ABSTRACT
Fusobacterium necrophorum is a Gram-negative, filamentous anaerobe prevalent in the mucosal flora of animals and humans. It causes necrotic infections in cattle, resulting in a substantial economic impact on the cattle industry. Although infection severity and management differ within F. necrophorum species, little is known about F. necrophorum speciation and the genetic virulence determinants between strains. To characterize the clinical isolates, we performed whole-genome sequencing of four bovine isolates (8L1, 212, B17, and SM1216) and one human isolate (MK12). To determine the phylogenetic relationship and evolution pattern and investigate the presence of antimicrobial resistance genes (ARGs) and potential virulence genes of F. necrophorum, we also performed comparative genomics with publicly available Fusobacterium genomes. Using up-to-date bacterial core gene (UBCG) set analysis, we uncovered distinct Fusobacterium species and F. necrophorum subspecies clades. Pangenome analyses revealed a high level of diversity among Fusobacterium strains down to species levels. The output also identified 14 and 26 genes specific to F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme, respectively, which could be essential for bacterial survival under different environmental conditions. ClonalFrameML-based recombination analysis suggested that extensive recombination among accessory genes led to species divergence. Furthermore, the only strain of F. necrophorum with ARGs was F. necrophorum subsp. funduliforme B35, with acquired macrolide and tetracycline resistance genes. Our custom search revealed common virulence genes, including toxins, adhesion proteins, outer membrane proteins, cell envelope, type IV secretion system, ABC (ATP-binding cassette) transporters, and transporter proteins. A focused study on these genes could help identify major virulence genes and inform effective vaccination strategies against fusobacterial infections.
IMPORTANCE Fusobacterium necrophorum is an anaerobic bacterium that causes liver abscesses in cattle with an annual incidence rate of 10% to 20%, resulting in a substantial economic impact on the cattle industry. The lack of definite biochemical tests makes it difficult to distinguish F. necrophorum subspecies phenotypically, where genomic characterization plays a significant role. However, due to the lack of a good reference genome for comparison, F. necrophorum subspecies-level identification represents a significant challenge. To overcome this challenge, we used comparative genomics to validate clinical test strains for subspecies-level identification. The findings of our study help predict specific clades of previously uncharacterized strains of F. necrophorum. Our study identifies both general and subspecies-specific virulence genes through a custom search-based analysis. The virulence genes identified in this study can be the focus of future studies aimed at evaluating their potential as vaccine targets to prevent fusobacterial infections in cattle.
KEYWORDS: Fusobacterium necrophorum, virulence genes, phylogeny, pangenome, recombination, virulence factors, subspecies
INTRODUCTION
The genus Fusobacterium is a Gram-negative, anaerobic, non-spore-forming bacillus commonly found in the oral, intestinal, respiratory, and genital tracts of animals and humans. Fusobacterium produces volatile fatty acids, including butyrate, acetate, and propionate, as major metabolic end products (1). Of the many species in this genus, Fusobacterium necrophorum is an important opportunistic pathogen in both human and veterinary medicine. In humans, F. necrophorum is a causative agent in a variety of localized necrotic throat infections, such as tonsillitis, and systemic infections, such as Lemierre’s syndrome (2, 3). In animals, it causes severe cases of calf diphtheria, liver abscesses, and foot rot (4–7). As a consequence, F. necrophorum is responsible for substantial economic losses in the feedlot industry (8). F. necrophorum is divided into two main subspecies, F. necrophorum subsp. necrophorum (biotype A) and F. necrophorum subsp. funduliforme (biotype B), based on differences in cellular morphology, biochemical characterization, and differences in DNA level in 16S rRNA and DNA repeats (9–12). F. necrophorum subsp. necrophorum is more virulent and has thus been isolated more frequently from infections than F. necrophorum subsp. funduliforme (13).
Complete F. necrophorum subsp. funduliforme genome sequences have been published in recent years. However, the genome sequences of F. necrophorum subsp. necrophorum are not well characterized. Although there are a few additional genomes of F. necrophorum publicly available, the subspecies have not been properly defined. The lack of reference genome sequences makes the classification and phylogeny of F. necrophorum particularly challenging. Because there is no established biochemical methodology for subspecies differentiation, the phenotypic characterization of F. necrophorum is exclusively dependent on the output of the Rapid Ana II system (14). Consequently, genomic characterization of these clinical isolates could help validate the phenotypic characterizations. Also, to determine the phylogenetic relationship and evolution pattern and explore potential virulence genes of F. necrophorum, we genetically characterized five clinical isolates of F. necrophorum and confirmed the phenotypic representation of those isolates using genetic approaches. This study adds more reference genomes for additional F. necrophorum subspecies.
