Abstract
Obesity and aging predispose to numerous, yet overlapping chronic diseases. For example, metabolic abnormalities, including insulin resistance (IR) and type 2 diabetes (T2D) are important causes of morbidity and mortality. Low-grade chronic inflammation of tissues, such as the liver, visceral adipose tissue and neurological tissues, is considered a significant contributor to these chronic diseases. Thus, it is becoming increasingly important to understand what drives this inflammation in affected tissues. Recent evidence, especially in the context of obesity, suggests that the intestine plays an important role as the gatekeeper of inflammatory stimuli that ultimately fuel low-grade chronic tissue inflammation. In addition to metabolic diseases, abnormalities in the intestinal mucosal barrier have been linked to a range of other chronic inflammatory conditions, such as neurodegeneration and aging. The flow of inflammatory stimuli from the gut is in part controlled by local immunological inputs impacting the intestinal barrier. Here, we will review the impact of obesity and aging on the intestinal immune system and its downstream consequences on gut barrier function, which is strongly implicated in the pathogenesis of obesity and age-related diseases. In particular, we will discuss the effects of age-related intestinal dysfunction on neurodegenerative diseases.
Keywords: intestine, immune system, barrier function, obesity, aging
Graphical Abstract
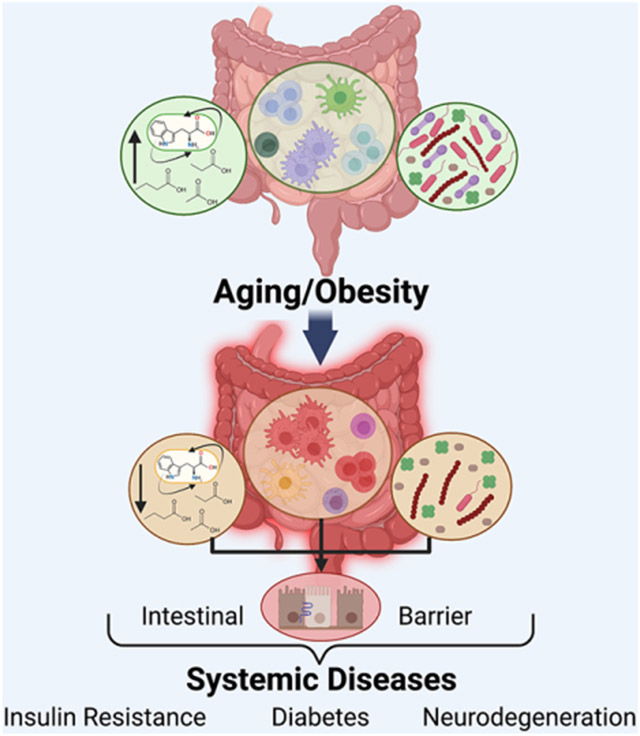
The intestine is the site of the largest compartment of the immune system. This gut immune system is altered in obesity and aging, driven by changes in microbiota, nutritional cues, and metabolites. These changes impact intestinal inflammatory mediators and gut barrier function, ultimately facilitating systemic chronic inflammation. Thus, the gut immune system is an emerging focal point contributing to multiple chronic inflammatory diseases linked to obesity and aging.
Intestinal Immune System Overview.
The intestinal immune system is composed of innate and adaptive constituents. The intestinal innate immune system includes many cells, such as innate lymphoid cells (ILC), macrophages, dendritic cells (DCs), and eosinophils, in addition to epithelial and associated cells with dynamic immune functional activities. Some of these dynamic immune functioning cells include intestinal epithelial cells (IECs)[1], goblet cells[2], Paneth cells[3], and microfold (M) cells[4]. Through secretion of factors that influence the gut microbiome and control the intrusion of pathogens, these cells contribute to intestinal barrier defense. Indeed, goblet cells produce mucus, which serves as a physical barrier defense mechanism[5]. Goblet cells also secrete molecules including trefoil factor peptides (TFF), mucins, particularly mucin-2, Fc-binding protein (Fcgbp), and resistin-like molecules (RELM) that are essential to intestinal defense and the goblet cell response to infection[6, 7]. Paneth cells also have a significant role in intestinal defense against pathogens. They are found in intestinal crypts and produce antimicrobial peptides (AMPs), such as α-defensins, lysozyme, and antimicrobial lectins (RegIIIβ and RegIIIγ)[8]. IECs can also secrete AMPs, including β-defensins[9].
Although ILCs exhibit T-cell-like characteristics, they react independently of antigen specificity[10]. Nonetheless, the three subsets of ILCs, ILC1, 2, and 3, each share transcription factors and cytokine secretion profiles with the various T helper (Th) cell subsets[10]. Indeed, transcription factor, T-bet is expressed by ILC1s, which secrete IFN-γ and TNF-α, resembling the profile of Th1 cells. On the other hand, transcription factor GATA3 is expressed by ILC2s, which secrete cytokines IL-5 and IL-13, akin to Th2 cells. Lastly, transcription factor RORC is expressed by ILC3s, which secrete IL-17 and/or IL-22, similar to Th17 and Th22 cells[10, 11]. The functional differences among the three ILC subsets, as well as their antigen-independent activation enable them to rapidly respond and provide defense against intestinal pathogens. Overall, immune cell barrier responses are frequently triggered by the recognition of commensal or pathogenic gut flora expressing microbe-associated molecular patterns (MAMPs), but are also shaped by the cytokine milieu. IECs and immune cells in the gut express pattern recognition receptors (PRRs), such as toll-like receptors (TLRs) and nuclear oligomerization domain-like receptors (NLRs), which recognize MAMPs to orchestrate immunity and barrier responses[9].
M cells of the follicle-associated epithelia’s (FAE) Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs) capture antigens from the gut lumen that are then transcytosed to be presented to a DC or macrophage in the gut[12]. DCs then migrate to mesenteric lymph nodes (MLN) via CCR7[13, 14], where they orchestrate adaptive immunity with an impact on the intestinal barrier, and in maintaining tolerance to commensal bacteria and food antigens[15].
Macrophages and DCs are dominant innate immune cells in the gut, with a diverse array of broad subtypes that are still being refined. CD11c−CD11b+F4/80+MHCII+ macrophages are typically anti-inflammatory under homeostatic conditions[16] and can stimulate Foxp3+ regulatory T (Treg) cell differentiation, IL-10, and suppress IFNγ-expressing CD4+ T cells[17]. The most abundant lamina propria (LP) myeloid cells are likely the CD11c+CD11b+CD103+CX3CR1− DCs. CD103+ DCs are tolerogenic, including for induction of oral tolerance[14, 18]. Through their elevated production of retinoic acid and TGFβ in the MLN, these DCs can induce naïve CD4+T cells to develop into Foxp3-expressing Treg cells[19]. These Treg cells then express gut-homing factors, particularly CCR9 and integrin α4β7, and can, in turn, migrate to the LP to reduce inflammation locally[13, 17, 20, 21]. Moreover, via TLR5 dependent processes, CD103+ DCs can modulate Th1 and Th17 cell function[22]. Th1 cell-derived IFNγ, facilitates macrophage phagocytosis of pathogens, while Th17 derived IL-17 and IL-22 enable AMP production[22]. Finally, after encountering a pathogen, CD103+ DCs migrate to the MLN to assist in production of a highly specific immunoglobulin A (IgA)[15]. All of these functions have the potential to impact gut inflammation and barrier function.
CD11c+CD11b+CD103−CX3CR1+ LP cells possess features of DCs and macrophages, but their characterization is more challenging, as they do not migrate to the MLN and possess limited antigen-presenting abilities[23]. It is possible that the primary role of these cells is to phagocytose pathogens[23]. Some CX3CR1hiLP cells can extend dendrites between epithelial cells to sample antigens in the intestinal lumen. They do so without perturbing the intestinal barrier, as it was shown that they express tight junction (TJ) proteins to prevent barrier disturbance[24]. These macrophage-like cells can transfer antigens onto DCs, which in turn migrate to MLNs in the small intestine[25]. Other CX3CR1 LP cells, like the CD11chiCD11b+CD103−CX3CR1int LP cells[21], may act as pro-inflammatory DCs with the ability to secrete IL-12, iNOS, TNFα, IL-6, and TGF-β, which stimulate Th1[21] and Th17[26] cells. Furthermore, novel intestine-resident mononuclear phagocytes were recently characterized in the colon. Indeed, subsets of IRF4-dependent CD14+CD24+ macrophages and DCs were found to be essential in Th17-mediated intestinal immunity[27]. This study highlights the regional differences of immune cell populations.
Regions of the intestine, entitled the gut-associated lymphoid tissue, or GALT, are comprised of ILFs, PPs, and MLNs, in which different types of adaptive immune cells including T cells, B cells and plasma cells are situated. Within the gut, Treg cells can either originate from the thymus (Helios+GATA3+ Treg) or have the ability to differentiate upon interaction with the gut microbiome (Helios−RORγt+ Treg)[28]. The latter process is dependent on c-Maf and is critical in maintaining Th17 cell homeostasis particularly[28, 29]. Conventional intestinal T cells express TCRαβ and CD4 or CD8αβ, while nonconventional intestinal T cells will express either TCRαβ or TCRγδ, and can express CD8αα homodimers[19]. T cells migrate to the intestine from the periphery via gut homing molecules, including the integrin α4β7 and the chemokine receptor CCR9[30]. Integrin α4β7 binds to the mucosal address in cell adhesion molecule-1 (MAdCAM-1), which facilitates homing to GALT. The integrin αEβ7 is increased on a fraction of infiltrating lymphocytes within the mucosa, and it facilitates lymphocyte retention at the epithelial layer via interaction with E cadherin [31]. Once in the gut, microbial and food antigens, as well as metabolites, can further shape T cell subset generation and inflammatory effect[19, 32].
Some T cells can be present in between IECs and are known as intraepithelial lymphocytes (IELs). Although IELs can be grossly categorized into TCR+ IEL and TCR− IELs, little is known about the latter group, as they contribute little to the overarching IEL population[33]. TCR+ IELs are comprised of induced (type-α) and natural (type-β) IELs[33]. Induced IELs (CD4+TCRαβ+ and CD8αβ+TCRαβ+) are activated through antigen stimulation on their path from the GALT and peripheral lymphoid tissue to the intestinal epithelium. CD4+TCRαβ+ IELs resemble Th cells, while CD8αβ+TCRαβ+ IELs share similarities with cytotoxic T cells[33, 34]. In contrast, natural IELs (TCRγδ+, including CD8αα+TCRγδ+ and CD8αα+TCRαβ+ IELs) are found within the intestinal epithelium and are thought to contribute to the regulation of intestinal immunity, as they secrete IL-10 and TGF-β [34]. Overall, it is thought that induced IELs possess a more active, protective role in the defense against pathogens, while natural IELs seem to be more tolerogenic[33].
Finally, fully differentiated B cells migrate from MLNs and intestinal GALT, where isotype switching from IgM to IgA, a major antibody in the gut likely has occurred[35, 36], into circulation. From circulation, IgA B cells reach the LP of either the small intestine or colon, based on their chemokine receptor expression, with CCR9 homing to the small intestine and CCR10/CXCR4 to the colon[37]. Inside the intestine, plasma cell (PC) terminal differentiation occurs. IgA is then produced locally in the intestine where it is shuttled through the epithelial layer to the gut lumen, rendering plasma cells indispensable in preserving intestinal barrier function. A newly elucidated mechanism by which IgA can reach the lumen is through transcytosis via integrin αEβ7 that occurs upon direct contact between IgA+ PCs that express αEβ7 and IECs that express E-cadherin[38]. In the gut lumen, IgA is typically found in a dimeric state and is a first line of defense against pathogenic gut bacteria, but is also secreted in response to commensal bacteria, providing an important role in the maintenance of tolerance[39].
Intestinal Immune Cells in Diet-Induced Obesity and in Aging.
Changes in Composition of Intestinal Immune Cells during Diet-Induced Obesity
Obesity also alters the intestinal microbiome composition, known as dysbiosis[40] and increases intestinal barrier permeability, allowing seepage of gut microbial products or MAMPs into circulation[41]. One of these MAMPs is bacterial lipopolysaccharide (LPS) that can pass through the gut barrier and into circulation, raising plasma levels of LPS, known as metabolic endotoxemia[41, 42]. Indeed, early studies in mice have linked obesity to a nonspecific low-grade intestinal inflammatory tone, accompanied by activation of pro-inflammatory signaling pathways, such as NF-κB, and enhanced expression of cytokines including IL-1β, TNFα, and IL-12p40[43, 44]. Interestingly, recent work shows that in addition to bacterial products entering metabolic tissues, bacterial colonies themselves may also impact metabolic tissue homeostasis during obesity[45].
