Abstract
With the emergence of the severe acute respiratory syndrome 2 (SARS-CoV-2) B.1.1.529/BA.1 (Omicron) variant in early 2022, Israel began vaccinating individuals 6o years of age or older with a fourth BNT162b2 vaccine. While the decision was based on little experimental data, longer follow-up showed clinical effectiveness of the fourth dose with reduction in the number of severely affected individuals. However, the immune response to fourth vaccine dose in this age group was not yet characterized, and little is known about the immunogenicity of repeated vaccine dosing in this age group. We therefore aimed to evaluate the humoral and cellular immune response pre- and 3-week post- the fourth vaccine dose in patients age 60 years or older. For this purpose, blood samples were collected from donors age 60 years or older, all received their 3rd vaccine dose 5 months prior. Serum samples were evaluated for the presence of anti-Spike protein (anti-S) antibodies (N = 133), and peripheral blood mononuclear cells (PBMCs) were evaluated by flow cytometry for their ability to respond to the SARS-CoV-2 wild type Spike-glycoprotein peptide mix, Membrane-glycoprotein (M) peptide mix and to the mutated Spike-regions of the Omicron variant (N = 34). Three weeks after the fourth vaccine dose, 24 out of 34 donors (70.5%) showed significant increase in the number of cells responding to the wild type S-peptide mix. Of note, out of 34 donors, 11 donors (32.3%) had pre-boost anti-M T-cell response, none of which had history of confirmed COVID-19, suggesting possible asymptomatic exposure. Interestingly, in M non-responding individuals, no statistically significant increase in the cellular response was observed following stimulation with omicron S-mutated regions. While there are limited data regarding the longevity of the observed response, our results are in accordance with the described clinical efficacy, provide mechanistic evidence to support it and argue against vaccine-induced or age-related immunosenescence.
Keywords: COVID-19, Fourth-dose, Second-booster, BNT162b2 age-related immunosenescence, Age-related immunosenescence
1. Introduction
Since the emergence of the COVID-19 pandemic in early 2020, the world has experienced several SARS-CoV-2 waves with surges in the number of new COVID-19 cases, and spikes in hospitalization rates. While the future of the pandemic and the burden of novel variants yet to appear are still not known, it seems that the combination of large-scale vaccination and high rates of exposure to previous variants, might increase population immunity and limit the severity of future waves. Despite that, there are several populations which might still be at risk for developing more severe disease, including immunocompromised patients and elderly individuals. The older age group is of special interest as it was shown that COVID-19 fatality risk directly correlates with age [1]. While this age-related mortality could be explained by higher incidence of background comorbidities [2], [3], there are other possible immune related mechanisms to explain increased mortality risk in older individuals. As such, it was shown that the incidence of neutralizing anti Type-I interferons autoantibodies increases with age, and that their presence confers a significant risk for severe COVID-19 and mortality [4], [5], [6]. In addition, it possible that age related immunosenescence, characterized mainly by reduced B and T cell response, results in impaired response to vaccines and prevents adequate immune protection despite repeated dosing. Specifically, it has been shown that B cells of elderly individuals have reduced ability to raise and sustain an effective antibody response against neo-antigens [7], [8], and that T cells show impaired ability to activate, proliferate and differentiate [8], [9]. Although such abnormal responses against different non-COVID-19 vaccines have been documented [10], [11], [7], [12], data on the immunogenicity of anti-SARS-CoV-2 vaccines in elderly is still accumulating, with some reports suggesting waning immunity [13] or reduced humoral and cellular immune response compared to young individuals [14], [15], while other showing long lasting immunogenicity [16]. Therefore, questions could be raised regarding the immunogenicity of repeated boosting.
With the emergence of the SARS-CoV-2 B.1.1.529/BA.1 (Omicron) variant in early 2022, Israel began offering a fourth vaccine dose (2nd booster) to individuals age 60 year or older, who received their 3rd vaccine dose at least 3 months prior. This approach of using the same vaccine to boost against a novel emerging variant was accepted with some skepticism. However, real world data showed at least a short-term benefit of protecting against severe disease and reducing mortality [17], [18], [19], [20], [21]. In this study we aimed to evaluate the immune response of individuals age 60 years and older, following a fourth BNT162b2 vaccine dose, focusing on the cellular immune response to different SARS-CoV-2 peptide mixes.
2. Methods
2.1. Study subjects
This study was approved by the Institutional Review Board of the Tel-Aviv Sourasky Medical Center (IRB #0576-21-TLV). After providing written informed consent, health care workers and hospital retirees age 60 years or older, who received 3 vaccine doses and did not have COVID-19, were offered the opportunity to join the study. Participating donors received the fourth dose (2nd booster) of the mRNA-based Pfizer-BioNTech COVID19 vaccine 5 months after the third vaccine dose (third dose was administered at the beginning of August 2021, and fourth dose in early January 2022). For cellular response (N = 34), blood samples were collected at two time points: immediately prior to the fourth vaccine dose, and three weeks after. For humoral response, serum samples were collected at 3 time points: two months prior to the fourth vaccine dose (and 3 months after the third dose)(N = 84), immediately prior to the fourth vaccine dose (N = 131), and three weeks after (N = 133). At all three time points, interested individuals (3-time vaccinated, age > 60 and COVID-19 naïve) were voluntarily enrolled. The decision to collect samples two months prior was made without knowing that a fourth dose will be recommended.
