Abstract
A study on the production performance of Giant African Land snails was carried out within the Joint Initiative Group of Agro-pastoral Producers United Hands of Foungwo-Santchou (CIG-PAMUFOSA). The general objective of this study was to contribute to a better knowledge of the production performance of the Giant African Land Snail in captivity in Cameroon. For this purpose, the snails were monitored for two months and data on growth and reproductive performance were collected. Growth parameters were estimated from 183 snails aged between 6 and 8 months as a starting number. The results obtained show that the average daily weight gain was 0.06 ± 0.07 g and the shell length and diameter gain was 0.0019 ± 0.0077 mm/day and 0.016 ± 0.0012 mm/day, respectively. Food intake was 1.86 ± 0.17 g/day with a consumption index of 25.87 ± 2.60 and a mortality rate of 40%. Reproductive characteristics were estimated a starting number of 1095 adult snails aged between 1.5 and 2.5 years as it appears that the age at sexual maturity was greater than or equal to 11 months of age. For an average breeding weight of 93.09 ± 25.03 g, they produce clutches averaging 5.66 ± 1.80 eggs per clutch with an average weight of 8.18 ± 3.30 g. The weight of an egg was 1.44 ± 0.35 g, the individual large and small diameter of the eggs were 14.97 ± 1.45 mm and 11.28 ± 1.13 mm, respectively. The incubation period was 35.62 ± 8.16 days and hatching percentage was 76.08%. The factors influencing reproduction and growth performance of Giant African Land Snail would be: housing conditions (high density, breeding substrate), incubation condition, feed quality, and neglect. For an improvement in production performance on this farm, it would be wise to feed the animals with concentrated feed and to follow the strict standard guidelines regarding stocking density.
Keywords: Achatiniculture, Food security, Production performance, Stocking density, Santchou
Achatiniculture; Food security; Production performance; Stocking density; Santchou
1. Introduction
The problem of food insecurity remains a major concern in sub-Saharan Africa in general and in Cameroon in particular (Sasson, 2012). Indeed, in sub-Saharan Africa, we went from 32.6 million undernourished people in 2015 to nearly 237 million in 2019; and in Cameroon from 3 million in 2015 to 3.9 million in 2019 (FAO, 2019). The main cause is thought to be insufficient agro-pastoral production and a surge in the price of basic foodstuffs (FAO, 2019) causing a food crisis characterized by a quantitative and qualitative food deficit in animal protein (Mpupu, 2012). Indeed, the production of conventional meat (pork, beef, poultry, etc.) in Cameroon during the last decade has been proven very insufficient to satisfy the needs of a population with an annual increase estimated to nearly 3% (INS, 2015). Thus, there is need for the promotion of small breeding, which is the rational and controlled exploitation of small-sized wild animals such as snails, cricetomas, grasscutters, guinea pigs, frogs, and compost worms (Otchoumou et al., 2004). It seems to be an alternative solution to complement conventional production (Ebenso, 2002).
The giant African land snail is one of the species with great zootechnical potential (Awohouedji, 2010). It is a gastropod animal that is often found under plant debris, in certain agricultural areas, and everywhere in the tropics (Adeyemo and Borire, 2002; Sodjinou et al., 2003). Snail meat is a very valuable source of animal protein (Ajayi et al., 1978) for rural populations who cannot afford meat from conventional breeding (Imevbore, 1990). It has a nutritional value superior to that of other meat sources (Adeyeye, 1996; Afolayan and Ejidike, 2000) and has by-products (slime and shell) which contribute to the profits of the breeders. Indeed, the flesh of Giant African Land Snails is very low in lipids (3.4% against 11.5% in beef and 12% in chicken), rich in proteins of high nutritional value (64 to 76%) and in mineral substances (6 to 7%) (Tchowan et al., 2018). In addition, the number of bacteria is relatively low (85,000 germs per gram on average) in raw meat, lower than that of other meat sources (chicken, 300,000 germs; sausage, 1 million germs) (Tchowan et al., 2018).
However, the main source of snail flesh supply in Cameroon remains the collection, which is insufficient due to various factors, namely the seasonality of collection (only in the rainy season), the destruction of their habitat due to human population expansion, accidental bushfires, slash-and-burn agriculture, land pesticide use (Sika et al., 2014). The survival of gastropods is threatened in certain regions of Cameroon such as the Sudano-Guinean zone where the giant snail is practically endangered and represented almost exclusively by species of the genus Achatina (Dafem et al., 2008). The development of controlled snail breeding is proving necessary to resolve the problem of decreasing endangered wild populations, while increasing the quantities available on the market and creating a source of income (Stievenart and Hardouin, 1990). It is with this in mind that many Giant African Land Snail breeding initiatives have been undertaken in some localities of Cameroon such as Souza, Loum, Melon, Mbanga, Edéa, etc; and have almost all failed because of the lack of information on the animal, training, technical supervision of breeders and above all the lack of sufficient data on techniques for breeding animals in captivity (Adeola et al., 2010; Karamoko et al., 2011; Deudjui, 2015).
