ABSTRACT.
Autochthonous leishmaniasis cases have been increasing continuously in Thailand over the years. We report multiple presentations of leishmaniasis in a 47-year-old patient with HIV/AIDS from Chiang Rai Province, northern Thailand. Physical examination showed multiple ulcerated papules, nodules, and plaques in a sporotrichoid distribution. Firm mucosal nodules on the hard palate and nasal opening, hepatosplenomegaly, and bilateral inguinal lymphadenopathy were found. Histopathological examination of the biopsies revealed an inflammatory infiltrate containing intramacrophage amastigotes compatible with Leishmania infection. In addition, Leishmania promastigotes were isolated successfully from the palatal biopsy and assigned the code MHOM/TH/2022/CULE6. Using internal transcribed spacer 1 polymerase chain reaction and sequence analysis, the causative parasite was identified as Leishmania martiniquensis. A definitive diagnosis of multiform leishmaniasis with disseminated cutaneous, mucocutaneous, and visceral involvement was established. The patient was administered intravenous amphotericin B 1 mg/kg/d for 2 weeks, followed by oral itraconazole 400 mg daily. At the 2-month follow-up, the cutaneous and mucosal lesions had improved significantly. To our knowledge, this is the first report of mucocutaneous involvement caused by L. martiniquensis in an immunocompromised patient with HIV/AIDS. In addition, we provide a literature review of leishmaniasis cases, reported formally in Thailand, resulting from this autochthonous parasite.
INTRODUCTION
Leishmaniasis is a neglected infectious disease with a varied clinical spectrum that can be categorized into cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis (VL).1,2 The variation of clinical presentation depends primarily on the infecting species and host immune status.3 Leishmania martiniquensis is an obligatory intramacrophage trypanosomatid that belongs to the newly identified subgenus Mundinia.4 This parasite has been reported previously in horses with CL in the United States and Central Europe, and in bovines in Switzerland.5–7 Since 1996, leishmaniasis cases in Thailand have been increasing continuously, mostly in northern and southern provinces, and most of these autochthonous cases were identified as L. martiniquensis.8 Autochthonous leishmaniasis caused by L. martiniquensis can exhibit various clinical manifestations, including localized cutaneous leishmaniasis, disseminated or diffuse cutaneous leishmaniasis (DCL), and VL.8–17 However, MCL has not yet been reported in leishmaniasis cases diagnosed with L. martiniquensis. We present the first confirmed case of a patient with HIV with multiform presentations of DCL, MCL, and VL caused by L. martiniquensis from northern Thailand. More importantly, we compile all clinical literature data on indigenous leishmaniasis resulting from this parasite in patients reported formally, which will help clinicians to diagnose accurately and provide more effective treatment of this emerging parasitic disease, especially in patients with HIV/AIDS.
CASE DESCRIPTION
A 47-year-old woman with HIV from Chiang Rai Province presented with multiple ulcerated plaques surrounded by a roll-edged border on both shins for 3 months. The lesions first appeared on her groins 5 years earlier and then spread progressively to her torso, extremities, and face. She denied a history of traveling abroad. Her current antiretroviral medications included tenofovir, nevirapine, and lamivudine.
