Abstract
Following a request from the European Commission, EFSA was asked to deliver a scientific opinion on the safety and efficacy of an essential oil obtained from gum resin of Ferula assa‐foetida L. (asafoetida oil), when used as a sensory additive (flavouring) in feed for dogs and cats. The EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) concluded that the use of asafoetida oil is safe at the proposed conditions of use of 1.5 mg/kg complete feed for dogs and 0.2 mg/kg complete feed for cats. The additive under assessment should be considered as irritant to skin and eyes, and as a dermal and respiratory sensitiser. Since F. assa‐foetida and its preparations are recognised to flavour food, and its function in feed would be essentially the same as that in food, no further demonstration of efficacy was considered necessary.
Keywords: sensory additives, flavouring compounds, Ferula assa‐foetida L., asafoetida oil, safety, component‐based approach
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/2003 1 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7. In addition, Article 10(2) of that Regulation specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, within a maximum of 7 years after the entry into force of this Regulation.
The European Commission received a request from Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG) 2 for authorisation/re‐evaluation of 29 preparations (namely dill herb oil, dill seed extract, dill tincture, dong quai tincture, celery seed oil, celery seed extract (oleoresin), celery tincture, hares ear tincture, caraway seed oil, caraway oleoresin/extract, coriander oil, cumin oil, taiga root extract (solvent‐based, sb), taiga root tincture, fennel oil, fennel tincture, common ivy extract (sb), opoponax oil, ginseng tincture, parsley oil, parsley tincture, anise oil, anise tincture, ajowan oil, Ferula assa‐foetida oil, anise star oil, anise star tincture, anise star terpenes and omicha tincture) belonging to botanically defined group (BDG) 02 – Apiales/Austrobaileyales when used as feed additives for all animal species (category: sensory additives; functional group: flavourings). During the assessment, the applicant withdrew the application for nine preparations (namely dill seed extract, celery seed extract (oleoresin), caraway oleoresin/extract, opoponax oil, 3 parsley oil, hares ear tincture, taiga root extract (sb), ajowan oil 4 and celery tincture 5 ). These preparations were deleted from the register of feed additives. 6 During the course of the assessment, this application was split and the present opinion covers only one out of the 20 remaining preparations under application: asafoetida oil from the gum resin of Ferula assa‐foetida L. 7 for all animal species. During the assessment, the applicant requested a change in the species limiting the application for authorisation to dogs and cats. 8
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive) and under Article 10(2) (re‐evaluation of an authorised feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 24 June 2019.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals and user and on the efficacy of the product asafoeida oil (F. assa‐foetida), when used under the proposed conditions of use (see Section 3.2.4).
The remaining 19 preparations belonging to botanically defined group (BDG) 02 – Apiales/Austrobaileyales under application are assessed in separate opinions.
1.2. Additional information
The additive is currently authorised as a feed additive according to the entry in the European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003 (2b natural products – botanically defined). It has not been assessed as a feed additive in the EU.
There is no specific EU authorisation for any F. assa‐foetida L. preparation when used to provide flavour in food.
Many of the individual components of the essential oil have been already assessed as chemically defined flavourings for use in feed and food by the FEEDAP Panel, the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) and the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). The list of flavouring compounds currently authorised for food 9 and feed 10 uses together with the EU Flavour Information System (FLAVIS) number, the chemical group as defined in Commission Regulation (EC) No 1565/2000 11 and the corresponding EFSA opinion are given in Table 1.
Table 1.
Flavouring compounds already assessed by EFSA as chemically defined flavourings, grouped according to the chemical group (CG) as defined in Commission Regulation (EC) No 1565/2000, with indication of the EU Flavour Information System (FLAVIS) number and the corresponding EFSA opinion
| CG | Chemical group | Product – EU register name (common name) | FLAVIS no | EFSA* or JECFA opinion, year |
|---|---|---|---|---|
| 07 | Primary alicyclic saturated and unsaturated alcohols, aldehydes, acids, acetals esters with esters containing alicyclic alcohols | Myrtenyl acetate (1) | 09.302 | 2017, CEF |
| 08 | Secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols | Bornyl acetate | 09.017 | 2016a |
| Fenchyl acetate | 09.269 | |||
| 20 | Aliphatic and aromatic mono‐ and di‐thiols and mono‐, di‐, tri‐, and polysulphides with or without additional oxygenated functional groups | Dipropyl disulfide | 12.014 | 2013 |
| 25 | Phenol derivatives containing ring‐alkyl, ring‐alkoxy and side‐chains with an oxygenated functional group | 2‐Methoxy‐4‐vinylphenol | 04.009 | 2012a |
| 29 | Thiazoles, thiophene, thiazoline and thienyl derivatives | 3,4‐Dimethylthiophene (1) | 15.065 | WHO (2012a,b) (JECFA) |
| 31 |
Aliphatic and aromatic hydrocarbons and acetals containing saturated aldehydes |
Limonene (1) , (2) | 01.001 | 2008, EFSA (AFC) |
| 1‐Isopropyl‐4‐methylbenzene (p‐cymene) | 01.002 | 2015 | ||
| Terpinolene | 01.005 | |||
| α‐Phellandrene | 01.006 | |||
| α‐Terpinene | 01.019 | |||
| γ‐Terpinene | 01.020 | |||
| Pin‐2(10)‐ene (β‐pinene) | 01.003 | 2016b | ||
| Pin‐2(3)‐ene (α‐pinene) | 01.004 | |||
| β‐Caryophyllene | 01.007 | |||
| Myrcene | 01.008 | |||
| Camphene | 01.009 | |||
| δ‐Cadinene (1) , (3) | 01.021 | 2011, CEF | ||
| β‐Bisabolene (1) | 01.028 | |||
| 3,7,10‐Humulatriene (1) , (3) | 01.043 | |||
| β‐Phellandrene (1) , (3) | 01.055 | |||
| α‐Farnesene (1) | 01.040 | 2015a, CEF | ||
| β‐Farnesene (1) | 01.041 | |||
| Sabinene (4(10)‐thujene) (1) | 01.059 | 2015b, CEF | ||
| cis‐β‐Ocimene (1) | 01.064 | |||
| 32 | Epoxides | β‐Caryophyllene epoxide (1) | 16.043 | 2014, CEF |
FEEDAP opinion unless otherwise indicated.
Evaluated for use in food. According to Regulation (EC) 1565/2000, flavourings evaluated by JECFA before 2000 are not required to be re‐evaluated by EFSA.
JECFA and EFSA evaluated d‐limonene [01.045] (EFSA, 2008). d‐Limonene [01.045] and l‐limonene [01.046] were also evaluated for use in feed (EFSA FEEDAP Panel, 2015).
Evaluated applying the ‘Procedure’ described in the Guidance on the data required for the risk assessment of flavourings to be used in or on food (EFSA CEF Panel, 2010). No longer authorised for use as flavours in food, as the additional toxicity data requested (EFSA CEF Panel, 2011) were not submitted and the CEF Panel was unable to complete its assessment.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier 12 in support of the authorisation request for the use of asafoetida oil from F. assa‐foetida as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts' knowledge, to deliver the present output.
