ABSTRACT
Many cities across the nation are plagued by lead contamination in drinking water. As such, many drinking water utilities have undertaken lead service line (LSL) replacement to prevent further lead contamination. However, given the urgency of lead mitigation, and the socioeconomic challenges associated with LSL replacement, cities have used phosphate-based corrosion inhibitors (i.e., orthophosphate) alongside LSL replacement. While necessary to ensure public health protection from lead contamination, the addition of orthophosphate into an aging and leaking drinking water system may increase the concentration of phosphate leaching into urban streams characterized by century-old failing water infrastructure. Such increases in phosphate availability may cascade into nutrient and microbial community composition shifts. The purpose of this study was to determine how this occurs and to understand whether full-scale distribution system orthophosphate addition impacts the microbial ecology of urban streams. Through monthly collection of water samples from five urban streams before and after orthophosphate addition, significant changes in microbial community composition (16S rRNA amplicon sequencing) and in the relative abundance of typical freshwater taxa were observed. In addition, key microbial phosphorus and nitrogen metabolism genes (e.g., two component regulatory systems) were predicted to change via BugBase. No significant differences in the absolute abundances of total bacteria, Cyanobacteria, and “Candidatus Accumulibacter” were observed. Overall, the findings from this study provide further evidence that urban streams are compromised by unintentional hydrologic connections with drinking water infrastructure. Moreover, our results suggest that infiltration of phosphate-based corrosion inhibitors can impact urban streams and have important, as-yet-overlooked impacts on urban stream microbial communities.
IMPORTANCE Elevated lead levels in drinking water supplies are a public health risk. As such, it is imperative for cities to urgently address lead contamination from aging drinking water supplies by way of lead service line replacements and corrosion control methods. However, when applying corrosion control methods, it is also important to consider the chemical and microbiological effects that can occur in natural settings, given that our water infrastructure is aging and more prone to leaks and breaks. Here, we examine the impacts on the microbial ecology of five urban stream systems before and after full-scale distribution system orthophosphate addition. Overall, the results suggest that infiltration of corrosion inhibitors may impact microbial communities; however, future work should be done to ascertain the true impact to protect both public and environmental health.
KEYWORDS: phosphate-based corrosion control, stream water microbiology, microbial community composition, aging infrastructure
INTRODUCTION
Since the drinking water lead crises in Washington, DC, in 2001, and more recently in Flint, MI, in 2014 (1–3), drinking water utilities across the United States have been required to address and prevent lead contamination in drinking water supplies. The city of Pittsburgh, like many other cities in the United States, experienced its own lead drinking water problems as regulatory compliance testing found drinking water lead concentrations to be above the U.S. Environmental Protection Agency (EPA)’s Lead and Copper Rule (LCR) action level of 15 ppb in over 10% of the homes sampled in 2016 (4). To combat drinking water lead issues, Pittsburgh’s drinking water utility opted for lead service line (LSL) replacement; however, economic constraints make complete LSL replacement a slow process. Given the urgency of lead mitigation, Pittsburgh, like many other urban centers, chose to introduce corrosion inhibitors (e.g., orthophosphate [PO43−]), which form a protective scale on pipe surfaces, to provide a quicker and more economical solution to control lead release while awaiting completed LSL replacement (3).
Phosphate-based corrosion inhibitors, namely, polyphosphates and orthophosphates, have been widely used by drinking water utilities for decades and have had varied success in inhibiting corrosion from copper, lead, and iron plumbing materials (5–12). Although there has been much success in corrosion inhibition using phosphate-based corrosion inhibitors across the United States, the city of Pittsburgh had been using soda ash as its main form of corrosion control until a year-long study found that orthophosphate was more effective at reducing lead corrosion. The introduction and continual application of up to 1.8 mg/L of PO43– into Pittsburgh’s drinking water distribution system (DS), which previously had nondetectable PO43–, will likely provide key nutrients for biota, resulting in increased microbial regrowth in the DS (13–18). Moreover, decaying water infrastructure could also cause changes in the microbial community composition of both the DS and nearby urban streams with further impacts to groundwater and riverine receiving waters (19–22).
Given the strong nutrient limitations for organisms in water systems, increased abundance of Cyanobacteria and other eutrophication-related taxa could be a likely observation due to an increase in biologically available phosphate to be used for growth and cell maintenance (23, 24) in the urban stream waters. In addition, recent metagenomics studies in the UK drinking water DS found an increase in microorganisms related to enhanced phosphate metabolism (e.g., polyphosphate accumulating taxa such as “Candidatus Accumulibacter”) (13, 17, 25) after increased phosphate addition. With the aging drinking water infrastructure across the nation that contributes millions of gallons per day in lost treated drinking water (through unmetered buildings and pipe breaks) (26), it is important to consider the impacts of such additions on urban stream microbiomes and nutrient limitations. Prior to PO43– addition into the DS, the five urban streams examined in this work (S1 to S5; Fig. 1) were either phosphorus limited or nitrogen-phosphorus colimited (27), similar to most aquatic systems (e.g., streams, drinking water networks) (28). As such, any shifts in urban stream nutrient limitations caused by PO43– addition into the DS can cause eutrophication (14, 16, 29–35) and alter food web composition (36). This study aims to assess for the first time whether changes in the DS impact the microbiome of urban streams suspected to be hydrologically connected to the DS (receiving water from breaks, leaks, etc., from an aging DS infrastructure) and add to the limited body of knowledge surrounding the impacts of full-scale PO43− corrosion inhibitor application on the changes in microbial community composition in urban stream networks.
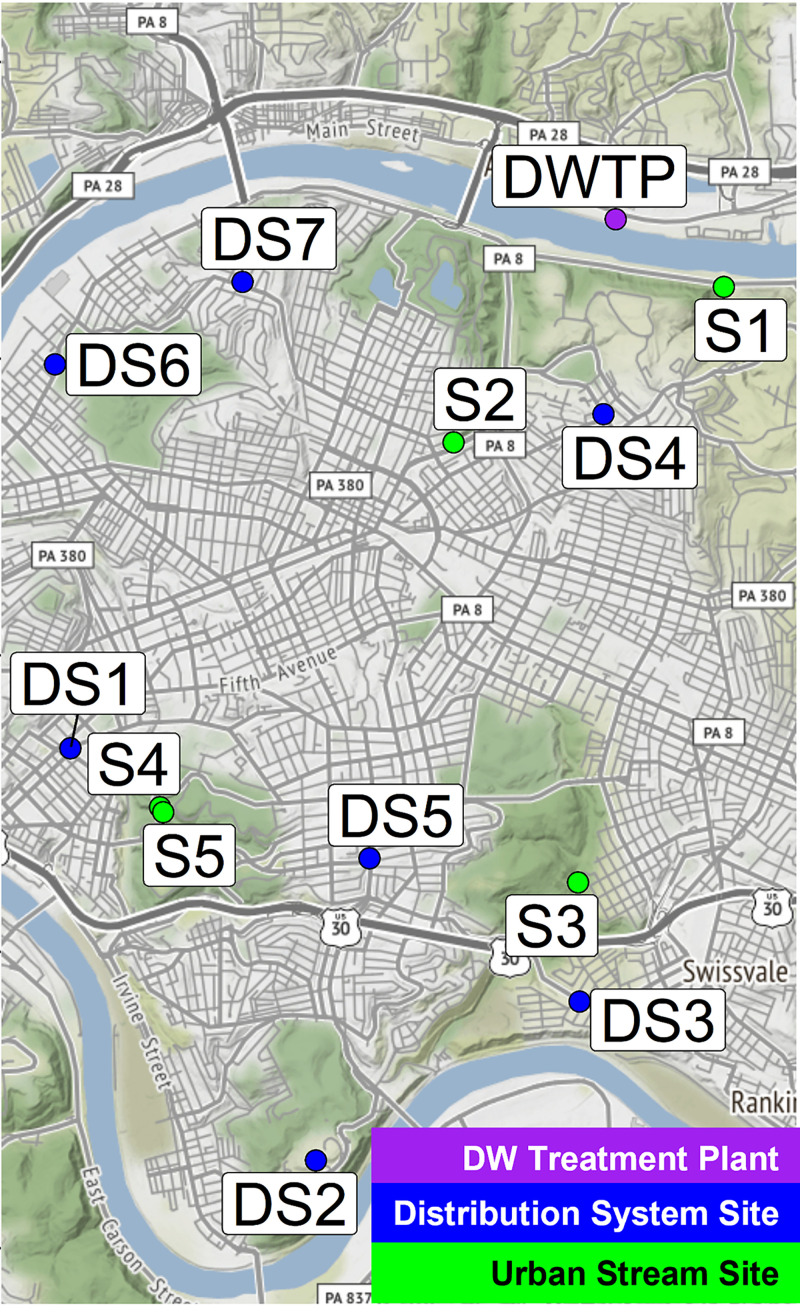
FIG 1.
