Abstract
Objective:
Develop an online estimator that accurately predicts bronchopulmonary dysplasia (BPD) severity or death using readily-available demographic and clinical data.
Design:
Retrospective analysis of data entered into a prospective registry.
Setting:
Infants cared for at centers of the United States Neonatal Research Network between 2011 and 2017.
Patients:
Infants 501–1250g birth weight and 23 0/7–28 6/7 weeks’ gestation.
Interventions:
None.
Main outcome measures:
Separate multinomial regression models for postnatal days 1, 3, 7, 14, and 28 were developed to estimate the individual probabilities of death or BPD severity (no BPD, grade 1 BPD, grade 2 BPD, grade 3 BPD) defined according to the mode of respiratory support administered at 36 weeks postmenstrual age.
Results:
Among 9181 included infants, birth weight was most predictive of death or BPD severity on postnatal day 1, while mode of respiratory support was the most predictive factor on days 3, 7, 14, and 28. The predictive accuracy of the models increased at each time period from postnatal day 1 (C-statistic: 0.674) to postnatal day 28 (C-statistic 0.741). We used these results to develop a web-based model that provides predicted estimates for BPD by postnatal day.
Conclusions:
The probability of BPD or death in extremely preterm infants can be estimated with reasonable accuracy using a limited amount of readily available clinical information. This tool may aid clinical prognostication, future research, and center-specific quality improvement surrounding BPD prevention.
Keywords: chronic lung disease, neonate, estimator, premature
Introduction
Bronchopulmonary dysplasia (BPD) is the most common chronic pulmonary morbidity associated with prematurity, affecting 30–50% of infants born extremely preterm.1,2 Preterm infants with BPD are more likely to die during early childhood or survive with severe developmental disability.3–6 While mortality and many other neonatal morbidities have decreased over time, BPD in large multicenter reports remains steady.7 The prevalence of BPD varies widely across centers,8 as do center and individual clinician practices that may influence BPD risk over time.9
In 2011, the first web-based BPD Outcome Estimator was developed using infant data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) Benchmarking Trial.10 This Estimator accurately quantified probability of BPD (per the 2001 NIH consensus definition)11 or death based on risk factors present on postnatal days 1 (day of birth), 3, 7, 14, 21, and 28, and has been used as a tool for epidemiologic research and clinical trials.12–14 An updated Estimator is needed for two main reasons: 1) respiratory care in very preterm infants continues to evolve as goal oxygen saturation targets, ventilator management strategies, and medication use change over time2,15–17; and 2) the recent development of a new, outcome-informed definition of BPD.18 This new definition is considered the best predictor of childhood respiratory and neurodevelopmental outcomes, categorizing BPD severity into 3 grades based on mode of respiratory support at 36 weeks’ postmenstrual age, regardless of prior or current oxygen therapy. We examined respiratory and clinical data from a cohort of infants born between 2011 and 2017 to develop an updated BPD Outcome Estimator that estimates an individual infant’s risk of developing the new outcome-driven definition of BPD or death at multiple time points in the first month after birth.
Subjects and Methods
Subjects
This was a retrospective analysis of data entered into a prospective registry of high-risk preterm infants maintained by the NRN.19 Infants studied were born between January 1, 2011 and December 31, 2017 and were included if they had a birth weight of 501–1250 g and gestational age of 23 0/7–28 6/7 weeks. Infants with gestational age <23 weeks were not included, due to insufficient sample size to provide accurate risk assessment. Exclusion criteria were: death ≤12 hours after birth, major congenital anomalies, transferred prior to 36 weeks postmenstrual age (PMA), remained hospitalized at 36 weeks PMA but missing data to determine BPD status, and admission to a neonatal intensive care unit (NICU) with <20 infants meeting inclusion criteria during the study period. While most NRN centers are comprised of multiple NICUs, each individual NICU was considered separately for study purposes. We excluded small NICUs so that the results would be generalizable to institutions routinely caring for these infants and to facilitate comparisons of outcomes’ prevalence among NICUs. The institutional review board at each center approved participation in the registry.
