Abstract
Background
Therapeutic exercise for gait function using an exoskeleton-assisted Body Weight Supported Treadmill Training (BWSTT) has been identified as a potential intervention that allows for task-based repetitive training with appropriate kinematics while adjusting the amount of body weight support (BWS). Nonetheless, its effect on gait in patients with stroke in the chronic phase are yet to be clarified. The primary aim of this scoping review was to present the status of effectiveness of exoskeleton-assisted BWSTT in patients with chronic stroke. The secondary aims were to summarise intervention protocols, types and functions of BWSTT exoskeletal robotic devices currently used clinically.
Method and results
Articles were accessed and collected from PubMed, Ovid MEDLINE, Cochrane Central Register of Controlled Trials, and Web of Science databases, which were completed in October 2020. Articles were included if the subjects were adults with stroke in the chronic phase (onset ≥ 6 months) and if they utilised a robotic exoskeleton with treadmill and body weight support and investigated the efficacy of gait exercise. A total of 721 studies were identified, of which 11 randomised controlled trials were selected. All included studies were published from 2008 to 2020. Overall, 309 subjects were enrolled; of these, 241 (156 males, 85 females) participated. Walking outcome measures were used more often to evaluate the functional aspects of gait than to evaluate gait independence. In 10 of 11 studies, showed the effectiveness of exoskeleton robot-assisted BWSTT in terms of outcomes contributing to improved gait function. Two studies reported that exoskeleton-assisted BWSTT with combination therapy was significantly more effective in improving than exoskeleton-assisted BWSTT alone. However, no significant difference was identified between the groups; compared with therapist-assisted BWSTT groups, exoskeleton-assisted BWSTT groups did not exhibit significant change.
Conclusion
This review suggests that exoskeleton-assisted BWSTT for patients with chronic stroke may be effective in improving walking function. However, the potential may be “to assist” and not because of using the robot. Further studies are required to verify its efficacy and strengthen evidence on intervention protocols.
Keywords: Robot-assisted gait training, Chronic stroke, Gait exercise, Body Weight-Supported Treadmill Training, Exoskeleton, Scoping review
Background
It is estimated that 15 million people worldwide have suffered from a stroke, with approximately 5 million living with permanent disability [1]. Almost 50% of stroke patients are unable to walk after stroke onset, and, even after intensive rehabilitation, 30–40% of these patients still have limited ability to walk [2–4]. Exoskeleton-assisted Body Weight Supported Treadmill Training (BWSTT) has gained attention over the past few decades as a popular method of post-stroke gait training due to its advantages for task-based repetitive training [5–10]. However, for patients with stroke in the chronic phase, improvements in gait, its outcome measures and specific intervention protocols have yet to be clarified.
Chronic stroke
In this review, we defined chronic phase in stroke as equal to or more than 6 months after stroke onset. It has been suggested that the majority of functional recovery after stroke onset occurs in the acute phase and plateaus from 3 to 6 months after onset. However, previous studies have shown that specialised and intensive training can improve motor function in patients with chronic stroke who have motor dysfunction [11–14]. Moreover, the degree and amount of improvement in motor function has been reported to be correlated with the intensity and frequency of rehabilitation [15–17].
Robotic devices in gait rehabilitation
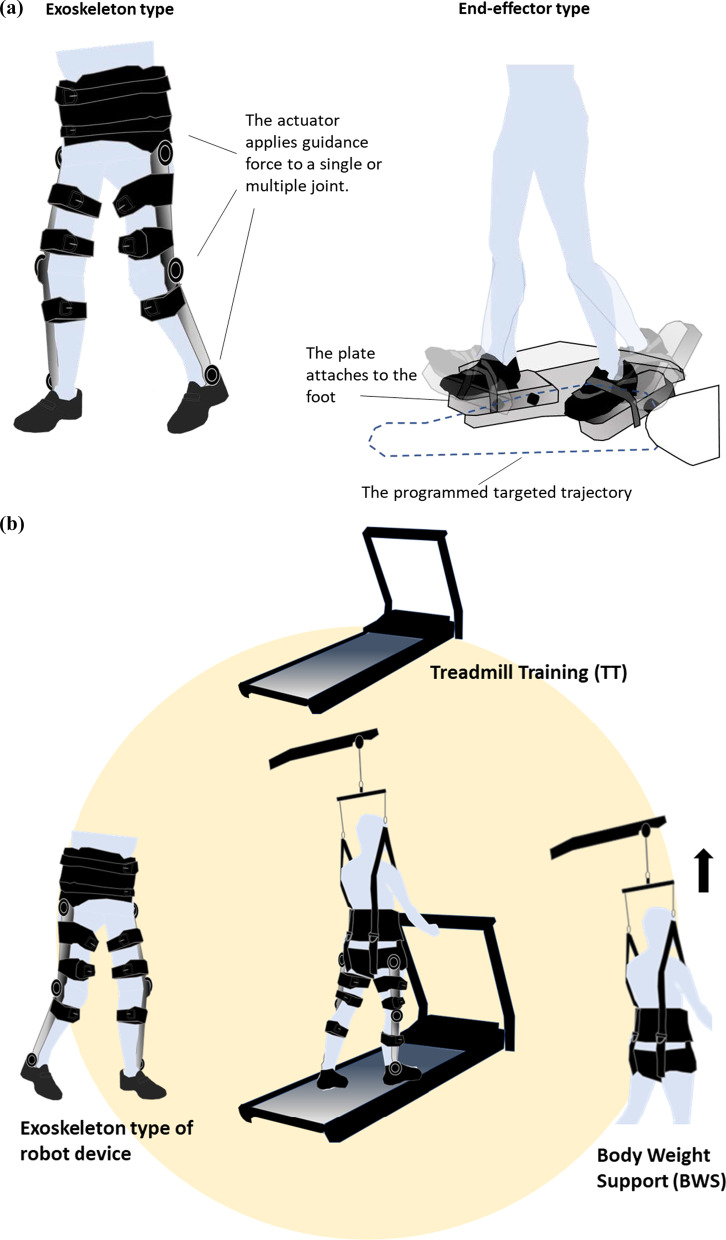
A search in the PubMed for articles related to gait-assist robots used in rehabilitation yielded no results prior to 1989, although the number of articles rapidly increased from 2003 to 2020 as the field was recognised in rehabilitation. The application of robotic technology to rehabilitation has substantially increased in recent years [18–22] and several gait-assistive robotic devices are already available on the market [5–10, 23, 24]. The available assistive robot systems include HAL (CYBERDYNE Inc., Japan), Welwalk (Toyota Motor Corporation, Japan), Lokomat (Hocoma AG, Switzerland), Ekso (Ekso Bionics, USA), and many others are currently undergoing development. All these devices possess one or more of the following functions: a body-weight-support device, a treadmill, or an overground walking system. The devices used for gait exercises utilise electromechanically actuated motors that control movement and exert force on the joints or parts of the lower limbs. These are categorised as exoskeleton type, end-effector type and other [25] (Table 1, Fig. 1a, b). The exoskeleton type is wearable and assists the patient by applying output torque directly at the targeted lower limb joints during gait training. The timing and intensity of the assistance is programmed and given during the entire gait cycle or in a specific phase. The end-effector type is a device placed on the plantar that provides assistance of the ideal gait trajectory to the foot (peripheral). Types stated as other included powered walking frames, powered ankle foot orthosis or non-exoskeleton wire-driven types. Unlike end-effector type robots, which have a fixed end and input programmed motion trajectory, exoskeletons are intended to compensate for lost gait function, characterised by dynamic assistance or control of the rotational motion of the target joints. In equipment associated with exoskeletons (Fig. 1b), the adjustable body weight support (BWS) function, which prevents falls, may safely accommodate patients with a wide range of gait function levels from Functional Ambulation Categories (FAC) 0–4 [26]. The treadmill also facilitates speed adjustment and repetitive gait input in a set position. BWSTT has been reported to significantly improve balance, gait speed, and endurance in stroke patients [27, 28]. Furthermore, it has been reported that gait training with adjusted weight bearing instead of full weight improves walking speed and endurance on level ground, leading to improved gait [29, 30]. BWSTT is highly effective in improving gait in patients who have suffered subacute stroke, but its effectiveness is not clear in chronic stroke [27, 29, 31]. It has been reported that gait-assistive robot training has an impact on gait improvement [27]. On the other hand, literature comparing the effects in terms of differences by type of gait-assist robot and stage of the target patients is limited, and effects on specific endpoints are not yet clear [25, 32, 33]. Moreover, results may differ depending on the clinical trial design and intervention protocols.