Map of urban stream sampling locations. Each urban stream was chosen such that it was close to a routine monitoring site in the DS to maximize chances of DS infiltration.
RESULTS AND DISCUSSION
Impacts of PO43– addition on urban stream phosphorus.
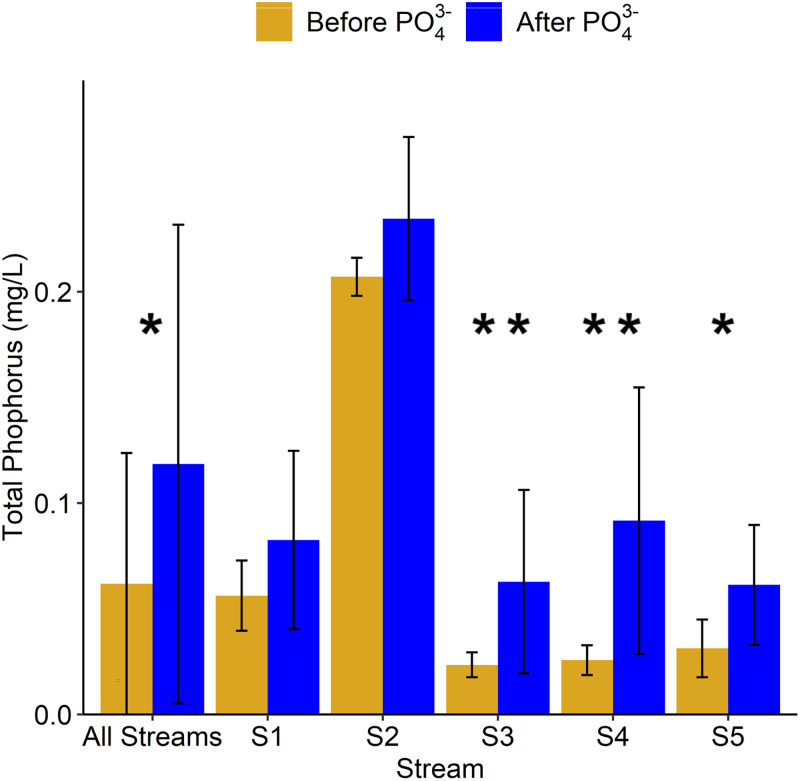
The aggregate (all five streams) average total phosphorus concentration significantly increased after the introduction of PO43– into the DS (Fig. 2), suggesting that these urban streams may be receiving PO43– from leaking DS infrastructure and are hydrologically connected. The significant increase in the aggregate average total phosphorus concentration was driven by significant increases in three urban streams: S3, S4, and S5. All three of these streams exist within areas of medium development intensity (see Table SA1 in the supplemental material), with S4 having the highest human population density surrounding it, further suggesting potential impacts from urban water infrastructure. Previous work has looked at the spatial distribution of potential deteriorating water infrastructure in Pittsburgh and documented that 71% of existing streams across the City of Pittsburgh are located in a potential leakage zone (37). Further work was also done to examine the impacts of PO43– addition on phosphorus and nitrogen limitations in the five urban streams (27). Four of the five streams (S1, S3, S4, and S5) were either phosphorus limited or nitrogen-phosphorus colimited prior to PO43– addition into the DS. At 2 months after the PO43– addition, the four streams shifted completely to phosphorus-nitrogen colimitation (S1, S3, and S5) or nitrogen limitation (S4). However, 12 months after PO43– addition, nutrient limitations had shifted back to phosphorus limitation or nitrogen-phosphorus colimitation, suggesting that PO43– addition in the DS caused a temporary shift in urban stream nutrient limitations, further highlighting that these streams are hydrologically connected to the DS.
FIG 2.
Total phosphorus concentration (average ± the standard deviation) in each urban stream before and after PO43– addition into the DS. Statistical significances between concentrations were analyzed between samples collected before and after PO43– addition into the distribution system (**, P < 0.01; *, P < 0.05).
Impacts of PO43– addition on microbial community composition in urban streams.
To assess changes in microbial community composition in urban streams, nonmetric multidimensional scaling analysis (NMDS) was performed, and the relative abundances of urban stream taxa and the community alpha diversity were determined.
NMDS analysis.
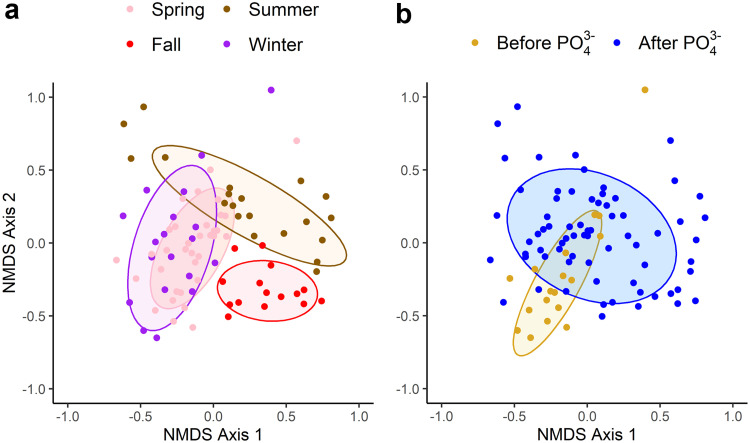
NMDS and permutational multivariate analysis of variance (as implemented in Adonis) at the 97% sequence similarity cutoff showed significant seasonal variation in the urban stream microbial community composition (Fig. 3a). In addition, significant variation in the urban stream microbial community composition was observed between stream samples collected before and after PO43– addition into the DS (Fig. 3b). Interestingly, however, no significant differences were observed in the microbial community composition between the five urban steams (see Fig. SA1) despite differences in land development types, population densities, and chemistries. Previous work has observed seasonal differences in urban stream microbial community composition (38), but this is the first work to the authors knowledge that has observed the impacts of PO43– addition on stream microbial ecology.
FIG 3.
(a and b) NMDS plots of Bray-Curtis distances for the five urban stream sites sampled seasonally (a) and before and after orthophosphate corrosion control addition (b). The ellipses represent the 95% confidence intervals of the distribution from the centroid of the cluster points.
Relative and absolute abundance analysis.