Definitions
BPD severity was defined at 36 weeks postmenstrual age (PMA) according to the outcome-driven diagnostic criteria developed by NRN investigators. This definition categorizes disease severity according to the mode of respiratory support utilized at 36 weeks’ PMA, regardless of the use of supplemental oxygen.18 No BPD was defined as breathing in room air at 36 weeks’ PMA; grade 1 BPD as receipt of nasal cannula ≤2L/min (or hood O2); grade 2 BPD as nasal cannula >2L/min, nasal continuous positive airway pressure (CPAP), or nasal intermittent positive pressure ventilation; and grade 3 BPD as invasive mechanical ventilation. For infants discharged home prior to 36 weeks PMA, respiratory status at discharge was used to determine BPD. Surgical NEC was defined as modified Bell’s stage IIIB.20 Sepsis was defined as a blood and/or cerebrospinal fluid culture growing a recognized bacterial or fungal pathogen if the infant was administered antibiotics for ≥5 days or until death.
Statistical Analysis
We compared demographic and clinical characteristics among infants with no BPD, grade 1 BPD, grade 2 BPD, grade 3 BPD, and death prior to 36 weeks using chi-square tests for categorical variables and Wilcoxon tests for continuous variables. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
We performed a multistage approach to select covariates for inclusion into the final multinomial regression models used to estimate the individual probabilities of death or BPD severity level at the following five time points: postnatal day 1 (day of birth), 3, 7, 14, and 28. Candidate covariates determined a priori were: gestational age, birth weight, race, ethnicity, sex, receipt of antenatal steroids, receipt of postnatal steroids, highest mode of respiratory support on the day of interest (high-frequency ventilation, conventional ventilation, non-invasive positive pressure ventilation, CPAP, nasal cannula, or hood oxygen), maximum fraction of inspired oxygen on the day of interest, sepsis, and surgical NEC. Sepsis was only considered for models estimating BPD risk on days 7, 14, and 28. We excluded race as a covariate from the models because it is a social construct (not a biological risk factor) and did not materially improve model prediction. We excluded receipt of postnatal steroids because of variable use across centers and because postnatal steroids are more often considered as treatment for developing BPD rather than a risk factor. Sepsis and surgical NEC were coded as “yes” if occurring prior to or on the day of interest. Site was not included because we hoped to develop a model that would be broadly applicable to any NICU. We performed stepwise forward selection of covariates using p<0.2 for entry into separate multinomial regression models for each day of interest to generate preliminary models for a 5-level outcome: no BPD, grade 1 BPD, grade 2 BPD, grade 3 BPD, and death. Final models were selected after exclusion of covariates with a p-value >0.01.
Predictive performance of our multinomial outcome models was assessed using a C-statistic, which corresponds to the area under the receiver-operating characteristic curve. C-statistics were calculated after adding each covariate to the models. To estimate the optimism of the overall C-statistic from each of the models, the regression models were repeated on 100 bootstrap samples drawn with replacement from the corresponding cohort of infants who survived to the day of the model; the sample size for the bootstrap samples matched the sample size of the corresponding regression model. The difference between the full cohort and bootstrap C-statistic is an estimate of the optimism of the model performance.21 The average optimism over the 100 samples was subtracted from the full cohort C-statistic to obtain the internally-validated C-statistic.22
Results
Sample Description
A total of 9181 infants from 38 NICUs met all inclusion and exclusion criteria (Figure 1). The mean (SD) birth weight and gestational age overall were 850 g (192) and 25.9 weeks (1.57), respectively. Among the entire cohort, 11% died prior to 36 weeks PMA, 35% survived without BPD, 30% developed grade 1 BPD, 17% developed grade 2 BPD, and 6% developed grade 3 BPD (Table 1). Multiple clinical factors were significantly associated with grade of BPD (Table 1). Center differences in outcomes were substantial; for example, the combined prevalence of grade 2 or 3 BPD or death ranged from 6–67% among the NICUs included in the study. Infants with more invasive respiratory support and those with higher fraction of inspired oxygen were more likely to die or have higher grades of BPD (Figure 2, Supplemental Table 1). Over time, there were trends toward increased use of high-frequency ventilation and nasal ventilation or CPAP (Supplemental Figure 1).
Figure 1.
