ABSTRACT.
Several arboviruses have emerged or reemerged into the New World during the past several decades, causing outbreaks of significant proportion. In particular, the outbreaks of Dengue virus (DENV), Zika virus, and Chikungunya virus (CHIKV) have been explosive and unpredictable, and have led to significant adverse health effects. These viruses are considered the leading cause of acute undifferentiated febrile illnesses in Colombia. However, Venezuelan equine encephalitis virus (VEEV) is endemic in Colombia, and arboviruses such as the Mayaro virus (MAYV) and the Oropouche virus (OROV) cause febrile illnesses in neighboring countries. Yet, evidence of human exposure to MAYV and OROV in Colombia is scarce. In this study, we conducted a serosurvey study in healthy individuals from the Cauca Department in Colombia. We assessed the seroprevalence of antibodies against multiple arboviruses, including DENV serotype 2, CHIKV, VEEV, MAYV, and OROV. Based on serological analyses, we found that the overall seroprevalence for DENV serotype 2 was 30%, 1% for MAYV, 2.6% for CHIKV, 4.4% for VEEV, and 2% for OROV. This study provides evidence about the circulation of MAYV and OROV in Colombia, and suggests that they—along with VEEV and CHIKV—might be responsible for cases of acute undifferentiated febrile illnesses that remain undiagnosed in the region. The study results also highlight the need to strengthen surveillance programs to identify outbreaks caused by these and other vector-borne pathogens.
INTRODUCTION
Arthropod-borne viruses (arboviruses) continue to be significant causes of mild to severe illness around the world. Among the arboviruses, Dengue virus (DENV), which belongs to the family Flaviviridae, is the most prevalent arbovirus affecting humans in the tropics.1 However, as a result of the similarity of signs and symptoms with many other tropical infectious diseases, and the lack of affordable and available laboratory diagnostics in many locations, DENV has become an “umbrella” under which many other arboviral diseases remain hidden. Surveillance studies for febrile illness have shown that typically only about one third of presumptive dengue cases are caused by DENV, whereas the remainder is caused by a variety of other arboviral etiologies and other infectious agents.2,3 Examples include emerging and reemerging arboviruses such as Zika virus (ZIKV; Flaviviridae family) and Chikungunya virus (CHIKV; Togaviridae family), which have caused explosive outbreaks resulting in a substantial economic impact and public health burden in recent years. There are several factors that are believed to be responsible for the emergence of these arboviruses, including deforestation, urbanization, climate change that causes the expansion of mosquito populations, lack of effective mosquito control strategies, globalization of air transport, and overall poor socioeconomic conditions in low-income countries.4,5
As a result of the extensive outbreaks caused by DENV,6,7 ZIKV,8 and CHIKV,9 most acute undifferentiated febrile illnesses (AUFIs) in tropical regions are now being reported as caused by these viruses.3 Nevertheless, other arboviruses of significant importance in these areas include Venezuelan equine encephalitis virus (VEEV),10 Mayaro virus (MAYV),11,12 both from the Togaviridae family; and Oropouche virus (OROV), which belongs to the family Peribunyaviridae.13 In the case of VEEV, it is estimated to cause tens of thousands of cases of febrile illness annually throughout Latin America from enzootic spillovers as well as during periodic equine-amplified outbreaks that can spread across continents.2,14,15 Some of the most massive VEEV outbreaks on record occurred in Colombia in 1967 and 1968, when more than 200,000 human cases and more than 100,000 equine deaths were recorded.16 Another arbovirus of significant importance is MAYV, which is considered the second most important alphavirus responsible for febrile illness in certain areas of South America.17,18 A recent report17 has shown that 54% of patients infected with MAYV develop persistent, debilitating arthralgia affecting the major joints, very similar to what is seen with CHIKV infection. Because surveillance activities in tropical regions are mainly based on symptoms, MAYV cases are likely misdiagnosed as CHIKV infections as a result of their similarities. It is also possible that the geographic range of MAYV circulation extends beyond the few countries that have reported cases. Last, OROV causes a significant portion of AUFIs in Latin America.2 OROV is capable of infecting hundreds of thousands of people.2,19 The segmented nature of its genome has allowed the emergence of new viruses capable of causing human illness, even in individuals infected previously with OROV.20,21
Colombia is a subtropical country in South America known for its biodiversity. It is also an endemic country for the transmission of arboviruses such as DENV, ZIKV, and CHIKV.22 As in most low- and middle-income countries, only a handful of probable dengue, nonmalarial cases are confirmed by laboratory testing.23 A past study24 conducted in Colombia found that only 38% of the possible dengue, nonmalarial cases were confirmed as dengue by laboratory testing, whereas the agents responsible for 62% of the cases remained unknown. These findings highlight the need to intensify efforts to identify the pathogens responsible for AUFIs and to enhance the diagnostic capacity of researchers and health professionals working in the area.
In this study, we present the results of a population-based survey of neutralizing antibodies against MAYV, CHIKV, OROV, VEEV, and DENV serotype 2 (DENV-2) to understand more completely the exposure to these viruses in residents from the Cauca Department, Colombia. The main objective was to determine the circulation of these arboviruses in the human population from Colombia, and to identify their seroprevalence and eco-epidemiological risk factors associated with previous viral infections.
MATERIALS AND METHODS
Study site.
