Abstract
Actinobacillus actinomycetemcomitans, the etiologic agent for localized juvenile periodontitis and certain other human infections, such as endocarditis, expresses a leukotoxin that acts on polymorphonuclear leukocytes and macrophages. Leukotoxin is a member of the highly conserved repeat toxin (RTX) family of bacterial toxins expressed by a variety of pathogenic bacteria. While the RTX toxins of other bacterial species are secreted, the leukotoxin of A. actinomycetemcomitans is thought to remain associated with the bacterial cell. We have examined leukotoxin production and localization in rough (adherent) and smooth (nonadherent) strains of A. actinomycetemcomitans. We found that leukotoxin expressed by the rough, adherent, clinical isolate CU1000N is indeed cell associated, as expected. However, we were surprised to find that smooth, nonadherent strains of A. actinomycetemcomitans, including Y4, JP2 (a strain expressing a high level of toxin), and CU1060N (an isogenic smooth variant of CU1000N), secrete an abundance of leukotoxin into the culture supernatants during early stages of growth. After longer times of incubation, leukotoxin disappears from the supernatants, and its loss is accompanied by the appearance of a number of low-molecular-weight polypeptides. The secreted leukotoxin is active, as evidenced by its ability to kill HL-60 cells in vitro. We found that the growth phase and initial pH of the growth medium significantly affect the abundance of secreted leukotoxin, and we have developed a rapid (<2 h) method to partially purify large amounts of leukotoxin. Remarkably, mutations in the tad genes, which are required for tight nonspecific adherence of A. actinomycetemcomitans to surfaces, cause leukotoxin to be released from the bacterial cell. These studies show that A. actinomycetemcomitans has the potential to secrete abundant leukotoxin. It is therefore appropriate to consider a possible role for leukotoxin secretion in the pathogenesis of A. actinomycetemcomitans.
Actinobacillus actinomycetemcomitans is a gram-negative, capnophilic, facultatively anaerobic bacterium responsible for several human diseases, including localized juvenile periodontitis and infective endocarditis (14, 47, 63). Clinical isolates of A. actinomycetemcomitans form colonies that appear rough, autoaggregate, and adhere tenaciously to surfaces, such as glass, plastic, and hydroxyapatite (13). These properties are associated with the expression of characteristic long fibrils, whose presence is dependent on the tadABCDEFG genes (23). Spontaneous smooth-colony, nonadherent variants arise readily during subculture (13, 62), but it is not known if these variants have any clinical relevance. A. actinomycetemcomitans has also been reported to express several potential virulence factors (14), of which the best studied is the 116-kDa cytotoxic leukotoxin (26, 34, 35).
Leukotoxin is a member of the RTX family of toxins (33, 60, 61), which include the Escherichia coli α-hemolysin (12), Pasteurella haemolytica leukotoxin (36), Bordetella pertussis bifunctional adenylate cyclase hemolysin (17), and other related toxins in a wide range of pathogens. The toxins of the RTX family are large (>100 kDa), basic proteins that contain C-terminal glycine-rich repeats. The repeats are responsible for binding divalent calcium, which is required for toxin activity (7, 8, 9, 21). In addition, they all share the unique characteristic of being modified by lipid acylation, the only example of such a protein modification in the prokaryotic world (52). With the apparent exception of A. actinomycetemcomitans leukotoxin, which is thought to be entirely cell associated, all other RTX toxins are secreted from the bacterial cell via type I secretion (6, 29, 57, 61). The adenylate cyclase of B. pertussis is both cell associated and released into the culture medium (37). While the cell target specificity of the RTX bacterial toxins is generally broad, that of A. actinomycetemcomitans leukotoxin is highly specific for the polymorphonuclear leukocytes (PMNs) and macrophages of humans and monkeys (53, 54).
Two models for the mechanism of RTX toxin-induced cell death have been proposed. The first model proposes toxin insertion into the membrane of the target cell to cause rapid cell lysis (at high doses) or apoptosis (at low doses) (4, 5, 25, 31, 38, 49). This model holds that the protein toxin forms a pore that allows passage of small molecules through the cell membrane. Other studies have led to a second model, in which the toxin does not pass completely through the target cell membrane (2, 18, 43, 48, 49). Rather, the toxin remains in the outer leaflet of the lipid bilayer. By displacing lipid molecules in the outer leaflet, the toxin causes cell death by lateral pressure and subsequent monolayer collapse (49).
The toxin biosynthetic genes in the various bacteria are present in identically arranged operons of four genes in the order CABD (26, 35, 52, 60, 61). The primary structures of the proteins encoded by the different organisms are significantly related, and their functions are thought to be conserved. The structural gene for the RTX toxin is the second gene of the operon (e.g., hlyA in E. coli). The proteins responsible for the maturation and secretion of E. coli α-hemolysin (HlyA) are among the best studied. The acyltransferase required to modify α-hemolysin is encoded by hlyC, the first gene of the operon (20, 21, 22, 51), whereas the hlyB and hlyD gene products are involved in type I secretion of toxin from the bacterial cell (27–30, 44, 59). A third protein, TolC, whose gene lies outside the toxin operon, is also required for toxin secretion (58).
It is generally accepted that A. actinomycetemcomitans is unique among the RTX toxin-producing bacteria because it does not secrete its leukotoxin (LtxA). Instead, the toxin remains associated with the bacterial cell, possibly within membranous vesicles or electrostatically associated with nucleic acids bound to the cell surface (3, 32, 41, 57). This property implies that leukotoxin-induced killing requires target cells to be in direct contact with the bacteria.