Many of the components of the essential oil under assessment have been already evaluated by the FEEDAP Panel as chemically defined flavourings (CDGs). The applicant submitted a written agreement to reuse the data submitted for the assessment of chemically defined flavourings (dossiers, publications and unpublished reports) for the risk assessment of preparations belonging to BDG 2. 13
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the active substance/agent in animal feed. The evaluation report is related to the methods of analysis for each feed additive included the group BDG 02 (Apiales and Austrobaileyales). In particular, for the determination of the phytochemical marker (E)‐sec‐butyl propenyl disulfide in ferula assa‐foetida oil the EURL recommended a method based on gas chromatography coupled with flame ionisation detection (GC‐FID). 14
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of asafoetida oil from F. assa‐foetida is in line with the principles laid down in Regulation (EC) No 429/2008 15 and the relevant guidance documents: Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements (EFSA SC, 2009), Compendium of botanicals that have been reported to contain toxic, addictive, psychotropic or other substances of concern (EFSA, 2012), Guidance for the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012b), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018) Guidance document on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals (EFSA SC, 2019a), Statement on the genotoxicity assessment of chemical mixtures (EFSA SC, 2019b), Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment (EFSA SC, 2019).
3. Assessment
The additive under assessment, asafoetida oil, is obtained from the gum resin of Ferula assa‐foetida L. It is intended for use as a sensory additive (functional group: flavouring compounds) in feed for cats and dogs.
3.1. Origin and extraction
Ferula assa‐foetida L. is a perennial herb belonging to the family Apiaceae. It is native to Southern Iran, Afghanistan and India which remain the areas in which the plant is found. Various parts of the plant are consumed locally as food (e.g. rhizome/roots, leaves and young shoots and the cabbage‐like head of the growing plant, but only after boiling or steeping in water to reduce its characteristic smell and taste). More typically the plant is harvested as a source of asafoetida, a milky exudate of the cut rhizome. Traditionally, stems are removed from the rhizome of 4‐ to 5‐year‐old plants just before flowering and cuts made to the large rhizome allowing collection of the exudate. As the exposed surface of the rhizome dries new cuts are made; a process which may be repeated over several months. The exudate or gum resin is then dried. The dried gum resin (asafoetida) is widely used as a food flavour and has long been valued for its medicinal properties. The Ayurvedic traditional medicine, for example, used asafoetida for the treatment of digestive disorders.
Asafoetida largely derives from wild collections, and it should be noted that there are several other Ferula species growing in the same or similar habitats able to produce exudates with similar characteristics, notably Ferula foetida (Bunge) Regel.
The additive is extracted from the dried gum resin by steam distillation. The volatile constituents are condensed and then separated from the aqueous phase by decantation.
3.2. Characterisation
3.2.1. Characterisation of asafoetida oil
Asafoetida oil is a liquid, with a characteristic aroma. In three recent batches of the additive (originating from Afghanistan, India or Iran, 2020), the refractive index (20°C) ranged between 1.533 and 1.537, the density (20°C) between 1.001 and 1.012 kg/m3, the optical rotation (20°C) between 43.64° and 51.16°. 16 Asafoetida oil is identified with the single Chemical Abstracts Service (CAS) number 9000‐04‐8, the European Inventory of Existing Chemical Substances (EINECS) number 289‐863‐4, 17 the Flavor Extract Manufacturers Association (FEMA) 2,108 and the Council of Europe (CoE) 196.
No international standard is available for the essential oil obtained by steam distillation of the gum resin from F. assa‐foetida. The product specifications were set based on the concentrations of the main volatile components, analysed by GC‐FID and expressed as % of gas chromatographic peak area (% GC area) 18 and on the available literature on asafoetida oils (Tisserand and Young, 2014; Zomorodian et al., 2018; Pavela et al., 2020). Four components contribute to the specification as shown in Table 2, with (E)‐sec‐butyl propenyl disulfide selected as the phytochemical marker. The applicant provided the full characterisation of the volatile constituents in three batches obtained by gas chromatography–mass spectrometry (GC–MS). The four compounds account for about 72.9% on average (range 70.0%–78.4%) of % GC area (Table 2). 18 Twenty‐seven sulfur compounds were detected in the additive and identified with numbers (1–27, according to the numbering in Table 4).
Table 2.
Major constituents of the essential oil from the gum resin of Ferula assa‐foetida L.: specifications and batch to batch variation based on the analysis of three batches. The content of each constituent is expressed as the area per cent of the corresponding chromatographic peak (% GC area), assuming the sum of chromatographic areas of all detected peaks as 100%
| Constituent | % GC area | |||
|---|---|---|---|---|
| EU register name (a) | CAS no | Specification | Mean (b) | Range |
| (E)‐2‐Butyl 3‐(methylthio)‐2‐propenyl disulfide (1) | – | 20–45 | 35.5 | 31.8–40.1 |
| (E)‐sec‐Butyl propenyl disulfide (2) | 24351‐71‐1 | 8–25 | 14.4 | 12.9–16.0 |
| (Z)‐sec‐Butyl propenyl disulfide (3) | 24351‐70‐0 | 8–24 | 13.9 | 12.8–15.5 |
| di‐sec‐Butyl disulfide (4) | 5943‐30‐6 | 4–16 | 9.1 | 7.0–12.6 |
| Total | 72.9 | 70.0–78.4 | ||
EU: European Union; CAS No: Chemical Abstracts Service number.
Only sulfur compounds were identified with numbers (1–27). For the numbering of the sulfur compounds and their structure see Table 4.
Mean calculated on three batches.
Table 4.