The CIG-PAMUFOSA farm specializing in the production and marketing of the Giant African Land Snail was created to meet the growing demand for its flesh and various by-products (slime and shell). To date, no study evaluating growth and reproductive performance has yet been conducted on this farm. However, knowing these performances is essential to effectively consider improving them. Thus the general objective of this study was to contribute to improving the performance of Giant African Land Snails (Archachatina marginata) in captivity. More specifically, the work aimed at the CIG-PAMUFOSA farm was to assess the performance of growth and reproduction.
2. Materials and methods
2.1. Period and study area
This study was carried out between April and May 2021 at the CIG-PAMUFOSA farm located in Santchou Sub-division of the Menoua division, West Region Cameroon (5° 17′30″ North attitude; 9° 57′ 12″ East longitude) at an average altitude of about 720 m (Fig. 1). The prevailing climate is the Equatorial type characterized by a short dry season (mid-October to mid-March) and a long rainy season (mid-March to mid-October). Precipitation averages 363.3 mm per year and the average relative humidity is 80% per year (www.climatestotravel.com/climate/cameroon). The average annual temperature is 23 °C (www.climatestotravel.com/climate/cameroon). The soils are distributed according to the altitude. They are hydromorphic in the low altitude zone, ferralitic in the high altitude zone and humus in the medium altitude zone. The vegetation is made up of three main strata (herbaceous, shrub and tree), with a varied flora made up of various plant species.
Figure 1.
Study area map.
2.1.1. Name and history of the host structure
2.1.1.1. Denomination
On 27 April 2020 in Bafoussam, the Common Initiative Group of Agropastoral Producers of Foungwo-Santchou (CIG-PAMUFOSA) was created, governed by the provisions of law N° 92/006 of 14/08/92 and its application decree N° 92/45/PM of 23/11/92, modified and completed by decree N° 98/300/PM of 9 September 1998 and N° 2006/0762/PM of 09 June 2006.
2.1.1.2. History
The Livestock initiative started in 2016 when the promoter traveled to Nigeria for vocational training and discovered the achatiniculture. He took the opportunity to train for a period of two weeks, but it was in 2020 that breeding took off with the creation of the CIG-PAMUFOSA which won the first price for snail breeding at the agro-pastoral show of West Cameroon (2020 edition).
2.1.2. Objective of CIG-PAMUFOSA
The objectives of CIG are among others:
-
-
Training and strengthening of the technical and managerial capacities of the population;
-
-
The popularization of achatiniculture.
2.1.3. CIG activities
The main activity of the CIG is the breeding of snails and the processing of products and by-products (transformation of the shell into powder and the use of slime for the manufacture of soaps).
2.2. Management
2.2.1. Animal material
The CIG-PAMUFOSA farm has a livestock of around 5,000 snails represented mainly by the species Archachatina marginata (around 1095 adult snails, 2827 juvenile snails, 600 newly hatched snails), and secondarily by the Achatina achatina and Achatina fulica species. For this study, the species Archachatina marginata was used because it is commonly raised and consumed by the population.
The study was approved by the Ethical committee of the Department of Animal Science of the University of Dschang (ECDAS-UDS 20/03/2017/UDS/FASA/DSAES) and was in conformity with the internationally accepted standard ethical guidelines for Laboratory animal use and care as described in the European community guidelines; EEC Directive 86/609/EEC, of the 24th November 1986.
2.2.2. Accommodation and equipments
The snails were housed in concrete cages (24) of dimension 1.75 m × 0.60 m × 0.5 m in a building (of dimension 70 m2 for 10 m long and 7 m wide) built in local materials (bamboo) whose roof was covered with straw (Fig. 2). The bottom of each cage was covered with 5-10 cm thick loose soil substrate. The snails were distributed in the different cages according to their size.
Figure 2.

Internal view of livestock building.
Each cage was equipped with a feeder (kitchen tray) and a drinker (kitchen plate, seal cover). A watering was also used to moisten and disinfect the litter. Buckets and basins were used to transport food, water, snails and waste from cleaning the various cages. Collecting and transporting the eggs was done using stainless steel spoons and small plastic strainers. Knives and several pairs of gloves were used respectively to slice food (fruits and vegetables) and to perform different tasks at the achatinery.
2.2.3. Feeding
The animals on the farm received a very diverse diet. They were fed ad libitum with green leaves (pawpaw, cabbage, bean, black nightshade, macabo, okra, Talinun leaf) and fruits (ripe and unripe pawpaw, banana, watermelon, and eggplant). Additional food included “water fufu” fresh cassava flour, corn flour and spent grain.
