SUMMARY
Osteoarticular mycoses are chronic debilitating infections that require extended courses of antifungal therapy and may warrant expert surgical intervention. As there has been no comprehensive review of these diseases, the International Consortium for Osteoarticular Mycoses prepared a definitive treatise for this important class of infections. Among the etiologies of osteoarticular mycoses are Candida spp., Aspergillus spp., Mucorales, dematiaceous fungi, non-Aspergillus hyaline molds, and endemic mycoses, including those caused by Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides species. This review analyzes the history, epidemiology, pathogenesis, clinical manifestations, diagnostic approaches, inflammatory biomarkers, diagnostic imaging modalities, treatments, and outcomes of osteomyelitis and septic arthritis caused by these organisms. Candida osteomyelitis and Candida arthritis are associated with greater events of hematogenous dissemination than those of most other osteoarticular mycoses. Traumatic inoculation is more commonly associated with osteoarticular mycoses caused by Aspergillus and non-Aspergillus molds. Synovial fluid cultures are highly sensitive in the detection of Candida and Aspergillus arthritis. Relapsed infection, particularly in Candida arthritis, may develop in relation to an inadequate duration of therapy. Overall mortality reflects survival from disseminated infection and underlying host factors.
KEYWORDS: mycoses, antifungal therapy, aspergillosis, candidiasis, coccidioidomycosis, cryptococcosis, histoplasmosis, mucormycosis, osteomyelitis, phaeohyphomycosis
INTRODUCTION
Fungi are uncommon causes of osteomyelitis and septic arthritis. Fungal osteoarticular (OA) infections are chronic debilitating and challenging diseases that cause considerable morbidity worldwide. Osteoarticular mycoses, caused by most major medically important fungi, may affect both immunocompromised and immunocompetent patients. These infections are difficult to diagnose and require protracted courses of antifungal therapy and, often, expert surgical intervention.
DEFINITIONS
The definitions that are used in this review are delineated in Table 1 and have also been utilized extensively in previous studies of osteoarticular mycoses (1, 2). The definitions in this review are utilized for epidemiological purposes. For a case of radiologically suspected osteomyelitis for which the only organism recovered from a contiguous focus was a fungal pathogen, we would consider this a probable case for investigational purposes. One would need more detail for the clinical management of patients with suspected osteomyelitis. For example, we would qualify that in the case of a traumatic injury with an open fracture and polymicrobial cultures yielding one or more fungi adjacent to the area of radiologically suspected osteomyelitis, the diagnosis should be confirmed, if possible, by biopsy and culture of the infected bone. One should also perform a biopsy or aspiration of a radiologically suspected region if the organism from a contiguous focus is thought to be a fungal contaminant. A diagnosis of suspected fungal arthritis would require culture of the synovial fluid in the clinical setting of symptoms and signs of arthritis.
TABLE 1.
Definitions
| Term | Definition |
|---|---|
| Mechanisms of infection | |
| Direct inoculation | Seeding of bone tissue or synovial fluid of the joint by trauma or surgical manipulation |
| Hematogenous inoculation | Seeding of bone tissue or synovial fluid of the joint by the blood-borne route |
| Contiguous inoculation | Seeding of bone tissue or synovial fluid of the joint from an adjacent site of fungal infection |
| Probability of infection | |
| Proven osteoarticular mycosis | All patients with osteoarticular fungal infection with evidence of a positive culture and/or histology from bone tissue, synovial fluid, or metal hardware |
| Probable osteoarticular mycosis | All patients with osteoarticular fungal infection with evidence of a positive culture and/or histology from adjacent tissue or operative samples (other than bone, tissue, synovial fluid, or metal hardware specimens, such as pus, disc, cartilage, adjacent abscess, blood, central venous catheter, skin, and mycotic aneurysm sac) with compatible clinical and radiological features |
| Emergence of infection in relation to antifungal therapy | |
| De novo osteoarticular mycosis | Patients who were not receiving systemic antifungal therapy when the episode of osteoarticular fungal infection occurred |
| Breakthrough osteoarticular mycosis | Patients receiving systemic antifungal agents before/at the onset of osteoarticular fungal infection |
| Response to antifungal therapy | |
| Complete response | Complete resolution of clinical and radiological findings of osteoarticular fungal infection |
| Partial response | Partial resolution of clinical and/or radiological findings of osteoarticular fungal infection or partial clinical improvement without availability of radiological data |
PHARMACOKINETICS AND PHARMACODYNAMICS OF ANTIFUNGAL THERAPY FOR OSTEOARTICULAR MYCOSES
This section reviews the pharmacokinetic and pharmacodynamic characteristics of antifungal agents used to treat osteoarticular mycoses, synthesizes data on synovial fluid and bone penetration, and discusses antifungal activity against fungal biofilms. Although new antifungal agents have been developed during the last decade, managing fungal bone and joint infections remains challenging. Indeed, the susceptibility of some fungi to antifungal agents is low, host defenses are often decreased, and knowledge about the pharmacokinetics and pharmacodynamics of antifungal agents in bones is sparse. There are limited preclinical and clinical studies of the bone penetration of licensed antifungal agents. Only three studies of bone penetration by antifungal agents (ketoconazole, 5-fluorocytosine [5-FC], and voriconazole) in humans have been published (3–5). The isavuconazole bone tissue concentration was measured in rats after single and repeated oral administrations (6). Bone concentrations of amphotericin B (AmB) from lipid formulations have been studied in rabbits (7). Table 2 summarizes the penetration of systemically administered antifungal agents into the bone, synovium, and nucleus pulposus (5, 7–15).
TABLE 2.
Concentrations of antifungal agents in bone and synoviuma
| Antifungal agent | Concn(s) in bone and bone marrow | Concn in synovium | Concn in nucleus pulposus |
|---|---|---|---|
| Amphotericin B deoxycholate | Bone marrow, 2.7 μg/g (dosed at 0.6 mg/kg), 8.0 ± 1.7 μg/g | 0.29 μg/mL; 40% of plasma concn | Not detected |
| ABLC | Bone marrow, 35.4 ± 12.7 μg/g | No data available | Not detected |
| ABCD | Bone marrow, 7.5 μg/g (dosed at 0.6 mg/kg), 9.9 μg/g (dosed at 1.2 mg/kg), 3.3 μg/g (dosed at 2.5 mg/kg), 96 μg/g (dosed at 5.0 mg/kg) | No data available | |
| Liposomal amphotericin B | Bone marrow, 39.5 ± 4.7 μg/g (dosed at 5.0 mg/kg) | 0.79 μg/mL; 140% of plasma concn | |
| Fluconazole | Bone, 33% of serum concn | 14.19 ± 5.07 μg/mL (horse) | 10.86 ± 21.42 μg/g; median value, 3.7 μg/g |
| Itraconazole | 470% of plasma concn | ||
| Voriconazole | Medullary bone, 20.3 μg/g; cortical bone, 1.9 μg/g | 30% of plasma concn (human); 60% of plasma concn (horse) | |
| Posaconazole | No data available | No data available | |
| Isavuconazole | Bone, 0.048–0.070 μg (eq/g); bone marrow, 0.054–0.822 μg (eq/g) ([14C]isavuconazonium sulfate orally at 5 mg/kg as a single dose to rats) | No data available | |
| Caspofungin | No data available | No data available | |
| Micafungin | No data available | No data available | |
| Anidulafungin | 4,830 ng/mL (male rats at postnatal day 4 dosed at 10 mg/kg once), 9,600 ng/mL (female rates at postnatal day 4 dosed at 10 mg/kg once), 7,220 ng/mL (all rats at postnatal day 8 dosed at 10 mg/kg once), 6,050 ng/mL (male rats at postnatal day 4 dosed at 10 mg/kg once, 7,800 ng/mL (male rates at postnatal day 8 after multiple doses at 10 mg/kg) | No data available | |
| 5-Fluorocytosine | 30% of max serum concn, 15% at 2 h postinjection, 6% at 4 h postinjection | 41% of max serum concn |
Several studies have reported concentrations of antifungal agents in the synovial fluid (5, 11, 16–21). These reports demonstrate that synovial fluid concentrations of antifungal agents usually approximate those in serum.
Biofilm formation in prosthetic joint infections (PJIs) and chronic osteomyelitis is an important mechanism by which pathogens protect themselves from antimicrobial agents and innate host defenses (22). Fungal pathogens embedded in biofilms have reduced growth, and their metabolic state confers inherent resistance to antimicrobial agents. Therefore, the complete removal of the foreign body and extensive excision of the infected tissues are generally required. The use of an antifungal agent with preserved activity against biofilms, such as echinocandins, warrants further study in the treatment of biofilms (23, 24). One multicenter retrospective study on Candida prosthetic joint infections (25) concluded that antifungal agents with antibiofilm activity, such as echinocandins and polyenes, seem to have a beneficial effect on therapeutic outcomes. Although the number of these patients was relatively small, the authors recommend these echinocandins, rather than triazoles, as first-line therapy. Further studies are needed to confirm these conclusions.
Antifungal susceptibility determinations of MICs should be performed, if possible, on all isolates that are recovered from the bone, synovium, cartilage, and contiguous foci. Moreover, to optimize the efficacy and tolerability of antifungal therapy, therapeutic drug monitoring (TDM) for voriconazole, itraconazole, posaconazole, and flucytosine (5-FC) should be utilized for the treatment of each patient.
THERAPY FOR BONE AND JOINT INFECTIONS: MAIN ANTIFUNGAL CLASSES
Polyenes: Amphotericin B and Its Formulations
Amphotericin B (AmB) (amphotericin B deoxycholate), a polyene antibiotic, is a product of Streptomyces nodosus. It acts on the fungal cell membrane by binding to ergosterol, causing membrane disruption, leakage of cell contents, and cell death. The affinity of amphotericin B is higher for ergosterol-rich fungal cell membranes than for cholesterol-rich human cell membranes, but in the kidney, where the drug is concentrated, it causes toxicity to the distal tubular cell membranes, leading to renal insufficiency and hypokalemia (26). Lipid amphotericin B formulations have lower renal concentrations, resulting in reduced nephrotoxicity (27, 28). Other mechanisms of the reduced nephrotoxicity of lipid formulations of amphotericin B include preferential binding by high-density lipoproteins, which are processed in the liver. In comparison, deoxycholate amphotericin B (DAmB) is bound preferentially by low-density lipoproteins that tend to accumulate in the kidney.
Amphotericin B displays concentration-dependent fungicidal activity (29, 30). Its spectrum of activity is broad, including most Candida spp., most Aspergillus spp., Mucorales, and many dimorphic and dematiaceous fungi. In vitro and in vivo models show that amphotericin B has potent activity against Candida albicans embedded within biofilms (31, 32).
The tissue distribution of AmB is highly variable, with the highest concentrations being found in the liver and the spleen, followed by the lung, the kidney, and the heart (29). The administration of lipid-based formulations results in high drug concentrations in the liver and spleen (33). Groll and colleagues studied the distributions of DAmB, liposomal amphotericin B (LAmB), and amphotericin B lipid complex (ABLC) in the bone marrow of noninfected rabbits (7). Animals received either 1 mg/kg of body weight/day of DAmB or 5 mg/kg/day of LAmB or ABLC for 7 doses. All formulations distributed well into the bone marrow, while LAmB and ABLC at the higher dosages also achieved proportionally higher tissue concentrations than those of DAmB (7). However, concentrations of DAmB in cortical bone are unknown. Concentrations of DAmB in synovial fluid approximate those in serum (11, 18–21).
Amphotericin B is excreted unchanged in the urine (21%) and the feces (42%). Its half-life is long, approximately 127 h for the conventional deoxycholate formulation. In a retrospective cohort study, Bates et al. showed that the frequently severe nephrotoxicity of DAmB leads to increased mortality (34). These results were instrumental in leading clinicians to use lipid formulations of amphotericin B as safer alternatives.
Amphotericin B and its lipid formulations are now an alternative choice for the treatment of bone and joint infections caused by Candida, Aspergillus, or Cryptococcus species. Antifungal triazoles have the advantages of oral formulations and favorable safety profiles for prolonged ambulatory treatment durations.
Amphotericin B lipid formulations remain the first choice for the treatment of Mucorales bone and joint infections (35, 36). Combination antifungal therapy has been recommended by some authors (36–39). However, combination therapy for mucormycosis was found to have no therapeutic benefit over lipid formulations of amphotericin B as a single agent in patients with hematological malignancies (40).
Triazoles
Triazole antifungal agents target ergosterol biosynthesis by inhibiting a fungal cytochrome P450-dependent enzyme (lanosterol-14-α-demethylase) that converts lanosterol to ergosterol, the principal fungal cell membrane sterol. Inhibition of ergosterol biosynthesis disrupts cell membrane structure and function. However, triazoles also exert collateral inhibition of human hepatic cytochrome-dependent enzymes and are responsible for important drug-drug interactions. Their activity is essentially fungistatic, and the area under the concentration-time curve (AUC)/MIC ratio seems to be the best predictor of treatment responses (25, 29, 41).
The spectrum of activity varies according to the specific triazole (1). Fluconazole has the narrowest spectrum, with in vitro and in vivo activity against Cryptococcus neoformans and Candida spp., with exceptions of Candida krusei and C. auris, which have intrinsic resistance, and C. glabrata, which is variably resistant, depending upon the isolate. Voriconazole, a derivative of fluconazole, has a wider spectrum of activity. It is active against yeasts, has excellent activity against Aspergillus, and is also active against endemic dimorphic fungi and some isolates of Fusarium species. Posaconazole also has a wide spectrum of activity, including against Aspergillus species, yeasts, dimorphic fungi, dematiaceous fungi, and some isolates of the Mucorales.
The antifungal activities of triazoles against Candida biofilms are reduced for posaconazole (32) and negligible for fluconazole (42, 43). A study on the voriconazole resistance of Aspergillus fumigatus biofilms demonstrated that this fungal complex expressed increased efflux pump activity that was induced by the triazole and provided evidence of voriconazole treatment failures (44).
The degree of drug absorption varies considerably among triazoles; the bioavailability of fluconazole and voriconazole exceeds 90%, that of itraconazole is approximately 50%, and the bioavailability of posaconazole in suspension is approximately 50%, while in the extended-release formulation, it exceeds 80% (29).
Data on the synovial and bone diffusion of triazoles are limited. The levels of fluconazole in synovial fluid reported in one article approximated those in serum (20). Another case report describes voriconazole synovial diffusion varying from 0.25 to 0.6 μg/mL (5). In this same case report, voriconazole bone levels were 10 to 20 times higher in medullar bone and 1 to 2 times higher in cortical bone than in serum.
Fluconazole and posaconazole are poorly metabolized. Elimination is predominantly urinary for fluconazole and biliary for posaconazole. Itraconazole and voriconazole are principally metabolized by the cytochrome P450 systems. Voriconazole metabolism is affected by polymorphisms of hepatic CYP3A4, CYP2C9, and CYP2C19 (45, 46). There are 35 estimated polymorphisms of CYP2C19, and their distribution may vary within different populations, which has been discussed in detail elsewhere (https://www.pharmgkb.org/vip/PA166169770). These genetic polymorphic differences in metabolism play an important role in the wide interpatient variability in voriconazole serum levels. Drug monitoring is therefore required for voriconazole, itraconazole, and posaconazole (29, 47–49). Most patients who are receiving isavuconazole do not require therapeutic drug monitoring.
For the treatment of osteoarticular mycoses, the most frequently used agents are fluconazole and voriconazole. Fluconazole is used as a first-line treatment for infections caused by susceptible Candida spp. and for nonmeningeal disease due to Cryptococcus neoformans. Voriconazole is currently the first-line therapy for Aspergillus osteomyelitis (48, 50, 51) and arthritis, as an extrapolation from its activity in pulmonary aspergillosis (52) as well as its safety profile and extended treatment duration with oral administration.
The most recently introduced triazole, isavuconazole, has in vitro, in vivo, and clinical activities against most Aspergillus species and Mucorales isolates (53). As the randomized clinical trial of isavuconazole against invasive pulmonary aspergillosis fulfilled noninferiority criteria but was safer and better tolerated than voriconazole, with more practicable pharmacokinetics (54), it may be a suitable agent for the extended treatment of Aspergillus osteoarticular infections. At this time, however, there are no published data supporting the use of isavuconazole for the treatment of osteoarticular mycoses.
Echinocandins
The echinocandin class of antifungal agents targets the fungal cell wall by competitively inhibiting the enzyme complex that is responsible for the synthesis of (1→3)-β-d-glucan (BDG) polymers. Inhibition of this essential component of the cell wall leads to osmotic cell lysis and death. Echinocandins, which include caspofungin, micafungin, and anidulafungin, exhibit concentration-dependent fungicidal activity (maximum concentration of the drug in serum [Cmax]/MIC ratio) against Candida species and are fungistatic against Aspergillus species (29).
Echinocandins showed potent antifungal activity in Candida biofilm-associated infection models in vitro (32, 42, 55, 56). These compounds are available only as intravenous (i.v.) formulations. Data for concentrations of echinocandins in synovial fluid and bone are lacking. Caspofungin undergoes extensive hepatic metabolism into inactive metabolites. Micafungin is metabolized into three metabolites, initially by aryl sulfatase and secondarily by catechol-O-methyltransferase; the third phase of hydrolysis by CYP3A is relatively limited. Anidulafungin is degraded slowly by peptide hydrolysis to an open chain. Some agents that are transported through P-glycoprotein transporters and/or hepatically metabolized may require an adjustment of the dosage when used concomitantly with caspofungin. In comparison, there are few drug-drug interactions with micafungin or anidulafungin (29, 30).
Treatment experience with echinocandins for bone and joint infections is limited. Case reports of Candida arthritis treated successfully with caspofungin, alone or combined with triazoles, have been reported (57–60). Among the 211 cases of Candida osteomyelitis reported by Slenker et al. (61) and the 53 cases of Candida osteomyelitis reported by Neofytos et al. (62), amphotericin B and triazoles were the most frequently used antifungal agents, with a few cases being treated with echinocandins. The paucity of patients who were treated with echinocandins in these two papers may be related to the studies being published in 2012 and 2014 and the relatively few patients being treated with these agents before 2000.
A Spanish retrospective multicenter study reported 43 Candida prosthetic joint infections. Nine patients were treated with echinocandins, which were combined with triazoles in 8 cases (25). Patients treated with the removal of the prosthesis and echinocandin or amphotericin B combination therapy (n = 6), agents that are active against biofilms, demonstrated a trend toward better outcomes (success for 5/6) than those treated with triazoles alone (success for 8/13). Data from additional patients treated with echinocandins as the initial single-agent therapy are needed to better understand the efficacy of these agents in the management of Candida prosthetic joint infections.
Although there is insufficient clinical evidence that the use of biofilm-active antifungals has an additional benefit, we recommend that these agents may be used where possible as initial therapy for the treatment of Candida osteoarticular infections. Well-designed preclinical studies in predictive animal models and correlative clinical trials are needed to further define the role of biofilm-active echinocandins in the treatment of Candida osteoarticular infections.
Other Antifungal Agents: Flucytosine (5-Fluorocytosine)
Flucytosine (5-FC) is a synthetic fluorinated analogue of cytosine. It is taken up by 2 fungus-specific enzymes and converted in the fungal cell to 5-fluorouracil, and it acts as an antimetabolite causing RNA miscoding (63). It has a narrow spectrum of activity, including yeasts and some dematiaceous fungi. Monotherapy with flucytosine is not recommended because it often leads to fungal resistance. It is used with other antifungal agents, usually amphotericin B, for the treatment of systemic mycoses. Flucytosine displays a concentration-independent pattern of activity (64). The best predictor of antifungal activity against Candida albicans is a time above the MIC of 20 to 40% (65). We found no data on its activity against biofilms.
The absorption of the drug is excellent, with a bioavailability of 76 to 89%. Due to its small size and minimal serum protein binding, tissue penetration is good in most body sites (cerebral, vitreous, and peritoneal fluids). A sole case report mentioned flucytosine synovial fluid levels, which approximated those found in serum (66). Bone concentrations of 5-FC were measured by Fuzibet et al. (3). Given its short half-life (3 to 4 h) in patients with normal renal function, 5-FC is administered 4 times a day at 100 mg/kg/day. Concentrations in bone marrow were 41% of those in serum. Flucytosine is eliminated via glomerular filtration and is only minimally metabolized in the liver. The dosage must be adjusted for patients with renal impairment, and drug monitoring is recommended to avoid toxicity, which includes myelosuppression and hepatotoxicity.
According to two previously published guidelines (67, 68), 5-FC combination therapy with amphotericin B is indicated for the treatment of cryptococcal meningoencephalitis or severe or disseminated non-central nervous system (CNS) cryptococcal infections. The role of 5-FC in the treatment of osteoarticular mycoses is not well defined.
THERAPEUTIC DRUG MONITORING
Triazole antifungal drugs may exhibit marked interpatient variability in serum drug concentrations due to differences in the absorption of the oral formulation, variations in hepatic metabolism via cytochrome P450, and drug-drug interactions. TDM is therefore an important tool to optimize dosing for voriconazole, posaconazole, itraconazole, and 5-FC (68). Nonetheless, there is a paucity of cases or case series describing the relationship between TDM and outcomes for osteoarticular mycoses (69).
The analytical methods most commonly utilized to measure serum drug concentrations are high-performance liquid chromatography (HPLC) and liquid chromatography-tandem mass spectroscopy (LC-MS/MS) because of their high sensitivity and rapid time to completion of the assay (49, 70, 71). As a comprehensive discussion of TDM is beyond the scope of this review, see several sources that discuss its important role in optimizing the outcomes of invasive mycoses (47, 48, 70, 72–80).
The arsenal of antifungal agents has increased during the last 3 decades with the development of new triazoles and the emergence of a new antifungal family, the echinocandins. Despite this evolution, very little is known about the pharmacokinetics of antifungal agents in bones. The management of fungal bone and joint infections remains a therapeutic challenge. Despite these gaps in knowledge, one may reasonably infer from sound pharmacokinetic and pharmacodynamic principles that the optimization of bone penetration is best achieved through the use of high dosages with prolonged treatment. As these treatment modalities can lead to long-term drug toxicity, TDM and close clinical and biological surveillance in these difficult-to-treat infections are important components of the strategy to optimize drug efficacy while minimizing toxicity. The use of antifungal agents such as echinocandins or the new orally bioavailable agent ibrexafungerp, which exert potent activity against Candida biofilms, may improve outcomes, especially in foreign-body-associated infections. Further preclinical and clinical data are necessary to support this hypothesis.
PATHOGENESIS OF BIOFILM FORMATION AND OSTEOARTICULAR MYCOSES
Many pathogenic bacteria and fungi grow in two fundamentally different forms, free-floating organisms (single-cell planktonic forms) and biofilms, a surface-adherent community of microorganisms embedded in a self-produced hydrated extracellular polymeric material, matrix. Biofilms can consist of one or more types of organisms (bacteria and/or fungi), but even monospecies biofilms may contain organisms with different phenotypic or genotypic characteristics. Biofilms are a major public health concern since they account for a preponderance of chronic and recurrent bacterial and fungal infections. The biofilm concept explains why some organisms initially considered “harmless” or “normal” flora become pathogens in the presence of foreign material, accounting for chronic infections being untreatable even when they are caused by a pathogen that is not resistant to chemotherapeutic agents (81–83).
The most frequent types of chronic osteomyelitis can be seen as characteristic examples of biofilm-mediated infections; these include chronic hematogenous osteomyelitis, progression to chronic osteomyelitis from deep soft tissue infection, diabetic foot osteomyelitis, osteomyelitis with sacral pressure ulcer, sternal wound osteomyelitis poststernotomy, persistent chronic osteomyelitis associated with internal fracture fixation, and prosthetic joint infection (PJI) (84, 85). Gram-positive bacteria predominate within the microbiological spectrum of osteoarticular biofilm-related infections, while fungal pathogens are less frequent. Particularly, in PJIs, fungi have been isolated in fewer than 1% and Candida spp. are the etiological agents of approximately 80% of these diseases, whereas dimorphic fungi, Aspergillus spp., and other filamentous fungi are considerably less common (86, 87). Although less common than bacterial musculoskeletal infections, fungal biofilm-related diseases, including osteoarticular mycoses, are challenging to treat and associated with increased mortality (88).
Biofilm formation is a dynamic process that includes consecutive stages of maturation, with each stage involving several physiological changes that have important clinical impacts. Overall, these changes constitute the biofilm “life cycle,” which can continue in perpetuity. Biofilm development is a stepwise process that can occur in four stages.
Stage 1: Attachment
In the attachment stage, planktonic, free-floating cells attach to a surface. The attachment surface can be an “abiotic” one, such as the surface of an indwelling device or prosthesis, or “biotic,” like bone tissue or a periprosthetic surface (89). Initially (at 0 to 2 h), during Candida biofilm temporal development, the majority of fungal cells are present as blastospores, and later (at 3 to 4 h), distinct microcolonies appear on the surfaces (90). Surface properties (charge, hydrophobicity, protein coating, and physiochemical properties such as surface roughness and porosity) are decisive factors in microbial adhesion and subsequent biofilm development. After device implantation, a conditioning film, composed of host proteins such as fibrinogen and fibronectin, is formed on the device’s surface (91, 92). This conditioning film alters the surface properties while the attached proteins serve to anchor the biofilm cells. Investigations have illustrated that surface modification of biomaterials can be a viable strategy against biofilm-related infections (93). For the establishment of an infection in the presence of prosthetic material, the inoculum size seems to be of less importance (94). Once the fungal cells are adherent to the substrate, genes that encode proteins involved in secretion and adhesion are upregulated. These properties in Candida albicans are exemplified by the significantly upregulated expression of genes encoding agglutinin-like sequence (ALS) family proteins, hyphal wall protein (HWP), and adhesion factor (EAP1) (95–97).
Stage 2: Accumulation
Following the attachment of the organism to a surface, fungal cells begin to proliferate and become progressively established on the colonized surface at approximately 12 to 14 h. At this phase, cells change their morphology, begin to elongate, and develop hyphae. Fungi, particularly C. albicans, excrete a number of enzymes like proteinases, lipases, esterases, hemolysins, and phospholipases that enable the developing biofilm to invade the substrate surface (host or medical device surface) (98). Early biofilms are relatively unstable and susceptible to host defense and antifungal agents.
Stage 3: Maturation
Mature fungal biofilms, as demonstrated by confocal laser scanning microscopy images, consist of a complex network of fungi in different developmental stages (conidia, hyphae, and pseudohyphae) encased in a well-developed matrix. As demonstrated in in vitro studies of biofilm temporal development, mature biofilms appear after approximately 38 to 72 h (90). Microorganisms in a mature biofilm are densely contiguous so that they can interact through quorum sensing and, possibly, the exchange of virulence or resistance genes. Throughout the biofilm ultrastructure, the prevailing physiological conditions differ as microorganisms have different access to nutrients and oxygen. This is particularly prominent in the biofilm core, where microorganisms fall into a dormant, nondividing stage, forming so-called “persister” cells (99). The extracellular polymeric material, which forms the scaffold for the three-dimensional architecture of the biofilm and provides a protective barrier for the embedded organisms, is one of the defining traits of biofilms at this stage. The nature of the individual components of the matrix can vary greatly and depends on the constellation of microorganisms within the biofilm, the surrounding environment (availability of nutrients, shear forces, or temperature), the substrate, and host factors. The biofilm matrix composition is complex and universally is comprised of proteins, nucleic acids, carbohydrates, lipids, and other polymers (100). For several fungal pathogens, the matrix components differ significantly from those of the cell wall that are recognized by immune cells, while the biofilm-embedded organisms are in the planktonic mode of growth (101–105). At this maturation phase, fungal biofilms are resistant to antifungal agents and host defenses.
In the phagocyte-fungus-antifungal agent interplay, drugs may either interact with phagocytes, leading to altered antifungal activities, or, through modulation of fungal virulence, initiate different immune response programs in phagocytes (43). Experiments studying host-Candida biofilm interactions have shown that micafungin, by increasing inflammatory responses, has a beneficial effect on innate immune cells in the host defense against C. albicans biofilms but a blunt effect on Candida parapsilosis biofilms (23). The factors that regulate differential species-dependent interactions between biofilms and host immune cells and the molecular events of interchange among biofilms, host components, and antifungal drugs should be elucidated, as there is an urgent need for alternative therapeutic approaches to treat several types of candidiasis.
In vitro studies demonstrate that human leukocytes remain viable while trying to penetrate Candida species biofilms but do not exhibit any significant phagocytic function while also displaying an altered cytokine profile. Characteristically, Candida species biofilms downregulate the production of tumor necrosis factor alpha (TNF-α) compared to their planktonic counterparts (106). The phagocytic impairment of human phagocytic cells by biofilms is so robust that it persists despite priming by interferon gamma (IFN-γ) or granulocyte colony-stimulating factor (G-CSF) (106–109).
Stage 4: Dispersal
The mature biofilm releases nonadherent or detached cells, which can colonize another surface and create a niche for another biofilm, thus perpetuating the biofilm’s “life cycle.” This last phase of biofilm development has important clinical significance as the dispersed cells display distinct phenotypic properties that are associated with increased virulence (110). Dispersion during biofilm development constitutes a major mechanism for disseminated fungal infection and/or the creation of a new biofilm in a remote area. Notably, there are in vivo observations that show that the detachment of fungal cells can occur very early in biofilm development; maximum numbers of cells are released when the biofilm proliferates rapidly during the intermediate phase (5 to 12 h), and during the stationary phase of a mature biofilm, the number of released cells decreases (110). Thus, an established focus of Candida osteomyelitis may propagate extensively through this mechanism of dispersal to involve the adjacent trabecular and canalicular bone matrices.
While important advances in understanding the pathogenesis of biofilms, including those caused by Candida spp., have been achieved, the matrices on which they have been studied are largely in vitro plastic or in vivo catheters and prosthetic material. Although the data from these studies can be reasonably extrapolated to biofilms of osteoarticular tissues, little is known about the impact of their unique mineral composition and unique tissue architecture on fungal biofilm formation and pathogenesis.
Diagnosing biofilm-related osteoarticular infections is challenging because usually they are indolent, they give few clinical signs and symptoms, and conventionally used microbiological methods often fail to detect the biofilm-embedded organisms. The difficulty in the diagnosis and treatment of chronic or prosthetic-device-related osteoarticular fungal infections is best understood within the context of biofilm pathogenesis. The Musculoskeletal Infection Society, the American Academy of Orthopedic Surgeons, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Biofilms, and the International Consensus Meeting on Musculoskeletal Infection have established criteria for diagnosing biofilm-related infections and provide answers on important aspects regarding the management of osteoarticular biofilm-related infections (111–114). Strategies to overcome diagnostic limitations are being developed with technologies like metagenomics shotgun sequencing, which seem to have the potential to enhance our diagnostic yield with difficult-to-detect pathogens (115).
CANDIDA OSTEOMYELITIS
History
The first well-defined case of Candida osteomyelitis was described in 1928 by Connor (116). The patient was a 19-year-old immunocompetent female with a 4-year history of recurrent humerus osteomyelitis. Later, she developed buttock abscesses. Tissue specimens from the affected humerus and buttock pus disclosed an identical histopathological appearance consistent with Candida infection. Of note, there was no report of previous trauma or disseminated fungal infection.
Since then, and until 2012, 215 well-described cases (1, 11, 60, 66, 117–255) of Candida osteomyelitis were analyzed by Gamaletsou et al. (1). Since 2012, there have been 36 notable publications on Candida osteomyelitis (61, 62, 139, 256–290).
Epidemiology
The age of onset of Candida osteomyelitis is widely distributed but trends toward a younger population (median age of 35 years) than what is observed for other osteoarticular mycoses. Similar to other invasive mycoses, males predominate in frequency.
Most patients who develop Candida osteomyelitis are not pharmacologically immunosuppressed. Instead, the key risk factors for these patients are surgical procedures, illicit intravenous drug use, orthopedic devices/prostheses, trauma or open wounds, as well as conditions that are associated with candidemia, such as central venous catheters and total parenteral nutritional support. Nonetheless, pharmacological immunosuppression is also a known risk factor in cases of Candida osteomyelitis with leukemia, lymphoma, renal transplantation, and liver transplantation. Candida costochondritis and sternoclavicular infections have been reported as distinct distributions of infection in patients with a history of illicit intravenous drug use (226, 291).
Pathogenesis
There are three mechanisms by which fungi infect bones: hematogenous dissemination, direct inoculation, and contiguous infection. Of the presumed mechanisms of Candida osteomyelitis observed among reported cases, the most common is hematogenous dissemination (n = 138 [67%]), followed by direct inoculation (n = 51 [25%]) and, less commonly, contiguous infection (n = 18 [9%]) (1). However, Candida osteomyelitis caused by contiguous infection in the setting of decubitus ulcers may also be an underreported mechanism. See above for a more detailed discussion of biofilm formation.
Clinical Manifestations
Patients typically complain of an insidious onset over several weeks to months of local pain with erythema, tenderness, and edema as the most common presenting clinical manifestations. However, fever is present in fewer than 1/3 of patients. Approximately 1/3 of patients present with a limited range of movement of the involved extremity. Physical examination may reveal sinus tracts with draining pus (1).
Gamaletsou et al. reported that Candida osteomyelitis was the first site of clinically overt deep infection in nearly one-half of patients. Candida osteomyelitis most commonly presents as a de novo infection in more than 70% of patients (1). The remainder of patients with Candida osteomyelitis develop breakthrough infections while receiving antifungal therapy.
Candida osteomyelitis may also occur in the setting of candidemia. For example, approximately one-half of patients with Candida osteomyelitis may have candidemia or another form of candidiasis as the initial manifestation of Candida infection. Indeed, candidemia and osteomyelitis may coexist and have been diagnosed simultaneously in 14% of cases (1).
Candida osteomyelitis is distributed as a polyosseous infection in more than 80% of cases. The most commonly infected sites are vertebrae, in approximately 50% of cases, followed by the femora, ribs, sternum, and humeri (1). Among the joints that are frequently infected concomitantly are intervertebral discs, in approximately 40% of cases. Other nonsynovial joints that are concomitantly infected include costoclavicular, costosternal, and costochondral joints in 10% of cases. The most common synovial joints infected are the knee (11%) and hip joint (7%) (1).
As a reflection of the age-dependent differences in the pathogenesis of Candida osteomyelitis, there are clear distinctions in osseous distributions between pediatric and adult populations (1). In descending order, the most frequently infected sites in adults are the vertebrae, ribs, and sternum. In comparison, the most frequently infected sites in patients ≤18 years of age are the femur, humerus, and vertebrae. While vertebrae are the most common osseous sites in adult patients, femoral bones are more commonly infected in the pediatric population. Despite these differences in osseous distributions between pediatric and adult patients, the local symptomatologies (i.e., pain, erythema, and edema), along with the limitation of function, are similar.
Diagnostic Approaches
Among the diagnostic strategies used for Candida osteomyelitis, percutaneous closed guided biopsy or open biopsy should be performed in order to establish a definitive diagnosis. Specimens obtained by these procedures are submitted to the relevant laboratories for direct culture and, when feasible, histopathology with special stains, particularly periodic acid-Schiff (PAS) and Gomori methenamine silver (GMS) stains.
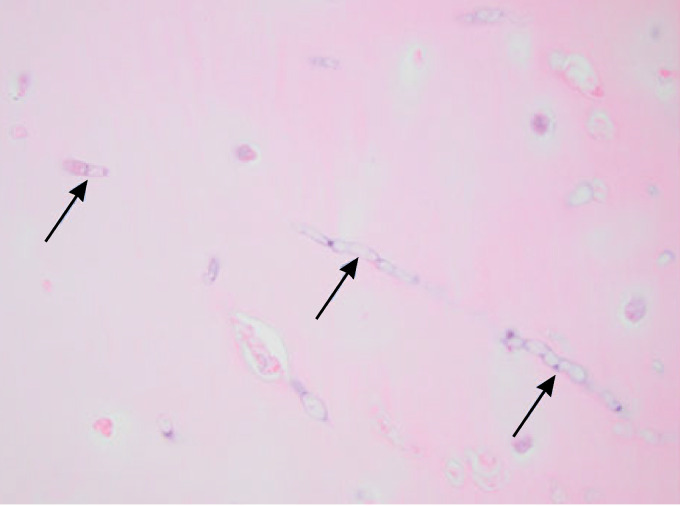
Diagnostic Mycology
Candida albicans is the most common cause of Candida osteomyelitis, followed in descending order by C. tropicalis, C. glabrata, and C. parapsilosis. One must also be aware of mixed fungal and bacterial infections in Candida osteomyelitis; e.g., Gamaletsou et al. (1) reported 12% of cases of Candida osteomyelitis with concomitant bacterial infections, the most common etiology of which was Staphylococcus aureus. Coinfection with bacteria also occurred in cases of osteoarticular infections caused by Aspergillus spp.
Inflammatory Markers
The white blood cell (WBC) counts in patients with Candida osteomyelitis are typically mildly to moderately elevated, with a median count of 10,500 cells/mm3 and a range from 900 to 36,000 cells/mm3, with a left shift toward neutrophil predominance in the differential count. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are often elevated, with median levels of 61 mm/h (range, 3 to 150 mm/h) for the ESR and 6.3 mg/dL for CRP (range, 1.2 to 46 mg/dL). However, as some patients with Candida osteomyelitis may have a minimal to no elevation of inflammatory biomarkers, the presence of normal values of white blood cell counts, ESR, and CRP does not exclude the diagnosis (Table 3).
TABLE 3.
Comparative analysis of inflammatory biomarkers in osteoarticular mycoses
| Type of infection (reference[s]) | No. of cases | Median ESR (mm/h) (range) | Median CRP concn (mg/dL) (range)a | Median WBC count (cells/μL) (range) |
|---|---|---|---|---|
| Candida osteomyelitis (1; case in this report) | 208 | 65 (3–150) | 8.8 (1.2–46) | 10,100 (2,650–36,000) |
| Candida arthritis (2) | 112 | 56 (10–118) | 28.9 (0.5–141) | 10,750 (160–36,500) |
| Candida bursitis (292–294, 296, 297) | 12 | 79 (48–106) | 5.4 (1.2–6.7) | 6,810 (500–11,700) |
| Aspergillus osteomyelitis (550) | 180 | 86 (10–148) | 51 (1.5–151) | 8,740 (100–37,000) |
| Aspergillus arthritis (572; case in this report) | 32 | 91 (46–148) | 3.7 (2.6–4.6) | 9,700 (1,300–12,700) |
| Non-Aspergillus mold osteomyelitis (1232) | 148 | 76 (32–120) | 45 (1.1–362) | 9,850 (1,900–33,500) |
| Osteoarticular mucormycosis (739; cases in this report) | 35 | 69 (40–107) | NA | 16,150 (500–29,400) |
NA, not applicable.
Diagnostic Imaging
Among 207 previously reported cases of Candida osteomyelitis (1), radiological manifestations included osteolytic destruction and soft tissue extension. Imaging of the spine demonstrated that Candida vertebral osteomyelitis yields decreased intervertebral space and complications of the type of epidural abscess.
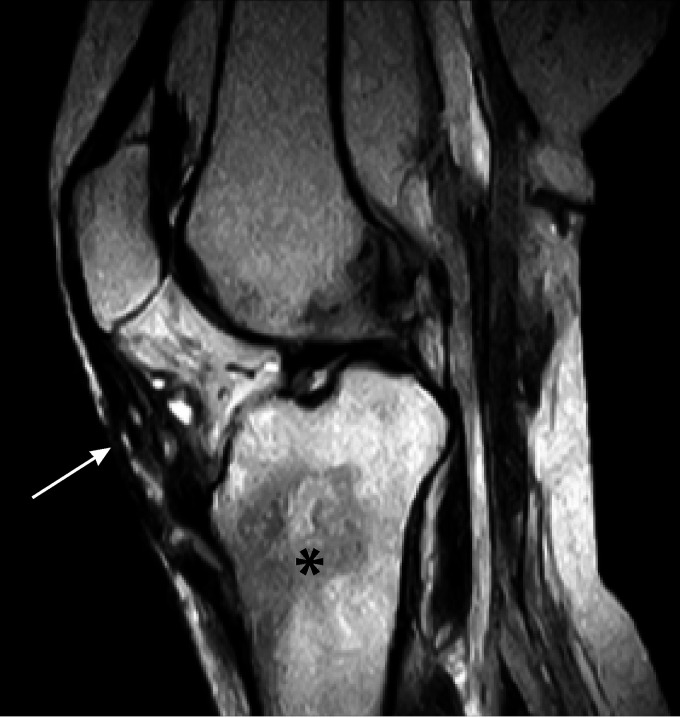
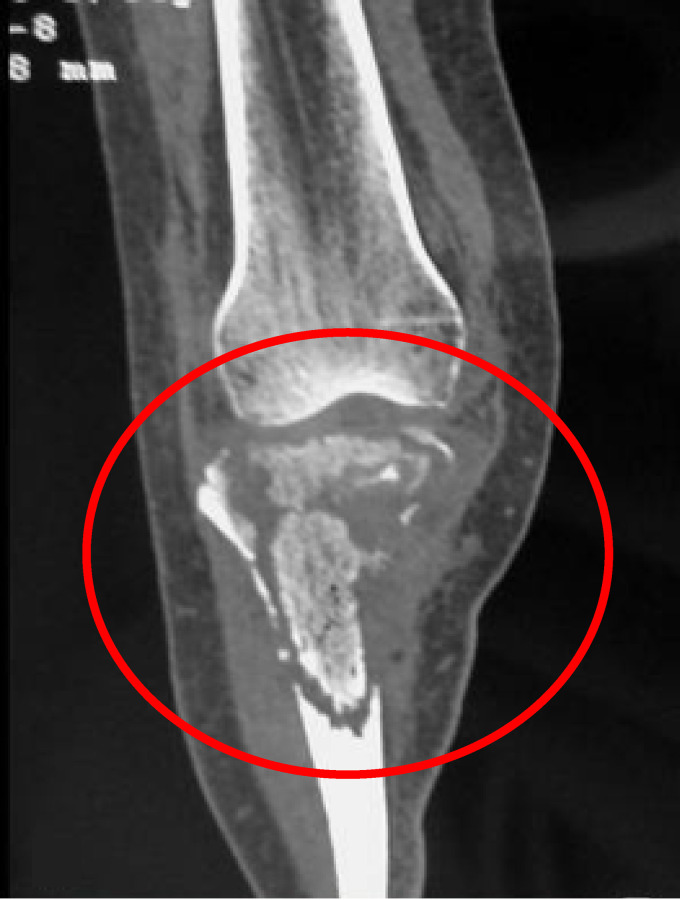
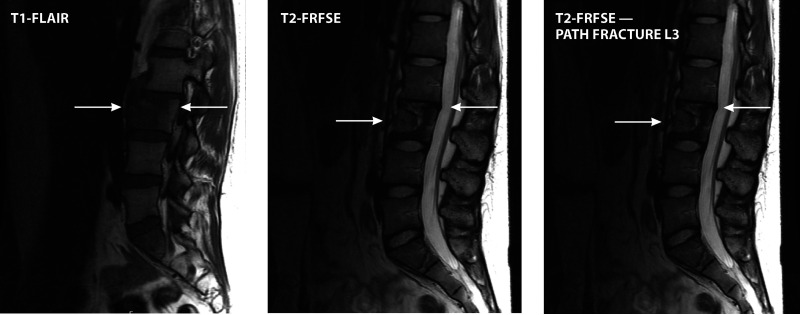
Magnetic resonance imaging (MRI) has emerged as the preferred diagnostic imaging modality for osteomyelitis because of its lack of ionizing radiation, high sensitivity for detecting early infection, and excellent anatomical detail. An illustrative case of Candida osteomyelitis of the spine is shown in Fig. 1. This paper and others (1, 139) found that MRI of lesions of Candida osteomyelitis displays increased signal intensities on T2-weighted images and decreased signal intensities on T1-weighted images. Radionuclide 99Tc scans demonstrate increased radionuclide scan uptake. Although indium-labeled white blood cell scans are a useful imaging tool for the detection of bacterial osteomyelitis, there are minimal data for Candida osteoarticular infections. The conventional radiological, MRI, and radionuclide findings of Candida osteomyelitis are not sufficiently distinct to permit differentiation from those of bacterial infections. Moreover, there may also be similarities between the imaging findings and those of lytic neoplastic processes. Thus, biopsy of suspicious lesions is warranted in the absence of supportive culture data for other sites.
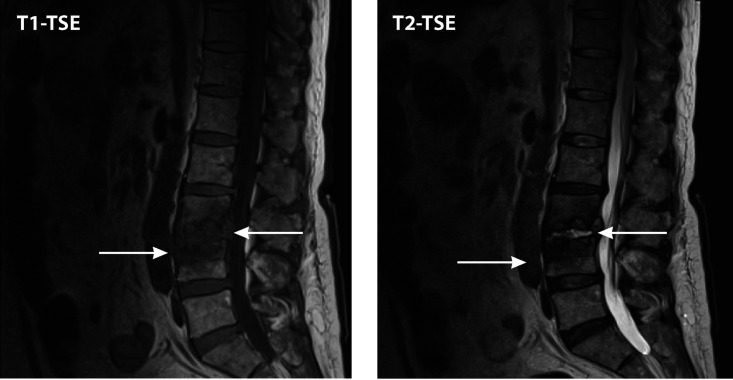
FIG 1.
Candida osteomyelitis/discitis with psoas abscess. A 62-year-old male with diffuse large B cell lymphoma (stage 4) and decompensated cirrhosis developed Candida albicans fungemia and chorioretinitis after the first cycle of chemotherapy and was treated with fluconazole, micafungin, and intravitreal antifungals. After 2 additional cycles of chemotherapy, he developed worsening back pain, tenderness at the lumbar spine, and limited mobility. MRI showed L3-L4 osteomyelitis/discitis with psoas abscess (left, T1-weighted scan; right, T2-weighted scan). Cultures of specimens from CT-guided biopsy of the vertebral body and soft tissue specimens grew C. albicans, and histology showed yeast. TSE, turbo spin echo.
Staging of infection with 18-fluoro-2-deoxy-d-glucose positron emission tomography combined with computed tomography (PET-CT) is another valuable imaging modality for characterizing the distribution and, possibly, assessing the microbiological burden of infection. A study of experimental Candida osteomyelitis demonstrated that the signal intensities of 18-fluoro-2-deoxy-d-glucose PET-CT scan varied directly with the tissue burden of Candida osteomyelitis (290).
Treatment and Outcome
As there are no randomized or controlled clinical therapeutic trials of Candida osteomyelitis, a statement about the advantages of one form of therapy over another is inevitably limited. Complete and partial responses as well as durations of therapy for Candida osteomyelitis are shown in Table 4. Data from the current literature and our updated database indicate that there were similar overall favorable outcomes of a complete response and a partial response in 89 (90%) of 99 patients receiving combination medical-surgical therapy versus 90 (97%) of 93 patients receiving antifungal therapy alone for the treatment of Candida osteomyelitis.
TABLE 4.
Comparative analysis of treatments and outcomes of osteoarticular mycoses
| Type of infection (reference[s]) | No. of cases | No. (%) of cases with outcome |
Median duration of therapy (days) (range) | ||
|---|---|---|---|---|---|
| Complete response | Partial response | Relapse | |||
| Candida osteomyelitis (1; this study) | 208 | 66 (32) | 124 (60) | 60 (29) | 90 (7–720) |
| Candida arthritis (2) | 112 | 87 (78) | 21 (19) | 18 (16) | 64 (14–436) |
| Candida bursitis (292–294, 296, 297) | 12 | 7 (58) | 4 (33) | 6 (50) | 21 (14–70) |
| Aspergillus osteomyelitis (550) | 180 | 106 (59) | 58 (32) | 17 (10) | 90 (10–772) |
| Aspergillus arthritis (572; this study) | 32 | 22 (69) | 6 (19) | 5 (16) | 219 (30–545) |
| Dimorphic fungal osteomyelitis (834) | 222 | 135 (72) | 34 (18) | NA | 180 (70–365) |
| Non-Aspergillus mold osteomyelitis (1232) | 148 | 94 (63) | 28 (19) | NA | 115 (5–730) |
| Osteoarticular mucormycosis (739; this study) | 35 | 18 (51) | 12 (20) | NA | 45 (5–573) |
The lack of an apparent impact of surgery on favorable outcomes of Candida osteomyelitis may be attributed to the greater complexity of cases requiring surgery. Alternatively, these data may also indicate that antifungal agents may exert a favorable outcome independent of surgery. For patients treated with an amphotericin B formulation or an echinocandin, there would be a direct impact on Candida biofilms that could preclude the need for surgery. For patients treated with protracted courses of fluconazole over weeks of therapy, there may be an impact on viable organisms in combination with innate host responses to eradicate the infection. These data also suggest that a prospective clinical trial is warranted for medical therapy alone for selected patients with Candida osteomyelitis.
The duration of therapy plays a key role in the outcome of Candida osteomyelitis irrespective of whether antifungal therapy is combined with surgery. For example, with combination of antifungal therapy-surgery, more than 40% of patients suffered a relapse following the premature discontinuation of therapy; however, with the reinstitution of medical therapy, an overall favorable response was achieved in most patients. Thus, relapsed Candida osteomyelitis is not necessarily an indication for surgery; instead, a longer course of antifungal therapy may be needed.
The management of Candida osteomyelitis needs clearer benchmarks for the potential discontinuation of antifungal therapy as well as a strategy for the use of medical therapy in more patients as the primary means of management. Among the possible benchmarks for the length of therapy, resolution of ESR and CRP may not be sufficiently sensitive to allow the discontinuation of therapy. In comparison, serial sampling of serum (1→3)-β-d-glucan and Candida mannan may offer higher sensitivity and specificity for the therapeutic monitoring of Candida osteomyelitis. Substantiating the potential role of serial serum (1→3)-β-d-glucan and Candida mannan diagnostic testing, the rabbit model of Candida osteomyelitis showed the expression of these biomarkers with infection limited to the tibia (290).
PET-CT scan is another option for the assessment of the therapeutic response of Candida osteomyelitis (290). Data from the rabbit model of Candida osteomyelitis on days 7, 14, and 21 of infection demonstrated significant inoculum-dependent and time-dependent increases in [18F]fluorodeoxyglucose ([18F]FDG) signals. Nonetheless, further validation of serial sampling of serum (1→3)-β-d-glucan and Candida mannan and PET-CT scan are required for the elucidation of the utilities of these promising modalities for the measurement of the therapeutic response. We therefore propose a clinical trial dedicated to the treatment of Candida osteomyelitis that would incorporate serial sampling of serum (1→3)-β-d-glucan and Candida mannan and PET-CT scan for initial diagnosis and subsequent therapeutic monitoring in the context of primarily medical management of the selected patient, with surgery held in reserve for those not responding to the initial antifungal therapy.
CANDIDA BURSITIS
Septic bursitis is most commonly caused by Staphylococcus aureus and Streptococcus pyogenes (292–294). Prototheca wickerhamii, an achlorophyllous alga that may mimic a yeast in culture, is a less common but also well-established cause of septic bursitis, particularly of the olecranon bursa (295). Individual case reports indicate that Candida bursitis can be a painful and debilitating osteoarticular mycosis (296). Among 12 previously published cases of Candida bursitis, the first reported case (296) was that of a 77-year-old hospitalized patient who, following “transient” candidemia due to C. tropicalis after bilateral ureteral catheterization, developed de novo arthritis of the right shoulder and, 1 week later, left-olecranon bursitis. Candida tropicalis was isolated from the cultures of fluids aspirated from both sites. Treatment with amphotericin B failed to eradicate the bursal infection, and the patient underwent bursectomy of the left elbow.
Demographic Features, Comorbidities, and Possible Risk Factors
Among the 12 previously reported cases of Candida bursitis, the median age was 66 years (range, 32 to 77 years), and 7 patients (58%) were male (Table 5) (271, 285, 296–305). With underlying comorbidities, possible direct inoculation may have occurred in 8 cases (67%) through bursal and joint injections of corticosteroids in 4 cases (33%) and 2 prior orthopedic surgical procedures (17%).
TABLE 5.
Comparative analysis of demographics in osteoarticular mycoses
| Type of infection (reference[s]) | No. of cases | No. of male patients | Median age (yrs) | Age range (yrs) | No. of cases with immunosuppression (%) |
|---|---|---|---|---|---|
| Candida osteomyelitis (1; case in this report) | 208 | 165 | 30 | <1–88 | 47 (23) |
| Candida arthritis (2) | 112 | 69 | 40 | <1–84 | 41 (37) |
| Candida bursitis (292–294, 296, 297) | 12 | 7 | 66 | 32–77 | 8 (67) |
| Aspergillus osteomyelitis (550) | 180 | 127 | 48 | 1–87 | 103 (57) |
| Aspergillus arthritis (572; case in this report) | 32 | 28 | 50 | 1–83 | 27 (84) |
| Dimorphic fungal osteomyelitis (834) | 222 | 178 | 43 | <1–75 | 35 (16) |
| Non-Aspergillus mold osteomyelitis (1232) | 148 | 117 | 42 | 16–92 | 82 (55) |
| Osteoarticular mucormycosis | 35 | 23 | 41 | <1–73 | 13 (37) |
As possible risk factors, the majority of patients received systemic corticosteroids, local corticosteroids, or other forms of pharmacological immunosuppression. Six patients (55%) received systemic corticosteroids, and two received anti-TNF-α therapy. Four patients had an autoimmune disease (two with systemic lupus erythematosus [SLE] and two with rheumatoid arthritis [RA]), three had a neoplastic disease (two with solid tumors and one with chronic lymphocytic leukemia), two had trauma to the bursal site, one had diabetes mellitus, and one had hemodialysis. Another patient had Candida albicans peripheral thrombophlebitis and candidemia followed by hematogenous Candida subacromial bursitis. As a general comment comparing immunosuppression as a risk factor for osteoarticular mycoses caused by different organisms (Table 5), Aspergillus and possibly non-Aspergillus molds caused more osteoarticular infections in immunosuppressed patients than did Candida species.
Clinical Manifestations
The onset and development of symptoms of Candida bursitis were typically indolent. The most common localizing symptoms were pain (75%), edema (75%), erythema (50%), and limitation of movement (25%), whereas only 17% of the patients presented with fever. The distribution of Candida bursitis was predominantly in the upper extremities. The most frequent sites of Candida bursitis were olecranon (50%), shoulder (25%), and humerus (17%) bursae. Six patients had concomitant arthritis and/or osteomyelitis. Among the 12 cases, 2 patients (17%) had candidemia.
Laboratory Features
One Candida species was isolated from the synovial and/or bursal fluid in each of the 12 patients. Candida parapsilosis was the most frequently recovered species (n = 6), followed by C. albicans (n = 2), C. tropicalis (n = 2), C. glabrata (n = 1), and C. lusitaniae (n = 1). One patient had a blood culture that was positive for C. albicans. Gram staining of the fluid revealed yeast-like cells in 25% of cases. Synovial/bursal fluid white blood cell (WBC) counts displayed a wide range of distributions from mildly to highly elevated counts, with a differential neutrophil count ranging from 41 to 98%.
Among systemic markers of the inflammatory response, the WBC count was mostly within normal limits, at 6,810 (500 to 11,700) cells/mm3, while ESR and CRP were moderately elevated, at 79 (48 to 106) mm/h and 5.4 (1.2 to 6.7) mg/L, respectively (Table 3).
Diagnostic Imaging Studies
In patients with Candida bursitis, nonspecific soft tissue swelling of the bursae was the predominant radiological finding. However, extrabursal radiological manifestations also included joint effusion, bone destruction, and extension into soft tissues, followed by periosteal reaction/synovitis, decreased articular space, increased CT signal density, increased PET-CT uptake, as well as increased contrast-enhanced T1- and T2-weighted signals.
Pathogenesis
Direct inoculation was the predominant mechanism of infection in 8 cases (67%), possibly resulting from direct corticosteroid injection, direct traumatic implantation, or prior surgical contamination. Contiguous infection was another mechanism of infection in two patients in the setting of adjacent septic arthritis and/or osteomyelitis. Hematogenous Candida bursitis developed in the setting of antecedent candidemia in two cases (Table 6).
TABLE 6.
Comparative analysis of mechanisms of osteoarticular mycoses
| Type of infection (reference[s]) | No. of cases | No. of cases with mechanism of osteoarticular mycosis (%) |
||
|---|---|---|---|---|
| Hematogenous | Trauma/surgery | Contiguous | ||
| Candida osteomyelitis (1; case in this report) | 208 | 139 (67) | 51 (25) | 18 (8) |
| Candida arthritis (2) | 112 | 91 (81) | 21 (19) | 0 (0) |
| Candida bursitis (292–294, 296, 297) | 12 | 2 (17) | 8 (67) | 2 (17) |
| Aspergillus osteomyelitis | 180 | 80 (44) | 42 (23) | 58 (33) |
| Aspergillus arthritis (572; case in this report) | 32 | 17 (53) | 12 (38) | 3 (9) |
| Non-Aspergillus mold osteomyelitis (1232) | 148 | 38 (26) | 80 (54) | 30 (20) |
| Osteoarticular mucormycosis (739; cases in this report) | 35 | 1 (3) | 21 (60) | 13 (37) |
Septic bursitis mainly affected the olecranon and subacromial bursae. Trauma or orthopedic interventions predispose to a subcutaneous localization and may subsequently lead to infection. Most cases of septic bursitis are related to the patient’s daily activities (roofing, gardening, plumbing, and other causes of local trauma to the bursae), but surgical interventions (aspiration, intrabursal injection, and intra-articular injections of corticosteroids) are among other possible causes (297).
The role of corticosteroid injections as a possible vehicle for the introduction of Candida into the bursa warrants further discussion. As only 1 of 6 patients with Candida bursitis had concomitant candidemia, the route of infection of the remaining 5 patients may have been through direct inoculation via corticosteroid injection. The introduction of corticosteroids into the joint region would also compromise local innate host defenses and further increase the propensity for the localized proliferation of Candida within the tissue. Once introduced via corticosteroid injection, Candida could extend into surrounding bursal, joint, and osseous tissues.
That Candida parapsilosis was the most common etiological agent (50%) of Candida bursitis is also consistent with the pathogenesis of direct inoculation being the most common mechanism of infection. We hypothesize that as Candida parapsilosis colonizes the glabrous skin, especially of the hands, preferentially over C. albicans, this propensity for skin colonization is also compatible with contamination from procedures for corticosteroid injection or trauma. Further underscoring the role of the direct inoculation of C. parapsilosis into bursal tissue is the observation that C. albicans is the most common cause of Candida osteomyelitis (65%) and Candida arthritis (63%), while C. parapsilosis caused 7% of cases of Candida osteomyelitis and 11% of cases of Candida arthritis. The pathogenesis of Candida osteomyelitis and Candida arthritis in these studies was predominantly through hematogenous dissemination, in comparison to direct inoculation for Candida bursitis. Also supporting the hypothesis of the role of direct inoculation in the pathogenesis of Candida bursitis, only one patient (8%) had a blood culture that was positive for Candida species (C. albicans) in this series, while hematogenous dissemination occurred in 67% of cases of Candida osteomyelitis and 81% of cases of Candida arthritis (1, 2). This hypothesis of the pathogenesis of Candida bursitis should be tempered by the consideration of the small number of only 12 reported cases.
Treatment and Outcome
Candida bursitis was treated most commonly with a combination of antifungal therapy and surgery, with successful outcomes (Table 4). Surgical procedures included drainage (8 cases), debridement (6), bursectomy (4), irrigation (2), decompression (1), stabilization (1), and arthrodesis (1). Five patients received fluconazole, two received amphotericin B, and one each received caspofungin or the combination of fluconazole plus 5-FC. One patient had an initial induction of amphotericin B followed by an antifungal triazole, while another received caspofungin followed by fluconazole. For 7 patients for whom data were available, the median duration of antifungal therapy was 21 days (range, 14 to 70 days). Two additional patients who also had contiguous Candida osteomyelitis and septic arthritis were reported to have received indefinite courses of antifungal therapy.
For timely diagnosis and proper management, the differential diagnosis of pyogenic bursitis should include Candida bursitis. If clinical evaluation or MRI findings demonstrate concomitant arthritis, a diagnosis of Candida arthritis should also be considered. The duration of antifungal therapy was considerably shorter than that for Candida osteomyelitis and Candida arthritis (1, 2). Nonetheless, the presence of concomitant osteomyelitis or arthritis may justify longer durations of antifungal therapy. Antifungal therapy should be administered following the surgical procedure for suspected Candida bursitis to prevent local progression and disseminated candidiasis, especially in immunocompromised patients (298).
We recommend an echinocandin or lipid formulation of amphotericin B where feasible as initial therapy. Either initial treatment regimen could then be followed by fluconazole for ambulatory management. The duration of therapy should be individualized according to the clinical response but should be completed at least through the median duration of therapy of 21 days found in this series.
CANDIDA ARTHRITIS
Candida arthritis is a debilitating infection that is included in the broad differential diagnosis of septic and inflammatory arthritis. A review of cases and subsequently reported patients reveals demographic characteristics and clinical manifestations of Candida arthritis that are similar to those of bacterial arthritis (2).
History
The first reported case of Candida arthritis in 1967 (118) was an infection of the left hip and vertebral bodies associated with a fatal case of disseminated candidiasis of C. albicans arising from septic emboli in a 20-year-old female following open-heart surgery for aortic stenosis 4 months before the onset of clinical manifestations. Subsequently, more than 100 cases of documented Candida arthritis have been reported in Medline (11, 18, 19, 58–60, 66, 158, 183, 194, 195, 205, 207, 217, 218, 221, 223, 232, 250, 305–394).
Epidemiology
A previously published study of Candida arthritis (2) found that the median age for this infection was 40 years, with a wide range from <1 month to 84 years. The underlying conditions, which are typically those of disseminated candidiasis, include surgery, hematological malignancies, diabetes mellitus, solid-organ transplantation, trauma, open wounds, and hemodialysis. These patients were also receiving prior broad-spectrum antibiotics and may have had central venous catheters. However, most patients were not pharmacologically immunocompromised.
Pathogenesis
Candida arthritis is established in most cases (approximately 80%) through hematogenous dissemination. A smaller percentage (approximately 20%) develops following direct traumatic inoculation.
Clinical Manifestations
The majority of patients (>80%) complain of local pain and tenderness; regional edema is present in approximately 70%, while localized erythema occurs in fewer than 25%. A limited range of motion may occur in approximately 40% of patients. Fever is notably absent in most patients at the time of presentation of local symptoms.
Candidemia or another form of invasive candidiasis is a common early clinical manifestation of Candida arthritis. Indeed, Candida arthritis may emerge during the course of antifungal therapy in patients being treated for antecedent candidemia. Finally, Candida arthritis may present simultaneously with the development of candidemia in a minority (approximately 11%) of cases.
The greater diagnostic challenge is for those patients who present de novo with septic arthritis and no prior evidence of invasive candidiasis. This group constitutes approximately 26% of previously reported cases.
Candida arthritis presents in most patients as a monoarticular infection in approximately 70% of cases, consistent with the original case reported in 1967. In patients with Candida arthritis, the knee is the most frequently infected site (approximately 75%), as commonly observed for bacterial arthritis, followed by the hip and shoulder joints. Contiguous osteomyelitis is seen, with the femur, tibia, and humerus being the most commonly infected sites.
Candida septic arthritis has been reported to be present in atypical circumstances. For example, failure of primary antibacterial treatment of a patient with staphylococcal septic arthritis of a native hip joint was caused by the development of secondary Candida joint infection (381). Several reports describe patients suffering from rheumatoid arthritis or other autoimmune diseases with affected joints, which necessitated arthroplasty or the initiation of biological agents, which was further complicated by Candida arthritis (305, 387, 390, 392).
Diagnostic Approaches
A definitive diagnosis of Candida arthritis requires needle aspiration, open biopsy, or arthroscopic surgery for the acquisition of synovial fluid or tissue. Candida species are recovered in most patients with Candida arthritis via cultures of synovial fluid by needle aspiration. Less commonly, Candida may be identified by histopathology of infected synovial tissue or adjacent bone.
Diagnostic Mycology
The most common cause of Candida arthritis is C. albicans, occurring in approximately 60% of cases. The most frequently recovered non-albicans Candida spp. are C. tropicalis and C. parapsilosis.
Inflammatory Markers
Similar to that in Candida osteomyelitis, the median peripheral WBC count in Candida arthritis is only mildly elevated, with a range from neutropenia (<500 cells/mm3) to leukocytosis (>20,000 cells/mm3). The median ESR and CRP values in Candida arthritis tend to be moderately elevated but with a wide range. For example, in the case series reported by Gamaletsou et al. (2), the median ESR value was 56 mm/h, with a range from 10 mm/h to 118 mm/h, while the median CRP value was 28.9 mg/dL, with a range from 0.5 mg/dL to 141 mg/dL. The WBC count in the synovial fluid typically demonstrated neutrophilic leukocytosis. For instance, the median synovial WBC count in a series of cases of Candida arthritis was 27,500 cells/mm3, with a range from 100 cells/mm3 to 220,000 cells/mm3, with medians of 90% neutrophils, approximately 60 mg/dL of glucose, and 5 g/dL of protein.
Diagnostic Imaging
Among patients with Candida arthritis, the most frequently observed abnormalities by diagnostic imaging modalities are bone destruction and joint effusion with soft tissue extension, decreased articular space, periosteal reaction, synovitis, and underlying osteoarthritis.
Treatment and Outcome
Most patients with Candida arthritis are treated successfully with antifungal therapy alone (2). This study found that there was no significant difference between patients treated with antifungal therapy only and those managed with surgery plus antifungal therapy.
The types of antifungal agents reported to treat osteoarticular mycoses vary widely, from amphotericin B to triazoles to combination therapy, with no apparent advantage of a specific modality. Although there has been less experience with an echinocandin, we advocate for the initial administration of micafungin, caspofungin, or anidulafungin followed by an antifungal triazole as initial therapy, pending in vitro antifungal susceptibility data. Depending on the clinical response and susceptibility, fluconazole could be initiated for transition to ambulatory therapy. The median duration of therapy is approximately 2 months, with a wide range, including treatment durations of up to 1 year. A complete response is achievable in approximately 80% of patients with Candida arthritis, and a partial response is achievable in nearly 20%. Although relapses are not infrequent, they are usually related to inadequate durations of therapy. Reinitiation of antifungal therapy usually achieves a successful response.
Analogous to Candida osteomyelitis, one could consider that antifungal monotherapy with or without minimally invasive arthroscopic surgery for Candida arthritis may be sufficient for most patients to achieve a complete response with the proper duration of treatment. Monitoring of the therapeutic response with biomarkers and imaging may provide a more individual or personalized approach for guiding the duration of therapy.
In conclusion, in cases of septic arthritis, the clinician should have a high index of clinical suspicion to consider osteoarticular candidiasis when the patient does not respond adequately to the initial standard antibacterial treatment (381).
ASPERGILLUS OSTEOMYELITIS
Aspergillus osteomyelitis is a painful, immobilizing, and debilitating form of invasive aspergillosis. A comprehensive review of the literature (50, 51, 184, 222, 253, 395–549) indicated that most cases arise in immunocompromised patients, with Aspergillus osteomyelitis appearing as the initial manifestation of invasive aspergillosis in approximately 80% of cases. Aspergillus osteomyelitis of the ribs and vertebrae arises usually by invasion from adjacent foci of pulmonary aspergillosis. Spondylodiscitis is the predominant manifestation of vertebral aspergillosis. Approximately one-half of the reported cases progress to spinal cord compression. Cranial aspergillosis is a distinctly localized but potentially lethal infection. Successful outcomes can be achieved with antifungal therapy and individualized surgery.
History
The first case of Aspergillus osteomyelitis was reported in 1947 (395). Cawley described a 5-year-old child with a history of pleuritis of unknown origin who presented with a tender, fluctuant mass, approximately the size and shape of a child’s hand, located at the lower costal margin in the left anterior axillary line. A radiograph showed a localized periosteal lesion involving the sternal portion of the left seventh rib and underlying the soft tissue tumor. Culture of purulent material aspirated from the lesion on two occasions resulted in the growth of a mold, subsequently identified as A. fumigatus. Two years later, the child died from disseminated aspergillosis. Since that time, there have been more than 180 well-documented cases of Aspergillus osteomyelitis in the English literature.
Epidemiology
Aspergillus osteomyelitis occurs principally in immunocompromised patients. Most patients with Aspergillus osteomyelitis are immunocompromised as the result of corticosteroids, neutropenia, and primary immunodeficiency, most frequently chronic granulomatous disease (CGD). Patients are predominantly male. Pediatric patients, most of whom have CGD, constitute approximately 20% of cases. This finding alone warrants the consideration of chronic granulomatous disease in the differential diagnosis of underlying host defects for patients with unexplained Aspergillus osteomyelitis.
Prior orthopedic surgery was also an apparent predisposing factor for patients with Aspergillus vertebral osteomyelitis, while patients with Aspergillus cranial osteomyelitis had diabetes mellitus and/or prior head and neck surgery as risk factors. Prior thoracic surgery may be a risk factor for the development of sternal osteomyelitis. Aspergillus osteomyelitis most commonly presents de novo in patients who are not receiving antifungal therapy at the time of symptom onset.
Pathogenesis
Most cases of Aspergillus osteomyelitis arise from hematogenous dissemination, usually from a pulmonary source, in immunocompromised patients. The next most common mechanism of infection is contiguous infection from a pneumonic focus invading the ribs or vertebrae. Direct inoculation via trauma or prior surgery is another mechanism by which Aspergillus osteomyelitis is established.
Clinical Manifestations
Aspergillus osteomyelitis most commonly presents as pain and tenderness at the local osseous site. Fever is an infrequent manifestation of Aspergillus osteomyelitis. Purulent drainage and sinus tracts may be present in long bones infected by Aspergillus. Neurological and cranial nerve deficits are other clinical manifestations of Aspergillus osteomyelitis, caused by vertebral spinal cord compression and basilar skull infection, respectively.
The most frequently infected bones in Aspergillus osteomyelitis are the vertebrae, cranial bones, and ribs. The tibia is the most commonly infected long bone. With vertebral involvement, spondylodiscitis developed in nearly one-half of patients. Approximately one-half of patients with Aspergillus vertebral osteomyelitis suffer from spinal cord compression with neurological deficits. Reflecting its mechanism of hematogenous dissemination, Aspergillus osteomyelitis manifests as ≥2 nonadjacent infected bones in the majority of cases. Among patients with costal aspergillosis, the majority have a primary immunodeficiency, usually chronic granulomatous diseases, with a contiguous focus of invasive pulmonary aspergillosis.
Similar to the findings for Candida osteomyelitis, the osseous distribution of Aspergillus osteomyelitis reflects a distinct age-related pattern. As a reflection of primary immunodeficiency, especially CGD, infection of the ribs occurs more frequently in pediatric patients. Rib infection develops more commonly in this population as the result of contiguous pulmonary disease.
Cranial aspergillosis is a distinctive category of Aspergillus osteomyelitis. Patients suffer most frequently from a contiguous infection, including Aspergillus invasive externa otitis, otitis media, as well as mastoiditis. Other patients may have previous trauma or prior surgery that serves as a focus of contiguous infection.
Aspergillosis of the Ribs and/or Sternum
The two most common sites of infection for Aspergillus osteomyelitis are the ribs and/or sternum and the vertebrae plus ribs (550). Aspergillosis of the ribs and sternum occurs among children and adolescents in 36% of reported patients. This propensity for Aspergillus osteomyelitis of the ribs in pediatric patients is related mostly (57%) to the presence of a primary immunodeficiency, particularly chronic granulomatous disease. Among patients with a rib and/or sternal infection, an underlying primary immunodeficiency was present in nearly one-half (48%). Thoracic surgery was the other major risk factor for 30% of the patients, especially associated with the development of sternal osteomyelitis.
Diagnostic Approaches
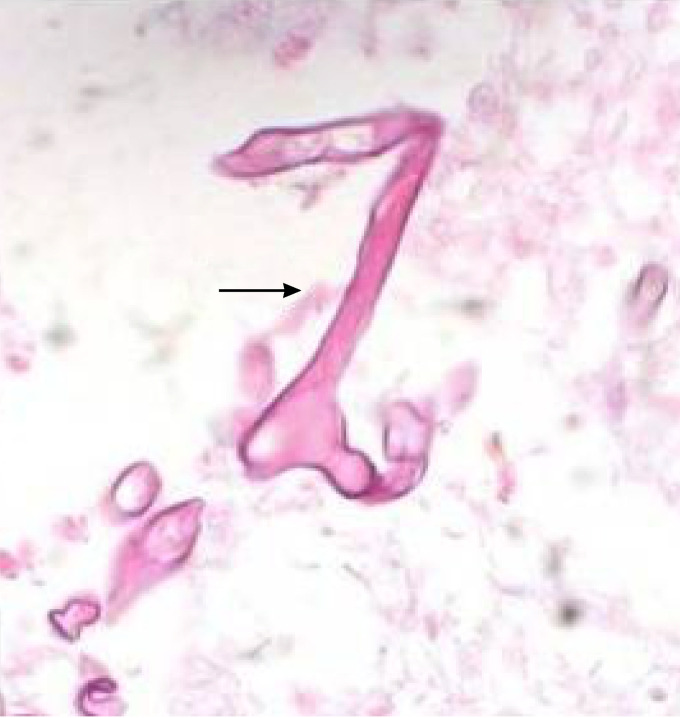
Diagnostic recognition of Aspergillus osteomyelitis is established preferably by open biopsy or percutaneous biopsy. The identification of Aspergillus may then be established by direct culture and/or histopathology of morphologically compatible organisms with angular dichotomously branching septate hyphae. As other septate molds may also histologically resemble Aspergillus spp. in tissue, a definitive diagnosis of Aspergillus osteomyelitis cannot be established by histology alone.
Laboratory Diagnosis
Aspergillus fumigatus, followed by Aspergillus flavus, is the most common species recovered from patients with bone infections. The serum galactomannan index (GMI) is elevated in only a minority of cases. Among 6 cases for whom the GMI was reported, only 1 had an increased GMI, which ranged from 5.6 to 6.3. This patient had chronic pulmonary aspergillosis complicated by Aspergillus fumigatus disseminated infection to C3-T2 spondylodiscitis, spondylolysis, and neurological deficits related to epidural abscess (50).
Among patients with Aspergillus osteomyelitis, bacteria and other fungi may be simultaneously cultured from the same focus of infection. Staphylococcus species are the bacteria most frequently cocultured with Aspergillus spp., whereas Pseudomonas aeruginosa and Klebsiella pneumoniae are among the less commonly recovered Gram-negative bacilli. Among other fungi, Cryptococcus neoformans and Candida parapsilosis may be recovered simultaneously.
Inflammatory Markers
C-reactive protein levels and ESRs are increased in most patients; e.g., the median CRP level was 86 mm/h, and the median ESR was 51 mg/dL. In comparison, the median WBC counts and absolute neutrophil counts are usually within normal limits in the majority of patients. Notable exceptions occur in patients with hematological malignancies who are neutropenic as well as those with primary immunodeficiencies and systemic corticosteroid use who may have leukocytosis (>10,000 cells/mm3).
Diagnostic Imaging
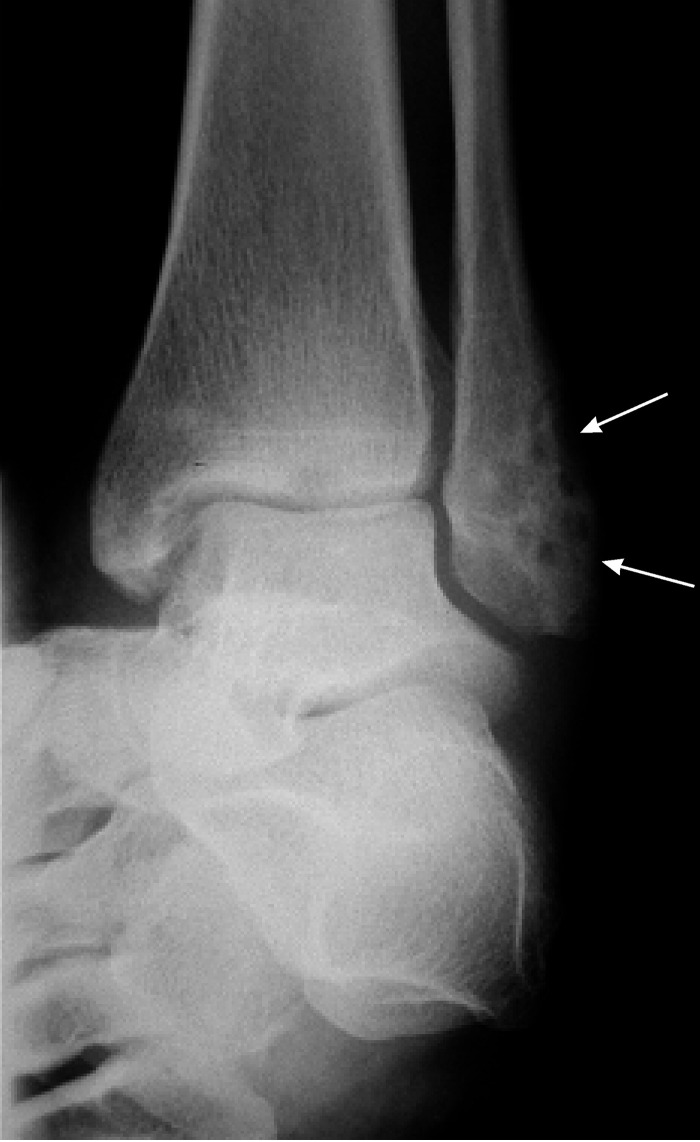
The vertebral bodies are the most frequently infected sites found in Aspergillus osteomyelitis. Osteolytic bone destruction, bony erosion, and extension into soft tissue are the most common radiological manifestations of Aspergillus osteomyelitis. Periosteal reactions and sequestra are less commonly observed. Aspergillus vertebral osteomyelitis may demonstrate decreased intervertebral space, spondylolisthesis, epidural or subdural abscesses, spinal cord compression, and paraspinal abscesses. Magnetic resonance imaging typically reveals increased signal intensities on T2-weighted imaging modalities and reduced signal intensities on T1-weighted modalities. The intensity of T1-weighted images may be enhanced by gadolinium contrast.
Treatment and Outcome
The majority (approximately 70%) of reported patients with Aspergillus osteomyelitis are managed with a combination of antifungal therapy and surgery. The remaining reported cases are managed with antifungal therapy only. Patients seldom undergo surgery without the need for antifungal therapy. The duration of medical therapy varies widely, from approximately 1 week to more than 2 years, with a median length of 3 months.
Surgical procedures usually consist of debridement or drainage. Vertebral osteomyelitis may be further treated by bone grafting and vertebral fusion to restore the stability of the spinal column. Spinal cord decompression is used in those with neurological deficits. Special surgical approaches to vertebral aspergillosis in these patients may include bone grafting, spinal stabilization, decompression, and intervertebral body fusion.
When comparing the outcome of antifungal therapy with surgery to that of antifungal therapy alone, the percentages of complete and partial responses at the end of therapy in the study by Gamaletsou et al. (550) are strikingly similar between the two groups (57% and 30% [n = 121] versus 52 and 27% [n = 44], respectively). These data raise an important question as to whether new strategies may be utilized to provide medical therapy alone as the primary option, reserving surgical intervention for advanced cases, including those with neurological symptoms. However, this approach must be tempered with the understanding that the relapse rate for patients treated with antifungal therapy only (30%) is significantly higher than that for patients managed with the combination of antifungal therapy plus surgery (8%). Relapses are typically due to the premature discontinuation of antifungal therapy. However, when antifungal therapy is reinstituted, most patients can be successfully treated.
Considering the optimal selection of an antifungal agent, the response rates for Aspergillus osteomyelitis seem to be similar for itraconazole, voriconazole, and amphotericin B. There are a few reported cases of posaconazole being used for the treatment of Aspergillus osteomyelitis (435, 438). Nonetheless, posaconazole may be especially useful in the treatment of Aspergillus osteomyelitis in cases where voriconazole is inducing adverse effects such as visual hallucinations or in situations where therapeutic serum concentrations of voriconazole are not achievable.
There are no reported cases of the use of isavuconazole for the treatment of Aspergillus osteomyelitis. Our own unpublished experience includes a patient successfully treated with isavuconazole for Aspergillus osteomyelitis of the clivus.
A more recent study published after the initial submission of the manuscript reported a series of 186 documented cases of Aspergillus osteoarticular infections (551). In assessing the overall therapeutic response, that study combined patient populations with Aspergillus osteomyelitis and Aspergillus arthritis. The report found that among 107 patients who underwent combined medical and surgical interventions versus 79 with medical therapy alone, the resolution rate was 70% versus 40% (P < 0.001). The study found that the rates of complete resolution were similar with amphotericin B (58%) and voriconazole (59%). The differences between the therapeutic outcomes analyzed in the studies by Gamaletsou et al. (550) and Tsantes et al. (551) may be related to the latter study combining cases of osteomyelitis and arthritis as well as the different definitions of a successful response.
The combination of an echinocandin and a mold-active triazole for the treatment of invasive pulmonary aspergillosis has been shown to be additive or synergistic in several preclinical studies (552) and to improve the outcomes in a randomized clinical trial of voriconazole plus anidulafungin (553). Although there are no preclinical or clinical studies that have systematically investigated the combination of a mold-active triazole and an echinocandin for the treatment of Aspergillus osteoarticular infections, we suggest that this combination may provide an optimal initial medical therapy for such diseases.
In conclusion, the management of Aspergillus osteomyelitis is typically focused on prolonged courses of antifungal therapy. Surgical intervention is usually required for complete resolution, especially in the setting of refractory Aspergillus osteomyelitis or infection due to Aspergillus nidulans or other poorly responsive molds (554).
ASPERGILLUS ARTHRITIS
History
The first well-documented case of septic arthritis caused by Aspergillus spp. was reported in 1976 (253). In this case, a 12-month-old boy with idiopathic aplastic anemia developed cutaneous aspergillosis on the leg, directly over the point of contact with a paper-covered board used to immobilize the leg for intravenous infusions. Over the next months, the lesion progressed to a necrotic ulcer, while radiographs showed a lytic lesion in the tibia underlying the ulcer. Cultures from both the skin and the bone grew A. flavus. Subsequently, multiple osteolytic lesions developed in the skull, humerus, and ribs, and arthritis was noted in the left knee. The patient died, and at autopsy, A. fumigatus was grown from the left-knee fluid.
Epidemiology
Since that first report in 1976, more than 30 well-described cases (5, 403, 406, 416, 438, 453, 463, 479, 484, 489, 502, 513, 526, 527, 555–571) of Aspergillus arthritis have been reported in the English literature. Among 31 cases of Aspergillus arthritis analyzed by Gamaletsou et al. (572), 27 patients (87%) were male. The median age of this entire population was 50 years, with a range from 1 to 83 years. The most common underlying conditions observed in patients with Aspergillus arthritis included orthopedic surgery, hematological malignancies, and solid-organ transplantation. Corticosteroid use and neutropenia were documented as possible risk factors in 12 (39%) and 6 (19%) patients with Aspergillus arthritis in that study, respectively. Among four pediatric patients with Aspergillus arthritis, a 12-month-old infant with acute leukemia developed cutaneous aspergillosis that disseminated to involve three joints and bones. Among the three children, two had acute lymphoblastic leukemia, and one had CGD.
Pathogenesis
The most common mechanism for the development of Aspergillus arthritis is hematogenous dissemination from an extrapulmonary site. Among the 31 patients previously reported, 16 (52%) had hematogenous dissemination as a mechanism for the development of Aspergillus arthritis. Direct inoculation was the second most common mechanism of infection, in 12 cases (39%), with contiguous inoculation in only 3 (10%). The preponderance of cases (>80%) of Aspergillus arthritis developed as a de novo infection.
Clinical Manifestations
Pain and tenderness over the infected joint are the most common clinical manifestations, occurring in nearly 90% of cases of Aspergillus arthritis. Other features that are typical of septic arthritis, such as fever, erythema, swelling, and decreased range of motion, occur less frequently (≤26%).
Approximately one-half of cases of Aspergillus arthritis manifest as a single-joint infection. The remaining cases of polyarticular infection consist of approximately one-quarter of patients demonstrating ≥3 joints involved as a disseminated process. The knee is the most commonly infected joint, followed by intervertebral discs (spondylodiscitis) and the hip. Consistent with the knee being the most commonly infected joint in Aspergillus arthritis, the tibia and femur are the most frequently infected adjacent bones.
Previously published cases from areas of endemicity of coinfection of the joint with Aspergillus spp. and Mycobacterium tuberculosis (571) or Aspergillus arthritis mimicking tuberculosis (TB) (568) underline the dynamic interplay between these two pathogens. Immunocompromised patients, particularly those receiving corticosteroids or TNF-α inhibitors, are at risk for the development of invasive aspergillosis or tuberculosis. Chronic pulmonary tuberculosis also commonly complicates the course of patients with cavitary pulmonary tuberculosis (573). Finally, reports of Aspergillus septic arthritis secondary to pulmonary aspergillosis (569) or otitis externa (570) in the immunocompetent host further expand the clinical spectrum of this disease.
Diagnostic Approaches
The diagnosis of Aspergillus arthritis is most commonly established by arthrocentesis. Open surgery or biopsy of an adjacent bone is an alternative or adjunct procedure.
Laboratory Diagnostic Mycology
Synovial fluid cultures grew Aspergillus spp. in the laboratory diagnosis of all 17 reported cases of Aspergillus arthritis. Culture of bone adjacent to a septic joint was also positive in 13 of the 14 reported cases. A culture of synovial fluid or adjacent bone in a patient with suspected septic arthritis should not be dismissed as being contaminated pending further clinical correlation.
Aspergillus fumigatus is the most frequently recovered species. A positive culture for Aspergillus spp. from the synovial fluid or bone specimen should not be considered contaminated until Aspergillus arthritis is completely excluded. While cytology of synovial fluid and histology of adjacent bone tissue, when available, should additionally be performed, these methods appear to be less sensitive than culture.
Biomarkers of Inflammation
The values of inflammatory biomarkers in Aspergillus arthritis have a wide range of distributions. The median values tend to demonstrate only mild to moderate elevations. For example, the median white blood cell count in the blood of the reported cases of Aspergillus arthritis was 9,700 WBCs/μL, with a median of 71% neutrophils. Similarly, the median erythrocyte sedimentation rate in the same series was also only moderately increased to 90 mm/h, while the median CRP level was 3.6 mg/dL.
The synovial fluid white blood cell count values also demonstrate a wide distribution, with the median showing only a moderate elevation of 17,200 WBCs/μL and a differential count of 61 to 92% neutrophils.
Diagnostic Imaging
Lytic bone destruction is the most common diagnostic imaging abnormality in Aspergillus arthritis. Adjacent osteomyelitis with extension into soft tissues occurs in approximately one-half of cases. MRI scans demonstrate increased signal intensities on T2-weighted modalities and decreased signal intensities on T1-weighted modalities. An illustrative case is shown in Fig. 2.
FIG 2.
Aspergillus osteomyelitis and arthritis. A 52-year-old male with lymphoblastic lymphoma and a history of pulmonary aspergillosis developed pain in the left hip. MRI showed a femoral head lesion and avascular necrosis. After another cycle of chemotherapy, he was admitted to the hospital with a worsening of chronic left-hip pain and limited range of motion. (Left) A plain radiograph showed bone destruction, osteolysis of the left femoral head, and a decrease in the left articular space. (Middle and right) MRI showed a 3.8- by 2.1-cm oval mass in the anterior medial inferior left femoral head with a low T1 intensity (middle) and a high signal intensity with T2 (right), extending into the neck of the femur, with a well-defined border. There was moderate left-hip effusion and abnormal T2 hyperintensity in muscles around the left hip. A left-hip aspirate smear demonstrated hyphae by microscopy and grew Aspergillus flavus in culture.
Treatment and Outcome
Aspergillus arthritis is a therapeutically challenging disease, with most patients undergoing protracted antifungal therapy and surgical debridement. In a series of Aspergillus arthritis cases (572), 19 cases (61%) were treated with antifungal therapy plus surgery, while 10 (32%) were managed with antifungal therapy only. Amphotericin B and itraconazole were the two most commonly used agents, while the use of voriconazole for the treatment of Aspergillus arthritis was reported in only 2 cases (7%). The median duration of treatment in this series was 219 days. Surgical interventions most commonly consisted of debridement and drainage. These approaches resulted in a complete response rate of 71% and a partial response rate of 16% of the 31 cases. Relapse may occur due to the inadequate duration of therapy. Aspergillus arthritis is associated with a mortality rate of 35% in relation to disseminated infection, skull-based osteomyelitis, and serious underlying diseases.
OSTEOARTICULAR HYALOHYPHOMYCOSIS
Epidemiology
Hyaline molds such as Scedosporium spp. and Fusarium spp. are commonly found worldwide in polluted waters, decaying organic material, contaminated produce, and soil. Fusarium spp. are also important plant pathogens. Respiratory sites (574–576) and integumentary sites are the two most common portals of entry (577). Localized osteoarticular infections in otherwise previously healthy patients result from the direct traumatic inoculation of fungal elements into bones or joints. In comparison, disseminated infections and infection of bones by the hematogenous route are more commonly observed in immunocompromised patients (578).
Cases of osteoarticular hyalohyphomycosis have been rarely reported from all over the world. The demographic characteristics of 80 patients were reviewed for the period from 1976 to 2017 (407, 574, 579–651), including 2 unpublished cases and 72 cases described previously (652). Among the 80 patients with bone and joint infections, male subjects predominated (85%) (Table 7). The mean age of patients with osteoarticular hyalohyphomycosis was 35.9 ± 21.4 years.
TABLE 7.
Demographic and clinical characteristics and anatomical distribution of cases of osteoarticular infections caused by hyalohyphomycosis and phaeohyphomycosise
| Parameter | Value for group |
P value | |
|---|---|---|---|
| Hyalohyphomycosis (n = 80) | Phaeohyphomycosis (n = 57) | ||
| Mean age (yrs) ± SD | 35.9 ± 21.4 | 35 ± 22.2 | 0.837 |
| No. of male patients (%) | 68 (85) | 41 (74.5) | 0.13 |
| No. of patients with underlying condition (%) | |||
| Diabetes | 16 (20) | 3 (5.6) | 0.019 |
| Trauma | 41 (51.9) | 26/54 (48.1) | 0.671 |
| Prior surgery | 20 (26) | 18 (33.3) | 0.361 |
| Prosthesis | 2/78 (2.6) | 1/54 (1.9) | 0.999 |
| Immunocompromised | 42/80 | 22/55 | 0.153 |
| Solid cancer | 1/76 (1.3) | 2/54 (3.7) | 0.57 |
| Hematological malignancy | 8/78 (10.3) | 2/54 (3.7) | 0.19 |
| Neutropenia (ANCf < 500 cells/mm3) | 5/77 (6.5) | 4/54 (7.4) | 0.999 |
| Solid-organ transplant | 6/78 (7.7) | 3/54 (5.6) | 0.737 |
| Chronic granulomatous disease | 4/78 (5.1) | 1/54 (1.8) | 0.029 |
| HIV/AIDS | 1/76 (1.3) | 3/54 (5.6) | 0.307 |
| Corticosteroid use | 17/77 (22.1) | 7/54 (13) | 0.11 |
| No. of patients with other medical conditions (%) | |||
| Total | 75 | 50 | |
| Road accident | 7 | 0 | 0.029 |
| Penetrating puncture/injection | 22 (29.3) | 16 (32) | 0.751 |
| History of laceration/wound/ulcer | 10 (13.3) | 3 | 0.188 |
| Traumatic injury/fall/fracture | 4 | 10 (20) | 0.011 |
| No. of patients with bone infected (%) | |||
| Total | 58 | 34 | |
| Vertebra | 17 (29.3) | 3 (8.8) | 0.443 |
| Foot | 14 (24.1) | 10 (29.4) | |
| Tibia | 8 (13.8) | 4 (11.8) | |
| Ilium/crest/wing | 1 (1.7) | 2 (5.9) | |
| Fibula | 0 | 2 (5.9) | |
| Femur | 3(5.2) | 4 (11.8) | |
| Cranium | 5 (8.6) | 2 (5.9) | |
| Pterygoid/mandibular (others) | 2 (3.4) | 1 (2.9) | |
| Sternum/ribs | 2 (3.4) | 1 (2.9) | |
| Humerus | 1 (1.7) | 0 | |
| Hand | 2 (3.4) | 3 (8.8) | |
| Ulna | 1 (1.7) | 0 | |
| Multiple bone infections | 1 (1.7) | 1 (2.9) | |
| No. of patients with joint involvement (%) | |||
| Total | 29 | 27 | |
| Knee | 18 (62.1) | 15 (55.5) | 0.443 |
| Osteoarthritis | 5 (17.3) | 5 (18.5) | |
| Othersa | 6 (20.6) | 7 (25.9) | |
| No. of patients with mechanism of infection (%) | |||
| Total | 73 | 48 | |
| Direct inoculation | 39 (53.4) | 30 (62.5) | 0.614 |
| Hematogenous | 21 (28.8) | 11(22.9) | |
| Contiguous | 13 (17.8) | 7 (14.6) | |
| No. of patients with clinical symptom (%)b | |||
| Total | 80 | 57 | |
| Pain/tenderness | 59 (73.7) | 36 (63.1) | 0.185 |
| Swollen | 27 (33.7) | 25 (43.8) | 0.230 |
| Drainage/sinus/abscess | 23 (28.7) | 12 (21) | 0.310 |
| Erythema/cellulitis | 17 (21.25) | 5 (8.77) | 0.050 |
| Restricted movement | 47 (58.8) | 28 (49.1) | 0.265 |
| Warmness | 4 (5) | 2 (3.5) | 0.714 |
| Fatigue/wt loss | 3 (3.7) | 2 (3.5) | 0.962 |
| Neurological deficits | 9 (11.25) | 2 (3.5) | 0.179 |
| Impaired vision/blindness | 1 (1.2) | 1 (1.75) | 0.998 |
| Fever | 24 (30) | 16 (28.1) | 0.807 |
| Mean inflammatory marker value ± SD (no. of patients) | |||
| CRP (mg/dL) | 62.58 ± 76.18 (23) | 58.14 ± 44.218 (15) | 0.840 |
| ESR | 76 ± 34.78 (28) | 86.6 ± 37.65 (17) | 0.340 |
| WBCs (cells/mm3) | 11,025.9 ± 6,491.8 (27) | 8,109 ± 4,042.8 (11) | 0.176 |
| Treatment(s) and outcome | |||
| Total no. of patients | 79 | 57 | |
| No. of patients with type treatment(s) (%) | |||
| Medical and surgical | 50 (63.3) | 43 (75.4) | 0.552 |
| Medical | 20 (25.3) | 11 (19.3) | |
| Surgical | 3 (3.8) | 3 (5.2) | |
| Mean diagnostic delay (days) ± SD | 206.3 ± 301 | 156.3 ± 242.8 | 0.352 |
| Mean duration of treatment (days) ± SD | 189.12 ± 180.7 | 196.46 ± 174.9 | 0.834 |
| No. of patients with complete response/total no. of patients (%) | 56/74 (75.7) | 47/55 (85.5) | 0.171 |
| No. of patients with partial response (%) | 12 (15.6) | 4 (7) | 0.149 |
| Crude mortality [no. of patients (%)] | 6 (5.6) | 4 (7) | 0.898 |
| Attributable mortality [no. of patients (%)] | 3 (3.8) | 1 (1.7) | 0.882 |
| No. of patients administered antifungal agent (%) | |||
| Total | 72 | 53 | |
| Amphotericin B | 29 (40.2) | 19 (35.8) | 0.615 |
| Voriconazole | 20 (27.7) | 15 (28.3) | 0.949 |
| Other azoles | 11 (15.3) | 4 (7.4) | 0.189 |
| Others | 12 (16.66) | 15 (28.3) | 0.118 |
| Drug in association | 16 (20.8) | 22 (39.3) | 0.020 |
| Switch therapy | 19 (26) | 13 (23.6) | 0.814 |
| No. of patients with surgical intervention (%) | |||
| Total | 55 | 45 | |
| Laminectomy/discectomy | 5 (9) | 3 (6.66) | 0.948 |
| Debridement | 16 (29) | 19 (42.22) | |
| Amputation | 9 (16.3) | 4 (8.88) | |
| Drainage | 5 (9) | 5 (11.11) | |
| Excision | 4 (7.27) | 5 (11.11) | |
| Bone grafting/autotransplantation/fixation | 4 (7.27) | 2 (4.44) | |
| Joint proceduresc | 11 (20) | 6 (13.33) | |
| Insertion of prosthesis | 1 (0.2) | 1 (2.22) | |
| No. of patients with radiological feature (%) | |||
| MRI | |||
| Total | 48 | 36 | |
| Osteolytic lesion | 18 (37.5) | 15 | 0.998 |
| Compression | 2 (4.16) | 1 | |
| T1 low intensity | 6 (12.5) | 4 | |
| T2 enhancement | 10 (20.8) | 6 | |
| Effusion/fluid collection | 3 (6.25) | 3 | |
| Abscessd | 7 (14.6) | 5 | |
| Discitis | 1 (2.1) | 1 | |
| Malformation | 1 (2.1) | 1 | |
| Conventional radiography | |||
| Total | 42 | 30 | |
| Osteolytic destruction | 28 (66.7) | 15 (50) | 0.977 |
| Lucency | 4 (9.5) | 4 (13.3) | |
| Soft tissue swelling | 5 (11.9) | 5 (16.7) | |
| “Honeycomb” appearance | 1 (2.4) | 2 (6.6) | |
| Necrosis | 1 (2.4) | 1 (3.3) | |
| Decreased space | 2 (4.8) | 2 (6.6) | |
| Effusion | 1 (2.4) | 1 (3.3) | |
Metacarpophalangeal (n = 1), hip (n = 4), ankle (n = 5), cuneiform (n = 1), sternoclavicular (n = 1), and elbow (n = 1).
Some cases had more than one symptom.
Arthrotomy/synovectomy/arthrocentesis/arthroscopy/aspiration.
Epidural, paraspinal, psoas, and paravertebral.
See reference 1232. All data are expressed in absolute numbers (percentages) unless otherwise specified. Yates-corrected and Fisher’s exact chi-square tests were applied for low expected frequencies. The total numbers in several categories may be smaller than the total number of cases due to incomplete information in individual published reports.
ANC, absolute neutrophil count.
Bone and joint infections may occur among otherwise healthy individuals, usually following penetrating trauma after an injury or a road accident (581, 583, 586, 591, 594, 598, 600, 613, 623, 624, 627, 634, 640) and prior surgery (582, 597, 643), while severely immunocompromised patients represent 52.5% of the population with hyalohyphomycosis (Table 7), including those with hematological malignancies (574, 599, 611, 616, 632); chronic granulomatous disease (592, 617, 620, 653); solid-organ transplantation (589, 642, 644, 650); bone marrow transplantation (638), especially when associated with neutropenia; and HIV (615). The number of diabetic patients with hyalohyphomycosis of the bones and joints was significantly higher (20%) (P = 0.019) than the number of patients with phaeohyphomycosis (5.6%) (Table 7).
Pathogenesis
Diseases caused by hyaline fungi are clinically classified as hyalohyphomycosis. The presence of hyaline hyphae devoid of melanin in the cell wall in tissue is a characteristic feature of this disease. Osteoarticular infections in apparently immunocompetent patients develop following traumatic inoculation with tissue injury. In comparison, infection in immunocompromised patients occurs in the setting of an impaired qualitative or quantitative neutrophil response to these fungi.
Risk factors for infection are the same as those described for other opportunistic fungi and include significant exposure to pathogens, severe and prolonged immunosuppression, and organ dysfunction.
The organisms causing osteoarticular hyalohyphomycosis are phylogenetically varied and include Fusarium species (574, 579–581), Scedosporium spp. (599–601, 605), Acremonium spp. (638–640), Paecilomyces spp. (653), Phialemonium spp. (633, 653), and Chrysosporium species (620). The descriptive term “hyalohyphomycosis” describes a fungal disease where septate hyphae devoid of pigment within their cell wall are identified in tissue. This term serves as a counterpart to “phaeohyphomycosis,” where pigmented septate hyphae are observed in tissue. Other agents of hyalohyphomycosis include Aspergillus species (the disease caused by this pathogen is described above).
Infection occurs mostly because of the traumatic inoculation of a saprobic hyaline fungus into host tissue. The infection progresses, slowly leading to osteoarticular disease, which is an indolent form of infection. The most common and important hyaline fungus involved in bone and joint infections is Scedosporium apiospermum, comprising 33 (41%) cases of the total hyalohyphomycosis bone and joint infections. Other fungal genera occasionally causing bone and joint infections are Fusarium spp. (n = 16 [20%]); some species are common plant pathogens, and Fusarium solani is the species most often associated with osteoarticular human infections. Five cases (6%) of Acremonium species infections were reported to cause vertebral osteomyelitis, septic arthritis, and osteomyelitis of the lower tibia. Paecilomyces variotii has been reported in a single case of a chronic granulomatous disease patient (653).
Clinical Presentation
Hyalohyphomycetes can infect a wide variety of human bones and joints in immunocompromised and immunocompetent patients, with no real preference for the site of infection. These organisms caused 14% of cases of tibial osteomyelitis (591, 622, 643, 649), 5% of cases of osteomyelitis of the femur (595, 635), and tibiofemoral osteomyelitis with septic arthritis after a penetrating wound or knee injury (586, 598, 607, 613, 624, 627, 628, 636, 637) in healthy hosts. Osteomyelitis of the foot occurs mostly in diabetic foot or following a penetrating injury (582–585, 603, 618, 632); it may cause painful restricted movement or may progress to amputation following persistent osteomyelitis with F. solani in a patient with diabetes (584). An illustrative case of Fusarium osteoarticular infection in a patient with hematological malignancy is shown in Fig. 3. Hematogenous dissemination to bones in adults is less common and, when it does occur, usually leads to vertebral infection. Vertebral osteomyelitis constitutes 29% of bone and joint infections by hyaline fungi and may occur in immunocompromised and immunocompetent individuals (588, 591, 601, 604, 605, 608–611, 619, 626, 646, 651).
FIG 3.
Fusarium osteomyelitis of the head of the 4th and 5th metacarpal bones with soft tissue involvement and septic arthritis of the 4th and 5th metacarpophalangeal (MCP) joints. A 68-year-old leukemic male presented with left-shin ulceration and multiple subcutaneous nodules while on posaconazole prophylaxis after two cycles of chemotherapy for acute myelogenous leukemia. Skin biopsy specimen and blood cultures grew Fusarium spp. He then developed pain/swelling/tenderness of the left 5th MCP joint and left metacarpal. MRI showed bony destruction of the head of the 5th metacarpal and soft tissue involvement with extension into the left 5th MCP. Intraoperative findings were consistent with osteomyelitis and septic arthritis.
Mechanisms of infection most commonly include direct inoculation following trauma or instrumentation (53%) in nonimmunosuppressed individuals, followed by hematogenous dissemination in 29% of the cases, mostly in immunocompromised patients, whereas the rate of contiguous infections has been reported to be 18%. Mechanisms of direct inoculation include penetrating wounds, prior surgeries, motor vehicle accidents, and work injuries, whereas the risk factors for hematogenous dissemination are hematological malignancies, chronic granulomatous disease, and, occasionally, diabetes mellitus and cystic fibrosis.
Laboratory Features
The lack of laboratory serological tests and specific radiological findings may result in a delayed diagnosis of hyalohyphomycosis. As the “gold standard” is the isolation of the etiological agent from bone tissue and joint fluids, culture identification is essential due to similarities between hyalohyphomycosis and aspergillosis in histopathological sections. In addition, hyalohyphomycosis can be differentiated from phaeohyphomycosis by staining with Fontana-Masson stain, which detects the hyphal elements of melanized fungi in histological sections.
The proper approach for diagnosing osteomyelitis caused by hyalohyphomycosis is bone biopsy for culture and histopathology sections, which consist of infected fragments submitted to a microbiology laboratory for fungal culture and embedded in paraffin. The embedded tissue is used for the preparation of histological sections. The fungal etiological agent and tissue can be stained with periodic acid-Schiff (PAS), hematoxylin and eosin (H&E), and Gomori methenamine silver (GMS) stains. Features of chronic inflammation, necrosis, and hyphal elements can be visualized in tissue samples stained by using H&E, GMS, and PAS stains. A combination of histological, H&E, PAS, and GMS stains may be used to confirm both the tissue reaction and the fungal etiology. A specimen stained by H&E shows mixed chronic inflammatory changes and advanced bone tissue necrosis. In the sections stained with GMS and PAS stains, fungal hyphae invading bone tissue with fungal balls were observed (Fig. 4). Fungal hyphae were scattered throughout the tissue; these were septate and branched at 45° angles, a feature characteristic of Scedosporium species.
FIG 4.
Hyalohyphomycosis-related osteomyelitis. Shown is the histopathology of the left lateral malleolus depicted in Fig. 6 in a patient with osteomyelitis caused by Scedosporium spp. (A) Gomori methenamine-stained fungal balls and fungal hyphae branched at 45° invading bone tissue (magnification, ×400). (B) Periodic acid-Schiff-stained fungal balls with peripheral zonation and septate hyphae (magnification, ×400).
Fusarium is another opportunistic mold that can easily be recovered from a clinical specimen. The morphology of hyphae in histological sections is similar to those of Aspergillus and Scedosporium hyphae (654). Yeast-like structures and fungal septate and branched hyphae at 45° angles are morphologically compatible with Fusarium species in resected clinical specimens (Fig. 5) (647).
FIG 5.
Hyalohyphomycosis-related septic arthritis. Shown is a histological section of the articular cartilage within a lesion containing multiple septate hyaline hyphae from a trans-metatarsal amputation in a patient with Fusarium osteoarticular infection of the left foot, some of which are indicated by arrows (H&E staining) (magnification, ×600). (Reproduced from reference 647 with permission from Oxford University Press.)
Inflammatory markers in osteoarticular mycoses were elevated, with mean CRP and ESR values of 62.6 ± 76.2 mg/dL and 76 ± 34.8 mm/h, respectively. In comparison, the WBC values in these patients were only slightly elevated (11,026 ± 6,492 cells/mm3) (Table 7).
Diagnostic Imaging
Plain radiographs have limitations due to their low sensitivity and specificity for the detection of acute osteomyelitis (655). However, they remain the first imaging procedure for suspected bone infection. Plain radiographs are also useful for assessing the progression of the disease by comparing changes seen on follow-up films with the initial radiograph (656). Plain radiographs showed osteolytic lesions/destruction and bone erosion in 66.7% of the cases (574, 581, 584, 595, 603, 604, 606, 618, 628, 635, 637, 640, 641, 643, 651, 657). Lucency (4%) and soft tissue swelling (5%) may also be evident upon conventional radiography (624, 628, 636).
A plain radiograph of the left lateral malleolus with osteomyelitis due to Scedosporium apiospermum showed osteolytic lesions in a 57-year-old immunocompetent male patient (Fig. 6).
FIG 6.
Hyalohyphomycosis-related osteomyelitis. A plain radiograph shows osteolytic lesions of the left lateral malleolus in a patient with Scedosporium osteomyelitis (arrows).
MRI is the modality of choice for the diagnosis of osteomyelitis because of its high sensitivity for the detection of early infection and minimal ionizing radiation. The MRI findings in patients with hyalohyphomycotic osteomyelitis (Table 7) were osteolytic lesions in 37.5% of the cases (592, 596, 601, 610, 611, 614, 632, 658). MRI showed low signal intensities on T1-weighted images and patches of high signal intensity on T2-weighted images in a few cases (599, 601, 604, 614, 632, 651).
In a 45-year-old male with vertebral osteomyelitis following the Japanese tsunami, T2-weighted MRI showed a high-signal-intensity area at the L3-L4 intervertebral disc. In a case of left-knee osteomyelitis in a 27-year-old immunocompetent female after the Indonesian tsunami of 2004 (598), MRI of the left knee showed a progressive signal alteration of bone with ovoid osteolytic areas (Fig. 7).
FIG 7.
Scedosporium osteomyelitis. An MRI coronal T2 image shows the progressive signal alteration of the tibial cancellous bone in the metaphyseal region (black asterisk) associated with an abnormal cystic lesion (grains) in the soft tissue (white arrow) from an immunocompetent patient with tsunami-related Scedosporium apiospermum osteomyelitis. (Reproduced from reference 598 with permission from the International Society for Infectious Diseases.)
Treatment and Outcome
Bone and joint infections caused by Fusarium and Scedosporium are difficult to diagnose and often refractory to conventional antifungal treatment, particularly in immunosuppressed patients, where recovery from immunosuppression is a critical factor for a favorable outcome. In comparison, these infections in immunocompetent patients can be treated by systemic antifungal therapy and surgical debridement, with favorable prognoses in most cases. Considering the optimal treatment strategy for patients with osteoarticular hyalohyphomycosis, we understand that there are limitations due to the lack of clinical trials and a relative paucity of published case reports (581, 582, 598, 599, 649, 650, 657).
Various antifungal agents that may be effective against bone and joint infections are available. Both amphotericin B and voriconazole are available in i.v. formulations for seriously ill patients and constitute the mainstays of therapy for osteoarticular fungal infections in severely ill patients. Most patients have been treated with amphotericin B (n = 29 [40%]), voriconazole (n = 20 [28%]), or combination antifungal therapy (n = 16 [21%]) (Table 7). The variability in the antifungal susceptibilities of hyalohyphomycetous fungi requires laboratory testing against most active antifungal drugs as a guide for directed therapy. However, in the European Society of Clinical Microbiology and Infectious Diseases (ESCMID)-European Confederation of Medical Mycology (ECMM) joint guidelines for the treatment of invasive infections caused by Fusarium and Scedosporium species, the first-line treatment is voriconazole followed by amphotericin B (659).
A review of previously published global guidelines for the diagnosis and management of rare mold infections found that the response rates in patients treated with combination therapy with voriconazole and amphotericin B were not significantly different from those in patients treated with monotherapy (659). Although initial empirical therapy using both agents, pending the determination of in vitro susceptibility profiles, is a common clinical approach, selection of the single most active agent is recommended for the treatment of osteoarticular hyalohyphomycosis, especially that caused by Fusarium spp.
The duration of therapy is generally determined by the achievement of complete eradication of the infection and ranges from several weeks to several months or longer, with a mean value of 189 ± 181 days.
A complete response was achieved in 56/74 (76%) patients, and 12 (16%) patients had a partial response. The diversity of the pathogens and hosts makes it impossible to find an optimal therapeutic strategy; amphotericin B was the therapeutic agent in 19 (36%) patients, and voriconazole was the agent in 15 (28%). These were the most commonly used drugs for the treatment of osteoarticular infections due to hyalohyphomycosis.
Debridement was the most common surgical intervention (n = 16 [29%]), followed by amputation (n = 9 [16%]) and laminectomy/discectomy and drainage (n = 5 [9% each]), whereas multiple procedures were performed in 11 (20%) patients. The overall mortality rate was 5.6%. Irrespective of the antifungal treatment, death was attributed to osteoarticular hyalohyphomycosis in 3 (3.8%) patients.
OSTEOARTICULAR PHAEOHYPHOMYCOSIS
Epidemiology
Phaeohyphomycosis is defined as an infection caused by a heterogeneous group of dematiaceous or darkly pigmented fungi. These organisms are characterized by darkly pigmented hyphae due to the presence of melanin within their cell walls. Melanin is considered a virulence factor of these fungi. Their conidia are commonly detected during outdoor air sampling (660). These uncommon fungi may infect both immunosuppressed and immunocompetent individuals (661). The rate of human infections caused by dematiaceous fungi increases in warmer climates and lower latitudes (662). Fungi such as Rhinocladiella (formerly Ramichloridium) mackenziei are geographically restricted to the Middle East and are known to cause fatal cerebral infections (663). In 2010, the first case of Rhinocladiella infection outside the Middle East was reported (664).
Phaeohyphomycosis is increasingly being diagnosed in a wide diversity of patients, such as transplant recipients in relation to prolonged immunosuppressive therapy (665). These fungi accounted for 2.6% of all fungal infections, particularly in hematopoietic cell transplant (HCT) and solid-organ transplant (SOT) recipients (662). The development of these infections may be caused by the inhalation of conidia or by inoculation from an adjacent inapparent traumatic surface.
Although rare, these infections are increasingly being seen in a variety of clinical syndromes in both immunocompromised and healthy individuals. Transplant patients are especially at risk due to their prolonged immunosuppression (665). In the Transnet database, phaeohyphomycosis accounted for 2.6% of all fungal infections seen, and the cases were evenly divided between HCT and SOT patients (662). Exposure is thought to be via inhalation or minor trauma, which may not even be initially noticed by the patient. Since these fungi are widespread in the environment, individuals are constantly exposed to them, although they rarely cause disease.
As the number of immunocompromised patients increases, additional species are being reported as causes of human disease, creating a long list of potential pathogens (666, 667). Trauma is the main cause of osteomyelitis due to melanized fungi (668) and other non-Aspergillus filamentous fungi (652). Cases are reported from all over the world and are generally associated with minor trauma or other environmental exposures (669). Most osteoarticular infections due to these unusual fungi are rather seen in apparently immunocompetent patients. In a review comprising 55 cases of osteoarticular infections caused by melanized fungi (582, 666, 670–711), 79.2% of the patients were immunocompetent, and most of the infections (52%) were caused by the direct inoculation of the pathogen into the host. It seems that localized infections presenting as septic arthritis and osteomyelitis are more common in immunocompetent patients. Risk factors in immunocompetent patients were direct inoculation secondary to trauma/open fracture (670, 673, 679, 682, 701) and penetrating injuries (671, 672, 679, 681, 682, 685, 686, 690, 691, 696, 702, 703, 711, 712).
Phaeohyphomycosis has been related to inoculation injuries in association with steroid injections; hydrocortisone and local anesthetic injections provide a portal of entry and a locally immunodeficient environment for the infection to develop, as with cases of septic arthritis and osteomyelitis due to Lomentospora prolificans (711). Such infections may occur months to years (685, 705) after the injection procedure. Local immunosuppressive effects associated with invasive steroid treatments such as hydrocortisone and local anesthetic injections may increase susceptibility to these infections (713).
Immunosuppressed patients are especially vulnerable to osteoarticular phaeohyphomycosis, including solid-organ transplant (676, 694, 710) or bone marrow transplant (674, 714) recipients. Other susceptible subjects are neutropenic patients (689), individuals with autoimmune diseases such as chronic granulomatous disease (692), and HIV/AIDS patients (706, 709).
Pathogenesis
Fungi causing phaeohyphomycoses are characterized by the presence of dark hyphae of the etiological agent in human tissue (668, 715). Such conditions are observed in superficial cutaneous and deeply invasive and disseminated infections. Clinical characteristics of cutaneous and subcutaneous lesions may vary with the immune status of the patient (716). The majority of cases are reported as subcutaneous phaeohyphomycosis, including mycetoma, which may be caused by a wide range of dematiaceous fungi (668, 669). The portal of entry is the skin because of traumatic inoculation of environmental fungi that progress deeply into the subcutaneous tissue. The disease is indolent and progresses slowly in immunocompetent patients, leading to osteoarticular infection (652).
Disseminated disease may occur in immunosuppressed individuals but also may arise in patients without apparent immune dysfunction (717). In the latter context, underlying immunodeficiencies, including CARD9 mutations, should be suspected. The most common and important melanized fungus involved in bone and joint infections was L. prolificans (46.4%), comprising 26 cases of total bone and joint phaeohyphomycosis; 85% of these infections were in individuals with no apparent immune dysfunction. Other fungal genera occasionally causing bone and joint infections were Alternaria alternata, Myceliophthora thermophila, Phialophora richardsiae, and Cladophialophora bantiana.
The pathogenic mechanisms by which phaeohyphomycetes can cause disease are not well defined. Invasive diseases caused by these opportunistic fungi are considered uncommon in immunocompetent individuals. Melanin within the cell wall is considered to be an important virulence factor of dematiaceous fungi. The disruption of specific genes responsible for melanin production leads to significantly reduced virulence in animal models (718, 719). Cell wall melanin may protect fungi against host responses by scavenging free radicals and hypochlorite ions that are produced by phagocytic cells in their oxidative burst (720). The functional loss of the immunoregulatory role of the CARD9 protein due to gene mutations has been reported to be associated with an increased risk of disseminated infection caused by Exophiala species in two otherwise healthy adults (721).
Clinical Presentation
Deep infections by phaeohyphomycetes are life-threatening and may affect any organ. In particular, phaeohyphomycosis encompasses many clinical osteoarticular syndromes due to a wide diversity of dematiaceous fungi (687, 690, 695, 706, 714). A variety of bone and joint infections can be seen with these fungi; tibial and femoral osteomyelitis (671, 677, 679, 688, 690, 692, 710) may cause painful restricted movement and discomfort or swelling and discharge. Penetrating trauma or multiple injuries may progress to septic and joint arthritis, particularly with infections due to certain species, such as L. prolificans (676, 678, 682, 703). These infections may result in extremity amputation after knee arthritis due to penetrating trauma (682). Infection may result in amputation due to persistent osteomyelitis in renal and bone marrow transplant patients (710, 714). Patients may also experience fever as in spondylodiscitis and vertebral osteomyelitis (675, 706). Most patients who developed osteoarticular infections were subjected to direct inoculation (65%) because of injury, penetrating trauma, history of laceration, puncture, fracture after an accident, and prior surgery (670–673, 678, 679, 681–683, 685, 686, 690, 691, 696, 698, 702), whereas hematological dissemination occurred in 17.5% of patients, particularly in bone marrow transplant (674), renal transplant (676, 694), chronic granulomatous disease (692), and HIV/AIDS (695, 706) patients. Other groups of patients were infected through the contiguous route (17.5%), mainly after a surgical procedure infection (675, 680, 684, 697, 700).
Markers of inflammation were also detectable in patients with osteoarticular phaeohyphomycosis. The CRP level was moderately elevated, with a median of 43 mg/dL (range, 2.8 to 134 mg/dL), compared to the ESR, with a median of 90 mm/h (range, 10 to 150 mm/h), whereas the WBC counts were determined to be within the normal range, with a median value of 7,850 WBCs/μL (range, 3,500 to 15,500 WBCs/μL).
Laboratory Features
The laboratory diagnosis of phaeohyphomycosis relies on culture and pathological examination of clinical specimens. An illustrative case of phaeohyphomycete hyphae is shown in Fig. 8. In routine diagnostics, these fungi are characterized by the presence of dark hyphae in the infected tissue (668).
FIG 8.
Phaeohyphomycotic osteomyelitis. Shown are hyphae with intercalary and terminal chlamydoconidium swellings as seen by the direct preparation of infected tissue using Blankophor P fluorescent stain (magnification, ×400).
Growth in cultures occasionally requires the expertise of a clinical mycologist for unusual or newly described pathogens. Bone and tissue biopsies are the gold standard for determining proven cases of infection by the visualization of darkly stained hyphae, which are characteristic of phaeohyphomycosis. Phaeohyphomycete hyphae are characterized by chlamydoconidia as terminal or intercalary swelling structures, which are evident using the Blankophor P fluorescence staining technique (Fig. 8). In another report, numerous swollen cells with hyphae constricted at the septa could be observed in histopathology preparations (654). Fontana-Masson stain, which detects melanin, may confirm the presence of dematiaceous hyphae in histopathological sections (722).
Culture morphology and direct microscopy are the most common diagnostic procedures for fungal identification. PCR methods are not routinely available to identify these fungi to the species level. However, with the use of molecular typing methods for pathogenic phaeohyphomycetes, the number of recognized species and genera has increased markedly (667). These methods identified eight nonsporulating clinical isolates to the species or genus level by a combination of internal transcribed spacer (ITS) and D1/D2 typing (723). The use of antigen-based tests has not been very valuable, with variable results. Antigen tests used for Aspergillus and Candida, such as galactomannan and β-d-glucan, may occasionally be cross-reactive with this group of fungi, but this is not consistent (724, 725).
Diagnostic Imaging
Radiological abnormalities can be viewed by different radiological techniques; plain radiographs showed osteolytic lesions/destruction and bone erosion in 25% of the cases (676, 677, 679, 681, 682, 685, 686, 688, 694, 701–703, 726); lucencies were diagnosed to a lesser extent (678, 684, 705, 727). In adult individuals, vertebral compressions were evident on MRI in a 42-year-old female patient with vertebral osteomyelitis who became neutropenic following one cycle of chemotherapy (704). Postoperative spondylodiscitis with epidural abscess has been reported in a 62-year-old female (675). Osteolytic lesions and bone destruction after vertebral osteomyelitis were seen in a 53-year-old female bone marrow transplant recipient (674), and an increase in radionuclide uptake was seen in a 6-year-old child (682) and in adult patients (691, 703, 706). MRI showed decreased signal intensities on T1-weighted images (685, 696, 697) as well as increased signal intensities on T2-weighted images (672, 673, 691, 697).
Treatment and Outcome
While there are no randomized controlled trials or standardized therapeutic approaches for the management of osteoarticular infections caused by phaeohyphomycetes, guidelines sponsored by the ESCMID, the ECMM, and the International Society for Human and Animal Mycology (ISHAM) provide evidence-based strategies for the diagnosis and treatment of these diseases (728, 729). Although antifungal susceptibility testing is standardized for some molds, there is a paucity of dematiaceous molds for which robust epidemiological cutoff data are available. Moreover, there are no interpretive breakpoints for any antifungal agents or correlative clinical outcome data for these organisms. Antifungal triazoles, including itraconazole, voriconazole, and posaconazole, all have excellent in vitro activity against most dematiaceous fungi, with the notable exception of Lomentospora prolificans, which has elevated MICs for all of the available triazoles.
The most commonly used antifungal agents for the treatment of osteoarticular infections are amphotericin B (n = 19 [35.8%]) and voriconazole (n = 15 [28.3%]), alone and in combination (Table 7). Both amphotericin B and voriconazole are available in i.v. formulations for patients who are unable to tolerate oral therapy. The length of therapy is generally based on the clinical response and ranges from several weeks to several months or longer (mean value of 196 ± 175 days). A complete response was achieved in 47/55 (86%) patients. In the absence of randomized clinical trials, the diversity of these pathogens and hosts with phaeohyphomycotic osteoarticular infections warrants an individualized approach.
Among the causes of phaeohyphomycotic bone and joint infections, Lomentospora prolificans (previously Scedosporium prolificans) is the most common (n = 27/57 [47.4%]). Infections by this fungus are difficult to treat, despite aggressive therapy, due to its pattern of high-level resistance to the most commonly used antifungal agents (730). The treatment of L. prolificans osteomyelitis usually includes surgical debridement with antifungal therapy. The prognosis is more favorable with a combination of antifungal treatment and aggressive surgical debridement; the most common successful combination was voriconazole and terbinafine (672, 674, 676, 678). In a previous report, the oral antileishmanial drug hexadecylphosphocholine (miltefosine) was added to voriconazole and terbinafine, resulting in the successful treatment of osteomyelitis of the acetabulum and right iliac wing in an immunocompetent child (673).
Among 41 patients with L. prolificans infections reported by Jenks et al. from eight different countries, 4 had bone disease (731). This important report found that therapeutic regimens that included terbinafine were associated with more successful outcomes (P = 0.012), particularly when combined with voriconazole (P = 0.054). These data and other reports led to the recommendation of the combination of voriconazole plus terbinafine as the preferred initial regimen for the treatment of L. prolificans infections by the ECMM/ISHAM Guidelines Committee (732). Among investigational antifungal agents, olorofim, an inhibitor of the fungal dihydroorotate synthase pathway, has potent in vitro activity and promising therapeutic effects against infections caused by L. prolificans.
Olorofim, a recently developed investigational antifungal agent that targets the dihydroorotate synthesis (pyrimidine salvage) pathway, is a promising option for the treatment of Lomentospora osteoarticular infections. Olorofim may be an important therapeutic advance against serious L. prolificans diseases, pending the outcome of a currently ongoing clinical trial (733).
Surgery plays an important role in the management of most patients with phaeohyphomycotic osteoarticular infections (Table 7). Surgical intervention and/or medical therapy was reported for 57 patients with phaeohyphomycotic osteoarticular mycoses. Most patients (n = 43 [75%]) were treated with antifungal agents and surgery, 11 patients (19%) were treated with antifungal agents only, and 3 patients (5.2%) received surgical treatment only. Debridement was the most common surgical intervention (n = 19 [42%]); in 4 cases (8.9%), amputation was performed.
OSTEOARTICULAR MUCORMYCOSIS
Epidemiology
Mucormycosis is a devastating disease with an incidence of approximately 1.7 cases per 1,000,000 population per year, which means that there are an estimated 500 patients per year in the United States (734). Postmortem evaluation of the presence of fungi responsible for mucormycosis showed that mucormycosis is 10- to 50-fold less frequent than candidiasis or aspergillosis, corresponding to 1 to 5 cases per 10,000 autopsies (683, 723, 724, 735).
Osteomyelitis caused by Mucorales may affect any bone. However, the incidence is difficult to be estimated from previous case series due to the small numbers of patients suffering from osteoarticular mucormycosis. Nevertheless, it has been reported to constitute 15.8% of the total bone and joint infections caused by non-Aspergillus filamentous fungi (652). Most publications on osteoarticular mucormycosis have heretofore been limited to individual case descriptions and small case series. In order to address these limited data, colleagues of the International Consortium for Osteoarticular Mycoses prepared a comprehensive review of reported cases of these devastating infections (46, 736).
Fungi that belong to the Mucorales are ubiquitous in nature, compromising saprophytes that inhabit soil and decompose matter; they are thermotolerant (737). Cases of osteoarticular mucormycosis have been reported throughout the world. The demographic characteristics of 39 patients were reviewed for the period from 1978 to 2017 (738–771), including 34 cases described previously (736) and an unpublished case in this study. Male subjects predominated (69%) among the 39 patients in this series. Osteoarticular mucormycoses are uncommon diseases in children. Among 39 patients, 95% were adults (≥18 years of age). Although the main risk factor for the development of mucormycosis is diabetic ketoacidosis (772), other conditions in immunocompetent patients included trauma, vehicular accidents with fracture (741, 742, 751), prior surgery (740, 755, 760, 765), and puncture or penetrating wounds (747, 748, 757). The risk factors identified for most patients included prior surgery and disruption of mucocutaneous barriers by catheters and other devices (41%), trauma (21%), corticosteroids (21%), and diabetes mellitus (18%). Severely immunocompromised patients, including those with hematological malignancies and bone marrow/stem cell transplantation, especially when associated with neutropenia and graft-versus-host disease in hematological patients (738, 752, 764, 767, 768), solid-organ transplantation (746, 750, 769), and HIV/AIDS (743), accounted for 35% of the cases.
The high reported number of cases of osteoarticular mucormycosis observed within the last decade suggests an expanding number of vulnerable hosts. The exact reason is most likely any of the following: more adequate access to radiological imaging allowing more accurate modalities for the diagnosis of complications caused by osteoarticular mucormycosis, an increase in the frequency of complex osteoarticular procedures currently performed, and increased awareness of osteoarticular infections by physicians.
Pathogenesis
The pathogenesis of osteoarticular mucormycosis has not been well studied. Host defense against inhaled fungal elements occurs primarily through macrophages that inhibit the germination of sporangiospores of the Mucorales, whereas neutrophils use the oxidative burst to kill proliferating hyphal elements. Therefore, individuals who have defects in the function of one or both of these two lines of the host are at an increased risk of infection (737). The fungus tends to invade the vascular system and occlude arterial blood flow, causing rapid thrombosis, ischemia, and necrosis of the structures supplied by the affected vessels. Invasion rarely involves the bone. Increased susceptibility to infection is mediated by the enhanced availability of iron in tissue or serum, which promotes the aggressive invasive growth of the acquired Mucorales spores (773).
Ketoacidosis in diabetic patients produces macrophage and neutrophil dysfunction, leading to an increased risk of mucormycosis. As anticipated, chemotherapy and stem cell transplantation have evolved in the past 2 decades as major risk factors for invasive bone mucormycosis. In addition, these fungi are increased when iron is present in the host. During the initial phases of the infection, there is edema, but as the hyphae invade blood vessels, the tissue undergoes necrosis, which may develop into osteomyelitis.
The principal mechanism of infection caused by these fungi occurs through direct inoculation into bone and joints, accounting for 56% of cases. Such a mechanism especially occurs in individuals subjected to prior trauma or previous surgery. The infections may proceed to hematogenous dissemination in 24% of cases, especially in patients with hematological malignancy and other types of immune dysfunction. Contiguous spread accounted for the remaining 21% of cases (736).
Clinical Presentation
The clinical features of mucormycosis depend on the site of involvement. The hallmark of this condition is angioinvasion and localized destruction of tissues (774). The symptoms of bone infection, or osteomyelitis by filamentous fungi, include signs of inflammation around the infected bone (550, 652, 736), and the local symptoms in most cases may demonstrate two or multiple signs. The most frequently reported clinical manifestations of Mucorales osteoarticular infections were local pain or tenderness (738–740, 745, 746, 749, 768, 770). Patients may experience fever (743, 747, 748, 756, 758), swelling (745, 746, 749, 750, 753, 760), cellulitis/ulcer/abscess (743, 746, 750, 753, 754, 757, 763), restricted movement (740, 741, 760, 765, 768), and infections, which may progress to neurological deficits, particularly in spondylodiscitis (756). Musculoskeletal symptoms are often neglected due to other comorbidities. Spinal involvement, as seen in reported cases (740, 756, 770), presented as localized pain and constitutional symptoms with associated numbness and weakness. In an unpublished case from this study, restricted movement and back pain caused by disseminated infection affected vertebrae (L3, L4, sacral S1, and ilium) in HCT patients with relapsed acute myeloid leukemia (AML). Hadgaonkar et al. (770) presented a case of spondylodiscitis as an isolated lesion with no other organs being involved, except for the spine, unlike most cases of mucormycosis, which commonly present as disseminated forms due to the angioinvasive nature of the disease.
Laboratory Features
The most common species of the order Mucorales that cause bone and joint infections are Rhizopus species, followed by Apophysomyces, Mucor, Cunninghamella, Lichtheimia (formerly Absidia), and Saksenaea. In some case studies, Rhizopus arrhizus, R. microsporus, and R. rhizopodiformis accounted for more than 47% of pathogenic isolates. All patients were infected with one fungal species; Mucorales were not identified to the species level in 8 cases. A total of 39 individual cases of osteoarticular infections were published in the English literature. Most cases were identified as “proven” in 31 patients (82%), with hyphae being observed in histopathological tissue sections, and “probable” in 7 patients (18%), for whom histopathology was not performed.
The early diagnosis of acute osteomyelitis is critical because prompt antifungal therapy may prevent the progression of the infection and necrosis of the bone. Morphological characteristics of the Mucorales genera can be seen in clinical specimens. An ideal approach for diagnosing osteomyelitis due to Mucorales incorporates accurate clinical observation, culture of properly collected specimens, and microscopic examination of the infected tissue specimen. Infectious disease physicians should alert the laboratory microbiologist to a suspected mucormycosis infection and request that the infected tissue specimen be gently minced in order to avoid homogenization, which may destroy viable nonseptate hyphae.
The identification of hyphae in tissue is a vital diagnostic tool because it distinguishes the presence of fungal pathogens in the clinical specimen from a culture contaminant. Genera of the order Mucorales are characterized by nonpigmented, broad (5- to 20-μm), thin-walled, ribbon-like, nonseptate hyphae with nondichotomous “right-angle” branching (Fig. 9). The hyphae may appear sparse or fragmented and vary in width (775). Staining of hyphae in histopathological sections using routine H&E staining shows only the cell wall without an internal structure. The most specific stains used to highlight the fungal wall are GMS and PAS stains, while GMS stain may faintly stain the hyphae of Mucorales, resulting in either an unclearly positive or a false-negative report. The right-angle branching and poor staining of hyphae with GMS stain should suggest mucormycosis, whereas the presence of abundant septation and acute-angle branching (45°) should suggest Aspergillus, Scedosporium, or Fusarium species infection, while yeasts with pseudohyphae should suggest Candida species infection.
FIG 9.
Osteoarticular mucormycosis. Mucorales hyphae are seen in a bone biopsy specimen from the tibial bone shown in Fig. 10. Typical hyphae are broad, thin walled, and pleomorphic (arrow). They vary in caliber and produce irregular branches that often arise from parent hyphae at right angles. Hematoxylin and eosin stain was used (magnification, ×400). (Reproduced from reference 761 with permission from the Association of Bone and Joint Surgeons.)
The laboratory detection of mucormycosis can be optimized in several ways to improve direct examination and yields of cultures (776). Sampling an infected tissue lesion at its advancing border may increase the yield of viable organisms. Alerting the clinical microbiology laboratory to the suspicion of mucormycosis may prompt the technologist who is processing the tissue specimen to avoid homogenization processes that would fragment the nonseptate hyphae and potentially diminish the yield of the cultures (776, 777). Direct examination of a minced tissue specimen under fluorescence microscopy using fluorescent dyes (calcofluor, Fungi-Fluor, and Blankophor) may demonstrate characteristic mucoralean hyphal structures despite negative cultures (778).
