Abstract
Aging increases the risk of atherosclerotic cardiovascular disease which is associated with arterial senescence; however, the mechanisms responsible for the development of cellular senescence in endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) remain elusive. Here, we study the effect of aging on arterial DNA damage and telomere dysfunction. Aging resulted in greater DNA damage in ECs than VSMCs. Further, telomere dysfunction–associated DNA damage foci (TAF: DNA damage signaling at telomeres) were elevated with aging in ECs but not VMSCs. Telomere length was modestly reduced in ECs with aging and not sufficient to induce telomere dysfunction. DNA damage and telomere dysfunction were greatest in atheroprone regions (aortic minor arch) versus non-atheroprone regions (thoracic aorta). Collectively, these data demonstrate that aging results in DNA damage and telomere dysfunction that is greater in ECs than VSMCs and elevated in atheroprone aortic regions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00681-6.
Keywords: Aging, DNA damage, Telomere dysfunction, Endothelial cell, Vascular smooth muscle cell
Introduction
Aging is the greatest risk factor for developing cardiovascular disease (CVD), which is the leading cause of death in elderly individuals [1]. Therefore, it is of critical importance to understand how aging increases the risk of CVD as the elderly population continues to grow. Advancing age results in arterial dysfunction that precedes the development of most age-related CVDs and predicts future CVD morbidity and mortality [2]. Aged arteries display elevated production of reactive oxygen species (ROS) and inflammation that contributes to arterial dysfunction and CVD progression [3–5]. Although the origin of this aged arterial phenotype remains incompletely understood, emerging evidence suggests that an increase in the number of resident senescent cells within arteries contributes to elevated burden of ROS and inflammation with advancing age [6].
Cellular senescence is defined as permanent cell cycle arrest and occurs due to a variety of stressors [7]. Aging increases senescence burden in whole arteries [8] and in endothelial cells (ECs) which is associated with impaired endothelial function [9]. Likewise, senescent ECs [10] and vascular smooth muscle cells (VSMCs) [11] have been identified at sites of atherosclerosis. However, evidence identifying the senescence-inducing stimuli that occurs in arteries with aging is lacking.
Two senescence inducing stimuli that likely contribute to arterial senescence and ultimately atherosclerotic CVD include total nuclear DNA damage [12] and telomere dysfunction [6, 13, 14]. Persistent DNA damage activates tumor suppressor pathways resulting in senescence, [15] and DNA damage contributes to vascular aging phenotypes and age-related CVD [12]. Telomeres are the ends of chromosomes comprised of repeated DNA sequences which are folded into a telomere (T)-loop by a protein complex known as shelterin, effectively capping the telomere [16]. Telomere capping protects chromosome ends, preventing recognition as damaged DNA and subsequent activation of the DNA damage response (DDR) [16, 17]. Loss of shelterin [18] or excessive telomere shortening which occurs with each cell division [19, 20] result in telomere uncapping. The telomere sequence contains many guanine triplets which are highly sensitive to oxidative modifications [21]. Oxidative damage at telomeres can lead to shortening or damage independent of telomere length [22–26]. Ultimately, telomere uncapping or damage to telomeres independent of length results in persistent DDR signaling at telomeres, known as telomere dysfunction-associated DDR foci (TAF) [26–28]. The susceptibility of telomeres to damage is important because TAF are highly predictive of senescence induction and thus are used as markers of senescence [8, 26, 27, 29, 30]. Based on these characteristics of telomere dynamics, telomeres likely act as cellular stress sensors, tracking lifetime replicative demands, and exposure to oxidative stress, ultimately signaling for senescence when cells have experienced excessive stress [18]. Importantly, the prevalence of DNA damage and telomere dysfunction with aging in ECs versus VSMCs and at sites prone to atherosclerotic CVD development is unknown.
Atherosclerosis preferentially develops at sites that experience low and oscillatory wall shear stress (WSS) in comparison to sites that experience high laminar WSS [31]. The predisposition of certain vascular regions to atherosclerosis likely stems from the cellular consequences of exposure to differing hemodynamic environments [32]. For example, ECs exposed to low and oscillatory WSS display elevated cell turnover and a pro-oxidant, pro-inflammatory phenotype akin to the canonical SASP [32, 33]. Evidence suggests that this cell turnover results in telomere attrition that is associated with atherosclerosis [34]. However, it is unclear whether this telomere attrition is sufficient to result in telomere dysfunction.
Here, we sought to determine if aging results in DNA damage and telomere dysfunction in ECs and VSMCs. We found that with aging, ECs have a greater DNA damage burden than VSMCs and that ECs but not VSMCs have dysfunctional telomeres that are not a result of telomere attrition. Furthermore, we evaluated atheroprone and non-atheroprone vascular regions. With aging, both DNA damage and telomere dysfunction were greatest in regions that are prone to atherosclerosis. These findings represent important insights into effects of aging on DNA damage and telomere dysfunction between vascular cell type and arterial regions.
Methods
Ethical approval and animals
All animal studies were in compliance with the Guide and Use of Laboratory Animals and were approved by the University of Utah, Veteran’s Affairs Medical Center-Salt Lake City (VAMC-SLC). Young 5.5 ± 0.5 mo C57BL/6 male mice were obtained from Charles River Inc. Old 25.6 ± 0.4 mo C57BL/6 mice were obtained from the National Institute of Aging colony maintained by Charles River Inc.
Aorta preparation
For aorta preparation, immunofluorescence-fluorescent in situ hybridization (IF-FISH), and imaging, samples were batched into groups of ≥ 3 young and ≥ 3 old animals (3 batches total). Mice were anesthetized with 2% isoflurane and the portal vein was cut. Mice were perfused through the left ventricle with saline for ~ 5 min until saline ran clear, followed by perfusion with 60 mL of paraformaldehyde (pH 7.4). The heart and connected aorta were carefully dissected down to the abdominal aorta and placed in cold physiological saline solution and pinned under a dissecting scope. Perivascular adipose tissue was gently removed from the thoracic aorta working towards the heart. The aorta was removed from the heart at the base and branches were gently cut off. The arch was then cut from the thoracic descending aorta and placed in 1 × PBS (pH 7.4, calcium and magnesium free, used for all steps where 1 × PBS was required) at 4 °C overnight.