2.2. PBMC isolation and stimulation
Peripheral Blood Mononuclear Cells (PBMCs) were isolated using Ficoll gradient density.
Following isolation, cells were stored at −80 °C for later use, including flowcytometry-based peptide-induced intracellular cytokine staining.
2.3. Evaluation of humoral response
The presence of anti-SARS-CoV-2 IgG antibodies was evaluated by using a commercial automated SARS-CoV-2 IgG assays. For the detection of anti-Spike glycoprotein (anti-S) antibodies, we used an automated qualitative and semi-quantitative indirect 2‐step sandwich chemiluminescent immunoassay (ADVIA Centaur® SARS-CoV-2 IgG (sCOVG) assay, Siemens Healthcare Diagnostics Inc, Tarrytown, NY, USA). The assay detects antibodies directed against the receptor-binding domain (RBD) of the SARS-CoV-2 S1 spike antigen. Results are reported in Index Values or U/mL, with a level of > 1.00U/mL considered positive and a maximum reported level of 100U/mL. For statistical analysis, samples with levels of > 100U/mL were considered as levels of 100U/mL.
A second chemiluminescent microparticle immunoassay (CMIA) was used for qualitative detection of anti-nucleocapsid (anti-N) antibodies (SARS-CoV-2 IgG, Cat# 6R86, Abbott, Ireland). Results were provided in relative light units (RLU) and anti-N antibody level of above 1.4RLU considered positive. This assay was used only for individuals who showed cellular reactivity against the membrane-glycoprotein (M) pooled-peptide mix, as will be explained in the results section.
2.4. Evaluation of cellular response
T-cell response was assessed by stimulating donor PBMCs with pooled peptide mixes in the presence of protein transport inhibitor, followed by staining for the activation marker CD154 and intracellular cytokines (TNFα and IFNγ). For this purpose, we used a SARS-CoV-2 T-Cell Analysis Kits for human PBMCs (Cat# 130-128-156, Miltenyi Biotec, Germany). Assay was performed according to manufacturer instructions. Briefly, donor PBMCs were plated in a 96-well plate at a concentration of 0.5X106 PBMCs/100 μL and incubated at 37 °C and 5% CO2 with 2 μL of different peptide mixes, including pooled Wuhan wild-type (WT) Spike glycoprotein (S)-peptide mix (Cat# 130–127-951, Miltenyi Biotec, Germany) covering the whole protein sequence of the S-glycoprotein, pooled Omicron-specific S-peptide mix (Cat# 130–129-928, Miltenyi Biotec, Germany) covering selectively only the mutated regions of the Omicron S-glycoprotein, and pooled M−peptide mix (Cat# 130–126-702, Miltenyi Biotec, Germany) covering the complete sequence of the membrane glycoprotein (M). CytoStim™ reagent was used for positive control and 10% DMSO in sterile water for negative control. After two hours, Brefeldin A was added to each well and cells were incubated for additional 4 h. Cells were then stained with viability dye, followed by fixation, permeabilization and staining for surface markers (CD3, CD20, CD14, CD4, CD8, CD154) and intracellular cytokines (TNFα and IFNγ). Following staining, samples were acquired using BD FACSCanto II, and 20,000 CD4+ events were collected for each sample.
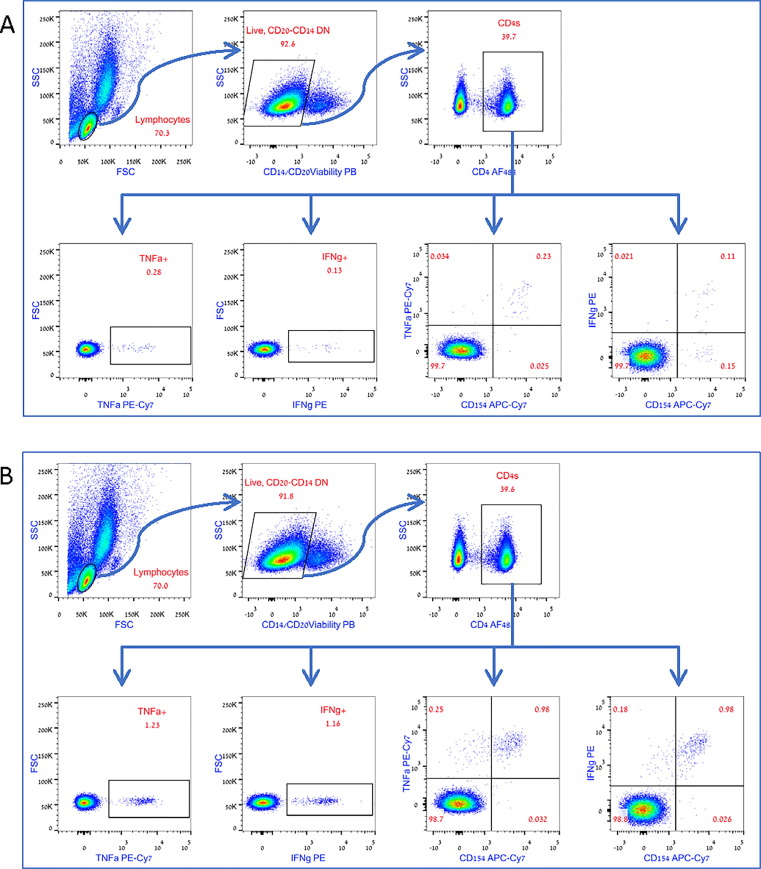
Analysis was performed on gated CD4+ T-cells, evaluating the absolute numbers of activated INFγ+ CD4s, TNFα+ CD4s, INFγ and TNFα double positive CD4s, INFγ and CD154 double positive CD4s or TNFα and CD154 double positive CD4s (gating strategy is shown in Fig. 2A). Recorded numbers were normalized for 1X106 CD4+ T-cells.
