ABSTRACT
Usutu virus (USUV, Flaviviridae) is an emerging mosquito-borne virus that has been implicated in neuroinvasive disease in humans and epizootic deaths in wild birds. USUV is maintained in an enzootic cycle between ornithophilic mosquitoes, primarily Culex spp., and wild birds, predominantly passerine species. However, limited experimental data exist on the species competent for USUV transmission. Here, we demonstrate that house sparrows are susceptible to multiple USUV strains. Our study also revealed that Culex quinquefasciatus mosquitoes are susceptible to USUV, with a significantly higher infection rate for the Netherlands 2016 USUV strain compared to the Uganda 2012 USUV strain at 50% and 19%, respectively. To assess transmission between avian host and mosquito vector, we allowed mosquitoes to feed on either juvenile chickens or house sparrows inoculated with USUV. Both bird models transmitted USUV to C. quinquefasciatus mosquitoes. Linear regression analyses indicated that C. quinquefasciatus infection rates were positively correlated with avian viremia levels, with 3 to 4 log10 PFU/mL representing the minimum avian viremia threshold for transmission to mosquitoes. Based on the viremia required for transmission, house sparrows were estimated to more readily transmit the Netherlands 2016 strain compared to the Uganda 2012 strain. These studies provide insights on a competent reservoir host of USUV.
IMPORTANCE Usutu virus (USUV) is a zoonotic mosquito-borne virus that can cause neuroinvasive disease, including meningitis and encephalitis, in humans and has resulted in hundreds of thousands of deaths in wild birds. The perpetuation of USUV in nature is dependent on transmission between Culex spp. mosquitoes and various avian species. To date, few experimental data exist for determining which bird species are important for the maintenance of USUV. Our studies showed that house sparrows can transmit infectious Usutu virus, indicating their role as a competent host species. By identifying reservoir species of USUV, we can predict areas of USUV emergence and mitigate its impacts on global human and wildlife health.
KEYWORDS: arbovirus, house sparrow, mosquito, transmission
INTRODUCTION
Usutu virus (USUV) is an emerging mosquito-borne virus that requires an ornithophilic mosquito vector, primarily Culex spp., and an avian host for its viral maintenance (1, 2). As a member of the Japanese encephalitis serocomplex of the Flaviviridae family, USUV shares similar antigenic properties with its other members, such as West Nile virus (WNV) and St. Louis encephalitis virus (SLEV) (3–5). Originally isolated in South Africa in 1959 (6), USUV is now emerging in Europe (7). USUV is zoonotic and is associated with neuroinvasive disease in humans. Acute USUV infections in humans have been reported in approximately 80 individuals in Europe and Africa (8–21).
Not only is USUV of significance for human public health, but it is also a major concern for wildlife health. USUV causes disease in wild bird populations, where the burden of death is greatest in the Eurasian blackbird (Turdus merula). The earliest indication of USUV circulating in European wild bird populations occurred in 2001 in Austria (22), though retrospective analysis suggests that USUV was circulating 5 years earlier in Italy (23). Since its establishment in Europe, USUV has been implicated in the decline of breeding bird populations (24, 25). In epizootic events between 2003 and 2012, blackbird die-offs ranged from roughly 1,000 in Italy (26) to approximately 50,000 in Austria (27), and to upwards of 400,000 in Germany (24). Deaths have also been reported in great gray owls (Strix nebulosa) (25) and house sparrows (Passer domesticus) (28). While it is apparent that birds in the orders Passeriformes or Strigiformes are more vulnerable to a fatal outcome, USUV has been detected in birds from 17 additional orders, representing over 100 different species found in Africa or Europe (1, 15, 29–32). Although USUV infection has been reported in a diverse array of species, the species which serve as reservoir hosts, critical for viral transmission, have yet to be determined.
Most studies documenting naturally infected mosquitoes in Africa and Europe have predominantly reported on Culex spp. mosquitoes, including C. neavei (33), C. modestus (34), C. perexiguus (35), C. univitattus (36), and C. pipiens (37, 38). USUV has also been detected in Aedes spp. (2), Ochlerotatus spp. (37), and Mansonia spp. (6) mosquitoes. European colonies of C. pipiens were shown to be highly competent for USUV when fed an infectious bloodmeal with the Bologna 2009 or Netherlands 2016 USUV strains (39, 40). C. pipiens and C. quinquefasciatus colonies from North America which fed on an infectious bloodmeal with the prototype USUV strain, South Africa 1959, were both susceptible to infection (41). Another study using a South African lab colony of C. quinquefasciatus reported transmission in saliva (42). USUV infection and transmission has also been described in other mosquito species, including C. neavei (43) and Aedes japonicus (44). While numerous studies have shed light on the important potential vector species competent for USUV using artificial bloodmeals, none to date have assessed their infection potential following exposure to an infected avian host.
Passerine species were identified as the major reservoir hosts for WNV and SLEV through experimentation demonstrating transmission from viremic birds to Culex spp. mosquitoes (45, 46). Due to the challenges associated with assessing viral transmission from wild avian species to mosquitoes, limited experimental data exist and thus models have been developed based on one or two species to estimate the likelihood that other avian species will serve as a reservoir host, per the reservoir competence index (45, 47). In particular, juvenile chickens have been used as models in flavivirus transmission experiments to determine the minimum infectious threshold of transmitting virus from avian host to mosquito (46, 48, 49). The reservoir competence index (C) is the product of three components: host susceptibility to infection (s); host infectiousness (i), or the estimated proportion of mosquitoes that become infected after feeding on a host; and the duration of host infectiousness (d), or how long the host sustains an infectious viremia level (50). Based on these studies, house sparrows and other passerine species have been defined as competent reservoir species for WNV (45).
The aim of this study was to determine whether North American house sparrows are competent for USUV. The house sparrow, an invasive species in the United States that was originally introduced from Europe, is a competent host for WNV (45). House sparrows experimentally inoculated with multiple strains of USUV were susceptible and reached high viremia levels. To determine the level of avian viremia required for transmission, C. quinquefasciatus mosquitoes were fed upon USUV-infected juvenile chickens or house sparrows. USUV was transmitted from birds to mosquitoes, with significantly higher predicted mosquito infection rates for a European USUV strain compared to an African USUV strain. These experiments provide much needed insights on identifying reservoir host species of USUV.
RESULTS
Susceptibility of C. quinquefasciatus mosquitoes to USUV.
The susceptibility and vector competence of C. quinquefasciatus for USUV was evaluated through artificial bloodmeals spiked with either Netherlands 2016 or Uganda 2012 USUV strains. Bloodmeals were back-titrated, indicating that mosquitoes were exposed to 7.5 and 7.4 log10 PFU/mL for Netherlands 2016 and Uganda 2012, respectively. Fifty percent (30/60) of mosquitoes exposed to Netherlands 2016 were infected, a significantly higher proportion compared to the 19% (13/68) of mosquitoes that were infected following exposure to Uganda 2012 (Fisher’s exact test, P = 0.0003, Fig. 1A). Additionally, Netherlands 2016-exposed mosquitoes had significantly higher titers in body homogenates relative to Uganda 2012-exposed mosquitoes (P = 0.0045, Fig. 1B). Both groups had comparable dissemination rates: 1.7% (1/60) of mosquitoes exposed to Netherlands 2016 and 1.5% (1/68) of those exposed to Uganda 2012 had detectable virus in the legs/wings (Fig. 1A) with similar titers (Fig. 1C). Limited transmission potential in saliva was observed. However, infectious virus was detected in one saliva sample (1.7%) from a Netherlands 2016-exposed mosquito, with a titer of 4.1 log10 PFU/mosquito, but not in Uganda 2012-exposed mosquito saliva samples (0/68) (Fig. 1A and D). Together these data indicate that C. quinquefasciatus mosquitoes are susceptible to USUV infection, though only a weakly competent vector.