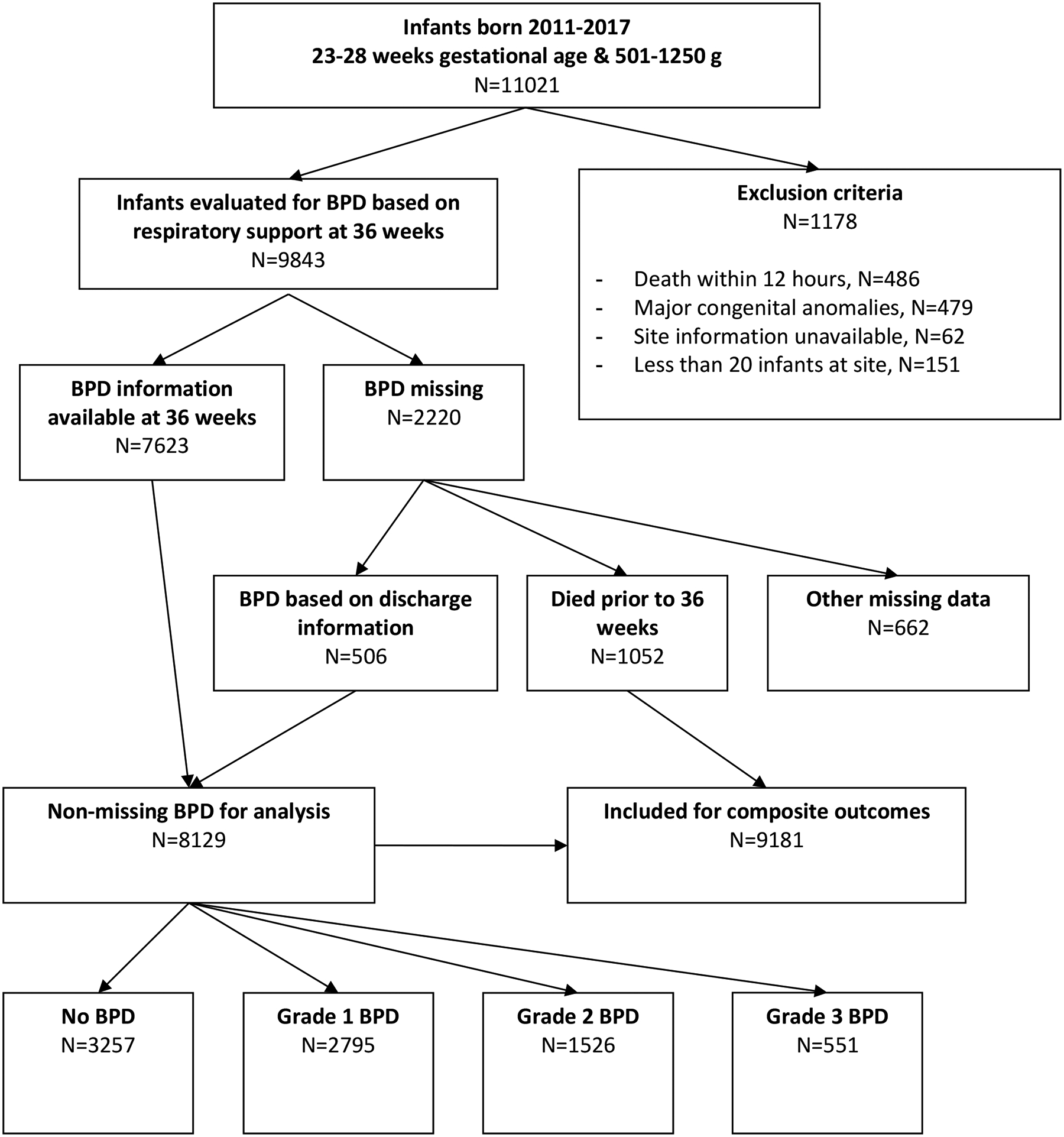
Study Flow Diagram
This figure displays the study population, from initial cohort, through exclusions, to the final study population.
Definition of abbreviations: BPD = bronchopulmonary dysplasia
Table 1.