Fig. 2.
Gating Strategy. The CD4+ population was gated based on positive staining for CD4, out of the live, CD14 negative, CD20 negative lymphocytes. Percent of intracellularly stained, cytokine-positive or cytokine-CD154 double positive cells was gated out of the CD4+ population. The absolute numbers of the recorded TNFα, IFNγ, or cytokine-CD154 double positive cells, was used to calculated the number of positive events per 1X106 CD4+ T-cells. A. Representative S-WT stimulated analysis. B. Representative M stimulated analysis. The same cell sample, from a presumed asymptomatic COVID-19 experienced patient, was used in both conditions. An enhanced response to M−peptide mix can be observed.
For each donor, samples were evaluated pre and 3-week post vaccine. In order to calculate the actual response rate, the calculated number of positive events (per 1X106 CD4+ T-cells) in the unstimulated negative controls was deducted from the calculated number of events (per 1X106 CD4+ T-cells) in the peptide-stimulated samples, as shown in the following formula:
(Calculated numbers are shown in Supp Table 1A-C).
In order to overcome possible variations between runs, the assay was performed at the same time on frozen cells collected at the different time points. For the purpose of this study, an absolute number of above 250 responding CD4s per 1X106 CD4s, and a fold increase of 1.5 or above in the number of responding cells in pre-booster samples, were arbitrary considered as the cutoff to define cellular responsiveness to the fourth vaccine dose.
2.5. Statistical analysis
Continuous variables were described as the mean, median, standard deviation and range of values, as applicable. Antibody titers were compared between patient groups using unpaired t test. Paired analysis of cellular response was performed using Wilcoxon signed-rank tests. A two-sided p value of < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism, version 9.4.0.
3. Results
3.1. Patient characteristics
The average age of the 133 donors who provided serum samples for serology was 69.79 (std ± 6.34) with male to female ratio of 0.6 (50 males and 83 females). Out of the 133, 39 (29.3%) were between the ages 60–65, 37 (27.8%) between the ages 66–70, 38 (28.6%) between the ages 71–75, 12 (9%) between 76 and 80 and 7 (5.3%) between the ages 81–92.
Of the 34 donors who provided blood samples for cellular response, the average age was 70.67 (std ± 6.67) with male to female ratio of 1 (17 males and 17 females). Out of the 34, 8 (23.5%) were between the ages 60–65, 9 (26.5%) between the ages 66–70, 10 (29.4%) between the ages 71–75, 5 (14.7%) between 76 and 80 and 2 (5.9%) between the ages 81–92.
None of the donors was receiving immunosuppressive drugs, and all donors reported being COVID-19 naïve and never tested positive for SARS-CoV-2.
3.2. Humoral immune response
Serum samples were evaluated for the presence of anti-S antibodies at 3 time points: two months prior to the fourth vaccine dose (and 3 months after the third dose)(N = 84), day of administration of the fourth vaccine dose (N = 131), and 3 weeks after it (N = 133). While all tested individuals, at all three time points, were considered positive (value of > 1U/mL), comparing the measured titers at the 3 timepoints showed a clear waning of the antibody titers between the first and the second time points, with a drop from an average value of 80.92 ± 32.42U/mL two month prior to the fourth dose, to an average value of 50.77 ± 34.83U/mL on the day of the fourth dose (p < 0.0001) (Fig. 1 A). In accordance, the percentage of the individuals with values of > 100U/mL showed similar decline from 67.86% at the first time point to 22.13% at the second timepoint (Fig. 1B). However, the 4th dose restored high antibody titers with an average value of 97.90 ± 9.2U/mL (vs 50.77 ± 34.83U/mL at the second timepoint, p < 0.0001; and vs 80.92 ± 32.42U/mL at the first time point, p < 0.0001) (Fig. 1A), with 93.2% of the samples showing a titer of 100U/mL (Fig. 1B). These results show the ability of a second booster to induced at least a short-term increase in anti-S antibodies, arguing against the possibility that repeated boosting could induce immune senescence or anergy.
