Abstract
Previous studies have suggested that the C-C chemokine C10 is involved in the chronic stages of host defense reactions. The present study addressed the role of C10 in a murine model of septic peritonitis, induced by cecal ligation and puncture (CLP). Unlike other C-C chemokines, C10 levels in the peritoneal wash were increased approximately 30-fold above baseline levels at 48 h after CLP surgery. Immunoneutralization of peritoneal C10 levels with polyclonal anti-C10 antiserum during CLP-induced peritonitis negatively impacted mouse survival over 4 days. In contrast, when 500 ng of recombinant murine C10 was administered immediately after CLP surgery, the 4-day survival rate increased from 20% to over 60%. The C10 therapy appeared to facilitate a rapid and significant enhancement of the levels of tumor necrosis factor alpha (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) and a later increase in interleukin-13 (IL-13) levels in the peritoneal cavity. In vitro studies showed that the combination of IL-1β and C10 markedly augmented TNF-α synthesis by peritoneal macrophages and that C10 synthesis was induced in these cells following their exposure to IL-13. At 24 h after CLP surgery, only 25% of C10-treated mice were bacteremic versus 85% of the control group that exhibited dissemination of bacteria into the circulation. The lack of bacteremia in C10-treated mice appeared to be related, in part, to in vitro evidence that C10 significantly enhanced the bacterial phagocytic activity of peritoneal macrophages. In addition, in vivo evidence suggested that C10 therapy significantly reduced the amount of material that leaked from the damaged gut. Taken together, the results of this study demonstrate that the C10 chemokine rapidly promotes disease resolution in the CLP model through its direct effects on the cellular events critically involved in host defense during septic peritonitis.
Sepsis and sepsis-like hyperinflammatory states are initiated after a host is exposed to microbes or microbial products such as lipopolysaccharide (LPS), a gram-negative bacterial cell wall component. The systemic inflammatory response, which follows, is mediated by a number of complex, interacting molecular networks, which include a large array of mediators such as lipid metabolites, reactive nitrogen and oxygen metabolites, lipids, nucleotides, and cytokines. Cytokines in particular appear to function as central soluble initiators and propagators of the septic inflammatory response. The systemic production of the early-response, proinflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) results in a number of inflammatory events, including widespread inflammatory-cell recruitment and activation. TNF-α and IL-1β instigate inflammation primarily by initiating cascades of downstream mediators, such as pro- and anti-inflammatory cytokines and chemokines (4). TNF-α and IL-1β presumably play a central role as proximal mediators of a wide range of vital downstream processes, which explains the failure of clinical therapies targeting these cytokines (1, 2, 8, 11, 12).
Chemokines are distal mediators of the septic inflammatory response and thus may offer novel avenues for therapy during sepsis. With the exception of a number of circumstantial observations showing that several chemokines are produced at higher levels in patients with sepsis (6), little is known about the specific functions of chemokines during this inflammatory disorder. However, several general characteristics of chemokines cast these molecules as potentially important participants in the septic response. By definition, chemokines display chemotactic activities for various immune/inflammatory populations. Furthermore, many of the chemokines also appear to activate the cell population(s) for which they are chemotactic. In this capacity, chemokines are intrinsically proinflammatory. On the other hand, chemokines have more recently been attributed a variety of regulatory roles in such diverse processes as fibrinogenesis, angiogenesis, and immune/inflammatory responses (21). We hypothesized that those chemokines that display unique immunostimulatory properties may be especially relevant during bacterial sepsis.
C10 chemokine displays amino acid sequence homology to a number of CCR1-binding chemokines, all of which are structurally large chemokines due to a genomic structure which contains an unique second exon (3, 13, 20, 27). Furthermore, the portion of the C10 molecule encoded by this extra exon is necessary for its biological activity (3). Interestingly, unlike many other chemokines, C10 is IL-4 inducible but not LPS inducible in macrophages and IL-4 stimulation of these cells results in de novo C10 synthesis (19). Upon appropriate cytokine stimulation, C10 production by various cell populations appears to peak between 24 and 48 h after cytokine stimulation (14, 19). In addition, C10 recruits a diverse array of cell populations including lymphocytes, macrophages, and eosinophils (14, 19). Thus, the present study explored the possibility that C10 may modulate the host defense response, thereby promoting disease resolution and survival in a clinically relevant experimental model of bacterial sepsis.
MATERIALS AND METHODS
CLP model.
Specific-pathogen-free female CD-1 mice, 6 to l2 weeks old (Charles River Breeding Labs), were caged in groups of five within the Specific-Pathogen-Free Unit of Laboratory Animal Medicine at the University of Michigan until the day of each experiment. The cecal ligation and puncture (CLP) model of acute sepsis was used as previously described (24). Briefly, mice were anesthetized by an intraperitoneal (i.p.) injection of 3.0 to 3.5 mg of ketamine HCl (Ketaset; Fort Dodge Laboratories) followed by inhaled methoxyflurane (Metafane; Pitman-Moore Inc.) as required for full anesthesia. A 1- to 2-cm longitudinal incision was made to the lower left quadrant of the abdomen. The cecum was exposed, and the distal one-third was ligated with 3-0 silk suture. The ligated cecum was punctured through and through with a 21-gauge needle. Finally, the cecum was replaced in the peritoneal cavity and the incision was closed with surgical staples. In sham controls, the cecum was exposed but not ligated or punctured and then was returned to the abdominal cavity. All mice were injected with 1 ml of sterile saline subcutaneously as a fluid resuscitation measure immediately following surgery.