While examining secreted proteins from rough and smooth strains of A. actinomycetemcomitans, we were surprised to observe an abundance of leukotoxin in the supernatants of young cultures of smooth strains. Here we present an analysis of leukotoxin production and secretion by different strains of A. actinomycetemcomitans and we show the effects of both environmental and genetic factors. Finally, we discuss the possible relevance of these findings to the role of leukotoxin in the pathogenesis by A. actinomycetemcomitans.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A. actinomycetemcomitans strains (Table 1) were grown in AAGM broth (13) containing 30 g of Trypticase soy broth (BBL) and 6 g of yeast extract (BBL) per liter, 0.75% glucose, and 0.4% NaHCO3. The glucose and NaHCO3 were added to the medium after autoclaving. AAGM plates were made similarly, except that 40 g of Trypticase agar was substituted for the Trypticase soy broth. When appropriate, media for A. actinomycetemcomitans were supplemented with 4 μg of chloramphenicol and 20 μg of nalidixic acid/ml. Plates were incubated at 37°C in a CO2-enriched environment in a sealed GasPak container (BBL) for 72 h. Broth cultures of A. actinomycetemcomitans were grown in screwcap plastic tubes at 37°C for approximately 24 h. In certain experiments, the pH of the medium was changed with either 1 M NaOH or 1 M HCl. The nalidixic acid-resistant strains were isolated by plating dense cell suspensions on medium containing nalidixic acid. In every case, AAGM broth was inoculated with a fresh, single, well-isolated colony, and never did we culture from broth to broth or from plate to plate. Cells were always streaked from frozen stocks to avoid passages of the strains. The E. coli strain used as the host to transfer plasmids to A. actinomycetemcomitans by conjugation was Top10 (InVitrogen). E. coli strains were grown on Luria-Bertani plates and broth overnight at 37°C with aeration. When needed, the following antibiotics were used for E. coli: kanamycin, 50 μg/ml; chloramphenicol, 50 μg/ml. All of the IncQ plasmids listed in Table 1 were mobilized from E. coli donor cells to A. actinomycetemcomitans recipients by the RK2 oriT-defective mutant plasmid, pRK21761, as previously described (56).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| A. actinomycetemcomitans strains | ||
| CU1000N | Spontaneous nalidixic acid-resistant mutant of rough clinical isolate CU1000 | 23 |
| CU1060 | Spontaneous smooth, nonadherent variant of CU1000 | 23 |
| CU1060N | Spontaneous nalidixic acid-resistant mutant of CU1060 | 23 |
| JP2 | High-level leukotoxin producer; nonadherent, smooth | 50 |
| Y4 | Nonadherent, smooth | 39 |
| AA1360 | Derivative of CU1000N; tadA2::IS903φkan; Kmr | 23 |
| AA1332 | Derivative of CU1000N; tadB7::IS903φkan; Kmr | 23 |
| AA1359 | Derivative of CU1000N; tadC3::IS903φkan; Kmr | 23 |
| AA1577 | Derivative of CU1000N; tadD19::IS903φkan; Kmr | 23 |
| Plasmids | ||
| pEK2 | pJAK16 with tadA from CU1000N expressed from tac promoter | 23 |
| pJAK16 | Broad-host-range mobilizable IncQ vector derived from pMMB67; Cmr, tac promoter, lacIq | 56 |
| pRK21761 | oriT mutant derivative of RK2 used to mobilize plasmids from E. coli to A. actinomycetemcomitans; Kmr AprtetA::lacZYA | 45 |
| pSK69 | pJAK16 with tadCD from CU1000N expressed from tac promoter | 23 |
| pSK98 | pJAK16 with tadC from CU1000N expressed from tac promoter | 23 |
| pSK128 | pJAK16 with tadB from CU1000N expressed from tac promoter | 23 |
Preparation of leukotoxin from A. actinomycetemcomitans.
The following small-scale method was used for all experiments in which leukotoxin was prepared only to compare its abundance and localization in different strains. After the strains were grown in broth (5 ml) for the desired time, 1 ml of cell suspension was removed and added to a sterile Eppendorf tube. For strains which are adherent, cells were first scraped from the wall. The tube was then centrifuged at 16,000 × g for 2 min to pellet cells. Five hundred microliters of supernatant was removed to a new Eppendorf tube, to which 1 ml of ice-cold 100% ethanol was added to precipitate protein (11). In initial experiments, the supernatant was further passed through a 0.22-μm-pore-size syringe filter (HT Tuffryn membrane; Pall Corporation, Ann Arbor, Mich.); however, no difference was ever observed. The supernatant-ethanol mixture was placed at −80°C for 5 min and then centrifuged as before at 4°C for 15 min. After centrifugation, the supernatant was removed and the resulting pellet was allowed to dry on the bench top for several minutes. Ten microliters of sodium dodecyl sulfate (SDS) sample buffer (0.0625 M Tris-HCl [pH 6.8], 10% glycerol, 5% β-mercaptoethanol, 2% SDS, 0.005% bromophenol blue) was added, and the pellets were resuspended. All 10 μl was added to each lane of the polyacrylamide gel. The original tube containing the cell pellet and remaining supernatant was inverted to remove all of the liquid. The cell pellet was resuspended directly in 100 μl of SDS sample buffer, and 10 μl of this was added per lane.
For the large-scale isolation of leukotoxin from CU1060N, cells were grown for 12 h in broth as before, except that colonies were inoculated into 10 ml of AAGM instead of 5 ml. Cultures were pooled and centrifuged at 10,000 × g for 10 min. The supernatant was then passed through a 0.22-μm-pore-size syringe filter to remove any remaining cells which could obstruct the concentrator apparatus. The filtered supernatant was then concentrated to 1/100 of the initial volume using a concentrator unit (Ultrafree PF-60 Biomax-50K membrane filter device; Millipore, Bedford, Mass.) according to the manufacturer's instructions. Briefly, the units were centrifuged for 1.5 h at 1,000 × g in a Sorvall RT6000B refrigerated centrifuge. The concentrated samples were stored either at −20 or 4°C.
SDS-polyacrylamide gel electrophoresis (PAGE) of protein samples.