FIG 1.
Culex quinquefasciatus mosquitoes are susceptible to Usutu virus (USUV) infection. (A) Proportion of mosquitoes found positive for infectious USUV out of the total number of mosquitoes that were fed on a bloodmeal containing the Netherlands 2016 or Uganda 2012 USUV strain. Mosquito bodies represent infection rates; legs and wings represent dissemination rates; saliva represents transmission rates. Fisher’s exact test was used to compare proportions. ***, P < 0.001. (B) Viral titers in mosquito bodies. (C) Viral titers in mosquito legs and wings. (D) Viral titers in mosquito saliva. Circles represent individual samples; horizontal lines represent the mean; error bars represent standard deviation. Limit of detection (LOD) is represented by the dashed line. Mann-Whitney test was used to compare viruses. **, P < 0.01.
A chicken model for USUV enzootic transmission.
To assess USUV transmission between C. quinquefasciatus and an avian host, mosquitoes were fed on individual low antibody response (LAS) chicks inoculated 2 days prior with 1,500 PFU USUV, an avian model of infection previously established by our lab (51). Chicks inoculated with Netherlands 2016 developed significantly lower viremia on 2 days postinoculation (dpi) than those inoculated with Uganda 2012, with means of 3.2 and 4.2 log10 PFU/mL, respectively (P = 0.0286, Fig. 2A). Transmission of USUV from chicks to mosquitoes was evident, with infectious virus detected in 6% (7/116) of mosquitoes that fed upon Netherlands 2016-infected birds and 8% (8/101) of those that fed upon Uganda 2012-infected birds (Fig. 2B). There was no significant difference in the mean titer of mosquito body homogenates between virus strains (Fig. 2C).
FIG 2.
Low antibody response (LAS) chicken model transmits USUV to C. quinquefasciatus mosquitoes. (A) Viremia in LAS chicks inoculated with Netherlands 2016 or Uganda 2012 USUV strain. Circles represent individual samples; horizontal lines represent the mean; error bars represent standard deviation. Limit of detection is represented by the dashed line. Mann-Whitney test was used to compare viruses. *, P < 0.05. (B) Proportion of mosquito bodies positive for infectious USUV out of the total number of mosquitoes fed on either Netherlands 2016-inoculated or Uganda 2012-inoculated LAS chicks. Fisher’s exact test was used to compare proportions; no significance was observed. (C) USUV titers in mosquito bodies fed on USUV-inoculated LAS chicks. Circles represent individual samples; horizontal lines represent the mean; error bars represent standard deviation. Limit of detection is represented by the dashed line. Mann-Whitney test was used to compare viruses; no significance was observed.
House sparrows are susceptible to African and European USUV strains.
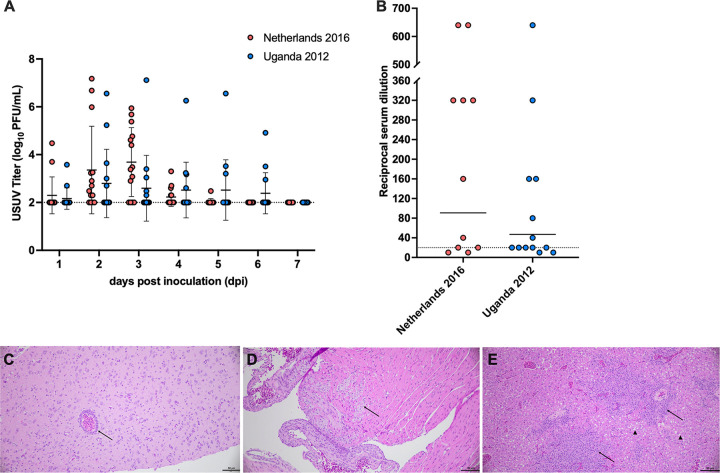
To better understand enzootic transmission of USUV, we established another avian model of infection using house sparrows. Wild-caught house sparrows, confirmed to be seronegative for WNV, were divided into two groups (n = 14) and subcutaneously inoculated with 1,500 PFU of USUV strain Netherlands 2016 or Uganda 2012. Viremia was measured daily for 7 days following inoculation; 89.3% of house sparrows became viremic. The mean peak titer for the Netherlands 2016-inoculated group occurred at 3 dpi and was 3.7 log10 PFU/mL (Fig. 3A). The mean peak titer for the Uganda 2012-inoculated group occurred on 2 dpi and was 2.8 log10 PFU/mL. Multiple individuals in both experimental groups had titers at or above 5 log10 PFU/mL at multiple time points. At 6 dpi, one individual from the Uganda 2012-inoculated group exhibited clinical signs of illness, including ruffled feathers, lethargy, and poor responsiveness, and was euthanized immediately.
FIG 3.
House sparrows are susceptible to African and European USUV strains. (A) Viremia in house sparrows inoculated with Netherlands 2016 or Uganda 2012 USUV strain. Circles represent individual samples; horizontal lines represent the mean; error bars represent standard deviation. Limit of detection is represented by the dashed line. Multiple Mann-Whitney tests using the Holm-Sidak method were used to compare viruses; no differences were observed. (B) Neutralizing antibody response was measured by plaque reduction neutralization test (PRNT); samples were considered positive if they reached the 90% reduction threshold (PRNT90). Highest reciprocal serum titer where 90% reduction was reached is shown; circles represent individual serum samples. The geometric mean titer (GMT) of each group is represented by a solid line. Samples that did not reach 90% reduction threshold were considered negative and graphed at half-LOD, 10. A Mann-Whitney test was used to compare GMTs; no significance was observed. (C) Brain tissue collected on 14 dpi from house sparrow inoculated with Netherlands 2016 USUV strain, with perivascular cuffing (arrow) (hematoxylin and eosin [H&E] stain). (D) Heart tissues collected on 14 dpi from house sparrow inoculated with Uganda 2012 USUV strain, with foci of inflammation and myocardial degeneration (arrow) (H&E stain). (E) Liver tissue from house sparrow inoculated with Uganda 2012 USUV strain, with lesions of lymphocytic inflammation (arrow) and hepatic degeneration (arrowhead) (H&E stain). Scale bars = 50 μm.
A final blood sample was collected on 14 dpi to assess for seroconversion by plaque reduction neutralization test (PRNT). One Uganda 2012-inoculated house sparrow never developed viremia, but did neutralize USUV at a titer of 20, indicating exposure to the virus. Thus, we observed a 92.9% infection rate in house sparrows. There was no significant difference in the 90% PRNT (PRNT90) titers between strains (Fig. 3B). These data indicate that, when exposed to USUV, house sparrows develop a robust neutralizing antibody response.
Tissue samples were collected from a subset of birds on 14 dpi to evaluate for microscopic evidence of USUV infection. Perivascular cuffing was observed in the brain of one house sparrow inoculated with the Netherlands 2016 USUV strain (Fig. 3C). A region of inflammation, as evidenced by lymphocytic infiltrates and myocardial degeneration, was observed in the heart tissue of a bird inoculated with the Uganda 2012 USUV strain (Fig. 3D). Multifocal lymphocytic infiltrates and hepatic degeneration were observed in a Uganda 2012-inoculated house sparrow (Fig. 3E). Together, these results demonstrate that house sparrows may be an appropriate model to investigate enzootic transmission between an avian host and mosquitoes.
