Abstract
Background
Control-arm mortality varies between acute respiratory distress syndrome (ARDS) RCTs.
Methods
We systematically reviewed ARDS RCTs that commenced recruitment after publication of the American–European Consensus (AECC) definition (MEDLINE, Embase, and Cochrane central register of controlled trials; January 1994 to October 2020). We assessed concordance of RCT inclusion criteria to ARDS consensus definitions and whether exclusion criteria are strongly or poorly justified. We estimated the proportion of between-trial difference in control-arm 28-day mortality explained by the inclusion criteria and RCT design characteristics using meta-regression.
Results
A literature search identified 43 709 records. One hundred and fifty ARDS RCTs were included; 146/150 (97.3%) RCTs defined ARDS inclusion criteria using AECC/Berlin definitions. Deviations from consensus definitions, primarily aimed at improving ARDS diagnostic certainty, frequently related to duration of hypoxaemia (117/146; 80.1%). Exclusion criteria could be grouped by rationale for selection into strongly or poorly justified criteria. Common poorly justified exclusions included pregnancy related, age, and comorbidities (infectious/immunosuppression, hepatic, renal, and human immunodeficiency virus/acquired immunodeficiency syndrome). Control-arm 28-day mortality varied between ARDS RCTs (mean: 29.8% [95% confidence interval: 27.0–32.7%; I2=88.8%; τ2=0.02; P<0.01]), and differed significantly between RCTs with different Pao2:FiO2 ratio inclusion thresholds (26.6–39.9 kPa vs <26.6 kPa; P<0.01). In a meta-regression model, inclusion criteria and RCT design characteristics accounted for 30.6% of between-trial difference (P<0.01).
Conclusions
In most ARDS RCTs, consensus definitions are modified to use as inclusion criteria. Between-RCT mortality differences are mostly explained by the Pao2:FiO2 ratio threshold within the consensus definitions. An exclusion criteria framework can be applied when designing and reporting exclusion criteria in future ARDS RCTs.
Keywords: ARDS, exclusion, inclusion, mortality, randomised controlled trial
Editor's key points.
-
•
In this systematic review and meta-analysis, the authors identified modifications to acute respiratory distress syndrome definitions that are used to specify trial inclusion criteria.
-
•
Variation in mortality between RCTs is accounted for by differences in selected Pao 2:FiO2 ratio thresholds.
-
•
Exclusion criteria between trials vary greatly, but can be adjudicated based on the rationale for selection.
-
•
This framework can be used to select exclusion criteria in future RCTs and when deciding whether results from an RCT are useful in patients excluded from the RCT.
Inclusion criteria in acute respiratory distress syndrome (ARDS) RCTs are usually based either on the American–European Consensus Conference (AECC) definition1 or the Berlin definition,2 which superseded the former. It is recognised that the difference in control-arm mortality between ARDS RCTs is related to the severity of hypoxaemia3 within the ARDS consensus definitions.1 , 2 However, there has been limited assessment of how components of the inclusion criteria that are not specified within the consensus definitions1 , 2 contribute towards the observed mortality differences between ARDS RCTs. These refinements of ARDS inclusion criteria are considered important for improving certainty of ARDS diagnosis. For example, the use of standardised ventilatory settings or confirmatory time periods before definitive diagnosis of ARDS has been proposed to select patients more likely to have ‘true’ ARDS.4 , 5
Furthermore, exclusion criteria vary between ARDS RCTs, excluded patients do not explain the difference in control-arm outcomes, and justification6 of exclusion criteria used in ARDS RCTs has not been assessed to date.
In this context, we tested the hypotheses that variations in inclusion criteria not specified within the ARDS consensus definitions could contribute to the differences in control-arm mortality in ARDS RCTs, and that developing an exclusion criteria justification framework for ARDS RCTs would inform design of future RCTs. Using a systematic review and meta-analysis of ARDS RCTs, we assessed the concordance of the inclusion criteria reported in RCTs with the ARDS consensus definitions,1 , 2 and estimated the proportion of between-trial variance in control-arm 28-day mortality explained by differences in the inclusion criteria and RCT characteristics. We then used the justification framework reported by Van Spall and colleagues6 to assess the exclusion criteria reported in ARDS RCTs.
Methods
Review protocol
This systematic review and meta-analysis was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42019089703) and conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations.7 The PRISMA checklist is available in the Supplementary data. We did not receive external funding.
Information sources
We searched MEDLINE, Embase, and the Cochrane central register of controlled trials from January 1, 1994 to October 31, 2020 with no language restrictions. We used subject headings and text-word terms to search for RCTs on ARDS and adults (Cochrane, McMaster, Robinson, and Dickersin clinical trial filters). The full MEDLINE electronic search strategy is presented in Supplementary Methods S1. We manually searched reference lists from published ARDS systematic reviews. Citations were saved in EndNote (Philadelphia, PA, USA) and duplicated records removed.
RCT selection
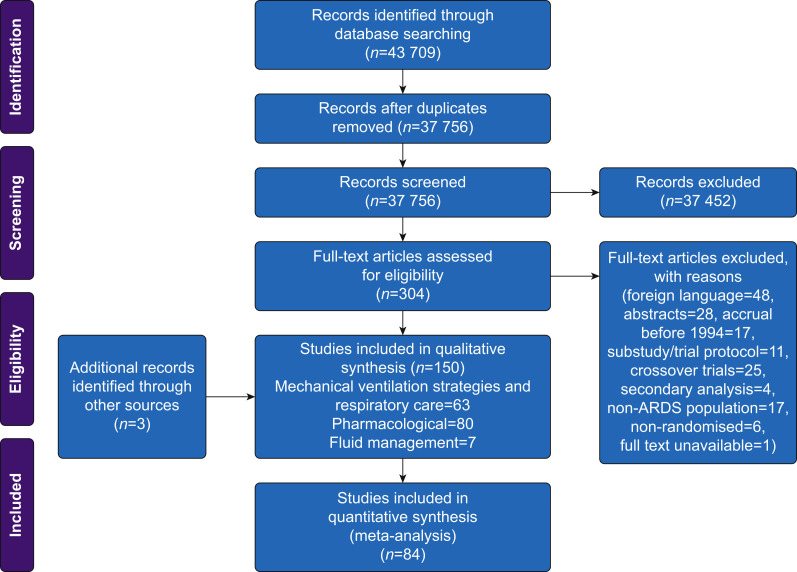
All citations were independently reviewed against our RCT selection criteria by at least two authors (RS, BA, and GM) using Rayyan QCRI.8 Potentially relevant full-text articles were reviewed and disagreements were resolved by consensus (RS, BA, and GM). We included RCTs in adult patients (>16 yr) with ARDS that commenced recruitment after publication of the AECC definition in March 1994. RCTs with a factorial design that were reported separately were considered as distinct RCTs. Crossover RCTs, where control-arm mortality could not be quantified, and articles published only in abstract form or foreign language were excluded. RCTs in patients with COVID-19 were also excluded. Further details of RCT selection criteria are available in Figure 1 .
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowsheet showing RCT selection process. ARDS, acute respiratory distress syndrome.
Data items
A preliminary data extraction form was piloted on 20 randomly selected RCTs by RS and BA. Based on feedback from this process, variables in the final data collection form were then amended for inter-observer reliability. Data were independently extracted by RS, BA, and GM from the following domains: RCT design, patient characteristics, inclusion criteria, exclusion criteria, intervention tested, and all reported mortality outcomes. RCT design characteristics included type of intervention, sponsorship and funding, number of participating centres, World Bank country income group (first author), year of publication, and number of patients in control group.
Inclusion criteria were defined as any variable used for the ARDS case definition by the RCT; these included consensus ARDS definition cited, radiographic criteria, assessment of cardiac involvement, Pao 2:FiO2 (P:F) ratio threshold, PEEP threshold, and inclusion of invasively or noninvasively ventilated patients. All time criteria that specified inclusion into the RCT based on the duration of ARDS or ventilation were also extracted (time since intubation, time since onset of ARDS, time since symptom onset, and time since admission). In RCTs, where ARDS inclusion criteria were not explicitly listed, we assumed that criteria corresponded to the ARDS definition cited in the body of the text or references. Criteria limiting RCT eligibility of patients were treated as exclusion criteria and were extracted from the RCTs.
Risk of bias
RCTs were assessed for risk of bias for control-arm mortality outcomes using the Cochrane risk-of-bias tool.9 A funnel plot of standard error against control-arm mortality was used to assess for evidence of publication bias related to control-arm mortality (Supplementary Fig. S1). The analysis was not subsequently adjusted for bias.
Synthesis of results
We extracted 28-day control-arm mortality using GraphClick10 from uncensored cumulative mortality/survival curves, if not reported. In addition, 30-day mortality, reported in two RCTs, was combined with 28-day mortality for meta-analysis.
We assessed concordance of every element of the ARDS inclusion criteria reported to the corresponding consensus definitions cited by the authors. A summary of clinical criteria used to diagnose ARDS in consensus definitions is available in the Supplementary data. The P:F ratio thresholds reported in RCTs were regrouped into two categories: 26.6–39.9 and <26.6 kPa.1 , 2
To inform an exclusion criteria framework for future ARDS RCTs, all exclusion criteria were categorised, as described previously by Van Spall and colleagues.6 Additional categories were specified for acute illness severity, and barotrauma, and an exclusion criteria category based on lower age limited was removed (in keeping with our RCT selection criteria). The category ‘related to female gender’ was recoded to ‘pregnancy related’. They were then classified into ‘strongly justified’ and ‘poorly justified’ based upon the rationale for their selection, as described by Van Spall and colleagues.6 Further details of the classification scheme are available in Supplementary Tables S3 and S4.
