Abstract
An unstable chromosomal element encoding multiple antibiotic resistance in Shigella flexneri serotype 2a was found to include sequences homologous to the csg genes encoding curli in Escherichia coli and Salmonella enterica serovar Typhimurium. As curli have been implicated in the virulence of serovar Typhimurium, we investigated the csg loci in all four species of Shigella. DNA sequencing and PCR analysis showed that the csg loci of a wide range of Shigella strains, of diverse serotypes and different geographical distributions, were almost universally disrupted by deletions or insertions, indicating the existence of a strong selective pressure against the expression of curli. Strains of enteroinvasive E. coli (EIEC), which share virulence traits with Shigella spp. and cause similar diseases in humans, also possessed insertions or deletions in the csg locus or were otherwise unable to produce curli. Since the production of curli is a widespread trait in environmental isolates of E. coli, our results suggest that genetic lesions that abolish curli production in the closely related genus Shigella and in EIEC are pathoadaptive mutations.
Bacillary dysentery is a severe diarrheal disease affecting hundreds of millions of people worldwide, leading to more than 500,000 deaths annually (11). The disease is caused by four bacterial species comprising the genus Shigella, i.e., Shigella flexneri, S. dysenteriae, S. sonnei, and S. boydii. Shigella spp. are transmitted to their hosts via the fecal-oral route and infect the colonic epithelium. Subsequent cell destruction, inflammation, and ulceration of the colon are responsible for the bloody, mucoid diarrhea that is characteristic of the disease. In recent years much has been learned about the sophisticated virulence mechanisms that allow Shigella to invade epithelial cells and spread to neighboring cells (5). However, nothing is known about the first step in the infection process, colonization of the host.
Bacterial colonization of the host intestine is generally mediated by fimbrial adhesins (2, 3, 8, 10). However, it is not clear what role fimbriae play in the virulence of Shigella (22). Over the last decade, Escherichia coli and Salmonella spp. have been found to express a surface structure termed thin aggregative fimbriae or curli (14, 20, 21). In E. coli, curli mediate the formation of biofilms on inert surfaces (26). However, in Salmonella enterica serovar Typhimurium, curli mediate bacterial attachment to mouse intestinal cells in vitro (25), and the expression of curli at 37°C is a phase-variable characteristic that is essential for full virulence in mice (24). These findings demonstrate that curli probably have a role in the colonization of the mouse intestine by serovar Typhimurium. Furthermore, curli are capable of mediating bacterial binding to a wide variety of tissues (15), cell matrix proteins, and plasma proteins (13, 14) and may therefore have additional roles in virulence.
During investigations of a deletable chromosomal element encoding multiple antibiotic resistance in S. flexneri serotype 2a (17, 18), members of our group discovered a locus with high sequence similarity to the csg gene clusters encoding curli in E. coli and serovar Typhimurium. This preliminary finding prompted us to investigate the presence of csg loci in a variety of Shigella strains.
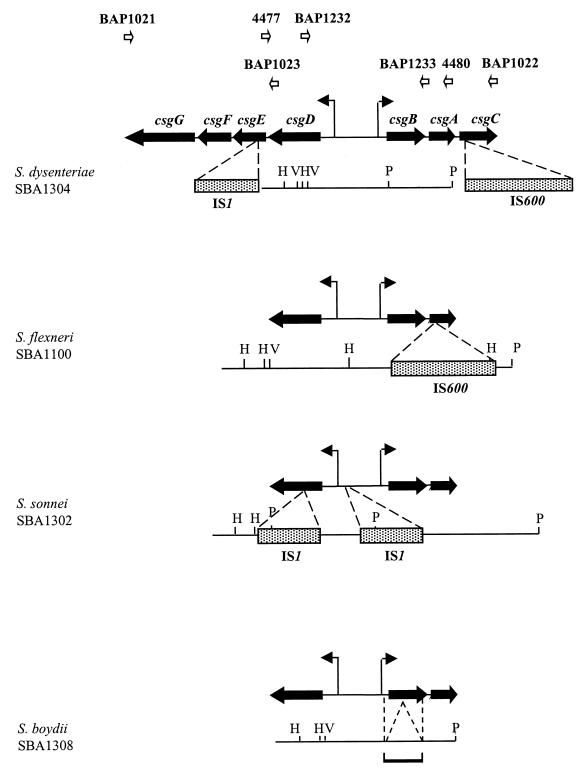
Restriction analysis and DNA sample sequencing of csg loci in Shigella.
