Abstract
Optimistic and pessimistic cognitive biases have been described in many animals and are related to the perceived valence of the environment. We, therefore, hypothesize that such cognitive bias can be adaptive depending on environmental conditions. In reward-rich environments, an optimistic bias would be favoured, whereas in harsh environments, a pessimistic one would thrive. Here, we empirically investigated the potential adaptive value of such bias using zebrafish as a model. We first phenotyped female zebrafish in an optimistic/pessimistic axis using a previously validated judgement bias assay. Optimistic and pessimistic females were then exposed to an unpredictable chronic stress protocol for 17 days, after which fish were euthanized and the sectional area of the different ovarian structures was quantified in both undisturbed and stressed groups. Our results show that zebrafish ovarian development responded to chronic stress, and that judgement bias impacted the relative area of the vitellogenic developmental stage, with pessimists showing higher vitellogenic areas as compared with optimists. These results suggest that pessimism maximizes reproductive investment, through increased vitellogenesis, indicating a relationship between cognitive bias and life-history organismal decisions.
Keywords: cognitive bias, optimism, pessimism, ovarian maturation, fitness, zebrafish
1. Introduction
Optimistic and pessimistic cognitive biases, defined as a higher expectation of a positive, or negative, respectively, outcome of a given event than the average, are widespread in animal and human decision-making processes [1,2]. In animals, these cognitive biases are usually measured in judgement bias tasks, in which subjects respond to ambiguous cues that are intermediate between two anchor cues that differ in the valence of an associated outcome (e.g. positive cue versus negative cue; or positive versus neutral cue), which was learned during a prior discrimination phase of the test [3]. This paradigm has been developed for a broad range of species [4–8], and the majority of these studies have focused on the modulation of judgement bias through situational or contextual factors that may influence the affective state on the animals. Consequently, judgement bias has been traditionally considered as a transient condition, that is as an organismal state. However, recent studies have reported a high correlation with other behaviours that are commonly used to assess specific behavioural traits [9,10], which suggests that judgement bias could also be considered as a trait that is relatively stable over time. Assuming that the animals' response to judgement bias tasks reflects evolved optimal behaviour selected to deal with environmental uncertainty, then these cognitive biases must have an adaptive value [11,12]. In general, experimental manipulations that alter perceived probabilities of reward and punishment and/or change the payoffs from reward and punishment tend to shift the response of the animals along the judgement bias, with environments enriched with reward opportunities promoting optimism and environments rich in punishment threats promoting pessimism [6,13–19]. Therefore, the evolutionary function of these judgement biases of ambiguous stimuli has been hypothesized to be the prioritization of the allocation of resources towards the current most relevant fitness-related activities [2].
A large body of evidence indicates that environmental cues are critical factors in predicting various correlated behaviours and outcomes, which include mating strategies, risky behaviours, reproductive development and investment, and health [20–22]. Considering life-history theory, these phenotypic variables are commonly conceptualized as indicators of individual differences along a fast–slow pace of life continuum [23,24]. Typically, fast life-history strategies dominate when the environment is harsh and/or unpredictable and involve the allocation of resources toward current reproduction and investment in offspring quantity. Individuals with fast LH tactics are therefore risk-takers that, in species with parental care, devote less time to their offspring [25,26]. Conversely, individuals living in safe and/or predictable environments adopt slow life-history strategies, by which they expend more effort investing in the quality of their offspring and somatic maintenance [25,27]. It can therefore be hypothesized that specific cognitive biases can provide a fitness benefit for the organism depending on the environment and that optimistic and pessimistic individuals may express different life-history strategies when exposed to specific environments.
As a first approach to the study of the relationship between cognitive bias and life-history strategies, in this study we tested if optimistic/pessimistic bias is associated with reproductive investment of female zebrafish exposed to harsh environments, which ultimately would affect their reproductive fitness. For this purpose, female zebrafish were first phenotyped in an optimistic/pessimistic axis using an already validated judgement bias assay for zebrafish. Afterwards, optimistic and pessimistic female zebrafish were exposed to an unpredictable chronic stress (UCS) protocol for 17 days. Finally, in order to assess reproductive investment, the sectional area of the different ovarian structures was quantified and the effects of optimistic/pessimistic bias, exposure to chronic stress, and their interaction was assessed.
