Abstract
Purpose
As a secondary report to elucidate the diverse spectrum of oncofertility practices for childhood cancer around the globe, we present and discuss the comparisons of oncofertility practices for childhood cancer in limited versus optimum resource settings based on data collected in the Repro-Can-OPEN Study Part I & II.
Methods
We surveyed 39 oncofertility centers including 14 in limited resource settings from Africa, Asia, and Latin America (Repro-Can-OPEN Study Part I), and 25 in optimum resource settings from the USA, Europe, Australia, and Japan (Repro-Can-OPEN Study Part II). Survey questions covered the availability of fertility preservation and restoration options offered in case of childhood cancer as well as their degree of utilization.
Results
In the Repro-Can-OPEN Study Part I & II, responses for childhood cancer and calculated oncofertility scores showed the following characteristics: (1) higher oncofertility scores in optimum resource settings than in limited resource settings for ovarian and testicular tissue cryopreservation; (2) frequent utilization of gonadal shielding, fractionation of anticancer therapy, oophoropexy, and GnRH analogs; (3) promising utilization of oocyte in vitro maturation (IVM); and (4) rare utilization of neoadjuvant cytoprotective pharmacotherapy, artificial ovary, in vitro spermatogenesis, and stem cells reproductive technology as they are still in preclinical or early clinical research settings.
Conclusions
Based on Repro-Can-OPEN Study Part I & II, we presented a plausible oncofertility best practice model to help optimize care for children with cancer in various resource settings. Special ethical concerns should be considered when offering advanced and innovative oncofertility options to children.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02679-7.
Keywords: Oncofertility, Childhood cancer, Fertility preservation, Best practice, Limited resource settings, Optimum resource settings
Introduction
Over the past decades, the number of childhood cancer survivors has steadily increased due to advances in diagnosis and treatment [1, 2]. The most common childhood cancers that may require aggressive gonadotoxic anticancer therapy and hence necessitate fertility preservation measures include leukemia, central nervous system cancers, lymphoma, and sarcomas [1, 2]. According to the most recent international guidelines and recommendations from the American Society of Clinical Oncology (ASCO) [3], the American Society for Reproductive Medicine (ASRM) [4], and the Pediatric Initiative Network (PIN) of the Oncofertility Consortium [5, 6], few established, debatable, and experimental oncofertility options can be offered to pre-pubertal children with cancer to preserve their reproductive potential. Established oncofertility options include ovarian tissue cryopreservation which was considered experimental by ASRM until 2019. Debatable options include gonadal shielding for irradiation, fractionation of chemotherapy and radiotherapy, oophoropexy, and GnRH analogs. Experimental options include testicular tissue cryopreservation, in vitro spermatogenesis, oocyte in vitro maturation (IVM), artificial ovary, neoadjuvant cytoprotective pharmacotherapy, stem cell reproductive technology, and others [3–6].
Despite recognition as official recommendations, oncofertility international guidelines face several challenges in practice especially for children with cancer. Over the past years, the Oncofertility Consortium has studied oncofertility practices in many countries within its Oncofertility Professional Engagement Network (OPEN) [7, 8]. Our previous studies identified a variety of standards and challenges in oncofertility practices worldwide [9–13]. Recently, in our Repro-Can-OPEN Study Part I & II, we proposed installation of specific oncofertility programs for childhood, breast, and blood cancers in limited versus optimum resource settings. The main objectives of Repro-Can-OPEN Study Part I & II included (a) empirical measurement of the availability and degree of utilization of oncofertility options provided by the surveyed centers, (b) identification of different styles of oncofertility practice for common cancers in limited and optimum resource settings, and (c) suggestion of best practice models for oncofertility care based on the results of the survey and the existing literature [14–16].
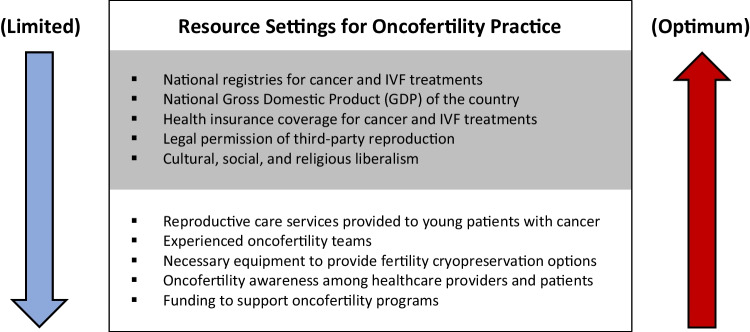
Limited resource settings for oncofertility practice include the following criteria especially as seen in low- and middle-income countries (Fig. 1): shortage of reproductive care services provided to young patients with cancer; lack of experienced oncofertility teams and necessary equipment; lack of national registries for in vitro fertilization (IVF) and/or cancer treatments; lack of awareness among providers and patients, cultural and religious constraints; partial or complete legal prohibition of third-party reproduction; lack of insurance coverage for IVF and/or cancer treatments resulting in high out-of-pocket costs for patients; and lack of funding to support oncofertility programs. Even in developed countries, a state of limited resource settings could be experienced in the absence of dedicated personnel or special lab protocols, or in case of sudden national disasters when most of public services including healthcare are negatively affected as occurred recently during COVID-19 pandemic and its related economic shutdown. Additionally, within developed countries, there may be specific regions that qualify as limited resource [14].
Fig. 1.
Limited versus optimum resource settings affecting oncofertility practice on national (grey) and local (white) levels
Optimum resource settings for oncofertility practice include the following criteria especially as seen in high-income countries (Fig. 1): availability of reproductive care services provided to young patients with cancer, availability of experienced oncofertility teams and necessary equipment, presence of national registries for IVF and cancer treatments, awareness among providers and patients, minimal cultural or religious constraints, legally allowed third-party reproduction, insurance coverage for IVF and cancer treatments, and availability of funding to support oncofertility programs [15].
As a secondary report to elucidate the diverse spectrum of oncofertility practices for childhood cancer around the globe, we present and discuss the comparisons of oncofertility practices for childhood cancer in limited versus optimum resource settings based on data collected in Repro-Can-OPEN Study Part I & II.
