Abstract
Despite the high prevalence of cutaneous infections, little is known about the role of host immune responsiveness during Staphylococcus aureus dermatitis. We have recently described a murine model of infectious dermatitis induced by superantigen-producing S. aureus. To assess the role of neutrophils in staphylococcal dermatitis, mice were given granulocyte-depleting monoclonal antibody prior to and on several occasions following intracutaneous inoculation with staphylococci. The granulocyte-depleted mice that had been intradermally inoculated with S. aureus developed crusted ulcerations which tended not to heal, whereas animals injected with control monoclonal antibody displayed only minor and transient skin lesions. The finding of severe ulcerations in neutropenic mice correlated with a significantly higher burden of bacteria in the blood and skin during the early phase of the infection. Importantly, while mice with an intact granulocyte population showed only limited skin infection, bacteremia occurred in the great majority of the neutrophil-depleted animals. As a consequence, the latter individuals exhibited significantly increased levels of the proinflammatory cytokine interleukin-6 and specific antibodies to staphylococcal cell wall components and toxic shock syndrome toxin-1 in the serum. Our data point to a crucial protective role of granulocytes in S. aureus dermatitis.
Neutrophils are host immune defense cells that are among the first to migrate into the skin in response to invading pathogens. These cells respond to chemotactic signals present at the site of infection. Among the roles played by neutrophils in inflammatory and immune responses are phagocytosis and killing of bacteria via the generation of reactive oxygen intermediates and the release of lytic enzymes stored in granules. In a recently described model of infectious dermatitis induced by the toxic shock syndrome toxin-1 (TSST-1)-producing Staphylococcus aureus strain LS-1 (12), healthy mice inoculated with high doses of staphylococci (108 CFU) displayed clinical and histopathological signs of local infection and inflammation within 48 h but lacked clinical or bacteriological signs of sepsis. The aim of this study was to evaluate the role of neutrophils in the induction, progression, and outcome of infectious dermatitis induced by intradermal injection of TSST-1-producing S. aureus. Our results show that depletion of neutrophil granulocytes both increases the severity of the skin lesion and gives rise to generalized infection, including bacteremia. These data demonstrate a crucial protective role for neutrophil granulocytes in S. aureus dermatitis.
MATERIALS AND METHODS
Mice.
Male BALB/c mice, 5 to 6 weeks old, were purchased from B&K Universal AB (Sollentuna, Sweden). They were housed in the animal facility of the Department of Rheumatology, University of Göteborg, under standard conditions of light and temperature and fed standard laboratory chow and water ad libitum.
Bacterial strain.
The BALB/c mice were inoculated intracutaneously with S. aureus strain LS-1, which is harbored naturally on the skin of many strains of mice (2). Strain LS-1 has been shown to produce large amounts of TSST-1 and is coagulase and catalase positive. The bacteria were kept frozen at −20°C in 5% bovine serum albumin and 10% dimethylsulfoxide (C2H6OS) in phosphate-buffered saline (PBS), pH 7.4, until they were used. Before use, the bacterial solution was thawed and washed in PBS. Viable counts were used to check the number of live bacteria in each bacterial solution.
Experimental protocol.
Two experiments were performed with four groups of BALB/c mice with 15 or 16 mice per group. The mice were inoculated intracutaneously with bacteria on the shaved back during a neurolept analgesia (Dormicum-Hypnorm). In experiment I, mice were inoculated with 0.1 ml of saline containing either 107 or 108 CFU of S. aureus. In experiment II, mice were inoculated with either 107 or 106 CFU of S. aureus. The mice were given intraperitoneal injections of either 1 mg of granulocyte-depleting monoclonal antibody (MAb) RB6-8C5 or 1 mg of rat immunoglobulin G (IgG) anti-ovalbumin MAb as a control 2 h before intracutaneous injection with bacteria. Intraperitoneal injection of the MAbs was repeated on days 3, 7, and 10. All mice were monitored individually and sacrificed by cervical dislocation at either 6, 24, or 48 h or 1 or 2 weeks after inoculation with bacteria. The mice were clinically evaluated for local inflammatory reaction, signs of sepsis, and weight development. In experiment I, skin as well as blood samples were obtained for bacterial analysis. Blood samples were also analyzed for granulocyte counts and total white blood cell counts. In experiment II, skin samples corresponding to the injection sites were dissected for histopathological evaluation at the indicated time intervals. Blood samples were obtained and analyzed for levels of interleukin-6 (IL-6), immunoglobulins (IgG1, IgG2a, IgG3, and IgM), and specific antibodies to staphylococcal cell walls and to TSST-1 (see below).
