ABSTRACT.
Dengue virus (DENV) reemerged in the Americas in the 1980s and 1990s, whereas chikungunya virus (CHIKV) emerged in 2014. Although CHIKV produced large epidemics from 2014 to 2017, dengue fever has been the prominent arboviral disease identified through passive surveillance, bringing to question the degree to which cases are misdiagnosed. To address this concern, we conducted an active household-based surveillance of arboviral-like illnesses in six rural and remote communities in northern coastal Ecuador from May 2019 to February 2020. Although passive surveillance conducted by the Ecuadorian Ministry of Health reported only DENV cases in the region, more than 70% of the arbovirus-like illnesses detected by active surveillance in our study were positive for CHIKV. These findings underline the need for active surveillance of arboviral infections with laboratory confirmation, especially in rural communities where arboviral illnesses are more likely to be underreported.
INTRODUCTION
Dengue virus (DENV), genus Flavivirus, family Flaviviridae, and chikungunya virus (CHIKV), genus Alphavirus, family Togaviridae, share many traits. Both viruses are transmitted to humans by Aedes aegypti and Aedes albopictus mosquitoes. Both have single-stranded positive RNA genomes. DENV has four distinct serotypes (DENV1–4), whereas CHIKV has one serotype with three genotypes known as West African, East/Central/South African (ECSA), and Asian.1,2
Dengue fever (DF) and chikungunya fever (CHIKF) share similar symptoms, including fever, myalgia, arthralgia, and rash. Most DF cases are self-limited but can progress to dengue with warning signs or severe disease, characterized by vascular leakage leading to shock, fluid accumulation, severe bleeding, and organ involvement.3,4 Likewise, most CHIKF cases are self-limited.5 In rare cases, musculoskeletal symptoms can cause a chronic polyarthritis, which may lead to severe polyarthralgia/polyarthritis.5,6 Differential diagnosis of DF and CHIKF, therefore, has been historically difficult. Given that there is less clinical awareness of CHIKF, and passive surveillance relies on symptoms due to the limited capacity for laboratory testing, underreporting CHIKF by passive surveillance is likely higher than for DF.7
Both DF and CHIKF are ancient diseases that have reemerged multiple times throughout history.8 DF case counts and geographic spread have increased 8-fold over the past 2 decades,3 whereas CHIKV’s global reach has increased since the early 2000s. An estimated 105 million dengue infections and 51 million dengue fever cases per year have been recorded in 128 countries,9 whereas an estimated 10 million Chikungunya cases were reported worldwide from 2005 to 2017.10
In the Americas, DF was introduced in the early 1980s, with a total of 2,733,635 cases of dengue reported in 201911; whereas CHIKF was introduced in the Americas in 2013, with more than 2.1 million cases reported.12 In Ecuador, dengue virus type I (DENV-1) emerged in 1988, followed by DENV-2 in 1990, DENV-4 in 1993, and DENV-3 10 years later. The four serotypes are now endemic, producing yearly outbreaks.13 The first CHIKF case was detected in Ecuador in 2014. By 2015, 33,619 cases were reported,14 and by 2019 CHIKF had subsided with only 2 cases reported nationwide.15 An entomological study suggested the circulation of CHIKV Asian lineage in Ecuador.16
The northwest Pacific Coast of Ecuador (Esmeraldas province) is one of the poorest regions in the country. In the 1980s, rapid deforestation, accompanied by the construction of a highway connecting previously remote communities to the coast and the Andes in the early 2000s, resulted in rapid socioecological change in the area.17 After this transition, the province underwent an etiologic transition in febrile diseases from malaria to arboviral infections in the 1990s, with periodic dengue outbreaks that were largely underreported.18 CHIKV was first reported in Esmeraldas in 2014. From 2014 to 2018, the Ecuadorian Ministry of Health (MOH) reported 10,791 CHIKF cases and 2,913 dengue cases. In 2019, the MOH reported 1,750 dengue cases and no chikungunya cases.15
Suspected arbovirus cases require mandatory notification to the Ecuadorian MOH, the national surveillance system of which includes passive surveillance of cases from state-run clinics and hospitals. Only a subset of suspected cases is confirmed for DENV infection using IgM antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA) or NS1 antigen ELISAs in health posts or and hospital diagnostic laboratories; a smaller subset of cases is sent to the national reference laboratory (the National Institute for Public Health Research) to be confirmed by reverse transcription polymerase chain reaction (RT-PCR) for etiology. In the case of malaria, the MOH performs microscopy for diagnosis.
Only a fraction of DENV cases is tested by the national system, and thus many cases are misclassified or misdiagnosed. Thus, it is necessary to integrate strategies involving surveillance and differential diagnosis supported by serological and virological laboratory testing of arboviral diseases at national, regional, and local levels. Here we use confirmation by real-time RT-PCR to describe a CHIKV outbreak in 2019. This outbreak was identified through an active household-based surveillance study of arbovirus-like illness in a semiurban town (Borbón) and five rural and remote communities in northern coastal Ecuador.
MATERIALS AND METHODS
Study site.
