Abstract
Background
Progression of Barrett esophagus (BE) to esophageal adenocarcinoma occurs among a minority of BE patients. To date, BE behavior cannot be predicted on the basis of histologic features.
Aims
We compared BE samples that did not develop dysplasia or carcinoma upon follow-up of ≥ 7 years (BE nonprogressed [BEN]) with BE samples that developed carcinoma upon follow-up of 3 to 4 years (BE progressed [BEP]).
Methods
The NanoString nCounter miRNA assay was used to profile 24 biopsy samples of BE, including 13 BENs and 11 BEPs. Fifteen samples were randomly selected for miRNA prediction model training; nine were randomly selected for miRNA validation.
Results
Unpaired t tests with Welch’s correction were performed on 800 measured miRNAs to identify the most differentially expressed miRNAs for cases of BEN and BEP. The top 12 miRNAs (P < .003) were selected for principal component analyses: miR-1278, miR-1301, miR-1304–5p, miR-517b-3p, miR-584–5p, miR-599, miR-103a-3p, miR-1197, miR-1256, miR-509–3–5p, miR-544b, miR-802. The 12-miRNA signature was first self-validated on the training dataset, resulting in 7 out of the 7 BEP samples being classified as BEP (100% sensitivity) and 7 out of the 8 BEN samples being classified as BEN (87.5% specificity). Upon validation, 4 out of the 4 BEP samples were classified as BEP (100% sensitivity) and 4 out of the 5 BEN samples were classified as BEN (80% specificity). Twenty-four samples were evaluated, and 22 cases were correctly classified. Overall accuracy was 91.67%.
Conclusion
Using miRNA profiling, we have identified a 12-miRNA signature able to reliably differentiate cases of BEN from BEP.
Keywords: Intestinal metaplasia, Barrett’s esophagus, Neoplasia, miRNA
Introduction
Esophageal adenocarcinoma (EAC) is an aggressive malignancy with an increasing incidence in the USA [1]. Chronic gastroesophageal reflux disease (GERD), which leads to Barrett esophagus (BE), is the most common risk factor for EAC development [2]. BE is a metaplastic process that occurs in the lower portion of the esophagus, in which the normal stratified squamous epithelium lining of the esophagus is replaced by simple columnar epithelium with goblet cells. BE is thought to represent an adaptation to chronic acid and bile exposure from GERD [3]. BE is found in 4% to 14% of patients who seek medical care for GERD. However, a large subgroup of patients with BE do not have any symptoms [4]; fewer than 10% of patients with GERD are likely to progress to BE within 5 years [5, 6].
BE is a premalignant condition associated with an increased risk of progression to EAC of 0.15% to 0.58% per patient annually [7, 8]. Progression of BE to EAC is a gradual process. Therefore, periodic esophageal biopsies are used to monitor patients with BE. Current endoscopic surveillance programs for early detection of BE progression to dysplasia involve 4 quadrant biopsies (every 1 to 2 cm) that are performed in accordance with the Seattle Protocol, including biopsies of any endoscopic mucosal abnormality [9]. Biopsies are pathologically classified into 5 general categories: nondysplastic, low-grade dysplasia, high-grade dysplasia (HGD), intramucosal adenocarcinoma, and invasive carcinoma [3, 10, 11]. In the absence of dysplasia, the American College of Gastroenterology recommends endoscopic surveillance at 3-year intervals [12]. If the biopsy shows evidence of low-grade dysplasia, then annual endoscopy is recommended. In the event of HGD, endoscopy is recommended every 3 months [12], followed by appropriate endoscopic treatments.
Several studies have shown that these surveillance programs do not reduce EAC-related mortality rates among patients with BE [13–15]. This suboptimal result is potentially due to sampling errors and/or poor adherence to the Seattle Protocol (as low as 30%) [16].
The management of BE is further complicated by pathologic inter- and intraobserver variability in diagnosing BE and in grading dysplasia [12]. Moreover, according to the British Society of Gastroenterology, the introduction of chromo-endoscopy, narrow-band imaging, and auto-fluorescence endoscopy has not significantly contributed to improved effectiveness of BE surveillance [17].
Although a small percentage of BE patients will progress to dysplasia and cancer, the majority will continue to have long-standing BE without progression [6, 11, 18]. The annual incidence of progression to EAC among patients with BE is between 0.15 and 0.58% per patient [7, 8], and the combined incidence of HGD and EAC is about 1% [6, 11]. Population-based studies of BE patient follow-ups have reported an annual risk of progression to EAC between 0.12 and 0.14% [18]. If HGD is present, then the risk of developing EAC is significantly increased to ≥ 10% per patient annually [11, 18, 19]. In the absence of dysplasia, the behavior of BE cannot be predicted on the basis of evaluation of histologic features alone.
Analyses of microRNAs (miRNAs) have demonstrated reliable patterns of upregulation and downregulation in different cancer types and have a regulatory role in cellular differentiation, proliferation, and apoptosis [20, 21]. Studies have consistently shown that alterations in expressions of specific miRNAs observed during progression to BE, dysplasia, and EAC can be used to reliably predict the histology of a given tissue on the basis of its corresponding miRNA expression profile. Indeed, miRNAs can be used to differentiate between specific evolutionary events in the progression of BE to dysplasia and cancer [22–27].
Although these studies have characterized the miRNAs involved in BE progression, no studies to our knowledge have tested the discriminatory role of miRNAs in differentiating between BE patients who are prone to progress to dysplasia/cancer (BEP) and those who are not (BEN). An miRNA panel capable of differentiating between cases of BEN and BEP would be of great clinical value for properly monitoring patients. Accurate risk stratification could direct preventive medical attention to patients at high risk of progression, thereby decreasing their time to follow-up. This risk stratification could also help to prevent unnecessary follow-up and testing for BE patients who are not at risk of progression to dysplasia and cancer. These potential clinical benefits motivated our development of a novel 12-miRNA signature assay that reliably differentiates cases of BEN from cases of BEP by using NanoString nCounter analyses of miRNA expression.