Diagnostic Imaging
The manifestations of bone and joint infections by Mucorales are heterogeneous, depending on several factors, the specific causative fungus involved, the anatomical area of involvement, the segment of the affected bone, the route or type of infection, host factors, and the presence of underlying comorbidities. One of the best diagnostic procedures for osteomyelitis is imaging. Imaging techniques play a key role in early diagnosis and follow-up (779).
Radiological abnormalities can be seen using different radiological techniques. X ray showed osteolytic lesions in a number of reported cases (739, 747, 759, 767), lucencies (741, 746), or multiple small erosions of the vertebral bodies (740), and bone destruction has been reported on CT scan for tibial osteomyelitis infection (Fig. 10) (761). Magnetic resonance imaging showed low signal intensities on T1-weighted images and patches of high signal intensity on T2-weighted images (751, 756, 769). An illustrative MRI image of Mucorales osteomyelitis of the spine is shown in Fig. 11. Bone destruction caused by mucormycosis occurred after the reconstruction of the anterior cruciate ligament of the affected tibia with allograft after radical debridement (760, 761). An increase in 99mTc scan radionuclide activity is observed in osteoarticular mucormycosis (738, 741, 751).
FIG 10.
Tibial mucormycotic osteomyelitis. A coronal view CT scan of the knee before radical debridement shows destruction of cortical and cancellous bone. (Reproduced from reference 761 with permission from the Association of Bone and Joint Surgeons.)
FIG 11.
Vertebral mucormycosis. A 22-year-old female after allogeneic HCT for relapsed AML and a history of pulmonary and CNS mucormycosis presented with new-onset back pain. MRI scan of the spine showed a pathological fracture of the L3 body, paravertebral enhancement, a complex nondisplaced fracture of S1, and a right-psoas abscess (FRFSE, fast recovery fast spin echo). The psoas abscess was drained, and hyphae were seen by microscopy. L1-L3 laminectomy and L3-L4 corpectomy were performed, and pathology showed osteomyelitis and hyphae.
Treatment and Outcome
Surgical intervention and/or medical therapy was reported for 38 (97%) patients. Most patients (n = 32 [82%]) were treated with antifungal agents and surgery, 6 patients (15%) were treated with antifungal agents only, and 1 received surgical treatment only. Amphotericin B was the most commonly used antifungal agent for all patients. Combinations of antifungal therapy were reported for 3 of the patients.
The overall response included 7 deaths; 2 were due to the progressive risk factors and infection, and 5 were attributable to advanced bone infection and treatment failure with amphotericin B. Combination therapy was provided to 3 patients, mainly due to treatment failure, and included azole combination treatment. Isavuconazole has been used successfully in the treatment of vertebral osteomyelitis in combination with micafungin and surgery (T. J. Walsh, unpublished data). Local irrigation with amphotericin B may also be used as an adjunctive modality with systemic antifungal therapy.
Debridement was the most common surgical intervention (n = 11 [38%]), followed by bone grafting/fixation procedures (n = 7); amputation and excision were reported for 5 patients each.
An overall response rate of 68% was achieved in 38 cases of osteoarticular mucormycosis. A partial response was achieved in 12 patients (32%), and 1 patient died before treatment. The survival rate was 71.8%, and the overall mortality rate was 28.2%. Irrespective of antifungal treatments, death was attributed to fungal osteoarticular infections in 8 (22%) adult patients.
We concluded that bone infections due to Mucorales are characterized by a high mortality rate. Guidelines for the treatment of Mucorales bone infection require antifungal therapy with amphotericin B and surgical intervention in most cases; as other novel antifungal modalities for Mucorales infection are still under trial, amphotericin B remains the gold standard for treatment (780).
Osteoarticular mucormycosis is a highly destructive infection with a poor prognosis if not diagnosed early. The possible mechanisms of infection that cause osteomyelitis or arthritis are also not well documented. The portal of entry and the ability to disseminate may differ for each group of fungi. Furthermore, many clinical, diagnostic, and therapeutic questions remain for these infections.
CRYPTOCOCCAL OSTEOMYELITIS
Epidemiology
The two causative agents of cryptococcal bone infection are Cryptococcus neoformans and Cryptococcus gattii (781). The former is an important pathogen that tends to cause severe illness and mortality in immunocompromised patients, while the latter tends to affect patients without immune impairment (782). Although C. neoformans is classically associated with human immunodeficiency virus and AIDS, this organism is also an important cause of disseminated disease in solid-organ transplant recipients and patients with hematological diseases (783, 784). C. gattii is endemic in the tropics and subtropics and has been found in the Pacific Northwest in British Columbia, Canada, and along with C. neoformans, it is an important cause of fungal osteomyelitis (785).
Epidemiological and host-pathogen-environment studies have yielded tremendous insights into the pathogenesis of cryptococcal bone infection (786). Cryptococcus is a spherical-to-oval, encapsulated, yeast-like fungus that is widespread in spoiled milk, soil, and bird droppings (787). Following the inhalation of the basidiospore form of the fungus, C. neoformans can cause focal pneumonitis that may or may not be symptomatic. Factors that promote symptomatic infection include the inoculum of the fungi and the virulence factors of the infecting strain. The host’s immune status is the most important determinant of whether this infection resolves or progresses to symptomatic dissemination to bone. Cryptococcal osteomyelitis is generally preceded by fungemia; however, in some cases, it is caused by the direct inoculation of the etiological agent (787).
Clinical Manifestations
Humans are routinely exposed to species of Cryptococcus; however, most infections are asymptomatic (788). If the host’s immune system becomes impaired, especially in cases of cell-mediated dysfunction, fungal organisms may emerge from the granulomatous complexes and cause disseminated or invasive disease (784, 789). CNS and pulmonary diseases are the most common clinical manifestations of cryptococcosis; in comparison, localized bone infection is uncommon, occurring in fewer than 10% of patients with disseminated disease (781, 790). Cryptococcal bone disease has been divided into two types: skeletal cryptococcosis, which is an aspect of disseminated cryptococcosis, and primary skeletal cryptococcosis, which does not involve other tissues (791). The vertebrae are the most common sites of osteoarticular infection, while septic arthritis is exceedingly rare (781). The prostate classically serves as a reservoir of cryptococcal infection, which organisms can reach via paravertebral veins draining the prostate (792).
Diagnostic Approaches
Fungal osteomyelitis should be considered in the differential diagnosis of osteolytic bone lesions (793). When Cryptococcus is recovered from bone, an evaluation for disseminated disease, including CNS infection, is necessary. Isolated bone infection in the absence of meningitis is uncommon, especially in HIV-infected patients (794). In some cases of cryptococcal osteomyelitis, the signs and symptoms may resemble those of malignancy, which underscores the need for rapid and reliable diagnostic options (795). As with most fungal infections, the gold standard for diagnosis involves the direct histopathological evaluation of the specimen, where GMS and PAS stains may reveal the mucopolysaccharide-containing capsule of Cryptococcus species (796). In cases where mycological evaluation is not possible following staining procedures, other diagnostic modalities, including non-culture-based methods, are utilized.
Additional diagnostic options include the identification of serum biomarkers by a lateral flow assay, multiplex PCR, and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (797). However, their use in the routine diagnosis of cryptococcal osteomyelitis has not been fully clarified. The detection of cryptococcal antigen (CrAg) in the cerebrospinal fluid (CSF) and serum is essential in the diagnostic evaluation of a patient with suspected or documented cryptococcal osteomyelitis (798).
Treatment and Outcome
As with other forms of fungal bone infection, optimal treatment involves a combination of appropriate antifungal therapy, reversal of immune impairment (when possible), and surgical resection (if necessary). Although cryptococcal osteomyelitis shares some features with other forms of invasive fungal disease, there are important distinctions in the approach to therapy.
The optimal antifungal treatment for cryptococcal bone disease is uncertain (798–800). While some providers recommend polyene therapy to treat bone disease, some now prefer a triazole for initial antifungal therapy (67). The dose and duration of antifungal therapy may differ for certain patient populations such as pregnant women, children, and people in resource-limited settings, while some patients may require surgery to remove focal disease (cryptococcomas) (67).
The duration of therapy varies but may last up to 1 year or longer (801). Current Infectious Diseases Society of America (IDSA) guidelines for the management of cryptococcal arthritis recommend that drainage of the joint is not necessary (67). However, the infrequency of this disease and the paucity of evidence warrant that patients should be assessed individually for this intervention. As with other types of native joint infections, lavage for the control of the infectious bioburden may be beneficial. There is currently no role for monitoring serum cryptococcal antigen to evaluate the response to therapy. However, some experts recommend this approach as an adjunctive strategy. Several new antifungal agents with activity against species of Cryptococcus are currently in development and may find utility in the treatment of fungal osteomyelitis (802).
OSTEOARTICULAR COCCIDIOIDOMYCOSIS
Epidemiology
Coccidioides species are endemic in the Sonoran life zones of arid and semiarid areas of the Americas, particularly in the Southwestern United States (Arizona, California, New Mexico, Nevada, Utah, and Texas) and northern Mexico, and smaller foci have been described in Central and South America (803). Although phenotypically identical, the genus Coccidioides is divided into two different species, Coccidioides immitis and Coccidioides posadasii, which have distinct geographical distributions (804). C. posadasii is more prevalent in Arizona, Texas, Mexico, and South America, whereas C. immitis is found in central and southern California. Since this literature review included cases from 1970, the genus Coccidioides was not identified to the species level for most of the cases.
Among 905 cases of coccidioidomycosis, 63 (7%) patients had a disseminated form, including 28% with bone or joint involvement (805). In another study including 207 patients with disseminated coccidioidomycosis, osteoarticular involvement occurred in 70 (34%) patients (806). Coccidioidomycosis may occur as an extrapulmonary form in children (807), with osteoarticular involvement in 15 to 44% of the cases (808, 809). Several case reports have provided a detailed description of osteoarticular involvement in children (810–820).
Men are more susceptible to the development of osteoarticular coccidioidomycosis than women, accounting for 72 to 89% of cases (821–825). Patients of races other than Caucasian are more susceptible to coccidioidomycosis (826) and have more bone or joint localizations (824, 827). Bone and joint localizations have been described in various immunocompromised conditions such as solid-organ transplantation (822, 828, 829), hemodialysis (830), autoimmune diseases necessitating long-term corticosteroids (831–833), insulin-dependent diabetes mellitus (822, 834, 835), cancer chemotherapy (822), and interleukin-12 (IL-12)/IFN-γ axis and STAT3 deficiencies (827). The risk of the development of disseminated coccidioidomycosis is higher during pregnancy; e.g., 10% (n = 5/52) of pregnant women with coccidioidomycosis in one study developed osteomyelitis (827).
Pathogenesis
An environment of alkaline soil and high temperature favors the growth of the mycelial form of Coccidioides species, which produces arthroconidia dispersed by the wind (836). Arthroconidia are inhaled by the host to initiate infection. Once in the lungs, dimorphic changes lead to the formation of spherules due to a temperature shift from 25°C to 37°C and interaction with leukocytes (837). Spherules subsequently differentiate to produce endospores that are released upon spherule rupture. Endospores are capable of disseminating hematogenously from the lungs and reinitiating the spherulation cycle in another location. Spherules may be sequestered in granulomas to prevent disease dissemination. Therefore, the disease may occur months to years after an individual returns from an area of endemicity (838–842). The longest recorded duration for developing osteoarticular infection since leaving an area of endemicity is 56 years (840).
Hematogenous dissemination from the lungs is a key pathophysiological feature of bone and joint lesions. Patients presenting with dissemination tend to lack a cellular immune response (843). Although trauma is rarely reported, direct inoculation of the fungus may be possible, leading to osteoarticular involvement. Indeed, in those cases of coccidioidomycosis related to trauma, the lesion is often uniquely localized at the site of the injury (810, 815, 816, 844–850).
Clinical Manifestations
Fever is frequent albeit inconstant. When a patient is symptomatic, the most common symptom is bone and/or joint pain (851, 852). Diagnosis is performed at a median of 120 days (853). In children, coccidioidal osteomyelitis is more frequent than arthritis (816, 823, 851). In adults, the distribution of clinical and radiological patterns of osteoarticular lesions is more variable.
Unifocal lesions of coccidioidal osteomyelitis have been described in long bones (811), flat bones (812, 817, 831, 850, 854, 855), feet (833, 848, 856–858), and hands (818). These lesions are sometimes associated with lung (812, 817) and cutaneous (845, 856) lesions, which should systematically be investigated. Contiguous ribs can be involved in pulmonary infection (815). Abscesses in the surrounding tissue can complicate osteomyelitis, sometimes with cutaneous sinus tracts (839).
The axial skeleton is involved in 43% of cases, representing the most commonly affected location (825). Osteomyelitis of the axial skeleton often affects multiple vertebrae (811, 820, 831, 838, 839, 849, 859–873), the pelvis (839, 863, 866, 867, 872, 874, 875), the skull (810, 831, 860, 871, 876, 877), and the ribs and sternum (815, 850, 864, 866, 877, 878). The thoracic and lumbar spines are more involved than the cervical spine (879, 880). Complications of coccidioidal vertebral osteomyelitis include destruction of the anterior part of the vertebral body by adjacent abscesses (861, 862, 865), spinal cord compression (838, 863, 873, 880), and vertebral collapse or fracture (864, 873).
Among possible locations in the appendicular skeleton, the knee is the first localization of arthritis as a unifocal lesion (658, 828, 834, 835, 840, 841, 844, 846, 847, 874, 876, 881–885) or in the setting of multiple involvements (859, 860, 876, 886). Other localizations such as the elbow (816, 886–888), wrist (829, 842, 886, 889, 890), hip (850, 891), and ankle (850, 886, 892) are rare. Shoulder arthritis has been described solely in association with multiple bone and joint lesions (859, 861). Small joints are usually not affected. Monoarthritis is associated with lung (828, 844, 850, 876, 889) and/or cutaneous (844, 889) involvement. Arthritis may begin as monoarticular or polyarticular arthritis, suggesting rheumatoid arthropathy (893). Osteoarthritis is often present, although it is not always investigated (816, 835, 840–842, 850, 874, 876, 881, 885, 890, 891). Arthritis may occur on a knee prosthesis (840, 841, 894).
One-third of the cases described in the literature had multiple bone and joint lesions (810, 814, 815, 819, 839, 842, 859–861, 863, 864, 866, 870–872, 874, 876, 878, 886). Osteoarticular lesions are combined less frequently with skin lesions (859, 860, 874, 878) than with lung involvement (834, 842, 859, 863, 864, 867, 870, 871, 875, 876, 878).
Laboratory Features
There are no specific laboratory features of osteoarticular coccidioidomycosis. White blood cell counts and C-reactive protein levels may be normal or elevated (851). Disseminated coccidioidomycosis associated with multiple-bone infection can cause hypercalcemia (859, 866, 878).
The detection of anticoccidioidal antibodies is useful for the diagnosis of the infection as well as during the follow-up and monitoring of the therapeutic response. The two most common serological tests are the tube precipitin assay, which detects IgM, and the complement fixation test, which detects IgG. The complement fixation antibody titer declines in response to effective antifungal therapy. In addition, various commercial enzyme immunoassays have become available. Finally, seropositivity for Coccidioides spp. is variable, depending on the presence of disseminated infection and the immunocompromised status (806). For example, in immunocompetent patients with vertebral osteomyelitis, the sensitivity of serological testing was 93%, whereas in immunocompromised patients such as HIV patients, the sensitivity was 67% (824). Blood cultures are seldom positive (813, 856).
When submitting tissue or fluid specimens suspected to be infected with Coccidioides spp., as well as Blastomyces spp. and Histoplasma spp., clinicians should forewarn their clinical microbiology laboratory staff so that the infected material can be processed and cultured under appropriate containment procedures. In cases of arthritis, arthrocentesis typically shows exudative synovial fluid (895). When performed, histopathology, by using GMS and PAS stains, is usually definitive, with rare exceptions (817, 828, 876, 881). Necrotic granulomatous tissue composed of epithelioid multinucleated giant cell granulomas encloses spherules that are 20 to 50 μm in diameter (831, 833, 838, 839). Spherules can usually be seen to contain endospores. Cultures of biopsied tissue specimens are usually positive, with a few exceptions, and grow mold at 35°C, usually within 7 days (846, 889). Molecular tools such as PCR are sufficiently sensitive and specific to be useful to identify Coccidioides to the species level (658, 839, 840, 856).
Diagnostic Imaging
Diagnostic imaging findings are generally nonspecific (822, 896). The lesions are punched-out lytic or permeative with or without a periosteal reaction (825). They can mimic metastatic cancer or myeloma, especially when there are multiple lesions. Destructive lytic lesions can be complicated by pathological fractures (839). Small bone lytic lesions have poorly defined borders (897). Cyst-like lesions with or without cortical breaks may rarely be observed and more accurately described on CT scan or MRI (825, 831, 833). A complete assessment of skeletal involvement is required if bone lesions are suspected. Nuclear imaging provides useful tools in cases of osteoarticular coccidioidomycosis (874, 898–900). In 13 patients, a 99mTc bone scan was 100% sensitive in identifying bone lesions (874). [18F]FDG PET-CT scan can also be useful for demonstrating osteoarticular lesions (875, 899). Although [18F]FDG PET-CT scan can be used to screen for vertebral lesions, MRI is the best imaging modality to accurately describe the anatomy. Noncontiguous multifocal foci of vertebral osteomyelitis are typically observed (895, 901). Epidural involvement was observed in 13 of 15 patients with vertebral osteomyelitis (901). Extension through paravertebral muscles is possible (861, 865). Although the disc height is preserved, MRI signal abnormalities are almost always present (901). In cases of coccidioidal arthritis, radiographs can be normal at an early stage of the disease. Synovial abnormalities, articular cartilage loss, and subchondral bone loss were observed in a case of active coccidioidal arthritis seen on MRI (902).
Treatment and Outcome
Two recommendations have been provided by the IDSA regarding the treatment of coccidioidal bone and/or joint infection (903). AmB is required for severe osseous disease, including vertebral osteomyelitis with cord compression and extensive or limb-threatening skeletal infection, whereas antifungal triazole therapy is recommended for all other cases.
In a randomized double-blind trial comparing fluconazole at 400 mg/day and itraconazole at 200 mg twice daily (BID) in patients with extrameningeal coccidioidomycosis, 50 patients had a skeletal infection (904). At 12 months of therapy, success rate was significantly higher with itraconazole than with fluconazole (47% versus 37% [P = 0.03]). However, the numbers of patients treated in each group were low, 23 and 27, respectively. Thus, the recommendation is to administer fluconazole at a dosage of 800 mg daily or itraconazole at 200 mg twice daily. TDM is necessary to monitor itraconazole serum concentrations to ensure drug efficacy.
The safety and tolerability of posaconazole capsules were evaluated in 20 patients with nonmeningeal disseminated coccidioidomycosis (905). A posaconazole suspension was well tolerated, and the initial response was favorable in 17/20 patients. Posaconazole plasma concentrations ranged from 960 ng/mL to 1,342 ng/mL in this study. Higher plasma concentrations may be achievable with sustained-release posaconazole tablets, which could provide higher and more stable plasma concentrations. Posaconazole and voriconazole have also been used with satisfactory response rates in patients with refractory coccidioidomycosis (852, 867, 906, 907). Ketoconazole should not be used due to its high rate of relapse (44%) and hepatotoxicity (908).
Combined medical and surgical therapy was required in 72% of cases (821). Surgery is recommended in cases of spinal instability, spinal cord or nerve root compression, or significant paraspinal abscesses (903). In a retrospective study of 27 patients treated surgically for vertebral osteomyelitis with neurological symptoms, no surgical complications were noted (852). In another retrospective study, among 39 cases of vertebral osteomyelitis, 67% required surgery with hardware placement (824). No patients required the removal of hardware for persistent or recurrent infection. Synovectomy and/or debridement is usually performed to treat unique joint arthritis (658, 816, 828, 844, 846, 876, 882, 883, 885, 889, 891). Surgery with spacer implantation with AmB cement has been attempted (861). However, spacer implantations with AmB have not been well studied in coccidioidomycosis (909). In vitro studies of AmB- and fluconazole-loaded cement have shown suboptimal characteristics for the treatment of deep-seated fungal infections (910). In addition, the management of prosthetic joint infection is difficult. Few cases have been reported so far (840, 841, 894). Primary infection occurring on prosthetic joints should be treated by prosthesis replacement (894). Knee replacement by prosthetic material during the course of coccidioidomycosis arthritis requires lifelong antifungal treatment to avoid a relapse (894).
Relapse may occur in cases of insufficient durations of treatment and low drug exposure (824, 844, 846, 882, 904). Relapse after the end of therapy may occur from 31 to 497 days (904). Long-term follow-up is thus required since relapse may occur years after the initial infection. In cases of persistent immunosuppression, lifelong therapy is mandatory to avoid a relapse (829). Sequelae such as residual lower extremity paralysis are seen with vertebral osteomyelitis (824).
Among 79 cases in the literature whose outcomes are reported, mortality was related to coccidioidomycosis in 9 (11%) patients (859, 860, 866, 870–872, 876, 878). All of these patients had disseminated disease with meningeal or vertebral involvement, and all were apparently immunocompetent. Dissemination is a known risk factor for mortality (806), especially in cases of associated meningeal coccidioidomycosis.
OSTEOARTICULAR PARACOCCIDIOIDOMYCOSIS
Epidemiology
Distributed mostly in Latin America (Brazil, Argentina, Colombia, Venezuela, Ecuador, Peru, and Mexico), paracoccidioidomycosis (PCM) is endemic in rural areas (911, 912). With the advancement of molecular biology, two species have now been identified: Paracoccidioides brasiliensis and Paracoccidioides lutzii (913, 914). However, molecular tools to properly classify Paracoccidioides to the subspecies level were not used in the case reports and case series in the literature. Epidemiological and clinical differences may exist between infections with both fungi but have not yet been studied sufficiently.
Paracoccidioides species are frequently isolated from armadillos endemic in Central and South America, representing one of its natural reservoirs (915, 916). They have been found in soil and air samples near armadillo burrows (917). Climatic events such as heavy rains may favor fungus maintenance as well as dispersion in soil (918, 919).
The incidence of PCM is higher among male adults, with sex ratios (male to female [M/F]) of 3:1 during the second and third decades and 15:1 after the fourth decade (920). In children before puberty, the incidence is equal between both genders; in 12- to 15-year-old-children, the sex ratio is 5:1 (921, 922). Osteoarticular lesions are thus rarely described in women (921, 923–926). In addition, osteoarticular involvement during the course of PCM is rare. Data collected from two different states of Brazil, São Paulo and Mato Grosso, comprising 2,319 patients with PCM showed an incidence of osteoarticular lesions of 1 to 4% (920, 927–929). When PCM is diagnosed in children, 18 to 20% have osteoarticular involvement (922, 930).
The known risk factor for PCM is direct contact with soil. Patients who develop osteoarticular PCM practice agricultural activities (931–936), pottery (931), and stonework (931). Alcohol abuse (931, 937) and diabetes mellitus (936) are rarely associated with osteoarticular PCM. Of note, 87.5% of patients with osteoarticular PCM are apparently immunocompetent (28, 850). Osteoarticular PCM may also occur in HIV-infected patients (932, 938, 939). In a retrospective series of 73 HIV-infected patients with paracoccidioidomycosis, 7% developed osteoarticular lesions in the setting of disseminated disease (940). However, HIV is not a risk factor for PCM per se. A case-control study involving 53 HIV-infected patients and 106 controls with PCM showed that there was no significant difference between the groups in terms of bone lesions (3/53 versus 1/106) (941).
Pathogenesis
The disease is acquired by soil aerosolization and the inhalation of conidia (942). Direct inoculation causing localized osteoarticular lesions is scarcely ever described (943–945). Paracoccidioides species have the ability to change their morphology from a multicellular filamentous form to a unicellular yeast-like form. The temperature is the only factor triggering P. brasiliensis dimorphism (911). However, depending on its concentration, estrogen inhibits the conversion to the yeast-like form (946). Therefore, the infrequency of the disease in females compared to males, but only during their reproductive years, as mentioned above, might be related to estrogen blockade of the transition of the fungus from a saprobic state to the invasive form (946).
A model of Paracoccidioides arthritis was developed in Wistar rats by the direct inoculation of 105 yeast-like cells of P. brasiliensis into the right knee (947). The arthritis course was then observed at day 15 and day 45 (948). The infection started as synovitis with granuloma formation at day 15 and then progressed to necrosis, articular destruction, and extension to the subchondral bone at day 45.
Clinical Manifestations
Two forms of PCM are classically described: acute/subacute and chronic (927). The acute/subacute form of PCM occurs more frequently in patients who are <30 years of age (927). Osteoarticular lesions may occur in both forms. In acute/subacute forms, bone and/or joint involvement is accompanied by other manifestations such as fever, anemia, weight loss, lymphadenopathies, hepatomegaly, splenomegaly, digestive symptoms, and cutaneous localizations (922). In these forms, pulmonary involvement is rare (949). In chronic forms, ulcerations of the oropharyngeal mucosa and larynx as well as infections of the central nervous system and lower respiratory tract are frequent (927).
Paracoccidioides arthritis is localized to the wrist (931, 950), knee (924, 927, 943, 944, 951, 952), hip (925, 926, 952, 953), and shoulder (931). Lytic osseous lesions of the adjacent bone are usually observed (927, 943, 944, 951, 952). Pulmonary lesions are often associated with arthritis (926, 927, 943, 950, 951). When assessing pulmonary involvement with chest diagnostic imaging, arthritis may be the sole localization of the disease (925). Paracoccidioides spp. may superinfect preexisting lesions, such as gouty arthritis (936). The median diagnostic delay is 150 days (interquartile range [IQR], 90 to 270 days). Associated clinical features include joint edema, pain, redness, local heat, and functional loss (931) or pain only (926).
Osteomyelitis is localized to the femur (922, 929, 931, 937, 954, 955), tibia (945), humerus (931), ulna (952), finger or toe (929, 956), foot (952), or sternum (934). Rib osteomyelitis may be associated with contiguous lung lesions (929, 932, 935). Vertebral osteomyelitis is rare and may develop during the course of disseminated disease (921, 952) or be isolated (933, 957). Osteomyelitis affecting long bones may mimic bone neoplasia (937, 955, 958). The median diagnostic delay is 120 days (IQR, 60 to 225 days). Polyosseous involvement may occur in children (921–923, 959) or severely immunocompromised patients (939). Bone lesions may be completely asymptomatic and detectable only by whole-body imaging such as technetium bone scan (922). Pulmonary (926, 929, 931, 932, 950, 951, 954), cutaneous (921, 934), or lung and skin (935, 939, 943) lesions are commonly associated with PCM osteoarticular infections.
Laboratory Features
In order to optimize the yield of cultures, several media are available with antibacterial agents incubated at room temperature. Blood cultures are rarely positive in the setting of osteoarticular PCM (931). Diverse serological tests with overall good sensitivities are available, particularly enzyme-linked immunosorbent assays (ELISAs) for the confirmation of the diagnosis, and may help to guide treatment and detect fungal recurrences (960, 961). However, there are major inconsistencies in PCM serology among reference center laboratories (962). Nevertheless, in an osteoarticular case series, the titers of antibodies were higher in cases of disseminated disease than in cases of isolated bone or joint lesions (963). Direct examination of the fungus from a bone biopsy or joint fluid sample usually establishes the diagnosis in 74% of cases (853). Bone or synovial biopsies, when performed, show granulomatous inflammation with epithelioid cells, multinucleated giant cells, lymphocytes, and fibroblasts. The cytoplasm of giant cells contains abundant fungal yeast-like cells. The centralized fungal cell may have multiple uniform buds, the typical “pilot wheel” configuration, which can be identified by argentic staining (925, 926, 937, 953, 955).
Diagnostic Imaging
Lytic bone lesions with or without a periosteal reaction are the main findings on standard radiographs (964). Computed tomography displays osteolytic lesions with perilesional reactional osteosclerosis and/or periostitis (952). The fungus may also destroy cortical bone and invade adjacent soft tissues. MRI can confirm lytic lesions and focal cortical bone destruction, with or without a periosteal reaction, as well as soft tissue edema (953, 958). MRI may be helpful in cases of a suspicion of a neoplasm and in characterizing musculoskeletal involvement (955, 963). The main features of arthritis by MRI are reductions of the joint space, joint edema, capsule thickening, bone destruction, and infiltration of adjacent soft tissues (958, 963).
Treatment and Outcome
Consensus guidelines on PCM treatment were published in 2006 (965). Itraconazole and trimethoprim-sulfamethoxazole are the two drugs recommended for mild to moderate PCM, with a duration depending on the severity of the disease. Trimethoprim-sulfamethoxazole treatment is generally longer than itraconazole treatment, 12 months versus 6 to 9 months for mild forms and 18 to 24 months versus 12 to 18 months for moderate forms, respectively. Intravenous trimethoprim-sulfamethoxazole or deoxycholate AmB is recommended for severe forms. The definition of a cure of PCM is based on clinical, radiological, and mycological improvement as well as stabilization of agar gel double-immunodiffusion results at 1:2, negative conversion of two samples within a 6-month interval after treatment, or normal inflammatory protein levels for 3 consecutive months (965).
The primary therapies in reported cases of PCM osteoarticular lesions were trimethoprim-sulfamethoxazole (925, 931, 933, 937, 950, 952), itraconazole (926, 936, 951, 954), deoxycholate AmB (921, 923, 931, 932, 935, 939), and ketoconazole (924, 945). Trimethoprim-sulfamethoxazole is usually the drug of choice for a switch from intravenous to oral therapy (921, 924, 931, 935, 945). Although the combination of deoxycholate AmB plus trimethoprim-sulfamethoxazole was used once (944), monotherapy is the rule, even in cases of disseminated disease.
Itraconazole is the most widely studied drug for the treatment of PCM (966, 967), including comparisons with trimethoprim-sulfamethoxazole (968, 969). The efficacy of itraconazole is dose dependent, and the drug seems to be more effective at a dosage of 200 mg/day in adult patients. Although a clinical cure is obtained faster with itraconazole, trimethoprim-sulfamethoxazole is as effective and safe as itraconazole (968). The outcome may depend on the species of the Paracoccidioides isolate. Thus, patients infected with P. lutzii isolates seem to respond better to trimethoprim-sulfamethoxazole therapy than patients infected with P. brasiliensis isolates (970).
Although not specifically assessed for Paracoccidioides osteoarticular infections, other drugs could be useful in cases of PCM osteoarticular infection. A randomized open-label study compared the safety and efficacy of itraconazole to those of voriconazole for the treatment of PCM in 53 adults (971). Voriconazole was as safe and effective as itraconazole. Very few data are available on isavuconazole, which was evaluated in 10 patients after a median of 180 days of treatment (972). One patient had a complete cure at the end of therapy, and 7 had partial success. Lipid formulations of AmB may be useful for severe forms of PCM or if intravenous treatment is required (973).
The adjunctive use of corticosteroids has not been studied in osteoarticular treatment. However, corticosteroids have been used with success in combination with antifungal treatment for different localizations (974). One should also note that paradoxical reactions to antifungal treatment may occur and can be treated with corticosteroids (975).
The in-hospital mortality rate for PCM in Brazil is estimated to be 5 to 8% (976). In case reports describing osteoarticular lesions, no death has been reported, whereas in relevant case series, the outcome was not even described (952, 963, 964).
OSTEOARTICULAR BLASTOMYCOSIS
Epidemiology
Blastomycosis is endemic in North America (977). Its main agent, Blastomyces dermatitidis, is responsible for infections in Canada along the St. Lawrence River and the Great Lakes (978–980); the Eastern and Midwestern United States, especially the states bordering the Ohio and Mississippi River Valleys; as well as the Mid-Atlantic (981–983). Since the molecular identification in 2013 of Blastomyces gilchristii, a cryptic species of the genus Blastomyces, it is unclear whether previous reports were actually reporting B. dermatitidis or B. gilchristii (984).
Osteoarticular involvement is the third localization of blastomycosis after the lungs and skin (985–988). Among 936 patients infected with B. dermatitidis, 18% had osteoarticular lesions (985). There is also a male predominance (68 to 78%) among adults infected by Blastomyces species (853, 980).
Regarding children, osteoarticular lesions seem to occur more frequently in this particular population. In a series of 114 children with blastomycosis, 47 (41%) presented with a bone fracture or joint pain (989). The identified risk factors for blastomycosis are contact with soil, especially during construction, excavation, or other outdoor activities (981, 986, 987, 990). In a case-control study with 118 controls and 112 patients, of whom 11 had bone involvement, the significant underlying conditions for the risk of blastomycosis were the use of immunosuppressive therapy, collagen vascular disease, and drug abuse (987). In two series of solid-organ transplant recipients comprising 19 patients, bone involvement was diagnosed in 2 patients (991, 992). In HIV-infected patients, blastomycosis is often disseminated (993, 994).
Pathogenesis
Host inhalation of conidia from soil is thought to be the common portal of entry of B. dermatitidis (977). Osteoarticular infection is acquired either by the dissemination of the fungus from the lungs or by direct inoculation following trauma. Few patients were reported to have previous trauma (980, 995–1003), and only two were considered to have no localization other than the osteoarticular one (998, 1001). The time to initial symptoms is difficult to estimate in cases of direct inoculation and may vary from a few days to weeks.
Clinical Manifestations
Osteoarticular lesions are seen in approximately one-fifth of patients with a diagnosis of blastomycosis. Malaise, fatigue, fever, and weight loss are unspecific symptoms observed in disseminated blastomycosis. Bone blastomycosis may mimic cancer (1004). A longer duration of symptoms is associated with extrapulmonary blastomycosis (986). More specifically, bone and joint blastomycosis diagnosis can be delayed, and the median reported time to diagnosis is 105 days (range, 54 to 210 days) (853). Although any bone can be involved, vertebrae are the most frequent localizations (997, 1005–1019), mainly in the context of disseminated disease. However, some cases have isolated vertebral osteomyelitis (1006, 1009, 1011, 1014, 1017). Infected vertebrae are mainly thoracic (1006, 1007, 1010, 1012, 1014–1016), lumbar (1005, 1008, 1009, 1011, 1014, 1017), and, rarely, cervical (997, 1013). Multiple vertebrae may be involved (1007, 1009, 1011, 1014, 1015). Discitis is usually present, except for a few cases (1008, 1012). Complications such as paravertebral or prevertebral abscesses (997, 1010, 1013, 1015, 1018, 1019), pathological fracture of the vertebral body (1016), and spinal cord compression (997, 1016, 1018) have also been reported. Vertebral body destruction may mimic metastatic disease (1020). Other bone localizations include the foot (1002, 1012, 1021–1024), tibia (1022, 1025–1027), fibula (998, 1028), femur (1025, 1029), radius (1025, 1030), humerus (1031), hand (1012, 1032), iliac bone (1029, 1033), sternum (1025, 1034), temporal bone (1035, 1036), rib following local pulmonary and mediastinal invasion (1037), mandible (1038), and skull base (1036).
Arthritis with (1001–1003, 1039–1043) or without (996, 999, 1000, 1032, 1042, 1044) bone lesions occurs less frequently than osteomyelitis. The affected joints are mainly the knee (996, 999, 1001, 1002, 1032, 1039, 1042) and elbow (996, 1000, 1003, 1012) and more rarely the wrist (1041, 1044), ankle (1042), and hip (1040). Arthritis may be complicated by tenosynovitis (1043) and purulent discharge by a sinus tract from the lesion to the skin (1045).
Multiple bone and joint lesions (995, 996, 1012, 1014, 1031, 1032, 1042, 1045–1055) are associated with lung involvement (1031, 1047, 1050), skin involvement (995, 1012, 1032, 1042), or both (996, 1014, 1045, 1046, 1048, 1052–1055). Osteoarticular lesions may be painless and may be discovered only via diagnostic imaging (1053).
Laboratory Features
Serological tests for blastomycosis have poor performances and are not routinely used for establishing a microbiological diagnosis (977). Data for antigen detection in urine for the diagnosis of osteoarticular blastomycosis are inconclusive. Therefore, direct examination or culture of the fungus from a pathological tissue specimen remains the gold standard for the diagnosis of blastomycosis.
Clinicians should apprise the staff of their clinical microbiology laboratory about infected material from patients with suspected blastomycosis so that the fluid or tissue can be handled under proper containment procedures. The typical histopathological feature of osseous blastomycosis is granulomatous inflammatory tissue with numerous Langerhans giant cells (1004). The granuloma surrounds large yeasts of 8 to 15 μm with broad-based buds. Necrotizing tissue with neutrophilic and eosinophilic infiltrates may also be seen (103, 1033, 1043). Rare yeasts may be missed by the histopathologist (1004).
For blastomycotic arthritis, draining pus or fluid aspirates generally contain yeasts (999). Synovial fluid could, however, remain sterile in culture, especially if the incubation time is too short or if the patient has already received antifungal drugs (1042). In 46 patients, 14 of whom had bone and joint localizations, histopathological examination correlated with culture results in 31 (67.4%) patients, whereas in 11 (24%) patients, cultures failed to isolate Blastomyces species (1056). Therefore, incubation of samples from patients suspected of having osteoarticular blastomycosis should be done for at least 4 weeks to allow the growth of the mold phase of the organism.
Diagnostic Imaging
Radiographic lesions are not specific for blastomycosis and may be easily mistaken for cancer (1004). In patients living in areas of endemicity and travelers returning from these areas, destructive bone lesions should be biopsied in order to resolve the differential diagnosis of blastomycosis versus cancer. Gehweiler et al. reported distinct radiographic patterns for 45 cases (1057). In the short bones, cystic areas of focal osteomyelitis with a sclerotic margin can be observed. In the flat or long bones, lytic lesions with a periosteal reaction may occur (1051, 1057). Vertebral osteomyelitis may mimic tuberculosis with the presence of paraspinal abscesses. Subchondral radiolucencies can evoke intraosseous abscesses (1043). MRI may help to further assess lesions of arthritis or vertebral osteomyelitis and may display intraosseous abscesses (980, 1043, 1045). Bone scintigraphy or [18F]fluorodeoxyglucose positron emission tomography (PET) scan may be useful to detect asymptomatic bone localizations (1034).
Treatment and Outcome
Surgery is not always required (1058). However, more than half of the reported cases of osteoarticular infections benefited from a combination of medical and surgical management. Regarding medical treatment, the two more commonly used treatments of itraconazole and AmB are recommended by the IDSA (1058). The success of this strategy was evaluated in a literature review (853). For osteoarticular blastomycosis, 97% of the cases were successfully treated with monotherapy with either itraconazole or AmB.
Posaconazole may be an alternative to itraconazole. One case was successfully treated with posaconazole for tibial and fibular osteomyelitis after having received itraconazole and voriconazole (998). Itraconazole never achieved optimal plasma concentrations, and voriconazole was responsible for hepatic side effects. Posaconazole was administered for 6 months. Although clinical breakpoints are not fully established, posaconazole and voriconazole have good in vitro activity against Blastomyces species (1059).
Fluconazole was used in a clinical trial at a high dose (800 mg daily) versus a low dose (400 mg) (1060). Among 39 randomized patients, 4 had osteoarticular involvement, and all of them were cured, regardless of the dose received. Currently, there are insufficient data to support the use of isavuconazole for the treatment of osteoarticular blastomycosis (972).
According to IDSA guidelines, patients with osteoarticular blastomycosis should receive at least a 12-month course of antifungal therapy due to frequent relapses (1058). When the data from 79 case reports were analyzed, the median duration of treatment was 225 days (range, 4 to 780 days), with only 2 relapses. Among the 65 patients for whom mortality data were available, 3 patients died (4.6%); only one death was attributable to blastomycosis. This result is consistent with the results of a retrospective study of 45 patients with bone and joint infections, where 4% of the patients died (980). Sequelae such as impaired musculoskeletal function were present in 24% of the patients (980).
OSTEOARTICULAR HISTOPLASMOSIS
Epidemiology
Histoplasma capsulatum var. capsulatum (HCC) is present worldwide (1061). Osteoarticular HCC infections have been described in the United States (1062–1070), Brazil (1071, 1072), French Guiana (1073), Paraguay (1074), Argentina (1075), Philippines (1076), China (1077, 1078), the Indian subcontinent (1079–1086), Morocco (1087), and Lao PDR (1088). Some of these infections occurred years after traveling to areas of endemicity (1084, 1088). Most of the patients with bone and joint involvement are adults, as children rarely develop this kind of lesion (1074, 1077, 1082). The prevalence of osteoarticular localizations is low. During an epidemic in Indianapolis, IN, HCC arthritis was reported in only 6 (1.6%) of 381 patients (1089).
HCC infection may occur in immunocompromised patients, and its link with the AIDS epidemic and corticosteroid use is well established (1090). Underlying comorbidities associated with osteoarticular localizations are chronic corticosteroid use, mostly for rheumatologic diseases (1066, 1071, 1073, 1084, 1088); HIV infection with fewer than 200 CD4+ T cells/mm3 (1068, 1075); polymyositis with chronic use of nonsteroid anti-inflammatory drugs (1064); and acute leukemia with neutropenia (1069).
Histoplasma capsulatum var. duboisii (HCD) bone and joint disease, also known as African histoplasmosis, is particularly located in sub-Saharan Africa. The fungus was reported in almost all African countries, including Madagascar Island (1091). A few cases are diagnosed in countries where the disease is not endemic in migrants but rarely in occasional travelers (1092). The prevalence of the disease is largely underestimated due to the weakness of public health services in some countries (1093). In Congo-Brazzaville, 14 cases were reported in a single institution in 10 years, with 3 having osseous involvement (1094). In 1972, Drouhet described 116 cases of African histoplasmosis (1095). Localized infection of bones was present in 42 patients (36%). When disseminated, the disease affected bones in almost all cases (n = 18/19). Notwithstanding HCD infection occurring in HIV-infected patients, no link between the two diseases has been proven (1096). In a series of 37 patients with and without HIV infection, bone lesions occurred in 17 (46%) cases, 12 in HIV-infected patients and 5 in non-HIV-infected patients (1096). In individual case reports on osteoarticular infections, only one involved an immunocompromised patient with systemic lupus erythematosus treated with corticosteroids and azathioprine (1097). The other cases were apparently immunocompetent. Cases are reported in children (1079, 1094, 1098–1109) as well as adults (1079, 1097, 1100, 1106, 1110–1124).
Pathogenesis
Regarding HCC, trauma is very rarely reported to be the cause of direct inoculation leading to bone lesions (1067). Microconidia are found in soil containing bird or rat droppings and may be inhaled by patients (1082). Primary lung lesions can be asymptomatic since only 4 of 26 patients in this review had documented lung involvement (1068, 1074, 1086, 1125). However, dissemination leading to secondary bone and joint infections can occur. HCC yeast-like forms may be phagocytized by macrophages, dendritic cells, and neutrophils (1126).
The environmental reservoir of HCD could be bat intestines since this fungus has been found in bat guano (1127). Inhalation of microconidia could be the main portal of entry. Direct inoculation of the fungus is rare; there were only two reported cases of posttrauma osteoarticular infection in children aged 4 and 5 years (1103, 1104). No experimental model of HCD infection exists to study its pathophysiology.
Clinical Manifestations
HCC osteomyelitis evolves very slowly (1082, 1086). The median time to diagnosis is 90 days (from a minimum of 7 days to a maximum of 3 years). Most of the osteoarticular infections described are isolated localizations. A few cases are accompanied by cutaneous lesions such as ulcers, erythema nodosum, papular lesions, or subcutaneous nodules (1076, 1082, 1089); hepatomegaly or lymphadenopathy (1085, 1086); or lung localizations (1068, 1074, 1086, 1125). Fever can be absent. HCC can infect long bones such as the humerus (1062), radius (1070), fibula (1077), tibia (1067), and femur (1083). Lesions can lead to pathological fractures (1083). Flat bone localizations are represented by maxillary (1062, 1065, 1072), sternum (1081), and sacrum (1087) involvements. Maxillary osteomyelitis occurs in immunocompetent patients and is frequently associated with oral ulcerations. Histoplasmosis of the extremities, including the hand (1063) and foot (1076), is less common. Isolated vertebral osteomyelitis is also possible (1071). Regarding arthritis, it occurs more frequently in the knee (1064, 1068, 1069, 1073, 1086) than in the hip (1066), wrist (1084), ankle (1088), and shoulder (1085). The differential diagnosis of HCC arthritis includes tuberculosis arthritis, which may be clinically and histologically indistinguishable, pending special stains and cultures of synovial fluid (1073, 1085). Interestingly, two cases of arthritis occurred in patients suffering from rheumatoid arthritis who were receiving tocilizumab and corticosteroids (1073, 1079, 1088). Multiple bone and joint localizations are rare and occur in children (1074, 1082) or immunocompromised patients (1075).
HCD infection has some characteristic clinical findings. Fever is not specific to HCD infection and can be absent. Bone tumefactions are generally painful (1120). Children do not have any particular features compared to adults (1104). The median time to diagnosis is 150 days (from a minimum of 30 to a maximum of 2,920 days). Multiple bone and/or joint infections occurred in 44% (n = 14/32) of the case reports described in this review (1097–1099, 1104, 1105, 1107, 1108, 1110, 1111, 1115, 1117, 1120, 1124, 1128). Isolated osseous localizations are encountered in long bones (1103, 1104, 1109, 1118, 1129), flat bones (1079, 1094, 1100–1102, 1106, 1114), and vertebrae (1100, 1116, 1123).
HCD infection of the face has been described to involve mostly maxillary bones (1062, 1079, 1101, 1106), skull (1100, 1114), and orbit (1094). Maxillary localizations lead to discussions about an extended dental infection, Burkitt’s lymphoma, or even ameloblastoma, pathologies that are commonly observed among young black people (1106). Osteoarthritis is exceptionally found alone, involving the shoulder in two patients (1106, 1122). When described in combination with other bone lesions, osteoarthritis can involve various localizations such as the knee (1098), ankle (1128), sternoclavicular articulation (1120), elbow (1095), wrist (1097), and hands (1105).
Complications of bone and/or joint involvement may occur. Vertebral osteomyelitis alone (1100, 1116, 1123) or in combination with other bone lesions (1097, 1117, 1123, 1124) may lead to fractures mimicking malignancy (1123) or spinal cord compression (1124). Spontaneous pathological fractures of long bones can occur (1103, 1110, 1129). Chronic cutaneous fistulae are a common finding that may occur in HCD bone lesions (1099, 1107, 1120). Although lung localization is rarely described during the course of osteoarticular HCD infection (1100, 1123), the major differential diagnosis is tuberculosis in countries of endemicity. Regarding 28 case reports, skin or mucosal lesions and lymphadenopathies were present in 54% (n = 15/28) of the cases (1097–1100, 1103–1105, 1109, 1111, 1115, 1120, 1123, 1129). Other cases have disseminated lesions without mucocutaneous or lymph node involvement (1079, 1094, 1102, 1106, 1107, 1110, 1114, 1116–1118, 1122, 1124).
Laboratory Features
Similar to cases of coccidioidomycosis and blastomycosis, clinicians should apprise their clinical microbiology laboratory when submitting tissue or fluid specimens suspected to be infected with Histoplasma species. Samples of bones or synovial fluids should be plated onto Sabouraud’s dextrose agar. Incubation is performed at 25°C to allow the growth of the mycelial phase of H. capsulatum (1090). The culture has to be kept for at least 6 to 8 weeks. Blood cultures are never positive.
Direct examination by Gram staining may show both intracellular and extracellular yeast forms of HCC (27, 1088). Histopathological examination of bone samples usually shows inflammatory granulation tissue with well-formed granulomas composed of epithelioid histiocytes, lymphocytes, plasma cells, and multinucleated giant cells (1072, 1082). Necrosis can be present without caseum (1073, 1078). Hematoxylin and eosin staining may show neutrophilic infiltrates in bone biopsy specimens (1076). Intracellular and extracellular yeasts are easily stained with Gomori methenamine silver stain. They are 2 to 4 μm in diameter and oval, with narrow-based buds (1130). PCR on tissue specimens could help to identify the species when a culture is positive (1073, 1088). The sensitivity of urinary antigen detection is highest in patients with disseminated or pulmonary histoplasmosis and immunocompromised patients (1131). Due to the paucity of cases with isolated bone and/or joint lesions, evaluations of this assay for osteoarticular histoplasmosis are inconclusive. Serology could be useful, albeit it has never been specifically tested in isolated osteoarticular infections (1130).
Regarding HCD, cultures of bone, synovial fluid, pus from lymphadenopathies, and cutaneous lesions can be negative. Direct examination of a simple smear of pus or synovial liquid may show yeasts (1120, 1123). In cases of disseminated disease, bone marrow cultures can be positive (1120). Upon histopathological examination of bone tissue, periodic acid-Schiff staining reveals yeasts larger than those of HCC, 6 to 12 μm in diameter, with a thick cell wall, a vacuolated cytoplasm, and narrow-based buds (1122, 1132). Granuloma formation is frequently observed with focal necrosis (1122). Yeast forms can be extra- and intracellular in the cytoplasm of multinucleated giant cells (1101). Grocott methenamine silver staining demonstrates the classic “hourglass” shape of HCD. The “parent” and “daughter” cells are of equal sizes and are joined by a narrow base (1132).
Quantitative PCR can identify HCD when performed on bone, respiratory, lymph node, and plasma samples (1122, 1133).
Diagnostic Imaging
None of the lesions are specific to one variety of H. capsulatum. Osteolysis with or without periostitis or cortical disruption is the most common bone lesion seen on plain radiographs (1120). MRI of the spine may show lysis of vertebrae, with a common complication of vertebral osteomyelitis (i.e., epiduritis and paravertebral abscesses) (1117). MRI is the best imaging modality to precisely describe osteoarthritis lesions. It can reveal a fistulous tract (1122), intraosseous abscesses, or necrosis (1073). Increased uptake of the lesions on PET-CT can be observed (1073, 1078).
Treatment and Outcome
Surgical treatment.
Surgery is usually not required for HCC and HCD infections. Surgery can be useful to drain a subcutaneous abscess adjacent to a focus of osteomyelitis (1103, 1118), to consolidate vertebrae by arthrodesis (1078), or in cases of arthritis with poor outcomes (1063, 1073, 1084, 1097). As for bacterial prosthesis infection, it is recommended that the prosthesis be replaced whenever possible. In cases of prosthesis retention, lifelong antifungal treatment may be required (1066). Plastic or functional surgery is sometimes needed to manage sequelae. Bone reconstruction can be performed after medical treatment (1101, 1129) or replacement arthroplasty (1073).
Medical treatment.
Among 21 patients with HCC osteoarticular lesions for whom clinical responses were evaluable, a complete response, defined as the absence of clinical symptoms and total healing of the lesions on imaging, was observed in 14 (66%) patients. The mean duration of treatment for a complete response was 223 days (from a minimum of 60 days to a maximum of 522 days). The mortality rate due to HCC infection was 9% (n = 2/22), linked to disseminated disease (1075, 1086).
The recommended drugs for disseminated HCC infection are itraconazole and amphotericin B (1134). Liposomal amphotericin B at 3.0 mg/kg daily is recommended for 1 to 2 weeks, followed by oral itraconazole at 200 mg 3 times daily for 3 days and then 200 mg twice daily for a total of at least 12 months (1134). Posaconazole can be used as an alternative therapy for disseminated HCC infection (1135). It could also be a good alternative for the treatment of osteoarticular lesions due to its good bone diffusion. Voriconazole use has been reported twice, with both success and failure: one case was successfully treated after debridement surgery of the fibula (1077), and another case failed to be treated for knee arthritis (1073). Fluconazole is another triazole used for isolated knee arthritis (1064). The HCC MIC for voriconazole in vitro can be as low as those for amphotericin B (1136, 1137). The MICs for fluconazole are usually higher than those for itraconazole or voriconazole (1136, 1137). Itraconazole was used as a first-line therapy in seven patients, one of whom had disseminated infection (1066, 1067, 1072–1074, 1078, 1084). Successful therapy occurred in six patients. Failure in one case was probably due to the low dosage and the lack of monitoring of itraconazole plasma concentrations (1084). Recurrence of the lesions can occur as long as 10 years later if medical treatment is inappropriate, especially with ketoconazole (1063). Immune reconstitution inflammatory syndrome may appear with HCC infection in the setting of AIDS or TNF-α blockade therapy (1075, 1138, 1139).
Among 24 patients with HCD infection for whom responses were evaluable, 15 (62.5%) achieved a complete response to treatment. The mean treatment duration for those patients was 214 days (from a minimum of 28 days to a maximum of 365 days). Treatment was shortened to 28 days in one case of a maxillary lesion, which was entirely surgically excised (1101). Among 25 patients for whom outcomes are known, 3 (12%) apparently immunocompetent patients died due to disseminated HCD infection (1104, 1123).
No international recommendations exist for the specific treatment of HCD infection. By analogy to HCC infection, AmB followed by itraconazole could be the best option for disseminated disease (1134). Posaconazole could be used as an alternative therapy for disseminated HCD infection (1140).
AmB was used as the first-line therapy in 13 osteoarticular cases and led to failure in only 1 case after 6 months of treatment with deoxycholate AmB for vertebral and sternal osteomyelitis (1100). Itraconazole was used as a first-line monotherapy in only four cases, three of whom had disseminated infection (1099, 1104, 1122). Success was reported once in a case of disseminated bone disease (1099). Due to poor-resource settings, monitoring of itraconazole plasma concentrations is never reported in osteoarticular case reports from Africa. One case of failure due to a low plasma level of itraconazole was documented in a Liberian patient living in the United States (1122). It is thus mandatory to monitor drug concentrations to ensure efficacy. Even if ketoconazole was used to treat some cases of osteoarticular HCD infection (1100, 1110, 1116–1118), it should no longer be used due to its hepatotoxicity. Fluconazole was used once for initial therapy and was associated with failure in a case of disseminated disease with vertebral osteomyelitis (1123). It was used with success as a second-line treatment combined with surgery for shoulder arthritis (1122).
OSTEOARTICULAR TALAROMYCOSIS
Epidemiology
Penicilliosis (talaromycosis) was due to the organism formerly termed Penicillium marneffei, the only dimorphic member of the genus Penicillium, now reclassified as Talaromyces marneffei. Penicilliosis is largely restricted to Southeast Asia. Although it was described in bamboo rats in the 1950s, the ecological niche of this fungus remains unknown (1141). T. marneffei is probably present in the environment, as infected patients more frequently reported an occupation involving exposure to plants or animals (1142). In addition, a peak of hospital admissions is frequently found during the rainy season (1143). Initially described in HIV-infected patients with CD4+ T cell counts below 50 cells/mm3, it has now been recognized in various immune disorders (1144). The incidence of bone and/or joint involvement ranges from 4 to 14% in patients with penicilliosis (1145–1148). HIV-uninfected adult patients seem to be more prone to the development of osteoarticular lesions. In three retrospective studies comparing HIV-infected with non-HIV-infected patients, all of the bone and joint lesions were seen in non-HIV-infected patients (1146–1148). Cases in HIV-infected adults were also reported (1149, 1150). In children, bone and/or joint lesions were described, regardless of the underlying condition (1151–1153). The main comorbidities associated with osteoarticular lesions are diabetes (1147, 1148), previous corticosteroid use (1147, 1148), β-thalassemia (1147), cancer (1147, 1148), Langerhans cell histiocytosis (1147), autoimmune disorders (1147–1149), idiopathic CD4+ lymphocytopenia (1154), common variable immunodeficiency (1155), and solid-organ transplantation (1156). Although no osteoarticular involvement has been described thus far in patients receiving monoclonal antibodies or kinase inhibitors, new biotherapies may increase the risk of penicilliosis (1157). Apparently, immunocompetent patients can also develop osteoarticular infections caused by T. marneffei (1147, 1148, 1150, 1158–1160).
Pathogenesis
Inhalation of airborne conidia is the predominant portal of entry, while direct inoculation of the fungus through the skin is considerably less frequent (1154). Dissemination via the monocyte/macrophage system follows contamination. At 37°C, the conidia undergo a phase of transition to a yeast-like form. The yeasts are very small (2 to 3 μm by 2 to 7 μm) and can be misdiagnosed as those of Histoplasma capsulatum (1161). The yeasts divide by fission, in contrast to those of H. capsulatum, which bud and have a central septum. T. marneffei is a facultative intracellular pathogen that is cleared by immune cells in immunocompetent hosts, especially T cells and macrophages. In immunocompromised hosts, yeasts are able to survive and replicate inside the phagosome (1141). Granulomas seem to be the structures that can control the infection by containing the fungus (1162–1164).
Clinical Manifestations
The main clinical features of disseminated penicilliosis are fever, weight loss, and anemia (1141). In addition, when bones and/or joints are involved, patients may suffer from ostealgia and joint paint with surrounding soft tissue swelling and erythema (1147). The classical cutaneous lesions of penicilliosis and the presence of pulmonary infiltrates are frequently associated with osteomyelitis or arthritis (853). The clinical presentation can be misdiagnosed as tuberculosis, leading to diagnostic delays of several weeks (853). Unmasking immune reconstitution inflammatory syndrome may reveal disseminated penicilliosis with osteoarticular infection (1153).
Laboratory Features
Osteoarticular penicilliosis seems to develop mainly by hematogenous dissemination from the lungs. Blood cultures are thus positive in approximately 40% of patients (1150, 1152, 1159). Histopathological examination of a synovial biopsy specimen from an apparently immunocompetent patient showed dense fibroblastic proliferation within large granulomatous foci of epithelioid cells, with a few scattered Langerhans giant cells and areas of caseous necrosis. Epithelioid cells may contain large phagosomes, each of which engulfs dozens of yeast-like fungi (1158). Caseous necrosis has already been described for lymphadenopathies of non-HIV-infected yet immunocompromised children (1165). Other histopathological features, observed in a patient with mixed connective tissue disease, consist of diffuse fibrosis of the intertrabecular space of a long bone and infiltration of histiocytes, without well-formed granulomas or giant cells (1149). T. marneffei can be seen in the bone samples with special staining. It can also be isolated from bone or joint fluid samples by culture (1149, 1159). Albeit not used specifically for the diagnosis of osteoarticular infections, the galactomannan antigen of Aspergillus may be useful for diagnosis and monitoring the therapeutic response as it cross-reacts with T. marneffei antigens and is positive in 95% of cases involving HIV-infected patients (1166).
Diagnostic Imaging
Radiological examinations display osteolytic lesions with or without a periosteal reaction (1147). Osteolysis may lead to fracture (1147). Vertebral osteomyelitis might present with multiple lucent defects or bone destruction of the vertebral body (1147, 1149). Multiple osteolytic lesions are very frequently observed and signify hematogenous dissemination, while arthritis may involve only one joint. [18F]FDG PET-CT shows increased uptake in bone lesions (1147).
Treatment and Outcome
The control of the immunodeficiency is one of the cornerstones of the treatment of penicilliosis. The introduction of highly active antiretroviral therapy to increase CD4+ T cell counts in HIV-infected patients is essential and has thus decreased the incidence of penicilliosis in this population (1144). Osteoarticular penicilliosis rarely requires surgery, as musculoskeletal involvement often reflects disseminated disease (1150).
AmB deoxycholate at a dosage of 0.6 mg/kg/day intravenously for 2 weeks followed by a 400-mg/day dosage of oral itraconazole for 10 weeks is the reference treatment for penicilliosis (1152). A regimen of up to 1 mg/kg/day of deoxycholate AmB has also been used to treat osteoarticular infections (1147). Additionally, liposomal AmB has been used with success (1156). The duration of therapy is not well established and ranges from 60 days to 18 months in cases of osteoarticular involvement (1147, 1150, 1159).
Voriconazole is an effective and well-tolerated treatment for penicilliosis (1167). No case has been reported regarding voriconazole use for the treatment of osteomyelitis or arthritis. However, voriconazole may be a valuable option as it showed the lowest MIC among several antifungal agents tested against 14 strains (1168).
The prognosis of osteoarticular penicilliosis is tightly linked to dissemination and correction of the underlying immunosuppression. Relapses are frequent at the end of treatment (1147, 1150). The attributable mortality rate is approximately 30% (853, 1147). Sequelae have not been evaluated in the literature.
OSTEOARTICULAR SPOROTRICHOSIS
Epidemiology
For a long time, Sporothrix schenckii was considered to be the sole agent responsible for sporotrichosis. In 2008, clinically relevant species of the S. schenckii complex were described: Sporothrix brasiliensis, Sporothrix globosa, Sporothrix luriei, Sporothrix mexicana, and Sporothrix schenckii sensu stricto. Since only 13 case reports dealing with osteoarticular localizations were published after 2008 (1169–1180), the identification of members of the S. schenckii complex to the species level had not been done before 2008. Of note, S. brasiliensis was described in 2015 as a species responsible for osteoarticular manifestations in two case reports (1172, 1180). Shortly after its initial description, most of the early cases were described in France.
The Sporothrix schenckii complex now has a worldwide distribution, occurring predominantly in the United States, Japan, and Central and South America (1181). Sporadic cases have been described in Europe. Among case series of sporotrichosis, bones and/or joints are the second localization after skin involvement (1182). A total of 118 cases with sufficient clinical data have been described in the literature. Whereas 52% of the patients who developed osteoarticular lesions had no known comorbidities, alcohol abuse was present in 30% of the patients (853). Diabetes mellitus (1170–1172, 1183–1190), hematological malignancy (1185, 1191–1194), HIV infection (1177, 1179, 1180, 1189, 1195–1200), solid tumors (1185, 1201, 1202), autoimmune diseases (1203), and kidney transplantation (1204) were other comorbidities less commonly associated with osteoarticular sporotrichosis. Males seem to represent 89% of the population with osteoarticular sporotrichosis (853).
The risk factors for Sporothrix transmission to humans include outdoor activities, gardening (“rose gardener’s disease”), or contact with cats. Exposure to cats is especially important for infections caused by Sporothrix brasiliensis. In the setting of suspected osteoarticular sporotrichosis, searching for a history of gardening, manual work, or contact with soil, dust, or wood is important. When occupations or activities were reported (n = 64/118), 81% (n = 52) were outdoor activities or manual work. A history of trauma with thorns, hay, wood splinters, needles, or blades; injury due to a fall; or cat bites or scratches was reported for 17% (n = 20) of the cases.
Pathogenesis
Bone and/or joint lesions can occur locally at the site of the direct inoculation of the fungus following a skin injury or through hematogenous dissemination. In a series of 118 cases, only 9 had positive blood cultures (1180, 1187, 1189, 1196, 1198, 1205–1208). The fungus leads to a granulomatous formation at the site of inoculation. Granuloma formation in cutaneous sporotrichosis is associated with a Th1 response (1209). Yeasts and conidia of the fungus can bind to extracellular matrix proteins such as type II collagen, fibronectin, and laminin (1210). This fungus also adheres to human endothelial cells in vitro (1211) and can transmigrate across cell monolayers in a time-dependent manner to disseminate through the bloodstream (1212).
Clinical Manifestations
In the setting of cutaneous sporotrichosis, a careful assessment of bone and joint localizations is required. Indeed, bone lesions can be asymptomatic and may be revealed only by bone scintigraphy by [18F]FDG PET-CT scan (1208, 1213). The median diagnostic delay is 240 days (853).
Isolated osteomyelitis represents approximately 10% of all cases of osteoarticular sporotrichosis. It is localized in long bones (1186, 1214), preferentially those of the lower limbs; flat bones (1203, 1214); and hands (847, 1169, 1171, 1179, 1199, 1215). Tenosynovitis may be associated with hand osteomyelitis (1199).
Arthritis (1170, 1176, 1187, 1189, 1194, 1196, 1216–1220) and osteoarticular infection (847, 1173, 1184, 1185, 1187, 1190–1192, 1201, 1205, 1215, 1216, 1219–1229) comprise the majority of the cases (48%) described. The knee is the main infected joint, with 38 cases being reported (847, 1170, 1173, 1176, 1185, 1187, 1189–1192, 1194, 1196, 1200, 1202, 1205, 1215, 1216, 1218–1222, 1224–1227, 1229). Other localizations include the wrist (1184, 1185, 1187, 1219, 1221, 1223), ankle (1221, 1228), elbow (1187, 1192, 1194, 1201), and metacarpophalangeal joints (1194, 1217). Hip lesions have never been described. The infected joint may be just swollen without erythema or warmth and without any general signs, mimicking degenerative arthrosis (1194). When a single joint is infected, skin nodules (1189, 1190, 1194, 1196, 1200, 1220, 1223) or lung localizations (1196, 1222) are rarely present. One case of prosthetic knee joint infection has been described (1224).
The remaining cases of osteoarticular sporotrichosis are disseminated cases (42%) with multiple bone and joint lesions (1172, 1174, 1175, 1177, 1179, 1180, 1183, 1185, 1187, 1188, 1191–1195, 1197, 1198, 1204, 1206–1208, 1213, 1220, 1221, 1230–1245). Isolated vertebral localizations have never been described in the literature. Vertebral osteomyelitis is always associated with other disseminated lesions (1207, 1213). Subcutaneous abscesses with bone fistulae and spontaneous purulent discharge may be observed by using MRI (1175).
Laboratory Features
All cases of osteoarticular sporotrichosis were cases proven by bone biopsy or analysis of the synovial fluid, except for 10 cases (1174, 1179, 1183, 1186, 1187, 1199, 1215, 1223, 1245). Although direct examination of the synovial fluid never showed fungi, cultures were always reported to be positive.
Tan-, brown-, and black-pigmented filamentous colonies grow at 25°C. Microscopic slide examination may reveal lateral conidiophores with clusters of pyriform conidia appearing as flowers or bouquets (1173). Synovial biopsy specimens usually reveal granulomatous lesions (1206, 1246) or unspecific inflammatory infiltrates of mononuclear cells with large numbers of plasmacytes (1176). Silver staining may display typical yeast-like round forms, and cigar-shaped bodies of 3 to 10 μm can be seen in synovial biopsy specimens (1195, 1229, 1232) or bone biopsy specimens (1203). The majority of Sporothrix cells are located within macrophages (as many as 10 per phagocytic cell). In bone tissue, there are foci of caseous necrosis marginated by epithelioid cells, with an absence of fungi (1203). The diagnostic value of intradermal tests to detect delayed hypersensitivity using sporotrichin or peptide-rhamnomannan antigen is not reliable for the diagnosis of bone/joint sporotrichosis in view of the significant variations in specificity and sensitivity.
Diagnostic Imaging
Standard radiographs show well-defined lytic lesions with sclerotic halos and periosteal reactions associated with the disruption of cortical bone (1174). MRI is more specific for the description of osteoarticular lesions. For instance, in a case of knee osteoarticular infection, MRI demonstrated large complex joint effusions and bone marrow edema within the femoral condyles and tibial plateaus consistent with osteomyelitis (1173, 1229). MRI is also useful for screening for local complications of osteomyelitis, such as tibia fistula drainage into the adjacent tissues or abscess formation (1175).
Treatment and Outcome
Although epidemiological cutoff values for MICs are now better described, clinical breakpoints that reliably predict the clinical response to therapy are not available for Sporothrix species (1247). Since 2007, the IDSA has recommended itraconazole at 200 mg BID for at least 12 months as the first-line option for osteoarticular sporotrichosis (1248). This recommendation relies on one study comprising 15 patients with osteoarticular involvement (1249). Liposomal AmB at a dose of 3 to 5 mg/kg/day is preferred for the treatment of disseminated or osteoarticular sporotrichosis (1248). In a previous literature review, according to IDSA guidelines, a favorable outcome was achieved in 77% of osteoarticular sporotrichosis cases (853). The median duration of treatment was 180 days (range, 52 to 355 days), compared to the 12-month duration recommended by the IDSA. Perhaps greater success could be achieved by global adherence to a standard of 12 months of treatment.
Regarding other antifungal drugs, a phase II study assessing the safety and efficacy of different doses of fluconazole for the treatment of lymphocutaneous/osteoarticular/visceral sporotrichosis failed to demonstrate any benefit in osteoarticular or visceral sporotrichosis (1250). A potassium iodide solution was used in 23 evaluable osteoarticular cases as a first-line therapy (1191, 1195, 1217, 1220, 1221, 1230, 1233, 1236, 1242, 1243). Failure was reported in 56% of the cases (n = 13). In the same way, almost all of the patients treated with ketoconazole as the first-line option failed to respond or relapsed early (847, 1183, 1185, 1240, 1244).
There are insufficient clinical data to support the use of posaconazole for osteoarticular sporotrichosis. Posaconazole has been used in two cases as rescue therapy for disseminated sporotrichosis, one of whom had osteoarticular involvement (1180, 1251). One patient failed to respond and died (1180), and the other was cured with posaconazole in combination with liposomal AmB (1251). However, its efficacy has been assessed in murine models with both S. schenckii stricto sensu and S. brasiliensis strains (1252, 1253). Posaconazole, alone or in combination with AmB, was effective in the treatment of experimental murine disseminated sporotrichosis. In the same murine models, voriconazole had only modest efficacy against S. schenckii and no efficacy against S. brasiliensis (1254). Voriconazole is thus not recommended for the treatment of human sporotrichosis.
Surgical debridement is not routinely needed (1248). Both medical and surgical treatments were used in 67% of cases of osteoarticular sporotrichosis (853). For joint prosthesis infections, the sole case described with late-onset infection received long-term therapy without prosthesis removal (1224). Of note, the optimal treatment for late chronic bacterial joint prosthesis infections is surgery with prosthesis removal.
The outcome is generally good, without sequelae. Five deaths were reported as being attributable to sporotrichosis among 118 osteoarticular cases. In cases where osteoarticular involvement evolved for many months, it led to bone deformation and chronic disability (1172). Arthrodesis is sometimes required for the management of sporotrichosis of the joints (1194).
Careful consideration should be given to HIV-infected patients with fewer than 200 CD4+ T cells during treatment. Immune reconstitution inflammatory syndrome during osteoarticular or disseminated sporotrichosis treatment has been described (1179).
SPECIAL POPULATIONS
Osteoarticular Fungal Infections in Pediatric Patients
Epidemiology.
Osteoarticular (OA) fungal infections are rare in infants and children. However, they have been described in the literature, usually taking the form of osteomyelitis and/or arthritis (1, 2, 550, 572, 652, 736). In the Osteoarticular Mycoses Consortium database, there were 153 cases that occurred in neonates, infants, or children (<18 years of age) (1, 2, 550, 572, 652, 736). A summary of the pediatric cases analyzed by the consortium in its various studies is included in Table 8 (1, 2, 550, 572, 652, 736).
TABLE 8.
Pediatric cases of osteoarticular fungal infections extracted from the various studies of the Osteoarticular Mycoses Consortiuma
| Authors (reference), yr | Type of infection | No. of pediatric patients (age [yrs]) | Underlying condition(s) (no. of cases [%]) | Mechanism(s) of infection (no. of cases) | 3 most common sites | No. of sites (no. of cases) | Clinical symptoms (no. of cases) | Therapies (no. of cases) | Outcome(s) (no. of cases) |
|---|---|---|---|---|---|---|---|---|---|
| Gamaletsou et al. (1), 2012 | Candida OM | 37 (<18) | Neonates (10), low birth wt (14), Hemato (7), HCT (2), solid tumor (2) | Hematogenous (29), contiguous (5), direct inoculation (3) | Femur (21), humorous (17), others (19) | 1 bone, (15), 2 bones (8), ≥3 bones (14) | Local (31), limitation of movement (13), fever (12), draining pus (3) | AFT (25), surgery (1), AFT + surgery (10), amphotericin (22), azoles (6), combination AFT (3) | CR (16), PR (17), R (9), D (5) |
| Gamaletsou et al. (270), 2012 | Candida AR | 40 (<18) | Neonates (11), Hemato (5), HCT (2) | Hematogenous (38), contiguous (0), direct inoculation (2) | Knee (31), hip (10), ankle (5) | 1 bone (20), 2 bones (5), ≥3 bones (15) | Pain (22), edema (31), erythema (6), limitation of movement (20), fever (7), draining pus (2) | AFT (28), surgery (0), AFT + surgery (11), debridement (4), amputation (1), drainage (5), lavage (4), amphotericin (29), azoles (9), 5-FC (14), echinocandins (2) | CR (35), PR (5), R (3), D (2) |
| Gamaletsou et al. (572), 2017 | Aspergillus AR | 4 (<18); 1 neonate and 3 children | Hemato (2), CGD (1) | Hematogenous (3) | Intervertebral sites (T1–T3), knee, ankle, carpal, hip | 1 bone (1), ≥3 bones (3) | Pain (4), tenderness (4), draining pus (1), sinus formation (1) | AFT (2), AFT + surgery (1) | CR (3), D (1) |
| Gamaletsou et al. (550), 2014 | Aspergillus OM | 37 (<18) | Hemato (9), CGD (20) | Hematogenous (23), contiguous (11), direct inoculation (3) | Ribs (12), vertebrae (11), cranium (8) | 1 bone (20), 2 bones (5), ≥3 bones (12) | Local (25), limitation of movement (5), fever (15), draining pus (8) | NR specifically in children | CR (18), PR (10), R (1), D (12) |
| Taj-Aldeen et al. (652), 2015 | Non-Aspergillus OA | 34 (≤15) | Trauma/wound (25 [73.5]), immunosuppression (16 [47]) | Direct inoculation (27), hematogenous (7) contiguous (0) | Bone (19), lower limbs (15/19); joint (18), knee (14/18) | 1 bone (21), 2 bones (4), ≥3 bones (1) | Pain (30), limitation of movement (23), fever (19), inflammation (26) | AFT + surgery (21), AFT (10), surgery (3), amphotericin (16), azoles (9), combination AFT (9) | CR (29), PR (4), D (1) |
| Taj-Aldeen et al. (736), 2017 | Mucorales OA | 1 (0.5) | Liver transplant | Contiguous | Ulna | 1 bone | NR | AFT + surgery, amphotericin, voriconazole | CR |
OM, osteomyelitis; AR, arthritis; OA, osteoarticular; AFT, antifungal therapy; Hemato, hematological malignancy; HCT, hematopoietic cell transplantation; CR, complete response; PR, partial response; NR, no response; R, relapsed; D, death; 5-FC, 5-fluorocytosine; CGD, chronic granulomatous disease.
There are certain differences between pediatric and adult patients (1, 2, 550, 572, 652, 736). The most important differences are the underlying conditions in pediatric compared to adult cases. The underlying conditions in pediatric patients frequently associated with OA fungal infections are (i) neonatal age, especially prematurity; (ii) primary immunodeficiencies, mainly chronic granulomatous disease (CGD); (iii) acquired immunodeficiencies following many conditions, including hematological malignancies, transplantation, and the administration of immunosuppressive agents; and (iv) various predisposing conditions in immunocompetent children, such as trauma and other types of injury (1, 2, 550, 572, 652, 736). The first two categories are relatively more unique in pediatric patients, whereas the latter two categories are no different from those for adults.
Pathogenesis and host defenses.
Pathogenesis and host defenses depend on the underlying condition. For example, the pathogenesis of osteoarticular Candida infection is different in neonates in whom the infection is in both joints and adjacent bones and takes the form of osteoarthritis (656). This usually happens because there are no strict blood circulation borders between bones and joints, and inflammation usually disseminates to both areas.
In infants and children with primary immunodeficiencies, the most frequent underlying immune impairment is underlying CGD, in which NADPH-dependent oxidative fungal killing does not function properly due to phagocyte NADPH oxidase defects (1255). In these cases, the most frequent fungi causing OA infections are Aspergillus species (540, 1256, 1257). Aspergillus nidulans is uniquely found to cause OA infections in patients with CGD (540, 1256). In the database of the Osteoarticular Mycoses Consortium, 73% of the pediatric patients with Aspergillus species osteomyelitis suffered from CGD (550). This rate is much higher than in the general population of the analysis (adults and children), of whom only 15% of the patients suffered from CGD and the majority of the patients suffered from other underlying diseases. For osteomyelitis caused by Candida spp. and Aspergillus spp., the hematogenous spread of infection is the most common mode of infection, while for non-Aspergillus osteoarticular infections, direct inoculation is most common (2, 550, 652).
Clinical manifestations.
There are some unique clinical characteristics of OA fungal infections in pediatric patients. In premature neonates, arthritis and osteomyelitis usually occur together and manifest as painful swelling of the corresponding extremity (2). In older pediatric patients, the majority of cases are accompanied by local symptoms, including limitation of movement, pain, swelling (inflammatory signs), and fever (1, 2, 550, 572, 652, 736). Specifically, fever has been significantly more frequently reported in pediatric patients than in adults with non-Aspergillus OA infections (61% versus 22% [P < 0.001]) (652).
Although Aspergillus osteomyelitis has been shown to be more frequent in pediatric CGD patients than in adults, there has been no difference in the specific analysis of the Osteoarticular Mycoses Consortium database in non-Aspergillus cases (652). The reasons for this are unclear. It may be that CGD patients present with non-Aspergillus OA infection in adulthood as well, and thus, there is no age-specific difference. The numbers of cases of non-Aspergillus OA infection, however, have been small in both children and adults with CGD.
There are a number of differences in Aspergillus and non-Aspergillus osteomyelitis cases between pediatric and adult patients in the consortium database (550, 572, 652). In the series of Aspergillus osteomyelitis cases, rib involvement was significantly more frequently present in infants, toddlers, and children (12/37 [32%]) than in adults (15/141 [11%]) (P = 0.003). This difference may be attributed to the association between primary immunodeficiency, particularly CGD, and Aspergillus osteomyelitis in children (550). In addition, direct inoculation caused by trauma-associated infections was reported more frequently in adults (35/141 [25%]) than in children (3/37 [8%]) (550).
In the series of non-Aspergillus OA infections, lower limb infections were significantly more frequently reported in children, while vertebral osteomyelitis (due to hematogenous spread from a site of pulmonary infection or direct inoculation) was more frequently reported in adults (5). Regarding septic arthritis caused by non-Aspergillus filamentous fungi, the knee was the most common involvement (68%) in both adults and children (652). Although there were no significant differences in the numbers of immunocompromised patients, adults had fewer clinical symptoms and signs, such as fever, limitation of movement, or local inflammatory signs, than children (652).
Laboratory diagnostics.
Nonspecific inflammatory indices, such as the erythrocyte sedimentation rate and C-reactive protein level, are not different between children and adults with OA fungal infections (1–6). Reviews of different diseases and fungi have shown that inflammatory indices are increased but in no unique manner (1, 2, 550, 572, 652, 736). However, specifically for non-Aspergillus OA infections, higher mean CRP values have been reported for pediatric than for adult patients (110 ± 130 mg/L versus 47 ± 39 mg/L [P = 0.034]) (652).
In comparison, among serum biomarkers, (1→3)-β-d-glucan (BDG) has not been proven to be useful for pediatric patients, in contrast to adults, where it has been shown to aid in diagnosis (1258, 1259).
On the other hand, serum galactomannan has been proven to be a useful biomarker in both children and adults, with angioinvasive aspergillosis being most frequently reported in neutropenic patients (1258, 1259). However, its role is minimal in both adults and children with nonangioinvasive Aspergillus infections, such as those occurring in patients with CGD, nonneutropenic patients with organ transplantation, or immunocompetent patients with trauma. Notably, the existence of galactomannan in joint fluid is indicative of arthritis due to Aspergillus in both adults and children.
Diagnostic imaging.
Differences in imaging between adults and children with invasive fungal infections exist mainly in the pulmonary findings in filamentous fungal infections. For example, halo and crescent signs are not as frequently shown as nonspecific nodules and opacities in the lungs of children as they are in the lungs of adults (1260, 1261). However, ultrasound, CT scan, or MRI as well as technetium radionuclide scan of bones and joints show the same findings for patients of all ages.
Treatment and outcome.
The general principles for the treatment of various types of OA fungal infections are the same regardless of age (47, 48, 1262). Of course, in neonates and young children, the osteoarticular tissues are in a growing state, and any damage can permanently impair bone and joint growth and formation. Thus, it is very important to start treatment as early as possible in young children and to remove any purulent material from the joint, if possible. The general principles of OA fungal infections are the cleaning and removal of the damaged tissue, administration of the appropriate antifungal treatment, and decrease of the immunosuppressive treatment for the underlying disease, if this is feasible (383).
With regard to antifungal treatment, there are some agents that are not allowed to be used or have to be used with caution in young patients (1263). For example, voriconazole is not approved for use in children <2 years of age (Table 9) (47, 48, 1263). In addition, the dosages of antifungal agents in children may be very different (usually higher) than those in adults. For example, in order to achieve similar levels in the serum in young children, the dosage of voriconazole is not 3 mg/kg BID, which is usually administered to adults, but 9 mg/kg BID due to the different pharmacokinetics of the drug. When it is administered to infants, the dosage may be even higher in order to achieve therapeutic drug levels. In contrast, while we do not know much about liposomal amphotericin B in young children, we can use it for patients of all ages, including premature infants.
TABLE 9.
Antifungal therapy of osteoarticular fungal infections in infants and childrena
| Type of infection | Recommended drug treatment(s) and duration(s) | Pediatric dose(s) | Alternative treatment | Pediatric dose(s) for alternative treatment | Description |
|---|---|---|---|---|---|
| Candida osteomyelitis | Fluconazole for 6–12 mo or echinocandin for 2 wks followed by fluconazole for 6–12 mo (IDSA, strong recommendation; low-quality evidence) | Fluconazole at 12 mg/kg; caspofungin with loading dose of 70 mg/m2 followed by 50 mg/m2/day; micafungin at 2 mg/kg/day with the option to increase the dose to 4 mg/kg/day in children weighing <40 kg; neonates, 10 mg/kg/day or higher | Lipid formulation of AmB for at least 2 wks followed by fluconazole daily for 6–12 mo (IDSA, weak recommendation; low-quality evidence) | Lipid formulation of AmB at 3–5 mg/kg/day | Surgical debridement indication in selected cases (strong recommendation; low-quality evidence) |
| Candida septic arthritis | Fluconazole for 6 wks or echinocandin for 2 wks followed by fluconazole for at least 4 wks (IDSA, strong recommendation; low-quality evidence) | Fluconazole at 12 mg/kg/day; caspofungin with a loading dose of 70 mg/m2 followed by 50 mg/m2/day; micafungin at 2 mg/kg/day, with the option to increase the dose to 4 mg/kg/day in children weighing <40 kg, and 10 mg/kg/day for neonates | Lipid formulation of AmB daily for at least 2 wks followed by fluconazole daily for 4 wks (IDSA, weak recommendation; low-quality evidence) | Lipid formulation of AmB at 3–5 mg/kg/day | Surgical drainage indication for all cases of septic arthritis (strong recommendation; moderate-quality evidence); prosthetic device removal is recommended (strong recommendation; moderate quality of evidence); alternative chronic suppression with fluconazole (6 mg/kg) if the strain is susceptible |
| Aspergillus osteomyelitis | Voriconazole for a durationb of a minimum of 6–12 wks depending on the degree/duration of immunosuppression, site of disease, and evidence of disease improvement; longer courses (>6 mo) are frequently necessary (strong recommendation; low-quality evidence) | Loading dose of 9 mg/kg BID followed by 8 mg/kg BID; oral dose of 9 mg/kg BID (max dose of 350 mg) | Alternative of liposomal AmB; salvage with ABLC, caspofungin, micafungin, posaconazole, Itraconazole | ABLC at 5 mg/kg/day i.v. (ECILc guidelines); posaconazole at 800 mg/day orally q6h or q12h in children aged ≤13 yrs (ECIL); itraconazole at 5 mg/kg/day orally (children aged ≤2 yrs) q12h | Surgical intervention is recommended where feasible (strong recommendation; moderate-quality evidence) |
| Aspergillus septic arthritis | Voriconazole for a durationb of a minimum of 6–12 wks depending on the degree/duration of immunosuppression, site of disease, and evidence of disease improvement; longer courses (>6 mo) are frequently necessary (strong recommendation; low-quality evidence) | Loading dose of 9 mg/kg q12h followed by 8 mg/kg q12h; oral dose of 9 mg/kg q12h (max dose of 350 mg) | Alternative of liposomal AmB; salvage with ABLC, caspofungin, micafungin, posaconazole, itraconazole | ABLC at 5 mg/kg/day i.v. (ECIL guidelines); posaconazole at 800 mg/day orally q6h or q12h in children aged ≤13 yrs (ECIL); itraconazole at 5 mg/kg/day orally (children aged ≤2 yrs) q12h | Surgical intervention is recommended where feasible (strong recommendation; moderate-quality evidence) |
With regard to antifungal prevention of fungal infections, the principles and the high-risk factors in children are similar to those in adults. The differences are in the type of antifungal agent to be used because not all antifungal agents have been approved for pediatric patients. For example, posaconazole is recommended for adults (>18 years) and older children ≥13 years of age with the need for antifungal prophylaxis, whereas it has not been approved for younger children. Itraconazole or posaconazole can be used for antifungal prophylaxis in pediatric patients with CGD.
Fungal Prosthetic Joint Infections
Epidemiology.
Over the lifetime of a prosthetic joint, the rate of developing a PJI is approximately 1 to 2%, of which fewer than 1% are fungal PJIs (270, 1264). The largest series of fungal PJIs from two North American medical centers over 10 years, reporting on 41 cases, found that 61% of PJIs were caused by C. albicans and noted that the recent receipt of antibiotics and prolonged postoperative wound drainage were risk factors for fungal PJIs (1265).
A review of over 25,000 total knee arthroplasties performed between 2005 and 2009 in South Korea identified only 30 instances of fungal PJI (for an incidence of 0.12%) (1266). A comprehensive literature review of 164 cases of fungal hip and knee PJIs in 2013 found moderate preponderances of women compared to men (64%) and infected knees compared to hips (57%) (1267).
Concomitant bacterial infection was common, complicating one-third of these cases. Two multicenter cohorts of 28 (1266) and 31 (1268) patients with fungal PJI each found a lower rate of bacterial coinfection of 16%. The age at presentation was not reported by Kuiper et al. (1267); however, similar literature reviews have reported average ages of presentation in the late 60s (382, 1269, 1270). Fungal infections of prosthetic joints other than the knee and hip have been reported but only in small numbers (1271, 1272).
While a wide variety of yeasts and molds have been reported to cause prosthetic joint infections, including dimorphic fungi (1066, 1273–1275), hyaline hyphomycetes (1276), and dematiaceous fungi (1268), Candida species seem to cause the large majority. As a result, any meaningful review of fungal PJIs will primarily provide insight into the nature of Candida prosthetic joint infections; non-Candida PJIs are too infrequently encountered to make any specific inferences regarding clinical presentation, risk factors, or treatment. The published experience with Candida PJIs is comprised largely of case reports and small case series. Multiple systematic reviews of the literature have found Candida infections to account for >80% of the reported fungal PJIs, with C. albicans, followed by C. parapsilosis, being the most commonly identified species (1267, 1269, 1270). In the largest single multicenter series of patients with fungal PJIs, a compilation of 31 patients were identified from a consortium of six medical centers between 1999 and 2006, 28 (90.3%) of whom had Candida species infections, with over two-thirds of the infections being identified as C. albicans infections (1268).
While a wide array of risk factors for fungal PJIs has been suggested, including rheumatoid arthritis and other immunocompromising conditions, diabetes, obesity, and a previous bacterial prosthetic joint infection (1277), many of these risk factors overlap those for bacterial PJIs. Thus, their specificity for fungal PJIs is unclear. Prior revision arthroplasty in patients with fungal PJIs has frequently been reported, documented in 65% of patients in one multicenter cohort (1268) and 44% of patients reported in a systemic literature review (1267).
The specific risk factors associated with invasive Candida infections, such as profound immunosuppression (hematological malignancy or organ transplant), the receipt of broad-spectrum antibiotics, the presence of central venous catheters, and hemodialysis (1278), are not as frequently encountered in patients with Candida PJIs. It is not unusual for Candida PJIs to arise in the absence of ordinary risk factors, with 30 to 50% of reported patients having no risk factor for invasive Candida infection (332, 382, 1269).
Upwards of 50% or more of patients with Candida PJIs have undergone a prior surgical revision of the affected arthroplasty (1279, 1280). Unlike Candida osteomyelitis and septic arthritis (1, 2), concurrent or prior candidemia, or infection via contiguous foci, is rarely encountered in Candida PJIs (382, 1281). Candida PJIs following therapy for candidemia are rare but have been clearly described (1282, 1283).
Specific risk factors for non-Candida fungal PJIs are even less well understood owing to their infrequent occurrence. Other than some of the dimorphic yeasts, most fungi cause osteoarticular infections in only profoundly immunosuppressed patients or in the setting of trauma (550). However, a variety of yeasts and molds have been reported to sporadically cause prosthetic joint infection in seemingly otherwise healthy patients, including Aspergillus, Trichosporon, and Rhodotorula species (1284–1286). As many of the dimorphic fungi have been reported to cause PJIs, travel history may help establish a diagnosis (841, 1066).
Pathogenesis.
The pathogenesis of fungal PJI is likely similar to that of bacterial PJI. The joint can become infected hematogenously, or infection can be acquired during implantation. Less commonly, the joint can become infected via a contiguous focus or penetrating trauma exposing the prosthesis. As with other foreign-body infections, the formation of a biofilm plays an integral role in allowing these infections to develop. Many of the medically important fungi, including Candida, Aspergillus, and Trichosporon, can readily form biofilms (1287). Similar to their bacterial counterparts, fungi that cease planktonic growth and form biofilms can develop higher levels of resistance to antifungal agents (1288, 1289). Biofilm formation also allows fungal prosthetic joint infections to develop years after hematogenous seeding (1282, 1283) or following treatment of Candida septic arthritis (320).
Clinical manifestations.
The presentation of fungal PJI is often indistinguishable from that of chronic bacterial joint infection. Of the 31 patients reported in a multi-institutional study, none were reported to have any systemic signs or symptoms, including fever (1268). In a review of 164 patients with fungal PJIs (1267), the most common presenting symptoms were pain (78% of patients) and swelling (65%), while symptoms of acute inflammation (redness and fever) were found in fewer than 10% of the patients. This case series also demonstrated wide ranges from the time of prosthesis implantation to the onset of fungal PJI. The average time from onset to diagnosis following the most recent arthroplasty was 27 months, but the range was from 2 weeks to 22 years. The time interval between the onset of symptoms and diagnosis is a reflection of the typically subacute clinical presentation of fungal PJIs and the relative insensitivity of techniques to detect the presence of yeasts and molds. Local findings such as draining sinuses can also be found on presentation (1266). As with bacteria, fungal PJI may manifest as a metastatic complication of a multifocal hematogenous infection.
Laboratory features.
Synovial fluid studies and cultures, histopathology, and clinical presentation are generally required to establish a diagnosis of fungal PJI; both the IDSA and the Musculoskeletal Infection Society (MSIS) have established guidelines to assist clinicians (1289, 1290). Some, like Candida species, can be identified via culture of the synovial fluid or tissue and may be identified using routinely employed manual and automated microbiological techniques, without the use of specialized media (1289). In a review of 73 cases of Candida prosthetic joint infection, 45% were identified in an aspirate culture alone, 22% were identified in a culture of intraoperative tissue, and 25% were identified in both. In order to prevent a delay in diagnosis, the significance of finding Candida in such specimens needs to be appreciated: among 84 cases for whom the interpretation of the initial cultures was reported, 21% of the cultures were considered to be contaminants (1267). Notably, Candida species should never be regarded as a contaminant in intraoperative cultures of normally sterile prostheses.
Other fungi, such as endemic dimorphic fungal pathogens, may require alternative culture techniques and media to be identified. Therefore, the diagnosis of fungal PJIs, particularly non-Candida infections, requires a heightened level of clinical suspicion as routine testing can fail to identify the pathogen. In up to one-third of the reported cases, more than one aspirate culture was required to confirm the diagnosis (1289). The role of molecular diagnostics in microbiology is rapidly advancing and will very likely contribute greatly toward identifying fungal pathogens (1276).
As seen with bacterial PJIs, routine blood testing is rarely helpful in the diagnosis of fungal PJIs (81). Even systemic inflammatory markers (ESR and CRP), which are incorporated into the MSIS criteria, are relatively nonspecific and insensitive for fungal PJIs (1269, 1270). Likewise, neither inflammatory markers nor synovial fluid cell counts helped to differentiate between fungal and bacterial infections (1291).
Diagnostic imaging.
In cases where radiographic data were available, the most commonly reported radiological finding on plain film was loosening of the prosthesis; much less commonly, osteolysis or bony destruction was detected (1269, 1270). However, the absence of any imaging abnormalities is common. As with bacterial PJIs, routine radiology studies rarely have a significant role in establishing a diagnosis (81). The role of more advanced imaging, such as CT and/or MRI or nuclear imaging studies, is likely similar to that in the setting of more routine bacterial PJIs, but the results of such imaging modalities in the specific setting of fungal PJIs have infrequently been reported (1292).
Treatment and outcome.
As with bacterial PJIs, the optimal treatment for fungal PJIs requires both surgical and medical treatment. A series of 31 fungal PJIs (80% of which were caused by Candida species) noted rates of survivorship free of reinfection of 38% and 76% for hips and knees, respectively (88). The use of either modality without the other is rarely successful, and as with other foreign-body-associated infections, the likelihood of a cure of a fungal PJI is determined largely by whether the prosthesis can be removed or must be left in situ.
(i) Surgical therapy.
While surgery is required to successfully treat fungal PJIs, the optimal surgical approach to treatment has not been fully realized. Current IDSA guidelines address fungal infections only briefly, noting that they are considered to be difficult to treat, and therefore, a two-stage approach is recommended (1290). Hwang et al., reporting on the outcomes of two-stage exchanges for 30 fungal knee infections done at 4 medical centers, found that infection recurred after reimplantation in only 2 of the cases, with a microbiological success rate of over 90% (1266). Delayed reimplantation was performed at a mean of 9.5 weeks (range, 6 to 24 weeks). Two of the patients required repeat debridement with spacer exchange because of uncontrolled infection, prior to undergoing reimplantation. The mean follow-up time for noninfected cases was 4.3 years. However, the results from a contemporary series reporting on 31 cases treated at 6 medical centers were less encouraging: fewer than one-third of the patients undergoing resection arthroplasty could be cured with the completion of the two-stage approach. Of the 29 patients who underwent resection arthroplasty and spacer placement, only 19 were able to undergo delayed reimplantation. The average time between the two stages was 28 weeks (range, 8 weeks to 56 weeks). Infection was eradicated in only 9 of the 19 patients; the remaining 10 had persistent or relapsing infection. This represents a much lower success rate than those of similar treatments for bacterial PJIs. These more dismal results were reflected in a systemic review of the literature, which analyzed the outcomes of 164 reported cases of fungal PJIs (1267). Of the 107 patients who underwent a two-stage exchange and had a 2-year follow-up, only 58% were cured.
Several case reports have documented successful treatment with a one-stage approach (1293, 1294). The largest case series to date comprises 10 patients with Candida PJIs treated at a single center in Germany, reporting a successful cure in 9 patients with a mean follow-up time of 7 years (1295). The patients were treated with intravenous antifungal agents for a mean of 10 days, followed by oral antifungal treatment for a mean of 5 weeks. Antifungal agents were not used in the orthopedic cement. Additional studies will be required to determine if this level of success can be duplicated at other centers.
There are reports of the successful retention of infected implants with debridement and systemic antifungal agents alone (332, 1296). Likewise, some successful outcomes with antifungal chemotherapy alone, without any surgical intervention, have been reported (1297–1299). On the other hand, two case reports detailed successful treatment with surgery only; no systemic antifungals were given (349, 1279). Overall, despite anecdotal reports, combination strategies with adequate source control and potent antifungal agents are likely to be the optimal approach for a patient with a fungal PJI. Attempts to treat the patient without the removal of the infected hardware generally meet with poor outcomes and are not recommended (1266–1268).
(ii) Antifungal chemotherapy.
The choice of antifungal agent is dependent primarily on the identity and pattern of susceptibility of the fungus in question. While the specific treatment of prosthetic joint infections is generally not directly addressed, the IDSA has published treatment guidelines for a number of invasive fungal infections, which can help guide the choice of antifungal agent and the duration of treatment, as summarized in Table 10 (48, 67, 267, 903, 1058, 1134, 1300).
TABLE 10.
IDSA guidelines for the treatment of osteoarticular mycosesa
| Pathogen(s) | Antifungal agent | Duration | Description |
|---|---|---|---|
| Candida spp. | Fluconazole | Septic arthritis, 6 wks; osteomyelitis, 6–12 mo | The choice of antifungal agent should be guided by susceptibility testing |
| Echinocandin (caspofungin, micafungin, or anidulafungin) for at least 2 wks followed by fluconazole or | |||
| Liposomal AmB for at least 2 wks followed by fluconazole | |||
| Aspergillus spp. | Primary, voriconazole | No fewer than 8 wks to >6 mo | Guidelines recommend following the same treatment protocols as the ones for invasive pulmonary aspergillosis, but note that there is little experience with echinocandins for the treatment of Aspergillus OA infection |
| Alternative, liposomal AmB | |||
| Salvage, ABLC, caspofungin, micafungin, posaconazole, itraconazole | |||
| Blastomyces dermatitidis | Mild-to-moderate disease, itraconazole | At least 12 mo | |
| Severe disease, liposomal AmB for 2 wks followed by itraconazole | |||
| Coccidioides immitis | Mild-to-moderate disease, fluconazole or itraconazole | 3 yrs to indefinite | |
| Severe disease, liposomal AmB for 3 mo followed by fluconazole or itraconazole | |||
| Cryptococcus neoformans | Fluconazole for patients without cryptococcemia and with a single site of infection and no immunosuppressive risk factors | Maintenance therapy of fluconazole for 6–12 mo; depending on the immune status, patients may require long-term secondary prophylaxis with fluconazole | Osteoarticular infections are not specifically addressed in current IDSA guidelines; recommendations for extrapulmonary non-CNS cryptococcosis in immunocompetent patients are to follow the treatment protocol for CNS disease listed here (see the guidelines for separate recommendations for HIV-positive patients and transplant recipients) |
| Otherwise, (i) induction with AmB plus flucytosine for 4 wks, AmB for 6 wks; liposomal AmB or ABLC combined with flucytosine, if possible, for 4 wks; or AmB plus flucytosine for 2 wks (for patients at low risk for therapeutic failure [see guidelines]) or (ii) consolidation therapy with fluconazole (400–800 mg/day) for 8 wks | |||
| Histoplasma capsulatum | Mild-to-moderate disease, itraconazole | At least 12 mo | Histoplasma osteoarticular infections usually occur in the setting of disseminated disease |
| Severe disease, liposomal AmB for 2–6 wks followed by itraconazole | |||
| Sporothrix schenckii | Preferred, itraconazole | At least 12 mo | Alternative, liposomal AmB with a change to itraconazole after a favorable response is achieved |
Refer to IDSA guidelines for dosing recommendations. Abbreviations: ABLC, amphotericin B lipid complex; AmB, amphotericin B.
For prosthetic joint infections specifically, no standardized guidelines have been published. The body of published literature focuses on the treatment of Candida PJIs, with the overwhelming majority of cases reporting the use of AmB or fluconazole (1267). Treatment with echinocandins has been reported as well but generally as part of a dual-therapy regimen (389). A series of 17 PJI patients had favorable outcomes after treatment with surgery and echinocandins (1301).
Antifungal treatment protocols have varied greatly, with >20% reporting the use of multidrug regimens (389, 1270). Most authors recommend a minimum of 6 weeks, while some advocate longer treatments (1267). In some cases, patients were managed with chronic antifungal suppression (841, 1273). Success in a series of cases using intraoperative lavage with an AmB solution has been reported (270).
(iii) Spacers.
Data addressing the effectiveness of antifungal spacers and depot formulations of antifungal agents in orthopedic surgery are limited. Temporary arthroplasty spacers serve two functions: (i) they maintain the anatomical relationship at the arthroplasty site, which improves the ease of reimplantation, and (ii) they also can elute antimicrobial agents, which may help eradicate local residual infection. Several studies have reported on biodegradable (hydroxyapatite [HAP] and calcium sulfate products) and nonbiodegradable (polymethylmethacrylate [PMMA] bone cement) materials loaded with antifungal agents as adjunctive treatments for fungal PJIs, in the form of both beads and spacers. Various in vitro models have also been used in attempts to better understand the stability and elution properties of the available antifungals and to compare them to each other. As when assessing the utility of an antibiotic for use in bone cement for the treatment of a bacterial PJI, the ideal antifungal must possess several qualities (910). The antifungal must elute from the cement in a predictable and prolonged manner at levels that are clinically meaningful. The antifungal must be heat stable in order to survive the polymerization process and maintain biological function (which is typically more of an issue with PMMA than with degradable materials). It must not impair the mechanical properties of the cement and should not cause local tissue or systemic toxicity. The antifungal should also be available in a powder form as the excess volume of aqueous formulations added to the cement can weaken the cement’s mechanical properties. To date, consensus as to which antifungal agents are suitable candidates for inclusion in bone cement is lacking.
There are fewer than two dozen published studies addressing the in vitro properties of antifungal-loaded bone cement. The majority of these studies address the use of AmB in PMMA. It has a broad spectrum of antifungal activity, making it an attractive candidate for treatment; local depot formulations might minimize the systemic toxicity associated with parenteral use while delivering high doses to the local tissues. While AmB has been shown to be heat stable, retaining its activity after undergoing the polymerization process, its elution properties vary widely among studies, with some investigators finding little to no release of AmB (909, 1302, 1303) and others citing prolonged elution at therapeutic doses (910, 1304). Differences in the percentages (by weight) of AmB used as well as variations in assays likely contribute to the inconsistent findings. Reports of the effects of AmB on the compressive strength of the cement have been conflicting as well. One study found that AmB increased the compressive strength of the cement, without affecting the compressive modulus or compressive strain to failure, leading to the postulation that AmB binds to the cement, preventing effective elution (1303). Other studies also demonstrating poor elution qualities have not confirmed this finding (909, 1305). The potential of the liposomal formulation of AmB (LAB) has been assessed only once (1305). In a direct comparison with AmB, LAB was found to elute in a larger amount than AmB, but the compressive strength of the cement was weakened below the strength recommendations for implant fixation. Furthermore, the compressive strength further deteriorated as LAB eluted over time; this was not observed with AmB. The impact of these structural differences may be less relevant when the cement is used in a temporary spacer as opposed to being used for implant fixation.
Both fluconazole and voriconazole have also been found to be heat stable and to retain their antifungal properties following polymerization. Two studies have evaluated fluconazole’s elution properties. Using agar diffusion plates, Silverberg et al. found that fluconazole PMMA discs generated zones of inhibition against 24- to 28-h agar cultures of Candida parapsilosis (1302). Sealy et al. detected the elution of fluconazole from PMMA beads for 33 to 42 days and from hydroxyapatite for 18 to 22 days before becoming undetectable, compared to the elution of amphotericin from both cements for over 110 days (910).
Three experimental models of voriconazole elution have been reported, with varying results. Grimsrud et al. reported the detection of the stable elution of voriconazole (30%, by weight) from both PMMA and calcium sulfate beads over a 2-week period (1306), while Rouse et al. found that detectable levels in PMMA beads containing voriconazole at 7.5% (by weight) lasted only approximately 10 h (1307). In addition to the composition (by weight) of voriconazole in the cement, these studies also had important methodological differences, making a true comparison difficult. In a third study, using both 300 mg and 600 mg of voriconazole per batch of Simplex P acrylic cement, Miller et al. (1308) found the cumulative release of voriconazole to be dose dependent, with the rate of elution dropping rapidly after 7 days. This was the only one of the three studies to assess compressive strength. A significant loss of compressive strength with voriconazole was noted over time: by the end of 30 days, the compressive strength of the 600-mg model fell below that found in the commonly used antibacterial-loaded cement.
There are few data available regarding other antifungal agents. Neither Sealy et al. (910) nor Rouse et al. (1307) detected any elution of anidulafungin from PMMA beads, although Sealy et al. (910) did find that anidulafungin eluted from HAP beads for 18 to 22 days. Sealy et al. (910) assessed several other antifungal agents. No elution was detected from either PMMA or HAP beads infused with micafungin or terbinafine. Flucytosine performed comparably to fluconazole, with detectable elution from both PMMA and HAP beads for approximately 3 weeks. However, Silverberg et al. (1302) did not detect any zone of inhibition when using flucytosine-loaded PMMA discs, suggesting that flucytosine did not retain its activity following polymerization.
The published literature evaluating the clinical aspects of antifungal-loaded bone cement is sparse. A 2014 review of the literature identified seven cases of Candida PJIs where the outcomes of the use of antifungal-loaded PMMA were reported (1309). One patient underwent single-stage exchange arthroplasty using fluconazole-loaded cement to fix the prosthesis, and the remaining six underwent placement of antifungal-loaded cement as part of a two-stage exchange arthroplasty, two with beads and four with spacers. Successful outcomes were reported for five of these seven patients, including the patient who underwent the single-stage exchange, although the follow-up was limited to a mean of 17 months. AmB was used in three patients with spacers, and fluconazole was used in the other four. In three cases, two with fluconazole beads (1310) and one with an AmB spacer (1311), the concentration of the antifungal used was postoperatively assayed in the wound drainage fluid for several days, and therapeutic levels were noted. Following this literature review, three single-center case series reporting on the use of antifungal-loaded cement spacers have emerged; in each series, AmB was used in the spacer (1312–1314). In addition, a case report detailed the successful use of a dual-antifungal spacer to treat a C. albicans-infected total hip prosthesis. Voriconazole and AmB were used together in the cement, with detectable levels of each antifungal being found in the wound fluid over the first 72 h (1315).
Overall, the rarity of the diagnosis and heterogeneity in methods make it difficult to reach conclusions about the optimal formulations and strategies of depot antifungals for PJI therapy. Virtually no studies have explored depot antifungal cement for non-Candida fungal prosthetic joint infections, other than limited case reports including Blastomyces (1275) and histoplasmosis (1316).
(iv) Other modalities for antimicrobial therapy of PJI.
Given the high morbidity rates and limited success of conventional therapy for bacterial PJIs, some centers have investigated the use of intra-articular antibiotics instilled directly into the joint for a prolonged treatment course (1317). Whether this modality is effective for the treatment of fungal PJIs is not clear; the published literature on intra-articular treatment is limited to a single interesting report (1318) of two patients who were successfully treated with one-stage prosthesis exchange and 6 weeks of intra-articular amphotericin B.
Autoimmune Diseases and Osteoarticular Mycoses
Anti-TNF-α blockade and fungal infections.
TNF-α inhibitors are considered a milestone therapeutic strategy to control autoimmune rheumatic diseases (ARDs). The TNF-α pathway exerts a potent regulatory role on the innate immune system against infectious diseases by inducing a cascade of cytokines and interacting with inflammatory cells, leading to cell apoptosis. The risk of developing disseminated tuberculosis (TB) or activating latent tuberculosis increases dramatically when using anti-TNF-α blockers (1319). Histoplasmosis may also develop in patients from areas of endemicity (1320). Both infections elicit a granulomatous reaction. In contrast, the risk of developing other invasive fungal infections remains relatively low. A blockage in protection against invasive fungal disease and phagocyte recruitment will downregulate the Th1 response, compromising T cell activation, which is responsible for the innate immune reaction against fungi (1321).
The vast majority of fungal infections related to anti-TNF-α therapeutic components refer to patients with rheumatoid arthritis (RA), notably pertaining to the respiratory system (1321).
Candida osteoarticular mycoses and anti-TNF-α blockade.
A. Huang et al. reported a case of an 11-year-old patient with C. parapsilosis vertebral osteoarticular infection following infliximab administration for refractory Crohn’s disease (283). He was admitted to the hospital with worsening lumbosacral spine pain and urinary incontinence causing limitation of movement. There was no history of trauma, use of injected drugs, or preceding use of broad-spectrum antibiotics. MRI disclosed an abnormal enhancement of the first sacral body, compatible with sacral osteomyelitis. Infliximab administration was discontinued. Culture of a specimen obtained by CT-guided bone biopsy grew C. parapsilosis. Following fluconazole initiation, the clinical symptomatology improved rapidly, while lumbosacral spine MRI showed complete radiological resolution a few months later (283).
Candida osteomyelitis and autoimmune rheumatic diseases.
For patients suffering from ARDs, the affected bones and joints necessitate a broad differential diagnosis to include osteoarticular fungal infections. Osteomyelitis due to Candida spp. is being reported at an increasing frequency (1). A total of five well-described cases of Candida osteomyelitis in patients with ARD have been identified in the literature (1, 130, 175, 241, 244). Three patients suffered from systemic lupus erythematosus (SLE), one suffered from Bechet’s disease, and one suffered from RA (1).
(i) History.
The first case of Candida vertebral osteomyelitis and SLE was diagnosed in a 30-year-old female who presented with local pain and edema in the lumbar spine in association with radiological evidence of osteolysis, osteosclerosis, and extension into soft tissues. Candida albicans was repeatedly isolated from blood cultures (periphery and tip of the subclavian catheter) and bone specimens obtained via needle biopsy of the third lumbar vertebra (L3). The patient suffered from SLE-nephritis and had received glucocorticosteroids and parenteral nutrition in the preceding period. She achieved a complete response following treatment with ketoconazole for 210 days (241).
(ii) Epidemiology and clinical manifestations.
There were four female patients and one male patient (age range, 30 to 64 years). Three patients were receiving long-term corticosteroids, and one patient had neutropenia. The most common localizing symptoms were pain (5/5), limitation of movement (3/5), and fever (2/5). The infection affected the spine in four cases and the femur adjacent to the prosthetic hip joint, along with concomitant arthritis, in one case. Spinal involvement affected thoracic vertebrae (1 case) and lumbar vertebrae (3 cases). All cases were de novo infections.
(iii) Laboratory features.
Candida species in this patient population were isolated from cultures of bone tissue samples obtained through percutaneous, imaging-guided biopsy (all cases) and blood (one case). All patients had positive bone histology. In the patient with a fungal prosthetic joint infection, Candida glabrata had also been isolated from the urine and synovial fluid. In two patients, there were coinfections with other pathogens (namely, S. aureus and enterococci). Among the Candida species, C. albicans was identified in 3 patients, and C. glabrata was identified in 2. Three patients had moderately elevated ESR values, and two patients had mild leukocytosis.
(iv) Diagnostic imaging studies.
All four patients with spinal involvement had imaging (conventional radiography, CT, and/or MRI) findings compatible with spondylitis or spondylodiscitis, including osteolysis, erosion, and destruction of the affected vertebrae along with reduced intervertebral space. Other findings were osteonecrosis and sequestra. Two patients had paraspinal and/or soft tissue abscesses due to local extension of the infection.
(v) Pathogenesis.
Although candidemia had been documented in only one case, the pattern of dissemination in all four cases of spondylitis was considered hematogenous. Direct inoculation into the synovial fluid, development of septic arthritis, and extension of the infection to the adjacent femur were the mechanisms of infection in the patient with PJI.
(vi) Treatment and outcome.
Three patients were treated with a combination of surgery and antifungal therapy, while two patients were treated with antifungal agents only. Surgical techniques comprised debridement, stabilization, and drainage of paraspinal abscesses. The patient with fungal PJI underwent the removal of the prosthesis and replacement in two stages. Antifungal regimens in these five patients included AmB followed by fluconazole for 54 to 330 days, amphotericin B plus flucytosine for 30 days, AmB plus flucytosine and ketoconazole for 150 days, miconazole plus ketoconazole for 210 days, and AmB plus fluconazole and caspofungin for 54 days. Three patients achieved a complete response, while the remaining two patients had a partial response and relapse.
Candida bursitis and autoimmune rheumatic diseases.
Individual case reports indicate that Candida bursitis can be a painful and debilitating osteoarticular mycosis (1). Among 12 well-defined cases of Candida bursitis, 55% received systemic corticosteroids, and 2 received anti-TNF-α therapy (1). Four patients had an autoimmune rheumatic disease: two of them suffered from SLE, and the other two individuals had RA (298, 302, 1322). Another patient with long-standing RA and olecranon bursitis due to C. parapsilosis has also been reported (305).
(i) Epidemiology and clinical manifestations.
All five cases were de novo infections, with ages ranging from 59 to 73 years (mean, 65 years) and with a female predominance (female, 4/5). Two patients were receiving biological agents, two were receiving methotrexate, and one was receiving a combination of methotrexate and infliximab, while one patient was not receiving immunosuppressants at all. The onset of symptoms was typically slow and indolent. None of the patients had a fever; in contrast, all of them had local pain, edema, erythema, and limitation of movement. The most frequent sites of Candida bursitis were the olecranon bursae (3 cases), and the subacromial bursa and the wrist bursa were affected in one case each. Further complicating the differential diagnosis, two patients had concomitant arthritis and/or osteomyelitis, while in one case, there was an extension of the infection to the adjacent muscles.
(ii) Laboratory features.
Candida species were recovered from the synovial and/or bursal fluid from each of the 5 patients. Candida parapsilosis was isolated in three patients, and C. lusitaniae and C. albicans were isolated in one patient each. In one case, there was bacterial coinfection with S. aureus. Only one patient had a blood culture that was positive for C. albicans. Synovial/bursal fluid WBC counts were available for two patients and ranged from 3,100 cells/mm3 to 12,500 cells/mm3, with a differential neutrophil count ranging from 41% to 80%. Indices of inflammation, which were available for only one patient, were as follows: a WBC counts 7,000 cells/mm3 (neutrophils, 56%; lymphocytes, 33%), an ESR value of 48 mm/h, and a CRP level of 6.7 mg/L (0 to 1 mg/L).
(iii) Diagnostic imaging studies.
Imaging findings were available for two patients, with nonspecific soft tissue swelling of the bursae being the predominant radiological finding. However, in one patient, there was osteomyelitis with bone destruction, and in the other, there were extrabursal findings, including joint effusion, decreased articular space, and extension into adjacent soft tissues.
(iv) Pathogenesis.
Consistent with the recovery of Candida parapsilosis, direct inoculation was the predominant mechanism of infection in 3 cases (75%), as there was a history of preceding trauma, orthopedic surgical intervention, or direct corticosteroid injection. In on patient, there was hematogenous infection, as Candida bursitis developed after an episode of candidemia.
(v) Treatment and outcome.
The majority of patients (4/5) were treated with a combination of antifungal therapy and surgery, while one patient received antifungal therapy only. One patient received amphotericin B for 2 weeks, one received caspofungin and fluconazole for 20 days, one was treated initially with amphotericin B for 1 week and oral fluconazole for an additional week, and finally, one was treated with the combination of fluconazole plus 5-FC for 56 days. The latter patient, who also had contiguous Candida osteomyelitis and septic arthritis, had a persistent infection and reportedly received lifetime fluconazole. Contrary to the favorable outcomes of Candida bursitis reported by Gamaletsou et al. (1323), the outcomes for the five patients with ARD were ominous, as only two patients achieved a complete response, one after an initial relapse. Two patients had a partial response and relapse, while one patient died after a relapse.
Candida arthritis and autoimmune rheumatic diseases.
Among the 112 well-documented cases of Candida arthritis described by M. N. Gamaletsou et al. (2), 2 had RA as an underlying condition (313, 356). There have been four additional well-described cases of Candida arthritis in patients with ARD published since that time (389, 1283, 1324). Notably, in a study of the preoperative presence of microorganisms in affected knee joints of RA patients who needed total knee arthroplasty, in 2 out of 47 patients (53 knees), Candida spp. were isolated from intraoperative tissue cultures, while the patients had no signs of infection (387).
(i) Epidemiology and clinical manifestations.
The ages of the patients ranged from 17 to 77 years, with a female predominance (female/male ratio of 5:1). Four patients had RA, and two had SLE. Four patients were receiving chronic corticosteroids, two were receiving methotrexate, and one was receiving mycophenolate mofetil. Three patients had a hip infection, and three had a knee joint infection. All patients presented with symptoms and signs of septic arthritis, including pain, redness, edema, and limitation of function, and in two patients, a sinus tract draining pus was present.
(ii) Laboratory features.
For all patients, arthrocentesis produced cloudy synovial fluid, with a WBC count ranging from 5,995 cells/μL to 38,000 cells/μL and a predominance of neutrophils. CRP levels ranged from 2.25 mg/dL to 19.8 mg/dL, and ESR values ranged from 98 mm/h to 120 mm/h. Culture of the synovial fluid yielded C. albicans in two patients, C. glabrata in three, and C. orthopsilosis in one. None of the patients had a positive blood culture.
(iii) Pathogenesis.
All six cases were de novo infections. Four out of the six patients had Candida prosthetic joint infections, and two had Candida arthritis through direct inoculation via a contaminated corticosteroid injection.
Although direct inoculation seems to be the predominant pathogenic mechanism of Candida arthritis, an interesting theory suggests that Candida infection may lead to a cascade of autoimmune responses similar to those in RA (338). The joint synovium in post-Candida arthritis is similar to that of the rheumatoid “pannus.” When Candida affects the joints, it leads to CD4+ T cell activation targeting Candida epitopes within the infected joint, and this stimulates local inflammatory responses like those in RA. This persistent fungal stimulation may lead to severe joint degeneration and, ultimately, the development of an autoimmune profile reaction against Candida antigens.
(iv) Treatment and outcome.
Three of the four patients with Candida PJI had the prosthesis removed and an antibiotic spacer placed, and one underwent irrigation and debridement. All six patients received protracted antifungal therapy, with the duration of treatment ranging from 6 weeks to 12 months. The antifungal regimens included monotherapy with fluconazole, caspofungin, amphotericin B, and miconazole or combinations of voriconazole along with intra-articular amphotericin B or caspofungin plus fluconazole.
Regarding outcomes, the two patients with arthritis had a partial or no response during the follow-up period, and in one patient with PJI, above-knee amputation was necessary due to the persistence of the infection.
In summary, for patients suffering from ARD with signs and symptoms of septic arthritis, Candida arthritis should be considered in the differential diagnosis before a final clinical decision is reached, especially when severe immunosuppressive agents are considered in the therapeutic plan.
Dimorphic fungal osteoarticular infections and autoimmune rheumatic diseases.
(i) Talaromyces marneffei osteomyelitis and autoimmune rheumatic diseases.
Talaromyces marneffei infection of bones and joints is relatively rare and occurs in areas of endemicity such as Southeast Asia and southern China. There is only one well-described case of Talaromyces marneffei osteomyelitis in a patient with ARD (1149). The patient was a 30-year-old Filipino woman with a history of mixed connective tissue disease who developed multifocal Talaromyces marneffei osteomyelitis of the axial skeleton along with multiple soft tissue abscesses.
(ii) Osteoarticular histoplasmosis and autoimmune rheumatic diseases.
There is one well-described case of septic arthritis due to H. capsulatum in a patient with RA (1325). An 86-year-old female patient presented at the hospital with fever, malaise, and symptoms of single-shoulder joint arthritis (pain, erythema, and edema) following a history of recent trauma. The patient suffered from RA and was administered weekly methotrexate. Magnetic resonance imaging of the shoulder revealed effusion in the subacromial and subdeltoid bursae, whereas the glenohumeral joint had no evidence of adjacent osteomyelitis.
FUTURE DIRECTIONS
Osteoarticular mycoses are uncommon and debilitating infections that warrant investigation into several key areas: pathogenesis, clinical epidemiology, laboratory diagnosis, and antifungal therapeutics. Systematic investigations of the in vitro and in vivo innate host defenses and biofilm formation are needed to understand the basic pathogenesis of osteoarticular mycoses. The epidemiology of osteoarticular mycoses may be best understood through a multicenter, multinational, prospective registry, along with matched controls, to explore the epidemiology, risk factors, comparative effectiveness, and outcomes. Laboratory diagnosis can be strengthened through the integration of advanced imaging studies, such as [18F]FDG PET scanning technology, with molecular biomarkers to establish rapid noninvasive techniques that would lead to earlier treatment. Similar technologies can be applied for determining the optimal duration of therapy, as guided by the resolution of imaging and biomarker signals.
ACKNOWLEDGMENTS
This research was supported in part by grants to Thomas J. Walsh from the Save Our Sick Kids Foundation and the Henry Schueler 41&9 Foundation. This work also was supported in part by the Intramural Research Program of the National Institutes of Health (NIH) Clinical Center.
The opinions expressed in this article are the authors’ own and do not reflect the view of the NIH, NIH Clinical Center, the Department of Health and Human Services, or the U.S. government.
For Fig. 1 through 3 and 11, Sanjeet S. Dadwal photographed the images, prepared them for the manuscript, and wrote the legends for this report. Saad J. Taj-Aldeen photographed, prepared, and wrote the legends for Fig. 4, 6, and 8 and obtained the licenses and permissions for the reproduction of Fig. 5, 7, 9, and 10 in this report.
Biographies