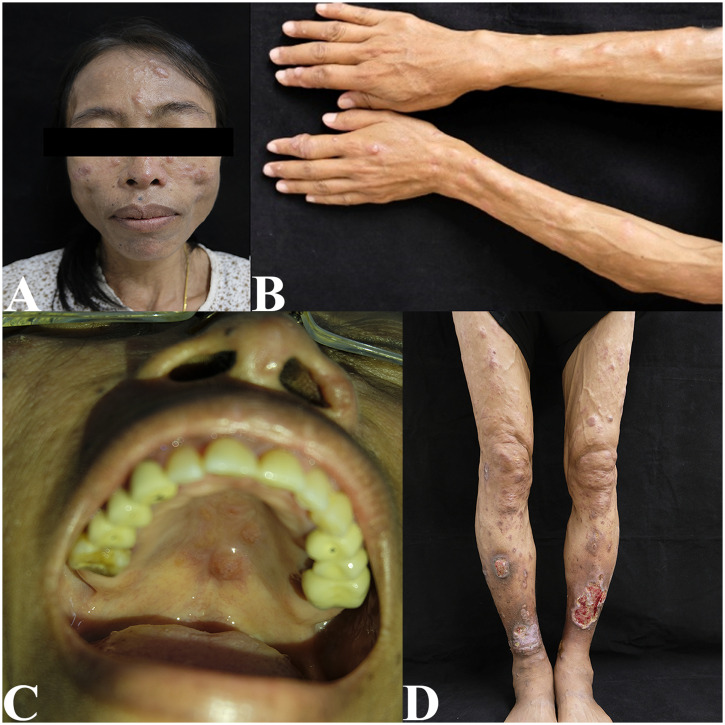
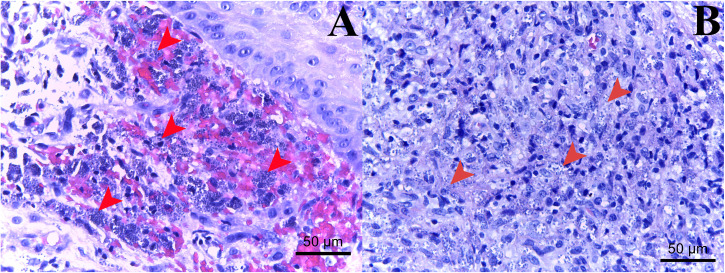
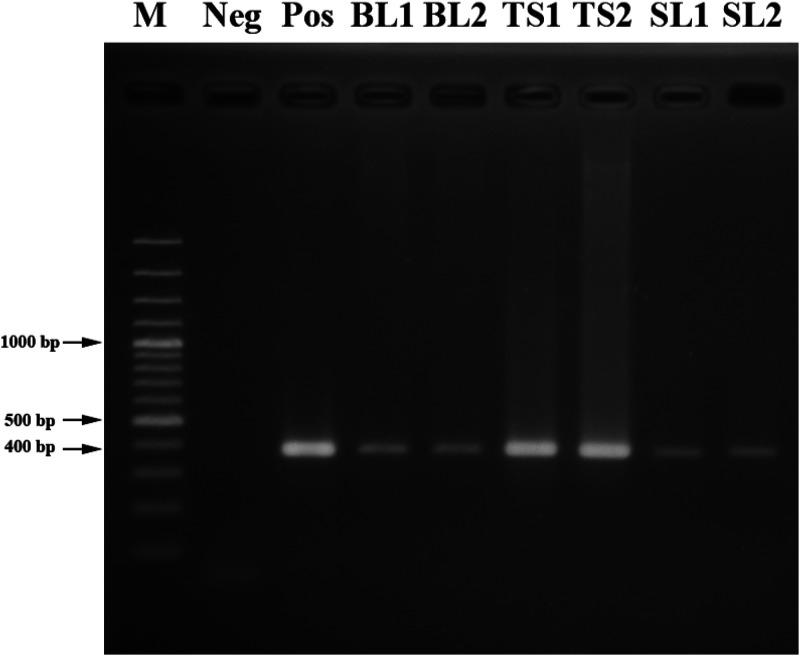
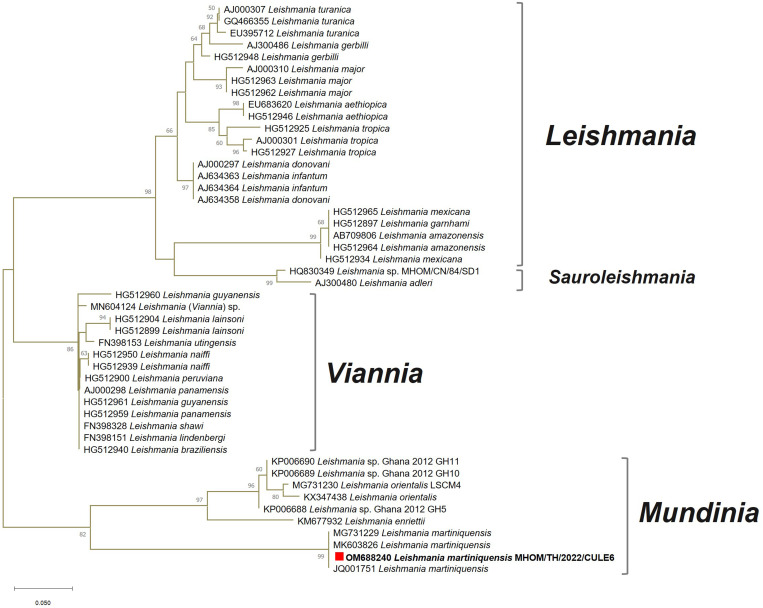
Physical examination showed multiple ulcerated papules, nodules, and plaques all over the face and body. The lesions on both the upper and lower extremities were arranged in a sporotrichoid pattern. Multiple firm nodules on the hard palate mucosa and nasal opening, hepatosplenomegaly, and bilateral lymphadenopathy on both sides of her groins were noted (Figure 1). Biopsies from the papulonodular lesion on her left leg, palatal nodule, and right inguinal lymph node revealed an inflammatory infiltrate consisting of lymphocytes and macrophages with numerous intracellular amastigotes, suggestive of leishmaniasis (Figure 2). Laboratory investigations revealed a CD4 count of 185 cells/mm3, a hemoglobin level of 10.9 g/dL, a white blood cell count of 2,700 cells/mm3 (neutrophils, 68.6%; lymphocytes, 22%; monocytes, 7.5%; eosinophils, 1.3%; and basophils, 0.6%), a platelet count of 100,000 platelets/mm3, and normal liver function test. Computed tomography of the whole abdomen also showed hepatosplenomegaly without focal lesions, and multiple subcutaneous nodules along the posterior back and bilateral groin nodes. Leishmania martiniquensis DNA was detected molecularly in her saliva, cutaneous nodular biopsy, and whole blood specimens using Leishmania-specific polymerase chain reaction (PCR) targeting the internal transcribed spacer 1 (ITS1) region, as described previously18 (Figure 3). Furthermore, Leishmania promastigotes were cultivated successfully from the palatal nodule specimens. Our culture isolate was given the WHO code MHOM/TH/2022/CULE6. Sanger sequencing and a nucleotide BLAST (BLASTn) search indicated that the retrieved ITS1 sequence was 100% identical to the L. martiniquensis reference in the GenBank. Phylogenetic analysis also reaffirmed that the obtained sequence clustered closely with other L. martiniquensis sequences available in the database (Figure 4). The identified sequence was submitted to GenBank under accession no. OM688240.
