Abstract
OBJECTIVE
To determine long-term outcomes for islet-alone and islet-after-kidney transplantation in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia.
RESEARCH DESIGN AND METHODS
This was a prospective interventional and observational cohort study of islet-alone (n = 48) and islet-after-kidney (n = 24) transplant recipients followed for up to 8 years after intraportal infusion of one or more purified human pancreatic islet products under standardized immunosuppression. Outcomes included duration of islet graft survival (stimulated C-peptide ≥0.3 ng/mL), on-target glycemic control (HbA1c <7.0%), freedom from severe hypoglycemia, and insulin independence.
RESULTS
Of the 48 islet-alone and 24 islet-after-kidney transplantation recipients, 26 and 8 completed long-term follow-up with islet graft function, 15 and 7 withdrew from follow-up with islet graft function, and 7 and 9 experienced islet graft failure, respectively. Actuarial islet graft survival at median and final follow-up was 84% and 56% for islet-alone and 69% and 49% for islet-after-kidney (P = 0.007) with 77% and 49% of islet-alone and 57% and 35% of islet-after-kidney transplantation recipients maintaining posttransplant HbA1c <7.0% (P = 0.0017); freedom from severe hypoglycemia was maintained at >90% in both cohorts. Insulin independence was achieved by 74% of islet-alone and islet-after-kidney transplantation recipients, with more than one-half maintaining insulin independence during long-term follow-up. Kidney function remained stable during long-term follow-up in both cohorts, and rates of sensitization against HLA were low. Severe adverse events occurred at 0.31 per patient-year for islet-alone and 0.43 per patient-year for islet-after-kidney transplantation.
CONCLUSIONS
Islet transplantation results in durable islet graft survival permitting achievement of glycemic targets in the absence of severe hypoglycemia for most appropriately indicated recipients having impaired awareness of hypoglycemia, with acceptable safety of added immunosuppression for both islet-alone and islet-after-kidney transplantation.
Introduction
Type 1 diabetes is caused by autoimmune destruction of pancreatic islet β-cells that renders affected individuals dependent on insulin administered by multiple daily injections or continuous subcutaneous infusion for survival. Despite increasing use of new technologies such as continuous glucose monitoring over the last decade, only approximately one in five adults with type 1 diabetes receiving specialized diabetes care can achieve or maintain glycosylated hemoglobin (HbA1c) <7.0% (53 mmol/mol), recommended for prevention and mitigation of vascular complications of diabetes (1). Moreover, ∼7% of adults with type 1 diabetes report experiencing a severe hypoglycemia episode resulting in seizure or loss of consciousness in the previous 3 months, irrespective of the level of HbA1c (1). The Clinical Islet Transplantation (CIT) Consortium designed two pivotal trials registered with the U.S. Food and Drug Administration of a purified human pancreatic islet (PHPI) product for treatment of individuals with type 1 diabetes experiencing severe hypoglycemia (2) or already receiving immunosuppression following a previous kidney transplant (3). Both trials of islet-alone and islet-after-kidney transplantation met their criteria for safety and efficacy over the initial 2- and 3-year planned follow-up, respectively, including achievement and maintenance of a composite outcome of HbA1c <7.0% in the absence of severe hypoglycemia episodes by most islet transplant recipients. Uncertainty remains over the long-term effectiveness of islet transplantation and concerning the long-term safety of chronic immunosuppression on renal function. There are also concerns related to possible sensitization of islet recipients to donor HLA that could limit availability of compatible organs should a future transplant be required. The objective of the current study was to determine the duration of sustained islet graft function assessed according to mixed-meal tolerance test (MMTT)-stimulated C-peptide, glycemic control (HbA1c and severe hypoglycemia events), and insulin requirements, consistent with consensus recommendations for defining islet graft function (4), as well as renal function, the development of donor-specific alloantibodies, cardiovascular events, and serious adverse events (SAEs) over up to 8 years of posttransplant follow-up.
Research Design and Methods
Study Design
The CIT Consortium conducted a longitudinal cohort study to provide extended follow-up after receipt of a PHPI product for up to 8 years (extended follow-up study protocol in Supplementary Material, Section 2). The local institutional review boards of each participating consortium center approved the study protocol. Participants provided written informed consent prior to study entry.
Participants
All participants who completed the U.S. Food and Drug Administration–registered phase 3 CIT Consortium islet-alone (n = 48) (CIT-07) (2) or islet-after-kidney (n = 24) (CIT-06) (3) transplantation studies with continued PHPI graft function were invited to enroll in the extended follow-up study (CIT-08). Subjects who participated in phase 2 CIT Consortium islet-alone studies (CIT-02, CIT-03, CIT-04, or CIT-05) could also enroll in CIT-08 but were not included in the long-term analysis of the phase 3 studies. Enrollment occurred between 2010, when the first PHPI recipient completed 2 years of follow-up in the islet-alone trial, and 2017, when the last PHPI recipient completed 3 years of follow-up in the islet-after-kidney trial and the CIT Consortium program ended. All subjects had documented histories (2,3) of impaired awareness of hypoglycemia and had experienced severe hypoglycemia episodes before PHPI transplantation. Subjects were excluded for intent to procreate, unwillingness to use effective contraceptive measures, or receipt of an islet or pancreas transplant outside a CIT Consortium study.
Intervention
Each subject received an initial intraportal infusion of a PHPI product containing ≥5,000 islet equivalents (IEQ)/kg body wt of recipient manufactured from a single deceased donor pancreas as previously described (5). Individuals who remained insulin dependent after 75 (islet-alone) or 30 (islet-after-kidney) days from receiving an initial PHPI product could receive one or two additional PHPI products each containing ≥4,000 IEQ/kg body wt within 240 days of the initial transplant. For immunosuppression we followed the methodology first described by Hering et al. (6) for islet-alone transplants, modified for islet-after-kidney transplants to allow substitution of mycophenolate mofetil for sirolimus and cyclosporine for tacrolimus if already used for the kidney transplant.