2.2.4. Prophylaxis
The CIG-PAMUFOSA farm has a well-defined sanitary prophylaxis program and biosecurity rules and guidelines. Cleaning of cages and service corridors took place daily using brooms and bucket to transport droppings, waste and dead animals. The substrate was disinfected every three months with a mixture of water and ash (1 kg of ash for 10 liters of water). The animals were washed every two weeks to rid them of parasites according to the breeder. To wash them, the snails were placed in a basin containing water from a well. After cleaning the snails, they were returned directly to their respective cages.
2.3. Breeding management
At the CIG-PAMUFOSA farm, the snails were divided into cages according to their size. Thus, a cage could house animals whose age difference could be 1 to 2 months. Density varied depending on the availability of cages.
After hatching, the newly hatched snails were collected and then immersed in a bucket of water for two minutes to make them active before being placed in cages with densities varying between 400 and 500 per square meter (Cobbinah et al., 2008). When the young snails became three months old, they were distributed in new cages at a density of 200 to 250/m2 until sexual maturity is reached. They were then taken to reproduction pits at a density of 100 to 150/m2 to replace those that are or will be taken for sale.
Snail's food was washed, sliced for fruit and served ad libitum every day or every other day (except for those less than two months old or the duration may exceed) depending on the food served and availability of food. Water was also provided to them ad libitum and the watering of the substrate was done every two to three times a week depending on the ambient temperature. All the animals on the farm receive the same feed regardless of their ages and physiological stages, but in varying amounts depending on the stocking density.
For egg collection and incubation, egg collection was done every two weeks before being transported to the incubation pit. The substrate was gently turned by a hoe for the possible discovery of the eggs. Each time a clutch was discovered; eggs were removed with a stainless steel spoon and then placed in a plastic sieve containing a little damp soil. After searching all the cages, the eggs were counted and then incubated in heaps depending on the number of eggs and space available in the incubation cage. The incubation substrate was watered every 2 weeks. After the emergence of the first newly hatched snails from the soil, the substrate was searched to remove the newly hatched snails and the action repeated once a week to remove any hatched potential.
2.4. Data collection
2.4.1. Growth characteristics
A cage containing 181 juvenile snails (Archachatina marginata) was randomly selected from the different juvenile snail cages. At the beginning of the study, then every 2 weeks, the snails were weighed and the shell measurements were recorded respectively using an electronic scale of precision 0.01 g and an electronic caliper of precision 0.01 mm to evaluate the growth characteristics. Snail's Food was weighed daily and left over were collected and weighed to assess food consumption. The animals were monitored for a period of 8 weeks.
2.4.2. Reproductive characteristics
Every 2 weeks, the breeding substrate (1095) was thoroughly stirred with a hoe to collect the eggs in order to determine the egg laying characteristics (number of eggs per clutch, clutch weight). The morphometric characteristics of egg (weight (g), length (mm) and diameter (mm)) were determined by measurements performed on 10% of the eggs collected. The eggs were then placed 4 cm deep in the loose soil substrates until they hatched. Unhatched eggs were opened and embryonic development status was observed. This was to determine the early embryonic mortality rate (number of eggs with a dead embryo without shell) and the late embryonic mortality rate (number of eggs with a dead shelled embryo) (Dafem et al., 2008).
2.5. Studied parameters
2.5.1. Growth characteristics
The growth characteristics studied are:
-
-Food consumption (FC)
-
-Weight gain (WG)
-
-Average daily gain (ADG)
-
-Shell length gain (SLG)
-
-Shell diameter gain (SDL)
-
-Consumption index (CI)
-
-Mortality rate (Tm)
2.5.2. Reproduction parameters
-
-
Incubation period = time taken (days) for the eggs to hatch
-
-
Fertilization rate = (Number of embryonated eggs/Number of eggs laid) × 100 (Dafem et al., 2008)
-
-
Hatching percentage = (Number of eggs hatched/Number of eggs laid) × 100
-
-
Average weight of newly hatched snails = newly hatched snails weight
-
-
Early embryonic mortality rate = (Number of eggs with a dead embryo without a shell/Number of eggs laid) × 100
-
-
Late embryonic mortality rate = Number of eggs with a dead embryo with shell/Number of eggs laid) × 100
-
-
Adult mortality rate (Number of adult snails that died during the breeding season/total number of adult snails) × 100 (Dafem et al., 2008).
2.6. Statistical analyzes
The collected data was analyzed for descriptive statistics using an Excel. Our data was expressed as mean ± standard deviation and as percentage.
3. Results
3.1. Growth characteristics
The growth characteristics of Archachatina marginata at the CIG-PAMUFOSA farm are summarized in Table 1. It appears that the average shell length and shell diameter gain were lower compared to those obtained by Koudande et al. (2006) and Kouassi et al. (2007a) respectively. The mortality rate observed during the study was 40%. The value of average daily weight gain was lower compared to value observed by Daouda (1995).
