SUMMARY
HIV-1 DNA exists in nonintegrated linear and circular episomal forms and as integrated proviruses. In patients with plasma viremia, most peripheral blood mononuclear cell (PBMC) HIV-1 DNA consists of recently produced nonintegrated virus DNA while in patients with prolonged virological suppression (VS) on antiretroviral therapy (ART), most PBMC HIV-1 DNA consists of proviral DNA produced months to years earlier. Drug-resistance mutations (DRMs) in PBMCs are more likely to coexist with ancestral wild-type virus populations than they are in plasma, explaining why next-generation sequencing is particularly useful for the detection of PBMC-associated DRMs. In patients with ongoing high levels of active virus replication, the DRMs detected in PBMCs and in plasma are usually highly concordant. However, in patients with lower levels of virus replication, it may take several months for plasma virus DRMs to reach detectable levels in PBMCs. This time lag explains why, in patients with VS, PBMC genotypic resistance testing (GRT) is less sensitive than historical plasma virus GRT, if previous episodes of virological failure and emergent DRMs were either not prolonged or not associated with high levels of plasma viremia. Despite the increasing use of PBMC GRT in patients with VS, few studies have examined the predictive value of DRMs on the response to a simplified ART regimen. In this review, we summarize what is known about PBMC HIV-1 DNA dynamics, particularly in patients with suppressed plasma viremia, the methods used for PBMC HIV-1 GRT, and the scenarios in which PBMC GRT has been used clinically.
KEYWORDS: antiviral therapy, DNA sequencing, HIV-1, peripheral blood mononuclear cells, provirus, adaptive mutations, drug resistance evolution
INTRODUCTION
People with HIV-1 who have attained virological suppression (VS) on antiretroviral therapy (ART) often require changes to their therapy to avoid drug toxicity, intolerance, drug-drug interactions, and to improve adherence. In these patients, standard genotypic resistance testing (GRT) of plasma virus is not possible. HIV-1 DNA GRT of peripheral blood mononuclear cells (PBMCs) has increasingly been studied for the optimization of ART in patients with VS or low plasma virus levels, and in other scenarios in which plasma virus GRT might be insensitive for detecting drug-resistance mutations (DRMs). PBMC GRT results, however, are difficult to interpret because there are fewer data demonstrating the predictive value of PBMC GRT compared with plasma virus GRT.
Retrospective studies have shown that the presence of HIV-1 DRMs in plasma virus before the start of a new ART regimen are independent predictors of the virologic response to that regimen (1–8). Prospective and retrospective studies have shown that patients whose care providers have access to plasma virus GRT respond better to therapy than those whose providers do not (9–13). The accumulation of such retrospective and prospective data has led to the routine use of plasma GRT in the management of people with HIV-1 (14–16). Whereas it is unlikely that prospective clinical trials will be performed to evaluate the use of PBMC GRT, a critical mass of retrospective data that provides insight into scenarios in which PBMC GRT may be useful could be applied to clinical practice.
The topic of PBMC GRT intersects with recent research conducted to understand the replication-competent HIV-1 reservoir. Therefore, this review will begin with several sections summarizing recent studies on PBMC HIV-1 dynamics particularly in patients with suppressed plasma viremia, as understanding this foundational topic is important for the optimal use of PBMC GRT in clinical decision-making.
HIV DNA POPULATIONS
HIV-1 DNA exists in nonintegrated linear and circular episomal forms and as integrated proviruses. The biological significance of the nonintegrated forms of HIV-1 DNA is poorly understood. Both nonintegrated linear and episomal forms have short half-lives ranging from several days to weeks, although episomal forms can persist for longer periods of time (17–22). Consequently, within several months of VS, PBMC HIV-1 DNA consists primarily of integrated proviral DNA (23–27). Although PBMC HIV-1 GRT is often referred to as proviral DNA sequencing, PBMC GRT assays rarely distinguish between integrated and nonintegrated HIV-1 DNA. Additionally, the terms proviral DNA compartment and latent HIV reservoir have been used interchangeably because there is no way to determine which parts of the proviral DNA compartment are responsible for virological rebound in patients who discontinue ART.
The vast majority (>90%) of HIV-1-infected CD4+ T cells from peripheral blood and lymph node tissue contain only one integrated HIV-1 DNA molecule (28, 29). In the absence of therapy, HIV-1 DNA is present at a level ranging between 2.5 to 3.5 log copies/106 PBMCs (20). Elite controllers and the rare posttreatment controllers who maintain VS off ART have been shown to average approximately 1.5 log copies/106 PBMCs (20, 30–32). In research studies, GRT can be performed using sorted CD4+ lymphocytes. However, in clinical settings, GRT is performed using the complete PBMC population, which includes multiple cell types not infected by HIV-1, including monocytes, B lymphocytes, CD8+ T lymphocytes, and natural killer cells.
Complete proviral DNA genomic sequencing has revealed that approximately 90% to 95% of integrated HIV-1 genomes have major sequence abnormalities incompatible with viral replication, including large deletions and apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC)-mediated G-to-A hypermutation (23, 33, 34). The deletions result from replication errors as the reverse transcriptase (RT) enzyme switches between paired RNA template genomes during reverse transcription. G-to-A hypermutation often results in lethal mutations, such as premature stop codons and mutations at highly conserved positions.
The frequency of replication-competent viruses that can be cultured in quantitative virus outgrowth assays (QVOAs) is nearly 100-fold lower than the number of nondefective proviral genomes (33). However, QVOAs provide only a minimal estimate of the number of nondefective proviral genomes because the single round of T cell stimulation performed in these assays is insufficient to activate all replication-competent proviruses and because not all activated proviruses are capable of establishing a spreading infection (33, 35). Thus, only a small proportion of the proviruses that appear genetically intact contribute to the replication-competent viral reservoir. Many additional proviruses may be epigenetically silenced as a result of repressive chromatin states (23) and transcriptional interference (36, 37). Moreover, some proviruses without obvious genetic defects may contain subtle genetic changes associated with reduced replication capacity.
PROVIRAL DNA DYNAMICS DURING ACTIVE VIRAL REPLICATION
Although activated CD4+ lymphocytes are most vulnerable to HIV-1 infection, they are also the cells most vulnerable to being eliminated following infection as a result of virus cytopathic effect, cytotoxic T cell (CTL) killing, or apoptosis. In contrast, memory CD4+ lymphocytes, the main reservoirs of latent virus, are less vulnerable to infection (38, 39). Based on these observations, it has been hypothesized that the latent virus reservoir is established when HIV-1 infects activated CD4+ T lymphocytes that transition to memory CD4+ lymphocytes before they are destroyed in what has been termed an “effector-to-memory transition” (23, 39).
In patients with acute infection, proviral DNA levels and sequence diversity increase during the first few years after infection (40–43). In the absence of ART, proviral viral DNA levels eventually reach an equilibrium between processes that increase and decrease their levels. Proviral DNA levels are increased when CD4+ lymphocytes are infected during periods of active virus replication and during clonal proliferation of latently infected cells. Clonal proliferation (Sidebar) has been reported primarily in patients with VS because proviral DNA sequence homogeneity is most readily detected in patients in whom active viral replication is not occurring (44–47). Proviral DNA levels decrease when viruses reactivate, resulting either in viral cytopathic effects or host cytolytic effects (48, 49). Nonviral causes of cell death, such as senescence, are also likely to reduce proviral DNA levels (50).
In untreated patients, the proviral DNA reservoir undergoes continuous evolution (51–54). In several studies, the plasma virus quasispecies in ART-naive patients at multiple time points over periods ranging from 3 to 10 years before ART initiation were compared with the PBMC virus quasispecies following ART-induced VS. One study specifically sequenced the replication-competent proviral DNA reservoir (51) while others examined the nondefective PBMC HIV-1 DNA population (52–54). In two studies, most proviral DNA sequences were closely related to the pretherapy sequences obtained in the months prior to ART initiation (51, 52), while in three other studies a significant proportion of proviral DNA sequences was closely related to earlier pretherapy sequences (53–55). Together, these studies indicate that in the absence of ART, the proviral DNA population is constantly evolving. The likelihood of detecting older circulating variants within this population is not a linear function of time likely because of the stochasticity with which proviral DNA sequences are eliminated by virus activation and CTL killing as opposed to being amplified by clonal proliferation.
PROVIRAL DNA DYNAMICS FOLLOWING ART INITIATION
In most patients initiating highly effective ART regimens, CD4+ lymphocytes no longer become infected (51, 52, 56–60). PBMC DNA levels decline approximately 0.5 to 1.0 log10 copies/106 cells within weeks to months because of a decline in linear and, to some extent, episomal DNA but then plateau after one to 2 years (19, 43, 61–63). Following ART initiation, the turnover of the proviral DNA reservoir also slows. The estimated half-life of approximately 1 year observed for this compartment in untreated patients (51–54) lengthens to approximately 3 to 4 years in virologically suppressed patients (64–66). This slower turnover is believed to result from the progressive increase over time in the proportion of latently infected cells undergoing clonal proliferation (67) and from changes in the immunological environment, as both immune activation and CD4+ lymphocyte killing are reduced following VS (68).
In virologically suppressed patients, clonal proliferation of latently infected cells helps to stabilize the size of the proviral DNA reservoir, even though CD4+ lymphocytes are no longer being infected. Clonally expanded cells often eventually dominate the latent reservoir (67–72). Although clonal expansion of memory CD4+ lymphocytes containing both replication-competent and defective proviruses have been reported (23, 46, 69, 70, 73), there is likely to be a progressive enrichment of defective viruses since these are less likely to reactivate and lead to viral or cytotoxic T-cell killing (46, 71, 74, 75).
In patients with active virus replication and those with stable VS, the PBMC HIV-1 DNA population appears to be in equilibrium with the plasma virus RNA population and with the lymphoid HIV-1 DNA population (59, 60, 76, 77). Several studies have also shown that in patients who discontinue therapy, the rebounding plasma viral RNA population is closely related to the nondefective PBMC HIV-1 DNA population (55, 71, 78).
PBMC GRT METHODS
To perform PBMC GRT, DNA is extracted either from whole blood or, following density gradient centrifugation, from the fraction of the blood that contains lymphocytes and monocytes. The extracted DNA is then amplified using nested PCR (PCR). The number of viral genomes able to be sampled depends on the PBMC virus load, the number of PBMCs from which DNA is extracted, and the efficiency of DNA extraction and PCR. The number of sampled genomes may not be linearly related to the number of PBMCs used in an assay because the efficiency of PCR has been reported to be reduced when the amount of DNA in the assay is increased (79). Although PBMC HIV DNA levels are predictors of the response to therapy independent of the CD4+ count and plasma VL (20, 80, 81), these levels are rarely quantified when PBMC HIV GRT is performed. Additionally, in most publications, the number of PBMCs used for GRT is not reported.
PBMC HIV-1 DNA GRT has been performed using both dideoxy-terminator Sanger sequencing and next-generation sequencing (NGS) technologies. In a clinical sample, Sanger sequencing can detect the presence of mutations at levels of approximately 20% in the viral population (82–84). In contrast, the proportion at which variants can be detected by NGS depends on the selected threshold. Although mutation-detection thresholds as low as 1% have been used in research studies, most clinical applications of NGS have used thresholds between 5% and 10% in part to avoid the increased risk of sequence artifact that occurs at lower thresholds (85–87). Indeed, the widely used commercial PBMC GRT assay developed by Monogram Biosciences called the GenoSure Archive assay uses NGS with a mutation-detection threshold of about 10% (88–91).
There appears to be greater intrasample stochastic variation in the detection of DRMs in PBMC than in plasma, probably because DRMs are more likely to coexist with ancestral wild-type virus populations in PBMCs than in plasma (92–94). Indeed, a recent study in which two to four GenoSure Archive assays were performed on 90 samples from 70 patients with VS indicated that the mean reproducibility at detecting DRMs was approximately 80% (93). In another study of 55 patients who underwent three PBMC GRTs at different times also using the GenoSure Archive assay, the overall redetection rate for 178 DRMs detected at the first time point was also 80% (89). Sampling bias is likely to affect redetection rates particularly when the copy number of DRMs is low.
The interpretation of PBMC GRT results requires an approach that considers the near universal presence of APOBEC-mediated G-to-A hypermutation at some level within PBMC HIV-1 DNA (Sidebar). APOBEC3F and 3G are host enzymes that cripple viral genomes by indiscriminately mutating the dinucleotides GG→AG and GA→AA, respectively (95). DRMs resulting from G-to-A hypermutation (rather than drug selection pressure) are likely to be present in nonfunctional viruses that also contain stop codons and other crippling mutations. Ignoring all DRMs that could have resulted from a GG→AG or GA→AA change, however, would reduce the sensitivity for detecting HIV drug resistance (HIVDR), as nearly 20 DRMs arise in one of these two dual nucleotide contexts: most commonly the nucleoside RT inhibitor (NRTI)-resistance mutation M184I, the protease inhibitor (PI)-resistance mutation M46I, the nonnucleoside RT inhibitor (NNRTI)-resistance mutations E138K and G190S, and the integrase strand transfer inhibitor (INSTI)-resistance mutations G118R, G140S, and R263K. Several of the DRMs that are commonly caused by G-to-A hypermutation, such as the rilpivirine-associated mutation M230I and the cabotegravir-associated mutation G140R, are extremely rare even in the presence of selective drug pressure (96–98). Two approaches to minimize reporting of APOBEC-mediated DRMs can be applied using NGS: (i) excluding sequence reads that appear to be hypermutated; and (ii) determining the proportion of sequences that appear to be hypermutated and then reporting only those APOBEC-context DRMs that occur above this proportion (99).
PBMC DNA GRT DURING ACTIVE REPLICATION
Several studies have compared the results of PBMC and plasma virus GRT in persons with detectable viremia. Table 1 shows data from six of the largest studies of ART-naive individuals undergoing simultaneous PBMC and plasma virus GRT. There was complete concordance between PBMC and plasma for the one study in which all patients were acutely infected (100). In four studies, DRMs were more likely to be detected only by PBMC GRT (98, 101–103). However, in one of these four studies, eight of the nine additional DRMs detected by PBMC GRT occurred in an APOBEC dinucleotide context, including four instances of the otherwise extremely rare DRM M230I (98). The finding that PBMC GRT was usually more sensitive than plasma virus GRT at detecting DRMs in ART-naive persons with transmitted drug resistance (TDR) is consistent with the established observation that many transmitted DRMs are out-competed in plasma by more fit wild-type revertants (104, 105).
TABLE 1.
Comparison of HIV-1 PBMC and plasma virus GRT in ART-naive patients
| Study author and year | No. of patients | Patient characteristics | Genes | Sequencing method | No. of patients with DRMs and no. of DRMsa | No. of DRMs detected by plasma and PBMC GRT | No. of DRMs detected by plasma GRT alone | No. of DRMs detected by PBMC GRT alone |
|---|---|---|---|---|---|---|---|---|
| Ghosn 2006 (100) | 44 | Acutely infected; known transmitted drug resistance. | PR/RT | Sanger sequencing | 44 patients with 150 DRMs | 150 (100%) | 0 (0%) | 0 (0%) |
| Vicenti 2007 (219) | 169 | Most chronically infected. | PR/RT | Sanger sequencing (in GenBank) | 24 patients with 65 DRMs | 53 (81%) | 5 (8%) | 7 (11%) |
| Parisi 2007 (101) | 288 | 80% chronically and 20% recently infected. | PR/RT | Sanger sequencing | 80 patients with 170 DRMs | 71 (42%) | 39 (23%) | 60 (35%) |
| Bon 2007 (102) | 31 | Most chronically infected. | PR/RT | Sanger sequencing | 17 patients with 30 DRMs | 16 (53%) | 0 (0%) | 14 (47%) |
| Kabamba-Mukadi 2010 (103) | 44 | Most chronically infected. | PR/RT | Sanger sequencing | 8 patients with 11 DRMs | 4 (36%) | 1 (9%) | 6 (55%) |
| Huruy 2018 (98)b | 41 | Most chronically infected. | PR/RT | Sanger sequencing (in GenBank) | 11 patients with 15 DRMs | 4 (27%) | 2 (13%) | 9 (60%) |
Whenever possible the drug-resistance mutations (DRMs) included the predominantly nonpolymorphic mutations used by the Stanford HIV Drug Resistance Database (HIVDB) sequence interpretation program. Data for Parisi 2007 were obtained based on the presence of 8 major NRTI DRMs, 4 major NNRTI DRMs, and 6 major PI DRMs.
Although the sequences did not have evidence for G-to-A hypermutation, 8 of the 9 DRMs detected only in PBMCs in Huruy 2018 occurred in an APOBEC context, including the otherwise rare mutation M230I which was detected in 4 patients. One study that performed 454 NGS on plasma and PBMC samples from 20 patients with evidence for TDR by Sanger sequencing and which reported extensive G-to-A hypermutation in the PBMC sequences is not shown (220). Studies comparing the prevalence of DRMs in plasma and PBMCs using point-mutation assays are not shown (221, 222).
Table 2 shows data from 13 of the largest studies of ART-experienced persons undergoing simultaneous PBMC and plasma virus GRT. In contrast to the scenario in ART-naive patients, PBMC GRT can be less sensitive than plasma virus GRT for detecting DRMs in ART-experienced patients because there is often a lag of weeks to months before circulating DRMs reach sufficiently high levels in PBMCs to become detectable. This lag is especially prolonged in patients with low plasma HIV-1 RNA levels. Indeed, three smaller studies (not included in Table 2) that reported the results of simultaneous PBMC and plasma virus GRT at multiple time points supported this observation. In one of these studies in which nine patients received nonsuppressive therapy with a lamivudine-containing regimen, the lamivudine-resistance mutation M184V required 24 weeks to reach levels in PBMC between 10% and 80% (106). In a second study of 107 sequential samples from 22 patients with multiple virological failures (VFs) on a PI-containing regimen, 53 of 58 PI-associated DRMs emerged first in plasma (107). Furthermore, plasma virus GRT detected these DRMs 425 days earlier than PBMC GRT when plasma HIV-1 RNA levels were <4.0 log copies/mL compared with 225 days when levels were ≥4.0 log copies/mL (107). In a third study in which NGS was used to detect INSTI-associated DRMs in four patients with VF on a raltegravir-containing regimen, DRMs were detected by NGS at levels of 0%, 1%, 11%, and 36% in PBMCs versus 78% to 100% in plasma between 6 to 12 months following VF (108).
TABLE 2.
Comparison of HIV-1 PBMC and plasma virus GRT in ART-experienced patients
| Study author and year | No. of patients | Patient characteristics | Genes | Sequencing Methoda | Total no. of DRMsb | No. of DRMs detected by plasma and PBMC GRT | No. of DRMs detected by plasma GRT | No. of DRMs detected by PBMC GRT |
|---|---|---|---|---|---|---|---|---|
| Devereux 2000 (223) | 12 | Multiple past ART regimens. | PR/RT | Sanger sequencing (in GenBank) | 44 | 39 (89%) | 1 (2%) | 4 (9%) |
| Venturi 2002 (116) | 58 | First ART VF. | PR/RT | Sanger sequencing (in GenBank) | 129 | 78 (60.5%) | 44 (34.1%) | 7 (5.4%) |
| Chew 2005 (224) | 34 | ART history not described. | PR/RT | Sanger sequencing | 127 | 83 (65%) | 35 (28%) | 9 (7%) |
| Turriziani 2007 (225) | 95 | Mean ART history: 7 yrs. | PR/RT | Sanger sequencing | 85 patients had 676 DRMs | 483 (71%) | 122 (18%) | 71 (11%) |
| Saracino 2008 (226) | 73 | First and second ART VF. | PR/RT | Sanger sequencing (in GenBank) | 438 | 179 (41%) | 235 (54%) | 24 (5%) |
| Diallo 2012 (227) | 76 | Median ART history: 3 yrs. | PR/RT | Sanger sequencing (in GenBank) | 416 | 380 (91%) | 15 (4%) | 21 (5%) |
| Derache 2015 (228) | 18 | Median ART history: 4 yrs. | PR/RT | Sanger sequencing (in GenBank) | 8 patients had 31 DRMs | 25 (81%) | 6 (19%) | 0 (0%) |
| Lubke 2015 (229) | 30 | ART history not described. | PR/RT | Sanger sequencing | 25 patients had 169 DRMs | 128 (76%) | 39 (23%) | 2 (1%) |
| Michelini 2016 (164) | 12 | Median ART history: 12 yrs. | PR/RT | Sanger sequencing | 136 | 90 (66%) | 18 (13%) | 28 (21%) |
| Sotillo 2018 (109)c | 12 | ART history not described. | PR/RT | Sanger historical sequencing; single genome sequencing | 79 | 38 (48%) | 29 (37%) | 12 (15%) |
| Milne 2019 (230)d | 26 | History of PMTCT. | RT | NGS at a 10% mutation-detection threshold for PBMC | 13 patients had 29 DRMs | 25 (86%) | 1 (4%) | 3 (10%) |
| Curanovic 2020 (90) | 89 | ART history not described. | PR/RT/IN | NGS at a 10% mutation-detection threshold | 676 | 505 (75%) | 43 (6%) | 128 (19%) |
| Armenia 2022 (110) | 20 | Multiple past ART regimens. | PR/RT/IN | NGS at a 1% mutation-detection threshold | 255 | 169 (66%) | 16 (6%) | 70 (27%) |
NGS was performed using Illumina technology. Curanovic 2020 used the Monogram Biosciences GenoSure Archive assay.