Figure 1.
(A) Multiple cutaneous nodules on the face. (B and D) Disseminated papules, nodules, and plaques were arranged on the extremities in a sporotrichoid manner. (C) Mucocutaneous nodules were also observed on the hard palate.
Figure 2.
Histopathological examination of the tissue biopsies from the cutaneous papulonodule (A) and the hard palate nodule (B) demonstrates a chronic lymphohistiocytic infiltrate of lymphocytes and heavily parasitized macrophages containing numerous Leishmania amastigotes (arrowheads).
Figure 3.
Agarose gel (1.5%) electrophoresis shows internal transcribed spacer 1 polymerase chain reaction products with a size of 379 bp, amplified from the clinical specimens (whole blood, nodule biopsy, and saliva) of this patient. BL1/2 = whole blood samples 1 and 2, respectively; M = 100-bp ladder marker; Neg = negative control; Pos = Leishmania martiniquensis positive control; (Pos); SL1/2 = saliva samples 1 and 2, respectively; TS1/2 = tissue biopsies 1 and 2 respectively.
Figure 4.
Phylogenetic analysis of enrolled Leishmania internal transcribed spacer 1 sequences from four Leishmania subgenera identified our clinical isolate as Leishmania martiniquensis in the Mundinia clade.
Therefore, we established a definitive diagnosis of autochthonous multiform leishmaniasis, including DCL, MCL, and VL, resulting from L. martiniquensis in our patient. She was treated with amphotericin B deoxycholate 1 mg/kg/d for the first 2 weeks, followed by 400 mg daily of oral itraconazole. The mucosal nodules resolved during the 2-month follow-up, and cutaneous patches and papulonodules improved significantly.
