SUMMARY
Paracoccidioidomycosis (PCM), initially reported in 1908 in the city of São Paulo, Brazil, by Adolpho Lutz, is primarily a systemic and neglected tropical mycosis that may affect individuals with certain risk factors around Latin America, especially Brazil. Paracoccidioides brasiliensis sensu stricto, a classical thermodimorphic fungus associated with PCM, was long considered to represent a monotypic taxon. However, advances in molecular taxonomy revealed several cryptic species, including Paracoccidioides americana, P. restrepiensis, P. venezuelensis, and P. lutzii, that show a preference for skin and mucous membranes, lymph nodes, and respiratory organs but can also affect many other organs. The classical diagnosis of PCM benefits from direct microscopy culture-based, biochemical, and immunological assays in a general microbiology laboratory practice providing a generic identification of the agents. However, molecular assays should be employed to identify Paracoccidioides isolates to the species level, data that would be complemented by epidemiological investigations. From a clinical perspective, all probable and confirmed cases should be treated. The choice of treatment and its duration must be considered, along with the affected organs, process severity, history of previous treatment failure, possibility of administering oral medication, associated diseases, pregnancy, and patient compliance with the proposed treatment regimen. Nevertheless, even after appropriate treatment, there may be relapses, which generally occur 5 years after the apparent cure following treatment, and also, the mycosis may be confused with other diseases. This review provides a comprehensive and critical overview of the immunopathology, laboratory diagnosis, clinical aspects, and current treatment of PCM, highlighting current issues in the identification, treatment, and patient follow-up in light of recent Paracoccidioides species taxonomic developments.
KEYWORDS: Paracoccidioides, endemic mycosis, dimorphic fungi, Paracoccidioides brasiliensis, Paracoccidioides lutzii, epidemiology, diagnostics, mycology
INTRODUCTION
The first unquestionable case presenting as paracoccidioidomycosis (PCM) was reported in 1908 from the city of São Paulo, Brazil, by the physician Adolpho Lutz. He described the clinical and pathological aspects of pseudococcidic infections observed in two patients with severe mucocutaneous lesions (1). Morphological similarities with other infections reported in the Americas led Lutz to classify such pseudococcidic infection as American hyphoblastomycosis (1). Later on, Alfonso Splendore documented four new PCM cases based on histopathological observations (2). Through his work, he was able to reisolate the fungus from biological samples and proceeded to classify it as Zymonema brasiliensis. It was only in 1930 that Floriano de Almeida, after careful observation of the differences between Coccidioides immitis and the newly described fungus, demonstrated that PCM and coccidioidomycosis were two distinct pathologies caused by different fungi (3). This led him to classify the agent as Paracoccidioides brasiliensis. Although there were other attempts to name it, none of these names prevailed after Almeida’s classification (3–5). Finally, in 1971, during the Paracoccidioidomycosis Symposium in Medellin, Colombia, the organism was consensually recognized as Paracoccidioides brasiliensis (6, 7).
Paracoccidioides was long considered to represent a monotypic taxon, epitypified by P. brasiliensis (https://www.mycobank.org/page/Name%20details%20page/name/Paracoccidioides%20brasiliensis) (MycoBank accession number MB258811), the only representative of its kind since its description (3). Almeida meticulously described the micro- and macromorphological characteristics of this organism (3). Recently, however, molecular studies carried out with this fungus in recent years have provided a more reliable taxonomic classification by including descriptions of numerous cryptic Paracoccidioides species (8–12).
The high genetic diversity (13–17) and virulence attributes (14, 18, 19) of Paracoccidioides spp. have been recognized since the 1990s (12, 13).
Based on nuclear gene sequencing and subsequent phylogenetic analyses of different Latin American isolates, at least four phylogenetic species have been proposed for P. brasiliensis. They have been named S1 (species 1), PS2 (phylogenetic species 2), PS3 (phylogenetic species 3), and PS4 (phylogenetic species 4) (8, 20). Among these phylogenetic species, S1 comprises a monophyletic and recombinant population, whereas the other three species, PS2, PS3, and PS4, are formed by paraphyletic and clonal populations. Also, the two phylogenetic species S1 and PS2 are sympatric but reproductively isolated, and the cryptic species PS3 (Colombia) and PS4 (Venezuela) are allopatric, suggesting geographic isolation (8, 20, 21).
After discovering distinct species belonging to the Paracoccidioides species complex, Carrero and colleagues (11) described an isolate, initially named Pb01-like, that is phylogenetically separated from the other cryptic Paracoccidioides species. Hence, it was proposed that Pb01 could represent a new taxon (11). However, more Pb01-like specimens need to be found to validate this hypothesis. Teixeira and coworkers (10) confirmed the existence of a new species of Paracoccidioides based on analyses of the phylogenetic data, molecular recombination, and morphology of 17 isolates genetically similar to the Pb01-like isolate (10). Finally, in 2014, this group of isolates, divergent from the cryptic species described previously, was named Paracoccidioides lutzii in honor of the physician Adolpho Lutz, a pioneer in the description of PCM (22). Along with genetic characteristics, the distinction between the species P. brasiliensis and P. lutzii was supported by significant antigenic differences, having implications for the serological diagnosis of the disease (23–26).
The application of multilocus sequence analysis led Turissini and collaborators (9) to propose four cryptic species formed by the S1, PS2, PS3, and PS4 genetic clusters. The genetic divergence of Paracoccidioides spp. is supported by nuclear gene genealogies but not by mitochondrial loci, where it is assumed that mitochondrial introgression has occurred. In this scenario, P. brasiliensis sensu stricto comprises the S1 group, while the remaining clusters, PS2, PS3, and PS4, were named P. americana, P. restrepiensis, and P. venezuelensis, respectively (9).
In this review, we have adopted the term Paracoccidioides spp. to refer to phylogenetic groups S1, PS2, PS3, and PS4 (P. brasiliensis sensu stricto, P. americana, P. restrepiensis, and P. venezuelensis). This adoption was based on the taxonomic concept that defines a species complex as a cluster of closely related microorganisms that share an ancestor and that are phenotypically very similar to the point where the boundaries are generally unclear (27, 28). Therefore, the P. brasiliensis complex denomination is an appropriate term to refer to a cluster of cryptic species showing no differences in their clinical attributes or their virulence characteristics among them thus far (26, 29–31). Nonetheless, they exhibit striking genetic features that distinguish them (32). Therefore, the relevance of the separation is molecular, based on DNA sequencing of different gene regions (9) or the analysis of their complete genomes (33, 34).
Ecology of Paracoccidioides Species
The genus Paracoccidioides (Ascomycota, Onygenales) belongs to the family Ajellomycetaceae along with the other clinically relevant genera, Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis, and Histoplasma, and therefore, they share characteristics, being saprophytic, thermodimorphic, and associated with warm-blooded vertebrate hosts (35, 36). Molecular studies suggest that the nonculturable pathogen Lacazia loboi also belongs to the Ajellomycetaceae and is phylogenetically related to Paracoccidioides spp. (37, 38).
Fungi of the Onygenales are present in the soil, and such a substratum certainly does not act as a passive propagule reservoir (39). In soil, Paracoccidioides species inhabit a complex environment with amoeboid predators (e.g., Acanthamoeba spp., Allovahlkampfia spelaea, and Vermamoeba vermiformis), all of which may exert selective pressure guiding the evolution of fungal virulence (40). The Paracoccidioides species mycelium form is influenced by environmental conditions such as temperature and humidity variations as well as competition with other microorganisms. During the infectious stage, Paracoccidioides species changes into a yeast phase while going through a significant process of adaptation to conditions such as increased temperature, hormonal influences, and attacks by the host immune system (41).
Although PCM has been known for over a century, the exact niche of the agent and the ecology of its habit remain puzzling due to difficulties in the isolation of the agent from the environment. Paracoccidioides spp. have rarely been isolated from environmental samples such as soil, possibly due to their low concentrations in such localities.
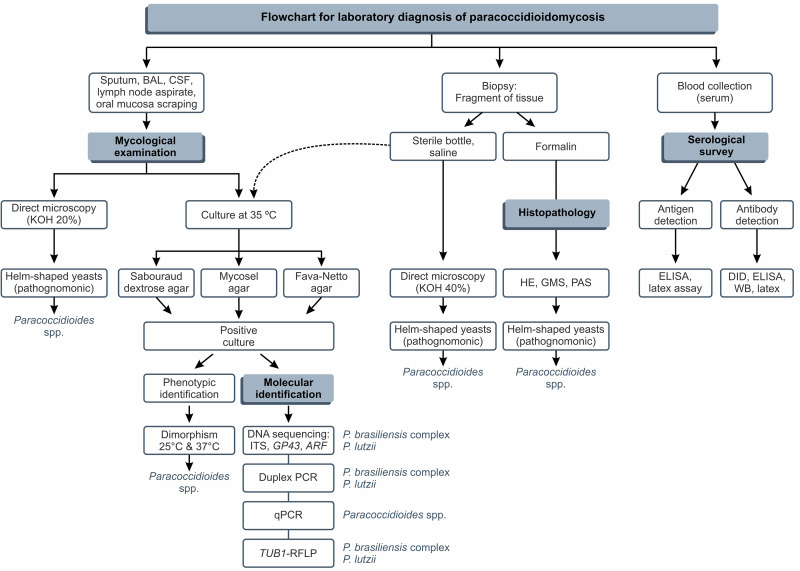
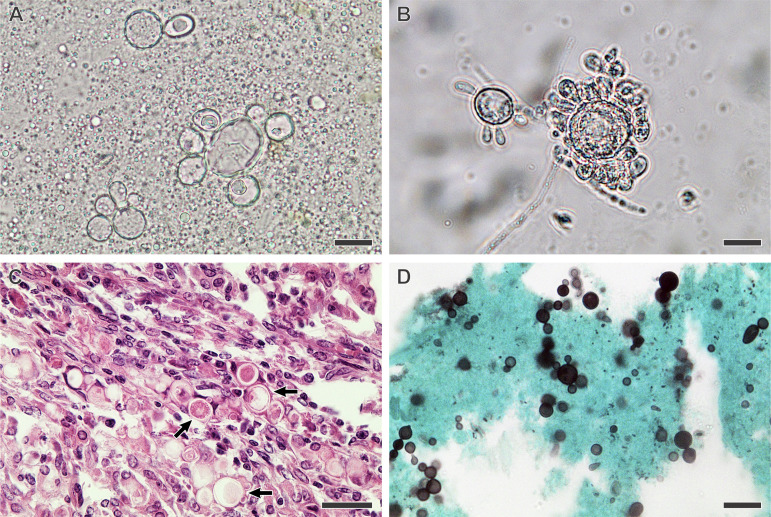
The suspected diagnosis of PCM occurs through clinical and epidemiological data, but confirmation is done primarily by the identification of the etiological agent using fresh-tissue examinations, cultures, and histopathological preparations, which are considered the gold standards for the definition of the disease, being known as direct techniques for the diagnosis of PCM. In addition, Paracoccidioides spp. are very slow-growing fungi, as in culture, it may take up to 30 days for colonies to become apparent (42). In enriched culture media, e.g., Fava-Netto agar (43, 44) or Sabouraud dextrose agar, fungal colonies are easily covered by other fast-growing soil fungi (e.g., Aspergillus, Cladosporium, Penicillium, and Mucorales).
An exciting result was found by Hahn et al., where higher positivity was found for culture identification (97.1%) than for direct mycological examination (88.2%) (25, 26). This result is not typical in mycology laboratories, given the difficulty observed due to the fastidious growth of Paracoccidioides spp. in vitro and the probability of contamination by other microorganisms that grow more quickly.
In our experience, to increase the chance of isolating Paracoccidioides spp. in the mycological routine, seeding should be carried out in 2 to 3 tubes for each culture medium, namely, Sabouraud dextrose agar, Mycosel, and Fava-Netto agar. For clinical materials such as sputum, bronchial lavage fluid, and biopsy specimens, we consider it important to add 150 mg/L of chloramphenicol to Fava-Netto agar and Sabouraud dextrose agar to prevent and/or minimize the growth of contaminating fungi and bacteria.
Generally, for biopsy specimens, we use previous washing of tissue fragments in sterile petri dishes containing a chloramphenicol solution at a concentration of 150 mg/L. Considering other clinical materials such as lymph node aspirates and cerebrospinal fluid (CSF), the isolation of Paracoccidioides spp., without contaminating microorganisms, is observed in the laboratory routine.
Despite sparse reports of environmental isolation, Paracoccidioides spp. have been obtained from soil, environmental aerosols, penguin feces, dog food, and animals such as armadillos, dogs, and bats (45–54).
The casual and nonrepetitive characteristics of these isolates, the lack of epidemic outbreaks, and the long latency period of infection before it becomes a disease pose a challenge in determining the precise fungal environment. However, based on reports of other systemic mycoses caused by dimorphic fungi, there is a consensus that Paracoccidioides spp. live saprophytically in soil, water, and plants (55–57). In this scenario, molecular biology tools have elegantly demonstrated the presence of the fungi in soil and aerosols, especially in a niche characterized by animal burrows or places with medium to high humidity protected by arboreal coverage (53, 54, 58). Thus, to promote infection in humans and animals, the fungi must be released from their reservoirs but survive in their filamentous stage and release infectious propagules (e.g., conidia) (59).
The application of geoprocessing methods to both associate PCM with climatic factors and make a clinical diagnosis of PCM has demonstrated that Paracoccidioides spp. should preferably occur in soil locations characterized by frequent and high rates of rainfall, good soil permeability, high relative humidity, and temperatures varying between 18°C and 28°C (60).
Recently, do Valle and colleagues (61) reported a cluster of eight PCM cases among members of a highway construction crew working from December 2015 to December 2016 in the Rio de Janeiro metropolitan area. P. brasiliensis sensu stricto and P. americana were reported to be the primary agents of these cases, supporting the sympatry of S1 and PS2. Interestingly, this highway crosses a native Atlantic forest area, indicating that deforestation involving soil disturbance and people’s migration may have been involved in fungal transmission and might represent a risk factor for the emergence of paracoccidioidomycosis (61).
Classical seroepidemiological studies demonstrated the presence of circulating Paracoccidioides species anti-gp43 (43-kDa glycoprotein) antibodies, suggesting that the fungus has a wide variety of mammalian hosts, such as dogs (62), horses (63), cats (64), monkeys (65), sheep (66), and armadillos (67), among other animals living in areas where the disease is endemic. The nine-banded armadillo (Dasypus novemcinctus) has been identified as an important host in the epidemiology of Paracoccidioides spp. The geographical distribution of armadillos is widespread and clearly overlaps the areas where PCM is endemic (68). Armadillos have a habit of being intense excavators to obtain food and build burrows, where they are likely to become infected with Paracoccidioides species propagules, which also aids in the environmental dispersal of fungal propagules. In addition, Paracoccidioides spp. have been identified in aerosols and soil from armadillo burrows, thus opening perspectives to study the possible role of this animal in the natural cycle of Paracoccidioides spp. (53, 54, 58).
Epidemiology of Paracoccidioides Species and Paracoccidioidomycosis
Neglected tropical diseases (NTDs) comprise a diverse group of transmissible diseases that are prevalent under tropical and subtropical conditions and affect more than a billion humans annually, creating an extremely high financial burden on the economies of developing countries (69). PCM is a neglected tropical mycosis of great importance in Latin American countries, affecting mainly rural workers. In Brazil, the annual incidence of PCM is estimated to be 3 to 4 new cases/million inhabitants, and in major areas of endemicity, it is estimated to be up to 10 to 30 new cases/million inhabitants per year. Brazil stands out for reporting 80% of PCM cases in Latin America (70, 71). Regrettably, PCM is not a notifiable disease, which hinders the determination of the real magnitude of its occurrence and its epidemiology and the implementation of public policies for fungal containment.
Epidemiological data have shown that tropical and subtropical regions, with average temperatures of 14°C to 20°C, average rates of rainfall of 800 to 2,000 mm, and high relative humidity, represent the main areas of endemicity (72). P. brasiliensis sensu stricto (S1) is the principal human agent in these areas and is widely distributed in Latin America. It is found in Argentina, Bolivia, Brazil, Guadeloupe Island, Paraguay, Peru, Uruguay, and Venezuela (9, 10, 21, 32, 34, 35, 73). Isolates from animals have also been recovered, mainly from armadillos (34, 53, 74).
Other cryptic species within the Paracoccidioides species complex present irregular and generally restricted geographical occurrences. P. americana (PS2) was isolated from human cases in the southeastern part of Brazil, Argentina, Uruguay, and Caracas, Venezuela (9, 10, 32, 34, 35, 73), as well as in armadillo samples in the region of endemicity of Botucatu, São Paulo, Brazil. P. restrepiensis (PS3) has been isolated mostly from human cases in Colombia (9, 10, 32, 34, 35), with scattered cases in Argentina, Brazil, Peru, and Uruguay (73). P. venezuelensis has been obtained only from clinical samples from Venezuela (21), with a single strain being reported from São Paulo, Brazil (73). Epidemiological data point toward midwestern Brazil as being the epicenter of P. lutzii PCM cases (26). However, culture-proven human cases have been less frequently found in the northern (75) and southeastern (76) parts of Brazil. Interestingly, P. lutzii has never been isolated from animals (74), although anti-P. lutzii antibodies have been detected by enzyme-linked immunosorbent assays (ELISAs) in domestic and wild animals in southern Brazil (77). The sole P. lutzii isolate reported from Ecuador most likely represents a case from a migrant (10). The epidemiological data recently reported for Paracoccidioides spp. reveal the urgent need to improve genetic surveillance in areas of endemicity (73).
It remains unknown which geographical barrier separated P. lutzii from the cryptic species of P. brasiliensis S1 and PS2. Since the geographic distance between these species is limited, it is believed that reproductive isolation occurred, leading to a divergence between them. A likely explanation for the speciation of the PS3 genetic group may be major geological events that occurred in South America, such as the rise of the Andes and marine incursions, resulting in their geographical isolation (32).
During the past years, an increase in PCM case reports has been seen from different regions of Brazil. Generally, epidemiological findings indicate that the infection is acquired mainly in the first 2 decades of life, with peak incidence in the second decade. The appearance of clinical manifestations or disease development is uncommon in this group, occurring more frequently in adults 30 to 50 years of age when the latent endogenous focus reactivates (78). Men constitute the leading affected group (79–83); however, a series of acute cases affecting children and adolescents in regions of endemicity was reported (84).
The PCM mortality rate in Brazil was determined previously (85), based on a study of 3,181 deceased subjects between 1980 and 1995. The average annual PCM mortality rate was 1.45 deaths per million inhabitants, and the different Brazilian regions were not homogeneously distributed, as the southern states had the highest regional rate, and the southeast showed a decreasing trend. The mortality rate was higher among males, comprising 84.75% of all deaths; individuals aged 30 to 59 years were the most affected, with a mortality rate of 562 men versus 100 women with PCM (85). Recent data (1998 to 2006) show a trend in the number of cases toward a slight increase in the temporal distribution of hospital admissions due to PCM, a disorder responsible for the most significant number of hospitalizations among systemic mycoses (71). Traditional areas in southern and southeastern Brazil cover 60% of hospitalizations, although increasing numbers of admissions in northern and midwestern regions are noticeable (71).
IMMUNOPATHOLOGY OF PARACOCCIDIOIDOMYCOSIS
Historical Perspective of the Knowledge on the Immunopathology of PCM
Although considered a frequently reported neglected fungal disease, the knowledge on PCM has greatly advanced during the past 40 years. In the 1980s, research on and the understanding of PCM were far behind those of other mycoses. The research developed in the last decades among various countries, mainly Brazil and various South American countries, increased the scientific knowledge of immune mechanisms involved in PCM in the scope of the most modern scientific data produced (78).
Overview of Classical Concepts of the Role of the Immune Response in PCM
Resistance to PCM seemed to be dependent on the integrity of the cellular immune response. Cutaneous tests performed on PCM patients showed the existence of generalized specific immunosuppression. This could be partially reversed with antifungal therapy, while its persistence was associated with severe forms of PCM. Therefore, it was generally accepted that the severity of PCM disease depended on the immune status of the affected host. High levels of specific anti-Paracoccidioides species antibodies were found in the sera of PCM patients. However, a protective role of such antibodies in this disease has not been demonstrated. On the contrary, an inverse correlation between high antibody titers and severe clinical manifestations of PCM was often observed (86).
The standing concept since 1990 was that PCM manifests as different clinical forms and may occur as an acute or chronic disease. Benign forms may also occur; they are characterized by the development of a cellular immune response marked by phagocyte activation, humoral immunosuppression, and subsequent disease control. Severe forms are characterized by hypergammaglobulinemia and depressed cellular immunity with a reduced activation of phagocytes; their course evolves into a disease with a poor prognosis (85, 87). A solid body of evidence points out that the immunosuppression of the cell-mediated response observed in patients presenting severe clinical forms of PCM is associated with the severity of the disease (87–90).
Understanding the Events That Lead to Polar Responses in PCM
As initially organized by the 1986 Medellin meeting and expertly summarized in a review on consensus in PCM by multiple expert authors (91), the characteristics observed in its various clinical presentations were cataloged according to their immunopathogenic manifestations. The above-described findings began to be understood in the light of the discovery by Mosmann and Coffman of the existence of T-helper (Th) lymphocyte subpopulations that secrete different cytokines in response to antigenic stimuli (92). It was observed that Th1 lymphocytes secrete interleuken-2 (IL-2) and interferon gamma (IFN-γ) and constitute the cell population responsible for the cellular immune response to intracellular pathogens and delayed-type hypersensitivity reactions. On the other hand, the overall cytokines produced by Th2 lymphocytes (IL-4, IL-5, and IL-10) suppress cellular immunity and exacerbate humoral immune responses, for which this cell population is responsible. In some infections, the activation of each of these cell populations constitutes two mutually antagonistic immunological pathways, which promote resistance or susceptibility to infections, respectively (88).
Therefore, the clinical manifestations can be summarized as follows.
Controlled disease, with no clinical manifestations, is characterized by the development of a Th1 pattern of acquired immunity, in which the cytokines preferentially produced activate macrophages and lymphocytes, mainly of the CD4+ T-cell subset but also of the CD8+ T-cell subset, leading to the formation of a few compact granulomas that control the proliferation of Paracoccidioides spp. Mild forms of the disease (chronic unifocal or multifocal PCM) show a partially involved Th1 immunity pattern sufficient to allow granulomas to form, with a marked presence of neutrophils in which Paracoccidioides spp. are partially engulfed, with the disease being under control. Severe disease (either severe disseminated chronic or acute/subacute juvenile) characteristically presents a Th2 pattern of an acquired immune response, characterized by the cytokines IL-4, IL-5, IL-10, and transforming growth factor β (TGF-β) activating B lymphocytes that secrete high levels of specific anti-Paracoccidioides species antibodies, often of the IgE or IgG4 isotype, which leads to the formation of numerous disseminated granulomas and the conspicuous presence of eosinophils, with the disease being out of control.
The contributions of many authors working with patients or using experimental, mainly murine, models have been fundamental in establishing the roles of Th1 and Th2 lymphocytes in the immune response to Paracoccidioides spp., which were verified in human studies (89–94) and experimental models (95, 96).
The Immune Response Is Important: Cellular and Humoral Immunity
Paracoccidioides species can invade primary lymphoid organs. Yeast-like forms were found in the bone marrow (94) and the thymus (95) of infected mice. The presence of the fungus may subvert the immune response and skew it toward a latent state. In fact, acute infection with Paracoccidioides spp. promotes thymic alterations leading to a defective repertoire of peripheral T cells and also major alterations in the maturation of many cell populations, including neutrophils, during hematopoiesis. As for most systemic mycoses, PCM seems to cause more severe disease in athymic than in euthymic mice (97, 98).
It was shown that after the intraperitoneal (i.p.) inoculation of a virulent Paracoccidioides species isolate in athymic and euthymic mice, the former group exhibited a more severe disease presentation during follow-up observations. All nude mice died at between 54 and 77 days postinfection, whereas no deaths were recorded in control nu/+ mice until 120 days postinfection, and marked differences were found in fungal dissemination (99).
Participation of B Cells and T Cells
The protective role of specific antibodies against Paracoccidioides spp. is controversial. The first evidence of antibody-mediated protection was shown with the passive transfer of murine monoclonal antibodies (MAbs) against a glycoprotein of 70 kDa, which is recognized by 96% of sera from PCM patients (100). This effective protection was demonstrated by the reduced numbers of viable fungi and the sizes of granulomatous lesions in the lungs of experimentally infected mice (101).
Studies on the effect of monoclonal antibodies on the major diagnostic antigen gp43 provided additional insights into the role of antibody protection in PCM, showing that gp43 played a part. Reduced fungal burdens and decreased pulmonary inflammation were achieved by the passive administration of some monoclonal antibodies against this epitope. When protection was achieved, it was associated with enhanced IFN-γ levels in the lungs and increased fungal phagocytosis, leading to higher levels of nitric oxide (NO) production by macrophages. Travassos and Taborda (102) reported that gp43 and a derivative, the peptide P10, contain a CD4+ T-cell epitope that elicits an IFN-γ-mediated Th1 immune response, which effectively protects against Paracoccidioides spp. in a murine model of PCM.
More recent data have refuted the long-accepted concept that specific anti-Paracoccidioides species antibodies are associated with severe PCM. Tristão and colleagues (103) showed that B-cell knockout mice were more susceptible than their wild-type controls, presenting a higher mortality rate, increased numbers of viable Paracoccidioides species fungi in the lungs, and larger granulomatous lesions. The passive transfer of immune serum from controls to knockout mice caused a decrease in the inflammatory infiltrate and the formation of better-organized pulmonary granulomas, thus demonstrating the increased susceptibility of B-cell-deficient mice to PCM (103).
The strong in vitro inhibitory activity of monoclonal antibodies against a Paracoccidioides species glycolipid antigen was demonstrated by Toledo and colleagues (104). Batista and coworkers (105) showed that polyclonal antibodies to a Paracoccidioides species component opsonized yeast forms in vitro, increased phagocytosis, and reduced the fungal burden in infected animals; those authors demonstrated these effects using both prophylactic and therapeutic protocols in a murine intratracheal infection model.
Also, working with polyclonal antibodies against Paracoccidioides species components, Bueno et al. (106) showed an in vitro opsonizing effect on yeast forms, with consequent increases in phagocytosis, reductions of the fungal burden, and the formation of well-organized granulomas.
Protection against P. lutzii was achieved using monoclonal antibodies raised against Histoplasma capsulatum, with enhanced phagocytosis by macrophage cells and a reduction of the pulmonary fungal burden compared to those in mice treated with irrelevant monoclonal antibodies (107).
Experimental Models for the Study of Paracoccidioidomycosis
Although PCM is primarily a human disease, animals are capable of developing P. brasiliensis infections similar to those of patients. The evolution of the disease in animals depends on the infecting species, the strain, the route of infection, and the host response.
A murine PCM model was established to mimic the clinical forms of human PCM. One isogenic mouse strain is susceptible to Paracoccidioides species infection, simulating patients with severe chronic forms (CFs) of the disease, and another isogenic mouse strain is resistant to this infection and reproduces what is observed in patients with the less severe, localized chronic forms of the disease. Susceptible mice infected intraperitoneally present inefficient polymorphonuclear neutrophil and macrophage activation, anergy in delayed-type hypersensitivity reactions, high levels of specific antibodies (mainly of the IgG2b, IgA, and IgG1 isotypes) and the monokine TGF-β, low levels of the monokine tumor necrosis factor alpha (TNF-α), the production of the cytokines IL-4 and IL-10, the polyclonal activation of B cells, low levels of glycemia, and progressive disease. On the other hand, resistant mice present efficient polymorphonuclear neutrophil and macrophage activation, adequate delayed-type hypersensitivity responses, relatively low levels of specific antibodies (mainly of the IgG2a and IgG3 isotypes) and TNF-α, the production of the cytokines IFN-γ and IL-2, normal levels of glycemia, and an absence of the polyclonal activation of B cells, resulting in the resolution of the infectious process. Similar results were obtained when the intratracheal or intravenous route of infection was used.
Certainly, the knowledge acquired since the discovery of Th1 and Th2 3 decades ago shows that the phenomena occurring are much more complex and involve several other cell populations, cytokines, and mechanisms than what was described at that time. In later years, the involvement of other T lymphocytes such as Th9, Th17, Th22, and regulatory T (Treg) cells and the numerous cytokines that they secrete has been addressed, with their participation in PCM immunopathology being defined.
The spectrum of clinical manifestations of PCM is associated with the acquired immune responses that develop. Asymptomatic PCM is considered to be ruled by a pure Th1 response; chronic mild forms (unifocal chronic form) are considered to be ruled by a preserved cellular immune response, with high levels of production of a mixture of Th17 and Th22 cytokines and also of Th1 and Th2 cytokines and low antibody titers; and the severe and disseminated forms (acute juvenile clinical form and multifocal chronic form) are considered to be ruled by an impaired cellular immune response, eosinophilia, the production of high levels of a mixture of Th2 (including IL-33, an alarmin) and Th9 cytokines, and a very marked participation of Treg lymphocytes, whereas IL-37 plays an essential role as an antagonist of inflammation. Th1/Th17-mediated immunity seems to mount a protective response to PCM effectively in this context.
Events in the Innate Immune Response to PCM Infections: Effects on the Outcome of Acquired Immunity
In recent years, the concept has developed that the innate immunity cells that are the first to contact the invading pathogen, in our case the fungus P. brasiliensis, play roles not only in the elimination of all possible pathogens but also in controlling the type of acquired immunity to be developed consequently.
Numerous authors have reported on this concept thoroughly in many top papers, signaling the importance of the first steps in the pathogen-host interaction through the involvement of pattern recognition receptors (PRRs) such as Toll-like NOD and dectin via fungal surface components, pathogen-associated molecular patterns (PAMPs).
Depending on which receptor is activated, different metabolic pathways would be set in motion, with the synthesis of different cytokines, and each unique combination would in turn affect the type of acquired immune response to be developed and, consequently, define the outcome of the infection.
Receptors
The concept that most phenomena ruling the immunopathology of PCM are dependent on the interactions of Paracoccidioides spp. and numerous receptors present on cells from the innate and adaptive immune responses is relatively recent, and the corresponding contributions are abundant. The most relevant receptors in host-Paracoccidioides species interactions are reviewed below.
Dectin. Since the original description by Brown and Gordon (108) of dectin-1, a C-type-lectin-like cell surface receptor present in many cells of the immune system, including neutrophils and macrophages, all of which bind specifically to the β-1,3-glucans on the fungal cell wall, much has been learned about its roles in the activation of innate immunity cells, mainly phagocytes; the phagocytosis-accelerated respiratory burst; and the production of reactive oxygen species, all of which result in the production of inflammatory cytokines. Therefore, these processes are fundamental for the induction of innate and adaptive immunity once fungal recognition is achieved.
Dectin-1 participates in the recognition of Paracoccidioides spp. (109–112). Romagnolo et al. (113) showed using human cells that the linkage of Paracoccidioides spp. to the dectin-1 receptor triggers both cellular activation and modulation through the production of cytokines such as TNF-α, IL-1β, IL-18, IL-12, IL-8, IL-17, and IL-10. Using human monocytes and neutrophils, de Quaglia e Silva and colleagues (114) demonstrated that treatments leading to enhanced dectin-1 expression caused a decline in the number of viable Paracoccidioides species cells. On the contrary, treatments inhibiting the expression of this receptor caused increased fungal recovery, showing the positive role of dectin-1 in fungal killing by these cells.
TLRs. Toll-like receptors (TLRs) comprise the best-characterized PRR family, which are able to activate distinct immune responses depending on the receptor/adaptor set assembled. TLRs such as TLR-2, TLR-4, and TLR-9 and their signaling abilities were important during Paracoccidioides species infection. However, the role of endosomal TLR-3 in experimental PCM remains to be determined.
TLRs are PPRs (115) expressed in innate and acquired immune cells that recognize PAMPs, as shown in numerous microorganisms, and upon recognition, they become responsible for activating signals for various immune responses, including cytokine production. Kawai and Akira showed that TLRs recognize fungi (115), and Bonfim and colleagues demonstrated that TLRs also recognize P. brasiliensis (116).
TLR-2, TLR-4, and TLR-9 are the main TLRs involved in sensing fungal components, and TLR-2 and TLR-4 bind to Paracoccidioides spp. (116, 117). It has been reported that mice lacking the signaling adaptor MyD88 (molecule myeloid differentiation primary response protein 88) showed an increase in fungal infections. Specifically, when considering the MyD88-dependent pathway, Paracoccidioides spp. are recognized by TLR-2 and TLR-4 (116, 117), with the engagement of these receptors with pathogens triggering the activation of macrophages, resulting in the production of inflammatory cytokines. Importantly, MyD88−/− mice infected by Paracoccidioides spp. demonstrated high susceptibility but produced significant amounts of cytokines.
Loures and colleagues (118) demonstrated that TLR-2 is able to recognize Paracoccidioides spp. but has a nonprotective role through interactions with Th17. In normal mice, while pulmonary infection was more severe than that in TLR-2−/− mice, the level of production of Kc, a neutrophil-attracting chemokine, was higher, suggesting that this receptor inhibits the activation of a Th17 immune pathway.
Preferential TLR-2 expression and IL-10 production were observed in susceptible mice compared to their resistant counterparts. Therefore, it was proposed that TLR-2 had a nonprotective role in Paracoccidioides species infection (119). For TLR-3, it was observed that it had a negative effect on protection against experiment Paracoccidioides species infection (120). Here, wild-type mouse macrophages had diminished fungicidal activity and decreased NO production compared to macrophages obtained from TLR-3−/− mice. Those authors also showed that various effector pathways of cytotoxic CD8+ T lymphocytes were less effective in normal mice than in the knockout ones.
NOD-like receptors (NLRs). Inactive IL-1β is synthesized by phagocytes when exposed to microorganisms. The presence of such microorganisms elicits phagocytes to synthesize inactive IL-1βs. As in the complement system, enzymatic activity is necessary to obtain the active form, for which the participation of a cytosolic multiprotein complex called the inflammasome is required (121, 122). These phenomena of inflammasome activation and active IL-1β production were associated with resistance to fungal infection. In experimental PCM, several studies demonstrated that infection with Paracoccidioides spp. resulted in inflammasome activation and the production of IL-1β via the NLRP3 inflammasome (123, 124).
Participation of Cell Populations
Dendritic cells. Human plasmacytoid dendritic cells (pDCs) are involved in Paracoccidioides species recognition and appear to play an essential role in innate and adaptive immunity against this fungal pathogen. The balance between CD4+ T cells and Treg cells may result from antigen presentation by one of two dendritic cell subpopulations, namely, inflammatory dendritic cells (DCs), which activate both Th17 and Th2 lymphocytes by signaling pathways involving the receptor TLR and the adaptor MyD88, and tolerogenic DCs, which activate Th1 Treg lymphocytes through the signaling adaptor TRIF (125). Using an experimental model, Ferreira and colleagues (119) showed that Paracoccidioides spp. induce regulatory DCs in susceptible mice, thus promoting IL-10 production and contributing to mouse susceptibility to Paracoccidioides species infection.
Another experimental model that used resistant and susceptible mouse strains showed that Paracoccidioides species infection induced both myeloid and plasmacytoid dendritic cells in resistant mice, while in susceptible mice, a population of myeloid dendritic cells was observed, resulting in different cytokine secretion patterns. Resistant mice produced both proinflammatory and anti-inflammatory cytokines, while susceptible mice produced a homogeneous proinflammatory cytokine pattern (126).
Notch signaling, which has an important role in DC maturation, contributes effectively to the maturation of such cells and the DC-mediated activation of the T-cell response to Paracoccidioides species infections (127).
Eosinophils. Paracoccidioidomycosis is usually a systemic illness with marked eosinophilia only upon the initial diagnosis. However, in severe forms of the disease, a high degree of eosinophilia may be observed (128).
Lymphocyte subpopulations. The relative participation of the helper and cytotoxic T-lymphocyte subpopulations was studied in an experimental model of PCM. Chiarella and colleagues (129) observed that in mice with resistant and intermediate patterns, CD4+ T lymphocytes exert protection, but in mice with a susceptible pattern, these cells are either anergic or absent. Helper T lymphocytes are also responsible for regulating effective delayed-type hypersensitivity reactions and specific antibody synthesis.
The participation of CD8+ T lymphocytes in experimental PCM has been demonstrated. The depletion of this cell population resulted in more severe infection (130). It was shown that CD8+ T lymphocytes were responsible for controlling the fungal load in both resistant and susceptible mice (129, 131). The critical role of CD8+ T lymphocytes in experimental PCM was shown by the cytotoxicity of CD8+ T cells against Paracoccidioides species infection mediated by granzymes and perforins (132).
The participation of cell populations in establishing innate immune responses plays an essential role in protective immunity to PCM. In this context, Th1/Th17-mediated immunity seems to result in protective immunity to PCM. An adverse effect of Treg cells in a murine model of PCM was reported (133, 134), during both initial and subsequent infections, which resulted in a more severe infection, as verified by the morphology of the granulomatous lesions as well as the increased fungal burdens and mortality.