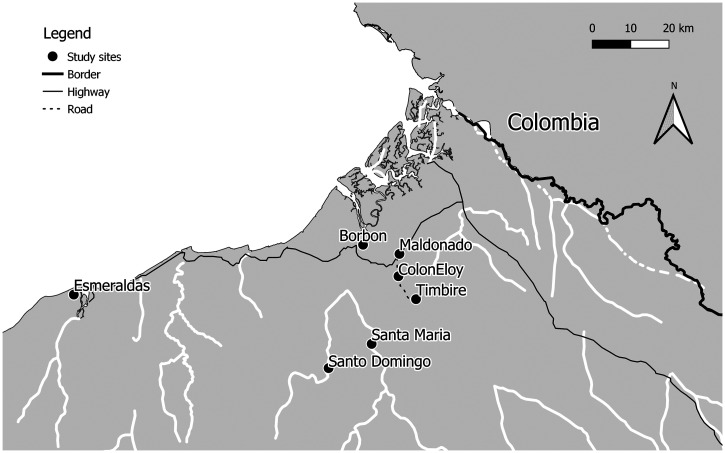
Located in the northwestern coastal Ecuadorian province of Esmeraldas, in Eloy Alfaro County, our study communities—Borbón, Colón Eloy, Maldonado, Santa María, Santo Domingo, and Timbiré—lie along three rivers that flow toward the Pacific Ocean (Figure 1). The communities were selected to represent different levels of remoteness as defined by our previously published remoteness metric,17 which we have been shown to be associated with regional-scale patterns of human movement,19 community cohesion, and different antimicrobial resistance and enteric infection risk.17,20 The population in our study site primarily self-identifies as Afro-Ecuadorian, Indigenous Chachi, and mestizo; the majority group is Afro-Ecuadorian.21
Figure 1.
Map of the study area. The towns of Borbón, Maldonado, Colon Eloy, Timbire, Santa Maria, and Santo Domingo are located approximately 100 kilometers north of the provincial capital of Esmeraldas.
Study design and population.
We developed an active household-based surveillance system for arboviral-like illness within our six study communities. This surveillance includes an annual census of each study community to generate a record of the age, sex, occupation, and other key demographic and socioeconomic characteristics of individuals living in each household. Cases were identified through weekly household visits during the rainy season (June–October) when there is a higher risk for dengue virus transmission. During the low-risk dry season (November–December), household visits decreased to every 2 weeks. A trained study team of community residents, called brigadistas, began household-based fever surveillance in May 2019. For this analysis, we include household surveillance data collected from May 2019 until the end of February 2020, at which time household visits were shifted to phone-based surveillance due to the COVID-19 pandemic and movement restrictions.
The brigadistas visit involved a symptom survey administrated to the household key informant, asking whether there were any cases of fever, red eyes, or rash in the home. When a positive case was identified, the brigadista notified a study nurse who visited the household within 24 hours to complete a follow-up survey with the symptomatic participant to determine whether the participant’s symptoms were consistent with an arbovirus-like illness. Participants were excluded from further follow-up if the nurse considered their symptoms to be associated with an upper respiratory or gastrointestinal infection (on the basis of the presence of diarrhea, bloody stool, cough, or nasal congestion and confirmed by the nurse’s clinical judgment). For those with an arbovirus-like illness, a serum sample was collected, and an aliquot was used for onsite testing with Dengue DUO NS1 IgG/IgM rapid test (Standard Diagnostic Inc., Suwon, Korea; data not shown). Another aliquot was frozen in liquid nitrogen and transported for further testing at the Institute of Microbiology at Universidad San Francisco de Quito (USFQ).
The study was approved by the Bioethics committee of the Universidad San Francisco de Quito, the University of Michigan, and the Ecuadorian Ministry of Health, Ecuador.
RNA extraction, PCR detection, and metagenomic analysis.
Viral RNA was extracted from a total of 182 febrile serum samples using the Qiagen viral RNA mini kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany) and was tested using a triplex real time RT-PCR Zika-Dengue-Chikungunya (ZDC) assay to confirm infection and etiology.22 Samples positive for dengue were next analyzed for their serotype using a conventional multiplex RT-PCR following the Harris et al. (1998) protocol.23 Primers are shown in Supplemental Table 1. An additional screening for Oropouche,24 Mayaro,25 and Leptospira spp.26 was conducted for samples that were negative for dengue, Zika, and chikungunya. Primers and probes for each pathogen are shown in Supplemental Table 1.
PCR detection for CHIKV.
A conventional RT-PCR protocol for Chikungunya confirmation was developed by our team for a subset of positive samples, primers are shown in Supplemental Table 1, using the kit Superscript III one step RT-PCR system. The reaction mix contained: 12.5 μL 2× reaction mix, 1-μL primers CHIKV F 10011, CHIKV R 10396 at a concentration of 10 µM, 80 U SuperScript III Taq Platinum mix (Invitrogen, Waltham, MA), and 5 μL of Rnase-free water. The amplification protocol was used as follows: 50°C for 30 minutes, 95°C for 15 minutes, 40 cycles of 94°C for 1 minute, 52°C for 1 minute, 72°C for 1 minute, and a final extension of 72°C for 10 minutes. For additional confirmation, the amplicons were sent for sequencing by Sanger.
We conducted a metagenomic analysis for two randomly selected febrile samples that were negative for DENV, CHIKV, and ZIKV using the cDNA preparation protocol from Public Health England Genomics laboratory (RNA viral metagenomics MINION one-pot)27 and the Oxford Nanopore Technologies Ligation kit protocol (SQK-LSK109; Oxford Nanopore Technologies, Oxford, United Kingdom) and Native barcoding kit (NB-114) following manufacturer’s instructions for library preparation. This library was sequenced using MinKNOW version 4.05 for 24 hours. Base calling was performed using Guppy v. 3.4.5. Porechop v. 0.2.4 was performed for demultiplexing and to remove adapters and barcodes. The taxonomic profile of the sequences of each sample was classified with the Kaiju web server platform.28 The database “NCBI Blast nr + euk” was selected (includes bacteria, archaea, fungi, viruses, and eukaryotes), the running method used was the MEM algorithm (maximum exact matches), and the rest of the parameters were kept by default. For the analysis of the taxonomic profiles, the number of assigned readings and the generated Krona charts were considered.
