Abstract
Background:
Human papillomavirus (HPV) vaccines were first licensed as a three-dose series. Two doses are now widely recommended in some age groups; there are data suggesting high efficacy with one dose. We updated a systematic literature review of HPV vaccine effectiveness by number of doses in observational studies.
Methods:
We searched Medline and Embase databases from January 1, 2007, through September 29, 2021. Data were extracted and summarized in a narrative synthesis. We also conducted quality assessments for bias due to selection, information, and confounding.
Results:
Overall, 35 studies were included; all except one were conducted within the context of a recommended three-dose schedule. Evaluations were in countries that used bivalent HPV vaccine (seven), quadrivalent HPV vaccine (27) or both (one). Nine evaluated effectiveness against HPV infection, ten anogenital warts, and 16 cervical abnormalities. All studies were judged to have moderate or serious risk of bias. The biases rated as serious would likely result in lower effectiveness with fewer doses. Investigators attempted to control for or stratify by potentially important variables, such as age at vaccination. Eight studies evaluated impact of buffer periods (lag time) for case counting and 10 evaluated different intervals between doses for two-dose vaccine recipients. Studies that stratified by vaccination age found higher effectiveness with younger age at vaccination, although differences were not all formally tested. Most studies found highest estimates of effectiveness with three doses; significant effectiveness was found among 28/29 studies that evaluated three doses, 19/29 that evaluated two doses, and 18/30 that evaluated one dose. Some studies that adjusted or stratified analyses by age at vaccination found similar effectiveness with three, two and one doses.
Conclusion:
Observational studies of HPV vaccine effectiveness have many biases. Studies examining persons vaccinated prior to sexual activity and using methods to reduce sources of bias are needed for valid effectiveness estimates.
Keywords: human papillomavirus, HPV, HPV vaccine, vaccine effectiveness
1.0. BACKGROUND
All currently available human papillomavirus (HPV) vaccines were originally evaluated in clinical trials, licensed and recommended as a three-dose schedule (0, 1–2 and 6 months). The high efficacy and immunogenicity observed in those trials, as well as a post hoc analysis of one clinical trial, stimulated interest in whether fewer doses would result in similar high efficacy.[1] Subsequently, immunogenicity studies designed to compare two and three doses showed non-inferior antibody response after two doses, administered 6 to 12 months apart, in young adolescent females compared with three doses in women in the age group in which efficacy was demonstrated in clinical trials.[2, 3] These data led to regulatory approval of a two-dose schedule for younger age groups. In 2014 and 2016, respectively, the World Health Organization and the U.S. Advisory Committee on Immunization Practices changed dosing recommendations to two doses for girls starting the series at age 9 through 14 years.[4, 5] Some data that raised interest in two-dose schedules also suggested that one dose might provide long lasting immunity;[6] the biologic plausibility for this has been summarized.[7] Trials were designed to rigorously evaluate the efficacy and immunogenicity of single dose HPV vaccination; results from one were reported in early 2022.[8, 9]
We previously conducted a systematic review of the literature of post-licensure observational studies to summarize evidence regarding effectiveness of HPV vaccination by number of doses.[10] In that 2018 report, there were 14 studies; most found highest effectiveness with three doses. However, there were many biases in the studies and most included mainly women who had been vaccinated during catch-up vaccination programs at an age older than the routine target age group. We updated our previous report for several reasons: 1) the number of studies examining effectiveness of HPV vaccination by number of doses has increased considerably since our first review; 2) more recent publications include girls vaccinated at a younger age, a population less likely to be affected by biases due to differences between dose groups in the likelihood of prevalent infection at the time of vaccination; and 3) we conducted an extensive quality assessment of all studies to explore the main limitations and challenges of estimating HPV vaccine effectiveness by number of doses using observational studies.
2.0. METHODS
2.1. Study selection
Studies were eligible for inclusion if they fulfilled the following criteria: 1) reported effectiveness of HPV vaccination against infection, anogenital warts, or cervical abnormalities (based on cytological or histological outcomes); 2) assessed effectiveness of HPV vaccination by number of doses received (one, two, or three). We excluded studies if vaccination was administered as part of a clinical trial (e.g., post hoc evaluations of clinical trials).
In the previously published review, we first searched Medline and Embase databases from January 1, 2007, through June 15, 2017. For this review, we updated the search through September 29, 2021. We used the same methodology in the original and updated searches, which was a combination of Medical Subject Headings (MeSH) terms, title or abstract words, without restriction on language of publications: (“papillomavirus vaccines”, “HPV vaccine”, “HPV vaccination”, “papillomavirus vaccine”, or “papillomavirus vaccination”) and (“program evaluation”, “immunization programs”, “population surveillance”, “sentinel surveillance”, “incidence”, “prevalence”, “rate”, “rates”, “effectiveness”, “doses”) and (“papillomavirus infections”, “HPV”, “uterine cervical neoplasms”, “cervical intraepithelial neoplasia”, “HPV related diseases”, “condylomata acuminata”, “genital warts”). The selection of eligible articles was performed first by NP on title and abstract, and second by NP and MD on the full-text article.
2.2. Data extraction
Three authors (NP, LM, RL) independently extracted main study characteristics and outcomes using standardized forms. Discrepancies were resolved by a fourth author (MD). The main study characteristics included country, study design, age at vaccination and at outcome assessment, sample size according to the number of doses received, case definition, and statistical analyses (procedure used to assign the number of doses and adjustment for potential confounders).
2.3. Quality assessment
Two teams that were part of this review (Université Laval and Centers for Disease Control and Prevention (CDC)) independently assessed quality; discrepancies were resolved by consensus. We assessed included studies for selection bias, information bias, and confounding based on the Risk Of Bias In Non-Randomized Studies - of Interventions (ROBINS-I). We used the same main sources of biases and ratings as the ROBINS-I, but adapted questions to consider the particularities of reduced dose observational studies. For selection bias, we examined whether selection of participants could be influenced by participants’ characteristics or outcome. For information bias, we examined potential biases in measurement of intervention (e.g., validity of data sources to determine dose groups, sufficient interval between first and second dose among two-dose recipients) and in measurement of outcome (e.g., validity of the algorithm used to identify outcomes, use of lag time or buffer period between time of vaccination and counting of outcome to exclude outcomes originating from prevalent infections at a given dose). For confounding, we examined the likelihood of differences between dose groups in: 1) prevalence of HPV infection at first dose, 2) risk of HPV acquisition during study follow-up, and 3) immunogenicity (for studies with formal comparisons between three, two and one doses). We also examined methods used to control for these potential confounders.
For each domain, possible ratings were “Low”: confident that there is little chance for bias; “Moderate”: unlikely that there is a substantial bias, but a slight bias is possible; “Serious”: significant possibility of a substantial bias; “Critical”: confident that a substantial bias exists. The risk of bias in each category was assigned based on the highest (worst) domain rating within that category. If more than one age group was used in the analyses, we rated the age group with the lowest risk of bias. Because one aim of this systematic review was to discuss study limitations, no studies were excluded on the basis of methodological quality. Quality assessment findings were compiled in a descriptive synthesis.
2.4. Data synthesis
A narrative synthesis was conducted using data reported in the publications. The main outcome was effectiveness of HPV vaccination comparing the incidence or prevalence of HPV-related endpoints between individuals vaccinated with different numbers of doses (three vs none, two vs none, one vs none, three vs two, three vs one, two vs one) of quadrivalent HPV vaccine (Gardasil, 4vHPV) or bivalent HPV vaccine (Cervarix, 2vHPV). Results are presented as crude or adjusted risk ratios (RR), hazard ratios (HR), incidence rate ratios (IRR), prevalence ratios (PR), or odds ratios (OR). Because eligible studies used different buffer periods or age groups at vaccination and at outcome assessment, heterogeneity between studies was significant. Therefore, it was not possible to pool results from the different studies.
3.0. RESULTS
3.1. Search results
In the updated search from June 16, 2017, through September 29, 2021, 3784 additional articles were identified, 116 full text articles were assessed and 21 included. Overall, there were 35 articles in the review, 14 from the initial review[11–24] and 21 from the update.[25–45] (Figure 1). The 35 articles were from 12 countries: United States (eleven), Scotland (six), Australia (four), Denmark (three), Sweden (two), Canada (two), and one each from Belgium, Denmark/Sweden, Italy, Mongolia, Netherlands, Spain, and New Zealand (Table 1, Table 2, Table S1).
Figure 1.
Flow diagram of study selection
Table 1.