For those studies in GenBank or for which mutations are present in tables, DRM totals are based on those DRMs used by the Stanford HIV Drug Resistance Database interpretation program. A subset of samples in two studies (90, 225) included several highly polymorphic mutations comprising approximately 10% and 30% of mutations in these studies, respectively.
In Sotillo 2018, 5 of the 12 patients had undetectable VL at the time of PBMC GRT.
However, in studies of highly treatment-experienced patients, PBMC GRT can be nearly as sensitive or more sensitive than simultaneous plasma virus GRT for detecting DRMs, particularly when NGS is used (90, 109, 110). In one of these studies, which employed a mutation-detection threshold of 5%, many of the PBMC GRT DRMs were detected on historical plasma virus GRTs but not in simultaneously performed plasma virus GRT even at the same threshold (110) (Table 2).
In one clinical trial that is not shown in Table 2, 492 ART-naive patients were randomized to undergo PBMC GRT using an oligonucleotide ligation assay to detect three NNRTI and two NRTI mutations, and 495 were randomized to undergo standard-of-care which did not involve GRT (111). Participants randomized to the GRT arm initiated ART with a ritonavir-boosted lopinavir (lopinavir/r)-containing regimen if they were found to have any of the five tested mutations. The remaining participants in the GRT arm and all of the participants in the standard-of-care arm were randomized to a first line NNRTI-containing regimen. PBMC GRT was found to reduce the risk of VF at 12 months in the subset of participants with TDR. Although simultaneous plasma sequencing was not performed, this trial indicates that there are scenarios in which clinical investigators have chosen to perform PBMC GRT on ART-naive patients possibly because of the potential increased sensitivity of detecting DRMs in PBMC in patients with TDR or because PBMC samples are easier to transport.
ART Discontinuation
In ART-experienced patients who develop VF as a result of HIVDR and discontinue therapy, plasma viruses containing DRMs are usually rapidly outcompeted by ancestral wild-type viruses established in viral reservoirs prior to ART initiation because wild-type viruses replicate better in the absence of ART (112–118). Indeed, by 2 to 3 months after ART discontinuation of therapy, approximately 60% to 90% of nonpolymorphic DRMs are no longer detectable in plasma by Sanger sequencing (112, 114, 116, 117). Limited data suggest DRMs may be detectable for a slightly longer period of time by PBMC compared with plasma virus GRT using Sanger sequencing (112, 114–117). Indeed, in one study of 66 patients who had wild-type plasma viremia after discontinuing ART, only 20% had DRMs detectable by PBMC GRT using NGS at a mutation-detection threshold of 10% (91). In this study, the time since the patients had discontinued therapy was not reported. One likely reason the sensitivity of PBMC GRT might show little improvement over that of plasma virus GRT in this scenario is because in the absence of therapy, there is a rapid accumulation of recently produced wild-type linear and episomal viral DNA within PBMC to some extent obscuring the proviral DNA population. Moreover, if the time since discontinuing therapy was more than several months, the proviral DNA population may have also undergone significant turnover.
Low-Level Viremia
A confirmed plasma HIV-1 RNA level between 200 and 1,000 copies/mL is associated with an increased risk of further elevations in virus load and the emergence of HIVDR (119–128). An increasing number of studies have also reported that patients with persistent plasma HIV-1 RNA levels between 50 to 200 copies/mL, referred to as low-level viremia, are also at an increased risk of VF (123, 125, 129–132).
Low-level viremia in patients receiving ART may result from two distinct processes that have different implications for patient management. In some patients, low-level viremia results from ongoing viral replication and is associated with the risk of emergent HIVDR and further elevations in virus load (133). In other patients, it results from the activation of virus in latently infected, clonally proliferated cells (134–136). Although neither plasma virus nor PBMC GRT can definitively distinguish between these two processes, the detection of HIVDR by GRT by either method could prompt a change in therapy to reduce the risk of further elevations in virus load (137–139). The proportion of patients for whom plasma virus GRT is successful is reduced at plasma virus levels below 1,000 copies/mL and is usually less than 70% at levels below 200 copies/mL (137–143).
Most studies of PBMC GRT in patients with low-level viremia pooled GRT results from patients who had low-level viremia with those who had complete VS (144–149). Only small numbers of PBMC GRT results in patients with low-level viremia have been correlated with either historical (145, 146, 148, 149) or simultaneous plasma virus GRT results (145). In these studies, approximately half of the mutations identified by historical plasma virus GRT and a slightly higher proportion of the mutations identified by simultaneous plasma virus GRT were detected by PBMC GRT (145, 146, 148, 149). In addition, in one of the smaller studies, PBMC GRT did not detect DRMs associated with patients’ most recent regimens, suggesting that if DRMs had developed, they were not yet detectable in PBMCs (147). These findings are consistent with the time lag of weeks to months before emergent DRMs become detectable by PBMC GRT in patients with low plasma virus levels (107, 108). Nonetheless, detecting DRMs in unintegrated viral DNA may be possible as these are more likely to reflect recently circulating viruses (20, 150–152).
PBMC DNA GRT DURING STABLE VIROLOGICAL SUPPRESSION
In patients with stable VS, several studies have assessed the concordance between PBMC and historical plasma virus GRT. Table 3 shows those studies that compared the frequency with which PBMC and historical plasma virus GRT detected DRMs that were detected by either approach (94, 97, 146, 149, 153–155). Table 4 shows those studies that compared the frequency with which PBMC GRT detected M184V/I in patients for whom this mutation was previously detected by historical plasma virus GRT (156–161). In the studies shown in Table 3, PBMC GRT was less sensitive than historical plasma virus GRT for detecting HIV-1 DRMs in five of seven studies (94, 97, 146, 149, 153). The sensitivity of PBMC GRT was higher in patients with a greater number of past VFs and with VFs of longer duration (146, 154, 161), as well as in patients with a higher plasma VL at the time of VF and a higher PBMC VL at the time of PBMC GRT (161). The one study in which PBMC GRT detected more DRMs than historical plasma virus GRT was a clinical trial involving patients who had not experienced VF and who therefore had TDR for which PBMC GRT is more sensitive than plasma virus GRT (155).
TABLE 3.
Comparison of HIV-1 PBMC GRT with historical plasma GRT in ART-experienced patients with VSa
|
Study author and year |
No. of patients | Characteristics | ART history (yrs) | VS duration (yrs)b | Genes | Sequencing methodc | Plasma DRMsd | PBMC DRMse |
|---|---|---|---|---|---|---|---|---|
| Verhofstede 2004 (154) | 11 | History of HIVDR on suboptimal ART. | 8.5 | 5 | RT | Sanger sequencing of 3 to 8 limiting-dilution clones (in GenBank) | 47 (96%) | 45 (92%) |
| Wirden 2011 (149) | 151 | Heavily treated; VS (70%) or LLV (30%); median 3 historical GRTs. | NA | NA | PR/RT | Sanger sequencing | NA (94%) | NA (61%) |
| Delaugerre 2012 (153)f | 121 | Heavily treated patients in the ANRS EASIER Trial; median 4 historical GRTs. | 13.6 | 0.25 | PR/RT | Sanger sequencing | 1408 (NA) | 889 (NA) |
| Zaccarelli 2016 (146) | 149 | VS (~60%) or LLV (~40%) with ≥ 2 historical GRTs. | 8 | NA | PR/RT/IN | Sanger sequencing | 677 (94%) | 304 (42%) |
| Lambert-Niclot 2016 (97)g | 114 | History of VF. | 3.1 | ≥1 | RT | Sanger sequencing | 101 (NA) | 71 (NA) |
| Porter 2016 (155) | 51 | Clinical trial participants with transmitted K103N without a history of VF. | 2.4 | ≥0.5 | RT | NGS at a 10% mutation-detection threshold | 46 (69%) | 62 (92%) |
| Hoffmann 2022 (94)h | 96 | Patients with history of DRMs associated with ≥3 ART classes. | NA | 9 | PR/RT/IN | NGS at a 15% mutation-detection threshold | 872 (93%) | 610 (65%) |
| NGS at a 1% mutation detection threshold | 872 (84%) | 831 (80%) |
NA, details not available or not provided in the study.
For all studies, VS was defined as a plasma HIV-1 RNA level <50 copies/mL except for Delaugerre 2012 which included patients with levels <400 copies/mL.
Illumina NGS technology was used for Porter 2016 and Hoffmann 2022. The Monogram Biosciences GenoSure Archive assay, which uses a mutation-detection threshold of 10%, was used for Porter 2016.
Number of DRMs detected in plasma with the percentage indicating the number DRMS detected in plasma/(number of DRMs detected in plasma + number of DRMs detected in PBMCs).
Number of DRMs detected in PBMC with percentage indicating the number of DRMs detected in PBMC/(number of DRMs detected in PBMC + number of DRMs detected in plasma). The plasma DRMs percentage was below 100% when PBMC GRT detected DRMs that were not present by historical plasma virus GRT.
Successful genotyping of both PR and RT in 121 patients; RT alone in 128 patients; and PR alone in 156 patients. A total of 34 nonpolymorphic RT and PR mutations were evaluated.
Only DRMs associated with tenofovir, emtricitabine, and rilpivirine were reported.
PBMC NGS was performed at two time points (2017, 2020) and compared to historical plasma GRT; percentage is based on total number of DRMs identified at all time points assessed. In an additional study, PBMC GRT and historical plasma virus GRT were compared for their ability to detect resistance to seven ARVs but data on DRMs were not provided (148).
TABLE 4.
HIV-1 PBMC GRT in ART-experienced patients with VS and M184V/I by historical plasma virus GRTa
| Study author and year | No. of patients with past M184V/Ib | Patient characteristics | ART history (yrs) | VS duration (yrs)c | Sequencing methodd | Detection of M184V/I by PBMC GRT |
|---|---|---|---|---|---|---|
| Charpentier 2017 (156) | 8 of 18 tested | Heavily treated; DOLULAM cohort; 18 of 27 had undergone historical plasma virus GRT. | 18 | 4.3 | NGS at mutation-detection threshold of 1% | 38% |
| DeMiguel 2020 (157) | 21 of 41 | ART-PRO single-arm trial. | 18 | 6 | NGS at 20%, 10%, 5%, 1% mutation-detection thresholds | 33%, 52%, 67%, and 95% at 20%, 10%, 5%, and 1% mutation-detection thresholds, respectively. |
| Margot 2020 (158) | 84 | GS-US-282-1824 EVG/c/F/TAF trial participants with history of M184V/I. | 17 | 7 | NGS at 10% mutation-detection threshold | 48% |
| Montejano 2021 (159) | 52 | ART-experienced GEN-PRO cohort with history of M184V/I. | 19 | 8.7 | Sanger sequencing | 27% |
| NGS at 20%, 10%, 5%, 1% mutation-detection thresholds | 48%, 65%, 75%, and 88% at 20%, 10%, 5%, and 1% mutation-detection thresholds, respectively. | |||||
| Jimenez de Ory 2021 (160) | 12 | Perinatally infected youth with history of M184V. | 13.5 | 4 | Sanger sequencing | 25% |
| Delaugerre 2021 (161, 234) | 242 of 252 | MOBIDIP participants with history NRTI/NNRTI resistance on WHO 1st-line ART with VS on 2nd-line PI-based ART. | 7.5 | 3 | NGS at 5% and 1% mutation-detection thresholds | 72% and 81% at 5% and 1% mutation-detection thresholds, respectively. |
Historical plasma virus GRT was performed using Sanger sequencing in all cases.
Jimenez De Ory 2021 did not include M184I.
Plasma HIV-1 RNA level <50 copies/mL and for ≥6 months.
Illumina technology was used for all NGS studies. Margot 2020 used the Monogram Biosciences GenoSure Archive assay.
In the studies shown in Table 4, PBMC GRT using Sanger sequencing detected M184V/I in about 25% of those in whom it had been detected by historical plasma virus GRT. In contrast, when PBMC GRT was performed using NGS, M184V/I was detected in 40% to 90% of patients, with higher sensitivities reported at the lower mutation-detection thresholds.
Sequential PBMC GRT in the setting of stable VS has been described in at least four studies (Table 5). In one study, there was a marked reduction in the detection of DRMs in PBMCs over a period of 5 years (162). In this study, the number of NRTI-associated DRMs decreased from 40 to 14 in 10 patients receiving an NRTI-sparing suppressive regimen and the number of NNRTI-associated DRMs decreased from 10 to 0 in 11 patients receiving an NNRTI-sparing regimen. The reduced detectability of DRMs in this population may reflect the fact that in nine of the patients with clearance of PBMC DRMs, there were periods of residual viremia that may have generated new linear and episomal DNA DRMs that obscured persistent proviral DNA DRMs. In two of the remaining three studies, there were modest reductions in the detectability of PBMC DRMs over a period of 24 to 72 months (163, 164). In a fourth study of 20 heavily treated patients who possessed a mean of 12 RT and protease DRMs detected by PBMC GRT prior to VS, there was little change in the number of DRMs after 18 months of VS (165).
TABLE 5.
Studies Tracking DRMs at multiple time points by PBMC GRT in patients with VS
| Study author and year | No. of patients | Patient characteristics | VS (yrs) | Genes | Sequencing methoda | No. of baseline DRMsb | No. of follow-up DRMsb | Notes |
|---|---|---|---|---|---|---|---|---|
| Falasca 2013 (165) | 20 | VS after salvage ART with a DRV/r-based regimen. | 1.5 | PR/RT | Sanger sequencing | ~240 | ~240 | In 6 patients, the no. of DRMs increased. In 3 patients, the no. of DRMs decreased. The specific DRMs were not reported. |
| Gantner 2016 (163) | 10 | VS after salvage ART (INNOVE and ANRS 123 ETOILE studies). | 2–6 | PR/RT | NGS at a mutation-detection threshold of 1% | 69 | 44 | In 3 patients, all 23 baseline DRMs were no longer detected after 48–72 mo. In 1 patient, 6 new DRMs were detected. No meaningful change observed in 6 patients. |
| Michelini 2016 (164) | 12 | VS after salvage ART. | 2 | PR/RT | Sanger sequencing | 118 | 100 | In 6 patients, 22 baseline DRMs were no longer detected. In 3 patients, 4 new DRMs were detected. |
| Nouchi 2018 (162)c | 21 | VS on an NRTI- (n = 10) or NNRTI-sparing (n = 11) regimen. | 5 | RT | Sanger sequencing and NGS at a 1% mutation-detection threshold | 57 | 21 | In 10 patients receiving an NRTI-sparing regimen, the no. of detectable NRTI DRMs with a proportion >10% decreased from 40 to 14. In 11 patients receiving an NNRTI-sparing regimen, the no. of detectable nonpolymorphic NNRTI DRMs with a proportion >10% decreased from 10 to 0. |
454 technology was used for Gantner 2016 and Illumina NGS technology was used for Nouchi 2018.
In studies for which mutations were present in tables, the DRM totals were based on those DRMs used by the Stanford HIV Drug Resistance Database interpretation program.
In Nouchi 2018, residual viremia was found at least once during the 5 years in 13 patients of whom 9 showed progressive clearance of archived DRMs. DRV/r, ritonavir-boosted darunavir.
Genotypic testing has also been performed to determine HIV-1 tropism and the likely response to the CCR5 inhibitor maraviroc. Such testing involves sequencing the HIV-1 envelope gp120 V3 loop often in combination with other parts of gp120 (166). The detection of envelope variants that are associated with CXCR4 tropism is a contraindication to prescribing maraviroc, even if these variants represent only a small proportion of a patient’s virus population. Although phenotyping is more sensitive for detecting minor populations of CXCR4 tropic viruses, NGS GRT is more widely available and is considered an acceptable substitute for phenotypic testing (166). The determination of tropism by simultaneous plasma virus and PBMC sequencing have been about 85% concordant (167–169). Furthermore, the determination of tropism by PBMC sequencing has been used as an inclusion criterion for clinical trials that have involved switching patients with VS to a maraviroc-containing regimen (170).
CLINICAL SIGNIFICANCE OF PBMC DNA GRT DURING STABLE VIROLOGICAL SUPPRESSION
The first clinical trial which involved changing therapy in patients with VS randomized patients with plasma HIV-1 RNA levels below 200 copies/mL for 16 to 24 weeks who were receiving zidovudine/lamivudine/indinavir to either zidovudine/lamivudine alone, indinavir alone, or continued three-drug therapy (171). Patients who were randomized to zidovudine/lamivudine or indinavir were more likely to experience VF than those who continued three drug-therapy (23% versus 4%; P < 0.001). Although PBMC GRT was not performed in this trial, one of two other subsequent studies that involved switching patients with VS on a PI-containing regimen to a triple NRTI-containing regimen containing abacavir/lamivudine/zidovudine reported that the detection of NRTI-associated mutations by PBMC GRT increased the risk of VF (172, 173) (Table 6).
TABLE 6.
Studies describing the predictive value of PBMC GRT in patients with VS switching ART regimens
| Study author and year | No. of patients | Patient characteristics | Sequencing methoda | PBMC GRT results and virologic response |
|---|---|---|---|---|
| Opravil 2002 (173) | 84 | Patients with VS on a PI-containing ART regimen who were switched to a triple NRTI regimen (ABC/3TC/AZT). | Sanger sequencing (PR/RT) | 13 (15%) patients experienced VF. 6/13 had ≥1 ABC-resistance mutation. It was not possible to determine whether these mutations increased the risk of VF. |
| Pellegrin 2003 (172) | 55 | Patients with VS on a PI-containing ART regimen who were switched to a triple NRTI regimen (ABC/3TC/AZT). | Sanger sequencing (PR/RT) | 9/15 (60%) patients with and 8/34 (24%) without ≥1 ABC-resistance mutations experienced VF. The no. of ABC-resistance mutation (P = 0.009) and the PBMC virus load (P = 0.006) were each associated with an increased risk of VF. |
| Porter 2016 (155) | 51 | Patients with VS and no history VF or historical plasma virus resistance to TDF, FTC, or RPV who switched from a PI-containing regimen to TDF/FTC/RPV. Subset of 24 patients with and 27 without K103N history who had stored PBMC samples (SPIRIT trial). | NGS at 10% mutation-detection threshold (PR/RT) | Of 4 patients with VF and emergent HIVDR, 1 had Y181C and M184I detected by PBMC GRT at baseline. E138A (n = 4), E138G (n = 1), E138K (n = 1), and E138Q (n = 1) were present at baseline in patients who did not develop VF. |
| Armenia 2018 (204) | 227 | ART-experienced patients with a history of VS for a median of 4 yrs who underwent PBMC GRT and switched ART. | Sanger sequencing (PR/RT/IN) | 24 mo after switch, 28% of patients with intermediate or full resistance to one of the drugs used at the time of the switch experienced VF compared with 14% lacking genotypic resistance. |
| Ellis 2020 (205) | 83 | Clinic patients undergoing PBMC GRT. 66 switched ART and 59 had postswitch follow-up. 76% had VS and 15% had LLV. 46% had been on ≥2 regimens; 34% lacked a complete ART history. Only 9 had historical plasma virus GRTs. | NGS at 10% mutation-detection threshold (PR/RT/IN) | Among the 24 patients not switching ART, 4 chose not to switch because of the presence of multiclass resistance by PBMC GRT. In a logistic regression model, switching therapy did not appear to increase the risk of subsequent VF. |
| Meybeck 2020 (148) | 185 | Clinic patients undergoing PBMC GRT. 73 switched ART most commonly to an INSTI- (70%) or RPV- (8%) based regimen. The mean no. of previous ART regimens was 5.4. Approx, one-half had historical plasma virus GRTs. | NA | After 6 mo, those who switched therapy had a lower risk of VF than those who continued their original regimen (19% vs. 5%). Other predictors of VF were higher PBMC virus load, lower CD4 nadir, and shorter time of VS. |
| Rodriguez 2021 (206) | 72 | STRUCTR trial participants with VS while on EVG/c/TAF/FTC or EVG/c/TDF/FTC. Patients were switched to ABC/3TC/DTG if PBMC GRT did not detect DRMs associated with this regimen. | NGS at 10% mutation-detection threshold (PR/RT/IN) | 9 of 72 screening patients were found to have resistance to ABC and/or 3TC and therefore did not switch therapy. 50 patients were switched to ABC/3TC/DTG. 44 of 44 evaluable patients maintained VS at wk 48. |
| Overton 2021 (184) | 1045 | Of 1,045 ATLAS-2M participants, 10 had confirmed VF. Patients had been VS for ≥6 mo and had no history of VF or baseline NNRTI or INSTI resistance by plasma virus GRT associated with cabotegravir or RPV, the drugs used for ART simplification. | NGS at 10% mutation-detection threshold (PR/RT/IN) | 5 of 10 patients with confirmed VF had NNRTI resistance by PBMC GRT that was not detected by historical plasma virus GRT, including 3 with major NNRTI-resistance DRMs (Y181C+H221Y; Y188L; Y188L) and 2 with E138A. |
Illumina was used for NGS in each study. ABC, abacavir; 3TC, lamivudine; AZT, zidovudine; TDF, tenofovir DF; FTC, emtricitabine; RPV, rilpivirine; EVG/c, elvitegravir/cobicistat; DTG, dolutegravir; NA, details not available or not provided in the study.