Meta-analysis model
To study the variability in control-arm mortality between RCTs, we used a random-effects meta-analysis model with control-arm 28-day mortality as the dependent variable and a random intercept for each RCT. Distribution of control-arm mortality was assessed for normality using a quantile–quantile plot (Supplementary Fig. S3). As control-arm mortality was not normally distributed, proportions were transformed using the Freeman–Tukey double arc-sine method. Each RCT was weighted by the inverse of the sampling variance. A maximum likelihood estimator was used to estimate mean mortality (random-effects pooled estimate), between-trial standard deviation attributable to heterogeneity (τ), and the percentage of variance attributable to heterogeneity rather than chance (I 2). Subgroup analyses were conducted for all ARDS RCT inclusion criteria (listed in Table 1 ) and specific RCT characteristics (year of publication, single vs multicentre, first author World Bank country income group, and sample size). To estimate the proportion of between-trial variance (R 2) explained by ARDS inclusion criteria, they were all included as predictors in a meta-regression model. The RCT characteristics were treated as categorical predictors (as specified in Table 1) and were added to estimate additional impact.11 Variance inflation factors were calculated for all predictors in the model to exclude multicollinearity. Model robustness was tested using a permutation test.
Table 1.
RCT design characteristics and specified inclusion criteria. ARDS, acute respiratory distress syndrome; CXR, chest radiograph; LA, left atrial; PAWP, pulmonary artery wedge pressure; P:F, Pao2:FiO2. ∗Inclusion criteria were not assessed individually for the four RCTs that used the composite Murray score to include patients.
|
A. RCT design characteristics |
Number (%) of RCTs, n=150 |
| Type of intervention | |
| Mechanical ventilation strategies and respiratory care | 63 (42.0) |
| Pharmacological RCTs | 80 (53.3) |
| Fluid management strategies | 7 (4.7) |
| Sponsorship and funding | |
| Government or institutional funding | 80 (53.3) |
| Partial/complete industry funding | 36 (24.0) |
| Unknown | 34 (22.7) |
| Number of participating centres | |
| Single | 67 (44.7) |
| Multiple | 83 (55.3) |
| National | 31 (20.6) |
| International | 52 (34.7) |
| World Bank country income group (first author) | |
| Middle | 37 (24.7) |
| High | 113 (75.3) |
| Year of publication | |
| 1996–2000 | 15 (10.0) |
| 2001–2005 | 24 (16.0) |
| 2006–2010 | 33 (22.0) |
| 2011–2015 | 38 (25.3) |
| 2016–2020 | 40 (26.7) |
| Patients in control group | |
| 0–25 | 61 (40.7) |
| 26–50 | 33 (22.0) |
| 51–100 | 16 (10.7) |
| 101–200 | 20 (13.3) |
| >200 | 20 (13.3) |
| ARDS definition | |
| American–European Consensus Conference | 118 (78.7) |
| Berlin | 28 (18.7) |
| Murray score | 4 (2.6) |
| Invasively ventilated patients only | |
| Yes | 142 (94.7) |
| No | 6 (4.0) |
| Unclear |
2 (1.3) |
|
B. Inclusion criteria∗ |
|
| P:F ratio (maximum threshold; kPa) | |
| 26.6–39.9 | 64 (43.8) |
| <39.9 | 4 (2.7) |
| <250 | 1 (0.7) |
| <225 | 63 (42.5) |
| <26.6 | 1 (0.7) |
| <26.6 | 11 (8.2) |
| <22.6 | 1 (0.7) |
| <20.0 | 1 (0.7) |
| <6.7 for >3 h or P:F <10.6 for >6 h | |
| <26.6 or 39.9 depending on PEEP | |
| Minimum P:F ratio threshold specified | 10 (6.9) |
| Minimum PEEP (cm H2O) specified | |
| 5 | 40 (24.7) |
| >5 | 10 (7.5) |
| Description of radiographic findings | |
| Bilateral infiltrates on CXR | 112 (76.7) |
| In three or four quadrants | 6 (4.1) |
| Bilateral infiltrates on CXR or CT | 27 (18.5) |
| Non-aerated lung parenchyma on CT | 1 (0.7) |
| Exclusion of cardiac involvement | |
| No clinical evidence of LA hypertension or PAWP <18 mm Hg | 121 (82.9) |
| Not explained by cardiac failure or overload | 25 (17.1) |
| Illness duration before enrolment specified | |
| Time since symptom onset | 34 (23.3) |
| Time since ARDS onset | 66 (45.2) |
| Time since intubation (maximum) | 29 (19.9) |
| Time since intubation (minimum) | 7 (4.8) |
| Time since admission | 3 (2.1) |
| Confirmatory time period specified | 20 (13.7) |
| FiO2 specified | 12 (8.2) |
Sensitivity analysis
We report three sensitivity analyses. First, to assess whether lung-protective strategy was independently associated with mortality, we included this variable in our meta-regression model. As RCTs did not always explicitly state the use of a lung-protective strategy, we assumed that RCTs commencing recruitment after the publication of the ARDS Network lower vs higher tidal volume study used a lung-protective strategy and vice versa. Second, we repeated the meta-regression with alternative mortality time points (i.e. ICU, hospital, and 60-day mortality) as an independent variable. Third, we excluded RCTs that did not specifically use a P:F ratio inclusion threshold of 26.6 or 39.9 kPa.
For all analyses, a P-value of <0.05 was considered significant. Analyses were performed in R version 3.4.212 (R Foundation for Statistical Computing, Vienna, Austria) using the tidyr,13 dplyr,14 meta,15 and forestplot 16 packages.
Results
RCT selection
The bibliographic database search identified 43 709 records, as of October 31, 2020. After excluding duplicates, amongst the 37 756 records screened, 304 records were eligible for full-text evaluation. After full-text evaluation, we excluded 157 records and included 147 RCTs from the database search and three further RCTs from hand searching published review articles, resulting in 150 unique ARDS RCTs published between 1994 and 2020 that met our selection criteria (Fig. 1). A summary of RCT characteristics is reported in Table 1.
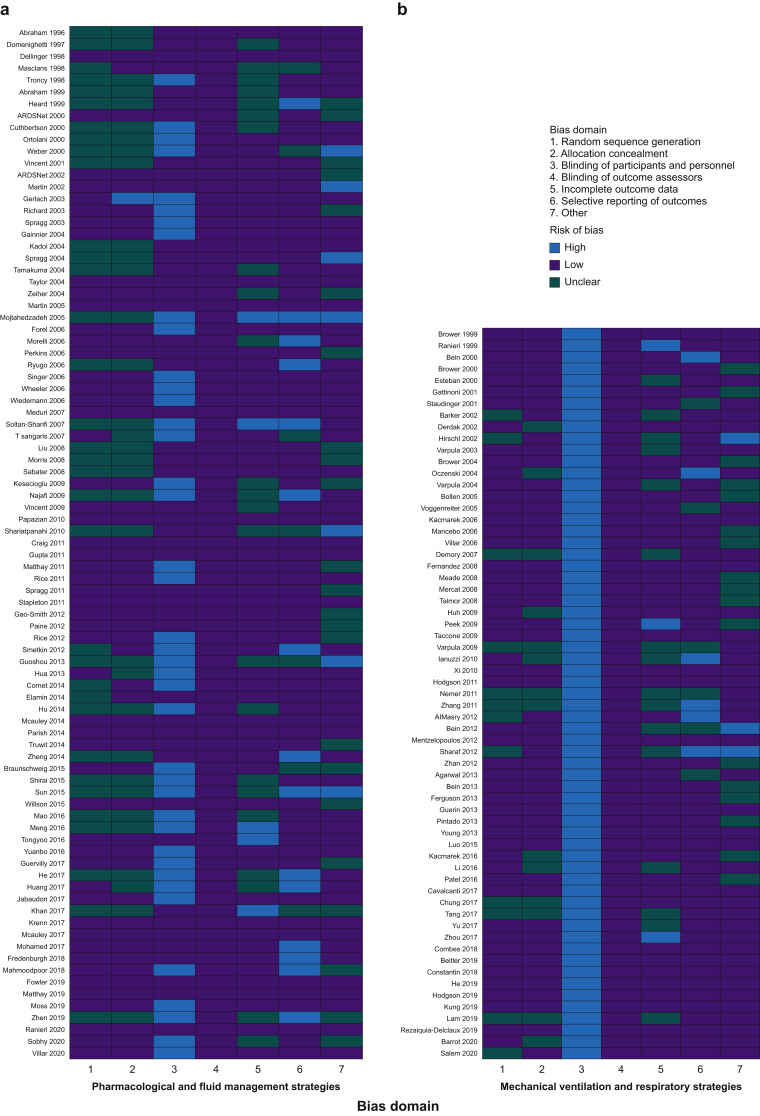
Risk of bias within RCTs
One hundred and four (69.3%) of 150 RCTs, including all RCTs of mechanical ventilation and respiratory care, were at high risk of bias attributable to inadequate blinding of participants or personnel. Although we included only RCTs in our study, 58 (38.7%) of 150 RCTs either did not or inadequately described the methods for allocation concealment and random sequence generation. Risk of bias was unclear in these RCTs. Lack of blinding of outcome assessors was not deemed to introduce bias, as mortality is an objective outcome. Twenty-one (14.0%) RCTs that did not report mortality outcomes were adjudicated to have a high risk of reporting bias, whereas 13 (8.7%) RCTs that did not specify the mortality time point were felt to be at unclear risk (Fig. 2 ).