Experimental protocols.
Changes in endogenous C10, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and MIP-2 levels in the peritoneum after CLP were initially examined. At 15 min and 6, 24, and 48 h after CLP surgery, groups of five mice were killed and peritoneal fluid was obtained from each mouse by lavaging the peritoneal cavity with 2 ml of sterile saline. Cell-free peritoneal samples were stored at −20°C prior to enzyme-linked immunosorbent assay (ELISA) analysis.
The first survival experiment addressed the contribution of endogenous C10 to mouse survival after CLP surgery. Accordingly, groups of 12 mice received either 0.5 ml of anti-C10 antiserum or normal rabbit preimmune serum approximately 2 h prior to the CLP surgery. As described previously (10), anti-C10 polyclonal antiserum was generated in New Zealand White rabbits by multiple-site immunization with adjuvant and recombinant murine C10 (R & D Systems, Minneapolis, Minn.). Anti-C10 polyclonal antibody was titrated, and its specificity was verified by direct ELISA. The endotoxin content in recombinant C10, preimmune rabbit serum, and anti-C10 antiserum was consistently below the limit of detection (<0.05 EU/ml) (PYROGENT [BioWhittaker, Walkersville, Md.] was used to detect endotoxin). Significant depletion of peritoneal levels of C10 was still apparent on day 4 after anti-C10 antiserum administration and CLP surgery. The second survival experiment assessed the effect of recombinant murine C10 on mouse survival after CLP surgery. Groups of 12 mice received an i.p. injection of 0.1 ml of normal saline alone or containing 500 ng of C10 chemokine (R&D Systems). Mouse survival was monitored for up to 4 days after CLP surgery in both experiments. Both survival studies were performed on three separate occasions with similar group sizes, and the data were pooled.
The effects of recombinant C10 on cytokine and chemokine levels in the peritoneum after CLP surgery were examined in additional experiments. At 15 min and 6, 24 and 48 h after the i.p. injections, groups of five mice in both treatment groups were anesthetized with Metafane and whole blood was drained from the retro-orbital sinus of each mouse. Peritoneal fluid was then obtained from each mouse by lavaging the peritoneal cavity with 2 ml of sterile saline. Serum was derived from the blood samples by centrifugation, and fresh serum was used to assess the presence of bacteria (see below). Cell-free peritoneal samples were stored at −20°C prior to ELISA analysis.
Other groups of 10 mice were used to address the effect of recombinant C10 therapy on the leakage of material from the gut after CLP surgery. Mice received 5 mg of fluorescein isothiocyanate (FITC)-labeled dextran (mean molecular mass, 40,000 kDa) per os at 24 and 48 h prior to CLP surgery. At 24 h after the induction of sepsis by CLP surgery, the mice were sacrificed and peritoneal lavages were performed with a total volume of 1 ml of sterile saline. Neat and 1:10 dilutions of the lavage fluid (100 μl) were added to a Costar flat-bottom, 96-well microtiter plate (Corning Inc., Corning, N.Y.). The plates were read at 485 nm in a fluorescent plate reader. Standards were 1/2-log-unit dilutions of FITC-dextran from 1 ng/ml to 1 μg/ml.
Determination of bacteremia.
Serum samples were aseptically collected 24 h after CLP surgery. A 10-μl volume of each sample was plated on soy base blood agar plates (Difco). The plates were then incubated at 37°C for 24 h. Colony formation was taken as an indication of bacteremia.
Murine cytokine ELISA.
Cell-free peritoneal lavage samples were subjected to cytokine ELISA. Murine C10, MCP-1, MIP-1α, MIP-2, TNF-α, IL-13, and IL-10 were quantified using a modified double-ligand assay as previously described (10). Briefly, flat-bottom 96-well microtiter plates (Nunc Immuno-Plate I 96-F) were coated at 50 μl/well with rabbit antibody against the various cytokines (1 μg/ml in 0.6 M NaCl–0.26 M H3BO4–0.08 M NaOH [pH 9.6]) for 16 h at 4°C and then washed with phosphate-buffered saline (PBS) (pH 7.5)–0.05% Tween 20 (wash buffer). Nonspecific binding sites were blocked with 2% bovine serum albumin BSA in PBS and incubated for 90 min at 37°C. The plates were rinsed four times with wash buffer. Diluted (neat and 1:10) cell-free supernatants (50 μl) in duplicate were added, and the plates were incubated for 1 h at 37°C and washed four times. Then biotinylated rabbit antibodies against the specific cytokines (3.5 μg/ml in PBS [pH 7.5]–0.05% Tween 20–2% fetal calf serum) were added at 50 μl/well. After being incubated at 37°C for 30 min, the plates were washed four times. Streptavidin-peroxidase conjugate (Bio-Rad Laboratories, Richmond, Calif.) was added, after which, the plates were again incubated at 37°C for 30 min. The plates were washed four times prior to the addition of chromogen substrate (Bio-Rad Laboratories) and incubated at room temperature to the desired extinction, and the reaction was terminated with 50 μl of 3 M H2SO4 solution per well. The plates were read at 490 nm in an ELISA reader. Standards were 1/2-log-unit dilutions of recombinant murine cytokines from 1 pg/ml to 100 ng/ml. ELISA specificity was confirmed for each cytokine and chemokine measured.