Chemical Abstract System (CAS) number, molecular formula and chemical structure of the sulfur compounds present in the additive
| No | Constituent EU register name | CAS no | Molecular formula | Chemical structure |
|---|---|---|---|---|
| 1 | (E)‐2‐Butyl 3‐(methylthio)‐2‐propenyl disulfide | 56019‐36‐4* | C8H16S3 |
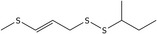
|
| 2 | (E)‐sec‐Butyl‐propenyl disulfide | 24351‐71‐1 | C7H14S2 |
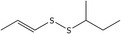
|
| 3 | (Z)‐sec‐Butyl‐propenyl disulfide | 24351‐70‐0 | C7H14S2 |
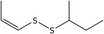
|
| 4 | di‐sec‐Butyl disulfide | 5943‐30‐6 | C8H18S2 |
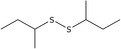
|
| 5 | (Z)‐1‐(Methylthio)propyl 1‐propenyl disulfide | 56019‐32‐0 | C7H14S3 |
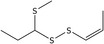
|
| 6 | (E)‐1‐(Methylthio)propyl 1‐propenyl disulfide | 56019‐35‐3 | C7H14S3 |
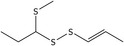
|
| 7 | n‐Propyl‐sec‐butyl disulfide | 59849‐54‐6 | C7H16S2 |
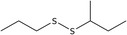
|
| 8 | (E)‐1‐(But‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane | 110690‐24‐9 | C8H16S2 |
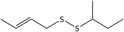
|
| 9 | 1‐(1‐(Methylthio)propyl)‐2‐propyldisulfane | 126876‐22‐0 | C7H16S3 |
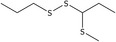
|
| 10 | (Z)‐2‐Butyl 3‐(methylthio)‐2‐propenyl disulfide | 56019‐34‐2* | C8H16S3 |
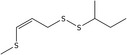
|
| 11 | Rutadisulfid A | – | C9H16O2S2 |
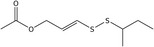
|
| 12 | Isomer | – | C7H14S2 | – |
| 13 | (Z)‐1‐(But‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane | 110690‐23‐8 | C8H16S2 |
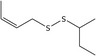
|
| 14 | Isomer | – | C8H16S3 | – |
| 15 | Isomer | – | C8H16S2 | – |
| 16 | (Z)‐1‐(But‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane | 110690‐21‐6 | C8H16S2 |
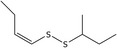
|
| 17 | Methyl sec‐butyl disulfide | 67421‐87‐8 | C5H12S2 |
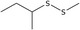
|
| 18 | (E)‐1‐(But‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane | 110690‐22‐7 | C8H16S2 |
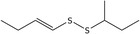
|
| 19 | Allyl tert‐butyl sulfide |
37850‐75‐2 2867‐05‐2* |
C7H14S |
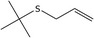
|
| 20 | (E)‐1‐Propenyl methyl disulfide |
23838‐19‐9 2179‐60‐4* |
C4H8S2 |
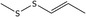
|
| 21 | Dipropyl disulfide | 629‐19‐6 | C6H14S2 |
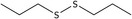
|
| 22 | 2,3,4‐Trimethylthiophene | 1795‐04‐6 | C7H10S |
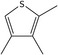
|
| 23 | (Z)‐1‐Propenyl methyl disulfide | 23838‐18‐8 | C4H8S2 |
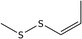
|
| 24 | 3,4‐Dimethylthiophene | 632‐15‐5 | C6H8S |
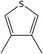
|
| 25 | 2‐(Ethenyldisulfanyl)butane | 110690‐20‐5 | C6H12S2 |
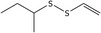
|
| 26 | Disulfide, ethyl 1‐methylpropyl | 54166‐53‐9 | C6H14S2 |
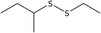
|
| 27 | 3‐Ethyl‐1,2‐dithi‐4‐ene | 126790‐02‐1 | C6H10S2 |
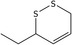
|
: CAS number given by the applicant.
In total, up to 62 constituents were detected, 59 of which were identified and accounted on average for 99.5% (99.1%–99.9%) of the GC area. Besides the four compounds indicated in the product specifications, seven other compounds were detected at individual levels > 0.5% and are listed in Table 3. These 11 compounds > 0.5% together, account on average for 93.9% (92.7%–94.9%) of the % GC area. The remaining 51 compounds (ranging between 0.003% and 0.5%) and accounting for 4.9% are listed in the footnote. 19 Based on the available data on the characterisation, asafoetida oil is considered a fully defined mixture.
Table 3.
Other constituents of the essential oil from the gum resin of F. assa‐foetida L. accounting on average for > 0.5% of the composition (based on the analysis of three batches) not included in the specification. The content of each constituent is expressed as the area per cent of the corresponding chromatographic peak (% GC area), assuming the sum of chromatographic areas of all detected peaks as 100%
| Constituent | % GC area | |||
|---|---|---|---|---|
| EU register name (a) | CAS no | FLAVIS no | Mean (b) | Range |
| (Z)‐1‐(Methylthio)propyl 1‐propenyl disulfide (5) | 56019‐32‐0 | – | 8.08 | 4.52–10.5 |
| (E)‐1‐(Methylthio)propyl 1‐propenyl disulfide (6) | 56019‐35‐3 | – | 5.68 | 3.34–7.07 |
| n‐Propyl sec‐butyl disulfide (7) | 59849‐54‐6 | – | 2.01 | 0.90–2.88 |
| (E)‐1‐(But‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane (8) | 110690‐24‐9 | – | 1.98 | 1.51–2.78 |
| β‐Caryophyllene | 87‐44‐5 | 01.007 | 1.25 | 0.97–1.67 |
| 1‐(1‐(Methylthio)propyl)‐2‐propyldisulfane (9) | 126876‐22‐0 | – | 1.21 | 0.51–1.60 |
| (Z)‐2‐Butyl 3‐(methylthio)‐2‐propenyl disulfide (10) | – | – | 0.77 | 0.63–0.91 |
| Total | 21.0 | 14.3–24.6 | ||
EU: European Union; CAS No: Chemical Abstracts Service number; FLAVIS No: EU Flavour Information System numbers.
Only sulfur compounds were identified with numbers (1–27). For the numbering of the sulfur compounds and their structure see Table 4.
Mean calculated on three batches.
The molecular formulae and the molecular structures of the 27 sulfur compounds present in the additive under assessment are shown in Table 4.
Twenty‐five out of the 27 compounds belong to chemical group 20. Twenty‐four are aliphatic disulfides (five named as aliphatic disulfanes), one is a monosulfide (19), three (12, 14 and 15, accounting together for 0.74% of the % GC area) were not completely identified, however according to the chemical composition they seem to be isomers of identified compounds. Six out of the 21 identified aliphatic disulfides (4, 7, 9, 17, 26 and 21, accounting together for 12.4% of the % GC area) are saturated aliphatic disulfides. Fifteen (accounting for 82% of the % GC area) contain a double bond in the aliphatic chain. All compounds except one, which is a cyclic disulfide (27), are aliphatic acyclic derivatives. Two additional sulfur compounds in Table 4 are thiophene derivatives (22 and 24) and belong to CG 29.
The applicant performed a literature search regarding substances of concern and chemical composition of the plant species F. assa‐foetida and its preparations. 20 No substances of concern were identified.
3.2.2. Impurities
The applicant makes reference to the ‘periodic testing’ of some representative flavourings premixtures for mercury, cadmium, lead, arsenic, fluoride, dioxins and polychlorinated biphenyls (PCBs), organo‐chloride pesticides, organo‐phosphorous pesticides, aflatoxins B1, B2, G1, G2 and ochratoxin A. However, no data have been provided. Since asafoetida oil is produced by steam distillation, the likelihood of any measurable carry‐over of all the above‐mentioned elements is low except for mercury.
3.2.3. Shelf life
The typical shelf‐life of asafoetida oil is stated to be at least 12 months, when stored in tightly closed containers under standard conditions (in a cool, dry place protected from light). 21 However, no data supporting this statement were provided.
3.2.4. Conditions of use
Asafoetida oil is intended to be added to feed for cats and dogs. The maximum proposed use level in complete feed is 1.5 mg/kg for dogs and 0.2 mg/kg for cats.
3.3. Safety
The assessment of safety of asafoetida oil is based on the maximum use levels proposed by the applicant.
Among the major components included in Tables 2 and 3, only β‐caryophyllene [01.007] has been previously assessed for use as flavouring. Several minor components of asafoetida oil, accounting for about 2.2% of the % GC peak areas, have been previously assessed and considered safe for use as flavourings, and are currently authorised for use in food 9 without limitations and for use in feed10 at individual use levels higher than those resulting from the intended use of the essential oil in feed. The list of the compounds already evaluated by the EFSA Panels is given in Table 1 (see Section 1.2).
