Abstract
In addition to eliciting antigen specific T-cell-mediated immunity, Cryptococcus neoformans possesses a mitogen (CnM) that activates naive T cells to proliferate. This mechanism of T-cell activation is accessory cell dependent and major histocompatibility complex unrestricted. CnM-induced T-cell proliferation correlates with internalization of the organism, suggesting that intracellular processing is required to liberate CnM prior to presentation to T cells. To determine whether phagocytosis and processing are required, various inhibitors of accessory cell uptake and processing were used. C. neoformans was observed within the accessory cells. Paraformaldehyde fixation of the accessory cell abrogated presentation of CnM to T cells, indicating that a dynamic accessory cell surface was required. A lysosomotropic agent abrogated the response to CnM but had no effect on a control stimulus that did not require processing. Both aspartic acid and cysteine protease inhibitors blocked effective processing of CnM, so that it was unable to stimulate T cells. Finally, an inhibitor of microfilament polymerization abrogated proliferation to CnM. These results indicate that the mitogenic activity of C. neoformans requires phagocytosis of the organism, lysosomal or endosomal processing, proteolytic activity, and microfilament polymerization and intracellular transport as a prerequisite for T-cell proliferation.
Cryptococcus neoformans is a pathogenic yeast that causes one of the leading fatal mycoses in AIDS (9, 12, 18, 38). This clinical observation, in addition to animal studies, has made it clear that T-cell-mediated immunity is of paramount importance in host defense against C. neoformans (1, 7, 15, 19, 20, 22, 28, 31). T-cell immunity is antigen specific; however, we have recently shown that T cells are also capable of responding to C. neoformans by an alternate mechanism of activation (36). Specifically, when T cells from a previously unexposed individual are placed in culture with C. neoformans, the organism induces a mitogenic response. The mitogenic T-cell response is accessory cell (AC) dependent but not major histocompatibility complex restricted (36). Since the whole organism is capable of mediating this mitogenic effect, it raises questions about how the C. neoformans mitogen (CnM) might be liberated or displayed prior to T-cell activation.
We had previously shown that CnM was confined to the cell wall of the organism (32). Since CnM may be displayed on the cell wall, it is possible that it cross-links surface molecules on the T cell and AC analogous to superantigens. Alternately, the AC might produce an enzyme that results in extracellular degradation and release of CnM, with liberation of the molecule into the surrounding milieu, where it exerts its mitogenic effect. Finally, we considered the possibility that the organism must be taken up by the AC and degraded internally, with subsequent presentation of CnM by the AC to the T cell. We have previously shown that lymphocyte proliferation in response to C. neoformans correlates with the magnitude of phagocytosis by AC (48), which suggests that the organism must be taken up by AC prior to presentation of CnM to T cells. Because of this finding, we wanted to investigate the possibility that processing of the organism is required to liberate CnM.
To determine whether processing of the cryptococcal mitogen was required for lymphocyte proliferation, AC were fixed to determine whether a dynamic AC membrane was required. To investigate the nature of the processing, a lysosomotropic agent was used to neutralize acid-dependent processing in acidic organelles. Specific elements of proteolysis were studied by using protease inhibitors. Finally, the effect of cytochalasin B was analyzed to understand the importance of microfilament polymerization in processing and presentation of CnM.
MATERIALS AND METHODS
Preparation of C. neoformans.
C. neoformans strain 68 (ATCC 24064, lightly encapsulated, serotype A), and strain 67 (ATCC 52817, acapsular mutant) were obtained from the American Type Culture Collection (Rockville, Md.). The organisms were maintained as previously described (35) on Sabouraud's slants (Difco, Detroit, Mich.) and passaged to fresh slants bimonthly. The organisms were killed by autoclaving at 121°C for 15 min and stored at 4°C for up to 3 months or were killed by incubation in a 56°C water bath for 30 min. In some experiments, live C. neoformans was used. For these experiments, organisms were grown to the plateau phase in Sabouraud's medium containing 1% neopeptone and 2% dextrose (Difco), placed at 4°C, and used within 72 h.
Isolation of PBMC.
Human peripheral blood was obtained by venipuncture from healthy adults who had no history of cryptococcosis and had not worked with C. neoformans. Blood was anticoagulated by adding 10 U of heparin (Organon-Teknika-Cappel, Scarborough, Ontario, Canada) per ml. Fetal umbilical cord blood was collected in 8-ml heparinized tubes (Becton-Dickinson, Rutherford, N.J.). Peripheral blood mononuclear cells (PBMC) or fetal blood mononuclear cells were isolated by centrifugation (800 × g for 20 min.) over a Ficoll-Hypaque density gradient (C-Six Diagnostics Inc., Mequon, Wis.) and washed three times in Hanks' balanced salt solution (Gibco, Burlington, Ontario, Canada). Cells were then counted and suspended in complete medium consisting of RPMI 1640 medium (Gibco) containing 5% heat-inactivated pooled human AB serum (BioWhittaker, Walkersville, Md.), 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), amphotericin B (0.25 μg/ml), 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids (all from Gibco).
Preparation of T cells and AC.
T lymphocytes were purified by nonadherence to plastic and rosetting to 2-aminoethyl-isothiouronium bromide (AET) (Sigma, St. Louis, Mo.)-treated sheep red blood cells (SRBC) (Cedarlane, Hornby, Ontario, Canada), followed by nylon wool nonadherence as previously described (43, 49). T cells isolated by this technique were routinely greater than 95% CD3+ by fluorescence-activated cell sorter analysis.