Maria N. Gamaletsou, M.D., Ph.D., M.P.H., graduated Summa Cum Laude from the Medical School of the National and Kapodistrian University of Athens, Greece. She completed her Internal Medicine training at the Athens University Hospital Laikon and the New York Presbyterian Hospital, Weill Cornell Medical Center, Cornell University. She then completed a 2-year fellowship in Infectious Diseases at the Athens University Hospital Laikon. She completed her Ph.D. thesis at the Athens University Medical School and her master’s in Public Health at the School of Public Health, Athens, Greece. She served for 2 years as Specialist Registrar in two United Kingdom hospitals, the National Aspergillosis Centre, University Hospital of South Manchester, and St James’s University Hospital, Leeds. Currently, she has the position of Senior Consultant and serves as a member of the Infection Control Committee at the Athens University Hospital Laikon. She is a founding member of the International Osteoarticular Mycoses Study Consortium.

Blandine Rammaert, M.D., Ph.D., is a Professor of infectious and tropical diseases at Poitiers University in France. She is a senior researcher at INSERM U1070, advisor for infectious diseases at Poitiers Cancer Center, and head assistant of the Department of Infectious and Tropical Diseases of Poitiers University Hospital, France. She did a clinical fellowship at the Department of Infectious and Tropical Diseases at the Hôpital Necker-Enfants Malades in Paris (2010 to 2012) and a research fellowship at the epidemiology unit of the Institut Pasteur in Cambodia (2009 to 2010). She obtained a Ph.D. in experimental research on mucormycosis, cryptococcosis, and aspergillosis at University Paris Diderot, France (2015). Her major professional interests are invasive fungal infections and infectious diseases in cancer patients. She is the principal investigator of two national research programs funded by the French Ministry of Health on chronic disseminated candidiasis and antibiotherapy in febrile low-risk neutropenic patients involving French cancer centers.

