ABSTRACT
The accumulation of autotoxins in soil causes continuous cropping obstacle stress in crops, and the bioremediation of autotoxins by microorganisms is an efficient process. In this study, strain ZH07 was isolated from the peanut rhizosphere and was found to be utilizing multiple autotoxins as its carbon sources. Based on its genomic characteristics and a phylogenetic analysis, ZH07 represents a member of Klebsiella variicola subsp. variicola. A comparative genomic analysis exhibited evolutionary dynamics exhibited by mobile genetic elements (MGEs), strain-specific genes, potential horizontal genes, and evolutionary constraints driven by purifying selection, which facilitated its genomic adaptation to rhizosphere soil. Genome mining revealed the potential genomic properties associated with plant growth promotion, such as nitrogen fixation, indole acetic acid synthesis, phosphonate solubilization and assimilation, siderophore production, and secondary metabolite synthesis. Moreover, abundant genes putatively responsible for the biodegradation of aromatic xenobiotics, including benzoic acid, cinnamic acid, vanillic acid, protocatechuic acid, phenylacetic acid, and p-hydroxybenzoic acid were also observed in the ZH07 genome. Compared to autotoxin stress alone, the combination of ZH07 application promoted peanut germination and seedling growth. Our analysis revealed the genetic adaptation of ZH07 to the rhizosphere environment and the potential genetic basis and effectiveness of the isolate to serve as a plant growth stimulator.
IMPORTANCE Continuous cropping obstacles reduce the production and quality of agricultural products, and the application of rhizosphere beneficial microbes is an important strategy. Strain ZH07 showed autotoxin-degrading and plant growth-promoting capacities. The objectives of this study were to characterize its genomic evolution and the potential genetic basis of the autotoxin degradation and plant growth promotion. ZH07 represents a member of Klebsiella variicola subsp. variicola, based on genomic and phylogenetic analyses. Its genomic components have undergone different degrees of purifying selection, and the disparity in the evolutionary rate may be associated with its niche adaptation. A systematic analysis of the ZH07 genome identified the potential genetic basis that contributes to plant growth promotion and to aromatic xenobiotic biodegradation. This study demonstrates that plant growth-promoting rhizobacteria (PGPR) play important roles in autotoxin biodegradation and can be used as biofertilizers to enhance the growth of peanuts in response to continuous cropping obstacle stress.
KEYWORDS: autotoxin degradation, comparative genomics, Klebsiella, peanut, plant growth-promoting rhizobacteria
INTRODUCTION
Continuous cropping obstacles are caused by planting patterns in which the same or a similar crop is cultivated in the same soil, year after year (1). It reduces the production and quality of agricultural products, seriously restricting the development of modern comprehensive and sustainable agriculture. Importantly, autotoxicity results from the accumulation of autotoxins, which can be released into the environment through root secretion and plant residue decomposition (2–4). The accumulation of autotoxins in soil is harmful to conspecific plants (5–9). Autotoxins can inhibit germination, resulting in oxidative stress in seedling roots, the disruption of membrane permeability, damage to DNA and proteins, the alteration of enzyme activities, and the alteration of mineral uptake (10–14). In addition to the direct impact on plants, the accumulation of autotoxins in the rhizosphere soil can also indirectly promote root disease by changing the microbial community structure, resulting in continuous cropping obstacles (15–17).
Some methods have been used to alleviate autotoxicity, including the selection of resistant varieties, crop rotation and the provision of a proper fallow period for the decomposition of autotoxins (18–21). Notably, the application of environment-friendly, beneficial microbes with highly efficient autotoxin degradation capabilities is increasingly being applied for the suppression of continuous cropping obstacles (22, 23). Among them, plant growth-promoting rhizobacteria (PGPR), as strong root colonizers, represent beneficial functions, including autotoxin degradation, plant growth promotion, and soilborne disease suppression (24–26). A large number of autotoxin-degrading PGPRs have been isolated, including Bacillus, Azotobacter, Pseudomonas, Staphylococcus, Acinetobacter, and Exiguobacterium, among others. Some of them are capable of degrading multiple types of autotoxins in combination with a high degradation efficiency (16, 21, 27, 28). A plant usually produces more than one kind of autotoxin. Hence, the strains with the ability to degrade multiple autotoxins will have a greater practical value. The application of autotoxin-degrading PGPR could be an ecofriendly bioremediation method by which to increase the productivity of plants. For example, Acinetobacter calcoaceticus CSY-P13 and Streptomyces canus GLY-P2 have been reported to rapidly degrade ferulic acids and p-hydroxybenzoic acids and reduce their autotoxicity to cucumbers (16, 28). Moreover, Pseudomonas putida and Pseudomonas hunanensis strains show high efficiency in the degradation of ferulic acid, p-hydroxybenzoic acid, and syringic acid, and they also reduce the inhibition of lily, watermelon, poplar, and strawberry seedling growth (21).
Peanut, as an important oilseed crop worldwide, is sensitive to continuous cropping obstacles. Benzoic acid, p-hydroxybenzoic acid, p-coumaric acid, and several other phenolic acids are the major autotoxins in peanut root exudation (15, 29). In this study, PGPR strain ZH07 isolated from the peanut rhizosphere displayed highly efficient abilities to degrade multiple autotoxins. Whole-genome sequencing (WGS) has offered a tremendous advantage in determining the evolutionary relationship, genetic diversity, and biotechnological properties (30–32). Hence, the genome of ZH07 was sequenced for a comparative genomic analysis. The phylogenetic relationships and evolutionary dynamics were explored to elucidate the evolution of this strain. The key genetic characteristics (e.g., nitrogen fixation, indole acetic acid synthesis, phosphate solubilization and assimilation, siderophore synthesis and transport, secondary metabolite biosynthesis gene clusters, and potential autotoxin degradation pathways) were investigated to reveal the underlying genetic basis of plant growth promotion and autotoxin degradation in the ZH07 genome. The effects of inoculation of ZH07 on peanut seed germination and seedling growth was also evaluated. Our results will provide a new choice for the biological removal of autotoxins and for the alleviation of peanut continuous cropping obstacles.
RESULTS
Isolation, identification, and characterization of ZH07.
Using benzoic acid as the sole carbon source, 31 strains were isolated from the peanut rhizosphere as potential autotoxin-degrading strains. The strain ZH07 showed outstanding abilities to degrade autotoxins and promote plant growth. Therefore, it was selected for further studies. Growth of ZH07 occurred at 20 to 50°C (optimum: 30°C) with 0 to 10% NaCl (optimum: 1%) and a pH of 5.0 to 9.0 (optimum: pH 7.0) (Table 1; Fig. S1).
TABLE 1.
Characterization of Klebsiella ZH07
| Characteristic | Klebsiella variicola subsp. variicola ZH07 |
|---|---|
| Source | Rhizosphere soil of peanut |
| Growth conditiona | |
| Temp range | 20 to 50°C (30°C) |
| NaCl range | 0 to 10% (1%) |
| pH range | 5 to 9 (7) |
| IAA production | 8.33 + 0.15 (mg/L) |
| Siderophore productionb | + |
| Phosphate solubilizationb | + |
| Ammonia productionb | + |
| Potassium solubilizationb | + |
| Antibiotic susceptibility testc | Em+, Amp+, Zeo+, Hyg+, Gm−, Sm−, Cm−, Kan−, Sh−, Tc− |
Values in parentheses indicate the optimal growth condition.
+, strain has the related capacity; −, strain does not have the related capacity.
+, resistant; −, sensitive; Em, erythromycin, 10 ppm; Amp, ampicillin, 100 ppm; Zeo, Zeocin, 20 ppm; Hyg, hygromycin, 30 ppm; Gm, gentamycin, 50 ppm; Sm, streptomycin, 50 ppm; Cm, chloramphenicol, 10 ppm; Kan, kanamycin, 30 ppm; Sh, spectinomycin, 100 ppm; Tc, tetracycline, 15 ppm.
The plant growth-promoting abilities of ZH07 were evaluated, including nitrogen fixation, phosphorus solubilization, potassium dissolution, and siderophore and indole acetic acid (IAA) production (Table 1). In the qualitative assay, ZH07 could grow well in nitrogen-free Ashby medium (Fig. S2A), form a solubilization halo on an inorganic phosphorus medium (Fig. S2B), an egg yolk medium (Fig. S2C), and an Alexandrov medium (Fig. S2D). It also formed orange halo zones, indicating siderophore production (Fig. S2E). Moreover, ZH07 produced 8.33 ± 0.15 mg·L−1 of IAA after 4 days of incubation with 200 μg·L−1 of l-tryptophan at 30°C (Table 1). In our results, ZH07 showed the capacity of nitrogen fixation, phosphorus solubilization, potassium dissolution, siderophore production, and IAA synthesis, suggesting that ZH07 has the potential to promote plant growth.
Autotoxin-degrading properties of ZH07.