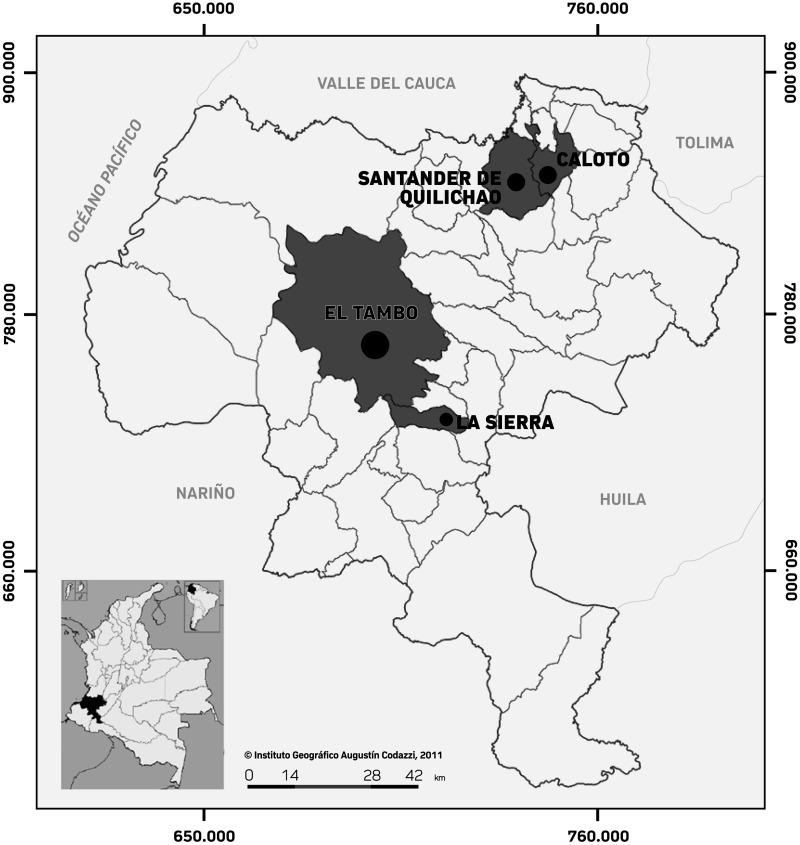
The study site was the Cauca Department, located in the southwestern region of Colombia, with ∼1.24 million inhabitants in 2018.25 It is located near the Pacific Ocean at an elevation of 1,738 m. The annual average temperature is ∼19°C, with irregular periods of precipitation, and relative humidity that ranges between 80% and 85%. There were four municipalities chosen for this study: Caloto, with 17,642 habitants; Santander de Quilichao, with 96,500 habitants; El Tambo, with 53,769 habitants; and La Sierra, with 10,643 habitants (Figure 1). The sites were selected based on the information given by the Cauca Health Secretary regarding problems with arthropod vectors and accessibility to the armed conflict–affected area. Most people in those locations live in poverty and work primarily in agriculture and livestock production, forestry, mineral extraction, fishing, and trade.25
Figure 1.
Location map of the Cauca department in which the municipalities included in the study are visible. Modified from the Instituto Geográfico Agustín Codazzi (www.igac.gov.co) and Wikimedia commons (Colombia departamentos https://commons.wikimedia.org/wiki/File:Colombia_departamentos_otros.svg).
Study population.
The study subjects were older than 18 years who lived in the chosen municipalities and agreed to participate in the study. Minors were not included in the study because of institutional review board regulations. Demographic and clinical information was obtained from each individual at the time of voluntary enrollment, along with signed consent. Demographic data included information about gender, age, occupation, housing material, period of residence in the area, episodes of fever recorded during the past year, and previous history of being diagnosed with DENV, ZIKV, CHIKV, or malaria. Blood samples were collected and transported to the Pontificia Universidad Javeriana (Bogota, Colombia). Serum samples were then aliquoted and stored at –20°C before being transported to The Galveston National Laboratory, University of Texas Medical Branch, Galveston, TX, for further processing.
Plaque reduction neutralization testing.
Plaque reduction neutralization testing (PRNT) was conducted as described previously.26–28 Briefly, Vero cells were cultured in Minimal Essential Medium Eagle (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and maintained at 37°C under 5% carbon dioxide. The human serum samples collected previously were diluted in culture media and heat-inactivated at 56°C for 30 minutes to be tested for the detection of antibodies against the following viruses: MAYV (strain FPI00179), CHIKV (vaccine strain 181/clone 25), VEEV (vaccine strain TC-83), OROV (FSE 0812), and DENV-2 (strain New Guinea C). All virus work was performed in a biosafety level 2 laboratory except for OROV, which was handled in a high-containment laboratory (biosafety level 3 laboratory). All samples were screened initially at 1:20 dilution, and positive samples were titrated further (except for DENV-2). The highest serum dilution reducing plaque numbers by 80% was determined.
Data analysis.
Descriptive statistics counts and percentages are presented in Tables 1 through 4. Along with descriptive statistics we looked at running a generalized linear model for the binomial family model adjusted by municipality to find possible risk factors for neutralizing antibodies against MAYV, CHIKV, VEEV, and OROV considering the hypothesis that seroprevalence correlates with clinical and sociodemographic factors (e.g., gender, age, occupation) (Table 1). Because of the small number of individuals with neutralizing antibodies against MAYV, CHIKV, VEEV, and OROV, we combined the results, regardless of the pathogen evaluated, into one dependent variable. Our first outcome was having an infection with at least one virus, and we evaluated possible risk factors with crude and adjusted prevalence ratios (PRs) from all questions surveyed. We used a stepwise forward variable selection technique to find significant risk factors according to the researchers’ criteria. Considering the exploratory nature of this study, we set the stopping rule to be a P value < 0.1 to enter the model and a P value > 0.15 to leave the model, and an alpha value of 0.05 for a 95% level of confidence. This analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
Table 1.