Fig. 1.
Humoral response. Anti-S IgG titers showing a significant decline over time, with a clear boosting effect of the fourth vaccine doe. A. Changes in antibody titers over time, starting at 3 months prior to the fourth dose, on the day of the fourth dose and 3 weeks after. Lines are showing trends for each individual. B. Bar plot showing the percent of patients with a maximal anti-S IgG titer of 100U/mL. **** p < 0.0001.
3.3. Cellular immune response
Cellular reactivity was evaluated using flow-cytometry, by stimulating donor cells with peptide mixes, and staining for surface CD154 and intracellular INFγ and TNFα. Cells were stimulated with one of three peptide mixes: 1) Pooled Wuhan wild-type S-glycoprotein (WT-S) peptide mix. This mix was used to evaluate the cellular response against the wild type strain. 2) Pooled Omicron (BA.1 SARS-CoV-2 B.1.1.529 lineage)-specific S-peptide mix, covering selectively only the mutated regions of the Omicron S-glycoprotein. This mix was used to evaluate the ability of the vaccine to induce cell reactivity against Omicron variant. Of note, since this mix covers only the mutated regions of the variant, the induced cellular response is expected to be lower and cannot be directly compared to the response to the WT-S peptide mix which covers the whole S-glycoprotein. 3) Pooled M−peptide mix. This mix was used to evaluate possible prior exposure to the virus. Since the vaccine includes the RNA sequence for the S-glycoprotein only, reactivity against the M−protein suggests prior exposure to the virus and has the potential to identify symptomatic or asymptomatic convalescent individuals.
Out of the 34 donors, 24 (70.5%) responded to WT-S peptide pool in all parameters (INFγ, TNFα and CD154), with a significant increase in the average number of INFγ+ CD4s (from 619.11 ± 598.8 to 1315.88 ± 995.72, p < 0.0001), TNFα+ CD4s (from 1018.23 ± 915.79 to 2080.29 ± 1510.14, p < 0.0001), INFγ and TNFα double positive CD4s (from 544.41 ± 579.15 to 1183.52 ± 954.3, p < 0.0001), INFγ and CD154 double positive CD4s (from 542.35 ± 479.1 to 1189.41 ± 808.0, p < 0.0001), and TNFα and CD154 double positive CD4s (from 908.52 ± 751.69 to 1889.11 ± 1294.4, p < 0.0001) (Fig. 3 A).
Fig. 3.
WT-S and Omi-S Cellular response. Evaluation of cellular response shows statistically significant increase in the number CD4+ T-cells responding to WT-S peptide mix stimulation, 3 weeks after the fourth vaccine dose (A). In addition, evaluation of the response to the Omicron variant, showed some reactivity against Omicron-specific peptides, with statistically significant increase in most parameters, including the number of TNFa/CD154 double positive CD4s, INFg/TNFa double positive CD4s, INFg+ CD4s and INFg/CD154 double positive CD4s, but only a trend toward statistically significant increase in the number of TNFa+ CD4s. (B)(N = 34). ns – nonsignificant; * p < 0.05; ** p < 0.01; *** p < 0.001.
When evaluating the response to the Omicron variant, CD4+ T-cells showed some reactivity against Omicron-specific peptides, with statistically significant increase in most parameters, including the number of TNFα/CD154 double positive CD4s (from 184 ± 221.3 to 291.47 ± 259.40, p = 0.0015), INFγ/TNFα double positive CD4s (from 114.06 ± 178.32 to 197.65 ± 192.08, p = 0.0199), INFγ+ CD4s (from 152.5 ± 185.59 to 233.53 ± 212.19, p = 0.0457) and INFγ/CD154 double positive CD4s (from 122.81 ± 170.39 to 187.05 ± 188.6, p = 0.008), but only a trend toward statistically significant increase in the number of TNFα+ CD4s only (from 180 ± 246.73 to 352.35 ± 294.76, p = 0.084)(Fig. 3B).
It should be noted that while the number of responding CD4s was significantly lower following stimulation with Omicron-specific peptide compared to stimulation with WT-S peptide pool, these assays cannot be directly compared, as the WT-S peptide pool covers the whole S-glycoprotein, while the Omicron-specific pool covers only the Omicron-unique protein sequences.
Finally, we did not see statistical correlation between age or gender and the number of responding cells for either WT-S or Omicron-S stimulation.
3.4. M−positive individuals
Our previous experience evaluating recovered individuals showed that the cellular response to M−peptide mix is more robust compared with S-peptide mix response [22]. Therefore, we assume that M−responding donors can be considered convalescent individuals, even in the absence of clear history suggesting COVID-19. Interestingly, 11 out of 34 donors (32.4%) showed significant anti-M cellular response.
