ABSTRACT
Two-component system (TCS) plays a vital role in modulating target gene expression in response to the changing environments. Pseudomonas aeruginosa is a ubiquitous opportunistic pathogen that can survive under diverse stress conditions. The great adaptability of P. aeruginosa relies heavily on the abundant TCSs encoded by its genome. However, most TCSs in P. aeruginosa have not been well-characterized. CzcS/CzcR is a metal responsive TCS which displays multiple regulatory functions associated with metal hemostasis, quorum sensing activity and antibiotic resistance. In this study, we found that swimming motility of P. aeruginosa was completely abolished during zinc (Zn2+) stress when the czcR gene from the TCS CzcS/CzcR was deleted. Noticeably, CzcR was dispensable for swimming without the stress of Zn2+ excess. CzcR was shown to be activated by Zn2+ stress possibly through inducing its expression level and triggering its phosphorylation to positively regulate swimming which was abolished by Zn2+ stress in a CzcR-independent manner. Further TEM analyses and promoter activity examinations revealed that CzcR was required for the expression of genes involved in flagellar biosynthesis during Zn2+ stress. In vitro protein-DNA interaction assay showed that CzcR was capable of specifically recognizing and binding to the promoters of operons flgBCDE, flgFGHIJK, and PA1442/FliMNOPQR/flhB. Together, this study demonstrated a novel function of CzcR in regulating flagellar gene expression and motility in P. aeruginosa when the pathogen encounters Zn2+ stress conditions.
IMPORTANCE The fitness of bacterial cells depends largely on their ability to sense and respond quickly to the changing environments. P. aeruginosa expresses a great number of signal sensing and transduction systems that enable the pathogen to grow and survive under diverse stress conditions and cause serious infections at different sites in many hosts. In addition to the previously characterized functions to regulate metal homeostasis, quorum sensing activity, and antibiotic resistance, here we report that CzcR is a novel regulator essential for flagellar gene expression and swimming motility in P. aeruginosa during Zn2+ stress. Since swimming motility is important for the virulence of P. aeruginosa, findings in this study might provide a new target for the treatment of P. aeruginosa infections with Zn2+-based antimicrobial agents in the future.
KEYWORDS: Pseudomonas aeruginosa, TCS, CzcR, swimming, zinc stress
INTRODUCTION
Bacteria frequently encounter a wide variety of challenges from ever-changing environmental stresses such as the shift of temperature, pH, metal concentration, nutrient availability, and antimicrobial threat. Rapid reprograming of gene expression profiles and adapting to various environmental stresses are crucial for bacteria to survive, which relies on the cooperation of abundant regulatory systems and complicated signal transduction networks in the cell (1). Among them, two-component system (TCS) represents one of the most ubiquitous mechanisms employed by bacteria to sense environmental stresses and control physiological behaviors accordingly (2). An increasing number of studies have reported that many TCSs are indispensable for the expression of genes associated with virulence and antibiotic resistance (3, 4). Thus, TCSs have been recognized as a group of important targets for antimicrobial therapies (5).
A typical TCS is composed of a membrane-bound sensor histidine kinase (HK) that detects specific signals and a cognate response regulator (RR) which regulates downstream target gene expression (6). A typical HK is a homodimeric integral membrane protein that contains two transmembrane (TM) helices flanking a sensor domain in the periplasmic space and one of the TM helices connected a HAMP (commonly found in Histidine kinases, Adenylyl cyclases, Methyl-accepting chemotaxis proteins, and Phosphatases) domain, a DHp (dimerization and histidine phosphorylation) domain, and a C-terminal CA (catalytic and ATP-binding) domain in the cytosol (7). RR generally contains a REC receiver domain and a linked effector domain (8). When the HK detects a specific environmental stimulus, signal transduction occurs through the ATP-dependent phosphorylation of a histidine (H) residue in the DHp domain by the CA domain. Subsequently, the REC domain in RR catalyzes the transfer of the phosphoryl group from the HK to its aspartate (D) residues, triggering the conformational changes of RR, and leading to activation of the linked effector domain to modulate downstream gene expression (6).
Pseudomonas aeruginosa is a ubiquitous environmental bacterium commonly found in soil and water (9). It is also an opportunistic human pathogen that can produce numerous virulence factors to cause nosocomial acute and chronic infections (10). Genome analysis indicated that P. aeruginosa encodes abundant TCSs. There are at least 64 HKs and 72 RRs expressed by the PAO1 genome (11, 12). Although a lot of them remain uncharacterized, studies have shown the importance of TCSs in regulating many cellular behaviors related to its pathogenicity and antibiotic resistance in P. aeruginosa in response to diverse environmental cues (4, 13). For instance, PhoR/PhoB senses inorganic phosphate to regulate the production of quorum-sensing-controlled virulence factors such as pyocyanin (PYO) (14). CbrA/CbrB senses various carbon sources and modulates motility, biofilm formation, and antibiotic resistance (15).
Zinc (Zn2+) is the second most abundant transition metal ion in bacteria and serves as an important cofactor in many enzymatic reactions while excessive Zn2+ is cytotoxic. P. aeruginosa has developed a delicate Zn2+ homeostatic network to support its growth under Zn2+-depleted and high Zn2+ conditions by coordinating the processes of Zn2+ uptake, storage, and extrusion (16). CzcS/CzcR was previously identified as a Zn2+-responsive TCS, which plays an essential role in maintaining homeostasis and elevating tolerance of Zn2+ and other metal ions such as cadmium and cobalt by upregulating the expression of the efflux system CzcCBA (17–19). The response regulator CzcR in P. aeruginosa was also found to repress the expression of OprD, which is a porin for the entry of carbapenem antibiotics and therefore induces the recalcitrance of P. aeruginosa to these antibiotics (17, 20). Moreover, CzcR was reported to mediate the repression of PYO production and biofilm formation in the presence of excessive Zn2+ or ZnO nanoparticles (21). Further experiments demonstrated that CzcR modulates quorum sensing and antibiotic resistance by directly binding with the promoters of genes such as lasI, phzA1, and oprD (20). These findings exhibited the versatility of CzcS/CzcR to control both virulence and antibiotic resistance in P. aeruginosa. However, it remains unexplored whether CzcS/CzcR plays additional regulatory roles and participates in other physiological processes.
Flagellum-dependent swimming motility is known as an important virulence factor in P. aeruginosa for the pathogen to rapidly disseminate from initial infection sites to different tissues and establish biofilms to cause life-threatening infections (22–26). Studies have revealed that P. aeruginosa has complicated regulatory systems, including several TCSs such as PilS/PilR and FleS/FleR, to modulate flagellar biosynthesis and swimming motility (27, 28). In the present study, we showed that CzcS/CzcR is essential for flagellar gene expression and swimming motility in P. aeruginosa during Zn2+ stress. Deletion of czcR in P. aeruginosa completely inactivates its swimming motility during Zn2+ stress. Findings in this study not only expanded our understanding in the regulon of CzcS/CzcR in P. aeruginosa but also provided implications for antipseudomonal therapies by targeting this TCS.
