Abstract
The Omicron variant is sweeping the world, which displays striking immune escape potential through mutations at key antigenic sites on the spike protein, making broad-spectrum SARS-CoV-2 prevention or therapeutical strategies urgently needed. Previously, we have reported a hACE2-targeting neutralizing antibody 3E8, which could efficiently block both prototype SARS-CoV-2 and Delta variant infections in prophylactic mouse models, having the potential of broad-spectrum to prevent SARS-CoV-2. However, preparation of monoclonal neutralizing antibodies is severely limited by the time-consuming process and the relative high cost. Here, we utilized a modified VEEV replicon with two subgenomic (sg) promoters engineered to express the light and heavy chains of the 3E8 mAb. The feasibility and protective efficacy of replicating mRNA encoding 3E8 against Omicron infection in the hamster were demonstrated through the lung targeting delivery with the help of VEEV-VRP. Overall, we developed a safe and cost-effective platform of broad-spectrum to prevent SARS-CoV-2 infection.
Keywords: Omicron, VEEV VRP, 3E8 antibody, Replicable mRNA
Introduction
The unprecedented coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in ∼63.3 million infections with more than 6 million deaths since the end of 2019 as of November 17th, 2022 (https://covid19.who.int). SARS-CoV-2 evolves rapidly and has generated multiple variants, some of which that have been shown to enhance the viral transmissibility, adaptability, infectivity, and/or escape from the host's immune response were defined as variant of concern (VOC) by WHO (Plante et al., 2021). Since the beginning of pandemic, five VOCs including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529) have been declared (Tao et al., 2021). Currently, the Omicron variant is sweeping the world with a high risk of infection and reinfection even in fully vaccinated populations, raising concerns about the protective effect of the vaccine- and infection-induced immunity against SARS-CoV-2 variants.
Entry of SARS-CoV-2 into host cells is mediated by the binding of the spike protein to angiotensin-converting enzyme 2 (ACE2) expressed on host cells (Hoffmann et al., 2020; Walls et al., 2020). The S protein is a homotrimer and each consists of two subunits, S1 and S2. The S1 subunit binds to ACE2 receptor, and S2 is responsible for membrane fusion (Walls et al., 2020). The N-terminal domain (NTD) and the receptor-binding domain (RBD) within the S1 subunit, are the major targets of neutralizing antibodies as well as small molecule antivirals (Dejnirattisai et al., 2022; Iketani et al., 2022; VanBlargan et al., 2022; Westendorf et al., 2022b). However, the frequent mutations within these regions along with the evolution of SARS-CoV-2 posed significant challenges to the inhibitory effects of these antibodies or compounds, with some variants showing resistance to them. The sera of patients who had the previous COVID-19 infection and those who had immunized with the prototype SARS-CoV-2 vaccine showed 3 to 9-fold reduction of neutralizing activity against the Beta and Gamma variants (Tao et al., 2021; Wang et al., 2021a, 2021b), and the Omicron variant can largely escape from vaccination, convalescent sera and most approved monoclonal antibodies (Lu et al., 2021; Planas et al., 2021). Recently, emerging subvariants of the Omicron strain such as BA.2.12.1 and BA.4/5 exhibit stronger resistance to vaccines compared with original Omicron variant (Qu et al., 2022). Therefore, it is imperative to develop an effective and broad-spectrum therapy against various VOCs of SARS-CoV-2.
Human angiotensin-converting enzyme 2 (hACE2) receptor is a potential broad-spectrum target against COVID-19, since it is the main receptor for the SARS-CoV-2 entry into host cells. We have previously reported a neutralizing antibody targeting hACE2, 3E8 mAb, which could block the S1-subunits binding to hACE2 and prevent pseudo-typed viruses infection from multiple SARS-CoV-2 variants in hACE2-expressing cells, including VOCs Gamma, Beta and Delta, without affecting the physiological activities of hACE2 or causing severe toxicities in hACE2 “knock-in” mice (Chen et al., 2021; Ou et al., 2022). Importantly, 3E8 also efficiently blocked both prototype SARS-CoV-2 and Delta variant infections in prophylactic mouse models of COVID-19 (Chen et al., 2021; Ou et al., 2022). These results demonstrate that the 3E8 mAb is a potential broad-spectrum therapy and could be theoretically utilized in managing current Omicron variants for prevention and treatment.
Alphavirus is positive sense, single-stranded RNA virus in Togaviridae family. The alphavirus replicon particles (VRPs) are efficient vectors for gene delivery and have been applied to studies of vaccine development, gene therapy and cell transduction. The self-replicating replicon RNA encodes viral replicase proteins (nsP1-nsP4) and translates the gene of interest in place of viral structural protein genes (Polo et al., 1999). By providing viral structural proteins in trans, the replicon RNA can be packaged into VRPs for in vitro and in vivo gene delivery (Perri et al., 2003). Among the alphavirus VRPs, Venezuelan equine encephalitis virus (VEEV)-VRP is an ideal delivery system as it has a broad range of susceptible host cells and high expression level of cytoplasmic proteins.
Here, in the present study, we explored feasibility of VEEV-VRP to deliver the neutralizing antibody of hACE2 (3E8) against SARS-CoV-2 Omicron variant infection. Our results demonstrated that VEEV-VRP could efficiently deliver 3E8 mAb both in vitro and in vivo. And lung-targeted delivery of VEEV-VRP encoding 3E8 mAb could remarkably protect the hamsters against the Omicron BA.1 infection.
2. Results
2.1. Characterization of 3E8 antibody produced by expression plasmids
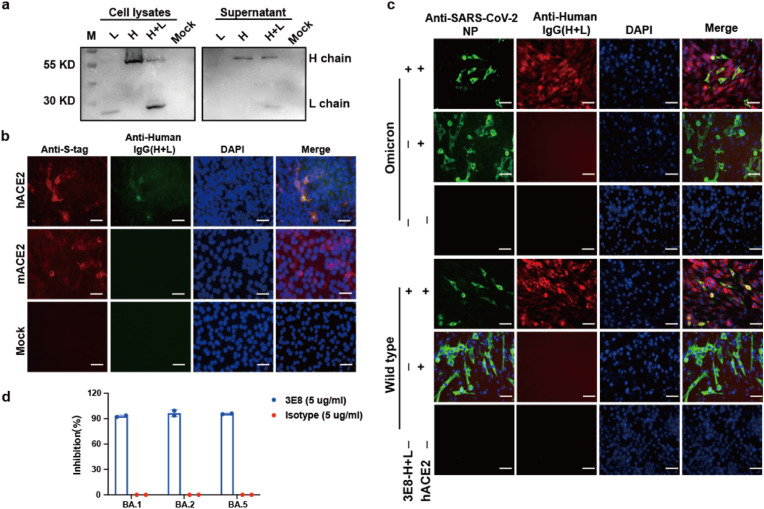
To verify the feasibility of expressing 3E8 antibody with self-replicating VEEV-replicon RNA, we firstly examined whether 3E8 antibodies could be successfully assembled by co-transfecting 3E8 heavy- and light-chain expressing plasmids in BHK-21 cells. The variable regions of the heavy (VH) and light (VL) chains of hACE2 neutralizing antibody 3E8 were cloned into human IgG4 backbones as described previously (Chen et al., 2021). BHK-21 cells were co-transfected with heavy chain and light chain expressing plasmids. At 48 h post transfection (hpt), both cell lysates and supernatants were subjected to Western blotting (WB) using goat anti-human IgG (H + L) antibody conjugated with HRP. As shown in Fig. 1 a, the heavy and light chains could be simultaneously detected in both cell lysates and supernatants from the cells co-transfected with two plasmids, whereas only a single heavy or light chain was detected in cells transfected with the corresponding plasmid alone. This result indicated the assembly and secretion of recombinant 3E8 antibody with co-expression of heavy and light chains.
