Abstract
Human serum at low concentrations inhibits the growth of Cryptococcus neoformans in vitro. Fractionation of serum yielded a purified inhibitory protein with a molecular mass of ∼81.8 kDa, a pI of ∼6.2, and an amino acid sequence that matched that of human transferrin. The inhibitory activity and that of apotransferrin and 5% human serum were reversed by 10 μM freshly prepared FeCl3.
Human serum inhibits the growth of Cryptococcus neoformans (1, 11). Early attempts to identify an inhibitory component(s) were limited by the technology available (12, 18). Here a sequential method was used for characterization of the anticryptococcal activity.
C. neoformans isolates CDC 9759 and 46545 were grown on Sabouraud's dextrose agar or blood agar plates at 35°C for 48 h. Yeast cells were washed in saline and suspended in RPMI 1640 (GIBCO, Grand Island, N.Y.) with or without human serum fractions and dispensed, 0.1 ml per microtest plate well, in sets of quadruplicate cultures. In other experiments, C. neoformans was cultured in iron-free human transferrin (siderophilin) (Sigma Chemical Company, St. Louis, Mo.).
Cultures were incubated at 37°C in 5% CO2–95% air for 24 h, harvested, and washed with sterile distilled water. Dilutions of harvested material were plated on blood agar plates, CFU were enumerated after 48 h at 35°C. Percent inhibition was calculated by the formula [1 − (experimental CFU/control CFU)] × 100. CFU from RPMI 1640 cultures constituted the control. This definition measures the sum of events during incubation where some cells may be static, some multiply, and others die.
Serum from healthy donors and human AB serum (GIBCO) were stored at −80°C.
For anion-exchange gel chromatography, DEAE-Sephacel (Pharmacia LKB, Uppsala, Sweden) columns (2.5 by 6.5 cm and 3 by 12 cm) were prepared. Four milliliters of serum was applied to the smaller column, and 12 ml was applied to the larger. Columns were eluted sequentially with Tris-HCl (0.1 M, pH 8.5) buffer and then 0.1, 0.2, and 0.3 M NaCl in buffer. Five-milliliter fractions were collected, and the protein concentration of fractions was estimated by absorbance at 280 nm. Fractions that formed a protein peak were pooled, lyophilized, dialyzed against distilled water, and filter sterilized.
Sephadex G-200 (Pharmacia, Piscataway, N.J.) columns (2.5 by 90 cm) were used for molecular sieve chromatography. Samples were eluted from the column with phosphate-buffered saline diluted 1:10. Fractions were processed as described above.
A precast Bis-Tris polyacrylamide minigel electrophoresis system, which runs at neutral pH, 7.0 (NuPAGE; NOVEX, San Diego, Calif.), was used for one-dimensional gel electrophoresis. Electrophoresis was done on 4 to 12% gels using a 3-(N-morpholino)propanesulfonic acid-sodium dodecyl sulfate buffer in an Xcell-II apparatus. Proteins were fixed, stained with Coomassie blue, photographed with a digital camera, and printed with a graphic printer.
NOVEX isoelectric focusing (IEF) gels, pH 3 to 7, nondenaturing (no urea), containing 5% polyacrylamide and 2% ampholytes, were used for pI determination and confirmation of isoforms or purified products. The gels were stained with Coomassie blue, and images were printed as described above.
Purified protein was analyzed for amino acid sequences at the protein facility, Beckman Center, Stanford University, using automated cycles of the Edman degradation reaction. Fourteen three-step cycles were used.
Data were analyzed for statistical significance (set at P < 0.05) using Student's t test. Bonferroni's adjustment was used for multiple comparisons against a single control.
A representative protein elution profile from three experiments where 3 to 4 ml of fresh or AB human serum was chromatographed on a 2.5- by 6.5-cm (20-ml) DEAE-Sephacel column is shown in Fig. 1. When 10 to 12 ml of AB serum was fractionated on 3- by 11-cm (60-ml) DEAE-Sephacel columns, similar elution profiles were obtained.
FIG. 1.
Fractionation of AB serum on a DEAE-Sephacel anion-exchange column. AB serum (3.5 ml) was applied to a 2.5- by 6.5-cm (20-ml) DEAE-Sephacel column. Proteins were eluted (fractions 1 to 5) with Tris-HCl buffer (0.1 M, pH 8.5) and then with 0.1, 0.2, and 0.3 M NaCl in buffer (fractions 6 to 10, 11 to 15, and 16 to 20, respectively). Protein concentrations in 5-ml fractions were estimated by absorbance at 280 nm as given on the vertical axis.