Demographics and Clinical Characteristics
| No BPD | Grade 1 BPD | Grade 2 BPD | Grade 3 BPD | Death prior to 36 weeks | p-value* | |
|---|---|---|---|---|---|---|
| N | 3257 | 2795 | 1526 | 551 | 1052 | |
| Birth weight, g, mean ± SD | 951 ± 173 | 838 ± 176 | 778 ± 173 | 752 ± 168 | 722 ± 161 | <0.0001 |
| Gestational age, weeks, mean ± SD | 26.7 ± 1.24 | 25.8 ± 1.49 | 25.5 ± 1.51 | 25.1 ± 1.52 | 24.7 ± 1.49 | <0.0001 |
| Male, n (%) | 1480 (45%) | 1420 (51%) | 896 (59%) | 315 (57%) | 613 (58%) | <0.0001 |
| Race | <0.0001 | |||||
| Black, n (%) | 1576 (50%) | 973 (36%) | 511 (35%) | 237 (44%) | 415 (41%) | |
| White, n (%) | 1399 (44%) | 1531 (57%) | 870 (59%) | 267 (50%) | 533 (52%) | |
| Other, n (%) | 198 (6%) | 196 (7%) | 100 (7%) | 32 (6%) | 73 (7%) | |
| Hispanic ethnicity, n (%) | 447 (14%) | 439 (16%) | 228 (15%) | 48 (9%) | 142 (14%) | 0.0004 |
| Patent ductus arteriosus, n (%) | 971 (30%) | 1445 (52%) | 950 (62%) | 356 (65%) | 419 (40%) | <0.0001 |
| Sepsis, n (%) | 378 (12%) | 624 (22%) | 444 (29%) | 253 (46%) | 339 (41%) | <0.0001 |
| Surgical necrotizing enterocolitis, n (%) | 46 (1%) | 61 (2%) | 51 (3%) | 63 (11%) | 156 (15%) | <0.0001 |
| Antenatal corticosteroids, n (%) | 2990 (92%) | 2543 (91%) | 1379 (90%) | 515 (93%) | 904 (86%) | <0.0001 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; SD = standard deviation
Figure 2.
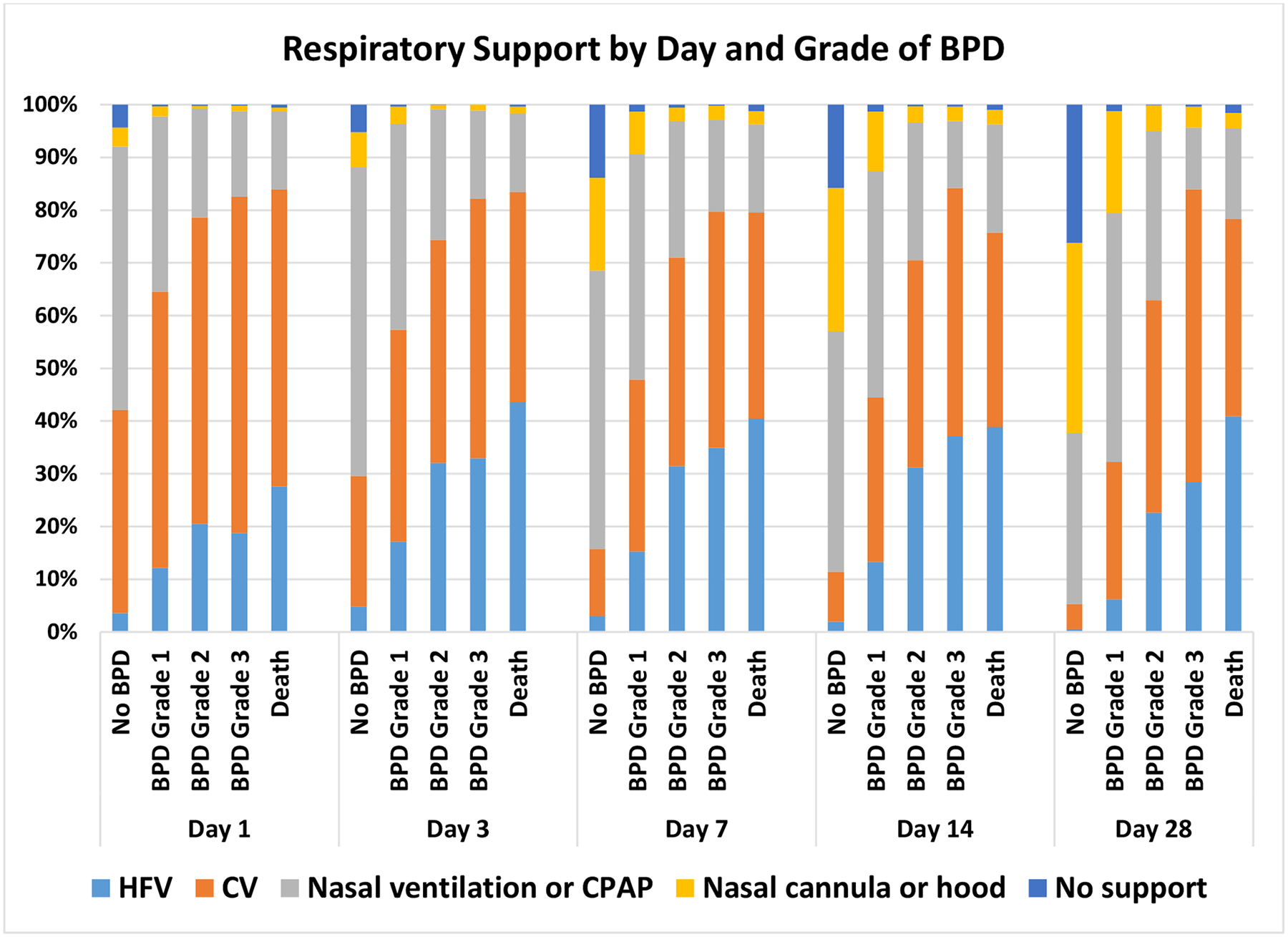
Respiratory Support by Day and Grade of BPD
Infants who were more likely to die or have a higher grade of BPD had more invasive respiratory support and a higher fraction of inspired oxygen.
Definition of abbreviations: BPD = bronchopulmonary dysplasia
Prediction Models
Five risk factors were identified for inclusion in the final multinomial models at each time point: birth weight, gestational age, sex, mode of respiratory support, and fraction of inspired oxygen. Treatment with antenatal steroids was included in the day 1 model only; surgical NEC was included on days 14 and 28 (Table 2). Birth weight was the covariate that explained the most variation in outcome risk on day 1. For all subsequent days, mode of respiratory support was the most predictive. Validated C-statistics produced via bootstrap