Three compounds, δ‐cadinene [01.021], 3,7,10‐humulatriene [01.043] and β‐phellandrene [01.055] have been evaluated in Flavouring Group Evaluation 25, Revision 2 (FGE25.Rev2) by applying the procedure described in the Guidance on the data required for the risk assessment of flavourings to be used in or on food (EFSA CEF Panel, 2010). For these compounds, for which there is no concern for genotoxicity, EFSA requested additional subchronic toxicity data (EFSA CEF Panel, 2011). In the absence of such data, the EFSA CEF Panel was unable to complete its assessment. As a result, these compounds are not authorised for use as flavours in food. For these compounds, the FEEDAP Panel applies the approach recommended in the Guidance document on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals (EFSA SC, 2019a).
The FEEDAP Panel notes that 94.5% of GC % area is accounted by 25 aliphatic sulfides structurally related to flavourings already assessed in CG 20, three of which were tentatively identified as isomers of the identified compounds. With the exception of dipropyl disulfide [12.014], they have not been previously evaluated for use as flavourings.
The genotoxic potential for the 21 identified aliphatic sulfides not yet evaluated 22 was predicted by the applicant using the Quantitative Structure–Activity Relationship (QSAR) Toolbox. 23 No structural alerts were found for allyl tert‐butyl sulfide (19), (Z)‐1‐(methylthio)propyl 1‐propenyl disulfide (5) and (E)‐1‐(methylthio)propyl 1‐propenyl disulfide (6). For the other compounds, structural alerts were due to the presence of disulfides, which is also present for dipropyl disulfide [12.014]. They are therefore expected to have a similar metabolic and toxicological profile and not to be genotoxic as well. The same conclusions apply to the three unidentified compounds which are structurally related to the compounds screened by QSAR and to dipropyl disulfide [12.014].
For two additional sulfur compounds, 3,4‐dimethylthiophene and 2,3,4‐trimethylthiophene belonging to CG 29, structural alerts were due to the presence of heterocyclic ring systems (thiophenes). The mutagenicity (Ames test) prediction for 2,3,4‐trimethylthiophene was made by read‐across analyses of data available for similar substances (i.e. analogues obtained by categorisation). Categories were defined using general mechanistic and endpoint profilers, as well as empirical profilers. Mutagenicity read‐across‐based predictions were found consistently negative for all categories of analogues. On this basis, the alerts raised for 2,3,4‐trimethylthiophene were discounted. Read‐across can also be extended from 2,3,4‐trimethylthiophene to 3,4‐dimethylthiophene. The FEEDAP Panel notes that these compounds share the same heterocyclic ring structure of thiophene [15.106], 2‐methylthiophene [15.091], 3‐methylthiophene [15.092] and other thiophenes evaluated in FGE.21. For these compounds, for which there is no concern for genotoxicity, EFSA requested additional subchronic toxicity data (EFSA CEF Panel, 2011). In the absence of such data, the EFSA CEF Panel was unable to complete its assessment. As a result, these compounds are not authorised for use as flavours in food.
For xanthoxylin belonging to CG 26, structural alerts were due to the presence of aromatic carbonyl. The mutagenicity (Ames test) prediction was made by read‐across analyses of data available for similar substances (i.e. analogues obtained by categorisation). Categories were defined using general mechanistic and endpoint profilers as well as empirical profilers. Mutagenicity read‐across‐based predictions were found consistently negative for all categories of analogues. On this basis, the alerts raised for xanthoxylin were discounted.
In addition, 11 compounds (accounting for 0.7% of the GC % area) are aliphatic mono‐ or sesquiterpenes structurally related to flavourings already assessed in CG 31 for which a similar metabolic and toxicological profile is expected. 24 These lipophilic compounds are expected to be rapidly absorbed from the gastrointestinal tract, oxidised to polar oxygenated metabolites, conjugated and excreted (EFSA FEEDAP Panel, 2015, 2016b).
3.3.1. Safety for the target species
Tolerance studies with the target species and/or toxicological studies in laboratory animals made with the essential oil under application were not submitted.
In the absence of these data, the approach to the safety assessment of a mixture whose individual components are known is based on the safety assessment of each individual component (component‐based approach). This approach requires that the mixture is sufficiently characterised. The individual components can be grouped into assessment groups, based on structural and metabolic similarity. The combined toxicity can be predicted using the dose addition assumption within an assessment group, taking into account the relative toxic potency of each component (EFSA SC, 2019a).
As the additive under assessment is a fully defined mixture (> 99.5% of the components were identified, see Section 3.2.1), the FEEDAP Panel applied a component‐based approach to assess the safety for target species of the essential oil.
Based on considerations related to structural and metabolic similarities, the components were allocated to nine assessment groups, corresponding to the chemical groups (CGs) 7, 8, 20, 25, 26, 29, 31 and 32, as defined in Annex I of Regulation (EC) No 1565/2000. For chemical group 31 (‘aliphatic and aromatic hydrocarbons’), the application of sub‐assessment groups as defined in FGE.25 and FGE.78 is applied (EFSA CEF Panel, 2015a,b). The allocation of the components to the (sub‐)assessment groups is shown in Tables 5 and 6 and in the corresponding footnotes.
Table 5.
Compositional data, intake values (calculated for dogs at 1.5 mg/kg complete feed), reference points and margin of exposure (MOE) for the individual components of asafoetida oil classified according to assessment groups
| Essential oil composition | Exposure | Hazard characterisation | Risk characterisation | ||||
|---|---|---|---|---|---|---|---|
| Assessment group | Highest conc. in the oil | Highest feed conc. | Daily intake (a) | Cramer class (b) | NOAEL (c) | MOE | MOET |
| Constituent | % | mg/kg | mg/kg bw/day | – | mg/kg bw/day | – | – |
| CG 20 | |||||||
| (E)‐2‐Butyl 3‐(methylthio)‐2‐propenyl disulfide | 40.10 | 0.602 | 0.0114 | (III) | 7.3 | 641 | |
| (E)‐sec‐Butyl propenyl disulfide | 16.00 | 0.240 | 0.0045 | (III) | 7.3 | 1,606 | |
| (Z)‐sec‐Butyl propenyl disulfide | 15.50 | 0.233 | 0.0044 | (III) | 7.3 | 1,658 | |
| di‐sec‐Butyl disulfide | 12.60 | 0.189 | 0.0036 | (I) | 7.3 | 2,039 | |
| (Z)‐1‐(Methylthio)propyl 1‐propenyl disulfide | 10.50 | 0.158 | 0.0030 | (III) | 7.3 | 2,447 | |
| (E)‐1‐(Methylthio)propyl 1‐propenyl disulfide | 7.07 | 0.106 | 0.0020 | (III) | 7.3 | 3,635 | |
| n‐Propyl sec‐butyl disulfide | 2.88 | 0.043 | 0.0008 | (III) | 7.3 | 8,922 | |
| (E)‐1‐(But‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane | 2.78 | 0.042 | 0.0008 | (III) | 7.3 | 9,243 | |
| 1‐(1‐(Methylthio)propyl)‐2‐propyldisulfane | 1.60 | 0.024 | 0.0005 | (III) | 7.3 | 16,060 | |
| (Z)‐2‐butyl 3‐(methylthio)‐2‐propenyl disulfide | 0.91 | 0.014 | 0.0003 | (III) | 7.3 | 28,393 | |
| Rutadisulfide A | 0.52 | 0.008 | 0.0001 | (III) | 7.3 | 49,895 | |
| Sulfur compound C7H14S2 | 0.54 | 0.008 | 0.0002 | (III) | 7.3 | 47,322 | |
| MOET CG 20 | 232 | ||||||
| CG 29 | |||||||
| 2,3,4‐Trimethylthiophene | 0.03 | 0.0007 | 0.00001 | III | 0.15 | 10,776 | |
| 3,4‐Dimethylthiophene | 0.01 | 0.0005 | 0.00001 | III | 0.15 | 15,086 | |
| MOET CG 29 | 6,286 | ||||||
Intake calculations for the individual components are based on the use level of 1.5 mg/kg in feed for dog. The MOE for each component is calculated as the ratio of the reference point (NOAEL) to the intake. The combined margin of exposure (MOET) is calculated for each assessment group as the reciprocal of the sum of the reciprocals of the MOE of the individual substances.