House sparrows are competent hosts of USUV and exhibit a higher predicted mosquito infectiousness for a European USUV strain.
In a similar experimental setup to that for the LAS chickens, mosquitoes were fed upon USUV-inoculated house sparrows on 2 dpi to assess USUV transmission. There was a 22.2% (6/27) infection rate in mosquitoes that fed upon Netherland 2016-inoculated house sparrows and a 29.4% (5/17) infection rate in those that fed upon Uganda 2012-inoculated house sparrows (Fig. 4A). No significant difference in the mean titers of mosquito body homogenates was detected between groups, and titers were consistent with the mean body titers of mosquitoes that fed on LAS chicks (Fig. 4B). These results indicate that the house sparrow is a competent species for USUV transmission.
FIG 4.

House sparrows transmit USUV to C. quinquefasciatus mosquitoes. (A) Proportion of mosquito bodies positive for USUV out of the total number of mosquitoes fed on either Netherlands 2016-inoculated or Uganda 2012-inoculated house sparrows. Fisher’s exact test was used to compare proportions; no significance was observed. (B) USUV titers in mosquito bodies fed on USUV-inoculated house sparrows. Circles represent individual samples; horizontal lines represent the mean; error bars represent standard deviation. Limit of detection is represented by the dashed line. Mann-Whitney test was used to compare viruses; no significant difference was observed.
To determine whether there was a correlation between USUV titers in avian blood samples and mosquito infection rates, a linear regression analysis was performed (see Materials and Methods). Chicken and house sparrow data were grouped by virus strain to compare avian viremia titers with their respective mosquito infection rates. For both virus strains, we found a positive relationship between avian viremia titer and mosquito infection rate (Fig. 5A, P = 0.0095; Fig. 5B, P = 0.0097). Furthermore, we observed that birds with a minimum of 2.7 log10 PFU/mL viral titer and those with a minimum of 3.7 log10 PFU/mL viral titer were capable of transmitting Netherlands 2016 and Uganda 2012, respectively, to C. quinquefasciatus mosquitoes, although this difference was not significant (P = 0.44). Together, these data suggest that avian viremia levels play a crucial role in determining the proportion of infected vectors and ultimately the likelihood of USUV transmission.
FIG 5.
Estimated USUV infectiousness relative to avian viremia level. (A) Linear regression analysis of Netherlands 2016 avian viremia titer (log10 PFU/mL) compared to C. quinquefasciatus mosquito USUV infection rates. (B) Linear regression analysis of Uganda 2012 avian viremia titer (log10 PFU/mL) compared to C. quinquefasciatus mosquito USUV infection rates. Open circles represent mosquitoes that fed on LAS chicks; closed circles represent mosquitoes that fed on house sparrows. Dashed lines represent 95% confidence intervals. (C) Estimated daily infectiousness of house sparrows inoculated with either Netherlands 2016 or Uganda 2012 over the course of 7 days. Circles represent individual samples; horizontal lines represent the mean; error bars represent standard deviation. Multiple Mann-Whitney tests using the Holm-Sidak method were used to compare viruses. **, P < 0.01.
The estimated daily mosquito infectiousness, or the proportion of mosquitoes that are predicted to become infected as a result of feeding on a host, was calculated for all house sparrows based on the linear regression equation (45, 47). There was a significantly higher estimated daily infectiousness for Netherlands 2016-inoculated house sparrows compared to Uganda 2012-inoculated house sparrows on 3 dpi (Fig. 5C, P = 0.0036). This indicates that, when applied to animals on a population level, Uganda 2012-inoculated house sparrows are predicted to infect fewer mosquitoes due to lower average viremias. To further understand the role of house sparrows as USUV reservoir hosts, we calculated the overall reservoir competence index (the product of host susceptibility, host infectiousness, and duration of host infectiousness) (50). The reservoir competence index was 0.27 for house sparrows inoculated with Netherlands 2016 and 0.17 for those inoculated with Uganda 2012. This indicates that 27% of mosquitoes that feed on house sparrows infected with Netherlands 2016 are expected to become infected, while 17% of mosquitoes that feed on house sparrows infected with Uganda 2012 are expected to become infected.
DISCUSSION
Here, we showed that house sparrows are susceptible to multiple strains of USUV and can transmit it to Culex spp. mosquitoes, indicating their potential role as a reservoir host for the virus. Moreover, we showed that a 2.7 to 3.7 log10 PFU/mL viremia was sufficient to transmit USUV to C. quinquefasciatus mosquitoes. Finally, we found that house sparrows infected with the Netherlands 2016 strain are predicted to be more infectious to mosquitoes than house sparrows infected with Uganda 2012 strain.
Our results suggest that the house sparrow has the potential to serve as a reservoir host for USUV due to its susceptibility to infection and ability to transmit multiple USUV strains to Culex spp. mosquitoes. USUV infection has been reported in naturally infected house sparrows in Austria (52), Switzerland (28), and recently in the United Kingdom, where viral antigen was detected in neurons and endothelial cells in the brain and in renal tubular epithelial cells in the kidney (53). In our study, microscopic evidence of USUV disease in house sparrows, including inflammatory and immune cell infiltrates in the brain, heart, and liver, is consistent with reports of disease in USUV-positive wild birds in Europe (28, 54, 55). Therefore, experimentally inoculated house sparrows present similar signs of disease as naturally infected house sparrows. Additionally, the lack of clinical signs in our experimentally inoculated house sparrows is supportive of reservoir host potential. Furthermore, house sparrows have been implicated as epidemiologically important species for SLEV and WNV (56, 57). With Culex spp. mosquitoes serving as competent vectors for USUV (39) and house sparrows serving as a common bloodmeal source of Culex mosquitoes in the United States (58–60), it is possible that house sparrows could maintain USUV transmission in locations where Culex spp. are present. Thus, we have identified the house sparrow as a relevant USUV reservoir species.
Our study demonstrated a significant relationship between mosquito infection rate and viral load in avian host blood. This correlation has been demonstrated in other bird-mosquito enzootic transmission systems. For example, the infection rates of C. pipiens fed upon WNV-inoculated juvenile chickens (48), C. tarsalis fed upon WNV-inoculated house sparrows, and C. quinquefasciatus fed upon SLEV-inoculated juvenile chickens (46) were dependent on avian viremia titers. Additionally, artificial bloodmeal titers have a dose-dependent effect on Culex spp. mosquito infection rates, as has been reported for USUV (43), WNV (46, 61), and SLEV (46). It is evident that viral blood titer influences the proportion of Culex spp. mosquitoes which acquire infection. One study demonstrated that the proportion of infected mosquitoes was typically greater after feeding on live avian hosts compared to that after feeding on an artificial bloodmeal, at similar or even lower titers (46), which is consistent with our study. One caveat to our study is that we did not measure the transmission potential of USUV in saliva from mosquitoes fed on infected birds. Additional experimentation to determine whether feeding on live birds increases transmission rates in mosquitoes is warranted, which has also been demonstrated by Reisen et al. (46).