Fig 2.
Risk of bias for mortality outcomes in ARDS RCTs. Domains were specified and adjudicated as per the Cochrane risk-of-bias tool. RCTs were divided by intervention type and ordered by year of publication. ARDS, acute respiratory distress syndrome.
Assessment of concordance of inclusion criteria to ARDS consensus definitions
The AECC definition was used in 117 of 150 (78.0%),17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133 the Berlin definition in 29 of 150 (19.3%),134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162 and the composite Murray score163 in four RCTs164, 165, 166, 167 as ARDS inclusion criteria, resulting in 146 RCTs, where components of inclusion criteria were assessed further. Only three of 32131, 132, 133 RCTs that commenced recruitment of patients after publication of the Berlin definition did not use it to specify RCT inclusion criteria. The most commonly reported P:F ratio inclusion thresholds, below which patients were included in the RCT, were ≤26.6 kPa (63/146; 43.2%) and ≤39.9 kPa (64/146; 43.8%) (Table 1). The P:F ratio maximum threshold was concordant with the Berlin definition in 26/29 (89.7%) RCTs134, 135, 136 , 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149 , 151 , 152 , 154, 155, 156, 157, 158, 159, 160, 161 compared with 101/117 (86.3%) RCTs18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 , 36 , 38, 39, 40, 41, 42, 43, 44 , 46 , 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 , 76 , 78, 79, 80, 81, 82, 83, 84, 85, 86 , 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109 , 111 , 112 , 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130 , 133 , 137 that used the AECC definition. Bilateral chest infiltrates on the chest radiograph (CXR) were used in 118/146 (80.8%) RCTs.17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112 , 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133 , 137 , 138 The CXR or CT findings (as per the Berlin definition) were used in 27/146 (18.5%) of RCTs.134, 135, 136 , 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162 Exclusion of cardiac involvement conformed to the AECC definition (absence of clinical evidence of left atrial hypertension or pulmonary arterial wedge pressure >18 mm Hg) in 121/146 (82.9%) RCTs,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133 , 137, 138, 139 , 143 and was consistent with the Berlin definition (not explained by cardiac failure or overload) in the remaining 25/146 (17.1%) RCTs.134, 135, 136 , 140, 141, 142 , 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162 In accordance with the Berlin definition, a minimum PEEP of 5 cm H2O was specified in all 29 RCTs using this definition. The AECC definition does not specify a minimum PEEP value, and 96/117 (82.1%) RCTs17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 , 39, 40, 41, 42, 43 , 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 , 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 , 78, 79, 80, 81, 82, 83, 84, 85, 86 , 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102 , 106 , 107 , 109 , 111, 112, 113, 114, 115, 116, 117, 118, 119 , 121, 122, 123, 124 , 127, 128, 129, 130 , 132 , 133 that used this definition did not specify a minimum PEEP value. Time since symptom onset was specified in 34/146 (23.3%) RCTs,22 , 27 , 42 , 75 , 80 , 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162 including all 29 RCTs, where the Berlin definition was used (median: 7 days; range: 3–28 days).
Assessment of deviation of inclusion criteria from ARDS consensus definitions
Deviations from the consensus criteria for P:F ratio were P:F <20.0 kPa (11/146; 7.5%),35 , 37 , 45 , 47, 48, 49 , 77 , 87 , 113 , 150 , 153 P:F <33.3 kPa (4/146; 2.7%),75 , 110 , 132 , 162 P:F <26.6 kPa with PEEP >5 cm H2O or P:F <39.9 with PEEP >10 cm H2O (1/146; 0.7%),46 P:F <29.9 kPa (1/146; 0.7%),18 P:F <22.6 kPa (1/146; 0.7%),105 and P:F <6.7 kPa for >3 h or P:F <10.6 kPa for >6 h (1/146; 0.7%).131
Ten of 146 (6.8%) RCTs specified an additional minimum P:F ratio threshold below which patients were excluded.25 , 53 , 57 , 88 , 104 , 106 , 128 , 139 , 145 , 157 This minimum threshold varied from 8.0 to 26.6 kPa. Twelve (8.2%) RCTs specified standardised FiO2 settings that ranged between 0.5 and 1.0.36 , 42 , 47 , 48 , 53 , 61 , 62 , 110 , 125 , 131 , 139 , 158
Deviation from radiological criteria was noted in six (4.1%) RCTs17 , 18 , 52 , 53 , 67 , 81 that stipulated the presence of infiltrates in more than three or four CXR quadrants, and one (0.7%) RCT113 that used CT criteria only. Four (2.7%) RCTs137, 138, 139 , 143 that used the Berlin definition instead used criteria from the AECC definition to exclude cardiac involvement. Amongst RCTs using the AECC definition, 22/117 (18.8%)35, 36, 37 , 45, 46, 47, 48, 49 , 61 , 77 , 87 , 103 , 104 , 110 , 120 , 125 , 126 , 131 specified a minimum value for PEEP. In RCTs using the Berlin definition, 3/29 (10.3%)147 , 150 , 158 specified PEEP >5 cm H2O. One hundred and seventeen of 146 RCTs (80.1%) specified inclusion criteria related to illness duration before enrolment. Time since ARDS onset was specified in 66/146 (45.2%; median: 2 days; range: 1–7) RCTs.17, 18, 19, 20, 21 , 28, 29, 30, 31, 32, 33, 34 , 36 , 42, 43, 44, 45 , 48 , 49 , 52 , 53 , 61 , 62 , 64, 65, 66, 67 , 71, 72, 73, 74, 75, 76, 77, 78 , 81 , 84 , 85 , 87 , 89 , 92 , 93 , 96 , 102, 103, 104 , 106 , 107 , 110 , 112 , 118 , 119 , 122 , 123 , 130 , 135, 136, 137, 138 , 141 , 142 , 147 , 148 , 150 , 153 , 161 Maximum time since intubation was specified in 29/146 (19.9%; median: 3 days; range: 1–10) RCTs.24 , 35 , 44 , 47 , 53 , 54 , 60 , 61 , 63 , 64 , 90 , 92 , 93 , 105 , 113, 114, 115, 116 , 124 , 126 , 127 , 131 , 132 , 134 , 136 , 141 , 145 , 150 , 152 , 162 Minimum time since intubation was specified in seven (4.8%; median: 1 day; range: 0.33–1) RCTs.35 , 69 , 70 , 91 , 105 , 113 , 140 Time since admission was specified in three (2.1%; median: 2 days; range: 2–2) RCTs.51 , 56 , 59
Twenty (13.7%) RCTs24 , 25 , 31 , 37 , 42 , 48 , 49 , 90 , 94 , 98 , 105 , 114, 115, 116, 117 , 120 , 131 , 132 , 153 , 158 stipulated a confirmatory time period, between 30 min and 24 h, during which ARDS needed to persist for enrolment into the trial. Eight of these RCTs reported a confirmatory time period between 12 and 24 h. Although 94.7% (142/150) of RCTs were conducted in invasively ventilated patients, six (4.0%) included patients receiving noninvasive ventilation33 , 57 , 128 , 139 , 151 and two (1.3%) did not clearly state how patients were being ventilated.79 , 133
Assessment of between-trial variance (I2) in control-arm 28-day mortality and impact of differences in inclusion criteria
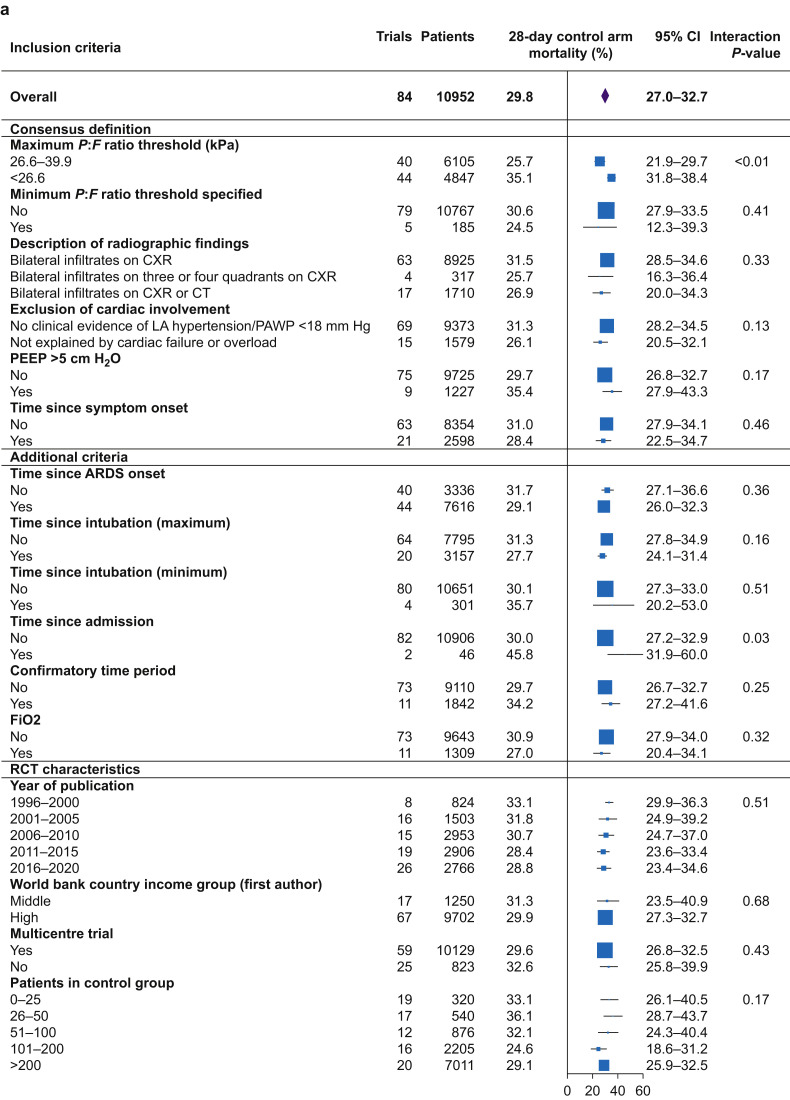
Mortality outcomes were reported in 125 of 146 (85.6%) RCTs (Supplementary Fig. S2). The 28-day mortality was reported in 73 (50.0%) RCTs, 30-day mortality in two, and extrapolated from a further nine RCTs using GraphClick, resulting in 84 RCTs with primary outcome for meta-analysis. There was significant variance in control-arm mortality at 28 days between RCTs, with a random effects estimated mean mortality of 29.8% (95% confidence interval [CI]: 27.0–32.7%; range: 3.6–69.7%; I 2=88.8% (84.4–92.3%); τ2=0.015 (0.011–0.023); P<0.01) (Fig. 3 a; Supplementary Fig. S4).