Immunofluorescence–fluorescent in situ hybridization
Whole thoracic aorta and aortic arch sections were left intact during IF-FISH, which was performed in 0.2 mL PCR tubes. Subsequent steps were performed by transferring samples between tubes containing solutions. Samples were placed in 100% methanol at − 15 °C for 15 min and then rehydrated in 1 × PBS for 5 min at room temperature. Samples were placed in blocking solution containing 1 mg/mL bovine serum albumin, 3% goat serum, 0.1% Triton X-100, 1 mM EDTA, and all in 1 × PBS for 30 min at room temperature. Next, samples were placed in 1:500 53BP1 (Novus Biologicals, NB100-0304, Rabbit) antibody in blocking solution for 1 h at room temperature. Samples were washed 3 × 5 min in 1 × PBS, followed by incubation in 1:500 Alexa Fluor 555 (Invitrogen, A-21429, Goat anti-Rabbit) in blocking solution for 1 h at room temperature. Samples were washed 3 × 5 min in 1 × PBS, followed by dehydration in 70%, 95%, and 100% ethanol at room temperature for 5 min each. Samples were allowed to dry very briefly and placed in hybridization solution containing 2% Tris HCL, 60% formamide, and 5% blocking reagent from a 10% Roche stock (blocking reagent for nucleic acid hybridization, stock dissolved in maleic acid buffer containing 100 mM maleic acid, 150 mM NaCl, pH 7.4, Millipore Sigma), 1:200 Tel Probe (Integrated DNA Technologies, 5Alex488N/CC CTA ACC CTA ACC CTA A, purification: HPLC) all in diH2O. The PCR tubes were placed in a thermocycler and warmed to 60° for 10 min, followed by 10-min denaturation at 85 °C, and 2 h hybridization at 37 °C. Samples were placed in a washing solution containing 2 × saline-sodium citrate (SSC) buffer and 0.1% Tween 20 in diH2O for 2 × 10 min at 60 °C followed by a brief incubation in room temperature washing solution to bring the samples down to room temperature. Finally, samples were washed in 2 × SSC followed by 1 × SSC followed by diH2O for 10 min at room temperature. Under a dissecting scope, the aortic arch was cut laterally to separate the major arch from the minor arch. Thoracic aortas were cut open longitudinally. Samples were placed onto a cover slip containing DAPI Fluoromount G (VWR-Catalog #102,092–102) with the endothelial side facing down and gently slid into the DAPI using forceps. A glass slide was placed onto the smooth muscle side of the artery and mounted. Samples were weighed down by 1 kg weight for 5 min and stored in a dark container at 4 °C until imaging.
Imaging and analysis
Samples were imaged on an Olympus Fluoview FV1000 Confocal microscope at 100 × zoom, and the same settings were used for all samples. 1 μM Z-slices were taken through each nucleus. Images were converted to 16-bit images and analyzed using the Telometer Plugin for Image J (https://demarzolab.pathology.jhmi.edu/telometer/index.html). Telomere length was ascertained from mean telomere fluorescence intensity, which is calculated by the Telometer as the sum of all fluorescent intensity values for all pixels of the object divided by the object’s area. DNA damage was identified as distinct 53BP1 foci, and TAF were identified as 53BP1 foci which colocalized with telomere signal. Telomere length for each batch was normalized to the mean value of all telomere signals from the thoracic aorta of young animals from the respective batch.
Statistical analysis
Statistical analysis was performed in GraphPad Prism version 9.2.0 except for cell frequency distributions which were analyzed using Stata / BE 17.0. For comparisons between young and old ECs and VSMCs, two-way analysis of variance (ANOVA) models that included an interaction term were performed with least significant difference post-hoc tests to assess differences in age groups, cell types and vascular region. In the ANOVA models, we used one observation per mouse, which represented the mean of multiple cells. For outcomes related to vascular region, we fit a two-way ANOVA for each of the three vascular region subgroups. Our planned sample size of N = 11 mice/group provided 80% power to detect differences in main effect terms of the model, but no attempt was made to adequately power for interaction terms. Pearson correlation analysis was performed to assess correlations between ECs and VSMCs. Cell frequency distributions were analyzed using a multilevel linear regression to account for clustering of cells within animals. Data are presented as mean ± SEM. Significance was set as p < 0.05.
Results
Aging increases arterial DNA damage to a greater extent in ECs than VSMCs
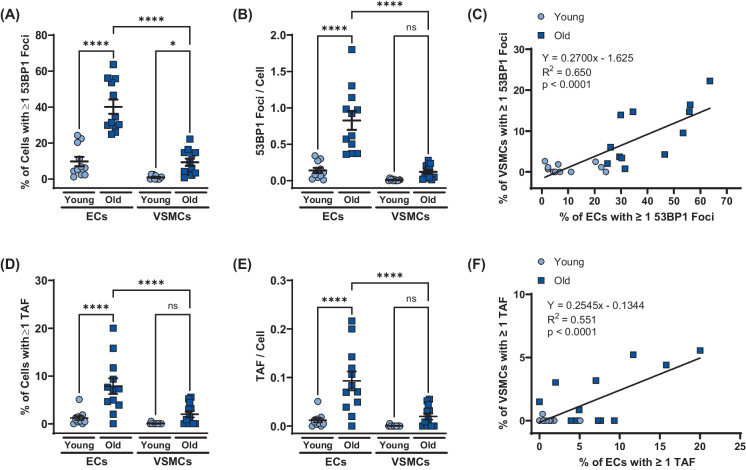
To examine whether aging results in arterial DNA damage and in which cell types, we quantified 53BP1 foci in ECs and VSMCs from aortas of young (5.5 ± 0.5 mo, N = 11, 1658 ECs, 1453 VSMCs) and old (25.6 ± 0.4 mo, N = 12, 1506 ECs, 1061 VSMCs) male mice. Aging increased the percentage of cells containing one or more 53BP1 in both ECs (p < 0.0001) and VSMCs (p < 0.05; Fig. 1A). The number of 53BP1 foci per cell was elevated with aging in ECs (p < 0.0001) but not VSMCs (p > 0.05; Fig. 1B). ECs from old mice displayed a higher percentage of cells with one or more 53BP1 foci and a greater number of 53BP1 per cell compared to VMSCs (both p < 0.0001; Fig. 1A and B). The percentage of ECs and VSMCs containing one or more 53BP1 foci was positively correlated (R2 = 0.650, p < 0.0001; Fig. 1C). These observations suggest that aging results in DNA damage in ECs and VSMCs; however, the DNA damage burden is greater in ECs as compared with VSMCs. Furthermore, the amount of DNA damage incurred is associated between the two cell types.
