Abstract
The early role of natural killer cells and gamma delta T cells in the development of protective immunity to the blood stage of nonlethal Plasmodium yoelii infection was studied. Splenic cytokine levels were measured 24 h after infection of natural killer cell-depleted immunodeficient and littermate mice or transiently T-cell-depleted normal mice. Splenic gamma interferon levels were significantly increased above background in immunodeficient and littermate mice 24 h after infection. Depletion of natural killer cells resulted in markedly depressed gamma interferon levels and poor control of parasitemia, particularly in severe combined immunodeficient mice. In the littermates, gamma interferon levels were partially reduced, but parasitemias were resolved normally. However, in athymic mice, natural killer cell depletion had no effect on gamma interferon production. Levels of tumor necrosis factor alpha were increased in all animals 24 h after infection, and responses were not affected by natural killer cell depletion. However, in T-cell-depleted animals, both gamma interferon and tumor necrosis factor alpha levels were decreased 24 h after infection, and depleted mice were unable to control their parasitemia. These results suggest that the early production of both cytokines is important in the early control of parasitemia and that both natural killer and gamma delta T cells contribute equally towards their production. The data also suggest that the subsequent resolution of infection requires early production of gamma interferon, which might act by switching on the appropriate T-helper-cell subsets and other essential parasitotoxic effector mechanisms.
Nonspecific immune responses have long been recognized as essential first-line defenses against pathogenic microorganisms. However, their ability to activate specific immunological effector mechanisms, via the release of cytokines, is just beginning to receive recognition. Two cell types now known to participate in such mechanisms include nonspecific killer (NK), partly specific NK 1.1+ T cells, and gamma delta T cells (γδT cells) (reviewed by Schaible et al. [22]). Early NK cell-mediated gamma interferon (IFN-γ) production has been implicated in the control of viral (9), bacterial (20), fungal (24), and parasite (23, 29) infections, including blood-stage Plasmodium chabaudi malaria (11), and NK1.1+ T-cell activity has been reported in protective immunity against sporozoite-induced nonlethal Plasmodium yoelii (NLPY) infection (16). γδT cells also play an important role in antimicrobial immunity (reviewed by Salerno and Dieli [18]), and their activation is required for the control of P. chabaudi (26) and NLPY infections (10). However, they may also contribute to immunopathology, as seen in murine cerebral malaria (31) and in Mycobacterium avium infections in mice (21).
We previously showed that infections with the nonlethal malaria parasites NLPY and P. chabaudi were associated with an early burst of IFN-γ activity 24 h after challenge (5). This response was dose dependent and appeared to be partially dependent on NK cells, since mice depleted of their NK cells by treatment with anti-asialo GM-1 (ASGM-1) antibody showed a reduced IFN-γ response 24 h after challenge with parasitized red blood cells (pRBC). Furthermore, this response was markedly lower in nude mice than in their littermates. Lethal parasite infections with P. yoelii strain YM and Plasmodium berghei did not induce this early cytokine response, which appears to be important for the elimination of parasitemia (1, 8, 11, 25).
In the present study, we have further investigated the role of NK cells and also the possible role of γδT cells, by conducting similar studies in SCID, nude, and normal (BALB/c × C57BL/6)F1 mice. Our data suggest that both NK and γδT cells contribute to the early IFN-γ and tumor necrosis factor alpha (TNF-α) responses 24 h after NLPY challenge. While both cytokines are necessary for the initial control of parasitemia, IFN-γ may be important for subsequent T helper cell (Th) subset activation and possibly activation of other mechanisms essential for resolution of infection.
MATERIALS AND METHODS
Mice.
(BALB/c × C57BL/6)F1 mice were bred at Biological Services, University College London Medical School, from parental stocks obtained from the National Institute for Medical Research, Mill Hill, London, United Kingdom. Mice (8–12 weeks old) of both sexes were used in all experiments.
BALB/c athymic nude (Nu/Nu) and littermate (Nu/+) mice from Harlan Olac and CB17 SCID mice, from Biological Services, London School of Hygiene and Tropical Medicine, London, United Kingdom, were housed in filter-topped cages in ventilated racks.
Parasite and infection.
The nonlethal strain of P. yoelii (NLPY) was obtained from N. Wedderburn, Royal College of Surgeons, London, United Kingdom. Groups of animals were injected intravenously (i.v.) with either 106 pRBC for analysis of cytokines or 104 pRBC for estimation of parasitemia.