All of the protein samples were boiled for 5 min and separated by electrophoresis through a 12% polyacrylamide gel with a stacking gel of 5% (1). The gel was run at 70 to 100 V until the dye ran to the end of the gel. Gels were stained overnight with 0.5% Coomassie blue and destained in 50% methanol–10% acetic acid for 1 to 2 h. Densitometric analysis was done using Kodak Digital Science 1D image analysis software.
In-gel digestion and MALDI analysis of leukotoxin.
Identification of leukotoxin from the rough and smooth strains was performed by the Columbia University Protein Chemistry Core Facility. Gels were prepared for digestion by staining them with Coomassie blue and excising the bands with a clean blade. The gel bands were placed in acid-washed microcentrifuge tubes and lightly crushed with a tissue grinder and then washed with 400 μl of 0.05 M Tris (pH 8.5)–50% acetonitrile for 20 min with shaking. The supernatant was discarded, and the wash was repeated. The washed gel pieces were dried for 30 min in a Speed-Vac concentrator. Forty microliters of digestion buffer (0.025 M Tris, pH 8.5), containing 0.1 μg of endoproteinase Lys-C (Boehringer Mannheim; sequencing grade), was added to the tube containing the dried gel, and the tube was incubated for 20 h at 32°C. When digestion was complete, peptides were extracted by adding 100 μl of 50% acetonitrile–0.1% trifluoroacetic acid (TFA), shaking the tube for 30 min, and removing the supernatant to a clean tube. The extraction was repeated, and the combined supernatants were dried in a Speed-Vac concentrator. Matrix solution for matrix-assisted laser desorption ionization (MALDI) analysis was prepared by making a 10-mg/ml solution of 4-hydroxy-a-cyanocinnamic acid in 50% acetonitrile–0.1% TFA and adding an internal standard (angiotensin) to the matrix. The dried digest was dissolved in 4 μl of matrix-standard solution, and 0.8 μl was spotted onto the sample plate. MALDI-mass spectrometry (MALDI-MS) was performed on the digest using a PerSeptive Voyager DE-RP mass in spectrometer in the linear mode.
Cell culture and flow cytometry.
HL-60 cells were grown as previously described (25). Fluorescence-activated cell sorting (FACS) analysis was performed essentially as described by Karakelian et al. (25) at the Columbia University Flow Cytometry Facility using a FACScan instrument (Becton Dickinson). Approximately 30 μg of leukotoxin and 100 μg of propidium iodide (PI) were added to each 6-ml suspension of HL-60 cells at time zero, and the suspension was allowed to incubate for the desired time at 37°C. At each time point, 0.5 ml was removed and analyzed. Cells that were PI positive were scored as dead. During each sampling, 20,000 events (cells) were recorded by the FACS instrument. A control of uninoculated medium, which was taken through the whole leukotoxin purification scheme, was included with every experiment.
RESULTS
Appearance of abundant leukotoxin in culture supernatants of smooth strains.
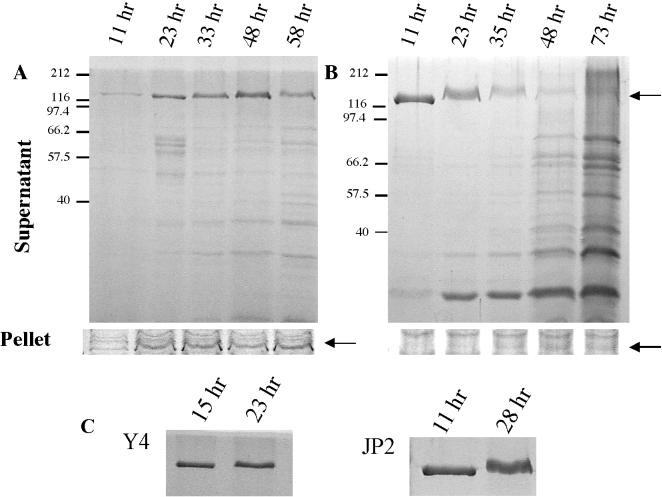
While comparing proteins from culture supernatants of a rough clinical isolate of A. actinomycetemcomitans (CU1000N) and an isogenic smooth variant (CU1060N), we noted an intensely staining species of approximately 120 kDa from the supernatant of a young culture of the smooth strain (Fig. 1B). This species was also present in the supernatant of the rough strain, but in a much smaller amount (Fig. 1A). To attempt to identify the protein, the band from the polyacrylamide gel was extracted and analyzed by MALDI-MS. The protein was identified unequivocally as A. actinomycetemcomitans leukotoxin (LtxA) (GenBank accession no. 79293).
FIG. 1.
Localization of leukotoxin from the rough and smooth strains. (A and B) Visualization of cell-free (supernatant) and cell-associated (pellet) leukotoxin from rough strain CU1000N (A) and smooth strain CU1060N (B). Arrows, leukotoxin band (approximately 120 kDa). (C) Leukotoxin in the culture supernatants of the commonly used A. actinomycetemcomitans smooth strains Y4 (left) and JP2, a high-level-leukotoxin producer (right). The times after inoculation at which the samples were taken are noted above each lane. Polypeptides were separated by SDS-PAGE and stained with Coomassie blue. The identity of the leukotoxin band was confirmed by MALDI-MS.
To characterize further the difference in leukotoxin localization by rough and smooth strains, we examined the cell pellets and supernatants from cultures of different ages (Fig. 1). At all times observed, leukotoxin from the rough strain remained cell associated (Fig. 1A, pellet). Only at later times (23 h) did leukotoxin become detectable in the supernatant (Fig. 1A, supernatant). MALDI-MS was again used to confirm that the cell-associated band was leukotoxin.
