Abstract
Background
Most biopharmaceuticals are developed in liquid dosage forms that are less stable than solid forms. To ensure the stability of biopharmaceuticals, it is critical to use an effective drying technique in the presence of an appropriate stabilizing excipient. Various drying techniques are available for this purpose, such as freeze drying or lyophilization, spray drying, spray freeze-drying, supercritical fluid drying, particle replication in nonwetting templates, and fluidized bed drying.
Area covered
In this review, we discuss drying technologies and their applications in the production of stable solid-state biopharmaceuticals, providing examples of commercially available products or clinical trial formulations. Alongside this, we also review how different analytical methods may be utilized in the evaluation of aerosol performance and powder characteristics of dried protein powders. Finally, we assess the protein integrity in terms of conformational and physicochemical stability and biological activity.
Expert opinion
With the aim of treating either infectious respiratory diseases or systemic disorders, inhaled biopharmaceuticals reduce both therapeutic dose and cost of therapy. Drying methods in the presence of optimized protein/stabilizer combinations, produce solid dosage forms of proteins with greater stability. A suitable drying method was chosen, and the process parameters were optimized based on the route of protein administration. With the ongoing trend of addressing deficiencies in biopharmaceutical production, developing new methods to replace conventional drying methods, and investigating novel excipients for more efficient stabilizing effects, these products have the potential to dominate the pharmaceutical industry in the future.
Keywords: Stability, Biopharmaceuticals, Characterization, Solid-dosage form, Drying
Introduction
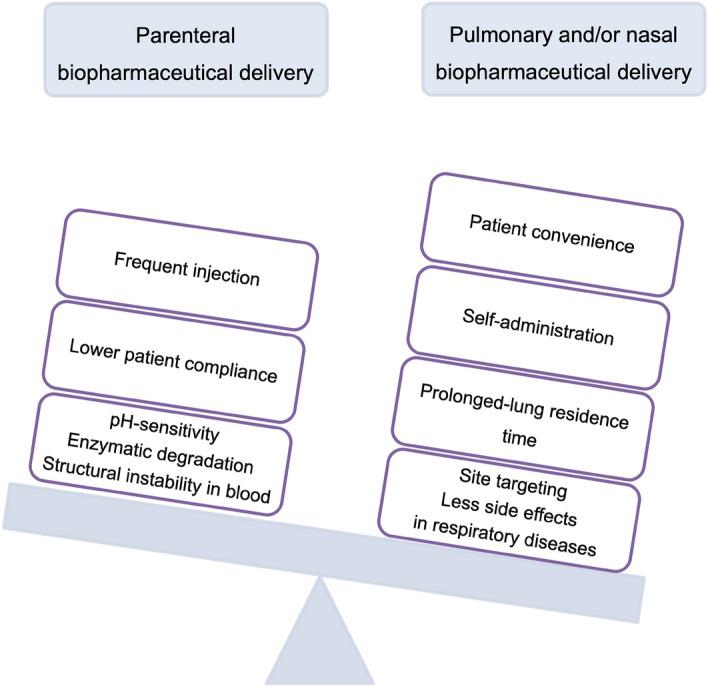
In recent decades, biopharmaceuticals, including peptides, proteins, vaccines, genes, hormones, and enzymes (Sharma et al. 2021; O’Sullivan et al. 2022a, b) have experienced rapid growth in their production (Chen et al. 2021a, b). Biopharmaceutical drugs have accounted for 35% of all FDA-approved products over the past 16 years (Vass et al. 2019), and it is projected that by 2025, this market will be worth $395 billion (Guo et al. 2020). Accordingly, overcoming the challenges in formulating these biopharmaceuticals is of immense importance. The large size, complex structure, and susceptibility of therapeutic proteins to environmental stress result in several physicochemical degradations, such as deamidation, oxidation, hydrolysis, racemization, isomerization, β-elimination, disulfide exchange, aggregation, precipitation, and denaturation (Filipe et al. 2013). Given the low oral bioavailability of these formulations and their limited transport through the epithelium, parenteral administration is an intriguing prospect (Anselmo et al. 2019). The most common route of administration is parenteral administration, which frequently uses a liquid dosage form for delivery (Zhang et al. 2021a, b); however, more stable products can be obtained in solid dosage forms (Fig. 1) (Chen et al. 2021a, b). Thus, to retain the physicochemical integrity and intrinsic activity of the product (Vass et al. 2019; Mutukuri et al. 2021), methods of solid-state formulation must be developed (Mutukuri et al. 2021).
Fig. 1.
The schematic figure compares biopharmaceuticals delivery via different routes of administration and shows how pulmonary and/or nasal delivery outweighs parenteral (Kunde et al. 2022)
In liquid form, biomolecule instability can lead to permanent or reversible changes in drug during the storage, transportation, and administration (Vass et al. 2019; O’Sullivan et al. 2022a, b). Various methods have been applied to stabilize biopharmaceuticals, such as glycosylation (O’Sullivan et al. 2022a, b), lipidation (Egli et al. 2021), and the incorporation of new ingredients. However, many applied stabilization technologies are expensive and lead to unwanted effects on the structure and specificity of some biomolecules. To improve stability, drying techniques (O’Sullivan et al. 2022a, b) such as freeze drying (FD), spray drying (SD), spray freeze drying (SFD), supercritical fluid drying (SCFD), and critical fluid SD, can be applied to eliminate water and provide the product in a more stable state in powder form (Vass et al. 2019) with a longer shelf life (Keil et al. 2019). Drying methods result in more convenient handling of the final products and minimize the expense of supplying a cold chain (2 to 8 °C) or sometimes freezing (− 20 to − 80 °C) during transportation and storage (O’Sullivan et al. 2022a, b). In our previous study, we reported different drying technologies and stabilizing excipients available to stabilize biopharmaceuticals (Emami et al. 2018a, b). In this review, we discuss the recent drying techniques used to produce dry powder formulations of biopharmaceuticals, as well as characterization tests for therapeutic proteins. Alongside this, we also review that dried powder inhalers have mainly focused on local drug delivery for treating respiratory diseases. Aside from patients suffering from pulmonary diseases benefiting from local drug delivery through inhalation, those patients with diabetes, osteoporosis, and hormone deficiency (Keyhan shokouh et al. 2021) could enjoy the simplicity and non-invasiveness of such administration way (Emami et al. 2018a, b; Emami et al. 2019; Dhahir et al. 2021).
Drying techniques
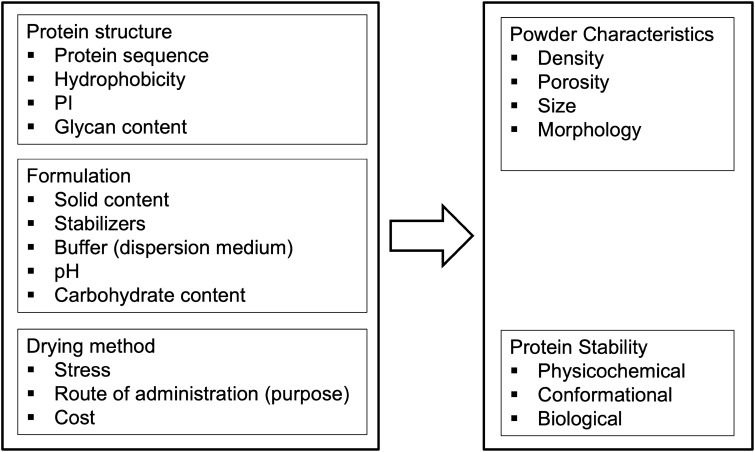
Choosing a suitable drying technique from various methods depends on the native features of the given biomolecules, desired route of their administration, and the expenses of the drying procedure (Vass et al. 2019) (Fig. 2).
Fig. 2.
Summary of the parameters influencing the characteristics of dried proteins
Freeze drying (FD)
FD, lyophilization, is the most common applied dehydration technology consisting of three main steps (1) freezing, (2) primary drying, and (3) secondary drying. Included variables in the FD process from the rate of decreasing temperature in the freezing stage and increasing temperature to a certain point in sublimation to the rate at which temperature reduces and the frequency of temperature cycles can affect the final product characteristics such as crystallinity, crystal size, pore size, and even structural stability (O’Sullivan et al. 2022a, b). Despite its shortfalls such as high energy and time consumption (from days to weeks to complete) (Vass et al. 2019), induced stresses in freezing and thawing stages, and lack of control over the size of the resultant particles (O’Sullivan et al. 2022a, b), FD is widely applied to produce more than half of the present biopharmaceuticals in today's market (Vass et al. 2019). Freeze dried products include monoclonal antibodies (mAbs) for either inhalation or injection purpose (Hickey et al. 2022), high protein concentration products for targeted delivery systems (Butreddy et al. 2021), vaccines [DNA (Chen et al. 2021a, b), mRNA (Cohen 2022), siRNA- and mRNA (Zhao et al. 2020) based therapeutics (Rehman et al. 2021; Tang et al. 2021)], and room-temperature stable mAb solutions (Zhang et al. 2021a, b). Conventional excipient, with low glass transition (Tg) and low collapse temperature (Tc) such as sucrose can lead to a long and expensive lyophilization cycle, which could be substituted with new excipients, e.g., dextran (Haeuser et al. 2020).
Thin-film freeze drying (TFFD) is a cryogenic technology applying an intermediate freezing rate from 102 to 103 k/s somewhere between SFD (102 k/s) and lyophilization (1 k/s) (Engstrom et al. 2008) to engineer aerosolized dry powder from the liquid form to deliver drugs to the lungs (Hufnagel et al. 2022). At a relatively high freezing rate, such as in TFFD, dissolved solute particle aggregation and precipitation as any form of dispersion can be avoided. Moreover, this high yield technique can produce homogenous particle size distribution, and a low-density brittle matrix composed of microparticle-sized nanoaggregates is favored for the respiratory delivery system (Sahakijpijarn et al. 2020). TFFD has been applied to formulate dry powders of small molecules, proteins, and vaccines (Wang et al. 2021).
Hufnagel et al. applied TFFD to formulate mAbs, immunoglobulin G (IgG) and anti-programmed cell death protein 1 (anti-PD-1), into dried powders and investigated their aerosol performance. The efficacy of the optimal ratios of two applied excipient groups, trehalose/leucine (75:25 w/w) and lactose/leucine (60:40 w/w), as well as water or phosphate-buffered saline (PBS) as the TFFD solvent were investigated in their studies. In their study, the mAb loadings percentages were 0.5% and 1% IgG, in which 1% IgG with lactose/leucine (60:40 w/w) in PBS showed the best aerosol performance among others; in terms of the solvents, PBS rather than water improved reproducibility and aerosols performance. While trehalose/leucine (75:25 w/w) incorporating 1% IgG formulation displayed a significantly reduced fine particle fraction (FPF) compared to the half of that loaded mAb. Preparing 1% of Anti-PD-1 mAb with the same optimal ratio of lactose/leucine (60:40 w/w) resulted in a dry powder formulation almost as aerosolizable as IgG1-LL-PBS [the recovered FPF for Anti-PD-1 91.38 ± 1.89 and recovered FPF for IgG 92.64 ± 1.311). Furthermore, anti-tumor necrosis factor α (anti-TNF-α) mAb was formulated at 1% w/w (trehalose/leucine 75:25), and interestingly its recovery was reported as a full one, and the monomer content did not change while in the Anti-PD-1 mAb formulation, the FPF experienced a fall to 79.2% from 96% following TFFD process (Hufnagel et al. 2022).
The time and money-consuming drying step in FD, primary drying, are inevitable with formulations including sucrose as their lyo- and cryoprotectant ingredient. Haeuser et al. investigated how to improve mAbs stability at high temperatures of storage. They utilized different weights of a polysaccharide, dextrans (1, 10, 40, 150, and 500 kDa) alone and in combination with sucrose at different ratios. At higher molecular weight (MW) of dextran (40–500) and dextran to sucrose weight ratios, an increased temperature of 20 °C in Tc compared to sucrose was observed. It caused longer reconstitutions and more viscosities. Moreover, by elevating the dextran weight fraction, a higher degree of cracking of cakes was seen, amplified if a higher weight dextran was used. Adding dextran also resulted in higher Tg values while residual moisture level experienced a decline. Protein stability analysis for mAb1 and mAb2 formulations with pure dextran or dextran/sucrose 1:1 mixture was conducted for two weeks at 40 °C regarding the soluble aggregate formation. According to the results, directly after FD, 100% dextran formulations showed a slight increase of high molecular weight species (HMWs) 1–3%, under 40 °C storage conditions, all formulations showed a remarkable amount of HMWs. In terms of HMWs% in pure dextran formulations, higher MW dextrans showed less HMWs% although they were larger in size compared to HMWs in formulations with lower MW dextrans. mAb1(IgG1, pI ~ 9.4, 148 kDa) and mAb2 (IgG1 pI ~ 8.2, 149 kDa) were formulated with 1:1 dextran/sucrose mixture. Their protein stability analysis displayed no change in HMWs% immediately after FD, although at 40 °C storage, 10–500 kDa dextran containing formulations showed five times lower HMWs% than pure dextran ones. Free terminal glucose of dextrans was the reason for glycation, and a higher percentage of HMWs was noticeably reduced by adding sucrose (Haeuser et al. 2020).
High concentrated therapeutic proteins have a concentration of 50 to 150 mg/mL. These proteins include plasma-derived immunoglobulins with the typical concentrations of 50–100 mg/mL of IV immunoglobulin. Duraliu et al. assessed the stability of freeze-dried plasma-derived IgG during a 6 to 12 month span under accelerated and real-time storage conditions. (− 20 °C ~ < 5% relative humidity (RH); 20 °C, ~ 40% RH; and 45 °C ~ 10% RH). A range of IgG concentrations from 10 to 200 mg/mL were prepared and in combination with sucrose its concentration was fixed at 10 mg/mL while sucrose/IgG molar ratio varied as follows: 1% IgG, 439:1; 5% IgG, 88:1; 10% IgG, 44:1; 20% IgG 22:1. At the end of the 12 months of storage at − 20 °C, 20 °C, and 45 °C temperatures, all IgG concentrations showed a higher percentage of moisture content, especially at higher temperatures (20 °C and 45 °C). While 10 mg/mL IgG had a moisture content around 3–4% at elevated temperatures, increased concentrations of IgG resulted in less moisture, 1–2% w/w. At 20 °C and 45 °C, the monomer loss was experienced with all IgG concentrations in which the higher concentration have shown the greater monomer loss. Considering binding activity measured by ELISA and by the application of anti-diphtheria and anti-tetanus IgG as markers for IgG, at concentrations of 50 and 100 mg/mL, reduced monomer content with proper binding activity was observed at high temperatures and after 12-month storage. At the same time, the monomer content was decreased under the same condition. The higher IgG concentration (200 mg/mL) had variable ELISA results due to the lack of full reconstitution and over two hours duration for being dissolved (Duralliu et al. 2020).
