Abstract
OBJECTIVES:
The Texas Hepatocellular Carcinoma Consortium cohort study investigates risk factors of hepatocellular carcinoma (HCC) and biomarkers for early HCC detection in patients with liver cirrhosis.
METHODS:
Adult patients with liver cirrhosis are enrolled at 5 clinical centers from 3 cities in Texas, with a target of 5,000 patients. Clinical history, risk factor questionnaires, liver imaging, laboratory data, and blood samples were collected at enrollment and at each 6-month follow-up visit.
RESULTS:
The primary outcome was the development of HCC. Biomarkers were tested in banked blood samples using prospective specimen collection, retrospective blinded evaluation design.
CONCLUSIONS:
We describe study design, eligibility criteria, recruitment, study cores, and sample size and analysis considerations.
INTRODUCTION
Hepatocellular carcinoma (HCC) has the fastest increasing cancer-related mortality in the United States. Texas has the highest HCC incidence and mortality rates in the nation (1). Texas residents have high prevalence of hepatitis C virus (HCV), hepatitis B virus (HBV), alcoholic liver disease, and emerging HCC risk factors such as nonalcoholic fatty liver disease (NAFLD).
Although most cases of HCC (90%) arise in the background of cirrhosis, which is the main predisposing condition for HCC, only 2%–3% of patients with cirrhosis develop HCC annually (2). For the primary prevention and early detection of HCC, there is need to better risk-stratify cirrhosis patients for HCC and also reliable and accurate biomarkers.
The goal of the Texas Hepatocellular Carcinoma Consortium (THCCC), funded by the Cancer Prevention Research Institute of Texas, is to reduce the burden of HCC. The objectives are (i) developing risk stratification algorithms based on demographic, clinical, molecular, and epidemiological risk profiles to identify patients with cirrhosis who might benefit from chemoprevention or intensive surveillance; and (ii) identifying and validating blood-based biomarkers to improve early HCC detection. We used data and specimens collected from a prospective cohort of 5,000 patients with cirrhosis recruited at 5 institutions in 3 Texas cities.
METHODS
Study design and variables for HCC risk prediction
The primary outcome of this prospective cohort study is the development of new HCC, defined as cases detected at least 1 month after an HCC-free baseline assessment. Hepatocellular carcinoma will be defined by histopathological (3) or radiological criteria. Exposures of interest include risk factors and features of metabolic syndrome (e.g., obesity, diabetes, physical activity, HbA1c, triglycerides, medications, pro- and anti-inflammatory markers, and germline genetic markers). Other risk factors include HCC risk factors such as HCV, HBV, hemochromatosis, and confounders including demographics, severity of liver disease (e.g., Model for End-Stage Liver Disease (MELD) score), and history of tobacco smoking, alcohol use, and family and personal history of cancer.
Study design and variables for testing HCC biomarkers
The THCCC is a phase-3 biomarker study, which is a critical evaluation of early detection biomarkers to determine how early a biomarker can detect HCC before clinical symptoms appear (4). The study defines the test-positive rule and estimate the sensitivity and specificity of the decision rule before it can be used to trigger a diagnostic workup in prospective cohort (phase 4) studies. Our analytic design used the prospective specimen collection, retrospective blinded evaluation design (5), in which biologic specimens are collected prospectively in the absence of knowledge about patient outcomes. After HCC status is ascertained, patients with HCC and control subjects without HCC are randomly selected from the cohort, and their specimens assayed for the biomarker. Prospective specimen collection, retrospective blinded evaluation design avoids extrapolation, spectrum, and overfitting biases inherent to case–control studies. The measurements will be performed in a blinded fashion for the Food and Drug Administration–approved HCC biomarkers and several novel biomarkers, including osteopontin (6), collagens, and fatty acids.
THCCC study population
Study coordinators at each site (Houston VA, Baylor Clinic, UT Southwestern, Parkland, UT San Antonio) screened liver clinic schedules to identify eligible participants with cirrhosis, and during the clinic visit, administer informed consent and collect study data and blood samples. Enrollment took place during routine medical care encounters. Study blood draw was combined whenever possible with blood draw for clinical examinations within 8 weeks of the baseline study visit. Cirrhosis diagnosis was based on histology; radiology, liver elastography, or serum biomarkers. Patients with hepatic decompensation, previous HCC, or current non-melanoma skin primary cancer were excluded. Follow-up continued until the participant’s development of HCC, death, or the end of the study. Most patients were seen every 6 months for routine management of liver disease, which provides efficient follow-up.