To test whether the csg locus was present in all four species of Shigella, oligonucleotide primers were designed for the PCR amplification of an internal portion of the csg locus. Primer 4477 was homologous to a 5′-terminal sequence of the csgE gene of E. coli K-12, while primer 4480 was homologous to a 3′-terminal sequence of csgA (Fig. 1). PCR amplification of the csg internal fragment from the E. coli control strain, DH5α (9), generated a DNA fragment of 2.4 kb, the length predicted from sequence analysis of the E. coli K-12 csg locus (GenBank accession no. X90754). Similarly, a 2.4-kb fragment was amplified from S. dysenteriae serotype 3 strain SBA1304. However, fragments of 3.6, 4.0, and 2.15 kb were amplified from S. flexneri serotype 2a (SBA1100), S. sonnei (SBA1302), and S. boydii serotype 3 (SBA1308), respectively. These results implied that the csg locus was present in all four species of Shigella but had acquired insertions or undergone internal duplications in S. flexneri and S. sonnei, while the S. boydii csg locus appeared to have undergone a deletion.
FIG. 1.
Deletion and insertion mutations in the csg loci of Shigella spp. Restriction maps of the internal portion of the csg locus, spanning the 5′ terminus of csgE and the 3′ terminus of csgA, are shown for four strains of Shigella. The positions of ISs and deletions in relation to the csg genes are indicated by dashed lines. The 250-bp deletion in the S. boydii (SBA1308) csg gene lies within a 350-bp region indicated by a horizontal bracket. The positions of promoters for the csgBAC and csgDEFG operons are indicated by bent arrows. The binding sites for primers are indicated by open arrows. Restriction sites include HindIII (H), PstI (P), and EcoRV (V). Primer sequences are as follows: 4477, 5′-GCGGCGAACAGAAATTCTGCC-3′; 4480, 5′-GTTACCAAAGCCAACCTGAGTCACG-3′; BAP1021, 5′-TCAGGATTCCGGTGGAAC-3′; BAP1022, 5′-TTAAGACTTTTCTGAAGAGG-3′; BAP1023, 5′-CAGCGAAAATACGGTTAAAACGC-3′; BAP1232, 5′-TCCTGCTCAAAGTATCCTGCC-3′); and BAP1233, GATTGCTGCAATTGCTGCTAC-3′.
To investigate the basis for the variation in length of the csg PCR products, each fragment was characterized by restriction mapping (Fig. 1) and sample sequencing. The 3.6-kb PCR product from S. flexneri contained sequences with homology to the csgD, csgB, and csgA genes of E. coli K-12 and serovar Typhimurium. However, an IS600 element had inserted downstream of nucleotide 108 of the csgA gene (Fig. 1).
Sample sequencing of the 4-kb internal csg fragment from S. sonnei strain SBA1302 also revealed the presence of csgD, csgB, and csgA homologues. In addition, two distinct IS1 elements had inserted into two separate locations in the csg locus. The first IS1 element had interrupted the csgD gene at a position approximately 0.25 kb downstream of the start of the gene. The second IS1 element had inserted into the intergenic region between the divergently transcribed csgD and csgB genes, 255 bp upstream of csgD.
The PCR-amplified csg fragment from S. boydii SBA1308 was shorter than the 2.4-kb fragment amplified from DH5α, suggesting that a deletion had occurred in the S. boydii csg locus. In order to locate the site of the predicted 250-bp deletion, the 2.15-kb PCR product was sequenced with primers 4477 and 4480 and the two internal primers BAP1232 and BAP1233 (Fig. 1). Sequencing showed that the deletion had occurred within a 350-bp region which included most of the csgB open reading frame (Fig. 1).
Although no deletions or insertions were evident from PCR analysis of the S. dysenteriae serotype 3 csg locus, the primers used did not encompass the entire csg locus. This left open the possibility that mutations existed in other parts of the locus. To address this question, primers BAP1021 and BAP1022 were used to PCR amplify the entire csg locus of S. dysenteriae (Fig. 1). PCR products of 4.4 kb, the expected length of the intact E. coli csg locus, were amplified from DH5α and the curliated E. coli strains YMel (19) and χ7122 (16). However, a product of approximately 6 kb was amplified from S. dysenteriae serotype 3, suggesting that one or more IS elements may have inserted into the locus, outside of the region previously investigated. To determine the sites of the proposed insertion, the 6-kb PCR product was sequenced with primers BAP1021, BAP1022, and BAP1023 (Fig. 1). Sequence analysis showed that an IS1 element had inserted downstream of nucleotide 98 in the csgE gene, while an IS600 element had inserted downstream of nucleotide 71 of the csgC gene.