analysis differed from the full-model C-statistics by 0.005 or less. Using the final regression models, we developed a web-based BPD Outcome Estimator that provides individual predicted estimates for the probabilities of death or BPD by severity grade at postnatal days 1, 3, 7, 14, and 2823 (Supplemental Tables 2–6 show model odds ratios and p-values).
Table 2.
Multinomial Regression Model Prediction C-statistics with the Addition of Individual Variables for Postnatal Days 1, 3, 7, 14, and 28*
| Day 1 | Day 3 | Day 7 | Day 14 | Day 28 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | C statistic | Variable | C statistic | Variable | C statistic | Variable | C statistic | Variable | C statistic |
| Birth weight | 0.629 | Respiratory support | 0.629 | Respiratory support | 0.654 | Respiratory support | 0.669 | Respiratory support | 0.709 |
| Respiratory support | 0.655 | Birth weight | 0.664 | Birth weight | 0.674 | FIO2 | 0.688 | FIO2 | 0.728 |
| Gestational age | 0.660 | FIO2 | 0.678 | FIO2 | 0.686 | Birth weight | 0.694 | Birth weight | 0.731 |
| FIO2 | 0.668 | Gestational age | 0.682 | Male | 0.690 | Male | 0.696 | Surgical NEC | 0.737 |
| Male | 0.672 | Male | 0.686 | Gestational age | 0.692 | Surgical NEC | 0.699 | Male | 0.738 |
| Antenatal steroids | 0.674 | Gestational age | 0.702 | Gestational age | 0.741 | ||||
The C-statistic for each row corresponds to the model with the variable on that row and the variables above that row.
Definition of abbreviations: FIO2 = fraction of inspired oxygen; NEC = necrotizing enterocolitis
Discussion
We examined >9000 hospitalized very preterm infants from 38 NICUs, more than twice the number included in the development of the original NRN BPD risk estimator. Our models accurately estimated BPD and death grades at multiple time points in the first 28 postnatal days, with reasonable accuracy after the first postnatal week. Accurate prediction of BPD is critical for multiple reasons. Understanding an individual infant’s risk can help inform parents and the neonatal care team about prognosis. Knowledge of risk can also advance BPD research and clinical care by contributing to a deeper understanding of what factors affect prevalence.