Our data suggests that the USUV minimum infectious threshold for C. quinquefasciatus mosquitoes is 3 to 4 log10 PFU/mL, which is similar to the minimum infectious thresholds of 4 to 5 log10 PFU/mL and 2 to 3 log10 PFU/mL for WNV (45) and SLEV (46), respectively. However, the competence of house sparrows for USUV appears to be lower than that for WNV (45, 47). We may have underestimated the magnitude of the host competence of house sparrows for USUV due to the use of North American C. quinquefasciatus mosquitoes, which have weak vector competence for USUV. Other groups have also found low vector competence for C. quinquefasciatus mosquitoes (41, 42). Other mosquitoes that are more competent for USUV should be tested in future studies. For example, while we observed a 1.7% transmission rate in C. quinquefasciatus mosquitoes feeding on an infectious bloodmeal containing Netherlands 2016 USUV strain, a European C. pipiens colony was shown to have a 15% transmission rate feeding on the same strain at a comparable bloodmeal titer (5 × 106 TCID50 [50% tissue culture infective dose]/mL) (40). C. pipiens feeding on an infectious bloodmeal containing the Bologna 2009 strain demonstrated a much higher transmission rate, with infectious saliva detected in 69% of mosquitoes (39). Using a more competent Culex spp. mosquito will be essential in future experiments to obtain an accurate measurement of host competence. To further elucidate the species of importance for USUV maintenance, assessing the susceptibility of other potential host species is necessary. Our minimum infectious threshold can then be applied to additional species to determine reservoir competence. However, there are limitations to all models: for example, a minimum infectious threshold model may underestimate the impact abundant hosts with lower viremia levels have on the maintenance of virus in the environment (62). Nonetheless, identifying potential reservoir species serves as a tool for predicting possible spread and emergence of USUV.
By comparing virus strains from Europe and Africa, our results can shed light on the possible impact of viral evolution on transmission. A significant difference between the estimated mosquito infectiousness on 3 dpi was observed between viruses in house sparrows, suggesting that Netherlands 2016-infected house sparrows are more infectious to C. quinquefasciatus on 3 dpi than Uganda 2012-infected house sparrows. Other flaviviruses have evolved to increase potential mosquito infectiousness in birds, including WNV, as evidenced by increased viremia in American crows (Corvus brachyrhynchos) (63, 64) and house sparrows (65), and Japanese encephalitis virus, as evidenced by increased viremia in poultry (66). Our group has previously identified 23 amino acid differences between these two USUV strains (67). Many of the amino acid distinctions occur in the structural proteins, with 8 found in the envelope protein. Residues in the envelope protein have been characterized as virulence factors for numerous flaviviruses (68–70). However, a recent experiment that designed chimeric viruses using segments of the structural and non-structural genes of WNV and SLEV found that the structural genes were not key in regulating differences in virulence and replication in multiple bird species (71). As such, nonstructural genes have also been implicated as virulence factors in flaviviruses (72). Netherlands 2016 and Uganda 2012 also had amino acid differences in NS1, NS2A, NS3, NS4B, and NS5. A previous study demonstrated that at least one site in the NS1, NS3, and NS5 genes in USUV had undergone positive selection (73). Therefore, determining which amino acid differences account for increased viremia is an important avenue of research to explore in order to understand flavivirus emergence and USUV genetic determinants of transmission.
Understanding the geographic range of house sparrows and Culex spp. mosquitoes is essential to predicting where potential USUV transmission may occur. Native to Europe, Asia, and North Africa, house sparrows are now found on every continent except Antarctica and are considered the most widely distributed bird species (74, 75). Mosquitoes in the C. pipiens complex, including C. pipiens and C. quiquefasciatus, are also found on every continent except Antarctica, with C. pipiens being the predominant species in Europe, C. quinquefasciatus predominating in South America, and a mix of both species occuring in North America, Africa, and Asia (76). As such, house sparrows and mosquitoes in the C. pipiens complex are ubiquitous and coupled with the ever-increasing occurrence of flaviviruses across the globe (77), it will be vital to continue monitoring USUV transmission globally.
MATERIALS AND METHODS
Viruses and cells.
The following USUV isolates were used throughout these experiments: TMNetherlands (Netherlands 2016, Europe 3 lineage, MN813490, passage 5, isolated from Turdus merula) (25) and UG09615 (Uganda 2012, Africa 3 lineage, MN813491, passage 3 to 4, isolated from Culex spp.) (36). These strains were previously sequenced by our laboratory (67, 78). Vero cells were cultured in Dulbecco’s modified Eagle medium (Corning) supplemented with 5% fetal bovine serum (FBS, VWR International) and 1% penicillin-streptomycin (Gibco); cells were maintained at 37°C with 5% CO2.
Mosquito experiments.
(i) Mosquito rearing. Culex quinquefasciatus (Say) came from a laboratory colony originally collected in Sebring County, Florida in 1988 (79). Larvae were reared on Tropical First Bites fish food (Hikari Sales USA, Inc.). Adult mosquitoes were provided 10% sucrose ad libitum for maintenance or defibrinated sheep blood (Colorado Serum Company) and 50% sucrose (5% sucrose in total volume) for egg production. Mosquitoes were maintained in an environmental chamber at 26°C to 27°C on a 16:8 light:dark cycle at 60% to 70% relative humidity.
(ii) Artificial infectious blood feeds. Adult C. quinquefasciatus mosquitoes, 6 to 8 days post-emergence, were sorted into cartons with 50 females and 10 males. Mosquitoes were starved for 16 to 18 h prior to an infectious bloodmeal, which was dispensed onto a cotton ball. Mosquitoes were exposed to the cotton ball with 3 mL of blood containing 7.5 and 7.4 log10 PFU/mL of Netherlands 2016 or Uganda 2012, respectively, for 14 to 16 h. Engorged mosquitoes were sorted under cold anesthesia and returned to the carton; unfed mosquitoes were discarded. Mosquitoes were maintained for 14 days with 10% sucrose ad libitum. On day 14 post-exposure, surviving mosquitoes were dissected under cold anesthesia. Legs and wings were removed and stored in 300 μL of mosquito diluent (RPMI 1640 with l-glutamine + 25 mM HEPES [Gibco], 2% FBS [VWR], 50 μg/mL gentamicin [Genesee Scientific], and 2.5 μg/mL amphotericin B [Gibco]). Forced salivation assays (80) were performed as follows. The mosquito proboscis was inserted into the cut end of a pipette tip containing 10 μL of immersion oil. Mosquitoes were salivated for 60 to 80 min. Following salivation, bodies were removed and stored in 500 μL of mosquito diluent, and the salivary excretions were dispensed into 50 μL of mosquito diluent. All mosquito body, leg, wing, and saliva samples were frozen at −80°C until further processing. Artificial blood feed experiments were performed twice independently.
Chicken experiments.
(i) LAS chicken inoculations. The LAS line, previously characterized as susceptible to USUV infection (51), originated from a common White Leghorn founder population and has been selected for >40 generations for a single trait: low antibody response against sheep red blood cells (81, 82). Ten 1-day-old chicks were randomized into two groups. Groups (n = 5) of LAS chicks were subcutaneously inoculated with 1,500 PFU of the Netherlands 2016 or Uganda 2012 USUV strain. A blood sample was collected by jugular venipuncture on day 2 following inoculation, prior to the mosquito feed.
(ii) Mosquito-LAS chicken infectious feed. Adult C. quinquefasciatus mosquitoes, 7 to 10 days post emergence, were sorted into cartons with 35 to 50 females and 7 to 10 males each. Mosquitoes were starved for 16 to 18 h prior to exposure to USUV-infected chicks.
On 2 dpi, each chick was paired with a carton of mosquitoes. At the time of feeding, chicks were gently restrained against the mesh of the mosquito carton; mosquitoes were allowed to feed on them for 30 min (45, 83). Engorged mosquitoes were sorted under cold anesthesia and returned to the carton. Unfed mosquitoes were discarded. Mosquitoes were maintained for 14 days under previously described conditions. On day 14 post-exposure, surviving mosquito bodies were collected in 500 μL of mosquito diluent and held at −80°C until further processing. Following the mosquito feed, chicks were euthanized by CO2 asphyxiation followed by cervical dislocation.