With rapid advances in sequencing methods, whole-genome sequencing technology has been widely used to predict pathogens’ phenotypic traits, such as virulence and antimicrobial resistance. Sequence analysis offers better insight into strain-specific genetic variation, disease propensity, phylogenetic relationships, virulence patterns, potential virulence genes, and diversity among subspecies and strains (15, 16). This critical information is lacking for F. necrophorum, an opportunistic pathogen that is one of the main causes of liver abscesses (17). Therefore, in this study, we used whole-genome sequence analysis to understand F. necrophorum virulence by determining the genetic content of virulence and antimicrobial resistance genes (ARGs). We concentrated on understanding the phylogenetic relationship, evolution pattern, and potential virulence genes of F. necrophorum at the subspecies level.
RESULTS
Phylogenetic analyses of Fusobacterium and F. necrophorum.
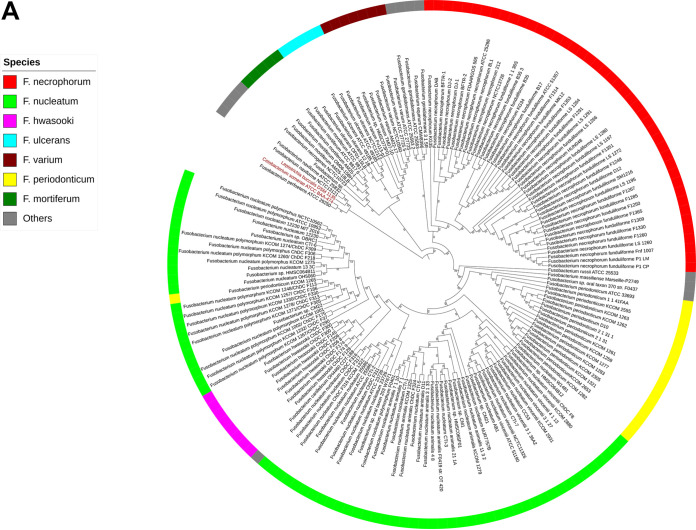
(i) Phylogenetic distribution and relationship of Fusobacterium genus. We studied genomic evolution by assembling dissimilarity matrices within the core genomes of 167 Fusobacterium strains (162 latest [July 2019] RefSeq sequences plus five test strains) and illustrated the results in Fig. 1A based on phylogenetic analyses of the 92 core genes (up-to-date bacterial core genes [UBCGs]). Two distinct strains, Leptotrichia buccalis and Cetobacterium somerae, were used as an outgroup (Fig. 1A, red font).
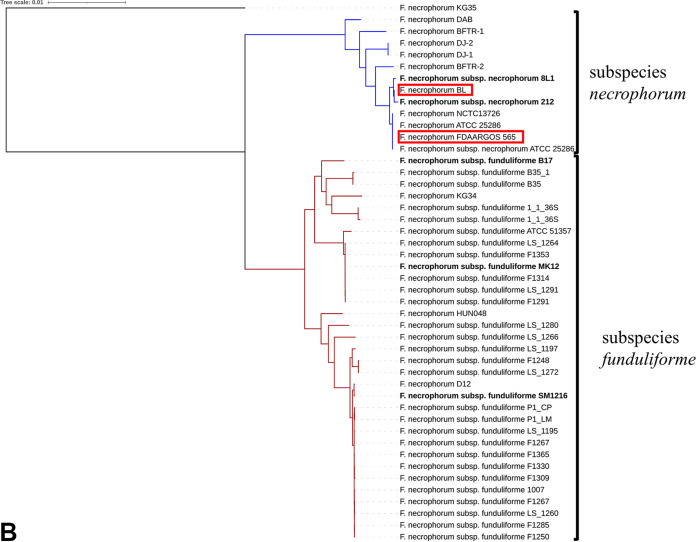
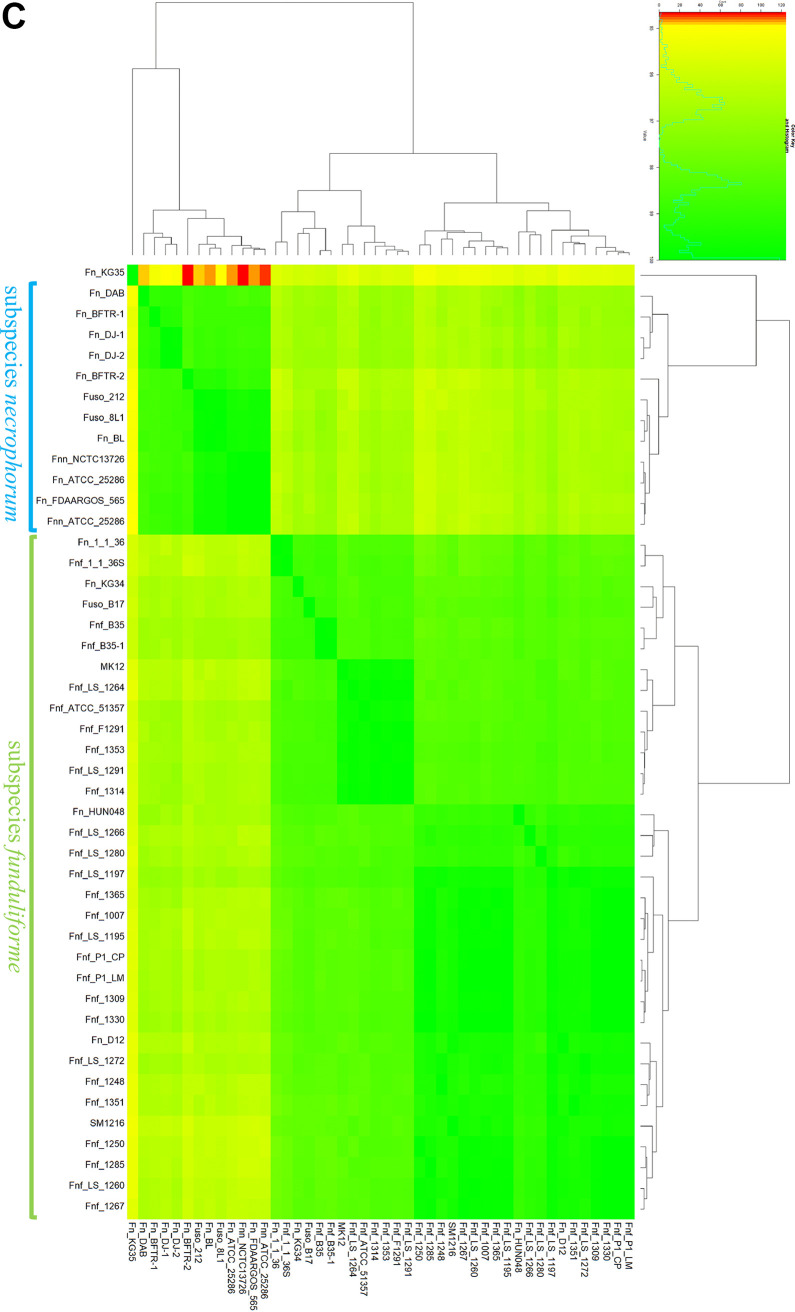
FIG 1.