Table 1.
Types of robotic devices for therapeutic gait rehabilitation
| Type of device | BWS device | Treadmill | Examples of some representative product | |
|---|---|---|---|---|
| Exoskeleton | BWSTT Exoskeleton | Yes | Yes | Lokomat (Hocoma AG, Switzerland), Welwalk (Toyota Motor Corporation, Japan), Walkbot (P&S Mechanics Co. Ltd., Korea) |
| Overground Exoskeleton | No | No | HAL(Cyberdyne, Japan), Ekso-GT(Ekso Bionics, USA) | |
| End-effector | Yes | N/A | G-EO System (Reha Technology, Swissland), LokoHelp(Woodway, USA) | |
| Other | – | – | Powered AFO, Walking aids with electric assist functions |
BWSTT Body Weight Supported and Treadmill Training, BWS Body Weight Support
Fig. 1.
a Major types of robotic devices for robot-assisted therapeutic gait rehabilitation. The figure shows the Exoskeleton type (left) and the End-effector type (right). b Exoskeleton type of robot device with Body Weight Supported Treadmill Training (BWSTT)
Gait exercise for stroke
Currently, several forms of intervention in gait training have been proposed for the management of stroke. Conventional physiotherapy for walking after stroke generally includes muscle strengthening, functional task practise, symmetrical movement practise (including weight bearing and shifting training), stepping and single-leg standing targets for practising specific gait phases, circuit training, and neurodevelopmental techniques [34]. Most conventional gait retraining is undertaken with hands-on assistance, which is potentially physically taxing for the therapist.
Frequent intense gait training interventions have been shown to result in higher overall functional improvements in patients with stroke in chronic phase [5–10]. However, the physical burden and time/cost required to maintain such functions are a key challenge amongst therapists, as well as patients in the chronic phase after stroke.
The use of technology-enhanced gait training for rehabilitation which gives mechanically assisted task-based repetitive training is expanding; nonetheless, its competence is still being argued. A previous study reported that individuals who received electromechanical-assisted gait training in combination with physiotherapy after stroke were more likely to achieve independent walking than people who received gait training without these devices [25, 31]. In another study, therapist-assisted locomotor training was superior to robotic-assisted locomotor training amongst ambulatory survivors with chronic stroke [35]. In addition to refining the application of devices and determining patients who may benefit from robot-assisted training, the identification of an effective combination therapy crossover is essential.
In summary, although significant research has been done on exoskeleton robotic rehabilitation, only minimal research has been conducted on its application in patients with chronic stroke. Moreover, investigations on the efficacy of devices used in robotic gait exercise in the chronic phase are limited, and the current situation is unclear. At the same time, while various types of robotic assistive devices have been developed to date, the trends in equipment, design, and functional requirements specifically for the chronic phase are still not well known [27, 29, 31].
However, recent randomised control trials (RCT) focusing on the efficacy of exoskeleton-assisted BWSTT (Fig 1b) for chronic stroke have reported positive improvements in gait [35–45], indicating that there is a potential value in further research to clarify this finding.
Therefore, this study aimed to review and describe the effectiveness of exoskeleton assisted BWSTT in patients with chronic stroke. And the second objectives were to summarise intervention protocols and the types and functions of BWSTT exoskeletal robotic devices currently used clinically.
Methods
The literature review protocol was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [46], and with reference to the work conducted by Arksey and O'Malley [47], Ferrari [48] and Peters [49].
Articles were accessed and collected from PubMed, Ovid MEDLINE, Cochrane Central Register of Controlled Trials, and Web of Science databases, which were completed on October 27, 2020.
The primary search was conducted using combined terms: (robot* OR exoskeleton OR “powered gait orthosis” OR PGO OR HAL OR “hybrid assistive limb” OR ReWalk OR Ekso OR Indego OR lower-extremity robot OR robotic-assisted OR electromechanical OR mechanically assisted OR powered assisted OR robotic device OR Welwalk OR electromechanical-assisted OR robotic OR end-effector OR assist robot OR GEAR OR robotic orthosis OR rehabilitation robotics OR orthotic devices OR Lokomat) AND (stroke OR post-stroke OR CVA OR “cerebrovascular accident” OR “cerebral infarct” OR “cerebral haemorrhage” OR hemiplegia OR hemiparesis) AND (gait OR walk OR walking OR ambulation OR gait training) AND (chronic OR community OR at home). Additionally, the following parameters were used: clinical trial/ RCT and scientific articles written in English, with its full text available to all the authors. Date was not restricted. Additional references were also identified by manual search, and duplicates were removed.
The inclusion criteria were as follows:
Interventions, allocated subject group, and outcome measures that refer to the efficacy and/or effectiveness of gait exercise and exoskeleton-assisted BWSTT
Human subjects: post-stroke, hemiplegia in the chronic phase with onset at ≥ 6 months
Type of electromechanical robotic exoskeleton used in gait exercise that facilitates movement or exerts force on the hip, knee, or ankle joints
The exclusion criteria were as follows:
The stated devices were not for use on the lower limbs (upper limbs, hand robots, devices for controlling pelvic motion, etc.)
The disease stage was not clearly stated or there were stages other than chronic in the target group
Included healthy participants or children aged < 18 years
Included participants with mixed diagnosis
Used only general braces such as ankle foot orthosis and had a drive source that was not controllable electro mechanically
Used only the following interventions for the subject group: BWS device on a treadmill, electromyographically driven neuromuscular electrical stimulation, and virtual reality (VR)/augmented reality
Did not apply force on the limb nor was movement facilitated by the device
Used the end-effector type of device or exoskeleton for overground walking
Reported only technology development
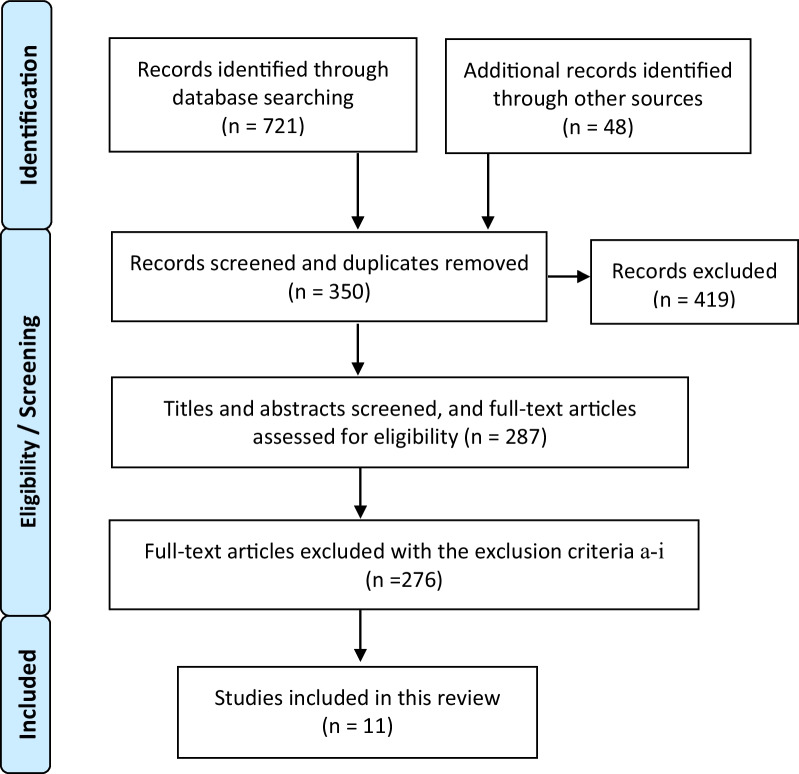
The selection process is shown in the flow diagram in Fig. 2 [46].
Fig. 2.
A flow diagram of the selection process. The PRISMA flow diagram [46] of the process of the database searches, the number of abstracts and full texts screened assessed
With respect to the reference selection process and the inclusion and exclusion criteria above, the titles and abstracts of potential articles were screened by two reviewers to remove irrelevant studies. Potentially eligible studies were chosen from the remainder if full texts were available.