(iii) Ethics. All experiments were performed in accordance with the Virginia Tech Institutional Animal Care and Use Committee (IACUC no. 21-048). Throughout the experiments, commercial feed and fresh water were provided ad libitum. Chicks were monitored daily for clinical signs by animal care staff and research personnel. If clinical signs including lethargy, ruffled feathers, poor responsiveness, or weight loss of ≥5% were observed, the chick was euthanized via CO2 inhalation followed by cervical dislocation.
House sparrow experiments.
(i) House sparrow inoculations. Twenty-eight house sparrows (Passer domesticus) of mixed sex and age were trapped with mist nets in Blacksburg, VA during 2019 and 2021. House sparrows were housed at up to 7 birds per cage (76.2 cm length × 45.7 cm width × 45.7 cm height). A blood sample was obtained prior to the start of the experiment to determine previous WNV exposure by plaque reduction neutralization test. In groups of 14, birds were subcutaneously inoculated with 1,500 PFU of USUV strain Netherlands 2016 or Uganda 2012. A blood sample was collected daily for 7 days through jugular venipuncture or lateral wing vein. A final blood sample was collected on 14 dpi, upon which all birds were euthanized. House sparrow inoculations were performed in two independent experiments.
(ii) Mosquito-house sparrow infectious feed. Adult C. quinquefasciatus mosquitoes, 4 to 12 days post emergence, were sorted into 6 cartons with 50 females and 10 males each. Mosquitoes were starved for 16 to 18 h prior to exposure to USUV-infected house sparrows. There was high overnight mosquito mortality, so group sizes feeding on infected house sparrows were smaller than expected.
On 2 dpi, a subset of house sparrows from each experimental group (n = 3) was paired with a carton of mosquitoes. House sparrows were gently restrained against the mesh of the mosquito carton and mosquitoes were allowed to feed on them for 30 min. Engorged mosquitoes were sorted under cold anesthesia and returned to the carton and unfed individuals were discarded. Mosquitoes were held in the environmental chamber under previously described conditions for 14 days. On day 14 post-exposure, surviving whole mosquitoes were collected in 500 μL of mosquito diluent and held at −80°C until further processing.
(iii) Ethics. All experiments were performed in accordance with the Virginia Tech Institutional Animal Care and Use Committee (IACUC no. 21-048) and state scientific collection permit (VADGIF permit no. 070947). Throughout the experiments, commercial wild bird feed supplemented with meal worms and fresh water were provided ad libitum. House sparrows were monitored daily for clinical signs by animal care staff and research personnel. If clinical signs such as ruffled feathers, lethargy, or poor responsiveness were observed, the bird was euthanized by CO2 inhalation followed by cervical dislocation.
Viral quantification assays.
Mosquito bodies and legs and wings suspended in mosquito diluent were homogenized in a Qiagen TissueLyserLT at 50 oscillations/sec for 3 min. Samples were clarified by centrifugation at 18,000 rpm for 3 min. Viral titers of serum, mosquito bodies, legs and wings, and saliva were quantified through a Vero cell plaque assay.
PRNT assays.
Blood collected from house sparrows prior to inoculation and at 14 dpi was assayed by plaque reduction neutralization test. Sera were heat-inactivated at 56°C for 30 min and incubated with approximately 100 PFU of WNV or the homologous USUV strain for 1 h at 37°C before plating on Vero cells. Neutralization activity was defined by plaque reduction at a 90% threshold (84).
Histopathology.
After euthanasia, a subset of house sparrows was necropsied on 14 dpi to collect tissues, including brain, heart, and liver. Tissues were kept in 10% neutral-buffered formalin prior to routine processing and paraffin embedding. Sections were cut at 5 μm and stained with hematoxylin and eosin for histopathological analysis. Slides were analyzed by a board-certified veterinary pathologist.
Infectiousness and reservoir competence index.
Using our experimental data from feeding mosquitoes on birds, we calculated a linear regression for the avian blood titer and mosquito infection rate. For Netherlands 2016-inoculated house sparrows, the equation was calculated. For Uganda 2012-inoculated house sparrows, the equation was calculated. The linear regression equation was then applied to the viremia of all house sparrows to estimate the proportion of C. quinquefasciatus mosquitoes predicted to become infected after feeding on each house sparrow, i.e., the estimated daily infectiousness, as described previously (45, 47). The mean estimated daily infectiousness was then determined for each time point (1 to 7 dpi) across all house sparrows, and these values were then summed to calculate the reservoir competence index of house sparrows, as described by Kilpatrick et al. (47).
Statistical analysis.
Data were checked for normality using the Shapiro-Wilk test. Viral titers were compared using a Mann-Whitney test. House sparrow viremia data and estimated daily infectiousness values were analyzed via multiple Mann-Whitney tests using the Holm-Sidak method to adjust for multiple comparisons. Fisher’s exact test was used to compare mosquito infection, dissemination, and transmission rates. Geometric mean titers were also compared using a Mann-Whitney test. All data was analyzed and graphed using GraphPad Quick Calcs and GraphPad Prism 9 (GraphPad Software, San Diego, CA).
ACKNOWLEDGMENTS
Funding for this project was provided by NIH NIAD R21 AI156322 and the Virginia-Maryland College of Veterinary Medicine (VMCVM) Summer Veterinary Student Research Program.
We thank VT Laboratory Animal Research staff for contributions to animal care and husbandry. We thank the VMCVM ViTALS laboratory for tissue processing and staining for microscopy. A special thank you to Greg Ebel for providing the C. quinquefasciatus mosquito colony. Many thanks to Cara C. Drehoff, Nicholas Pietrobono, and Pallavi Rai with assistance during house sparrow and mosquito experiments.
Contributor Information
Nisha K. Duggal, Email: nduggal@vt.edu.
Shirit Einav, Stanford University School of Medicine.