Table 1.
Growth characteristics of Archachatina marginata at the PAMUFOSA farm.
| Characteristics | Values (mean ± standard deviation) |
|---|---|
| Initial live weight (g) | 45.75 ± 10.14 |
| Final live weight (g) | 49.12 ± 11.61 |
| Food consumption (g/day) | 1.86 ± 0.17 |
| Total weight gain (g) | 3.38 ± 1.47 |
| Average daily weight gain (g/day) | 0.06 ± 0.07 |
| Consumption index | 25.87 ± 2.60 |
| Average shell length gain (mm/day) | 0.019 ± 0.77 |
| Average shell diameter gain (mm/day) | 0.016 ± 0.12 |
| Mortality rate (%) | 40 |
3.2. Food consumption, live weight, shell length, and shell diameter
Irrespective of the study period (Fig. 3), our results showed that, the curve of food consumption (Fig. 3a), live weight (Fig. 3b), shell length (Fig. 3c), and shell diameter (Fig. 3d) of Archachatina marginata evolved irregularly (sawtooth between the 4th and the 8th week).
Figure 3.
Food consumption (a), live weight (b), shell length (c), and shell diameter (d) of Archachatina marginata during an eight weeks period of its development.
When we consider the study period, the significantly highest values of food consumption and live weight of Archachatina marginata during an eight weeks period were recorded during the fourth week and the lowest value during the sixth week. In the contrary, the significantly highest values of shell length and shell diameter were registered during the last week of the study and the lowest value at the sixth week.
3.3. Mortality rate
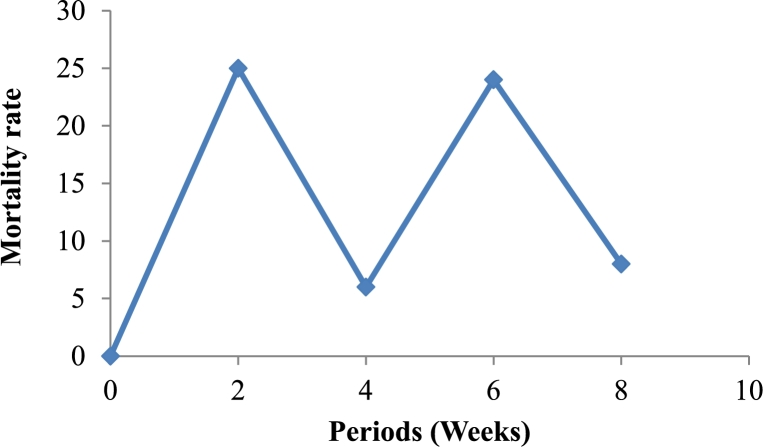
Irrespective of the study period (Fig. 4), the mortality rate evolved in an irregular manner. When we consider the study period, the significantly highest values of mortality rate were observed in the second and sixth week and the lowest values in the fourth and eighth week.
Figure 4.
Mortality rate of Archachatina marginata during an eight weeks period of its development.
3.4. Reproductive characteristics
Our results (Table 2) showed that Archachatina marginata reaches sexual maturity at an age greater than or equal to 11 months at an average weight of 93.09 ± 25.03 g. The clutches vary from 1 to 9 eggs in the substrate. Egg weights are relatively the same within the same clutch but vary greatly from one to another.
Table 2.
Reproductive characteristics of Archachatina marginata at the PAMUFOSA farm.
| Reproductive Characteristics | Numbers | Values |
|---|---|---|
| Age of onset of maturity (months) | - | ≥11 |
| Breeding weight (g) | 397 | 93.09 ± 25.03 |
| Number of eggs per clutch | 47 | 5.66 ± 1.80 |
| Clutch weight (g) | 47 | 8.18 ± 3.30 |
| Egg weight (g) | 266 | 1.44 ± 0.35 |
| Large egg diameter (mm) | 266 | 14.97 ± 1.45 |
| Small egg diameter (mm) | 266 | 11.28 ± 1.13 |
| Incubation period (days) | 30 | 31.62 ± 4.16 |
| Newly hatched snails weight (g) | 101 | 1.23 ± 0.33 |
| Shell length of newly hatched snails (mm) | 101 | 14.86 ± 1.79 |
| Shell diameter of newly hatched snails (mm) | 101 | 11.88 ± 1.07 |
| Fertilization rate (%) | 393 | 90.34 |
| Early embryonic mortality rate (%) | 393 | 7.38 |
| Late embryonic mortality rate (%) | 393 | 6.88 |
| Hatching percentage (%) | 393 | 76.08 |
| Breeding mortality rate (%) | 1095 | 32.49 |
For an incubation period of 35.62 days, an unhatched egg percentage of 23.92% was recorded for Archachatina marginata. The analysis of the latter revealed 38.46% of unembryonic eggs as well as 7.38 and 6.88% of early and late embryonic mortalities, respectively.