DISCUSSION
Leishmaniasis is a multispectral disease that can manifest clinical polymorphisms including CL, MCL, and VL, depending on the infecting species and host immune responses.1–3 In general, clinical manifestations of CL can vary from localized to disseminated or diffuse cutaneous papules, plaques, and nodules with central ulceration, typically surrounded by roll-edged borders. In addition, severe CL in the head region may progress into the so-called leonine face.1,2 Our patient presented with disseminated papulonodular lesions distributed linearly along the lymphatic system of the extremities, exhibiting a sporotrichoid pattern. This pathognomonic manifestation suggests lymphocutaneous involvement, most likely a result of various infectious diseases (e.g., atypical mycobacteria infection, sporotrichosis, nocardiosis, and leishmaniasis) and noninfectious causes (e.g., lymphoma and lymphocutaneous metastasis).19 Microscopic examination of the cutaneous lesion and groin node biopsies also revealed a chronic inflammatory infiltrate containing lymphocytes and heavily parasitized macrophages. In addition, pancytopenia, hepatosplenomegaly, and lymphadenopathy were found, consistent with VL.
More interestingly, this patient also presented with nodules on the hard palate mucosa and nasal opening (Figure 1C), prompting a clinical suspicion of MCL. MCL, or so-called espundia, is typically caused by members of the Viannia and Leishmania subgenera, including Leishmania (Viannia) braziliensis, Leishmania (Viannia) guyanensis, Leishmania (Viannia) panamensis, and Leishmania (Leishmania) amazonensis.20 The progression of mucocutaneous involvement usually depends on the host immune status and the infecting Leishmania species.20,21 This manifestation could result from either a direct extension or hematological spreading to the upper respiratory tract. If left untreated, the nasal septum can be perforated, resulting in tissue ulceration and deformation of the nasal bridge, also known as tapir nose.21 However, MCL in patients with HIV needs to be diagnosed differentially with other opportunistic infections, such as tuberculosis, syphilis, and paracoccidioidomycosis as well as HIV-associated cancers such as Kaposi sarcoma.20–22 In our patient, a palatal nodule was also sampled for histopathological examination and showed a lymphohistiocytic infiltrate with intramacrophage amastigotes. In addition, Leishmania promastigotes could be isolated successfully from the palatal biopsy, confirming MCL. Therefore, our patient is the first case of leishmaniasis with mucocutaneous involvement that has ever been reported in Thailand.
In our patient, histopathological examination, promastigote isolation, Leishmania ITS1-specific PCR, and DNA sequencing helped ensure the accurate diagnosis of autochthonous multiform leishmaniasis. Positive ITS1 amplification was shown in all clinical samples, including whole blood, tissue biopsy, and saliva. Based on the BLASTn result, L. martiniquensis was diagnosed. Consistently, our retrieved sequence was clustered phylogenetically with other L. martiniquensis ITS1 sequences derived from the cutaneous lesion of a patient with HIV and DCL from Lamphun Province, northern Thailand (accession no. MG731229); the bone marrow of a patient with HIV and VL (accession no. JQ001751); and Sergentomyia khawi sandfly (accession no. MK603826) from Songkhla Province, southern Thailand with very high bootstrap support.12,16,23 Also, the ITS1 phylogenetic analysis revealed the identification of L. martiniquensis, including our isolate, as a member of the same Mundinia clade as L. enriettii, L. orientalis, and Leishmania sp. Ghana.
As reviewed in Table 1, most of the autochthonous leishmaniasis cases infected by L. martiniquensis in the country are people with HIV who have a CD4 count of < 200 cells/mm3, indicative of WHO clinical stage 4 of HIV infection or AIDS.24 It was noted that VL was common in all reported patients, whereas combined DCL and VL were diagnosed exclusively in most HIV-infected cases. In addition, DCL has been reported in an HIV-seronegative Burmese male who was treated with prednisolone.14 Thus, this indicates that a severe form of CL caused by L. martiniquensis is mainly associated with immunosuppression, especially resulting from HIV infection and steroid therapy. Previous studies24,25 claimed that Leishmania can induce HIV replication, consequently accelerating the AIDS status. In addition, stimulation of the T-helper type 1 (Th1) immune response, which is the main protective mechanism against leishmaniasis, was found to decrease significantly in patients with coinfection of HIV and VL.26 HIV infection also induces the T-helper type 2 (Th2) response that provokes the disease progression of leishmaniasis.27 Therefore, suppressed Th1 and activated Th2 immune responses in patients coinfected with HIV and Leishmania can affect the progression of disease severity and cause a high relapse rate.24–28 In our patient, we also speculated that atypical disease progression to MCL was most likely a result of her severely immunodeficient status. Furthermore, nephritis & nephrotic syndrome has been reported10 previously as a complication in a male patient with VL coinfected with HIV and L. martiniquensis from Chanthaburi Province, eastern Thailand.