Material and Methods
Patients and Sample Selection
This retrospective study was approved by the H. Lee Moffitt Cancer Center and Research Institute (MCC) Institutional Review Board (Pro00014706). We evaluated esophageal biopsy samples taken from patients with BE who developed EAC over a period of 3 to 4 years and compared them with esophageal biopsy samples taken from patients who did not develop dysplasia or carcinoma over a period of ≥ 7 years. Patients who progressed to carcinoma included 6 males with a median age of 70 years (range, 49–79 years), and patients who did not progress to dysplasia or carcinoma included 3 females and 7 males with a median age of 72 years (range, 55–88 years). All patients had endoscopic appearance of BE of ≥ 1 cm within the esophagus (proximal to the top of the gastric folds), and goblet cells intestinal metaplasia were present in their endoscopic biopsies, thereby meeting the criteria for BE as defined by the American College of Gastroenterology [12]. Multiple biopsies, taken during the same procedure from different areas of the Barrett’s mucosa, were performed on 4 of the patients studied (2 BEN and 2 BEP) and included in the study. For each patient, biopsies were taken following the Seattle Protocol as per ACG clinical guidelines [28].
The tissue collection and review processes were regulated by specific standard operating procedures and quality assurance/quality control protocols. Overall, we studied 24 samples of Barrett’s mucosa (13 BEN and 11 BEP). A total of 24 patient samples (13 BENs/11 BEPs) were profiled by using the NanoString nCounter miRNA assay (NanoString Technologies Inc, Seattle, WA). The BEP patient samples were identified by selecting patients with EAC from completed MCC clinical trials (MCC 15,464; MCC 16,464; MCC18099 and MCC 18,739). Patient clinical records were retrospectively reviewed to identify the initial esophageal biopsies that were taken when the patients harbored BE without dysplasia. The BEN patient samples were identified from a pathology laboratory database of a community gastroenterologist (coauthor FSC). A retrospective chart review was performed by using PowerChart/PathNet at MCC and Greenway database software at the gastroenterologist office.
Pathologic Evaluation of Patient Samples
Selected hematoxylin and eosin stain slides underwent a comprehensive histopathologic review by 2 board-certified senior pathologists (DC and KN). Ten 1 μm sections were cut from each selected formalin-fixed paraffin-embedded (FFPE) tissue block. Each BE lesional area was marked on the slides using permanent markers and carefully examined to measure cellular composition. To ensure BE tissue purity for each specimen, the areas containing the marked BE mucosa were macrodissected, excluding other tissue components (esophageal squamous mucosa, nonintestinalized columnar mucosa, muscularis mucosae, and submucosal tissue). The samples were then submitted to the MCC Genomic Core, where RNA was extracted and subjected to NanoString analyses.
RNA Extraction
Total RNA, including miRNA, was extracted from the ten 1 μm FFPE tissue blocks by using the Recover All Total Nucleic Acid Isolation Kit (Life Technologies, cat # AM1975). After macrodissection of the selected FFPE block areas, each sample contained ≤ 80% of BE mucosa.
NanoString Analyses
Total RNA samples were processed in accordance with the nCounter Human miRNA Expression Assay V2 (NanoString, Seattle, WA) user manual. We used 100 ng of RNA extracted from each sample as input. Mature miRNAs were ligated to a species-specific tag sequence (miRtag) by using a thermally controlled splinted ligation. Unligated miRtags were removed by enzymatic purification, and miR-tagged mature miRNAs were then hybridized overnight at 65 °C with an nCounter Human (V2) miRNA Expression Assay CodeSet containing 800 miRNA probes. The unhybridized CodeSet was removed by automated purification that was performed on an nCounter Prep Station, and the remaining target probe complexes were transferred and bound to an imaging surface. Absolute counts of the reporter probes were tabulated for each sample by using the nCounter Digital Analyzer, and raw data output was imported into nSolver (http://www.nanostring.com/products/nSolver). Positive control probes in the CodeSet were tested for their linearity, with a correlation between the concentration of the added target and the resulting count; the correlation was ≥ 0.95, indicating high-quality data. The limit of detection for each assay was confirmed by using the positive and negative controls. Six negative controls were used for each sample to measure the levels of nonspecific binding. The mean value with 2 standard deviations was calculated for each sample and subtracted from the miRNA count. Every value equal to or less than that of the background noise was not considered during the qualitative and quantitative analyses.
Statistical Methods
A total of 24 samples were used to build miRNA signatures to predict progression from BE to malignancy. The 24 samples included 13 BENs and 11 BEPs. Sixty percent of the samples (n = 15) were randomly selected for training purposes, and the remaining 40% (n = 9) were used for validation purposes.
nSolver Analysis Software 4.0 by NanoString was used to process miRNA data. A background correction was performed using geometric means of negative controls, and between-sample normalization was performed using geometric means of positive controls and housekeeping genes. The normalized data were log-transformed before analyses.
Mann–Whitney U tests were performed to detect the most significant differentially expressed miRNAs between BENs and BEPs. The false discovery rate was calculated to correct for multiple comparisons. Confidence intervals were also calculated for miRNA selection purposes. The top 12 miRNAs with a false discovery rate < 0.15 and both confidence limits, which fell on one side of 0 (i.e., both confidence limits were > 0, or both confidence limits were < 0), were selected to build the model.