When a NOAEL value is available or read‐across is applied, the allocation to the Cramer class is put into parentheses.
Values in italics are the 5th percentile of the distribution of NOAELs of the corresponding Cramer Class.
Table 6.
Compositional data, intake values (calculated for cats at 0.2 mg/kg complete feed), reference points and margin of exposure (MOE) for the individual components of asafoetida oil classified according to assessment groups
| Essential oil composition | Exposure | Hazard characterisation | Risk characterisation | ||||
|---|---|---|---|---|---|---|---|
| Assessment group | Highest conc. in the oil | Highest feed conc. | Daily intake (a) | Cramer class (b) | NOAEL | MOE | MOET |
| Constituent | % | mg/kg | mg/kg bw/day | – | mg/kg bw/day | – | – |
| CG 20 | |||||||
| (E)‐2‐Butyl 3‐(methylthio)‐2‐propenyl disulfide | 40.10 | 0.080 | 0.0018 | (III) | 7.3 | 4,005 | |
| (E)‐sec‐Butyl propenyl disulfide | 16.00 | 0.032 | 0.0007 | (III) | 7.3 | 10,038 | |
| (Z)‐sec‐Butyl propenyl disulfide | 15.50 | 0.031 | 0.0007 | (III) | 7.3 | 10,361 | |
| di‐sec‐Butyl disulfide | 12.60 | 0.025 | 0.0006 | (I) | 7.3 | 12,746 | |
| (Z)‐1‐(Methylthio)propyl 1‐propenyl disulfide | 10.50 | 0.021 | 0.0005 | (III) | 7.3 | 15,295 | |
| (E)‐1‐(Methylthio)propyl 1‐propenyl disulfide | 7.07 | 0.014 | 0.0003 | (III) | 7.3 | 22,716 | |
| MOET CG 20 | 1,578 | ||||||
Intake calculations for the individual components are based on the use level of 0.2 mg/kg in feed for cat. The MOE for each component is calculated as the ratio of the reference point (NOAEL) to the intake. The combined margin of exposure (MOET) is calculated for each assessment group as the reciprocal of the sum of the reciprocals of the MOE of the individual substances.
When a NOAEL value is available or read‐across is applied, the allocation to the Cramer class is put into parentheses.
For each component in the assessment group, exposure of target animals was estimated considering the use levels in feed, the percentage of the component in the oil and the default values for feed intake according to the guidance on the safety of feed additives for target species (EFSA FEEDAP Panel, 2017b). Default values on body weight are used to express exposure in terms of mg/kg body weight (bw) per day. The intake levels of the individual components calculated for dog and cat are shown in Tables 5 and 6, respectively.
For hazard characterisation, each component of an assessment group was first assigned to the structural class according to Cramer classification. For some components in the assessment group, toxicological data were available to derive no observed adverse effect level (NOAEL) values. Structural and metabolic similarity among the components in the assessment groups were assessed to explore the application of read‐across. If justified, extrapolation from a known NOAEL of a component of an assessment group to the other components of the group with no available NOAEL was made. If sufficient evidence was available for the members of a (sub‐)assessment group, a (sub‐)assessment group NOAEL was derived.
Toxicological data for subchronic studies, from which NOAEL values could be derived, were available for 2‐methoxy‐4‐vinylphenol [04.009] (EFSA FEEDAP Panel, 2012a), myrcene [01.008], limonene [01.045], 1‐isopropyl‐4‐benzene [01.002] and β‐caryophyllene [01.007] in CG 31 (EFSA FEEDAP Panel, 2015, 2016a), and β‐caryophyllene oxide in CG 32 (EFSA CEF Panel, 2014).
For dipropyl disulfide [12.014] a NOAEL was identified by JECFA to be 7.29 mg/kg bw per day, the highest dose tested in a 90‐day oral toxicity study in rats (Posternak et al., 1969). The same NOAEL was applied by JECFA to di‐sec‐butyldisulfide and other disulfide compounds (WHO, 2010). The NOAEL determined by Posternak et al. (1969) was derived from a single dose experiment. The dose was selected from the calculation of the average human exposure by multiplication with a factor of 100. It can be expected that this value is very conservative. It seems therefore justifiable to extend the NOAEL of 7.3 mg/kg bw per day to all aliphatic disulfides present in the additive.
For the remaining compounds, toxicity studies and NOAEL values performed with the compounds under assessment were not available and read‐across was not possible. Therefore, the threshold of toxicological concern (TTC) approach was applied (EFSA FEEDAP Panel, 2017b).
As the result of the hazard characterisation, a reference point was identified for each component in the assessment group based on the toxicity data available (NOAEL from in vivo toxicity study or read‐across) or from the 5th percentile of the distribution of NOAELs of the corresponding Cramer Class (i.e. 3, 0.91 and 0.15 mg/kg bw per day, respectively, for Cramer Class I, II and III compounds).
For risk characterisation, the margin of exposure (MOE) was calculated for each component as the ratio between the reference point and the exposure. For each assessment group, the combined (total) margin of exposure (MOET) was calculated as the reciprocal of the sum of the reciprocals of the MOE of the individual substances (EFSA SC, 2019a). A MOET > 100 allowed for interspecies differences and intra‐individual variability (as in the default 10 × 10 uncertainty factor). The compounds resulting individually in an MOE > 50,000 were not further considered in the assessment group as their contribution to the MOE(T) is negligible. They are listed in the footnote. 25
The approach to the safety assessment of asafoetida oil for dogs is summarised in Table 5.
As shown in Table 5, for all the assessment groups, the MOET was ≥ 232. For cat, the corresponding calculations are shown in Table 6. 26
For cats, a MOET > 500 is considered adequate, considering their unusually low capacity for glucuronidation of compounds (Court and Greenblatt, 1997; Lautz et al., 2021).
3.3.1.1. Conclusions on safety for the target species
Asafoetida oil is safe up to the maximum proposed use level of 1.5 mg/kg complete feed for dog and 0.2 mg/kg for cat.
3.3.2. Safety for the user
No specific data were provided by the applicant regarding the safety of the additive for users.
The applicant produced a safety data sheet 27 for asafoetida oil, where hazards for users have been identified. The Panel notes that the additive contains a variety of compounds, known to cause allergic reactions in sensitive persons. Therefore, sensitisation may occur in users handling the additive.