Measurements and Procedures
Primary Outcome
The primary outcome was the duration of sustained islet graft function defined prospectively as fasting or MMTT-stimulated serum C-peptide ≥0.3 ng/mL (0.1 pmol/mL, assay sensitivity 0.05 ng/mL) at 60 or 90 min following a standardized liquid meal (6 mL/kg up to 360 mL BOOST High Protein). Fasting C-peptide was assessed monthly during the 1st year posttransplant, quarterly until year 2 in the islet-alone study (2), and until year 3 in the islet-after-kidney study (3) and then yearly during extended follow-up. MMTTs were performed at posttransplant months 2.5, 6, 9, and 12 and then biannually until year 2 in the islet-alone study; quarterly until year 3 in the islet-after-kidney study; and yearly in both studies during extended follow-up. If any fasting C-peptide value was <0.3 ng/mL, a MMTT was performed to confirm PHPI functional status; if this was not available the islet graft was considered to have failed. All C-peptide levels were measured centrally. Because subjects entered CIT-08 at variable times from their first islet infusion depending on whether a subsequent islet infusion was performed and whether their primary study follow-up was planned for 2 (CIT-07) or 3 (CIT-06) years, duration of exposure was from the day of initial PHPI transplant to the day of islet graft failure or the day of completion of participation in both the primary and long-term CIT Consortium studies without islet graft failure.
Secondary Outcomes
Maintenance of glycemic control was assessed as the duration of HbA1c <7.0% (7) or ≤6.5% (48 mmol/mol) (8). HbA1c was measured centrally every 3 months until year 2 in the islet-alone study and until year 3 in the islet-after-kidney study and then yearly during extended follow-up. Loss of glycemic control was defined post hoc as the day on and following which no HbA1c met the above criteria, the day of PHPI graft failure, or the end of CIT Consortium study participation. Episodes of severe hypoglycemia involved neuroglycopenic symptoms requiring third party assistance associated with blood glucose level <54 mg/dL (<3.0 mmol/L) or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration (9). The duration of freedom from severe hypoglycemia was assessed starting on day 28 following initial PHPI transplant (to permit adjustment of insulin dosing post–initial PHPI transplant) until the earliest of the first subsequent severe hypoglycemia episode, PHPI graft failure, or the end of CIT Consortium study participation. Severe hypoglycemia episodes were considered reportable events captured and entered as soon as possible via a specific electronic case report form.
Insulin use was recorded daily and reported at each study visit. Insulin independence was defined post hoc as more than seven consecutive days of no insulin use (10) with HbA1c <7.0% at the most recent and/or subsequent assessment starting from the initial PHPI transplant. Temporary use of insulin to maintain normoglycemia and protect transplanted islets from metabolic exhaustion during illness or physiologic stress (11) for up to six consecutive days was permitted. Evaluation of insulin independence was censored at the earlier of PHPI graft failure or the end of CIT Consortium study participation.
Safety Outcomes
Evaluation of safety outcomes was extended to the end of subject treatment in CIT and up to 30 days following PHPI graft failure. Adverse events (AEs) occurring during the first 2 years in the islet-alone and 3 years in the islet-after-kidney study have been reported (2,3). AEs reporting during extended follow-up were limited to SAEs, severe hypoglycemia, renal insufficiency, hepatic cirrhosis, malignancy, and major adverse cardiovascular events. Renal function was assessed with the estimated glomerular filtration rate (eGFR) derived from serum creatinine with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (12). Creatinine was measured centrally every month during the 1st year posttransplant, quarterly until year 2 in the islet-alone study (2), and until year 3 in the islet-after-kidney study (3) and then yearly during extended follow-up. Donor-specific alloantibodies were assessed centrally with at least two different Luminex bead assays including Luminex single antigen bead assays at posttransplant months 2.5, 6, 9, and 12; biannually until year 2 in the islet-alone study; quarterly until year 2; and then biannually until year 3 in the islet-after-kidney study and 3 months after islet graft failure (and for islet-alone subjects, tapering of immunosuppression) during extended follow-up.
Statistical Analyses
Total PHPI dose and tacrolimus levels were log transformed, and HbA1c values underwent a Box-Cox transformation, to improve normality. For time-to-event outcome comparisons between islet-alone and islet-after-kidney subjects we used Kaplan-Meier (univariate) and proportional hazards (multivariate) methods. To avoid “immortal bias,” in survival analyses we treated total IEQ/kg body wt dose as a time-dependent covariate. The relationships of tacrolimus trough concentrations and HbA1c values with graft outcome were analyzed with Bayesian joint analysis (13). The median subject HbA1c over follow-up time was modeled by Bayesian joint analysis followed by prediction of subject-specific longitudinal trajectory (13). A semi-Markov model was constructed (14) to calculate the fraction of subjects in one of four states over the follow-up period: not yet insulin independent, insulin independent, functioning graft/resumed daily insulin, or islet graft failure. Renal function was assessed with eGFR over two time periods: change of eGFR from pretransplant to 1 year following the first PHPI transplant, reflecting the impact of the transplant procedures and initiation (or adjustments for islet-after-kidney) of immunosuppression, and the subsequent slope of eGFR after the 1st year. Further details of analytic and statistical methods are presented in relevant sections of Supplementary Material.