A principal component analysis was performed on the 12 miRNAs. The first principle component (PC1), which captures the largest proportion of the variance, was selected as the signature score. The cutoff was set to 0, which maximized the sum of sensitivity and specificity. Samples with PC1 < 0 were classified as BEP, and samples with PC1 ≥ 0 were classified as BEN.
The signature was first self-validated on the training dataset, resulting in a sensitivity of 100% (7 BEPs were all classified as BEPs) and a specificity of 84.5 (7/8 BENs were classified as BEN).
The signature was then tested on the validation dataset, resulting in a sensitivity of 100% (4 BEPs were classified as BEP) and a specificity of 80% (4/5 BENs were classified as BEN).
Results
We define BEP as BE that progressed to EAC over a period of 3 to 4 years and BEN as BE that did not progress to dysplasia or carcinoma over a period of ≥ 7 years.
Differential Expression of miRNA Between BEN and BEP
The expression levels of 800 miRNAs were evaluated. Initially, 60% of samples (15 [8 BENs and 7 BEPs]) were randomly selected for training purposes, and 40% (9 [5 BENs and 4 BEPs]) were randomly selected for validation purposes. Twenty-two miRNAs were found to be differentially expressed between BEN and BEP samples with a P < 0.01. Validation of these 22 miRNA signatures, using a cutoff of PC1 > 1 for BEP and PC1 ≤ 1 for BEN, gave a sensitivity of 75% and a specificity of 80%, with a total accuracy of 92% ([24-2]/24) (Fig. 1 and Table 1). As some of these miRNA biomarkers shared correlated predictive information, we then used P < 0.005 to select the most significantly differentiated miRNAs from the initial 22 differentially expressed miRNAs. Twelve were identified and selected: miR-1278, miR-1301, miR-1304–5p, miR-517b-3p, miR-584–5p, miR-599, miR-103a-3p, miR-1197, miR-1256, miR-509–3-5p, miR-544b, miR-802 (Table 1). Using the same cutoff of PC1 > 1 for BEP and PC1 ≤ 1 for BEN, the final 12 miRNA models showed a high prediction performance with a sensitivity of 100% and a specificity of 100% at training and a sensitivity of 75% and a specificity of 80% at validation, with a total rate of accuracy of 92% ([24-2]/24) (Fig. 2). Batch effect was not observed between cases selected from MCC and those selected from the community pathology laboratory database.
Fig. 1.
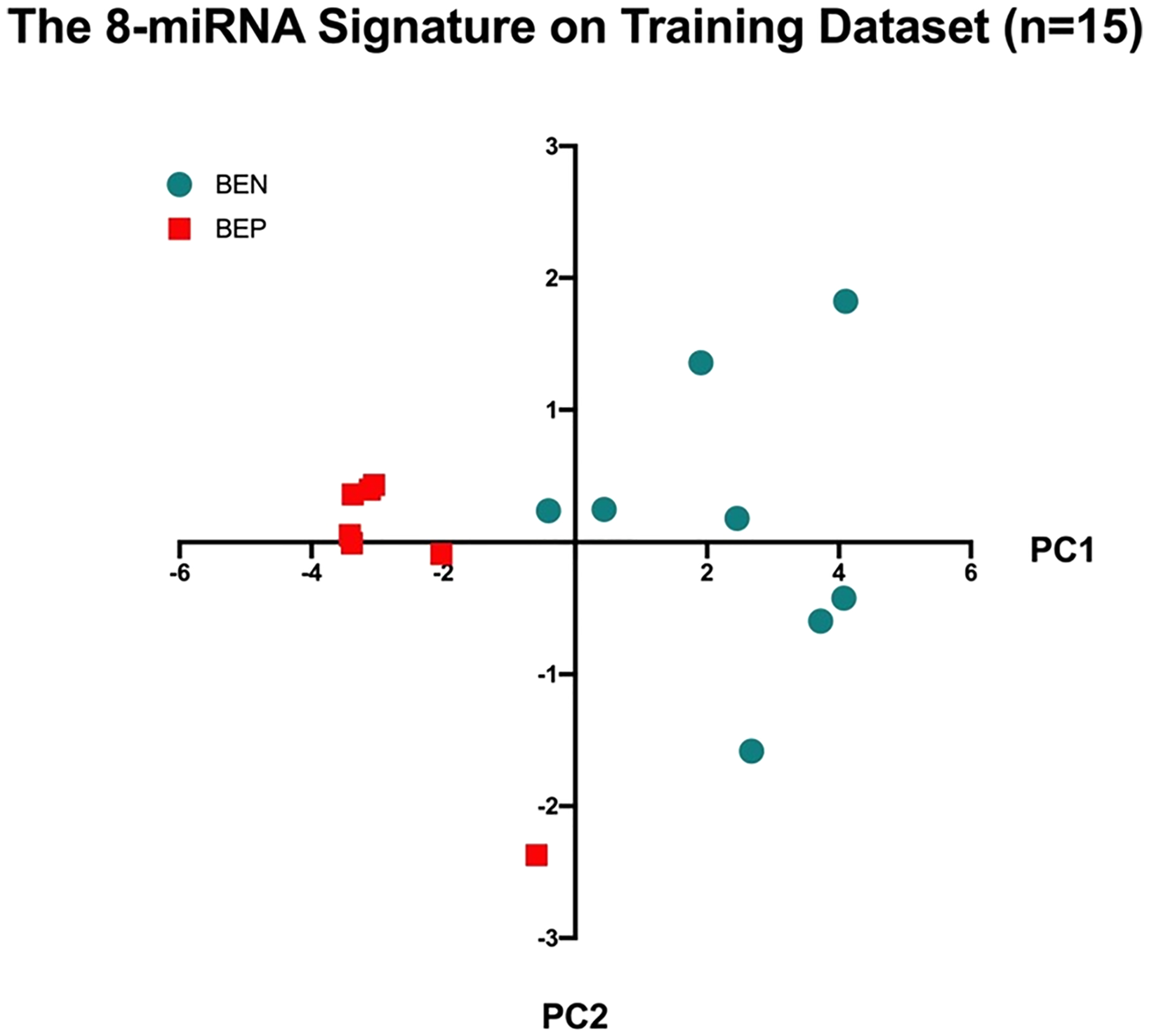
Self-validation of the 12 miRNA signature on training dataset (n = 15). Abbreviations: BEN, Barrett esophagus non progressed; BEP, Barrett esophagus progressed; MDS, multidimensional scaling; PC1, first principal component
Table 1.