REFERENCES
- 1.Clé M, Beck C, Salinas S, Lecollinet S, Gutierrez S, Van de Perre P, Baldet T, Foulongne V, Simonin Y. 2019. Usutu virus: A new threat? Epidemiol Infect 147:e232. doi: 10.1017/S0950268819001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calzolari M, Bonilauri P, Bellini R, Albieri A, Defilippo F, Maioli G, Galletti G, Gelati A, Barbieri I, Tamba M, Lelli D, Carra E, Cordioli P, Angelini P, Dottori M. 2010. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS One 5:e14324. doi: 10.1371/journal.pone.0014324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis 11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curren EJ, Lindsey NP, Fischer M, Hills SL. 2018. St. Louis encephalitis virus disease in the United States, 2003–2017. Am J Trop Med Hyg 99:1074–1079. doi: 10.4269/ajtmh.18-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmonds P, Becher P, Bukh J, Gould EA, Meyers G, Monath T, Muerhoff S, Pletnev A, Rico-Hesse R, Smith DB, Stapleton JT, Ictv Report Consortium. 2017. ICTV Virus Taxonomy Profile: Flaviviridae. J Gen Virol 98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MC, Simpson DIH, Haddow AJ, Knight EM. 1964. The Isolation of West Nile Virus from Man and of Usutu Virus from the Bird-Biting Mosquito Mansonia Aurites (Theobald) in the Entebbe Area of Uganda. Ann Trop Med Parasitol 58:367–374. doi: 10.1080/00034983.1964.11686258. [DOI] [PubMed] [Google Scholar]
- 7.Vilibic-Cavlek T, Petrovic T, Savic V, Barbic L, Tabain I, Stevanovic V, Klobucar A, Mrzljak A, Ilic M, Bogdanic M, Benvin I, Santini M, Capak K, Monaco F, Listes E, Savini G. 2020. Epidemiology of Usutu Virus: The European Scenario. Pathogens 9:699. doi: 10.3390/pathogens9090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavrini F, Gaibani P, Longo G, Pierro AM, Rossini G, Bonilauri P, et al. 2009. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Euro Surveill. 14 PubMed PMID: 20070935. doi: 10.2807/ese.14.50.19448-en. [DOI] [PubMed] [Google Scholar]
- 9.Cavrini F, Della Pepa ME, Gaibani P, Pierro AM, Rossini G, Landini MP, Sambri V. 2011. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J Clin Virol 50:221–223. doi: 10.1016/j.jcv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Pecorari M, Longo G, Gennari W, Grottola A, Sabbatini A, Tagliazucchi S, et al. 2009. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Euro Surveill. 14 PubMed PMID: 20070936. doi: 10.2807/ese.14.50.19446-en. [DOI] [PubMed] [Google Scholar]
- 11.Grottola A, Marcacci M, Tagliazucchi S, Gennari W, Di Gennaro A, Orsini M, Monaco F, Marchegiano P, Marini V, Meacci M, Rumpianesi F, Lorusso A, Pecorari M, Savini G. 2017. Usutu virus infections in humans: a retrospective analysis in the municipality of Modena, Italy. Clin Microbiol Infect 23:33–37. doi: 10.1016/j.cmi.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Vilibic-Cavlek T, Savic V, Sabadi D, Peric L, Barbic L, Klobucar A, Miklausic B, Tabain I, Santini M, Vucelja M, Dvorski E, Butigan T, Kolaric-Sviben G, Potocnik-Hunjadi T, Balenovic M, Bogdanic M, Andric Z, Stevanovic V, Capak K, Balicevic M, Listes E, Savini G. 2019. Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the 'One health' context, 2018. Transbound Emerg Dis 66:1946–1957. doi: 10.1111/tbed.13225. [DOI] [PubMed] [Google Scholar]
- 13.Santini M, Vilibic-Cavlek T, Barsic B, Barbic L, Savic V, Stevanovic V, Listes E, Di Gennaro A, Savini G. 2015. First cases of human Usutu virus neuroinvasive infection in Croatia, August-September 2013: clinical and laboratory features. J Neurovirol 21:92–97. doi: 10.1007/s13365-014-0300-4. [DOI] [PubMed] [Google Scholar]
- 14.Simonin Y, Sillam O, Carles MJ, Gutierrez S, Gil P, Constant O, Martin MF, Girard G, Van de Perre P, Salinas S, Leparc-Goffart I, Foulongne V. 2018. Human Usutu Virus Infection with Atypical Neurologic Presentation, Montpellier, France, 2016. Emerg Infect Dis 24:875–878. doi: 10.3201/eid2405.171122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolay B, Diallo M, Boye CS, Sall AA. 2011. Usutu virus in Africa. Vector Borne Zoonotic Dis 11:1417–1423. doi: 10.1089/vbz.2011.0631. [DOI] [PubMed] [Google Scholar]
- 16.Pacenti M, Sinigaglia A, Martello T, De Rui ME, Franchin E, Pagni S, Peta E, Riccetti S, Milani A, Montarsi F, Capelli G, Doroldi CG, Bigolin F, Santelli L, Nardetto L, Zoccarato M, Barzon L. 2019. Clinical and virological findings in patients with Usutu virus infection, northern Italy, 2018. Eurosurveillance 24:1900180. doi: 10.2807/1560-7917.ES.2019.24.47.1900180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caracciolo I, Mora-Cardenas E, Aloise C, Carletti T, Segat L, Burali MS, Chiarvesio A, Totis V, Avšič-Županc T, Mastrangelo E, Manfroni G, D'Agaro P, Marcello A. 2020. Comprehensive response to Usutu virus following first isolation in blood donors in the Friuli Venezia Giulia region of Italy: Development of recombinant NS1-based serology and sensitivity to antiviral drugs. PLoS Negl Trop Dis 14:e0008156. doi: 10.1371/journal.pntd.0008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percivalle E, Cassaniti I, Sarasini A, Rovida F, Adzasehoun KMG, Colombini I, Isernia P, Cuppari I, Baldanti F. 2020. West Nile or Usutu virus? A three-year follow-up of humoral and cellular response in a group of asymptomatic blood donors. Viruses 12:157. doi: 10.3390/v12020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy A, Mezei E, Nagy O, Bakonyi T, Csonka N, Kaposi M, Koroknai A, Szomor K, Rigó Z, Molnár Z, Dánielisz Á, Takács M. 2019. Extraordinary increase in West Nile virus cases and first confirmed human Usutu virus infection in Hungary, 2018. Euro Surveill. 24:1900038. doi: 10.2807/1560-7917.ES.2019.24.28.1900038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaaijer HL, Slot E, Molier M, Reusken CBEM, Koppelman MHGM. 2019. Usutu virus infection in Dutch blood donors. Transfusion 59:2931–2937. doi: 10.1111/trf.15444. [DOI] [PubMed] [Google Scholar]
- 21.Carletti F, Colavita F, Rovida F, Percivalle E, Baldanti F, Ricci I, De Liberato C, Rosone F, Messina F, Lalle E, Bordi L, Vairo F, Capobianchi MR, Ippolito G, Cappiello G, Spanò A, Meschi S, Castilletti C. 2019. Expanding Usutu virus circulation in Italy: detection in the Lazio region, central Italy, 2017 to 2018. Euro Surveill. 24:1800649. doi: 10.2807/1560-7917.ES.2019.24.3.1800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissenbock H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. 2002. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis 8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissenbock H, Bakonyi T, Rossi G, Mani P, Nowotny N. 2013. Usutu virus, Italy, 1996. Emerg Infect Dis 19:274–277. doi: 10.3201/eid1902.121191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lühken R, Jöst H, Cadar D, Thomas SM, Bosch S, Tannich E, Becker N, Ziegler U, Lachmann L, Schmidt-Chanasit J. 2017. Distribution of Usutu virus in Germany and its effect on breeding bird populations. Emerg Infect Dis 23:1994–2001. doi: 10.3201/eid2312.171257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijks JM, Kik ML, Slaterus R, Foppen R, Stroo A, Ijzer J, Stahl J, Gröne A, Koopmans M, van der Jeugd HP, Reusken C. 2016. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Euro Surveill. 21:30391. doi: 10.2807/1560-7917.ES.2016.21.45.30391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savini G, Monaco F, Terregino C, Di Gennaro A, Bano L, Pinoni C, De Nardi R, Bonilauri P, Pecorari M, Di Gialleonardo L, Bonfanti L, Polci A, Calistri P, Lelli R. 2011. Usutu virus in Italy: an emergence or a silent infection? Vet Microbiol 151:264–274. doi: 10.1016/j.vetmic.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Chvala S, Bakonyi T, Bukovsky C, Meister T, Brugger K, Rubel F, Nowotny N, Weissenböck H. 2007. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Vet Microbiol 122:237–245. doi: 10.1016/j.vetmic.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz HW, Bakonyi T, Weissenböck H, Hatt J-M, Eulenberger U, Robert N, Hoop R, Nowotny N. 2011. Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland: Genomic and pathologic comparison to other central European outbreaks. Vet Microbiol 148:207–212. doi: 10.1016/j.vetmic.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Benzarti E, Linden A, Desmecht D, Garigliany M. 2019. Mosquito-borne epornitic flaviviruses: an update and review. J Gen Virol 100:119–132. doi: 10.1099/jgv.0.001203. [DOI] [PubMed] [Google Scholar]
