ABSTRACT
Geobacter sulfurreducens is a ubiquitous iron-reducing bacterium in soils, and in engineered systems, it can respire an electrode to produce measurable electric current. Its unique metabolism, heavily dependent on an extensive network of cytochromes, requires a unique cell composition. In this work, we used metallomics, cell fraction and elemental analyses, and transcriptomics to study and analyze the cell composition of G. sulfurreducens. Elemental composition studies (C, H, O, N, and ash content) showed high C:O and H:O ratios of approximately 1.7:1 and 0.25:1, indicative of more reduced cell composition that is consistent with high lipid content. Our study shows that G. sulfurreducens cells have a large amount of iron (2 ± 0.2 μg/g dry weight) and lipids (32 ± 0.5% dry weight/dry weight) and that this composition does not change whether the cells are grown with a soluble or an insoluble electron acceptor. The high iron concentration, higher than similar microorganisms, is attributed to the production of cytochromes that are abundant in transcriptomic analyses in both solid and soluble electron acceptor growth. The unique cell composition of G. sulfurreducens must be considered when growing this microorganism for lab studies and commercial applications.
IMPORTANCE Geobacter sulfurreducens is an electroactive microorganism. In nature, it grows on metallic minerals by transferring electrons to them, effectively “breathing” metals. In a manmade system, it respires an electrode to produce an electric current. It has become a model organism for the study of electroactive organisms. There are potential biotechnological applications of an organism that can bridge the gap between biology and electrical signal and, as a ubiquitous iron reducer in soils around the world, G. sulfurreducens has an impact on the global iron cycle. We measured the concentrations of metals, macromolecules, and basic elements in G. sulfurreducens to define this organism’s composition. We also used gene expression data to discuss which proteins those metals could be associated with. We found that G. sulfurreducens has a large amount of lipid and iron compared to other bacteria—these observations are important for future microbiologists and biotechnologists working with the organism.
KEYWORDS: Geobacter sulfurreducens, cell composition, iron reduction, metalloproteins
INTRODUCTION
Anode-respiring electroactive bacteria, such as Geobacter sulfurreducens, have been studied for almost 2 decades for their capability to produce electrical current from metabolic respiration of organic compounds while in multilayered biofilms (1–3). A unique feature of these biofilms is the extracellular matrix that allows the transport of electrons over tens of micrometers (4–6). As part of this extracellular matrix, several components have been proposed to be crucial in achieving extracellular electron transport (EET). The transport of electrons starts at the inner membrane and travels across the periplasm and outer membrane before it reaches the extracellular environment. Cytochromes at these locations are known to play an important role in delivering electrons outside the cell (7–10). Microbial nanowires, now also identified as cytochrome polymers (4, 6), are the main path by which electrons are thought to be conducted in the extracellular environment, reaching a solid electron acceptor. Extracellular polymeric substances have also been proposed to play a role in EET in G. sulfurreducens (3, 11). On the other hand, Shewanella oneidensis MR-1 has been shown to produce outer membrane and periplasmic extensions, which are lipid bilayers and contain extracellular cytochromes (12, 13). In both cases, it is clear that the EET mechanism creates an extra metabolic burden to electroactive organisms and that it can alter their cell composition and nutrient requirements compared to microorganisms performing respiration of soluble electron acceptors. These nutrient requirements, however, have not been assessed in a systematic way.
Transcriptomic and proteomic studies in G. sulfurreducens have highlighted the importance of respiratory and EET proteins for their growth on anodes and metal oxides. Extracellular and outer membrane proteins, including pili, outer membrane channels, and c-type membrane cytochromes, are essential to the metabolism of G. sulfurreducens (9, 14–26). The high abundance of these proteins and other possible extracellular components may result in unique cellular composition. For example, each cytochrome contains one or more iron-containing heme complexes that can influence the iron content of the cell. An analysis of cell composition can provide insights into the composition of the extracellular matrix and EET-related components.
Several studies have provided insights into the cellular composition of G. sulfurreducens. For example, the lipid fraction of Geobacter sulfurreducens was reported as 15% wt/wt by Mahadevan et al. (27), where an additional 4% lipopolysaccharides fraction was assumed. In comparison, the lipid in Escherichia coli has been reported to be 9.1% weight (28) while cyanobacterium Synechocystis sp. PCC6803, known to produce thylakoid membranes, has been reported to be as high as 14% weight (29) and the lipid-rich microalgae Schizochytrium sp. can contain up to 30% lipid (30). The high lipid content of G. sulfurreducens has potential implications for biotechnology applications.
To our knowledge, few studies have performed a metallomic analysis in G. sulfurreducens, and the existing literature has conflicting results. Previous research has shown that G. sulfurreducens, when grown on fumarate as an electron acceptor, has similar metal content to that of E. coli. On the other hand, the closely related organism Geobacter metallireducens grown on iron citrate showed an order of magnitude higher iron content; however, the possible formation of inorganic precipitates was reported to be a possible hindrance to the measurement (31). Another study found that G. sulfurreducens had a per-cell iron content an order of magnitude higher than E. coli and that limiting growth medium iron content inhibited EET in G. sulfurreducens (15).
In this study, we hypothesized that G. sulfurreducens has a significantly different cellular composition compared to other cells performing soluble respiratory metabolisms. The differences that stem out of the EET requirements can help explain how EET develops. Understanding these characteristics can lead to better growth and maintenance of these microorganisms in laboratory and applied systems. We performed a metallomic analysis on G. sulfurreducens grown on an anode versus fumarate as electron acceptors and compared it to E. coli K-12. The use of an anode allows us to study EET and eliminates the interference of possible iron oxides reported by Budhraja et al. (31). We also performed an elemental analysis (C, H, O, N, and ash content) and a fraction analysis (protein, carbohydrates, and proteins) to obtain a comprehensive cell composition and determine if the previously observed lipid fractions measured in fumarate samples are also observed during anodic respiration. The results are complemented with a transcriptomic analysis of G. sulfurreducens grown under similar conditions.