Production and purification of MAbs.
MAb RB6-8C5 is a rat IgG2b antibody that selectively binds to and depletes mature mouse neutrophils and eosinophils. The hybridoma cells secreting RB6-8C5 were a kind gift from R. Coffman, DNAX Research Institute, Palo Alto, Calif. The hybridoma cells were expanded in Iscove's medium (Gibco, Paisley, United Kingdom) supplemented with 5% heat-inactivated fetal calf serum (Seralab, Crawley Down, United Kingdom), 50 μg of gentamicin/ml, 2 mM l-glutamine, and 5 × 10−5 M β-mercaptoethanol. The cells were grown to maximal density, and the immunoglobulin fraction was precipitated with 50% saturated ammonium sulfate, dialyzed against PBS, and filter sterilized. The concentration of immunoglobulin fraction was determined by the radial immunodiffusion method (10). As a control, immunoglobulin class-matched anti-ovalbumin MAbs were used (kindly provided by E. Telemo, Department of Clinical Immunology, University of Göteborg, Sweden).
Histopathological examination.
Skin samples from 63 mice were examined histopathologically after routine fixation and staining with hematoxylin-eosin. Blinded microscopic evaluation was done to characterize the inflammatory infiltrates.
Bacterial culture.
Skin and blood samples from 63 mice were used for bacterial analysis. After disinfection with 70% alcohol, skin samples corresponding to the injection sites were deposited in sterile plastic bags, homogenized, and suspended in 10 ml of PBS. The skin suspensions were plated in appropriate dilutions on agar plates containing 5% horse blood and incubated at 37°C for 24 h. The number of CFU per skin sample and per 100 μl of blood were determined, and the bacterial colonies were tested for coagulase and catalase activity.
Serological analyses. (i) IL-6 assay.
Cell line B13.29, which is dependent on IL-6 for growth, has been described previously (1, 6, 8). For IL-6 determinations, the more sensitive subclone B9 was used. B9 cells were harvested from tissue culture flasks, seeded into microtiter plates (Nunc, Roskilde, Denmark) at a concentration of 5,000 per well, and cultured in Iscove's medium supplemented with 5 × 10−5 M 2-mercaptoethanol, 10% fetal calf serum, gentamycin (50 μg/ml), and l-glutamine, and serum samples were added. [3H] thymidine was added after 68 h of culturing, and the cells were harvested 4 h later. Each sample was tested in twofold dilutions and compared with a recombinant IL-6 standard. B9 cells were previously shown not to react with several recombinant cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-5, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor alpha, and gamma interferon. There was only weak reactivity with IL-4 (6).
(ii) Immunoglobulins.
Total levels of IgG1, IgG2a, IgG3, and IgM in serum were measured by the radial immunodiffusion technique (10). Antisera and immunoglobulin standards specific for IgG1, IgG2a, IgG3, and IgM were purchased from Sigma.
(iii) Antibodies to cell walls of S. aureus.