Fig. 1.
Characterization of recombinant 3E8 antibody produced by expression plasmids a) BHK-21 cells were transfected with heavy chain or light chain expression plasmid alone or co-transfected with both plasmids. At 48 h after transfection, cells and supernatant from plasmids- or mock-transfected cells were subjected to western blotting using the HRP conjugated goat anti-human IgG (H + L) antibody.
b) BHK-21 cells were transfected with 1 μg pCAGGS-hACE2-S-tag or pCAGGS-mACE2-S-tag, respectively, and mock transfected cells as the negative control. At 48 h after transfection, cells were fixed following stained for IFA. Recombinant 3E8 antibody produced by 3E8-L and 3E8-H expression plasmids co-transfected cells and anti-S-tag mAb (red) were as the primary antibody, respectively. Nuclei were stained with DAPI (blue). Scale bars represent 50 μm.
c) BHK-21 cells were transfected with pCAGGS-hACE2-S-tag alone, or co-transfected with pCAGGS-hACE2-S-tag with hACE2 and 3E8 expression plasmids, and mock transfected cells as the negative control. 24 h later after transfection, all the cells were infected with prototype or Omicron SARS-CoV-2 at an MOI of 0.01, respectively. At 24 hpi, the cells were fixed and stained for SARS-CoV-2 NP protein (green) and 3E8 mAb (red), respectively. Nuclei were stained with DAPI (blue). Scale bars represent 50 μm
d) 3E8 mAb potent against Omicron subvariants. Vero-E6 cells infected with Omicron BA.1 (MOI = 0.001), BA.2 (MOI = 0.001) and BA.5 (MOI = 0.0001) were treated with 3E8 and Isotype antibodies at a concentration of 5 mg/ml, respectively. At 48 h after infection, the viral RNAs in cell culture media were quantified with quantitative real-time RT-PCR (qRT-PCR) assay.
Next, we evaluated the specificity of recombinant 3E8 antibody recognizing hACE2 receptors. BHK-21 cells were transfected with the S tag fused hACE2 or mouse ACE2 (mACE2) expression plasmids, and immunofluorescence assay (IFA) was performed at 24 hpt using the anti-S tag antibody and the supernatant collected from BHK-21 cells co-transfected with 3E8 heavy and light chains expression plasmids as the primary antibodies respectively. The expression of mACE2 and hACE2 was confirmed by S tag-positive cells observed after transfection. However, the recombinant 3E8 antibody could specifically recognize hACE2 but not mACE2 (Fig. 1b), demonstrating the recombinant 3E8 antibody is functional as the parental antibody.
After verifying the ability of recombinant 3E8 antibody recognizing hACE2 receptor, we then evaluated the protective efficacy of recombinant 3E8 antibody against SARS-CoV-2 infection. BHK-21 cells were co-transfected with hACE2-and 3E8-expressing plasmids at 24 h prior to the infection of prototype SARS-CoV-2 or Omicron BA.1. The efficiency of SARS-CoV-2 infection was evaluated by measuring the expression of the NP antigen through IFA in the cells at 24 h after virus infection (Fig. 1c). Compared with the hACE2-transfected BHK-21 cells without 3E8 expression, in which approximately 60% cells were positive for SARS-CoV-2 NP protein, much fewer NP-positive cells were observed in BHK-21 cells transduced with 3E8 expression after the infection of either SARS-CoV-2 prototype or Omicron BA.1. As a negative control, there were no viral antigen-positive cells observed in naïve BHK-21 cells infected with prototype SARS-CoV-2 or Omicron BA.1 (Fig. 1c). These results suggested that by specifically binding to the hACE2 receptor, the recombinant 3E8 antibody could efficiently block the infection of different SARS-CoV-2 variants in vitro. Recently, with the continued emergence of Omicron subvariants, we also tested the 3E8 potent against Omicron subvariants of BA.2 and BA.5 (Fig. 1d). Briefly, Vero-E6 cells infected with Omicron BA.1 (MOI = 0.001), BA.2 (MOI = 0.001) and BA.5 (MOI = 0.0001) were treated with 3E8 and Isotype antibodies at a concentration of 5 mg/mL, respectively. At 48 h after infection, the viral RNAs in cell culture media were quantified with quantitative real-time RT-PCR (qRT-PCR) assay. The result showed that the inhibition rates of 3E8 against BA.1, BA.2 and BA.5 were more than 90%, further demonstrating the broad-spectrum potent of 3E8 antibody against SARS-CoV-2.
2.2. Construction of VEEV replicon for 3E8 mAb expression (VEEV-Rep-3E8)
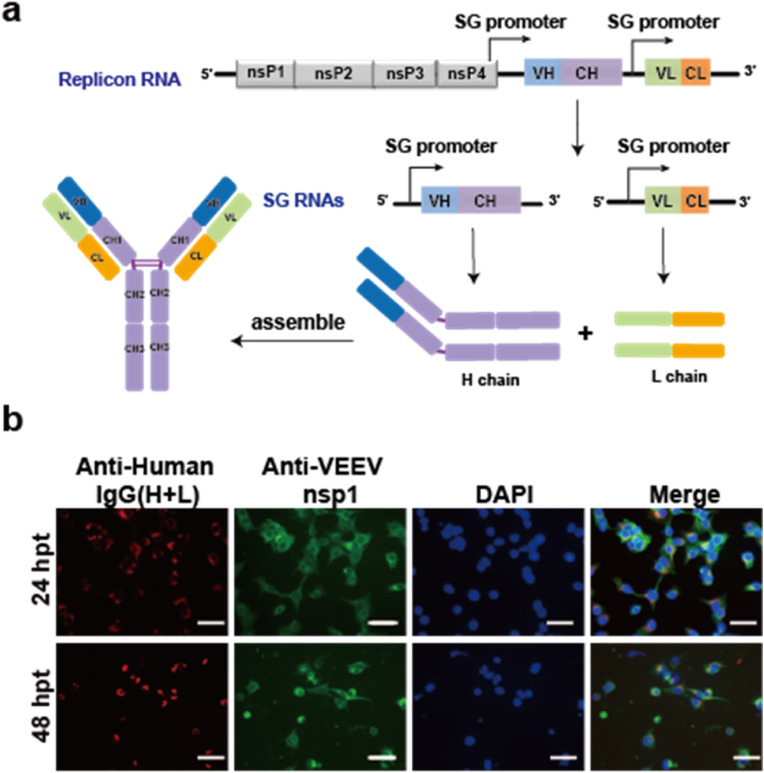
The coding sequences of the heavy and light chains of 3E8 neutralizing antibody were inserted to a modified VEEV replicon under the control of two independent sg promoters (designated as VEEV-Rep-3E8) for the neutralizing antibody expression as described in our previous study (Li et al., 2021) (Fig. 2 a). BHK-21 cells were transfected with VEEV-Rep-3E8 RNA, the expression and assembly of 3E8 mAb in transfected cells was determined by IFA using the goat anti-human IgG (H + L) conjugated with Alexa Fluor 568 dyes. To verify the replication of the VEEV-Rep-3E8 mRNA, the expression of VEEV non-structural protein nsP1 was detected at different time points post transfection. As expected, the expression of 3E8 was observed in VEEV-Rep-3E8 transfected cells, which co-localized with VEEV nsP1 protein (Fig. 2b). The result indicated that VEEV-Rep-3E8 mRNA could efficiently express 3E8 neutralizing antibody.