RESULTS AND DISCUSSION
Elemental analysis.
We analyzed the trace metals and macronutrients of dried G. sulfurreducens and E. coli grown in the lab with different environments (Table S3 in the supplemental material; Table 1; Fig. 1). We found significant differences between the two species in the mass fraction of several trace metals (Fig. 1; Table S3; Fig. S1). Media compositions were different in each growth condition, matching each organism’s requirements (Table S2). There were a few differences between anode-grown and fumarate-grown G. sulfurreducens. Significant differences in Mn and Fe content were observed in E. coli that were growing in M9 medium versus Geobacter medium. Given the low abundance of nutrients in M9’s minimal medium, significant increases in Cu, Fe, Mn, and Se were observed when E. coli was grown in Geobacter medium. We use the latter condition as a point of reference when comparing G. sulfurreducens and E. coli.
TABLE 1.
Cell compositions of G. sulfurreducens and E. coli in different growth conditionsa
| Growth condition | G. sulf. electrode | G. sulf. fumarate | E. coli M9 medium |
|---|---|---|---|
| Proteins (mg body surface area/g dry weight) | 215 (±7) | 262 (±56) | 284 (±15) |
| Crude lipids (mg/g dry weight) | 323 (±45) | 321 (±35) | 243 (±36) |
| Carbohydrates (mg glucose/g dry weight) | 193 (±11) | 87 (±24) | 68 (±19) |
| Elements | |||
| C (%) | 47.0 (±1.1) | 46.8 (±1.8) | 46.9 (±0.7) |
| H (%) | 7.2 (±0.6) | 7.4 (±0.1) | 7.4 (±1.0) |
| N (%) | 9.5 (±0.2) | 8.3 (±0.3) | 12.5 (±0.0) |
| O (%) | 26.4 (±3.3) | 28.6 (±3.9) | 24.8 (±2.0) |
| Ash (%) | 9.9 (±3.0) | 9.0 (±3.3) | 8.4 (±1.3) |
Error is the sample standard deviation.
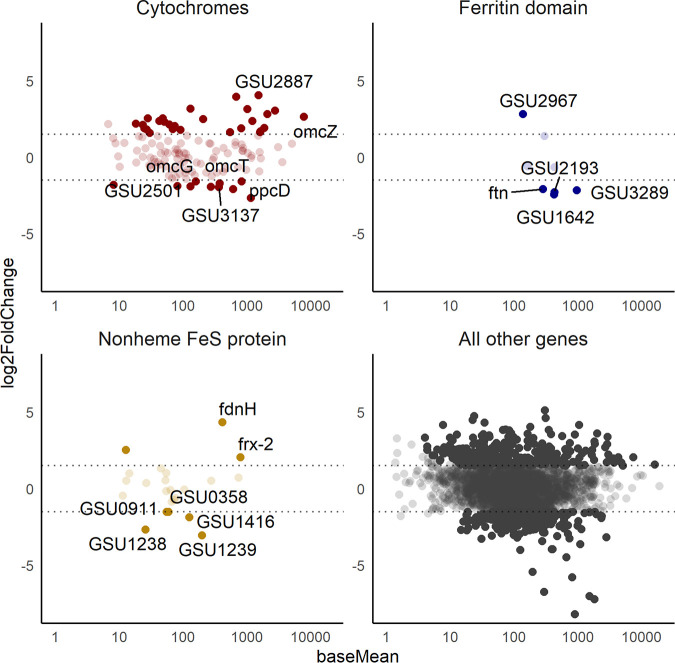
FIG 1.
Relevant differences in metal concentrations between E. coli-GS (Geobacter medium), E. coli-M9 (M9 medium), G. sulf-A (biofilm grown on an electrode), and G. sulf-F (planktonic cells using fumarate as the electron acceptor). See Fig. S1 for more comparisons. *, P < 0.05, pairwise t test with multiple comparison correction performed with the Benjamini-Hochberg method. We chose to omit alkali metals from this figure, but lithium did have significant differences as well (Table S1).
Several metals were present at higher concentrations in anode- and fumarate-grown G. sulfurreducens compared to E. coli, but only a subset of them had statistically significant differences between the organisms (Table S3; Fig. 1).
Metals used as cofactors.
Fe is a required trace metal for cellular respiration in many organisms as the cofactor in cytochromes. In both growth conditions for G. sulfurreducens, Fe was much higher than in E. coli, which suggests a much higher abundance of Fe-containing metalloproteins and other iron-containing biomolecules (Fig. 1).
In previous studies, listed in Table 2, anaerobic bacteria are reported to have more iron per cell biomass than aerobic bacteria. Phototrophic bacteria (Rhodospirillum, Rhodopseudomonas, Chromatium) and facultative bacteria (Escherichia, Enterobacter) have 150 to 500 μg/g dry weight Fe. Desulfovibrio vulgaris, an anaerobic Deltaproteobacterium like G. sulfurreducens, has over 900 μg/g dry weight. Not included in our analysis are iron-oxidizing bacteria, whose Fe precipitates can lead to Fe concentrations over 2% by dry mass (32). To our knowledge, G. sulfurreducens has the highest Fe content among bacteria studied, with almost twice the content of D. vulgaris. This is consistent with their production of heme-containing cytochromes in much higher abundance than other microorganisms, leading to not only cytoplasmic and membrane metalloproteins but also an extensive abundance of extracellular cytochromes. Growing G. sulfurreducens on the anode versus fumarate did not change the total Fe amount, suggesting a similar abundance of Fe metalloproteins. OmcS nanowires have been isolated from fumarate culture (6), indicating that G. sulfurreducens does not necessarily downregulate its EET metabolism when it is not needed. Our gene expression data also show a high expression of cytochromes associated with EET in fumarate and anode biofilm culture (Fig. 2).
TABLE 2.