Characteristics of studies that evaluated HPV vaccine effectiveness by number of doses
| Endpoint/vaccine/authors | Country | Study design | Study population | Vaccination | Case definition | Statistical analyses | Overall risk of bias assessmentb | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Age (years) at Vaccination Outcome | N by dose number | Assignment of dose number | Buffer periodsa (months) | Adjustment or stratification | ||||||
|
| ||||||||||
| Vaccine-type HPV infection | ||||||||||
|
| ||||||||||
| Quadrivalent vaccine | ||||||||||
|
| ||||||||||
| Chandler 2018 | United States | Cross-sectional study using self-reported data - men | ≤26c | 14–26 | 0: 82 1: NA 2: NA 3: NA |
HPV 6,11,16, or 18 DNA positivity in self-collected penile and perianal/anal swabsd | Final status | 0 | None | Serious (2/3) |
|
| ||||||||||
| Widdice 2019 | United States | Cross-sectional study using self-reported data - men | 16.2 wave 1 (mean); 15.1 wave 2 (mean) |
13–26 | 0: 471 1: 58 2: 37 3: 143 |
HPV 6,11,16, or 18 DNA positivity in genital and perianal/anal swabsd | Final status | 0 | Age at vaccination, sexual initiation before or after vaccination | Serious (1/3) |
|
| ||||||||||
| Sonawane 2019 | United States | Cross-sectional study of a nationally representative sample | <26b | 18–26 | 0: 1,004 1: 106 2: 126 3: 384 |
HPV 6,11,16, or 18 DNA positivity in self-collected cervicovaginal samplesd | Final status | 0 | Attained age, race/ethnicity, age at sexual debut, lifetime number of male sexual partners | Serious (2/3) |
|
| ||||||||||
| Markowitz 2020 | United States | Cross-sectional study of women enrolled in an integrated health-care delivery system | <29b | 20–29 | 0: 1,052 1: 303 2: 304 3: 2,610 |
HPV 6,11,16, or 18 DNA positivity in liquid-based cytology samplesd | Final status | 1 | Age at vaccination, screening year, race/ethnicity, attained age | Moderate (2/3) |
|
| ||||||||||
| Batmunkh 2020 | Mongolia | Cross-sectional study of women | 11–17 | 16–26 | 0: 357 1: 118 |
HPV 16 or 18 DNA positivity in self-collected swabse | Final status | 0 | Attained age at assessment, sexual behavior, education, income, employment status, tobacco and alcohol use, pregnancy | Moderate (2/3) |
|
| ||||||||||
| Bivalent vaccine | ||||||||||
|
| ||||||||||
| Kavanagh 2014 | Scotland | Cross-sectional study using screening registry data | 15–17 | 20–21 | 0: 3,418 1: 55 2: 106 3: 1,100 |
HPV 16 or 18 DNA positivity in liquid-based cytology samplesf | Final status | 0 | Birth year cohort, deprivation score | Serious (1/3) |
|
| ||||||||||
| Cuschieri 2016 | Scotland | Cross-sectional study using screening registry data with additional sampling of those with <3 doses | 15–>18 | 20–21 | 0: 3,619 1: 177 2: 300 3: 1,853 |
HPV 16 or 18 DNA positivity in liquid-based cytology samplesg | Final status | 0 | Birth year cohort, deprivation score, age at first dose | Serious (1/3) |
|
| ||||||||||
| Kavanagh 2017 | Scotland | Cross-sectional study using screening registry data | 12–>18 | 20–21 | 0: 4,008 1: 223 2: 391 3: 3,962 |
HPV 16 or 18 DNA positivity in liquid-based cytology samplesg | Final status | 0 | Age at vaccination, birth year cohort, deprivation score | Moderate (2/3) |
|
| ||||||||||
| Hoes 2021 | Netherlands | Prospective cohort study | 12–13 | 14–17 | 0: 929 2: 1098h |
HPV 16 or 18 incident DNA positivity in self-collected vaginal swabsi | Final status | 0 | Attained age, ethnicity, ever had sex, ever used contraception | Moderate (1/3) |
|
| ||||||||||
| Anogenital warts | ||||||||||
|
| ||||||||||
| Quadrivalent vaccine | ||||||||||
|
| ||||||||||
| Herweijer2014 | Sweden | Retrospective cohort study using population-based health registries | 10–19 | 10–24 | 0: 1,045,157 1: 115,197 2: 107,338 3: 89,836 |
First observed diagnosis: ICD-10 code A63.0 or podophyllotoxin / imiquimod prescription | Time-dependent Final status | 0 to 12 | Age at first vaccination, age at outcome, parental education | Serious (1/3) |
|
| ||||||||||
| Blomberg 2015 | Denmark | Retrospective cohort study using population-based health national registries | 12–27 | 12–27 | 0: 188,956 1: 55,666 2: 93,519 3: 212,549 |
First diagnosis: ICD-10 code A63.0 or podophyllotoxin prescription | Time-dependent | 1 | Attained age, age at vaccination, maternal education disposable income, calendar year | Serious (2/3) |
|
| ||||||||||
| Dominiak-Felden 2015 | Belgium | Retrospective cohort study using sick-fund/insurance reimbursement database | 10–21 | 16–23 | 0: 63,180 1: 4,020 2: 3,587 3: 35,792 |
First prescription of imiquimod and reimbursement | Time-dependent | 1 | Age at first dose | Serious (2/3) |
|
| ||||||||||
| Perkins 2017 | United States | Retrospective cohort study using commercial claims database | 9–25 | 9–25 | 0: 201,933 1: 30,438 2: 36,583 3: 118,962 |
ICD-9 and CPT codes and prescriptionsj | Final status | 0, 12 | Age at start of exposure period, regions, SES indicators, calendar year, differential observation periods | Serious (1/3) |
|
| ||||||||||
| Navarro-Illana 2017 | Spain | Retrospective cohort study using national registries | 14 | 14–19 | 0: 607,006 1: 18,142 2: 31,420 3: 153,296 (person-yrs) |
First diagnosis of ICD-9-CM code 078.11 | Time-dependent | 0 | Attained age (time varying), calendar year, health department | Serious (1/3) |
|
| ||||||||||
| Lamb 2017 | Sweden | Retrospective cohort study using national registries | 10–19 | 10–27 | 2: 79,042 3: 185,456 |
First diagnosis of ICD-10 code A63.0 or podophyllotoxin / imiquimod prescription | Time-dependent | 0 | Attained age at outcome, age at vaccination time between doses | Serious (1/3) |
|
| ||||||||||
| Hariri 2018 | United States | Retrospective cohort study in integrated health-care delivery systems | 16–17 (mean) | 11–28 | 0: 31,563 1: 5,864 2: 5,459 3: 21,631 |
ICD-9 code (078.10, 078.11, 078.19), specialty of diagnosing provider, and STI tests ordered | Final status | 6 from last dose 12 from first dose | Race/ethnicity, health plan, age at enrollment in health plan, age at beginning of study period, evidence of sexual activity (as defined by composite measure), age at first evidence of sexual activity, age at first dose, continuous enrollment indicator, months enrolled in health plan, Medicaid enrollment | Moderate (3/3) |
|
| ||||||||||
| Zeybek 2018 | United States | Matched retrospective cohort study using health insurance claims databases (males and females) | 9–26 | 10–31 | 0: 286,963 1: 54,280 2: 55,632 3: 177,051 |
ICD-9-CM or 10 code 078.11 or A63.0 | Final status | 3 | Age group (based on age at last dose) sex, region of residence, history of STDs, enrollment history. | Serious (1/3) |
|
| ||||||||||
| Willows 2018 | Canada | Matched retrospective cohort study using linked vaccine registry and claims and population-based databases | 9–26 | 10–33 | 0: 94,327 1: 3,521 2: 6,666 3: 21,277 |
ICD-9-CM or 10 code 078.11 or A63.0 and related procedure code | Final status | 0 | Age at vaccination, place of residence, area-level income, birth date, previous hospitalizations and physician visits, history of chronic diseases, sexual activity (based on evidence using a composite measure) | Serious (2/3) |
|
| ||||||||||
| Baandrup, 2021 | Denmark | Retrospective cohort study using population-based health national registries | 12–30 | 12–30 | 0: 1,904,895 1: 235,653 2: 460,978 3: 1,934,589 (person-yrs) |
First diagnosis: ICD-10 code A63.0 or podophyllotoxin prescription | Time-dependent | 1 | Attained age, age at vaccination, maternal education, calendar time | Serious (1/3) |
|
| ||||||||||
| Cervical abnormalities | ||||||||||
|
| ||||||||||
| Quadrivalent vaccine | ||||||||||
|
| ||||||||||
| Gertig 2013 | Australia | Retrospective cohort study using linked data from registries | 12–19 | 12–21 | 0: 14,085 1: 1,422 2: 2,268 3: 21,151 |
Histology: CIN3/AIS, CIN2, CIN1, any high grade Cytology: low grade and high grade | Time-dependent Final status | 0 | Age at first screen, remoteness area, SES | Serious (2/3) |
|
| ||||||||||
| Crowe 2014 | Australia | Case control study using linked data from registries | 12–26 | 11–31 | 0: 60,282 1: 10,879 2: 12,073 3: 25,119 |
Histology: CIN2+/AIS | Final status | 0, 1, 6, 12 | Year of birth, remoteness area, SES, follow-up time | Serious (2/3) |
|
| ||||||||||
| Brotherton 2015 | Australia | Retrospective cohort study using linked regional data registries | 12–26 | 12–30 | 0: 133,055 1: 20,659 2: 27,500 3: 108,264 |
Histology: CIN3/AIS, CIN2, any high grade Cytology: low grade and high grade | Final status | 0, 1, 6, 12, 24 | Age in 2007, remoteness, SES, screening start (before or after vaccination) | Serious (1/3) |
|
| ||||||||||
| Hofstetter 2016 | United States | Retrospective cohort study using medical center records | 11–20 | 11–27 | 0: 1,632 1: 695 2: 604 3: 1,196 |
Cytology: any abnormal and high gradej | Final status | 1 | Age at vaccination initiation or first missed opportunity for vaccination for unvaccinated, insurance, language, clinic type, CT screening, and baseline cytology | Serious (2/3) |
|
| ||||||||||
| Kim 2016 | Canada | Nested case-control study using linked data from registries | 10–15 | 18–21 | 0: 5,712 1: 327 2: 490 3: 3,675 |
Cytology: low grade and high gradek | Final status | 0 | Attained age, urban/rural, laboratory site, neighborhood income | Serious (2/3) |
|
| ||||||||||
| Silverberg 2018 | United States | Nested case-control study of women enrolled in an integrated health-care delivery system | 14–26 | 18–34 | 0: 23,293 1: 756 2: 554 3: 1,527 |
Histology: CIN2+/AIS | Final status | 6 | Smoking, parity, recent outpatient visits, race/ethnicity, STDs, hormonal contraceptives, immunosuppression | Serious (2/3) |
|
| ||||||||||
| Dehlendorff 2018 | Denmark& Sweden | Retrospective cohort study using linked national registry data | 13–29 | 13–30 | 0: 2,091,579 1: NA 2: NA 3: NA |
Histology: CIN2+/AIS | Time-dependent | 0 | Attained age, age at vaccination, maternal education | Serious (1/3) |
|
| ||||||||||
| Brotherton 2019 | Australia | Retrospective cohort study using linked regional data registries | ≤13–22 | 15–22 | 0: 48,845 1: 8,618 2: 18,190 3: 174, 995 |
Histology: CIN2+CIN3+ | Final status (time-varying as a sensitivity analysis) | 0, 12, 24 | Birth cohort, age at study entry, area of residence, socioeconomic status, attained age (time varying) | Serious (1/3) |
|
| ||||||||||
| Verdoodt 2020 | Denmark | Retrospective cohort study using linked national registry data | 12–16 | 17–25 | 0: 374,327 1: 10,480 2: 30,259 3: 174,532 |
Histology: CIN2+CIN3+ | Time-dependent (final status for the comparison between doses) | 0 6 in secondary analysis | Attained age, maternal education | Serious (1/3) |
|
| ||||||||||
| Johnson Gargano 2020 | United States | Case control study using medical records data from 5 US sites; test negative design | 12–26 | 18–39 | 0: 2,731 1: 136 2: 108 3: 325 |
Histology: HPV type-specific CIN2+ | Final status | 1, 12, 24, 36 | Birth cohort, geographic site, race/ethnicity, insurance status, age at vaccination | Moderate (3/3) |
|
| ||||||||||
| Rodriguez 2020 | United States | Retrospective matched cohort study using health insurance claims database | 9–26 | 9–31 | 0: 66,541 1: 13,630 2: 14,088 3: 38,823 |
Histology: CIN2/3 Cytology: HSIL/ASC-H | Final status | 12 | Age at vaccination, region, history of STDs and pregnancy, length of enrollment, history and results of pap test, US census region, age at beginning of follow-up | Serious (1/3) |
|
| ||||||||||
| Innes 2020 | New Zealand | Retrospective cohort study using linked national registry data | 14–21 | 20–24 | 0: 47,283 1 or 2: 8,317 3: 48,713 |
Histology: CIN1 CIN2+ | Final status | 0 | Age at first dose, birth year cohort | Serious (1/3) |
|
| ||||||||||
| Bivalent vaccine | ||||||||||
|
| ||||||||||
| Pollock 2014 | Scotland | Retrospective cohort study using linked national registry data | 15->18 | 20–21 | 0: 76,114 1: 1,315 2: 2,725 3: 25,898 |
Histology: CIN1, CIN2, CIN3 | Final status | 0 | Attained age, birth year cohort year, deprivation score | Serious (2/3) |
|
| ||||||||||
| Cameron 2017 | Scotland | Retrospective cohort study using linked national registry data | 14->18 | 20–21 | 0: 75,683 1: 2,258 2: 4,462 3: 55,303 |
Histology: CIN1, CIN2, CIN3 | Final status | 0 | Deprivation score, birth year cohort | Serious (2/3) |
|
| ||||||||||
| Palmer 2019 | Scotland | Retrospective cohort study using linked national registry data | 12->18 | 20 | 0: 64,026 1: 2,051 2: 4,135 3: 68,480 |
Histology: CIN1, CIN2, CIN3 Cytology: Low grade, moderate grade, severe grade | Final status | 0 | Age at vaccination, deprivation score, rurality | Serious (2/3) |
|
| ||||||||||
| Acuti Martellucci 2021 l | Italy | Retrospective cohort study using administrative data | 14->30 | 17–32 | 0: 7,394 1: 212 2: 83 3: 96 |
Cytology: Any abnormal cytology, low and high grade | Final status | 1, 6, 12 | Year of birth, residential area, country of birth, screening test kit, number of screens | Serious (2/3) |
Abbreviations: CT, chlamydia trachomatis; SES, socioeconomic status, STD, sexually transmitted disease or infection; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2 or worse; AIS, adenocarcinoma in situ; ICD-9, International Classification of Disease, ninth revision; ICD-10, International Classification of Disease, tenth revision; NA, not available
Note: Crowe et al (2014) reported on an additional outcome defined using cytology and histology data for classification, results are not included in this paper
Buffer period is the lag time between vaccination and counting of outcomes.