Spray drying (SD)
Despite several advantages of the FD process to produce dry powders, FD has some limitations. The restriction of FD includes consuming a tremendous amount of time, and energy and resources. In addition, FD has a difficulty in processing massive material quantities, which is critical in pharmaceutical industries. Moreover, handling the characteristics of particles and cake in FD is difficult. These challenges are drivers to look for an alternative drying technique, SD (Pinto et al. 2021).
Dried powder production via SD technique involves atomizing protein solution into a drying chamber to be dried at once and to form solid particles, all happening in one step. Regarding functional excipients, both techniques share the same stabilizers (Massant et al. 2020). However, "continues processing" assigned to the SD makes it an outstanding technique in the pharmaceutical industry to manufacture a large volume of stable, dried powders (Pinto et al. 2021). During SD, the temperature and the atomizing gas flow rate are the essentials parameters responsible for particles properties, such as their size, morphology, and residual moisture content (Wu et al. 2019). Numerous spray-dried products are either approved or commercially available. Since 2006 when inhaled spray-dried insulin, Exubera®(Pfizer), became commercially available, many investigations have been conducted to produce spray-dried biopharmaceuticals (endocrine hormones, bacteriophage viruses, therapeutic enzymes, etc.) to treat diseases (Pinto et al. 2021).
Considering excipients and their ratio on storage stability of dried powder and reconstitution time, Massnat et al. used two IgG4 mAbs (mAb1 and mAb2) as the model protein, sugars (trehalose, sucrose), and a range of amino acids including glycine (Gly), alanine (Ala), proline (Pro), serine (Ser), valine (Val), leucine (Leu), isoleucine (Ile), glutamine (Gln), histidine (His), lysine (Lys), arginine (Arg), phenylalanine (Phe), tryptophan (Trp) as their formulation stabilizers. According to the initial results, small neutral and basic as in a sugary basis (sucrose, trehalose) showed reduced reconstitution time and improved stability. In the second part of their study, a design of experiment (DOE) based trial, incorporating 16 formulations, in which an optimum ratio of trehalose/amino acid by employing two groups of essential amino acids (Lys and Arg) and neutral amino acids (Gly and Pro) was put into test. In such a trial, trehalose concentration started from 30 up to 120 mM, and for amino acids, it ranged between 50 and 150 mM. Storage condition was set at 25 °C and 40 °C for 13 consecutive weeks. All formulations preserved their amorphous state except for those containing high Lys or Gly content and low trehalose content. Arg remarkably reduced reconstitution time among tested amino acids and increased storage stability. Whereas, in other formulations with amino acids, the trehalose concentration and the sugar component, played crucial roles in improving stability and reconstitution time. Indeed, the stabilizing effect of Arg was so strong that there was no need to add a sugar stabilizer to enhance the stability. In addition, the weight ratio of mAb to stabilizer should be at least 1:1 (w/w) to obtain the satisfactory stability and reconstitution time. In their study, HMWs, relative humidity (RH), and turbidity results were utilized to evaluate the efficacy of stabilizers on aggregations and storage stability (Massant et al. 2020).
Wu et al. assessed the efficacy of two significant stresses, thermal and shear, on the chemical integrity, biological activity, yield, and size of particles during SD of enhanced green fluorescent protein-specific short interfering RNA (EGFP-siRNAs) solutions. Accordingly, in terms of siRNA content, all spray-dried powders showed a range of entrapment from 77 to 93%, indicating some extent of siRNA loss during the process, though with an increase in inlet temperature, the entrapment was decreased. A correlation between measured yield and atomization gas flow rate was observed by measuring production yield. However, the effect of inlet air temperature on yield production is variable and dependent on the flow rate. Unfortunately, the chemical integrity of spray-dried siRNAs could be hugely affected by the thermal and shear stress that particles were exposed during the SD process. Based on the results obtained from exposing siRNA solutions to the degrees of temperature close to the outlet temperature, 92 °C, the percentage of decomposed siRNA experienced a 52.9% enhancement and reached 66% when the heating time was changed from 10 to 120 min. Even though the outlet temperatures in the current SD process were as high as 92 °C and 125 °C, short-time exposure of siRNAs and quick evaporation of the solvent resulted in decomposition as low as 20%. According to the transfection efficiency tests, all spray-dried siRNA experienced a lower biological activity than non-spray-dried formulations. Moreover, further experiments did not reveal any correlation between transfection efficiency and chemical integrity. In other words, a small extent of chemical integrity does not compromise the biological activity of spray-dried siRNAs (Wu et al. 2019).
Spray freeze drying (SFD)
SFD is an attractive drying technique comprising of atomizing feeding solution into a cryogenic liquid, e.g., O2, N2, or Ar container, and drying the frozen droplets. Formed particles because of some unique characteristics including homogenous size distribution, porous structure, and improved shelf stability are desirable for pulmonary drug delivery. This method of drying has proved its functionality in enhancing biopharmaceutics classification system (BSC) class II drugs' solubility (Adali et al. 2020), producing inhalable dry powders of drugs other than those of the previous group (Liao et al. 2020; Faghihi et al. 2021), remarkably enhancing dissolution (Hu et al. 2019). Although SFD is highly favorable for drying thermo-sensitive materials (Adali et al. 2020), induced stresses such as dehydration, cold denaturation, and ice crystallization on mAbs and other biopharmaceuticals. Therefore, applying appropriate excipients to preserve their stability becomes essential.
Therapeutic proteins, including antibodies and antibody–drug conjugates, made their way to the market by having the first antibody–drug (muromonab-CD3 (Orthoclone OKT3, Janssen-Cilag)) approved by the Food and Drug Administration (FDA) in 1986. Since then, many antibody-based drugs have been approved and commercialized (Kim et al. 2016a, b; Singh et al. 2018). Given that their stability during the manufacturing process and their storage are of utmost importance and the fact that any single mAb requires its optimal production process parameters, e.g., excipients' type, combination, and concentration (Emami et al. 2018a, b; Emami et al. 2019) (Fig. 1).
Emami et al. constructed spray freeze-dried IgG microparticles using different amino acids including Leu, Phe, Arg, Gly, and cysteine (Cys) in the presence of trehalose to investigate the effect of the amino acids on the stability of IgG through SFD method. Pure spray freeze-dried-IgG resulted in the formation of 14% of IgG aggregates, however; IgG formulations stabilized using Leu, Phe, or Gly in the presence of trehalose have demonstrated aggregates < 2.2%. Combination of Phe and trehalose was most effective in stabilizing IgG against different verities of stresses during SFD. Arg and Cys were destabilizers representing aggregation and fragmentation of IgG, respectively. The IgG formulations prepared with Leu, Phe, or Gly in the presence of trehalose showed good stability (40 °C and 75% relative humidity for two months). Thus, a combination of the trehalose and uncharged, nonpolar amino acids have demonstrated the most stabilizing effects for spray freeze-dried-IgG formulations (Emami et al. 2018a, b).
In another study by Milani and colleagues, hydroxypropyl beta-cyclodextrin (HPβCD), renowned for its water-replacement, vitrification, and surfactant-like effects, was added to IgG formulations along with trehalose in different ratios ranging from the upper and lower ratios of 1:2 and 1:0.05 IgG: HpβCD, respectively. Among combinations with varying ratios, two formulations with the following ratios 1:2:0.25 (IgG: trehalose: HPβCD) and 1:2:0.05 had the minimum aggregation constants (0.46 ± 0.02 and 0.58 ± 0.01, respectively) after one month of storage at 45 °C and 60% of relative humidity. Measuring induced soluble aggregates after SFD and 1 and 2 months post SFD showed intriguing results as follows: all formulations' aggregations ranged between 0.01% and 0.1%, with a 1:2:2 ratio hitting the minuscule amount (0.01%), 0.25 ± 0.05 and 0.28 ± 0.02% of aggregation reported as the lowest levels belonging to 1:2:0.25, 1:2:0.05 in 2 months storage post SFD. Tracking chemical degradation in all samples after storage revealed no visible fragments of IgG molecules. The percentage of the dominant secondary structure of IgG molecules, beta-sheet structure, ranged between 65.13% and 77.82% following SFD. Beta-sheet content after storage conditions were slightly higher (66.32–78.15%). Finally, FPF and ED were measured as representatives of formulations' aerosol performance. For chosen formulations, 1:2:0.25 and 1:2:0.05 ratios, FPF values were 56.43 and 48.12%, respectively and their respective ED values were 93.15 and 91.23% (Milani et al. 2020).
Supercritical fluid drying (SCFD)
Among various existing drying methods spoken of so far, SCFD technique is an intriguing one due to its unique features, including being non-toxic, non-flammable, inert, recyclable, and readily removable by reducing the pressure (Costa et al. 2021) and being ecofriendly (Xu et al. 2018) and applying supercritical carbon dioxide (scCO2) as a benign solvent to process polymers under such harsh conditions (temperature: 31.1 °C, pressure: 73.8 bar). This technology's feasibility in producing polymer-based porous microparticles with a minimum amount of residual organic solvent is noticeable (Xu et al. 2018). With solvent remnants in the product, storage stability and drug administration could be compromised (Park et al. 2019). Moreover, particle morphology is a subject under control through this process, and uniformed particle size is also achievable (Kankala et al. 2018). Based on applying scCO2, various drying techniques are available such as rapid expansion of supercritical solvent (RESS), particle from gas saturated solution (PGSS), carbon dioxide-assisted nebulization with a bubble dryer (CAN-BD), supercritical assisted atomization (SAA)/ supercritical CO2 assisted spray drying (SASD), depressurization of an expanded liquid organic solution (DELOS) and supercritical CO2 as anti-solvent. Regarding the biological products, the gas anti-solvent method is the most popular among other methods based on supercritical drying (Wilson et al. 2018).
In one study, Wu et al. fabricated HPβCD particles by employing scCO2 as the spraying medium and ethanol as the solvent in the supercritical assisted atomization (SAA) process. They aimed to investigate the effects of ethanol as the solvent on morphology and the size of inhalable particles and to optimize the involved parameters of SAA in producing those particles. Moreover, their formulations were entirely designed by adding the proper amount of Leu as an ingredient known to affect dry powders' aerosol performance positively. In their study, several parameters including solvent's and HPβCD solution's concentrations (W/W), precipitator and saturator temperatures, and the flow rate ratio of CO2/HPβCD were investigated. In addition to eighteen tests run for determining the most practical value among all varying ones, six tests were run to determine the effect of different concentration of Leu. Measuring particle size showed that enhanced ethanol concentration resulted in more atomization and reduced particle size. Notably, adding ethanol to the binary mixture of water and CO2 increased CO2 solubility by nine-fold. Despite the positive effect of increased ethanol concentration on micronization, its undesired effect on the shape is noticeable. In order to avoid irregular or shell particles and benefit from micronization, the ethanol concentration of 54.2% (w/w%) was chosen. Other optimal parameters were reported as precipitator (TP) and saturator (Ts) temperatures of 373.2 K and 353.2 K, respectively. Following set optimal parameters of SAA process TP: 373.2 K, Ts: 353.2 K, flow ratio of CO2/HPβCD: 1.8, and fixed concentration of the HPβCD solution 10 mg/mL, Leu was added to investigate formulations' aerosol performance. The mass concentration of 13% achieved a FPF value of 27.8 ± 0.4. whereas the augmented concentration of Leu to 16.7% resulted in agglomerations and reduced FPF value (Wu et al. 2021).
In another study conducted by Xu et al., the fabrication of nano-embedded porous microparticles (NEPMs) to deliver both multi-drug resistance protein 1 (MRP1) siRNA and doxorubicin (DOX) in order to overcome multi-drug resistance (MDR) observed in the lung cancer, e.g., small cell lung cancer, was reported. By using a supercritical antisolvent process (SAS) and CO2 as an antisolvent in the process, they could encapsulate DOX and prefabricated siRNA-chitosan (siRNA-CS) nanoparticles into polymer-based, poly-L-lactide (PLLA) to form (siRNA-CS-DOX-PLLA PMs) considered as NEPMs. Characterization the physical properties of the siRNA-CS nanoparticles displayed spherical structures with a narrow size distribution, an average diameter of 100 nm, and loading efficiency of 77.4%, which is remarkable. These particles could sustainably release ~ 60% of siRNA in 24 h. The final microstructures were rough over the surface, indicating the excellent attachment of NPs, and highly porous having an average geometric particle size of 16.86 µm. Considering the reported aerodynamic properties of NEPM, increasing DOX content had no significant effect on measured values of Dg or Da, yet all features were at an optimal level for pulmonary delivery (10 µm < Dg, 1 < Da < 5 µm, FPF > 50%). Visual observation of the NEPM's aerosolization behavior was achieved in 0.12 s post actuation, which is considered good mobility. Releasing 60% to 80% of the entrapped DOX from NEPMs took place as slowly as in 60 h. The subcellular localization of NEPMs in H69AR cancer cells was investigated as an indicator of their delivery efficiency. Based on the results, cellular uptake of siRNA-CS nanoparticles was high, and these nanoparticles mediated siRNA release was via the escape from the cytosol. Performing anticancer efficacy tests revealed that NEPMs had the strength to lower cell viability to ~ 46%, while the results of other formulations (DOX, DOX-PLLA PMs, and NC NEPMs) were > 80% (Xu et al. 2018).
Promising drying techniques
So far, several methods to produce dried powders of biopharmaceuticals have been discussed, yet there are other potential techniques worthy of being named at least.
The particle replication in non-wetting templates (PRINT®) technology is a lithography-based method with the potential to precisely control the size and the shape of particles and their monodispersity (El-Hammadi et al. 2022) by adjusting mold dimensions (Shah et al. 2022). Applying PRINT technology makes the possibility to load a wide range of drugs from hydrophobic to hydrophilic ones. Produced poly-lactic-co-glycolic acid (PLGA) nanoparticles could be in the shape of a needle or a cylinder (El-Hammadi et al. 2022). This method has been applied to micro mold proteins into high-performance dry powders (Wilson et al. 2018) to synthetize lipid-polymer hybrid nanocarriers (Shah et al. 2022) for delivery of influenza vaccine antigens (Rana 2021).