From the final selected studies, data on the study design and subjects, equipment used for the interventions, gait exercise treatment protocol, evaluation tools, and reporting of information on the effectiveness of clinical gait exercise were extracted from the selected articles to provide information on the results and effects that would be useful in clinical practise (Table 2). Additionally, data on the characteristics, type and functional requirements of the gait assistive robots as devices used in the selected literature were summarised to analyse them for use in the chronic stage (Table 3).
Table 2.
Literature summary
| Author, year Study design | Participants | Participants' walking ability at baseline | Training period and protocol (duration of the intervention, session) | Apparatus and its setting | Outcome |
|---|---|---|---|---|---|
| Hornby et al. (2008) [35] RCT | 48 patients with chronic stroke IG: robotic LT group (n = 24) CG: therapist-assisted LT group (n = 24) | > 10 min overground without assistance at WS ≤ 0.8 m/s at SSV, using assistive device | IG: with guided symmetrical locomotor assistance by robot CG: with guided symmetrical locomotor assistance by therapist manually 12 sessions 30 min per session | Device: Lokomat BWS: weight support% is 25 ± 6.7% for robotic- assisted and 21 ± 7.5% for therapist-assisted WS: up to 3.0 kmph decreased throughout training | Outcome measures: WS (SSV and FV), 6MD at SSV, mEFAP, BBS, SF-36, MMT, MAS, CES-D Result: In therapist-assisted LT, greater improvements in speed and single limb stance time on the impaired leg compared with those in the robotic LT group were found |
| Belas Dos Santos et al. (2018) [36] RCT | 15 patients with chronic stroke IG: n = 7 CG: n = 8 | Baseline and outcome evaluation: BBS, FIM, TUG | IG: 2 sessions of PT and 1 session of RAGT CG: 2 sessions of PT and 1 session of TAGT 60 min per session 5-month protocol | Device: Lokomat BWS: gradually reduced from 50% of patient weight at the start of the protocol treatment until a minimum of 10% at the end WS: low speed between 0.8 kph and 1.5 kph | Outcome measures: BBS, TUG, FIM, SARA Functional scale scores mean values differed significantly between groups. No significant difference for the between-group comparison at both baseline and after treatment |
| Seo et al (2018) [37] RCT | 12 patients with chronic stroke IG: n = 6 CG: n = 6 | Able to walk at least 10 m independently Asymmetrical gait with a step length asymmetry ratio > 1.1 FAC Group 1: 3.3 ± 0 Group 2: 3 3.7 ± 0.2 | IG: assist-as-needed RAGT for the unaffected limb and fully assisted RAGT for the affected limb CG: assist-as-needed RAGT for the affected limb and fully assisted RAGT for the unaffected limb 20 sessions 2 times per week 45 min per session | Device: Walkbot BWS: bodyweight support was reduced as the subject's function improved (no clear description present) WS: the treadmill speed was increased to a maximum of 2.2 km/h as the subject's function improved | Outcome measures: NIHSS, FMLE, FAC, MI, and the TCT Assessed at the baseline (T0) and after 10 (T1) and 20 (T2) training sessions Result: In IG, FMLE, FAC, and MI scores significantly improved at T2 compared to T0. The unaffected limb's step length asymmetry ratio and hip maximal extension moment significantly improved In CG, only FMLE score improved significantly at T2 compared to T0 No significant differences in the between groups analysis was found |
| Lewek et al. (2009) [38] RCT | 19 participants with chronic stroke IG: therapist-assisted LT (n = 9) CG: robotic-assisted LT (n = 10) | Able to walk at least 10 min overground without assistance and at SSV of 0.8 m/s | IG: therapist-assisted LT CG: robotic-assisted LT 12 sessions 3 times per week 60 min per session | Device: Lokomat BWS: 40% BWS at the first session and reduced as tolerated WS: speed was gradually increased during the first session, remaining at 3.0 km/h (0.83 m/s) for the duration of the intervention | Outcome measures: Maximum joint angles, Excursions of the hip, knee, and ankle joints, Hip and knee ACC Result: Robotic-assisted LT did not demonstrate a significant increase in SSV, hip and knee ACC. Therapist assisted LT resulted in significant improvements in both outcome measures |
| Westlake et al. (2009) [39] RCT | 16 volunteers with chronic stroke IG: Lokomat (n = 8) CG: manual BWSTT (n = 8) | At least unlimited household ambulators (WS > 0.3 m/s) | IG: RAGT with Lokomat CG: manual BWSTT The fast WS group and slow WS group were assigned to the IG and CG, respectively 12 sessions 3 times per week ≤ 60 min per session | Device: Lokomat BWS: 35% at first, reduced in increments of 5% as long as gait quality was maintained WS: maintained below 0.69 m/s (2.5 km/h) in the slow groups and above 0.83 m/s (3 km/h) in the fast groups | Outcome measures: SSV, paretic step length ratio, FV, 6MD Result: IG group, SSV and paretic step length ratio, and four of the six secondary measures improved. Group differences between fast and slow training groups were not stated |
| Danzl et al. (2013) [40] RCT | 8 subjects with chronic stroke IG: active tDCS with RGO CG: sham tDCS with RGO | N/A | IG: active tDCS with identical locomotor training with a robotic gait orthosis (RGO) CG: sham tDCS with identical locomotor training with a robotic gait orthosis (RGO) 12 sessions 3 times per week | Device: Lokomat BWS: 40–50% at the beginning of session and then reduced to 20% at the end of protocol training WS: Initially at comfortable speed, followed by progressive, rapid decrease to a speed slow enough to allow subject to initiate movement, thereby increasing engagement with the task and appropriate attentional demands | Outcome measures: 10MWT, BBS, FAC, SIS-16 Result: the active tDCS group showed greater improvement than the sham group in all measures except BBS |
| Bae et al. (2014) [41] RCT | 20 subjects with chronic stroke IG: RAGT with FES (n = 10) CG: RAGT only (n = 10) | Able to walk for 10 m with or without an assistive device | IG: robot-assisted gait training and functional electrical stimulation on the ankle dorsiflexor of the affected side CG: robot-assisted gait training only 15 sessions 3 times per week 30 min per session with regular 30-min PT session in both groups | Device: Lokomat BWS: reduced from 40 to 0% according to the patient’s gait pattern WS: from 1.2 km/h up to the maximum speed at which patients could adapt | Outcome measures: MMAS, TUG, BBS, gait parameters, WS, cadence, step length, stride length, double support, pelvic, hip, knee, ankle joint angle Result: In IG, Step length and maximal knee extension were significantly greater than those before training and Maximal Knee flexion showed a significant difference between the in IG. The MMAS, BBS, and TUG scores improved significantly after training compared with before training in both groups |
| Ogino et al (2020) [42] RCT | 19 patients with chronic stroke IG: GEAR group (n = 8) CG: treadmill group (n = 11) | All subjects were required to walk on the ground or a treadmill without physical assistance using assistive devices | IG: GEAR training CG: using only the treadmill function of GEAR 20 sessions 5 times per week 60 min per session Visual and audio feedback was used when needed | Device: GEAR system (Welwalk) BWS: - WS: the treadmill speed was increased from overground maximum speed. The treadmill speed was increased by 10% in the next session, if it was judged safe to do so, and reduced by 10% if it was not | Outcome measures: Abnormal gait pattern, Spatiotemporal gait parameters Result: In IG, step length and maximal knee extension were significantly greater than those before training Maximal knee flexion showed a significant difference between the experimental and control groups |
| Ogino et al. (2020) [43] | 19 participants IG: GEAR group (n = 8) CG: treadmill group (n = 11) | Independent gait overground without physical assistance, using assistive devices, and bracing below the knee as needed | IG: GEAR training CG: The treadmill group performed gait training using only the treadmill function of GEAR 20 sessions 5 times per week 60 min per session Visual and audio feedback was used when needed | Device: GEAR system (Welwalk) BWS: - WS: the treadmill speed was increased from overground maximum speed. The treadmill speed was increased by 10% in the next session, if it was judged safe to do so, and reduced by 10% if it was not | Outcome measures: 10MWT, TUG, 6MD, SF-8, and GRC scales Result: In both groups, the MMAS, BBS, and TUG scores showed significant difference before and after training. In IG, WS was significantly increased at completion of training and 1-mo follow-up compared with baseline and GRC scales were significantly increased at completion of training, 1 month follow-up, and 3-month follow-up compared with baseline TUG and 6 min walk were significantly greater in IG than CG at completion of training compared to baseline |
| Bae et al. (2016) [44] RCT | 34 patients IG: HRR-guided high-intensity RAGT group (n = 17) CG: RPE-guided high-intensity RAGT group (n = 17) | Able to walk but with difficulty | IG: HRR-guided high-intensity RAGT; RAGT at 70% of HRR CG: RPE-guided high-intensity RAGT group; RAGT at RPE of 15 All participants: additional regular 30-min PT 18 sessions 3 days per week 30 min per session, | Lokomat BWS: from 40 to 0% according to individual ability WS: controlled gait speed (from 1.2 km/h up to) | Outcome measures: FMLE, 10MWT, gait parameters (walking speed, cadence, step length, stride length, swing time, stance time, double, support rate, single support rate, and symmetrical ratio index) Result: The FMLE was significantly higher than that before the intervention in both groups. IG improved significantly more than CG. The value of 10 MWT was significantly higher than that before the intervention in both groups |
| Erbil et al. (2018) [45] RCT | IG: RAT (n = 29) CG: control (n = 14) | Ambulatory patients with or without assistive devices and patients with BBS score ≥ 20 points | IG: 30 min of RAT (RoboGait®) plus 60 min of PT CG: 90 min of PT 3 weeks during weekdays In both groups, BoNT‐A injections were applied | Device: RAT (RoboGait®) BWS: Body weight support range 0 to approximately 100 kg (220.5 lbs), continuously adjustable without training interruption WS:0.2–3.2 km/h | Outcome measures: MAS and Tardieu Scale, BBS, TUG, and RVGA Result: In both groups significant improvements were determined regarding spasticity, balance, and gait functions In IG, at post‐treatment Weeks 6 and 12, change from baseline TUG, BBS, RVGA were significantly higher than CG |
BBS Berg balance scale, BSW body weight support, BWSTT Body-Weight Supported Treadmill Training, CG control group, FAC Family Assistance Centre, FIM Functional Independence Measure, FMLE Functional Mobilisation Lower Extremities, IG intervention group, MMAS Modified Motor Assessment Scale, NIHSS National Institutes of Health Stroke Scale, PT Physiotherapy, SSV Self-Selected Velocity, SARA Scale for Assessment and Rating of Ataxia, TUG Timed Up and Go, RAGT Robot assisted gait training, RVGA Rivermead Visual Gait Assessment, WS walking speed
Table 3.