In 2010, the SWITCHMRK I and II trials reported that patients with VS (defined as having a plasma HIV-1 RNA level <50 or <75 copies/mL for three or more months) on a lopinavir/r-containing regimen who substituted raltegravir for lopinavir/r had a 6.2% increased risk of VF at week 24 (95% CI: 1.3%–11.2%) compared to those who continued lopinavir/r (174). Although baseline PBMC GRT was not performed in this trial, the finding that the increased risk of VF was confined to patients who had a history of VF led to the hypothesis that pretherapy NRTI-resistance was likely responsible for the increased risk of VF in the raltegravir arms.
During the past 10 years, there have been many clinical trials in patients with stable VS that involved changing therapy from a PI-containing regimen to an NNRTI- or INSTI-containing regimen. The inclusion criteria of most of these trials were designed to minimize the risk of VF and emergent HIVDR. Indeed, tenofovir/emtricitabine/rilpivirine (155), dolutegravir/lamivudine (175–180), dolutegravir/rilpivirine (181), and long-acting cabotegravir/rilpivirine (182, 183) were usually studied in patients without a history of VF or DRMs associated with lamivudine, rilpivirine, or INSTI resistance. Although VFs were rare with these regimens, the few patients with VF and emergent HIVDR appeared to be more likely to have had preexisting DRMs detectable by PBMC GRT than those without VF (155, 184, 185) (Table 6). In contrast, several three-drug regimens, including tenofovir/emtricitabine/bictegravir (186), tenofovir/emtricitabine/elvitegravir/cobicistat (187), and abacavir/lamivudine/dolutegravir (188) were shown to be efficacious at maintaining VS even in patients with a history of lamivudine resistance.
During the past 5 years, there have been four clinical trials in which dolutegravir monotherapy has been investigated for ART simplification. In a meta-analysis of these trials, the risk of VF among patients randomized to dolutegravir monotherapy was 7% (16/227) compared with 0% (0/189; P < 0.001) for patients continuing their previous ART regimen (189). Among 15 patients who underwent integrase sequencing at the time of VF, seven (3.1% of the total) had newly developed INSTI-resistance mutations. Four factors were identified as risk factors for VF, including a PBMC virus level ≥2.7 copies/mL, a CD4+ count <350 copies/mL, a plasma HIV-1 RNA PCR signal at baseline despite the absence of quantifiable RNA levels and having begun ART more than 3 months after HIV-1 infection (189). Baseline PBMC GRT was not performed in any of the trials in this meta-analysis.
Although dolutegravir monotherapy is not considered an acceptable option for maintaining VS in patients with stable VS (14), dolutegravir/lamivudine has been widely studied to maintain VS even in patients with a history of the lamivudine-resistance mutations M184V/I by historical plasma virus GRT or by preswitch PBMC GRT. Table 7 summarizes the findings from two clinical trials and eight retrospective cohort studies of dolutegravir/lamivudine simplification in about 3,700 patients of whom approximately 480 had M184V/I detected by historical plasma virus GRT and 40 had M184V/I detected by PBMC GRT just prior to starting dolutegravir/lamivudine (156, 157, 180, 190–198). In one open-label trial of 100 patients, in which 50 were stratified based on a history of M184V/I, switching to dolutegravir/lamivudine was shown to be equally effective in those with and without M184V/I (199). In another clinical trial of 493 patients with VS randomized to receive dolutegravir/lamivudine or to continue three-drug ART, a post hoc analysis of preswitch PBMC GRT demonstrated the presence of M184V in 5 of the 192 patients randomized to dolutegravir/lamivudine (180). In the eight retrospective studies, treatment discontinuation rates over a follow-up period of one or more years were consistently below 5% to 10%, and protocol-defined VF rates were even lower, regardless of the patient’s M184V/I history. Although a history of M184V/I in combination with a shorter time of VS was associated with an increased risk of VF (193, 195), there were no reports of emergent INSTI resistance in any of these studies.
TABLE 7.
Studies of dolutegravir (DTG)/lamivudine (3TC) in patients with and without M184V/I by historical plasma virus GRTa
| Study author and year | No. of patients | Patient characteristics | ART (yrs) | VS (yrs) | RT184 genotypes | Outcome |
|---|---|---|---|---|---|---|
| Charpentier 2017 (156) | 27 | Heavily treated; DOLULAM substudy; no INSTI DRMs. | 18 | 6 | 8 of 18 with historical plasma virus GRT had M184V. 3 of these 8 and 7 additional patients had M184V/I detected by PBMC GRT. | No cases of VF occurred during the first yr of ART. |
| Gagliardini 2018 (191) | 436 | Retrospective study of patients switching to DTG/3TC (n = 126) or bPI/3TC (n = 310). | 7.8 | 4.4 | 87 (20%) had M184V by historical plasma virus GRT, including 21 of those switched to DTG/3TC. | VF occurred in 12% with and 8% without M184V. GRT at VF was available only for 8 patients who switched to bPI/3TC. |
| De Miguel 2020 (157, 235) | 41 | Single-arm pilot trial; ART-PRO; INSTI-naive patients switched to DTG/3TC. | 18 | 6 | 21 had history of plasma virus M184V/I of whom 7 (33%), 11 (52%), 14 (67%), and 20 (95%) had M184V/I detected by PBMC GRT at 20%, 10%, 5%, and 1% mutation-detection thresholds. | No patient developed protocol-defined VF or emergent DRMs. |
| Galizzi 2020 (192) | 374 | Retrospective study of VS patients switching to DTG/3TC (n = 307) or DTG/RPV (n = 67). 45% had previous VF. | 15 | 5.1 | 220 patients had a historical plasma virus GRT, including 174 in the 3TC group and 46 in the RPV group. 60 (27%) had a history of M184V/I and 35 had a history of an RPV-resistance mutation. | In the entire 3TC group, 17 patients developed VF after a median of 1.7 yrs. 0/8 patients with GRT at VF developed new INSTI resistance DRMs. |
| Blick 2021 (199) | 100 | SOLAR 3D open label trial. | 22 | 12 | 50 had history of plasma virus M184V/I which was a stratification criterion. | At wk 48, 1 patient in the M184V/I arm and 3 in the non-M184V/I arm had RNA ≥50 copies/mL. None had confirmed VF or emergent DRMs. |
| Borghetti 2021 (190, 193, 194) | 669 | Retrospective study of patients switching to DTG/3TC during VS. 14 patients had previous VF on an INSTI regimen. | 12 | 8 | 48 had history of plasma virus M184V/I. | 23 cases of VF after a median follow-up period of 1.9 yrs. No patients developed HIVDR. A history of M184V/I and a history of VF on an INSTI regimen were associated with an increased risk of VF. |
| Ciccullo 2021 (195, 236) | 785 | Retrospective study of patients switching to DTG/3TC during VS. | 11.5 | 2.5 | 33 had history of plasma virus M184V/I. | 18 cases of VF after a median follow-up period of 2.2 yrs. No patients developed HIVDR. A history of M184V/I combined with a shorter period of VS (<7.3 yrs) was associated with an increased risk of VF. |
| Santoro 2022 (198) | 712 | Retrospective study of patients switching to DTG/3TC during VS (LAMRES cohort). | 7 | 5.2 | 60 (8.4%) had history of past M184V before DTG/3TC switch: 48 (80.0%) only in HIV-RNA GRTs, 10 (16.7%) exclusively in HIV-DNA, and 2 (3.3%) in both HIV-DNA and -RNA. | 22 cases of VF after 1 yr of follow-up. 1 patient developed M184V after 4 mo on DTG/3TC. There was an increased risk of VF in those with M184V and a duration of VS ≤3.5 yrs (M184V+VS ≤3.5 yrs = 22.7%; M184V+VS >3.5 yrs = 7.8%; no mutation + VS ≤3.5 yrs = 9.0%; no mutation + VS >3.5 yrs = 4.9%). |
| Deschanvres 2021, Hocqueloux 2021 (196, 237) | 695 | Retrospective study of patients (Dat’AIDS cohort) switching to DTG/3TC or DTG/FTC. | 10.6 | 6.3 | 105 had historical plasma virus M184V/I a median of 10 yrs before switching therapy. | 15 cases of VF after a median follow-up of 1.2 yrs. No patients developed HIVDR. |
| Underwood 2022 (180) | 192 | Post-hoc analysis to determine proportion of baseline samples with PBMC DRMs (SALSA); patients had VS for ≥6 mo with no history of VF. | 5.5 | NA | 5 patients in DTG/3TC arm had M184V by PBMC GRT at a mutation-detection threshold of 15%. | None of the 5 patients with baseline M184V by PBMC GRT developed VF. |
Patients were HBsAg-negative. Except for Underwood 2022, past VF was allowed and had occurred in a significant proportion of persons in all studies. VF and/or treatment discontinuation were considered outcome variables. Several of the above studies may contain overlapping patients as they are from the same medical centers. bPI, boosted darunavir, lopinavir, or atazanavir; RPV, rilpivirine. NA, details not available or not provided in the study.
It has been hypothesized that in persons with a history of M184V/I or with M184V/I detectable by PBMC GRT, the combination of dolutegravir/lamivudine is more active than dolutegravir monotherapy because M184V/I is associated with reduced virus replication (200–202). Additionally, the success of dolutegravir/lamivudine despite genotypic evidence for lamivudine resistance may be because the risk of reactivation of viruses containing M184V/I is low in patients with prolonged VS and that the number of reactivating viruses with M184V/I may be insufficient to overcome the high genetic barrier to dolutegravir resistance.
Another study which highlighted the importance of baseline PBMC virus load prior to switching to a regimen with a low genetic barrier to resistance was the TRULIGHT trial (203). It found that in patients with VS with an HIV-1 DNA level <2.7 log10 copies/106 PBMC and no history of VF, a regimen containing tenofovir/emtricitabine alone was noninferior at week 48 to a regimen containing tenofovir/emtricitabine plus a third antiretroviral agent (203). Nonetheless, VF was more common in the tenofovir/emtricitabine arm with 6 of 113 patients developing VF compared with 2 of 110 in the control arm. In the tenofovir/emtricitabine arm, one person developed the DRM K65R and one developed the DRM M184V. In contrast, no DRMs developed in the control arm (203). Baseline PBMC GRT was not performed in this trial.
Three additional studies provide anecdotal evidence that PBMC GRT might be useful in clinical practice. One involved 227 heavily treated clinic patients with VS for a median of 4 years who underwent PBMC GRT prior to ART simplification (204). In this analysis, 28% of patients with intermediate or full resistance to one of the drugs used at the time of the switch were likely to experience VF compared with 14% lacking genotypic resistance. In two other clinic-based studies, the use of PBMC GRT was reported to assist clinicians in switching therapy in patients with VS, but the predictive value of DRMs detected by PBMC GRT was not reported (205, 206) (Table 6).
The success of certain regimens, such as dolutegravir or boosted darunavir in combination with lamivudine despite the presence of lamivudine resistance by PBMC GRT, suggests that regimen-specific interpretations may be necessary as regimens anchored by a single fully active drug with a high genetic barrier to resistance may be successful despite the presence of resistance to one or more companion drugs. The success of such regimens, particularly in patients with prolonged VS or low PBMC HIV-1 DNA levels may be part of a spectrum that includes posttreatment controllers who experience prolonged periods of VS following the discontinuation of ART (30, 207–209).
CONCLUSIONS
The main scenario for which PBMC GRT is likely to be most useful in patient management is for modifying therapy in patients with VS on a stable ART regimen. However, there are three additional scenarios in which PBMC GRT has also been studied, albeit less often: (i) modifying therapy in patients with persistent low-level viremia; (ii) detecting DRMs in patients with VF on an ART regimen who recently discontinued therapy; and (iii) detecting DRMs in patients who may have been infected with a drug-resistant virus. In each of these scenarios, there are limitations to the sensitivity of PBMC GRT that must be appreciated to appropriately use PBMC GRTs and to interpret their results.
NGS is preferable to Sanger sequencing for PBMC GRT because it has greater sensitivity for detecting DRMs present in a mixed virus population and because it can be used to reduce the risk of reporting DRMs resulting from G-to-A hypermutation. However, when a low mutation-detection threshold is used, the specific threshold at which each mutation is detected should be reported, as mutations detected at low levels are at increased risk of being PCR or sequence artifact. Moreover, viruses containing mutations present at low levels may be less likely to reactivate than those present at high levels.
In patients with VS, PBMC GRT will often not detect DRMs that were once detected by historical plasma virus GRT. This is particularly the case if previous episodes of VF and emergent drug resistance were either not prolonged or not associated with high levels of plasma viremia (Fig. 1). In contrast, in patients who have had multiple VFs but who infrequently underwent genotypic testing during past episodes of VF, PBMC GRT may be more sensitive than historical plasma virus GRTs. However, in either case, the results of PBMC GRT should be interpreted in conjunction with the results of historical plasma virus GRTs and careful consideration of the drug resistance likely to have emerged during past episodes of VF.
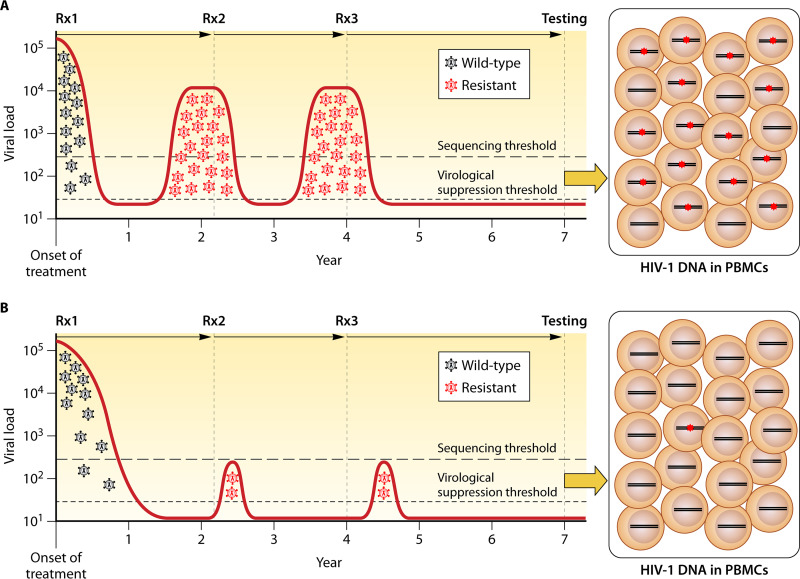
FIG 1.
Sensitivity of peripheral blood mononuclear cell (PMBC) genotypic resistance testing (GRT) in patients with plasma virus suppression while receiving antiretroviral therapy (ART). In the first scenario (A), in which previous episodes of virological failure (VF) were prolonged and associated with high plasma HIV-1 RNA levels, the likelihood of detecting drug-resistance mutations (DRMs) in proviral DNA will be high. In the second scenario (B), in which previous episodes of VF were short and associated with low plasma HIV-1 RNA levels, the likelihood of detecting DRMs in proviral DNA will be low. Rx1, Rx2, and Rx3 followed by an arrow indicate periods of antiviral therapy. Proviral DNA containing DRMs are indicated by red asterisks in the boxes at the right side of the figure.
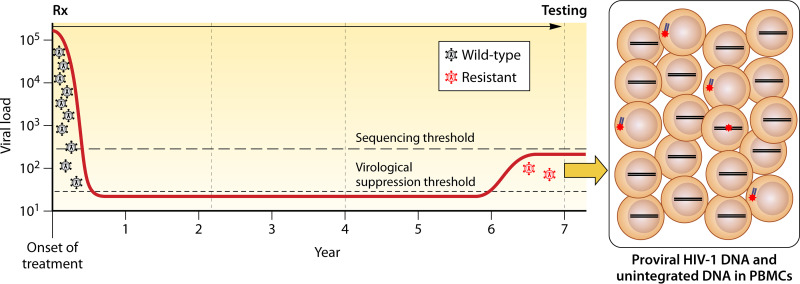
In patients with persistent low-level viremia, PBMC GRT is likely to be insensitive at detecting emergent drug resistance because it can take several months for newly emergent DRMs to become detectable (Fig. 2). Moreover, there are few studies of PBMC GRT in this scenario. If the plasma virus level is high enough, plasma virus GRT should be initially attempted. PBMC GRT may be useful because unintegrated linear and episomal DNA are in equilibrium with plasma virus and may be detectable, although there are no published studies to support this conjecture.
FIG 2.
Sensitivity of PMBC GRT in patients with the recent development of low-level viremia while receiving ART. Rx followed by an arrow indicates the period of antiviral therapy. The dashed line indicates a threshold of between 200 and 500 copies/mL below which plasma virus GRT is often not successful. The likelihood of detecting DRMs in proviral DNA is likely to be low. Detecting DRMs in unintegrated linear and episomal viral DNA may be possible because these are more likely to reflect recently circulating viruses. Proviral and unintegrated viral DNA containing DRMs are indicated by red asterisks. In the boxed figure on the right, unintegrated linear viral DNA is shown outside the nucleus. Episomal DNA is not shown; its dynamics are likely to be closer to unintegrated linear DNA than proviral DNA.
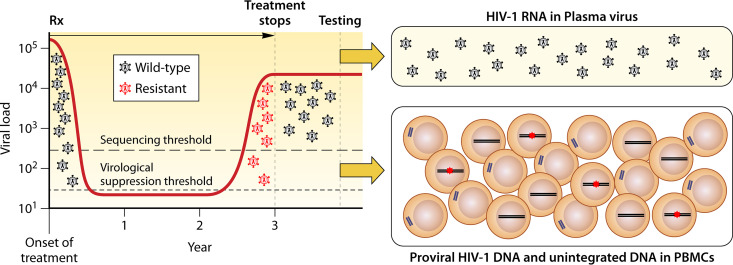
Plasma virus GRT is most valuable when performed while a patient experiencing VF is still taking antiretroviral drugs or within 4 weeks after discontinuing therapy (14). If a patient has been off therapy for a longer period and plasma virus GRT does not explain the cause of VF, PBMC GRT may be considered (Fig. 3). However, the sensitivity of PBMC GRT in this scenario may not be high if the duration of viremia while receiving ART was not prolonged. Moreover, within several months following ART discontinuation, there will be an accumulation of recently produced nonintegrated HIV-1 DNA genomes and a partial turnover of the proviral DNA population.
FIG 3.