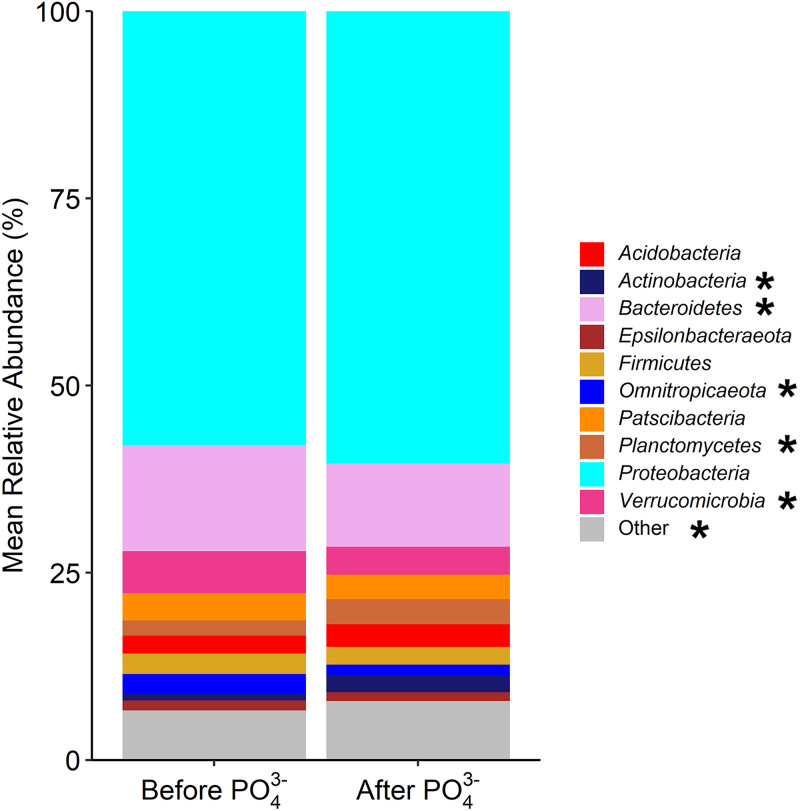
The collective urban stream network (all five streams) microbial community was primarily comprised of 10 phyla: Acidobacteria, Actinobacteria, Bacteroidetes, Epsilonbacteraeota, Firmicutes, Omnitrophicaeota, Patescibacteria, Planctomycetes, Proteobacteria, and Verrucomicrobia, with Proteobacteria dominating in all urban stream networks, making up 52 to 66% of the community (Fig. 4). The presence and abundances of these phyla are consistent with previous work which has highlighted their ubiquitous presence in both urbanized and forested stream networks (39–41). Previous studies of streams and rivers have found that in-stream microbial populations are related to taxa typically found in lakes and other freshwater environments (40, 42). Proteobacteria, Bacteroidetes, Verrucomicrobia, and Actinobacteria are all considered typical freshwater lake phyla and have been studied extensively (42). Of the remaining phyla, members of the Planctomycetes, Acidobacteria, and Firmicutes phyla are commonly found in freshwater sediments (42), while more recent work has shown the abundance of both Omnitrophicaeota and Patescibacteria in fresh- and groundwater systems (43). Overall, significant changes in the relative abundances of Actinobacteria, Bacteroidetes, Omnitrophicaeota, Planctomycetes, Verrucomicrobia, and less-abundant phyla, such as Chlamydiae, were observed after PO43– addition into the DS (Fig. 4). Previous work has observed an association between each of these phyla and phosphorus uptake or utilization in sediment and marine environments (44–46); however, to the best of our knowledge, no study has detailed an association with phosphorus in freshwater environments. The observed significant changes in these phyla could be indicative of the potential impacts of PO43– addition on urban stream networks, but additional long-term studies are needed to confirm this.
FIG 4.
Top 10 most abundant phyla in all five urban streams before and after PO43– addition in the distribution system. Proteobacteria dominated the urban stream microbial communities under both conditions. Significant changes in the top 10 phyla were observed after PO43– addition into the distribution system (indicated by an asterisk [*]).
Examining each stream individually, streams S1, S3, and S5 all had the same top 10 phyla present over the course of the study, while streams S2 and S4 differed in their top 10 phyla with Elusimicrobia replacing Actinobacteria in stream S2 (see Fig. SA2) and chlamydiae replacing Epsilonbacteraeota in stream S4 (see Fig. SA3). Chlamydiae is an environmentally ubiquitous phylum of chlamydia-like organisms that is a part of the Planctomycetes-Verrucomicrobia-Chlamydiae (PVC) superphylum. Although this phylum appeared in low proportions (1 to 4%) in stream S4, it is important to note that a combined sewer overflow outfall exists in the immediate vicinity of this stream and could be a potential source for these taxa, further highlighting the connection between the urban streams and urban water infrastructure. Recent work examining taxonomic shifts in urban streams found an increase in Chlamydiae after rainfall events and suggests that the increase is likely driven by combined sewer overflow contributions (47). Furthermore, although not studied in the context of surface waters extensively, recent work in the marine space has identified one lineage of the Chlamydiae phylum that could uptake inorganic phosphate (48). As such, the prospect of phosphate impacts on the Chlamydiae phylum (and other members of the PVC-superphylum) are interesting for future consideration as potential eutrophication biomarkers in aquatic systems.
No significant changes in Cyanobacteria or “Ca. Accumulibacter” relative abundance were observed after PO43– addition into the DS, nor were they present in the top 10 taxa of any stream compared to urban stream samples collected before PO43– addition into the DS. Members of the “Ca. Accumulibacter” genus are commonly found in wastewater treatment plants that perform enhanced biological phosphorus removal (49), so the low relative abundance in more dynamic freshwater systems, where parameters affecting their survival can vary depending on hydrologic conditions, is expected. The lack of significant changes in Cyanobacteria, however, was an unexpected result, since an increased abundance of Cyanobacteria and other eutrophication-related taxa has been associated with an increase in biologically available phosphate to be used for growth and cell maintenance (23, 24). Certain Cyanobacteria are known to have the functional trait for the high affinity phosphate uptake system, called the “phosphate-specific transport system” (50). However, many of these functional traits are only induced under low phosphate concentration conditions, which these streams were not since they were above the 1986 EPA recommendation of 0.1 mg/L (51). Collectively between the five urban streams studied, Cyanobacteria had an average relative abundance of 0.87% ± 1.1% before PO43– addition into the DS, and 0.65% ± 0.62% after PO43– addition. Combined with the results from the eutrophication assays (27), it is likely that the low abundance of Cyanobacteria is due to a combination of elevated phosphorus requirements and a natural occurrence in this set of urban streams. Future studies should include continued examination of Cyanobacteria and specific taxa, including known contributors to harmful algal blooms such as Microcystis.
Overall, the lack of significant changes relative abundance in both Cyanobacteria and “Ca. Accumulibacter” could be due to the dynamic nature of urban streams (52). For example, seasonal changes in hydrologic conditions (i.e., stream flow and groundwater table) and environmental parameters can impact necessary (or inhibitory) nutrients for certain organisms (53), which in turn would impact and shift microbial growth rates. As such, future studies should consider and explore the impacts of seasonal and event based hydrological dynamics on microbial nutrient availability. Furthermore, it is known that PO43– binds with lead in pipe networks, so although DS pipe leaks could contribute additional phosphorus to urban stream networks, the amount may not have been enough to cause significant changes in cyanobacterial or “Ca. Accumulibacter” abundance given other temporal and seasonal changes in the streams.
The lack of significant differences in the relative abundances of both Cyanobacteria and “Ca. Accumulibacter” was further confirmed by the lack of significant differences in the absolute abundance analyses. No significant difference in the absolute abundance of total bacteria, Cyanobacteria (eutrophication indicator phylum), or “Ca. Accumulibacter (polyphosphate accumulating genus) was observed after PO43– addition into the DS (see Fig. SA9 to SA11 in the supplemental material). The lack of significant differences in absolute abundance combined with the significant changes in the relative abundance of different taxa in the urban streams suggests that PO43– may be causing urban stream microbial communities to respond differently rather than changing the total number of organisms present in the urban streams. Different microorganisms have different nutrient requirements and, as such, the amount of PO43– reaching the streams from the DS may be enough for some members, while not enough for others. This conclusion is also supported by the observation of increased phosphorus requirements for Cyanobacteria in these urban streams from eutrophication assays conducted by Balangoda et al. (27). As such, it is imperative that future studies examine the impacts of long-term PO43– addition on urban streams as lower dosages are used for scale maintenance in the DS and continued infiltration occurs.
Alpha diversity analysis.