DNA extraction and PCR detection for Plasmodium.
DNA was extracted from negative serum samples using the kit pure link genomic DNA following the manufacturer’s instructions (Life Technologies, Carlsbad, CA). These samples were tested with a nested PCR for Plasmodium falciparum detection following the Snounou (1996) protocol.29 Primers are shown in Supplemental Table 1.
Passive surveillance.
The Ecuadorian MOH reports dengue cases using a standardized case report form. The MOH provided us with the case report on all dengue cases reported from January 2019 to the end of February 2020 including data on cases living in the six communities in our study. For each case, the MOH also reported demographic and symptoms data.
Statistical analysis.
Outcome variables.
Active surveillance samples were considered positive for DENV or CHIKV if they were positive by qPCR. Our laboratory also uses other diagnostic methods to define a dengue case DENV (e.g., NS1 and IgM); however, for this analysis, these results were not included. Moreover, a coinfection was defined as two pathogens detected with the triplex real time RT-PCR ZDC in the same serum sample.
Comparing DF and CHIKF clinical symptoms.
To analyze differences in the prevalence of reported symptoms for CHIKF versus DF, we performed two-sided t tests, excluding samples coinfected with DENV and CHIKV. Differences at the P < 0.05 were considered statistically significant. We used radar plots to visualize differences in symptom prevalence between dengue with and without warning signs and between DENV and CHIKV cases. We included those symptoms that were present in at least 5% of the cases.
Risk factor analysis.
To compare characteristics of individuals with a symptomatic DENV or CHIKV infection to the study population overall, we calculated the incidence of each virus (symptomatic cases per 10,000 person-years). We then developed bivariate and multivariate logistic models to test the association between common arboviral risk factors and symptomatic dengue and chikungunya. The risk factors we considered were age, sex, ethnicity, community of residence, occupation, and whether the individual had ever lived outside of their current community.
Comparison of active and passive surveillance.
On the basis of reported symptoms, dengue cases from the MOH passive surveillance were divided into dengue with and without warning signs. We compared the number of episodes of dengue and chikungunya reported by passive and active surveillance. We also compared the prevalence of symptoms reported in our study active surveillance and the MOH passive surveillance. For a subset of individuals whose case episode was captured by both active and passive surveillance, we compared laboratory results between the two systems. All analyses were conducted in R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) or Stata version 16.1 (Stata Corp., LLC, College Station, TX).
RESULTS
DENV and CHIKV cases were detected in all six rural communities.
From May 2019 to the end of February 2020, the active surveillance study collected 182 serum samples from 172 unique individuals with acute arbovirus-like illness; 10 individuals had two illness episodes (mean = 129 days apart; range = 32–229 days). A distinct episode was considered when the individual presented fever separated by at least 15 fever-free days. The blood draw was completed, on average, 3 days after symptoms reportedly began (mean = 3.2, SD = 2.2).
Laboratory testing was completed for 174 of 182 episodes. More than 80% of these episodes were diagnosed with a CHIKV, DENV, or CHIKV-DENV coinfection (145 of 174). More than half of these 145 pathogen-positive episodes (61%) were diagnosed with a CHIKV infection only (N = 88), 30% with DENV infection only (N = 42), and 10% with a coinfection (N = 15). The conventional RT-PCR for CHIKV performed in 11 randomly selected positive samples and the amplicon sequences, confirmed the presence of CHIKV (Accession IDs: ON959484–ON959494). All DENV infections were identified as the DENV-1 serotype.
Of the 29 samples that were negative for DENV and CHIKV, 10 samples (all from 2019) were screened for additional viral pathogens by qPCR: these were found to be negative for ZIKV, Oropouche, Mayaro, and Leptospira spp. Two of these negative samples were randomly selected and subjected to metagenomic analysis and showed Plasmodium spp nucleotide sequences (Accession ID: SAMN27568483, SAMN27568586); nested PCR confirmed the presence of Plasmodium falciparum in these samples.
Symptom analysis.