Diet-induced obesity (DIO) also induces important changes to the cellular make-up of the intestines (fig. 1). For example, mice that are fed a high-fat diet (HFD) display a relevant reduction in the quantities of colonic Nkp46+CD4− ILC3s, which produce IL-22, a cytokine that regulates intestinal epithelial cells and maintenance of the gut barrier[46]. Interestingly, as opposed to IL-22 producing ILC3s, small intestinal ILC2s may actually have a pathogenic role in obesity through an IL-2 related mechanism, and adoptive transfer of intestinal ILC2s can worsen parameters linked to diet-induced obesity in mice[47]. Other innate immune cells also show changes in composition. In the colon of HFD-fed mice, pro-inflammatory macrophages increase in abundance in a CCL2-dependent manner after 4 and 12 weeks of HFD feeding[48], while eosinophils decrease after as little as one week after HFD initiation[49]. These changes are associated with an overall reduction in the number of anti-inflammatory CX3CR1 macrophages (CD11b+CD11c+ in particular) and in the frequency of CD103+CD11b+CD11c+ DCs in the colon of mice during diet-induced obesity[50]. These changes are consistent with the obesity-related skewed inflammatory potential of immune cell constituents. Such skewing may be conserved across species. For example, in humans, obesity has been associated with increased systemic inflammation and changes in the innate immune system, including increases in jejunal macrophages, mature DCs, and natural killer cells[51].
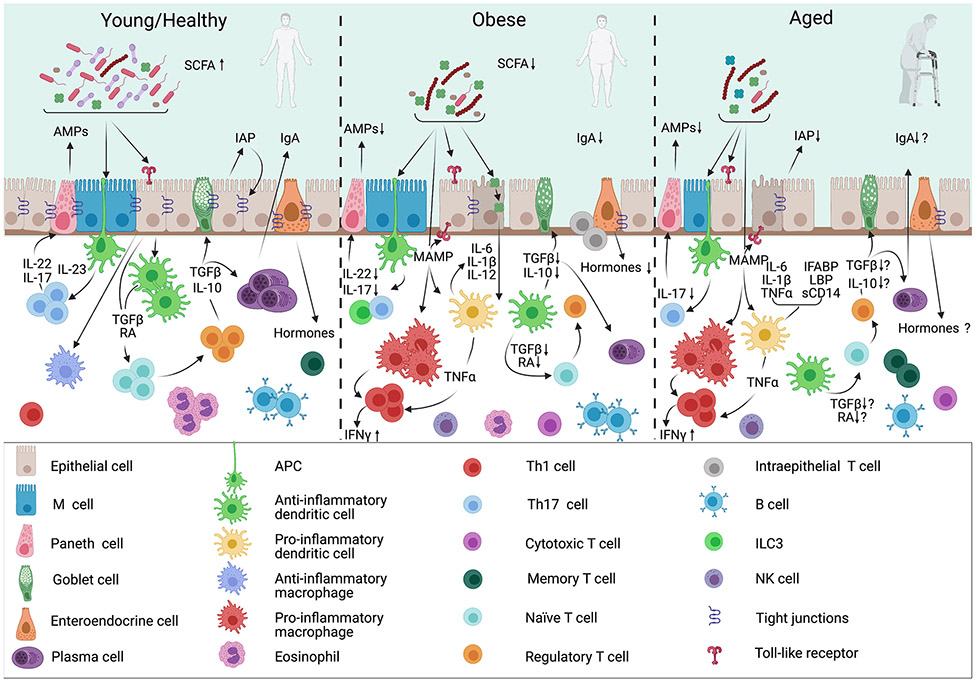
Figure 1. Summary of Young/Healthy vs. Obese vs. Aged Gut Immune Cell Composition and Function.
In obese and aged guts, a shift towards pro-inflammatory changes in immune cells contributes to increased gut permeability, as well as decreased microbial diversity. These changes are better documented in the obese setting compared to the aged environment, an emerging area of research. Leaked bacterial components act as microbe-associated molecular patterns (MAMPs) and further exacerbate pro-inflammatory activities of macrophages, dendritic cells and T cells. This effect creates an imbalance between tolerogenic anti-inflammatory and defensive pro-inflammatory cytokine and chemokine secretion along with consequent immune cell differentiation. These local changes in the intestinal digestive and barrier functions can in part drive similar changes in immune responses systemically. RA, retinoic acid. AMP, antimicrobial peptides. IAP, intestinal alkaline phosphatase. IgA, Immunoglobulin A. I-FABP, intestinal fatty acid-binding protein. LBP, LPS-binding protein.
The composition of the innate like cells in the gut is also changed during obesity. Innate-like T cells that express an invariant TCR-α chain, known as mucosal-associated invariant T cells (MAIT), are found in lower abundance in patients with obesity and type 1 diabetes in several studies[52-54]. A reduction in MAIT cells leads to microbial dysbiosis and gut integrity loss, and can cause M1-like macrophage polarization in the gut resulting in increased inflammation in mice and humans[52, 54, 55]. In mice with diet-induced obesity, absolute numbers of MAIT cells within the small intestine were also reduced[52]. However, the MAIT cells that persist have an active CD44+ phenotype, providing a means by which these cells can promote or exacerbate to intestinal inflammation and IR[52].
Moreover, DIO promotes skewing of adaptive immune cell populations, which contributes to chronic low-grade inflammation. For example, work has shown reductions in IgA+ B cells in the gut, especially in the colon and MLNs[50], as well as in the small intestine and LP in mice[56]. Other studies have shown that the proportions of IFNγ-producing Th1 cells and CD8+ T cells increased in the small intestinal lamina propria (SILP) during HFD feeding in mice, while the proportion of immunosuppressive Treg cells are reduced[46, 57-59]. Moreover, DIO mice were reported to not only exhibit a shift in T cell phenotype from naïve to effector-memory, but also to display T cell senescence within their GALT[60]. In humans, there is an increase in intestinal IFNγ-producing CD8+ T cells, which is especially seen in the IEL fraction. These cells display a switch from CD8αα to CD8αβ co-receptor expression in the jejunum, which was associated with greater expression of pro-inflammatory cytokines and insulin resistance[51]. Similarly, Tbet+ cells (including nonspecifically, cells like ILC1s and Th1 cells), are also found in greater number in the small intestine and colon of obese patients when compared to lean human control subjects[46], most likely contributing to the inflammatory profile of the gut [61] as a result of DIO. Interestingly, in mice it was found that Th1 skewing during obesity is concomitant with reduced numbers of intestinal IL-17 producing T cells (Th17 cells). These studies showed that this imbalance of Th1/Th17 cells promotes intestinal inflammation[57, 58]. Conversely, adoptive transfer of Th17 cells in obese mice was associated with improved metabolic homeostasis and protection from obesity by promoting microbiota associated with leanness, suggesting the adaptive immune system may be targeted for therapeutic purposes to treat diet-induced obesity[58].
Changes in Composition of Intestinal Immune Cells during Aging
Aging of the immune system, known as immunosenescence, compromises innate and adaptive responses, leading to an overall impairment in immunity and increased risk of inflammatory conditions that occur with age[62, 63]. Aging in the gut is linked to increased risk of developing GI cancers, chronic constipation, gut infections, dysphagia, reflux, and anorexia, among other co-morbidities of intestinal immunosenescence[64, 65]. Immune changes with age may also be linked to modulation of intestinal barrier function and these changes could have implications in aging-related insulin resistance.
On a cellular level, the intestinal innate immune system seems to be impacted by aging. It has been reported that intestinal aging brings about an elevation of pro-inflammatory mediators, such as IL-6,TNFα, IFNγ, IL-1β and C-reactive protein (CRP) locally and in visceral tissues, serum, and in the enteric nervous system (ENS) [66, 67] contributing to systemic chronic low-grade inflammation with age[68-70] (fig. 1). These inflammatory and “pro-inflammaging” cytokines also connect to poor cognitive performance, and high plasma IL-6 and CRP concentrations are linked to cognitive decline in older adults[66, 71, 72]. In rhesus macaques, aging was associated with increased expression of intestinal fatty acid-binding protein (I-FABP), LPS-binding protein (LBP), and sCD14 in circulation, which are biomarkers of leaky gut[68]. Moreover, aged macaques also showed a decline in CD161+ cells (NK, T cells, and ILCs) leading to reduced expression of IL-22 and IL-17, cytokines that protect against intestinal permeability, and enhanced Th1-like pro-inflammatory cytokine phenotype[68]. Thus, this study revealed a link between aging, gut immune cell changes, and increased barrier permeability.
Metabolic endotoxemia, as well as an age-associated increase in the paracellular permeability of the microbiome, has also been found to be associated with excessive production of IL-6 in old mice, as well as increased tissue and circulatory levels of IL-6 and TNFα[69]. Overall, these changes show similarities at the cytokine level between diet-induced obesity and aging within the gut. In aging mice, gut macrophages may show reduced phagocytosis, but inside MLN they have been shown to increase co-stimulatory markers, including CD80 and CD86[69, 73]. Inside the small intestinal muscularis, aging induces a shift towards a pro-inflammatory M1-like macrophage profile, seemingly related to decreased FoxO3 levels in mice with age[67]. This pro-inflammatory shift in macrophages is also linked to a premature aging phenotype of the ENS[67]. In mice, total CD11c+ DCs, especially inside PPs, may be reduced with age in the intestine[74]. Similarly, goblet cell density was decreased in aged mice due to apoptosis in colonic crypts, resulting in decreased mucus production[75]. Paneth cell function also seems to be altered with age, as transcript levels of an α-defensin and lysozyme are significantly decreased in the ileum of aged mice[76]. The number of ILC3s has been reported to be decreased in the gut of aged mice [77] while the numbers of ILC2s increase in humans[78]. Finally, intestinal murine and human stem cells have been found to have a significantly reduced regenerative ability with age, through decreased canonical Wnt signaling[79]. Collectively, these data show that aging may impact both the function and composition of the innate immune system in the intestine.
Similarly, the adaptive immune system in the intestine also changes with age[74, 80]. Several studies emphasize the age-related impairment of DC function and the diminished ability for naïve T cells to differentiate, particularly in their ability to induce CD4+CD25+LAP+ Tregs[73, 80, 81]. One study observed that the proportion of T cells increased overall with age in mice, including unique CD4+CD8αα+ cells in the ILFs[82]. As well, several studies found a reduction in the number of CD3, CD4 and cytotoxic T cells[75, 80, 83], an increase of CD8+CD45RA− memory T cells[67], and a decrease of CD8+CD45RA+CD28+ naïve T cells[80, 83, 84] with age. In addition, expression of the co-inhibitory receptor, CTLA-4, is reduced in human colonic LP CD4+ and CD8+ T cells from older individuals[85], highlighting a potential link to local inflammation induced with age in the mediation of immune responses. However, the status of Tregs and conventional Th cell subsets with age are controversial. One study showed a reduction in the proportion of Tregs in the GALT and MLN in older patients[86] and another observed a reduction in Th cells in mice[83] indicating that gut Tregs and Th cells regress with age[86]. However, a third group noted an increase in CD4+CD25+Foxp3+, CD4+CD25+LAP+ cells (Tregs), and CD4+CD44+ T cells, and a decrease in naive CD4+CD62L+ T cells in the MLN and PP of old mice, coupled with an increase in both Th cells and T memory cells in the LP[73]. The latter group[73] also observed a decrease in the number of IELs possessing regulatory functions, such as TCRγδ+ and TCRαβ+CD8αα+, in aged mice.
With age, the number of PPs does not vary, but they are significantly smaller and lighter in older mice, weighing almost half the weight of PPs from younger mice, which is likely due to a reported reduction in cell number in older mice PPs[70, 83, 87]. In addition, there is a substantial decrease of mature M cell number[87] and of transcytosis of pathogens with age in mice[70, 87]. This decrease has been attributed to Spi-B, a factor involved in the maturation of M cells, and CCL20, which draws CCR6 expressing lymphocytes towards the FAE[70, 87]. The reduced expression of CCL20, as well as CXCL13, and receptors CCR6 and CXCR5[82], has been associated with the decreased number of B cells in PP of aging mice[82, 83, 87].
Age may also limit clonal diversity of B cells[80] in the ILF and PP, with increased VH1 usage[70, 82]. With age, changes to IgA are controversial, with some studies showing increases and other studies showing no changes in the stool of mice[82, 88-90]. One study demonstrated that cells expressing IgA were doubled in PPs, but decreased by more than half in the LP of aged rats, which they attribute to a significant reduction in α4β7 and MAdCAM-1 in the LP, impairing IgA homing to the LP[91]. Likewise, a recent study demonstrated that IgA and a number of immunoglobulin chains were down-regulated transcriptionally in the ileum and/or colon of old mice[75]. Interestingly, this paper also showed downregulation of CTLA-4 pathways within the ileum as the as well global downregulation of processes related to adaptive immunity in both colon and ileum of old mice. Thus, it will be necessary to better map out ways in which the affinity of IgA and other antibody responses change with age, particularly in humans. Finally, studies have linked an increase in apoptosis with a steady drop in CD8α+ blood MAIT cells in circulation of elderly persons[92, 93], though more work is needed to map out how these cells change within the intestine with age.