We have assembled study-specific cores (administrative, statistical, cohort samples). The Statistical Coordinating Center (SCC) implemented a Web-accessible data collection system, with controlled access to allow authorized site staff to register participants, report eligibility and consent dates, and enter data. Source documents (medical records, radiology, and laboratory reports) are made available for periodic review and monitoring. We developed the manual of operations, and SCC created the data collection elements in the electronic database system. Training sessions were performed with study coordinators of all the sites. It took 12 months to launch the study and start recruiting patients. Monthly teleconference calls with the sites were initiated to review enrollment. The SCC performs data monitoring on a continuous basis and on-site audits of sample collection steps and a random sample of source documents. The Cohort Samples Core provides training to maximize enrollment and follow-up, performs audits with SCC, and resolves screening and eligibility questions. All study samples were collected, transported, and stored at and according to the validated protocol developed by the Population Sciences Biorepository at Baylor College of Medicine.
Statistical methods
Deriving a risk prediction index involves model building, assessing performance, and validation. Predictive factors are derived from demographics, survey, and clinical data including blood-based tests. With our enrollment target of 5,000 patients, an average HCC annual incidence of 3%, and an average 2 years of follow-up, we anticipate that at least 300 participants will develop HCC during the study.
For biomarker analyses, we planned a case–control design with randomly selected 150 HCC patients and 300 control subjects for the initial panel development and a similar number for validation. We selected a model and a cutoff to optimize sensitivity at a 10% false-positive rate, that is, receiver operating characteristic (ROC) curve (0.1). This has the potential to “rule in” patients for computed tomography or MRI surveillance for the diagnosis of HCC. The study had >87% power to select a panel for validation if its ROC (0.1) was >0.65 against a null hypothesis of ROC (0.1) of ≤0.5 (7).
DISCUSSION
This will be the largest modern cirrhosis cohort with rigorous data and specimen collection and follow-up, focusing on primary and secondary prevention of HCC. Most cases of new HCC among patients with cirrhosis occur in a current modern hepatology practice within the settings of cured HCV, adequately suppressed HBV, or NAFLD. The cohort will provide an adequate sample size to ensure sufficiently precise estimations of the effects of risk factors, including metabolic syndrome and NAFLD, in the cirrhosis population and also biomarker sensitivities and specificities. The data collection methods ensure uniform and granular information on risk factors and concurrent imaging.
