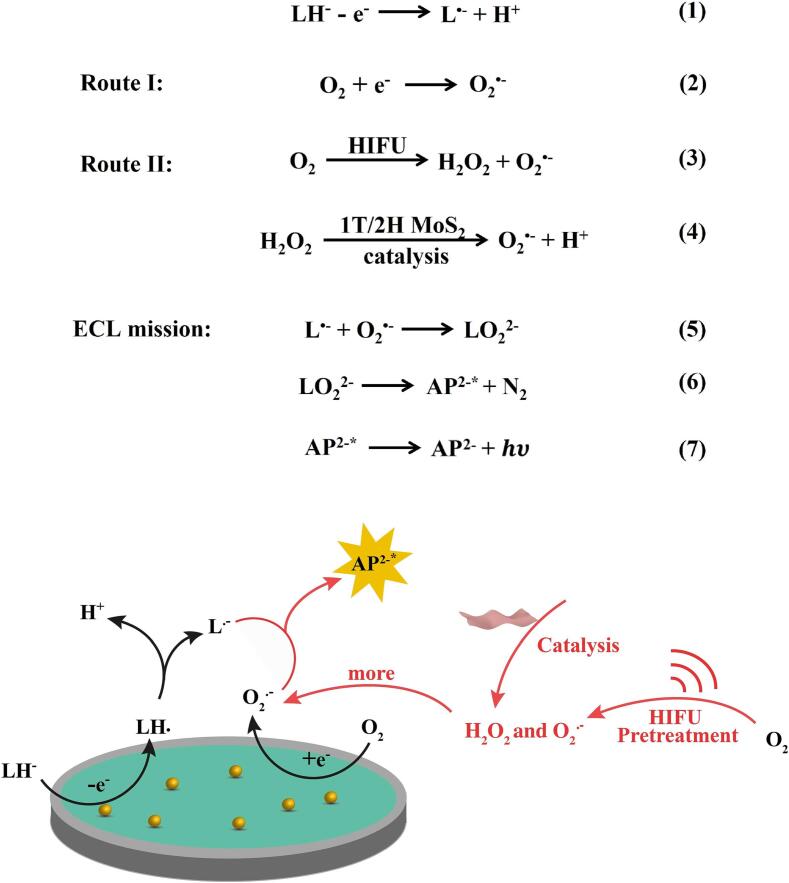
Graphical abstract
High-intensity focused ultrasound pretreatment as a non-invasive method could generate H2O2 and O2•− in situ, triggering and boosting the ECL signal of luminol-O2 system. Meanwhile, 1T/2H MoS2 could catalyze the H2O2 formed in situ to enhance the ECL response.
Keywords: Electrochemiluminescence, High-intensity focused ultrasound, Pretreatment, 1T/2H MoS2, Luminol-O2
Highlights
-
•
A novel strategy combining ultrasound with nanomaterial was designed.
-
•
High-intensity focused ultrasound (HIFU) could generate H2O2 and O2•− in situ.
-
•
1T/2H MoS2 could catalyze the H2O2 to boost the ECL signal of luminol-O2 system.
-
•
The ECL biosensor was successfully applied to the determination of miRNA-155.
Abstract
In the luminol-O2 ECL system, O2 as an endogenous coreactant has the advantages of non-toxicity and stability. Improving the efficiency to generate radicals of O2 is a challenge currently. In this work, a strategy combining physical method - ultrasound and nanomaterial with unique physicochemical properties was designed to enhance the ECL signal of luminol-O2 system. Specifically, high-intensity focused ultrasound (HIFU) pretreatment as a non-invasive method could generate ROS (H2O2, O2•−, OH•, 1O2) in situ, triggering and boosting the ECL signal of luminol. In addition, 1T/2H MoS2 with excellent catalytic activity could catalyze the H2O2 produced in situ, accelerate the oxidation of luminol and further enhance the ECL response. At the same time, combined with the catalytic hairpin assembly (CHA) reaction, the constructed ECL biosensing platform showed excellent performance for the detection of miRNA-155. The concentration range of 0.1 fM ∼ 1 nM with the detection limit as low as 0.057 fM were obtained. Furthermore, the ECL biosensor was also successfully applied to the determination of miRNA-155 in human serum samples. The established ECL sensing platform opens up a promising method for the detection of clinical biomarkers.
1. Introduction
Electrochemiluminescence (ECL) combines both electrochemical methods and chemiluminescence, which has the advantages of low background signal, wide dynamic range, high sensitivity, simple instrument and low cost [1], [2], [3], [4], [5]. Luminol has been extensively used in the field of ECL sensing because of its high luminous rate, non-toxic and low cost [6], [7], [8]. O2 as an endogenous coreactant has the characteristics of mild reaction and convenient operation, and the derived radicals could increase the ECL efficiency of luminol based system [9]. However, the low decomposition rate of O2 usually could not produce adequate intermediate reactive oxygen species (ROS) to obtain the ideal signal in luminol-O2 ECL system [10]. Researchers have introduced a third substance, coreaction promoter, to improve the free radical yield of O2. For example, (i) Fe@Fe2O3 nanowires [11], CeO2/SnS2 heterostructure [12], ZnO nanostars [13], Cu-doped TiO2 [14] and other nanomaterials were introduced into lumino-O2 system as the coreaction promoter to amplify the ECL signal; (ii) high-entropy oxides containing five metal components (Ni, Co, Cr, Cu and Fe) have made important contributions in catalyzing the conversion of dissolved O2 and improving the ECL efficiency of luminol [15]. Despite the extensive work that has been done so far, it remains challenging to develop a simple and efficient method to improve ECL signal in the luminol-O2 system.