Methods
Data collection
This is a secondary report based on data collected in Repro-Can-OPEN Study Part I & II. As a pilot survey within its network, the Oncofertility Consortium sent the Repro-Can-OPEN Study questionnaire via email to 39 oncofertility centers in total; 14 oncofertility centers with limited resource settings from Africa, Asia, and Latin America in Repro-Can-OPEN Study Part I (2019–2020); and 25 oncofertility centers with optimum resource settings from the USA, Europe, Australia, and Japan in Repro-Can-OPEN Study Part II (2020–2021) (Table 1). The Repro-Can-OPEN Study questionnaire included questions on the availability of fertility preservation options provided to children with cancer (females and males), and whether these options are always, commonly, occasionally, or rarely used. Responses by the leading oncofertility medical teams from surveyed centers were collected, reviewed, and analyzed. For more details about our questionnaire and the collected data, please see the supplementary material (A).
Table 1.
The 39 surveyed oncofertility centers in Repro-Can-OPEN Study Part I & II
| Surveyed oncofertility centers with limited resource settings (Repro-Can-OPEN Study Part I) (n = 14) | |
| 1 | National Research Center, Cairo, Egypt |
| 2 |
Aziza Othmana Hospital of Tunis, Tunisia FERTILLA, Clinique la Rose, Tunis, Tunisia |
| 3 | Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil |
| 4 |
Laboratorio de Biología Reproductiva y Preservación de la Fertilidad, Laboratorios de Investigación y Desarrollo, Universidad Peruana Cayetano Heredia, Lima, Peru Unidad de Oncología Pediátrica, Hospital Edgardo Rebagliati Martins, Lima, Peru |
| 5 | Panama Fertility, Sistema Nacional de Investigadores, Panama City, Panama |
| 6 |
Pregna Medicina Reproductiva, Buenos Aires, Argentina Hospital de Niños Ricardo Gutierrez, Buenos Aires, Argentina Procrearte, Buenos Aires, Argentina Hospital de Niños Victor J. Vilela. Rosario, Santa Fe, Argentina |
| 7 | Centro de Reproduccion Humana, Facultad de Medicina, Universidad de Valparaiso, Valparaiso, Chile |
| 8 | Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico |
| 9 |
Fertility Preservation Centre, Department of Clinical Embryology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India Department of Medical Oncology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India Mother and Child Hospital, New Delhi, India Dr. Patil's Fertility and Endoscopy Clinic, Bangalore, India Hospital Institute of Medical Sciences & SRCC children’s Hospital, Mumbai, India |
| 10 |
Vitalab Fertility Centre, Johannesburg, South Africa Department Medical Oncology, University of Witwatersrand, Johannesburg, South Africa Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa |
| 11 |
Instituto Nacional de Cancerología, Bogota, Colombia FERTIVIDA Fertility Center, Bogota, Colombia |
| 12 | Instituto Guatemalteco de Seguridad Social (IGSS), Guatemala City, Guatemala |
| 13 | Thuriah Medical Center, Riyadh, Kingdom of Saudi Arabia |
| 14 |
The Oncology and Fertility Centres of Ekocorp Plc, Eko Hospitals, Lagos, Nigeria Kingswill Specialist Hospital, Lagos, Nigeria |
| Surveyed Oncofertility Centers with Optimum Resource Settings (Repro-Can-OPEN Study Part II): (n = 25) | |
| 1 |
Oncofertility Consortium, Feinberg School of Medicine, Northwestern University, Chicago, IL 60,611, USA Ann & Robert H. Lurie Children’s Hospital of Chicago, 225 East Chicago Ave, Box 63, Chicago IL, 60,611, USA |
| 2 | Yale Fertility Center and Yale Fertility Preservation program, 200 West Campus Dr., Orange, CT 06,477, USA |
| 3 | Karolinska Institutet, Department of Oncology-Pathology and Karolinska University Hospital, Department of Reproductive Medicine, Division of Gynecology and Reproduction, SE-14186, Stockholm, Sweden |
| 4 | Department of Obstetrics and Gynecology, St. Marianna University School of Medicine, 2–16-1, Sugao, Miyamae-ku, Kawasaki, Kanagawa, Japan |
| 5 |
Department of Medical Oncology, UOC Clinica di Oncologia Medica, IRCCS Ospedale Policlinico San Martino, Genova, Italy Department of Internal Medicine and Medical Specialties (DiMI), School of Medicine, University of Genova, Genova, Italy |
| 6 |
Fertility Preservation Service, Reproductive Services Unit, Royal Women’s Hospital, Parkville, 3051, Australia Fertility Preservation Service, Melbourne IVF, East Melbourne, 3002, Australia |
| 7 | Children’s National Hospital, 111 Michigan Avenue NW, Washington, DC 20,010, USA. (ZIA# HD008985) |
| 8 | Center for Reproductive Medicine, Michigan Medicine, 475 Market Place, Building 1, Suite B, Ann Arbor, MI 48,108, USA |
| 9 | Fertility Research Centre, Royal Hospital for Women, Barker Street, Sydney, Australia |
| 10 | Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA, USA |
| 11 |
University of Edinburgh, Edinburgh, UK Royal Infirmary of Edinburgh and Royal Hospital for Children and Young People, Little France Crescent, Edinburgh, UK |
| 12 | Nationwide Children’s Hospital, 700 Children's Dr., Columbus, OH 43,205, USA |
| 13 | University of Pennsylvania, Division of Reproductive Endocrinology & Infertility, 3701 Market Street, Suite 8000, Philadelphia, PA 19,104, USA |
| 14 | New York University, NYU Langone Fertility Center, 660 First Ave, 5th Floor, New York, NY 10,016, USA |
| 15 | UniKiD—Center for Reproductive Medicine, UniCareD—Center for Fertility Preservation, Düsseldorf University Hospital, Moorenstrasse 5, D-40225 Düsseldorf, Germany |
| 16 | Laboratory of Reproductive Biology, Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, Blegdamsvej 9, DK-2100 Copenhagen, Denmark |
| 17 | Fertility Preservation Service, The Royal Children’s Hospital, Flemington Rd, Parkville, Melbourne, Vic 3054, Australia |
| 18 | University of California, San Diego, 3855 Health Sciences Drive, La Jolla, CA 92,039–0901, USA |
| 19 |