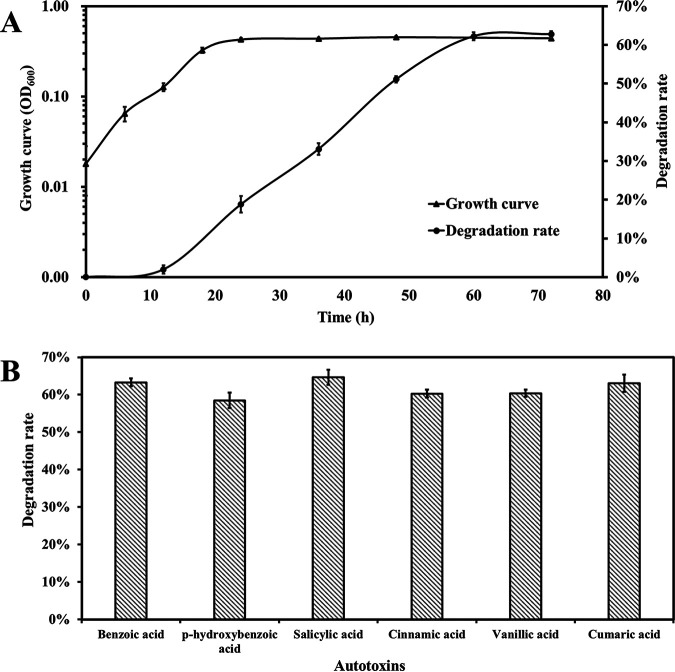
To evaluate its potential for alleviating continuous cropping obstacle stress, the degradation of a range of autotoxins by ZH07 was assessed. As shown in Fig. 1A and in Fig. S3, ZH07 has the ability to grow in MSM medium with benzoic acid as its sole carbon source. It could utilize more than 60% benzoic acid within ~21 h of incubation, and it exhibited maximum growth within 59 h of incubation (Fig. 1A). Moreover, ZH07 was also able to utilize p-hydroxybenzoic acid, salicylic acid, cinnamic acid, vanillic acid, and cumaric acid with more than 50% utilization (Fig. 1B). Hence, our results revealed that ZH07 could efficiently degrade multiple autotoxins, indicating the mitigation effects of ZH07 on autotoxin stress in peanuts.
FIG 1.
(A) Cell growth in semilogarithmic coordinates and the benzoic acid degradation rate of strain ZH07 in MSM liquid medium. (B) The degradation rates of six phenolic acids by ZH07.
Genomic characteristics of ZH07.
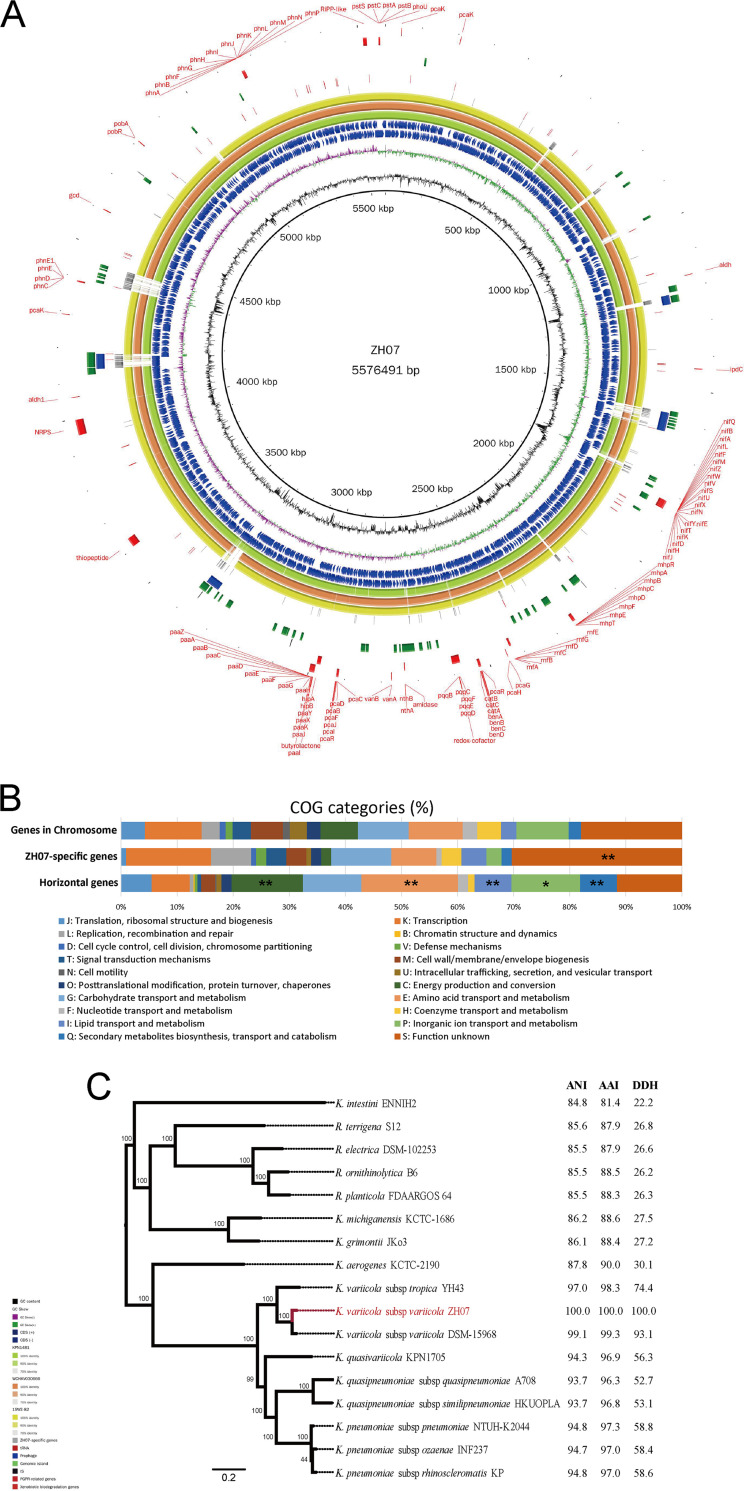
The above biological properties indicated that the genomic evolution and underlying genetic basis of ZH07 deserve further systematic study. Therefore, the complete genome sequence of ZH07 with one plasmid pZH07 was sequenced. The genome was estimated to have 100% completeness with 1% contamination. The ZH07 chromosome was comprised of 5,576,491 bp with a guanine-cytosine (GC) content of 57.4% and 5,404 rapid antimicrobial susceptibility testing (RAST)-predicted coding sequences (CDSs) (Fig. 2A). Based on the Clusters of Orthologous Genes (COG) functional assignment, a total of 4,886 (90.4%) CDSs were classified into 20 COG categories. “K: transcription” (541 CDSs), “G: Carbohydrate transport and metabolism” (485 CDSs), “E: Amino acid transport and metabolism” (515 CDSs), and “P: Inorganic ion transport and metabolism” (498 CDSs) were the most enriched functional categories in the chromosome (Fig. 2B). About 27.4% of the CDSs were poorly characterized (“S: Functional unknown”: 962 CDSs; “no homologs identified”: 518 CDSs).
FIG 2.
Genome features of the ZH07 chromosome. (A) Circular representation of the ZH07 chromosome. Rings represent the following features labeled from inside to outside: ring 1, GC content; ring 2, GC-skew, where green and purple correspond to above-average and below-average GC-skew, respectively; rings 3 and 4, blue arrows correspond to plus-strand CDS and minus-strand CDS; rings 5 to 7, circular comparison of KPN1481, WCHKV030666, and 15WZ-82, respectively; rings 8 to 14, blocks correspond to ZH07-specific genes, tRNA, prophages, GIs, ISs, secondary metabolite-related biosynthesis gene clusters, and xenobiotic biodegradation genes, respectively. (B) Distribution of COG categories for chromosome genes, ZH07-specific genes, and potential horizontal genes, respectively. *, Fisher’s exact test P value < 0.05; **, Fisher’s exact test P value < 0.01. (C) Phylogenetic tree based on SNPs across 2,529 single-copy core gene families shared by ZH07 and on 16 complete genomes of other Klebsiella species that was constructed via the maximum likelihood method with 100 replicates.
The plasmid pZH07 was comprised of 174,413 bp, which made up 3.0% of the ZH07 genome. It harbored 230 RAST-predicted CDSs and presented with a 50.8% GC content. A Nucleotide Basic Local Alignment Search Tool (BLASTN) search of the National Center for Biotechnology Information (NCBI) nr database, using the full plasmid sequence, showed that pZH07 exhibits a backbone similar to those of some described plasmids (e.g., pKLVA-1, pLMG 23571, p15WZ-82_res, pKP91, and pKp5-1) in other K. variicola strains (Fig. S4A). The plasmid pZH07 carries a variety of functional genes, including genes related to “K: transcription” (22 CDSs), “L: Replication, recombination and repair” (28 CDSs), and “S: Function unknown” (44 CDSs) (Fig. S4B). Furthermore, one conjugal transfer region (tra operon) and one CRISPR locus with 13 different spacer sequences and no cas genes was found in pZH07 (Fig. S4A).