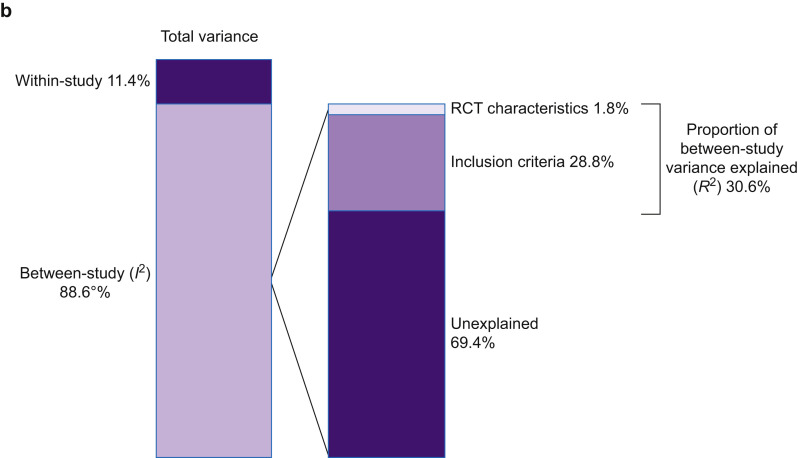
Fig 3.
(a) Univariate, weighted analysis of the impact of inclusion criteria and RCT characteristics on 28-day mortality. (b) Results of a meta-regression model of the impact of inclusion criteria and RCT characteristics on 28-day mortality. Total variance is made up of between- and within-study variance. Within-study variance cannot be explained using study-level factors. I2 is the proportion of variance in ARDS RCTs that is attributable to between-trial variance. The proportion of between-trial variance that can be explained (R2) is 30.6%; inclusion criteria (28.8%), and RCT design characteristics (1.8%); 69.4% of between-trial variance remained unexplained. ARDS, acute respiratory distress syndrome; CI, confidence interval; CXR, chest radiograph; P:F, Pao2:FiO2; LA, left atrial; PAWP, pulmonary artery wedge pressure.
The 28-day mortality in RCTs that used a P:F ratio inclusion threshold of ≤26.6 kPa (35.1%; 95% CI: 31.8–38.4%) was higher compared with RCTs using a P:F ratio inclusion threshold between 26.6 and 39.9 kPa (25.7%; 95% CI: 21.9–29.7%). Mean mortality did not significantly differ between RCTs with other additional inclusion criteria differences: specification of minimum P:F ratio threshold below which patients were excluded, definition of imaging findings, definition for exclusion of cardiac involvement, time windows for inclusion (symptom onset, ARDS onset, intubation, admission, and confirmatory time period), and specification of PEEP >5 cm H2O or FiO2 (Fig. 3a).
Within-trial variance that cannot be explained by study-level variables accounted for 11.4% (95% CI: 7.7–16.0%) of total variance. Therefore, 88.6% (95% CI: 84.0–92.3%) of total variance was a result of between-trial variance (I 2). A meta-regression model, including all inclusion criteria variables and RCT design characteristics, accounted for a total of 30.6% (95% CI: 17.7–43.5%) of the between-trial variance (R 2).
The P:F ratio inclusion threshold was the only variable in the model significantly associated with control-arm 28-day mortality (P<0.01). Therefore, 69.4% (95% CI: 56.5–82.3%) of the between-trial variance remained unexplained by inclusion criteria differences (Fig. 3b; Supplementary Tables S5 and S6).
Assessment of exclusion criteria and justification framework
Of 150 RCTs, 141 (94.0%) reported exclusion criteria. Of the nine RCTs that did not report exclusion criteria, five were published after the Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines.168 Definitions of exclusion criteria varied greatly between RCTs (Supplementary Table S4). For example, hepatic disease was variably defined within RCTs based on specific factors, including bilirubin level, transaminase concentration, Child–Pugh grade, and evidence of cirrhosis, or non-specifically as chronic liver disease or acute liver failure (Supplementary Table S4).
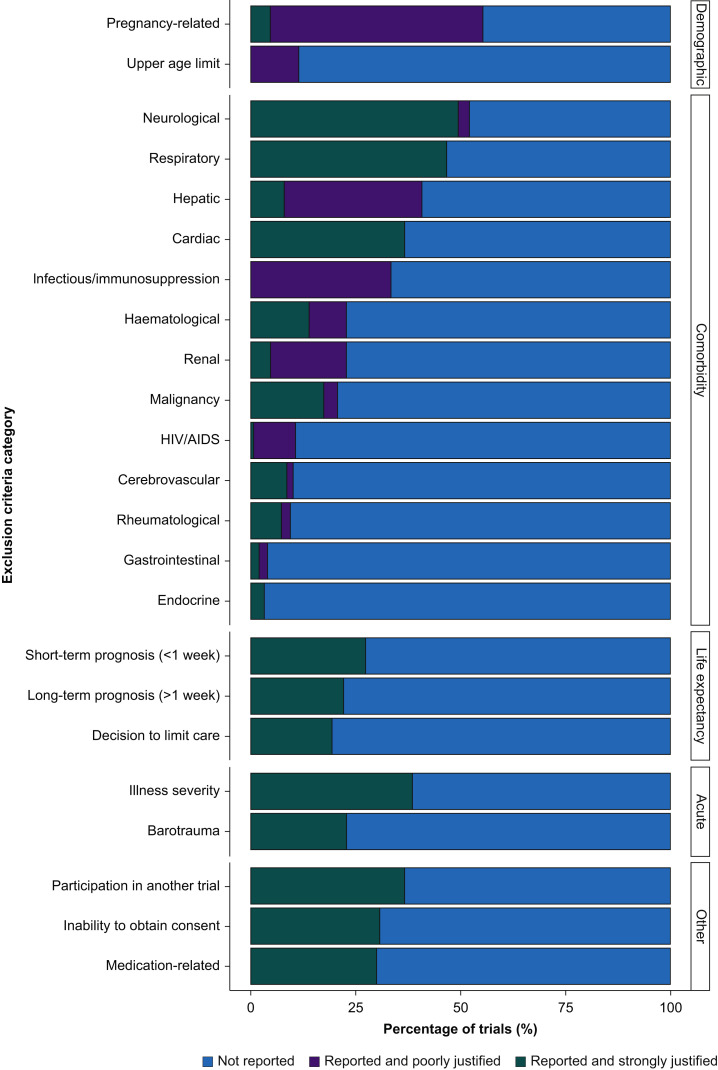
The exclusion criteria reported in ARDS RCTs were then categorised based on the justification framework proposed by Van Spall and colleagues6 (Fig. 4 ). To do this evaluation, we had to modify this framework in four ways: (i) by including two additional but important ARDS specific categories: acute illness severity and barotrauma; (ii) changing the domain ‘related to female gender’ to ‘pregnancy related’ to avoid conflating the two issues; (iii) by not including a lower age limit category, as we were assessing adult ARDS RCTs; and (iv) by not including the ‘potentially justified’ category. As defined by Van Spall and colleagues,6 potentially justified criteria relate to potential patient non-adherence to intervention or follow-up that cannot be classified as poorly/strongly justified. We did not identify any potentially justified criteria in ARDS RCTs, and therefore opted to simplify the justification framework.
Fig 4.
Categories of exclusion criteria reported in ARDS RCTs. ARDS, acute respiratory distress syndrome; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome.
The exclusion criteria in RCTs with strong justification were comorbidities neurological (73/78; 93.6%), respiratory (70/70; 100%), cardiac (55/55; 100%), life expectancy (long-term prognosis [33/33; 100%]; short-term prognosis [41/41; 100%]), acute illness related (acute illness severity [58/58; 100.0%]; barotrauma [34/34; 100%]), participation in another trial (55/55; 100%), inability to obtain consent (46/46; 100%), and medication related (45/45; 100%) (Fig. 4; Supplementary Table S3). The exclusion criteria in RCTs with poor justification were pregnancy related (76/83 [80.5%]; upper age limit [17/17; 100%]) and the following comorbidities: infectious/immunosuppression (50/50; 100%), hepatic (49/61; 80.3%), renal (27/34; 79.4%), and human immunodeficiency virus/acquired immunodeficiency syndrome (15/16; 93.8%) (Fig. 4; Supplementary Table S4).