As expected, and since the mRNA vaccine encodes for the S-glycoprotein only, within those 11 M−responding individuals, we did not see significant boosting effect of the anti-M cellular response by the fourth vaccine, and the 11 showed similar M−stimulated pre- and post-vaccine average numbers of INFγ+ CD4s (5296.36 ± 4278.07 pre, 7435.45 ± 5587.2 post, p = 0.0742), TNFα+ CD4s (5618.18 ± 4475.54 pre, 7802.73 ± 5587.21 post, p = 0.0593), INFγ/TNFα double positive CD4s (5160 ± 4255.62 pre, 7318.18 ± 5538.07 post, p = 0.0623), INFγ/CD154 double positive CD4s (4197.27 ± 3212.73 pre, 6035.45 ± 5417.90 post, p = 0.045), and TNFα/CD154 double positive CD4s (4199.09 ± 3137.58 pre, 6118.19 ± 4528.06, p = 0.0508) (Fig. 4 A).
Fig. 4.
M Cellular response. Analysis of the 11 donors who responded to stimulation with M−peptide mix showed marginal statistically significant increase in the number CD4+ T cells responding to M−peptide mix stimulation, 3 weeks after the fourth vaccine dose (N = 11)(A). Despite that, the intensity of the cellular response to M−peptide mix was much higher compared to the WT-S induced response, even when comparing the pre-vaccine M−induced T-cell response (N = 11) with the post-vaccination WT-S response (N = 34) (B). ns – non-significant; * p < 0.05; **** p < 0.0001.
However, these numbers were significantly higher compared with those observed for WT-S stimulated cells, even when comparing the pre-booster anti-M cellular response with the enhanced post-booster anti-WT-S cellular response. As such, the average numbers of INFγ+ CD4s was 1315.88 ± 995.72 for WT-S and 7435.45 ± 5587.2 for M (p < 0.0001); 2080.29 ± 1510.14 vs 7802.73 ± 5587.21 (p < 0.0001) for TNFα+ CD4s; 1138.53 ± 954.30 vs 7318.18 ± 5538.07 (p < 0.0001) for INFγ/TNFα double positive CD4s; 1189.41 ± 808.0 vs 6035.45 ± 5417.90 (p < 0.0001) for INFγ/CD154 double positive CD4s, and 1889.11 ± 1294.4 vs 6118.19 ± 4528.06 (p < 0.0001) for TNFα/CD154 double positive CD4s (Fig. 4B and Fig. 2B).
Of note, following these results, all M−responding donors were questioned again about previous SARS-CoV-2 exposure. All denied previous exposure or history of COVID-19, although some described remote respiratory illnesses in the previous year. This was completed by measuring anti-N antibody titers, and all had a level of < 0.2RLU (level of above 1.4RLU considered positive). Together, these results suggest that significant percent of participating donors could be asymptomatic convalescents, and that their infection occurred long prior, to explain declining anti-N antibodies [23], [24].
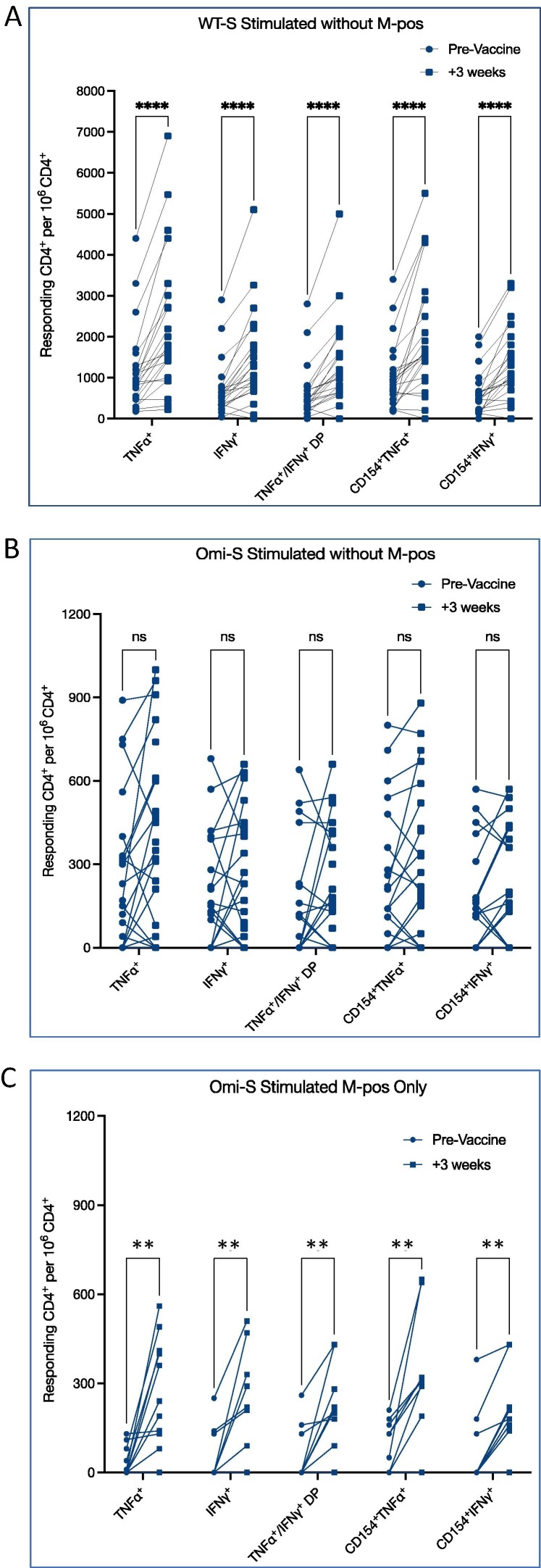
Due the high rate of M−responsive individuals, we re-analyzed the WT-S and Omicron-specific cellular response, in order to see whether the observed pre- and post-vaccine differences were affected by responding samples. Interestingly, even after excluding the 11 M−responding individuals, there was still a clear boosting effect on the anti-WT-S cellular response in all parameters (INFγ, TNFα and CD154), with a significant increase in the average number of INFγ+ CD4s (from 711.74 ± 665.44 to 1450.87 ± 1126.61, p < 0.0001), TNFα+ CD4s (from 1170.0 ± 1020.94 to 2270.87 ± 1700.57, p < 0.0001), INFγ and TNFα double positive CD4s (from 620.0 ± 652.87 to 1304.35 ± 1082.21, p < 0.0001), INFγ and CD154 double positive CD4s (from 585.21 ± 531.39 to 1287.39 ± 905.21, p < 0.0001), and TNFα and CD154 double positive CD4s (from 1001.74 ± 827.54 to 2032.609 ± 1453.42, p < 0.0001) (Fig. 5 A).
Fig. 5.