Overall risk of bias assessment considers 3 categories: selection, information bias and confounding (ratings are low, moderate, serious, critical) and is based on the worst rating. If different objectives have different overall assessments, this table includes the bias rating for 1 vs 0 doses when available. In parentheses is number of categories of bias (out of 3) with the worst rating. More information is provided in supplementary material.
not explicitly stated in paper.
By Roche Linear Array assay detecting 37 types.
By Xpert HPV assay and Anypex II detecting 28 types.
By multimetrix HPV assay detecting 24 types.
By Optiplex HPV assay detecting 24 types.
Numbers in first study year;
By HPV-LIPA25 detecting 25 types.
Three possible scenarios: a) ≥ 1 diagnosis of ICD-9 code 078.1; b) ≥ 1 diagnosis of ICD-9 code 078.1, 078.10, 078.19 plus destruction/excision procedure or ICD-9 code 211.4, 216.5, 221.8, 222.9; c) ≥ 1 prescription for anogenital warts plus destruction/excision procedure or ICD-9 code 211.4, 216.5, 221.8, 222.9.
Low-grade cytology defined as atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. High-grade cytology defined as atypical squamous cells, cannot rule out a high-grade lesion, or high-grade squamous intraepithelial lesion.
Either bivalent or quadrivalent HPV vaccine.
Table 2.
Studies that evaluated HPV vaccine effectiveness by number of doses: analyses and main findings
| Endpoint/vaccine/authors | Study population age (years) at vaccination and outcome | Buffera (months) | Sensitivity analyses by age group/ buffer/dose intervalb | Comparison with unvaccinated | Formal comparison between doses | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Effect (95% CI) | Comments | ||||||
|
| |||||||
| Vaccine-type HPV infection | |||||||
|
| |||||||
| Quadrivalent vaccine | |||||||
|
| |||||||
| Chandler 2018 | ≤26c | 14–26 | 0 | No/No/No | No significant effectiveness for ≥1 dose | 1 vs 3 doses OR = 0.99 (0.33–2.96) 2 vs 3 doses OR = 0.60 (0.17–2.12) |
|
|
| |||||||
| Widdice 2019 | 16.2 wave 1 (mean) 15.1 wave 2 (mean) |
13–26 | 0 | Yes/No/No | No significant effectiveness for ≥1 dose | • Similar results for the analysis restricted to men vaccinated at ≥15 years and men vaccinated before sexual initiation, and men vaccinated after sexual initiation | Number of doses (0,1,2,3) not associated with ≥1 vaccine-type HPV or HPV16 and/or 18 |
|
| |||||||
| Sonawane 2019 | ≤26c | 18–26 | 0 | No/No/No | Difference in predicted probability 3: aPD = −4.3 (−4.6, −4.0) 2: aPD = −1.7 (−2.4, −0.1) 1: aPD = −5.0 (−5.6, −4.5) |
1 vs 3 doses p-value = 0.70 2 vs 3 doses p-value = 0.40 1 vs 2 doses p-value = 0.12 |
|
|
| |||||||
| Markowitz 2020 | ≤29c | 20–29 | 1 | Yes/No/No | Overall results 3: aPR = 0.17 (0.11–0.26) 2: aPR = 0.15 (0.05–0.47) 1: aPR = 0.25 (0.10–0.62) Results for those with first dose at age ≤18 years 3: aPR = 0.08 (0.04–0.15) 2: aPR = 0.07 (0.01–0.47) 1: aPR = 0.08 (0.01–0.54) |
• Similar results for unadjusted analyses and controlling for race/ethnicity and age at screening | 3 vs 1 dose PR = 1.06 (0.14–8.09) 3 vs 2 doses PR = 1.17 (0.15–8.96) 2 vs 1 dose PR = 0.90 (0.06–14.36) |
|
| |||||||
| Batmunkh 2020 | 11–17 | 16–26 | 0 | No/No/No | 1: aPR = 0.08 (0.01–0.56) | • Adjusted for income and employment status | No |
|
| |||||||
| Bivalent vaccine | |||||||
|
| |||||||
| Kavanagh 2014 | 15–17 | 20–21 | 0 | Yes/No/No | 3: aOR = 0.43 (0.34–0.55) 2: aOR = 0.68 (0.42–1.12) 1: aOR = 0.95 (0.51–1.76) |
• Differences by number of doses still observed when stratified by age at vaccination | No |
|
| |||||||
| Cuschieri 2016 | 15–17 | 20–21 | 0 | No/No/No | 3: aOR = 0.27 (0.20–0.36) 2: aOR = 0.45 (0.29–0.69) 1: aOR = 0.52 (0.31–0.83) |
No | |
|
| |||||||
| Kavanagh 2017 | 12->18 | 20–21 | 0 | Yes/No/No | 3: aOR = 0.40 (0.33–0.48) 2: aOR = 0.75 (0.57–0.99) 1: aOR = 0.89 (0.63–1.25) |
• When stratified by age at first dose, 3-dose effectivness was highest in the youngest group and lower with age, but all were significant (range: 28.9%–89.1%) | No |
|
| |||||||
| Hoes 2021 | 12–13 | 14–17 | 0 | No/No/No | 2: aHR = 0.16 (0.035–0.73) | • Study conducted when routine 2-dose vaccination program recommended | No |
|
| |||||||
| Anogenital warts | |||||||
|
| |||||||
| Quadrivalent vaccine | |||||||
|
| |||||||
| Herweijer 2014 | 10–19 | 10–24 | 3 | Yes/Yes/No | 3: aIRR = 0.20 (0.17–0.23) 2: aIRR = 0.32 (0.26–0.40) 1: aIRR = 0.54 (0.43–0.68) |
• Similar results for age groups 10–16 and 17–19 • Similar results for buffers of 0–12 months, except effectiveness for 1 dose was not significant among those vaccinated at 17–19 using buffers of 0 and 1 month(s) |
3 vs 1 dose aIRR = 0.37 (0.28–0.48) 3 vs 2 doses aIRR = 0.63 (0.48–0.82) 2 vs 1 dose aIRR = 0.59 (0.43–0.81) • With buffer periods >4 months, no significant difference between 3 and 2 doses |
|
| |||||||
| Blomberg 2015 | 12–27 | 12–27 | 1 | Yes/No/Yes | 1: IRR = 0.51 (0.46–0.56) | 3 vs 2 doses IRR = 0.46 (0.39–0.54) 2 vs 1 doses IRR = 0.44 (0.37–0.51) • With dose interval >4 months, no significant difference for 3 vs 2 doses • Similar results when stratified by age at vaccination |
|
|
| |||||||
| Dominiak-Felden 2015 | 10–21 | 16–23 | 1 | Yes/No/No | 3: aIRR = 0.12 (0.07– 0.21) 2: aIRR = 0.34 (0.14–0.83) 1: aIRR = 0.63 (0.35–1.16) |
• 3 dose effectiveness estimates were higher for those vaccinated at age <15 and 15–17 years than ≥18 years • 3 dose effectiveness estimates higher with buffers >1 year |
No |
|
| |||||||
| Perkins 2017 | 9–25 | 9–25 | 0 | No/Yes/Yes | 3: aIRR = 0.53 (0.46–0.60) | 3 vs 1 doses aIRR = 0.82 (0.71–0.95) 3 vs 2 doses aIRR = 0.89 (0.78–1.03) • With 1 yr buffer period, no change in findings (data not shown) • Similar results with interval >5 months for 2 doses |
|
|
| |||||||
| Navarro-Illana 2017 | 14 | 14–19 | 0 | No/No/No | 3: aRR = 0.24 (0.15–0.34) 2: aRR = 0.36 (0.14–0.68) 1: aRR = 0.39 (0.13–0.80) |
No | |
|
| |||||||
| Lamb 2017 | 10–19 | 10–27 | 0 | Yes/No/Yes | No analyses of 3, 2 or 1 doses compared to 0 | • Higher effectiveness of 3 vs 2 doses, when 1st and 2nd doses administered 0–3 or >8 months apart but not 4–7 months • Similar results stratified by age at vaccination |
|
|
| |||||||
| Hariri 2018 | 16–17 (mean) | 11–28 | 6 from last dose 12 from first dose |
No/Yes/Yes | 6 month buffer from last dose 3: aHR = 0.23 (0.17–0.31) 2d: aHR = 0.32 (0.17–0.59) 1: aHR = 0.81 (0.60–1.08) 12 month buffer from first dose 3: aHR = 0.20 (0.15–0.27) 2d: aHR = 0.24 (0.13– 0.44) 1: aHR = 0.32 (0.20–0.52) |
6 month buffer from last dose 3 vs 1 dose aHR = 0.29 (0.20–0.42) 3 vs 2d doses aHR = 0.74 (0.38–1.43) 2d vs 1 dose aHR = 0.39 (0.20–0.76) 12 month buffer from first dose 3 vs 1 dose aHR = 0.63 (0.37–1.09) 2 vs 1 dose aHR = 0.74 (0.35–1.60) |
|
|
| |||||||
| Zeybek 2018 | 9–26 | 10–31 | 3 from last dose | Yes/No/Yes | Results for 15–19 yr-olds 3: aRR = 0.58 (0.49–0.70) 2: aRR = 0.67 (0.51–0.89) 1: aRR = 0.65 (0.49–0.85) |
• No significant effect in older or younger age groups • Similar results with 2-dose interval <6 or ≥6 months |
• No significant differences for 3 vs 1, 3 vs 2, or 2 vs 1 doses (only p-values reported) |
|
| |||||||
| Willows 2018 | 9–26 | 10–33 | 0 | Yes/No/No | Results for those vaccinated at age 9–18 years 3: aHR = 0.4 (0.3–0.7) 2: aHR = 1.4 (0.6–3.3) 1: aHR = 0.6 (0.2–1.8) |
• No significant effect in those vaccinated at older ages | No |
|
| |||||||
| Baandrup 2021 | 12–30 | 12–30 | 1 | Yes/No/No | Results for first dose at age 12–14 years 3: aIRR = 0.16 (0.15–0.18) 2: aIRR = 0.22 (0.18–0.26) 1: aIRR = 0.29 (0.22–0.38) |
• Results presented for 4 age at vaccination groups; in oldest age, ≥19 years, significant effect only with 3 doses • Significant but lower effectiveness for 3, 2 and 1 doses in 15–16 and 17–18 age groups than 12–14 years |
Results for first dose at age 12–14 years 3 vs 1 dose: aIRR = 0.56 (0.43–0.73) 2 vs 1 dose: aIRR = 0.76 (0.56–1.03) |
|
| |||||||
| Cervical abnormalities e | |||||||
|
| |||||||
| Quadrivalent vaccine | |||||||
|
| |||||||
| Gertig 2013 | 12–19 | 12–21 | 0 | No/No/No | Outcome summarized: CIN2+ 3: aHR = 0.61 (0.48–0.78) 2: aHR = 1.02 (0.68–1.53) 1: aHR = 1.47 (0.97–2.23) Outcome summarized: CIN3/AIS 3: aHR = 0.53 (0.36–0 .77) 2: aHR = 0.87 (0.46–1.67) 1: aHR = 1.40 (0.75–2.61) |
• Similar results for CIN2 as an outcome | No |
|
| |||||||
| Crowe 2014 | 12–26 | 11–31 | 0 | Yes/Yes/No | Outcome summarized: high grade histological lesions 3: aOR = 0.54 (0.43–0.67) 2: aOR = 0.79 (0.64–0.98) 1: aOR = 0.95 (0.77–1.16) |
• Buffer periods from 1 to 12 months - no consistent impact on estimates • Similar results among those vaccinated at ages 15–18 and 19–22 years |
No |
|
| |||||||