NanoString data showing the 12 most significantly differentiated miRNAs between BEPs and BENs
| miRNA | BEN, median | BEP, median | P value | FDR | Lower 95% CL | Upper 95% CL |
|---|---|---|---|---|---|---|
| hsa-miR-1278 | 3.5573 | 0.8875 | .0012 | 0.1451 | 1.0765 | 4.4791 |
| hsa-miR-1301 | 4.0910 | 0.8875 | .0012 | 0.1451 | 1.1195 | 4.2912 |
| hsa-miR-1304-5p | 2.8979 | 0.8875 | .0012 | 0.1451 | 0.9484 | 3.2899 |
| hsa-miR-517b-3p | 3.1299 | 0.8875 | .0012 | 0.1451 | 1.0412 | 2.9330 |
| hsa-miR-584-5p | 3.0542 | 0.7570 | .0012 | 0.1451 | 1.1135 | 3.2899 |
| hsa-miR-599 | 3.3670 | 1.4005 | .0012 | 0.1451 | 1.0570 | 3.0911 |
| hsa-miR-103a-3p | 3.0808 | 0.7570 | .0022 | 0.1451 | 0.8387 | 2.8286 |
| hsa-miR-1197 | 5.7902 | 4.8768 | .0022 | 0.1451 | 0.4979 | 2.8116 |
| hsa-miR-1256 | 4.6010 | 1.4005 | .0022 | 0.1451 | 1.0789 | 4.1753 |
| hsa-miR-509–3-5p | 3.0808 | 0.8875 | .0022 | 0.1451 | 0.8474 | 3.2357 |
| hsa-miR-544b | 2.8152 | 0.7570 | .0022 | 0.1451 | 1.0789 | 2.9941 |
| hsa-miR-802 | 5.5812 | 3.2032 | .0022 | 0.1451 | 1.1223 | 3.9482 |
BEN, Barrett esophagus that has not progressed to dysplasia/carcinoma; BEP, Barrett
Fig. 2.
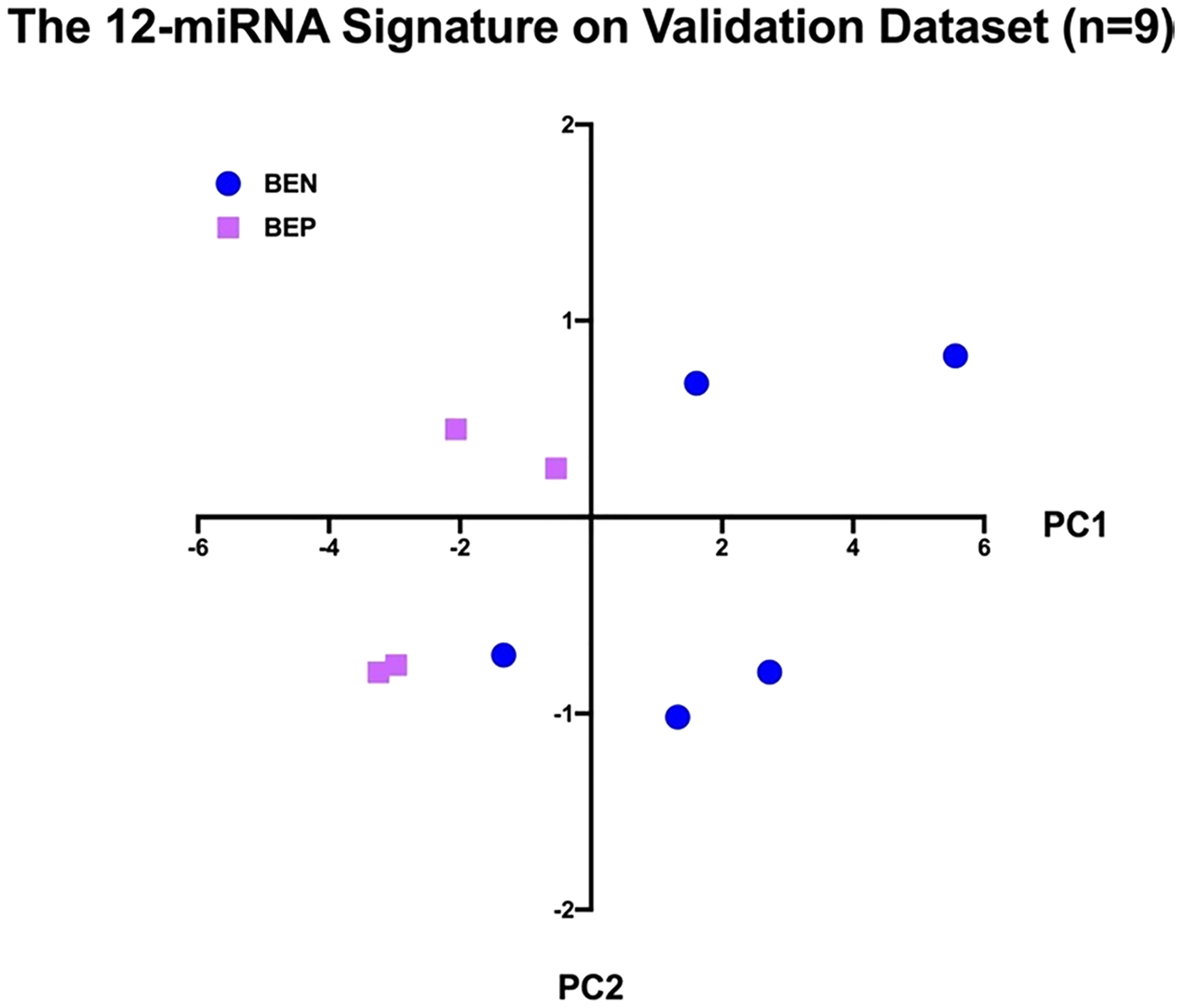
The 12 miRNA Signature on Validation Dataset (n = 9). Abbreviations: BEN, Barrett esophagus nonprogressed; BEP, Barrett esophagus progressed; MDS, multidimensional scaling; PC1, first principal component
Statistical Analyses
The 12 most statistically significant dysregulated miRNAs (P < 0.003) were selected for principal components analyses. The 12-miRNA signature was first self-validated on the training dataset itself, resulting in 7 out of the 7 BEP samples being classified as BEP (100% sensitivity) and 8 out of the 8 BEN samples being classified as BEN (100% specificity). The 12-miRNA signature was then validated by using the 9-sample validation dataset. To validate the 12-miRNA signature, we extracted the expression data of the 12 miRNAs from each sample of the validation dataset and standardized the gene expressions by the mean and standard deviation of the training dataset. We then multiplied the standardized gene expressions by the loading factors of the 12 miRNAs, the result of which was a weighted sum for each sample. Finally, we applied the same cutoff (PC1 > 0) to the weighted sums and categorized each sample as BEN or BEP, resulting in 3 out of the 4 BEP samples being classified as BEP (75% sensitivity) and 4 out of the 6 BEN samples being classified as BEN (80% specificity). A total of 24 samples were evaluated, and 22 cases were correctly classified. The overall accuracy was 91.67%. Samples taken from the same patient expressed a similar miRNA profile, with high concordance.
Discussion
We compared the miRNA expression profiles of 800 miRNAs between BEN and BEP samples. Our data showed that a 12-miRNA signature is capable of differentiating between BEN and BEP in biopsies taken from patients during initial visits. This signature may allow for a better stratification of BE patients and provide them with an optimized personalized follow-up schedule.