Microbial alpha diversity (calculated based on the operational taxonomic units [OTU] at 97% sequence similarity) analysis revealed significant increases in the three alpha diversity indices measured in stream S4 and a significant increase in Chao’s species richness in stream S5 after PO43– addition into the DS (Table 1). This result is not surprising given the locations of streams S4 and S5, since one of these streams receives water from a neighboring suburb, and the other receives water from a neighboring golf course, and stream S5 has the highest population density of all the streams studied (see Table SA1 in the supplemental material). The increase in alpha diversity in streams S4 and S5 coincides with the increases in total phosphorus (Fig. 2) and changes in relative abundance, suggesting that these changes could have been driven by PO43– leakage from the DS.
TABLE 1.
Urban stream alpha diversity before and after PO43– addition to the DSa
| Stream | Shannon diversity |
Chao’s richness |
Pielou’s evenness |
|||
|---|---|---|---|---|---|---|
| Before PO43– | After PO43– | Before PO43– | After PO43– | Before PO43– | After PO43– | |
| S1 | 5.92 | 6.48 | 460 | 805 | 0.97 | 0.98 |
| S2 | 6.31 | 6.62 | 797 | 940 | 0.98 | 0.98 |
| S3 | 6.44 | 6.34 | 773 | 738 | 0.98 | 0.98 |
| S4 | 5.92 | 6.4* | 456 | 756* | 0.97 | 0.98* |
| S5 | 5.99 | 6.39 | 488 | 732* | 0.97 | 0.97 |
*, Significant difference in the alpha diversity metric (P < 0.05).
Environmental factors impacting microbial community composition.
Detailed analysis of the urban streams revealed that 21% of the variance in the microbial community composition could be explained by the geographic location of the streams, the season, and the presence or absence of orthophosphate addition into the DS (Table 2). Other parameters that impacted community composition included total and dissolved iron concentration, nitrogen concentration, phosphorous concentration, and human population density (Table 2).
TABLE 2.
Parameters that explain the variance in urban stream microbial community composition
| Parameter | Variance (%)a | P |
|---|---|---|
| Stream location | 6.37 | 0.001 |
| Season | 5.18 | 0.001 |
| Total phosphorus | 1.96 | 0.001 |
| DS PO43– addition | 1.70 | 0.001 |
| Total nitrogen | 1.68 | 0.001 |
| Human population density | 1.55 | 0.001 |
| Total iron concn | 1.50 | 0.001 |
| Dissolved iron concn | 1.47 | 0.003 |
That is, the percent variance of urban stream microbial community composition.
The presence or absence of PO43– in the DS and the total phosphorus concentration of the streams were significant factors in explaining community composition, likely due to these two parameters being related. Combined with the significant increase in the average total phosphorus concentrations after PO43– addition into the DS (Fig. 2), this result provides further evidence that PO43– may be reaching the urban streams via leaks from the DS. In addition, iron (a historically common pipe material and environmentally present metal) is an important biogeochemical element that can serve as a cofactor in several biological processes, hence its significance in explaining variance in the microbial community is understandable. Microbial iron reduction has been shown to play a critical role in several nutrient cycles, including those for the nutrients nitrogen and phosphorus (54), and iron-oxidizing bacteria are often abundant in urban streams where groundwater infiltration occurs (55). Geographic location and seasonality have been previously shown to impact the microbial community composition at the drinking water treatment plant and in the DS (40, 41, 56), but to our knowledge, this is the first study to document that DS phosphate addition impacted the microbial community composition in urban streams.
Impacts of PO43– addition on predicted stream microbial phosphorus and nitrogen functional traits.
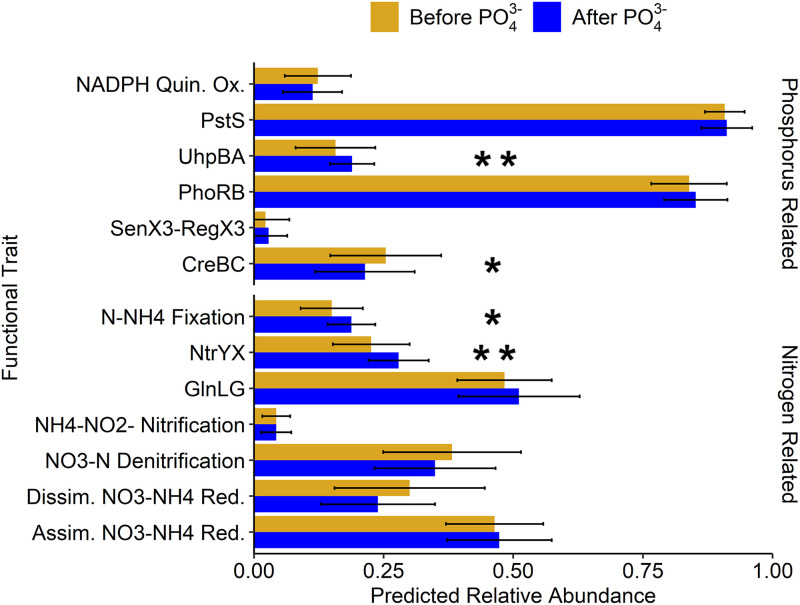
Of the default phenotypes, no predicted significant changes in the relative abundance of the selected traits in urban streams were observed after PO43– addition into the DS; however, significant differences in phenotypic traits were observed among seasons (57–59) and among urban stream locations (39), which have been previously observed. Predicted significant differences in phosphate uptake phenotypic traits including bacterial two-component regulatory systems (i.e., CreB-CreC and UhpB-UhpA) were observed in the urban streams after PO43– addition (Fig. 5; see also Fig. SA4, SA7, and SA8 in the supplemental material). The predicted changes in two-component regulatory systems are not surprising given that they are bacterial response mechanisms to changes in environmental conditions (60) and they result in a cascade of different gene expressions (60, 61). Here, a predicted significant decrease in the relative abundance of the CreC-CreB phosphate regulation system (CreBC) in the collective urban stream network was observed after PO43– addition (Fig. 5). Examining the streams on an individual basis revealed that these changes were driven by significant changes in streams S1, S4, and S5 (see Fig. A4, A7, and A8, respectively). The CreBC system is a conserved regulatory system that has been observed in a myriad of Gram-negative bacteria, including Escherichia coli and Pseudomonas aeruginosa (62). In these microorganisms, CreBC is responsible for the global regulation of gene expression for nine genes dealing with mediation of growth, adaptation, and biofilm formation and is directly related to carbon source and energy metabolism (63). In addition, a predicted significant increase in the UhpB-UhpA hexose phosphate system was observed, which has been linked to the ability to take up a broad range of organic phosphates (64). Since temporal and spatial fluctuations occurred in stream waters (significant increases in total phosphorus in three of the urban streams after 1 year of DS PO43– addition [Fig. 2]), it is expected that nutrient sources for microbial communities likewise change; therefore, since phosphorus concentrations were elevated after PO43– introduction into the DS (Fig. 2), it is possible that the carbon/nitrogen/phosphorus (CNP) ratio changed, impacting the relative abundance of microorganisms that express this system. As such, total nitrogen/total phosphorus (TN:TP) ratios were calculated and compared before and after PO43– addition in the DS (27). No significant difference in TN:TP ratios were observed after PO43– addition into the DS; however, a seasonal pattern was observed. The lack of significant differences in TN:TP ratios could be due to the complex dynamics of phosphorus species in streams (27). As such, future studies should also measure the carbon concentration to gain a better understanding of nutrient dynamics and the interactions between microbial communities as the CNP ratio can vary greatly in planktonic communities depending on a number of environmental factors (65, 66).
FIG 5.
Predicted relative abundance of phosphorus- and nitrogen-related phenotypes in urban streams before and after PO43– addition into the DS. Predicted significant differences in bacterial two-component regulatory systems and nitrogen fixation were observed across three of the five urban streams examined (*, P < 0.05; **, P < 0.01).
