Abstract
In several gram-negative bacterial pathogens, autoagglutination (AAG) activity is a marker for interaction with host cells and virulence. Campylobacter jejuni strains also show AAG, but this property varies considerably among strains. To examine the characteristics of C. jejuni AAG, we developed a quantitative in vitro assay. For strain 81-176, which shows high AAG, activity was optimal for cells grown for ≤24 h, was independent of growth temperature, and was best measured for cells suspended in phosphate-buffered saline at 25°C for 24 h. AAG activity was heat labile and was abolished by pronase or acid-glycine (pH 2.2) treatment but not by lipase, DNase, or sodium metaperiodate. Strain 4182 has low AAG activity, but extraction with water increased AAG, suggesting the loss of an inhibitor. Strain 6960 has weak AAG with no effect due to water extraction. Our study with clinical isolates suggests that C. jejuni strains may be grouped into three AAG phenotypes. A variant derived from strain 81116 that is flagellate but immotile showed the strong AAG exhibited by the parent strain, suggesting that motility per se is not necessary for the AAG activity. AAG correlated with both bacterial hydrophobicity and adherence to INT407 cells. Mutants which lack flagella (flaA, flaB, and flbA) or common cell surface antigen (peb1A) were constructed in strain 81-176 by natural transformation-mediated allelic exchange. Both AAG activity and bacterial hydrophobicity were abolished in the aflagellate mutants but not the peb1A mutant. In total, these findings indicate that C. jejuni AAG is highly associated with flagellar expression.
Campylobacter jejuni now is recognized as an important enteropathogenic bacterium of humans (51, 57). C. jejuni colonizes the intestinal tract of domesticated animals, and ingestion of contaminated water, milk, or meat products and direct contact with infected pets are considered the most important sources of infection in humans (3, 53). Although there have been many reports about virulence-related factors for C. jejuni such as toxin production (17, 28, 34, 45, 59), adherence to tissue (5, 8, 18, 29, 43), and invasion of tissue (19, 20, 22, 23, 38, 47, 60), their contribution to pathogenesis has not been clearly understood. Although tests for these virulence markers are available for presumptive determination of the pathogenic potential of C. jejuni isolates (20, 26, 33), as described for other enteropathogens, these tests often are difficult and expensive to perform and results cannot be immediately obtained in most cases. Despite the recent determination of the C. jejuni genomic sequence (41), simple and rapid methods for assessing pathogenicity are not yet available and are needed.
Autoagglutination (AAG) activity is known to be a marker of virulence in several gram-negative bacterial pathogens, including Vibrio cholerae (4), Bordetella pertussis (30), Neisseria gonorrhoeae (54), and Yersinia (25, 46) and Aeromonas (16, 21, 42) species. Pilins (4, 54) or outer membrane proteins (52) of these bacteria have been demonstrated to be autoagglutinins. AAG of C. jejuni has been previously described (3, 27, 39, 62) as a property preventing the determination of coaggulutination type, serotype, or lectin type; however, further characterization of this activity was not done. In the present study, we developed a quantitative assay system for AAG of C. jejuni and then examined the AAG characteristics of these organisms. We sought to develop a simple and reproducible system for measuring AAG, to examine the factors influencing AAG activity, and to determine whether AAG correlated with other virulence-associated phenotypes such as hydrophobicity (41) and the ability to adhere to intestinal cells (41). We also sought to determine the effects of flagellin on AAG activity and bacterial hydrophobicity by creating aflagellate and control mutants.
MATERIALS AND METHODS
Bacterial strains.
A total of 25 C. jejuni strains isolated from humans with campylobacteriosis were used in this study. Clinical isolate 81116 and its two variants, flagellate and immotile (F+ M−) and aflagellate and immotile (F− M−) (2), were included to examine the effect of flagellation and motility on AAG. The strains were isolated from patients in the United States, United Kingdom, or Japan and had been suspended in brucella broth (BBL Microbiology Systems, Cockeysville, Md.) containing 15% glycerol (Sigma Chemical Co., St. Louis, Mo.) and stored at −70°C until testing. For the AAG assays, thawed bacteria were cultured microaerobically (10% CO2, 5% O2, 85% N2) on Trypticase soy agar plus 5% sheep blood (TSAS) (BBL) at 37°C for 24 h, unless otherwise stated.
AAG assay.
The AAG assay involved suspending a standard inoculum of bacterial cells in an aqueous medium and assessing the optical density after incubation at a fixed temperature for a fixed time. The bacterial cells were harvested from TSAS plates with a sterile cotton swab into 10 mM phosphate-buffered saline (PBS) (pH 7.2), and the absorbance at 600 nm (A600) of the suspension was adjusted to approximately 1.0. PBS was initially used until the most appropriate assay solution was determined. The suspension (2 ml) was poured into sterile glass tubes (13 by 100 mm), and after a predetermined incubation period, 1.0 ml of the upper aqueous phase was carefully aspirated and the A600 was measured. Bacterial cells that strongly agglutinate do not remain in the aqueous phase, and the A600 diminishes. Each assay was done at least in quadruplicate for each experimental condition studied.
Effect of assay conditions on AAG.
To determine the optimal conditions for detecting AAG activity, C. jejuni strains 81-176 (1), 4182, and 6960 were used. The following conditions were varied, and the AAG activity was observed: culture temperature (37 and 42°C), culture age (18 to 72 h), in vitro passage number on TSAS plates (one to four times), observation period (6 to 48 h), observation temperature (4 to 37°C), and diluents for the bacterial suspension (distilled water [DW], 10 mM phosphate buffer [PB] [pH 7.2], 10 mM PBS [pH 7.2], 1 mM MgCl2, 1 mM CaCl2, or both 1 mM MgCl2 and CaCl2).
Effect of physical and chemical treatments on AAG activity.
AAG was measured after the following physical and chemical treatment of cells. The bacterial suspension was heated at 65°C for 30 min and then cooled to 25°C. For treatment of C. jejuni with enzymes, the test cells were harvested and washed twice in DW. The A600 of the bacterial suspension was adjusted to 1.5, and the cells were incubated at 37°C for 2 h with rotation at 15 rpm with either DW alone or DW plus 1 mg of pronase (Boehringer GmbH, Mannheim, Germany) per ml, 10 U of lipase (Boehringer) per ml, or 1 mg of DNase I (Sigma) per ml and then washed twice in PBS. Alternatively, after being washed twice in DW, the bacterial cells were treated with 10 mM sodium metaperiodate (Sigma) in 50 mM sodium acetate (pH 4.5) at 25°C for 1 h in a dark room and then washed twice in PBS. Control bacteria were treated with 50 mM sodium acetate (pH 4.5) alone. Finally, C. jejuni cells were harvested in 0.2 M glycine buffer at either pH 2.2 or 4.0 and incubated with shaking for 30 min at 25°C, essentially as described by Kervella et al. (18), and then washed twice with PBS.