The regulatory role of miRNAs in normal cellular processes and their dysregulation in cancer development are known [29–31]. Prior studies on miRNA among BE patients used paired tissues to compare the miRNA expression profiles of patients with normal esophageal squamous mucosa to those with BE without dysplasia/EAC and those with BE with dysplasia/EAC [22–27]. Although these studies have shown that specific miRNAs are consistently altered during BE progression, an miRNA signature that is able to discriminate between BEN and BEP has yet to be identified. We focused on identifying an optimal miRNA signature for identifying patients with BE who are at high risk of progression to dysplasia and carcinoma.
Down-regulated miRNAs that lead to oncogenesis are considered tumor suppressor miRNAs (TSmiRs). If select TSmiRs are down-regulated in a significantly consistent number of BEPs compared to BENs, then these TSmiRs may have utility in predicting BEPs that would require a higher level of surveillance.
Of the 12 miRNAs identified in the optimal signature; all were downregulated in the BEP cases compared to those of BEN. Interestingly, some of the 12 miRNAs selected for our analyses have previously been reported to play a role in the regulation of carcinogenic pathways of other tumor types [31–44]. For example, miRNA-1301 has been associated with the inhibition of glioma cell proliferation by directly targeting N-Ras as well as the inhibition of hepatocellular carcinoma cell migration, invasion, and angiogenesis by decreasing Wnt/β-catenin signaling through the targeting of BCL9 [45, 46].
MiR-1304 reportedly behaves as a TSmiR through the regulation of heme oxygenase-1 post-transcriptionally in NSCLC cells [47]. miR-517b-3p has been shown to have lower levels of expression in mesothelioma [48].
MiR-584–5p is downregulated in medulloblastoma through the inhibition of histone deacetylase 1, eukaryotic translation initiation factor 4e family member 3, and C-MYC [49]. MiR-584–5p behaves as a TSmiR in renal cell carcinoma, glioma, and neuroblastoma [50–52].
MiR-599 behaves as a TSmiR via inhibition of cell migration, invasion, metastasis, and epithelial–mesenchymal transition in the inhibition of EIF5A2 in gastric cancer [53]. MiR-599 also behaves as a TSmiR via inhibition of MYC in hepatocellular carcinoma and serves as a TSmiR in anaplastic thyroid cancer by activating the T-cell intracellular antigen 1, which is an established tumor suppressor [54, 55].
MiR-103a-3p has target sites in the 5′UTR of GPRC5A, which consequently acts as a TSG in some cancer types and as an oncogene in others [56]. MiR-544 similarly operates as an oncomiR in gastric cancer and osteosarcoma by downregulating AXIN2 but operates as a TSmiR in gliomas through the inhibition of PARK7, which in turn inhibits glioma proliferation, invasion, and migration while inducing cell apoptosis [57–59].