High-intensity focused ultrasound (HIFU), as a non-invasive means, has attracted more and more attention in clinical application [16], [17], [18]. The cavitation bubble is generated by the high negative pressure in the focal region of HIFU, and then oscillated and collapsed by HIFU. When the bubble shrinks, water molecules or O2 are thermically decomposed due to high temperature and pressure, and then the ROS are generated containing H2O2, superoxide anion radical (O2•−), hydroxyl radical (OH•), singlet oxygen (1O2) [19]. ROS produced by HIFU is highly oxidized, which can induce apoptosis of cancer cells and treat cancer safely and effectively [20], [21]. So far, there have been only a few studies on HIFU/ECL published, while continuous low-frequency ultrasound has been applied to ECL. For example, it has been proved that ultrasound irradiation could markedly enhance the ECL emission of arylacetate [22]. In addition, ECL response of Ru(bpy)32+/lidocaine system was also obviously improved with ultrasound irradiation [23]. Inspired by the above, HIFU pretreatment, as a non-invasive pretreatment means, was introduced into the lumino-O2 ECL system to promote the conversion of dissolved O2 to ROS in situ and enhance ECL signal firstly in our group [24].
MoS2, as one of the most typical transition-metal disulfides (TMDCs) with two-dimensional ultrathin atomic layer structure, has been widely investigated in supercapacitor and catalysis due to its excellent layered structure and electronic property [25], [26], [27]. For example, the narrow band gap of MoS2 endows it have photoelectric effect [28], thus, it is promising in photocatalysis [29], [30]. Furthermore, MoS2 shows electrocatalytic activity because of unique electronic structure, and it has been expected to be a wonderful catalyst for hydrogen evolution reaction (HER) [31], CO2 [32] or N2 [33] reduction reactions. MoS2 has various crystal phase structures due to different coordination of atoms [34]. 2H MoS2 crystal structure have poor conductivity, while 1T MoS2 is a metallic phase with a higher catalytic performance than the 2H MoS2 owing to that it has active sites at the base plane and edge. Nonetheless, 1T MoS2 has some problems such as strict synthesis conditions and poor stability. Therefore, the catalytic activity of 1T/2H MoS2 is higher than 2H MoS2, and its thermochemical stability is better than that of 1T MoS2, which has great research potential.
In the luminol-H2O2 system, the electrocatalytic activity of MoS2 nanosheets was developed for the decomposition of H2O2, which could accelerate the oxidation of luminol and enhance the ECL emission. Jia’s group synthesized MoS2-PEI-Au nanocomposites which significantly amplified the ECL sensing signal [35]. Wei’s group used the good catalytic effect of MoS2 nanoflowers to accelerate the decomposition of H2O2, increase the ECL intensity of luminol, and improve the sensitivity of quenching sandwich-type immunosensor [36]. Notably, 1T/2H MoS2 that has higher electrocatalytic activity is rarely applied in ECL field.
In view of the above, we constructed a novel ECL biosensor combined the HIFU pretreatment and 1T/2H MoS2 for sensitive detection of miRNA-155. HIFU pretreatment promotes the conversion of dissolved O2 to ROS through the well-known cavitation effect, which could accelerate the oxidation of luminol and improve the ECL response. 1T/2H MoS2 with excellent catalytic performance accelerates the decomposition of H2O2 generated in situ, thus further enhancing the ECL emission of luminol. Scheme 1 is the design of the ECL sensor. 1T/2H MoS2 was modified by sDNA as a probe (Scheme 1A). Ti3C2-Pt nanomaterial was modified on glassy carbon electrode (GCE) surface to bind H1 via the Pt-S bonds. The catalytic hairpin assembly (CHA) reaction was carried out to realize the miRNA-155 cycle. Then, the modified electrode was incubated in 1T/2H MoS2 nanoprobe solution (Scheme 1B). Finally, ECL detection was carried out with the assistance of HIFU pretreatment (Scheme 1C). The designed ECL biosensor shows high sensitivity for miRNA-155 detection, which will provide a new prospect for the research and analysis of miRNA-155.
Scheme 1.
(A) Design of 1T/2H MoS2 nanoprobe. (B) Construction of the biosensor. (C) HIFU pretreatment and then the ECL detection.
2. Experimental section
The subsection of materials, instruments, the preparation of Ti3C2-Pt, 1T/2H MoS2 and 1T/2H MoS2 nanoprobe are shown in Supplementary Material.