AC were obtained by incubating PBMC on 100-mm2 plastic petri dishes for 2 h at 37°C in RPMI medium under 5% CO2 (52). Nonadherent cells were gently harvested, and the adherent population was resuspended in RPMI medium plus 0.1% human serum overnight at 37°C under 5% CO2. Eighteen hours later, the nonadherent cells were resuspended in complete medium and used as AC.
Lymphocyte proliferation assays.
C. neoformans (2 × 105 organisms/well) was added to 2 × 105 PBMC or 2 × 105 T cells plus 1 × 105 AC. The cells were incubated for 7 days in 96-well round-bottom plates (Falcon, Franklin Lakes, N.J.). At 18 h before the end of incubation, 1 μCi of [3H]thymidine ([3H]TdR) (ICN, Montreal, Quebec, Canada) was added. Cells were harvested on glass filters, and counts per minute (cpm) were determined using a liquid scintillation counter. The bacterial mitogen Staphylococcus enterotoxin B (SEB) (Toxin Technologies, Sarasota, Fla.) was used at 0.5 or 1.0 μg/ml and incubated for 5 days (the optimal time for lymphocyte proliferation in response to this stimulus). The mitogenic lectin concanavalin A (ConA) (Sigma) was used at 10 μg/ml and incubated for 3 days (the optimal time for response to this stimulus).
In some experiments the AC were lightly fixed (5 min) in 1% paraformaldehyde prior to being placed in T-cell cultures with C. neoformans. The cells were washed extensively prior to suspension in complete medium. In other experiments a lysosomotropic agent (0.5 to 1 mM NH4Cl) was added to cultures to inhibit lysosomal function as previously described (55).
To examine the role of proteases, various protease inhibitors were added to the cultures as previously described (40). Various concentrations of the cysteine and serine protease inhibitors such as antipain and leupeptin (1 to 100 μg/ml; Sigma) and the aspartic acid protease inhibitor pepstatin A (1 to 100 μg/ml; Sigma) were added to the cultures. In other experiments, microfilament polymerization was inhibited by adding cytochalasin B (0.1 to 100 μg/ml; Sigma) to the cultures.
Light microscopy.
The appearance of AC that were interacting with C. neoformans was examined by light microscopy. AC were incubated with C. neoformans for 18 h at 37°C on 12-mm coverslips (Nunc, Roskilde, Denmark) that had been inserted into 24-well plates (Falcon, Franklin Lakes, NJ). The coverslips were removed, and the cells were fixed with methanol and stained with Giemsa for examination by light microscopy.
Statistics.
Data are given as mean ± standard error of the mean (SEM). Each experiment was repeated with different donors on different days. [3H]TdR incorporation is expressed as the mean counts per minute ± SEM of quadruplicate wells. Statistical analysis was performed by one-way analysis of variance (Statview 512+; Brainpower Inc., Calabasas, Calif.). For these tests, P < 0.05 was considered significant.
RESULTS
Encapsulated strains of C. neoformans have mitogenic activity.
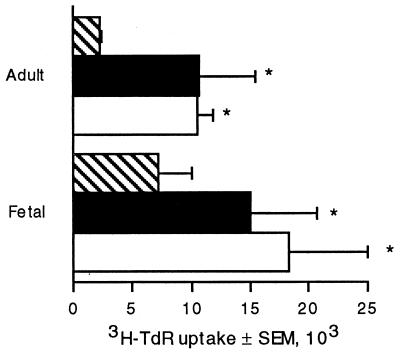
Previous studies have demonstrated that the cell wall of the acapsular strain 67 of C. neoformans contains a mitogen for T lymphocytes. To determine whether this mitogen is also present in a genetically distinct, unrelated encapsulated strain of C. neoformans, the T-lymphocyte response to the encapsulated strain 68 was assessed. Fetal lymphocytes proliferated in response to strain 68 of C. neoformans, indicating that it possesses CnM (Fig. 1). This extends the previous observation that healthy adults with no known exposure to C. neoformans respond to all strains of C. neoformans that have been tested (34). Having demonstrated that CnM is present in a completely distinct strain of C. neoformans, further experiments were performed with strain 67 to avoid the confounding effect of the polysaccharide capsule (48).
FIG. 1.
Fetal lymphocytes proliferate in response to encapsulated C. neoformans. Adult (top) or fetal (bottom) blood lymphocytes were left unstimulated (hatched bars) or stimulated with C. neoformans strain 68 (solid bars) or C. neoformans strain 67 (open bars) for 7 days, and [3H]TdR incorporation was determined during the last 18 h of culture. The data represents the mean values from four different experiments and SEM. ∗, P < 0.05 compared to unstimulated cultures using a paired t test.
Photomicrographs of binding and phagocytosis.
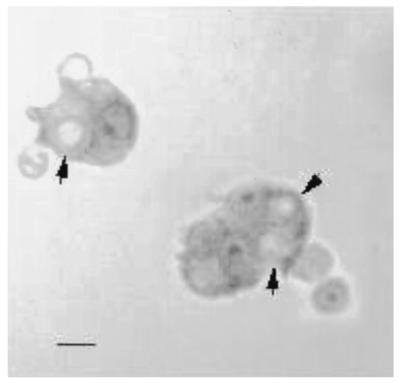
We had previously demonstrated that phagocytosis of C. neoformans by PBMC correlated with lymphocyte proliferation (48). To determine whether the AC where taking up C. neoformans, they were examined by light microscopy. C. neoformans was completely internalized in AC as early as 2 h after incubation. By 18 h, 24.9% ± 3.1% (n = 4 experiments) of the AC had phagocytosed the organism (Fig. 2).
