Abstract
Integrin receptors are established drug targets, but many of the drugs that have been developed act as partial agonists, inducing the receptor into a high-affinity, ligand-binding state. Lin et al. discovered a general mechanism to circumvent this problem—stabilizing a key water molecule that prevents receptor activation. Their findings are likely to impact future therapeutic development.
The 24 members of the integrin family of cell receptors are type 1 transmembrane heterodimers composed of one of 18 α chains and one of 8 β chains (Ley et al., 2016). They characteristically undergo activation to a high-affinity ligand binding state in response to internal signals (inside-out signaling), bind ligand, and then transmit additional signals back into the cell (outside-in signaling). Since the receptors play vital roles in a wide variety of physiological and pathologic processes and diseases—including development, immunity, hemostasis and thrombosis, inflammation, angiogenesis, tumor growth and metastasis, multiple sclerosis, inflammatory bowel disease, nephritis, osteoporosis, sickle cell disease, and fibrosis—it is not surprising that they have attracted attention as drug targets (Ley et al., 2016). The discovery that small peptides containing the Arg-Gly-Asp (RGD) sequence could block binding of ligand to a subset of the receptors (Pierschbacher and Ruoslahti, 1984) un-leashed efforts to develop small molecule antagonists based on this motif as therapeutics. It later became apparent that many of these compounds could induce the receptors to adopt a high-affinity ligand binding conformation as evidenced by the binding of monoclonal antibodies specific for these “ligand-induced binding sites (LIBS),” and under certain, admittedly artificial, experimental conditions paradoxically prime the receptor to bind ligand (Du et al., 1991; Frelinger et al., 1990). This drug-induced activation has been hypothesized to contribute to the failure to produce safe and effective oral integrin antagonists for long-term therapy (Ley et al., 2016). In this issue of Cell, Springer and his colleagues provide exciting new information on a key aspect of the mechanism of this activation and propose a way to produce small molecule antagonists for a broad range of integrin receptors that lock the receptor in its inactive conformation (Lin et al., 2022). This information will likely guide future pharmaceutical development.
The authors focused on the platelet integrin receptor αIIbβ3 (GPIIb/IIIa), which undergoes an activation-dependent change in its conformation that results in the binding of fibrinogen and von Willebrand factor, large multivalent glycoproteins that can span between platelets and produce platelet aggregation. The latter process is crucial for normal hemostasis, but also contributes to thrombotic diseases, including heart attack and stroke. αIIbβ3 was the first integrin receptor successfully targeted therapeutically, initially with a chimeric monoclonal antibody Fab fragment (abciximab) and later by two small-molecule antagonists, eptifibatide and tirofiban, variably patterned on the RGD sequence (Ley et al., 2016). These intravenous drugs, which are given for a period of just 12–24 h while patients undergo coronary interventions and stenting, reduce the risk of death or heart attack by about 30% (Bosch et al., 2013). In contrast, several oral αIIbβ3 antagonists, when given for many months, failed to show a benefit and paradoxically were sometimes associated with increased mortality (Cox, 2004). The failure of these oral agents has been ascribed to their tendency to prime the receptor to bind fibrinogen and initiate platelet aggregation as they cycle on and off the receptor between doses (Cox, 2004). The αIIbβ3 antagonists also produce thrombocytopenia in rare instances, primarily by inducing a conformational change in the receptor that is then recognized by patients’ own pre-existing or induced antibodies (Aster, 2005). Thus, the conformational change induced by the drugs may limit both efficacy and safety.
In previous work, Springer and colleagues (Eng et al., 2011) showed that resting αIIbβ3 primarily adopts a bent conformation, but that a subpopulation of receptors undergoes extension of the headpiece from the lower leg region when activated with Mn2+. The extended integrin then undergoes a further dramatic conformational change when an RGD peptide binds, with swing-out of the hybrid domain in β3, resulting in opening the ligand-binding region and enhancing affinity for ligand (Figure 1A). Their crystal structures of the αIIbβ3 headpiece in complex with eptifibatide and tirofiban showed that they bind, as do RGD and fibrinogen peptides, by their positively charged group interacting with an Asp residue in the αIIb β-propeller and their carboxyls coordinating the metal ion in the metal-ion-dependent adhesion site (MIDAS) in the β3 βI domain (Springer et al., 2008; Xiao et al., 2004). Both drugs also induce the swing-out motion, apparently triggered by their negatively charged carboxyl oxygens also interacting with backbone nitrogens on the βI domain β1-α1 loop. This results in movement of the loop toward the MIDAS (Figure 1B), reorganization of the MIDAS and the nearby adjacent to MIDAS (ADMIDAS) metal-ion coordination residues, movement of the ADMIDAS metal ion toward the MIDAS, and swing-out of the hybrid domain. This conformational change converts the receptor from a “closed,” low-affinity state, to an “open,” high-affinity state.