Sensitivity of plasma virus and PMBC GRT in patients who discontinue ART following VF and emergent HIV drug resistance (HIVDR). Rx followed by an arrow indicates the period of antiviral therapy. Most DRMs will no longer be detectable by plasma virus GRT within 2 to 3 months following ART discontinuation because plasma viruses containing DRMs are often rapidly outcompeted by ancestral wild-type viruses established in viral reservoirs prior to ART initiation. Although recently emergent DRMs may have begun to seed the proviral DNA reservoir, their levels in this compartment will be low unless VF has been prolonged. In addition, the presence of unintegrated linear and episomal viral DNA, which are in equilibrium with circulating plasma virus, may also reduce the proportion of viral DNA molecules containing DRMs. In the boxed figure on the right, proviral DNA containing DRMs are indicated by red asterisks. Unintegrated linear viral DNA is shown outside the nucleus. Episomal DNA is not shown; its dynamics are likely to be closer to unintegrated linear DNA than proviral DNA.
PBMC GRT may be more sensitive than plasma virus GRT at detecting transmitted DRMs, as viruses containing these DRMs are often outcompeted in plasma by viruses containing wild-type revertants. Nonetheless, such testing has rarely been performed and the added clinical benefit of such testing is not known.
Limitations to the sensitivity of PBMC GRT are described above, however, there may also be limitations to its specificity. Although most laboratories performing PBMC GRT employ algorithms to minimize the reporting of DRMs resulting from APOBEC-mediated G-to-A hypermutation, these algorithms are imperfect. In addition, mutations present at low-levels within PBMC may represent PCR or sequence artifact. Finally, while there are many studies demonstrating the clinical relevance of DRMs detected by plasma virus GRT, there are few such studies for PBMC GRT at this time. Nonetheless, ART modifications in patients with sustained VS are being increasingly studied in clinical trials and performed in clinical practice. As premodification PBMC GRT will be increasingly performed prospectively or on stored samples in clinical trials, there will be an increased opportunity to determine the predictive value of PBMC GRT on the response to a new ART regimen.
Sidebars
Clonal expansion.
Around 2005, several groups observed that low-level viremia in patients receiving ART was often composed of viruses with identical env sequences (134, 135). The levels of viremia observed suggested that multiple cells from a clonal lineage were synchronously producing virions because single cells would be incapable of producing the observed quantities of viruses (210). Although clonal expansion of proviral DNA-containing cells may also be occurring in patients with ongoing virus replication, this phenomenon only became noticeable in patients with VS. This is in part because CD4+ lymphocytes are no longer becoming newly infected with diverse viral variants and in part because clonally proliferating cells appear to account for a higher proportion of circulating virus the longer a patient maintains VS (67, 210). To prove that identical viral sequences were due to the proliferation of infected cells expressing circulating viruses, several groups developed assays to demonstrate these identical viral sequences were being produced by proviruses integrated in the same human genomic location (72, 210–212).
APOBEC-mediated G-to-A hypermutation.
APOBEC3F and 3G are host cytidine deaminases that inhibit viral infections by causing extensive cytidine (C) to uridine (U) editing of negative-strand viral RNA in newly infected cells. This results in guanosine (G) to adenosine (A) hypermutations in plus-stranded cDNA (95). APOBEC 3F and 3G act in specific dinucleotide contexts. APOBEC3F causes GA to be mutated to AA and APOBEC3G causes GG to be mutated to GA. The HIV-1 Vif protein targets host APOBEC molecules for proteasomal destruction and usually blocks the actions of APOBEC3F and 3G. Nonetheless, for unknown reasons, APOBEC-mediated viral editing is frequently successful and results in defective nonviable integrated hypermutated genomes. These genomes often contain stop codons due to APOBEC3G editing of tryptophan (W): TGG → TAG or TGG → TGA or active site mutations in PR (D25N), RT (D110N, D185N, and D186N), and IN (D64N, D116N, and E152K) resulting from APOBEC3F editing of aspartic acid GAC/T (D) → AAC/T (N) or glutamic acid GAA/G (E) → AAA/G (K). PBMC sequences can be considered hypermutated if they have a global excess of G to A changes in the appropriate dinucleotide context (213) or if they contain two to three or more signature APOBEC mutations in a single sequence (99). In either scenario, any DRM that can arise in an APOBEC dinucleotide context should be regarded with caution. The list of such mutations includes D30N, M46I, G48S, and G73S in PR; D67N, E138K, M184I, G190ES, and M230I in RT; and G118R, E138K, G140RS, G163KR, D232N, and R263K in IN.
Tropism.
In addition to attaching to the CD4+ receptor, HIV-1 must bind one of two coreceptors, CCR5 or CXCR4. Most patients are primarily infected by CCR5-tropic HIV-1 variants, with CXCR4-tropic variants emerging during disease progression (128, 214). The CCR5 inhibitor maraviroc is inactive against CXCR4 variants. In patients receiving CCR5 inhibitors, the most common mechanism of VF is the expansion of preexisting CXCR4 tropic viruses intrinsically resistant to CCR5 inhibitors (215). CXCR4 tropism is most reliably detected phenotypically using pseudotyped viruses containing the HIV-1 env gene (216). It can also be detected genotypically as certain positively charged residues at positions 11 and 25 of the V3 loop of gp120 and several less common combinations of mutations primarily, but not exclusively, within the V3 loop are associated with CXCR4 tropism (217).
Next-generation sequencing and low-abundance variants.
Dideoxynucleotide Sanger sequencing of nonclonal PCR products (direct PCR sequencing) of plasma viral cDNA has been the standard approach to HIV-1 GRT for more than 25 years. Sanger sequencing usually detects HIV-1 variants present in proportions above 20% with a range of 10% to 30% depending on the nucleotide context (82–84). Next-generation sequencing (NGS) technologies have been used in research studies to detect variants present in proportions as low as 1% (218). However, mutation-detection thresholds of ≥5.0% have been recommended for use in clinical settings to reduce the risk of experimental artifact and increase reproducibility (15, 85, 86, 99). NGS is not necessarily considered superior to Sanger sequencing for plasma virus GRT. This is because the number of additional DRMs detected by plasma virus GRT at mutation-detection thresholds between 5% to 20% is not very high, as most DRMs emerge rapidly in plasma in patients experiencing VF (15, 87). However, the ability to detect variants below 20% is more important for PBMC GRT because the emergence of DRMs in PBMCs is slower than in plasma.
ACKNOWLEDGMENTS
LabCorp-Monogram Biosciences is a wholly owned subsidiary of Labcorp, Burlington, North Carolina, USA. R.W.S. was supported by a grant from the NIH/National Institute of Allergy and Infectious Diseases (NIAID), R24AI36618. C.C. was supported by a grant from the Health Resources and Services Administration (HRSA), 6 UIOHA30039.
Biographies

Carolyn Chu, MD, MSc is Professor of Clinical Family Community Medicine at the University of California San Francisco. She is a family physician and HIV specialist who completed undergraduate education at Case Western Reserve University, received her medical degree at the University of North Carolina at Chapel Hill, and completed a clinical research fellowship at Albert Einstein College of Medicine/Montefiore Medical Center in the Bronx, NY. She joined the AETC Program’s National Clinician Consultation Center in 2015 and currently serves as its PI/Chief Clinical Officer. From 2009–2015 she served as HIV Clinical Coordinator/Medical Director for various federally qualified health center networks in New York. Dr. Chu practices at the San Francisco General Hospital Family Health Center where she also helps support HIV training/education and care delivery initiatives.

Daniele Armenia, PhD, is an Associate Professor of Microbiology and Clinical Microbiology at the Saint Camillus International University of Health Sciences in Rome, Italy. He holds an undergraduate degree in Pharmaceutical Biotechnology from the University of Bologna, Italy, and a PhD in Medical Microbiology and Immunology from the University of Rome “Tor Vergata”, Italy. Dr. Armenia is Graduate Program Director of the Degree course in Biomedical Laboratory Techniques at the Saint Camillus International University of Health Sciences in Rome. He has been involved in studies on HIV-1 drug resistance since 2008 and has dedicated attention to the characterization (by both experimental and computational approaches) of structural and biological modifications of HIV integrase, reverse transcriptase and protease, and also on HIV-1 tropism determination. He has investigated the clinical relevance of HIV-1 resistance detected in plasma and PBMCs through Sanger and next-generation sequencing, in both viremic and virologically suppressed individuals.

Charles Walworth, MD completed his undergraduate studies and medical school at Georgetown University. He subsequently completed a fellowship in Infectious Diseases at the National Institutes of Health and served as the HIV Clinical Trials Physician in the NIAID Clinical Trials Unit. Dr. Walworth later entered private practice in Orange County, CA where he built a large HIV practice, conducted numerous clinical trials, and worked closely with HIV/AIDS service organizations. In 2008, he joined Monogram Biosciences in South San Francisco, CA, as Director of Global Medical Affairs. Dr. Walworth is now the Associate Vice President of Medical Affairs and Education at Monogram Biosciences, a wholly owned subsidiary of Labcorp, where he also serves as discipline director for molecular infectious diseases. Dr. Walworth has a strong interest in the clinical application of HIV DNA genotypic testing as an adjunct to plasma viral resistance testing.

Maria M. Santoro, PhD, is an Assistant Professor of Microbiology and Clinical Microbiology at the University of Rome “Tor Vergata”, Italy. She holds an undergraduate degree in Biology and attained a PhD in Medical Microbiology and Immunology at the University of Rome “Tor Vergata”, Italy. Dr. Santoro is a member of the Steering Committee of the ARCA Database (https://www.dbarca.net/), the ICONA Study group (fondazioneicona.org/_new2/), and the Italian PRESTIGIO Registry (https://ichgcp.net/clinical-trials-registry/NCT04098315). She was a panel member for the Italian guidelines on management of HIV-1 infected patients (2012–2017) and has been involved in HIV research since 2002. Her main interests are on viral genotyping (by Sanger and next-generation sequencing), viral quantification, HIV drug resistance, viral evolution and dynamics, and the structural and functional analysis of viral proteins. Dr. Santoro has been involved in several studies assessing the clinical relevance of HIV-1 drug resistance detected in plasma and PBMCs.

Robert W. Shafer, MD is a Professor of Medicine, and by courtesy, Pathology in the Division of Infectious Diseases at Stanford University. Dr. Shafer received his undergraduate education at Columbia University and his medical degree at New York University. He has studied the mechanisms and consequences of virus evolution with a focus on antiretroviral drug therapy and drug resistance. In 2000, he created the Stanford HIV Drug Resistance Database, a publicly available resource for those performing HIV drug resistance surveillance, interpreting HIV drug resistance tests, and developing new antiretroviral drugs. His work is focused on the clinical management, epidemiology, and laboratory science of HIV drug resistance. In 2020, Dr. Shafer created the SARS-CoV-2 Antiviral and Resistance Database to characterize the phenotypic effects of mutations responsible for SARS-CoV-2 antiviral and antibody resistance.
REFERENCES
- 1.DeGruttola V, Dix L, D'Aquila R, Holder D, Phillips A, Ait-Khaled M, Baxter J, Clevenbergh P, Hammer S, Harrigan R, Katzenstein D, Lanier R, Miller M, Para M, Yerly S, Zolopa A, Murray J, Patick A, Miller V, Castillo S, Pedneault L, Mellors J. 2000. The relation between baseline HIV drug resistance and response to antiretroviral therapy: re-analysis of retrospective and prospective studies using a standardized data analysis plan. Antivir Ther 5:41–48. 10.1177/135965350000500112. [DOI] [PubMed] [Google Scholar]
- 2.Lanier ER, Ait-Khaled M, Scott J, Stone C, Melby T, Sturge G, St Clair M, Steel H, Hetherington S, Pearce G, Spreen W, Lafon S. 2004. Antiviral efficacy of abacavir in antiretroviral therapy-experienced adults harbouring HIV-1 with specific patterns of resistance to nucleoside reverse transcriptase inhibitors. Antivir Ther 9:37–45. 10.1177/135965350400900102. [DOI] [PubMed] [Google Scholar]
- 3.Miller MD, Margot N, Lu B, Zhong L, Chen S-S, Cheng A, Wulfsohn M. 2004. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis 189:837–846. 10.1086/381784. [DOI] [PubMed] [Google Scholar]
- 4.Kempf DJ, Isaacson JD, King MS, Brun SC, Sylte J, Richards B, Bernstein B, Rode R, Sun E. 2002. Analysis of the virological response with respect to baseline viral phenotype and genotype in protease inhibitor-experienced HIV-1-infected patients receiving lopinavir/ritonavir therapy. Antivir Ther 7:165–174. 10.1177/135965350200700305. [DOI] [PubMed] [Google Scholar]
- 5.de Meyer S, Vangeneugden T, van Baelen B, de Paepe E, van Marck H, Picchio G, Lefebvre E, de Béthune M-P. 2008. Resistance profile of Darunavir: combined 24-week results from the POWER trials. AIDS Res Hum Retroviruses 24:379–388. 10.1089/aid.2007.0173. [DOI] [PubMed] [Google Scholar]
- 6.Rhee S-Y, Fessel WJ, Liu TF, Marlowe NM, Rowland CM, Rode RA, Vandamme A-M, Van Laethem K, Brun-Vezinet F, Calvez V, Taylor J, Hurley L, Horberg M, Shafer RW. 2009. Predictive value of HIV-1 genotypic resistance test interpretation algorithms. J Infect Dis 200:453–463. 10.1086/600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, Poizot-Martin I, Richmond G, Soriano V, Ait-Khaled M, Fujiwara T, Huang J, Min S, Vavro C, Yeo J, Group for the VS, Walmsley SL, Cox J, Reynes J, Morlat P, Vittecoq D, Livrozet J-M, Fernández PV, Gatell JM, DeJesus E, DeVente J, Lalezari JP, McCurdy LH, Sloan LA, Young B, LaMarca A, Hawkins T, for the VIKING Study Group . 2013. Safety and efficacy of Dolutegravir in treatment-experienced subjects with Raltegravir-resistant HIV type 1 infection: 24-Week results of the VIKING study. J Infect Dis 207:740–748. 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertagnolio S, Hermans L, Jordan MR, Avila-Rios S, Iwuji C, Derache A, Delaporte E, Wensing A, Aves T, Borhan ASM, Leenus A, Parkin N, Doherty M, Inzaule S, Mbuagbaw L. 2020. Clinical impact of pretreatment human immunodeficiency virus drug resistance in people initiating nonnucleoside reverse transcriptase inhibitor–containing antiretroviral therapy: a systematic review and meta-analysis. The J Infectious Diseases 224:377–388. 10.1093/infdis/jiaa683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxter JD, Mayers DL, Wentworth DN, Neaton JD, Hoover ML, Winters MA, Mannheimer SB, Thompson MA, Abrams DI, Brizz BJ, Ioannidis JPA, Merigan TC. 2000. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. AIDS 14:F83–F93. 10.1097/00002030-200006160-00001. [DOI] [PubMed] [Google Scholar]
- 10.Tural C, Ruiz L, Holtzer C, Schapiro J, Viciana P, González J, Domingo P, Boucher C, Rey-Joly C, Clotet B, Group and the HS . 2002. Clinical utility of HIV-1 genotyping and expert advice: the Havana trial. Aids 16:209–218. 10.1097/00002030-200201250-00010. [DOI] [PubMed] [Google Scholar]
- 11.Durant J, Clevenbergh P, Halfon P, Delgiudice P, Porsin S, Simonet P, Montagne N, Boucher C, Schapiro J, Dellamonica P. 1999. Drug-resistance genotyping in HIV-1 therapy: the VIRAD APT randomi sed controlled trial. Lancet 353:2195–2199. 10.1016/S0140-6736(98)12291-2. [DOI] [PubMed] [Google Scholar]
- 12.Cingolani A, Antinori A, Rizzo MG, Murri R, Ammassari A, Baldini F, Di Giambenedetto S, Cauda R, De Luca A. 2002. Usefulness of monitoring HIV drug resistance and adherence in individuals failing highly active antiretroviral therapy: a randomized study (ARGENTA). Aids 16:369–379. 10.1097/00002030-200202150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Palella FJ, Armon C, Buchacz K, Cole SR, Chmiel JS, Novak RM, Wood K, Moorman AC, Brooks JT, HOPS (HIV Outpatient Study) Investigators . 2009. The association of HIV susceptibility testing with survival among HIV-infected patients receiving antiretroviral therapy: a cohort study. Ann Intern Med 151:73–84. 10.7326/0003-4819-151-2-200907210-00003. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. 2021. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. US Department of Health and Human Services, Washington, DC. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/archive/AdultandAdolescentGL_2021_08_16.pdf.