DW extraction.
To determine the effect of DW extraction on AAG activity, hydrophobicity, or adhesion to INT 407 cells, 24-h cultures of C. jejuni on five TSAS plates were harvested in 15 ml of PBS. After centrifugation at 8,000 × g for 10 min, the supernatant was decanted and the bacterial pellet was vortex mixed in 15 ml of DW for 30 s. This DW extraction was repeated two or three times, and the cells were assayed as above.
Hydrophobicity.
Hydrophobicity of C. jejuni cells was determined by a salting-out method as described by Honda et al. with slight modifications (15). Serial twofold dilutions of 4 M ammonium sulfate were made with 2 mM sodium phosphate, to reach a final ammonium sulfate concentration of 3.9 mM. The bacteria were suspended in 2 mM sodium phosphate, and A600 was adjusted to approximately 1.0. Equal volumes (25 μl) of bacterial suspension and ammonium sulfate solution were mixed in U-bottom 96-well microtiter plates (Costar, Cambridge, Mass.). The plates were incubated at 25°C for 18 h without shaking. Assessment of hydrophobicity was based on the minimum concentration of ammonium sulfate permitting aggregation. In a preliminary test, C. jejuni strains used in this study did not show AAG activity in 2 mM sodium phosphate solution used as a diluent.
Adherence to INT407 cells.
INT 407 cells (human embryonic intestine cells [ATCC CCL 6]) were obtained from the American Type Culture Collection (Rockville, Md.) and maintained in Eagle's minimal essential medium (EMEM; GIBCO-BRL, Gaithersburg, Md.) supplemented with 15% newborn calf serum (NCS), 50 μg of streptomycin per ml, 200 U of penicillin per ml, 200 mM l-glutamine, and 0.075% sodium bicarbonate. Trypsinized cells were placed in a 24-well tissue culture plate (Costar) and cultivated at 37°C for 24 h in 5% CO2. Immediately before assay, the culture medium was replaced with prewarmed EMEM supplemented with 1% NCS but without antibiotics. The adhesion assay was performed by the method of Grant et al. (10), except that we did not centrifuge after inoculation of bacteria onto INT 407 cells. The bacteria were harvested in EMEM with 1% NCS. To examine the effect that extraction of the bacteria had on adherence to INT 407 cells, the bacterial cells were washed twice in sterile DW and then resuspended in EMEM with 1% NCS to achieve ratios of inoculated bacteria to epithelial cells of 10:1, 100:1, or 1,000:1.
Genetic techniques.
Bacteria were grown on blood agar plates for 48 h, and chromosomal DNA was prepared as described previously (9). Plasmids were isolated using the QIAprep Spin Plasmid kit (Qiagen Inc., Chatsworth, Calif.) as specified by the manufacturer. PCR products were purified with the QIAquick PCR purification kit (Qiagen). All other standard molecular genetic techniques were used as described elsewhere (49). Escherichia coli strain DH5α was grown in Luria (L) broth or on L plates (49). The oligonucleotide primers listed in Table 1 were synthesized with an ABI 392 DNA synthesizer (Applied Biosystems, Inc., Foster City, Calif.) in the Vanderbilt University Cancer Center Core Facility.
TABLE 1.
PCR primers used in this study
| Gene | Primera | Positions | Sequence | Reference |
|---|---|---|---|---|
| flbA | A9498 F | 895–915 | 5′-AGGTGATGGACTTGTATCTCA-3′ | 31 |
| A9499 R | 2117–2137 | 5′-AACTTCAGCCCTTAGAGTATC-3′ | ||
| flaA flaB | B6208 F | 569–590 | 5′-GCTACTCAATCTTCTAAAATCG-3′ | 13 |
| B6209 R | 1606–1627 | 5′-TGTAACTTGATTTTGTATAGAA-3′ | ||
| A8142 R | 1944–1965 | 5′-GCATCGAAAAGATTAAAGCAAG-3′ | ||
| A8141 F | 1944–1965 | 5′-CTTGCTTTAATCTTTTCGATGC-3′ | ||
| B6207 R | 3497–3518 | 5′-TGTACAGAACCAATATCTGCTC-3′ | ||
| peb1A | B6202 F | 1483–1504 | 5′-GATGAACCTACTTCAGCCCTTG-3′ | 43 |
| B6203 R | 2470–2491 | 5′-GTTCTTTTACAAAATCATCAAC-3′ | ||
| aphA | B6350 F | 35–56 | 5′-GGTAAGATTATACCGAGGTATG-3′ | 56 |
| B6351 R | 1396–1417 | 5′-AGACATCTAAATCTAGGTACTA-3′ |
F, forward; R, reverse.
Construction of aflagellate mutants.
To determine the effect of flagellin on AAG activity, isogenic aflagellate mutants were produced by insertion of a kanamycin resistance cassette (aphA) (56) into the flaA or flbA (alternative designation, flhA) (31, 32) loci of C. jejuni strain 81-176. To obtain a flaA fragment by PCR, primers B6208 (sense) and A8142 (antisense) were designed on the basis of the DNA sequence of flaA from C. coli strain VC167 (13). To obtain a flbA mutant, primers A9498 (sense) and A9499 (antisense) were designed based on the sequence of flbA from C. jejuni 81-176 (31). The antisense primer of flaA was located in the intergenic region between flaA and flaB, so that the amplicon includes flaA but not flaB. Using chromosomal DNA from strain 81-176 as the template, 1.4-kb flaA and 1.2-kb flbA fragments were amplified by PCR and each gene was cloned into pT7Blue T vector (Novagen, Madison, Wis.), yielding plasmids pTIC107 and pTIC109, respectively. A fragment containing aphA was purified from SmaI-digested pILL600 (24). The aphA cassette was inserted into the unique EcoRV site of flaA or into a NcoI site of flbA treated with Klenow polymerase to blunt the ends, yielding pTIC107::aphA or pTIC109::aphA, respectively. The orientation of the aphA insertion in the constructs was confirmed by PCR. Natural transformation of strain 81-176 was performed by the method of Wang and Taylor (58). Kanamycin-resistant colonies were screened on a semisolid agar plate composed of brucella broth containing 0.4% agar and 30 μg of kanamycin per ml. The compact, pinpoint colonies formed by nonmotile organisms were isolated, and the aflagellate phenotypes were confirmed by transmission electron microscopy of negatively stained cells or by immunoblotting analysis using antiflagellin antibody kindly provided by Trevor Trust. Characterization of chromosomal DNA from the mutants by PCR using the primers listed in Table 1 and Southern hybridization confirmed the correct insertion of the aphA cassette into the fla locus or into flbA. To determine the AAG activity and hydrophobicity of another flagellate mutant, a peb1A mutant (strain 98-311), constructed from strain 81-176 as described previously (43), was used. The peb1A gene encodes the PEB1 protein, which is a C. jejuni adhesin and is a conserved cell surface antigen (43).