The anticryptococcal activities of proteins in peak 1, peak 2, and peak 3 (Fig. 1) are given in Table 1. Peak 1 had some activity, but peak 2 had potent anticryptococcal activity, whereas peak 3 stimulated growth. We later found that overloading the columns with serum (4.5 ml on the small column or 12 ml on the large column) caused the activity to appear in peak 1.
TABLE 1.
Anticryptococcal activity of human AB serum fractions separated on DEAE-Sephacel anion-exchange columns
| Condition | Mean CFU ± SD (n = 4) | % Inhibition | Fold enhancement |
|---|---|---|---|
| Inoculum | 1,360 ± 140 | ||
| RPMI 1640 | |||
| Alone | 156,625 ± 20,138 | ||
| With 10% serum | 6,365 ± 725 | 96b | 0 |
| DEAE peaksa | |||
| 1 | 125,000 ± 26,450 | 24 | 0 |
| 2 | 10,533 ± 1,892 | 94b | 0 |
| 3 | 203,750 ± 34,247 | 0 | 1.3 |
Four hundred micrograms of protein per milliliter.
P < 0.01, compared with CFU in RPMI 1640 control cultures.
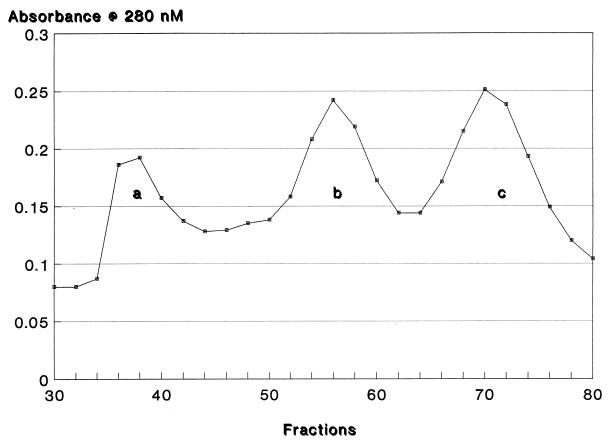
AB serum proteins in peak 2 from the DEAE-Sephacel column (Fig. 1) were fractionated on Sephadex G-200. The elution profile of peak 2 gave three major peaks. Only peak c (Fig. 2) inhibited C. neoformans growth (Table 2). Peaks a and b at similar concentrations significantly enhanced growth. The significance of growth-enhancing proteins remains to be determined.
FIG. 2.
Molecular sieve fractionation of peak 2 proteins from a DEAE-Sephacel column. Peak 2 proteins were fractionated on a Sephadex G-200 column (2.5 by 90 cm). The protein concentrations in eluted fractions, estimated by absorbance at 280 nm, are given on the vertical axis.
TABLE 2.
Anticryptococcal activity of DEAE-Sephacel peak 2 proteins fractionated on a Sephadex G-200 column
| Condition | CFUa (mean ± SD) | % Fungistasis | Fold enhancement |
|---|---|---|---|
| Inoculum | 740 ± 30 | ||
| RPMI 1640 | 69,000 ± 12,250 | ||
| 10% fresh serum | 4,440 ± 240 | 94b | 0 |
| G-200 peaks (μg/ml) | |||
| a (160) | 175,000 ± 25,000 | 0 | 2.5b |
| b (190) | 235,000 ± 10,000 | 0 | 3.4b |
| c (200) | 11,900 ± 700 | 84b | 0 |
Mean CFU ± standard deviation from the mean of quadruplicate 24-h cultures.
P < 0.01, compared with CFU in RPMI 1640 cultures.
When decreasing concentrations of serum inhibitory protein (SIP) in peak c (Fig. 2) were tested, there was not a concentration-dependent effect (Table 3). Comparable inhibition of growth was seen from 200 to 12.5 μg/ml. Lower concentrations did not inhibit C. neoformans growth. Peak c proteins from fresh frozen serum and a second isolation from AB serum gave similar activity.
TABLE 3.