Dietary Involvement in Diet-Induced Obesity and in Aging.
Nutrition and Microbial Metabolites in Intestinal Immunity and Gut Barrier Function in Obesity
As discussed, diet-induced obesity drives changes to the intestinal immune system, which can significantly compromise intestinal barrier function resulting in increased permeability. Within one month of HFD feeding, mice display overall reduced AMP levels that impair barrier function, specifically in the ileum[94]. The pro-inflammatory shift in intestinal immune cells observed during obesity is associated with increases in inflammatory cytokines, IFNγ and IL-1β, that exert detrimental effects on epithelial TJ proteins, occludin and zonula occludens-1 (ZO-1), which in turn increase barrier permeability[46, 95]. Diet-induced obesity may also worsen barrier function through decreased secretion of anti-inflammatory cytokines, such as IL-10, IL-17, IL-22, and TGF-β, attributed to the reduction in these reparative cytokine-producing intestinal immune cells,Th17 cells, ILC3s, Tregs, and eosinophils in mice[96]. These changes have been shown to impair intestinal barrier function, as IL-10 stimulates mucin production from goblet cells[97] and induces IgA secretion from plasma cells[98] while IL-22 drives IEC and Paneth cell AMP secretion[99, 100]. High-cholesterol diets also negatively alter murine intestinal immunity by suppressing IgA response to infection[101]. Moreover, murine consumption of simple sugars can stimulate the pathogenesis of colitis, as well as severely impact the gut microbiome, such as by altering the amounts of Akkermansia muciniphila and Bacteriodes fragilis, known to be mucus-degrading bacteria[102].
Since immune cells and inflammatory output can impact the gut barrier, identifying factors that control them in the gut will lend insight into the role of intestinal immunity during diet-induced obesity. Remarkably, obesity-induced immune changes in the intestine rely on dietary and bacterial signals (fig. 2). During diet-induced obesity, bacterial dysbiosis leads to microbial encroachment further into the epithelium and is also associated with leakage of bacterial products, such as LPS. These effects lead to PRR activation to promote inflammatory responses within the intestine[103]. Such processes are facilitated by the accompanying reduction in IgA levels alongside increased insulin resistance seen in diet-induced obesity[50]. The link between insulin resistance, inflammation and increased gut barrier permeability is also being uncovered in humans[104]. A recent study found that administration of an insulin receptor antagonist to mice, which leads to systemic insulin resistance, severely worsened barrier permeability, which was shown to be a result of a dramatically altered gut microbiome and loss of Paneth cell antimicrobial function[105]. The potential significant impact of insulin resistance on intestinal homeostasis emphasizes the effect of diet on this organ system and its diverse microbial population, which not only carries local repercussions, but also on an organism-wide scale.
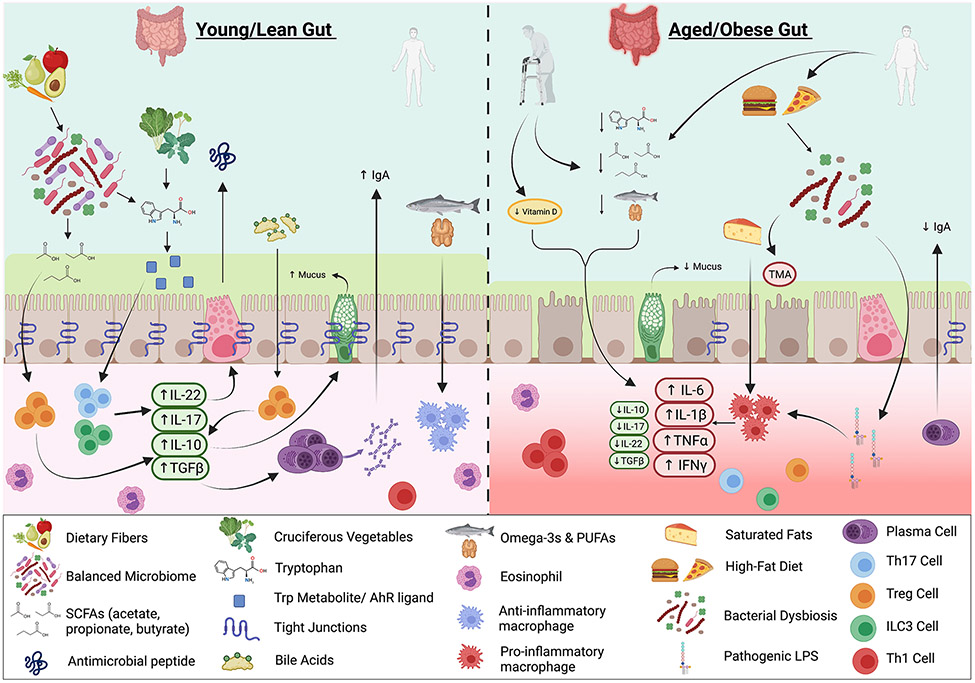
Figure 2. Nutritional and Microbial Metabolite Impacts on the Gut Immune System and Barrier Function in the Young/Healthy, Obese, and Elderly.
In the young/lean gut, intake of dietary fiber promotes homeostasis of the gut flora and favor production of short-chain fatty acids (SCFAs), which stimulate Treg cells to produce IL-10 and TGFβ. Intake of cruciferous vegetables leads to generation of tryptophan metabolites and aryl hydrocarbon receptor (AHR) ligands, which upon binding to AHR, stimulate Th17 cells and ILC3s to secrete IL-22 and IL-17, and antimicrobial peptides (AMP) release by Paneth cells. Bile acids stimulate Treg cells, which produce IL-10 and enable production of mucus by goblet cells. Healthy fats maintain anti-inflammatory macrophage populations. With aging, decreased intake of tryptophan metabolites, healthy fats, vitamin D and decreased SCFA production act to create a more inflammatory environment. With obesity, increased saturated fat intake polarizes macrophages to adopt a pro-inflammatory profile, while increased consumption of red meat leads to generation of trimethylamine (TMA) by the microbiome. In addition, obesity and potentially aging are characterized by heightened inflammation through elevated secretion of pro-inflammatory cytokines and stimulation of Th1 cells with simultaneous decrease in Treg, Th17 and ILC3 differentiation, and reduced Immunoglobulin A (IgA) secretion. These states also contribute to loosened tight junctions (TJ), increased membrane permeability and microbial dysbiosis, which exacerbate the inflammatory signature, in part attributable to leakage of bacterial lipopolysaccharide (LPS) and other microbial products across the barrier into tissues and circulation.
Specific nutritional factors possess the ability to shape intestinal immunity and can act directly on immune cells or interface with the gut bacteria to produce bioactive metabolites. Saturated fats were shown to activate intestinal macrophages, while omega-3 polyunsaturated fats may exert anti-inflammatory effects on these cells in mice[106, 107]. The effects of omega-6 polyunsaturated fats have been debated, with some murine and human studies showing that they can promote metabolic inflammation[108-111] but other studies, including meta-analyses, linking consumption of these fats to reduced overall mortality[112]. Additionally, the Standard American Diet is also associated with greater consumption of red and processed meat. Recent evidence suggests that metabolites found in these meats, including choline, betaine, and L-carnitine, are metabolized by the gut microbiota to produce trimethylamine (TMA), which is further oxidized by the liver to produce trimethylamine N-oxide (TMAO)[113]. Increased levels of TMAO can further promote inflammation in both humans and mice, and has been linked to higher incidence of cardiovascular disease, colorectal cancer, and hyperlipidemia acute pancreatitis[114-116]. Lastly, in addition to food metabolites, processed foods consumed in a Standard American Diet contain high amounts of food colorants, emulsifiers, oxazoles from thermal processing, and preservatives that can alter immune cell function, the intestinal flora, and promote intestinal and systemic inflammation [117-120].
However, unlike the Standard American Diet, diets rich in dietary fibers, fruits, and vegetables can be beneficial against metabolic disease. For example, dietary fibers are fermented by gut microbes to produce short-chain fatty acids (SCFA), such as acetate, propionate and butyrate. Propionate and butyrate can enhance insulin sensitivity in the tissues and decrease lipogenesis, lowering the risk of T2D and cardiovascular disease[121]. Butyrate, and inulin, which is a prebiotic fiber, were also found to improve barrier function in a HFD obesity-induced mouse model and in in vitro gut organoid cultures by upregulating AMPs and Paneth cell activity[122], the latter known to be dysfunctional as a result of HFD-induced obesity in humans and in mice[123]. On the other hand, acetate has shown mixed effects on obesity with some studies showing an inhibition of liver lipids and improved glucagon-like peptide (GLP-1) and peptide-YY levels, others have shown opposing effects, linking it elevated ghrelin and obesity risk[124, 125]. SCFA function in part through activation of the G-protein coupled receptors (GPCR). GPR43 is the most well-known SCFA target, as it is widely expressed in the intestine, adipose tissue, and in many immune cell populations. It plays a role in recruiting various immune cells during inflammatory reactions in vivo in mammals[126]. The intestinal barrier and immune system are generally reinforced by SCFAs, as they can regulate mucus production, Treg differentiation, IgA secretion, epithelial repair, and inhibition of NF-κB[127-129], which reduce inflammation and promote healthy gut tissue homeostasis. Consistently, patients with T2D typically exhibit reduced levels of SCFA-producing microbes[130]. Some sugar alcohols, such as erythritol, can also boost SCFA production during HFD feeding mice. Erythritol supplementation in mice fed a HFD dampened inflammation in the small intestine and related metabolic disorders, such as insulin resistance, dyslipidemia, and fatty liver disease through increasing SCFA concentration and number of ILC2s and ILC3s, in the small intestine and WAT respectively[131].
Certain nutrients, such as those derived from fruits, vegetables, and tryptophan-rich foods, can also be metabolized by particular intestinal bacteria to target the aryl hydrocarbon receptor (AHR). Cruciferous vegetables, like broccoli and other members of the Brassica genus, are enriched in AHR precursor compounds, such as glucobrassicin, and are broken down in the stomach to yield AHR modulators[106]. Th17, Treg, IELs, and ILC3 subsets in the murine gut express the AHR, which upon ligation, can promote IL-22 secretion, a cytokine that promotes AMP secretion and gut barrier repair mechanisms[132, 133]. Conversely, obesity mediated microbial dysbiosis in mice and potentially in humans hinders bacterial metabolism of tryptophan into AHR modulators, jeopardizing barrier function, lessening GLP-1 secretion[134, 135], and promoting inflammation[136].
Nutrition and Microbial Metabolites in Intestinal Immunity and Gut Barrier Function in Aging
Multiple studies have shown that aging, especially in mice, can increase intestinal permeability, promoting gut barrier leakage of bacterial components such as endotoxin or muramyl dipeptide (MDP), which is partly enabled by the downregulation of TJ and adherent junction proteins observed with age[70, 73, 137-142]. LPS activation of TLR4 can stimulate an inflammatory response that worsens the intestinal barrier and TJ permeability (fig. 2). Indeed, pro-inflammatory cytokines have been reported to increase TJ permeability. For example, increased levels of TNF-α and IFNγ have been shown to downregulate the expression of occludin and ZO-1 in various organisms[137, 143, 144]. Other cytokines like IL-6 may exhibit more variable effects on paracellular permeability[145].
Interestingly, two publications [146, 147] have reported additional mechanisms in mice and humans that may be involved in age-related barrier dysfunction: the stress polarity signaling (SPS) pathway and intestinal alkaline phosphatase (IAP) dysfunction. The SPS pathway is reported to decline with age and is dependent on AMPK and its effector G-α interacting vesicle–associated protein, GIV (also known as Girdin), which are important in preserving the gut barrier integrity by enforcing TJs and maintaining cell polarity during stress[146]. Metformin can restore this pathway to reinforce the gut barrier, in addition to its effects on the microbiome and mucin production by goblet cells[146, 148]. IAP is expressed by enterocytes in the small intestine and can detoxify a variety of bacterial factors like LPS, CpG DNA, and flagellin, and promote gut barrier and TJ function. IAP decline with age reduces TJ protein expression, increases pro-inflammatory cytokine expression and LPS in the portal and systemic blood, increases frailty, and shortens lifespan. Restoration of IAP through supplementation reversed these co-morbidities[147] (fig. 1). With age, it is likely that these same processes impact local immune populations within the intestine, which in turn contribute to the barrier phenotypes. The control of bacterial products into circulation then could impact downstream inflammation in metabolic tissues and insulin resistance with age. However, it is important to note that although many studies observe an increase in intestinal permeability with age, especially in mice, some studies do not observe the exact change, including in humans[149-152]. This concept in humans is still a controversial topic and differences between mice and humans could be related to differences in the microbiome or in assays used to measure permeability between species.