Another wide-spreading method is fluidized bed drying in which the drug incorporating solution gets sprayed onto the inert carrier beads in fluidized bed systems having hot gas. This method is usually of choice to formulate oral drug delivery systems of peptides (Vass et al. 2019) though its productions cover pharmaceutical and food powders to detergents and fertilizers (Orth et al. 2022). Accordingly, Tyagi et al. constructed a multi-unit particulate system with the purpose of targeted oral delivery of a glucagon like peptide-1 (GLP-1) agonist peptide. They used a fluidized bed system for drug layering, seal coating, sustained release coating, mucoadhesive and enteric coating (Tyagi et al. 2021).
Other methods, namely vacuum drying, microwave drying, and electrospinning are drawing attention as they show potential for being more efficient drying methods than conventional ones (Sharma et al. 2021).
Biopharmaceuticals in clinical trials
The market of biopharmaceutical products consists of monoclonal antibodies, purified proteins, vaccines, cell and gene therapies, synthetic immunomodulators, and recombinant biomolecules i.e., growth factors, proteins, hormones, as well as enzymes. This market with such diversity has the potential to see a $534.19 billion revenue in 2027 with a compound growth rate of 7.32% over the years 2022 to 2027 (Intelligence 2022).
Increasing interest for manufacturing these products comes from their higher efficacy in treating many diseases including chronic viral hepatitis, rheumatoid arthritis, psoriasis and some types of cancers. Since these molecules are large in weight and biologically instable, the usual administration route is parenteral and mostly in form of lyophilizates, diluted or concentrated solution and suspensions (Bjelošević et al. 2020). On the other hand, there are inhalable dried powders or solutions of biologic molecules, proteins, which are or were commercially available such as Pulomozyme®, Technosphere ™ Afrezza® and Exubera®. Moreover, many other products of this type have been undergoing clinical trials (Karimi et al. 2022). Table 1. shows a number of biopharmaceutical products with FDA approval and biopharmaceuticals in clinical trials since 2018. However, Resusix® clinical trial was terminated (Entegrion 2020).
Table 1.
A list of FDA-approved and clinical trials studies of biopharmaceuticals
| Biological | Brand/dosage form | Condition or disease | NCT No./STN | Production method | Excipients | Route of administration | References |
|---|---|---|---|---|---|---|---|
| ALVAC-HIV (vCP2438) (Canarypox virus), BIVALENT SUBTYPE C (2 recombinant monomeric proteins mixed with MF59® adjuvant) | NA/lyophilized vaccine | HIV | NCT02404311 | Freeze drying | NA | Intramuscular | National Institute of et al. (2018) |
| Coagulation factor VIIa (recombinant)-jncw | SevenFACT®/lyophilized powder | Hemophilia A or B with inhibitors | 125641 | Freeze drying | Arginine hydrocholoride, glycine, isoleucine, lysine hydrochloride, polysorbate 80, trisodium citrate dihydrate, hydrochloric acid and nitrogen | IV | FDA (2020) |
| Glu-Plasminogen | Ryplazim®/lyophilized powder | Plasminogen deficiency type I | 125659 | Freeze drying | sodium citrate, sodium chloride, glycine, and sucrose | IV | (FDA 2021a) |
| Levodopa | INBRIJA®/inhalation powder | Parkinson | 209184 | Spray drying | dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and sodium chloride | Inhalation | FDA (2021b), Sharma et al. (2021) |
| Live, attenuated BCG (Bacillus Calmette-Guerin Strain) | NA/lyophilized vaccine | COVID-19 | NCT04475302 | Freeze drying | NA | Intradermal | Tuberculosis Research Centre et al. (2021) |
| LMN-101 | NA/capsule | Campylobacter Jejuni infection | NCT04098263 | Spray drying | Spirulina biomass | Orally | Lumen Bioscience (2020) |
| NTD-RBD derived from S protein of Omicron variant (RH109) | NA/lyophilized mRNA vaccine | COVID-19 | NCT05366296 | Freeze drying | ALC-0315 (4- hydroxy butyl) azanediyl) bis (hexane-6,1-diyl) bis (2- hexyldecanoate), ALC-0159 (2- [(polyethylene glycol)-2000]-N, N-ditetradecylacetamide), DSPC [1,2- distearoyl-sn-glycero-3-phosphocholine], Cholesterol, Trometamol and Sucrose | Intramuscular | Wuhan Recogen Biotechnology Co (2022) |
| Recombinant human coagulation Factor VIIa (rFVIIa) | NOVOSEVEN® RT/ lyophilized powder | Congenital Hemophilia A or B with Inhibitors, Acquired Hemophilia, Congenital Factor VII Deficiency, Glanzmann’s Thrombasthenia | 103665 | Freeze drying | Sodium chloride, calcium chloride dihydrate, glycylglycine, poly dorbate80, mannitol, sucrose, methionine | Intravenous | FDA (2014), Nordisc (2021) |
| Resusix® (solvent/detergent treated plasma), FP24 (frozen plasma) | Resusix®/NA | Acquired coagulopathy | NCT03700723 | Spray drying | NA | Intravenous | Entegrion (2020) |
| Triptorelin pamoate | Trelstar ® LA/suspension for injection | Advanced prostate cancer | NA | Spray drying | PLGA, mannitol, carboxymethycellulose sodium, polysorbate 80 | Intramuscular | Sharma et al. (2021) |
Resusix® clinical trial was terminated
NTD-RBD N-terminal domain, receptor binding domain, HIV human immunodeficiency virus, MF59® an oil-in-water emulsion as adjuvant
Characterization of dried-powder proteins
The critical aspect to the success of therapeutic proteins involves improved methods to evaluate the processed particles and characterize these proteins. Characterization tests include physical powder characteristics, in vitro aerosol performance, and physicochemical stability tests of dried powders. Furthermore, for therapeutic proteins, the conformational stability and biological activity of dried active pharmaceutical ingredients (APIs) are required (Wanning et al. 2015) (Fig. 1).
Solid powder characteristics
Processed particles by different drying techniques have shown various sizes and morphologies. Powder characteristics have been categorized based on the required administration route of APIs, including parenteral, pulmonary, nasal, and epidermal drug delivery systems (Wanning et al. 2015). The analytical methods used to evaluate powders' physical characteristics are summarized in Table 2. A successfully dried powder inhalation has good powder dispersibility, influenced by powder characteristics. The formulation, particle size, bulk density, specific surface area, and flow properties should be considered to have a proper aerodynamic behavior of the processed powders (Maa et al. 1999).
Table 2.
Analytical techniques for physical powder characteristics
| Analytical methods | Principles | Consideration | Proteins & peptides | References |
|---|---|---|---|---|
| SEM, TEM | Size, Surface structure, Morphology | Solid | IgG, Lysozyme, Anti-IgE Mab, rhDNase, PTH, Anthrax vaccine, Influenza vaccine | Sarciaux et al. (1999), Jovanovic et al. (2008a, b), Ramezani et al. (2013), Faghihi et al. (2014), Emami et al. (2018a, b), (Jovanovic et al. (2006), Jovanovic et al. (2008a, b), Nuchuchua et al. (2014), (Maa et al. (1999), (Poursina et al. 2015), (Wang et al. 2012), (Maa et al. 2004) |
| BET | Specific surface area | IgG, Anti-IgE Mab, rhDNase | (Jocelyn (1967), Awotwe-Otoo et al. (2015), Maa et al. (1999) | |
| DLS | Hydrodynamic size (1–1000 nm) | Liquid & Suspension, Spherical particles | IgG | Emami et al. (2018a, b) |
| SLS | Size (1–1000 µm) Molecular weight | Suspension | IgG, Anti-IgE Mab, rhDNase, PTH, Anthrax vaccine, Influenza vaccine | Ramezani et al. (2013), Faghihi et al. (2014), Emami et al. (2018a, b), Maa et al. (1999), Poursina et al. (2015), Wang et al. (2012), Maa et al. (2004) |
| Flow imaging microscopy | Size (1–500 µm), Porosity | IgG, Lysozyme | Nuchuchua et al. (2014) | |
| NTA | Concentration, Arithmetic size (30–1000 nm) | IgG, Lysozyme | Nuchuchua et al. (2014) | |
| Karl- Fischer | Residual moisture | IgG, Lysozyme, Anti-IgE Mab, rhDNase, IFN-α-2a, Anthrax vaccine, BSA | Sarciaux et al. (1999), Ramezani et al. (2013), Awotwe-Otoo et al. (2015), Garidel et al. (2015), Jovanovic et al. (2006), Jovanovic et al. (2008a, b), Nuchuchua et al. (2014), Maa et al. (1999), Kumar et al. (2009), Wang et al. (2012), Imamura et al. (2003) | |
| TGA | Residual moisture | Lysozyme | Liao et al. (2004) | |
| Dynamic vapor sorption | Relative humidity (Hygroscopicity) | Solid | Influenza vaccine | Saluja et al. (2010) |
| MDSC solid & liquid state | Crystallinity, Glass transition | Solid & liquid | IgG, Lysozyme, Etanercept, Anthrax vaccine, BSA | Vermeer et al. (2000), Jovanovic et al. (2008a, b), Sahin et al. (2010), Awotwe-Otoo et al. (2015), Jovanovic et al. (2006), Jovanovic et al. (2008a, b), Nuchuchua et al. (2014), Kim et al. (2014), Kim et al. (2016a, b), Wang et al. (2012), Imamura et al. (2003) |
| X-ray diffraction | Crystallinity | Solid | IgG, Lysozyme | Jovanovic et al. (2008a, b), Faghihi et al. (2014), Emami et al. (2018a, b), Jovanovic et al. (2006), Jovanovic et al. (2008a, b) |
| Andersen-cascade impactor, Multi-stage impinge, Twin stage impinge, Next generation impactor | Aerodynamic behavior (FPF, Span) | Solid | IgG, Growth hormone, Anti-IgE Mab, rhDNase, Alkaline phosphatase, Adalimumab | Faghihi et al. (2014), Kim et al. (2016a, b), Maa et al. (1999), Li et al. (2010), Emami et al. (2019), Kim and Kim (2016) |
SEM scanning electron microscopy, TEM transmission electron microscopy, BET Brunauer–Emmett–Teller, DLS dynamic light scattering, SLS static light scattering, NTA Nanoparticle tracking analysis, TGA thermogravimetric analysis, MDSC modulated differential scanning calorimetry, PTH parathyroid hormone, BSA bovine serum albumin, IFN-α-2a interferon-alpha-2a
The surface morphology of the processed powders was evaluated by using electron microscopy. The most important electron microscopy techniques for powder analysis are scanning electron microscopy (SEM) and transmission electron microscopy (TEM). TEM and SEM methods were applied to evaluate the particles in the range of 1–10 µm (Merkus 2009). Both microscopic methods have high resolution, enabling detailed information about the particle structure. However, due to the limited photo area in the millimeter range, information about the particle morphologies is not representative of whole samples. SEM represents three-dimensional images of the surface of particles as well as particle porosity (Zolls et al. 2012). However, TEM micrographs have been performed to show the outer shell and internal structure of processed particles to assess porosity and drug loading. The content distribution of particles was evaluated by TEM (Chen et al. 2012).
The surface area is vital in the absorption of APIs in the pulmonary drug delivery system. Also, powder surface area has influenced the degradation of protein formulations during freezing and crystallization (Awotwe-Otoo et al. 2015). Brunauer–Emmett–Teller (BET) method determines the specific surface area per unit weight of dried particles by adsorption nitrogen gas (Maa et al. 1999). Liquid nitrogen is adsorbed as a monolayer on the surface of microparticles. Increasing the pressure pores at the surface of particles are filled with liquid nitrogen. Specific surface areas are estimated by considering the pressure fluctuation (Filipe et al. 2013). Specific surface areas was shown for IgG, which determines how the freezing rate affects the size and surface area of final freeze dried powders (Table 2) (Sarciaux et al. 1999; Awotwe-Otoo et al. 2015).
A laser diffraction analyzer (static light scattering) measured the particle size distribution of the processed powders in a liquid suspension (Maa et al. 1999). Particle size analysis was performed to size subvisible particles larger than 1 µm up to 1 mm (Hawe et al. 2009). The particle size measurement is based on the light scattering intensity. Protein formulation for pulmonary drug delivery must have an aerodynamic diameter of 1–5 μm. Poursina et al. prepared a parathyroid hormone (PTH) formulation for inhalation with a geometric size of 14–16 µm. However, because of the low density of porous particles processed by SFD, these peptide particles have the proper aerodynamic diameter for inhalation (Poursina et al. 2015).
On the other hand, dynamic light scattering (DLS) can determine particle size in the range of 1–10 µm. DLS, based on the diffusion of particles in solution, can measure intensity changes of laser light scattered by the sample. Hydrodynamic diameter is determined according to the diffusion coefficient of solutes in an aqueous solution. In DLS, because of the hydrodynamic layer, the measured particle size is larger than the size determined by TEM (Gaumet et al. 2008).
Flow imaging microscopy measures particle size without any isolation. The particles pass through an imaging field, which is lightened by a light source and imaged by a charge coupled device camera. Automated image analysis can determine the morphology, particles size, and concentration (Zolls et al. 2012).
Nanoparticle tracking analysis (NTA) is another method to measure the size and concentration of nanoparticles. NTA is a combination of laser light-scattering microscopy with a charge coupled device camera. NTA, in comparison to DLS, has peaks with higher separation capacity. In addition, information about the concentration and heterogeneity of particles by visualization is available. NTA and DLS can determine the aggregate percentage in solutions (Zolls et al. 2012).
After the drying procedure, the residual moisture content of dried protein powders was determined with a Karl-Fischer titrator. The Karl Fischer coulometer determines the sample's water content based on an iodine/iodide redox reaction, in which the remaining water in the protein sample reacts with iodine until all water is consumed (Garidel et al. 2015). The remaining water probably results from bound, free or bulk water in dried powder based on the protein product. Generally, based on Food and Drugs regulations, the maximum residual moisture content should be equal to or less than 3% to maintain the protein stability (Nuchuchua et al. 2014). The hygroscopicity of the powders was determined by dynamic vapor sorption (DVS). In the gravimetric sorption analyzer, the powders were subjected to 0–70% relative humidity at room temperature, and the weight change equal to water uptake was determined (Saluja et al. 2010).
Modulated differential scanning calorimetry (MDSC) by scanning different protein formulations has the potential to detect whether there are interactions between proteins and excipients at the molecular level or not (Tian et al. 2006). Also, the thermal behavior of dried protein formulation compared to the physical mixture or individual excipients and proteins was evaluated. The melting or crystallization transition in a protein formulation thermogram confirms the product's crystallinity, which is not preferable, and vice versa (Nuchuchua et al. 2017). The glass transition values and melting points for different solid formulations were determined using a differential scanning calorimetry (DSC) device. DSC was accomplished on powder samples. Typical glass transition peaks for SFD formulations of vaccine were not detected in all formulations scans due to interference from other thermal transitions (Wang et al. 2012). On the other hand, the Nano DSC can be done for liquid protein formulations to evaluate the structural stability of the protein (Kim et al. 2016a, b).