2.1. Construction of biosensor
The GCE was polished with 0.3 and 0.05 μm alumina obtaining a mirror-like surface. Electrode was ultrasonically treated in ethanol and deionized (DI) water, which was dried with nitrogen. Then, 6 μL Ti3C2-Pt was dropped onto the GCE surface and dried. Subsequently, Ti3C2-Pt/GCE was incubated in 40 μL H1 solution (2 μM) for 12 h to obtain H1/Ti3C2-Pt/GCE. After that, H1/Ti3C2-Pt/GCE was incubated in 40 μL MCH solution (0.5 mM) for 2 h to eliminate the non-specific binding effect and obtain MCH/H1/Ti3C2-Pt/GCE. Next, 20 μL H2 (2 μM) and 20 μL miRNA-155 were mixed, and CHA reaction was performed in the above mixed solution to obtain H2 + H1/MCH/Ti3C2-Pt/GCE. Finally, H2 + H1/MCH/Ti3C2-Pt/GCE was incubated in 1T/2H MoS2 nanoprobe solution for 2 h to obtain the 1T/2H MoS2/H2 + H1/MCH/Ti3C2-Pt/GCE.
2.2. HIFU pretreatment
HIFU pretreatment was performed in luminol solution (50 μM, pH 10.91) for 5 min with 7.5 W, and then removed before the ECL detection. Next, the modified electrode was immediately inserted into the above solution to obtain ECL emission.
3. Results and discussion
3.1. Characterization of Ti3C2 MXene and Ti3C2-Pt
To verify the successful synthesis of Ti3C2 MXene and Ti3C2-Pt, we performed transmission electron microscopy (TEM), dynamic light scattering (DLS), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) characterization. As shown in Fig. 1A, Ti3C2 MXene exhibits monodisperse sheet structure. The data measured by DLS (Fig. S1) proves that the size of Ti3C2 MXene is about 200 nm. Fig. S2 shows the XRD patterns of Ti3AlC2 (a), Ti3C2 (b) and Ti3C2-Pt (c). Comparing with the XRD pattern of Ti3AlC2, the (104) main peak of Ti3C2 disappears and the (002) peak moves to the left, indicating that Al is etched in Ti3AlC2. In addition, the diffraction peaks 2θ ≈ 39.9°, 46.4° and 67.7° correspond to the (111), (200) and (220) planes of Pt, respectively, thus proving the successful synthesis of Ti3C2-Pt. TEM characterization (Fig. 1B) also clearly shows that Pt NPs are deposited on Ti3C2 MXene nanosheets. Ti3C2 MXene and Ti3C2-Pt are further characterized by XPS. Elements Pt, C, Ti, O and F are observed in the XPS map of Ti3C2-Pt in Fig. 1C. C, Ti, O, and F are derived from Ti3C2, and Pt is derived from Pt NPs generated in situ. The XPS pattern of Ti element in Ti3C2 MXene (Fig. S3A) shows that the peaks located at 455.3, 456.3 and 461.6 eV correspond to Ti (II), Ti-C and Ti-O bonds, respectively. As shown in Fig. S3B, when Pt NPs are introduced, Ti element is transformed from Ti (II) to Ti (IV). Meanwhile, as shown in the Pt 4f orbital spectrum (Fig. S3C), the peaks located at 71.2 eV and 74.5 eV are attributed to Pt (0) bonds, while the peaks located at 70.2 eV and 73.5 eV correspond to Pt (II). The above results indicate that Ti3C2-Pt has been successfully prepared.
Fig. 1.
The TEM images of (A) Ti3C2 MXene and (B) Ti3C2-Pt. (C) XPS spectrum of the Ti3C2-Pt. (D) HRTEM image of 1T/2H MoS2. (E) The Raman spectra of bulk MoS2 and 1T/2H MoS2. (F) Zeta potential values of PEI (a), 1T/2H MoS2 (b) and 1T/2H MoS2-PEI (c).
3.2. Characterization of 1T/2H MoS2
The successful synthesis of 1T/2H MoS2 was demonstrated by scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), Raman and zeta characterization. 1T/2H MoS2 is a distinct sheet-like structure (Fig. S4). We chose HRTEM as a tool to detect the phase of 1T/2H MoS2 because of its high resolution and high definition. As shown in Fig. 1D, the 1T phase region shows the Mo-S octahedral coordination, while the 2H phase region exhibits the typical Mo-S trigonalprismatic coordination. In Raman spectrum (Fig. 1E), the bulk MoS2 before chemical intercalation is a completely 2H phase, thus it only shows two characteristic peaks at 380 cm−1 (E2g) and 404 cm−1 (A1g). In addition to characteristic peaks of 2H phase, exfoliated MoS2 has 1T structure in the Raman spectrum, which appears at around 150, 280 and 330 cm−1, corresponding J1, E1g, and J3 modes respectively [37]. The HRTEM image and Raman spectrum could prove that 1T/2H MoS2 was successful synthesized. 1T/2H MoS2 shows a negative zeta potential, while 1T/2H MoS2-PEI becomes electropositive of 11.01 mV, indicating the successful modification of the cationic polymer PEI (Fig. 1F).