Barry Brause, M.D., F.A.C.P., F.I.D.S.A., is a Professor of Clinical Medicine at Weill/Cornell University Medical College (W/CUMC), and he was Director of Infectious Diseases at the Hospital for Special Surgery (HSS) for 15 years (2006 to 2021). He has been a member of the Infectious Disease Division of W/CUMC since 1973. In collaboration with orthopedists at HSS, Dr. Brause designed the 2-stage protocol for treating prosthetic joint infections, which remains the most effective approach for eradicating prosthetic joint infections. He has authored chapters on infections with prostheses in bones and joints in four editions of Principles and Practice of Infectious Diseases and on osteomyelitis in three editions of Cecil Textbook of Medicine. He was president of the Musculoskeletal Infection Society (2018 to 2019), and he has been on its executive board for the past 5 years (2016 to 2021). He is a Fellow of both the American College of Physicians and the Infectious Diseases Society of America.

Marimelle A. Bueno, M.D., graduated from Far Eastern University-Dr. Nicanor Reyes Medical Foundation, Philippines. She did her research year at the NYP-Weill Cornell School of Medicine, New York, before completing 2 years of General Residency Surgery at St. Luke’s Medical Center, Global City, Philippines. She is very interested in Addiction and Preventive Medicine. She strongly believes that everything comes down to disease prevention. She is currently enjoying serving as a patient advocate while doing rotations in preparation for the 2023 residency match. She has completed at least 20 medical-surgical missions in underserved areas in the Philippines. In her downtime, you will find her in a choir for Sunday Mass, in one of the theaters in Times Square updating her Broadway to-do list, or in an art gallery staring at a Monet, Van Gogh, or Pollock.

Sanjeet S. Dadwal, M.D., F.A.C.P., is a Professor of Medicine and Chief of the Division of Infectious Diseases at the City of Hope National Medical Center in Duarte, CA, an NCI-designated Comprehensive Cancer Center. He is the head of the Antimicrobial Stewardship Program and the Infectious Disease lead for the Transplant Disease Team at City of Hope. Dr. Dadwal’s clinical and research interests include the management and outcomes of opportunistic infections in cancer patients, particularly recipients of hematopoietic stem cell transplant/cellular therapy. He serves on the steering committee and is cochair of the transplant infectious diseases special interest group of the American Society of Transplantation and Cellular Therapy.

Michael W. Henry, M.D., is an Assistant Attending Physician in Infectious Diseases and Internal Medicine at the Hospital for Special Surgery and an Assistant Professor of Clinical Medicine at Weill Cornell Medical School. Dr. Henry received his B.A. at Haverford College and his M.D. at the NYU School of Medicine. He then completed his internship in Internal Medicine at New York Hospital and his residency training and fellowship training at the NYU Medical Center. Dr. Henry provides infectious diseases consultative services to patients at HSS. His research focuses on the treatment and outcomes of orthopedic hardware infections, including prosthetic joint infections and fracture fixation hardware infections, as well as the prevention and management of prosthetic joint infections in patients with rheumatologic disease who are receiving immunosuppressive therapy.

Aspasia Katragkou, M.D., Ph.D., is an Assistant Professor of Pediatrics, Goryeb Children’s Hospital, Atlantic Health System, Thomas Jefferson University. She received her medical degree from the Aristotle University of Thessaloniki, Greece, and completed a residency in pediatrics in Greece and the United States. Her Pediatric Infectious Diseases fellowship was at Nationwide Children’s Hospital, Columbus, OH. Her primary area of interest is fungal and biofilm-related infections. Her research has contributed to our understanding of host-drug interactions and to the innovative utility of antimicrobial agents to combat serious life-threatening infections, including fungal biofilm-related infections.

Dimitrios P. Kontoyiannis, M.D., Sc.D., F.A.C.P., Ph.D. (Hon.), F.I.D.S.A., F.E.C.M.M., F.A.A.M., F.A.A.A.S., is the Robert C. Hickey Chair in Clinical Care and Deputy Head of the Division of Internal Medicine of the Division of Internal Medicine at the MD Anderson Cancer Center in Houston, TX. Dr. Kontoyiannis has authored over 650 peer-reviewed manuscripts and has given over 350 lectures at national/international conferences and academic institutions. He is considered the leading mycology expert worldwide (https://expertscape.com), with an H index of 116 and over 55,000 citations. His research group is credited for many sustained contributions to clinical, translational, and experimental mycology. He is the past president-elect of the Immunocompromised Host Society (2016 to 2018). He is the leader of the European Confederation of Medical Mycology (ECMM) Diamond Excellence in Mycology Center at the MD Anderson Cancer Center, the only U.S. center to receive such a designation by the ECMM.

Matthew W. McCarthy, M.D., is an Associate Professor of Medicine at Weill Cornell Medicine and a Staff Physician at New York Presbyterian Hospital. He has a degree in molecular biophysics and biochemistry from Yale and a medical degree from Harvard. Dr. McCarthy’s research focuses on novel antimicrobial agents for hospitalized patients.

Andy O. Miller, M.D., is a fellow of the Infectious Disease Society of America, an associate professor of clinical medicine at Weill Cornell Medicine, and Chief of the Division of Infectious Diseases at the Hospital for Special Surgery. He received a bachelor of science in biology from Yale College (1994) and a medical degree from Harvard Medical School (2001). He completed internal medicine training (2004) and infectious disease training (2006) at Columbia University and New York University, respectively. In addition to clinical care and administrative responsibilities, his research interests include orthopedic surgical infections and surgical outcomes in the COVID-19 era.

Brad Moriyama, Pharm.D., B.C.C.C.P., is a special volunteer with the NIH Clinical Center Critical Care Medicine Department in Bethesda, MD. He was previously an intensive care unit clinical pharmacist at the NIH Clinical Center Pharmacy Department in Bethesda, MD. He received his doctor of pharmacy degree from the University of Washington. He completed a general practice pharmacy residency at the Sacred Heart Medical Center in Spokane, WA, and a specialty pharmacy residency in infectious diseases/critical care at Henry Ford Hospital in Detroit, MI. He is a board-certified critical care pharmacist. His interests include antimicrobial pharmacotherapy in the critically ill patient.

Zoi Dorothea Pana, M.Sc., Ph.D., serves as Lecturer in Pediatrics at the School of Medicine of the European University of Cyprus. Dr. Pana completed her Ph.D. (2009 to 2012) in Genetic Immunodeficiencies and Infectious Diseases in Pediatrics, Medical Faculty, Aristotle University, Thessaloniki, Greece. Her dissertation was entitled Mannose Binding Lectin and Ficolin 2 Gene Polymorphisms and Infection Susceptibility in Children with Acute Lymphoblastic Leukemia. She holds two master’s degrees (M.Sc.), also through Aristotle University, in Medical Epidemiology and Methodology (2006 to 2008) and Nanotechnology and Nanosciences (2010 to 2014). She completed a Postdoctoral Fellowship in Infectious Disease (2016 to 2017) at Johns Hopkins Hospital, Hospital Epidemiology and Infection Control Department, Division of Infectious Diseases, Pediatric Infectious Diseases, and the Clinical Microbiology Department. Among her current research interests, Dr. Pana also serves as Principal Investigator conducting an ESPID-sponsored mycobiome multicenter study to evaluate the dynamic changes of the mycobiome in children with leukemia under exposure to chemotherapy and/or antimicrobial/antifungal agents.

Ruta Petraitiene, M.D., is an Assistant Professor of Research in Medicine at the Weill Medical College of Cornell University. She obtained her medical degree in the specialty of Obstetrician-Gynecologist at Kaunas Medical Institute (now the Lithuanian University of Health Sciences) in Kaunas, Lithuania. She completed the fellowship training program at the National Institutes of Health, NCI. Since 1996, she has been working in translational research in infectious diseases, with an emphasis on various areas of research in molecular diagnostics. She is also involved in developing new strategies for pharmacodynamically rational methods for the administration of existing antimicrobial agents as well as new compounds in development. With 26 years of direct laboratory experience in experimental work, she has authored 88 publications in peer-reviewed journals. She is a member of the ASM, ESCMID, MMSA, and IDSA.

Vidmantas Petraitis, M.D., is an Assistant Professor of Research in Medicine at the Weill Medical College of Cornell University. Dr. Petraitis obtained his medical degree in the specialty of Surgeon-Traumatologist at Kaunas Medical Institute (now the Lithuanian University of Health Sciences) in Kaunas, Lithuania. He completed the fellowship training program at the National Institutes of Health, NCI. He has been conducting translational research since 1996 in infectious diseases, with an emphasis on the pharmacology of antimicrobial agents. He is involved in developing new strategies for pharmacodynamically rational methods for the administration of existing antibacterial and antifungal agents as well as new compounds in development. With 26 years of direct laboratory experience in experimental pharmacology, Dr. Petraitis has authored more than 85 publications in peer-reviewed journals. He is a member of the ASM, ESCMID, MMSA, and IDSA.

Emmanuel Roilides, M.D., Ph.D., F.I.D.S.A., F.A.A.M., F.E.S.C.M.I.D., F.E.C.M.M., F.I.S.A.C., is Professor of Pediatrics-Infectious Diseases at the Aristotle University School of Medicine at Hippokration Hospital in Thessaloniki, Greece. He received his M.D. and Ph.D. from the University of Athens, Greece, and worked for 7 years at the NIH, Bethesda, MD. Since 1993, Professor Roilides has been a faculty member of Aristotle University. He currently directs the research laboratory and the Division of Infectious Diseases and chairs the 3rd Department of Pediatrics. He is a Board Member of the Special Unit for Biomedical Research and Education of the School of Medicine responsible for Basic and Translational Research. His research focuses on fungal infections and multiresistant Gram-negative bacteria. He is on the Editorial Boards of several international biomedical journals and the author of >640 peer-reviewed articles and book chapters (>440 in PubMed). He has been the coordinator or partner of several multicenter or multinational studies.

Jean-Pierre Sarkis, M.D., joined the Department of Infectious Disease at Weill Cornell Medicine (New York, NY) in 2018 as a Research Fellow, having graduated from St. George’s University in 2017. He has contributed to clinical research projects through data management, including drug-related clinical trials associated with the use of antifungal medications in critically ill and immunosuppressed patients. His interest in participating in the International Consortium for Osteoarticular Mycoses stems from the desire to synthesize multinational collaborations for the betterment of clinical practice in Infectious Disease and Microbiology. In his spare time, Dr. Sarkis enjoys taking in the beautiful sights, sounds, and activities of New York City.

Maria Simitsopoulou holds M.Sc. and Ph.D. degrees in Microbiology and Molecular Biology of Yeasts and is currently the head of the Infectious Diseases Laboratory of the 3rd Pediatrics Department at Aristotle University, Thessaloniki, Greece. She has strong expertise in the pharmacodynamics analysis of antimicrobial drugs against pathogens that cause systemic infections and in the immunomodulatory effects of drugs on host responses against fungal and bacterial biofilms. Her research interests also include molecular surveillance of resistance genes in preventing multidrug resistance in critical care units; she is active in projects on molecular diagnostics and infection control of resistant bacteria. She is involved in several European Union projects (NeoVanc, Neo IPC, and Value-Dx), providing expert opinion on molecular microbiology and diagnostics.

Nikolaos V. Sipsas, M.D., Ph.D., received his medical degree from the National and Kapodistrian University of Athens in Greece. He did his postdoctoral theses on the History of Medicine at the Medical School of Zurich University in Switzerland and on Infectious Diseases at the Athens University Medical School, Greece. He was then trained in Internal Medicine at the Athens Naval Hospital and the Athens University Laikon Hospital. He was subsequently trained as a research fellow in Infectious Diseases at Massachusetts General Hospital in Boston, MA. He is currently an attending physician at the Department of Medicine, head of the Infectious Disease-COVID Unit, and Chair of the Infection Control Committee at the Laikon General Hospital in Athens, Greece, and Professor at the Athens University Medical School. He is a reviewer in peer-reviewed journals in Infectious Diseases and Internal Medicine, and he has authored more than 130 peer-reviewed manuscripts.

Saad J. Taj-Aldeen, Ph.D., F.R.S.B., D.(A.B.M.M.), F.R.Cpath., is a Clinical Scientist in Medical Mycology at the Department of Laboratory Medicine and Pathology, Hamad Medical Corporation, Qatar. He received his Ph.D. from the University of Manchester, United Kingdom; American Board in Medical Microbiology and Infectious Diseases D.(A.B.M.M.); and Fellow of the Royal College of Pathologists (F.R.Cpath.). He developed the unit of Medical and Diagnostic Mycology. His research interests focus on the epidemiology, diagnosis, and fungal infections of immunocompromised individuals and antifungal susceptibility testing. He is appointed as Assistant Professor at Weill Cornell Medicine—Qatar. Dr. Taj-Aldeen has authored several journal articles, abstracts, and book chapters and is working closely with the International Osteoarticular Mycoses Consortium. He is a member of the Editorial Board of Medical Mycology, a member of ASM and ISHAM, and a Fellow of the Royal Society of Biology, London, United Kingdom. He is also serving as a guest editor for the journals Frontiers in Microbiology and Pathogens.

Valérie Zeller, M.D., F.E.S.C.M.I.D., is an Infectious Disease Physician and a Fellow of the European Society of Clinical Microbiology and Infectious Diseases. She received a medical degree from the Faculté de Médecine Paris Descartes in 1999 and is a specialist in Internal Medicine and Infectious Diseases. She is responsible for the Infectious Diseases Unit at the Groupe Hospitalier Diaconesses Croix Saint Simon in Paris. This multipurpose unit includes one of the National Reference Centres for Complex Osteoarticular Infections, which she has been managing with her orthopedic and microbiology colleagues since November 2002. This has enabled her to acquire significant expertise in this field. In addition to clinical and administrative tasks, she is involved in clinical research protocols and studies, teaching and continuing education in the field of bone and joint infections.

Olivier Lortholary, M.D., Ph.D., is an Exceptional Class Professor and Chair of the Department of Infectious and Tropical Diseases at the Hôpital Necker-Enfants Malades in Paris as well as the Section Head for Invasive Fungal Infections Faculty of 1000, part of the Infectious Diseases Faculty of the Institut Pasteur, where he serves as Deputy Director of the National Reference Center for Invasive Mycoses and Antifungals and codirector of the International Postgraduate School and MOOCS of Medical Mycology. He has contributed to several clinical trials and translational research studies, has authored numerous referenced publications (H factor of 110) and 3 books, and has participated in many plenary sessions at international microbiology or infectious diseases congresses. His key research interests include the pathophysiology, innovative diagnostic strategies, and new therapeutic approaches for invasive fungal infections in immunocompromised and neutropenic hosts and transplant patients, cryptococcosis, mucormycosis, and other infectious diseases. He is a member of various guideline expert panels.