(A) Gene content cladogram of genus Fusobacterium. The unweighted pair group method with arithmetic means (UPGMA) cladogram was constructed based on the dissimilarities among core gene sequence analyses using a UBCG set in 167 Fusobacterium strains. Leptotrichia buccalis DSM 1135 and Cetobacterium somerae ATCC BAA-474 (red font) were used as outgroups. Each colored strip denotes a different Fusobacterium species, as defined in the legend. Gene support index values are given at branching points. (B) Phylogenetic tree of Fusobacterium necrophorum species. Maximum-likelihood phylogenetic tree based on UBCG sequences of 41 RefSeq and five F. necrophorum test strains (bold). The clades are distinctly defined for each subspecies. The blue branch represents the clade for F. necrophorum subsp. necrophorum, and the red branch represents the clade for F. necrophorum subsp. funduliforme. strains BL and FDAARGOS 565 in the red boxes fall into the clade of F. necrophorum subsp. necrophorum, indicating that they are most likely F. necrophorum subsp. necrophorum. Bar, 0.01substitution per nucleotide position. (C) Heat map of ANI of whole-genome comparison of 46 (41 RefSeq sequences and five test strains) F. necrophorum strains. The row and column labels in the heat map correspond to the pairwise alignment of 46 F. necrophorum species. Cells in the heat map correspond to 95% identity and 70% coverage or greater. The identity match ranges from green to yellow to red as identity matches drop to 94.5%. F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme form each group represented in two distinct green blocks in the heat map and clustered together into separate clades in the phylogenetic tree. KG35 had a comparatively small ANI value, with the lowest-identity matches (some values were <95%), as indicated in the red grid. Strains are represented by their acronym and are provided in a supplementary material S1, see Data set S1.