2. Method
(a) . Fish and housing
All subjects used were 4-month-old female wild-type (TU) zebrafish (Danio rerio) (n = 72) bred and held at the Animal House Facility at the Instituto Gulbenkian de Ciência (IGC, Oeiras, Portugal). Fish were kept in mixed sex groups (10 adults per litre) in a recirculation system (Tecniplast®) at 28°C, 750 µS, pH 7 in 14 L : 10 D photoperiod and fed twice a day with freshly hatched Artemia salina in the morning and commercial food flakes (Gemma) in the afternoon. Details of husbandry protocols and health programme have been described previously [28]. All procedures described in this study were carried out in accordance with the relevant guidelines and regulations for animal experimentation, reviewed by the Instituto Gulbenkian de Ciência Ethics Committee, and approved by the competent Portuguese authority (Direcção Geral de Alimentação e Veterinária; permit number: 0421/000/000/2019).
(b) . Experimental design
Individual zebrafish were first categorized in an optimistic/pessimistic dimension following a validated protocol for measuring judgement bias in zebrafish [8,29]. In brief, a go/no-go task was designed in a half radial maze where individual zebrafish were trained to approach a positive cue (P; food reward) and to avoid a negative cue (N; punishment). Once fish were able to distinguish between P and N cues (as indicated by different latencies to enter each cued arm), their response to an ambiguous cue (an intermediate location/colour cue between the P and N locations/colour cues) was then tested (for a detailed description of the judgement bias protocol see electronic supplementary material). Video recordings of the judgement bias assay were analysed by using multi-event recorder software (The Observer XT, Noldus technology, version 9). A total of 48 (out of 64; electronic supplementary material, figure S1) individuals scoring lower (n = 24; optimists) and higher (n = 24; pessimists) in the JBS values were selected for the chronic stress experiment. Selected fish were individually tagged using a validated procedure for zebrafish [30]. After a recovery period of 4 days, tagged zebrafish (n = 48) were randomly assigned to one of two different groups: receiving UCS (stress group) or left undisturbed (control group). Fish assigned to each treatment were distributed across four tanks (replicates). JBS values were counterbalanced between the two treatments (stress versus control), and each tank (replicate) consisted of a mixed-phenotype group of six fish (i.e. three optimists and three pessimists). Four experimental treatments were therefore set-up: optimists control, pessimists control, optimists stressed and pessimists stressed (n = 12 individuals per group) (for a detailed description of the statistical calculation of sample sizes see electronic supplementary material). Fish were then exposed to an UCS protocol already validated for zebrafish [31]. In brief, the UCS group was stressed twice per day using 10 different stressors given in a random order across 17 days (electronic supplementary material, table S1). All fish of the same home tank were given the same stressor at the same time. Stressors included: alarm substance exposure, air-exposure, chasing fish with a hand net, changing fish between different tanks, lowering water level until the dorsal part of the fish is exposed to air, crowding, lowering the water temperature, social isolation, heating up water and restraint stress (for a detailed description of the UCS protocol see electronic supplementary material).
(c) . Histological preparation
The day after the UCS protocol ended, fish were collected from their home tank and euthanized using a lethal dose of MS-222 (1 g l−1; Sigma, MO, USA). Ovaries were dissected out and fixed for 72 h in 10% neutral-buffered formalin. After fixation, ovaries were dehydrated through a series of graded ethanol solutions (70–99.8%), cleared in xylene and embedded in paraffin. Each gonad was entirely sectioned into thin sections (3 µm thick) and stained with haematoxylin–eosin.
(d) . Histological and quantitative analysis
The sectional area of the different ovarian structures was quantified using the Visiopharm Integrator System software (VIS; Visiopharm A/S, Hoersholm, Denmark) and a NanoZoomer-SQ Digital slide scanner (Hamamatsu Photonics). For the quantitative measurements, 10 sections corresponding to the medial zone of each right ovarian lobe were selected. Sections were spaced 15 µm apart from one another. A systematic uniform random sampling (meander sampling) was carried out for each slide. Step-lengths of 1435 µm were used in both x- and y-directions, enabling the acquisition of 50% of the total area using an objective of ×10. The meander sampling generated an average of 60 fields for each slide, which were overlapped using a test system. A total of 64 grid points were regularly arranged, covering 16 095 µm2 per point (area per point; a/p). The sectional area of the ovarian structures was estimated by an unbiased, stereological technique based on point-counting [32], in which the total number of grid points in a section hitting the structures of interest (p structure) was calculated:
Results are therefore expressed as the average sectional area of each oocyte stage per section. In this study, four follicular stages of maturation were identified and counted from the zebrafish ovaries: (i) primary growth stage; (ii) cortical alveolus stage; (iii) vitellogenic stage and (iv) mature stage (electronic supplementary material, figure S2 for detailed description).