RESULTS
CzcR regulated PYO production and swimming motility in the presence of Zn2+ stress.
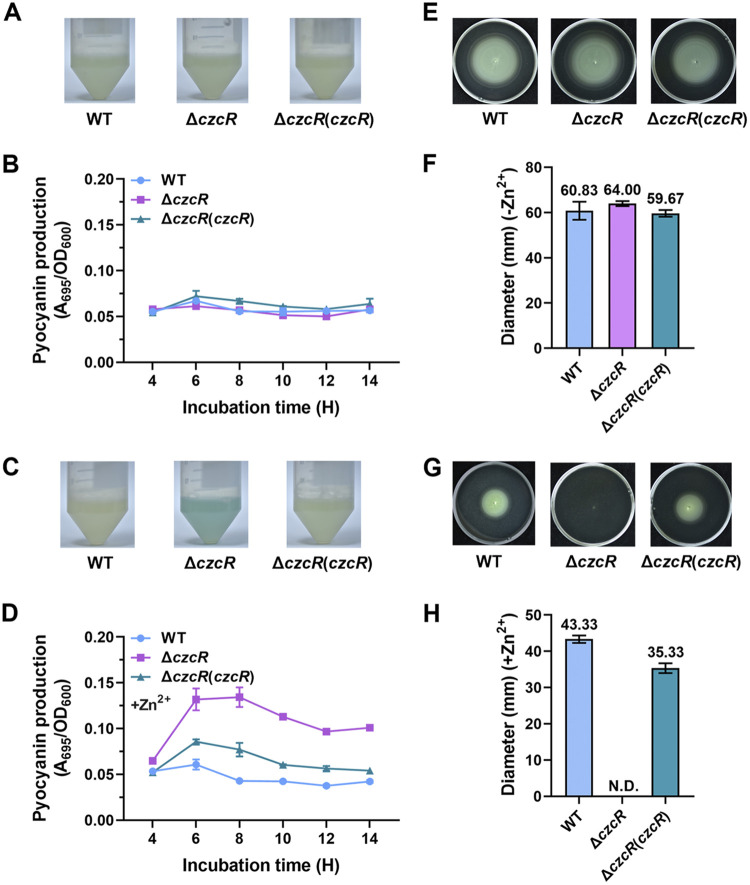
To explore physiological functions of the TCS CzcS/CzcR, we deleted the czcR gene and attempted to detect the phenotypical differences between the ΔczcR mutant and the PAO1 wild-type (WT) strain. Because PYO is a kind of blue/green pigment, its production level can be easily evaluated with the color of the liquid culture. However, in contrast to the previous report that deletion of the czcR gene in PAO1 led to a substantial accumulation of PYO (20), we did not observe any difference between the WT and ΔczcR strains when they were cultured in the liquid LB medium (Fig. 1A). PYO quantifications for the WT strain, ΔczcR mutant and the ΔczcR mutant complemented with czcR at different time points during growth in the liquid medium did not show any difference in PYO production as well (Fig. 1B). The discrepant results led us to speculate that CzcR in the PAO1 WT strain was inactive because these two strains were cultured in normal LB medium without supplementing any inducible elements for this TCS system. We then treated these strains with a stressed concentration (0.5 mM) of ZnSO4 (Zn2+). A slight decrease of PYO production was observed in the PAO1 WT strain when it was grown in the presence of Zn2+ stress compared to the absence of Zn2+ stress (Fig. 1A and C), which was in accordance with the previous study showing the inhibition of PYO production by ZnO nanoparticles (21). Interestingly, in the presence of Zn2+ stress, we observed a substantial accumulation of the blue/green-colored PYO in the medium of the ΔczcR strain, which was greatly higher than that in the WT strain and could be reduced nearly back to the WT level when the mutant was complemented with czcR (Fig. 1C). PYO accumulation in the ΔczcR strain was not observed by supplementing Na2SO4 instead of ZnSO4 (Fig. S1A), confirming that it was Zn2+ that induced the production of PYO in CzcR-independent pathways while concurrently Zn2+ activated CzcR to antagonize the induction of PYO biosynthesis. Further quantifications of PYO at different time points during cell growth showed that deletion of czcR resulted in a substantially increased PYO production after 6-h incubation compared to the WT strain (Fig. 1D).
FIG 1.
CzcR negatively regulates PYO biosynthesis and positively regulates swimming motility in the presence of Zn2+ stress. (A) Liquid culture of the strains of PAO1 WT, ΔczcR, and ΔczcR with complemented expression of czcR (ΔczcR[czcR]) when they were grown without the supplementation of Zn2+. (B) Quantification of PYO production at different time points during growth without the supplementation of Zn2+ in PAO1 WT, ΔczcR, and ΔczcR(czcR) strains. (C) Liquid culture of the PAO1 WT, ΔczcR, and ΔczcR(czcR) strains grown with the supplementation of 0.5 mM Zn2+. (D) Quantification of PYO production at different time points during growth with the supplementation of 0.5 mM Zn2+ in PAO1 WT, ΔczcR, and ΔczcR(czcR) strains. (E) Examination of swimming motility of the PAO1 WT, ΔczcR, and ΔczcR(czcR) strains on the plates without the supplementation of Zn2+. (F) Diameters of the swimming zone of the PAO1 WT, ΔczcR, and ΔczcR(czcR) strains grown without the supplementation of Zn2+. (G) Examination of swimming motility of the PAO1 WT, ΔczcR, and ΔczcR(czcR) strains on the plates with the supplementation of 0.5 mM Zn2+. (H) Diameters of the swimming zone of the PAO1 WT, ΔczcR, and ΔczcR(czcR) strains grown with the supplementation of 0.5 mM Zn2+. N.D., not detected.
In addition to the liquid culture, we also noticed an obvious difference in the colony morphology between PAO1 WT and ΔczcR strains when they were streaked on the solid agar plates. As with the PYO production, no difference of the colony phenotypes was observed between the WT and ΔczcR strains without supplementation of Zn2+ in the agar plates, while, interestingly, most colonies of the ΔczcR strain became smoother than the WT strain when 0.5 mM Zn2+ was supplemented in the agar plates (Fig. S2A). This was more evident when the colony edge was enlarged under a stereo microscope (Fig. S2B). It was reported that bacterial motility is different between smooth and rough colonies in other species (29). Thus, we suspected that CzcR might play a role in regulating motility besides its already known functions. To test this speculation, we examined the swimming motility of the PAO1 WT, ΔczcR, and ΔczcR(czcR) strains. As shown in Fig. 1E, still no difference was observed between these strains without the presence of Zn2+ stress with regard to their swimming motility. Although Zn2+ stress reduced swimming motility of the PAO1 WT strain by decreasing its swimming zone from 60.83 mm to 43.33 mm, swimming motility was completely abolished when czcR was deleted during Zn2+ stress and complementation of czcR restored the swimming motility of ΔczcR (Fig. 1E to H). As a control, Na2SO4 did not cause any difference in the swimming motility between PAO1 WT and ΔczcR strains (Fig. S1B). These results indicated that CzcR was essential for P. aeruginosa swimming during Zn2+ stress. As with the regulatory pattern of PYO production, it seemed that the swimming motility was inhibited by Zn2+ stress through CzcR-independent pathways, but CzcR was activated in the presence of Zn2+ stress to concurrently rescue the inhibited swimming motility. We further monitored the survival of the PAO1 WT and ΔczcR strains in the absence and presence of Zn2+ stress, respectively, to ensure that loss of CzcR did not influence cell viability. The result showed that cell growth on the agar plates containing Zn2+ was not influenced by the deletion of czcR (Fig. S3), excluding the possibility that swimming motility of the ΔczcR strain was abolished owing to its defective growth during Zn2+ stress. Since CzcR inhibits PYO production by repressing the quorum sensing system and the PYO biosynthetic gene cluster phz as previously reported (20), we next continued to focus on exploring how swimming motility was controlled by CzcR during Zn2+ stress.