Cliniques Universitaires Saint Luc, Université Catholique de Louvain, Avenue Hippocrate, 10, 1200 Brussels, Belgium Université Catholique de Louvain, Avenue Mounier 52, 1200 Brussels, Belgium |
| 20 | Fertility Clinic and Research Laboratory on Human Reproduction, CUB-Erasme Hospital, Université Libre de Bruxelles (ULB), 808 route de Lennik, 1070 Brussels, Belgium |
| 21 | Centre for Reproductive Medicine of UZ Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium |
| 22 | Gynecological Endocrinology and Reproductive Medicine Division, Obstetrics and Gynecology Department, Cologne University Hospital, Cologne, Germany |
| 23 | Center for Reproduction and Transplantation, Magee-Womens Hospital, University of Pittsburgh Medical Center, 300 Halket Street, Pittsburgh, PA 15,213, USA |
| 24 |
University of Cincinnati, Department of Obstetrics and Gynecology, Division for REI, Cincinnati, OH 45,229, USA Cincinnati Children’s Hospital Medical Center, Division of Pediatric Adolescent Gynecology Pediatric, Cincinnati, OH 45,229, USA |
| 25 |
Urology Department, UCSF Medical Center, University of California, San Francisco, CA 94,143, USA Obstetrics and Gynecology Department, UCSF Medical Center, University of California, San Francisco, CA 94,143, USA |
Data analysis
To analyze the collected data, we used descriptive statistics and developed a new scoring system, “the oncofertility score” [14–16]. As previously described, the oncofertility score, is a diagnostic tool to measure the availability and degree of utilization of oncofertility options for cancer patients in a treating center, country, or group of centers or countries. Although empirical, the oncofertility score could be also used as a prognostic tool to follow up on the development of oncofertility options and strategies provided to cancer patients over time especially in absence of accurate national oncofertility registries [14–16]. The oncofertility score is calculated as a percentile ratio between the actual and maximal points of utilization that an oncofertility option might have (Table 2 and Fig. 2). When a fertility preservation option is available and always used for cancer patients, it is given (Yes + + + +) that weighs 100 actual points (25 points per each +). When a fertility preservation option is available and commonly used for cancer patients, it is given (Yes + + +) that weighs 75 points (25 points per each +). When a fertility preservation option is available but occasionally used for cancer patients, it is given (Yes + +) that weighs 50 points (25 points per each +). When a fertility preservation option is available but rarely used or only used in research settings for cancer patients, it is given (Yes +) that weighs 25 points (25 points per each +). When a fertility preservation option is not available, it is given (No) that weighs 0 points. When the fertility preservation option is not available to cancer patients because it is still in the preclinical research stage, it is marked with (No*). The maximal points of utilization that an oncofertility option might have is 100 when it is available and always used for cancer patients and is given (Yes + + + +) (25 points per each +) [14–16].
Table 2.
Oncofertility score calculation
| Availability and utilization of an oncofertility option | Available and always used for cancer patients | Available and commonly used for cancer patients | Available but occasionally used for cancer patients | Available but rarely used or only used in research settings for cancer patients | Not available |
|---|---|---|---|---|---|
| Scale symbol | + + + + | + + + | + + | + | - |
|
Actual points (AP) (25 points per +) |
100 | 75 | 50 | 25 | 0 |
|
Maximal points (MP) (100 points per + + + +) |
100 | 100 | 100 | 100 | 100 |
|
Oncofertility score = AP/MP (%) |
100% | 75% | 50% | 25% | 0% |
Fig. 2.
Oncofertility score calculation
In our Repro-Can-OPEN Study Part I & II, the oncofertility score was calculated as a percentile ratio between the total actual points and the total maximal points of utilization that an oncofertility option might have. The total actual points for an oncofertility option equal the sum of actual points for this option in the surveyed centers. The total maximal points for an oncofertility option equal 100 points multiplied by the number of surveyed centers [14–16].
Results
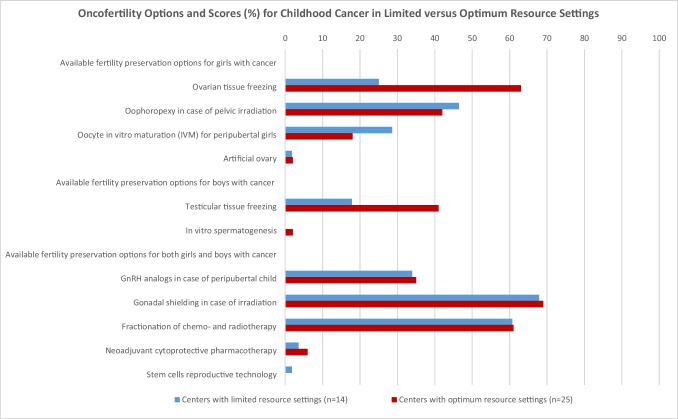
Based on data collected in the Repro-Can-OPEN Study Parts I and II, all 39 surveyed centers responded to all questions. The oncofertility scores (%) for options provided to children with cancer in the 14 centers with limited resource settings versus in the 25 centers with optimum resource settings respectively were as follows (Table 3 and Fig. 3).
Table 3.
Oncofertility options and scores (%) for childhood cancer in limited versus optimum resource settings, based on empirical data from 39 surveyed centers in Repro-Can-OPEN Study Part I & II [14, 15]
| Oncofertility options and scores (%) for childhood cancer | Centers with limited resource settings (Repro-Can-OPEN Study I) |
Centers with optimum resource settings (Repro-Can-OPEN Study II) |
|---|---|---|
| (n = 14) | (n = 25) | |
| Available fertility preservation options for girls with cancer | ||
| Ovarian tissue freezing | 25 | 63 |
| Oophoropexy in case of pelvic irradiation | 46.42 | 42 |
| Oocyte in vitro maturation (IVM) for peripubertal girls | 28.57 | 18 |
| Artificial ovary | 1.78 | 2 |
| Available fertility preservation options for boys with cancer | ||
| Testicular tissue freezing | 17.85 | 41 |
| In vitro spermatogenesis | 0 | 2 |
| Available fertility preservation options for both girls and boys with cancer | ||
| GnRH analogs in case of peripubertal child | 33.92 | 35 |
| Gonadal shielding in case of irradiation | 67.85 | 69 |
| Fractionation of chemo- and radiotherapy | 60.71 | 61 |
| Neoadjuvant cytoprotective pharmacotherapy | 3.57 | 6 |
| Stem cells reproductive technology | 1.78 | 0 |
Fig. 3.