(e) . Statistical analyses
For the analyses of the average sectional areas for each oocyte stage, we used the R software [33] packages ‘lme4’ [34] and ‘afex’ [35] for the generalized linear mixed effects (GLMM) models. Sectional areas for cortical alveolus and mature stages were log transformed. The other variables did not need transformations, confirmed with the Shapiro–Wilk test of normality. In all models, the fixed effects were the judgement bias phenotype (with two groups: optimists and pessimists) in interaction with treatment (with two groups: control and stress). The random effect was the tank identity, since the fish of the control and stress groups were distributed in four tanks (replicates) each. This procedure allowed controlling for a possible tank effect. Inspection of model residuals showed satisfactory normal distributions. All p-values are two-tailed.
3. Results
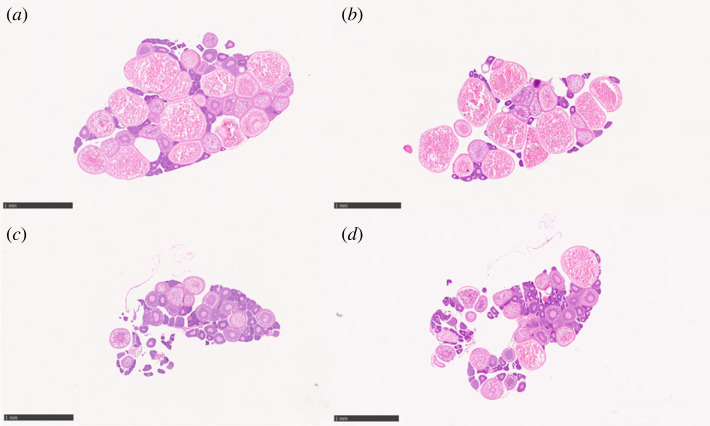
Histological examination of gonadal sections, from both control and stressed groups, revealed normal ovarian architecture (figure 1a–d). However, a higher occurrence of follicles in the early stages of development and a lower presence of vitellogenic and mature follicles was observed in the ovaries of stressed females (figure 1c,d). The quantitative study of the average sectional area of primary growth oocytes revealed that only stress had a significant main effect (table 1), indicating that exposure to chronic stress caused suppression of ovarian maturation, resulting in ovaries that exhibited a higher occurrence of follicles at the earliest stages of development (primary growth; figure 2a). Although a tendency for increased cortical alveolus area was observed for stressed pessimists, no significant differences were found between the stress and control groups neither for optimists nor for pessimists (table 1; figure 2b). Regarding follicles in advanced stages of development, a significant main effect of both treatment and phenotype (table 1) was detected for the vitellogenic area (table 1). This result reinforces the hypothesis that exposure to chronic stress caused suppression of ovarian maturation by inducing ovaries with lower occurrence of follicles at advanced stages of development (vitellogenic). Notably, these results also suggest that judgement bias is associated with the regulation of the ovarian stage of development and more specifically in the regulation of vitellogenic oocites, with pessimist females exhibiting a higher vitellogenic area than optimists (figure 2c). No statistical differences were observed in sectional area of mature stage between the different experimental groups (figure 2d). Given that there were no significant interactions between the two main effects (i.e. stress and judgement bias phenotype) we have not conducted post hoc tests to compare between specific treatments.
Figure 1.
Ovarian histology of zebrafish from the different experimental groups: (a) optimists control; (b) pessimists control; (c) optimists stressed and (d) pessimists stressed. Scale bar = 1 mm.
Table 1.