Neutrophils. The onset of the protective immune response against fungi, including Paracoccidioides spp., is mediated by neutrophils, which play an important role by directly killing fungi and interacting with other cell types to modulate the acquired protective immune response that may ensue.
Classically, PCM patients present with neutrophil-related deficiencies, which interfere with the capacity to effectively phagocytose and destroy the fungus (135–137). These studies propose that neutrophils from paracoccidioidomycosis patients are less effective than those from healthy individuals, suggesting that Paracoccidioides spp. exert some deactivating effect on this cell population. On the other hand, a feature of PCM is the conspicuous presence of neutrophils in the lesions, even in advanced stages of the disease, indicating that these phagocytes play a central role in protective immunity.
Previous in vivo results showed that intraperitoneal infection with a virulent isolate of Paracoccidioides species induces drastic changes in the hemopoiesis of neutrophils, mainly in a susceptible animal lineage with the production of a more immature population of neutrophils than that in resistant mice, showing a significantly higher level of production of mature neutrophils. Therefore, one of the consequences of Paracoccidioides species infection in susceptible subjects is the impairment of normal neutrophil maturation, with a relative inability to deal with Paracoccidioides species infection (138).
Previously, ex vivo studies observed that neutrophils from PCM-resistant mice were more efficient at lysing Paracoccidioides species cells than neutrophils from PCM-susceptible mice (139). Prominent neutrophilic infiltrates in paracoccidioidomycotic lesions have been observed in experimental animals as well as in tissue samples from patients. Polymorphonuclear neutrophils (PMNs) are conspicuous in PCM granulomatous lesions and lead to the altered morphology of nearby fungal cells (140). The role of neutrophils at this early stage is dual: they can help destroy Paracoccidioides spp. by phagocytosis and the subsequent generation of reactive oxygen species during the respiratory burst and release several pro- and anti-inflammatory cytokines that influence the acquired immune response that further develops.
Macrophages. Kashino and coworkers (141) showed that a blockade of macrophage function rendered experimental PCM more severe in both susceptible and resistant mouse lineages. It is known that phagocytosed Paracoccidioides species cells can multiply inside macrophages, but this proliferation can be inhibited when such macrophages are activated with IFN-γ (142). The essential role of cytokines in the activation of various phagocytic cell populations such as monocytes (143, 144), macrophages (142, 145), and neutrophils (117, 146) for the lysis of Paracoccidioides species yeasts has been consistently confirmed.
The contributions of Gonzalez and colleagues (147), working with cells from both patients and animal models, led to the understanding that a combination of inflammatory cytokines is more effective in activating phagocytes, rendering them more competent to lyse Paracoccidioides spp. More recently, newly discovered cytokines were inserted into this concept of the joint effects of proinflammatory cytokines such as TNF-α, IL-12, and IL-18 (146, 148).
The role of other cytokines was also addressed. Bannwart and colleagues (149) found that IL-15, a pleiotropic cytokine that regulates the proliferation and survival of many cell types and is produced by monocytes and macrophages, may induce Pb18 killing by human monocytes through the activation of oxidative metabolism dependent on TNF-α production, exerting a modulatory effect on pro- and anti-inflammatory cytokine production, oxidative metabolism, and the fungicidal activity of monocytes during Pb18 infection.
A correlation between high serum cytokine levels of the IL-1 family (IL-1β, IL-18, IL-37, IL-33, and IL-33) in the sera and lymph node lesions of patients with severe (acute) forms of PCM was observed (150). The serum levels of these cytokines diminished with antifungal therapy.
In an experimental pulmonary infection model, a negative role of the regulator cytokine IL-10 was demonstrated (151). A more positive outcome was seen in IL-10 knockout mice than in their controls. In the former, macrophages were more efficient in phagocytosing fungi and had better fungicidal abilities, increased production of proinflammatory mediators, early development of efficient T-cell responses, and increased control of fungal loads without causing excessive tissue pathology, resulting in lower rates of mortality of infected mice (151).
Pina and coworkers (126) showed that IL-6 plays an important role in the protective response during experimental Paracoccidioides species infection, thus demonstrating that, in fact, the participation of IL-6 together with IL-23 upregulates TNF-α and IFN-γ expression, resulting in a Th17 profile in which granulomas form, with the subsequent restriction of Paracoccidioides species multiplication.
Similar conclusions were made by Tristão and coworkers (152), who reported that the cytokines IL-6 and IL-23 exert a protective effect on experimental PCM by inducing a Th17 defensive response in which compact granulomas are formed. Indeed, the Th17 subpopulation of CD4+ T cells requires IL-1, IL-6, and TGF-β for differentiation and IL-23 for persistence in a murine model. Hence, Th17 is protective in experimental PCM.
Participation of Products of Oxygen and Nitrogen Oxidative Metabolites in Host Defense
Nitric oxide (NO) is an important reactive nitrogen intermediate produced by an oxidative mechanism involving the catabolism of l-arginine. The enzymatic action of inducible nitric oxide synthase (iNOS) generates NO, which represents one of the major microbicidal mechanisms of macrophages against pathogens, including Paracoccidioides spp. and other fungi. The role of NO in PCM has always been dichotomic: Nascimento and colleagues (153) suggested that NO exerted a protective role in the initial phases of experimental Paracoccidioides species infection. Nishikaku and colleagues (154) demonstrated that NO has an essential role in modulating the chronic inflammatory response by favoring tissue degradation and/or decreasing extracellular matrix synthesis, thus controlling inflammatory cytokines and matrix-degrading enzymes. The harmful effects of NO observed at the later stages of Paracoccidioides species infection appear to be seen with loose granulomas and extensive fungal dissemination resulting in disease progression. The above-mentioned facts indicate that the absence of NO leads to a better PCM outcome.
Participation of Granulomas in Host Defense
The above-described findings led to the understanding of the wider role of cytokines in PCM immunopathology, not only encompassing activated phagocytic cell populations but also influencing the overall immune response that is mounted against P. brasiliensis, including the development of type 0 granulomatous lesions. Many important contributions expanded our present knowledge of the interactions of an ever-increasing number of cytokines with P. brasiliensis (155). Efforts will be made to focus on each of the interacting cytokines. Souto et al. (156) showed high mortality rates among TNF-α-deficient mice, which were shown to be very susceptible to P. brasiliensis infection and unable to mount organized granulomas, with few fungi in the lesions.
PCM is characterized by the formation of loose and compact granulomas in susceptible and resistant mice, respectively (96). An important role of the cellular immune response is in the genesis of compact lesions, providing restrictive containment for Paracoccidioides spp. and contributing to PCM control, as suggested by studies employing euthymic (nu/+) and athymic (nu/nu) BALB/c mice. Both animal groups were shown to develop similar lesion patterns at the early stages of P. brasiliensis infection, with the presence of compact granulomas. At later stages, however, euthymic mice kept the same pattern observed earlier in the infection course, with compact granulomas accompanied by abundant collagen fibers charged with controlling fungal dissemination. In contrast, athymic mice developed expansive lesions with fewer collagen deposits and increased fungal loads (157, 158).
Xidieh and coworkers (140) performed a comparative histopathological analysis of the intraperitoneal lesions developed by a virulent Paracoccidioides species isolate in resistant and susceptible mice. Macrophages and plasmacytes were the predominant cells in all lesions of both lineages, followed by neutrophils and macrophages that differentiated into giant and epithelioid cells. However, the extracellular matrix of the granulomatous lesions showed differences between resistant and susceptible mice; in the former, two types of lesions were observed. The first type showed a well-defined enclosure composed mainly of type I collagen and an abundance of neutrophils in areas where massive fungal destruction was detected, with few apparently viable yeast cells. The second type had residual characteristics with sparse collagen deposits and the presence of xanthoma-like macrophages that had ingested degenerated fungi, thus indicating control of the infection. In the susceptible lineage, there were multiple small lesions with less tendency toward confinement and the presence of type III collagen fibers.
Comparisons of the presence of the fibrogenic cytokine TGF-β in resistant and susceptible mice were undertaken by Nishikaku and Burger (159), who observed that resistant mice, which developed mild disease, presented encapsulated granulomas, located around fibrotic and necrotic areas of confined and residual lesions containing lysed fungi. On the other hand, susceptible mice tended to develop fatal disease exhibiting many disseminated lesions that increased in size and number throughout the infection. In the omentum granulomas, the target organ of PCM in this experimental model, TGF-β was present on macrophages, giant cells, lymphocytes, and fibroblasts and absent on neutrophils. TGF-β was also detected in fibrotic and necrotic areas and appeared dispersed in the amorphous extracellular matrix, mostly in resistant mice. The same authors (160) studied the in situ localization of IFN-γ in the omentum granulomas of both resistant and susceptible mice inoculated i.p. with either a highly (Pb18) or a slightly (Pb265) virulent Paracoccidioides species isolate.
In a follow-up study of infection with a virulent Paracoccidioides species isolate, IFN-γ-positive cells with a lymphocyte appearance were localized mainly at the periphery of granulomas. At a later time postinfection, a significant increase in the number of positive cells was found in resistant mice showing compact granulomas. Significantly higher numbers of the above-described positive cells were detected in the resistant mice showing compact granulomas later on, and in comparison with susceptible mice, loose and multifocal granulomas were observed. At the onset of experimental infection with the low-virulence Paracoccidioides species isolate, the same localization pattern of IFN-γ was found. However, in both mouse strains, the frequency of such cells decreased due to the presence of only residual lesions, in comparison to observations during the initial stage of the infection. Later on, IFN-γ staining observed in the granulomas of resistant mice confirmed their importance in the control of fungal dissemination and suggested their importance in the development of such granulomas.
Nishikaku and colleagues (161) pinpointed the immunolocalization of matrix metalloproteinase 9 (MMP-9) in multinucleated giant cells, macrophages, and lymphocytes present in the granulomas of Paracoccidioides species-infected mice, suggesting that such that cells are the main cellular sources of MMP-9. The presence and gelatinolytic activity of matrix metalloproteinases, particularly MMP-9, in Paracoccidioides species infection suggested a possible influence on the organizational pattern of the granulomatous lesions and also fungal dissemination.
The broad spectrum of existing clinical forms and even of asymptomatic infection is characterized by the involvement, in each case, of different, very complex sets of cell populations and soluble factors pertaining to the innate and acquired immunity arms of the immune response. Finally, the best outcome for the host in Paracoccidioides species infection is conservatively by mounting a protective immune response strong enough to control the infection, if elimination of the fungus is not possible, by avoiding excessive inflammatory responses that may cause more severe immunopathological consequences than the fungus per se.
CLINICAL MANIFESTATIONS OF PCM
PCM usually involves all organs and systems and has a tendency to spread, thus resulting in a wide variety of clinical manifestations (162, 163). Table 1 shows the frequency of impairment in different organs in studies and in patients treated at a single clinic. Both the capacity for the spread of the disease as well as the predominance of the involvement of multiple organs (lungs, lymph nodes, and upper digestive mucosa) have been described. Skin lesions are frequently the reason why patients seek medical attention. Through autopsy, the widespread configuration of the mycosis was confirmed in patients who died despite antifungal treatment and in whom lesions in certain organs, such as the adrenal glands and liver, were noticed but were not detected upon clinical examination. In addition, the presence of multiple Paracoccidioides species-infected organs may not have been accompanied by clinical manifestations, probably due to the complexity of the disease where other more notorious manifestations prevail, and may not have induced major patient complaints; their detection would be possible only at necropsy (163).
TABLE 1.
Organs involved in 25 autopsied patients and clinical involvement in 273 patients with paracoccidioidomycosis who were seen at Botucatu Medical School, São Paulo State, Brazila
| Organ(s) | No. of patients with autopsy findings | % of patients with autopsy findings | No. of patients with clinical involvement | % of patients with clinical involvement |
|---|---|---|---|---|
| Lung | 24 | 96 | 200 | 73.3 |
| Lymph nodes | 18 | 72 | 95 | 34.8 |
| Oral mucosa, pharynx, larynx | 15 | 60 | 124 | 45.4 |
| Adrenal glands | 11 | 44 | 14 | 5.1 |
| Central nervous system | 9 | 36 | 3 | 1.1 |
| Liver | 8 | 32 | 1 | 0.4 |
| Spleen | 7 | 28 | ||
| Skin | 6 | 24 | 22 | 14.7 |
| Kidneys | 4 | 16 | ||
| Bone marrow | 3 | 12 | 1 | 0.4 |
| Heart | 3 | 12 | ||
| Digestive tract | 2 | 8 | 9 | 3.3 |
| Testis | 2 | 8 | 3 | 1.1 |
| Prostate | 2 | 8 | 1 | 0.4 |
| Eyes | 2 | 0.7 | ||
| Breast | 1 | 0.4 |
Updated from data reported by M. F. Franco et al. (163), with input from R. P. Mendes. Namely, data from 100 additional patients are included here relative to the data set in the original study.
Patients often report discomfort, generalized malaise, a reduced work capacity, anorexia, and weight loss, sometimes so intense that it may lead to cachexia. Fever is usually not present and can be considered a sign of severity.
Lungs
Pulmonary impairment is of great importance because the lung is the portal of entry for Paracoccidioides spp., in most cases due to the frequency and intensity of fibrotic and emphysematous sequelae of pulmonary impairment that ensue. Pulmonary involvement in PCM was first reported in 1911, and 8 years later, the first case of exclusively pulmonary involvement was described but without clinical evidence of extrapulmonary injury. However, pulmonary participation was valued only in 1946, when lung impairment was demonstrated in 84% of 25 autopsied cases (164). This study was followed by a plethora of reports focusing on the different aspects of pulmonary impairment.
Dyspnea is the most frequent complaint, and it is progressive, manifesting at first only after strenuous exercise but worsening over time. Cough is observed in 57% and mucus production is observed in 50% of patients. Sputum is bloody in 11% of the patients. Chest pain is generally not reported. On the other hand, pulmonary impairment can be asymptomatic, even when chest radiography reveals extensive lung lesions. Pulmonary signs are usually minor, and in a large number of patients, no abnormalities are observed, including in those with severe respiratory complaints or extensive radiological lesions, characterizing a clinical-semiological or radiological-semiological dissociation, respectively. In patients with no other cause of pulmonary impairment, chest inspection was normal in 64% of cases, palpation and percussion were normal in 46%, and auscultation was normal in 43% (165). Emphysematous thorax and hyperresonance at pulmonary percussion were observed in only 11% of cases, decreased thoracic-vocal fremitus was observed in 36% of cases, and submassive thoracic percussion (dullness) was observed in 46% of patients (165). Pulmonary function has been evaluated by some researchers and has generally been found to be impaired (166). An obstructive pattern is the most common finding, followed by the presence of a mixed (obstructive-restrictive) pattern, and in some cases, a restrictive pattern is present. Hypoxemia and increased alveolar-arterial oxygen differences are observed in almost all patients, reflecting a predominance of perfusion over ventilation. The spirometry findings suggest a predominance of lesions in the bronchial tree, especially at the level of bronchioles or peribronchial connective tissue, which would occur in the early as well as the progressive stages of the disease, independently of the action of smoking. The regression of radiological lesions, which is observed with treatment, is not accompanied by an improvement in lung function.
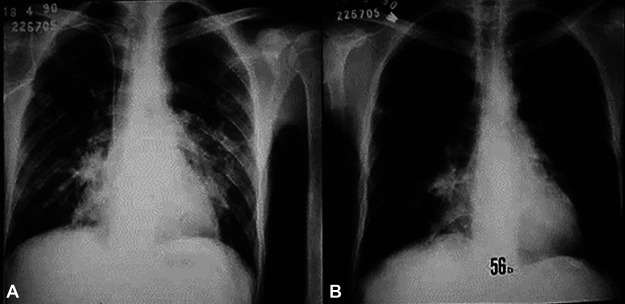
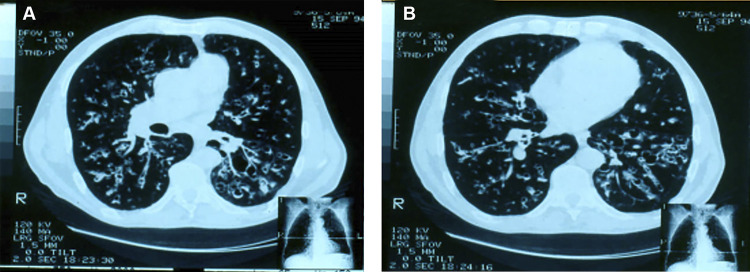
Chest X rays reveal bilateral, parahilar, and symmetrical lesions, most commonly located in the middle one-third of the lungs (Fig. 1). The upper one-third is affected in one-third of the cases, and the apex is involved in just under one-half of the cases and bilaterally. There is a higher prevalence of interstitial or mixed lesions with a predominance of interstitial patterns among interstitial reticulonodular lesions (167). Alveolar or mixed lesions, with a predominantly alveolar pattern, generally preserve the apexes, offering an image similar to that of a butterfly’s wings, which is very suggestive of PCM, although their prevalence is low. In addition to these patterns, tumoral, pneumonic, or cavitated masses have been observed. Sometimes, radiological alterations may simulate those of tuberculosis. Because the cavitation contains a viscous exudate and the cavities are pressured by neighboring tissues, they become tortuous slits that are difficult to see by chest radiography. However, they are easily identified by a computed axial tomography scan (CT scan). The involvement of hilar lymph nodes, confirmed by histopathological examination at autopsy, is rarely demonstrated on chest radiographs because of the intense parenchymal involvement in this area.
FIG 1.
Patient with the chronic form of paracoccidioidomycosis. (A) Chest radiograph showing bilateral symmetrical infiltrates of the mixed type, with a predominance of alveolar lesions. (B) The same patient after 56 days of itraconazole therapy.
CT scans show a diversity of pulmonary lesions and nodules, predominantly small ones, with septal thickening, thickened lines, alveolar opacities, fibrosis blocks, bronchial wall thickening, bronchiectasis, and cavities without liquid content (168). Although rare, pneumothorax (169) and pleural effusion (170) have been reported.
Lymph Nodes
Lymph node involvement was reported in Lutz’s pioneering report, which described submandibular adenopathy with the fungus in the tuberculoid granuloma. Paracoccidioides species lymphotropism was suggested in 1919, followed by the observation that patients with earlier and more intense lymph node involvement had a worse clinical prognosis.
The importance of lymphatic involvement can be assessed by its frequency in clinical and postmortem studies by identifying subclinical adenopathy and lymphatic involvement by contrast and anatomopathological examinations, especially by specific cellular immune depression caused by lymphoid tissue lesions. Subclinical adenopathy is characterized by the finding of paracoccidioidal lesions in lymph nodes considered normal upon clinical examination (171). It was demonstrated that lymph nodes are reached by drainage of the involved organs, regionally, with posterior diffusion by the lymphatic system. In addition, distant lymph nodes are reached by hematogenous dissemination.
Adenopathy can be the patient’s main complaint; it usually occurs in children, adolescents, and young adults who present the acute/subacute form (AF) of PCM, also called the juvenile form (Fig. 2 and Fig. 3A and B). The lymphatic chains of the cephalic segment are commonly involved, often followed by supraclavicular and axillary segments. In the cephalic segment, the submandibular lymph nodes and those of the anterior and posterior cervical chains are mostly affected. The submentonian, pre- and retroauricular, and suboccipital lymph nodes may also be involved. Less frequent is the involvement of the inguinal, intercostal, epitrochlear, and popliteal lymph nodes.
FIG 2.
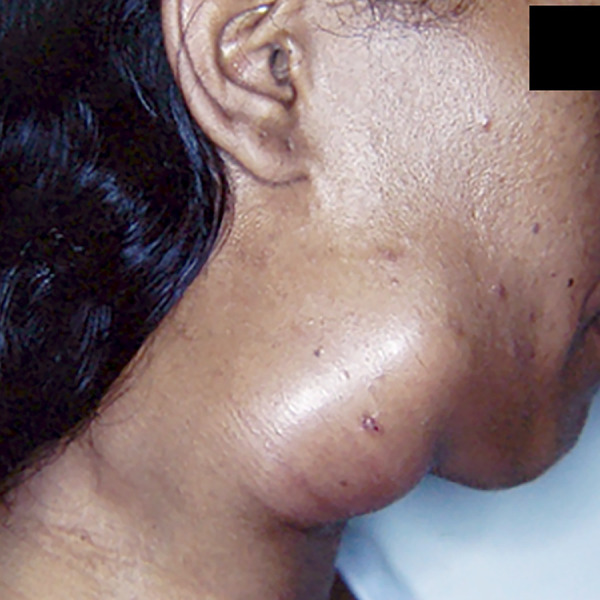
Young adult patient with an acute/subacute form of paracoccidioidomycosis (juvenile type). Shown is intense lymph node enlargement in the cervical and submandibular chains, of the suppurative type.
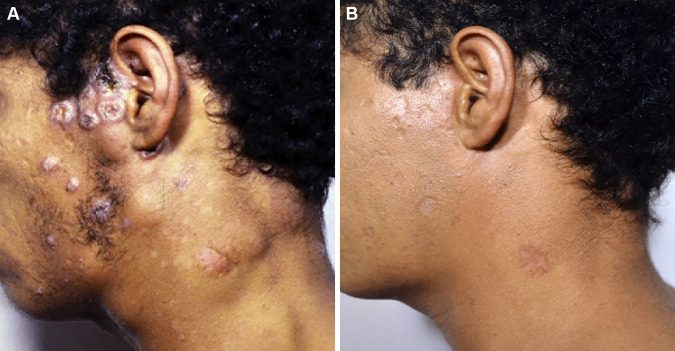
FIG 3.
Patient with the acute/subacute form (juvenile form) of paracoccidioidomycosis. (A) Very thin patient with intense adenopathy of the suppurative type and multiple nodular and papular skin lesions. (B) The same patient after 3 months of itraconazole therapy. Note the weight gain and the disappearance of the skin and lymph node lesions.
Initially described in 1915 and most commonly reported in midwestern Brazil, abdominal lymphatic involvement may lead to several clinical pictures, even simulating acute abdomen (172).
Many patients have large tumoral masses, simulating lymphoproliferative diseases. Abdominal adenopathy can compress hollow viscera, causing jaundice due to extrahepatic biliary tract compression, inferior vena cava compression syndrome, and intestinal subocclusion or occlusion (173–176). In addition, mesenteric lymphatic involvement may lead to protein and fat malabsorption syndrome, sometimes accompanied by chylous ascites (177–179).
It is important to note the frequent and sometimes intense involvement of the deep lymphatic system, revealed by bipedal lymphography, in patients with chronic PCM who do not present lymph node enlargement upon physical examination, demonstrating that the lymphatic system is compromised in almost all cases but is not always accompanied by clinical evidence. Deep lymph nodes can be studied by lymphography, ultrasound (US), or computed tomography (180–183).
The involvement of several lymph nodes in different ganglion chains of the same patient makes the overall characterization of lymphatic involvement complex. This difficulty can be overcome by using the clinical classification of adenopathy into three types based on lymph node diameter and suppuration (163): (i) nonsuppurative inflammatory, when the largest diameter of all lymph nodes is less than 2.0 cm, and none of them presents suppuration; (ii) tumoral, when the diameter of at least one of the lymph nodes is larger than 2.0 cm; and (iii) suppurative, when at least one lymph node presents fluctuation or fistulae, regardless of its diameter (Fig. 2 and Fig. 3A and B).
Patients with nonsuppurative inflammatory adenopathy generally have painless and noncoalescent lymph nodes, free from superficial and deep planes, without flushing and heat, while those with tumor-like adenopathy often reveal warm, flushed, and painful palpation lymph nodes fixed to the superficial planes.
Mucous Membranes of the Upper Aerodigestive Tract
Paracoccidioidal lesions of the nasal cavities, oral cavity, oropharynx, hypopharynx, and larynx constitute upper aerodigestive tract (UADT) involvement and are of major importance due to their prevalence, the ease of access to clinical material for diagnostic investigation, and, finally, the sequelae that remain even after appropriate treatment. The first systematic study of the mucosal lesions caused by Paracoccidioides spp. was carried out in 1936 when Aguiar Pupo described ulcerative mulberry-like (Latin morus) stomatitis, which was later named after him (Fig. 4). Several studies after that focused on mucosal involvement in paracoccidioidomycosis (184–187).
FIG 4.
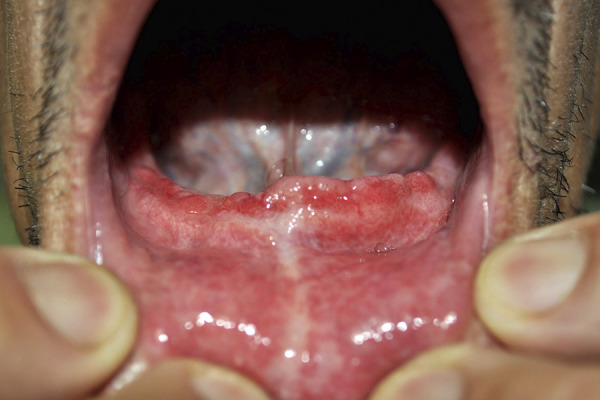
Patient with the chronic form of paracoccidioidomycosis. The oral mucous membrane lesions are characterized by an ulcerative-infiltrative process, with red points covered with a whitish secretion, called ulcerative mulberry-like stomatitis. (Latin morus, for mulberry fruit, leading to the term moriform).
Hoarseness, odynophagia, dysphagia, sore throat, and dyspnea are prevalent clinical manifestations. Mucosal lesions are sometimes painful, especially when hot food is consumed. Many patients report tooth softening, which is confirmed by physical examination, which also reveals periodontal injuries. This tissue is very rich in fungi, with various forms in reproduction, suggesting that the periodontium is an excellent environment for Paracoccidioides species proliferation.
The study of the UADT reveals that more than one body site is affected, with a predominance of the larynx, followed by the oropharynx, hypopharynx, and oral cavity. Also noteworthy is the predominance of bilateral lesions in the various regions evaluated. In the oral cavity, lesions are found more frequently in the hard palate and upper gingival region, with their presence being noted in contiguous regions.
In the oropharynx, soft palate involvement predominates, often followed by the pillars and lateral walls. The piriform sinus and anterior wall are the most engaged regions of the hypopharynx, followed by the lateral and posterior walls. In the larynx, lesions are found more frequently in the ventricular band, vocal cord, and arytenoid regions, although the free and laryngeal parts of the epiglottis, the erythenoepiglottic fold, the ventricular-laryngeal space, and the subepiglottic region are also frequently compromised.
The lesions have quite varied morphologies. Moriform stomatitis, described by Aguiar Pupo as a slowly evolving lesion whose bottom has the appearance of a fine granulation, very similar to that of a blackberry, is the most common type of lesion in the oral cavity (Fig. 4). Although not a pathognomonic sign, its detection is highly suggestive of PCM. Hyperemia predominates in the oropharynx and hypopharynx, whereas in the larynx, edema and then hyperemia are the most common lesions. In addition to these findings, ulcerations and vegetative and infiltrative lesions can be found. Oral cavity lesions are often accompanied by a thick secretion, which forms elastic bridges between different points of this region when patients open their mouths for a physical examination.
Skin
The importance of skin involvement is connected to its prevalence, its pleomorphism, the excess of lesions, and the ease of obtaining clinical samples for diagnostic investigation. The fungus reaches the skin via hematogenous spread or contiguity from mucosal or lymph node lesions. There have been very few cases in which the skin was considered the portal of entry for Paracoccidioides spp. The following conditions were observed: the appearance of paracoccidioidal injury within 2 weeks after local trauma, the presence of regional lymphadenopathy, and the absence of lung lesions upon chest X ray.
Skin lesions may be located on any segment of the body, but they are found more frequently on the face and rarely affect the genitals or palmar or plantar regions (162, 188, 189). They may be single or multiple and, in the latter case, may be sparse or grouped. Polymorphism is characterized by variations in color, size, and appearance. The lesions may appear as papules or nodules, which sometimes ulcerate and become crusted, or as abscesses, vegetations, or verrucous lesions (Fig. 3A). Erythematous plaques and nummular lesions are uncommon. Paracoccidioidal skin involvement may be confused with other diseases such as sarcoidosis, chromoblastomycosis, tuberculosis, and systemic lupus erythematosus, among others.
Adrenal Glands
The first report of adrenal gland involvement in Paracoccidioides species infection dates back to 1914, with autopsy findings of a patient with disseminated disease whose lungs revealed only fibrotic lesions. Despite the various studies that subsequently showed paracoccidioidal adrenal impairment, it was not until 1952 that the signs and symptoms presented by PCM patients and the clinical manifestations of chronic adrenal insufficiency were correlated (190–193). These include indisposition; easy fatigue; anorexia; weight loss; decreased blood pressure and postural hypotension; hyperpigmentation of the skin and mucous membranes, especially in regions of friction; nausea; vomiting; and decreased libido. Elevated serum potassium, calcium, and urea levels and decreased sodium and chloride levels are generally observed, although they may be absent.
In 1961, the Thorn test was used to assess adrenal function, showing a decreased reserve in 48% of patients, making this gland the third most compromised viscus in PCM. Low plasma cortisol and aldosterone levels in these patients and the absence of increment after stimulation with a semisynthetic adrenocorticotropic hormone (ACTH) were also observed, and the cortisol test was then employed in the laboratory evaluation of adrenal function. Many PCM patients show no clinical manifestation of Addison’s syndrome because they have minimal adrenal function, but they experience an adrenal crisis when under some kind of stress.
Adrenal glands can be assessed by imaging examinations such as US and CT scans (193). In a study with 15 PCM patients, US, CT scan, and measurements of plasma cortisol and aldosterone levels were performed after ACTH stimulation. The adrenal reserve was decreased in 53% of the patients. The CT scan was altered in 40% of the patients, showing contour irregularity and changes in the volume and density of the glands. The use of both imaging methods in parallel allowed the detection of alterations in 85% of the patients.
Digestive Tract
Digestive tract involvement was observed in the first autopsies of PCM patients, but this was considered uncommon. As specialized examinations developed, several cases were reported (185). However, few studies have evaluated a higher number of patients, and rare studies have evaluated the whole digestive tract.
More than 50% of patients reported digestive complaints in a prospective study when targeted interrogation was performed (194). Sialorrhea, dysphagia, halitosis, abdominal pain, bloating, burning, and changes in intestinal motility are more frequently reported, followed by regurgitation, vomiting, hiccups, and the presence of an abdominal mass.
Radiological evaluation of the digestive tract demonstrates functional or organic changes in almost all cases, with more than one segment in most of them (194). The ileus, stomach, duodenum, jejunum, ascending colon, and descending colon are the most commonly affected segments, while the esophagus and rectum are altered in a few cases. Thus, special attention should be given to the jejunum and the ileocecal region. The involvement of the appendix is exceptional.
Functional changes are detected more frequently than organic ones, with a predominance of hypersecretion, hypotonia, and decreased peristalsis, among the functional alterations (194). Mucosal fold thickening; dilation; extrinsic compression by the liver, spleen, or lymph nodes; stenosis; and stiffness were the predominant organic alterations. In some patients, intestinal lesions may lead to subocclusion or occlusion, constituting a surgical acute abdomen (Fig. 5).
FIG 5.
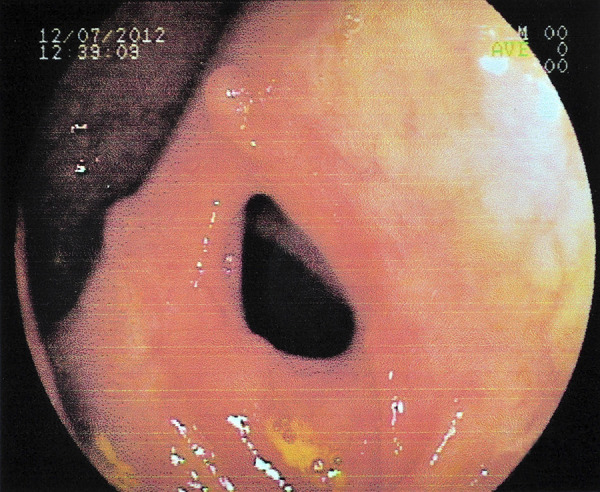
Patient with the acute/subacute form of paracoccidioidomycosis. An endoscopic view of the ascending colon shows luminal narrowing.
The impaired intestinal absorption of glucose, protein, and fat has been reported. Although the underlying cause is the obstruction of the lymphatic pathways, clinical manifestations related to the digestive tract justify its inclusion. Evaluations of d-xylose absorption, fecal fat excretion, and fecal excretion of labeled albumin have been used, among other tests, to confirm malabsorption syndrome (177, 178, 195).
Few studies analyzed hepatic impairment in PCM, suggested by the tropism of Paracoccidioides spp. for the mononuclear phagocytic system, autopsy findings, and the presence of hepatomegaly that is reduced with antifungal treatment. Liver lesions are usually asymptomatic, although there is a case report of severe jaundice and symptoms of severe liver failure, including terminal coma (196).
Liver biopsy has low sensitivity for the detection of paracoccidioidal impairment and may reveal anything from mild and nonspecific lesions characterized by the reactivity of the mononuclear phagocytic system to severe changes evidenced by the presence of portal and intrasinus granulomas (197).
Signs of portal hypertension are not present. Jaundice, observed in several juvenile patients, is generally due to the extrinsic compression of the bile ducts by lymph nodes of the hepatic hilum (173, 175).
Liver and biliary scintigraphy demonstrates the presence of intrahepatic cholestasis, more frequent in the juvenile form; choledochal obstruction; single or multiple focal defects; and heterogeneity of hepatic uptake (198). A quantitative study of radiopharmaceutical kinetics reveals normal uptake by liver cells but delayed excretion in several patients, sometimes very intense.
Although exceptional, pancreatic involvement has been reported, simulating pancreatic head neoplasia or abdominal mass (199, 200).
Bones and Joints
Knowledge of bone and joint involvement in PCM is based on case reports, a review of cases published until 1964, and three prospective systematic studies (201–203). These lesions are usually accompanied by the involvement of other organs but may be the only manifestation of the disease. A history of trauma before bone and/or joint manifestations should be interpreted as a triggering factor for infection at this site and not as the site of inoculation of the fungus, whose conditions were already presented when the skin theme was addressed.
Bone lesions begin at the medullary layer and reach the cortical layer and, finally, the periosteum (89, 204, 205). Bone involvement is usually asymptomatic, and in some cases, some superficial bone lesions may be palpated. In other cases, damage is found through a radiological examination or some other imaging examination. In contrast, joint involvement is accompanied by exuberant clinical manifestations characterized by pain and dysfunction. Upon physical examination, the joints reveal increased volumes and temperature.
Although Paracoccidioides spp. can attack any bone, the lesions predominate in the chest (ribs and sternum), shoulder waist (clavicle and scapula), and upper limbs, which can be evaluated by chest radiography (202, 203). These findings reinforce the hypothesis of PCM in cases of pulmonary involvement in which the suspicion of tuberculosis has been raised. Radiological examination reveals multiple lytic lesions, usually without a perifocal reaction, with a mild or absent periosteal reaction, and with sharp edges. The cortical layer is destroyed in just over one-half of the cases. Joint involvement is observed in about one-third of the cases where there is a bone lesion.
Fibrosis and bone neoformation are observed after the introduction of antifungal treatment, slowly and gradually modifying the characteristics of the lesions.
Scintigraphic methods, such as the use of the radiopharmaceutical technetium-99m methylene diphosphonate (99mTc-MDP), allow the evaluation of the entire skeleton and the identification of bone lesions (206). These procedures are noninvasive and easy to perform.
Fibrosis and bone neoformation are observed after the introduction of antifungal treatment, slowly and gradually modifying the characteristics of the lesions.
The Central Nervous System
The onset of convulsive syndrome in a patient with generalized paracoccidioidal skin lesions was the first indication that Paracoccidioides species infection could involve the central nervous system (CNS). Further studies confirmed this suspicion and showed that it might be more frequent than previously thought (207–211). However, its incidence is difficult to evaluate because autopsies are not always complete, especially concerning the spinal cord. In addition, these studies are representative of disseminated disease that progresses to death. On the other hand, clinical studies do not always include appropriate neurological evaluations, including laboratory tests. A prospective study focusing on CNS involvement revealed suggestive symptomatology in 25% of cases, usually associated with the involvement of other organs. Isolated CNS involvement, however, has already been observed.
Central lesions of PCM can be localized in the parenchyma or meninges, leading to clinical forms that can be considered polar: the parenchymatous or pseudotumoral form, which is the most prevalent, and the meningeal form. The granulomas may be single or multiple and, in this case, may be located in different regions, justifying the polymorphism of the clinical picture (Fig. 6). Symptoms of slow and progressive intracranial hypertension predominate, accompanied by signs of localization, such as sensory symptoms and motor deficits. Convulsive syndrome and papilledema are common. Granulomas are more commonly found in the brain than in the cerebellum or brain stem, and a spinal cord location is rare. These tumoral masses may evolve into abscesses of various sizes. Meningeal involvement, which predominates at the base of the brain, presents as a chronic condition, usually associated with brain involvement.