Fig. 1.
Effect of aging on double stranded DNA breaks and telomere dysfunction in aortic endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). A Percentage of ECs and VSMCs containing one or more 53BP1 foci. B Number of 53BP1 foci per EC and VSMC. C Positive relation between percentage of ECs and percentage of VSMCs containing one or more 53BP1 foci. D Percentage of ECs and VSMCs containing one or more telomere-associated DDR foci (TAF). E Number of telomere-associated DDR foci (TAFs) per EC and VSMC. F Positive relation between percentage of ECs and percentage of VSMCs containing one or more telomere-associated DDR foci (TAF) N = 11/12 per group. Data are mean ± SEM. *p < 0.05, ****p < 0.0001
Aging increases telomere dysfunction in ECs, but not VSMCs
To examine whether aging results in arterial telomere dysfunction and in which cell types it occurs, we quantified the number of TAF in ECs and VSMCs from aortas of young and old mice. The percentage of ECs containing one or more TAF as well as the number of TAF per cell was elevated in ECs from old compared to young mice (both p < 0.0001); however, aging did not result in increased TAF in VSMCs (Fig. 1D and E). Both the percentage of ECs containing one or more TAF and the number of TAF per cell were greater in ECs than VMSCs from old mice (both p < 0.0001; Fig. 1D and E). The percentage of ECs and VSMCs containing one or more TAF was positively correlated (R2 = 0.551, p < 0.0001; Fig. 1F). These observations suggest that aging results in telomere damage in ECs, but not VSMCs, yet the amount of TAF between the two cell types is correlated.
Aging-induced endothelial cell telomere dysfunction occurs independent of telomere length
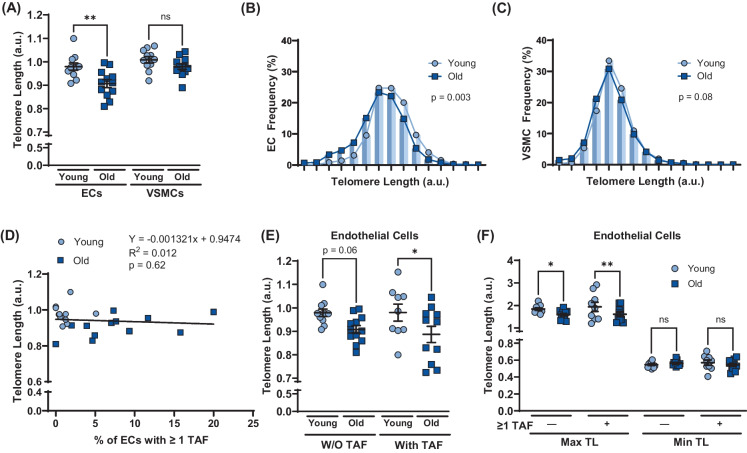
To determine if aging induced EC telomere dysfunction occurs as a result of telomere attrition and critically short telomeres, telomere length was quantified. Aging resulted in shorter telomere length in ECs (p < 0.01) but not VSMCs (p > 0.05; Fig. 2A). The frequency of ECs with short telomeres was greater in old versus young mice (p < 0. × 01; Fig. 2B). The frequency of VSMCs with short telomeres tended to be greater in old versus young mice but did not reach statistical significance (p = 0.08; Fig. 2C). There was no correlation between EC telomere length and the percentage of ECs containing one or more TAF (p > 0.05; Fig. 2D). Telomere length tended to be reduced with aging in ECs that did not contain TAF (p = 0.06); however, aging reduced telomere length in ECs that contained at least one TAF (p < 0.05; Fig. 2E). In old mice, telomere length in ECs and VSMCs with and without TAF was not different (Supplementary Fig. 1A). To examine if critically short telomeres were responsible for TAF, the maximum and minimum telomere length for each mouse in cells with and without TAF were quantified (Fig. 2F). Aging reduced maximal telomere length in cells with and without TAF; however, minimum telomere length was unaffected by aging independent of TAF (Fig. 2F). In old mice, maximum and minimum telomere length did not differ in ECs and VSMCs with and without TAF (Supplementary Fig. 1B and C). These data suggest that mean telomere length is reduced with aging in ECs but not VSMCs; however, telomere attrition is not responsible for age-related increases in telomere dysfunction because aging leads attrition of the longest, rather than the shortest telomeres.
Fig. 2.