In addition to plasmids, several other types of mobile genetic elements (MGEs) were identified in the ZH07 genome, including genomic islands (GIs), prophages, and insertion sequences (ISs) (Fig. 2A; Table S1). A total of 87 tRNA codons were scattered throughout the chromosome. 54 GIs were identified, covering a 541.5 kb (9.7%) region of the chromosome. The ZH07 chromosome harbored three intact prophages and one incomplete prophage that covered a 175.1 kb (3.1%) region of the chromosome. A total of 19 complete IS elements were identified, including 7 on the chromosome and 12 on the plasmid. The presence of diverse MGEs indicated the genetic plasticity of ZH07. We also identified 380 potential horizontal genes (Table S2) in the ZH07 chromosome that were significantly enriched in “C: Energy production and conversion”, “E: Amino acid transport and metabolism”, “I: Lipid transport and metabolism”, “Q: Secondary metabolites biosynthesis, transport and catabolism” (Fisher’s exact test P value < 0.01), and “P: Inorganic ion transport and metabolism” (Fisher’s exact test P value < 0.05).
Phylogenetic analysis revealed the evolutionary position of ZH07.
The complete 16S rRNA sequence (1,544 bp) of ZH07 was subjected to similarity-based searches against the taxonomically united 16S rRNA database in EzBioCloud (33). The result (Table S3) revealed up to 99.9% identity with Klebsiella variicola subsp. tropica SB5531T (accession number: MK040621.1), as well as high identity matches (>98.0%) with other Klebsiella species (Fig. S4C), indicating that strain ZH07 belongs to the Klebsiella genus. To further explore the taxonomic position of ZH07, a phylogenetic tree was created, based on the 2,529 single-copy core gene families shared by ZH07 and 16 complete genomes of other Klebsiella species (Table S3). The core genome tree identified ZH07 as a member of K. variicola subsp. variicola (Fig. 2C). The identification is further supported by average nucleotide identity (ANI), average amino acid identity (AAI), and in silico DNA-DNA hybridization analyses. The highest ANI (99.1%), AAI (99.3%), and in silico DDH (93.1) values were shared between ZH07 and K. variicola subsp. variicola DSM-15968T (Fig. 2C). Thus, our results indicated that ZH07 is a strain of K. variicola subsp. variicola.
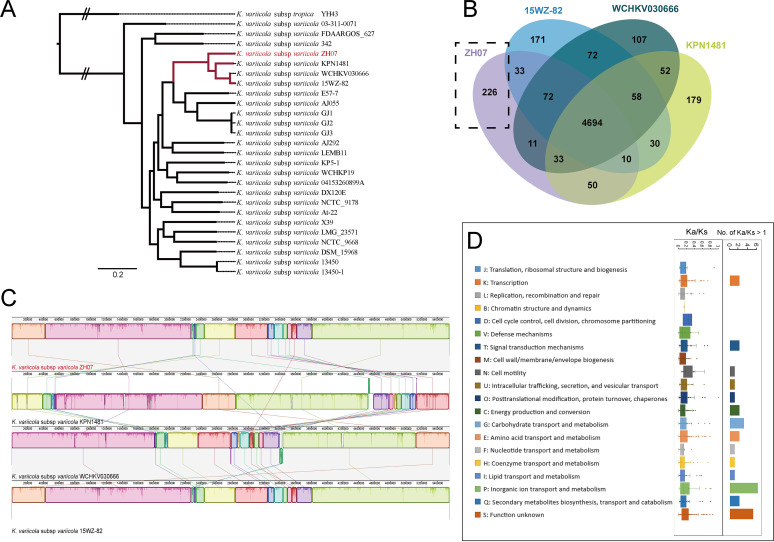
To further evaluate the evolutionary dynamics of ZH07, 25 publicly complete genomes of K. variicola subsp. variicola and one complete genome of K. variicola subsp. tropica as an outgroup were collected for subsequent analyses. A high-resolution phylogeny, based on 4,114 single-copy core gene families that shared all 27 genomes (Table S3), was generated. As shown in Fig. 3A, ZH07, together with KPN1481, WCHKV030666, and 15WZ-82, formed a monophyletic clade and were deeply nested within the tree, indicating their close evolutionary relationship. Unlike ZH07, which was isolated from the rhizosphere soil, KPN1481, WCHKV030666, and 15WZ-82 were isolated from Homo sapiens. Considering the difference in niches, a comparative genome analysis of these strains might provide insights into the specific adaptation and genomic evolution of ZH07.
FIG 3.
Comparative genomic analysis of ZH07 and closely related strains. (A) Phylogenetic tree based on SNPs across 4,114 single-copy core gene families shared by 27 genomes of K. variicola that was constructed via the maximum likelihood method with 100 replicates. (B) Venn diagram displaying overlaps and differences in orthologous gene families in ZH07 and closely related strains (KPN1481, WCHKV030666, and 15WZ-82). (C) Chromosome alignment of ZH07 and closely related strains. Synteny blocks are shown as identically colored regions and are linked across the sequences. (D) Distribution of the Ka/Ks rates of the orthologous mutational gene pairs from ZH07 and KPN1481 in COG functional categories.
Comparative genomic analysis of ZH07 with closely related species revealed its evolutionary dynamics.
We performed a comparative genome analysis of ZH07 with these closely related strains. A total of 226 ZH07-specific gene families (226 genes) were identified (Fig. 3B). As shown in Fig. 2A, additional features in these ZH07-specific genes, such as adjacent tRNAs and insertion sequences, as well as remnants of genomic islands and prophages, indicated the occurrence of horizontal gene transfers (HGTs). It can be assumed that HGTs were evolutionary forces that contributed to the genome-specific evolution of ZH07. The COG classification showed that the genes unique to ZH07 were significantly enriched in “S: Function unknown” (Fisher’s exact test P value < 0.01) (Fig. 2B). These unknown functional genes in the ZH07-specific genetic repertoires might play a role in the niche adaptation of ZH07 and in their biological functions, but this requires further research. To further elucidate the evolutionary dynamics of ZH07 with these closely related strains, chromosome rearrangement and synteny analysis were performed. Most regions of the ZH07 chromosome appeared as synteny blocks (n = 16), spanning 5,549,018 bp (99.5%) (Fig. 3C). We did not observe significant rearrangements and inversions occurring in the genomic evolution of these strains.
To explore the selective pressure of genetic function, the evolutionary signatures of 892 orthologous mutational gene pairs between ZH07 and KPN1481 were characterized by their nonsynonymous (Ka) and synonymous (Ks) substitution rates (Ka/Ks). Overall, the average Ka/Ks rate of these gene pairs was 0.213 ± 0.374. The Ka/Ks rates of most of the gene pairs (n = 866; 97.1%) were less than 1. Our analysis exhibited a predominant action of purifying selection. We further investigated the degree of purifying selection of each functional category. The genes related to “M: Cell wall/membrane/envelope biogenesis” (average Ka/Ks = 0.117 ± 0.106), “L: Replication, recombination and repair” (average Ka/Ks = 0.142 ± 0.149), and “F: Nucleotide transport and metabolism” (average Ka/Ks = 0.148 ± 0.232) exhibited stronger evolutionary constraints of purifying selection (Fig. 3D). In contrast, the genes involved in “N: Cell motility” (average Ka/Ks = 0.406 ± 0.709), “T: Signal transduction mechanisms” (average Ka/Ks = 0.315 ± 0.698), and “P: Inorganic ion transport and metabolism” (average Ka/Ks = 0.259 ± 0.441) underwent weaker evolutionary constraints of purifying selection (Fig. 3D). A total of 26 gene pairs were identified under positive selection (Ka/Ks > 1) (Table S4). Most of the positively selected genes were distributed in functional categories that were undergoing weaker purifying selection, such as “P: Inorganic ion transport and metabolism” (n = 6), “S: Function unknown” (n = 5), “T: Signal transduction mechanisms” (n = 2), and “N: Cell motility” (n = 1) (Fig. 3D). Considering the differences in the niches of the two strains (ZH07: rhizosphere soil; KPN1481: Homo sapiens), it can be inferred that the weaker functional constraints of “N: Cell motility” and “T: Signal transduction mechanisms” might promote adaptation during habitat conversion.
Genomic properties associated with plant growth-promotion.