Like with obesity, bacterial metabolites play a crucial role in controlling the immune system at the gut barrier with age. Older individuals are frail and have a less diverse microbiome and varies in which species predominate in the gut compared to younger individuals. This difference can be attributed to less mobility, reduced appetite, lifestyle and dietary changes, use of antibiotics, and weakened immune system, among many other factors[153-156]. It is widely accepted that there is a decreased frequency of commensal bacteria, like Clostridium cluster XIVa, and an increase in opportunistic bacteria in the elderly, like Proteobacteria[157-161]. This shift can decrease the amounts of bacteria that produce SCFAs. As discussed above, SCFAs play a role in anti-inflammatory responses, as they also possess histone deacetylase (HDAC) inhibitory abilities that decrease pro-inflammatory cytokine expression and can induce differentiation of IL-10 producing Foxp3+ Treg cells[19, 134, 159, 160, 162, 163]. Some of the commensal bacteria that are less prevalent in the gut of older individuals and affect such processes are Clostridium cluster XIVa, Bacteroides fragilis, Faecalibacterium prausnitzii, Firmicutes, Ruminococcaceae, and Akkermansia muciniphila[153, 157, 158, 164]. Overall, a reduction in SCFAs, which is also related to decreased dietary fiber intake in the elderly, provides less fuel for these commensals to grow and compromises Treg immunity at the barrier, leading to less IL-10 and mucin production, and an increased gut inflammatory tone[97, 165, 166] (fig. 2). Supplementation with dietary fibers, like inulin, or certain probiotic strains can increase SCFAs, reinforce the gut barrier and mucus layer, and strengthen TJs in various model organisms[167-170]. Another microbial metabolite, Urolithin B, derived from ellagitannin consumption (such as found in pomegranate juice), has also been reported to improve inflammation, barrier function and dysbiosis in the small intestine, associated with aging through repression of the HMGB1-TLR4-NF-κB pathway and boosting anti-oxidation capacity in mice in vivo and in human cells in vitro[171]. This work also points to the importance of targeting intestinal inflammatory parameters as well as oxidative stress within models of aging.
Like diet-induced obesity, intake of tryptophan-rich food decreases with age in different species[172-174]. Indoles are derived from tryptophan-rich foods and from commensal bacteria that assist in breaking down tryptophan to generate metabolites. Indoles created through this process exert a protective role for the host and have been shown to extend the healthspan of different species, acting through the AHR[173, 175]. Indeed, in mice, indoles have been shown to counteract some effects of colonic aging through increasing epithelial cell proliferation and goblet cell differentiation[175]. Under homeostatic conditions, the AHR is important in maintaining defenses against pathogens in the gut. Indeed, AHR can promote IL-22 and IL-17 production by ILC3s and Th17 cells, as well as increased IL-10 through CD8αα+ cells, and differentiation of Treg cells in mouse models[134, 176-178]. In fact, IL-22 production can induce AMP release to reinforce protection against pathogens, while IL-10 can promote mucin production[97, 134]. Moreover, tryptophan metabolism can also result in kynurenine production, which is also an AHR modulator. Reports associating aging in other tissues, like the brain, with the tryptophan catabolism pathway generally demonstrate an increase in kynurenine production with age, which has been associated with inflammation in animal models and humans[179, 180]. In the gut, intestinal AHR activation indirectly leads to other structures, like oxazoles, potentially through kynurenic and xanthurenic acid, which can sometimes promote gut inflammation in mice through a CD1d dependent pathway[118]. More work is needed to understand the balance between indole and kynurenine effects on the gut barrier with age [118, 179, 180].
NAD+ is an essential molecule for life that has been shown to decrease in aging tissues, as a consequence of aging-related inflammation and increased senescent cell burden[181-183]. Emerging evidence suggests decreased NAD+ levels are directly linked to age-related diseases, such as cancer and cognitive decline[184] via the dysregulation of multiple cellular processes associated with healthy aging including DNA repair, chromatin remodeling, stress response, and inflammation[185]. Thus, in the aging field there has been growing interest to assess, in both rodents and humans, whether restoring NAD+ to more youthful levels through the use of dietary NAD+ precursors, such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), could promote healthy aging. Indeed, a randomized control trial was published in which NMN supplementation effectively alleviated symptoms of metabolic dysfunction in overweight and obese patients through regulating insulin signaling[186]. While little is known on the influence of NAD+ levels on intestinal homeostasis during aging, a recent manuscript demonstrated that the administration of dietary NR to aging mice led to increased intestinal stems cells and colonic crypt formation, and a more efficient response to intestinal damage[187]. Interestingly, studies conducted on mice suggest that the ability of these NAD+ dietary precursors to restore NAD may be dependent on the gut microbiota[188], which has been found to be altered during aging resulting in dysbiosis. Conversely, lowering NAD+ levels may also be beneficial in some contexts. Another study found that NAD+ is required to fuel intestinal inflammation and that lower intestinal NAD+ levels with FK866, a drug that blocks the NAD+ salvage pathway, can ameliorate IBD by targeting macrophage inflammation in mice and humans[189]. Thus, while more studies are necessary to determine the role of NAD+ in the gut, these studies provide evidence that NAD+ augmentation may provide some benefits in both the aging gut and other inflammation-driven intestinal diseases.
Primary bile acids are produced in the liver and secreted into the upper intestinal tract to facilitate the absorption of lipids. The gut bacteria modify bile acids that get through to the colon to yield bioactive secondary bile acids[190]. Two important targets of secondary bile acids are the farnesoid X receptor (FXR)[191] and Takeda G protein-coupled receptor 5 (TGR5)[192]. Interestingly, a study demonstrated the involvement of TGR5 in inducing GLP-1 secretion from enteroendocrine cells in the murine intestine, heightened insulin and improved glucose tolerance[193]. Furthermore, bile acid activation of FXR in the intestine reduces expression of pro-inflammatory cytokines through modulating the inflammatory profile of macrophages, resulting in alleviation of Crohn’s disease pathology[194]. Thus, some of these bile acids are important in reducing inflammation in the gut. Indeed, bile acid metabolites have also been shown to suppress Th17 cell differentiation while promoting Foxp3+ Treg cell differentiation in mouse models (fig. 2), which appear to be reliant on the integrity of bile acid receptors, including the vitamin D receptor (VDR)[195, 196]. One group found several bile acids do so by inhibiting Th17 RORγt expression, while another found that bile acids relied on RORγ+ expression in Treg cells to promote their differentiation in the colon[195, 196]. Indeed, another study has uncovered that numerous gut bacteria belonging to the Actinobacteria and Firmicutes phyla convert the secondary bile acid lithocholic acid into 3-oxolithocholic acid and isolithocholic acid, which specifically inhibit Th17 differentiation by impeding RORγt[197]. 3-oxolithocolic acid can also be converted to isoallolithocholic by gut bacteria to enhance Treg formation by facilitating a permissive chromatin structure for Foxp3 expression[198]. The effects of bile acids on the intestinal immune system is still a relatively new area of research, and linking their effects from immunological changes to intestinal barrier function with age is an interesting avenue of future work.
Vitamin D insufficiency or deficiency, due to reduced sun exposure, reduced intake and absorption, is common among the elderly. Vitamin D deficiency is linked to elevated circulatory pro-inflammatory cytokines like IL-6 and TNFα (fig. 2). In the intestine, vitamin D deficiency was correlated with increased intestinal inflammation and permeability of the epithelial barrier, via NF-κB activation[199], as well as reduced expression of α-defensins from Paneth cells and loosening of TJ[200] and it is possible that similar mechanisms are at play with age. Along these lines, both vitamin E and plant polyphenol supplementation were reported to lower serum CRP levels and improve intestinal permeability in older individuals[201], while lycopene supplementation lowered circulating TNFα levels systemically[202]. How such compounds interface with the immune system gut barrier will reveal new mechanisms of action.
Finally, some studies have observed a link between intestinal barrier dysfunction and induction of insulin resistance with age. In Drosophila, increased barrier dysfunction correlated with increased expression of some AMPs and impaired the insulin signaling pathway[203]. Another study in mice showed that a decrease in SCFA-producing commensal bacteria, including Akkermansia muciniphilia, with age worsens gut permeability and enables inflammation via endotoxins. This activation of TLR4 by gram-negative bacterial products stimulates CCR2+ monocytes to differentiate B1a cells into 4–1BBL+ B1a cells (4BL cells), which can induce insulin resistance in mice and primates[164]. Thus, understanding the interplay between the microbiome, the gut barrier, and the immune system is critical to piecing together age-related chronic disease mechanisms. Indeed, alterations in the gut microbiome increase gut barrier permeability resulting in leakage of toxic bacterial products and immune activation, which have all been linked to the development of Alzheimer’s Disease (AD) and Parkinson’s Disease (PD)[204-207], major neurodegenerative diseases of aging. We will briefly highlight some of these connections in the next section.
Gut Microbial-Immune-Barrier Connection in Neurodegenerative Diseases
The gut microbiota interacts with the host to facilitate proper functioning of immunological and metabolic systems. However, the intestine and its microbiota can also impact most other systems in the body, including the nervous system, as well as associated neurodegenerative diseases[208]. Indeed, the gut-brain axis is a unique biochemical system that allows for dynamic bidirectional communication between the intestine, including the gut-residing microorganisms, and the central nervous system and controls a variety of physiological and pathological processes[209, 210].
Research has begun to elucidate underlying mechanisms by which the gut microbiome, the gut immune system, and gut barrier integrity contribute to the pathogenesis of neurodegenerative disorders, such as AD, PD, multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS)[211-214] (fig. 3). Increased intestinal permeability, resulting in leakage of bacterial products into circulation leads to systemic inflammation that skews the profile of immune cells towards a pro-inflammatory state[215, 216] and is also observed in mouse models of neurodegeneration. For example, Cunningham et al. demonstrated that administration of LPS to mice with prion disease not only increases secretion of pro-inflammatory cytokines (IL-1β, TNF-α, and IFN-β), but also worsens various cognitive functions, such as coordination and strength, and encourages disease progression[216]. Similarly, LPS priming, through intraperitoneal injection of LPS before intravenous administration of recombinant α-synuclein, resulted in heightened inflammation and greater dissemination of α-synuclein to the brain and spinal cord in PD mouse models[217]. In humans, one study showed that old patients with prodromal AD have higher plasma LPS, coupled with intestinal dysbiosis and possible barrier dysfunction[218]. Treatment of an APP/PS1 mouse model of AD with 27-Hydroxycholesterol (OHC), a pro-inflammatory oxysterol (a cholesterol metabolite), resulted in elevated inflammation characterized by increased levels of IL-1β, TNF-α, and IL-17 in the gut and in circulation, as well as an altered gut microbiome[219]. Changes in the gut included decreased abundance of the genera Roseburia, as well as reduced levels of SCFAs propionate and butyrate. In addition, 27-OHC also led to loss of intestinal barrier integrity caused by downregulation of TJ proteins occludin, claudin 1, claudin 5 and ZO-1, resulting in cognitive impairments and worsened AD pathology[219]. Other microbial metabolites, such as indole and phenol derivatives, can also impact neurological disease. For example, a recent study has demonstrated that exposure of rat neurons in vitro to MS patient-derived CSF induced neurotoxicity in part though the action of such metabolites[220]. This link was also found to be correlative in patients’ radiological and cognitive testing.