4. Discussion
The average food intake recorded in breeding was 1.86 g/day, a value lower than that of 3.80 g/day obtained by Kana et al. (2018) with a diet made from pawpaw only. Our value is otherwise close to the 1.89 g/day obtained by Sika et al. (2015) with a diversified plant diet: Carica papaya (pawpaw), Lactuva sativa (Lettuce), Brassica oleracea (common cabbage), Cecropia peltata (The trumpet wood or coulequin), Laportea aestuans (the West Indian woodnettle). On the other hand, Ogbolo et al. (2019) and Tchakounte et al. (2019) respectively obtained an average food intake of 1.27 g/day with a diet rich in protein (20%) and 0.40 g/day with diets rich in calcium (18%). In view of its results, it can be concluded that food consumption is strongly influenced by diet. This assertion corroborates that of Sika et al. (2015) who assert that snails consume much more forage to meet their needs because they are poorer in nutrients than compound feed. Also, due to the fact of their composition, concentrate-based diets are rich in dry matter and poor in water, whereas, the vegetable-based diets are poor in dry matter and rich in water, thus facilitating its consumption by the snails.
The value of the average daily weight gain recorded was 0.06 g/day with a vegetable-based feed; a value less than 0.22 g/day obtained by Kana et al. (2018) with a diet based on concentrate. This difference between our findings could be justified by the difference in the feed used since they used a diet based on concentrate while in this farm the snails were fed mainly with a diet based on plants which are relatively poor in nutrients, thus, resulting in poor performance. Due to a harmonious growth of snails, it is essential to note the synergistic effect of organic (protein, carbohydrate, lipids) and inorganic matter (vitamins, minerals) (Otchoumou et al., 2004) found in concentrated feed. This result corroborates with those of the work of Otchoumou et al. (2005), Kouassi (2007a), Adeola et al. (2010), Karamoko et al. (2015) and Sika et al. (2015) in A. achatina, A. fulica, A. marginata, A. ventricosa and L. flammea who all concluded that the use of plant feed such as green forages, fruits and tubers of certain plants results in relatively low and average growth performance.
The consumption index obtained was 25.87, a value far greater than the 2.33 obtained by Tchakounte et al. (2019) because of diets rich in calcium. A study by Pounde (2020) obtains a consumption index of 3.15 with a diet rich in protein; but is lower than the 29.05 reported by Kana et al. (2018) fed on diets made from plants (pawpaw only). These large differences could be explained by the types of feed provided because the consumption index is being used to measure the efficiency of conversion of a food into a given production by an animal. The increase of the consumption index could be explained by a poor conversion of feed due to the absence or the presence but in insufficient quantity of nutrient contained in the food ingested by the snails. The potentially different genetic characteristics of the species used by these authors as a result of existence of several unidentified subspecies (Hardouin et al., 1995) may have influenced this result.
At the CIG-PAMUFOSA farm, the shell length and diameter gains obtained were respectively 0.019 and 0.016 mm/day, relatively low values compared to those in Tchakounte et al. (2019). The same authors obtained 0.28 and 0.15 mm/day, respectively in average shell length and diameter gain from concentrated diets rich in dietary calcium (18%). This difference between their average shell gains (length and diameter) may have been strongly influenced by the calcium content in the snail diet. Since calcium is the main constituent of the shell (almost 90%), a low intake will cause a low shell gain; but also, this low shell gain could have been influenced by other factors like high density, feeding, and excessive handling of the snails.
The mortality rate in this study represented 40% of snails monitored, a result close to 37.78% obtained by Sika et al. (2015) with a diet based on plants (Carica papaya, Lactuva sativa, Brassica oleracea, Cecropia peltata, Phaulopsis falcisepala, Laportea aestuans and Leuceana leucocephalia) on Achatina fulica. But, our value is greater than 6.67-10% obtained by Ogbolo et al. (2019) on Archachatina marginata receiving a diet containing respectively soybean meal and fishmeal associated with soybean meal. However, Tchowan et al. (2018) obtained values ranging from 13.66 to 43.34% mortality in Achatina achatina subjected to the concentrated feed-associated with pawpaw leaves. These observations suggest an increase in the mortality rate with the low nutritional value in the diet. In fact, the snail needs calcium for the development and repair of its shell in case of breakage, which limits the mortalities due to broken shells commonly observed in farming (Karamoko, 2009). In addition, the high mortality rate could also be due to the high breeding density (181 snails/m2) to which they were exposed because according to Noumonvi et al. (2012), the mortality rate changes proportionally with density. Indeed this author obtained mortality rates of 0, 15.62, 29.17, and 34.37% respectively for densities of 50, 100, 150, and 200 m2 resulting in the fact that compliance with density standards is a very important parameter for improving the productivity of livestock. Also, these mortalities could be explained by the attack of the snails by some nematodes, such as Angiosloma (Angiosloma aspersa), whose larvae are lodged between the body and the shell of the snail, capable of separating the mantle from the shell (Tompa, 1984). The diseased snail presents a body either retracted into the shell or out of the latter but loose and/or swollen (Zongo, 1994).