Table 1.
Review of clinical data on indigenous leishmaniasis resulting from Leishmania martiniquensis formally recorded in Thailand from 2006 to date
| Year, province, part of the country | Age, gender | HIV status, CD4 count | Form of leishmaniasis | Clinical manifestations (duration) | First-line treatment | Relapse and retreatment |
|---|---|---|---|---|---|---|
| 2006, Phang Nga, South9 | 55, male | Negative | VL | Intermittent fever, anemia, weight loss, epistaxis, gum bleeding, hepatosplenomegaly, and pancytopenia (3 years) | Intravenous amphotericin B (100 mg daily) for 2 weeks | Relapse of VL after 2 months (retreatment information not available) |
| 2009, Chanthaburi, East10 | 37, male | Positive, 129 cells/mm3 | VL | Prolonged fever, hepatomegaly, anemia, thrombocytopenia, and nephritis & nephrotic syndrome (8 weeks) | Intravenous amphotericin B (2 mg/kg/d on alternate days) for 2 weeks followed by oral itraconazole (400 mg daily) | No relapse at 3-month follow-up |
| 2010, Stun, South11 | 7, female | Negative | VL | Hepatosplenomegaly, anemia, and thrombocytopenia (2 years) | Intravenous amphotericin B (1 mg/kg/d) for 3 weeks | Relapse of VL twice at 3 and 6 months; cured by amphotericin B (1 mg/kg/d) for 5 weeks with monthly prophylaxis 5 consecutive days for 6 months |
| 2011, Songkhla, South12,13,17 | 46, male | Positive, 175 cells/mm3 (2011); positive, 207 cells/mm3 (2014) | LCL, VL | A single, punched-out 3 × 3 cm ulcer on the left knee; left groin lymphadenopathy; and anemia (a few months before first admission) Hepatosplenomegaly and thrombocytopenia (4 weeks after receiving a high dose of steroids during the first admission) | Intravenous amphotericin B (1 mg/kg/d) for 2 weeks followed by oral itraconazole (400 mg daily) | First relapse after 2 months of itraconazole and retreated with intravenous amphotericin B (3 mg/kg/d) for 3 weeks followed by oral itraconazole (400 mg daily) Second relapse of multiple cutaneous nodules in 2014 and retreated with intravenous amphotericin B (1 mg/kg/d) for 4 weeks followed by oral itraconazole (300 mg daily) twice a day for 5 months |
| 2011, Trang, South12,17 | 30, male | Positive, 111 cells/mm3 (2011); positive, 110 cells/mm3 (2013) | DCL, VL | Multiple papules and plaques with ulcers at the trunk and lower extremities (4 years) Anemia, thrombocytopenia, and hepatosplenomegaly (1 month before admission) | Intravenous amphotericin B (1 mg/kg/d) for 2 weeks followed by oral itraconazole (400 mg daily) | No relapse at 3-month follow-up, but relapse of DCL with multiple papules and ulcers 2 years later (retreatment information not available) |
| 2012, Lamphun, North15 | 52, male | Negative | VL | Subacute fever, weight loss, splenomegaly, and pancytopenia (2 weeks) | Intravenous amphotericin B (1 mg/kg/d) for 3 weeks | Resolved |
| 2013, Chiang Mai, North16 | 48, male | Positive, 121 cells/mm3 | DCL, VL | Multiple, firm cutaneous nodules; hepatosplenomegaly; and pancytopenia (4 years) | Intravenous amphotericin B (1 mg/kg/d) for 20 days followed by oral itraconazole (400 mg daily) | Suspected relapse with multiple papules in December 2014 and continued long-term oral itraconazole |
| 2013, Lamphun, North16 | 38, male | Positive, 543 cells/mm3 | DCL, VL | Multiple hypopigmented papules and nodules, and pancytopenia (4 years) | Intravenous amphotericin B (1 mg/kg/d) for 2 weeks followed by oral itraconazole (200 mg daily) | Resolved |
| 2021, Chiang Rai, North (our patient) | 47, female | Positive, 185 cells/mm3 | DCL, MCL, VL | Multiple ulcerated papules, nodules, and plaques; firm mucosal nodules; lymphadenopathy; hepatosplenomegaly; and pancytopenia (5 years) | Intravenous amphotericin B (1 mg/kg/d) for 2 weeks followed by oral itraconazole (400 mg daily) | Improved significantly at 2-month follow-up |
DCL = disseminated or diffuse cutaneous leishmaniasis; LCL = localized cutaneous leishmaniasis; MCL = mucocutaneous leishmaniasis; VL = visceral leishmaniasis.