Survey of insertion and deletion mutations in the csg locus of Shigella strains.
Initial analysis of the four Shigella strains representing each species suggested that mutations within the csg locus are probably widespread phenomena. To test this hypothesis, 43 Shigella strains, representing a wide range of serotypes isolated over several years from patients in Australia and Japan, were surveyed by colony PCR with the primers BAP1021 and BAP1022, which flank the complete csg locus. The results (Table 1) demonstrated that insertions into the csg locus are widespread in Shigella spp. The size variation in the csg loci suggests that different types of insertion events have occurred. The smaller PCR products are consistent with the insertion of single IS elements, while the larger products (6.4 to 7.4 kb) are consistent with the insertion of multiple IS elements similar to those in S. dysenteriae serotype 3 strain SBA1304 and S. sonnei strain SBA1302 (Fig. 1). However, in many strains the csg locus was either partially deleted or not detected at all.
TABLE 1.
Presence and size of the csg locus in Shigella strains
| Strain | Serotype | Country of isolation | Size of csg PCR product (kb) |
|---|---|---|---|
| S. flexneri | |||
| SBA1316 | 2b | Australia | NPa |
| SBA1317 | 2a | Australia | 6.4 |
| SBA1318 | 2a | Australia | 6.4 |
| SBA1319 | 2a | Australia | 6.4 |
| SBA1320 | 2a | Australia | 5 |
| SBA1321 | 2a | Australia | 5 |
| SBA1322 | 2a | Australia | 6.4 |
| SBA1323 | 2a | Australia | 6.4 |
| SBA1173 | 1b | Australia | NP |
| SBA1387 | 5a | Japan | 5 |
| SBA1388 | 2a | Japan | 5 |
| SBA1389 | 3a | Japan | NP |
| SBA1390 | 2b | Japan | 5 |
| SBA1391 | 4a | Japan | 5 |
| SBA1392 | 6 | Japan | NP |
| SBA1401 | 2b | Australia | 6.4 |
| SBA1402 | 2a | Australia | 6.4 |
| SBA1403 | 4 | Australia | NP |
| SBA1404 | 2a | Australia | 5 |
| SBA1405 | 6 | Australia | NP |
| SBA1406 | 6 | Australia | NP |
| SBA1407 | 3a | Australia | 7.4 |
| SBA1308 | 4a | Australia | 6 |
| S. dysenteriae | |||
| SBA1393 | 1 | Japan | NP |
| SBA1394 | 3 | Japan | 6 |
| SBA1395 | 9 | Japan | 7 |
| SBA1396 | 6 | Japan | 6 |
| SBA1397 | 4 | Japan | NP |
| SBA1398 | 5 | Japan | 2.2 |
| S. boydii | |||
| SBA1381 | 1 | Japan | 6 |
| SBA1382 | 2 | Japan | 5.5 |
| SBA1383 | 3 | Japan | 3 |
| SBA1384 | 4 | Japan | 5.5 |
| SBA1385 | 7 | Japan | 4.4 |
| SBA1386 | 8 | Japan | 3 |
| S. sonnei | |||
| SBA1375 | Japan | 6.4 | |
| SBA1376 | Japan | 6 | |
| SBA1377 | Japan | 6.4 | |
| SBA1378 | Japan | 6 | |
| SBA1379 | Japan | 6 | |
| SBA1380 | Japan | 6 | |
| SBA1399 | Australia | 7 | |
| SBA1400 | Australia | 6.4 |
NP, no product.
Only a serotype 7 strain of S. boydii, SBA1385, produced a 4.4-kb fragment, suggesting that the csg locus may be intact. To test if this strain produced curli, SBA1385 and the positive control E. coli strains, YMel and χ7122, were grown on CFA agar (4) for 48 h at 25°C and were negatively stained with ammonium phosphotungstate for examination by electron microscopy as previously described (7). Although curli were clearly visible on the positive control E. coli strains, they were not produced by SBA1385 (data not shown). The most likely explanations for the absence of curli in SBA1385 include the possibility of point mutations or small deletions that were undetectable by agarose gel electrophoresis. Alternatively, the absence of curli may have been due to extragenic mutations such as those in rpoS, which are known to affect curli expression in E. coli (13).