Levels in serum of IgG and IgM antibodies specific for S. aureus cell wall constituents were estimated by an enzyme-linked immunosorbent assay using poly-l-lysine (25 μg/ml) to precoat the wells and 100 μl of whole, formalin-treated (4%; 20 min) S. aureus LS-1 cells (108/ml) to coat the wells. All sera were serially diluted in 0.5% PBS-bovine serum albumin and incubated in wells. To measure the level and class specificity of anti-cell-wall antibodies bound to the solid phase, affinity-purified and biotinylated F(ab′)2 fragments of goat anti-mouse IgG and IgM (Jackson Laboratories) diluted 1:3,000 in PBS-Tween 20 were added to the wells, followed stepwise by 0.5 μg of extravidin-horseradish peroxidase (Sigma)/ml and 2.5 mg of the enzyme substrate 2,2-azino-bis-(3-ethylbenzothiazoline sulfonic acid) (Sigma)/ml in citrate buffer (pH 4.2) containing 0.0075% H2O2. The A414 was measured in a Spectra Max Plus spectraphotometer (Molecular Devices). All optical density values were converted to antigen-specific arbitrary units with calibration curves based on the optical density values obtained from serial dilutions of a reference pool of sera. The calibration curves were constructed with a computer program based on weighted logit-log models (9, 14).
(iv) Anti-TSST-1 antibodies.
Serum levels of IgG antibodies to TSST-1 were estimated by an enzyme-linked immunosorbent assay using 0.5 μg of highly purified TSST-1 (Toxin Technology, Sarasota, Fla.)/ml as a solid-phase coating. The subsequent steps were similar to those described above.
Hematological analysis.
Total white blood cell counts were determined in a hemacytometer (Toa Medical Electronics, Kobe, Japan). Blood smears were prepared and stained by the May-Grunewald-Giemsa method for differential counts. In addition, flow cytometry (Becton Dickinson) was used to identify the granulocyte population.
Phagocytosis assay.
For determination of phagocytic activity, uptake of fluorescein isothiocyanate (FITC)-labeled bacteria by granulocytes in peripheral blood was examined using a test kit (Phagotest; Orpegen Pharma, Heidelberg, Germany). In brief, freshly drawn heparinized whole blood was incubated with the S. aureus LS-1 strain labeled with FITC (5) for 10 min at 37°C. The samples were then placed on ice to stop phagocytosis. The samples were treated with quenching solution to suppress fluorescence of the bacteria attached to the host cell membrane. The percentage of granulocytes showing phagocytosis (ingestion of one or more bacteria per cell) and the phagocytic activity (fluorescence intensity per cell) were determined by flow cytometry. At each time interval (day 2 and day 7), peripheral blood leukocytes were obtained from five mice pretreated with either granulocyte-depleting MAb (n = 10) or control antibody (n = 10).
Statistical evaluation.
The differences between mean values were tested for significance using the nonparametric Mann-Whitney U test.
RESULTS
Clinical evaluation.
The granulocyte-depleted mice inoculated with 108 CFU of S. aureus developed within 7 days crusted ulcerations at the inoculation sites which gradually increased in size, reaching maximal size at day 10. In contrast, mice treated with control MAb displayed less marked skin abnormalities with minor crusted ulcerations that tended to reach peak size after 7 days and resolved thereafter. The granulocyte-depleted mice showed significant weight loss compared to the controls (P < 0.0002). Two days after antibody treatment and inoculation with 108 CFU of S. aureus, the granulocyte-depleted mice had lost 13% of their original weight (P < 0.0001), whereas those in the control group inoculated with the same dose of S. aureus gained weight from day 1 onward. Granulocyte-depleted mice inoculated with 108 CFU of S. aureus never regained their original weight (Fig. 1). In contrast, mice inoculated with 107 CFU of S. aureus gained weight 14 days after bacterial inoculation (Fig. 1). Mice receiving 106 CFU of S. aureus showed minor ulcerations after 7 days which tended to resolve in both groups thereafter, with no clinical signs of inflammation in the control group after 14 days.
FIG. 1.
Changes in body weight following intracutaneous inoculation with S. aureus and repeated treatment with either anti-neutrophilic (anti-neutr) MAb RB6-8C5 or control anti-ovalbumin (anti-OVA) MAb. Each group consisted of 15 or 16 mice at the start of the experiment. At 6, 24, and 48 h, as well as at days 7 and 10, three or four mice in each group were killed and serum and skin samples were obtained for further analyses. All values represent means ± standard errors of the mean.
Microscopic evaluation.