Dose response of G-200 peak c protein
| Condition | CFU (mean ± SD) | % Fungistasis |
|---|---|---|
| Inoculum | 593 ± 34 | |
| RPMI 1640 | 51,000 ± 11,000 | 0 |
| 10% AB serum | 12,250 ± 1,500 | 76 |
| 10% fresh serum | 7,900 ± 460 | 85 |
| G-200 AB serum peak c (μg/ml) | ||
| 200 | 10,000 ± 1,250 | 80 |
| 100 | 12,256 ± 750 | 76 |
| 25 | 13,650 ± 1,000 | 74 |
| 12 | 13,500 ± 500 | 74 |
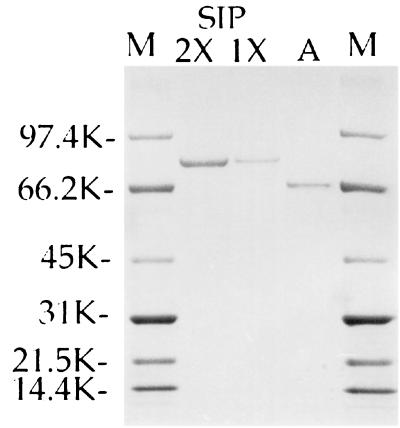
Peak c proteins, SIP, electrophoresed on NuPAGE gels gave a single sharp band (Fig. 3). The protein migrated to a point equidistant between 97.4- and 66.2-kDa molecular mass markers and was estimated to be ∼81.8 kDa. Under reduced conditions, an identical migration pattern was obtained. SIP and apotransferrin migrated the same distance and appear to have the same molecular mass.
FIG. 3.
NuPAGE of SIP from Sephadex G-200 column. Molecular weight markers (M) are shown in lanes 1 and 5. Migration of SIP undiluted (2×) and diluted 1:1 (1×) is shown in lanes 2 and 3, respectively. Human serum albumin (A) was run in lane 4.
Electrophoresis of SIP on IEF gels resulted in a single sharp protein band (Fig. 4). The protein migrated to a point just above the pI 6.0 marker, a pI estimated to be ∼6.2.
FIG. 4.
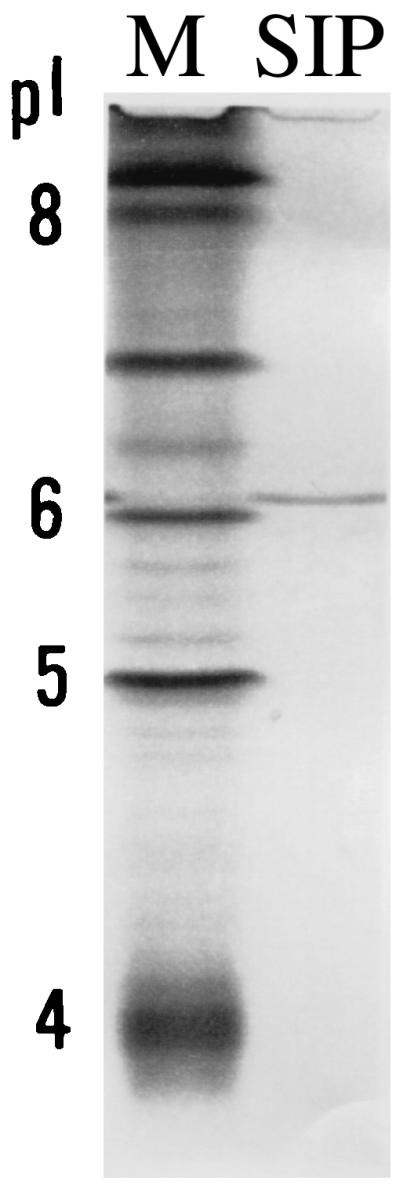
IEF of SIP. The migration of SIP on an IEF gel is shown in lane 2. Migration of pI markers (M) is shown in lane 1. The 5 and 4 pI markers are approximately at pI 5.1 and 4.4, respectively.
The 14 end-terminal amino acids of SIP matched those of human transferrin data stored in the data bank.