Results
Participants
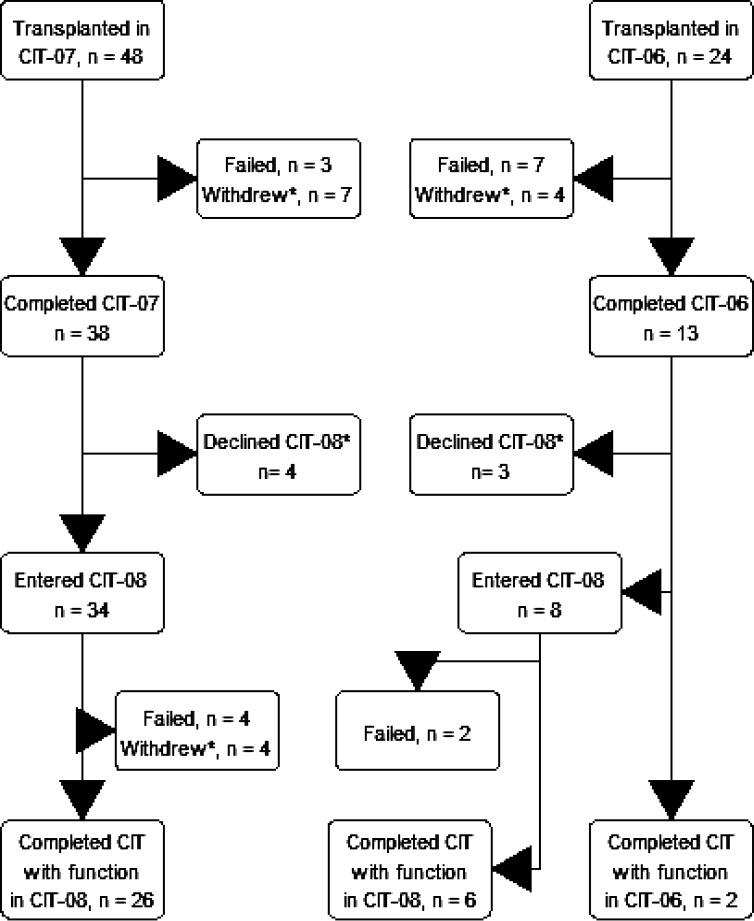
A total of 72 subjects, 48 with islet-alone and 24 islet-after-kidney transplantation, received one (n = 33 [46%]), two (n = 36 [50%]), or three (n = 3 [4%]) intraportal infusions of a PHPI product in the course of the primary phase 3 studies (2,3). Forty-two subjects entered the extended follow-up study (34 islet-alone and 8 islet-after-kidney), a median of 876 days (range 721–2,035) for islet-alone and 1,168 days (1,087–1,208) for islet-after-kidney following initial islet infusion. Two additional islet-after-kidney subjects completed the 3 years of follow-up in their primary study in 2017 simultaneously with completion of the entire CIT Consortium program (Fig. 1, Consolidated Standards of Reporting Trials [CONSORT] diagram).
Figure 1.
CONSORT diagram. Withdrew, subjects withdrawing with continued function. *Further information can be found in Supplementary Material, Section 3.
Baseline and pre–PHPI transplant covariates were similar between the cohorts of islet-alone and islet-after-kidney transplantation recipients except for marginally longer diabetes duration, higher HbA1c, and lower eGFR in the islet-after-kidney group (Table 1). There was no difference in baseline insulin requirement or PHPI dose administered between the cohorts. There was also no difference between the study cohorts in the number of islet infusions or time between first and second infusions for those receiving more than one islet infusion. Trough levels of tacrolimus were lower in islet-alone participants, who all initially received low-dose tacrolimus and sirolimus (15), than in islet-after-kidney participants, who more frequently received standard dose tacrolimus with mycophenolic acid (21 of 24 [87.5%]).
Table 1.
Baseline, pre–PHPI transplant, and treatment covariates and subject disposition by CIT Consortium study
| Islet-alone transplantation (n = 48) | Islet-after-kidney transplantation (n = 24) | P | |
|---|---|---|---|
| Baseline covariates | |||
| Sex (n female/n male) | 29/19 | 11/13 | 0.36 |
| Age (years) | 47.8 ± 11.5 | 51.8 ± 11.1 | 0.17 |
| Weight (kg) | 71.9 ± 13.7 | 69.4 ± 8.8 | 0.35 |
| BMI (kg/m2) | 24.9 ± 3.1 | 24.6 ± 3.1 | 0.64 |
| Diabetes duration (years) | 31.5 ± 11.0 | 37.0 ± 10.0 | 0.04 |
| Pre–PHPI transplant covariates* | |||
| Daily insulin (units/day) | 33.6 ± 11.1 | 36.0 ± 12.2 | 0.43 |
| Daily insulin/kg (units/kg/day) | 0.47 ± 0.14 | 0.51 ± 0.14 | 0.32 |
| eGFR (mL/min/1.73 m2) | 100.3 ± 14.2 | 75.8 ± 19.3 | <0.001 |
| HbA1c (mmol/mol) | 57.3 ± 9.9 | 63.0 ± 13.0 | 0.06 |
| HbA1c (%) | 7.39 ± 0.91 | 7.92 ± 1.19 | 0.06 |
| HbA1c <7.0%, n (%) | 18 (38) | 5 (21) | |
| HbA1c ≤6.5%, n (%) | 8 (17) | 3 (13) | |
| Treatment covariates | |||
| Total IEQ/kg body wt of recipient† | 11,278 ± 3,935 | 12,585 ± 6,191 | 0.77 |
| Mean tacrolimus trough (ng/mL)† | 6.17 ± 1.53 | 7.63 ± 2.00 | 0.01 |
| Median follow-up and subject disposition | |||
| Median follow-up (months) | 65.8 | 39.3 | |
| Failed | 7 | 9 | |
| Withdrew with function | 15 | 7 | |
| Completed with function | 26 | 8 |
Data are means ± SD unless otherwise indicated.
Values obtained during the subject’s time on the islet transplant waiting list while on intensive insulin therapy.
IEQ/kg body wt and tacrolimus were log transformed for t tests. IEQ/kg body wt was entered as a time-dependent covariate.
A total of 16 subjects (22%) experienced islet graft failure, 7 islet-alone and 9 islet-after-kidney, during participation in the primary studies or the extended follow-up study. Twenty-two subjects (31%) withdrew participation with continued islet graft function (15 islet-alone and 7 islet-after-kidney). Supplementary Table 3.1 lists reasons for subject withdrawal and subjects’ final C-peptide and HbA1c measures and insulin requirement and total time in follow-up. Thirty-four subjects (47% [26 islet-alone and 8 islet-after-kidney]) completed the CIT Consortium program with islet graft function confirmed at closeout visits.
