Abstract
Aging of the cardiovascular regulatory function manifests as an imbalance between the sympathetic and parasympathetic (vagal) components of the autonomic nervous system (ANS). The most characteristic change is sympathetic overdrive, which is manifested by an increase in the muscle sympathetic nerve activity (MSNA) burst frequency with age. Age-related changes that occur in vagal nerve activity is less clear. The resting tonic parasympathetic activity can be estimated noninvasively by measuring the increase in heart rate occurring in response to muscarinic cholinergic receptor blockade; animal study models have shown this to diminish with age. Humoral, cellular, and neural mechanisms work together to prevent non-resolving inflammation. This review focuses on the mechanisms underlying age-related alternations in the ANS and how an imbalance in the ANS, evaluated by MSNA and heart rate variability (HRV), potentially facilitates inflammation when the homeostatic mechanisms between reflex neural circuits and the immune system are compromised, particularly the dysfunction of the cholinergic anti-inflammatory reflex. Physiologically, the efferent arm of this reflex acts via the 7 nicotinic acetylcholine receptors expressed in macrophages, monocytes, dendritic cells, T cells, and endothelial cells to curb the release of inflammatory cytokines, in which inhibition of NF‑κB nuclear translocation and activation of a JAK/STAT-mediated signaling cascade in macrophages and other immune cells are implicated. This reflex is likely to become less adequate with advanced age. Consequently, a pro-inflammatory state induced by reduced vagus output with age is associated with endothelial dysfunction and may significantly contribute to the development and propagation of atherosclerosis, heart failure, and hypertension. The aim of this review is to summarize the relationship between ANS dysfunction, inflammation, and endothelial dysfunction in the context of aging. Meanwhile, this review also attempts to describe the role of HRV measures as a predictor of the level of inflammation and endothelial dysfunction in the aged population and explore the possible therapeutical effects of vagus nerve stimulation.
Keywords: Aging, Autonomic nervous system, Inflammation, Oxidative stress, Endothelial dysfunction
Introduction
Aging is commonly defined as the accumulation of diverse deleterious changes occurring in cells and tissues with advancing age that is responsible for the increased risk of disease and death [1]. Aging is an extremely complicated process that cannot be explained by one single cause. From the cellular standpoint, the most well-known theory is the free radical theory [2], which proposes that reactive oxygen species (ROS) result in cumulative damage and senescence [3]. Similar to every other organ and system in the human body, the autonomic nervous system is affected by advanced age leading to an imbalance between the sympathetic and parasympathetic systems [4–6].
Although a dysregulated innate immunity, cell senescence, and several other factors have been proposed as critical sources of the chronic inflammatory state in aging [7], the diminished parasympathetic activity appears to play another key role. Autonomic nervous system (ANS) dysfunction could facilitate a tilt towards a pro-inflammatory state via diminished parasympathetic activity. Due to the anti-inflammatory effects of the vagus nerve, disruption of the vagus nerve is documented to perpetuate the inflammatory state [8]. Inflammation and oxidative stress then interact to increase the risk for endothelial dysfunction and a variety of related cardiovascular disease processes [9–11]. In this review, we aim to collect current evidence pinpointing how the altered ANS activity functions as a supplemental source of chronic inflammation in aging through an impaired cholinergic anti-inflammatory reflex caused by a decreased activity in the parasympathetic nervous system. Reduced efferent output from the reflex circuit and reduced stimulation of the splenic and enteric macrophage α7-nicotinic receptors cause an increase in pro-inflammatory cytokine release. Heart rate variability (HRV) serves important clinical purposes when used as a risk stratification tool in predicting clinical outcome. Thus, we summarized the changes in HRV measures observed in age-related inflammation and endothelial dysfunction. We also illustrate the interaction between inflammation and ROS in endothelial dysfunction as well as summarize the dynamic relationship between age-related autonomic dysfunction, inflammation, and endothelial dysfunction.
Age-related dysfunction of the autonomic nervous system
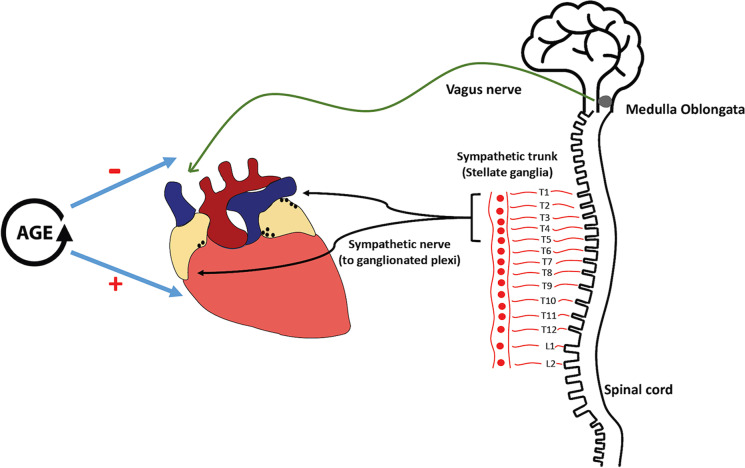
The extrinsic part of the ANS includes both sympathetic and parasympathetic components, which work in a collaborative yet counteractive way to modulate cardiac physiology [12]. Anatomically, the sympathetic component is regulated via the bulbospinal pathways originating from the medulla to activate the preganglionic synapses in the lateral column of the spinal cord mainly from T1 to T5 but may be extended to as low as T6/7 [13] (Fig. 1). Cell bodies of postganglionic neurons mainly reside in the paravertebral stellate ganglia, with their axons terminating on the myocardium [14, 15]. The parasympathetic preganglionic axons originate from the nucleus ambiguous in the medulla oblongata and the dorsal motor nucleus of the vagal nerve in the brainstem. Parasympathetic fibers then synapse within the cardiac ganglia located on the surface of the heart [12, 16, 17].
Fig. 1.
Anatomy and age-related changes of the cardiac autonomic nervous system. The vagus nerve originates from nuclei of the medulla oblongata. Parasympathetic fibers of the vagus nerve cardiac branches then synapse within the cardiac ganglia located on the surface of the heart. However, sympathetic nerve synapses within the paravertebral stellate ganglia and axons of postganglionic neurons mainly terminate on the myocardium. Weakened vagal activity and increased sympathetic tone are usually observed with aging
Increased sympathetic activity in aging
An increase in sympathetic activity is the characteristic functional change that occurs with aging. The neurography technique is the gold standard applied to demonstrate increased muscle sympathetic activity with age. In a cross-sectional study with the largest sample size to date discussing the effects of age and sex on resting muscle sympathetic nerve activity (MSNA), both the burst frequency and burst incidence of the common fibular nerve increase with age in both genders [4]. A direct measurement of adrenal sympathetic nerve activity in rats also showed that nerve activity frequency increases with age [18]. Venous plasma norepinephrine (NE) introduced by nerve-ending spillover provides a useful estimation of the average sympathetic outflow. In humans, an age-related increase in plasma NE is observed due to both an increased NE spillover into the circulation and decreased plasma NE clearance [19, 20]. Notably, both plasma epinephrine levels and clearance rate are not found to be different between younger and older subjects [21].
Electrocardiograms recorded under a controlled environment can be used to generate HRV measures, which is based on the RR interval. High-frequency (HF, 0.18–0.4 Hz) cyclic fluctuations of HRV are modulated by ventilation and mediated entirely by changes in vagal outflow [22]; the low frequency (LF, 0.04 to 0.15 Hz) component appears to be mediated by sympathetic activity with some associated parasympathetic influence [23, 24]. The very low-frequency (VLF, 0.0033–0.04 Hz) component represents long-term regulation, thermoregulation, and hormonal regulatory mechanisms [24]. Despite controversy [25, 26], the LF/HF ratio is still used as a measure of cardiac sympathovagal balance in clinical studies, wherein its increase reflects the predominance of sympathetic over parasympathetic activity. Some of the HRV measurements can reflect the parasympathetic modulation of heart rate in response to respiration, but none of them can represent tonic vagus nerve activity, and discrepancies have been found between HRV measurements and direct sympathetic/vagal activity recording [27, 28]. Nonetheless, HRV is frequently used in clinical studies to represent cardiac ANS activity. HRV serves an important clinical purpose when used as a risk stratification tool for endothelial function and inflammatory disease prognosis (Tables 1 and 2). Nevertheless, it is well established that HRV abnormalities in multiple time-domain frequency measures such as SDNN, SDANN, pNN50, and frequency measures such as HF power, LF power, VLF power, and LF/HF ratio correlate with advancing age [54–56] (Table 3).