FIG. 2.
C. neoformans is internalized by AC. AC were cultured with C. neoformans for 18 h, washed, fixed, stained with Giemsa, and examined by light microscopy. Arrows indicate internalized C. neoformans organisms. Bar, 5 μm.
T-lymphocyte proliferation in response to C. neoformans requires a metabolically active AC.
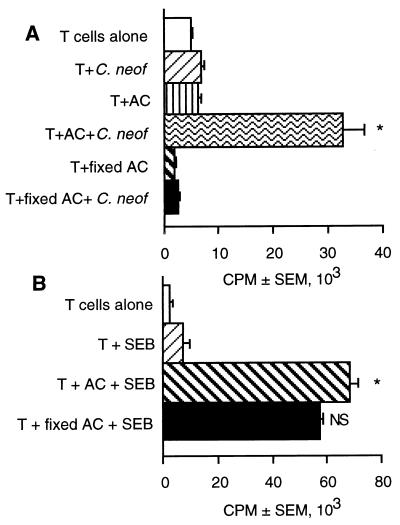
AC that are lightly fixed with paraformaldehyde are not capable of taking up and processing proteins but can present some mitogens and superantigens to T cells (27, 37). To determine if uptake and processing was required, the ability of fixed AC to present C. neoformans to T cells was examined. T cells, in the presence of purified, unfixed AC, were able to proliferate in response to C. neoformans. However, AC that had been fixed in 1% paraformaldehyde prior to being placed in T cell cultures with C. neoformans were incapable of supporting T-cell proliferation in response to C. neoformans (Fig. 3A). These fixed cells were functional as AC when presentation but not intracellular processing was required, such as for the presentation of SEB to T cells (Fig. 3B). These data suggests that intracellular processing is required to release CnM.
FIG. 3.
Paraformaldehyde fixation of AC inhibits lymphocyte proliferation in response to C. neoformans. C. neoformans (A) or SEB (B) was used to stimulate T cells in the presence of irradiated AC or AC that had been fixed with 1% paraformaldehyde for 5 min. [3H]TdR incorporation was determined during the last 18 h of culture. ∗, P < 0.05 compared to unstimulated cultures. NS, not significantly different compared to stimulated cells. The experiment was repeated twice with similar results.
An acidic compartment is required.
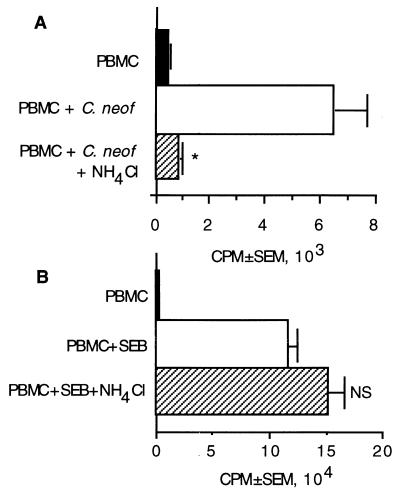
Having determined that internalization occurred and that a dynamic AC surface was required to generate a proliferative response, we investigated the contribution of lysosomal or endosomal processing. To do this, ammonium chloride, a lysosomotropic agent that prevents acidification of the vesicle, was added to the cultures. PBMC were plated with C. neoformans in the presence or absence of ammonium chloride. NH4Cl abrogated lymphocyte proliferation in response to C. neoformans (Fig. 4A) but had no effect on the response to the superantigen SEB (Fig. 4B), which does not require lysosomal processing (27). Thus, an acidified compartment is required for processing, suggesting that lysosomal or endosomal processing is required to liberate CnM.
FIG. 4.
An NH4Cl-sensitive pathway is used to process C. neoformans. PBMC were stimulated with C. neoformans (A) or SEB (B) in the presence or absence of NH4Cl (1 mM). ∗, P < 0.05 compared to stimulated PBMC, NS, not significant compared to stimulated PBMC. The experiment was repeated three times with similar results.
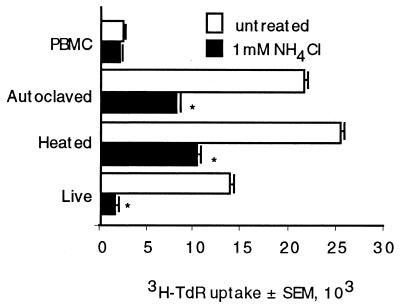
Previous experiments had been performed with autoclaved organisms. We considered the possibility that autoclaving might somehow liberate the mitogen, and therefore additional experiments were performed to determine whether live organisms or organisms that had been killed by gentle heating required processing. NH4Cl abrogated lymphocyte proliferation in response to autoclaved, heat-killed, and live C. neoformans (Fig. 5). Thus, the same mechanism is required to liberate CnM from live C. neoformans as from killed C. neoformans.
FIG. 5.
An NH4Cl-sensitive pathway is used to process live and heat-killed C. neoformans. PBMC were cultured alone or stimulated with autoclaved, heat-killed (56°C for 30 min), or live C. neoformans in the presence or absence of NH4Cl (1 mM). ∗, P < 0.05 compared to stimulated PBMC. The experiment was repeated three times with similar results.
Role of proteases in processing of CnM.