To date, MiR-1197 has not been known to function as a TSmiR. The literature suggests that miR-1197 plays a role as an oncomiR, as its downregulation inhibits proliferation and migration in human non-small cell lung cancer cells through the upregulation of the oncogene Homeobox C11 [60]. MiR-509–3-5p behaves as a TSmiR via inhibition of invasion and lymphatic metastasis by targeting PODXL in gastric cancer [61]. Its role as a TSmiR is also observed in its downregulation in breast cancer and targeting of superoxide dismutase 2 [62].
MiRNA-802 has been shown to target Rab-23, thereby suppressing migration and invasion of gastric cancer [38]. Presumably, its downregulation in BEP will have opposite effects. MiRNA-802 has also been shown to inhibit epithelial–mesenchymal transition targeting flotillin-2 in prostate cancer [39] and to promote proliferation of lung carcinoma and osteosarcoma by targeting the Menin and p27 genes, respectively [40, 41]. These findings suggest that the function of miRNA-802 may be tumor-type specific.
MiRNA-1256 is a recently discovered miRNA that is positioned at chromosome 1p36.12. The expression of miRNA-1256 was found to be reduced in non-small cell lung cancer cells, and its upregulation was found to suppress the proliferation and migration of 2 non-small cell lung cancer cell lines [42]. Such suppression is the result of tectonic family member 1 (TCTN1) downregulation by the upregulated MiR-1256 [42]. Dysregulation of miR-1256 was also reported in prostate [43] and colorectal cancers [44].
Given the rapid increase of EAC incidence during the past several decades in Western countries [53], this 12-miRNA signature may allow for high-risk BEP patients to be enrolled in a more stringent follow-up endoscopic program combined with earlier surgical or endoscopic intervention. Conversely, the BEN patients at low risk of progression would be saved the cost and inconvenience of unnecessary testing and could be followed-up at longer time intervals. We anticipate that using the 12-miRNA signature will improve the effectiveness of the BE patient surveillance program (Seattle Protocol). To date, this monitoring program has, unfortunately, not effectively reduced EAC patient mortality. In addition, the majority of BE patients never develop cancer, with risk of progression being approximately 0.15% to 0.58% per patient annually [7, 8]. Nevertheless, this population is subjected to routine endoscopic procedures with multiple biopsies that cost approximately $1000 for a single patient screening in the USA. Guidelines on the diagnosis and management of BE from the British Society of Gastroenterology include remarks on the high economic costs of this program [17].
Most studies have shown that surveillance has no effect or only results in an incremental cost-effectiveness ratio of approximately $90 000/quality-adjusted life years (QALY), which is above the threshold of being considered cost-effective [63–65]. Therefore, having a discriminatory tool to differentiate between BEP and BEN could have significant cost-saving advantages and help to identify BE individuals at high risk of progression to dysplasia and cancer, potentially facilitating the detection of BE early dysplastic lesions.
Conclusion
We have identified a novel 12-miRNA signature that can identify patients with BE who are at high risk of progression. Use of this signature could improve the effectiveness of current screening and surveillance programs focused on reducing the incidence of EAC through prevention of BE progression.
Acknowledgments
This work has been supported in part by the Total Cancer Care Consortium, the Tissue Core Facility, the Molecular Genomics Core, and the Department of Biostatistics and Bioinformatics at the H. Lee Moffitt Cancer Center and Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). We thank Daley Drucker and Paul Fletcher (H. Lee Moffitt Cancer Center and Research Institution) for editorial support. They were not compensated beyond their regular salaries. We also thank Anders Berglund, PhD, Dung-Tsa Chen, PhD, and Braydon Schaible, MS for their role in data analysis. They were not compensated beyond their regular salary.
Funding
MMG Jr. Faculty TSP Pilot Project: 09-33401-15-01.
Abbreviations
- BE
Barrett esophagus
- BEN
Barrett esophagus nonprogressed
- BEP
Barrett esophagus progressed
- EAC
Esophageal adenocarcinoma
- FFPE
Formalin-fixed paraffin-embedded
- GERD
Gastroesophageal reflux disease
- HGD
High-grade dysplasia
- MCC
H. Lee Moffitt Cancer Center and Research Institute
- miRNA
microRNA
- PC1
First principal component
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Lin JJ, Kennedy E, Sequist LV et al. Clinical Activity of Alectinib in Advanced RET-Rearranged Non-Small-Cell Lung Cancer Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016. [DOI] [PubMed] [Google Scholar]
- 2.Martinucci I, de Bortoli N, Russo S et al. Barrett’s esophagus in 2016: From pathophysiology to treatment World J Gastrointest Pharmacol Therapeut. 2016;7:190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naini BV, Souza RF, Odze RD. Barrett’s esophagus: a comprehensive and contemporary review for pathologists. Am J Surg Pathol. 2016;40:e45–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc. 2010;71:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Nocon M, Vieth M et al. Evolution of gastrooesophageal reflux disease over 5 years under routine medical care–the ProGERD study. Aliment Pharmacol Therapeut. 2012;35:154–164 [DOI] [PubMed] [Google Scholar]
- 6.Bhat S, Coleman HG, Yousef F et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischer DE, Odze R, Overholt BF et al. The case for endoscopic treatment of non-dysplastic and low-grade dysplastic Barrett’s esophagus. Digest Dis Sci. 2010;55:1918–1931. 10.1007/s10620-010-1218-1.pdf [DOI] [PubMed] [Google Scholar]