Amphotericin B has been considered the drug of choice for the treatment of VL in Thailand. This antileishmanial drug is highly effective, with a high cure rate in immunocompetent patients.29 However, a high incidence of relapses of VL and DCL after treatment with amphotericin B has been documented, especially in patients with HIV/AIDS.30 According to our literature review (Table 1), some indigenous cases in Thailand had an early relapse shortly within the first 6 months after first-line antileishmanial therapy, and the occurrence of relapse could last up to 3 years.9,11–13,16,17 As recorded, an extended course of amphotericin B with 6 consecutive months of prophylaxis was administered to a 7-year-old girl who was seronegative, with two episodes of relapse before a definitive cure was obtained.11 In addition, reduced efficacy of amphotericin B against L. martiniquensis CU1R1 isolated from a patient who experienced a relapse from Songkhla Province was reported,31 suggesting that clinicians might need to increase the dosage of chemotherapy, extend the duration of treatment, and monitor closely those patients who relapse. Hence, a combination of parasite virulence, host immune status, and drug resistance could affect treatment outcomes.31
Because of the continuous increase of autochthonous cases in Thailand, this situation has raised the question of which insects are the primary vectors responsible for disease transmission in the country. Phlebotomine sandflies have been widely accepted as the natural vectors of Leishmania parasites in the subgenera Viannia, Leishmania, and Sauroleishmania.32,33 However, the principal vectors and reservoirs of Leishmania species in the subgenus Mundinia remain unclear.34,35 In Thailand, S. khawi, Sergentomyia barraudi, Sergentomyia iyengari, and Phlebotomus stantoni have been previously proposed as possible vectors of L. martiniquensis.23,34,36,37 Furthermore, Culicoides mahasarakhamense biting midges collected from the CL transmission area in Lamphun Province recently showed positive for L. martiniquensis DNA, supporting a possible role of a leishmaniasis vector in northern Thailand.38 In addition, L. martiniquensis DNA was detected in the liver tissues of the black rat (Rattus rattus) from Songkhla Province, and the buffy coat of the black rat from Chiang Rai, implicating it as a natural reservoir in Thailand.34,37 Ultimately, molecular characterization of virulence in L. martiniquensis clinical isolates needs to be elucidated further to help us better understand this emerging parasite for developing effective treatment, prevention, and control of this parasitic transmission.
ACKNOWLEDGMENTS
We acknowledge the staff members of the Division of Dermatology, Department of Medicine, and Center of Excellence in Vector Biology and Vector-Borne Disease, Department of Parasitology, Faculty of Medicine, Chulalongkorn University for their assistance in histopathological, parasitological, and molecular investigations. We also gratefully give thanks to our patient for her contribution to this study.