The data revealed eight distinct clades of Fusobacterium species. In most clades, the Fusobacterium species within a clade shown in Fig. 1A with a given color belonged to the same species. In particular, F. necrophorum (Fig. 1A, red) formed a distinct clade, suggesting a close relationship with the species Fusobacterium gonidiaformans. The genome with the most sequences available, Fusobacterium nucleatum (Fig. 1A, green), formed a distinct clade. Other representative clades of Fusobacterium species included Fusobacterium hwasooki (Fig. 1A, pink), F. periodonticum (yellow), F. mortiferum (dark green), F. ulcerans (blue), and F. varium (dark red). Furthermore, the data revealed that several uncategorized Fusobacterium species (F. naviforme, F. perfoetens, F. gonidiaformans, F. russii, F. massiliense, and F. canifelinum) formed a separate clade (Fig. 1A, gray). In the resulting cladogram, F. naviforme and F. perfoetens ATCC 29250 were closest to the outgroup strains and belonged to the same clade.
(ii) Phylogenic relationships among Fusobacterium necrophorum subspecies. We used the whole-genome sequence to perform phylogenetic analysis based on a concatenated bacterial core gene set in a UBCG pipeline. The phylogeny implemented using RAxML revealed that all test strains (B17, SM1216, MK12, 8L1, and 212) clustered according to their respective subspecies (i.e., the F. necrophorum subsp. funduliforme and F. necrophorum subsp. necrophorum clades) (Fig. 1B). These findings cross-confirmed the phenotypic classification of the test strains. However, some of the RefSeq species in this study have not been identified to the subspecies level. These RefSeq species were placed in F. necrophorum subsp. necrophorum or F. necrophorum subsp. funduliforme on the phylogenetic tree based on the core genome (Fig. 1B), except for strain F. necrophorum KG35. Table 1 shows the predicted subspecies of these RefSeq strains based on the phylogenetic tree (Fig. 1B). For example, the RefSeq strains F. necrophorum BL and F. necrophorum FDAARGOS_565 clustered with the F. necrophorum subsp. necrophorum ATCC 25286 (the only complete published F. necrophorum subsp. necrophorum genome) and test strains 8L1 and 212 (phenotypically identified as F. necrophorum subsp. necrophorum). Therefore, UBCG analysis showed that out of 46 strains, 12 strains belonged to F. necrophorum subsp. necrophorum, while 34 belonged to F. necrophorum subsp. funduliforme.
TABLE 1.
Predicted subspecies of publicly available reference sequences of Fusobacterium necrophorum based on phylogenetic analysis
| Species, subspecies, and strain | Predicted subspecies | Type | Source of isolationa |
|---|---|---|---|
| F. necrophorum subsp. funduliforme ATCC 51357 | funduliforme | RefSeq | NA |
| F. necrophorum subsp. funduliforme 1_1_36S | funduliforme | RefSeq | NA |
| F. necrophorum subsp. funduliforme F1291 | funduliforme | RefSeq | Blood |
| F. necrophorum subsp. funduliforme F1260 | funduliforme | RefSeq | Blood |
| F. necrophorum FDAARGOS_565 | necrophorum | RefSeq | Environmental |
| F. necrophorum subsp. necrophorum ATCC 25286 | necrophorum | RefSeq | NA |
| F. necrophorum D12 | funduliforme | RefSeq | NA |
| F. necrophorum HUN048 | funduliforme | RefSeq | NA |
| F. necrophorum subsp. funduliforme 1007 | funduliforme | RefSeq | NA |
| F. necrophorum subsp. funduliforme B35 | funduliforme | RefSeq | Bovine liver abscess |
| F. necrophorum BL | necrophorum | RefSeq | Bovine liver abscess |
| F. necrophorum DJ-1 | necrophorum | RefSeq | Deer tongue/jaw |
| F. necrophorum BFTR-1 | necrophorum | RefSeq | Bovine foot rot |
| F. necrophorum DAB | necrophorum | RefSeq | Deer jaw abscess |
| F. necrophorum BFTR-2 | necrophorum | RefSeq | Bovine foot rot |
| F. necrophorum DJ-2 | necrophorum | RefSeq | Deer jaw |
| F. necrophorum subsp. funduliforme B35 | funduliforme | RefSeq | Bovine liver abscess |