Crystallinity is determined by X-Ray diffraction (XRD). XRD technique is applied to study the solid states of solutes in frozen aqueous solutions (Oetjen 2004). For the crystalline combination, a sharp peak appeared. However, the formulation in an amorphous state does not have a sharp peak. Amorphous protein/excipients because of better solubility and improved stability are desirable. Faghihi et al. prepared stable spray-dried IgG with Leu. However, the antibody has shown some aggregates in the presence of Gly that has a crystalline structure (Faghihi et al. 2014).
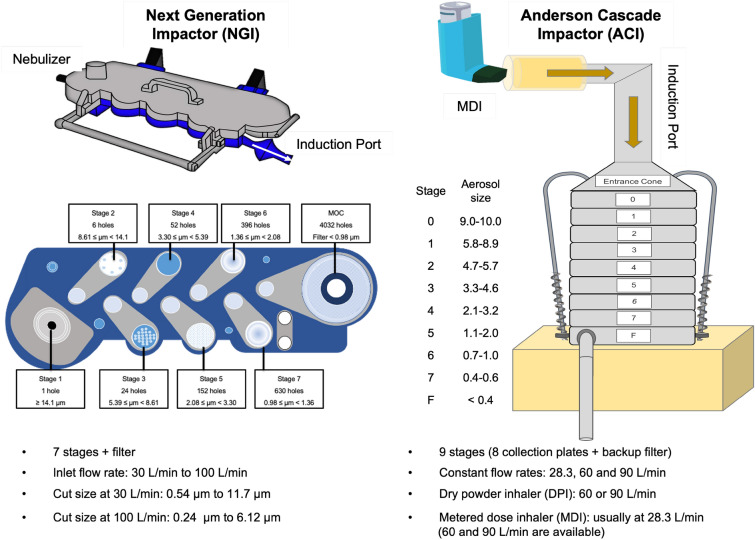
The aerodynamic behavior is important to have a successful inhalable dried powder, which influences drug deposition for inhalation (Ali et al. 2014). The dry powder's aerosol performance was evaluated using twin stage impinger, multi-stage liquid impinger, Anderson-cascade impactor (ACI), and the Next-generation impactor (NGI) (Wanning et al. 2015; Emami et al. 2019). Amounts of powders collected from different stages of these devices were determined, representing the aerodynamic particle size. Using these devices, we can estimate by inhalation how much the drug will reach the alveolar airways (Maa et al. 1999). A cascade impactor measures the particle size as it distributes via an opening with the use of aerosol. The ACI, manufactured by Copley, can predict the deposition profile of aerosol particles into different parts of airways respiratory system. ACI with eight collection plates and a filter stage has been designed for measuring the aerodynamic particle size distributed by dry-powder inhalers (DPIs) and metered-dose inhalers (MDIs) (Newton et al. 1977; Yoshida et al. 2017; Dechraksa et al. 2020). While the NGI is a high-performance cascade impactor with seven collection cups and different cut-off diameters for classifying aerosol particle into size fractions for testing MDIs and DPIs and other inhaled drug delivery devices including nasal sprays and nebulizers (Marple et al. 2003, 2004; Yoshida et al. 2017). The schematic figures of the impactors and some of their features are displayed in Fig. 3.
Fig. 3.
The schematic figure displays two impactors, Andersen cascade impactor (ACI) and Next generation impactor (NGI) as well as their features
Conformational stability
Due to the delicate nature of protein structure, secondary or tertiary structure of proteins are susceptible to changes during drying methods. Detection of variation in protein's secondary or tertiary structure can be challenging, especially if they involve only a tiny part of the therapeutic protein (Houde et al. 2014). Some examples of spectroscopy methods for particles engineered by different drying procedures are available (Table 3).
Table 3.
Analytical methods for evaluating the protein conformation
| Spectroscopy methods | Principle | Detection | Properties | Proteins & peptides | Reference |
|---|---|---|---|---|---|
| Far CD, Near CD, Spectroscopy | Aromatic amino acid environment, Peptide bond | Secondary structure, Tertiary structure, Quaternary structure | Fairly expensive, Liquid sample | IgG, Lysozyme, Etanercept, PTH, Anthrax vaccine | Vermeer et al. (2000), Schüle et al. (2007), Hawe et al. (2009), Sahin et al. (2010), Emami et al. (2018a, b), Jovanovic et al. (2006), Nuchuchua et al. (2014), Kim et al. (2014), Poursina et al. (2015), Wang et al. (2012) |
| FTIR | Peptide bond structure | Secondary structure | Cheap solid and liquid sample | IgG, Lysozyme, Etanercept, BSA, Anthrax vaccine | Schüle et al. (2007), Hawe et al. (2009), Awotwe-Otoo et al. (2015), Jovanovic et al. (2008a, b), Kim et al. (2014), Kim et al. (2016a, b), Imamura et al. (2003), Wang et al. (2012) |
| Fluorescence, Spectroscopy (Intrinsic & Extrinsic) | Aromatic amino acid environment | Tertiary structure, Quaternary structure | Liquid sample | IgG, PTH, Lysozyme | Hawe et al. (2009), Sahin et al. (2010), Emami et al. (2018a, b), Poursina et al. (2015), Jovanovic et al. (2006) |
| UV absorbance spectroscopy | Aromatic amino acid environment | Tertiary structure | Cheap liquid sample | IgG | Emami et al. (2018a, b) |
CD circular dichroism, FTIR fourier transforms infrared, BSA bovine serum albumin
In order to study the structure of processed proteins in the solid-state, Fourier transforms infrared (FTIR) spectroscopy is applied (Jovanovic et al. 2008a, b). FTIR spectra estimate the secondary structure elements in the solid-state. Each protein has a unique spectrum and the significant peaks in the second derivatives spectra are found, which are related to α-helix, β-sheet, turn, and a random coil of the protein structure (Schüle et al. 2007). After reconstitution, the protein conformation was further investigated using circular dichroism (CD) and fluorescence spectroscopy to determine whether the protein preserved its structure (Jovanovic et al. 2008a, b). CD is a valuable spectroscopy technique for evaluating the structure of proteins in solution. By absorption of radiation, CD signals appear, and spectral bands are related to distinct structural characteristics of a protein. From the intensity of spectral regions, complementary protein structural information can be provided (Kelly et al. 2005). Far-UV CD region with wavelength ranging from 190 to 250 nm, related to the peptide bond absorption and information on the protein secondary structure can be obtained. However, the near-UV CD chromophore, a wavelength range between 250 and 320 nm, reflects the aromatic amino acid residue and therefore gets information about the tertiary structure of a protein (Hawe et al. 2009) and broad absorption peaks with less intensity centered at 260 nm belong to disulfide bridges (Kelly et al. 2005). CD spectroscopy is suitable for determining whether a protein is folded, determining the percent of α-helix, β-sheet turns, and random structure in proteins, and comparing the secondary structure of proteins in different conditions, including temperature, pH, salt, protein concentration, ligands, etc. Also, CD spectroscopy is applied to measure the protein stability via thermal melts and denaturation studies, testing the structural integrity of site-directed mutations and the stability of domain structures (Kelly et al. 2005).
Fluorescence spectroscopy is a highly sensitive method for evaluating the conformation of the protein. Intrinsic protein fluorescence spectra are derivatives of the fluorescent amino acid, tryptophan, and tyrosine, which can provide information on changes in protein structure. On the other hand, extrinsic fluorescent dyes like 1-anilinonaphthalene-8-sulfonate (ANS), Bis-ANS, Nile Red, Thioflavin T, Congo Red can produce covalent linkage to proteins, e.g. via the ɛ-amino group of lysine, the α-amino group of the N-terminus, or the thiol group of cysteine and so on they perform protein analysis (Hawe et al. 2008).
Although CD and FTIR spectroscopy has high sensitivity with high resolution, Ultra-Violet (UV) absorption spectroscopy has gained attraction to study the protein conformational changes. UV absorbance as a zero-order or derivative analysis is a rapid, nondestructive, high resolution, and cheap alternative to other commonly used spectroscopic techniques for protein analysis (Engineers et al. 2003).
Nano DSC is a highly sensitive DSC with high-resolution to investigate the thermal stability of large biopharmaceuticals in liquid samples. The Nano DSC have a potential to characterize the thermal stability of their samples without the use of exogenous tags or dyes, thereby simplifying workflows with higher accuracy and more reproducibility. The Nano DSC determines the heat of reaction from tertiary and secondary structure changes that occur when a biomolecule unfolds. In addition, this instrument in one experimental set up, can measure the enthalpy, melting temperature, heat capacity, and calculate the free energy to evaluate the stability of sample (Spink 2008; Gill et al. 2010).
Physicochemical stability
Despite the attractiveness of different drying procedures, these processes generate a variety of freezing and dehydration stresses which are destructive for protein formulations (Tian et al. 2006). Because of complexity, variation of protein instability, and presence of aggregates with extended particle size range, more than one physicochemical technique was desired to characterize the instability (Table 4) (Houde et al. 2014). DLS, or quasi-elastic light scattering or photon correlation spectroscopy, is used to determine the hydrodynamic size of innate proteins (Zolls et al. 2012). In addition, DLS is highly sensitive method to detect dimers and large aggregates in the size ranging between 1 nm and 10 µm (Hawe et al. 2009; Emami et al. 2018a, b). In size exclusion chromatography (SEC), proteins are separated based on their hydrodynamic size. Generally, SEC is performed to identify and quantify protein monomers, fragments, and small aggregates. In SEC, the percent of soluble aggregates can be estimated by directly the peak area of aggregates and for insoluble aggregates as a loss area in the total peak area. UV absorbance, fluorescence, or refractive index are typical detectors for evaluating protein content. Light scattering detectors can estimate the molecular weight of the monomer and aggregates (Zolls et al. 2012; Emami et al. 2018a, b). Usually, SEC result has high precision; however, the nonspecific interaction of proteins and protein oligomers in the chromatography columns are existed (Engineers et al. 2003).
Table 4.
Analytical techniques for physicochemical characterization of protein
| Methods | Principles | Instability | Applications | Proteins & peptides | Reference |
|---|---|---|---|---|---|
| DLS | Hydrodynamic size, Polydispersity | Physical | Aggregation (1 nm–10 µm) | IgG, Etanercept | Sarciaux et al. (1999), Ahrer et al. (2006), Hawe et al. (2009), Menzen et al. (2014), Emami et al. (2018a, b), Kim et al. (2014), Kim et al. (2016a, b) |
| NTA | Concentration, Arithmetic size | Physical | Aggregation (30–1000 nm) | IgG, Lysozyme | Nuchuchua et al. (2014) |
| SLS (Light Obscuration) | Average Mw | Physical | Aggregation (1–600 µm) | IgG | Hawe et al. (2009), Emami et al. (2018a, b) |
| Turbidity | Optical density (λ ˃340 nm), Aggregation index | Physical | Aggregation | IgG, Lysozyme | Sarciaux et al. (1999), Ahrer et al. (2004), Jovanovic et al. (2008a, b), Hawe et al. (2009), Menzen et al. (2014), Jovanovic et al. (2006), Jovanovic et al. (2008a, b) |
| SEC | Hydrodynamic size, Mw | Chemical, Physical | Fragmentation, (Disulfide) oxidation, Aggregation, dimer (1–100 nm) | IgG, Lysozyme, Etanercept | Sarciaux et al. (1999), Ahrer et al. (2004), Schüle et al. (2007), Sahin et al. (2010), Garidel et al. (2015), Emami et al. (2018a, b), Nuchuchua et al. (2014), Kim et al. (2014), Kim et al. (2016a, b) |
| IEX | Charge | Chemical | Deamidation ((1–100 nm) | IgG | Wang et al. (2007) |
| RP-HPLC | Hydrophobicity | Chemical | Fragmentation, Deamidation, (Disulfide) Oxidation (1–100 nm) | Growth hormone | Kim et al. (2014), Kim et al. (2016a, b) |
| Analytical Ultracentrifuge | Mw | Physical | Aggregation | – | Jiskoot et al. (2005) |
| Electrophoresis, Native-PAGE, SDS-PAGE, CE-SDS, MCGE | Mw, Charge | Chemical, Physical | (Disulfide) oxidation, Fragmentation aggregation, Dimer, Isomerization | IgG, Phosphopeptide, Lysozyme, Anthrax vaccine, Influenza vaccine, Adalimumab | Alexander et al. (1995), Hawe et al. (2009), Awotwe-Otoo et al. (2015), Wang et al. (2015), Emami et al. (2018a, b), Ollikainen et al. (2016), Wang et al. (2007), Jovanovic et al. (2006), Wang et al. (2012), Maa et al. (2004), Emami et al. (2019) |
| LC–MS | Mass | Chemical | Deamidation | β-Lactoglobulins | Monaci et al. (2007) |
| Mass spectrometry, MALDI-TOF, ESI-TOF | Mass/Charge | Chemical, Physical | Fragmentation, Low Mw aggregation | IgG, Adalimumab | Alexander et al. (1995), Sandra et al. (2014), Zhang et al. (2014), Emami et al. (2019) |
DLS dynamic light scattering, SLS static light scattering, NTA nanoparticle tracking analysis, IEX ion exchange chromatography, SEC size exclusion chromatography, RP-HPLC reverse phase- high performance liquid chromatography, Native-PAGE native polyacrylamide gel electrophoresis, SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis, CE-SDS capillary electrophoresis sodium dodecyl sulfate, MCGE microchip capillary gel electrophoresis, Mw molecular weigth, LC–MS liquid chromatography-mass spectrometry, MALDI-TOF matrix-assisted laser desorption/ionization-time of flight, ESI-TOF time-of-flight mass analyzer with an electrospray ionization
Other chromatographic methods like reverse-phase-high-performance liquid chromatography (RP-HPLC) can recognize the chemical instability that caused hydrophobicity changes, including deamidatin, oxidation of disulfide bridge, β-elimination and isomerization of aspartic acid. On the other hand, ion-exchange chromatography (IEX) can separate with the principle of charge variation. In chemical reactions such as deamidation and β-elimination total charge of the degradation product is changed, which can be assessed by IEX method (Filipe et al. 2013).