3.3. Effects of HIFU pretreatment and 1T/2H MoS2 catalysis on luminol-O2 ECL
The effects of bulk MoS2 and 1T/2H MoS2 in luminol-O2 ECL system with HIFU pretreatment were investigated. In Fig. 2A, the lower ECL signals of bare GCE (a), bulk MoS2/GCE (b) and 1T/2H MoS2/GCE (c) were observed. After luminol solution was pretreated with HIFU, the ECL signal was significantly enhanced (curves d, e, f), and the signal value of 1T/2H MoS2/GCE (f) was higher than bulk MoS2/GCE (e). According to the above results, it is speculated that HIFU pretreatment could promote the conversion of O2 to ROS by cavitation effect, thus enhancing the ECL signal of luminol. Meanwhile, 1T/2H MoS2 catalyzes the conversion of H2O2 generated in situ to O2•− by virtue of its high catalytic activity, which further amplifies the ECL signal of luminol. In addition, we analyzed the stability of ECL signal value of 1T/2H MoS2/GCE in luminol-O2 with HIFU pretreatment by consecutive potential scans for 10 cycles (Fig. S5). The ECL intensity almost remained unchanged, indicating its excellent stability.
Fig. 2.
(A) ECL intensity of bare GCE (a), bulk MoS2/GCE (b) and 1T/2H MoS2/GCE (c) in luminol-O2 without HIFU pretreatment; ECL intensity of bare GCE (d), bulk MoS2/GCE (e) and 1T/2H MoS2/GCE (f) in luminol-O2 with HIFU pretreatment. (B) ECL intensity of 1T/2H MoS2/GCE in luminol with HIFU pretreatment variation in air-saturated (a), N2-saturated (b) and addition benzoquinone (BQ) (c) as radical scavengers. (C) The UV–vis analysis of the mixture solution of TMB and HRP (a), the mixture solution of TMB and HRP with HIFU pretreatment (b), and the inset is the photograph of a and b. (D) ECL intensity of 1T/2H MoS2/GCE in 50 μM luminol containing 30 μM H2O2 (a), 20 μM H2O2 (b) and 1T/2H MoS2/GCE in 50 μM luminol with HIFU pretreatment (c).
With aim of proving the speculation, we verified the importance of O2 firstly. In Fig. 2B, ECL intensity of 1T/2H MoS2/GCE in luminol-O2 with HIFU pretreatment was the highest (curve a). We continuously injected N2 into luminol solution to remove O2, 1T/2H MoS2/GCE obtained extremely low ECL response (curve b), which proved that O2 played a vital role in luminol ECL system. In order to further confirm which kind of ROS played a key role, benzoquinone (BQ) as a scavenger was used to capture O2•−. ECL signal was significantly reduced which indicating that O2•− produced by HIFU was crucial for the enhancement of luminol ECL (curve c). In addition, the in situ production of H2O2 by HIFU pretreatment was verified by colorimetric analysis. HRP could catalyze H2O2 to oxidation of substrate TMB into colored substance TMB*+, which the peak absorbance is about 650 nm. In Fig. 2C, the characteristic peak of TMB*+ was not shown at 650 nm in the mixed solution of TMB and HRP (curve a). Surprisingly, using the strategy of HIFU pretreatment, the color of the solution turned blue and the peak of 650 nm appeared (curve b), which suggested that the HIFU could generate H2O2 in the air saturated solution.
In the comparative experiment, H2O2 as an exogenous coreaction reagent was used in the luminol system without HIFU pretreatment (Fig. 2D). The results showed that the effect achieved by HIFU pretreatment was equivalent to the 20 ∼ 30 μM H2O2.
On this basis, a possible mechanism for luminol-O2 ECL system based on HIFU pretreatment and 1T/2H MoS2 catalyze could be proposed as follows Fig. 3 and Eqs. (1) ∼ (7). First, a large amount of luminol anions (LH−) generate luminol anion radical (L•−) by electrochemical oxidation. In route I, O2 in the system could obtain e− on the electrode to produce O2•−. In route II, HIFU pretreatment generates H2O2 and O2•− effectively through the cavitation effect, and 1T/2H MoS2 catalyzes the conversion of H2O2 produced in situ to more O2•−. Finally, O2•− interacts with the L•− to produce the excited-state intermediate (AP2−*), which returns to the ground state to obtain significant ECL emission.
Fig. 3.
The mechanism of luminol-O2 ECL system with HIFU pretreatment and 1T/2H MoS2 catalysis.
3.4. Electrochemical characterization of biosensor
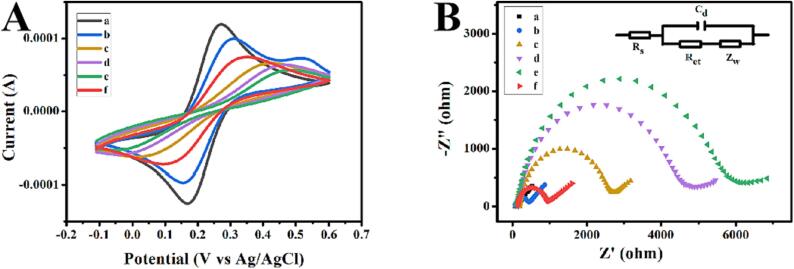
The construction of proposed ECL sensor was characterized by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). When Ti3C2-Pt was modified on the GCE, the electronic transmission of [Fe(CN)6]3−/4− on the GCE surface was impeded, and the peak current decreased (Fig. 4A, curve b). Moreover, the peak current continuously decreased with successive decoration of H1, MCH, and H2 on the electrode surface, which could be ascribed to their non-conductivity. Finally, when the 1T/2H MoS2 nanoprobe was modified (curve f), the redox peak current increased owing to good conductivity of 1T/2H MoS2. EIS is also a good method to characterize the electrode self-assembly process (Fig. 4B). The inset is the equivalent circuit of EIS, and the four elements are internal resistance of solution (Rs), charge-transfer resistance (Ret), Constant phase element (Cd) and Warburg resistances (Zw), respectively. With the assembly of the biosensor, the semicircle diameter gradually increased, indicating that the electron transfer resistance gradually increased. When the 1T/2H MoS2 nanoprobe was incubated, the resistance on the electrode surface decreased. These results correspond to those in CV, implying that this ECL sensor was constructed successfully.