| F. necrophorum subsp. funduliforme LS_1260 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme LS_1264 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme LS_1197 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme LS_1195 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme LS_1266 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme LS_1280 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme LS_1272 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme LS_1291 | funduliforme | RefSeq | Human blood |
| F. necrophorum subsp. funduliforme F1248 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1285 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1250 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1267 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1309 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1314 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1353 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1330 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme F1365 | funduliforme | RefSeq | Human throat swab |
| F. necrophorum subsp. funduliforme P1_CP | funduliforme | RefSeq | Colorectal tumor tissue |
| F. necrophorum subsp. funduliforme P1_LM | funduliforme | RefSeq | Liver metastasis tumor tissue |
| F. necrophorum KG34 | funduliforme | RefSeq | Cow uterine swab |
| F. necrophorum ATCC 25286 | necrophorum | RefSeq | Cow uterine swab |
| F. necrophorum subsp. funduliforme 1_1_36S | funduliforme | RefSeq | NA |
| F. necrophorum subsp. necrophorum NCTC13726 | necrophorum | RefSeq | Bovine liver abscess |
| F. necrophorum subsp. necrophorum 8L1 | necrophorum | WGS test | Bovine liver abscess |
| F. necrophorum subsp. necrophorum 212 | necrophorum | WGS test | Bovine foot rot |
| F. necrophorum subsp. funduliforme B17 | funduliforme | WGS test | Bovine liver abscess |
| F. necrophorum subsp. funduliforme MK12 | funduliforme | WGS test | Human tonsil |
| F. necrophorum subsp. funduliforme SM1216 | funduliforme | WGS test | Bovine foot rot |
NA, not available.
(iii) Average nucleotide identity analysis. To confirm our phylogenetic analysis, we also used average nucleotide identity (ANI) analysis, which gives the mean of nucleotide identity values for F. necrophorum strains. Using the FastANI results, we calculated pairwise ANI values among the F. necrophorum strains, which ranged from 94.64% to 99.99%. Next, we generated a heat map to visualize RefSeq and test strain clustering based on the distance matrix of ANI values (Fig. 1C). Each cell in the heat map corresponds to 95% identity and 70% coverage (indicating the same subspecies). As the comparison approaches 94.5%, the intensity of the color changes from green (>97% match) to yellow (95% to 97% match) to red (94% to 95% match). Within the heat map, there are defined clusters that separate clades for F. necrophorum subsp. funduliforme from those for F. necrophorum subsp. necrophorum.
All ANI values between F. necrophorum strains (including all test strains) were ≥95%, suggesting that the strains belong to F. necrophorum. However, the heat map showed similarities ranging from 94.645% to 96.028% for F. necrophorum KG35. The homology with ANI values <95% between the F. necrophorum strains KG35 and BFTR-2 (94.688%), KG35 and ATCC 25286 (94.739%), and KG35 and NCTC13726 (94.645%) is represented in the red grid in the heat map (Fig. 1C).
Pangenome analyses.
To understand the genomic diversity among F. necrophorum strains, we performed pangenome analyses to compare core and accessory genes. We performed pangenome analysis on 167 Fusobacterium strains (RefSeq strains plus five test strains), and we included two outgroups to evaluate genus-level diversity. The Fusobacterium genus pangenome contained 52,441 gene clusters (Fig. 2A, part 1). Of the 52,441 gene clusters, 29 (0.05%) constituted the core genes and 2 (0.004%) the soft core genes. There were 3,855 and 42,332 shell and cloud genes, respectively, indicating that 88.07% of the genes in the genome are accessory genes, suggesting that every species diverged enormously and formed new phenotypic traits. The set of 29 core genes included genes encoding cellular translation proteins, such as ribosomal proteins, and enzyme-encoding genes (see Data Set S7 in the supplemental material). Most of these core genes were also included in UBCG sets.
