Abstract
Introduction
In this study, clinical performance of a hydrophobic acrylic diffractive trifocal intraocular lens (IOL) with double C-loop haptics was evaluated in Japanese cataract eyes.
Methods
Twenty-three patients had bilateral cataract surgery with the implantation of a trifocal IOL with double C-loop haptics. Postoperative examinations at 6 months included assessing uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA) at 5 m, uncorrected intermediate visual acuity (UIVA), distance-corrected intermediate visual acuity (DCIVA) at 80 cm, uncorrected near visual acuity (UNVA) and distance-corrected near visual acuity (DCNVA) at 40 cm. Binocular defocus, contrast sensitivity, spectacle independence, symptoms of photic phenomena and quality of vision (QOV) were also observed.
Results
Twenty-three patients received 46 IOLs binocularly. Manifest refraction spherical equivalent was − 0.227 ± 0.385 D (mean ± standard deviation) at 6 months postoperatively. Binocular UDVA, binocular UIVA and binocular UNVA were − 0.101 ± 0.065, − 0.021 ± 0.079 and 0.022 ± 0.095 logMAR units, respectively. Binocular CDVA, binocular DCIVA and binocular DCNVA were − 0.151 ± 0.044, − 0.042 ± 0.067 and − 0.011 ± 0.080 logMAR, respectively. Binocular CDVA of 0.00 logMAR or better was obtained in the defocus from − 3.0 D until + 0.5 D. Only 8.7% of patients required the use of spectacles postoperatively. There were no symptoms of glare, halo and light disturbance in 78.3%, 56.5% and 69.6% of patients, respectively. QOV scores significantly improved postoperatively (P < 0.0001).
Conclusion
The hydrophobic acrylic trifocal IOL with double C-loop haptics provides good visual performance at all distances and produces high spectacle independence rate and patient satisfaction.
Trial Registration Number
NCT04699266 (Clinicaltrials.gov).
Keywords: Cataract, Double C-loop, Hydrophobic acrylic IOL, Multifocal IOL, POD F GF, Trifocal IOL
Key Summary Points
| Why carry out this study? |
| Clinical results of trifocal IOLs with double C-loop haptics are mostly reported from Europe using hydrophilic materials, and several clinical studies have reported on the POD F GF, a hydrophobic acrylic trifocal IOL. |
| There have been no clinical results reported so far for POD F GF in Asian eyes. |
| The purpose of the study was to evaluate the clinical performance of the POD F GF in the Japanese population. |
| What was learned from the study? |
| POD F GF provided excellent visual acuities at all distances in Japanese eyes and the postoperative visual acuity corresponded with previous results in monocular and binocular visions. |
| Visual acuity of 0.00 logMAR or better was obtained in the 3.5 D depth of focus range and an improvement in QOV was observed. |
Introduction
Currently, several types of presbyopia-correcting intraocular lenses (IOLs) are available. In bifocal IOLs, the spectacle-independent vision is provided at two distances (near and far), with the outcome of the intermediate vision being inherently inferior. This results in the frequent requirement of spectacles in daily routines at intermediate distance, such as during computer work. Trifocal IOLs were developed to overcome the limitations of bifocal IOLs at intermediate distances. Trifocal IOLs are designed to improve the intermediate vision by utilizing three or more focal points (near, intermediate and far vision).
A unique trifocal IOL that combines two diffractive patterns has been developed: one with + 3.5 D addition for near vision and the other with + 1.75 D for intermediate vision [1]. This trifocal IOL, named POD F GF (PhysIOL s.a., Liège, Belgium), is made of hydrophobic acrylic material. The optic is supported by double C-loop haptics.
The double C-loop design is one of the innovative design features of this IOL. Currently, most of the widely used IOL haptics are single C-loop design. Although the double C-loop design is expected to improve stability in the intraocular capsule, double C-loop IOLs are rarely used in Japan, and the only clinical reports on the use of double C-loop IOLs in Japan are on lenses made of hydrophilic materials [2].
Clinical outcomes in the use of POD F GF have been studied and favorable outcomes obtained at far, intermediate and near distances for presbyopia correction [3, 4]. However, the numbers of subjects in these studies were limited, and the studies were conducted only in EU countries.
To our knowledge, the clinical performance of POD F GF has not been evaluated in Asian eyes thus far. In this study, the clinical outcomes were observed in Japanese subjects implanted with POD F GF trifocal IOLs.