Amount of iron in different prokaryotic species
| Bacterial species | Iron (μg Fe/g dry weight) | References |
|---|---|---|
| Rhodospirillum rubrum | Light: 202 (±13) Dark: 198 (±10) |
|
| Rhodopseudomonas spheroides | Light: 163 (±21) Dark: 230 (±24) |
Kassner and Kamen (61)a |
| Chromatium | Light: 456 (±76) | |
| Desulfovibrio vulgaris | 952 | Lancaster et al. (62)b |
| Enterobacter cloacae | 154 | Lancaster et al. (62)b |
| Escherichia coli | 223 | Hartmann and Braun (63) |
| 280 | Abdul-Tehrani et al. (64) | |
| 300 | Abdul-Tehrani et al. (64) | |
| Micrococcus roseus | 200 | Rouf (32) |
| Bacillus cercus | 300~400 | Rouf (32) |
| Psudomonas aeruginosa | 0.1 | Ma et al. (65) |
| Shewanella oneidensis | 147c | Daly et al. (66) |
| Escherichia coli | G medium: 430 (±90) M9 medium: 130 (±50) |
This study |
| Geobacter sulfurreducens | Electrode: 1,970 (±226) Fumarate: 1,960 (±229) |
Photosynthetic bacteria were grown in different growth conditions with and without light exposure.
Iron in D. vulgaris and E. cloacae were estimated with the measured iron per total protein of the cells; we used a conversion factor of 0.55 to convert from protein to volatile solids (28).
S. oneidensis iron content was converted from nmol Fe/mg protein to μg Fe/g dry weight using 52.8% protein content as measured previously (67).
FIG 2.
MA plots of mRNA gene expression data comparing planktonic cells and anode biofilms. Positive log2 fold change indicates higher expression in the anode biofilm condition. Solid points indicate a log2 fold change >1.5 and an adjusted P value of <0.05.
Fe may be a limiting trace mineral in commonly used G. sulfurreducens media. Estevez-Canales et al. (15) found that a medium concentration of 2 μM Fe limits biomass culture in a chemostat growing G. sulfurreducens led to a culture with 1.9 × 10−6 ng of Fe per cell. If we assume the average cell dry weight of G. sulfurreducens is between 0.1 and 1 pg, as has been shown in E. coli (33), our results would give an iron content of 2 × 10−7 ng to 2 × 10−6 ng Fe per cell in G. sulfurreducens.
Nickel, cobalt, and chrome content were significantly higher in both G. sulfurreducens conditions relative to E. coli (Table S3; Fig. 1). Nickel is a cofactor in Ni-Fe hydrogenases, and the genome of G. sulfurreducens encodes several (34, 35). G. sulfurreducens is able to assimilate cobalt through its cobamide-synthesis pathways (36), but it may also be precipitated on the cell surface as a defense mechanism against cobalt toxicity (37).
Precipitating metals.
There are some metals that may be overrepresented in our G. sulfurreducens samples due to precipitation. G. sulfurreducens requires at least two multicopper proteins, OmpC and OmpB, to respire Fe (III) oxide (38), and while these and other metalloproteins are a likely reservoir of Cu in our samples, G. sulfurreducens is capable of reducing Cu(II) to Cu2S nanoparticles that associate with cells (39). This phenomenon makes it difficult to estimate how much copper was required for metalloproteins and how much may have been trapped as inorganic precipitates. G. sulfurreducens can also immobilize copper through dissimilatory reduction (40). In our data, the G. sulfurreducens samples were enriched in Cr compared to E. coli (Fig. 1), while differences in Cu were not statistically significant. Manganese was significantly lower in the E. coli grown with M9 medium compared to all other conditions, including E. coli grown with the G. sulfurreducens medium recipe because M9 medium does not contain manganese (Fig. 1; Table S2).
Based on the metal content of the G. sulfurreducens cells collected, we can estimate a maximum cell density from the available mineral content in the common Geobacter medium (ATCC 1957). Table S4 shows the estimated growth cell assuming cells require the observed metal concentrations and only have the medium as a source. As expected, Fe is the most limiting metal in the medium, allowing for only 0.10 g cells/L. Cu and Zn are also close to this limitation and could lead to a multinutrient limitation when growing G. sulfurreducens at ~0.1 g/L. This nutrient limitation can either limit cell density in cell suspensions or limit current generation in microbial electrochemical technologies when operated in batch mode. Assuming a current production of ~0.28 A/g protein (17) or 0.6 A/g cell (based on Table 1), 1 L of Geobacter medium can support enough G. sulfurreducens cells to produce 60 mA, an amount of current that is enough for most experimental setups but might be limiting in electrochemical cells with a high specific surface area.
Cell composition of G. sulfurreducens is different from an average bacterium.
We also studied the cell composition and elemental analysis (C, H, O, N, and ash) of G. sulfurreducens. Interestingly, the G. sulfurreducens cell showed a high abundance of lipids in both growth conditions (Table 1). The values of ~32% lipid content were much higher than previously reported and similar to lipid-accumulating algal cultures (41–43). We do not know the reason why G. sulfurreducens requires such a high lipid content. Their smaller diameter (~0.5 μm) and distinct morphology (44) compared to other rod-shaped bacteria certainly plays a role in the increased lipid content. Shewanella oneidensis, another electrogenic organism, is known to produce outer membrane extensions for electron transfer (13). While it is likely that our analysis captured some extracellular polymeric substances, the extracellular matrix of G. sulfurreducens has not been found to have a significant lipid component (45). Most microorganisms exhibiting this high lipid fraction have either lipid accumulation, as in the case of certain algal species (41–43), or have internal lipid structures that increase its relative fraction, as in the case of thylakoid membranes and intracytoplasmic membranes (46–48). Our method for lipid quantification would likely collect other nonpolar compounds like polyhydroxyalkanoates if they were present in the biofilm, and while G. sulfurreducens has not been shown to produce this type of carbon storage polymer, biofilms enriched in other Geobacter species do use some uncharacterized carbon storage mechanism (49). We anticipate that future studies may add context to the data we present. The lipid fraction in E. coli was lower than G. sulfurreducens and higher than has been previously reported at 24.3 ± 3.6%. However, there is a high variance in reported E. coli lipid content with values ranging from 9% to 19% by dry weight (50, 51).