The age at onset of sexual maturity was equal to or greater than 11 months or more. On the other hand, the experiment carried out by Kouassi et al. (2007a) led to the first egg-laying at the end of the 34th week, i.e. about 9 months on the substrate consisting of soil collected under a cassava plantation with 10% white sawdust. Zongo (1994) meanwhile with a diet containing 14.02% calcium and 17.36% nitrogenous matter, the snails would have reached sexual maturity at 30 weeks of age, i.e. around 7 months against 12 to 15 months in a natural environment where it consumes only plant products. Also, Otchoumou et al. (2003) showed that when reared at a density of 50/m2 Archachatina marginata reached sexual maturity at age of 9 months and that this age continued with increasing density. In view of the above, the age at sexual maturity could be influenced by the nature of the substrate, feed and stocking density.
For an average breeding weight of 93.09 g, the morphometric characteristics obtained were 1.44 g, 14.97 mm, and 11.28 mm respectively for the weight, large and small diameter of the egg. These results are higher than 1.3 g, 14.41 mm, and 11.36 mm obtained by Tchakounte et al. (2019) with an average breeding weight of 50 g. Our value is and less than 2.5 g, 1.66 mm, and 1.44 mm obtained by Ogbolo et al. (2019) with average weight breeders of 169.5 g. In view of these results, it would seem that the morphometric characteristics of the eggs are influenced by the average weight of the parental snails. Corroborously, Dafem et al. (2008) who stated that Archachatina marginata lays egg clutches of about 10% of their weight.
The incubation period obtained was 31.62 days for an egg hatch percentage of 76.08% with a loose soil substrate, unlike Tchakounte et al. (2019) who had a duration of 50 days for a 100% hatch rate on sawdust substrate. In view of its results, one could conclude that the duration and rate of hatching are influenced by the nature of the substrate, thus agreeing with the results of Dafem et al. (2008). The early and late embryonic mortality rates were 7.38 and 6.88%, respectively; lower than 10.69 and 9.92% obtained by Dafem et al. (2008). The variability of our results compared to those of the various authors cited could have been influenced by certain factors such as the relative humidity of the air, nature of the substrate and water supply (Kouassi et al., 2007b). The mortality rate of adult is close to 1/3 as reported by Thompson and Cheney (2004), and Dafem et al. (2008).
5. Conclusion
Given the aim of this study, we concluded that the values of growth characteristics such as food intake, total weight gain, average daily weight gain, and consumption index are lower than the standards recommended by several authors in the literature except for the mortality rate which was higher. At the level of reproduction, the values of the characteristics of the egg laying, the morphometrics of the eggs and fertility are on the whole close to the standards recommended by several authors (Dafem et al., 2008). This is exceptional for the Archachatina marginata hatching percentage, which was relatively low due to poor interventions on the various stages ranging from collection, incubation of eggs until hatching.
Declarations
Author contribution statement
Tchowan Guy Merlin: Conceived and designed the experiments, Wrote the paper; Zangho Junior: Conceived and designed the experiments, Performed data collection; Kouam Kenmogne Marc: Conceived and designed the experiments, Analyzed and interpreted the data; Ngoula Ferdinand: Conceived and designed the experiments, Performed the experiments; Tchoumboué Joseph: Conceived and designed the experiments, Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adeola A.J., Adeyemo A.I., Ogunjobi J.A., Alaye S.A., Adelakun K.M. Effects of natural and concentrate diets on proximate composition and sensory properties of giant land snail (Archachatina marginata) meat. J. Appl. Sci. Environ. 2010;5(2):185–189. [Google Scholar]
- Adeyemo A.I., Borire O.F. Response of giant snail (Archachatina marginata) to graded levels of yam peel meal-based diet. J. Niger. Soc. Anim. Prod. 2002;12:42–51. [Google Scholar]