Methods
This multi-center and single-arm clinical trial was approved by the investigational review boards at all participating institutes (Zengyo Suzuki Eye Clinic, Chukyo Eye Clinic, Tsukazaki Hospital and Miyata Eye Hospital), and a written informed consent was obtained from each patient prior to the study. The study adhered to the tenets of the Declaration of Helsinki and good clinical practice for a medical device study in Japan. This study was registered at ClinicalTrials.gov (ID: NCT04699266, January 7, 2021).
Participants
Twenty-three patients were enrolled. The inclusion criteria for patient selection were: 20 years of age or older, bilateral cataract surgery following the implantation of multifocal IOL, a potential postoperative corrected distance visual acuity (CDVA) of 0.3 logMAR or better and preoperative corneal astigmatism of < 1.0 D in keratometry. The exclusion criteria included patients with an ocular pathology influencing postoperative vision except for cataract, history of previous ocular surgery, corneal abnormalities or intraoperative complications.
Sample Size
This study was designed to examine the non-inferiority in CDVA and superiority in distance-corrected near (at 40 cm, DCNVA) and intermediate (at 80 cm, DCIVA) visual acuities at 6 months postoperatively compared with monofocal IOLs. Based on the previous studies of SN60AT [5] and SN60WF [6] IOLs (Alcon, Fort Worth, USA), mean postoperative DCVA, DCNVA and DCIVA of − 0.039, 0.529 and 0.23 in logMAR value, respectively, were considered as historical control. Sixteen patients or more were required with detection power > 90%, significance level of 2.5% (one sided test) for non-inferiority with a margin of 0.10 logMAR and the superiority in the use of trifocal IOLs. The discontinuation of 30% of individual subjects was anticipated considering the influence of COVID-19 during the study period. Thus, the sample size was planned to be 22 patients or more.
IOL and Surgery
Implanted IOLs were single-piece trifocal IOLs: POD F GF. The material of the IOL was a cross-linked, acrylate/methacrylate copolymer with blue light absorber (refractive index of 1.53). The diameter of the biconvex aspheric optic was 6.0 mm, and the overall length was 11.4 mm. On the anterior optic surface, there was a diffractive multifocal structure with the added powers of + 1.75 D and + 3.50 D for proving the intermediate and near vision. The added powers corresponded to foci around 83 and 42 cm. The unique haptics consisted of four C-loops for better centration [7].
Four surgeons from four surgical sites performed the surgeries. Powers of IOLs were determined for emmetropia using the SRK/T or Barrett Universal II formulas. The IOL was implanted in the capsule after cataract removal using phacoemulsification and aspiration procedure and with the use of a specific injector (Accuject, Medicel AG, Switzerland). The first eye with advanced cataract underwent surgery, and the surgery of the fellow eye was performed within 30 days after the postoperative examination of the first eye. All subjects received the standard regimens of preoperative, operative and postoperative medications.
Examination
Visual acuity was examined preoperatively, 1 month and 6 months postoperatively. Uncorrected distance visual acuity (UDVA) and CDVA were measured with a Landolt ring chart with illumination (Non-Glare Vision Chart, Takagi Seiko Co., Ltd., Japan/CV-500, Tomey, Japan) at a distance of 5 m under photopic condition. Uncorrected intermediate visual acuity (UIVA) and DCIVA was measured using a non-illuminated Landolt ring chart (TMI-V7080, Precision Vision, Woodstock, IL, USA) designed for the distance of 80 cm. Uncorrected near visual acuity (UNVA) and DCNVA were measured at 40 cm using a non-illuminated Landolt ring chart (TMI-V5040 chart, Precision Vision, USA). Intermediate and near visual acuities were measured under photopic conditions. Manifest refraction spherical equivalent (MRSE) was also recorded during monocular DCVA examinations. All visual acuities at 5 m, 80 cm and 40 cm were also examined binocularly.
At 6 months postoperatively, a binocular defocus curve was obtained. Under a distance-corrected condition, binocular visual acuities under defocus from − 5.0 D to 0.0 D in steps of 0.5 D were measured. Then, visual acuity was measured in the same manner by adding defocus from + 2.0 D to 0.0 D in steps of 0.5 D.
Binocular contrast sensitivity was measured using CSV-1000 (Vector Vision, Houston, TX, USA) under photopic (85 cd/m2) illuminations at spatial frequencies of 3, 6, 12 and 18 cycles per degree (cpd).
Spectacle usage, presence of photic phenomena and quality of vision (QOV) were also assessed. To assess the subjective symptoms of glare, halo and light disturbance at night, the intensity or severity was graded as none, mild, moderate (permissible in daily life) and severe. QOV was evaluated based on measurable patient-reported outcomes using the Visual Function Questionnaire 11-item Japanese version (VFQ-J11), which is validated using the item-response theory [8]. The response for each item was presented as an index between 0 and 100 (0 representing the worst possible score and 100 the best). The mean of all items was obtained as the score and compared with preoperative scores [9]. Sub-scores for near and distance activities using the VFQ-J11 were also obtained and compared in the same manner.