- 8.Wolf WA, Pasricha S, Cotton C et al. Incidence of esophageal adenocarcinoma and causes of mortality after radiofrequency ablation of barrett’s esophagus. Gastroenterology. 2015;149:e1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The practice parameters committee of the american college of gastroenterology. Am J Gastroenterol. 1998;93:1028–1032 [DOI] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet (London, England). 2009;373:850–861 [DOI] [PubMed] [Google Scholar]
- 11.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383 [DOI] [PubMed] [Google Scholar]
- 12.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG clinical guideline: diagnosis and management of barrett’s esophagus. Am J Gastroenterol. 2016;111:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Burgh A, Dees J, Hop WC, van Blankenstein M. Oesophageal cancer is an uncommon cause of death in patients with Barrett’s oesophagus. Gut. 1996;39:5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett’s oesophagus: observational study. BMJ Clin Res. 2000;321:1252–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curvers WL, Peters FP, Elzer B et al. Quality of Barrett’s surveillance in The Netherlands: a standardized review of endoscopy and pathology reports. Eur J Gastroenterol Hepatol. 2008;20:601–607 [DOI] [PubMed] [Google Scholar]
- 16.Boyce HW. Barrett esophagus: endoscopic findings and what to biopsy. J Clin Gastroenterol. 2003;36:S6–S18 ((discussion S26–18)). [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald RC, di Pietro M, Ragunath K et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42 [DOI] [PubMed] [Google Scholar]
- 18.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Internal Med. 2003;138:176–186 [DOI] [PubMed] [Google Scholar]
- 19.de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett’s oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–1036 [DOI] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297 [DOI] [PubMed] [Google Scholar]
- 21.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith CM, Watson DI, Michael MZ, Hussey DJ. MicroRNAs, development of Barrett’s esophagus, and progression to esophageal adenocarcinoma. World J Gastroenterol. 2010;16:531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kan T, Meltzer SJ. MicroRNAs in Barrett’s esophagus and esophageal adenocarcinoma. Curr Opin Pharmacol. 2009;9:727–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijnhoven BP, Hussey DJ, Watson DI, Tsykin A, Smith CM, Michael MZ. MicroRNA profiling of Barrett’s oesophagus and oesophageal adenocarcinoma. Br J Surg. 2010;97:853–861 [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Gu J, Wang KK et al. MicroRNA expression signatures in Barrett’s esophagus and esophageal adenocarcinoma. Clin Cancer Res. 2009;15:5744–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Ajani JA, Gu J et al. MicroRNA expression signatures during malignant progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer Prev Res Philadelphia, Pa. 2013;6:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fassan M, Volinia S, Palatini J et al. MicroRNA expression profiling in the histological subtypes of barrett’s metaplasia. Clin Transl Gastroenterol. 2013;4:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine DS, Blount PL, Rudolph RE, Reid BJ. Safety of a systematic endoscopic biopsy protocol in patients with Barrett’s esophagus. Am J Gastroenterol. 2000;95:1152–1157 [DOI] [PubMed] [Google Scholar]
- 29.Herrera-Merchan A, Cerrato C, Luengo G et al. miR-33-mediated downregulation of p53 controls hematopoietic stem cell self-renewal. Cell cycle (Georgetown, Tex). 2010;9:3277–3285 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Liu J, Zang D et al. Upregulation of miR-572 transcriptionally suppresses SOCS1 and p21 and contributes to human ovarian cancer progression. Oncotarget. 2015;6:15180–15193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Ma Y, Xu W et al. Association of microRNA-933 variant with the susceptibility to gastric cancer. J BUON. 2017;22:390–395 [PubMed] [Google Scholar]
- 32.Lu JH, Zuo ZX, Wang W et al. A two-microRNA-based signature predicts first-line chemotherapy outcomes in advanced colorectal cancer patients. Cell Death Discov. 2018;4:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin S, Collin J, Zhu L et al. A novel role for miR-1305 in regulation of pluripotency-differentiation balance, cell cycle, and apoptosis in human pluripotent stem cells. Stem Cells (Dayton, Ohio). 2016;34:2306–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian HW, Zhou Y, Jian ZH, Liu RZ. MiR-323–5p acts as a tumor suppressor by targeting the insulin-like growth factor 1 receptor in human glioma cells. Asian Pac J Cancer Prev APJCP. 2014;15:10181–10185 [DOI] [PubMed] [Google Scholar]
- 35.Qiu S, Lin S, Hu D, Feng Y, Tan Y, Peng Y. Interactions of miR-323/miR-326/miR-329 and miR-130a/miR-155/miR-210 as prognostic indicators for clinical outcome of glioblastoma patients. J Transl Med. 2013;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momen-Heravi F, Trachtenberg AJ, Kuo WP, Cheng YS. Genomewide study of salivary MicroRNAs for detection of oral cancer. J Dental Res. 2014;93:86s–93s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song L, Dai T, Xie Y et al. Up-regulation of miR-1245 by c-myc targets BRCA2 and impairs DNA repair. J Mol Cell Biol. 2012;4:108–117 [DOI] [PubMed] [Google Scholar]
- 38.Zhang XY, Mu JH, Liu LY, Zhang HZ. Upregulation of miR-802 suppresses gastric cancer oncogenicity via targeting RAB23 expression. Eur Rev Med Pharmacol Sci. 2017;21:4071–4078 [PubMed] [Google Scholar]