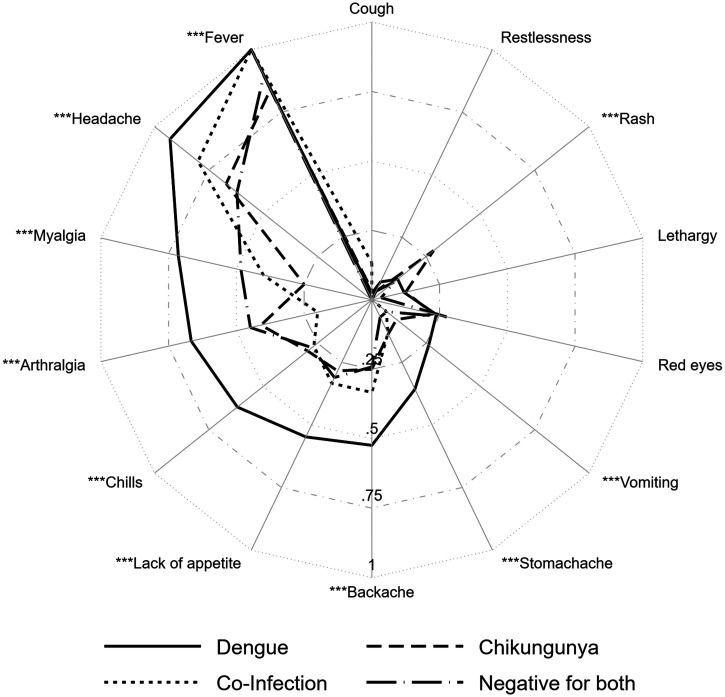
Cases positive for DENV reported more severe illness than cases positive for CHIKV; symptoms that were more frequent in DENV than CHIKV included fever, headache, myalgia, arthralgia, chills, anorexia, backache, stomachache, and vomiting. In contrast, CHIKV cases showed more frequently rash than DENV cases (28.4% versus 11.9%) (Figure 2). There were no statistically significant differences in the prevalence of reported symptoms by patient age for either illness (Supplemental Figures 1 and 2).
Figure 2.
Prevalence of reported symptoms from DENV+ and CHIKV+ arbovirus cases. The prevalence of symptoms reported by participants in active surveillance who were confirmed to have a dengue virus (DENV) infection, a chikungunya virus (CHIKV) infection, a coinfection, or neither a DENV nor a CHIKV infection. *** Statistically significant difference in symptom prevalence between DENV and CHIKV cases.
Risk factor analysis of dengue and chikungunya cases.
A total of 5,189 individuals were censused in 2018 and 2019 in our study. Unadjusted risk factors for symptomatic CHIKV infection (using logistic regression) were sex (female 1.65, 95% CI: 1.10–2.48), living in a more remote community (Santo Domingo odds ratio [OR] = 3.60, 95% CI: 1.89–6.85; Santa María OR = 3.88, 95% CI: 2.16–6.96 relative to living in Borbón), and occupation (Domestic OR = 11.63, 95% CI: 1.59–85.37 relative to individuals reporting that they were unemployed). After adjusting for sex and occupation, living in a more remote community remained statistically significant risk for symptomatic CHIKV infection (Santa Maria OR = 3.73, 95% CI: 2.06–6.74; Santo Domingo OR = 3.59, 95% CI: 1.87–6.88 relative to living in Borbón) (Table 1).
Table 1.
Community of residence and occupation are associated with symptomatic chikungunya virus infection
| Proportion of population | Incidence per 10,000 | OR | ||
|---|---|---|---|---|
| Bivariate | Multivariate | |||
| Age (years) | ||||
| 0–18 | 46.1% | 148.8 (108.4–205.6) | Ref | – |
| 19–36 | 25.2% | 214.3 (149.2–307.0) | 1.43 (0.88–2.33) (P = 0.145) | – |
| 37+ | 28.7% | 220.2 (157.7–306.9) | 1.47 (0.93–2.35) (P = 0.102) | – |
| Sex | – | |||
| Male | 49.2% | 139.6 (94.9–184.4) | Ref | – |
| Female | 50.8% | 230.3 (173.9–286.6) | 1.65 (1.10–2.48) (P = 0.016) | 1.63 (0.96–2.77) (P = 0.068) |
| Ethnicity | – | |||
| Afro-Ecuadorian | 72.8% | 193.9 (155.1–242.3) | Ref | – |
| Mestizo/other | 21.5% | 121.1 (71.8–203.7) | 0.62 (0.35–1.10) (P = 0.106) | – |
| Chachi | 5.8% | 302.9 (173.3–587.7) | 1.65 (0.86–3.20) (P = 0.134) | – |
| Community | ||||
| Borbón | 35.2% | 115.9 (76.4–175.6) | Ref | Ref |
| Colon Eloy | 15.6% | 83.3 (39.8–173.9) | 0.72 (0.31–1.68) (P = 0.447) | 0.69 (0.29–1.62) (P = 0.393) |
| Timbire | 11.6% | 128.5 (64.4–255.2) | 1.11 (0.49–2.49) (P = 0.803) | 1.08 (0.48–2.43) (P = 0.957) |
| Maldonado | 21.1% | 211.1 (141.8–313.4) | 1.82 (1.02–3.25) (P = 0.042) | 1.78 (0.99–3.18) (P = 0.052) |
| Sto Domingo | 7.1% | 417.4 (257.0–672.2) | 3.60 (1.89–6.85) (P < 0.001) | 3.59 (1.87–6.88) (P < 0.001) |
| Sta Maria | 9.5% | 449.5 (300.2–668.9) | 3.88 (2.16–6.96) (P < 0.001) | 3.73 (2.06–6.74) (P < 0.001) |
| Ever lived outside community | ||||
| No | 43.8% | 157.4 (114.2–216.6) | Ref | – |
| Yes | 56.2% | 207.9 (162.7–265.4) | 1.32 (0.88–1.98) (P = 0.179) | – |
| Occupation | ||||
| None | 6.3% | 29.6 (4.2–207.8) | Ref | Ref |
| Domestic | 16.2% | 344.5 (241.7–489.2) | 11.63 (1.59–85.37) (P = 0.016) | 8.75 (1.18–65.02) (P = 0.034) |
| Small child | 34.3% | 146.1 (100.3–212.3) | 4.93 (0.67–36.33) (P = 0.117) | 4.66 (0.63–34.33) (P = 0.131) |
| Student | 11.8% | 188.7 (107.4–329.9) | 6.37 (0.83–49.05) (P = 0.075) | 5.68 (0.74–43.72) (P = 0.094) |
| Teacher/works with children | 2.7% | 69.8 (9.8–481.1) | 2.36 (0.15–37.69) (P = 0.545) | 1.78 (0.11–28.58) (P = 0.683) |
| Manual labor | 16.0% | 209.3 (132.2–330.2) | 7.07 (0.94–54.98) (P = 0.057) | 8.00 (1.05–61.00) (P = 0.045) |
| Small business | 6.8% | 164.4 (74.0–361.8) | 5.55 (0.67–46.14) (P = 0.983) | 6.04 (0.72–50.41) (P = 0.097) |
| Government or healthcare | 2.8% | – | – | – |
| Other | 3.3% | 281.7 (117.6–661.8) | 9.51 (1.11–81.48) (P = 0.040) | 10.72 (1.25–92.16) (P = 0.031) |
OR = odds ratio; CI = confidence interval; Ref = reference.