Identification of which care practices and therapies have the most impact on BPD remains elusive. Over the past 20 years, a large number of studies have investigated the impact of multiple interventions on BPD, such as less invasive respiratory support,24 high frequency ventilation,25,26 inhaled nitric oxide,27 hydrocortisone,28 patent ductus arteriosus management,29 and minimally invasive surfactant therapy.30 Most of these studies have shown mixed results, with minimal to no effect on BPD, or the composite outcome of death or BPD. One recent trial of furosemide used the previous NRN BPD Outcome Estimator to calculate BPD risk at multiple time points as a secondary outcome.13 Such use of our new Estimator in future trials could detect differences in BPD risk that occur over the course of an intervention during the first 28 days of the NICU hospitalization, allowing researchers to estimate impact of potential therapies throughout the hospital course.
Our Estimator can also quantitatively stratify prospective trial participants into risk groups. For several therapies that have proven effective in the prevention of BPD, underlying BPD risk has been shown to be critical for effectiveness.31,32 For example, multiple clinical trials have demonstrated that postnatal corticosteroids improve lung function, but are associated with increased risk of cerebral palsy. A 2014 meta-analysis of 20 randomized clinical trials showed that when the risk of chronic lung disease was <33%, postnatal corticosteroids increased the chance of death or cerebral palsy, while when the risk of BPD was >60%, postnatal corticosteroids decreased the chance of both adverse outcomes.32 Likewise, risk of BPD appears to influence the impact of Vitamin A on the outcomes of BPD or death, with infants at lower risk showing a greater positive effect of Vitamin A therapy.31 Such examples demonstrate that using therapies without consideration for an individual’s outcomes risk may result in a potentially useful therapy at a quantifiable risk level being deemed ineffective or even harmful in clinical trial results. Center variation in outcomes remains a persistent finding in the field of neonatology. In our study, prevalence of grade 2/3 BPD or death was quite variable (6–67%). Our study was not designed to investigate the influence of population differences or treatment and care practices associated with these differences. However, these results underscore the importance of focusing on center care differences while trying to improve the overall BPD prevalence.
We found that risk factors for BPD or death were similar to those found for the previous Estimator; in particular, birth weight is the most important risk factor on postnatal day 1, while respiratory support becomes the most important factor as time progresses after the first postnatal day. For example, at postnatal day 7, a male 500-gram 24-week gestational age infant on 50% fraction of inspired oxygen on the high-frequency ventilator would have a 16% probability of grade 3 BPD, a 23% probability of death, and a 2% probability of no BPD or death, while the same infant administered the identical oxygen concentration on CPAP would have a 9% probability of grade 3 BPD, 18% probability of death, and 10% probability of no BPD or death, thereby demonstrating how postnatal management choices affecting respiratory support could have substantial impact on infant outcomes.
Our C-statistics were slightly lower than those for the previous Estimator (maximum C-statistic 0.741 vs. 0.854 for the previous Estimator, both on day 28).10 Hypothetically, the lower C-statistics in the current study are likely due to a combination of the following factors: 1) different methods used to estimate C-statistics; 2) different definitions of BPD; 3) inclusion of a larger number of centers (therefore introducing more variability); and 4) changes in patient population and care practices over time.
Our study has multiple strengths. We created a BPD Outcome Estimator with an online application, allowing widespread use for both clinical and research purposes. We validated our Estimator internally using a bootstrap method, which is more robust than a simple division of the cohort into development and validation cohorts. While we did not conduct external validation as a part of this study, the online availability of the Estimator will allow (and we encourage) any interested investigator to perform external validation using local or other multicenter cohorts. This external validation will be critical to understand the broader applicability of the Estimator. The use of the outcome-driven definition of BPD,18 which is pragmatic in its application because of its sole reliance on respiratory support (without the need for radiographs or inspired oxygen concentrations), will facilitate retrospective use of this Estimator for existing databases. Yet like any study of BPD using a clinical definition, the “BPD” predicted by our Estimator almost certainly represents multiple clinical phenotypes lumped together into one diagnosis, so any individual result should be interpreted with caution, especially when using individual results for prognostication. Center differences in BPD and death were marked. Centers that utilize substantially different care practices from NRN centers may find the Estimator to be less reliable. For example, different centers may have unique protocols for using high-frequency ventilation; for some, this might represent an escalation of care, but for others, it may be standard for infants of certain size and gestation. Individual center was intentionally not included as a covariate in our models since we hoped to create a tool usable at any center; however, a reasonable amount of variation remains that is unexplained by our model. The substantial center variation, although typical of BPD rates reported in previous multicenter studies,33,34 likely affected our model’s performance. Other factors not included in our model, such as condition at birth, medical interventions, and therapeutic management could also account for such variation. Practice changes in respiratory support over time could reflect population changes and perhaps affected the model’s performance. In the future, new practice changes may influence the Estimator’s validity.