Results of the GLMM to assess the effects of phenotype (optimists versus pessimists), treatment (control versus stress) and the double interaction among these variables. Asterisks (*) indicate a significant effect.
| main effects and interactions | F-value | p (>F) |
|---|---|---|
| primary growth stage | ||
| phenotype | F1,38 = 0.0007 | p = 0.9794 |
| treatment | F1,38 = 10.1848 | p = 0.0028** |
| phenotype × treatment | F1,38 = 2.9947 | p = 0.0916 |
| cortical alveolus stage | ||
| phenotype | F1,38.2 = 2.0807 | p = 0.1573 |
| treatment | F1,2.14 = 1.6572 | p = 0.3193 |
| phenotype × treatment | F1,38.2 = 0.7000 | p = 0.4080 |
| vitellogenic stage | ||
| phenotype | F1,41 = 7.3076 | p = 0.0099** |
| treatment | F1,41 = 29.1648 | p = 3.06 × 10−6*** |
| phenotype × treatment | F1,41 = 0.5230 | p = 0.4736 |
| mature stage | ||
| phenotype | F1,41 = 0.2498 | p = 0.6199 |
| treatment | F1,41 = 0.8198 | p = 0.3705 |
| phenotype × treatment | F1,41 = 0.0544 | p = 0.8167 |
Figure 2.
Sectional areas (µm) of each developmental oocyte stage, including primary growth (a), cortical alveolus (b), vitellogenic (c) and mature (d) oocyte stages in zebrafish from the different experimental groups (optimists control, optimists stressed pessimists control and pessimists stressed). Asterisks (*) indicate significant differences between pairs of experimental according to main effects. Data are expressed as mean ± s.e.m.
4. Discussion
To the best of our knowledge, this is the first study to evaluate the association of individual variation in judgement bias with reproductive investment, as measured by the relative areas of germ cells in ovaries, under harsh environmental conditions (i.e. chronic stress). Our results show that (i) females exposed to UCS have ovaries with a higher relative area of primary growth stage oocytes and a lower relative area of vitellogenic oocytes as compared with control ovaries, indicating that zebrafish ovaries respond to chronic stress and (ii) pessimist females have ovaries with higher vitellogenic areas than optimist females.
The general effects of chronic stress on ovarian maturation, irrespective of judgement bias phenotype, are not surprising, given the large body of literature on the effects of chronic exposure to stressors on reproductive outcomes, which include gonadal atresia, delayed ovulation, failure of gonadal maturation or low gonad mass, among others (e.g. [36–39]). According to this literature, difficult or challenging environmental conditions (i.e. stressful environments) lead to low reproductive fitness (i.e. low future reproductive success), an effect mediated by altered glucocorticoid activity.
The differential reproductive investment between optimistic and pessimistic individuals, with a higher investment by the latter, suggests that pessimism is associated with maximized current reproduction. The fact that the differences between pessimistic and optimistic females are only present in vitellogenic oocites is particularly interesting, since it suggests that these two phenotypes have differential regulation of vitellogenesis, which is the process of yolk formation via nutrients being deposited in the oocyte, hence requiring a significant resource investment from the females. From an evolutionary perspective, it could be therefore hypothesized that pessimistic strategies might have evolved to be ultimately advantageous in harsh (i.e. stressful) environments, where survival probability is diminished, whereas optimistic strategies would have higher success in reward-rich environments since they give excessive emphasis to doing well in positive circumstances. At a mechanistic level, such differential effects between optimists and pessimists could be mediated by a large number of hormones and neuromodulators (e.g. cortisol, dopamine, β-endorphin, enkephalins, dynorphin and endomorphins) that are involved in regulating both the stress response and reproduction [40]. Remarkably, the results of a meta-regression showed clear effects of some of the above-mentioned neurobiological systems (e.g. adrenergic, dopaminergic and glucocorticoid systems) in altering judgement bias in non-human animals [41], which suggest that the effect of judgement bias on the ovarian follicle development could be mediated through some of these neurohormones.
In summary, our results show that judgement bias impacts ovarian development, with pessimistic females showing higher vitellogenic areas as compared with optimistic females, hence suggesting that pessimism may maximize reproductive fitness under harsh environmental conditions. Future studies should complement this first evidence namely by using more in-depth physiological parameters (e.g. circulating oestradiol and vitellogenin levels) and by assessing fertility and fecundity under different environmental conditions (e.g. presence versus absence of stressors).
Acknowledgements
The authors thank the Fish Facility Platform of the Instituto Gulbenkian de Ciência (IGC) for animal care and the Histopathology Unit of the IGC for technical support of this work.