- Adeyeye E.I. Waste yield, proximative and mineral composition of three different types of land snails found in Nigeria. Int. J. Food Sci. Nutr. 1996;47(2):111–116. doi: 10.3109/09637489609012572. [DOI] [PubMed] [Google Scholar]
- Afolayan T.A., Ejidike B.N. Utilization of African giant land snail (Archachatina marginata) in the humid area of Nigeria. Trop. Agric. Trinidad. 2000;69(1):88–92. [Google Scholar]
- Ajayi S.S., Tewe O.O., Moriarty C., Awesu M.O. Observations on the biology and nutritive value of the African giant snail Archachatina marginata. East Afr. Wildl. J. 1978;16(2):85–95. [Google Scholar]
- Awohouedji D. Influence du substrat sur les performances des juveniles et le niveau de parasitisme des géniteurs d'Archachatina marginata en élevage contrôlé. Mémoire soutenu en vue de l'obtention du diplôme de master à l'Université d'Abomey-calavi. Nat. Sci. 2010:57. https://api.pageplace.de/preview/DT0400.9783668045620_A25741899/preview-9783668045620_A25741899.pdf Retrieved from. [Google Scholar]
- Cobbinah J.C., Vink A., Onwuka B. Série Agrodok No. 47. 2008. L'élevage d'escargots Production, transformation et commercialisation; p. 84.https://www.agromisa.org/wp-content/uploads/Agrodok-47-L%C3%A9levage-descargots.pdf [Google Scholar]
- Dafem R., Ngoula F., Teguia A., Kenfack A., Tchoumboué J. Performances de reproduction de l'escargot géant africain (Archachatina marginata) en captivité au Cameroun. Tropicultura. 2008;26(3):155–158. [Google Scholar]
- Daouda I.-haquou A. Le calcium dans l'alimentation de l'escargot géant d'Afrique Achatina achatina (Linné) Cah. Agric. 1995;4(6):444–448. (1) [Google Scholar]
- Deudjui G. 2015. Cameroun. Pourquoi l'élevage des escargots peine à décoller?http://ghide.overblog.com/2015/07/cameroun-pourquoi-l-elevage-des-escargots-peine-a-decoller.html Retrieved January 4, 2018. [Google Scholar]
- Ebenso I.E. Composition and sales of domesticated snails Archachatina marginata in rural southern Nigeria. Trop. Sci. 2002;42(4):185–187. [Google Scholar]
- F.A.O. L'État de la sécurité alimentaire et de la nutrition dans le monde. 2019. www.fao.org/3/ca5162fr/ca5162fr Retrieved from.
- Hardouin J., Steivenart C., Codjia J.T.C. L'Achatiniculture. Rev. Mond. Zootech. 1995;82(2):29–39. [Google Scholar]
- Imevbore E.A. Carcass evaluation and nutritive value of some popular edible molluscs in Nigeria. Nahrung. 1990;34(6):549–553. doi: 10.1002/food.19900340613. [DOI] [PubMed] [Google Scholar]
- Institut National de la Statistique (INS) 2015. Annuaire statistique du Cameroun; p. 59. [Google Scholar]
- Kana J.R., Tchakounte F.M., Meffowoet Chekam C.P. Effets du régime alimentaire sur la croissance et la valeur nutritive de la viande d'escargots géants africains Archachatina marginata. Livest. Res. Rural Dev. 2018;30 http://www.lrrd.org/lrrd30/3/kana30042.html Retrieved May 2, 2022, from. [Google Scholar]
- Karamoko M. Université de Cocody-Abidjan; 2009. Étude de la biologie, de l'écologie et du comportement d'un escargot terrestre d'intérêt économique, Limicolaria flammea (Müller, 1774), en milieu d'élevage; p. 184. Thèse de Doctorat unique. [Google Scholar]
- Karamoko M., Memel J.D., Kouassi K.D., Otchoumou A. Influence de la densité animale sur la croissance et la reproduction de l'escargot Limicolaria flammea (Müller) en conditions d'élevage. Acta Zool. Mex. 2011;27(2):393–406. [Google Scholar]
- Karamoko M., Sika N.A., Adou C.F.D., Otchoumou A., Kouassi K.P. Effets du calcium alimentaire sur les paramètres de croissance de l'escargot Limicolaria flammea (Müller, 1774), en élevage hors-sol. Int. J. Innov. Appl. Stud. 2015;11(1):231–240. [Google Scholar]
- Kouassi K.D., Otchoumou A., Dosso H. Effet de l'alimentation sur les performances biologiques chez l'escargot géant africain: Archachatina ventricosa (Gould, 1850) en élevage hors sol. Livest. Res. Rural Dev. 2007;19(5):16–20. [Google Scholar]
- Kouassi K.D., Otchoumou A., Dosso H. Les escargots comestibles de Côte d'Ivoire: Influence du substrat d'élevage sur les paramètres de croissance d'Archachatina ventricosa (Gould, 1850) en élevage hors sol. Tropicultura. 2007;25(1):16–20. [Google Scholar]
- Koudande O.D., Hountondji M.-C.S., Mensah G.A. Test de trois sources de calcium dans l'alimentation des achatines ou escargots géants africains (Archachatina sp) Bull. Rech. Agron. Bénin. 2006;3:18–21. [Google Scholar]