Table 1.
Correlation between heart rate variability measures and inflammatory markers and diseases
| Year | Authors | Subjects | Main findings |
|---|---|---|---|
| 2007 | Coruzzi P, et al. [29] | 26 ulcerative colitis patients and 26 Crohn’s disease patients | pNN50 and RMSSD were significantly lower in the ulcerative colitis group than both the Crohn’s disease group and the control group. However, Crohn’s disease patients showed comparable HRV to the controls |
| 2011 | Kox M, et al. [30] | 40 healthy volunteers receiving intravenous endotoxin | Acute inflammatory responses result in HRV changes (log SDNN, log LF/HF, LFnu, and HFnu), but the extent of the inflammatory response did not correlate with the HRV change magnitude |
| 2012 | Vlcek M, et al. [31] | 22 female RA patients < 40 years | Plasma epinephrine and norepinephrine were similar in RA patients and controls. No differences in HRV and blood pressure response to orthostasis were found |
| 2012 | Thayer JF, et al. [32] | 611 apparently healthy employees of a factory (545 male, 18–63 years) | Urinary norepinephrine was positively associated with WBC. An inverse association between indices of vagally mediated HRV (RMSSD and pNN50) and plasma levels of CRP was found |
| 2014 | Jarczok MN, et al. [33] | 106 nonsmoking adults (9% women; age 44 ± 8 years) | Higher HF-HRV was associated with lower levels of CRP at both baseline and 4-year follow-up |
| 2017 | Adlan AM, et al. [34] | RA-normotensive (n = 13), RA-HTN (n = 17), normotensive control (n = 17), HTN control (n = 16) | The RA, RA-HTN, and HTN groups have lower HRV time and frequency domain measures (rMSSD, pNN50, HF power, LF power, and total power) than the control group. Hs-CRP and reported pain were independently and inversely associated with time-domain rMMSD and pNN50 |
| 2017 | Aeschbacher S, et al. [35] | 2064 healthy adults (47% men, 25–41 years) | Significant inverse and linear associations of SDNN with hs-CRP, leukocytes, neutrophils, lymphocytes, and monocytes were found. Total power and normalized HF power were inversely associated with some of the markers |
| 2018 | Zawadka-Kunikowska M, et al. [36] | Crohn’s disease patients in remission (n = 30) | Disease duration was negatively associated with baroreflex sensitivity and positively correlated with normalized high-frequency HRV, LF/HF ratio at rest, and post-tilt Δ systolic blood pressure |
| 2018 | Hu MX, et al. [37] | 1774 subjects from the Netherlands Study of Depression and Anxiety | Higher CRP and IL-6 levels predicted lower respiratory sinus arrhythmia at follow-up |
| 2021 | Alen NV, et al. [38] | 836 community participants (450 female, 53 ± 13 years) | Robust inverse relations were found between HF power and IL-6, CRP, and fibrinogen. LF power was also inversely related to IL-6 and CRP |
CRP C-reactive protein, HF high frequency, HRV heart rate variability, hs-CRP high-sensitivity C-reactive protein, HTN hypertension, IL-6 interlukin-6, LF low frequency, pNN50 percentage of successive RR intervals that differ by more than 50 ms, RA rheumatic arthritis, RMSSD root mean square of successive RR interval differences, SDNN standard deviation of NN intervals, WBC white blood cell
Table 2.
A summary of clinical studies with respect to the association between heart rate variability and non-invasive endothelial function testing
| Year | Authors | Subjects | Main findings |
|---|---|---|---|
| HRV and FMD | |||
| 2007 | Kaufman CL, et al. [39] | 36 children (19 M, 17F, 11.5 ± 0.1 years) | FMD peak dilation was positively related to HFnu and negatively related to LF/HF ratio. The correlation is independent of fat mass, inflammation, and fasting insulin level |
| 2012 | Pinter A, et al. [40] | 46 healthy young males, (22 ± 6) years | RMSSD, pNN50, and lnHF power have positive correlations with normalized FMD |
| 2013 | Truccolo AB, et al. [41] | 13 patients with Chagas disease in its indeterminate phase, (59 ± 11) years | A positive correlation with FMD was observed in both normalized HF and LF spectral components, while LF/HF ratio was negatively correlated with FMD |
| 2013 | Watanabe S, et al. [42] | 47 patients with ischemic heart disease, (68 ± 7) years | FMD was correlated with SDNN and LF/HF. LF/HF was identified as the most powerful predictor of the magnitude of FMD |
| HRV and EndoPAT | |||
| 2021 | Tuttolomondo A, et al. [43] | 63 patients with diabetic foot (67 ± 10 years), 30 patients with diabetes and without ulcerative complications (61 ± 9 years), and 30 controls without diabetes (65 ± 6 years) | RHI measured by the Endo-PAT has a positive correlation with LF/HF ratio in subjects with type 2 diabetes mellitus with diabetic foot. RHI was negatively correlated with RMSDD and HF% in patients with diabetic foot |
| HRV and IMT | |||
| 2006 | Eller NH, et al. [44] | 84 healthy individuals (25 men, 43–63 years) | SDNN and LF/HF difference (stress test vs. sleep) were found to be negatively correlated to IMT progression four years after baseline |
| 2006 | Gottsäter A, et al. [45] | 61 type 2 diabetes patients (39 males, 45–69 years) | Mean IMT in the common carotid artery correlated with LF power |
| 2012 | Fakhrzadeh H, et al. [46] | 57 diabetic (51 ± 5 years) and 54 nondiabetics (49 ± 6 years) subjects free of coronary artery disease | Increased carotid IMT was inversely and independently associated with the total power of HRV in both groups |
| 2013 | Galetta F, et al. [47] | 32 elderly sedentary subjects (65 ± 4 years) and 32 age-matched endurance athletes (66 ± 4 years) | In both groups, SDNN was inversely related to IMT, while LF/HF ratio related positively to IMT |
| 2017 | Pereira VL Jr, et al. [48] | 101 subjects (60 ± 13 years) with an estimated 10-year atherosclerosis cardiovascular disease risk score of 16.4 ± 17.0 | A statistically significant association between SDNN and carotid IMT was found. IMT was also associated with coefficient of variation of RR intervals and dispersion of points along the line of identity (SD2) |
| 2020 | Hoshi RA, et al. [49] | 7256 apparently healthy adults (mean age 50 years) | An increased odds ratio for carotid IMT ≥ 75th percentile was verified within the lowest two quartiles of LF and HF, but significances did not remain after adjustments for anthropometric and clinical variables |
| HRV and EAT | |||
| 2014 | Balcioğlu AS, et al. [50] | 224 patients (56 ± 17 years) with premature ventricular beats complaining of palpitations | Significant correlations were found between EAT thickness and Holter findings, including SDNN, SDNN index, SDANN, RMSSD, and pNN50 |
| HRV and pulse wave velocity | |||
| 2004 | Nakao M, et al. [51] | 382 Japanese males (24–39 years) | PWV was positively associated with LF/HF ratio. LF/HF was an independent predictor of PWV after controlling for significant effects of age, systolic blood pressure, and plasma noradrenaline levels |
| 2014 | Chandra P, et al. [52] | 240 patients (mean age 60 years) with chronic kidney disease stage III–V | Several HRV measures (LF, VLF, LF/HF ratio, total power, and SDANN) were inversely correlated with PWV. However, the association was attenuated after adjustment for age and diabetes and no longer significant after adjustment for C-reactive protein |
| 2019 | Shah AS, et al. [53] | 397 patients (21 ± 3 years) enrolled in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study | Participants with ≥ 3 abnormal HRV indices that were greater or less than 2.5 standard deviations compared with control adolescents had greater pulse wave velocity compared with those without dysfunction |
EAT epicardial adipose tissue, FMD flow-mediated dilation, HF high frequency, HRV heart rate variability, IMT intima media thickness, LF low frequency, pNN50 percentage of successive RR intervals that differ by more than 50 ms, PWV pulse wave velocity, RHI reactive hyperactivity index, RMSSD root mean square of successive RR interval differences, SDANN standard deviation of the average NN intervals for each 5-min segment of a 24-h HRV recording, SDNN standard deviation of NN intervals, VLF very low frequency
Table 3.