In conclusion, birth weight was the most important risk factor for BPD or death on postnatal day 1, while respiratory support was most important on days 3, 7, 14, and 28. Future externally-validating studies would support clinicians using the new online tool to estimate risk of BPD or death in extremely preterm infants to guide treatment and inform discussions regarding prognosis.
Supplementary Material
What Is Already Known
Bronchopulmonary dysplasia (BPD) is the most common chronic pulmonary morbidity associated with prematurity, affecting 30–50% of infants who are born extremely preterm.
Preterm infants with BPD are more likely to die during early childhood or survive with severe developmental disability.
Infants with BPD who survive are more likely to experience impaired childhood health and quality of life, family stress and economic hardship, and increased healthcare costs.
What This Study Adds
Using respiratory and clinical data from a cohort of infants, we developed an updated BPD Outcome Estimator.
This tool estimates an infant’s risk of developing the new outcome-driven definition of BPD or death at multiple time points in the first month post-birth.
This tool may aid clinical prognostication, future research, and center-specific quality improvement surrounding BPD prevention.
Statement of financial support:
The National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (UG1 HD21364, UG1 HD21385, UG1 HD27851, UG1 HD27853, UG1 HD27856, UG1 HD27880, UG1 HD27904, UG1 HD34216, UG1 HD36790, UG1 HD40492, UG1 HD40689, UG1 HD53089, UG1 HD53109, UG1 HD68244, UG1 HD68263, UG1 HD68270, UG1 HD68278, UG1 HD68284, UG1 HD87226, UG1 HD87229, U10 HD21373, U10 HD27856, U10 HD53119, U10 HD53124, U10 HD27871), the National Center for Advancing Translational Sciences (UL1 TR6, UL1 TR41, UL1 TR42, UL1 TR83, UL1 TR93, UL1 TR105, UL1 TR142, UL1 TR442, UL1 TR454, UL1 TR1085, UL1 TR1108, UL1 TR1117, UL1 TR1425, UL1 TR1449), and the National Center for Research Resources (M01 RR30, M01 RR32, M01 RR44, M01 RR54, M01 RR59, M01 RR64, M01 RR70, M01 RR80, M01 RR750, M01 RR633, M01 RR8084) provided grant support for the Neonatal Research Network. Although the National Institute of Child Health and Human Development staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Access to data statement: Mr. McDonald and Dr. Das had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure statement: The authors have no conflicts of interest relevant to this article to disclose. RGG has received support from industry for research services (https://dcri.org/about-us/conflict-of-interest/) and consulting services for Tellus Therapeutics.
Data sharing statement: Data reported in this paper may be requested through a data use agreement. Further details are available at: https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
Clinical trial registration: ClinicalTrials.gov NCT00063063; https://clinicaltrials.gov/ct2/show/NCT00063063
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natarajan G, Pappas A, Shankaran S, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev 2012;88:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fily A, Pierrat V, Delporte V, Breart G, Truffert P. Factors associated with neurodevelopmental outcome at 2 years after very preterm birth: the population-based Nord-Pas-de-Calais EPIPAGE cohort. Pediatrics 2006;117:357–366. [DOI] [PubMed] [Google Scholar]
- 5.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 2000;105:1216–1226. [DOI] [PubMed] [Google Scholar]
- 6.Katz-Salamon M, Gerner EM, Jonsson B, Lagercrantz H. Early motor and mental development in very preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2000;83:F1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horbar JD, Edwards EM, Greenberg LT, et al. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA Pediatr 2017;171:e164396. [DOI] [PubMed] [Google Scholar]
- 8.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114:1305–1311. [DOI] [PubMed] [Google Scholar]
- 9.Mandell EW, Kratimenos P, Abman SH, Steinhorn RH. Drugs for the prevention and treatment of bronchopulmonary dysplasia. Clin Perinatol 2019;46:291–310. [DOI] [PubMed] [Google Scholar]
- 10.Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 2011;183:1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 12.Cuna A, Liu C, Govindarajan S, Queen M, Dai H, Truog WE. Usefulness of an online risk estimator for bronchopulmonary dysplasia in predicting corticosteroid treatment in infants born preterm. J Pediatr 2018;197:23–28.e2. [DOI] [PubMed] [Google Scholar]
- 13.ClinicalTrials.gov. NCT02527798: Safety of Furosemide in Premature Infants at Risk of Bronchopulmonary Dysplasia (BPD). Available at: https://clinicaltrials.gov/ct2/show/NCT02527798. Updated February 12,2021. Accessed September 16, 2021.