Demographic information of participants based on municipality
| Demographic characteristic | Municipality | No. of participants (N = 505) | |||
|---|---|---|---|---|---|
| El Tambo (n = 263) | Caloto (n = 50) | La Sierra (n = 40) | Santander de Quilichao (n = 152) | ||
| Age group, years | |||||
| 18–29 | 39 | 16 | 6 | 30 | 91 (18%) |
| 30–64 | 147 | 32 | 30 | 100 | 309 (61%) |
| >65 | 77 | 2 | 4 | 22 | 105 (21%) |
| Gender | |||||
| Male | 86 | 16 | 17 | 39 | 158 (31%) |
| Female | 177 | 34 | 23 | 113 | 347 (69%) |
| Occupation | |||||
| Agricultural activities | 74 | 21 | 30 | 35 | 160 (31.7%) |
| Homemakers | 147 | 14 | 4 | 80 | 245 (48.5%) |
| Elementary activities | 19 | 9 | 4 | 26 | 58 (11.5%) |
| Service workers and stores and market sellers | 6 | 0 | 2 | 2 | 10 (2.0%) |
| Scientific and intellectual professionals | 4 | 5 | 0 | 2 | 11 (2.2%) |
| Unemployed | 13 | 1 | 0 | 7 | 21 (4.2%) |
| Wall household material | |||||
| Brick or block concrete | 203 | 15 | 2 | 122 | 342 (68%) |
| Handcrafted (Bahereque) | 42 | 29 | 38 | 21 | 130 (26%) |
| Plant materials (Guadua) | 18 | 6 | 0 | 9 | 33 (6%) |
| Access to public services | |||||
| Water | 260 | 48 | 40 | 151 | 499 (99%) |
| Electricity | 261 | 49 | 40 | 150 | 500 (99%) |
| Additional information | |||||
| Report previous exposure to ZIKV, CHIKV, DENV, or malaria | 17 | 3 | 3 | 24 | 47 (9.3%) |
| Do not report previous exposure to ZIKV, CHIKV, DENV, or malaria | 243 | 46 | 37 | 126 | 452 (89/5%) |
| Does not know of previous exposure to ZIKV, CHIKV, DENV, or malaria | 3 | 1 | 0 | 2 | 6 (1.2%) |
| Animals on property | 239 | 47 | 34 | 133 | 453 (89.7%) |
CHIKV = Chikungunya virus; DENV = Dengue virus; ZIKV = Zika virus.
RESULTS
Study population.
Demographics of the study population are summarized in Table 1. A total of 505 participants were enrolled in this study and most of them resided in El Tambo (n = 263, 52.1%), followed by Santander de Quilichao (n = 152, 30.1%), Caloto (n = 50, 9.9%), and La Sierra (n = 40, 7.9%). The mean age of the participants was 48 years. There was a difference in gender distribution among the participants, with females represented more proportionally (n = 347, 69%) than males (n = 158, 31%). The principal occupations among the study population were homemaker (n = 245, 48.5%), and agricultural laborer (n = 160, 31.7%). The majority of the participants had access to water (n = 499, 99%) and electricity (n = 500, 99%), and lived with animals in their household (n = 453, 89.7%), including bovines (n = 52, 10.3%), horses (n = 79, 15.6%), dogs (n = 388, 76.8%), cats (n = 218, 43.2%), poultry (n = 155, 30.7%), wild birds (n = 4, 0.8%), pigs or sheep (n = 14, 2.8%), and rodents (n = 8, 1.6%). We also acquired information about past diagnoses of vector-borne diseases. Most individuals (n = 452, 89.5%) did not report a previous exposure to DENV, ZIKV, CHIKV, or malaria; only 9.3% (n = 47) reported being diagnosed previously with DENV, ZIKV, CHIKV, or malaria. Only 1.2% (n = 6) did not know about past exposure to these pathogens (Table 1).
Alphavirus neutralizing antibody prevalence.
A total of 505 human sera samples were tested for the presence of VEEV, CHIKV, and MAYV neutralizing antibodies. Laboratory analysis showed an overall prevalence for these alphaviruses as follows: VEEV, 4.4% (22 of 505; 95% CI, 2.8–6.4); CHIKV, 2.6% (13 of 505; 95% CI, 1.4–4.2); and MAYV, 1% (5 of 505; 95% CI, 0.4–2.2) (Table 2). Antibody titers against VEEV and CHIKV ranged between 40 and ≥ 640, and from 20 to ≥ 640 for MAYV (Table 3).
Table 2.
Overall prevalence against MAYV, CHIKV, VEEV, OROV, and DENV-2 in Cauca by municipalities
| Municipality | MAYV, n/N (%) | CHIKV, n/N (%) | VEEV, n/N (%) | OROV, n/N (%) | DENV-2, n/N (%) |
|---|---|---|---|---|---|
| Santander de Quilichao | 1/152 (0.7) | 7/152 (4.6) | 12/152 (7.9) | 1/152 (0.7) | 87/150 (58) |
| El Tambo | 3/263 (1.1) | 3/263 (1.1) | 5/263 (1.9) | 8/263 (3) | 45/262 (17.2) |
| La Sierra | 1/40 (2.5) | 1/40 (2.5) | 4/40 (10) | 1/40 (2.5) | 10/39 (25.6) |
| Caloto | 0/50 (0) | 2/50 (4) | 1/50 (2) | 0/50 (0) | 8/50 (16) |
| Total | 5/505 (1) | 13/505 (2.6) | 22/505 (4.4) | 10/505 (2) | 150/501 (29.9) |
CHIKV = Chikungunya virus; DENV-2 = Dengue virus serotype 2; MAYV = Mayaro virus; n = number of seropositive patients; N = total number of participants in each municipality; OROV = Oropouche virus; VEEV = Venezuelan equine encephalitis virus.