Depletion of T cells.
Mice were injected i.v. with one dose of 500 μg of anti-Thy 1.1 monoclonal antibody (MAb) purified from tissue culture supernatants of the YTS cell line (CAMR, Salisbury, U.K.) 4 days before infection with NLPY. T-cell depletion was judged by the ability of treated animals to mount a plaque-forming cell (PFC) response to a challenge with sheep red blood cells (SRBC). A separate group of anti-Thy 1.1-treated animals or phosphate-buffered saline (PBS)-treated control animals were injected i.v. with 106 SRBC, and 4 days later the animals were sacrificed and their spleens were harvested for analysis of PFC using a modified version of the Cunningham plaque assay (2), as described previously (4). The background level of PFC in normal, untreated animals was 600 ± 123 PFC/spleen (n = 3). While control animals pretreated with PBS developed 113,000 ± 2,093 PFC/spleen (n = 5), the group pretreated with the anti-Thy 1.1 MAb developed only 3,000 ± 167 PFC (n = 4). Hence, anti-Thy 1.1 MAb treatment reduced the normal PFC response by 97.6%.
The T-cell response of anti-Thy 1.1-depleted animals approached normal levels 10 days after treatment (equivalent to day 6 after infection) as judged by the PFC response (65,000 ± 19,200 PFC/spleen, (n = 3).
Depletion of NK cells.
Mice were injected i.v. with 50 μl of rabbit anti-asialo GM-1 (ASGM-1) antiserum (Wako Chemicals GmbH, Neuss, Germany) 2 days before infection with parasites. NK cell-mediated cytotoxicity of tumor cells in vitro is abolished from spleen cells of mice treated with 10 μl of this antibody (13). It has been demonstrated that mice treated with this antiserum have significantly reduced levels of NK cells but no reduction of T- or B-cell functions (7). Control mice were injected i.v. with 50 μl of PBS.
Cytokine extraction.
Endogenous levels of IFN-γ and TNF-α in the spleen were determined as described previously (3, 5). Individual spleens were weighed and homogenized in chilled RPMI medium containing 1% 3-[(cholamodopropyl)dimethylammonio]-1-propanesulfonate (CHAPS; Sigma, Poole, United Kingdom) in a Dounce tissue homogenizer, and 10% (wt/vol) homogenates were prepared. They were left on ice for 1 h, and insoluble debris was then removed by centrifugation at 2,000 × g for 20 min. The clear supernatants were stored in three aliquots at −80°C.
In some experiments the spontaneous secretion of cytokines in supernatants of cultured spleen cells in vitro was tested at 24 and 48 h. Individual spleen cell suspensions were prepared aseptically in RPMI medium supplemented with 5% heat-inactivated fetal calf serum, without the addition of challenge antigens. Cell concentrations were adjusted to 107 cells/ml, and triplicate cultures containing 2 ml/well were set up in 12-well Linbro plates (Nunc, GIBCO, Paisley, United Kingdom). Harvested supernatants were stored in three aliquots at −80°C.
Cytokine assays.
Standard capture enzyme-linked immunosorbent assays (ELISAs) were performed using Maxisorp (Nunc) plates as described before (3, 5). MAb pairs were used for the IFN-γ assay. Primary MAbs against IFN-γ (R46A2) and secondary biotinylated anti-mouse IFN-γ (XMG1.2) MAb (Pharmingen) was used with streptavidin peroxidase (Dako, Roskilde, Denmark) and 3,3′,5′5-tetramethylbenzidine (TMB) (liquid substitute system; Sigma) as the substrate. Recombinant mouse IFN-γ standards came from Pharmingen.
For TNF-α assays, a coating hamster MAb against murine TNF (TN3) (kindly provided by Celltech) was used with a polyclonal rabbit (detecting) antibody against murine TNF (Genzyme), peroxidase-conjugated anti-rabbit immunoglobulin G (IgG; Sigma), and TMB. A recombinant murine TNF (Genzyme) was used as a standard.
Cytokine production was calculated as the mean nanograms per spleen from spleens of at least six mice for total endogenous levels or as nanograms 107 cells per milliliter of culture supernatant from pooled data of supernatants from at least four test spleens per group. Cytokine assays were repeated three times on different aliquots of each sample.
Statistics.
Significance levels were determined by Student's t test for unpaired observations.