Significance of mutations in the csg loci of Shigella strains.
The insertion and precise excision of IS elements into genes have been described as a possible mechanism for the control of gene expression. However, this is only likely to be significant when insertion and excision occur at high frequencies. For example, the expression of exopolysaccharide synthesis in Pseudomonas atlantica is mediated by an IS element that excises from the eps locus at frequencies as high as 0.5 (1). This generates genetically distinct subpopulations that are preadapted to environmental change (1). In contrast, precise excision of IS1 in E. coli occurs at frequencies of less than 10−5 (12). Furthermore, since it appears that curli loci in Shigella are often interrupted by multiple IS elements, the restoration of curli expression by the simultaneous excision of more than one IS element seems unlikely. Rather than being involved in the control of curli expression, we propose that insertions into the csg locus are common because of a strong selection against the expression of curli in Shigella. This is supported by the finding that up to a quarter of Shigella strains may have partial or complete deletions of the csg locus.
Since curli are expressed in a wide variety of E. coli strains (13), we propose that the widespread loss of curli in the closely related genus Shigella may represent a pathoadaptive mutation. Such loss-of-function mutations overcome selections against an organism that is in transition from a free-living or commensal niche to a virulence niche (23). In this way, genes that hinder the colonization of or survival within the new virulence niche are lost in the process of evolution towards a pathogenic mode of life. It is possible that during the divergence of Shigella and E. coli, the expression of curli in the new virulence niche became a selective disadvantage in Shigella. Supporting this hypothesis is the observation that although the ability to express curli at 37°C increases the infectivity of serovar Typhimurium, the pathogen is cleared much more rapidly than are natural variants unable to express curli at body temperature (24), suggesting that curli are good targets for the host immune-clearance systems. In serovar Typhimurium the controlled expression of curli provides the pathogen with a net selective advantage, i.e., colonization of the host intestine (25), which outweighs the disadvantage of increased exposure to the host immune system. The ability to express curli is therefore maintained. We propose, however, that as Shigella and Salmonella have followed different evolutionary paths, the disadvantage of increased exposure to the host immune system has outweighed selective pressures to maintain curli in Shigella.
If host factors have selected against the expression of curli in Shigella, a similar phenomenon would be expected in enteroinvasive strains of E. coli (EIEC), which share common virulence determinants with Shigella and produce similar diseases in humans (6). To test this hypothesis, 11 EIEC strains isolated from patients in Australia, South Africa, and Japan were examined by PCR with primers BAP1021 and BAP1022 for insertions or deletions in the csg locus. Six strains had insertions ranging in size from 0.5 to 2.5 kb. The five remaining strains, however, appeared to have intact csg loci, as judged by agarose electrophoresis of the PCR products. These strains were tested for their ability to produce curli by electron microscopy of negatively stained cells. When grown on CFA agar at 25°C, the control E. coli strains, YMel and χ7122, clearly produced curli. In contrast, curli were not produced by any of the EIEC strains (data not shown). These results are consistent with the previous finding that EIEC strains do not bind fibronectin (13), a characteristic associated with curli in E. coli.
Our work demonstrates that there is a strong selective pressure against the maintenance of curli in EIEC, as in Shigella spp. The observation that curli are expressed in 60% of environmental isolates of E. coli (13) but are absent from all strains of the closely related genus Shigella and EIEC, two bacterial groups with very similar mechanisms of pathogenesis, supports the hypothesis that mutations abolishing curli expression in these strains are pathoadaptive.
Nucleotide sequence accession numbers.
Nucleotide sequences of the sites of IS element insertion into the csg locus have been deposited into the GenBank database under the accession numbers AF237724, AF237725, AF237726, and AF237727.
Acknowledgments
We are very grateful to D. Lightfoot for supplying Shigella strains, Roy Robbins-Browne for supplying EIEC strains, and Arne Olsen and Roy Curtiss for supplying curliated E. coli strains.
This work was supported by a grant from the National Health and Medical Research Council, Canberra, Australia.