Prevalence was established based on an initial screening at a 1:20 dilution followed by an end point titration (except for DENV). The n indicates the number of seropositive participants, and N refers to the total number of participants in each municipality.
Table 3.
End point titers for neutralizing antibodies against MAYV, CHIKV, VEEV, and OROV
| Sample | Report previous exposure to ZIKV, CHIKV, DENV, or malaria? | Neutralizing antibody titers (PRNT80) | PRNT positive | |||
|---|---|---|---|---|---|---|
| MAYV | CHIKV | VEEV | OROV | |||
| H017 | No | – | – | > 640 | – | VEEV |
| H019 | No | 320 | > 640 | – | – | MAYV, CHIKV |
| H025 | No | – | – | 40 | – | VEEV |
| H027 | No | – | – | > 640 | – | VEEV |
| H032 | No | – | – | > 640 | – | VEEV |
| H038 | No | – | – | – | > 640 | OROV |
| H045 | No | – | – | – | 20 | OROV |
| H048 | No | – | – | – | 40 | OROV |
| H058 | No | – | – | – | 20 | OROV |
| H074 | No | – | – | – | 20 | OROV |
| H079 | No | 320 | > 640 | – | – | MAYV, CHIKV |
| H082 | No | 20 | – | – | – | MAYV |
| H094 | No | – | – | – | 20 | OROV |
| H107 | No | – | – | 320 | – | VEEV |
| H127 | No | – | – | > 640 | – | VEEV |
| H131 | CHIKV | 40 | – | – | – | MAYV |
| H132 | No | – | – | > 640 | – | VEEV |
| H192 | No | – | 40 | – | 160 | CHIKV, OROV |
| H202 | No | – | – | – | 20 | OROV |
| H245 | Malaria | – | – | 160 | – | VEEV |
| H246 | Malaria | – | – | 160 | > 640 | VEEV, OROV |
| H272 | CHIKV | – | > 640 | – | – | CHIKV |
| H305 | No | – | – | > 640 | – | VEEV |
| H317 | No | – | 80 | – | – | CHIKV |
| H319 | DENV | – | > 640 | – | – | CHIKV |
| H329 | No | – | – | > 640 | – | VEEV |
| H333 | No | – | – | 40 | – | VEEV |
| H334 | No | – | – | – | 20 | OROV |
| H347 | No | – | – | > 640 | – | VEEV |
| H363 | No | – | – | 80 | – | VEEV |
| H384 | No | – | – | > 640 | – | VEEV |
| H387 | CHIKV | – | 80 | – | – | CHIKV |
| H403 | No | – | – | > 640 | – | VEEV |
| H408 | CHIKV | > 640 | 80 | – | – | MAYV, CHIKV |
| H412 | No | – | – | > 640 | – | VEEV |
| H419 | No | – | > 640 | – | – | CHIKV |
| H425 | DENV | – | – | 640 | – | VEEV |
| H429 | Malaria | – | – | 80 | – | VEEV |
| H435 | No | – | – | 320 | – | VEEV |
| H436 | No | – | – | 640 | – | VEEV |
| H441 | CHIKV | – | 640 | – | – | CHIKV |
| H450 | CHIKV | – | 640 | – | – | CHIKV |
| H470 | CHIKV | – | 640 | – | – | CHIKV |
| H474 | DENV | – | – | 640 | – | VEEV |
| H486 | CHIKV | – | 640 | – | – | CHIKV |
CHIKV = Chikungunya virus; DENV = Dengue virus; MAYV = Mayaro virus; OROV = Oropouche virus; PRNT = plaque reduction neutralization test; PRNT80 = plaque reduction neutralization test 80; VEEV = Venezuelan equine encephalitis virus; ZIKV = Zika virus.
Responses regarding past exposure to ZIKV, CHIKV, DENV, or malaria are included as a reference.
The VEEV PR across municipalities was 7.9% (12 of 152) in Santander de Quilichao, 1.9% (5 of 263) in El Tambo, 10% (4 of 40) in La Sierra, and 2% (1 of 50) in Caloto. The CHIKV PR was 4.6% (7 of 152) in Santander de Quilichao, 1.1% (3 of 263) in El Tambo, 2.5% (1 of 40) in La Sierra, and 4% (2 of 50) in Caloto. Last, MAYV seroprevalence was 0.7% (1 of 152) in Santander de Quilichao, 1.1% (3 of 263) in El Tambo and 2.5% (1 of 40) in La Sierra. Table 2 summarizes the alphavirus antibody seroprevalence in each municipality.
OROV neutralizing antibody prevalence.
The overall OROV seroprevalence among the study population was 2% (10 of 505; 95% CI, 1.0–3.5; Table 2). End point neutralizing antibody titers for OROV ranged between 20 and ≥ 640 (Table 3). The OROV PR across municipalities was 0.7% (1 of 152) in Santander de Quilichao, 3% (8 of 263) in El Tambo, and 2.5% (1 of 40) in La Sierra (Table 2).
DENV-2 neutralizing antibody prevalence.