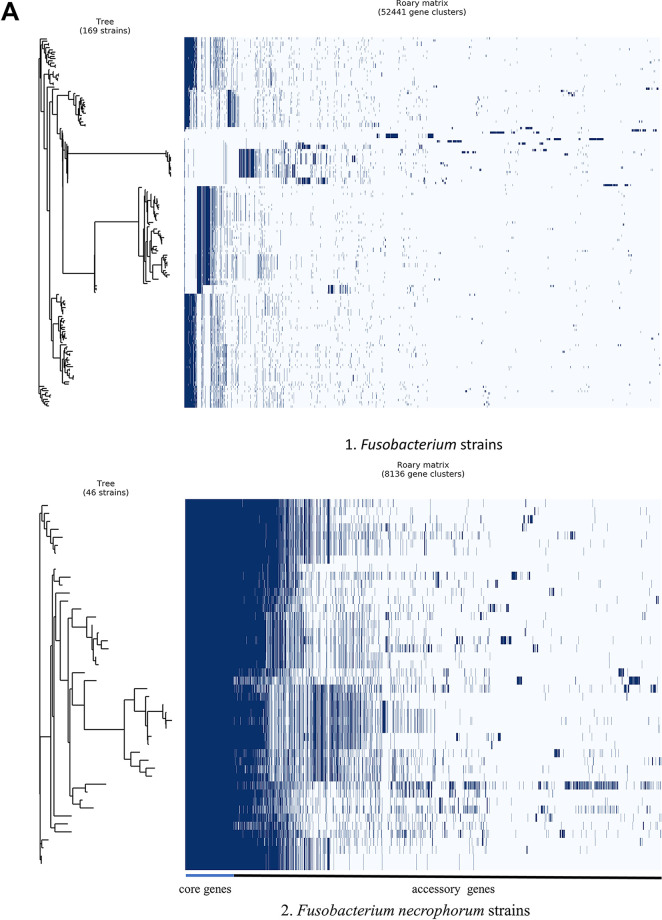
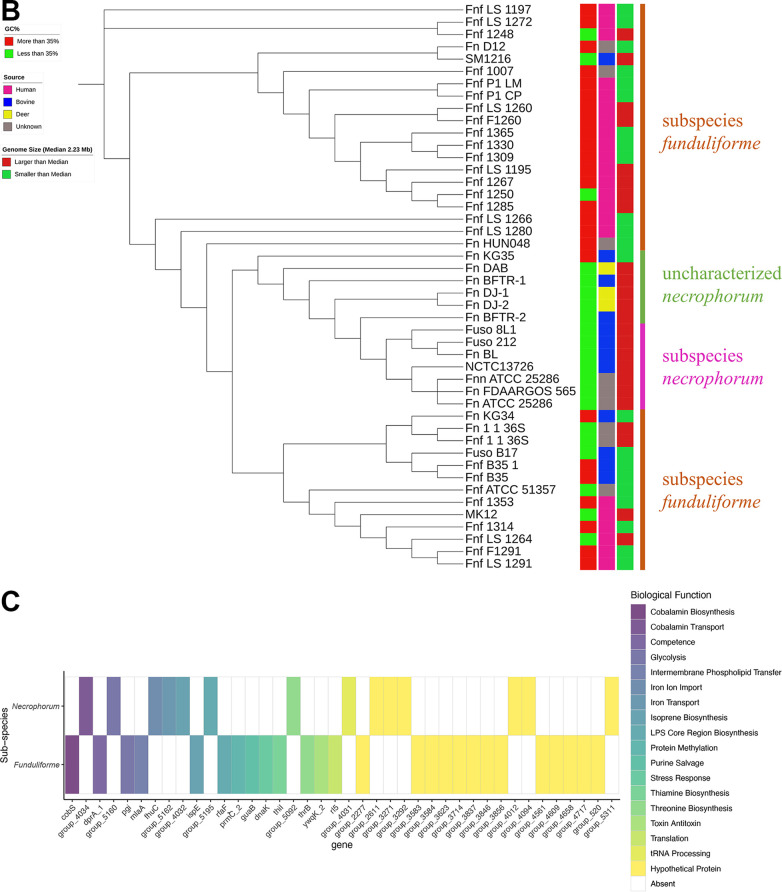
FIG 2.
(A) Pangenome alignment of Fusobacterium species. Pangenome analysis was performed using Roary. Pangenome analysis of Fusobacterium (genus level [part 1]) and F. necrophorum (species level [part 2]). The gene matrix shows the presence (blue blocks) and absence (gray areas) of core and accessory genes. In the matrix, genomes are shown as rows and gene clusters as columns. The dendrogram is based on the phylogenetic relatedness of the core genes and accessory gene clusters. (B) F. necrophorum population structure cladogram. The maximum-likelihood tree was constructed based on the pangenome analysis with 826 core genes and calculated based on the GTR+I+G4 model and 100 bootstrap replicates for 46 F. necrophorum strains. The right side of the cladogram includes three color-coded columns representing GC content, source, and genome size of the isolates. The cladogram forms distinct clades of F. necrophorum subsp. funduliforme, F. necrophorum subsp. necrophorum, and F. necrophorum not identified to the subspecies level. (C) F. necrophorum subsp. necrophorum- and F. necrophorum subsp. funduliforme-specific genes. Genes specific to F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme were separated by functional annotation. In the grid, color represents the presence and the absence of color shows the absence of genes. A total of 40 subspecies-specific genes with different biological functions were identified through the UniProt-based search. Nineteen hypothetical genes identified are shown in yellow.