FIG 6.
Magnetic resonance imaging with four views. Intense encephalic lesions in a patient with the chronic form of paracoccidioidomycosis are shown.
A study of 15 patients revealed pseudotumoral lesions in 11 of them, 6 of whom had multiple lesions and 5 of whom had single lesions (210). The locations of the lesions were cerebral in five patients, cerebellar in three, and cerebral associated with cerebellar in the other three. In the other four patients, the presentation was meningeal, in three of whom there were associated brain manifestations.
Spinal cord involvement is rare, and its clinical manifestations vary widely, depending on the injury site (212).
Cerebrospinal fluid (CSF) changes are not characteristic, with mild, moderate, or intense pleocytosis at the expense of mononuclear or polymorphonuclear cells and sometimes without a clear predominance of a cell type (211). High protein concentrations in the CSF at the expense of gamma globulin and hypoglycorrhachia are usually present. The fungus is exceptionally found in the CSF by direct examination or even cultivation. Specific antibodies cannot be demonstrated by routine serological methods such as agar gel precipitation but can be detected by more sensitive assays such as enzyme immunoassays.
Electroencephalograms and classic radiological examinations may reveal nonspecific alterations, which do not help in the diagnosis. CT scans and nuclear magnetic resonance imaging (MRI) have contributed significantly to the demonstration of paracoccidioidal lesions, as they are more sensitive to MRI (Fig. 6). CT scans reveal round lesions, with various localizations, without signs of neoformation or bone destruction, with a small amount of perifocal edema, and with a slight mass effect. After contrast administration, accumulation is observed only in the periphery of the lesion, which keeps its center free, characterizing ring accumulation.
The absence of specific CNS paracoccidioidal impairment by clinical manifestations and/or laboratory findings hinders confirmation of the etiological diagnosis. Thus, identifying the fungus in other organs or finding specific serum antibodies would support the diagnosis. Therefore, epidemiological antecedents and a thorough and careful clinical evaluation of cases in which the neurological condition is unique or dominant may lead to an etiological diagnosis, especially when there is only a slight involvement of the lungs, skin, UADT mucosa, or lymph nodes.
Eyes and Surrounding Structures
The first case of eye damage caused by Paracoccidioides spp. was reported in 1923, followed by reports of at least 50 cases in which other organs were also involved (213–215).
Eye involvement is unilateral, with no predominance of either eye. Eyelid and conjunctival lesions are detected most frequently, with rare cases of anterior uveitis or choroiditis. The eyelid lesion begins as a papule, usually near the edge, which grows and ulcerates in the center. The ulcer’s base reveals thin and raised hemorrhagic points with thick and hardened edges, reminiscent of the moriform aspect of Aguiar Pupo’s stomatitis (Fig. 7). At its onset, ocular paracoccidioidal lesions may simulate hordeolum or even bacterial blepharitis.
FIG 7.
Patient with the chronic form of paracoccidioidomycosis. Shown is unilateral eye involvement, reminiscent of the moriform aspect of Aguiar Pupo’s stomatitis.
Urogenital and Reproductive Systems
Urogenital involvement is seen almost exclusively in males in whom lesions become apparent. Rare are cases presenting urogenital manifestations as the main complaint, and exceptionally, they are the only manifestation (216–221). The epididymis, testicles, and prostate are most prevalently involved, with either an isolated or an associated presentation. Troublesome miction, polyuria, painful testicles, and epididymis are increased in both volume and consistency. An enlarged and hardened prostate is found with urogenital paracoccidioidal involvement. Kidney, ureter, bladder, and penis lesions have also been reported.
Breast involvement (222, 223) and placenta involvement (224, 225), although rare, count with a few reports.
Thyroid Gland
Thyroid involvement caused by Paracoccidioides species infection is a rare autopsy finding (163). The first study of thyroid function in PCM patients with severe disease revealed a higher frequency of reduced serum triiodothyronine (T3) levels among them (226). These results suggested a reduction in the peripheral conversion of T3 to thyroxine (T4) but did not indicate any form of hypothyroidism.
Other Organs
Impairments of the heart, vessels, pituitary and thymus glands, spleen, bone marrow, and striated muscle have been reported, confirming that any organ can be affected by PCM (227, 228). In many cases, no clinical manifestations were apparent, thus suggesting the involvement of such organs, and the diagnosis was established using autopsy findings.
Classification of Clinical Forms
The interactions between Paracoccidioides and humans can be characterized as infection or disease in different clinical forms (Table 2) (162).
TABLE 2.
Clinical forms of paracoccidioidomycosis in interactions between Paracoccidioides spp. and humansa
| Form of paracoccidioidal infection (paracoccidioidomycosis [disease]) |
|---|
| Regressive form |
| Progressive form |
| Acute/subacute form (juvenile form) |
| With superficial adenopathy (moderate and severe) |
| With abdominal or digestive involvement (severe) |
| With bone involvement (severe) |
| With other clinical manifestations (moderate or severe) |
| Chronic form (of the adult) |
| Mild |
| Moderate |
| Severe |
| Mixed form (severe) |
| Isolated organic form (mild or moderate) |
| Residual form (sequelae) |
See reference 162.
This classification is based on criteria established by a group of experts who gathered at the Third International Congress on PCM held in Medellin, Colombia (92), with some modifications based on studies of acute/subacute forms (229, 230); the introduction of the regressive form (231), well established in other systemic mycoses; severity characterization (163); the introduction of the mixed form (232); and finally, the introduction of the isolated organic form (162).
Paracoccidioidal infection is presented by healthy individuals who have had contact with the fungus and have mounted an effective cellular immune response that prevents disease progression. Infection is confirmed by the positivity of antigen-specific intradermal reactions and the finding of latent foci in autopsied individuals who died from other causes (233).
The regressive form of PCM represents the most benign type of disease, where the patient has only mild clinical manifestations, usually involving the lungs, a positive skin reaction to paracoccidioidin, and clinical regression even without treatment (228). This form has been poorly diagnosed due to a lack of knowledge of the ecological niche of Paracoccidioides spp., something that prevents the correlation of a suspicious contact occurring in an individual with self-limiting clinical manifestations, usually attributed to another cause.
The acute, chronic, mixed, and isolated organic forms constitute progressive disease and are characterized by signs and symptoms related to the impairment of one or more organs. The characterization of these clinical forms is based on the patient’s age, the duration of symptoms, clinical manifestations, the presence of associated diseases and aggravating factors, assessment of general and nutritional status, chest radiography, and serum levels of anti-Paracoccidioides species antibodies, determined by a double-agar gel immunodiffusion (DID) test (234).
The acute/subacute form (AF) of PCM, also called the juvenile form, generally affects children, adolescents, and young adults; its symptomatology is of a short duration, with a median of 2 months (162, 231). Additionally, it exhibits clinical manifestations compatible with the involvement of the mononuclear phagocytic system, that is, lymph node enlargement, hepatomegaly and/or splenomegaly, and, less frequently, bone marrow abnormalities. Adenomegaly is usually seen in several superficial and/or deep lymph node chains and is the dominant clinical manifestation of this form. In AF, mucosal involvement is uncommon, occurring in 17 to 20% of cases, and pulmonary involvement is even less common, being present in only 5 to 10% of patients (162) (Fig. 2 and Fig. 3A).
However, Paracoccidioides spp. may be isolated from the bronchoalveolar lavage (BAL) fluid of patients with the AF who do not present clinical manifestations or radiological alterations of pulmonary impairment (235). In these cases, the lungs constitute the only natural portal of entry of the fungi.
According to the predominant manifestations, the AF can be subdivided into four clinical presentations (230): (i) superficial lymphadenomegaly, (ii) abdominal or digestive tract involvement, (iii) bone involvement, and (iv) other clinical manifestations (Table 2).
On the other hand, there are PCM patients whose clinical expression is characterized as the AF but who are adults. In these patients, clinical manifestations correspond to acute/subacute PCM, also called the juvenile type (92). A recent study has shown that PCM patients under 30 years of age presenting the AF have a higher incidence of skin lesions, higher levels of eosinophilia, and elevated serum precipitating antibody titers than patients aged >29 years (236). These differences allow the characterization in AF patients of a clinical-laboratory pattern that corresponds to children, adolescents, and young adults and another pattern that corresponds to adults (236).
To establish the therapeutic approach and assess the prognosis, AF patients can be classified as moderate and severe cases. In either case, the possibility of mild damage to the host should not be considered due to a lower incubation period and an early and, finally, an intense impairment of the mononuclear phagocytic system, all contributing to the depression of the cellular immune response, resulting in a poor prognosis.
Table 3 presents the clinical and laboratory findings frequently observed in moderate and severe presentations of the AF. All of the findings listed in Table 3 must be present for a clinical presentation to be characterized as moderate; in contrast, the presence of only three of the listed characteristics is sufficient to define the severe form (162, 163).
TABLE 3.
Characterization of the acute/subacute forms of paracoccidioidomycosis regarding severity
| Finding(s) | Severity |
|
|---|---|---|
| Moderate (all findings) | Severe (≥3 findings) | |
| Worsening of general and nutritional conditionsa | Absent or present (mild) | Present (intense) |
| Site and type of adenopathy | Localized, inflammatory nonsuppurative | Generalized, tumoral or suppurative |
| Hepato- and/or splenomegaly | Absent or present (mild) | Present (intense) |
| Involvement of other organs | Absent | Present |
| Serum levels of specific antibodiesb | Low to moderate | High |
Worsening of nutritional status based on the usual weight: mild, <5%; moderate, 5 to 10%; intense, >10%.
Measured by the double-agar gel immunodiffusion test (162).
The chronic form (CF) of PCM usually affects adults over 30 years of age, with long-term symptomatology, often over 6 months. Although it may be absent in some cases, pulmonary involvement is the rule, and the involvement of the mucous membrane in the UADT is very common. Lymphadenomegaly is also observed but usually affects neck chains and is not a dominant finding.
Chronic forms are classified according to severity as mild, moderate, and severe. Patients with the mild chronic form have a normal general and nutritional status, with weight loss not exceeding 5% of their usual body weight. Pulmonary involvement, very frequent in chronic forms, is mild or may even be absent. The involvement of the UADT mucous membrane is mild or absent. Lymphadenomegaly, when present, is limited to the cephalic segment chains and is of the nonsuppurative inflammatory type. These patients do not exhibit an impairment of other organs, apparatuses, and systems. Serum anti-Paracoccidioides species antibody levels in these patients are low. Finally, it should be noted that all of the above-proposed criteria must be observed to characterize the chronic form as mild.
On the opposite side are patients with severe CF, with an intense impairment of their general and nutritional status and weight loss above 10% of their usual body weight. Respiratory manifestations are intense, and chest radiography reveals extensive pulmonary involvement. Lymphadenomegaly, when present, is not limited to cervical chains and may be tumoral or suppurative. Extensive skin lesions are usually present. Other organs, such as adrenal glands and the central nervous system, are frequently affected. These patients typically show increased serum anti-Paracoccidioides species antibody levels. It should be noted that meeting three of the above-mentioned criteria is sufficient to recognize the severity of these pathological processes.
Moderate CF cases occupy an intermediate position between the above-described two poles. Patients generally present a mild impairment of their general and nutritional status, with a loss of 5 to 10% of their usual body weight. These patients usually have no clinical manifestations of impairment in other organs, apparatuses, or systems such as the adrenal glands, central nervous system, digestive tract, and bones. Patients have moderate serum levels of specific antibodies.
The patients with this moderate clinical form are very heterogeneous. Some patients reveal almost all, but not all, of the criteria for inclusion in the mild affectation. There are patients with moderate affectation but very close to those with the mild form and therefore can be classified as presenting the mild-to-moderate form of the disease. On the other hand, some patients reveal only one or two of the criteria necessary to characterize the severe form. These individuals have a moderate form but appear closely related to those with the severe form and are classified as having a moderate-to-severe form of the disease. Finally, there is a group of patients whose severity criteria are equally distant from both the mild and severe forms, so their clinical status should be simply defined as moderate (162).
Table 4 shows the clinical and laboratory criteria that characterize the mild and severe forms of chronic PCM.
TABLE 4.
Characterization of the chronic forms of paracoccidioidomycosis regarding severity
| Finding | Severity |
|
|---|---|---|
| Mild (all findings) | Severe (≥3 findings) | |
| Worsening of general and nutritional conditionsc | Absent or present (mild) | Present (intense) |
| Pulmonary and/or mucocutaneous involvementa | Absent or present (mild) | Present (intense) |
| Type of adenopathy | Inflammatory nonsuppurative | Tumoral or suppurative |
| Involvement of other organs | Absent | Present (adrenal glands, central nervous system, digestive tract, and bones, among others)b |
| Serum levels of specific antibodiesd | Low | High |
The moderate forms present clinical and laboratory criteria of severity between those of the mild and severe forms.
The exclusive involvement of the central nervous system can characterize a severe form depending on the degree of dysfunction observed.
Worsening of the nutritional status based on the usual weight: mild, <5%; moderate, 5 to 10%; intense, >10%.
Measured by a double-agar gel immunodiffusion test (162).
Mixed forms. There are patients with some clinical manifestations common to the AF and others common to the CF, which hinders their classification. Such clinical presentations are classified as mixed forms and have been observed in patients with a significant depression of the cellular immune response, with widespread disease, a condition implied in the mixed designation (232).
Isolated organic form. There are rare PCM cases with clinical manifestations related to single-organ involvement but that do not fit into the AF or CF disease category. In these cases, the diagnosis is usually made after an invasive procedure and histopathological examination of biopsy samples, which sometimes reveals the involvement of contiguous structures, usually lymph nodes, implying asymptomatic primary pulmonary affectation. These cases should be classified being of as an isolated organic form, a name already used by pathologists (162).
Residual form. The clinical manifestations observed after effective treatment represent the sequelae resulting from the impairment of different organs. Among them, the following stand out: pulmonary, laryngeal, suprarenal, digestive, neurological, and cutaneous sequelae. Pulmonary sequelae consist of fibrosis and emphysema, which impair respiratory function at different levels, hindering a patient’s ability to carry out his usual activities. Residual pulmonary fibrosis, usually accompanied by bullous emphysema, directly correlates with the intensity of pulmonary infiltrates detected upon hospital admission (Fig. 8 and 9). The lung’s obstructive pattern is the most prevalent abnormality, with hypoxemia being very common (151, 166, 237, 238). The most frequent sequela in the oral cavity is the narrowing of the mouth, which may have cutaneous and/or mucosal involvement and may be so intense that it requires a corrective surgical procedure (239). Laryngeal manifestations are characterized by dysphonia, observed when the vocal cords have been severely impaired. Glottic stenosis, present in 33% of active cases, is usually caused by edema, a sequela caused by fibrotic scars and polypoid formations on the vocal cords or other laryngeal structures. In 16 patients, tracheostomy had to be performed, 8% of whom had UADT mucous membrane sequelae, with 3 fatalities due to asphyxia. Perceptual analysis of the voice revealed instabilities. Dysphonia was characterized by roughness and breathiness; some voices are not accepted by the Dr. Speech program. Jitter was increased in about one-third of cases. Endoscopy reveals that 80% of patients with laryngeal lesions presented two or more compromised structures: vocal folds, arytenoid cartilage, epiglottis, and vestibular folds are most frequently impaired (187). The patients’ voice-related quality of life was impaired, with poorer vocal quality upon self-assessment; on the other hand, dysphagic sequelae were not observed (240). Residual adrenal gland impairment manifested as Addison’s syndrome when active disease had already been suspected. Recovery of the adrenal reserve following antifungal treatment is rare (192, 241, 242). Digestive manifestations were related to symptoms of intestinal semiocclusion or occlusion, with protein and fat malabsorption syndrome becoming apparent (177, 195, 243). Residual neurological manifestations were quite variable and depended on the affected area. Sensitivity and motor manifestations, seizures, and gait disturbances have been reported (244). Scarring due to cutaneous manifestations has esthetic and functional importance and sometimes needs surgical procedures.
FIG 8.
Patients with the chronic form of paracoccidioidomycosis after efficacious treatment. Computed tomography scans of the thorax show pulmonary sequelae, including axial and peripheral interstitial sequelae, with septal, centrilobular, and subpleural thickening; fibrosis; and distortion of the parenchymal architecture. Dilatation and thickening of the bronchial wall can be seen. Bullae of emphysema are located at the periphery and interior of the parenchyma.
FIG 9.
Patients with the chronic form of paracoccidioidomycosis after efficacious treatment. Computed tomography scans of the thorax show pulmonary sequelae, including axial and peripheral interstitial sequelae, with septal, centrilobular, and subpleural thickening; fibrosis; and distortion of the parenchymal architecture. Bronchiectasis is present.
Characterization of the Severity of PCM
The clinical characterization of the severity of the disease is important because it guides the instauration of treatment based on two antifungal compounds, their route of administration, as well as complementary-care treatment during hospitalization, including nutritional support and hormone replacement treatment, among others (162, 163).
The acute/subacute form may be moderate or severe, as shown in Table 3, as the patient’s’ young age and the short incubation period may result in minor clinical manifestations. For the disease to be considered moderate, all clinical conditions need to be identified, while to be considered severe, at least three of them must be present. On the other hand, the clinical presentation of chronic forms may be mild, moderate, or severe. Table 4 presents the characteristics of mild and severe forms. Moderate conditions may be exhibited by cases with intermediate-to-extreme clinical manifestations. It should be noted that to characterize the disease as mild, all conditions must be met, while at least three of them must be present to define the severe form of the disease. Interestingly, moderate presentations are the most prevalent and show a wide distribution range, sometimes closer to mild and sometimes closer to severe forms, reflecting the host-fungus interaction.
In an epidemiological, observational, analytical, cross-sectional study performed to investigate the clinical and laboratory data from 44 PCM patients with a culture-proven P. lutzii infection at admission, 13 (31.7%) patients showed the disseminated multifocal chronic form of PCM, and 16 (36.4%) patients met the criteria for clinical severity. The treatment prescribed upon admission did not follow the recommendations of Brazilian guidelines for the clinical management of paracoccidioidomycosis in 26% of the severe PCM cases (245).
In the search for a biomarker that would complement clinical diagnosis regarding the criteria for PCM severity and that would concomitantly be common and feasible in any service unit, given the extensive geographical distribution of PCM in Brazil, the neutrophil/lymphocyte ratio (NLR) was evaluated as a diagnostic biomarker for PCM caused by P. lutzii in critically ill and non-critically-ill patients; it was concluded that the NLR represents a potential biomarker for the diagnosis of severe PCM (245).
Since it was first described, P. lutzii has been implicated in possibly worse clinical PCM prognoses (24). However, it is essential to note that so far, no differences have been reported between the clinical manifestations of PCM caused by P. lutzii and those caused by members of the P. brasiliensis complex or between the clinical manifestations caused by P. brasiliensis sensu stricto and those caused by P. americana (26, 29, 246).
The lack of objectivity in the definitions of the mixed form, the disseminated multifocal chronic form, the moderate form, and the severe acute form, even taking the Brazilian guidelines for the clinical management of paracoccidioidomycosis as a reference (78), makes bedside decision-making difficult, which leads to confusion regarding the implementation of individualized therapeutic recommendations according to the severity of the disease. Currently, there are no laboratory biomarkers available to support clinical decision-making when differentiating between severe and nonsevere cases of PCM, and this consequently culminates in the prescription of oral antifungal drugs for patients with nonsevere PCM at the time of admission or intravenous medication, which is associated with other forms of hospital support care, for critically ill patients (245).
Finally, it is essential to note that no differences were detected between the clinical manifestations of PCM caused by any the different members of the Paracoccidioides species complex (26, 29, 246).
Case Definition
The case definition comprises three possibilities: confirmed, probable, and possible (162).
Confirmed case. Patients presenting clinical manifestations compatible with PCM from whom typical yeast Paracoccidioides species forms have been identified in any clinical sample by direct mycological, cytopathological, or histopathological examination or by isolation in culture are confirmed cases.
Probable case. Patients presenting clinical manifestations compatible with PCM and with specific serum antibodies detected by a double-agar gel immunodiffusion (DID) test, counterimmunoelectrophoresis, or immunoblotting should be considered probable cases. These patients present no mycological diagnosis of PCM.
Possible case. Patients presenting one of the following conditions can be considered possible or suspected cases: (i) adults with a clinical picture of at least a 4-week duration characterized by asthenia, dry or productive cough, hoarseness, and skin or oral mucous membrane lesions, associated or not among them, for whom tuberculosis and neoplasia were not confirmed or had been disproven; (ii) children, adolescents, and young adults with a clinical picture with a duration of a few weeks to a few months, characterized by cervical or generalized lymph node enlargement, of the suppurative type or not, with or without hepato- and/or splenomegaly or abdominal mass, palpable or image demonstrated, for whom a diagnosis of infectious mononucleosis or lymphoproliferative disease has not been confirmed; and (iii) children with respiratory symptoms lasting more than one week whose viral and allergic etiologies are not probable or who did not respond to antibacterial or antituberculosis treatment.
Confirmed and probable cases must be treated, while possible cases can be treated provided that they are submitted to a careful evaluation of the clinical response.
LABORATORY DIAGNOSIS
PCM diagnosis is performed by identifying fungi in clinical specimens, conducting serological testing to demonstrate specific antibodies or antigens, and using molecular methods (Fig. 10) (247).
FIG 10.
Flowchart for the laboratory diagnosis of paracoccidioidomycosis. BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid; HE, hematoxylin and eosin; PAS, periodic acid-Schiff; GMS, Gomori methenamine silver; ELISA, enzyme-linked immunosorbent assay; DID, double immunodiffusion; WB, Western blotting.
Mycological Diagnosis
The identification of fungi can be carried out by the direct examination of clinical specimens, including sputum, lesion scrapings, BAL fluid, and fine-needle aspirates. An optical microscope permits their visualization as rounded elements ranging in size up to 30 or 40 μm, with a quite evident double-contoured wall and single or multiple buds. Identifying the typical yeast forms, the mother cell surrounded by multiple exosporulations, characterizing the Paracoccidioides species in its parasitic lifestyle, which is called the “helm-like” aspect, confirms the diagnosis. The “Mickey Mouse” aspect is not pathognomonic but is very suggestive of Paracoccidioides species and should be considered as a diagnostic finding (Fig. 11A and B).
FIG 11.
Morphological aspects of Paracoccidioides yeast cells. (A) Paracoccidioides species showing multiple buds with a “helm-like” aspect (lymph node aspirate [KOH]). (B) Positive direct mycological examination of pus showing large yeast cells (10 to 30 μm) that have a thick, birefringent cell wall with single or multiple buds. (C) Paracoccidioides species (arrow) in tissue stained with hematoxylin and eosin (HE). (D) Paracoccidioides species in tissue stained with Gomori methenamine silver (GMS) showing a Mickey Mouse aspect. Bars = 20 μm.
The sputum is the clinical material most frequently evaluated because the chronic form of PCM predominates, in about 80% of cases, and the involvement of the lungs is a rule in this clinical presentation. Direct mycological examination of fresh secretions can be performed by placing the sample between a slide and a coverslip. A 20% KOH solution should be placed between the slide and the coverslip, followed by light heating on the flame of a Bunsen burner (Fig. 10) (6, 247). Homogenized sputum increases the sensitivity of this diagnostic method and should be routinely carried out: the mixture of the sputum sample and an equal volume of 4% sodium hydroxide should be left in an incubator at 37°C for 30 to 60 min and then centrifuged at 3,000 rpm for 30 min, the supernatant should be eliminated, and the sediment should be examined by placing the sample between a slide and a coverslip (248). The sputum-digesting agents most frequently used are a pancreatin-trypsin solution, N-acetyl-l-cysteine, sodium hydroxide, and potassium hydroxide (249).
Finally, when the sputum samples cannot be immediately processed in the laboratory, they should be maintained at refrigerator temperature but not frozen.
The number of slides examined affects the success of mycological diagnosis regarding the finding of fungi in preparations with KOH (20 to 40%), depending on the clinical material to be examined. For inexperienced observers, the finding of yeasts with a birefringent wall of Paracoccidioides spp. but without unipolar, bipolar, or multipolar buds can often make detection in preparations with KOH difficult, leading to false-negative results (Fig. 10).
We suggest the mycological examination of sputum for three consecutive days, which identifies Paracoccidioides species in 93% of cases. New samples should be collected only when these evaluations are negative (250).
The isolation of Paracoccidioides spp. in culture should be performed using the following media: (i) Mycosel (Sabouraud agar plus cycloheximide and chloramphenicol), (ii) mycobiotic agar (dehydrated) (Difco), (iii) SABHI (Sabouraud dextrose plus brain heart infusion broth) agar (Bacto SABHI agar base; Difco), (iv) agar-yeast extract-phosphate, (v) agar-yeast extract, (vi) agar-yeast extract-penicillin plus streptomycin and cycloheximide; and (vii) Kelley medium with hemoglobin (6, 247). Fava-Netto medium is also useful for fungus isolation, especially considering Noble clinical materials (liquor and other biological liquids) (44). The sputum sample should be digested before sowing in an appropriate medium at room temperature. As the growth of the fungus is slow, the results should be evaluated about 4 weeks after the cultivation of the clinical material. Paracoccidioides species grow at room temperature in the form of white colonies resembling white rat fur. When examined under a microscope, the colonies show thin, septated mycelial filaments with a terminus of intercalated chlamydospores (6, 247). Transformation from the mycelial to the yeast phase, characteristic of these fungi, should be performed by sowing in Kelley medium, based on hemoglobin agar, maintaining the temperature at 35°C to 37°C. At these temperatures, cerebriform colonies are produced, and microscopy shows rounded cells similar to those observed in tissues. These phases are called the mycelial and yeast phases, respectively.
Judging from our expertise in the cultivation of this microorganism, it is important to highlight that the success of detection is linked to the following: (i) the use of new media (with good moisture content) favors the growth of Paracoccidioides species, (ii) sowing in two to three tubes containing culture medium (Sabouraud dextrose agar, Mycosel, and Fava-Netto agar) favors the isolation of the microorganism, and (iii) clinical materials such as sputum and bronchial lavage fluid should preferably be decontaminated before sowing, using a 100-mg/mL chloramphenicol solution. In clinical materials such as lymph node aspirates and cerebrospinal fluid from closed cavities, Paracoccidioides species tend to grow in a pure form without showing contamination by other bacterial or fungal microorganisms.
Cytopathological examination of sputum smears stained with Shorr, Leishman, or Gomori-Grocott stain is also useful for PCM diagnosis. Macrophages and neutrophils are observed in all patients, and giant cells are observed in 78% of them. The high frequency of neutrophils can lead to a misdiagnosis of a bacterial nontuberculosis etiology of the disease. Giant cells, lymphocytes, and eosinophils are observed at lower frequencies. The most general alteration of the respiratory epithelium is squamous metaplasia, which is present in 33% of cases (250, 251).
In addition, the cell block preparation of sputum, embedded in paraffin, shows high durability; permits the preparation of other slices specifically stained to investigate different etiologies of disease, such as Ziehl-Neelsen stain for tuberculosis; and can be sent to reference laboratories, including those in other countries (250, 251). Such preparations take longer and are more expensive than the usual procedures. When the tissue section is stained with Gomori methenamine silver (GMS), the walls of the fungal cells are intensely colored, indicating the organism’s true sizes and forms, which is a valuable help for diagnosis, mainly when an experienced mycologist or cytopathologist is not available. The sensitivity of this method showed a tendency toward being slightly lower for patients with pulmonary involvement of the pure interstitial type than for those with alveolar lesions detected by radiography. The sensitivity of the cell block preparation is higher than that of cytologic smears with the same staining. However, the evaluation of cells is lost with GMS stain.
The finding of small nonbudding cells (microforms) can be mistaken for the findings for other fungi such as Histoplasma capsulatum and capsule-deficient cryptococci (247, 252).
Interestingly, in cell block preparations stained with hematoxylin and eosin (HE) (Fig. 11C), alveolar macrophages predominate in 93% of cases, while lymphocytes were observed in only 10%, and neutrophils were observed in 3% (253).
Fungal identification in biopsied tissue samples is carried out by the histopathological examination of slides stained with HE and GMS. Part of these materials should be examined between a slide and a coverslip after being ground to a powder in sterile graal, and part should be cultured on Sabouraud broth containing 0.2% yeast extract. Histopathological examination of HE-stained tissue permits the identification of fungal cells and the evaluation of the patient’s immunological response, depending on the findings: compact granulomas in patients with less severe disease and loose granulomas in those showing intense immunosuppression. When the tissue section is stained with GMS, the walls of the fungal cells are intensely colored, indicating the true sizes and forms of the organisms, constituting a valuable aid for diagnosis (Fig. 11D). However, the histological architecture of the tissue is completely lost.
Diagnosis in Special Situations
Diagnosis is usually easy to perform, mainly when the site of the lesion is accessible or clinical materials are available, such as a mucous membrane or skin lesions and naturally released sputum. However, the involvement of the encephalon is an example of a site of a lesion that depends on the evaluation of the cerebrospinal fluid (CSF) or brain tissue to confirm the etiology of the disease. CSF puncture is sometimes contraindicated, and the identification of the Paracoccidioides species or its isolation in culture is frequently negative in this fluid. Moreover, the detection of specific antibodies by a DID test is usually nonreactive in CSF. In most of these cases, the involvement of the central nervous system is clinically demonstrated, but the diagnosis is considered confirmed by the identification of the etiological agent in other organs or by the detection of specific serum antibodies by a DID test. However, in immunosuppressed patients, serum antibodies are usually undetectable, and comorbidity is frequent, making the extrapolation of the diagnosis obtained using different clinical materials to the CNS dangerous. The detection of P. brasiliensis gp43 and gp70 antigens by an inhibition enzyme-linked immunosorbent assay (inh-ELISA) and anti-gp43 and anti-gp70 by a conventional ELISA can be used to diagnose these cases using CSF (247, 254).
Serodiagnosis of Paracoccidioidomycosis
Lately, special attention has been given to developing methods and techniques for the serodiagnosis of systemic mycoses, using sensitive and specific tests and higher-quality antigens, and carrying out epidemiological surveys to delimit the areas of endemicity of the main mycoses. The main systemic mycosis in Latin America and especially Brazil is PCM (78). Therefore, several groups are dedicated to improving the serological diagnosis and follow-up of patients during antifungal therapy.
In suspected fungal infections, no procedure is better than the isolation of the causative agent from biological material or its pathognomonic identification in clinical specimens. However, such ideal situations are not always feasible, and often, the procedures employed are based on serological testing. As the literature on the serology of PCM is extensive and diverse, we discuss the most significant advances to show what contributes to the immunodiagnosis of this important mycosis in Brazil (78, 255–257).
Antigens. For many years, various types of antigenic preparations have been used for PCM serodiagnosis, including different antigenic preparations, all of them obtained from fungal cells grown in different culture media and under different growth conditions (incubation time, growth temperature, initial inoculum size, and shaking or stationary culture). This implies that these antigenic preparations undergo considerable variations in antigen activity and quality due to the lack of standardization of different lots. On the other hand, different fungal strains may produce considerably different antigens. With all of these problems in fungal antigen production, it is not surprising that considerable disagreement regarding the sensitivity of these tests is found.
The most important contribution to Paracoccidioides species serology was the identification and purification of the 43-kDa glycoprotein (gp43), the main exocellular antigen secreted by the fungal yeast form (258).
The specificity of serological tests depends, in the first place, on the antigen used. The crude Paracoccidioides species antigen has components common to other fungi, and thus, serum samples from patients with other mycotic infections may cross-react. Efforts have been directed toward obtaining adequate antigenic preparations that may be used for highly specific and sensitive serological tests. After 7 days of growth, the exoantigen obtained from P. brasiliensis B-339 (ATCC 200273) proved ideal for immunodiffusion (ID) tests and was named antigen 7 (Ag7). The choice of the use of 7-day-old cultures is based on the knowledge that this period corresponds to the middle of the exponential growth phase. Using this protocol, the primary component of exoantigen is gp43, the immunodominant antigen. The methodology for preparing the exoantigen Ag7, which has 80 to 90% gp43 as its main component, has been described in detail by de Camargo et al. (234) (Fig. 12).
FIG 12.
SDS-PAGE of Paracoccidioides brasiliensis exoantigen showing the major antigen, a glycoprotein of 43,000 Da (gp43).
Immunodiffusion test. The ID test has been the method of choice for the initial diagnosis of patients with suspected PCM. Its specificity and sensitivity can vary from 65 to 100% depending on the type of antigen employed (259).
The Ag7 antigen was tested by a multinational group of researchers intending to provide laboratories in areas of endemicity with a simple and accurate PCM diagnostic test. The results of the ID tests showed 84.3% sensitivity and 98.9% specificity; thus, it was concluded that under these conditions, Ag7 can be considered an important instrument for PCM diagnosis (260).
There are numerous indications for implementing the procedures properly (234, 261, 262). In 2015, when P. lutzii was confirmed as another etiological agent of PCM in certain regions of Brazil, it was found that patients from these regions did not react with the gp43-containing antigen obtained from the regular antigen-producing Paracoccidioides species isolates. However, recognizing multiple species of the genus Paracoccidioides (presently 5) and their role as etiological agents of PCM in areas of endemicity poses a challenge when preparing sensitive and specific diagnostic antigens (Fig. 13) (22, 23, 34, 234, 246, 263).
FIG 13.
Immunodiffusion test. Paracoccidioides brasiliensis exoantigen (Ag) is in the center well; different serum samples from patients with paracoccidioidomycosis are in the peripherical wells (wells 1 to 6).
The tendency in PCM serology is to use a purified antigen. In this sense, we use purified gp43 in immunodiffusion assays and as a control for anti-gp43 rabbit hyperimmune serum. Although the gp43 purification process requires additional steps such as the use of affinity columns, the product obtained remains stable upon storage in a refrigerator and can be used for more than a year. The amount regularly used is approximately 1 μg of gp43 per reaction. In conclusion, the ID test is highly recommended for PCM diagnosis, defining the etiology of the mycosis in about 90% of cases, which is likely to reach 100% with further research (234, 261, 262).
ELISA. In the 1980s, the ELISA methodology began to be used with greater emphasis on PCM diagnosis, using crude antigens obtained from culture filtrates. Therefore, it was possible to estimate the level of anti-Paracoccidioides species antibodies in patient sera. Mainly due to cross-reactivity, the results of this test should be interpreted with particular caution if used for diagnostic purposes. These antigens consist predominantly of gp43, the immunodominant antigen (95, 264–267) (Fig. 14).
FIG 14.
Scheme of the ELISA for antibody (Ab) detection. Ag, antigen; OPD, o-phenylenediamine dihydrochloride; E, enzyme; S, substrate; P, product of substrate degradation.
Considering the ELISA reaction, several factors must be taken into account. First, the antigen concentration absorbable by the plate is of importance. This concentration should be such that there is no antigen shortage or excess, as failures may occur in the dosage of the desired antibody. Once the optimal concentration of the antigen preparation has been determined, the serum sample is to be dosed (264–266, 268, 269).
The main problem with ELISAs, especially using crude antigens, is cross-reactivity with agents of endemic systemic mycoses, mainly histoplasmosis and candidiasis. Serum absorption with Histoplasma capsulatum or Candida albicans antigens does not eliminate cross-reactions, and this procedure is not practical (265). For this reason, the ELISA is not used as a routine diagnostic technique. In our opinion, the ELISA for PCM may be helpful when the levels of serum anti-Paracoccidioides species antibodies need to be more precisely known, as in the case for the serological follow-up of patients taking antifungal therapy (264, 265).
Western blotting/immunoblotting technique. The Western blotting or immunoblotting technique is an immunoenzymatic reaction akin to an ELISA, supported this time by a nitrocellulose membrane. The difference between the conventional ELISA and Western blotting is that the former has an immunological reaction, where the antigenic components are fixed to the flat bottom of an ELISA plate, hindering the determination of the components being recognized by antibodies. On the other hand, in Western blotting, the nitrocellulose membrane components allow the precise discrimination of the recognized components (270).
In PCM patients, Paracoccidioides species exoantigens are used for both serological diagnosis and follow-up studies, which revealed anti-Paracoccidioides species antibodies (IgG) directed against its four major components (70, 52, 43, and 20 to 21 kDa). The predominant antigen is the 43-kDa glycoprotein, being recognized by 100% of patient serum samples. Although Western blotting is a highly sensitive technique and allows precise analysis, it is not accessible to all laboratories (270, 271) (Fig. 15).
FIG 15.
Western blotting of P. brasiliensis exoantigen. Lane 1, SDS-PAGE of P. brasiliensis exoantigen; lane 2, gp43 recognized by the serum of a patient with paracoccidioidomycosis.
Latex agglutination immunoassays. The latex particle agglutination test is based on an antigen-antibody agglutination reaction, that is, latex microparticles coated with specific antibodies or antigens. A positive reaction is triggered by rapid agglutination characterized by a homogeneous granularity pattern observed with the naked eye; otherwise, no changes occur (272, 273).