| Brotherton 2015 | 12–26 | 12–30 | 0 | Yes/Yes/Yes | Results for those vaccinated prior to screening Outcome summarized: CIN2+ 3: aHR = 0.71 (0.64–0.80) 2: aHR = 1.21 (1.02–1.44) 1: aHR = 1.19 (0.99–1.43) Outcome summarized: CIN3/AIS 3: aHR = 0.69 (0.58– 0.81) 2: aHR = 1.17 (0.92–1.48) 1: aHR = 1.41 (1.12–1.77) |
• Similar results for those vaccinated after screening, stratified by age at vaccination • With longer buffer periods, some effectiveness for 2 and 1 doses • No difference by interval between 2 doses |
No |
|
| |||||||
| Hofstetter 2016 | 11–20 | 11–27 | 1 | Yes/No/Yes | Outcome summarized: any abnormal cytology 3: aHR = 0.58 (0.48–0.69) 2: aHR = 0.81 (0.66–0.99) 1: aHR = 1.05 (0.88–1.26) |
• Similar results when stratified by age at vaccination, although 2 doses not always significant • Highest effectiveness, although only significant for 3 doses, for those vaccinated at ages 11–14 years |
No |
|
| |||||||
| Kim 2016 | 10–15 | 18–21 | 0 | No/No/No | Outcome summarized: high grade cytology 3: aOR = 0.48 (0.28–0.81) 2: aOR = 0.17 (0.02–1.20) 1: aOR = 0.45 (0.11–1.83) |
No | |
|
| |||||||
| Silverberg 2018 | 14–26 | 18–34 | 6 | Yes/No/No | Outcome summarized: CIN2+/AIS 3: aRR = 0.78 (0.66–0.91) 2: aRR = 1.02 (0.82–1.28) 1: aRR = 0.89 (0.73–1.09) Outcome summarized: CIN3+/AIS 3: aRR = 0.64 (0.48–0.84) 2: aRR = 0.97 (0.67–1.41) 1: aRR = 0.90 (0.65–1.24) |
• Highest 3 dose effectiveness among those vaccinated at youngest ages | No |
|
| |||||||
| Dehlendorff 2018 | 13–29 | 13–30 | 0 | Yes/No/Yes | Outcome summarized: CIN2+/AIS (age ≤16 years) 3: aIRR = 0.23 (0.11–0.49) 2: aIRR = 0.44 (0.10–2.03) 1: aIRR = 0.23 (0.01–5.24) |
• Similar results for age at vaccination 1719 years | 2 vs 3 doses aIRR = 1.60 (1.05–2.24) • No significant difference between 2 and 3 doses when interval between dose 1 and 2 >5 months and age at vaccination <20 years |
|
| |||||||
| Brotherton 2019 | ≤13–22 | 15–22 | 0 | Yes/No/Yes | Outcome summarized: CIN2+ 3: aHR = 0.59 (0.54–0.65) 2: aHR = 0.61 (0.52–0.72) 1: aHR = 0.65 (0.52–0.81) Outcome summarized: CIN3+/AIS 3: aHR = 0.43 (0.35–0.53) 2: aHR = 0.42 (0.27–0.64) 1: aHR = 0.66 (0.41–1.06) |
• Similar results for time-varying dose status, CIN3+, buffers of 0 and 12 months, and alternate status based on timing between 1 and 2 doses | Outcome summarized: CIN2+ 3 vs 1 doses aHR = 0.91 (0.74–1.13) 3 vs 2 doses aHR = 0.97 (0.83–1.14) 2 vs 1 doses aHR = 0.94 (0.73–1.21) Outcome summarized: CIN3+/AIS 3 vs 1 doses aHR = 0.66 (0.41–1.05) 3 vs 2 doses aHR = 1.04 (0.68–1.57) 2 vs 1 doses aHR = 0.64 (0.35–1.16) |
|
| |||||||
| Verdoodt 2020 | 12–16 | 17–25 | 0 (6 months for compari son betwee n doses) | Yes/No/No | Outcome summarized: CIN2+/AIS 3: aIRR = 0.43 (0.36–0.51) 2: aIRR = 0.49 (0.32–0.76) 1: aIRR = 0.34 (0.13–0.87) Outcome summarized: CIN3+/AIS 3: aIRR = 0.37 (0.30–0.45) 2: aIRR = 0.38 (0.22–0.66) 1: aIRR = 0.38 (0.14–0.98) |
• Similar results by age at vaccination, but only significant for <23 year-olds | Outcome summarized: CIN2+/AIS 3 vs 1 dose aIRR = 0.99 (0.64–1.53) 2 vs 1 doses aIRR = 1.00 (0.61–1.64) Outcome summarized: CIN3+/AIS 3 vs 1 dose aIRR = 0.95 (0.60–1.51) 2 vs 1 doses aIRR = 0.89 (0.53–1.52) |
|
| |||||||
| Johnson Gargano 2020 | 12–26 | 18–39 | 24 | Yes/Yes/No | Outcome summarized: CIN2+/AIS 3: aOR = 0.26 (0.20–0.35) 2: aOR = 0.45 (0.30–0.69) 1: aOR = 0.53 (0.37–0.76) |
• aORs were slightly higher using 1 and 12 month buffer periods and lower using a 36 month buffer period, but all showed significant effectiveness • Effectiveness was higher in earlier birth cohort and lower in later birth cohort |
3 vs 1 dose aOR = 0.61 (0.38–0.99) 3 vs 2 dose: aOR = 0.64 (0.39–1.05) 2 vs 1 dose aOR = 0.96 (0.55–1.68) |
|
| |||||||
| Rodriguez 2020 | 9–26 | 9–31 | 12 | Yes/No/Yes | Outcome summarized: CIN2/3 Age at first dose 15–19 years 3: aHR = 0.66 (0.55–0.80) 2: aHR = 0.72 (0.54–0.95) 1: aHR = 0.64 (0.47–0.88) |
• Study underpowered for <15 year-olds • No vaccine effectiveness against high-grade cytology or against CIN2/3 for those who received first dose at age >20 years |
No |
|
| |||||||
| Innes 2020 | 14–21 | 20–24 | 0 | Yes/No/No | Outcome summarized: high-grade histology (≥1 dose <18 years) 3: IRR = 0.66 (0.60–0.72) 2: IRR = 0.81 (0.63–1.03) 1: IRR = 1.10 (0.85–1.45) |
• No significant effectiveness against high-grade histology for ≥1 dose among women vaccinated at ≥18 years | No |
|
| |||||||
| Bivalent vaccine | |||||||
|
| |||||||
| Pollock 2014 | 15->18 | 20–21 | 0 | No/No/No | Outcome summarized: CIN3 3: aOR = 0.45 (0.35–0.58) 2: aOR = 0.77 (0.49–1.21) 1: aOR = 1.42 (0.89–2.28) |
No | |
|
| |||||||
| Cameron 2017 | 14->18 | 20–21 | 0 | No/No/No | Outcome summarized: CIN3 Significant effect only with 3 doses |
• Vaccinated in each deprivation category, compared with unvaccinated in most deprived | No |
|
| |||||||
| Palmer 2019 | 12–>18 | 20 | 0 | Yes/No/No | Outcome summarized: CIN3+ 2: aOR = 0.77 (0.48–1.24) 1: aOR = 1.19 (0.70–2.05) |
• Effect of 3-dose vaccination larger with younger age at vaccination ranging from 0.14 to 0.85 | No |
|
| |||||||
| Acuti Martellucci 2021 f | 14–>30 | 17–32 | 1,6,12 | Yes/Yes/No | Outcome summarized: Any abnormal cytology, youngest birth cohort (1990–1993), 1-month buffer duration 3: aOR = 0.44 (0.14–1.43) 2: aOR = 0.65 (0.20–2.16) 1: aOR = 0.43 (0.17–1.05) |
• Sensitivity analyses also for vaccine type (both bivalent and quadrivalent used), high and low grade cytology, and buffer duration | No |
Abbreviations: IRR, incidence rate ratio; aIRR, adjusted incidence rate ratio; RR, relative risk; aRR, adjusted RR; aOR, adjusted odds ratio; HR, hazard ratio; CI, confidence intervals; CIN3, cervical intraepithelial neoplasia grade 3; CIN2+, CIN grade 2 or worse; AIS, adenocarcinoma in situ; Significant, 95% CI does not include 1.
Buffer period is the lag time between vaccination and counting of outcomes. This column shows buffer period in main analysis.
Interval between doses for 2-dose vaccine recipients.
Not explicitly stated in paper.
Data presented for 2 doses are those with an interval ≥6 months between doses.
Some articles presented several outcomes for cervical cytological or histological abnormalities. In this table, we summarize results for the outcome most proximal to cervical cancer.
Either bivalent or quadrivalent vaccine.
All studies except one were conducted within the context of a recommended three-dose schedule. Evaluations were in countries that used 2vHPV (seven), 4vHPV (27) or both (one). Nine studies evaluated effectiveness for prevention of prevalent (detected at a single time point) or incident vaccine-type (4vHPV or 2vHPV) HPV infection,[11, 12, 25–31] ten anogenital warts,[13–18, 32–35] and 16 cervical cytological or histological abnormalities[19–24, 36–45].
3.2. Quality assessment
All 35 studies were determined to be at moderate or serious risk of bias (Table 1, Tables S2-S5, Figures S1–S6). No study had a domain rated as critical. Comparisons involving one and two doses were more likely to be affected by serious biases than three doses. Of seven potential sources of biases, three were more likely to be rated at serious risk of bias. These serious biases fell in two broad categories, i.e., information bias and confounding; each are likely to underestimate the effectiveness of one and two doses.
First, the majority of studies were deemed at moderate or serious risk of information bias for measurement of outcome (Figures S1, S4–S6). This is because prevalent infections at a given dose could cause an attribution of outcome to the wrong dose if outcomes are detected after a given dose but originate from an infection acquired before that dose. Studies using buffer periods to exclude outcomes originating from prevalent infections (≥12 months for infection, ≥six months for anogenital warts, ≥24 months for cervical abnormalities)[46–48] or restricting analyses to girls vaccinated at an age when they were less likely to have prevalent infections were deemed at lower risk of information bias. Of note, while buffer periods decrease the likelihood that a prevalent infection at the time of a given vaccination was responsible for the outcome detected, they do not guarantee this.
Second, studies examining two-dose effectiveness were deemed at serious risk of information bias in measurement of intervention if the interval between the two doses was less than five months (Figures S2, S4–S6).[2–4] Because the majority of studies were conducted when a three-dose schedule was recommended, the interval between the first and second dose was often only one or two months; longer intervals were found when individuals were late for the second dose in a recommended three-dose series.
