Abstract
The migratory properties of THP1 monocytes infected by Coxiella burnetii were determined in a transmigration assay across a human microvascular endothelial cell monolayer. Transendothelial migration of monocytes infected by virulent, but not avirulent, C. burnetii was inhibited. This inhibition was observed in spite of conserved adherence properties of infected monocytes.
Coxiella burnetii is a strictly intracellular bacterium responsible for Q fever. The acute form of Q fever has no symptoms or is characterized by hepatitis or pneumonia with a favorable prognosis, whereas the main manifestation of chronic Q fever is endocarditis (14). Monocytes are involved in the pathophysiology of Q fever. In vivo, C. burnetii inhabits monocytes/macrophages (3). Monocytes from patients suffering from Q fever endocarditis secrete elevated amounts of inflammatory cytokines, such as tumor necrosis factor (TNF) (5). Patient monocytes also allow the survival of C. burnetii (7). In addition, monocytes control the depression of specific T-cell responses, which is associated with Q fever endocarditis (12). Because of the strictly intracellular life of C. burnetii, the pathogenesis of Q fever endocarditis would require cellular vectorization of C. burnetii to target tissues rather than direct colonization of cardiac valves. The transmigration of monocytes through endothelium might thus be altered by C. burnetii infection. While the modulation of endothelium functions by intracellular bacteria has been extensively studied (8, 15), little is known about the interaction between infected monocytes and endothelium (16). In this report, we showed that C. burnetii infection of monocytes reduced their transmigration capacities through endothelial cells (EC).
The THP1 monocytic cell line was cultured as previously described (6). THP1 monocytes were infected with virulent C. burnetii (Nine Mile strain) at a bacterium-to-cell ratio of 200:1 for 24 h, leading to infection of about 65% of cells, as determined by Gimenez staining. Virulent C. burnetii organisms are highly infectious and are found in infected animals or humans (14). They were recovered from the spleens of infected mice and used within two passages in cell culture (6). The human microvascular EC line (HMEC-1) kindly provided by E. W. Ades was cultured as previously described (1). Transmigration assays were performed as follows (13). EC (8 × 104 per insert) were seeded in gelatin-coated cell culture inserts (8-μm pore size) (Costar, Cambridge, Mass.) in 24-well plates for 6 days until tight confluence, assessed by resistivity. Only confluent cultures with a resistivity of 960 ± 50 Ω/cm2 were used in transmigration assays. They were then stimulated or not stimulated with 100 ng of recombinant human TNF (Peprotech, Rocky Hill, N.J.) per ml for 24 h. THP1 cells (4 × 104 per well), infected or not infected by C. burnetii, were added to the EC monolayer. The viability of transmigrated monocytes, i.e., the number recovered from medium beneath inserts, was assessed by the trypan blue exclusion test. Only viable cells were counted in a Malassez hemocytometer. Statistical analysis was performed using the Mann-Whitney U test or linear regression analysis.
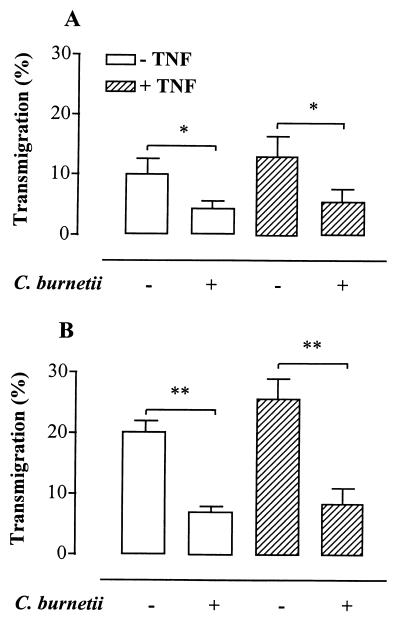
Transmigration of THP1 monocytes was detectable as early as 4 h after their addition onto EC monolayers. After 12 h, 10.2% ± 2.5% of control monocytes transmigrated across resting EC, whereas 4.4% ± 1.2% of C. burnetii-infected monocytes transmigrated (56.9% ± 2.3% inhibition; P < 0.02) (Fig. 1A). After 24 h, 20.3% ± 1.7% of control monocytes but only 7.2% ± 0.7% of infected monocytes transmigrated (62.7% ± 1.8% inhibition; P < 0.0001) (Fig. 1B). This inhibition of transmigration was not the consequence of a decrease in the number of viable infected cells (data not shown and reference 6). In addition, the transmigration process slightly decreased (less than 20%) the viability of both infected and noninfected monocytes. Monocyte transmigration across TNF-activated EC was slightly increased compared to control EC (Fig. 1). This result is consistent with the weak