Statistical Analysis
Visual acuities were measured in decimal visual acuity and converted to logMAR for analysis. Primary endpoints were the non-inferiority in CDVA and the superiority in DCNVA and DCIVA of POD F GF compared to the monofocal IOLs [5, 6] and were examined by using a mixed-effects models for repeated measures (MMRM) since visual acuity at 6 months postoperatively was measured in both eyes of patients; thus, the correlation between both eyes should be considered. With resultant least-squared mean and 95% confidence intervals (CI), the non-inferiority in the CDVA was determined if the upper limit of 95% CI was smaller than the mean visual acuity results in the use of monofocal IOLs [5, 6] with a margin of 0.10 logMAR. Similarly, the superiority in DCNVA and DCIVA was determined in similar manner except for the exclusion of 0.10 logMAR margin. P < 0.025 for one sided test was considered as statistically significant.
Visual acuities and refractive results were presented in the standard plots for primary outcomes in studies with presbyopia-correcting IOLs [10]. Mean MRSE was adjusted for infinity by adding − 0.20 D for 5 m examination. Postoperative MRSE was examined with the Shapiro-Wilk test for normality. Visual acuities were not normally distributed; the median was also calculated. For QOV, the scores of all items and sub-scores were compared preoperatively and at 6 months postoperatively using a paired t test. P < 0.05 was considered significant.
Results
In this clinical trial, 23 patients (46 eyes) were enrolled and followed up for 6 months. The study population consisted of 7 males and 16 females. The demographic data of subjects are listed in Table 1. No intraoperative complications occurred.
Table 1.
Demographic data of subjects
| Range | Mean (SD) | |
|---|---|---|
| Age, year | 56, 82 | 71.3 (5.9) |
| Axial length, mm | 22.15, 26.68 | 23.668 (1.049) |
| Corneal astigmatism, D | 0.12, 0.89 | 0.537 (0.215) |
| Preoperative pupil diameter, mm | 2.5, 4.9 | 3.58 (0.77) |
| Power of implanted IOL, D | 10.0, 26.0 | 20.54 (3.68) |
| Preoperative CDVA, logMAR | − 0.18, 0.40 | 0.071 (0.142) |
| Preoperative MRSE, D | − 8.83, 3.05 | 0.148 (2.577) |
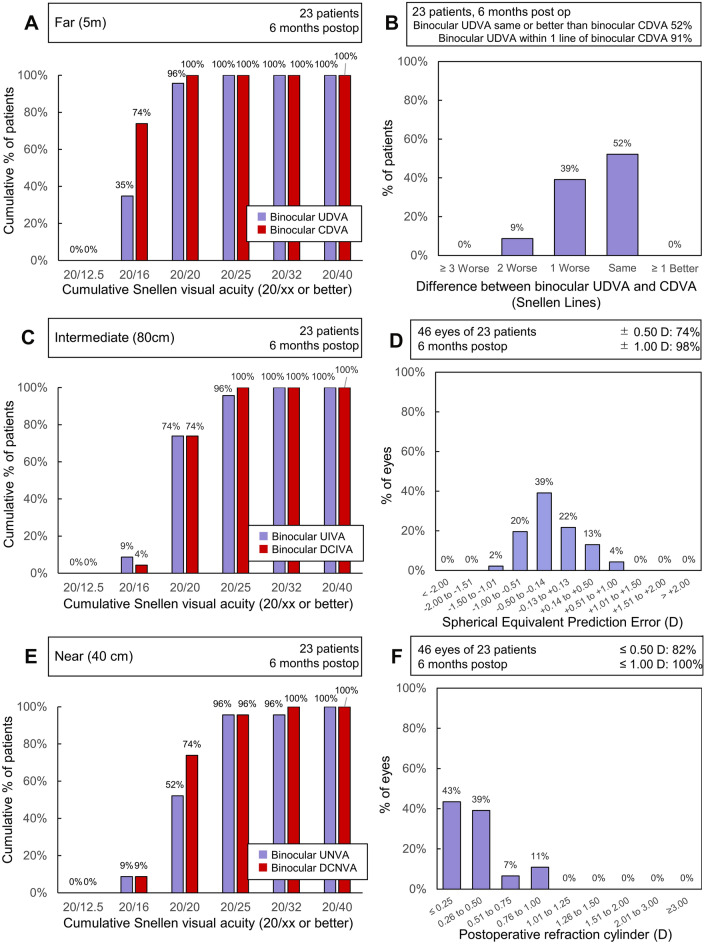
Table 2 showed postoperative MRSE and visual acuities. Cumulative binocular visual acuity (20× or better) at 6 months postoperatively at far, intermediate and near distances is plotted in Fig. 1. Under distance correction, all patients obtained binocular visual acuities of 0.00 logMAR (20/20 in Snellen notation) or better for far distance, 0.01 logMAR (20/25 in Snellen notation) or better for intermediate and 0.02 logMAR (20/32 in Snellen notation) or better for near distances. Difference between postoperative UDVA and CDVA, prediction error and postoperative refractive cylinder distribution were also shown in Fig. 1.