UV absorbance spectrum in wavelength between 240 to 350 nm is an alternative method to evaluate the aggregates. The aggregation index (AI) was estimated from optical densities (OD) in different wavelengths as OD340/(OD280-OD340) × 100. Protein aggregates, compared to native protein due to the higher light scattering, can cause increased turbidity at 340 nm wavelength. Therefore, AI is a good indicator for aggregate monitoring, which none of the intrinsic chromophores in protein structure absorb at this wavelength. AI values below 10 often indicate solutions with minor amounts of soluble aggregation (Emami et al. 2018a, b).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is a conventional technique to determine the molecular weight of proteins and protein aggregates and fragments (Filipe et al. 2013). The principle of separation in SDS–PAGE is the relative molecular size in the presence of SDS (Park et al. 2010). SDS-PAGE under reducing and non-reducing conditions was carried out to distinguish whether the protein aggregates were composed of covalently or non-covalently linked monomers (Hawe et al. 2009). For protein quantification capillary electrophoresis sodium dodecyl sulfate (CE-SDS) is an alternative method. CE-SDS, because of its high resolution, has received significant attention. However, this electrophoresis method was still time-consuming in sample preparation with low sensitivity (Park et al. 2010). Microchip capillary gel electrophoresis (MCGE) is based on Lab-on-a-chip technology and is recognized as a high-performance analytical method. Among the electrophoresis techniques, MCGE, because of its high precision and high sensitivity detector with laser-induced fluorescence, is preferable. Furthermore, MCGE is automated with fast sample analysis (10 samples within 25 min) and a minimum amount of protein (4 µL of protein sample) (Park et al. 2010; Park et al. 2015). Capillary isoelectric focusing (CIEF) is another electrophoresis method that can separate based on proteins' isoelectric points (pI). As two isoforms cannot separate based on their size and molecular weight, Na et al. have demonstrated that although mass spectrometry or SDS-PAGE cannot distinguish between two isoforms, CIEF can discriminate ricin isoform very well (Na et al. 2011).
Among analytical tools, mass spectrometry is a powerful option for assessing the physicochemical degradation of proteins. However, this method has limitation in the nature and concentration of additives and requires complex sample preparation for analysis. So, liquid chromatography- mass spectrometry (LC–MS) is a combination of chromatography and mass spectrometry which is a good alternative (Filipe et al. 2013). The liquid chromatographic method with electrospray ionization tandem mass spectrometry is a fast analytical method with high selectivity and sensitivity for quantification (Ji et al. 2011). Park et al. used developed liquid chromatography–tandem mass spectrometry to evaluate the stability of collagen pentapeptide, a subfragment of collagen with high precision and high accuracy (Park et al. 2012).
Biological stability
Each therapeutic protein or peptide after administration to patients has some efficacy associated with the biological activity of these drugs. Whenever the protein formulation is dried and formulated through a stressful procedure, it is critical to monitor the protein activity compared to unprocessed APIs. Potency assessment can be done by in-vitro and in-vivo tests to confirm the pharmacological efficacy of biologics is preserved significantly (Nuchuchua et al. 2017). Ramezani et al. prepared spray-dried trastuzumab and treated the human breast cancer cell line SKBr3, which confirmed that the bioactivity of the antibody was preserved after processing by SD (Ramezani et al. 2014).
Local and systemic delivery of dried-powder biopharmaceuticals
Respiratory diseases, asthma, chronic obstructive pulmonary disease (COPD) and lung cancer, as well as respiratory infectious diseases, pneumonia and tuberculosis (TB), have always been a serious issue to human’s society, not only threatening their lives but also negatively affecting their socioeconomic status (Parray et al. 2021). In the group of viral pathogens, we see corona virus, adenovirus, respiratory syncytial virus, influenza virus, rhinovirus, and measles, which are responsible for viral pneumonia (He et al. 2022). With the aim of treating either infectious respiratory diseases or systemic disorders, inhaled therapeutics reduce both applied dose and cost of therapy with benefiting patients to administer drugs individually or at a nursing home (Parray et al. 2021). Oligopeptides, cytokines, enzymes, vaccines, mAbs, genes and clotting factors are categorized as biopharmaceuticals (Osman et al. 2018). Pulmonary delivery of biopharmaceutics could be promising for preparing higher bioavailability than other routes while delivering lower dose of therapeutics (He et al. 2022). Local pulmonary system provides a rapid onset of action when it comes to low molecular weight drugs (Faghihi et al. 2021). Moreover, the efficacy of high molecular weight biomolecules due to the higher concentration of the biomolecules in respiratory organs is accelerated via inhalation. As in mAbs, it is reported that their accumulation in target organs, the lungs, is more when they are applied through inhalation. Therefore, other organs are less exposed to the therapeutic and related side effects such as toxicity and cytokine release syndrome are reduced (Parray et al. 2021). In addition, in terms of selectivity, protein biomolecules are superior to small drug molecules (Matthews et al. 2020). Although several trials in clinical and preclinical stages have been conducted, since 1993 the year in which Pulmozyme® was launched as an inhaled protein treatment for cystic fibrosis, no more such treatment has been introduced to the biopharmaceutical market (Matthews et al. 2020). Among many reported preclinical and clinical studies regarding local delivery of biomolecules as dried powders, infliximab as a mAb acting against TNF-α was utilized through respiratory system as a dry powder in Balbc/mice. According to results, secretion of TNF-α in mice’s lungs were remarkably reduced and resulted in locally inhibited inflammation (Faghihi et al. 2019). Clinically experimented inhaled proteins include anti-thymic stromal lymphopoietin (TSLP) antibody fragment (CSJ117), anti-interleukin (IL)-13 mAb fragment (VR942/CDP7766) (Liang et al. 2020) and a member of Anticalin® proteins acting as an IL-4Rα antagonist (AZD1402/PRS-060) all with efficacy against asthma (Matthews et al. 2020). Another clinical trial with focus on a rare autoimmune disease, pulmonary alveolar proteinosis, was conducted. The recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) was inhaled as a lyophilized formulation by patients suffering from mild to moderate form of the disease. Laboratory findings showed a modest but significant change in alveolar–arterial oxygen gradient in the target group of patients (Tazawa et al. 2019).
Aside from patients with respiratory disorders benefiting from local drug delivery through inhalation, those patients suffering from diseases including osteoporosis, diabetes, hormone deficiency and migraine (Keyhan shokouh et al. 2021) could enjoy the simplicity and non-invasiveness of such administration way (Dhahir et al. 2021). From a pharmacokinetic view, pulmonary delivery systems’ superiorities are large surface area (70–140 m2), fast absorption and less enzymatic activity than oral route; it also bypasses first pass metabolism of the liver (Miyamoto et al. 2021). Hence, an enhancing number of preclinical and clinical studies are trying to bring more biomolecules from bench to bedside. Moreover, in some cases e.g., postpartum hemorrhage (PPH), inhalation could save many lives. Currently, oxytocin injections are applied to cease the hemorrhage. Oxytocin products require cold chain transport and storage, which are hardly affordable in low- and middle-income countries (Carvalho et al. 2020; Rahman et al. 2021). In order to address such urgent need for dried powders of oxytocin, devoid of cold supply chain, some of which have undergone preclinical (McArthur et al. 2017) and clinical trials (GlaxoSmithKline 2015; Fernando et al. 2017) to test their viability. A powder formulation with ultrafine size of oxytocin was developed by Prankered et. al. They used a postpartum sheep model to assess the efficiency of the spray-dried powder of oxytocin for in vivo evaluations. Results showed an onset of uterine electromyographic (EMG) activity after 129 ± 18 s post inhalation, which is noticeably shorter than 275 ± 22 s post intramuscular administration. Despite the difference in the onset of action, both EMG responses were similar and closely resembled the natural EMG activity following delivery. Noticeably, oxytocin formulation was well tolerated in the airway showing no irritability or distress (Prankerd et al. 2013).
Myamoto and colleauges developed a dried powder formulation of another peptide, human Ghrelin (hGhrelin), which is responsible for enhancing hunger sensation. They aimed to change its administration from injection to inhalation. In this case, their powder achieved an FPF of 41.7 ± 3.8% and the bioavailability of optimum formulation tested in monkeys reached to a mark of 16.9 ± 2.6% (Miyamoto et al. 2021).
Pulmonary delivery of peptides and proteins
Due to the poor stability and limited permeability in gastrointestinal (GI), usually the parenteral administration of the therapeutic peptides and proteins are preferable (Zhu et al. 2021). Peptides and proteins have superior specificity due to highly selective receptor-binding that reduce off-target side effects (Osman et al. 2018). However, due to the high MW, hydrophilicity, instability, and subsequent limited absorption, therapeutic proteins and peptides have predominantly delivered through parenteral route. Short-circulatory half-life of peptides and proteins require frequent injections and subsequently lowering the patient compliance (Emami et al. 2018a, b; Osman et al. 2018). Aerosolized peptide and protein due to the patient compliance, bypassing hepatic first pass metabolism, and avoidance of the harsh proteolytic GI environment could be an appropriate alternative for local or systemic delivery (Emami et al. 2018a, b; Osman et al. 2018). Improved pharmacokinetics of peptide and protein due to rapid onset of action, the large surface area (100–140 m2) of the lungs, and vascularized lung epithelium are amongst the inhaler benefits (Osman et al. 2018).
Emami et al. investigated how the influence of different amino acids on the final formulation of adalimumab differs from one another in terms of the stability and aerosol performance. They processed adalimumab via SFD, benefiting from different types of amino acids including Leu, Phe, Gly, Arg, and trehalose in those formulations. All formulations, regardless of the type of the amino acids, showed highly porous and spherical particles. Regarding their size and size distribution, all amino acids-containing formulations showed small particles with a narrow span except for the Arg-containing formulation, which was not that satisfactory. Moreover, these stabilizers resulted in formulations in which the secondary structure of adalimumab was preserved. In term of stability, aggregation levels displayed how well amino acids stabilized adalimumab formulations during SFD, less than 4% aggregation, and under accelerated conditions (40 °C and 75% RH for three months), more than 95% intact adalimumab. In addition, data obtained from testing the biological activity of the formulations and their aerosol performance revealed more than 92% cell viability, comparable to the intact adalimumab, as well as FPF values of more than 50% with emitted dose (ED) figures more than 90%, excluding the Gly formulation (Emami et al. 2019).
Quarta et al. synthetized the excipient-free of insulin formulation through SD procedure for post-prandial glucose control. Physico-chemical properties of spray-dried insulin was preserved within the storage (6 months at room temperature). In addition, spray-dried powder of insulin has shown the successful in vitro aerodynamic behavior. Pharmacodynamic as well as pharmacokinetics in rats that received intratracheal insufflation of spray-dried insulin powders were determined and compared with Afrezza inhalation insulin. Spray-dried insulin has shown the fast absorbance (tmax 15 min and Cmax 4.9 ± 1.5 mU/mL) whereas, Afrezza had a slower absorption (tmax 30 min and Cmax of 1.8 ± 0.37 mU/mL). After glucose injection, spray-dried insulin caused a sudden reduction of glucose level, similar to Afrezza. The subcutaneous injection of insulin due to the slow absorption, showed the prolonged insulin circulation level and consequently a long-lasting hypoglycemic efficacy (Quarta et al. 2020).
Treatment efficacy against TB as an infectious respiratory disease, is enhanced via mucosal immunization induced by direct delivery of vaccines to the nose or lungs (Gomez et al. 2021). Gomez et al. constructed the thermostable formulation as an inhalable dry powder version of ID93 + GLA-SE, an adjuvanted subunit TB vaccine candidate, containing recombinant fusion protein ID93 and glucopyranosyl lipid A (GLA) in a squalene emulsion (SE) as an adjuvant system through SD procedure. Leu (20% w/w), trileucine (3%, 6% w/w), and pullulan (10%, 20% w/w) excipients in the presence of trehalose as a stabilizer was assessed. In the presence of Leu, the aerosol performance was enhanced, but induced aggregation of the emulsion droplets. Pullulan preserved emulsion droplet size; however, the antigen was not recognized after reconstitution. The trehalose-trileucine combination successfully stabilized the adjuvant system, with retention of the antigen, in an inhalable dry powder format for vaccine delivery (Gomez et al. 2021).
Pulmonary delivery of siRNA
Gene silencing specifically, short-interfering RNA (siRNA) through protein expression modification at the mRNA level by a sequence-specific posttranscriptional procedure known as RNA interference (RNAi) have a potential to inhibit the pathology pathways and introduce new treatment strategies for respiratory diseases (Ding et al. 2021; Zoulikha et al. 2021). The lung characteristics, including clear anatomy, accessibility, relative low enzyme activity, make a good target for local siRNAs therapy (Zoulikha et al. 2021). Inhalation-based delivery of siRNA for targeted delivery to specific lung cells holds great promise because it can reduce the overall dose required to treat pulmonary disorders in comparison to oral or parenteral routs. In addition, this reproducible and economical platform avoid first-pass metabolism, which reduce the dose and toxicity risk in diverse patient populations (Ding et al. 2021). In addition, siRNA as compared to other gene therapeutics, including the plasmid DNA (pDNA) and plasmid-based short RNAs (shRNA) could be easily constructed and avoids the gene therapy-related side effect (mutation and teratogenicity). Moreover, siRNA has a smaller size, higher transfection efficacy, potency and specificity, and lower immune response, making them the proper choice for RNAi therapeutics (Zoulikha et al. 2021).
On the other hand, targeted delivery of siRNA to the lung due to the instability of naked siRNA molecules in systemic circulation and the negative charge of siRNA is challenging (Bardoliwala et al. 2019). Naked siRNA or different verities of siRNA-containing carriers including liposomes, dendrimers, polypeptides, micelles, inorganic nanoparticles (gold nanoparticles, silica nanoparticles), polymeric nanoparticles, and exosomes were applied for siRNA pulmonary delivery (Ding et al. 2021).
Patisiran, the first approved siRNA therapy, stimulate the siRNA delivery to expand their scope of RNAi therapeutics to other diseases. Patisiran is a lipid nanoparticle containing the siRNA encapsulated with lipid excipients for delivery to hepatocytes. The nanoparticles are composed of ionizable cationic lipids (DLin-MC3-DMA), cholesterol, phospholipid (DSPC), and polyethylene glycol modified lipids (PEG2000-C-DMG). To enhance the physicochemical stability, the siRNA is modified with eleven 2′-methoxy-modified sugar residues and four 2′-deoxythymidine residues. GIVLAARI™ _(givosiran) is another RNAi medication for treatment of acute hepatic porphyria, which was approved in November 2019 (Ding et al. 2021).