Primary Outcome
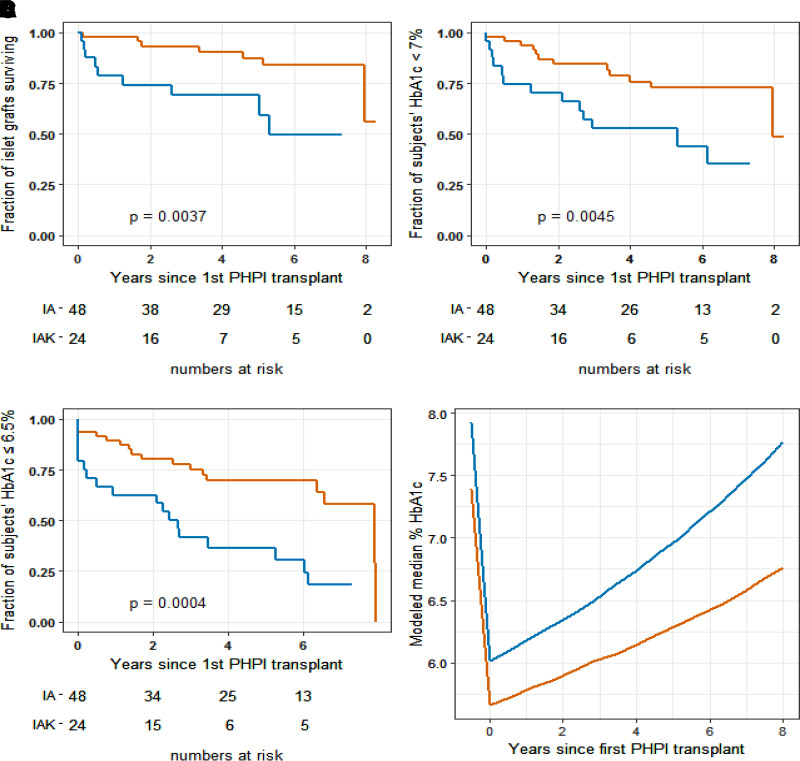
Islet-alone transplantation recipients had 56% actuarial survival of islet graft function at their maximum follow-up time of 8.3 years, while islet-after-kidney recipients had 49% actuarial survival at their maximum follow-up time of 7.3 years (P = 0.004) (Fig. 2A). A sensitivity analysis of the primary outcome imputing failure at the time of exit from follow-up (rather than censoring) of eight subjects who exited because of an AE (four subjects), noncompliance, poor graft function (two subjects), or loss to follow-up demonstrated similar results (Supplementary Fig. 3.1; details in Supplementary Material, Section 3). The models of evolution of fasting and post-MMTT simulated C-peptide levels revealed robust and sustained C-peptide levels in both islet-alone and islet-after-kidney subjects (Supplementary Fig. 4.1 and Supplementary Fig. 4.2; details in Supplemental Material, Section 4).
Figure 2.
Persistence of islet graft function and HbA1c control by cohort. Red lines, islet-alone transplantation (IA) recipients; blue lines, islet-after-kidney transplantation (IAK) recipients. Time 0 for all panels was the day of first PHPI transplant. Fraction of subjects with continued PHPI graft function by C-peptide criterion at median and final follow-up: islet-alone, 0.84 and 0.56, respectively; islet-after-kidney, 0.69 and 0.49) (A). Fraction of subjects with continued HbA1c <7% (American Diabetes Association criterion) at median and final follow-up: islet-alone, 0.77 and 0.49; islet-after-kidney, 0.57 and 0.35) (B). Fraction of subjects with continued HbA1c ≤6.5% (American Association of Clinical Endocrinologists criterion) at median and final follow-up: islet-alone, 0.73 and 0.00; islet-after-kidney, 0.59 and 0.17) (C). D: Median HbA1c at baseline and modeled median HbA1c by years following initial PHPI transplant.
Bivariate proportional hazards analysis of the baseline and treatment covariates together with the CIT Consortium study cohort showed no significant correlations of the covariates with islet graft survival, and no covariate meaningfully attenuated the islet graft survival difference between islet-alone and islet-after-kidney recipients. Joint analysis (13) of the impact of time-dependent tacrolimus trough levels on islet graft survival also showed no significant relationship between tacrolimus exposure and islet graft survival and no attenuation of the difference in outcomes between the islet transplant study groups (details in Supplemental Material, Section 5).
Secondary Outcomes
Glycemic control assessed as HbA1c <7.0% or ≤6.5% together with absence of severe hypoglycemia served as the primary outcomes at 1 year following initial PHPI transplant in the primary studies (2,3). In fact, at the time of the first HbA1c measurement at day 75 following the initial PHPI transplant, 42 of 48 (87.5%) of islet-alone and 17 of 24 (71%) of islet-after-kidney transplantation recipients already had achieved HbA1c <7.0% and 41 of 48 (85%) of islet-alone and 13 of 24 (54%) of islet-after-kidney recipients had HbA1c ≤6.5%. Throughout follow-up, 49% of islet-alone recipients maintained functioning grafts with HbA1c <7.0% (Fig. 2B), but none had levels ≤6.5% at the end of maximal follow-up at 8.3 years (Fig. 2C). For islet-after-kidney recipients, 35% maintained islet graft function with HbA1c <7.0% (P = 0.0017 vs. islet-alone) and 17% with HbA1c ≤6.5% (P < 0.0001 vs. islet-alone) at the end of maximal follow-up at 7.3 years. With use of Bayesian joint analysis (13), the modeled evolution of HbA1c in the islet-alone and islet-after-kidney study cohorts demonstrated an initial decline from medians of 7.4% and 7.9% to 5.7% and 6.0%, respectively, that gradually rose to 6.7% and 7.8% over 8 years (Fig. 2D). Thus, the projected median benefit of PHPI transplantation for glycemic control lasts >8 years for both islet-alone and islet-after-kidney recipients.
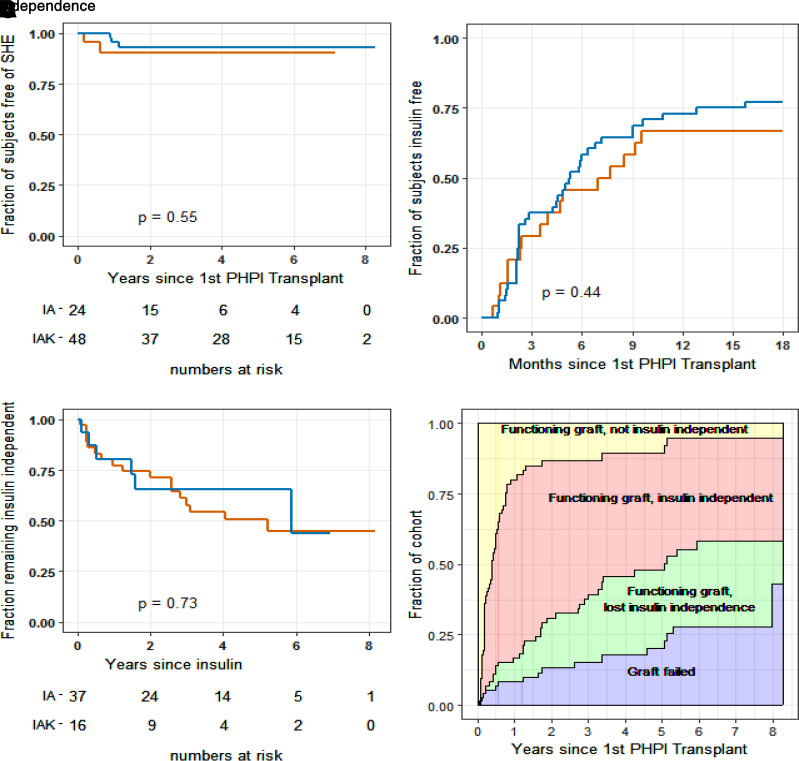
A total of 12 severe hypoglycemia episodes occurred in five subjects (7% [3 islet-alone and 2 islet-after-kidney]) over the course of the primary studies. There were no additional severe hypoglycemia episodes during the long-term follow-up study in any subject with islet graft function (Fig. 3A).