D51 was a potential residue in CzcR for phosphorylation to regulate swimming motility.
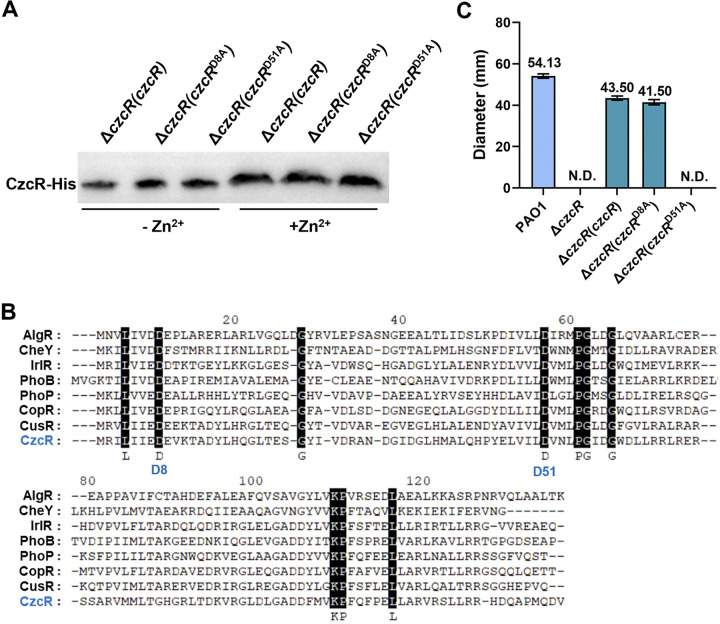
Because CzcR did not exhibit any regulatory function on PYO production and swimming motility in the absence of the inducible signal Zn2+, we speculated that CzcR requires Zn2+ to either promote its expression or induce its activity. Upregulated transcription of czcR by Zn2+ was previously reported (17). Here, we further demonstrated that presence of Zn2+ resulted in increased expression of CzcR at protein level by performing the Western blot assay (Fig. 2A). Given the signal transduction mode between the TCS pairs, we then attempted to investigate whether the regulatory function of the overexpressed CzcR to promote swimming motility was also associated with its phosphorylation status. Sequence alignment based on a set of well-characterized RRs using MEGA (version 11.0) led to the identification of two highly conserved aspartate residues, i.e., D8 and D51 (Fig. 2B) (30), which were potentially responsible for signal transduction by receiving the phosphoryl groups in CzcR. We therefore replaced these residues at D8 and D51 in the complemented CzcR with alanine (A) and tested the swimming motility of the ΔczcR mutant with these CzcR variants. It showed that replacement at the 8th position (D8A) did not affect the function of CzcR to promote swimming while replacement at the 51st position (D51A) completely prevented its activity to promote swimming without influencing the protein translation and stabilization (Fig. 2A and C). Consistent with this, D51 was the only aspartate residue detected in the conserved acidic pocket, which is the active site of RR containing the phosphorylatable aspartate residue using the ScanProsite tool (31). These results together suggested that the activity of CzcR was upregulated during Zn2+ stress and D51 was a key aspartate residue for CzcR to regulate swimming motility possibly serving as the residue for phosphorylation.
FIG 2.
Zn2+ induces the expression of CzcR and D51 in CzcR is a key residue to regulate swimming motility. (A) Translational levels of the complemented CzcR or its variants with D81A and D51A point mutations in the ΔczcR mutant grown in the absence or presence of Zn2+. (B) Sequence alignment of the receiver domain of CzcR with other TCS response regulators. Conserved amino acid residues were highlighted with black background and two conserved aspartate residues D8 and D51 were indicated. (C) Examination of swimming motility of the PAO1 WT strain, ΔczcR mutant, and the ΔczcR mutant complemented with czcR or its D8A and D51A variants grown on the plates with the supplementation of Zn2+. N.D., not detected.
CzcR activated the expression of genes involved in flagellar biosynthesis during Zn2+ stress.
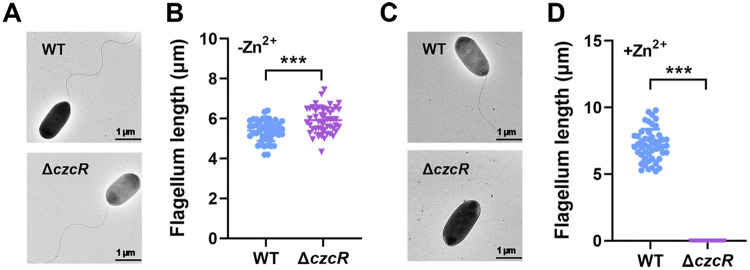
Given that swimming motility is a type of unicellular movement behavior in liquid or low-viscosity conditions that requires a functional flagellum (32), we first investigated whether deletion of czcR affected flagellar biosynthesis during Zn2+ stress. A transmission electron microscope (TEM) was applied to observe the flagellar morphology of the PAO1 WT and ΔczcR strains after they were cultured in the presence and absence of Zn2+ stress. TEM micrographs showed that the flagellar morphology of PAO1 WT and ΔczcR cells were similar but with a slight increase of the average flagellar length in the ΔczcR cells when they were grown in the absence of Zn2+ stress (Fig. 3A and B). However, in the presence of Zn2+ stress, flagella were not observed in the ΔczcR cells, whereas they were still intact in the PAO1 WT strain as shown in Fig. 3C and D. Consistent with the swimming pattern, this result demonstrated that flagellar biosynthesis was inhibited by Zn2+ stress in a CzcR-independent manner, and CzcR was induced and activated to stimulate flagellar biosynthesis.
FIG 3.
CzcR is required for flagellar biosynthesis during Zn2+ stress. (A) Observation of flagella in the PAO1 WT and ΔczcR strains grown in the absence of Zn2+ stress by TEM. Representative images for each sample were shown. (B) Flagellar length measured for the PAO1 WT and ΔczcR strains grown in the absence of Zn2+ stress. Fifty cells from each strain were selected for the measurement. (C) Observation of flagella in the PAO1 WT and ΔczcR strains grown in the presence of Zn2+ stress by TEM. (D) Flagellar length measured for the PAO1 WT and ΔczcR strains grown in the presence of Zn2+ stress. ***, P < 0.001 compared to WT based on Student's t test.
