Abstract
Elevated expression of β-amyloid (Aβ1-42) and tau are considered risk-factors for Alzheimer’s disease in healthy older adults. We investigated the effect of aging and cerebrospinal fluid levels of Aβ1-42 and tau on 1) frontal metabolites measured with proton magnetic resonance spectroscopy (MRS) and 2) cognition in cognitively normal older adults (n = 144; age range 50-85). Levels of frontal gamma aminobutyric acid (GABA+) and myo-inositol relative to creatine (mI/tCr) were predicted by age. Levels of GABA+ predicted cognitive performance better than mI/tCr. Additionally, we found that frontal levels of n-acetylaspartate relative to creatine (tNAA/tCr) were predicted by levels of t-tau. In cognitively normal older adults, levels of frontal GABA+ and mI/tCr are predicted by aging, with levels of GABA+ decreasing with age and the opposite for mI/tCr. These results suggest that age- and biomarker-related changes in brain metabolites are not only located in the posterior cortex as suggested by previous studies and further demonstrate that MRS is a viable tool in the study of aging and biomarkers associated with pathological aging and Alzheimer’s disease.
Keywords: Aging, Magnetic resonance spectroscopy, Alzheimer’s disease, Frontal cortex, Cerebrospinal fluid biomarkers, General cognition
1. Introduction
Accumulation of proteins β-amyloid (Aβ1-42) and tau are defining neuropathological hallmarks of Alzheimer’s disease (AD), but also occur in asymptomatic individuals (Jack Jr et al., 2018). Abnormally elevated expression of Aβ and tau are thus considered risk-factors for future development of AD in cognitively normal healthy older adults (Ittner & Götz, 2011; Jagust et al., 2021). On the one hand, elevated Aβ1-42 is associated with myelin pathology (Dean et al., 2017) and abnormal neuroinflammation (Heneka et al., 2015) on the other hand, elevated level of hyperphosphorylated tau is associated with reduced axonal function and cortical thinning, resulting in gradual degeneration and cognitive impairment (Donohue et al., 2017; LaPoint et al., 2017; Scholl et al., 2016). Although the interactions between age, Aβ1-42 and tau are complex and remain undeciphered, early detection of elevated levels of Aβ1-42 and tau in the healthy brain have become viewed as crucial for the eventual prevention and management of the disease. Thus, investigation of the neurochemical profile of healthy older adults with and without elevated levels of Aβ1-42 and tau is of primary interest in the study of normal and pathological human aging.
From this perspective, proton magnetic resonance spectroscopy (MRS) is a MR-based technique that noninvasively allows in vivo estimation of concentrations of various brain metabolites within a voxel of interest. Among the metabolites that may be investigated with MRS, myo-inositol (mI) is a compound frequently associated with neuroinflammation and glial proliferation (Brand et al., 1993; Glanville et al., 1989). Previous MRS results suggest that levels of mI increase with normal aging (Lind et al., 2020; Marjańska et al., 2017; Raininko & Mattsson, 2010) and in AD (Kantarci et al., 2000; Kantarci et al., 2003) in medial posterior regions of the cortex. MRS also allows the estimation of gamma-aminobutyric acid (GABA) levels, the main in-hibitory neurotransmitter in the mammalian brain. Previous studies have reported a decrease of GABA or GABA+ (GABA co-edited with macromolecules) levels in healthy older adults compared to young adults (Gao et al., 2013; Porges et al., 2017). Decreased GABA levels have also been reported in AD patients (Gueli & Taibi, 2013); however, it is still unclear whether this decrease is a product of AD pathology or a participating factor in its development (Li et al., 2016). Also measurable with MRS, glutamate+glutamine (Glu+Gln; Glx) is an index characterizing general glutamatergic metabolism. Although Glu and Gln can be identified at higher field strength, Glx is a more reliable metric at 3T. Previous MRS results have suggested that Glx levels may decrease with normal aging (Gao et al., 2013; Zahr et al., 2013) and in AD (Hattori et al., 2002), but results from the literature are not unanimous and others suggest an elevation in glutamatergic indices with aging (Cichocka & Berés, 2018). Finally, metabolites like creatine (Cr) and phosphocreatine (PCr), choline (Cho) and n-acetylaspartate (NAA), respectively reflecting cellular energy metabolism, membrane metabolism and neuronal integrity, can also be reliably estimated with MRS and are subject to changes with normal aging (Cichocka & Bereś, 2018; Haga et al., 2009) and AD pathology (Gao & Barker, 2014).
In sum, concentration levels of mIL and GABA and other metabolites assessed with MRS may be impacted by physiological brain aging, neuroinflammation and, more importantly, elevated levels of Aβ1-42 and tau. Importantly, the neurochemical characterization of the frontal cortex of healthy older adults expressing various levels of CSF Aβ1-42 and tau has not been extensively studied with MRS. Thus, we sought to investigate this in a cohort of healthy, cognitively normal, older adults expressing various levels of total tau (t-tau) and Aβ1-42 with MRS. This would allow for characterization of the effects of 1) aging and 2) expression of Aβ1-42 and tau on the concentration levels of frontal cortical metabolites in the aging brain. Additionally, we obtained neuropsychological test scores and observed their relationship with MRS metabolite levels and AD-related CSF biomarkers.
2. Materials and methods
Population:
Data were acquired from 43 healthy young (HY) and 144 healthy old (HO) (total n=187) (see Table 1 for subjects’ characteristics). The age range in the HO group was 50-85 years and 20-31 years in the HY group. Subjects were recruited as community-dwelling older adults without a diagnosis of mild cognitive impairment (MCI) or AD. Within the HO group, 39 were characterized as preclinical AD with ratio t-tau/Aβ1-42 > 0.18, a threshold determined by the Alzheimer’s Disease Neuroimaging Initiative (Blennow et al., 2019). Subjects with a history of neurological or psychiatric disorder and/or brain injury, renal dysfunction, any MRI contraindications (e.g., pacemaker, metal implant) or currently battling life-threatening illness (e.g., cancer) were excluded from the study. Informed and written consent was obtained from all subjects in accordance with procedures of the Emory University Institutional Review Board. All subjects are part of the ongoing Emory Brain Imaging Project embedded in the Emory Healthy Aging and Healthy Brain Studies (Goetz et al., 2019).