Figure 3.
Freedom from severe hypoglycemia and insulin requirements for islet transplant recipients. A–C: Red lines, islet-alone (IA) recipients; blue lines, islet-after-kidney (IAK) recipients. Fraction of subjects remaining free from severe hypoglycemia following initial PHPI transplant at both median and final follow-up: islet-alone, 0.90; islet-after kidney, 0.93 (A). B: Fraction of subjects achieving insulin independence following initial PHPI transplant. C: Fraction of subjects remaining insulin independent following achievement of insulin independence. D: Fraction of subjects in each of four states following initial PHPI transplant: functioning graft, not yet insulin independent; functioning graft, insulin independent; functioning graft, resumed daily insulin; and failed graft. Additional information about insulin use and glucose control at each state, 1–3, can be found in Supplementary Material, Section 6.
A total of 53 subjects (74% [37 of 48 islet-alone and 16 of 24 islet-after-kidney]) achieved a period of insulin independence with HbA1c maintained at <7.0%, with no difference in the proportion or time to outcome between the study cohorts (Fig. 3B). The range of time to achieve insulin independence (between 36 and 481 days) reflects individuals who became insulin independent after one (n = 20 [37.66%]), two (n = 30 [56.66%]), or three (n = 3 [5.66%]) PHPI infusions. Eleven subjects without insulin independence were not able to receive a subsequent islet infusion within the protocol-defined interval of 240 days from the initial transplant and so may have been underdosed for achievement of this outcome. Of the subjects who achieved insulin independence, thirty (57%) remained insulin independent throughout their duration of follow-up (20 of 37 islet-alone and 10 of 16 islet-after-kidney), with no difference in the duration of insulin independence observed between the study cohorts (Fig. 3C). Among those who achieved insulin independence at any time, 44% are projected to remain insulin independent for up to 8 years.
Figure 3D shows the proportions of subjects posttransplant as estimated with semi-Markov modeling (14) to be in each of four states (not yet insulin independent, insulin independent, functioning graft/resumed daily insulin, or islet graft failure). Additional methods, daily insulin dose, and HbA1c levels for states 1–3 are given in Supplementary Material, Section 6.
Safety Outcomes
PHPI transplants were generally well tolerated. There were no deaths during posttransplant follow-up. There were 104 SAEs (islet-alone, 71 over 226 patient-years [0.31/patient-year]; islet-after-kidney, 33 over 77 patient-years [0.43/patient-year]) among 43 of 72 subjects (60% [islet-alone, 28 of 48; islet-after-kidney, 15 of 24) over 303 years of total post-PHPI transplant follow-up in the primary and long-term follow-up studies (Supplementary Material, Section 7). Of these, 65 occurred during the primary islet-alone and islet-after-kidney trials, 36 within 101 patient-years for islet-alone (0.36/patient-year) and 29 within 66 patient-years for islet-after-kidney (0.44/patient-year), and were previously reported (2,3). An additional 39 SAEs occurred during the long-term follow-up CIT-08 study (islet-alone, 35 during 125 patient-years [0.28/patient-year]; islet-after-kidney, 4 during 11 patient-years [0.36/patient-year]) among 16 subjects (islet-alone, 14; islet-after-kidney, 2). One SAE during the long-term follow-up was related to islet infusion: an episode of severe abdominal pain from late migration of an embolization coil requiring surgical removal. Eleven were possibly related to immunosuppression: six posttransplant infections including one also with febrile leukopenia, one acute kidney injury, one hyperkalemia, one newly diagnosed lung cancer, and one episode of leukopenia in an islet-after-kidney subject remaining on immunosuppression in support of the kidney graft following PHPI graft failure. The other 27 long-term follow-up study SAEs were judged to be unrelated to study procedures. All SAEs except the lung cancer resolved with treatment, and none required accelerated reporting.
SAEs of Special Interest
Five malignancies were diagnosed in four subjects during total posttransplant follow-up in the primary and long-term studies, including one each with lung (above), breast, prostate, and small intestine carcinoma associated with celiac disease. This latter islet-after-kidney subject had resection of a posttransplant lymphoproliferative lesion identified at admission for and prior to PHPI transplant, therefore related to the prior kidney transplant. There were only two major adverse cardiovascular events during the 319 subject years of post-PHPI transplant follow-up: a cerebellar thrombosis stabilized following revascularization and a resuscitated cardiac arrest in a subject during an unrelated SAE 290 days following PHPI graft failure and return to insulin. There were no major pre- to posttransplant changes in cardiovascular risk factors (details in Supplemental Material, Section 8).
eGFR declined by 6.9 mL/min/1.73 m2 during the 1st year posttransplant in the islet-alone and by only 0.7 mL/min/1.73 m2 in the islet-after-kidney cohort (Table 2). Over longer-term follow-up from the 1st to up to 8 years, the slope of eGFR was only −1.27 mL/min/1.73 m2/year in the islet-alone cohort and was in fact positive in the islet-after-kidney cohort (Table 2). Further details of renal function methods and results are given in Supplementary Material, Section 9.
Table 2.
eGFR pre– and 1-year post–initial PHPI transplant and subsequent slope, by cohort
| Islet-alone transplantation | Islet-after-kidney transplantation | |||
|---|---|---|---|---|
| Median | MAD | Median | MAD | |
| Pre–PHPI transplant (mL/min/1.73 m2) | 99.50 | 13.45 | 81.37 | 17.45 |
| 1-year posttransplant estimate (mL/min/1.73 m2) | 89.52 | 17.52 | 80.11 | 21.30 |
| Step change (mL/min/1.73 m2) | −6.92 | 11.15 | −0.72 | 8.04 |
| Percent change | −7.30 | 10.69 | −0.89 | 9.77 |
| Slope after 1 year (mL/min/1.73 m2/year) | −1.27 | 1.37 | 0.55 | 1.12 |
MAD, median absolute deviation scaled to approximate SD.