Statistical analysis.
Comparisons of the absorbance values in the AAG assay were analyzed by Student's t test. Exponential regression analysis for comparison of AAG activity and bacterial hydrophobicity was performed by using CA-Cricket Graph III version 1.52 (Computer Associates International Inc., Islandia, N.Y.).
RESULTS
Effect of assay conditions on AAG activity.
Initially, we evaluated assay conditions to optimize the AAG assay by using strains 81-176, 4182, and 6960, which in preliminary studies had markedly different activities, For strain 81-176, the A600 values decreased substantially until 24 h after incubation at 25°C and then decreased slightly in the next 24 h. In contrast, strains 4182 and 6960 showed little AAG activity (Fig. 1A). Therefore, we selected 24 h as the period for incubation in subsequent assays. We then evaluated several incubation temperatures to use in conducting the AAG assays. The AAG activities did not change markedly with incubation temperature but were maximal at 25°C for strain 81-176 and at 37°C for strains 4182 and 6960 (Fig. 1B). Because of the relatively small differences and because the results at 25°C were the most representation of the entire temperature range, all subsequent incubations were performed at 25°C. To determine the appropriate diluent for the bacterial cells for observing AAG, seven different solutions were examined (Fig. 1C). Strain 81-176 showed strong AAG activity in sodium chloride, PBS, magnesium chloride, and calcium chloride solutions but not in PB or DW. In contrast, strains 4182 and 6960 did not show any substantial AAG activity in any diluent used in this experiment (Fig. 1C). Based on these experiments, we used PBS in all subsequent experiments.
FIG. 1.
Effect of assay conditions on the AAG activity of C. jejuni strains 81-176, 4182, and 6960. (A) Duration of incubation time. (B) Incubation temperatures. (C) Diluent composition. (D) Age of the culture on TSAS plates. Each assay was conducted by suspending the bacterial cells in PBS (except for panel C), at 25°C (except for panel B) after a 24-h incubation (except for panel A), and the measuring A600. Bacterial cells that strongly agglutinate do not remain in the aqueous phase, and A600 diminishes.
We next examined the effect of bacterial cultivation conditions on AAG activity (Fig. 1D). The AAG activity of strain 81-176 was greatly influenced by the age of the culture; cells grown for less than 24 h had high AAG activity, but the activity decreased after 48 h of incubation (Fig. 1D). Similarly, the AAG of strains 4182 and 6960 grown for 18 h was highest, but the differences related to variation in the incubation time were small. AAG activity of both strains was not substantially influenced by whether cells were cultured at 37 or at 42°C or by the number of in vitro passages (Table 2).
TABLE 2.
Characteristics of autoagglutination of C. jejuni strains 81-176, 4182, and 6960
| Treatment |
A600 (mean ±
SD)a
|
||
|---|---|---|---|
| 81-176 | 4182 | 6960 | |
| Cultivation tempb | |||
| 37°C | 0.218 ± 0.006 | 0.832 ± 0.013 | 0.779 ± 0.035 |
| 42°C | 0.279 ± 0.004 | 0.795 ± 0.015 | 0.750 ± 0.030 |
| Passage no.c | |||
| 1 | 0.250 ± 0.004 | 0.840 ± 0.020 | 0.740 ± 0.013 |
| 2 | 0.218 ± 0.012 | 0.723 ± 0.007 | 0.765 ± 0.056 |
| 3 | 0.227 ± 0.007 | 0.769 ± 0.015 | 0.718 ± 0.004 |
| 4 | 0.250 ± 0.011 | 0.713 ± 0.016 | 0.694 ± 0.010 |
| Heat stabilityd | |||
| Unheated | 0.218 ± 0.003 | 0.683 ± 0.009 | 0.745 ± 0.013 |
| 65°C | 0.619 ± 0.003j | 0.660 ± 0.003 | 0.845 ± 0.013 |
| Enzyme treatments | |||
| DW alonee | 0.193 ± 0.011 | 0.198 ± 0.006 | 0.686 ± 0.012 |
| Pronasef | 0.459 ± 0.014j | 0.510 ± 0.016j | 0.712 ± 0.014 |
| Lipasef | 0.117 ± 0.004 | 0.147 ± 0.006 | 0.775 ± 0.006 |
| DNasef | 0.195 ± 0.006 | 0.190 ± 0.007 | 0.748 ± 0.019 |
| Acid-glycine treatmentg | |||
| None | 0.218 ± 0.011 | 0.695 ± 0.017 | 0.732 ± 0.009 |
| pH 4.0 | 0.192 ± 0.004 | 0.707 ± 0.012 | 0.807 ± 0.013 |
| pH 2.2 | 0.674 ± 0.048j | 0.742 ± 0.015 | 0.839 ± 0.005 |
| Sodium metaperiodate treatment | |||
| Buffer aloneh | 0.093 ± 0.003 | 0.126 ± 0.005 | 0.708 ± 0.006 |
| NaIO4i | 0.111 ± 0.003 | 0.295 ± 0.005 | 0.725 ± 0.007 |
Bacterial cells were suspended in PBS, and then AAG activity was assayed after incubation for 24 h at 25°C.
Bacterial cells were cultured for 24 h at 37 or 42°C before being resuspended in PBS.
Bacterial cells were cultured for 24 h at 37°C one to four times before being resuspended in PBS.
Bacterial cells were cultured for 24 h at 37°C before being resuspended in PBS and then heated at 65°C for 30 min or not heated.
Bacterial were washed twice and then suspended in DW. The cell density was adjusted to an A600 of 1.5, and cells were incubated at 37°C for 2 h with rotation and then washed twice in PBS.