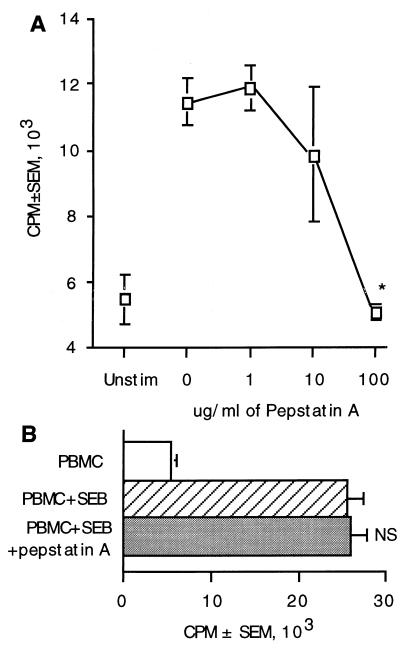
Proteases are required for the processing of most antigens and some mitogens (3, 10, 11, 27). To determine whether proteases were required for processing of CnM, protease inhibitors were used. A family of lysosomal enzymes called cathepsins is responsible for acid-dependent proteolytic processing of antigens prior to presentation to T cells. Pepstatin A is an aspartic acid protease inhibitor (6) that blocks the activity of cathepsin D (4, 45). When pepstatin A was added to the cultures, there was a dose-dependant reduction in lymphocyte proliferation in response to C. neoformans (Fig. 6). The threshold of inhibition occurred at 10 μg/ml, and lymphocyte proliferation was abrogated at 100 μg/ml (Fig. 6A). In control experiments, 100 μg of pepstatin A per ml had no effect on the response to the superantigen SEB (Fig. 6B), demonstrating that the effects on C. neoformans were not due to nonspecific toxicity. These results indicate that an aspartic acid protease is required and suggest that cathepsin D is involved in processing of C. neoformans.
FIG. 6.
Aspartic proteases are required for processing C. neoformans. PBMC were stimulated with C. neoformans (A) in the presence or absence of various concentrations of pepstatin A (0, 1, 10, or 100 μg/ml) for 7 days or were stimulated with SEB (B) in the presence or absence of 100 μg of pepstatin A per ml for 5 days. [3H]TdR incorporation was determined during the last 18 h of culture. ∗, P < 0.05 compared to stimulated PBMC in the absence of inhibitor. NS, not significantly different from stimulated PBMC in the absence of pepstatin A. The experiment was repeated twice with similar results.
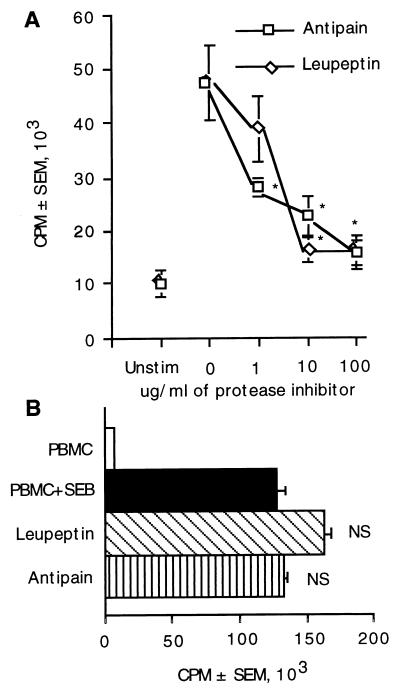
Leupeptin and antipain are cysteine and serine protease inhibitors that block the activity of cathepsins B, H, and L, while only antipain blocks the activity of cathepsin A (45). To examine the requirements for cysteine and serine proteases on the liberation of CnM, these inhibitors were individually put into culture with PBMC and C. neoformans. Both leupeptin and antipain reduced lymphocyte proliferation (Fig. 7A), showing that cysteine or serine proteases were required to liberate CnM. These inhibitors at 1 to 100 μg/ml showed a dose-dependent decrease in lymphocyte responses. In control experiments, neither of these protease inhibitors (at 100 μg/ml) were able to inhibit the responses to a superantigen (SEB) which does not require processing for a T-cell response (Fig. 7B). Together, these data demonstrate that C. neoformans requires the participation of aspartic acid and cysteine or serine proteases for complete processing and liberation of CnM.
FIG. 7.
Cysteine or serine proteases are required for processing of C. neoformans. (A) PBMC were stimulated with C. neoformans in the presence of various concentrations of the protease inhibitors leupeptin or antipain. (B) PBMC were also stimulated with SEB in the presence or absence of 100 μg of leupeptin or antipain per ml. ∗, P < 0.05 compared to stimulated PBMC in the absence of inhibitors. NS, not significant compared to stimulated PBMC in the absence of inhibitor. The experiment was repeated three times with similar results.
Role of microfilament polymerization in processing of C. neoformans.
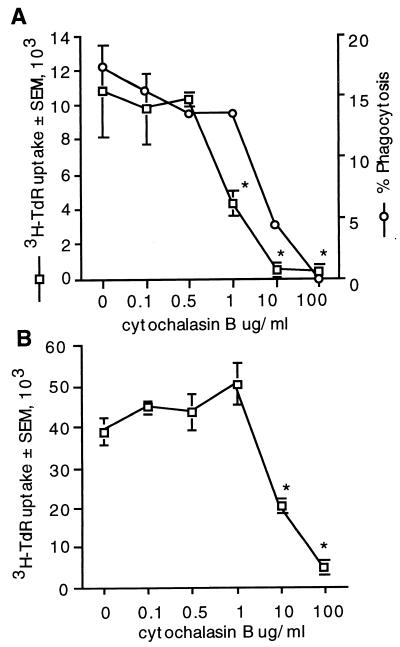
Cytochalasin B depolymerizes microfilaments, a process which has a number of effects that inhibit antigen uptake and processing (14, 17, 54). However, low concentrations of cytochalasin B enhance T-cell responses to mitogens such as ConA (17, 24, 47, 51). To determine whether cytochalasin B enhances or suppresses lymphocyte proliferation in response to CnM, different concentrations of cytochalasin B were added to the cultures. Cytochalasin B did not enhance T-cell proliferative responses in response to C. neoformans at any concentration tested, and it reduced T-cell proliferation in response to C. neoformans at 1 to 100 μg/ml (Fig. 8A). In control experiments low concentrations of cytochalasin B tended to augment proliferation in response to ConA while high concentrations abrogated responses (Fig. 8B), as previously described (17). Thus, microfilament polymerization is required for the proliferative response to CnM.