- 15.Günthard HF, Calvez V, Paredes R, Pillay D, Shafer RW, Wensing AM, Jacobsen DM, Richman DD. 2019. Human Immunodeficiency Virus drug resistance: 2018 recommendations of the international antiviral society-USA panel. Clin Infect Dis 68:177–187. 10.1093/cid/ciy463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandamme A-M, Camacho RJ, Ceccherini-Silberstein F, de Luca A, Palmisano L, Paraskevis D, Paredes R, Poljak M, Schmit J-C, Soriano V, Walter H, Sönnerborg A, European HIV Drug Resistance Guidelines Panel . 2011. European recommendations for the clinical use of HIV drug resistance testing: 2011 update. AIDS Rev 13:77–108. [PubMed] [Google Scholar]
- 17.Koelsch KK, Liu L, Haubrich R, May S, Havlir D, Günthard HF, Ignacio CC, Campos-Soto P, Little SJ, Shafer R, Robbins GK, D'Aquila RT, Kawano Y, Young K, Dao P, Spina CA, Richman DD, Wong JK. 2008. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J Infect Dis 197:411–419. 10.1086/525283. [DOI] [PubMed] [Google Scholar]
- 18.Blankson JN, Finzi D, Pierson TC, Sabundayo BP, Chadwick K, Margolick JB, Quinn TC, Siliciano RF. 2000. Biphasic decay of latently infected CD4+ T cells in acute Human Immunodeficiency Virus Type 1 infection. J Infect Dis 182:1636–1642. 10.1086/317615. [DOI] [PubMed] [Google Scholar]
- 19.Besson GJ, Lalama CM, Bosch RJ, Gandhi RT, Bedison MA, Aga E, Riddler SA, McMahon DK, Hong F, Mellors JW. 2014. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clinical Infectious Diseases 59:1312–1321. 10.1093/cid/ciu585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avettand-Fènoël V, Hocqueloux L, Ghosn J, Cheret A, Frange P, Melard A, Viard J-P, Rouzioux C. 2016. Total HIV-1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin Microbiol Rev 29:859–880. 10.1128/CMR.00015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, Siliciano RF. 2002. Intrinsic Stability of episomal circles formed during Human Immunodeficiency Virus Type 1 replication. J Virol 76:4138–4144. 10.1128/JVI.76.8.4138-4144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharkey M, Triques K, Kuritzkes DR, Stevenson M. 2005. In vivo evidence for instability of episomal Human Immunodeficiency Virus Type 1 cDNA. J Virol 79:5203–5210. 10.1128/JVI.79.8.5203-5210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta S, Siliciano RF. 2018. Targeting the latent reservoir for HIV-1. Immunity 48:872–895. 10.1016/j.immuni.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun T-W, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1:1284–1290. 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 25.Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–1295. 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 26.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 27.Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 Cure. Cell 155:540–551. 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josefsson L, King MS, Makitalo B, Brännström J, Shao W, Maldarelli F, Kearney MF, Hu W-S, Chen J, Gaines H, Mellors JW, Albert J, Coffin JM, Palmer SE. 2011. Majority of CD4+ T cells from peripheral blood of HIV-1–infected individuals contain only one HIV DNA molecule. Proc Natl Acad Sci USA 108:11199–11204. 10.1073/pnas.1107729108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josefsson L, Palmer S, Faria NR, Lemey P, Casazza J, Ambrozak D, Kearney M, Shao W, Kottilil S, Sneller M, Mellors J, Coffin JM, Maldarelli F. 2013. Single cell analysis of lymph node tissue from HIV-1 infected patients reveals that the majority of CD4+ T-cells contain one HIV-1 DNA molecule. PLoS Pathog 9:e1003432. 10.1371/journal.ppat.1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard J-P, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C, Group the AVS . 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog 9:e1003211. 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouzioux C, Avettand-Fenoël V. 2018. Total HIV DNA: a global marker of HIV persistence. Retrovirology 15:30. 10.1186/s12977-018-0412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwaa AK, Garliss CC, Ritter KD, Laird GM, Blankson JN. 2020. Elite suppressors have low frequencies of intact HIV-1 proviral DNA. AIDS 34:641–643. 10.1097/QAD.0000000000002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simonetti FR, White JA, Tumiotto C, Ritter KD, Cai M, Gandhi RT, Deeks SG, Howell BJ, Montaner LJ, Blankson JN, Martin A, Laird GM, Siliciano RF, Mellors JW, Siliciano JD. 2020. Intact proviral DNA assay analysis of large cohorts of people with HIV provides a benchmark for the frequency and composition of persistent proviral DNA. Proc Natl Acad Sci USA 117:18692–18700. 10.1073/pnas.2006816117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, Ho Y-C, Richman DD, Deeks SG, Siliciano JD, Siliciano RF. 2016. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 22:1043–1049. 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hataye JM, Casazza JP, Best K, Liang CJ, Immonen TT, Ambrozak DR, Darko S, Henry AR, Laboune F, Maldarelli F, Douek DC, Hengartner NW, Yamamoto T, Keele BF, Perelson AS, Koup RA. 2019. Principles governing establishment versus collapse of HIV-1 cellular spread. Cell Host Microbe 26:748–763.e20. 10.1016/j.chom.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenasi T, Contreras X, Peterlin BM. 2008. Transcriptional interference antagonizes proviral gene expression to promote HIV latency. Cell Host Microbe 4:123–133. 10.1016/j.chom.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Y, Lin YB, An W, Xu J, Yang H-C, O'Connell K, Dordai D, Boeke JD, Siliciano JD, Siliciano RF. 2008. Orientation-dependent regulation of integrated HIV-1 expression by host gene transcriptional readthrough. Cell Host Microbe 4:134–146. 10.1016/j.chom.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldauf H-M, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, König R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4+ T cells. Nat Med 18:1682–1688. 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan L, Deng K, Gao H, Xing S, Capoferri AA, Durand CM, Rabi SA, Laird GM, Kim M, Hosmane NN, Yang H-C, Zhang H, Margolick JB, Li L, Cai W, Ke R, Flavell RA, Siliciano JD, Siliciano RF. 2017. Transcriptional reprogramming during effector-to-memory transition renders CD4+ T Cells permissive for latent HIV-1iInfection. Immunity 47:766–775.e3. 10.1016/j.immuni.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasternak AO, Jurriaans S, Bakker M, Berkhout B, Lukashov VV. 2010. Steady increase in cellular HIV-1 load during the asymptomatic phase of untreated infection despite stable plasma viremia. Aids 24:1641–1649. 10.1097/QAD.0b013e32833b3171. [DOI] [PubMed] [Google Scholar]
- 41.Trémeaux P, Lenfant T, Boufassa F, Essat A, Mélard A, Gousset M, Delelis O, Viard J-P, Bary M, Goujard C, Rouzioux C, Meyer L, Avettand-Fenoel V, ANRS-SEROCO and PRIMO cohorts . 2019. Increasing contribution of integrated forms to total HIV DNA in blood during HIV disease progression from primary infection. EBioMedicine 41:455–464. 10.1016/j.ebiom.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, Epling L, Lee T-H, Busch MP, McCune JM, Pilcher CD, Hecht FM, Deeks SG. 2013. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. The J Infectious Diseases 208:1202–1211. 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray JM, McBride K, Boesecke C, Bailey M, Amin J, Suzuki K, Baker D, Zaunders JJ, Emery S, Cooper DA, Koelsch KK, Kelleher AD, Team on behalf of the PS . 2012. Integrated HIV DNA accumulates prior to treatment while episomal HIV DNA records ongoing transmission afterwards. AIDS 26:543–550. 10.1097/QAD.0b013e328350fb3c. [DOI] [PubMed] [Google Scholar]
- 44.Pinzone MR, VanBelzen DJ, Weissman S, Bertuccio MP, Cannon L, Venanzi-Rullo E, Migueles S, Jones RB, Mota T, Joseph SB, Groen K, Pasternak AO, Hwang W-T, Sherman B, Vourekas A, Nunnari G, O'Doherty U. 2019. Longitudinal HIV sequencing reveals reservoir expression leading to decay which is obscured by clonal expansion. Nat Commun 10:728. 10.1038/s41467-019-08431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy J-P, Haddad EK, Sékaly R-P. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Gurule EE, Brennan TP, Gerold JM, Kwon KJ, Hosmane NN, Kumar MR, Beg SA, Capoferri AA, Ray SC, Ho Y-C, Hill AL, Siliciano JD, Siliciano RF. 2018. Expanded cellular clones carrying replication-competent HIV-1 persist, wax, and wane. Proc Natl Acad Sci USA 115:E2575–E2584. 10.1073/pnas.1720665115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA 105:3879–3884. 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollack RA, Jones RB, Pertea M, Bruner KM, Martin AR, Thomas AS, Capoferri AA, Beg SA, Huang S-H, Karandish S, Hao H, Halper-Stromberg E, Yong PC, Kovacs C, Benko E, Siliciano RF, Ho Y-C. 2017. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 21:494–506.e4. 10.1016/j.chom.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White JA, Simonetti FR, Beg S, McMyn NF, Dai W, Bachmann N, Lai J, Ford WC, Bunch C, Jones JL, Ribeiro RM, Perelson AS, Siliciano JD, Siliciano RF. 2022. Complex decay dynamics of HIV virions, intact and defective proviruses, and 2LTR circles following initiation of antiretroviral therapy. Proc Natl Acad Sci USA 119. 10.1073/pnas.2120326119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bacchus-Souffan C, Fitch M, Symons J, Abdel-Mohsen M, Reeves DB, Hoh R, Stone M, Hiatt J, Kim P, Chopra A, Ahn H, York VA, Cameron DL, Hecht FM, Martin JN, Yukl SA, Mallal S, Cameron PU, Deeks SG, Schiffer JT, Lewin SR, Hellerstein MK, McCune JM, Hunt PW. 2021. Relationship between CD4 T cell turnover, cellular differentiation and HIV persistence during ART. PLoS Pathog 17:e1009214. 10.1371/journal.ppat.1009214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abrahams M-R, Joseph SB, Garrett N, Tyers L, Moeser M, Archin N, Council OD, Matten D, Zhou S, Doolabh D, Anthony C, Goonetilleke N, Karim SA, Margolis DM, Pond SK, Williamson C, Swanstrom R. 2019. The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci Transl Med 11. 10.1126/scitranslmed.aaw5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brodin J, Zanini F, Thebo L, Lanz C, Bratt G, Neher RA, Albert J. 2016. Establishment and stability of the latent HIV-1 DNA reservoir. Elife 5:e18889. 10.7554/eLife.18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones BR, Kinloch NN, Horacsek J, Ganase B, Harris M, Harrigan PR, Jones RB, Brockman MA, Joy JB, Poon AFY, Brumme ZL. 2018. Phylogenetic approach to recover integration dates of latent HIV sequences within-host. Proc Natl Acad Sci USA 115:E8958–E8967. 10.1073/pnas.1802028115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks K, Jones BR, Dilernia DA, Wilkins DJ, Claiborne DT, McInally S, Gilmour J, Kilembe W, Joy JB, Allen SA, Brumme ZL, Hunter E. 2020. HIV-1 variants are archived throughout infection and persist in the reservoir. PLoS Pathog 16:e1008378. 10.1371/journal.ppat.1008378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aamer HA, McClure J, Ko D, Maenza J, Collier AC, Coombs RW, Mullins JI, Frenkel LM. 2020. Cells producing residual viremia during antiretroviral treatment appear to contribute to rebound viremia following interruption of treatment. PLoS Pathog 16:e1008791. 10.1371/journal.ppat.1008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siliciano JD, Siliciano RF. 2013. Recent trends in HIV-1 drug resistance. Curr Opin Virol 3:487–494. 10.1016/j.coviro.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kearney MF, Wiegand A, Shao W, Coffin JM, Mellors JW, Lederman M, Gandhi RT, Keele BF, Li JZ. 2016. Origin of rebound plasma HIV includes cells with identical proviruses that are transcriptionally active before stopping of antiretroviral therapy. J Virol 90:1369–1376. 10.1128/JVI.02139-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joos B, Fischer M, Kuster H, Pillai SK, Wong JK, Böni J, Hirschel B, Weber R, Trkola A, Günthard HF, Study2 TSHC . 2008. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci USA 105:16725–16730. 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evering TH, Mehandru S, Racz P, Tenner-Racz K, Poles MA, Figueroa A, Mohri H, Markowitz M. 2012. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog 8:e1002506. 10.1371/journal.ppat.1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McManus WR, Bale MJ, Spindler J, Wiegand A, Musick A, Patro SC, Sobolewski MD, Musick VK, Anderson EM, Cyktor JC, Halvas EK, Shao W, Wells D, Wu X, Keele BF, Milush JM, Hoh R, Mellors JW, Hughes SH, Deeks SG, Coffin JM, Kearney MF. 2019. HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J Clin Invest 129:4629–4642. 10.1172/JCI126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayón-Gil Á, Puertas MC, Urrea V, Bailón L, Morón-López S, Cobarsí P, Brander C, Mothe B, Martinez-Picado J. 2020. HIV-1 DNA decay dynamics in early treated individuals: practical considerations for clinical trial design. J Antimicrob Chemother 75:2258–2263. 10.1093/jac/dkaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koelsch KK, Boesecke C, McBride K, Gelgor L, Fahey P, Natarajan V, Baker D, Bloch M, Murray JM, Zaunders J, Emery S, Cooper DA, Kelleher AD, Team for the P study . 2011. Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. Aids 25:2069–2078. 10.1097/QAD.0b013e32834b9658. [DOI] [PubMed] [Google Scholar]
- 63.Golob JL, Stern J, Holte S, Kitahata MM, Crane HM, Coombs RW, Goecker E, Woolfrey AE, Harrington RD. 2018. HIV DNA levels and decay in a cohort of 111 long-term virally suppressed patients. Aids 32:2113–2118. 10.1097/QAD.0000000000001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 65.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9:727–728. 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 66.Crooks AM, Bateson R, Cope AB, Dahl NP, Griggs MK, Kuruc JD, Gay CL, Eron JJ, Margolis DM, Bosch RJ, Archin NM. 2015. Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. J Infect Dis 212:1361–1365. 10.1093/infdis/jiv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner TA, McKernan JL, Tobin NH, Tapia KA, Mullins JI, Frenkel LM. 2013. An increasing proportion of monotypic HIV-1 DNA sequences during antiretroviral treatment suggests proliferation of HIV-infected cells. J Virol 87:1770–1778. 10.1128/JVI.01985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohn LB, Chomont N, Deeks SG. 2020. The biology of the HIV-1 latent reservoir and implications for cure strategies. Cell Host Microbe 27:519–530. 10.1016/j.chom.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bui JK, Sobolewski MD, Keele BF, Spindler J, Musick A, Wiegand A, Luke BT, Shao W, Hughes SH, Coffin JM, Kearney MF, Mellors JW. 2017. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog 13:e1006283. 10.1371/journal.ppat.1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DIS, Keele BF, Ho Y-C, Siliciano JD, Siliciano RF. 2017. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med 214:959–972. 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorenzi JCC, Cohen YZ, Cohn LB, Kreider EF, Barton JP, Learn GH, Oliveira T, Lavine CL, Horwitz JA, Settler A, Jankovic M, Seaman MS, Chakraborty AK, Hahn BH, Caskey M, Nussenzweig MC. 2016. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc Natl Acad Sci USA 113:E7908–E7916. 10.1073/pnas.1617789113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner TA, McLaughlin S, Garg K, Cheung CYK, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, Frenkel LM. 2014. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345:570–573. 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simonetti FR, Sobolewski MD, Fyne E, Shao W, Spindler J, Hattori J, Anderson EM, Watters SA, Hill S, Wu X, Wells D, Su L, Luke BT, Halvas EK, Besson G, Penrose KJ, Yang Z, Kwan RW, Van Waes C, Uldrick T, Citrin DE, Kovacs J, Polis MA, Rehm CA, Gorelick R, Piatak M, Keele BF, Kearney MF, Coffin JM, Hughes SH, Mellors JW, Maldarelli F. 2016. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci USA 113:1883–1888. 10.1073/pnas.1522675113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peluso MJ, Bacchetti P, Ritter KD, Beg S, Lai J, Martin JN, Hunt PW, Henrich TJ, Siliciano JD, Siliciano RF, Laird GM, Deeks SG. 2020. Differential decay of intact and defective proviral DNA in HIV-1–infected individuals on suppressive antiretroviral therapy. JCI Insight 5 10.1172/jci.insight.132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gandhi RT, Cyktor JC, Bosch RJ, Mar H, Laird GM, Martin A, Collier AC, Riddler SA, Macatangay BJ, Rinaldo CR, Eron JJ, Siliciano JD, McMahon DK, Mellors JW, AIDS Clinical Trials Group A5321 Team . 2021. Selective decay of intact HIV-1 proviral DNA on antiretroviral therapy. J Infect Dis 223:225–233. 10.1093/infdis/jiaa532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boritz EA, Darko S, Swaszek L, Wolf G, Wells D, Wu X, Henry AR, Laboune F, Hu J, Ambrozak D, Hughes MS, Hoh R, Casazza JP, Vostal A, Bunis D, Nganou-Makamdop K, Lee JS, Migueles SA, Koup RA, Connors M, Moir S, Schacker T, Maldarelli F, Hughes SH, Deeks SG, Douek DC. 2016. Multiple origins of virus persistence during natural control of HIV infection. Cell 166:1004–1015. 10.1016/j.cell.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin AR, Bender AM, Hackman J, Kwon KJ, Lynch BA, Bruno D, Martens C, Beg S, Florman SS, Desai N, Segev D, Laird GM, Siliciano JD, Quinn TC, Tobian AAR, Durand CM, Siliciano RF, Redd AD. 2021. Similar frequency and inducibility of intact Human Immunodeficiency Virus-1 proviruses in blood and lymph nodes. J Infect Dis 224:258–268. 10.1093/infdis/jiaa736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen YZ, Lorenzi JCC, Krassnig L, Barton JP, Burke L, Pai J, Lu C-L, Mendoza P, Oliveira TY, Sleckman C, Millard K, Butler AL, Dizon JP, Belblidia SA, Witmer-Pack M, Shimeliovich I, Gulick RM, Seaman MS, Jankovic M, Caskey M, Nussenzweig MC. 2018. Relationship between latent and rebound viruses in a clinical trial of anti–HIV-1 antibody 3BNC117. J Experimental Medicine 215:2311–2324. 10.1084/jem.20180936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, Spina CA, Woelk CH, Richman DD. 2013. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 8:e55943. 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsiara CG, Nikolopoulos GK, Bagos PG, Goujard C, Katzenstein TL, Minga AK, Rouzioux C, Hatzakis A. 2012. Impact of HIV Type 1 DNA levels on spontaneous disease progression: a meta-analysis. AIDS Res Hum Retroviruses 28:366–373. 10.1089/aid.2011.0032. [DOI] [PubMed] [Google Scholar]
- 81.Moh DR, Ntakpé J-B, Gabillard D, Yayo-Emieme AA, Badjé A, Kouame GM, d'Aquin TT, Danel C, Anglaret X, Eholié SP. 2022. Association of cellular HIV-1 DNA and virological success of antiretroviral treatment in HIV-infected sub-Saharan African adults. BMC Infect Dis 22:100. 10.1186/s12879-022-07082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woods CK, Brumme CJ, Liu TF, Chui CKS, Chu AL, Wynhoven B, Hall TA, Trevino C, Shafer RW, Harrigan PR. 2012. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol 50:1936–1942. 10.1128/JCM.06689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuurman R, Brambilla D, de Groot T, Huang D, Land S, Bremer J, Benders I, Boucher CAB, ENVA Working Group . 2002. Underestimation of HIV type 1 drug resistance mutations: results from the ENVA-2 genotyping proficiency program. AIDS Res and Human Retroviruses 18:243–248. 10.1089/088922202753472801. [DOI] [PubMed] [Google Scholar]
- 84.Mohamed S, Penaranda G, Gonzalez D, Camus C, Khiri H, Boulmé R, Sayada C, Philibert P, Olive D, Halfon P. 2014. Comparison of ultra-deep versus Sanger sequencing detection of minority mutations on the HIV-1 drug resistance interpretations after virological failure. AIDS 28:1315–1324. 10.1097/QAD.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 85.Huber M, Metzner KJ, Geissberger FD, Shah C, Leemann C, Klimkait T, Böni J, Trkola A, Zagordi O. 2017. MinVar: a rapid and versatile tool for HIV-1 drug resistance genotyping by deep sequencing. J Virol Methods 240:7–13. 10.1016/j.jviromet.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Trabaud M-A, Icard V, Ramière C, Tardy J-C, Scholtes C, André P. 2017. Comparison of HIV-1 drug-resistance genotyping by ultra-deep sequencing and sanger sequencing using clinical samples. J Med Virol 89:1912–1919. 10.1002/jmv.24872. [DOI] [PubMed] [Google Scholar]
- 87.Tzou PL, Ariyaratne P, Varghese V, Lee C, Rakhmanaliev E, Villy C, Yee M, Tan K, Michel G, Pinsky BA, Shafer RW. 2018. Comparison of an in vitro diagnostic next-generation sequencing assay with sanger sequencing for HIV-1 genotypic resistance testing. J Clin Microbiol 56. 10.1128/JCM.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang D, Lai J, Cai S, Toma J, Tan Y, Petropoulos C, Walworth C, Whitcomb J. 2019. HIV resistance associated mutations observed in cell-associated DNA sequencing assay. Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 89.Curanovic D, Cai S, Toma J, Petropoulos CJ, Walworth CM. 2020. 691. Results of repeat HIV-1 DNA resistance tests are highly concordant. Open Forum Infect Dis 7:S398–S399. 10.1093/ofid/ofaa439.883. [DOI] [Google Scholar]
- 90.Curanovic D, Martens S, Rodriguez M, Hammill H, Petropoulos C, Walworth C. 2020. HIV-1 DNA testing in viremic patients demonstrates a greater ability to detect drug resistance compared to plasma virus testing. Abstr PDB0402. International AIDS Conference 1. [Google Scholar]
- 91.Curanovic D, Martens SK, Rodriguez MA, Hammill HA, Petropoulos CJ, Walworth CM. 2020. HIV-1 DNA testing identifies drug resistance in viremic patients with pan-sensitive plasma virus. Open Forum Infect Dis 7:S510–S511. 10.1093/ofid/ofaa439.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Millière L, Bocket L, Tinez C, Robineau O, Veyer N, Wojciechowski F, Lambert V, Meybeck A, Huleux T, Ajana F, Hober D, Alidjinou EK. 2021. Assessment of intra-sample variability in HIV-1 DNA drug resistance genotyping. J Antimicrob Chemother 76:2143–2147. 10.1093/jac/dkab149. [DOI] [PubMed] [Google Scholar]
- 93.D’Antoni M, Andreatta K, Acosta R, Liu H, Shao Y, White K. HIV-1 DNA genotyping is often variable in repeat testing from single blood draws. Abstr 438. Conference on Retroviruses and Opportunistic Infections.