Third, the majority of studies were at moderate or serious risk of confounding as a result of differences between dose groups in prevalence of HPV infection at first dose or start of follow-up and/or in risk of HPV acquisition during follow-up (Figures S3–S6). The use of buffer periods or restriction of analysis to younger age groups could control for differences in prevalent infection at first dose between dose groups. More recent studies tend to include individuals vaccinated at a younger age and were less likely to be affected by biases related to prevalent infections at vaccination. Few studies were able to control for the potential difference in risk of HPV acquisition between dose groups by adjusting for sexual activity during follow-up (Tables S3–S5).
3.3. HPV infection
In the original review, two studies reported vaccine effectiveness for prevention of prevalent vaccine-type infection, both from Scotland where 2vHPV was introduced.[11, 12] In the updated review, seven additional studies were identified, two from countries were 2vHPV was used (Scotland and Netherlands[30, 31]), and five from countries where 4vHPV was used (four from the United States[25–28] and one from Mongolia[29]) (Table 1). In five of the nine studies, three-, two- and one-dose vaccine recipients were compared with those unvaccinated;[11, 12, 27, 28, 30] four included a formal comparison between those with different numbers of doses (Table 2).[25–28] One study was conducted after a 2vHPV two-dose schedule was implemented in the Netherlands.[31] None of the studies assessed effectiveness using different buffer periods or intervals between doses in two-dose vaccine recipients.
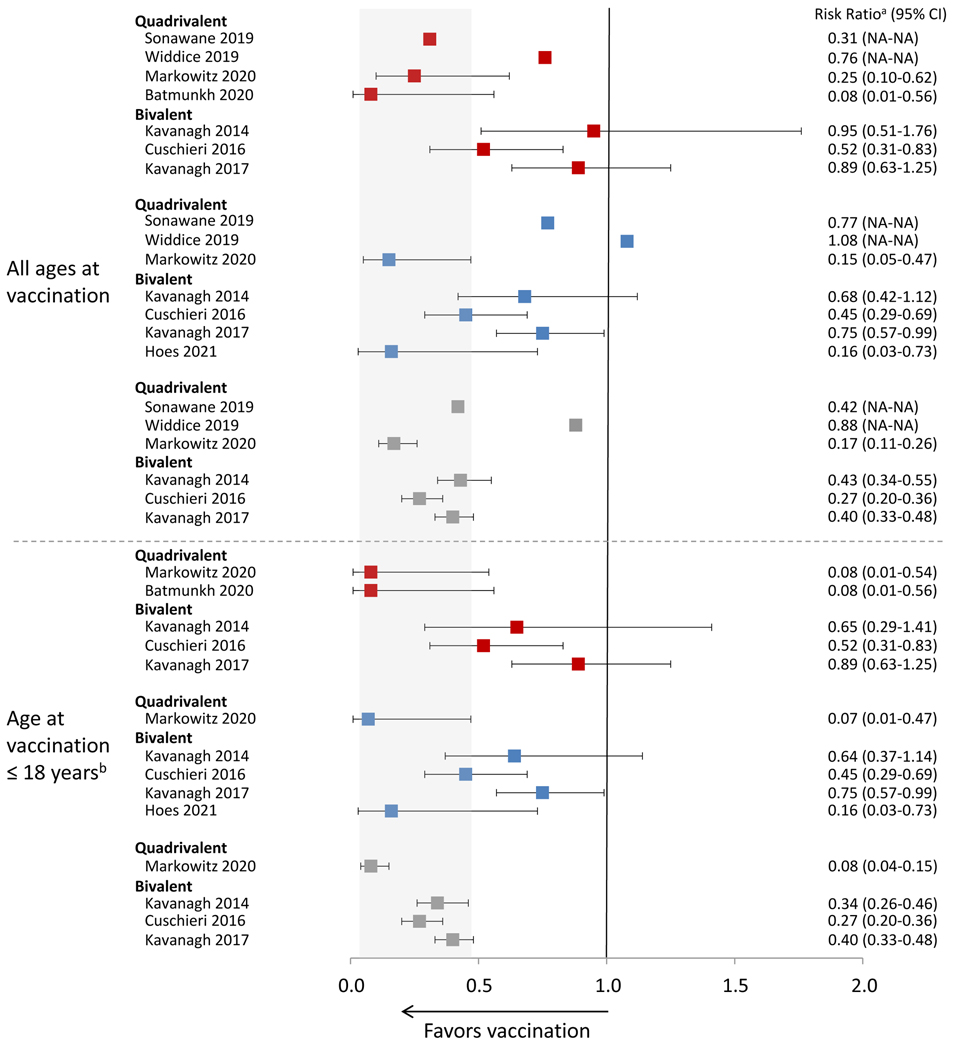
Figure 2 shows estimates from eight studies that provided vaccine effectiveness estimates against HPV infection, either overall or limited to persons vaccinated at ≤18 years. Of five studies that evaluated effectiveness with three doses (compared to no vaccination), all found significant effectiveness.[11, 12, 27, 28, 30] Five of six found some significant effectiveness with two doses,[12, 27, 28, 30, 31] and four of six with one dose.[12, 27–29] Six studies conducted analyses limited to persons vaccinated at a younger age or only included such persons in the study.[11, 12, 28–31] In general, effectiveness estimates were higher with younger age at vaccination. In the one study conducted in the setting of a routine 2vHPV two-dose schedule, girls who were vaccinated at age 12–13 years were followed prospectively with self-collected vaginal swabs for HPV DNA.[31] The adjusted VE of two doses against incident HPV16/18 infection was 84% (95% CI 27.0, 96.5).
Figure 2.
Effectiveness of HPV vaccination against HPV infection by number of doses and age at vaccination
 1 dose vs 0 doses;
1 dose vs 0 doses;  2 doses vs 0 doses;
2 doses vs 0 doses;  3 doses vs 0 doses
3 doses vs 0 doses
Data included in this analysis were extracted from original published articles.
NA, not available
Gray area indicates the range of the CIs from the published studies for effectiveness of 3 doses among girls aged 18 or younger when vaccinated.
aRisk ratio includes different measures depending on study, including incidence rate ratio, prevalence ratio, risk ratio, odds ratio or hazard ratio; for Widdice 2019 and Sonawane 2019, risk ratio was estimated from the prevalence of HPV infection among the different dose groups presented in the article, but the authors did not formally assess the effectiveness of 1,2,3 doses compared to 0 dose.
bAge at vaccination ≤18 years of age varied by study; for Markowitz 2020, ≤18 years; for Batmunkh 2020, age 11–17 years; for Kavanagh 2014, estimates from an analysis adjusted for birth cohort and there were few individuals vaccinated at age >18 years; for Cushieri 2016, estimates from an analysis adjusted by birth cohort and there were few individuals vaccinated at age >18 years; for Kavanagh 2017 estimates from an analysis adjusted for birth cohort and there were few individuals vaccinated at age >18 years.
The three reports from Scotland used the same data sources, specifically data from assessment of vaccine-type HPV prevalence in women attending their first cervical screen generally at age 20–21 years,[28] immunization records from the Scottish Immunization Recall System, and other national registries. The first Scottish study reported statistically significant effectiveness in adjusted analyses for three doses but not for two or one doses.[11] The two subsequent studies found significant effectiveness for three and two doses.[12, 30] Effectiveness of one dose was observed when one dose vaccinees were oversampled and in some adjusted analyses of a cohort that included girls vaccinated at routine ages as well as those vaccinated during catch-up.[12] The most recent report stratified by age at vaccination for three doses only; higher effectiveness was found with younger age at vaccination.[30] There was no formal comparison of three vs. fewer doses effectiveness in any report from Scotland. For 4vHPV, two studies from the United States both found similar effectiveness for three, two and one doses. One used data from a national survey that obtained vaccination history from self-report.[27] The other used data from women aged 20–29 years continuously enrolled in an integrated health care system with vaccination histories from medical records.[28] The later study found high and similar effectiveness with three, two and one doses when analyses were limited to women who received the first dose at age ≤18 years. A study from Mongolia included women who were part of a pilot 4vHPV vaccination campaign[29]; the analysis included 118 women who received only one dose at age 11–15 years compared with 357 unvaccinated women, frequency-matched on age. The adjusted PR was 0.08 (95% CI 0.01, 0.56).
Two articles reported effectiveness in men aged 14–26 years, both from the same U.S. study at different time points; there was no statistically significant effectiveness for prevention of genital or anal vaccine-type HPV among those who received ≥ one dose (compared to no vaccination) and no difference in effectiveness by number of doses.[25, 26] The number of vaccinated men was small in both, and almost half had initiated sexual activity at the same age as or before vaccination.