Table 1.
characteristics of subjects included in the study
| HY | HO | |
|---|---|---|
| Age (mean ± SD) | 25.7 ± 2.85 years | 65.5 ± 5.87 years |
| Total sample size | 43 | 144 |
| Sex | 28 Women | 107 Women |
| 15 Men | 37 Men | |
| Years of education (mean ± SD) | 16.79 ± 0.32 | 16.73 ± 0.21 |
Magnetic Resonance Imaging (MRI):
Anatomical images were obtained on a 3T Siemens Prisma (Siemens AG, Erlangen, GER) scanner with a 32-channel coil. The high-resolution T1-weighted images were acquired with a T1 (MPRAGE) sequence with TR 2300ms, TE 2.96ms, TI 900ms, slice thickness 1mm, 208 slices, field of view 256 × 256mm2, flip angle 9 degrees, isotropic resolution 1 × 1 × 1mm.
Magnetic Resonance Spectroscopy (MRS):
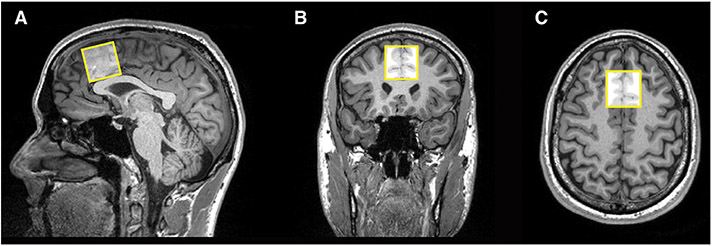
MRS was performed with 1) a Point-Resolved Spectroscopy (PRESS) single-voxel sequence with a short-echo time (TE 20ms, TR 20 0 0ms) with 100 averages, vector size 2048 points, flip angle 90 degrees, acquisition duration 1024ms, acquisition bandwidth 20 0 0Hz, water suppression bandwidth 135Hz, and 2) a Mescher-Garwood Point Resolved Spectroscopy (MEGA-PRESS) sequence (TE 68ms, TR 20 0 0ms) with 148 averages, vector size 2048 complex data points, flip angle 90 degrees, acquisition bandwidth 20 0 0Hz, acquisition duration 1024ms, editing pulse bandwidth 53Hz, ON editing pulse 1.90ppm, OFF editing pulse 7.50ppm, water suppression bandwidth 135Hz. Acquisition times were 3min for short-echo PRESS and 10min for MEGA-PRESS. Data were obtained from a 3 × 3 × 3cm 3 voxel positioned in the medial frontal cortex (MFC), superior to the genu of the corpus callosum and aligned with the shape of the corpus callosum, encompassing parts of Brodmann’ areas 24, 32 and 6 (see Fig. 1). Although frontal cortex often is thought to be targeted later in the development of AD pathology, frontal areas, including MFC, have been found to be more predictive of cognitive deficits and early disturbance of activities in daily living (Marshall et al., 2019; Stoeckel et al., 2013) and thus may be more sensitive to subclinical changes. Thus, it is possible that such subclinical changes could be attributable to changes in AD-related CSF biomarkers and frontal metabolites, a missing link in the literature since the frontal cortex is a less explored cerebral area in MRS studies in early AD. B0 shim was adjusted using the FASTESTMAP algorithm as implemented in the CMRR Spectroscopy package C2P (Gruetter, 1993; Gruetter & Tkáč, 2000), and frequency and eddy current corrections were performed prior to data processing. Spectra with poor water suppression, lipid contamination or movement artifact were excluded from further analyses.
Fig. 1.
Positioning of the MRS voxel on the medial frontal cortex (MFC) on the A) sagittal, B) coronal and C) axial planes.
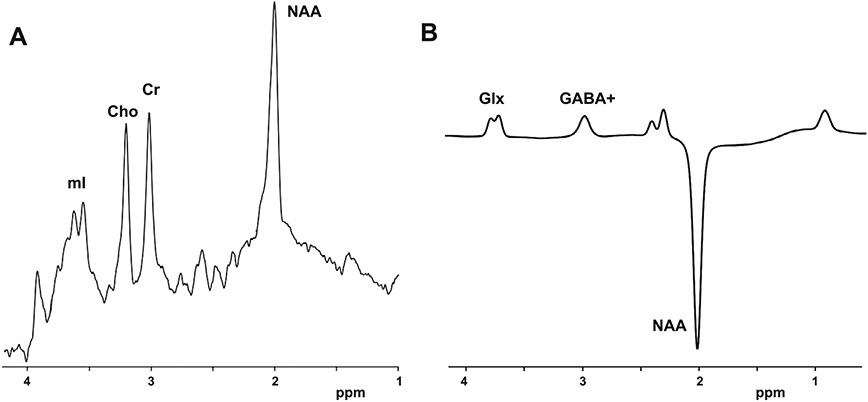
Concentration levels of metabolites from the PRESS sequence were estimated with LC Model (Provencher, 2001) and the MEGA-PRESS sequence with Gannet (Edden et al., 2014)(see Fig. 2). PRESS data were excluded when Cramer-Rao lower bounds (CRLBs) exceeded 10% and MEGA-PRESS data were excluded when normalized fitting residuals were below 10% to maintain a reliability standard in the estimation of metabolites. Metabolites were analyzed in absolute levels and normalized to total creatine (tCr). Normalization to tCr was utilized as internal control for changes in cellular metabolism within subject, and is frequently reported in MRS studies (Near et al., 2021). Estimation of absolute GABA levels with Gannet assumes a portion of macromolecules co-edited at 3ppm and is thus noted GABA+. Segmentation of the voxel was performed with SPM 12 on the MPRAGE images to obtain voxel tissue fraction of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF), and to perform partial volume correction. All MRS measurements were corrected for partial volume effects using a two-compartment model prior to statistical analyses (Harris et al., 2015).
Fig. 2.
Localization of metabolites of interest on sample MRS spectra from A) PRESS and B) MEGA-PRESS acquisition sequences.
CSF biomarkers:
Levels of biomarkers were obtained in HO with lumbar CSF sampling following the spinal tap procedure described in the multicentric study from Shaw and colleagues (Shaw et al., 2011). Concentration levels of Aβ1-42, t-tau and phosphorylated tau181 (p-tau) were obtained from one CSF sample per subject and estimated with an immunoassay biochemical test on a Luminex analytical platform (Luminex Corp., Austin, USA), run by AKESOgen Inc. (Atlanta, USA).
Neuropsychological assessments:
Cognitive measurements were obtained in HO subjects. General cognition was assessed with the Montreal Cognitive Assessment (MOCA) score (Nasreddine et al., 2005), short-term verbal memory with the Free Recall score (Grober & Buschke, 1987), and executive function with the Trail Making B score (Arnett & Labovitz, 1995).