Table 2.
Manifest refraction spherical equivalent (MRSE) and visual acuities at distance, intermediate (80 cm) and near (40 cm) at 1 and 6 months after surgery
| 1 month, monocular (N = 46) | 6 months monocular (N = 46) | 6 months, binocular (N = 23) | |
|---|---|---|---|
| MRSE, D |
− 0.243 (0.402) [− 1.20, 0.80] |
− 0.227 (0.385) [− 1.20, 0.80] |
NA |
| UDVA, logMAR |
0.001 (0.110) [− 0.18, 0.30] 0.000 |
− 0.038 (0.089) [− 0.18, 0.22] − 0.079 |
− 0.101 (0.065) [− 0.18, 0.05] − 0.079 |
| CDVA, logMAR |
− 0.087 (0.064) [− 0.18, 0.05] − 0.079 |
0.112 (0.052) [− 0.18, 0.00] − 0.079 |
− 0.151 (0.044) [− 0.18, − 0.08] − 0.176 |
| UIVA, logMAR |
0.071 (0.119) [− 0.08, 0.40] 0.046 |
0.073 (0.119) [− 0.08, 0.70] 0.046 |
− 0.021 (0.079) [− 0.18, 0.15] 0.000 |
| DCIVA, logMAR |
0.046 (0.105) [− 0.18, 0.30] 0.046 |
0.027 (0.084) [− 0.18, 0.22] 0.023 |
− 0.042 (0.067) [− 0.18, 0.10] − 0.079 |
| UNVA, logMAR |
0.116 (0.137) [− 0.08, 0.52] 0.097 |
0.085 (0.096) [− 0.08, 0.30] 0.046 |
0.022 (0.095) [− 0.18, 0.22] 0.000 |
| DCNVA, logMAR |
0.082 (0.118) [− 0.08, 0.40] 0.046 |
0.060 (0.095) [− 0.08, 0.40] 0.046 |
− 0.011 (0.080) [− 0.18, 0.15] 0.000 |
(Mean (SD) [Range] (Median)
Fig. 1.
Outcomes in studies with trifocal IOLs. Cumulative percentage of eyes achieving binocular CDVA and UDVA at A far, C intermediate and E near distances. B Difference on lines in far distance between postoperative UDVA and CDVA. D Spherical equivalent prediction error distribution; F postoperative refractive cylinder distribution
The non-inferiority in the CDVA and superiority in the DCNVA and DCIVA were examined. Table 3 shows the MMRM results of monocular distance-corrected visual acuities in logMAR. The upper limit of the 95% CI for the CDVA was lower than the margin (the mean value of control plus 0.10 logMAR). Thus, the CDVA was non-inferior to the use of monofocal IOLs (P < 0.025). For DCIVA and DCNVA, the upper limits of the 95% CIs wase better than the mean values of control, indicating superiority in intermediate and near visual acuity (P < 0.025).
Table 3.
Primary end points of distance-corrected monocular visual acuities at 6 months analyzed with a MMRM compared with the mean visual acuities of monofocal IOLs in the literature
| Visual acuity, (logMAR) |
POD F GF | Monofocal | Validated | ||
|---|---|---|---|---|---|
| LSM | 95% LCL | 95% UCL | Mean | ||
| CDVA | − 0.112 | − 0.136 | − 0.087 | − 0.039 [5] | Non-inferiority |
| DCIVA | 0.027 | − 0.013 | 0.068 | 0.23 [6] | Superiority |
| DCNVA | 0.060 | 0.022 | 0.099 | 0.529 [5] | Superiority |
LSM least squared mean, LCL lower confidence limit, UCL upper confidence limit
Figure 2 shows a binocular defocus curve. Under distance-correction, the binocular visual acuity of 0.00 logMAR or better was obtained in the defocus from − 3.0 D to + 0.5 D, indicating that the depth of focus was 3.5 D.