RESULTS
Splenic concentrations of IFN-γ and TNF-α were measured 24 h after infection of either normal or immunocompromised animals with 106 NLPY pRBC. This high dose of infection was previously shown to be ideal for the induction of cytokines soon after infection (5). A separate group of identical animals were challenged with 104 pRBC for monitoring the course of the infection. Spleen weights of mice increased significantly 24 h after infection with the high dose of inoculum, but there were no differences in the parasitemias (Table 1). Total cell numbers in spleen (±standard deviation) of these mice were as follows: for SCID, 4.8 (±1.2) × 106; for nude, 52 (±6.8) × 106; for littermate, 55 (±5.8) × 106; and for BALB/c, 62 (±3.5) × 106. There were no differences between spleen weights of the nondepleted animals and those depleted of their NK or T cells. However, total spleen cell numbers of the T-cell-depleted (BALB/c × C57BL6) F1 mice were markedly lower (70 [±13.0] × 106) after infection with 106 NLPY pRBC than those that were nondepleted (91.67 [±3.79] × 106).
TABLE 1.
Spleen weights of age-matched normal and infected mice and their parasitemias 24 h after NLPY infection
| Mouse strain | Mean spleen wt (g)a ± SD
|
% Parasitemia
|
||
|---|---|---|---|---|
| Preinfection | 24 h after infection (P) | Intact mice | T cell- or NK cell-depleted miceb | |
| (BALB/c × C57BL/6) F1 | 0.099 ± 0.01 | 0.13 ± 0.01 (<0.0001) | 0.15 ± 0.01 | 0.18 ± 0.06 |
| BALB/c | 0.11 ± 0.01 | 0.15 ± 0.01 (<0.005) | 0.16 ± 0.02 | 0.14 ± 0.02 |
| Nude | 0.10 ± 0.01 | 0.13 ± 0.01 (<0.005) | 0.17 ± 0.06 | 0.20 ± 0.02 |
| SCID | 0.06 ± 0.03 | 0.07 ± 0.01 (<0.02) | 0.19 ± 0.02 | 0.22 ± 0.09 |
| Littermates | 0.11 ± 0.01 | 0.15 ± 0.07 (<0.001) | 0.18 ± 0.03 | 0.17 ± 0.02 |
Values are means ± standard deviations for at least six spleens per group for the different mouse strains indicated. P values were calculated by Student's t test.
Parasitaemia after T-cell depletion in (BALB/c × C57BL/6)F1 mice or after NK cell depletion in the other strains 24 h after i.v. infection with 106 NLPY pRBC. Values are means ± standard deviations for six mice.
Early cytokine responses in NK cell-depleted nude and SCID mice.
To confirm and extend our previous observations of the role of NK cells in the early cytokine response to NLPY challenge, cytokine levels were analyzed in untreated control and ASGM-1-treated animals. Groups of SCID, nude, littermate, and normal BALB/c mice were pretreated with 50 μl of either physiological saline or ASGM-1 antibody 48 h prior to challenge with 106 NLPY; 24 h later, the animals were sacrificed and their spleens were assayed for the presence of IFN-γ and TNF-α. (Interleukin-4 [IL-4] responses were not measured, as they did not increase above background levels in previous studies [5].)
IFN-γ production.
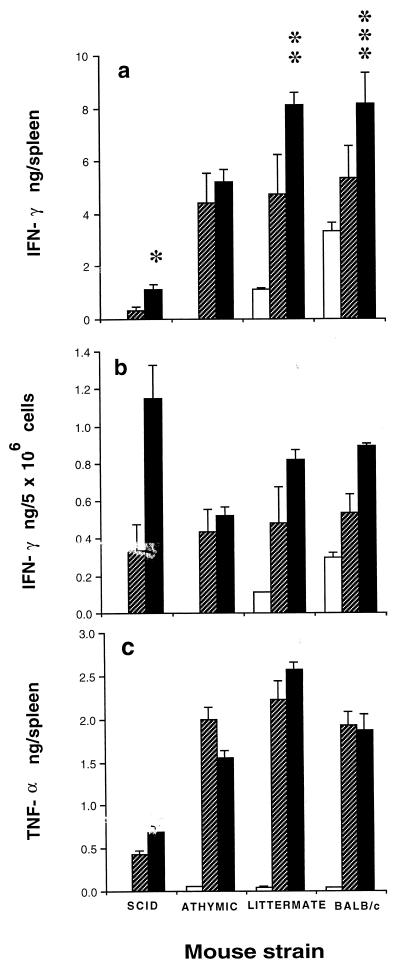
Background levels in nude and SCID mice were undetectable, while levels in the littermate (range, 0.05 to 0.09 ng/spleen) were significantly lower than those seen in normal BALB/c mice (range, 3.61 to 3.89 ng/spleen). NLPY challenge resulted in an early increase in IFN-γ levels in all mouse strains (Fig. 1a), and when the data were expressed in terms of cytokine levels per 5 × 106 cells, this increase was greatest in SCID mice (Fig. 1b). In the SCID and BALB/c mice, NK cell depletion led to significantly lower IFN-γ levels. This decrease was greater in the SCID mice than in the BALB/c mice, confirming the predominance of NK cells in this strain. However, NK cell depletion did not affect the response of nude mice, suggesting that another population of cells, possibly γδT cells, may be involved in these animals.