In contrast, the amount of cell-associated leukotoxin from the smooth strain was relatively insignificant at all times (Fig. 1B, pellet). Instead, leukotoxin was found in large quantity in the culture supernatants from the smooth strain at early incubation times (Fig. 1B, supernatant). At the earliest time examined (11 h), leukotoxin was the only polypeptide species readily detectable in the culture supernatant. For older cultures (35 to 73 h) of the smooth strain, the initially intense leukotoxin band disappeared, with a concomitant increase in lower-molecular-weight polypeptide species, possibly the result of toxin breakdown. Disappearance of leukotoxin from the supernatant of the rough strain was also observed at late times (Fig. 1A, 58 h), albeit less than from that of the smooth strain. The appearance of leukotoxin in the supernatants without detectable quantities of other cell-associated polypeptides indicates that leukotoxin is not released by cell lysis. We conclude that leukotoxin is secreted under these conditions.
The presence of abundant leukotoxin in the supernatant of the smooth strain was surprising, since it has been generally accepted that A. actinomycetemcomitans leukotoxin is the only RTX toxin that is cell associated and not secreted (3, 32, 41, 57). To determine if this is a property unique to CU1000N derivatives, we tested other commonly used smooth strains of A. actinomycetemcomitans. We found that leukotoxin is secreted from well-studied strain Y4 and from JP2, a strain expressing a high level of leukotoxin (50) (Fig. 1C). However, as observed for CU1060N, the extracellular leukotoxin was less abundant in older cultures of Y4 and JP2 (data not shown).
Large-scale purification of active leukotoxin.
Purification schemes for A. actinomycetemcomitans leukotoxin are long and tedious and yield relatively little protein. These protocols are designed to extract leukotoxin from the pellets of smooth strains. Our results suggest that this material represents only a small fraction of the leukotoxin that remains to be secreted. Furthermore, purification of leukotoxin from total cell extracts requires its separation from all other cellular proteins and consequently leads to a lower yield of toxin.
Our finding that young cultures of smooth A. actinomycetemcomitans strains secrete abundant leukotoxin made it possible to devise a simple method to prepare large quantities of leukotoxin. Using the supernatant from a 12-h culture of smooth strain CU1060N and a standard protein concentrator (Millipore), as described in Materials and Methods, we were able to isolate 4 to 10 μg of relatively pure toxin per ml of culture supernatant in less than 2 h. With a maximum volume capacity of 65 ml, each concentrator unit typically concentrated greater than 0.5 mg of total leukotoxin in less than a 500-μl volume. Hence, milligram amounts of leukotoxin were easily obtained. The quality of these preparations was typically that shown for the 11-h sample of Fig. 1B.
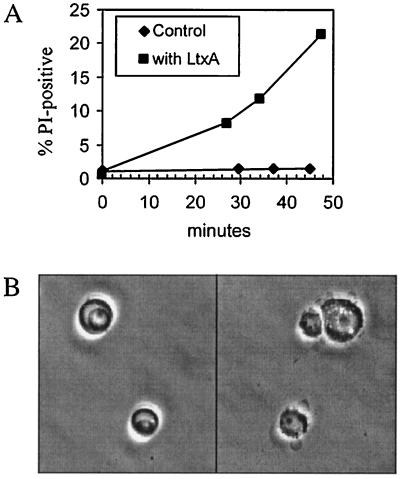
To determine whether the leukotoxin purified in this way was active, we assayed for leukotoxin-induced death of HL-60 cells, a human leukemia cell line commonly used for such assays (25, 64). Cell death, as measured by PI uptake, occurred in the presence of A. actinomycetemcomitans leukotoxin but not with the concentrated medium control (Fig. 2A). Furthermore, leukotoxin-treated HL-60 cells, examined by microscope, showed the characteristic membrane blebbing, nuclear breakdown, and cell destruction, in contrast to the untreated control cells (Fig. 2B). The estimated fraction of cells displaying blebbing was always significantly greater than the fraction of dead cells measured by PI uptake. Thus, leukotoxin prepared from culture supernatants exhibits the activities previously reported for leukotoxin prepared from cell extracts.
FIG. 2.
Activity of secreted leukotoxin on HL-60 cells. (A) HL-60 cells were treated with medium (control) or leukotoxin (LtxA) for various times in the presence of PI and assayed for PI uptake by FACS analysis as described in Materials and Methods. The percentages of cells that were PI positive (dead) are plotted versus the time of incubation. (B) Phase-contrast microscopy of leukotoxin-treated HL-60 cells. Cells were treated with medium (left) or leukotoxin (right) for 30 min at 37°C. Secreted leukotoxin was prepared from culture supernatants as described in Materials and Methods.
The abundance of cell-free leukotoxin is affected by pH.
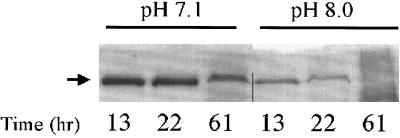
It has been proposed that various environmental conditions affect leukotoxin abundance, including pH, oxygen levels, growth rate, and temperature (42). Because the oral cavity is an environment of constantly changing pH and since laboratory growth media can vary in pH without an obvious defect in bacterial growth, we decided to test if the initial pH of the growth medium affects the abundance of supernatant leukotoxin from the smooth strain. Indeed, we found that pH has a striking effect on the amount of leukotoxin in the culture supernatants (Fig. 3). When CU1060N cultures were grown in medium with an initial pH of 7.1, abundant leukotoxin was present even after 61 h. Cultures grown in medium with an initial pH of 8.0 showed a nearly fourfold-lower level of leukotoxin at early times, as determined by densitometric analysis. Only a small amount of leukotoxin remained at 22 h, and by 61 h no leukotoxin was evident by Coomassie blue staining. These differences were highly reproducible, and, while differences in growth could theoretically account for such a variation, the cell densities and viable cell counts were essentially identical for the different pH conditions at each of the times tested (data not shown).
FIG. 3.