Because of the higher lipid content, G. sulfurreducens cells show a significantly lower protein content compared to other microorganisms (~22 to 26%; Table 1). Fumarate-grown cells had a larger protein fraction than anode-grown cells. On the other hand, total carbohydrates were approximately two times higher in the anode-grown cell; exopolysaccharide excreted from G. sulfurreducens to form a biofilm on the electrode probably increases the carbohydrate content in this growth condition.
Our elemental analysis of G. sulfurreducens cells is consistent with the low protein, high lipid content measured. Following equation 1, empirical cell biomass formulas of G. sulfurreducens, normalized to N, were calculated as C5.77H10.61O2.43N for electrode-grown and C6.58H12.48O3.02N for fumarate-grown cells. Compared with general formulas for bacterial biomass, such as C5H7O2N (52), G. sulfurreducens has a higher C:N ratio typical of low protein content. It also has a higher hydrogen content, due to the higher lipid content that has approximately a 1:2 for fumarate-grown cells.
We compared our cellular composition of G. sulfurreducens to that reported in Mahadevan et al. (27). The main difference between the fraction distributions reported here and those reported in Mahadevan et al. is the higher lipid content at the expense of lower protein content. We do not know the reason for the discrepancy, but in both cases the lipid content is significantly higher than E. coli and other bacterial cells.
Iron-containing genes are highly expressed.
Our analysis identified 434 genes that were differentially expressed between the anode biofilm samples and the planktonic fumarate cells out of 3,434 annotated genes detected at quantifiable levels. Two hundred five genes were more highly expressed in the anode biofilm, and 229 genes were more highly expressed in the planktonic samples. In Fig. 2, MA plots visualize the differential expression and highlight several types of iron-containing protein-coding genes. While a greater number of cytochromes were significantly upregulated in the anode biofilm than the number upregulated in the planktonic samples, most cytochromes were not differentially expressed. Ferritin domain-containing protein- and nonheme Fe-S domain protein-coding genes were also present among the differentially expressed genes. Our data show that iron-containing protein-coding genes are expressed in both planktonic fumarate cultures and anode biofilms but that there are specific iron-related genes whose expression depends on growth conditions. The abundance of expression of Fe-containing proteins is consistent with the high Fe abundance in both conditions. Our gene expression data are similar in overall patterns to the data in Otero et al. (7), with multiheme cytochromes like the PpcA, OmcZ, and CbcL among the most expressed genes overall, and only a subset of all cytochromes differentially expressed between electrode and fumarate conditions. We did, however, observe a greater number of cytochrome genes differentially expressed between electrode and fumarate conditions than Otero et al. (7), likely due to the lower electrode potential that we used. We also investigated the expression of lipid synthesis pathway genes and found that many of them were present in the transcriptome but not differentially expressed, although a few lipid-associated genes showed significant differences (Fig. S2).
Conclusions.
G. sulfurreducens is a bacterium with a complex system of electroactive proteins, and those electroactive proteins largely require iron. This may be a factor in the high iron concentration; we measured in G. sulfurreducens relative to nonelectroactive Gram-negative E. coli and values reported in literature for other bacteria. Our analysis complements previous work showing that restricting iron limits EET in G. sulfurreducens (15). This study estimates what the nutrient limitations might be for G. sulfurreducens, and this information is valuable for biotechnologists developing applications using this and similar organisms. The nearly identical composition between anode-grown and fumarate-grown cells supports the hypothesis that G. sulfurreducens is not adapted to efficiently grow on fumarate: it makes electron carriers for EET regardless of the electron acceptor if the nutrients are available. The lipid content measured in G. sulfurreducens was higher than what has been reported before and relatively high for a bacterium without lipidic storage. While all samples were taken from active biofilms or suspended cultures, we did not have a mechanism to separate dead cells from active cells, and it is probable that the composition of an individual cell may differ from the composition of the bulk samples analyzed. Compared to similar studies on other bacteria and E. coli in our study, we have shown that G. sulfurreducens has a unique composition to support its complex metabolism.
MATERIALS AND METHODS
Bacterial strain and culture media.
We subcultured G. sulfurreducens PCA (ATCC 51573) and E. coli K-12 from commercially available stocks. Medium compositions are listed in detail in Table S1 for four different cases: (i) G. sulfurreducens grown in microbial electrochemical cell (electrode), (ii) G. sulfurreducens grown in sodium fumarate-containing serum bottle (fumarate), (iii) E. coli grown in Geobacter medium, and (iv) E. coli grown in M9 medium. In brief, Geobacter medium contained sodium acetate (50 mM), NaHCO3 (30 mM), NH4Cl (20 mM), NaH2PO4 (4 mM), KCl (1 mM), vitamin mix (10 mL), and trace minerals (10 mL). Trace minerals contained nitrilotriacetic acid, trisodium salt (5.5 mM), MgSO4·7H2O (12 mM), MnSO4·H2O (2.9 mM), NaCl (17 mM), FeSO4·7H2O (0.36 mM), CaCl2·2H2O (0.68 mM), CoCl2·6H2O (0.42 mM), ZnCl2 (0.95 mM), CuSO4·5H2O (0.4 mM), AlK(SO4)2·12H2O (0.2 mM), H3BO4 (0.16 mM), Na2MoO4·H2O (0.01 mM), NiCl2·6H2O (0.01 mM), and Na2WO4·2H2O (8.5 μM). We provided a higher concentration of acetate (50 mM) than the previous studies (53–55) (10 mM), as we used a higher electrode surface area requiring more reduced electron donors. For G. sulfurreducens grown in serum bottles, sodium fumarate (100 mM) was added to the medium. We bubbled the media with N2/CO2 (80:20 vol/vol) to remove oxygen before autoclaving. After autoclaving, FeCl2·4H2O (20 μM), Na2S·9H2O (54 μM), sodium bicarbonate, and vitamins were added in the anaerobic glove box. E. coli medium (ATCC Medium 2511-M9 Minimal Broth) contained glucose (44 mM), Na2HPO4 (180 mM), KH2PO4 (44 mM), NaCl (17 mM), NH4Cl (37 mM), MgSO4·7H2O (0.2 mM), CaCl2 (10 μM), and thiamine (24 μM). Media for E. coli (M9 and Geobacter medium) were autoclaved without any gas sparging. Geobacter medium for E. coli contained the same ingredients of G. sulfurreducens medium for microbial electrochemical cells except for the electron donor; the same concentration of glucose as M9 (44 mM) was used instead of acetate.