FIG. 1.
Effect of NK cell depletion on cytokine production 24 h after NLPY infection. Normal and immunocompromised mice depleted of their NK cells by treatment with rabbit anti-ASGM-1 antibody (hatched bars) and controls treated with PBS (solid bars) were injected i.v. with 106 NLPY pRBC, and 24 h later splenic IFN-γ (a and b) and TNF-α (c) levels were assayed. Each bar represents the mean (+ standard error) cytokine concentration for groups of 6 to 10 mice from two separate experiments. The open bar represents baseline values for normal uninfected mice. ∗, P < 0.002; ∗∗, P < 0.02; and ∗∗∗, P < 0.003 compared with NK cell-depleted mice.
TNF-α production.
Background levels in SCID mice were undetectable, but levels in the nude (range, 0.059 to 0.061 ng/spleen), littermate (range, 0.050 to 0.061 ng/spleen), and normal BALB/c (range, 0.049 to 0.051 ng/spleen) mice were similar. These responses were markedly elevated in all strains 24 h after NLPY infection. There was no decrease in responsiveness after ASGM-1 treatment (Fig. 1c). In fact, in nude mice, the TNF-α levels were significantly greater (P < 0.006) in the NK cell-depleted than in the normal controls. Hence, the NK cells were clearly not responsible for TNF-α formation.
Effect of NK cell depletion on the course of NLPY infection.
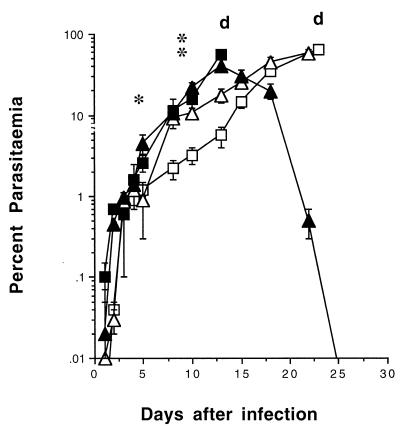
Since the effect of NK cell depletion on cytokine production was most marked in SCID mice, the effect of depletion on the development of parasitemia was assessed only in these mice. Animals were pretreated with 50 μl of ASGM-1 or PBS as a control 2 days before infection with 104 pRBC; peripheral blood parasite levels were monitored at intervals thereafter. Parasitemias in the NK cell-depleted SCID group were evident 1 day earlier than in their nondepleted counterparts; they were significantly higher between days 7 (P < 0.0l) and 12 (P < 0.0001), and the animals succumbed to the infection by day 15 (Fig. 2). The nondepleted SCID mice showed significantly lower parasitemias than all the other mice between days 10 and 15, although they were comparable to those of the nude mice later on. This early control of parasitemia, possibly attributable to the presence of NK cells, was only transient, as the animals died by day 25.
FIG. 2.
Course of NLPY infection in immunocompromised mice. SCID, nondepleted (□), and ASGM-1-depleted (▪) mice (details as in Fig. 1), and BALB/c nude (▵) and littermate (▴) mice were injected i.v. with 104 NLPY pRBC. Representative parasitemias (means ± standard errors) for groups of five to eight mice from two separate experiments are shown. d, day of death. For ASGM-1-treated SCID mice: ∗, P < 0.01, and ∗∗, P < 0.0001 compared with PBS-treated controls.
The nude and littermate mouse parasitemias were comparable up to day 15 (Fig. 2). Parasitemias in the nude mice continued to rise thereafter until they succumbed to the infection on day 25. By contrast, the littermates began to control their parasitemia, with almost complete resolution by day 26.
Early cytokine responses in anti-Thy 1.1-treated (BALB/c × C57BL/6)F1 mice.