enhancement of transendothelial migration of monocytes under inflammatory conditions (10). The inhibition of transmigration of infected monocytes was independent of the inflammatory status of endothelial cells, i.e., EC stimulated with TNF (Fig. 1). Hence, 25.9% ± 3.0% of control monocytes transmigrated after 24 h, whereas only 8.5% ± 2.4% of infected monocytes transmigrated across TNF-stimulated EC (73.2% ± 3.8% inhibition; P < 0.0001). Monocytes with different degrees of infection were also studied. THP1 cells were incubated with C. burnetii at a bacterium-to-cell ratio of 50:1, which led to the infection of about 35% of the cells. This resulted in a inhibition of transmigration of 37.8% ± 4%. The inhibitory effect of infection on transmigration was correlated to the percentage of infected cells (r2 = 0.99). Also, the majority of transmigrated monocytes (75% ± 5%) were noninfected by C. burnetii. Clearly, our results show that the inhibition of monocyte transmigration across EC was related to the infection of monocytes by C. burnetii.
FIG. 1.
Transmigration of infected monocytes. THP1 monocytes infected or not infected by C. burnetii (bacterium-to-cell ratio of 200:1) were allowed to transmigrate for 12 h (A) or 24 h (B) across resting or TNF-stimulated EC. Data were expressed as the percentage of monocytes that migrated through EC. Values are means ± standard errors of the means of five experiments performed in triplicate. ∗, P < 0.02; ∗∗, P < 0.0001.
To assess if the inhibition of monocyte transmigration is related to bacterial virulence, we used avirulent variants of C. burnetii. These variants are noninfectious and are only obtained after serial passages in cell culture or embryonated egg culture (6, 14). Since avirulent organisms are more efficiently phagocytosed than virulent bacteria (4), we incubated monocytes with avirulent bacteria at a bacterium-to-cell ratio of 50:1 to obtain the same percentage of infected monocytes, i.e., 65% of infected cells. This led to a slight inhibition of transmigration (20.4% ± 7.2%), whereas infection by virulent organisms led to a marked inhibition of 63.1% ± 2.1% (P < 0.0001). These results clearly show that the inhibition of transmigration of infected monocytes depends mainly on the virulence of C. burnetii.
Transendothelial migration of leukocytes is a complex phenomenon, including tethering and rolling of leukocytes at the site of tissue injury, firm adhesion, and a transmigration step (9). We wondered if C. burnetii infection affects adherence properties of monocytes or the transmigration step per se. Adherence assays were performed in gelatin-coated 96-well plates containing confluent EC. THP1 monocytes (2 × 104 per well), infected or not infected with virulent C. burnetii, were labeled with 10 μM calcein-AM (Molecular Probes, Eugene, Oreg.) during 30 min at 37°C. They were then washed and added to the endothelial monolayer for 30 min at 37°C (2). Fluorescence (excitation and emission filters of 485 and 530 nm, respectively) was measured in a spectrofluorometer before (total cell fluorescence) and after (fluorescence of bound monocytes) three washings under gentle shaking. C. burnetii infection did not alter adherence of monocytes on resting EC (P = 0.12) (Fig. 2). Infected monocytes exhibited higher levels of adhesion to TNF-stimulated endothelium than control monocytes (P < 0.005) (Fig. 2). Thus, the deficiency of C. burnetii-infected monocytes affected the step of transmigration but not the adherence step. This is in contrast with a recent report which demonstrates that mononuclear cells from patients infected by Salmonella enteritidis show an increase in both adherence and transmigration through EC (11).
FIG. 2.
Adherence of infected monocytes. Monocytes infected or not infected by C. burnetii were labeled with 10 μM calcein-AM. They were allowed to adhere for 30 min to resting or TNF-stimulated EC. The percentage of adherent monocytes was calculated as follows: (fluorescence of bound monocytes/total cell fluorescence) × 100. Values are means ± standard errors of the mean of five experiments performed in triplicate. ∗, P < 0.005.