A summary of clinical studies with respect to age-related changes in heart rate variability
| Year | Authors | Subjects | Main findings |
|---|---|---|---|
| 1998 | Umetani K, et al. [55] | 260 healthy subjects (112 male, 10–99 years old) | SDNN and SDANN decreased with aging in a quadratic regression pattern. The most marked decrease occurred between the second and third decades, after which time-domain declined only gradually. Beyond age 80, HRV again began to decline more rapidly |
| 2016 | Almeida-Santos MA, et al. [57] | 1743 community-based participants (40–100 years) | SDNN, SDANN, and SDNN index decreased linearly with age. U-shaped pattern for rMSSD and pNN50, with the nadir between 60 and 69 years for both genders |
| 2019 | Tan JPH, et al. [58] | 45 healthy participants (49–82 years) | There was no association between age and resting-state HRV (RMSSD, TP, HF spectral power), yet HRV in the 2 h preceding sleep was associated with age. Older participants showed greater HRV |
| 2020 | Geovanini GR, et al. [59] | 543 healthy participants (41% male, 40 ± 14 years) | RMSSD and pNN50 showed U-shaped distribution and reversal increase above 60 years old. SDNN and SDANN decreased linearly by age |
| 2020 | Choi J, et al. [54] | 291 healthy participants (144 men) aged 19–69 years | HRV indices (HF, LF, VLF, TP, SDNN, HRV index, and pNN50) show a decreasing trend with age in healthy Korean adults |
| 2020 | Hernández-Vicente A, et al. [60] | Young adults (n = 20; 21 ± 2 years), octogenarians (n = 18; 84 ± 3 years), centenarians (n = 17; 102 ± 2 years) | HF, LF, SDNN, and pNN50 all present an age-related reduction. SDNN showed a correlation with survival prognosis in centenarians |
HF high frequency, HRV heart rate variability, LF low frequency, pNN50 percentage of successive RR intervals that differ by more than 50 ms, RMSSD root mean square of successive RR interval differences, SDANN standard deviation of the average NN intervals for each 5-min segment of a 24-h HRV recording, SDNN standard deviation of NN intervals, TF total power, VLF very low frequency
The mechanism of age-related sympathetic overactivity is considered to be multifactorial and centrally mediated. Several molecular and cellular mediators have been proposed, including the presence of leptin, activation of the renin-angiotensin system, and cellular senescence, which all contribute to the common mediator of neuroinflammation and oxidative stress [61]. With neuroinflammation, inflammatory signals can be transmitted to the brain to increase sympathetic signaling via areas with blood–brain barrier leakages such as the circumventricular organs and area postrema as a part of the immune-to-brain communication to alter brain function [62]. Sympathetic activating signals are then relayed to the sympathetic preganglionic neurons via neurons in the hypothalamic paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM) of the brainstem [63], with which postganglionic neurons and effector organs are sequentially activated.
Diminished parasympathetic activity in aging
Less sufficient parasympathetic mechanisms co-exist with sympathetic activation in aging. What constitutes a valid non-invasive measurement of vagal tone still remains controversial. Muscarinic cholinergic receptor blockade is generally viewed as the gold standard for non-invasive cardiac parasympathetic activity evaluation [64]. Resting tonic parasympathetic activity can be estimated by measuring the increase in heart rate in response to muscarinic cholinergic blockers such as methylatropine [64]. A mouse model using the autonomic blockade methylscopolamine found diminished vagal modulation of heart rate in 19-month-old mice compared to 4-month-old mice [65]. However, other determinants such as the type of blocking agents, the route of administration, resting sympathetic nerve activity, and reactive changes of sympathetic activity may affect the test results. While direct single-unit vagus nerve activity recording was achieved in animal models and human studies with ultrasound-guided microneurography, there are no reports available on how human tonic vagal activity changes with age [66–68].
Some anatomical and physiological alternations are found in the parasympathetic nervous system with advanced age. The age-related loss of cardiac-projecting vagal preganglionic neurons in the medulla is observed in adult hypertensive rats compared to young hypertensive rats, and reductions in vagal neurons were most significant in the dorsal vagal nucleus and nucleus ambiguous on the right side of the medulla [69]. On the other hand, a peripheral mechanism involving a reduction of acetylcholine synthesis and an improper compensatory change of acetylcholine transporter was studied to explain age-related attenuation of parasympathetic control of the heart, as demonstrated by the blunted cardiac response to rostral severed vagal stimulation [70]. Reduced baroreflex sensitivity, or decreased baroreceptor firing, is a possible peripheral mechanism involved in the reduction of parasympathetic outflow [19]. Baroreflex sensitivity as assessed by the standard deviation of RR intervals in reaction to vasoactive agents was found to decrease with advancing age [5]. The mechanism behind baroreflex hyposensitivity is proposed to be multifactorial, such as stiffening of the carotid artery [71], inflammation and oxidative stress [72], and decreased cardiac cholinergic responsiveness [73].
The ANS and the vagal inflammatory reflex in aging
The effect of vagal tone on inflammation has been extensively discussed and summarized as the “cholinergic anti-inflammatory pathway” or the “vagal inflammatory reflex.” This concept was first proposed by Borovikova et al. two decades ago [74], where acetylcholine significantly attenuated the release of inflammatory cytokines in lipopolysaccharide-stimulated human macrophage cultures. The afferent arm of the vagal nerve responds to inflammatory mediators (i.e., cytokines) released by immune cells upon immune challenges. Neuronal interconnections between the brainstem nuclei integrate afferent signaling and conduct the efferent signaling output through the celiac ganglion and the splenic nerve to the spleen in order to regulate pro-inflammatory cytokine production and inflammation. The release of NE from the splenic nerve stimulates β2-adrenergic (β2-AR) receptors expressed by a selective population of T cells in the red pulp. Acetylcholine (ACh) released by these T cells dampens the release of inflammatory cytokines via activation of 7-nicotinic receptors on macrophages, dendritic cells, and other immune cells [75, 76]. This crosstalk between the brain and the immune system is vital for regulating inflammation, which provides a rationale for vagal nerve stimulation as a potential approach targeting the inflammatory process in age-related diseases.
Aging is accompanied by diminished vagal inflammatory reflex
Aging and inflammation often intertwine. Prolonged and persistent low-grade inflammation without overt infections observed in the elderly was coined as “inflammaging,” which involves age-related reshaping of inflammation-related transcription factors and elevated levels of pro-inflammatory cytokines [77]. Not only is inflammaging itself a risk factor for both morbidity and mortality in elderly adults, but the vast majority of all age-related diseases have some component inflammation, making inflammation a constitutional and ubiquitous element of aging and age-related diseases [78].
Age-related inflammation should be comprehensively illustrated at a molecular, cellular, and systemic level. On the cellular and molecular level, the activation of the innate immune system macrophages is induced as a result of cellular senescence, mitochondrial dysfunction, defective autophagy and mitophagy, dysregulation of the ubiquitin–proteasome system, activation of the DNA damage response, and dysbiosis [79]. On the systemic level, environmental and biological factors such as gender, diet, psychological stress, and genetics all affect the level of inflammation in individuals [80]. In this review, we propose that a loss of normal vagal tone and a subsequent disturbed anti-inflammatory reflex serve as a supplemental contributor to age-related chronic inflammation. As a result, there may be an exaggerated downstream immune dysregulation caused by otherwise harmless stimuli. The supporting evidence is summarized below.