The detection of Paracoccidioides species anti-gp43 antibodies by the latex agglutination test reveals high reactivity in PCM patients, with a sensitivity of 95% and a specificity of 92.59%.
Diluted sera should be used for the best results. The latex-gp43 test for anti-gp43 antibodies can be used for diagnostic purposes, and undiluted serum should be used for the best results (272).
Regarding antigen detection, studies of the latex agglutination test have shown that 96.4% of PCM patient serum samples were positive, with a sensitivity and specificity of 96.4% and 88.5%, respectively (272).
Overall, the latex tests for antibody or antigen detection show good sensitivity and specificity and are additional tests for the proper diagnosis of PCM patients.
The latex test has demonstrated several advantages, such as a short performance time, visual reading by the naked eye, low-complexity implementation, and sensitivity high enough to serve regular diagnostic needs (272).
Diagnosis of PCM Due to P. lutzii
For over a century, PCM was thought to be caused by a single etiological agent, P. brasiliensis. Even before the official description of the new species Paracoccidioides lutzii, the existence of regional strains with unique characteristics was suspected. This suspicion arose from the fact that sera from patients with PCM from midwestern Brazil, especially the State of Mato Grosso, fail to react with the traditional antigen obtained from the B-339 strain (P. brasiliensis sensu stricto), which is regularly used in Latin America as a standard for serological diagnostic methods (274).
In 2014, Teixeira et al. (22) described a new species, Paracoccidioides lutzii, previously named “Pb01-like,” based on phylogenetic data, comparative genomics, recombination analysis, and morphological characteristics. P. lutzii occurs mainly in midwestern Brazil (24) and was also described in the Amazonian region (25, 75).
The serological experience for PCM caused by P. lutzii is very recent and should be studied further before reaching definitive conclusions. The first studies carried out by Gegembauer et al. (23) showed that the best way to diagnose the disease is by immunodiffusion testing using cell-free antigen from P. lutzii (23). Freshly prepared cell-free antigens consist of noncovalently binding proteins expressed during the yeast phase that are attached to the surface of the Paracoccidioides cell wall (271).
Immunoenzymatic assays such as ELISAs and Western blotting can also be used to detect anti-P. lutzii antibodies, but they still require additional development.
In summary, the detection of specific antibodies against systemic mycosis agents by serological testing has long been used as a diagnostic aid, and some of these methods show high sensitivity. Unfortunately, most serological tests employ crude antigens, which contributes to the low specificity of the results. Consequently, cross-reactions occur to various degrees among sera from patients with diverse systemic mycoses and mostly crude antigens. All of the above-described issues limit the value of serological tests based on crude antigenic mixtures. Currently, the specific antigens from the main fungi causing systemic mycoses are already known, and they can be purified and tested for their use for immunodiagnosis. For example, the P. brasiliensis 43-kDa antigen and the H. capsulatum 94- and 120-kDa molecules are being used and tested in their native and chemically treated forms, with promising results (23, 265, 275–279). However, there is no consensus among laboratories on the use of a particular test, and each laboratory utilizes the most convenient methodology for its diagnostic service. The same goes for the choice of the antigen to be used for the reactions. Thus, it is not possible to precisely determine the best parameters to be used for diagnostic tests and/or serological follow-up (280–282).
With more precise and elaborate technological knowledge, the current trend is to use purified and specific antigens from each fungal species to obtain specific results. Other techniques are also helpful; however, they should be standardized to assist in diagnosis and/or serological follow-up. The issue of the antigen to be used is of prime importance, and it should be an appropriate preparation in both quality and quantity for each specific technique.
Immunoproteomics Studies of Paracoccidioidomycosis
The lack of high-quality diagnostic tests for PCM infection/disease results in deficient diagnoses and a poor prognosis for this neglected mycosis. Due to the multiple and diverse clinical manifestations of PCM, confirmatory diagnostic tests are needed to identify humans infected or affected by this fungus (283, 284). In this scenario, immunoproteomics studies provide a unique opportunity to refine diagnostic tools for this important neglected systemic mycosis by uncovering the significant antigens in human PCM. Immunoproteomics analysis revealed a diverse antigenic panel of 16 and 25 immunoreactive proteins in the P. lutzii and P. brasiliensis immunoproteomes, respectively, and 29 were proven to be novel antigens, including 7 uncharacterized proteins (285). PCM antigens are involved mainly in metabolic pathways, carbon metabolism, and the biosynthesis of secondary metabolites (e.g., triosephosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, fructose-bisphosphate aldolase 1, HSP72-like protein, transketolase, enolase, and mitochondrial HSP60) (285). A recent study involving a murine model of PCM helped to increase the number of potential diagnostic targets (286). Besides, an essential panel of B-cell-immunogenic peptides was proposed (285, 286), which will undoubtedly benefit a new generation of diagnostic tests prepared from various B-cell epitope lineages, mapped in silico from Paracoccidioides species antigens identified in immunoproteomics studies (285, 286).
Despite significant improvements, gp43 remains an important antigen embedded in the Paracoccidioides species complex (287). Although common gp43 epitopes between P. lutzii and other Paracoccidioides species exist, anti-gp43 MAbs (MAb3e, MAb17c, MAb19g, MAb21f, or MAb32) do not recognize the recombining orthologous P. lutzii protein (288), nor do antibodies from patients infected with P. lutzii recognize the native B-339 antigen (23), thus supporting the idea that B-339-derived gp43 cannot be used for the diagnosis of disease caused by P. lutzii (288). On the other hand, Rodrigues et al. demonstrated that the main antigen of P. lutzii is a surface-associated enolase, which is present as six isoforms in the range of 54 kDa; therefore, new antigenic preparations should include this molecule, especially in areas where there is a predominance of P. lutzii infection (285).
Therefore, these novel Paracoccidioides species immunoproteomes should be employed to develop sensitive and affordable point-of-care diagnostic assays and an effective vaccine to prevent infection and the development of human PCM.
Molecular Assays for the Detection and Identification of Paracoccidioides Species
The identification of Paracoccidioides isolates to the species level based solely on phenotypic methods is a challenge, as the morphological characteristics overlap those of phylogenetically related species, such as those within the P. brasiliensis complex (289). Hence, molecular technologies offer an effective solution for PCM diagnostics and should be used in the laboratory as a complement or an alternative to classical morphological methods (290). Most molecular assays developed for PCM diagnostics employ DNA extracted from cultures (8–10, 32, 291). Sample preparation is a critical step in the molecular detection of PCM, and only a few methods have been developed to detect Paracoccidioides directly from sputum; pus; biopsied fresh tissue or formalin-fixed, paraffin-embedded (FFPE) tissue; bronchoalveolar lavage fluid; and cerebrospinal fluid (283, 292) (Table 5). This subject has been reviewed by Pinheiro et al. (283).
TABLE 5.
Molecular and proteomic methods used to identify Paracoccidioides isolates to the species levele
| Method | Sample type used in molecular assays |
Identification power |
Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|
| Culture | Biopsy specimena | Biological fluidsb | Soil | Genus level | Species complex levelc | 5-species systemd | ||
| DNA barcoding (ITS1/2 + 5.8S) | Yes | No | No | No | Yes | Yes | No | 313 |
| DNA sequencing (MLSA) | Yes | No | No | No | Yes | Yes | Yes | 8–10, 32, 291 |
| Single-plex PCR | Yes | Yes | Yes | Yes | Yes | No | No | 10, 294–300 |
| Duplex PCR | Yes | Yes | Yes | Yes | Yes | Yes | No | 284 |
| Nested PCR | Yes | Yes | Yes | Yes | Yes | No | No | 54, 301–306 |
| qPCR | Yes | Yes | Yes | Yes | Yes | Yes | No | 32, 309–312 |
| RAPD | Yes | No | No | No | Yes | No | No | 17 |
| PCR-RFLP | Yes | No | No | No | Yes | Yes | No | 293 |
| AFLP | Yes | No | No | No | Yes | Yes | Yes | 293 |
| Microsatellites (SSR) | Yes | No | No | No | Yes | Yes | No | 20 |
| SNaPshot | Yes | No | No | No | Yes | Yes | No | 32 |
| LAMP | Yes | Yes | Yes | No | Yes | No | No | 307, 308 |
| FISH/ISH | Yes | No | No | Yes | Yes | Yes | No | 53, 318, 319 |
| Whole-genome sequencing | Yes | No | No | No | Yes | Yes | Yes | 12, 34, 316, 322 |
| MALDI-TOF MS | Yes | No | No | No | Yes | Yes | No | 321 |
Fresh tissue or formalin-fixed, paraffin-embedded (FFPE) tissue.
Biological samples include sputum, bronchoalveolar lavage fluid, and serum, etc.
Differentiation between the P. brasiliensis complex (S1, PS2, PS3, and PS4) and P. lutzii.
Five species of Paracoccidioides: S1 (P. brasiliensis sensu stricto), PS2 (P. americana), PS3 (P. restrepiensis), PS4 (P. venezuelensis), and P. lutzii.
MLSA, multilocus sequence analysis; qPCR, quantitative real-time PCR; RAPD, random amplified polymorphic DNA; PCR-RFLP, restriction fragment length polymorphism; AFLP, amplified fragment length polymorphism; SSR, simple-sequence repeat; LAMP, loop-mediated isothermal amplification; FISH, fluorescence in situ hybridization; MALDI-TOF MS, matrix-assisted laser desorption ionization–time of flight mass spectrometry.
The advent of molecular diagnostics has provided the clinical laboratory with a wealth of alternatives for routine Paracoccidioides identification, notably those techniques based on PCR, such as random amplified polymorphic DNA (RAPD) (17), PCR-RFLP (restriction fragment length polymorphism) (293), species-specific PCR (10, 294–300), nested PCR (54, 301–306), microsatellite markers (20), SNaPshot (32), loop-mediated isothermal amplification (LAMP) (307, 308), and quantitative real-time PCR (qPCR) (32, 309–312). Nevertheless, most of these methods were developed before the recognition of cryptic species of Paracoccidioides. Thus, judging from recent taxonomic changes, these methods allow only generic or partial characterizations of Paracoccidioides isolates as the culprits of disease.
Among the PCR-based techniques, we highlight the recent use of a duplex PCR assay targeting exon 2 of the GP43 gene as a promising strategy for the molecular diagnosis of PCM based on both cultures and clinical material such as FFPE tissues (284). The duplex PCR assay covers all species within the P. brasiliensis complex and differentiates them from P. lutzii. This is a highly relevant approach, as classical serology-based detection of anti-gp43 antibodies cannot detect P. lutzii, leading to false-negative results (23, 26).
Sequencing followed by phylogenetic analysis is considered the gold standard for the species identification of Paracoccidioides, but it may present limitations depending on the locus used (1). DNA barcoding is relevant for identification, allowing differentiation between members of the P. brasiliensis complex and P. lutzii (313). DNA barcoding targets the partial amplification of ribosomal DNA (internal transcribed spacer 1/2 [ITS1/2] and 5.8S) using universal primers such as ITS1 and ITS4, as proposed by White et al. (314). The GP43 gene may be used as a secondary barcode marker (8, 315). Differentiation using a five-species system can be achieved by multilocus sequence analysis (MLSA) based on a scheme that includes GP43, ARF, and TUB1 (8, 10, 24, 32). The introduction of other loci can increase the resolution of MLSA, which includes targets such as partial regions of the mitochondrial genome (COB2, ATP6, COX3, RNS, and RNL) or the nuclear genome, including coding (PRP8, CHS2, CDC42, FKS) and noncoding (ORN1, 11b12b, 15b16b, AB, KL, MN, R56, TUB, III-IV, and XI-XII) regions (8–10, 32, 291).
Amplified fragment length polymorphism (AFLP) markers provide a source of robust, fast, and inexpensive genetic data that can be directly applied to identify clinical and environmental isolates of Paracoccidioides to the species level, as recently demonstrated by Roberto et al. (73). Moreover, in nonmodel organisms with scarce genomic resources, such as Paracoccidioides, AFLP markers allow the exploration of more polymorphic loci than with microsatellites (20) or DNA sequencing, increasing the resolution of the method to identify Paracoccidioides in a five-species system correctly.
The advent of whole-genome sequencing opens important avenues for exploring new diagnostic and epidemiological tools, allowing a better understanding of Paracoccidioides species boundaries (12, 34, 316). However, the high cost associated with the need for expertise to analyze and interpret large data sets this tool away from the reality of most clinical laboratories in Latin America, where PCM is an endemic, neglected disease associated with socioeconomically underprivileged populations (317).
Fluorescence in situ hybridization (FISH) with sequence-specific complementary probes targeting the ribosomal DNA (ITS region) helped identify members of the P. brasiliensis complex and P. lutzii from cultures of the microorganisms in fresh mountings of clinical and environmental samples (53, 318, 319). However, FISH employs DNA probes and methods that require local expertise/availability of the appropriate technology (320).
Nucleic acid-independent molecular assays such as matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) have emerged as important tools in differentiating the P. brasiliensis complex from P. lutzii, but so far, the species incorporated into the P. brasiliensis complex (S1, PS2, PS3, and PS4) cannot be discriminated (321). In summary, it is essential to highlight that not all available molecular diagnostic tests can differentiate Paracoccidioides down to a five-species system. In many cases, a combination of two or more techniques is recommended to obtain highly discriminatory results. A critical gap for PCM diagnostics is the development and application of point-of-care testing, also known as near-patient testing (322), which will allow faster and more effective diagnoses, positively impacting the early treatment and clinical outcomes of patients affected by PCM.
TREATMENT
All confirmed cases should be treated. Suspected or probable cases may be treated provided that the response to therapy is carefully observed, considering that the definitive diagnosis could be different from that of PCM.
PCM treatment should include support measures such as controlling the triad of malnutrition-immunodepression-infection (323). Malnutrition impairs the cellular immune response, aggravating existing antigen-specific immunosuppression (150).
Support Measures
Support measures include rest; treatment of associated diseases, among which worm infections are frequently found; as well as the control of aggravating conditions. The control of both alcohol intake and smoking, so common among PCM patients, greatly contributes to their recovery.
An impaired nutritional status in patients may have several causes, such as a poor diet, as expected in a low-income population; anorexia, depending on the infectious process; the inability to ingest food due to upper digestive tract mucous membrane damage (oral cavity, oropharynx, and hypopharynx); and impaired absorption, which often becomes a syndrome of its own, mainly for fat and protein. Malnutrition is treated with an appropriate diet, usually hyperproteic and hypercaloric; vitamin supplementation; and, when indicated, parenteral nutrition.
Medications That Are Active against the Action of Paracoccidioides Species
Sulfamethoxazole (SMX)-trimethoprim (TMP), amphotericin B (AmB), and their lipid formulations, plus azole derivatives, are recommended for PCM treatment. Table 6 presents the formulations, total daily doses, routes of administration, dose spacing, as well as interactions between food intake and the oral route of administration.
TABLE 6.
Antifungal compounds available for the treatment of paracoccidioidomycosisd
| Antifungal compound | Formulation | Total daily dose | Interval between doses | Route of administration | Method of drug intake (empty or full stomach) |
|---|---|---|---|---|---|
| Azole compounds | |||||
| Ketoconazole | Tablet | 400 mg | q24h | Oral | Empty |
| Solutionc | 5–8 mg/kg | q24h | Oral | Empty | |
| Itraconazole | Capsule | 200 mg | q24h | Oral | Full |
| Capsule | 200 mg | q12h | Oral | Full | |
| Fluconazole | Tablet | 400 mg | q12h | Oral | Without regard to meals |
| Glass bottle | 400 mg | q12h | Intravenous | ||
| Voriconazolea | Tablet | 400 mg | q12h | Oral | 1 h before or 1 h after a meal |
| Glass bottle | 400 mg | q12h | Intravenous | ||
| Amphotericin B | |||||
| AmB-db | Glass bottle | 50 mg | q48h | Intravenous | |
| ABLC | Glass bottle | 3.5 mg/kg | q24h | Intravenous | |
| TMP-SMX combination | Tablet | 480–2,400 mg | q12h | Oral | Without regard to meals |
| Suspensionc | 16–20 mg/kg TMP | q12h | Oral | Without regard to meals | |
| Glass bottle | 480–2,400 mg | q12h | Intravenous | ||
Four hundred milligrams twice on the first day is usually given as a loading dose.
AmB-d (amphotericin B deoxycholate) should be used at increasing doses up to 1.0 mg/kg of body weight, not exceeding 50 mg/dose.
Dosage for children.
ABLC, amphotericin B lipid complex; TMP-SMX, trimethoprim-sulfamethoxazole, also called co-trimoxazole; q24h, every 24 h.
Sulfonamide-trimethoprim combination. The combination of a sulfonamide derivative and trimethoprim is very effective in treating PCM (324–329), although its mechanism of action remains unknown. The most commonly used preparation is the combination of sulfamethoxazole-trimethoprim (400 mg SMX–80 mg TMP), called co-trimoxazole (CMX), which is available in Brazil for oral, intravenous, and intramuscular use.
When using the intravenous route, each 5.0 mL of co-trimoxazole should be diluted in 125 mL of a 5% glucose saline solution and administered dropwise within 60 to 90 min. Fast or bolus infusions should be avoided. On the other hand, after co-trimoxazole is diluted in a glycosylated solution, the solution should not be refrigerated and must be used within 6 h. In water-restricted patients, each 5.0 mL of the drug should be diluted in 75 mL of a 5% glycosylated solution. Under this condition, the solution should be prepared immediately before administration, which should be made within no more than 2 h.
In patients with impaired renal function, the dose of co-trimoxazole should be adjusted for endogenous creatinine clearance, maintained when clearance is above 30 mL/min and halved when it is between 15 and 30 mL/min. When clearance is below 15 mL/min, co-trimoxazole should be contraindicated.
Cerebrospinal fluid concentrations of TMP and SMX are about 50 and 40%, respectively, of their concomitant serum concentrations.
Serum sulfa-free levels should also be measured in these patients by adjusting the daily doses of co-trimoxazole or co-trimazine to be maintained above 70 μg/mL during initial treatment and above 50 μg/mL during consolidation treatment.
The sulfonamide base medication is effective and well tolerated (324, 329, 330). After oral administration, there is often a complaint of gastric intolerance, which rarely requires the replacement of the medication. Hepatotoxicity is generally revealed only by slightly (maximum of 3-fold the upper limit of normal for bilirubins [direct bilirubin {DBil}]) increased serum aminotransferase levels (aspartate aminotransferase [AST] = 17.3%; alanine aminotransferase [ALT] = 28.6%), bilirubins (DBil = 7.4%; total bilirubin [TBil] = 1.8%), alkaline phosphatase (10.2%), and γ-glutamyltransferase (14.3%) (330). Hepatic impairment caused by CMX is hepatocellular and regresses despite medication maintenance (330). However, severe cases of hepatitis have been observed (331).
In addition, 26% of patients have slight and transient increases in serum urea and creatinine levels without any associated clinical manifestation, and 7.5% have mild leukopenia (325). Low platelet counts and anemia, although rare, have also been reported. Thus, in cases of spinal cord depression, characterized by thrombocytopenia, leukopenia, and megaloblastic anemia, folinic acid (leucovorin) should be administered at a daily dose of 3.0 to 6.0 mg intramuscularly for 3 days or until hematopoiesis is restored.
CMX is contraindicated during full-term pregnancy because it passes through the placental barrier and reaches high fetal plasma levels, leading to severe encephalopathy known as kernicterus, and as it is excreted in breast milk during breastfeeding, sufficient serum levels can be reached in the newborn to trigger kernicterus.
Amphotericin B. AmB, which alters the permeability of the fungal cytoplasmic membrane, subsequently leading to increased proton influx and potassium efflux, was used in 1958 for the treatment of four PCM patients with resistance to sulfonamide derivatives (332), and 1 year later, two studies on a larger number of cases and with longer-term follow-up of patients formed the basis for the treatment of PCM with AmB (333, 334).
Over the years, isolates of Paracoccidioides spp. remained responsive to AmB. Reaching fungicidal levels is a slow process as AmB should be administered in increasing doses. Its elimination occurs especially by metabolic conversion and biliary excretion, with a short average life of a few days. Amphotericin B is not dialyzable, and its CSF concentration is very low after intravenous administration. The pharmacokinetics of this antifungal do not change in anuric or nephrectomized patients (335, 336).
Because it is a very slightly soluble antibiotic, which precipitates when in contact with a saline solution, AmB should be administered intravenously, suspended in 500 mL of a 5% glycosylated solution, protected from light, as it decomposes after 6 h of exposure (335).
Treatment should be initiated at increasing doses starting at 5.0 mg, increasing by 10.0 mg with each new administration until 1.0 mg/kg of body weight is attained, taking care not to exceed 50 mg/kg for adults and 25.0 mg for children at each administration.
Administrations should be done every other day, as the serum levels obtained 48 h after an infusion of 1.2 mg/kg of body weight (337) are higher than the MIC of AmB for Paracoccidioides spp. (338). The infusion time should not be less than 6 h to minimize undesirable effects.
Immediate side effects are characterized as fever, generalized discomfort, severe and lasting chills, tachycardia, tachypnea, and high blood pressure, which are partly caused by the release of prostaglandin E2 (339). These effects can be avoided or minimized by administering 5.0 mL of Y-linked dipyrone to the AmB-infusing equipment. When these effects manifest during the administration of dipyrone, the antimycotic drip is temporarily decreased or even suppressed, with dipyrone being increased. After the side effects cease, the initial rates of infusion are resumed. Over the course of treatment, the undesirable effects described above tend to disappear.
The oral administration of 500 mg acetylsalicylic acid, 2 h and then 30 min before the start of the AmB infusion, also helps counteract this antibiotic’s immediate side effects by inhibiting prostaglandin E2 synthesis (162).
Phlebitis is quite frequently observed and should be treated by applying a hot-water bag and topical anti-inflammatory drugs. It is sometimes so intense that it becomes difficult to puncture the vein for further antimycotic infusions or to draw blood for tests (323).
Special care should be given to renal function, as AmB is very nephrotoxic, causing decreased glomerular filtration rates, hypokalemia, hypomagnesemia, renal tubular acidosis, and nephrocalcinosis. It has been suggested that AmB interferes with tubuloglomerular feedback. This is the mechanism by which an increased demand for chlorine ions from the distal tubular dense macula determines a rapid decline in the glomerular filtration rate, probably due to the increased vascular resistance of the afferent arteriole. Tubuloglomerular feedback is potentiated by sodium deprivation and suppressed by a previous sodium overload. These observations were confirmed by the recovery or preservation of renal function in patients receiving AmB following the administration of sodium overload (340).
In patients taking AmB, the serum creatinine level and endogenous creatinine clearance should be determined at least once a week. Although the proposed doses given on alternate days are far less nephrotoxic than daily dosing schedules, impaired renal function is virtually a rule. In such cases, the AmB dose should be decreased as a function of endogenous creatinine clearance as this procedure preserves renal function while maintaining treatment efficacy (341). Table 7 lists the correction factors (f) that should multiply the dose at which nephrotoxicity is observed (D) as a function of endogenous creatinine clearance (323). Thus, if a patient taking 50 mg AmB has creatinine clearance reduced to 76 mL/min, the antibiotic dose should be corrected (Dc) to 40 mg, which corresponds to the 50-mg dose multiplied by 0.80, the correction factor for creatinine clearance values of between 75 and 79 mL/min. Thus, the formula Dc = f × D allows the correction of the AmB dose to be administered, depending on the endogenous creatinine clearance values (323, 341, 342).
TABLE 7.
Correction factors for different stages of renal functiona
| Clearance of endogenous creatinine (mL/min) | f |
|---|---|
| 20–24 | 0.30 |
| 25–29 | 0.35 |
| 30–35 | 0.40 |
| 36–40 | 0.45 |
| 41–46 | 0.50 |
| 47–52 | 0.55 |
| 53–57 | 0.60 |
| 58–63 | 0.65 |
| 64–68 | 0.70 |
| 69–74 | 0.75 |
| 75–79 | 0.80 |
| 80–85 | 0.85 |
| 86–90 | 0.90 |
| 91–96 | 0.95 |
| >97 | 1.00 |
The formula for calculating the doses of amphotericin B according to the clearance of endogenous creatinine is Dc = D × f, where Dc is the corrected dose, D is the dose planned to be administered, and f is the correction factor (323).
Renal function was believed to return to normal in most cases within a few weeks or months after the interruption of treatment. A subsequent study showed that the incidence of hypertension among patients with PCM treated with AmB was 3 times higher than that in the general population in the same region (343). The assessment of renal function in these patients was made by measuring serum endogenous creatinine levels and assessing the average life of [51Cr]EDTA and the glomerular filtration rate determined by the above-described test. The results showed that patients who were treated with AmB in the above-described follow-up but who did not receive creatinine clearance dose correction demonstrated a very high frequency of impaired renal function. In contrast, among those who received AmB with the care mentioned above and dose correction of creatinine clearance, the frequency of hypertension was much lower, equal to that of the population in the same region.
AmB can cause hypokalemia due to its nephrotoxicity and as a consequence of its mechanism of action. On host cells, there is potassium efflux followed by its excretion. Hypokalemia care begins with the prophylactic administration of 500 mL of orange juice with two ampoules of 19.1% potassium chloride, which should be taken daily. If persistent, potassium replacement should be performed by the oral or intravenous administration of potassium chloride (336).
Electrocardiographic changes observed in patients with PCM during AmB administration reveal sinus node stimulation with an increased heart rate, a decreased atrioventricular conduction rate, and increased atrial and ventricular automaticity, leading to the appearance of extrasystoles, especially in patients above 45 years of age (342, 344). However, the most crucial effect is related to ventricular repolarization. The T wave can become symmetrical, of low voltage, isoelectric, or even negative. The appearance of an increased U wave even fused with the T wave could be observed. These changes are similar to those observed in metabolic or electrolyte disorders such as hypokalemia, although serum calcium, sodium, potassium, and alkaline phosphatase levels, evaluated in many cases, have been normal. Considering that many patients also have AmB-induced hypokalemia, these effects may add up. It should be noted, however, that these changes are transient, disappearing when the medication is discontinued. In addition, electrocardiographic changes, associated with an increased cardiac area, have been observed in some patients taking AmB and corticosteroids.
Hematocrit may decrease due to an AmB-induced decrease in erythrocyte production, which requires periodic control.
Other side effects of AmB can be observed although at a very low frequency. Hypomagnesemia, liver dysfunction, thrombocytopenia, and peripheral arteriole constriction are among the side effects with the total dose of an AmB series, and the dose should not exceed 30 mg/kg of body weight. Although many patients received this dose, less severe cases respond to lower doses, while some critically ill patients appeared to require higher doses (323).
Amphotericin B is the most effective drug available for PCM treatment, especially in severe cases and those resistant to other antifungal compounds. An evaluation of patients treated with AmB and monitored for up to 14 years revealed fully satisfactory results in 54% of them (345). Cases that did not respond to treatment could be explained by the short duration of the treatment, around 3 months, which is an insufficient amount of time to recover the cellular immune response, which is responsible for patient protection after treatment discontinuation.
In conclusion, AmB, when indicated, should be used with caution, accompanied by careful clinical and laboratory evaluations. Serum sodium, potassium, and creatinine levels and endogenous creatinine clearance should be evaluated at least once a week, while a complete hemogram and an electrocardiogram are to be performed at longer intervals.
AmB has great efficacy but important toxicity, and research is oriented toward obtaining a less toxic but equally effective derivative. When incorporated into liposomes, which are phospholipid vesicles, AmB increases ergosterol binding by about 15-fold, becoming more effective and decreasing its binding to cholesterol, thus reducing its toxicity (346).
The amphotericin B lipid complex was used to treat 28 PCM patients undergoing their initial (onset) treatment, lasting an average of 18 days, followed by consolidation treatment with CMX for 14 months or itraconazole (ITC) for 19 months on average (347). The patients were monitored for 12 months after treatment discontinuation and showed no relapses during this period. The main side effects observed were hypomagnesemia, chills, and anemia in more than 50% of cases and acute renal failure in 11% of them.
Azole derivatives. Azole derivatives have broad-spectrum antifungal activity, including the Paracoccidioides genus. They inhibit cytochrome P450 depending on 14-α-demethylase, a key enzyme involved in converting lanosterol to ergosterol; the latter is the primary cell membrane component of these fungi.
Several azole derivatives are active against Paracoccidioides spp. Among the imidazoles, miconazole, never marketed in Brazil, and ketoconazole (KTC) are effective both in vitro and in patients. Miconazole showed anti-Paracoccidioides species activity both in vitro and in animal models (348). Short intravenous miconazole treatment of six patients proved effective in all of them, although two relapsed 3 to 5 months later, one remained in remission, and the other three were given sulfonamides after miconazole discontinuation (349). The efficacy of orally administered miconazole was demonstrated in only five patients (350). The response to treatment was deemed adequate, and its administration by the oral route facilitated patient compliance. Several studies evaluating ketoconazole in PCM patients proved that this treatment was effective (351, 352). Among the triazoles, ITC was the most extensively evaluated antifungal in PCM patients (353–361), followed by fluconazole (FLC) (362–364) and voriconazole (VRC) (365).
These antifungal compounds also inhibit the host cell cytochrome system, explaining the possible effects on bile, fatty acid, thromboxane, and hormone synthesis; prostaglandin and leukotriene metabolism; as well as testosterone (366, 367) and cortisol (366–368) synthesis. Itraconazole has a higher affinity for fungal cytochrome P450 while presenting a lower affinity for the mammalian cell membrane cytochrome P450 (367).
KTC is generally well tolerated; side effects such as gastric intolerance and minor changes in serum aminotransferase, bilirubin, alkaline phosphatase, and γ-glutamyltransferase levels are uncommon and transient, disappearing with treatment discontinuation (369, 370). However, although very unusual, more severe cases of hepatotoxicity, including fulminant hepatitis, have been reported with KTC (346, 370, 371). Although rare, there are cases of severe and generalized KTC-induced pruritus.
KTC, at a daily dose of 400 or 600 mg, blocks cortisol synthesis for 8 to 16 h (366, 368) but without clinical repercussions. However, patients should be monitored for their adrenal function. At daily doses of up to 400 mg, KTC may block testosterone synthesis, usually resulting in decreased serum levels, which remain within the normal range and are not accompanied by clinical complaints (366–368). In daily doses at concentrations three times higher than that used as an antifungal compound, KTC leads to decreased cholesterol serum levels, attributed to low-density lipoprotein (LDL) cholesterol. At the same time, high-density lipoprotein (HDL) cholesterol and triglyceride concentrations do not change significantly (372). PCM patients undergoing KTC treatment have reduced glucose-6-phosphate dehydrogenase and glutathione reductase activities (4). One patient presented an episode of low-intensity hemolysis not requiring treatment interruption but warranting hematological follow-up treatment. KTC is effective for PCM treatment, including for PCM patients with relapsing disease (373), although failures have been reported (374, 375). Although KTC allows outpatient treatment and despite its tolerance and efficacy, KTC has been replaced by ITC.
An in vitro study with ITC showed anti-Paracoccidioides species activity that was 10 to 100 times higher than that of KTC (376); in recent years, it has been introduced as the treatment of choice for PCM (356). Although ITC is better absorbed when capsules are taken after a meal, its absorption is still irregular, and its bioavailability is variable. Effective PCM treatment results have been observed with 100 or 200 mg given as a single daily dose (353–356). Hepatotoxicity is the main ITC side effect and is generally characterized by elevated serum levels of biochemical parameters, presented as prevalence (percent) and intensity (number of times the upper limit of normal), respectively, for aminotransferases (AST, 6.3% and 4.0; ALT, 21.9% and 1.8), bilirubins (DBil, 32.3% and 2.4; TBil, 12.9% and 1.4), alkaline phosphatase (6.3% and 1.1), and β-glutamyltransferase (16.1% and 2.2) (330). However, some cases of severe hepatitis have been observed, with jaundice and intense elevations of serum levels of these enzymes and bilirubin, which required discontinuation of the medication. ITC hepatic impairment is of a mixed source (cholestatic-hepatocellular) of mild intensity persisting with medication maintenance. ITC hepatotoxicity is less frequent and less intense than that observed with KTC. Although uncommon, rashes, dizziness, gastric intolerance, and hypokalemia are also observed with ITC treatment (329).
Finally, cases of congestive heart failure were observed in patients taking ITC (166). Thus, considering previous pharmacological studies indicating a negative ITC inotropic effect, an association between this antifungal and the observed congestive heart failure has been suggested. Consequently, ITC is contraindicated for patients with evidence of ventricular dysfunction, and additionally, its use in older patients should be accompanied by careful cardiac monitoring. New ITC formulations using cyclodextrins as carriers are available for oral administration as a suspension, thus offering increased bioavailability and intravenous use; however, such formulations are not yet available in many countries where PCM is endemic.
Fluconazole is a water-soluble triazole derivative, well absorbed after oral administration, which diffuses into the cerebrospinal fluid, reaching high CNS levels; it is excreted by the kidneys in an active form, and it causes very low hepatotoxicity. Its efficacy in treating PCM appears to be lower than those of KTC and ITC (355). As in some countries, FLC has been licensed to treat only candidiasis and cryptococcosis, its indication for PCM treatment should be restricted to rare cases where AmB, CMX, KTC, and ITC are contraindicated, according to patient consent.
A comparative study of VRC and ITC evaluated their efficacy and tolerability in PCM treatment (365). Both antifungal compounds resulted in equal prevalences of partial or complete responses (VRC = 94.4%; ITC = 88.6% [P > 0.05]). Overall, adverse effects were detected more frequently with VRC than with ITC (82.9% versus 55.6% [P < 0.05]). Photosensitivity reaction, abnormal vision, and color blindness predominated in patients treated with VRC, while the frequency of bradycardia was higher with ITC than with VRC (33.3% versus 5.7% [P = 0.012]). The diffusion of VRC in the CNS was higher than that of ITC, which was confirmed by the excellent treatment outcome with VRC in a patient with a paracoccidioidal brain injury (365).
Other antifungal compounds. In a phase 3 study performed to determine the safety and efficacy of isavuconazole, a triazole derivative, 10 PCM patients were treated for a median of 180 days (range, 27 to 182 days), showing complete remission in 1 case, a partial response in 7 patients, and progressive disease in 2 patients, who died on days 27 and 91 after the start of therapy (377). In a PCM patient (62-year-old man with perianal, perineal, and scrotal lesions and mild infiltrations on chest X rays) with the diagnosis confirmed by direct and histopathological examinations as well as by isolation in culture, terbinafine, an allylamine antifungal agent, was orally administered to the patient at 250 mg twice daily for 6 months. He was monitored for 2 years after treatment discontinuation, at the time when his pulmonary abnormalities had disappeared, and no signs of disease activity were detected (378). Repeat chest X rays were normal, and no residual fibrosis was seen. Terbinafine had previously shown in vitro activity against Paracoccidioides spp., with MICs higher than those observed for ITC. The MIC of terbinafine was defined as the lowest drug concentration inhibiting 80% of fungal growth, as determined by comparison (as described above) to the growth control. MICs, in micrograms per milliliter, were as follows: 0.015 to 1.0 (geometric mean, 0.1188) for terbinafine and 0.007 to 0.5 (geometric mean, 0.03165) for itraconazole. These results indicate that favorable in vitro MICs were indeed predictive of clinical success with terbinafine, demonstrating that terbinafine has potent antifungal activity against Paracoccidioides spp. in vitro (379).
Choice of Antifungal
In choosing medication to treat PCM, consideration should be given to the mycotic process-involved organs, severity, history of previous treatment failures, the possibility of administering oral medications, associated diseases, pregnancy, and then patient compliance with the proposed treatment regimen (162, 323). Serious cases should be treated with the most effective drug available, with the intravenous route being preferred at the beginning of treatment to ensure medication bioavailability. The presence of associated diseases should be taken into consideration to avoid collateral damage resulting from the originally prescribed antifungals. Therefore, AmB should be avoided in patients with impaired renal function and in elderly individuals with peripheral arteriopathy. Azole derivatives, especially KTC and, to a lesser extent, ITC and CMX, are hepatotoxic and should be used cautiously in patients with liver damage. The high incidence of alcoholism among PCM patients requires liver function testing during treatment with these antifungals (346).
Patients with associated diseases are also treated with other medications, and consequently, the possibility of multiple interactions should be analyzed (Table 8) (380). For example, for a patient with tuberculosis and PCM under specific treatment with rifampicin and KTC, in this case, rifampicin will stimulate KTC metabolism, decreasing the serum level of the latter below therapeutic concentrations, thus compromising treatment. The KTC dose should be increased, or KTC should be replaced with CMX or AmB. A medication that after proper administration has been found to be ineffective due to low availability or fungal resistance should not be used. Nonetheless, irregularities in following medical prescriptions and even treatment are reported more frequently than low bioavailability or resistance to the antifungal compound. The immediate side effects and, more importantly, AmB nephrotoxicity may lead to systemic arterial hypertension, thus stressing its use only in patients with extremely serious disease for whom the risk of death appears evident (75). Although rare, PCM may affect pregnant or breastfeeding women.