The essential oil under assessment should be considered as irritant to skin and eyes, and as a dermal and respiratory sensitiser.
3.4. Efficacy
Both the gum resin (asafoetida) and the derived essential oil are listed in Fenaroli's Handbook of Flavour Ingredients (Burdock, 2009) and by FEMA with the reference numbers of 2107 (gum) and 2,108 (asafoetida oil).
Since F. assa‐foetida and its oil are recognised to flavour food and their function in feed would be essentially the same as that in food, no further demonstration of efficacy is considered necessary.
4. Conclusions
The use of asafoetida oil from F. assa‐foetida is safe at the proposed conditions of use of 1.5 mg/kg complete feed for dogs and 0.2 mg/kg complete feed for cats.
The essential oil under assessment should be considered as irritant to skin and eyes, and as a dermal and respiratory sensitiser.
Asafoetida oil is recognised to flavour food. Since its function in feed would be essentially the same as that in food, no further demonstration of efficacy is considered necessary.
Documentation provided to EFSA/Chronology
| Date | Event |
|---|---|
| 28/10/2010 | Dossier received by EFSA. Botanically defined flavourings from Botanical Group 02 – Apiales and Austrobaileyales for all animal species and categories. Submitted by Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG) |
| 09/11/2010 | Reception mandate from the European Commission |
| 26/02/2013 | EFSA informed the applicant (EFSA ref. 7150727) that, in view of the workload, the evaluation of applications on feed flavourings would be re‐organised by giving priority to the assessment of the chemically defined feed flavourings, as agreed with the European Commission |
| 24/06/2015 | Technical hearing during risk assessment with the applicant according to the “EFSA's Catalogue of support initiatives during the life‐cycle of applications for regulated products”: data requirement for the risk assessment of botanicals |
| 17/06/2016 | Technical hearing during risk assessment with the applicant according to the “EFSA's Catalogue of support initiatives during the life‐cycle of applications for regulated products”. Discussion on the ongoing work regarding the pilot dossiers BDG08 and BDG 09 |
| 27/04/2017 | Trilateral meeting organised by the European Commission with EFSA and the applicant FEFANA on the assessment of botanical flavourings: characterisation, substances of toxicological concern present in the botanical extracts, feedback on the pilot dossiers |
| 27/02/2019 | Partial withdrawal by applicant (EC was informed) for the following additives: dill seed extract, celery seed extract (oleoresin), caraway oleoresin/extract, and opoponax oil |
| 24/06/2019 | Application validated by EFSA – Start of the scientific assessment |
| 03/07/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterization, safety for the target species, safety for the consumer, safety for the user, safety for the environment |
| 30/09/2019 | Comments received from Member States |
| 13/07/2021 | Reception of supplementary information from the applicant (partial dataset on asafoetida oil) – Scientific assessment remains suspended |
| 24/06/2022 | The application was split and a new EFSA‐Q‐2022‐00404 was assigned to the preparation included in the present assessment |
| 31/10/2022 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives – Scientific assessment re‐started |
| 22/11/2022 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment for the preparation included in the present assessment. The assessment of other preparations is still ongoing |
Abbreviations
- AFC
EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- BDG
botanically defined group
- bw
body weight
- CAS
Chemical Abstracts Service
- CDG
chemically defined group
- CEF
EFSA Scientific Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CG
chemical group
- CoE
Council of Europe
- EINECS
European Inventory of Existing Chemical Substances
- EURL
European Union Reference Laboratory
- FAO
Food Agricultural Organization
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- FEMA
Flavor Extract Manufacturers Association
- FFAC
Feed Flavourings authorisation Consortium of FEFANA (EU Association of Specialty Feed Ingredients and their Mixtures)
- FGE
food group evaluation
- FLAVIS
The EU Flavour Information System
- FL‐No
FLAVIS number
- GC
gas chromatography
- GC‐FID
gas chromatography‐flame ionisation detection
- GC–MS
gas chromatography–mass spectrometry
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MOE
margin of exposure
- MOET
combined margin of exposure
- NOAEL
no observed adverse effect level
- PCBs
polychlorinated biphenyls
- QSAR
Quantitative Structure–Activity Relationship
- sb
solvent‐based
- SC
EFSA Scientific Committee
- TTC
threshold of toxicological concern
- UF
uncertainty factor
- WHO
World Health Organization
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Fašmon Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brantom P, Chesson A, Westendorf J, Manini P, Pizzo F and Dusemund B, 2022. Scientific Opinion on the safety and efficacy of a feed additive consisting of an essential oil from the gum resin of Ferula assa‐foetida L. (asafoetida oil) for use in dogs and cats (FEFANA asbl). EFSA Journal 2022;20(12):7688, 19 pp. 10.2903/j.efsa.2022.7688
Requestor European Commission
Question number EFSA‐Q‐2010‐01286 (new EFSA‐Q‐2022‐00404)
Panel members Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Panel wishes to thank the following for the support provided to this scientific output (in alphabetical order of the last name): Jaume Galobart and Daniel Pagés Plaza.
Adopted: 22 November 2022
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
On 13/03/2013, EFSA was informed by the applicant that the applicant company changed to FEFANA asbl, Avenue Louise 130 A, Box 1, 1,050 Brussels, Belgium.
On 27 February 2019, EFSA was informed by the applicant about the withdrawal of the applications on dill seed extract, celery seed extract (oleoresin), caraway oleoresin/extract, and opoponax oil.
On 2 April 2020, EFSA was informed by the applicant about the withdrawal of the applications on parsley oil, hares ear tincture, taiga root extract (sb), ajowan oil.
On 9 December 2020, the applicant informed EFSA about the withdrawal of the application on celery tincture.
Register of feed additives, Annex II, withdrawn by OJ L162, 10.05.2021, p. 5.
Accepted name: Ferula assa‐foetida L., synonym Ferula foetida St.‐Lag.
Technical dossier/Supplementary information July 2021/SIn_reply_asafoetida_oil.
Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1.
European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed-eu-reg-comm_register_feed_additives_1831-03.pdf.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96 of the European Parliament and of the Council. OJ L 180, 19.7.2000, p. 8.
FEED dossier reference: FAD‐2010‐0221.
Technical dossier/Supplementary information/Letter dated 29/04/2021.
The full report is available on the EURL website: https://joint-research-centre.ec.europa.eu/publications/fad-2010-0221_en.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Supplementary information July 2021/Annex_II_asafoetida_oil_CoA_chrom.
EINECS No associated to ‘Ferula assa‐foetida, ext.’ (Extractives and their physically modified derivatives such as tinctures, concretes, absolutes, essential oils, oleoresins, terpenes, terpene‐free fractions, distillates, residues, etc., obtained from Ferula assa‐foetida, Umbelliferae.)
Technical dossier/Supplementary information July 2021/Annex_II_ asafoetida_oil_CoA_chromatogram.