In granulocyte-depleted mice, microscopic evaluation of the affected skin in those inoculated with 107 CFU of S. aureus revealed bacterial foci in the deep dermis and around the superficial musculature with surrounding edema and a sparse, diffuse infiltrate of predominantly mononuclear cells at 6 h and 1 and 2 days after bacterial inoculation. Ulcerating necrotic areas involving the whole dermis were prominent 2 days after bacterial inoculation. The inflammatory component was most evident 1 week after inoculation, with a rich infiltrate of mononuclear as well as polymorphonuclear cells surrounding ulcerated, deep necrotic areas with underlying panniculitis. Declining numbers of polymorphonuclear cells were seen 2 weeks after inoculation. In contrast, the affected skin of control mice inoculated with staphylococci displayed a heavy inflammatory infiltrate consisting of both mononuclear and polymorphonuclear cells within 6 h after inoculation and a sparse inflammatory infiltrate consisting mainly of mononuclear cells at later times. Bacterial foci were evident at all time intervals in the neutrophil-depleted mice in contrast to control mice, where no visible bacterial foci were noted at 1 or 2 weeks after bacterial inoculation. In mice receiving 106 CFU of S. aureus, the findings in the granulocyte-depleted group were comparable to those in mice receiving the higher dose of S. aureus, whereas the control mice inoculated with 106 CFU of S. aureus displayed only sparse inflammation at all time intervals tested.
Bacterial cultures.
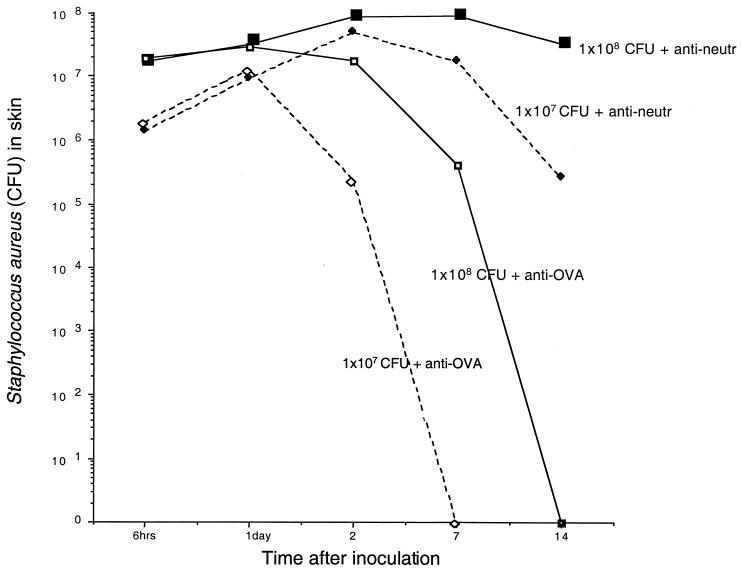
Bacterial cultures from the skins of mice in the granulocyte-depleted group revealed high numbers of catalase- and coagulase-positive bacteria at all time intervals in contrast to control mice, where no bacteria were found after 7 (107 CFU) or 14 (108 CFU) days. In all mice, an initial rise in the number of recovered bacteria, compared to the inoculation dose, was noted (Fig. 2). Blood samples showed growth of S. aureus in the neutrophil-depleted group after 6 h (one of six mice) and after 48 h (five of six mice). In contrast, control mice inoculated with S. aureus and injected with irrelevant MAb did not display bacterial growth in blood at any time interval.
FIG. 2.
Numbers of S. aureus in skin samples recovered at defined time intervals (n = 3 or 4 for each time period and inoculation) after initial intracutaneous inoculation with either 108 or 107 CFU of S. aureus and repeated treatment with either anti-neutrophilic (anti-neutr) MAb or control anti-ovalbumin (anti-OVA) MAb. All the values represent means.
Serological analyses.
(i) IL-6. Serum IL-6 levels in granulocyte-depleted animals were significantly higher at 24 and 48 h after bacterial inoculation with either 107 or 106 CFU of S. aureus (P < 0.05) compared to controls. For example, in neutrophil-depleted mice inoculated with 107 CFU of S. aureus, IL-6 peaked at levels exceeding 12,000 pg/ml 1 day after inoculation with bacteria whereas anti-ovalbumin MAb-pretreated mice showed a minor increase, with peak values around 500 pg/ml 6 h after bacterial inoculation (Fig. 3). Similar results were obtained in mice inoculated with 106 CFU of S. aureus (results not shown).