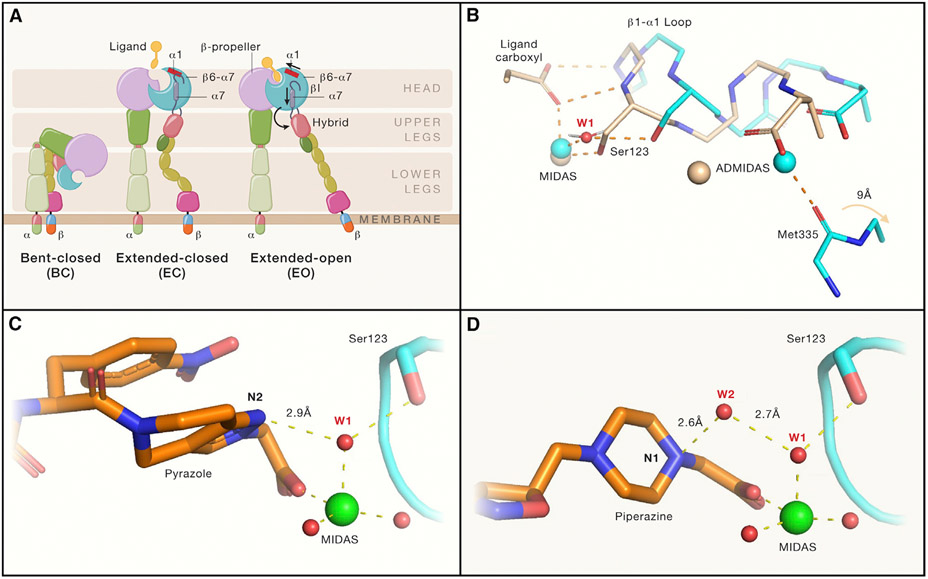
Figure 1. Integrin αIIb(β3 conformational states and mechanisms by which “closing” αIIb(β3 antagonists stabilize the closed conformation.
(A) Integrin domain organization and conformational states. Resting integrin receptors primarily adopt a bent-closed conformation, and with activation and ligand binding transition to extended-closed and extended-open conformations. Integrin headpiece opening increases affinity for biological ligands and is accomplished by ligand binding, which causes α1-helix pistoning (red bar) of the βI domain, leading to pistoning of the α7-helix (purple bar), and swing-out of the hybrid domain (curved arrow), accompanied by (B) rearrangement of loops at the ligand-binding site.
(B) Shows that the resting integrin adopts a closed conformation (cyan carbons and metals; PDB 3T3P) in which a tightly bound water molecule (water 1; W1) mediates the interaction of β3 Ser123 with the MIDAS metal ion. With the binding of the fibrinogen peptide (wheat carbons and metals; PDB 2VDO), the ligand carboxyl pulls the backbone nitrogens on the β1-α1 loop toward the MIDAS, resulting in the Ser123 now directly coordinating the MIDAS metal ion and freeing the Met335 on the β6-α7 loop to move ~9 Å (out of the frame) as part of the swing-out motion of the hybrid domain. Thus, opening of the receptor requires displacement of water 1.
(C and D) (C) The hydrogen acceptor pyrazole nitrogen of the “closing” antagonist UR-2922 stabilizes water 1 and (D) the hydrogen donor piperidine nitrogen of “closing” compound BMS4 stabilizes water 2 (W2), which in turn stabilizes water 1. Thus, both compounds prevent the displacement of water 1, which is required for reorganization of the ligand binding region and initiation of the swing-out motion. Figure adapted from Lin et al. (2022) and made with the assistance of Dr. Deena Oren of the Rockefeller University Structural Biology Resource Center.