Given the relatively low seroprevalence for MAYV, VEEV, CHIKV, and OROV, we proceeded to investigate the seroprevalence of DENV, which is the most prevalent arbovirus affecting humans in the tropics.1 For the DENV seroprevalence studies, we selected DENV-2 for the analysis because this dengue serotype has been circulating in Colombia since at least the 1960s, and the virus is isolated yearly in Colombia from patients with febrile illness, representing the major DENV serotype in circulation in Colombia. The overall DENV-2 seroprevalence in the study population was 30% (150 of 501; 95% CI, 26–34). Santander de Quilichao had the highest seroprevalence, with 58% of the study population presenting neutralizing antibodies against DENV-2 (Table 2).
Risk factors analysis.
The bivariate analyses for arbovirus seropositivity, excluding DENV-2, suggest an association with gender, age, bovines on the property, rats or mice near the property, outdoor occupations, not showering daily, infrequent handwashing, time of residence in the municipality, having a fever in the past year, and previous diagnoses of DENV, CHIKV, ZIKV, or malaria. In the multivariate analysis with a binomial model adjusted by municipality, seropositivity to MAYV, CHIKV, VEEV, and OROV was associated with gender, age, and presence of bovines and rats or mice in the property. Specifically, men (PR, 1.68) and participants older than 47 years (PR, 2.28) were at a greater risk of infection with MAYV, CHIKV, VEEV, and OROV. The presence of bovines (PR, 1.88) and rats or mice near the property (PR, 2.37), and the absence of canines in the property (PR, 1.63) were also associated significantly with MAYV, CHIKV, VEEV, and OROV seropositivity. Table 4 summarizes the results of the risk factor analyses.
Table 4.
Description of the variables by outcome of MAYV, CHIKV, OROV, and VEEV, including the crude and adjusted PR from the multivariate analysis adjusted for municipality (N = 505)
| Variable | Outcome mixed MAYV, CHIKV, OROV, and VEEV (N = 505) | ||||||
|---|---|---|---|---|---|---|---|
| n (%) | Crude PR (95% CI) | P value | Model 1 | Null model | |||
| Adjusted PR (95% CI) | P value | ||||||
| Gender | |||||||
| Female | 24 (4.75) | Reference | Reference | – | |||
| Male | 21 (4.16) | 1.92 (1.27–2.90) | 0.002 | 1.68 (1.08–2.61) | 0.022 | ||
| Age, years | |||||||
| 18–46 | 13 (2.59) | Reference | Reference | ||||
| ≥ 47 | 32 (6.37) | 2.42 (1.70–3.46) | < 0.001 | 2.28 (1.65–3.14) | < 0.001 | ||
| Dogs on the property | |||||||
| Yes | 30 (5.94) | Reference | Reference | ||||
| No | 15 (2.97) | 1.66 (1.40–1.95) | < 0.001 | 1.63 (1.38–1.92) | < 0.001 | ||
| Bovines on the property | |||||||
| No | 37 (7.33) | Reference | Reference | ||||
| Yes | 8 (1.58) | 1.88 (1.05–3.36) | 0.033 | 1.88 (1.25–2.82) | 0.002 | ||
| Rats or mice on the property | |||||||
| No | 9 (1.78) | Reference | Reference | ||||
| Yes | 36 (7.13) | 2.21 (1.43–3.44) | < 0.001 | 2.37 (1.59–3.53) | < 0.001 | ||
| Occupation | |||||||
| Indoors | 22 (4.36) | Reference | – | ||||
| Outdoors | 23 (4.55) | 1.27 (0.96–1.69) | 0.098 | ||||
| Years in residence | |||||||
| < 5 | 8 (1.58) | Reference | |||||
| ≥ 5 | 37 (7.33) | 1.44 (1.04–2.00) | 0.03 | ||||
| Frequency of bathing | |||||||
| Daily | 35 (6.93) | Reference | |||||
| Not daily | 10 (1.98) | 2.54 (1.55–4.18) | < 0.001 | ||||
| Frequency of handwashing | |||||||
| Often (> 4 times a day) | 34 (6.73) | Reference | |||||
| Occasionally (< 3 times a day) | 11 (2.18) | 1.60 (1.51–1.69) | < 0.001 | ||||
| Presence of fever in the past year | |||||||
| No | 27 (5.35) | Reference | |||||
| Yes | 18 (3.56) | 1.4 (0.97–2.0) | 0.069 | ||||
| Previous diagnosis of DENV, CHIKV, ZIKV, or malaria | |||||||
| No | 31 (6.14) | Reference | |||||
| Yes | 14 (2.77) | 4.3 (1.96–9.6) | < 0.001 | ||||
| – | – | – | – | BIC* | 297.74 | BIC | 309.7 |
| – | – | – | – | AIC* | 285.09 | AIC | 305.48 |
AIC = Akaike information criterion; BIC = Bayesian information criterion; CHIKV = Chikungunya virus; MAYV = Mayaro virus; OROV = Oropouche virus; PR = prevalence ratio; VEEV = Venezuelan equine encephalitis virus.
The smaller BIC of AIC value indicates the better model.
DISCUSSION
Colombia is one of the countries in South America with the highest level of AUFIs in the region, where the incidence of dengue-like illnesses has increased dramatically in recent years. In 2019, there were ∼124,989 probable dengue cases reported to Colombia’s National Health Institute, and 50.8% (n = 63,497) presented with warning signs requiring hospitalization.29 Because of limitations in diagnostic capacity, only a few cases were confirmed by laboratory testing as being caused by DENV, ZIKV, or CHIKV, and therefore most of the cases remain undiagnosed or diagnosed erroneously as “dengue.” Thus, efforts to identify agents responsible for causing AUFIs are needed to develop proper public health control strategies.