WT-S and Omi-S Cellular response, excluding M−responsive individuals. Evaluation of cellular response shows statistically significant increase in the number CD4+ T-cells responding to WT-S peptide mix stimulation, 3 weeks after the fourth vaccine dose, even after excluding M−responsive individuals (N = 23)(A). In contrast, the enhanced Omicron specific response was not seen when excluding M−responsive samples (B) and was limited to M−responding individuals (N = 11)(C). ns – nonsignificant; ** p < 0.01; **** p < 0.0001.
However, the Omicron-specific cellular response was limited to M−responding individuals only. As such, there was no statistically significant response to Omicron-S in the 23 M−non−responding individuals with and average number of INFγ+ CD4s (207.62 ± 200.97 pre, vs 249.13 ± 226.61 post, p = 0.54), TNFα+ CD4s (256.67 ± 274.18 pre, vs 390.435 ± 332.641 post, p = 0.99), INFγ and TNFα double positive CD4s (147.62 ± 204.25 pre, vs 203.48 ± 210.23 post, p = 0.37), INFγ and CD154 double positive CD4s (154.29 ± 185.62 pre, vs 191.74 ± 207.69 post, p = 0.28), and TNFα and CD154 double positive CD4s (240.0 ± 250.80 pre, vs 300.0 ± 278.16 post, p = 0.13) (Fig. 5B).
In contrast, when analyzing the 11 M−responding only, we did see a statistically significant anti Omicron-S response, with an increase in the average number of INFγ+ CD4s (from 47.27 ± 86.26 to 200.91 ± 184.04, p = 0.0078), TNFα+ CD4s (from 33.64 ± 49.65 to 272.73 ± 181.61, p = 0.0039), INFγ and TNFα double positive CD4s (from 50.0 ± 90.89 to 185.45 ± 155.84, p = 0.0078), INFγ and CD154 double positive CD4s (from 62.73 ± 122.65 to 177.27 ± 150.54, p = 0.0078), and TNFα and CD154 double positive CD4s (78.18 ± 84.36 to 273.64 ± 226.55, p = 0.0078) (Fig. 5C).
This differential response between M−responding and M−non−responding individuals, could theoretically suggest that pre-exposed individuals can further benefit from a booster vaccine dose and develop cellular response against overlapping S-variants.
4. Discussion
In this study we focused on patient age 60 years and older, and evaluated their immune response to the fourth vaccine dose. This population is of special interest as there were questions regarding potential age-related immunosenescence and efficacy of repeated dosing. While real-world data showed significant short-term efficacy of the second booster in preventing severe disease [17], [18], [19], [20], [21], there is limited data regarding the induced enhanced immunogenicity, especially when focusing on the cellular immune response [25].
Similar to other studies [17], [25] our results show significant increase in the level of IgG antibodies directed against the wild-type S1 RBD, and restoration of pre-vaccine anti-S antibody titers. In addition, we show significant increase in the number of WT-specific CD4+ T-cells, three weeks after the fourth vaccine dose. Despite that, our T-cell response evaluation did not show an increase in the number of CD4+ T-cells directed against the mutated regions of the Omicron (BA.1 SARS-CoV-2B.1.1.529 lineage) S-glycoprotein in all individuals, but only in those who showed T-cell reactivity against M−peptide mix. As we did not compare this response to the response against a peptide mix covering the same limited region of the Wuhan WT-S (we used a peptide mix covering the whole WT-S amino-acid sequence), we do not know what part these specific regions played in the enhanced post vaccine response. However, the absence of increase in the number of Omicron-specific CD4+ T-cells suggests that using the same mRNA vaccine sequence did not induce additional variant-specific T-cell reactivity in COVID-19 naïve donors without anti-M cellular response.