- Mpupu B. Guide pratique et scientifique de l'élevage de poule pondeuse et de poulet de chair. Harmattan; Paris; 2012. p. 116. [Google Scholar]
- Noumonvi C.G.R., Lougbegnon O.T., Dahouda M., Codjia J.T.C. Influence de la densité de populations sur les performances de croissance d'Archachatina marginata (Swainson) en élevage contrôlé. Bull. Rech. Agron. Bénin. 2012;5:1–8. [Google Scholar]
- Ogbolo E., Toukourou Y., Sanni W.H.S., Assogba B.G.C., Alkoiret T.I. Effet de la variation du niveau en protéine sur les performances zootechniques de l'escargot Archachatina marginata (Swainson, 1821) et la morphologie des naissains en élevage hors-sol au nord-Bénin. Afrique Sci. 2019;15(6):1–10. [Google Scholar]
- Otchoumou A., Dosso H., Fantodji A. The edible African giant snails: fertility of Achatina achatina (Linné 1758) Achatina fulica (Bowdich, 1820) and Archachatina ventricosa (Gould 1850) in humid forest; influence of animal density and photoperiod on fertility in breeding. Boll. Malacol. 2003;39(9–12):185–190. [Google Scholar]
- Otchoumou A., Dupont-Nivet M., Dosso H. Les escargots comestibles de Côte d'Ivoire: effets de quelques plantes, d'aliments concentrés et de la teneur en calcium alimentaire sur la croissance d'Archachatina ventricosa (Gould, 1850) en élevage hors-sol en bâtiment. Tropicultura. 2004;22(3):127–133. [Google Scholar]
- Otchoumou A., Dupont N.M., N'Da K., Dosso H. L'élevage des escargots comestibles Africains: Effets de la qualité du régime et du taux de calcium alimentaires sur les performances de reproduction d'Achatina fulica (Bowdich 1820) Livest. Res. Rural Dev. 2005;17 http://www.lrrd.org/lrrd17/10/otch17118.htm Retrieved September 10, 2018, from. [Google Scholar]
- Pounde Z.M. Mémoire en vue d'obtenir le Master of Science à la FASA. Université de Dschang; 2020. Effets du niveau de protéines alimentaires sur les performances de croissance de l'escargot géant Archachatina marginata en captivité; p. 71. [Google Scholar]
- Sasson A. Food security for Africa: an urgent global challenge. Agric. Food Secur. 2012;1(2):2–16. [Google Scholar]
- Sika P.N.A., Karamoko M., Adou C.F.D., Otchoumou A., Kouassi P.K. Effet du régime et de la teneur en protéines brutes alimentaires sur le rendement en viande de l'escargot Achatina fulica (Bowdich, 1720) Int. J. Biol. Chem. Sci. 2014;8(5):2296–2305. [Google Scholar]
- Sika P.N.A., Karamoko M., Bouye T.R., Otchoumou A., Kouassi K.P. Effet de la teneur en protéines alimentaires sur la croissance de l'escargot Achatina fulica (Bowdich, 1720) Int. J. Innov. Appl. Stud. 2015;13(1):85–93. [Google Scholar]
- Sodjinou E., Biaou G., Codjia J-C. Caractérisation du marché des escargots géants africains (achatines) dans les départements de l'Atlantique et du littoral au Sud-Benin. Tropicultura. 2003;20(2):83–88. [Google Scholar]
- Stievenart C., Hardouin J. Centre Technique de Coopération Agricole et Rural; Pays-Bas: 1990. Manuel des escargots géants africains sous les tropiques; p. 35. [Google Scholar]
- Tchakounte F., Kana J., Azine P., Meffowoet P., Djuidje V. Growth and reproductive performances of African giant snail (Archachatina marginata) as affected by dietary calcium levels. J. Anim. Res. Vet. Sci. 2019;8(1):263–271. [Google Scholar]
- Tchowan G.M., Ngoula F., Kenfack A., Tchoumboue J. Effect of protein levels on growth performance of giant African land snails (Achatina achatina) J. Agric. Sci. 2018;10(4):278–286. [Google Scholar]
- Thompson R., Cheney S. National Agricultural Library; Baltimore: 2004. Raising snails Alternative Farming Systems Information Center.http://www.aphis.usda.gov/ppq/permits/plantpest/rearing.pdf Retrieved from. [Google Scholar]
- Tompa A.S. In: The Mollusca 7. Reproduction. Tompa A.S., Verdonk N.H., Van Den Biggelaar J.A.M., editors. Academic Press; Orlando, San Diego, San Francisco, New York, London, Sydney, Tokyo, Sao Paulo: 1984. Land snails (Stylommatophora) pp. 47–140. [Google Scholar]
- Zongo D. Fiche technique N°2 ENSA/LACENA. 1994. L'élevage des escargots, une source insoupçonnée de protéines de hautes valeurs nutritionnelles; pp. 5–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.