The THCCC was conceived to address current hypothesis-driven studies to produce specific and timely results that have translational potential to impact clinical practice, while having a forward outlook of building a scientific infrastructure in the form of well-characterized cohorts with relevant biosamples and clinical and epidemiological data. Our approach is highly relevant for Texas, but our studies address hypotheses that are highly relevant and informative for HCC prevention anywhere.
Figure 1.
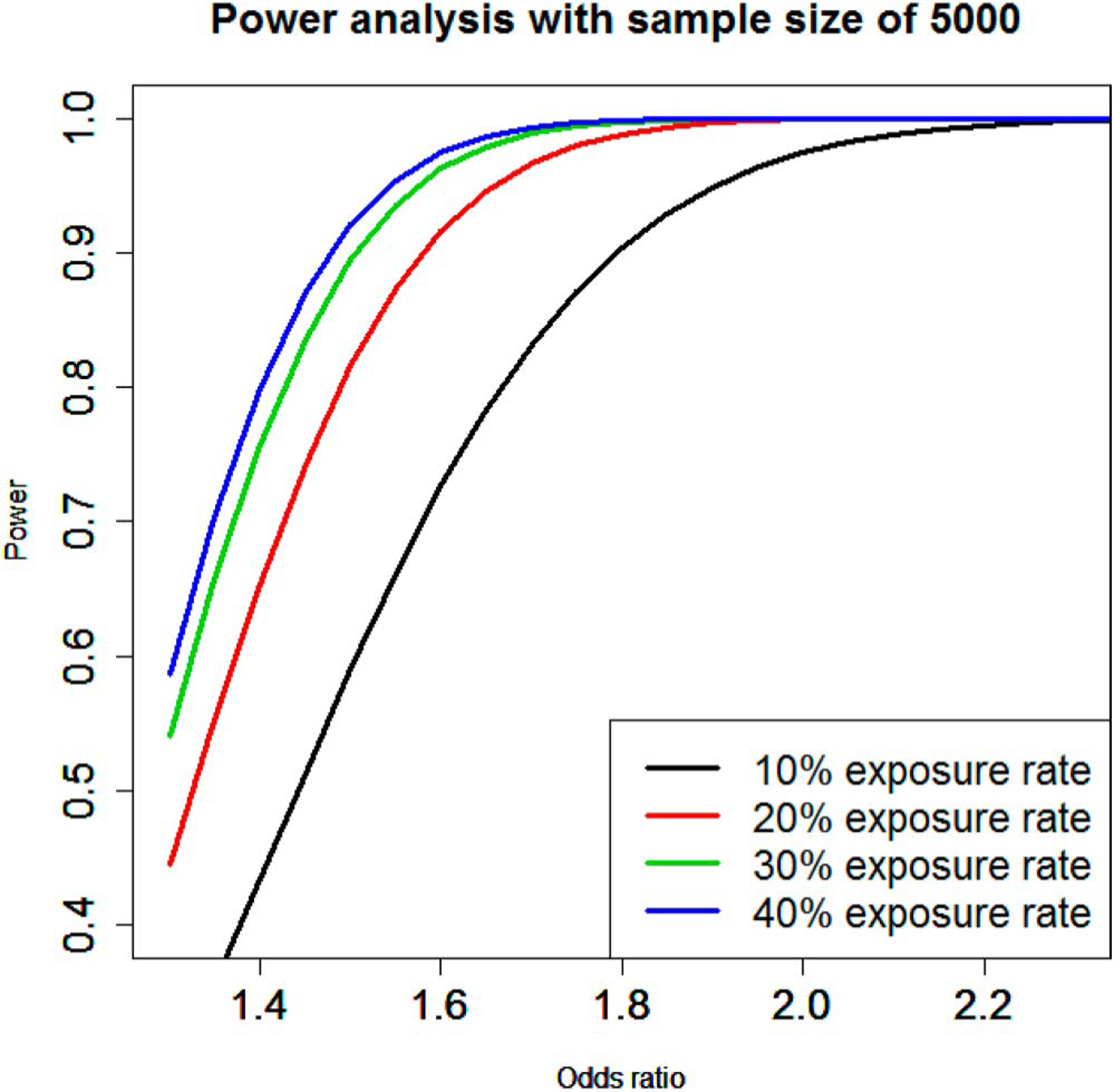
Statistical power with cohort size of 5,000 patients for various assumptions of effect size (odds ratio) and exposure levels.
Table 1. Data collection schedules.
| Data forms/source | Event interval | Submission schedule |
|---|---|---|
| Recruitment data | Baseline | At the time of initial subject contact |
| Informed consent data and source | Baseline | Within 14 d after subject registration |
| Eligibility data | Baseline | At the time of registration |
| Baseline data and source | Baseline | Within 14 d after subject registration |
| Follow-up data | Every 6 mo for years 1–5 | Within 14 d after each assessment |
| Post-HCC follow-up data | 1 yr after diagnosis | Within 14 d after each assessment |
| Histologic diagnosis data/source (pathology report) | When applicable | Within 14 d after becoming aware of the diagnosis |
| Adverse event data | When applicable | Within 5 d after becoming aware of the event |
| Off-study data/source | Off study | Within 14 d after off-study date |
HCC, hepatocellular carcinoma
Financial support:
Cancer Prevention & Research Institute of Texas grant (RP150587). The works is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Footnotes
Guarantor of the article: Hashem B. El-Serag.
CONFLICTS OF INTEREST
Potential competing interests: Research grant funding for investigator initiated research from Gilead, Wako, and Merck to H.B.E-S.
REFERENCES
- 1.White DL, Thrift AP, Kanwal F, et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: Translating knowledge into practice. Clin Gastroenterol Hepatol 2015;13:2140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clavien PA, Lesurtel M, Bossuyt PM, et al. , OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol 2012;13:e11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001;93:1054–61. [DOI] [PubMed] [Google Scholar]
- 5.Pepe MS, Feng Z, Janes H, et al. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J Natl Cancer Inst 2008;100:1432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang S, Plymoth A, Ge S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 2012;55: 483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bantis L, Feng Z. Comparison of two correlated ROC curves at a given specificity or sensitivity level. Stat Med 2016;35:4352–67. [DOI] [PMC free article] [PubMed] [Google Scholar]