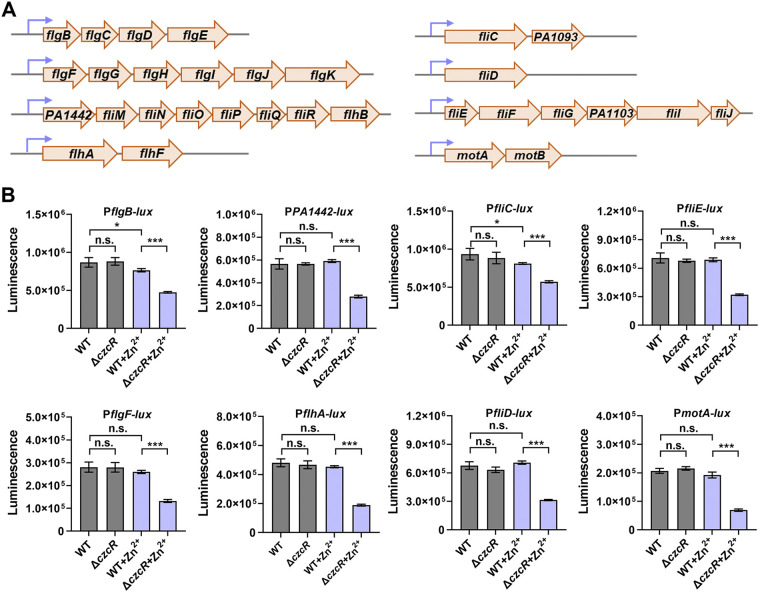
P. aeruginosa flagellum is composed of three elements, including a membrane complex, a hook, and a flagellin filament (33). These compositions are mainly transcribed from the operons or genes of flgBCDE, flgFGHIJKL, PA1442/FliMNOPQR/flhB, flhAF, fliC/PA1093, fliD, fliEFG/PA1103/fliIJ, and motAB based on the annotations in the Pseudomonas Genome Database (https://www.pseudomonas.com/) (Fig. 4A) (34). To measure the expression of these operons or genes, promoter-lux transcriptional fusions were constructed for all these promoters and luminescence reporter assay was performed to evaluate their activity. As shown in Fig. 4B, all the promoters, i.e., PflgBCDE (PflgB-lux), PflgFGHIJKL (PflgF-lux), PPA1442/FliMNOPQR/flhB (PPA1442-lux), PflhAF (PflhA-lux), PfliC/PA1093 (PfliC-lux), PfliD (PfliD-lux), PfliEFG/PA1103/fliIJ (PfliE-lux), and PmotAB (PmotA-lux), were significantly repressed with the deletion of czcR during Zn2+ stress, suggesting that the abolished swimming motility in ΔczcR during Zn2+ stress was associated with the repression of genes involved in flagellar biosynthesis. Consistent with the swimming pattern (Fig. 1E), deletion of czcR did not influence the transcriptional activity of these promoters in the absence of Zn2+ stress (Fig. 4B). These results implied that Zn2+ stress repressed the expression of genes involved in flagellar biosynthesis probably in a CzcR-independent manner, while CzcR was activated concurrently to rescue the expression of these genes. Notably, promoter activities of PflgBCDE and PfliC/PA1093 were slightly but significantly lower in the PAO1 WT strain when it was cultured during Zn2+ stress compared to that without Zn2+ stress, which suggested that CzcR-based activation of both promoters failed to fully antagonize the repression caused by Zn2+ with the CzcR-independent pathway. This result might also account for the inhibited swimming motility as observed in the PAO1 WT strain during Zn2+ stress (Fig. 1E and G).
FIG 4.
CzcR regulates the expression of genes involved in flagellar biosynthesis during Zn2+ stress. (A) Major genes or operons involved in flagellar biosynthesis in P. aeruginosa. (B) Promotor activities of genes or operons involved in flagellar biosynthesis in PAO1 WT and ΔczcR strains grown in the absence or presence of Zn2+ stress. n.s., not significant, *, P < 0.05, ***, P < 0.001 based on Student's t test.
CzcR regulated flagellar biosynthetic gene operons by directly interacting with some promoters.
Bacterial flagellar biosynthesis is controlled by numerous regulatory systems and the cyclic diguanylate (c-di-GMP) is a global bacterial second messenger that serves as a key modulator connecting flagellar motility to different regulatory pathways in P. aeruginosa and many other microbes (35–37). Since all the flagellar biosynthetic gene operons were activated by CzcR, we wondered whether flagellar gene expression and swimming motility regulated by CzcR in P. aeruginosa was mediated by the global regulatory signal c-di-GMP. To answer this, intracellular c-di-GMP contents in the PAO1 WT and ΔczcR strains were measured using LC-MS after they were grown in the absence or presence of Zn2+ stress. Unexpectedly, deletion of czcR did not alter the intracellular c-di-GMP content in both the presence and absence of Zn2+ stress (Fig. S4A). Consistently, deletion of czcR did not influence the expression of cdrA, whose expression was regarded as an indicator of c-di-GMP levels in P. aeruginosa (38, 39) (Fig. S4B). These results suggested that CzcR modulated flagellar gene expression and swimming motility of P. aeruginosa was independent of c-di-GMP.
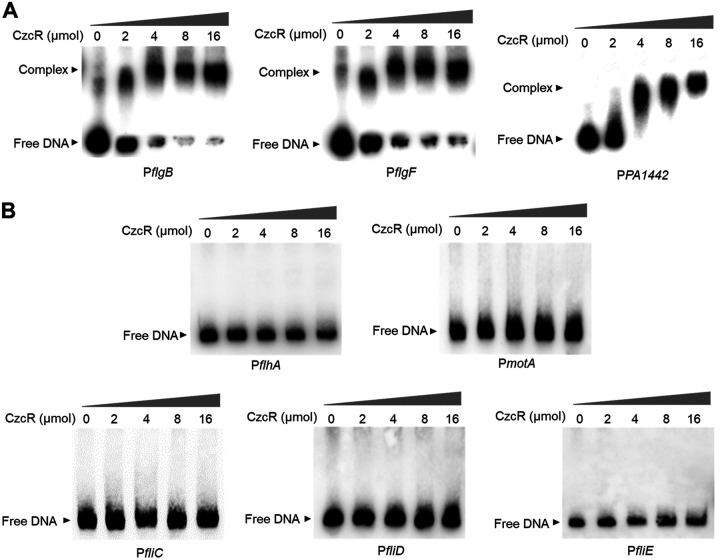
CzcR, a typical response regulator belonging to the TCS CzcS/CzcR, was predicted to contain a REC receiver domain and a linked DNA-binding Trans_reg_C domain (40). Because it was previously demonstrated to regulate PYO production by directly binding to the promoter of the phzA1 gene (20), we then moved to explore if CzcR could recognize the flagellar biosynthetic gene promoters as well and regulate their expression directly. After the His6-tagged CzcR protein was purified, EMSA was performed to examine the binding potentials of CzcR with the promoters of flagellar biosynthetic genes and operons which included flgBCDE (PflgB), flgFGHIJKL (PflgF), PA1442/FliMNOPQR/flhB (PPA1442), flhAF (PflhA), fliC/PA1093 (PfliC), fliD (PfliD), fliEFG/PA1103/fliIJ (PfliE), and motAB (PmotA). As shown in Fig. 5A, CzcR was found to be capable of binding with the promoter sequences of flgBCDE (PflgB), flgFGHIJKL (PflgF), and PA1442/FliMNOPQR/flhB (PPA1442). As a negative control, using an irrelevant 126-bp DNA sequence from the recA gene did not show a CzcR-DNA complex (Fig. S5), meaning that the purified CzcR protein could specifically recognize the above promoter sequences. However, no interaction was observed between CzcR and the promoters of flhAF (PflhA), fliC/PA1093 (PfliC), fliD (PfliD), fliEFG/PA1103/fliIJ (PfliE), and motAB (PmotA) (Fig. 5B). These results indicated that CzcR could regulate the expression of operons flgBCDE, flgFGHIJKL, and PA1442/FliMNOPQR/flhB by directly interacting with their promoters but other flagellar biosynthetic genes or operons indirectly might through additional regulators.