Effect of aging on telomere length and influence of telomere length on telomere dysfunction in aortic endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). A Mean telomere length in ECs and VSMCs. B Frequency distribution of ECs based on mean telomere length. C Frequency distribution of VSMCs based on mean telomere length. D Relation between percentage of ECs containing one or more telomereassociated DDR foci (TAF) and telomere length. E Mean telomere length in ECs and VSMCs with and without (W/O) one or more telomere-associated DDR foci (TAF). F Maximum and minimum telomere length in ECs and VSMCs with and without (W/O) one or (TIF). A–D N = 11/12 per group. E–F N = 9/11 per group. Data are mean ± SEM. *p < 0.05, **p < 0.01 more uncapped telomere
Age-related increases in DNA damage are exacerbated in atheroprone regions
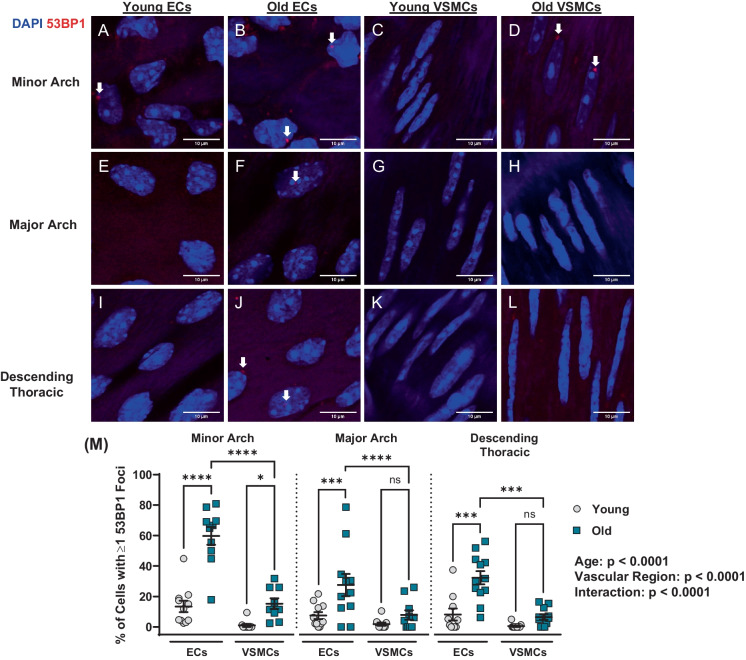
To determine if arterial DNA damage burden differs between atheroprone and non-atheroprone aortic regions, 53BP1 foci were compared at the minor arch, the major arch, and the descending thoracic aorta (Fig. 3A–M). Aging increased the percentage of cells with one or more 53BP1 foci (p < 0.0001; Fig. 3M). Likewise, there was a main effect for region (p < 0.0001) and an interaction between age and region (p < 0.0001; Fig. 3M). Post hoc analysis indicated that the percentage of cells with DNA damage was elevated with aging in ECs (p < 0.0001) and VSMCs (p < 0.05) at the minor arch; however, old ECs had more DNA damage than VSMCs at the same region (p < 0.0001; Fig. 3M). At the minor arch, the number of 53BP1 foci per cell was elevated with aging in ECs (p < 0.0001) but not VSMCs (p > 0.05;Supplementary Fig. 2A). Old ECs had more 53BP1 foci per cell than VSMCs at the minor arch (p < 0.0001; Supplementary Fig. 2A). At the major arch and the descending thoracic aorta, aging increased the percentage of ECs with one or more 53BP1 foci (p < 0.001; Fig. 3M), as well as the number of 53BP1 foci per EC (p < 0.01; Supplementary Fig. 2A). In old mice at the major arch and descending thoracic aorta, there was more cells with DNA damage and more DNA damage per cell in ECs versus VSMCs (p < 0.001; Fig. 3M; p < 0.01; Supplementary Fig. 2A). These data suggest that aging increases DNA damage in both ECs and VSMCs; however, DNA damage is more common in ECs versus VSMCs and in atheroprone regions versus non-atheroprone regions.
Fig. 3.
Effect of aging on double stranded DNA breaks in aortic endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) at atheroprone and non-atheroprone regions. Representative images of ECs (A–B) and VSMCs (C–D) from minor arch. Representative images of ECs (E–F) and VSMCs (G–H) from major arch. Representative images of ECs (I–J) and VSMCs (K–L) from descending thoracic aorta. M Percentage of ECs and VSMCs containing one or more 53BP1 foci. N = 11/12 per group. Data are mean ± SEM. Dapi — blue, 53BP1 — red (shown by white arrows). *p < 0.05, ***p < 0.001, ****p < 0.0001
Aging results in endothelial cell telomere dysfunction at atheroprone regions
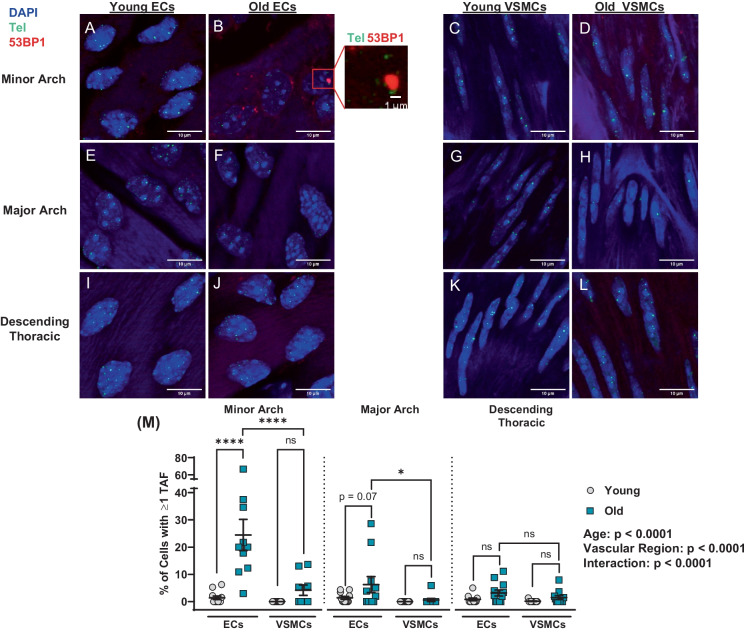
To determine if age, vascular region, and cell type affect telomere dysfunction, TAF were compared at the minor arch, the major arch, and the descending thoracic aorta (Fig. 4A–M). Aging increased the percentage of cells with one or more TAF (p < 0.0001; Fig. 4M). Likewise, there was an effect for region (p < 0.0001) and an interaction between age and region (p < 0.0001; Fig. 4M). Post hoc analysis indicated that the percentage of cells with one or more TAF was elevated with aging in ECs at the minor arch (p < 0.0001), but was not different in VSMCs (p > 0.05; Fig. 4M). Similarly, the number of TAF per cell was elevated in ECs with aging at the minor arch (p < 0.0001) but not VSMCs (p > 0.05), and the number of TAF per cell in old ECs was greater than old VSMCs (p < 0.0001, Supplementary Fig. 3A). In the major arch, the percentage of cells with one or more TAF was greater in old ECs versus VSMCs; however, aging did not increase telomere damage in either cell type in the major arch or descending thoracic aorta (both p > 0.05; Fig. 4M). These data indicate that aging results in telomere damage in ECs at atheroprone regions but not at non-atheroprone regions or VSMC independent of region.