Ethics
All procedures described in this study were carried out in accordance with the relevant guidelines and regulations for animal experimentation, reviewed by the Instituto Gulbenkian de Ciência Ethics Committee, and approved by the competent Portuguese authority (Direcção Geral de Alimentação e Veterinária permit number: 0421/000/000/2019).
Data accessibility
Original data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1jwstqjxv [42].
The data are provided in the electronic supplementary material [43].
Authors' contributions
F.E.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, writing—original draft and writing—review and editing; M.V.A.: conceptualization, investigation and writing—review and editing; P.F.: formal analysis, investigation and writing—review and editing; D.A.-T.: investigation and writing—review and editing; R.F.O.: conceptualization, funding acquisition, project administration, resources, supervision, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by a grant from Fundação para a Ciência e a Tecnologia (FCT, PTDC/BIA-COM/31010/2017 awarded to F.E. and R.F.O.). F.E. was supported by a Marie Skłodowska-Curie Actions—Individual Fellowship (H2020-MSCA-IF/703285) under the Horizon 2020 Framework Programme (H2020).
References
- 1.Mendl M, Burman OH, Paul ES. 2010. An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B 277, 2895-2904. ( 10.1098/rspb.2010.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateson M. 2016. Optimistic and pessimistic biases: a primer for behavioural ecologists. Curr. Opin. Behav. Sci. 12, 115-121. ( 10.1016/j.cobeha.2016.09.013) [DOI] [Google Scholar]
- 3.Roelofs S, Boleij H, Nordquist RE, Van der Staay FJ. 2016. Making decisions under ambiguity: judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 10, 119. ( 10.3389/fnbeh.2016.00119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendl M, Brooks J, Basse C, Burman O, Paul E, Blackwell E, Casey R. 2010. Dogs showing separation-related behaviour exhibit a ‘pessimistic’ cognitive bias. Curr. Biol. 20, R839-R840. ( 10.1016/j.cub.2010.08.030) [DOI] [PubMed] [Google Scholar]
- 5.Enkel T, Gholizadeh D, Von Bohlen und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, Gass P, Vollmayr B. 2010. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology 35, 1008-1015. ( 10.1038/npp.2009.204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateson M, Desire S, Gartside SE, Wright GA. 2011. Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 21, 1070-1073. ( 10.1016/j.cub.2011.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zidar J, Campderrich I, Jansson E, Wichman A, Winberg S, Keeling L, Løvlie H. 2018. Environmental complexity buffers against stress-induced negative judgement bias in female chickens. Sci. Rep. 8, 1-14. ( 10.1038/s41598-018-23545-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espigares F, Abad-Tortosa D, Varela SAM, Ferreira MG, Oliveira RF. 2021. Short telomeres drive pessimistic judgement bias in zebrafish. Biol. Lett. 17, 20200745. ( 10.1098/rsbl.2020.0745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jardim V, Verjat A, Féron C, Châline N, Rödel HG. 2021. Is there a bias in spatial maze judgment bias tests? Individual differences in subjects' novelty response can affect test results. Behav. Brain Res. 407, 113262. ( 10.1016/j.bbr.2021.113262) [DOI] [PubMed] [Google Scholar]
- 10.Horback KM, Parsons TD. 2022. Judgement bias of group housed gestating sows predicted by behavioral traits, but not physical measures of welfare. PLoS ONE 17, e0264258. ( 10.1371/journal.pone.0264258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNamara JM, Trimmer PC, Eriksson A, Marshall JA, Houston AI. 2011. Environmental variability can select for optimism or pessimism. Ecol. Lett. 14, 58-62. ( 10.1111/j.1461-0248.2010.01556.x) [DOI] [PubMed] [Google Scholar]
- 12.Marshall JA, Trimmer PC, Houston AI, McNamara JM. 2013. On evolutionary explanations of cognitive biases. Trends Ecol. Evol. 28, 469-473. ( 10.1016/j.tree.2013.05.013) [DOI] [PubMed] [Google Scholar]
- 13.Doyle RE, Fisher AD, Hinch GN, Boissy A, Lee C. 2010. Release from restraint generates a positive judgement bias in sheep. Appl. Anim. Behav. Sci. 122, 28-34. ( 10.1016/j.applanim.2009.11.003) [DOI] [Google Scholar]
- 14.Brydges NM, Leach M, Nicol K, Wright R, Bateson M. 2011. Environmental enrichment induces optimistic cognitive bias in rats. Anim. Behav. 81, 169-175. ( 10.1016/j.anbehav.2010.09.030) [DOI] [Google Scholar]