A list of islet donor–specific alloantibodies that appeared during the primary studies and affected 2 of 48 islet-alone and 5 of 24 islet-after-kidney recipients (2,3) is presented in Supplementary Material, Section 10. One additional islet-alone recipient developed islet donor–specific alloantibodies at 3 years, with islet graft failure occurring 5 years following initial PHPI transplant. One islet-alone subject developed islet donor–specific alloantibodies following graft failure and cessation of immunosuppression. There were no additional de novo islet or kidney donor–specific alloantibodies in islet-after-kidney recipients during extended follow-up and no episodes of kidney rejection in any of the islet-after-kidney recipients.
Conclusions
Transplantation of a standardized PHPI product by portal vein infusion in individuals with type 1 diabetes complicated by severe hypoglycemia resulted in sustained islet graft survival in >80% of islet-alone and ∼50% of islet-after-kidney transplantation recipients at the median follow-up of 6 years posttransplant. A greater proportion of islet-alone compared with islet-after-kidney recipients maintained HbA1c <7.0 and ≤6.5%, while in both study cohorts >90% were free from severe hypoglycemia episodes posttransplant. Insulin independence was achieved by ∼75% of recipients in both studies, with more than one-half of these maintaining insulin independence during long-term follow-up. The up to 8 years’ worth of prospective posttransplant follow-up reported here provides robust long-term estimates for anticipated duration of both insulin freedom and islet graft survival. While the loss to follow-up of subjects with graft failure or early withdrawal may bias the conclusion about the fraction of subjects with long-term islet graft survival/function, results of a sensitivity analysis with imputation of graft failure rather than censoring at the end of follow-up for those subjects who withdrew for AEs, with marginal islet graft function, or for unknown reasons are similar to those of the primary analysis. Importantly, islet graft survival is associated with the maintenance of recommended glycemic targets without severe hypoglycemia (4) that was problematic for all recipients prior to transplantation.
There was a meaningful difference in the duration of islet graft survival and maintenance of glycemic control between the islet-alone and islet-after-kidney study cohorts. In this study we cannot determine whether baseline metabolic differences in individuals with type 1 diabetes and preserved versus replaced kidney function or the initial use of the mTOR inhibitor sirolimus with the calcineurin inhibitor tacrolimus in the islet-alone cohort versus ongoing mycophenolic acid use with higher-dose tacrolimus in the islet-after-kidney cohort might explain these differences. In a single-center prospective cohort study of islet-alone and islet-after-kidney transplantation under sirolimus and tacrolimus maintenance of immunosuppression (16), investigators reported similar long-term islet graft survival and metabolic control in both groups, similar to those reported here for islet-alone recipients, suggesting a possible metabolic or immunologic benefit to sirolimus use in the setting of islet transplantation.
In the prospective cohort study reported by Vantyghem et al. (16), all 28 recipients received islets isolated from two or three donor pancreases, whereas 33 of 72 subjects reported here received islets isolated from a single donor pancreas. The similar long-term islet graft survival and metabolic control observed in both studies when sirolimus was initially used in combination with tacrolimus suggests that more efficient islet engraftment and survival was achieved in the CIT Consortium trials. This may be explained by use of the Edmonton protocol for induction of immunosuppression by Vantyghem et al. (16) versus the combination of T cell depletion using thymoglobulin and TNFα inhibition using etanercept for induction immunosuppression in the CIT Consortium (2,3). One CIT Consortium site demonstrated achievement of significantly greater β-cell secretory capacity, a measure of engrafted islet β-cell mass, in subjects from the islet-alone CIT Consortium trial reported here compared with previous subjects transplanted under the Edmonton protocol (17). This evidence of more efficient islet engraftment and survival likely explains the higher rate of insulin independence, even with use of a single islet donor, in the CIT Consortium trials compared with results reported with the Edmonton protocol (18) or T cell depletion without TNFα inhibition (19).
Transplantation of the PHPI product was generally well tolerated, as previously reported (2,3), with few related problems developing during the extended follow-up and a remarkably low rate of major adverse cardiovascular events. The initial decline in eGFR observed in the islet-alone cohort is expected following the initiation of calcineurin inhibitor–based immunosuppression that can induce glomerular afferent arteriole vasoconstriction (20) but may also reflect reduced glomerular hyperfiltration with normalization of glycemia, since eligible islet-alone participants had rather high baseline eGFR (mean 100 mL/min/1.73 m2). Renal function remained stable during long-term follow-up and especially in the islet-after-kidney cohort where eGFR appears to have improved over time. The stability of kidney function following islet transplantation is consistent with other reports involving smaller cohorts of islet-alone (16,21) and islet-after-kidney (16) recipients. There were no episodes of kidney transplant rejection, and the rate of sensitization to islet donor–specific alloantibodies remained low during extended follow-up and was lower than that reported for pancreas transplantation (22,23).
This study has several limitations including the nonrandomized design of the primary trials. Inclusion of a randomized control group was not feasible due to the availability of clinically reimbursed pancreas-alone and pancreas-after-kidney transplantation outside of the CIT Consortium. Withholding access to an alternatively available form of β-cell replacement as recommended in current guidelines (24) would be unethical. Indeed, increased hypoglycemia mortality has been reported in individuals referred for but not receiving islet transplantation (25), while islet transplant recipients followed for 20 years have demonstrated high rates of survival (26). The results of one randomized trial of islet transplantation versus intensive insulin therapy confirmed the metabolic control benefits of islet transplantation over a period limited to 6 months, after which those initially assigned to intensive insulin therapy underwent islet transplantation. However, one patient initially assigned to intensive insulin therapy died of hypoglycemia while waiting for an islet transplant (27).
The withdrawal of subjects prior to study end or islet graft failure raises the possibility of indication bias. Four subjects ended participation in CIT prior to its completion in 2017 following AEs. Eighteen elected to end participation early, nine of whom had access to clinical care in the Canadian Health System where islet transplantation is approved. Results of a sensitivity analysis suggested that the impact of bias was small. Finally, participants were only followed for 3 months following islet graft failure. It is possible that additional islet-alone subjects may have been sensitized to islet donor HLA later following discontinuation of immunosuppression (28).