Next, we performed pangenome analysis on 46 F. necrophorum strains (41 NCBI RefSeq strains plus five test strains) to compare unique genes present at the species level. This pangenome contained 8,136 gene clusters. Of these 8,136 gene clusters, a total of 826 (10.15%) constituted the core genes and 404 (0.0004%) the soft core genes. Notably, the proportions of shell and cloud genes were higher, 1,856 (22.81%) and 5,050 (62.06%), respectively. Using whole-genome alignment with comparisons of core and ancillary genes (generated using Roary), we examined the genetic relatedness of the 46 strains (Fig. 2A, part 2). We made further comparisons by clustering F. necrophorum species based on the alignment of 826 core genes obtained from the 46 F. necrophorum strains (Fig. 2B). In the resulting cladogram, test strains B17, MK12, and SM1216 fell into the F. necrophorum subsp. funduliforme clade. Likewise, test strains 8L1 and 212 formed a separate clade along with some of the unspecified F. necrophorum strains and the known F. necrophorum subsp. necrophorum. These findings demonstrate that F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme formed a distinct clade (Fig. 2B).
Based on the pangenome-wide association analysis, F. necrophorum subsp. funduliforme had 26 subspecies-specific genes and F. necrophorum subsp. necrophorum has 14 subspecies-specific genes. The list of genes with their biological functions is provided in Data Set S4. The subspecies-level unique genes are shown in a binary matrix group and defined by their biological function in Fig. 2C. We observed differences in the biological functional group between the subspecies. These included genes that encode transferases, transporters, an antitoxin YwqK in F. necrophorum subsp. funduliforme and enzymes for various pathways such as vitamin biosynthetic (thiamine and cobalamin) and metabolic pathways (transferases and dehydrogenases). There are some unique genes in F. necrophorum subsp. necrophorum. These genes are for iron transport and biosynthetic pathways. Overall, 19 of 40 genes have not been annotated and are labeled as hypothetical genes.
Distribution of virulence genes and antimicrobial resistance genes.
(i) Virulence genes. Because the widely used virulence factor database did not have the Fusobacterium reference genome (18), we created a custom database with 46 potential virulence genes to analyze. This database was used to determine potential virulence genes in F. necrophorum RefSeq strains and the five test strains. Based on a customized search approach, we identified 13 different virulence genes (Fig. 3A). These virulence genes encoded the following traits: toxins (leukotoxin and hemolysin), adhesion proteins (FadA, YadA C-terminal domain-containing protein, and trimeric autotransporter adhesins), outer membrane proteins (OmpA and -H), invasive capability (VapD), cell envelope (CreD), type IV secretion system, ATP-binding cassette (ABC) transporters, hemolysin activation protein (HecB), and transporter proteins (ShlB and FhaC). The presence of these virulence genes in the test strains is shown in Fig. 3A, and percent sequence similarity is shown in Data Set S6.