Description of devices used in the selected literature
| Studies and exoskeletons | Design | Type of actuator | Assist joint | Device description |
|---|---|---|---|---|
| Lokomat [35, 36, 38–41, 44] (Hocoma AG, Switzerland) | Bilateral | Electric Actuation [50] (DC motor) | Hip and knee (ankle passive) |
System consisting of a robotic lower-extremity orthosis with: ••••A dynamic body weight support system that supports vertical/lateral centre-of-gravity movement ••••Pelvic position is fixed ••••Adjustable level of assist, gait speed, and guidance force (from 100 to 0%) ••••Computer-regulated motors for individual joints ••••Synchronised treadmill and visual feedback utilities |
| Walkbot [37] (P&S Mechanics, South Korea) | Bilateral | Electric Actuation [51] (DC brushless motor) | Hip, knee, and ankle |
System consisting of a robotic lower-extremity orthosis with: ••••A dynamic body weight support system that supports vertical centre-of-gravity movement ••••Pelvic position is fixed ••••Synchronisation of the hip, knee, and ankle to assist patients in learning correct gait patterns, impedance control: patient's voluntary efforts are detected and patients are allowed to influence gait patterns during rehabilitation, automatic adjustment of leg length, motion analysis: kinetic and kinematic data reconstructed as a 3D image ••••Synchronised treadmill |
| RoboGait [45] (Bama Technology, Turkey) | Bilateral | Electric Actuation [52] (Linear motor) | Hip and knee |
System consisting of a robotic lower-extremity orthosis with: ••••Body weight supported system and pelvic position is fixed ••••Eight force sensors measuring in pairs in each joint and four force sensor amplifiers ••••Synchronised treadmill and biofeedback utilities |
| GEAR system/Welwalk [42, 43] (Toyota Motor Corporation, Japan) | Unilateral | Electric Actuation [53] | Knee |
System consisting of a robotic lower-extremity orthosis with: ••••Body weight supported system ••••Gait phase is calculated using data from the pressure sensor ••••Adjustable level of assist, gait speed, and guidance force (from 100 to 0%) ••••Real-time feedback system for gait characteristics on the monitor screen ••••Recording of quantitative data during intervention with biofeedback system |
Results
The PRISMA flow diagram for this study is presented in Fig. 2. Overall, 721 studies were identified through database search, and 48 additional potentially relevant studies were found through manual search. From a total of 769 studies, 350 were retrieved by screening the studies’ written language and article type. Additionally, duplicate records were removed. After assessing the eligibility of articles based on the title, abstract, and full text, 11 were selected. All included studies were published from 2008 to 2020. Six studies were published between 2015 and 2020 and five studies were published before 2014.
Study design
All included studies were RCTs or randomised crossover trials. Most studies included fewer than 30 participants and were recognised as small-scale pilot studies except for three RCTs [35, 44, 45] which included 34, 48, and 48 participants.
Participants
A total of 309 subjects were enrolled in all included studies; of these, 241 (156 males, 85 females) participated. All participants were patients with chronic stroke with onset at ≥ 6 months. The mean age was 57.8 ± 7.0 years. The subjects were community residents or recruited from the outpatient department in three studies [42, 43, 45], were hospitalised in four studies [36, 37, 41, 44], and belonged to other categories or were unknown in four studies [35, 38–40].
In addition, in a few studies, the subjects were conditioned to a certain level of walking prior to the intervention (a level of independence in which the subjects were able to walk for more than 10 m without a walking assistive device or without receiving walking assistance). Few studies also involved the use of a cane, orthotic device, or walker to walk for more than 10 m.
Three reports specified a walking speed of 0.3 m/s [39] and 0.8 m/s or more [35, 38]. Furthermore, four studies used the Berg Balance Scale (BBS) [36, 45], Timed Up and Go Test [36], Functional Independence Measure [36], Modified Ashworth Scale [42, 43], etc. at the functional level.
Training period
The duration of intervention and the duration of a session differed in each study. In the included studies, the duration of intervention ranged from 3 weeks [45] to 5 months [36], and the number of training sessions varied from nine [45] to 12 sessions [35, 38–40], 18 sessions [44], and 20 sessions [36, 41, 42].
The duration of one session of lower-limb robotic gait training was 30 min [38, 39, 41, 44, 45], 45 min [37], and 60 min [36, 42, 43], with 30 min being set in approximately half of the studies. In addition, the frequency range was, in descending order of days, five days a week [43, 43], three days a week [38–41, 44, 45], and two days a week [41]. Most of the studies were conducted five or three times a week.
Training protocol and subject group characteristics
All selected studies utilised gait training on a treadmill and included BWSTT robot-assisted gait training as the intervention. The characteristics of the training protocol and comparison groups were as follows: the most frequent comparisons were robot-assisted gait training groups vs. therapist-assisted groups [35, 36, 38, 39], and the second most frequent comparisons were robot-assisted gait training with combination therapy groups vs. robot-assisted gait training groups. Combination therapy was described as transcranial direct current stimulation (tDCS) [40] or functional electrical stimulation (FES) [41]. Robot-assisted gait training with conventional therapy was compared with robot-assisted gait training alone in one study [45], the effects of robot-assisted gait training were compared between the affected and non-affected sides in another study [37], and the intervention was evaluated in BWSTT robot-assisted gait training groups vs. a robot-free group in two studies [42, 43].
Additional studies compared an exercise loading index, heart rate reserve, and rating of perceived exertion for robot-assisted gait training [44] and compared the effects of different walking speeds between robot-assisted groups [54].
Outcome measures
Walking outcome measures were used more often to evaluate the functional aspects of gait than to evaluate gait independence. Two studies evaluated the Functional Ambulation Category [37, 40] and Rivermead Visual Gait Assessment [45] as the measures of gait and mobility independence. Two studies either measured the walking speed or utilised the 10-m walk test as a quantitative assessment of gait function [40, 44]. In addition, two studies [35, 43] assessed gait endurance using the 6-min walk test, whereas four studies [36, 39, 40, 43] assessed it using the Timed Up and Go Test. Four studies [36, 40–42] assessed balance ability using the BBS. Three studies evaluated spatiotemporal parameters such as stance time and stride length, floor reaction force data, and kinematic gait parameters such as angular changes in each joint were measured using a 3D motion capture system [38, 42, 44].