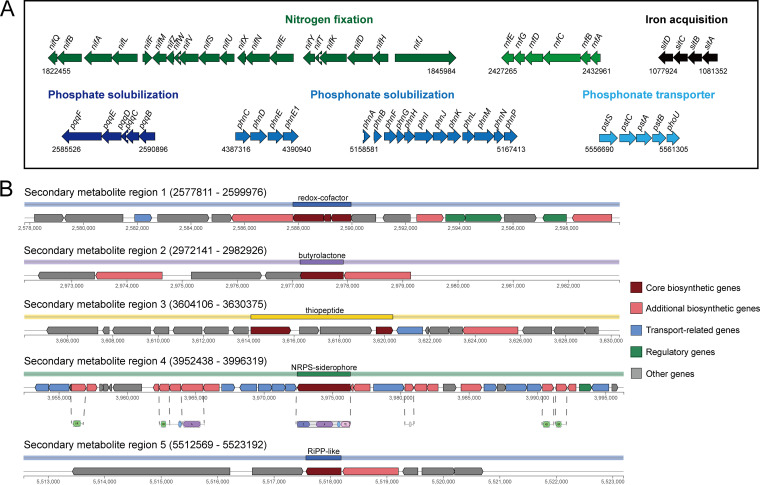
Regarding nitrogen fixation, the ZH07 genome harbored a nif operon, consisting of 20 genes (nifQBALFMZWVSUXNEYTKDHJ), arranged within a 23.5 kb region in the chromosome (Table S5; Fig. 4A). Most of them were shown to be required for the synthesis and activity of nitrogenase, the key enzyme in nitrogen fixation (34). The rnf operon (rnfABCDGE), encoding a membrane-bound protein complex related to the transport of electrons to nitrogenase, was also found in the ZH07 chromosome (Table S5; Fig. 4A) (35). Strain ZH07 has the ability to produce IAA (Table 1). Bacterial IAA synthesis is mainly from tryptophan via four main alternative pathways, including the indole-3-pyruvic acid (IPyA), indole-3-acetamide (IAM), indole-3-acetonitrile (IAN), and tryptamine (TAM) pathways (36). In ZH07, we identified ipdC (encoding indolepyruvate decarboxylase, a key enzyme in the IPyA pathway), aldh (encoding aldehyde dehydrogenase, converting Indole-3-acetaldehyd to IAA), nthAB (encoding nitrile hydratase, converting IAN to IAM), and amiE (encoding amidase, converting IAM to IAA) (Table S5).
FIG 4.
Genomic characteristics of ZH07 associated with plant growth promotion. (A) Genetic organization of the related gene clusters in the ZH07 chromosome. (B) Genetic organization of the gene clusters for secondary metabolism in the ZH07 chromosome.
Relating to phosphate solubilization and assimilation, rhizobacteria could produce organic acids (mainly gluconic acid) to solubilize insoluble or poorly soluble mineral phosphates (37). Gluconic acid dehydrogenase encoded by gad and cofactor pyrrolo-quinolone quinine (PQQ) encoded by the pqq operon play a key role in the synthesis of gluconic acid (38, 39). In ZH07, the pqq operon (pqqBCDEF) and the gcd gene were identified. We also found two phn-related operons (phnCDEE and phnABFGHIJKLMNP) in the chromosome, which were reported to be responsible for the uptake and degradation of phosphonate (organophosphorus molecules) (40). Furthermore, the pst operon (pstSCAB), encoding a high-affinity free-phosphate transport system, was present in ZH07 (41).
Our genomic analysis also identified five putative biosynthesis gene clusters (BGC 1 to 5) associated with the synthesis of secondary metabolites (Fig. 4B; Fig. S5; Table S6). BGC-1 was predicted to synthesize the redox-cofactor and exhibited similarity to the biosynthesis regions of lankacidin C, which exhibit antimicrobial activities (42). BGC-2 might be responsible for butyrolactone biosynthesis, as butyrolactone derivatives were reported to show pronounced antimicrobial activities (43). BGC-3 might be associated with the synthesis of thiopeptides (a class of natural product antibiotics) (44). BGC-5 was predicted to synthesize RiPP-like peptides (BGC-5). RiPPs had various biological functions, such as antibacterial, antifungal, and cytotoxic activities (45). Thus, it can be inferred that these BGCs might play a possible role in ZH07 in plant disease biocontrol. BGC-4 harbored several genes encoding the synthesis and transport of siderophores, such as entABCDEHFS for the synthesis and transport of enterobactin and fepABCDG encoding siderophores receptors (46, 47). Indeed, our results indicated that strain ZH07 could synthesize siderophores. Furthermore, ZH07 also carried iron acquisition genes, such as sitABCD (Fig. 4A) (48).
Genomic insight into the biodegradation of aromatic xenobiotics in ZH07.
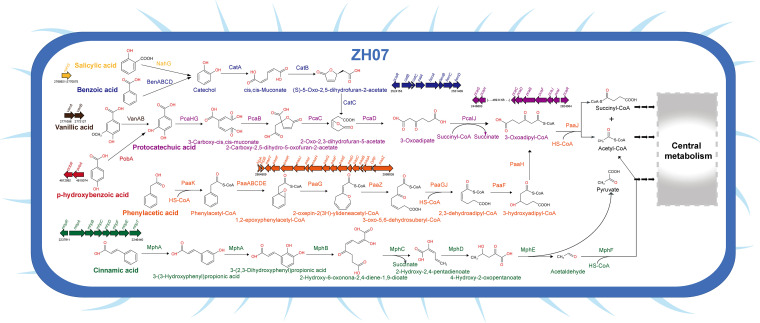
The ZH07 genome was further analyzed with respect to the genetic elements potentially involved in the aromatic xenobiotic biodegradation. In total, several genetic loci determined in the chromosome played a putative role in the biodegradation of several aromatic compounds, including protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, benzoic acid, salicylic acid, phenylacetic acid, and cinnamic acid. A variety of aromatic compounds can be converted to central intermediates, including protocatechuate or catechol. Herein, the ZH07 genome possessed the complete protocatechuate branch of the β-ketoadipate pathway, which consists of the pcaI (pcaHG) and pcaII (pcaCDBFJIR) operons, which encode enzymes for the catabolism of protocatechuic acid to succinate and 3-oxoadipyl-CoA (Fig. 5) (49). Environmental bacteria could use the protocatechuate branch of the β-ketoadipate pathway for the degradation of various aromatic pollutants. In ZH07, we identified the van (vanAB) and pob (pobRA) operons, which mediated the degradation of vanillic acid and p-hydroxybenzoic acid, respectively (Table S7). The two-component vanillate O-demethylase encoded by vanAB transforms vanillic acid to protocatechuic acid (50). The pobA gene encoded a hydroxylase that converts p-hydroxybenzoic acid to protocatechuic acid (51). The adjacent pobR gene encoded an AraC family transcriptional regulator PobR as a transcriptional activator of pobA (52). Furthermore, three open reading frames (ORFs) (pcaK) encoding a major facilitator superfamily (MFS) transporter (AAHS family) were found in the ZH07 chromosome, and these could play a potential role in the transport of p-hydroxybenzoic acid (53).
FIG 5.
Genomic characteristics of ZH07 related to aromatic xenobiotic biodegradation. The cell cartoon was constructed from the genome annotation of ZH07. The features putatively involved in the aromatic xenobiotic biodegradation are shown. The genetic organization of the related gene clusters in the ZH07 chromosome is shown.
The catechol branch of the β-ketoadipate pathway was also identified in ZH07, including the benABCD and catBCA operons. In the catechol branch, benzoic acid is converted into 1,2-dihydro-1,2-dihydroxybenzoic acid by benzoate-1,2-dioxygenase (benABC) and is then transformed into catechol by cis-diol dehydrogenase (benD) (Fig. 5; Table S7) (54). Subsequently, the metabolites of each process in the catechol branch undergo a series of transformations by functional enzymes encoded by catA, catB, and catC and are finally converted into 2-oxo-2,3-dihydrofuran-5-acetate that can be further catabolized by enzymes from the pca operon (Fig. 5) (55). Furthermore, the predicted protein encoded by the nahG gene (ORF2729) exhibited high identity (98.7%) with the annotated salicylate hydroxylase (AHM85232) from K. pneumoniae 30660. You et al. have reported that salicylate hydroxylase catalyzed the catabolism of salicylic acid through its conversion to catechol (56).
In addition to the β-ketoadipate pathway, other aromatic compound degradation-related elements were also identified in ZH07. Phenylacetate is a major intermediate in the degradation of the diverse aromatic compounds of environmental bacteria. Herein, the paa operon (paaYXKJIHGFEDCBAZ), comprising the phenylacetic acid catabolic pathway, was arranged within a 13.5 kb region in the ZH07 chromosome (Table S7). These genes encode functional enzymes for the transformation of phenylacetic acid to succinyl-CoA and acetyl-CoA, which are subsequently fed to the central metabolism (Fig. 5) (57). Regarding cinnamic acid degradation, we also identified the mhp operon (mhpRABCDEFET) encoding functional enzymes that mediate the degradation of 3-(3-hydroxyphenyl)propionic acid to pyruvate and acetyl-CoA (Fig. 5) (58). Based on the KEGG annotation (M00545 trans-cinnamate degradation), trans-cinnamic acid could be converted to 3-(3-hydroxyphenyl)propionic acid (Reaction: R06781) by hydroxylase MphA (K05712). However, recent research indicated that MphA in Escherichia coli K-12 showed no activity in response to trans-cinnamic acid and cis-cinnamic acid (59). Thus, the cinnamic acid catabolic pathway in ZH07 deserves further study in future work. Overall, the existence of these genes, carried by the ZH07 genome, provides a potential genomic basis for xenobiotic degradation, whereas the detailed elucidation of how they function requires further transcription and mutation studies.