Peritoneal macrophage isolation and phagocytosis assay.
Mice not subjected to CLP surgery were used for the isolation of peritoneal macrophages. The peritoneal cavity was exposed, and approximately 10 ml of sterile saline solution containing 0.05 M EDTA was injected into the peritoneal cavity. The EDTA-saline solution was withdrawn using a 21-gauge needle. This procedure was repeated approximately three times per mouse. Upon removal from the peritoneal cavity, the cell-containing EDTA saline solution was immediately placed on ice. The cells were pelleted by centrifugation at 1,500 rpm, after which the red blood cells were lysed using an ammonium chloride red blood cell lysis buffer. The cells were washed twice in RPMI, after which they were resuspended in RPMI 1640 containing 10% fetal bovine serum. The generation of C10 by peritoneal macrophages was evaluated in cultures of 106 cells exposed for 24 h to either supplemented RPMI alone or RPMI containing LPS, IL-1β, or IL-13. The generation of TNF-α by peritoneal macrophages was determined in cultures of 106 cells exposed for 6 h to IL-1β alone or IL-1β plus C10. Unless otherwise stated, LPS was used at 1 μg/ml and all cytokines were used at 100 ng/ml. Macrophage phagocytic potential was evaluated, using a modification of a previously described method (23). Peritoneal macrophages were incubated for 1 h at 37°C in Hanks' balanced salt solution in eight-well Labteks plates (Nunc Inc., Naperville, Ill.). Escherichia coli cells (106) were added to each well, and the plates were incubated on a shaker at 37°C for 1 h. The supernatants were removed, and the cells were washed in Hanks' balanced salt solution. The gasket was removed, and the slides were allowed to air dry, after which a Diff-Quik (Baxter, McGraw Park, Ill.) staining procedure was performed. Two hundred cells per well were counted to determine the mean number of intracellular E. coli cells per well.
Statistical analysis.
Analysis of variance (ANOVA) followed by Dunnett's test was used in all experiments in which multiple experimental groups were compared to a single control group. A two-tailed Student t test was utilized to assess significance for experiments comparing a single experimental group to a single control. Survival curves were analyzed by the log rank test. All calculations were performed using Prism 2.0 (Graphpad Software, Inc., San Diego, Calif.) or Primer of Biostatistics 3.01 (McGraw-Hill). Significance was assigned for P < 0.05.
RESULTS
CLP surgery augments and sustains C10 levels above other C-C chemokines in the peritoneal cavity.
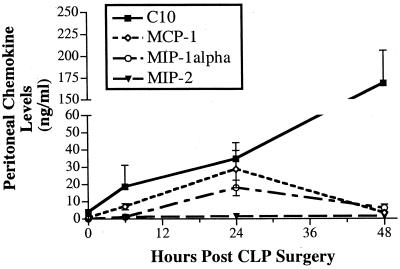
Previous studies have documented that chemokine levels are markedly increased during the course of septic peritonitis after CLP surgery (15, 26). In the present study, we first examined whether changes in peritoneal levels of C10 mirrored those of other C-C chemokines such as MCP-1, MIP-1α, and MIP-2 after CLP. To this end, peritoneal levels of all four chemokines were measured by ELISA. Compared to baseline peritoneal levels, C10 levels in the peritoneal cavity were markedly elevated at all times after CLP surgery (Fig. 1). At 48 h after CLP surgery, peritoneal fluids contained 30-fold-higher levels of C10 than that measured in the peritoneum prior to surgery. In contrast, MCP-1, MIP-1α, and MIP-2 were detected at lower concentrations in peritoneal fluid before and at all times after CLP. Furthermore, peak levels of MCP-1 and MIP-1α were measured at 24 h after CLP and the levels of both chemokines were near baseline levels at 48 h after CLP surgery. The dramatic and sustained increase of C10 levels after CLP surgery clearly suggested that C10 played a prominent role during septic peritonitis.
FIG. 1.
CLP surgery enhances C10, MCP-1, MIP-1α, and MIP-2 levels in the peritoneum. CLP surgeries were performed, and peritoneal lavage samples were collected at 6, 24, and 48 h after surgery. Peritoneal washes were also conducted on mice that did not undergo CLP surgery (i.e., T = 0 values). Chemokine concentrations were measured by specific ELISA. Five mice per time point were used. The error bars indicate the SEM.
Anti-C10 antiserum augments whereas exogenous C10 protein prevents CLP-induced mortality.