- 94.Hoffmann C, Wolf E, Braun P, Bickel M, Stoehr A, Heldwein S, Knechten H, Esser S, Mayr C, Wyen C, Müller M, Wasmuth J-C, Münchhoff M, Thielen A, Däumer M. 2022. Temporal variability of multi-class resistant HIV-1 in proviral DNA. Abstr 513. Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 95.Malim MH. 2009. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci 364:675–687. 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, Etemad B, Dele-Oni R, Sharaf R, Gao C, Lichterfeld M, Li JZ. 2021. Drug resistance mutations in HIV provirus are associated with defective proviral genomes with hypermutation. Aids 35:1015–1020. 10.1097/QAD.0000000000002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lambert-Niclot S, Allavena C, Grude M, Flandre P, Sayon S, Andre E, Wirden M, Rodallec A, Jovelin T, Katlama C, Calvez V, Raffi F, Marcelin A-G. 2016. Usefulness of an HIV DNA resistance genotypic test in patients who are candidates for a switch to the rilpivirine/emtricitabine/tenofovir disoproxil fumarate combination. J Antimicrob Chemother 71:2248–2251. 10.1093/jac/dkw146. [DOI] [PubMed] [Google Scholar]
- 98.Huruy K, Mulu A, Liebert UG, Melanie M. 2018. HIV-1C proviral DNA for detection of drug resistance mutations. PLoS One 13:e0205119. 10.1371/journal.pone.0205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tzou PL, Kosakovsky PS, Avila-Rios S, Holmes SP, Kantor R, Shafer RW. 2020. Analysis of unusual and signature APOBEC-mutations in HIV-1 pol next-generation sequences. PLoS One 15:e0225352. 10.1371/journal.pone.0225352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosn J, Pellegrin I, Goujard C, Deveau C, Viard J-P, Galimand J, Harzic M, Tamalet C, Meyer L, Rouzioux C, Chaix M-L, for the FPCSG (ANRS Co 06) . 2006. HIV-1 resistant strains acquired at the time of primary infection massively fuel the cellular reservoir and persist for lengthy periods of time. AIDS 20:159–170. 10.1097/01.aids.0000199820.47703.a0. [DOI] [PubMed] [Google Scholar]
- 101.Parisi SG, boldrin C, Cruciani M, Nicolini G, Cerbaro I, Manfrin V, Dal Bello F, Franchin E, Franzetti M, Rossi MC, Cattelan AM, Romano L, Zazzi M, Andreoni M, Palù G. 2007. Both Human Immunodeficiency Virus Cellular DNA sequencing and plasma RNA sequencing are useful for detection of drug resistance mutations in blood samples from antiretroviral-drug-naive patients. J Clin Microbiol 45:1783–1788. 10.1128/JCM.00056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bon I, Gibellini D, Borderi M, Alessandrini F, Vitone F, Schiavone P, Re MC. 2007. Genotypic resistance in plasma and peripheral blood lymphocytes in a group of naive HIV-1 patients. J Clinical Virology 38:313–320. 10.1016/j.jcv.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 103.Kabamba-Mukadi B, Duquenne A, Henrivaux P, Musuamba F, Ruelle J, Yombi J-C, Bodéus M, Vandercam B, Goubau P. 2010. HIV-1 proviral resistance mutations: usefulness in clinical practice. HIV Med 11:483–492. 10.1111/j.1468-1293.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 104.Jain V, Sucupira MC, Bacchetti P, Hartogensis W, Diaz RS, Kallas EG, Janini LM, Liegler T, Pilcher CD, Grant RM, Cortes R, Deeks SG, Hecht FM. 2011. Differential persistence of transmitted HIV-1 drug resistance mutation classes. J Infect Dis 203:1174–1181. 10.1093/infdis/jiq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castro H, Pillay D, Cane P, Asboe D, Cambiano V, Phillips A, Dunn DT, Resistance for the UCG, on HD Aitken C, Asboe D, Webster D, Cane P, Castro H, Chadwick D, Churchill D, Clark D, Collins S, Delpech V, Geretti AM, Goldberg D, Hale A, Hué S, Kaye S, Kellam PLL, Leigh-Brown A, Mackie N, Orkin C, Rice P, Pillay D, Smit E, Templeton K, Tilston P, Tong W, Williams I, Zhang H, Zuckerman M, Greatorex J, Wildfire A, O’Shea S, Mullen J, Mbisa T, Cox A, Tandy R, Hale T, Fawcett T, Hopkins M, Ashton L, Garcia-Diaz A, Shepherd J, SchmidUK Collaborative Group on HIV Drug Resistance , et al. 2013. Persistence of HIV-1 transmitted drug resistance mutations. J Infect Dis 208:1459–1463. 10.1093/infdis/jit345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strain MC, Günthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, Opravil M, Levine H, Bacheler L, Spina CA, Richman DD, Wong JK. 2003. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci USA 100:4819–4824. 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bi X, Gatanaga H, Ida S, Tsuchiya K, Matsuoka-Aizawa S, Kimura S, Oka S. 2003. Emergence of protease inhibitor resistance–associated mutations in plasma HIV-1 precedes that in proviruses of peripheral blood mononuclear cells by more than a year. J Acquir Immune Defic Syndr 34:1–6. 10.1097/00126334-200309010-00001. [DOI] [PubMed] [Google Scholar]
- 108.Lee GQ, Swenson LC, Poon AFY, Martin JN, Hatano H, Deeks SG, Harrigan PR. 2012. Prolonged and substantial discordance in prevalence of Raltegravir-resistant HIV-1 in plasma versus PBMC samples revealed by 454 “deep” sequencing. PLoS One 7:e46181. 10.1371/journal.pone.0046181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sotillo A, Sierra O, Martínez-Prats L, Gutiérrez F, Zurita S, Pulido F, Rubio R, Delgado R. 2018. Analysis of drug resistance mutations in whole blood DNA from HIV-1 infected patients by single genome and ultradeep sequencing analysis. J Virol Methods 260:1–5. 10.1016/j.jviromet.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 110.Armenia D, Santoro MM, Bellocchi MC, Carioti L, Galli L, Galli A, Scutari R, Salsi E, Mussini C, Sterrantino G, Calza L, Rossetti B, Zazzi M, Castagna A, PRESTIGIO Registry Study Group . 2022. Viral resistance burden and APOBEC editing correlate with virological response in heavily treatment-experienced people living with multi-drug resistant HIV. Int J Antimicrob Agents 59:106492. 10.1016/j.ijantimicag.2021.106492. [DOI] [PubMed] [Google Scholar]
- 111.Chung MH, McGrath CJ, Beck IA, Levine M, Milne RS, So I, Andersen N, Dross S, Coombs RW, Chohan B, Yatich N, Kiptinness C, Sakr SR, Kiarie JN, Frenkel LM. 2020. Evaluation of the management of pretreatment HIV drug resistance by oligonucleotide ligation assay: a randomised controlled trial. Lancet HIV 7:e104–e112. 10.1016/S2352-3018(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Devereux HL, Youle M, Johnson MA, Loveday C. 1999. Rapid decline in detectability of HIV-1 drug resistance mutations after stopping therapy. Aids 13:F123–127. [PubMed] [Google Scholar]
- 113.Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, Barbour JD, Hellmann NS, Petropoulos CJ, McCune JM, Hellerstein MK, Grant RM. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med 344:472–480. 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 114.Bi X, Gatanaga H, Koike K, Kimura S, Oka S. 2007. Reversal periods and patterns from drug-resistant to wild-type HIV type 1 after cessation of anti-HIV therapy. AIDS Res Hum Retroviruses 23:43–50. 10.1089/aid.2005.0029. [DOI] [PubMed] [Google Scholar]
- 115.Kijak GH, Simon V, Balfe P, Vanderhoeven J, Pampuro SE, Zala C, Ochoa C, Cahn P, Markowitz M, Salomon H. 2002. Origin of uman Immunodeficiency Virus type 1 quasispecies emerging after antiretroviral treatment interruption in patients with therapeutic failure. J Virol 76:7000–7009. 10.1128/JVI.76.14.7000-7009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Venturi G, Romano L, Carli T, Corsi P, Pippi L, Valensin PE, Zazzi M. 2002. Divergent distribution of HIV-1 drug-resistant variants on and off antiretroviral therapy. Antivir Ther 7:245–250. 10.1177/135965350200700403. [DOI] [PubMed] [Google Scholar]
- 117.Pellegrin I, Thiébaut R, Blanco P, Viallard J-F, Schrive M-H, Merel P, Chêne G, Fleury H, Moreau J-F, Pellegrin J-L. 2005. Can highly active antiretroviral therapy be interrupted in patients with sustained moderate HIV RNA and > 400 CD4+ cells/μl? Impact on immunovirological parameters. J Med Virol 77:164–172. 10.1002/jmv.20452. [DOI] [PubMed] [Google Scholar]
- 118.Wang D, Hicks CB, Goswami ND, Tafoya E, Ribeiro RM, Cai F, Perelson AS, Gao F. 2011. Evolution of drug-resistant viral populations during interruption of antiretroviral therapy. J Virol 85:6403–6415. 10.1128/JVI.02389-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vardhanabhuti S, Taiwo B, Kuritzkes DR, Eron JJ, Bosch RJ. 2015. Phylogenetic evidence of HIV-1 sequence evolution in subjects with persistent low-level viraemia. Antivir Ther 20:73–76. 10.3851/IMP2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li JZ, Gallien S, Do TD, Martin JN, Deeks S, Kuritzkes DR, Hatano H. 2012. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother 56:5998–6000. 10.1128/AAC.01217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Delaugerre C, Gallien S, Flandre P, Mathez D, Amarsy R, Ferret S, Timsit J, Molina J-M, de Truchis P. 2012. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One 7:e36673. 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.von Wyl V, Yerly S, Böni J, Shah C, Cellerai C, Klimkait T, Battegay M, Bernasconi E, Cavassini M, Furrer H, Hirschel B, Vernazza PL, Ledergerber B, Günthard HF, the Swiss HIV Cohort Study . 2012. Incidence of HIV-1 drug resistance among antiretroviral treatment–naive individuals starting modern therapy combinations. Clinical Infectious Diseases 54:131–140. 10.1093/cid/cir728. [DOI] [PubMed] [Google Scholar]
- 123.Taiwo B, Gallien S, Aga E, Ribaudo H, Haubrich R, Kuritzkes DR, Eron JJ. Jr.. 2011. Antiretroviral drug resistance in HIV-1–infected patients experiencing persistent low-level viremia during first-line therapy. The J Infectious Diseases 204:515–520. 10.1093/infdis/jir353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amstutz A, Nsakala BL, Vanobberghen F, Muhairwe J, Glass TR, Namane T, Mpholo T, Battegay M, Klimkait T, Labhardt ND. 2020. Switch to second-line versus continued first-line antiretroviral therapy for patients with low-level HIV-1 viremia: an open-label randomized controlled trial in Lesotho. PLoS Med 17:e1003325. 10.1371/journal.pmed.1003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Laprise C, de Pokomandy A, Baril J-G, Dufresne S, Trottier H. 2013. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 57:1489–1496. 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 126.Boillat-Blanco N, Darling KE, Schoni-Affolter F, Vuichard D, Rougemont M, Fulchini R, Bernasconi E, Aouri M, Clerc O, Furrer H, Günthard HF, Cavassini M, Aubert V, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Gorgievski M, Günthard H, Haerry D, Hasse B, Hirsch HH, Hoffmann M, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kouyos R, Kovari H, Ledergerber B, Martinetti G, Martinez DTB, Metzner K, Müller N, Nadal D, Nicca D, Pantaleo G, Rauch A, Regenass S, the Swiss HIV Cohort Study , et al. 2015. Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antivir Ther 20:165–175. 10.3851/IMP2815. [DOI] [PubMed] [Google Scholar]
- 127.Esber A, Polyak C, Kiweewa F, Maswai J, Owuoth J, Maganga L, Adamu Y, Hickey PW, Ake JA, Crowell TA. 2019. Persistent low-level viremia predicts subsequent virologic failure: is it time to change the third 90? Clin Infect Dis 69:805–812. 10.1093/cid/ciy989. [DOI] [PubMed] [Google Scholar]
- 128.Vandenhende M-A, Ingle S, May M, Chene G, Zangerle R, Van Sighem A, Gill MJ, Schwarze-Zander C, Hernandez-Novoa B, Obel N, Kirk O, Abgrall S, Guest J, Samji H, D'Arminio Monforte A, Llibre JM, Smith C, Cavassini M, Burkholder GA, Shepherd B, Crane HM, Sterne J, Morlat P, Antiretroviral Therapy Cohort Collaboration (ART-CC) . 2015. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 29:373–383. 10.1097/QAD.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 129.Fleming J, Mathews WC, Rutstein RM, Aberg J, Somboonwit C, Cheever LW, Berry SA, Gebo KA, Moore RD, Network for the HR . 2019. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS 33:2005–2012. 10.1097/QAD.0000000000002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vandenhende M-A, Perrier A, Bonnet F, Lazaro E, Cazanave C, Reigadas S, Chêne G, Morlat P, Groupe d’Epidémiologie Clinique du SIDA en Aquitaine . 2015. Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study). Antivir Ther 20:655–660. 10.3851/IMP2949. [DOI] [PubMed] [Google Scholar]
- 131.Gaifer Z, Boulassel M-R. 2020. Low-level viremia predicts virological failure in HIV-infected Omani patients receiving antiretroviral therapy. J Int Assoc Provid AIDS Care 19:232595822097981. 2325958220979817. 10.1177/2325958220979817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Joya C, Won SH, Schofield C, Lalani T, Maves RC, Kronmann K, Deiss R, Okulicz J, Agan BK, Ganesan A. 2019. Persistent Low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis 69:2145–2152. 10.1093/cid/ciz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lorenzo-Redondo R, Fryer HR, Bedford T, Kim E-Y, Archer J, Kosakovsky PS, Chung Y-S, Penugonda S, Chipman JG, Fletcher CV, Schacker TW, Malim MH, Rambaut A, Haase AT, McLean AR, Wolinsky SM. 2016. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530:51–56. 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, McKernan JL, Pawluk DM, Mohan KM, Lewis PF, Mullins JI, Frenkel LM. 2005. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 79:9625–9634. 10.1128/JVI.79.15.9625-9634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, Persaud D, Gallant JE, Cofrancesco J, Quinn TC, Wilke CO, Ray SC, Siliciano JD, Nettles RE, Siliciano RF. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 80:6441–6457. 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Halvas EK, Joseph KW, Brandt LD, Guo S, Sobolewski MD, Jacobs JL, Tumiotto C, Bui JK, Cyktor JC, Keele BF, Morse GD, Bale MJ, Shao W, Kearney MF, Coffin JM, Rausch JW, Wu X, Hughes SH, Mellors JW. 2020. HIV-1 viremia not suppressible by antiretroviral therapy can originate from large T cell clones producing infectious virus. J Clin Invest 130:5847–5857. 10.1172/JCI138099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Swenson LC, Min JE, Woods CK, Cai E, Li JZ, Montaner JSG, Harrigan PR, Gonzalez-Serna A. 2014. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. Aids 28:1125–1134. 10.1097/QAD.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Santoro MM, Fabeni L, Armenia D, Alteri C, Di Pinto D, Forbici F, Bertoli A, Di Carlo D, Gori C, Carta S, Fedele V, D'Arrigo R, Berno G, Ammassari A, Pinnetti C, Nicastri E, Latini A, Tommasi C, Boumis E, Petrosillo N, D'Offizi G, Andreoni M, Ceccherini-Silberstein F, Antinori A, Perno CF. 2014. Reliability and clinical relevance of the HIV-1 drug resistance test in patients with low viremia levels. Clinical Infectious Diseases 58:1156–1164. 10.1093/cid/ciu020. [DOI] [PubMed] [Google Scholar]
- 139.Gonzalez-Serna A, Min JE, Woods C, Chan D, Lima VD, Montaner JSG, Harrigan PR, Swenson LC. 2014. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis 58:1165–1173. 10.1093/cid/ciu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Assoumou L, Charpentier C, Recordon-Pinson P, Grudé M, Pallier C, Morand-Joubert L, Fafi-Kremer S, Krivine A, Montes B, Ferré V, Bouvier-Alias M, Plantier J-C, Izopet J, Trabaud M-A, Yerly S, Dufayard J, Alloui C, Courdavault L, Le Guillou-Guillemette H, Maillard A, Amiel C, Vabret A, Roussel C, Vallet S, Guinard J, Mirand A, Beby-Defaux A, Barin F, Allardet-Servent A, Ait-Namane R, Wirden M, Delaugerre C, Calvez V, Chaix M-L, Descamps D, Reigadas S, on behalf of the ANRS AC-11 Resistance Study Group. 2017. Prevalence of HIV-1 drug resistance in treated patients with viral load >50 copies/mL: a 2014 French nationwide study. J Antimicrob Chemother 72:1769–1773. 10.1093/jac/dkx042. [DOI] [PubMed] [Google Scholar]
- 141.Gallien S, Delaugerre C, Charreau I, Braun J, Boulet T, Barrail-Tran A, de Castro N, Molina J-M, Kuritzkes DR. 2011. Emerging integrase inhibitor resistance mutations in raltegravir-treated HIV-1-infected patients with low-level viremia. AIDS 25:665–669. 10.1097/QAD.0b013e3283445834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Armenia D, Fabeni L, Alteri C, Di Pinto D, Di Carlo D, Bertoli A, Gori C, Carta S, Fedele V, Forbici F, D'Arrigo R, Svicher V, Berno G, Pizzi D, Nicastri E, Sarmati L, Pinnetti C, Ammassari A, D'Offizi G, Latini A, Andreoni M, Antinori A, Ceccherini-Silberstein F, Perno CF, Santoro MM. 2015. HIV-1 integrase genotyping is reliable and reproducible for routine clinical detection of integrase resistance mutations even in patients with low-level viraemia. J Antimicrob Chemother 70:1865–1873. dkv029. 10.1093/jac/dkv029. [DOI] [PubMed] [Google Scholar]
- 143.Fokam J, Ngoufack Jagni Semengue E, Armenia D, Takou D, Dambaya B, Teto G, Chenwi CA, Nka AD, Beloumou GA, Ndjeyep SCD, Tchouaket MCT, Fainguem N, Sosso SM, Colizzi V, Perno C-F, Ndjolo A, Ceccherini-Silberstein F, Santoro MM. 2022. High performance of integrase genotyping on diverse HIV-1 clades circulating in Cameroon: toward a successful transition to dolutegravir-based regimens in low and middle-income countries. Diagn Microbiol Infect Dis 102:115574. 10.1016/j.diagmicrobio.2021.115574. [DOI] [PubMed] [Google Scholar]
- 144.Villalobos C, Ceballos ME, Ferrés M, Palma C. 2020. Drug resistance mutations in proviral DNA of HIV-infected patients with low level of viremia. J Clin Virol 132:104657. 10.1016/j.jcv.2020.104657. [DOI] [PubMed] [Google Scholar]
- 145.Boukli N, Boyd A, Collot M, Meynard J-L, Girard P-M, Morand-Joubert L. 2018. Utility of HIV-1 DNA genotype in determining antiretroviral resistance in patients with low or undetectable HIV RNA viral loads. J Antimicrob Chemother 73:3129–3136. 10.1093/jac/dky316. [DOI] [PubMed] [Google Scholar]
- 146.Zaccarelli M, Santoro MM, Armenia D, Borghi V, Gennari W, Gori C, Forbici F, Bertoli A, Fabeni L, Giannetti A, Cicalini S, Bellagamba R, Andreoni M, Mastroianni CM, Mussini C, Ceccherini-Silberstein F, Perno CF, Antinori A. 2016. Genotypic resistance test in proviral DNA can identify resistance mutations never detected in historical genotypic test in patients with low level or undetectable HIV-RNA. J Clin Virol 82:94–100. 10.1016/j.jcv.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 147.Kao S-W, Liu Z-H, Wu T-S, Ku SW-W, Tsai C-L, Shie S-S, Huang P-Y, Wu Y-M, Hsiao Y-H, Chen N-Y. 2020. Prevalence of drug resistance mutations in HIV-infected individuals with low-level viraemia under combination antiretroviral therapy: an observational study in a tertiary hospital in Northern Taiwan, 2017–19. J Antimicrob Chemother 100:722–728. 10.1093/jac/dkaa510. [DOI] [PubMed] [Google Scholar]