Sensitivity analysis
Between-trial and overall relationships between inclusion criteria and mortality remained consistent and unchanged after the inclusion of lung-protective strategy as a variable in the meta-regression model. Similarly, meta-regression with mortality time points (28-day/60-day/ICU/hospital mortality) as an independent categorical variable, or exclusion of RCTs that used a P:F ratio inclusion threshold other than 26.6 or 39.9 did not alter findings. Univariate and multivariate models for these analyses are reported in Supplementary Figures S5–S7.
Discussion
Although 97% of ARDS RCTs use consensus definitions for inclusion criteria, we observed deviations from the consensus definitions that were primarily aimed to increase the certainty of ARDS diagnosis, such as duration of hypoxaemia after ventilation, in more than 80% of ARDS RCTs. Importantly, the transition from the AECC definition to the Berlin definition has improved overall concordance between individual elements of the RCT inclusion criteria and the consensus definition. As predicted, there was significant variance in control-arm mortality. However, these additional refinements of ARDS inclusion criteria did not explain the observed variance, over and above the effect of severity of hypoxaemia on control-arm mortality. We illustrate that the justification framework reported by Van Spall and colleagues6 is a feasible tool to assess the exclusion criteria reported in ARDS RCTs, the tool requires modification, and that many exclusion criteria in ARDS RCTs were poorly justified. We provide a novel assessment of the concordance of inclusion criteria in ARDS RCTs to the ARDS consensus definitions and its impact on control-arm mortality.
For assessing the impact of inclusion criteria differences, we explored how consensus definitions were modified as ARDS RCT inclusion criteria and assessed individual contributions to outcome. Our finding that there is significant heterogeneity in control-arm mortality is consistent with a previous meta-analysis that included RCTs between 1984 and 2006.3 Importantly, deviations from the consensus criteria, such as use of standardised ventilator settings for PEEP and FiO2 when defining ARDS,5 , 169 , 170 delayed reassessment of patients to confirm ARDS diagnosis (confirmatory period),171 , 172 and classification based on time of onset into early or late ARDS173 was thought to improve ARDS diagnosis or enrich for adverse outcome. These had negligible impact on 28-day mortality over and above the P:F ratio strata. Our analyses highlight that severity of hypoxaemia is the only inclusion criterion that reliably stratifies ARDS patients by risk of death, which is consistent with the predictive validity analyses reported in the Berlin ARDS definition.
We also highlight that the justification framework proposed by Van Spall and colleagues6 requires modification for use in ARDS RCTs. Based on our analysis, we propose two classes of exclusion criteria, namely, strongly justified and poorly justified, and that exclusion criteria could be linked to the intervention tested. This proposal has validity, as exclusion criteria that we categorised as poorly justified in ARDS RCTs have also been highlighted in non-critical care RCTs.6 , 174 , 175 For example, instead of blanket exclusion of women of reproductive age attributable to pregnancy-related risk, the framework we propose would consider whether excluding pregnant patients was strongly or poorly justified for the specific intervention that is being trialled. If the intervention does not have teratogenic properties (e.g. mechanical ventilation), then pregnancy need not be an exclusion. Extending this argument, we could also consider whether results from a trial are useful when managing patients with an exclusion criterion in that trial. This issue is seldom considered during clinical practise.
Aside from eligibility criteria, heterogeneity in mortality outcomes between RCTs can be attributed to setting and design characteristics, such as geographic and socio-economic population differences,176, 177, 178 unreported exclusion of patients,179 and differences in post-randomisation care between RCTs.180
Strengths and limitations
We report the first assessment of ARDS RCT eligibility criteria as a reason for between-trial differences in control-arm mortality. We pre-registered with PROSPERO and reported in accordance with PRISMA recommendations. Our exclusion criteria framework could be used for RCT design and prospective reporting. This would inform whether the results of a specific ARDS RCT would apply to patients with one or more of the RCT exclusion criteria, but without a known risk of harm from the intervention.
We applied the Cochrane risk-of-bias tool to explore bias between RCTs, but did not alter our analysis to account for this. This was because the primary outcome, control-arm mortality, is unlikely to be influenced by risk of bias. As we do not know which exclusion criteria were specifically met by patients excluded from RCTs, we were unable to assess their impact on mortality. We used year of publication rather than year of enrolment as the time variable in our analysis because the latter was not always reported. In a proportion of RCTs, where individual components of the inclusion criteria were not specified, inclusion criteria were assumed to correspond to the stated definition. We specifically focused on 28-day mortality, which was not available in 66 RCTs, and grouped RCTs by P:F ratio inclusion threshold (26.6–39.9 and <26.6) to assess concordance with consensus definitions. Both issues are addressed within our sensitivity analyses, which were consistent with our main analysis.
We need to ascertain whether our modified exclusion criteria framework is implementable in future ARDS RCTs. Furthermore, it will be important to consider how to record multiple exclusion criteria in a single patient, within the CONSORT framework, which can be explored using a clinical database. Various definitions of exclusion criteria can be simulated to examine their impact upon an RCT population.
Conclusion
In most ARDS RCTs, the consensus definitions are modified to use as inclusion criteria. Most of the between-RCT differences in mortality are accounted for by the P:F ratio threshold within the consensus definitions. Exclusion criteria definitions were different between RCTs. We provide a simplified exclusion criteria framework to be applied when designing and reporting exclusion criteria in future ARDS RCTs.
Authors' contributions
Study conception/design: RS, MS-H
Development of search strategy: RS, MS-H
Literature review: RS, BA, GM
Data extraction: RS, BA, GM
Data analysis: RS
Data interpretation: all authors
Drafting of paper: RS, MS-H
Critical revision and approval of paper: all authors
All authors confirm the accuracy and integrity of the work. RS takes responsibility for the integrity of the work as a whole, from inception to published paper.
Acknowledgements
The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care.
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.02.027.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Wellcome Trust to CS; Medical Research Council to CS; National Institute of Health Research Cambridge Biomedical Research Centre to CS; National Institutes of Health to CS; British Heart Foundation to CS; GlaxoSmithKline to CS; AstraZeneca to CS; Bristol Myers Squibb to CS; National Institute of Health Research Clinician Scientist Award (NIHR-CS-2016-16-011) to MS-H.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bernard G.R., Artigas A., Brigham K.L., et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Force A.D.T., Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Phua J., Badia J.R., Adhikari N.K.J., et al. Has mortality from acute respiratory distress syndrome decreased over time? Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 4.Villar J., Perez-Mendez L., Belda J., et al. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;176:795–804. doi: 10.1164/rccm.200610-1534OC. [DOI] [PubMed] [Google Scholar]
- 5.Villar J., Perez-Mendez L., Blanco J., et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting—a prospective, multicenter validation study. Intensive Care Med. 2013;39:583–592. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 6.Van Spall H.G., Toren A., Kiss A., Fowler R.A. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 8.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J.P., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flower A., McKenna J.W., Upreti G. Validity and reliability of GraphClick and DataThief III for data extraction. Behav Modif. 2016;40:396–413. doi: 10.1177/0145445515616105. [DOI] [PubMed] [Google Scholar]
- 11.Borenstein M., Hedges L., Higgins J., Rothstein H. Wiley; Chichester: 2009. Introduction to meta-analysis. [Google Scholar]
- 12.R Foundation for Statistical Computing . 2019. R Development Core Team. R: a language and environment for statistical computing.http://www.R-project.org/ [Google Scholar]
- 13.Wickham H, Henry L. tidyr: tidy messy data. R package version 100. https://gforge.se/packages/. Last accessed: 23 Dec 2020.