- 15.Doyle RE, Lee C, Deiss V, Fisher AD, Hinch GN, Boissy A. 2011. Measuring judgement bias and emotional reactivity in sheep following long-term exposure to unpredictable and aversive events. Physiol. Behav. 102, 503-510. ( 10.1016/j.physbeh.2011.01.001) [DOI] [PubMed] [Google Scholar]
- 16.Douglas C, Bateson M, Walsh C, Bédué A, Edwards SA. 2012. Environmental enrichment induces optimistic cognitive biases in pigs. Appl. Anim. Behav. Sci. 139, 65-73. ( 10.1016/j.applanim.2012.02.018) [DOI] [Google Scholar]
- 17.Neave HW, Daros RR, Costa JH, von Keyserlingk MA, Weary DM. 2013. Pain and pessimism: dairy calves exhibit negative judgement bias following hot-iron disbudding. PLoS ONE 8, e80556. ( 10.1371/journal.pone.0080556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateson M, Emmerson M, Ergün G, Monaghan P, Nettle D. 2015. Opposite effects of early-life competition and developmental telomere attrition on cognitive biases in juvenile European starlings. PLoS ONE 10, e0132602. ( 10.1371/journal.pone.0132602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy E, Kraak L, van den Broek J, Nordquist RE, van der Staay FJ. 2015. Decision-making under risk and ambiguity in low-birth-weight pigs. Anim. Cogn. 18, 561-572. ( 10.1007/s10071-014-0825-1) [DOI] [PubMed] [Google Scholar]
- 20.Brumbach BH, Figueredo AJ, Ellis BJ. 2009. Effects of harsh and unpredictable environments in adolescence on development of life history strategies. Hum. Nat. 20, 25-51. ( 10.1007/s12110-009-9059-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belsky J, Schlomer GL, Ellis BJ. 2012. Beyond cumulative risk: distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Dev. Psychol. 48, 662-673. ( 10.1037/a0024454) [DOI] [PubMed] [Google Scholar]
- 22.McCullough ME, Pedersen EJ, Schroder JM, Tabak BA, Carver CS. 2013. Harsh childhood environmental characteristics predict exploitation and retaliation in humans. Proc. R. Soc. B 280, 20122104. ( 10.1098/rspb.2012.2104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Promislow DE, Harvey PH. 1990. Living fast and dying young: a comparative analysis of life-history variation among mammals. J. Zool. 220, 417-437. ( 10.1111/j.1469-7998.1990.tb04316.x) [DOI] [Google Scholar]
- 24.Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. 2009. Fundamental dimensions of environmental risk: the impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Hum. Nat. 20, 204-268. ( 10.1007/s12110-009-9063-7) [DOI] [PubMed] [Google Scholar]
- 25.Simpson JA, Griskevicius V, Kuo SI, Sung S, Collins WA. 2012. Evolution, stress, and sensitive periods: the influence of unpredictability in early versus late childhood on sex and risky behavior. Dev. Psychol. 48, 674. ( 10.1037/a0027293) [DOI] [PubMed] [Google Scholar]
- 26.Griskevicius V, Ackerman JM, Cantú SM, Delton AW, Robertson TE, Simpson JA, Thompson ME, Tybur JM. 2013. When the economy falters, do people spend or save? Responses to resource scarcity depend on childhood environments. Psychol. Sci. 24, 197-205. ( 10.1177/0956797612451471) [DOI] [PubMed] [Google Scholar]
- 27.Figueredo AJ, Vásquez G, Brumbach BH, Schneider SM. 2007. The K-factor, covitality, and personality: a psychometric test of life history theory. Hum. Nat. 18, 47-73. ( 10.1007/BF02820846) [DOI] [PubMed] [Google Scholar]
- 28.Borges AC, Pereira N, Franco M, Vale L, Pereira M, Cunha MV, Amaro A, Albuquerque T, Rebelo M. 2016. Implementation of a zebrafish health program in a research facility: a 4-year retrospective study. Zebrafish 13, S-115. ( 10.1089/zeb.2015.1230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espigares F, Martins RR, Oliveira RF. 2022. A behavioural assay to investigate judgment bias in zebrafish. Bio-protocol 12, e4327. ( 10.21769/BioProtoc.4327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teles MC, Oliveira RF. 2016. Zebrafish: methods and protocols (methods in molecular biology, 1451). New York, NY: Humana. [DOI] [PubMed] [Google Scholar]
- 31.Piato ÂL, Capiotti KM, Tamborski AR, Oses JP, Barcellos LJ, Bogo MR, Lara DR, Vianna MR, Bonan CD. 2011. Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 35, 561-567. ( 10.1016/j.pnpbp.2010.12.018) [DOI] [PubMed] [Google Scholar]
- 32.Howard CV, Reed MG. 2004. Unbiased stereology. Three-dimensional measurements in microscopy, 2nd edn. London, UK: Garland Science. [Google Scholar]
- 33.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 34.Singmann H, Bolker B, Westfall J, Aust F, Ben-Shachar MS. 2020. afex: Analysis of Factorial Experiments. R package version 0.27-2. See https://CRAN.R-project.org/package=afex.