Effect of initial pH on abundance of secreted leukotoxin. CU1060N cells were grown in medium with an initial pH of 7.1 or 8.0. Leukotoxin was prepared from culture supernatants at the times indicated, separated by SDS-PAGE, and visualized by Coomassie blue as described in Materials and Methods.
Leukotoxin is released from nonadherent tad mutants.
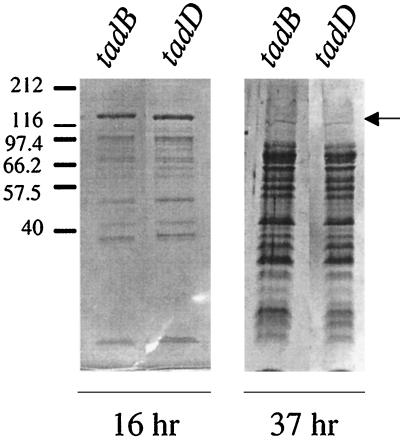
Recently, we reported a cluster of genes (tadABCDEFG) required for the tight adherence of A. actinomycetemcomitans (23). A mutation in any of the seven tad genes causes a change in colony morphology from rough to smooth. The tad mutants are unable to adhere to surfaces, autoaggregate, or form the bundled fibrils typical of the parental rough strain, CU1000N. We examined four tad mutants (tadA, tadB, tadC, and tadD mutants) for secretion of leukotoxin; unexpectedly, all four exhibited an altered leukotoxin phenotype. The tad mutants reproducibly demonstrated a greatly diminished amount of cell-associated leukotoxin and a corresponding release of leukotoxin into the culture medium (Fig. 4). In this regard, the tad mutants resembled the spontaneous smooth variant CU1060N. However, the protein composition of the culture supernatants from the tad mutants over time was different from that for CU1060N (Fig. 5). SDS-PAGE of the supernatants of relatively young (16- to 37-h) cultures of tad mutants revealed many background protein bands, very similar to the supernatants of older (48- to 73-h) cultures of the spontaneous smooth strain (Fig. 1B). The tad mutant cultures appeared to grow normally beyond 24 h, and viable counts and microscopic examination of the bacterial cells revealed no signs of cell death, disruption, or lysis. The mutations were complemented by supplying the wild-type genes in trans. As seen in the rough strain CU1000N, all the complemented mutants retained leukotoxin (Fig. 4), and the smear of background protein bands in the supernatant was absent (data not shown).
FIG. 4.
Cell-associated leukotoxin in tad mutants. tad mutant strains were grown for 23 h. Cell-associated proteins were prepared and separated by SDS-PAGE and stained with Coomassie blue as described in Materials and Methods. The cell-associated protein is shown for each mutant containing the plasmid vector control (pJAK16) or the appropriate complementing plasmid. The tad mutant is noted below the lanes. Leukotoxin (arrow) was confirmed by MALDI-MS.
FIG. 5.
Extracellular proteins from tad mutants. Polypeptides from culture supernatants were prepared, separated by SDS-PAGE, and stained with Coomassie blue as described in Materials and Methods. (Left) Leukotoxin is present in 16-h supernatants of tadB and tadD mutants along with lower-molecular-weight polypeptides. (Right) Leukotoxin is no longer visible in 37-h supernatants of tadB and tadD mutants, which show abundant lower-molecular-weight polypeptides.
DISCUSSION
Early studies on A. actinomycetemcomitans leukotoxin indicated that the protein was not secreted but instead remained associated with the bacterial cell (3, 32, 41, 57). This generally accepted observation stands in contrast to the findings with the other RTX toxin-producing bacteria, all of which secrete their toxins in soluble form (60). In this study, we have shown that A. actinomycetemcomitans leukotoxin is indeed secreted in large quantities by spontaneous, nonadherent, smooth strains, by nonadherent tad mutants, and to a lesser degree by a rough, adherent strain. The supernatants of young cultures of smooth strains contained high levels of leukotoxin in relatively pure form. We also found that a significant amount of cell-associated leukotoxin is retained by the rough strain but not by the smooth strain. These results may have implications with respect to A. actinomycetemcomitans pathogenesis. Furthermore, the ability to obtain large amounts of pure A. actinomycetemcomitans leukotoxin should greatly facilitate structure-function studies of leukotoxin.
We wished to identify factors that affect leukotoxin abundance, as these may be important, not only for purification of the toxin but also for understanding the disease process in humans. We found that several factors affect the abundance and purity of leukotoxin in the supernatant. (i) Addition of fresh sodium bicarbonate to the medium just before inoculation results in higher levels of leukotoxin (data not shown). (ii) Inoculation with a fresh colony from a plate just removed from a CO2 environment, rather than one that had been exposed to air, gives the most abundant and pure leukotoxin (data not shown). (iii) pH also affects the quality of leukotoxin in the supernatant. Growth of the smooth strain in culture medium with an initial pH of 7.1 yields more supernatant leukotoxin over a longer time than growth culture media with higher initial pH. (iv) The age of the culture is also important, with very young cultures having the highest levels of leukotoxin in the medium. Because A. actinomycetemcomitans is relatively fastidious and slow growing, it is likely that investigators grow their cultures for 2 or 3 days to maximize cell density before harvesting the toxin. However, our results demonstrate that this strategy is counterproductive. For purification of large amounts of leukotoxin, we have found that it is best to inoculate a fresh colony into new medium at pH 6.5 to 7.0 and harvest toxin from the supernatant after approximately 12 h of incubation. The reason for the disappearance of leukotoxin from the supernatant of older cultures is currently unknown, although a likely explanation is that leukotoxin is being degraded by a protease. Our initial attempts to identify such a protease in the supernatants of older cultures have thus far been unsuccessful. Whether the breakdown of secreted leukotoxin is relevant to the pathogenesis of A. actinomycetemcomitans will require further work.