Fig. 4.
Cyclic voltammograms (A) and electrochemical impedance spectra (B) of bare GCE (a), Ti3C2-Pt/GCE (b), H1/Ti3C2-Pt/GCE (c), MCH/H1/Ti3C2-Pt/GCE (d), H2 + H1/MCH/Ti3C2-Pt/GCE (e) and 1T/2H MoS2/H2 + H1/MCH/Ti3C2-Pt/GCE (f) in 5 mM [Fe(CN)6]3−/4− containing 0.1 M KCl solution. The concentration of miRNA-155 is 1 pM. The inset is the equivalent circuit of EIS.
3.5. ECL behavior of biosensor
The effects of HIFU pretreatment and the 1T/2H MoS2 were demonstrated, and shown in Fig. 5. Compared with H2 + H1/MCH/Ti3C2-Pt/GCE (a) and 1T/2H MoS2/H2 + H1/MCH/Ti3C2-Pt/GCE (b) without HIFU pretreatment, the higher ECL signal in luminol-O2 solution with HIFU pretreatment was observed at the H2 + H1/MCH/Ti3C2-Pt/GCE (c) and 1T/2H MoS2/H2 + H1/MCH/Ti3C2-Pt/GCE (d). This is mainly attributed to the fact that HIFU could generate ROS effectively by virtue of its excellent cavitation effect. Comparing with (c), (d) showed higher ECL emission, which thanks to its catalytic effect of 1T/2H MoS2 on H2O2. The synergistic effects of the HIFU pretreatment and the 1T/2H MoS2 result in amplification for ECL signal in luminol-O2 system.
Fig. 5.
ECL intensity H2 + H1/MCH/Ti3C2-Pt/GCE (a), 1T/2H MoS2/H2 + H1/MCH/Ti3C2-Pt/GCE (b) in luminol-O2 without HIFU pretreatment; H2 + H1/MCH/Ti3C2-Pt/GCE (c) and 1T/2H MoS2/H2 + H1/MCH/Ti3C2-Pt/GCE (d) in luminol-O2 with HIFU pretreatment, the concentration of miRNA-155 is 1 pM.
3.6. Optimization of experimental conditions
We optimized the CHA reaction time and probe incubation time in a view to obtaining the best experimental results. As displayed in Fig. S6A, the strongest ECL signal was obtained when CHA reaction time reached 2 h, indicating that the CHA reaction had reached saturation. If we continue to increase the reaction time, ECL signal will decrease instead. The incubation time of the 1T/2H MoS2 probe was carried out (Fig. S6B), when the probe incubation time was 2 h, the ECL intensity reached the maximum, thus proving the hybridization reaction of H2 reached saturation at 2 h. In the experiment, the optimal time for CHA reaction is 2 h, and the optimal time for probe incubation is 2 h.
3.7. ECL detection of miRNA-155
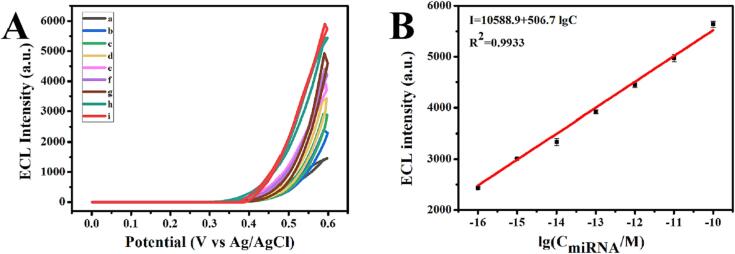
Upon the above optimization conditions, the developed detection strategy was applied for quantitative analysis of miRNA-155. As indicated from Fig. 6A, the ECL intensity was continuously amplified with the increase of target concentration. A satisfying linear relationship between the concentration of miRNA-155 from 0.1 fM to 100 pM and the ECL signal was obtained (Fig. 6B). The linear regression equation is IECL = 10588.9 + 506.7lgCmiRNA-155, and the correlation coefficient is 0.9933. Meanwhile, the limit of detection (LOD) is 0.057 fM calculated by LOD = 3σ/K (σ represents the background standard deviation obtained from 10 parallel experiments and K represents the slope of the regression equation). In addition, we summarized the analytical performance of the reported methods and listed in the Table S1. The comparison shows that our work has a higher sensitivity and wider linear range.
Fig. 6.
(A) ECL intensity-potential curves for various concentrations of miRNA-155 (0 fM, 0.1 fM, 1.0 fM, 10 fM, 100 fM, 1.0 pM, 10 pM, 100 pM, 1 nM (from a to i)). (B) The linear relationship between the ECL intensity and the logarithmic value of miRNA-155 concentration.