Electrochemical setup and operation.
Single-chamber microbial electrochemical cells were constructed in 500-mL bottles with rubber stoppers located on top having PTFE tubing for gas inflow and outflow, a carbon anode as working electrode, a nickel wire cathode as counter electrode, and a reference electrode. We used two square graphite electrodes to grow biofilms of G. sulfurreducens with a surface area of ~20.9 cm2 and an Ag/AgCl reference electrode (BASi, West Lafayette, IN). We mixed the chambers with magnetic stirrer bars at 180 rpm and flushed humidified N2/CO2 gas (80:20 vol/vol) continuously. Before filling up the media in the anaerobic glove box, electrochemical cells were autoclaved for sterilization. We set −0.3 V versus Ag/AgCl (−0.03 V versus SHE) as the fixed anode potential using a VMP3 digital potentiostat (Bio-Logic USA, Knoxville, TN). This is at the lower end of the range of electrode potentials that G. sulfurreducens can respire. Fumarate-grown G. sulfurreducens reactors were set up in 250-mL serum bottles. Both electrochemical cells and serum bottles were in a temperature-controlled room at 30°C. In addition, the media filling along with inoculation was performed in the anaerobic glove box. We used 250-mL flasks for E. coli cultivation with M9 and Geobacter media. Geobacter medium (G medium) for E. coli was used to compare the cell composition with G. sulfurreducens. After filling the media and inoculation for E. coli, we placed the flasks in an incubator with shaking at 180 rpm and temperature at 37°C.
Sample preparation.
For the determination of carbohydrate, protein, lipid, metal, and element per dried cell, we collected the G. sulfurreducens grown on anodes in the microbial electrochemical cells and in serum bottle reactors and the E. coli grown in flasks. G. sulfurreducens biofilms grown on the anodes were scraped off with a needle in an anaerobic glove box. Grown cells of G. sulfurreducens and E. coli from the serum bottles and flasks were separated by centrifugation (Eppendorf Centrifuge 5810 R, USA) at 4,000 rpm in microcentrifuge tubes. Cells were washed once with a Ringer’s solution (25% strength) and centrifuged again (Table S1). The cells dried overnight at 105°C in plastic tubes, and the cool pellets were then broken up with a sterile stainless-steel spatula.
Analytical methods.
We measured proteins by bicinchoninic acid protein assay (56). In brief, dried cell biomass (2 to 3 mg) was treated with 0.1 N NaOH at 90°C for 30 min, the lysate was resuspended and centrifuged the lysate, and 0.1 mL of supernatant was used for the assay. Carbohydrates were measured by a colorimetric method (57). In brief, dried cell biomass (2 to 3 mg) was acidified in sulfuric acid with sonication for 2 h, and dissolved samples (0.5 mL) were added into the test tubes with distilled water (0.5 mL), phenol (50 μL), and sulfuric acid (5 mL) for overnight reaction. Concentrations of proteins and carbohydrates were determined using a calibration curve with bovine serum albumin and glucose with absorbance at wavelengths of 485 and 562 nm, respectively. Crude lipids were extracted from the dried cell biomass using the Folch method (58). The dried biomass (~15 mg) was sonicated for 1 h and vortexed for 1 h with Folch solution (chloroform-methanol, 2:1, vol/vol) at room temperature. Solvent extracts were obtained after removing the biomass by centrifugation at 4,000 rpm. The crude lipid weight was determined by evaporating in the water bath (60°C) and weighing the tube before and after the evaporation of lipids. For metal extraction, we added dried cell biomass (3 to 5 mg) to glass vials along with hydrochloric acid (12 M) and sonicated at 60°C for 2 h. The dissolved metals in acid were analyzed by an inductively coupled plasma-optical emission spectrometer (ICP-OES, Thermo iCAP6300). Carbon, hydrogen, and nitrogen in the dried cell biomass (~2 mg) were measured using CHN Elemental Analyzer (PE2400). Instrumentation for ICP-OES and CHN Elemental Analyzer was done in Goldwater Environmental Laboratory at Arizona State University. For oxygen estimation, we measured the ash content of the dried cell biomass (~10 mg) following the previous method (59), burning the biomass at 600°C in an alumina crucible. Then, we subtracted the fraction of C, H, N, and ash content from the dried cell biomass (100%) for O estimation. Based on the elemental analysis data (%C, %H, %O, and %N), we obtained the empirical biomass formula followed by equation 1 below (52),
| (1) |
where , , , and
and
Transcriptomic analysis.
We sequenced reverse transcribed RNA from G. sulfurreducens grown in a single chamber microbial electrochemical cell as an anode biofilm with an anode poised at −0.28 V versus Ag/AgCl as the electron acceptor and in anaerobic test tubes with fumarate as the electron acceptor. Both conditions were collected in biological triplicate. We extracted RNA with the Qiagen/MOBIO PowerMicrobiome RNA extraction kit and used the ThermoFisher MicrobExpress bacterial mRNA enrichment kit to reduce the fraction of rRNA following the manufacturer’s recommendations. RNA library preparation and sequencing were performed by the genomics core facility at Arizona State University (ASU). We mapped the reads to the RefSeq assembly for G. sulfurreducens PCA and used DESeq2 in R for differential expression analysis. The cutoff determination for differential expression was set as a log2 fold change of at least 1.5 and a multiple comparison adjusted P value of <0.05.
Data availability.
The sequencing data we used here are a subset of data analyzed in a previous publication, and raw sequence data are available from NCBI under accession number GSE200066 (60).