In Colombia, no comprehensive studies on the circulation of arboviruses, other than DENV, ZIKV, and CHIKV, have been carried out. Therefore, the objective of our study was to determine the level of exposure to other arboviruses among the adult population living in the Cauca Department. This region was selected because it has a vast forest habitat in a subtropical climate, with a set of conditions that make this place ideal for the circulation of vector-borne pathogens.
In our study, 505 sera samples collected in 2017 from El Tambo, Santander de Quilichao, Caloto, and La Sierra were tested for the presence of neutralizing antibodies against VEEV, MAYV, CHIKV, OROV, and DENV-2. The overall seroprevalence for DENV-2 was 30%, which confirmed the relatively high exposure to DENV in the study population. We selected DENV-2 for the analyses because this serotype has been circulating in Colombia since the 1960s and cases are reported continuously on a yearly basis in this country.30 However, we acknowledge that because we only conducted PRNT using a DENV-2 strain, it is possible that the overall DENV seroprevalence is greater than 30%. In addition, given the substantial high level of PRNT cross-reactivity among the DENV serotypes and other flaviviruses, we also acknowledge that detailed serological analyses are needed to assess properly the DENV and flavivirus seroprevalence in the study population. However, we believe those investigations fall outside the scope of our study because our focus was VEEV, MAYV, CHIKV, and OROV.
In general, the results of our study show an overall low seroprevalence for VEEV, MAYV, OROV, and CHIKV. Nevertheless, our study confirmed human exposure to VEEV and CHIKV in the study population and, more importantly, it provides evidence of the circulation of as well as human exposure to MAYV and OROV in Cauca, Colombia. Our study also identified risk factors associated with MAYV, CHIKV, VEEV, and OROV infection. Specifically, the multivariate model identified gender as a risk factor, with men as having a greater risk of infection than females, possibly because of their agricultural and livestock activities. Another risk factor is age, with individuals 47 years or older having significantly greater odds of infection compared with those younger than 47 years. The presence of bovines and rodents near homes is also a risk factor of infection, and may be related to occupational exposure and ecological conditions favoring arbovirus transmission. Overall, our findings are consistent with the results of prior studies conducted in other endemic areas in South America.20,31,32 In Colombia, DENV, CHIKV, and VEEV are of mandatory notification, whereas MAYV and OROV are not considered as part of the routine diagnosis. Cases of CHIKV were first recognized after the introduction of CHIKV in South America in 2013, with ∼106,592 clinical cases reported by Colombia’s National Health Institute and only 1.3% cases confirmed by laboratory testing.33 Interestingly, in our study, although the 13 individuals seropositive for CHIKV reported previous episodes of fever, only eight indicated having a previous diagnosis of DENV, ZIKV, or CHIKV infection. The data indicate that cases of CHIKV are underreported in Colombia (Table 3).
In Colombia, VEEV is mainly considered a pathogen of veterinary importance, even though it is the second alphavirus responsible for AUFIs in South America.2 Although VEEV is of mandatory notification, VEEV is mostly overlooked and is only considered as a potential cause of AUFIs when equine encephalitis cases are reported to the health authorities. For instance, in 2016, 86 clusters of equine encephalitis were recognized in the departments of Magdalena, Cauca, Meta, Santander, Casanare, and Cesar, among others. That same year, the NIH received a report of 20 possible VEEV cases in Santander, Cordoba, Cesar, and Antioquia, but no possible cases were reported from Cauca.34 In our study, we found an overall VEEV seroprevalence of 4.4% (22 of 505), providing evidence of human exposure to VEEV in Cauca. Notably, 17 individuals did not report previous infection with DENV, ZIKV, CHIKV, or malaria whereas five indicated past infection with DENV or malaria. The data confirm VEEV is underreported in Colombia. Given the importance of VEEV as a veterinary and human pathogen, studies are needed to determine whether VEEV is a significant cause of AUFIs in this area and to elucidate completely the public health impact of VEEV in Cauca and other endemic regions in Colombia.
Data concerning MAYV in Colombia was initially reported by Groot et al.35 in 1959, when MAYV and other viruses were isolated from field-collected mosquitoes in San Vicente de Chucurí. Another study conducted in the population from this area found an overall MAYV positivity of 3.3% (3 of 90) based on the results of hemagglutination inhibition (HI) testing.36 In another study from Colombia,37 159 sera samples obtained between 2001 and 2004 were tested for the presence of antibodies against several viruses by HI. Approximately 15.6% (10 of 64) of samples provided by the NIH were positive for MAYV.36,37 Unfortunately, the high cross-reactivity among alphaviruses by HI testing made it difficult to confirm human exposure to MAYV in Colombia based on those studies. By using the gold standard confirmatory serological assay, our data provide confirmatory evidence about human exposure to MAYV in Colombia and highlight the need to intensify efforts to determine whether MAYV is a significant cause of AUFIs in Colombia. A previous study38 suggested that individuals exposed to alphaviruses developed cross-reactive neutralizing antibody responses. Nevertheless, in our study we found individuals with specific neutralizing antibodies for CHIKV (n = 10) or MAYV (n = 2) as well as individuals with neutralizing antibodies against both pathogens (n = 3, Table 3). In addition, a recent study39 conducted with samples collected from MAYV-infected individuals in Peru before the arrival of the CHIKV to the Americas did not find evidence supporting the development of cross-reactive neutralizing antibody responses between CHIKV and MAYV. Thus, PRNT continues to be the gold standard test to distinguish between human exposure to CHIKV or MAYV.40 The evidence supports our conclusions about the circulation and human exposure to MAYV in Colombia.