Except for those mentioned above, with respect to performance-based outcome measures that are directly related to gait assessment, the Modified Ashworth Scale [45] was investigated as a score of spasticity and a scale of sensorimotor function in stroke. In another study using combination therapy, robot-assisted gait training was undertaken with neurophysiological assessment using an electroencephalogram (EEG). Furthermore, four of the 11 studies included self-reported assessments for depression and satisfaction with treatment (Hamilton Rating Scale for Depression, Center for Epidemiological Studies-Depression Scale [35, 36], Global Rating of Change [42]).
Types of exoskeletons and their control methods
In all selected studies, exoskeletons utilised a combination of a treadmill and BWS system. In this review, the most utilised exoskeleton was Lokomat, which was used in seven studies [35, 36, 38–41, 44]. The Wellwalk prototype was used in two studies. [42, 43]. Other exoskeletons used were Walkbot [37] and RoboGait [45]. Wellwalk was the only unilateral type; the other exoskeletons had a bilateral set-up.
Lower-limb robots utilised the following joint assistance: Walkbot: hip knee and ankle tri-joint control [37]; Lokomat and RoboGait: hip and knee bi-joint control [35]; and Gait Exercise Assist Robot (GEAR) system/Wellwalk: knee and ankle joint bi-joint control [42, 43]. These four lower-limb robotic devices have an integrated BWS system and treadmill. Of these, Lokomat [35, 36, 38–41, 44], Walkbot [37], and RoboGait [45] have a pelvic immobilisation device in addition to the assisted joint to fix the trajectory of the centre-of-gravity movement in the pelvis. In the GEAR system/Welwalk [42, 43], the pelvic movement is not fixed and is relatively free. All the above-mentioned devices are equipped with visual and auditory feedback functions and the ability to see the patient’s gait.
Table 3 provides further details about all four devices, including their design and type of actuator, and describes these devices, including control strategies and their function.
Walking speed and BWS in treadmill gait training
The setting criteria for the treadmill walking speed varied. Some studies did not state a numerical speed but specified it as the maximum speed that the patients could achieve [42, 43]. Alternatively, some studies used an individual patient’s comfortable speed that decreased to the appropriate speed [40] or set a constant speed (2.5 km/h to 3.0 km/h [39], 0.2 km/h to 3.2 km/h [45]). In addition, some had a fixed starting speed, with gradual increases in speed in accordance with the patients’ maximal effort and improvement (starting at 0.8 km/h to 1.5 km/h [36] or 1.2 km/h [41, 44]). Others had a fixed maximum speed, and the speed gradually increased within the upper limit of its maximum value (up to 2.2 km/h [37] and up to 3.0 km/h [35, 38]).
The criteria for BWS also varied widely. In some studies, BWS at the beginning of the protocol was set as the percentage of each patient’s body weight and was gradually decreased based on the patient’s ability or improvement [36, 38, 40, 41, 44]. In particular, most studies were based on a gradual decrease in the upper limit of BWS from 40% [38, 40, 41, 44] and from 50% [36] at the start of the protocol. The lower limit was set in the range of 0–20%, depending on the patient’s improvement and change in ability [36, 40, 41, 44].
Efficacy of BWSTT exoskeleton-assisted gait training and results of individual studies
In all 11 RCTs, the effects of exoskeletal robot-assisted training varied due to different intervention protocols, intervention periods, and lower-limb robotic devices used (Table 2). The results were classified according to the characteristics of the subject groups as follows:
Four studies [35, 36, 38, 39] were categorised into BWSTT-robot assisted gait training (BWSTT-RAGT) vs BWSTT- therapist assisted gait training (BWSTT- TAGT) (Table 4, 4.1). In all three studies [35, 36, 39], both groups showed improvement in gait outcome measures (Table 4) when comparing within each group. However, no significant between-group difference was observed [35, 36, 39]. In one study [35], the BWSTT-RAGT group had a lower improvement in walking speed (self-selected velocity and fast velocity) than the BWSTT-TAGT group. In addition, the results of one study [38] indicated that robot-assisted gait training groups did not show a significant change within and between groups.
Table 4.
Summary of efficacy of BWSTT exoskeleton-assisted gait training
| 4.1 BSWTT-RAGT vs BSWTT-TAGT | |||||
|---|---|---|---|---|---|
| Author | Hornby et al. [35] | Belas Dos Santos et al. [36] | Lewek et al. [38] | Westlake et al. [39] | |
| Additional treatment provided | N/A | Conventional PT | N/A | N/A | |
| Results in BSWTT-RAGT groups (pre, post change, p < 0.05) | Body function/structure level |
SSV + 0.07, d = 0.29 FV + 0.06, d = 0.19 |
SARA − 3.5, d = 0.49 BBS + 5.8, d = 0.31 TUG − 0:19 s, d = 0.64 |
No change |
FMLE + 2.6, d = 0.56, BBS + 1.4, d = 0.2 SS + 0.01 m/s, d = 0.29 FV + 0.09 m/s, d = 0.15 SLR (abs) − 0.16, d = 0.31 |
| Activity level | – | FIM + 4.6, d = 0.34 | – | – | |
| Results between groups (p < 0.05) | BWSTT-TAGT group showed greater improvements in SSV + 0.06 m/s, d = 0.65, FV + 0.07 m/s, d = 0.69, Single limb stance time at FV: + 2.4 ± 3.7%, d = 0.91 | No significant difference | No significant difference | No significant difference | |
| 4.2 BSWTT-RAGT vs BSWTT-RAGT with combination therapy | |||
|---|---|---|---|
| Author | Danzl et al. [40] | Bae et al. [41] | |
| Additional treatment provided | tDCS for experimental group |
FES for experimental group Conventional PT |
|
| Results in BSWTT-RAGT groups (pre, post change, p < 0.05) | Body function/structure level | 10MWT + improved |
MAS + 1.92, d = 0.27 TUG − 5.63 s, d = 0.38 BBS + 3.43, d = 0.41 Gait speed + 0.007 m/s, d = 0.47 Step length + 0.05, d = 0.43 Stride length + 0.33, d = 0.33 Maximal Knee flexion + 18.747 d = 1.07 Maximal Knee flexion + 6.904 d = 0.58 |
| Activity level |
FAC + improved SIS-16 + improved |
– | |
| Results between groups (p < 0.05) | BSWTT-RAGT with active tDCS group showed greater improvement than the sham group in 10MWT, FAC, and SIS-16 measures except BBS | BSWTT-RAGT with FES group showed a significantly greater in Maximal Knee flexion + 8.97, d = 0.56 | |
| 4.3 BSWTT-RAGT vs BWSTT | |||
|---|---|---|---|
| Author | Ogino et al. [42] | Ogino et al. [43] | |
| Additional treatment provided | N/A | N/A | |
| Results in BSWTT-RAGT groups (pre, post change, p < 0.05) | Body function/structure level |
GRC scale (change of gait) + improved |
10MWT + 0.09 m/s |
| Activity level | – | – | |
| Results between groups (p < 0.05) | No significant difference | BSWTT-RAGT group were significantly improved in TUG (r = 0.57), 6-min walk (r = 0.51) and score of general health in SF-8 (r = 0.49) | |
| 4.4 Other | ||||
|---|---|---|---|---|
| Author | Assist unaffected limb vs affected limb | HRR vs RPE guided BSWTT-RAGT | BSWTT-RAGT vs Conventional PT | |
| Seo et al. [37] | Bae et al. [44] | Erbil et al. [45] | ||
| Additional treatment provided | N/A | N/A |
Conventional PT BoNT-A |
|
| Results in BSWTT-RAGT groups (pre, post change, p < 0.05) | Body function/structure level |
Assist US: FMLE + 3.2, d = 1.18 MI + 11.7, d = 2.32 Step length asymmetry ratio -0.2, d = 2.0 Hip maximal extension moment (US) -0.5, d = 1.79 Assist AS: FMLE + 2.7, d = 1.29 Ankle maximal dorsiflexion angle (US) -8.9, d = 3.26 |
HRR guided: FMLE + 3.67, d = 0.23, 10MWT + 0.22 m/s, d = 0.80, WS + 0.20 m/s, d = 1.53 And Improved in Stride length, Cadence, Single and Double support rate, Swing time, Stance time, Step length, and Symmetrical index RPE guided: FMLE + 2.20, d = 0.63, 10MWT + 0.13 m/s, d = 0.41, WS + 0.14 m/s, d = 0.14 And Improved in Stride length, Cadence, Single support rate Single and Double support rate, Swing time, Stance time, Step length, Symmetrical index |
MAS − 1.5, d = 2.94 Tardieu Scale (spasticity grade) − 0.2, d = 0.44 BBS + 2.7, d = 0.29 TUG + 5.7, d = 0.66 |
| Activity level | Assist US: FAC + 0.7, d = 2.33 | – | RVGA + 5.3, d = 1.0 | |
| Results between groups (p < 0.05) | No significant difference | HRR-guided group showed significantly improved in compared to RPE-guided group in FMLE, 10MWT, WS, Stride length, Cadence, Single support rate, Single and Double support rate, Swing time, Symmetrical index | BSWTT-RAGT group is significantly higher in TUG, BBS, and RVGA | |