Thomas J. Walsh, M.D., Ph.D. (Hon.), F.I.D.S.A., F.C.C.P., F.A.A.M., F.A.A.A.S., F.E.C.M.M., is Cofounder of the International Consortium for Osteoarticular Mycoses (ICOM). He and his colleagues Maria N. Gamaletsou (Greece), Olivier Lortholary (France), Blandine Rammaert (France), Nicholas V. Sipsas (Greece), and Barry Brause (United States) established ICOM in 2012. During the past decade, the co-founders and other esteemed colleagues, including the coauthors of this review, from 8 countries participated in the Consortium to collaborate in conducting a groundbreaking series of studies of human osteoarticular mycoses: Candida osteomyelitis, Candida arthritis, Candida bursitis, Aspergillus osteomyelitis, Aspergillus arthritis, osteoarticular mycoses caused by endemic dimorphic fungi, osteoarticular mucormycosis, and osteoarticular mycoses caused by hyaline and dematiaceous fungi in adult and pediatric patients. The Consortium then collaborated to write this definitive and comprehensive review of all major osteoarticular mycoses, which we believe will serve as a valuable landmark resource for understanding and managing these debilitating invasive fungal diseases.
REFERENCES
- 1.Gamaletsou MN, Kontoyiannis DP, Sipsas NV, Moriyama B, Alexander E, Roilides E, Brause B, Walsh TJ. 2012. Candida osteomyelitis: analysis of 207 pediatric and adult cases (1970-2011). Clin Infect Dis 55:1338–1351. 10.1093/cid/cis660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gamaletsou MN, Rammaert B, Bueno MA, Sipsas NV, Moriyama B, Kontoyiannis DP, Roilides E, Zeller V, Taj-Aldeen SJ, Miller AO, Petraitiene R, Lortholary O, Walsh TJ. 2016. Candida arthritis: analysis of 112 pediatric and adult cases. Open Forum Infect Dis 3:ofv207. 10.1093/ofid/ofv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuzibet JG, Squara P, Verdier JM, Lapalus P, Gratecos N, Cassuto JP, Chichmanian RM, Dujardin P. 1982. Candida albicans spondylitis—a case report with a study of bone penetration of 5-fluorocytosine and a review of the literature. Ann Med Interne (Paris) 133:410–415. [PubMed] [Google Scholar]
- 4.Brass C, Galgiani JN, Blaschke TF, Defelice R, O’Reilly RA, Stevens DA. 1982. Disposition of ketoconazole, an oral antifungal, in humans. Antimicrob Agents Chemother 21:151–158. 10.1128/AAC.21.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denes E, Boumediene A, Durox H, Oksman A, Saint-Marcoux F, Darde M-L, Gaulier J-M. 2007. Voriconazole concentrations in synovial fluid and bone tissues. J Antimicrob Chemother 59:818–819. 10.1093/jac/dkm023. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt-Hoffmann AH, Kato K, Townsend R, Potchoiba MJ, Hope WW, Andes D, Spickermann J, Schneidkraut MJ. 2017. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother 61:e01292-17. 10.1128/AAC.01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groll AH, Mickiene D, Piscitelli SC, Walsh TJ. 2000. Distribution of lipid formulations of amphotericin B into bone marrow and fat tissue in rabbits. Antimicrob Agents Chemother 44:408–410. 10.1128/AAC.44.2.408-410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fielding RM, Singer AW, Wang LH, Babbar S, Guo LS. 1992. Relationship of pharmacokinetics and drug distribution in tissue to increased safety of amphotericin B colloidal dispersion in dogs. Antimicrob Agents Chemother 36:299–307. 10.1128/AAC.36.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polak A. 1979. Pharmacokinetics of amphotericin B and flucytosine. Postgrad Med J 55:667–670. 10.1136/pgmj.55.647.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felton T, Troke PF, Hope WW. 2014. Tissue penetration of antifungal agents. Clin Microbiol Rev 27:68–88. 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evdoridou J, Roilides E, Bibashi E, Kremenopoulos G. 1997. Multifocal osteoarthritis due to Candida albicans in a neonate: serum level monitoring of liposomal amphotericin B and literature review. Infection 25:112–116. 10.1007/BF02113589. [DOI] [PubMed] [Google Scholar]
- 12.Latimer FG, Colitz CM, Campbell NB, Papich MG. 2001. Pharmacokinetics of fluconazole following intravenous and oral administration and body fluid concentrations of fluconazole following repeated oral dosing in horses. Am J Vet Res 62:1606–1611. 10.2460/ajvr.2001.62.1606. [DOI] [PubMed] [Google Scholar]
- 13.Conaughty JM, Khurana S, Banovac K, Martinez OV, Eismont FJ. 2004. Antifungal penetration into normal rabbit nucleus pulposus. Spine (Phila Pa 1976) 29:E289–E293. 10.1097/01.brs.0000131210.59316.2d. [DOI] [PubMed] [Google Scholar]
- 14.Ripp SL, Aram JA, Bowman CJ, Chmielewski G, Conte U, Cross DM, Gao H, Lewis EM, Lin J, Liu P, Schlamm HT. 2012. Tissue distribution of anidulafungin in neonatal rats. Birth Defects Res B Dev Reprod Toxicol 95:89–94. 10.1002/bdrb.20347. [DOI] [PubMed] [Google Scholar]
- 15.Visser GW, Boele S, Knops GH, Herscheid JD, Hoekstra A. 1985. Synthesis and biodistribution of [18F]-5-fluorocytosine. Nucl Med Commun 6:455–459. 10.1097/00006231-198508000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Heykants J, Van Peer A, Van de Velde V, Van Rooy P, Meuldermans W, Lavrijsen K, Woestenborghs R, Van Cutsem J, Cauwenbergh G. 1989. The clinical pharmacokinetics of itraconazole: an overview. Mycoses 32(Suppl 1):67–87. 10.1111/j.1439-0507.1989.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 17.Cushing RD, Fulgenzi WR. 1997. Synovial fluid levels of fluconazole in a patient with Candida parapsilosis prosthetic joint infection who had an excellent clinical response. J Arthroplasty 12:950. 10.1016/S0883-5403(97)90166-2. [DOI] [PubMed] [Google Scholar]
- 18.Noyes FR, McCabe JD, Fekety FR, Jr.. 1973. Acute Candida arthritis. Report of a case and use of amphotericin B. J Bone Joint Surg Am 55:169–176. [PubMed] [Google Scholar]
- 19.Farrell JB, Person DA, Lidsky MD, Hopfer RL, Musher DM. 1978. Candida tropicalis arthritis—assessment of amphotericin B therapy. J Rheumatol 5:267–271. [PubMed] [Google Scholar]
- 20.Silveira LH, Cuellar ML, Citera G, Cabrera GE, Scopelitis E, Espinoza LR. 1993. Candida arthritis. Rheum Dis Clin North Am 19:427–437. 10.1016/S0889-857X(21)00195-2. [DOI] [PubMed] [Google Scholar]
- 21.Kohli R, Hadley S. 2005. Fungal arthritis and osteomyelitis. Infect Dis Clin North Am 19:831–851. 10.1016/j.idc.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Birt MC, Anderson DW, Bruce Toby E, Wang J. 2017. Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop 14:45–52. 10.1016/j.jor.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simitsopoulou M, Chlichlia K, Kyrpitzi D, Walsh TJ, Roilides E. 2018. Pharmacodynamic and immunomodulatory effects of micafungin on host responses against biofilms of Candida parapsilosis in comparison to those of Candida albicans. Antimicrob Agents Chemother 62:e00478-18. 10.1128/AAC.00478-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simitsopoulou M, Peshkova P, Tasina E, Katragkou A, Kyrpitzi D, Velegraki A, Walsh TJ, Roilides E. 2013. Species-specific and drug-specific differences in susceptibility of Candida biofilms to echinocandins: characterization of less common bloodstream isolates. Antimicrob Agents Chemother 57:2562–2570. 10.1128/AAC.02541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escola-Verge L, Rodriguez-Pardo D, Lora-Tamayo J, Morata L, Murillo O, Vilchez H, Sorli L, Carrion LG, Barbero JM, Palomino-Nicas J, Bahamonde A, Jover-Saenz A, Benito N, Escudero R, Sampedro MF, Vidal RP, Gomez L, Corona PS, Almirante B, Ariza J, Pigrau C. Study Group on Osteoarticular Infections of the Spanish Society of Clinical Microbiology and Infectious Diseases (GEIO-SEIMC), Spanish Network for Research in Infectious Pathology (REIPI). 2018. Candida periprosthetic joint infection: a rare and difficult-to-treat infection. J Infect 77:151–157. 10.1016/j.jinf.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Loo AS, Muhsin SA, Walsh TJ. 2013. Toxicokinetic and mechanistic basis for the safety and tolerability of liposomal amphotericin B. Expert Opin Drug Saf 12:881–895. 10.1517/14740338.2013.827168. [DOI] [PubMed] [Google Scholar]
- 27.Adler-Moore J, Lewis RE, Bruggemann RJM, Rijnders BJA, Groll AH, Walsh TJ. 2019. Preclinical safety, tolerability, pharmacokinetics, pharmacodynamics, and antifungal activity of liposomal amphotericin B. Clin Infect Dis 68:S244–S259. 10.1093/cid/ciz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groll AH, Rijnders BJA, Walsh TJ, Adler-Moore J, Lewis RE, Bruggemann RJM. 2019. Clinical pharmacokinetics, pharmacodynamics, safety and efficacy of liposomal amphotericin B. Clin Infect Dis 68:S260–S274. 10.1093/cid/ciz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis RE. 2011. Current concepts in antifungal pharmacology. Mayo Clin Proc 86:805–817. 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellmann R. 2007. Clinical pharmacokinetics of systemically administered antimycotics. Curr Clin Pharmacol 2:37–58. 10.2174/157488407779422311. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee PK, Long L, Kim HG, Ghannoum MA. 2009. Amphotericin B lipid complex is efficacious in the treatment of Candida albicans biofilms using a model of catheter-associated Candida biofilms. Int J Antimicrob Agents 33:149–153. 10.1016/j.ijantimicag.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Tobudic S, Lassnigg A, Kratzer C, Graninger W, Presterl E. 2010. Antifungal activity of amphotericin B, caspofungin and posaconazole on Candida albicans biofilms in intermediate and mature development phases. Mycoses 53:208–214. 10.1111/j.1439-0507.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 33.Vogelsinger H, Weiler S, Djanani A, Kountchev J, Bellmann-Weiler R, Wiedermann CJ, Bellmann R. 2006. Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J Antimicrob Chemother 57:1153–1160. 10.1093/jac/dkl141. [DOI] [PubMed] [Google Scholar]
- 34.Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Dasbach EJ, Platt R. 2001. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis 32:686–693. 10.1086/319211. [DOI] [PubMed] [Google Scholar]
- 35.Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Flörl C, Lortholary O, Meis JF, Meletiadis J, Muñoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, van Diepeningen A, Verweij P, Petrikkos G. European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 20(Suppl 3):5–26. 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 36.Koehler P, Tacke D, Cornely OA. 2016. Bone and joint infections by Mucorales, Scedosporium, Fusarium and even rarer fungi. Crit Rev Microbiol 42:158–171. 10.3109/1040841X.2014.910749. [DOI] [PubMed] [Google Scholar]
- 37.Cornely OA, Vehreschild JJ, Ruping MJGT. 2009. Current experience in treating invasive zygomycosis with posaconazole. Clin Microbiol Infect 15(Suppl 5):77–81. 10.1111/j.1469-0691.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 38.Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, Kontoyiannis DP, Walsh TJ. 2012. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis 54:S73–S78. 10.1093/cid/cir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagano L, Cornely OA, Busca A, Caira M, Cesaro S, Gasbarrino C, Girmenia C, Heinz WJ, Herbrecht R, Lass-Florl C, Nosari A, Potenza L, Racil Z, Rickerts V, Sheppard DC, Simon A, Ullmann AJ, Valentini CG, Vehreschild JJ, Candoni A, Vehreschild MJGT. 2013. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: a report from the SEIFEM and FUNGISCOPE registries. Haematologica 98:e127–e130. 10.3324/haematol.2012.083063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyvernitakis A, Torres HA, Jiang Y, Chamilos G, Lewis RE, Kontoyiannis DP. 2016. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect 22:811.e1–811.e8. 10.1016/j.cmi.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Howard SJ, Lestner JM, Sharp A, Gregson L, Goodwin J, Slater J, Majithiya JB, Warn PA, Hope WW. 2011. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J Infect Dis 203:1324–1332. 10.1093/infdis/jir023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uppuluri P, Srinivasan A, Ramasubramanian A, Lopez-Ribot JL. 2011. Effects of fluconazole, amphotericin B, and caspofungin on Candida albicans biofilms under conditions of flow and on biofilm dispersion. Antimicrob Agents Chemother 55:3591–3593. 10.1128/AAC.01701-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatzimoschou A, Katragkou A, Simitsopoulou M, Antachopoulos C, Georgiadou E, Walsh TJ, Roilides E. 2011. Activities of triazole-echinocandin combinations against Candida species in biofilms and as planktonic cells. Antimicrob Agents Chemother 55:1968–1974. 10.1128/AAC.00959-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G. 2011. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother 55:2092–2097. 10.1128/AAC.01189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott SA, Agundez J, Wingard JR, McLeod HL, Klein TE, Cross SJ, Caudle KE, Walsh TJ. 2017. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharmacol Ther 102:45–51. 10.1002/cpt.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh TJ, Moriyama B, Penzak SR, Klein TE, Caudle KE. 2018. Response to “Impact of CYP3A4 genotype on voriconazole exposure: new insights into the contribution of CYP3A4*22 to metabolism of voriconazole.” Clin Pharmacol Ther 103:187. 10.1002/cpt.811. [DOI] [PubMed] [Google Scholar]
- 47.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:E1–E50. 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patterson TF, Thompson GR, III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-AH, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:E1–E60. 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 53:24–34. 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mouas H, Lutsar I, Dupont B, Fain O, Herbrecht R, Lescure F-X, Lortholary O. Voriconazole/Bone Invasive Aspergillosis Study Group. 2005. Voriconazole for invasive bone aspergillosis: a worldwide experience of 20 cases. Clin Infect Dis 40:1141–1147. 10.1086/428734. [DOI] [PubMed] [Google Scholar]
- 51.Studemeister A, Stevens DA. 2011. Aspergillus vertebral osteomyelitis in immunocompetent hosts: role of triazole antifungal therapy. Clin Infect Dis 52:e1–e6. 10.1093/cid/ciq039. [DOI] [PubMed] [Google Scholar]
- 52.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann J-W, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer, Global Aspergillus Study Group. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415. 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs SE, Petraitis V, Small CB, Walsh TJ. 2017. Orphan drugs for the treatment of aspergillosis: focus on isavuconazole. Orphan Drugs Res Rev 7:37–46. 10.2147/ODRR.S126518. [DOI] [Google Scholar]
- 54.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee DG, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, III, Lee M, Maher RM, Schmitt-Hoffmann AH, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387:760–769. 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira JAG, Carr JH, Starling CEF, de Resende MA, Donlan RM. 2009. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob Agents Chemother 53:4377–4384. 10.1128/AAC.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiori B, Posteraro B, Torelli R, Tumbarello M, Perlin DS, Fadda G, Sanguinetti M. 2011. In vitro activities of anidulafungin and other antifungal agents against biofilms formed by clinical isolates of different Candida and Aspergillus species. Antimicrob Agents Chemother 55:3031–3035. 10.1128/AAC.01569-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lejko-Zupanc T, Mozina E, Vrevc F. 2005. Caspofungin as treatment for Candida glabrata hip infection. Int J Antimicrob Agents 25:273–274. 10.1016/j.ijantimicag.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Sim JPY, Kho BCS, Liu HSY, Yung R, Chan JCW. 2005. Candida tropicalis arthritis of the knee in a patient with acute lymphoblastic leukaemia: successful treatment with caspofungin. Hong Kong Med J 11:120–123. [PubMed] [Google Scholar]
- 59.Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. 2006. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis 38:728–730. 10.1080/00365540500447192. [DOI] [PubMed] [Google Scholar]
- 60.Yang SC, Shao PL, Hsueh PR, Lin KH, Huang LM. 2006. Successful treatment of Candida tropicalis arthritis, osteomyelitis and costochondritis with caspofungin and fluconazole in a recipient of bone marrow transplantation. Acta Paediatr 95:629–630. 10.1080/08035250500491629. [DOI] [PubMed] [Google Scholar]
- 61.Slenker AK, Keith SW, Horn DL. 2012. Two hundred and eleven cases of Candida osteomyelitis: 17 case reports and a review of the literature. Diagn Microbiol Infect Dis 73:89–93. 10.1016/j.diagmicrobio.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Neofytos D, Huprikar S, Reboli A, Schuster M, Azie N, Franks B, Horn D. 2014. Treatment and outcomes of Candida osteomyelitis: review of 53 cases from the PATH Alliance registry. Eur J Clin Microbiol Infect Dis 33:135–141. 10.1007/s10096-013-1939-0. [DOI] [PubMed] [Google Scholar]
- 63.Francis P, Walsh TJ. 1992. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis 15:1003–1018. 10.1093/clind/15.6.1003. [DOI] [PubMed] [Google Scholar]
- 64.Hope WW, Warn PA, Sharp A, Howard S, Kasai M, Louie A, Walsh TJ, Drusano GL, Denning DW. 2006. Derivation of an in vivo drug exposure breakpoint for flucytosine against Candida albicans and impact of the MIC, growth rate, and resistance genotype on the antifungal effect. Antimicrob Agents Chemother 50:3680–3688. 10.1128/AAC.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vermes A, Guchelaar HJ, Dankert J. 2000. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother 46:171–179. 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 66.Weisse ME, Person DA, Berkenbaugh JT, Jr.. 1993. Treatment of Candida arthritis with flucytosine and amphotericin B. J Perinatol 13:402–404. [PubMed] [Google Scholar]
- 67.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anonymous. 2018. WHO guidelines approved by the Guidelines Review Committee, guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 69.Miyakis S, van Hal SJ, Ray J, Marriott D. 2010. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect 16:927–933. 10.1111/j.1469-0691.2009.02990.x. [DOI] [PubMed] [Google Scholar]
- 70.Stott KE, Hope WW. 2017. Therapeutic drug monitoring for invasive mould infections and disease: pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother 72:i12–i18. 10.1093/jac/dkx029. [DOI] [PubMed] [Google Scholar]
- 71.Balakrishnan I, Shorten RJ. 2016. Therapeutic drug monitoring of antimicrobials. Ann Clin Biochem 53:333–346. 10.1177/0004563215618981. [DOI] [PubMed] [Google Scholar]
- 72.John J, Loo A, Mazur S, Walsh TJ. 2019. Therapeutic drug monitoring of systemic antifungal agents: a pragmatic approach for adult and pediatric patients. Expert Opin Drug Metab Toxicol 15:881–895. 10.1080/17425255.2019.1671971. [DOI] [PubMed] [Google Scholar]
- 73.Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, Song KH, Choe PG, Kim NJ, Jang IJ, Oh MD, Yu KS. 2012. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis 55:1080–1087. 10.1093/cid/cis599. [DOI] [PubMed] [Google Scholar]
- 74.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Brüggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux J-P, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Löffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinkó J, Skiada A, et al. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24(Suppl 1):e1–e38. 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Jin H, Wang T, Falcione BA, Olsen KM, Chen K, Tang H, Hui J, Zhai S. 2016. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother 71:1772–1785. 10.1093/jac/dkw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dekkers BGJ, Bakker M, van der Elst KCM, Sturkenboom MGG, Veringa A, Span LFR, Alffenaar JC. 2016. Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep 10:51–61. 10.1007/s12281-016-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dolton MJ, Ray JE, Marriott D, McLachlan AJ. 2012. Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob Agents Chemother 56:2806–2813. 10.1128/AAC.05900-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ. 2018. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother 62:e00585-18. 10.1128/AAC.00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhagavatula S, Vale L, Evans J, Carpenter C, Barnes RA. 2014. Scedosporium prolificans osteomyelitis following penetrating injury: a case report. Med Mycol Case Rep 4:26–29. 10.1016/j.mmcr.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69:1162–1176. 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 83.Bjarnsholt T. 2013. The role of bacterial biofilms in chronic infections. APMIS Suppl 121:1–58. 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 84.Zimmerli W, Sendi P. 2017. Orthopaedic biofilm infections. APMIS 125:353–364. 10.1111/apm.12687. [DOI] [PubMed] [Google Scholar]
- 85.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 52:13–22. 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 86.Dutronc H, Dauchy FA, Cazanave C, Rougie C, Lafarie-Castet S, Couprie B, Fabre T, Dupon M. 2010. Candida prosthetic infections: case series and literature review. Scand J Infect Dis 42:890–895. 10.3109/00365548.2010.498023. [DOI] [PubMed] [Google Scholar]
- 87.Bartalesi F, Fallani S, Salomoni E, Marcucci M, Meli M, Pecile P, Cassetta MI, Latella L, Bartoloni A, Novelli A. 2012. Candida glabrata prosthetic hip infection. Am J Orthop (Belle Mead NJ) 41:500–505. [PubMed] [Google Scholar]
- 88.Brown TS, Petis SM, Osmon DR, Mabry TM, Berry DJ, Hanssen AD, Abdel MP. 2018. Periprosthetic joint infection with fungal pathogens. J Arthroplasty 33:2605–2612. 10.1016/j.arth.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Darouiche RO. 2001. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis 33:1567–1572. 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 90.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183:5385–5394. 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nett JE, Zarnowski R, Cabezas-Olcoz J, Brooks EG, Bernhardt J, Marchillo K, Mosher DF, Andes DR. 2015. Host contributions to construction of three device-associated Candida albicans biofilms. Infect Immun 83:4630–4638. 10.1128/IAI.00931-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rochford ET, Richards RG, Moriarty TF. 2012. Influence of material on the development of device-associated infections. Clin Microbiol Infect 18:1162–1167. 10.1111/j.1469-0691.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- 93.Chandra J, Patel JD, Li J, Zhou G, Mukherjee PK, McCormick TS, Anderson JM, Ghannoum MA. 2005. Modification of surface properties of biomaterials influences the ability of Candida albicans to form biofilms. Appl Environ Microbiol 71:8795–8801. 10.1128/AEM.71.12.8795-8801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Southwood RT, Rice JL, McDonald PJ, Hakendorf PH, Rozenbilds MA. 1985. Infection in experimental hip arthroplasties. J Bone Joint Surg Br 67:229–231. 10.1302/0301-620X.67B2.3980532. [DOI] [PubMed] [Google Scholar]
- 95.O’Connor L, Lahiff S, Casey F, Glennon M, Cormican M, Maher M. 2005. Quantification of ALS1 gene expression in Candida albicans biofilms by RT-PCR using hybridisation probes on the LightCycler. Mol Cell Probes 19:153–162. 10.1016/j.mcp.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 96.Blankenship JR, Mitchell AP. 2006. How to build a biofilm: a fungal perspective. Curr Opin Microbiol 9:588–594. 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B. 2011. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One 6:e17046. 10.1371/journal.pone.0017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsui C, Kong EF, Jabra-Rizk MA. 2016. Pathogenesis of Candida albicans biofilm. Pathog Dis 74:ftw018. 10.1093/femspd/ftw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis K. 2010. Persister cells. Annu Rev Microbiol 64:357–372. 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 100.Flemming HC, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8:623–633. 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 101.Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E, Philippe B, Kauffmann-Lacroix C, Latge JP, Beauvais A. 2010. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol 12:405–410. 10.1111/j.1462-5822.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 102.Baillie GS, Douglas LJ. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother 46:397–403. 10.1093/jac/46.3.397. [DOI] [PubMed] [Google Scholar]
- 103.Hawser SP, Baillie GS, Douglas LJ. 1998. Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol 47:253–256. 10.1099/00222615-47-3-253. [DOI] [PubMed] [Google Scholar]
- 104.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj Sahraoui A, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR. 2014. Novel entries in a fungal biofilm matrix encyclopedia. mBio 5:e01333-14. 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez LR, Casadevall A. 2007. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol 73:4592–4601. 10.1128/AEM.02506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katragkou A, Kruhlak MJ, Simitsopoulou M, Chatzimoschou A, Taparkou A, Cotten CJ, Paliogianni F, Diza-Mataftsi E, Tsantali C, Walsh TJ, Roilides E. 2010. Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. J Infect Dis 201:1941–1949. 10.1086/652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Katragkou A, Simitsopoulou M, Chatzimoschou A, Georgiadou E, Walsh TJ, Roilides E. 2011. Effects of interferon-gamma and granulocyte colony-stimulating factor on antifungal activity of human polymorphonuclear neutrophils against Candida albicans grown as biofilms or planktonic cells. Cytokine 55:330–334. 10.1016/j.cyto.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. 2007. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect Immun 75:2612–2620. 10.1128/IAI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Katragkou A, Chatzimoschou A, Simitsopoulou M, Georgiadou E, Roilides E. 2011. Additive antifungal activity of anidulafungin and human neutrophils against Candida parapsilosis biofilms. J Antimicrob Chemother 66:588–591. 10.1093/jac/dkq466. [DOI] [PubMed] [Google Scholar]
- 110.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, Kadosh D, Lopez-Ribot JL. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 6:e1000828. 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. 2011. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469:2992–2994. 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Della Valle C, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, III, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K, American Academy of Orthopaedic Surgeons. 2011. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am 93:1355–1357. 10.2106/JBJS.9314ebo. [DOI] [PubMed] [Google Scholar]
- 113.Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Hola V, Imbert C, Kirketerp-Moller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C, ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli. 2015. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21(Suppl 1):S1–S25. 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 114.Saeed K, McLaren AC, Schwarz EM, Antoci V, Arnold WV, Chen AF, Clauss M, Esteban J, Gant V, Hendershot E, Hickok N, Higuera CA, Coraca-Huber DC, Choe H, Jennings JA, Joshi M, Li WT, Noble PC, Phillips KS, Pottinger PS, Restrepo C, Rohde H, Schaer TP, Shen H, Smeltzer M, Stoodley P, Webb JCJ, Witso E. 2019. 2018 International Consensus Meeting on Musculoskeletal Infection: summary from the Biofilm Workgroup and consensus on biofilm related musculoskeletal infections. J Orthop Res 37:1007–1017. 10.1002/jor.24229. [DOI] [PubMed] [Google Scholar]
- 115.Thoendel MJ, Jeraldo PR, Greenwood-Quaintance KE, Yao JZ, Chia N, Hanssen AD, Abdel MP, Patel R. 2018. Identification of prosthetic joint infection pathogens using a shotgun metagenomics approach. Clin Infect Dis 67:1333–1338. 10.1093/cid/ciy303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Connor CL. 1928. Monilia from osteomyelitis. J Infect Dis 43:108–116. 10.1093/infdis/43.2.108. [DOI] [Google Scholar]
- 117.Weingart JS, Wirtz DC, Irving NW. 1942. Monilia osteomyelitis: report of a case resulting from thrush. Am J Clin Pathol 12:597–600. 10.1093/ajcp/12.12.597. [DOI] [Google Scholar]
- 118.Newsom SW, Lee WR, Rees JR. 1967. Fatal fungal infection following open-heart surgery. Br Heart J 29:457–460. 10.1136/hrt.29.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lehrer RI, Cline MJ. 1969. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest 48:1478–1488. 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Keating PM. 1932. Fungus infection of bone and joint. South Med J 25:1072–1076. [Google Scholar]
- 121.Trueta J, Agerholm M. 1948. Acute haematogenous osteomyelitis; diagnosis and treatment. Postgrad Med J 24:229–240. 10.1136/pgmj.24.271.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wiley AM, Trueta J. 1959. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg Br 41-B:796–809. 10.1302/0301-620X.41B4.796. [DOI] [PubMed] [Google Scholar]
- 123.Guri JP. 1946. Pyogenic osteomyelitis of the spine: differential diagnosis through clinical and roentgenographic observations. J Bone Joint Surg 28:29–39. [PubMed] [Google Scholar]
- 124.Stone DB, Bonfiglio M. 1963. Pyogenic vertebral osteomyelitis. A diagnostic pitfall for the internist. Arch Intern Med 112:491–500. 10.1001/archinte.1963.03860040087007. [DOI] [PubMed] [Google Scholar]
- 125.Curtiss PH, Klein L. 1965. Destruction of articular cartilage in septic arthritis. 2. In vivo studies. J Bone Joint Surg Am 47:1595–1604. 10.2106/00004623-196547080-00013. [DOI] [PubMed] [Google Scholar]
- 126.Gathe JC, Jr, Harris RL, Garland B, Bradshaw MW, Williams TW, Jr.. 1987. Candida osteomyelitis. Report of 5 cases and review of the literature. Am J Med 82:927–937. 10.1016/0002-9343(87)90154-9. [DOI] [PubMed] [Google Scholar]
- 127.Collignon P. 1987. Candida osteomyelitis. Am J Med 83:1173. 10.1016/0002-9343(87)90970-3. [DOI] [PubMed] [Google Scholar]
- 128.Hendrickx L, Van Wijngaerden E, Samson I, Peetermans WE. 2001. Candidal vertebral osteomyelitis: report of 6 patients, and a review. Clin Infect Dis 32:527–533. 10.1086/318714. [DOI] [PubMed] [Google Scholar]
- 129.Bellini C, Antonini P, Ermanni S, Dolina M, Passega E, Bernasconi E. 2003. Malignant otitis externa due to Aspergillus niger. Scand J Infect Dis 35:284–288. 10.1080/00365540310000247. [DOI] [PubMed] [Google Scholar]
- 130.Dailey NJM, Young EJ. 2011. Candida glabrata spinal osteomyelitis. Am J Med Sci 341:78–82. 10.1097/MAJ.0b013e3181f6c6ea. [DOI] [PubMed] [Google Scholar]
- 131.Chia SL, Tan BH, Tan CT, Tan SB. 2005. Candida spondylodiscitis and epidural abscess: management with shorter courses of anti-fungal therapy in combination with surgical debridement. J Infect 51:17–23. 10.1016/j.jinf.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 132.Burton MJ, Shah P, Swiatlo E. 2011. Misidentification of Candida parapsilosis as C. famata in a clinical case of vertebral osteomyelitis. Am J Med Sci 341:71–73. 10.1097/MAJ.0b013e3181f54dab. [DOI] [PubMed] [Google Scholar]
- 133.Matta RF, El Hajje M-J, Safadieh L, Salem G, Hmaimess G, Korkomaz R, Diab NA. 2010. Primary sternal osteomyelitis: a report of two cases with literature review. Pediatr Infect Dis J 29:976–978. 10.1097/inf.0b013e3181e0c928. [DOI] [PubMed] [Google Scholar]
- 134.Wellinghausen N, Moericke A, Bundschuh S, Friedrich W, Schulz AS, Gatz SA. 2009. Multifocal osteomyelitis caused by Candida dubliniensis. J Med Microbiol 58:386–390. 10.1099/jmm.0.003970-0. [DOI] [PubMed] [Google Scholar]
- 135.Metcalfe S, Morgan-Hough C. 2009. Cervical epidural abscess and vertebral osteomyelitis following non-traumatic oesophageal rupture: a case report and discussion (vol 18, pg S224, 2009). Eur Spine J 18:1394. 10.1007/s00586-009-1101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhogal RH, Nayeemuddin M, Akhtar I, Grainger M, Downing R. 2008. Continued lumbar spinal erosion after repair of chronic contained rupture of a mycotic abdominal aortic aneurysm. Surg Infect (Larchmt) 9:475–480. 10.1089/sur.2007.054. [DOI] [PubMed] [Google Scholar]
- 137.Ozdemir N, Celik L, Oguzoglu S, Yildirim L, Bezircioglu H. 2008. Cervical vertebral osteomyelitis and epidural abscess caused by Candida albicans in a patient with chronic renal failure. Turk Neurosurg 18:207–210. [PubMed] [Google Scholar]
- 138.Yener S, Topcu A, Manisali M, Comlekci A, Yesil S. 2009. Candida albicans osteomyelitis in a diabetic foot ulcer. J Diabetes Complications 23:137–138. 10.1016/j.jdiacomp.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 139.Cha J-G, Hong H-S, Koh Y-W, Kim H-K, Park J-M. 2008. Candida albicans osteomyelitis of the cervical spine. Skeletal Radiol 37:347–350. 10.1007/s00256-007-0429-9. [DOI] [PubMed] [Google Scholar]
- 140.Sica G, Meissner S, Dawas K, Maynard N. 2007. Candida osteo-chondromyelitis complicating thoraco-abdominal esophageal surgery. Surg Infect (Larchmt) 8:479–482. 10.1089/sur.2006.022. [DOI] [PubMed] [Google Scholar]
- 141.Schilling A, Seibold M, Mansmann V, Gleissner B. 2008. Successfully treated Candida krusei infection of the lumbar spine with combined caspofungin/posaconazole therapy. Med Mycol 46:79–83. 10.1080/13693780701552996. [DOI] [PubMed] [Google Scholar]
- 142.Ghersin E, Lessick J, Agmon Y, Engel A, Kophit A, Adler Z. 2007. Candida prosthetic valve endocarditis: the complementary role of multidetector computed tomography and transoesophageal echocardiography in preoperative evaluation. Australas Radiol 51:B231–B234. 10.1111/j.1440-1673.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 143.Torres-Ramos FM, Botwin K, Shah CP. 2004. Candida spondylodiscitis: an unusual case of thoracolumbar pain with review of imaging findings and description of the clinical condition. Pain Physician 7:257–260. [PubMed] [Google Scholar]
- 144.Gursel T, Kaya Z, Kocak U, Erbaş G, Akyurek N, Tali ET. 2005. Candida vertebra osteomyelitis in a girl with factor X deficiency. Haemophilia 11:629–632. 10.1111/j.1365-2516.2005.01148.x. [DOI] [PubMed] [Google Scholar]
- 145.Tietz HJ, Czaika V, Sterry W. 1999. Osteomyelitis caused by high resistant Candida guilliermondii. Mycoses 42:577–580. 10.1046/j.1439-0507.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- 146.Khazim RM, Debnath UK, Fares Y. 2006. Candida albicans osteomyelitis of the spine: progressive clinical and radiological features and surgical management in three cases. Eur Spine J 15:1404–1410. 10.1007/s00586-005-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaikh Z, Shaikh S, Pujol F, Trauber D, Sam M. 2005. Candida tropicalis osteomyelitis: case report and review of literature. Am J Med 118:795–798. 10.1016/j.amjmed.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 148.Cone LA, Dreisbach L, Dreisbach P, Wuesthoff M. 2005. Another patient with Candida vertebral osteomyelitis treated with liposomal amphotericin B. Surg Neurol 63:592. 10.1016/j.surneu.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 149.Cone LA, Byrd RG, Potts BE, Wuesthoff M, Sotelo J. 2004. Diagnosis and treatment of Candida vertebral osteomyelitis: clinical experience with a short course therapy of amphotericin B lipid complex. Surg Neurol 62:234–237. 10.1016/j.surneu.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 150.Arias F, Mata-Essayag S, Landaeta ME, de Capriles CH, Perez C, Nunez MJ, Carvajal A, Silva M. 2004. Candida albicans osteomyelitis: case report and literature review. Int J Infect Dis 8:307–314. 10.1016/j.ijid.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 151.Lerch K, Kalteis T, Schubert T, Lehn N, Grifka J. 2003. Prosthetic joint infections with osteomyelitis due to Candida albicans. Mycoses 46:462–466. 10.1046/j.0933-7407.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 152.Seravalli L, Van Linthoudt D, Bernet C, de Torrente A, Marchetti O, Porchet F, Genne D. 2003. Candida glabrata spinal osteomyelitis involving two contiguous lumbar vertebrae: a case report and review of the literature. Diagn Microbiol Infect Dis 45:137–141. 10.1016/s0732-8893(02)00497-2. [DOI] [PubMed] [Google Scholar]
- 153.Petrikkos G, Skiada A, Sabatakou H, Antoniadou A, Dosios T, Giamarellou H. 2001. Case report. Successful treatment of two cases of post-surgical sternal osteomyelitis, due to Candida krusei and Candida albicans, respectively, with high doses of triazoles (fluconazole, itraconazole). Mycoses 44:422–425. 10.1046/j.1439-0507.2001.00673.x. [DOI] [PubMed] [Google Scholar]
- 154.Boix V, Tovar J, Martin-Hidalgo A. 1990. Candida spondylodiscitis. Chronic illness due to heroin analgesia in an HIV positive person. J Rheumatol 17:563–564. [PubMed] [Google Scholar]
- 155.Parry MF, Grant B, Yukna M, Adler-Klein D, McLeod GX, Taddonio R, Rosenstein C. 2001. Candida osteomyelitis and diskitis after spinal surgery: an outbreak that implicates artificial nail use. Clin Infect Dis 32:352–357. 10.1086/318487. [DOI] [PubMed] [Google Scholar]
- 156.Miller DJ, Mejicano GC. 2001. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis 33:523–530. 10.1086/322634. [DOI] [PubMed] [Google Scholar]
- 157.Arranz-Caso JA, Lopez-Pizarro VM, Gomez-Herruz P, Garcia-Altozano J, Martinez-Martinez J. 1996. Candida albicans osteomyelitis of the zygomatic bone. A distinctive case with a possible peculiar mechanism of infection and therapeutic failure with fluconazole. Diagn Microbiol Infect Dis 24:161–164. 10.1016/0732-8893(96)00012-0. [DOI] [PubMed] [Google Scholar]
- 158.Harris MC, Pereira GR, Myers MD, Cardin AJ, Razdan B, Pleasure J, Bell LM. 2000. Candidal arthritis in infants previously treated for systemic candidiasis during the newborn period: report of three cases. Pediatr Emerg Care 16:249–251. 10.1097/00006565-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 159.Hayes WS, Berg RA, Dorfman HD, Freedman MT. 1984. Case report 291. Diagnosis: Candida discitis and vertebral osteomyelitis at L1-L2 from hematogenous spread. Skeletal Radiol 12:284–287. 10.1007/BF00349511. [DOI] [PubMed] [Google Scholar]
- 160.Eisen DP, MacGinley R, Christensson B, Larsson L, Woods ML. 2000. Candida tropicalis vertebral osteomyelitis complicating epidural catheterisation with disease paralleled by elevated D-arabinitol/L-arabinitol ratios. Eur J Clin Microbiol Infect Dis 19:61–63. 10.1007/s100960050013. [DOI] [PubMed] [Google Scholar]
- 161.Jonnalagadda S, Veerabagu MP, Rakela J, Kusne S, Randhawa P, Rabinovitz M. 1996. Candida albicans osteomyelitis in a liver transplant recipient: a case report and review of the literature. Transplantation 62:1182–1184. 10.1097/00007890-199610270-00028. [DOI] [PubMed] [Google Scholar]
- 162.Curran MP, Lenke LG. 1996. Torulopsis glabrata spinal osteomyelitis involving two contiguous vertebrae. A case report. Spine (Phila Pa 1976) 21:866–870. 10.1097/00007632-199604010-00019. [DOI] [PubMed] [Google Scholar]
- 163.Kaji M, Shoji H, Oizumi K. 1998. Intractable meningitis and intracranial abscess following sinusitis due to Candida species. Kurume Med J 45:279–281. 10.2739/kurumemedj.45.279. [DOI] [PubMed] [Google Scholar]
- 164.Sanz-Rodriguez C, Hernandez-Surmann F, Bueno AG, Goizueta C, Noguerado A. 1998. Candida and bacterial mandibular osteomyelitis in an AIDS patient. Eur J Clin Microbiol Infect Dis 17:531–532. 10.1007/s100960050123. [DOI] [PubMed] [Google Scholar]
- 165.McCullers JA, Flynn PM. 1998. Candida tropicalis osteomyelitis: case report and review. Clin Infect Dis 26:1000–1001. 10.1086/517629. [DOI] [PubMed] [Google Scholar]
- 166.Clancy CJ, Nguyen MH, Morris AJ. 1997. Candidal mediastinitis: an emerging clinical entity. Clin Infect Dis 25:608–613. 10.1086/513770. [DOI] [PubMed] [Google Scholar]
- 167.Dorigo B, Cameli AM, Trapani M, Raspanti D, Torri M, Mosconi G. 1995. Efficacy of femoral intra-arterial administration of teicoplanin in gram-positive diabetic foot infections. Angiology 46:1115–1122. 10.1177/000331979504601207. [DOI] [PubMed] [Google Scholar]
- 168.Sorrell TC, Dunlop C, Collignon PJ, Harding JA. 1984. Exogenous ocular candidiasis associated with intravenous heroin abuse. Br J Ophthalmol 68:841–845. 10.1136/bjo.68.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Connell CJ, Cherry AV, Zoll JG. 1973. Letter. Osteomyelitis of cervical spine: Candida guilliermondii. Ann Intern Med 79:748. 10.7326/0003-4819-79-5-748_1. [DOI] [PubMed] [Google Scholar]
- 170.Tang C. 1993. Successful treatment of Candida albicans osteomyelitis with fluconazole. J Infect 26:89–92. 10.1016/0163-4453(93)97064-5. [DOI] [PubMed] [Google Scholar]
- 171.Turner DL, Johnson SA, Rule SA. 1999. Successful treatment of candidal osteomyelitis with fluconazole following failure with liposomal amphotericin B. J Infect 38:51–53. 10.1016/s0163-4453(99)90032-4. [DOI] [PubMed] [Google Scholar]
- 172.Munk PL, Lee MJ, Poon PY, O’Connell JX, Coupland DB, Janzen DL, Logan PM, Dvorak MF. 1997. Candida osteomyelitis and disc space infection of the lumbar spine. Skeletal Radiol 26:42–46. 10.1007/s002560050189. [DOI] [PubMed] [Google Scholar]
- 173.Mullins RF, Still JM, Savage J, Davis JB, Law EJ. 1993. Osteomyelitis of the spine in a burn patient due to Candida albicans. Burns 19:174–176. 10.1016/0305-4179(93)90045-A. [DOI] [PubMed] [Google Scholar]
- 174.Diament MJ, Weller M, Bernstein R. 1982. Candida infection in a premature infant presenting as discitis. Pediatr Radiol 12:96–98. 10.1007/BF00972443. [DOI] [PubMed] [Google Scholar]
- 175.Neale TJ, Muir JC, Mills H, Horne JG, Jones MR. 1987. Candida albicans vertebral osteomyelitis in chronic renal failure. Postgrad Med J 63:695–698. 10.1136/pgmj.63.742.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hirschmann JV, Everett ED. 1976. Candida vertebral osteomyelitis: case report and review of literature. J Bone Joint Surg Am 58:573–575. [PubMed] [Google Scholar]
- 177.Hennequin C, Bouree P, Hiesse C, Dupont B, Charpentier B. 1996. Spondylodiskitis due to Candida albicans: report of two patients who were successfully treated with fluconazole and review of the literature. Clin Infect Dis 23:176–178. 10.1093/clinids/23.1.176. [DOI] [PubMed] [Google Scholar]
- 178.Kashimoto T, Kitagawa H, Kachi H. 1986. Candida tropicalis vertebral osteomyelitis and discitis. A case report and discussion on the diagnosis and treatment. Spine (Phila Pa 1976) 11:57–61. 10.1097/00007632-198601000-00016. [DOI] [PubMed] [Google Scholar]
- 179.Friedman BC, Simon GL. 1987. Candida vertebral osteomyelitis: report of 3 cases and a review of the literature. Diagn Microbiol Infect Dis 8:31–36. 10.1016/0732-8893(87)90044-7. [DOI] [PubMed] [Google Scholar]
- 180.Armstrong N, Schurr M, Helgerson R, Harms B. 1998. Fungal sacral osteomyelitis as the initial presentation of Crohn’s disease of the small bowel: report of a case. Dis Colon Rectum 41:1581–1584. 10.1007/BF02237311. [DOI] [PubMed] [Google Scholar]
- 181.Rowe IF, Wright ED, Higgens CS, Burnie JP. 1988. Intervertebral infection due to Candida albicans in an intravenous heroin abuser. Ann Rheum Dis 47:522–525. 10.1136/ard.47.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smilack JD, Gentry LO. 1976. Candida costochondral osteomyelitis. Report of a case and review of the literature. J Bone Joint Surg Am 58:888–890. [PubMed] [Google Scholar]
- 183.Ward RM, Sattler FR, Dalton AS, Jr.. 1983. Assessment of antifungal therapy in an 800-gram infant with candidal arthritis and osteomyelitis. Pediatrics 72:234–238. 10.1542/peds.72.2.234. [DOI] [PubMed] [Google Scholar]
- 184.Simpson MB, Jr, Kurlinski JP, Solomon MH, Merz WG. 1977. Opportunistic mycotic osteomyelitis: bone infections due to Aspergillus and Candida species. Medicine (Baltimore) 56:475–482. [PubMed] [Google Scholar]
- 185.Gustke KA, Wu KK. 1981. Torulopsis glabrata osteomyelitis: report of a case. Clin Orthop Relat Res 154:197–200. 10.1097/00003086-198101000-00029. [DOI] [PubMed] [Google Scholar]
- 186.Shaikh BS, Appelbaum PC, Aber RC. 1980. Vertebral disc space infection and osteomyelitis due to Candida albicans in a patient with acute myelomonocytic leukemia. Cancer 45:1025–1028. . [DOI] [PubMed] [Google Scholar]
- 187.Dan M, Priel I. 1994. Failure of fluconazole therapy for sternal osteomyelitis due to Candida albicans. Clin Infect Dis 18:126–127. 10.1093/clinids/18.1.126. [DOI] [PubMed] [Google Scholar]
- 188.Belzunegui J, Gonzalez C, Lopez L, Plazaola I, Maiz O, Figueroa M. 1997. Osteoarticular and muscle infectious lesions in patients with the human immunodeficiency virus. Clin Rheumatol 16:450–453. 10.1007/BF02238936. [DOI] [PubMed] [Google Scholar]
- 189.Imahori SC, Papademetriou T, Ogliela DM. 1987. Torulopsis glabrata osteomyelitis. A case report. Clin Orthop Relat Res 219:214–220. 10.1097/00003086-198706000-00031. [DOI] [PubMed] [Google Scholar]
- 190.Sugar AM, Saunders C, Diamond RD. 1990. Successful treatment of Candida osteomyelitis with fluconazole: a noncomparative study of 2 patients. Diagn Microbiol Infect Dis 13:517–520. 10.1016/0732-8893(90)90084-9. [DOI] [PubMed] [Google Scholar]
- 191.Corso FA, Shaul DB, Wolfe BM. 1995. Spinal osteomyelitis after Tpn catheter-induced septicemia. JPEN J Parenter Enteral Nutr 19:291–295. 10.1177/0148607195019004291. [DOI] [PubMed] [Google Scholar]
- 192.Ackerman G, Bayley JC. 1990. Candida albicans osteomyelitis in a vertebral body previously infected with Serratia marcescens. Spine (Phila Pa 1976) 15:1362–1363. 10.1097/00007632-199012000-00024. [DOI] [PubMed] [Google Scholar]
- 193.Chmel H, Grieco MH, Zickel R. 1973. Candida osteomyelitis: report of a case. Am J Med Sci 266:299–304. 10.1097/00000441-197310000-00008. [DOI] [PubMed] [Google Scholar]
- 194.Freeman JB, Wienke JW, Soper RT. 1974. Candida osteomyelitis associated with intravenous alimentation. J Pediatr Surg 9:783–784. 10.1016/0022-3468(74)90119-5. [DOI] [PubMed] [Google Scholar]
- 195.Svirsky-Fein S, Langer L, Milbauer B, Khermosh O, Rubinstein E. 1979. Neonatal osteomyelitis caused by Candida tropicalis. Report of two cases and review of the literature. J Bone Joint Surg Am 61:455–459. [PubMed] [Google Scholar]
- 196.Weber ML, Abela A, de Repentigny L, Garel L, Lapointe N. 1987. Myeloperoxidase deficiency with extensive candidal osteomyelitis of the base of the skull. Pediatrics 80:876–879. [PubMed] [Google Scholar]
- 197.Owen PG, Willis BK, Benzel EC. 1992. Torulopsis glabrata vertebral osteomyelitis. J Spinal Disord 5:370–373. 10.1097/00002517-199209000-00018. [DOI] [PubMed] [Google Scholar]
- 198.Edwards JE, Turkel SB, Elder HA, Rand RW, Guze LB. 1975. Hematogenous Candida osteomyelitis. Report of three cases and review of the literature. Am J Med 59:89–94. 10.1016/0002-9343(75)90325-3. [DOI] [PubMed] [Google Scholar]
- 199.Ferra C, Doebbeling BN, Hollis RJ, Pfaller MA, Lee CK, Gingrich RD. 1994. Candida tropicalis vertebral osteomyelitis: a late sequela of fungemia. Clin Infect Dis 19:697–703. 10.1093/clinids/19.4.697. [DOI] [PubMed] [Google Scholar]
- 200.Williams RL, Fukui MB, Meltzer CC, Swarnkar A, Johnson DW, Welch W. 1999. Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. AJNR Am J Neuroradiol 20:381–385. [PMC free article] [PubMed] [Google Scholar]
- 201.Flanagan PG, Barnes RA. 1997. Hazards of inadequate fluconazole dosage to treat deep-seated or systemic Candida albicans infection. J Infect 35:295–297. 10.1016/s0163-4453(97)93270-9. [DOI] [PubMed] [Google Scholar]
- 202.Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA. 1983. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am 65:19–29. [PubMed] [Google Scholar]
- 203.Holzman RS, Bishko F. 1971. Osteomyelitis in heroin addicts. Ann Intern Med 75:693–696. 10.7326/0003-4819-75-5-693. [DOI] [PubMed] [Google Scholar]
- 204.Glower DD, Douglas JM, Jr, Gaynor JW, Jones RN, Oldham HN, Jr.. 1990. Candida mediastinitis after a cardiac operation. Ann Thorac Surg 49:157–163. 10.1016/0003-4975(90)90382-G. [DOI] [PubMed] [Google Scholar]
- 205.Lertratanakul Y, Glassford GH, Rubinstein HM. 1977. Arthritis and osteomyelitis due to Candida albicans: a case report. J Rheumatol 4:317–320. [PubMed] [Google Scholar]
- 206.Fogarty M. 1983. Candidial osteomyelitis: a case report. Aust N Z J Surg 53:141–143. 10.1111/j.1445-2197.1983.tb02415.x. [DOI] [PubMed] [Google Scholar]
- 207.Lafont A, Olivé A, Gelman M, Roca-Burniols J, Cots R, Carbonell J. 1994. Candida albicans spondylodiscitis and vertebral osteomyelitis in patients with intravenous heroin drug addiction. Report of 3 new cases. J Rheumatol 21:953–956. [PubMed] [Google Scholar]
- 208.Morrow JD, Manian FA. 1986. Vertebral osteomyelitis due to Candida glabrata. A case report. J Tenn Med Assoc 79:409–410. [PubMed] [Google Scholar]
- 209.Wang YC, Lee ST. 2001. Candida vertebral osteomyelitis: a case report and review of the literature. Chang Gung Med J 24:810–815. [PubMed] [Google Scholar]
- 210.Bortel DT. 1993. Candida osteomyelitis pubis following a Marshall-Marchetti procedure. Orthopedics 16:1353–1355. 10.3928/0147-7447-19931201-12. [DOI] [PubMed] [Google Scholar]
- 211.Munoz Fernandez S, Quiralte J, del Arco A, Balsa A, Cardenal A, Pena JM, Vazquez JJ, Gijon Banos J. 1991. Osteoarticular infection associated with the human immunodeficiency virus. Clin Exp Rheumatol 9:489–493. [PubMed] [Google Scholar]
- 212.Thomas FE, Jr, Martin CE, Fisher RD, Alford RH. 1977. Candida albicans infection of sternum and costal cartilages: combined operative treatment and drug therapy and 5-fluorocytosine. Ann Thorac Surg 23:163–166. 10.1016/S0003-4975(10)64093-3. [DOI] [PubMed] [Google Scholar]
- 213.Berant M, Kristal C, Wagner Y. 1979. Candida osteomyelitis as a complication of parenteral nutrition in an infant. Successful treatment with flucytosine. Helv Paediatr Acta 34:155–160. [PubMed] [Google Scholar]
- 214.Gallo WJ, Shapiro DN, Moss M. 1976. Suppurative candidiasis: review of the literature and report of case. J Am Dent Assoc 92:936–939. 10.14219/jada.archive.1976.0104. [DOI] [PubMed] [Google Scholar]
- 215.Nissen TP, Lehman CR, Otsuka NY, Cerruti DM. 2001. Fungal osteomyelitis of the distal femoral epiphysis. Orthopedics 24:1083–1084. 10.3928/0147-7447-20011101-23. [DOI] [PubMed] [Google Scholar]
- 216.Bannatyne RM, Clarke HM. 1989. Ketoconazole in the treatment of osteomyelitis due to Candida albicans. Can J Surg 32:201–202. [PubMed] [Google Scholar]
- 217.Adler S, Plotkin SA, Randall J. 1972. Candidal osteomyelitis and arthritis in a neonate. Am J Dis Child 123:595–596. 10.1001/archpedi.1972.02110120119017. [DOI] [PubMed] [Google Scholar]
- 218.Lasday SD, Jay RM. 1994. Candida osteomyelitis. J Foot Ankle Surg 33:173–176. [PubMed] [Google Scholar]
- 219.Kerr J. 1994. Fungal osteomyelitis of the temporal bone: a review of reported cases. Ear Nose Throat J 73:339. [PubMed] [Google Scholar]
- 220.Cooper P, Schofield B, Lennox DW, Ebert-Smith T. 1991. Candida albicans osteomyelitis in a patient with avascular necrosis of the hip. Orthopedics 14:352–355. [PubMed] [Google Scholar]
- 221.Noble HB, Lyne ED. 1974. Candida osteomyelitis and arthritis from hyperalimentation therapy. Case report. J Bone Joint Surg Am 56:825–829. [PubMed] [Google Scholar]
- 222.Hanna E, Hughes G, Eliachar I, Wanamaker J, Tomford W. 1993. Fungal osteomyelitis of the temporal bone: a review of reported cases. Ear Nose Throat J 72:532, 537–541. 10.1177/014556139307200807. [DOI] [PubMed] [Google Scholar]
- 223.Edelstein H, McCabe R. 1991. Candida albicans septic arthritis and osteomyelitis of the sternoclavicular joint in a patient with human immunodeficiency virus infection. J Rheumatol 18:110–111. [PubMed] [Google Scholar]
- 224.Oliverson TJ, Joshi A, Nana A, Lindsey RW. 2002. Chronic tibial osteomyelitis caused by Candida parapsiliosis [sic]. Orthopedics 25:763–764. 10.3928/0147-7447-20020701-19. [DOI] [PubMed] [Google Scholar]
- 225.Hashimoto Y, Tanioka H. 1991. Vertebral osteomyelitis associated with disseminated candidiasis in an oral cancer patient. J Oral Maxillofac Surg 49:901–903. 10.1016/0278-2391(91)90026-I. [DOI] [PubMed] [Google Scholar]
- 226.Dupont B, Drouhet E. 1985. Cutaneous, ocular, and osteoarticular candidiasis in heroin addicts: new clinical and therapeutic aspects in 38 patients. J Infect Dis 152:577–591. 10.1093/infdis/152.3.577. [DOI] [PubMed] [Google Scholar]
- 227.Bruns J, Hemker T, Dahmen G. 1986. Fungal spondylitis. A case of Torulopsis glabrata and Candida tropicalis infection. Acta Orthop Scand 57:563–565. 10.3109/17453678609014795. [DOI] [PubMed] [Google Scholar]
- 228.Pennisi AK, Davis DO, Wiesel S, Moskovitz P. 1985. CT appearance of Candida diskitis. J Comput Assist Tomogr 9:1050–1054. 10.1097/00004728-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 229.Herzog W, Perfect J, Roberts L. 1989. Intervertebral diskitis due to Candida tropicalis. South Med J 82:270–273. 10.1097/00007611-198902000-00029. [DOI] [PubMed] [Google Scholar]
- 230.Liudahl KJ, Limbird TJ. 1987. Torulopsis glabrata vertebral osteomyelitis. Case report and review of the literature. Spine (Phila Pa 1976) 12:593–595. 10.1097/00007632-198707000-00017. [DOI] [PubMed] [Google Scholar]
- 231.Heald AH, O’Halloran DJ, Richards K, Webb F, Jenkins S, Hollis S, Denning DW, Young RJ. 2001. Fungal infection of the diabetic foot: two distinct syndromes. Diabet Med 18:567–572. 10.1046/j.1464-5491.2001.00523.x. [DOI] [PubMed] [Google Scholar]
- 232.Meberg A, Langslet A, Sovde A, Kolstad A. 1977. Candida septicemia with chorioretinitis, osteomyelitis and arthritis treated with systemic miconazole and intraarticular amphotericin B. Mykosen 20:257–260. 10.1111/j.1439-0507.1977.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 233.Thurston AJ, Gillespie WJ. 1981. Torulopsis glabrata osteomyelitis of the spine: a case report and review of the literature. Aust N Z J Surg 51:374–376. 10.1111/j.1445-2197.1981.tb04972.x. [DOI] [PubMed] [Google Scholar]
- 234.Kraus WE, Valenstein PN, Corey GR. 1988. Purulent pericarditis caused by Candida: report of three cases and identification of high-risk populations as an aid to early diagnosis. Rev Infect Dis 10:34–41. 10.1093/clinids/10.1.34. [DOI] [PubMed] [Google Scholar]
- 235.Frederickson B, Yuan H, Olans R. 1978. Management and outcome of pyogenic vertebral osteomyelitis. Clin Orthop Relat Res 131:160–167. [PubMed] [Google Scholar]
- 236.Garbino J, Schnyder I, Lew D, Bouchuiguir-Wafa K, Rohner P. 2003. An unusual cause of vertebral osteomyelitis: Candida species. Scand J Infect Dis 35:288–291. 10.1080/00365540310000067. [DOI] [PubMed] [Google Scholar]
- 237.Tchang FKM, Gilardi GL. 1973. Osteomyelitis due to Torulopsis inconspicua. Report of a case. J Bone Joint Surg Am 55:1739–1743. 10.2106/00004623-197355080-00018. [DOI] [PubMed] [Google Scholar]
- 238.Almekinders LC, Greene WB. 1991. Vertebral Candida infections. A case report and review of the literature. Clin Orthop Relat Res 267:174–178. [PubMed] [Google Scholar]
- 239.El-Zaatari MM, Hulten K, Fares Y, Baassiri A, Balkis M, Almashhrawi A, El-Zaatari FA. 2002. Successful treatment of Candida albicans osteomyelitis of the spine with fluconazole and surgical debridement: case report. J Chemother 14:627–630. 10.1179/joc.2002.14.6.627. [DOI] [PubMed] [Google Scholar]
- 240.Brill PW, Winchester P, Krauss AN, Symchych P. 1979. Osteomyelitis in a neonatal intensive care unit. Radiology 131:83–87. 10.1148/131.1.83. [DOI] [PubMed] [Google Scholar]
- 241.Dijkmans BA, Koolen MI, Mouton RP, Falke TH, van den Broek PJ, van der Meer JW. 1982. Hematogenous Candida vertebral osteomyelitis treated with ketoconazole. Infection 10:290–292. 10.1007/BF01640877. [DOI] [PubMed] [Google Scholar]
- 242.Machi T, Kitagawa S, Hamaoka H, Akasaki T, Miyamoto Y. 1994. Postoperative Candida osteomyelitis in femoral fracture: a case report. Kansenshogaku Zasshi 68:1122–1125. 10.11150/kansenshogakuzasshi1970.68.1122. [DOI] [PubMed] [Google Scholar]
- 243.Oleinik EM, Della-Latta P, Rinaldi MG, Saiman L. 1993. Candida lusitaniae osteomyelitis in a premature infant. Am J Perinatol 10:313–315. 10.1055/s-2007-994749. [DOI] [PubMed] [Google Scholar]
- 244.Pohjola-Sintonen S, Ruutu P, Tallroth K. 1984. Hematogenous Candida spondylitis. A case report. Acta Med Scand 215:85–87. 10.1111/j.0954-6820.1984.tb04974.x. [DOI] [PubMed] [Google Scholar]
- 245.Dwyer K, McDonald M, Fitzpatrick T. 1999. Presentation of Candida glabrata spinal osteomyelitis 25 months after documented candidaemia. Aust N Z J Med 29:571–572. 10.1111/j.1445-5994.1999.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 246.Collignon PJ, Sorrell TC. 1983. Disseminated candidiasis: evidence of a distinctive syndrome in heroin abusers. Br Med J (Clin Res Ed) 287:861–862. 10.1136/bmj.287.6396.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Aryan HE, Lu DC, Acosta FL, Jr, Ames CP. 2007. Corpectomy followed by the placement of instrumentation with titanium cages and recombinant human bone morphogenetic protein-2 for vertebral osteomyelitis. J Neurosurg Spine 6:23–30. 10.3171/spi.2007.6.1.23. [DOI] [PubMed] [Google Scholar]
- 248.Quindos G, Rowe IF, Higgens CS, Ponton J, Cisterna R, Mackenzie DW. 1990. Candidal infection of bone. Assessment of serologic tests in diagnosis and management. Diagn Microbiol Infect Dis 13:297–302. 10.1016/0732-8893(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 249.Potasman I, Leibovitz Z, Sharf M. 1991. Candida sepsis in pregnancy and the postpartum period. Rev Infect Dis 13:146–149. 10.1093/clinids/13.1.146. [DOI] [PubMed] [Google Scholar]
- 250.Yousefzadeh DK, Jackson JH. 1980. Neonatal and infantile candidal arthritis with or without osteomyelitis: a clinical and radiographical review of 21 cases. Skeletal Radiol 5:77–90. 10.1007/BF00347327. [DOI] [PubMed] [Google Scholar]
- 251.Pittard WB, III, Thullen JD, Fanaroff AA. 1976. Neonatal septic arthritis. J Pediatr 88:621–624. 10.1016/S0022-3476(76)80022-4. [DOI] [PubMed] [Google Scholar]
- 252.Malani PN, McNeil SA, Bradley SF, Kauffman CA. 2002. Candida albicans sternal wound infections: a chronic and recurrent complication of median sternotomy. Clin Infect Dis 35:1316–1320. 10.1086/344192. [DOI] [PubMed] [Google Scholar]
- 253.Prystowsky SD, Vogelstein B, Ettinger DS, Merz WG, Kaizer H, Sulica VI, Zinkham WH. 1976. Invasive aspergillosis. N Engl J Med 295:655–658. 10.1056/NEJM197609162951206. [DOI] [PubMed] [Google Scholar]
- 254.Basu S, Kumar A. 2011. Osteomyelitis as a manifestation of perinatal human immunodeficiency virus disease. J Infect 63:163–166. 10.1016/j.jinf.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 255.Estrov Z, Resnitzky P, Shenker Y, Berrebi A, Hurwitz N. 1984. Candidemia and sternal Candida albicans osteomyelitis in a patient with chronic lymphatic leukemia. Isr J Med Sci 20:711–714. [PubMed] [Google Scholar]
- 256.Richaud C, De Lastours V, Panhard X, Petrover D, Bruno F, Lefort A. 2017. Candida vertebral osteomyelitis (CVO) 28 cases from a 10-year retrospective study in France. Medicine (Baltimore) 96:e7525. 10.1097/MD.0000000000007525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schiedo RM, Lavelle W, Sun MH. 2017. Lumbar spinal Candida glabrata treated without surgical intervention: a case report. Cureus 9:e1371. 10.7759/cureus.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Orlowski HLP, McWilliams S, Mellnick VM, Bhalla S, Lubner MG, Pickhardt PJ, Menias CO. 2017. Imaging spectrum of invasive fungal and fungal-like infections. Radiographics 37:1119–1134. 10.1148/rg.2017160110. [DOI] [PubMed] [Google Scholar]
- 259.Ma L, Tong H, Ruan L, Ling Z, Ren Y, Zhou X. 2016. Successful treatment of Candida tropicalis osteomyelitis with micafungin in a leukemia patient. IDCases 6:109–111. 10.1016/j.idcr.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dietl M, Schwabegger A, Grimm M, Ruttmann E, Kompatscher P. 2017. First line treatment of Candida osteomyelitis of the sternum: is there really a need for radical surgical debridement and reconstructive surgery in Candida osteomyelitis of the sternum after cardiac surgery? J Plast Reconstr Aesthet Surg 70:291–292. 10.1016/j.bjps.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 261.Kulcheski AL, Graells XS, Benato ML, Santoro PG, Sebben AL. 2015. Fungal spondylodiscitis due to Candida albicans: an atypical case and review of the literature. Rev Bras Ortop 50:739–742. 10.1016/j.rboe.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gopinathan A, Kumar A, Rao SN, Kumar K, Karim S. 2016. Candidal vertebral osteomyelitis in the midst of renal disorders. J Clin Diagn Res 10:DD03–DD05. 10.7860/JCDR/2016/18134.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Giger A, Yusuf E, Manuel O, Clerc O, Trampuz A. 2016. Polymicrobial vertebral osteomyelitis after oesophageal biopsy: a case report. BMC Infect Dis 16:141. 10.1186/s12879-016-1471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jones N, Garcez T, Newman W, Denning D. 2016. Endogenous Candida endophthalmitis and osteomyelitis associated with CARD9 deficiency. BMJ Case Rep 2016:bcr2015214117. 10.1136/bcr-2015-214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kankare J, Lindfors NC. 2016. Reconstruction of vertebral bone defects using an expandable replacement device and bioactive glass S53P4 in the treatment of vertebral osteomyelitis: three patients and three pathogens. Scand J Surg 105:248–253. 10.1177/1457496915626834. [DOI] [PubMed] [Google Scholar]
- 266.Magano R, Cortez J, Ramos E, Trindade L. 2015. Candida albicans osteomyelitis as a cause of chest pain and visual loss. BMJ Case Rep 2015:bcr2015211327. 10.1136/bcr-2015-211327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kauffman CA. 2015. Complications of candidemia in ICU patients: endophthalmitis, osteomyelitis, endocarditis. Semin Respir Crit Care Med 36:641–649. 10.1055/s-0035-1562891. [DOI] [PubMed] [Google Scholar]
- 268.Abchee A, Elias E, Al Garnawee M, Ayoub C, Kanj S, Skaf G. 2017. Injury to hypopharynx by a foreign body with subsequent Candida osteomyelitis of the upper cervical spine. J Neurosurg Sci 61:449–452. 10.23736/S0390-5616.16.03275-6. [DOI] [PubMed] [Google Scholar]
- 269.Ganesh D, Gottlieb J, Chan S, Martinez O, Eismont F. 2015. Fungal infections of the spine. Spine (Phila Pa 1976) 40:E719–E728. 10.1097/BRS.0000000000000903. [DOI] [PubMed] [Google Scholar]
- 270.Miller AO, Gamaletsou MN, Henry MW, Al-Hafez L, Hussain K, Sipsas NV, Kontoyiannis DP, Roilides E, Brause BD, Walsh TJ. 2015. Successful treatment of Candida osteoarticular infections with limited duration of antifungal therapy and orthopedic surgical intervention. Infect Dis (Lond) 47:144–149. 10.3109/00365548.2014.974207. [DOI] [PubMed] [Google Scholar]
- 271.Taneja AK, Torriani M, Simeone FJ. 2014. Septic arthritis and osteomyelitis of the hip by Candida albicans. J Rheumatol 41:2270. 10.3899/jrheum.140354. [DOI] [PubMed] [Google Scholar]
- 272.Lopez R, Hunter AR, Geoghegan O, Demertzi E. 2014. Candida parapsilosis osteomyelitis. BMJ Case Rep 2014:bcr2014206520. 10.1136/bcr-2014-206520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Brembilla C, Lanterna LA, Risso A, Bonaldi G, Gritti P, Resmini B, Viscone A. 2014. Cervical bone graft Candida albicans osteomyelitis: management strategies for an uncommon infection. Case Rep Orthop 2014:986393. 10.1155/2014/986393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Toki S, Hibino N, Sairyo K, Takahashi M, Yoshioka S, Yamano M, Henmi T. 2014. Osteomyelitis caused by Candida glabrata in the distal phalanx. Case Rep Orthop 2014:962575. 10.1155/2014/962575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. 2014. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg 22:390–401. 10.5435/JAAOS-22-06-390. [DOI] [PubMed] [Google Scholar]
- 276.Tan AC, Parker N, Arnold M. 2014. Candida glabrata vertebral osteomyelitis in an immunosuppressed patient. Int J Rheum Dis 17:229–231. 10.1111/1756-185X.12113. [DOI] [PubMed] [Google Scholar]
- 277.Eves S, Sayeed R, Potter M. 2014. A case of rib fungal osteomyelitis. J Plast Reconstr Aesthet Surg 67:e81–e83. 10.1016/j.bjps.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 278.Oksi J, Finnila T, Hohenthal U, Rantakokko-Jalava K. 2014. Candida dubliniensis spondylodiscitis in an immunocompetent patient. Case report and review of the literature. Med Mycol Case Rep 3:4–7. 10.1016/j.mmcr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Prevost N, English JC, III.. 2013. Candida rib osteomyelitis: erythema and nodule in midline scar. J Am Acad Dermatol 69:e205–e207. 10.1016/j.jaad.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 280.Bali R, Sharma P, Gupta P, Gaba S. 2013. Chronic candidal osteomyelitis of mid face: a therapeutic dilemma. J Oral Biol Craniofac Res 3:151–153. 10.1016/j.jobcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pan N, Herzog R, Blanco JS, Nauseef WM, Jenkins S, Kovanlikaya A, Salvatore CM, Toussi SS. 2013. Candida albicans osteomyelitis in an infant: a case report and literature review. J Pediatr Orthop B 22:491–497. 10.1097/BPB.0b013e3283613313. [DOI] [PubMed] [Google Scholar]
- 282.Seguchi O, Fujita T, Murata Y, Yanase M, Higashi M, Toda K, Nakatani T. 2013. Bone-destroying Candida infection following left ventricular assist device explant. J Artif Organs 16:258–262. 10.1007/s10047-013-0696-2. [DOI] [PubMed] [Google Scholar]
- 283.Huang A, Huang C, Kugathasan S. 2013. Vertebral osteomyelitis due to Candida parapsilosis in a child with Crohn disease while receiving anti-TNF therapy. J Pediatr Gastroenterol Nutr 56:E23–E26. 10.1097/MPG.0b013e31827ecbda. [DOI] [PubMed] [Google Scholar]
- 284.Phan TQ, Depner C, Theodorou P, Lefering R, Perbix W, Spilker G, Weinand C. 2013. Failure of secondary wound closure after sternal wound infection following failed initial operative treatment: causes and treatment. Ann Plast Surg 70:216–221. 10.1097/SAP.0b013e31823b67ec. [DOI] [PubMed] [Google Scholar]
- 285.Lim KB, Kwak YG, Kim YS, Park KR. 2012. Shoulder joint infectious arthritis and acromioclavicular joint osteomyelitis due to Candida. Ann Rehabil Med 36:573–577. 10.5535/arm.2012.36.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Grimes CL, Tan-Kim J, Garfin SR, Nager CW. 2012. Sacral colpopexy followed by refractory Candida albicans osteomyelitis and discitis requiring extensive spinal surgery. Obstet Gynecol 120:464–468. 10.1097/AOG.0b013e318256989e. [DOI] [PubMed] [Google Scholar]
- 287.Yuste JR, Alfonso M, Bustos C, Quintana J, Rubio M, Villas C, Del Pozo JL. 2012. Iliac bone Candida albicans osteomyelitis in a patient with iliac crest bone autograft: a case report and review of the literature. Infection 40:445–449. 10.1007/s15010-012-0276-z. [DOI] [PubMed] [Google Scholar]
- 288.Kaldau NC, Brorson S, Jensen PE, Schultz C, Arpi M. 2012. Bilateral polymicrobial osteomyelitis with Candida tropicalis and Candida krusei: a case report and an updated literature review. Int J Infect Dis 16:e16–e22. 10.1016/j.ijid.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 289.Fleming L, Ng A, Paden M, Stone P, Kruse D. 2012. Fungal osteomyelitis of calcaneus due to Candida albicans: a case report. J Foot Ankle Surg 51:212–214. 10.1053/j.jfas.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 290.Gamaletsou MN, Meletiadis J, Chatziioannou S, Panayiotides IG, Agadakos E, Katsimpoulas M, Kostomitsopoulos N, Petraitis V, Walsh TJ, Sipsas NV, International Consortium of Osteoarticular Mycoses. 2019. Experimental Candida albicans osteomyelitis: microbiologic, antigenic, histologic, and 18FDG-PET-CT imaging characteristics in a newly established rabbit model. Med Mycol 57:1011–1017. 10.1093/mmy/myz001. [DOI] [PubMed] [Google Scholar]
- 291.Crawford SJ, Swan CD, Boutlis CS, Reid AB. 2016. Candida costochondritis associated with recent intravenous drug use. IDCases 4:59–61. 10.1016/j.idcr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Aaron DL, Patel A, Kayiaros S, Calfee R. 2011. Four common types of bursitis: diagnosis and management. J Am Acad Orthop Surg 19:359–367. 10.5435/00124635-201106000-00006. [DOI] [PubMed] [Google Scholar]
- 293.Sayegh ET, Strauch RJ. 2014. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg 134:1517–1536. 10.1007/s00402-014-2088-3. [DOI] [PubMed] [Google Scholar]
- 294.Reilly D, Kamineni S. 2016. Olecranon bursitis. J Shoulder Elbow Surg 25:158–167. 10.1016/j.jse.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 295.Lass-Florl C, Mayr A. 2007. Human protothecosis. Clin Microbiol Rev 20:230–242. 10.1128/CMR.00032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Murray HW, Fialk MA, Roberts RB. 1976. Candida arthritis. A manifestation of disseminated candidiasis. Am J Med 60:587–595. 10.1016/0002-9343(76)90728-2. [DOI] [PubMed] [Google Scholar]
- 297.Skedros JG, Keenan KE, Trachtenberg JD. 2013. Candida glabrata olecranon bursitis treated with bursectomy and intravenous caspofungin. J Surg Orthop Adv 22:179–182. 10.3113/jsoa.2013.0179. [DOI] [PubMed] [Google Scholar]
- 298.Miyamoto H, Miura T, Morita E, Morizaki Y, Uehara K, Ohe T, Tanaka S. 2012. Fungal arthritis of the wrist caused by Candida parapsilosis during infliximab therapy for rheumatoid arthritis. Mod Rheumatol 22:903–906. 10.1007/s10165-012-0594-0. [DOI] [PubMed] [Google Scholar]
- 299.Jeong YM, Cho HY, Lee S-W, Hwang YM, Kim Y-K. 2013. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol 14:465–469. 10.3348/kjr.2013.14.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wall BA, Weinblatt ME, Darnall JT, Muss H. 1982. Candida tropicalis arthritis and bursitis. JAMA 248:1098–1099. [PubMed] [Google Scholar]
- 301.Schlesinger N, Hoffman BI. 1995. Fungal bursitis: olecranon bursitis caused by Candida parapsilosis with review of the literature. J Clin Rheumatol 1:232–235. [PubMed] [Google Scholar]
- 302.Roschmann RA, Bell CL. 1987. Septic bursitis in immunocompromised patients. Am J Med 83:661–665. 10.1016/0002-9343(87)90895-3. [DOI] [PubMed] [Google Scholar]
- 303.Behar SM, Chertow GM. 1998. Olecranon bursitis caused by infection with Candida lusitaniae. J Rheumatol 25:598–600. [PubMed] [Google Scholar]
- 304.Jimenez-Palop M, Corteguera M, Ibanez R, Serrano-Heranz R. 2002. Olecranon bursitis due to Candida parapsilosis in an immunocompetent adult. Ann Rheum Dis 61:279–281. 10.1136/ard.61.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gamarra-Hilburn CF, Rios G, Vila LM. 2016. Olecranon bursitis caused by Candida parapsilosis in a patient with rheumatoid arthritis. Case Rep Rheumatol 2016:2019250. 10.1155/2016/2019250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Larru B, Barrett DM, Gerber JS. 2013. Candida krusei arthritis in an adolescent with acute myelogenous leukemia. Pediatr Infect Dis J 32:1142–1144. 10.1097/INF.0b013e318294b012. [DOI] [PubMed] [Google Scholar]
- 307.Le Calloch R, Ianotto J-C, Guillerm G, Tonnelier JM. 2013. Fungal arthritis of the hip in patient with aplastic anaemia. BMJ Case Rep 2013:bcr2013008902. 10.1136/bcr-2013-008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lee GW, Kim TH, Son JH. 2012. Primary Candida guilliermondii infection of the knee in a patient without predisposing factors. Case Rep Med 2012:375682. 10.1155/2012/375682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lu H, Marengo MF, Mihu CN, Garcia-Manero G, Suarez-Almazor ME. 2012. Rare case of septic arthritis caused by Candida krusei: case report and literature review. J Rheumatol 39:1308–1309. 10.3899/jrheum.111348. [DOI] [PubMed] [Google Scholar]
- 310.Kim SY, Lim JS, Kim DH, Lee HJ, Cho JB, Lee JA, Kim DH. 2011. Candida tropicalis arthritis of the elbow in a patient with Ewing’s sarcoma that successfully responded to itraconazole. Korean J Pediatr 54:385–388. 10.3345/kjp.2011.54.9.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Radike K, Kunzmann S, Abele-Horn M, Beer M, Hebestreit H. 2011. Osteoarticular infection by Candida albicans in an infant with cystic fibrosis. J Med Microbiol 60:1542–1545. 10.1099/jmm.0.031757-0. [DOI] [PubMed] [Google Scholar]
- 312.Lee CH, Oh JM, Oh SR, Yoo M, Lee MS. 2010. Candida arthritis after arthroscopic arthroplasty in a patient without predisposing factors. Open Rheumatol J 4:7–9. 10.2174/1874312901004010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bland CM, Thomas S. 2009. Micafungin plus fluconazole in an infected knee with retained hardware due to Candida albicans. Ann Pharmacother 43:528–531. 10.1345/aph.1L508. [DOI] [PubMed] [Google Scholar]
- 314.Kathresal A, Biundo J, Blais CM, Morse S, Reisin E. 2008. A rare case of Candida arthritis in a hemodialysis patient. Am J Med Sci 336:437–440. 10.1097/MAJ.0b013e31815fa556. [DOI] [PubMed] [Google Scholar]
- 315.Masoud M, Nasser NJ, Karban A, Edelstein S. 2008. Candida parapsilosis septic arthritis in a renal transplant patient. J Clin Rheumatol 14:56. 10.1097/RHU.0b013e318163ccec. [DOI] [PubMed] [Google Scholar]
- 316.Bariola JR, Saccente M. 2008. Candida lusitaniae septic arthritis: case report and review of the literature. Diagn Microbiol Infect Dis 61:61–63. 10.1016/j.diagmicrobio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 317.Sili U, Yilmaz M, Ferhanoglu B, Mert A. 2007. Candida krusei arthritis in a patient with hematologic malignancy: successful treatment with voriconazole. Clin Infect Dis 45:897–898. 10.1086/521253. [DOI] [PubMed] [Google Scholar]
- 318.Jeragh A, Ahmad S, Naseem J, Khan ZU. 2007. Candida lusitaniae arthritis in an intravenous drug user. Mycoses 50:430–432. 10.1111/j.1439-0507.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 319.Wang HP, Yen YF, Chen WS, Chou YL, Tsai CY, Chang HN, Chou CT. 2007. An unusual case of Candida tropicalis and Candida krusei arthritis in a patient with acute myelogenous leukemia before chemotherapy. Clin Rheumatol 26:1195–1197. 10.1007/s10067-006-0336-9. [DOI] [PubMed] [Google Scholar]
- 320.Lazzarini L, Manfrin V, De Lalla F. 2004. Candidal prosthetic hip infection in a patient with previous candidal septic arthritis. J Arthroplasty 19:248–252. 10.1016/S0883-5403(03)00407-8. [DOI] [PubMed] [Google Scholar]
- 321.Vicari P, Feitosa Pinheiro R, Chauffaille MDLLF, Yamamoto M, Figueiredo MS. 2003. Septic arthritis as the first sign of Candida tropicalis fungaemia in an acute lymphoid leukemia patient. Braz J Infect Dis 7:426–428. 10.1590/s1413-86702003000600012. [DOI] [PubMed] [Google Scholar]
- 322.Kawanabe K, Hayashi H, Miyamoto M, Tamura J, Shimizu M, Nakamura T. 2003. Candida septic arthritis of the hip in a young patient without predisposing factors. J Bone Joint Surg Br 85:734–735. 10.1302/0301-620X.85B5.13879. [DOI] [PubMed] [Google Scholar]
- 323.Turgut B, Vural O, Demir M, Kaldir M. 2002. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol 81:529–531. 10.1007/s00277-002-0503-2. [DOI] [PubMed] [Google Scholar]
- 324.Hacimustafaoğlu M, Cil E, Sarisözen B, Zincirci M, Ildirim I. 2001. Bilateral septic arthritis of the knee joint in three children caused by unusual infectious agents. Pediatr Int 43:697–700. 10.1046/j.1442-200x.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 325.Choi IS, Kim SJ, Kim BY, Joh JW, Kim YI, Lee SK, Huh WS, Oh HY, Kim DJ, Kim YG, Kim MK, Ko YH, Lee BB. 2000. Candida polyarthritis in a renal transplant patient: case report of a patient successfully treated with amphotericin B. Transplant Proc 32:1963–1964. 10.1016/S0041-1345(00)01513-X. [DOI] [PubMed] [Google Scholar]
- 326.Weigl JA. 2000. Candida arthritis in a premature infant treated successfully with oral fluconazole for six months. Ann Acad Med Singap 29:253–255. [PubMed] [Google Scholar]
- 327.Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. 1999. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol 18:406–409. 10.1007/s100670050127. [DOI] [PubMed] [Google Scholar]
- 328.Gumbo T, Isada CM, Muschler GF, Longworth DL. 1999. Candida (Torulopsis) glabrata septic arthritis. Clin Infect Dis 29:208–209. 10.1086/520160. [DOI] [PubMed] [Google Scholar]
- 329.Trowbridge J, Ludmer LM, Riddle VD, Levy CS, Barth WF. 1999. Candida lambica polyarthritis in a patient with chronic alcoholism. J Rheumatol 26:1846–1848. [PubMed] [Google Scholar]