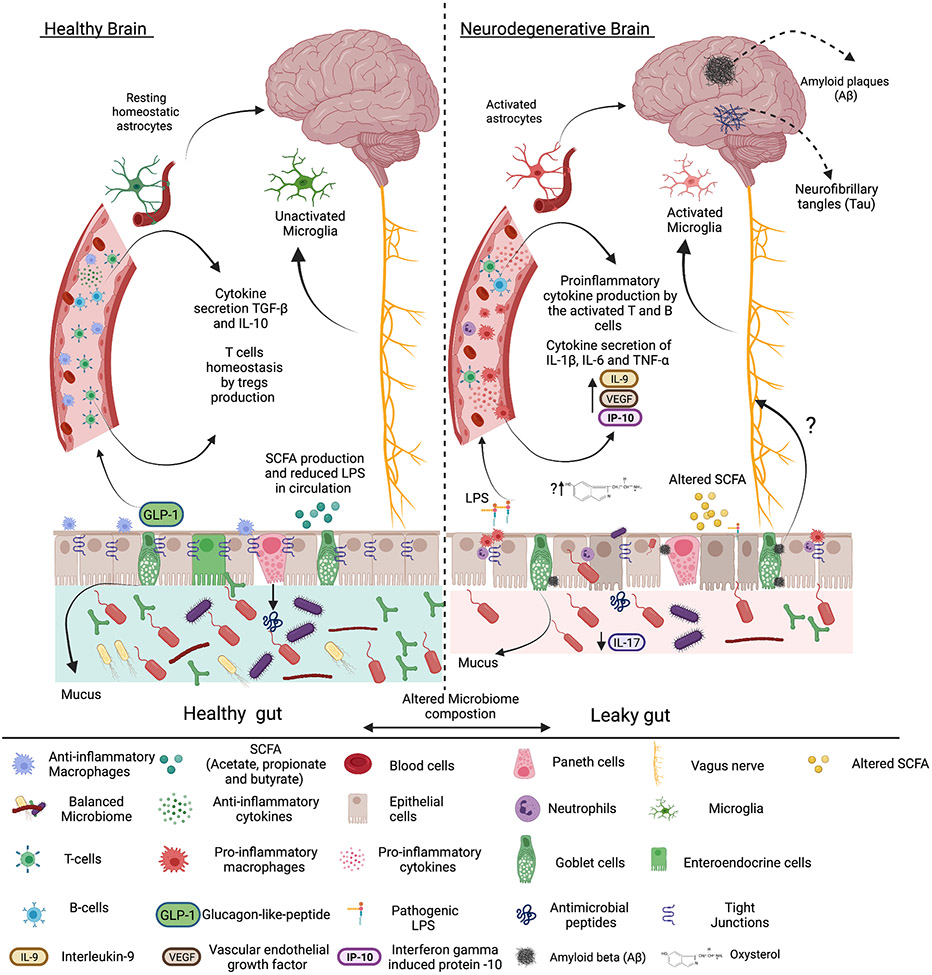
Figure 3. The proposed brain-gut-microbiome axis hypothesis.
Intestinal dysbiosis and increased gut barrier permeability enable bacterial lipopolysaccharide (LPS) translocation systemically, leading to activation of pro-inflammatory immune cells and astrocyte stimulation. Sustained systemic circulation of LPS induces secretion of pro-inflammatory cytokines and activation of microglia. These events contribute to systemic, and potentially local, activation of T cells and B cells to produce pro-inflammatory cytokines and increased inflammation. Inflammation in age-related neurodegenerative disease (Alzheimer’s Disease shown) may also be potentiated by inflammatory oxysterols, such as 27-Hydroxycholesterol (OHC), which can weaken the gut barrier or possibly enable the propagation of protein aggregates within the enteric nervous system to the brain. These effects are further exacerbated by changes in the intestinal microbiome leading to a reduction in short-chain fatty acid (SCFA) production, a reduction in protective IL-17, and increased intestinal permeability and inflammation.
Surprisingly, a recent study investigating the consequences of HFD feeding on cognition and the neurological system found that mice fed a HFD for as little as two days exhibited heightened neuroinflammation and a more permeable blood-brain barrier[221]. Within one week of HFD feeding, mice began to display cognitive impairments, owing to hippocampal dysfunction[221]. After four weeks on this diet, the mice demonstrated severely dysfunctional mitochondria within their hippocampal cells, as well as astrogliosis within their hippocampus[221]. Daily treatment of APP/PS1 mice with Akkermansia muciniphila fed a HFD for six months resulted in improved glucose tolerance, lipid homeostasis, intestinal barrier function, and cognition, alongside lowered levels of soluble amyloid β (Aβ) 40-42[222]. In addition to diet, metabolites and gut barrier dysfunction, host-derived signals during chronic intestinal inflammation also can impact neuroinflammation. Accordingly, a study in wild type mice (C57BL/6 and CD-1) with chronic DSS colitis found that chronic intestinal inflammation impaired hippocampal activity, and long-term memory specifically, by induction of neuroinflammation[223]. This process occurred potentially through HMGB1 release from the intestine, coupled with low levels of LPS, inducing caspase-1, caspase-11, IL-1β and gasdermin in the brain[223]. Ultimately, these studies strengthen the link between metabolites, intestinal barrier function, intestinal and systemic inflammation, and neurodegeneration.
Intestinal dysbiosis has been identified in AD, PD, MS, and ALS[224-227]. Given that nutritional intake plays a significant role in modulating the intestinal milieu, an emerging field of study is aimed at elucidating the connection between microbial dysbiosis and these diseases[228]. One study found that a common mouse model of AD, 5xFAD mice, possess GALT with increased CD4+ cells, though with a reduced amount of IL-17 in PP and MLN cells, likely fueling the dysbiosis observed in AD[229]. Moreover, another study [230] conducted on Tg2576 AD mice found that AD pathology may preemptively occur in the gut, accompanied by microbial dysbiosis and a damaged intestinal barrier. Indeed, Aβ deposits were found in the intestinal epithelial barrier before they appeared in the brain. This observation occurred alongside increased expression of IL-9, VEGF, and IP-10 in the plasma and disrupted absorption of nutrients[230]. The same study also observed Aβ aggregation in the intestinal epithelium of patients with AD, which reinforces the importance of the gut-brain axis in the pathogenesis of neurodegenerative diseases. In fact, a study recently has found that Aβ-like peptides synthesized from the gut microbiota (Clostridiales order) were actually capable of activating AD-related pathways in neural cells in vitro, due to their homology to Aβ, although whether this phenomenon occurs in vivo remains to be seen[231]. Furthermore, it has been reported that the fecal microbiota composition of patients with AD is significantly different. Indeed, Bacteroidetes, Ruminococcus, Actinobacteria, Lachnospiraceae, and Selenomonadales were shown to be reduced in AD patients[232]. Recently, novel strategies in the design of treatments for AD aim to target the gut microbiome. Indeed, sodium oligomannate was approved in China as a therapeutic treatment for AD. According to the study by Wang et al., the oligosaccharide improved, and even reversed cognitive impairment and Th1-related cerebral inflammation by altering the gut microbiome and dampening the pro-inflammatory effects of amino acids, phenylalanine and isoleucine[233].
Moreover, a study conducted in mice reported the effects of a low-fiber diet on the gut-brain axis over time[234]. In fact, the administered long-term, low-fiber diet induced heightened intestinal permeability, gut microbial dysbiosis, cognitive impairment, neuroinflammation and was even shown to damage hippocampal synapses, while re-introduction of SCFAs through oral supplementation was found to ameliorate this dysfunction[234]. Likewise, supplementation of propionate in a mouse model of MS, experimental autoimmune encephalomyelitis (EAE), rescued the damage induced by a HFD and resulting rise in Th17-related inflammation[235]. Recent work has illustrated how Th17 cells can be essential to maintaining intestinal homeostasis, but also contribute to systemic autoimmunity, as it is observed in EAE. During homeostasis and in EAE mice, intestinal homeostatic stem-like TCF1+ IL-17+ SLAMF6+ Th17 cells are maintained by the gut microbiome and can be converted to pathogenic GM-CSF+ IFN-γ+ CXCR6+ Th17 cells through IL-23 receptor signaling. This signaling may be occurring in the spleen, ultimately leading to potentially pathogenic cells which can migrate to the CNS and induce autoimmunity[236]. Whether a similar phenomenon occurs in age related neurodegenerative diseases, such as AD or PD, remains to be seen.
Most patients with PD present gastrointestinal symptoms, which has led the scientific community to suspect the intestine as an important site of pathological changes in PD[237-239]. For example, calprotectin, a marker of inflammation in the gut, and zonulin, a marker of impaired intestinal barrier function, are reported to be significantly elevated in the feces and serum of patients with PD compared to healthy individuals[240, 241]. Upon further investigation, the gut microbiome tends to differ between individuals with and without PD. Indeed, a large meta-analysis elucidated that the main differences consist of an increase in abundance of Lactobacillus, Akkermansia, and Bifidobacterium alongside a reduced abundance of Lachnospiraceae and Faecalibacterium in patients with PD[242]. The latter two microbes both generate SCFAs, so their significant reduction could greatly impact gut microbial homeostasis[242]. Furthermore, one group was able to identify a gut microbial marker of PD, Proteus mirabilis. When they administered it to other mice, this treatment induced neuroinflammation, dopaminergic neuronal death and motor impairment, as well as enhanced aggregation of α-synuclein[243]. Thus, the intestinal microbiome seems to be critical in the exacerbation of PD pathogenesis. To understand the implications PD progression on the GI system, rotenone, a natural pesticide that leads to symptoms of PD in mice, was administered to germ-free and conventionally-raised mice[239]. Surprisingly, rotenone caused colonic epithelial permeability and induction of motor symptoms similar to those observed in PD only in the conventionally-raised mice, suggesting that the induction of PD symptoms relies on the presence of the gut microbiome[239]. These findings emphasize the importance of the gut microbiota in the development of PD motor dysfunction.
Although many studies have investigated the impact of the intestinal microbiome on age-related neurodegenerative diseases, studies focusing on the intestinal immune system within this field are still lacking. Further research is also required to shed light on the importance of metabolites, their immune activation, and their potential role in ameliorating neurodegenerative diseases.
Model Overview and Conclusion
There is likely much overlap in the effects of diet-induced obesity and aging on the gut immune system, which in turn influences intestinal permeability and hallmarks of related diseases. A young, lean individual eating a balanced diet including fruits, vegetables and dietary fibers will possess increased intestinal microbial diversity, producing numerous metabolites from the ingested foods, including SCFAs and AHR ligands. These metabolites can fuel immune cells in the intestine, like Tregs, IELs, IL-22-producing ILC3, and Th17 cells, which are primarily associated with the production of mucus, AMPs, and/or epithelial repair programs to maintain the intestinal barrier.
In diet-induced obesity, increased intake of saturated fat coupled with reduced consumption of dietary fibers, fruits, and vegetables alter bacterial metabolites, decrease immune populations that maintain barrier integrity, and increase inflammatory cytokines that disrupt intestinal barrier TJ[20, 103]. With aging, a similar decline in barrier function occurs in mice, linked to changes in their gut microbiome, metabolites, and the promotion of an inflammatory-skewed intestinal environment. However, this effect appears to be more controversial in aging humans.
Collectively, the observed changes lead to intestinal barrier dysfunction along with metabolic endotoxemia. Leakage of bacterial products can reach visceral fat, the liver, and even neurological tissues, where it acts as an adjuvant to promote low-grade tissue inflammation[244, 245]. Metabolic tissue inflammation and associated IR lead to increased free fatty acids and hyperglycemia, ultimately further disrupting intestinal barrier function[246]. Studies have demonstrated the benefits of a healthy, fiber-rich diet on maintenance of barrier integrity with age and on reducing systemic inflammation[167, 202, 247, 248]. However, it remains to be shown whether these changes in barrier permeability can significantly impact insulin sensitivity with age. These findings may also be important in the context of age-related neurodegenerative disease, which show a close relationship between intestinal dysbiosis, barrier function, inflammation and neurological disease. Thus, the intestinal barrier is a critical gateway to the development obesity-related IR and represents an important target for future therapeutic design, but more work is needed to better map out its role in manifestation of aging and in age-related disease.
Acknowledgements
This work was funded in part through funds derived from the Buck Institute for Research on Aging (D.A.W.) and the National Institutes of Health (R01DK128435) (D.A.W.). The graphical abstract and figures of this manuscript were created with BioRender.com.