- 14.Wickham H., François R., Henry L., Müller K. 2019. dplyr: a grammar of data manipulation. R package version 083.https://github.com/tidyverse/dplyr [Google Scholar]
- 15.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. https://github.com/guido-s/meta/ [Google Scholar]
- 16.Gordon M., Lumley T. 2019. forestplot: advanced forest plot using ‘grid’ graphics. R package version 1.9.https://gforge.se/packages/ [Google Scholar]
- 17.Abraham E., Baughman R., Fletcher E., et al. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med. 1999;27:1478–1485. doi: 10.1097/00003246-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Abraham E., Park Y.C., Covington P., Conrad S.A., Schwartz M. Liposomal prostaglandin E 1 in acute respiratory distress syndrome: a placebo-controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med. 1996;24:10–15. doi: 10.1097/00003246-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 19.The ARDS Network Authors for the ARDS Network Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 20.The ARDS Clinical Trials Network National Heart, Lung, and Blood Institute, National Institutes of Health. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R., Srinivasan A., Aggarwal A.N., Gupta D. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology. 2013;18:1108–1115. doi: 10.1111/resp.12126. [DOI] [PubMed] [Google Scholar]
- 23.Al Masry A., Boules M., Boules N., Ebied R. Optimal method for selecting PEEP level in ALI/ARDS patients under mechanical ventilation. J Egypt Soc Parasitol. 2012;42:359–372. doi: 10.12816/0006323. [DOI] [PubMed] [Google Scholar]
- 24.Bein T., Weber-Carstens S., Goldmann A., et al. Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bein T., Zimmermann M., Schiewe-Langgartner F., et al. Continuous lateral rotational therapy and systemic inflammatory response in posttraumatic acute lung injury: results from a prospective randomised study. Injury. 2012;43:1892–1897. doi: 10.1016/j.injury.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Bein T.H., Reber A., Ploner F., Taeger K., Jauch K.W. Continuous axial rotation and pulmonary fluid balance in acute lung injury. Clin Intensive Care. 2000;11:307–310. [Google Scholar]
- 27.Bollen C.W., van Well G.T., Sherry T., et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669] Crit Care. 2005;9:R430–R439. doi: 10.1186/cc3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braunschweig C.A., Sheean P.M., Peterson S.J., et al. Intensive nutrition in acute lung injury: a clinical trial (INTACT) JPEN J Parenter Enteral Nutr. 2015;39:13–20. doi: 10.1177/0148607114528541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brower R.G., Lanken P.N., MacIntyre N., et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 30.Brower R.G., Shanholtz C.B., Fessler H.E., et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27:1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Cavalcanti A.B., Suzumura E.A., Laranjeira L.N., et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung F.T., Lee C.S., Lin S.M., et al. Alveolar recruitment maneuver attenuates extravascular lung water in acute respiratory distress syndrome. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornet A.D., Groeneveld A.B., Hofstra J.J., et al. Recombinant human activated protein C in the treatment of acute respiratory distress syndrome: a randomized clinical trial. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig T.R., Duffy M.J., Shyamsundar M., et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (the HARP study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 35.Cuthbertson B.H., Galley H.F., Webster N.R. Effect of inhaled nitric oxide on key mediators of the inflammatory response in patients with acute lung injury. Crit Care Med. 2000;28:1736–1741. doi: 10.1097/00003246-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Dellinger R.P., Zimmerman J.L., Taylor R.W., et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Demory D., Michelet P., Arnal J.M., et al. High-frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenation. Crit Care Med. 2007;35:106–111. doi: 10.1097/01.CCM.0000251128.60336.FE. [DOI] [PubMed] [Google Scholar]
- 38.Derdak S., Mehta S., Stewart T.E., et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 39.Domenighetti G., Suter P.M., Schaller M.-D., Ritz R., Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care. 1997;12:177–182. doi: 10.1016/s0883-9441(97)90029-0. [DOI] [PubMed] [Google Scholar]
- 40.Elamin E.M., Miller A.C., Ziad S. Immune enteral nutrition can improve outcomes in medical-surgical patients with ARDS: a prospective randomized controlled trial. J Nutr Disord Ther. 2012;2:109. doi: 10.4172/2161-0509.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esteban A., Alia I., Gordo F., et al. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest. 2000;117:1690–1696. doi: 10.1378/chest.117.6.1690. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson N.D., Cook D.J., Guyatt G.H., et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez R., Trenchs X., Klamburg J., et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med. 2008;34:1487–1491. doi: 10.1007/s00134-008-1119-3. [DOI] [PubMed] [Google Scholar]
- 44.Forel J.M., Roch A., Marin V., et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 45.Gainnier M., Roch A., Forel J.M., et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113–119. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 46.Gattinoni L., Tognoni G., Pesenti A., et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 47.Gerlach H., Keh D., Semmerow A., et al. Dose–response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med. 2003;167:1008–1015. doi: 10.1164/rccm.2108121. [DOI] [PubMed] [Google Scholar]
- 48.Guerin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 49.Guervilly C., Forel J.M., Hraiech S., Roch A., Talmor D., Papazian L. Effect of high-frequency oscillatory ventilation on esophageal and transpulmonary pressures in moderate-to-severe acute respiratory distress syndrome. Ann Intensive Care. 2016;6:84. doi: 10.1186/s13613-016-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guoshou Z., Chengye Z., Zhihui L., Jinlong L. Effects of high dose of anisodamine on the respiratory function of patients with traumatic acute lung injury. Cell Biochem Biophys. 2013;66:365–369. doi: 10.1007/s12013-012-9475-6. [DOI] [PubMed] [Google Scholar]
- 51.Gupta A., Govil D., Bhatnagar S., et al. Efficacy and safety of parenteral omega 3 fatty acids in ventilated patients with acute lung injury. Indian J Crit Care Med. 2011;15:108–113. doi: 10.4103/0972-5229.83019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heard S.O., Longtine K., Toth I., Puyana J.C., Potenza B., Smyrnios N. The influence of liposome-encapsulated prostaglandin E1 on hydrogen peroxide concentrations in the exhaled breath of patients with the acute respiratory distress syndrome. Anesth Analg. 1999;89:353–357. doi: 10.1097/00000539-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 53.Hirschl R.B., Croce M., Gore D., et al. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:781–787. doi: 10.1164/ajrccm.165.6.2003052. [DOI] [PubMed] [Google Scholar]
- 54.Hodgson C.L., Tuxen D.V., Davies A.R., et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15:R133. doi: 10.1186/cc10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu W., Lin C.W., Liu B.W., Hu W.H., Zhu Y. Extravascular lung water and pulmonary arterial wedge pressure for fluid management in patients with acute respiratory distress syndrome. Multidiscip Respir Med. 2014;9:3. doi: 10.1186/2049-6958-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hua F., Wang X., Zhu L. Terlipressin decreases vascular endothelial growth factor expression and improves oxygenation in patients with acute respiratory distress syndrome and shock. J Emerg Med. 2013;44:434–439. doi: 10.1016/j.jemermed.2012.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang S.R., Ma A.Y., Liu Y., Qu Y. Effects of inflammatory factors including plasma tumor necrosis factor-alpha in the clinical treatment of acute respiratory distress syndrome. Oncol Lett. 2017;13:5016–5020. doi: 10.3892/ol.2017.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huh J.W., Jung H., Choi H.S., Hong S.B., Lim C.M., Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13:R22. doi: 10.1186/cc7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iannuzzi M., De Sio A., De Robertis E., Piazza O., Servillo G., Tufano R. Different patterns of lung recruitment maneuvers in primary acute respiratory distress syndrome: effects on oxygenation and central hemodynamics. Minerva Anestesiol. 2010;76:692–698. [PubMed] [Google Scholar]
- 60.Kacmarek R.M., Villar J., Sulemanji D., et al. Open lung approach for the acute respiratory distress syndrome. Crit Care Med. 2016;44:32–42. doi: 10.1097/CCM.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 61.Kacmarek R.M., Wiedemann H.P., Lavin P.T., Wedel M.K., Tutuncu A.S., Slutsky A.S. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:882–889. doi: 10.1164/rccm.200508-1196OC. [DOI] [PubMed] [Google Scholar]
- 62.Kadoi Y., Hinohara H., Kunimoto F., et al. Pilot study of the effects of ONO-5046 in patients with acute respiratory distress syndrome. Anesth Analg. 2004;99:872–877. doi: 10.1213/01.ANE.0000129996.22368.85. [DOI] [PubMed] [Google Scholar]
- 63.Kesecioglu J., Beale R., Stewart T.E., et al. Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180:989–994. doi: 10.1164/rccm.200812-1955OC. [DOI] [PubMed] [Google Scholar]
- 64.Khan A., Benthin C., Zeno B., et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krenn K., Lucas R., Croize A., et al. Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: a phase IIa randomized placebo-controlled trial. Crit Care. 2017;21:194. doi: 10.1186/s13054-017-1795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu K.D., Levitt J., Zhuo H., et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mancebo J., Fernández R., Blanch L., et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 68.Mao Z., Wang H. Effects of Xuanbai Chengqi decoction on lung compliance for patients with exogenous pulmonary acute respiratory distress syndrome. Drug Des Devel Ther. 2016;10:793–798. doi: 10.2147/DDDT.S93165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin G.S., Mangialardi R.J., Wheeler A.P., Dupont W.D., Morris J.A., Bernard G.R. Albumin and furosemide therapy in hypoproteinemic patients with acute lung injury. Crit Care Med. 2002;30:2175–2182. doi: 10.1097/00003246-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Martin G.S., Moss M., Wheeler A.P., Mealer M., Morris J.A., Bernard G.R. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33:1681–1687. doi: 10.1097/01.ccm.0000171539.47006.02. [DOI] [PubMed] [Google Scholar]
- 71.Masclans J., Iglesia R., Bermejo B., Picó M., Rodriguez-Roisin R., Planas M. Gas exchange and pulmonary haemodynamic responses to fat emulsions in acute respiratory distress syndrome. Intensive Care Med. 1998;24:918–923. doi: 10.1007/s001340050690. [DOI] [PubMed] [Google Scholar]