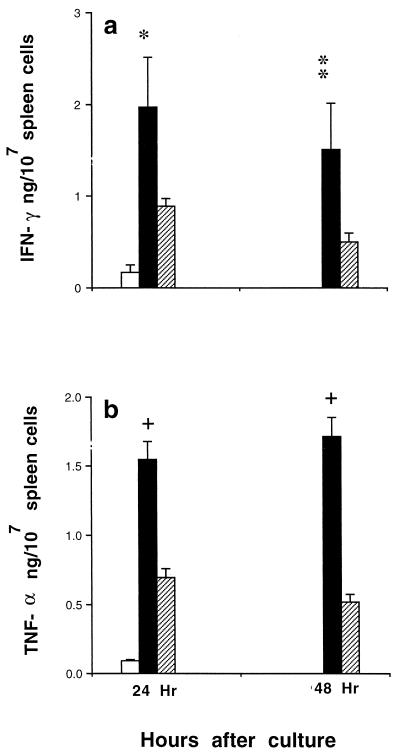
To see if γδT cells were revolved in IFN-γ production soon after NLPY infection, normal (BALB/c × C57BL/6)F1 mice were treated with a single dose of 500 μg of an anti-Thy 1.1 MAb 4 days before challenge with 106 NLPY-pRBC. As an alternative to specific anti-γδT cell MAbs, which were not available at the time, we used an anti-Thy 1.1 MAb for transient depletion of T cells in normal (BALB/c × C57BL/6)F1 mice. Treatment was started 4 days before pRBC challenge, so that the effect of T-cell loss would be greatest during the first 24 to 48 h after NLPY infection. The half-life of this antibody is approximately 10 days, as judged by the SRBC PFC response of treated animals. Hence, the T-cell pool should have been approaching normal levels by day 4 after infection. In these series of experiments, cytokine responses from in vitro-cultured spleen cell supernatants were analyzed. There were no significant differences between cytokine levels of the 24- and 48-h supernatants. Background IFN-γ and TNF-α levels in tissue culture supernatants of uninfected control spleen cells were 0.17 ± 0.08 and 0.09 ± 0.01 ng/ml, respectively. Levels of both cytokines were significantly higher in infected animals, but this response was reduced by approximately 50% in anti-Thy 1.1-treated animals (Fig. 3). This reduced response is likely to be due to the loss of γδT cells, since antigen-specific T cells were unlikely to be present 24 h after infection in anti-Thy 1.1-treated animals.
FIG. 3.
Effect of T-cell depletion on cytokine production 24 h after NLPY infection. (BALB/c × C57BL/6)F1 mice depleted of their T cells by treatment with an anti-Thy 1.1 MAb (hatched bars) and controls treated with PBS (solid bars) were injected i.v. with 106 NLPY pRBC, and 24 h later splenic IFN-γ (a) and TNF-α (b) levels were assayed. Each bar represents the mean (+ standard error) cytokine concentration in 24- and 48-h tissue culture supernatants from four to six individual spleens from two separate experiments. The open bar represents baseline values for normal uninfected mice. ∗, P < 0.006; ∗∗, P < 0.001, +, P < 0.0001 compared with T-cell-depleted mice.
Effect of anti-Thy 1.1 deletion on the course of NLPY infection in (BALB/c × C57BL/6)F1 mice.
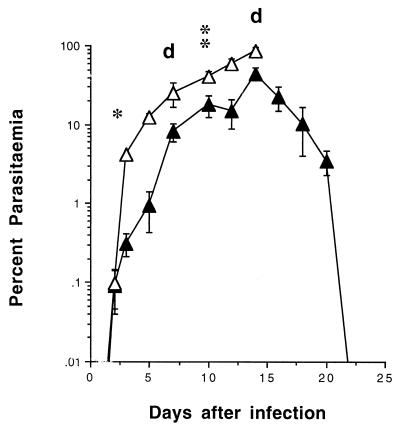
NLPY infections in (BALB/c × C57BL/6)F1 mice peaked between days 12 and 15 but then declined steadily and were resolved by day 21 to 25 (Fig. 4). Transient anti-Thy 1.1 treatment resulted in significantly higher parasitemias, particularly between days 3 (P < 0.0001) and 10 (P < 0.002); by day 7 two mice had died, and four other severely moribund animals had to be culled humanely on day 15.
FIG. 4.