- 14.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr 2014;164:966–972.e6. [DOI] [PubMed] [Google Scholar]
- 15.Chavez TA, Lakshmanan A, Iyer N, et al. Resource utilization patterns using non-invasive ventilation in neonates with respiratory distress syndrome. J Perinatol 2018;38:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg RG, Gayam S, Savage D, et al. Furosemide exposure and prevention of bronchopulmonary dysplasia in premature infants. J Pediatr 2019;208:134–140.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darlow BA, Vento M, Beltempo M, et al. Variations in oxygen saturation targeting, and retinopathy of prematurity screening and treatment criteria in neonatal intensive care units: an international survey. Neonatology 2018;114:323–331. [DOI] [PubMed] [Google Scholar]
- 18.Jensen EA, Dysart K, Gantz MG, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants: an evidence-based approach. Am J Respir Crit Care Med 2019;200:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov. Generic Database of Very Low Birth Weight Infants (GDB). ClinicalTrials.gov web site. https://www.clinicaltrials.gov/ct2/show/NCT00063063. Updated October 29, 2021. Accessed January 11, 2022.
- 20.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Harrell FE Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 22.Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol 2014;180:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Neonatal BPD outcome estimator (2021): infants with GA 23–28 weeks and birth weight 501–1250g. Available at: https://neonatal.rti.org/index.cfm?fuseaction=BPD_Calculator2.start&passphrase=86245b4d-357a-421b-b347-bed8110a3b2d. Accessed September 16, 2021.
- 24.Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010;362:1970–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med 2002;347:643–652. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AH, Peacock JL, Greenough A, et al. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med 2002;347:633–642. [DOI] [PubMed] [Google Scholar]
- 27.Van Meurs KP, Wright LL, Ehrenkranz RA, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 2005;353:13–22. [DOI] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov. NCT01353313: Hydrocortisone for BPD. Available at: https://clinicaltrials.gov/ct2/show/NCT01353313. Updated January 7, 2021. Accessed September 16, 2021.
- 29.Jensen EA, Foglia EE, Schmidt B. Association between prophylactic indomethacin and death or bronchopulmonary dysplasia: a systematic review and meta-analysis of observational studies. Semin Perinatol 2018;42:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta BK, Saha AK, Mukherjee S, Saha B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation-a randomized controlled trial. Eur J Pediatr 2020;179:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rysavy MA, Bell EF, Li L, et al. Heterogeneity of effect of vitamin A therapy for death or bronchopulmonary dysplasia among very low birth weight infants: re-analysis of a clinical trial. Abstract # 3930.528. PAS 2020 Meeting (Cancelled) Online Program Guide, 2020. Available at: https://plan.core-apps.com/pas2020/abstract/6edec56c63f592adb37f205ea9458220. Accessed September 16, 2021. [Google Scholar]
- 32.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatr 2014;165:1258–1260. [DOI] [PubMed] [Google Scholar]
- 33.Walsh M, Laptook A, Nadya Kazzi S, et al. , A cluster-randomized trial of benchmarking and multimodal quality improvement to improve rates of survival free of bronchopulmonary dysplasia for infants with birth weights of less than 1250 grams. Pediatrics 2007;119:876–890. [DOI] [PubMed] [Google Scholar]
- 34.Avery ME, Tooley WH, Keller JB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79:26–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