Risk of symptomatic DENV was associated with individuals who reported indigenous Chachi ethnicity (compared with Afro-Ecuadorian) and who lived in the remote community of Santa Maria (compared with Borbón) (OR = 6.29, 95% CI: 3.45–11.46); (OR = 50.68, 95% CI: 15.69–163.66), respectively (Table 2). These results were due to a DENV outbreak in the Chachi neighborhood of Santa Maria.
Table 2.
Community of residence is associated with symptomatic dengue virus infection
| Proportion of population | Incidence per 10,000 | IRR | ||
|---|---|---|---|---|
| Bivariate | Multivariate | |||
| Age, years | ||||
| 0–18 | 46.1% | 108.9 (74.8–158.5) | Ref | – |
| 19–36 | 25.2% | 88.7 (50.4–155.6) | 0.81 (0.41–1.61) (P = 0.552) | – |
| 37+ | 28.7% | 103.6 (63.6–168.6) | 0.95 (0.51–1.76) (P = 0.873) | – |
| Sex | – | |||
| Male | 49.2% | 83.0 (54.7–125.8) | Ref | – |
| Female | 50.8% | 120.6 (85.9–169.3) | 1.45 (0.85–2.49) (P = 0.175) | – |
| Race | ||||
| Afro-Ecuadorian | 72.8% | 81.7 (57.8–115.3) | Ref | Ref |
| Mestizo/other | 21.5% | 60.6 (28.9–126.6) | 0.74 (0.3–1.68) (P = 0.474) | 0.88 (0.38–2.00) (P = 0.753) |
| Chachi | 5.8% | 513.4 (316.4–824.2) | 6.29 (3.45–11.46) (P < 0.001) | 0.59 (0.31–1.12) (P = 0.106) |
| Community | ||||
| Borbón | 35.2% | 15.8 (5.1–48.9) | Ref | Ref |
| Colon Eloy | 15.6% | – | – | – |
| Timbire | 11.6% | 48.2 (15.5–148.5) | 3.05 (0.62–15.10) (P = 0.172) | 2.93 (0.59–14.65) (P = 0.191) |
| Maldonado | 21.1% | 70.4 (35.2–140.2) | 4.45 (1.18–16.78) (P = 0.027) | 4.36 (1.16–16.48) (P = 0.030) |
| Santo Domingo | 7.1% | – | – | – |
| Santa Maria | 9.5% | 801. 3 (594.4–1073.4) | 50.68 (15.69–163.66) (P < 0.001) | 61.85 (18.60–205.69) (P < 0.001) |
| Ever lived outside community | ||||
| No | 43.8% | 59.6 (35.3–100.4) | Ref | – |
| Yes | 56.2% | 135.3 (99.8–183.4) | 0.99 (0.98–1.02) (P = 0.858) | – |
| Occupation | – | |||
| None | 6.3% | 29.6 (4.2–207.8) | Ref | – |
| Domestic | 16.2% | 126.3 (70.1–226.9) | 4.26 (0.55–33.02) (P = 0.165) | – |
| Small child | 34.3% | 81.2 (49.0–134.3) | 2.74 (0.36–20.73) (P = 0.329) | – |
| Student | 11.8% | 188.7 (107.4–329.9) | 6.37 (0.83–48.98) (P = 0.075) | – |
| Teacher/works with children | 2.7% | 139.5 (34.9–543.5) | 4.71 (0.43–51.93) (P = 0.206) | – |
| Manual labor | 16.0% | 127.9 (70.9–229.7) | 4.32 (0.56–33.44) (P = 0.161) | – |
| Small business | 6.8% | 27.4 (3.9–192.3) | 0.92 (0.06–14.78) (P = 0.959) | – |
| Government or healthcare | 2.8% | 65.2 (9.2–450.6) | 2.20 (0.14–35.19) (P = 0.577) | – |
| Other | 3.3% | 56.3 (7.9–390.7) | 1.90 (0.12–30.40) (P = 0.650) | – |
IRR = incidence rate ratio; Ref = reference.
Comparison of dengue and chikungunya infections from the active versus passive surveillance.