- 30.Nikolay B. 2015. A review of West Nile and Usutu virus co-circulation in Europe: how much do transmission cycles overlap? Trans R Soc Trop Med Hyg 109:609–618. doi: 10.1093/trstmh/trv066. [DOI] [PubMed] [Google Scholar]
- 31.Benzarti E, Garigliany M, Hauman D, Paternostre J, Linden A, Franssen M, Sarlet M, Cassart D, Desmecht D. 2019. First evidence of fatal Usutu virus natural infections in an Anatidae, the Common Scoter (Melanitta nigra). Vector Borne Zoonotic Dis 19:777–780. doi: 10.1089/vbz.2019.2460. [DOI] [PubMed] [Google Scholar]
- 32.Weidinger P, Kolodziejek J, Bakonyi T, Brunthaler R, Erdélyi K, Weissenböck H, Nowotny N. 2020. Different dynamics of Usutu virus infections in Austria and Hungary, 2017–2018. Transbound Emerg Dis 67:298–307. doi: 10.1111/tbed.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ndiaye EH, Diallo D, Fall G, Ba Y, Faye O, Dia I, Diallo M. 2018. Arboviruses isolated from the Barkedji mosquito-based surveillance system, 2012–2013. BMC Infect Dis 18:642. doi: 10.1186/s12879-018-3538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolf I, Bakonyi T, Šebesta O, Mendel J, Peško J, Betášová L, Blažejová H, Venclíková K, Straková P, Nowotny N, Hubálek Z. 2015. Co-circulation of Usutu virus and West Nile virus in a reed bed ecosystem. Parasit Vectors 8:520. doi: 10.1186/s13071-015-1139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mannasse B, Mendelson E, Orshan L, Mor O, Shalom U, Yeger T, Lustig Y. 2017. Usutu virus RNA in mosquitoes, Israel, 2014–2015. Emerg Infect Dis 23:1699–1702. doi: 10.3201/eid2310.171017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mossel EC, Crabtree MB, Mutebi J-P, Lutwama JJ, Borland EM, Powers AM, Miller BR. 2017. Arboviruses isolated from mosquitoes collected in Uganda, 2008–2012. J Med Entomol 54:1403–1409. doi: 10.1093/jme/tjx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancini G, Montarsi F, Calzolari M, Capelli G, Dottori M, Ravagnan S, Lelli D, Chiari M, Santilli A, Quaglia M, Quaglia M, Federici V, Monaco F, Goffredo M, Savini G. 2017. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet Ital 53:97–110. doi: 10.12834/VetIt.114.933.4764.2. [DOI] [PubMed] [Google Scholar]
- 38.Busquets N, Alba A, Allepuz A, Aranda C, Ignacio Nuñez J. 2008. Usutu virus sequences in Culex pipiens (Diptera: Culicidae), Spain. Emerg Infect Dis 14:861–863. doi: 10.3201/eid1405.071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fros JJ, Miesen P, Vogels CB, Gaibani P, Sambri V, Martina BE, Koenraadt CJ, van Rij RP, Vlak JM, Takken W, Pijlman GP. 2015. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health 1:31–36. doi: 10.1016/j.onehlt.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Abbo SR, Visser TM, Westenberg M, Geertsema C, Fros JJ, Koenraadt CJM, Pijlman GP. 2020. Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes. Emerg Microbes Infect 9:2642–2652. doi: 10.1080/22221751.2020.1854623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook CL, Huang Y-JS, Lyons AC, Alto BW, Unlu I, Higgs S, Vanlandingham DL. 2018. North American Culex pipiens and Culex quinquefasciatus are competent vectors for Usutu virus. PLoS Negl Trop Dis 12:e0006732. doi: 10.1371/journal.pntd.0006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bates TA, Chuong C, Rai P, Marano J, Waldman A, Klinger A, Reinhold JM, Lahondère C, Weger-Lucarelli J. 2021. American Aedes japonicus japonicus, Culex pipiens pipiens, and Culex restuans mosquitoes have limited transmission capacity for a recent isolate of Usutu virus. Virology 555:64–70. doi: 10.1016/j.virol.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Nikolay B, Diallo M, Faye O, Boye CS, Sall AA. 2012. Vector competence of Culex neavei (Diptera: Culicidae) for Usutu virus. Am J Trop Med Hyg 86:993–996. doi: 10.4269/ajtmh.2012.11-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbo SR, Visser TM, Wang H, Göertz GP, Fros JJ, Abma-Henkens MHC, Geertsema C, Vogels CBF, Koopmans MPG, Reusken CBEM, Hall-Mendelin S, Hall RA, van Oers MM, Koenraadt CJM, Pijlman GP. 2020. The invasive Asian bush mosquito Aedes japonicus found in the Netherlands can experimentally transmit Zika virus and Usutu virus. PLoS Negl Trop Dis 14:e0008217. doi: 10.1371/journal.pntd.0008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reisen WK, Fang Y, Martinez VM. 2005. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol 42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- 47.Kilpatrick AM, LaDeau SL, Marra PP. 2007. Ecology of West Nile virus transmission and its impact on birds in the Western Hemisphere. The Auk 124:1121–1136. doi: 10.1093/auk/124.4.1121. [DOI] [Google Scholar]
- 48.Turell MJ, Guinn M, Oliver J. 2000. Potential for New York mosquitoes to transmit West Nile virus. Am J Trop Med Hyg 62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- 49.Tiawsirisup S, Platt KB, Evans RB, Rowley WA. 2005. A Comparision of West Nile Virus Transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse). Vector Borne Zoonotic Dis 5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- 50.Komar N, Dohm DJ, Turell MJ, Spielman A. 1999. Eastern equine encephalitis virus in birds: relative competence of European starlings (Sturnus vulgaris). Am J Trop Med Hyg 60:387–391. doi: 10.4269/ajtmh.1999.60.387. [DOI] [PubMed] [Google Scholar]
- 51.Kuchinsky SC, Frere F, Heitzman-Breen N, Golden J, Vázquez A, Honaker CF, Siegel PB, Ciupe SM, LeRoith T, Duggal NK. 2021. Pathogenesis and shedding of Usutu virus in juvenile chickens. Emerg Microbes Infect 10:725–738. doi: 10.1080/22221751.2021.1908850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weissenböck H, Kolodziejek J, Fragner K, Kuhn R, Pfeffer M, Nowotny N. 2003. Usutu virus activity in Austria, 2001–2002. Microbes Infect 5:1132–1136. doi: 10.1016/S1286-4579(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 53.Folly AJ, Lawson B, Lean FZ, McCracken F, Spiro S, John SK, Heaver JP, Seilern-Moy K, Masters N, Hernández-Triana LM, Phipps LP, Nuñez A, Fooks AR, Cunningham AA, Johnson N, McElhinney LM. 2020. Detection of Usutu virus infection in wild birds in the United Kingdom, 2020. Eurosurveillance 25:2001732. doi: 10.2807/1560-7917.ES.2020.25.41.2001732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chvala S, Kolodziejek J, Nowotny N, Weissenböck H. 2004. Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J Comp Pathol 131:176–185. doi: 10.1016/j.jcpa.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Manarolla G, Bakonyi T, Gallazzi D, Crosta L, Weissenböck H, Dorrestein GM, Nowotny N. 2010. Usutu virus in wild birds in northern Italy. Vet Microbiol 141:159–163. doi: 10.1016/j.vetmic.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 56.McLean RG, Kirk LJ, Shriner RB, Townsend M. 1993. Avian hosts of St. Louis encephalitis virus in Pine Bluff, Arkansas, 1991. Am J Trop Med Hyg 49:46–52. doi: 10.4269/ajtmh.1993.49.46. [DOI] [PubMed] [Google Scholar]