ACKNOWLEDGMENTS
The funding for this work was provided by the Office of Naval Research (ONR awards N0014-15-1-2702 and N0014-20-1-2269).
We also thank Roy Erickson and Adam Smith for the assistance with ICP-OES and elemental analysis (Goldwater Environmental Laboratory at ASU) and the Genomics Core at ASU for sequencing.
Footnotes
Supplemental material is available online only.
Contributor Information
César I. Torres, Email: cit@asu.edu.
Jeffrey A. Gralnick, University of Minnesota
REFERENCES
- 1.Torres CI. 2014. On the importance of identifying, characterizing, and predicting fundamental phenomena towards microbial electrochemistry applications. Curr Opin Biotechnol 27:107–114. doi: 10.1016/j.copbio.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR. 2006. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72:7345–7348. doi: 10.1128/AEM.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond DR, Strycharz-Glaven SM, Tender LM, Torres ICI. 2012. On electron transport through Geobacter Biofilms. ChemSusChem 5:1099–1105. doi: 10.1002/cssc.201100748. [DOI] [PubMed] [Google Scholar]
- 4.Lovley DR, Walker DJF. 2019. Geobacter protein nanowires. Front Microbiol 10:2078. doi: 10.3389/fmicb.2019.02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadwick GL, Jiménez F, Gralnick JA, Bond DR, Orphan VJ, Otero FJ, Gralnick JA, Bond DR, Orphan VJ, Jiménez F, Gralnick JA, Bond DR, Orphan VJ. 2019. NanoSIMS imaging reveals metabolic stratification within current-producing biofilms. Proc Natl Acad Sci USA 116:20716–20724. doi: 10.1073/pnas.1912498116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Coureuil M, Osinski T, Orlova A, Altindal T, Gesbert G, Nassif X, Egelman EH, Craig L. 2017. Cryoelectron microscopy reconstructions of the Pseudomonas aeruginosa and Neisseria gonorrhoeae type IV Pili at sub-nanometer resolution. Structure 25:1423–1435.e4. doi: 10.1016/j.str.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otero FJ, Chi Ho Chan DRB, Jiménez Otero F, Chan CH, Bond DR. 2018. Identification of different putative outer membrane electron conduits necessary for Fe(III) citrate, Fe(III) oxide, Mn(IV) oxide, or electrode reduction by Geobacter sulfurreducens. J Bacteriol 200:e00347-18. doi: 10.1128/jb.00347-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levar CE, Hoffman CL, Dunshee AJ, Toner BM, Bond DR. 2017. Redox potential as a master variable controlling pathways of metal reduction by Geobacter sulfurreducens. ISME J 11:741–752. doi: 10.1038/ismej.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacharoff L, Chan CH, Bond DR. 2016. Reduction of low potential electron acceptors requires the CbcL inner membrane cytochrome of Geobacter sulfurreducens. Bioelectrochemistry 107:7–13. doi: 10.1016/j.bioelechem.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Pessanha M, Morgado L, Louro RO, Londer YY, Pokkuluri PR, Schiffer M, Salgueiro CA. 2006. Thermodynamic characterization of triheme cytochrome PpcA from Geobacter sulfurreducens: evidence for a role played in e−/H+ energy transduction. Biochemistry 45:13910–13917. doi: 10.1021/bi061394v. [DOI] [PubMed] [Google Scholar]
- 11.Rollefson JB, Stephen CS, Tien M, Bond DR. 2011. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J Bacteriol 193:1023–1033. doi: 10.1128/JB.01092-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian P, Pirbadian S, El-Naggar MY, Jensen GJ. 2018. Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc Natl Acad Sci USA 115:E3246–E3255. doi: 10.1073/pnas.1718810115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirbadian S, Barchinger SE, Leung KM, Byun HS, Jangir Y, Bouhenni RA, Reed SB, Romine MF, Saffarini DA, Shi L, Gorby YA, Golbeck JH, El-Naggar MY. 2014. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc Natl Acad Sci USA 111:12883–12888. doi: 10.1073/pnas.1410551111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BC, Leang C, Ding YHR, Glaven RH, Coppi MV, Lovley DR. 2005. OmcF, a putative c-type monoheme outer membrane cytochrome required for the expression of other outer membrane cytochromes in Geobacter sulfurreducens. J Bacteriol 187:4505–4513. doi: 10.1128/JB.187.13.4505-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estevez-Canales M, Kuzume A, Borjas Z, Füeg M, Lovley D, Wandlowski T, Esteve-Núñez A. 2015. A severe reduction in the cytochrome C content of Geobacter sulfurreducens eliminates its capacity for extracellular electron transfer. Environ Microbiol Rep 7:219–226. doi: 10.1111/1758-2229.12230. [DOI] [PubMed] [Google Scholar]
- 16.Peng L, Zhang Y. 2017. Cytochrome OmcZ is essential for the current generation by Geobacter sulfurreducens under low electrode potential. Electrochim Acta 228:447–452. doi: 10.1016/j.electacta.2017.01.091. [DOI] [Google Scholar]
- 17.Jiménez Otero F, Chadwick GL, Yates MD, Mickol RL, Saunders SH, Glaven SM, Gralnick JA, Newman DK, Tender LM, Orphan VJ, Bond DR. 2021. Evidence of a streamlined extracellular electron transfer pathway from biofilm structure, metabolic stratification, and long-range electron transfer parameters. Appl Environ Microbiol 87:e0070621. doi: 10.1128/AEM.00706-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi K, Chan CH, Bond DR. 2021. Geobacter sulfurreducens inner membrane cytochrome CbcBA controls electron transfer and growth yield near the energetic limit of respiration. Mol Microbiol 116:1124–1139. doi: 10.1111/mmi.14801. [DOI] [PubMed] [Google Scholar]