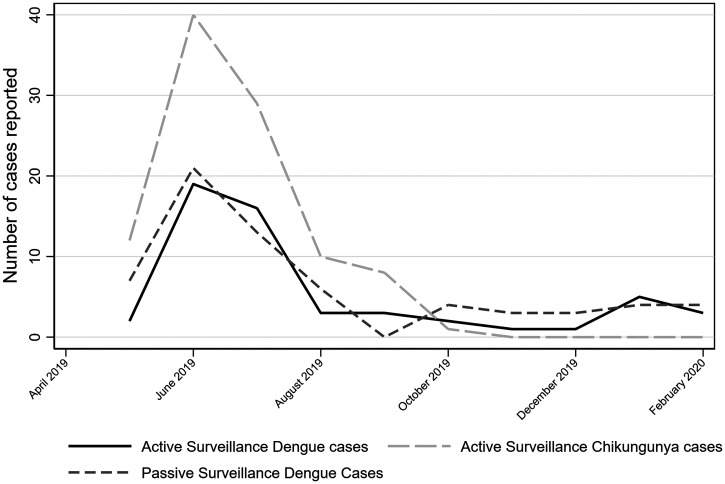
During the same period as the active surveillance (May 2019–February 2020), the MOH passive surveillance system identified 65 cases of dengue in our six study communities. No CHIKF, Zika, Oropouche, or Mayaro cases were identified by the MOH during this time (Figure 3).
Figure 3.
Number of dengue cases reported according the passive surveillance from the Ministry of Health; number of Dengue and Chikungunya cases reported by active Surveillance from May 2019 to February 2020.
The highest number of dengue (N = 19) and chikungunya (N = 40) cases, identified through active surveillance, was reported during the month of June (Figure 3). Similarly, the MOH also reported the greatest number of dengue cases in June (21 cases).
Symptoms reported by dengue cases captured by the MOH were more severe than dengue and chikungunya cases identified through active community surveillance (Table 3). Twenty cases were identified by both the MOH surveillance and the study active surveillance, and 18 of these had complete laboratory results. Of these 18 cases, 14 cases were DENV+, one was CHIKV+, two were DENV-CHIKV coinfections, and one was PCR negative for both viruses.
Table 3.
Demographic/clinical characteristics of individuals with dengue virus/chikungunya virus stratified by passive vs. active surveillance
| Variable | Active surveillance dengue cases N = 80 (%) | Active surveillance chikungunya cases N = 96 (%) | Passive surveillance dengue cases N = 152 |
|---|---|---|---|
| Age, median years (IQR) | 18.5 (11.0–36.5) | 26.5 (13.0–40.2) | 19 (12.0–28.5) |
| Female sex | 47 (58.8) | 59 (61.5) | 67 (44.1) |
| Clinical characteristics | |||
| Fever | 77 (96.2) | 82 (85.4) | 141 (92.8) |
| Headache | 70 (87.5) | 66 (68.8) | 99 (65.1) |
| Myalgia | 43 (53.8) | 26 (27.1) | 88 (57.8) |
| Arthralgia | 39 (48.8) | 35 (36.5) | 98 (64.5) |
| Abdominal pain | 21 (26.2) | 13 (13.5) | 24 (15.8) |
| Rash | 10 (12.5) | 23 (24.0) | 5 (3.3) |
| Diarrhea | 1 (1.3) | 1 (1.0) | 6 (3.9) |
IQR = interquartile range.
DISCUSSION
We found that CHIKV infections went undetected in our study region in northern coastal Ecuador. In 2019 and early 2020, DENV and CHIKV co-circulated and were responsible for approximately 85% of febrile cases captured by our active surveillance (70% of these arbovirus cases were caused by CHIKV). As expected, reported symptoms were similar between the two illnesses, reinforcing the importance of arboviral screening using molecular tools such as real-time PCR as a method for detection of multiple arboviral diseases. Given that molecular screening is limited in many settings with high arbovirus burden and given that DENV infections are assumed to be the primary arbovirus, CHIKV circulation may be underappreciated globally.
In this study, 14.5% (N = 10) of arbovirus-like illnesses were negative for DENV, CHIKV, and other common important viral pathogens in our study area. We found that two arbitrarily selected negative samples were positive for Plasmodium falciparum. The MOH reported 146 cases of Plasmodium falciparum in 2019 in the region.15,30 In a previous study, Oropouche virus was identified in a dengue negative serum sample from our affiliated hospital in Esmeraldas.31 These results indicate that although DENV is common cause of febrile disease, diverse tropical pathogens with common fevers affect these communities. Our reports underline the need of a multiplex approach for febrile diseases detection because different therapeutic regimes and public health measures are necessary to control these pathogens.
Our analysis likely underestimates the true prevalence of DENV in these communities because we used only PCR-confirmed cases and excluded cases that were positive by NS1 (Standard Diagnostic Inc.) and confirmed by IgM ELISA (PanBio, Abbott, Brisbane, Australia). This choice was made to maximize our ability to differentiate between DENV and CHIKV; however, as a result, prevalence estimates are slightly lower compared with other reports by our study team.32
Febrile diseases like DF and CHIKF show similar clinical presentation.33 We found that individuals with DENV infections reported a higher symptoms, with the exception of rash, which was more often reported in CHIKV cases. Other studies have also suggested that although rash may be present in both illnesses, it is more common in early chikungunya cases.34 On the other hand, the prevalence of arthralgia was higher among DENV cases than CHIKV cases. This finding is in accordance with Kuno’s 2015 review reporting more arthralgia among severe dengue cases due to consecutive outbreaks of different dengue serotypes or chikungunya.35 This result is, however, in contrast with many previous studies that have shown that arthralgia is often more prominent in patients with chikungunya.36 Our active surveillance identified cases an average of 3 days after symptom onset; therefore, recall bias was unlikely to account for this unexpected finding.