Fig. 4.
Effect of aging on telomere dysfunction in aortic endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) at atheroprone and non-atheroprone regions. Representative images of ECs (A–B) and VSMCs (C–D) from minor arch. Representative images of ECs (E–F) and VSMCs (G–H) from major arch. Representative images of ECs (I–J) and VSMCs (K–L) from descending thoracic aorta. M Percentage of ECs and VSMCs containing one or more telomere-associated DDR foci (TAF). N = 11/12 per group. Data are mean ± SEM. *p < 0.05, ****p < 0.0001
Aging results in endothelial cell telomere shortening at atheroprone and non-atheroprone regions that does not lead to telomere dysfunction
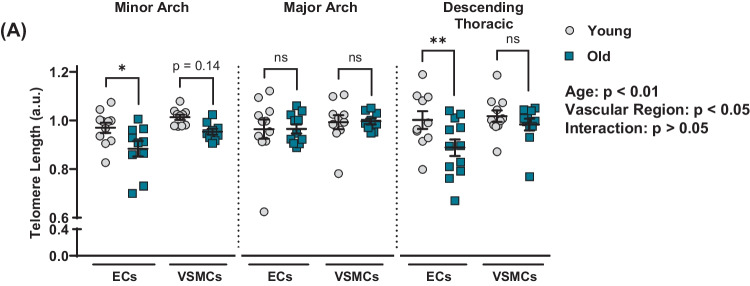
To examine whether aging results in telomere shortening in atheroprone versus non-atheroprone aortic regions, telomere length was quantified at the minor arch, the major arch, and the descending thoracic aorta. There was an effect of age (p < 0.01) and vascular region (p < 0.05) on telomere length, but no interaction between the two (p > 0.05; Fig. 5A). Post hoc analysis indicated that aging resulted in telomere attrition in ECs at the minor arch (p < 0.05) and descending thoracic aorta (p < 0.01; Fig. 5A). Aging did not affect telomere length in ECs at the major arch nor in VSMCs at any vascular region (p > 0.05; Fig. 5A).
Fig. 5.
Effect of aging on telomere length in aortic endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) at atheroprone and non-atheroprone regions. A Mean telomere length in ECs and VSMCs. N = 9 – 12 per group. Data are mean ± SEM. *p < 0.05, **p < 0.01
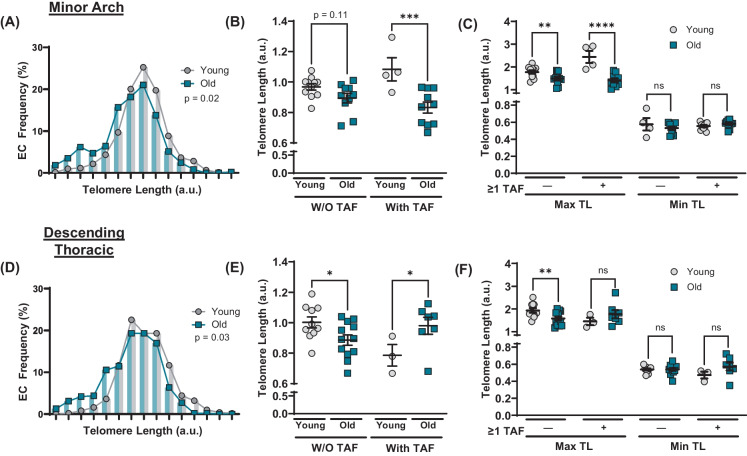
To explore the relationship between age-related EC telomere attrition and telomere dysfunction in atheroprone versus non-atheroprone aortic regions, we evaluated telomere length and its influence on telomere dysfunction at the minor arch and the descending thoracic aorta. In the minor arch, old mice had a higher frequency of ECs with short telomeres (p < 0.05; Fig. 6A). With aging, telomere length tended to be reduced in ECs without TAF (p = 0.11; Fig. 6B). In cells that contained one or more TAF, aging resulted in shorter telomeres (p < 0.001; Fig. 6B), suggesting that telomere dysfunction may be related to decreases in telomere length. However, detailed analysis of how telomere attrition occurred revealed that the maximum telomere length in each mouse was reduced in both cells with and without TAF (p < 0.0001 vs. p < 0.01, respectively), whereas minimum telomere length was unaltered regardless of TAF status (p > 0.05; Fig. 6C). Furthermore, in old mice at the minor arch, telomere length in ECs with and without TAF was not different (p > 0.05; Supplementary Fig. 4A). In the descending thoracic aorta where telomere damage did not increase with aging (Fig. 4M), telomere length was still reduced, as old animals had a greater percentage of ECs with short telomeres (p < 0.05; Fig. 6D). Telomere length was reduced with aging in cells without TAF (p < 0.05) and increased with aging in cells with one or more TAF (p < 0.05; Fig. 6E). Decreases in mean telomere length (Fig. 5A) and cell telomere length frequency (Fig. 6D) were driven by reductions in maximum telomere length in cells without TAF (p < 0.01), as there were no differences in maximum or minimum telomere length in cells with TAF (p > 0.05; Fig. 6F). Additionally, in old mice at the descending thoracic aorta, telomere length in ECs with and without TAF was not different (p > 0.05; Supplementary Fig. 4B). In the major arch, there were no differences in EC telomere length frequency, telomere length in cells with and without TAF, or maximum or minimum telomere length regardless of TAF status (p > 0.05; Supplementary Fig. 5A-C). In total, these observations suggest that telomere length is reduced in ECs with aging at the minor arch and descending thoracic aorta; however, telomere attrition is not sufficient to result in telomere dysfunction.
Fig. 6.