Bacteria were treated exactly as above, except that the DW contained 1 mg of pronase per ml, 10 U of lipase per ml, or 1 mg of DNase per ml.
Bacterial cells were cultured for 24 h at 37°C before being resuspended in acid-glycine buffer at pH 4 or 2.2 at 25°C for 30 min and then washed twice in PBS.
Bacteria were washed twice in DW, suspended in 50 mM sodium acetate (pH 4.5) at 25°C for 1 h in a dark room, and washed twice in PBS.
Bacteria were treated exactly as above except that the sodium accetate solution contained 10 mM sodium metaperiodate.
P < 0.001 compared with control.
Based on these observations, we adopted the following standard conditions for all subsequent AAG assays. The C. jejuni cells were grown at 37°C on TSAS plates for 24 h and then suspended in PBS at 25°C, and AAG activity was observed at 24 h. Under these conditions, C. jejuni cells had minimal or absent viability by 24 h.
Alteration of AAG by physical and chemical treatment of cells.
We next examined the stability of the strong AAG activity of cells of strain 81-176 following each of several treatments of the cells. Heating the cells to 65°C abolished AAG activity (Table 2). The AAG activity of strain 81-176 was abolished by pronase or acid-glycine (pH 2.2) treatment but not by treatment with lipase, DNase, or sodium metaperiodate (Table 2). These findings suggested that the AAG of strain 81-176 was strongly influenced by a surface protein rather than by a polysaccharide-, DNA-, or lipid-containing moiety. The AAG of strain 4182 was markedly enhanced by treatment with DW alone; treatment with pronase, but not lipase or DNase, reduced the AAG effect of DW. Although the AAG of strain 4182 was as strong in these experiments as that of strain 81-176, this phenomenon is addressed in the next section. In contrast, the AAG of strain 6960 was not changed by any physical or chemical treatments (Table 2).
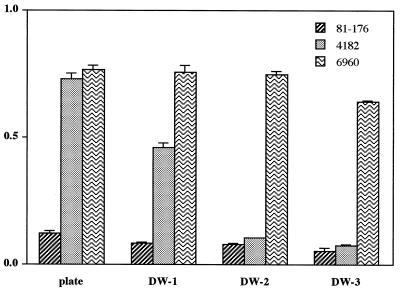
Effect of water extraction on AAG.
Because strain 4182 did not show substantial AAG activity even under conditions optimal for strain 81-176, we then considered the possibility that cells of strain 4182 have intrinsic AAG but that there also might be an inhibitor present. Based on previous studies of other Campylobacter species (63), we sought to determine whether extraction of the cells in DW might release a superficial component of the cells, external to the outer membrane. We found that for strains 81-176 and 6960, sequential water extraction had little effect on the substantial AAG (Fig. 2). In contrast, for strain 4182, after two extractions with DW, the AAG activity increased to the same level as that of strain 81-176. These findings suggest the loss of an inhibitor of AAG from cells of strain 4182 (Fig. 2). The effects of DW extraction on strain 4182 explain the higher AAG activity when the cells were suspended in DW with enzymes or in the presence of sodium metaperiodate (Table 2).
FIG. 2.
Autoagglutination of cells of C. jejuni strains 81-176, 4182, and 6960 after sequential extraction with DW. The AAG assay was conducted in PBS at 25°C after 24-h incubation.
Relationship between AAG and hydrophobicity or adhesion to INT 407 cells.
For other bacterial pathogens, including V. cholerae (4), H. pylori (37), Aeromonas species (16, 21, 42), and Yersinia species (25, 46), AAG is associated with virulence. Therefore, we sought to determine whether the same may be true for C. jejuni, especially since strain 81-176 is a highly virulent strain in both human (1) and monkey (48) challenges. To assess whether C. jejuni AAG might correlate with other virulence markers (20, 26), we determined the hydrophobicity of cells of 22 clinical C. jejuni isolates including the three well-studied strains (81-176, 4182, and 6960). The bacterial hydrophobicity was measured both before and after water extraction of the bacterial cells, because of the presence of the inhibitor affecting AAG of strain 4182, as described above (Fig. 2). We found that AAG was strongly associated (r > 0.9, P < 0.001) with hydrophobicity, both for cells without extraction (Fig. 3A) and after extraction in distilled water (Fig. 3B). Our studies suggest that C. jejuni strains may be grouped into three AAG phenotypes, (i) substantially strong AAG (e.g., strain 81-176); (ii) weak AAG, which increases after water extraction of cells (e.g., strain 4182); and (iii) substantially weak AAG (e.g., strain 6960), with no effect due to water extraction (Fig. 3). Next, we determined the adherence to INT 407 cells of three strains (81-176, 4182, and 6960) showing the different AAG phenotypes. Strain 81-176, which had highest AAG activity, adhered better to INT 407 cells than did strain 4182 or 6960 under every condition tested (Table 3). Extraction of cells of strains 81-176 and 4182 in DW, which increased AAG activity (Table 2 and Fig. 2), also increased adherence (Table 3). Nevertheless, even with extraction, cells of strain 6960 adhered 1 to 2 log10 units less well than did strains 81-176 and 4182 (Table 3).
FIG. 3.
Relationship between AAG activity and bacterial hydrophobicity, measured as minimum ammonium sulfate concentration permitting aggregation among 22 clinical C. jejuni isolates. Data are shown for cells before (A) and after (B) extraction with DW. For preextraction, r = 0.909 and P < 0.001, and for postextraction, r = 0.926 and P < 0.001. The circles indicate three phenotypes: ●, substantially strong AAG and hydrophobicity (e.g., strain 81-176); ○, weak AAG and hydrophobicity, which increases after water extraction of cells (e.g., strain 4182); , substantially weak AAG and hydrophobicity, not affected by water extraction (e.g., strain 6960).
TABLE 3.
Effect of DW extraction on C. jejuni adherence to INT 407 cells
| Treatment time | Inoculation ratioa | Log10 CFU of
adherent bacteria per wellb
|
||
|---|---|---|---|---|
| 81-176 | 4182 | 6960 | ||
| Preextraction | 1,000:1 | 4.72 ± 0.17 | 3.30 ± 0.20 | 1.40 ± 0.17 |
| 100:1 | 3.37 ± 0.32 | 2.85 ± 0.06 | 0.43 ± 0.75 | |
| 10:1 | 2.52 ± 0.24 | 1.84 ± 0.47 | NDc | |
| Postextractiond | 1,000:1 | 5.13 ± 0.13 | 4.32 ± 0.08 | 3.23 ± 0.15 |
| 100:1 | 4.34 ± 0.52 | 3.52 ± 0.16 | 2.51 ± 0.07 | |
| 10:1 | 3.06 ± 0.15 | 3.06 ± 0.17 | 1.63 ± 0.54 | |
Ratio of C. jejuni CFU to INT 407 cells.
