
Physical activity* Yes No |
14 (22.2) 49 (77.8) |
|
Direct professional contact with patients Yes No |
41 (53.9) 35 (46.1) |
| BMI | 26.6 ± 5.0 (26.3) |
|
Smoker history (active or past) * Yes No |
42 (55.3) 34 (44.7) |
|
Working Night shifts* Yes No |
49 (72.1) 19 (27.9) |
BMI, body mass index. * Self-reported by participants.
Fig. 3.
Dynamics of IgG anti-S levels geometric means over time Vertical bars correspond to 25–75 % percentiles and are crossed by the dashed line at the mean. Each circles represents a single individual measurement. Sera were diluted x20 since anti-S levels were over the assay's threshold of 400AU/ml for all participants in the 5th time point (after the third vaccine dose), and for some participants in the 6th time point. Antibody levels at the last, 6th, measurement were significantly higher than at measurements 1–4, p < 0.001. There was a significant difference in IgG anti-S levels between all consecutive measurements, p < 0.001.
4. Discussion
A third dose of BNT162b2 was associated with a meaningful increase in Ab levels in HCW, which remained significantly higher than previous measurements for up to 5–6 months of follow-up, despite declining. The profound humoral response after a third vaccine dose has been previously described, and correlates with immune protection from COVID-19 [3], [4]. Yet, the durability of this response is currently unknown. More importantly, vaccine effectiveness in terms of preventing transmission, symptomatic disease, and severe COVID-19 from variants of concerns, are debatable. In a recently published huge retrospective study, mRNA-based vaccines were significantly more effective in preventing symptomatic disease caused by the Delta than by the Omicron SARS-CoV-2 variant, although protection from COVID-19 related hospitalizations and mortality remained high [7].
We are unaware of data regarding a negative correlation between patient contact and antibody levels post-vaccination. Such an association should be assessed in future trials.
Study limitations include the small sample size, requirement for dilution of the sera after the third vaccine dose, and the measurement of Quant anti-S only after the third dose. Large reports measured peak antibody titers of 8,000–22,000AU/ml after two doses with the Quant assay, which rapidly decline [8], [9]. Thus, we can safely assume that measurements after the third dose in our study are indeed significantly higher. We chose to exclude HCW with previous SARS-CoV-2 infection, since it is associated with significantly increased humoral response following vaccination. However, universal, routine screening with RT-PCR swabs was not employed, and anti-N titers may decline after infection. Thus, some HCW with undetected infection might have been included. However, we believe that high awareness of HCW to undergo testing in cases of exposure or symptoms, together with routine measurement of anti-N antibodies would probably prevent this. Antibody titers are commonly used as surrogate markers for immune protection, yet the correlation between antibody titers and clinical vaccine efficiency against SARS-CoV-2 infection is imperfect. We didn't measure neutralizing antibodies, which may better represent immune protection from infection. It has been reported that post-vaccination anti-spike antibody titers correlate with neutralizing capacity and with immune protection from COVID-19 [10], [11], [12], including in-vitro neutralization efficiency against the Omicron variant of concern [13]. In addition, real-life studies reported that low antibody levels were associated with breakthrough infections among HCW [2] and dialysis patients [14], [15]. Thus, the fact that high IgG anti-S titers were maintained months after the third vaccine dose, implies that protection from infection and severe disease might also be preserved. A protective threshold of IgG anti-S antibodies, from either infection or symptomatic disease, however, is undefined.
5. Conclusions
A third vaccine dose of BN162b2 led to a profound increase in IgG anti-S antibodies among HCW. Antibody levels decreased afterwards yet remained higher than previous measurement for up to 6 months.
Funding
No funding was received to assist with the preparation of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliakim-Raz N., Leibovici-Weisman Y., Stemmer A., Ness A., Awwad M., Ghantous N., et al. Antibody Titers Before and After a Third Dose of the SARS-CoV-2 BNT162b2 Vaccine in Adults Aged ≥60 Years. JAMA. 2021;326(21):2203. doi: 10.1001/jama.2021.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krutikov M., Palmer T., Tut G., Fuller C., Azmi B., Giddings R., et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev. 2022;3(1):e13–e21. doi: 10.1016/S2666-7568(21)00282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison [published correction appears in Lancet Infect Dis. 2020 Dec;20(12):e298]. Lancet Infect Dis. 2020;20(12):1390-1400. [DOI] [PMC free article] [PubMed]
- 7.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., AlMukdad S., Yassine H.M., Al-Khatib H.A., et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N Engl J Med. 2022;386(19):1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira-Silva J., Reis T., Lopes C., Batista-Silva R., Ribeiro R., Marques G., et al. Humoral response to the SARS-CoV-2 BNT162b2 mRNA vaccine: Real-world data from a large cohort of healthcare workers. Vaccine. 2022;40(4):650–655. doi: 10.1016/j.vaccine.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassilaki N., Gargalionis A.N., Bletsa A., Papamichalopoulos N., Kontou E., Gkika M., et al. Impact of Age and Sex on Antibody Response Following the Second Dose of COVID-19 BNT162b2 mRNA Vaccine in Greek Healthcare Workers. Microorganisms. 2021;9(8):1725. doi: 10.3390/microorganisms9081725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 12.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Beltran WF, St Denis KJ, Hoelzemer Aet al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022: S0092-8674(21)01496-3. [DOI] [PMC free article] [PubMed]
- 14.Anand S., Montez-Rath M.E., Han J., Garcia P., Cadden LinaCel, Hunsader P., et al. SARS-CoV-2 Vaccine Antibody Response and Breakthrough Infection in Patients Receiving Dialysis [published online ahead of print, 2021 Dec 14] Ann Intern Med. 2022;175(3):371–378. doi: 10.7326/M21-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wand O., Nacasch N., Fadeela A., Shashar M., Grupper A., Benchetrit S., et al. Humoral response and breakthrough infections with SARS-CoV-2 B.1.617.2 variant in vaccinated maintenance hemodialysis patients [published online ahead of print, 2022 Feb 17] J Nephrol. 2022;35(5):1479–1487. doi: 10.1007/s40620-022-01245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.