In summary, among nine studies of effectiveness against HPV infection, three, all from Scotland, reported highest point estimates with three doses.[11, 12, 30] Two studies among women in the United States reported similar effectiveness regardless of number of doses.[27, 28] A study from Mongolia only reported single-dose effectiveness (92%),[29] and a study from the Netherlands only reported two-dose effectiveness (84%).[31] Two studies among men included a small number of vaccinated participants; no significant effectiveness was reported with ≥ one dose.[25, 26]
3.2. Anogenital warts
The original review included six studies of anogenital wart outcomes.[13–18] In the updated review, four additional studies were identified; one included men and women.[32–35] The 10 studies were from six countries that had introduced 4vHPV. In nine of ten studies, analyses were adjusted or stratified for age at vaccination; all 10 were able to adjust for markers of socioeconomic status, and several attempted to adjust for differences in sexual behavior using composite measures (Table 1).[32–34] Nine studies analyzed at least one of the vaccine dose groups compared with no vaccination; seven conducted a formal comparison of effectiveness between number of doses (Table 2).[13, 14, 16, 18, 32, 33, 35] Three included assessment of effectiveness using different buffer periods,[13, 16, 32] and five evaluated different intervals between doses in two-dose vaccine recipients.[14, 16, 18, 32, 33]
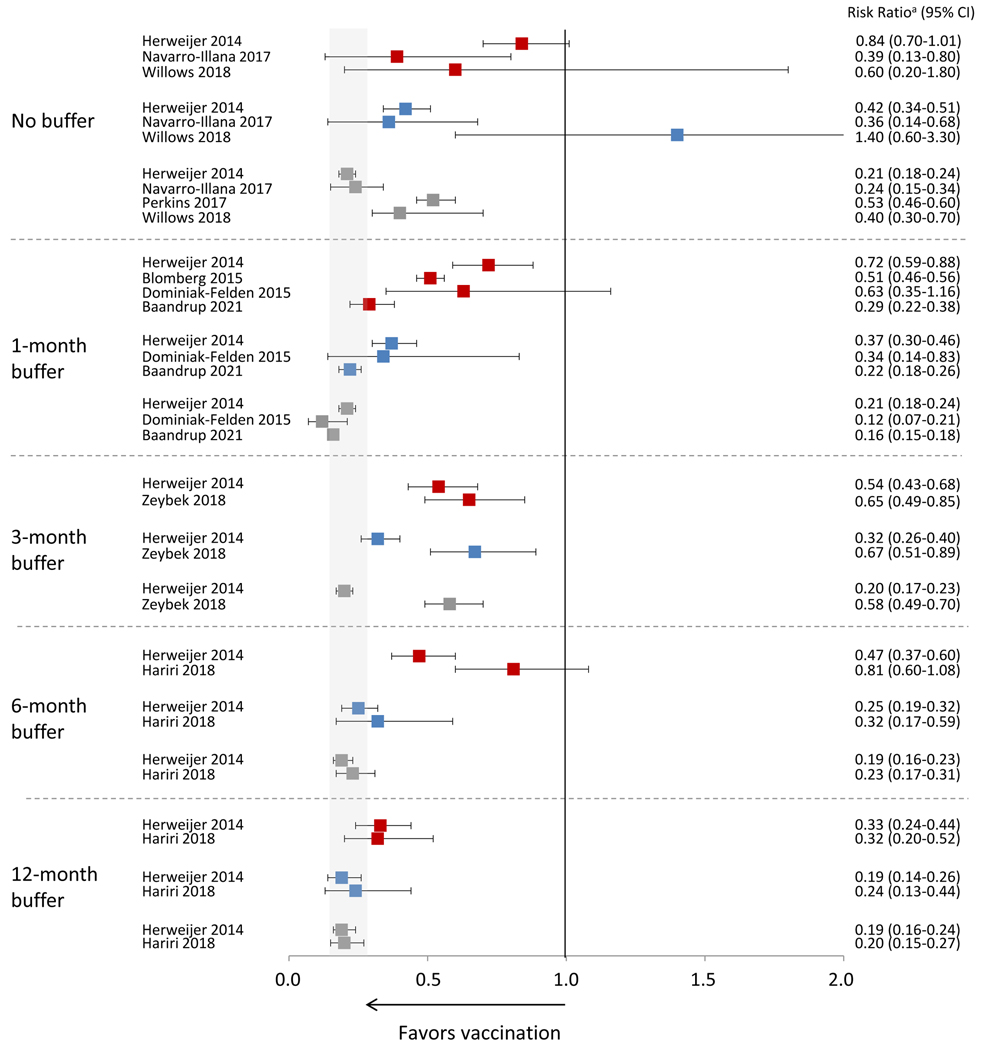
Figure 3 shows estimates from nine studies that provided vaccine effectiveness estimates against anogenital warts and buffer periods used. Of eight studies that formally evaluated effectiveness with three doses (compared to no vaccination), all found significant effectiveness. Six of seven found some significant effectiveness with two doses,[13, 15, 17, 32, 33, 35] and six of eight with one dose.[13, 14, 17, 32, 33, 35] Effectiveness estimates of one and two doses were generally higher when using buffer periods of longer duration. In the three studies that examined different buffer periods,[13, 16, 32] two found that a longer buffer period decreased differences by number of doses. Herweijer et al, used a three-month buffer period in the primary analysis.[13] In analyses adjusted for attained age and parental education, there was statistically significant effectiveness for three, adjusted IRR (aIRR)=0.20 (95% CI 0.17, 0.23); two, aIRR=0.32 (95% CI 0.26, 0.40); and one doses, aIRR=0.54 (95% CI 0.43, 0.68). In analyses stratified by age at vaccination (10–16 years and 17–19 years), with a buffer period > four months, there was no statistically significant difference between three and two doses, regardless of age at first vaccine dose. With a 12-month buffer period, there was no difference in effectiveness for three (aIRR=0.19 [95% CI 0.14, 0.24]), two (aIRR=0.19 [95% CI 0.13, 0.29]) or one (aIRR=0.24 [95% CI 0.15, 0.39]) doses among those who initiated vaccination at age 10–16 years. In a U.S. study, using data from electronic medical records and chart review, the adjusted HR of a single dose with a 6-month buffer from the last received dose was 0.81 (95% CI 0.60, 1.08) and with a 12-month buffer from the first dose was 0.32 (95% CI 0.20, 0.52).[32]
Figure 3.
Effectiveness of HPV vaccination against anogenital warts by number of doses and duration of buffer period for studies using the quadrivalent vaccine
 1 dose vs 0 doses;
1 dose vs 0 doses;  2 doses vs 0 doses;
2 doses vs 0 doses;  3 doses vs 0 doses
3 doses vs 0 doses
Data included in this analysis were extracted from original published articles.
Gray area indicates the range of the CIs from the published studies for effectiveness of 3 doses using the longest buffer period.
Perkins reports using a buffer period but does not report results in the article.
a Risk ratio includes different measures depending on study, including incidence rate ratio, prevalence ratio, risk ratio, odds ratio or hazard ratio.
Seven studies that stratified by age at vaccination found higher vaccine effectiveness with younger compared to older age at vaccination, although differences were not formally tested in all.[13–15, 18, 33–35] Four studies evaluated vaccine effectiveness for three, two and one doses stratified by age at vaccination.[13, 33–35] Herweijer et al conducted the most detailed analysis, stratifying by age at vaccination (10–16 years and 17–19 years) and using different buffer periods.[13] They found higher effectiveness within the younger age at vaccination group, particularly for one-dose recipients when no or short buffer periods were used. Willows et al found higher effectiveness for all dose groups in those vaccinated at younger ages (9–18 years vs ≥19 years),[34] but differences by number of doses remained in all age at vaccination groups. In contrast, Zeybek et al found similar effectiveness by number of doses with younger age at vaccination.[33] A study by Navarro-Illana et al was limited mainly to girls vaccinated at age 14 years due to the national vaccination program in Spain; that study reported similar point estimates of effectiveness regardless of number of doses.[17]
In the five studies that explored the interval between doses in two-dose vaccine recipients,[14, 16, 18, 32, 33] three found that a longer interval changed effectiveness estimates or resulted in no difference between three and two doses.[14, 18, 32] For example, in a large study in Denmark where 70% of girls had an interval of two months between doses one and two, effectiveness was significantly higher with three doses than two doses in overall analyses.[14] However, there was no statistically significant difference between three and two doses with an interval > four months between doses and the IRR was close to one with an interval of six months.
In summary, among the ten studies evaluating 4vHPV effectiveness against anogenital warts, a range of age groups and different sensitivity analyses were included. Most studies reported highest effectiveness with three doses in the primary analyses. Sensitivity analyses suggested biases that could result from differences in age at vaccination across dose groups. Some found that differences between three and fewer doses decreased in analyses limited to persons vaccinated at younger ages, with longer buffer periods, or with longer intervals between two doses.
3.4. Cervical cytological and histological abnormalities
The original review included six studies that evaluated vaccine effectiveness for prevention of cervical cytological or histological abnormalities. In the updated review, ten additional studies were included (Tables 1 and 2).[36–45] Of the 16 studies, 12 were from countries that had introduced mainly 4vHPV, three 2vHPV, and one both. By number of doses, eight studies evaluated histological outcomes only, three cytological outcomes only,[22, 23, 45] and five both histological and cytological outcomes.[19–21, 41, 44] Histological abnormalities included cervical intraepithelial neoplasia (CIN) grade 1, 2, 3, CIN2+ (CIN grade 2 or higher or adenocarcinoma in situ [AIS]), and CIN3/AIS. Characteristics of women, including age at first vaccine dose, differed across dose groups in most studies. All studies except one evaluated cytological or histological outcomes irrespective of HPV type.[40]
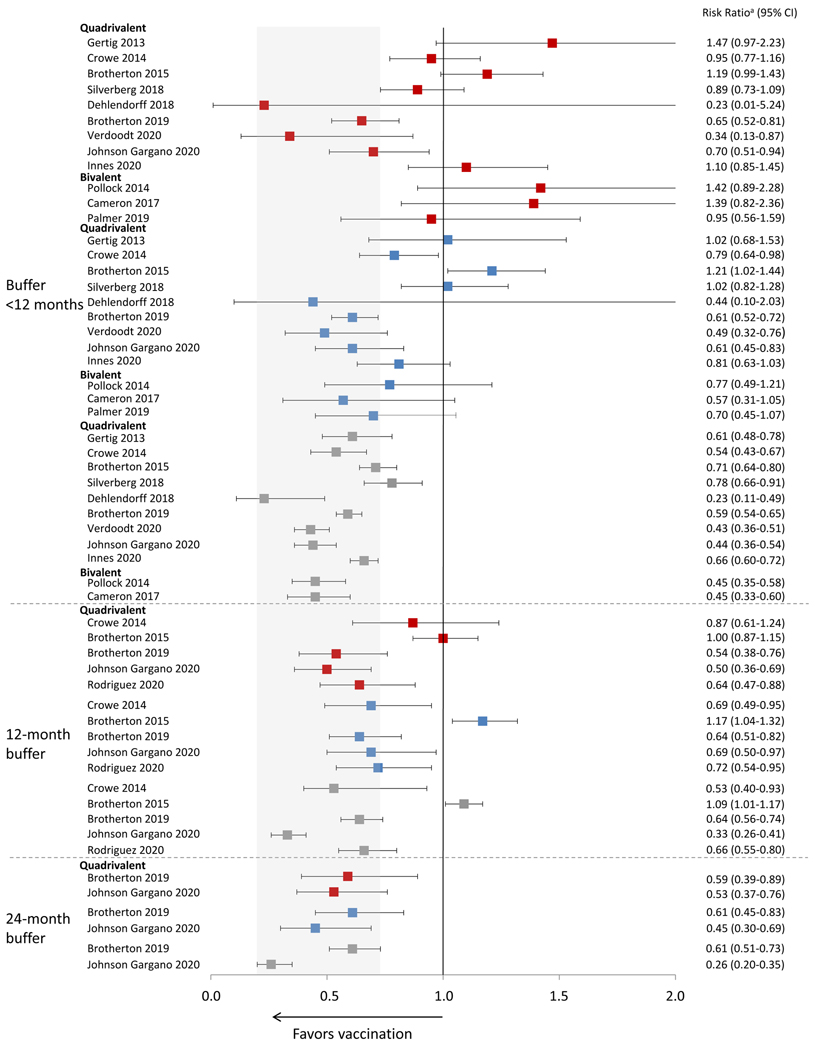
Among the 16 studies, 15 found significant effectiveness for three doses. Figure 4 shows estimates from 13 studies that provide estimates of vaccine effectiveness against CIN2+. Either in the main analysis, in analyses restricted to certain age groups, or those using longer buffer periods, all 13 found significant effectiveness for prevention of high grade lesions (CIN2+ or CIN3+) with three doses; five of these 13 studies found significant effectiveness with two doses;[20, 38–41] and five with one dose.[20, 38–41] In general, analyses using longer buffer periods found higher effectiveness estimates and smaller differences by number of doses.
Figure 4.
Effectiveness of HPV vaccination against CIN2+ by number of doses and duration of buffer period
 1 dose vs 0 doses;
1 dose vs 0 doses;  2 doses vs 0 doses;
2 doses vs 0 doses;  3 doses vs 0 doses
3 doses vs 0 doses
Data included in this analysis were extracted from original published articles.
Gray area indicates the range of the CIs from the published studies for effectiveness of 3 doses using the longest buffer period.
CIN2+, cervical intraepithelial neoplasia grade 2, 3, or worse or adenocarcinoma in situ
a Risk ratio includes different measures depending on study, including incidence rate ratio, prevalence ratio, risk ratio, odds ratio, or hazard ratio.