Fig. 2.
The expression of recombinant 3E8 antibody using the replicable VEEV replicon as vector. a) Schematic of the replicable VEEV replicon expressing 3E8-L and 3E8-H
b) BHK-21 cells were transfected with 1 μg VEEV-rep-3E8 mRNA, and the transfected cells were fixed and stained for the detection of 3E8 mAb (red) and VEEV-nsP1 (green) using Alexa Fluor™ 568 conjugated goat anti human IgG (H + L) and anti-VEEV nsP1 mouse serum, respectively, at 24 and 48 h post-transfection (hpt). Nuclei were stained with DAPI (blue). Scale bars represent 50 μm.
2.3. VEEV replicon particles (VEEV-VRP-3E8) assembly for 3E8 antibody delivery
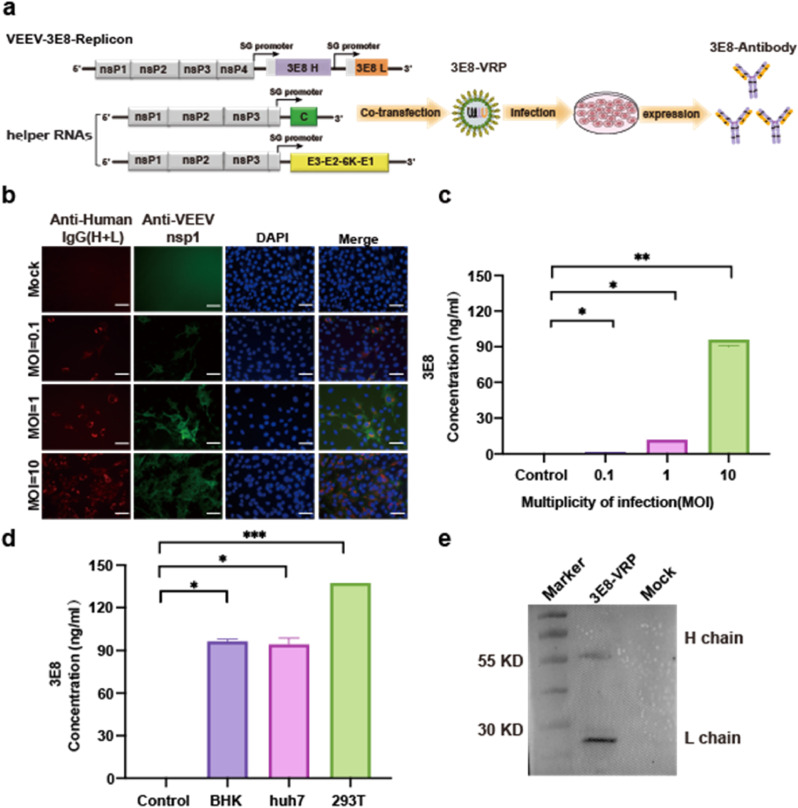
For delivery of the 3E8 mAb both in vitro and in vivo, the VEEV-Rep-3E8 was assembled into VEEV replicon particles, named VEEV-VRP-3E8, by co-transfection of VEEV-Rep-3E8 and two helper RNAs encoding VEEV capsid and envelope proteins (Fig. 3 a). To determine the delivery efficacy of 3E8 mAb in vitro, BHK-21 cells were firstly infected with VEEV-VRP-3E8 at MOIs of 0.1, 1, and 10 respectively, as shown in Fig. 3b, increasing IFA positive cells of 3E8 mAb and VEEV nsP1 were observed within VRP-infected cells at 24 h post-infection (hpi) in a dose-dependent manner, whereas no expression of 3E8 and nsP1 was detected in the uninfected cells. Similarly, increasing levels of 3E8 expression were detected in the supernatants of VRP-infected cells through enzyme-linked immunosorbent assay (ELISA) using the purified human ACE2 protein as antigen (Fig. 3c), implying the expression, assembly and secretion of 3E8 mAb after VRP infection. Besides BHK-21 cells, VEEV-VRP-3E8 could infect multiple cell lines with the highest production (up to 130 ng/mL) achieved in 293T cells (Fig. 3d). In addition, the integrity of 3E8 mAb was confirmed by WB analysis using goat anti human IgG (H + L) antibody conjugated with HRP (Fig. 3e).
Fig. 3.
The expression of 3E8 with VEEV-VRP-3E8 delivery in vitro a) Schematic representation of the packaging system of VEEV-VRP-3E8 including the VEEV-rep-3E8, and two helper RNAs as described previously.
b) BHK-21 cells were infected with VEEV-VRP-3E8 at different MOIs (0.1/1/10), the cells were fixed and stained for the expression of 3E8 mAb (red) and VEEV-nsP1 (green) using Alexa Fluor™ 568 conjugated goat anti-human IgG (H + L) and anti-VEEV nsP1 mouse serum respectively at 24 h post-infection (hpi). The mock-infected BHK-21 cells were used as a negative control. Nuclei were stained with DAPI (blue). Scale bars represent 50 μm.
c) Quantification of 3E8 expression levels by hACE2-specific ELISA using the supernatants collected at 48 hpi from the VEEV-VRP-3E8 infected BHK cells with different MOIs (0.1/1/10) and mock treated BHK-21 cells. The antibody titers were determined in triplicate. Statistical analysis was performed by Ordinary one-way ANOVA. *P < 0.05, **P < 0.01.
d) Quantification of 3E8 expression levels by antigen (hACE2)-specific ELISA using the supernatants collected at 48 hpi from the VEEV-VRP-3E8 infected different cells (BHK/293T/Huh7). The antibody titers were determined in triplicate. Statistical analysis was performed by Ordinary one-way ANOVA. *P < 0.05, ***P < 0.001.
e) The VEEV-VRP-3E8 infected BHK-21 cells (MOI = 1) and the mock treated cells were lysed with RIPA at 48 hpi and subjected to western blotting using the HRP conjugated goat anti-human IgG (H + L) and the anti-β-actin antibody.