The Chikungunya risk factors we identified in our study were female, reside in a more rural community and reporting an occupation involving domestic work (i.e., being a housewife) or manual labor. These findings are similar to a previous study in a rural Malaysian cohort in which sex and rural occupancy were predictors of seropositivity for CHIKV, estimating that rural occupancy had 3.9 higher odds than urban occupancy.37 Fred et al.38 reported that outdoor activities such as farm work was a risk factor for CHIKV. Similarly, in a cross-sectional study performed in Quinindé, Esmeraldas, found that women were more likely to have CHIKF than men.39
Our active surveillance data indicated that chikungunya cases went undetected by the passive surveillance system operated by the Ecuadorian Ministry of Public Health. A major challenge with the use of passive surveillance data is the reliance of clinical symptoms for diagnosis, which results in greater underreporting of the diseases that present with broad symptoms similar to other more known diseases.40 Typically, active community-based surveillance captures significantly more arboviral disease including mild cases, like dengue, when compared with passive surveillance studies that focus on more severe cases.41 Despite research demonstrating that active surveillance is an important tool for estimating disease burden, the expense and logistics required for active surveillance posed a challenge to its implementation.42
Therefore, active surveillance data from sentinel sites and research studies around the world like our study provide key insights into the dynamics of arboviral diseases such as dengue and chikungunya; however, for many countries, active surveillance is not feasible for multiple arboviruses given resource limitations. This study demonstrated that active surveillance of febrile illness in remote rural sites is key for the identification of possible sources and routes of transmission for infectious diseases that may circulate endemically. Here we show that CHIKV is still prevalent in rural localities of northwestern Ecuador despite significant CHIKV circulation not been reported since 2018 in Esmeraldas province. Moreover, our data demonstrate that active surveillance using molecular characterization is able to differentiate dengue and chikungunya cases with similar symptoms that are often misdiagnosed clinically. These findings highlight the value of active surveillance data from sentinel sites and prospective research studies to provide key epidemiological insights into dynamics of arboviral diseases like dengue and chikungunya in Ecuador.
Supplemental files
ACKNOWLEDGMENTS
We thank Veronica Barragan for running the polymerase chain reaction (PCR) test to detect Leptospira, Fabián Saenz for his PCR protocol test for Plasmodium falciparum detection, and Lesly Simbaña for helping in metagenomic analysis.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1. Wijesinghe C, Gunatilake J, Kusumawathie PHD, Sirisena PDNN, Daulagala SWPL, Iqbal BN, Noordeen F, 2021. Circulating dengue virus serotypes and vertical transmission in Aedes larvae during outbreak and inter-outbreak seasons in a high dengue risk area of Sri Lanka. Parasit Vectors 14: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harapan H, Michie A, Mudatsir M, Nusa R, Yohan B, Wagner AL, Sasmono RT, Imrie A, 2019. Chikungunya virus infection in Indonesia: a systematic review and evolutionary analysis. BMC Infect Dis 19: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization , 2022. Dengue and severe dengue. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed November 1, 2021.
- 4. Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB, 2016. Dengue infection. Nat Rev Dis Primers 2: 16055. [DOI] [PubMed] [Google Scholar]
- 5. McFee RB, 2018. Selected mosquito-borne illnesses-Chikungunya. Dis Mon 64: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pathak H, Mohan MC, Ravindran V, 2019. Chikungunya arthritis. Clin Med 19: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carabali M, Jaramillo-Ramirez GI, Rivera VA, Possu NJM, Restrepo BN, Zinszer K, 2021. Assessing the reporting of dengue, chikungunya and zika to the national surveillance system in Colombia from 2014–2017: a capture-recapture analysis accounting for misclassification of arboviral diagnostics. PLoS Negl Trop Dis 15: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X, 2017. Emerging arboviruses: why today? One Health 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM, 2020. Mapping global variation in dengue transmission intensity. Sci Transl Med 12: eaax4144. [DOI] [PubMed] [Google Scholar]
- 10. de Brito CAA, Freitas ARR, Said RF, Falcão MB, da Cunha RV, Siqueira AM, Teixeira MG, Ribeiro GS, de Brito MCM, Cavalcanti LP de G, 2020. Classification of chikungunya cases: a proposal. Rev Soc Bras Med Trop 53: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan-American Health Organization/World Health Organization , 2019. Epidemiological update. Available at: https://www3.paho.org/hq/index.php?option=com_docman&view=download&category_slug=dengue-2217&alias=50963-11-november-2019-dengue-epidemiological-update-1&Itemid=270&lang=en. Accessed January 8, 2022.
- 12. de Lima STS. et al. , 2021. Fatal outcome of chikungunya virus infection in Brazil. Clin Infect Dis 73: e2436–e2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Real J, Regato M, Burgos V, Jurado E, 2017. Evolución del virus dengue en el Ecuador: Período 2000 a 2015 [Evolution of dengue virus in Ecuador 2000–2015]. An Fac Med (Perú) 78: 29–35. [Google Scholar]
- 14. Ministerio de Salud Publica , 2021. Gaceta vectores SE 12.
- 15. Ministerio de Salud Publica , 2019. Gaceta vectores SE 52. Available at: https://www.salud.gob.ec/wp-content/uploads/2020/02/GACETA-VECTORES-SE-52.pdf. Accessed January 10, 2022.