Abbreviations:
- IR
insulin resistance
- T2D
type 2 diabetes
- ILC
innate lymphoid cells
- DCs
dendritic cells
- IECs
intestinal epithelial cells
- M cells
microfold cells
- TFF
trefoil factor peptides
- Fcgbp
Fc-binding protein
- RELM
resistin-like molecules
- AMPs
antimicrobial peptides
- Th
T helper
- MAMPs
microbe-associated molecular patterns
- PRRs
pattern recognition receptors
- TLRs
toll-like receptors
- NLRs
nuclear oligomerization domain-like receptors
- FAE
follicle-associated epithelia
- PPs
Peyer’s patches
- ILFs
isolated lymphoid follicles
- MLN
mesenteric lymph nodes
- Treg
regulatory T
- LP
lamina propria
- IgA
immunoglobulin A
- GALT
gut-associated lymphoid tissue
- TJ
tight junction
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- IELs
intraepithelial lymphocytes
- PC
plasma cell
- LPS
lipopolysaccharide
- DIO
diet-induced obesity
- HFD
high-fat diet
- MAIT
mucosal-associated invariant T
- CRP
C-reactive protein
- ENS
enteric nervous system
- ZO-1
zonula occludens-1
- TMA
trimethylamine
- TMAO
trimethylamine N-oxide
- SCFA
short-chain fatty acids
- GLP-1
glucagon-like peptide
- GPCR
G-protein coupled receptors
- WAT
white adipose tissue
- AHR
aryl hydrocarbon receptor
- MDP
muramyl dipeptide
- SPS
stress polarity signaling
- IAP
intestinal alkaline phosphatase
- NAD
nicotinamide adenine dinucleotide
- NR
nicotinamide riboside
- NMN
nicotinamide mononucleotide
- FXR
farnesoid X receptor
- TGR5
Takeda G protein-coupled receptor 5
- VDR
vitamin D receptor
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- MS
multiple sclerosis
- ALS
amyotrophic lateral sclerosis
- OHC
Hydroxycholesterol
- Aβ
amyloid β
- DSS
dextran sodium sulfate
- EAE
experimental autoimmune encephalomyelitis
- CNS
central nervous system
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- 1.Goto Y, Epithelial Cells as a Transmitter of Signals From Commensal Bacteria and Host Immune Cells. Front Immunol, 2019. 10: p. 2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knoop KA and Newberry RD, Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol, 2018. 11(6): p. 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wehkamp J and Stange EF, Paneth cells and the innate immune response. Curr Opin Gastroenterol, 2006. 22(6): p. 644–50. [DOI] [PubMed] [Google Scholar]
- 4.Dillon A and Lo DD, M Cells: Intelligent Engineering of Mucosal Immune Surveillance. Front Immunol, 2019. 10: p. 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson ME and Hansson GC, Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol, 2016. 16(10): p. 639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S and Yu M, Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J Inflamm Res, 2021. 14: p. 3171–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelaseyed T, et al. , The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev, 2014. 260(1): p. 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers HC and Bevins CL, Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol, 2013. 75: p. 289–311. [DOI] [PubMed] [Google Scholar]
- 9.Peterson LW and Artis D, Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol, 2014. 14(3): p. 141–53. [DOI] [PubMed] [Google Scholar]
- 10.Ryu S, et al. , Reduction of circulating innate lymphoid cell progenitors results in impaired cytokine production by innate lymphoid cells in patients with lupus nephritis. Arthritis Res Ther, 2020. 22(1): p. 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivier E, The discovery of innate lymphoid cells. Nat Rev Immunol, 2021. 21(10): p. 616. [DOI] [PubMed] [Google Scholar]
- 12.Janeway CA Jr, T.P., Walport M, et al. , Immunobiology: The Immune System in Health and Disease. , in The mucosal immune system. 2001, Garland Science: New York. [Google Scholar]
- 13.Pabst O and Bernhardt G, The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur J Immunol, 2010. 40(8): p. 2107–11. [DOI] [PubMed] [Google Scholar]
- 14.Farache J, et al. , Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity, 2013. 38(3): p. 581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinnebrew MA and Pamer EG, Innate immune signaling in defense against intestinal microbes. Immunol Rev, 2012. 245(1): p. 113–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isidro RA and Appleyard CB, Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol, 2016. 311(1): p. G59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denning TL, et al. , Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol, 2007. 8(10): p. 1086–94. [DOI] [PubMed] [Google Scholar]
- 18.Jaensson E, et al. , Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med, 2008. 205(9): p. 2139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H, Tao W, and Zhu S, T lymphocytes in the intestinal mucosa: defense and tolerance. Cell Mol Immunol, 2019. 16(3): p. 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winer DA, et al. , The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab, 2016. 23(3): p. 413–26. [DOI] [PubMed] [Google Scholar]
- 21.Rivollier A, et al. , Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med, 2012. 209(1): p. 139–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uematsu S, et al. , Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol, 2008. 9(7): p. 769–76. [DOI] [PubMed] [Google Scholar]
- 23.Schulz O, et al. , Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med, 2009. 206(13): p. 3101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rescigno M, et al. , Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol, 2001. 2(4): p. 361–7. [DOI] [PubMed] [Google Scholar]
- 25.Mazzini E, et al. , Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity, 2014. 40(2): p. 248–61. [DOI] [PubMed] [Google Scholar]
- 26.Atarashi K, et al. , ATP drives lamina propria T(H)17 cell differentiation. Nature, 2008. 455(7214): p. 808–12. [DOI] [PubMed] [Google Scholar]
- 27.Huang HI, et al. , Th17 Immunity in the Colon Is Controlled by Two Novel Subsets of Colon-Specific Mononuclear Phagocytes. Front Immunol, 2021. 12: p. 661290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann C, Blume J, Roy U, Teh PP, Vasanthakumar A, Beller A, Liao Y, Heinrich F, Arenzana TL, Hackney JA, Eidenschenk C, Gálvez E, Stehle C, Heinz GA, Maschmeyer P, Sidwell T, Hu Y, Amsen D, Romagnani C, Chang HD, … Scheffold A, c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host-microbiota homeostasis. Nature Immunology 2019. 20(4): p. 471–481. [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, Galan C, Belkaid Y, Bonneau R, & Littman DR , c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature, 2018. 554(7692): p. 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Calisto J, et al. , T-cell homing to the gut mucosa: general concepts and methodological considerations. Methods Mol Biol, 2012. 757: p. 411–34. [DOI] [PubMed] [Google Scholar]
- 31.Dotan I, et al. , The role of integrins in the pathogenesis of inflammatory bowel disease: Approved and investigational anti-integrin therapies. Med Res Rev, 2020. 40(1): p. 245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omenetti S, et al. , The Intestine Harbors Functionally Distinct Homeostatic Tissue-Resident and Inflammatory Th17 Cells. Immunity, 2019. 51(1): p. 77–89 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma H, Qiu Y, and Yang H, Intestinal intraepithelial lymphocytes: Maintainers of intestinal immune tolerance and regulators of intestinal immunity. J Leukoc Biol, 2021. 109(2): p. 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Y, et al. , Role of the intestinal cytokine microenvironment in shaping the intraepithelial lymphocyte repertoire. J Leukoc Biol, 2015. 97(5): p. 849–857. [DOI] [PubMed] [Google Scholar]
- 35.Barone F, et al. , Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol, 2009. 2(6): p. 495–503. [DOI] [PubMed] [Google Scholar]
- 36.Bergqvist P, et al. , T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J Immunol, 2010. 184(7): p. 3545–53. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, et al. , Function and dysfunction of plasma cells in intestine. Cell Biosci, 2019. 9: p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guzman M, et al. , An integrin alphaEbeta7-dependent mechanism of IgA transcytosis requires direct plasma cell contact with intestinal epithelium. Mucosal Immunol, 2021. 14(6): p. 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato LM, et al. , The role of the adaptive immune system in regulation of gut microbiota. Immunol Rev, 2014. 260(1): p. 67–75. [DOI] [PubMed] [Google Scholar]
- 40.Turnbaugh PJ, et al. , An obesity-associated gut microbiome with increased capacity for energy harvest. Nature, 2006. 444(7122): p. 1027–31. [DOI] [PubMed] [Google Scholar]
- 41.Cani PD, et al. , Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes, 2007. 56(7): p. 1761–72. [DOI] [PubMed] [Google Scholar]
- 42.Cani PD, et al. , Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes, 2008. 57(6): p. 1470–81. [DOI] [PubMed] [Google Scholar]
- 43.Ding S, et al. , High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One, 2010. 5(8): p. e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, et al. , Intestinal, adipose, and liver inflammation in diet-induced obese mice. Metabolism, 2008. 57(12): p. 1704–10. [DOI] [PubMed] [Google Scholar]
- 45.Anhê FF, Jensen BAH, Varin TV et al. , Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nature Metabolism, 2020. 2: p. 233–242. [DOI] [PubMed] [Google Scholar]
- 46.Luck H, et al. , Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab, 2015. 21(4): p. 527–42. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki T, et al. , Innate Lymphoid Cells in the Induction of Obesity. Cell Rep, 2019. 28(1): p. 202–217 e7. [DOI] [PubMed] [Google Scholar]
- 48.Kawano Y, et al. , Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab, 2016. 24(2): p. 295–310. [DOI] [PubMed] [Google Scholar]
- 49.Johnson AM, et al. , High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLoS One, 2015. 10(4): p. e0122195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luck H, et al. , Gut-associated IgA(+) immune cells regulate obesity-related insulin resistance. Nat Commun, 2019. 10(1): p. 3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monteiro-Sepulveda M, et al. , Jejunal T Cell Inflammation in Human Obesity Correlates with Decreased Enterocyte Insulin Signaling. Cell Metab, 2015. 22(1): p. 113–24. [DOI] [PubMed] [Google Scholar]
- 52.Toubal A, et al. , Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun, 2020. 11(1): p. 3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouxel O, et al. , Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol, 2017. 18(12): p. 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magalhaes I, et al. , Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest, 2015. 125(4): p. 1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carolan E, et al. , Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol, 2015. 194(12): p. 5775–80. [DOI] [PubMed] [Google Scholar]
- 56.Sakamoto Y, et al. , High-Fat Diet and Age-Dependent Effects of IgA-Bearing Cell Populations in the Small Intestinal Lamina Propria in Mice. Int J Mol Sci, 2021. 22(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garidou L, et al. , The Gut Microbiota Regulates Intestinal CD4 T Cells Expressing RORgammat and Controls Metabolic Disease. Cell Metab, 2015. 22(1): p. 100–12. [DOI] [PubMed] [Google Scholar]
- 58.Hong CP, et al. , Gut-Specific Delivery of T-Helper 17 Cells Reduces Obesity and Insulin Resistance in Mice. Gastroenterology, 2017. 152(8): p. 1998–2010. [DOI] [PubMed] [Google Scholar]
- 59.Garidou L, et al. , The Gut Microbiota Regulates Intestinal CD4 T Cells Expressing RORγt and Controls Metabolic Disease. Cell Metab, 2015. 22(1): p. 100–12. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, et al. , High-fat diet and dyslipidemia synergistically contribute to T cell senescence in gut associated lymphoid tissue. Exp Gerontol, 2021. 151: p. 111404. [DOI] [PubMed] [Google Scholar]
- 61.Bernink JH, et al. , Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol, 2013. 14(3): p. 221–9. [DOI] [PubMed] [Google Scholar]
- 62.Aiello A, et al. , Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front Immunol, 2019. 10: p. 2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feehan J, Tripodi N, and Apostolopoulos V, The twilight of the immune system: The impact of immunosenescence in aging. Maturitas, 2021. 147: p. 7–13. [DOI] [PubMed] [Google Scholar]
- 64.Chassaing B, et al. , Mammalian gut immunity. Biomed J, 2014. 37(5): p. 246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saffrey MJ, Aging of the mammalian gastrointestinal tract: a complex organ system. Age (Dordr), 2014. 36(3): p. 9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chambers ES and Akbar AN, Can blocking inflammation enhance immunity during aging? J Allergy Clin Immunol, 2020. 145(5): p. 1323–1331. [DOI] [PubMed] [Google Scholar]
- 67.Becker L, et al. , Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut, 2018. 67(5): p. 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker EM, et al. , Inflammaging phenotype in rhesus macaques is associated with a decline in epithelial barrier-protective functions and increased pro-inflammatory function in CD161-expressing cells. Geroscience, 2019. 41(6): p. 739–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thevaranjan N, et al. , Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe, 2017. 21(4): p. 455–466 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mabbott NA, et al. , Aging and the mucosal immune system in the intestine. Biogerontology, 2015. 16(2): p. 133–45. [DOI] [PubMed] [Google Scholar]
- 71.Lin T, et al. , Systemic Inflammation Mediates Age-Related Cognitive Deficits. Frontiers in aging neuroscience, 2018. 10: p. 236–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradburn S, Sarginson J, and Murgatroyd CA, Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Frontiers in aging neuroscience, 2018. 9: p. 438–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santiago AF, et al. , Aging correlates with reduction in regulatory-type cytokines and T cells in the gut mucosa. Immunobiology, 2011. 216(10): p. 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato H, et al. , Lack of oral tolerance in aging is due to sequential loss of Peyer's patch cell interactions. Int Immunol, 2003. 15(2): p. 145–58. [DOI] [PubMed] [Google Scholar]
- 75.Sovran B, et al. , Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci Rep, 2019. 9(1): p. 1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tremblay S, et al. , Ileal antimicrobial peptide expression is dysregulated in old age. Immun Ageing, 2017. 14: p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yudanin NA, Schmitz F, Flamar AL, Thome J, Tait Wojno E, Moeller JB, Schirmer M, Latorre IJ, Xavier RJ, Farber DL, Monticelli LA, & Artis D , Spatial and Temporal Mapping of Human Innate Lymphoid Cells Reveals Elements of Tissue Specificity. Immunity, 2019. 50(2): p. 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D'Souza SS, Shen X, Fung I, Ye L, Kuentzel M, Chittur SV, Furuya Y, Siebel CW, Maillard IP, Metzger DW, & Yang Q, Compartmentalized effects of aging on group 2 innate lymphoid cell development and function. . Aging Cell, 2019. 18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nalapareddy K, Nattamai KJ, Kumar RS, Karns R, Wikenheiser-Brokamp KA, Sampson LL, Mahe MM, Sundaram N, Yacyshyn MB, Yacyshyn B, Helmrath MA, Zheng Y, & Geiger H , Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Rep, 2017. 18(11): p. 2608–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato S, Kiyono H, and Fujihashi K, Mucosal Immunosenescence in the Gastrointestinal Tract: A Mini-Review. Gerontology, 2015. 61(4): p. 336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agrawal S, et al. , Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging (Albany NY), 2016. 8(6): p. 1223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McDonald KG, et al. , Aging impacts isolated lymphoid follicle development and function. Immun Ageing, 2011. 8(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawanishi H and Kiely J, Immune-related alterations in aged gut-associated lymphoid tissues in mice. Dig Dis Sci, 1989. 34(2): p. 175–84. [DOI] [PubMed] [Google Scholar]