2.4. Protection efficacy of VEEV-VRP-3E8 against SARS-CoV-2 infection in vivo
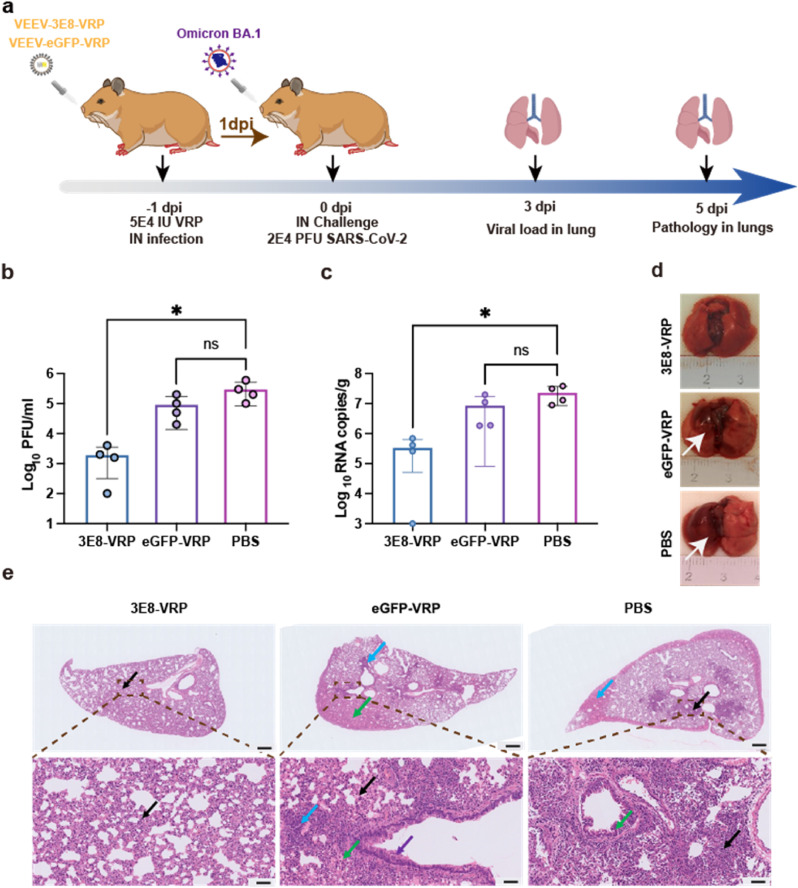
Since the Omicron BA.1 could efficiently propagate and induce serious damage in lungs in golden (Syrian) hamster model, we evaluated protection efficacy of VEEV-VRP-3E8 in the hamsters. Groups of 4–6 weeks old hamsters were first inoculated with 5 × 104 IU VEEV-VRP-3E8 or 5 × 104 IU VEEV-VRP-eGFP or DMEM in a volume of 100 μL through intranasal route. After 24 h, the hamsters from different groups were administered intranasally with 2 × 104 PFU Omicron BA.1 (Fig. 4 a). No obvious clinical symptoms were observed in all groups of hamsters after Omicron BA.1 infections as described in our previous study (Zhang et al., 2022). The viral loads in lungs at 3 days after Omicron BA.1 infection were quantified through plaque assay and quantitative real-time RT-PCR (qRT-PCR). Much lower levels of viral titers were found in VEEV-VRP-3E8-treated hamsters in comparison with DMEM or VEEV-VRP-eGFP treated hamsters (Fig. 4b). Similarly, lower viral RNA copies were also observed in VEEV-VRP-3E8 transduced hamsters (Fig. 4c). Consistent with the results of viral loads, homogeneously pink lung lobes without gross pathological changes were observed in VEEV-VRP-3E8 treated hamsters at 5 dpi in contrast to the lungs of DMEM or VEEV-VRP-eGFP treated hamsters with obvious gross pathological changes characterized by apparent focal or multifocal dark red discoloration (Fig. 4d). Moreover, we performed pathological analysis of the lung tissues at 5 dpi by hematoxylin-eosin (H&E) staining. The results further demonstrated that only alveolar septal thickening and a small amount of inflammatory cell infiltration (black arrow) were visible in the hamsters treated with VEEV-VRP-3E8. In contrast, typical interstitial pneumonia with large number of inflammatory cell infiltration around blood vessels (black arrow), pulmonary edema (blue arrow) and much tissue exudate in the alveoli (green arrow) as well as exfoliated epithelial cells in the bronchial (purple arrow) displayed in VEEV-VRP-eGFP or DMEM-treated hamsters (Fig. 4e). Taken together, our results indicated that pulmonary targeted delivery of VRP encoding 3E8 mAb could efficiently forestall the Omicron BA.1 infection from the outset.
Fig. 4.
VEEV-VRP-3E8 infection could protect hamster against SARS-CoV-2 Omicron variant challenge. a) The outline of the experimental design of the in vivo protection evaluation of VEEV-VRP-3E8. Four-to-six-week-old golden (Syrian) hamsters were intranasally administered with 5 × 104 IU VEEV-VRP-3E8, VEEV-VRP-eGFP or DMEM per hamster in a total volume of 100 μL after anesthetization with avertin (250 mg/kg). Twenty-four hours later, the hamsters from different groups were intranasally challenged with 2E4 PFU Omicron strain in a total volume of 100 μL. Lung samples were collected at 3 days after SARS-CoV-2 infection for determination of the viral loads and lung samples at 5 dpi were collected for the pathological analysis.
b) RNA copies of lung samples at 3 dpi after SARS-CoV-2 infection (n = 4) were determined by qRT-PCR. Statistical analysis was performed by T-test. *P < 0.05.
c) Viral titers of lung samples at 3 dpi after SARS-CoV-2 infection (n = 4 hamsters per group) were determined by plaque assays. Statistical analysis was performed by T-test. *P < 0.05.
d) Pathological changes of the lung samples at 5 dpi after SARS-CoV-2 infection (n = 3). Obvious gross pathology changes in the lungs were depicted by white arrows
e) H&E analysis of lung samples at 5 dpi after SARS-CoV-2 infection. Scale bar represents 500 μm and 50 μm (n = 3), respectively. Inflammatory cell infiltration (black arrow), pulmonary edema (blue arrow) and much tissue exudate in the alveoli (green arrow) as well as exfoliated epithelial cells in the bronchial (purple arrow) were indicated.