Course of NLPY infection in (BALB/c × C57BL/6)F1 mice. Intact, nondepleted (▴) or T-cell-depleted (▵) animals (details as in Fig. 3) were injected i.v. with 104 NLPY pRBC. Representative parasitemias (means ± standard errors) in groups of 6 to 10 mice from two separate experiments are shown. d, day of death of T-cell-depleted mice. ∗, P < 0.0001; ∗∗, P < 0.002 compared with PBS-treated controls.
Taken together, the data suggest that both NK and γδT cells are responsible for the early IFN-γ production and that control of the early parasitemia is associated with high levels of both TNF-α and IFN-γ. However, for complete resolution of infection, IFN-γ-mediated activation of specific T-helper-cell subsets and other effector mechanisms appears to be necessary.
DISCUSSION
Two clear conclusions can be drawn from these studies. First, both IFN-γ and TNF-α are essential for the early control of NLPY parasitemia. Second, these cytokines are the products of both NK and γδT-cell activation, as evidenced by ASGM-1 and anti-Thy 1.1 depletion experiments, respectively. Furthermore, these findings also suggest that control of the later parasitemia, which is likely to be due to antigen-specific T helper cell activation, is probably driven by the IFN-γ that is produced during the early phase of infection. Thus, the early phase is perhaps the key rate-limiting step in the evolution of the disease: without it, later control cannot be elicited.
Our studies in SCID mice clearly show that NK cells play a vital role in damping down the early parasitemia. One possible mechanism for this is macrophage activation (as judged by the small but significant increase in TNF-α production), which could be attributed to IFN-γ produced by NK cells. However, during the later phase of the infection, the IFN-γ produced by NK cells was not sufficient to prevent a fatal outcome.
In the anti-Thy 1.1-treated animals, parasitemias were higher initially and reached lethal levels early. Since this is associated with lower levels of both IFN-γ and TNF-α, these findings are also consistent with the notion that IFN-γ production is important for early activation of macrophages as well as for subsequent activation of specific T helper cells. The low level of IFN-γ that is seen in these T-cell-depleted animals after pRBC challenge was probably due to NK cell activity and was clearly not high enough to contain the infection.
These findings resemble those seen in some other malaria models in which the early presence of IFN-γ, whether induced by IL-12 (8, 12, 25) or administered together with TNF-α (1), provided strong protective immunity against infection. We are currently testing this hypothesis further by administering IFN-γ to these T-cell-depleted animals at various times between days 2 and 4 after infection in an attempt to document the timing and kinetics of any IFN-γ-mediated T-cell activation.
Both γδT cells (26) and NK cell cytokine production (11) have been implicated in protection against P. chabaudi, and γδT cells have been shown to increase in number during NLPY infections (10). It is also evident that P. chabaudi infections in delta-chain knockout mice are resolved by an antibody-mediated mechanism, although the animals cannot mount a cell-mediated immune response against the parasite (30). Our data, which focus on the early activation of both γδT cells and NK cells, suggest that both cell types play a role in the early control of NLPY infections. It is possible, however, that under normal circumstances the contribution of the γδT cells is predominant, in view of the reduced levels of both IFN-γ and TNF-α in our T-cell-depleted animals, that is associated with higher parasitemias during both the early and later phases of the infection.
If this is correct, then it would support a two-stage hypothesis about the natural history of the disease. In this hypothesis, parasite antigens would stimulate macrophages to produce IL-12 and TNF-α, leading to NK cell activation with subsequent production of IFN-γ in the early phase. Later a combination of NK cell- but mainly γδT-cell-derived IFN-γ primes specific T helper cells for the induction of the necessary parasitotoxic effector mechanisms. The activation of Th1 cells would result in further production of IFN-γ and further activation of macrophages, which would be beneficial in controlling parasitemia. This latter response means that the early γδT cell production of IFN-γ is superseded by other sources of the cytokine, which is itself a protective mechanism because excess γδT-cell activation may itself be associated with immunopathology, e.g., murine cerebral malaria (31).
Evidence in support of the two-stage hypothesis comes from studies with the P. chabaudi malaria model, in which the first stage is marked by macrophage activation in the spleen, as judged by early production of both IL-12 p70 (19) and TNF-α (27). In the second stage, a CD4+ Th1 response associated with IFN-γ and TNF-α together contributes to resolution of infection in resistant C57BL/6 mice (28).