The function and innervation of the ANS was found to be altered in many chronic inflammatory diseases, such as Crohn’s disease [36], osteoarthritis [81], and rheumatic arthritis [82], which led to the hypothesis that a low vagal tone has pro-inflammatory effect. ANS dysfunction is a potential etiology of inflammation instead of a consequence, where a decremental vagal tone leads to reduced stimulation of macrophage 7-nicotinic receptors and an incremental increase in pro-inflammatory cytokines. There are several downstream inflammatory pathways that are involved. NF-κB, which functions as a modulator of the expression of multiple genes involved in cytokine release, cell survival, apoptosis, and cell proliferation [83], is found to be downregulated by vagus nerve stimulation (VNS). Specifically, VNS suppress NF-κB/NLRP3 inflammasome activation, which promotes the secretion of Interleukin (IL)-1β and IL-18 [84]. These cytokines induce pro-inflammatory cell death by activating Bax. Moreover, HMGB1, which mediates a plethora of downstream effects within the inflammatory cascade, is observed to be downregulated by VNS and cholinergic agonists [85]. Meanwhile, the JAK/STAT/ERK signaling pathways can also be mediated by the vagus nerve [86, 87]. Although the mechanistic connection between 7nAchR and eNOS production is not thoroughly studied, increased eNOS expression and nitrate/nitrite production are also observed in chronic VNS. Melanocortin analog (Nle4, D-Phe7)-α-melanocyte-stimulating hormone (NDP-α-MSH) is associated with the over-expression of the pro-survival proteins heme oxygenase-1 (HO-1) and Bcl-XL in the heart. Furthermore, the phosphatidylinositol-3 kinase (PI3K)/AKT signaling pathway, which is another highly conserved pathway involved in various biological process including apoptosis and myocardial hypertrophy was reported to be involved in the anti-arrhythmic effects of low-level VNS [88, 89].
An intact vagus nerve is essential to containing inflammation, and stimulatory effects on the vagus nerve have been consistently described as a method to inhibit the inflammatory cytokine release. For example, it was found that bilateral vagotomy exacerbated ventilator-induced lung injury in mice while vagal stimulation attenuated lung ischemia/reperfusion injury in rats [90]. Neuroimmune regulation is a potential therapeutic target for not only inflammatory diseases but also other conditions associated with inflammation such as diabetes mellites, obesity, and hypertension. Similarly, in healthy aging, an example of the association between ANS function and inflammation is that baroreflex sensitivity is independently inversely associated with both white blood cell (WBC) count and C reactive protein level, and HRV is independently inversely associated with WBC count [91]. Further studies linking resting tonic parasympathetic activity with specific molecular pathways and circulatory inflammatory markers are warranted.
The effects of inflammation on the ANS
When an inflammatory process is present in the body, inflammatory cells such as macrophages, neutrophils, and mast cells are recruited to the site of inflammation, followed by the secretion of various cytokines. Peripheral sympathetic nerve fibers with the corresponding receptors are prone to nerve fiber repulsion caused by nerve repellent factors Sema3C and Sema3F secreted by macrophages and fibroblasts in the neurite outgrowth assay [92, 93]. This loss of innervation is a specific process that mainly affects the sympathetic nerves fibers [94].
Asides from the loss of sympathetic innervation, a centrally controlled systemic increase in sympathetic nervous system (SNS) activity is also a basic response to inflammation. However, SNS activity contributes to both pro-inflammatory and anti-inflammatory mechanisms. The local promotion of regulatory B cells subsides inflammation, and β2-adrenoceptor stimulation inhibits TNF production [95]. On the other hand, stimulation via α2-adrenoceptors is pro-inflammatory [96]. Therefore, the net outcome of stimulating adrenoceptors on immune cells is not entirely predictable.
Chronic inflammation as a source of endothelial dysfunction in the context of aging
Atherosclerotic cardiovascular disease and hypertension, the two major sources of health burden in the elderly, are both adverse outcomes of endothelial dysfunction [97]. The normal functioning of various organs and tissues is supported by the integrity of the vascular endothelium. Endothelial dysfunction, characterized by the reduced bioavailability of endothelial nitric oxide (NO), is accompanied by permanent disruption of the permeability of the endothelial barriers and is generally considered as an inflammatory response to noxious stimuli [98]. When challenged with certain pro-inflammatory cytokines or bacterial products (e.g., endotoxins), endothelial cells undergo a coordinated program of altered gene expression, which shifts many of their metabolic properties [99]. Vascular inflammation is a major part of inflammaging and a significant contributing factor to endothelial dysfunction.
It has been established that age-related oxidative stress is the main contributor to endothelial dysfunction. Although less evidence is available, chronic inflammation is proposed to be another inducing factor for endothelial dysfunction. Based on clinical observation, the risks for endothelial dysfunction and atherosclerosis are elevated for inflammatory bowel disease even in patients without traditional cardiovascular risk factors [100]. Similarly, increased cardiovascular risks are seen in patients with systemic lupus erythematosus, rheumatoid arthritis, and severe psoriasis [101], while reduced levels of plasma endothelial dysfunction biomarkers including intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion protein 1 (VCAM-1), and E-selectin are found in patients with rheumatoid arthritis after anti-TNF-α therapy [102].
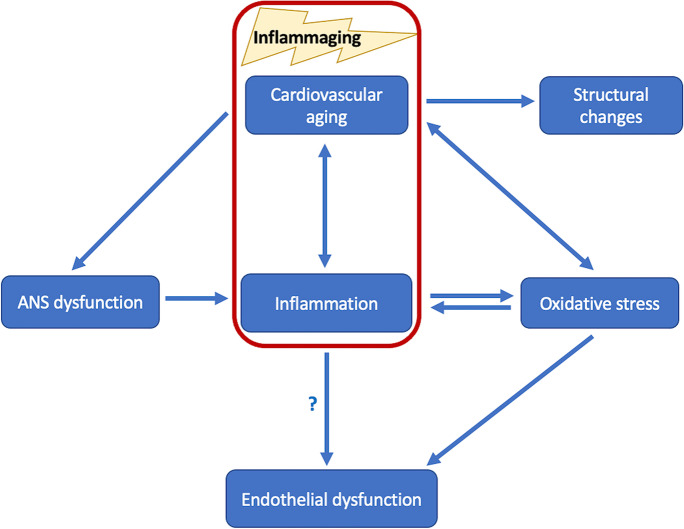
Circulating inflammatory markers such as homocysteine and von Willebrand factor have strong correlations with endothelial dysfunction and immune cell infiltration into blood vessels [103]. However, whether or not inflammation has a direct contribution to vascular dysfunction or only indirectly contributes to endothelial dysfunction by inducing oxidative stress has not been well established (Fig. 2). There is plenty of evidence to suggest that excessive or unregulated free radical production not only induces inflammatory responses but also occasionally serves as a response to inflammatory cytokines [104, 105]. Early in vitro studies showed that neutrophils from an inflammatory response may directly produce ROS such as superoxide [105]. It has also been recently discovered that there is crosstalk between important inflammatory cytokines and ROS. TNF-induced necroptosis itself generate ROS, and TNF signaling leads to NADPH oxidase 2 activation to produce ROS [9, 10]. IL-6 induces oxidative stress in vascular smooth muscle cells through upregulation of the angiotensin II type 1 receptor [11]. Meanwhile, the production of ROS can reciprocally activate the IL-6/STAT3 signaling pathway to induce cellular senescence [106]. Independent of ROS level, IL-17 is an important example of how cytokines can directly affect endothelial function. IL-17 is one of the most important cytokines involved in neutrophil recruitment and macrophage activation [107, 108]. Dendritic cells in the vascular wall produce IL-6, IL-1, and TGF- to activate CD4+ T cells, which then transform to Th-17 cells. IL-17A produced by Th-17 cells then activates T cells themselves, endothelial cells, smooth muscle cells, and macrophages. When activated, endothelial cells produce IL-6 and G-CSF to further promote the inflammatory process in the vascular wall, which involves neutrophil activation and recruitment to the vessel wall, altering the structure of the vessel wall and leading to vascular function impairment [109]. Aside from C reactive protein (CRP), TNF- can directly disrupt the biosynthesis of NO by downregulating the expression of endothelial nitric oxide synthase (eNOS) [110, 111]. On the contrary, it has been shown that mice without B and T cells (RAG-1-/-) show a less pronounced production of superoxide and a less severe development of hypertension in response to angiotensin II; ablation of myelomonocytic cells generated the same effects [112, 113].
Fig. 2.