Miwata et al. have demonstrated that intratracheal administration of siRNA dry powder, vascular endothelial growth factor-specific siRNA (VEGFsiRNA), through the suppression of specific genes expressed in cancerous lung tissue was a successful therapeutic strategy for lung cancers. VEGFsiRNA inhaler was administered intratracheally to mice with metastatic lung cancers consisting of B16F10 melanoma cells or Lewis lung carcinoma cells. A single intratracheal dose of VEGF-siRNA diminished the VEGF expression levels in lung tumor tissue and bronchoalveolar lavage fluid. In addition, repeated intake of intratracheal VEGF-siRNA reduced the number of visible metastatic foci on the lung surface and tumor area in lung tissues (Miwata et al. 2018).
Nasal delivery of vaccines
In addition to the FDA-approved nasal vaccine, which is a liquid vaccine form, there is a growing interest in gel-based and dry powder vaccine formulations (Tai et al. 2022). Although liquid form of vaccines has limitations including the chemical, physical, and thermal instability, solid dosage form of vaccines have the advantages of improved physicochemical stability, which omit the role of preservatives and cold chain circulation (Wang et al. 2012; Tai et al. 2022). Different devices must be applied to actively deliver the dry powder vaccine into the nasal cavity. Until now, there is no FDA-approved dry powder vaccine for intranasal administration (Tai et al. 2022), but an increasing number of preclinical and clinical studies are undergoing to investigate new strategies for dry powder delivery of vaccines (Wang et al. 2012; Tai et al. 2022). Intranasal vaccination using dry powder vaccines is an attractive, non-invasive strategy with improved stability and enhanced protection at the mucosal surfaces (Thakkar et al. 2018). The immunization through injection usually fails to induce mucosal immunity or induce weaker immunity responses (Mato 2019). Nasal administration constitutes an alternative and promising strategy for vaccine delivery. Mucosal routes have several advantages supporting their selective use for different pathologies. Currently, many efforts are being made to develop effective drug formulations and novel devices for nasal delivery (Mato 2019). Thakkar et al. induced local and systemic immunity responses by intranasal immunization of a dry powder vaccine adjuvanted with an insoluble aluminum salt. The dry powder vaccine was prepared by thin-film FD of a model antigen, ovalbumin, adsorbed on an adjuvant (aluminum hydroxide). The dried vaccine have shown the proper aerodynamic behavior, good flow properties, and uniformly distribution of vaccine in dry powder. An in vitro nasal deposition study using nasal casts of humans showed that most of the dried powder was deposited in the nasal cavity (~ 90%). Intranasal immunization of rats with the dry powder vaccine stimulated a serum antibody response as well as specific IgA responses in the nose and lung secretions of the rats (Thakkar et al. 2018).
Conclusion
In conclusion, using drying methods, protein formulations in the presence of stabilizers produce a solid dosage form with greater stability. Cryoprotectants and lyoprotectants maintain the stability and bioactivity of biopharmaceutical APIs. Based on the route of protein administration, a proper drying method was chosen, and the process parameters were optimized. Finally, formulation optimization was performed to determine the stable protein/stabilizer combinations. In addition, physical powder characterization and possible analytical technologies were employed to evaluate protein stability. Because each characterization test has strengths and limitations, a comprehensive analysis is required to recognize the cause of instability. Regarding the ongoing trend towards addressing current defects in biopharmaceutical production, searching for new drying methods as substitutes for conventional ones, and investigating novel excipients for more efficient stabilizing effects, these products could dominate the future pharmaceutical industry.
Declarations
Conflict of interest
All authors (F. Emami, M. Keihan Shokooh, and S.J. Mostafavi Yazdi) declare that they have no conflict of interest.
Research involving in human and animal participants
This article does not contain any studies with human and animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fakhrossadat Emami, Mahsa Keihan Shokooh, Seyed Jamaleddin Mostafavi Yazdi have equally contributed to this work.
References
- Adali MB, Barresi AA, Boccardo G, Pisano R. Spray freeze-drying as a solution to continuous manufacturing of pharmaceutical products in bulk. Processes. 2020;8:709. doi: 10.3390/pr8060709. [DOI] [Google Scholar]
- Ahrer K, Buchacher A, Iberer G, Jungbauer A. Detection of aggregate formation during production of human immunoglobulin G by means of light scattering. J Chromatogr A. 2004;1043:41–46. doi: 10.1016/j.chroma.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Ahrer K, Buchacher A, Iberer G, Jungbauer A. Thermodynamic stability and formation of aggregates of human immunoglobulin G characterised by differential scanning calorimetry and dynamic light scattering. J Biochem Biophys Methods. 2006;66:73–86. doi: 10.1016/j.jbbm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Alexander AJ, Hughes DE. Monitoring of IgG antibody thermal stability by micellar electrokinetic capillary chromatography and matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 1995;67:3626–3632. doi: 10.1021/ac00116a002. [DOI] [PubMed] [Google Scholar]
- Ali ME, Lamprecht A. Spray freeze drying for dry powder inhalation of nanoparticles. Eur J Pharm Biopharm. 2014;87:510–517. doi: 10.1016/j.ejpb.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Anselmo AC, Gokarn Y, Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discovery. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- Awotwe-Otoo D, Agarabi C, Read EK, Lute S, Brorson KA, Khan MA. Product and process understanding to relate the effect of freezing method on glycation and aggregation of lyophilized monoclonal antibody formulations. Int J Pharm. 2015;490:341–350. doi: 10.1016/j.ijpharm.2015.03.056. [DOI] [PubMed] [Google Scholar]
- Bardoliwala D, Patel V, Javia A, Ghosh S, Patel A, Misra A. Nanocarriers in effective pulmonary delivery of siRNA: current approaches and challenges. Ther Deliv. 2019;10:311–332. doi: 10.4155/tde-2019-0012. [DOI] [PubMed] [Google Scholar]
- Bjelošević M, Zvonar Pobirk A, Planinšek O, Ahlin Grabnar P. Excipients in freeze-dried biopharmaceuticals: contributions toward formulation stability and lyophilisation cycle optimisation. Int J Pharm. 2020;576:119029. doi: 10.1016/j.ijpharm.2020.119029. [DOI] [PubMed] [Google Scholar]
- Butreddy A, Janga KY, Ajjarapu S, Sarabu S, Dudhipala N. Instability of therapeutic proteins—an overview of stresses, stabilization mechanisms and analytical techniques involved in Lyophilized proteins. Int J Biol Macromol. 2021;167:309–325. doi: 10.1016/j.ijbiomac.2020.11.188. [DOI] [PubMed] [Google Scholar]
- Carvalho N, Hoque ME, Oliver VL, Byrne A, Kermode M, Lambert P, Mcintosh MP, Morgan A. Cost-effectiveness of inhaled oxytocin for prevention of postpartum haemorrhage: a modelling study applied to two high burden settings. BMC Med. 2020;18:201. doi: 10.1186/s12916-020-01658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AZ, Li L, Wang SB, Lin XF, Liu YG, Zhao C, Wang GY, Zhao Z. Study of Fe 3 O 4–PLLA–PEG–PLLA magnetic microspheres based on supercritical CO 2: preparation, physicochemical characterization, and drug loading investigation. J Supercrit Fluids. 2012;67:139–148. doi: 10.1016/j.supflu.2012.04.009. [DOI] [Google Scholar]
- Chen Y, Liao Q, Chen T, Zhang Y, Yuan W, Xu J, Zhang X. Freeze-drying formulations increased the adenovirus and poxvirus vaccine storage times and antigen stabilities. Virol Sin. 2021;36:365–372. doi: 10.1007/s12250-020-00250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mutukuri TT, Wilson NE, Zhou Q. Pharmaceutical protein solids: drying technology, solid-state characterization and stability. Adv Drug Deliv Rev. 2021;172:211–233. doi: 10.1016/j.addr.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. First self-copying mRNA vaccine proves itself in pandemic trial. Science. 2022;376:446–446. doi: 10.1126/science.abq7232. [DOI] [PubMed] [Google Scholar]
- Costa C, Casimiro T, Corvo ML, Aguiar-Ricardo A. Solid dosage forms of biopharmaceuticals in drug delivery systems using sustainable strategies. Molecules. 2021;26:7653. doi: 10.3390/molecules26247653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechraksa J, Suwandecha T, Srichana T. Deposition pattern of polydisperse dry powders in andersen cascade impactor-aerodynamic assessment for inhalation experimentally and In silico. Turk J Pharm Sci. 2020;17:20. doi: 10.4274/tjps.galenos.2018.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahir RK, Al-Nima AM, Al-Bazzaz FY. Dry powder inhalers: main characteristics, classifications and novel drug delivery systems review. Lat Am J Pharm. 2021;40:34–43. [Google Scholar]
- Ding L, Tang S, Wyatt TA, Knoell DL, Oupický D. Pulmonary siRNA delivery for lung disease: review of recent progress and challenges. J Control Release. 2021;330:977–991. doi: 10.1016/j.jconrel.2020.11.005. [DOI] [PubMed] [Google Scholar]
- Duralliu A, Matejtschuk P, Stickings P, Hassall L, Tierney R, Williams DR. The influence of moisture content and temperature on the long-term storage stability of freeze-dried high concentration immunoglobulin G (IgG) Pharmaceutics. 2020;12:303. doi: 10.3390/pharmaceutics12040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli J, Esposito C, Müri M, Riniker S, Wennemers H. Influence of lipidation on the folding and stability of collagen triple helices—an experimental and theoretical study. J Am Chem Soc. 2021;143:5937–5942. doi: 10.1021/jacs.1c01512. [DOI] [PubMed] [Google Scholar]
- El-Hammadi MM, Arias JL. Recent advances in the surface functionalization of PLGA-based nanomedicines. Nanomaterials (basel) 2022;12:354. doi: 10.3390/nano12030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami F, Vatanara A, Najafabadi AR, Kim Y, Park EJ, Sardari S, Na DH. Effect of amino acids on the stability of spray freeze-dried immunoglobulin G in sugar-based matrices. Eur J Pharm Sci. 2018;119:39–48. doi: 10.1016/j.ejps.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Emami F, Vatanara A, Park EJ, Na DH. Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics. 2018;10:131. doi: 10.3390/pharmaceutics10030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami F, Vatanara A, Vakhshiteh F, Kim Y, Kim TW, Na DH. Amino acid-based stable adalimumab formulation in spray freeze-dried microparticles for pulmonary delivery. J Drug Deliv Sci Technol. 2019;54:101249. doi: 10.1016/j.jddst.2019.101249. [DOI] [Google Scholar]
- Engineers AIOC & Committee AIOCEETP . Spray dryers: a guide to performance evaluation. Wiley; 2003. [Google Scholar]
- Engstrom JD, Lai ES, Ludher BS, Chen B, Milner TE, Williams RO, Kitto GB, Johnston KP. Formation of stable submicron protein particles by thin film freezing. Pharm Res. 2008;25:1334–1346. doi: 10.1007/s11095-008-9540-4. [DOI] [PubMed] [Google Scholar]
- Entegrion I. Comparison of the efficacy & safety of resusix with FP24 in patients with acquired coagulopathy. US National Library of Medicine; 2020. [Google Scholar]
- Faghihi H, Vatanara A, Najafabadi AR, Ramezani V, Gilani K. The use of amino acids to prepare physically and conformationally stable spray-dried IgG with enhanced aerosol performance. Int J Pharm. 2014;466:163–171. doi: 10.1016/j.ijpharm.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Faghihi H, Najafabadi AR, Daman Z, Ghasemian E, Montazeri H, Vatanara A. Respiratory administration of infliximab dry powder for local suppression of inflammation. AAPS PharmSciTech. 2019;20:128. doi: 10.1208/s12249-019-1308-0. [DOI] [PubMed] [Google Scholar]
- Faghihi H, Darabi M, Mirmoeini M, Vatanara A. Formulation and evaluation of inhalable microparticles of Rizatriptan Benzoate processed by spray freeze-drying. J Drug Deliv Sci Technol. 2021;62:102356. doi: 10.1016/j.jddst.2021.102356. [DOI] [Google Scholar]
- FDA (2014) NovoSeven® RT, coagulation factor VIIa (recombinant) for intravenous use only. Lyophilized powder for solution for injection. https://www.fda.gov/media/70442/download
- FDA (2020) SEVENFACT® [coagulation factor VIIa (recombinant)-jncw] Lyophilized powder for solution, for intravenous use. https://www.fda.gov/media/136610/download.
- FDA (2021a) RYPLAZIM (plasminogen, human-tvmh) lyophilized powder for reconstitution, for intravenous use. https://www.fda.gov/media/149806/download
- FDA (2021b) INBRIJA™ (levodopa inhalation powder), for oral inhalation use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209184s000lbl.pdf
- Fernando D, Siederer S, Singh S, Schneider I, Gupta A, Powell M, Richards D, Mcintosh MP, Lambert P, Fowles S. Safety, tolerability and pharmacokinetics of single doses of oxytocin administered via an inhaled route in healthy females: randomized, single-blind, phase 1 study. EBioMedicine. 2017;22:249–255. doi: 10.1016/j.ebiom.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe V, Hawe A, Carpenter JF, Jiskoot W. Analytical approaches to assess the degradation of therapeutic proteins. TrAC, Trends Anal Chem. 2013;49:118–125. doi: 10.1016/j.trac.2013.05.005. [DOI] [Google Scholar]
- Garidel P, Pevestorf B, Bahrenburg S. Stability of buffer-free freeze-dried formulations: a feasibility study of a monoclonal antibody at high protein concentrations. Eur J Pharm Biopharm. 2015;97:125–139. doi: 10.1016/j.ejpb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech JBT. 2010;21:167. [PMC free article] [PubMed] [Google Scholar]
- Glaxosmithkline (2015) Safety, tolerability and pharmacokinetics (PK) study of oxytocin (GR121619) administered Via an inhaled route in healthy female volunteers
- Gomez M, Mccollum J, Wang H, Ordoubadi M, Jar C, Carrigy NB, Barona D, Tetreau I, Archer M, Gerhardt A. Development of a formulation platform for a spray-dried, inhalable tuberculosis vaccine candidate. Int J Pharm. 2021;593:120121. doi: 10.1016/j.ijpharm.2020.120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Tu H, Atouf F. Measurement of macro- and micro-heterogeneity of glycosylation in biopharmaceuticals: a pharmacopeia perspective. Future Sci. 2020 doi: 10.4155/fdd-2020-0021. [DOI] [Google Scholar]
- Haeuser C, Goldbach P, Huwyler J, Friess W, Allmendinger A. Impact of dextran on thermal properties, product quality attributes, and monoclonal antibody stability in freeze-dried formulations. Eur J Pharm Biopharm. 2020;147:45–56. doi: 10.1016/j.ejpb.2019.12.010. [DOI] [PubMed] [Google Scholar]
- Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 2008;25:1487–1499. doi: 10.1007/s11095-007-9516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawe A, Kasper JC, Friess W, Jiskoot W. Structural properties of monoclonal antibody aggregates induced by freeze–thawing and thermal stress. Eur J Pharm Sci. 2009;38:79–87. doi: 10.1016/j.ejps.2009.06.001. [DOI] [PubMed] [Google Scholar]
- He S, Gui J, Xiong K, Chen M, Gao H, Fu Y. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. J Nanobiotechnol. 2022;20:1–22. doi: 10.1186/s12951-022-01307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey AJ, Stewart IE. Inhaled antibodies: quality and performance considerations. Hum Vaccin Immunother. 2022;18:1940650. doi: 10.1080/21645515.2021.1940650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde DJ, Berkowitz SA. Biophysical characterization of proteins in developing biopharmaceuticals. Newnes; 2014. [Google Scholar]
- Hu Y, Ma C, Sun M, Guo C, Shen J, Wang J, Nie F, Gao B. Preparation and characterization of nano amitriptyline hydrochloride particles by spray freeze drying. Nanomedicine (lond) 2019;14:1521–1531. doi: 10.2217/nnm-2018-0116. [DOI] [PubMed] [Google Scholar]
- Hufnagel S, Xu H, Sahakijpijarn S, Moon C, Chow LQ, Williams Iii RO, Cui Z. Dry powders for inhalation containing monoclonal antibodies made by thin-film freeze-drying. Int J Pharm. 2022;618:121637. doi: 10.1016/j.ijpharm.2022.121637. [DOI] [PubMed] [Google Scholar]
- Imamura K, Ogawa T, Sakiyama T, Nakanishi K. Effects of types of sugar on the stabilization of protein in the dried state. J Pharm Sci. 2003;92:266–274. doi: 10.1002/jps.10305. [DOI] [PubMed] [Google Scholar]
- Intelligence M (2022) Biopharmaceutical market-growth, trends, COVID-19 impact, and forecast (2019–2027). https://www.mordorintelligence.com/industry-reports/global-biopharmaceuticals-market-industry.