Fig. 2.
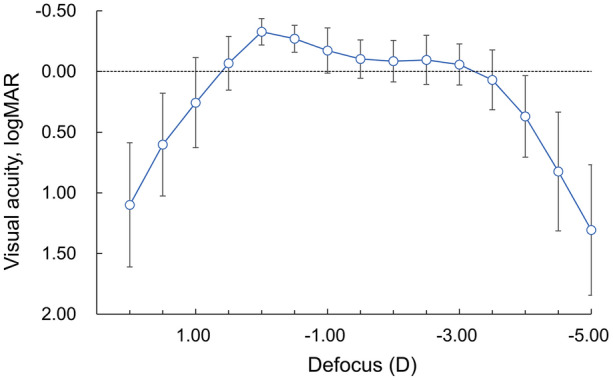
Binocular defocus curve. Dotted line depicts visual acuity of 0.00 logMAR
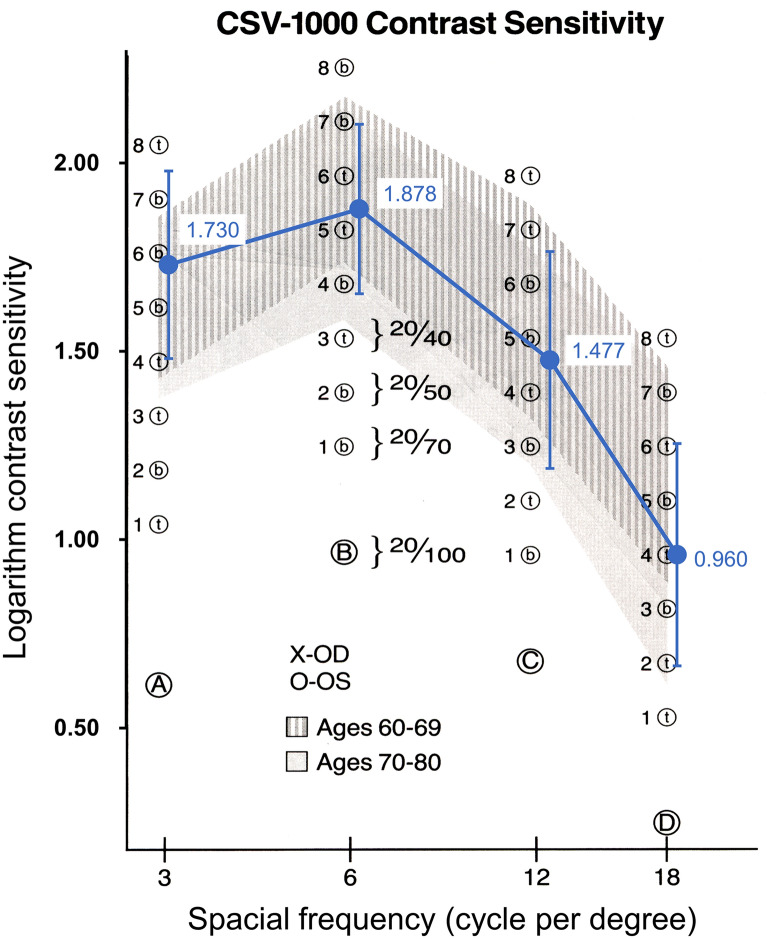
Binocular contrast sensitivity is shown in Fig. 3. Considering the mean age of patients was 71.3 years, contrast sensitivities at 3, 6, 12 and 18 cpd were within the level of normal.
Fig. 3.
Binocular contrast sensitivity
Spectacle independence was achieved in 21 of 23 patients (91.3%), while the remaining 2 patients used reading glasses only. Regarding photic symptoms, 18 patients (78.3%) had no symptoms of glare, while 4 patients and 1 patient claimed mild and moderate glare, respectively. Thirteen patients (56.5%) reported no halo, nine reported mild halo and one reported moderate halo. Symptoms of light disturbance at night was not found in 16 patients (69.6%); mild and moderate symptoms were reported in 6 patients and 1 patient, respectively. No severe symptoms were reported, and none of the patients required further intervention related to the observed photic symptoms.
Table 4 shows the VFQ-J11 scores preoperatively and 6 months postoperatively. The total score, sub-scores on distance and near vision, and the postoperative scores were significantly improved (P < 0.0001, paired t test).
Table 4.