Statistical analyses:
Metabolite levels were compared between the HY and HO groups with a two-sample t-test or Wilcoxon test when normality of distribution was not verified. Within the HO group, multiple linear regression analyses were performed to evaluate the effect of age (as predicting variable) on metabolite levels (as dependent variables), the predicting effect of GABA+ and mI/tCr on cognitive scores, and the predicting effect of levels of Aβ1-42,p-tau and t-tau on levels of GABA+ and mI/tCr and cognitive scores. Results are reported significant at α=0.05 after correcting for false discovery rate with the Hochberg-Benjamini procedure (Benjamini & Hochberg, 1995). Multiple regression models were controlled for age and sex. Adjusted R2 accounting for multiple predictors and covariates are reported. Linear analyses were not performed on HY, as the focus of the study was on the older group. Mediation analyses for the effects of age and metabolites on cognitive scores were performed with 1000 samples bootstrapping procedure, unstandardized indirect effects were computed for each of the bootstrapped samples, and the 95% confidence interval was computed by the indirect effects at the 2.5th and 97.5th percentiles. All analyses were performed using R (R Foundation for Statistical Computing, Vienna, AUS).
3. Results
3.1. Group-based comparisons of demographic variables
Age and MRS voxel tissue fraction of GM, WM and CSF were compared in HY and HO. For age, as expected, the two-sample t-test was significant (p < 0.001)(means ± standard deviations: HY 25.7 ± 2.85 years; HO 65.5 ± 5.87 years).
3.2. GABA+ and mI/tCr concentrations as a function of age
For voxel tissue fraction of GM, WM and CSF, the two-sample t-test was significantly different for GM (p < 0.001; HY 0.51 ± 0.03, HO 0.44 ± 0.04) and CSF fraction (p < 0.001; HY 0.14 ± 0.03, HO 0.19 ± 0.05) but not for WM (p = 0.274; HY 0.36 ± 0.04, HO 0.36 ± 0.06).
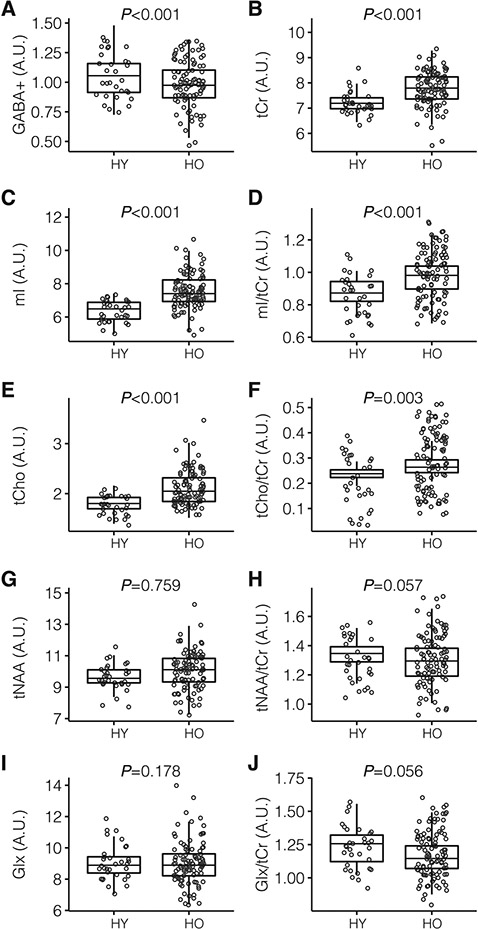
MRS metabolites were also compared in HY and HO. The two-sample t-test showed levels of GABA+ (P < 0.001; HY 1.05 ± 0.17, HO 0.94± 0.17), Ins (p < 0.001; HY 6.38 ± 0.65, HO 7.60 ± 1.15), tCho ( p < 0.001; HY 1.80 ± 0.17, HO 2.10 ± 0.35), tCr (p < 0.001; HY 7.25 ± 0.50, HO 7.73 ± 0.79) were significantly different. Levels of Glx (p = 0.178; HY 9.33± 1.30, HO 8.74 ± 1.04) and tNAA (p = 0.759; HY 9.66 ± 0.83, HO 9.75 ± 1.24) did not reach significance. When normalizing metabolites to tCr, levels of mI/tCr (p < 0.001; HY 0.89 ± 0.08, HO 0.98 ± 0.11) and tCho/tCr (p = 0.0032; HY 0.248 ± 0.022, HO 0.268 ± 0.035) were significantly different, but levels of tNAA/tCr (P = 0.057; HY 1.34 ± 0.11, HO 1.30 ± 0.15) were not (see Fig. 3 and Table 2 for summary).
Fig. 3.
Box and whisker diagrams representing distribution of A) GABA+, B) tCr, C) mI, D) mI/tCr, E) Cho, F) tCho/tCr, G) tNAA, H) tNAA/tCr, I) Glx, J) Glx/tCr, in HY and HO groups. Results are given in arbitrary units (A.U.).
Table 2.
summary of measurements obtained in each group (mean ± SD)
| HY | HO | ||
|---|---|---|---|
| MRS measurement | GABA+ | 1.05 ± 0.17 | 0.94 ± 0.17 |
| N = 38 | N = 105 | ||
| GABA+/ tCr | 0.15 ± 0.025 | 0.12 ± 0.025 | |
| N = 38 | N = 105 | ||
| tCr | 7.25 ± 0.50 | 7.73 ± 0.79 | |
| N = 38 | N = 105 | ||
| mI | 6.38 ± 0.65 | 7.60 ± 1.15 | |
| N = 38 | N = 105 | ||
| mI/tCr | 0.89 ± 0.08 | 0.98 ± 0.11 | |
| N = 38 | N = 105 | ||
| tCho | 1.80 ± 0.17 | 2.10 ± 0.35 | |
| N = 38 | N = 105 | ||
| tCho/tCr | 0.248 ± 0.022 | 0.268 ± 0.035 | |
| N = 38 | N = 105 | ||
| tNAA | 9.66 ± 0.83 | 9.75 ± 1.24 | |
| N = 38 | N = 105 | ||
| tNAA/tCr | 1.34 ± 0.11 | 1.30 ± 0.15 | |
| N = 38 | N = 105 | ||
| Glu+Gln (Glx) | 9.33 ± 1.30 | 8.74 ± 1.04 | |
| N = 40 | N = 105 | ||
| Glx/tCr | 1.28 ± 0.36 | 1.18 ± 0.43 | |
| N = 40 | N = 105 | ||
| MRS voxel tissue fraction | GM | 0.51 ± 0.03 | 0.44 ± 0.04 |
| N = 40 | N = 105 | ||
| WM | 0.36 ± 0.04 | 0.36 ± 0.06 | |
| N = 40 | N = 105 | ||
| CSF | 0.14 ± 0.03 | 0.19 ± 0.05 | |
| N = 40 | N = 105 |
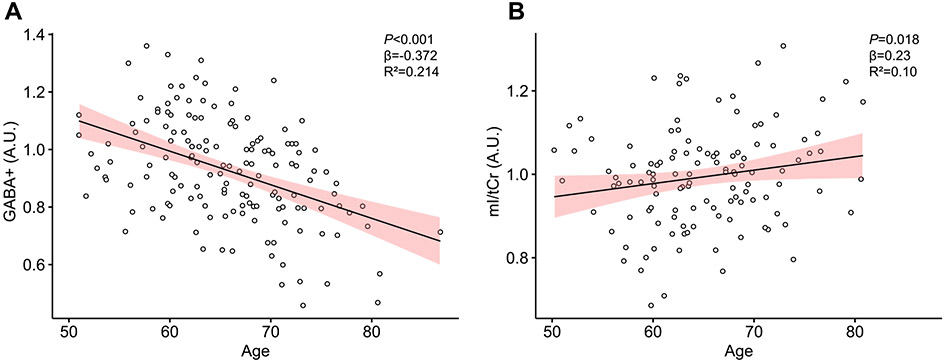
With the use of multiple linear regression models with age as predictor and sex as covariate, higher age in HO significantly predicted lower levels of GABA+ (R2=0.214; F2,128=21.36; p <0.001) and higher levels of mI/tCr (R2 = 0.10; F2,117=7.636; p = 0.018).