Effects of ZH07 application on peanut seed germination and seedling growth.
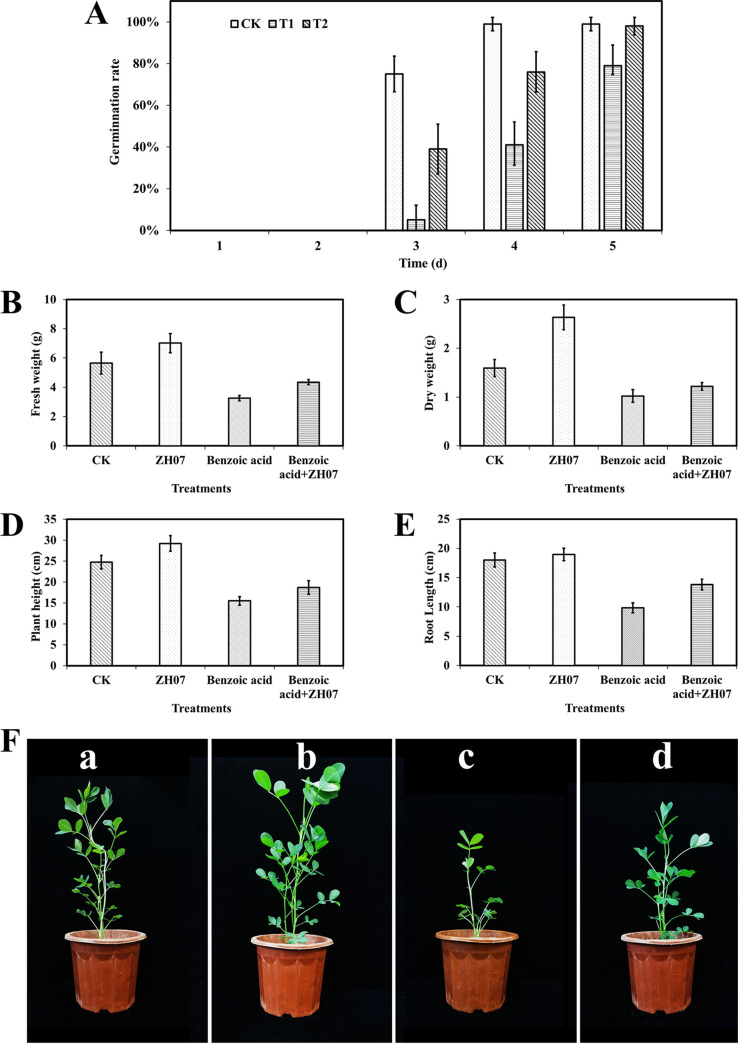
The toxicity of benzoic acid and the detoxification effect of ZH07 were evaluated using peanut seeds (Fig. 6A). On the third day, when peanut seeds were treated with distilled water (CK), the germination rate was 75% at 3 d, but the germination rate of seeds exposed to benzoic acid was reduced by 72% (T1) (t test, P < 0.01); however, when the peanut seeds were exposed to benzoic acid inoculated with strain ZH07 (T2), the germination rate increased by 36% compared with T1 (t test, P < 0.01).
FIG 6.
Bioassay on peanut of benzoic acid and the detoxification effect by strain ZH07. (A) Effects of ZH07 and benzoic acid application on peanut seed germination. CK, the surface-sterilized seeds were treated with distilled water; T1, the surface-sterilized seeds were treated with 200 mg·L−1 of benzoic acid; T2, the surface-sterilized seeds were treated with 200 mg·L−1 of benzoic acid and ZH07 suspensions (108 cells·mL−1). Effects of ZH07 and benzoic acid on peanut seeding on fresh weight (B), dry weight (C), plant height (D), and root length (E). (F) Pot experiment. (F, panel a) CK, plants were just watered with Murashige Skoog (MS) medium. (F, panel b) ZH07, watered with 20 mL of ZH07 suspensions (108 cells·mL−1). (F, panel c) Benzoic acid, watered with 20 mL of benzoic acid solution (4 g·L−1) to make the benzoic acid concentration reach 200 μg·g−1 soil. (F, panel d) Benzoic acid+ZH07 treatment group, the seedlings were watered with autoclaved MS liquid medium.
The growth-promoting capacity of strain ZH07 was further studied in a pot experiment. Compared to a control, the fresh weight (Fig. 6B), dry weight (Fig. 6C), plant height (Fig. 6C), and root length (Fig. 6D) of peanut seedilngs were increased significantly in the ZH07 treatment by 24.3% (t test, P value < 0.01), 65.4% (t test, P value < 0.01), 18% (t test, P value < 0.01), and 5.2%, respectively, but they decreased when exposed to benzoic acid by 42.4% (t test, P value < 0.01), 35.8% (t test, P value < 0.01), 37.4% (t test, P value < 0.01), and 45.4% (t test, P value < 0.01), respectively. Meanwhile, under benzoic acid conditions, strain ZH07 could also increase the fresh weight, dry weight, plant height, and root length by 33.8% (t test, P value < 0.01), 19.4%, 20.8% (t test, P value < 0.01), and 40.7% (t test, P value < 0.01), respectively. Pot experiments further confirmed that strain ZH07 could promote the growth of peanuts (Fig. 6F) and exhibited a significant detoxification effect.
DISCUSSION
Conclusions.
This study provided a comprehensive understanding of a novel PGPR strain, ZH07, that was isolated from peanut rhizosphere soil. This strain could not only degrade benzoic acid but also exhibit efficient p-hydroxybenzoic acid, salicylic acid, cinnamic acid, vanillic acid, and cumaric acid degradation. Moreover, in a plant growth-promoting attributes assay, ZH07 showed the capacities of nitrogen fixation, phosphorus solubilization, potassium dissolution, siderophore production, and IAA synthesis. We report the complete genome and comprehensive comparative analysis of this strain. The phylogenetic analysis of the 16S rRNA and the core genome in combination with the ANI, AAI, and in silico DDH values determined the accurate evolutionary position of ZH07, which represents a member of K. variicola subsp. variicola. A comparative genomic analysis of ZH07 with closely related strains showed the evolutionary dynamics exhibited by the existence of strain-specific genes related to diverse MGEs (e.g., ISs, plasmids, prophages, and GIs) as well as a predominant action of purifying selection. Different degrees of purifying selection occurred in the gene families with different functional categories. Furthermore, abundant genes putatively responsible for plant growth promotion and aromatic xenobiotic biodegradation were determined. Meanwhile, we also demonstrated that the inoculation of ZH07 could promote peanut germination and seedling growth against the toxic effects of benzoic acid. These results provide a comprehensive understanding of the genomic evolution of ZH07 and provide insights into the adaptive evolution of the PGPR strain in rhizosphere habitats. This study provided a theoretical reference for PGPR commercialization as a potential candidate for various bioremediation applications.
MATERIALS AND METHODS
Isolation of the strain ZH07.
Rhizosphere soil was collected from an agricultural field at Qingdao County, Shandong Province, China (36°34' and 120°12'E), where peanuts have been continuously planted for 12 years. Mineral salt medium (MSM) (1L: K2HPO4, 58 g; (NH4)2SO4, 20 g; KH2PO4, 45 g; CaCl2, 0.002 g; MgCl2, 0.16 g; FeCl3, .00018 g; Na2MoO4·2H2O, .00024 g; MnCl2,·2H2O, 0.00015 g; pH 7.0) containing benzoic acid as the sole carbon source was used to isolate degrading bacteria. One gram of a rhizosphere soil sample was added to 100 mL MSM medium supplemented with 1 g·L−1 benzoic acid and then incubated at 30°C and 180 rpm for 7 days for enrichment. Then, the strain ZH07 was isolated from the enriching medium and stored in the China General Microbiology Culture Collection Center (CGMCC accession number: 21261).
Autotoxin degradation properties of ZH07.
ZH07 was grown at 30°C in Luria-Bertani (LB) medium (1L: peptone, 10 g; yeast extract, 5 g; NaCl, 5 g; pH 7.0) solidified with 2.5% agar. A single colony from the LB plate was inoculated into liquid MSM and incubated at 30°C and 180 rpm to the logarithmic phase, and the bacterial cells were collected, washed with liquid MSM, and resuspended in liquid MSM to an OD600 of 1.0. Then, 1 mL of this culture was inoculated into 100 mL MSM containing 1 g·L−1 of benzoic acid and incubated for 72 h at 30°C and 180 rpm. The control group was uninoculated, and then the benzoic acid residue was quickly analyzed using a microplate reader (SpectraMax 190, Molecular Devices Corporation, American) at 227 nm. The degradation rate was calculated by using the light absorptance value compared to the control, and the tests of the other autotoxin degradation abilities of ZH07 (p-hydroxybenzoic acid, salicylic acid, cinnamic acid, vanillic acid, cumaric acid) were tested using previously described methods (60).
Plant growth-promoting attributes.