- 330.Azaceta G, Olave T, de los Martires LD, Delgado C, Gutierrez M, Palomera L. 1999. Successful lipid-complexed amphotericin B treatment of Candida arthritis in a lymphoma patient. Rev Rhum Engl Ed 66:434–435. [PubMed] [Google Scholar]
- 331.Calvo Romero JM, Alvarez Vega JL, Salazar Vallinas JM, Ortega Alberdi R. 1998. Candida arthritis in an immunocompetent patient without predisposing factors. Clin Rheumatol 17:393–394. 10.1007/BF01450899. [DOI] [PubMed] [Google Scholar]
- 332.Brooks DH, Pupparo F. 1998. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty 13:707–712. 10.1016/s0883-5403(98)80017-x. [DOI] [PubMed] [Google Scholar]
- 333.Perez-Gomez A, Prieto A, Torresano M, Diez E, Mulero J, Labiano I, Andreu JL. 1998. Role of the new azoles in the treatment of fungal osteoarticular infections. Semin Arthritis Rheum 27:226–244. 10.1016/s0049-0172(98)80003-6. [DOI] [PubMed] [Google Scholar]
- 334.Hacimustafaoglu M, Ener B, Tarim O, Kilic S, Tanritanir A, Ildirim I. 1997. Systemic candidiasis with acute Epstein-Barr virus infection. Acta Paediatr 86:1267–1270. 10.1111/j.1651-2227.1997.tb14861.x. [DOI] [PubMed] [Google Scholar]
- 335.Fukasawa N, Shirakura K. 1997. Candida arthritis after total knee arthroplasty—a case of successful treatment without prosthesis removal. Acta Orthop Scand 68:306–307. 10.3109/17453679708996709. [DOI] [PubMed] [Google Scholar]
- 336.Weers-Pothoff G, Havermans JF, Kamphuis J, Sinnige HA, Meis JF. 1997. Candida tropicalis arthritis in a patient with acute myeloid leukemia successfully treated with fluconazole: case report and review of the literature. Infection 25:109–111. 10.1007/BF02113588. [DOI] [PubMed] [Google Scholar]
- 337.Swanson H, Hughes PA, Messer SA, Lepow ML, Pfaller MA. 1996. Candida albicans arthritis one year after successful treatment of fungemia in a healthy infant. J Pediatr 129:688–694. 10.1016/S0022-3476(96)70151-8. [DOI] [PubMed] [Google Scholar]
- 338.Rovinsky D, Williams GR, Jr, Iannotti JP, Ragsdale BD. 1995. Autoimmune arthritis caused by Candida septic arthritis. J Shoulder Elbow Surg 4:472–476. 10.1016/S1058-2746(05)80041-2. [DOI] [PubMed] [Google Scholar]
- 339.Cuende E, Barbadillo C, E-Mazzucchelli R, Isasi C, Trujillo A, Andreu JL. 1993. Candida arthritis in adult patients who are not intravenous drug addicts: report of three cases and review of the literature. Semin Arthritis Rheum 22:224–241. 10.1016/0049-0172(93)80071-M. [DOI] [PubMed] [Google Scholar]
- 340.Barbara JA, Clarkson AR, LaBrooy J, McNeil JD, Woodroffe AJ. 1993. Candida albicans arthritis in a renal allograft recipient with an interaction between cyclosporin and fluconazole. Nephrol Dial Transplant 8:263–266. [PubMed] [Google Scholar]
- 341.Christensson B, Ryd L, Dahlberg L, Lohmander S. 1993. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand 64:695–698. 10.3109/17453679308994601. [DOI] [PubMed] [Google Scholar]
- 342.Nouyrigat P, Baume D, Blaise D, Revillon D, Gabus R, Miquel M, Maraninchi D. 1993. Candida arthritis treated with intra-articular amphotericin B. Eur J Med 2:124–125. [PubMed] [Google Scholar]
- 343.Marcus J, Grossman ME, Yunakov MJ, Rappaport F. 1992. Disseminated candidiasis, Candida arthritis, and unilateral skin lesions. J Am Acad Dermatol 26:295–297. 10.1016/0190-9622(92)70038-h. [DOI] [PubMed] [Google Scholar]
- 344.Pizarro S, Barile L, Fraga A, Medina F. 1992. Favorable outcome with ketoconazole in Candida septic arthritis. J Rheumatol 19:328. [PubMed] [Google Scholar]
- 345.Hermann E, Mayet WJ, Klein O, Lohse AW, Trautwein C, Michiels I, Poralla T, Meyer zum Buschenfelde KH. 1991. Candida arthritis: cellular immune responses of synovial fluid and peripheral blood lymphocytes to Candida albicans. Ann Rheum Dis 50:697–701. 10.1136/ard.50.10.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.O’Meeghan T, Varcoe R, Thomas M, Ellis-Pegler R. 1990. Fluconazole concentration in joint fluid during successful treatment of Candida albicans septic arthritis. J Antimicrob Chemother 26:601–602. 10.1093/jac/26.4.601. [DOI] [PubMed] [Google Scholar]
- 347.Meyer RD, Gaut PL. 1990. Candidal pyarthrosis in an AIDS patient. Scand J Infect Dis 22:607–610. 10.3109/00365549009027104. [DOI] [PubMed] [Google Scholar]
- 348.Campen DH, Kaufman RL, Beardmore TD. 1990. Candida septic arthritis in rheumatoid arthritis. J Rheumatol 17:86–88. [PubMed] [Google Scholar]
- 349.Lambertus M, Thordarson D, Goetz MB. 1988. Fungal prosthetic arthritis: presentation of two cases and review of the literature. Rev Infect Dis 10:1038–1043. 10.1093/clinids/10.5.1038. [DOI] [PubMed] [Google Scholar]
- 350.Levine M, Rehm SJ, Wilde AH. 1988. Infection with Candida albicans of a total knee arthroplasty. Case report and review of the literature. Clin Orthop Relat Res 1988:235–239. [PubMed] [Google Scholar]
- 351.De Clerck L, Dequeker J, Westhovens R, Hauglustaine D. 1988. Candida parapsilosis in a patient receiving chronic hemodialysis. J Rheumatol 15:372–374. [PubMed] [Google Scholar]
- 352.Nguyen VQ, Penn RL. 1987. Candida krusei infectious arthritis. A rare complication of neutropenia. Am J Med 83:963–965. 10.1016/0002-9343(87)90660-7. [DOI] [PubMed] [Google Scholar]
- 353.Bisbe J, Vilardell J, Valls M, Moreno A, Brancos M, Andreu J. 1987. Transient fungemia and Candida arthritis due to Candida zeylanoides. Eur J Clin Microbiol 6:668–669. 10.1007/BF02013067. [DOI] [PubMed] [Google Scholar]
- 354.Smith SM, Lee EY, Cobbs CJ, Eng RH. 1987. Unusual features of arthritis caused by Candida parapsilosis. Arch Pathol Lab Med 111:71–73. [PubMed] [Google Scholar]
- 355.Thobani SU, George RH. 1986. Candida albicans arthritis in an infant. J Infect 13:163–165. 10.1016/S0163-4453(86)93009-4. [DOI] [PubMed] [Google Scholar]
- 356.Gambrioli PL, Maggi F, Minola R. 1986. Candida arthritis of the knee. Case report. Ital J Orthop Traumatol 12:245–252. [PubMed] [Google Scholar]
- 357.Katzenstein D. 1985. Isolated Candida arthritis: report of a case and definition of a distinct clinical syndrome. Arthritis Rheum 28:1421–1424. 10.1002/art.1780281216. [DOI] [PubMed] [Google Scholar]
- 358.Mandel DR, Segal AM, Wysenbeek AJ, Calabrese LH. 1984. Two unusual strains of Candida arthritis. Am J Med Sci 288:25–27. 10.1097/00000441-198407000-00005. [DOI] [PubMed] [Google Scholar]
- 359.Duquesnoy B, Fournier E, Berniere L, Delcambre B. 1984. Ketoconazole for treatment of Candida arthritis. J Rheumatol 11:105–107. [PubMed] [Google Scholar]
- 360.Pope TL, Jr. 1982. Pediatric Candida albicans arthritis: case report of hip involvement with a review of the literature. Prog Pediatr Surg 15:271–283. [PubMed] [Google Scholar]
- 361.Arnold HJ, Dini A, Jonas G, Zorn EL. 1981. Candida albicans arthritis in a healthy adult. South Med J 74:84–85. 10.1097/00007611-198101000-00033. [DOI] [PubMed] [Google Scholar]
- 362.Gerster JC, Glauser MP, Delacretaz F, Nguyen T. 1980. Erosive Candida arthritis in a patient with disseminated candidiasis. J Rheumatol 7:911–914. [PubMed] [Google Scholar]
- 363.Rao HK, Myers GJ. 1979. Candida meningitis in the newborn. South Med J 72:1468–1471. 10.1097/00007611-197911000-00036. [DOI] [PubMed] [Google Scholar]
- 364.Ginzler E, Meisel AD, Munters M, Kaplan D. 1979. Candida arthritis secondary to repeated intra-articular corticosteroids. N Y State J Med 79:392–394. [PubMed] [Google Scholar]
- 365.Yarchoan R, Davies SF, Fried J, Mahowald ML. 1979. Isolated Candida parapsilosis arthritis in a heroin addict. J Rheumatol 6:447–450. [PubMed] [Google Scholar]
- 366.Bayer AS, Guze LB. 1978. Fungal arthritis. I. Candida arthritis: diagnostic and prognostic implications and therapeutic considerations. Semin Arthritis Rheum 8:142–150. 10.1016/0049-0172(78)90016-1. [DOI] [PubMed] [Google Scholar]
- 367.Imbeau SA, Hanson J, Langejans G, D’Alessio D. 1977. Flucytosine treatment of Candida arthritis. JAMA 238:1395–1396. 10.1001/jama.1977.03280140073026. [DOI] [PubMed] [Google Scholar]
- 368.Specht EE. 1977. Candida pyarthrosis of the hip and renal homotransplant. Report of a case treated by femoral head and neck resection and 5-fluorocytosine. Clin Orthop Relat Res 126:176–177. [PubMed] [Google Scholar]
- 369.Ide A, Jacobelli S, Zenteno G. 1977. Candida arthritis associated with positive birefringent crystals without chondrocalcinosis. J Rheumatol 4:327–328. [PubMed] [Google Scholar]
- 370.Businco L, Iannaccone G, Del Principe D, Lucarelli S, Cardi E, Rezza E. 1977. Disseminated arthritis and osteitis by Candida albicans in a two month old infant receiving parenteral nutrition. Acta Paediatr Scand 66:393–395. 10.1111/j.1651-2227.1977.tb07914.x. [DOI] [PubMed] [Google Scholar]
- 371.Keller MA, Sellers BB, Jr, Melish ME, Kaplan GW, Miller KE, Mendoza SA. 1977. Systemic candidiasis in infants: a case presentation and literature review. Am J Dis Child 131:1260–1263. 10.1001/archpedi.1977.02120240078017. [DOI] [PubMed] [Google Scholar]
- 372.Marmor L, Peter JB. 1976. Candida arthritis of the knee joint. Clin Orthop Relat Res 118:133–135. [PubMed] [Google Scholar]
- 373.Fitzgerald E, Lloyd-Still J, Gordon SL. 1975. Candida arthritis. A case report and review of the literature. Clin Orthop Relat Res 106:143–147. 10.1097/00003086-197501000-00021. [DOI] [PubMed] [Google Scholar]
- 374.Poplack DG, Jacobs SA. 1975. Candida arthritis treated with amphotericin B. J Pediatr 87:989–990. 10.1016/s0022-3476(75)80926-7. [DOI] [PubMed] [Google Scholar]
- 375.Hill HR, Mitchell TG, Matsen JM, Quie PG. 1974. Recovery from disseminated candidiasis in a premature neonate. Pediatrics 53:748–752. 10.1542/peds.53.5.748. [DOI] [PubMed] [Google Scholar]
- 376.Umber J, Chapman MW, Drutz DJ. 1974. Candida pyarthrosis. Report of a case and results of treatment with 5-fluorocytosine. J Bone Joint Surg Am 56:1520–1524. [PubMed] [Google Scholar]
- 377.Lindstrom FD, Lindholm T. 1973. Candida albicans arthritis treated with flucytosine. Ann Intern Med 79:131. 10.7326/0003-4819-79-1-131_1. [DOI] [PubMed] [Google Scholar]
- 378.Klein JD, Yamauchi T, Horlick SP. 1972. Neonatal candidiasis, meningitis, and arthritis: observations and a review of the literature. J Pediatr 81:31–34. 10.1016/S0022-3476(72)80369-X. [DOI] [PubMed] [Google Scholar]
- 379.Lachman RS, Yamauchi T, Klein J. 1972. Neonatal systemic candidiasis and arthritis. Radiology 105:631–632. 10.1148/105.3.631. [DOI] [PubMed] [Google Scholar]
- 380.Pruitt AW, Achord JL, Fales FW, Patterson JH. 1969. Glucose-galactose malabsorption complicated by monilial arthritis. Pediatrics 43:106–110. [PubMed] [Google Scholar]
- 381.Matthews S, Sloan S, McCaffrey D, Ruiz A. 2017. Septic arthritis of the hip complicated by secondary fungal superinfection. J Orthop Case Rep 7:46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Cobo F, Rodriguez-Granger J, Sampedro A, Aliaga-Martinez L, Navarro-Mari JM. 2017. Candida prosthetic joint infection. A review of treatment methods. J Bone Jt Infect 2:114–121. 10.7150/jbji.17699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Henry MW, Miller AO, Walsh TJ, Brause BD. 2017. Fungal musculoskeletal infections. Infect Dis Clin North Am 31:353–368. 10.1016/j.idc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 384.Simmons SC, Budavari AI, Kusne S, Zhang N, Vikram HR, Blair JE. 2017. Culture-proven thorn-associated infections in Arizona: 10-year experience at Mayo Clinic. Open Forum Infect Dis 4:ofx017. 10.1093/ofid/ofx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tucker A, Matthews S, Wilson A. 2016. Mycotic septic arthritis of the ankle joint. Am J Orthop (Belle Mead NJ) 45:E478–E480. [PubMed] [Google Scholar]
- 386.Cevik R, Tekin R, Gem M. 2016. Candida arthritis in a patient diagnosed with spondyloarthritis. Rev Soc Bras Med Trop 49:793–795. 10.1590/0037-8682-0089-2016. [DOI] [PubMed] [Google Scholar]
- 387.Luo JM, Guo L, Chen H, Yang PF, Xiong R, Peng Y, Yang L. 2017. A study of pre-operative presence of micro-organisms in affected knee joints of rheumatoid arthritis patients who need total knee arthroplasty. Knee 24:409–418. 10.1016/j.knee.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 388.Walker JW, Hennrikus WL. 2016. Septic arthritis of the pediatric shoulder: from infancy to adolescence. Int J Pediatr 2016:3086019. 10.1155/2016/3086019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Cobo F, Rodriguez-Granger J, Lopez EM, Jimenez G, Sampedro A, Aliaga-Martinez L, Navarro-Mari JM. 2017. Candida-induced prosthetic joint infection. A literature review including 72 cases and a case report. Infect Dis (Lond) 49:81–94. 10.1080/23744235.2016.1219456. [DOI] [PubMed] [Google Scholar]
- 390.Saunte DM, Mrowietz U, Puig L, Zachariae C. 2017. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol 177:47–62. 10.1111/bjd.15015. [DOI] [PubMed] [Google Scholar]
- 391.Bono KT, Samora JB, Klingele KE. 2015. Septic arthritis in infants younger than 3 months: a retrospective review. Orthopedics 38:e787–e793. 10.3928/01477447-20150902-56. [DOI] [PubMed] [Google Scholar]
- 392.Heslop OD, De Ceulaer K, Rainford L, Nicholson AM. 2015. A case of Candida orthopsilosis associated septic arthritis in a patient with systemic lupus erythematosus (SLE). Med Mycol Case Rep 7:1–3. 10.1016/j.mmcr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Sharma S, Gangwal K. 2014. Neonatal Candida arthritis. Indian J Orthop 48:339–342. 10.4103/0019-5413.132533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.d’Aleo F, Bonanno R, Midiri A, Mancuso G, Cordaro S, Warm A, Verduci E, Beninati C, Biondo C. 2017. A case of Candida septic arthritis with rice body formation in a 2-month-old infant. Infez Med 25:374–376. [PubMed] [Google Scholar]
- 395.Cawley EP. 1947. Aspergillosis and the Aspergilli; report of a unique case of the disease. Arch Intern Med (Chic) 80:423–434. 10.1001/archinte.1947.00220160002001. [DOI] [PubMed] [Google Scholar]
- 396.Horn D, Sae-Tia S, Neofytos D. 2009. Aspergillus osteomyelitis: review of 12 cases identified by the Prospective Antifungal Therapy Alliance Registry. Diagn Microbiol Infect Dis 63:384–387. 10.1016/j.diagmicrobio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 397.Severino M, Liyanage S, Novelli V, Cheesborough B, Saunders D, Gunny R, Rossi A. 2012. Skull base osteomyelitis and potential cerebrovascular complications in children. Pediatr Radiol 42:867–874. 10.1007/s00247-011-2340-8. [DOI] [PubMed] [Google Scholar]
- 398.Tew CW, Han FC, Jureen R, Tey BH. 2009. Aspergillus vertebral osteomyelitis and epidural abscess. Singapore Med J 50:e151–e154. [PubMed] [Google Scholar]
- 399.Vinas FC, King PK, Diaz FG. 1999. Spinal Aspergillus osteomyelitis. Clin Infect Dis 28:1223–1229. 10.1086/514774. [DOI] [PubMed] [Google Scholar]
- 400.Abu Jawdeh L, Haidar R, Bitar F, Mroueh S, Akel S, Nuwayri-Salti N, Dbaibo GS. 2000. Aspergillus vertebral osteomyelitis in a child with a primary monocyte killing defect: response to GM-CSF therapy. J Infect 41:97–100. 10.1053/jinf.2000.0673. [DOI] [PubMed] [Google Scholar]
- 401.Allen D, Ng S, Beaton K, Taussig D. 2002. Sternal osteomyelitis caused by Aspergillus fumigatus in a patient with previously treated Hodgkin’s disease. J Clin Pathol 55:616–618. 10.1136/jcp.55.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Al-Tawfiq JA, Al-Abdely HM. 2010. Vertebral osteomyelitis due to Aspergillus fumigatus in a patient with chronic granulomatous disease successfully treated with antifungal agents and interferon-gamma. Med Mycol 48:537–541. 10.3109/13693780903325290. [DOI] [PubMed] [Google Scholar]
- 403.Alvarez L, Calvo E, Abril C. 1995. Articular aspergillosis: case report. Clin Infect Dis 20:457–460. 10.1093/clinids/20.2.457. [DOI] [PubMed] [Google Scholar]
- 404.Amonoo-Kuofi K, Tostevin P, Knight JR. 2005. Aspergillus mastoiditis in a patient with systemic lupus erythematosus: a case report. Skull Base 15:109–112. 10.1055/s-2005-870595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Andaluz N, Zuccarello M. 2008. Multidrug-resistant, progressive, invasive diffuse spinal aspergillosis: case report and review of the literature. J Neurosurg Sci 52:49–53. [PubMed] [Google Scholar]
- 406.Anderson J, Kron IL. 1984. Treatment of Aspergillus infection of the proximal aortic prosthetic graft with associated vertebral osteomyelitis. J Vasc Surg 1:579–581. 10.1016/0741-5214(84)90048-X. [DOI] [PubMed] [Google Scholar]
- 407.Assaad W, Nuchikat PS, Cohen L, Esguerra JV, Whittier FC. 1994. Aspergillus discitis with acute disc abscess. Spine (Phila Pa 1976) 19:2226–2229. 10.1097/00007632-199410000-00019. [DOI] [PubMed] [Google Scholar]
- 408.Attah CA, Cerruti MM. 1979. Aspergillus osteomyelitis of sternum after cardiac surgery. N Y State J Med 79:1420–1421. [PubMed] [Google Scholar]
- 409.Nandeesh BN, Kini U, Alexander B. 2010. Vertebral osteomyelitis with a rare etiology diagnosed by fine-needle aspiration cytology. Diagn Cytopathol 38:360–363. 10.1002/dc.21212. [DOI] [PubMed] [Google Scholar]
- 410.Barnwell PA, Jelsma LF, Raff MJ. 1985. Aspergillus osteomyelitis. Report of a case and review of the literature. Diagn Microbiol Infect Dis 3:515–519. 10.1016/s0732-8893(85)80008-0. [DOI] [PubMed] [Google Scholar]
- 411.Barzaghi N, Emmi V, Mencherini S, Minzioni G, Marone P, Minoli L. 1994. Sternal osteomyelitis due to Aspergillus fumigatus after cardiac surgery. Chest 105:1275–1277. 10.1378/chest.105.4.1275. [DOI] [PubMed] [Google Scholar]
- 412.Beluffi G, Bernardo ME, Meloni G, Spinazzola A, Locatelli F. 2008. Spinal osteomyelitis due to Aspergillus flavus in a child: a rare complication after haematopoietic stem cell transplantation. Pediatr Radiol 38:709–712. 10.1007/s00247-008-0789-x. [DOI] [PubMed] [Google Scholar]
- 413.Bhatt YM, Pahade N, Nair B. 2013. Aspergillus petrous apicitis associated with cerebral and peritubular abscesses in an immunocompetent man. J Laryngol Otol 127:404–407. 10.1017/S0022215113000315. [DOI] [PubMed] [Google Scholar]
- 414.Bianchi R, Chekikian G, Ciboddo G, Ciceri F, Fioretti ES, Rugarli C. 1994. Primary sternal osteomyelitis by Aspergillus fumigatus. Br J Rheumatol 33:994–995. 10.1093/rheumatology/33.10.994. [DOI] [PubMed] [Google Scholar]
- 415.Bickley LS, Betts RF, Parkins CW. 1988. Atypical invasive external otitis from Aspergillus. Arch Otolaryngol Head Neck Surg 114:1024–1028. 10.1001/archotol.1988.01860210090023. [DOI] [PubMed] [Google Scholar]
- 416.Brandt SJ, Thompson RL, Wenzel RP. 1985. Mycotic pseudoaneurysm of an aortic bypass graft and contiguous vertebral osteomyelitis due to Aspergillus fumigatus. Am J Med 79:259–262. 10.1016/0002-9343(85)90019-1. [DOI] [PubMed] [Google Scholar]
- 417.Bridwell KH, Campbell JW, Barenkamp SJ. 1990. Surgical treatment of hematogenous vertebral Aspergillus osteomyelitis. Spine (Phila Pa 1976) 15:281–285. 10.1097/00007632-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 418.Brodsky JW, Seidenfeld SM, Brooks B, Shabat S. 2005. Aspergillus osteomyelitis and lymphangitis in immunocompromised patient after toenail clipping. Foot Ankle Int 26:576–578. 10.1177/107110070502600715. [DOI] [PubMed] [Google Scholar]
- 419.Brown DL, Musher DM, Taffet GE. 1987. Hematogenously acquired Aspergillus vertebral osteomyelitis in seemingly immunocompetent drug addicts. West J Med 147:84–85. [PMC free article] [PubMed] [Google Scholar]
- 420.Bryce GE, Phillips P, Lepawsky M, Gribble MJ. 1997. Invasive Aspergillus tympanomastoiditis in an immunocompetent patient. J Otolaryngol 26:266–269. [PubMed] [Google Scholar]
- 421.Bujak JS, Kwon-Chung KJ, Chusid MJ. 1974. Osteomyelitis and pneumonia in a boy with chronic granulomatous disease of childhood caused by a mutant strain of Aspergillus nidulans. Am J Clin Pathol 61:361–367. 10.1093/ajcp/61.3.361. [DOI] [PubMed] [Google Scholar]
- 422.Byrd BF, III, Weiner MH, McGee ZA. 1982. Aspergillus spinal epidural abscess. JAMA 248:3138–3139. 10.1001/jama.1982.03330230050031. [DOI] [PubMed] [Google Scholar]
- 423.Cartoni C, Capua A, Damico C, Potente G. 1992. Aspergillus osteomyelitis of the rib: sonographic diagnosis. J Clin Ultrasound 20:217–220. 10.1002/jcu.1870200311. [DOI] [PubMed] [Google Scholar]
- 424.Casscells SW. 1978. Aspergillus osteomyelitis of the tibia. A case report. J Bone Joint Surg Am 60:994–995. [PubMed] [Google Scholar]
- 425.Chi C-Y, Fung C-P, Liu C-Y. 2003. Aspergillus flavus epidural abscess and osteomyelitis in a diabetic patient. J Microbiol Immunol Infect 36:145–148. [PubMed] [Google Scholar]
- 426.Cimerman M, Gunde-Cimerman N, Zalar P, Perkovic T. 1999. Femur osteomyelitis due to a mixed fungal infection in a previously healthy man. J Clin Microbiol 37:1532–1535. 10.1128/JCM.37.5.1532-1535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Convent L, Van de Mierop L, Blijweert D. 1979. A case of vertebral aspergillosis. Acta Orthop Belg 45:141–150. [PubMed] [Google Scholar]
- 428.Corrado ML, Cleri D, Fikrig SM, Phillips JC, Ahonkhai VI. 1980. Aspergillosis in chronic granulomatous disease: therapeutic considerations. Am J Dis Child 134:1092–1094. 10.1001/archpedi.1980.02130230070021. [DOI] [PubMed] [Google Scholar]
- 429.Corrall CJ, Merz WG, Rekedal K, Hughes WT. 1982. Aspergillus osteomyelitis in an immunocompetent adolescent: a case report and review of the literature. Pediatrics 70:455–461. 10.1542/peds.70.3.455. [DOI] [PubMed] [Google Scholar]
- 430.Cosgarea AJ, Tejani N, Jones JA. 1993. Carpal Aspergillus osteomyelitis: case report and review of the literature. J Hand Surg Am 18:722–726. 10.1016/0363-5023(93)90327-Y. [DOI] [PubMed] [Google Scholar]
- 431.Cunningham M, Yu VL, Turner J, Curtin H. 1988. Necrotizing otitis externa due to Aspergillus in an immunocompetent patient. Arch Otolaryngol Head Neck Surg 114:554–556. 10.1001/archotol.1988.01860170084024. [DOI] [PubMed] [Google Scholar]
- 432.Dayan L, Sprecher H, Hananni A, Rosenbaum H, Milloul V, Oren I. 2007. Aspergillus vertebral osteomyelitis diagnosed by a novel panfungal in chronic leukocyte leukemia patient polymerase chain reaction method. Spine J 7:615–617. 10.1016/j.spinee.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 433.De Bock R, Schrijvers D, Peetermans M. 1991. Pulmonary aspergillosis complicated by osteomyelitis. Acta Clin Belg 46:397–400. 10.1080/17843286.1991.11718196. [DOI] [PubMed] [Google Scholar]
- 434.Garazzino S, Maiello A, De Rosa FG, Aprato A, Di Perri G. 2008. Post-traumatic osteomyelitis due to Aspergillus flavus successfully treated with voriconazole: a case report. J Chemother 20:524–526. 10.1179/joc.2008.20.4.524. [DOI] [PubMed] [Google Scholar]
- 435.Hodiamont CJ, Dolman KM, Ten Berge IJM, Melchers WJG, Verweij PE, Pajkrt D. 2009. Multiple-azole-resistant Aspergillus fumigatus osteomyelitis in a patient with chronic granulomatous disease successfully treated with long-term oral posaconazole and surgery. Med Mycol 47:217–220. 10.1080/13693780802545600. [DOI] [PubMed] [Google Scholar]
- 436.Kumashi PR, Safdar A, Chamilos G, Chemaly RF, Raad II, Kontoyiannis DP. 2006. Fungal osteoarticular infections in patients treated at a comprehensive cancer centre: a 10-year retrospective review. Clin Microbiol Infect 12:621–626. 10.1111/j.1469-0691.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 437.Kuruvilla G, Job A, Mathew J, Ayyappan AP, Jacob M. 2006. Septate fungal invasion in masked mastoiditis: a diagnostic dilemma. J Laryngol Otol 120:250–252. 10.1017/S0022215106000326. [DOI] [PubMed] [Google Scholar]
- 438.Lodge BA, Ashley ED, Steele MP, Perfect JR. 2004. Aspergillus fumigatus empyema, arthritis, and calcaneal osteomyelitis in a lung transplant patient successfully treated with posaconazole. J Clin Microbiol 42:1376–1378. 10.1128/JCM.42.3.1376-1378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Martinez M, Lee AS, Hellinger WC, Kaplan J. 1999. Vertebral Aspergillus osteomyelitis and acute diskitis in patients with chronic obstructive pulmonary disease. Mayo Clin Proc 74:579–583. 10.4065/74.6.579. [DOI] [PubMed] [Google Scholar]
- 440.Parize P, Chandesris MO, Lanternier F, Poiree S, Viard JP, Bienvenu B, Mimoun M, Mechai F, Mamzer MF, Herman P, Bougnoux ME, Lecuit M, Lortholary O. 2009. Antifungal therapy of Aspergillus invasive otitis externa: efficacy of voriconazole and review. Antimicrob Agents Chemother 53:1048–1053. 10.1128/AAC.01220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Pollack IF, Pang D, Schuit KE. 1987. Chronic granulomatous disease with cranial fungal osteomyelitis and epidural abscess. Case report. J Neurosurg 67:132–136. 10.3171/jns.1987.67.1.0132. [DOI] [PubMed] [Google Scholar]
- 442.Shelton JC, Antonelli PJ, Hackett R. 2002. Skull base fungal osteomyelitis in an immunocompetent host. Otolaryngol Head Neck Surg 126:76–78. 10.1067/mhn.2002.120699. [DOI] [PubMed] [Google Scholar]
- 443.Stodulski D, Kowalska B, Stankiewicz C. 2006. Otogenic skull base osteomyelitis caused by invasive fungal infection. Case report and literature review. Eur Arch Otorhinolaryngol 263:1070–1076. 10.1007/s00405-006-0118-7. [DOI] [PubMed] [Google Scholar]
- 444.Vaishya S, Sharma MS. 2004. Spinal Aspergillus vertebral osteomyelitis with extradural abscess: case report and review of literature. Surg Neurol 61:551–555. 10.1016/j.surneu.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 445.Verghese S, Chellamma T, Cherian KM. 2009. Osteomyelitis of the rib caused by Aspergillus flavus following cardiac surgery. Mycoses 52:91–93. 10.1111/j.1439-0507.2008.01541.x. [DOI] [PubMed] [Google Scholar]
- 446.Vourexakis Z, Kos MI, Guyot JP. 2010. Atypical presentations of malignant otitis externa. J Laryngol Otol 124:1205–1208. 10.1017/S0022215110000307. [DOI] [PubMed] [Google Scholar]
- 447.Watanabe C, Yajima S, Taguchi T, Toya K, Fujii Y, Hongo T, Ohzeki T. 2001. Successful unrelated bone marrow transplantation for a patient with chronic granulomatous disease and associated resistant pneumonitis and Aspergillus osteomyelitis. Bone Marrow Transplant 28:83–87. 10.1038/sj.bmt.1703086. [DOI] [PubMed] [Google Scholar]
- 448.Weclawiak H, Garrouste C, Kamar N, Linas M-D, Tall P, Dambrin C, Durand D, Rostaing L. 2007. Aspergillus fumigatus-related spondylodiscitis in a heart transplant patient successfully treated with voriconazole. Transplant Proc 39:2627–2628. 10.1016/j.transproceed.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 449.Witzig RS, Greer DL, Hyslop NE, Jr.. 1996. Aspergillus flavus mycetoma and epidural abscess successfully treated with itraconazole. J Med Vet Mycol 34:133–137. 10.1080/02681219680000201. [DOI] [PubMed] [Google Scholar]
- 450.de la Cruz R, Jain M, Hsu K, Lim DT. 1983. Intrinsic polymorphonuclear chemotactic defect in a boy with chronic granulomatous disease. Allergol Immunopathol (Madr) 11:457–464. [PubMed] [Google Scholar]
- 451.De Vuyst D, Surmont I, Verhaegen J, Vanhaecke J. 1992. Tibial osteomyelitis due to Aspergillus flavus in a heart transplant patient. Infection 20:48–49. 10.1007/BF01704898. [DOI] [PubMed] [Google Scholar]
- 452.Denning DW, Tucker RM, Hanson LH, Stevens DA. 1989. Treatment of invasive aspergillosis with itraconazole. Am J Med 86:791–800. 10.1016/0002-9343(89)90475-0. [DOI] [PubMed] [Google Scholar]
- 453.D’Hoore K, Hoogmartens M. 1993. Vertebral aspergillosis. A case report and review of the literature. Acta Orthop Belg 59:306–314. [PubMed] [Google Scholar]
- 454.Dietz R, Huber G, Thetter O, Volkmer I. 1982. Aspergillosis of the lung with osteoclasis and paraplegia. Neuroradiology 23:219–221. 10.1007/BF00342546. [DOI] [PubMed] [Google Scholar]
- 455.Diop EM, Schachern PA, Paparella MM. 1998. Acquired immunodeficiency syndrome with massive Aspergillus fumigatus infection. Otolaryngol Head Neck Surg 118:283–285. 10.1016/S0194-5998(98)80034-X. [DOI] [PubMed] [Google Scholar]
- 456.Dotis J, Panagopoulou P, Filioti J, Winn R, Toptsis C, Panteliadis C, Roilides E. 2003. Femoral osteomyelitis due to Aspergillus nidulans in a patient with chronic granulomatous disease. Infection 31:121–124. 10.1007/s15010-002-2167-1. [DOI] [PubMed] [Google Scholar]
- 457.Elahi MM, Mitra A, Spears J, McClurken JB. 2005. Recalcitrant chest wall Aspergillus fumigatus osteomyelitis after coronary artery bypass grafting: successful radical surgical and medical management. Ann Thorac Surg 79:1057–1059. 10.1016/j.athoracsur.2003.09.119. [DOI] [PubMed] [Google Scholar]
- 458.Ersoy A, Akdag I, Akalin H, Sarisozen B, Ener B. 2007. Aspergillosis osteomyelitis and joint infection in a renal transplant recipient. Transplant Proc 39:1662–1663. 10.1016/j.transproceed.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 459.Faure BT, Biondi JX, Flanagan JP, Clarke R. 1990. Aspergillar osteomyelitis of the acetabulum. A case report and review of the literature. Orthop Rev 19:58–64. [PubMed] [Google Scholar]
- 460.Ferris B, Jones C. 1985. Paraplegia due to aspergillosis. Successful conservative treatment of 2 cases. J Bone Joint Surg Br 67:800–803. 10.1302/0301-620X.67B5.3902849. [DOI] [PubMed] [Google Scholar]
- 461.Finer G, Greenberg D, Leibovitz E, Leiberman A, Shelef I, Kapelushnik J. 2002. Conservative treatment of malignant (invasive) external otitis caused by Aspergillus flavus with oral itraconazole solution in a neutropenic patient. Scand J Infect Dis 34:227–229. 10.1080/00365540110077137. [DOI] [PubMed] [Google Scholar]
- 462.Fisher MS. 1992. Case report 750: aspergillosis of the chest wall in an apparently immunocompetent host. Skeletal Radiol 21:410–413. 10.1007/BF00241824. [DOI] [PubMed] [Google Scholar]
- 463.Flynn PM, Magill HL, Jenkins JJ, III, Pearson T, Crist WM, Hughes WT. 1990. Aspergillus osteomyelitis in a child treated for acute lymphoblastic leukemia. Pediatr Infect Dis J 9:733–736. 10.1097/00006454-199010000-00010. [DOI] [PubMed] [Google Scholar]
- 464.Gettleman LK, Shetty AK, Prober CG. 1999. Posttraumatic invasive Aspergillus fumigatus wound infection. Pediatr Infect Dis J 18:745–747. 10.1097/00006454-199908000-00026. [DOI] [PubMed] [Google Scholar]
- 465.Ghotaslou R, Parvizi R, Safaei N, Yousefi S. 2008. A case of Aspergillus fumigatus mediastinitis after heart surgery in Madani Heart Center, Tabriz, Iran. Prog Cardiovasc Nurs 23:133–135. 10.1111/j.1751-7117.2008.00003.x. [DOI] [PubMed] [Google Scholar]
- 466.Glotzbach RE. 1982. Aspergillus terreus infection of pseudoaneurysm of aortofemoral vascular graft with contiguous vertebral osteomyelitis. Am J Clin Pathol 77:224–227. 10.1093/ajcp/77.2.224. [DOI] [PubMed] [Google Scholar]
- 467.Gordon G, Giddings NA. 1994. Invasive otitis externa due to Aspergillus species: case report and review. Clin Infect Dis 19:866–870. 10.1093/clinids/19.5.866. [DOI] [PubMed] [Google Scholar]
- 468.Govender S, Rajoo R, Goga IE, Charles RW. 1991. Aspergillus osteomyelitis of the spine. Spine (Phila Pa 1976) 16:746–749. 10.1097/00007632-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 469.Grossman M. 1975. Aspergillosis of bone. Br J Radiol 48:57–59. 10.1259/0007-1285-48-565-57. [DOI] [PubMed] [Google Scholar]
- 470.Hall PJ, Farrior JB. 1993. Aspergillus mastoiditis. Otolaryngol Head Neck Surg 108:167–170. 10.1177/019459989310800210. [DOI] [PubMed] [Google Scholar]
- 471.Heinrich SD, Finney T, Craver R, Yin LL, Zembo MM. 1991. Aspergillus osteomyelitis in patients who have chronic granulomatous disease. Case report. J Bone Joint Surg Am 73:456–460. [PubMed] [Google Scholar]
- 472.Holmes PF, Osterman DW, Tullos HS. 1988. Aspergillus discitis. Report of two cases and review of the literature. Clin Orthop Relat Res 226:240–246. [PubMed] [Google Scholar]
- 473.Hovi L, Saarinen UM, Donner U, Lindqvist C. 1996. Opportunistic osteomyelitis in the jaws of children on immunosuppressive chemotherapy. J Pediatr Hematol Oncol 18:90–94. 10.1097/00043426-199602000-00018. [DOI] [PubMed] [Google Scholar]
- 474.Hughes WT. 1966. Generalized aspergillosis. A case involving the central nervous system. Am J Dis Child 112:262–265. 10.1001/archpedi.1966.02090120130017. [DOI] [PubMed] [Google Scholar]
- 475.Hummel M, Schuler S, Weber U, Schwertlick G, Hempel S, Theiss D, Rees W, Mueller J, Hetzer R. 1993. Aspergillosis with Aspergillus osteomyelitis and diskitis after heart transplantation: surgical and medical management. J Heart Lung Transplant 12:599–603. [PubMed] [Google Scholar]
- 476.Ingwer I, McLeish KR, Tight RR, White AC. 1978. Aspergillus fumigatus epidural abscess in a renal transplant recipient. Arch Intern Med 138:153–154. 10.1001/archinte.1978.03630250105029. [DOI] [PubMed] [Google Scholar]
- 477.Kaneko J, Sugawara Y, Makuuchi M. 2002. Aspergillus osteomyelitis after liver transplantation. Liver Transpl 8:1073–1075. 10.1053/jlts.2002.35778. [DOI] [PubMed] [Google Scholar]
- 478.Kawashima A, Kuhlman JE, Fishman EK, Tempany CM, Magid D, Lederman HM, Winkelstein JA, Zerhouni EA. 1991. Pulmonary Aspergillus chest wall involvement in chronic granulomatous disease: CT and MRI findings. Skeletal Radiol 20:487–493. 10.1007/BF00194242. [DOI] [PubMed] [Google Scholar]
- 479.Kline MW, Bocobo FC, Paul ME, Rosenblatt HM, Shearer WT. 1994. Successful medical therapy of Aspergillus osteomyelitis of the spine in an 11-year-old boy with chronic granulomatous disease. Pediatrics 93:830–835. 10.1542/peds.93.5.830. [DOI] [PubMed] [Google Scholar]
- 480.Kolbe AB, McKinney AM, Kendi AT, Misselt D. 2007. Aspergillus meningitis and discitis from low-back procedures in an immunocompetent patient. Acta Radiol 48:687–689. 10.1080/02841850701342153. [DOI] [PubMed] [Google Scholar]
- 481.Korovessis P, Repanti M, Katsardis T, Stamatakis M. 1994. Anterior decompression and fusion for Aspergillus osteomyelitis of the lumbar spine associated with paraparesis. Spine (Phila Pa 1976) 19:2715–2718. [PubMed] [Google Scholar]
- 482.Kountakis SE, Psifidis A, Chang CJ, Stiernberg CM. 1997. Risk factors associated with hearing loss in neonates. Am J Otolaryngol 18:90–93. 10.1016/s0196-0709(97)90093-4. [DOI] [PubMed] [Google Scholar]
- 483.Lang EW, Pitts LH. 1996. Intervertebral disc space infection caused by Aspergillus fumigatus. Eur Spine J 5:207–209. 10.1007/BF00395517. [DOI] [PubMed] [Google Scholar]
- 484.Langlois RP, Flegel KM, Meakins JL, Morehouse DD, Robson HG, Guttmann RD. 1980. Cutaneous aspergillosis with fatal dissemination in a renal transplant recipient. Can Med Assoc J 122:673–676. [PMC free article] [PubMed] [Google Scholar]
- 485.Lenzi J, Agrillo A, Santoro A, Marotta N, Cantore GP. 2004. Postoperative spondylodiscitis from Aspergillus fumigatus in immunocompetent subjects. J Neurosurg Sci 48:81–85. [PubMed] [Google Scholar]
- 486.Liu Z, Hou T, Shen Q, Liao W, Xu H. 1995. Osteomyelitis of sacral spine caused by Aspergillus versicolor with neurologic deficits. Chin Med J (Engl) 108:472–475. [PubMed] [Google Scholar]
- 487.Mamishi S, Zomorodian K, Saadat F, Gerami-Shoar M, Tarazooie B, Siadati SA. 2005. A case of invasive aspergillosis in CGD patient successfully treated with amphotericin B and INF-gamma. Ann Clin Microbiol Antimicrob 4:4. 10.1186/1476-0711-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Mawk JR, Erickson DL, Chou SN, Seljeskog EL. 1983. Aspergillus infections of the lumbar disc spaces. Report of three cases. J Neurosurg 58:270–274. 10.3171/jns.1983.58.2.0270. [DOI] [PubMed] [Google Scholar]
- 489.McGregor A, McNicol D, Collignon P. 1992. Aspergillus-induced discitis. A role for itraconazole in therapy? Spine (Phila Pa 1976) 17:1512–1514. 10.1097/00007632-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 490.McKee DF, Barr WM, Bryan CS, Lunceford EM, Jr.. 1984. Primary aspergillosis of the spine mimicking Pott’s paraplegia. J Bone Joint Surg Am 66:1481–1483. [PubMed] [Google Scholar]
- 491.Menachof MR, Jackler RK. 1990. Otogenic skull base osteomyelitis caused by invasive fungal infection. Otolaryngol Head Neck Surg 102:285–289. 10.1177/019459989010200315. [DOI] [PubMed] [Google Scholar]
- 492.Mershon JC, Samuelson DR, Layman TE. 1968. Left ventricular “fibrous body” aneurysm caused by Aspergillus endocarditis. Am J Cardiol 22:281–285. 10.1016/0002-9149(68)90236-1. [DOI] [PubMed] [Google Scholar]
- 493.Michelson JB, Freedman SD, Boyden DG. 1982. Aspergillus endophthalmitis in a drug abuser. Ann Ophthalmol 14:1051–1054. [PubMed] [Google Scholar]
- 494.Myhre AP, Jarosz TJ, Hunter JC, Richardson ML. 2006. Postoperative bone graft displacement: an unusual sign of infection following posterior spinal fusion. Radiol Case Rep 1:21–23. 10.2484/rcr.v1i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Nasca RJ, McElvein RB. 1985. Aspergillus fumigatus osteomyelitis of the thoracic spine treated by excision and interbody fusion. Spine (Phila Pa 1976) 10:848–850. 10.1097/00007632-198511000-00013. [DOI] [PubMed] [Google Scholar]
- 496.Natesan S, Abraham G, Mathew M, Lalitha MK, Srinivasan CN. 2007. Secondary sternal Aspergillus osteomyelitis in a diabetic hemodialysis patient with previous allograft rejection. Hemodial Int 11:403–405. 10.1111/j.1542-4758.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- 497.Nusair A, Smith PW. 2007. Aspergillus vertebral osteomyelitis in an immunocompetent host treated with voriconazole. Infect Dis Clin Pract 15:122–124. 10.1097/01.idc.0000236976.97075.f5. [DOI] [Google Scholar]
- 498.Ohki M, Ito K, Ishimoto S. 2001. Fungal mastoiditis in an immunocompetent adult. Eur Arch Otorhinolaryngol 258:106–108. 10.1007/s004050100322. [DOI] [PubMed] [Google Scholar]
- 499.Parker KM, Nicholson JK, Cezayirli RC, Biggs PJ. 1996. Aspergillosis of the sphenoid sinus: presentation as a pituitary mass and postoperative gallium-67 imaging. Surg Neurol 45:354–358. 10.1016/0090-3019(95)00484-x. [DOI] [PubMed] [Google Scholar]
- 500.Pasic S, Abinun M, Pistignjat B, Vlajic B, Rakic J, Sarjanovic L, Ostojic N. 1996. Aspergillus osteomyelitis in chronic granulomatous disease: treatment with recombinant gamma-interferon and itraconazole. Pediatr Infect Dis J 15:833–834. 10.1097/00006454-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 501.Perlmutter I, Perlmutter D, Hyams PJ. 1980. Fungal infection of the brain: an increasing threat. South Med J 73:499–501. 10.1097/00007611-198004000-00025. [DOI] [PubMed] [Google Scholar]
- 502.Peters-Christodoulou MN, de Beer FC, Bots GT, Ottenhoff TM, Thompson J, van‘t Wout JW. 1991. Treatment of postoperative Aspergillus fumigatus spondylodiscitis with itraconazole. Scand J Infect Dis 23:373–376. 10.3109/00365549109024325. [DOI] [PubMed] [Google Scholar]
- 503.Phillips P, Bryce G, Shepherd J, Mintz D. 1990. Invasive external otitis caused by Aspergillus. Rev Infect Dis 12:277–281. 10.1093/clinids/12.2.277. [DOI] [PubMed] [Google Scholar]
- 504.Plazanet F, Hira M, Ferrand E, Rahbari F, Crevel J, Bontoux D. 1998. Aspergillus osteomyelitis. Report of a case investigated by magnetic resonance imaging. Rev Rhum Engl Ed 65:76–77. [PubMed] [Google Scholar]
- 505.Rajaram T, Mahapatra AK, Sarkar C, Roy S. 1991. Aspergillosis of spine. A case report. J Neurosurg Sci 35:117–120. [PubMed] [Google Scholar]
- 506.Ranjan R, Mishra S, Ranjan S. 2010. Aspergillus vertebral osteomyelitis in an immunocompetent person. Neurol India 58:806–808. 10.4103/0028-3886.72196. [DOI] [PubMed] [Google Scholar]
- 507.Rassa M. 1977. Vertebral aspergillosis with preservation of the disc. Br J Radiol 50:918–920. 10.1259/0007-1285-50-600-918. [DOI] [PubMed] [Google Scholar]
- 508.Redmond A, Carre IJ, Biggart JD, Mackenzie DW. 1965. Aspergillosis (Aspergillus nidulans) involving bone. J Pathol Bacteriol 89:391–395. 10.1002/path.1700890147. [DOI] [PubMed] [Google Scholar]
- 509.Richards RH, Priaulx LR. 1988. A case of Aspergillus osteomyelitis complicating an open fracture of the tibia. Injury 19:129–130. 10.1016/0020-1383(88)90094-0. [DOI] [PubMed] [Google Scholar]
- 510.Robinson MF, McGregor R, Collins R, Cheung K. 1982. Combined neutrophil and T-cell deficiency: initial report of a kindred with features of the hyper-IgE syndrome and chronic granulomatous disease. Am J Med 73:63–70. 10.1016/0002-9343(82)90927-5. [DOI] [PubMed] [Google Scholar]
- 511.Rodriguez-Hernandez MJ, Jimenez-Mejias ME, Montero JM, Regordan C, Ferreras G. 2001. Aspergillus fumigatus cranial infection after accidental traumatism. Eur J Clin Microbiol Infect Dis 20:655–656. 10.1007/s100960100579. [DOI] [PubMed] [Google Scholar]
- 512.Roselle GA, Baird IM. 1979. Aspergillus flavipes group osteomyelitis. Arch Intern Med 139:590–592. [PubMed] [Google Scholar]
- 513.Sachs MK, Paluzzi RG, Moore JH, Jr, Fraimow HS, Ost D. 1990. Amphotericin-resistant Aspergillus osteomyelitis controlled by itraconazole. Lancet 335:1475. 10.1016/0140-6736(90)91513-A. [DOI] [PubMed] [Google Scholar]
- 514.Salloum A, Rao S, Havasi A, Miljkovic G, Amoateng-Adjepong Y. 2004. Aspergillus rib and vertebral osteomyelitis in a former intravenous drug user. Am J Med 116:208–209. 10.1016/j.amjmed.2003.05.006. [DOI] [PubMed] [Google Scholar]
- 515.Salvalaggio PRO, Bassetti M, Lorber MI, Micheletto GC, Friedman AL, Andriole VT, Basadonna GP. 2003. Aspergillus vertebral osteomyelitis after simultaneous kidney-pancreas transplantation. Transpl Infect Dis 5:187–190. 10.1111/j.1399-3062.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- 516.Santos AB, Llamas P, Gadea I, Roman A, Subira D, Prieto E, Tomas JF. 2004. Aspergillus fumigatus: a rare cause of vertebral osteomyelitis. Haematologica 89:ECR10. [PubMed] [Google Scholar]
- 517.Schubert M, Schar G, Curt A, Dietz V. 1998. Aspergillus spondylodiscitis in an immunocompetent paraplegic patient. Spinal Cord 36:800–803. 10.1038/sj.sc.3100645. [DOI] [PubMed] [Google Scholar]
- 518.Seligsohn R, Rippon JW, Lerner SA. 1977. Aspergillus terreus osteomyelitis. Arch Intern Med 137:918–920. [PubMed] [Google Scholar]
- 519.Seres JL, Benner EJ, Ono H. 1972. Aspergillosis presenting as spinal cord compression. Case report. J Neurosurg 36:221–224. 10.3171/jns.1972.36.2.0221. [DOI] [PubMed] [Google Scholar]
- 520.Shouldice E, Fernandez C, McCully B, Schmidt M, Fraser R, Cook C. 2003. Voriconazole treatment of presumptive disseminated Aspergillus infection in a child with acute leukemia. J Pediatr Hematol Oncol 25:732–734. 10.1097/00043426-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 521.Slack CL, Watson DW, Abzug MJ, Shaw C, Chan KH. 1999. Fungal mastoiditis in immunocompromised children. Arch Otolaryngol Head Neck Surg 125:73–75. 10.1001/archotol.125.1.73. [DOI] [PubMed] [Google Scholar]
- 522.Sonin AH, Stern SH, Levi E. 1996. Primary Aspergillus osteomyelitis in the tibia of an immunosuppressed man. AJR Am J Roentgenol 166:1277–1279. 10.2214/ajr.166.6.8633431. [DOI] [PubMed] [Google Scholar]
- 523.Stanley RJ, McCaffrey TV, Weiland LH. 1988. Fungal mastoiditis in the immunocompromised host. Arch Otolaryngol Head Neck Surg 114:198–199. 10.1001/archotol.1988.01860140096030. [DOI] [PubMed] [Google Scholar]
- 524.Stratov I, Korman TM, Johnson PDR. 2003. Management of Aspergillus osteomyelitis: report of failure of liposomal amphotericin B and response to voriconazole in an immunocompetent host and literature review. Eur J Clin Microbiol Infect Dis 22:277–283. 10.1007/s10096-003-0909-3. [DOI] [PubMed] [Google Scholar]
- 525.Strauss M, Fine E. 1991. Aspergillus otomastoiditis in acquired immunodeficiency syndrome. Am J Otol 12:49–53. [PubMed] [Google Scholar]
- 526.Tack KJ, Rhame FS, Brown B, Thompson RC, Jr.. 1982. Aspergillus osteomyelitis. Report of four cases and review of the literature. Am J Med 73:295–300. 10.1016/0002-9343(82)90192-9. [DOI] [PubMed] [Google Scholar]
- 527.Taillandier J, Alemanni M, Cerrina J, Le Roy Ladurie F, Dartevelle P. 1997. Aspergillus osteomyelitis after heart-lung transplantation. J Heart Lung Transplant 16:436–438. [PubMed] [Google Scholar]
- 528.Tang TJ, Janssen HL, van der Vlies CH, de Man RA, Metselaar HJ, Tilanus HW, de Marie S. 2000. Aspergillus osteomyelitis after liver transplantation: conservative or surgical treatment? Eur J Gastroenterol Hepatol 12:123–126. 10.1097/00042737-200012010-00022. [DOI] [PubMed] [Google Scholar]
- 529.Tsumura N, Akasu Y, Yamane H, Ikezawa S, Hirata T, Oda K, Sakata Y, Shirahama M, Inoue A, Kato H. 1999. Aspergillus osteomyelitis in a child who has p67-phox-deficient chronic granulomatous disease. Kurume Med J 46:87–90. 10.2739/kurumemedj.46.87. [DOI] [PubMed] [Google Scholar]
- 530.van ‘t Wout JW, Raven EJ, van der Meer JW. 1990. Treatment of invasive aspergillosis with itraconazole in a patient with chronic granulomatous disease. J Infect 20:147–150. 10.1016/0163-4453(90)93418-R. [DOI] [PubMed] [Google Scholar]
- 531.van Tol A, van Rijswijk J. 2009. Aspergillus mastoiditis, presenting with unexplained progressive otalgia, in an immunocompetent (older) patient. Eur Arch Otorhinolaryngol 266:1655–1657. 10.1007/s00405-008-0877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Wagner DK, Varkey B, Sheth NK, DaMert GJ. 1985. Epidural abscess, vertebral destruction, and paraplegia caused by extending infection from an aspergilloma. Am J Med 78:518–522. 10.1016/0002-9343(85)90349-3. [DOI] [PubMed] [Google Scholar]
- 533.Walker WA, Pate JW. 1991. Primary Aspergillus osteomyelitis of the sternum. Ann Thorac Surg 52:868–870. 10.1016/0003-4975(91)91232-k. [DOI] [PubMed] [Google Scholar]
- 534.Wellens F, Potvliege C, Deuvaert FE, Primo G. 1982. Aspergillus osteochondritis after median sternotomy. Combined operative treatment and drug therapy with amphotericin B. Thorac Cardiovasc Surg 30:322–324. 10.1055/s-2007-1022417. [DOI] [PubMed] [Google Scholar]
- 535.Winslow CP, Dichard A, McGuire KA. 2001. Osteomyelitis of the temporomandibular joint. Am J Otolaryngol 22:142–145. 10.1053/ajot.2001.22577. [DOI] [PubMed] [Google Scholar]
- 536.Pasqualotto AC, Denning DW. 2006. Post-operative aspergillosis. Clin Microbiol Infect 12:1060–1076. 10.1111/j.1469-0691.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 537.Yates PD, Upile T, Axon PR, de Carpentier J. 1997. Aspergillus mastoiditis in a patient with acquired immunodeficiency syndrome. J Laryngol Otol 111:560–561. 10.1017/s0022215100137909. [DOI] [PubMed] [Google Scholar]
- 538.Yuen JC, Puri SK, Feng Z. 2002. Scalp necrotizing fasciitis with osteomyelitis of the skull from Aspergillus. J Craniofac Surg 13:762–764. 10.1097/00001665-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 539.Sun L, Zhang L, Wang K, Wang W, Tian M. 2012. Fungal osteomyelitis after arthroscopic anterior cruciate ligament reconstruction: a case report with review of the literature. Knee 19:728–731. 10.1016/j.knee.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 540.Dotis J, Roilides E. 2011. Osteomyelitis due to Aspergillus species in chronic granulomatous disease: an update of the literature. Mycoses 54:E686–E696. 10.1111/j.1439-0507.2010.02001.x. [DOI] [PubMed] [Google Scholar]
- 541.Kirby A, Hassan I, Burnie J. 2006. Recommendations for managing Aspergillus osteomyelitis and joint infections based on a review of the literature. J Infect 52:405–414. 10.1016/j.jinf.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 542.Dotis J, Roilides E. 2004. Osteomyelitis due to Aspergillus spp. in patients with chronic granulomatous disease: comparison of Aspergillus nidulans and Aspergillus fumigatus. Int J Infect Dis 8:103–110. 10.1016/j.ijid.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 543.Chen DS, Lalwani AK, House JW, Choo D. 1999. Aspergillus mastoiditis in acquired immunodeficiency syndrome. Am J Otol 20:561–567. [PubMed] [Google Scholar]
- 544.Aguado JM, Valle R, Arjona R, Ferreres JC, Gutierrez JA. 1992. Aortic bypass graft infection due to Aspergillus: report of a case and review. Clin Infect Dis 14:916–921. 10.1093/clinids/14.4.916. [DOI] [PubMed] [Google Scholar]
- 545.Segal BH, DeCarlo ES, Kwon-Chung KJ, Malech HL, Gallin JI, Holland SM. 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 77:345–354. 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 546.Lortholary O, Meyohas MC, Dupont B, Cadranel J, Salmon-Ceron D, Peyramond D, Simonin D, Frottier J, Gilquin J, Armengaud M, Chouaid C, Delzant G, Detruchis P, Dournan E. 1993. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. French Cooperative Study Group on Aspergillosis in AIDS. Am J Med 95:177–187. 10.1016/0002-9343(93)90258-Q. [DOI] [PubMed] [Google Scholar]
- 547.Sambatakou H, Dupont B, Lode H, Denning DW. 2006. Voriconazole treatment for subacute invasive and chronic pulmonary aspergillosis. Am J Med 119:527.e7–527.e14. 10.1016/j.amjmed.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 548.Galluzzo ML, Hernandez C, Davila MTG, Perez L, Oleastro M, Zelazko M, Rosenzweig SD. 2008. Clinical and histopathological features and a unique spectrum of organisms significantly associated with chronic granulomatous disease osteomyelitis during childhood. Clin Infect Dis 46:745–749. 10.1086/527446. [DOI] [PubMed] [Google Scholar]
- 549.Koehler P, Tacke D, Cornely OA. 2014. Aspergillosis of bones and joints: a review from 2002 until today. Mycoses 57:323–335. 10.1111/myc.12165. [DOI] [PubMed] [Google Scholar]
- 550.Gamaletsou MN, Rammaert B, Bueno MA, Moriyama B, Sipsas NV, Kontoyiannis DP, Roilides E, Zeller V, Prinapori R, Taj-Aldeen SJ, Brause B, Lortholary O, Walsh TJ. 2014. Aspergillus osteomyelitis: epidemiology, clinical manifestations, management, and outcome. J Infect 68:478–493. 10.1016/j.jinf.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Tsantes AG, Papadopoulos DV, Markou E, Zarokostas K, Sokou R, Trikoupis I, Mavrogenis AF, Houhoula D, Piovani D, Bonovas S, Tsantes AE, Tsakris A, Vrioni G. 2022. Aspergillus spp. osteoarticular infections: an updated systematic review on the diagnosis, treatment and outcomes of 186 confirmed cases. Med Mycol 60:myac052. 10.1093/mmy/myac052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.McCarthy MW, Petraitis V, Walsh TJ. 2017. Combination therapy for the treatment of pulmonary mold infections. Expert Rev Respir Med 11:481–489. 10.1080/17476348.2017.1325322. [DOI] [PubMed] [Google Scholar]
- 553.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee DG, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR, Maertens JA. 2015. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 162:81–89. 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 554.Falcone EL, Holland SM. 2012. Invasive fungal infection in chronic granulomatous disease: insights into pathogenesis and management. Curr Opin Infect Dis 25:658–669. 10.1097/QCO.0b013e328358b0a4. [DOI] [PubMed] [Google Scholar]
- 555.Golmia R, Bello I, Marra A, Hamerschlak N, Osawa A, Scheinberg M. 2011. Aspergillus fumigatus joint infection: a review. Semin Arthritis Rheum 40:580–584. 10.1016/j.semarthrit.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 556.Saba R, Beköz H, Karadoğan I, Inan D, Bilgin-Uğur A, Oğünç D, Cevikol C, Temizkan K, Timurağaoğlu A, Undar L. 2004. Septic arthritis due to Aspergillus treated with amphotericin B lipid complex and surgical debridement. J Chemother 16:218–220. 10.1179/joc.2004.16.2.218. [DOI] [PubMed] [Google Scholar]
- 557.Mekan SF, Saeed O, Khan JA. 2004. Invasive aspergillosis with polyarthritis. Mycoses 47:518–520. 10.1111/j.1439-0507.2004.01031.x. [DOI] [PubMed] [Google Scholar]
- 558.Sohail MR, Smilack JD. 2004. Aspergillus fumigatus septic arthritis complicating intra-articular corticosteroid injection. Mayo Clin Proc 79:578–579. 10.4065/79.4.578. [DOI] [PubMed] [Google Scholar]
- 559.Steinfeld S, Durez P, Hauzeur JP, Motte S, Appelboom T. 1997. Articular aspergillosis: two case reports and review of the literature. Br J Rheumatol 36:1331–1334. 10.1093/rheumatology/36.12.1331. [DOI] [PubMed] [Google Scholar]
- 560.Panigrahi S, Nagler A, Or R, Wolf DG, Slavin S, Shapira MY. 2001. Indolent Aspergillus arthritis complicating fludarabine-based non-myeloablative stem cell transplantation. Bone Marrow Transplant 27:659–661. 10.1038/sj.bmt.1702853. [DOI] [PubMed] [Google Scholar]
- 561.Motte S, Bellens B, Rickaert F, Serruys E, Thys JP, Dereume JP. 1993. Vascular graft infection caused by Aspergillus species: case report and review of the literature. J Vasc Surg 17:607–612. [PubMed] [Google Scholar]
- 562.Garcia-Porrua C, Blanco FJ, Atanes A, Torres P, Galdo F. 1997. Septic arthritis by Aspergillus fumigatus: a complication of corticosteroid infiltration. Br J Rheumatol 36:610–611. 10.1093/rheumatology/36.5.610. [DOI] [PubMed] [Google Scholar]
- 563.Gunsilius E, Lass-Florl C, Mur E, Gabl C, Gastl G, Petzer AL. 1999. Aspergillus osteoarthritis in acute lymphoblastic leukemia. Ann Hematol 78:529–530. 10.1007/s002770050551. [DOI] [PubMed] [Google Scholar]
- 564.Cassuto-Viguier E, Mondain JR, Van Elslande L, Bendini JC, Gaid H, Franco M, Gari-Toussaint M. 1995. Fatal outcome of Aspergillus fumigatus arthritis in a renal transplant recipient. Transplant Proc 27:2461. [PubMed] [Google Scholar]
- 565.Franco M, Van Elslande L, Robino C, Gari-Toussaint M, Bendini C, Barillon JR, Mondain D, Bracco J, Padovani B, Cassuto-Viguier E. 1995. Aspergillus arthritis of the shoulder in a renal transplant recipient. Failure of itraconazole therapy. Rev Rhum Engl Ed 62:215–218. [PubMed] [Google Scholar]
- 566.Lagier R. 1990. A case of hip osteoarthrosis contaminated by fungi. A histological study. Arch Orthop Trauma Surg 109:113–116. 10.1007/BF00439391. [DOI] [PubMed] [Google Scholar]
- 567.Austin KS, Testa NN, Luntz RK, Greene JB, Smiles S. 1992. Aspergillus infection of total knee arthroplasty presenting as a popliteal cyst. Case report and review of the literature. J Arthroplasty 7:311–314. 10.1016/0883-5403(92)90055-u. [DOI] [PubMed] [Google Scholar]
- 568.Tiwari V, Khatri K, Khan SA, Nath D. 2014. Disseminated Aspergillus flavus following septic arthritis in an immunocompetent patient: a case report. BMC Res Notes 7:709. 10.1186/1756-0500-7-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Yoon PW, Song JH, Yoon KS, Chang JS, Kim HJ, Rhyu KH. 2015. Aspergillus septic arthritis of the hip in an immunocompetent middle-aged female with undiagnosed recurrent pulmonary aspergillosis. Hip Pelvis 27:196–200. 10.5371/hp.2015.27.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Varghese L, Chacko R, Varghese GM, Job A. 2015. Septic arthritis of the temporomandibular joint caused by Aspergillus flavus infection as a complication of otitis externa. Ear Nose Throat J 94:E24–E26. [PubMed] [Google Scholar]
- 571.Kumar M, Thilak J, Zahoor A, Jyothi A. 2016. Septic arthritis due to tubercular and Aspergillus co-infection. Indian J Orthop 50:327–330. 10.4103/0019-5413.181783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Gamaletsou MN, Rammaert B, Bueno MA, Sipsas NV, Moriyama B, Kontoyiannis DP, Roilides E, Zeller V, Taj-Aldeen SJ, Henry M, Petraitis V, Denning DW, Lortholary O, Walsh TJ, International Osteoarticular Mycoses Consortium. 2017. Aspergillus arthritis: analysis of clinical manifestations, diagnosis, and treatment of 31 reported cases. Med Mycol 55:246–254. 10.1093/mmy/myw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Page ID, Byanyima R, Hosmane S, Onyachi N, Opira C, Richardson M, Sawyer R, Sharman A, Denning DW. 2019. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J 53:1801184. 10.1183/13993003.01184-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Brint JM, Flynn PM, Pearson TA, Pui CH. 1992. Disseminated fusariosis involving bone in an adolescent with leukemia. Pediatr Infect Dis J 11:965–968. 10.1097/00006454-199211110-00012. [DOI] [PubMed] [Google Scholar]
- 575.Anaissie E, Kantarjian H, Ro J, Hopfer R, Rolston K, Fainstein V, Bodey G. 1988. The emerging role of Fusarium infections in patients with cancer. Medicine (Baltimore) 67:77–83. 10.1097/00005792-198803000-00001. [DOI] [PubMed] [Google Scholar]
- 576.Rombaux P, Eloy P, Bertrand B, Delos M, Doyen C. 1996. Lethal disseminated Fusarium infection with sinus involvement in the immunocompromised host: case report and review of the literature. Rhinology 34:237–241. [PubMed] [Google Scholar]
- 577.Boutati EI, Anaissie EJ. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood 90:999–1008. 10.1182/blood.V90.3.999. [DOI] [PubMed] [Google Scholar]
- 578.Nucci M, Varon AG, Garnica M, Akiti T, Barreiros G, Trope BM, Nouer SA. 2013. Increased incidence of invasive fusariosis with cutaneous portal of entry, Brazil. Emerg Infect Dis 19:1567–1572. 10.3201/eid1910.120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Edupuganti S, Rouphael N, Mehta A, Eaton M, Heller JG, Bressler A, Brandt M, O’Donnell K. 2011. Fusarium falciforme vertebral abscess and osteomyelitis: case report and molecular classification. J Clin Microbiol 49:2350–2353. 10.1128/JCM.02547-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Sierra-Hoffman M, Paltiyevich-Gibson S, Carpenter JL, Hurley DL. 2005. Fusarium osteomyelitis: case report and review of the literature. Scand J Infect Dis 37:237–240. 10.1080/00365540410021036. [DOI] [PubMed] [Google Scholar]
- 581.Bourguignon RL, Walsh AF, Flynn JC, Baro C, Spinos E. 1976. Fusarium species osteomyelitis. Case report. J Bone Joint Surg Am 58:722–723. [PubMed] [Google Scholar]
- 582.Page JC, Friedlander G, Dockery GL. 1982. Postoperative Fusarium osteomyelitis. J Foot Surg 21:174–176. [PubMed] [Google Scholar]
- 583.Nuovo MA, Simmonds JE, Chacho MS, McKitrick JC. 1988. Fusarium solani osteomyelitis with probable nosocomial spread. Am J Clin Pathol 90:738–741. 10.1093/ajcp/90.6.738. [DOI] [PubMed] [Google Scholar]
- 584.Bader M, Jafri AK, Krueger T, Kumar V. 2003. Fusarium osteomyelitis of the foot in a patient with diabetes mellitus. Scand J Infect Dis 35:895–896. 10.1080/00365540310016565. [DOI] [PubMed] [Google Scholar]
- 585.Wu CY, Chen GS, Lan CCE. 2009. Onychomycosis caused by Fusarium solani in a woman with diabetes. Clin Exp Dermatol 34:E772–E774. 10.1111/j.1365-2230.2009.03498.x. [DOI] [PubMed] [Google Scholar]
- 586.Gradon JD, Lerman A, Lutwick LI. 1990. Septic arthritis due to Fusarium moniliforme. Rev Infect Dis 12:716–717. 10.1093/clinids/12.4.716. [DOI] [PubMed] [Google Scholar]
- 587.Moschovi M, Trimis G, Anastasopoulos J, Kanariou M, Raftopoulou A, Tzortzatou-Stathopoulou F. 2004. Subacute vertebral osteomyelitis in a child with diabetes mellitus associated with Fusarium. Pediatr Int 46:740–742. 10.1111/j.1442-200x.2004.01994.x. [DOI] [PubMed] [Google Scholar]
- 588.Keynan Y, Sprecher H, Weber G. 2007. Acremonium vertebral osteomyelitis: molecular diagnosis and response to voriconazole. Clin Infect Dis 45:e5–e6. 10.1086/518700. [DOI] [PubMed] [Google Scholar]
- 589.Beaudreuil S, Buchler M, Al Najjar A, Bastides F, Francois M, Duong TH, Nivet H, Richard-Lenoble D, Lebranchu Y. 2003. Acute septic arthritis after kidney transplantation due to Acremonium. Nephrol Dial Transplant 18:850–851. 10.1093/ndt/gfg040. [DOI] [PubMed] [Google Scholar]
- 590.Noble RC, Salgado J, Newell SW, Goodman NL. 1997. Endophthalmitis and lumbar diskitis due to Acremonium falciforme in a splenectomized patient. Clin Infect Dis 24:277–278. 10.1093/clinids/24.2.277. [DOI] [PubMed] [Google Scholar]
- 591.Hell M, Neureiter J, Wojna A, Presterl E, Willinger B, de Hoog GS, Lackner M. 2011. Post-traumatic Pseudallescheria apiosperma osteomyelitis: positive outcome of a young immunocompetent male patient due to surgical intervention and voriconazole therapy. Mycoses 54:43–47. 10.1111/j.1439-0507.2011.02106.x. [DOI] [PubMed] [Google Scholar]
- 592.Gompels MM, Bethune CA, Jackson G, Spickett GP. 2002. Scedosporium apiospermum in chronic granulomatous disease treated with an HLA matched bone marrow transplant. J Clin Pathol 55:784–786. 10.1136/jcp.55.10.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Porte L, Khatibi S, Hajj LE, Cassaing S, Berry A, Massip P, Linas MD, Magnaval JF, Sans N, Marchou B. 2006. Scedosporium apiospermum mycetoma with bone involvement successfully treated with voriconazole. Trans R Soc Trop Med Hyg 100:891–894. 10.1016/j.trstmh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 594.Stripeli F, Pasparakis D, Velegraki A, Lebessi E, Arsenis G, Kafetzis D, Tsolia M. 2009. Scedosporium apiospermum skeletal infection in an immunocompetent child. Med Mycol 47:441–444. 10.1080/13693780802695470. [DOI] [PubMed] [Google Scholar]
- 595.Gottesman-Yekutieli T, Shwartz O, Edelman A, Hendel D, Dan M. 2011. Pseudallescheria boydii infection of a prosthetic hip joint—an uncommon infection in a rare location. Am J Med Sci 342:250–253. 10.1097/MAJ.0b013e31821f9691. [DOI] [PubMed] [Google Scholar]
- 596.Lindsley MD, Guarro J, Khairy RN, Williams J, Iqbal N, Pancholi P. 2008. Pseudallescheria fusoidea, a new cause of osteomyelitis. J Clin Microbiol 46:2141–2143. 10.1128/JCM.00205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Kanafani ZA, Comair Y, Kanj SS. 2004. Pseudallescheria boydii cranial osteomyelitis and subdural empyema successfully treated with voriconazole: a case report and literature review. Eur J Clin Microbiol Infect Dis 23:836–840. 10.1007/s10096-004-1233-2. [DOI] [PubMed] [Google Scholar]
- 598.Angelini A, Drago G, Ruggieri P. 2013. Post-tsunami primary Scedosporium apiospermum osteomyelitis of the knee in an immunocompetent patient. Int J Infect Dis 17:E646–E649. 10.1016/j.ijid.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 599.Sydnor MK, Kaushik S, Knight TE, Jr, Bridges CL, McCarty JM. 2003. Mycotic osteomyelitis due to Scedosporium apiospermum: MR imaging-pathologic correlation. Skeletal Radiol 32:656–660. 10.1007/s00256-003-0695-0. [DOI] [PubMed] [Google Scholar]
- 600.Piper JP, Golden J, Brown D, Broestler J. 1990. Successful treatment of Scedosporium apiospermum suppurative arthritis with itraconazole. Pediatr Infect Dis J 9:674–675. [PubMed] [Google Scholar]
- 601.Levine NB, Kurokawa R, Fichtenbaum CJ, Howington JA, Kuntz C. 2002. An immunocompetent patient with primary Scedosporium apiospermum vertebral osteomyelitis. J Spinal Disord Tech 15:425–430. 10.1097/00024720-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 602.Dellestable F, Kures L, Mainard D, Pere P, Gaucher A. 1994. Fungal arthritis due to Pseudallescheria boydii (Scedosporium apiospermum). J Rheumatol 21:766–768. [PubMed] [Google Scholar]
- 603.Malekzadeh M, Overturf GD, Auerbach SB, Wong L, Hirsch M. 1990. Chronic, recurrent osteomyelitis caused by Scedosporium inflatum. Pediatr Infect Dis J 9:357–359. 10.1097/00006454-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 604.Mesfin FB, Tobin E, Adamo MA, DiRisio D. 2008. Fungal vertebral osteomyelitis due to Scedosporium apiospermum after near-drowning. Case report. J Neurosurg Spine 9:58–61. 10.3171/SPI/2008/9/7/058. [DOI] [PubMed] [Google Scholar]
- 605.German JW, Kellie SM, Pai MP, Turner PT. 2004. Treatment of a chronic Scedosporium apiospermum vertebral osteomyelitis. Case report. Neurosurg Focus 17:E9. 10.3171/foc.2004.17.6.9. [DOI] [PubMed] [Google Scholar]
- 606.Taj-Aldeen SJ, Taj-Aldeen WS, Guarro J, Cano JF, El Shafie S. 2008. Osteomyelitis caused by Scedosporium apiospermum in an immunocompetent patient. J Invasive Fungal Infect 2:96–99. [Google Scholar]
- 607.Ginter G, de Hoog GS, Pschaid A, Fellinger M, Bogiatzis A, Berghold C, Reich EM, Odds FC. 1995. Arthritis without grains caused by Pseudallescheria boydii. Mycoses 38:369–371. 10.1111/j.1439-0507.1995.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 608.Ong A, Blyth CC, Bency R, Vicaretti M, Harun A, Meyer W, Shingde M, Gilroy N, Chapman J, Chen SCA. 2011. Fatal mycotic aneurysms due to Scedosporium and Pseudallescheria infection. J Clin Microbiol 49:2067–2071. 10.1128/JCM.02615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Guignard S, Hubert D, Dupont B, Anract P, Alioua D, Guerini H, Paugam A, Dougados M. 2008. Multifocal Scedosporium apiospermum spondylitis in a cystic fibrosis patient. J Cyst Fibros 7:89–91. 10.1016/j.jcf.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 610.Lonser RR, Brodke DS, Dailey AT. 2001. Vertebral osteomyelitis secondary to Pseudallescheria boydii. J Spinal Disord 14:361–364. 10.1097/00002517-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 611.Ochiai N, Shimazaki C, Uchida R, Fuchida SI, Okano A, Ashihara E, Inaba T, Fujita N, Nakagawa M. 2003. Disseminated infection due to Scedosporium apiospermum in a patient with acute myelogenous leukemia. Leuk Lymphoma 44:369–372. 10.1080/1042819021000029957. [DOI] [PubMed] [Google Scholar]
- 612.Hung LHY, Norwood LA. 1993. Osteomyelitis due to Pseudallescheria boydii. South Med J 86:231–234. 10.1097/00007611-199302000-00020. [DOI] [PubMed] [Google Scholar]
- 613.Tirado-Miranda R, Solera-Santos J, Brasero JC, Haro-Estarriol M, Cascales-Sanchez P, Igualada JB. 2001. Septic arthritis due to Scedosporium apiospermum: case report and review. J Infect 43:210–212. 10.1053/jinf.2001.0866. [DOI] [PubMed] [Google Scholar]
- 614.Vasoo S, Yeo SB, Lim PL, Ang BS, Lye DC. 2008. Efficacy of voriconazole for Scedosporium apiospermum skull base osteomyelitis: case report and literature review. Int J Antimicrob Agents 31:184–185. 10.1016/j.ijantimicag.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 615.Busaba NY, Poulin M. 1997. Invasive Pseudallescheria boydii fungal infection of the temporal bone. Otolaryngol Head Neck Surg 117:S91–S94. 10.1016/S0194-59989770067-6. [DOI] [PubMed] [Google Scholar]
- 616.Gatto J, Paterson D, Davis L, Lockwood L, Allworth A. 1997. Vertebral osteomyelitis due to Pseudallescheria boydii. Pathology 29:238–240. 10.1080/00313029700169964. [DOI] [PubMed] [Google Scholar]
- 617.Bassiri-Jahromi S, Doostkam A. 2011. Actinomyces and Nocardia infections in chronic granulomatous disease. J Glob Infect Dis 3:348–352. 10.4103/0974-777X.91056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Vanhooteghem O, Gillard P, Dezfoulian B, de la Brassinne M. 2009. Scedosporium apiospermum septicemia following a wedge excision of an ingrown toenail. Int J Dermatol 48:1137–1139. 10.1111/j.1365-4632.2008.03790.x. [DOI] [PubMed] [Google Scholar]
- 619.Frazier DD, Campbell DR, Garvey TA, Wiesel S, Bohlman HH, Eismont FJ. 2001. Fungal infections of the spine. Report of eleven patients with long-term follow-up. J Bone Joint Surg Am 83:560–565. 10.2106/00004623-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 620.Roilides E, Sigler L, Bibashi E, Katsifa H, Flaris N, Panteliadis C. 1999. Disseminated infection due to Chrysosporium zonatum in a patient with chronic granulomatous disease and review of non-Aspergillus fungal infections in patients with this disease. J Clin Microbiol 37:18–25. 10.1128/JCM.37.1.18-25.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Dan M, Yossepowitch O, Hendel D, Shwartz O, Sutton DA. 2006. Phialemonium curvatum arthritis of the knee following intra-articular injection of a corticosteroid. Med Mycol 44:571–574. 10.1080/13693780600631883. [DOI] [PubMed] [Google Scholar]
- 622.Stillwell WT, Rubin BD, Axelrod JL. 1984. Chrysosporium, a new causative agent in osteomyelitis. A case report. Clin Orthop Relat Res 184:190–192. [PubMed] [Google Scholar]
- 623.Lutwick LI, Galgiani JN, Johnson RH, Stevens DA. 1976. Visceral fungal infections due to Petriellidium boydii (Allescheria boydii). In vitro drug sensitivity studies. Am J Med 61:632–640. 10.1016/0002-9343(76)90141-8. [DOI] [PubMed] [Google Scholar]
- 624.Hayden G, Lapp C, Loda F. 1977. Arthritis caused by Monosporium apiospermum treated with intraarticular amphotericin B. Am J Dis Child 131:927. 10.1001/archpedi.1977.02120210105026. [DOI] [PubMed] [Google Scholar]
- 625.Fernandez-Guerrero ML, Ruiz Barnes P, Ales JM. 1987. Postcraniotomy mycetoma of the scalp and osteomyelitis due to Pseudallescheria boydii. J Infect Dis 156:855. 10.1093/infdis/156.5.855. [DOI] [PubMed] [Google Scholar]
- 626.Hung CC, Chang SC, Yang PC, Hsieh WC. 1994. Invasive pulmonary pseudallescheriasis with direct invasion of the thoracic spine in an immunocompetent patient. Eur J Clin Microbiol Infect Dis 13:749–751. 10.1007/BF02276059. [DOI] [PubMed] [Google Scholar]
- 627.Haapasaari J, Essen RV, Kahanpää A, Kostiala AA, Holmberg K, Ahlqvist J. 1982. Fungal arthritis simulating juvenile rheumatoid arthritis. Br Med J (Clin Res Ed) 285:923–924. 10.1136/bmj.285.6346.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Kemp HBS, Bedford AF, Fincham WJ. 1982. Petriellidium boydii infection of the knee: a case report. Skeletal Radiol 9:114–117. 10.1007/BF00360494. [DOI] [PubMed] [Google Scholar]
- 629.Dirschl DR, Henderson RC. 1991. Patellar overgrowth after infection of the knee. A case report. J Bone Joint Surg Am 73:940–941. [PubMed] [Google Scholar]
- 630.Halpern AA, Nagel DA, Schurman DJ. 1977. Allescheria boydii osteomyelitis following multiple steroid injections and surgery. Clin Orthop Relat Res 126:232–234. [PubMed] [Google Scholar]
- 631.Lang AG, Peterson HA. 1976. Osteomyelitis following puncture wounds of the foot in children. J Trauma 16:993–999. 10.1097/00005373-197612000-00010. [DOI] [PubMed] [Google Scholar]
- 632.Charles JF, Eberle C, Daikh DI, Rooney T. 2011. Resolution of recurrent fusarium arthritis after prolonged antifungal therapy. J Clin Rheumatol 17:44–45. 10.1097/RHU.0b013e318205669d. [DOI] [PubMed] [Google Scholar]
- 633.Magnon KC, Jalbert M, Padhye AA. 1993. Osteolytic phaeohyphomycosis caused by Phialemonium obovatum. Arch Pathol Lab Med 117:841–843. [PubMed] [Google Scholar]
- 634.Jakle C, Leek JC, Olson DA, Robbins DL. 1983. Septic arthritis due to Fusarium solani. J Rheumatol 10:151–153. [PubMed] [Google Scholar]
- 635.Kooijman CM, Kampinga GA, de Hoog GS, Goudswaard WB, Reijnen MM. 2007. Successful treatment of Scedosporium aurantiacum osteomyelitis in an immunocompetent patient. Surg Infect (Larchmt) 8:605–610. 10.1089/sur.2006.038. [DOI] [PubMed] [Google Scholar]
- 636.McCall RE. 1981. Maduromycosis “Allescheria boydii” septic arthritis of the knee: a case report. Orthopedics 4:1144–1146. 10.3928/0147-7447-19811001-07. [DOI] [PubMed] [Google Scholar]
- 637.Drouhet E, Dupont B. 1983. Laboratory and clinical assessment of ketoconazole in deep-seated mycoses. Am J Med 74:30–47. 10.1016/0002-9343(83)90512-0. [DOI] [PubMed] [Google Scholar]
- 638.Miyakis S, Velegraki R, Delikou S, Parcharidou A, Papadakis V, Kitra V, Papadatos I, Polychronopoulou S. 2006. Invasive Acremonium strictum infection in a bone marrow transplant recipient. Pediatr Infect Dis J 25:273–275. 10.1097/01.inf.0000202107.73095.ad. [DOI] [PubMed] [Google Scholar]
- 639.Szombathy SP, Chez MG, Laxer RM. 1988. Acute septic arthritis due to Acremonium. J Rheumatol 15:714–715. [PubMed] [Google Scholar]
- 640.Brabender W, Ketcherside J, Hodges GR, Rengachary S, Barnes WG. 1985. Acremonium kiliense osteomyelitis of the calvarium. Neurosurgery 16:554–556. [PubMed] [Google Scholar]
- 641.Tadros TS, Workowski KA, Siegel RJ, Hunter S, Schwartz DA. 1998. Pathology of hyalohyphomycosis caused by Scedosporium apiospermum (Pseudallescheria boydii): an emerging mycosis. Hum Pathol 29:1266–1272. 10.1016/s0046-8177(98)90255-6. [DOI] [PubMed] [Google Scholar]
- 642.Talbot TR, Hatcher J, Davis SF, Pierson RN, III, Barton R, Dummer S. 2002. Scedosporium apiospermum pneumonia and sternal wound infection in a heart transplant recipient. Transplantation 74:1645–1647. 10.1097/00007890-200212150-00028. [DOI] [PubMed] [Google Scholar]
- 643.Galgiani JN, Stevens DA, Graybill JR, Stevens DL, Tillinghast AJ, Levine HB. 1984. Pseudallescheria boydii infections treated with ketoconazole. Clinical evaluations of 7 patients and in vitro susceptibility results. Chest 86:219–224. 10.1378/chest.86.2.219. [DOI] [PubMed] [Google Scholar]
- 644.Lichtman DM, Johnson DC, Macks GR, Lack EE. 1978. Maduromycosis (Allescheria boydii) infection of hand. Case report. J Bone Joint Surg Am 60:546–548. [PubMed] [Google Scholar]
- 645.Travis LB, Roberts GD, Wilson WR. 1985. Clinical significance of Pseudallescheria boydii: a review of 10 years experience. Mayo Clin Proc 60:531–537. 10.1016/s0025-6196(12)60571-0. [DOI] [PubMed] [Google Scholar]
- 646.Foster MR, Friedenberg ZB, Passero F. 1994. Lumbar Petriellidium boydii osteomyelitis with a systemic presentation. J Spinal Disord 7:356–360. [PubMed] [Google Scholar]
- 647.Hiebert RM, Welliver RC, Yu ZX. 2016. Fusarium osteomyelitis in a patient with Pearson syndrome: case report and review of the literature. Open Forum Infect Dis 3:ofw183. 10.1093/ofid/ofw183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Jalava-Karvinen P, Nyman M, Gardberg M, Harju I, Hohenthal U, Oksi J. 2016. Scedosporium apiospermum as a rare cause of central skull base osteomyelitis. Med Mycol Case Rep 11:28–30. 10.1016/j.mmcr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Mosqueira JR, Soto LM, Bustamante AB, Cáceres J, Soria J, Rivera GMF. 2017. Septic arthritis due to underlying Scedosporium apiospermum chronic osteomyelitis. Report of one case and brief review of the literature. J Microbiol Infect Dis 6:192–196. 10.5799/jmid.vi.328943. [DOI] [Google Scholar]
- 650.Denton EJ, Smibert O, Gooi J, Morrissey CO, Snell G, McGiffin D, Paraskeva M. 2016. Invasive Scedosporium sternal osteomyelitis following lung transplant: cured. Med Mycol Case Rep 12:14–16. 10.1016/j.mmcr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Shimizu J, Yoshimoto M, Takebayashi T, Ida K, Tanimoto K, Yamashita T. 2014. Atypical fungal vertebral osteomyelitis in a tsunami survivor of the Great East Japan Earthquake. Spine (Phila Pa 1976) 39:E739–E742. 10.1097/BRS.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 652.Taj-Aldeen SJ, Rammaert B, Gamaletsou M, Sipsas NV, Zeller V, Roilides E, Kontoyiannis DP, Miller AO, Petraitis V, Walsh TJ, Lortholary O, International Osteoarticular Mycoses Consortium. 2015. Osteoarticular infections caused by non-Aspergillus filamentous fungi in adult and pediatric patients: a systematic review. Medicine (Baltimore) 94:e2078. 10.1097/MD.0000000000002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Cohen-Abbo A, Edwards KM. 1995. Multifocal osteomyelitis caused by Paecilomyces varioti in a patient with chronic granulomatous disease. Infection 23:55–57. 10.1007/BF01710060. [DOI] [PubMed] [Google Scholar]
- 654.Schell WA. 2000. Histopathology of fungal rhinosinusitis. Otolaryngol Clin North Am 33:251–276. 10.1016/s0030-6665(00)80004-3. [DOI] [PubMed] [Google Scholar]
- 655.Lee YJ, Sadigh S, Mankad K, Kapse N, Rajeswaran G. 2016. The imaging of osteomyelitis. Quant Imaging Med Surg 6:184–198. 10.21037/qims.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Offiah AC. 2006. Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children. Eur J Radiol 60:221–232. 10.1016/j.ejrad.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 657.Sutton DA, Timm WD, Morgan-Jones G, Rinaldi MG. 1999. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis saccardo 1905: criteria for identification, case history, and therapy. J Clin Microbiol 37:807–811. 10.1128/JCM.37.3.807-811.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Ong ATL, Mahajan H, Chen SC-A, Halliday C, Watts MR, Brighton R, Ralph AP. 2012. Coccidioidal septic arthritis: lessons learned from a clinical and laboratory perspective. Med J Aust 196:705–706. 10.5694/mja11.10533. [DOI] [PubMed] [Google Scholar]
- 659.Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Munoz P, Johnson E, Meletiadis J, Pana Z-D, Lackner M, Verweij P, Freiberger T, Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Guinea J, Guarro J, de Hoog S, Hope W, Kathuria S, Lortholary O, Meis JF, Ullmann AJ, Petrikkos G, Lass-Florl C, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 20(Suppl 3):27–46. 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]
- 660.Shelton BG, Kirkland KH, Flanders WD, Morris GK. 2002. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl Environ Microbiol 68:1743–1753. 10.1128/AEM.68.4.1743-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Okeke CN, Gugnani HC. 1986. Studies on pathogenic dematiaceous fungi. 1. Isolation from natural sources. Mycopathologia 94:19–25. 10.1007/BF00437257. [DOI] [PubMed] [Google Scholar]
- 662.McCarty TP, Baddley JW, Walsh TJ, Alexander BD, Kontoyiannis DP, Perl TM, Walker R, Patterson TF, Schuster MG, Lyon GM, Wingard JR, Andes DR, Park BJ, Brandt ME, Pappas PG, TRANSNET Investigators. 2015. Phaeohyphomycosis in transplant recipients: results from the Transplant Associated Infection Surveillance Network (TRANSNET). Med Mycol 53:440–446. 10.1093/mmy/myv018. [DOI] [PubMed] [Google Scholar]