We also note that leukotoxin can be lost by adsorption during the isolation procedure. It is important to filter culture supernatants using low-protein-binding membranes (e.g., HT Tuffryn). Filters that were not low protein binding (e.g., cellulose acetate) yielded little or no leukotoxin (data not shown). In addition, low levels of leukotoxin were obtained when bacteria were cultured in certain tissue culture vessels (data not shown). This result is similar to the findings of Daefler (10), who reported that SipC and InvJ proteins of Salmonella enterica serovar Typhimurium could only be recovered from culture supernatants after the tissue culture dishes were coated with bovine calf serum, which prevents nonspecific binding of proteins to the culture dishes. It is possible that the early experiments that led to the conclusion that leukotoxin is not secreted from A. actinomycetemcomitans may have been done under one or more of the unfavorable conditions described here.
The physiological relevance of the effect of pH on leukotoxin abundance in the supernatant may be related to the unique specificity of A. actinomycetemcomitans leukotoxin for PMNs. When PMNs accumulate at the site of infection to attack a pathogen, a decrease in pH accompanies the inflammatory response (16, 46, 55). Since PMNs are the target of leukotoxin, it is logical to propose that it would be to the advantage of the pathogen to have more-abundant leukotoxin in the presence of host defenses. A pH of lower than 7.0 may increase either the stability or expression of leukotoxin, while a higher pH may signal that leukotoxin is no longer required and that leukotoxin can be restored to the basal level. These possibilities will require further investigation, and it will be of considerable interest to identify other environmental and cellular signals that regulate the cytotoxicity of A. actinomycetemcomitans.
Because leukotoxin was previously thought not to be secreted, the functions of the putative toxin secretion proteins, LtxB and LtxD, have remained undefined (19, 32). In E. coli the function of HlyB (the LtxB homolog) is to supply energy for the secretion process via its ATPase activity (27, 30). HlyD (the LtxD homolog) is proposed to serve as the transmembrane channel through which the α-hemolysin is secreted (44). A. actinomycetemcomitans ltxB and ltxD mutants gave rise to subtle phenotypes in which the amount of intracellular leukotoxin was slightly decreased relative to that of the wild-type strains. However, because it was not known that A. actinomycetemcomitans leukotoxin could be secreted, the effect of these mutations on the production of soluble leukotoxin was not examined. Our present findings lead to the obvious prediction that LtxB and LtxD are indeed required for leukotoxin secretion, as they are in other RTX toxin-expressing bacteria, and that the mutations will block the accumulation of leukotoxin in culture supernatants.
We were surprised to find that the tad mutant strains do not retain leukotoxin, but rather release it into the culture medium, just as the spontaneous nonadherent, smooth strains do. The tad genes are required for tight adherence to surfaces, autoaggregration, and the production of long bundled fibers. On the basis of phylogenetic analysis and functional analysis, we have proposed that the Tad proteins constitute a novel secretion system required for the production of fibrils (23). The simplest explanation for the leukotoxin phenotype of tad mutants is that the Tad system is required to secrete a factor that allows leukotoxin to remain cell associated, possibly the fibrils themselves. Ohta et al. (40, 41) found that A. actinomycetemcomitans leukotoxin can be associated with the bacterial cell by electrostatic interactions with nucleic acids bound to the cell surface. While it is possible that the Tad proteins are also involved in the export or binding of nucleic acid to the cell surface, there is currently no evidence to support this idea. Lipopolysaccharide (LPS) has also been reported to differ between rough and smooth strains (13), but we have not yet determined if LPS is affected by the tad genes. At present, the reasons for the observed leukotoxin phenotype in the tad mutants remain unknown. Nevertheless, it is interesting that both the leukotoxin-encoding region (24) and the tadABCDEFG region (23) of the A. actinomycetemcomitans genome have identical G+C contents of 36%, which is significantly different from the 48% G+C content of the rest of the genome. Furthermore, neither region contains any copies of the 11-bp putative DNA uptake sequence that we recently identified in the genome of A. actinomycetemcomitans (56; P. Planet, S. Kachlany, and D. Figurski, unpublished results). Given these observations and the phenotypes of the tad mutants, it is intriguing to consider the possibility that these two regions were recently acquired and that there is a functional or regulatory relationship between them.
ACKNOWLEDGMENTS
We thank Andrew K. Joe for help with tissue culture techniques, and we are grateful to Helen Schreiner, David Furgang, Jeff Kaplan, Paul Planet, and Mrinal Bhattacharjee for their comments.