- 72.Matthay M.A., Brower R.G., Carson S., et al. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McAuley D.F., Laffey J.G., O’Kane C.M., et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 74.McAuley D.F., Cross L.M., Hamid U., et al. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir Med. 2017;5:484–491. doi: 10.1016/S2213-2600(17)30171-6. [DOI] [PubMed] [Google Scholar]
- 75.Meade M.O., Cook D.J., Guyatt G.H., et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 76.Meduri G.U., Golden E., Freire A.X., et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 77.Mentzelopoulos S.D., Malachias S., Zintzaras E., et al. Intermittent recruitment with high-frequency oscillation/tracheal gas insufflation in acute respiratory distress syndrome. Eur Respir J. 2012;39:635–647. doi: 10.1183/09031936.00158810. [DOI] [PubMed] [Google Scholar]
- 78.Mercat A., Richard J.C., Vielle B., et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 79.Mojtahedzadeh M., Vazin A., Najafi A., Khalilzadeh A., Abdollahi M. The effect of furosemide infusion on serum epidermal growth factor concentration after acute lung injury. J Infus Nurs. 2005;28:188–193. doi: 10.1097/00129804-200505000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Morelli A., Teboul J.-L., Maggiore S.M., et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med. 2006;34:2287–2293. doi: 10.1097/01.CCM.0000230244.17174.4F. [DOI] [PubMed] [Google Scholar]
- 81.Morris P.E., Papadakos P., Russell J.A., et al. A double-blind placebo-controlled study to evaluate the safety and efficacy of L-2-oxothiazolidine-4-carboxylic acid in the treatment of patients with acute respiratory distress syndrome. Crit Care Med. 2008;36:782–788. doi: 10.1097/CCM.0B013E318164E7E4. [DOI] [PubMed] [Google Scholar]
- 82.Najafi A., Mojtahedzadeh M., Mahmoodpoor A., et al. Effect of N-acetylcysteine on microalbuminuria in patients with acute respiratory distress syndrome. Arch Med Sci. 2009;5:408–414. [Google Scholar]
- 83.Nemer S.N., Caldeira J.B., Azeredo L.M., et al. Alveolar recruitment maneuver in patients with subarachnoid hemorrhage and acute respiratory distress syndrome: a comparison of 2 approaches. J Crit Care. 2011;26:22–27. doi: 10.1016/j.jcrc.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Oczenski W., Hörmann C., Keller C., et al. Recruitment maneuvers after a positive end-expiratory pressure trial do not induce sustained effects in early adult respiratory distress syndrome. Anesthesiology. 2004;101:620–625. doi: 10.1097/00000542-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Ortolani O., Conti A., Gaudio A., Masoni M., Novelli G. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock. 2000;13:14–18. doi: 10.1097/00024382-200013010-00003. [DOI] [PubMed] [Google Scholar]
- 86.Paine R., 3rd, Standiford T.J., Dechert R.E., et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012;40:90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Papazian L., Forel J.M., Gacouin A., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 88.Parish M., Valiyi F., Hamishehkar H., et al. The effect of omega-3 fatty acids on ARDS: a randomized double-blind study. Adv Pharm Bull. 2014;4:555–561. doi: 10.5681/apb.2014.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perkins G.D., Gates S., Park D., et al. The beta agonist lung injury trial prevention. A randomized controlled trial. Am J Respir Crit Care Med. 2014;189:674–683. doi: 10.1164/rccm.201308-1549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pintado M.C., de Pablo R., Trascasa M., et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58:1416–1423. doi: 10.4187/respcare.02068. [DOI] [PubMed] [Google Scholar]
- 91.Ranieri V.M., Suter P.M., Tortorella C., et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 92.Rice T.W., Wheeler A.P., Thompson B.T., et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rice T.W., Wheeler A.P., Thompson B.T., et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richard C., Warszawski J., Anguel N., et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290:2713–2720. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- 95.Ryugo M., Sawa Y., Takano H., et al. Effect of a polymorphonuclear elastase inhibitor (sivelestat sodium) on acute lung injury after cardiopulmonary bypass: findings of a double-blind randomized study. Surg Today. 2006;36:321–326. doi: 10.1007/s00595-005-3160-y. [DOI] [PubMed] [Google Scholar]
- 96.Sabater J., Masclans J., Sacanell J., Chacon P., Sabin P., Planas M. Effects on hemodynamics and gas exchange of omega-3 fatty acid-enriched lipid emulsion in acute respiratory distress syndrome (ARDS): a prospective, randomized, double-blind, parallel group study. Lipids Health Dis. 2008;7:39. doi: 10.1186/1476-511X-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soltan-Sharifi M.S., Mojtahedzadeh M., Najafi A., et al. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Hum Exp Toxicol. 2007;26:697–703. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- 98.Sharaf M., El-Hantery M., Noaman M., Abel-Salam Y. Biphasic intermittent positive airway pressure ventilation versus conventional ventilation in acute respiratory distress syndrome and acute lung injury. Trends Med Res. 2012;7:43–52. [Google Scholar]
- 99.Shariatpanahi Z.V., Taleban F.A., Mokhtari M., Shahbazi S. Ginger extract reduces delayed gastric emptying and nosocomial pneumonia in adult respiratory distress syndrome patients hospitalized in an intensive care unit. J Crit Care. 2010;25:647–650. doi: 10.1016/j.jcrc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 100.Shirai K., Yoshida S., Matsumaru N., Toyoda I., Ogura S. Effect of enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with sepsis-induced acute respiratory distress syndrome. J Intensive Care. 2015;3:24. doi: 10.1186/s40560-015-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singer P., Theilla M., Fisher H., Gibstein L., Grozovski E., Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 102.Smetkin A.A., Kuzkov V.V., Gaidukov K.M., Bjertnaes L.J., Kirov M.Y. Derecruitment test and surfactant therapy in patients with acute lung injury. Crit Care Res Pract. 2012;2012:428798. doi: 10.1155/2012/428798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spragg R.G., Lewis J.F., Walmrath H.D., et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 104.Spragg R.G., Lewis J.F., Wurst W., et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562–1566. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 105.Spragg R.G., Taut F.J., Lewis J.F., et al. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med. 2011;183:1055–1061. doi: 10.1164/rccm.201009-1424OC. [DOI] [PubMed] [Google Scholar]
- 106.Stapleton R.D., Martin T.R., Weiss N.S., et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–1662. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Staudinger T., Kofler J., Müllner M., et al. Comparison of prone positioning and continuous rotation of patients with adult respiratory distress syndrome: results of a pilot study. Crit Care Med. 2001;29:51–56. doi: 10.1097/00003246-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 108.Taccone P., Pesenti A., Latini R., et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302:1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 109.Talmor D., Sarge T., Malhotra A., et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taylor R.W., Zimmerman J.L., Dellinger R.P., et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 111.Tongyoo S., Permpikul C., Mongkolpun W., et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care. 2016;20:329. doi: 10.1186/s13054-016-1511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Truwit J.D., Bernard G.R., Steingrub J., et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsangaris I., Galiatsou E., Kostanti E., Nakos G. The effect of exogenous surfactant in patients with lung contusions and acute lung injury. Intensive Care Med. 2007;33:851. doi: 10.1007/s00134-007-0597-z. [DOI] [PubMed] [Google Scholar]
- 114.Varpula T., Jousela I., Niemi R., Takkunen O., Pettila V. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiol Scand. 2003;47:516–524. doi: 10.1034/j.1399-6576.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 115.Varpula T., Valta P., Markkola A., et al. The effects of ventilatory mode on lung aeration assessed with computer tomography: a randomized controlled study. J Intensive Care Med. 2009;24:122–130. doi: 10.1177/0885066608330098. [DOI] [PubMed] [Google Scholar]
- 116.Varpula T., Valta P., Niemi R., Takkunen O., Hynynen M., Pettilä V. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2004;48:722–731. doi: 10.1111/j.0001-5172.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 117.Villar J., Kacmarek R.M., Perez-Mendez L., Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34:1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 118.Vincent J.L., Artigas A., Petersen L.C., Meyer C. A multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial assessing safety and efficacy of active site inactivated recombinant factor VIIa in subjects with acute lung injury or acute respiratory distress syndrome. Crit Care Med. 2009;37:1874–1880. doi: 10.1097/CCM.0b013e31819fff2c. [DOI] [PubMed] [Google Scholar]
- 119.Vincent J.L., Brase R., Santman F., et al. A multi-centre, double-blind, placebo-controlled study of liposomal prostaglandin E1 (TLC C-53) in patients with acute respiratory distress syndrome. Intensive Care Med. 2001;27:1578–1583. doi: 10.1007/s001340101077. [DOI] [PubMed] [Google Scholar]
- 120.Voggenreiter G., Aufmkolk M., Stiletto R.J., et al. Prone positioning improves oxygenation in post-traumatic lung injury—a prospective randomized trial. J Trauma. 2005;59:333–341. doi: 10.1097/01.ta.0000179952.95921.49. discussion 41–3. [DOI] [PubMed] [Google Scholar]
- 121.Weber T., Tschernich H., Sitzwohl C., et al. Tromethamine buffer modifies the depressant effect of permissive hypercapnia on myocardial contractility in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;162:1361–1365. doi: 10.1164/ajrccm.162.4.9808092. [DOI] [PubMed] [Google Scholar]
- 122.Wheeler A.P., Bernard G.R., Thompson B.T., et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 123.Wiedemann H.P., Wheeler A.P., Bernard G.R., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 124.Willson D.F., Truwit J.D., Conaway M.R., Traul C.S., Egan E.E. The adult calfactant in acute respiratory distress syndrome trial. Chest. 2015;148:356–364. doi: 10.1378/chest.14-1139. [DOI] [PubMed] [Google Scholar]
- 125.Xi X.-M., Jiang L., Zhu B., RM Group Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl) 2010;123:3100–3105. [PubMed] [Google Scholar]
- 126.Young D., Lamb S.E., Shah S., et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 127.Zeiher B.G., Artigas A., Vincent J.L., et al. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32:1695–1702. doi: 10.1097/01.ccm.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]