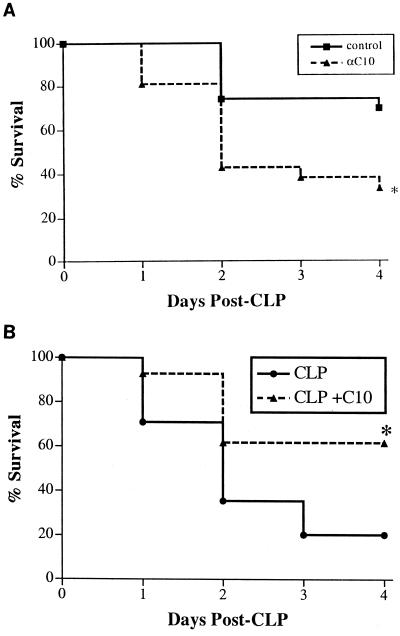
Several studies showing a correlation between chemokine production and sepsis-associated mortality have demonstrated that chemokines function as deleterious (MIP-2 [26]) and beneficial (MCP-1 [15]) inflammatory mediators during sepsis. We attempted to determine whether increased C10 levels in the peritoneal cavity contributed to sepsis-induced mortality by neutralizing C10 in the context of CLP surgery. Normal rabbit preimmune serum or anti-C10 antiserum was administered i.p. 2 h prior to CLP surgery. The administration of normal rabbit preimmune serum to mice prior to CLP surgery was protective since more than 60% of these mice were alive on day 4 after CLP surgery (Fig. 2A), consistent with previous observations in this CLP model (15). The protective effect of normal rabbit serum in the CLP model appears to be a consequence of the macrophage activating factors present within this biological fluid (A. Matsukawa, unpublished data). In addition, we have never detected any cytokine or chemokine in rabbit serum that cross-reacts with murine C10 chemokine. In contrast, when mice received the same volume of rabbit serum containing polyclonal anti-C10 antibodies, only 35% of these mice were alive on day 4 after CLP (Fig. 2A). These data suggested that endogenous C10 was required for mouse survival during CLP-induced sepsis.
FIG. 2.
Effects of anti-C10 antiserum (A) and C10 therapy (B) on the survival of mice after CLP surgery. Immunoneutralization of endogenous C10 increased whereas exogenous C10 reduced mortality associated with CLP. Mice underwent CLP surgery. Experimental mice received 500 ng of C10 immediately after surgery. Time zero corresponds to the time of CLP surgery. Survival was assessed every 24 h for 4 days. Survival curves were analyzed using the log rank test. Both survival studies were performed on three separate occasions with similar group sizes (n = 12 mice per group), and the data were pooled. ∗, P < 0.005.
Given that the immunoneutralization of C10 during CLP-induced sepsis was clearly detrimental, we subsequently explored the possibility that the exogenous administration of C10 after CLP would confer a survival advantage. A pilot study revealed that a minimum of 500 ng of recombinant C10 was required for a significant survival effect following CLP surgery. In addition, doses of 1 and 2 μg of C10 did not markedly enhance mouse survival above that observed with the lower C10 dose. Thus, in all subsequent experiments, a dose of 500 ng of C10 was used. A bolus i.p. injection of recombinant C10 protein (500 ng in 100 μl of normal saline) or normal saline alone was administered immediately after CLP surgery. While C10-treated mice displayed a substantial improvement in survival by 24 h after CLP surgery (93 versus 71%), C10 treatment resulted in an impressive 300% improvement in survival at 4 days after CLP surgery (62 versus 20%) (Fig. 2B). These data clearly suggested that the exogenous administration of C10 protein after CLP surgery can substantially abrogate mortality associated with septic peritonitis.
Recombinant C10 therapy significantly enhances MCP-1, TNF-α, and IL-13 levels in the peritoneum after CLP surgery.
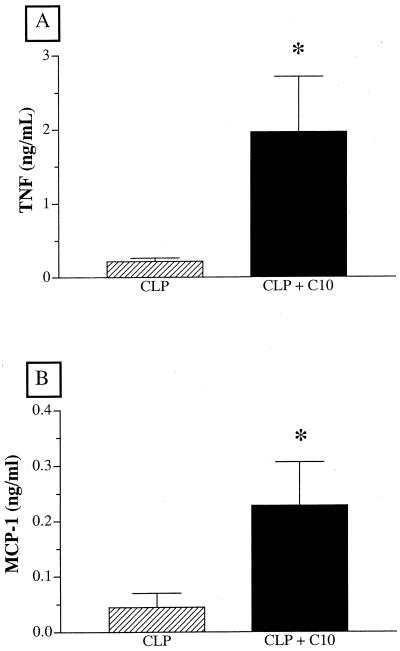
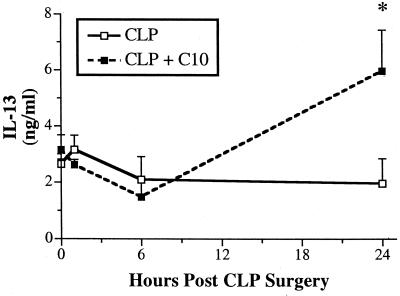
Emerging evidence suggests that C10 may modulate the production of chemokines such MCP-1 and cytokines such as IL-13 (14). Furthermore, TNF-α (9) and MCP-1 (15) have major immune-enhancing and protective effects in CLP models. Recent studies in this laboratory have shown that endogenous IL-13 is required for mouse survival following CLP surgery, because of the immunomodulatory effects of this cytokine (16). These previous findings provided the impetus to examine the effect of C10 therapy on the peritoneal chemokine and cytokine profile associated with CLP-induced sepsis. In C10-treated mice, peritoneal levels of TNF-α and MCP-1 were significantly elevated at 15 min after CLP surgery compared with those in CLP controls at this time (Fig. 3). Peritoneal levels of TNF-α and MCP-1 were similar in control and C10-treated mice at 6 h after CLP surgery (data not shown). Figure 4 depicts the temporal changes in IL-13 levels in peritoneal washes from control and C10-treated mice before and 6 and 24 h after CLP surgery and shows that peritoneal IL-13 levels were significantly elevated in C10-treated mice compared with control mice at 24 h after CLP surgery. Thus, the exogenous addition of C10 after CLP surgery rapidly increased levels of two major proinflammatory mediators that promote the clearance of bacteria from the septic peritoneum. In addition, this therapy promoted the production of IL-13 in the septic peritoneal cavity, suggesting that immunomodulatory processes were activated by C10 during sepsis.