This work was supported in part by NIH research grants (to D. H. Figurski and D. H. Fine) and an NIH traineeship (to S.C.K.).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 2.Bakas L, Ostolaza H, Vaz W L, Goni F M. Reversible adsorption and nonreversible insertion of Escherichia coli α-hemolysin into lipid bilayers. Biophys J. 1996;71:1869–1876. doi: 10.1016/S0006-3495(96)79386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthold P, Forti D, Kieba I R, Rosenbloom J, Taichman N S, Lally E T. Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiol Immunol. 1992;7:24–27. doi: 10.1111/j.1399-302x.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi S, Mackman N, Nicaud J M, Holland I B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi S, Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- 6.Binet R, Letoffe S, Ghigo J M, Delepelaire P, Wandersman C. Protein secretion by gram-negative bacterial ABC exporters—a review. Gene. 1997;192:7–11. doi: 10.1016/s0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 7.Boehm D F, Welch R A, Snyder I S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990;58:1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm D F, Welch R A, Snyder I S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coote J G. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol Rev. 1992;8:137–161. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 10.Daefler S. Type III secretion by Salmonella typhimurium does not require contact with a eukaryotic host. Mol Microbiol. 1999;31:45–51. doi: 10.1046/j.1365-2958.1999.01141.x. [DOI] [PubMed] [Google Scholar]
- 11.Englard S, Seifter S. Precipitation techniques. In: Deutscher M, editor. Guide to protein purification. San Diego, Calif: Academic Press; 1990. pp. 285–300. [Google Scholar]
- 12.Felmlee T, Pellett S, Welch R A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine D H, Furgang D, Schreiner H C, Goncharoff P, Charlesworth J, Ghazwan G, Fitzgerald-Bocarsly P, Figurski D H. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology. 1999;145:1335–1347. doi: 10.1099/13500872-145-6-1335. [DOI] [PubMed] [Google Scholar]
- 14.Fives-Taylor P M, Meyer D H, Mintz K P, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 15.Fürste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Tsao G, Conn H O, Lerner E. The diagnosis of bacterial peritonitis: comparison of pH, lactate concentration and leukocyte count. Hepatology. 1985;5:91–96. doi: 10.1002/hep.1840050119. [DOI] [PubMed] [Google Scholar]
- 17.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goni F M, Ostolaza H. E. coli α-hemolysin: a membrane-active protein toxin. Braz J Med Biol Res. 1998;31:1019–1034. doi: 10.1590/s0100-879x1998000800002. [DOI] [PubMed] [Google Scholar]
- 19.Guthmiller J M, Kolodrubetz D, Kraig E. Mutational analysis of the putative leukotoxin transport genes in Actinobacillus actinomycetemcomitans. Microb Pathog. 1995;18:307–321. doi: 10.1006/mpat.1995.0028. [DOI] [PubMed] [Google Scholar]
- 20.Hardie K R, Issartel J P, Koronakis E, Hughes C, Koronakis V. In vitro activation of Escherichia coli prohaemolysin to the mature membrane-targeted toxin requires HlyC and a low molecular-weight cytosolic polypeptide. Mol Microbiol. 1991;5:1669–1679. doi: 10.1111/j.1365-2958.1991.tb01914.x. [DOI] [PubMed] [Google Scholar]
- 21.Hughes C, Stanley P, Koronakis V. E. coli hemolysin interactions with prokaryotic and eukaryotic cell membranes. Bioessays. 1992;14:519–525. doi: 10.1002/bies.950140804. [DOI] [PubMed] [Google Scholar]
- 22.Issartel J P, Koronakis V, Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991;351:759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- 23.Kachlany S C, Planet P J, Bhattacharjee M K, Kollia E, DeSalle R, Fine D H, Figurski D H. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in Bacteria and Archaea. J Bacteriol. 2000;182:6169–6176. doi: 10.1128/jb.182.21.6169-6176.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan J B, Fine D H. Codon usage in Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1998;163:31–36. doi: 10.1111/j.1574-6968.1998.tb13022.x. [DOI] [PubMed] [Google Scholar]
- 25.Karakelian D, Lear J D, Lally E T, Tanaka J C. Characterization of Actinobacillus actinomycetemcomitans leukotoxin pore formation in HL60 cells. Biochim Biophys Acta. 1988;1406:175–187. doi: 10.1016/s0925-4439(98)00002-7. [DOI] [PubMed] [Google Scholar]
- 26.Kolodrubetz D, Dailey T, Ebersole J, Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989;57:1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koronakis E, Hughes C, Milisav I, Koronakis V. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol Microbiol. 1995;16:87–96. doi: 10.1111/j.1365-2958.1995.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 28.Koronakis V, Hughes C. Bacterial signal peptide-independent protein export: HlyB-directed secretion of hemolysin. Semin Cell Biol. 1993;4:7–15. doi: 10.1006/scel.1993.1002. [DOI] [PubMed] [Google Scholar]
- 29.Koronakis V, Hughes C. Synthesis, maturation and export of the E. coli hemolysin. Med Microbiol Immunol (Berlin) 1996;185:65–71. doi: 10.1007/s004300050016. [DOI] [PubMed] [Google Scholar]
- 30.Koronakis V, Hughes C, Koronakis E. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol Microbiol. 1993;8:1163–1175. doi: 10.1111/j.1365-2958.1993.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 31.Korostoff J, Wang J, Kieba I, Miller M, Shenker B, Lally E. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect Immun. 1998;66:4474–4483. doi: 10.1128/iai.66.9.4474-4483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lally E T, Golub E E, Kieba I R, Taichman N S, Decker S, Berthold P, Gibson C W, Demuth D R, Rosenbloom J. Structure and function of the B and D genes of the Actinobacillus actinomycetemcomitans leukotoxin complex. Microb Pathog. 1991;11:111–121. doi: 10.1016/0882-4010(91)90004-t. [DOI] [PubMed] [Google Scholar]
- 33.Lally E T, Hill R B, Kieba I R, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- 34.Lally E T, Kieba I R. Molecular biology of Actinobacillus actinomycetemcomitans leukotoxin. In: Genco R, Hamada S, Lehner T, McGhee J, Mergenhagen S, editors. Molecular pathogenesis of periodontal disease. Washington, D.C.: American Society for Microbiology; 1994. pp. 69–82. [Google Scholar]
- 35.Lally E T, Kieba I R, Demuth D R, Rosenbloom J, Golub E E, Taichman N S, Gibson C W. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem Biophys Res Commun. 1989;159:256–262. doi: 10.1016/0006-291x(89)92431-5. [DOI] [PubMed] [Google Scholar]