- Ji HY, Oh SR, Lee HK, Na DH, Lee HS. Liquid chromatography–electrospray ionization tandem mass spectrometry for the quantification of styraxlignolide A in rat plasma. J Sep Sci. 2011;34:1828–1833. doi: 10.1002/jssc.201100272. [DOI] [PubMed] [Google Scholar]
- Jiskoot W, Crommelin D. Methods for structural analysis of protein pharmaceuticals. Springer Science & Business Media; 2005. [Google Scholar]
- Jocelyn P. The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur J Biochem. 1967;2:327–331. doi: 10.1111/j.1432-1033.1967.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Jovanovic N, Bouchard A, Hofland GW, Witkamp GJ, Crommelin DJ, Jiskoot W. Distinct effects of sucrose and trehalose on protein stability during supercritical fluid drying and freeze-drying. Eur J Pharm Sci. 2006;27:336–345. doi: 10.1016/j.ejps.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Jovanovic N, Bouchard A, Hofland GW, Witkamp GJ, Crommelin DJ, Jiskoot W. Stabilization of IgG by supercritical fluid drying: optimization of formulation and process parameters. Eur J Pharm Biopharm. 2008;68:183–190. doi: 10.1016/j.ejpb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jovanovic N, Bouchard A, Sutter M, Van Speybroeck M, Hofland GW, Witkamp GJ, Crommelin DJ, Jiskoot W. Stable sugar-based protein formulations by supercritical fluid drying. Int J Pharm. 2008;346:102–108. doi: 10.1016/j.ijpharm.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Kankala RK, Lin XF, Song HF, Wang SB, Yang DY, Zhang YS, Chen AZ. Supercritical fluid-assisted decoration of nanoparticles on porous microcontainers for codelivery of therapeutics and inhalation therapy of diabetes. ACS Biomater Sci Eng. 2018;4:4225–4235. doi: 10.1021/acsbiomaterials.8b00992. [DOI] [PubMed] [Google Scholar]
- Karimi M, Kamali H, Mohammadi M, Tafaghodi M. Evaluation of various techniques for production of inhalable dry powders for pulmonary delivery of peptide and protein. J Drug Deliv Sci Technol. 2022;69:103186. doi: 10.1016/j.jddst.2022.103186. [DOI] [Google Scholar]
- Keil TWM, Feldmann DP, Costabile G, Zhong Q, Da Rocha S, Merkel OM. Characterization of spray dried powders with nucleic acid-containing PEI nanoparticles. Eur J Pharm Biopharm. 2019;143:61–69. doi: 10.1016/j.ejpb.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim Biophys Acta Proteins Proteom. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Keyhan Shokouh M, Faghihi H, Darabi M, Mirmoeini M, Vatanara A. Formulation and evaluation of inhalable microparticles of Rizatriptan Benzoate processed by spray freeze-drying. J Drug Deliv Sci Technol. 2021;62:102356. doi: 10.1016/j.jddst.2021.102356. [DOI] [Google Scholar]
- Kim SJ, Kim CW. Development and characterization of sodium hyaluronate microparticle-based sustained release formulation of recombinant human growth hormone prepared by spray-drying. J Pharm Sci. 2016;105:613–622. doi: 10.1016/j.xphs.2015.11.046. [DOI] [PubMed] [Google Scholar]
- Kim NA, Lim DG, Lim JY, Kim KH, Jeong SH. Comprehensive evaluation of etanercept stability in various concentrations with biophysical assessment. Int J Pharm. 2014;460:108–118. doi: 10.1016/j.ijpharm.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Kim NA, Hada S, Thapa R, Jeong SH. Arginine as a protein stabilizer and destabilizer in liquid formulations. Int J Pharm. 2016;513:26–37. doi: 10.1016/j.ijpharm.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park EJ, Na DH. Antibody–drug conjugates for targeted anticancer drug delivery. J Pharm Investig. 2016;46:341–349. doi: 10.1007/s40005-016-0254-z. [DOI] [Google Scholar]
- Kumar V, Sharma VK, Kalonia DS. In situ precipitation and vacuum drying of interferon alpha-2a: development of a single-step process for obtaining dry, stable protein formulation. Int J Pharm. 2009;366:88–98. doi: 10.1016/j.ijpharm.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Kunde SS, Ghosh R, Wairkar S. Emerging trends in pulmonary delivery of biopharmaceuticals. Drug Discovery Today. 2022;27:1474. doi: 10.1016/j.drudis.2022.02.003. [DOI] [PubMed] [Google Scholar]
- Li HY, Song X, Seville PC. The use of sodium carboxymethylcellulose in the preparation of spray-dried proteins for pulmonary drug delivery. Eur J Pharm Sci. 2010;40:56–61. doi: 10.1016/j.ejps.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Liang W, Pan HW, Vllasaliu D, Lam JK. Pulmonary delivery of biological drugs. Pharmaceutics. 2020;12:1025. doi: 10.3390/pharmaceutics12111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YH, Brown MB, Martin GP. Investigation of the stabilisation of freeze-dried lysozyme and the physical properties of the formulations. Eur J Pharm Biopharm. 2004;58:15–24. doi: 10.1016/j.ejpb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Liao Q, Lam IC, Lin HH, Wan LT, Lo JC, Tai W, Kwok PC, Lam JK. Effect of formulation and inhaler parameters on the dispersion of spray freeze dried voriconazole particles. Int J Pharm. 2020;584:119444. doi: 10.1016/j.ijpharm.2020.119444. [DOI] [PubMed] [Google Scholar]
- Lumen Bioscience, Inc (2020) A phase 1 randomized, double-blind, placebo-controlled, dose-escalation, safety and pharmacokinetic study of LMN-101 in healthy volunteers. Clinicaltrials.gov. July 3, 2020. https://clinicaltrials.gov/ct2/show/NCT04098263
- Maa YF, Nguyen PA, Sweeney T, Shire SJ, Hsu CC. Protein inhalation powders: spray drying vs spray freeze drying. Pharm Res. 1999;16:249–254. doi: 10.1023/A:1018828425184. [DOI] [PubMed] [Google Scholar]
- Maa YF, Ameri M, Shu C, Payne LG, Chen D. Influenza vaccine powder formulation development: spray-freeze-drying and stability evaluation. J Pharm Sci. 2004;93:1912–1923. doi: 10.1002/jps.20104. [DOI] [PubMed] [Google Scholar]
- Marple VA, Roberts DL, Romay FJ, Miller NC, Truman KG, Van Oort M, Olsson B, Holroyd MJ, Mitchell JP, Hochrainer D. Next generation pharmaceutical impactor (a new impactor for pharmaceutical inhaler testing). Part I: design. J Aerosol Med. 2003;16:283–299. doi: 10.1089/089426803769017659. [DOI] [PubMed] [Google Scholar]
- Marple VA, Olson BA, Santhanakrishnan K, Roberts DL, Mitchell JP, Hudson-Curtis BL. Next generation pharmaceutical impactor: a new impactor for pharmaceutical inhaler testing. Part III. Extension of archival calibration to 15 L/min. J Aerosol Med. 2004;17:335–343. doi: 10.1089/jam.2004.17.335. [DOI] [PubMed] [Google Scholar]
- Massant J, Fleurime S, Batens M, Vanhaerents H, Van Den Mooter G. Formulating monoclonal antibodies as powders for reconstitution at high concentration using spray-drying: Trehalose/amino acid combinations as reconstitution time reducing and stability improving formulations. Eur J Pharm Biopharm. 2020;156:131–142. doi: 10.1016/j.ejpb.2020.08.019. [DOI] [PubMed] [Google Scholar]
- Mato YL. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharm. 2019;572:118813. doi: 10.1016/j.ijpharm.2019.118813. [DOI] [PubMed] [Google Scholar]
- Matthews AA, Ee PLR, Ge R. Developing inhaled protein therapeutics for lung diseases. Mol Biomed. 2020;1:11. doi: 10.1186/s43556-020-00014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcarthur AJ, Oliver VL, Lambert P, French E, Harker J, Mcintosh MP. A design of experiments approach to optimising spray drying yield and production efficiency of a model inhaled powder for global health applications. Iran J Pharm Res. 2017;16:74. [Google Scholar]
- Menzen T, Friess W. Temperature-ramped studies on the aggregation, unfolding, and interaction of a therapeutic monoclonal antibody. J Pharm Sci. 2014;103:445–455. doi: 10.1002/jps.23827. [DOI] [PubMed] [Google Scholar]
- Merkus HG. Particle size measurements: fundamentals, practice, quality. Springer Science & Business Media; 2009. [Google Scholar]
- Milani S, Faghihi H, Roulholamini Najafabadi A, Amini M, Montazeri H, Vatanara A. Hydroxypropyl beta cyclodextrin: a water-replacement agent or a surfactant upon spray freeze-drying of IgG with enhanced stability and aerosolization. Drug Dev Ind Pharm. 2020;46:403–411. doi: 10.1080/03639045.2020.1724131. [DOI] [PubMed] [Google Scholar]
- Miwata K, Okamoto H, Nakashima T, Ihara D, Horimasu Y, Masuda T, Miyamoto S, Iwamoto H, Fujitaka K, Hamada H. Intratracheal administration of siRNA dry powder targeting vascular endothelial growth factor inhibits lung tumor growth in mice. Mol Ther Nucl Acids. 2018;12:698–706. doi: 10.1016/j.omtn.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Ishibashi Y, Akita T, Yamashita C. Systemic delivery of hGhrelin derivative by lyophilizate for dry powder inhalation system in monkeys. Pharmaceutics. 2021;13:233. doi: 10.3390/pharmaceutics13020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaci L, Van Hengel AJ. Effect of heat treatment on the detection of intact bovine β-lactoglobulins by LC mass spectrometry. J Agric Food Chem. 2007;55:2985–2992. doi: 10.1021/jf063083x. [DOI] [PubMed] [Google Scholar]
- Mutukuri TT, Wilson NE, Taylor LS, Topp EM, Zhou QT. Effects of drying method and excipient on the structure and physical stability of protein solids: freeze drying vs. spray freeze drying. Int J Pharm. 2021;594:120169. doi: 10.1016/j.ijpharm.2020.120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DH, Park EJ, Kim MS, Cho CK, Woo BH, Lee HS, Lee KC. Characterization of two ricin isoforms by sodium dodecyl sulfate-capillary gel electrophoresis and capillary isoelectric focusing. Bull Korean Chem Soc. 2011;32:4253–4257. doi: 10.5012/bkcs.2011.32.12.4253. [DOI] [Google Scholar]
- National Institute of A, Infectious D, Network HIVVT, Bill, Melinda Gates F, Medical Research C, Sanofi Pasteur ASC, Glaxosmithkline (2018) A safety and immune response study of 2 experimental HIV vaccines. https://ClinicalTrials.gov/show/NCT02404311
- Newton G, Raabe O, Mokler B. Cascade impactor design and performance. J Aerosol Sci. 1977;8:339–347. doi: 10.1016/0021-8502(77)90021-0. [DOI] [Google Scholar]
- Nordisc N (2021) NovoSeven® RT, coagulation factor Viia (recombinant)for intravenous use only. Lyophilized powder for solution for injection. https://www.novosevenrt.com/?&utm_source=bing&utm_medium=cpc&utm_term=novoseven%20rt&utm_campaign=1_All_Shared_BR_Branded&utm_content=-dc_pcrid_73804982819897_pkw_novoseven%20rt_pmt_be_slid__&msclkid=d3ecfd548314163065e59feeb1ca8e40&gclid=d3ecfd548314163065e59feeb1ca8e40&gclsrc=3p.ds
- Nuchuchua O, Every HA, Hofland GW, Jiskoot W. Scalable organic solvent free supercritical fluid spray drying process for producing dry protein formulations. Eur J Pharm Biopharm. 2014;88:919–930. doi: 10.1016/j.ejpb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Nuchuchua O, Nejadnik MR, Goulooze SC, Lješković NJ, Every HA, Jiskoot W. Characterization of drug delivery particles produced by supercritical carbon dioxide technologies. J Supercrit Fluids. 2017;128:244. doi: 10.1016/j.supflu.2017.06.002. [DOI] [Google Scholar]
- O’sullivan A, Ryan KM, Padrela L. Production of biopharmaceutical dried-powders using supercritical co2 technology. J Supercrit Fluids. 2022;187:105645. doi: 10.1016/j.supflu.2022.105645. [DOI] [Google Scholar]
- O’sullivan A, Ryan KM, Padrela L. Production of biopharmaceutical dried-powders using supercritical CO2 technology. J Supercrit Fluids. 2022;187:105645. doi: 10.1016/j.supflu.2022.105645. [DOI] [Google Scholar]
- Oetjen GW. Freeze-drying. Wiley; 2004. [Google Scholar]
- Ollikainen E, Bonabi A, Nordman N, Jokinen V, Kotiaho T, Kostiainen R, Sikanen T. Rapid separation of phosphopeptides by microchip electrophoresis–electrospray ionization mass spectrometry. J Chromatogr A. 2016;1440:249–254. doi: 10.1016/j.chroma.2016.02.063. [DOI] [PubMed] [Google Scholar]
- Orth M, Kieckhefen P, Pietsch S, Heinrich S. Correlating granule surface structure morphology and process conditions in fluidized bed layering spray granulation. KONA Powder Part J. 2022;39:230. doi: 10.14356/kona.2022016. [DOI] [Google Scholar]