Influence of telomere length on telomere dysfunction in aortic endothelial cells (ECs) at atheroprone and non-atheroprone region. A Frequency distribution of ECs from minor arch based on mean telomere length. N = 10/11 per group. B Mean telomere length in ECs from minor arch with and without (W/O) one or more telomere-associated DDR foci (TAF). N = 4–11 per group. C Maximum and minimum telomere length in ECs from minor arch with and without (W/O) one or more telomere-associated DDR foci (TAF). N = 4–11 per group. D Frequency distribution of ECs from descending thoracic aorta based on mean telomere length. N = 10 / 12 per group. E Mean telomere length in ECs from descending thoracic aorta with and without (W/O) one or more telomere-associated DDR foci (TAF). N = 3–12 per group. F Maximum and minimum telomere length in ECs from descending thoracic aorta with and without (W/O) one or more telomere-associated DDR foci (TAF). N = 3–12 per group. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
The key novel findings of the present study are as follows. First, aging results in DNA damage that is more abundant in ECs than VSMCs. Second, aging increases telomere dysfunction in ECs, but not VSMCs. Third, aging induced EC telomere dysfunction occurs independent of critically short telomeres. Fourth, at atheroprone aortic regions DNA damage is elevated in ECs and VSMCs, and telomere dysfunction occurs independent of critically short telomeres exclusively in ECs. To our knowledge, this is the first report of the effect of aging on DNA damage and telomere dysfunction in ECs versus VSMCs and at atheroprone versus non-atheroprone vascular regions. Interestingly, the findings of the present study suggest DNA damage and abundance of telomere dysfunction is greater in ECs than VSMCs and is elevated in atheroprone regions suggesting this may be a foundational alteration that promotes arterial dysfunction, inflammation, and subsequent atherosclerosis in those regions.
Influence of aging on EC and VSMC DNA damage
DNA damage is thought to contribute directly to age-related arterial dysfunction [12]. The present study represents the first comprehensive examination of arterial DNA damage burden in the context of healthy aging. We found that aging increases the abundance of cells with DNA damage in both ECs and VSMCs; however, the number of DNA damage foci per cell is elevated in ECs from old mice but not VSMCs. Interestingly, arterial DNA damage burden appears to be unique to each mouse, as the percent of cells containing DNA damage foci was associated between ECs and VSMCs. Senescence, a potential consequence of DNA damage, is increasingly heterogeneous in old versus young animals [35]. Despite significant heterogeneity amongst individuals, our findings suggest that at least in the aorta, DNA damage burden is comparable across cell types. Whether this extends beyond cells in large arteries is unknown but holds important implications as it is technically challenging to survey DNA damage specifically in ECs as compared with whole artery samples.
Understanding the effect of advancing age on arterial DNA damage burden is important because existing evidence suggests that it contributes to arterial dysfunction and CVD. For example, mice with reduced DNA damage repair capacity display an accelerated vascular aging phenotype [36, 37]. Furthermore, in humans, accelerated aging syndromes caused by mutations in genes involved in DNA repair and maintenance often lead to CVD [38]. Likewise, DNA damage is elevated in age-related CVD such as atherosclerosis [11, 39], and patients treated with genotoxic chemotherapies have higher rates of CVD [40]. Taken together, these findings suggest an important role for DNA damage in age-related arterial dysfunction and CVD. Thus, our study provides critical insight into the frequency (% of cells) and quantity (no. of foci per cell) of arterial DNA damage in the context of healthy aging, and more specifically, identifies which cell types and vascular regions it is occurring in.
Influence of aging on EC and VSMC telomere dysfunction
DNA damage is an established mediators of senescence [15]. However, in VSMCs and other cell types, DNA damage may be repaired when DDR signaling is intact [24, 26, 27, 30]. This is contrast to DNA damage signaling at telomeres (i.e., TAF) which is persistent and resists repair, likely due to the presence of shelterin proteins that inhibit DDR to prevent chromosome fusions [22–24]. Due to the sustained nature of DNA damage signaling at uncapped telomeres, TAF are highly predictive of senescence induction in as much as they are used as a marker of senescence [8, 26, 27, 29]. Interestingly, in the present study, we found that aging increases the percentage of cells with TAF and the number of TAF per cell in ECs but not VSMCs. Utilizing IF-FISH allowed us to determine the contribution of telomere shortening to telomere uncapping with single-cell resolution. Aging resulted in telomere attrition in ECs but not VSMCs due to a greater frequency of ECs containing short telomeres. However, in ECs, telomere length was not associated with TAF status and reductions in length were driven by changes in maximum rather than minimum telomere length (an important determinant of telomere dysfunction [41]), supporting the idea that aging does not result in critically short telomeres in arteries [6, 8, 13].
Prior studies from our group demonstrated that in older adults, arterial telomere dysfunction occurs irrespective of telomere length and is associated with senescence [8]. The present study builds upon this work by demonstrating the contributions of various arterial cell types. While mice have longer telomeres than humans, our data suggest that the rate of shortening between ECs (6.7%) and VSMCs (3.0%) from 5.5 to 25.6 months in mice equates to a similar net loss of telomeric DNA as seen in human arteries. In human arteries, telomere attrition equates to an approximately 2.9 kb reduction in mean telomere length in older adults (70.9 years old) compared with younger adults (31.2 years old) [8]. Given a starting length of 40 kb (a low estimate for C57BL/6 mice), we found a 2.7 kb reduction in telomere length in ECs from young mice compared with that in old animals. Importantly, this would result in an average mean telomere length of 37.3 kb, whereas the reduction in telomere length required to induce endothelial dysfunction and senescence is far greater (estimated at ~ 64.5% or 25.8 kb reduction in length) [42]. In total, these data support findings in human arterial tissue and indicate that aging results in EC telomere dysfunction that occurs independent of telomere length. Furthermore, aging does not impact telomere dysfunction or telomere length in VSMCs.
Influence of aging on DNA damage and telomere dysfunction at atheroprone versus non-atheroprone aortic regions
Hemodynamics dictate preferential development of atherosclerosis at certain vascular regions [32]. For example, regions that experience low WSS such as the minor arch of the aorta are more likely to develop atherosclerosis [32]. In the present study, we demonstrated for the first time that healthy aging increases both DNA damage and telomere dysfunction at atheroprone vascular regions. Additionally, we demonstrated that telomere attrition at sites of disturbed flow does not contribute to telomere dysfunction, as maximum telomere length was reduced with aging while minimum telomere lengths were not different.