- 84.Dock J, et al. , Distinct aging profiles of CD8+ T cells in blood versus gastrointestinal mucosal compartments. PLoS One, 2017. 12(8): p. e0182498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dillon SM, et al. , Reduced immune-regulatory molecule expression on human colonic memory CD4 T cells in older adults. Immun Ageing, 2021. 18(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Senda T, et al. , Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol, 2019. 12(2): p. 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi A, et al. , The functional maturation of M cells is dramatically reduced in the Peyer's patches of aged mice. Mucosal Immunol, 2013. 6(5): p. 1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Senda S, Cheng E, and Kawanishi H, Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scand J Immunol, 1988. 27(2): p. 157–64. [DOI] [PubMed] [Google Scholar]
- 89.Szewczuk MR, Campbell RJ, and Jung LK, Lack of age-associated immune dysfunction in mucosal-associated lymph nodes. J Immunol, 1981. 126(6): p. 2200–4. [PubMed] [Google Scholar]
- 90.Santiago AF, et al. , Role of mesenteric lymph nodes and aging in secretory IgA production in mice. Cell Immunol, 2008. 253(1-2): p. 5–10. [DOI] [PubMed] [Google Scholar]
- 91.Schmucker DL, et al. , Basis for the age-related decline in intestinal mucosal immunity. Clin Dev Immunol, 2003. 10(2–4): p. 167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novak J, et al. , The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol, 2014. 80(4): p. 271–5. [DOI] [PubMed] [Google Scholar]
- 93.Chen P, et al. , Circulating Mucosal-Associated Invariant T Cells in a Large Cohort of Healthy Chinese Individuals From Newborn to Elderly. Frontiers in Immunology, 2019. 10(260). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomas J, et al. , High-fat diet modifies the PPAR-gamma pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci U S A, 2016. 113(40): p. E5934–E5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Sadi RM and Ma TY, IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol, 2007. 178(7): p. 4641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X, et al. , Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature, 2014. 514(7521): p. 237–41. [DOI] [PubMed] [Google Scholar]
- 97.Hasnain SZ, et al. , IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology, 2013. 144(2): p. 357–368 e9. [DOI] [PubMed] [Google Scholar]
- 98.Gutzeit C, Magri G, and Cerutti A, Intestinal IgA production and its role in host-microbe interaction. Immunol Rev, 2014. 260(1): p. 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng Y, et al. , Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med, 2008. 14(3): p. 282–9. [DOI] [PubMed] [Google Scholar]
- 100.Sonnenberg GF, et al. , Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science, 2012. 336(6086): p. 1321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trindade BC, et al. , The cholesterol metabolite 25-hydroxycholesterol restrains the transcriptional regulator SREBP2 and limits intestinal IgA plasma cell differentiation. Immunity, 2021. 54(10): p. 2273–2287 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan S, Waliullah S, Godfrey V, Khan M, Ramachandran RA, Cantarel BL, Behrendt C, Peng L, Hooper LV, & Zaki H , Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med, 2020. 12(567). [DOI] [PubMed] [Google Scholar]
- 103.Winer DA, et al. , Immunologic impact of the intestine in metabolic disease. J Clin Invest, 2017. 127(1): p. 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mkumbuzi L, et al. , Insulin Resistance is Associated with Gut Permeability Without the Direct Influence of Obesity in Young Adults. Diabetes Metab Syndr Obes, 2020. 13: p. 2997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gueddouri D, et al. , Insulin resistance per se drives early and reversible dysbiosis-mediated gut barrier impairment and bactericidal dysfunction. Mol Metab, 2022. 57: p. 101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Veldhoen M and Brucklacher-Waldert V, Dietary influences on intestinal immunity. Nat Rev Immunol, 2012. 12(10): p. 696–708. [DOI] [PubMed] [Google Scholar]
- 107.Oh DY, et al. , A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med, 2014. 20(8): p. 942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaliannan K, et al. , Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun Biol, 2019. 2: p. 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simopoulos AP, An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients, 2016. 8(3): p. 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaliannan K, et al. , A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep, 2015. 5: p. 11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee HC, et al. , A high linoleic acid diet exacerbates metabolic responses and gut microbiota dysbiosis in obese rats with diabetes mellitus. Food Funct, 2019. 10(2): p. 786–798. [DOI] [PubMed] [Google Scholar]
- 112.Li J, et al. , Dietary intake and biomarkers of linoleic acid and mortality: systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Z, et al. , Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 2011. 472(7341): p. 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janeiro MH, et al. , Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients, 2018. 10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu R, Wang Q, and Li L, A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genomics, 2015. 16 Suppl 7: p. S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang G and Zhang X, Trimethylamine N-oxide promotes hyperlipidemia acute pancreatitis via inflammatory response. Can J Physiol Pharmacol, 2021: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 117.He Z, et al. , Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab, 2021. 33(7): p. 1358–1371 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Iyer SS, et al. , Dietary and Microbial Oxazoles Induce Intestinal Inflammation by Modulating Aryl Hydrocarbon Receptor Responses. Cell, 2018. 173(5): p. 1123–1134 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chassaing B, et al. , Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 2015. 519(7541): p. 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paula Neto HA, et al. , Effects of Food Additives on Immune Cells As Contributors to Body Weight Gain and Immune-Mediated Metabolic Dysregulation. Front Immunol, 2017. 8: p. 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Canfora EE, Jocken JW, and Blaak EE, Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol, 2015. 11(10): p. 577–91. [DOI] [PubMed] [Google Scholar]
- 122.Beisner J, et al. , Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front Immunol, 2021. 12: p. 678360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu TC, et al. , Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe, 2021. 29(6): p. 988–1001 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wong JM, et al. , Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol, 2006. 40(3): p. 235–43. [DOI] [PubMed] [Google Scholar]
- 125.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, & Shulman GI, Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature, 2016. 534(7606): p. 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun M, et al. , Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol, 2017. 52(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koh A, et al. , From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell, 2016. 165(6): p. 1332–1345. [DOI] [PubMed] [Google Scholar]
- 128.Thorburn AN, Macia L, and Mackay CR, Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity, 2014. 40(6): p. 833–42. [DOI] [PubMed] [Google Scholar]
- 129.Fernandes MF, de Oliveira S, Portovedo M, Rodrigues PB, & Vinolo M, Effect of Short Chain Fatty Acids on Age-Related Disorders. Advances in experimental medicine and biology, 2020. 1260: p. 85–105. [DOI] [PubMed] [Google Scholar]
- 130.Le Chatelier E, et al. , Richness of human gut microbiome correlates with metabolic markers. Nature, 2013. 500(7464): p. 541–6. [DOI] [PubMed] [Google Scholar]
- 131.Kawano R, et al. , Erythritol Ameliorates Small Intestinal Inflammation Induced by High-Fat Diets and Improves Glucose Tolerance. Int J Mol Sci, 2021. 22(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JS, et al. , AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol, 2011. 13(2): p. 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Veldhoen M, et al. , The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature, 2008. 453(7191): p. 106–9. [DOI] [PubMed] [Google Scholar]
- 134.Lin YH, et al. , Aryl hydrocarbon receptor agonist indigo protects against obesity-related insulin resistance through modulation of intestinal and metabolic tissue immunity. Int J Obes (Lond), 2019. 43(12): p. 2407–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Natividad JM, et al. , Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab, 2018. 28(5): p. 737–749 e4. [DOI] [PubMed] [Google Scholar]
- 136.Postal BG, Ghezzal S, Aguanno D, André S, Garbin K, Genser L, Brot-Laroche E, Poitou C, Soula H, Leturque A, Clément K, & Carrière V , AhR activation defends gut barrier integrity against damage occurring in obesity. Molecular Metabolism, 2020. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Man AL, Gicheva N, and Nicoletti C, The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol, 2014. 289(1-2): p. 112–8. [DOI] [PubMed] [Google Scholar]
- 138.Tran L and Greenwood-Van Meerveld B, Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci, 2013. 68(9): p. 1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilson QN, et al. , Greater Microbial Translocation and Vulnerability to Metabolic Disease in Healthy Aged Female Monkeys. Sci Rep, 2018. 8(1): p. 11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pasternak JA, et al. , Claudin-4 undergoes age-dependent change in cellular localization on pig jejunal villous epithelial cells, independent of bacterial colonization. Mediators Inflamm, 2015. 2015: p. 263629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu A, et al. , Aging Increases the Severity of Colitis and the Related Changes to the Gut Barrier and Gut Microbiota in Humans and Mice. J Gerontol A Biol Sci Med Sci, 2020. 75(7): p. 1284–1292. [DOI] [PubMed] [Google Scholar]
- 142.Amsterdam D and Ostrov BE, The Impact of the Microbiome on Immunosenescence. Immunol Invest, 2018. 47(8): p. 801–811. [DOI] [PubMed] [Google Scholar]
- 143.Mankertz J, et al. , Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci, 2000. 113 (Pt 11): p. 2085–90. [DOI] [PubMed] [Google Scholar]
- 144.Lee B, Moon KM, and Kim CY, Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J Immunol Res, 2018. 2018: p. 2645465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suzuki T, Yoshinaga N, and Tanabe S, Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem, 2011. 286(36): p. 31263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghosh P, et al. , The stress polarity signaling (SPS) pathway serves as a marker and a target in the leaky gut barrier: implications in aging and cancer. Life Sci Alliance, 2020. 3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kuhn F, et al. , Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight, 2020. 5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ahmadi S, et al. , Metformin Reduces Aging-Related Leaky Gut and Improves Cognitive Function by Beneficially Modulating Gut Microbiome/Goblet Cell/Mucin Axis. J Gerontol A Biol Sci Med Sci, 2020. 75(7): p. e9–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wilms E, et al. , Intestinal barrier function is maintained with aging - a comprehensive study in healthy subjects and irritable bowel syndrome patients. Sci Rep, 2020. 10(1): p. 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.An R, et al. , Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut, 2018. 67(12): p. 2213–2222. [DOI] [PubMed] [Google Scholar]
- 151.Wilms E, et al. , The Impact of Pectin Supplementation on Intestinal Barrier Function in Healthy Young Adults and Healthy Elderly. Nutrients, 2019. 11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Valentini L, et al. , Small intestinal permeability in older adults. Physiol Rep, 2014. 2(4): p. e00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takiishi T, Fenero CIM, and Camara NOS, Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers, 2017. 5(4): p. e1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Remond D, et al. , Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget, 2015. 6(16): p. 13858–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johnson KO, et al. , Differences in circulating appetite-related hormone concentrations between younger and older adults: a systematic review and meta-analysis. Aging Clin Exp Res, 2020. 32(7): p. 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Norman K, Hass U, and Pirlich M, Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients, 2021. 13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Biagi E, et al. , Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One, 2010. 5(5): p. e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Round JL and Mazmanian SK, Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A, 2010. 107(27): p. 12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fransen F, et al. , Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front Immunol, 2017. 8: p. 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gao J, et al. , Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol, 2018. 8: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Adriansjach J, Baum ST, Lefkowitz EJ, Van Der Pol WJ, Buford TW, & Colman RJ , Age-Related Differences in the Gut Microbiome of Rhesus Macaques. The journals of gerontology. Series A, Biological sciences and medical sciences, 2020. 75(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Arpaia N, et al. , Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature, 2013. 504(7480): p. 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jin UH, et al. , Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci Rep, 2017. 7(1): p. 10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bodogai M, et al. , Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med, 2018. 10(467). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elderman M, et al. , The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLoS One, 2017. 12(9): p. e0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shan M, et al. , Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science, 2013. 342(6157): p. 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matt SM, et al. , Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Front Immunol, 2018. 9: p. 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Beek AA, et al. , Supplementation with Lactobacillus plantarum WCFS1 Prevents Decline of Mucus Barrier in Colon of Accelerated Aging Ercc1(-/Delta7) Mice. Front Immunol, 2016. 7: p. 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang S, et al. , Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: from C. elegans to mice. Geroscience, 2020. 42(1): p. 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim KW, et al. , Lipoteichoic Acid of Probiotic Lactobacillus plantarum Attenuates Poly I:C-Induced IL-8 Production in Porcine Intestinal Epithelial Cells. Front Microbiol, 2017. 8: p. 1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen P, et al. , Gut microbial metabolite urolithin B attenuates intestinal immunity function in vivo in aging mice and in vitro in HT29 cells by regulating oxidative stress and inflammatory signalling. Food Funct, 2021. 12(23): p. 11938–11955. [DOI] [PubMed] [Google Scholar]