- 128.Zhan Q., Sun B., Liang L., et al. Early use of noninvasive positive pressure ventilation for acute lung injury. Crit Care Med. 2012;40:455–460. doi: 10.1097/CCM.0b013e318232d75e. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J.G., Chen X.J., Liu F., Zeng Z.G., Qian K.J. Lung recruitment maneuver effects on respiratory mechanics and extravascular lung water index in patients with acute respiratory distress syndrome. World J Emerg Med. 2011;2:201–205. doi: 10.5847/wjem.j.1920-8642.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao Smith F., Perkins G.D., Gates S., et al. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 132.Kung S.-C., Hung Y.-L., Chen W.-L., Wang C.-M., Chang H.-C., Liu W.-L. Effects of stepwise lung recruitment maneuvers in patients with early acute respiratory distress syndrome: a prospective, randomized, controlled trial. J Clin Med. 2019;8:231. doi: 10.3390/jcm8020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhen G., Shan Y., Qiao H., Wang W., Gai L., Li Z. Efficacy of Xuebijing injection in the adjunctive therapy of acute respiratory distress syndrome caused by sepsis. Int J Clin Exp Med. 2019;12:10029–10038. [Google Scholar]
- 134.Barrot L., Asfar P., Mauny F., et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 135.Beitler J.R., Sarge T., Banner-Goodspeed V.M., et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321:846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Constantin J.-M., Jabaudon M., Lefrant J.-Y., et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7:870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 137.Fowler A.A., 3rd, Truwit J.D., Hite R.D., et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fredenburgh L.E., Perrella M.A., Barragan-Bradford D., et al. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight. 2018;3 doi: 10.1172/jci.insight.124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He H., Sun B., Liang L., et al. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit Care. 2019;23:300. doi: 10.1186/s13054-019-2575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.He J., Si X., Ji M., et al. Effect of rhubarb on extravascular lung water in patients with acute respiratory distress syndrome. Rev Assoc Med Bras (1992) 2017;63:435–440. doi: 10.1590/1806-9282.63.05.435. [DOI] [PubMed] [Google Scholar]
- 141.Hodgson C.L., Cooper D.J., Arabi Y., et al. Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP). A phase II, multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200:1363–1372. doi: 10.1164/rccm.201901-0109OC. [DOI] [PubMed] [Google Scholar]
- 142.Jabaudon M., Boucher P., Imhoff E., et al. Sevoflurane for sedation in acute respiratory distress syndrome. A randomized controlled pilot study. Am J Respir Crit Care Med. 2017;195:792–800. doi: 10.1164/rccm.201604-0686OC. [DOI] [PubMed] [Google Scholar]
- 143.Lam N.N., Hung T.D., Hung D.K. Impact of “opening the lung” ventilatory strategy on burn patients with acute respiratory distress syndrome. Burns. 2019;45:1841–1847. doi: 10.1016/j.burns.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 144.Li J.Q., Li N., Han G.J., et al. Clinical research about airway pressure release ventilation for moderate to severe acute respiratory distress syndrome. Eur Rev Med Pharmacol Sci. 2016;20:2634–2641. [PubMed] [Google Scholar]
- 145.Luo J., Wang M.Y., Liang B.M., et al. Initial synchronized intermittent mandatory ventilation versus assist/control ventilation in treatment of moderate acute respiratory distress syndrome: a prospective randomized controlled trial. J Thorac Dis. 2015;7:2262–2273. doi: 10.3978/j.issn.2072-1439.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mahmoodpoor A., Hamishehkar H., Shadvar K., et al. The effect of intravenous selenium on oxidative stress in critically ill patients with acute respiratory distress syndrome. Immunol Invest. 2019;48:147–159. doi: 10.1080/08820139.2018.1496098. [DOI] [PubMed] [Google Scholar]
- 147.Matthay M.A., Calfee C.S., Zhuo H., et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meng J.B., Lai Z.Z., Xu X.J., Ji C.L., Hu M.H., Zhang G. Effects of early continuous venovenous hemofiltration on E-selectin, hemodynamic stability, and ventilatory function in patients with septic-shock-induced acute respiratory distress syndrome. Biomed Res Int. 2016;2016:7463130. doi: 10.1155/2016/7463130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mohamed H.S., Meguid M.M. Effect of nebulized budesonide on respiratory mechanics and oxygenation in acute lung injury/acute respiratory distress syndrome: randomized controlled study. Saudi J Anaesth. 2017;11:9–14. doi: 10.4103/1658-354X.197369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.The National Heart, Lung. Blood Institute PETAL Clinical Trials Network Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patel B.K., Wolfe K.S., Pohlman A.S., Hall J.B., Kress J.P. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ranieri V.M., Pettilä V., Karvonen M.K., et al. Effect of intravenous interferon β-1a on death and days free from mechanical ventilation among patients with moderate to severe acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2020;323:725–733. doi: 10.1001/jama.2019.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rezaiguia-Delclaux S., Laverdure F., Genty T., et al. Neuromuscular blockade monitoring in acute respiratory distress syndrome: randomized controlled trial of clinical assessment alone or with peripheral nerve stimulation. Anesth Analg. 2020 doi: 10.1213/ANE.0000000000005174. Advance Access published on September 23. [DOI] [PubMed] [Google Scholar]
- 154.Salem M.S., Eltatawy H.S., Abdelhafez A.A., Alsherif SE-dI. Lung ultrasound- versus FiO2-guided PEEP in ARDS patients. Egypt J Anaesth. 2020;36:31–37. [Google Scholar]
- 155.Sobhy A., Hussiny A., Kamal M. Evaluation of the use of hypertonic saline 3% nebulizer versus intravenous hypertonic saline 3% to attenuate the manifestations of acute respiratory distress syndrome. Open Anesth J. 2020;15:9. [Google Scholar]
- 156.Sun R., Li Y., Chen W., Zhang F., Li T. Total ginsenosides synergize with ulinastatin against septic acute lung injury and acute respiratory distress syndrome. Int J Clin Exp Pathol. 2015;8:7385–7390. [PMC free article] [PubMed] [Google Scholar]
- 157.Tang K.Q., Yang S.L., Zhang B., et al. Ultrasonic monitoring in the assessment of pulmonary recruitment and the best positive end-expiratory pressure. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Villar J., Ferrando C., Martínez D., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 159.Yu S., Hu T.-X., Jin J., Zhang S. Effect of protective lung ventilation strategy combined with lung recruitment maneuver in patients with acute respiratory distress syndrome (ARDS) J Acute Dis. 2017;6:163–168. [Google Scholar]
- 160.Yuanbo Z., Jin W., Fei S., et al. ICU management based on PiCCO parameters reduces duration of mechanical ventilation and ICU length of stay in patients with severe thoracic trauma and acute respiratory distress syndrome. Ann Intensive Care. 2016;6:113. doi: 10.1186/s13613-016-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zheng G., Huang L., Tong H., et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou Y., Jin X., Lv Y., et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43:1648–1659. doi: 10.1007/s00134-017-4912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 164.Peek G.J., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 165.Barker M., Adams S. An evaluation of a single chest physiotherapy treatment on mechanically ventilated patients with acute lung injury. Physiother Res Int. 2002;7:157–169. doi: 10.1002/pri.252. [DOI] [PubMed] [Google Scholar]
- 166.Tamakuma S., Ogawa M., Aikawa N., et al. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Ther. 2004;17:271–279. doi: 10.1016/j.pupt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 167.Troncy E., Collet J.P., Shapiro S., et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998;157:1483–1488. doi: 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 168.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Villar J., Blanco J., del Campo R., et al. Assessment of PaO2/FiO2 for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Villar J., Fernandez R.L., Ambros A., et al. A clinical classification of the acute respiratory distress syndrome for predicting outcome and guiding medical therapy. Crit Care Med. 2015;43:346–353. doi: 10.1097/CCM.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 171.Yehya N., Thomas N.J., Khemani R.G. Risk stratification using oxygenation in the first 24 hours of pediatric acute respiratory distress syndrome. Crit Care Med. 2018;46:619–624. doi: 10.1097/CCM.0000000000002958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Madotto F., Pham T., Bellani G., et al. Resolved versus confirmed ARDS after 24 h: insights from the LUNG SAFE study. Intensive Care Med. 2018;44:564–577. doi: 10.1007/s00134-018-5152-6. [DOI] [PubMed] [Google Scholar]
- 173.Zhang R., Wang Z., Tejera P., et al. Late-onset moderate to severe acute respiratory distress syndrome is associated with shorter survival and higher mortality: a two-stage association study. Intensive Care Med. 2017;43:399–407. doi: 10.1007/s00134-016-4638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lichtman S.M., Harvey R.D., Damiette Smit M.A., et al. Modernizing clinical trial eligibility criteria: recommendations of the American society of clinical oncology-friends of cancer research organ dysfunction, prior or concurrent malignancy, and comorbidities working group. J Clin Oncol. 2017;35:3753–3759. doi: 10.1200/JCO.2017.74.4102. [DOI] [PubMed] [Google Scholar]
- 175.Bourgeois F.T., Orenstein L., Ballakur S., Mandl K.D., Ioannidis J.P.A. Exclusion of elderly people from randomized clinical trials of drugs for ischemic heart disease. J Am Geriatr Soc. 2017;65:2354–2361. doi: 10.1111/jgs.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laffey J.G., Madotto F., Bellani G., et al. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med. 2017;5:627–638. doi: 10.1016/S2213-2600(17)30213-8. [DOI] [PubMed] [Google Scholar]
- 177.Rush B., McDermid R.C., Celi L.A., Walley K.R., Russell J.A., Boyd J.H. Association between chronic exposure to air pollution and mortality in the acute respiratory distress syndrome. Environ Pollut. 2017;224:352–356. doi: 10.1016/j.envpol.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ike J., Kempker J., Kramer M., Martin G. The association between acute respiratory distress syndrome mortality and annual hospital ARDS case volumes from 2002-2011 in the nationwide inpatient sample. Am J Respir Crit Care Med. 2017;195:A1819. [Google Scholar]
- 179.Laursen P.N., Holmvang L., Lonborg J., et al. Unreported exclusion and sampling bias in interpretation of randomized controlled trials in patients with STEMI. Int J Cardiol. 2019;289:1–5. doi: 10.1016/j.ijcard.2019.04.064. [DOI] [PubMed] [Google Scholar]
- 180.Zhang Z., Spieth P.M., Chiumello D., et al. Declining mortality in patients with acute respiratory distress syndrome: an analysis of the Acute Respiratory Distress Syndrome Network trials. Crit Care Med. 2019;47:315–323. doi: 10.1097/CCM.0000000000003499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.