Each study was categorised according to the characteristics of the comparison group under investigation. The results of BWSTT-RAGT intervention groups in pre-post change (p < 0.05) and results compared to the control group are shown. Descriptive values are presented as the mean change and d describes effect size. Results were categorised as Body function/structure level and Activity level [55, 56]. RAGT Robot-assisted gait training, TAGT Therapist-assisted gait training, BWSTT Body-Weight Supported Treadmill Training, PT Physiotherapy, AS Affected side, US Unaffected side, BBS Berg balance scale, BWS body weight support, FAC Family Assistance Centre, FIM Functional Independence Measure, FMLE, Functional Mobilisation Lower Extremities; MMAS Modified Motor Assessment Scale, SARA Scale for Assessment and Rating of Ataxia, TUG Timed Up and Go, RVGA Rivermead Visual Gait Assessment, WS walking speed, FES functional electrical stimulation, GRC scale Global rating of change scale
In BWSTT-RAGT vs BWSTT-RAGT with combination therapy (Table 4, 4.2), two studies [40, 41] reported that robot-assisted BWSTT-RAGT with combination therapy was significantly more effective in improving gait mainly in activity level of outcome within and between groups than BWSTT-RAGT alone. Furthermore, in a study using tDCS as combination therapy [40], the BWSTT-RAGT with active tDCS group showed greater improvements in 10 MWT (10 Metre Walk Test), FAC, and SIS-16 (Stroke Impact Scale-16) measures except for the BBS than the sham group. In another trial investigating the effects of an intervention combining robot-assisted gait training and FES, maximal knee flexion during gait was significantly greater than that before training in the BWSTT-RAGT with FES group [41].
Table 4, 4.3 shows studies comparing BWSTT-RAGT and BWSTT, two of which were applicable [42, 43]. In these two studies, effects were found within the BWSTT-RAGT group for activity level measures, including the 10MWT. There were no significant differences between the groups regarding kinetics and gait pattern changes [42]. However, quantitative measures of gait, such as TUG and 6-min walk, in the BWSTT-RAGT were higher than in the BWSTT group [43].
In other categories summarised in Table 4, 4.4, one study [45] which compared with conventional physiotherapy showed greater improvement in gait function between group in BSWTT-RAGT group. One of the studies compared the effect of robot assistance on the unaffected limb or affected limb during BWSTT-RAGT [37], and others compared the method of guiding the target of BWSTT-RAGT [38]. Both studies [37, 44] showed significant improvement in outcome measures both in body function/structure and activity level in both conducted BWSTT-RAGT groups.
Discussion
The purpose of this review was to present and assess the status of effectiveness of robot-assisted BWSTT. Eleven studies were included, indicating that only a small number of RCTs on this topic have been published. As the date of publishing ranged from 2008 to 2020, it could be said that this is a relatively new field. Additionally, more positive improvements in walking in the acute to subacute phase have been reported [26, 31, 57]. There exist few reviews about BWSTT robot-assisted gait exercise that focus on the chronic phase after stroke; hence, its efficacy remains unclarified.
Effect of robot-assisted gait training in the chronic phase after stroke
There is an expectation that robotic rehabilitation will lead to a paradigm shift in work due to the therapeutic effect on the patient and the reduced burden on the therapist. From this perspective, the results of this review lead to the conclusion that it is not possible to conclude that BWSTT-RAGT is significantly more effective.
We have identified from the study protocol that it is relevant to address papers that present the results of the target intervention group which investigate the effect of exoskeleton used RAGT from the perspective of scoping the current RAGT.
Within the BWSTT-RAGT group, pre- and post-intervention results demonstrated that 10 out of 11 studies [35–37, 39–45] showed a significant improvement in some gait function outcome. Furthermore, there was no significant worsening of gait function in all selected studies [35–45].
The four of the 11 selected studies compared BWSTT-RAGT with BWSTT-TAGT, indicated that there was either no significant difference between the groups [36, 38, 39] or a predominant change in the therapist-assisted group as compared to that in the group receiving conventional gait exercise and the group receiving robot-assisted gait exercise [35]. Therefore, we did not reach the conclusion that the robot was more effective than the therapist for chronic stroke patients.
On other hand, those comparing the BWSTT-RAGT only group to the BWSTT-RAGT with combination therapy group (tDCS [40], FES [41]) have reported significant effects on improving gait outcome measures in between groups. Further research is encouraged, as BWSTT-RAGT with combination therapy may further enhance the efficacy of BWSTT-RAGT. Furthermore, there are studies excluded from the conditions for acceptance, although the following studies have been reported. In studies reporting on improvements in brain function levels that may be involved in the improvement of gait function and an RCT focusing on robot assisted gait training with combination therapy using visual stimulation with VR had shown and identified three main areas of brain activity, as measured by electroencephalography that were significantly evident in the robot assist with VR group [58]. Another RCT [59] of BSWTT-RAGT reported improvements in cognitive flexibility and shifting skills, selective attention/visual research, and quality of life.
The results of two out of 11 references [42, 43], which were from the same research group, and BWSTT-RAGT was effective between the groups, depending on the outcome. These indicate that BWSTT-RAGT in addition to BWSTT alone may be more effective than BWSTT alone in improving dynamic balance, speed and endurance during gait [43], but the actual changes in gait pattern in kinetics are not yet cleared [44]. Additionally, in a comparison of BSWTT-RAGT and Conventional Physiotherapy [45], the BSWTT-RAGT group showed a significant improvement between groups.
To summarise these results, some showed that BWSTT-RAGT was more effective than the target group, while others showed no significance. These results indicate that BWSTT-RAGT seems to be more effective than gait training with BWSTT alone, whether the robot or the therapist provides the assistance. Regarding gait assistance, it is unclear whether it is worthwhile to use the current exoskeletal robot. Nevertheless, from the viewpoint of dependency of neural plasticity and training dose, it is impractical for therapists to provide long-term assistance in gait rehabilitation, therefore the use of robots should be advantageous from a clinical point of view. In addition, the significance of combination therapy together with BWSTT-RAGT has been demonstrated to be more effective than conventional physiotherapy, indicating that exoskeleton robot-assisted training has potential, with further research expected in the future.
Types of exoskeleton design
Amongst the selected studies, there were four BWSTT-type exoskeletal robots: Lokomat, Walkbot, RoboGait, and GEAR system. The GEAR system is a prototype of the Welwalk and is already in clinical use as of 2021. The features of the devices and the details of assistance methods and intervention protocols vary. The differences in gait exercise effects between them are also not yet clear [60].
Lokomat and Walkbot are characterised by restricted motion of the pelvic girdle in the sagittal plane. The pelvic girdle’s semi-fixation in a certain position may reduce the abnormal gait pattern of the lower-limb joints [61]. With Lokomat, the timing of each muscle’s activation during gait is changed by adjusting the speed and guidance force [61]. On the other hand, with Welwalk, the pelvis is not fixed by the device, and there is more freedom in the direction of movement as compared to that with Lokomat and Walkbot. Other than the feature of BWS on the treadmill, the Welwalk can be used in situations closer to overground walking.
Regarding the protocol for adjusting the assist, there are a wide variety of possible assist trajectories, assist volumes, torque values at the joint, and control strategies. All included studies employed assist-as-needed approaches tailored to the gait of individual patients [35–45]. In all studies reviewed, these settings were applied and adapted to the subjects’ gait using an exploratory and experimental approach. For comparisons of effectiveness, the details of the settings of these elements need to be considered.