FIG. 8.
Microfilament polymerization is required for lymphocyte proliferation in response to C. neoformans. PBMC were stimulated with C. neoformans (A) or with ConA (B) in the presence or absence of various concentrations of cytochalasin B. ∗, P < 0.05 compared to cells stimulated in the absence of cytochalasin B. Phagocytosis is expressed as the mean of duplicate determinations. The experiment was repeated twice with similar results.
DISCUSSION
Taken together, there are two major observations from these studies. (i) The mitogenic response to C. neoformans requires uptake and processing of the organism. (ii) Processing of CnM, prior to presentation, requires an acidified compartment and participation of aspartic acid and cysteine and serine proteases and intracellular transport that involves microfilament rearrangement.
We have previously demonstrated that C. neoformans contains a mitogen for T lymphocytes (36). This conclusion was based on the observation that C. neoformans stimulates fetal blood lymphocytes and adult CD45RA+ T lymphocytes to proliferate, indicating that it activates naive T cells. The T-cell response to C. neoformans was dependent on the presence of AC, but allogeneic cells, which would be unable to present cognate antigens to T cells, were sufficient for AC function. While most mitogens simply bind to the surface of the responding cells, recent studies have indicated that the T-cell response to C. neoformans in vitro is highly dependent on phagocytosis of the organism (48). These observations suggested that liberation of CnM might require uptake and processing of the organism, similar to the mitogens of Mycoplasma arthritidis (3) or exotoxin A from Pseudomonas aeruginosa (27).
Experiments were performed to determine whether a dynamic AC surface was required for processing and presentation of the cryptococcal mitogen to T cells. These experiments demonstrated that fixation, which cross-links lysine residues by reacting with free amino groups on membrane-bound structural or receptor proteins (39), rendered the cells inactive and abrogated proliferation. There are a number of possible mechanisms by which fixation might abrogate the mitogenic effect. It is possible that the AC requirements for the CnM are similar to those for mitogenic lectins. Mitogenic lectins require a dynamic AC membrane to provide accessory signals, which are abrogated by fixation (16, 21, 30). Fixation could also have abrogated the activity of an enzyme that resulted in extracellular degradation of the organism, with liberation of CnM into the surrounding milieu to produce lymphocyte proliferation. This possibility is unlikely, since the present studies demonstrate that AC internalize the organism, previous studies indicated that phagocytosis of C. neoformans correlates with lymphocyte proliferation (48), and the present studies implicate lysosomal processing. Finally, it is possible and most likely that fixation exerts its major effect by abrogating uptake of the organism. What is clear from these studies, however, is that CnM does not directly cross-link the surface of AC and T cells as do most superantigens and mitogens.
The mitogenic effect correlates with internalization of C. neoformans. Once a phagocytic particle has been internalized, the phagosome fuses with a lysosome. The phagolysosome is acidified by activation of vacuolar ATPase proton pumps (46), which are required for acid hydrolysis, and pH-dependent proteases, which are responsible for degrading the phagolysosomal contents. Lysosomotropic agents such as NH4Cl produce an alkaline environment in the lysosome and therefore prevent acid-dependent hydrolysis and protease activation (55). Importantly, NH4Cl inhibits protein degradation without affecting uptake or ingestion by AC (55). Since NH4Cl inhibits lymphocyte proliferation in response to CnM, acid hydrolysis or acid-dependent proteases appear to be critical in the liberation of CnM within the AC.
Within the phagolysosome, proteases activated by low pH degrade proteins to liberate immunogenic peptides. The requirements for proteases can be quite restricted. For example, cathepsin B, which is inhibited by leupeptin but not by pepstatin, is sufficient for processing of myoglobin (50). By contrast, Pseudomonas exotoxin A is a T-cell mitogen that requires processing by an aspartic acid protease but not cysteine or serine proteases (27). Due to the complex nature of the cryptococcal cell wall, we reasoned that the processing requirements for liberating CnM from C. neoformans might be different from the processing requirements of peptide antigens.
Proteolytic processing occurs through the actions of cathepsins, which are endosomal and lysosomal enzymes (13). These enzymes are acid-optimal proteases that belong to two major families, the aspartic acid and cysteine proteases (23). The major cathepsins in antigen processing are cathepsins D and E, which are aspartic proteases, and cathepsins B, L, H, A, and S, which are cysteine proteases. Leupeptin is a specific inhibitor of cysteine and serine proteases including cathepsins B, H, and L. Antipain is also a cysteine and serine protease inhibitor that inhibits the activity of cathepsins B and A. Pepstatin A is an aspartic acid protease inhibitor that blocks the activity of cathepsin D (4, 45). Because all three inhibitors block the response, sequential action of aspartic and cysteine or serine cathepsins in the processing of CnM is implied.