Oropouche fever is a disease of public health concern in South America, and several outbreaks have occurred in Brazil, Panama, Ecuador, Peru, Trinidad, and Tobago since the 1960s. The circulation of OROV in Colombia has been poorly studied, although antibodies in humans were detected in a previous study,13 and OROV was isolated from mosquitoes by Groot et al.35 and from a patient with AUFI.41 Our findings confirm that human exposure to OROV occurs in Colombia, and studies are needed to determine more fully the extent of OROV activity in this country.
One of the major limitations of this study is the relatively small sample size; however, the number of samples acquired are very valuable because of the challenges of having access to the municipalities in the Cauca Department, an armed-conflict area. In addition, our study population was not balanced with respect to gender, because females were more present at home during sampling and were more inclined to participate in the research. Thus, it is likely that the prevalence for these viruses might be greater than that reported in our study.
Nevertheless, our study provides evidence about the circulation of MAYV and OROV in this region, and confirms human exposure to these pathogens. Our study highlights the need to consider these pathogens as causes of AUFIs in Colombia and to determine further their impact on public health in Colombia. Altogether, our study highlights the need to strengthen surveillance to improve the diagnosis of AUFIs in the region and to perform programs of vector control for the prevention of future arbovirus outbreaks.
ACKNOWLEDGMENTS
We acknowledge Pontificia Universidad Javeriana ID 7277 for the physical and human resources provided, and Sandra Vargas for helping with digital image editing of the figure.
REFERENCES
- 1. Guzman MG, Harris E, 2015. Dengue. Lancet 385: 453–465. [DOI] [PubMed] [Google Scholar]
- 2. Forshey BM. et al. , 2010. Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Negl Trop Dis 4: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreira J, Bressan CS, Brasil P, Siqueira AM., 2018. Epidemiology of acute febrile illness in Latin America. Clin Microbiol Infect 24: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasconcelos PFC, Travassos da Rosa APA, Rodrigues SG, Travassos da Rosa ES, Dégallier N, Travassos da Rosa JFS, 2001. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad Saude Publica 17: S155–S164. [DOI] [PubMed] [Google Scholar]
- 5. Muñoz M, Navarro JC, 2012. Mayaro: a re-emerging arbovirus in Venezuela and Latin America. Biodmedica 32: 286–302. [DOI] [PubMed] [Google Scholar]
- 6. Istúriz RE, Gubler DJ, del Castillo JB, 2000. Dengue and dengue hemorrhagic fever in Latin America and the Caribbean. Infect Dis Clin North Am 14: 121–140. [DOI] [PubMed] [Google Scholar]
- 7. Ramos-Castañeda J, Barreto dos Santos F, Martínez-Vega R, Galvão de Araujo JM, Joint G, Sarti E, 2017. Dengue in Latin America: systematic review of molecular epidemiological trends. PLoS Negl Trop Dis 11: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song B-H, Yun S-I, Woolley M, Lee Y-M, 2017. Zika virus: history, epidemiology, transmission, and clinical presentation. J Neuroimmunol 308: 50–64. [DOI] [PubMed] [Google Scholar]
- 9. Yactayo S, Staples JE, Millot V, Cibrelus L, Ramon-Pardo P, 2016. Epidemiology of Chikungunya in the Americas. J Infect Dis 214: S441–S445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guzmán-Terán C, Calderón-Rangel A, Rodriguez-Morales A, Mattar S, 2020. Venezuelan equine encephalitis virus: the problem is not over for tropical America. Ann Clin Microbiol Antimicrob 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Acosta-Ampudia Y, Monsalve DM, Rodríguez Y, Pacheco Y, Anaya J-M, Ramírez-Santana C, 2018. Mayaro: an emerging viral threat? Emerg Microbes Infect 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mota MT de O, Ribeiro MR, Vedovello D, Nogueira ML, 2015. Mayaro virus: a neglected arbovirus of the Americas. Future Virol 10: 1109–1122. [Google Scholar]
- 13. Romero-Alvarez D, Escobar LE, 2018. Oropouche fever, an emergent disease from the Americas. Microbes Infect 20: 135–146. [DOI] [PubMed] [Google Scholar]
- 14. Aguilar PV. et al. , 2004. Endemic Venezuelan equine encephalitis in northern Peru. Emerg Infect Dis 10: 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aguilar PV, Estrada-Franco JG, Navarro-Lopez R, Ferro C, Haddow AD, Weaver SC, 2011. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol 6: 721–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Groot H, 1972. The health and economic importance of Venezuelan equine encephalitis (VEE). Pan Am Health Org 243: 7–16. [Google Scholar]
- 17. Halsey ES, Siles C, Guevara C, Vilcarromero S, Jhonston EJ, Ramal C, Aguilar PV, Ampuero JS, 2013. Mayaro virus infection, Amazon Basin region, Peru, 2010–2013. Emerg Infect Dis 19: 1839–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azevedo RSS, Silva EVP, Carvalho VL, Rodrigues SG, Nunes-Neto JP, Monteiro H, Peixoto VS, Chiang JO, Nunes MRT, Vasconcelos PFC, 2009. Mayaro fever virus, Brazilian Amazon. Emerg Infect Dis 15: 1830–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baisley KJ, Watts DM, Munstermann LE, Wilson ML, 1998. Epidemiology of endemic Oropouche virus transmission in upper Amazonian Peru. Am J Trop Med Hyg 59: 710–716. [DOI] [PubMed] [Google Scholar]
- 20. Aguilar PV. et al. , 2011. Iquitos virus: a novel reassortant Orthobunyavirus associated with human illness in Peru. PLoS Negl Trop Dis 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ladner JT. et al. , 2014. Genomic and phylogenetic characterization of viruses included in the Manzanilla and Oropouche species complexes of the genus Orthobunyavirus, family Bunyaviridae. J Gen Virol 95: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgan J, Strode C, Salcedo-Sora JE, 2021. Climatic and socio-economic factors supporting the co-circulation of dengue, Zika and chikungunya in three different ecosystems in Colombia. PLoS Negl Trop Dis 15: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carabali M, Jaramillo-Ramirez GI, Rivera VA, Mina Possu N-J, Restrepo BN, Zinszer K, 2021. Assessing the reporting of Dengue, Chikungunya and Zika to the National Surveillance System in Colombia from 2014–2017: a capture–recapture analysis accounting for misclassification of arboviral diagnostics. PLoS Negl Trop Dis 15: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dirección Seccional de Salud y Protección Social de Antioquia , 2012. Enfermedades Transmitidas por Vectores. Gobernacion de Antioquia, Antioquia, Colombia.