In their current study, Lin et al. (2022) used complementary biochemical, immunological, and crystallographic methods to divide a large number of αIIbβ3 antagonists into three groups: those that stabilize opening of the β3 headpiece, those that stabilize the closed form, and those that are conformationally neutral. The eight compounds that promoted opening of the β3 headpiece varied in ionic requirements, with some active in Mg2+/Ca2+ buffer and others only producing opening with a Mn2+/Ca2+ buffer. One compound was conformationally neutral and has a novel structure, lacking a carboxyl group, and a novel mechanism of binding in that it displaces the MIDAS metal ion (Li et al., 2014). Two compounds were judged to promote closing because they not only did not increase the hydrodynamic radius of the αIIbβ3 headpiece, but they actually reversed the effect of Mn2+. By detailed analysis of the crystal structures of the compounds, focusing on the tightly bound water molecule that mediates the interaction of β3 Ser-123 to the MIDAS metal ion in the closed conformation but is lost in the open conformation as the Ser-123 oxygen directly coordinates the MIDAS metal ion, they were able to identify a key difference between the opening and closing compounds. In their words, “The inspirational moment finally came when we noticed that closing compounds either directly hydrogen bonded water 1 (W1), which bridges the MIDAS metal ion to the sidechain of MIDAS residue Ser-123; or indirectly hydrogen bonded to water 1 through water 2 (W2).” Thus, the key to designing a closing compound is to introduce a polar atom that is positioned to either accept a hydrogen bond from water 1 (Figure 1C) or to donate a hydrogen bond to water 2, which in turn accepts a hydrogen bond from water 1 (Figure 1D). They went on to identify an inhibitor to α4β1, an integrin receptor composed of different α and β subunits, that also produced closing of the receptor by the same mechanism, indicating the generalizability of their discovery. Further confirmation was obtained by demonstrating that (1) In accord with ensemble analysis, opening compounds bind with higher affinity to recombinant αIIbβ3 expressed on the surface of cells in the extended, open conformation than the bent, closed conformation, whereas closing compounds bind with higher affinity to the bent, closed conformation than the extended, open conformation, and (2) opening compounds induce LIBS epitopes on αIIbβ3 receptors on platelets whereas closing ones do not and, moreover, can actually suppress LIBS exposure.
Springer and colleagues’ findings are likely to stimulate new research to repurpose existing closing integrin receptor antagonists and develop new ones that utilize the mechanism they have elegantly defined. Other approaches to developing integrin antagonists that do not induce the active conformation have included identifying compounds that only bind to αIIb, making compounds that displace the MIDAS metal ion, and making compounds that prevent the movement of the β1-α1 loop toward the MIDAS by interacting with the 122-Tyr on the loop (Ley et al., 2016). Still lacking, however, is clinical evidence that closing or conformationally neutral integrin antagonists will improve patient outcomes. This paper brings us closer to testing that important hypothesis.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant HL19278 and National Center for Advancing Translational Sciences (NCATS) grant UL1TR001866, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
Footnotes
DECLARATION OF INTERESTS
The author is an inventor of the integrin αIIbβ3 antagonist antiplatelet drugs abciximab and zalunfiban (RUC-4). In accord with federal law and the policies of the Research Foundation of the State University of New York, he received royalties on the sale of abciximab. In accord with federal law and the policies of the Rockefeller University, he has a royalty interest in zalunfiban, which is in advanced phase clinical testing. He is also a founder of CeleCor Therapeutics, Inc., which is developing zalunfiban, as well as an equity owner and scientific advisor to CeleCor. Zalunfiban is one of the compounds studied by Lin et al.
REFERENCES
- Aster RH (2005). Immune thrombocytopenia caused by glycoprotein IIb/IIIa inhibitors. Chest 127, 53S–59S. [DOI] [PubMed] [Google Scholar]
- Bosch X, Marrugat J, and Sanchis J (2013). Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. Cochrane Database Syst. Rev 11, CD002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D (2004). Oral GPIIb/IIIa antagonists: what went wrong? Curr Pharm Des 10, 1587–1596. [DOI] [PubMed] [Google Scholar]
- Du XP, Plow EF, Frelinger AL III, O’Toole TE, Loftus JC, and Ginsberg MH (1991). Ligands "activate" integrin αIIbβ3 (platelet GPIIb-IIIa). Cell 65, 409–416. [DOI] [PubMed] [Google Scholar]
- Eng ET, Smagghe BJ, Walz T, and Springer TA (2011). Intact alphaIIbbeta3 integrin is extended after activation as measured by solution X-ray scattering and electron microscopy. J. Biol. Chem 286, 35218–35226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger AL III, Cohen I, Plow EF, Smith MA, Roberts J, Lam SC, and Ginsberg MH (1990). Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J. Biol. Chem 265, 6346–6352. [PubMed] [Google Scholar]
- Ley K, Rivera-Nieves J, Sandborn WJ, and Shattil S (2016). Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat. Rev. Drug Discov 15, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Vootukuri S, Shang Y, Negri A, Jiang JK, Nedelman MA, Diacovo TG, Filizola M, Thomas CJ, and Coller BS (2014). RUC-4: A novel alphaIIbbeta3 antagonist for pre-hospital therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol 34, 2321–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F-Y, Li J, Xie Y, Zhu J, Nguyen TTH, Zhang Y, Zhu J, and Springer TA (2022). A general chemical principle for creating closure-stabilizing integrin inhibitors. Cell 185, 3533–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, and Ruoslahti E (1984). Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33. [DOI] [PubMed] [Google Scholar]
- Springer TA, Zhu J, and Xiao T (2008). Structural basis for distinctive recognition of fibrinogen gammaC peptide by the platelet integrin alphaIIbbeta3. J. Cell Biol 182, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Coller BS, Wang J, and Springer TA (2004). Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]