FIG. 3.
Serum IL-6 levels (pg/ml) at specified time intervals following intracutaneous inoculation with 107 CFU of S. aureus and repeated treatment with either anti-neutrophilic (anti-neutr) MAb or control anti-ovalbumin (anti-OVA) MAb. SEM, standard error of the mean; w, week; n.s., not significant.
(ii) B-lymphocyte responses.
Since IL-6 is an efficient B-lymphocyte-stimulating factor, we decided to analyze the levels in serum of immunoglobulins and specific antibodies to staphylococcal components. Serum IgG1 levels were increased 40-fold (16 g/liter) 2 weeks after inoculation of bacteria in neutrophil-depleted mice, as well as in the controls. Serum IgG2a levels were significantly higher (P < 0.05) compared to controls, displaying a threefold increase (1 g/liter) in neutrophil-depleted mice receiving 107 CFU of S. aureus. Serum IgG3 levels were increased twofold in neutrophil-depleted mice receiving 107 CFU of S. aureus (not significant) and increased somewhat less in mice receiving 106 CFU of S. aureus (P < 0.05) compared to the controls. IgM levels were not significantly different among the groups (results not shown).
Levels of S. aureus cell wall-specific antibodies of the IgG class in serum were significantly increased 2 weeks after bacterial inoculation, with a 200-fold increase in mice inoculated with 106 CFU of S. aureus. However, there were no significant differences between experimental and control groups. The levels of IgM anti-S. aureus antibodies showed a slight but not significant increase 2 weeks after bacterial inoculation (data not shown).
A significant increase in serum levels of IgG class-specific antibodies for TSST-1 was noted in all groups, but there were no significant differences between experimental and control groups (data not shown).
Hematological analysis.
Analysis of blood smears showed that the MAb RB6-8C5 depletes granulocytes to a significant degree. Thus, 48 h after bacterial inoculation with 108 CFU of S. aureus, the blood smears from the mice pretreated with anti-granulocyte MAb exhibited a significantly lower (P < 0.05) frequency of granulocytes than did those from the control mice (Table 1). Interestingly, when blood smears and the total blood cell count were analyzed 1 and 2 weeks after pretreatment with MAb RB6-8C5 in the group of mice receiving 108 CFU, the levels of circulating granulocytes in the RB6-8C5-treated group were significantly higher than those in the control group.
TABLE 1.
Percentage of circulating granulocytes in BALB/c micea
| Treatment | No. of CFU | % Granulocytes (± SEM) after:
|
||||
|---|---|---|---|---|---|---|
| 6 h (n = 3) | 24 h (n = 3) | 48 h (n = 3) | 1 week (n = 3) | 2 weeks (n = 4) | ||
| RB6-8C5 | 108 | 10.0 ± 1.2 | 12.3 ± 5.4 | 4.7 ± 1.7 | 64.3 ± 2.0 | 59.0 ± 6.5 |
| Rat IgG | 108 | 53.0 ± 2.3 | 32.7 ± 7.3 | 44.0 ± 4.0 | 29.3 ± 2.4 | 31.2 ± 4.1 |
| RB6-8C5 | 107 | 14.0 ± 5.0 | 10.7 ± 3.5 | 6.3 ± 3.0 | 56.7 ± 1.3 | 63.3 ± 2.3 |
| Rat IgG | 107 | 55.3 ± 8.7 | 46.0 ± 9.7 | 40.3 ± 5.0 | 20.7 ± 3.2 | 29.0 ± 3.6 |
After intracutaneous inoculation with S. aureus and repeated treatment with either MAb RB6-8C5 or control rat IgG. The granulocytes were monitored using differential counts on blood smears stained with May-Grunewald-Giemsa stain.
Phagocytosis test.