FIG. 3.
C10 therapy significantly augments peritoneal TNF-α and MCP-1 levels. Mice underwent CLP surgery and received either 500 ng of C10 protein or normal saline alone via i.p. injection immediately after surgery. Peritoneal cytokine concentrations were determined by analyzing peritoneal lavage fluids by cytokine-specific ELISA at 15 min after the CLP surgery. A two-tailed t test was used to assess significance. Three separate experiments were performed (n = 12 mice per group), and the data were pooled. ∗, P < 0.01.
FIG. 4.
C10 therapy significantly augments peritoneal IL-13 levels. Mice underwent CLP surgery and received 500 ng of C10 protein or normal saline alone via i.p. injection immediately after surgery. Peritoneal cytokine concentrations were determined by analyzing peritoneal lavage fluids by cytokine-specific ELISA at 15 min and 6 and 24 h after CLP surgery. Peritoneal washes were also conducted on mice that did not undergo CLP surgery (i.e., T = 0 values). A two-tailed t test was used to assess significance. Three separate experiments were performed (n = five mice per time point), and the data were pooled. ∗, P < 0.01.
The combination of IL-1 and C10 induces TNF-α production by peritoneal macrophages.
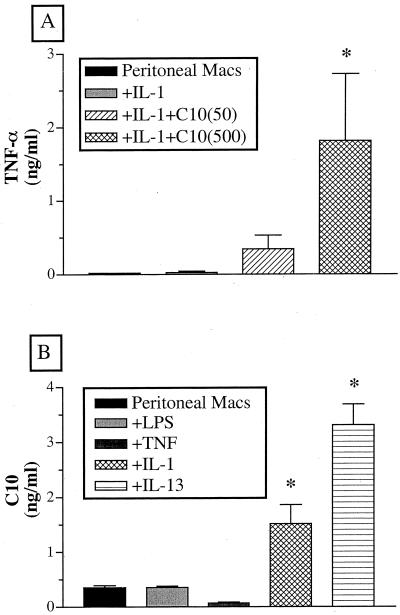
To determine whether C10 induced TNF-α production after CLP surgery, we costimulated peritoneal macrophages in vitro with septic inflammatory mediators and C10. Neither IL-1β nor C10 alone induced the release of TNF-α by peritoneal macrophages incubated in vitro for 6 h. However, in the presence of IL-1β, C10 induced TNF-α production in a dose-dependent manner, which was evident after 6 h of culture (Fig. 5A). These data suggested that peritoneal macrophages, in the septic inflammatory setting, probably respond to C10 therapy by producing TNF-α.
FIG. 5.
(A) C10 induces peritoneal macrophage TNF-α production after costimulation with IL-1. Freshly isolated peritoneal macrophages were stimulated with IL-1 (50 ng/ml) with or without C10 (50 and 500 ng/ml) and cultured in 10% RPMI for 6 h. A TNF-α-specific ELISA was used to measure TNF-α concentrations. Significance was assessed using ANOVA followed by Dunnett's test. ∗, P < 0.05. (B) Peritoneal macrophages produce increased C10 levels upon stimulation with IL-1 or IL-13. Freshly isolated peritoneal macrophages were stimulated with various cytokines (100 ng/ml) or LPS (1 μg/ml) and cultured in 10% RPMI for 24 or 48 h. A C10-specific ELISA was used to measure C10 concentrations. Significance was assessed using ANOVA followed by Dunnett's test. ∗, P < 0.05.
IL-13 is a potent inducer of C10 production by peritoneal macrophages.
C10 is a unique C-C chemokine in that it is induced by IL-4 but not by LPS (19). However, IL-4 is not normally associated with innate immune responses elicited by septic peritonitis, and we did not detect it at any time after the CLP-mediated induction of sepsis in the present study. Therefore, we examined whether other cytokines associated with CLP, namely, IL-1β (18) and IL-13 (16), affected the synthesis of C10 by peritoneal macrophages. Additional impetus for this study came from the present in vivo studies showing that IL-13 was constitutively present and induced in the peritoneal cavity after CLP surgery. Compared with cultures exposed to medium alone, LPS, TNF-α, or IL-1β, the most potent inducer of C10 production by peritoneal macrophages cultured in vitro was IL-13, as demonstrated by the presence of more than 3 ng of C10 per ml after 24 h (Fig. 5B).
C10 protein therapy attenuates bacterial infiltration of the systemic circulation during CLP-induced sepsis: effects on macrophage phagocytosis and gut leakage.