For GABA+, age was a significant predictor (β = −0.372; p <0.001) and sex (β = 0.138; p = 0.089) was not significant. For mI/tCr, the effect of age was significant but more modest (β = 0.23; p = 0.0138) and sex was not significant (β = −0.109; p = 0.266) (see Fig. 4).
Fig. 4.
Scatterplots representing A) the association of age and GABA+ levels, and B) the association of age and mI/tCr levels. Age is given in years and metabolite levels in arbitrary units (A.U.). Confidence interval are 95% for the regression lines.
3.3. Cognitive performance as a function of age, GABA+ and mI/tCr concentrations
Age significantly predicted Free Recall (R2=0.061; F1,95=7.212; β = −0.27; pP = 0.009) and Trail Making B (R2 = 0.081; F1,108=10.57; β = 0.29; pP = 0.002) scores, but not MOCA scores.
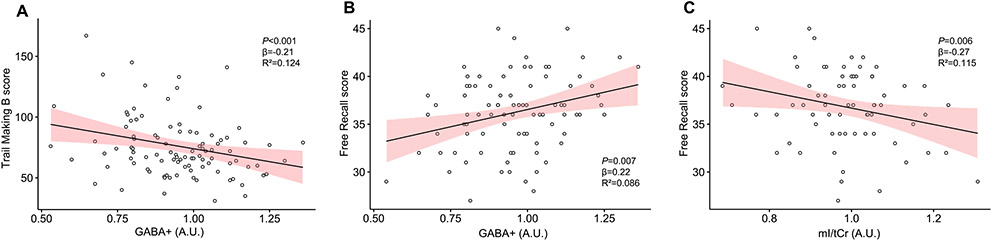
With the use of multiple linear regression models with GABA+ as predictor, Trail Making B performance was significantly predicted by levels of GABA+ (R2 = 0.124; F3,95=3.655; β = −0.21; p <0.001) where lower levels of GABA+ predicted worse performance. Age and sex were not significantly associated with scores (see Fig. 5A).
Fig. 5.
Scatterplot representing the association of A) levels of GABA+ and Trail Making B score, B) levels of GABA+ and Free Recall score and C) levels of mI/tCr and Free Recall score. Metabolite levels are given in arbitrary units (A.U.). Confidence interval is 95% for the regression line.
Levels of GABA+ (R2 = 0.086; F3,82=3.335; β=0.22; p = 0.007) positively predicted higher Free Recall scores and mI/tCr (R2 = 0.115; F3,66=3.983; β =−0.27; p = 0.006) predicted lower Free Recall scores. In both models, age and sex were not significant (see Fig. 5B and 5C).
Levels of GABA+ and mI/tCr were examined as mediators of the relation between age and scores for Free Recall and Trail Making B. Mediation analyses showed the effect of age on Free Recall scores was significantly mediated by levels of GABA+ (indirect effect = −0.059, CI(−0.1294; −0.01), p= 0.018) but not mI/tCr (P=0.068). Trail Making B scores were not significantly mediated by either metabolite.
3.4. MRS metabolites as a function of AD-related CSF biomarkers
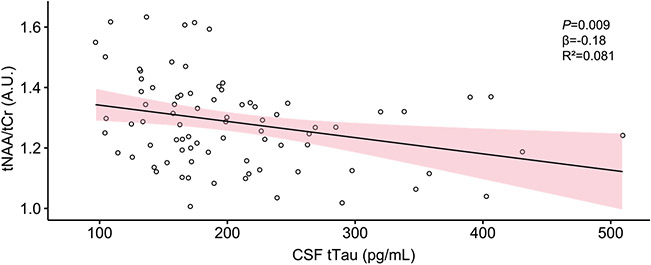
With the use of multiple linear regression, we found that concentration of tNAA/tCr was significantly predicted by levels of CSF t-tau (R2 = 0.081; F3,77=3.541; p= 0.009), where elevated levels of t-tau predicted lower levels of tNAA/tCr (β = −0.18); age and sex were not significantly associated (see Fig. 6). There were no other MRS metabolites predicted by any CSF biomarkers.
Fig. 6.
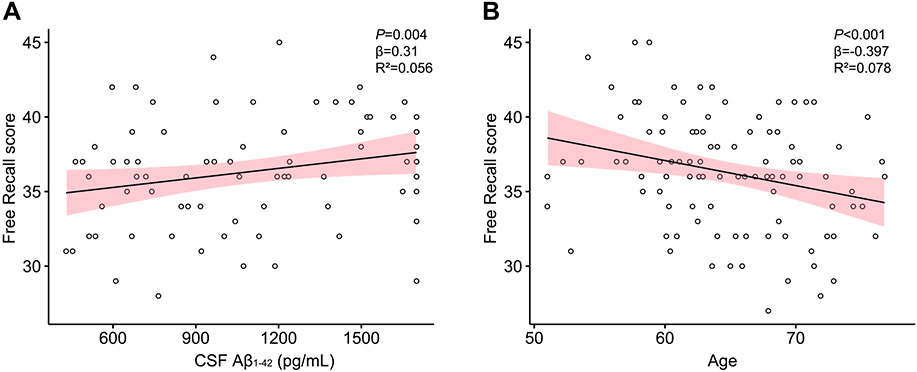
Scatterplots representing A) the association of CSF Aβ1-42 levels and Free Recall score, and B) the association of age and Free Recall scores. CSF Aβ1-42 levels are given in picogram per milliliter (pg/mL) and age in years. Confidence intervals are 95% for the regression lines.