3. Discussion
The Omicron variant, as the most heavily mutated VOC of SARS-CoV-2 cause a substantial reduction of neutralizing activity of sera from BNT162b2 (Pfizer/Biotech) or ChAdOx1 nCoV-19 (AstraZeneca) vaccine recipients and totally or partially enable neutralization escape by potent neutralizing mAbs including most monoclonal antibodies that obtained emergency use authorization (Cao et al., 2022; Dejnirattisai et al., 2022; Iketani et al., 2022; Liu et al., 2022; Planas et al., 2022; VanBlargan et al., 2022; Westendorf et al., 2022a). Although several evidences have demonstrated that some of mAbs, such as LY-CoV1404 (Westendorf et al., 2022a), REGN10987 (Cao et al., 2022) and S2K146 (Cameroni et al., 2022), which bind to epitopes on the RBD with partial overlap with the ACE2 binding site, were found to retain neutralization potency against Omicron strain, further research is still needed to compare the in vivo protective effect of these broadly neutralizing antibodies. However, these clues suggest that the treatment targeting the hACE2 receptor probably is a more effective strategy against the continuing evolution of SARS-CoV-2.
mRNA-based antibody platform offers time-saving strategy for in vivo antibody expression over massive antibody production and purification. Recently, we developed a safe and cost-effective platform to express a SARS-CoV-2 neutralizing antibody CB6 in the lung with self-replicating mRNA based on VEEV-VRP delivery system, which could efficiently prevent SARS-CoV-2 infection (Li et al., 2021). In this study, using the similar expression strategy, we constructed the VEEV replicon that encodes a hACE2-targeting neutralizing antibody 3E8 and evaluated its protective efficacy against the SARS-CoV-2 Omicron BA.1 infection by intranasal VEEV-VRP delivery in vivo. Our present data clearly demonstrate that the VEEV-Rep-3E8 mRNA is fully active in preventing the Omicron BA.1 replication and reducing lung damage in the prophylactic hamster model.
The 3E8 mAb blocks the prototype SARS-CoV-2 infection by competing with RBD at the binding interface with hACE2. According to crystal-structure analysis, 3E8 mAb causes gaps between hACE2 receptor and prototype RBD, and further analysis demonstrates that Gln24 and His34 of hACE2 are two overlapped sites for RBD and 3E8 binding (Li et al., 2021). While the Omicron is seriously mutated in RBD, the Omicron RBD-hACE2 complex exhibits an overall similar conformation with prototype and Delta RBD-hACE2 complex through X-ray crystallography and cryo-EM analysis. Mutations within Omicron RBD mainly result in substitutions on the hACE2 recognizing interface (Han et al., 2022). This is the reason why 3E8 is still efficiently resistant to Omicron BA.1, BA.2 and BA.5 (Fig. 1d). The ongoing evolution of SARS-CoV-2 may lead to continuous emergence of antigenic drift variants, however, as long as the hACE2 interface binding to RBD remains stable, 3E8 mAb will maintain its protective property against SARS-CoV-2.
In terms of viral vector mediated antibody encoding gene delivery strategies, many groups utilized the adeno-associated virus (AAV) platform. However, as a DNA virus, the risk of integration raises a safety concern about their clinical application. Currently, LNP-encapsulated mRNA platforms are commonly used for antibody delivery (Kose et al., 2019; Pardi et al., 2017; Thran et al., 2017; Tiwari et al., 2018), and the safety profiles in clinical studies were all well-documented (Alameh et al., 2020). Compared to the DNA virus vectors, the alphavirus VRP-mediated antibody delivery is more efficient as the replicon mRNA bypasses the endonuclear transcription process and directly translates the antibody in the cytoplasm after transferred into cells. Furthermore, the replicon mRNA circumvents the risk of genomic integration, increasing the safety profile of this strategy. The alphavirus VRP systems have been extensively used in the studies of gene transduction, cancer therapy and vaccine development, and their efficacies and safety have been well evaluated pre-clinically in different animal models including various rodents and non-human primates(Ljungberg and Liljestrom, 2015). Several clinical trials of the VRP-based vaccines against infectious diseases or cancers have been conducted, and only mild or moderate adverse events with the patterns of response similar with the placebo groups occurred (Singh et al., 2019), confirming the safety and tolerability of the alphavirus VRP platform, and encouraging the clinical transformation of VRP-vectored antibody delivery approach.
In addition, we delivered the self-replicating VEEV replicon RNA encoding 3E8 mAb through intranasal route. In contrast to routinely used administration routes of mAb, like intravenous (i.v) infusion or intramuscular (i.m) injection, which always require large amounts of antibodies, our results showed that local delivery of mRNA a single low dose (5 × 104 IU) could display an effective protective effect against Omicron variant infection. In our previous study, we have demonstrated that intranasal antibody delivery based on the VRP system caused the competent expression of mAb in the majority cell types within the lung of mice, and the mAbs could be stably maintained for at least 5 days (Li et al., 2021). Therefore, the mAb VRP delivery strategy has a distinct advantage in the treatment of respiratory diseases.
Although self-replicating replicon RNA have presented their attractive and promising features, there is some disadvantages. In the case of replicon RNA, which demands careful handling and requires RNA encapsulation in VRPs or LNPs for improved stability and delivery. Moreover, the replicon RNA not only takes time to expression, but also renders the heterologous gene expression transient for their rapid degradation (Lundstrom, 2021). Thus, using replicon RNA platform for antibody expression could not provide instant protection. Recently, intranasal delivery of monoclonal antibody has been developed by Thai researchers and got Thai FDA approval to use in human, unlike mRNA-based delivery, intranasal delivery of monoclonal antibodies can provide immediate effect to protect the individual from infection. Even so, the strategy that a self-replicating mRNA encoding the known hACE2-targeting neutralizing mAb (3E8), delivered with help of VRP delivery system through nasal inoculation provides an effective and economic approach against the evolving SARS-CoV-2 and it has great potential for translation into human applications.
4. Materials and methods
4.1. Cell lines, viruses and antibodies
Baby hamster kidney (BHK-21) cells, Vero-E6 cells (ATCC CRL-1686), Huh7 cells and 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) containing 10% (v/v) fetal bovine serum (FBS, Gibco), 1% (v/v) penicillin and streptomycin (Beyotime). The Omicron variant BA.1 (CCPM-B-V-049-2112-18) was isolated from pharyngeal swab of infected patients in Hong Kong by the Institute of Experimental Animals, Chinese Academy of Medical Sciences. The Omicron variant BA.2 (CSTR: 16533.06.IVCAS 6.7617) and BA.5 (CSTR: 16533.06.IVCAS 6.8981) were provided by Microorganisms & Viruses Culture Collection Center, Wuhan Institute of Virology, Chinese Academy of Sciences. All above viruses were propagated and titrated by plaque assays using Vero-E6 cells. All obtained viruses were stored aliquots at −80 °C for subsequent experiments. The antibody against RP3-CoV NP and S-tag for hACE2/mACE2 detection were kindly provided by Prof. Zheng-li Shi and Prof. Bing Yan at Wuhan Institute of Virology. FITC-conjugated goat anti-rabbit/mouse IgG and Alexa Fluor 568-anti mouse/human IgG were purchased from Proteintech, China.