Oncofertility options and scores (%) for childhood cancer in limited versus optimum resource settings, based on empirical data from 39 surveyed centers in Repro-Can-OPEN Study Part I & II [14, 15]
Oncofertility options and scores (%) for girls with cancer in limited versus optimum resource settings
Ovarian tissue freezing (25 vs 63), oophoropexy in case of pelvic irradiation (46.42 vs 42), oocyte in vitro maturation (IVM) for peripubertal girls (28.57 vs 18), and artificial ovary (1.78 vs 2).
Oncofertility options and scores (%) for boys with cancer in limited versus optimum resource settings
Testicular tissue freezing (17.85 vs 41), and in vitro spermatogenesis (0 vs 2).
Oncofertility options and scores (%) for both girls and boys with cancer in limited versus optimum resource settings
GnRH analogs in case of peripubertal child (33.92 vs 35), gonadal shielding in case of irradiation (67.85 vs 69), fractionation of chemo- and radiotherapy (60.71 vs 61), neoadjuvant cytoprotective pharmacotherapy (3.57 vs 6), and stem cells reproductive technology (1.78 vs 0).
Discussion
Oncofertility options and scores for childhood cancer in limited versus optimum resource settings
In our Repro-Can-OPEN Study Part I & II, the responses for childhood cancer and their calculated oncofertility scores (Table 3 and Fig. 3) showed the following characteristics: (1) higher oncofertility scores in optimum resource settings than in limited resource settings for ovarian and testicular tissue cryopreservation; (2) frequent utilization of gonadal shielding in case of irradiation, fractionation of chemo- and radiotherapy, oophoropexy, and GnRH analogs; (3) promising utilization of oocyte in vitro maturation (IVM) for peripubertal girls; and (4) rare utilization of neoadjuvant cytoprotective pharmacotherapy, artificial ovary, in vitro spermatogenesis, and stem cells reproductive technology as they are still in preclinical or early clinical research settings.
Counseling considerations for oncofertility care of childhood cancer
Fertility counseling and preservation in the pediatric population is particularly challenging from both a clinical and ethical perspective. Recently in 2019, the American Society for Reproductive Medicine Committee Opinion on fertility preservation in patients undergoing gonadotoxic therapies stated that ovarian tissue freezing and autotransplantation should be considered an established medical procedure and no longer considered experimental [4]. However, in prepubertal females, there are limited outcome data for ovarian tissue freezing, and ongoing data collection is still needed as recommended recently by the Pediatric Initiative Network (PIN) of the Oncofertility Consortium [5]. Testicular tissue freezing, oocyte in vitro maturation (IVM), in vitro spermatogenesis, artificial ovary technology, neoadjuvant cytoprotective pharmacotherapy, and stem cell reproductive technology are still considered experimental, and it is essential that they are offered only under clear ethical regulations. Obtaining ethical approval from the Institutional Review Board (IRB) or the equivalent ethics committee is required, as is obtaining informed consent from the child’s parents or the legal guardians and assent from children older than 7 [17]. Informed consent for experimental medical treatments and interventions should include the explanation of the procedures, benefits, risks, alternative treatments, and information about the expected outcome and costs. Several oncofertility options are expensive and not fully covered by health insurance in many states and countries [18], leaving many parents or legal guardians under acute financial pressure at the time of a life-altering cancer diagnosis.
Fertility preservation decision making can be particularly challenging in pediatric patients, where future oriented thinking is limited and both parents and providers have an important influence [19]. Parents may experience considerable decisional conflict in the face of suboptimal counseling, which can lead to future decision regret [20]. In such complex situations, doctors, and patient navigators as well as patient support and advocacy organizations can play an important role in reassuring and guiding cases [21–23]. The recommended approach to facilitate complex healthcare decisions where there is more than one reasonable choice, and where patients may value benefits and risks differently, is to use an evidence-based decision aid which is a psychoeducational tool providing high-quality unbiased information on options, and values clarification tools to facilitate reasoned and deliberate choices aligned with the user’s own values [24].
Practical considerations for oncofertility care of childhood cancer
Based on the responses and their oncofertility scores in our Repro-Can-OPEN Study Part I & II (Table 3 and Fig. 3) and existing literature, we propose to design and suggest plausible oncofertility programs for childhood cancer as an extrapolation for a best practice model (Table 4). Early referral of patients to highly specialized oncofertility centers for timely discussions and exploration of fertility preservation options is strongly recommended. Immediately after a child is diagnosed with cancer, we recommend early referral to the oncofertility team to review the cancer therapy plan and estimate the related risk of gonadotoxicity and subsequent fertility loss. The risk of anticancer therapy induced gonadotoxicity and fertility loss depends mainly on the type and stage of the disease, type, and dose of anticancer therapy as well as the age of the child at the time of treatment [6]. If the risk of gonadotoxicity and fertility loss is estimated or even unknown, a comprehensive multidisciplinary oncofertility strategy should be offered before, during and after anticancer therapy.
Table 4.