In conclusion, islet transplantation can result in long-term achievement of glycemic targets in the absence of severe hypoglycemia for many recipients with type 1 diabetes and impaired awareness of hypoglycemia. Glycemic control and islet graft survival may be superior with islet-alone than with islet-after-kidney transplantation, although in both groups >50% of those who achieved insulin independence remained insulin free after 5 years. In balancing the long-term risk of the required immunosuppression with the metabolic benefit of a PHPI transplant, the added risk is less for individuals already receiving maintenance of immunosuppression in support of a kidney transplant, and despite the use of induction immunosuppression in both cohorts, incidence and types of malignancy were not different than expected for this age-group. These results support the consideration of islet transplantation as a less invasive alternative to existing pancreas-alone and pancreas-after-kidney transplantation (24) in appropriate individuals with type 1 diabetes.
Article Information
Acknowledgments. The authors are indebted to the individuals with type 1 diabetes who participated in these studies. The authors thank Dr. Santica Marcovina of the University of Washington Northwest Lipid Metabolism and Diabetes Research Laboratories for performance of the central biochemical and immunoassays and Dr. Malek Kamoun of the University of Pennsylvania Clinical Immunology and Histocompatibility Laboratory for conducting the HLA typing of donors and recipients and Luminex bead assays for assessment of alloantibodies.
Funding. The long-term analyses were supported by JDRF grant 1-SRA-2019-728-A-N (to L.G.H. and M.R.R.). Study conduct was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants to the University of Pennsylvania (U01DK070430), University of Iowa (U01DK070431), University of Miami (U01DK070460), and University of California, San Francisco (U01DK085531), and National Institute of Allergy and Infectious Diseases grants to the University of Alberta (U01AI065191), Uppsala University (U01AI065192), University of Minnesota (U01AI065193), Northwestern University (U01AI089316), and Emory University (U01AI089317). In addition, the study was supported in part by National Center for Research Resources and National Center for Advancing Translational Sciences grants to Emory University (UL1TR000454), Northwestern University (UL1RR025741 and UL1TR000150), University of California, San Francisco (UL1TR000004), University of Illinois, Chicago (UL1TR000050), University of Miami (UL1TR000460), University of Minnesota (M01RR000400 and UL1TR000114), and University of Pennsylvania (M01RR00040 and UL1TR000003).
Duality of Interest. B.J.H. holds equity in and serves as a paid executive officer and director of Diabetes Free, Inc., a company that may commercially benefit from the results of this research. This interest has been reviewed and managed by the University of Minnesota in accordance with its conflicts of interest policies. M.R.R. reports consulting fees from Sernova Corp. and Vertex Pharmaceuticals, R.A. reports consulting fees from Vertex Pharmaceuticals, iTolerance, and eGenesis, and J.F.M. reports consulting fees from Vertex Pharmaceuticals, all outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.R.R., T.L.E., R.A., N.D.B., B.J.H., J.F.M., P.A.S., and L.G.H. developed the study concept and design and provided supervision. M.R.R., T.L.E., L.B., J.C.Q., R.A., N.D.B., B.J.H., J.F.M., P.A.S., and L.G.H. acquired, analyzed, and/or interpreted data. L.B., J.C.Q., and L.G.H. conducted the statistical analysis. T.L.E., L.B., and J.C.Q. provided administrative, technical, and/or material support. M.R.R. and L.G.H. obtained funding and drafted the manuscript. T.L.E., R.A., N.D.B., B.J.H., J.F.M., and P.A.S. made critical revisions to the manuscript for important intellectual content. M.R.R. and L.G.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 19th American Transplant Congress, Boston, MA, 1–5 June 2019; the 17th World Congress of the International Pancreas and Islet Transplant Association, Lyon, France, 2–5 July 2019; and the 20th American Transplant Congress, Philadelphia, PA, 30 May–3 June 2020.
Footnotes
Clinical trial reg. nos. NCT00434811, NCT00468117, and NCT01369082, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.21120631.
A list of additional members of the Clinical Islet Transplantation Consortium who contributed to this study can be found in the supplementary material online.
Contributor Information
Clinical Islet Transplantation Consortium:
Jose Avila, Beth Begley, Jose Cano, Sallie Carpentier, Elizabeth Holbrook, Jennifer Hutchinson, Christian P. Larsen, Johanna Moreno, Marti Sears, Nicole A. Turgeon, Dasia Webster, Christian Berne, Carl Jorns, Torbjörn Lundgren, Mikael Rydén, Enrico Cagliero, Kerry Crisalli, S. Deng, Ji Lei, James F. Markmann, David Nathan, Patrice Al-Saden, Jason Battle, Xioajuan Chen, Angela Hecyk, Dixon B. Kaufman, Herman Kissler, Xunrong Luo, Mark Molitch, Natalie Monson, Elyse Stuart, Amisha Wallia, Lingjia Wang, Shusen Wang, Xiaomin Zhang, Nancy D. Bridges, Christine W. Czarniecki, Julia S. Goldstein, Tomeka Granderson, Yvonne Morrison, Allison Priore, Gerry Putz, Mark A. Robien, Elizabeth Schneider, Guillermo Arreaza, Thomas L. Eggerman, Neal Green, David L. Bigam, Patricia Campbell, Parastoo Dinyari, Sharleen Imes, Tatsuya Kin, Norman M. Kneteman, Angela Koh, James Lyon, Andrew Malcolm, Doug O’Gorman, Chris Onderka, Richard Owen, Rena Pawlick, Brad Richer, Shawn Rosichuk, Edmond A. Ryan, Donna Sarman, Adam Schroeder, Peter A. Senior, A.M. James Shapiro, Lana Toth, Vali Toth, Wendy Zhai, Kristina Johnson, Joan McElroy, Andrew M. Posselt, Marissa Ramos, Tara Rojas, Peter G. Stock, Gregory Szot, Barbara Barbaro, Leelama George, Joan Martellotto, Jose Oberholzer, Meirigeng Qi, Yong Wang, Levent Bayman, Kathryn Chaloner, William R. Clarke, Joseph S. Dillon, Cynthia Diltz, Gregory C. Doelle, Dixie