4.2. Construction of a VEEV replicon expressing the light and heavy chains of 3E8
VEEV-3E8 replicon was constructed by inserting the cassette encoding light and heavy chains of 3E8 antibody into the cDNA clone of VEEV replicon (TC-83 strain) with the entire deletion of the structural protein genes. The light and heavy chains sequences were amplified and cloned into pACYC VEEV replicon by unique restriction enzyme Asc I/Pac I and Pac I/Mfe I, successively. VEEV replicon-control carrying an eGFP cassette was also generated as a negative control. The cDNA clones of two helper RNAs were constructed as previous study described, encoding the structural proteins of TC-83 strain, respectively.
The resulting VEEV-3E8 and two helper replicons cDNA clones were linearized and purified by phenol/chloroform extraction. The corresponding RNAs were transcribed in vitro by using the mMESSAGE mMACHINE™ T7 Transcription Kit (Invitrogen) according to the manufacturer's instructions.
4.3. Transient transfection and indirect immunofluorescence assay (IFA)
The BHK-21 cells were seeded on a Chamber Slide (Nalge Nunc) in 6-well plate. After adherence for 1 days, the cells were transfected with 2 μg plasmids expressing mACE2 or hACE2 as manufacturer's Instructions (Promega Corporation) prior to SARS-CoV-2 WT or Omicron variant infection at an MOI of 0.01. At time points of sample collection, the cells were fixed with cold (−20 °C) 5% acetone in methanol at room temperature for 10 min, washed three times with PBS and fixed with 3.7% formaldehyde for 24h. For the detection of viral replication, the cells were incubated with the antibodies against RP3-CoV NP and S-tag for 1 h. After washing with PBS three times, the cells were incubated with FITC-conjugated goat anti-rabbit IgG and Alexa Fluor 568-anti mouse/human IgG at room temperature for 1 h. The nuclei were stained with DAPI. Following by PBS washing, the slides were mounted with 95% glycerol and analyzed under a Zeiss fluorescence microscope.
4.4. Production and titration of VRP expressing 3E8 (3E8-VRP)
To generate the 3E8-VRP and eGFP-VRP, equal replicon RNAs (10:10:10 μg) were co-electroporated into BHK cells (1.2 × 107) using a GenePulser apparatus (Bio-Rad) at 850 V and 25 μF in a regimen of three pulses with 3 s interval. The transfected-cells were acclimated at room temperature for 10 min, resuscitated with pre-heated medium in a T75 flask and cultured at 37 °C.
After incubation for 24 and 48 h, cell supernatant was harvested, centrifuged, and filtered by 0.2 μm membrane to remove the cell debris. The resulting VRPs were stored aliquots at −80 °C for subsequent experiments and quantified by infectious center assay. Briefly, VRPs were 10-fold diluted and added onto BHK cell slides (1 × 105 per well) in 24-well plates at 37 °C for 1 h. VRP dilutions were replaced by fresh 2% FBS DMEM and cultured for another 24 h. The slides were fixed and analyzed by IFA for VRPs titration, expressed as infectious unit (IU) per mL.
4.5. Enzyme-linked immunosorbent assay (ELISA)
The hACE2-Fc protein (0.2 μg/mL) was coated on 96-well plates (100 μL/well) overnight at 4 °C. The plates were then blocked by 5% skimmed milk at 37 °C for 2 h, and treated with the supernatant dilutions in 4-fold. After another 2 h incubation and PBST washing, HRP-conjugated goat anti-human Kappa antibody (1:5000 dilution) was added for 1 h at 37 °C. The plates were colorized with a mixed substrate (two-component 3, 3′, 5, 5′-tetramethylbenzidine (TMB) color development kit, Beyotime) and terminated by the addition of 1 M H2SO4. The optical density at 450 nm was analyzed by a multimode microplate reader (Varioskan Flash; Thermo Fisher)
4.6. Western blotting assay (WB)
The expressing of 3E8 antibody was analyzed by western blotting. Briefly, the cells lysed in RIPA buffer (Beyotime) and the supernatant were denatured at 95 °C for 15 min. The samples were electrophoretized and transferred onto 0.2 μm PVDF membrane (Bio-Rad). The membrane was blocked by 5% skimmed milk for 2 h, and incubated with HRP-conjugated anti-human IgG (H + L) antibody for another 1 h. The proteins were detected under a chemiluminescence system (Chemi-Doc, Bio-Rad) with Immobilon western chemiluminescent HRP substrate (Millipore).
4.7. Plaque assay
Infectious virus titration of SARS-CoV-2 was conducted by monolayer plaque assay as described previously. Briefly, 8 × 104 Vero-E6 cells per well were seeded into 24-well plate for one day cultivation. The SARS-CoV-2 supernatant was then 10-fold diluted and added onto the cells to allow the viral entry at 37 °C for 1 h. After treatment, the monolayer was overlaid with a DMEM mixture containing 0.8% methylcellulose 2% FBS, 1%penicillin and streptomycin with the dilutions removed, which required 4–5 days to form the clear plaque. The monolayer was fixed with 10% formaldehyde solution for 24 h and stained with 1% crystal violet in water for 10 min. Plaques were counted after rinsing.
4.8. Golden Syrian hamsters challenge
4-6-week-old female Golden Syrian hamsters were divided into three groups (n = 4). Groups of hamsters received 5 × 104 IU 3E8-VRP, eGFP-VRP or PBS via intranasal route in a total volume of 100 μL, respectively. One day after VRPs infection, all hamsters were intranasally challenged with 2 × 104 PFU SARS-CoV-2 Omicron variant. On day 3 after challenge, hamsters were sacrificed for the quantification of viral RNA and infectious virus in the nasal turbinates and left lung.
On day 5 after challenge, the rest hamsters were further sacrificed for the observation and H&E staining analysis of lung lesions after one day fixation.
4.9. RNA extraction and quantitative real-time PCR (qRT-PCR)
Viral RNA was extracted by executing the manufacturer's protocol provided by QIAamp viral RNA mini kit (52,906, Qiagen). Briefly, the lungs and nasal turbinates of hamsters were homogenized in DMEM (1 mL volume) and centrifuged at 10, 000 rpm for 10 min. The viral supernatant was lysed and the viral RNA was further purified by adsorption column in a total volume of 50 μL qRT-PCR were performed using Luna® Universal Probe One-Step RT-PCR Kit (E3006). The primer pair and probe based on SARS-CoV-2 S gene was used:
RBD-qF1: 5′- CAATGGTTTAACAGGCACAGG-3’;
RBD-qR1: 5′-CTCAAGTGTCTGTGGATCACG-3’;
Probe: ACAGCATCAGTAGTGTCAGCAATGTCTC.