Of the phenotypes relating to nitrogen utilization/uptake (see Table SA6 in the supplemental material), there was a predicted significant increase in the relative abundance of the NtrY-NtrX nitrogen two-component regulation system (NtrYX) and nitrogen-ammonia fixation in the collective urban stream networks after PO43– addition into the DS (Fig. 5), with overall predicted significant changes driven by predicted changes in stream S4 (see Fig. SA7). The NtrYX system is found in a variety of Proteobacteria and has been observed to be linked to several cellular processes, including responses to oxygen stress, biofilm formation, and nitrogen fixation in the environment (67–70). As such, the predicted significant changes in both traits are expected since organisms that can fix atmospheric nitrogen (e.g., Planctomycetes [71]) have been observed to express the NtrYX system (70). Likewise, their increases after PO43– addition could be indicative of a response due to increased total phosphorus loading in the stream (Fig. 2) as the relative abundance of phyla such as Planctomycetes increased after PO43– addition in the DS (Fig. 4). This observation matches with the results from a previous study in which the NtrX gene was upregulated in a high-phosphate environment (72); however, future studies should also confirm this change in the presence of extended PO43– use in the DS.
Overall, while predicted significant changes in both functional traits relating to phosphorus or nitrogen utilization were observed, these results should be interpreted with caution. To better assess the active expression of functional traits in the urban stream microbial community, future studies should consider examining the impacts of prolonged PO43– addition in the DS on the active microbial community by utilizing RNA-based approaches rather than DNA. In addition, conducting droplet digital PCR (ddPCR) on specific functional traits of interest over time would be a helpful addition to elucidating the impacts of PO43– addition into the DS on urban stream microbial community functionality. Furthermore, as PO43– continues to be added into the DS as a form of corrosion control, future studies should also examine the impacts of extended low dosages of PO43– on urban stream microbial community functionality to ensure no adverse effects occur.
Conclusion.
The addition of PO43– as a lead corrosion control mechanism is a widely utilized and effective option; however, the effects it can have on the microbial ecology of urban streams has not been documented. As many cities draw water from and release water to freshwater sources, this work examined the potential impacts of increasing the amount of phosphorus in the DS and its residual effects in surrounding urban stream networks. Significant increases in the total phosphorus concentration in the urban streams were observed after PO43– addition into the DS, suggesting that the streams are hydrologically connected to the DS. In addition, varying changes in the microbial community composition and predicted changes in nitrogen and phosphorus functional traits were observed after PO43– addition into the DS. Overall, the observations presented in this study suggest that while phosphate-based corrosion inhibitors are an effective tool in mitigating lead corrosion, their infiltration into local water bodies through leaks and breaks may result in changes in the existing stream microbial community. It should, however, be stressed that the impacts of such changes are unknown, but due to the degree of microbial functional redundancy in aquatic ecosystems, it is possible that ecosystem impacts would not occur or would only be apparent over a longer time frame than the year of study discussed here. Future work should carefully monitor how the microbial ecology of hydrologically connected urban streams change over longer periods of time when using phosphate-based corrosion inhibitors to ascertain the true urban stream impact.
MATERIALS AND METHODS
Orthophosphate application in the DS.
PO43– is applied at three different locations in the DS: once directly after treatment in the treatment plant, once after treated water is sent to a pump station for further distribution, and another after treated water is transported from one of the storage reservoirs. PO43– was applied in a step-down methodology over the course of 6 months, starting at 3.0 mg/L PO43– in April 2019 to help scale formation. As of September 2019, PO43– has been dosed at 1.8 mg/L PO43– for scale maintenance.
Urban stream sampling information.
Between February 2019 and June 2020, samples were collected monthly from five urban stream locations in Pittsburgh, PA (Fig. 1). This sampling range represents three time points before and 14 time points after PO43− addition to the DS. The five above-ground urban stream locations range in development intensity from open forestry to high-intensity development (see Table SA1).
Sample collection.
Stream samples were collected by submerging a sample bottle into the stream. All water samples were stored on ice after collection, with 1 L filtered within 1 h through 0.2-μm-pore-size polycarbonate filters (Isopore Membrane Filters; EMD Millipore, Billerica, MA), and the resulting 0.2-μm filters were stored at −20°C. Prior to filtration, two 100-mL aliquots of water samples were removed and stored for water quality analyses.
Water quality analysis.
A total of 22 water quality parameters (see Table SA2) were measured according to standard methods (73). The temperature and pH were monitored on site using a YSI multiparameter sonde (Yellow Spring Instruments, Yellow Springs, OH). Total and dissolved metal concentrations were analyzed using inductively coupled plasma mass spectrometry (NexION 300×; Perkin-Elmer, Waltham, MA).
Droplet digital PCR and sequencing.
DNA was extracted from the stored filters using the FastDNA Spin kit (MP Biomedicals, Solon, OH) and stored at −20°C until use. The abundance of total bacteria was determined using droplet digital PCR (ddPCR) targeting the 16S rRNA gene using primers previously studied (74). ddPCR assays were also used to target phylum- and genus-specific genes for Cyanobacteria (16S rRNA) (75) and the polyphosphate accumulating genus “Candidatus Accumulibacter” (16S rRNA) (76) using previously published primers (see Table SA3).
ddPCR reactions were performed for all DNA samples (n = 90), alongside negative controls (ddPCR negative controls, filtration controls, and extraction controls) and positive controls (gblocks of the target amplicons provided by Integrated DNA Technologies, Inc., Coralville, IA). Then, 22-μL reactions contained 11 μL of 2× ddPCR Supermix (Bio-Rad Laboratories, Inc., Hercules, CA), 0.4 μM concentrations of all primers (Integrated DNA Technologies), 0.55 μL of bovine serum albumin (Invitrogen Corporation, Waltham, MA), and 2 μL of DNA template. Droplets were generated to a 20-μL reaction volume using the automated droplet generation oil for Sybr (Bio-Rad Laboratories), and the plate was sealed. PCR was performed on the C1000 Touch thermal cycler (Bio-Rad Laboratories) within 15 min of droplet generation using the reaction conditions presented in Table SA4 in the supplemental material. Plates were run on the droplet reader within 1 h of PCR completion. Thresholds were set for each ddPCR assay (see Table SA5) using Quantasoft v1.0.596 to determine the absolute abundance of the target taxa according to the method described by Lievens et al. (77).
16S rRNA gene amplicon library preparation and sequencing were performed on 90 samples at Argonne National Laboratory following the Illumina Earth Microbiome Protocol (78). Samples were sequenced on an Illumina HiSeq 2500 with a total of 5,063,434 raw reads generated from 90 samples after quality assurance and control, the average quality score was 94% with a median of 29,000 reads per sample. Microbiome analysis was performed using QIIME2 with quality filtering performed using the method described in Bolyen et al. (79). Reads were assigned to operational taxonomic units (OTU) using a 97% cutoff using the closed reference OTU-picking protocol in QIIME2 (v2020.2) using the Silva (v132.5) and Greengenes (v13.5) databases. The OTU generated from the Silva database were used in microbiome analysis, while the OTU from the Greengenes database were used specifically in BugBase (80) to determine phenotypes present in the samples.
Phenotypic prediction using BugBase.
Representative sequences of OTU generated by QIIME2 (v2020.2) were assigned taxonomic identities according to the Greengenes (v13.5) database. Annotated OTU matrices were then uploaded to the online BugBase database for phenotype prediction (80). The default analysis analyzes nine common traits to most prokaryotic organisms, including aerobic and anaerobic respiration, Gram-negative and Gram-positive delineation, pathogenic presence, and stress tolerance. Additional traits relating to phosphate or nitrogen metabolism were chosen from a BugBase compatible Kyoto Encyclopedia of Genes and Genomes (KEGG) list (see Table SA6) and also analyzed.
Statistical analyses.