There are a number of possible mechanisms by which peptide processing might liberate or activate a mitogen. T-cell antigens have been classified into those that do not require processing, those that require unfolding, and those that require cleavage into smaller peptide fragments (2). In some circumstances, producing smaller peptides may not be the critical event. To exert its effect, the protein might only need to be unfolded, but the simplest way to do this might be to cut it, as suggested by Berzofsky (4). A T-cell antigen that does not require processing is exemplified by fibrinogen (26). Myoglobin is an example of a T-cell antigen that requires unfolding (45), while cytochrome c requires proteolytic cleavage and production of smaller peptide fragments (25). Since the mitogenic effect of C. neoformans is likely to require contact with more than one T cell or AC surface molecule, we speculate that complexity would be required. Thus, proteases are more likely to unfold CnM (possibly to allow refolding into an active conformation), rather than producing small peptide fragments, so that it can activate T cells.
Protease inhibitors have been used to block the processing of peptide antigens (3, 27, 50). The protease inhibitors used are highly specific for classes of proteases, but because cathepsins have a broad spectrum of activity that cleaves proteins at multiple sites, only a limited amount of information about CnM can be obtained from the data. All cathepsins are endoproteases, while cathepsin B is also a pH-dependent exopeptidase. Cysteine proteases form a complex between the enzyme and the substrate (cysteine, histidine, and asparagine residues). An acyl enzyme complex is formed that is resolved by acid hydrolysis (41). Cathepsin L targets leucine and aromatic amino acids at P-1 and P-1′ (amino acids relative to the target bond). The enzyme cleavage pattern of cathepsin B is complex, with the best substrates containing basic amino acid residues. Aspartic acid proteases act in a similar fashion, although the carboxyl active site is not nucleophilic. Instead, a hydrogen-bonded water molecule attacks the carbonyl carbon (13). Cathepsins D and E cleave peptide bonds between hydrophobic amino acids (13). Cathepsin D specifically targets leucine and aromatic amino acids at P-2 and P-1 (8). Since all of these classes of proteases are implicated in the processing of the cryptococcal mitogen, it is likely that CnM possesses these features. All of these endoproteases function optimally at acidic pH, with the exception of cathepsin S, which retains its activity at pH 8 (5). Since NH4Cl abrogated the mitogenic response to C. neoformans, cathepsin S may not be solely responsible for processing, which provides additional support for the idea that a sequence of cathepsins is involved.
In addition to their role in producing antigenic peptides, lysosomal proteases can destroy linear antigenic epitopes (53) and conformational epitopes (29). In particular, cathepsins B and L have been implicated in the destruction of epitopes (42). Because mitogens are even more likely to require a critical conformation than an antigen, they might be even more susceptible to destruction. We were interested in determining whether the protease inhibitors might enhance the response to CnM by preventing the enzymatic destruction of an important conformational structure. We failed to see any enhancement in proliferation with any of the protease inhibitors that were tested, suggesting that proteases do not destroy a critical conformation.
Microfilaments play a role in protein processing by their involvement in intracellular transport in addition to their effects on uptake of particles. They are required for delivery of proteins to appropriate sites of degradation and to the site of peptide antigen loading. Microfilament-mediated vesicular transport of peptide antigen, major histocompatibility complex class II, and other cofactors is required for antigen processing (44). Because of the critical role of microfilaments in intracellular transport, we wondered whether they might also be involved in the processing and liberation of CnM. Cytochalasin B is an inhibitor of microfilament polymerization that inhibits intracellular transport and can inhibit antigen processing (17). It affects microfilament-dependent transfer of peptide antigens, which is a late step in antigen processing, but does not inhibit uptake or delivery of endocytosed protein to lysosomes, which is an early stage of antigen processing (44). Experiments were performed to determine whether cytochalasin B inhibited phagocytosis and to determine whether it inhibited proliferation at concentrations lower than the concentration that inhibited phagocytosis. As expected, cytochalasin B at 10 and 100 μg/ml inhibited phagocytosis. However, it inhibited proliferation when present at a 10-fold-lower concentration, implicating microfilament-mediated intracellular transport in the liberation and display of CnM to T cells.
Previous studies have demonstrated that low concentrations of cytochalasin B enhance lymphocyte proliferation in response to mitogens such as phytohemagglutinin and ConA (24, 47, 54). Because of this observation, experiments were performed to determine whether low concentrations of cytochalasin B enhanced lymphocyte proliferation in response to CnM. The mechanism by which cytochalasin B enhances lymphocyte proliferation is by enhancing the expression of interleukin-2 receptor α (IL-2Rα) (24) and enhancing the production of IL-2 (17). We have previously studied the role of IL-2 in lymphocyte proliferation in response to C. neoformans (33). While we found that IL-2 and IL-15 were required for T-lymphocyte proliferation, we failed to observe any increase in proliferation when additional IL-2 was added to the cultures (33). This suggests that optimal IL-2 is present during the lymphocyte proliferative response, in keeping with the observation that cytochalasin B-enhanced IL-2 signaling does not enhance lymphocyte proliferation in response to CnM.