It is well established in bacterial sepsis that the development of bacteremia is often a prequel to death (7). Thus, we examined the incidence of bacteremia after CLP surgery and in the context of C10 protein therapy. Mice underwent CLP surgery, and experimental animals received a 500-ng i.p. injection of C10 immediately after surgery. Bacteremia was assessed 24 h after CLP surgery by incubating serum samples from experimental and control animals on a nonselective growth medium. C10 protein therapy convincingly attenuated bacterial infiltration of the systemic circulation, as manifested by a significant (P ≤ 0.05) reduction in the percentage of bacteremic mice from 85% in the control group (n = 13) to 25% in the C10-treated group (n = 12).
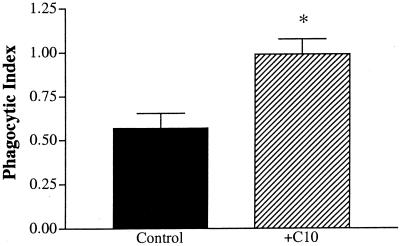
The peritoneal macrophage represents an important line of defense once an invading pathogen has infiltrated the peritoneal cavity. To examine the functional impact of C10 on this resident phagocytic cell line, peritoneal macrophages were stimulated in vitro with C10 for 1 h, after which their phagocytic potential was evaluated by direct challenge with viable E. coli cells. C10 was clearly able to augment peritoneal macrophage function, as evidenced by an approximate twofold increase in the mean number of E. coli cells per peritoneal macrophage after C10 stimulation (Fig. 6).
FIG. 6.
C10 stimulation augments the phagocytic capacity of peritoneal macrophages. Peritoneal macrophages were pretreated with C10 (500 ng/ml) for 1 h prior to the addition of E. coli (106 cells). After a 1-h exposure to the bacterial challenge, the experiment was terminated and the cells were stained. Intracellular bacteria were counted in a sample population of 200 cells per well. Graphed values represent the mean number of E. coli per cell. Statistics were analyzed using a two-tailed t test. Three separate experiments were performed, and the data were pooled. ∗, P < 0.05.
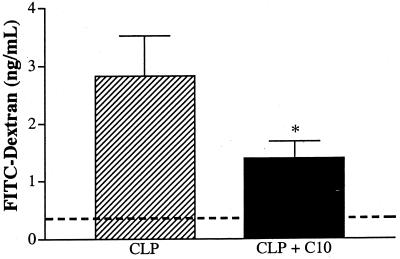
The septic inflammatory response often compromises the integrity of the bowel wall (22), and the importance of bowel barrier function to mouse survival after CLP surgery was recently highlighted in work published from this laboratory (24). The histologic examination of various portions of the bowel walls of CLP mice treated with C10 revealed a general improvement in the integrity of the intestinal epithelium (data not shown) and provided the impetus to further examine intestinal containment function after C10 therapy. Bowel barrier function was evaluated by measuring the ability of the septic bowel to contain FITC-labeled dextran. Briefly, mice were given 5 mg of FITC-labeled dextran (40,000 kDa) orally 24 and 48 h prior to CLP surgery. Experimental animals received a bolus i.p. injection of 500 ng of C10 protein immediately after CLP surgery. At 24 h after surgery, all the mice were sacrificed and peritoneal lavages were performed. Mice treated with C10 after CLP surgery displayed a 50% reduction in the leakage of FITC-dextran across the intestinal wall (Fig. 7), suggesting that C10 therapy attenuated the loss of bowel integrity associated with septic peritonitis.
FIG. 7.
C10 therapy improves intestinal wall barrier function during CLP-induced sepsis. Mice were lavage-fed 2 mg of FITC-labeled dextran (40,000 kDa) 24 and 48 h prior to CLP surgery. Experimental mice were given 500 ng of C10 protein immediately after surgery. At 24 h after CLP surgery, the mice were sacrificed and peritoneal lavages were performed. The concentration of dextran that had leaked from the intestinal tract into the peritoneal cavity was determined by measuring the fluorescent intensity of the peritoneal lavage fluid. The dashed line at 0.356 ng/ml denotes the baseline dextran leakage as determined from a mouse that received FITC-labeled dextran but did not suffer from CLP-induced sepsis. A two-tailed t test was used to compare the two groups (n = 10). ∗, P < 0.05.
DISCUSSION
Clinically, the diagnosis of sepsis must satisfy two general requirements (5). The patient must present with symptoms of systemic hyperinflammation, also known as the systemic inflammatory response syndrome. Additionally, the diagnosis of sepsis requires documented evidence of infection. These diagnostic criteria highlight the challenge that the immune system faces during a septic episode. The host must rein in the hyperinflammatory response while simultaneously containing the bacterial infection. Similarly, the design of novel therapeutics must reflect the paradoxical features of sepsis syndrome. Thus, these defining features of clinical sepsis guided our further exploration of the therapeutic mechanism and clinical relevance of C10 therapy in sepsis.