4.10. Ethics declarations
4-6-week-old female Golden Syrian hamsters were purchased from Wuhan Institute of Biological Products Co. Ltd. All the hamsters were cared following the recommendations of National Institutes of Health Guidelines for the Care and Use of Experimental Animals. Studies related to virus infection were performed in biosafety level 3 (BSL-3) facility at Wuhan Institute of Virology under a protocol approved by the Laboratory Animal Ethics Committee of Wuhan Institute of Virology, Chinese Academy of Sciences (Permit number: WIVA26202108).
4.11. Statistical analysis
All data were analyzed using GraphPadPrism 8.0.2 software and expressed as mean ± standard deviation (SD). The statistical significance was assigned when P values were <0.05. Student's T-test was used to analyze the differences between two groups, and significant differences between groups were determined using a one-way analysis of variance (ANOVA).
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
This work was supported by National Natural Science Foundation of China (32200132) and China Postdoctoral Science Foundation (2022M720895). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We thank the National Virus Resource center for making Omicron available. We are grateful to the staff from the Zhengdian BSL-3 laboratory (Hong-Ping Wei and Tao Du), Center for Animal Experiment (Yan-feng Yao, Ge Gao, Yun Peng and Miao-yu Chen) and the running team of the laboratory for their helpful support during the work.
Data availability
The data that has been used is confidential.
References
- Alameh M.G., Weissman D., Pardi N. Messenger RNA-based vaccines against infectious diseases. Curr. Top. Microbiol. Immunol. 2020 doi: 10.1007/82_2020_202. [DOI] [PubMed] [Google Scholar]
- Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., Pinto D., VanBlargan L.A., De Marco A., di Iulio J., Zatta F., Kaiser H., Noack J., Farhat N., Czudnochowski N., Havenar-Daughton C., Sprouse K.R., Dillen J.R., Powell A.E., Chen A., Maher C., Yin L., Sun D., Soriaga L., Bassi J., Silacci-Fregni C., Gustafsson C., Franko N.M., Logue J., Iqbal N.T., Mazzitelli I., Geffner J., Grifantini R., Chu H., Gori A., Riva A., Giannini O., Ceschi A., Ferrari P., Cippa P.E., Franzetti-Pellanda A., Garzoni C., Halfmann P.J., Kawaoka Y., Hebner C., Purcell L.A., Piccoli L., Pizzuto M.S., Walls A.C., Diamond M.S., Telenti A., Virgin H.W., Lanzavecchia A., Snell G., Veesler D., Corti D. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., Wang J., Wang Y., Niu X., Yang S., Liang H., Sun H., Li T., Yu Y., Cui Q., Liu S., Yang X., Du S., Zhang Z., Hao X., Shao F., Jin R., Wang X., Xiao J., Wang Y., Xie X.S. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang Y.N., Yan R., Wang G., Zhang Y., Zhang Z.R., Li Y., Ou J., Chu W., Liang Z., Wang Y., Chen Y.L., Chen G., Wang Q., Zhou Q., Zhang B., Wang C. ACE2-targeting monoclonal antibody as potent and broad-spectrum coronavirus blocker. Signal Transduct. Targeted Ther. 2021;6:315. doi: 10.1038/s41392-021-00740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Huo J., Zhou D., Zahradnik J., Supasa P., Liu C., Duyvesteyn H.M.E., Ginn H.M., Mentzer A.J., Tuekprakhon A., Nutalai R., Wang B., Dijokaite A., Khan S., Avinoam O., Bahar M., Skelly D., Adele S., Johnson S.A., Amini A., Ritter T.G., Mason C., Dold C., Pan D., Assadi S., Bellass A., Omo-Dare N., Koeckerling D., Flaxman A., Jenkin D., Aley P.K., Voysey M., Costa Clemens S.A., Naveca F.G., Nascimento V., Nascimento F., Fernandes da Costa C., Resende P.C., Pauvolid-Correa A., Siqueira M.M., Baillie V., Serafin N., Kwatra G., Da Silva K., Madhi S.A., Nunes M.C., Malik T., Openshaw P.J.M., Baillie J.K., Semple M.G., Townsend A.R., Huang K.A., Tan T.K., Carroll M.W., Klenerman P., Barnes E., Dunachie S.J., Constantinides B., Webster H., Crook D., Pollard A.J., Lambe T., Consortium O., Consortium I.C., Paterson N.G., Williams M.A., Hall D.R., Fry E.E., Mongkolsapaya J., Ren J., Schreiber G., Stuart D.I., Screaton G.R. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484 e415. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Li L., Liu S., Wang Q., Zhang D., Xu Z., Han P., Li X., Peng Q., Su C., Huang B., Li D., Zhang R., Tian M., Fu L., Gao Y., Zhao X., Liu K., Qi J., Gao G.F., Wang P. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185:630–640 e610. doi: 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iketani S., Liu L., Guo Y., Liu L., Chan J.F., Huang Y., Wang M., Luo Y., Yu J., Chu H., Chik K.K., Yuen T.T., Yin M.T., Sobieszczyk M.E., Huang Y., Yuen K.Y., Wang H.H., Sheng Z., Ho D.D. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose N., Fox J.M., Sapparapu G., Bombardi R., Tennekoon R.N., de Silva A.D., Elbashir S.M., Theisen M.A., Humphris-Narayanan E., Ciaramella G., Himansu S., Diamond M.S., Crowe J.E., Jr. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Q., Zhang Z.R., Zhang H.Q., Zhang Y.N., Zeng X.Y., Zhang Q.Y., Deng C.L., Li X.D., Zhang B., Ye H.Q. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice. Signal Transduct. Targeted Ther. 2021;6:369. doi: 10.1038/s41392-021-00783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Iketani S., Guo Y., Chan J.F., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., Yu J., Chik K.K., Yuen T.T., Yoon C., To K.K., Chen H., Yin M.T., Sobieszczyk M.E., Huang Y., Wang H.H., Sheng Z., Yuen K.Y., Ho D.D. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- Ljungberg K., Liljestrom P. Self-replicating alphavirus RNA vaccines. Expet Rev. Vaccine. 2015;14:177–194. doi: 10.1586/14760584.2015.965690. [DOI] [PubMed] [Google Scholar]