- 57.Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge R, Langevin SA, Gates R, Lamonte KM, Lambert A, Lanciotti RS, Blackmore CGM, Loyless T, Stark L, Oliveri R, Conti L, Komar N. 2005. West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis 5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- 58.Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. 2009. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg 80:268–278. doi: 10.4269/ajtmh.2009.80.268. [DOI] [PubMed] [Google Scholar]
- 59.Thiemann TC, Wheeler SS, Barker CM, Reisen WK. 2011. Mosquito host selection varies seasonally with host availability and mosquito density. PLoS Negl Trop Dis 5:e1452. doi: 10.1371/journal.pntd.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komar N, Panella NA, Young GR, Brault AC, Levy CE. 2013. Avian hosts of West Nile virus in Arizona. Am J Trop Med Hyg 89:474–481. doi: 10.4269/ajtmh.13-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richards SL, Mores CN, Lord CC, Tabachnick WJ. 2007. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis 7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lord CC, Rutledge CR, Tabachnick WJ. 2006. Relationships between host viremia and vector susceptibility for arboviruses. J Med Entomol 43:623–630. doi: 10.1093/jmedent/43.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brault AC, Huang CY-H, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM, Miller BR. 2007. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet 39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nielsen CF, Reisen WK. 2007. West Nile virus-infected dead corvids increase the risk of infection in Culex mosquitoes (Diptera: Culicidae) in domestic landscapes. J Med Entomol 44:1067–1073. doi: 10.1093/jmedent/44.6.1067. [DOI] [PubMed] [Google Scholar]
- 65.Duggal NK, Bosco-Lauth A, Bowen RA, Wheeler SS, Reisen WK, Felix TA, Mann BR, Romo H, Swetnam DM, Barrett ADT, Brault AC. 2014. Evidence for co-evolution of West Nile virus and house sparrows in North America. PLoS Negl Trop Dis 8:e3262. doi: 10.1371/journal.pntd.0003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan Y-C, Liang J-J, Chen J-M, Lin J-W, Chen Y-Y, Su K-H, Lin C-C, Tu W-C, Chiou M-T, Ou S-C, Chang G-JJ, Lin Y-L, Chiou S-S. 2019. NS2B/NS3 mutations enhance the infectivity of genotype I Japanese encephalitis virus in amplifying hosts. PLoS Pathog 15:e1007992. doi: 10.1371/journal.ppat.1007992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuchinsky SC, Hawks SA, Mossel EC, Coutermarsh-Ott S, Duggal NK. 2020. Differential pathogenesis of Usutu isolates in mice. PLoS Negl Trop Dis 14:e0008765. doi: 10.1371/journal.pntd.0008765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beasley DWC, Whiteman MC, Zhang S, Huang CY-H, Schneider BS, Smith DR, Gromowski GD, Higgs S, Kinney RM, Barrett ADT. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol 79:8339–8347. doi: 10.1128/JVI.79.13.8339-8347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Yang H, Li Z, Wang W, Lin H, Liu L, Ni Q, Liu X, Zeng X, Wu Y, Li Y. 2017. Envelope protein mutations L107F and E138K are important for neurovirulence attenuation for Japanese encephalitis virus SA14-14–2 strain. Viruses 9:20. doi: 10.3390/v9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindqvist R, Rosendal E, Weber E, Asghar N, Schreier S, Lenman A, Johansson M, Dobler G, Bestehorn M, Kröger A, Överby AK. 2020. The envelope protein of tick-borne encephalitis virus influences neuron entry, pathogenicity, and vaccine protection. J Neuroinflammation 17:284. doi: 10.1186/s12974-020-01943-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maharaj PD, Bosco-Lauth AM, Langevin SA, Anishchenko M, Bowen RA, Reisen WK, Brault AC. 2018. West Nile and St. Louis encephalitis viral genetic determinants of avian host competence. PLoS Negl Trop Dis 12:e0006302. doi: 10.1371/journal.pntd.0006302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wicker JA, Whiteman MC, Beasley DWC, Davis CT, Zhang S, Schneider BS, Higgs S, Kinney RM, Barrett ADT. 2006. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology 349:245–253. doi: 10.1016/j.virol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Engel D, Jöst H, Wink M, Börstler J, Bosch S, Garigliany M-M, Jöst A, Czajka C, Lühken R, Ziegler U, Groschup MH, Pfeffer M, Becker N, Cadar D, Schmidt-Chanasit J. 2016. Reconstruction of the evolutionary history and dispersal of Usutu virus, a neglected emerging arbovirus in Europe and Africa. mBio 7:e01938-15. doi: 10.1128/mBio.01938-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moulton MP, Cropper WP, Avery ML, Moulton LE. 2010. The earliest house sparrow introductions to North America. Biol Invasions 12:2955–2958. doi: 10.1007/s10530-010-9692-0. [DOI] [Google Scholar]
- 75.Anderson T. 2007. Biology of the ubiquitous house sparrow: from genes to populations. Oxford University Press, Oxford, United Kingdom. doi: 10.1093/acprof:oso/9780195304114.001.0001. [DOI] [Google Scholar]
- 76.Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. 2011. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol 11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pierson TC, Diamond MS. 2020. The continued threat of emerging flaviviruses. Nat Microbiol 5:796–812. doi: 10.1038/s41564-020-0714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salgado R, Hawks SA, Frere F, Vázquez A, Huang CY-H, Duggal NK. 2021. West Nile virus vaccination protects against Usutu virus disease in mice. Viruses 13:2352. doi: 10.3390/v13122352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weger-Lucarelli J, Rückert C, Chotiwan N, Nguyen C, Garcia Luna SM, Fauver JR, Foy BD, Perera R, Black WC, Kading RC, Ebel GD. 2016. Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl Trop Dis 10:e0005101. doi: 10.1371/journal.pntd.0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith DR, Carrara AS, Aguilar PV, Weaver SC. 2005. Evaluation of methods to assess transmission potential of Venezuelan equine encephalitis virus by mosquitoes and estimation of mosquito saliva titers. Am J Trop Med Hyg 73:33–39. doi: 10.4269/ajtmh.2005.73.33. [DOI] [PubMed] [Google Scholar]
- 81.Siegel PB, Gross WB. 1980. Production and persistence of antibodies in chickens to sheep erythrocytes. 1. Directional selection. Poult Sci 59:1–5. doi: 10.3382/ps.0590001. [DOI] [PubMed] [Google Scholar]
- 82.Gross WG, Siegel PB, Hall RW, Domermuth CH, DuBoise RT. 1980. Production and persistence of antibodies in chickens to sheep erythrocytes. 2. Resistance to infectious diseases. Poult Sci 59:205–210. doi: 10.3382/ps.0590205. [DOI] [PubMed] [Google Scholar]
- 83.Styer LM, Bernard KA, Kramer LD. 2006. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am J Trop Med Hyg 75:337–345. doi: 10.4269/ajtmh.2006.75.337. [DOI] [PubMed] [Google Scholar]
- 84.Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang G-JJ, Bowen RA, Brault AC. 2017. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep 18:1751–1760. doi: 10.1016/j.celrep.2017.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]