Disease development in atheroprone regions (e.g., minor arch) versus non-atheroprone regions (e.g., thoracic aorta) likely stems from the cellular consequences of exposure to differing hemodynamics [32, 43]. In culture, exposing endothelial cells to disturbed flow results in oxidation of DNA and telomere attrition, likely due to increased cell turnover [33]. In human tissue samples, both senescent ECs and VSMCs have been identified in atherosclerotic lesions at a higher proportion than in non-atherosclerotic regions from the same individuals [10, 44]. These studies implicated telomere attrition as a potential cause of senescence [10, 44], which is supported by findings that telomere attrition in the intima and medial layer is associated with atherosclerotic lesion grade exclusively in regions that experience low and oscillatory WSS [34]. However, when adjusted for age, this association is no longer significant [34]. Importantly, these studies did not demonstrate that telomere attrition results in critically short telomeres. Therefore, it is possible that telomere attrition is a byproduct of cellular replication.
Preclinical rodent models provide insight into whether telomere attrition or dysfunction independent of length contributes more to atherosclerosis. Mice bred to have critically short telomeres have reduced atherosclerotic plaque size [45], whereas disruption of the telomere capping protein TRF2 in either ECs [46] or VSMCs [11] promotes atherosclerosis. Additionally, non-telomeric DNA damage is found in human atherosclerotic plaques, increases arterial senescence, and accelerates the development of atherosclerosis [39, 43, 47, 48]. These studies indicate that activation of the DDR both at telomeres and in non-telomeric regions, rather than telomere shortening, contributes to the development of atherosclerotic CVD. In the present study, we demonstrated that telomere attrition occurs at atheroprone regions. Importantly, however, we found that telomere attrition does not contribute to telomere dysfunction. The increases in telomere dysfunction independent of length may in part be explained by recent evidence that suggests that dividing cells (as is seen at sites of oscillatory but not laminar flow) are susceptible to oxidation of telomeric DNA that induces TAFs and senescence independent of telomere length by impairing telomere replication [49]. Furthermore, in the present study, we found that advancing age results in DNA damage and telomere dysfunction that is elevated at atheroprone vascular regions and therefore may explain the increased senescence burden that is seen in and contributes to atherosclerosis.
Experimental considerations
We recognize several limitations of our study that may be addressed in future studies. In the present study, we utilized a single marker for DNA damage (53BP1) and assessment of telomere dysfunction (IF-FISH). Future studies could include assessment of DNA damage using γH2A.X to improve our understanding of the DDR at telomeres in ECs and VSMCs. Additionally, there are inherent limitations to assessing telomere dynamics with IF-FISH, including that it may be possible for chromosomes to have an insufficient number of telomere repeats for hybridization [50], and additional methods should be utilized in future studies. However, at present, IF-FISH represents the best available technique for use in inbred mouse strains with long telomeres, and provides important information about damage burden at telomeres that is not acquired using other telomere length measurement techniques. Furthermore, our understanding that critically short telomeres, rather than exclusively mean telomere length, are important was uncovered using this technique [41]. Moreover, IF-FISH allows quantitation of telomere length and damage within a population of cells, including cells from fixed tissues that are difficult to study such as the endothelial monolayer. Other techniques often require greater starting material that may be hard to obtain when studying the endothelium and therefore require culturing of cells, confounding the ability to quantify the extent of DNA damage and telomere dysfunction as they exist in vivo. Future studies may utilize cell culture methods to expand upon the findings of the current study. For example, it will be of interest to determine if aging increases susceptibility to hemodynamic stress such as that experienced in atheroprone regions that may explain the greater DNA damage and telomere dysfunction at the aortic minor arch, or if persistent exposure to hemodynamic stress across the lifespan is responsible. Additional studies may also examine if DNA damage and TAF at atheroprone vs. non-atheroprone regions occur in atherosclerotic regions. Finally, it will be important to determine if the findings of the present study in male mice are comparable to female mice.
Conclusions
Here, we demonstrate that aging results in a greater abundance of DNA damage in ECs than in VSMCs. Further, aging results in telomere dysfunction in ECs but not VSMCs. Importantly, EC telomere dysfunction occurs independent of telomere length. Finally, we found that cells at atheroprone sites experience greater DNA damage and telomere dysfunction than in regions not typically associated with the development of atherosclerosis. It is possible that lifetime exposure to hemodynamic stress at atheroprone aortic regions is responsible for the greater burden of DNA damage and telomere dysfunction in ECs versus VSMCs. In summary, we believe that these findings represent important observations about the age-related cellular and molecular processes within ECs and VSMCs that lead to senescence and ultimately atherosclerotic CVD.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
SIB and AJD designed the study. SIB and JRT performed the experiments. SIB, JRT, JL, TGT, and GJS analyzed data. SIB, LAL, and AJD interpreted the data. SIB and AJD wrote the manuscript. All authors read and approved of the final version of the manuscript.