- 148.Meybeck A, Alidjinou EK, Huleux T, Boucher A, Tetart M, Choisy P, Bocket L, Ajana F, Robineau O. 2020. virological outcome after choice of antiretroviral regimen guided by proviral HIV-1 DNA genotyping in a real-life cohort of HIV-infected patients. AIDS Patient Care and STDs 34:51–58. 10.1089/apc.2019.0198. [DOI] [PubMed] [Google Scholar]
- 149.Wirden M, Soulie C, Valantin M-A, Fourati S, Simon A, Lambert-Niclot S, Bonmarchand M, Clavel-Osorio C, Marcelin A-G, Katlama C, Calvez V. 2011. Historical HIV-RNA resistance test results are more informative than proviral DNA genotyping in cases of suppressed or residual viraemia. J Antimicrob Chemother 66:709–712. 10.1093/jac/dkq544. [DOI] [PubMed] [Google Scholar]
- 150.Donovan RM, Bush CE, Smereck SM, Baxa DM, Markowitz NP, Saravolatz LD. 1994. Rapid decrease in unintegrated Human Immunodeficiency Virus DNA after the initiation of nucleoside therapy. The J Infectious Diseases 170:202–205. 10.1093/infdis/170.1.202. [DOI] [PubMed] [Google Scholar]
- 151.Buzón M, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, Palmer S, Stevenson M, Clotet B, Blanco J, Martinez-Picado J. 2010. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 16:460–465. 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 152.Giron LB, Tenore SB, Janini LMR, Sucupira MCA, Diaz RS. 2019. Laboratory surrogate markers of residual HIV replication among distinct groups of individuals under antiretroviral therapy. PLoS One 14:e0217502. 10.1371/journal.pone.0217502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Delaugerre C, Braun J, Charreau I, Delarue S, Nere ML, de Castro N, May T, Marchou B, Simon F, Molina JM, Aboulker JP. 2012. Comparison of resistance mutation patterns in historical plasma HIV RNA genotypes with those in current proviral HIV DNA genotypes among extensively treated patients with suppressed replication. HIV Medicine 13:517–525. [DOI] [PubMed] [Google Scholar]
- 154.Verhofstede C, Noë A, Demecheleer E, De Cabooter N, Van Wanzeele F, Van Der Gucht B, Vogelaers D, Plum J. 2004. Drug-resistant variants that evolve during nonsuppressive therapy persist in HIV-1–infected peripheral blood mononuclear cells after long-term highly active antiretroviral therapy. J Acquir Immune Defic Syndr 35:473–483. 10.1097/00126334-200404150-00005. [DOI] [PubMed] [Google Scholar]
- 155.Porter DP, Toma J, Tan Y, Solberg O, Cai S, Kulkarni R, Andreatta K, Lie Y, Chuck SK, Palella F, Miller MD, White KL. 2016. Clinical outcomes of virologically-suppressed patients with pre-existing HIV-1 drug resistance mutations switching to rilpivirine/emtricitabine/tenofovir disoproxil fumarate in the SPIRIT study. HIV Clin Trials 17:29–37. 10.1080/15284336.2015.1115585. [DOI] [PubMed] [Google Scholar]
- 156.Charpentier C, Montes B, Perrier M, Meftah N, Reynes J. 2017. HIV-1 DNA ultra-deep sequencing analysis at initiation of the dual therapy dolutegravir + lamivudine in the maintenance DOLULAM pilot study. J Antimicrob Chemother 72:2831–2836. 10.1093/jac/dkx233. [DOI] [PubMed] [Google Scholar]
- 157.De Miguel R, Rial-Crestelo D, Dominguez-Dominguez L, Montejano R, Esteban-Cantos A, Aranguren-Rivas P, Stella-Ascariz N, Bisbal O, Bermejo-Plaza L, Garcia-Alvarez M, Alejos B, Hernando A, Santacreu-Guerrero M, Cadiñanos J, Mayoral M, Castro JM, Moreno V, Martin-Carbonero L, Delgado R, Rubio R, Pulido F, Arribas JR, ART-PRO, PI16/00837-PI16/00678 study group . 2020. Dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: 48-week results of a non-randomized, pilot clinical trial (ART-PRO). EBioMedicine 55:102779. 10.1016/j.ebiom.2020.102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Margot N, Ram R, McNicholl I, Haubrich R, Callebaut C. 2020. Differential detection of M184V/I between plasma historical HIV genotypes and HIV proviral DNA from PBMCs. J Antimicrob Chemother 75:2249–2252. [DOI] [PubMed] [Google Scholar]
- 159.Montejano R, Dominguez-Dominguez L, de Miguel R, Rial-Crestelo D, Esteban-Cantos A, Aranguren-Rivas P, García-Álvarez M, Alejos B, Bisbal O, Santacreu-Guerrero M, Hernando A, Bermejo-Plaza L, Cadiñanos J, Mayoral M, Castro JM, Moreno V, Martin-Carbonero L, Rodés B, Delgado R, Rubio R, Pulido F, Arribas JR. 2021. Detection of archived lamivudine-associated resistance mutations in virologically suppressed, lamivudine-experienced HIV-infected adults by different genotyping techniques (GEN-PRO study). J Antimicrob Chemother 76:3263–3271. 10.1093/jac/dkab323. [DOI] [PubMed] [Google Scholar]
- 160.Jiménez de Ory S, Beltrán-Pavez C, Gutiérrez-López M, Santos MDM, Prieto L, Sainz T, Guillen S, Aguilera-Alonso D, Díez C, Bernardino JI, Mellado MJ, Ramos JT, Holguín Á, Navarro M, Mellado MJ, Escosa L, Hortelano MG, Sainz T, Rojo P, Prieto-Tato L, Epalza C, Ramos JT, Illán M, Guillén S, Navarro ML, Saavedra J, Santos M, Santiago B, de Ory SJ, Carrasco I, Berzosa A, Aguilera D, Muñoz-Fernández MA, Roa MÁ, Penín M, Martínez J, Badillo K, Jiménez AB, Navas A, Oñate E, Pocheville I, Garrote E, Colino E, Sirvent JG, Garzón M, Román V, Angulo R, Neth O, Falcón L, Terol P, Paediatric Cohort of the Spanish National AIDS Network (CoRISpe and CoRISpe-FARO) , et al. 2021. Prevalence of M184V and K65R in proviral DNA from PBMCs in HIV-infected youths with lamivudine/emtricitabine exposure. J Antimicrob Chemother 76:1886–1892. 10.1093/jac/dkab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Delaugerre C, Nere M-L, Eymard-Duvernay S, Armero A, Ciaffi L, Koulla-Shiro S, Sawadogo A, Ngom Gueye NF, Ndour CT, Mpoudi Ngolle M, Amara A, Chaix M-L, Reynes J, the ANRS 12286/MOBIDIP study group . 2021. Deep sequencing analysis of M184V/I mutation at the switch and at the time of virological failure of boosted protease inhibitor plus lamivudine or boosted protease inhibitor maintenance strategy (substudy of the ANRS-MOBIDIP trial). J Antimicrob Chemother 76:1286–1293. 10.1093/jac/dkab002. [DOI] [PubMed] [Google Scholar]
- 162.Nouchi A, Nguyen T, Valantin MA, Simon A, Sayon S, Agher R, Calvez V, Katlama C, Marcelin AG, Soulie C. 2018. Dynamics of drug resistance-associated mutations in HIV-1 DNA reverse transcriptase sequence during effective ART. J Antimicrob Chemother 73:2141–2146. 10.1093/jac/dky130. [DOI] [PubMed] [Google Scholar]
- 163.Gantner P, Morand-Joubert L, Sueur C, Raffi F, Fagard C, Lascoux-Combe C, Salmon D, Amiel C, Lambert-Niclot S, Fofana DB, Viard J-P, Fafi-Kremer S, Rouzioux C, Avettand-Fenoel V, Ghosn J. 2016. Drug resistance and tropism as markers of the dynamics of HIV-1 DNA quasispecies in blood cells of heavily pretreated patients who achieved sustained virological suppression. J Antimicrob Chemother 71:751–761. 10.1093/jac/dkv395. [DOI] [PubMed] [Google Scholar]
- 164.Michelini Z, Galluzzo CM, Pirillo MF, Francisci D, Antoni Ad, Vivarelli A, Ladisa N, Cirioni O, Weimer LE, Fragola V, Cara A, Floridia M, Baroncelli S. 2016. HIV-1 DNA dynamics and variations in HIV-1 DNA protease and reverse transcriptase sequences in multidrug-resistant patients during successful raltegravir-based therapy. J Med Virol 88:2115–2124. 10.1002/jmv.24581. [DOI] [PubMed] [Google Scholar]
- 165.Falasca F, Montagna C, Maida P, Bucci M, Fantauzzi A, Mezzaroma I, Antonelli G, Turriziani O. 2013. Analysis of intracellular human immunodeficiency virus (HIV)-1 drug resistance mutations in multi-failed HIV-1-infected patients treated with a salvage regimen: 72-week follow-up. Clin Microbiol Infect 19:E318–E321. 10.1111/1469-0691.12175. [DOI] [PubMed] [Google Scholar]
- 166.Vandekerckhove LPR, Wensing AMJ, Kaiser R, Brun-Vézinet F, Clotet B, De Luca A, Dressler S, Garcia F, Geretti AM, Klimkait T, Korn K, Masquelier B, Perno CF, Schapiro JM, Soriano V, Sönnerborg A, Vandamme A-M, Verhofstede C, Walter H, Zazzi M, Boucher CAB, European Consensus Group on clinical management of tropism testing . 2011. European guidelines on the clinical management of HIV-1 tropism testing. Lancet Infect Dis 11:394–407. 10.1016/S1473-3099(10)70319-4. [DOI] [PubMed] [Google Scholar]
- 167.Seclén E, del Mar González M, De Mendoza C, Soriano V, Poveda E. 2010. Dynamics of HIV tropism under suppressive antiretroviral therapy: implications for tropism testing in subjects with undetectable viraemia. J Antimicrob Chemother 65:1493–1496. 10.1093/jac/dkq156. [DOI] [PubMed] [Google Scholar]
- 168.Paar C, Geit M, Stekel H, Berg J. 2011. Genotypic prediction of Human Immunodeficiency Virus type 1 tropism by use of plasma and peripheral blood mononuclear cells in the routine clinical laboratory. J Clin Microbiol 49:2697–2699. 10.1128/JCM.00336-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fabeni L, Berno G, Svicher V, Ceccherini-Silberstein F, Gori C, Bertoli A, Mussini C, Lichtner M, Zaccarelli M, Ammassari A, Pinnetti C, Cicalini S, Mastroianni CM, Andreoni M, Antinori A, Perno CF, Santoro MM. 2015. Genotypic tropism testing in HIV-1 proviral DNA can provide useful information at low-level viremia. J Clin Microbiol 53:2935–2941. 10.1128/JCM.00893-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pett SL, Amin J, Horban A, Andrade-Villanueva J, Losso M, Porteiro N, Sierra Madero J, Belloso W, Tu E, Silk D, Kelleher A, Harrigan R, Clark A, Sugiura W, Wolff M, Gill J, Gatell J, Fisher M, Clarke A, Ruxrungtham K, Prazuck T, Kaiser R, Woolley I, Arnaiz JA, Cooper D, Rockstroh JK, Mallon P, Emery S, Kelleher A, Merlin K, Yeung J, Fsadni B, Marks K, Suzuki K, Rismanto N, Salomon H, Rubio AE, Chibo D, Birch C, Harrigan R, Swenson L, Chan D, Berg T, Obermeier M, Kaiser R, Schuelter E, Sierra Aragon S, Luebke N, Coughlan S, Dean J, for the Maraviroc Switch (MARCH) Study Group. for the Maraviroc Switch (MARCH) Study Group, et al. 2016. Maraviroc, as a switch option, in HIV-1–infected individuals with stable, well-controlled HIV replication and R5-tropic virus on their first nucleoside/nucleotide reverse transcriptase inhibitor plus ritonavir-boosted protease inhibitor regimen: week 48 results of the randomized, multicenter MARCH study. Clinical Infectious Diseases 63:122–132. 10.1093/cid/ciw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Havlir DV, Marschner IC, Hirsch MS, Collier AC, Tebas P, Bassett RL, Ioannidis JPA, Holohan MK, Leavitt R, Boone G, Richman DD. 1998. Maintenance antiretroviral therapies in HIV-infected subjects with undetectable plasma HIV RNA after triple-drug therapy. N Engl J Med 339:1261–1268. 10.1056/NEJM199810293391801. [DOI] [PubMed] [Google Scholar]
- 172.Pellegrin I, Caumont A, Garrigue I, Merel P, Schrive M-H, Fleury H, Dupon M, Pellegrin J-L, Ragnaud J-M. 2003. Predictive Value of Provirus Load and DNA Human Immunodeficiency Virus Genotype for Successful Abacavir-Based Simplified Therapy. J Infect Dis 187:38–46. 10.1086/345860. [DOI] [PubMed] [Google Scholar]
- 173.Opravil M, Hirschel B, Lazzarin A, Furrer H, Chave J-P, Yerly S, Bisset LR, Fischer M, Vernazza P, Bernasconi E, Battegay M, Ledergerber B, Günthard H, Howe C, Weber R, Perrin L, Swiss HIV Cohort Study . 2002. A randomized trial of simplified maintenance therapy with Abacavir, Lamivudine, and Zidovudine in Human Immunodeficiency Virus infection. The J Infectious Diseases 185:1251–1260. 10.1086/340312. [DOI] [PubMed] [Google Scholar]
- 174.Eron JJ, Young B, Cooper DA, Youle M, Dejesus E, Andrade-Villanueva J, Workman C, Zajdenverg R, Fätkenheuer G, Berger DS, Kumar PN, Rodgers AJ, Shaughnessy MA, Walker ML, Barnard RJO, Miller MD, Dinubile MJ, Nguyen B-Y, Leavitt R, Xu X, Sklar P, SWITCHMRK 1 and 2 investigators . 2010. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 375:396–407. 10.1016/S0140-6736(09)62041-9. [DOI] [PubMed] [Google Scholar]
- 175.van Wyk J, Ajana F, Bisshop F, De Wit S, Osiyemi O, Portilla J, Routy J-P, Wyen C, Ait-Khaled M, Nascimento MC, Pappa KA, Wang R, Wright J, Tenorio AR, Wynne B, Aboud M, Gartland MJ, Smith KY. 2020. Efficacy and safety of switching to Dolutegravir/Lamivudine fixed-dose two-drug regimen versus continuing a Tenofovir Alafenamide-based three- or four-drug regimen for maintenance of virologic suppression in adults With HIV-1: phase 3, randomized, non-inferiority TANGO study. Clin Infect Dis 10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Joly V, Burdet C, Landman R, Vigan M, Charpentier C, Katlama C, Cabié A, Benalycherif A, Peytavin G, Yeni P, Mentre F, Argoud A-L, Amri I, Descamps D, Yazdanpanah Y, Goujard C, Joly V, Phung B, Viard JP, Weiss L, Duvivier C, Katlama C, Girard PM, Molina JM, Morlat P, Jacomet C, Piroth L, Cabié A, Poizot-Martin I, Reynes J, Allavena C, Billaud E, Boutouille D, Raffi F, Reliquet V, Roenthal E, Naqvi A, Aumaitre H, Souala F, Bernard L, Biezunski N, Ajana F, Miailhes P, Amat K, Benalicherif A, Sylla B, LAMIDOL Study Group . 2019. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL). J Antimicrob Chemother 74:739–745. 10.1093/jac/dky467. [DOI] [PubMed] [Google Scholar]
- 177.Taiwo BO, Marconi VC, Berzins B, Moser CB, Nyaku AN, Fichtenbaum CJ, Benson CA, Wilkin T, Koletar SL, Colasanti J, Acosta EP, Li JZ, Sax PE. 2018. Dolutegravir plus Lamivudine maintains Human Immunodeficiency Virus-1 suppression through week 48 in a pilot randomized trial. Clinical Infectious Diseases 66:1794–1797. 10.1093/cid/cix1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li JZ, Sax PE, Marconi VC, Fajnzylber J, Berzins B, Nyaku AN, Fichtenbaum CJ, Wilkin T, Benson CA, Koletar SL, Lorenzo-Redondo R, Taiwo BO. 2019. No significant changes to residual viremia after switch to dolutegravir and Lamivudine in a randomized trial. Open Forum Infect Dis 6. 10.1093/ofid/ofz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Osiyemi O, De Wit S, Ajana F, Bisshop F, Portilla J, Routy J-P, Wyen C, Ait-Khaled M, Leone P, Pappa KA, Wang R, Wright J, George N, Wynne B, Aboud M, van Wyk J, Smith KY. 2022. Efficacy and safety of switching to Dolutegravir/Lamivudine (dtg/3tc) versus continuing a Tenofovir Alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with HIV-1: results through week 144 from the phase 3, non-inferiority tango randomized trial. Clin Infect Dis ciac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Underwood M, Osiyemi O, Rubio R, Hocqueloux L, Porteiro N, Degen O, Oyee J, Horton J, Parry C, Wang R, Sithamparanathan M, Van Wyk J, Wynn B, Man C, Blair E. 2022. Archived resistance and response to <40 C/ML & TND – DTG/3TC FDC at week 48 in SALSA [Abstract 481]. CROI. [Google Scholar]
- 181.Llibre JM, Hung C-C, Brinson C, Castelli F, Girard P-M, Kahl LP, Blair EA, Angelis K, Wynne B, Vandermeulen K, Underwood M, Smith K, Gartland M, Aboud M. 2018. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 391:839–849. 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 182.Swindells S, Andrade-Villanueva J-F, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, Latiff G, Pokrovsky V, Bredeek F, Smith G, Cahn P, Kim Y-S, Ford SL, Talarico CL, Patel P, Chounta V, Crauwels H, Parys W, Vanveggel S, Mrus J, Huang J, Harrington CM, Hudson KJ, Margolis DA, Smith KY, Williams PE, Spreen WR. 2020. Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression. N Engl J Med 382:1112–1123. 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 183.Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard P-M, Oka S, Walmsley S, Bettacchi C, Brinson C, Philibert P, Lombaard J, St Clair M, Crauwels H, Ford SL, Patel P, Chounta V, D'Amico R, Vanveggel S, Dorey D, Cutrell A, Griffith S, Margolis DA, Williams PE, Parys W, Smith KY, Spreen WR. 2020. Long-acting Cabotegravir and Rilpivirine after oral induction for HIV-1 infection. N Engl J Med 382:1124–1135. 10.1056/NEJMoa1909512. [DOI] [PubMed] [Google Scholar]
- 184.Overton ET, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, Bredeek F, García DM, Swindells S, Andrade-Villanueva JF, Wong A, Khuong-Josses M-A, Van Solingen-Ristea R, van Eygen V, Crauwels H, Ford S, Talarico C, Benn P, Wang Y, Hudson KJ, Chounta V, Cutrell A, Patel P, Shaefer M, Margolis DA, Smith KY, Vanveggel S, Spreen W. 2020. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 396:1994–2005. 10.1016/S0140-6736(20)32666-0. [DOI] [PubMed] [Google Scholar]
- 185.Cutrell AG, Schapiro JM, Perno CF, Kuritzkes DR, Quercia R, Patel P, Polli JW, Dorey D, Wang Y, Wu S, Van Eygen V, Crauwels H, Ford SL, Baker M, Talarico CL, Clair MS, Jeffrey J, White CT, Vanveggel S, Vandermeulen K, Margolis DA, Aboud M, Spreen WR, van Lunzen J. 2021. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. Aids 35:1333–1342. 10.1097/QAD.0000000000002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Andreatta K, Willkom M, Martin R, Chang S, Wei L, Liu H, Liu Y-P, Graham H, Quirk E, Martin H, White KL. 2019. Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J Antimicrob Chemother 74:3555–3564. 10.1093/jac/dkz347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perez-Valero I, Llibre JM, Castagna A, Pulido F, Molina J-M, Esser S, Margot N, Shao Y, Temme L, Piontkowsky D, McNicholl IR, Haubrich R. 2021. Switching to Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in adults with HIV and M184V/I mutation. J Acquir Immune Defic Syndr 86:490–495. 10.1097/QAI.0000000000002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jary A, Marcelin A-G, Charpentier C, Wirden M, Lê MP, Peytavin G, Descamps D, Calvez V. 2020. M184V/I does not impact the efficacy of abacavir/lamivudine/dolutegravir use as switch therapy in virologically suppressed patients. J Antimicrob Chemother 75:1290–1293. 10.1093/jac/dkaa019. [DOI] [PubMed] [Google Scholar]
- 189.Fournier AL, Hocqueloux L, Braun DL, Metzner KJ, Kouyos RD, Raffi F, Briant AR, Martinez E, De Lazzari E, Negredo E, Rijnders B, Rokx C, Günthard HF, Parienti J-J. 2022. Dolutegravir monotherapy as maintenance strategy: a meta-analysis of individual participant data from randomized controlled trials. Open Forum Infect Dis 9:ofac107. 10.1093/ofid/ofac107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baldin G, Ciccullo A, Borghetti A, Di Giambenedetto S. 2019. Virological efficacy of dual therapy with lamivudine and dolutegravir in HIV-1-infected virologically suppressed patients: long-term data from clinical practice. J Antimicrob Chemother 74:1461–1463. 10.1093/jac/dkz009. [DOI] [PubMed] [Google Scholar]