Suggested best practice model: plausible fertility preservation, protection, and restoration strategies for children with cancer
| Cancer patients | Before anticancer therapy (fertility preservation) |
During anticancer therapy (fertility protection) |
After anticancer therapy and later after puberty (fertility restoration) |
|---|---|---|---|
|
Childhood cancer (♀ & ♂) Leukemias, central nervous system cancers, lymphomas and sarcomas |
- Freezing of gonadal tissue - In vitro maturation and vitrification of gametes (promising in research but not yet clinically proven in children) - Oophoropexy in case of female pelvic radiation - Artificial gonads technology (promising in research but not yet clinically proven) |
- Gonadal shielding - Fractionation of chemo- and radiotherapy - GnRH analogs in case of peripubertal child (widely debated) - Neoadjuvant cytoprotective pharmacotherapy (promising in research but not yet clinically proven) |
- IVF/ICSI of frozen in-vitro-matured gametes - Autotransplantation of frozen gonadal tissue (should be utilized with caution in leukemia) - Stem cells (promising in research but not yet clinically proven) |
From a practical point of view, an effective oncofertility strategy should be individualized and tailored to the patient’s circumstances, and it may integrate various established, debatable, and experimental options after proper counseling and obtaining informed consent from the parents or the legal guardians of the child. It is recommended that a proposed oncofertility strategy for children should discuss the feasibility of gonadal tissue cryopreservation when indicated. While it may be challenging for centers with low resource settings to offer gonadal tissue cryopreservation for children, at the very least a dedicated program to ensure counsel on gonadotoxicity risk should be offered. After complete cure or extended remission from cancer, and when the patient becomes an adult and wishes to have biological children, a new assessment of reproductive function should be performed. If anticancer therapy induced premature gonadal failure, fertility restoration may be achieved by using frozen gonadal tissue or in vitro-matured gametes.
Installing oncofertility programs for children with cancer
Unique medical challenges in oncofertility programs for childhood cancer exist and include the following: (1) freezing of gonadal tissues is the only feasible cryopreservation option before puberty and (2) autotransplantation of frozen gonadal tissue may carry the risk of reintroducing malignant cells, especially in leukemia which is the most common childhood cancer [25–31].
According to the aforementioned unique medical challenges, as well as the responses and their oncofertility scores in our Repro-Can-OPEN Study Part I & II (Table 3 and Fig. 3), we suggest developing the following oncofertility programs for childhood cancer as a best practice model (Table 4). Before initiation of anticancer therapy, discussion of gonadotoxicity risk should be paramount. Options to freeze prepubertal gonadal tissues (ovarian or testicular tissue) should be considered when possible [32, 33]. In vitro maturation and further vitrification of gametes (oocytes or spermatozoa) and artificial gonad technology (ovary or testis) are still experimental and cannot be relied upon as effective oncofertility options in children. Although experimental, these emerging technologies of in vitro maturation of gametes and artificial gonads aim to provide safe alternatives to avoid future gonadal tissue autotransplantation and potential reintroduction of malignant cells. Some reports highlighted the current developments and future possibilities of artificial gonads to restore fertility in cancer patients [34–37]. Oophoropexy before female pelvic irradiation should be attempted when possible. During anticancer therapy, gonadal shielding in case of irradiation should be attempted. Fractionation of chemo- and radiotherapy could be attempted whenever deemed feasible by the oncologist. The use of GnRH analogs to preserve fertility during chemotherapy in case of peripubertal children is widely debated and needs additional research to inform evidence-based practice [38, 39]. Neoadjuvant cytoprotective pharmacotherapy is still experimental and not yet clinically proven as an effective oncofertility option. After anticancer therapy, gonadal function should be monitored to ensure appropriate growth, pubertal development, and reproductive function, with hormone replacement therapy introduced for those with gonadal failure. Furthermore, regular follow-up during survivorship offers a window of opportunity for interval fertility and sexual healthcare, linking patients in with the tissue storage laboratory, and discussing expectations around relationships, pregnancy, and parenthood [40].
When the patient becomes an adult and wishes to have biological children, fertility restoration may be possible using frozen gonadal tissue or in vitro-matured gametes. Autotransplantation of frozen gonadal tissue may carry the risk of reintroducing malignant cells, especially in leukemia which is the most common childhood cancer. Proper gonadal tissue assessment is mandatory to reduce the risk of reintroducing malignant cells with autotransplantation. To date, only two live births have been reported after autotransplantation of ovarian tissue frozen during childhood [41, 42]. Testicular tissue autotransplantation is promising in xenografting and animal research but it has not entered human clinical trials yet [43]. Stem cells reproductive technology may be promising in research settings, but it is not yet clinically proven as an effective oncofertility option [44] (Table 4). Some reports highlighted the advances and potentials of stem cells reproductive technology to restore fertility in boys [45] and girls with cancer [46].
Follow-up on oncofertility programs for children with cancer
After installation of such specific oncofertility programs for childhood cancer, we encourage using the “oncofertility score” as a prognostic tool to follow up on the development of these new oncofertility programs over time. In the event where there is limited availability for oncofertility counseling during the time of diagnosis, centers with low resource settings may wish to develop patient facing materials, designed for low literacy, to convey potential risks and available options. Other alternatives include telehealth consultation or peer-to-peer support. The oncofertility consortium provides for free through its website (https://oncofertility.msu.edu/) a variety of helpful resources to oncofertility patients and healthcare professionals. In addition to several educational materials, free online webinars, courses, and workshops are also provided on the oncofertility consortium website to help train healthcare professionals on different aspects of oncofertility practice. Such free online resources enable oncofertility consortium to reach out to patients, healthcare professionals, and scholars around the globe more effectively and efficiently.
In cases where oncofertility options are rejected, contraindicated, infeasible, unsuccessful, or unavailable, other options can be offered later in adulthood such as family building alternatives including adoption and third-party reproduction, such as sperm, egg, and embryo donation and surrogacy [11].
Limitations of Repro-Can-OPEN Study Part I & II
Limitations of Repro-Can-OPEN Study Part I & II included the small sample size (14 vs 25 surveyed centers with limited and optimum resource settings, respectively) making assessment of statistical significance infeasible, and lack of data on success rates of the oncofertility options due to absence of national registries for cancer and IVF treatments in many developing countries involved in the study [14, 15]. For more details about our questionnaire and the collected data, please see the supplementary material (A). Despite challenges, many opportunities exist to improve oncofertility practice in limited resource settings and create potential for the future including improved cancer survival rates and improved success rates of several oncofertility options as well as emergence of new promising technologies. The Oncofertility Consortium will continue to engage more stakeholders from the USA and abroad [47] to help build a sustainable oncofertility core competency worldwide according to the Oncofertility Consortium Vision 2030 [48].