Mean ± standard deviation from three replicate determinations.
ND, not detected (<1 CFU/well).
After extraction twice with DW.
AAG activity in the immotile variants.
To determine the effect of motility on AAG activity, one spontaneous flagellate and immotile variant (F+ M−) and one aflagellate and immotile variant (F− M−) derived from strain 81116 were used. As described previously (2), no differences in protein profile between the variants and the parent strain were detected except for the absent flagellar band in the F− M− strain. Both the parent strain and the F+ M− variant revealed strong AAG and hydrophobicity, but the F− M− variant had neither activity (Table 4). These studies indicated that AAG and hydrophobicity are not related to motility and suggest a possible relationship with flagellation.
TABLE 4.
Comparison of autoagglutination of C. jejuni strain 81116 and its spontaneous variants
| Strain | Phenotypea | AAG activitybA600 |
|---|---|---|
| 81116 | F+ M+ | 0.047 ± 0.005 |
| 83-84 | F+ M− | 0.039 ± 0.005 |
| 83-86 | F− M− | 0.765 ± 0.006c |
F, flagella; M, motility. Symbols: +, positive; −, negative.
Bacterial cells were suspended in PBS after extraction twice with DW, and AAG activity was assayed after incubation for 24 h at 25°C.
P < 0.001 compared with F+ strains.
Characterization of aflagellated mutants.
Since AAG activity was related to a pronase-sensitive, superficial structure, and from the above studies using the 81116 variants, an important possibility is that the major autoagglutinin is flagellin. To test this hypothesis, we made a series of mutants of C. jejuni strain 81-176. After introduction of the mutated flaA allele in pTIC107 into C. jejuni strain 81-176 by natural transformation, a total of 22 kanamycin-resistant colonies were isolated. When motility was examined using the soft agar method, 7 of 22 isolates were nonmotile. We then analyzed chromosomal DNA obtained from these nonmotile isolates and the wild-type strain by PCR with specific primers for flaA, flaB, or aphA and Southern hybridization with flaA and aphA probes. As expected, we found that aphA was inserted in flaA of the nonmotile mutants (data not shown). However, strain 98-248 showed different results in Southern hybridization and PCR analyses from those of the other flaA mutants (data not shown). For 98-248, the band detected in Southern hybridizations using the flaA probe decreased to approximately 2.0 kb compared with 5 kb for the wild-type strain, in which chromosomal DNA was digested with BglII. A pair of primers (A8141 and B6207) specific for flaB did not amplify a 1.5-kb band in 98-248 which was present in the wild-type strain. These results indicate that strain 98-248 was a flaA flaB mutant due to a recombinational event that replaced both adjacent genes with the mutated flaA.
A total of 10 kanamycin-resistant colonies were isolated after the mutated flbA allele in pTIC109 was introduced into C. jejuni by natural transformation. All kanamycin-resistant colonies were nonmotile (data not shown). Strain 98-255 was characterized further by Southern hybridization with probes for flbA or aphA. As expected, insertion of the aphA cassette into strain 98-255 caused a 1.4-kb increase in the size of the 7.0-kb flbA-containing BglII restriction fragment (data not shown). To further characterize these presumed aflagellate mutants, motility and flagellin expression were determined by the soft agar assay and Western immunoblotting, respectively. As expected, both the flaA flaB (98-248) and flbA (98-255) mutant strains were nonmotile and produced no flagellin, in contrast to the wild-type strain and peb1A mutant (Fig. 4). Consistent with our findings by electron microscopy (data not shown), these results indicated that both mutants were aflagellate.
FIG. 4.
Phenotypic characterization of the wild-type C. jejuni strain (81-176), the flaAflaB isogenic mutant (strain 98-248), the flbA isogenic mutant (strain 98-255), and the peb1A isogenic mutant (strain 98-311). (A) Motility analysis by semisolid agar assay. (B) Western blotting analysis of whole-cell extracts with rabbit polyclonal anti-flagellin antibody. Fla indicates the flagellin band present in strains 81-176 and 98-311 but absent from strains 98-248 and 98-255 as expected.
AAG activity and hydrophobicity in the isogenic mutants.
We used these mutant strains to examine the effect of flagella on cell surface properties. To determine (and maximize) the AAG activity and bacterial hydrophobicity of C. jejuni strain 81-176 and its two different aflagellate mutants (98-248 and 98-255) and the peb1A mutant 98-311, the bacterial cells first were washed twice in DW, as described above. Both aflagellate mutants had significantly decreased AAG and hydrophobicity in comparison with the wild-type strain (Table 5). In contrast, the peb1A mutant had similar AAG activity to the wild type strain.
TABLE 5.
Comparison of AAG and hydrophobicity of C. jejuni strain 81-176 and its isogenic mutants
| Strain | Genotype | AAG activitya | Hydrophobicityb |
|---|---|---|---|
| 81-176 | Wild type | 0.031 ± 0.003 | 0.031 |
| 98-248 | flbA | 0.654 ± 0.004c | 4.0c |
| 98-255 | flaA flaB | 0.731 ± 0.008c | 4.0c |
| 98-311 | peb1A | 0.065 ± 0.002 | 0.031 |
Bacterial cells were suspended in PBS after extraction twice with DW, and AAG activity was assayed after incubation for 24 h at 25°C. Values are given in AAG optical density units (A600).
Minimum concentration (molar) of ammonium sulfate permitting agglutination. Results shown are the mean of three determinations.
P < 0.001 compared with wild type.
DISCUSSION
Although the phenomenon of AAG in C. jejuni strains has been observed previously (3, 27, 39, 44, 62), there has been little recent investigation. AAG activities ranging from 3.7 to 50% were described as factors preventing the determination of typing by coagglutination assays using antisera or lectins (3, 27, 39, 62). However, previous investigators did not attempt to characterize the factor(s) responsible for AAG. We first examined the variables influencing the expression and measurement of AAG activity in C. jejuni cells. The age of the bacterial culture and the nature of the diluent used for suspending the bacterial cells were the most important factors influencing AAG detection. Although the AAG activity of cells from 18-h cultures was stronger than for those from 24-h cultures, we used the longer (24-h) culture to obtain sufficient cells to allow reproducible assays. Similarly, although the AAG activity of strain 4182 observed at 37°C was stronger than that at 25°C, we chose to observe AAG at 25°C because the difference between strain 81-176 and 4182 was maximal at 25°C (Fig. 1). AAG occurred in the presence of either monovalent or divalent cations but not in DW, and we adopted PBS as the diluent for the AAG assay because it is a widely available buffer. In total, these studies permit the use of a simple, quantitative, and reproducible assay for AAG of C. jejuni cells.