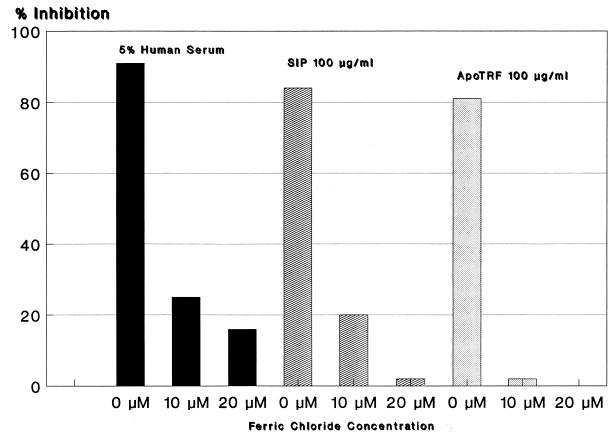
Transferrin binds ferric iron strongly, and bound iron is not available to most microorganisms, whereas human cells have receptors for transferrin and transport iron into the cell. With iron overload (iron in excess of what transferrin can bind), it may be possible to reverse the inhibition of growth which results from transferrin sequestration of iron. When FeCl3 was added to cultures containing 5% serum, apotransferrin, or SIP, the anticryptococcal activity of these agents was reversed (Fig. 5). Cultures with 5% human serum in RPMI 1640 contain 125 to 150 μg of transferrin per ml, and the inhibitory activity (90%) was reduced to 25% by 10 μM and to 16% by 20 μM FeCl3. SIP and apotransferrin (100 μg/ml) inhibited growth of C. neoformans 86 and 83%, respectively (Fig. 5), and this activity was completely reversed by 20 μM FeCl3. Dilution studies with apotransferrin showed the same lack of concentration dependence, until low concentrations (<6.25 μg/ml) are reached, as described above for SIP.
FIG. 5.
Reversal of C. neoformans growth inhibition by iron overload. C. neoformans growth inhibition by 5% human serum, SIP, and apotransferrin (ApoTRF), compared to growth in RPMI 1640 alone, is indicated on the vertical axis. Ferric chloride (horizontal axis) reversed the serum, SIP, and apotransferrin inhibition of C. neoformans growth.
Inhibition (86%) of C. neoformans growth by 1% human serum was completely reversed by 10 μM FeCl3. Growth of C. neoformans was enhanced in cultures containing 10 μM FeCl3 with or without 1% serum compared to growth in RPMI 1640 alone. These results indicate that RPMI 1640 has enough ferric iron to support growth of C. neoformans, but ferric iron can be growth limiting.
Others have studied anticryptococcal activity of human serum (1, 2, 10–12, 14, 16, 18) but were not able to characterize the inhibitory factor. In preliminary studies, we used molecular sieve followed by anion-exchange chromatography to isolate a protein that inhibited C. neoformans (5). Due to the small quantity of purified protein obtained, characterization could not be studied.
Here we reversed the procedure by fractionating first the protein on DEAE-Sephacel and then the active peak on Sephadex G-200. This procedure yielded sufficient highly purified protein for characterization.
Characterization of SIP was dependent on verification of sample purity, aided by the use of an improved polyacrylamide gel electrophoresis system. Amino acid analysis plus data bank access permitted identification.
There were some indications in the literature that the C. neoformans-inhibitory activity of serum was due to the iron binding capacity of serum (9, 10, 17). Reversal by FeCl3 is demonstrable with freshly prepared (3, 5) but not stored (9) reagent, presumably due to absorption, reduction, or complexing of ferric ion in stored material. C. neoformans can use ferric (15) or ferrous (13) iron via transport systems with different avidities. The ferric iron added in FeCl3 saturates transferrin; thus, transferrin cannot deny C. neoformans the available and critical iron, preventing the inhibition. This iron-denying system presumably represents an innate, first line of defense against C. neoformans in humans, coming into play when outer defenses are breached and before humoral and cellular immunity may be fully engaged.
Cryptococcosis has been frequently seen in patients with Hodgkin's disease (8) or cancer of the reticuloendothelial and lymphatic system (4, 7). It was observed previously that many serum samples, especially from patients with Hodgkin's disease, had little inhibitory activity against C. neoformans growth (1, 7). Since we demonstrated that anticryptococcal activity of normal human serum can be reversed by iron overload, it is possible that sera from patients with low inhibitory activity for C. neoformans may have overload. This is consistent with elevated serum iron and low unbound transferrin in cord blood serum (6) and low inhibitory activity against C. neoformans (18). Alternatively, in some patients low transferrin levels may be responsible for low inhibitory activity. Gadebusch (7) correlated the decreased serum activity in hematologic dyscrasias with liver involvement and found decreased activity in hepatic cirrhosis. Transferrin is synthesized in the liver.