TABLE 8.
Some drug interactions involving azole antifungal compoundsa
| Effect of interaction | Drug(s) interacting with: |
|||
|---|---|---|---|---|
| Ketoconazole | Itraconazole | Fluconazole | Voriconazole | |
| Decreased absorption of azoles | Antacids | Antiacids | ||
| H2 receptor antagonists | H2 receptor antagonists | |||
| Proton pump inhibitors | Proton pump inhibitors | |||
| Sucralfate | ||||
| Decreased plasma levels of azoles due to metabolism | Rifampicin | Rifampicin | Rifampicin | Rifampicin |
| Rifabutin | Rifabutin | Rifabutin | ||
| Phenytoin | Phenytoin | Phenytoin | ||
| Isoniazid | Isoniazid | |||
| Carbamazepine | Carbamazepine | |||
| Nevirapine | ||||
| Phenobarbital | ||||
| Increased plasma levels of the coadministered drug | Warfarin | Warfarin | Warfarin | Warfarin |
| Sulfonylureas | Sulfonylureas | Sulfonylureas | Sulfonylureas | |
| Cyclosporine | Cyclosporine | Cyclosporine | Cyclosporine | |
| Tacrolimus | Tacrolimus | Tacrolimus | Tacrolimus | |
| Phenytoin | Phenytoin | Phenytoin | Phenytoin | |
| Midazolam | Midazolam | Midazolam | Midazolam | |
| Triazolam | Triazolam | Triazolam | Triazolam | |
| Alprazolam | Alprazolam | Alprazolam | Alprazolam | |
| Corticosteroids | Felodipine | Theophylline | Statins | |
| Theophylline | Verapamil | Zidovudine | Omeprazole | |
| Ritonavir | Pimozide | Rifabutin | Vinca alkaloids | |
| Saquinavir | Statins | |||
| Ritonavir | ||||
| Saquinavir | ||||
| Indinavir | ||||
| Digoxin | ||||
See reference 380.
Due to the potential teratogenic activity of azole derivatives, they are contraindicated in pregnancy. However, a prospective ITC study failed to reveal a significant risk of malformation (381). In addition, CMX is contraindicated in the last month of gestation as it may lead to kernicterus (330).
For this reason, AmB should be chosen for the treatment of pregnant women with PCM, as although it crosses the placental barrier, it is not teratogenic (178). CMX and ITC are the antifungal compounds most commonly used for PCM treatment (329). Although its efficacy and effectiveness did not differ during initial treatment, the period needed to achieve clinical cure was shorter with ITC, especially in chronic form (CF) patients. Neither the efficiency nor effectiveness of the consolidation treatment differed, but serological cure (diminishing or disappearing antibody levels) was achieved in a shorter period with ITC. The prevalence of clinically manifested side effects was much lower with ITC (6.4%) than with CMX (20.0%). Only two other studies comparing ITC and CMX for the treatment of PCM patients have been performed (382, 383). In both studies, ITC was considered more effective, although in one of the studies (383), severe cases were not treated with this triazole. Thus, considering the relationship between efficacy and toxicity and the ease of use of an oral single-dose daily administration, ITC is the drug of choice to treat the vast majority of PCM cases in regions where it is freely distributed. In other cases, the choice should be CMX. In addition, PCM patients with central nervous system involvement should be treated with CMX, FLC, or VRC, medications that achieve higher concentrations in cerebrospinal fluid and brain tissue.
Immune Stimulants
The beneficial effect of immune stimulants on PCM has been demonstrated in animal models (384, 385) and was assessed in only one clinical trial performed on patients taking β-glucan in addition to antifungal drugs (386). β-1,3-glucan, which is a β-1,3-polyglucan, was extracted from Saccharomyces cerevisiae and used at a dose of 10 mg intravenously or intramuscularly once a week for the first month and then once a month for a year. Patients treated with antifungal-associated β-glucan showed better outcomes than those not receiving immune stimulation when clinical manifestations, erythrocyte sedimentation rates (ESRs), and serological outcomes were evaluated. In BALB/c mice, β-glucan has been shown to be a potent inducer of TNF-α and IFN-γ production in BALB/c mice, findings that may explain its adjuvant effect on PCM treatment (387). Further clinical studies need to be performed to assess the efficacy and safety of β-glucan as an adjuvant in treating patients with serious PCM forms. High serum TNF-α levels should be monitored closely as they are harmful to patients (386).
THERAPEUTIC REGIMENS FOR THE CONTROL OF PROGRESSIVE FORMS OF PCM
Patients with mild and moderate forms of PCM can receive outpatient treatment, which should be conducted with CMX or ITC. Patients with a severe or moderate-to-severe form of PCM should be hospitalized in order to receive the required general care and, in most cases, intravenous antifungal compounds. After evident clinical improvement, the therapeutic regimen should be changed to oral administration. The patient should then be discharged from the hospital and continue treatment on an outpatient basis. Hospitalized patients receiving oral antifungal compounds should be hospitalized only long enough to recover their nutritional status and be assessed for the initial response to treatment (162, 323).
PCM treatment consists of induction and consolidation treatments (162, 323, 326). The first stage begins with the administration of the antifungal agent until the patient presents a clinical cure and the erythrocyte sedimentation rate normalizes. The consolidation treatment, which then follows, should be continued until there is a persistent 1-year negative reaction by a double-agar gel immunodiffusion (DID) test (Fig. 13). During the initial treatment and with outpatient visits, regular checks should be performed every month until clinical cure and mycological cure are achieved and the erythrocyte sedimentation rate normalizes (162, 323). During this period, patients should be submitted to careful clinical, mycological, and laboratory controls, to monitor the initially altered values as well as to control the toxicity of the antifungals, along with chest X rays and immunological testing. During the consolidation treatment, patients should be reevaluated every 3 months. In addition to a complete clinical observation, a chest X ray should be performed initially since pulmonary fibrosis is a common side effect, and serum anti-Paracoccidioides species antibodies should be measured. After treatment is discontinued, the patient should be evaluated every 3 months, according to the same protocol as the one described above for consolidation treatment, to assess whether the patient has recovered the specific cellular immunity responsible for keeping him or her healthy in the absence of antifungal treatment. Patients who have recovered and remained negative for 2 years in the absence of antifungal treatment are considered to have an apparent cure and can be permanently discharged. Exceptions are patients with sequelae that require periodic reevaluations, such as Addison’s syndrome and severe chronic obstructive pulmonary disease (COPD).
Cure Criteria
PCM cure is defined based on five criteria: clinical, mycological, radiological, immunological, and apparent cure (Fig. 16) (162, 323).
FIG 16.
Treatment regimen and follow-up of paracoccidioidomycosis patients according to data described previously by Mendes et al. (180, 323). DID, double-agar gel immunodiffusion (levels are the inverse of the dilution); ESR, erythrocyte sedimentation rate.
Nonetheless, the word “cure” does not imply that the fungus has been completely eradicated, as it could well become latent in several body organs (323).
Clinical cure. A patient is clinically cured when the signs and symptoms of the disease have disappeared, noticed in a relatively short time, giving the impression that a cure had been obtained, thus reducing the patient’s adherence to treatment (Fig. 3B). Therefore, professionals should contemplate the possibility of relapse and, consequently, the patient’s need to fully adhere to prolonged treatment and periodic clinical, radiological, and laboratory checks.
Mycological cure. Mycological cure can be contemplated when no fungi are found upon extensive mycological examination, as should occur after effective treatment. Therefore, this criterion refers only to the disappearance of Paracoccidioides spp. from clinical materials where the fungus was previously identified but not from the whole body.
This extensive search should be especially focused on pulmonary samples.
Radiological cure. Radiological cure refers to the radiological evaluation of the lung, as about 80% of patients have a chronic form, in which pulmonary involvement is almost always present. Initially, radiological changes include scar lesions (fibrosis) during and after treatment, when a predominance of fibrosis and emphysema is noticed (Fig. 1A). Following effective treatment, radiological cure is achieved when the patient’s X rays maintain the same scarring lesions on at least three radiographs taken at regular intervals over a year.
No new infiltrates should be noticed once treatment is concluded.
Alveolar lesions disappear faster than interstitial lesions, which regress slowly and behave differently. While small nodules disappear with treatment, large nodules usually persist, even when respiratory manifestations and serum anti-Paracoccidioides species antibodies are no longer present. The most frequently detected residual lesions are pulmonary and emphysematous, where parenchymal bands, fibrotic nodules, and diffuse or bullous emphysema can be observed.
Finally, complete normalization of the chest radiograph is observed in only rare cases, as the initial lesions generally persist as sequelae.
Immunological cure. Immunological evaluation includes humoral immunity, assessed by determining anti-Paracoccidioides species serum antibody levels and, if possible, specific cell-mediated immunity.
Specific serum antibody levels decrease with treatment, becoming negative by DID tests and sometimes by counterimmunoelectrophoresis (CIE), or stabilize at values less than or equal to a 1/8 dilution or are considered to be serological scars by this test (330).
Cellular immunity has rarely been studied after treatment has begun, explaining why routine testing has not been standardized (91, 167). The lymphoproliferative response, the intradermal reaction to paracoccidioidin, and the balance between serum cytokine levels in the Th1 (IFN-γ) and Th2 (IL-10) arms are restored after successful treatment (91, 388, 389).
A direct correlation of the above-described findings has been demonstrated for decreasing serum anti-Paracoccidioides species antibody levels determined by CIE, with decreases in IL-10 levels and increases in IFN-γ levels. The progression of the antibody concentration, already incorporated into regular laboratory testing, allows us to infer the recovery of the cellular immune response. Such a treatment response should control the not-eliminated fungi in a latent state (dormant state) (323).
Apparent cure. Apparent cure refers to patients who have shown clinical, mycological, radiological, and immunological cures for 2 years in the absence of treatment. The term apparent cure is preferred over the term cure so that it is not inferred that there was a radical cure, that is, eradication of the fungi from the host, as latent foci persist, even after effective treatment.
A study of patients with chronic PCM revealed that the recovery of cellular immunity, assessed by the quantification of mononuclear cell subpopulations and functional tests, occurred only in patients who had an apparent cure (388). Thus, the use of the term apparent cure implies the recovery of specific cellular immunity.
Treatment Duration
The duration of treatment varies depending on several factors, such as the degree of antigen-specific immunosuppression, the presence and intensity of the patient’s malnutrition, fungal virulence, the inoculum size of Paracoccidioides spp., antifungal medication, the therapeutic regimen administered, and, finally, the patient’s compliance (162, 323, 390, 391). All of these factors can hardly be adequately assessed and vary from patient to patient, explaining why these criteria have been established as applying to all patients while individualizing the duration of treatment.
Treatment control should be based on clinical, mycological, erythrocyte sedimentation rate (ESR), radiological, and serological evaluations. Since serological parameters are the last to normalize, the duration of treatment should be controlled by specific precipitin tests (DID or CIE). Thus, treatment should be discontinued only when the DID reaction is persistently negative or CIE reveals stable antibody levels equal to or less than a 1/8 dilution. This means that at least four serological evaluations indicating treatment discontinuation should be observed within a year before interrupting medication, as the patient often presents a momentarily negative DID test, which turns positive in subsequent evaluations. Having this in mind, early discontinuation of treatment can be avoided.
If it is impossible to carry out DID or CIE controls, patients with mild forms of the disease should be treated with ITZ for at least 6 to 9 months, and those with moderate forms should be treated for at least 12 to 18 months. When CMX is indicated, the durations of treatment should be at least 12 months for mild forms and at least 18 to 24 months for moderate forms (78). The duration of treatment for severe and moderately severe forms should be individualized and fulfill the available cure criteria. In such cases, treatment needs to be continued even longer. It should be noted, however, that treatment is to be discontinued only if the cure criteria have been achieved. On the other hand, based on clinical discretion, a dose reduction of either CMX or ITZ should be considered after 1 year of treatment (328, 329).
Treatment of Residual Forms (Sequelae)
Treatment of residual forms of PCM aims to correct scar lesions whenever possible and prevent complications that could occur. Nasal septum lesions are rare, and perforation of the septum is even less frequent, and no standardized procedures for these cases are available. Evaluation by an ear, nose, and throat doctor (ENT) and/or a plastic surgeon is indicated. Microstomy treatment should be performed by surgical correction for anatomical, esthetic, and functional recovery of the mouth. Laryngeal lesions remain a challenge due to the voice alterations caused by the disease, with all of their consequences for the patient’s social life, and the persistence of vocal cord closure defects, which facilitates the aspiration of saliva to the bronchial tree and, therefore, could lead to pneumonia (187). Treatment for these sequelae has not yet been defined. Tracheal lesions could lead to stenosis, suspected to be caused by the presence of dyspnea not explainable by pulmonary impairment, and wheezing in the upper respiratory system, sometimes triggered by neck rotation to one side. Diagnosis is made by bronchoscopic examination, and treatment is performed by the surgical resection of the obstructed tracheal rings if their number allows tracheotracheal anastomosis. In the case of more extensive lesions, one patient underwent laser photoresection neodymium-doped yttrium aluminum garnet (Nd-YAG) of the stenotic tracheal area with further implantation of a silicone prosthesis through rigid bronchoscopy associated with initial corticosteroid therapy. This patient was monitored for 13 years and had no problems related to the trachea (238–240). Another patient from the service received a Montgomery T tube and is being monitored, with no complaints.
Pulmonary sequelae characterized by fibrosis, emphysema, and mild-to-severe obstructive-type functional problems are significant in many cases because of the frequency and severity of clinical manifestations (Fig. 8 and 9). These patients should receive special care: practicing bronchial hygiene, fighting against smoking addiction, changing the work environment if exposed to dust, avoiding exposure to early-morning and late-evening low temperatures on cold days, and being vaccinated for influenza and pneumococcal diseases, even for those under 60 years of age. A sudden onset or increase of dyspnea and cough and an increase in the volume or a change in the color of sputum indicate acute bacterial infection, and therefore, antimicrobial treatment should be prescribed.
Patients with an exacerbation of dyspnea should receive treatment with β2-adrenergic agonists and/or anticholinergics. In some cases, combination treatment with corticosteroids for a fixed period may be indicated.
However, despite all of these precautions, some patients manifest such intense COPD that it is necessary to consider home oxygen therapy. The goals for these patients are to improve their psychological state and their quality of sleep, increase their exercise tolerance, decrease hematocrit and hemoglobin levels, and decrease or stabilize pulmonary artery pressure. All of this care may lead to reduced hospitalizations, increased survival, and an improved quality of life. Long-term home oxygen therapy is indicated for patients with a partial oxygen pressure of <56 mm Hg or with oxygen saturation of <89%. In addition to these criteria, patients with a partial oxygen pressure of between 56 and 59 mm Hg or oxygen saturation above 88% should receive this treatment when these conditions are associated with edema caused by cardiac decompensation, cor pulmonale, and/or hematocrit higher than 55%.
The duration of oxygen therapy should not be less than 15 h a day, although ideally, a duration of 24 h a day is recommended.
Clinical manifestations of paracoccidioidal adrenal insufficiency have been recorded, and usually, the abnormality manifests when the signs and symptoms of the disease have become apparent. However, there are cases in which clinical evidence of chronic adrenal insufficiency manifests after antifungal treatment has begun. Most patients with Addison’s syndrome remain dependent on hormone replacement therapy, characteristic of PCM in the residual form. Treatment should consist of prednisone at daily doses of 5.0 mg in the morning and 2.5 mg in the afternoon. Initial doses may be higher and, with normalization of blood pressure and electrolytes, should be reduced to the lowest dose possible to keep the patient balanced. Some patients are admitted to the hospital with a potentially fatal adrenal crisis and require prompt and appropriate treatment to reverse hypotension and correct dehydration and electrolyte disturbances. In addition to an infusion of 0.9% saline and 10% dextrose, 100 mg of intravenous hydrocortisone should be administered every 6 h. Once the patient is stabilized, the hydrocortisone dose should be reduced to 50 mg every 6 h. After about a week, prednisone should be administered orally at daily doses equivalent to those of hydrocortisone. After that, daily doses may be reduced slowly until the patient’s lowest effective dose is determined. In some cases, the pressure-electrolyte balance is achieved only with the combination with fludrocortisone at a daily dose of 0.05 or 0.10 mg, taken once orally; the maximum daily dose is 0.20 mg (335).
Digestive tract sequelae are generally related to intestinal occlusion or subocclusion. Clinical manifestations related to these conditions can be observed when signs and symptoms of the disease appear. However, in many cases, they manifest after treatment is started due to the fibrosis that follows. Surgical resection should be indicated as soon as the patient can undergo surgery, the results of which have been quite satisfactory.
Impairment of the abdominal lymphatic system may lead to hypertension and, sometimes, the leakage of lymph into the intestinal lumen, with the loss of lipids, gamma globulin, albumin, and lymphocytes. Therefore, these patients suffer malabsorption of fat and fat-soluble vitamins and a loss of protein and lymphocytes. Digestive tract ulcerations also contribute to the protein loss observed in this type of enteropathy. This condition usually manifests during the prodromal phase of the disease but may persist or even worsen with the introduction of antifungal therapy. Treatment includes a low-fat diet; parenteral vitamin B12 administration; supplementation with medium-chain triglyceride, as it is directly absorbed into the portal circulation; dietary supplementation with short-chain amino acid for its better absorption, calcium, a multivitamin complex, and mineral salts; and the use of antispasmodics to combat diarrhea when present. The participation of a nutrition specialist is essential during follow-up for these patients whose prognosis is not encouraging.
In addition, some patients present obstructive jaundice during the prodromal stage due to the extrinsic compression of the extrahepatic biliary tract, which is reduced by treatment. However, many of these patients present obstructive jaundice again when treatment is completed due to compression of the same pathways, now by residual fibrosis. In such cases, surgical clearance and Roux-en-Y are indicated.
Neurological sequelae, related to localized compression and intracranial hypertension due to CSF blockade, can be treated when located in an accessible area and require a ventricular shunt.
Treatment of Immunosuppressed Patients and Coinfections
PCM is sometimes diagnosed in patients immunosuppressed by AIDS, neoplastic disease, and/or drugs that induce cellular immune response depression, such as corticosteroids and cytostatic drugs (232, 392). As a rule, anti-Paracoccidioides species treatment should be maintained while the depression of the patient’s cellular immune response persists. In patients with AIDS-PCM coinfection, a CD4+ T-cell count below 200 cells/mL, a high viral load, and low serum levels of cytokines of the Th1 arm indicate the need for continued antifungal treatment. In these patients, the dose of CMX indicated as pneumocystosis prophylaxis may be sufficient for anti-Paracoccidioides species activity. In addition, consideration should be given to the possibility of antifungal drug interactions with antiretrovirals and other drugs used by AIDS patients (Table 7). On the other hand, discontinuation of antifungal treatment may be considered in patients with undetectable viral loads and CD4+ T-lymphocyte counts of >200 cells/mL provided that PCM cure criteria have been achieved (78). In patients with neoplastic diseases and/or who are treated with drugs that induce cellular immunosuppression, anti-Paracfdsoccidioides species treatment should be maintained for as long as immunosuppression persists, especially during periods of chemotherapy or radiotherapy. In such cases, careful monitoring of PCM cure criteria is critical for defining the duration of treatment.
Tuberculosis may manifest in patients with active PCM, which requires the simultaneous treatment of both diseases. In such cases, there is a great risk that therapeutic levels of azole derivatives will not be achieved, as their metabolism is stimulated by the action of rifampicin on cytochrome CYP3A4, which may require the use of higher daily doses of the antifungal such as 600 mg instead of 400 mg KTC, the administration of antituberculosis compounds separately (12 h after KTC), or replacement with another medication (393). In addition, hepatotoxicity may be induced either by drugs that make up the specific chemotherapy for tuberculosis or by azole derivatives. These patients should start treatment under hospitalization and careful clinical and laboratory observations to evaluate the toxicity and efficacy of the instituted treatments. On the other hand, CMX can be used for PCM treatment, and in this case, liver damage should be monitored, as CMX is potentially hepatotoxic.
PCM patients with pulmonary impairment may present associated acute bacterial pneumonia, which is challenging to diagnose, especially when no data from a prior radiographic or tomographic evaluation are available. The finding of leukocytosis with neutrophilia suggests this coinfection, as this finding is not frequent in PCM, which facilitates the indication for pneumonia treatment. Fistulized lymph nodes sometimes present an associated acute bacterial infection, which should be treated with antimicrobial agents that act on Staphylococcus aureus, its frequent etiology. Intestinal parasitosis should always be treated due to the frequency with which it occurs and the severity of some cases, especially strongyloidiasis cases.
Relapse Management and Serological Reactivation
The possibility of PCM relapse should always be taken into account due to the persistence of latent foci, even after appropriate treatment. Relapses are usually late, about 5 years after an apparent cure, and can be confused with other diseases (394). Relapse is rarely accompanied by suggestive clinical manifestations, the identification of Paracoccidioides spp. in clinical materials, and increased serum antibody levels, making it a more straightforward diagnosis. However, the sensitivity of the DID reaction is much lower in patients with relapse than in patients treated for the first time, and the use of the enzyme-linked immunosorbent assay only slightly increases the efficacy of serological tests (394). Therefore, a safe relapse diagnosis is possible only with fungal identification. Radiological characterization of reactivation is easy when new lesions are alveolar, but it becomes difficult when they are interstitial and occur in the pulmonary parenchyma with residual fibrosis, bands, and/or nodules. In confirmed cases of relapse, treatment should be instituted with all of the above-mentioned precautions right from the beginning. However, when only serum antibody levels are positive, without clinical manifestations in any organ or a chest X ray suggestive of activity, serological reactivation is characterized; antifungal treatment should be restarted as a consolidation treatment regimen. The patient should be reevaluated within 30 days; in the case of a satisfactory response, the conduct should be maintained, and reevaluations should be performed every 3 months. However, the absence of a response or a resurgence of symptoms requires a new treatment series, considering the observed therapeutic failure when choosing an antifungal drug.
PROPHYLAXIS
The lack of knowledge about the ecological niche of Paracoccidioides spp. hinders the taking of prophylactic measures that prevent infection of the population most exposed to the fungus, as occurs in histoplasmosis. The recommendation not to use vegetable leaves for anal hygiene may be the only measure with some practical value for the population at risk for paracoccidioidal infection. This measure is not related to the local inoculation of Paracoccidioides spp., which is a very remote possibility, but aims to avoid the fixation of fungi that may be in the bloodstream since the sequelae of lesions in these locations can be very serious, especially if the rectum is affected. Finally, laboratory technicians working with Paracoccidioides species cultures should be careful in handling them. In the event of a perforating accident with a risk of infection, the exposed area should be thoroughly washed with soap and water. In addition, the injured individual should be screened for anti-Paracoccidioides species serum antibodies and receive a daily dose of 200 mg itraconazole as a single dose after breakfast for 1 month. If neither clinical manifestations, characterized by lesions at the site of probable inoculation and regional lymph node enlargement, nor positive DID reactions are observed, the medication can be discontinued, but the patient should be clinically and serologically evaluated for the following 2 months. If the clinical manifestations mentioned above remain absent and the serological evaluation continues to be negative, the case should be closed. On the other hand, in the presence of paracoccidioidal lesions or serological DID-positive results, antifungal treatment should be maintained and conducted according to the regimen described above.
ACKNOWLEDGMENTS
We acknowledge financial support granted by the Mato Grosso Research Foundation (FAPEMAT 0505753/2017), the São Paulo Research Foundation (FAPESP 2017/27265-5 and FAPESP 2018/21460-3), the National Council for Scientific and Technological Development (CNPq 429594/2018-6 and CNPq 309917/2020-4), and the Coordination for the Improvement of Higher Education Personnel (CAPES 88887.177846/2018-00). A.M.R. is a CNPq Research Productivity Fellow (CNPq 304902/2020-9). The funders had no role in the study design, data collection, analysis, decision to publish, or manuscript preparation.
Biographies

Rosane Christine Hahn holds an M.Sc. (1998) and a Ph.D. in Biological Sciences (2002) from the Federal University of Minas Gerais (UFMG). She completed a postdoctoral fellowship at the Federal University of São Paulo (UNIFESP) (2018), São Paulo, Brazil. Currently, Dr. Hahn is the head of the Mycology Laboratory of the Faculty of Medicine, Federal University of Mato Grosso, Brazil, and Júlio Muller University Hospital, Federal University of Mato Grosso (UFMT), MT, Brazil. She is currently performing work in the Postgraduate Program Sciences of Health (PPGCS), Faculty of Medicine, UFMT. She was the president of the Brazilian Society of Mycology (2014 to 2019). Her experience includes over 25 years in medical mycology, and her research interests are surveillance studies to characterize the epidemiology, clinical features, and molecular identification of endemic mycoses, emphasizing paracoccidioidomycosis.

Ferry Hagen, Ph.D., F.E.C.M.M., F.E.S.C.M.I.D., is currently the group leader in Medical Mycology at the Westerdijk Fungal Biodiversity Institute in Utrecht, The Netherlands, and is working in the areas of fungal diagnostics, molecular epidemiology, and genomics. After he received his Ph.D. from the University of Utrecht, he was trained as a medical molecular microbiologist and thereafter became a team leader in molecular diagnostics at the Canisius-Wilhelmina Hospital in Nijmegen, The Netherlands. During this period, he further specialized in molecular mycology with a focus on outbreak investigation. In 2017, he joined the Westerdijk Fungal Biodiversity Institute as a group leader to head the Department of Medical Mycology. His research focuses are emerging yeast pathogens and endemic mycoses.

Rinaldo Poncio Mendes, M.D., Ph.D., graduated from the School of Medical and Biological Sciences of Botucatu, now São Paulo State University-UNESP Botucatu, School of Medicine, São Paulo State, Brazil (UNESP-SM), in 1968, with specialization in Tropical Diseases at the Tropical Medicine Institute, School of Medicine, University of São Paulo, Brazil, in 1982. He is Head Professor of Infectious and Parasitic Diseases (UNESP-SM) and Permanent Professor in the Grad Program on Tropical Diseases (UNESP-SM) and the Grad Program on Infectious and Parasitic Diseases, Federal University of Mato Grosso do Sul, UFMS Campo Grande, School of Medicine. His lines of research are systemic mycoses, especially paracoccidioidomycosis, which is hyperendemic in the Botucatu Region. He is the leader of the Research Group on Systemic Mycoses, accredited by the Brazilian National Council for Scientific and Technological Development (CNPq).

Eva Burger holds a bachelor’s degree in Pharmaceutical Sciences, a Ph.D. in Sciences, and an Associate Professor degree from São Paulo University (USP). As an Associate Professor at USP for 35 years and at Alfenas Federal University (UNIFAL) since 2011, she has been the team leader of the Immunopathology of Mycoses research group. She has experience in science administration, having served on ISHAM and research support foundation boards and as dean of research and postgraduation at UNIFAL. Her scientific interest is anti-infectious immunity, mainly protective immunity against pathogenic fungi, especially Paracoccidioides brasiliensis. In this context, she has published more than a hundred papers in peer-reviewed journals and given over 200 presentations at international congresses in the field of Mycology and Immunology.

Andreia Ferreira Nery, M.D., M.Sc., Ph.D., is currently a Professor of Infectious Diseases at the Federal University of Mato Grosso, Department of Internal Medicine, and infectious diseases physician at the Hospital Universitário Júlio Muller, where she coordinates the outpatient clinic of emerging fungal infections. Dr. Nery completed her M.Sc. (2015) and Ph.D. (2020) in Health Sciences with an area of expertise in infectious diseases from the Federal University of Mato Grosso, Brazil. Her current research interests are centered on caring for patients with emerging fungal diseases and promoting improvements in diagnostic and therapeutic approaches, with a view to improving medical care and scientific knowledge offered to people affected by neglected diseases.

Nathan Pereira Siqueira, B.Sc., M.Sc., received an M.Sc. in Health Sciences from the Federal University of Mato Grosso, Brazil. He is currently a Ph.D. student under the guidance of Professor Rosane Christine Hahn. He has experience in clinical analysis teaching professional training at technical and higher levels in the health area. His research interests are fungal infections in humans, including ecoepidemiology, taxonomy, clinic, pathogenesis, diagnosis, and treatment, with an emphasis on paracoccidioidomycosis and cryptococcosis.

Armando Guevara, M.D., M.Sc., Ph.D., received his medical degree from Eastern University, Venezuela; his M.Sc. in Clinical Microbiology from the University of the Andes, Venezuela; and his Ph.D. in Health Sciences from the Federal University of Mato Grosso, Brazil. He is currently head of the Department of Infectious Diseases and Microbiology at the Ruiz and Paez University Hospital in Ciudad Bolívar, Venezuela. His research interests reside in clinical mycology, with emphasis on chromoblastomycosis, paracoccidioidomycosis, molecular epidemiology, and health care-associated infections.

Anderson Messias Rodrigues, B.Sc., M.Sc., Ph.D., is currently a Professor of Medical Mycology in the Department of Microbiology, Immunology, and Parasitology (DMIP), Escola Paulista de Medicina (EPM), Federal University of São Paulo (UNIFESP), and the group leader of the Laboratory of Emerging Fungal Pathogens at EPM-UNIFESP. Dr. Rodrigues completed his M.Sc. (2010) and Ph.D. (2015) in Microbiology and Immunology at the Federal University of São Paulo, Brazil, under the supervision of Professor Zoilo Pires de Camargo. Dr. Rodrigues’ primary areas of interest include endemic mycoses, taxonomy, molecular diagnostics, epidemiology, and the evolution of human-pathogenic fungi. His current research interests are centered around studying emerging infectious fungal diseases and developing and integrating innovative epidemiological and diagnostics approaches, comparative genomics, and host-pathogen models to advance human health.

Zoilo Pires de Camargo, M.Sc., Ph.D., is currently a full Professor of Medical Mycology in the Department of Microbiology, Immunology, and Parasitology (DMIP), Escola Paulista de Medicina (EPM), Federal University of São Paulo (UNIFESP). Dr. de Camargo completed his M.Sc. (1975) and Ph.D. (1978) in Microbiology and Immunology at the Federal University of São Paulo, Brazil. He was subsequently trained in medical mycology and serology at the Institut Pasteur, Paris, France, under the supervision of Professor Edouard Drouhet and thereafter became team leader of medical mycology diagnostics in the 1980s at the EPM-UNIFESP, Brazil. During this period, he has published >230 peer-reviewed papers. His research interests include the classical, serological, and molecular diagnostics of medically relevant fungi and the molecular epidemiology of endemic mycoses in Latin America, focusing on paracoccidioidomycosis investigation.
Contributor Information
Rosane Christine Hahn, Email: rchahn@terra.com.br.
Zoilo Pires de Camargo, Email: zpcamargo1@gmail.com.