Eight studies used no buffer or buffer periods <12 months when evaluating prevention of high grade histological lesions, and five used an explicit buffer ≥12 months.[20, 21, 39–41] Four explored different buffer periods;[20, 21, 39, 40] of these, two found that longer buffer periods increased estimates of effectiveness for all dose groups.[21, 40] Brotherton et al found that point estimates for effectiveness of three and one doses were similar with a 12-month buffer but not with shorter buffers.[21] Another study using a 12-month buffer found that among those vaccinated at age 15–19 years, effectiveness was similar for three, two and one doses. Although Johnson et al found that effectiveness estimates increased with longer buffer periods, that study also formally compared three and one doses; a significant difference remained even with a 24-month buffer.[40]
Overall, 12 of 16 studies presented data stratified by age at vaccination or birth year group.[20–22, 36–42, 44, 45] Most found higher point estimates of vaccine effectiveness against cytological or histological outcomes with younger age at vaccination or later birth year, although the differences were not all formally tested. In eight studies that evaluated high grade histological lesions by number of doses stratified by age at vaccination, or in studies limited to women vaccinated at younger ages,[20, 21, 37–42] three found similar and significant effectiveness regardless of number of doses.[38, 39, 41] These three studies evaluated women vaccinated at age ≤16 years in Denmark, age <16 years in Australia and age 15–19 years in the United States. A study from Sweden and Demark also reported similar point estimates by number of doses among those vaccinated at age ≤16 years and 17–19 years, but only the the effectiveness estimate for three doses was significant among those vaccinated at ≤16 years.[37]
Most two-dose vaccine recipients received doses at a one- or two-month interval because they received vaccination under recommendations for a three-dose schedule. Four studies using histological outcomes examined intervals between two doses; three found no impact of interval on the estimate of effect,[21, 39, 41] and one found similar effectiveness with three and two doses with an interval ≥ five months but not < five months between doses in those vaccinated at age ≤16 years.[37]
In summary, we identified 16 studies evaluating vaccine effectiveness for prevention of cervical abnormalities. In many studies, baseline characteristics of women who received fewer than three doses were different than three-dose vaccine recipients, and investigators conducted stratified and/or adjusted analyses to control for these differences. All studies except one found effectiveness with three doses compared to no vaccination. While many of the earliest published studies reported highest efficacy with three doses and no significant effectiveness with fewer doses, more recently published studies have reported effectiveness with three, two and one doses, and three of five reported similar effectiveness by number of doses.[38, 39, 41] Limiting analyses to persons vaccinated at younger ages and using buffer periods, factors that impact several different domains in the quality assessments, resulted in higher effectiveness estimates.
4.0. DISCUSSION
Vaccine effectiveness studies are valuable for evaluating vaccination programs and vaccines in real world settings. Because not all persons who start the HPV vaccination schedule complete the series, data from observational studies have been used to provide information on effectiveness with reduced dose schedules. However, differences in persons who complete a recommended three-dose series and those who do not, as well as other methodologic limitations, can result in substantial biases. In this updated systematic review of HPV vaccination effect by number of doses, we included 35 observational studies that evaluated outcomes of vaccine-type HPV infection, anogenital warts or cervical abnormalities. Among 29 studies that evaluated three doses, 28 found evidence of significant effectiveness.[11–13, 15–17, 19–24, 27, 28, 30, 32–37, 39–44] Among 29 studies that evaluated two doses, 19 found significant effectiveness.[12, 13, 15, 17, 19–22, 27, 28, 30–33, 35, 38–41] In 18 of 30 studies, significant effectiveness was observed for one dose in some or all analyses.[12–14, 17, 19–21, 27–29, 32, 33, 35, 38–41, 45] Across all endpoints (infection, anogenital warts, and cervical abnormalities), variation in effectiveness by number of doses was observed in most of the earliest published studies; highest effectiveness was found with three doses. Few studies directly compared three, two, and one doses and some effectiveness estimates had wide confidence intervals due to the small number of outcomes in one- and two-dose vaccine recipients.
In this review we formally assessed the risk of bias; almost all studies were assessed to have serious risk for at least one type of bias or confounding. The most common types of bias identified as serious were information bias, in measurement of the intervention or outcome, and confounding due to differences by number of doses in HPV infection at the time of vaccination and acquisition during follow-up. These multiple potential biases should be considered when interpreting the findings. Except for one study, all post-licensure studies were conducted in settings of a three-dose recommendation; for most two-dose vaccine recipients there was only a one- or two-month interval between doses one and two, leading to information bias in measurement of the intervention. Importantly, girls who received one or two doses differed from those completing the recommended schedule. In countries with catch-up vaccination policies, studies included persons vaccinated in the catch-up age group. Girls who received fewer than three doses were often older at the time of vaccination than three-dose vaccine recipients, had lower socioeconomic status, and/or had indicators of earlier sexual exposure.[19, 21, 28, 32, 34, 38–41], resulting in these studies being at risk of both information bias related to measurement of the outcome, as misattribution of an outcome to the wrong dose was likely, and bias due to confounding. While some risk factors were measured and analyses adjusted, it is likely that unmeasured confounding remained, particularly for risk of HPV acquisition during the study follow-up. These would likely bias results towards greater effectiveness of three doses compared to one or two.
Although most studies found highest point estimates of effectiveness with three doses, the variation in effectiveness by number of doses was diminished or eliminated in studies when analyses were stratified by age at vaccination. Overall, five studies that found similar effectiveness for three, two and one doses, three evaluating cervical outcomes,[38, 39, 41] one evaluating anogenital warts[33] and one prevalent vaccine-type infection,[28] limited analyses to mainly those vaccinated in teenage years. However, other studies limited to persons vaccinated at ages 12–17 years did not report similar findings.[30, 42]
As more vaccinated persons age into age groups where outcomes are detected, more recent studies have been able to stratify by age at vaccination or limit studies to persons vaccinated at younger ages, which, among other things, improved the overall quality of studies. While less than 50% of studies published before 2019 were rated at only moderate risk of bias or had only one category rated at serious risk of bias, 77% (10/13) of studies published in 2019 or later were rated as such (Table 1).[26, 28, 29, 31, 35, 38–42] Among these ten studies, eight provided effectiveness estimates for three, two, and one doses, and six showed significant effectiveness for all three dose groups.[28, 35, 38–41] Of five studies that compared effectiveness between one and three doses,[28, 35, 38–40] three found similar point estimates with no significant difference in effectiveness.[28, 38, 39] Limiting studies to persons vaccinated at younger ages decreases the likelihood that a detected outcome is due to an infection present at the time of vaccination and minimizes potential biases due to differences in age at vaccination between dose groups.
In these types of observational studies, it is not possible to determine who was infected at the time of vaccination and if the outcome detected was due to infection already present at vaccination. Buffer periods, used in some studies to delay case counting, attempt to address this problem, as the use of buffers makes it more likey that the outcome detected was due to infection acquired after vaccination. Based on HPV natural history, longer buffer periods might be more important for evaluation of vaccine effectiveness against CIN2+ than anogenital warts because progression from infection to disease is shorter for anogenital warts. In our quality assessments, we considered different buffer periods for each outcome: 12 months for infection, six months for anogenital warts and 24 months for cervical abnormalities. In addition, buffer periods could be of greater importance with older age at vaccination because there is a higher likelihood of prevalent infection with increasing age through the twenties. Therefore, the impact of buffer periods would likely vary across studies. Among eight studies that conducted sensitivity analyses using different buffer periods,[13, 16, 20, 21, 32, 39, 40, 45] five found that estimates changed with longer buffer periods including higher effectiveness for one or two doses and a decrease in differences by number of doses.[13, 20, 21, 32, 40] While helpful to reduce some bias, buffer periods also can reduce the number of person-years with one or two doses, which is small in some studies.
Because most post-licensure studies published to date were conducted in settings of a national three-dose recommendation, the majority of individuals vaccinated with two doses received doses with a one-month or two-month interval, not the interval recommended for a two-dose series. Immunogenicity studies leading to approval of a two-dose schedule found non-inferior results with two doses compared to three doses when the doses were separated by at least five or six months.[2, 3] Although the number of girls vaccinated with two doses separated by at least six months was small in studies identified for this review, ten evaluated interval between doses.[14, 16, 18, 21, 22, 32, 33, 37, 39, 41] Three of five studies evaluating anogenital wart outcomes[14, 18, 32] and two of five evaluating cervical outcomes[22, 37] found that longer intervals increased effectiveness estimates. The findings of higher effectiveness with a longer interval between two doses in some observational studies could be due to the longer interval functioning as a buffer period and not related to the spacing between doses. The inconsistent findings by interval between doses across studies also could be due to differing importance of buffer periods for the endpoints and age groups evaluated.
The accuracy of vaccination history is important for vaccine effectiveness studies. Incomplete vaccination histories could lead to overestimating effectiveness of fewer than three doses. While many studies in this review were conducted in countries with high quality vaccination registries, underreporting of vaccinations to registries can occur.[20, 21] In countries without registries, use of claims or insurance data is preferable to use of self-reported vaccination, but these sources could be incomplete if persons moved or changed insurers during the vaccination series. Several studies limited evaluation to persons continuously enrolled in insurance plans or integrated health care systems, resulting in a much higher likelihood of complete vaccination data collection.[28, 32, 33]
In conclusion, most post-licensure observational studies report highest effectiveness with three doses; some, particularly those limited to persons who received vaccine at younger ages, those able to stratify by age at vaccination, or those using longer buffer periods, found smaller or no statistically significant differences by number of doses. There are several biases in currently available data that impact effectiveness estimates, with most biasing two- and one-dose results. Nevertheless, observational studies are increasingly showing effectiveness with fewer than three doses and some show similar effectiveness with three, two and one doses. Studies examining persons vaccinated prior to sexual activity and using methods to reduce potential sources of bias are needed for more valid interpretation.
Clinical trials designed to examine single-dose vaccination as well as long term follow-up of post-hoc analyses from three-dose clinical trials, in which not all women completed the schedule, are now available; data show high efficacy with a single dose and suggest good duration of protection.[6, 9, 49] These and other trials have provided important data for policy considerations.[50] Disparate results from observational effectiveness studies using data from national programs should be interpreted with an understanding of the inherent biases discussed in this review.