Summary of future research questions
Based on the results of this review, future research questions and directions are discussed. Firstly, are there purpose-specific combinations of exoskeleton-assisted BWSTT and effective combination therapies for use in patients with chronic hemiplegia? If there are, what are these purpose-specific combinations? Secondly, do the effects of various robotic devices on gait training differ among patients with chronic hemiplegia?
Currently, the clinical use of exoskeleton-assisted BWSTT in patients with chronic stroke remains unclear due to a lack of evidence. Large RCTs in which patient recruitment, numerical assisted adjustments, treadmill speed, and details of intervention protocols that are compared with a control group will be needed in the future. This may aid in determining the appropriate applications of exoskeleton-assisted BWSTT.
Limitations
The quality of evidence has not been assessed in the literature. A greater range of intervention methodologies and non-specific selection of case types need to be included. The type and severity of subjects’ disability, as well as intervention methodologies and protocols, are not considered and included. To address these limitations in the future, a high-quality systematic review with an expanded scope is necessary to be conducted.
Conclusions
This review suggests that exoskeletal robot-assisted BWSTT for patients with chronic stroke may be effective in improving walking function as 10 out of 11 studies showed the effectiveness of exoskeleton robot-assisted BWSTT in terms of outcomes contributing to improved gait function. However, the potential may be “to assist” and not because of using the robot. In other words, the effect could be attributed to assisting, irrespective of whether it is due to a robot or therapist.
Further studies are required to verify the effectiveness of BWSTT exoskeletal robotic training in patients with chronic stroke, strengthen the evidence on intervention protocols, and provide detailed information regarding the application of different robot types to enable best practise for the benefit of patients.
Acknowledgements
None.
Abbreviations
- BBS
Berg balance scale
- BWS
Body weight support
- BWSTT
Body Weight-Supported Treadmill Training
- BWSTT-RAGT
BWSTT-robot assisted gait training
- BWSTT-TAGT
BWSTT-therapist assisted gait training
- EEG
Electroencephalogram
- FAC
Functional ambulation categories
- FES
Functional electrical stimulation
- GEAR
Gait exercise assist robot
- 10MWT
10 Metre walk test
- RCTs
Randomised controlled trials
- tDCS
Transcranial direct current stimulation
- VR
Virtual reality
Author contributions
RY developed the framework, performed the search and study selection, evaluated the candidate studies, and drafted the manuscript. SS evaluated the candidate studies during the study selection process and revised the manuscript. FK supervised the process of this review, participated in the selection process, and revised the manuscript. WK and MK revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by AMED under Grant Number JP19he2302006 and JP19he2202005.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rieko Yamamoto, Email: r-yamamoto@cehs.hokudai.ac.jp.
Fuminari Kaneko, Email: f-kaneko@tmu.ac.jp.
References
- 1.The world health report. 2002. https://www.who.int/whr/2002/en. Accessed 12 Dec 2021
- 2.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76(1):27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 3.Kollen B, Kwakkel G, Lindeman E. Longitudinal robustness of variables predicting independent gait following severe middle cerebral artery stroke. A prospective cohort study. Clin Rehabil. 2006;20(3):262–268. doi: 10.1191/0269215506cr910oa. [DOI] [PubMed] [Google Scholar]
- 4.Jang SH. The recovery of walking in stroke patients: a review. Int J Rehabil Res. 2010;33(4):285–289. doi: 10.1097/MRR.0b013e32833f0500. [DOI] [PubMed] [Google Scholar]
- 5.Cooke EV, Mares K, Clark A, Tallis RC, Pomeroy VM. The effects of increased dose of exercise-based therapies to enhance motor recovery after stroke: a systematic review and meta-analysis. BMC Med. 2010;8:60. doi: 10.1186/1741-7015-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvin R, Murphy B, Cusack T, Stokes E. The impact of increased duration of exercise therapy on functional recovery following stroke–what is the evidence? Top Stroke Rehabil. 2008;15(4):365–377. doi: 10.1310/tsr1504-365. [DOI] [PubMed] [Google Scholar]
- 7.Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disabil Rehabil. 2006;28(13–14):823–830. doi: 10.1080/09638280500534861. [DOI] [PubMed] [Google Scholar]
- 8.Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, Miller K, Lincoln N, Partridge C, Wellwood I, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke. 2004;35(11):2529–2539. doi: 10.1161/01.STR.0000143153. [DOI] [PubMed] [Google Scholar]
- 9.Veerbeek JM, Koolstra M, Ket JC, van Wegen EE, Kwakkel G. Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke: a meta-analysis. Stroke. 2011;42(11):3311–3315. doi: 10.1161/STROKEAHA.111.623819. [DOI] [PubMed] [Google Scholar]
- 10.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE. 2014;9(2):e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann JF, DeLateur BJ, Fowler RS, Jr, Warren CG, Arnhold R, Schertzer G, Hurka R, Whitmore JJ, Masock AJ, Chambers KH. Stroke: does rehabilitation affect outcome? Arch Phys Med Rehabil. 1975;56(9):375–382. [PubMed] [Google Scholar]
- 12.Tangeman PT, Banaitis DA, Williams AK. Rehabilitation of chronic stroke patients: changes in functional performance. Arch Phys Med Rehabil. 1990;71(11):876–880. [PubMed] [Google Scholar]
- 13.Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74(4):347–354. [PubMed] [Google Scholar]
- 14.Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. Am J Phys Med Rehabil. 1996;75(2):114–120. doi: 10.1097/00002060-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Langhorne P, Wagenaar R, Partridge C. Physiotherapy after stroke: more is better? Physiother Res Int. 1996;1(2):75–88. doi: 10.1002/pri.6120010204. [DOI] [PubMed] [Google Scholar]
- 16.Nugent JA, Schurr KA, Adams RD. A dose-response relationship between amount of weight-bearing exercise and walking outcome following cerebrovascular accident. Arch Phys Med Rehabil. 1994;75(4):399–402. doi: 10.1016/0003-9993(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 17.Smith DS, Goldenberg E, Ashburn A, Kinsella G, Sheikh K, Brennan PJ, Meade TW, Zutshi DW, Perry JD, Reeback JS. Remedial therapy after stroke: a randomised controlled trial. Br Med J (Clin Res Ed) 1981;282(6263):517–520. doi: 10.1136/bmj.282.6263.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang S, Lee S, Shin D, Baek I, Ham S, Kim W. Development of a prototype overground pelvic obliquity support robot for rehabilitation of hemiplegia gait. Sensors (Basel) 2022;22(7):2462. doi: 10.3390/s22072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covaciu F, Pisla A, Iordan AE. Development of a virtual reality simulator for an intelligent robotic system used in ankle rehabilitation. Sensors (Basel) 2021;21(4):1537. doi: 10.3390/s21041537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisla D, Nadas I, Tucan P, Albert S, Carbone G, Antal T, Banica A, Gherman B. Development of a control system and functional validation of a parallel robot for lower limb rehabilitation. Actuators. 2021;10:277. doi: 10.3390/act10100277. [DOI] [Google Scholar]
- 21.Shihomi K, Koji O, Tadao T, Yuichi S, Yoshiyuki H: 2017. Development of new rehabilitation robot device that can be attached to the conventional Knee-Ankle-Foot-Orthosis for controlling the knee in individuals after stroke. In: 2017 International Conference on Rehabilitation Robotics (ICORR). IEEE Press, 304–307. 10.1109/ICORR.2017.8009264 [DOI] [PubMed]
- 22.Jin-Gang J, Xue-Feng M, Biao H, Yong-De Z, Xiao-Yang Y. Recent advances on lower limb exoskeleton rehabilitation robot. Recent Patents Eng . 2017 doi: 10.2174/1872212111666170614111623. [DOI] [Google Scholar]
- 23.Satoh Y, Yamada T, Arai Y, Shimamura R, Hirosawa M, Yamakawa R, Takagi S. The immediate effect of the Honda Walking Assist Device on foot and ankle function in hemiplegic stroke patients. J Phys Ther Sci. 2020;32(6):405–409. doi: 10.1589/jpts.32.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chien A, Chang FC, Meng NH, Yang PY, Huang C, Chou LW. Clinical efficacy of a new robot-assisted gait training system for acute stroke patients. J Med Biol. 2021;41:99–107. doi: 10.1007/s40846-020-00590-z. [DOI] [Google Scholar]