3.5. Cognitive performance as a function of AD-related CSF biomarkers
Finally, with the use of multiple linear regression models with CSF Aβ1-42 as predictor with age and sex as covariates, Free Recall scores were significantly (R2=0.188; F3,72=9.682; p< 0.001) predicted by CSF Aβ1-42 (β = 0.31, p = 0.0043) and age (β = −0.397, pP <0.001), such that higher levels of CSF Aβ1-42 and lower age predicted better cognitive performance. Sex was not significantly associated (see Fig. 7).
Fig. 7.
Scatterplot representing CSF t-tau and tNAA/tCr levels. CSF t-tau levels are given in picogram per milliliter (pg/mL) and metabolites in arbitrary units (A.U.). Confidence interval is 95% for the regression line.
4. Discussion
Numerous studies have demonstrated the importance of proteins Aβ1-42 and tau in the pathogenesis of AD, although their respective physiological roles are still not completely understood. We sought to investigate the relationship between 1) chronological aging and 2) asymptomatic AD pathology based on expression of CSF Aβ1-42, p-tau and t-tau in a sample of healthy older adults using multiple MRS sequences (i.e. PRESS and MEGA-PRESS) in a single voxel located in the medial frontal cortex. Additionally, we obtained neuropsychological test scores and observed the relationship of MRS metabolite levels and AD-related CSF biomarkers to cognitive performance.
We found that normal aging is associated with a decrease in levels of GABA+ and an increase in levels of mI/tCr. These results were obtained in group comparisons between HY and HO and within the HO group with an age range of 50-80 years. More importantly, we found that levels of frontal GABA+ predicted behavior changes with age better than other metabolites.
Our results show a decrease in levels of GABA+ with chronological aging, similarly to previous reports in the literature (Gao et al., 2013; Porges et al., 2017). Porges and colleagues (2017) have shown that GABA+ decreases as a function of age in healthy older subjects and lower levels of frontal GABA+ are correlated to lower cognitive performance. Moreover, studies suggest that GABA levels are further decreased in AD patients compared to older healthy subjects (Bai et al., 2015) and this may be caused by Aβ1-42 damaging the membrane and presynaptic terminals in GABA neurons (Marttinen et al., 2015). We did not find any significant relationship between AD-related CSF biomarkers and levels of GABA+, thus not supporting a correlation of asymptomatic AD pathology on actual levels of GABA+ in the MFC. The present results suggest that in the current sample, decrease in frontal GABA+ could be strictly age-related.
Based on previous literature, these results also support the idea of an elevation of neuroinflammation in healthy older adults, as reflected by the elevation of mIL, mIL/tCr, Cho and tCho/tCr. Indeed, ml is frequently associated with glial proliferation and neuroinflammation, although that relationship remains to be demonstrated more clearly (Rae, 2014). Previous MRS studies have shown that levels of mI are elevated in older compared to younger subjects in the frontal white matter (Ross et al., 2006), supraventricular white matter (Raininko & Mattsson, 2010) and posterior cingulate cortex (Marjańska et al., 2017; Nedelska et al., 2017). Increases in posterior mI have also been reported with elevated levels of CSF Aβ1-42 (Murray et al., 2014), and with risk of progression to MCI (Kantarci et al., 2013). Recent evidence also reported elevated levels of mI in the ACC in older subjects (Lind et al., 2020). Our results show that this may also be the case in the MFC of healthy older adults, suggesting the possibility that this is an age-related phenomenon and not solely ascribable to the expression of proteins Aβ42 and tau in the current sample. Further, when considering the previous literature, expression of mI/tCr in the MFC and posterior cortex suggest the relationships are systemic rather than localized.
Our results also show an elevation of tCho/tCr with age when comparing HY and HO. Prior MRS studies show inconsistent findings with Cho and aging: studies have reported both increases (Marjańska et al., 2017) and decreases (Maudsley et al., 2012). Cerebral Cho is concentrated in phospholipids and changes in its concentration are regularly associated with membrane turnover (Rae, 2014). Cho levels could also be impacted by AD pathology; authors have suggested that an elevation of Cho could represent demyelination (Tartaglia et al., 2002). Thus, it is possible that changes in Cho could be a collateral of Aβ42 - or tau-associated neuronal damage seen in later, symptomatic stages of AD and were not possible to detect in our sample.
With the use of multiple linear regression models predicting performance at three cognitive tasks, we found that levels of frontal mI/tCr and GABA+ differently predict performance at a memory task and, more importantly, levels of frontal GABA+ predict behavior changes with age better than other metabolites. Indeed, our results suggest that GABA+ is the metabolite that most strongly predicts cognitive performance. When controlled for age and sex, levels of GABA+ predicted performance at the Free Recall and Trail Making B tasks. The relationship between levels of GABA+ and MOCA scores was trending, even when controlled for age and sex, but failed to reach significance with adjustment for multiple comparisons (unadjusted p = 0.027; R2 = 0.071). These results are in line with previous works that have observed GABA+ decreases as a function of age in healthy older subjects (Gao et al., 2013; Porges et al., 2017) and that lower levels of frontal GABA+ are associated with lower cognitive performance on the MOCA (Porges et al., 2017). Additionally, our results from the mediation analysis show that the direct effect of age on memory performance may be partly mediated by levels of frontal GABA+. Previous works have elicited the importance of GABA in working memory (Michels et al., 2012) and cognition in general. Our results strengthen previous findings and suggest that frontal GABA levels may be sensitive enough to predict performance on several tests indicative of various cognitive processes.
Our results also demonstrate that levels of mIL/Cr were negatively correlated with Free Recall, supporting the idea that elevation of mIrelative to tCr may reflect a detrimental process on cognition in chronological aging, or metabolic differences associated with early decline in performance in Free Recall. Previous results have shown that poorer MMSE scores were correlated to greater mIL levels in the posterior cingulate cortex (Voevodskaya et al., 2019). Our results, taken in the context of previous literature, support the idea that this elevation in mI may be systemic and affect several cerebral structures.