The association between autonomic nervous system dysfunction, inflammation, oxidative stress, and endothelial dysfunction. ANS, autonomic nervous system. Aging-induced autonomic nervous system dysfunction is a possible inciting factor of inflammation. Inflammation and oxidative stress then enhance each other to induce endothelial dysfunction in the elderly. However, whether inflammation has a direct contribution to endothelial dysfunction is not established
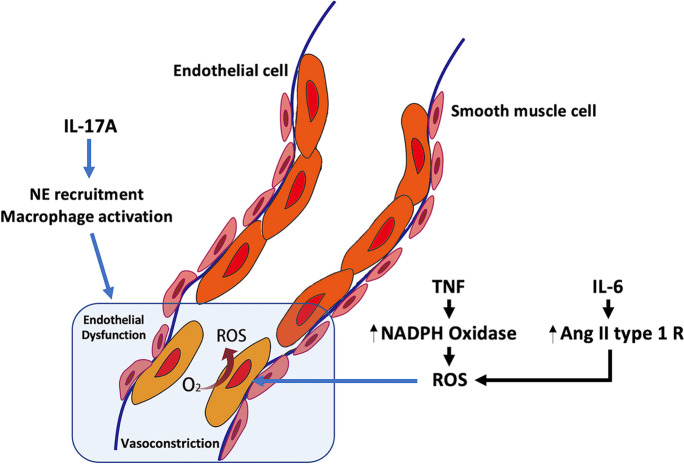
On the other hand, ROS activate pro-inflammatory transcription factors such as Nrf2, NF B, and AP1 to modulate the expression of genes encoding chemokines and adhesion molecules [114]. Radicals also signal to directly upregulate the expression of neutrophil recruiter cytokine IL-8 via MAPK [115]. Nevertheless, ROS-mediated cell damage is a major source of local and systemic inflammation. Damage-associated molecular patterns (DAMPs), such as chromatin-associated protein high mobility group box1, heat shock proteins, and purine metabolites (ATP, uric acid), are released from damaged cells. Extracellular matrix fragments also release extracellular DAMPs. DAMPs go on to exert their pro-inflammatory effects via pattern recognition receptors (e.g., Toll-like receptors) and non-pattern recognition receptors (e.g., receptor for advanced glycation end-product, RAGE) [116]. When combined, all these factors further propel inflammation. The downstream signal transduction pathways, including NF B, PI3K, JAK [116], STAT, and MAPK are then activated by inflammatory cytokines. Finally, a pro-inflammatory, pro-vasoconstrictive, and prothrombotic endothelial cell phenotype is induced by the interaction between inflammation mediators and ROS (Fig. 3).
Fig. 3.
Inflammation contributes to endothelial dysfunction via or independent of oxidative stress. Ang II type 1 R, angiotensin II type 1 receptor; IL, interleukin. TNF signaling leads to NADPH oxidase 2 activation to produce ROS. IL-6 induces oxidative stress through upregulation of the angiotensin II type 1 receptor. Independent of oxidative stress, IL-17 produced by Th17 cells is one of the most important cytokines involved in neutrophil recruitment and macrophage activation to cause vascular inflammation and endothelial dysfunction
Sex differences in vascular function
Sex is an important biological variable in a variety of physiological and pathophysiological processes. Men and women lose the normal anti-inflammatory and antithrombotic properties of their endothelial cells at different paces. While the decline in endothelial function occur earlier in men, women have a higher rate of decline than men later in life, especially around the stage of menopause [117, 118]. Sex hormone profiles contribute to endothelial functional differences between men and women. When activated, estrogen receptors promote NO release via eNOS while engagement of androgen receptors result in impaired NO release. In addition, differences in cardiovascular risk factors such as cigarette smoking, hypertension and dyslipidemia constitute a worse risk profile in men. On the other hand, sex differences in systemic and plaque inflammation is not well-determined. Female sex is associated with enhanced systemic inflammation suggested by higher CRP levels in women [119]. On the contrary, male sex is associated with increased inflammatory plaque infiltrates found in endarterectomy specimens [120]. Similar to the trends seen with endothelial dysfunction, younger women tend to have a decreased development of cardiovascular disease and experience lower rates of myocardial infarction relative to men; it should be noted that women catch up to men at the age of 60–79 years and surpass men by the age of 80 years [121]. When discussing vascular function in the geriatric population, gender differences factor should not be neglected.
The protective effects of VNS against endothelial dysfunction
Diminished vagal tone contributes to a pro-inflammatory status with associated sequelae, as evidenced by impaired endothelial function with age and the beneficial effects of experimental VNS, possibly through both direct protective effects and curbed systemic inflammation [122, 123]. VNS is documented to attenuate vascular endothelial impairments in ovariectomized rats [122]. Similar protective effects were found in a high-salt diet-induced endothelial dysfunction model [124]. VNS is associated with attenuated lung microvascular endothelial cell damage such as swelling, basement membrane defects, as well as necrosis and cavitation caused by LPS administration and burn injury [125, 126]. In a rat myocardial ischemia/reperfusion injury model, the mesenteric artery microscopic structure was preserved after VNS [127]. In humans, acute non-invasive low-level tragus stimulation is observed to improve endothelial function assessed by flow-mediated dilatation in the brachial artery and cutaneous microcirculation with laser speckle contrast imaging in the hand and nail bed [128]. Non-invasive neuromodulation aiming to regain balance of ANS is a promising therapeutic option to modulate the neuroimmune axis in a variety of inflammatory and cardiovascular conditions.
Conclusions
Aging is related to numerous dysfunctions and homeostasis disturbances. Along with other established contributors to inflammaging, ANS dysfunction characterized by sympathetic overdrive and reduced vagal activity as assessed by MSNA and HRV may lead to a weakened vagal anti-inflammatory reflex and chronic low-degree inflammatory state in the elderly, whereas VNS exerts protective effects against both inflammation and oxidative stress in order to counteract endothelial damage. Inflammation interacts with oxidative stress and acts independently to induce endothelial dysfunction. In an effort to establish a weakened anti-inflammatory reflex as a source of chronic inflammation, preclinical models might be required to investigate the activity of inflammatory pathways in aged animals. Furthermore, the molecular and cellular level investigation into the complete pathway of autonomic dysregulation-inflammation-endothelial dysfunction is warranted.
Funding
This work was supported by grants from the Oklahoma Center for the Advancement of Science and Technology and the Cellular and Molecular GeroScience CoBRE (P20GM125528).