- 663.Taj-Aldeen SJ, Almaslamani M, Alkhal A, Al Bozom I, Romanelli AM, Wickes BL, Fothergill AW, Sutton DA. 2010. Cerebral phaeohyphomycosis due to Rhinocladiella mackenziei (formerly Ramichloridium mackenziei): a taxonomic update and review of the literature. Med Mycol 48:546–556. 10.3109/13693780903383914. [DOI] [PubMed] [Google Scholar]
- 664.Badali H, Chander J, Bansal S, Aher A, Borkar SS, Meis JF, De Hoog GS. 2010. First autochthonous case of Rhinocladiella mackenziei cerebral abscess outside the Middle East. J Clin Microbiol 48:646–649. 10.1128/JCM.01855-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Revankar SG, Sutton DA. 2012. Melanized fungi in human disease. Clin Microbiol Rev 25:720. 10.1128/CMR.00069-12. (Erratum). 10.1128/CMR.00069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Mohan AK, Cote TR, Siegel JN, Braun MM. 2003. Infectious complications of biologic treatments of rheumatoid arthritis. Curr Opin Rheumatol 15:179–184. 10.1097/00002281-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 667.Ahmed SA, Desbois N, Quist D, Miossec C, Atoche C, Bonifaz A, de Hoog GS. 2015. Phaeohyphomycosis caused by a novel species, Pseudochaetosphaeronema martinelli. J Clin Microbiol 53:2927–2934. 10.1128/JCM.01456-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clin Microbiol Rev 23:884–928. 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Revankar SG. 2015. Phaeohyphomycosis in transplant patients. J Fungi (Basel) 2:2. 10.3390/jof2010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Cetrulo CL, Barone AAL, Jordan K, Chang DS, Louie K, Buntic RF, Brooks D. 2012. A multi-disciplinary approach to the management of fungal osteomyelitis: current concepts in post-traumatic lower extremity reconstruction. A case report. Microsurgery 32:144–147. 10.1002/micr.20956. [DOI] [PubMed] [Google Scholar]
- 671.Studahl M, Backteman T, Stalhammar F, Chryssanthou E, Petrini B. 2003. Bone and joint infection after traumatic implantation of Scedosporium prolificans treated with voriconazole and surgery. Acta Paediatr 92:980–982. 10.1080/08035250310004595. [DOI] [PubMed] [Google Scholar]
- 672.Steinbach WJ, Schell WA, Miller JL, Perfect JR. 2003. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J Clin Microbiol 41:3981–3985. 10.1128/JCM.41.8.3981-3985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Kesson AM, Bellemore MC, O’Mara TJ, Ellis DH, Sorrell TC. 2009. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with a novel agent, hexadecylphospocholine (miltefosine), in combination with terbinafine and voriconazole: a case report. Clin Infect Dis 48:1257–1261. 10.1086/597772. [DOI] [PubMed] [Google Scholar]
- 674.Howden BP, Slavin MA, Schwarer AP, Mijch AM. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur J Clin Microbiol Infect Dis 22:111–113. 10.1007/s10096-002-0877-z. [DOI] [PubMed] [Google Scholar]
- 675.Garcia-Vidal C, Cabellos C, Ayats J, Font F, Ferran E, Fernandez-Viladrich P. 2009. Fungal postoperative spondylodiscitis due to Scedosporium prolificans. Spine J 9:e1–e7. 10.1016/j.spinee.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 676.Li JYZ, Yong TY, Grove DI, Coates PTH. 2008. Successful control of Scedosporium prolificans septic arthritis and probable osteomyelitis without radical surgery in a long-term renal transplant recipient. Transpl Infect Dis 10:63–65. 10.1111/j.1399-3062.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 677.Matlani M, Kaur R, Shweta. 2013. A case of Scedosporium prolificans osteomyelitis in an immunocompetent child, misdiagnosed as tubercular osteomyelitis. Indian J Dermatol 58:80–81. 10.4103/0019-5154.105319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Dalton PA, Munckhof WJ, Walters DW. 2006. Scedosporium prolificans: an uncommon cause of septic arthritis. ANZ J Surg 76:661–663. 10.1111/j.1445-2197.2006.03796.x. [DOI] [PubMed] [Google Scholar]
- 679.Pickles RW, Pacey DE, Muir DB, Merrell WH. 1996. Experience with infection by Scedosporium prolificans including apparent cure with fluconazole therapy. J Infect 33:193–197. 10.1016/s0163-4453(96)92249-5. [DOI] [PubMed] [Google Scholar]
- 680.Holmes NE, Trevillyan JM, Kidd SE, Leong TY. 2013. Locally extensive angio-invasive Scedosporium prolificans infection following resection for squamous cell lung carcinoma. Med Mycol Case Rep 2:98–102. 10.1016/j.mmcr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Gosbell IB, Toumasatos V, Yong J, Kuo RS, Ellis DH, Perrie RC. 2003. Cure of orthopaedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses 46:233–236. 10.1046/j.1439-0507.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 682.Wilson CM, O’Rourke EJ, McGinnis MR, Salkin IF. 1990. Scedosporium inflatum: clinical spectrum of a newly recognized pathogen. J Infect Dis 161:102–107. 10.1093/infdis/161.1.102. [DOI] [PubMed] [Google Scholar]
- 683.Wood GM, McCormack JG, Muir DB, Ellis DH, Ridley MF, Pritchard R, Harrison M. 1992. Clinical features of human infection with Scedosporium inflatum. Clin Infect Dis 14:1027–1033. 10.1093/clinids/14.5.1027. [DOI] [PubMed] [Google Scholar]
- 684.Menon S, Edwards JC. 1994. Mycotic arthritis of the knee due to Madurella grisea. Br J Rheumatol 33:292–295. 10.1093/rheumatology/33.3.292. [DOI] [PubMed] [Google Scholar]
- 685.Capoor MR, Khanna G, Nair D, Hasan A, Rajni, Deb M, Aggarwal P. 2007. Eumycetoma pedis due to Exophiala jeanselmei. Indian J Med Microbiol 25:155–157. 10.4103/0255-0857.32726. [DOI] [PubMed] [Google Scholar]
- 686.Roncoroni AJ, Smayevsky J. 1988. Arthritis and endocarditis from Exophiala jeanselmei infection. Ann Intern Med 108:773. 10.7326/0003-4819-108-5-773_1. [DOI] [PubMed] [Google Scholar]
- 687.Lim A, Speers D, Inderjeeth C. 2013. Cladophialophora (Xylohypha) bantiana—an unusual cause of septic arthritis. Rheumatology (Oxford) 52:958–959. 10.1093/rheumatology/kes317. [DOI] [PubMed] [Google Scholar]
- 688.Karuppal R, Kumaran CM, Marthya A, Kumar CVM, Narayanan MP, Raman RV, Thomas S. 2009. Tibial osteomyelitis due to Fonsecaea pedrosoi in an immunocompetent patient: case report. J Foot Ankle Surg 48:569–572. 10.1053/j.jfas.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 689.Sridhar S, Cheong D, Fontaine J-P, Sandin RL, Greene JN. 2013. Alternaria-infected sternoclavicular joint. Infect Dis Clin Pract 21:e21–e23. 10.1097/IPC.0b013e3182769261. [DOI] [Google Scholar]
- 690.Destino L, Sutton DA, Helon AL, Havens PL, Thometz JG, Willoughby RE, Jr, Chusid MJ. 2006. Severe osteomyelitis caused by Myceliophthora thermophila after a pitchfork injury. Ann Clin Microbiol Antimicrob 5:21. 10.1186/1476-0711-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Dewar CL, Sigler L. 2010. Fungal arthritis of the knee caused by Mycoleptodiscus indicus. Clin Rheumatol 29:1061–1065. 10.1007/s10067-010-1448-9. [DOI] [PubMed] [Google Scholar]
- 692.Shigemura T, Agematsu K, Yamazaki T, Eriko K, Yasuda G, Nishimura K, Koike K. 2009. Femoral osteomyelitis due to Cladophialophora arxii in a patient with chronic granulomatous disease. Infection 37:469–473. 10.1007/s15010-009-8238-9. [DOI] [PubMed] [Google Scholar]
- 693.Khan SA. 2007. Calcaneal osteomyelitis caused by Exophiala jeanselmei in an immunocompetent child. J Bone Joint Surg Am 89:2547. 10.2106/00004623-200711000-00031. [DOI] [PubMed] [Google Scholar]
- 694.O’Riordan E, Denton J, Taylor PM, Kerr J, Short CD. 2002. Madura foot in the U.K.: fungal osteomyelitis after renal transplantation. Transplantation 73:151–153. 10.1097/00007890-200201150-00029. [DOI] [PubMed] [Google Scholar]
- 695.Morio F, Le Berre JY, Garcia-Hermoso D, Najafzadeh MJ, de Hoog S, Benard L, Michau C. 2012. Phaeohyphomycosis due to Exophiala xenobiotica as a cause of fungal arthritis in an HIV-infected patient. Med Mycol 50:513–517. 10.3109/13693786.2011.648218. [DOI] [PubMed] [Google Scholar]
- 696.Katsolis JG, Sudduth EJ, Chen N, Brumble LM. 2012. Alternaria osteomyelitis in an immunocompetent host treated with voriconazole. Infect Dis Clin Pract 20:164–166. 10.1097/IPC.0b013e3182302719. [DOI] [Google Scholar]
- 697.Lee DK, Schwartz AK. 2007. Primary mycetoma osteomyelitis of the calcaneus with active subcutaneous nodules. J Foot Ankle Surg 46:302–306. 10.1053/j.jfas.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 698.Woollons A, Darley CR, Pandian S, Arnstein P, Blackee J, Paul J. 1996. Phaeohyphomycosis caused by Exophiala dermatitidis following intra-articular steroid injection. Br J Dermatol 135:475–477. 10.1046/j.1365-2133.1996.d01-1026.x. [DOI] [PubMed] [Google Scholar]
- 699.Lespessailles E, Kerdraon R, Michenet P, Barthez JP, Mille C, Benhamou CL. 1999. Alternaria infection of the skin and joints. A report of two cases involving the hand. Rev Rhum Engl Ed 66:509–511. [PubMed] [Google Scholar]
- 700.Murtagh J, Smith JW, Mackowiak PA. 1987. Alternaria osteomyelitis: eight years of recurring disease requiring cyclic courses of amphotericin B for cure. Am J Med Sci 293:399–402. 10.1097/00000441-198706000-00010. [DOI] [Google Scholar]
- 701.Yangco BG, TeStrake D, Okafor J. 1984. Phialophora richardsiae isolated from infected human bone: morphological, physiological and antifungal susceptibility studies. Mycopathologia 86:103–111. 10.1007/BF00436495. [DOI] [PubMed] [Google Scholar]
- 702.Kaell AT, Weitzman I. 1983. Acute monoarticular arthritis due to Phialophora parasitica. Am J Med 74:519–522. 10.1016/0002-9343(83)91001-x. [DOI] [PubMed] [Google Scholar]
- 703.Toy EC, Rinaldi MG, Savitch CB, Leibovitch ER. 1990. Endocarditis and hip arthritis associated with Scedosporium inflatum. South Med J 83:957–960. 10.1097/00007611-199008000-00025. [DOI] [PubMed] [Google Scholar]
- 704.Ratnayake G, Judson IR, Scurr M, Thway K, Fisher C, Jones RL. 2011. Fungal spinal cord compression in metastatic synovial sarcoma. Acta Oncol 50:158–159. 10.3109/0284186X.2010.498830. [DOI] [PubMed] [Google Scholar]
- 705.Koppang HS, Olsen I, Stuge U, Sandven P. 1991. Aureobasidium infection of the jaw. J Oral Pathol Med 20:191–195. 10.1111/j.1600-0714.1991.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 706.Uberti-Foppa C, Fumagalli L, Gianotti N, Viviani AM, Vaiani R, Gieho E. 1995. First case of osteomyelitis due to Phialophora richardsiae in a patient with HIV infection. AIDS 9:975–976. [PubMed] [Google Scholar]
- 707.Beeram V, Challa S, Vannemreddy P. 2008. Cerebral mycetoma with cranial osteomyelitis. J Neurosurg Pediatr 1:493–495. 10.3171/PED/2008/1/6/493. [DOI] [PubMed] [Google Scholar]
- 708.Sadigursky D, Nogueira EFL, Moreno de Oliveira Correa L. 2016. Phaeohyphomycosis infection in the knee. Rev Bras Ortop 51:231–234. 10.1016/j.rboe.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.van Hougenhouck-Tulleken WG, Mathole G, Karstaedt A, Govind N, Moodley M, Seetharam S, Govender NP, Menezes CN. 2016. Disseminated fungal infection in an HIV-infected patient due to Aureobasidium pullulans. S Afr J Infect Dis 31:71–73. 10.4102/sajid.v31i3.79. [DOI] [Google Scholar]
- 710.Desmet S, Smets L, Lagrou K, Derdelinckx I, Neyt J, Maertens J, Sciot R, Demaerel P, Bammens B. 2016. Cladophialophora bantiana osteomyelitis in a renal transplant patient. Med Mycol Case Rep 12:17–20. 10.1016/j.mmcr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Daniele L, Le M, Parr AF, Brown LM. 2017. Scedosporium prolificans septic arthritis and osteomyelitis of the hip joints in an immunocompetent patient: a case report and literature review. Case Rep Orthop 2017:3809732. 10.1155/2017/3809732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Pace CS, Frankenhoff JA, Isaacs JE. 2017. Scedosporium prolificans septic arthritis. J Hand Microsurg 9:37–38. 10.1055/s-0036-1597553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Kaspar S, de Beer JD. 2005. Infection in hip arthroplasty after previous injection of steroid. J Bone Joint Surg Br 87:454–457. 10.1302/0301-620X.87B4.15546. [DOI] [PubMed] [Google Scholar]
- 714.Mohan M, Shalin SC, Kothari A, Rico JCC, Caradine K, Burgess M. 2016. Lasiodiplodia species fungal osteomyelitis in a multiple myeloma patient. Transpl Infect Dis 18:761–764. 10.1111/tid.12573. [DOI] [PubMed] [Google Scholar]
- 715.Rossmann SN, Cernoch PL, Davis JR. 1996. Dematiaceous fungi are an increasing cause of human disease. Clin Infect Dis 22:73–80. 10.1093/clinids/22.1.73. [DOI] [PubMed] [Google Scholar]
- 716.Isa-Isa R, Garcia C, Isa M, Arenas R. 2012. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol 30:425–431. 10.1016/j.clindermatol.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 717.Bonifaz A, Davoudi MM, de Hoog GS, Padilla-Desgarennes C, Vazquez-Gonzalez D, Navarrete G, Meis JF, Badali H. 2013. Severe disseminated phaeohyphomycosis in an immunocompetent patient caused by Veronaea botryosa. Mycopathologia 175:497–503. 10.1007/s11046-013-9632-5. [DOI] [PubMed] [Google Scholar]
- 718.Casadevall A, Rosas AL, Nosanchuk JD. 2000. Melanin and virulence in Cryptococcus neoformans. Curr Opin Microbiol 3:354–358. 10.1016/s1369-5274(00)00103-x. [DOI] [PubMed] [Google Scholar]
- 719.Feng B, Wang X, Hauser M, Kaufmann S, Jentsch S, Haase G, Becker JM, Szaniszlo PJ. 2001. Molecular cloning and characterization of WdPKS1, a gene involved in dihydroxynaphthalene melanin biosynthesis and virulence in Wangiella (Exophiala) dermatitidis. Infect Immun 69:1781–1794. 10.1128/IAI.69.3.1781-1794.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Jacobson ES. 2000. Pathogenic roles for fungal melanins. Clin Microbiol Rev 13:708–717. 10.1128/CMR.13.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Lanternier F, Barbati E, Meinzer U, Liu LY, Pedergnana V, Migaud M, Heritier S, Chomton M, Fremond ML, Gonzales E, Galeotti C, Romana S, Jacquemin E, Angoulvant A, Bidault V, Canioni D, Lachenaud J, Mansouri D, Mahdaviani SA, Adimi P, Mansouri N, Jamshidi M, Bougnoux ME, Abel L, Lortholary O, Blanche S, Casanova JL, Picard C, Puel A. 2015. Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J Infect Dis 211:1241–1250. 10.1093/infdis/jiu412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Rinaldi MG. 1996. Phaeohyphomycosis. Dermatol Clin 14:147–153. 10.1016/s0733-8635(05)70335-1. [DOI] [PubMed] [Google Scholar]
- 723.Santos DWCL, Padovan ACB, Melo ASA, Goncalves SS, Azevedo VR, Ogawa MM, Freitas TVS, Colombo AL. 2013. Molecular identification of melanised non-sporulating moulds: a useful tool for studying the epidemiology of phaeohyphomycosis. Mycopathologia 175:445–454. 10.1007/s11046-012-9608-x. [DOI] [PubMed] [Google Scholar]
- 724.Cuetara MS, Alhambra A, Moragues MD, Gonzalez-Elorza E, Ponton J, del Palacio A. 2009. Detection of (1→3)-β-d-glucan as an adjunct to diagnosis in a mixed population with uncommon proven invasive fungal diseases or with an unusual clinical presentation. Clin Vaccine Immunol 16:423–426. 10.1128/CVI.00009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. 2009. Utility of galactomannan enzyme immunoassay and (1,3) β-d-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol 47:129–133. 10.1128/JCM.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Khan SA, Hasan AS, Capoor MR, Varshney MK, Trikha V. 2007. Calcaneal osteomyelitis caused by Exophiala jeanselmei in an immunocompetent child. J Bone Joint Surg Am 89:859–862. 10.2106/00004623-200704000-00024. [DOI] [PubMed] [Google Scholar]
- 727.Ansari RA, Hindson DA, Stevens DL, Kloss JG. 1987. Pseudallescheria boydii arthritis and osteomyelitis in a patient with Cushing’s disease. South Med J 80:90–92. 10.1097/00007611-198701000-00020. [DOI] [PubMed] [Google Scholar]
- 728.Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, Arikan-Akdagli S, Akova M, Boekhout T, Caira M, Guinea J, Chakrabarti A, Dannaoui E, van Diepeningen A, Freiberger T, Groll AH, Hope WW, Johnson E, Lackner M, Lagrou K, Lanternier F, Lass-Florl C, Lortholary O, Meletiadis J, Munoz P, Pagano L, Petrikkos G, Richardson MD, Roilides E, Skiada A, Tortorano AM, Ullmann AJ, Verweij PE, Cornely OA, Cuenca-Estrella M, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect 20(Suppl 3):47–75. 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 729.Hoenigl M, Gangneux J-P, Segal E, Alanio A, Chakrabarti A, Chen SC-A, Govender N, Hagen F, Klimko N, Meis JF, Pasqualotto AC, Seidel D, Walsh TJ, Lagrou K, Lass-Flörl C, Cornely OA, European Confederation of Medical Mycology (ECMM). 2018. Global guidelines and initiatives from the European Confederation of Medical Mycology to improve patient care and research worldwide: new leadership is about working together. Mycoses 61:885–894. 10.1111/myc.12836. [DOI] [PubMed] [Google Scholar]
- 730.Elizondo-Zertuche M, Montoya AM, Robledo-Leal E, Garza-Veloz I, Sanchez-Nunez AL, Ballesteros-Elizondo R, Gonzalez GM. 2017. Comparative pathogenicity of Lomentospora prolificans (Scedosporium prolificans) isolates from Mexican patients. Mycopathologia 182:681–689. 10.1007/s11046-017-0137-5. [DOI] [PubMed] [Google Scholar]
- 731.Jenks JD, Seidel D, Cornely OA, Chen S, van Hal S, Kauffman C, Miceli MH, Heinemann M, Christner M, Jover Sáenz A, Burchardt A, Kemmerling B, Herbrecht R, Steinmann J, Shoham S, Gräber S, Pagano L, Deeren D, Slavin MA, Hoenigl M. 2020. Clinical characteristics and outcomes of invasive Lomentospora prolificans infections: analysis of patients in the FungiScope registry. Mycoses 63:437–442. 10.1111/myc.13067. [DOI] [PubMed] [Google Scholar]
- 732.Hoenigl M, Salmanton-García J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, Lackner M, Sprute R, Al-Hatmi AMS, Bassetti M, Carlesse F, Freiberger T, Koehler P, Lehrnbecher T, Kumar A, Prattes J, Richardson M, Revankar S, Slavin MA, Stemler J, Spiess B, Taj-Aldeen SJ, Warris A, Woo PCY, Young J-AH, Albus K, Arenz D, Arsic-Arsenijevic V, Bouchara J-P, Chinniah TR, Chowdhary A, de Hoog GS, Dimopoulos G, Duarte RF, Hamal P, Meis JF, Mfinanga S, Queiroz-Telles F, Patterson TF, Rahav G, Rogers TR, Rotstein C, Wahyuningsih R, Seidel D, Cornely OA. 2021. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 21:e246–e257. 10.1016/S1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]
- 733.Wiederhold NP. 2021. Review of T-2307, an investigational agent that causes collapse of fungal mitochondrial membrane potential. J Fungi (Basel) 7:130. 10.3390/jof7020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL. 1998. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin Infect Dis 27:1138–1147. 10.1093/clinids/27.5.1138. [DOI] [PubMed] [Google Scholar]
- 735.Bouza E, Munoz P, Guinea J. 2006. Mucormycosis: an emerging disease? Clin Microbiol Infect 12:7–23. 10.1111/j.1469-0691.2006.01604.x. [DOI] [Google Scholar]
- 736.Taj-Aldeen SJ, Gamaletsou MN, Rammaert B, Sipsas NV, Zeller V, Roilides E, Kontoyiannis DP, Henry M, Petraitis V, Moriyama B, Denning DW, Lortholary O, Walsh TJ, International Osteoarticular Mycoses Consortium. 2017. Bone and joint infections caused by mucormycetes: a challenging osteoarticular mycosis of the twenty-first century. Med Mycol 55:691–704. 10.1093/mmy/myw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Ribes JA, Vanover-Sams CL, Baker DJ. 2000. Zygomycetes in human disease. Clin Microbiol Rev 13:236–301. 10.1128/CMR.13.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Moore PH, Jr, McKinney RG, Mettler FA, Jr.. 1978. Radiographic and radionuclide findings in Rhizopus osteomyelitis. Radiology 127:665–666. 10.1148/127.3.665. [DOI] [PubMed] [Google Scholar]
- 739.Echols RM, Selinger DS, Hallowell C, Goodwin JS, Duncan MH, Cushing AH. 1979. Rhizopus osteomyelitis: case report and review. Am J Med 66:141–145. 10.1016/0002-9343(79)90505-9. [DOI] [PubMed] [Google Scholar]
- 740.Buruma OJS, Craane H, Kunst MW. 1979. Vertebral osteomyelitis and epidural abcess [sic] due to mucormycosis: case report. Clin Neurol Neurosurg 81:39–44. 10.1016/S0303-8467(79)80005-0. [DOI] [PubMed] [Google Scholar]
- 741.Maliwan N, Reyes CV, Rippon JW. 1984. Osteomyelitis secondary to cutaneous mucormycosis. Report of a case and a review of the literature. Am J Dermatopathol 6:479–481. 10.1097/00000372-198410000-00011. [DOI] [PubMed] [Google Scholar]
- 742.Pierce PF, Wood MB, Roberts GD, Fitzgerald RH, Jr, Robertson C, Edson RS. 1987. Saksenaea vasiformis osteomyelitis. J Clin Microbiol 25:933–935. 10.1128/jcm.25.5.933-935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Mostaza JM, Barbado FJ, Fernandez-Martin J, Pena-Yanez J, Vazquez-Rodriguez JJ. 1989. Cutaneoarticular mucormycosis due to Cunninghamella bertholletiae in a patient with AIDS. Rev Infect Dis 11:316–318. 10.1093/clinids/11.2.316. [DOI] [PubMed] [Google Scholar]
- 744.Huffnagle KE, Southern PM, Jr, Byrd LT, Gander RM. 1992. Apophysomyces elegans as an agent of zygomycosis in a patient following trauma. J Med Vet Mycol 30:83–86. 10.1080/02681219280000111. [DOI] [PubMed] [Google Scholar]
- 745.Buhl MR, Joseph TP, Snelling BE, Buhl L. 1992. Temporofacial zygomycosis in a pregnant woman. Infection 20:230–232. 10.1007/BF02033066. [DOI] [PubMed] [Google Scholar]
- 746.Chaudhuri R, McKeown B, Harrington D, Hay RJ, Bingham JB, Spencer JD. 1992. Mucormycosis osteomyelitis causing avascular necrosis of the cuboid bone: MR imaging findings. AJR Am J Roentgenol 159:1035–1037. 10.2214/ajr.159.5.1414771. [DOI] [PubMed] [Google Scholar]
- 747.Weinberg WG, Wade BH, Cierny G, III, Stacy D, Rinaldi MG. 1993. Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin Infect Dis 17:881–884. 10.1093/clinids/17.5.881. [DOI] [PubMed] [Google Scholar]
- 748.Eaton ME, Padhye AA, Schwartz DA, Steinberg JP. 1994. Osteomyelitis of the sternum caused by Apophysomyces elegans. J Clin Microbiol 32:2827–2828. 10.1128/jcm.32.11.2827-2828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Meis JFGM, Kullberg BJ, Pruszczynski M, Veth RPH. 1994. Severe osteomyelitis due to the zygomycete Apophysomyces elegans. J Clin Microbiol 32:3078–3081. 10.1128/jcm.32.12.3078-3081.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Shaw CJ, Thomason AJS, Spencer JD. 1994. Fungal osteomyelitis of the foot. A report of an unusual case. J Bone Joint Surg Br 76:137–139. 10.1302/0301-620X.76B1.8300658. [DOI] [PubMed] [Google Scholar]
- 751.Fortun J, Cobo J, Canal J, Martinez-San Millan J. 1995. Post-traumatic cranial mucormycosis in an immunocompetent patient. J Oral Maxillofac Surg 53:1099–1102. 10.1016/0278-2391(95)90132-9. [DOI] [PubMed] [Google Scholar]
- 752.Oo MM, Kutteh LA, Koc ON, Strauss M, Lazarus HM. 1998. Mucormycosis of petrous bone in an allogeneic stem cell transplant recipient. Clin Infect Dis 27:1546–1547. 10.1086/517749. [DOI] [PubMed] [Google Scholar]
- 753.Stevanovic MV, Mirzayan R, Holtom PD, Schnall SB. 1999. Mucormycosis osteomyelitis in the hand. Orthopedics 22:449–450. 10.3928/0147-7447-19990401-16. [DOI] [PubMed] [Google Scholar]
- 754.Holtom PD, Obuch AB, Ahlmann ER, Shepherd LE, Patzakis MJ. 2000. Mucormycosis of the tibia: a case report and review of the literature. Clin Orthop Relat Res 381:222–228. 10.1097/00003086-200012000-00026. [DOI] [PubMed] [Google Scholar]
- 755.Burke WV, Zych GA. 2002. Fungal infection following replacement of the anterior cruciate ligament. A case report. J Bone Joint Surg Am 84:449–453. 10.2106/00004623-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 756.Chen F, Lu G, Kang Y, Ma Z, Lu C, Wang B, Li J, Liu J, Li H. 2006. Mucormycosis spondylodiscitis after lumbar disc puncture. Eur Spine J 15:370–376. 10.1007/s00586-005-1025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Adler N, Seitz IA, Gottlieb LJ. 2008. Acute wound closure and reconstruction following head zygomycosis: presentation of two cases and review of literature. J Reconstr Microsurg 24:507–513. 10.1055/s-0028-1088233. [DOI] [PubMed] [Google Scholar]
- 758.Parra-Ruiz J, Pena-Monje A, Tomas-Jimenez C, Antelo-Lorenzo R, Escobar-Lara T, Hernandez-Quero J. 2008. Septic arthritis due to Absidia corymbifera in a patient with HIV-1 infection. Infection 36:279–281. 10.1007/s15010-007-6297-3. [DOI] [PubMed] [Google Scholar]
- 759.Jones NF, Shin EK, Eo S, Starzl TE. 2008. Successful salvage of mucormycosis infection of the forearm and osteomyelitis of the ulna. Hand (N Y) 3:332–336. 10.1007/s11552-008-9119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Wilkins RM, Hahn DB, Blum R. 2009. Bread mold osteomyelitis in the femur. Orthopedics 32:362. 10.3928/01477447-20090501-21. [DOI] [PubMed] [Google Scholar]
- 761.Muscolo DL, Carbo L, Aponte-Tinao LA, Ayerza MA, Makino A. 2009. Massive bone loss from fungal infection after anterior cruciate ligament arthroscopic reconstruction. Clin Orthop Relat Res 467:2420–2425. 10.1007/s11999-009-0714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Oswal NP, Gadre PK, Sathe P, Gadre KS. 2012. Mucormycosis of mandible with unfavorable outcome. Case Rep Dent 2012:257940. 10.1155/2012/257940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Dinasarapu CR, Auerbach J, Levi MH, Corpuz M. 2010. Mucormycosis as a pathogen in polymicrobial necrotizing fasciitis. Infect Dis Clin Pract 18:417–418. 10.1097/IPC.0b013e3181e85dfb. [DOI] [Google Scholar]
- 764.Harrasser N, Banke IJ, Hauschild M, Lenze U, Prodinger PM, Toepfer A, Peschel C, von Eisenhart-Rothe R, Ringshausen I, Verbeek M. 2014. Clinical challenge: fatal mucormycotic osteomyelitis caused by Rhizopus microsporus despite aggressive multimodal treatment. BMC Infect Dis 14:488. 10.1186/1471-2334-14-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Garces Zarzalejo C, Fakkas Fernandez M, Fernandez Sampedro M, Estelles Marcos L, De la Red Gallegos M, Garcia Granja S. 2014. Femoral distal mucormycosis after a knee meniscectomy. J Knee Surg Rep 1:012–016. 10.1055/s-0034-1370900. [DOI] [Google Scholar]
- 766.Mandelia A, Garg R, Agarwala S, Kale SS. 2015. Vertebral osteomyelitis and epidural abscess due to mucormycosis in a neonate with esophageal atresia. J Clin Neonatol 4:271–274. [Google Scholar]
- 767.Arockiaraj J, Balaji G, Ashok A, Kokil G. 2012. Amphotericin B cement beads: a good adjunctive treatment for musculoskeletal mucormycosis. Indian J Orthop 46:369–372. 10.4103/0019-5413.96370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Vashi N, Avedian R, Brown J, Arai S. 2012. Successful surgical and medical treatment of Rhizopus osteomyelitis following hematopoietic cell transplantation. Orthopedics 35:e1556–e1561. 10.3928/01477447-20120919-30. [DOI] [PubMed] [Google Scholar]
- 769.Navanukroh O, Jitmuang A, Chayakulkeeree M, Ngamskulrungroj P. 2014. Disseminated Cunninghamella bertholletiae infection with spinal epidural abscess in a kidney transplant patient: case report and literature review. Transpl Infect Dis 16:658–665. 10.1111/tid.12251. [DOI] [PubMed] [Google Scholar]
- 770.Hadgaonkar S, Shah K, Bhojraj S, Nene A, Shyam A. 2015. Isolated mucormycotic spondylodiscitis of lumbar spine—a rare case report. J Orthop Case Rep 5:55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Stephen S, Subashini B, Thomas R, Philip A, Sundaresan R. 2016. Skull base osteomyelitis caused by an elegant fungus. J Assoc Physicians India 64:70–71. [PubMed] [Google Scholar]
- 772.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 773.Ibrahim AS, Kontoyiannis DP. 2013. Update on mucormycosis pathogenesis. Curr Opin Infect Dis 26:508–515. 10.1097/QCO.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Prabhu RM, Patel R. 2004. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect 10:31–47. 10.1111/j.1470-9465.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 775.Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE. 1997. Pathology of infectious diseases, vol 1, p 1113–1119. Stamford, Appleton & Lange Co, Hong Kong. [Google Scholar]
- 776.Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. 2012. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 54(Suppl 1):S55–S60. 10.1093/cid/cir868. [DOI] [PubMed] [Google Scholar]
- 777.Walsh TJ, Hayden RT, Larone DH. 2018. Larone’s medically important fungi: a guide to identification, 6th ed. ASM Press, Washington, DC. [Google Scholar]
- 778.McDermott NE, Barrett J, Hipp J, Merino MJ, Richard Lee C-C, Waterman P, Domingo DL, Walsh TJ. 2010. Successful treatment of periodontal mucormycosis: report of a case and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:e64–e69. 10.1016/j.tripleo.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 779.Pineda C, Vargas A, Rodríguez AV. 2006. Imaging of osteomyelitis: current concepts. Infect Dis Clin North Am 20:789–825. 10.1016/j.idc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 780.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE, Mellinghoff SC, Mer M, Pana ZD, Seidel D, Sheppard DC, Wahba R, Akova M, Alanio A, Al-Hatmi AMS, Arikan-Akdagli S, Badali H, Ben-Ami R, Bonifaz A, Bretagne S, Castagnola E, Chayakulkeeree M, Colombo AL, Corzo-León DE, Drgona L, Groll AH, Guinea J, Heussel C-P, Ibrahim AS, Kanj SS, Klimko N, Lackner M, Lamoth F, Lanternier F, Lass-Flörl C, Lee D-G, Lehrnbecher T, Lmimouni BE, Mares M, Maschmeyer G, Meis JF, Meletiadis J, Morrissey CO, Nucci M, Oladele R, Pagano L, et al. 2019. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19:e405–e421. 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Medaris LA, Ponce B, Hyde Z, Delgado D, Ennis D, Lapidus W, Larrison M, Pappas PG. 2016. Cryptococcal osteomyelitis: a report of 5 cases and a review of the recent literature. Mycoses 59:334–342. 10.1111/myc.12476. [DOI] [PubMed] [Google Scholar]
- 782.Chayakulkeeree M, Perfect JR. 2006. Cryptococcosis. Infect Dis Clin North Am 20:507–544. 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 783.Poenaru SM, Rofaiel R, Hosseini-Moghaddam SM. 2017. Osteomyelitis and intramuscular abscess in a liver transplant patient. BMJ Case Rep 2017:bcr2017221650. 10.1136/bcr-2017-221650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Chen WA, Emory CL, Graves BR. 2018. Disseminated cryptococcal osteomyelitis to the hand in an immunosuppressed lymphoma patient. J Hand Surg Am 43:291.e1–291.e6. 10.1016/j.jhsa.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 785.Molter CM, Zuba JR, Papendick R. 2014. Cryptococcus gattii osteomyelitis and compounded itraconazole treatment failure in a Pesquet’s parrot (Psittrichas fulgidus). J Zoo Wildl Med 45:127–133. 10.1638/2013-0042R1.1. [DOI] [PubMed] [Google Scholar]
- 786.Schmertmann LJ, Stalder K, Hudson D, Martin P, Makara M, Meyer W, Malik R, Krockenberger MB. 2018. Cryptococcosis in the koala (Phascolarctos cinereus): pathogenesis and treatment in the context of two atypical cases. Med Mycol 56:926–936. 10.1093/mmy/myx146. [DOI] [PubMed] [Google Scholar]
- 787.Corral JE, Lima S, Quezada J, Samayoa B, Arathoon E. 2011. Cryptococcal osteomyelitis of the skull. Med Mycol 49:667–671. 10.3109/13693786.2011.558124. [DOI] [PubMed] [Google Scholar]
- 788.de Aguiar PADF, Pedroso RDS, Borges AS, Moreira TDA, de Araujo LB, Roder DVDDB. 2017. The epidemiology of cryptococcosis and the characterization of Cryptococcus neoformans isolated in a Brazilian university hospital. Rev Inst Med Trop Sao Paulo 59:e13. 10.1590/S1678-9946201759013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Wood L, Miedzinski L. 1996. Skeletal cryptococcosis: case report and review of the literature. Can J Infect Dis 7:125–132. 10.1155/1996/102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Behrman RE, Masci JR, Nicholas P. 1990. Cryptococcal skeletal infections: case report and review. Rev Infect Dis 12:181–190. 10.1093/clinids/12.2.181. [DOI] [PubMed] [Google Scholar]
- 791.Zhou HX, Lu L, Chu T, Wang T, Cao D, Li F, Ning G, Feng S. 2014. Skeletal cryptococcosis from 1977 to 2013. Front Microbiol 5:740. 10.3389/fmicb.2014.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Ndimbie OK, Dekker A, Martinez AJ, Dixon B. 1994. Prostatic sequestration of Cryptococcus neoformans in immunocompromised persons treated for cryptococcal meningoencephalitis. Histol Histopathol 9:643–648. [PubMed] [Google Scholar]
- 793.Ahn JH, Park C, Lee CW, Kim YC. 2017. Cryptococcal osteomyelitis of the first metatarsal head in an immunocompetent patient. J Am Podiatr Med Assoc 107:248–252. 10.7547/16-067. [DOI] [PubMed] [Google Scholar]
- 794.Burch KH, Fine G, Quinn EL, Eisses JF. 1975. Cryptococcus neoformans as a cause of lytic bone lesions. JAMA 231:1057–1059. 10.1001/jama.1975.03240220037017. [DOI] [PubMed] [Google Scholar]
- 795.Joo HS, Ha JK, Hwang CJ, Lee DH, Lee CS, Cho JH. 2015. Lumbar cryptococcal osteomyelitis mimicking metastatic tumor. Asian Spine J 9:798–802. 10.4184/asj.2015.9.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Chen J, Liu S, Xiong Z, Yang Y, Tan X, Luo Q, Peng J, Chen H, Jiang Q. 2015. Cryptococcal infection of the femoral bone similar with pathologic features of vascular tumors: a case report and review of literature. Int J Clin Exp Pathol 8:8551–8554. [PMC free article] [PubMed] [Google Scholar]
- 797.McCarthy MW, Walsh TJ. 2016. PCR methodology and applications for the detection of human fungal pathogens. Expert Rev Mol Diagn 16:1025–1036. 10.1080/14737159.2016.1219253. [DOI] [PubMed] [Google Scholar]
- 798.Abassi M, Boulware DR, Rhein J. 2015. Cryptococcal meningitis: diagnosis and management update. Curr Trop Med Rep 2:90–99. 10.1007/s40475-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Nathan CF. 1974. Cryptococcal osteomyelitis treated with 5-fluorocytosine. Am Rev Respir Dis 110:78–81. [DOI] [PubMed] [Google Scholar]
- 800.Murphy SN, Parnell N. 2005. Fluconazole treatment of cryptococcal rib osteomyelitis in an HIV-negative man. A case report and review of the literature. J Infect 51:e309–e311. 10.1016/j.jinf.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 801.Zhang Y, Yu YS, Tang ZH, Zang GQ. 2012. Cryptococcal osteomyelitis of the scapula and rib in an immunocompetent patient. Med Mycol 50:751–755. 10.3109/13693786.2012.670277. [DOI] [PubMed] [Google Scholar]
- 802.McCarthy MW, Kontoyiannis DP, Cornely OA, Perfect JR, Walsh TJ. 2017. Novel agents and drug targets to meet the challenges of resistant fungi. J Infect Dis 216:S474–S483. 10.1093/infdis/jix130. [DOI] [PubMed] [Google Scholar]
- 803.Hector RF, Laniado-Laborin R. 2005. Coccidioidomycosis—a fungal disease of the Americas. PLoS Med 2:e2. 10.1371/journal.pmed.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Teixeira MM, Barker BM. 2016. Use of population genetics to assess the ecology, evolution, and population structure of Coccidioides. Emerg Infect Dis 22:1022–1030. 10.3201/eid2206.151565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Rosenstein NE, Emery KW, Werner SB, Kao A, Johnson R, Rogers D, Vugia D, Reingold A, Talbot R, Plikaytis BD, Perkins BA, Hajjeh RA. 2001. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995-1996. Clin Infect Dis 32:708–715. 10.1086/319203. [DOI] [PubMed] [Google Scholar]
- 806.Adam RD, Elliott SP, Taljanovic MS. 2009. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med 122:770–777. 10.1016/j.amjmed.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 807.Sondermeyer GL, Lee LA, Gilliss D, Vugia DJ. 2016. Coccidioidomycosis-associated deaths in California, 2000-2013. Public Health Rep 131:531–535. 10.1177/0033354916662210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Dimitrova D, Ross L. 2016. Coccidioidomycosis: experience from a children’s hospital in an area of endemicity. J Pediatric Infect Dis Soc 5:89–92. 10.1093/jpids/piu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.McCarty JM, Demetral LC, Dabrowski L, Kahal AK, Bowser AM, Hahn JE. 2013. Pediatric coccidioidomycosis in central California: a retrospective case series. Clin Infect Dis 56:1579–1585. 10.1093/cid/cit114. [DOI] [PubMed] [Google Scholar]
- 810.Arnold MG, Arnold JC, Bloom DC, Brewster DF, Thiringer JK. 2004. Head and neck manifestations of disseminated coccidioidomycosis. Laryngoscope 114:747–752. 10.1097/00005537-200404000-00029. [DOI] [PubMed] [Google Scholar]
- 811.Kushwaha VP, Shaw BA, Gerardi JA, Oppenheim WL. 1996. Musculoskeletal coccidioidomycosis. A review of 25 cases. Clin Orthop Relat Res 332:190–199. 10.1097/00003086-199611000-00026. [DOI] [PubMed] [Google Scholar]
- 812.Waterman BR, Waterman SM, McCoy AC, Cameron CD. 16 April 2010. Coccidioidal osteomyelitis of the patella. Orthopedics . 10.3928/01477447-20100225-30. [DOI] [PubMed]
- 813.Bickel KD, Press BH, Hovey LM. 1993. Successful treatment of coccidioidomycosis osteomyelitis in an infant. Ann Plast Surg 30:462–465. 10.1097/00000637-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 814.Bried JM, Speer DP, Shehab ZM. 1987. Coccidioides immitis osteomyelitis in a 12-month-old child. J Pediatr Orthop 7:328–330. 10.1097/01241398-198705000-00016. [DOI] [PubMed] [Google Scholar]
- 815.Homans JD, Spencer L. 2010. Itraconazole treatment of nonmeningeal coccidioidomycosis in children: two case reports and review of the literature. Pediatr Infect Dis J 29:65–67. 10.1097/INF.0b013e3181b20ebd. [DOI] [PubMed] [Google Scholar]
- 816.Sheppard JE, Switlick DN. 2008. Coccidioides immitis osteomyelitis of the radius presenting as Ewing’s sarcoma. Orthopedics 31:607. [PubMed] [Google Scholar]
- 817.Sieber OF, Jr, Larter W, Smith PJ, Crowe P, Jr, Pitt M. 1977. Coccidioides immitis osteomyelitis of the mandible in an infant. J Oral Surg 35:721–725. [PubMed] [Google Scholar]
- 818.Thorpe CD, Spjut HJ. 1985. Coccidioidal osteomyelitis in a child’s finger. A case report. J Bone Joint Surg Am 67:330–332. [PubMed] [Google Scholar]
- 819.Gillespie R. 1986. Treatment of cranial osteomyelitis from disseminated coccidioidomycosis. West J Med 145:694–697. [PMC free article] [PubMed] [Google Scholar]
- 820.Islam S, Hsieh CJ, Lee BP. 2012. A teenage male with acute worsening of chronic back pain. Disseminated coccidioidomycosis. Pediatr Infect Dis J 31:1209, 1214–1215. 10.1097/INF.0b013e31825c6f9a. [DOI] [PubMed] [Google Scholar]
- 821.Rammaert B, Gamaletsou MN, Zeller V, Elie C, Prinapori R, Taj-Aldeen SJ, Roilides E, Kontoyiannis DP, Brause B, Sipsas NV, Walsh TJ, Lortholary O. 2014. Dimorphic fungal osteoarticular infections. Eur J Clin Microbiol Infect Dis 33:2131–2140. 10.1007/s10096-014-2149-0. [DOI] [PubMed] [Google Scholar]
- 822.Bried JM, Galgiani JN. 1986. Coccidioides immitis infections in bones and joints. Clin Orthop Relat Res 211:235–243. [PubMed] [Google Scholar]
- 823.Torres-Nájera M, de la Garza-Galván S, Cerda-Flores RM, Nocedal-Rustrián FC, Calderón-Garcidueñas AL. 2006. Osteoarticular coccidioidomycosis. Clinical and pathological study of 36 Mexican patients. Rev Invest Clin 58:211–216. (In Spanish.) [PubMed] [Google Scholar]
- 824.Szeyko LA, Taljanovic MS, Dzioba RB, Rapiejko JL, Adam RD. 2012. Vertebral coccidioidomycosis: presentation and multidisciplinary management. Am J Med 125:304–314. 10.1016/j.amjmed.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 825.Zeppa MA, Laorr A, Greenspan A, McGahan JP, Steinbach LS. 1996. Skeletal coccidioidomycosis: imaging findings in 19 patients. Skeletal Radiol 25:337–343. 10.1007/s002560050092. [DOI] [PubMed] [Google Scholar]
- 826.Brown J, Benedict K, Park BJ, Thompson GR. 2013. Coccidioidomycosis: epidemiology. Clin Epidemiol 5:185–197. 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Odio CD, Marciano BE, Galgiani JN, Holland SM. 2017. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 23:308–311. 10.3201/eid2302.160505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Holt CD, Winston DJ, Kubak B, Imagawa DK, Martin P, Goldstein L, Olthoff K, Millis JM, Shaked A, Shackleton CR, Busuttil RW. 1997. Coccidioidomycosis in liver transplant patients. Clin Infect Dis 24:216–221. 10.1093/clinids/24.2.216. [DOI] [PubMed] [Google Scholar]
- 829.Blair JE. 2004. Coccidioidal pneumonia, arthritis, and soft-tissue infection after kidney transplantation. Transpl Infect Dis 6:74–76. 10.1111/j.1399-3062.2004.00058.x. [DOI] [PubMed] [Google Scholar]
- 830.Yoshino MT, Hillman BJ, Galgiani JN. 1987. Coccidioidomycosis in renal dialysis and transplant patients: radiologic findings in 30 patients. AJR Am J Roentgenol 149:989–992. 10.2214/ajr.149.5.989. [DOI] [PubMed] [Google Scholar]
- 831.Antony SJ, Parikh MS, Friedman G. 2015. Coccidioidomycosis involving the cranium: a case report and review of current literature. Infect Disord Drug Targets 15:202–206. 10.2174/1871526515666150724101823. [DOI] [PubMed] [Google Scholar]
- 832.El Abd OH, Fusco HN, Gomba L, Lew M, Jenis L. 2012. Coccidioidomycosis infection presenting with thoracic spinal pain. PM R 4:450–455. 10.1016/j.pmrj.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 833.Fishco WD, Blocher KS. 2000. Disseminated coccidioidomycosis masquerading as tendinitis. J Am Podiatr Med Assoc 90:508–511. 10.7547/87507315-90-10-508. [DOI] [PubMed] [Google Scholar]
- 834.Acree T, Abreo F, Bagby J. 1998. Coccidioidomycosis of the knee diagnosed by fine-needle aspiration: a case report. Diagn Cytopathol 19:110–112. . [DOI] [PubMed] [Google Scholar]
- 835.Ellerbrook L, Laks S. 2015. Coccidioidomycosis osteomyelitis of the knee in a 23-year-old diabetic patient. Radiol Case Rep 10:1034. 10.2484/rcr.v10i1.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.Del Rocío Reyes-Montes M, Pérez-Huitrón MA, Ocaña-Monroy JL, Frías-De-León MG, Martínez-Herrera E, Arenas R, Duarte-Escalante E. 2016. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infect Dis 16:550. 10.1186/s12879-016-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Galgiani JN, Hayden R, Payne CM. 1982. Leukocyte effects on the dimorphism of Coccidioides immitis. J Infect Dis 146:56–63. 10.1093/infdis/146.1.56. [DOI] [PubMed] [Google Scholar]
- 838.Reach P, Paugam A, Kahan A, Allanore Y, Wipff J. 2010. Coccidioidomycosis of the spine in an immunocompetent patient. Joint Bone Spine 77:611–613. 10.1016/j.jbspin.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 839.Arora NP, Taneja V, ReyesSacin C, Bhanot R, Natesan SK. 2012. Coccidioidomycosis masquerading as malignancy. BMJ Case Rep 2012:bcr1220115357. 10.1136/bcr.12.2011.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Arbeloa-Gutierrez L, Kuberski T, Johnson SM, Sagastibelza I, Alaez JI, Pappagianis D. 2016. Reactivation of coccidioidomycosis: a prosthetic joint infection in Spain. Eur J Clin Microbiol Infect Dis 35:183–186. 10.1007/s10096-015-2526-3. [DOI] [PubMed] [Google Scholar]
- 841.Austen S, van der Weegen W, Verduin CM, van der Valk M, Hoekstra HJ. 2013. Coccidioidomycosis infection of a total knee arthroplasty in a nonendemic region. J Arthroplasty 28:375.e13–375.e15. 10.1016/j.arth.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 842.Capoor MR, Sen B, Varshney P, Verghese M, Shivaprakash MR, Chakrabarti A. 2014. Coccidioidomycosis masquerading as skeletal tuberculosis: an imported case and review of coccidioidomycosis in India. Trop Doct 44:25–28. 10.1177/0049475513512641. [DOI] [PubMed] [Google Scholar]
- 843.Drutz DJ, Catanzaro A. 1978. Coccidioidomycosis. Part II. Am Rev Respir Dis 117:727–771. [DOI] [PubMed] [Google Scholar]
- 844.Greenman R, Becker J, Campbell G, Remington J. 1975. Coccidioidal synovitis of the knee. Arch Intern Med 135:526–530. [PubMed] [Google Scholar]
- 845.Ho L, Schnall S, Schiller F, Holtom P. 2011. Metacarpal coccidioidal osteomyelitis. Am J Orthop (Belle Mead NJ) 40:34–36. [PubMed] [Google Scholar]
- 846.Rettig AC, Evanski PM, Waugh TR, Prietto CA. 1978. Primary coccidioidal synovitis of the knee: a report of four cases and review of the literature. Clin Orthop Relat Res 132:187–192. [PubMed] [Google Scholar]
- 847.Horsburgh CR, Jr, Cannady PB, Jr, Kirkpatrick CH. 1983. Treatment of fungal infections in the bones and joints with ketoconazole. J Infect Dis 147:1064–1069. 10.1093/infdis/147.6.1064. [DOI] [PubMed] [Google Scholar]
- 848.Mishra DD, Mohanty A. 1991. Coccidioidal osteomyelitis of a metatarsal. A case report. Int Orthop 15:323–324. 10.1007/BF00186870. [DOI] [PubMed] [Google Scholar]
- 849.Taxy JB, Kodros S. 2005. Musculoskeletal coccidioidomycosis: unusual sites of disease in a nonendemic area. Am J Clin Pathol 124:693–696. 10.1309/KRNY-U4RN-7Q12-WEYD. [DOI] [PubMed] [Google Scholar]
- 850.Caraway NP, Fanning CV, Stewart JM, Tarrand JJ, Weber KL. 2003. Coccidioidomycosis osteomyelitis masquerading as a bone tumor. A report of 2 cases. Acta Cytol 47:777–782. 10.1159/000326605. [DOI] [PubMed] [Google Scholar]
- 851.Ho AK, Shrader MW, Falk MN, Segal LS. 2014. Diagnosis and initial management of musculoskeletal coccidioidomycosis in children. J Pediatr Orthop 34:571–577. 10.1097/BPO.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 852.Kakarla UK, Kalani MYS, Sharma GK, Sonntag VKH, Theodore N. 2011. Surgical management of coccidioidomycosis of the spine: clinical article. J Neurosurg Spine 15:441–446. 10.3171/2011.5.SPINE10596. [DOI] [PubMed] [Google Scholar]
- 853.Koutserimpas C, Naoum S, Raptis K, Vrioni G, Samonis G, Alpantaki K. 2022. Skeletal infections caused by Coccidioides species. Diagnostics (Basel) 12:714. 10.3390/diagnostics12030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Hammoudeh ZS, Lettieri S. 2016. Management of temporomandibular joint coccidioidomycosis. Cranio 34:402–405. 10.1080/08869634.2015.1106812. [DOI] [PubMed] [Google Scholar]
- 855.Li Y-C, Calvert G, Hanrahan CJ, Jones KB, Randall RL. 2014. Coccidiomycosis infection of the patella mimicking a neoplasm—two case reports. BMC Med Imaging 14:8. 10.1186/1471-2342-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Sandoval JJ, Shank JR, Morgan SJ, Agudelo JF, Price CS. 2006. Midfoot coccidioidal osteomyelitis. A case report and review of the literature. J Bone Joint Surg Am 88:861–865. 10.2106/JBJS.E.00176. [DOI] [PubMed] [Google Scholar]
- 857.Stein SR, Leukens CA, Bagg RJ. 1975. Treatment of coccidioidomycosis infection of bone with local amphotericin B suction-irrigation. Report of a case. Clin Orthop Relat Res 108:161–164. 10.1097/00003086-197505000-00025. [DOI] [PubMed] [Google Scholar]
- 858.Khalid A, Boken DJ, Nelson CA, Totten VY. 2017. A case of osteomyelitis of the toe caused by coccidioidomycosis in a 17 year-old with diabetes insipidus. IDCases 9:14–16. 10.1016/j.idcr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859.Bernreuter WK. 1989. Coccidioidomycosis of bone: a sequela of desert rheumatism. Arthritis Rheum 32:1608–1610. 10.1002/anr.1780321219. [DOI] [PubMed] [Google Scholar]
- 860.McGahan JP, Graves DS, Palmer PE. 1980. Coccidioidal spondylitis: usual and unusual radiographic manifestations. Radiology 136:5–9. 10.1148/radiology.136.1.7384522. [DOI] [PubMed] [Google Scholar]
- 861.McConnell MF, Shi A, Lasco TM, Yoon L. 2017. Disseminated coccidioidomycosis with multifocal musculoskeletal disease involvement. Radiol Case Rep 12:141–145. 10.1016/j.radcr.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Copeland B, White D, Buenting J. 2003. Coccidioidomycosis of the head and neck. Ann Otol Rhinol Laryngol 112:98–101. 10.1177/000348940311200118. [DOI] [PubMed] [Google Scholar]
- 863.Delaney P, Niemann B. 1982. Spinal cord compression by Coccidioides immitis abscess. Arch Neurol 39:255–256. 10.1001/archneur.1982.00510160061015. [DOI] [PubMed] [Google Scholar]
- 864.Mirochnik BD, Lev S, Weingarten EP. 2014. Case 209: disseminated coccidioidal spondylodiskitis. Radiology 272:914–918. 10.1148/radiol.14112328. [DOI] [PubMed] [Google Scholar]
- 865.Elgafy H, Miller J, Meyers S, Assaly R. 2014. Disseminated coccidioidomycosis of the spine in an immunocompetent patient. Am J Orthop (Belle Mead NJ) 43:E181–E184. [PubMed] [Google Scholar]
- 866.Westphal SA. 1998. Disseminated coccidioidomycosis associated with hypercalcemia. Mayo Clin Proc 73:893–894. 10.4065/73.9.893. [DOI] [PubMed] [Google Scholar]
- 867.Prabhu RM, Bonnell M, Currier BL, Orenstein R. 2004. Successful treatment of disseminated nonmeningeal coccidioidomycosis with voriconazole. Clin Infect Dis 39:e74–e77. 10.1086/424019. [DOI] [PubMed] [Google Scholar]
- 868.Santos GH, Cook WA. 1972. Vertebral coccidioidomycosis: unusual polymorphic disease. N Y State J Med 72:2784–2785. [PubMed] [Google Scholar]
- 869.Wesselius LJ, Brooks RJ, Gall EP. 1977. Vertebral coccidioidomycosis presenting as Pott’s disease. JAMA 238:1397–1398. 10.1001/jama.1977.03280140075028. [DOI] [PubMed] [Google Scholar]
- 870.Wilde GE, Emery C, Lally JF. 2008. Radiological reasoning: miliary disease, vertebral osteomyelitis, and soft-tissue abscesses. AJR Am J Roentgenol 190:S11–S17. 10.2214/AJR.07.7013. [DOI] [PubMed] [Google Scholar]
- 871.Alfreijat M, Wilhelmi B. 2017. A case of a positive Coccidioides stool culture in an immunocompetent patient with disseminated coccidioidomycosis. IDCases 8:89–91. 10.1016/j.idcr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Verghese S, Arjundas D, Krishnakumar KC, Padmaja P, Elizabeth D, Padhye AA, Warnock DW. 2002. Coccidioidomycosis in India: report of a second imported case. Med Mycol 40:307–309. 10.1080/mmy.40.3.307.309. [DOI] [PubMed] [Google Scholar]
- 873.Herron LD, Kissel P, Smilovitz D. 1997. Treatment of coccidioidal spinal infection: experience in 16 cases. J Spinal Disord 10:215–222. [PubMed] [Google Scholar]
- 874.Holley K, Muldoon M, Tasker S. 2002. Coccidioides immitis osteomyelitis: a case series review. Orthopedics 25:827–832. 10.3928/0147-7447-20020801-13. [DOI] [PubMed] [Google Scholar]
- 875.Foerter J, Sundell J, Vroman P. 2016. PET/CT: first-line examination to assess disease extent of disseminated coccidioidomycosis. J Nucl Med Technol 44:212–213. 10.2967/jnmt.115.170449. [DOI] [PubMed] [Google Scholar]
- 876.Bayer AS, Yoshikawa TT, Galpin JE, Guze LB. 1976. Unusual syndromes of coccidioidomycosis: diagnostic and therapeutic considerations; a report of 10 cases and review of the English literature. Medicine (Baltimore) 55:131–152. 10.1097/00005792-197603000-00003. [DOI] [PubMed] [Google Scholar]
- 877.Baddley JW, Cobbs CS, Pappas PG. 2004. Surgical treatment of multiple skull abscesses associated with coccidioidomycosis. Mycoses 47:69–71. 10.1046/j.0933-7407.2003.00938.x. [DOI] [PubMed] [Google Scholar]
- 878.Lee JC, Catanzaro A, Parthemore JG, Roach B, Deftos LJ. 1977. Hypercalcemia in disseminated coccidioidomycosis. N Engl J Med 297:431–433. 10.1056/NEJM197708252970808. [DOI] [PubMed] [Google Scholar]
- 879.Martinez-Del-Campo E, Kalb S, Rangel-Castilla L, Moon K, Moran A, Gonzalez O, Soriano-Baron H, Theodore N. 2017. Spinal coccidioidomycosis: a current review of diagnosis and management. World Neurosurg 108:69–75. 10.1016/j.wneu.2017.08.103. [DOI] [PubMed] [Google Scholar]
- 880.Dubey D, Narayan RN, Motiwala A, Gupta P. 2014. Teaching NeuroImages. Spherules in spine: vertebral coccidioidomycosis. Neurology 83:e158. 10.1212/WNL.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 881.Bried JM, Benjamin JB, Galgiani JN. 1990. Coccidioides immitis: an unusual presentation. Orthopedics 13:345–347. 10.3928/0147-7447-19900301-14. [DOI] [PubMed] [Google Scholar]
- 882.Lantz B, Selakovich WG, Collins DN, Garvin KL. 1988. Coccidioidomycosis of the knee with a 26-year follow-up evaluation. A case report. Clin Orthop Relat Res 234:183–187. [PubMed] [Google Scholar]
- 883.Sung JP, Grendahl JG, Levine HB. 1977. Intravenous and intrathecal miconazole therapy for systemic mycoses. West J Med 126:5–13. [PMC free article] [PubMed] [Google Scholar]
- 884.Wascher DC, Hartman GP, Salka C, Mertz GJ. 1998. Coccidiomycosis presenting as a popliteal cyst. Arthroscopy 14:99–102. 10.1016/s0749-8063(98)70130-6. [DOI] [PubMed] [Google Scholar]
- 885.Bayer AS, Guze LB. 1979. Fungal arthritis. II. Coccidioidal synovitis: clinical, diagnostic, therapeutic, and prognostic considerations. Semin Arthritis Rheum 8:200–211. 10.1016/s0049-0172(79)80008-6. [DOI] [PubMed] [Google Scholar]
- 886.Cloninger P, Thrupp LD, Granger GA, Novey HS. 1974. Immunotherapy with transfer factor in disseminated coccidioidal osteomyelitis and arthritis. West J Med 120:322–325. [PMC free article] [PubMed] [Google Scholar]
- 887.Kumar KSS, Narasimhan A, Gopalakrishnan R, Geetha N, Thirunarayanan MA, Suryanarayanan P. 2011. Coccidioidomycosis in Chennai. J Assoc Physicians India 59:122–124. [PubMed] [Google Scholar]
- 888.Dimitrova D, Mason WH, Shaham B. 2014. Monoarticular coccidioidal synovitis in a pediatric patient. Pediatr Infect Dis J 33:100–102. 10.1097/01.inf.0000438261.02917.49. [DOI] [PubMed] [Google Scholar]
- 889.Winter WG, Jr, Larson RK, Honeggar MM, Jacobsen DT, Pappagianis D, Huntington RW, Jr.. 1975. Coccidioidal arthritis and its treatment—1975. J Bone Joint Surg Am 57:1152–1157. [PubMed] [Google Scholar]
- 890.Al-Ani M, Parperis KM. 2016. Erosive monoarthritis of the wrist secondary to Coccidioides immitis infection. Arthritis Rheumatol 68:1550. 10.1002/art.39625. [DOI] [PubMed] [Google Scholar]
- 891.Pankovich AM, Jevtic MM. 1973. Coccidioidal infection of the hip. A case report. J Bone Joint Surg Am 55:1525–1528. [PubMed] [Google Scholar]
- 892.Sprinkle RL, Kosova LM, Tougas T, Morales LM, DeUgarte R. 1989. Disseminated coccidioidomycosis of an ankle joint. A case study. J Am Podiatr Med Assoc 79:300–305. 10.7547/87507315-79-6-300. [DOI] [PubMed] [Google Scholar]
- 893.Akin JR. 2001. Diagnostic dilemma. Musculoskeletal coccidioidomycosis involving the left wrist. Am J Med 111:236, 239. 10.1016/S0002-9343(01)00801-4. [DOI] [PubMed] [Google Scholar]
- 894.Kuberski T, Ianas V, Ferguson T, Nomura J, Johnson R. 2011. Treatment of prosthetic joint infections associated with coccidioidomycosis. Infect Dis Clin Pract 19:252–255. 10.1097/IPC.0b013e31820fc869. [DOI] [Google Scholar]
- 895.Blair JE. 2007. State-of-the-art treatment of coccidioidomycosis skeletal infections. Ann N Y Acad Sci 1111:422–433. 10.1196/annals.1406.000. [DOI] [PubMed] [Google Scholar]
- 896.Taljanovic MS, Adam RD. 2011. Musculoskeletal coccidioidomycosis. Semin Musculoskelet Radiol 15:511–526. 10.1055/s-0031-1293497. [DOI] [PubMed] [Google Scholar]
- 897.Dalinka MK, Greendyke WH. 1971. The spinal manifestations of coccidioidomycosis. J Can Assoc Radiol 22:93–99. [PubMed] [Google Scholar]
- 898.Armbuster TG, Goergen TG, Resnick D, Catanzaro A. 1977. Utility of bone scanning in disseminated coccidioidomycosis: case report. J Nucl Med 18:450–454. [PubMed] [Google Scholar]
- 899.Dryden JR, Starsiak MD, Johnston MJ, Silverman ED. 2017. Bone scan, PET-CT, and MRI in disseminated coccidioidomycosis. Clin Nucl Med 42:319–322. 10.1097/RLU.0000000000001570. [DOI] [PubMed] [Google Scholar]
- 900.Moreno AJ, Weisman IM, Rodriguez AA, Henry CD, Turnbull GL. 1987. Nuclear imaging in coccidioidal osteomyelitis. Clin Nucl Med 12:604–609. 10.1097/00003072-198708000-00005. [DOI] [PubMed] [Google Scholar]
- 901.Olson EM, Duberg AC, Herron LD, Kissel P, Smilovitz D. 1998. Coccidioidal spondylitis: MR findings in 15 patients. AJR Am J Roentgenol 171:785–789. 10.2214/ajr.171.3.9725317. [DOI] [PubMed] [Google Scholar]
- 902.Lund PJ, Chan KM, Unger EC, Galgiani TN, Pitt MJ. 1996. Magnetic resonance imaging in coccidioidal arthritis. Skeletal Radiol 25:661–665. 10.1007/s002560050154. [DOI] [PubMed] [Google Scholar]
- 903.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, Johnson RH, Kusne S, Lisse J, MacDonald JD, Meyerson SL, Raksin PB, Siever J, Stevens DA, Sunenshine R, Theodore N. 2016. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 63:E112–E146. 10.1093/cid/ciw360. [DOI] [PubMed] [Google Scholar]
- 904.Galgiani JN, Catanzaro A, Cloud GA, Johnson RH, Williams PL, Mirels LF, Nassar F, Lutz JE, Stevens DA, Sharkey PK, Singh VR, Larsen RA, Delgado KL, Flanigan C, Rinaldi MG. 2000. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann Intern Med 133:676–686. 10.7326/0003-4819-133-9-200011070-00009. [DOI] [PubMed] [Google Scholar]
- 905.Catanzaro A, Cloud GA, Stevens DA, Levine BE, Williams PL, Johnson RH, Rendon A, Mirels LF, Lutz JE, Holloway M, Galgiani JN. 2007. Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis. Clin Infect Dis 45:562–568. 10.1086/519937. [DOI] [PubMed] [Google Scholar]
- 906.Kim MM, Vikram HR, Kusne S, Seville MT, Blair JE. 2011. Treatment of refractory coccidioidomycosis with voriconazole or posaconazole. Clin Infect Dis 53:1060–1066. 10.1093/cid/cir642. [DOI] [PubMed] [Google Scholar]
- 907.Stevens DA, Rendon A, Gaona-Flores V, Catanzaro A, Anstead GM, Pedicone L, Graybill JR. 2007. Posaconazole therapy for chronic refractory coccidioidomycosis. Chest 132:952–958. 10.1378/chest.07-0114. [DOI] [PubMed] [Google Scholar]
- 908.Galgiani JN, Stevens DA, Graybill JR, Dismukes WE, Cloud GA. 1988. Ketoconazole therapy of progressive coccidioidomycosis. Comparison of 400- and 800-mg doses and observations at higher doses. Am J Med 84:603–610. 10.1016/0002-9343(88)90143-x. [DOI] [PubMed] [Google Scholar]
- 909.Kweon C, McLaren AC, Leon C, McLemore R. 2011. Amphotericin B delivery from bone cement increases with porosity but strength decreases. Clin Orthop Relat Res 469:3002–3007. 10.1007/s11999-011-1928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 910.Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. 2009. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother 43:1606–1615. 10.1345/aph.1M143. [DOI] [PubMed] [Google Scholar]
- 911.San-Blas G, Nino-Vega G, Iturriaga T. 2002. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med Mycol 40:225–242. 10.1080/mmy.40.3.225.242. [DOI] [PubMed] [Google Scholar]
- 912.López-Martínez R, Hernández-Hernández F, Méndez-Tovar LJ, Manzano-Gayosso P, Bonifaz A, Arenas R, Padilla-Desgarennes MDC, Estrada R, Chávez G. 2014. Paracoccidioidomycosis in Mexico: clinical and epidemiological data from 93 new cases (1972-2012). Mycoses 57:525–530. 10.1111/myc.12190. [DOI] [PubMed] [Google Scholar]
- 913.Teixeira MDM, Theodoro RC, de Oliveira FFM, Machado GC, Hahn RC, Bagagli E, San-Blas G, Soares Felipe MS. 2014. Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol 52:19–28. 10.3109/13693786.2013.794311. [DOI] [PubMed] [Google Scholar]
- 914.Marques SA. 2013. Paracoccidioidomycosis: epidemiological, clinical, diagnostic and treatment up-dating. An Bras Dermatol 88:700–711. 10.1590/abd1806-4841.20132463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Corredor GG, Castano JH, Peralta LA, Diez S, Arango M, McEwen J, Restrepo A. 1999. Isolation of Paracoccidioides brasiliensis from the nine-banded armadillo Dasypus novemcinctus, in an endemic area for paracoccidioidomycosis in Colombia. Rev Iberoam Micol 16:216–220. [PubMed] [Google Scholar]
- 916.Nishikaku AS, Peracoli MTS, Bagagli E, Sugizaki MF, Sartori A. 2008. Experimental infections with Paracoccidioides brasiliensis obtained from armadillos: comparison to clinical isolates. Braz J Infect Dis 12:57–62. 10.1590/s1413-86702008000100013. [DOI] [PubMed] [Google Scholar]
- 917.Arantes TD, Theodoro RC, Teixeira MDM, Bosco SDMG, Bagagli E. 2016. Environmental mapping of Paracoccidioides spp. in Brazil reveals new clues into genetic diversity, biogeography and wild host association. PLoS Negl Trop Dis 10:e0004606. 10.1371/journal.pntd.0004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Barrozo LV, Benard G, Silva ME, Bagagli E, Marques SA, Mendes RP. 2010. First description of a cluster of acute/subacute paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl Trop Dis 4:e643. 10.1371/journal.pntd.0000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 919.Barrozo LV, Mendes RP, Marques SA, Benard G, Silva ME, Bagagli E. 2009. Climate and acute/subacute paracoccidioidomycosis in a hyper-endemic area in Brazil. Int J Epidemiol 38:1642–1649. 10.1093/ije/dyp207. [DOI] [PubMed] [Google Scholar]
- 920.Paniago AMM, Aguiar JIA, Aguiar ES, da Cunha RV, Pereira GRDOL, Londero AT, Wanke B. 2003. Paracoccidioidomycosis: a clinical and epidemiological study of 422 cases observed in Mato Grosso do Sul. Rev Soc Bras Med Trop 36:455–459. (In Portuguese.) 10.1590/s0037-86822003000400004. [DOI] [PubMed] [Google Scholar]
- 921.Benard G, Orii NM, Marques HH, Mendonca M, Aquino MZ, Campeas AE, del Negro GB, Durandy A, Duarte AJ. 1994. Severe acute paracoccidioidomycosis in children. Pediatr Infect Dis J 13:510–515. 10.1097/00006454-199406000-00009. [DOI] [PubMed] [Google Scholar]
- 922.Pereira RM, Bucaretchi F, Barison EDM, Hessel G, Tresoldi AT. 2004. Paracoccidioidomycosis in children: clinical presentation, follow-up and outcome. Rev Inst Med Trop Sao Paulo 46:127–131. 10.1590/s0036-46652004000300002. [DOI] [PubMed] [Google Scholar]
- 923.Nogueira SA, Guedes AL, Wanke B, Capella S, Rodrigues K, Abreu TF, Morais JC, Lambert JS. 2001. Osteomyelitis caused by Paracoccidioides brasiliensis in a child from the metropolitan area of Rio de Janeiro. J Trop Pediatr 47:311–315. 10.1093/tropej/47.5.311. [DOI] [PubMed] [Google Scholar]
- 924.Silvestre MT, Ferreira MS, Borges AS, Rocha A, de Souza GM, Nishioka SA. 1997. Monoarthritis of the knee as an isolated manifestation of paracoccidioidomycosis. Rev Soc Bras Med Trop 30:393–395. (In Portuguese.) 10.1590/s0037-86821997000500008. [DOI] [PubMed] [Google Scholar]
- 925.Picado CHF, Garcia FL, Barbieri CH. 2003. Isolated hip infection by Paracoccidioides brasiliensis: a case report. Hip Int 13:193–195. 10.1177/112070000301300312. [DOI] [Google Scholar]
- 926.Da Rocha FF, Campos MG. 2007. Monoarthritis manifestations of paracoccidioidomycoses. Rev Panam Infectol 9:31–33. [Google Scholar]
- 927.Bellissimo-Rodrigues F, Bollela VR, Da Fonseca BAL, Martinez R. 2013. Endemic paracoccidioidomycosis: relationship between clinical presentation and patients’ demographic features. Med Mycol 51:313–318. 10.3109/13693786.2012.714529. [DOI] [PubMed] [Google Scholar]
- 928.Trad HS, Trad CS, Elias Junior J, Muglia VF. 2006. Radiological review of 173 consecutive cases of paracoccidioidomycosis. Radiol Bras 39:175–179. 10.1590/S0100-39842006000300005. [DOI] [Google Scholar]
- 929.Severo LC, Agostini AA, Londero AT. 1996. Bone involvement in chronic disseminated paracoccidioidomycosis. A report on the first cases in Rio Grande do Sul. Rev Soc Bras Med Trop 29:241–244. (In Portuguese.) 10.1590/s0037-86821996000300004. [DOI] [PubMed] [Google Scholar]
- 930.Nogueira MG, Andrade GM, Tonelli E. 2006. Clinical evolution of paracoccidioidomycosis in 38 children and teenagers. Mycopathologia 161:73–81. 10.1007/s11046-005-3653-7. [DOI] [PubMed] [Google Scholar]
- 931.Amstalden EM, Xavier R, Kattapuram SV, Bertolo MB, Swartz MN, Rosenberg AE. 1996. Paracoccidioidomycosis of bones and joints. A clinical, radiologic, and pathologic study of 9 cases. Medicine (Baltimore) 75:213–225. 10.1097/00005792-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 932.de Freitas RS, Dantas KC, Garcia RSP, Magri MMC, de Andrade HF, Jr.. 2010. Paracoccidioides brasiliensis causing a rib lesion in an adult AIDS patient. Hum Pathol 41:1350–1354. 10.1016/j.humpath.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 933.Alvarenga JA, Martins DE, Kanas M, Elizeche HG, Dell’Aquila AM, Fernandes EA, Wajchenberg M, Puertas EB. 2016. Paracoccidioidomycosis in the spine: case report and review of the literature. Sao Paulo Med J 134:263–267. 10.1590/1516-3180.2015.02691801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934.Marchiori E, Dalston M, Zanetti G, Hochhegger B. 2012. Paracoccidioidomycosis: another cause of sternal osteomyelitis. Joint Bone Spine 79:323–324. 10.1016/j.jbspin.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 935.Pereira PMR, Akel PBD, de Lima LL, Kimura EN, Jalkh AP. 2011. Multifocal paracoccidioidomycosis: a diagnostic challenge due to late cutaneous manifestation. An Bras Dermatol 86:149–152. 10.1590/S0365-05962011000100024. [DOI] [PubMed] [Google Scholar]
- 936.Bonilla-Abadia F, Velez JD, Zarate-Correa LC, Carrascal E, Guarin N, Castaneda-Ramirez CR, Canas CA. 2012. Overinfection by Paracoccidioides brasiliensis in gouty crystal arthritis. Case Rep Med 2012:128103. 10.1155/2012/128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Correa-de-Castro B, Pompilio MA, Odashiro DN, Odashiro M, Arao-Filho A, Paniago AM. 2012. Unifocal bone paracoccidioidomycosis, Brazil. Am J Trop Med Hyg 86:470–473. 10.4269/ajtmh.2012.11-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938.Miranda Aires E, Costa Alves CA, Ferreira AV, Moreira IM, Pappalardo MC, Peluso D, Silva RJ. 1997. Bone paracoccidioidomycosis in an HIV-positive patient. Braz J Infect Dis 1:260–265. [PubMed] [Google Scholar]
- 939.Castro G, Martinez R. 2006. Images in clinical medicine. Disseminated paracoccidioidomycosis and coinfection with HIV. N Engl J Med 355:2677. 10.1056/NEJMicm053465. [DOI] [PubMed] [Google Scholar]
- 940.Benard G, Duarte AJ. 2000. Paracoccidioidomycosis: a model for evaluation of the effects of human immunodeficiency virus infection on the natural history of endemic tropical diseases. Clin Infect Dis 31:1032–1039. 10.1086/318146. [DOI] [PubMed] [Google Scholar]
- 941.Morejon KM, Machado AA, Martinez R. 2009. Paracoccidioidomycosis in patients infected with and not infected with human immunodeficiency virus: a case-control study. Am J Trop Med Hyg 80:359–366. 10.4269/ajtmh.2009.80.359. [DOI] [PubMed] [Google Scholar]
- 942.Franco M, Bagagli E, Scapolio S, da Silva Lacaz C. 2000. A critical analysis of isolation of Paracoccidioides brasiliensis from soil. Med Mycol 38:185–191. 10.1080/mmy.38.3.185.191. [DOI] [PubMed] [Google Scholar]
- 943.Safe IP, do Valle FF, Maia DCC, Agonio B, Monte RL, Araujo JDR, Cordeiro-Santos M. 2014. Extra-pulmonary manifestations of paracoccidioidomycosis associated with acquired immunodeficiency syndrome: a case report. An Bras Dermatol 89:150–153. 10.1590/abd1806-4841.20142768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944.Castaneda OJ, Alarcon GS, Garcia MT, Lumbreras H. 1985. Paracoccidioides brasiliensis arthritis. Report of a case and review of the literature. J Rheumatol 12:356–358. [PubMed] [Google Scholar]
- 945.Rosario Filho NA, Telles Filho FQ, Costa O, Marinoni LP. 1985. Paracoccidioidomycosis in children with different skeletal involvement. Rev Inst Med Trop Sao Paulo 27:337–340. 10.1590/s0036-46651985000600007. [DOI] [PubMed] [Google Scholar]
- 946.Shankar J, Restrepo A, Clemons KV, Stevens DA. 2011. Hormones and the resistance of women to paracoccidioidomycosis. Clin Microbiol Rev 24:296–313. 10.1128/CMR.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947.Loth EA, Biazin SK, Paula CR, Simão RDCG, de Franco MF, Puccia R, Gandra RF. 2012. Experimental model of arthritis induced by Paracoccidioides brasiliensis in rats. Mycopathologia 174:187–191. 10.1007/s11046-012-9537-8. [DOI] [PubMed] [Google Scholar]
- 948.Loth EA, Biazim SK, Dos Santos JHFF, Puccia R, Brancalhão RC, Chasco LDF, Gandra RF, Simão RDCG, de Franco MF. 2014. Dose response effect of Paracoccidioides brasiliensis in an experimental model of arthritis. Rev Inst Med Trop Sao Paulo 56:259–264. 10.1590/s0036-46652014000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949.Wanke B, Aide MA. 2009. Chapter 6—paracoccidioidomycosis. J Bras Pneumol 35:1245–1249. 10.1590/s1806-37132009001200013. [DOI] [PubMed] [Google Scholar]
- 950.Mendonca JA, Peron Filho F, Schincariol NB, Vierhout CV, Provenza JR. 2016. Musculoskeletal ultrasound findings in paracoccidioidomycosis. Rev Bras Reumatol Engl Ed 56:75–78. 10.1016/j.rbre.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 951.Fulciniti F, Troncone G, Fazioli F, Vetrani A, Zeppa P, Manco A, Palombini L. 1996. Osteomyelitis by Paracoccidioides brasiliensis (South American blastomycosis): cytologic diagnosis on fine-needle aspiration biopsy smears. A case report. Diagn Cytopathol 15:442–446. . [DOI] [PubMed] [Google Scholar]
- 952.Lima Junior FVA, Savarese LG, Monsignore LM, Martinez R, Nogueira-Barbosa MH. 2015. Computed tomography findings of paracoccidiodomycosis in musculoskeletal system. Radiol Bras 48:1–6. 10.1590/0100-3984.2014.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 953.Michelan MS, Fernandes EDA, Freitas LF, Ribeiro RH, Milano MM, Monteiro SS. 2012. Osteomyelitis and pyoarthritis resulting from local paracoccidioidomycosis in an immunocompetent patient: a case report. J Med Case Rep 6:342. 10.1186/1752-1947-6-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Borgia G, Reynaud L, Cerini R, Ciampi R, Schioppa O, Dello Russo M, Gentile I, Piazza M. 2000. A case of paracoccidioidomycosis: experience with long-term therapy. Infection 28:119–120. 10.1007/s150100050060. [DOI] [PubMed] [Google Scholar]
- 955.Valera ET, Mori BM, Engel EE, Costa IS, Brandão DF, Nogueira-Barbosa MH, Queiroz RGDP, Silveira VDS, Scrideli CA, Tone LG. 2008. Fungal infection by Paracoccidioides brasiliensis mimicking bone tumor. Pediatr Blood Cancer 50:1284–1286. 10.1002/pbc.21499. [DOI] [PubMed] [Google Scholar]
- 956.Lambertucci JR, Botelho JS, Melo FH. 2002. Osteomyelitis by Paracoccidioides brasiliensis. Rev Soc Bras Med Trop 35:271–272. (In Portuguese.) 10.1590/s0037-86822002000300015. [DOI] [PubMed] [Google Scholar]
- 957.Milazzo LC, Veloso GA. 1992. Localized form of paracoccidioidomycosis in the spine. Rev Bras Ortop 27:150–152. [Google Scholar]
- 958.Picado CHF, Garcia FL, Marcondes CRR. 2006. Late outcome of Paracoccidioides brasiliensis isolated infection on the hip. Acta Ortop Bras 14:97–99. 10.1590/S1413-78522006000200008. [DOI] [Google Scholar]
- 959.Doria AS, Taylor GA. 1997. Bony involvement in paracoccidioidomycosis. Pediatr Radiol 27:67–69. 10.1007/s002470050067. [DOI] [PubMed] [Google Scholar]
- 960.Do Valle AC, Costa RL, Fialho Monteiro PC, Von Helder J, Muniz MM, Zancope-Oliveira RM. 2001. Interpretation and clinical correlation of serological tests in paracoccidioidomycosis. Med Mycol 39:373–377. 10.1080/mmy.39.4.373.377. [DOI] [PubMed] [Google Scholar]
- 961.Cano LE, Restrepo A. 1987. Predictive value of serologic tests in the diagnosis and follow-up of patients with paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo 29:276–283. 10.1590/s0036-46651987000500003. [DOI] [PubMed] [Google Scholar]
- 962.Vidal MS, Del Negro GM, Vicentini AP, Svidzinski TI, Mendes-Giannini MJ, Almeida AM, Martinez R, de Camargo ZP, Taborda CP, Benard G. 2014. Serological diagnosis of paracoccidioidomycosis: high rate of inter-laboratorial variability among medical mycology reference centers. PLoS Negl Trop Dis 8:e3174. 10.1371/journal.pntd.0003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Savarese LG, Monsignore LM, de Andrade Hernandes M, Martinez R, Nogueira-Barbosa MH. 2015. Magnetic resonance imaging findings of paracoccidioidomycosis in the musculoskeletal system. Trop Med Int Health 20:1346–1354. 10.1111/tmi.12552. [DOI] [PubMed] [Google Scholar]
- 964.Monsignore LM, Martinez R, Simao MN, Teixeira SR, Elias J, Jr, Nogueira-Barbosa MH. 2012. Radiologic findings of osteoarticular infection in paracoccidioidomycosis. Skeletal Radiol 41:203–208. 10.1007/s00256-011-1214-3. [DOI] [PubMed] [Google Scholar]
- 965.Shikanai-Yasuda MA, Telles Filho FDQ, Mendes RP, Colombo AL, Moretti ML. 2006. Guidelines in paracoccidioidomycosis. Rev Soc Bras Med Trop 39:297–310. (In Portuguese.) 10.1590/s0037-86822006000300017. [DOI] [PubMed] [Google Scholar]
- 966.Naranjo MS, Trujillo M, Munera MI, Restrepo P, Gomez I, Restrepo A. 1990. Treatment of paracoccidioidomycosis with itraconazole. J Med Vet Mycol 28:67–76. 10.1080/02681219080000091. [DOI] [PubMed] [Google Scholar]
- 967.Restrepo A, Gomez I, Robledo J, Patino MM, Cano LE. 1987. Itraconazole in the treatment of paracoccidioidomycosis: a preliminary report. Rev Infect Dis 9(Suppl 1):S51–S56. 10.1093/clinids/9.supplement_1.s51. [DOI] [PubMed] [Google Scholar]
- 968.Cavalcante RD, Sylvestre TF, Levorato AD, de Carvalho LR, Mendes RP. 2014. Comparison between itraconazole and cotrimoxazole in the treatment of paracoccidiodomycosis. PLoS Negl Trop Dis 8:e2793. 10.1371/journal.pntd.0002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969.Borges SRC, da Silva GMS, Chambela MDC, de Oliveira RDVC, Costa RLB, Wanke B, do Valle ACF. 2014. Itraconazole vs. trimethoprim-sulfamethoxazole: a comparative cohort study of 200 patients with paracoccidioidomycosis. Med Mycol 52:303–310. 10.1093/mmy/myt012. [DOI] [PubMed] [Google Scholar]
- 970.Hahn RC, Macedo AM, Fontes CJ, Batista RD, Santos NL, Hamdan JS. 2003. Randomly amplified polymorphic DNA as a valuable tool for epidemiological studies of Paracoccidioides brasiliensis. J Clin Microbiol 41:2849–2854. 10.1128/JCM.41.7.2849-2854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Queiroz-Telles F, Goldani LZ, Schlamm HT, Goodrich JM, Espinel-Ingroff A, Shikanai-Yasuda MA. 2007. An open-label comparative pilot study of oral voriconazole and itraconazole for long-term treatment of paracoccidioidomycosis. Clin Infect Dis 45:1462–1469. 10.1086/522973. [DOI] [PubMed] [Google Scholar]
- 972.Thompson GR, III, Rendon A, Ribeiro Dos Santos R, Queiroz-Telles F, Ostrosky-Zeichner L, Azie N, Maher R, Lee M, Kovanda L, Engelhardt M, Vazquez JA, Cornely OA, Perfect JR. 2016. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis 63:356–362. 10.1093/cid/ciw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Pecanha PM, de Souza S, Falqueto A, Grao-Veloso TR, Lirio LV, Ferreira CU, Jr, Santos AR, Costa HG, de Souza LR, Tuon FF. 2016. Amphotericin B lipid complex in the treatment of severe paracoccidioidomycosis: a case series. Int J Antimicrob Agents 48:428–430. 10.1016/j.ijantimicag.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 974.Benard G, Campos AF, Netto LC, Goncalves LG, Machado LR, Mimicos EV, Franca FO, Gryschek RC. 2012. Treatment of severe forms of paracoccidioidomycosis: is there a role for corticosteroids? Med Mycol 50:641–648. 10.3109/13693786.2011.654135. [DOI] [PubMed] [Google Scholar]
- 975.Gryschek RC, Pereira RM, Kono A, Patzina RA, Tresoldi AT, Shikanai-Yasuda MA, Benard G. 2010. Paradoxical reaction to treatment in 2 patients with severe acute paracoccidioidomycosis: a previously unreported complication and its management with corticosteroids. Clin Infect Dis 50:e56–e58. 10.1086/652290. [DOI] [PubMed] [Google Scholar]
- 976.Coutinho ZF, Wanke B, Travassos C, Oliveira RM, Xavier DR, Coimbra CEA. 2015. Hospital morbidity due to paracoccidioidomycosis in Brazil (1998-2006). Trop Med Int Health 20:673–680. 10.1111/tmi.12472. [DOI] [PubMed] [Google Scholar]
- 977.Saccente M, Woods GL. 2010. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev 23:367–381. 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978.Litvinov IV, St-Germain G, Pelletier R, Paradis M, Sheppard DC. 2013. Endemic human blastomycosis in Quebec, Canada, 1988-2011. Epidemiol Infect 141:1143–1147. 10.1017/S0950268812001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.St-Germain G, Murray G, Duperval R. 1993. Blastomycosis in Quebec (1981-90): report of 23 cases and review of published cases from Quebec. Can J Infect Dis 4:89–94. 10.1155/1993/249823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Oppenheimer M, Embil JM, Black B, Wiebe L, Limerick B, MacDonald K, Trepman E. 2007. Blastomycosis of bones and joints. South Med J 100:570–578. 10.1097/SMJ.0b013e3180487a92. [DOI] [PubMed] [Google Scholar]
- 981.Habte-Gabr E, Smith IM. 1973. North American blastomycosis in Iowa: review of 34 cases. J Chronic Dis 26:585–594. 10.1016/0021-9681(73)90063-5. [DOI] [PubMed] [Google Scholar]
- 982.Borchardt SM. 2005. Awareness of blastomycosis in Illinois needs to increase. Ill Infect Dis Rep I:1–2. [Google Scholar]
- 983.Proctor ME, Davis JP. 1996. Blastomycosis—Wisconsin, 1986-1995. From the Centers for Disease Control and Prevention. JAMA 276:444. [PubMed] [Google Scholar]
- 984.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. 2013. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One 8:e59237. 10.1371/journal.pone.0059237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Lemos LB, Guo M, Baliga M. 2000. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 4:391–406. 10.1053/adpa.2000.20755. [DOI] [PubMed] [Google Scholar]
- 986.Crampton TL, Light RB, Berg GM, Meyers MP, Schroeder GC, Hershfield ES, Embil JM. 2002. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis 34:1310–1316. 10.1086/340049. [DOI] [PubMed] [Google Scholar]