In analyses exploring the relationships of AD-related biomarkers and MRS brain metabolites, we found that levels of t-tau significantly predicted levels of tNAA/tCr, and the relationship re-ained significant when controlled for age and sex. This showed that increased accumulation of t-tau is correlated to lower levels of tNAA/tCr. NAA being regularly associated with general neuronal health, this result suggests that in this sample, levels of tau and not Aβ1-42 may be detrimental to neurological health. Similar results have been reported with a voxel located in the precuneus, where levels of tNAA/tCr were negatively correlated to levels of CSF t-tau (Voevodskaya et al., 2019). Previous reports have suggested that decreases in NAA/Cr are thought to appear later in the development of AD pathology and usually after increases of mIL/Cr are detected (Kantarci et al., 2007). Our result suggests that this relationship may be detectable in the frontal cortex as well suggesting more systemic brain changes and, interestingly, that this may be observable in cognitively normal healthy older adults. Levels of tNAA/tCr were not correlated with cognitive scores in our sample, suggesting that t-tau has a physiological effect, although not predictive of cognitive performance at this stage. This is supported by several reports suggesting that tau impacts cognition later on in MCI and AD pathology (Johnson et al., 2016). Interestingly, we did not observe a significant decrease in tNAA/tCr in older subjects compared to HY, something reported in prior MRS studies, although not unanimously (Cleeland et al., 2019). Such changes are associated with normal aging of gray matter (Cleeland et al., 2019) and AD (Kantarci et al., 2013; Murray et al., 2014).
Finally, in analyses exploring the relationship of AD-related biomarkers to MRS metabolites on cognitive performance, we found that levels of CSF Aβ1-42 predicted performance at the Free Recall memory task but not the other tasks. When controlled for age and sex, the relationship remained significant and suggested that higher CSF Aβ1-42 correlated with better performance at the task; age as a covariate shared half of the variance, suggesting an important concurrent aging effect. This is in line with previous works showing levels of CSF- and PET-Aβ associated with higher risk of cognitive decline in healthy older individuals (Donohue et al., 2017), with memory encoding impairments in cognitively normal older adults (Edelman et al., 2017; Song et al., 2016), and with impaired default network and memory function in asymptomatic older adults (Sperling et al., 2009). It also suggests that elevated CSF Aβ1-42 could be an additional determining factor in cognitive performance in the present sample, with advanced chronological age and decreased concentration of frontal GABA+. This complements previous findings in the way that chronological age predicts levels of GABA+ (Porges et al., 2017), but differential expression of Aβ1-42 could be an additional determining factor for cognitive performance. These results suggest an important role for elevated brain expression of Aβ1-42 in healthy older adults that may be related to neuroinflammation. In our sample, however, there was no significant correlation between levels of mIL/tCr and Aβ1-42, limiting our interpretation in healthy older adults. Although we do know that Aβ1-42 is essential in the development of AD, it also requires certain physiological conditions to provoke cell toxicity; the most important one thought to be the presence of tau pathology via overexpression of phosphorylated tau (Ittner & Götz, 2011). However, prior works suggest that Aβ42 (Pereira et al., 2018), not t-tau (Franzmeier et al., 2020), is detectable in the frontal cortex in early aging. Other key factors like mitochondrial dysfunction, cytokine trafficking, lipid processing and cellular pH are thought to hold secondary roles in igniting AD-related cell toxicity (Marttinen et al., 2015). In this perspective, it may be possible that healthy older adults with elevated expression of t-tau and Aβ42 are more vulnerable to development of AD pathology though it is not yet impacting levels of most brain metabolites detectable with MRS at 3T Table 3.
Table 3.
summary of cognitive and CSF biomarker measurements obtained in the HO group
| Cognitive measurement | MOCA | 26.07 ± 2.63 |
| N = 112 | ||
| Free Recall | 36.28 ± 3.87 | |
| N = 97 | ||
| Trail Making B | 78.94 ± 36.31 | |
| N = 112 | ||
| CSF biomarker measurement (pg/mL) | Aβ1-42 | 1093.6 ± 418.0 |
| N = 117 | ||
| p-tau | 19.69 ± 12.18 | |
| N = 117 | ||
| t-tau | 214.73 ± 114.79 | |
| N = 117 |
Taken together, our results closely support previous MRS and behavioural evidence obtained from similar cohorts from voxels located in the posterior regions of the cortex. This suggests that MRS is sensitive enough to discriminate physiological differences ascribable to normal healthy aging and pathological aging, in the frontal cortex. In the context of previous literature, our results also suggest that in healthy aging, changes in mIL and GABA may be systemic and independent of early expression of t-tau and Aβ1-42 . This highlights the need for additional research combining MRS with PET or CEST, in order to correlate spatial distributions of AD-related biomarkers t-tau and Aβ1-42 and MRS metabolites.
Some limitations in our work need to be acknowledged. A technical limitation of this study is that we did not control for T2 relaxation times of water and metabolites when comparing HY and HO subjects. A previous study shows T2 changes with age at a gradual rate (Kirov et al., 2008). As another limitation, we did not collect a separate spectrum of macromolecules and instead utilized the built-in macromolecular basis set from LCModel. Recent literature suggests that macromolecules may increase with age (Marjańska et al., 2018), indicating that a participant-specific macromolecule set should be collected and utilized during analysis to improve precision of quantification of all metabolites. Our results show a decrease of GABA+ with age, which is a replication of previous studies (Gao et al., 2013; Porges et al., 2017). Because macromolecular content increases with age and GABA+ contains some contribution of macromolecules, our aging-related decrease in GABA+ is potentially underestimated, but it is unlikely that GABA+ is constant with aging, a notion strongly supported by recent metanalytic evidence (Porges et al., 2021). Further, our results show that mIL/tCr increases with age, which is in accordance with a recent report that also showed elevation in mI at 7T (Lind et al., 2020). Future work is needed to improve metabolite quantification in order to build consensus in the directionality of aging-related mIL changes. Indeed, the link between mI with normal age-related or pathological neuroinflammation needs to be established more robustly. Although early MRS studies have associated mI with glial activation through observation of elevated levels of mIL in cultured astrocytes (Brand et al., 1993; Glanville et al., 1989), other in vivo reports suggest it may not be related to gliosis or glial proliferation (Kim et al., 2005). From that perspective, correlating MRS-obtained levels of mIL with serum-based markers of inflammation like cytokines and inflammatory complexes (e.g., NF-κB) is warranted and could prove beneficial for further aging research. Another limitation is the variation in sample sizes for our different groups; larger cohorts and a longitudinal design with several MRS acquisitions are warranted for more robust statistical interpretation. Also, the use of CSF measurements for Aβ1-42 and tau offers no spatial specificity in the brain compared to other methods (e.g., PET) and has lower accuracy for diagnosis of AD and MCI (Ritchie et al., 2017), although this is beyond the scope of this work. Finally, it has to be stated that our cohort is composed of mostly women, although sex has been controlled in statistical analyses. We did not obtain information about menstrual cycle because the average age for menopause in the US is 49 years (McKnight et al., 2011). Although our HO cohort is older than 50 years of age, this may have impacted levels of GABA+ measured in females from the HY cohort and should be considered in future works.