The ability of neutrophilic granulocytes to phagocytize FITC-labeled S. aureus was not decreased in mice treated with MAb RB6-8C5 compared to the control mice. The total number of neutrophils, as well as the number of phagocytizing neutrophils, was increased after repeated inoculation with MAb RB6-8C5 compared to that of the control (Table 2).
TABLE 2.
Quantification of phagocytic activity of granulocytesa
| Treatment | No. of PMNC (P)
|
No. of phagocytizing PMNC (P)
|
Fluorescence intensity (P)
|
|||
|---|---|---|---|---|---|---|
| 2 days | 7 days | 2 days | 7 days | 2 days | 7 days | |
| RB6-8C5 | 7.2 ± 1.4 (<0.02) | 239.4 ± 74 (<0.009) | 0.4 ± 0.1 (<0.02) | 58.8 ± 9 (<0.009) | 563 ± 23 (<0.014) | 218 ± 10 (NS)b |
| Rat IgG | 81 ± 16.5 | 64.4 ± 5.5 | 22.8 ± 4.4 | 18.4 ± 2.9 | 273 ± 16.8 | 224 ± 13 |
Phagocytic activity of granulocytes in heparinized whole blood of noninfected mice 2 days after a single inoculation and 7 days after repeated inoculations with either MAb RB6-8C5 (n = 10) or rat IgG (n = 10). The number of leukocytes was evaluated with a hemacytometer, and flow cytometry was used to calculate the proportion of polymorphonuclear cells (PMNC). The values are given as 107 PMNC/liter ± standard error of the mean.
NS, not significant.
DISCUSSION
The major aim of the present study was to assess the role of neutrophil granulocytes during S. aureus-triggered dermatitis. Neutrophils are known to be important effector cells in the defense against bacteria and are rapidly recruited to infected sites, both by chemotactic factors generated by the bacteria themselves and by inflammatory mediators generated at the site of infection. Once there, they phagocytize and kill the invaders (4). Although essential to host defense, neutrophils also display tissue-destructive properties through several independent mechanisms, including extracellular release of microbicidal products of the NADPH oxidase system (11).
In this study, depletion of neutrophils was accomplished via injection of MAb RB6-8C5, directed against differentiation antigens on myeloid cells (7), resulting in substantial early neutropenia. The efficiency and selectivity of this procedure has recently been reported (15). At least 90% of peripheral blood granulocytes were depleted at 6, 24, and 48 h. Despite the fact that the granulocyte-depleting MAb was administered repeatedly every third or fourth day, the levels of circulating granulocytes in the RB6-8C5-treated group increased significantly in the later stages of the experiment. This finding was reported earlier (15) and may be due to formation of neutralizing anti-idiotypic antibodies to RB6-8C5.
Notably, whereas bacteremia is not detectable when staphylococci are intradermally injected in immunocompetent mice, granulocyte depletion led to the appearance of bacteria in the bloodstream. The finding of blood-borne bacteria after intracutaneous administration of staphylococci occurred typically between 6 and 48 h. The clearance of bacteria in the RB6-8C5-treated mice was defective due to the lack of phagocytizing neutrophils, and this resulted in a peak bacterial burden in the skin after 48 h and significantly increased numbers of bacteria in skin samples 2 weeks later. The cutaneous infection in the neutrophil-depleted mice was more extensive, with deep necrotizing ulcers, and persisted longer than that in control mice. This outcome might have been due to increased release of tissue-destructive bacterial products.
In addition, the granulocyte-depleted mice exhibited generalized signs of inflammation as monitored by raised levels of the proinflammatory cytokine IL-6, which was triggered early and produced in large amounts during the first 2 days after bacterial inoculation. IL-6 is produced by macrophages and T lymphocytes and is known to promote B-cell differentiation. Indeed, serum IgG2 and IgG3 levels were significantly increased 2 weeks after bacterial inoculation in the mouse group receiving granulocyte-depleting antibody compared to those in the controls. Elevated production of proinflammatory cytokines, including IL-6, has previously been reported to correlate with the severity of the infectious process (13) and has also been correlated with increased mortality rates both in patients and in animal models of sepsis (3).