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5(5):557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Keir DA, Badrov MB, Tomlinson G, Notarius CF, Kimmerly DS, Millar PJ, et al. Influence of sex and age on muscle sympathetic nerve activity of healthy normotensive adults. Hypertension. 2020;76(3):997–1005. doi: 10.1161/HYPERTENSIONAHA.120.15208. [DOI] [PubMed] [Google Scholar]
- 5.Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol. 1992;263(3 Pt 2):H798–803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB. Differential changes of autonomic nervous system function with age in man. Am J Med. 1983;75(2):249–258. doi: 10.1016/0002-9343(83)91201-9. [DOI] [PubMed] [Google Scholar]
- 7.Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, et al. Source of Chronic Inflammation in Aging. Front Cardiovasc Med. 2018;5:12. doi: 10.3389/fcvm.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihaylova S, Schweighofer H, Hackstein H, Rosengarten B. Effects of anti-inflammatory vagus nerve stimulation in endotoxemic rats on blood and spleen lymphocyte subsets. Inflamm Res. 2014;63(8):683–690. doi: 10.1007/s00011-014-0741-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sriram S, Subramanian S, Sathiakumar D, Venkatesh R, Salerno MS, McFarlane CD, et al. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-kappaB. Aging Cell. 2011;10(6):931–948. doi: 10.1111/j.1474-9726.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 12.Hasan W. Autonomic cardiac innervation: development and adult plasticity. Organogenesis. 2013;9(3):176–193. doi: 10.4161/org.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wink J, van Delft R, Notenboom RGE, Wouters PF, DeRuiter MC, Plevier JWM, et al. Human adult cardiac autonomic innervation: controversies in anatomical knowledge and relevance for cardiac neuromodulation. Auton Neurosci. 2020;227:102674. doi: 10.1016/j.autneu.2020.102674. [DOI] [PubMed] [Google Scholar]
- 14.Armour JA. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm. 2010;7(7):994–996. doi: 10.1016/j.hrthm.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Chadda KR, Ajijola OA, Vaseghi M, Shivkumar K, Huang CL, Jeevaratnam K. Ageing, the autonomic nervous system and arrhythmia: from brain to heart. Ageing Res Rev. 2018;48:40–50. doi: 10.1016/j.arr.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Johnson PI, Lee RE, Orfei E, Lonchyna VA, Sullivan HJ, et al. Topography of cardiac ganglia in the adult human heart. J Thorac Cardiovasc Surg. 1996;112(4):943–953. doi: 10.1016/S0022-5223(96)70094-6. [DOI] [PubMed] [Google Scholar]
- 17.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part I. Headache. 2016;56(1):71–78. doi: 10.1111/head.12647. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Sato A, Sato Y, Suzuki H. Increases in adrenal catecholamine secretion and adrenal sympathetic nerve unitary activities with aging in rats. Neurosci Lett. 1986;69(3):263–268. doi: 10.1016/0304-3940(86)90491-X. [DOI] [PubMed] [Google Scholar]
- 19.Rowe JW, Troen BR. Sympathetic nervous system and aging in man. Endocr Rev. 1980;1(2):167–179. doi: 10.1210/edrv-1-2-167. [DOI] [PubMed] [Google Scholar]
- 20.Wallin BG, Sundlof G, Eriksson BM, Dominiak P, Grobecker H, Lindblad LE. Plasma noradrenaline correlates to sympathetic muscle nerve activity in normotensive man. Acta Physiol Scand. 1981;111(1):69–73. doi: 10.1111/j.1748-1716.1981.tb06706.x. [DOI] [PubMed] [Google Scholar]
- 21.Morrow LA, Linares OA, Hill TJ, Sanfield JA, Supiano MA, Rosen SG, et al. Age differences in the plasma clearance mechanisms for epinephrine and norepinephrine in humans. J Clin Endocrinol Metab. 1987;65(3):508–511. doi: 10.1210/jcem-65-3-508. [DOI] [PubMed] [Google Scholar]
- 22.Billman GE, Dujardin JP. Dynamic changes in cardiac vagal tone as measured by time-series analysis. Am J Physiol. 1990;258(3 Pt 2):H896–902. doi: 10.1152/ajpheart.1990.258.3.H896. [DOI] [PubMed] [Google Scholar]
- 23.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 24.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81. [PubMed]
- 25.Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013;4:26. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96(9):3224–3232. doi: 10.1161/01.CIR.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 27.Marmerstein JT, McCallum GA, Durand DM. Direct measurement of vagal tone in rats does not show correlation to HRV. Sci Rep. 2021;11(1):1210. doi: 10.1038/s41598-020-79808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBeck LD, Petersen SR, Jones KE, Stickland MK. Heart rate variability and muscle sympathetic nerve activity response to acute stress: the effect of breathing. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R80–91. doi: 10.1152/ajpregu.00246.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coruzzi P, Castiglioni P, Parati G, Brambilla V, Brambilla L, Gualerzi M, et al. Autonomic cardiovascular regulation in quiescent ulcerative colitis and Crohn’s disease. Eur J Clin Invest. 2007;37(12):964–970. doi: 10.1111/j.1365-2362.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 30.Kox M, Ramakers BP, Pompe JC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Interplay between the acute inflammatory response and heart rate variability in healthy human volunteers. Shock. 2011;36(2):115–120. doi: 10.1097/SHK.0b013e31821c2330. [DOI] [PubMed] [Google Scholar]
- 31.Vlcek M, Rovensky J, Eisenhofer G, Radikova Z, Penesova A, Kerlik J, et al. Autonomic nervous system function in rheumatoid arthritis. Cell Mol Neurobiol. 2012;32(5):897–901. doi: 10.1007/s10571-012-9805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med. 2009;265(4):439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 33.Jarczok MN, Koenig J, Mauss D, Fischer JE, Thayer JF. Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. J Intern Med. 2014;276(6):667–671. doi: 10.1111/joim.12295. [DOI] [PubMed] [Google Scholar]
- 34.Adlan AM, Veldhuijzen van Zanten J, Lip GYH, Paton JFR, Kitas GD, Fisher JP. Cardiovascular autonomic regulation, inflammation and pain in rheumatoid arthritis. Auton Neurosci. 2017;208:137-45. [DOI] [PMC free article] [PubMed]
- 35.Aeschbacher S, Schoen T, Dorig L, Kreuzmann R, Neuhauser C, Schmidt-Trucksass A, et al. Heart rate, heart rate variability and inflammatory biomarkers among young and healthy adults. Ann Med. 2017;49(1):32–41. doi: 10.1080/07853890.2016.1226512. [DOI] [PubMed] [Google Scholar]
- 36.Zawadka-Kunikowska M, Slomko J, Klopocka M, Liebert A, Tafil-Klawe M, Klawe JJ, et al. Cardiac and autonomic function in patients with Crohn’s disease during remission. Adv Med Sci. 2018;63(2):334–340. doi: 10.1016/j.advms.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Hu MX, Lamers F, Neijts M, Willemsen G, de Geus EJC, Penninx B. Bidirectional prospective associations between cardiac autonomic activity and inflammatory markers. Psychosom Med. 2018;80(5):475–482. doi: 10.1097/PSY.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 38.Alen NV, Parenteau AM, Sloan RP, Hostinar CE. Heart rate variability and circulating inflammatory markers in midlife. Brain Behav Immun Health. 2021;15. [DOI] [PMC free article] [PubMed]
- 39.Kaufman CL, Kaiser DR, Steinberger J, Dengel DR. Relationships between heart rate variability, vascular function, and adiposity in children. Clin Auton Res. 2007;17(3):165–171. doi: 10.1007/s10286-007-0411-6. [DOI] [PubMed] [Google Scholar]
- 40.Pinter A, Horvath T, Sarkozi A, Kollai M. Relationship between heart rate variability and endothelial function in healthy subjects. Auton Neurosci. 2012;169(2):107–112. doi: 10.1016/j.autneu.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Truccolo AB, Dipp T, Eibel B, Ribeiro RA, Casali KR, Irigoyen MC, et al. Association between endothelial function and autonomic modulation in patients with Chagas disease. Arq Bras Cardiol. 2013;100(2):135–140. doi: 10.5935/abc.20130026. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe S, Amiya E, Watanabe M, Takata M, Ozeki A, Watanabe A, et al. Simultaneous heart rate variability monitoring enhances the predictive value of flow-mediated dilation in ischemic heart disease. Circ J. 2013;77(4):1018–1025. doi: 10.1253/circj.CJ-12-1043. [DOI] [PubMed] [Google Scholar]
- 43.Tuttolomondo A, Del Cuore A, La Malfa A, Casuccio A, Daidone M, Maida CD, et al. Assessment of heart rate variability (HRV) in subjects with type 2 diabetes mellitus with and without diabetic foot: correlations with endothelial dysfunction indices and markers of adipo-inflammatory dysfunction. Cardiovasc Diabetol. 2021;20(1):142. doi: 10.1186/s12933-021-01337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eller NH, Malmberg B, Bruhn P. Heart rate variability and intima media thickness. Int J Behav Med. 2006;13(3):201–213. doi: 10.1207/s15327558ijbm1303_3. [DOI] [PubMed] [Google Scholar]