5. Conclusion
Identifying biomarkers impacted by the expression of proteins Aβ1-42 and tau is critical in the early identification of AD in healthy older adults and in the broad understanding of the neurophysiology of AD. In this study, we have found that normal chronological aging predicts a decrease in frontal levels of GABA+ and an increase in levels of mI and mI/tCr. These results were obtained in group comparisons between HY and HO and within the HO group with an age range of 50-80 years. More importantly, we found that levels of frontal GABA+ predict cognitive changes with age better than other metabolites. Additionally, we have found a significant correlation between expression of t-tau and tNAA/tCr, suggesting that tau may impact neuronal health early on in cognitively normal older adults. Taken together, our findings suggest that MRS is a viable tool in the investigation of biomarkers depicting aging and AD-associated risk factors. We believe the investigation with MRS of mIL/tCr and neuroinflammatory compounds in relation to levels of CSF AD biomarkers Aβ1-42 and tau in healthy older subjects should be replicated with a more robust sample size, with longitudinal measurements and additional sequences to estimate other metabolites of interest.
Acknowledgement
The authors wish to mention the support of the Goizueta Foundation in the Emory Brain Imaging Project and Emory Brain Health Study.
Funding
AHB is supported by a research fellowship from the Fonds de Recherche en Santé du Québec. BC is supported by a Senior Research Career Scientist Award #B6364-L from the US Department of Veterans Rehabilitation Research & Development Service.
Footnotes
Disclosure statement
The authors have no conflict of interest.
References
- Arnett JA, Labovitz SS, 1995. Effect of physical layout in performance of the trail making test. Psychol Assess.,t 7 (2), 220. [Google Scholar]
- Bai X, Edden RA, Gao F, Wang G, Wu L, Zhao B, Wang M, Chan Q, Chen W, Barker PB, 2015. Decreased γ -aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J Magn Reson Imaging. 41 (5), 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 57 (1), 289–300. [Google Scholar]
- Blennow K, Shaw LM, Stomrud E, Mattsson N, Toledo JB, Buck K, Wahl S, Eichenlaub U, Lifke V, Simon M, 2019. Predicting clinical decline and conversion to Alzheimer’s disease or dementia using novel Elecsys Aβ (1–42). pTau and tTau CSF immunoassays. Scientific reports 9 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D, 1993. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 15 (3-5), 289–298. [DOI] [PubMed] [Google Scholar]
- Cichocka M, Beres A, 2018. From fetus to older age: a review of brain metabolic changes across the lifespan. Ageing Res Rev. 46, 60–73. [DOI] [PubMed] [Google Scholar]
- Cleeland C, Pipingas A, Scholey A, White D, 2019. Neurochemical changes in the aging brain: a systematic review. Neurosci Biobehav Rev. 98, 306–319. [DOI] [PubMed] [Google Scholar]
- Dean DC, Hurley SA, Kecskemeti SR, O’Grady JP, Canda C, Davenport-Sis NJ, Carlsson CM, Zetterberg H, Blennow K, Asthana S 2017. Association of amyloid pathology with myelin alteration in preclinical Alzheimer disease. JAMA Neurol. 74 (1), 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MC, Sperling RA, Petersen R, Sun C-K, Weiner MW, Aisen PS, Initiative, A.s.D.N., 2017. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. Jama 317 (22), 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ, 2014. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 40 (6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman K, Tudorascu D, Agudelo C, Snitz B, Karim H, Cohen A, Mathis C, Price J, Weissfeld L, Klunk W, 2017. Amyloid-beta deposition is associated with increased medial temporal lobe activation during memory encoding in the cognitively normal elderly. Am J Geriatr Psychiatry. 25 (5), 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N, Neitzel J, Rubinski A, Smith R, Strandberg O, Ossenkoppele R, Hansson O, Ewers M, 2020. Functional brain architecture is associated with the rate of tau accumulation in alzheimer’s disease. Nat Commun. 11 (1), 1–17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Barker PB, 2014. Various MRS application tools for Alzheimer disease and mild cognitive impairment. Am J Neuroradiol. 35 (6), S4–S11 suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N, Byers D, Cook H, Spence M, Palmer FSC, 1989. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1004 (2), 169–179. [DOI] [PubMed] [Google Scholar]
- Goetz ME, Hanfelt JJ, John SE, Bergquist SH, Loring DW, Quyyumi A, Clifford GD, Vaccarino V, Goldstein F, Johnson TM 2nd, 2019. Rationale and design of the emory healthy aging and emory healthy brain studies. Neuroepidemiology. 53 (3-4), 187–200. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, 1987. Genuine memory deficits in dementia. Dev Neuropsychol. 3 (1), 13–36. [Google Scholar]
- Gruetter R, 1993. Automatic, localized in vivo adjustment of all first-and second-order shim coils. Magn Reson Med. 29 (6), 804–811. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkáč I, 2000. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 43 (2), 319–323. [DOI] [PubMed] [Google Scholar]
- Gueli MC, Taibi G, 2013. Alzheimer’s disease: amino acid levels and brain metabolic status. Neurol Sci. 34 (9), 1575–1579. [DOI] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM, 2009. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 30 (3), 353–363 . [DOI] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Edden RA, 2015. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 42 (5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Abe K, Sakoda S, Sawada T, 2002. Proton MR spectroscopic study at 3 Tesla on glutamate/glutamine in alzheimer’s disease. Neuroreport. 13 (1), 183–186. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14 (4), 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Götz J, 2011. Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 12 (2), 67–72. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, 2018. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14 (4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Initiative, A.s.D.N., 2021. Temporal dynamics of β-amyloid accumulation in aging and alzheimer disease. Neurol. 96 (9), e1347–e1357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, 2016. Tau positron emission tomographic imaging in aging and early A lzheimer disease. Ann Neurol. 79 (1), 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Jack C, Xu Y, Campeau N, O’Brien P, Smith G, Ivnik R, Boeve B, Kokmen E, Tangalos E, 2000. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurol. 55 (2), 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Reynolds G, Petersen RC, Boeve BF, Knopman DS, Edland SD, Smith GE, Ivnik RJ, Tangalos EG, Jack CR, 2003. Proton MR spectroscopy in mild cognitive impairment and Alzheimer disease: comparison of 1.5 and 3 T. Am J Neuroradiol. 24 (5), 843–849. [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Weigand SD, Petersen RC, Boeve BF, Knopman DS, Gunter J, Reyes D, Shiung M, O’Brien PC, Smith GE, 2007. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. v Neurobiol Aging. 28 (9), 1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Weigand SD, Przybelski SA, Preboske GM, Pankratz VS, Vemuri P, Senjem ML, Murphy MC, Gunter JL, Machulda MM, 2013. MRI and MRS predictors of mild cognitive impairment in a population-based sample. Neurol. 81 (2), 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JP, Lentz MR, Westmoreland SV, Greco JB, Ratai EM, Halpern E, Lackner AA, Masliah E, González RG, 2005. Relationships between astrogliosis and 1H MR spectroscopic measures of brain choline/creatine and myo-inositol/creatine in a primate model. Am J Neuroradiol. 26 (4), 752–759. [PMC free article] [PubMed] [Google Scholar]