Next steps and future directions of Repro-Can-OPEN Studies
In our next Repro-Can-OPEN studies, we are planning to investigate in detail the oncofertility programs offered to leukemia and lymphoma patients according to their gender and age groups. We are planning also to investigate other cancers as well as other patient groups (e.g., LGBTQ population: lesbian, gay, bisexual, transgender, and queer or questioning) who were not included in our previous studies. We will provide further discussions on the advanced and the emerging oncofertility options and highlight the recent achievements in the related preclinical research.
Conclusion
Offering fertility preservation services to the pediatric cancer patients is particularly challenging from both a clinical and ethical perspective. Based on data collected from 39 surveyed oncofertility centers in our Repro-Can-OPEN Study Part I & II and the existing literature, this manuscript presents up-to-date recommendations and a plausible best practice model to help optimize oncofertility care for children with cancer in various resource settings.
We surveyed 14 oncofertility centers with limited resource settings from Africa, Asia, and Latin America in our Repro-Can-OPEN Study Part I and 25 oncofertility centers with optimum resource settings from the United States, Europe, Australia, and Japan in our Repro-Can-OPEN Study Part II. In our Repro-Can-OPEN Study Part I and II, the responses for childhood cancer and their calculated oncofertility scores showed the following characteristics: (1) higher oncofertility scores in optimum resource settings than in limited resource settings for ovarian and testicular tissue cryopreservation; (2) frequent utilization of gonadal shielding in case of irradiation, fractionation of chemo- and radiotherapy, oophoropexy, and GnRH analogs; (3) promising utilization of oocyte in vitro maturation (IVM); (4) rare utilization of neoadjuvant cytoprotective pharmacotherapy, artificial ovary, in vitro spermatogenesis, and stem cells reproductive technology as they are still in preclinical or early clinical research settings. Special ethical concerns should be considered when offering experimental oncofertility options to children. Although challenging, oncofertility teams working with children in limited resource settings should be supported to learn more about ovarian and testicular tissue cryopreservation as they are the only feasible fertility cryopreservation options before puberty.
Despite its limitations, this is the first report to date that presents comparisons between oncofertility programs for childhood cancer in limited versus optimum resource settings according to 39 surveyed centers. Dissemination of our comparisons, up-to-date recommendations, and best practice model may provide efficient oncofertility edification and modelling to pediatric oncofertility teams and related healthcare providers around the globe and help them offer the best care possible to their children with cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Collaborators of Repro-Can-OPEN Study Part I
Salama M, Ataman-Millhouse L, Braham M, Berjeb K, Khrouf M, Rodrigues JK, Reis FM, Silva TC, Sánchez F, Romero S, Smitz J, Vásquez L, Vega M, Sobral F, Terrado G, Lombardi MG, Scarella A, Bourlon MT, Verduzco-Aguirre H, Sánchez AM, Adiga SK, Tholeti P, Udupa KS, Mahajan N, Patil M, Dalvi R, Venter C, Demetriou G, Geel J, Quintana R, Rodriguez G, Quintana T, Viale L, Fraguglia M, Coirini M, Remolina-Bonilla YA, Noguera JAR, Velásquez JC, Suarez A, Arango GD, Pineda JID, Aldecoa MDC, Javed M, Al Sufyan H, Daniels N, Oranye BC, Ogunmokun AA, Onwuzurigbo KI, Okereke CJ, Whesu TC, Woodruff TK.
Collaborators of Repro-Can-OPEN Study Part II
Salama M, Laronda MM, Rowell E, Erickson L, Goldman K , Smith K, Pavone M, Duncan FE, Brannigan R, Ataman-Millhouse L, Patrizio P, Rodriguez-Wallberg KA, Okutsu-Horage Y, Suzuki N, Lambertini M, Stern C, Gomez-Lobo V, Maher JY, Hsieh MH, Moravek MB, Anazodo A, Westphal LM, Anderson RA, Wallace WH, Mitchell RT, Nahata L, Whiteside S, Senapati S, Shah DK, Gracia C, Fino ME, Blakemore JK, Quinn GP, Krüssel JS, Baston-Büst DM, Liebenthron J, Andersen CY, Kristensen SG, Mamsen LS, Jayasinghe Y, Su HI, Dolmans MM, Amorim CA, Demeestere I, De Vos M, Van Moer E, Isachenko V, Isachenko E, Mallmann P, Rahimi G, Valli-Pulaski H, Steimer SR, McMahon KV, Orwig KE, Rios JS, Smith JF, Mok-Lin E, Woodruff TK.
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
M Salama, Email: salamam2@msu.edu.