It is difficult to know whether the fourth vaccine dose was able to enhance cell-mediated reactivity against the Omicron variant or against other variant of concern. However, in contrast to vaccine-induced anti-SARS-CoV-2 antibodies, which were shown to have reduced neutralizing activity against more recent variants [26], [27], T-cells reactivity is directed against processed viral peptides presented upon MHC molecules [28], [29], and can therefore be less affected by mutations and by mild structural changes in viral antigens [30]. In other words, as the neutralizing antibody activity depends on their ability to bind the S1-receptor binding domain (RBD) and block RBD:ACE2 interaction, changes in the RBD are predicted to negatively affect this activity. In contracts, since T-cell activity is directed against complexes of MHC and processed SARS-CoV-2 peptides presented on already infected cells, this reactivity should be less affected by minor changes, and is not required for virus neutralization. Therefore, the observed increase in the number of CD4s responding to WT-S peptides following a fourth vaccine dose, could explain the observed clinical efficacy, even in the presence of diminished neutralizing antibody activity. Our observation of an increase in the number of Omicron-S specific T-cells only in M−responding individuals, is in line with this theory.
As mentioned above, our T-cell assay identified significant T-cell reactivity against M−peptide mix in 11/34 donors (32.4%). Since the used mRNA vaccine encodes only for the SARS-CoV-2 S-glycoprotein, reactivity against M−peptide mix suggests prior exposure. Of note, all M−positive individuals denied history of COVID-19 and were tested negative for the presence of anti-N antibodies. While in theory such reactivity could be due to cross reactivity between circulating ‘‘common cold’’ coronaviruses and SARS-CoV-2 [28], the intensity of the observed response argues against it. Therefore, these results could support the possibility of high-rate remote asymptomatic SARS-CoV-2 infection, even in elderly individuals.
Beyond that, and similar to previous studies showing a robust post COVID-19 anti-M cellular response [28], [31], evaluation of the cellular response showed that the number of M responding CD4s was significantly higher than the number of WT-S responding CD4s. This robust response cannot be explained by the size of the glycoprotein, as the Membrane glycoprotein (222 amino acids in length) is much smaller than the Spike glycoprotein (1273 amino acids in length) [32]. While it might be explained by higher intracellular level of the translated viral proteins in infected cells, M−epitopes have been shown to induce robust cellular response [33], and the observed enhanced response could have an implication for future vaccine development to include additional viral proteins.
Our study has several limitations including small size and short-term post vaccine evaluation. In addition, since reduced neutralizing activity against Omicron was already shown before, we chose to focus on the cellular response (including anti-Omicron cellular response), while evaluating only the general anti-WT-S humoral response. Thus, our humoral response evaluation is limited in several ways. The serologic assay we used tested only antibody binding to the RBD domain and not neutralization. In addition, a level of > 100U/mL describes an analytical limit of the assay and should not be translated to increased protection. Finally, while most likely increasing the validity of our findings, an upper limit of 100U/mL, and the fact that for statistical analysis level of above 100U/mL was considered as 100, probably resulted in lower means of calculated anti-S antibody titers than the actual ones.
Despite that, our study clearly shows the ability of the fourth vaccine to further boost humoral and cellular reactivity, arguing against age-related immunosenescence or theoretical risk of anergy induced by repeated dosing. Whether the same vaccine should be used with the emergence of novel variants of concern, how often should the vaccine mRNA sequence be updated, and whether future vaccines should include additional viral targets such as M−protein, are only several questions awaiting to be answered.
5. Conclusions
Evaluation of the humoral and cellular immune response shows that the fourth vaccine dose is able to induce significant response in elderly individuals, and there is no short-term evidence for age-related or repeated-dosing induced immunosenescence or anergy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank all participants for their enthusiasm and involvement in the study. The authors have no relevant financial or non-financial interests to disclose.