The two stage hypothesis, which conforms with current thinking about the close relationship between innate and adaptive immunity (reviewed by Schaible et al. [22]), may have important implications for the design of effective vaccination strategies against infectious diseases. If the early interaction between NLPY and nonspecific innate mechanisms is crucial to the subsequent outcome of the infection, then the type of parasite antigen responsible for inducing this part of the response may be an important component of a vaccine. Our earlier observation that lethal parasites are weaker inducers of the early IFN-γ response (5) supports the concept further. Conversely, in preliminary studies (unpublished data), we have shown that the vaccine delivery systems that induce the strongest protective immunity against blood-stage infections are also those that trigger an early IFN-γ response. Furthermore, vaccination (irradiated sporozoite or DNA vaccines) against sporozoite-induced lethal P. yoelii initially activates CD8+ T cells, which in turn activate NK cells, and this will in turn generate both IL-12 and IFN-γ, which are essential for the induction of protective immunity (6).
Conceivably, this early response is dependent on particular parasite antigens that might initially influence the release of transforming growth factor beta (TGF-β), now considered an important regulatory cytokine associated with protective immunity to malaria (14). High levels of TGF-β have been associated with protection and low levels with lethality (15). Early production of IFN-γ (5) and IL-12, as discussed above, is another distinguishing feature of infection with nonlethal stains of parasite. Lethal strains such as P. berghei fail to stimulate IL-12 during the early phase; not surprisingly, therefore, recombinant IL-12 pretreatment significantly delays the onset of parasitemia in infected mice, apparently due to enhanced IFN-γ production (32). Likewise, lethality may be overcome by IL-12 pretreatment of susceptible A/J mice prior to infection with P. chabaudi (12, 28). Pretreatment with IL-12 also induced sterile protection against sporozoite-induced nonlethal infections in both mice (25) and monkeys (8).
The underlying mechanism of this early response is reminiscent of findings from other forms of immune activation. It is interesting that in our earlier work we noted that there was a strong correlation between protective immunity in vaccinated mice and delayed-type hypersensitivity T-cell reactions (4, 17). This work may extend the analogy further. It has been argued that the delayed-type hypersensitivity response at 48 to 72 h can only be properly understood if triggered by the early phase. We suggest that it is this similar early phase, in the natural history of malaria parasitemia and morbidity, which has been highlighted by our studies.
ACKNOWLEDGMENTS
This work was supported by a UCL discretionary grant. H. R. Choudhury was supported by a grant from the foundation established by the late Jean Shanks.
We thank M. Quinlan and S. Sabir for assistance with cytokine assays.
REFERENCES
- 1.Clark I A, Hunt N H, Butcher G A, Cowden W B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-γ or tumour necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987;139:3493–3496. [PubMed] [Google Scholar]
- 2.Cunningham A J, Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968;14:599–600. [PMC free article] [PubMed] [Google Scholar]
- 3.de Souza J B, Ling I T, Ogun S A, Holder A A, Playfair J H L. Cytokines and antibody subclass associated with protective immunity against blood-stage malaria in mice vaccinated with the C terminus of merozoite surface protein 1 plus a novel adjuvant. Infect Immun. 1996;64:3532–3536. doi: 10.1128/iai.64.9.3532-3536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Souza J B, Playfair J H L. Immunization of mice against blood-stage Plasmodium yoelii malaria with isoelectrically focused antigens and correlation of immunity with T-cell priming in vivo. Infect Immun. 1988;56:88–92. doi: 10.1128/iai.56.1.88-91.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Souza J B, Williamson K E, Otani T, Playfair J H L. Early interferon gamma responses in lethal and non-lethal murine blood-stage malaria. Infect Immun. 1997;65:1593–1598. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doolan D L, Hoffman S L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 7.Ghayur T, Xenocostas A, Seemayer T A, Lapp W S. Induction, specificity and elimination of asialo-GM1+ graft-versus-host effector cells of donor origin. Scand J Immunol. 1991;34:497–508. doi: 10.1111/j.1365-3083.1991.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman S L, Crutcher J M, Puri S K, Ansari A A, Villinger F, Franke E D, Singh P P, Finkelman F, Gately M K, Dutta G P, Sedegah M. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat Med. 1997;3:80–83. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 9.Hussell T, Openshaw P J. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–2601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 10.Kopacz J, Kumar N. Murine gamma delta T lymphocytes elicited during Plasmodium yoelii infection respond to Plasmodium heat shock proteins. Infect Immun. 1999;67:57–63. doi: 10.1128/iai.67.1.57-63.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan K, Moulin P, Stevenson M M. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–4998. [PubMed] [Google Scholar]