- 39.Wang D, Lu G, Shao Y, Xu D. microRNA-802 inhibits epithelial-mesenchymal transition through targeting flotillin-2 in human prostate cancer Biosci Rep. 2017;37. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Wang LQ, Chen G, Liu XY, Liu FY, Jiang SY, Wang Z. micro-RNA802 promotes lung carcinoma proliferation by targeting the tumor suppressor menin. Mol Med Rep. 2014;10:1537–1542 [DOI] [PubMed] [Google Scholar]
- 41.Cao ZQ, Shen Z, Huang WY. MicroRNA-802 promotes osteosarcoma cell proliferation by targeting p27. Asian Pac J Cancer Prev APJCP. 2013;14:7081–7084 [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Wan X, Mu Z et al. MiR-1256 suppresses proliferation and migration of non-small cell lung cancer via regulating TCTN1. Oncol Lett. 2018;16:1708–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Kong D, Ahmad A, Bao B, Dyson G, Sarkar FH. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics. 2012;7:940–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu ZY, Yang L, Chang HY. Clinicopathologic and prognostic relevance of miR-1256 in colorectal cancer: a preliminary clinical study. Eur Rev Med Pharmacol Sci. 2018;22:7704–7709 [DOI] [PubMed] [Google Scholar]
- 45.Zhi T, Jiang K, Zhang C et al. MicroRNA-1301 inhibits proliferation of human glioma cells by directly targeting N-Ras. Am J Cancer Res City;2017:982–998. [PMC free article] [PubMed] [Google Scholar]
- 46.Yang C, Xu Y, Cheng F et al. miR-1301 inhibits hepatocellular carcinoma cell migration, invasion, and angiogenesis by decreasing Wnt/β-catenin signaling through targeting BCL9. Cell Death Dis. 2017;8:e2999–e2999 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Li CG, Pu MF, Li CZ et al. MicroRNA-1304 suppresses human non-small cell lung cancer cell growth in vitro by targeting heme oxygenase-1. Acta Pharmacol Sin. 2017;38:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felley-Bosco E. Mesothelioma Heterogeneity: Potential Mechanisms. City: MDPI AG; 2019. [Google Scholar]
- 49.Abdelfattah N, Rajamanickam S, Timilsina S, Subbarayalu P, Onyeagucha B, Rao M. Abstract A45: tumor suppressor miR-584–5p regulates MYC and sensitizes MYC-amplified medulloblastoma to vincristine and ionizing radiation. Cancer Res. 2018;78:A45–A45 [Google Scholar]
- 50.Ueno K, Hirata H, Shahryari V et al. Tumour suppressor micro-RNA-584 directly targets oncogene Rock-1 and decreases invasion ability in human clear cell renal cell carcinoma. Br J Cancer. 2011;104:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XP, Deng XL, Li LY. MicroRNA-584 functions as a tumor suppressor and targets PTTG1IP in glioma. Int J Clin Exp Pathol. 2014;7:8573–8582 [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang X, Mei H, Qu H et al. miRNA-584–5p exerts tumor suppressive functions in human neuroblastoma through repressing transcription of matrix metalloproteinase. Biochim et Biophys Acta BBA Mol Basis Dis 2015;1852:1743–1754 [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Jin Y, Zhang H, Huang X, Zhang Y, Zhu J. Micro-RNA-599 inhibits metastasis and epithelial-mesenchymal transition via targeting EIF5A2 in gastric cancer. Biomed Pharm Biomed Pharm. 2018;97:473–480 [DOI] [PubMed] [Google Scholar]
- 54.Tian J, Hu X, Gao W et al. Identification a novel tumor-suppressive hsa-miR-599 regulates cells proliferation, migration and invasion by targeting oncogenic MYC in hepatocellular carcinoma. Am J Transl Res. 2016;8:2575–2584 [PMC free article] [PubMed] [Google Scholar]
- 55.Bi JW, Zou YL, Qian JT, Chen WB. MiR-599 serves a suppressive role in anaplastic thyroid cancer by activating the T-cell intracellular antigen. Exp Ther Med. 2019;18:2413–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou H, Rigoutsos I. MiR-103a-3p targets the 5’ UTR of GPRC5A in pancreatic cells. RNA New York, NY. 2014;20:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhi Q, Guo X, Guo L et al. Oncogenic miR-544 is an important molecular target in gastric cancer. Anti-Cancer Agents Med Chem. 2012;13. [DOI] [PubMed] [Google Scholar]
- 58.Jin S, Dai Y, Li C, Fang X, Han H, Wang D. MicroRNA-544 inhibits glioma proliferation, invasion and migration but induces cell apoptosis by targeting PARK7. Am J Transl Res. 2016;8:1826–1837 [PMC free article] [PubMed] [Google Scholar]
- 59.Chen M, Liu YY, Zheng MQ et al. microRNA-544 promoted human osteosarcoma cell proliferation by downregulating AXIN2 expression. Oncol Lett. 2018;15:7076–7082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun B, Hua J, Cui H, Liu H, Zhang K, Zhou H. MicroRNA-1197 downregulation inhibits proliferation and migration in human non-small cell lung cancer cells by upregulating HOXC11. Biomed Pharmacother. 2019;117:109041 [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Zhu Z, Sheng J et al. miR-509–3-5P inhibits the invasion and lymphatic metastasis by targeting PODXL and serves as a novel prognostic indicator for gastric cancer. Oncotarget. 2017;8:34867–34883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song YH, Wang J, Nie G et al. MicroRNA-509–5p functions as an anti-oncogene in breast cancer via targeting SOD2. Eur Rev Med Pharmacol Sci. 2017;21:3617–3625 [PubMed] [Google Scholar]
- 63.Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053 [DOI] [PubMed] [Google Scholar]
- 64.Somerville M, Garside R, Pitt M, Stein K. Surveillance of Barrett’s oesophagus: is it worthwhile? Eur J Cancer Oxford, Engl 1999. 2008;44:588–599 [DOI] [PubMed] [Google Scholar]
- 65.Sonnenberg A, Soni A, Sampliner RE. Medical decision analysis of endoscopic surveillance of Barrett’s oesophagus to prevent oesophageal adenocarcinoma. Aliment Pharmacol Therapeut. 2002;16:41–50 [DOI] [PubMed] [Google Scholar]