QOV scores preoperatively and 6 months postoperatively
| Preoperative | 6 months postoperative | |
|---|---|---|
| Total score | 78.3 (16.2) [32.7, 95.9] | 97.1 (3.2) [84.5, 100.0] |
| Sub-scale on distance vision | 75.2 (24.6) [0.0, 100.0] | 97.1 (6.5) [75.0, 100.0] |
| Sub-scale on near vision | 72.8 (22.5) [8.33, 100.0] | 95.7 (7.9) [75.0, 100.0] |
Mean (SD) [range]
Discussion
Postoperative distance-corrected visual acuities at 6 months after the implantations of POD F GF IOLs indicated non-inferiority CDVA and superiority in the DCNVA and DCIVA compared with monofocal IOLs.
Table 5 compares the current results with the previous evaluations of the same types of IOLs in EU countries. Postoperative DCVA, DCIVA and DCNVA coincided well with the current results in monocular and binocular vision acuity [3, 4].
Table 5.
Comparison of postoperative distance-corrected visual acuities
| Monocular visual acuities | ||
|---|---|---|
| Study | Nagy [3] (25 eyes of 25 patients) | Current |
| CDVA, logMAR | − 0.04 (0.08) at 4 m | − 0.112 (0.052) |
| DCIVA, logMAR | 0.04 (0.09) at 70 cm | 0.027 (0.084) |
| DCNVA, logMAR | 0.04 (0.07) at 35 cm | 0.060 (0.095) |
| Binocular visual acuities | ||
| Study | Poyales [4] (52 eyes of 26 patients) | Current |
|---|---|---|
| CDVA, logMAR | − 0.04 (0.04) at 4 m | − 0.151 (0.044) |
| DCIVA, logMAR | 0.09 (0.10) at 80 cm | − 0.042 (0.067) |
| DCNVA, logMAR | 0.10 (0.09) at 40 cm | − 0.011 (0.080) |
Mean (SD)
Table 6 shows binocular distance-corrected visual acuity of trifocal IOLs with the diffractive optic technology as reported in Asian eyes in the literature [11, 12]. Among the three types of trifocal IOLs shown in Table 6, preferable distance-corrected visual acuities were obtained in the range of near-to-far distances. Tri-focality of the current IOLs was also confirmed in the defocus curves. Bissen-Miyajima et al. recently reported the visual performance of diffractive trifocal IOL TFNT00 (Alcon, Fort Worth, TX, USA) in Japanese eyes, showing that the logMAR value of 0.00 was achieved at an extended range of distance, with approximately + 0.5 to − 3.0 D of defocus [11]. The depth of focus with POD F GF in the current study was similar to that of TFNT00 in the reported study.
Table 6.
Binocular distance-corrected visual acuities in eye with other multifocal IOLs
| Study | Bissen–Miyajima [11] (67 patients) | Liu [12] (25 patients) | Current |
|---|---|---|---|
| IOL | TFNT00 | AT LISA tri839MP | POD F GF |
| CDVA, logMAR | − 0.197 (0.076) | − 0.04 (0.07) | − 0.151 (0.044) |
| DCIVA, logMAR | − 0.094 (0.130) | 0.06 (0.08) | − 0.042 (0.067) |
| DCNVA, logMAR | − 0.073 (0.111) | 0.07 (0.08) | − 0.011 (0.080) |
Mean (SD)
Decrease in contrast sensitivity is the well-known side effect of multifocal IOLs [13, 14]. The splitting of light among multiple focal points leads to a fraction of the light entering the lens being used to produce an image at a given distance, resulting in reduced contrast on the retinal image [15]. A previous meta-analysis reported [16] contrast sensitivity with trifocal IOLs improved in contrast sensitivity compared with bifocal IOLs. Under photopic conditions, trifocal IOLs had better performance at 3, 6 and 12 cpd than bifocal IOLs. In the current study, contrast sensitivity at 3, 6, 12 and 18 cpd was within the normal range. There is a report that indicates the trifocal IOL reduces the loss of light compared to bifocal IOLs [1]. However, under mesopic conditions, Jonker et al. reported contrast sensitivity was significantly better for 6 cpd in the bifocal group compared to the trifocal group [17]. More studies will be needed to evaluate contrast sensitivity performance of trifocal IOLs under mesopic conditions.
There were no symptoms of glare, halo and light disturbance at night in 78.3%, 56.5% and 69.6% of patients, respectively. In the use of TFNT00 IOL, glare and halo were reported in 67.2% and 71.6% of Japanese patients [11]. It was speculated that convolution of two diffractive optics together with apodization would be effective to reduce light energy for near vision.