- 25.Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD006185.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morone G, Paolucci S, Cherubini A, De Angelis D, Venturiero V, Coiro P, Iosa M. Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr Dis Treat. 2017;15(13):1303–1311. doi: 10.2147/NDT.S114102.PMID:28553117;PMCID:PMC5440028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrholz J, Thomas S, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD002840.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonan IV, Yelnik AP, Colle FM, Michaud C, Normand E, Panigot B, Roth P, Guichard JP, Vicaut E. Reliance on visual information after stroke Part II: Effectiveness of a balance rehabilitation program with visual cue deprivation after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85(2):274–278. doi: 10.1016/j.apmr.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava A, Taly AB, Gupta A, Kumar S, Murali T. Bodyweight-supported treadmill training for retraining gait among chronic stroke survivors: a randomized controlled study. Ann Phys Rehabil Med. 2016;59(4):235–241. doi: 10.1016/j.rehab.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Ullah MA, Shafi H, Khan GA, Malik AN, Amjad I. The effects of gait training with body weight support (BWS) with no body weight support (no-BWS) in stroke patients. J Pak Med Assoc. 2017;67(7):1094–1096. [PubMed] [Google Scholar]
- 31.Mehrholz J, Thomas S, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD006185.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Ma H, Qin LY, Gao F, Chan KM, Law SW, Qin L, Liao WH. Recent developments and challenges of lower extremity exoskeletons. J Orthop Translat. 2016;5:26–37. doi: 10.1016/j.jot.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruni MF, Melegari C, De Cola MC, Bramanti A, Bramanti P, Calabrò RS. What does best evidence tell us about robotic gait rehabilitation in stroke patients: a systematic review and meta-analysis. J Clin Neurosci. 2018;48:11–17. doi: 10.1016/j.jocn.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Rev Neurother. 2007;7(10):1417–1436. doi: 10.1586/14737175.7.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39(6):1786–1792. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 36.Belas dos Santos M, Barros de Oliveira C, Dos Santos A, Garabello Pires C, Dylewski V, Arida RM: A comparative study of conventional physiotherapy versus robot-assisted gait training associated to physiotherapy in individuals with ataxia after stroke. Behav Neurol 2018. 10.1155/2018/2892065. [DOI] [PMC free article] [PubMed]
- 37.Seo JS, Yang HS, Jung S, Kang CS, Jang S, Kim DH. Effect of reducing assistance during robot-assisted gait training on step length asymmetry in patients with hemiplegic stroke: a randomized controlled pilot trial. Medicine (Baltimore) 2018;97(33):e11792. doi: 10.1097/MD.0000000000011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89(8):829–839. doi: 10.2522/ptj.20080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil. 2009;6:18. doi: 10.1186/1743-0003-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation. 2013;33(1):67–76. doi: 10.3233/NRE-130929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae YH, Ko YJ, Chang WH, Lee JH, Lee KB, Park YJ, Ha HG, Kim YH. Effects of robot-assisted gait training combined with functional electrical stimulation on recovery of locomotor mobility in chronic stroke patients: a randomized controlled trial. J Phys Ther Sci. 2014;26(12):1949–1953. doi: 10.1589/jpts.26.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogino T, Kanata Y, Uegaki R, Yamaguchi T, Morisaki K, Nakano S, Uchiyama Y, Domen K. Improving abnormal gait patterns by using a gait exercise assist robot (GEAR) in chronic stroke subjects: a randomized, controlled, pilot trial. Gait Posture. 2020;82:45–51. doi: 10.1016/j.gaitpost.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Ogino T, Kanata Y, Uegaki R, Yamaguchi T, Morisaki K, Nakano S, Domen K. Effects of gait exercise assist robot (GEAR) on subjects with chronic stroke: a randomized controlled pilot trial. J Stroke Cerebrovasc Dis. 2020;29(8):104886. doi: 10.1016/j.jstrokecerebrovasdis.2020. [DOI] [PubMed] [Google Scholar]
- 44.Bae Y-H, Kim Y-H, Fong SSM. Comparison of heart rate reserve-guided and ratings of perceived exertion-guided methods for high-intensity robot-assisted gait training in patients with chronic stroke: focused on the motor function and gait ability. Top Geriatr Rehabil. 2016;32(2):119–126. doi: 10.1097/TGR.0000000000000098. [DOI] [Google Scholar]
- 45.Erbil D, Tugba G, Murat TH, Melike A, Merve A, Cagla K, Mehmetali ÇC, Akay Ö, Nigar D. Effects of robot-assisted gait training in chronic stroke patients treated by botulinum toxin-a: a pivotal study. Physiother Res Int. 2018;23(3):e1718. doi: 10.1002/pri.1718. [DOI] [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 48.Ferrari R. Writing narrative style literature reviews. Med Writing. 2015;24(4):230–235. doi: 10.1179/2047480615Z.000000000329. [DOI] [Google Scholar]
- 49.Perry Y, Petrie K, Buckley H, Cavanagh L, Clarke D, Winslade M, Hadzi-Pavlovic D, Manicavasagar V, Christensen H. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015 doi: 10.1097/XEB.0000000000000050. [DOI] [Google Scholar]
- 50.Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev. 2000;37(6):693–700. [PubMed] [Google Scholar]
- 51.Park C, Oh-Park M, Bialek A, Friel K, Edwards D, You JSH. Abnormal synergistic gait mitigation in acute stroke using an innovative ankle-knee-hip interlimb humanoid robot: a preliminary randomized controlled trial. Sci Rep. 2021;11(1):22823. doi: 10.1038/s41598-021-01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ertop TE. Realization of virtual fluid environment on a robotic gait trainer for therapeutic purposes [M.S.—Master of Science]. Middle East Technical University. 2017
- 53.TOYOTA webpage. https://global.toyota/jp/newsroom/corporate/30609537.html. Accessed 20 December 2021
- 54.Rodrigues TA, Goroso DG, Westgate PM, Carrico C, Batistella LR, Sawaki L. Slow versus fast robot-assisted locomotor training after severe stroke: a randomized controlled trial. Am J Phys Med Rehabil. 2017;96(10 Suppl 1):S165–s170. doi: 10.1097/PHM.0000000000000810. [DOI] [PubMed] [Google Scholar]
- 55.The World Health Organization’s International Classification of Functioning, Disability and Health (WHO ICF). https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health. Accessed 10 Oct 2022.
- 56.Demers M, Levin M. Do activity level outcome measures commonly used in neurological practice assess upper-limb movement quality? Neurorehabil Neural Repair. 2017;31(7):623–637. doi: 10.1177/1545968317714576. [DOI] [PubMed] [Google Scholar]
- 57.Louie DR, Eng JJ. Powered robotic exoskeletons in post-stroke rehabilitation of gait: a scoping review. J Neuroeng Rehabil. 2016;13(1):53. doi: 10.1186/s12984-016-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calabrò RS, Naro A, Russo M, Leo A, De Luca R, Balletta T, Buda A, La Rosa G, Bramanti A, Bramanti P. The role of virtual reality in improving motor performance as revealed by EEG: a randomized clinical trial. J Neuroeng Rehabil. 2017;14(1):53. doi: 10.1186/s12984-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manuli A, Maggio MG, Latella D, Cannavò A, Balletta T, De Luca R, Naro A, Calabrò RS. Can robotic gait rehabilitation plus Virtual Reality affect cognitive and behavioural outcomes in patients with chronic stroke? A randomized controlled trial involving three different protocols. J Stroke Cerebrovasc Dis. 2020;29(8):104994. doi: 10.1016/j.jstrokecerebrovasdis.2020. [DOI] [PubMed] [Google Scholar]
- 60.Haarman JA, Reenalda J, Buurke JH, van der Kooij H, Rietman JS. The effect of 'device-in-charge' versus 'patient-in-charge' support during robotic gait training on walking ability and balance in chronic stroke survivors: a systematic review. J Rehabil Assist Technol Eng. 2016;3:2055668316676785. doi: 10.1177/2055668316676785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hidler JM, Wall AE. Alterations in muscle activation patterns during robotic-assisted walking. Clin Biomech (Bristol, Avon) 2005;20(2):184–193. doi: 10.1016/j.clinbiomech.2004.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.