Funding
D.H is funded by the Alrov Foundation and the Joint Tel-Aviv Sourasky Medical Center and The Weizmann Institute of Science Research Grant
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.12.035.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.O'Driscoll M., Ribeiro Dos Santos G., Wang L., et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 2.Dadras O., SeyedAlinaghi S., Karimi A., et al. COVID-19 mortality and its predictors in the elderly: a systematic review. Health Sci Rep. 2022;5(3):e657. doi: 10.1002/hsr2.657. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Jung C., Flaatten H., Fjolner J., et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021;25(1):149–221. doi: 10.1186/s13054-021-03551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370(6515). doi: 10.1126/science.abd4585. Epub 2020 Sep 2doi: eabd4585 [pii]. [DOI] [PMC free article] [PubMed]
- 5.Bastard P, Gervais A, Le Voyer T, et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol. 2021;6(62). doi: 10.1126/sciimmunol.abl4340. doi: eabl4340 [pii]. [DOI] [PMC free article] [PubMed]
- 6.Manry J., Bastard P., Gervais A., et al. The risk of COVID-19 death is much greater and age-dependent with type I IFN autoantibodies. Res Sq. 2022 doi: 10.1073/pnas.2200413119. rs.3.rs-1225906 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner A., Garner-Spitzer E., Jasinska J., et al. Age-related differences in humoral and cellular immune responses after primary immunisation: Indications for stratified vaccination schedules. Sci Rep. 2018;8(1):9825–10018. doi: 10.1038/s41598-018-28111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustafson C.E., Kim C., Weyand C.M., Goronzy J.J. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145(5):1309–1321. doi: 10.1016/j.jaci.2020.03.017. S0091-6749(20)30421-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boraschi D, Aguado MT, Dutel C, et al. The gracefully aging immune system. Sci Transl Med 2013;5(185):185ps8. doi: 10.1126/scitranslmed.3005624 [doi]. [DOI] [PubMed]
- 10.Hainz U., Jenewein B., Asch E., Pfeiffer K.P., Berger P., Grubeck-Loebenstein B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23(25):3232–3235. doi: 10.1016/j.vaccine.2005.01.085. S0264-410X(05)00124-6 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Weinberger B., Keller M., Fischer K.H., et al. Decreased antibody titers and booster responses in tick-borne encephalitis vaccinees aged 50–90 years. Vaccine. 2010;28(20):3511–3515. doi: 10.1016/j.vaccine.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg A., Lazar A.A., Zerbe G.O., et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010;201(7):1024–1030. doi: 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Alos L., Armenteros J.J.A., Madsen J.R., et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat Commun. 2022;13(1):1614–1622. doi: 10.1038/s41467-022-29225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collier D.A., Ferreira I.A.T.M., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Tong Y., Li D., Li J., Li Y. The impact of age difference on the efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.758294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HK, Knabl L, Knabl L, et al. Robust immune response to the BNT162b mRNA vaccine in an elderly population vaccinated 15 months after recovery from COVID-19. medRxiv. 2021. doi: 2021.09.08.21263284 [pii].
- 17.Regev-Yochay G., Gonen T., Gilboa M., et al. Efficacy of a fourth dose of covid-19 mRNA vaccine against omicron. N Engl J Med. 2022;386(14):1377–1380. doi: 10.1056/NEJMc2202542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection by a fourth dose of BNT162b2 against omicron in israel. N Engl J Med. 2022;386(18):1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbel R, Sergienko R, Friger M, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. [DOI] [PubMed]
- 20.Gazit S., Saciuk Y., Perez G., Peretz A., Pitzer V.E., Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in israel: retrospective, test negative, case-control study. BMJ. 2022;377:e071113–e72022. doi: 10.1136/bmj-2022-071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magen O., Waxman J.G., Makov-Assif M., et al. Fourth dose of BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagin D., Freund T., Navon M., et al. Immunogenicity of pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148(3):739–749. doi: 10.1016/j.jaci.2021.05.029. S0091-6749(21)00887-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krutikov M., Palmer T., Tut G., et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in england. Lancet Healthy longevity. 2022;3(1):e13–e21. doi: 10.1016/S2666-7568(21)00282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Elslande J., Oyaert M., Ailliet S., et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;136 doi: 10.1016/j.jcv.2021.104765. S1386-6532(21)00032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro A.P.S., Feng S., Janani L., et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): A multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00271-7. S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edara V, Manning KE, Ellis M, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. bioRxiv: the preprint server for biology. 2021. [DOI] [PMC free article] [PubMed]
- 27.Sievers BL, Chakraborty S, Xue Y, et al. Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against beta and omicron pseudoviruses. Sci Transl Med 2022;14(634):eabn7842. [DOI] [PMC free article] [PubMed]
- 28.Grifoni A., Weiskopf D., Ramirez S.I., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 30.Jordan S.C., Shin B., Gadsden T.M., et al. T cell immune responses to SARS-CoV-2 and variants of concern (alpha and delta) in infected and vaccinated individuals. Cell Mol Immunol. 2021;18(11):2554–2556. doi: 10.1038/s41423-021-00767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thieme C.J., Anft M., Paniskaki K., et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorkhali R., Koirala P., Rijal S., Mainali A., Baral A., Bhattarai H.K. Structure and function of major SARS-CoV-2 and SARS-CoV proteins. Bioinform Biol Insights. 2021;15 doi: 10.1177/11779322211025876. 11779322211025876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heide J., Schulte S., Kohsar M., et al. Broadly directed SARS-CoV-2-specific CD4 T cell response includes frequently detected peptide specificities within the membrane and nucleoprotein in patients with acute and resolved COVID-19. PLoS Pathog. 2021;17(9):e1009842. doi: 10.1371/journal.ppat.1009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.