L. Nahata, Email: Leena.nahata@nationwidechildrens.org
Y. Jayasinghe, Email: Yasmin.jayasinghe@unimelb.edu.au
V. Gomez-Lobo, Email: Veronica.gomez-lobo@nih.gov
MM. Laronda, Email: mlaronda@luriechildrens.org
MB. Moravek, Email: mpenderg@med.umich.edu
LR. Meacham, Email: illian.meacham@emory.edu
MS. Christianson, Email: mchris21@jhmi.edu
M. Lambertini, Email: matteo.lambertini@unige.it
A. Anazodo, Email: Antoinette.anazodo@health.nsw.gov.au
GP. Quinn, Email: gwendolyn.quinn@nyulangone.org
TK. Woodruff, Email: tkw@msu.edu
References
- 1.Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of the American Society for Reproductive Medicine (ASRM) Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112(6):1022–1033. doi: 10.1016/j.fertnstert.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Nahata L, Woodruff TK, Quinn GP, Meacham LR, Chen D, Appiah LC, Finlayson C, Orwig KE, Laronda MM, Rowell EE, Anazodo A, Frias O, Rios JS, Whiteside S, Gomez-Lobo V, Dwiggins M, Childress KJ, Hoefgen HR, Levine JM, Jayasinghe Y, Moravek M. Ovarian tissue cryopreservation as standard of care: what does this mean for pediatric populations? J Assist Reprod Genet. 2020;37(6):1323–1326. doi: 10.1007/s10815-020-01794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meacham LR, Burns K, Orwig KE, Levine J. Standardizing risk assessment for treatment-related gonadal insufficiency and infertility in childhood adolescent and young adult cancer: the pediatric initiative network risk stratification system. J Adolesc Young Adult Oncol. 2020;9(6):662–666. doi: 10.1089/jayao.2020.0012. [DOI] [PubMed] [Google Scholar]
- 7.Oncofertility Consortium - Michigan State University [Internet]. [cited 2022 July 30]. Available from: <http://oncofertility.msu.edu/>
- 8.Oncofertility Professional Engagement Network (OPEN) - Michigan State University [Internet]. [cited 2022 July 30]. Available from: <https://oncofertility.msu.edu/about/oncofertility-professional-engagement-network/>
- 9.Ataman LM, Rodrigues JK, Marinho RM, et al. Creating a global community of practice for oncofertility. J Glob Oncol. 2016;2(2):83–96. doi: 10.1200/JGO.2015.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashedi A, de Roo SF, Ataman L, et al. A survey of fertility preservation options available to cancer patients around the globe. J Glob Oncol. 2018;4:1–16. doi: 10.1200/JGO.2016.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashedi A, de Roo SF, Ataman L, et al. A survey of third-party parenting options associated with fertility preservation available to patients with cancer around the globe. J Glob Oncol. 2018;4:1–7. doi: 10.1200/JGO.2017.009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salama M, Ataman L, Taha T, et al. Building oncofertility core competency in developing countries: experience from Egypt, Tunisia, Brazil, Peru, and Panama. J Glob Oncol. 2018;4:1–11. doi: 10.1200/JGO.17.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salama M, Ataman-Millhouse L, Sobral F, et al. Barriers and opportunities of oncofertility practice in nine developing countries and the emerging oncofertility professional engagement network. J Glob Oncol. 2018;4:1–7. doi: 10.1200/JGO.18.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salama M, Ataman-Millhouse L, Braham M, et al. Installing oncofertility programs for common cancers in limited resource settings (Repro-Can-OPEN Study): an extrapolation during the global crisis of Coronavirus (COVID-19) pandemic. J Assist Reprod Genet. 2020;37(7):1567–1577. doi: 10.1007/s10815-020-01821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Practice Committee of the Oncofertility Consortium Installing oncofertility programs for common cancers in optimum resource settings (Repro-Can-OPEN Study Part II): a committee opinion. J Assist Reprod Genet. 2021;38(1):163–176. doi: 10.1007/s10815-020-02012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama M, Lambertini M, Christianson MS, Jayasinghe Y, Anazodo A, De Vos M, Amant F, Stern C, Appiah L, Woodard TL, Anderson RA, Westphal LM, Leach RE, Rodriguez-Wallberg KA, Patrizio P, Woodruff TK. Installing oncofertility programs for breast cancer in limited versus optimum resource settings: empirical data from 39 surveyed centers in Repro-Can-OPEN Study Part I & II. J Assist Reprod Genet. 2022;39(2):505–516. doi: 10.1007/s10815-022-02394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDougall RJ, Gillam L, Delany C, Jayasinghe Y. Ethics of fertility preservation for prepubertal children: should clinicians offer procedures where efficacy is largely unproven? J Med Ethics. 2018;44(1):27–31. doi: 10.1136/medethics-2016-104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sax MR, Pavlovic Z, DeCherney AH. Inconsistent mandated access to fertility preservation: a review of relevant state legislation. Obstet Gynecol. 2020;135(4):848–851. doi: 10.1097/AOG.0000000000003758. [DOI] [PubMed] [Google Scholar]
- 19.Klosky JL, Wang F, Russell KM, Zhang H, Flynn JS, Huang L, Wasilewski-Masker K, Landier W, Leonard M, Albritton KH, Gupta AA, Casillas J, Colte P, Kutteh WH, Schover LR. Prevalence and predictors of sperm banking in adolescents newly diagnosed with cancer: examination of adolescent, parent, and provider factors influencing fertility preservation outcomes. J Clin Oncol. 2017;35(34):3830–3836. doi: 10.1200/JCO.2016.70.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayasuriya S, Peate M, Allingham C, Li N, Gillam L, Zacharin M, Downie P, Moore P, Super L, Orme L, Agresta F, Stern C, Jayasinghe Y. Satisfaction, disappointment and regret surrounding fertility preservation decisions in the paediatric and adolescent cancer population. J Assist Reprod Genet. 2019;36(9):1805–1822. doi: 10.1007/s10815-019-01536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, Bradford N, Cohn R, Birdsall M, Barr R, Suzuki N, Takae S, Marinho R, Xiao S, Qiong-Hua C, Mahajan N, Patil M, Gunasheela D, Smith K, Sender L, Melo C, Almeida-Santos T, Salama M, Appiah L, Su I, Lane S, Woodruff TK, Pacey A, Anderson RA, Shenfield F, Ledger W, Sullivan E. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. 2019;25(2):159–179. doi: 10.1093/humupd/dmy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, Bradford N, Cohn R, Birdsall M, Barr R, Suzuki N, Takae S, Marinho R, Xiao S, Chen QH, Mahajan N, Patil M, Gunasheela D, Smith K, Sender L, Melo C, Almeida-Santos T, Salama M, Appiah L, Su I, Lane S, Woodruff TK, Pacey A, Anderson RA, Shenfield F, Sullivan E, Ledger W. The development of an international oncofertility competency framework: a model to increase oncofertility implementation. Oncologist. 