In summary, we have demonstrated that CnM is not displayed directly by the organism. During the course of infection, the organisms would be taken up and exposed to a series of degradative events in the phagolysosome prior to presentation and stimulation of T cells by a mitogenic mechanism.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council of Canada and the Canadian Foundation for AIDS Research. R.M.S. was supported by a studentship from the National Health Research and Development Program. C.H.M. is a Scholar of the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Abrahams J, Gilleran T G. Studies on actively acquired resistance to experimental cryptococcosis in mice. J Immunol. 1960;85:629–635. [Google Scholar]
- 2.Allen P M, Babbitt B P, Unanue E R. T cell recognition of lysozyme: the biochemical basis of presentation. Immunol Rev. 1987;98:171–187. doi: 10.1111/j.1600-065x.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 3.Bauer A, Rutenfranz I, Kirchner H. Processing requirements for T cell activation by Mycoplasma arthritidis derived mitogen. Eur J Immunol. 1988;18:2109–2112. doi: 10.1002/eji.1830181239. [DOI] [PubMed] [Google Scholar]
- 4.Berzofsky J A, Brett S J, Streicher H Z, Takahashi H. Antigen processing for presentation to T lymphocytes: function, mechanisms, and implications for the T-cell repertoire. Immunol Rev. 1988;106:5–31. doi: 10.1111/j.1600-065x.1988.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 5.Bromme D, Steinert A, Friebe S, Fittkau S, Wiederanders B, Kirschke H. The specificity of bovine spleen cathepsin S. A comparison with rat liver cathepsins L and B. Biochem J. 1989;264:475–81. doi: 10.1042/bj2640475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buus S, Werdelin O. A group-specific inhibitor of lysosomal cysteine proteinases selectively inhibits both proteolytic degradation and presentation of the antigen dinitrophenyl-poly-l-lysine by guinea pig accessory cells to T cells. J Immunol. 1986;136:452–458. [PubMed] [Google Scholar]
- 7.Cauley L K, Murphy J W. Response of congenially athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infections. Infect Immun. 1979;23:644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman H A. Endosomal proteolysis and MHC class II function. Curr Opin Immunol. 1998;10:93–102. doi: 10.1016/s0952-7915(98)80038-1. [DOI] [PubMed] [Google Scholar]
- 9.Coker R J. Cryptococcal infection in AIDS. Int J STD AIDS. 1992;3:168–172. doi: 10.1177/095646249200300303. [DOI] [PubMed] [Google Scholar]
- 10.Demotz S, Matricardi P M, Irle C, Panina P, Lanzavecchia A, Corradin G. Processing of tetanus toxin by human antigen-presenting cells. Evidence for donor and epitope-specific processing pathways. J Immunol. 1989;143:3881–3886. [PubMed] [Google Scholar]
- 11.Demotz S, Peleraux A. Processing of DR1-restricted determinants from the fusion protein of measles virus following two distinct pathways. Mol Immunol. 1996;33:387–397. doi: 10.1016/0161-5890(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 12.Dismukes W E. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1988;157:624–628. doi: 10.1093/infdis/157.4.624. [DOI] [PubMed] [Google Scholar]
- 13.Fineschi B, Miller J. Endosomal proteases and antigen processing. Trends Biochem Sci. 1997;22:377–382. doi: 10.1016/s0968-0004(97)01116-x. [DOI] [PubMed] [Google Scholar]
- 14.Freed B M, Lempert N, Lawrence D A. The inhibitory effects of N-ethylmaleimide, colchicine and cytochalasins on human T-cell functions. Int J Immunopharmacol. 1989;11:459–465. doi: 10.1016/0192-0561(89)90174-4. [DOI] [PubMed] [Google Scholar]
- 15.Fung P Y S, Murphy J W. In vitro interactions of immune lymphocytes and C. neoformans. Infect Immun. 1982;36:1128–1138. doi: 10.1128/iai.36.3.1128-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher R B, Whelan A, Feighery C. Studies on the accessory requirement for T lymphocyte activation by concanavalin A. Clin Exp Immunol. 1986;66:118–125. [PMC free article] [PubMed] [Google Scholar]
- 17.Geppert T D, Lipsky P E. Regulatory role of microfilaments in the induction of T4 cell proliferation and interleukin 2 production. Cell Immunol. 1990;131:205–218. doi: 10.1016/0008-8749(90)90247-o. [DOI] [PubMed] [Google Scholar]
- 18.Grant I H, Armstrong D. Fungal infections in AIDS. Cryptococcosis. Infect Dis Clin North Am. 1988;2:457–464. [PubMed] [Google Scholar]
- 19.Graybill J R, Alford R H. Cell mediated immunity in cryptococcosis. Cell Immunol. 1974;14:12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- 20.Hill J O, Harmsen A G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med. 1991;173:755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirayama Y, Inaba K, Komatsubara S, Yoshida K, Kawai J, Naito K, Muramatsu S. Accessory cell functions of dendritic cells and macrophages in the thymic T-cell response to Con A. Immunology. 1987;62:393–399. [PMC free article] [PubMed] [Google Scholar]
- 22.Huffnagle G B, Yates J L, Lipscomb M F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirschke H, Barrett A J, Rawlings N D. Proteinases. 1. Lysosomal cysteine proteinases. Protein Profile. 1995;2:1581–1643. [PubMed] [Google Scholar]
- 24.Komada H, Nakabayashi H, Idota M, Hara M, Takahashi T, Takanari H, Izutsu K. Cytochalasin B enhances T cell mitogenesis by promoting expression of an interleukin 2 receptor. Cell Struct Funct. 1987;12:281–285. doi: 10.1247/csf.12.281. [DOI] [PubMed] [Google Scholar]
- 25.Kovac Z, Schwartz R H. The molecular basis of the requirement for antigen processing of pigeon cytochrome c prior to T cell activation. J Immunol. 1985;134:3233–3240. [PubMed] [Google Scholar]
- 26.Lee P, Matsueda G R, Allen P M. T cell recognition of fibrinogen. A determinant on the A alpha-chain does not require processing. J Immunol. 1988;140:1063–1068. [PubMed] [Google Scholar]