The alteration of transmigration of C. burnetii-infected monocytes described here might interfere with the vectorization of this bacterium into target tissues. It may affect the formation of granuloma, a process which is consistent with the poor granulomatous response observed in Q fever endocarditis patients.
Acknowledgments
J. Dellacasagrande and P. A. Moulin contributed equally to this work.
REFERENCES
- 1.Ades E W, Candal F J, Swerlick R A, George V G, Summers S, Bosse D C, Lawley T J. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Investig Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 2.Akeson A L, Woods C W. A fluorometric assay for the quantitation of cell adherence to endothelial cells. J Immunol Methods. 1993;163:181–185. doi: 10.1016/0022-1759(93)90121-m. [DOI] [PubMed] [Google Scholar]
- 3.Baca O G, Paretsky D. Q fever and Coxiella burnetii: a model for host-parasite interaction. Microbiol Rev. 1983;47:127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capo C, Lindberg F P, Meconi S, Zaffran Y, Tardei G, Brown E J, Raoult D, Mege J L. Subversion of monocyte functions by Coxiella burnetii: impairment of the cross-talk between αvβ3 integrin and CR3. J Immunol. 1999;163:6078–6085. [PubMed] [Google Scholar]
- 5.Capo C, Zugun F, Stein A, Tardei G, Lepidi H, Raoult D, Mege J L. Upregulation of tumor necrosis factor alpha and interleukin-1β in Q fever endocarditis. Infect Immun. 1996;64:1638–1642. doi: 10.1128/iai.64.5.1638-1642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellacasagrande J, Capo C, Raoult D, Mege J L. IFN-γ-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J Immunol. 1999;162:2259–2265. [PubMed] [Google Scholar]
- 7.Dellacasagrande J, Ghigo E, Capo C, Raoult D, Mege J L. Coxiella burnetii survives in monocytes from patients with Q fever endocarditis: involvement of tumor necrosis factor. Infect Immun. 2000;68:160–164. doi: 10.1128/iai.68.1.160-164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dignat-George F, Teysseire N, Mutin M, Bardin N, Lesaule G, Raoult D, Sampol J. Rickettsia conorii infection enhances vascular cell adhesion molecule-1- and intercellular adhesion molecule-1-dependent mononuclear cell adherence to endothelial cells. J Infect Dis. 1997;175:1142–1152. doi: 10.1086/520353. [DOI] [PubMed] [Google Scholar]
- 9.Imhof B A, Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- 10.Issekutz A C, Chuluyan H E, Lopes N. CD11/CD18-independent transendothelial migration of human polymorphonuclear leukocytes and monocytes: involvement of distinct and unique mechanisms. J Leukoc Biol. 1995;57:553–561. doi: 10.1002/jlb.57.4.553. [DOI] [PubMed] [Google Scholar]
- 11.Kirveskari J, Jalkanen S, Maki-Ikola O, Granfors K. Increased synovial endothelium binding and transendothelial migration of mononuclear cells during Salmonella infection. Arthritis Rheum. 1998;41:1054–1063. doi: 10.1002/1529-0131(199806)41:6<1054::AID-ART12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Koster F T, Williams J C, Goodwin J S. Cellular immunity in Q fever: modulation of responsiveness by a suppressor T cell-monocyte circuit. J Immunol. 1985;135:1067–1072. [PubMed] [Google Scholar]
- 13.Lou J, Gasche Y, Zheng L, Giroud C, Morel P, Clements J, Ythier A, Grau G E. Interferon-β inhibits activated leukocyte migration through human brain microvascular endothelial cell monolayer. Lab Investig. 1999;79:1015–1025. [PubMed] [Google Scholar]
- 14.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molestina R E, Miller R D, Ramirez J A, Summersgill J T. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67:1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuorela M, Tohka S, Granfors K, Jalkanen S. Monocytes that have ingested Yersinia enterocolitica serotype O:3 acquire enhanced capacity to bind to nonstimulated vascular endothelial cells via P-selectin. Infect Immun. 1999;67:726–732. doi: 10.1128/iai.67.2.726-732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]