It appeared likely that the major autoagglutinin is a surface protein(s) because it is heat labile and is removed from C. jejuni cells by either pronase or acid-glycine (pH 2.2) treatment but not by lipase or sodium metaperiodate treatment. Other investigators suggested that C. jejuni AAG activity was due to the release of extracellular material including DNA (27, 62); however, in our study, AAG activity was not abolished by DNase treatment. O'Sullivan et al. (39) reported that AAG of C. jejuni was observed among 4.5% of isolates after bacteria were heated in boiling water for 1 h for lectin typing. However, the present study showed that AAG of C. jejuni strain 81-176 is due to a heat-labile material. Interestingly, two separate AAG phenomena, spontaneous pelleting and pelleting only after boiling, have been observed in mesophilic aeromonads (16). Thus, AAG properties may vary considerably among strains and may have different mechanisms even for a single bacterial species.
We next determined the effect of C. jejuni motility on AAG activity. We used C. jejuni strain 81116 and its two variants (83-84 and 83-86) because the profiles of the proteins expressed on the cell surface have been well characterized (2). One flagellate variant lost motility but retained AAG, suggesting that the motility per se is not necessary for AAG. That the aflagellate variant did not have AAG activity also suggested a role for flagella in AAG.
Flagellar expression is critical for C. jejuni virulence in vivo (35, 61). To directly determine the effect of flagellin on AAG activity, we constructed aflagellate mutants by insertional inactivation into either the fla locus or flbA, which controls assembly and surface expression of the mature flagella (31). Mutational analysis of C. coli has shown that flaB inactivation does not affect flagellar length but significantly diminishes motility while flaA inactivation leads to both truncated flagella and reduced motility (11, 12). Thus, to produce an aflagellate strain, mutations in both flaA and flaB are needed. One of the kanamycin-resistant mutants of strain 81-176 that we selected (strain 98-248) was nonmotile, and analyses showed that the insertion into flaA resulted in its detection within flaB as well.
Our mutagenesis studies clearly indicate that flagellar expression on the C. jejuni cell surface plays a major role in the AAG activity observed. The use of the flbA mutant also indicates that production of flagella is necessary for AAG activity. However, since the insertion used in the mutagenesis experiments could be polar on the downstream genes, it cannot conclusively stated that the observed phenotype results solely from the inactivation of those genes. The kanamycin resistance cassette was confirmed by PCR to be in the same transcriptional orientation as the target genes (data not shown). When the cassette is inserted in the same orientation as the target genes, it has been reported to be nonpolar on downstream genes because it lacks a transcriptional terminator (50). Complementation of these mutants in trans should determine whether flagella per se are necessary for the AAG activity (55). However, although many attempts were made to subclone the intact copy of the flaA, flaB, or flbA fragment onto pRY112, a chloramphenicol-resistant campylobacter shuttle vector (64), these fragments were unstable in E. coli cells (data not shown); thus, complementation was not possible.
In several pathogenic bacteria (4, 16, 25), type IV pili are highly associated with expression of adhesins and are responsible for AAG. Loss of AAG is the standard phenotype used to detect nonpilated variants in N. gonorrhoeae and V. cholerae (4). Furthermore, genetic analysis of spontaneous nonautoagglutinating mutants of N. gonorrhoeae revealed that single amino acid changes in the N terminus of PilE abolish AAG (4). Since C. jejuni strain 6960 is both flagellate and motile (data not shown), its low-level agglutination could reflect a type of flagella that is less efficient in inducing AAG. For C. jejuni and the closely related C. coli, the regulation of flagellar biosynthesis is complex; phase variations in both antigenic (13) and flagellar (6, 36) expression have been observed; a reversible frameshift mutation in C. coli flhA confers phase variability to flagellin gene expression (40). It is possible that differences in the level of expression or in posttranslational modifications (7) may explain the C. jejuni AAG activity variation observed under different bacterial growth conditions or in different strains.
Strains 81-176 and 4182 differed markedly in AAG. However, that the AAG of strain 4182 increased after extraction in water suggests that strain 4182 could possess an inhibitor that was removed by extraction. Although the inhibitor affecting AAG has not been characterized, it may be a halophilic matrix on the cell surface because the inhibitor reduces bacterial hydrophobicity. Thus, future studies of C. jejuni AAG should examine both the autoagglutinin and its inhibitor(s). Furthermore, assessment of AAG activity, hydrophobicity, or cell adherence should be determined after water extraction to remove any such inhibitors.
The AAG activities of C. jejuni strains could be grouped into three phenotypes, and the AAG phenotype also correlated with bacterial hydrophobicity and adhesion to INT 407 cells. That water extraction increased each of these phenomena for most strains suggests conserved characteristics affecting each of the phenotypes. These findings suggest that AAG of C. jejuni may provide a simple indicator to facilitate the analysis of adherence in vitro. Recent studies showing that early events in the interaction of C. jejuni and intestinal epithelial cells (including INT 407) are critical for induction and release of the proinflammatory cytokine interleukin-8 (14) further suggest the utility of understanding the basis for AAG.
In conclusion, the AAG assay for C. jejuni developed here is simple and inexpensive to perform and yields results rapidly. In the absence of suitable animal models of disease, simple in vitro systems such as adhesion to tissue culture cells, hydrophobicity, and AAG analyses can be used to develop hypotheses related to C. jejuni virulence. Although the ability to express flagella on the C. jejuni surface is the critical determinant for AAG, studies to further define the basis for strain differences should be conducted.
ACKNOWLEDGMENTS
These studies were supported in part by the Medical Research Service of the Department of Veterans Affairs and by the Iris and Horner Akers Fellowship in Infectious Disease (to N.M.).