2019;24(12):e1450–e1459. doi: 10.1634/theoncologist.2019-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salama M, Woodruff TK. Anticancer treatments and female fertility: clinical concerns and role of oncologists in oncofertility practice. Expert Rev Anticancer Ther. 2017;17(8):687–692. doi: 10.1080/14737140.2017.1335199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allingham C, Gillam L, McCarthy M, Zacharin M, Jayasuriya S, Heloury Y, Orme L, Sullivan M, Peate M, Jayasinghe Y. Fertility preservation in children and adolescents with cancer: pilot of a decision aid for parents of children and adolescents with cancer. JMIR Pediatr Parent. 2018;1(2):e10463. doi: 10.2196/10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salama M, Isachenko V, Isachenko E, Rahimi G, Mallmann P. Updates in preserving reproductive potential of prepubertal girls with cancer: systematic review. Crit Rev Oncol Hematol. 2016;103:10–21. doi: 10.1016/j.critrevonc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 2015;34(4):807–822. doi: 10.1007/s10555-015-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corkum KS, Rhee DS, Wafford QE, et al. Fertility and hormone preservation and restoration for female children and adolescents receiving gonadotoxic cancer treatments: a systematic review. J Pediatr Surg. 2019;54(11):2200–2209. doi: 10.1016/j.jpedsurg.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Goossens E, Jahnukainen K, Mitchell RT, van Pelt A, Pennings G, Rives N, Poels J, Wyns C, Lane S, Rodriguez-Wallberg KA, Rives A, Valli-Pulaski H, Steimer S, Kliesch S, Braye A, Andres MM, Medrano J, Ramos L, Kristensen SG, Andersen CY, Bjarnason R, Orwig KE, Neuhaus N, Stukenborg JB. Fertility preservation in boys: recent developments and new insights. Hum Reprod Open. 2020;2020(3):hoaa016. doi: 10.1093/hropen/hoaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stukenborg JB, Wyns C. Fertility sparing strategies for pre- and peripubertal male cancer patients. Ecancermedicalscience. 2020;14:1016. doi: 10.3332/ecancer.2020.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgström B, Fridström M, Gustafsson B, Ljungman P, Rodriguez-Wallberg KA. A prospective study on the long-term outcome of prepubertal and pubertal boys undergoing testicular biopsy for fertility preservation prior to hematologic stem cell transplantation. Pediatr Blood Cancer. 2020;67(9):e28507. doi: 10.1002/pbc.28507. [DOI] [PubMed] [Google Scholar]
- 31.Ho WLC, Bourne H, Gook D, et al. A short report on current fertility preservation strategies for boys. Clin Endocrinol (Oxf) 2017;87(3):279–285. doi: 10.1111/cen.13377. [DOI] [PubMed] [Google Scholar]
- 32.Wikander I, Lundberg FE, Nilsson H, Borgström B, Rodriguez-Wallberg KA. A prospective study on fertility preservation in prepubertal and adolescent girls undergoing hematological stem cell transplantation. Front Oncol. 2021;11:692834. doi: 10.3389/fonc.2021.692834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borgström B, Fridström M, Gustafsson B, Ljungman P, Rodriguez-Wallberg KA. A prospective study on the long-term outcome of prepubertal and pubertal boys undergoing testicular biopsy for fertility preservation prior to hematologic stem cell transplantation. Pediatr Blood Cancer. 2020;67(9):e28507. doi: 10.1002/pbc.28507. [DOI] [PubMed] [Google Scholar]
- 34.Salama M, Woodruff TK. From bench to bedside: current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet Gynecol Scand. 2019;98(5):659–664. doi: 10.1111/aogs.13552. [DOI] [PubMed] [Google Scholar]
- 35.Chiti MC, Donnez J, Amorim CA, Dolmans MM. From isolation of human ovarian follicles to the artificial ovary: tips and tricks. Minerva Ginecol. 2018;70(4):444–455. doi: 10.23736/S0026-4784.18.04231-4. [DOI] [PubMed] [Google Scholar]
- 36.Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: from research to clinic. Hum Reprod. 2013;28(4):897–907. doi: 10.1093/humrep/det039. [DOI] [PubMed] [Google Scholar]
- 37.Onofre J, Baert Y, Faes K, Goossens E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. 2016;22(6):744–761. doi: 10.1093/humupd/dmw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bangalore Krishna K, Fuqua JS, Rogol AD, et al. Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr. 2019;91(6):357–372. doi: 10.1159/000501336. [DOI] [PubMed] [Google Scholar]
- 39.Allen NG, Krishna KB, Lee PA. Use of gonadotropin-releasing hormone analogs in children. Curr Opin Pediatr. 2021;33(4):442–448. doi: 10.1097/MOP.0000000000001026. [DOI] [PubMed] [Google Scholar]
- 40.Jayasinghe Y, Wallace W, Anderson R. Ovarian function, fertility and reproductive lifespan in cancer patients. Expert Endocrinol Reviews. 2018;13(3):125–136. doi: 10.1080/17446651.2018.1455498. [DOI] [PubMed] [Google Scholar]
- 41.Demeestere I, Simon P, Dedeken L, Moffa F, Tsépélidis S, Brachet C, Delbaere A, Devreker F, Ferster A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30(9):2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 42.Matthews SJ, Picton H, Ernst E, Andersen CY. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. 2018;70(4):432–435. doi: 10.23736/S0026-4784.18.04240-5. [DOI] [PubMed] [Google Scholar]
- 43.Wyns C, Kanbar M, Giudice MG, Poels J. Fertility preservation for prepubertal boys: lessons learned from the past and update on remaining challenges towards clinical translation. Hum Reprod Update. 2021;27(3):433–459. doi: 10.1093/humupd/dmaa050. [DOI] [PubMed] [Google Scholar]
- 44.Vermeulen M, Giudice MG, Del Vento F, Wyns C. Role of stem cells in fertility preservation: current insights. Stem Cells Cloning. 2019;12:27–48. doi: 10.2147/SCCAA.S178490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David S, Orwig KE. Spermatogonial stem cell culture in oncofertility. Urol Clin North Am. 2020;47(2):227–244. doi: 10.1016/j.ucl.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunlop CE, Telfer EE, Anderson RA. Ovarian stem cells–potential roles in infertility treatment and fertility preservation. Maturitas. 2013;76(3):279–283. doi: 10.1016/j.maturitas.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Ataman LM, Laronda MM, Gowett M, et al. A synopsis of global frontiers in fertility preservation. J Assist Reprod Genet. 2022;39(8):1693–1712. doi: 10.1007/s10815-022-02570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodruff TK, Ataman-Millhouse L, Acharya KS, et al. A View from the past into our collective future: the oncofertility consortium vision statement. J Assist Reprod Genet. 2021;38(1):3–15. doi: 10.1007/s10815-020-01983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).