3.8. Selectivity and stability of biosensor
Selectivity and stability play an important role in evaluating ECL sensing performance. In Fig. 7A, the ECL responses of the biosensor for blank solution, non-complementary DNA (100 pM), non-complementary RNA (100 pM) and single-base difference RNA (100 pM) were weak, while it was strong to miRNA-155 (10 pM), demonstrating the wonderful selectivity of the ECL biosensor. The stability of the sensing platform was analyzed by consecutive potential scans for 10 cycles (Fig. 7B). The ECL intensity had no obvious variation and the RSD was 0.80%, thus indicating the excellent stability for the proposed ECL sensing platform.
Fig. 7.
(A) Selectivity of the designed ECL biosensor: blank (a), noncomplementary DNA (b), noncomplementary RNA (c), single-base difference RNA (d) and miRNA-155 (e). (B) Stability test of the designed ECL biosensor, the concentration of miRNA-155 is 1 pM.
3.9. Analysis of miRNA-155 in actual samples
We performed a recovery test in human serum to study the feasibility of the designed ECL biosensor. By calculation, the recovery rates were 97.72 % ∼ 104.71 % with the RSD in the range of 2.70 % ∼ 7.27 % (Table 1). Thus, the designed ECL biosensing platform could be used in human samples to determine RNA, and have a good prospect in clinical application.
Table 1.
Determination of miRNA-155 in human serum samples.
| Sample | Added (M) | Found (M) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| 1 | 1.00×10−15 | 1.05×10−15 | 3.96 | 104.71 |
| 2 | 1.00×10−14 | 1.02×10−14 | 7.27 | 101.98 |
| 3 | 1.00×10−13 | 9.77×10−14 | 6.75 | 97.72 |
| 4 | 1.00×10−12 | 9.91×10−13 | 2.70 | 99.08 |
4. Conclusion
In summary, we constructed a highly sensitive ECL biosensing platform, which combined the HIFU pretreatment, 1T/2H MoS2 and CHA reaction to detect and analyze miRNA-155. In this strategy, HIFU pretreatment generates ROS in situ to improve the ECL signal of luminol, meanwhile 1T/2H MoS2 has good conductivity and electrocatalytic activity further catalyze the H2O2 generated in situ to enhance the ECL response. The ECL biosensing platform has excellent selectivity, stability, reproducibility, and sensitivity. This study combining ultrasound with physical materials proposes a new way to improve the ECL response of luminol-O2 system, and also brings a new strategy for miRNA-155 ultra-sensitive detection, which will inspire the development of novel ECL platforms in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Shandong Province (ZR2020MB063), the Taishan Scholar Program of Shandong Province (ts201511027), Key Project of Innovative Teaching Laboratory of Qingdao University in 2020 (CXSYZD202004). We thank Dr. Lili Lv, Xiyue Cao and Huiqi Wang from Instrumental Analysis Center of Qingdao University for their help in TEM, SEM and XPS measurements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106264.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Ma C., Cao Y., Gou X., Zhu J.-J. Recent progress in electrochemiluminescence sensing and imaging. Analytical Chemistry. 2020;92(1):431–454. doi: 10.1021/acs.analchem.9b04947. [DOI] [PubMed] [Google Scholar]
- 2.Feng M., Dauphin A.L., Bouffier L., Zhang F., Wang Z., Sojic N. Enhanced cathodic electrochemiluminescence of luminol on iron electrodes. Analytical Chemistry. 2021;93(49):16425–16431. doi: 10.1021/acs.analchem.1c03139. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H., Wang Z., Wang F., Zhang Y., Wang H., Liu Y. In situ formation of gold nanoparticles decorated Ti3C2 MXenes nanoprobe for highly sensitive electrogenerated chemiluminescence detection of exosomes and their surface proteins. Analytical Chemistry. 2020;92(7):5546–5553. doi: 10.1021/acs.analchem.0c00469. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Zhang H., Zhuang T., Liu J., Sojic N., Wang Z. Sensitive electrochemiluminescence biosensing of polynucleotide kinase using the versatility of two-dimensional Ti3C2Tx MXene nanomaterials. Analytica Chimica Acta. 2022;1191:339346. doi: 10.1016/j.aca.2021.339346. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H.X., Wang Z.H., Zhang Q.X., Wang F., Liu Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosensors and Bioelectronics. 2018;124:184–190. doi: 10.1016/j.bios.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Cheng S., Xu R., Yang F., Huang J., Sun X., Huang X., Li H.e., Li F., Guo Y., Hasanzadeh M., Zhu Y. Novel sandwich-type electrochemiluminescence aptasensor based on luminol functionalized aptamer as signal probe for kanamycin detection. Bioelectrochemistry. 2022;147:108174. doi: 10.1016/j.bioelechem.2022.108174. [DOI] [PubMed] [Google Scholar]