Although the production of various chemokines from both the C-C and CxC superfamilies has been correlated with the pathologic changes associated with septic peritonitis (25, 26), the C-C chemokine C10 has not previously been examined in the context of an acute inflammatory response like sepsis. Unlike many other chemokines, C10 is IL-1β, IL-4, and IL-13 but not LPS inducible (19). Thus, we were interested in determining whether an acute inflammatory response to bacterial pathogens would result in upregulated C10 production. Interestingly, mice that had undergone CLP surgery clearly produced more C10 than other C-C chemokines at each time point examined (6, 24, and 48 h after CLP surgery). In addition, although other C-C chemokines were at or near baseline levels at 48 h after CLP surgery, the C10 levels remained 30-fold higher than the baseline levels. These findings are consistent with those of Wu et al. (28), who recently showed that C10 accumulation was sustained for 10 days after the intraperitoneal introduction of thioglycolate. In this sterile peritonitis model, the pattern of expression of C10 was unique since MIP-1α expression occurred early and was transient (28). Thus, there is a growing consensus that C10 is involved in the later stages of the inflammatory process, although its precise role is still under investigation.
The observed pattern of C10 production following CLP surgery suggested two divergent possibilities. (i) Because CLP-induced C10 production at 48 h after CLP surgery greatly exceeded production at earlier time points, septic mice might have suffered from an acute deficiency in C10. (ii) Because C10 production during CLP-induced sepsis correlated with sepsis-induced death, C10 might have functioned as a deleterious mediator of sepsis. The former possibility was addressed by the immunoneutralization of C10 during CLP-induced sepsis, which resulted in a substantial increase in sepsis-related mortality. In contrast, the administration of recombinant C10 protein to mice immediately after CLP surgery yielded an impressive therapeutic effect, improving survival from 20 to 60%. Thus, these data suggest that C10 production during CLP-induced sepsis constitutes a necessary host response. Subsequent experimentation was aimed at determining the therapeutic mechanism and potential clinical significance of C10 therapy for sepsis.
We analyzed the temporal production of a number of inflammatory mediators by mice that had undergone CLP surgery and treatment with C10 protein, in order to evaluate the effect of C10 therapy on the septic inflammatory state. The administration of C10 significantly increased peritoneal TNF-α and MCP-1 levels at 15 min after CLP surgery. TNF-α has been previously shown to exert a major protective effect during CLP-induced septic peritonitis (9), whereas MCP-1 stimulates local immunity by augmenting macrophage microbicidal and phagocytic function and by facilitating neutrophil and macrophage recruitment (17). To further dissect the ability of C10 to stimulate TNF-α synthesis, peritoneal macrophages were stimulated with C10 concomitant with other proinflammatory cytokines commonly produced during a septic response. In the presence of IL-1β, C10 induced TNF-α production by peritoneal macrophages in a dose-dependent manner. While C10 therapy appeared to rapidly enhance the peritoneal production of proinflammatory mediators such as TNF-α and MCP-1, by 24 h after CLP surgery the predominant cytokine in peritoneal washes from C10-treated mice was IL-13. Although IL-13 is an established Th2-type anti-inflammatory cytokine, our laboratory has also demonstrated a beneficial role for IL-13 in the setting of CLP-induced sepsis (16), and results of the present study suggested that it was a potent stimulus of C10 production by isolated peritoneal macrophages. Taken together, these findings suggested that C10 rapidly induced immune events in the peritoneal cavity that involved the production of cytokines relevant to a beneficial outcome after CLP surgery.
We further examined the therapeutic potential of C10 by assessing whether the controlled enhancement of local inflammation positively affected clinically relevant parameters of bacterial containment. Bacteria play a central yet paradoxical role in the pathogenesis of sepsis, since bacterial components initiate the overwhelming inflammatory response that characterizes sepsis. Yet, an impotent host inflammatory response can result in uncontrolled bacterial growth, leading to bacterial infiltration of the systemic circulation. Clearly, the development of bacteremia is a common prequel to death in many septic cases (7). The administration of recombinant C10 chemokine to mice significantly reduced the incidence of bacteremia at 24 h after CLP surgery. This impressive maintenance of circulatory sterility after C10 therapy probably resulted from some enhancement in bacterial containment within the peritoneum. This hypothesis was supported by in vitro experiments showing that the stimulation of peritoneal macrophages with C10 enhanced their phagocytic capacity. During clinical and experimental sepsis, the bowel wall represents an important barrier to the overwhelming infiltration of intestinal microbes into the peritoneal cavity and subsequently into the systemic circulation (24). However, once pathogenic agents have subverted the intestinal barrier function, the resident peritoneal macrophage represents the first and perhaps most important line of defense. Likewise, the administration of C10 to animals that had undergone CLP surgery significantly attenuated the leakage of FITC-labeled dextran from the bowel into the intestine. While the effect of C10 on the host defense response requires further investigation, these data suggest that C10 enhances bacterial containment in septic mice by enabling the peritoneal macrophage to better control the bacteria that do subvert the intestinal barrier and by attenuating the leakage of intestinal microbes into the peritoneal cavity.
In conclusion, C10 displayed a therapeutically potent yet focused enhancement of inflammatory parameters, which enabled septic mice to contain microbial invaders of the peritoneum while preventing the exacerbation of systemic inflammation. Due to the ever-increasing incidence of antibiotic-resistant bacteria, it is becoming increasingly important to develop methods of safely enhancing the host immune function during a bacterial or septic insult. The unique resolution-promoting properties of the chemokine C10 during murine bacterial sepsis validate an additional examination of the therapeutic potential of human C10 homologues for the treatment of human sepsis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants IP50HL56402, HL35276, HL31963, AI36302, IP50HL60289, and CA66180.