The lack of mortality and lower morbidity associated with bacteria administered intradermally compared to the rates with intravenous administration reflect the ability of the skin to act as an efficient barrier against invading pathogens. However, as demonstrated by the present study, resident mononuclear phagocytes (i.e., macrophages) alone are not sufficiently effective to clear invading bacteria. The recruitment of neutrophils and phagocytosis in the initial stages of S. aureus infection are critical for the outcome of staphylococcal infection in the skin. Interestingly, a later increase in the number of neutrophilic granulocytes does not seem to be effective with regard to bacterial clearance. The phagocytosis test was performed in order to measure the phagocytic activity of the large number of neutrophils appearing after treatment with granulocyte-depleting antibody RB6-8C5 as a rebound phenomenon (Table 2). Impaired phagocytic activity was not seen in this group compared to the controls, and diminished phagocytosis is probably not the cause of the high bacterial load at later times. Changes in the local microenvironment, including tissue necrosis, may be important. It is, however, more likely that the initial deficiency of neutrophils allows the staphylococci to expand in situ to high numbers, leading to an uncontrollable course of infection, and thus, despite adequate numbers of neutrophils at later time intervals, the skin infection continues.
ACKNOWLEDGMENTS
We thank Lena Svensson and Ing-Marie Jousson for excellent technical assistance.
This study was supported by grants from the Göteborg Medical Society, Swedish Medical Research Council, the A. M. E. Wolff Foundation, the Welander Foundation, and the University of Göteborg (LUA).
REFERENCES
- 1.Aarden L A, De Groot E R, Schaap O L, Lansdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Bremell T, Lange S, Svensson L, Jennische E, Grondahl K, Carlsten H, Tarkowski A. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990;33:1739–1744. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- 3.Brouckaert P, Fiers W. Tumor necrosis factor and the systemic inflammatory response syndrome. Curr Top Microbiol Immunol. 1996;216:167–187. doi: 10.1007/978-3-642-80186-0_8. [DOI] [PubMed] [Google Scholar]
- 4.Campbell P A. The neutrophil, a professional killer of bacteria, may be controlled by T cells. Clin Exp Immunol. 1990;79:141–143. doi: 10.1111/j.1365-2249.1990.tb05169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentry M J, Snitily M U, Preheim L C. Phagocytosis of Streptococcus pneumoniae measured in vitro and in vivo in a rat model of carbon tetrachloride-induced liver cirrhosis. J Infect Dis. 1995;171:350–355. doi: 10.1093/infdis/171.2.350. [DOI] [PubMed] [Google Scholar]
- 6.Helle M, Boeije L, Aarden L A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- 7.Hestdal K, Ruscetti F W, Ihle J N, Jacobsen S E, Dubois C M, Kopp W C, Longo D L, Keller J R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 8.Lansdorp P M, Aarden L A, Calafat J, Zeiljemaker W P. A growth-factor dependent B-cell hybridoma. Curr Top Microbiol Immunol. 1986;132:105–113. doi: 10.1007/978-3-642-71562-4_14. [DOI] [PubMed] [Google Scholar]
- 9.Lue C, Tarkowski A, Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J Immunol. 1988;140:3793–3800. [PubMed] [Google Scholar]
- 10.Mancini G, Carbonara A O, Heremans J F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 11.McCord J M. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987;46:2402–2406. [PubMed] [Google Scholar]
- 12.Molne L, Tarkowski A. An experimental model of cutaneous infection induced by superantigen-producing Staphylococcus aureus. J Investig Dermatol. 2000;114:1120–1125. doi: 10.1046/j.1523-1747.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- 13.Rozalska B, Wadström T. Interferon-γ, interleukin-1 and tumor necrosis factor-α synthesis during experimental murine staphylococcal infection. FEMS Immunol Med Microbiol. 1993;7:145–152. doi: 10.1111/j.1574-695X.1993.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 14.Russell M W, Brown T A, Radl J, Haaijman J J, Mestecky J. Assay of human IgA subclass antibodies in serum and secretions by means of monoclonal antibodies. J Immunol Methods. 1986;87:87–93. doi: 10.1016/0022-1759(86)90347-9. [DOI] [PubMed] [Google Scholar]
- 15.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]