REFERENCES
- 1.Lutz A. 2004. Uma micose pseudococcídica localizada na boca e observada no Brasil. Contribuição ao conhecimento das hifoblastomicoses americanas, p 483–494. In Benchimol JL, Sá MR (ed), Adolpho Lutz obra completa, vol 1. Dermatalogia e micologia. FIOCRUZ, Rio de Janeiro, Brazil. [Google Scholar]
- 2.Splendore A. 1912. Zymonematosi con localizzazione nella cavita della bocca, osservata in Brasile. Bull Soc Pathol Exot 5:313–319. [Google Scholar]
- 3.de Almeida F. 1930. Estudos comparativos do granuloma coccidióidico nos Estados Unidos e no Brasil. Novo gênero para o parasito brasileiro. An Fac Med Sao Paulo 5:125–141. [Google Scholar]
- 4.Moore M. 1935. A new species of the Paracoccidioides Almeida. Rev Biol Hig 6:148–162. [Google Scholar]
- 5.Gezuele E. 1989. Aislamiento de Paracoccidioides sp. de heces de pinguino de la Antartida. In Proceedings of the IV International Symposium on Paracoccidioidomycosis. Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela. [Google Scholar]
- 6.da Silva Lacaz C, Porto E, Martins JEC, Heins-Vaccari EM, Takahashi de Melo N. 2002. Tratado de micologia médica, 9th ed. Sarvier, São Paulo, Brazil. [Google Scholar]
- 7.Ajello L. 1971. Paracoccidioidomycosis: a historical review, p 3–10. In Paracoccidioidomycosis. Proceedings of the First Pan American Symposium, Medellin, Colombia. Pan American Health Organization, Washington, DC. [Google Scholar]
- 8.Matute DR, McEwen JG, Puccia R, Montes BA, San-Blas G, Bagagli E, Rauscher JT, Restrepo A, Morais F, Niño-Vega G, Taylor JW. 2006. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol 23:65–73. 10.1093/molbev/msj008. [DOI] [PubMed] [Google Scholar]
- 9.Turissini DA, Gomez OM, Teixeira MM, McEwen JG, Matute DR. 2017. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet Biol 106:9–25. 10.1016/j.fgb.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira MM, Theodoro RC, de Carvalho MJA, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E, San-Blas G, Felipe MSS. 2009. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol 52:273–283. 10.1016/j.ympev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Carrero LL, Niño-Vega G, Teixeira MM, Carvalho MJA, Soares CMA, Pereira M, Jesuino RSA, McEwen JG, Mendoza L, Taylor JW, Felipe MS, San-Blas G. 2008. New Paracoccidioides brasiliensis isolate reveals unexpected genomic variability in this human pathogen. Fungal Genet Biol 45:605–612. 10.1016/j.fgb.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Mavengere H, Mattox K, Teixeira MM, Sepúlveda VE, Gomez OM, Hernandez O, McEwen J, Matute DR. 2020. Paracoccidioides genomes reflect high levels of species divergence and little interspecific gene flow. mBio 11:e01999-20. 10.1128/mBio.01999-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares CM, Madlun EE, da Silva SP, Pereira M, Felipe MS. 1995. Characterization of Paracoccidioides brasiliensis isolates by random amplified polymorphic DNA analysis. J Clin Microbiol 33:505–507. 10.1128/jcm.33.2.505-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molinari-Madlum EE, Felipe MS, Soares CM. 1999. Virulence of Paracoccidioides brasiliensis isolates can be correlated to groups defined by random amplified polymorphic DNA analysis. Med Mycol 37:269–276. 10.1046/j.1365-280X.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 15.Niño-Vega GA, Calcagno AM, San-Blas G, San-Blas F, Gooday GW, Gow NAR. 2000. RFLP analysis reveals marked geographical isolation between strains of Paracoccidioides brasiliensis. Med Mycol 38:437–441. 10.1080/mmy.38.6.437.441. [DOI] [PubMed] [Google Scholar]
- 16.Hahn RC, Macedo AM, Santos NL, Resende JCDP, Hamdan JS. 2002. Characterization of Paracoccidioides brasiliensis atypical isolates by random amplified polymorphic DNA analysis. Rev Iberoam Micol 19:49–51. [PubMed] [Google Scholar]
- 17.Hahn RC, Macedo AM, Fontes CJF, Batista RD, Santos NL, Hamdan JS. 2003. Randomly amplified polymorphic DNA as a valuable tool for epidemiological studies of Paracoccidioides brasiliensis. J Clin Microbiol 41:2849–2854. 10.1128/JCM.41.7.2849-2854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurokawa CS, Lopes CR, Sugizaki MF, Kuramae EE, Franco MF, Peraçoli MTS. 2005. Virulence profile of ten Paracoccidioides brasiliensis isolates: association with morphologic and genetic patterns. Rev Inst Med Trop Sao Paulo 47:257–262. 10.1590/s0036-46652005000500004. [DOI] [PubMed] [Google Scholar]
- 19.Borba CDM, Correia J, Vinhas E, Martins A, Alves BCA, Unkles S, Kinghorn JR, Lucena-Silva N. 2008. Genetic characterization of morphologically variant strains of Paracoccidioides brasiliensis. Mem Inst Oswaldo Cruz 103:306–309. 10.1590/s0074-02762008005000013. [DOI] [PubMed] [Google Scholar]
- 20.Matute DR, Sepulveda VE, Quesada LM, Goldman GH, Taylor JW, Restrepo A, McEwen JG. 2006. Microsatellite analysis of three phylogenetic species of Paracoccidioides brasiliensis. J Clin Microbiol 44:2153–2157. 10.1128/JCM.02540-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira MM, Theodoro RC, Nino-Vega G, Bagagli E, Felipe MSS. 2014. Paracoccidioides species complex: ecology, phylogeny, sexual reproduction, and virulence. PLoS Pathog 10:e1004397. 10.1371/journal.ppat.1004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira MDM, Theodoro RC, de Oliveira FFM, Machado GC, Hahn RC, Bagagli E, San-Blas G, Soares Felipe MS. 2014. Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol 52:19–28. 10.3109/13693786.2013.794311. [DOI] [PubMed] [Google Scholar]
- 23.Gegembauer G, Araujo LM, Pereira EF, Rodrigues AM, Paniago AMM, Hahn RC, de Camargo ZP. 2014. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis 8:e2986. 10.1371/journal.pntd.0002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn RC, Rodrigues AM, Fontes CJF, Nery AF, Tadano T, Queiroz Júnior LDP, de Camargo ZP. 2014. Fatal fungemia due to Paracoccidioides lutzii. Am J Trop Med Hyg 91:394–398. 10.4269/ajtmh.13-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queiroz Júnior LDP, de Camargo ZP, Tadano T, Rodrigues AM, Takarara DT, Gegembauer G, Araujo LM, Hahn RC. 2014. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp. from central western Brazil. Mycoses 57:466–472. 10.1111/myc.12183. [DOI] [PubMed] [Google Scholar]
- 26.Hahn RC, Rodrigues AM, Della Terra PP, Nery AF, Hoffmann-Santos HD, Góis HM, Fontes CJF, de Camargo ZP. 2019. Clinical and epidemiological features of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis 13:e0007437. 10.1371/journal.pntd.0007437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Hoog GS, Haase G, Chaturvedi V, Walsh TJ, Meyer W, Lackner M. 2013. Taxonomy of medically important fungi in the molecular era. Lancet Infect Dis 13:385–386. 10.1016/S1473-3099(13)70058-6. [DOI] [PubMed] [Google Scholar]
- 28.de Hoog GS, Chaturvedi V, Denning DW, Dyer PS, Frisvad JC, Geiser D, Gräser Y, Guarro J, Haase G, Kwon-Chung K-J, Meis JF, Meyer W, Pitt JI, Samson RA, Taylor JW, Tintelnot K, Vitale RG, Walsh TJ, Lackner M, ISHAM Working Group on Nomenclature of Medical Fungi . 2015. Name changes in medically important fungi and their implications for clinical practice. J Clin Microbiol 53:1056–1062. 10.1128/JCM.02016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Macedo PM, Teixeira MDM, Barker BM, Zancopé-Oliveira RM, Almeida-Paes R, Francesconi do Valle AC. 2019. Clinical features and genetic background of the sympatric species Paracoccidioides brasiliensis and Paracoccidioides americana. PLoS Negl Trop Dis 13:e0007309. 10.1371/journal.pntd.0007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.do Amaral CC, Fernandes GF, Rodrigues AM, Burger E, de Camargo ZP. 2019. Proteomic analysis of Paracoccidioides brasiliensis complex isolates: correlation of the levels of differentially expressed proteins with in vivo virulence. PLoS One 14:e0218013. 10.1371/journal.pone.0218013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa PF, Fernandes GF, dos Santos PO, Amaral CC, Camargo ZP. 2010. Characteristics of environmental Paracoccidioides brasiliensis isolate. Mycopathologia 169:37–46. 10.1007/s11046-009-9228-2. [DOI] [PubMed] [Google Scholar]
- 32.Theodoro RC, Teixeira MDM, Felipe MSS, Paduan KDS, Ribolla PM, San-Blas G, Bagagli E. 2012. Genus Paracoccidioides: species recognition and biogeographic aspects. PLoS One 7:e37694. 10.1371/journal.pone.0037694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz JF, Gallo JE, Misas E, Priest M, Imamovic A, Young S, Zeng Q, Clay OK, McEwen JG, Cuomo CA. 2014. Genome update of the dimorphic human pathogenic fungi causing paracoccidioidomycosis. PLoS Negl Trop Dis 8:e3348. 10.1371/journal.pntd.0003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñoz JF, Farrer RA, Desjardins CA, Gallo JE, Sykes S, Sakthikumar S, Misas E, Whiston EA, Bagagli E, Soares CMA, Teixeira MDM, Taylor JW, Clay OK, McEwen JG, Cuomo CA. 2016. Genome diversity, recombination, and virulence across the major lineages of Paracoccidioides. mSphere 1:e00213-16. 10.1128/mSphere.00213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, Soares Felipe MS. 2013. Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol 8:1177–1191. 10.2217/fmb.13.68. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Dukik K, Muñoz JF, Sigler L, Schwartz IS, Govender NP, Kenyon C, Feng P, van den Ende BG, Stielow JB, Stchigel AM, Lu H, de Hoog S. 2018. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers 90:245–291. 10.1007/s13225-018-0403-y. [DOI] [Google Scholar]
- 37.Vilela R, Rosa PS, Belone AFF, Taylor JW, Diório SM, Mendoza L. 2009. Molecular phylogeny of animal pathogen Lacazia loboi inferred from rDNA and DNA coding sequences. Mycol Res 113:851–857. 10.1016/j.mycres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Herr RA, Tarcha EJ, Taborda PR, Taylor JW, Ajello L, Mendoza L. 2001. Phylogenetic analysis of Lacazia loboi places this previously uncharacterized pathogen within the dimorphic Onygenales. J Clin Microbiol 39:309–314. 10.1128/JCM.39.1.309-314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajello L. 1956. Soil as natural reservoir for human pathogenic fungi. Science 123:876–879. 10.1126/science.123.3203.876. [DOI] [PubMed] [Google Scholar]
- 40.Albuquerque P, Nicola AM, Magnabosco DAG, Derengowski LDS, Crisóstomo LS, Xavier LCG, Frazão SDO, Guilhelmelli F, de Oliveira MA, Dias JDN, Hurtado FA, Teixeira MDM, Guimarães AJ, Paes HC, Bagagli E, Felipe MSS, Casadevall A, Silva-Pereira I. 2019. A hidden battle in the dirt: soil amoebae interactions with Paracoccidioides spp. PLoS Negl Trop Dis 13:e0007742. 10.1371/journal.pntd.0007742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagagli E, Theodoro RC, Bosco SMG, McEwen JG. 2008. Paracoccidioides brasiliensis: phylogenetic and ecological aspects. Mycopathologia 165:197–207. 10.1007/s11046-007-9050-7. [DOI] [PubMed] [Google Scholar]
- 42.Restrepo A, Correa I. 1972. Comparison of two culture media for primary isolation of Paracoccidioides brasiliensis from sputum. Sabouraudia 10:260–265. 10.1080/00362177285190491. [DOI] [PubMed] [Google Scholar]
- 43.Fava-Netto C. 1961. Contribuição para o estudo imunológico da blastomicose de Lutz. Rev Inst Adolfo Lutz 21:99–194. [Google Scholar]
- 44.Fava-Netto C, Vegas VS, Sciannaméa IM, Guarnieri DB. 1969. The polysaccharidic antigen from Paracoccidioides brasiliensis. Study of the time of cultivation necessary for the preparation of the antigen. Rev Inst Med Trop Sao Paulo 11:177–181. [PubMed] [Google Scholar]
- 45.Grose E, Tamsitt JR. 1965. Paracoccidioides brasiliensis recovered from the intestinal tract of three bats (Artibeus lituratus) in Colombia, S.A. Med Mycol 4:124–125. 10.1080/00362176685190281. [DOI] [PubMed] [Google Scholar]
- 46.Naiff RD, Ferreira LC, Barrett TV, Naiff MF, Arias JR. 1986. Paracoccidioidomicose enzoótica em tatus (Dasypus novemcinctus) no estado do Pará. Rev Inst Med Trop Sao Paulo 28:19–27. 10.1590/s0036-46651986000100005. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira MS, Freitas LH, Lacaz CDS, Del Negro GMB, de Melo NT, Garcia NM, de Assis CM, Salebian A, Heins-Vaccari EM. 1990. Isolation and characterization of a Paracoccidioides brasiliensis strain from a dogfood probably contaminated with soil in Uberlândia, Brazil. J Med Vet Mycol 28:253–256. 10.1080/02681219080000311. [DOI] [PubMed] [Google Scholar]
- 48.Garcia NM, Del Negro GMB, Heins-Vaccari EM, de Melo NT, de Assis CM, Lacaz CDS. 1993. Paracoccidioides brasiliensis, nova amostra isolada de fezes de um pinguim (Pygoscelis adeliae). Rev Inst Med Trop Sao Paulo 35:227–235. 10.1590/s0036-46651993000300003. [DOI] [PubMed] [Google Scholar]
- 49.Bagagli E, Sano A, Coelho KI, Alquati S, Miyaji M, de Camargo ZP, Gomes GM, Franco M, Montenegro MR. 1998. Isolation of Paracoccidioides brasiliensis from armadillos (Dasypus noveminctus) captured in an endemic area of paracoccidioidomycosis. Am J Trop Med Hyg 58:505–512. 10.4269/ajtmh.1998.58.505. [DOI] [PubMed] [Google Scholar]
- 50.Silva-Vergara ML, Martinez R, Camargo ZP, Malta MHB, Maffei CML, Chadu JB. 2000. Isolation of Paracoccidioides brasiliensis from armadillos (Dasypus novemcinctus) in an area where the fungus was recently isolated from soil. Med Mycol 38:193–199. 10.1080/mmy.38.3.193.199. [DOI] [PubMed] [Google Scholar]
- 51.Silva-Vergara ML, Martinez R, Chadu A, Madeira M, Freitas-Silva G, Leite Maffei CM. 1998. Isolation of a Paracoccidioides brasiliensis strain from the soil of a coffee plantation in Ibiá, State of Minas Gerais, Brazil. Med Mycol 36:37–42. 10.1046/j.1365-280X.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- 52.Corte AC, Gennari SM, Labruna MB, Camargo LMA, Itano EN, Freire RL, Camargo ZP, Ono MA. 2012. Paracoccidioides brasiliensis infection in dogs from western Brazilian Amazon. Pesqi Vet Bras 32:649–652. 10.1590/S0100-736X2012000700011. [DOI] [Google Scholar]
- 53.Arantes TD, Theodoro RC, Teixeira MDM, Bosco SDMG, Bagagli E. 2016. Environmental mapping of Paracoccidioides spp. in Brazil reveals new clues into genetic diversity, biogeography and wild host association. PLoS Negl Trop Dis 10:e0004606. 10.1371/journal.pntd.0004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arantes TD, Theodoro RC, Da Graça Macoris SA, Bagagli E. 2013. Detection of Paracoccidioides spp. in environmental aerosol samples. Med Mycol 51:83–92. 10.3109/13693786.2012.698444. [DOI] [PubMed] [Google Scholar]
- 55.Restrepo A. 1985. The ecology of Paracoccidioides brasiliensis: a puzzle still unsolved. Sabouraudia 23:323–334. 10.1080/00362178585380481. [DOI] [PubMed] [Google Scholar]
- 56.Brummer E, Castaneda E, Restrepo A. 1993. Paracoccidioidomycosis: an update. Clin Microbiol Rev 6:89–117. 10.1128/CMR.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Restrepo A, McEwen JG, Castañeda E. 2001. The habitat of Paracoccidioides brasiliensis: how far from solving the riddle? Med Mycol 39:233–241. 10.1080/mmy.39.3.233.241. [DOI] [PubMed] [Google Scholar]
- 58.Theodoro RC, Candeias JMG, Araújo JP, Jr, Bosco SDMG, Macoris SADG, Padula LOJ, Franco M, Bagagli E. 2005. Molecular detection of Paracoccidioides brasiliensis in soil. Med Mycol 43:725–729. 10.1080/13693780500129418. [DOI] [PubMed] [Google Scholar]
- 59.Conti Días IA. 2007. On the unknown ecological niche of Paracoccidioides brasiliensis: our hypothesis of 1989. Present status and perspectives. Rev Inst Med Trop Sao Paulo 49:131–134. 10.1590/s0036-46652007000200014. [DOI] [PubMed] [Google Scholar]
- 60.Barrozo LV, Benard G, Silva MES, Bagagli E, Marques SA, Mendes RP. 2010. First description of a cluster of acute/subacute paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl Trop Dis 4:e643. 10.1371/journal.pntd.0000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.do Valle ACF, Marques de Macedo P, Almeida-Paes R, Romão AR, Lazéra MDS, Wanke B. 2017. Paracoccidioidomycosis after highway construction, Rio de Janeiro, Brazil. Emerg Infect Dis 23:1917–1919. 10.3201/eid2311.170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teles AJ, Klafke GB, Cabana ÂL, Albano APN, Xavier MO, Meireles MCA. 2016. Serological investigation into Paracoccidioides brasiliensis infection in dogs from southern Rio Grande do Sul, Brazil. Mycopathologia 181:323–328. 10.1007/s11046-015-9972-4. [DOI] [PubMed] [Google Scholar]
- 63.Albano APN, Klafke GB, Brandolt TM, Da Hora VP, Nogueira CEW, Xavier MO, Meireles MCA. 2015. Seroepidemiology of Paracoccidioides brasiliensis infection in horses from Rio Grande do Sul, Brazil. Braz J Microbiol 46:513–517. 10.1590/S1517-838246246220140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Oliveira GG, Belitardo DR, Balarin MRS, Freire RL, de Camargo ZP, Ono MA. 2013. Serological survey of paracoccidioidomycosis in cats. Mycopathologia 176:299–302. 10.1007/s11046-013-9681-9. [DOI] [PubMed] [Google Scholar]
- 65.Corte AC, Svoboda WK, Navarro IT, Freire RL, Malanski LS, Shiozawa MM, Ludwig G, Aguiar LM, Passos FC, Maron A, Camargo ZP, Itano EN, Ono MA. 2007. Paracoccidioidomycosis in wild monkeys from Paraná State, Brazil. Mycopathologia 164:225–228. 10.1007/s11046-007-9059-y. [DOI] [PubMed] [Google Scholar]
- 66.Oliveira GG, Navarro IT, Freire RL, Belitardo DR, Silveira LH, Camargo ZP, Itano EN, Ono MA. 2012. Serological survey of paracoccidioidomycosis in sheep. Mycopathologia 173:63–68. 10.1007/s11046-011-9463-1. [DOI] [PubMed] [Google Scholar]
- 67.Fernandes GF, Deps P, Tomimori-Yamashita J, Camargo ZP. 2004. IgM and IgG antibody response to Paracoccidioides brasiliensis in naturally infected wild armadillos (Dasypus novemcinctus). Med Mycol 42:363–368. 10.1080/13693780310001658748. [DOI] [PubMed] [Google Scholar]
- 68.Corredor GG, Castaño JH, Paralta LA, Díez S, Arango M, McEwen J, Restrepo A. 1999. Isolation of Paracoccidioides brasiliensis from the nine-banded armadillo Dasypus novemcinctus, in an endemic area for paracoccidioidomycosis in Colombia. Rev Iberoam Micol 16:216–220. [PubMed] [Google Scholar]
- 69.World Health Organization. 2019. Neglected tropical diseases. World Health Organization, Geneva, Switzerland. https://www.who.int/neglected_diseases/diseases/en/. [Google Scholar]
- 70.Bellissimo-Rodrigues F, Machado AA, Martinez R. 2011. Paracoccidioidomycosis epidemiological features of a 1,000-cases series from a hyperendemic area on the southeast of Brazil. Am J Trop Med Hyg 85:546–550. 10.4269/ajtmh.2011.11-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coutinho ZF, Wanke B, Travassos C, Oliveira RM, Xavier DR, Coimbra CEA. 2015. Hospital morbidity due to paracoccidioidomycosis in Brazil (1998–2006). Trop Med Int Health 20:673–680. 10.1111/tmi.12472. [DOI] [PubMed] [Google Scholar]
- 72.de Góes AM, da Silva LSS, de Araújo SA, da Cruz SG, Siqueira WC, Pedroso ERP. 2014. Paracoccidioidomycosis disease (Lutz-Splendore-Almeida): etiology, epidemiology, and pathogenesis. Rev Med Minas Gerais 24:61–66. [Google Scholar]
- 73.Roberto TN, de Carvalho JA, Beale MA, Hagen F, Fisher MC, Hahn C, de Camargo ZP, Rodrigues AM. 2021. Exploring genetic diversity, population structure, and phylogeography in Paracoccidioides species using AFLP markers. Stud Mycol 100:100131. 10.1016/j.simyco.2021.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hrycyk MF, Garcia Garces H, Bosco SDMG, de Oliveira SL, Marques SA, Bagagli E. 2018. Ecology of Paracoccidioides brasiliensis, P. lutzii and related species: infection in armadillos, soil occurrence and mycological aspects. Med Mycol 56:950–962. 10.1093/mmy/myx142. [DOI] [PubMed] [Google Scholar]
- 75.Marques-da-Silva SH, Rodrigues AM, de Hoog GS, Silveira-Gomes F, de Camargo ZP. 2012. Occurrence of Paracoccidioides lutzii in the Amazon region: description of two cases. Am J Trop Med Hyg 87:710–714. 10.4269/ajtmh.2012.12-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takayama A, Itano EN, Sano A, Ono MA, Kamei K. 2010. An atypical Paracoccidioides brasiliensis clinical isolate based on multiple gene analysis. Med Mycol 48:64–72. 10.3109/13693780902718065. [DOI] [PubMed] [Google Scholar]
- 77.Mendes JF, Klafke GB, Albano APN, Cabana ÂL, Teles AJ, de Camargo ZP, Xavier MO, Meireles MCA. 2017. Paracoccidioidomycosis infection in domestic and wild mammals by Paracoccidioides lutzii. Mycoses 60:402–406. 10.1111/myc.12608. [DOI] [PubMed] [Google Scholar]
- 78.Shikanai-Yasuda MA, Mendes RP, Colombo AL, de Queiroz-Telles F, Kono ASG, Paniago AMM, Nathan A, do Valle ACF, Bagagli E, Benard G, Ferreira MS, Teixeira MDM, Silva-Vergara ML, Pereira RM, Cavalcante RDS, Hahn R, Durlacher RR, Khoury Z, de Camargo ZP, Moretti ML, Martinez R. 2017. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop 50:715–740. 10.1590/0037-8682-0230-2017. [DOI] [PubMed] [Google Scholar]
- 79.Paniago AMM, Aguiar JIA, Aguiar ES, da Cunha RV, Pereira GRDOL, Londero AT, Wanke B. 2003. Paracoccidioidomicose: estudo clínico e epidemiológico de 422 casos observados no Estado de Mato Grosso do Sul. Rev Soc Bras Med Trop 36:455–459. 10.1590/s0037-86822003000400004. [DOI] [PubMed] [Google Scholar]
- 80.Loth EA, de Castro SV, da Silva JR, Gandra RF. 2011. Occurrence of 102 cases of paracoccidioidomycosis in 18 months in the Itaipu Lake region, western Paraná. Rev Soc Bras Med Trop 44:636–637. 10.1590/s0037-86822011000500023. [DOI] [PubMed] [Google Scholar]
- 81.de Matos WB, dos Santos GMC, da Silva VEB, Gonçalves EDGDR, da Silva AR. 2012. Paracoccidioidomycosis in the state of Maranhão, Brazil: geographical and clinical aspects. Rev Soc Bras Med Trop 45:385–389. 10.1590/S0037-86822012000300020. [DOI] [PubMed] [Google Scholar]
- 82.Vieira GDD, Alves TDC, de Lima SMD, Camargo LMA, de Sousa CM. 2014. Paracoccidioidomycosis in a western Brazilian Amazon state: clinical-epidemiologic profile and spatial distribution of the disease. Rev Soc Bras Med Trop 47:63–68. 10.1590/0037-8682-0225-2013. [DOI] [PubMed] [Google Scholar]
- 83.de Souza SP, Jorge VM, Orzechowski Xavier M. 2014. Paracoccidioidomycosis in southern Rio Grande do Sul: a retrospective study of histopathologically diagnosed cases. Braz J Microbiol 45:243–247. 10.1590/s1517-83822014000100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romaneli MTDN, Tardelli NR, Tresoldi AT, Morcillo AM, Pereira RM. 2019. Acute-subacute paracoccidioidomycosis: a paediatric cohort of 141 patients, exploring clinical characteristics, laboratorial analysis and developing a non-survival predictor. Mycoses 62:999–1005. 10.1111/myc.12984. [DOI] [PubMed] [Google Scholar]
- 85.Coutinho ZF, da Silva D, Lazéra M, Petri V, de Oliveira RM, Sabroza PC, Wanke B. 2002. Paracoccidioidomycosis mortality in Brazil (1980-1995). Cad Saude Publica 18:1441–1454. 10.1590/s0102-311x2002000500037. [DOI] [PubMed] [Google Scholar]
- 86.Calich VLG, Fazioli RA, Teixeira HC, Russo M, Singer-Vermes LM, Burger E, Vaz CAC. 1988. Mechanisms of host-resistance to Paracocidioides brasiliensis, p 154–157. In Torres-Rodrigues JM (ed), Proceedings of the X Congress of the International Society for Human and Animal Mycology. International Society for Human and Animal Mycology, Barcelona, Spain. [Google Scholar]
- 87.Musatti CC, Rezkallah MT, Mendes E, Mendes NF. 1976. In vivo and in vitro evaluation of cell-mediated immunity in patients with paracoccidioidomycosis. Cell Immunol 24:365–378. 10.1016/0008-8749(76)90220-3. [DOI] [PubMed] [Google Scholar]
- 88.Biagioni L, Souza MJ, Chamma LG, Mendes RP, Marques SA, Mota NGS, Franco M. 1984. Serology of paracoccidioidomycosis. II. Correlation between class-specific antibodies and clinical forms of the disease. Trans R Soc Trop Med Hyg 78:617–621. 10.1016/0035-9203(84)90220-7. [DOI] [PubMed] [Google Scholar]
- 89.Shikanai-Yasuda MA, Higaki Y, Uip D, Mori N, Del Negro G, Melo N, Hutzler R, Amato Neto V. 1992. Comprometimento da medula óssea e eosinofilia na paracoccidioidomicose. Rev Inst Med Trop Sao Paulo 34:85–90. 10.1590/S0036-46651992000200002. [DOI] [PubMed] [Google Scholar]
- 90.Benard G, Mendes-Giannini MJS, Juvenale M, Miranda ET, Duarte AJS. 1997. Immunosuppression in paracoccidioidomycosis: T cell hyporesponsiveness to two Paracoccidioides brasiliensis glycoproteins that elicit strong humoral immune response. J Infect Dis 175:1263–1267. 10.1086/593694. [DOI] [PubMed] [Google Scholar]
- 91.Benard G, Romano CC, Cacere CR, Juvenale M, Mendes-Giannini MJS, Duarte AJS. 2001. Imbalance of IL-2, IFN-γ and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine 13:248–252. 10.1006/cyto.2000.0824. [DOI] [PubMed] [Google Scholar]
- 92.Mosmann TR, Coffman RL. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173. 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 93.Franco M, Montenegro MR, Mendes RP, Marques SA, Dillon NL, Mota NGDS. 1987. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev Soc Bras Med Trop 20:129–132. 10.1590/s0037-86821987000200012. [DOI] [PubMed] [Google Scholar]
- 94.Oliveira SJ, Mamoni RL, Musatti CC, Papaiordanou PMO, Blotta MHSL. 2002. Cytokines and lymphocyte proliferation in juvenile and adult forms of paracoccidioidomycosis: comparison with infected and non-infected controls. Microbes Infect 4:139–144. 10.1016/S1286-4579(01)01521-0. [DOI] [PubMed] [Google Scholar]
- 95.Mamoni RL, Blotta MHSL. 2006. Flow-cytometric analysis of cytokine production in human paracoccidioidomycosis. Cytokine 35:207–216. 10.1016/j.cyto.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Calich VLG, Vaz CAC, Burger E. 1998. Immunity to Paracoccidioides brasiliensis infection. Res Immunol 149:407–417. 10.1016/S0923-2494(98)80764-5. [DOI] [PubMed] [Google Scholar]
- 97.Di Gangi R, Alves da Costa T, Thomé R, Peron G, Burger E, Verinaud L. 2016. Paracoccidioides brasiliensis infection promotes thymic disarrangement and premature egress of mature lymphocytes expressing prohibitive TCRs. BMC Infect Dis 16:209. 10.1186/s12879-016-1561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyaji M, Nishimura K. 1983. Granuloma formation and killing functions of granuloma in congenitally athymic nude mice infected with Blastomyces dermatitidis and Paracoccidioides brasiliensis. Mycopathologia 82:129–141. 10.1007/BF00439218. [DOI] [PubMed] [Google Scholar]
- 99.Kerr IB, Mendes da Silva AM, Drouhet E, de Oliveira P, da Costa SCG. 1988. Paracoccidioidomycosis in nude mice: presence of filamentous forms of the fungus. Mycopathologia 101:3–11. 10.1007/BF00455663. [DOI] [PubMed] [Google Scholar]
- 100.de Mattos Grosso D, de Almeida SR, Mariano M, Lopes JD. 2003. Characterization of gp70 and anti-gp70 monoclonal antibodies in Paracoccidioides brasiliensis pathogenesis. Infect Immun 71:6534–6542. 10.1128/IAI.71.11.6534-6542.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burger E, Vaz CC, Sano A, Calich VL, Singer LM, Xidieh CF, Kashino SS, Nishimura K, Miyaji M. 1996. Paracoccidioides brasiliensis infection in nude mice: studies with isolates differing in virulence and definition of their T cell-dependent and T cell-independent components. Am J Trop Med Hyg 55:391–398. 10.4269/ajtmh.1996.55.391. [DOI] [PubMed] [Google Scholar]
- 102.Travassos LR, Taborda CP. 2012. New advances in the development of a vaccine against paracoccidioidomycosis. Front Microbiol 3:212. 10.3389/fmicb.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tristão FSM, Panagio LA, Rocha FA, Cavassani KA, Moreira AP, Rossi MA, Silva JS. 2013. Cell-deficient mice display enhanced susceptibility to Paracoccidioides brasiliensis infection. Mycopathologia 176:1–10. 10.1007/s11046-013-9671-y. [DOI] [PubMed] [Google Scholar]
- 104.Toledo MS, Tagliari L, Suzuki E, Silva CM, Straus AH, Takahashi HK. 2010. Effect of anti-glycosphingolipid monoclonal antibodies in pathogenic fungal growth and differentiation. Characterization of monoclonal antibody MEST-3 directed to Manpα1→3Manpα1→2IPC. BMC Microbiol 10:47. 10.1186/1471-2180-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Batista VG, Toledo MS, Straus AH, Mendes-Giannini MJS, Duarte AJS, Takahashi HK, Benard G. 2014. Glycolipid sensing and innate immunity in paracoccidioidomycosis. Mycopathologia 178:153–162. 10.1007/s11046-014-9783-z. [DOI] [PubMed] [Google Scholar]
- 106.Bueno RA, Thomaz L, Muñoz JE, da Silva CJ, Nosanchuk JD, Pinto MR, Travassos LR, Taborda CP. 2016. Antibodies against glycolipids enhance antifungal activity of macrophages and reduce fungal burden after infection with Paracoccidioides brasiliensis. Front Microbiol 7:74. 10.3389/fmicb.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomaz L, Nosanchuk JD, Rossi DCP, Travassos LR, Taborda CP. 2014. Monoclonal antibodies to heat shock protein 60 induce a protective immune response against experimental Paracoccidioides lutzii. Microbes Infect 16:788–795. 10.1016/j.micinf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Brown GD, Gordon S. 2001. A new receptor for β-glucans. Nature 413:36–37. 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 109.Balderramas HA, Penitenti M, Rodrigues DR, Bachiega TF, Fernandes RK, Ikoma MRV, Dias-Melicio LA, Oliveira SL, Soares ÂMVC. 2014. Human neutrophils produce IL-12, IL-10, PGE2 and LTB4 in response to Paracoccidioides brasiliensis. Involvement of TLR2, mannose receptor and dectin-1. Cytokine 67:36–43. 10.1016/j.cyto.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 110.Feriotti C, Bazan SB, Loures FV, Araújo EF, Costa TA, Calich VLG. 2015. Expression of dectin-1 and enhanced activation of NALP3 inflammasome are associated with resistance to paracoccidioidomycosis. Front Microbiol 6:913. 10.3389/fmicb.2015.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loures FV, Araújo EF, Feriotti C, Bazan SB, Calich VLG. 2015. TLR-4 cooperates with dectin-1 and mannose receptor to expand Th17 and Tc17 cells induced by Paracoccidioides brasiliensis stimulated dendritic cells. Front Microbiol 6:261. 10.3389/fmicb.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bachiega TF, Dias-Melicio LA, Fernandes RK, de Almeida Balderramas H, Rodrigues DR, Ximenes VF, de Campos Soares AMV. 2016. Participation of dectin-1 receptor on NETs release against Paracoccidioides brasiliensis: role on extracellular killing. Immunobiology 221:228–235. 10.1016/j.imbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 113.Romagnolo AG, de Quaglia e Silva JC, Della Coletta AM, Gardizani TP, Martins ATL, Romagnoli GG, Kaneno R, de Campos Soares AMV, De Faveri J, Dias-Melicio LA. 2018. Role of dectin-1 receptor on cytokine production by human monocytes challenged with Paracoccidioides brasiliensis. Mycoses 61:222–230. 10.1111/myc.12725. [DOI] [PubMed] [Google Scholar]
- 114.de Quaglia e Silva JC, Della Coletta AM, Gardizani TP, Romagnoli GG, Kaneno R, Dias-Melicio LA. 2019. Involvement of the dectin-1 receptor upon the effector mechanisms of human phagocytic cells against Paracoccidioides brasiliensis. J Immunol Res 2019:1529189. 10.1155/2019/1529189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 116.Bonfim CV, Mamoni RL, Lima Blotta MHS. 2009. TLR-2, TLR-4 and dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis. Med Mycol 47:722–733. 10.3109/13693780802641425. [DOI] [PubMed] [Google Scholar]
- 117.Acorci-Valério MJ, Bordon-Graciani AP, Dias-Melicio LA, de Assis Golim M, Nakaira-Takahagi E, de Campos Soares AMV. 2010. Role of TLR2 and TLR4 in human neutrophil functions against Paracoccidioides brasiliensis. Scand J Immunol 71:99–108. 10.1111/j.1365-3083.2009.02351.x. [DOI] [PubMed] [Google Scholar]
- 118.Loures FV, Pina A, Felonato M, Calich VLG. 2009. TLR2 negative regulator of TH17 cells and tissue pathology in a pulmonary model of fungal infection. J Immunol 183:1279–1290. 10.4049/jimmunol.0801599. [DOI] [PubMed] [Google Scholar]
- 119.Ferreira KS, Bastos KR, Russo M, Almeida SR. 2007. Interaction between Paracoccidioides brasiliensis and pulmonary dendritic cells induces interleukin-10 production and Toll-like receptor-2 expression: possible mechanisms of susceptibility. J Infect Dis 196:1108–1115. 10.1086/521369. [DOI] [PubMed] [Google Scholar]
- 120.Jannuzzi GP, de Almeida JRF, Amarante-Mendes GP, Romera LMD, Kaihami GH, Vasconcelos JR, Ferreira CP, de Almeida SR, Ferreira KS. 2019. TLR3 is a negative regulator of immune responses against Paracoccidioides brasiliensis. Front Cell Infect Microbiol 8:426. 10.3389/fcimb.2018.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinon F, Burns K, Tschopp J. 2002. The inflammasome. Mol Cell 10:417–426. 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 122.Franchi L, Muñoz-Planillo R, Reimer T, Eigenbrod T, Núñez G. 2010. Inflammasomes as microbial sensors. Eur J Immunol 40:611–615. 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 123.Tavares AH, Magalhães KG, Almeida RDN, Correa R, Burgel PH, Bocca AL. 2013. NLRP3 inflammasome activation by Paracoccidioides brasiliensis. PLoS Negl Trop Dis 7:e2595. 10.1371/journal.pntd.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ketelut-Carneiro N, Silva GK, Rocha FA, Milanezi CM, Cavalcanti-Neto FF, Zamboni DS, Silva JS. 2015. IL-18 triggered by the NLRP3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J Immunol 194:4507–4517. 10.4049/jimmunol.1402321. [DOI] [PubMed] [Google Scholar]
- 125.Romani L. 2011. Immunity to fungal infections. Nat Rev Immunol 11:275–288. 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 126.Pina A, de Araujo EF, Felonato M, Loures FV, Feriotti C, Bernardino S, Barbuto JAM, Calich VLG. 2013. Myeloid dendritic cells (DCs) of mice susceptible to paracoccidioidomycosis suppress T cell responses whereas myeloid and plasmacytoid DCs from resistant mice induce effector and regulatory T cells. Infect Immun 81:1064–1077. 10.1128/IAI.00736-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jannuzzi GP, de Almeida JRF, Dos Santos SS, de Almeida SR, Ferreira KS. 2018. Notch signaling is required for dendritic cell maturation and T cell expansion in paracoccidioidomycosis. Mycopathologia 183:739–749. 10.1007/s11046-018-0276-3. [DOI] [PubMed] [Google Scholar]
- 128.Braga FG, Ruas LP, Pereira RM, Lima XT, Antunes E, Mamoni RL, Blotta MHSL. 2017. Functional and phenotypic evaluation of eosinophils from patients with the acute form of paracoccidioidomycosis. PLoS Negl Trop Dis 11:e0005601. 10.1371/journal.pntd.0005601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chiarella AP, Arruda C, Pina A, Costa TA, Ferreira RCV, Calich VLG. 2007. The relative importance of CD4+ and CD8+ T cells in immunity to pulmonary paracoccidioidomycosis. Microbes Infect 9:1078–1088. 10.1016/j.micinf.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 130.Cano LE, Singer-Vermes LM, Costa TA, Mengel JO, Xidieh CF, Arruda C, Andre DC, Vaz CAC, Burger E, Calich VLG. 2000. Depletion of CD8+ T cells in vivo impairs host defense of mice resistant and susceptible to pulmonary paracoccidioidomycosis. Infect Immun 68:352–359. 10.1128/IAI.68.1.352-359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loures FV, Araújo EF, Feriotti C, Bazan SB, Costa TA, Brown GD, Calich VLG. 2014. Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary paracoccidioidomycosis. J Infect Dis 210:762–773. 10.1093/infdis/jiu136. [DOI] [PubMed] [Google Scholar]
- 132.Jannuzzi GP, Tavares AHFP, Kaihami GH, de Almeida JRF, de Almeida SR, Ferreira KS. 2015. scFv from antibody that mimics gp43 modulates the cellular and humoral immune responses during experimental paracoccidioidomycosis. PLoS One 10:e0129401. 10.1371/journal.pone.0129401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Felonato M, Pina A, de Araujo EF, Loures FV, Bazan SB, Feriotti C, Calich VLG. 2012. Anti-CD25 treatment depletes Treg cells and decreases disease severity in susceptible and resistant mice infected with Paracoccidioides brasiliensis. PLoS One 7:e51071. 10.1371/journal.pone.0051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galdino NAL, Loures FV, de Araújo EF, da Costa TA, Preite NW, Calich VLG. 2018. Depletion of regulatory T cells in ongoing paracoccidioidomycosis rescues protective Th1/Th17 immunity and prevents fatal disease outcome. Sci Rep 8:16544. 10.1038/s41598-018-35037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goihman-Yahr M, Pereira J, Isturiz G, Viloria N, Carrasquero M, Saavedra N, De Gomez MH, Roman A, San Martin B, Bastardo de Albornoz MC, de Fernandez B, Avila-Millan E. 1992. Relationship between digestive and killing abilities of neutrophils against Paracoccidioides brasiliensis. Mycoses 35:269–274. 10.1111/j.1439-0507.1992.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 136.Goihman-Yahr M, Essenfeld-Yahr E, De Albornoz MC, Yarzábal L, De Gómez MH, San Martín B, Ocanto A, Gil F, Convit J. 1980. Defect of in vitro digestive ability of polymorphonuclear leukocytes in paracoccidioidomycosis. Infect Immun 28:557–566. 10.1128/iai.28.2.557-566.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kurita N, Oarada M, Ito E, Miyaji M. 1999. Antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol 37:261–267. 10.1080/j.1365-280X.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- 138.Sperandio FF, Fernandes GP, Mendes ACSC, Bani GMDAC, Calich VLG, Burger E. 2015. Resistance to P. brasiliensis experimental infection of inbred mice is associated with an efficient neutrophil mobilization and activation by mediators of inflammation. Mediators Inflamm 2015:430525. 10.1155/2015/430525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meloni-Bruneri LH, Campa A, Abdalla DSP, Calich VLG, Lenzi HL, Burger E. 1996. Neutrophil oxidative metabolism and killing of P. brasiliensis after air pouch infection of susceptible and resistant mice. J Leukoc Biol 59:526–533. 10.1002/jlb.59.4.526. [DOI] [PubMed] [Google Scholar]
- 140.Xidieh CF, Lenzi HL, Calich VLG, Burger E. 1999. Influence of the genetic background on the pattern of lesions developed by resistant and susceptible mice infected with Paracoccidioides brasiliensis. Med Microbiol Immunol 188:41–49. 10.1007/s004300050103. [DOI] [PubMed] [Google Scholar]
- 141.Kashino SS, Fazioli RDA, Moscardi-Bacchi M, Franco M, Singer-Vermes LM, Burger E, Calich VLG. 1995. Effect of macrophage blockade on the resistance of inbred mice to Paracoccidioides brasiliensis infection. Mycopathologia 130:131–140. 10.1007/BF01103095. [DOI] [PubMed] [Google Scholar]
- 142.Moscardi-Bacchi M, Brummer E, Stevens DA. 1994. Support of Paracoccidioides brasiliensis multiplication by human monocytes or macrophages: inhibition by activated phagocytes. J Med Microbiol 40:159–164. 10.1099/00222615-40-3-159. [DOI] [PubMed] [Google Scholar]
- 143.Calvi SA, Peraçoli MTS, Mendes RP, Marcondes-Machado J, Fecchio D, Marques SA, Soares AMVC. 2003. Effect of cytokines on the in vitro fungicidal activity of monocytes from paracoccidioidomycosis patients. Microbes Infect 5:107–113. 10.1016/S1286-4579(02)00078-3. [DOI] [PubMed] [Google Scholar]
- 144.Carmo JPM, Dias-Melicio LA, Calvi SA, Peraçoli MTS, Soares AMVC. 2006. TNF-α activates human monocytes for Paracoccidioides brasiliensis killing by an H2O2-dependent mechanism. Med Mycol 44:363–368. 10.1080/13693780500536885. [DOI] [PubMed] [Google Scholar]
- 145.Cano LE, Arango R, Salazar ME, Brummer E, Stevens DA, Restrepo A. 1992. Killing of Paracoccidioides brasiliensis conidia by pulmonary macrophages and the effect of cytokines. J Med Vet Mycol 30:161–168. 10.1080/02681219280000211. [DOI] [PubMed] [Google Scholar]
- 146.Rodrigues DR, Dias-Melicio LA, Calvi SA, Peraçoli MTS, Soares AMVC. 2007. Paracoccidioides brasiliensis killing by IFN-γ, TNF-α and GM-CSF activated human neutrophils: role for oxygen metabolites. Med Mycol 45:27–33. 10.1080/13693780600981676. [DOI] [PubMed] [Google Scholar]
- 147.Gonzalez A, Sahaza JH, Ortiz BL, Restrepo A, Cano LE. 2003. Production of pro-inflammatory cytokines during the early stages of experimental Paracoccidioides brasiliensis infection. Med Mycol 41:391–399. 10.1080/13693780310001610038. [DOI] [PubMed] [Google Scholar]
- 148.Moreira AP, Dias-Melicio LA, Peraçoli MTS, Calvi SA, Victoriano de Campos Soares AM. 2008. Killing of Paracoccidioides brasiliensis yeast cells by IFN-γ and TNF-α activated murine peritoneal macrophages: evidence of H2O2 and NO effector mechanisms. Mycopathologia 166:17–23. 10.1007/s11046-007-9046-3. [DOI] [PubMed] [Google Scholar]
- 149.Bannwart CF, Martins RAR, Nakaira-Takahashi É, Dias-Melício LA, Soares ÂMC, Peraçoli MTS. 2010. Interleukin-15 augments oxidative metabolism and fungicidal activity of human monocytes against Paracoccidioides brasiliensis. Mem Inst Oswaldo Cruz 105:866–872. 10.1590/s0074-02762010000700005. [DOI] [PubMed] [Google Scholar]
- 150.Alves ABRM, David MA, de Castro LF, da Silva RM, Longhi LNA, Blotta MHDSL, Mamoni RL. 2018. Differential production of interleukin-1 family cytokines (IL-1β, IL-18, IL-33 and IL-37) in patients with paracoccidioidomycosis: correlation with clinical form and antifungal therapy. Med Mycol 56:332–343. 10.1093/mmy/myx050. [DOI] [PubMed] [Google Scholar]
- 151.Costa TA, Bazan SB, Feriotti C, Araújo EF, Bassi ÊJ, Loures FV, Calich VLG. 2013. In pulmonary paracoccidioidomycosis IL-10 deficiency leads to increased immunity and regressive infection without enhancing tissue pathology. PLoS Negl Trop Dis 7:e2512. 10.1371/journal.pntd.0002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tristão FSM, Rocha FA, Carlos D, Ketelut-Carneiro N, Souza COS, Milanezi CM, Silva JS. 2017. Th17-inducing cytokines IL-6 and IL-23 are crucial for granuloma formation during experimental paracoccidioidomycosis. Front Immunol 8:949. 10.3389/fimmu.2017.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nascimento FRF, Calich VLG, Rodríguez D, Russo M. 2002. Dual role for nitric oxide in paracoccidioidomycosis: essential for resistance, but overproduction associated with susceptibility. J Immunol 168:4593–4600. 10.4049/jimmunol.168.9.4593. [DOI] [PubMed] [Google Scholar]
- 154.Nishikaku AS, Molina RFS, Ribeiro LC, Scavone R, Albe BP, Cunha CS, Burger E. 2009. Nitric oxide participation in granulomatous response induced by Paracoccidioides brasiliensis infection in mice. Med Microbiol Immunol 198:123–135. 10.1007/s00430-009-0113-x. [DOI] [PubMed] [Google Scholar]
- 155.Burger E, Nishikaku AS, Gameiro J, Francelin C, Camargo ZP, Verinaud L. 2013. Cytokines expressed in the granulomatous lesions in experimental paracoccidioidomycosis: role in host protective immunity and as fungal virulence factor. J Clin Cell Immunol S1:010. 10.4172/2155-9899.S1-010. [DOI] [Google Scholar]
- 156.Souto JT, Figueiredo F, Furlanetto A, Pfeffer K, Rossi MA, Silva JS. 2000. Interferon-γ and tumor necrosis factor-α determine resistance to Paracoccidioides brasiliensis infection in mice. Am J Pathol 156:1811–1820. 10.1016/s0002-9440(10)65053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lenzi HL, Calich VL, Miyaji M, Sano A, Nishimura K, Burger E. 1994. Fibrosis patterns of lesions developed by athymic and euthymic mice infected with Paracoccidioides brasiliensis. Braz J Med Biol Res 27:2301–2308. [PubMed] [Google Scholar]
- 158.Lenzi HL, Sano A, Burger E, Calich VLG, Miyaji M, Nishimura K. 1996. Histopathology of paracoccidioidomycotic infection in athymic and euthymic mice: a sequential study. Am J Trop Med Hyg 55:235–242. 10.4269/ajtmh.1996.55.235. [DOI] [PubMed] [Google Scholar]
- 159.Nishikaku AS, Burger E. 2003. Immunohistochemical demonstration of TGF-β and decorin in paracoccidioidal granulomas. Braz J Med Biol Res 36:1073–1078. 10.1590/s0100-879x2003000800014. [DOI] [PubMed] [Google Scholar]
- 160.Nishikaku AS, Molina RFS, Albe BP, Cunha CDS, Scavone R, Pizzo CRP, de Camargo ZP, Burger E. 2011. Immunolocalization of IFN-gamma in the lesions of resistant and susceptible mice to Paracoccidioides brasiliensis infection. FEMS Immunol Med Microbiol 63:281–288. 10.1111/j.1574-695X.2011.00851.x. [DOI] [PubMed] [Google Scholar]
- 161.Nishikaku AS, Scavone R, Molina RFS, Albe BP, Cunha CDS, Burger E. 2009. Osteopontin involvement in granuloma formation and in the severity of Paracoccidioides brasiliensis infection. Med Mycol 47:495–507. 10.1080/13693780802342537. [DOI] [PubMed] [Google Scholar]
- 162.Mendes RP, Cavalcante RDS, Marques SA, Marques MEA, Venturini J, Sylvestre TF, Paniago AMM, Pereira AC, da Silva JDF, Fabro AT, Bosco SDMG, Bagagli E, Hahn RC, Levorato AD. 2017. Paracoccidioidomycosis: current perspectives from Brazil. Open Microbiol J 11:224–282. 10.2174/1874285801711010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Franco MF, Mendes R, Moscardi-Bacchi M, Rezkallah-Iwasso M, Montenegro M. 1989. Paracoccidioidomycosis. Baillieres Clin Trop Med Commun Dis 4:185–220. [Google Scholar]
- 164.Fialho AS. 1946. Localizações pulmonares da “micose de Lutz.” Anatomia patológica e patogenia. Importância de seu estudo na patologia pulmonar. PhD thesis. Faculdade Nacional de Medicina, Universidade do Brasil, Rio de Janeiro, Brazil. [Google Scholar]
- 165.Lima F. 1952. Contribuição ao estudo clínico da blastomicose pulmonar. Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil. [Google Scholar]
- 166.Afonso JE, Nery LE, Romaldini H, Bogossian M, Ribeiro-Ratto O. 1979. Função pulmonar na paracoccidioidomicose (blastomicose sul-americana). Rev Inst Med Trop Sao Paulo 21:269–280. [PubMed] [Google Scholar]
- 167.Morceli J, Mendes RP, Marcondes J, Meira DA. 1982. Estudo das alterações do raio x de tórax de doentes com paracoccidioidomicose, abstr F1. Abstr An Congr Soc Bras Med Trop, Ribeirão Preto, São Paulo, Brazil.