Taxonomic and OTU tables generated for the samples were transformed using the Hellinger transformation due to the data set having many zeros and or low relative abundances (81). The transformed OTU data were then used to calculate pairwise dissimilarities between samples based on the Bray-Curtis dissimilarity index, with the resulting matrices examined for temporal and spatial patterns in the bacterial community structure by NMDS as implemented in the Vegan package in R (82). Significant differences in the microbial community compositions (Hellinger transformed OTU) of the urban streams before and after PO43– addition were determined by using nonparametric multivariate analysis of variance using Adonis, as implemented in the Vegan package in R (83). Shannon diversity index, Chao’s richness, Pielou’s evenness, and rarefaction curves were calculated on rarefied samples at a 3% genetic distance. The relationships between environmental parameters and patterns in bacterial community structure were examined by canonical correspondence analysis with significance tested by analysis of variance after reducing the overall suite of environmental variables with a stepwise Akaike information criterion model. In addition, significant differences in the absolute bacterial abundance before and after PO43– addition were determined by nonparametric Wilcoxon testing and the functional relationships between water quality parameters and bacterial groups were analyzed by stepwise multivariate forward/reverse regression analysis. All statistical analyses were performed in R (v4.0.2) (84) with significance set at P < 0.05.
Data availability.
Environmental and sequencing data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.6480526 under reference number 6480526.
ACKNOWLEDGMENTS
The National Science Foundation provided funding (grant 1929843) used to conduct this study. I.S.-W. is supported by a Pitt STRIVE fellowship from the University of Pittsburgh.
We thank the Pittsburgh Water and Sewer Authority, particularly Faith, Mike, Frank, and all sample collectors, for collecting samples and providing information about the PO43– addition deployment. We also thank Catherine Armbruster for help in setting up and troubleshooting our sequencing analysis pipeline. We thank the University of Pittsburgh High-Throughput Cluster Computational Services for computational processing of our sequencing data. Finally, we thank Kate Zidar for providing the population density information for the urban stream networks included in the study, Mark Rivers for contributions to the overall study design, Daniel Bain for contributions to the analysis of our total and dissolved metal concentrations and discussions that helped us better understand the observations presented, and Niyousha Mohammadshafie for initial sample collection.
Footnotes
Supplemental material is available online only.
Contributor Information
Sarah-Jane Haig, Email: sjhaig@pitt.edu.
Jeffrey A. Gralnick, University of Minnesota
REFERENCES
- 1.Pieper KJ, Tang M, Edwards MA. 2017. Flint Water crisis caused by interrupted corrosion control: investigating “ground zero” home. Environ Sci Technol 51:2007–2014. doi: 10.1021/acs.est.6b04034. [DOI] [PubMed] [Google Scholar]
- 2.Goovaerts P. 2017. The drinking water contamination crisis in flint: modeling temporal trends of lead level since returning to Detroit water system. Sci Total Environ 581-582:66–79. doi: 10.1016/j.scitotenv.2016.09.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson TM, Wax M, Yonts J, Heidecorn K, Haig S-J, Yeoman D, Hayes Z, Raskin L, Ellis BR. 2017. Forensic estimates of lead release from lead service lines during the water crisis in Flint, Michigan. Environ Sci Technol Lett 4:356–361. doi: 10.1021/acs.estlett.7b00226. [DOI] [Google Scholar]
- 4.US EPA. 2021. 40 CFR part 141 subpart I: control of lead and copper. USEPA, Washington, DC. https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-141/subpart-I. Accessed 29 September 2021. [Google Scholar]
- 5.Becker A. 2002. The effect of corrosion inhibitors in drinking water installations of copper. Mater Corros 53:560–567. doi:. [DOI] [Google Scholar]
- 6.McNeill LS, Edwards M. 2002. Phosphate inhibitor use at US utilities. J Am Water Works Assoc 94:57–63. doi: 10.1002/j.1551-8833.2002.tb09506.x. [DOI] [Google Scholar]
- 7.Edwards M, McNeill LS. 2002. Effect of phosphate inhibitors on lead release from pipes. J Am Water Works Assoc 94:79–90. doi: 10.1002/j.1551-8833.2002.tb09383.x. [DOI] [Google Scholar]
- 8.Yohai L, Schreiner WH, Vázquez M, Valcarce MB. 2013. Phosphate ions as inhibiting agents for copper corrosion in chlorinated tap water. Mater Chem Phys 139:817–824. doi: 10.1016/j.matchemphys.2013.02.037. [DOI] [Google Scholar]
- 9.Comber S, Cassé F, Brown B, Martin J, Hillis P, Gardner M. 2011. Phosphate treatment to reduce plumbosolvency of drinking water also reduces discharges of copper into environmental surface waters. Water Environ J 25:266–270. doi: 10.1111/j.1747-6593.2010.00219.x. [DOI] [Google Scholar]
- 10.Cantor AF, Denig-Chakroff D, Vela RR, Oleinik MG, Lynch DL. 2000. Use of polyphosphate in corrosion control. J Am Water Works Assoc 92:95–102. doi: 10.1002/j.1551-8833.2000.tb08820.x. [DOI] [Google Scholar]
- 11.Bae Y, Pasteris JD, Giammar DE. 2020. The ability of phosphate to prevent lead release from pipe scale when switching from free chlorine to monochloramine. Environ Sci Technol 54:879–888. doi: 10.1021/acs.est.9b06019. [DOI] [PubMed] [Google Scholar]
- 12.Schock MR. 1996. Corrosion Inhibitor Applications in Drinking Water Treatment: Conforming to the Lead and Copper Rule. Paper presented at the CORROSION 96, Denver, Colorado. https://onepetro.org/NACECORR/proceedings/CORR96/All-CORR96/NACE-96231/113862. [Google Scholar]
- 13.Douterelo I, Dutilh BE, Calero C, Rosales E, Martin K, Husband S. 2020. Impact of phosphate dosing on the microbial ecology of drinking water distribution systems: fieldwork studies in chlorinated networks. Water Res 187:116416. doi: 10.1016/j.watres.2020.116416. [DOI] [PubMed] [Google Scholar]
- 14.Batté M, Koudjonou B, Laurent P, Mathieu L, Coallier J, Prevost M. 2003. Biofilm responses to aging and to a high phosphate load in a bench-scale drinking water system. Water Res 37:1351–1361. doi: 10.1016/S0043-1354(02)00476-1. [DOI] [PubMed] [Google Scholar]
- 15.Batté M, Mathieu L, Laurent P, Prévost M. 2003. Influence of phosphate and disinfection on the composition of biofilms produced from drinking water, as measured by fluorescence in situ hybridization. Can J Microbiol 49:741–753. doi: 10.1139/w03-094. [DOI] [PubMed] [Google Scholar]
- 16.Appenzeller BM, Batté M, Mathieu L, Block JC, Lahoussine V, Cavard J, Gatel D. 2001. Effect of adding phosphate to drinking water on bacterial growth in slightly and highly corroded pipes. Water Res 35:1100–1105. doi: 10.1016/S0043-1354(00)00337-7. [DOI] [PubMed] [Google Scholar]
- 17.Del Olmo G, Ahmad A, Jensen H, Karunakaran E, Rosales E, Calero Preciado C, Gaskin P, Douterelo I. 2020. Influence of phosphate dosing on biofilms development on lead in chlorinated drinking water bioreactors. NPJ Biofilms Microbiomes 6:43. doi: 10.1038/s41522-020-00152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang H-J, Choi Y-J, Ro H-M, Ka J-O. 2012. Effects of phosphate addition on biofilm bacterial communities and water quality in annular reactors equipped with stainless steel and ductile cast iron pipes. J Microbiol 50:17–28. doi: 10.1007/s12275-012-1040-x. [DOI] [PubMed] [Google Scholar]