REFERENCES
- 1. Pearson RD, Sousa AQ, 1996. Clinical spectrum of leishmaniasis. Clin Infect Dis 22: 1–13. [DOI] [PubMed] [Google Scholar]
- 2. McGwire BS, Satoskar AR, 2014. Leishmaniasis: clinical syndromes and treatment. QJM 107: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burza S, Croft SL, Boelaert M, 2018. Leishmaniasis. Lancet 392: 951–970. [DOI] [PubMed] [Google Scholar]
- 4. Espinosa OA, Serrano MG, Camargo EP, Teixeira MMG, Shaw JJ, 2016. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology 145: 430–442. [DOI] [PubMed] [Google Scholar]
- 5. Müller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, Hilbe M, Gottstein B, Frey CF, Geyer C, von Bomhard W, 2009. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet Parasitol 166: 346–351. [DOI] [PubMed] [Google Scholar]
- 6. Reuss SM, Dunbar MD, Calderwood Mays MB, Owen JL, Mallicote MF, Archer LL, Wellehan JF, Jr, 2012. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg Infect Dis 18: 1545–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lobsiger L, Müller N, Schweizer T, Frey CF, Wiederkehr D, Zumkehr B, Gottstein B, 2010. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol 169: 408–414. [DOI] [PubMed] [Google Scholar]
- 8. Leelayoova S, Siripattanapipong S, Manomat J, Piyaraj P, Tan-Ariya P, Bualert L, Mungthin M, 2017. Leishmaniasis in Thailand: a review of causative agents and situations. Am J Trop Med Hyg 96: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sukmee T. et al. , 2008. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol 38: 617–622. [DOI] [PubMed] [Google Scholar]
- 10. Suankratay C, Suwanpimolkul G, Wilde H, Siriyasatien P, 2010. Autochthonous visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient: the first in Thailand and review of the literature. Am J Trop Med Hyg 82: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osatakul S, Mungthin M, Siripattanapipong S, Hitakarun A, Kositnitikul R, Naaglor T, Leelayoova S, 2014. Recurrences of visceral leishmaniasis caused by Leishmania siamensis after treatment with amphotericin B in a seronegative child. Am J Trop Med Hyg 90: 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chusri S, Hortiwakul T, Silpapojakul K, Siriyasatien P, 2012. Consecutive cutaneous and visceral leishmaniasis manifestations involving a novel Leishmania species in two HIV patients in Thailand. Am J Trop Med Hyg 87: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phumee A, Chusri S, Kraivichian K, Wititsuwannakul J, Hortiwakul T, Thavara U, Silpapojakul K, Siriyasatien P, 2014. Multiple cutaneous nodules in an HIV-infected patient. PLoS Negl Trop Dis 8: e3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noppakun N, Kraivichian K, Siriyasatien P, 2014. Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am J Trop Med Hyg 91: 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, Supparatpinyo K, Bates MD, Kwakye-Nuako G, Bates PA, 2014. First isolation of Leishmania from northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl Trop Dis 8: e3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiewchanvit S, Tovanabutra N, Jariyapan N, Bates MD, Mahanupab P, Chuamanochan M, Tantiworawit A, Bates PA, 2015. Chronic generalized fibrotic skin lesions from disseminated leishmaniasis caused by Leishmania martiniquensis in two patients from northern Thailand infected with HIV. Br J Dermatol 173: 663–670. [DOI] [PubMed] [Google Scholar]
- 17. Siriyasatien P, Chusri S, Kraivichian K, Jariyapan N, Hortiwakul T, Silpapojakul K, Pym AM, Phumee A, 2016. Early detection of novel Leishmania species DNA in the saliva of two HIV-infected patients. BMC Infect Dis 16: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spanakos G, Piperaki ET, Menounos PG, Tegoa N, Flemetakis A, Vakalis NC, 2008. Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans R Soc Trop Med Hyg 102: 46–53. [DOI] [PubMed] [Google Scholar]
- 19. Tobin EH, Jih WW, 2001. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician 63: 326–332. [PubMed] [Google Scholar]
- 20. Abadías-Granado I, Diago A, Cerro PA, Palma-Ruiz AM, Gilaberte Y, 2021. Cutaneous and mucocutaneous leishmaniasis. Acta Dermosifiliogr 112: 601–618. [DOI] [PubMed] [Google Scholar]