- 19.Leang C, Adams LA, Chin KJ, Nevin KP, Methé BA, Webster J, Sharma ML, Lovley DR. 2005. Adaptation to disruption of the electron transfer pathway for Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol 187:5918–5926. doi: 10.1128/JB.187.17.5918-5926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd JR, Leang C, Hodges Myerson AL, Coppi MV, Cuifo S, Methe B, Sandler SJ, Lovley DR. 2003. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem J 369:153–161. doi: 10.1042/bj20020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta T, Coppi MV, Childers SE, Lovley DR. 2005. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl Environ Microbiol 71:8634–8641. doi: 10.1128/AEM.71.12.8634-8641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelobolina ES, Coppi MV, Korenevsky AA, DiDonato LN, Sullivan SA, Konishi H, Xu H, Leang C, Butler JE, Kim BC, Lovley DR. 2007. Importance of c-type cytochromes for U(VI) reduction by Geobacter sulfurreducens. BMC Microbiol 7:1–15. doi: 10.1186/1471-2180-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nevin KP, Kim B-C, Glaven RH, Johnson JP, Woodard TL, Methé BA, DiDonato RJ, Covalla SF, Franks AE, Liu A, Lovley DR. 2009. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS One 4:e5628. doi: 10.1371/journal.pone.0005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aklujkar M, Coppi MV, Leang C, Kim BC, Chavan MA, Perpetua LA, Giloteaux L, Liu A, Holmes DE. 2013. Proteins involved in electron transfer to Fe(III) and Mn(IV) oxides by Geobacter sulfurreducens and Geobacter uraniireducens. Microbiology (Reading) 159:515–535. doi: 10.1099/mic.0.064089-0. [DOI] [PubMed] [Google Scholar]
- 25.Levar CE, Chan CH, Mehta-Kolte MG, Bond DR. 2014. An inner membrane cytochrome required only for reduction of high redox potential extracellular electron acceptors. mBio 5:e02034. doi: 10.1128/mBio.02034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Fredrickson JK, Zachara JM, Shi L. 2015. Direct involvement of ombB, omaB, and omcB genes in extracellular reduction of Fe(III) by Geobacter sulfurreducens PCA. Front Microbiol 6:1075. doi: 10.3389/fmicb.2015.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahadevan R, Bond DR, Butler JE, Esteve-Nuñez A, Coppi MV, Palsson BO, Schilling CH, Lovley DR. 2006. Characterization of metabolism in the Fe (III)-reducing organism Geobacter sulfurreducens by constraint-based modeling. Appl Environ Microbiol 72:1558–1568. doi: 10.1128/AEM.72.2.1558-1568.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neidhart FC, Ingraham JL, Schaechter M. 1990. Physiology of the bacterial cell: a molecular approach. Sinauer Associates, Sunderland, MA. [Google Scholar]
- 29.Cuellar-Bermudez SP, Romero-Ogawa MA, Vannela R, Lai YJS, Rittmann BE, Parra-Saldivar R. 2015. Effects of light intensity and carbon dioxide on lipids and fatty acids produced by Synechocystis sp. PCC6803 during continuous flow. Algal Res 12:10–16. doi: 10.1016/j.algal.2015.07.018. [DOI] [Google Scholar]
- 30.Byreddy AR, Barrow CJ, Puri M. 2016. Bead milling for lipid recovery from thraustochytrid cells and selective hydrolysis of Schizochytrium DT3 oil using lipase. Bioresour Technol 200:464–469. doi: 10.1016/j.biortech.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Budhraja R, Ding C, Walter P, Wagner S, Reemtsma T, Gary Sawers R, Adrian L. 2019. The impact of species, respiration type, growth phase and genetic inventory on absolute metal content of intact bacterial cells. Metallomics 11:925–935. doi: 10.1039/c9mt00009g. [DOI] [PubMed] [Google Scholar]
- 32.Rouf MA. 1964. Spectrochemical analysis of inorganic elements in bacteria. J Bacteriol 88:1545–1549. doi: 10.1128/jb.88.6.1545-1549.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loferer-Krössbacher M, Klima J, Psenner R. 1998. Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl Environ Microbiol 64:688–694. doi: 10.1128/AEM.64.2.688-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coppi MV. 2005. The hydrogenases of Geobacter sulfurreducens: a comparative genomic perspective. Microbiology (Reading) 151:1239–1254. doi: 10.1099/mic.0.27535-0. [DOI] [PubMed] [Google Scholar]
- 35.Tremblay PL, Lovley DR. 2012. Role of the NiFe hydrogenase hya in oxidative stress defense in Geobacter sulfurreducens. J Bacteriol 194:2248–2253. doi: 10.1128/JB.00044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan J, Ritalahti KM, Wagner DD, Löffler FE. 2012. Unexpected specificity of interspecies cobamide transfer from geobacter spp. to organohalide-respiring dehalococcoides mccartyi strains. Appl Environ Microbiol 78:6630–6636. doi: 10.1128/AEM.01535-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dulay H, Tabares M, Kashefi K, Reguera G. 2020. Cobalt resistance via detoxification and mineralization in the iron-reducing bacterium Geobacter sulfurreducens. Front Microbiol 11:1–17. doi: 10.3389/fmicb.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes DE, Mester T, O'Neil RA, Perpetua LA, Larrahondo MJ, Glaven R, Sharma ML, Ward JE, Nevin KP, Lovley DR. 2008. Genes for two multicopper proteins required for Fe(III) oxide reduction in Geobacter sulfurreducens have different expression patterns both in the subsurface and on energy-harvesting electrodes. Microbiology (Reading) 154:1422–1435. doi: 10.1099/mic.0.2007/014365-0. [DOI] [PubMed] [Google Scholar]