Patient’s QOV represented by the VFQ-J11 scores improved after trifocal IOL implantation in the study. Normally, the QOV improves with cataract surgery owing to removal of the opacified crystalline lens. Another study with monofocal IOLs reported the mean VFQ-J11 score before and after surgery to be 65.12 and 87.28, respectively, representing a change of 22.16 [9]. In the current study, while the change of VFQ-J11 score was 18.8, the postoperative score was 10 points higher compared to the results with monofocal IOLs. These findings demonstrate cataract surgery with this particular trifocal IOL increases patient’s QOV. In addition, the small MRSE resulted in most patients obtaining uncorrected visual acuities close to 20/20 under distance correction (Fig. 1) and high spectacle independence (91.3%), which would improve postoperative QOV.
There were some limitations to this study as it was designed as a single-arm trial without a direct comparison to a monofocal IOL. Comparison with monofocal IOL implantation was difficult in the current clinical environment, so we performed a comparison with historical results. Next, the follow-up period was 6 months. Multifocal IOLs can be sensitive to mild degradation of ocular optics, such as posterior capsule opacification (PCO). Owing to the unique design with double C-loop haptics, the development of PCO is a factor of interest. A previous observation of monofocal IOL: POD AY GF (PhysIOL s.a., Liège, Belgium) employing a similar platform indicated no PCO in 66% of eyes at 3 years postoperatively [18]. Long-term evaluation would be recommended to confirm these results in the IOL observed in this study.
Conclusions
Twenty-three Japanese patients were implanted bilaterally with POD F GF IOL, providing good visual acuities at all distances with low levels of photic phenomena observed and high spectacle independence rate and patient satisfaction.
Acknowledgements
We thank the participants of the study.
Funding
This clinical trial and the journal’s Rapid Service Fee were supported by Beaver-Visitec International Japan KK. All authors received research support from Beaver-Visitec International Japan KK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
Naoyuki Maeda was involved in the design of the study and analysis of data. Yosai Mori, Kazunori Miyata, Hisaharu Suzuki, Santaro Noguchi and Kazuo Ichikawa were involved in conduct, data collection and management of the study. All authors were involved in preparation, review and final approval of the manuscript.
Disclosures
Yosai Mori reports speaker honorarium from Alcon, Johnson & Johnson, HOYA and Santen Pharmaceutical. Kazunori Miyata reports financial support from Alcon and Beaver-Visitec International, Johnson & Johnson and HOYA, speaker honorarium from Alcon, Johnson & Johnson, HOYA and Santen Pharmaceutical. Hisaharu Suzuki reports research grants from Alcon and consulting fee from NIDEK. Santaro Noguchi reports speaker honorarium from Alcon, Johnson & Johnson, HOYA and HANITA. Kazuo Ichikawa reports financial support and consulting fee from Alcon, personal financial interest from Santen Pharmaceutical and STAAR Surgical, speaker honorarium from Alcon and Santen Pharmaceutical. Naoyuki Maeda reports consulting fee from Beaver-Visitec International, speaker honorarium from Alcon, HOYA, Johnson & Johnson and Santen Pharmaceutical.
Compliance with Ethics Guidelines
This clinical trial was approved by the investigational review boards at all participating institutes prior to the initiation of the trial (Zengyo Suzuki Eye Clinic, Chukyo Eye Clinic, Tsukazaki Hospital and Miyata Eye Hospital). The study adhered to tenets of the Declaration of Helsinki and Good Clinical Practice for a medical device study in Japan. This study was registered at ClinicalTrials.gov (ID: NCT04699266, January 7, 2021).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Gatinel D, Pagnoulle C, Houbrechts Y, Gobin L. Design and qualification of a diffractive trifocal optical profile for intraocular lenses. J Cataract Refract Surg. 2011;37(11):2060–2067. doi: 10.1016/j.jcrs.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura K, Bissen-Miyajima H, Hirasawa M, Ota Y, Oki S, Tanaka M, Minami K. Clinical results of trifocal intraocular lens (Pod F, Pod FT) J Jpn Ophthalmol Soc. 2018;122:281–286. [Google Scholar]