Funding
This investigation was supported by the University of Utah Population Health Research (PHR) Foundation, with funding in part from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and The National Institutes of Health awards UL1TR002538 (formerly 5UL1TR001067-05, 8UL1TR000105 and UL1RR025764), R01 AG060395 (AJD), R01 AG050238 (AJD), R44 AG053131 (AJD), R01 AG048366 (LAL), F31AG076312 (SIB), T32HL007576 (JL) and Veteran’s Affairs Merit Review Award I01 BX004492 (LAL) from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Disclaimer
The contents do not represent the views of the US Department of Veterans Affairs, the National Institutes of Health, or the US Government.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Center for Health S. Health, United States. Health, United States, 2016: with chartbook on long-term trends in health. Hyattsville: National Center for Health Statistics (US); 2017. [PubMed]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51(6):1651–1657. doi: 10.1161/hypertensionaha.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 5.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123(7):825–848. doi: 10.1161/circresaha.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Bloom SI, Donato AJ. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation. 2019;26(2):e12487. doi: 10.1111/micc.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 8.Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, et al. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013;305(2):H251–H258. doi: 10.1152/ajpheart.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, et al. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313(5):H890–H895. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, et al. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation. 2015;132(20):1909–1919. doi: 10.1161/circulationaha.115.016457. [DOI] [PubMed] [Google Scholar]
- 12.Bautista-Niño PK, Portilla-Fernandez E, Vaughan DE, Danser AH, Roks AJ. DNA damage: a main determinant of vascular aging. Int J Mol Sci. 2016;17(5). 10.3390/ijms17050748. [DOI] [PMC free article] [PubMed]
- 13.Morgan RG, Donato AJ, Walker AE. Telomere uncapping and vascular aging. Am J Physiol Heart Circ Physiol. 2018;315(1):H1–h5. doi: 10.1152/ajpheart.00008.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RG, Walker AE, Trott DW, Machin DR, Henson GD, Reihl KD, et al. Induced Trf2 deletion leads to aging vascular phenotype in mice associated with arterial telomere uncapping, senescence signaling, and oxidative stress. J Mol Cell Cardiol. 2019;127:74–82. doi: 10.1016/j.yjmcc.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326(5955):948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roake CM, Artandi SE. Control of cellular aging, tissue function, and cancer by p53 downstream of telomeres. Cold Spring Harb Perspect Med. 2017;7(5). 10.1101/cshperspect.a026088. [DOI] [PMC free article] [PubMed]
- 18.Victorelli S, Passos JF. Telomeres and cell senescence — size matters not. EBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225(4):951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 20.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 21.Oikawa S, Kawanishi S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453(3):365–368. doi: 10.1016/s0014-5793(99)00748-6. [DOI] [PubMed] [Google Scholar]
- 22.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 23.Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019;38(23):e101982. doi: 10.15252/embj.2019101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019;38(5). 10.15252/embj.2018100492. [DOI] [PMC free article] [PubMed]
- 25.Lagnado A, Leslie J, Ruchaud-Sparagano MH, Victorelli S, Hirsova P, Ogrodnik M, et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021;40(9):e106048. doi: 10.15252/embj.2020106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14(4):355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossiello F, Jurk D, Passos JF, d’Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135–47. doi: 10.1038/s41556-022-00842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González-Gualda E, Baker AG, Fruk L, Muñoz-Espín D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021;288(1):56–80. doi: 10.1111/febs.15570. [DOI] [PubMed] [Google Scholar]
- 30.Uryga AK, Grootaert MOJ, Garrido AM, Oc S, Foote K, Chappell J, et al. Telomere damage promotes vascular smooth muscle cell senescence and immune cell recruitment after vessel injury. Commun Biol. 2021;4(1):611. doi: 10.1038/s42003-021-02123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24(1):12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 32.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotla S, Vu HT, Ko KA, Wang Y, Imanishi M, Heo KS, et al. Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight. 2019;4(9). 10.1172/jci.insight.124867. [DOI] [PMC free article] [PubMed]
- 34.Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152(2):391–398. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 35.Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 2013;152(1–2):340–351. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bautista-Niño PK, Portilla-Fernandez E, Rubio-Beltrán E, van der Linden JJ, de Vries R, van Veghel R, et al. Local endothelial DNA repair deficiency causes aging-resembling endothelial-specific dysfunction. Clin Sci (Lond) 2020;134(7):727–746. doi: 10.1042/cs20190124. [DOI] [PubMed] [Google Scholar]
- 37.Ataei Ataabadi E, Golshiri K, van der Linden J, de Boer M, Duncker DJ, Jüttner A, et al. Vascular ageing features caused by selective DNA damage in smooth muscle cell. Oxid Med Cell Longev. 2021;2021:2308317. doi: 10.1155/2021/2308317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capell BC, Collins FS, Nabel EG. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res. 2007;101(1):13–26. doi: 10.1161/circresaha.107.153692. [DOI] [PubMed] [Google Scholar]
- 39.Shah A, Gray K, Figg N, Finigan A, Starks L, Bennett M. Defective base excision repair of oxidative DNA damage in vascular smooth muscle cells promotes atherosclerosis. Circulation. 2018;138(14):1446–1462. doi: 10.1161/circulationaha.117.033249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–3131. doi: 10.1161/01.cir.0000133187.74800.b9. [DOI] [PubMed] [Google Scholar]
- 41.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 42.Bhayadia R, Schmidt BM, Melk A, Hömme M. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci. 2016;71(2):161–169. doi: 10.1093/gerona/glv008. [DOI] [PubMed] [Google Scholar]
- 43.Bloom SI, Islam MT, Lesniewski LA, Donato AJ. Mechanisms and consequences of endothelial cell senescence. Nat Rev Cardiol. 2022 doi: 10.1038/s41569-022-00739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99(2):156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 45.Poch E, Carbonell P, Franco S, Díez-Juan A, Blasco MA, Andrés V. Short telomeres protect from diet-induced atherosclerosis in apolipoprotein E-null mice. FASEB J. 2004;18(2):418–420. doi: 10.1096/fj.03-0710fje. [DOI] [PubMed] [Google Scholar]
- 46.Honda S, Ikeda K, Urata R, Yamazaki E, Emoto N, Matoba S. Cellular senescence promotes endothelial activation through epigenetic alteration, and consequently accelerates atherosclerosis. Sci Rep. 2021;11(1):14608. doi: 10.1038/s41598-021-94097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercer JR, Cheng KK, Figg N, Gorenne I, Mahmoudi M, Griffin J, et al. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circ Res. 2010;107(8):1021–1031. doi: 10.1161/circresaha.110.218966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, et al. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4(5):377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Barnes RP, de Rosa M, Thosar SA, Detwiler AC, Roginskaya V, Van Houten B, et al. Telomeric 8-oxo-guanine drives rapid premature senescence in the absence of telomere shortening. Nat Struct Mol Biol. 2022;29(7):639–652. doi: 10.1038/s41594-022-00790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai TP, Wright WE, Shay JW. Comparison of telomere length measurement methods. Philos Trans R Soc Lond B Biol Sci. 2018;373(1741). 10.1098/rstb.2016.0451. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.