- Kirov II, Fleysher L, Fleysher R, Patil V, Liu S, Gonen O, 2008. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3 T. Magn Reson Med. 60 (4), 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPoint MR, Chhatwal JP, Sepulcre J, Johnson KA, Sperling RA, Schultz AP, 2017. The association between tau PET and retrospective cortical thinning in clinically normal elderly. Neuroimage. 157, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H, 2016. Implications of GABAergic neurotransmission in Alzheimer’s disease. Front Aging Neurosci. 8, 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind A, Boraxbekk C-J, Petersen ET, Paulson OB, Siebner HR, Marsman A, 2020. Regional myo-inositol, creatine, and choline levels are higher at older age and scale negatively with visuospatial working memory: a cross-sectional proton MR spectroscopy study at 7 tesla on normal cognitive ageing. J Neurosci. 40 (42), 8149–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanska M, Deelchand DK, Hodges JS, McCarten JR, Hemmy LS, Grant A, Terpstra M, 2018. Altered macromolecular pattern and content in the aging human brain. NMR in Biomedicine. 31 (2), e3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjańska M, McCarten JR, Hodges J, Hemmy LS, Grant A, Deelchand DK, Terpstra M, 2017. Region-specific aging of the human brain as evidenced by neurochemical profiles measured noninvasively in the posterior cingulate cortex and the occipital lobe using 1H magnetic resonance spectroscopy at 7 T. Neurosci. 354, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GA, Gatchel JR, Donovan NJ, Muniz MC, Schultz AP, Becker JA, Chhatwal JP, Hanseeuw BJ, Papp KV, Amariglio RE, 2019. Regional tau correlates of instrumental activities of daily living and apathy in mild cognitive impairment and Alzheimer’s disease dementia. J Alzheimer’s Dis. 67 (2), 757–768 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marttinen M, Kurkinen KM, Soininen H, Haapasalo A, Hiltunen M, 2015. Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Mol Neurodegener. 10 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley AA, Govind V, Arheart K, 2012. Associations of age, gender and body mass with 1H MR-observed brain metabolites and tissue distributions. NMR in Biomedicine 25 (4), 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight KK, Wellons MF, Sites CK, Roth DL, Szychowski JM, Halanych JH, Cushman M, Safford MM, 2011. Racial and regional differences in age at menopause in the United States: findings from the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Obstet Gynecol. 205 (4), 353 e351–353. e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, Lüchinger R, Brandeis D, O’Gorman RL, 2012. Frontal GABA levels change during working memory. PloS one 7 (4), e31933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Przybelski SA, Lesnick TG, Liesinger AM, Spychalla A, Zhang B, Gunter JL, Parisi JE, Boeve BF, Knopman DS, 2014. Early Alzheimer’s disease neuropathology detected by proton MR spectroscopy. J Neurosci. 34 (49), 16247–16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53 (4), 695–699. [DOI] [PubMed] [Google Scholar]
- Near J, Harris AD, Juchem C, Kreis R, Marjańska M, Öz G, Slotboom J, Wilson M, Gasparovic C, 2021. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts’ consensus recommendations. NMR in Biomed. 34 (5), e4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelska Z, Przybelski SA, Lesnick TG, Schwarz CG, Lowe VJ, Machulda MM, Kremers WK, Mielke MM, Roberts RO, Boeve BF, 2017. 1H-MRS metabolites and rate of β-amyloid accumulation on serial PET in clinically normal adults. Neurol. 89 (13), 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Strandberg TO, Palmqvist S, Volpe G, Van Westen D, Westman E, Hansson O, Initiative, A.s.D.N., 2018. Amyloid network topology characterizes the progression of Alzheimer’s disease during the predementia stages. Cereb Cortex. 28 (1), 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Jensen G, Foster B, Puts NA, 2021. The trajectory of cortical GABA across the lifespan, an individual participant data meta-analysis of edited MRS studies. Elife. 10, e62575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, 2017. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn. 2 (1), 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CD, 2014. A guide to the metabolic pathways and function of metabolites observed in human brain 1 H magnetic resonance spectra. Neurochem Res. 39 (1), 1–36 . [DOI] [PubMed] [Google Scholar]
- Raininko R, Mattsson P, 2010. Metabolite concentrations in supraventricular white matter from teenage to early old age: a short echo time 1H magnetic resonance spectroscopy (MRS) study. Acta Radiol. 51 (3), 309–315 . [DOI] [PubMed] [Google Scholar]
- Ritchie C, Smailagic N, Noel-Storr AH, Ukoumunne O, Ladds EC, Martin S, 2017. CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Sachdev PS, Wen W, Brodaty H, 2006. Longitudinal changes during aging using proton magnetic resonance spectroscopy. The J. Gerontol A Biol Sci Med Sci. 61 (3), 291–298 . [DOI] [PubMed] [Google Scholar]
- Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, 2016. PET imaging of tau deposition in the aging human brain. Neuron 89 (5), 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean RA, 2011. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol Commun. 121 (5), 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, McDonough IM, Liu P, Lu H, Park DC, 2016. Cortical amyloid burden and age moderate hippocampal activity in cognitively-normal adults. NeuroImage Clin. 12, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, 2009. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 63 (2), 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Stewart CC, Griffith HR, Triebel K, Okonkwo OC, Den Hollander JA, Martin RC, Belue K, Copeland JN, Harrell LE, 2013. MRI volume of the medial frontal cortex predicts financial capacity in patients with mild Alzheimer’s disease. Brain Imaging Behav 7 (3), 282–292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Narayanan S, De Stefano N, Arnaoutelis R, Antel S, Francis S, Santos A, Lapierre Y, Arnold D, 2002. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J Neurol. 249 (10), 1382–1390. [DOI] [PubMed] [Google Scholar]
- Voevodskaya O, Poulakis K, Sundgren P, van Westen D, Palmqvist S, Wahlund L-O, Stomrud E, Hansson O, Westman E, Group, S.B.S., 2019. Brain myoinositol as a potential marker of amyloid-related pathology: a longitudinal study. Neurol.y 92 (5), e395–e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Chanraud S, Gu M, Sullivan EV, Pfefferbaum A, 2013. In vivo glutamate measured with magnetic resonance spectroscopy: behavioral correlates in aging. Neurobiol Aging. 34 (4), 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]