- 36.Lo R Y, Strathdee C A, Shewen P E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987;55:1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masure H, Storm D. Characterization of the bacterial cell-associated calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1989;28:438–442. doi: 10.1021/bi00428a005. [DOI] [PubMed] [Google Scholar]
- 38.Menestrina G, Dalla Serra M, Pederzolli C, Bregante M, Gambale F. Bacterial hemolysins and leukotoxins affect target cells by forming large exogenous pores into their plasma membrane: Escherichia coli hemolysin A as a case example. Biosci Rep. 1995;15:543–551. doi: 10.1007/BF01204356. [DOI] [PubMed] [Google Scholar]
- 39.Newman M, Socransky S S, Savitt E D, Propos D A, Crawford A. Studies on the microbiology of periodontosis. J Periodontol. 1976;47:373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- 40.Ohta H, Hara H, Fukui K, Kurihara H, Murayama Y, Kato K. Association of Actinobacillus actinomycetemcomitans leukotoxin with nucleic acids on the bacterial cell surface. Infect Immun. 1993;61:4878–4884. doi: 10.1128/iai.61.11.4878-4884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohta H, Kato K, Kokeguchi S, Hara H, Fukui K, Murayama Y. Nuclease-sensitive binding of an Actinobacillus actinomycetemcomitans leukotoxin to the bacterial cell surface. Infect Immun. 1991;59:4599–4605. doi: 10.1128/iai.59.12.4599-4605.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohta H, Miyagi A, Kato K, Fukui K. The relationships between leukotoxin production, growth rate and the bicarbonate concentration in a toxin-production-variable strain of Actinobacillus actinomycetemcomitans. Microbiology. 1996;142:963–970. doi: 10.1099/00221287-142-4-963. [DOI] [PubMed] [Google Scholar]
- 43.Ostolaza H, Bartolome B, Ortiz de Zarate I, de la Cruz F, Goni F M. Release of lipid vesicle contents by the bacterial protein toxin α-haemolysin. Biochim Biophys Acta. 1993;1147:81–88. doi: 10.1016/0005-2736(93)90318-t. [DOI] [PubMed] [Google Scholar]
- 44.Schulein R, Gentschev I, Mollenkopf H J, Goebel W. A topological model for the haemolysin translocator protein HlyD. Mol Gen Genet. 1992;234:155–163. doi: 10.1007/BF00272357. [DOI] [PubMed] [Google Scholar]
- 45.Sia E A, Kuehner D M, Figurski D H. Mechanism of retrotransfer in conjugation: prior transfer of the conjugative plasmid is required. J Bacteriol. 1996;178:1457–1464. doi: 10.1128/jb.178.5.1457-1464.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmen H P, Blaser J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am J Surg. 1993;166:24–27. doi: 10.1016/s0002-9610(05)80576-8. [DOI] [PubMed] [Google Scholar]
- 47.Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontology 2000. 1999;20:82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 48.Soloaga A, Ramirez J M, Goni F M. Reversible denaturation, self-aggregation, and membrane activity of Escherichia coli α-hemolysin, a protein stable in 6 M urea. Biochemistry. 1998;37:6387–6393. doi: 10.1021/bi9730994. [DOI] [PubMed] [Google Scholar]
- 49.Soloaga A, Veiga M P, Garcia-Segura L M, Ostolaza H, Brasseur R, Goni F M. Insertion of Escherichia coli α-haemolysin in lipid bilayers as a non-transmembrane integral protein: prediction and experiment. Mol Microbiol. 1999;31:1013–1024. doi: 10.1046/j.1365-2958.1999.01225.x. [DOI] [PubMed] [Google Scholar]
- 50.Spitznagel J J, Kraig E, Kolodrubetz D. Regulation of leukotoxin in leukotoxic and nonleukotoxic strains of Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:1394–1401. doi: 10.1128/iai.59.4.1394-1401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanley P, Koronakis V, Hardie K, Hughes C. Independent interaction of the acyltransferase HlyC with two maturation domains of the Escherichia coli toxin HlyA. Mol Microbiol. 1996;20:813–822. doi: 10.1111/j.1365-2958.1996.tb02519.x. [DOI] [PubMed] [Google Scholar]
- 52.Stanley P, Koronakis V, Hughes C. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol Mol Biol Rev. 1998;62:309–333. doi: 10.1128/mmbr.62.2.309-333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taichman N S, Dean R T, Sanderson C J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980;28:258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taichman N S, Simpson D L, Sakurada S, Cranfield M, DiRienzo J, Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 55.Tapper H. The secretion of preformed granules by macrophages and neutrophils. J Leukoc Biol. 1996;59:613–622. doi: 10.1002/jlb.59.5.613. [DOI] [PubMed] [Google Scholar]
- 56.Thomson V J, Bhattacharjee M K, Fine D H, Derbyshire K M, Figurski D H. Direct selection of IS903 transposon insertions by use of a broad-host-range vector: isolation of catalase-deficient mutants of Actinobacillus actinomycetemcomitans. J Bacteriol. 1999;181:7298–7307. doi: 10.1128/jb.181.23.7298-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai C C, Shenker B J, DiRienzo J M, Malamud D, Taichman N S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984;43:700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wandersman C, Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci USA. 1990;87:4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R C, Seror S J, Blight M, Pratt J M, Broome-Smith J K, Holland I B. Analysis of the membrane organization of an Escherichia coli protein translocator, HlyB, a member of a large family of prokaryote and eukaryote surface transport proteins. J Mol Biol. 1991;217:441–454. doi: 10.1016/0022-2836(91)90748-u. [DOI] [PubMed] [Google Scholar]
- 60.Welch R, Bauer M, Kent A, Leeds J, Moayeri M, Regassa L, Swenson D. Battling against host phagocytes: the wherefore of the RTX family of toxins? Infect Agents Dis. 1995;4:254–272. [PubMed] [Google Scholar]
- 61.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 62.Wyss C. Selected low-cohesion variants of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus lack distinct antigens recognized by human antibodies. Arch Microbiol. 1989;151:133–136. doi: 10.1007/BF00414427. [DOI] [PubMed] [Google Scholar]
- 63.Zambon J J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 64.Zambon J J, DeLuca C, Slots J, Genco R J. Studies of leukotoxin from Actinobacillus actinomycetemcomitans using the promyelocytic HL-60 cell line. Infect Immun. 1983;40:205–212. doi: 10.1128/iai.40.1.205-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]