REFERENCES
- 1.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light R B, Poole L, Allred R, Constant J, Pennington J, Porter S. Double blind randomized controlled trial of monoclonal antibody to human tumor necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 2.Abraham E, Wunderink R, Silverman H, Perl T M, Nasraway S, Levy H, Bone R, Wenzel R P, Balk R, Allred R, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- 3.Berger M S, Taub D D, Orlofsky A, Kleyman T R, Coupaye-Gerard B, Eisner D, Cohen S A. The chemokine C10: immunological and functional analysis of the sequence encoded by the novel second exon. Cytokine. 1996;8:439–447. doi: 10.1006/cyto.1996.0060. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell T, Christman J. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 5.Bone R C, Balk R A, Cerra F B, Dellinger R P, Fein A M, Knaus W A, Schein R M H, Sibbald W J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 6.Bossink A W, Paemen L, Jansen P M, Hack C E, Thijs L G, Van Damme J. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood. 1995;86:3841–3847. [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Leptoutre A, Mercier J C, Offenstadt G, Regnier B. Incidence, risk factors and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. JAMA. 1995;274:968–974. [PubMed] [Google Scholar]
- 8.Cohen J, Carlet J. INTERSEPT: An international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Echtenacher B, Mannel D N, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 10.Evanoff H, Burdick M, Moore S, Kunkel S, Strieter R. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1) Immunol Investig. 1992;21:39–45. doi: 10.3109/08820139209069361. [DOI] [PubMed] [Google Scholar]
- 11.Fisher C J, Jr, Agosti J M, Opal S M, Lowry S F, Balk R A, Sadoff J C, Abraham E, Schein R M, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 12.Fisher C J, Jr, Dhainaut J F, Opal S M, Pribble J P, Balk R A, Slotman G J, Iberti T J, Rackow E C, Shapiro M J, Greenman R L, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 13.Hara T, Bacon K B, Cho L C, Yoshimura A, Morikawa Y, Copeland N G, Gilbert D J, Jenkins N A, Schall T J, Miyajima A. Molecular cloning and functional characterization of a novel member of the C-C chemokine family. J Immunol. 1995;155:5352–5358. [PubMed] [Google Scholar]
- 14.Hogaboam C M, Gallinat C S, Taub D D, Strieter R M, Kunkel S L, Lukacs N W. Immunomodulatory role of C10 chemokine in a murine model of allergic bronchopulmonary aspergillosis. J Immunol. 1999;162:6071–6079. [PubMed] [Google Scholar]
- 15.Matsukawa A, Hogaboam C M, Lukacs N W, Lincoln P M, Strieter R M, Kunkel S L. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- 16.Matsukawa A, Hogaboam C M, Lukacs N W, Lincoln P M, Evanoff H L, Strieter R M, Kunkel S L. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J Immunol. 2000;164:2738–2744. doi: 10.4049/jimmunol.164.5.2738. [DOI] [PubMed] [Google Scholar]
- 17.Nakano Y, Kasahara T, Mukaida N, Ko Y C, Nakano M, Matsushima K. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect Immun. 1994;62:377–383. doi: 10.1128/iai.62.2.377-383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Reilly M, Silver G M, Davis J H, Gamelli R L, Hebert J C. Interleukin 1 beta improves survival following cecal ligation and puncture. J Surg Res. 1992;52:518–522. doi: 10.1016/0022-4804(92)90321-p. [DOI] [PubMed] [Google Scholar]
- 19.Orlofsky A, Lin E Y, Prystowsky M B. Selective induction of the beta chemokine C10 by IL-4 in mouse macrophages. J Immunol. 1994;152:5084–5091. [PubMed] [Google Scholar]
- 20.Pardigol A, Forssmann U, Zucht H D, Loetscher P, Schulz-Knappe P, Baggiolini M, Forssmann W G, Magert H J. HCC-2, a human chemokine: gene structure, expression pattern, and biological activity. Proc Natl Acad Sci USA. 1998;95:6308–6313. doi: 10.1073/pnas.95.11.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 22.Sartor R B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995;24:475–507. [PubMed] [Google Scholar]
- 23.Steinhauser M L, Hogaboam C M, Kunkel S L, Lukacs N W, Strieter R M, Standiford T J. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162:392–399. [PubMed] [Google Scholar]
- 24.Steinhauser M L, Hogaboam C M, Lukacs N W, Strieter R M, Kunkel S L. Multiple roles for IL-12 in a model of acute septic peritonitis. J Immunol. 1999;162:5437–5443. [PubMed] [Google Scholar]
- 25.Walley K, Lukacs N, Standiford T, Strieter R, Kunkel S. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847–3851. doi: 10.1128/iai.65.9.3847-3851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Bacon K B, Oldham E R, Schall T J. Molecular cloning and functional characterization of human MIP-1 delta, a new C-C chemokine related to mouse CCF-18 and C10. J Clin Immunol. 1998;18:214–222. doi: 10.1023/a:1020535106684. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Prystowsky M B, Orlofsky A. Sustained high-level production of murine chemokine C10 during chronic inflammation. Cytokine. 1999;11:523–530. doi: 10.1006/cyto.1998.0436. [DOI] [PubMed] [Google Scholar]