Nitrogen fixation activity was tested on N-free Ashby medium (1L: mannitol, 10.0 g; KH2PO4, 0.2 g; MgSO4, 0.2 g; NaCl, 0.2 g; CaSO4, 0.1 g; CaCO3, 5 g; pH 7.0, solidified with 2.5% agar). ZH07 was grown at 30°C on Ashby medium for 7 days, and then the growth of the colony was observed.
The mineral phosphate solubilization activity was assayed on inorganic phosphorus medium (1L: glucose, 10 g; (NH4)2SO4, 0.5 g; NaCl, 0.3 g; KCl, 0.3 g; FeSO4,·7H2O 0.003 g; MnSO4·4H2O, 0.003 g; MgSO4·7H2O, 0.3 g; Ca(PO4)2, 5 g; pH 7.0; solidified with 2.5% agar). The organic phosphate solubilization potential was assayed on egg yolk medium (1L: glucose, 10 g; (NH4)2SO4, 0.5 g; NaCl, 0.3 g; KCl, 0.3 g; FeSO4·7H2O, 0.003 g; MnSO4·4H2O, 0.003 g; egg yolk lecithin, 0.2 g; CaCO3, 5 g; yeast extract, 5 g; pH 7.0; solidified with 2.5% agar). ZH07 was grown at 30°C on inorganic phosphorus medium or egg yolk medium for 7 days. The phosphate solubilization capacity was characterized by a clear halo around the bacterial colonies.
The potassium dissolution activity was assayed on Alexandrov medium (61) (1L: glucose, 5 g; Na2HPO4, 2 g; MgSO4·7H2O, 0.5 g; FeCl3, 0.005 g; CaCO3, 0.1 g; potassium feldspar powder, 1 g; pH 7.0; solidified with 2.5% agar). ZH07 was grown at 30°C on Alexandrov medium for 7 days. The potassium dissolution capacity was characterized by a clear halo around the bacterial colonies.
The siderophore production activity was tested on chrome azurol S assay medium (1L: CAS, 60.5 mg; HDTMA, 72.9 mg; FeCl·6H2O 2.645 mg; NaH2PO4·2H2O, 295.25 mg; Na2HPO4·12H2O, 1213.5 mg; NH4Cl, 125 mg; KH2PO4, 37.5 mg; NaCl, 62.5 mg; pH 7.0; solidified with 2.5% agar) (62). ZH07 was grown at 30°C on chrome azurol S assay medium for 7 days. The siderophore production capacity was characterized by orange color-change reactions on the blue agar around the bacterial colonies.
Indole acetic acid (IAA) production was studied according to Salkowski’s method (63). ZH07 were incubated in LB medium containing 200 μg·L−1 l-tryptophan at 30°C and 180 rpm for 4 days. The supernatant was collected by centrifugation at 4°C and 8,000 rpm for 10 min. Then, 2 mL of the supernatant was mixed with 2 mL of Salkowski reagent (50 mL, 35% of perchloric acid, 1 mL 0.5 mol·L−1 FeCl3 solution) and then incubated in darkness for 30 min and quickly analyzed using the spectrophotometer at OD530. The levels of IAA were calculated via comparison with the standard curve.
Seed germination.
Seed germination tests were carried out using peanut seeds cultivar Huayu-20. Peanut seeds were surface sterilized using 5% sodium hypochlorite for 10 min and then washed with sterilized distilled water three times. ZH07 was grown in LB liquid medium at 30°C for an OD600 value of 1.0 and then centrifuged at 4°C and 8,000 rpm to obtain cell pellets. The pellets were washed with sterilized distilled water three times and resuspended in the same volume of sterile distilled water. The experiment includes three treatments: CK, the surface-sterilized seeds were treated with distilled water; T1, the surface-sterilized seeds were treated with 200 mg·L−1 of benzoic acid; T2, the surface-sterilized seeds were treated with 200 mg·L−1 of benzoic acid and ZH07 suspensions (108 cells·mL−1). Peanut seeds were placed in a 9 cm diameter petri dish that contained two layers of sterile filter paper. 10 seeds were placed in each dish, and each treatment was replicated five times.
Pot experiment.
Peanut (Huayu-20) seeds were surface-sterilized and grown in nursery cups and then transplanted to pots filled with 400 g of soil-less growth media (Klasmann-Deilmann Base Substrate, Recipe-No. 422, blended with sterile vermiculite, 1:1) at four true leaves of age. The experiment includes four treatments: CK, plants were watered with Murashige Skoog (MS) medium; ZH07, ZH07 treatment group, watered with 20 mL of ZH07 suspensions (108 cells·mL−1); Benzoic acid, benzoic acid treatment group, watered with 20 mL of benzoic acid solution (4 g·L−1) so as to make the benzoic acid concentration reach 200 μg·g−1 soil; Benzoic acid + ZH07 treatment group, the seedlings were watered with autoclaved MS liquid medium and grown under conditions of 70% humidity with 25 ± 2°C during the day (12 h, 600 μmol·m−2·s−1) and 22 ± 2°C during the night (12 h) for 15 days.
Genome sequencing and bioinformatics analysis.
The ZH07 genome was sequenced using a PacBio RS II platform and an Illumina HiSeq 4000 platform at the Beijing Genomics Institute (BGI, Shenzhen, China). Draft genomic contigs were assembled using a Celera Assembler against a high-quality corrected circular consensus sequence subreads set. The estimates for genome completeness and contamination were performed using CheckM (64). The genome sequence was annotated using the Rapid Annotations using Subsystems Technology (RAST) (65). The genome data were deposited in the NCBI GenBank (accession number: GCA_020995405.1). The features of chromosomes and plasmids, as well as the comparisons thereof, were performed using the BLAST Ring Image Generator (BRIG) (66). The IslandViewer 4 was used for the genomic islands analysis (67). The prophage regions were predicted using the PHAge Search Tool - Enhanced Release (PHASTER) (68). The annotations of the insertion sequences were obtained using the ISFinder (69). The Clusters of Orthologous Groups (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases were used for the general functional annotations (70). The ZH07 chromosome was analyzed for the presence of horizontal genes using HGTector (71). Klebsiella (rank, genus; taxon ID: 570) and Enterobacteriaceae (rank, family; taxon ID: 543) were set as the self-group and close-group, respectively. A syntenic analysis was conducted via ProgressiveMauve (72), which enabled the identification of locally colinear blocks (LCBs) using default parameters. For the selection pressure analysis, ParaAT was used to codon-based align the orthologous genes between ZH07 and K. variicola subsp. variicola KPN1481 (73). The KaKs_Calculator 2.0 was used to compute the nonsynonymous (Ka) and synonymous (Ks) substitution rates (Ka/Ks) (74). The gene cluster that was related to secondary metabolism was identified and analyzed using antiSMASH (75).
Phylogenetic analysis.
The 16S rRNA sequence of ZH07 was compared with the taxonomically united 16S rRNA database in EzBioCloud (33) to identify similar species, and the 16S rRNA and genome sequences of these species were collected for subsequent analyses. The collected genomes were reannotated using the RAST (65). The average nucleotide identity, amino acid identity, and in silico DNA-DNA hybridization were calculated using JSpecies 1.2.1 (76), CompareM, and the genome-to genome distance calculator 3.0 (GGDC) (77), respectively. For the core genome phylogeny, orthologous groups of protein families were delimited using the OrthoFinder software package (78). The single-copy core gene families were extracted from the OrthoFinder output files. The nucleotide sequences of the single-copy core gene families were extracted according to the protein accession numbers and were then aligned using the MAFFT software package (79). The core genome tree was constructed using the set of single-nucleotide polymorphisms (SNPs) that were present in the single-copy core gene families. The set of SNPs was integrated according to the arrangement of the genes on the ZH07 chromosome. The putative recombinational regions were identified and removed from the set of SNPs using the ClonalFrameML software package (80). The maximum likelihood (ML) tree was constructed using the MEGA X software package (81) with the general time-reversible (GTR) model with 100 bootstrap replicates.
Statistical analysis.
Data were expressed as means ± standard errors. All analyses were performed using the SPSS v22.0 software package, and the differences were analyzed by using a one-way analysis of variance (ANOVA) and the least significant difference (LSD) test. The statistical significance of differences (P < 0.05) was assessed.
Data availability.
The genome data of ZH07 were deposited in NCBI GenBank (accession number GCA_020995405.1).
ACKNOWLEDGMENTS
This work was funded by the NSFC-Shandong Joint Fund Key Projects (U1806206), the Shandong Provincial Natural Science Foundation (ZR2021QC175, ZR2021QC208), the National Natural Science Foundation of China (31870020), the Research and Development Plan in Shandong Province (2021CXGC010804) and the Scientific Research Foundation of Shandong Agricultural University (010/72091, 010/72094, 010/72100).
Footnotes
Supplemental material is available online only.
Contributor Information
Zhiqiu Yin, Email: yzq7873728@126.com.
Zhihong Xie, Email: zhihongxie211@163.com.