- Osman N, Kaneko K, Carini V, Saleem I. Carriers for the targeted delivery of aerosolized macromolecules for pulmonary pathologies. Expert Opin Drug Deliv. 2018;15:821–834. doi: 10.1080/17425247.2018.1502267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Na DH. Microchip electrophoresis for monitoring covalent attachment of poly (ethylene glycol) to proteins. Anal Biochem. 2010;400:304–306. doi: 10.1016/j.ab.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Park EJ, Kim MS, Choi YL, Shin YH, Lee HS, Na DH. Liquid chromatography–tandem mass spectrometry to determine the stability of collagen pentapeptide (KTTKS) in rat skin. J Chromatogr B. 2012;905:113–117. doi: 10.1016/j.jchromb.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Park EJ, Sun Kim M, Suk Lee H, Choon Lee K, Hee Na D. Differences in electrophoretic behavior between linear and branched PEG-conjugated proteins. Electrophoresis. 2015;36:918–923. doi: 10.1002/elps.201400539. [DOI] [PubMed] [Google Scholar]
- Park HJ, Yoon TJ, Son WS, Lee CJ, Kim SN, Song SU, Lee YW. Precipitation of VEGF from mesenchymal stem cell culture supernatant using the PCA process. J Supercrit Fluids. 2019;151:40–48. doi: 10.1016/j.supflu.2019.05.008. [DOI] [Google Scholar]
- Parray HA, Shukla S, Perween R, Khatri R, Shrivastava T, Singh V, Murugavelu P, Ahmed S, Samal S, Sharma C, Sinha S, Luthra K, Kumar R. Inhalation monoclonal antibody therapy: a new way to treat and manage respiratory infections. Appl Microbiol Biotechnol. 2021;105:6315–6332. doi: 10.1007/s00253-021-11488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JT, Faulhammer E, Dieplinger J, Dekner M, Makert C, Nieder M, Paudel A. Progress in spray-drying of protein pharmaceuticals: literature analysis of trends in formulation and process attributes. Drying Technol. 2021;39:1415–1446. doi: 10.1080/07373937.2021.1903032. [DOI] [Google Scholar]
- Poursina N, Vatanara A, Rouini MR, Gilani K, Rouholamini Najafabadi A. Systemic delivery of parathyroid hormone (1–34) using spray freeze-dried inhalable particles. Pharm Dev Technol. 2015;2015:1–7. doi: 10.3109/10837450.2015.1125924. [DOI] [PubMed] [Google Scholar]
- Prankerd RJ, Nguyen TH, Ibrahim JP, Bischof RJ, Nassta GC, Olerile LD, Russell AS, Meiser F, Parkington HC, Coleman HA, Morton DA, Mcintosh MP. Pulmonary delivery of an ultra-fine oxytocin dry powder formulation: potential for treatment of postpartum haemorrhage in developing countries. PLoS ONE. 2013;8:e82965. doi: 10.1371/journal.pone.0082965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta E, Chierici V, Flammini L, Tognolini M, Barocelli E, Cantoni AM, Dujovny G, Probst SE, Sonvico F, Colombo G. Excipient-free pulmonary insulin dry powder: pharmacokinetic and pharmacodynamics profiles in rats. J Control Release. 2020;323:412–420. doi: 10.1016/j.jconrel.2020.04.015. [DOI] [PubMed] [Google Scholar]
- Rahman A, Austin A, Begum T, Anwar I. Post-partum hemorrhage is still killing mothers in Bangladesh: inhaled oxytocin (IHO) can help. Research. 2021 doi: 10.21203/rs.3.rs-402427/v1. [DOI] [Google Scholar]
- Ramezani V, Vatanara A, Najafabadi AR, Gilani K, Nabi-Meybodi M. Screening and evaluation of variables in the formation of antibody particles by spray drying. Powder Technol. 2013;233:341–346. doi: 10.1016/j.powtec.2012.07.038. [DOI] [Google Scholar]
- Ramezani V, Vatanara A, Najafabadi AR, Shokrgozar MA, Khabiri A, Seyedabadi M. A comparative study on the physicochemical and biological stability of IgG 1 and monoclonal antibodies during spray drying process. DARU J Pharm Sci. 2014;22:1. doi: 10.1186/2008-2231-22-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana MM. Polymer-based nano-therapies to combat COVID-19 related respiratory injury: progress, prospects, and challenges. J Biomater Sci Polym Ed. 2021;32:1219–1249. doi: 10.1080/09205063.2021.1909412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AU, Busignies V, Coelho Silva Ribeiro M, Almeida Lage N, Tchoreloff P, Escriou V, Charrueau C. Fate of tableted freeze-dried siRNA lipoplexes in gastrointestinal environment. Pharmaceutics. 2021;13:1807. doi: 10.3390/pharmaceutics13111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakijpijarn S, Moon C, Koleng JJ, Christensen DJ, Williams Iii RO. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics. 2020;12:1002. doi: 10.3390/pharmaceutics12111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Grillo AO, Perkins MD, Roberts CJ. Comparative effects of pH and ionic strength on protein–protein interactions, unfolding, and aggregation for IgG1 antibodies. J Pharm Sci. 2010;99:4830–4848. doi: 10.1002/jps.22198. [DOI] [PubMed] [Google Scholar]
- Saluja V, Amorij JP, Kapteyn JC, De Boer AH, Frijlink HW, Hinrichs WL. A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. J Control Release. 2010;144:127–133. doi: 10.1016/j.jconrel.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Sandra K, Vandenheede I, Sandra P. Modern chromatographic and mass spectrometric techniques for protein biopharmaceutical characterization. J Chromatogr A. 2014;1335:81–103. doi: 10.1016/j.chroma.2013.11.057. [DOI] [PubMed] [Google Scholar]
- Sarciaux JM, Mansour S, Hageman MJ, Nail SL. Effects of buffer composition and processing conditions on aggregation of bovine IgG during freeze-drying. J Pharm Sci. 1999;88:1354–1361. doi: 10.1021/js980383n. [DOI] [PubMed] [Google Scholar]
- Schüle S, Frieß W, Bechtold-Peters K, Garidel P. Conformational analysis of protein secondary structure during spray-drying of antibody/mannitol formulations. Eur J Pharm Biopharm. 2007;65:1–9. doi: 10.1016/j.ejpb.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Shah S, Famta P, Raghuvanshi RS, Singh SB, Srivastava S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Coll Interf Sci Commun. 2022;46:100570. [Google Scholar]
- Sharma A, Khamar D, Cullen S, Hayden A, Hughes H. Innovative drying technologies for biopharmaceuticals. Int J Pharm. 2021;609:121115. doi: 10.1016/j.ijpharm.2021.121115. [DOI] [PubMed] [Google Scholar]
- Singh S, Tank NK, Dwiwedi P, Charan J, Kaur R, Sidhu P, Chugh VK. Monoclonal antibodies: a review. Curr Clin Pharmacol. 2018;13:85–99. doi: 10.2174/1574884712666170809124728. [DOI] [PubMed] [Google Scholar]
- Spink CH. Differential scanning calorimetry. Methods Cell Biol. 2008;84:115–141. doi: 10.1016/S0091-679X(07)84005-2. [DOI] [PubMed] [Google Scholar]
- Tai J, Han M, Lee D, Park IH, Lee SH, Kim TH. Different methods and formulations of drugs and vaccines for nasal administration. Pharmaceutics. 2022;14:1073. doi: 10.3390/pharmaceutics14051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Hattori Y. Effect of using amino acids in the freeze-drying of siRNA lipoplexes on gene knockdown in cells after reverse transfection. Biomed Rep. 2021;15:1–7. doi: 10.3892/br.2021.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa R, Ueda T, Abe M, Tatsumi K, Eda R, Kondoh S, Morimoto K, Tanaka T, Yamaguchi E, Takahashi A. Inhaled GM-CSF for pulmonary alveolar proteinosis. N Engl J Med. 2019;381:923–932. doi: 10.1056/NEJMoa1816216. [DOI] [PubMed] [Google Scholar]
- Thakkar SG, Warnken ZN, Alzhrani RF, Valdes SA, Aldayel AM, Xu H, Williams Iii RO, Cui Z. Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine. J Control Release. 2018;292:111–118. doi: 10.1016/j.jconrel.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Sane S, Rytting JH. Calorimetric investigation of protein/amino acid interactions in the solid state. Int J Pharm. 2006;310:175–186. doi: 10.1016/j.ijpharm.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Tuberculosis Research Centre I, Icmr-National Institute for Research in Tuberculosis CTN, All India Institute of Medical Sciences ND, National Institute for Research in Environmental Health BMP, National Institute of Occupational Health AG, King Edward Memorial H & National Institute for Implementation Research on Non-Communicable D. (2021).
- Tyagi P, Trivedi R, Pechenov S, Patel C, Revell J, Wills S, Huang Y, Rosenbaum AI, Subramony JA. Targeted oral peptide delivery using multi-unit particulates: drug and permeation enhancer layering approach. J Control Release. 2021;338:784–791. doi: 10.1016/j.jconrel.2021.09.002. [DOI] [PubMed] [Google Scholar]
- Vass P, Démuth B, Hirsch E, Nagy B, Andersen SK, Vigh T, Verreck G, Csontos I, Nagy ZK, Marosi G. Drying technology strategies for colon-targeted oral delivery of biopharmaceuticals. J Control Release. 2019;296:162–178. doi: 10.1016/j.jconrel.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Vermeer AW, Norde W. The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophys J. 2000;78:394–404. doi: 10.1016/S0006-3495(00)76602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability, and formulation. J Pharm Sci. 2007;96:1–26. doi: 10.1002/jps.20727. [DOI] [PubMed] [Google Scholar]
- Wang SH, Kirwan SM, Abraham SN, Staats HF, Hickey AJ. Stable dry powder formulation for nasal delivery of anthrax vaccine. J Pharm Sci. 2012;101:31–47. doi: 10.1002/jps.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liu YD, Cai B, Huang G, Flynn GC. Investigation of antibody disulfide reduction and re-oxidation and impact to biological activities. J Pharm Biomed Anal. 2015;102:519–528. doi: 10.1016/j.jpba.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Wang JL, Hanafy MS, Xu H, Leal J, Zhai Y, Ghosh D, Williams Iii RO, Smyth HDC, Cui Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int J Pharm. 2021;596:120215. doi: 10.1016/j.ijpharm.2021.120215. [DOI] [PubMed] [Google Scholar]
- Wanning S, Süverkrüp R, Lamprecht A. Pharmaceutical spray freeze drying. Int J Pharm. 2015;488:136–153. doi: 10.1016/j.ijpharm.2015.04.053. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Luft JC, Desimone JM. Formulation of high-performance dry powder aerosols for pulmonary protein delivery. Pharm Res. 2018;35:195. doi: 10.1007/s11095-018-2452-z. [DOI] [PubMed] [Google Scholar]
- Wu J, Wu L, Wan F, Rantanen J, Cun D, Yang M. Effect of thermal and shear stresses in the spray drying process on the stability of siRNA dry powders. Int J Pharm. 2019;566:32–39. doi: 10.1016/j.ijpharm.2019.05.019. [DOI] [PubMed] [Google Scholar]
- Wu HT, Chuang YH, Lin HC, Chien LJ. Characterization and aerosolization performance of hydroxypropyl-beta-cyclodextrin particles produced using supercritical assisted atomization. Polymers. 2021;13:2260. doi: 10.3390/polym13142260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhan Recogen Biotechnology Co L (2022) Safety, reactogenicity, and immunogenicity study of a lyophilized COVID-19 mRNA vaccine. https://ClinicalTrials.gov/show/NCT05366296
- Xu PY, Kankala RK, Pan YJ, Yuan H, Wang SB, Chen AZ. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int J Nanomed. 2018;13:4685–4698. doi: 10.2147/IJN.S169399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Kuwana A, Shibata H, Izutsu KI, Goda Y. Comparison of aerodynamic particle size distribution between a next generation impactor and a cascade impactor at a range of flow rates. AAPS PharmSciTech. 2017;18:646–653. doi: 10.1208/s12249-016-0544-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cui W, Gross ML. Mass spectrometry for the biophysical characterization of therapeutic monoclonal antibodies. FEBS Lett. 2014;588:308–317. doi: 10.1016/j.febslet.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Gong L, Zhang Y, Shen Y, Shen L, Cao L, Han G, Hu F, Zhao F, Chen Z. Room-temperature-storable chemiluminescence freeze-drying mixes for detection of SARS-CoV-2 neutralizing antibody. Dry Technol. 2021;2021:1–8. [Google Scholar]
- Zhang Y, Davis DA, Aboulfotouh K, Wang J, Williams D, Bhambhani A, Zakrewsky M, Maniruzzaman M, Cui Z, Williams RO. Novel formulations and drug delivery systems to administer biological solids. Adv Drug Deliv Rev. 2021;172:183–210. doi: 10.1016/j.addr.2021.02.011. [DOI] [PubMed] [Google Scholar]
- Zhao P, Hou X, Yan J, Du S, Xue Y, Li W, Xiang G, Dong Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact Mater. 2020;5:358–363. doi: 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Chen Z, Paul PK, Lu Y, Wu W, Qi J. Oral delivery of proteins and peptides: challenges, status quo and future perspectives. Acta Pharm Sinica B. 2021;11:2416–2448. doi: 10.1016/j.apsb.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolls S, Tantipolphan R, Wiggenhorn M, Winter G, Jiskoot W, Friess W, Hawe A. Particles in therapeutic protein formulations, Part 1: overview of analytical methods. J Pharm Sci. 2012;101:914–935. doi: 10.1002/jps.23001. [DOI] [PubMed] [Google Scholar]
- Zoulikha M, Xiao Q, Boafo GF, Sallam MA, Chen Z, He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm Sinica B. 2021;12:600. doi: 10.1016/j.apsb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]