REFERENCES
- 1.Bartlett D H, Wright M, Silverman M. Variable expression of extracellular polysaccharide in the marine bacterium Pseudomonas atlantica is controlled by genome rearrangement. Proc Natl Acad Sci USA. 1988;85:3923–3927. doi: 10.1073/pnas.85.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 3.Darwin K H, Miller V L. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev. 1999;12:405–428. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans D G, Evans D J, Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977;18:330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floderus E, Pal T, Karlsson K, Lindberg A A. Identification of Shigella and enteroinvasive Escherichia coli strains by a virulence-specific, monoclonal antibody-based enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1995;14:111–117. doi: 10.1007/BF02111868. [DOI] [PubMed] [Google Scholar]
- 7.Froehlich B J, Karakashian A, Sakellaris H, Scott J R. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect Immun. 1995;63:4849–4856. doi: 10.1128/iai.63.12.4849-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaastra W, Svennerholm A M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz S L. Diseases of importance in developing countries. Vol. 2. Washington, D.C.: National Academy Press; 1986. p. 165. [Google Scholar]
- 12.Lu S D, Lu D, Gottesman M. Stimulation of IS1 excision by bacteriophage P1 ref function. J Bacteriol. 1989;171:3427–3432. doi: 10.1128/jb.171.6.3427-3432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen A, Arnqvist A, Hammar M, Sukupolvi S, Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol. 1993;7:523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 14.Olsen A, Jonsson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 15.Olsén A, Wick M J, Mörgelin M, Björck L. Curli, fibrous surface proteins of Escherichia coli, interact with major histocompatibility complex class I molecules. Infect Immun. 1998;66:944–949. doi: 10.1128/iai.66.3.944-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provence D L, Curtiss R., III Role of crl in avian pathogenic Escherichia coli: a knockout mutation of crl does not affect hemagglutination activity, fibronectin binding, or curli production. Infect Immun. 1992;60:4460–4467. doi: 10.1128/iai.60.11.4460-4467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajakumar K, Bulach D, Davies J, Ambrose L, Sasakawa C, Adler B. Identification of a chromosomal Shigella flexneri multi-antibiotic resistance locus which shares sequence and organizational similarity with the resistance region of the plasmid NR1. Plasmid. 1997;37:159–168. doi: 10.1006/plas.1997.1280. [DOI] [PubMed] [Google Scholar]
- 18.Rajakumar K, Sasakawa C, Adler B. A spontaneous 99-kb chromosomal deletion results in multi-antibiotic susceptibility and an attenuation of contact hemolysis in Shigella flexneri 2a. J Med Microbiol. 1996;45:64–75. doi: 10.1099/00222615-45-1-64. [DOI] [PubMed] [Google Scholar]
- 19.Richenberg H V, Lester G. The preferential synthesis of beta-galactosidase in Escherichia coli. J Gen Microbiol. 1955;13:279–284. doi: 10.1099/00221287-13-2-279. [DOI] [PubMed] [Google Scholar]
- 20.Römling U, Bian Z, Hammar M, Sierralta W D, Normark S. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J Bacteriol. 1998;180:722–731. doi: 10.1128/jb.180.3.722-731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjobring U, Pohl G, Olsen A. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA) Mol Microbiol. 1994;14:443–452. doi: 10.1111/j.1365-2958.1994.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 22.Snellings N J, Tall B D, Venkatesan M M. Characterization of Shigella type 1 fimbriae: expression, FimA sequence, and phase variation. Infect Immun. 1997;65:2462–2467. doi: 10.1128/iai.65.6.2462-2467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokurenko E V, Hasty D L, Dykhuizen D E. Pathoadaptive mutations: gene loss and variation in bacterial pathogens. Trends Microbiol. 1999;7:191–195. doi: 10.1016/s0966-842x(99)01493-6. [DOI] [PubMed] [Google Scholar]
- 24.Sukupolvi S, Edelstein A, Rhen M, Normark S J, Pfeifer J D. Development of a murine model of chronic Salmonella infection. Infect Immun. 1997;65:838–842. doi: 10.1128/iai.65.2.838-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukupolvi S, Lorenz R G, Gordon J I, Bian Z, Pfeifer J D, Normark S J, Rhen M. Expression of thin aggregative fimbriae promotes interaction of Salmonella typhimurium SR-11 with mouse small intestinal epithelial cells. Infect Immun. 1997;65:5320–5325. doi: 10.1128/iai.65.12.5320-5325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidal O, Longin R, Prigent-Combaret C, Dorel C, Hooreman M, Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]