- 168.Marchiori E, de Moraes HP, Muniz MAS, Santos MDO, Capone D. 2000. Paracoccidioidomicose: correlação da tomografia computadorizada de alta resolução com a anatomopatologia. Radiol Bras 33:333–340. [Google Scholar]
- 169.Cabrera LG, Santos AF, Andrade UV, Guedes CIA, Oliveira SM, Chang MR, Mendes RP, Paniago AM. 2017. Spontaneous pneumothorax in paracoccidioidomycosis patients from an endemic area in midwestern Brazil. Mycoses 60:124–128. 10.1111/myc.12571. [DOI] [PubMed] [Google Scholar]
- 170.Severo CB, Bello AGD, de Mattos Oliveira F, Guazzelli LS, Tregnago R, Haas M, Hochhegger B, Severo LC. 2013. Pleural effusion an unusual feature of paracoccidioidomycosis: report of two new cases with a systematic review of the literature. Mycopathologia 175:323–330. 10.1007/s11046-013-9617-4. [DOI] [PubMed] [Google Scholar]
- 171.Padilha-Gonçalves A. 1964. Subclinical adenopathy in Lutz’s mycosis. Minerva Dermatol 39:32.14135782 [Google Scholar]
- 172.Barbosa W, Daher R, de Oliveira AR. 1968. Forma linfático-abdominal da blastomicose sul-americana. Rev Inst Med Trop Sao Paulo 10:16–27. [PubMed] [Google Scholar]
- 173.Boccalandro I, Mello FJ, Albuquerque E. 1960. Jaundice and liver damage in South American blastomycosis. Apropos of 10 cases. Bibliographical review. Rev Paul Med 56:350–366. [PubMed] [Google Scholar]
- 174.Carvalho SA, Cerri GG, Shiroma M, Shikanai-Yasuda MA, Barone AA, Amato Neto V. 1986. Inferior vena cava compression syndrome in paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo 28:61–64. 10.1590/s0036-46651986000100011. [DOI] [PubMed] [Google Scholar]
- 175.Colombo AL, Hadad DJ, Camargo ZP, Mendes RP. 1994. Icterícia obstrutiva como apresentação de paracoccidioidomicose. Arq Bras Pediatr 1:100–110. [Google Scholar]
- 176.Gonçalves A, Pinto A, Mansur Filho J, Silva Filho MC. 1983. Paracoccidioidomicose—formas linfaticoabdominal pseudotumoral e disseminada com abdomen agudo e manifestacoes neurologicas. Arq Bras Med 57:7–10. [Google Scholar]
- 177.Bettarello A, Magaldi C, Amato Netto V. 1972. Síndrome de malabsorção na blastomicose sul americana: relato de um caso. Rev Hosp Clin Fac Med Sao Paulo 27:245–250. [PubMed] [Google Scholar]
- 178.de Andrade DR, Hutzler RU, de Carvalho SA, Rosenthal C, de Carvalho MA, Ferreira JM. 1976. Hypoproteinemia in patients with paracoccidioidomycosis of the digestive tract and the abdominal lymphatic system. Review of autopsy cases and report of a case with protein losing enteropathy. Rev Hosp Clin Fac Med Sao Paulo 31:174–179. [PubMed] [Google Scholar]
- 179.Troncon LEDA, Martinez R, Meneghelli UG, de Oliveira RB, Iazigi N. 1981. Perda intestinal de proteinas na paracoccidioidomicose. Rev Hosp Clin Fac Med Sao Paulo 36:172–178. [PubMed] [Google Scholar]
- 180.Mendes RP, Marcondes J, Souza LR, Barravieira B, Pereira PC. 1986. Estudo clínico do comprometimento linfático na paracoccidioidomicose. Rev Soc Bras Med Trop 19:93. [Google Scholar]
- 181.De Arruda PR, Castro RM. 1965. Lymphography in South American blastomycosis. An Bras Dermatol 40:7–14. [PubMed] [Google Scholar]
- 182.Cerri GG, Del Negro G, Magalhães Júnior A, Amato Neto V, Magalhães A. 1983. Use of ultrasonography and lymphography in the lymphatic form of paracoccidioidomycosis. Rev Hosp Clin Fac Med Sao Paulo 38:160–163. [PubMed] [Google Scholar]
- 183.Simão C. 1975. Lymphatic radiologic changes in South American blastomycosis. Rev Inst Med Trop Sao Paulo 17:242–246. [PubMed] [Google Scholar]
- 184.Bicalho RN, Santo MFDE, de Aguiar MCF, Santos VR. 2001. Oral paracoccidioidomycosis: a retrospective study of 62 Brazilian patients. Oral Dis 7:56–60. 10.1034/j.1601-0825.2001.70111.x. [DOI] [PubMed] [Google Scholar]
- 185.Fonseca JB. 1963. Blastomicose sul-americana. Estudo das lesões dentais e paradentais sob o ponto de vista clínico e histopatológico. Rev Bras Fac Odontol Sao Paulo 1:1–31. [Google Scholar]
- 186.Lauand F, Lia R, Paino M. 1975. Blastomicose sul-americana. Estudo clínico das lesões bucais. Rev Fac Farm Odontol Araraquara 9:243–251. [Google Scholar]
- 187.Weber SAT, Brasolotto A, Rodrigues L, Marcondes-Machado J, Padovani CR, Carvalho LR, Mendes RP. 2006. Dysphonia and laryngeal sequelae in paracoccidioidomycosis patients: a morphological and phoniatric study. Med Mycol 44:219–225. 10.1080/13693780500340320. [DOI] [PubMed] [Google Scholar]
- 188.Marques SA. 2012. Paracoccidioidomycosis. Clin Dermatol 30:610–615. 10.1016/j.clindermatol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 189.Marques SA, Cortez DB, Lastória JC, de Camargo RMP, Marques MEA. 2007. Paracoccidioidomicose: freqüência, morfologia e patogênese de lesões tegumentares. An Bras Dermatol 82:411–417. 10.1590/S0365-05962007000500003. [DOI] [Google Scholar]
- 190.Del Negro G, Melo EHL, Rodbard D, Melo MR, Layton J, Wachslicht-Rodbard H. 1980. Limited adrenal reserve in paracoccidioidomycosis: cortisol and aldosterone responses to 1-24 ACTH. Clin Endocrinol (Oxf) 13:553–559. 10.1111/j.1365-2265.1980.tb03423.x. [DOI] [PubMed] [Google Scholar]
- 191.Del Negro G, Wajchenberg B, Pereira V, Shnaider J, Cintra A, De Assis L, Sampaio S. 1961. Addison’s disease associated with South American blastomycosis. Ann Intern Med 54:189–197. [Google Scholar]
- 192.do Valle ACF, Guimaraes MRC, Cuba J, Wanke B, Tendrich M. 1993. Recovery of adrenal function after treatment of paracoccidioidomycosis. Am J Trop Med Hyg 48:626–629. 10.4269/ajtmh.1993.48.626. [DOI] [PubMed] [Google Scholar]
- 193.Tendrich M, De Luca V, Tourinho EK, Wanke B, Cuba J, Buescu A, Vaisman M, Pereira AA, El-Andere W, Wajchenberg BL. 1991. Computed tomography and ultrasonography of the adrenal glands in paracoccidioidomycosis. Comparison with cortisol and aldosterone responses to ACTH stimulation. Am J Trop Med Hyg 44:83–92. 10.4269/ajtmh.1991.44.83. [DOI] [PubMed] [Google Scholar]
- 194.Morceli J, Mendes RP, Mendes EF, Maia D. 1983. Estudo do tubo digestivo de doentes com paracoccidioidomicose. Influência do tratamento sobre as alterações encontradas, p 25. Abstr An Congr Soc Bras Med Trop, Rio de Janeiro, Brazil.
- 195.Laudanna A, Bettarello A, Van Bellen B, Kieffer J. 1975. South American blastomycosis as a cause of malabsorption and protein-losing enteropathy. Arch Gastroenterol 12:195–198. [Google Scholar]
- 196.Raphael A, Campana AO, Waiman J. 1964. Hepatic coma in South America blastomycosis. Rev Assoc Med Bras 10:151–154. [PubMed] [Google Scholar]
- 197.de Brito T, Castro RM, Shiroma M. 1968. Liver biopsy in South American blastomycosis. Rev Inst Med Trop Sao Paulo 10:188–191. [PubMed] [Google Scholar]
- 198.Mendes RP, Oliveira BGV, Kiy Y, Marcondes-Machado J, Pereira PCM. 1990. Cintilografia hepato-biliar e hepato-esplênica em doentes com paracoccidioidomicose, p 284. Abstr Proc Congr Soc Bras Med Trop, Natal, Brazil.
- 199.Forattini O. 1947. Blastomicose da região pancreática. Rev Paul Med 31:165–172. [Google Scholar]
- 200.Forattini OP. 1946. Um caso de blastomicose com localização pancreática. Rev Med 30:515–520. [Google Scholar]
- 201.Cerutti P. 1964. Mario Truffi, 1872-1963. Minerva Dermatol 39:32–33. [PubMed] [Google Scholar]
- 202.Nanni L, Pereira ICMR. 1988. Aspectos radiográficos da paracoccidioidomicose óssea. Radiol Bras 21:101–106. [Google Scholar]
- 203.Paixao U, Guimaraes NA. 1964. Lesōes ósseas de blastomicose Sul-Americana. Revisão do assunto e apresentação de um caso. Hospital (Rio J) 65:1253–1266. [PubMed] [Google Scholar]
- 204.Resende LS, Mendes RP, Bacchi MM, Marques SA, Barraviera B, Souza LR, Meira DA, Niéro-Melo L. 2009. Bone marrow necrosis related to paracoccidioidomycosis: the first eight cases identified at autopsy. Histopathology 54:486–489. 10.1111/j.1365-2559.2009.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Resende L, Mendes RP, Bacchi M, Marques SA, Barraviera B, Souza L, Meira D, Niéro-Melo L. 2006. Infiltrative myelopathy by paracoccidioidomycosis. A review and report of nine cases with emphasis on bone marrow morphology. Histopathology 48:377–386. 10.1111/j.1365-2559.2006.02354.x. [DOI] [PubMed] [Google Scholar]
- 206.Moriguchi SM, Marcondes-Machado J, Viterbo BG, Souza LR, Meira DA, Barraviera B, Pereira PCM, Mendes RPM. 2001. Avaliação cintilográfica do esqueleto com MDP-99MTc em pacientes com paracoccidioidomicose (PBM). Rev Soc Bras Med Trop 34(Suppl 1):455–456. [Google Scholar]
- 207.Magalhães AC, Bacheschi LA, Caramelli P, Lo LS, de Menezes Neto JR, Shikanai-Yasuda MA, Magalhães A. 1993. Paracoccidioidomycosis of the central nervous system: study of 5 cases by magnetic resonance. Rev Hosp Clin Fac Med Sao Paulo 48:94–97. [PubMed] [Google Scholar]
- 208.Mendes RP, Ueda AK, Agapejev S, Oliveira SR, Marcondes J, Pereira PC. 1985. Paracoccidioidomicose do sistema nervoso central. Apresentação de seis casos, p 148. Abstr An Congr Soc Bras Med Trop, São Paulo, Brazil.
- 209.Minguetti G. 1983. Computerized tomography of blastomycotic granuloma of the brain. Rev Inst Med Trop Sao Paulo 25:99–107. [PubMed] [Google Scholar]
- 210.Nobrega JPS, Spina-Franca Netto A. 1994. Neuroparacoccidioidomycosis, p 321–330. In Franco M, Lacaz CDS, Restrepo-Moreno A, Del Nigro G (ed), Paracoccidioidomycosis, 1st ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 211.Raphael A. 1966. Localização nervosa da blastomicose sul-americana. Arq Neuropsiquiatr 24:69–90. 10.1590/S0004-282X1966000200001. [DOI] [Google Scholar]
- 212.Rocha FC, Souza LR, Coelho KI, Mendes RP. 1998. Spinal cord involvement by Paracoccidioides brasiliensis. Rev Soc Bras Med Trop 31:102–103. [Google Scholar]
- 213.Arruda WO, Canto MAS, Loddo G, Rebuffi VF, Cardoso MDA. 1986. Paracoccidioidomicose ocular: relato de um caso com coriorretinite posterior. Rev Inst Med Trop Sao Paulo 28:190–193. 10.1590/s0036-46651986000300010. [DOI] [PubMed] [Google Scholar]
- 214.Silva MRBDM, Mendes RP, Lastória JC, Barraviera B, Marques SA, Kamegasawa A. 1988. Paracoccidioidomycosis: study of six cases with ocular involvement. Mycopathologia 102:87–96. 10.1007/BF00437445. [DOI] [PubMed] [Google Scholar]
- 215.Terra FS. 1923. Três casos de blastomicose. Bras Med 32:41–44. [Google Scholar]
- 216.Fava Netto C, Del Negro G. 1954. Localização testículo-epididimaria da blastomicose Sul-Americana. Rev Assoc Med Bras 1:210–213. [PubMed] [Google Scholar]
- 217.Londero AT, Fabricio R. 1966. Genital localization of South American blastomycosis. A new case. Mycopathol Mycol Appl 30:253–256. 10.1007/BF02053155. [DOI] [PubMed] [Google Scholar]
- 218.Cechella MS, Melo CR, Melo IS, Londero AT, Barreto SM, Gaiger AM. 1982. Paracoccidioidomicose genital masculina. Rev Inst Med Trop Sao Paulo 24:240–245. [PubMed] [Google Scholar]
- 219.Mendes RP, Coelho KI, Marcondes-Machado J, Oliveira EC, Menezes I, Morceli J. 2000. Paracoccidioidomycosis with adrenal and urogenital involvement. Rev Soc Bras Med Trop 33(Suppl 1):458–459. [Google Scholar]
- 220.Severo LC, Kauer CL, Oliveira FDM, Rigatti RA, Hartmann AA, Londero AT. 2000. Paracoccidioidomycosis of the male genital tract. Report of eleven cases and a review of Brazilian literature. Rev Inst Med Trop Sao Paulo 42:38–40. 10.1590/s0036-46652000000100006. [DOI] [PubMed] [Google Scholar]
- 221.Marques SA, Tangoda LK, de Camargo RMP, Stolf HO, Marques MEA. 2012. Paracoccidioidomycosis of external genitalia: report of six new cases and review of the literature. An Bras Dermatol 87:235–240. 10.1590/s0365-05962012000200007. [DOI] [PubMed] [Google Scholar]
- 222.Nohmi N, Gazinelli Sobrinho R. 1981. Paracoccidioidomicose de localização mamária. Rev Assoc Med Minas Gerais 32:41–42. [Google Scholar]
- 223.Mendes RP, Pereira PC, Ueda AK, Franco M. 1994. Breast involvement in paracoccidioidomycosis (PBM). Rev Soc Bras Med Trop 21:20. [Google Scholar]
- 224.Blotta MHSL, Altemani AM, Amaral E, Silva LJ, Camargo ZP. 1993. Placental involvement in paracoccidioidomycosis. J Med Vet Mycol 31:249–257. 10.1080/02681219380000301. [DOI] [PubMed] [Google Scholar]
- 225.Braga LFC, Kasting G, Franke HJ. 1989. Placentite intervilosa paracoccidioidomicótica. Rev Bras Ginecol Obstet 11:117–120. [Google Scholar]
- 226.Kiy Y, Machado JM, Mendes RP, Barraviera B, Pereira PCM, Cury PR. 1988. Paracoccidioidomycosis in the region of Botucatu (state of São Paulo, Brazil). Evaluation of serum thyroxine (T4) and triiodothyronine (T3) levels and of the response to thyrotropin releasing hormone (TRH). Mycopathologia 103:3–9. 10.1007/BF00437215. [DOI] [PubMed] [Google Scholar]
- 227.Del Negro G, Lacaz CDS, Fiorillo AM. 1982. Paracoccidioidomicose (blastomicose sul-americana), p 229–243. Sarvier, EDUSP, São Paulo, Brazil. [Google Scholar]
- 228.Mendes RP, Morceli J, Marques M, Santos AG. 1996. Infiltration of pancreas in paracoccidioidomycosis (PBM) simulating carcinoma. Rev Soc Bras Med Trop 29:115. [Google Scholar]
- 229.Londero AT, Melo IS. 1983. Paracoccidioidomycosis in childhood. A critical review. Mycopathologia 82:49–55. 10.1007/BF00436946. [DOI] [PubMed] [Google Scholar]
- 230.Londero AT, Rios-Gonçalves AJ, Florim Terra GM, Nogueira SA. 1996. Paracoccidioidomycosis in Brazilian children. A critical review (1911-1994). Arq Bras Med 70:197–204. [Google Scholar]
- 231.López R, Restrepo A. 1983. Spontaneous regression of pulmonary paracoccidioidomycosis. Report of a case. Mycopathologia 83:187–189. 10.1007/BF00437027. [DOI] [PubMed] [Google Scholar]
- 232.Benard G, Duarte AJ. 2000. Paracoccidioidomycosis: a model for evaluation of the effects of human immunodeficiency virus infection on the natural history of endemic tropical diseases. Clin Infect Dis 31:1032–1039. 10.1086/318146. [DOI] [PubMed] [Google Scholar]
- 233.Severo L, Geyer G, Londero A, Porto N, Rizzon C. 1979. The primary pulmonary lymph node complex in paracoccidioidomycosis. Mycopathologia 67:115–118. 10.1007/BF00440683. [DOI] [PubMed] [Google Scholar]
- 234.de Camargo ZP, Unterkircher C, Campoy SP, Travassos LR. 1988. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J Clin Microbiol 26:2147–2151. 10.1128/jcm.26.10.2147-2151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Restrepo A, Trujillo M, Gomez I. 1989. Inapparent lung involvement in patients with the subacute juvenile type of paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo 31:18–22. 10.1590/s0036-46651989000100004. [DOI] [PubMed] [Google Scholar]
- 236.Mendes RP, Souza LR, Marcondes-Machado J, Meira DA, Barraviera B, Pereira PC. 2001. Evaluation of the acute or subacute form of paracoccidioidomycosis (PBM). Rev Soc Bras Med Trop 34:75–76. [Google Scholar]
- 237.Lemle A, Wanke B, Mandel MB. 1983. Pulmonary localization of paracoccidioidomycosis: lung function studies before and after treatment. Rev Inst Med Trop Sao Paulo 25:73–78. [PubMed] [Google Scholar]
- 238.Tobon AM, Agudelo CA, Osorio ML, Alvarez DL, Arango M, Cano LE, Restrepo A. 2003. Residual pulmonary abnormalities in adult patients with chronic paracoccidioidomycosis: prolonged follow-up after itraconazole therapy. Clin Infect Dis 37:898–904. 10.1086/377538. [DOI] [PubMed] [Google Scholar]
- 239.do Valle ACF, Aprigliano Filho F, Moreira JS, Wanke B. 1995. Clinical and endoscopic findings in the mucosae of the upper respiratory and digestive tracts in post-treatment follow-up of paracoccidioidomycosis patients. Rev Inst Med Trop Sao Paulo 37:407–413. 10.1590/s0036-46651995000500005. [DOI] [PubMed] [Google Scholar]
- 240.Pissurno NSCA, Esteves LDM, Benedito JM, Giglio VP, de Carvalho LR, Mendes RP, Paniago AMM. 2020. Impact of laryngeal sequelae on voice- and swallowing-related outcomes in paracoccidioidomycosis. J Venom Anim Toxins Incl Trop Dis 26:e20200008. 10.1590/1678-9199-JVATITD-2020-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Abad A, Velez P, Gomez I, Restrepo A. 1986. Adrenal function in paracoccidioidomycosis: a prospective study in patients before and after ketoconazole therapy. Infection 14:22–26. 10.1007/BF01644805. [DOI] [PubMed] [Google Scholar]
- 242.Osa SR, Peterson RE, Roberts RB. 1981. Recovery of adrenal reserve following treatment of disseminated South American blastomycosis. Am J Med 71:298–301. 10.1016/0002-9343(81)90131-5. [DOI] [PubMed] [Google Scholar]
- 243.Meyer TN. 1982. Obstrução duodenal por blastomicose sul-americana. AMB Rev Assoc Med Bras 28:73–74. [PubMed] [Google Scholar]
- 244.Pedroso VSP, Lyon AC, Araújo SA, Veloso JMR, Pedroso ERP, Teixeira AL. 2012. Paracoccidioidomycosis case series with and without central nervous system involvement. Rev Soc Bras Med Trop 45:586–590. 10.1590/s0037-86822012000500009. [DOI] [PubMed] [Google Scholar]
- 245.Nery AF, de Camargo ZP, Rodrigues AM, Portela TF, Hoffmann-Santos HD, Pinheiro BG, Possa AP, Cavalcante LRDS, Hagen F, Hahn RC. 2021. Puzzling paracoccidioidomycosis: factors associated with the severity of Paracoccidioides lutzii infections. Int J Infect Dis 107:284–290. 10.1016/j.ijid.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 246.Pereira EF, Gegembauer G, Chang MR, de Camargo ZP, Nunes TF, Ribeiro SM, de Carvalho LR, Maldonado BM, Mendes RP, Paniago AM. 2020. Comparison of clinico-epidemiological and radiological features in paracoccidioidomycosis patients regarding serological classification using antigens from Paracoccidioides brasiliensis complex and Paracoccidioides lutzii. PLoS Negl Trop Dis 14:e0008485. 10.1371/journal.pntd.0008485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.de Camargo ZP, Rodrigues AM. 2020. Paracoccidioides complex, p 125–134. In Cordeiro RDA (ed), Pocket guide to mycological diagnosis, 1st ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 248.Lopes OS. 1955. Descrição de uma técnica de concentração para pesquisa do Paracoccidioides brasiliensis no escarro. Hospital (Rio J) 47:69–79. [Google Scholar]
- 249.Moreto TC, Marques MEA, de Oliveira MLSC, Moris DV, de Carvalho LR, Mendes RP. 2011. Accuracy of routine diagnostic tests used in paracoccidioidomycosis patients at a university hospital. Trans R Soc Trop Med Hyg 105:473–478. 10.1016/j.trstmh.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 250.Iwama de Mattos MCF, Mendes RP, Marcondes-Machado J, Meira DA, Morceli J, Pereira PCM, Barraviera B. 1991. Sputum cytology in the diagnosis of pulmonary paracoccidioidomycosis. Mycopathologia 114:187–191. 10.1007/BF00437213. [DOI] [PubMed] [Google Scholar]
- 251.Iwama de Mattos MCF. 1979. Cell-block preparation for cytodiagnosis of pulmonary paracoccidioidomycosis. Chest 75:212. 10.1378/chest.75.2.212. [DOI] [PubMed] [Google Scholar]
- 252.Londero AT, Severo LC, Ramos CD. 1980. Small forms and hyphae of Paracoccidioides brasiliensis in human tissue. Mycopathologia 72:17–19. 10.1007/BF00443046. [DOI] [PubMed] [Google Scholar]
- 253.Calvi SA, Soares AMVC, Peraçoli MTS, Franco M, Ruiz RL, Jr, Marcondes-Machado J, Fecchio D, Mattos MCI, Mendes RP. 2003. Study of bronchoalveolar lavage fluid in paracoccidioidomycosis: cytopathology and alveolar macrophage function in response to gamma interferon; comparison with blood monocytes. Microbes Infect 5:1373–1379. 10.1016/j.micinf.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 254.da Silva SHM, Colombo AL, Blotta MHSL, Queiroz-Telles F, Lopes JD, de Camargo ZP. 2005. Diagnosis of neuroparacoccidioidomycosis by detection of circulating antigen and antibody in cerebrospinal fluid. J Clin Microbiol 43:4680–4683. 10.1128/JCM.43.9.4680-4683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.de Camargo ZP, de Franco MF. 2000. Current knowledge on pathogenesis and immunodiagnosis of paracoccidioidomycosis. Rev Iberoam Micol 17:41–48. [PubMed] [Google Scholar]
- 256.Restrepo A, Benard G, de Castro CC, Agudelo CA, Tobón AM. 2008. Pulmonary paracoccidioidomycosis. Semin Respir Crit Care Med 29:182–197. 10.1055/s-2008-1063857. [DOI] [PubMed] [Google Scholar]
- 257.Bethlem EP, Capone D, Maranhao B, Carvalho CRR, Wanke B. 1999. Paracoccidioidomycosis. Curr Opin Pulm Med 5:319–325. 10.1097/00063198-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 258.Puccia R, Schenkman S, Gorin P, Travassos LR. 1986. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect Immun 53:199–206. 10.1128/iai.53.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Niño-Vega GA, Camacho E, Moreno ÁR, Tobón AM, Gómez BL, Teixeira MM, Barker BM. 2017. Paracoccidioides spp. and paracoccidioidomycosis, p 281–308. In Mora-Montes HM, Lopes-Bezerra LM (ed), Current progress in medical mycology. Springer, Cham, Switzerland. [Google Scholar]
- 260.Restrepo A. 1992. Report of activities of the Committee on Paracoccidioidomycosis Serodiagnosis. ISHAM Mycoses Newsl 59:4. [Google Scholar]
- 261.Taborda CP, Camargo ZP. 1994. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J Clin Microbiol 32:554–556. 10.1128/jcm.32.2.554-556.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Camargo ZP, Berzaghi R, Amaral CC, Silva SHM. 2003. Simplified method for producing Paracoccidioides brasiliensis exoantigens for use in immunodiffusion tests. Med Mycol 41:539–542. 10.1080/13693780310001615358. [DOI] [PubMed] [Google Scholar]
- 263.Machado GC, Moris DV, Arantes TD, Silva LRF, Theodoro RC, Mendes RP, Vicentini AP, Bagagli E. 2013. Cryptic species of Paracoccidioides brasiliensis: impact on paracoccidioidomycosis immunodiagnosis. Mem Inst Oswaldo Cruz 108:637–643. 10.1590/0074-0276108052013016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.de Camargo ZP, Guesdon J, Drouhet E, Improvisi L. 1984. Enzyme-linked immunosorbent assay (ELISA) in the paracoccidioidomycosis. Comparison with counterimmunoelectrophoresis and erythro-immunoassay. Mycopathologia 88:31–37. 10.1007/BF00439292. [DOI] [PubMed] [Google Scholar]
- 265.Albuquerque CF, da Silva SM, Camargo ZP. 2005. Improvement of the specificity of an enzyme-linked immunosorbent assay for diagnosis of paracoccidioidomycosis. J Clin Microbiol 43:1944–1946. 10.1128/JCM.43.4.1944-1946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.de Camargo ZP, Guesdon JL, Drouhet E, Improvisi L. 1984. Magnetic enzyme-linked immunosorbent assay (MELISA) for determination of specific IgG in paracoccidioidomycosis. Med Mycol 22:291–299. 10.1080/00362178485380491. [DOI] [PubMed] [Google Scholar]
- 267.Kamikawa CM, Mendes RP, Vicentini AP. 2017. Standardization and validation of dot-ELISA assay for Paracoccidioides brasiliensis antibody detection. J Venom Anim Toxins Incl Trop Dis 23:11. 10.1186/s40409-017-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Guesdon JL, Chevrier D, Fadel R, Avrameas S. 1988. Immunoenzyme assay for histamine. Allerg Immunol (Paris) 20:336–338, 340–342. [PubMed] [Google Scholar]
- 269.Muñoz C, Nieto A, Gayá A, Martínez J, Vives J. 1986. New experimental criteria for optimization of solid-phase antigen concentration and stability in ELISA. J Immunol Methods 94:137–144. 10.1016/0022-1759(86)90226-7. [DOI] [PubMed] [Google Scholar]
- 270.de Camargo ZP, Unterkircher C, Travassos LR. 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J Med Vet Mycol 27:407–412. 10.1080/02681218980000531. [DOI] [PubMed] [Google Scholar]
- 271.de Camargo ZP, Taborda CP, Rodrigues EG, Travassos LR. 1991. The use of cell-free antigens of Paracoccidioides brasiliensis in serological tests. J Med Vet Mycol 29:31–38. 10.1080/02681219180000061. [DOI] [PubMed] [Google Scholar]
- 272.dos Santos PO, Rodrigues AM, Fernandes GF, da Silva SHM, Burger E, de Camargo ZP. 2015. Immunodiagnosis of paracoccidioidomycosis due to Paracoccidioides brasiliensis using a latex test: detection of specific antibody anti-gp43 and specific antigen gp43. PLoS Negl Trop Dis 9:e0003516. 10.1371/journal.pntd.0003516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Silveira-Gomes F, Sarmento DN, Pinto TM, Pimentel RF, Nepomuceno LB, Espírito Santo EPT, Mesquita-da-Costa M, Camargo ZP, Marques-da-Silva SH. 2011. Development and evaluation of a latex agglutination test for the serodiagnosis of paracoccidioidomycosis. Clin Vaccine Immunol 18:604–608. 10.1128/CVI.00130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Batista J, Jr, de Camargo ZP, Fernandes GF, Vicentini AP, Fontes CJF, Hahn RC. 2010. Is the geographical origin of a Paracoccidioides brasiliensis isolate important for antigen production for regional diagnosis of paracoccidioidomycosis? Mycoses 53:176–180. 10.1111/j.1439-0507.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 275.Guimarães AJ, Nosanchuk JD, Zancopé-Oliveira RM. 2006. Diagnosis of histoplasmosis. Braz J Microbiol 37:1–13. 10.1590/S1517-83822006000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.McHardy IH, Dinh B-TN, Waldman S, Stewart E, Bays D, Pappagianis D, Thompson GR, III.. 2018. Coccidioidomycosis complement fixation titer trends in the age of antifungals. J Clin Microbiol 56:e01318-18. 10.1128/JCM.01318-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Saubolle MA. 2007. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann N Y Acad Sci 1111:301–314. 10.1196/annals.1406.049. [DOI] [PubMed] [Google Scholar]
- 278.Haydour Q, Hage CA, Carmona EM, Epelbaum O, Evans SE, Gabe LM, Knox KS, Kolls JK, Wengenack NL, Prokop LJ, Limper AH, Murad MH. 2019. Diagnosis of fungal infections. A systematic review and meta-analysis supporting American Thoracic Society practice guideline. Ann Am Thorac Soc 16:1179–1188. 10.1513/AnnalsATS.201811-766OC. [DOI] [PubMed] [Google Scholar]
- 279.Alvarado P, Pérez-Rojas Y, Zambrano EA, Gonzatti MI, Roschman-González A. 2020. Improved serodiagnosis of histoplasmosis by use of deglycosylated extracellular released antigens of Histoplasma capsulatum. J Microbiol Methods 175:105981. 10.1016/j.mimet.2020.105981. [DOI] [PubMed] [Google Scholar]
- 280.Del Negro GMB, Pereira CN, Andrade HF, Palacios SA, Vidal MMS, Charbel CE, Benard G. 2000. Evaluation of tests for antibody response in the follow-up of patients with acute and chronic forms of paracoccidioidomycosis. J Med Microbiol 49:37–46. 10.1099/0022-1317-49-1-37. [DOI] [PubMed] [Google Scholar]
- 281.Martins R, Marques S, Alves M, Fecchio D, de Franco MF. 1997. Serological follow-up of patients with paracoccidioidomycosis treated with itraconazole using dot-blot, ELISA and Western-blot. Rev Inst Med Trop Sao Paulo 39:261–270. 10.1590/s0036-46651997000500004. [DOI] [PubMed] [Google Scholar]
- 282.Marques da Silva SH, de Mattos Grosso D, Lopes JD, Colombo AL, Blotta MHSL, Queiroz-Telles F, Pires de Camargo Z. 2004. Detection of Paracoccidioides brasiliensis gp70 circulating antigen and follow-up of patients undergoing antimycotic therapy. J Clin Microbiol 42:4480–4486. 10.1128/JCM.42.10.4480-4486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pinheiro BG, Hahn RC, de Camargo ZP, Rodrigues AM. 2020. Molecular tools for detection and identification of Paracoccidioides species: current status and future perspectives. J Fungi (Basel) 6:293. 10.3390/jof6040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pinheiro BG, Pôssa AP, Della Terra PP, de Carvalho JA, Ricci G, Nishikaku AS, Hahn RC, de Camargo ZP, Rodrigues AM. 2021. A new duplex PCR-assay for the detection and identification of Paracoccidioides species. J Fungi (Basel) 7:169. 10.3390/jof7030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rodrigues AM, Kubitschek-Barreira PH, Pinheiro BG, Teixeira-Ferreira A, Hahn RC, de Camargo ZP. 2020. Immunoproteomic analysis reveals novel candidate antigens for the diagnosis of paracoccidioidomycosis due to Paracoccidioides lutzii. J Fungi (Basel) 6:357. 10.3390/jof6040357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Moreira ALE, Oliveira MAP, Silva LOS, Inácio MM, Bailão AM, Parente-Rocha JA, Cruz-Leite VRM, Paccez JD, de Almeida Soares CM, Weber SS, Borges CL. 2020. Immunoproteomic approach of extracellular antigens from Paracoccidioides species reveals exclusive B-cell epitopes. Front Microbiol 10:2968. 10.3389/fmicb.2019.02968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cisalpino PS, Puccia R, Yamauchi LM, Cano MIN, da Silveira JF, Travassos LR. 1996. Cloning, characterization, and epitope expression of the major diagnostic antigen of Paracoccidioides brasiliensis. J Biol Chem 271:4553–4560. 10.1074/jbc.271.8.4553. [DOI] [PubMed] [Google Scholar]