REFERENCES
- 1.Baum G L, Artis D. Growth inhibition of Cryptococcus neoformans by cell free human serum. J Med Sci. 1961;241:613–616. doi: 10.1097/00000441-196105000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Baum G L, Artis D. Characterization of the growth inhibition factor for Cryptococcus neoformans in human serum. Am J Med. 1963;87:53–57. doi: 10.1097/00000441-196307000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Brummer E. Human defenses against Cryptococcus neoformans: an update. Mycopathologia. 1999;145:121–125. doi: 10.1023/a:1006905331276. [DOI] [PubMed] [Google Scholar]
- 4.Collins V P, Gellhorn A, Trimble J R. The coincidence of cryptococcosis and disease of the reticuloendothelial system. Cancer. 1951;4:883–889. doi: 10.1002/1097-0142(195107)4:4<883::aid-cncr2820040426>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Duvvuru S, Brummer E, Morelli R, Stevens D A. Isolation of a human serum protein that inhibits the growth of Cryptococcus neoformans. Mycopathologia. 1999;144:1–7. doi: 10.1023/a:1006905010363. [DOI] [PubMed] [Google Scholar]
- 6.Esterly N B, Brammer S R, Crounse R G. The relationship of transferrin and iron to serum inhibition of Candida albicans. J Investig Dermatol. 1967;49:437–442. [PubMed] [Google Scholar]
- 7.Gadebusch H H. Anticryptococcal serum factors in malignant reticuloendothelioses in man. 1965. pp. 640–648. . Antimicrob. Agents Chemother. 1964. [PubMed] [Google Scholar]
- 8.Gendel B R, Ende M, Norman S L. Cryptococcosis: a review with special reference to apparent association with Hodgkin's disease. Am J Med. 1950;9:343–355. doi: 10.1016/0002-9343(50)90430-x. [DOI] [PubMed] [Google Scholar]
- 9.Grover D D, Brummer E, Stevens D A. Study of the role of iron in the anticryptococcal activity of human serum and fluconazole. Mycopathologia. 1996;133:71–77. doi: 10.1007/BF00439116. [DOI] [PubMed] [Google Scholar]
- 10.Hendry A T, Bakerspigel A. Factors affecting serum inhibited growth of Candida albicans and Cryptococcus neoformans. Sabouraudia. 1969;7:219–229. [PubMed] [Google Scholar]
- 11.Howard D H. Some factors which affect the initiation of growth of Cryptococcus neoformans. J Bacteriol. 1961;82:340–345. doi: 10.1128/jb.82.3.430-435.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igel J H, Bolande R P. Humoral defense mechanisms in cryptococcosis: substances in normal human serum, saliva, and cerebrospinal fluid affecting the growth of Cryptococcus neoformans. J Infect Dis. 1966;116:75–83. doi: 10.1093/infdis/116.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson E S, Goodner A P, Nyhus K J. Ferrous iron uptake in Cryptococcus neoformans. Infect Immun. 1998;66:4169–4175. doi: 10.1128/iai.66.9.4169-4175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nassar F, Brummer E, Stevens D A. Different components in human serum inhibit multiplication of Cryptococcus neoformans and enhance fluconazole activity. Antimicrob Agents Chemother. 1995;39:2490–2493. doi: 10.1128/aac.39.11.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyhus K J, Wilborn A T, Jacobson E S. Ferric iron reduction by Cryptococcus neoformans. Infect Immun. 1997;65:434–438. doi: 10.1128/iai.65.2.434-438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiss F, Szilagyi G, Mayer E. Immunological studies of the anticryptococcal factor of normal human serum. Mycopathologia. 1975;55:175–178. doi: 10.1007/BF00491504. [DOI] [PubMed] [Google Scholar]
- 17.Shiraishi A, Arai T. Antifungal activity of transferrin. Sabouraudia. 1979;17:79–83. doi: 10.1080/00362177985380101. [DOI] [PubMed] [Google Scholar]
- 18.Szilagyi G, Reiss F, Smith J C. The anticryptococcal factor of blood serum. J Investig Dermatol. 1966;46:306–308. doi: 10.1038/jid.1966.47. [DOI] [PubMed] [Google Scholar]