- 39.Nanoparticles CS, Kimber RL, Bagshaw H, Smith K, Buchanan DM, Coker VS, Cavet JS, Lloyd JR. 2020. Biomineralization of Cu2S nanoparticles by Geobacter sulfurreducens. Appl Environ Microbiol 86:e00967-20. doi: 10.1128/AEM.00967-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong Y, Werth CJ, He Y, Su Y, Zhang Y, Zhou X. 2018. Intracellular versus extracellular accumulation of Hexavalent chromium reduction products by Geobacter sulfurreducens PCA. Environ Pollut 240:485–492. doi: 10.1016/j.envpol.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 41.Ma C, Wen H, Xing D, Pei X, Zhu J, Ren N, Liu B. 2017. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol Biofuels 10:1–13. doi: 10.1186/s13068-016-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahidin S, Idris A, Shaleh SRM. 2013. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11. doi: 10.1016/j.biortech.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 43.Piligaev AV, Sorokina KN, Shashkov MV, Parmon VN. 2018. Screening and comparative metabolic profiling of high lipid content microalgae strains for application in wastewater treatment. Bioresour Technol 250:538–547. doi: 10.1016/j.biortech.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 44.Caccavo F, Lonergan DJ, Lovley DR, Davis M, Stolz JF, McInerney MJ. 1994. Geobacter Sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol 60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stöckl M, Teubner NC, Holtmann D, Mangold KM, Sand W. 2019. Extracellular polymeric substances from Geobacter sulfurreducens biofilms in microbial fuel cells. ACS Appl Mater Interfaces 11:8961–8968. doi: 10.1021/acsami.8b14340. [DOI] [PubMed] [Google Scholar]
- 46.Davies SL, Whittenbury R. 1970. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol 61:227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- 47.Murray RG, Watson SW. 1965. Structure of Nitrosocystis oceanus and comparison with Nitrosomonas and Nitrobacter. J Bacteriol 89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greening C, Lithgow T. 2020. Formation and function of bacterial organelles. Nat Rev Microbiol 18:677–689. doi: 10.1038/s41579-020-0413-0. [DOI] [PubMed] [Google Scholar]
- 49.Kubannek F, Schröder U, Krewer U. 2018. Revealing metabolic storage processes in electrode respiring bacteria by differential electrochemical mass spectrometry. Bioelectrochemistry 121:160–168. doi: 10.1016/j.bioelechem.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Damoglou AP, Dawes EA. 1968. Studies on the lipid content and phosphate requirement of glucose-and acetate-grown Escherichia coli. Biochem J 110:775–781. doi: 10.1042/bj1100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S, Jeon E, Yun HS, Lee J. 2011. Improvement of fatty acid biosynthesis by engineered recombinant Escherichia coli. Biotechnol Bioproc E 16:706–713. doi: 10.1007/s12257-011-0034-6. [DOI] [Google Scholar]
- 52.McCarty PL, Rittmann BE. 2001. Environmental biotechnology: principles and applications. McGraw-Hill, New York. [Google Scholar]
- 53.Snider RM, Strycharz-Glaven SM, Tsoi SD, Erickson JS, Tender LM. 2012. Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc Natl Acad Sci USA 109:15467–15472. doi: 10.1073/pnas.1209829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strycharz-Glaven SM, Snider RM, Guiseppi-Elie A, Tender LM. 2011. On the electrical conductivity of microbial nanowires and biofilms. Energy Environ Sci 4:4366–4379. doi: 10.1039/c1ee01753e. [DOI] [Google Scholar]
- 55.Yates MD, Strycharz-Glaven SM, Golden JP, Roy J, Tsoi S, Erickson JS, El-Naggar MY, Barton SC, Tender LM. 2016. Measuring conductivity of living geobacter sulfurreducens biofilms. Nat Nanotechnol 11:910–913. doi: 10.1038/nnano.2016.186. [DOI] [PubMed] [Google Scholar]
- 56.Brown RE, Jarvis KL, Hyland KJ. 1989. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem 180:136–139. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- 57.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 58.Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 59.Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D. 2008. Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory, Golden, CO. [Google Scholar]
- 60.Howley E, Krajmalnik-brown R, Torres CI. 2022. Cytochrome expression shifts in Geobacter sulfurreducens to maximize energy conservation in response to changes in redox conditions. bioRxiv. doi: 10.1101/2022.05.22.492868. [DOI] [PubMed]
- 61.Kassner RJ, Kamen MD. 1968. Trace metal composition of photosynthetic bacteria. Biochim Biophys Acta 153:270–278. doi: 10.1016/0005-2728(68)90169-2. [DOI] [PubMed] [Google Scholar]
- 62.Lancaster WA, Menon AL, Scott I, Poole FL, Vaccaro BJ, Thorgersen MP, Geller J, Hazen TC, Hurt RA, Brown SD, Elias DA, Adams MWW. 2014. Metallomics of two microorganisms relevant to heavy metal bioremediation reveal fundamental differences in metal assimilation and utilization. Metallomics 6:1004–1013. doi: 10.1039/c4mt00050a. [DOI] [PubMed] [Google Scholar]
- 63.Hartmann A, Braun V. 1981. Iron uptake and iron limited growth of Escherichia coli K-12. Arch Microbiol 130:353–356. doi: 10.1007/BF00414599. [DOI] [PubMed] [Google Scholar]
- 64.Abdul-Tehrani H, Hudson AJ, Chang YS, Timms AR, Hawkins C, Williams JM, Harrison PM, Guest JR, Andrews SC. 1999. Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J Bacteriol 181:1415–1428. doi: 10.1128/JB.181.5.1415-1428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma JF, Ochsner UA, Klotz MG, Nanayakkara VK, Howell ML, Johnson Z, Posey JE, Vasil ML, Monaco JJ, Hassett DJ. 1999. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J Bacteriol 181:3730–3742. doi: 10.1128/JB.181.12.3730-3742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 67.Pinchuk GE, Hill EA, Geydebrekht OV, de Ingeniis J, Zhang X, Osterman A, Scott JH, Reed SB, Romine MF, Konopka AE, Beliaev AS, Fredrickson JK, Reed JL. 2010. Constraint-based model of Shewanella oneidensis MR-1 metabolism: a tool for data analysis and hypothesis generation. PLoS Comput Biol 6:e1000822-8. doi: 10.1371/journal.pcbi.1000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.02593-22-s0001.pdf, PDF file, 0.3 MB (276.5KB, pdf)
Data Availability Statement
The sequencing data we used here are a subset of data analyzed in a previous publication, and raw sequence data are available from NCBI under accession number GSE200066 (60).