Jannell V. Bazurto, University of Minnesota
REFERENCES
- 1.Yin C, Casa Vargas JM, Schlatter DC, Hagerty CH, Hulbert SH, Paulitz TC. 2021. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 9:86. doi: 10.1186/s40168-020-00997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XL, Pan ZG, Zhou XF, Ni WZ. 2007. Autotoxicity and continuous cropping obstacles: a review. Chinese J Soil Sci 38:781–784. [Google Scholar]
- 3.Tan G, Liu Y, Peng S, Yin H, Meng D, Tao J, Gu Y, Li J, Yang S, Xiao N, Liu D, Xiang X, Zhou Z. 2021. Soil potentials to resist continuous cropping obstacle: three field cases. Environ Res 200:111319. doi: 10.1016/j.envres.2021.111319. [DOI] [PubMed] [Google Scholar]
- 4.Ranran Z, Wang Y, Tian M, Shah Jahan M, Shu S, Sun J, Li P, Ahammed GJ, Guo S. 2021. Mixing of biochar, vinegar and mushroom residues regulates soil microbial community and increases cucumber yield under continuous cropping regime. Appl Soil Ecol 161:103883. [Google Scholar]
- 5.Null I, Duke SO. 2003. Ecophysiological aspects of allelopathy. Planta 217:529–539. doi: 10.1007/s00425-003-1054-z. [DOI] [PubMed] [Google Scholar]
- 6.Li ZH, Wang Q, Ruan X, Pan CD, Jiang DA. 2010. Phenolics and plant allelopathy. Molecules 15:8933–8952. doi: 10.3390/molecules15128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Null I, Wardle DA, Karban R, Callaway RM. 2011. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26:655–662. doi: 10.1016/j.tree.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Kong CH, Xuan TD, Khanh TD, Tran HD, Trung NT. 2019. Allelochemicals and signaling chemicals in plants. Molecules 24:2737. doi: 10.3390/molecules24152737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Liu Y, Yuan L, Weber E, van Kleunen M. 2021. Effect of allelopathy on plant performance: a meta-analysis. Ecol Lett 24:348–362. doi: 10.1111/ele.13627. [DOI] [PubMed] [Google Scholar]
- 10.Baziramakenga R, Leroux GD, Simard RR. 1995. Effects of benzoic and cinnamic acids on membrane permeability of soybean roots. J Chem Ecol 21:1271–1285. doi: 10.1007/BF02027561. [DOI] [PubMed] [Google Scholar]
- 11.Nilsen ET, Walker JF, Miller OK, Semones SW, Lei TT, Clinton BD. 1999. Inhibition of seedling survival under Rhododendron maximum (Ericaceae): could allelopathy be a cause? Am J Bot 86:1597–1605. doi: 10.2307/2656796. [DOI] [PubMed] [Google Scholar]
- 12.Prati D, Bossdorf O. 2004. Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91:285–288. doi: 10.3732/ajb.91.2.285. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Gu M, Shi K, Zhou YH, Yu JQ. 2010. Effects of aqueous root extracts and hydrophobic root exudates of cucumber (Cucumis sativus L.) on nuclei DNA content and expression of cell cycle-related genes in cucumber radicles. Plant Soil 327:455–463. doi: 10.1007/s11104-009-0075-1. [DOI] [Google Scholar]
- 14.Mohammadkhani N, Servati M. 2018. Nutrient concentration in wheat and soil under allelopathy treatments. J Plant Res 131:143–155. doi: 10.1007/s10265-017-0981-x. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Zhang T, Wang X, Hua K, Zhao L, Han Z. 2013. The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int J Biol Sci 9:164–173. doi: 10.7150/ijbs.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu F, An YQ, An Y, Wang XJ, Cheng ZY, Zhang Y, Hou X, Chen CX, Wang L, Bai JG. 2018. Acinetobacter calcoaceticus CSY-P13 mitigates stress of ferulic and p-hydroxybenzoic acids in cucumber by affecting antioxidant enzyme activity and soil bacterial community. Front Microbiol 9:1262. doi: 10.3389/fmicb.2018.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao XL, Zhang XS, Lu XH, Qin R, Bi YM, Gao WW. 2019. Effects of maize rotation on the physicochemical properties and microbial communities of American ginseng cultivated soil. Sci Rep 9:8615. doi: 10.1038/s41598-019-44530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asao T, Kitazawa H, Motoki S, Hashimoto Y, Ban T. 2007. Amelioration of autotoxic soil constraints to lettuce and mat-rush growths by activated charcoals. Environ Control Biol 45:33–38. doi: 10.2525/ecb.45.33. [DOI] [Google Scholar]
- 19.Mullins E. 2015. Engineering for disease resistance: persistent obstacles clouding tangible opportunities. Pest Manag Sci 71:645–651. doi: 10.1002/ps.3930. [DOI] [PubMed] [Google Scholar]
- 20.Lv H, Cao H, Nawaz MA, Sohail H, Huang Y, Cheng F, Kong Q, Bie Z. 2018. Wheat intercropping enhances the resistance of watermelon to fusarium wilt. Front Plant Sci 9:696. doi: 10.3389/fpls.2018.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhang W, Zhang Z, Wang W, Xu S, He X. 2021. Isolation, identification and characterization of phenolic acid-degrading bacteria from soil. J Appl Microbiol 131:208–220. doi: 10.1111/jam.14956. [DOI] [PubMed] [Google Scholar]
- 22.Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL. 2018. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9:1473. doi: 10.3389/fpls.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao C, Li R, Xiong W, Shen Z, Liu S, Wang B, Ruan Y, Geisen S, Shen Q, Kowalchuk GA. 2020. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 8:137. doi: 10.1186/s40168-020-00892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu B, Wang W, Yuan Z, Sederoff RR, Sederoff H, Chiang VL, Borriss R. 2020. Microbial interactions within multiple-strain biological control agents impact soil-borne plant disease. Front Microbiol 11:585404. doi: 10.3389/fmicb.2020.585404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J. 2020. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ 743:140682. doi: 10.1016/j.scitotenv.2020.140682. [DOI] [PubMed] [Google Scholar]
- 26.Yin Z, Wang X, Hu Y, Zhang J, Li H, Cui Y, Zhao D, Dong X, Zhang X, Liu K, Du B, Ding Y, Wang C. 2022. Metabacillus dongyingensis sp. nov. is represented by the plant growth-promoting bacterium BY2G20 isolated from saline-alkaline soil and enhances the growth of Zea mays L. under salt stress. mSystems 7:e0142621. doi: 10.1128/msystems.01426-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalid M, Hassani D, Bilal M, Asad F, Huang D. 2017. Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot Stud 58:35. doi: 10.1186/s40529-017-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F, Shi Q, Wang XJ, Sun ZT, Wang W, Li X, Guo LY, Bai JG. 2019. Streptomyces canus GLY-P2 degrades ferulic and p-hydroxybenzoic acids in soil and affects cucumber antioxidant enzyme activity and rhizosphere bacterial community. Plant Soil 436:71–89. doi: 10.1007/s11104-018-03911-z. [DOI] [Google Scholar]
- 29.Liu J, Wang X, Zhang T, Li X. 2017. Assessment of active bacteria metabolizing phenolic acids in the peanut (Arachis hypogaea L.) rhizosphere. Microbiol Res 205:118–124. doi: 10.1016/j.micres.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Du Y, Ma J, Yin Z, Liu K, Yao G, Xu W, Fan L, Du B, Ding Y, Wang C. 2019. Comparative genomic analysis of Bacillus paralicheniformis MDJK30 with its closely related species reveals an evolutionary relationship between B. paralicheniformis and B. licheniformis. BMC Genomics 20:283. doi: 10.1186/s12864-019-5646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin Z, Zhang S, Wei Y, Wang M, Ma S, Yang S, Wang J, Yuan C, Jiang L, Du Y. 2020. Horizontal gene transfer clarifies taxonomic confusion and promotes the genetic diversity and pathogenicity of Plesiomonas shigelloides. mSystems 5. doi: 10.1128/mSystems.00448-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin Z, Liu X, Qian C, Sun L, Pang S, Liu J, Li W, Huang W, Cui S, Zhang C, Song W, Wang D, Xie Z. 2022. Pan-Genome analysis of Delftia tsuruhatensis reveals important traits concerning the genetic diversity, pathogenicity, and biotechnological properties of the species. Microbiol Spectr 10:e0207221. doi: 10.1128/spectrum.02072-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. 2017. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold W, Rump A, Klipp W, Priefer UB, Pühler A. 1988. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol 203:715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- 35.Biegel E, Schmidt S, Müller V. 2009. Genetic, immunological and biochemical evidence for a Rnf complex in the acetogen Acetobacterium woodii. Environ Microbiol 11:1438–1443. doi: 10.1111/j.1462-2920.2009.01871.x. [DOI] [PubMed] [Google Scholar]