- 12.Mohan K, Sam H, Stevenson M M. Therapy with a combination of low doses of interleukin 12 and chloroquine completely cures blood-stage malaria, prevents severe anemia, and induces immunity to reinfection. Infect Immun. 1999;67:513–519. doi: 10.1128/iai.67.2.513-519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakane A, Numata A, Chen Y, Minagawa T. Endogenous gamma interferon-independent host resistance against Listeria monocytogenes infection in CD4+ T cell- and asialo GM1 cell-depleted mice. Infect Immun. 1991;59:3439–3445. doi: 10.1128/iai.59.10.3439-3445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omer F M, Kurtzhals J A L, Riley E M. Maintaining the immunological balance in parasitic infections: a role for TGF-β? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 15.Omer F M, Riley E M. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, Cazenave P A. Liver CD4-CD8 NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol. 2000;164:1463–1469. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- 17.Playfair J H L, de Souza J B. Recombinant gamma inteferon is a potent adjuvant for a malaria vaccine in mice. Clin Exp Immunol. 1987;67:5–10. [PMC free article] [PubMed] [Google Scholar]
- 18.Salerno A, Dieli F. Role of gamma delta T lymphocytes in immune response in humans and mice. Crit Rev Immunol. 1998;18:327–357. doi: 10.1615/critrevimmunol.v18.i4.30. [DOI] [PubMed] [Google Scholar]
- 19.Sam H, Stevenson M M. Early IL-12 p70, but not p40, production by splenic macrophages correlates with host resistance to blood-stage Plasmodium chabaudi AS malaria. Clin Exp Immunol. 1999;117:343–349. doi: 10.1046/j.1365-2249.1999.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santanirand P, Harley V S, Dance D A, Drasar B S, Bancroft G J. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect Immun. 1999;67:3593–3600. doi: 10.1128/iai.67.7.3593-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders B M, Frank A A, Cooper A M, Orme I M. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect Immun. 1998;66:5508–5514. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaible U E, Collins H L, Kaufmann S H. Confrontation between intracellular bacteria and the immune system. Adv Immunol. 1999;71:267–377. doi: 10.1016/s0065-2776(08)60405-8. [DOI] [PubMed] [Google Scholar]
- 23.Scharton-Kersten T, Nakajima H, Yap G, Sher A, Leonard W J. Infection of mice lacking the common cytokine receptor gamma-chain (gamma(c)) reveals an unexpected role for CD4+ T lymphocytes in early IFN-gamma-dependent resistance to Toxoplasma gondii. J Immunol. 1998;160:2565–2569. [PubMed] [Google Scholar]
- 24.Schelenz S, Smith D A, Bancroft G J. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus:obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med Mycol. 1999;37:183–194. doi: 10.1046/j.1365-280x.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- 25.Sedegah M, Finkelman F, Hoffman S L. Interleukin 12 induction of interferon γ-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;25:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seixas E M, Langhorne J. Gammadelta T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J Immunol. 1999;162:2837–2841. [PubMed] [Google Scholar]
- 27.Stevenson M M, Huang D Y, Podoba J E, Nowotarski M E. Macrophage activation during Plasmodium chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice. Infect Immun. 1992;60:1193–1201. doi: 10.1128/iai.60.3.1193-1201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 29.Vester B, Muller K, Solbach W, Laskay T. Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infect Immun. 1999;67:3155–3159. doi: 10.1128/iai.67.6.3155-3159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weidanz W P, Kemp J R, Batchelder J M, Cigel F K, Sandor M, Heyde H C. Plasticity of immune responses suppressing parasitemia during acute Plasmodium chabaudi malaria. J Immunol. 1999;162:7383–7388. [PubMed] [Google Scholar]
- 31.Yanez D M, Batchelder J, van der Heyde H C, Manning D D, Weidanz W P. Gamma delta T-cell function in pathogenesis of cerebral malaria in mice infected with Plasmodium berghei ANKA. Infect Immun. 1999;67:446–448. doi: 10.1128/iai.67.1.446-448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimoto T, Yoneto T, Waki S, Nariuchi H. Interleukin-12-dependent mechanisms in the clearance of blood-stage murine malaria parasite Plasmodium berghei XAT, an attenuated variant of P. berghei NK65. J Infect Dis. 1998;177:1674–1681. doi: 10.1086/515301. [DOI] [PubMed] [Google Scholar]