- 191.Gagliardini R, Ciccullo A, Borghetti A, Maggiolo F, Bartolozzi D, Borghi V, Pecorari M, Di Biagio A, Callegaro AP, Bruzzone B, Saladini F, Paolucci S, Maserati R, Zazzi M, Di Giambenedetto S, De Luca A, Group AS, Mellace V, Capetti A, Rita Gismondo M, Luisa BM, Mussini C, Pecorari M, Gianotti N, Sacchini D, Parruti G, Polilli E, Baldelli F, Zanussi S, Nerli A, Lenzi L, Calzetti C, Vivarelli A, Maserati R, Baldanti F, Poletti F, Mondino V, Malena M, Cascio A, Filice G, Magnani G, Zerbini A, Lombardi F, Di Giambenedetto S, Andreoni M, Montano M, Vullo V, Turriziani O, Zazzi M, Gonnelli A, et al. 2018. Impact of the M184V resistance mutation on virological efficacy and durability of lamivudine-based dual antiretroviral regimens as maintenance therapy in individuals with suppressed HIV-1 RNA: a cohort study. Open Forum Infect Dis 5:ofy113. 10.1093/ofid/ofy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Galizzi N, Poli A, Galli L, Muccini C, Mastrangelo A, Dell'Acqua R, Maillard M, Bossolasco S, Cinque P, Lazzarin A, Castagna A, Gianotti N. 2020. Retrospective study on the outcome of two-drug regimens based on dolutegravir plus one reverse transcriptase inhibitor in virologically-suppressed HIV-infected patients. Int J Antimicrobial Agents 55:105893. 10.1016/j.ijantimicag.2020.105893. [DOI] [PubMed] [Google Scholar]
- 193.Borghetti A, Giacomelli A, Borghi V, Ciccullo A, Dusina A, Fabbiani M, Rusconi S, Zazzi M, Mussini C, Di Giambenedetto S. 2021. nucleoside reverse-transcriptase inhibitor resistance mutations predict virological failure in Human Immunodeficiency Virus-positive patients during Lamivudine plus Dolutegravir maintenance therapy in clinical practice. Open Forum Infect Dis 8. 10.1093/ofid/ofab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Borghetti A, Lombardi F, Gagliardini R, Baldin G, Ciccullo A, Moschese D, Emiliozzi A, Belmonti S, Lamonica S, Montagnani F, Visconti E, De Luca A, Di Giambenedetto S. 2019. Efficacy and tolerability of lamivudine plus dolutegravir compared with lamivudine plus boosted PIs in HIV-1 positive individuals with virologic suppression: a retrospective study from the clinical practice. BMC Infect Dis 19:59. 10.1186/s12879-018-3666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ciccullo A, Borghi V, Giacomelli A, Cossu MV, Sterrantino G, Latini A, Giacometti A, De Vito A, Gennari W, Madeddu G, Capetti A, d’Ettorre G, Mussini C, Rusconi S, Di Giambenedetto S, Baldin G. 2021. Five years with Dolutegravir plus Lamivudine as a switch strategy: much more than a positive finding. JAIDS J Acquired Immune Deficiency Syndromes 88:234–237. 10.1097/QAI.0000000000002787. [DOI] [PubMed] [Google Scholar]
- 196.Deschanvres C, Reynes J, Lamaury I, Rey D, Palich R, Bani-Sadr F, Robineau O, Duvivier C, Hocqueloux L, Cuzin L, Joly V, Raffi F, Cabie A, Allavena C, Chirouze C, Drobacheff-Thiébaut C, Foltzer A, Bouiller K, Hustache- Mathieu L, Lepiller Q, Bozon F, Babre O, Brunel AS, Muret P, Chevalier E, Jacomet C, Laurichesse H, Lesens O, Vidal M, Mrozek N, Aumeran C, Baud O, Corbin V, Goncalvez E, Mirand A, Brebion A, Henquell C, Lamaury I, Fabre I, Curlier E, Ouissa R, Herrmann-Storck C, Tressieres B, Receveur MC, Boulard F, Daniel C, Clavel C, Roger PM, Markowicz S, Chellum Rungen N, Dat’AIDS Study Group , et al. 2021. Dolutegravir-based dual maintenance regimens combined with lamivudine/emtricitabine or rilpivirine: risk of virological failure in a real-life setting. J Antimicrob Chemother 77:196–204. 10.1093/jac/dkab367. [DOI] [PubMed] [Google Scholar]
- 197.Fabbiani M, Rossetti B, Ciccullo A, Oreni L, Lagi F, Celani L, Colafigli M, De Vito A, Mazzitelli M, Dusina A, Durante M, Montagnani F, Rusconi S, Capetti A, Sterrantino G, D’Ettorre G, Di Giambenedetto S, Zanelli G, Baldin G, Borghetti A, Latini A, Mastroianni C, Borghi V, Mussini C, Cossu MV, Giacomelli A, Formenti T, Trecarichi EM, Torti C, Madeddu G, Vecchiet J, Vignale F, Giacometti A, Group for the OS . 2021. Efficacy and durability of two- vs. three-drug integrase inhibitor-based regimens in virologically suppressed HIV-infected patients: data from real-life ODOACRE cohort. HIV Med 22:843–853. 10.1111/hiv.13146. [DOI] [PubMed] [Google Scholar]
- 198.Santoro MM, Armenia D, Teyssou E, Santos JR, Charpentier C, Lambert-Niclot S, Antinori A, Katlama C, Descamps D, Perno CF, Calvez V, Paredes R, Ceccherini-Silberstein F, Marcelin AG. 2022. Virological efficacy of switch to DTG plus 3TC in a retrospective observational cohort of suppressed HIV-1 patients with or without past M184V – The LAMRES Study. J Glob Antimicrob Resist. 10.1016/j.jgar.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 199.Blick G, Ceretta E, Mancini G, Cosenza A, Fang L. SOLAR 3D: a prospective comparative study switching from 3- or 4-drug ART to DTG/3TC for maintenance of viral suppression in the setting of current and historical M184V/I and prior virologic failures: 48 week primary endpoint results. 18th European AIDS Conference, October 27–30, 2021. London. [Google Scholar]
- 200.Eron JJ, Benoit SL, Jemsek J, MacArthur RD, Santana J, Quinn JB, Kuritzkes DR, Fallon MA, Rubin M. 1995. Treatment with Lamivudine, Zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N Engl J Med 333:1662–1669. 10.1056/NEJM199512213332502. [DOI] [PubMed] [Google Scholar]
- 201.Campbell TB, Shulman NS, Johnson SC, Zolopa AR, Young RK, Bushman L, Fletcher CV, Lanier ER, Merigan TC, Kuritzkes DR. 2005. Antiviral activity of Lamivudine in salvage therapy for multidrug-resistant HIV-1 infection. Clin Infect Dis 41:236–242. 10.1086/430709. [DOI] [PubMed] [Google Scholar]
- 202.Diallo K, Götte M, Wainberg MA. 2003. Molecular impact of the M184V mutation in human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother 47:3377–3383. 10.1128/AAC.47.11.3377-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Prazuck T, Verdon R, Le Moal G, Ajana F, Bernard L, Sunder S, Roncato-Saberan M, Ponscarme D, Etienne M, Viard J-P, Pasdeloup T, Darasteanu I, Pialoux G, de la Blanchardière A, Avettand-Fènoël V, Parienti J-J, Hocqueloux L, Fourmy A, Mureau E, Juteau N, Giraudeau B, Dargere S, Michon J, Baldoli A, the TRULIGHT Study Team . 2021. Tenofovir disoproxil fumarate and emtricitabine maintenance strategy in virologically controlled adults with low HIV-1 DNA: 48 week results from a randomized, open-label, non-inferiority trial. J Antimicrob Chemother 76:1564–1572. 10.1093/jac/dkab038. [DOI] [PubMed] [Google Scholar]
- 204.Armenia D, Zaccarelli M, Borghi V, Gennari W, Di Carlo D, Giannetti A, Forbici F, Bertoli A, Gori C, Fabeni L, Pinnetti C, Marocco R, Latini A, Ceccherini-Silberstein F, Mastroianni CM, Mussini C, Antinori A, Perno CF, Santoro MM. 2018. Resistance detected in PBMCs predicts virological rebound in HIV-1 suppressed patients switching treatment. J Clinical Virology 104:61–64. 10.1016/j.jcv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 205.Ellis KE, Nawas GT, Chan C, York L, Fisher J, Connick E, Zangeneh TT. 2020. Clinical outcomes following the use of archived proviral HIV-1 dna genotype to guide antiretroviral therapy adjustment. Open Forum Infect Dis 7:ofz533. 10.1093/ofid/ofz533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rodriguez MA, Mills A, Stoker A, Cai S, Curanovic D, Petropoulos CJ, Walworth C. 2021. HIV-1 DNA resistance testing informs the successful switch to a single tablet regimen. J Acquir Immune Defic Syndr Clin Res 12:1. [Google Scholar]
- 207.Assoumou L, Weiss L, Piketty C, Burgard M, Melard A, Girard P-M, Rouzioux C, Costagliola D, ANRS 116 SALTO study group . 2015. A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. Aids 29:2003–2007. 10.1097/QAD.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 208.Martin GE, Frater J. 2018. Post-treatment and spontaneous HIV control. Curr Opin HIV AIDS 13:402–407. 10.1097/COH.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 209.Sharaf R, Lee GQ, Sun X, Etemad B, Aboukhater LM, Hu Z, Brumme ZL, Aga E, Bosch RJ, Wen Y, Namazi G, Gao C, Acosta EP, Gandhi RT, Jacobson JM, Skiest D, Margolis DM, Mitsuyasu R, Volberding P, Connick E, Kuritzkes DR, Lederman MM, Yu XG, Lichterfeld M, Li JZ. 2018. HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J Clin Invest 128:4074–4085. 10.1172/JCI120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mullins JI, Frenkel LM. 2017. Clonal expansion of human immunodeficiency virus–infected cells and human immunodeficiency virus persistence during antiretroviral therapy. J Infect Dis 215:S119–S127. 10.1093/infdis/jiw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ikeda T, Shibata J, Yoshimura K, Koito A, Matsushita S. 2007. Recurrent HIV-1 integration at the BACH2 locus in resting CD4+ T cell populations during effective highly active antiretroviral therapy. J Infect Dis 195:716–725. 10.1086/510915. [DOI] [PubMed] [Google Scholar]
- 212.Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH. 2014. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345:179–183. 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rose PP, Korber BT. 2000. Detecting hypermutations in viral sequences with an emphasis on G → A hypermutation. Bioinformatics 16:400–401. 10.1093/bioinformatics/16.4.400. [DOI] [PubMed] [Google Scholar]
- 214.Eshleman SH, Husnik M, Hudelson S, Donnell D, Huang Y, Huang W, Hart S, Jackson B, Coates T, Chesney M, Koblin B. 2007. Antiretroviral drug resistance, HIV-1 tropism, and HIV-1 subtype among men who have sex with men with recent HIV-1 infection. AIDS 21:1165–1174. 10.1097/QAD.0b013e32810fd72e. [DOI] [PubMed] [Google Scholar]
- 215.Westby M, Lewis M, Whitcomb J, Youle M, Pozniak AL, James IT, Jenkins TM, Perros M, van der Ryst E. 2006. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist Maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol 80:4909–4920. 10.1128/JVI.80.10.4909-4920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Whitcomb JM, Huang W, Fransen S, Limoli K, Toma J, Wrin T, Chappey C, Kiss LDB, Paxinos EE, Petropoulos CJ. 2007. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob Agents Chemother 51:566–575. 10.1128/AAC.00853-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hartley O, Klasse PJ, Sattentau QJ, Moore JP. 2005. V3: HIV’s switch-hitter. AIDS Res Hum Retroviruses 21:171–189. 10.1089/aid.2005.21.171. [DOI] [PubMed] [Google Scholar]
- 218.Pou C, Noguera-Julian M, Pérez-Álvarez S, García F, Delgado R, Dalmau D, Álvarez-Tejado M, Gonzalez D, Sayada C, Chueca N, Pulido F, Ibáñez L, Rodríguez C, Casadellà M, Santos JR, Ruiz L, Clotet B, Paredes R. 2014. Improved prediction of salvage antiretroviral therapy outcomes using ultrasensitive HIV-1 drug resistance testing. Clin Infect Dis 59:578–588. 10.1093/cid/ciu287. [DOI] [PubMed] [Google Scholar]
- 219.Vicenti I, Razzolini F, Saladini F, Romano L, Zazzi M. 2007. Use of peripheral blood DNA for genotype antiretroviral resistance testing in drug-naive HIV-infected subjects. Clin Infect Dis 44:1657–1661. 10.1086/518287. [DOI] [PubMed] [Google Scholar]
- 220.Dauwe K, Staelens D, Vancoillie L, Mortier V, Verhofstede C. 2016. Deep sequencing of HIV-1 RNA and DNA in newly diagnosed patients with baseline drug resistance showed no indications for hidden resistance and is biased by strong interference of hypermutation. J Clin Microbiol 54:1605–1615. 10.1128/JCM.00030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Trabaud M-A, Leriche-Guerin K, Regis C, Bordes I, Cotte L, Detmer J, Kolberg J, Ritter J, Trépo C. 2000. Prevalence of primary resistance to zidovudine and lamivudine in drug-naive human immunodeficiency virus type-1 infected patients: high proportion of reverse transcriptase codon 215 mutant in circulating lymphocytes and free virus. J Med Virol 61:352–359. . [DOI] [PubMed] [Google Scholar]
- 222.Ellis GM, Page LC, Burman BE, Buskin S, Frenkel LM. 2009. Increased detection of HIV-1 drug resistance at time of diagnosis by testing viral DNA with a sensitive assay. JAIDS J Acquired Immune Deficiency Syndromes 51:283–289. 10.1097/QAI.0b013e3181a9972c. [DOI] [PubMed] [Google Scholar]
- 223.Devereux HL, Loveday C, Youle M, Sabin CA, Burke A, Johnson M. 2000. Substantial correlation between HIV type 1 drug-associated resistance mutations in plasma and peripheral blood mononuclear cells in treatment-experienced patients. AIDS Res Hum Retroviruses 16:1025–1030. 10.1089/08892220050075273. [DOI] [PubMed] [Google Scholar]
- 224.Chew CB, Potter SJ, Wang B, Wang YM, Shaw CO, Dwyer DE, Saksena NK. 2005. Assessment of drug resistance mutations in plasma and peripheral blood mononuclear cells at different plasma viral loads in patients receiving HAART. J Clin Virol 33:206–216. 10.1016/j.jcv.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 225.Turriziani O, Bucci M, Stano A, Scagnolari C, Bellomi F, Fimiani C, Mezzaroma I, D'Ettorre G, Brogi A, Vullo V, Antonelli G. 2007. Genotypic resistance of archived and circulating viral strains in the blood of treated HIV-infected individuals. J Acquir Immune Defic Syndr 44:518–524. 10.1097/QAI.0b013e3180315515. [DOI] [PubMed] [Google Scholar]
- 226.Saracino A, Gianotti N, Marangi M, Cibelli DC, Galli A, Punzi G, Monno L, Lazzarin A, Angarano G, on behalf of the Mutations, Salvage (MuSa) Study Group. 2008. Antiretroviral genotypic resistance in plasma RNA and whole blood DNA in HIV-1 infected patients failing HAART. J Medical Virology 80:1695–1706. 10.1002/jmv.21261. [DOI] [PubMed] [Google Scholar]
- 227.Diallo K, Murillo WE, de Rivera IL, Albert J, Zhou Z, Nkengasong J, Zhang G, Sabatier JF, Yang C. 2012. Comparison of HIV-1 resistance profiles in plasma RNA versus PBMC DNA in heavily treated patients in Honduras, a resource-limited country. Int J Mol Epidemiol Genet 3:56–65. [PMC free article] [PubMed] [Google Scholar]
- 228.Derache A, Shin H-S, Balamane M, White E, Israelski D, Klausner JD, Freeman AH, Katzenstein D. 2015. HIV drug resistance mutations in proviral dna from a community treatment program. PLoS One 10:e0117430. 10.1371/journal.pone.0117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lübke N, Cristanziano VD, Sierra S, Knops E, Schülter E, Jensen B, Oette M, Lengauer T, Kaiser R, Group on behalf of the RS . 2015. Proviral DNA as a target for HIV-1 resistance analysis. INT 58:184–189. [DOI] [PubMed] [Google Scholar]
- 230.Milne RS, Silverman RA, Beck IA, Mckernan-Mullin J, Deng W, Sibley TR, Dross S, Kiarie JN, Sakr SR, Coombs RW, Chung MH, Frenkel LM. 2019. Minority and majority pretreatment HIV-1 drug resistance associated with failure of first-line nonnucleoside reverse-transcriptase inhibitor antiretroviral therapy in Kenyan women. Aids 33:941–951. 10.1097/QAD.0000000000002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang YM, Dyer WB, Workman C, Wang B, Peng NK, Lachireddy K, Chew CB, Sullivan J, Saksena NK. 2007. Drug resistance and viral evolution in plasma and peripheral blood cells during structured treatment interruption (STI) and non-interrupted HAART. Curr HIV Res 5:235–250. 10.2174/157016207780077039. [DOI] [PubMed] [Google Scholar]
- 232.Sarmati L, Nicastri E, Uccella I, D'Ettorre G, Parisi SG, Palmisano L, Galluzzo C, Concia E, Vullo V, Vella S, Andreoni M. 2003. Drug-associated resistance mutations in plasma and peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected patients for whom highly active antiretroviral therapy is failing. J Clin Microbiol 41:1760–1762. 10.1128/JCM.41.4.1760-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Debiaggi M, Bruno R, Sacchi P, Achilli G, Romero E, Filice G. 2002. Distinct mutational drug resistance profiles of HIV-1 RNA in plasma and culture isolates of patients receiving antiretroviral therapy. Int 45:52–55. [DOI] [PubMed] [Google Scholar]
- 234.Ciaffi L, Koulla-Shiro S, Sawadogo AB, Ndour CT, Eymard-Duvernay S, Mbouyap PR, Ayangma L, Zoungrana J, Gueye NFN, Diallo M, Izard S, Bado G, Kane CT, Aghokeng AF, Peeters M, Girard PM, Le Moing V, Reynes J, Delaporte E, MOBIDIP study group . 2017. Boosted protease inhibitor monotherapy versus boosted protease inhibitor plus lamivudine dual therapy as second-line maintenance treatment for HIV-1-infected patients in sub-Saharan Africa (ANRS12 286/MOBIDIP): a multicentre, randomised, parallel, open-label, superiority trial. Lancet HIV 4:e384–e392. 10.1016/S2352-3018(17)30069-3. [DOI] [PubMed] [Google Scholar]
- 235.Rial-Crestelo D, de Miguel R, Montejano R, Dominguez-Dominguez L, Aranguren-Rivas P, Esteban-Cantos A, Bisbal O, Santacreu-Guerrero M, Garcia-Alvarez M, Alejos B, Hernando A, Bermejo-Plaza L, Cadiñanos J, Mayoral M, Castro JM, Moreno V, Martin-Carbonero L, Delgado R, Rubio R, Pulido F, Arribas JR, Arribas JR, Buckley RDM, Montejano R, Esteban-Cantos A, Stella-Ascariz N, Cadiñanos J, Mayoral M, Castro JM, Moreno V, Martin-Carbonero L, Valencia E, Bernardino I, Busca C, Micán R, Pérez-Valero I, González J, Montes ML, Centeno JR, Pulido F, Rial-Crestelo D, Dominguez-Dominguez L, Aranguren-Rivas P, Bisbal O, Plaza LB, Garcia-Alvarez M, Santacreu-Guerrero M, de Lagarde M, Matarranz M, Luzckoviak J, ART-PRO, PI16/00837-PI16/00678 study group , et al. 2021. Long-term efficacy of dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: week 96 results of ART-PRO pilot study. J Antimicrob Chemother 76:738–742. 10.1093/jac/dkaa479. [DOI] [PubMed] [Google Scholar]
- 236.Baldin G, Ciccullo A, Rusconi S, Capetti A, Sterrantino G, Colafigli M, d'Ettorre G, Giacometti A, Cossu MV, Borghetti A, Gennari W, Mussini C, Borghi V, Di Giambenedetto S. 2019. Long-term data on the efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multi-centre cohort of HIV-1-infected, virologically suppressed patients. Int J Antimicrob Agents 54:728–734. 10.1016/j.ijantimicag.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 237.Hocqueloux L, Allavena C, Secher S, Makinson A, Rey D, Huleux T, Palich R, Bani-Sadr F, Cotte L, For the DAT’AIDS Study Group . Archived mutation M184V does not increase virologic failure during maintenance therapy with dolutegravir + lamivudine in the French DAT’AIDS cohort. 18th European AIDS Conference, October 27–30, 2021. London. [Google Scholar]