Ecklund, Holly Ernst, Deb Feddersen, Eric Foster, Lawrence G. Hunsicker, Carol Jasperson, David-Erick Lafontant, Karen McElvany, Tina Neill-Hudson, Deb Nollen, Julie Qidwai, Traci Schwieger, Beth Shields, Jamie Willits, Jon Yankey, Rodolfo Alejandro, A. Alvarez, Andrea Curry Corrales, Raquel Faradji, Tatiana Froud, Ana Alvarez Gil, Eva Herrada, H. Ichii, Luca Inverardi, Norma Kenyon, Aisha Khan, Elina Linetsky, J. Montelongo, Eduardo Peixoto, K. Peterson, Camillo Ricordi, J. Szust, X. Wang, Xiumin Xu, Muhamad H. Abdulla, J. Ansite, A.N. Balamurugan, Melena D. Bellin, Mary Brandenburg, T. Gilmore, James V. Harmon, Bernhard J. Hering, Raja Kandaswamy, Gopal Loganathan, Kate Mueller, Klearchos K. Papas, Jayne Pedersen, Joshua J. Wilhelm, Jean Witson, Aksel Foss, Trond Jenssen, Cornelia Dalton-Bakes, Hongxing Fu, Malek Kamoun, Jane Kearns, Yanjing Li, Chengyang Liu, Eline Luning-Prak, Yanping Luo, Eileen Markmann, Zaw Min, Ali Naji, Maral Palanjian, Michael R. Rickels, Richard Shlansky-Goldberg, Kumar Vivek, Amin Sam Ziaie, Peter Chebleck, Juan Sebastian Danobeitia, Luis Fernandez Dixon B. Kaufman, Jon Odorico, Kristi Schneider, Laura Zitur, D. Brandhorst, A. Friiberg, Olle Korsgren, Bo Nilsson, Gunnar Tufveson, Bengt von Zur-MΈhlen, and Irene Feurer
References
- 1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hering BJ, Clarke WR, Bridges ND, et al.; Clinical Islet Transplantation Consortium . Phase 3 Trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Markmann JF, Rickels MR, Eggerman TL, et al. Phase 3 trial of human islet-after-kidney transplantation in type 1 diabetes. Am J Transplant 2021;21:1477–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rickels MR, Stock PG, de Koning EJP, et al. Defining outcomes for β-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA Opinion Leaders Workshop. Transplantation 2018;102:1479–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ricordi C, Goldstein JS, Balamurugan AN, et al. National Institutes of Health–sponsored Clinical Islet Transplantation Consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes 2016;65:3418–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830–835 [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Standards of medical care in diabetes—2008. In Clinical Practice Recommendations, 2008. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 8. Rodbard HW, Blonde L, Braithwaite SS, et al.; AACE Diabetes Mellitus Clinical Practice Guidelines Task Force . American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007;13(Suppl. 1):1–68 [DOI] [PubMed] [Google Scholar]
- 9. Seaquist ER, Anderson J, Childs B, et al.; American Diabetes Association; Endocrine Society . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab 2013;98:1845–1859 [DOI] [PubMed] [Google Scholar]
- 10. Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev 2019;40:631–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodrich B, Gabry J, Ali I, Brilleman S. Bayesian applied regression modeling via Stan. R package version 2.21.1, 2020. Accessed 4 May 2022. Available from https://mc-stan.org/rstanarm
- 14. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–2430 [DOI] [PubMed] [Google Scholar]
- 15. McAlister VC, Gao Z, Peltekian K, Domingues J, Mahalati K, MacDonald AS. Sirolimus-tacrolimus combination immunosuppression. Lancet 2000;355:376–377 [DOI] [PubMed] [Google Scholar]
- 16. Vantyghem MC, Chetboun M, Gmyr V, et al.; Ten-year outcome of islet alone or islet after kidney transplantation in type 1 diabetes: a prospective parallel-arm cohort study. Diabetes Care 2019;42:2042–2049 [DOI] [PubMed] [Google Scholar]
- 17. Rickels MR, Liu C, Shlansky-Goldberg RD, et al. Improvement in β-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013;62:2890–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lablanche S, Borot S, Wojtusciszyn A, et al.; GRAGIL Network . Ten-year outcomes of islet transplantation in patients with type 1 diabetes: data from the Swiss-French GRAGIL network. Am J Transplant 2021;21:3725–3733 [DOI] [PubMed] [Google Scholar]
- 19. Forbes S, Flatt AJ, Bennett D, et al. The impact of islet mass, number of transplants, and time between transplants on graft function in a national islet transplant program. Am J Transplant 2022;22:154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009;4:481–508 [DOI] [PubMed] [Google Scholar]
- 21. Thompson DM, Meloche M, Ao Z, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation 2011;91:373–378 [DOI] [PubMed] [Google Scholar]
- 22. Mittal S, Page SL, Friend PJ, Sharples EJ, Fuggle SV. De novo donor-specific HLA antibodies: biomarkers of pancreas transplant failure. Am J Transplant 2014;14:1664–1671 [DOI] [PubMed] [Google Scholar]
- 23. Parajuli S, Alagusundaramoorthy S, Aziz F, et al. Outcomes of pancreas transplant recipients with de novo donor-specific antibodies. Transplantation 2019;103:435–440 [DOI] [PubMed] [Google Scholar]
- 24. Choudhary P, Rickels MR, Senior PA, et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care 2015;38:1016–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee MH, Ward GM, MacIsaac RJ, et al. Mortality in people with type 1 diabetes, severe hypoglycemia, and impaired awareness of hypoglycemia referred for islet transplantation. Transplant Direct 2018;4:e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lemos JRN, Baidal DA, Ricordi C, Fuenmayor V, Alvarez A, Alejandro R. Survival after islet transplantation in subjects with type 1 diabetes: twenty-year follow-up. Diabetes Care 2021;44:e67–e68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lablanche S, Vantyghem MC, Kessler L, et al.; TRIMECO trial investigators . Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2018;6:527–537 [DOI] [PubMed] [Google Scholar]
- 28. Hilbrands R, Gillard P, Van der Torren CR, et al. Predictive factors of allosensitization after immunosuppressant withdrawal in recipients of long-term cultured islet cell grafts. Transplantation 2013;96:162–169 [DOI] [PubMed] [Google Scholar]