Supplementary Material
Figure S1. Summary of quality assessment ratings regarding selection bias for studies about A) HPV infection, B) anogenital warts, and C) cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias
Figure S2. Summary of quality assessment ratings regarding information bias for studies about A) HPV infection, B) anogenital warts, and C) cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias
Figure S3. Summary of quality assessment ratings regarding confounding for studies about A) HPV infection, B) anogenital warts, and C) cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias
Figure S4. Specific quality assessment ratings of studies examining HPV infection
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias; Gray – not applicable / no information
Figure S5. Specific quality assessment ratings of studies examining anogenital warts
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias; Gray – not applicable / no information
Figure S6. Specific quality assessment ratings of studies examining cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias; Gray – not applicable / no information
Table S1. Main results of studies that evaluated effectiveness of HPV vaccine by number of doses for each outcome of interest
Table S2. Description of potential biases and ratings
Table S3. Quality assessment of post-licensure studies examining the effectiveness of HPV vaccination by number of doses against HPV infection
Table S4. Quality assessment of post-licensure studies examining the effectiveness of HPV vaccination by number of doses against anogenital warts
Table S5. Quality assessment of post-licensure studies examining the effectiveness of HPV vaccination by number of doses against cervical abnormalities
Funding
Financial support for this project was provided in part by PATH on behalf of the Single-Dose HPV Vaccine Evaluation Consortium, which includes Harvard University, London School of Hygiene & Tropical Medicine, PATH, US National Cancer Institute, University of British Columbia, Canada, CHU de Québec-Université Laval, Quebec, University of Witwatersrand Reproductive Health and HIV Institute, and the US Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
All authors attest they meet the ICMJE criteria for authorship.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–21. [DOI] [PubMed] [Google Scholar]
- [3].Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–802. [DOI] [PubMed] [Google Scholar]
- [4].WHO. Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec. 2014;89:465–91. [PubMed] [Google Scholar]
- [5].Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–8. [DOI] [PubMed] [Google Scholar]
- [6].Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, et al. Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. J Natl Cancer Inst. 2020;112:1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schiller J, Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36:4768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stanley M, Dull P. HPV single-dose vaccination: Impact potential, evidence base and further evaluation. Vaccine. 2018;36:4759–60. [DOI] [PubMed] [Google Scholar]
- [9].Barnabas R, Brown E, Onono M, Bukusi E, Njoroge B, Winer R, et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid 2022;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Markowitz LE, Drolet M, Perez N, Jit M, Brisson M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018;36:4806–15. [DOI] [PubMed] [Google Scholar]
- [11].Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110:2804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cuschieri K, Kavanagh K, Moore C, Bhatia R, Love J, Pollock KG. Impact of partial bivalent HPV vaccination on vaccine-type infection: a population-based analysis. Br J Cancer. 2016;114:1261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herweijer E, Leval A, Ploner A, Eloranta S, Simard JF, Dillner J, et al. Association of varying number of doses of quadrivalent human papillomavirus vaccine with incidence of condyloma. JAMA. 2014;311:597–603. [DOI] [PubMed] [Google Scholar]
- [14].Blomberg M, Dehlendorff C, Sand C, Kjaer SK. Dose-Related Differences in Effectiveness of Human Papillomavirus Vaccination Against Genital Warts: A Nationwide Study of 550,000 Young Girls. Clin Infect Dis. 2015;61:676–82. [DOI] [PubMed] [Google Scholar]
- [15].Dominiak-Felden G, Gobbo C, Simondon F. Evaluating the Early Benefit of Quadrivalent HPV Vaccine on Genital Warts in Belgium: A Cohort Study. PLoS One. 2015;10:e0132404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Perkins RB, Lin M, Wallington SF, Hanchate A. Impact of number of human papillomavirus vaccine doses on genital warts diagnoses among a National Cohort of U.S. Adolescents. Sex Trans Dis. 2017;44:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Navarro-Illana E, Lopez-Lacort M, Navarro-Illana P, Vilata JJ, Diez-Domingo J. Effectiveness of HPV vaccines against genital warts in women from Valencia, Spain. Vaccine. 2017;35:3342–6. [DOI] [PubMed] [Google Scholar]
- [18].Lamb F, Herweijer E, Ploner A, Uhnoo I, Sundstrom K, Sparen P, et al. Timing of two versus three doses of quadrivalent HPV vaccine and associated effectiveness against condyloma in Sweden: a nationwide cohort study. BMJ Open. 2017;7:e015021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013;11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Crowe E, Pandeya N, Brotherton JM, Dobson AJ, Kisely S, Lambert SB, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ. 2014;348:g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brotherton J, Malloy M, Budd A, Saville M, Drennan K, Gertig D. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: Observational cohort of young women in Australia. Papillomavirus Res 2015;1:59–73. [Google Scholar]
- [22].Hofstetter AM, Ompad DC, Stockwell MS, Rosenthal SL, Soren K. Human Papillomavirus Vaccination and Cervical Cytology Outcomes Among Urban Low-Income Minority Females. JAMA Pediatr. 2016;170:445–52. [DOI] [PubMed] [Google Scholar]
- [23].Kim J, Bell C, Sun M, Kliewer G, Xu L, McInerney M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. CMAJ 2016;188:E281–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pollock KG, Kavanagh K, Potts A, Love J, Cuschieri K, Cubie H, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer. 2014;111:1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chandler E, Ding L, Gorbach P, Franco EL, Brown DA, Widdice LE, et al. Epidemiology of Any and Vaccine-Type Anogenital Human Papillomavirus Among 13–26-Year-Old Young Men After HPV Vaccine Introduction. J Adolesc Health. 2018;63:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Widdice LE, Bernstein DI, Franco EL, Ding L, Brown DR, Ermel AC, et al. Decline in vaccine-type human papillomavirus prevalence in young men from a Midwest metropolitan area of the United States over the six years after vaccine introduction. Vaccine. 2019;37:6832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sonawane K, Nyitray AG, Nemutlu GS, Swartz MD, Chhatwal J, Deshmukh AA. Prevalence of Human Papillomavirus Infection by Number of Vaccine Doses Among US Women. JAMA Netw Open. 2019;2:e1918571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Markowitz LE, Naleway AL, Klein NP, Lewis RM, Crane B, Querec TD, et al. Human Papillomavirus Vaccine Effectiveness Against HPV Infection: Evaluation of One, Two, and Three Doses. J Infect Dis. 2020;221:910–8. [DOI] [PubMed] [Google Scholar]
- [29].Batmunkh T, Dalmau MT, Munkhsaikhan ME, Khorolsuren T, Namjil N, Surenjav U, et al. A single dose of quadrivalent human papillomavirus (HPV) vaccine is immunogenic and reduces HPV detection rates in young women in Mongolia, six years after vaccination. Vaccine. 2020;38:4316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL, Watt C, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17:1293–302. [DOI] [PubMed] [Google Scholar]
- [31].Hoes J, King AJ, Schurink-van ‘t Klooster TM, Berkhof PJ, Bogaards JA, de Melker HE. Vaccine effectiveness following routine immunization with bivalent HPV vaccine: Protection against incident genital HPV infections from a reduced-dosing schedule. J Infect Dis. 2021. doi: 10.1093/infdis/jiab250. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hariri S, Schuler MS, Naleway AL, Daley MF, Weinmann S, Crane B, et al. Human Papillomavirus Vaccine Effectiveness Against Incident Genital Warts Among Female Health-Plan Enrollees, United States. Am J Epidemiol. 2018;187:298–305. [DOI] [PubMed] [Google Scholar]
- [33].Zeybek B, Lin YL, Kuo YF, Rodriguez AM. The Impact of Varying Numbers of Quadrivalent Human Papillomavirus Vaccine Doses on Anogenital Warts in the United States: A Database Study. JLGTD. 2018;22:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Willows K, Bozat-Emre S, Righolt CH, Kliewer EV, Mahmud SM. Early Evidence of the Effectiveness of the Human Papillomavirus Vaccination Program Against Anogenital Warts in Manitoba, Canada: A Registry Cohort Study. Sex Transm Dis. 2018;45:254–9. [DOI] [PubMed] [Google Scholar]
- [35].Baandrup L, Dehlendorff C, Kjaer SK. One-Dose Human Papillomavirus Vaccination and the Risk of Genital Warts: A Danish Nationwide Population-based Study. Clin Infect Dis. 2021;73:e3220–e6. [DOI] [PubMed] [Google Scholar]
- [36].Silverberg MJ, Leyden WA, Lam JO, Gregorich SE, Huchko MJ, Kulasingam S, et al. Effectiveness of catch-up human papillomavirus vaccination on incident cervical neoplasia in a US health-care setting: a population-based case-control study. Lancet Child Adolesc Health. 2018;2:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dehlendorff C, Sparen P, Baldur-Felskov B, Herweijer E, Arnheim-Dahlstrom L, Ploner A, et al. Effectiveness of varying number of doses and timing between doses of quadrivalent HPV vaccine against severe cervical lesions. Vaccine. 2018;36:6373–8. [DOI] [PubMed] [Google Scholar]
- [38].Verdoodt F, Dehlendorff C, Kjaer SK. Dose-related Effectiveness of Quadrivalent Human Papillomavirus Vaccine Against Cervical Intraepithelial Neoplasia: A Danish Nationwide Cohort Study. Clin Infect Dis. 2020;70:608–14. [DOI] [PubMed] [Google Scholar]
- [39].Brotherton JM, Budd A, Rompotis C, Bartlett N, Malloy MJ, Andersen RL, et al. Is one dose of human papillomavirus vaccine as effective as three?: A national cohort analysis. Papillomavirus Res. 2019;8:100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Johnson Jones ML, Gargano JW, Powell M, Park IU, Niccolai LM, Bennett NM, et al. Effectiveness of 1, 2, and 3 Doses of Human Papillomavirus Vaccine Against High-Grade Cervical Lesions Positive for Human Papillomavirus 16 or 18. Am J Epidemiol. 2020;189:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rodriguez AM, Zeybek B, Vaughn M, Westra J, Kaul S, Montealegre JR, et al. Comparison of the long-term impact and clinical outcomes of fewer doses and standard doses of human papillomavirus vaccine in the United States: A database study. Cancer. 2020;126:1656–67. [DOI] [PubMed] [Google Scholar]
- [42].Innes CR, Williman JA, Simcock BJ, Hider P, Sage M, Dempster-Rivett K, et al. Impact of human papillomavirus vaccination on rates of abnormal cervical cytology and histology in young New Zealand women. N Z Med J. 2020;133:72–84. [PubMed] [Google Scholar]
- [43].Cameron RL, Kavanagh K, Cameron Watt D, Robertson C, Cuschieri K, Ahmed S, et al. The impact of bivalent HPV vaccine on cervical intraepithelial neoplasia by deprivation in Scotland: reducing the gap. J Epidemiol Comm Health. 2017;71:954–60. [DOI] [PubMed] [Google Scholar]
- [44].Palmer T, Wallace L, Pollock KG, Cuschieri K, Robertson C, Kavanagh K, et al. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: retrospective population study. BMJ. 2019;365:l1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Acuti Martellucci C, Nomura S, Yoneoka D, Ueda P, Brotherton J, Canfell K, et al. Human papillomavirus vaccine effectiveness within a cervical cancer screening programme: cohort study. BJOG. 2021;128:532–9. [DOI] [PubMed] [Google Scholar]
- [46].Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. [DOI] [PubMed] [Google Scholar]
- [47].Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. [DOI] [PubMed] [Google Scholar]
- [48].Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Eng J Med. 2007;356:1928–43. [DOI] [PubMed] [Google Scholar]
- [49].Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. 2021;22:1518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Meeting of the Strategic Advisory Group of Experts on Immunization, April 2022: conclusions and recommendations. WER 2022;24:261–76. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary of quality assessment ratings regarding selection bias for studies about A) HPV infection, B) anogenital warts, and C) cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias
Figure S2. Summary of quality assessment ratings regarding information bias for studies about A) HPV infection, B) anogenital warts, and C) cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias
Figure S3. Summary of quality assessment ratings regarding confounding for studies about A) HPV infection, B) anogenital warts, and C) cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias
Figure S4. Specific quality assessment ratings of studies examining HPV infection
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias; Gray – not applicable / no information
Figure S5. Specific quality assessment ratings of studies examining anogenital warts
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias; Gray – not applicable / no information
Figure S6. Specific quality assessment ratings of studies examining cervical abnormalities
Green – low risk of bias; Yellow – moderate risk of bias; Red – serious risk of bias; Gray – not applicable / no information
Table S1. Main results of studies that evaluated effectiveness of HPV vaccine by number of doses for each outcome of interest
Table S2. Description of potential biases and ratings
Table S3. Quality assessment of post-licensure studies examining the effectiveness of HPV vaccination by number of doses against HPV infection
Table S4. Quality assessment of post-licensure studies examining the effectiveness of HPV vaccination by number of doses against anogenital warts
Table S5. Quality assessment of post-licensure studies examining the effectiveness of HPV vaccination by number of doses against cervical abnormalities