- 35.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01)36. [DOI] [Google Scholar]
- 36.Campbell PM, Pottinger TG, Sumpter JP. 1992. Stress reduces the quality of gametes produced by rainbow trout. Biol. Reprod. 47, 1140-1150. ( 10.1095/biolreprod47.6.1140) [DOI] [PubMed] [Google Scholar]
- 37.Campbell PM, Pottinger TG, Sumpter JP. 1994. Preliminary evidence that chronic confinement stress reduces the quality of gametes produced by brown and rainbow trout. Aquaculture 120, 151-169. ( 10.1016/0044-8486(94)90230-5) [DOI] [Google Scholar]
- 38.Eriksen MS, Bakken M, Espmark Å, Braastad BO, Salte R. 2006. Prespawning stress in farmed Atlantic salmon Salmo salar: maternal cortisol exposure and hyperthermia during embryonic development affect offspring survival, growth and incidence of malformations. J. Fish Biol. 69, 114-129. ( 10.1111/j.1095-8649.2006.01071.x) [DOI] [Google Scholar]
- 39.Eriksen MS, Poppe TT, McCormick M, Damsgård B, Salte R, Braastad BO, Bakken M. 2015. Simulated maternal pre-spawning stress affects offspring's attributes in farmed Atlantic salmon Salmo salar (Linnaeus, 1758). Aquac. Res. 46, 1480-1489. ( 10.1111/are.12301) [DOI] [Google Scholar]
- 40.Ganesh CB. 2021. The stress–reproductive axis in fish: the involvement of functional neuroanatomical systems in the brain. J. Chem. Neuroanat. 112, 101904. ( 10.1016/j.jchemneu.2020.101904) [DOI] [PubMed] [Google Scholar]
- 41.Neville V, Nakagawa S, Zidar J, Paul ES, Lagisz M, Bateson M, Løvlie H, Mendl M. 2020. Pharmacological manipulations of judgement bias: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 108, 269-286. ( 10.1016/j.neubiorev.2019.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espigares F, Alvarado MV, Faísca P, Abad-Tortosa D, Oliveira RF. 2022. Data from: Pessimistic cognitive bias is associated with enhanced reproductive investment in female zebrafish. Dryad Digital Repository. ( 10.5061/dryad.1jwstqjxv) [DOI] [PMC free article] [PubMed]
- 43.Espigares F, Alvarado MV, Faísca P, Abad-Tortosa D, Oliveira RF. 2022. Pessimistic cognitive bias is associated with enhanced reproductive investment in female zebrafish. Figshare. ( 10.6084/m9.figshare.c.6316824) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Espigares F, Alvarado MV, Faísca P, Abad-Tortosa D, Oliveira RF. 2022. Data from: Pessimistic cognitive bias is associated with enhanced reproductive investment in female zebrafish. Dryad Digital Repository. ( 10.5061/dryad.1jwstqjxv) [DOI] [PMC free article] [PubMed]
- Espigares F, Alvarado MV, Faísca P, Abad-Tortosa D, Oliveira RF. 2022. Pessimistic cognitive bias is associated with enhanced reproductive investment in female zebrafish. Figshare. ( 10.6084/m9.figshare.c.6316824) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Original data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1jwstqjxv [42].
The data are provided in the electronic supplementary material [43].