REFERENCES
- 1.Black R E, Levine M M, Clements M L, Hughes T P, Blaser M J. Experimental Campylobacter jejuniinfection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 2.Blaser M J, Hopkins J A, Perez-Perez G I, Cody H J, Newell D E. Antigenicity of Campylobacter jejuniflagella. Infect Immun. 1986;53:47–52. doi: 10.21236/ada265460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butzler J P. Infection with campylobacters. In: Williams J D, editor. Modern topics in infection. London, United Kingdom: Heinemann; 1978. pp. 214–239. [Google Scholar]
- 4.Chiang S L, Taylor R K, Koomey M, Mekakanos J J. Single amino acid substitutions in the N-terminus of Vibrio choleraeTcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- 5.De Melo M A, Pechere J C. Identification of Campylobacter jejunisurface proteins that bind to eucaryotic cells in vitro. Infect Immun. 1990;58:1749–1756. doi: 10.1128/iai.58.6.1749-1756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diker K S, Hascelik G, Akan M. Reversible expression of flagella in Campylobacterspp. FEMS Microbiol Lett. 1992;78:261–264. doi: 10.1016/0378-1097(92)90037-o. [DOI] [PubMed] [Google Scholar]
- 7.Doig P, Kinsella N, Guerry P, Trust T J. Characterization of a post-translational modification of Campylobacterflagellin: identification of a sero-specific glycosyl moiety. Mol Microbiol. 1996;19:379–387. doi: 10.1046/j.1365-2958.1996.370890.x. [DOI] [PubMed] [Google Scholar]
- 8.Fauchere J L, Kervella M, Rosenau A, Mohanna K, Veron M. Adhesion to HeLa cells of Campylobacter jejuni and C. coliouter membrane components. Res Microbiol. 1989;140:379–392. doi: 10.1016/0923-2508(89)90014-4. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto S, Allos B M, Misawa N, Patton C M, Blaser M J. Restriction fragment length polymorphism analysis and random amplified polymorphic DNA analysis of Campylobacter jejuni strains isolated from patients with Guillain-Barrésyndrome. J Infect Dis. 1997;176:1105–1108. doi: 10.1086/516522. [DOI] [PubMed] [Google Scholar]
- 10.Grant C C R, Konkel M E, Cieplak W, Jr, Tompkins L S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuniin nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerry P, Alm R A, Power M E, Logan S M, Trust T J. Role of two flagellin genes in Campylobactermotility. J Bacteriol. 1991;173:4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerry P, Alm R A, Power M E, Trust T J. Molecular and structural analysis of Campylobacter flagellin. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 267–281. [Google Scholar]
- 13.Guerry P, Logan S M, Thorton S, Trust T J. Genomic organization and expression of Campylobacterflagellin genes. J Bacteriol. 1990;172:1853–1860. doi: 10.1128/jb.172.4.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey T E, Baqar S, Bourgeols A L, Ewing C P, Guerry P. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect Immun. 1999;67:88–93. doi: 10.1128/iai.67.1.88-93.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honda T, Khan M M A, Takeda Y, Miwatani T. Grouping of enterotoxigenic Escherichia coliby hydrophobicity and its relation to hemagglutination and enterotoxin productions. FEMS Microbiol Lett. 1983;17:273–276. [Google Scholar]
- 16.Janda J M, Oshiro L S, Abbott S L, Duffey P S. Virulence markers of mesophilic aeromonads: association of the autoagglutination phenomenon with mouse pathogenicity and the presence of a peripheral cell-associated layer. Infect Immun. 1987;55:3070–3077. doi: 10.1128/iai.55.12.3070-3077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson W M, Lior H. Cytotoxic and cytotonic factors produced by Campylobacter jejuni, Campylobacter coli, and Campylobacter laridis. J Clin Microbiol. 1986;24:275–281. doi: 10.1128/jcm.24.2.275-281.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kervella M, Pages J-M, Pei Z, Grollier G, Blaser M J, Fauchere J-L. Isolation and characterization of two Campylobacterglycine-extracted proteins that bind to HeLa cell membranes. Infect Immun. 1993;61:3440–3448. doi: 10.1128/iai.61.8.3440-3448.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ketley J M. Virulence of Campylobacterspecies: a molecular genetic approach. J Med Microbiol. 1995;42:312–327. doi: 10.1099/00222615-42-5-312. [DOI] [PubMed] [Google Scholar]
- 20.Klipstein F A, Engert R F, Short H B. Enzyme-linked immunosorbent assays for virulence properties of Campylobacter jejuniclinical isolates. J Clin Microbiol. 1986;23:1039–1043. doi: 10.1128/jcm.23.6.1039-1043.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokka R P, Janda J M. Isolation and identification of autoagglutinating serogroup O:11 Aeromonasstrains in the clinical laboratory. J Clin Microbiol. 1990;28:1297–1299. doi: 10.1128/jcm.28.6.1297-1299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konkel M, Cieplak W. Altered synthetic response of Campylobacter jejunito cocultivation with human epithelial cells is associated with enhanced internalization. Infect Immun. 1992;92:4945–4949. doi: 10.1128/iai.60.11.4945-4949.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konkel M E, Babakhani F, Joens L A. Invasive-related antigens of Campylobacter jejuni. J Infect Dis. 1990;162:888–895. doi: 10.1093/infdis/162.4.888. [DOI] [PubMed] [Google Scholar]
- 24.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laird W J, Cavanaigh D C. Correlation of autoagglutination and virulence of Yersiniae. J Clin Microbiol. 1980;11:430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindblom G B, Cervantes L-E, Sjogren E, Kaijser B, Ruiz-Palacios G M. Adherence, enterotoxigenicity, invasiveness and serogroups in Campylobacter jejuni and Campylobacter colistrains from adult humans with acute enterocolitis. APMIS. 1990;98:179–184. [PubMed] [Google Scholar]
- 27.Lior H, Woodward D L, Edgar J A, Laroche L J, Gill P. Serotyping of Campylobacter jejuniby slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 1982;15:761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahajan S, Rodgers F G. Isolation, characterization, and host cell-binding properties of a cytotoxin from Campylobacter jejuni. J Clin Microbiol. 1990;28:1314–1320. doi: 10.1128/jcm.28.6.1314-1320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McSweegan E, Walker R I. Identification and characterization of two Campylobacter jejuni: adhesins for cellular and mucous substrates. Infect Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menozzi F D, Boucher P E, Riveau G, Gantiez C, Locht C. Surface-associated filamentous hemagglutinin induces autoagglutination of Bordetella pertussis. Infect Immun. 1994;62:4261–4269. doi: 10.1128/iai.62.10.4261-4269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller S, Pesci E C, Pickett C L. A Campylobacter jejuni homolog of the lcrD/flbFfamily of proteins id necessary for flagella biogenesis. Infect Immun. 1993;61:2930–2936. doi: 10.1128/iai.61.7.2930-2936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller S, Pesci E C, Pickett C L. Genetic organization of the region upstream from the Campylobacter jejuni flagellar gene flhA. Gene. 1994;19:31–38. doi: 10.1016/0378-1119(94)90830-3. [DOI] [PubMed] [Google Scholar]