FIG 5.
CzcR specifically binds to the promoters of operons flgBCDE, flgFGHIJKL, PA1442/FliMNOPQR/flhB. (A) EMSA examination showing the binding of CzcR to the promoters of flgBCDE (PflgB), flgFGHIJKL (PflgF), and PA1442/FliMNOPQR/flhB (PPA1442). (B) EMSA examination showing the incapable binding of CzcR to the promoters of flhAF (PflhA), fliC/PA1093 (PfliC), fliD (PfliD), fliEFG/PA1103/fliIJ (PfliE), and motAB (PmotA).
DISCUSSION
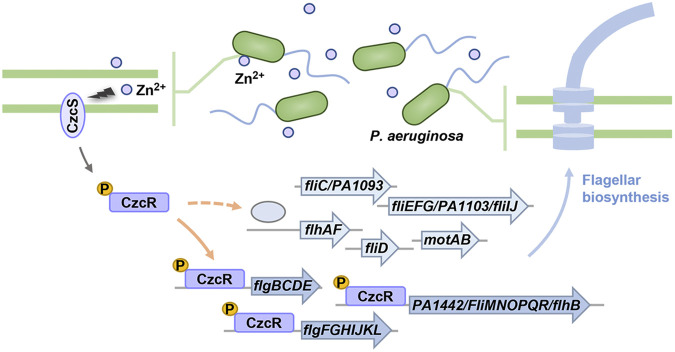
TCSs are an important group of regulatory systems in P. aeruginosa, which are employed by the pathogen to sense environmental changes and respond accordingly to maintain their ordinary growth and control production of virulence factors. However, the regulatory mechanisms and physiological roles of most TCSs are still unexplored. Characterization of these systems will facilitate the development of targeted antimicrobial strategies. CzcS/CzcR is a metal-responsive system that has been demonstrated to play important roles in modulating not only the intracellular metal homeostasis but also antibiotic resistance and quorum sensing activity (17, 20). In this study, we further reported that CzcR in this system is induced and activated during Zn2+ stress to upregulate expression of genes associated with flagellar biosynthesis and promote swimming motility in P. aeruginosa (Fig. 6). Indeed, it seemed that CzcR was induced and activated by Zn2+ to rescue the swimming motility that was abolished during Zn2+ stress in P. aeruginosa with unknown mechanisms.
FIG 6.
A schematic diagram illustrating the regulation of the Zn2+-responsive TCS CzcS/CzcR on swimming motility in P. aeruginosa. The CzcR protein is upregulated and phosphorylated to activate the expression of genes involved in flagellar biosynthesis directly (solid orange arrow) by interacting with their promoters or indirectly (dashed orange arrow) through other unknown regulators, which is essential for flagellar biosynthesis and swimming motility of P. aeruginosa during Zn2+ stress.
We confirmed the role of CzcR in reducing PYO production, but loss of CzcR did not change the level of PYO production in the absence of Zn2+. Noticeably, unchanged PYO production between the PAO1 WT and ΔczcR strains was not in accordance with the previous study which reported that ΔczcR could produce increased PYO production even in the absence of any inducible signals, including Zn2+ (20). Several possibilities might result in this discrepancy. For example, this might be caused by the different growth conditions or different compositions of the culture media (41). In addition, variations in the genetic background of the PAO1 strains could be another possible factor leading to the discrepant regulatory patterns because dramatic genetic and phenotypic diversity was reported among PAO1 sublines even from the same laboratory (42). Although CzcR was activated by Zn2+ to repress PYO production, it was also noted that Zn2+ could significantly induce the PYO production without the presence of CzcR. Unfortunately, how Zn2+ directly or indirectly induce PYO biosynthesis remains unclear.
P. aeruginosa is a motile microorganism that is mainly driven by its polar monotrichous flagellum or type IV pili (43, 44). The swimming motility of P. aeruginosa is controlled by a number of regulatory pathways such as the signaling cascade Gac/Rsm, chemotaxis system Chp, chemosensory pathway Wsp, and the HptB pathway (45–47). Increasing studies have shown that swimming motility regulated in P. aeruginosa is broadly mediated by the global second messenger c-di-GMP (48, 49). Here, we reported that CzcR is a novel regulator which is essential for the PAO1 strain to swim during Zn2+ stress. It was further shown that swimming motility regulated by CzcR did not rely on the signaling molecule c-di-GMP and CzcR was able to induce the expression of genes or operons involved in flagellar biosynthesis during Zn2+ stress by directly interacting with some promoters or indirectly through other mediators. Indirect regulation of CzcR on these genes or operons, including flhAF (PflhA), fliC/PA1093 (PfliC), fliD (PfliD), fliEFG/PA1103/fliIJ (PfliE), and motAB (PmotA), might be mediated by other flagellar biosynthesis-associated regulators such as FleN and FliA owing that strong interaction of CzcR with the promoter of the operon containing fleN and fliA genes was observed (Fig. S6). Nonetheless, more detailed mechanisms await investigations.
The fluctuating concentration of transition metals is a common stress to pathogens, including P. aeruginosa, during infection (50). Metal ions such as iron, copper, and Zn2+ are indispensable for many biological processes in bacteria but are also detrimental to bacterial cells when in excess (51). Thus, overloading or limiting transition metals at the host-pathogen interface is a common strategy of innate immunity in human and many other mammalian hosts to combat bacterial infections (52, 53). Regarding to Zn2+, its accumulation within macrophages or infection sites at different tissues serves as an important line of host defense against bacterial infections (54–58). Zn2+ is an important transition metal ion that is associated with numerous biological processes in all kingdoms of life. Although it is known that a large percentage of proteins in bacterial cells require Zn2+ as their structural or catalytic factor (59), excessive Zn2+ exhibits toxicity by frequently competing with other metals and causing mismetallation of metalloproteins in bacteria. In the present study, we further showed that Zn2+ stress can inhibit expression of P. aeruginosa flagellar genes and completely abolish its swimming motility, which is known as an important virulence factor when CzcR is absent. This study indicated that the TCS CzcS/CzcR could be a promising target for precise prevention of the dissemination of P. aeruginosa using Zn2+-based antimicrobial agents in the future.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and growth conditions.