- 288.Leitão NP, Jr, Vallejo MC, Conceição PM, Camargo ZP, Hahn R, Puccia R. 2014. Paracoccidioides lutzii Plp43 is an active glucanase with partial antigenic identity with P. brasiliensis gp43. PLoS Negl Trop Dis 8:e3111. 10.1371/journal.pntd.0003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cordeiro RDA (ed). 2020. Pocket guide to mycological diagnosis, 1st ed. CRC Press, Boca Raton, FL. https://www.taylorfrancis.com/books/9781351677677. Accessed 25 February 2022. [Google Scholar]
- 290.Wickes BL, Wiederhold NP. 2018. Molecular diagnostics in medical mycology. Nat Commun 9:5135. 10.1038/s41467-018-07556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Theodoro RC, Bagagli E, Oliveira C. 2008. Phylogenetic analysis of PRP8 intein in Paracoccidioides brasiliensis species complex. Fungal Genet Biol 45:1284–1291. 10.1016/j.fgb.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 292.Ricci G, Campanini EB, Nishikaku AS, Puccia R, Marques M, Bialek R, Rodrigues AM, Batista WL. 2022. PbGP43 genotyping using paraffin-embedded biopsies of human paracoccidioidomycosis reveals a genetically distinct lineage in the Paracoccidioides brasiliensis complex. Mycopathologia 187:157–168. 10.1007/s11046-021-00608-3. [DOI] [PubMed] [Google Scholar]
- 293.Roberto TN, Rodrigues AM, Hahn RC, de Camargo ZP. 2016. Identifying Paracoccidioides phylogenetic species by PCR-RFLP of the alpha-tubulin gene. Med Mycol 54:240–247. 10.1093/mmy/myv083. [DOI] [PubMed] [Google Scholar]
- 294.Gomes GM, Cisalpino PS, Taborda CP, de Camargo ZP. 2000. PCR for diagnosis of paracoccidioidomycosis. J Clin Microbiol 38:3478–3480. 10.1128/JCM.38.9.3478-3480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.San-Blas G, Niño-Vega G, Barreto L, Hebeler-Barbosa F, Bagagli E, Olivero de Briceño R, Mendes RP. 2005. Primers for clinical detection of Paracoccidioides brasiliensis. J Clin Microbiol 43:4255–4257. 10.1128/JCM.43.8.4255-4257.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Díez S, Garcia EA, Pino PA, Botero S, Corredor GG, Peralta LA, Castaño JH, Restrepo A, McEwen JG. 1999. PCR with Paracoccidioides brasiliensis specific primers: potential use in ecological studies. Rev Inst Med Trop Sao Paulo 41:351–358. 10.1590/s0036-46651999000600004. [DOI] [PubMed] [Google Scholar]
- 297.Sandhu GS, Aleff RA, Kline BC, da Silva Lacaz C. 1997. Molecular detection and identification of Paracoccidioides brasiliensis. J Clin Microbiol 35:1894–1896. 10.1128/jcm.35.7.1894-1896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Motoyama AB, Venancio EJ, Brandão GO, Petrofeza-Silva S, Pereira IS, Soares CMA, Felipe MSS. 2000. Molecular identification of Paracoccidioides brasiliensis by PCR amplification of ribosomal DNA. J Clin Microbiol 38:3106–3109. 10.1128/JCM.38.8.3106-3109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Imai T, Sano A, Mikami Y, Watanabe K, Aoki FH, Branchini MLM, Negroni R, Nishimura K, Miyaji M. 2000. A new PCR primer for the identification of Paracoccidioides brasiliensis based on rRNA sequences coding the internal transcribed spacers (ITS) and 5.8S regions. Med Mycol 38:323–326. 10.1080/mmy.38.4.323.326. [DOI] [PubMed] [Google Scholar]
- 300.Alves FL, Ribeiro MA, Hahn RC, de Melo Teixeira M, de Camargo ZP, Cisalpino PS, Marini MM. 2015. Transposable elements and two other molecular markers as typing tools for the genus Paracoccidioides. Med Mycol 53:165–170. 10.1093/mmy/myu074. [DOI] [PubMed] [Google Scholar]
- 301.Bialek R, Ibricevic A, Aepinus C, Najvar LK, Fothergill AW, Knobloch J, Graybill JR. 2000. Detection of Paracoccidioides brasiliensis in tissue samples by a nested PCR assay. J Clin Microbiol 38:2940–2942. 10.1128/JCM.38.8.2940-2942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ricci G, Zelck U, Mota F, Lass-Flörl C, Franco MF, Bialek R. 2008. Genotyping of Paracoccidioides brasiliensis directly from paraffin embedded tissue. Med Mycol 46:31–34. 10.1080/13693780701488373. [DOI] [PubMed] [Google Scholar]
- 303.Gaviria M, Rivera V, Muñoz-Cadavid C, Cano LE, Naranjo TW. 2015. Validation and clinical application of a nested PCR for paracoccidioidomycosis diagnosis in clinical samples from Colombian patients. Braz J Infect Dis 19:376–383. 10.1016/j.bjid.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Correia J, de Moraes Borba C, Reis B, Martins A, Unkles S, Kinghorn JR, Lucena-Silva N. 2010. The ceja-1 sequence as a potential new molecular marker for Paracoccidioides brasiliensis infection. Mycoses 53:130–137. 10.1111/j.1439-0507.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- 305.Koishi AC, Vituri DF, Dionízio Filho PSR, Sasaki AA, Felipe MSS, Venancio EJ. 2010. A semi-nested PCR assay for molecular detection of Paracoccidioides brasiliensis in tissue samples. Rev Soc Bras Med Trop 43:728–730. 10.1590/s0037-86822010000600026. [DOI] [PubMed] [Google Scholar]
- 306.Pitz ADF, Koishi AC, Tavares ER, de Andrade FG, Loth EA, Gandra RF, Venancio EJ. 2013. An optimized one-tube, semi-nested PCR assay for Paracoccidioides brasiliensis detection. Rev Soc Bras Med Trop 46:783–785. 10.1590/0037-8682-1625-2013. [DOI] [PubMed] [Google Scholar]
- 307.Endo S, Komori T, Ricci G, Sano A, Yokoyama K, Ohori A, Kamei K, Franco M, Miyaji M, Nishimura K. 2004. Detection of gp43 of Paracoccidioides brasiliensis by the loop-mediated isothermal amplification (LAMP) method. FEMS Microbiol Lett 234:93–97. 10.1016/j.femsle.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 308.Tatibana BT, Sano A, Uno J, Kamei K, Igarashi T, Mikami Y, Miyaji M, Nishimura K, Itano EN. 2009. Detection of Paracoccidioides brasiliensis gp43 gene in sputa by loop-mediated isothermal amplification method. J Clin Lab Anal 23:139–143. 10.1002/jcla.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Buitrago MJ, Merino P, Puente S, Gomez-Lopez A, Arribi A, Zancopé-Oliveira RM, Gutierrez MC, Rodriguez-Tudela JL, Cuenca-Estrella M. 2009. Utility of real-time PCR for the detection of Paracoccidioides brasiliensis DNA in the diagnosis of imported paracoccidioidomycosis. Med Mycol 47:879–882. 10.3109/13693780802713208. [DOI] [PubMed] [Google Scholar]
- 310.Rocha-Silva F, Gomes LI, Gracielle-Melo C, Goes AM, Caligiorne RB. 2017. Real time polymerase chain reaction (rt-PCR): a new patent to diagnostic purposes for paracoccidioidomycosis. Recent Pat Endocr Metab Immune Drug Discov 10:143–149. 10.2174/1872214810666160905150958. [DOI] [PubMed] [Google Scholar]
- 311.Costa MV, Landgraf TN, Corrêa PC, Souza IEL, Fernandes FF, Panunto-Castelo A. 2018. Quantitation of pulmonary fungal burden in Paracoccidioides brasiliensis-infected mice by real-time PCR. Rev Inst Med Trop Sao Paulo 61:e2. 10.1590/S1678-9946201961002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Semighini CP, de Camargo ZP, Puccia R, Goldman MHS, Goldman GH. 2002. Molecular identification of Paracoccidioides brasiliensis by 5′ nuclease assay. Diagn Microbiol Infect Dis 44:383–386. 10.1016/S0732-8893(02)00472-8. [DOI] [PubMed] [Google Scholar]
- 313.Irinyi L, Serena C, Garcia-Hermoso D, Arabatzis M, Desnos-Ollivier M, Vu D, Cardinali G, Arthur I, Normand A-C, Giraldo A, da Cunha KC, Sandoval-Denis M, Hendrickx M, Nishikaku AS, de Azevedo Melo AS, Merseguel KB, Khan A, Parente Rocha JA, Sampaio P, da Silva Briones MR, e Ferreira RC, de Medeiros Muniz M, Castañón-Olivares LR, Estrada-Barcenas D, Cassagne C, Mary C, Duan SY, Kong F, Sun AY, Zeng X, Zhao Z, Gantois N, Botterel F, Robbertse B, Schoch C, Gams W, Ellis D, Halliday C, Chen S, Sorrell TC, Piarroux R, Colombo AL, Pais C, de Hoog S, Zancopé-Oliveira RM, Taylor ML, Toriello C, de Almeida Soares CM, Delhaes L, Stubbe D, et al. 2015. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database—the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med Mycol 53:313–337. 10.1093/mmy/myv008. [DOI] [PubMed] [Google Scholar]
- 314.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc, New York, NY. [Google Scholar]
- 315.Morais FV, Barros TF, Fukada MK, Cisalpino PS, Puccia R. 2000. Polymorphism in the gene coding for the immunodominant antigen gp43 from the pathogenic fungus Paracoccidioides brasiliensis. J Clin Microbiol 38:3960–3966. 10.1128/JCM.38.11.3960-3966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Teixeira MDM, Cattana ME, Matute DR, Muñoz JF, Arechavala A, Isbell K, Schipper R, Santiso G, Tracogna F, Sosa MDLÁ, Cech N, Alvarado P, Barreto L, Chacón Y, Ortellado J, de Lima CM, Chang MR, Niño-Vega G, Yasuda MAS, Felipe MSS, Negroni R, Cuomo CA, Barker B, Giusiano G. 2020. Genomic diversity of the human pathogen Paracoccidioides across the South American continent. Fungal Genet Biol 140:103395. 10.1016/j.fgb.2020.103395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Griffiths J, Lopes Colombo A, Denning DW. 2019. The case for paracoccidioidomycosis to be accepted as a neglected tropical (fungal) disease. PLoS Negl Trop Dis 13:e0007195. 10.1371/journal.pntd.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.De Brito T, Sandhu GS, Kline BC, Aleff RA, Sandoval MP, Santos RT, Brandão AA, Lacaz CS. 1999. In situ hybridization in paracoccidioidomycosis. Med Mycol 37:207–211. 10.1080/j.1365-280X.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- 319.Arantes TD, Theodoro RC, Teixeira MDM, Bagagli E. 2017. Use of fluorescent oligonucleotide probes for differentiation between Paracoccidioides brasiliensis and Paracoccidioides lutzii in yeast and mycelial phase. Mem Inst Oswaldo Cruz 112:140–145. 10.1590/0074-02760160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Moter A, Göbel UB. 2000. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods 41:85–112. 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 321.Nobrega de Almeida J, Del Negro GMB, Grenfell RC, Vidal MSM, Thomaz DY, de Figueiredo DSY, Bagagli E, Juliano L, Benard G. 2015. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for differentiation of the dimorphic fungal species Paracoccidioides brasiliensis and Paracoccidioides lutzii. J Clin Microbiol 53:1383–1386. 10.1128/JCM.02847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT. 2015. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol 33:692–705. 10.1016/j.tibtech.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 323.Mendes RP, Negroni R, Arechavala A. 1994. Treatment and control of cure, p 373–392. In Franco M, Lacaz CDS, Restrepo-Moreno A, Del Nigro G (ed), Paracoccidioidomycosis, 1st ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 324.Barbosa W, Vasconcelos WMDP. 1973. Ação da sulfametoxazol associada ao trimetoprim na terapêutica da blastomicose sul-americana. Rev Patol Trop 2:329–339. [Google Scholar]
- 325.Pedrosa PN, Wanke B, Coura JR. 1974. Emprego da associação sulfametoxazol + trimetoprim no tratamento da paracoccidioidose (blastomicose Sul-Americana). Rev Soc Bras Med Trop 8:159–165. 10.1590/S0037-86821974000300004. [DOI] [Google Scholar]
- 326.Lopes C. 1976. Tratamento da blastomicose sul-americana com associacao sulfametoxazol-trimetoprim. An Bras Dermatol 51:207–213. [Google Scholar]
- 327.Barraviera B, Mendes RP, Machado JM, Pereira PCM, de Souza MJ, Meira DA. 1989. Evaluation of treatment of paracoccidioidomycosis with cotrimazine (combination of sulfadiazine and trimethoprim): preliminary report. Rev Inst Med Trop Sao Paulo 31:53–55. 10.1590/s0036-46651989000100011. [DOI] [PubMed] [Google Scholar]
- 328.Nery AF, Crepaldi NP, Rossi SB, Tadano T, Leal-Santos FA, Hahn RC, Menezes VM, Fontes CJF. 2017. Therapeutic response in adult patients with nonsevere chronic paracoccidioidomycosis treated with sulfamethoxazole-trimethoprim: a retrospective study. Am J Trop Med Hyg 97:556–562. 10.4269/ajtmh.16-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cavalcante RDS, Sylvestre TF, Levorato AD, de Carvalho LR, Mendes RP. 2014. Comparison between itraconazole and cotrimoxazole in the treatment of paracoccidiodomycosis. PLoS Negl Trop Dis 8:e2793. 10.1371/journal.pntd.0002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Levorato AD, Moris DV, Cavalcante RDS, Sylvestre TF, de Azevedo PZ, de Carvalho LR, Mendes RP. 2018. Evaluation of the hepatobiliary system in patients with paracoccidioidomycosis treated with cotrimoxazole or itraconazole. Med Mycol 56:531–540. 10.1093/mmy/myx080. [DOI] [PubMed] [Google Scholar]
- 331.Björnsson E, Jerlstad P, Bergqvist A, Olsson R. 2005. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 40:1095–1101. 10.1080/00365520510023846. [DOI] [PubMed] [Google Scholar]
- 332.Lacaz CDS, Sampaio SDA. 1958. Tratamento da blastomicose sul-americana com anfotericina B. Resultados preliminares. Rev Paul Med 52:443–450. [Google Scholar]
- 333.Veronesi R, Albuquerque FJM, Del Negro G, Sampaio SDA, Ferreira JM, Meira DA. 1959. Resultados terapeuticos obtidos com o emprego da anfotericina B em formas superficiais e profundas da blastomicose sul-americana. Rev Hosp Clin 14:231–248. [Google Scholar]
- 334.Miranda JL, Machado Filho J. 1959. Considerações em torno da blastomicose sul-americana. Sobre a ação da anfotericina B. Hospital (Rio J) 56:93–115.14419412 [Google Scholar]
- 335.Polak A. 1979. Pharmacokinetics of amphotericin B and flucytosine. Postgrad Med J 55:667–670. 10.1136/pgmj.55.647.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Feldman HA, Hamilton JD, Gutman RA. 1973. Amphotericin B therapy in an anephric patient. Antimicrob Agents Chemother 4:302–305. 10.1128/AAC.4.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Battock DJ, Grausz H, Bobrowsky M, Littman ML. 1968. Alternate-day amphotericin B therapy in the treatment of rhinocerebral phycomycosis (mucormycosis). Ann Intern Med 68:122–137. 10.7326/0003-4819-68-1-122. [DOI] [PubMed] [Google Scholar]
- 338.Lacaz C, Ulson C, Sampaio S. 1959. Ação in vitro da anfotericina B sobre o P. brasiliensis. Rev Paul Med 54:357–360. [PubMed] [Google Scholar]
- 339.Gigliotti F, Shenep JL, Lott L, Thornton D. 1987. Induction of prostaglandin synthesis as the mechanism responsible for the chills and fever produced by infusing amphotericin B. J Infect Dis 156:784–789. 10.1093/infdis/156.5.784. [DOI] [PubMed] [Google Scholar]
- 340.Heidemann HT, Gerkens JF, Spickard WA, Jackson EK, Branch RA. 1983. Amphotericin B nephrotoxicity in humans decreased by salt repletion. Am J Med 75:476–481. 10.1016/0002-9343(83)90353-4. [DOI] [PubMed] [Google Scholar]
- 341.Marcondes J, Barravieira B, Meira D, Mendes R. 1982. Emprego da anfotericina B corrigida pelo clearence da creatinina em doentes com paracoccidioidomicose, abstr F-19. Abstr An XVIII Congr Soc Bras Med Trop, Ribeirão Preto, São Paulo, Brazil.
- 342.Tranchesi J, Campana CL, Sampaio SAP. 1960. Alterações eletrocardiogralicas observadas durante o tratamento da blastomicose pela anfotericina B. Rev Hosp Clin Fac Med Sao Paulo 15:126–130. [Google Scholar]
- 343.Barraviera SRCS, Yoshio K, Dillon NL, Marques SA, Lastória JC, Curi PR. 1985. Avaliaçäo tardia da funçäo renal em doentes com paracoccidioidomicose tratados com anfotericina B por meio da determinaçäo do ritmo de filtraçäo glomerular com EDTA Cr51. An Bras Dermatol 65:293–297. [Google Scholar]
- 344.Butler WT, Bennett JE, Hill GJ, Szwed CF, Cotlove E. 1964. Electrocardiographic and electrolyte abnormalities caused by amphotericin B in dog and man. Proc Soc Exp Biol Med 116:857–863. 10.3181/00379727-116-29391. [DOI] [PubMed] [Google Scholar]
- 345.Dillon N. 1972. Tratamento da paracoccidioidomicose pela anfotericina B. Avaliação de 119 doentes num período de 14 anos. PhD thesis. University of São Paulo, São Paulo, Brazil. [Google Scholar]
- 346.Lewis RE. 2011. Current concepts in antifungal pharmacology. Mayo Clin Proc 86:805–817. 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Peçanha PM, de Souza S, Falqueto A, Grão-Veloso TR, Lírio LV, Ferreira CUG, Jr, Santos AR, Costa HG, de Souza LRM, Tuon FF. 2016. Amphotericin B lipid complex in the treatment of severe paracoccidioidomycosis: a case series. Int J Antimicrob Agents 48:428–430. 10.1016/j.ijantimicag.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 348.Van Cutsem JM, Thienpont D. 1972. Miconazole, a broad-spectrum antimycotic agent with antibacterial activity. Chemotherapy 17:392–404. 10.1159/000220875. [DOI] [PubMed] [Google Scholar]
- 349.Stevens DA, Restrepo A, Cortés A, Betancourt J, Galgiani JN, Gómez I. 1978. Paracoccidioidomycosis (South American blastomycosis): treatment with miconazole. Am J Trop Med Hyg 27:801–807. 10.4269/ajtmh.1978.27.801. [DOI] [PubMed] [Google Scholar]
- 350.Lima N, Teixeira G, Miranda J. 1974. Tratamento da blastomicose sul-americana pelo miconazol oral. An Bras Dermatol 49:327–344. [Google Scholar]
- 351.Restrepo A, Stevens DA, Leiderman E, Fuentes J, Arana A, Angel R, Mejia G, Gomez I. 1980. Ketoconazole in paracoccidioidomycosis: efficacy of prolonged oral therapy. Mycopathologia 72:35–45. 10.1007/BF00443049. [DOI] [PubMed] [Google Scholar]
- 352.Lima NDS, do Valle ACS. 1987. Ketoconazol na paracoccidioidomicose com longo seguimento pós-terapêutico. An Bras Dermatol 62:205–207. [Google Scholar]
- 353.Negroni R, Palmieri O, Koren F, Tiraboschi IN, Galimberti RL. 1987. Oral treatment of paracoccidioidomycosis and histoplasmosis with itraconazole in humans. Clin Infect Dis 9:S47–S50. 10.1093/clinids/9.Supplement_1.S47. [DOI] [PubMed] [Google Scholar]
- 354.Restrepo A, Gómes I, Robledo J, Patiño MM, Cano LE. 1987. Itraconazole in the treatment of paracoccidioidomycosis: a preliminary report. Rev Infect Dis 9:S51–S56. 10.1093/clinids/9.supplement_1.s51. [DOI] [PubMed] [Google Scholar]
- 355.Negroni R, Robles AM, Arechavala A, Tiraboschi IN. 1987. Resultados del tratamento com itraconazol por via oral em la paracoccidioidomicosis. Rev Argent Micol 10:27–32. [Google Scholar]
- 356.Naranjo MS, Trujillo M, Munera MI, Restrepo P, Gomez I, Restrepo A. 1990. Treatment of paracoccidioidomycosis with itraconazole. J Med Vet Mycol 28:67–76. 10.1080/02681219080000091. [DOI] [PubMed] [Google Scholar]
- 357.Mendes RP, Barraviera B, Souza LR, Pereira PCM, Marcondes-Machado J, Franco MF, Meira DA. 1992. Evaluation of itraconazole in the treatment of paracoccidioidomycosis (PBM). Rev Argent Micol 15:86. [Google Scholar]
- 358.Negroni R, Taborda A, Arechavala A, Robles AM. 1992. Experiencia con itraconazol en el tratamiento de la paracoccidioidomicosis. Rev Argent Micol 15:83. [Google Scholar]
- 359.Queiroz-Telles F, Bendhack L, Hagi NT, Purim KS, Lameira RP, Bordgnon GF. 1992. Itraconazole in the therapy of paracoccidioidomycosis. Rev Argent Micol 15:T6/P. [Google Scholar]
- 360.Vargas FJ. 1992. El itraconazol en la paracoccidioidomicosis (experiencia en 40 casos bolivianos). Rev Argent Micol 15:85. [Google Scholar]
- 361.Marques S, Camargo RP, Lastória JC, Machado NL, Dillon NL. 1992. Itraconazol: resultados terapêuticos na paracoccidioidomicose. Rev Argent Micol 15:10–15. [Google Scholar]
- 362.Stevens DA, Brummer E, McEwen JG, Perlman A. 1988. Efficacy of fluconazole, a new oral triazole, in blastomycosis and paracoccidioidomycosis and in comparison with ketoconazole. Rev Iber Micol 5(Suppl 1):26. [Google Scholar]
- 363.Mendes RP, Souza LR, Marcondes-Machado J, Meira DA. 1992. Evaluation of fluconazole in the initial treatment of paracoccidioidomycosis (PBM) preliminary results. Rev Argent Micol 15:84. [Google Scholar]
- 364.Castro LGM, del Negro G, Belda W, Cucé LC, Sampaio SAP. 1992. Fluconazole no tratamento da paracoccicioidomicose, resultados preliminares. Rev Argent Micol 15:85. [Google Scholar]
- 365.Telles FQ, Goldani LZ, Schlamm HT, Goodrich JM, Ingroff AE, Yasuda MAS. 2007. An open-label comparative pilot study of oral voriconazole and itraconazole for long-term treatment of paracoccidioidomycosis. Clin Infect Dis 45:1462–1469. 10.1086/522973. [DOI] [PubMed] [Google Scholar]
- 366.Pont A, Williams PL, Loose DS, Feldman D, Reitz RE, Bochra C, Stevens DA. 1982. Ketoconazole blocks adrenal steroid synthesis. Ann Intern Med 97:370–372. 10.7326/0003-4819-97-3-370. [DOI] [PubMed] [Google Scholar]
- 367.Pont A, Williams PL, Azhar S, Reitz RE, Bochra C, Smith ER, Stevens DA. 1982. Ketoconazole blocks testosterone synthesis. Arch Intern Med 142:2137–2140. 10.1001/archinte.1982.00340250097015. [DOI] [PubMed] [Google Scholar]
- 368.Pont A, Graybill JR, Craven PC, Galgiani JN, Dismukes WE, Reitz RE, Stevens DA. 1984. High-dose ketoconazole therapy and adrenal and testicular function in humans. Arch Intern Med 144:2150–2153. 10.1001/archinte.1984.04400020052007. [DOI] [PubMed] [Google Scholar]
- 369.Mendes R, Morceli J, Pereira P, Marcondes-Machado J, Franco M, Brandão N. 1988. Treatment of paracoccidioidomycosis with ketoconazole in short period scheme. Clinical, radiological and endocrinological evaluation. Rev Iber Micol 5:87. [Google Scholar]
- 370.Vilela MP, Ferraz ML, Franco DR. 1989. Toxic hepatitis caused by ketoconazole: a report of 4 cases. Rev Paul Med 107:57–58. [PubMed] [Google Scholar]
- 371.Tkach JR, Rinaldi MG. 1982. Severe hepatitis associated with ketoconazole therapy for chronic mucocutaneous candidiasis. Cutis 29:482–484. [PubMed] [Google Scholar]
- 372.Kraemer FB, Pont A. 1986. Inhibition of cholesterol synthesis by ketoconazole. Am J Med 80:616–622. 10.1016/0002-9343(86)90816-8. [DOI] [PubMed] [Google Scholar]
- 373.Restrepo A, Gomez I, Robledo MA. 1982. Eficacia del ketoconazol en pacientes con paracoccidioidomicosis recidivante. Rev Inst Med Trop Sao Paulo 24:173–179. [PubMed] [Google Scholar]
- 374.Marcondes J, Meira DA, Mendes RP, Pereira PCM, Barraviera B, Mota NGDS, Morceli J. 1984. Avaliação do tratamento da paracoccidioidomicose com o ketoconazol. Rev Inst Med Trop Sao Paulo 26:113–121. 10.1590/s0036-46651984000200008. [DOI] [PubMed] [Google Scholar]
- 375.Cucé LC, Belda Junior W, de Oliveira VM. 1987. Paracoccidioidomicose resistente ao ketoconazol: relato de três casos. An Bras Dermatol 62:203–204. [Google Scholar]
- 376.Borgers M, Van de Ven MA. 1987. Degenerative changes in fungi after itraconazole treatment. Rev Infect Dis 9(Suppl 1):S33–S42. 10.1093/clinids/9.supplement_1.s33. [DOI] [PubMed] [Google Scholar]
- 377.Thompson GR, Rendon A, Ribeiro Dos Santos R, Queiroz-Telles F, Ostrosky-Zeichner L, Azie N, Maher R, Lee M, Kovanda L, Engelhardt M, Vazquez JA, Cornely OA, Perfect JR. 2016. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis 63:356–362. 10.1093/cid/ciw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ollague JM, de Zurita AM, Calero G. 2000. Paracoccidioidomycosis (South American blastomycosis) successfully treated with terbinafine: first case report. Br J Dermatol 143:188–191. 10.1046/j.1365-2133.2000.03614.x. [DOI] [PubMed] [Google Scholar]
- 379.Hahn RC, Fontes CJF, Batista RD, Hamdan JS. 2002. In vitro comparison of activities of terbinafine and itraconazole against Paracoccidioides brasiliensis. J Clin Microbiol 40:2828–2831. 10.1128/JCM.40.8.2828-2831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Baddley JW, Pappas PG. 2005. Antifungal combination therapy: clinical potential. Drugs 65:1461–1480. 10.2165/00003495-200565110-00002. [DOI] [PubMed] [Google Scholar]
- 381.Bar-Oz B, Moretti ME, Bishai R, Mareels G, Van Tittelboom T, Verspeelt J, Koren G. 2000. Pregnancy outcome after in utero exposure to itraconazole: a prospective cohort study. Am J Obstet Gynecol 183:617–620. 10.1067/mob.2000.105962. [DOI] [PubMed] [Google Scholar]
- 382.Queiroz-Telles F, Carvalho V, Schulman D, Nucci M. 1999. Efetividade comparative de cotrimoxazol e itraconazol no tratamento da paracoccidioidomicose (PCM). Rev Soc Bras Med Trop 32:244–250. [Google Scholar]
- 383.Borges SRC, Sperandio da Silva GM, da Costa Chambela M, de Oliveira RDVC, Braga Costa RL, Wanke B, do Valle ACF. 2014. Itraconazole vs. trimethoprim-sulfamethoxazole: a comparative cohort study of 200 patients with paracoccidioidomycosis. Med Mycol 52:303–310. 10.1093/mmy/myt012. [DOI] [PubMed] [Google Scholar]
- 384.Rezkallah-Iwasso MT, Mota NGS, Gomes MCG, Montenegro MR. 1984. Effect of levamisole on experimental paracoccidioidomycosis in the Syrian hamster: immunologic and histopathologic correlation. Mycopathologia 84:171–180. 10.1007/BF00436529. [DOI] [PubMed] [Google Scholar]
- 385.Peraçoli MTS, Montenegro MR, Soares AMVC, Mota NGS. 1990. Transfer of cell-mediated immunity to Paracoccidioides brasiliensis in hamsters with dialysable leukocyte extracts. J Med Vet Mycol 28:35–46. 10.1080/02681219080000051. [DOI] [PubMed] [Google Scholar]
- 386.Meira DA, Pereira PCM, Marcondes-Machado J, Mendes RP, Barraviera B, Pellegrino J, Jr, Rezkallah-Iwasso MT, Peracoli MTS, Castilho LM, Thomazini I, da Silva CL, Foss NT, Curi PR. 1996. The use of glucan as immunostimulant in the treatment of paracoccidioidomycosis. Am J Trop Med Hyg 55:496–503. 10.4269/ajtmh.1996.55.496. [DOI] [PubMed] [Google Scholar]
- 387.Marcondes-Machado J. 2001. Avaliação dos níveis séricos de IL-12p40, α-TNF e 1/-IFN em camundongos BALB/c tratados pela b-1,3 poliglicose, com ou sem infecção aguda pelo Toxoplasma gondii. PhD thesis. Universidade do Estado de São Paulo, Botucatu, São Paulo, Brazil. [Google Scholar]
- 388.Peraçoli MT, Soares AM, Mendes RP, Marques SA, Guastale H, Meira DA. 1988. Cell-mediated immunity in patients with the chronic form of paracoccidioidomycosis. Early and late evaluation after treatment. Rev Iber Micol 5:69. [Google Scholar]
- 389.Venturini J, Cavalcante RS, Sylvestre TF, dos Santos RF, Moris DV, Carvalho LR, de Arruda MSP, Golim MDA, Mendes RP. 2017. Increased peripheral blood TCD4+ counts and serum SP-D levels in patients with chronic paracoccidioidomycosis, during and after antifungal therapy. Mem Inst Oswaldo Cruz 112:748–755. 10.1590/0074-02760170046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Singer-Vermes LM, Burger E, Calich VL, Modesto-Xavier LH, Sakamoto TN, Sugizaki MF, Meira DA, Mendes RP. 1994. Pathogenicity and immunogenicity of Paracoccidioides brasiliensis isolates in the human disease and in an experimental murine model. Clin Exp Immunol 97:113–119. 10.1111/j.1365-2249.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Andrade UV, do Valle Leone de Oliveira SM, Chang MR, Pereira EF, Marques APDC, de Carvalho LR, Mendes RP, Paniago AMM. 2019. Treatment compliance of patients with paracoccidioidomycosis in central-west Brazil. J Bras Pneumol 45:e20180167. 10.1590/1806-3713/e20180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Ruiz e Resende LS, Yasuda AG, Mendes RP, Marques SA, Niéro-Melo L, Defaveri J, Domingues MAC. 2015. Paracoccidioidomycosis in patients with lymphoma and review of published literature. Mycopathologia 179:285–291. 10.1007/s11046-014-9851-4. [DOI] [PubMed] [Google Scholar]
- 393.Engelhard D, Stutman HR, Marks MI. 1984. Interaction of ketoconazole with rifampin and isoniazid. N Engl J Med 311:1681–1683. 10.1056/NEJM198412273112606. [DOI] [PubMed] [Google Scholar]
- 394.Sylvestre TF, Silva LRF, Cavalcante RDS, Moris DV, Venturini J, Vicentini AP, de Carvalho LR, Mendes RP. 2014. Prevalence and serological diagnosis of relapse in paracoccidioidomycosis patients. PLoS Negl Trop Dis 8:e2834. 10.1371/journal.pntd.0002834. [DOI] [PMC free article] [PubMed] [Google Scholar]