- 987.Choptiany M, Wiebe L, Limerick B, Sarsfield P, Cheang M, Light B, Hammond G, MacDonald K, Trepman E, Pappas P, Embil JM. 2009. Risk factors for acquisition of endemic blastomycosis. Can J Infect Dis Med Microbiol 20:117–121. 10.1155/2009/824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988.Kralt D, Light B, Cheang M, MacNair T, Wiebe L, Limerick B, Sarsfield P, Hammond G, MacDonald K, Trepman E, Embil JM. 2009. Clinical characteristics and outcomes in patients with pulmonary blastomycosis. Mycopathologia 167:115–124. 10.1007/s11046-008-9163-7. [DOI] [PubMed] [Google Scholar]
- 989.Frost HM, Anderson J, Ivacic L, Meece J. 2017. Blastomycosis in children: an analysis of clinical, epidemiologic, and genetic features. J Pediatric Infect Dis Soc 6:49–56. 10.1093/jpids/piv081. [DOI] [PubMed] [Google Scholar]
- 990.Carlos WG, Rose AS, Wheat LJ, Norris S, Sarosi GA, Knox KS, Hage CA. 2010. Blastomycosis in Indiana: digging up more cases. Chest 138:1377–1382. 10.1378/chest.10-0627. [DOI] [PubMed] [Google Scholar]
- 991.Grim SA, Proia L, Miller R, Alhyraba M, Costas-Chavarri A, Oberholzer J, Clark NM. 2012. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl Infect Dis 14:17–23. 10.1111/j.1399-3062.2011.00658.x. [DOI] [PubMed] [Google Scholar]
- 992.Gauthier GM, Safdar N, Klein BS, Andes DR. 2007. Blastomycosis in solid organ transplant recipients. Transpl Infect Dis 9:310–317. 10.1111/j.1399-3062.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 993.Pappas PG, Pottage JC, Powderly WG, Fraser VJ, Stratton CW, McKenzie S, Tapper ML, Chmel H, Bonebrake FC, Blum R, Shafer RW, King C, Dismukes WE. 1992. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med 116:847–853. 10.7326/0003-4819-116-10-847. [DOI] [PubMed] [Google Scholar]
- 994.Witzig RS, Hoadley DJ, Greer DL, Abriola KP, Hernandez RL. 1994. Blastomycosis and human immunodeficiency virus: three new cases and review. South Med J 87:715–719. 10.1097/00007611-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 995.Yocum J, Seligson D. 1991. Blastomycosis of the knee and skull after arthroscopy. Am J Sports Med 19:670–672. 10.1177/036354659101900622. [DOI] [PubMed] [Google Scholar]
- 996.Vanĕk J, Schwarz J, Hakim S. 1970. North American blastomycosis: a study of ten cases. Am J Clin Pathol 54:384–400. 10.1093/ajcp/54.3.384. [DOI] [PubMed] [Google Scholar]
- 997.Patel KR, Szczodry M, Neckrysh S, Siemionow K. 2015. Anterior cervical corpectomy and fusion for blastomycosis causing destruction of C6 vertebra: a case report. J Med Case Rep 9:271. 10.1186/s13256-015-0762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 998.Day SR, Weiss DB, Hazen KC, Moore CC. 2014. Successful treatment of osseous blastomycosis without pulmonary or disseminated disease and review of the literature. Diagn Microbiol Infect Dis 79:242–244. 10.1016/j.diagmicrobio.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 999.Emer JJ, Spear JB. 2009. Primary cutaneous blastomycosis as a cause of acute respiratory distress syndrom [sic]: case report and literature review. J Clin Aesthet Dermatol 2:22–30. [PMC free article] [PubMed] [Google Scholar]
- 1000.Meyer KC, McManus EJ, Maki DG. 1993. Overwhelming pulmonary blastomycosis associated with the adult respiratory distress syndrome. N Engl J Med 329:1231–1236. 10.1056/NEJM199310213291704. [DOI] [PubMed] [Google Scholar]
- 1001.Veligandla SR, Hinrichs SH, Rupp ME, Lien EA, Neff JR, Iwen PC. 2002. Delayed diagnosis of osseous blastomycosis in two patients following environmental exposure in nonendemic areas. Am J Clin Pathol 118:536–541. 10.1309/JEJ0-3N98-C3G8-21DE. [DOI] [PubMed] [Google Scholar]
- 1002.Fountain FF, Jr. 1973. Acute blastomycotic arthritis. Arch Intern Med 132:684–688. 10.1001/archinte.1973.03650110040008. [DOI] [PubMed] [Google Scholar]
- 1003.McClain CM, Van Horn GT, Chappell JD, Stratton CW. 2012. Coccidioides, Cryptococcus, or Blastomyces? A diagnostic dilemma encountered during frozen section evaluation. Pediatr Dev Pathol 15:71–75. 10.2350/11-01-0973-CR.1. [DOI] [PubMed] [Google Scholar]
- 1004.Jain R, Singh K, Lamzabi I, Harbhajanka A, Gattuso P, Reddy VB. 2014. Blastomycosis of bone: a clinicopathologic study. Am J Clin Pathol 142:609–616. 10.1309/AJCPG2CFGHZ4URLN. [DOI] [PubMed] [Google Scholar]
- 1005.Arvin MC, Gehring RL, Crecelius JL, Curfman MF. 1991. Man with progressive lower back pain. Indiana Med 84:554–556. [PubMed] [Google Scholar]
- 1006.Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. 1980. Vertebral North American blastomycosis. Surg Neurol 13:311–312. [PubMed] [Google Scholar]
- 1007.Guler N, Palanduz A, Ones U, Ozturk A, Somer A, Salman N, Yalcin I. 1995. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J 14:816–818. 10.1097/00006454-199509000-00022. [DOI] [PubMed] [Google Scholar]
- 1008.Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. 1998. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J 7:416–421. 10.1007/s005860050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009.Hardjasudarma M, Willis B, Black-Payne C, Edwards R. 1995. Pediatric spinal blastomycosis: case report. Neurosurgery 37:534–536. 10.1227/00006123-199509000-00027. [DOI] [PubMed] [Google Scholar]
- 1010.Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. 1994. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis 26:111–115. 10.3109/00365549409008601. [DOI] [PubMed] [Google Scholar]
- 1011.Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. 2008. Nonsurgical treatment of lumbosacral blastomycosis involving L2-S1: a case report. Spine (Phila Pa 1976) 33:E442–E446. 10.1097/BRS.0b013e3181760fe2. [DOI] [PubMed] [Google Scholar]
- 1012.Moore RM, Green NE. 1982. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am 64:1097–1101. [PubMed] [Google Scholar]
- 1013.Morris SK, Brophy J, Richardson SE, Summerbell R, Parkin PC, Jamieson F, Limerick B, Wiebe L, Ford-Jones EL. 2006. Blastomycosis in Ontario, 1994-2003. Emerg Infect Dis 12:274–279. 10.3201/eid1202.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. 1998. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis 26:413–418. 10.1086/clinids/26.2.413. [DOI] [PubMed] [Google Scholar]
- 1015.O’Guinn DJ, Serletis D, Kazemi N. 2016. Fungal osteomyelitis with vertebral re-ossification. Int J Surg Case Rep 19:1–3. 10.1016/j.ijscr.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.Ortega-Loayza AG, Nguyen T. 2013. Cutaneous blastomycosis: a clue to a systemic disease. An Bras Dermatol 88:287–289. 10.1590/S0365-05962013000200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017.Gottlieb JR, Eismont FJ. 2006. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am 88:854–856. 10.2106/JBJS.E.00650. [DOI] [PubMed] [Google Scholar]
- 1018.Dimar JR, II, Puno RM, Nowacki MR, Carreon LY. 2014. Surgery for blastomycosis of the spine. Am J Orthop (Belle Mead NJ) 43:E266–E271. [PubMed] [Google Scholar]
- 1019.Nokes SR, Adametz J, Gardner G, Beaton JN. 1995. Radiological case of the month. Blastomycosis osteomyelitis with epidural and retropharyngeal abscess. J Ark Med Soc 92:253–254. [PubMed] [Google Scholar]
- 1020.Farmer C, Stanley MW, Bardales RH, Korourian S, Shah H, Bradsher R, Klimberg VS. 1995. Mycoses of the breast: diagnosis by fine-needle aspiration. Diagn Cytopathol 12:51–55. 10.1002/dc.2840120112. [DOI] [PubMed] [Google Scholar]
- 1021.Riegler HF, Goldstein LA, Betts RF. 1974. Blastomycosis osteomyelitis. Clin Orthop Relat Res 100:225–231. [PubMed] [Google Scholar]
- 1022.MacDonald PB, Black GB, MacKenzie R. 1990. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am 72:860–864. [PubMed] [Google Scholar]
- 1023.Embil JM, Wiens JL, Oppenheimer M, Trepman E. 2006. Foot ulcer and osteomyelitis. CMAJ 174:35–36. 10.1503/cmaj.051058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024.Mollano AV, Shamsuddin H, Suh J-S. 2005. Systemic blastomycosis with osseous involvement of the foot: case report. Iowa Orthop J 25:53–56. [PMC free article] [PubMed] [Google Scholar]
- 1025.Saiz P, Gitelis S, Virkus W, Piasecki P, Bengana C, Templeton A. 2004. Blastomycosis of long bones. Clin Orthop Relat Res 421:255–259. 10.1097/01.blo.0000126305.87452.82. [DOI] [PubMed] [Google Scholar]
- 1026.Saylor HL, III, Van Demark RE, Sr, Hartmann AE, Patterson LE, DeMarco L. 1984. Blastomycosis of the tibia: case report. S D J Med 37:5–8. [PubMed] [Google Scholar]
- 1027.Weber CL, Bartley D, Al-Thaqafi A, Embil JM. 2007. Blastomyces dermatitidis osteomyelitis of the tibia. Am J Orthop (Belle Mead NJ) 36:29–32. [PubMed] [Google Scholar]
- 1028.Jahangir AA, Heck RK. 2010. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop (Belle Mead NJ) 39:E22–E24. [PubMed] [Google Scholar]
- 1029.Rein MF, Fischetti JL, Sande MA. 1974. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis 109:286–289. [DOI] [PubMed] [Google Scholar]
- 1030.Monsanto EH, Johnston AD, Dick HM. 1986. Isolated blastomycotic osteomyelitis: a case simulating a malignant tumor of the distal radius. J Hand Surg Am 11:896–898. 10.1016/s0363-5023(86)80246-5. [DOI] [PubMed] [Google Scholar]
- 1031.Schutze GE, Hickerson SL, Fortin EM, Schellhase DE, Darville T, Gubbins PO, Jacobs RF. 1996. Blastomycosis in children. Clin Infect Dis 22:496–502. 10.1093/clinids/22.3.496. [DOI] [PubMed] [Google Scholar]
- 1032.Mauriello CT, Raustol OA, Aguiar MA, Cunnion KM. 2012. An 11-year-old male with refractory osteomyelitis. Case Rep Pediatr 2012:285980. 10.1155/2012/285980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1033.Miller-Catchpole R, Rippon JW. 1984. Case report: blastomycosis causing sciatic neuritis. Mycopathologia 86:185–190. 10.1007/BF00441131. [DOI] [PubMed] [Google Scholar]
- 1034.Even-Sapir E, Martin RH, Marrie TJ. 1993. Gallium-67-citrate and bone scintigraphy in disseminated North American blastomycosis. J Nucl Med 34:458–461. [PubMed] [Google Scholar]
- 1035.Farr RC, Gardner G, Acker JD, Brint JM, Haglund LF, Land M, Schweitzer JB, West BC. 1992. Blastomycotic cranial osteomyelitis. Am J Otol 13:582–586. [PubMed] [Google Scholar]
- 1036.Blackledge FA, Newlands SD. 2001. Blastomycosis of the petrous apex. Otolaryngol Head Neck Surg 124:347–349. 10.1067/mhn.2001.114250. [DOI] [PubMed] [Google Scholar]
- 1037.Arabi Y, Abbas M, Fairfax MR. 1996. Dissemination of pulmonary blastomycosis after thoracotomy. Diagn Microbiol Infect Dis 26:35–37. 10.1016/s0732-8893(96)00176-9. [DOI] [PubMed] [Google Scholar]
- 1038.Wagner DK, Varkey B, Head MD. 1985. Blastomycotic osteomyelitis of the mandible: successful treatment with ketoconazole. Oral Surg Oral Med Oral Pathol 60:370–371. 10.1016/0030-4220(85)90257-9. [DOI] [PubMed] [Google Scholar]
- 1039.Liggett AS, Silberman Z. 1970. Blastomycosis of the knee joint. Case report. J Bone Joint Surg Am 52:1445–1449. [PubMed] [Google Scholar]
- 1040.Blais RE, Cesani F, Ali S, Calhoun JH, Mader J. 1997. Blastomycotic osteomyelitis of the pelvis: a case report. Am Surg 63:414–416. [PubMed] [Google Scholar]
- 1041.DeLeon MA, Johnson A. 2001. Pathological case of the month. Blastomyces dermatitidis. Arch Pediatr Adolesc Med 155:91–92. 10.1001/archpedi.155.1.91. [DOI] [PubMed] [Google Scholar]
- 1042.George AL, Jr, Hays JT, Graham BS. 1985. Blastomycosis presenting as monoarticular arthritis. The role of synovial fluid cytology. Arthritis Rheum 28:516–521. 10.1002/art.1780280508. [DOI] [PubMed] [Google Scholar]
- 1043.Federer AE, Haughom BD, Levy DM, Riff AJ, Nho SJ. 2015. Blastomyces tenosynovitis of the foot and ankle: a case report and review of the literature. J Foot Ankle Surg 54:1183–1187. 10.1053/j.jfas.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 1044.Albert MC, Zachary SV, Alter S. 1995. Blastomycosis of the forearm synovium in a child. Clin Orthop Relat Res 317:223–226. [PubMed] [Google Scholar]
- 1045.Bernstein S, Brunner HI, Summerbell R, Allen U, Babyn P, Richardson SE. 2002. Blastomycosis acquired by three children in Toronto. Can J Infect Dis 13:259–263. 10.1155/2002/906757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046.Abril A, Campbell MD, Cotten VR, Jr, Steckleberg JM, El-Azhary RA, O’Duffy JD. 1998. Polyarticular blastomycotic arthritis. J Rheumatol 25:1019–1021. [PubMed] [Google Scholar]
- 1047.Claflin K, Milbauer J, Sullivan B. 1983. Ketoconazole and blastomycotic osteomyelitis. Ann Intern Med 98:260–261. 10.7326/0003-4819-98-2-260_2. [DOI] [PubMed] [Google Scholar]
- 1048.Head AJ, Myers LK, Thompson JD, Buckingham SC, Skinner RB, Jr.. 2005. Disseminated blastomycosis presenting as oligoarticular septic arthritis in a 12-year-old girl. Arthritis Rheum 53:138–141. 10.1002/art.20916. [DOI] [PubMed] [Google Scholar]
- 1049.Muniz AE, Evans T. 2000. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med 19:245–248. 10.1016/S0736-4679(00)00243-2. [DOI] [PubMed] [Google Scholar]
- 1050.Robert ME, Kauffman CA. 1988. Blastomycosis presenting as polyarticular septic arthritis. J Rheumatol 15:1438–1442. [PubMed] [Google Scholar]
- 1051.Hamann JC, Marberry K. 2010. Sentinel presentation of disseminated Blastomyces dermatitidis infection as hip pain in a young adult: a case report. J Bone Joint Surg Am 92:469–472. 10.2106/JBJS.I.00212. [DOI] [PubMed] [Google Scholar]
- 1052.Polcari IC, Reindel R, Chadwick E, Bogard A, Chou P, Chamlin S. 2013. Skin ulcers and bone pain in a healthy 6-year-old female. Pediatr Dermatol 30:749–750. 10.1111/pde.12011. [DOI] [PubMed] [Google Scholar]
- 1053.Brick KE, Drolet BA, Lyon VB, Galbraith SS. 2013. Cutaneous and disseminated blastomycosis: a pediatric case series. Pediatr Dermatol 30:23–28. 10.1111/j.1525-1470.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- 1054.Bernstein JM, Bacheller CD. 2006. It’s on the tip of my tongue. Skinmed 5:142–145. 10.1111/j.1540-9740.2006.05027.x. [DOI] [PubMed] [Google Scholar]
- 1055.Koh H, Demos TC, Lomasney LM, O’Keefe JP. 2011. Radiologic case study. Blastomycosis of bone. Orthopedics 34:409, 486. 10.3928/01477447-20110427-34. [DOI] [PubMed] [Google Scholar]
- 1056.Patel AJ, Gattuso P, Reddy VB. 2010. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol 34:256–261. 10.1097/PAS.0b013e3181ca48a5. [DOI] [PubMed] [Google Scholar]
- 1057.Gehweiler JA, Capp MP, Chick EW. 1970. Observations on the roentgen patterns in blastomycosis of bone. A review of cases from the Blastomycosis Cooperative Study of the Veterans Administration and Duke University Medical Center. Am J Roentgenol Radium Ther Nucl Med 108:497–510. 10.2214/ajr.108.3.497. [DOI] [PubMed] [Google Scholar]
- 1058.Chapman SW, Dismukes WE, Proia LA, Bradsher RW, Pappas PG, Threlkeld MG, Kauffman CA, Infectious Diseases Society of America. 2008. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 46:1801–1812. 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 1059.Dukik K, Al-Hatmi AMS, Curfs-Breuker I, Faro D, de Hoog S, Meis JF. 2018. Antifungal susceptibility of emerging dimorphic pathogens in the family Ajellomycetaceae. Antimicrob Agents Chemother 62:e01886-17. 10.1128/AAC.01886-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1060.Pappas PG, Bradsher RW, Kauffman CA, Cloud GA, Thomas CJ, Campbell GD, Jr, Chapman SW, Newman C, Dismukes WE. 1997. Treatment of blastomycosis with higher doses of fluconazole. The National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis 25:200–205. 10.1086/514539. [DOI] [PubMed] [Google Scholar]
- 1061.Bahr NC, Antinori S, Wheat LJ, Sarosi GA. 2015. Histoplasmosis infections worldwide: thinking outside of the Ohio River Valley. Curr Trop Med Rep 2:70–80. 10.1007/s40475-015-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1062.Akin L, Herford AS, Cicciù M. 2011. Oral presentation of disseminated histoplasmosis: a case report and literature review. J Oral Maxillofac Surg 69:535–541. 10.1016/j.joms.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 1063.Care SB, Lacey SH. 1998. Recurrent histoplasmosis of the wrist: a case report. J Hand Surg Am 23:1112–1114. 10.1016/S0363-5023(98)80025-7. [DOI] [PubMed] [Google Scholar]
- 1064.Darouiche RO, Cadle RM, Zenon GJ, Weinert MF, Hamill RJ, Lidsky MD. 1992. Articular histoplasmosis. J Rheumatol 19:1991–1993. [PubMed] [Google Scholar]
- 1065.Dobleman TJ, Scher N, Goldman M, Doot S. 1989. Invasive histoplasmosis of the mandible. Head Neck 11:81–84. 10.1002/hed.2880110114. [DOI] [PubMed] [Google Scholar]
- 1066.Fowler VG, Jr, Nacinovich FM, Alspaugh JA, Corey GR. 1998. Prosthetic joint infection due to Histoplasma capsulatum: case report and review. Clin Infect Dis 26:1017. 10.1086/517643. [DOI] [PubMed] [Google Scholar]
- 1067.Palmgren BA, Buhr BR. 2005. Histoplasmosis of the tibia. Orthopedics 28:67–68. 10.3928/0147-7447-20050101-17. [DOI] [PubMed] [Google Scholar]
- 1068.Weinberg JM, Ali R, Badve S, Pelker RR. 2001. Musculoskeletal histoplasmosis. A case report and review of the literature. J Bone Joint Surg Am 83:1718–1722. [PubMed] [Google Scholar]
- 1069.Jones PG, Rolston K, Hopfer RL. 1985. Septic arthritis due to Histoplasma capsulatum in a leukaemic patient. Ann Rheum Dis 44:128–129. 10.1136/ard.44.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1070.McCabe MP, Heck RK. 2010. Histoplasma osteomyelitis simulating giant-cell tumor of the distal part of the radius: a case report. J Bone Joint Surg Am 92:708–714. 10.2106/JBJS.H.01507. [DOI] [PubMed] [Google Scholar]
- 1071.de Morais SS, Mafra MDO, Canterle EM, de Lima LL, Ribeiro SLE. 2008. Histoplasmosis mimicking tuberculosis spondylodiscitis in a patient with rheumatoid arthritis. Acta Reumatol Port 33:360–363. (In Portuguese.) [PubMed] [Google Scholar]
- 1072.Figueira JA, Camilo Júnior D, Biasoli É, Miyahara GI, Bernabé DG. 2017. Oral ulcers associated with bone destruction as the primary manifestation of histoplasmosis in an immunocompetent patient. J Eur Acad Dermatol Venereol 31:e429–e430. 10.1111/jdv.14257. [DOI] [PubMed] [Google Scholar]
- 1073.Gaume M, Marie-Hardy L, Larousserie F, Lavielle M, Roux C, Leclerc P, Paugam A, Archambeau D, Eyrolle L, Gauzit R, Lortholary O, Anract P, Epelboin L, Salmon D. 2017. Histoplasma capsulatum bone and joint infection. Med Mal Infect 47:554–557. (In French.) 10.1016/j.medmal.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 1074.Verhaert K, Rodriguez M, Mendoza G, Delgadillo J-L, Casaer P. 2002. Polyarthritis and humeral epiphysial separation in an infant with acute disseminated histoplasmosis. Pediatr Infect Dis J 21:352–353. 10.1097/00006454-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 1075.Marianelli LG, Frassone N, Marino M, Debes J. 2014. Immune reconstitution inflammatory syndrome as histoplasmosis osteomyelitis in South America. AIDS 28:1848–1850. 10.1097/QAD.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 1076.Azar MM, Malinis MF. 2016. Disseminated histoplasmosis with skin lesions and osteomyelitis in a patient from the Philippines. Am J Trop Med Hyg 95:70–74. 10.4269/ajtmh.16-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1077.Huang L, Wu Y, Miao X. 2013. Localized Histoplasma capsulatum osteomyelitis of the fibula in an immunocompetent teenage boy: a case report. BMC Infect Dis 13:132. 10.1186/1471-2334-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1078.Liu B, Qu L, Zhu J, Yang Z, Yan S. 2017. Histoplasmosis mimicking metastatic spinal tumour. J Int Med Res 45:1440–1446. 10.1177/0300060517708530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1079.Adekeye EO, Edwards MB, Williams HK. 1988. Mandibular African histoplasmosis: imitation of neoplasia or giant-cell granuloma? Oral Surg Oral Med Oral Pathol 65:81–84. 10.1016/0030-4220(88)90197-1. [DOI] [PubMed] [Google Scholar]
- 1080.Houtman PM, Marck KW, Hol C. 1999. Histoplasmosis of the wrist: a case report. Rheumatology (Oxford) 38:906–907. 10.1093/rheumatology/38.9.906. [DOI] [PubMed] [Google Scholar]
- 1081.Karunanithi S, Kumar G, Sharma SK, Jain D, Gupta A, Kumar R. 2015. Staging and response of sternal histoplasmosis by 18F-FDG PET/CT. Clin Nucl Med 40:231–233. 10.1097/RLU.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 1082.Mathews DM, John R, Verghese V, Parmar H, Chaudhary N, Mishra S, Mathew L. 2016. Histoplasma capsulatum infection with extensive lytic bone lesions mimicking LCH. J Trop Pediatr 62:496–499. 10.1093/tropej/fmw040. [DOI] [PubMed] [Google Scholar]
- 1083.Quraishi NA, Davidson RN, Steele N, Grand F. 2003. Histoplasmosis as the cause of a pathological fracture. J Bone Joint Surg Br 85:732–733. [PubMed] [Google Scholar]
- 1084.Schasfoort RA, Marck KW, Houtman PM. 1999. Histoplasmosis of the wrist. J Hand Surg Br 24:625–627. 10.1054/jhsb.1999.0273. [DOI] [PubMed] [Google Scholar]
- 1085.Shukla A, Shah C, Hardik P, Gupte P. 2015. A probable case of histoplasmosis presenting as portal hypertension and bone lesion in a case of common variable immunodeficiency syndrome. J Postgrad Med 61:49–50. 10.4103/0022-3859.147054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1086.Van der Schee AC, Dinkla BA, Festen JJ. 1990. Gonarthritis as only manifestation of chronic disseminated histoplasmosis. Clin Rheumatol 9:92–94. 10.1007/BF02030251. [DOI] [PubMed] [Google Scholar]
- 1087.Tazi EM, Essadi I, Serraj K, Ichou M, Errihani H. 2009. Sacrum histoplasmosis 10 years after NHL of the sacrum: a case report. Cancer Radiother 13:337–339. (In French.) 10.1016/j.canrad.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 1088.Jordan AS, Chavada R, Nagendra V, McNeil HP, Kociuba K, Gibson KA. 2013. A budding surprise from the joint. Med J Aust 199:700–701. 10.5694/mja13.10504. [DOI] [PubMed] [Google Scholar]
- 1089.Rosenthal J, Brandt KD, Wheat LJ, Slama TG. 1983. Rheumatologic manifestations of histoplasmosis in the recent Indianapolis epidemic. Arthritis Rheum 26:1065–1070. 10.1002/art.1780260902. [DOI] [PubMed] [Google Scholar]
- 1090.Kauffman CA. 2007. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 20:115–132. 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1091.Oladele RO, Ayanlowo OO, Richardson MD, Denning DW. 2018. Histoplasmosis in Africa: an emerging or a neglected disease? PLoS Negl Trop Dis 12:e0006046. 10.1371/journal.pntd.0006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.Diongue K, Diallo MA, Badiane AS, Seck MC, Ndiaye M, Ndoye NW, Ndiaye YD, Dieye B, Déme A, Ndiaye IM, Ndir O, Ndiaye D. 2015. Nondermatophytic and noncandidal fungi isolated in Le Dantec University Hospital of Dakar in 2014: epidemiological, clinical and mycological study. J Mycol Med 25:181–190. (In French.) 10.1016/j.mycmed.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 1093.Darré T, Saka B, Mouhari-Touré A, Dorkenoo AM, Amégbor K, Pitche VP, Napo-Koura G. 2017. Histoplasmosis by Histoplasma capsulatum var. duboisii observed at the Laboratory of Pathological Anatomy of Lomé in Togo. J Pathog 2017:2323412. 10.1155/2017/2323412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094.Carme BHM, Ngaporo Itoua A, Ngolet A, Darozzin F, Moyikoua A, Ndeli D, Lehenaff YM, Obengui, Gombe Mbalawa C. 1993. African histoplasmosis due to Histoplasma duboisii (Histoplasma capsulatum var duboisii). Fourteen cases observed in Congo during 10 years (1981-1990). J Mycol Med 3:67–73. [Google Scholar]
- 1095.Drouhet E. 1972. Clinical aspects of African histoplasmosis. Ann Soc Belg Med Trop 52:391–405. (In French.). [PubMed] [Google Scholar]
- 1096.Loulergue P, Bastides F, Baudouin V, Chandenier J, Mariani-Kurkdjian P, Dupont B, Viard JP, Dromer F, Lortholary O. 2007. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis 13:1647–1652. 10.3201/eid1311.070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097.Moulin E, Hauser PM, Rey L-E, Zaugg T, Barcena C, Meylan P, Giulieri SG. 2015. Photo quiz. A 53-year-old woman with an unusual etiology of joint pain. Clin Infect Dis 61:76, 129–131. 10.1093/cid/civ136. [DOI] [PubMed] [Google Scholar]
- 1098.André C, Badoual J, Kalifa G, Dubousset J. 1984. African histoplasmosis. A case. Arch Fr Pediatr 41:429–431. (In French.). [PubMed] [Google Scholar]
- 1099.Bankolé Sanni R, Denoulet C, Coulibaly B, Nandiolo R, Kassi E, Honde M, Mobiot ML. 1998. Apropos of 1 Ivoirian case of osseus and cutaneous histoplasmosis by Histoplasma capsulatum var. duboisii. Bull Soc Pathol Exot 91:151–153. (In French.) [PubMed] [Google Scholar]
- 1100.Chandenier J, Goma D, Moyen G, Samba-Lefèbvre MC, Nzingoula S, Obengui, Mbitsi A, Nkiwabonga L, Ngaporo AI. 1995. African histoplasmosis due to Histoplasma capsulatum var. duboisii: relationship with AIDS in recent Congolese cases. Sante 5:227–234. (In French.) [PubMed] [Google Scholar]
- 1101.Daramola JO, Ajagbe HA, Abioye AA, Ogunba EO. 1979. Maxillary African histoplasmosis mimicking malignant jaw tumour. Br J Oral Surg 16:241–247. 10.1016/0007-117x(79)90030-1. [DOI] [PubMed] [Google Scholar]
- 1102.Khalil MA, Hassan AW, Gugnani HC. 1998. African histoplasmosis: report of four cases from north-eastern Nigeria. Mycoses 41:293–295. 10.1111/j.1439-0507.1998.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 1103.Kotloff KL, Vial PA, Young JW, Smith AG. 1987. Histoplasma duboisii infection in a Liberian girl. Pediatr Infect Dis J 6:202–205. 10.1097/00006454-198702000-00012. [DOI] [PubMed] [Google Scholar]
- 1104.Mabiala Babela JR, Mboutol Mandavo C, Nika Evrard R, Ossibi Ibara B, Lamah L, Ollandzobo Ikobo LC, Mouko A, Peko JF. 2017. African Histoplasmosis. A report of three pediatric cases. J Mycol Med 27:133–138. (In French.) 10.1016/j.mycmed.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 1105.Minta DK, Sylla M, Traoré AM, Soukho-Kaya A, Coulibaly I, Diallo K, Théra MA, Sidibé AT, Sidibé S, Traoré HA, Pichard E, Chabasse D. 2014. Malian first observation of disseminated African histoplasmosis with predominant bone localizations in an HIV-negative child in Bamako (Mali). Review of the literature. J Mycol Med 24:152–157. (In French.) 10.1016/j.mycmed.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 1106.N’Golet A, M’Bitsi A, Moyikoua A, Casiraghi O. 2005. An unusual scapular infectious arthritis. Ann Pathol 25:331–332. (In French.) 10.1016/S0242-6498(05)80140-3. [DOI] [PubMed] [Google Scholar]
- 1107.Pitche P, Dossim A, Mijiyawa M, Napo-Koura G, Tchangaï-Walla K. 1995. Multiple bone lesions of a type of disseminated African histoplasmosis in a Togolese immunocompetent child. Rev Chir Orthop Reparatrice Appar Mot 81:745–748. (In French.) [PubMed] [Google Scholar]
- 1108.Seriki O, Aderele WI, Johnson A, Smith JA. 1975. Disseminated histoplasmosis due to Histoplasma capsulatum in two Nigerian children. J Trop Med Hyg 78:248–255. [PubMed] [Google Scholar]
- 1109.Thompson EM, Ellert J, Peters LL, Ajdukiewicz A, Mabey D. 1981. Histoplasma duboisii infection of bone. Br J Radiol 54:518–521. 10.1259/0007-1285-54-642-518. [DOI] [PubMed] [Google Scholar]
- 1110.Akinyoola AL, Onayemi O, Famurewa OC. 2006. African histoplasmosis—masquerading as a malignant bone tumour. Trop Doct 36:250–251. 10.1258/004947506778604922. [DOI] [PubMed] [Google Scholar]
- 1111.Gentilini M, Brucker G, Danis M, Lebas J, Mogahed A, Felix H. 1980. Histoplasmosis: clinical, biological, and therapeutic aspects in ten cases (author’s transl). Ann Med Interne (Paris) 131:209–212. (In French.) [PubMed] [Google Scholar]
- 1112.Hiltenbrand C, Antoine HM, Durosoir JL, Misson R, Guénard C, Garrel J, Millet P. 1971. African histoplasmosis of cutaneous, osseous, lymph node and pulmonary form (apropos of a case). Bull Soc Fr Dermatol Syphiligr 78:600–602. (In French.) [PubMed] [Google Scholar]
- 1113.Hiltenbrand C, Antoine HM, Laurens A, Juillet Y, Du Bourguet F, Carlet J. 1972. Imported pathology: African histoplasmosis due to Histoplasma duboisii. Ann Med Interne (Paris) 123:573–578. (In French.) [PubMed] [Google Scholar]
- 1114.Ige AO, Nwosu SO, Odesanmi WO. 1992. African histoplasmosis (Duboisii) of the skull with neurological complication—a case report and review of literature. Afr J Med Med Sci 21:19–21. [PubMed] [Google Scholar]
- 1115.Márton K, Márton-Ecsi E, Lama A, Dialio O. 1974. 1st case of cutano-osseous histoplasmosis observed in Guinea. Int J Dermatol 13:190–196. (In French.) 10.1111/j.1365-4362.1974.tb01792.x. [DOI] [PubMed] [Google Scholar]
- 1116.N’dri Oka D, Varlet G, Kakou M, Zunon-Kipre Y, Broalet E, Ba Zeze V. 2001. Spondylodiscitis due to Histoplasma duboisii. Report of two cases and review of the literature. Neurochirurgie 47:431–434. (In French.) [PubMed] [Google Scholar]
- 1117.N’dri Oka D, Mbende AS, Sissoko D. 2016. Spinal cord compression caused by multifocal histoplasmosis treated conservatively: case report and literature review. Open J Mod Neurosurg 60:20–24. [Google Scholar]
- 1118.Onwuasoigwe O, Gugnani HC. 1998. African histoplasmosis: osteomyelitis of the radius. Mycoses 41:105–107. 10.1111/j.1439-0507.1998.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 1119.Rodríguez González DP, Valdés Sánchez AF, Jekin Savariego E, Gómez Bacallao M, Castillo Otero E. 1982. Thoracic bone lesions in African histoplasmosis. Rev Cubana Med Trop 34:82–87. (In Spanish.) [PubMed] [Google Scholar]
- 1120.Simon F, Chouc PY, Hervé V, Branquet D, Jeandel P. 1994. Bone and joint sites of African histoplasmosis (Histoplasma duboisii). Apropos of a case and review of the literature. Rev Rhum Ed Fr 61:829–838. (In French.) [PubMed] [Google Scholar]
- 1121.Valmary J, Bauduceau B, Lartisien D, Flechaire A, Debord T, Daly JP, Laverdant C. 1984. Disseminated Histoplasma duboisii histoplasmosis in a female inhabitant of the Ivory Coast. Med Trop (Mars) 44:369–373. (In French.) [PubMed] [Google Scholar]
- 1122.Young H, Tomsick S, Storfa A, Jenkins TC. 2011. Orthopaedic case of the month: arm pain and fistulous tract in a 45-year-old Liberian woman. Clin Orthop Relat Res 469:1800–1803. 10.1007/s11999-011-1835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1123.Zida A, Niamba P, Barro-Traoré F, Korsaga-Somé N, Tapsoba P, Briegel J, Guiguemdé RT. 2015. Disseminated histoplasmosis caused by Histoplasma capsulatum var. duboisii in a non-HIV patient in Burkina Faso: case report. J Mycol Med 25:159–162. 10.1016/j.mycmed.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 1124.Ndri D, Adou N, Drogba L, Konan L, Ba Zeze V. 2013. Compression médullaire causée par un granulome d’histoplasmose à Histoplasma duboisii et capsulatum. Neurochirurgie 59:262–263. 10.1016/j.neuchi.2013.10.121. [DOI] [Google Scholar]
- 1125.Adkins T, Rees M, Simeone F, Isaacs R, Halsell A, Flowers ER. 1994. Case records of the Department of Medicine University of Mississippi Medical Center. Histoplasma capsulatum osteomyelitis humerus, metastatim pulmonary histoplasmosis. J Miss State Med Assoc 35:59–64. [PubMed] [Google Scholar]
- 1126.Mittal J, Ponce MG, Gendlina I, Nosanchuk JD. 2019. Histoplasma capsulatum: mechanisms for pathogenesis. Curr Top Microbiol Immunol 422:157–191. 10.1007/82_2018_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Gugnani HC, Muotoe-Okafor FA, Kaufman L, Dupont B. 1994. A natural focus of Histoplasma capsulatum var. duboisii is a bat cave. Mycopathologia 127:151–157. 10.1007/BF01102915. [DOI] [PubMed] [Google Scholar]
- 1128.Shoroye A, Oyedeji GA. 1982. African histoplasmosis presenting as a facial tumour in a child. Ann Trop Paediatr 2:147–149. 10.1080/02724936.1982.11748248. [DOI] [PubMed] [Google Scholar]
- 1129.Ngatse-Oko A, Péko JF, Ntsiba H, Ngolet A, Kokolo J, Ondzoto M, Carme B, Moyikoua A. 2006. Pathological fracture revealing an osseous histoplasmosis. A case report on a 60-year patient. Bull Soc Pathol Exot 99:227–229. (In French.) [PubMed] [Google Scholar]
- 1130.Azar MM, Hage CA. 2017. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 55:1612–1620. 10.1128/JCM.02430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131.Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, Hammoud K, Alapat D, Babady NE, Parker M, Fuller D, Noor A, Davis TE, Rodgers M, Connolly PA, El Haddad B, Wheat LJ. 2011. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 53:448–454. 10.1093/cid/cir435. [DOI] [PubMed] [Google Scholar]
- 1132.Klassen-Fischer M, McEvoy P, Neafie RC, Nelson AM. 2004. Accurate diagnosis of infection with Histoplasma capsulatum var. duboisii. Clin Infect Dis 38:595. (Reply, 38:595–596, 10.1086/381457.) [DOI] [PubMed] [Google Scholar]
- 1133.Valero C, Gago S, Monteiro MC, Alastruey-Izquierdo A, Buitrago MJ. 2018. African histoplasmosis: new clinical and microbiological insights. Med Mycol 56:51–59. 10.1093/mmy/myx020. [DOI] [PubMed] [Google Scholar]
- 1134.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA, Infectious Diseases Society of America. 2007. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 45:807–825. 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 1135.Restrepo A, Tobón A, Clark B, Graham DR, Corcoran G, Bradsher RW, Goldman M, Pankey G, Moore T, Negroni R, Graybill JR. 2007. Salvage treatment of histoplasmosis with posaconazole. J Infect 54:319–327. 10.1016/j.jinf.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 1136.Brilhante RSN, Fechine MAB, Mesquita JRL, Cordeiro RA, Rocha MFG, Monteiro AJ, Lima RAC, Caetano É, Pereira JF, Castelo-Branco DSCM, Camargo ZP, Sidrim JJC. 2012. Histoplasmosis in HIV-positive patients in Ceará, Brazil: clinical-laboratory aspects and in vitro antifungal susceptibility of Histoplasma capsulatum isolates. Trans R Soc Trop Med Hyg 106:484–488. 10.1016/j.trstmh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 1137.Brilhante RSN, Guedes GMDM, da Silva MLQ, Castelo-Branco DSCM, Cordeiro RDA, Sidrim JJC, Rocha MFG. 2018. A proposal for antifungal epidemiological cut-off values against Histoplasma capsulatum var. capsulatum based on the susceptibility of isolates from HIV-infected patients with disseminated histoplasmosis in northeast Brazil. Int J Antimicrob Agents 52:272–277. 10.1016/j.ijantimicag.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 1138.Breton G, Adle-Biassette H, Therby A, Ramanoelina J, Choudat L, Bissuel F, Huerre M, Dromer F, Dupont B, Lortholary O. 2006. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. AIDS 20:119–121. 10.1097/01.aids.0000199014.66139.39. [DOI] [PubMed] [Google Scholar]
- 1139.Hage CA, Bowyer S, Tarvin SE, Helper D, Kleiman MB, Wheat LJ. 2010. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis 50:85–92. 10.1086/648724. [DOI] [PubMed] [Google Scholar]
- 1140.Gonçalves D, Ferraz C, Vaz L. 2013. Posaconazole as rescue therapy in African histoplasmosis. Braz J Infect Dis 17:102–105. 10.1016/j.bjid.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1141.Vanittanakom N, Cooper CR, Jr, Fisher MC, Sirisanthana T. 2006. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev 19:95–110. 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1142.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Praparattanapan J, Nelson KE. 1997. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin Infect Dis 24:1080–1086. 10.1086/513649. [DOI] [PubMed] [Google Scholar]
- 1143.Le T, Wolbers M, Chi NH, Quang VM, Chinh NT, Lan NP, Lam PS, Kozal MJ, Shikuma CM, Day JN, Farrar J. 2011. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis 52:945–952. 10.1093/cid/cir028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1144.Chan JFW, Lau SKK, Yuen K-Y, Woo PCY. 2016. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 5:e19. 10.1038/emi.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1145.Duong TA. 1996. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis 23:125–130. 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 1146.Kawila R, Chaiwarith R, Supparatpinyo K. 2013. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in northern Thailand: a retrospective study. BMC Infect Dis 13:464. 10.1186/1471-2334-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1147.Qiu Y, Zhang J, Liu G, Zhong X, Deng J, He Z, Jing B. 2015. Retrospective analysis of 14 cases of disseminated Penicillium marneffei infection with osteolytic lesions. BMC Infect Dis 15:47. 10.1186/s12879-015-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1148.Wong SS, Wong KH, Hui WT, Lee SS, Lo JY, Cao L, Yuen KY. 2001. Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J Clin Microbiol 39:4535–4540. 10.1128/JCM.39.12.4535-4540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1149.Pun TS, Fang D. 2000. A case of Penicillium marneffei osteomyelitis involving the axial skeleton. Hong Kong Med J 6:231–233. [PubMed] [Google Scholar]
- 1150.Louthrenoo W, Thamprasert K, Sirisanthana T. 1994. Osteoarticular penicilliosis marneffei. A report of eight cases and review of the literature. Br J Rheumatol 33:1145–1150. 10.1093/rheumatology/33.12.1145. [DOI] [PubMed] [Google Scholar]
- 1151.Lee PPW, Chan K-W, Lee T-L, Ho MH-K, Chen X-Y, Li C-H, Chu K-M, Zeng H-S, Lau Y-L. 2012. Penicilliosis in children without HIV infection—are they immunodeficient? Clin Infect Dis 54:e8–e19. 10.1093/cid/cir754. [DOI] [PubMed] [Google Scholar]
- 1152.Sirisanthana V, Sirisanthana T. 1993. Penicillium marneffei infection in children infected with human immunodeficiency virus. Pediatr Infect Dis J 12:1021–1025. 10.1097/00006454-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 1153.Sudjaritruk T, Sirisanthana T, Sirisanthana V. 2012. Immune reconstitution inflammatory syndrome from Penicillium marneffei in an HIV-infected child: a case report and review of literature. BMC Infect Dis 12:28. 10.1186/1471-2334-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1154.Hung CC, Hsueh PR, Chen MY, Hsiao CH, Chang SC, Luh KT. 1998. Invasive infection caused by Penicillium marneffei: an emerging pathogen in Taiwan. Clin Infect Dis 26:202–203. 10.1086/517068. [DOI] [PubMed] [Google Scholar]
- 1155.Lin WC, Dai YS, Tsai MJ, Huang LM, Chiang BL. 1998. Systemic Penicillium marneffei infection in a child with common variable immunodeficiency. J Formos Med Assoc 97:780–783. [PubMed] [Google Scholar]
- 1156.Lin J-N, Lin H-H, Lai C-H, Wang J-L, Yu T-J. 2010. Renal transplant recipient infected with Penicillium marneffei. Lancet Infect Dis 10:138. 10.1016/S1473-3099(10)70005-0. [DOI] [PubMed] [Google Scholar]
- 1157.Chan JFW, Chan TSY, Gill H, Lam FYF, Trendell-Smith NJ, Sridhar S, Tse H, Lau SKP, Hung IFN, Yuen KY, Woo PCY. 2015. Disseminated infections with Talaromyces marneffei in non-AIDS patients given monoclonal antibodies against CD20 and kinase inhibitors. Emerg Infect Dis 21:1101–1106. 10.3201/eid2107.150138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1158.Chan YF, Woo KC. 1990. Penicillium marneffei osteomyelitis. J Bone Joint Surg Br 72:500–503. 10.1302/0301-620X.72B3.2341456. [DOI] [PubMed] [Google Scholar]
- 1159.Jayanetra P, Nitiyanant P, Ajello L, Padhye AA, Lolekha S, Atichartakarn V, Vathesatogit P, Sathaphatayavongs B, Prajaktam R. 1984. Penicilliosis marneffei in Thailand: report of five human cases. Am J Trop Med Hyg 33:637–644. 10.4269/ajtmh.1984.33.637. [DOI] [PubMed] [Google Scholar]
- 1160.Liu GN, Huang JS, Zhong XN, Zhang JQ, Zou ZX, Yang ML, Deng JM, Bai J, Li MH, Mao CZ, He ZY. 2014. Penicillium marneffei infection within an osteolytic lesion in an HIV-negative patient. Int J Infect Dis 23:1–3. 10.1016/j.ijid.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 1161.Cooper CR, Vanittanakom N. 2008. Insights into the pathogenicity of Penicillium marneffei. Future Microbiol 3:43–55. 10.2217/17460913.3.1.43. [DOI] [PubMed] [Google Scholar]
- 1162.Sisto F, Miluzio A, Leopardi O, Mirra M, Boelaert JR, Taramelli D. 2003. Differential cytokine pattern in the spleens and livers of BALB/c mice infected with Penicillium marneffei: protective role of gamma interferon. Infect Immun 71:465–473. 10.1128/IAI.71.1.465-473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Cui J, Tanaka R, Taguchi H, Sano A, Ito E, Fukushima K, Takeo K, Yoshida S, Nishimura K, Miyaji M. 1997. Histopathological and electron microscopical studies on experimental Penicillium marneffei infection in mice. J Med Vet Mycol 35:347–353. [PubMed] [Google Scholar]
- 1164.Wong KF. 2010. Marrow penicilliosis: a readily missed diagnosis. Am J Clin Pathol 134:214–218. 10.1309/AJCPWVBQCW13DJLO. [DOI] [PubMed] [Google Scholar]
- 1165.Zeng W, Qiu Y, Lu D, Zhang J, Zhong X, Liu G. 2015. A retrospective analysis of 7 human immunodeficiency virus-negative infants infected by Penicillium marneffei. Medicine (Baltimore) 94:e1439. 10.1097/MD.0000000000001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Zheng J, Gui X, Cao Q, Yang R, Yan Y, Deng L, Lio J. 2015. A clinical study of acquired immunodeficiency syndrome associated Penicillium marneffei infection from a non-endemic area in China. PLoS One 10:e0130376. 10.1371/journal.pone.0130376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1167.Supparatpinyo K, Schlamm HT. 2007. Voriconazole as therapy for systemic Penicillium marneffei infections in AIDS patients. Am J Trop Med Hyg 77:350–353. [PubMed] [Google Scholar]
- 1168.Liu DH, Liang L, Chen JM. 2013. In vitro antifungal drug susceptibilities of Penicillium marneffei from China. J Infect Chemother 19:776–778. 10.1007/s10156-012-0511-7. [DOI] [PubMed] [Google Scholar]
- 1169.Mahajan VK, Sharma NL, Shanker V, Gupta P, Mardi K. 2010. Cutaneous sporotrichosis: unusual clinical presentations. Indian J Dermatol Venereol Leprol 76:276–280. 10.4103/0378-6323.62974. [DOI] [PubMed] [Google Scholar]
- 1170.Xavier MO, Bittencourt LR, da Silva CM, Vieira RS, Pereira HCP. 2013. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop 46:116–118. 10.1590/0037-868215282013. [DOI] [PubMed] [Google Scholar]
- 1171.de Carvalho Aguinaga F, Trope BM, Fernandes NC, Engel DC, Ramos ESM. 2014. Sporotrichosis with bone involvement: an alert to an occupational disease. Case Rep Dermatol 6:114–118. 10.1159/000362184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1172.Freitas DF, Santos SS, Almeida-Paes R, de Oliveira MM, do Valle AC, Gutierrez-Galhardo MC, Zancope-Oliveira RM, Nosanchuk JD. 2015. Increase in virulence of Sporothrix brasiliensis over five years in a patient with chronic disseminated sporotrichosis. Virulence 6:112–120. 10.1080/21505594.2015.1014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1173.Lederer HT, Sullivan E, Crum-Cianflone NF. 2016. Sporotrichosis as an unusual case of osteomyelitis: a case report and review of the literature. Med Mycol Case Rep 11:31–35. 10.1016/j.mmcr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1174.Ribeiro BN, Ribeiro RN, Penna CR, Frota AC. 2015. Bone involvement by Sporothrix schenckii in an immunocompetent child. Pediatr Radiol 45:1427–1430. 10.1007/s00247-015-3299-7. [DOI] [PubMed] [Google Scholar]
- 1175.Ferreira LC, Barroso PF, Tonomura E, Akiti T, Rodrigues KM. 2016. Osteomyelitis caused by Sporothrix schenckii in an immunocompetent patient. Rev Soc Bras Med Trop 49:527–529. 10.1590/0037-8682-0354-2015. [DOI] [PubMed] [Google Scholar]
- 1176.Costa RO, de Mesquita KC, Damasco PS, Bernardes-Engemann AR, Dias CMP, Silva IC, Lopes-Bezerra LM. 2008. Infectious arthritis as the single manifestation of sporotrichosis: serology from serum and synovial fluid samples as an aid to diagnosis. Rev Iberoam Micol 25:54–56. 10.1016/S1130-1406(08)70014-7. [DOI] [PubMed] [Google Scholar]
- 1177.Bustamante B, Lama JR, Mosquera C, Soto L. 2009. Sporotrichosis in human immunodeficiency virus infected Peruvian patients: two case reports and literature review. Infect Dis Clin Pract 17:78–83. 10.1097/IPC.0b013e31818add36. [DOI] [Google Scholar]
- 1178.Gutierrez-Galhardo MC, Zancope-Oliveira RM, Monzon A, Rodriguez-Tudela JL, Cuenca-Estrella M. 2010. Antifungal susceptibility profile in vitro of Sporothrix schenckii in two growth phases and by two methods: microdilution and E-test. Mycoses 53:227–231. 10.1111/j.1439-0507.2009.01701.x. [DOI] [PubMed] [Google Scholar]
- 1179.Freitas DFS, Hoagland BDS, do Valle ACF, Fraga BB, de Barros NB, Schubach ADO, de Almeida-Paes R, Cuzzi T, Rosalino CMV, Zancope-Oliveira RM, Gutierrez-Galhardo MC. 2012. Sporotrichosis in HIV-infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med Mycol 50:170–178. 10.3109/13693786.2011.596288. [DOI] [PubMed] [Google Scholar]
- 1180.Paixao AG, Galhardo MCG, Almeida-Paes R, Nunes EP, Goncalves MLC, Chequer GL, Lamas CDC. 2015. The difficult management of disseminated Sporothrix brasiliensis in a patient with advanced AIDS. AIDS Res Ther 12:16. 10.1186/s12981-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1181.Barros MBDL, de Almeida Paes R, Schubach AO. 2011. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev 24:633–654. 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1182.Rowe JG, Amadio PC, Edson RS. 1989. Sporotrichosis. Orthopedics 12:981–985. 10.3928/0147-7447-19890701-11. [DOI] [PubMed] [Google Scholar]
- 1183.Arenas R, Latapi F. 1984. Generalized sporotrichosis. Study of a Mexican case treated with amphotericin B and ketoconazole. Bull Soc Pathol Exot Filiales 77:385–391. (In French.) [PubMed] [Google Scholar]
- 1184.Janes PC, Mann RJ. 1987. Extracutaneous sporotrichosis. J Hand Surg Am 12:441–445. 10.1016/s0363-5023(87)80020-5. [DOI] [PubMed] [Google Scholar]
- 1185.Calhoun DL, Waskin H, White MP, Bonner JR, Mulholland JH, Rumans LW, Stevens DA, Galgiani JN. 1991. Treatment of systemic sporotrichosis with ketoconazole. Rev Infect Dis 13:47–51. 10.1093/clinids/13.1.47. [DOI] [PubMed] [Google Scholar]
- 1186.Mogavero GT, Fishman EK, Magid D. 1991. Osseous sporotrichosis: CT appearance. Case report. Clin Imaging 15:56–58. 10.1016/0899-7071(91)90049-2. [DOI] [PubMed] [Google Scholar]
- 1187.Winn RE, Anderson J, Piper J, Aronson NE, Pluss J. 1993. Systemic sporotrichosis treated with itraconazole. Clin Infect Dis 17:210–217. 10.1093/clinids/17.2.210. [DOI] [PubMed] [Google Scholar]
- 1188.Jones N. 1999. Photo quiz. Osteoarticular sporotrichosis. Clin Infect Dis 29:59, 202–203. 10.1086/520181. [DOI] [PubMed] [Google Scholar]
- 1189.Edwards C, Reuther WL, III, Greer DL. 2000. Disseminated osteoarticular sporotrichosis: treatment in a patient with acquired immunodeficiency syndrome. South Med J 93:803–806. 10.1097/00007611-200093080-00013. [DOI] [PubMed] [Google Scholar]
- 1190.Kohler LM, Hamdan JS, Ferrari TC. 2007. Successful treatment of a disseminated Sporothrix schenckii infection and in vitro analysis for antifungal susceptibility testing. Diagn Microbiol Infect Dis 58:117–120. 10.1016/j.diagmicrobio.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 1191.Lynch PJ, Voorhees JJ, Harrell ER. 1970. Systemic sporotrichosis. Ann Intern Med 73:23–30. 10.7326/0003-4819-73-1-23. [DOI] [PubMed] [Google Scholar]
- 1192.Crout JE, Brewer NS, Tompkins RB. 1977. Sporotrichosis arthritis: clinical features in seven patients. Ann Intern Med 86:294–297. 10.7326/0003-4819-86-3-294. [DOI] [PubMed] [Google Scholar]
- 1193.Manhart JW, Wilson JA, Korbitz BC. 1970. Articular and cutaneous sporotrichosis. JAMA 214:365–367. [PubMed] [Google Scholar]
- 1194.Howell SJ, Toohey JS. 1998. Sporotrichal arthritis in south central Kansas. Clin Orthop Relat Res 346:207–214. [PubMed] [Google Scholar]
- 1195.Shaw JC, Levinson W, Montanaro A. 1989. Sporotrichosis in the acquired immunodeficiency syndrome. J Am Acad Dermatol 21:1145–1147. 10.1016/s0190-9622(89)70318-2. [DOI] [PubMed] [Google Scholar]
- 1196.Heller HM, Fuhrer J. 1991. Disseminated sporotrichosis in patients with AIDS: case report and review of the literature. AIDS 5:1243–1246. [PubMed] [Google Scholar]
- 1197.Oscherwitz SL, Rinaldi MG. 1992. Disseminated sporotrichosis in a patient infected with human immunodeficiency virus. Clin Infect Dis 15:568–569. [PubMed] [Google Scholar]
- 1198.Al-Tawfiq JA, Wools KK. 1998. Disseminated sporotrichosis and Sporothrix schenckii fungemia as the initial presentation of human immunodeficiency virus infection. Clin Infect Dis 26:1403–1406. 10.1086/516356. [DOI] [PubMed] [Google Scholar]
- 1199.Gutierrez-Galhardo MC, do Valle ACF, Fraga BLB, Schubach AO, Hoagland BRDS, Monteiro PCF, Barros MBDL. 2010. Disseminated sporotrichosis as a manifestation of immune reconstitution inflammatory syndrome. Mycoses 53:78–80. 10.1111/j.1439-0507.2008.01655.x. [DOI] [PubMed] [Google Scholar]
- 1200.Ware AJ, Cockerell CJ, Skiest DJ, Kussman HM. 1999. Disseminated sporotrichosis with extensive cutaneous involvement in a patient with AIDS. J Am Acad Dermatol 40:350–355. 10.1016/S0190-9622(99)70484-6. [DOI] [PubMed] [Google Scholar]
- 1201.Levinsky WJ. 1972. Sporotrichial arthritis. Report of a case mimicking gout. Arch Intern Med 129:118–119. 10.1001/archinte.129.1.118. [DOI] [PubMed] [Google Scholar]
- 1202.Koeter S, Jackson RW. 2006. Successful total knee arthroplasty in the presence of sporotrichal arthritis. Knee 13:236–237. 10.1016/j.knee.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 1203.Streeten BW, Rabuzzi DD, Jones DB. 1974. Sporotrichosis of the orbital margin. Am J Ophthalmol 77:750–755. 10.1016/0002-9394(74)90544-3. [DOI] [PubMed] [Google Scholar]
- 1204.Gullberg RM, Quintanilla A, Levin ML, Williams J, Phair JP. 1987. Sporotrichosis: recurrent cutaneous, articular, and central nervous system infection in a renal transplant recipient. Rev Infect Dis 9:369–375. 10.1093/clinids/9.2.369. [DOI] [PubMed] [Google Scholar]
- 1205.Birnbaum MA, Walter NE. 1987. Case report of sporotrichosis arthritis. Orthop Rev 16:637–641. [PubMed] [Google Scholar]
- 1206.Friedman SJ, Doyle JA. 1983. Extracutaneous sporotrichosis. Int J Dermatol 22:171–176. 10.1111/j.1365-4362.1983.tb03358.x. [DOI] [PubMed] [Google Scholar]
- 1207.Morgan MA, Cockerill FR, III, Cortese DA, Roberts GD. 1984. Disseminated sporotrichosis with Sporothrix schenckii fungemia. Diagn Microbiol Infect Dis 2:151–155. 10.1016/0732-8893(84)90011-7. [DOI] [PubMed] [Google Scholar]
- 1208.Patange V, Cesani F, Phillpott J, Villanueva-Meyer J. 1995. Three-phase bone and Ga-67 scintigraphy in disseminated sporotrichosis. Clin Nucl Med 20:909–912. 10.1097/00003072-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 1209.Koga T, Duan H, Furue M. 2002. Immunohistochemical detection of interferon-gamma-producing cells in granuloma formation of sporotrichosis. Med Mycol 40:111–114. 10.1080/mmy.40.2.111.114. [DOI] [PubMed] [Google Scholar]
- 1210.Lima OC, Figueiredo CC, Pereira BAS, Coelho MGP, Morandi V, Lopes-Bezerra LM. 1999. Adhesion of the human pathogen Sporothrix schenckii to several extracellular matrix proteins. Braz J Med Biol Res 32:651–657. 10.1590/s0100-879x1999000500020. [DOI] [PubMed] [Google Scholar]
- 1211.Figueiredo CC, de Lima OC, de Carvalho L, Lopes-Bezerra LM, Morandi V. 2004. The in vitro interaction of Sporothrix schenckii with human endothelial cells is modulated by cytokines and involves endothelial surface molecules. Microb Pathog 36:177–188. 10.1016/j.micpath.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 1212.Figueiredo CC, Deccache PM, Lopes-Bezerra LM, Morandi V. 2007. TGF-beta1 induces transendothelial migration of the pathogenic fungus Sporothrix schenckii by a paracellular route involving extracellular matrix proteins. Microbiology (Reading) 153:2910–2921. 10.1099/mic.0.2006/005421-0. [DOI] [PubMed] [Google Scholar]
- 1213.Kumar R, van der Smissen E, Jorizzo J. 1984. Systemic sporotrichosis with osteomyelitis. J Can Assoc Radiol 35:83–84. [PubMed] [Google Scholar]
- 1214.Govender S, Rasool MN, Ngcelwane M. 1989. Osseous sporotrichosis. J Infect 19:273–276. 10.1016/S0163-4453(89)90829-3. [DOI] [PubMed] [Google Scholar]
- 1215.Chang AC, Destouet JM, Murphy WA. 1984. Musculoskeletal sporotrichosis. Skeletal Radiol 12:23–28. 10.1007/BF00373171. [DOI] [PubMed] [Google Scholar]
- 1216.Appenzeller S, Amaral TN, Amstalden EMI, Bertolo MB, Neto JFM, Samara AM, Fernandes SRM. 2006. Sporothrix schenckii infection presented as monoarthritis: report of two cases and review of the literature. Clin Rheumatol 25:926–928. 10.1007/s10067-005-0095-z. [DOI] [PubMed] [Google Scholar]
- 1217.Dehaven KE, Wilde AH, O’Duffy JD. 1972. Sporotrichosis arthritis and tenosynovitis. Report of a case cured by synovectomy and amphotericin B. J Bone Joint Surg Am 54:874–877. 10.2106/00004623-197254040-00018. [DOI] [PubMed] [Google Scholar]
- 1218.Downs NJ, Hinthorn DR, Mhatre VR, Liu C. 1989. Intra-articular amphotericin B treatment of Sporothrix schenckii arthritis. Arch Intern Med 149:954–955. [PubMed] [Google Scholar]
- 1219.Marrocco GR, Tihen WS, Goodnough CP, Johnson RJ. 1975. Granulomatous synovitis and osteitis caused by Sporothrix schenckii. Am J Clin Pathol 64:345–350. 10.1093/ajcp/64.3.345. [DOI] [PubMed] [Google Scholar]
- 1220.Parker JD, Sarosi GA, Tosh FE. 1970. Treatment of extracutaneous sporotrichosis. Arch Intern Med 125:858–863. [PubMed] [Google Scholar]
- 1221.Altner PC, Turner RR. 1970. Sporotrichosis of bones and joints. Review of the literature and report of 6 cases. Clin Orthop Relat Res 68:138–148. 10.1097/00003086-197001000-00026. [DOI] [PubMed] [Google Scholar]
- 1222.Brook CJ, Ravikrishnan KP, Weg JG. 1977. Pulmonary and articular sporotrichosis. Am Rev Respir Dis 116:141–143. [DOI] [PubMed] [Google Scholar]
- 1223.Campos-Macias P, Arenas R, Vega-Memije M, Kawasaki M. 2006. Sporothrix schenckii type 3D (mtDNA-RFLP): report of an osteoarticular case. J Dermatol 33:295–299. 10.1111/j.1346-8138.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 1224.DeHart DJ. 1995. Use of itraconazole for treatment of sporotrichosis involving a knee prosthesis. Clin Infect Dis 21:450. 10.1093/clinids/21.2.450. [DOI] [PubMed] [Google Scholar]
- 1225.Kreft E, Amihood S. 1972. Sporotrichosis of the knee joint. S Afr Med J 46:1329–1332. [PubMed] [Google Scholar]
- 1226.Serstock DS, Zinnerman HH. 1975. Pulmonary and articular sporotrichosis. Report of two cases. JAMA 233:1291–1293. [PubMed] [Google Scholar]
- 1227.Yacobucci GN, Santilli MD. 1986. Sporotrichosis of the knee. A case report. Orthopedics 9:387–390. 10.3928/0147-7447-19860301-11. [DOI] [PubMed] [Google Scholar]
- 1228.Yao J, Penn RG, Ray S. 1986. Articular sporotrichosis. Clin Orthop Relat Res 204:207–214. [PubMed] [Google Scholar]
- 1229.Zacharias J, Crosby LA. 1997. Sporotrichal arthritis of the knee. Am J Knee Surg 10:171–174. [PubMed] [Google Scholar]
- 1230.Atdjian M, Granda JL, Ingberg HO, Kaplan BL. 1980. Systemic sporotrichosis polytenosynovitis with median and ulnar nerve entrapment. JAMA 243:1841–1842. [PubMed] [Google Scholar]
- 1231.Badley AD, van Scoy RE. 1996. Long-term follow-up of multifocal osteoarticular sporotrichosis treated with itraconazole. Clin Infect Dis 23:394–395. 10.1093/clinids/23.2.394. [DOI] [PubMed] [Google Scholar]
- 1232.Chowdhary G, Weinstein A, Klein R, Mascarenhas BR. 1991. Sporotrichal arthritis. Ann Rheum Dis 50:112–114. 10.1136/ard.50.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1233.Gladstone JI, Littman M. 1971. Osseous sporotrichosis. Failure of treatment with potassium iodide and sulfadimethoxine and success with amphotericin B. Am J Med 51:121–133. 10.1016/0002-9343(71)90329-9. [DOI] [PubMed] [Google Scholar]
- 1234.Gordhan A, Ramdial PK, Morar N, Moodley SD, Aboobaker J. 2001. Disseminated cutaneous sporotrichosis: a marker of osteoarticular sporotrichosis masquerading as gout. Int J Dermatol 40:717–719. 10.1046/j.1365-4362.2001.01300.x. [DOI] [PubMed] [Google Scholar]
- 1235.Halverson PB, Lahiri S, Wojno WC, Sulaiman AR. 1985. Sporotrichal arthritis presenting as granulomatous myositis. Arthritis Rheum 28:1425–1429. 10.1002/art.1780281217. [DOI] [PubMed] [Google Scholar]
- 1236.Khan MI, Goss G, Gotsman A, Asvat MS. 1983. Sporotrichosis arthritis. A case presentation and review of the literature. S Afr Med J 64:1099–1101. [PubMed] [Google Scholar]
- 1237.Lesperance M, Baumgartner D, Kauffman CA. 1988. Polyarticular arthritis due to Sporothrix schenckii. Mycoses 31:599–603. [PubMed] [Google Scholar]
- 1238.Lipstein-Kresch E, Isenberg HD, Singer C, Cooke O, Greenwald RA. 1985. Disseminated Sporothrix schenckii infection with arthritis in a patient with acquired immunodeficiency syndrome. J Rheumatol 12:805–808. [PubMed] [Google Scholar]
- 1239.Molstad B, Strom R. 1978. Multiarticular sporotrichosis. JAMA 240:556–557. [PubMed] [Google Scholar]
- 1240.Purvis RS, Diven DG, Drechsel RD, Calhoun JH, Tyring SK. 1993. Sporotrichosis presenting as arthritis and subcutaneous nodules. J Am Acad Dermatol 28:879–884. 10.1016/0190-9622(93)70124-c. [DOI] [PubMed] [Google Scholar]
- 1241.Shelley WB, Sica PA, Jr.. 1983. Disseminate sporotrichosis of skin and bone cured with 5-fluorocytosine: photosensitivity as a complication. J Am Acad Dermatol 8:229–235. 10.1016/s0190-9622(83)70029-0. [DOI] [PubMed] [Google Scholar]
- 1242.Weitzner R, Mak E, Lertratanakul Y. 1977. Articular sporotrichosis. Ann Intern Med 87:382. 10.7326/0003-4819-87-3-382_1. [DOI] [PubMed] [Google Scholar]
- 1243.Wilson SD, Grossheim R, Blaine JW, Ferguson RB. 1988. Case report of synovial sporotrichosis involving both wrists. J Med Vet Mycol 26:307–309. 10.1080/02681218880000421. [DOI] [PubMed] [Google Scholar]
- 1244.Castro RM, de Sabogal MF, Cuce LC, Salebian A. 1981. Disseminate sporotrichosis—report of a clinical case with mucocutaneous, osteo-articular, and ocular lesions. Mykosen 24:92–96. 10.1111/j.1439-0507.1981.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 1245.Anees A, Ali A, Fordham EW. 1986. Abnormal bone and gallium scans in a case of multifocal systemic sporotrichosis. Clin Nucl Med 11:663–664. 10.1097/00003072-198609000-00018. [DOI] [PubMed] [Google Scholar]
- 1246.Bayer AS, Scott VJ, Guze LB. 1979. Fungal arthritis. III. Sporotrichal arthritis. Semin Arthritis Rheum 9:66–74. 10.1016/0049-0172(79)90003-9. [DOI] [PubMed] [Google Scholar]
- 1247.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Cordoba S, Gonzalez GM, Govender NP, Guarro J, Johnson EM, Kidd SE, Pereira SA, Rodrigues AM, Rozental S, Szeszs MW, Balleste Alaniz R, Bonifaz A, Bonfietti LX, Borba-Santos LP, Capilla J, Colombo AL, Dolande M, Isla MG, Melhem MSC, Mesa-Arango AC, Oliveira MME, Panizo MM, Pires de Camargo Z, Zancope-Oliveira RM, Meis JF, Turnidge J. 2017. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother 61:e01057-17. 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1248.Kauffman CA, Bustamante B, Chapman SW, Pappas PG, Infectious Diseases Society of America. 2007. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 45:1255–1265. 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 1249.Sharkey-Mathis PK, Kauffman CA, Graybill JR, Stevens DA, Hostetler JS, Cloud G, Dismukes WE. 1993. Treatment of sporotrichosis with itraconazole. NIAID Mycoses Study Group. Am J Med 95:279–285. 10.1016/0002-9343(93)90280-3. [DOI] [PubMed] [Google Scholar]
- 1250.Kauffman CA, Pappas PG, McKinsey DS, Greenfield RA, Perfect JR, Cloud GA, Thomas CJ, Dismukes WE. 1996. Treatment of lymphocutaneous and visceral sporotrichosis with fluconazole. Clin Infect Dis 22:46–50. 10.1093/clinids/22.1.46. [DOI] [PubMed] [Google Scholar]
- 1251.Bunce PE, Yang L, Chun S, Zhang SX, Trinkaus MA, Matukas LM. 2012. Disseminated sporotrichosis in a patient with hairy cell leukemia treated with amphotericin B and posaconazole. Med Mycol 50:197–201. 10.3109/13693786.2011.584074. [DOI] [PubMed] [Google Scholar]
- 1252.Fernandez-Silva F, Capilla J, Mayayo E, Guarro J. 2012. Efficacy of posaconazole in murine experimental sporotrichosis. Antimicrob Agents Chemother 56:2273–2277. 10.1128/AAC.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1253.Mario DN, Guarro J, Santurio JM, Alves SH, Capilla J. 2015. In vitro and in vivo efficacy of amphotericin B combined with posaconazole against experimental disseminated sporotrichosis. Antimicrob Agents Chemother 59:5018–5021. 10.1128/AAC.00052-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1254.Fernandez-Silva F, Capilla J, Mayayo E, Guarro J. 2014. Modest efficacy of voriconazole against murine infections by Sporothrix schenckii and lack of efficacy against Sporothrix brasiliensis. Mycoses 57:121–124. 10.1111/myc.12112. [DOI] [PubMed] [Google Scholar]
- 1255.King J, Pana Z-D, Lehrnbecher T, Steinbach WJ, Warris A. 2017. Recognition and clinical presentation of invasive fungal disease in neonates and children. J Pediatric Infect Dis Soc 6:S12–S21. 10.1093/jpids/pix053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1256.Henriet S, Verweij PE, Holland SM, Warris A. 2013. Invasive fungal infections in patients with chronic granulomatous disease. Adv Exp Med Biol 764:27–55. 10.1007/978-1-4614-4726-9_3. [DOI] [PubMed] [Google Scholar]
- 1257.Antachopoulos C, Walsh TJ, Roilides E. 2007. Fungal infections in primary immunodeficiencies. Eur J Pediatr 166:1099–1117. 10.1007/s00431-007-0527-7. [DOI] [PubMed] [Google Scholar]
- 1258.Pana ZD, Vikelouda K, Roilides E. 2016. Diagnosis of invasive fungal diseases in pediatric patients. Expert Rev Anti Infect Ther 14:1203–1213. 10.1080/14787210.2016.1242413. [DOI] [PubMed] [Google Scholar]
- 1259.Huppler AR, Fisher BT, Lehrnbecher T, Walsh TJ, Steinbach WJ. 2017. Role of molecular biomarkers in the diagnosis of invasive fungal diseases in children. J Pediatric Infect Dis Soc 6:S32–S44. 10.1093/jpids/pix054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1260.Groll AH, Schrey D, Tragiannidis A, Bochennek K, Lehrnbecher T. 2013. Invasive aspergillosis in children and adolescents. Curr Pharm Des 19:3545–3568. 10.2174/13816128113199990311. [DOI] [PubMed] [Google Scholar]
- 1261.Roilides E, Pana ZD. 2012. Application of diagnostic markers to invasive aspergillosis in children. Ann N Y Acad Sci 1272:1–8. 10.1111/j.1749-6632.2012.06828.x. [DOI] [PubMed] [Google Scholar]
- 1262.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Herbrecht R, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 18(Suppl 7):38–52. 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 1263.Pana ZD, Kougia V, Roilides E. 2015. Therapeutic strategies for invasive fungal infections in neonatal and pediatric patients: an update. Expert Opin Pharmacother 16:693–710. 10.1517/14656566.2015.1013936. [DOI] [PubMed] [Google Scholar]
- 1264.Peel TN, Cheng AC, Buising KL, Choong PF. 2012. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother 56:2386–2391. 10.1128/AAC.06246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1265.Riaz T, Tande AJ, Steed LL, Demos HA, Salgado CD, Osmon DR, Marculescu CE. 2020. Risk factors for fungal prosthetic joint infection. J Bone Jt Infect 5:76–81. 10.7150/jbji.40402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1266.Hwang BH, Yoon JY, Nam CH, Jung KA, Lee SC, Han CD, Moon SH. 2012. Fungal peri-prosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br 94:656–659. 10.1302/0301-620X.94B5.28125. [DOI] [PubMed] [Google Scholar]
- 1267.Kuiper JWP, van den Bekerom MPJ, van der Stappen J, Nolte PA, Colen S. 2013. 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections. Acta Orthop 84:517–523. 10.3109/17453674.2013.859422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1268.Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, Bozic K, Della Valle C, Pulido L, Barrack R. 2009. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am 91(Suppl 6):142–149. 10.2106/JBJS.I.00574. [DOI] [PubMed] [Google Scholar]
- 1269.Jakobs O, Schoof B, Klatte TO, Schmidl S, Fensky F, Guenther D, Frommelt L, Gehrke T, Gebauer M. 2015. Fungal periprosthetic joint infection in total knee arthroplasty: a systematic review. Orthop Rev (Pavia) 7:5623. 10.4081/or.2015.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1270.Schoof B, Jakobs O, Schmidl S, Klatte TO, Frommelt L, Gehrke T, Gebauer M. 2015. Fungal periprosthetic joint infection of the hip: a systematic review. Orthop Rev (Pavia) 7:18–22. 10.4081/or.2015.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271.Lichtman EA. 1983. Candida infection of a prosthetic shoulder joint. Skeletal Radiol 10:176–177. 10.1007/BF00357775. [DOI] [PubMed] [Google Scholar]
- 1272.Skedros JG, Keenan KE, Updike WS, Oliver MR. 2014. Failed reverse total shoulder arthroplasty caused by recurrent Candida glabrata infection with prior Serratia marcescens coinfection. Case Rep Infect Dis 2014:142428. 10.1155/2014/142428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1273.Shah NB, Shoham S, Nayak S. 2015. Cryptococcus neoformans prosthetic joint infection: case report and review of the literature. Mycopathologia 179:275–278. 10.1007/s11046-014-9847-0. [DOI] [PubMed] [Google Scholar]
- 1274.Johannsson B, Callaghan JJ. 2009. Prosthetic hip infection due to Cryptococcus neoformans: case report. Diagn Microbiol Infect Dis 64:76–79. 10.1016/j.diagmicrobio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 1275.MacLean IS, Day SR, Moore CC, Browne JA. 2015. Blastomycosis infection of the knee treated with staged total knee arthroplasty. Knee 22:669–671. 10.1016/j.knee.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 1276.Lackner M, De Man FH, Eygendaal D, Wintermans RGF, Kluytmans JA, Klaassen CH, Meis JF. 2011. Severe prosthetic joint infection in an immunocompetent male patient due to a therapy refractory Pseudallescheria apiosperma. Mycoses 54(Suppl 3):22–27. 10.1111/j.1439-0507.2011.02107.x. [DOI] [PubMed] [Google Scholar]
- 1277.Gebauer M, Frommelt L, Achan P, Board TN, Conway J, Griffin W, Heidari N, Kerr G, McLaren A, Nelson SB, Nijhof M, Zahar A. 2014. Management of fungal or atypical periprosthetic joint infections. J Orthop Res 32(Suppl 1):S147–S151. 10.1002/jor.22559. [DOI] [PubMed] [Google Scholar]
- 1278.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 1279.Phelan DM, Osmon DR, Keating MR, Hanssen AD. 2002. Delayed reimplantation arthroplasty for candidal prosthetic joint infection: a report of 4 cases and review of the literature. Clin Infect Dis 34:930–938. 10.1086/339212. [DOI] [PubMed] [Google Scholar]
- 1280.Kim SJ, Huh J, Odrobina R, Kim JH. 2015. Systemic review of published literature on Candida infection following total hip arthroplasty. Mycopathologia 179:173–185. 10.1007/s11046-014-9852-3. [DOI] [PubMed] [Google Scholar]
- 1281.Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. 1989. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis 11:89–96. 10.1093/clinids/11.1.89. [DOI] [PubMed] [Google Scholar]
- 1282.Nayeri F, Cameron R, Chryssanthou E, Johansson L, Soderstrom C. 1997. Candida glabrata prosthesis infection following pyelonephritis and septicaemia. Scand J Infect Dis 29:635–638. 10.3109/00365549709035912. [DOI] [PubMed] [Google Scholar]
- 1283.Gaston G, Ogden J. 2004. Candida glabrata periprosthetic infection: a case report and literature review. J Arthroplasty 19:927–930. 10.1016/j.arth.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 1284.Langer P, Kassim RA, Macari GS, Saleh KJ. 2003. Aspergillus infection after total knee arthroplasty. Am J Orthop (Belle Mead NJ) 32:402–404. [PubMed] [Google Scholar]
- 1285.Zuo Q, Dong L, Mu W, Zhou L, Hu T, Zhang H. 2015. Trichosporon asahii infection after total knee arthroplasty: a case report and review of the literature. Can J Infect Dis Med Microbiol 26:47–51. 10.1155/2015/458670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1286.Cutrona AF, Shah M, Himes MS, Miladore MA. 2002. Rhodotorula minuta: an unusual fungal infection in hip-joint prosthesis. Am J Orthop (Belle Mead NJ) 31:137–140. [PubMed] [Google Scholar]
- 1287.Fanning S, Mitchell AP. 2012. Fungal biofilms. PLoS Pathog 8:e1002585. 10.1371/journal.ppat.1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1288.Desai JV, Mitchell AP, Andes DR. 2014. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb Perspect Med 4:a019729. 10.1101/cshperspect.a019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1289.Gebauer M, Frommelt L, Achan P, Board TN, Conway J, Griffin W, Heidari N, Kerr G, McLaren A, Nelson SB, Nijhof M, Zahar A. 2014. Management of fungal or atypical periprosthetic joint infections. J Arthroplasty 29:112–114. 10.1016/j.arth.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 1290.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 1291.Bracken CD, Berbari EF, Hanssen AD, Mabry TM, Osmon DR, Sierra RJ. 2014. Systemic inflammatory markers and aspiration cell count may not differentiate bacterial from fungal prosthetic infections. Clin Orthop Relat Res 472:3291–3294. 10.1007/s11999-014-3631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1292.Kinuya S, Yokoyama K, Haji K, Konishi S, Hwang E-H, Michigishi T, Tonami N. 1996. In-111 labeled leukocyte scintigraphy of infected prosthesis with Candida palapsilosis [sic] in a patient without predisposing risk factors. Clin Nucl Med 21:885–886. 10.1097/00003072-199611000-00017. [DOI] [PubMed] [Google Scholar]
- 1293.Selmon GP, Slater RN, Shepperd JA, Wright EP. 1998. Successful 1-stage exchange total knee arthroplasty for fungal infection. J Arthroplasty 13:114–115. 10.1016/s0883-5403(98)90086-9. [DOI] [PubMed] [Google Scholar]
- 1294.Jenny J-Y, Goukodadja O, Boeri C, Gaudias J. 2016. May one-stage exchange for Candida albicans peri-prosthetic infection be successful? Orthop Traumatol Surg Res 102:127–129. 10.1016/j.otsr.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 1295.Klatte TO, Kendoff D, Kamath AF, Jonen V, Rueger JM, Frommelt L, Gebauer M, Gehrke T. 2014. Single-stage revision for fungal peri-prosthetic joint infection. Bone Joint J 96-B:492–496. 10.1302/0301-620X.96B4.32179. [DOI] [PubMed] [Google Scholar]
- 1296.Artiaco S, Ferrero A, Boggio F, Colzani G. 2013. Pseudotumor of the hip due to fungal prosthetic joint infection. Case Rep Orthop 2013:502728. 10.1155/2013/502728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1297.Simonian PT, Brause BD, Wickiewicz TL. 1997. Candida infection after total knee arthroplasty. Management without resection or amphotericin B. J Arthroplasty 12:825–829. 10.1016/s0883-5403(97)90015-2. [DOI] [PubMed] [Google Scholar]
- 1298.Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. 2001. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect 42:208–209. 10.1053/jinf.2001.0819. [DOI] [PubMed] [Google Scholar]
- 1299.Kelesidis T, Tsiodras S. 2010. Candida albicans prosthetic hip infection in elderly patients: is fluconazole monotherapy an option? Scand J Infect Dis 42:12–21. 10.3109/00365540903253510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1300.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg B-J, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1301.Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. 2019. Prosthetic joint infections caused by Candida species: a systematic review and a case series. Mycopathologia 184:23–33. 10.1007/s11046-018-0286-1. [DOI] [PubMed] [Google Scholar]
- 1302.Silverberg D, Kodali P, Dipersio J, Acus R, Askew M. 2002. In vitro analysis of antifungal impregnated polymethylmethacrylate bone cement. Clin Orthop Relat Res 403:228–231. 10.1097/00003086-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 1303.Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. 2007. Elution and mechanical properties of antifungal bone cement. J Arthroplasty 22:902–908. 10.1016/j.arth.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 1304.Buranapanitkit B, Oungbho K, Ingviya N. 2005. The efficacy of hydroxyapatite composite impregnated with amphotericin B. Clin Orthop Relat Res 437:236–241. 10.1097/01.blo.0000165851.81386.6a. [DOI] [PubMed] [Google Scholar]
- 1305.Cunningham B, McLaren AC, Pauken C, McLemore R. 2012. Liposomal formulation increases local delivery of amphotericin from bone cement: a pilot study. Clin Orthop Relat Res 470:2671–2676. 10.1007/s11999-012-2317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1306.Grimsrud C, Raven R, Fothergill AW, Kim HT. 2011. The in vitro elution characteristics of antifungal-loaded PMMA bone cement and calcium sulfate bone substitute. Orthopedics 34:e378–e381. 10.3928/01477447-20110627-05. [DOI] [PubMed] [Google Scholar]
- 1307.Rouse MS, Heijink A, Steckelberg JM, Patel R. 2011. Are anidulafungin or voriconazole released from polymethylmethacrylate in vitro? Clin Orthop Relat Res 469:1466–1469. 10.1007/s11999-010-1643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1308.Miller RB, McLaren AC, Pauken C, Clarke HD, McLemore R. 2013. Voriconazole is delivered from antifungal-loaded bone cement. Clin Orthop Relat Res 471:195–200. 10.1007/s11999-012-2463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1309.Goff TAJ, Rambani R, Ng AB. 2014. Current concepts in the management of periprosthetic fungal joint infection using antifungal bone cement. Curr Orthop Pract 25:169–174. 10.1097/BCO.0000000000000074. [DOI] [Google Scholar]
- 1310.Bruce AS, Kerry RM, Norman P, Stockley I. 2001. Fluconazole-impregnated beads in the management of fungal infection of prosthetic joints. J Bone Joint Surg Br 83:183–184. 10.1302/0301-620x.83b2.11444. [DOI] [PubMed] [Google Scholar]
- 1311.Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, Jewesson PJ. 2001. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg 44:383–386. [PMC free article] [PubMed] [Google Scholar]
- 1312.Ueng SWN, Lee C-Y, Hu C, Hsieh P-H, Chang Y. 2013. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res 471:3002–3009. 10.1007/s11999-013-3007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1313.Wang QJ, Shen H, Zhang XL, Jiang Y, Wang Q, Chen YS, Shao JJ. 2015. Staged reimplantation for the treatment of fungal peri-prosthetic joint infection following primary total knee arthroplasty. Orthop Traumatol Surg Res 101:151–156. 10.1016/j.otsr.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 1314.Kim JK, Lee DY, Kang DW, Ro DH, Lee MC, Han HS. 2018. Efficacy of antifungal-impregnated cement spacer against chronic fungal periprosthetic joint infections after total knee arthroplasty. Knee 25:631–637. 10.1016/j.knee.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 1315.Deelstra JJ, Neut D, Jutte PC. 2013. Successful treatment of Candida albicans-infected total hip prosthesis with staged procedure using an antifungal-loaded cement spacer. J Arthroplasty 28:374.e5–374.e8. 10.1016/j.arth.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 1316.Nowbakht C, Garrity K, Webber N, Eraso J, Ostrosky-Zeichner L. 2017. Prosthetic joint infection due to Histoplasma capsulatum complicating a total knee arthroplasty. Open Forum Infect Dis 4:ofx118. 10.1093/ofid/ofx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1317.Whiteside LA, Roy ME. 2017. One-stage revision with catheter infusion of intraarticular antibiotics successfully treats infected THA. Clin Orthop Relat Res 475:419–429. 10.1007/s11999-016-4977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1318.Cooper LG, Heydemann J, Misenhimer G, Antony S. 2017. Use of intra-articular amphotericin B in the treatment of Candida parasilosis [sic] and albicans in prosthetic joint infections (PJI): a novel approach to this difficult problem. Infect Disord Drug Targets 17:36–42. 10.2174/1871526516666161026125606. [DOI] [PubMed] [Google Scholar]
- 1319.Winthrop KL. 2005. Update on tuberculosis and other opportunistic infections associated with drugs blocking tumour necrosis factor alpha. Ann Rheum Dis 64(Suppl 4):iv29–iv30. 10.1136/ard.2005.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1320.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. 2004. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 38:1261–1265. 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 1321.Tsiodras S, Samonis G, Boumpas DT, Kontoyiannis DP. 2008. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc 83:181–194. [PubMed] [Google Scholar]
- 1322.Zimmermann B, III, Mikolich DJ, Ho G, Jr.. 1995. Septic bursitis. Semin Arthritis Rheum 24:391–410. 10.1016/S0049-0172(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 1323.Gamaletsou M, Sipsas N, Bueno M, Kontoyiannis D, Petraitiene R, Roilides E, Rammaert B, Zeller V, Prinapori R, Taj-Aldeen S, Brause B, Lortholary O, Walsh TJ. 2015. Candida bursitis: systematic analysis of mechanisms, manifestations, microbiology, treatment, and outcome, abstr EV0990. Abstr 25th Eur Congr Clin Microbiol Infect Dis, Copenhagen, Denmark.
- 1324.Hall RL, Frost RM, Vasukutty NL, Minhas H. 2012. Candida glabrata: an unusual fungal infection following a total hip replacement. BMJ Case Rep 2012:bcr-2012-006491. 10.1136/bcr-2012-006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1325.Compton J, Vander Voort W, Willey M, Sekar P. 2018. A case of Histoplasma capsulatum variety capsulatum septic arthritis successfully treated with surgery, systemic antifungals, and local amphotericin cement beads. Int J Infect Dis 77:23–25. 10.1016/j.ijid.2018.09.023. [DOI] [PubMed] [Google Scholar]