- 25. Departamento Administrativo Nacional de Estadística , 2019. Resultados Censo Nacional de Población y Vivienda 2018. Popayán, Cauca. Available at: https://www.dane.gov.co. Accessed November 4, 2022.
- 26. Hontz RD. et al. , 2015. Itaya virus, a novel Orthobunyavirus associated with human febrile illness, Peru. Emerg Infect Dis 21: 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santiago FW, Halsey ES, Siles C, Vilcarromero S, Guevara C, Silvas JA, Ramal C, Ampuero JS, Aguilar PV, 2015. Long-term arthralgia after Mayaro virus infection correlates with sustained pro-inflammatory cytokine response. PLoS Negl Trop Dis 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aguilar PV. et al. , 2007. Endemic eastern equine encephalitis in the Amazon region of Peru. Am J Trop Med Hyg 76: 293–298. [PubMed] [Google Scholar]
- 29. Instituto Nacional de Salud , 2019. Informe de Evento Dengue, Colombia, 2019. Available at: https://www.ins.gov.co/buscador-eventos/Informesdeevento/DENGUE_2019.pdf. Accessed November 4, 2022.
- 30. Gutierrez-Barbosa H, Medina-Moreno S, Zapata JC, Chua JV, 2020. Dengue infections in Colombia: epidemiological trends of a hyperendemic country. Trop Med Infect Dis 5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morrison AC. et al. , 2008. Venezuelan equine encephalitis virus in Iquitos, Peru: urban transmission of a sylvatic strain. PLoS Negl Trop Dis 2: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aguilar PV. et al. , 2010. Guaroa virus infection among humans in Bolivia and Peru. Am J Trop Med Hyg 83: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Instituto Nacional de Salud , 2015. Chikungunya en Colombia, Año 2014: Informe Quincenal Epidemiologico Nacional 20. Available at: https://www.ins.gov.co/buscador-eventos/IQEN/IQEN%20vol%2020%202015%20num%205.pdf. Accessed November 4, 2022.
- 34. Instituto Nacional de Salud , 2016. Boletin Epidemiologico Semanal: Semana Epidemiológica Número 52 de 2016. Instituto Nacional de Salud Dirección de Vigilancia y Análisis del Riesgo en Salud Pública. Available at: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2016%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%2052%20-.pdf. Accessed November 4, 2022.
- 35. Groot H, Morales A, Vidales H, 1961. Virus isolations from forest mosquitoes in San Vicente de Chucuri, Colombia. Am J Trop Med Hyg 10: 397–402. [DOI] [PubMed] [Google Scholar]
- 36. Groot H, Kerr JA, Sanmartin C, Vidales H, 1959. Antibodies to yellow fever and other arthropod-borne viruses in human residents of San Vicente de Chucuri, Santander, Colombia. Am J Trop Med Hyg 8: 175–189. [DOI] [PubMed] [Google Scholar]
- 37. Roberto S, Ricardo S, Hidalgo M, Vesga JF, Castañeda E, Niño N, Valbuena G, Orejuela L, 2008. Las Rickettsias como Agentes Etiológicas de Entidades Febriles no Diagnosticadas en Colombia. Ediciones Uniandes, Bogota, Colombia. [Google Scholar]
- 38. Martins KA. et al. , 2019. Neutralizing antibodies from convalescent Chikungunya virus patients can cross-neutralize Mayaro and Una viruses. Am J Trop Med Hyg 100: 1541–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bopp NE, Jencks KJ, Siles C, Guevara C, Vilcarromero S, Fernández D, Halsey ES, Ampuero JS, Aguilar PV, 2021. Serological responses in patients infected with Mayaro virus and evaluation of cross-protective responses against Chikungunya virus. Am J Trop Med Hyg 106: 607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fischer C. et al. , 2020. Robustness of serologic investigations for Chikungunya and Mayaro viruses following coemergence. MSphere 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gómez-Camargo DE, Egurrola-Pedraza JA, Cruz CD, Popuche D, Ochoa-Díaz MM, Guevara C, Silva M, Abente EJ, Ampuero JS, 2021. Evidence of Oropouche Orthobunyavirus infection, Colombia, 2017. Emerg Infect Dis 27: 1756–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]