- 27.Legaard P K, LeGrand R D, Misfeldt M L. The superantigen Pseudomonas exotoxin A requires additional functions from accessory cells for T lymphocyte proliferation. Cell Immunol. 1991;135:372–382. doi: 10.1016/0008-8749(91)90282-g. [DOI] [PubMed] [Google Scholar]
- 28.Lim T S, Murphy J W. Transfer of immunity to cryptococcosis by T-enriched spleen lymphocytes form Cryptococcus neoformans-sensitized mice. Infect Immun. 1980;30:5–9. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills K H, Skehel J J, Thomas D B. Conformational-dependent recognition of influenza virus hemagglutinin by murine T helper clones. Eur J Immunol. 1986;16:276–280. doi: 10.1002/eji.1830160312. [DOI] [PubMed] [Google Scholar]
- 30.Mincheff M S, Meryman H T. Costimulatory signals necessary for induction of T cell proliferation. Transplantation. 1990;49:768–772. doi: 10.1097/00007890-199004000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Mody C H, Lipscomb M F, Toews G B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990;144:1472–1477. [PubMed] [Google Scholar]
- 32.Mody C H, Sims K L, Wood C J, Syme R M, Spurrell J C, Sexton M M. Proteins in the cell wall/membrane of Cryptococcus neoformans stimulate both adult and fetal cord blood lymphocyte to proliferate. Infect Immun. 1996;64:4811–4819. doi: 10.1128/iai.64.11.4811-4819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mody C H, Spurrell J C L, Wood C J. Interleukin 15 (IL-15) induces antimicrobial activity after release by Cryptococcus neoformans-stimulated monocytes. J Infect Dis. 1998;178:803–814. doi: 10.1086/515381. [DOI] [PubMed] [Google Scholar]
- 34.Mody C H, Syme R M. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464–469. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mody C H, Toews G B, Lipscomb M F. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect Immun. 1988;56:7–12. doi: 10.1128/iai.56.1.7-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mody C H, Wood C J, Syme R M, Spurrell J C. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect Immun. 1999;67:936–941. doi: 10.1128/iai.67.2.936-941.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno J, Lipsky P E. Differential ability of fixed antigen-presenting cells to stimulate nominal antigen-reactive and alloreactive T4 lymphocytes. J Immunol. 1986;136:3579–3587. [PubMed] [Google Scholar]
- 38.Murray J F, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Am Rev Respir Dis. 1990;141:1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. , 1582–1598. [DOI] [PubMed] [Google Scholar]
- 39.Pancake S J, Nathenson S G. Selective loss of H-2 antigenic reactivity after chemical modification. J Immunol. 1973;111:1086–1092. [PubMed] [Google Scholar]
- 40.Puri J, Factorovich Y. Selective inhibition of antigen presentation to cloned T cells by protease inhibitors. J Immunol. 1988;141:3313–3317. [PubMed] [Google Scholar]
- 41.Rawlings N D, Barrett A J. Structure of membrane glutamate carboxypeptidase. Biochim Biophys Acta. 1997;1339:247–252. doi: 10.1016/s0167-4838(97)00008-3. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez G M, Diment S. Destructive proteolysis by cysteine proteases in antigen presentation of ovalbumin. Eur J Immunol. 1995;25:1823–1827. doi: 10.1002/eji.1830250705. [DOI] [PubMed] [Google Scholar]
- 43.Saxon A J, Robins R A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12:285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- 44.Soreng K M, Weber D A, Joshi H, Moore J C, Jensen P E. A role for microfilaments but not microtubules in processing soluble antigens. Cell Immunol. 1995;166:25–34. doi: 10.1006/cimm.1995.0004. [DOI] [PubMed] [Google Scholar]
- 45.Streicher H Z, Berkower I J, Busch M, Gurd F R N, Berzofsky J A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci USA. 1984;81:6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 47.Sundqvist K G, Wanger L, Ensgstom W. Permissive effect of cytochalasin B on DNA synthesis in concanavalin-A-treated lymphocytes. J Cell Sci. 1984;66:155–166. doi: 10.1242/jcs.66.1.155. [DOI] [PubMed] [Google Scholar]
- 48.Syme R M, Bruno T F, Kozel T R, Mody C H. The capsule of Cryptococcus neoformans reduces T lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect Immun. 1999;67:4620–4627. doi: 10.1128/iai.67.9.4620-4627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syme R M, Wong H, Wood C J, Mody C H. Both CD4+ and CD8+ human lymphocytes are activated and proliferate to Cryptococcus neoformans. Immunology. 1997;92:194–200. doi: 10.1046/j.1365-2567.1997.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi H, Cease K B, Berzofsky J A. Identification of proteases that process distinct epitopes on the same protein. J Immunol. 1989;142:2221–2229. [PubMed] [Google Scholar]
- 51.Valentine M A, Vaughan J A. Augmentation of interleukin-2 release by cytochalasins. Cell Immunol. 1986;101:651–658. doi: 10.1016/0008-8749(86)90175-9. [DOI] [PubMed] [Google Scholar]
- 52.Van Voorhis W C, Hair L S, Steinman R M, Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982;155:1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidard L, Rock K L, Benacerraf B. The generation of immunogenic peptides can be selectively increased or decreased by proteolytic enzyme inhibitors. J Immunol. 1991;147:1786–1791. [PubMed] [Google Scholar]
- 54.Yoshinaga M, Yoshinaga A, Waksman B H. Regulation of lymphocyte responses in vitro: potentiation and inhibition of rat lymphocyte responses to antigen and mitogens by cytochalasin B. Proc Natl Acad Sci USA. 1972;69:3251–3255. doi: 10.1073/pnas.69.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziegler H K, Unanue E R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]