- 36.Shao J, Li S, Zhang N, Cui X, Zhou X, Zhang G, Shen Q, Zhang R. 2015. Analysis and cloning of the synthetic pathway of the phytohormone indole-3-acetic acid in the plant-beneficial Bacillus amyloliquefaciens SQR9. Microb Cell Fact 14:130. doi: 10.1186/s12934-015-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werra P, Péchy-Tarr M, Keel C, Maurhofer M. 2009. Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl Environ Microbiol 75:4162–4174. doi: 10.1128/AEM.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagh J, Shah S, Bhandari P, Archana G, Kumar GN. 2014. Heterologous expression of pyrroloquinoline quinone (pqq) gene cluster confers mineral phosphate solubilization ability to Herbaspirillum seropedicae Z67. Appl Microbiol Biotechnol 98:5117–5129. doi: 10.1007/s00253-014-5610-1. [DOI] [PubMed] [Google Scholar]
- 39.Xie J, Shi H, Du Z, Wang T, Liu X, Chen S. 2016. Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci Rep 6:21329. doi: 10.1038/srep21329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metcalf WW, Wanner BL. 1993. Evidence for a fourteen-gene, phnC to phnP locus for phosphonate metabolism in Escherichia coli. Gene 129:27–32. doi: 10.1016/0378-1119(93)90692-v. [DOI] [PubMed] [Google Scholar]
- 41.Nikata T, Sakai Y, Shibat K, Kato J, Kuroda A, Ohtake H. 1996. Molecular analysis of the phosphate-specific transport (pst) operon of Pseudomonas aeruginosa. Mol Gen Genet 250:692–698. doi: 10.1007/BF02172980. [DOI] [PubMed] [Google Scholar]
- 42.Arakawa K. 2014. Genetic and biochemical analysis of the antibiotic biosynthetic gene clusters on the Streptomyces linear plasmid. Biosci Biotechnol Biochem 78:183–189. doi: 10.1080/09168451.2014.882761. [DOI] [PubMed] [Google Scholar]
- 43.Chen M, Wang KL, Liu M, She ZG, Wang CY. 2015. Bioactive steroid derivatives and butyrolactone derivatives from a gorgonian-derived Aspergillus sp. fungus. Chem Biodivers 12:1398–1406. doi: 10.1002/cbdv.201400321. [DOI] [PubMed] [Google Scholar]
- 44.Chan DCK, Burrows LL. 2021. Thiopeptides: antibiotics with unique chemical structures and diverse biological activities. J Antibiot (Tokyo) 74:161–175. doi: 10.1038/s41429-020-00387-x. [DOI] [PubMed] [Google Scholar]
- 45.Harwood CR, Mouillon J-M, Pohl S, Arnau J. 2018. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol Rev 42:721–738. doi: 10.1093/femsre/fuy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rusnak F, Sakaitani M, Drueckhammer D, Reichert J, Walsh CT. 1991. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entF gene, expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry 30:2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- 47.Shea CM, McIntosh MA. 1991. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol 5:1415–1428. doi: 10.1111/j.1365-2958.1991.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 48.Sabri M, Léveillé S, Dozois CM. 2006. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology (Reading) 152:745–758. doi: 10.1099/mic.0.28682-0. [DOI] [PubMed] [Google Scholar]
- 49.Kowalchuk GA, Hartnett GB, Benson A, Houghton JE, Ngai KL, Ornston LN. 1994. Contrasting patterns of evolutionary divergence within the Acinetobacter calcoaceticus pca operon. Gene 146:23–30. doi: 10.1016/0378-1119(94)90829-x. [DOI] [PubMed] [Google Scholar]
- 50.Upadhyay P, Lali A. 2021. Protocatechuic acid production from lignin-associated phenolics. Prep Biochem Biotechnol 51:979–984. doi: 10.1080/10826068.2021.1881908. [DOI] [PubMed] [Google Scholar]
- 51.Quinn JA, McKay DB, Entsch B. 2001. Analysis of the pobA and pobR genes controlling expression of p-hydroxybenzoate hydroxylase in Azotobacter chroococcum. Gene 264:77–85. doi: 10.1016/s0378-1119(00)00599-0. [DOI] [PubMed] [Google Scholar]
- 52.Bertani I, Kojic M, Venturi V. 2001. Regulation of the p-hydroxybenzoic acid hydroxylase gene (pobA) in plant-growth-promoting Pseudomonas putida WCS358. Microbiology (Reading) 147:1611–1620. doi: 10.1099/00221287-147-6-1611. [DOI] [PubMed] [Google Scholar]
- 53.Pernstich C, Senior L, MacInnes KA, Forsaith M, Curnow P. 2014. Expression, purification and reconstitution of the 4-hydroxybenzoate transporter PcaK from Acinetobacter sp. ADP1. Protein Expr Purif 101:68–75. doi: 10.1016/j.pep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neidle E, Hartnett C, Ornston LN, Bairoch A, Rekik M, Harayama S. 1992. cis-diol dehydrogenases encoded by the TOL pWW0 plasmid xylL gene and the Acinetobacter calcoaceticus chromosomal benD gene are members of the short-chain alcohol dehydrogenase superfamily. Eur J Biochem 204:113–120. doi: 10.1111/j.1432-1033.1992.tb16612.x. [DOI] [PubMed] [Google Scholar]
- 55.Kukor JJ, Olsen RH, Ballou DP. 1988. Cloning and expression of the catA and catBC gene clusters from Pseudomonas aeruginosa PAO. J Bacteriol 170:4458–4465. doi: 10.1128/jb.170.10.4458-4465.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You IS, Ghosal D, Gunsalus IC. 1991. Nucleotide sequence analysis of the Pseudomonas putida PpG7 salicylate hydroxylase gene (nahG) and its 3’-flanking region. Biochemistry 30:1635–1641. doi: 10.1021/bi00220a028. [DOI] [PubMed] [Google Scholar]
- 57.Teufel R, Mascaraque V, Ismail W, Voss M, Perera J, Eisenreich W, Haehnel W, Fuchs G. 2010. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc Natl Acad Sci USA 107:14390–14395. doi: 10.1073/pnas.1005399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrández A, Garciá JL, Díaz E. 1997. Genetic characterization and expression in heterologous hosts of the 3–(3-hydroxyphenyl) propionate catabolic pathway of Escherichia coli K-12. J Bacteriol 179:2573–2581. doi: 10.1128/jb.179.8.2573-2581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Y, Zhou N-Y. 2020. MhpA is a hydroxylase catalyzing the initial reaction of 3–(3-hydroxyphenyl)propionate catabolism in Escherichia coli K-12. Appl Environ Microbiol 86. doi: 10.1128/AEM.02385-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahboubifar M, Sobhani Z, Dehghanzadeh G, Javidnia K. 2011. A comparison between UV spectrophotometer and high-performance liquid chromatography method for the analysis of sodium benzoate and potassium sorbate in food products. Food Anal Methods 4:150–154. doi: 10.1007/s12161-010-9158-0. [DOI] [Google Scholar]
- 61.Rajawat M, Singh S, Tyagi SP, Saxena AK. 2016. A modified plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere 26:768–773. doi: 10.1016/S1002-0160(15)60080-7. [DOI] [Google Scholar]
- 62.Milagres A, Machuca A, Napoleão D. 1999. Detection of siderophore production from several fungi and bacteria by a modification of chrome Azurol S (CAS) agar plate assay. J Microbiol Methods 37:1–6. doi: 10.1016/s0167-7012(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 63.Glickmann E, Dessaux Y. 1995. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Little RF, Hertweck C. 2022. Chain release mechanisms in polyketide and non-ribosomal peptide biosynthesis. Nat Prod Rep 39:163–205. doi: 10.1039/d1np00035g. [DOI] [PubMed] [Google Scholar]
- 65.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertelli C, Laird MR, Williams KP, Lau BY, Hoad G, Winsor GL, Brinkman FSL, Simon Fraser University Research Computing Group . 2017. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res 45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y, Wishart DS. 2016. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res 44:W16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanehisa M, Sato Y, Morishima K. 2016. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol 428:726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Q, Kosoy M, Dittmar K. 2014. HGTector: an automated method facilitating genome-wide discovery of putative horizontal gene transfers. BMC Genomics 15:717. doi: 10.1186/1471-2164-15-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Xiao J, Wu J, Zhang H, Liu G, Wang X, Dai L. 2012. ParaAT: a parallel tool for constructing multiple protein-coding DNA alignments. Biochem Biophys Res Commun 419:779–781. doi: 10.1016/j.bbrc.2012.02.101. [DOI] [PubMed] [Google Scholar]
- 74.Wang D, Zhang Y, Zhang Z, Zhu J, Yu J. 2010. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genomics Proteomics Bioinformatics 8:77–80. doi: 10.1016/S1672-0229(10)60008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, van Wezel GP, Medema MH, Weber T. 2021. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 49:W29–W35. doi: 10.1093/nar/gkab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol 20:238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Didelot X, Wilson DJ. 2015. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLoS Comput Biol 11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5. Download spectrum.00846-22-s0001.pdf, PDF file, 1.0 MB (1MB, pdf)
Tables S1 to S7. Download spectrum.00846-22-s0002.xlsx, XLSX file, 0.07 MB (73.5KB, xlsx)
Data Availability Statement
The genome data of ZH07 were deposited in NCBI GenBank (accession number GCA_020995405.1).