- 172.van Beek AA, et al. , Frontline Science: Tryptophan restriction arrests B cell development and enhances microbial diversity in WT and prematurely aging Ercc1(-/Delta7) mice. J Leukoc Biol, 2017. 101(4): p. 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sonowal R, et al. , Indoles from commensal bacteria extend healthspan. Proc Natl Acad Sci U S A, 2017. 114(36): p. E7506–E7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Badawy AA, Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology, 2017. 112(Pt B): p. 248–263. [DOI] [PubMed] [Google Scholar]
- 175.Powell DN, Swimm A, Sonowal R, Bretin A, Gewirtz AT, Jones RM, & Kalman D, Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proceedings of the National Academy of Sciences of the United States of America, 2020. 117(35): p. 21519–21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zelante T, et al. , Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity, 2013. 39(2): p. 372–85. [DOI] [PubMed] [Google Scholar]
- 177.Chen W, et al. , Aryl hydrocarbon receptor activation modulates CD8alphaalpha(+)TCRalphabeta(+) IELs and suppression of colitis manifestations in mice. Biomed Pharmacother, 2017. 87: p. 127–134. [DOI] [PubMed] [Google Scholar]
- 178.Marsland BJ, Regulating inflammation with microbial metabolites. Nat Med, 2016. 22(6): p. 581–3. [DOI] [PubMed] [Google Scholar]
- 179.Capuron L, et al. , Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry, 2011. 70(2): p. 175–82. [DOI] [PubMed] [Google Scholar]
- 180.Kaiser H, Parker E, and Hamrick MW, Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp Gerontol, 2020. 130: p. 110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Covarrubias AJ, et al. , Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages. Nat Metab, 2020. 2(11): p. 1265–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chini CCS, et al. , CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD(+) and NMN levels. Nat Metab, 2020. 2(11): p. 1284–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yoshino J, Baur JA, and Imai SI, NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab, 2018. 27(3): p. 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Verdin E, NAD(+) in aging, metabolism, and neurodegeneration. Science, 2015. 350(6265): p. 1208–13. [DOI] [PubMed] [Google Scholar]
- 185.Covarrubias AJ, et al. , NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol, 2021. 22(2): p. 119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yoshino M, et al. , Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science, 2021. 372(6547): p. 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Igarashi M, et al. , NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell, 2019. 18(3): p. e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lynn-Jee Kim TJC, Smith Greg C., Das Abhirup, Poon Eric Wing Keung, Wang Jun, Tucker Simon P., Sinclair David A., Quek Lake-Ee, Wu Lindsay E., Nicotinamide mononucleotide (NMN) deamidation by the gut microbiome and evidence for indirect upregulation of the NAD+ metabolome. bioRxiv, 2020. [Google Scholar]
- 189.Gerner RR, et al. , NAD metabolism fuels human and mouse intestinal inflammation. Gut, 2018. 67(10): p. 1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jia W X. G , Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol , 2018. 15: p. 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, … Evans RM , Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. . Nat. Med, 2015. 21: p. 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chiang JYL and Ferrell JM, Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol, 2020. 318(3): p. G554–G573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thomas C, et al. , TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab, 2009. 10(3): p. 167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vavassori P, Mencarelli A, Renga B, Distrutti E, & Fiorucci S, The bile acid receptor FXR is a modulator of intestinal innate immunity. . J Immunol, 2009. 183(10): p. 6251–6261. [DOI] [PubMed] [Google Scholar]
- 195.Hang S, et al. , Bile acid metabolites control TH17 and Treg cell differentiation. Nature, 2019. 576(7785): p. 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Song X, et al. , Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature, 2020. 577(7790): p. 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Paik D, et al. , Human gut bacteria produce TauEta17-modulating bile acid metabolites. Nature, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li W, et al. , A bacterial bile acid metabolite modulates Treg activity through the nuclear hormone receptor NR4A1. Cell Host Microbe, 2021. 29(9): p. 1366–1377 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Goncalves de Carvalho CM and Ribeiro SM, Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr, 2017. 71(4): p. 434–440. [DOI] [PubMed] [Google Scholar]
- 200.Zeng Y, Luo M, Pan L, Chen Y, Guo S, Luo D, Zhu L, Liu Y, Pan L, Xu S, Zhang R, Zhang C, Wu P, Ge L, Noureddin M, Pandol SJ, & Han YP , Vitamin D signaling maintains intestinal innate immunity and gut microbiota: potential intervention for metabolic syndrome and NAFLD. . American journal of physiology. Gastrointestinal and liver physiology, 2020. 318(3): p. 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Del Bo' C, Bernardi S, Cherubini A, Porrini M, Gargari G, Hidalgo-Liberona N, González-Domínguez R, Zamora-Ros R, Peron G, Marino M, Gigliotti L, Winterbone MS, Kirkup B, Kroon PA, Andres-Lacueva C, Guglielmetti S, & Riso P , A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: The MaPLE randomised controlled trial. . Clinical Nutrition 2021. 40(5): p. 3006–3018. [DOI] [PubMed] [Google Scholar]
- 202.Calder PC, et al. , Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev, 2017. 40: p. 95–119. [DOI] [PubMed] [Google Scholar]
- 203.Rera M, Clark RI, and Walker DW, Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A, 2012. 109(52): p. 21528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Askarova S, et al. , The Links Between the Gut Microbiome, Aging, Modern Lifestyle and Alzheimer's Disease. Front Cell Infect Microbiol, 2020. 10: p. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sochocka M, et al. , The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer's Disease-a Critical Review. Mol Neurobiol, 2019. 56(3): p. 1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Forsyth CB, et al. , Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One, 2011. 6(12): p. e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao Y, Jaber V, and Lukiw WJ, Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer's Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front Cell Infect Microbiol, 2017. 7: p. 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maiuolo J, et al. , The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front Neurosci, 2021. 15: p. 616883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee Y and Kim YK, Understanding the Connection Between the Gut-Brain Axis and Stress/Anxiety Disorders. Curr Psychiatry Rep, 2021. 23(5): p. 22. [DOI] [PubMed] [Google Scholar]
- 210.Morais LH, Schreiber H.L.t., and Mazmanian SK, The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol, 2021. 19(4): p. 241–255. [DOI] [PubMed] [Google Scholar]
- 211.Ceppa FA, et al. , Human Gut-Microbiota Interaction in Neurodegenerative Disorders and Current Engineered Tools for Its Modeling. Front Cell Infect Microbiol, 2020. 10: p. 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roy Sarkar S and Banerjee S, Gut microbiota in neurodegenerative disorders. J Neuroimmunol, 2019. 328: p. 98–104. [DOI] [PubMed] [Google Scholar]
- 213.Santacroce L, et al. , Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front Biosci (Landmark Ed), 2021. 26(6): p. 135–148. [DOI] [PubMed] [Google Scholar]
- 214.Wu ML, et al. , Age-related cognitive decline is associated with microbiota-gut-brain axis disorders and neuroinflammation in mice. Behav Brain Res, 2021. 402: p. 113125. [DOI] [PubMed] [Google Scholar]
- 215.Brenchley JM and Douek DC, Microbial translocation across the GI tract. Annu Rev Immunol, 2012. 30: p. 149–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cunningham C, et al. , Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry, 2009. 65(4): p. 304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Peralta Ramos JM, et al. , Peripheral Inflammation Regulates CNS Immune Surveillance Through the Recruitment of Inflammatory Monocytes Upon Systemic α-Synuclein Administration. Front Immunol, 2019. 10: p. 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Duan M, et al. , Preoperative Microbiomes and Intestinal Barrier Function Can Differentiate Prodromal Alzheimer's Disease From Normal Neurocognition in Elderly Patients Scheduled to Undergo Orthopedic Surgery. Front Cell Infect Microbiol, 2021. 11: p. 592842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang Y, et al. , 27-Hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J Neuroinflammation, 2020. 17(1): p. 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ntranos A, et al. , Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.de Paula GC, et al. , Hippocampal Function Is Impaired by a Short-Term High-Fat Diet in Mice: Increased Blood-Brain Barrier Permeability and Neuroinflammation as Triggering Events. Front Neurosci, 2021. 15: p. 734158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ou Z, et al. , Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer's disease. Nutr Diabetes, 2020. 10(1): p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mitchell J, et al. , Chronic Intestinal Inflammation Suppresses Brain Activity by Inducing Neuroinflammation in Mice. Am J Pathol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang F, et al. , Altered gut microbiota in Parkinson's disease patients/healthy spouses and its association with clinical features. Parkinsonism & Related Disorders, 2020. 81: p. 84–88. [DOI] [PubMed] [Google Scholar]
- 225.Zhang L, et al. , Altered Gut Microbiota in a Mouse Model of Alzheimer's Disease. J Alzheimers Dis, 2017. 60(4): p. 1241–1257. [DOI] [PubMed] [Google Scholar]
- 226.Jangi S, et al. , Alterations of the human gut microbiome in multiple sclerosis. Nat Commun, 2016. 7: p. 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zeng Q, et al. , The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci Rep, 2020. 10(1): p. 12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Frausto DM, et al. , Dietary Regulation of Gut-Brain Axis in Alzheimer's Disease: Importance of Microbiota Metabolites. Front Neurosci, 2021. 15: p. 736814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Saksida T, et al. , Impaired IL-17 Production in Gut-Residing Immune Cells of 5xFAD Mice with Alzheimer's Disease Pathology. J Alzheimers Dis, 2018. 61(2): p. 619–630. [DOI] [PubMed] [Google Scholar]
- 230.Honarpisheh P, et al. , Dysregulated Gut Homeostasis Observed Prior to the Accumulation of the Brain Amyloid-beta in Tg2576 Mice. Int J Mol Sci, 2020. 21(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Blanco-Miguez A, et al. , Microbiota-Derived beta-Amyloid-like Peptides Trigger Alzheimer's Disease-Related Pathways in the SH-SY5Y Neural Cell Line. Nutrients, 2021. 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhuang ZQ, et al. , Gut Microbiota is Altered in Patients with Alzheimer's Disease. J Alzheimers Dis, 2018. 63(4): p. 1337–1346. [DOI] [PubMed] [Google Scholar]
- 233.Wang X, et al. , Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res, 2019. 29(10): p. 787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shi H, et al. , A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome, 2021. 9(1): p. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Haase S, et al. , Propionic Acid Rescues High-Fat Diet Enhanced Immunopathology in Autoimmunity via Effects on Th17 Responses. Front Immunol, 2021. 12: p. 701626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schnell A, et al. , Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell, 2021. 184(26): p. 6281–6298 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Poirier AA, et al. , Gastrointestinal Dysfunctions in Parkinson's Disease: Symptoms and Treatments. Parkinsons Dis, 2016. 2016: p. 6762528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sampson TR, et al. , Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell, 2016. 167(6): p. 1469–1480 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bhattarai Y, et al. , Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson's disease. Gut Microbes, 2021. 13(1): p. 1866974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schwiertz A, et al. , Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson's disease. Parkinsonism Relat Disord, 2018. 50: p. 104–107. [DOI] [PubMed] [Google Scholar]
- 241.Dumitrescu L, et al. , Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson's Disease. Front Neurosci, 2021. 15: p. 689723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Romano S, et al. , Meta-analysis of the Parkinson's disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis, 2021. 7(1): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Choi JG, et al. , Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Scientific reports, 2018. 8(1): p. 1275–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Caesar R, et al. , Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab, 2015. 22(4): p. 658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ghazarian M, et al. , Type I Interferon Responses Drive Intrahepatic T cells to Promote Metabolic Syndrome. Sci Immunol, 2017. 2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Thaiss CA, et al. , Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science, 2018. 359(6382): p. 1376–1383. [DOI] [PubMed] [Google Scholar]
- 247.Wang S, et al. , Functions of Macrophages in the Maintenance of Intestinal Homeostasis. J Immunol Res, 2019. 2019: p. 1512969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Desai MS, et al. , A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell, 2016. 167(5): p. 1339–1353 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]