- 45.Gottsater A, Ahlgren AR, Taimour S, Sundkvist G. Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin Auton Res. 2006;16(3):228–234. doi: 10.1007/s10286-006-0345-4. [DOI] [PubMed] [Google Scholar]
- 46.Fakhrzadeh H, Yamini-Sharif A, Sharifi F, Tajalizadekhoob Y, Mirarefin M, Mohammadzadeh M, et al. Cardiac autonomic neuropathy measured by heart rate variability and markers of subclinical atherosclerosis in early type 2 diabetes. ISRN Endocrinol. 2012;2012:168264. doi: 10.5402/2012/168264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galetta F, Franzoni F, Tocchini L, Camici M, Milanesi D, Belatti F, et al. Effect of physical activity on heart rate variability and carotid intima-media thickness in older people. Intern Emerg Med. 2013;8(Suppl 1):S27–S29. doi: 10.1007/s11739-013-0919-9. [DOI] [PubMed] [Google Scholar]
- 48.Pereira VL, Jr, Dobre M, Dos Santos SG, Fuzatti JS, Oliveira CR, Campos LA, et al. Association between carotid intima media thickness and heart rate variability in adults at increased cardiovascular risk. Front Physiol. 2017;8:248. doi: 10.3389/fphys.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoshi RA, Santos IS, Dantas EM, Andreao RV, Mill JG, Goulart AC, et al. Relationship between heart rate variability and carotid intima-media thickness in the Brazilian Longitudinal Study of Adult Health - ELSA-Brasil. Clin Physiol Funct Imaging. 2020;40(2):122–130. doi: 10.1111/cpf.12613. [DOI] [PubMed] [Google Scholar]
- 50.Balcioglu AS, Cicek D, Akinci S, Eldem HO, Bal UA, Okyay K, et al. Arrhythmogenic evidence for epicardial adipose tissue: heart rate variability and turbulence are influenced by epicardial fat thickness. Pacing Clin Electrophysiol. 2015;38(1):99–106. doi: 10.1111/pace.12512. [DOI] [PubMed] [Google Scholar]
- 51.Nakao M, Nomura K, Karita K, Nishikitani M, Yano E. Relationship between brachial-ankle pulse wave velocity and heart rate variability in young Japanese men. Hypertens Res. 2004;27(12):925–931. doi: 10.1291/hypres.27.925. [DOI] [PubMed] [Google Scholar]
- 52.Chandra P, Sands RL, Gillespie BW, Levin NW, Kotanko P, Kiser M, et al. Relationship between heart rate variability and pulse wave velocity and their association with patient outcomes in chronic kidney disease. Clin Nephrol. 2014;81(1):9–19. doi: 10.5414/CN108020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah AS, El Ghormli L, Vajravelu ME, Bacha F, Farrell RM, Gidding SS, et al. Heart Rate Variability and cardiac autonomic dysfunction: prevalence, risk factors, and relationship to arterial stiffness in the treatment options for type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care. 2019;42(11):2143–2150. doi: 10.2337/dc19-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi J, Cha W, Park MG. Declining trends of heart rate variability according to aging in healthy asian adults. Front Aging Neurosci. 2020;12:610626. doi: 10.3389/fnagi.2020.610626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593–601. doi: 10.1016/S0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 56.Yeragani VK, Sobolewski E, Kay J, Jampala VC, Igel G. Effect of age on long-term heart rate variability. Cardiovasc Res. 1997;35(1):35–42. doi: 10.1016/S0008-6363(97)00107-7. [DOI] [PubMed] [Google Scholar]
- 57.Almeida-Santos MA, Barreto-Filho JA, Oliveira JL, Reis FP, da Cunha Oliveira CC, Sousa AC. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr. 2016;63:1–8. doi: 10.1016/j.archger.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Tan JPH, Beilharz JE, Vollmer-Conna U, Cvejic E. Heart rate variability as a marker of healthy ageing. Int J Cardiol. 2019;275:101–103. doi: 10.1016/j.ijcard.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Geovanini GR, Vasques ER, de Oliveira AR, Mill JG, Andreao RV, Vasques BK, et al. Age and sex differences in heart rate variability and vagal specific patterns - Baependi Heart Study. Glob Heart. 2020;15(1):71. doi: 10.5334/gh.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernandez-Vicente A, Hernando D, Santos-Lozano A, Rodriguez-Romo G, Vicente-Rodriguez G, Pueyo E, et al. Heart rate variability and exceptional longevity. Front Physiol. 2020;11:566399. doi: 10.3389/fphys.2020.566399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balasubramanian P, Hall D, Subramanian M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. Geroscience. 2019;41(1):13–24. doi: 10.1007/s11357-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. NeuroImmunoModulation. 1995;2(4):241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 63.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 64.Chapleau MW, Sabharwal R. Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev. 2011;16(2):109–127. doi: 10.1007/s10741-010-9174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piantoni C, Carnevali L, Molla D, Barbuti A, DiFrancesco D, Bucchi A, et al. Age-Related changes in cardiac autonomic modulation and heart rate variability in mice. Front Neurosci. 2021;15:617698. doi: 10.3389/fnins.2021.617698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paintal AS. The conduction velocities of respiratory and cardiovascular afferent fibres in the vagus nerve. J Physiol. 1953;121(2):341–359. doi: 10.1113/jphysiol.1953.sp004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ottaviani MM, Wright L, Dawood T, Macefield VG. In vivo recordings from the human vagus nerve using ultrasound-guided microneurography. J Physiol. 2020;598(17):3569–3576. doi: 10.1113/JP280077. [DOI] [PubMed] [Google Scholar]
- 68.Silverman HA, Stiegler A, Tsaava T, Newman J, Steinberg BE, Masi EB, et al. Standardization of methods to record vagus nerve activity in mice. Bioelectron Med. 2018;4:3. doi: 10.1186/s42234-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corbett EK, Mary DA, McWilliam PN, Batten TF. Age-related loss of cardiac vagal preganglionic neurones in spontaneously hypertensive rats. Exp Physiol. 2007;92(6):1005–1013. doi: 10.1113/expphysiol.2007.038216. [DOI] [PubMed] [Google Scholar]
- 70.Freeling JL, Li Y. Age-related attenuation of parasympathetic control of the heart in mice. Int J Physiol Pathophysiol Pharmacol. 2015;7(3):126–135. [PMC free article] [PubMed] [Google Scholar]
- 71.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol. 2009;587(Pt 9):2049–2057. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernardes N, da Silva DD, Stoyell-Conti FF, de Oliveira B-M, Malfitano C, Caldini EG, et al. Baroreflex impairment precedes cardiometabolic dysfunction in an experimental model of metabolic syndrome: role of inflammation and oxidative stress. Sci Rep. 2018;8(1):8578. doi: 10.1038/s41598-018-26816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R3–R12. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- 74.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 75.Deng J, Jiang H. Role of nicotinic acetylcholine receptors in cardiovascular physiology and pathophysiology: current trends and perspectives. Curr Vasc Pharmacol. 2021;19(4):370–378. doi: 10.2174/1386207323666200917104920. [DOI] [PubMed] [Google Scholar]
- 76.Deng J, Wang M, Guo Y, Fischer H, Yu X, Kem D, et al. Activation of alpha7nAChR via vagus nerve prevents obesity-induced insulin resistance via suppressing endoplasmic reticulum stress-induced inflammation in Kupffer cells. Med Hypotheses. 2020;140:109671. doi: 10.1016/j.mehy.2020.109671. [DOI] [PubMed] [Google Scholar]
- 77.Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23(9):2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 78.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 79.Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol. 2013;9(4):228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- 80.Bachmann MC, Bellalta S, Basoalto R, Gomez-Valenzuela F, Jalil Y, Lepez M, et al. The challenge by multiple environmental and biological factors induce inflammation in aging: their role in the promotion of chronic disease. Front Immunol. 2020;11:570083. doi: 10.3389/fimmu.2020.570083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Wu D, Song F, Zhu C, Hui Y, Zhu Q, et al. Activation of alpha7 nicotinic acetylcholine receptors prevents monosodium iodoacetate-induced osteoarthritis in rats. Cell Physiol Biochem. 2015;35(2):627–638. doi: 10.1159/000369724. [DOI] [PubMed] [Google Scholar]