- 3.Nagy ZZ, Popper-Sachetti A, Kiss HJ. Comparison of visual and refractive outcomes between hydrophilic and hydrophobic trifocal intraocular lenses sharing the same optical design. J Cataract Refract Surg. 2019;45(5):553–561. doi: 10.1016/j.jcrs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Poyales F, Pérez R, López-Brea I, Zhou Y, Rico L, Garzón N. Comparison of visual performance and patient satisfaction outcomes with two trifocal IOLs with similar optical design but different materials. Clin Ophthalmol. 2020;14:3237–3247. doi: 10.2147/OPTH.S273641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modi S, Lehmann R, Maxwell A, Solomon K, Cionni R, Thompson V, Horn J, Caplan M, Fisher B, Hu JG, Yeu E. Visual and patient-reported outcomes of a diffractive trifocal intraocular lens compared with those of a monofocal intraocular lens. Ophthalmology. 2021;128(2):197–207. doi: 10.1016/j.ophtha.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi K, Manabe S, Hayashi H. Visual acuity from far to near and contrast sensitivity in eyes with a diffractive multifocal intraocular lens with a low addition power. J Cataract Refract Surg. 2009;35:2070–2076. doi: 10.1016/j.jcrs.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Bozukova D, Werner L, Mamalis N, et al. Double-C loop platform in combination with hydrophobic and hydrophilic acrylic intraocular lens materials. J Cataract Refract Surg. 2015;41:1490–1502. doi: 10.1016/j.jcrs.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Fukuhara S, Wakita T, Yamada M, Hiratsuka Y, Green J, Oki K. Development of a short version of the visual function questionnaire using item-response theory. PLoS One. 2013;8(9):e73084. doi: 10.1371/journal.pone.0073084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiratsuka Y, Yamada M, Akune Y, Murakami A, Okada AA, Yamashita H, Ohashi Y, Yamagishi N, Tamura H, Fukuhara S, Takura T. Assessment of vision-related quality of life among patients with cataracts and the outcomes of cataract surgery using a newly developed visual function questionnaire: the VFQ-J11. Jpn J Ophthalmol. 2014;58(5):415–422. doi: 10.1007/s10384-014-0335-3. [DOI] [PubMed] [Google Scholar]
- 10.Fernández J, Ribeiro FJ, Rodríguez-Vallejo M, Dupps WJ, Jr, Werner L, Srinivasan S, Kohnen T. Standard for collecting and reporting outcomes of IOL-based refractive surgery: update for enhanced monofocal, EDOF, and multifocal IOLs. J Cataract Refract Surg. 2022;48(11):1235–1241. doi: 10.1097/j.jcrs.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 11.Bissen-Miyajima H, Ota Y, Hayashi K, Igarashi C, Sasaki N. Results of a clinical evaluation of a trifocal intraocular lens in Japan. Jpn J Ophthalmol. 2020;64(2):140–149. doi: 10.1007/s10384-019-00712-4. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Xie L, Huang Y. Comparison of the visual performance after implantation of bifocal and trifocal intraocular lenses having an identical platform. J Refract Surg. 2018;34(4):273–280. doi: 10.3928/1081597X-20180214-01. [DOI] [PubMed] [Google Scholar]
- 13.Cillino S, Casuccio A, Di Pace F, Morreale R, Pillitteri F, Cillino G, Lodato G. One-year outcomes with new-generation multifocal intraocular lenses. Ophthalmology. 2008;115:1508–1516. doi: 10.1016/j.ophtha.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Zeng M, Liu Y, Liu X, Yuan Z, Luo L, Xia Y, Zeng Y. Aberration and contrast sensitivity comparison of aspherical and monofocal and multifocal intraocular lens eyes. Clin Exp Ophthalmol. 2007;35:355–360. doi: 10.1111/j.1442-9071.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 15.Cao K, Friedman DS, Jin S, Yusufu M, Zhang J, Wang J, Hou S, Zhu G, Wang B, Xiong Y, Li J, Li X, He H, Chai L, Wan XH. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv Ophthalmol. 2019;64:647–658. doi: 10.1016/j.survophthal.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Cao D, Chen X, Wu S, Wang X, Wu Q. Comparison of clinical performance between trifocal and bifocal intraocular lenses: a meta-analysis. PLoS ONE. 2017;12:e0186522. doi: 10.1371/journal.pone.0186522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonker SMR, Bauer NJC, Makhotkina NY, Berendschot TTJM, Van Den Biggelaar FJHM, Nuijts RMMA. Comparison of a trifocal intraocular lens with a +3.0 D bifocal IOL: results of a prospective randomized clinical trial presented at the XXXI Congress of the European Society of Cataract and Refractive Surgeons, Amsterdam, the Netherlands, October 2013. J Cataract Refract Surg. 2015;41:1631–1640. doi: 10.1016/j.jcrs.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Chassain C, Chamard C. Posterior capsule opacification, glistenings and visual outcomes: 3 years after implantation of a new hydrophobic IOL. J Fr Ophtalmol. 2018;41:513–520. doi: 10.1016/j.jfo.2017.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.