- 21. Miranda Lessa M, Andrade Lessa H, Castro TWN, Oliveira A, Scherifer A, Machado P, Carvalho EM, 2007. Mucosal leishmaniasis: epidemiological and clinical aspects. Rev Bras Otorrinolaringol (Engl Ed) 73: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchez IM, DiTommaso LE, Tsoukas MM, 2019. Oral Kaposi sarcoma. JAMA Dermatol 155: 370. [DOI] [PubMed] [Google Scholar]
- 23. Srisuton P, Phumee A, Sunantaraporn S, Boonserm R, Sor-Suwan S, Brownell N, Pengsakul T, Siriyasatien P, 2019. Detection of Leishmania and Trypanosoma DNA in field-caught sand flies from endemic and non-endemic areas of leishmaniasis in southern Thailand. Insects 10: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J, 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21: 334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garg R, Barat C, Ouellet M, Lodge R, Tremblay MJ, 2009. Leishmania infantum amastigotes enhance HIV-1 production in cocultures of human dendritic cells and CD4 T cells by inducing secretion of IL-6 and TNF-alpha. PLoS Negl Trop Dis 3: e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. d’Ettorre G, Ceccarelli G, Carnevalini M, Forcina G, Zaffiri L, Massetti AP, Mastroianni CM, Vullo V, 2006. Central role of interleukin-15 in human immunodeficiency virus (HIV)-infected patients with visceral leishmaniasis. Acta Trop 99: 83–87. [DOI] [PubMed] [Google Scholar]
- 27. Clerici M, Shearer GM, 1993. A TH1 → TH2 switch is a critical step in the etiology of HIV infection. Immunol Today 14: 107–111. [DOI] [PubMed] [Google Scholar]
- 28. Monge-Maillo B, López-Vélez R, 2016. Treatment options for visceral leishmaniasis and HIV coinfection. AIDS Rev 18: 32–43. [PubMed] [Google Scholar]
- 29. Paila YD, Bhaskar Saha B, Chattopadhyay A, 2010. Amphotericin B inhibits entry of Leishmania donovani into primary macrophages. Biochem Biophys Res Commun 399: 429–433. [DOI] [PubMed] [Google Scholar]
- 30. Grabmeier-Pfistershammer K, Poeppl W, Brunner PM, Rappersberger K, Rieger A, 2012. Clinical challenges in the management of Leishmania/HIV coinfection in a nonendemic area: a case report. Case Rep Infect Dis 2012: 787305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phumee A, Jariyapan N, Chusri S, Hortiwakul T, Mouri O, Gay F, Limpanasithikul W, Siriyasatien P, 2020. Determination of anti-leishmanial drugs efficacy against Leishmania martiniquensis using a colorimetric assay. Parasite Epidemiol Control 9: e00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D, 2016. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis 10: e0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maroli M, Gramiccia M, Gradoni L, Ready PD, Smith DF, Aquino C, 1988. Natural infections of phlebotomine sandflies with Trypanosomatidae in central and south Italy. Trans R Soc Trop Med Hyg 82: 227–228. [DOI] [PubMed] [Google Scholar]
- 34. Chusri S, Thammapalo S, Chusri S, Thammapalo S, Silpapojakul K, Siriyasatien P, 2014. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J Trop Med Public Health 45: 13–19. [PubMed] [Google Scholar]
- 35. Toontong P, Sunantaraporn S, Tiawsirisup S, Pengsakul T, Boonserm R, Phumee A, Siriyasatien P, Preativatanyou K, 2022. First report of anuran Trypanosoma DNA in flat-tailed house geckos (Reptilia: Gekkonidae) collected from southern Thailand: no evidence as a reservoir for human trypanosomatids. Pathogens 11: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siripattanapipong S, Leelayoova S, Ninsaeng U, Mungthin M, 2018. Detection of DNA of Leishmania siamensis in Sergentomyia (Neophlebotomus) iyengari (Diptera: Psychodidae) and molecular identification of blood meals of sand flies in an affected area, southern Thailand. J Med Entomol 55: 1277–1283. [DOI] [PubMed] [Google Scholar]
- 37. Sriwongpan P. et al. , 2021. Prevalence and associated risk factors of Leishmania infection among immunocompetent hosts: a community-based study in Chiang Rai, Thailand. PLoS Negl Trop Dis 15: e0009545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sunantaraporn S, Thepparat A, Phumee A, Sor-Suwan S, Boonserm R, Bellis G, Siriyasatien P, 2021. Culicoides Latreille (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Negl Trop Dis 15: e0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]