- 82.Aydemir M, Yazisiz V, Basarici I, Avci AB, Erbasan F, Belgi A, et al. Cardiac autonomic profile in rheumatoid arthritis and systemic lupus erythematosus. Lupus. 2010;19(3):255–261. doi: 10.1177/0961203309351540. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168(1–2):37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang Y, Dong X, Chen G, Ye W, Kang J, Tang Y, et al. Vagus Nerve stimulation attenuates early traumatic brain injury by regulating the NF-kappaB/NLRP3 signaling pathway. Neurorehabil Neural Repair. 2020;34(9):831–843. doi: 10.1177/1545968320948065. [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 86.Ottani A, Giuliani D, Neri L, Calevro A, Canalini F, Vandini E, et al. NDP-alpha-MSH attenuates heart and liver responses to myocardial reperfusion via the vagus nerve and JAK/ERK/STAT signaling. Eur J Pharmacol. 2015;769:22–32. doi: 10.1016/j.ejphar.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 87.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Q, Lu Y, Bian H, Guo L, Zhu H. Activation of the alpha7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am J Transl Res. 2017;9(3):971–985. [PMC free article] [PubMed] [Google Scholar]
- 89.Stavrakis S, Scherlag BJ, Fan Y, Liu Y, Mao J, Varma V, et al. Inhibition of atrial fibrillation by low-level vagus nerve stimulation: the role of the nitric oxide signaling pathway. J Interv Card Electrophysiol. 2013;36(3):199–208. doi: 10.1007/s10840-012-9752-8. [DOI] [PubMed] [Google Scholar]
- 90.dos Santos CC, Shan Y, Akram A, Slutsky AS, Haitsma JJ. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med. 2011;183(4):471–482. doi: 10.1164/rccm.201002-0314OC. [DOI] [PubMed] [Google Scholar]
- 91.Ulleryd MA, Prahl U, Borsbo J, Schmidt C, Nilsson S, Bergstrom G, et al. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One. 2017;12(4):e0174974. doi: 10.1371/journal.pone.0174974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kunath J, Delaroque N, Szardenings M, Neundorf I, Straub RH. Sympathetic nerve repulsion inhibited by designer molecules in vitro and role in experimental arthritis. Life Sci. 2017;168:47–53. doi: 10.1016/j.lfs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 93.Wei Y, Liang Y, Lin H, Dai Y, Yao S. Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J Neuroinflammation. 2020;17(1):80. doi: 10.1186/s12974-020-01752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller LE, Justen HP, Scholmerich J, Straub RH. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J. 2000;14(13):2097–2107. doi: 10.1096/fj.99-1082com. [DOI] [PubMed] [Google Scholar]
- 95.Stanojevic S, Dimitrijevic M, Kustrimovic N, Mitic K, Vujic V, Leposavic G. Adrenal hormone deprivation affects macrophage catecholamine metabolism and beta2-adrenoceptor density, but not propranolol stimulation of tumour necrosis factor-alpha production. Exp Physiol. 2013;98(3):665–678. doi: 10.1113/expphysiol.2012.070524. [DOI] [PubMed] [Google Scholar]
- 96.Deo SH, Jenkins NT, Padilla J, Parrish AR, Fadel PJ. Norepinephrine increases NADPH oxidase-derived superoxide in human peripheral blood mononuclear cells via alpha-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2013;305(10):R1124–R1132. doi: 10.1152/ajpregu.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haybar H, Shokuhian M, Bagheri M, Davari N, Saki N. Involvement of circulating inflammatory factors in prognosis and risk of cardiovascular disease. J Mol Cell Cardiol. 2019;132:110–119. doi: 10.1016/j.yjmcc.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 98.Sun HJ, Wu ZY, Nie XW, Bian JS. Role of Endothelial Dysfunction in Cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 2019;10:1568. doi: 10.3389/fphar.2019.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Assad TR, Hemnes AR. Metabolic dysfunction in pulmonary arterial hypertension. Curr Hypertens Rep. 2015;17(3):20. doi: 10.1007/s11906-014-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ozturk K, Guler AK, Cakir M, Ozen A, Demirci H, Turker T, et al. Pulse Wave velocity, intima media thickness, and flow-mediated dilatation in patients with normotensive normoglycemic inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(6):1314–1320. doi: 10.1097/MIB.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, et al. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174(12):1591–1619. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steyers CM, 3rd, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15(7):11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaiser D, Weise G, Moller K, Scheibe J, Posel C, Baasch S, et al. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol Commun. 2014;2:169. doi: 10.1186/s40478-014-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hishikawa K, Luscher TF. Felodipine inhibits free-radical production by cytokines and glucose in human smooth muscle cells. Hypertension. 1998;32(6):1011–1015. doi: 10.1161/01.HYP.32.6.1011. [DOI] [PubMed] [Google Scholar]
- 105.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52(3):741–4. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Liu Y, Chen J, Hu C, Teng M, Jiao K, et al. The ROS-mediated activation of IL-6/STAT3 signaling pathway is involved in the 27-hydroxycholesterol-induced cellular senescence in nerve cells. Toxicol In Vitro. 2017;45(Pt 1):10–18. doi: 10.1016/j.tiv.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 107.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188(12):6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, et al. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol. 2012;42(3):726–736. doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carter AM. Inflammation, thrombosis and acute coronary syndromes. Diab Vasc Dis Res. 2005;2(3):113–121. doi: 10.3132/dvdr.2005.018. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116(3):219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh U, Devaraj S, Vasquez-Vivar J, Jialal I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. 2007;43(6):780–791. doi: 10.1016/j.yjmcc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124(12):1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 114.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fu Y, Luo N, Lopes-Virella MF. Upregulation of interleukin-8 expression by prostaglandin D2 metabolite 15-deoxy-delta12, 14 prostaglandin J2 (15d-PGJ2) in human THP-1 macrophages. Atherosclerosis. 2002;160(1):11–20. doi: 10.1016/S0021-9150(01)00541-X. [DOI] [PubMed] [Google Scholar]
- 116.Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81(1):102–113. doi: 10.1002/1097-4644(20010401)81:1<102::AID-JCB1027>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 117.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 118.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692–4700. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 120.Wendorff C, Wendorff H, Pelisek J, Tsantilas P, Zimmermann A, Zernecke A, et al. Carotid plaque morphology is significantly associated with sex, age, and history of neurological symptoms. Stroke. 2015;46(11):3213–3219. doi: 10.1161/STROKEAHA.115.010558. [DOI] [PubMed] [Google Scholar]
- 121.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 122.Li P, Liu H, Sun P, Wang X, Wang C, Wang L, et al. Chronic vagus nerve stimulation attenuates vascular endothelial impairments and reduces the inflammatory profile via inhibition of the NF-kappaB signaling pathway in ovariectomized rats. Exp Gerontol. 2016;74:43–55. doi: 10.1016/j.exger.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 123.Deng J, Jiang Y, Wang M, Shao L, Deng C. Activation of vagovagal reflex prevents hepatic ischaemia-reperfusion-induced lung injury via anti-inflammatory and antioxidant effects. Exp Physiol. 2021;106(11):2210–2222. doi: 10.1113/EP089865. [DOI] [PubMed] [Google Scholar]
- 124.Chapleau MW, Rotella DL, Reho JJ, Rahmouni K, Stauss HM. Chronic vagal nerve stimulation prevents high-salt diet-induced endothelial dysfunction and aortic stiffening in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2016;311(1):H276–H285. doi: 10.1152/ajpheart.00043.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen C, Zhang Y, Du Z, Zhang M, Niu L, Wang Y, et al. Vagal efferent fiber stimulation ameliorates pulmonary microvascular endothelial cell injury by downregulating inflammatory responses. Inflammation. 2013;36(6):1567–1575. doi: 10.1007/s10753-013-9701-4. [DOI] [PubMed] [Google Scholar]
- 126.Krzyzaniak MJ, Peterson CY, Cheadle G, Loomis W, Wolf P, Kennedy V, et al. Efferent vagal nerve stimulation attenuates acute lung injury following burn: The importance of the gut-lung axis. Surgery. 2011;150(3):379–389. doi: 10.1016/j.surg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao M, He X, Bi XY, Yu XJ, Gil Wier W, Zang WJ. Vagal stimulation triggers peripheral vascular protection through the cholinergic anti-inflammatory pathway in a rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2013;108(3):345. doi: 10.1007/s00395-013-0345-1. [DOI] [PubMed] [Google Scholar]
- 128.Dasari TW, Csipo T, Amil F, Lipecz A, Fulop GA, Jiang Y, et al. Effects of Low-Level Tragus Stimulation on Endothelial Function in Heart Failure With Reduced Ejection Fraction. J Card Fail. 2021;27(5):568–576. doi: 10.1016/j.cardfail.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]