Additional constituents: constituents (n = 14) between < 0.5% and ≥ 0.1%: rutadisulfide A (11), sulfur compound (C7H14S2) (12, isomer of 2 and 3), pin‐2(3)‐ene (α‐pinene), pin‐2(10)‐ene (β‐pinene), α‐farnesene, (Z)‐1‐(but‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane (13), limonene, sulfur compound (C8H16S3) (14, isomer of 1 and 10), trans‐3,7‐dimethyl‐1,3,6‐octatriene, sulfur compound (C8H16S2) (15, cis‐isomer of 8), (Z)‐1‐(but‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane (16), β‐bisabolene, (Z,E)‐α‐farnesene and 3,7,10‐humulatriene; constituents (n = 33) between < 0.1% and ≥ 0.01%: fenchyl acetate, cis‐3,7‐dimethyl‐1,3,6‐octatriene, β‐farnesene, 2‐methoxy‐4‐vinylphenol, β‐curcumene, β‐caryophyllene epoxide, methyl sec‐butyl disulphide (17), α‐selinene, δ‐cadinene, (E)‐1‐(but‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane (18), β‐cedrene, myrtenyl acetate, allyl tert‐butyl sulphide (19), α‐curcumene, xanthoxylin, (E)‐1‐propenyl methyl disulfide (20), dipropyl disulfide (21), β‐selinene, α‐phellandrene, (E)‐γ‐bisabolene, γ‐cadinene, bornyl acetate, β‐phellandrene, α‐acoradiene, β‐bazzanene, 2,3,4‐trimethylthiophene (22), γ‐terpinene, 1‐isopropyl‐4‐methylbenzene, myrcene, (Z)‐1‐propenyl methyl disulfide (23), 3,4‐dimethylthiophene (24), 2‐(ethenyldisulfanyl)butane (25) and camphene; constituents (n = 5) > 0.01%: disulfide, ethyl 1‐methylpropyl (26), 3‐ethyl‐1,2‐dithi‐4‐ene (27), α‐terpinene, 4(10)‐thujene and terpinolene.
Technical dossier/Supplementary information July 2021/Literature search_asafoetida_oil.
Technical dossier/Section II.
Twenty‐one compounds, (E)‐2‐butyl 3‐(methylthio)‐2‐propenyl disulfide, (E)‐sec‐butyl propenyl disulfide, (Z)‐sec‐butyl propenyl disulfide, di‐sec‐butyl disulfide, (Z)‐1‐(methylthio)propyl 1‐propenyl disulfide, (E)‐1‐(methylthio)propyl 1‐propenyl disulfide, n‐propyl sec‐butyl disulfide, (E)‐1‐(but‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane, 1‐(1‐(methylthio)propyl)‐2‐propyldisulfane, (Z)‐2‐butyl 3‐(methylthio)‐2‐propenyl disulfide, rutadisulfide A, (Z)‐1‐(but‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane, (Z)‐1‐(but‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane, (E)‐1‐propenyl methyl disulfide, methyl sec‐butyl disulfide, (E)‐1‐(but‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane, allyl tert‐butyl sulfide, (Z)‐1‐propenyl methyl disulfide, ethyl 1‐methylpropyl disulfide, 2‐(ethenyldisulfanyl)butane and 3‐ethyl‐1,2‐dithi‐4‐ene.
Technical dossier/Supplementary information July 2021/Annex_V_Sin reply_asafoetida_oil_QSAR.
Eleven components (trans‐β‐ocimene, (Z,E)‐α‐farnesene, (E)‐γ‐bisabolene, α‐curcumene, β‐curcumene, β‐cedrene, α‐acoradiene, β‐selinene, α‐selinene, γ‐cadinene and β‐bazzanene) representing about 0.7% of the % GC area are allocated to CG 31 and are structurally related to compounds already authorised for use in food and feed as flavourings.
Compounds included in the assessment groups but not reported in the table: myrtenyl acetate (CG 7); fenchyl acetate and bornyl acetate (CG 8); (Z)‐1‐(but‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane, (Z)‐1‐(but‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane, (E)‐1‐propenyl methyl disulfide, methyl sec‐butyl disulfide, (E)‐1‐(but‐1‐en‐1‐yl)‐2‐(sec‐butyl)disulfane, allyl tert‐butyl sulfide, dipropyl disulfide, (Z)‐1‐propenyl methyl disulfide, ethyl 1‐methylpropyl disulfide, 2‐(ethenyldisulfanyl)butane, 3‐ethyl‐1,2‐dithi‐4‐ene (CG 20); 2‐methoxy‐4‐vinylphenol (CG 25); xanthoxylin (CG 26); myrcene, cis‐β‐ocimene, trans‐‐β‐ocimene, β‐farnesene, (Z,E)‐α‐farnesene and α‐farnesene (CG 31, II); α‐phellandrene, α‐terpinene, limonene, β‐phellandrene, γ‐terpinene, terpinolene, β‐bisabolene and (E)‐β‐bisabolene (CG 31, III); α‐pinene, camphene, sabinene, β‐pinene, β‐cedrene, β‐caryophyllene, α‐acoradiene, β‐selinene, α‐selinene, γ‐cadinene, δ‐cadinene and β‐bazzanene (CG 31, V); 3,7,10‐humulatriebne (CG 31, VI); β‐caryophyllene oxide (CG 32).
Additional compounds included in the assessment groups but not reported in the table: n‐propyl sec‐butyl disulfide, (E)‐1‐(but‐2‐en‐1‐yl)‐2‐(sec‐butyl)disulfane and 1‐(1‐(methylthio)propyl)‐2‐propyldisulfane (CG 20); 3,4‐dimethylthiophene and 2,3,4‐trimethylthiopehen (CG 29).
Technical dossier/ Supplementary Information July 2021/Annex_VII_SIn reply_asafoerida oil_MSDS_4. Aspiration hazard (H304, category 1), Hazards for skin corrosion/irritation (H315, category 2), skin sensitisation (H317, category 1).