Bacterial strains and plasmids used in this study are summarized in Table S1. Primers used in this study are summarized in Table S2. LB Broth Base and LB Agar (Invitrogen) were used to prepare medium for bacterial culture. Antibiotics were supplemented in the medium when necessary: tetracycline, 15 μg/mL; gentamicin, 20 μg/mL; kanamycin, 50 μg/mL; ampicillin, 100 μg/mL for E. coli DH5α, and tetracycline, 50 μg/mL; gentamicin, 50 μg/mL for P. aeruginosa PAO1.
Construction of PAO1 mutants and gene complementation.
A SacB-based counterselection method was employed to knockout genes in PAO1 (60). In brief, about 600 to 1,000-bp upstream and downstream sequences flanking the target gene were amplified and cloned into the BamHI and HindIII sites of pK18mobsacB, yielding a pK18-gene plasmid for gene knockout. The pK18-gene plasmid was then delivered into P. aeruginosa PAO1 via triparental mating with the help of the E. coli pRK2013 strain. Recovered colonies were selected on LB agar plates containing 10% sucrose. Mutants were confirmed by colony PCR and sanger sequencing. Complementation assay for czcR was achieved by the mini-Tn7 system (61). Briefly, sequence of the czcR coding region with a His tag and its native promoter was amplified from the genomic DNA of PAO1 and cloned into the BamHI and HindIII sites located on the pUC18T-mini-Tn7T-Gm plasmid, generating the pTn7-czcR plasmid. pTn7-czcR was then integrated into PAO1 genome via coelectroporation with the helper plasmid pTNS2.
Construction of promoter-lux reporter strains and measurement of promoter activity.
Mini-CTX-lux system was used to measure promoter activities (62). Promoter regions of target genes were amplified from PAO1 genome and cloned into the KpnI and BamHI sites on the mini-CTX-lux plasmid. The resultant plasmids were integrated into PAO1 genome by biparental mating. Luminescence was measured to indicate the activity of corresponding promoters. Briefly, overnight culture of each strain carrying the promoter-lux fusions was adjusted to OD600 of 1.0 and then 1:100 diluted into 2 mL fresh LB medium with or without the supplementation of 0.5 mM ZnSO4. Luminescence was monitored after 8 h growth at 37°C in a microplate reader (BioTek).
Pyocyanin production assays.
Pyocyanin production measurement was performed as previously described (63). The overnight culture was adjusted to the OD600 of 1.0 and then 1:100 diluted into 2 mL fresh LB medium with or without the supplementation of 0.5 mM ZnSO4. A 1.5 mL culture was collected every 2 h during growth and centrifuged at 13,000 rpm for 2 min. A 1 mL supernatant was transferred to a 24-well plate and the absorbance at A695 was measured in a microplate reader (BioTek).
Swimming motility assay.
The plates used for swimming assay was prepared as follows: 10 g/L bacto-peptone, 5 g/L NaCl with 0.2% (wt/vol) bacto-agar. The overnight culture was adjusted to the OD600 of 1.0 and 2 μL cell culture was inoculated on the center of the swimming plates with or without the supplementation of 0.5 mM ZnSO4. The plates were incubated at 37°C for 14 h and diameters of the swimming zones were measured.
RNA extraction and quantitative real-time PCR.
Overnight culture of PAO1 strains was 1:100 diluted into 2-mL fresh LB medium with or without the supplementation of 0.5 mM ZnSO4 and grown for 8 h. One mL bacterial culture was harvested, and total RNA were extracted using the RNA extraction kit (Omega) following the manufacturer’s instructions. cDNA was reverse transcribed using the TranScript one-step gDNA removal and cDNA Synthesis kit (TransGen Biotech). qPCR was performed using the 2 × SYBR green Master Mix Reagent (Yeasen) in an ABI QuantStudioTM6 Flex system (Applied Biosystems). The recA gene was selected as the internal reference control and the 2-ΔΔCt method was used to calculate the relative expression of the target genes (64). The result was displayed as the mean of biological triplicates.
Western blotting.
Overnight culture of the ΔczcR mutants with complementation of His6-tagged czcR or czcR variants (D8A and D51A) was 1:100 diluted into 5-mL fresh LB medium and subcultured with or without the supplementation of 0.5 mM ZnSO4 until OD600 was 1.0. Approximately 2.0 × 109 cells were harvested and lysed by 40 μL xTractor Buffer (TaKaRa) supplemented with protease inhibitor. After centrifugation, supernatant was mixed with SDS-loading buffer. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes by electroblotting. Membranes were blocked with Blocking buffer (5% nonfat milk PBST) followed by immunoblotting using anti-His6 antibody (Abcam) and horseradish peroxidase-conjugated goat anti-rabbit antibody (TransGen Biotech). Proteins were detected using the ECL kit (Bio-Rad) according to the manufacturer’s protocol.
Transmission electron microscopy.
P. aeruginosa PAO1 WT and ΔczcR strains were harvested after they were cultured on the center of agar plates (10 g/L bacto-peptone, 5 g/L NaCl, 0.5% [wt/vol] bacto-agar) with or without the supplementation of 0.5 mM ZnSO4. Cells were spread in a drop of MilliQ H2O and covered with Parlodion (Mallinckrodt) carbon-coated grid (300 mesh). Then, the cells were fixed with 0.5% uranyl acetate. Samples were examined by the Field Emission Transmission Electron Microscopy (Talos F200S) and flagellar length was measured by Image J (version: 1.52v).
Protein purification and electrophoretic gel mobility shift assay (EMSA).
Protein purification was performed as previously described with slight modifications (65). The czcR coding region was amplified from the PAO1 genome and cloned into the BamHI and XhoI sites on the pET28a vector, generating pET28a-czcR. The plasmid was transformed into E. coli BL21(DE3) and the strain containing pET28a-czcR was incubated until OD600 of 0.6~0.8. Expression of the His6-tagged CzcR was induced with 0.5 mM isopropyl-β-d-thiogalactoside (IPTG) at 18°C for 16 h. Purification of the His6-tagged CzcR protein was conducted using a Ni2+-affinity column. For EMSA, promoter sequences were amplified by PCR using the primers in Table S2 from the PAO1 genome and tagged with biotin using a DNA labeling kit (Thermo Scientific). The EMSA kit (Thermo Scientific) was used to test the interaction of CzcR with promoter sequences according to the manufacturer's instructions.
c-di-GMP measurement.