- 33.Misawa N, Ohnishi T, Itoh K, Takahashi E. Cytotoxin detection in Campylobacter jejunistrains of human and animal origin with three tissue culture assay systems. J Med Microbiol. 1995;43:354–359. doi: 10.1099/00222615-43-5-354. [DOI] [PubMed] [Google Scholar]
- 34.Misawa N, Ohnishi T, Itoh K, Takahashi E. Detection of serum dependent cytotoxic activity of Campylobacter jejuniand its characteristics. J Vet Med Sci. 1996;58:91–96. doi: 10.1292/jvms.58.91. [DOI] [PubMed] [Google Scholar]
- 35.Nachamkin I, Yang X-H, Stern N J. Role of Campylobacter jejuniflagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl Environ Microbiol. 1993;59:1269–1273. doi: 10.1128/aem.59.5.1269-1273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuijten P J, Marquez-Magana L, van der Zeijst B A. Analysis of flagellin gene expression in flagellar phase variants of Campylobacter jejuni81116. Antonie Leeuwenhoek. 1995;67:377–383. doi: 10.1007/BF00872938. [DOI] [PubMed] [Google Scholar]
- 37.Odenbreit S, Till M, Hass R. Optimized BlaM-transposon shuttle mutagenesis of Helicobacter pyloriallows the identification of novel genetic loci involved in bacterial virulence. Mol Microbiol. 1996;20:361–373. doi: 10.1111/j.1365-2958.1996.tb02623.x. [DOI] [PubMed] [Google Scholar]
- 38.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Sullivan N, Benjamin J, Skirrow M B. Lectin typing of Campylobacterisolates. J Clin Pathol. 1990;43:957–960. doi: 10.1136/jcp.43.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S F, Purdy D, Leach S. Localized reversible frameshift mutation in the flhA gene confers phase variability to flagellin gene expression in Campylobacter coli. J Bacteriol. 2000;182:207–210. doi: 10.1128/jb.182.1.207-210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkhill J, Wren B W, Mungall K, Ketley M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M-A, Rutherford K M, van Vliet A H M, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejunireveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 42.Paula S J, Duffey P S, Abbott S L, Kokka R P, Oshiro L S, Janda J M, Shimada T, Sakazaki R. Surface properties of autoagglutinating mesophilic aeromonads. Infect Immun. 1998;56:2658–2665. doi: 10.1128/iai.56.10.2658-2665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pei Z, Blaser M J. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in gram-negative nutrient transport systems. J Biol Chem. 1993;268:18717–18725. [PubMed] [Google Scholar]
- 44.Penner J L, Hennessy J N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejunion the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Perez G I, Cohn D L, Guerrant R L, Patton C M, Reller L B, Blaser M J. Clinical and immunologic significance of cholera-like toxin and cytotoxin production by Campylobacterspecies in patients with acute inflammatory diarrhea in the USA. J Infect Dis. 1989;160:460–468. doi: 10.1093/infdis/160.3.460. [DOI] [PubMed] [Google Scholar]
- 46.Roggenkamp A, Neuberger H-R, Flugel A, Schmoll T, Heesemann J. Substitution of two histidine residues in YadA protein of Yersinia enterocoliticaabrogates collagen binding, cell adherence and mouse virulence. Mol Microbiol. 1995;16:1207–1219. doi: 10.1111/j.1365-2958.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 47.Russell R G, Blake D C., Jr Cell association and invasion of Caco-2 cells by Campylobacter jejuni. Infect Immun. 1994;62:3773–3779. doi: 10.1128/iai.62.9.3773-3779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell R G, Blaser M J, Sarimento J I, Fox J. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect Immun. 1989;57:1438–1444. doi: 10.1128/iai.57.5.1438-1444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J E, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 50.Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbAgene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skirrow M B, Blaser M J. Clinical and epidemiologic considerations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni. Current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 3–8. [Google Scholar]
- 52.Skurnik M, Bolin I, Heikkinen H, Piha S, Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersiniaspp. J Bacteriol. 1984;158:1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stern N J. Reservoirs for Campylobacter jejuni and approaches for intervention in poultry. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni. Current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 49–60. [Google Scholar]
- 54.Swanson J, Kraus S J, Gotschlich E C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971;134:886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szymanski, C. M., R. Yao, C. P. Ewing, T. J. Trust, and P. Guerry. 1999. Evidence for a system of general protein glycosylation in Campylobacter jejuni. 32:1022–1030. [DOI] [PubMed]
- 56.Trieu-Cuot P, Lambert T, Courvalin P. In vivotransfer of genetic information between gram-positive bacteria. EMBO J. 1985;16:3538–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker R I, Caldwell M B, Lee E C, Guerry P, Trust J, Ruiz-Palacios G M. Pathophysiology of Campylobacterenteritis. Microbiol Rev. 1986;50:81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Taylor D E. Natural transformation in Campylobacterspecies. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wassenaar T. Toxin production by Campylobacterspp. Clin Microbiol Rev. 1997;10:466–476. doi: 10.1128/cmr.10.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wassenaar T M, Bleumink-Pluym N M C, van der Zeijst B A M. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaBis required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wassenaar T M, van der Zeijst B A M, Ayling R, Newell D G. Colonization of chicks by motility mutants of Campylobacter jejunidemonstrates the importance of flagellin. J Gen Microbiol. 1993;139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]
- 62.Wong K H, Skelton S K, Patton C M, Feeley J C, Morris G. Typing of heat-stable and heat-labile antigens of Campylobacter jejuni and Campylobacter coliby coagglutination. J Clin Microbiol. 1985;21:702–707. doi: 10.1128/jcm.21.5.702-707.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang L, Pei Z, Fujimoto S, Blaser M J. Reattachment of surface array proteins to Campylobacter fetuscells. J Bacteriol. 1992;174:1258–1267. doi: 10.1128/jb.174.4.1258-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao R, Alm R A, Trust T J, Guerry P. Construction of new Campylobactercloning vectors and a new mutational cat cassette. Gene. 1993;130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]