- 16. Cevallos V, Ponce P, Waggoner JJ, Pinsky BA, Coloma J, Quiroga C, Morales D, Cardenas MJ, 2018. Zika and Chikungunya virus detection in naturally infected Aedes aegypti in Ecuador. Acta Trop 177: 74–80. [DOI] [PubMed] [Google Scholar]
- 17. Eisenberg JNS. et al. , 2012. In-roads to the spread of antibiotic resistance: regional patterns of microbial transmission in northern coastal Ecuador. J R Soc Interface 9: 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cifuentes SG, Trostle J, Trueba G, Milbrath M, Baldeon ME, Coloma J, Eisenberg JNS, 2013. Transition in the cause of fever from malaria to dengue, northwestern Ecuador, 1990–2011. Emerg Infect Dis 19: 1642–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zelner JL, Trostle J, Goldstick JE, Cevallos W, House JS, Eisenberg JNS, 2012. Social connectedness and disease transmission: social organization, cohesion, village context, and infection risk in rural Ecuador. Am J Public Health 102: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisenberg JNS. et al. , 2006. Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proc Natl Acad Sci USA 103: 19460–19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sierra R, 1999. Traditional resource-use systems and tropical deforestation in a multi-ethnic region in north-west Ecuador. Environ Conserv 26: 136–145. [Google Scholar]
- 22. Waggoner JJ. et al. , 2016. Single-reaction multiplex reverse transcription PCR for detection of Zika, chikungunya, and dengue viruses. Emerg Infect Dis 22: 1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris E, Roberts TG, Smith L, Selle J, Kramer LD, Valle S, Sandoval E, Balmaseda A, 1998. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol 36: 2634–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wise EL. et al. , 2020. Oropouche virus cases identified in Ecuador using an optimised qRT-PCR informed by metagenomic sequencing. PLoS Negl Trop Dis 14: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powers ARJ, 2010. Diagnostic Virology Protocols, 2nd edition. Totowa, NJ: Humana Press. [Google Scholar]
- 26. Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR, 2009. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis 64: 247–255. [DOI] [PubMed] [Google Scholar]
- 27. Kafetzopoulou LE. et al. , 2018. Assessment of metagenomic Nanopore and Illumina sequencing for recovering whole genome sequences of chikungunya and dengue viruses directly from clinical samples. Euro Surveill 23: 1800228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menzel P, Ng KL, Krogh A, 2016. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat Commun 7: 11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snounou G, 1996. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol Biol 50: 263–291. [DOI] [PubMed] [Google Scholar]
- 30. Vera-Arias CA, Castro LE, Gómez-Obando J, Sáenz FE, 2019. Diverse origin of Plasmodium falciparum in northwest Ecuador. Malar J 18: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wise EL. et al. , 2018. Isolation of Oropouche virus from febrile patient, Ecuador. United States. [DOI] [PMC free article] [PubMed]
- 32. Lee GO. et al. , 2021. A dengue outbreak in a rural community in Northern Coastal Ecuador: an analysis using unmanned aerial vehicle mapping. PLoS Negl Trop Dis 15: e0009679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beltrán-Silva SL, Chacón-Hernández SS, Moreno-Palacios E, Pereyra-Molina JÁ, 2018. Clinical and differential diagnosis: dengue, chikungunya and Zika. Rev Med Hosp Gen (Mex) 81: 146–153. [Google Scholar]
- 34. Mohd Zim MA, Sam I-C, Omar SFS, Chan YF, AbuBakar S, Kamarulzaman A., 2013. Chikungunya infection in Malaysia: comparison with dengue infection in adults and predictors of persistent arthralgia. J Clin Virol 56: 141–145. [DOI] [PubMed] [Google Scholar]
- 35. Kuno G, 2015. A re-examination of the history of etiologic confusion between dengue and Chikungunya. PLoS Negl Trop Dis 9: e0004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laoprasopwattana K, Kaewjungwad L, Jarumanokul R, Geater A, 2012. Differential diagnosis of Chikungunya, dengue viral infection and other acute febrile illnesses in children. Pediatr Infect Dis J 31: 459–463. [DOI] [PubMed] [Google Scholar]
- 37. Vong S. et al. , 2010. Dengue incidence in urban and rural Cambodia: results from population-based active fever surveillance, 2006–2008. PLoS Negl Trop Dis 4: e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fred A. et al. , 2018. Individual and contextual risk factors for chikungunya virus infection: the SEROCHIK cross-sectional population-based study. Epidemiol Infect 146: 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chis Ster I, Rodriguez A, Romero NC, Lopez A, Chico M, Montgomery J, Cooper P, 2020. Age-dependent seroprevalence of dengue and chikungunya: inference from a cross-sectional analysis in Esmeraldas province in coastal Ecuador. BMJ Open 10: e040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chow A, Ho H, Win M-K, Leo Y-S, 2017. Assessing sensitivity and specificity of surveillance case definitions for Zika virus disease. Emerg Infect Dis 23: 677–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarti E, L’Azou M, Mercado M, Kuri P, Siqueira JBJ, Solis E, Noriega F, Ochiai RL., 2016. A comparative study on active and passive epidemiological surveillance for dengue in five countries of Latin America. Int J Infect Dis 44: 44–49. [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization , 2018. A Toolkit for National Dengue Burden Estimation. Geneva, Switzerland: WHO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.