Quantifications of c-di-GMP in P. aeruginosa strains was performed as described previously with modifications (37). In brief, P. aeruginosa overnight culture was 1:100 diluted and incubated in LB medium containing 0.5 mM ZnSO4 or not for 8 h. One mL cell culture was harvested, and cells were lysed with perchloric acid (70% vol/vol). After neutralization with 2.5 M KHCO3, supernatants were collected for c-di-GMP measurement by liquid chromatography-mass spectrometry (LC-MS). LC-MS was performed using a Q Exactive Focus Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific) with a 1.8 μm, 100 × 2.1 mm high-strength silica (HSS) T3 column (Waters). The m/z 691 > 248 transition was used for quantification.
Statistical analysis.
Experimental data were analyzed by either Student's t test or one-way analysis of variance (ANOVA) as indicated in the figure legends using GraphPad Prism software (version 8). Differences with P < 0.05 were considered statistically significant. *, **, and *** indicated P < 0.05, P < 0.01, and P < 0.001, respectively.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 32100020), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515010194), Guangdong Forestry Science and Technology Innovation Project (2018KJCX009, 2020KJCX009), and the Key Realm R&D Program of Guangdong Province (2020B020209001).
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Lian-Hui Zhang, Email: lhzhang01@scau.edu.cn.
Zeling Xu, Email: zelingxu@scau.edu.cn.
Beile Gao, South China Sea Institute of Oceanology.
REFERENCES
- 1.Bleuven C, Landry CR. 2016. Molecular and cellular bases of adaptation to a changing environment in microorganisms. Proc R Soc B 283:20161458. doi: 10.1098/rspb.2016.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groisman EA. 2016. Feedback control of two-component regulatory systems. Annu Rev Microbiol 70:103–124. doi: 10.1146/annurev-micro-102215-095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beier D, Gross R. 2006. Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol 9:143–152. doi: 10.1016/j.mib.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Bhagirath AY, Li Y, Patidar R, Yerex K, Ma X, Kumar A, Duan K. 2019. Two component regulatory systems and antibiotic resistance in Gram-negative pathogens. Int J Mol Sci 20:1781. doi: 10.3390/ijms20071781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiwari S, Jamal SB, Hassan SS, Carvalho PVSD, Almeida S, Barh D, Ghosh P, Silva A, Castro TLP, Azevedo V. 2017. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: an overview. Front Microbiol 8:1878. doi: 10.3389/fmicb.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casino P, Rubio V, Marina A. 2010. The mechanism of signal transduction by two-component systems. Curr Opin Struct Biol 20:763–771. doi: 10.1016/j.sbi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Jacob-Dubuisson F, Mechaly A, Betton J-M, Antoine R. 2018. Structural insights into the signalling mechanisms of two-component systems. Nat Rev Microbiol 16:585–593. doi: 10.1038/s41579-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 8.Gao R, Bouillet S, Stock AM. 2019. Structural basis of response regulator function. Annu Rev Microbiol 73:175–197. doi: 10.1146/annurev-micro-020518-115931. [DOI] [PubMed] [Google Scholar]
- 9.Crone S, Vives-Flórez M, Kvich L, Saunders AM, Malone M, Nicolaisen MH, Martínez-García E, Rojas-Acosta C, Catalina Gomez-Puerto M, Calum H, Whiteley M, Kolter R, Bjarnsholt T. 2020. The environmental occurrence of Pseudomonas aeruginosa. APMIS 128:220–231. doi: 10.1111/apm.13010. [DOI] [PubMed] [Google Scholar]
- 10.Gellatly SL, Hancock REW. 2013. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 11.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FSL, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GKS, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock REW, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 12.Gooderham WJ, Hancock REW. 2009. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol Rev 33:279–294. doi: 10.1111/j.1574-6976.2008.00135.x. [DOI] [PubMed] [Google Scholar]
- 13.Francis VI, Stevenson EC, Porter SL. 2017. Two-component systems required for virulence in Pseudomonas aeruginosa. FEMS Microbiology Lett 364:fnx104. doi: 10.1093/femsle/fnx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng X, Ahator Stephen D, Zhang LH. 2020. Molecular Mmechanisms of phosphate stress activation of Pseudomonas aeruginosa quorum sensing systems. mSphere 5:e00119-20. doi: 10.1128/mSphere.00119-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung AT, Bains M, Hancock RE. 2011. The sensor kinase CbrA is a global regulator that modulates metabolism, virulence, and antibiotic resistance in Pseudomonas aeruginosa. J Bacteriol 193:918–931. doi: 10.1128/JB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez MR, Ducret V, Leoni S, Perron K. 2019. Pseudomonas aeruginosa zinc homeostasis: key issues for an opportunistic pathogen. Biochim Biophys Acta Gene Regul Mech 1862:722–733. doi: 10.1016/j.bbagrm.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Perron K, Caille O, Rossier C, van Delden C, Dumas J-L, Köhler T. 2004. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J Biol Chem 279:8761–8768. doi: 10.1074/jbc.M312080200. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Chen W, Huang S, He Y, Liu X, Hu Q, Wei T, Sang H, Gan J, Chen H. 2017. Structural basis of Zn(II) induced metal detoxification and antibiotic resistance by histidine kinase CzcS in Pseudomonas aeruginosa. PLoS Pathog 13:e1006533. doi: 10.1371/journal.ppat.1006533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducret V, Abdou M, Goncalves Milho C, Leoni S, Martin–Pelaud O, Sandoz A, Segovia Campos I, Tercier-Waeber M-L, Valentini M, Perron K. 2021. Global analysis of the zinc homeostasis network in Pseudomonas aeruginosa and its gene expression dynamics. Front Microbiol 12:739988. doi: 10.3389/fmicb.2021.739988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieppois G, Ducret V, Caille O, Perron K. 2012. The transcriptional regulator CzcR modulates antibiotic resistance and quorum sensing in Pseudomonas aeruginosa. PLoS One 7:e38148. doi: 10.1371/journal.pone.0038148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J-H, Kim Y-G, Cho MH, Lee J. 2014. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol Res 169:888–896. doi: 10.1016/j.micres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun 66:43–51. doi: 10.1128/IAI.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.Kühn MJ, Talà L, Inclan YF, Patino R, Pierrat X, Vos I, Al-Mayyah Z, Macmillan H, Negrete J, Engel JN, Persat A. 2021. Mechanotaxis directs Pseudomonas aeruginosa twitching motility. Proc Natl Acad Sci USA 118:e2101759118. doi: 10.1073/pnas.2101759118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josenhans C, Suerbaum S. 2002. The role of motility as a virulence factor in bacteria. Int J Med Microbiol 291:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 26.Lyczak JB, Cannon CL, Pier GB. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 27.Kilmury SL, Burrows LL. 2018. The Pseudomonas aeruginosa PilSR two-component system regulates both twitching and swimming motilities. mBio 9:e01310-18. doi: 10.1128/mBio.01310-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou T, Huang J, Liu Z, Xu Z, Zhang L. 2021. Molecular mechanisms underlying the regulation of biofilm formation and swimming motility by FleS/FleR in Pseudomonas aeruginosa. Front Microbiol 12:707711. doi: 10.3389/fmicb.2021.707711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett EM, Sekulovic O, Wetzel D, Jones JB, Edwards AN, Vargas-Cuebas G, McBride SM, Tamayo R. 2019. Phase variation of a signal transduction system controls Clostridioides difficile colony morphology, motility, and virulence. PLoS Biol 17:e3000379. doi: 10.1371/journal.pbio.3000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Stecher G, Kumar S. 2021. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. 2006. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res 34:W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad JC, Gibiansky ML, Jin F, Gordon VD, Motto DA, Mathewson MA, Stopka WG, Zelasko DC, Shrout JD, Wong GC. 2011. Flagella and pili-mediated near-surface single-cell motility mechanisms in P aeruginosa. Biophys J 100:1608–1616. doi: 10.1016/j.bpj.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouteiller M, Dupont C, Bourigault Y, Latour X, Barbey C, Konto-Ghiorghi Y, Merieau A. 2021. Pseudomonas flagella: generalities and Specificities. Int J Mol Sci 22:3337. doi: 10.3390/ijms22073337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman Fiona SL. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. 2010. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Fang X, Gomelsky M. 2010. A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol Microbiol 76:1295–1305. doi: 10.1111/j.1365-2958.2010.07179.x. [DOI] [PubMed] [Google Scholar]
- 37.Lin S, Chen S, Li L, Cao H, Li T, Hu M, Liao L, Zhang LH, Xu Z. 2022. Genome characterization of a uropathogenic Pseudomonas aeruginosa isolate PA_HN002 with cyclic di-GMP-dependent hyper-biofilm production. Front Cell Infect Microbiol 12:956445. doi: 10.3389/fcimb.2022.956445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borlee BR, Goldman AD, Murakami K, Samudrala R, Wozniak DJ, Parsek MR. 2010. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol Microbiol 75:827–842. doi: 10.1111/j.1365-2958.2009.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moscoso JA, Jaeger T, Valentini M, Hui K, Jenal U, Filloux A. 2014. The diguanylate cyclase SadC is a central player in Gac/Rsm-mediated biofilm formation in Pseudomonas aeruginosa. J Bacteriol 196:4081–4088. doi: 10.1128/JB.01850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letunic I, Khedkar S, Bork P. 2021. SMART: recent updates, new developments and status in 2020. Nucleic Acids Res 49:D458–D460. doi: 10.1093/nar/gkaa937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun S, Zhou L, Jin K, Jiang H, He YW. 2016. Quorum sensing systems differentially regulate the production of phenazine-1-carboxylic acid in the rhizobacterium Pseudomonas aeruginosa PA1201. Sci Rep 6:30352. doi: 10.1038/srep30352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Ahator SD, Wang H, Feng Q, Xu Y, Li C, Zhou X, Zhang LH. 2022. Microevolution of the mexT and lasR reinforces the bias of quorum sensing system in laboratory strains of Pseudomonas aeruginosa PAO1. Front Microbiol 13:821895. doi: 10.3389/fmicb.2022.821895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toutain CM, Zegans ME, O'Toole GA. 2005. Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. J Bacteriol 187:771–777. doi: 10.1128/JB.187.2.771-777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol 66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 45.Xin L, Zeng Y, Sheng S, Chea RA, Liu Q, Li HY, Yang L, Xu L, Chiam KH, Liang ZX. 2019. Regulation of flagellar motor switching by c-di-GMP phosphodiesterases in Pseudomonas aeruginosa. J Biol Chem 294:13789–13799. doi: 10.1074/jbc.RA119.009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfe AJ, Visick KL. 2008. Get the message out: cyclic-Di-GMP regulates multiple levels of flagellum-based motility. J Bacteriol 190:463–475. doi: 10.1128/JB.01418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter SL, Wadhams GH, Armitage JP. 2011. Signal processing in complex chemotaxis pathways. Nat Rev Microbiol 9:153–165. doi: 10.1038/nrmicro2505. [DOI] [PubMed] [Google Scholar]
- 48.Baker AE, Diepold A, Kuchma SL, Scott JE, Ha DG, Orazi G, Armitage JP, O'Toole GA, Silhavy TJ. 2016. PilZ domain protein FlgZ mediates cyclic Di-GMP-dependent swarming motility control in Pseudomonas aeruginosa. J Bacteriol 198:1837–1846. doi: 10.1128/JB.00196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuchma SL, Delalez NJ, Filkins LM, Snavely EA, Armitage JP, O'Toole GA, Silhavy TJ. 2015. Cyclic Di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa PA14 requires the MotAB stator. J Bacteriol 197:420–430. doi: 10.1128/JB.02130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schalk IJ, Cunrath O. 2016. An overview of the biological metal uptake pathways in Pseudomonas aeruginosa. Environ Microbiol 18:3227–3246. doi: 10.1111/1462-2920.13525. [DOI] [PubMed] [Google Scholar]
- 51.Porcheron G, Garenaux A, Proulx J, Sabri M, Dozois C. 2013. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:90. doi: 10.3389/fcimb.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandrangsu P, Rensing C, Helmann JD. 2017. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 15:338–350. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haase H, Rink L. 2014. Multiple impacts of zinc on immune function. Metallomics 6:1175–1180. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 55.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. 2011. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ong C-l, Gillen CM, Barnett TC, Walker MJ, McEwan AG. 2014. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J Infect Dis 209:1500–1508. doi: 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- 57.Na-Phatthalung P, Min J, Wang F. 2021. Macrophage-mediated defensive mechanisms involving zinc homeostasis in bacterial infection. Infectious Microbes & Diseases 3:175–182. doi: 10.1097/IM9.0000000000000058. [DOI] [Google Scholar]
- 58.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. 2011. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andreini C, Banci L, Bertini I, Rosato A. 2006. Zinc through the three domains of life. J Proteome Res 5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- 60.Zhou T, Huang J, Liu Z, Lin Q, Xu Z, Zhang L-H, Alexandre G. 2022. The two-component system FleS/FleR represses H1-T6SS via cyclic di-GMP signaling in Pseudomonas aeruginosa. Appl Environ Microbiol 88:e01655-21. doi: 10.1128/AEM.01655-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi K-H, Schweizer HP. 2006. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 62.Hoang TT, Kutchma AJ, Becher A, Schweizer HP. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- 63.Fothergill JL, Panagea S, Hart CA, Walshaw MJ, Pitt TL, Winstanley C. 2007. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol 7:45. doi: 10.1186/1471-2180-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 65.Xu Z, Wang P, Wang H, Yu ZH, Au-Yeung HY, Hirayama T, Sun H, Yan A. 2019. Zinc excess increases cellular demand for iron and decreases tolerance to copper in Escherichia coli. J Biol Chem 294:16978–16991. doi: 10.1074/jbc.RA119.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.02846-22-s0001.pdf, PDF file, 0.4 MB (470.5KB, pdf)