- 19.Forgrave R, Elliott EM, Bain DJ. 2022. Event scale hydrograph responses highlight impacts of widespread stream burial and urban infrastructure failures. Hydrol Processes 36:e14584. 10.1002/hyp.14584. [DOI] [Google Scholar]
- 20.Divers MT, Elliott EM, Bain DJ. 2014. Quantification of nitrate sources to an urban stream using dual nitrate isotopes. Environ Sci Technol 48:10580–10587. doi: 10.1021/es404880j. [DOI] [PubMed] [Google Scholar]
- 21.Divers MT, Elliott EM, Bain DJ. 2013. Constraining nitrogen inputs to urban streams from leaking sewers using inverse modeling: implications for dissolved inorganic nitrogen (DIN) retention in urban environments. Environ Sci Technol 47:1816–1823. doi: 10.1021/es304331m. [DOI] [PubMed] [Google Scholar]
- 22.Kaushal SS, Groffman PM, Band LE, Elliott EM, Shields CA, Kendall C. 2011. Tracking nonpoint source nitrogen pollution in human-impacted watersheds. Environ Sci Technol 45:8225–8232. doi: 10.1021/es200779e. [DOI] [PubMed] [Google Scholar]
- 23.Jankowiak J, Hattenrath-Lehmann T, Kramer BJ, Ladds M, Gobler CJ. 2019. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol Oceanogr 64:1347–1370. doi: 10.1002/lno.11120. [DOI] [Google Scholar]
- 24.Tiwari B, Singh S, Kaushik MS, Mishra AK. 2015. Regulation of organophosphate metabolism in cyanobacteria: a review. Microbiology 84:291–302. doi: 10.1134/S0026261715030200.26263689 [DOI] [Google Scholar]
- 25.Rosales E, Del Olmo G, Calero Preciado C, Douterelo I. 2020. Phosphate dosing in drinking water distribution systems promotes changes in biofilm structure and functional genetic diversity. Front Microbiol 11:599091. doi: 10.3389/fmicb.2020.599091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alawadhi A, Tartakovsky D. 2020. Bayesian update and method of distributions: application to leak detection in transmission mains. Water Resour Res 56. doi: 10.1029/2019WR025879. [DOI] [Google Scholar]
- 27.Balangoda A, Elliott EM, Dabundo R, Spencer-Williams I, Haig S-J. 2022. Assessing nutrient limitation in urban streams following the addition of orthophosphate-based corrosion control to drinking water. Frontiers in Environmental Science, Biogeochemical Dynamics. [Google Scholar]
- 28.Robson BJ. 2014. State of the art in modeling of phosphorus in aquatic systems: review, criticisms and commentary. Environ Model Softw 61:339–359. doi: 10.1016/j.envsoft.2014.01.012. [DOI] [Google Scholar]
- 29.Thingstad TF, Zweifel UL, Rassoulzadegan F. 1998. P limitation of heterotrophic bacteria and phytoplankton in the northwest Mediterranean. Limnol Oceanogr 43:88–94. doi: 10.4319/lo.1998.43.1.0088. [DOI] [Google Scholar]
- 30.Camacho A, De Wit R. 2003. Effect of nitrogen and phosphorus additions on a benthic microbial mat from a hypersaline lake. Aquat Microb Ecol 32:261–273. doi: 10.3354/ame032261. [DOI] [Google Scholar]
- 31.Fang W, Hu JY, Ong SL. 2009. Influence of phosphorus on biofilm formation in model drinking water distribution systems. J Appl Microbiol 106:1328–1335. doi: 10.1111/j.1365-2672.2008.04099.x. [DOI] [PubMed] [Google Scholar]
- 32.Schindler DW. 1977. Evolution of phosphorus limitation in lakes. Science 195:260–262. doi: 10.1126/science.195.4275.260. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter SR. 2008. Phosphorus control is critical to mitigating eutrophication. Proc Natl Acad Sci USA 105:11039–11040. doi: 10.1073/pnas.0806112105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 35.US EPA. 2018. Nutrient pollution: the issue. US EPA, Washington, DC. https://www.epa.gov/nutrientpollution/issue. Accessed 8 January 2021. [Google Scholar]
- 36.Barbiero RP, Lesht BM, Warren GJ, Rudstam LG, Watkins JM, Reavie ED, Kovalenko KE, Karatayev AY. 2018. A comparative examination of recent changes in nutrients and lower food web structure in Lake Michigan and Lake Huron. J Great Lakes Res 44:573–589. doi: 10.1016/j.jglr.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkins KG, Bain DJ. 2018. Research note: mapping spatial patterns in sewer age, material, and proximity to surface waterways to infer sewer leakage hot spots. Landsc Urban Plan 170:320–324. doi: 10.1016/j.landurbplan.2017.04.011. [DOI] [Google Scholar]
- 38.Vignale FA, Rey DB, Pardo AM, Almasqué FJ, Ibarra JG, Do Porto DF, Turjanski AG, López NI, Helman RJM, Iustman LJR. 2022. Spatial and seasonal variations in the bacterial community of an anthropogenic impacted urban stream. Microb Ecol 10.1007/s00248-022-02055-z. [DOI] [PubMed] [Google Scholar]
- 39.Hosen JD, Febria CM, Crump BC, Palmer MA. 2017. Watershed urbanization linked to differences in stream bacterial community composition. Front Microbiol 8:1452. doi: 10.3389/fmicb.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassell N, Tinker KA, Moore T, Ottesen EA. 2018. Temporal and spatial dynamics in microbial community composition within a temperate stream network. Environ Microbiol 20:3560–3572. doi: 10.1111/1462-2920.14311. [DOI] [PubMed] [Google Scholar]
- 41.Staley C, Gould TJ, Wang P, Phillips J, Cotner JB, Sadowsky MJ. 2015. Species sorting and seasonal dynamics primarily shape bacterial communities in the upper Mississippi River. Sci Total Environ 505:435–445. doi: 10.1016/j.scitotenv.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S. 2011. A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49. doi: 10.1128/MMBR.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiedler CJ, Schönher C, Proksch P, Kerschbaumer DJ, Mayr E, Zunabovic-Pichler M, Domig KJ, Perfler R. 2018. Assessment of microbial community dynamics in river bank filtrate using high-throughput sequencing and flow cytometry. Front Microbiol 9:2887. doi: 10.3389/fmicb.2018.02887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faria M, Bordin N, Kizina J, Harder J, Devos D, Lage OM. 2018. Planctomycetes attached to algal surfaces: insight into their genomes. Genomics 110:231–238. doi: 10.1016/j.ygeno.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Bergkemper F, Schöler A, Engel M, Lang F, Krüger J, Schloter M, Schulz S. 2016. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ Microbiol 18:1988–2000. doi: 10.1111/1462-2920.13188. [DOI] [PubMed] [Google Scholar]
- 46.Hu Y, Duan C, Fu D, Wu X, Yan K, Fernando E, Karunarathna SC, Promputtha I, Mortimer PE, Xu J. 2020. Structure of bacterial communities in phosphorus-enriched rhizosphere soils. Appl Sci 10:6387. doi: 10.3390/app10186387. [DOI] [Google Scholar]
- 47.Chaudhary A, Kauser I, Ray A, Poretsky R. 2018. Taxon-driven functional shifts associated with storm flow in an urban stream microbial community. mSphere 3:e00194-18. doi: 10.1128/mSphere.00194-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dharamshi JE, Tamarit D, Eme L, Stairs CW, Martijn J, Homa F, Jørgensen SL, Spang A, Ettema TJG. 2020. Marine sediments illuminate chlamydiae diversity and evolution. Curr Biol 30:1032–1048. doi: 10.1016/j.cub.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Welles L, Abbas B, Sorokin DY, Lopez-Vazquez CM, Hooijmans CM, van Loosdrecht MCM, Brdjanovic D. 2016. Metabolic response of “Candidatus Accumulibacter phosphatis” clade II C to changes in influent P/C ratio. Front Microbiol 7:2121. doi: 10.3389/fmicb.2016.02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dignum M, Matthijs HC, Pel R, Laanbroek HJ, Mur LR. 2005. Nutrient limitation of freshwater cyanobacteria, p 65–86. In Harmful cyanobacteria. Springer, New York, NY. [Google Scholar]
- 51.Litke D.W. 1999. Review of phosphorus control measures in the united states and their effects on water quality. USGS, Reston, VA. 10.3133/wri994007. [DOI] [Google Scholar]