- 7.Xia H., Zheng X., Li J., Wang L., Xue Y., Peng C., Han Y., Wang Y., Guo S., Wang J., Wang E. Identifying luminol electrochemiluminescence at the cathode via single-atom catalysts tuned oxygen reduction reaction. Journal of the American Chemical Society. 2022;144(17):7741–7749. doi: 10.1021/jacs.2c00865. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang T., Zhang H., Wang L., Yu L., Wang Z. Anchoring luminol based on Ti3C2-mediated in situ formation of Au NPs for construction of an efficient probe for miRNA electrogenerated chemiluminescence detection. Analytical and Bioanalytical Chemistry. 2021;413(28):6963–6971. doi: 10.1007/s00216-021-03651-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y., Liao H., Chai Y., Yuan R. Electrochemiluminescence from a biocatalysis accelerated N-(aminobutyl)-N-(ethylisoluminol)/dissolved O2 system for microRNA detection. Microchimica Acta. 2021;188(6) doi: 10.1007/s00604-021-04854-6. [DOI] [PubMed] [Google Scholar]
- 10.Pu Y., Zhou M., Wang P., Wu Q., Liu T., Zhang M. An ultrasensitive electrochemiluminescence sensor based on luminol functionalized AuNPs@Fe-Co-Co nanocomposite as signal probe for glutathione determination. Journal of Electroanalytical Chemistry. 2020;873:114374. [Google Scholar]
- 11.Liu J.-L., Yang R., Chai Y.-Q., Yuan R. Versatile luminol/dissolved oxygen/Fe@Fe2O3 nanowire ternary electrochemiluminescence system combined with highly efficient strand displacement amplification for ultrasensitive microRNA detection. Analytical Chemistry. 2021;93(39):13334–13341. doi: 10.1021/acs.analchem.1c03102. [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Jia Y., Wu D., Zhang Y., Ju H., Du Y.u., Ma H., Wei Q. Synthesis and application of CeO2/SnS2 heterostructures as a highly efficient coreaction accelerator in the luminol-dissolved O2 system for ultrasensitive biomarkers immunoassay. Analytical Chemistry. 2019;91(21):14066–14073. doi: 10.1021/acs.analchem.9b03796. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X.L., Li W.M., Zhou Y., Chai Y.Q., Yuan R. An ultrasensitive electrochemiluminescence biosensor for MicroRNA detection based on luminol-functionalized Au NPs@ZnO nanomaterials as signal probe and dissolved O2 as coreactant. Biosensors and Bioelectronics. 2019;135:8–13. doi: 10.1016/j.bios.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Bushira F.A., Kitte S.A., Wang Y., Li H., Wang P., Jin Y. Plasmon-boosted Cu-doped TiO2 oxygen vacancy-rich luminol electrochemiluminescence for highly sensitive detection of alkaline phosphatase. Analytical Chemistry. 2021;93(45):15183–15191. doi: 10.1021/acs.analchem.1c03842. [DOI] [PubMed] [Google Scholar]
- 15.Bushira F.A., Wang P., Jin Y. High-entropy oxide for highly efficient luminol-dissolved oxygen electrochemiluminescence and biosensing applications. Analytical Chemistry. 2022;94(6):2958–2965. doi: 10.1021/acs.analchem.1c05005. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y., She D., Huang H., Lin L., Chen S., Lu Y., Liu L.i., Pang Z., Yin B.o. Versatile nanocomposite augments high-intensity focused ultrasound for high-efficacy sonodynamic therapy of glioma. Nano Research. 2022;15(10):9082–9091. [Google Scholar]
- 17.Li X.L., Zhu X.G., He S.L., Jiang Z.Y., Li H.H., Tian X.B., Long W.X., Xue M., Deng X.L., Ye M.Z. High-intensity focused ultrasound in the management of adenomyosis: long-term results from a single center. International Journal of Hyperthermia. 2021;38:241–247. doi: 10.1080/02656736.2021.1886347. [DOI] [PubMed] [Google Scholar]
- 18.Gao H., Wang Z.X., Tan M.X., Liu W.W., Zhang L., Huang J., Cao Y., Li P., Wang Z.G., Wen J.X., Shang T.T., Ran H.T. pH-responsive nanoparticles for enhanced antitumor activity by high-intensity focused ultrasound therapy combined with sonodynamic therapy. International Journal of Nanomedicine. 2022;17:333–350. doi: 10.2147/IJN.S336632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukahara K., Umemura S.-I., Yoshizawa S. Effect of ultrasonic intensity and intervals of ultrasonic exposure on efficiency of sonochemiluminescence in gel phantom for sonodynamic therapy. Japanese Journal of Applied Physics. 2021;60 [Google Scholar]
- 20.Ning Z.Y., Zhu Z.F., Wang H.Y., Zhang C.Y., Xu L.T., Zhuang L.P., Yan X., Wang D., Wang P., Meng Z.Q. High-intensity focused ultrasound enhances the effect of bufalin by inducing apoptosis in pancreatic cancer cells. Oncotargets and Therapy. 2019;12:1161–1170. doi: 10.2147/OTT.S185953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloney E., Hwang J.H. Emerging HIFU applications in cancer therapy. International Journal of Hyperthermia. 2015;31:302–309. doi: 10.3109/02656736.2014.969789. [DOI] [PubMed] [Google Scholar]