References
- Burdock GA, 2009. Fenaroli's handbook of flavor ingredients. 6th Edition. CRC Press, Taylor & Francis Group, Boca Raton, FL. pp. 119–120. 10.1201/9781439847503 [DOI] [Google Scholar]
- Court MH and Greenblatt DJ, 1997. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes. Biochemical Pharmacology, 53, 1041–1047. 10.1016/s0006-2952(97)00072-5 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2008. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from Commission on Flavouring Group Evaluation 87, (FGE.87) bicyclic secondary alcohols, ketones and related esters. EFSA Journal 2008;6(12):918, 109 pp. 10.2903/j.efsa.2008.918 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Compendium of botanicals reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements. EFSA Journal 2012;10(5):2663, 60 pp. 10.2903/j.efsa.2012.2663 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2010. Guidance on the data required for the risk assessment of flavourings. EFSA Journal 2010;8(6):1623, 38 pp. 10.2093/j.efsa.2010.1623 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2011. Scientific Opinion on Flavouring Group Evaluation 25, Revision 2 (FGE.25Rev2): aliphatic hydrocarbons from chemical group 31. EFSA Journal 2011;9(6):2177, 126 pp. 10.2903/j.efsa.2011.2177 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2014. Scientific Opinion on Flavouring Group Evaluation 82, Revision 1 (FGE.82Rev1): consideration of epoxides evaluated by the JECFA (65th meeting). EFSA Journal 2014;12(6):3708, 32 pp. 10.2903/j.efsa.2014.3708 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2015a. Scientific Opinion on Flavouring Group Evaluation 78, Revision 2 (FGE.78Rev2): consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic hydrocarbons evaluated by EFSA in FGE.25Rev3. EFSA Journal 2015;13(4):4067, 72 pp. 10.2903/j.efsa.2015.4067 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2015b. Scientific Opinion on Flavouring Group Evaluation 25, Revision 3 (FGE.25Rev3): aliphatic hydrocarbons from chemical group 31. EFSA Journal 2015;13(4):4069, 116 pp. 10.2903/j.efsa.2015.4069 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , Silano V, Bolognesi C, Castle L, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Husøy T, Karenlampi S, Mennes W, Milana MR, Penninks A, Smith A, de Fátima Tavares Poças M, Tlustos C, Wölfle D, Zorn H, Zugravu C‐A, Binderup M‐L, Marcon F, Marzin D, Mosesso P, Anastassiadou M, Carfí M, Saarma S and Gurtler R, 2017. Scientific Opinion on Flavouring Group Evaluation 208 Revision 2 (FGE.208Rev2): consideration of genotoxicity data on alicyclic aldehydes with α,β‐unsaturation in ring/side‐chain and precursors from chemical subgroup 2.2 of FGE.19. EFSA Journal 2017;15(5):4766, 44 pp. 10.2903/j.efsa.2017.4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012a. Scientific Opinion on the safety and efficacy of phenol derivatives containing ring‐alkyl, ring‐alkoxy and side‐chains with an oxygenated functional group (chemical group 25) when used as flavourings for all species. EFSA Journal 2012;10(2):2573, 19 pp. 10.2903/j.efsa.2012.2573 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012b. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.FWHO2012.2534 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2013. Scientific Opinion on the safety and efficacy of aliphatic and aromatic mono‐ and di‐thiols and mono‐, di‐, tri‐, and polysulphides with or without additional oxygenated functional groups (chemical group 20) when used as flavourings for all animal species. EFSA Journal 2013;11(5):3208, 34 pp. 10.2903/j.efsa.2013.3208 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2015. Scientific Opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical group 31) when used as flavourings for all animal species. EFSA Journal 2015;13(3):4053, 22 pp. 10.2903/j.efsa.2015.4053 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2016a. Scientific opinion on the safety and efficacy of secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols from chemical group 8 when used as flavourings for all animal species. EFSA Journal 2016;14(6):4475, 26 pp. 10.2903/j.efsa.2016.4475 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2016b. Scientific opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical group 31) when used as flavourings for all animal species and categories. EFSA Journal 2016;14(1):4339, 17 pp. 10.2903/j.efsa.2016.4339 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA SC (EFSA Scientific Committee ), 2009. Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, on request of EFSA. EFSA Journal 2009;7(9):1249, 19 pp. 10.2093/j.efsa.2009.1249 [DOI] [Google Scholar]
- EFSA SC (EFSA Scientific Committee ), More SJ, Hardy A, Bampidis V, Benford D, Bennekou SH, Bragard C, Boesten J, Halldorsson TI, Hernandez‐Jerez AF, Jeger MJ, Knutsen HK, Koutsoumanis KP, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Nielsen SS, Schrenk D, Solecki R, Turck D, Younes M, Benfenati E, Castle L, Cedergreen N, Laskowski R, Leblanc JC, Kortenkamp A, Ragas A, Posthuma L, Svendsen C, Testai E, Dujardin B, GEN Kass, Manini P, Zare Jeddi M, Dorne J‐LCM and Hogstrand C, 2019a. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA SC (EFSA Scientific Committee ), More S, Bampidis V, Benford D, Boesten J, Bragard C, Halldorsson T, Hernandez‐Jerez A, Hougaard‐Bennekou S, Koutsoumanis K, Naegeli H, Nielsen SS, Schrenk D, Silano V, Turck D, Younes M, Aquilina G, Crebelli R, Gürtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, Solecki R, Carfì M, Martino C, Maurici D, Parra Morte J and Schlatter J, 2019b. Statement on the genotoxicity assessment of chemical mixtures. EFSA Journal 2019;17(1):5519, 11 pp. 10.2903/j.efsa.2019.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA SC (EFSA Scientific Committee) , More SJ, Bampidis V, Benford D, Bragard C, Halldorsson TI, Hernandez‐Jerez AF, Hougaard BS, Koutsoumanis KP, Machera K, Naegeli H, Nielsen SS, Schlatter JR, Schrenk D, Silano V, Turck D, Younes M, Gundert‐Remy U, Kass GEN, Kleiner J, Rossi AM, Serafimova R, Reilly L and Wallace HM, 2019. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 2019;17(6):5708, 17 pp. 10.2903/j.efsa.2019.5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautz LS, Jeddi MZ, Girolami F, Nebbia C and Dorne JLCM, 2021. Metabolism and pharmacokinetics of pharmaceuticals in cats (Felix sylvestris catus) and implications for the risk assessment of feed additives and contaminants. Toxicology Letters, 338, 114–127. 10.1016/j.toxlet.2020.11.014 [DOI] [PubMed] [Google Scholar]
- Pavela R, Morshedoo MR, Lupidi G, Carolla G, Barboni L, Quassinti L, Bramucci M, Vitali LA, Petrelli D, Kavallieratos NG, Boukouvala MC, Ntalli N, Kontodimas DC, Maggi F, Canale A and Benelli G, 2020. The volatile oils from the oleo‐gum‐resins of Ferula assa‐foetida and Ferula gummosa: a comprehensive investigation of their insecticidal activity and eco‐toxicological effects. Food and Chemical Toxicology, 140, 111312. 10.1016/j.fct.2020.111312 [DOI] [PubMed] [Google Scholar]
- Posternak JM, Linder A and Vodoz CA, 1969. Summaries of toxicological data. Toxicological tests and flavouring matters. Food and Cosmetic Toxicology, 7, 405–407. [DOI] [PubMed] [Google Scholar]
- Tisserand R and Young R, 2014. Essential oil safety. A guide for health care professionals. Chapter 13. Essential oil profiles. 2nd edn. Elsevier Ltd. pp. 199–200. 10.1016/C2009-0-52351-3 [DOI] [Google Scholar]
- Zomorodian K, Saharkhiz J, Pakshir K, Immeripour Z and Sadatsharifi A, 2018. The composition, antibiofilm and antimicrobial activities of essential oil of Ferula assa‐foetida oleo‐gum‐resin. Biocatalysis an Agricultiral Biotechnology, 14, 300–304. 10.1016/j.bcab.2018.03.014 [DOI] [Google Scholar]
- WHO (World Health Organization) , 2010. WHO technical report series 960. Evaluation of certain food additives and contaminants. Seventy‐third report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 8–17 June 2010. https://www.who.int/publications/i/item/9789241209601
- WHO (World Health Organization) , 2012a. WHO Food Additives Series: 67. Safety evaluation of certain food additives. Prepared by the Seventy‐sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva, 5–14 June 2012. Available online: https://apps.who.int/iris/handle/10665/77763
- WHO (World Health Organization) , 2012b. WHO Technical Report Series 974. Evaluation of certain food additives and contaminants. Seventy‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 5–14 June 2012. Available online: https://www.who.int/publications/i/item/9789241209748