- 52.Zeglin LH. 2015. Stream microbial diversity in response to environmental changes: review and synthesis of existing research. Front Microbiol 6:454. doi: 10.3389/fmicb.2015.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones EF, Griffin N, Kelso JE, Carling GT, Baker MA, Aanderud ZT. 2020. Stream microbial community structured by trace elements, headwater dispersal, and large reservoirs in sub-alpine and urban ecosystems. Front Microbiol 11:491425. doi: 10.3389/fmicb.2020.491425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Yu S, Strong J, Wang H. 2012. Are the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus driven by the “FeIII–FeII redox wheel” in dynamic redox environments? J Soils Sediments 12:683–693. doi: 10.1007/s11368-012-0507-z. [DOI] [Google Scholar]
- 55.Paul MJ, Meyer JL. 2001. Streams in the urban landscape. Annu Rev Ecol Syst 32:333–365. doi: 10.1146/annurev.ecolsys.32.081501.114040. [DOI] [Google Scholar]
- 56.Hu A, Yang X, Chen N, Hou L, Ma Y, Yu C-P. 2014. Response of bacterial communities to environmental changes in a mesoscale subtropical watershed, southeast China. Sci Total Environ 472:746–756. doi: 10.1016/j.scitotenv.2013.11.097. [DOI] [PubMed] [Google Scholar]
- 57.Wilhelm SW, LeCleir GR, Bullerjahn GS, McKay RM, Saxton MA, Twiss MR, Bourbonniere RA. 2014. Seasonal changes in microbial community structure and activity imply winter production is linked to summer hypoxia in a large lake. FEMS Microbiol Ecol 87:475–485. doi: 10.1111/1574-6941.12238. [DOI] [PubMed] [Google Scholar]
- 58.Samad MS, Bertilsson S. 2017. Seasonal variation in abundance and diversity of bacterial methanotrophs in five temperate lakes. Front Microbiol 8:142. doi: 10.3389/fmicb.2017.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hullar MAJ, Kaplan LA, Stahl DA. 2006. Recurring seasonal dynamics of microbial communities in stream habitats. Appl Environ Microbiol 72:713–722. doi: 10.1128/AEM.72.1.713-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim IM, Puthiyaveetil S, Allen JF. 2016. A two-component regulatory system in transcriptional control of photosystem stoichiometry: redox-dependent and sodium ion-dependent phosphoryl transfer from cyanobacterial histidine kinase Hik2 to response regulators Rre1 and RppA. Front Plant Sci 7:137. doi: 10.3389/fpls.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu Rev Biochem 69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 62.Huang H-H, Chen W-C, Lin C-W, Lin Y-T, Ning H-C, Chang Y-C, Yang T-C. 2017. Relationship of the CreBC two-component regulatory system and inner membrane protein CreD with swimming motility in Stenotrophomonas maltophilia. PLoS One 12:e0174704. doi: 10.1371/journal.pone.0174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avison MB, Horton RE, Walsh TR, Bennett PM. 2001. Escherichia coli CreBC is a global regulator of gene expression that responds to growth in minimal media. J Biol Chem 276:26955–26961. doi: 10.1074/jbc.M011186200. [DOI] [PubMed] [Google Scholar]
- 64.Verhamme DT, Arents JC, Postma PW, Crielaard W, Hellingwerf KJ. 2001. Glucose-6-phosphate-dependent phosphoryl flow through the Uhp two-component regulatory system. Microbiology (Reading) 147:3345–3352. doi: 10.1099/00221287-147-12-3345. [DOI] [PubMed] [Google Scholar]
- 65.Cleveland CC, Liptzin D. 2007. C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252. doi: 10.1007/s10533-007-9132-0. [DOI] [Google Scholar]
- 66.They NH, Amado AM, Cotner JB. 2017. Redfield ratios in inland waters: higher biological control of C:N:P ratios in tropical semi-arid high water residence time lakes. Front Microbiol 8:1505. doi: 10.3389/fmicb.2017.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemmer KC, Alberge F, Myers KS, Dohnalkova AC, Schaub RE, Lenz JD, Imam S, Dillard JP, Noguera DR, Donohue TJ. 2020. The NtrYX two-component system regulates the bacterial cell envelope. mBio 11:e00957-20. doi: 10.1128/mBio.00957-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carrica MdC, Fernandez I, Martí MA, Paris G, Goldbaum FA. 2012. The NtrY/X two-component system of Brucella spp. acts as a redox sensor and regulates the expression of nitrogen respiration enzymes. Mol Microbiol 85:39–50. doi: 10.1111/j.1365-2958.2012.08095.x. [DOI] [PubMed] [Google Scholar]
- 69.Carrica MdC, Fernandez I, Sieira R, Paris G, Goldbaum FA. 2013. The two-component systems PrrBA and NtrYX coordinately regulate the adaptation of Brucella abortus to an oxygen-limited environment. Mol Microbiol 88:222–233. doi: 10.1111/mmi.12181. [DOI] [PubMed] [Google Scholar]
- 70.Calatrava-Morales N, Nogales J, Ameztoy K, van Steenbergen B, Soto MJ. 2017. The NtrY/NtrX system of Sinorhizobium meliloti GR4 regulates motility, EPS I production, and nitrogen metabolism but is dispensable for symbiotic nitrogen fixation. Mol Plant Microbe Interact 30:566–577. doi: 10.1094/MPMI-01-17-0021-R. [DOI] [PubMed] [Google Scholar]
- 71.Delmont TO, Quince C, Shaiber A, Esen ÖC, Lee ST, Rappé MS, McLellan SL, Lücker S, Eren AM. 2018. Nitrogen-fixing populations of planctomycetes and proteobacteria are abundant in surface ocean metagenomes. Nat Microbiol 3:804–813. doi: 10.1038/s41564-018-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernández I, Sycz G, Goldbaum FA, del Carrica M. 2018. Acidic PH triggers the phosphorylation of the response regulator NtrX in Alphaproteobacteria. PLoS One 13:e0194486. doi: 10.1371/journal.pone.0194486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cleseri LS, Greenberg AE, Eaton AD. 2005. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC. [Google Scholar]
- 74.Fierer N, Jackson JA, Vilgalys R, Jackson RB. 2005. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nübel U, Garcia-Pichel F, Muyzer G. 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol 63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He S, Gall DL, McMahon KD. 2007. “Candidatus Accumulibacter” population structure in enhanced biological phosphorus removal sludges as revealed by polyphosphate kinase genes. Appl Environ Microbiol 73:5865–5874. doi: 10.1128/AEM.01207-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lievens A, Jacchia S, Kagkli D, Savini C, Querci M. 2016. Measuring digital PCR quality: performance parameters and their optimization. PLoS One 11:e0153317. doi: 10.1371/journal.pone.0153317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ward T, Larson J, Meulemans J, Hillmann B, Lynch J, Sidiropoulos D, Spear J.R, Caporaso G, Blekhman R, Knight R, Fink R, Knights D. 2017. BugBase predicts organism-level microbiome phenotypes. bioRxiv. https://www.biorxiv.org/content/10.1101/133462v1.
- 81.Legendre P, Gallagher ED. 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. doi: 10.1007/s004420100716. [DOI] [PubMed] [Google Scholar]
- 82.Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos PH, Stevens MH, Szoecs E. 2020. Vegan: Community Ecology Package. https://cran.r-project.org/web/packages/vegan/vegan.pdf. [Google Scholar]
- 83.Haig S-J, Quince C, Davies RL, Dorea CC, Collins G. 2015. The relationship between microbial community evenness and function in slow sand filters. mBio 6:e00729-15. doi: 10.1128/mBio.00729-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.R Core Team. 2020. R: the R project for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.02158-22-s0001.pdf, PDF file, 1.2 MB (1.2MB, pdf)
Data Availability Statement
Environmental and sequencing data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.6480526 under reference number 6480526.