- Lu L., Mok B.W., Chen L.L., Chan J.M., Tsang O.T., Lam B.H., Chuang V.W., Chu A.W., Chan W.M., Ip J.D., Chan B.P., Zhang R., Yip C.C., Cheng V.C., Chan K.H., Jin D.Y., Hung I.F., Yuen K.Y., Chen H., To K.K. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom K. Vol. 9. Vaccines; Basel: 2021. (Self-Replicating RNA Viruses for Vaccine Development against Infectious Diseases and Cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Zhang Y., Wang Y., Zhang Z., Wei H., Yu J., Wang Q., Wang G., Zhang B., Wang C. ACE2-Targeting antibody suppresses SARS-CoV-2 Omicron and Delta variants. Signal Transduct. Targeted Ther. 2022;7:43. doi: 10.1038/s41392-022-00913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N., Secreto A.J., Shan X., Debonera F., Glover J., Yi Y., Muramatsu H., Ni H., Mui B.L., Tam Y.K., Shaheen F., Collman R.G., Kariko K., Danet-Desnoyers G.A., Madden T.D., Hope M.J., Weissman D. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 2017;8 doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri S., Greer C.E., Thudium K., Doe B., Legg H., Liu H., Romero R.E., Tang Z., Bin Q., Dubensky T.W., Jr., Vajdy M., Otten G.R., Polo J.M. An alphavirus replicon particle chimera derived from venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003;77:10394–10403. doi: 10.1128/JVI.77.19.10394-10403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., Bolland W.H., Porrot F., Staropoli I., Lemoine F., Pere H., Veyer D., Puech J., Rodary J., Baele G., Dellicour S., Raymenants J., Gorissen S., Geenen C., Vanmechelen B., Wawina-Bokalanga T., Marti-Carreras J., Cuypers L., Seve A., Hocqueloux L., Prazuck T., Rey F.A., Simon-Loriere E., Bruel T., Mouquet H., Andre E., Schwartz O. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., Prot M., Gallais F., Gantner P., Velay A., Le Guen J., Kassis-Chikhani N., Edriss D., Belec L., Seve A., Courtellemont L., Pere H., Hocqueloux L., Fafi-Kremer S., Prazuck T., Mouquet H., Bruel T., Simon-Loriere E., Rey F.A., Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Plante J.A., Mitchell B.M., Plante K.S., Debbink K., Weaver S.C., Menachery V.D. The variant gambit: COVID-19's next move. Cell Host Microbe. 2021;29:508–515. doi: 10.1016/j.chom.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo J.M., Belli B.A., Driver D.A., Frolov I., Sherrill S., Hariharan M.J., Townsend K., Perri S., Mento S.J., Jolly D.J., Chang S.M., Schlesinger S., Dubensky T.W., Jr. Stable alphavirus packaging cell lines for Sindbis virus and Semliki Forest virus-derived vectors. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4598–4603. doi: 10.1073/pnas.96.8.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P., Faraone J., Evans J.P., Zou X., Zheng Y.M., Carlin C., Bednash J.S., Lozanski G., Mallampalli R.K., Saif L.J., Oltz E.M., Mohler P.J., Gumina R.J., Liu S.L. N Engl J Med; 2022. Neutralization of the SARS-CoV-2 Omicron BA.4/5 and BA.2.12.1 Subvariants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Koutsoumpli G., van de Wall S., Daemen T. An alphavirus-based therapeutic cancer vaccine: from design to clinical trial. Cancer Immunol. Immunother. 2019;68:849–859. doi: 10.1007/s00262-018-2276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K., Tzou P.L., Nouhin J., Gupta R.K., de Oliveira T., Kosakovsky Pond S.L., Fera D., Shafer R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021;22:757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thran M., Mukherjee J., Ponisch M., Fiedler K., Thess A., Mui B.L., Hope M.J., Tam Y.K., Horscroft N., Heidenreich R., Fotin-Mleczek M., Shoemaker C.B., Schlake T. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017;9:1434–1447. doi: 10.15252/emmm.201707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P.M., Vanover D., Lindsay K.E., Bawage S.S., Kirschman J.L., Bhosle S., Lifland A.W., Zurla C., Santangelo P.J. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat. Commun. 2018;9:3999. doi: 10.1038/s41467-018-06508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBlargan L.A., Errico J.M., Halfmann P.J., Zost S.J., Crowe J.E., Jr., Purcell L.A., Kawaoka Y., Corti D., Fremont D.H., Diamond M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022;28:490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., Ho D.D. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–751 e744. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Graham B.S., Mascola J.R., Chang J.Y., Yin M.T., Sobieszczyk M., Kyratsous C.A., Shapiro L., Sheng Z., Huang Y., Ho D.D. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Westendorf K., Zentelis S., Wang L., Foster D., Vaillancourt P., Wiggin M., Lovett E., van der Lee R., Hendle J., Pustilnik A., Sauder J.M., Kraft L., Hwang Y., Siegel R.W., Chen J., Heinz B.A., Higgs R.E., Kallewaard N.L., Jepson K., Goya R., Smith M.A., Collins D.W., Pellacani D., Xiang P., de Puyraimond V., Ricicova M., Devorkin L., Pritchard C., O'Neill A., Dalal K., Panwar P., Dhupar H., Garces F.A., Cohen C.A., Dye J.M., Huie K.E., Badger C.V., Kobasa D., Audet J., Freitas J.J., Hassanali S., Hughes I., Munoz L., Palma H.C., Ramamurthy B., Cross R.W., Geisbert T.W., Menacherry V., Lokugamage K., Borisevich V., Lanz I., Anderson L., Sipahimalani P., Corbett K.S., Yang E.S., Zhang Y., Shi W., Zhou T., Choe M., Misasi J., Kwong P.D., Sullivan N.J., Graham B.S., Fernandez T.L., Hansen C.L., Falconer E., Mascola J.R., Jones B.E., Barnhart B.C. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. bioRxiv. 2022 doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf K., Zentelis S., Wang L., Foster D., Vaillancourt P., Wiggin M., Lovett E., van der Lee R., Hendle J., Pustilnik A., Sauder J.M., Kraft L., Hwang Y., Siegel R.W., Chen J., Heinz B.A., Higgs R.E., Kallewaard N.L., Jepson K., Goya R., Smith M.A., Collins D.W., Pellacani D., Xiang P., de Puyraimond V., Ricicova M., Devorkin L., Pritchard C., O'Neill A., Dalal K., Panwar P., Dhupar H., Garces F.A., Cohen C.A., Dye J.M., Huie K.E., Badger C.V., Kobasa D., Audet J., Freitas J.J., Hassanali S., Hughes I., Munoz L., Palma H.C., Ramamurthy B., Cross R.W., Geisbert T.W., Menachery V., Lokugamage K., Borisevich V., Lanz I., Anderson L., Sipahimalani P., Corbett K.S., Yang E.S., Zhang Y., Shi W., Zhou T., Choe M., Misasi J., Kwong P.D., Sullivan N.J., Graham B.S., Fernandez T.L., Hansen C.L., Falconer E., Mascola J.R., Jones B.E., Barnhart B.C. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.N., Zhang Z.R., Zhang H.Q., Li N., Zhang Q.Y., Li X.D., Deng C.L., Deng F., Shen S., Zhu B., Zhang B. Different pathogenesis of SARS-CoV-2 Omicron variant in wild-type laboratory mice and hamsters. Signal Transduct. Targeted Ther. 2022;7:62. doi: 10.1038/s41392-022-00930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.