- 22.Walton D.J., Phull S.S., Colton D., Richards P., Chyla A., Javed T., Clarke L., Lorimer J.P., Mason T.J. Ultrasongic enhancement of electrochemiluminescence from arylaceate electrooxidation. Ultrasonics Sonochemistry. 1994 [Google Scholar]
- 23.Takahashi F., Shimizu R., Nakazawa T., Jin J. Potential-modulated electrochemiluminescence of a tris(2,2'-bipyridine)ruthenium(II)/lidocaine system under 430 kHz ultrasound irradiation. Ultrasonics Sonochemistry. 2020;63 doi: 10.1016/j.ultsonch.2019.104947. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H.X., Du L., Wei Z.H., Wang X.M., Sojic N., Zhou X., Wang Z.H. Boosting the electrochemiluminescence of luminol-O2 system by high-intensity focused ultrasound. Analytical and Bioanalytical Chemistry. 2022;414:8309–8315. doi: 10.1007/s00216-022-04365-0. [DOI] [PubMed] [Google Scholar]
- 25.Ge R., Huo J., Sun M., Zhu M., Li Y., Chou S., Li W. Surface and interface engineering: Molybdenum carbide-based nanomaterials for electrochemical energy conversion. Small. 2021;17 doi: 10.1002/smll.201903380. [DOI] [PubMed] [Google Scholar]
- 26.Primo A., He J., Jurca B., Cojocaru B., Bucur C., Parvulescu V.I., Garcia H. CO2 methanation catalyzed by oriented MoS2 nanoplatelets supported on few layers graphene. Applied Catalysis B: Environmental. 2019;245:351–359. [Google Scholar]
- 27.Gong S., Zhao G.Y., Lyu P.B., Sun K.N. A pseudolayered MoS2 as Li-ion intercalation host with enhanced rate capability and durability. Small. 2018;14 doi: 10.1002/smll.201803344. [DOI] [PubMed] [Google Scholar]
- 28.Miao Y.P., Li Y., Fang Q.L., Huang Y.H., Sun Y.J., Xu K.W., Ma F., Chu P.K. Effects of dopant separation on electronic states and magnetism in monolayer MoS2. Applied Surface Science. 2018;428:226–232. [Google Scholar]
- 29.Zhang X.Y., Fu K., Su Z.Q. Fabrication of 3D MoS2-TiO2@PAN electro-spun membrane for efficient and recyclable photocatalytic degradation of organic dyes. Materials Science and Engineering B-advanced Functional Solid-state Materials. 2021;269 [Google Scholar]
- 30.Liu J.F., Lin H., He Y.H., Dong Y.B., E.r. Gueret Yadiberet Menzembere, Novel CoS2/MoS2@Zeolite with excellent adsorption and photocatalytic performance for tetracycline removal in simulated wastewater. Journal of Cleaner Production 260. 2020 [Google Scholar]
- 31.Singh V.K., Gupta U., Mukherjee B., Chattopadhyay S., Das S. MoS2 nanosheets on MoNi4/MoO2 nanorods for hydrogen evolution. ACS Applied Nano Materials. 2021;4:886–896. [Google Scholar]
- 32.Huang W., Zhou D.J., Yang H., Liu X.J., Luo J. Dual-doping promotes the carbon dioxide electroreduction activity of MoS2 nanosheet array. ACS Applied Energy Materials. 2021;4:7492–7496. [Google Scholar]
- 33.Zhang L., Ji X.Q., Ren X., Ma Y.J., Shi X.F., Tian Z.Q., Asiri A.M., Chen L., Tang B., Sun X.P. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: Theoretical and experimental studies. Advanced Materials. 2018;30 doi: 10.1002/adma.201800191. [DOI] [PubMed] [Google Scholar]
- 34.Liang Z.Q., Xue Y.J., Guo Y.C., Zhang G.S., Cui H.Z., Tian J. Rationalizing and controlling the phase transformation of semi-metallic 1T'-phase and semi-conductive 2H-phase MoS2 as cocatalysts for photocatalytic hydrogen evolution. Chemical Engineering Journal. 2020;396 [Google Scholar]
- 35.Zhang X., Guo W.W., Wang Z.M., Ke H., Zhao W.J., Zhang A., Huang C.S., Jia N.Q. A sandwich electrochemiluminescence immunosensor for highly sensitive detection of alpha fetal protein based on MoS2-PEI-Au nanocomposites and Au@BSA core/shell nanoparticles. Sensors and Actuators B: Chemical. 2017;253:470–477. [Google Scholar]
- 36.Li X.J., Wu D., Ma H.M., Wang H., Wang Y.G., Fan D.W., Du B., Wei Q., Zhang N. Ultrasensitive amyloid-beta proteins detection based on curcumin conjugated ZnO nanoparticles quenching electrochemiluminescence behavior of luminol immobilized on Au@MoS2/Bi2S3 nanorods. Biosensors and Bioelectronics. 2019;131:136–142. doi: 10.1016/j.bios.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 37.Park S., Kim C., Park S.O., Oh N.K., Kim U., Lee J., Seo J., Yang Y.J., Lim H.Y., Kwak S.K., Kim G., Park H. Phase engineering of transition metal dichalcogenides with unprecedentedly high phase purity, stability, and scalability via molten-metal-assisted intercalation. Advanced Materials. 2020;32 doi: 10.1002/adma.202001889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.