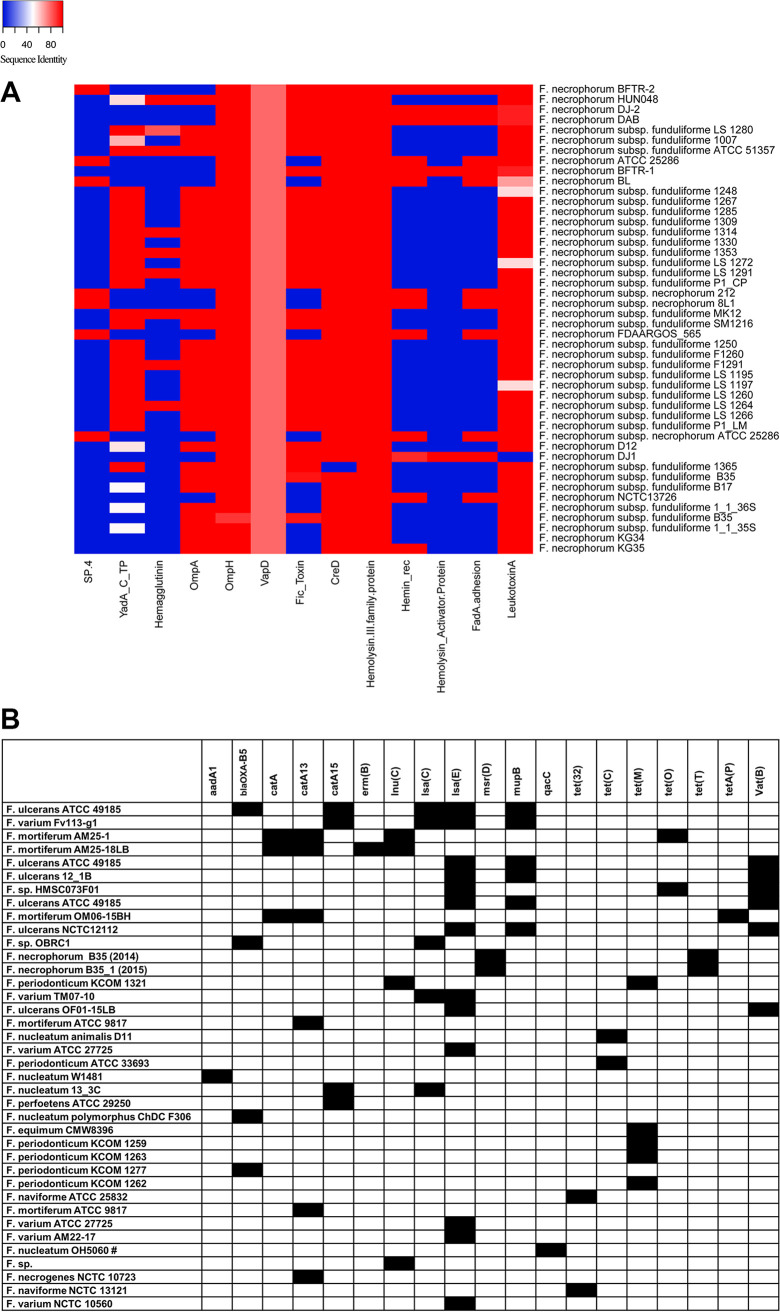
FIG 3.
(A) Virulence genes detected in Fusobacterium necrophorum. The heat map shows the percent sequence identity of 13 potential virulence genes compared to the 46 genes in the custom database. The names of F. necrophorum strains are shown on the right, and the virulence genes are below the heat map. Blue indicates ≤40%, white indicates 40 to 50%, and red indicates >50 to 100% sequence identity. Virulence gene acronyms are defined at the bottom. (B) ARGs detected in Fusobacterium. The gene matrix shows the presence and absence of genes detected in each Fusobacterium genome (indicated on the left) using ABRicate. Black indicates the presence and white indicates the absence of antimicrobial resistance genes shown at the tops of the columns. aadA1, aminoglycoside; blaOXA-85, beta-lactam; catA, catA13, catA15, chloramphenicol; erm(B), macrolide; lnu(C), lincosamide; lsa(C), ABC efflux/lincomycin, clindamycin, tiamulin; lsa(E), pleuromutilin, lincosamide, streptogramin A; msr(D), macrolide; mupB, mupirocin; qacC, quaternary ammonium compound; tet(32), tet(C), tet(M), tet(O), tet(T), tetA(P), tetracycline; vat(B), streptogramin.
In particular, several virulence genes, including those for the type IV secretion system, ABC transporter system, and FadA adhesion protein, were unique to F. necrophorum subsp. necrophorum and not present in F. necrophorum subsp. funduliforme. All phenotypically characterized F. necrophorum subsp. necrophorum strains, including F. necrophorum BL, F. necrophorum FDAARGOS_565, F. necrophorum ATCC 25286, and test strains 8L1 and 212, clustered in the same clade in the phylogenetic tree (Fig. 1B, UBCG tree). A notable exception was one of the RefSeq strains, NCTC13726, as the type IV secretion system was absent despite the strain’s belonging to the same F. necrophorum subsp. necrophorum clade.
Similarly, some virulence genes, such as genes encoding OmpA and Fic toxin, were present in all except F. necrophorum subsp. necrophorum. Furthermore, our genomic analyses identified specific virulence genes, including leukotoxin, hemolysin, ompH, vapD, and creD, that are present in almost all species of F. necrophorum.
(ii) Antimicrobial resistance genes. To identify the presence of ARGs, we screened both the RefSeq sequences and the test strains using ABRicate. This prediction tool identified several acquired ARGs in 38 Fusobacterium genomes, and the list of Fusobacterium species with different ARGs harbored by them is shown in Fig. 3B. We detected 19 acquired ARGs in the tested Fusobacterium strains (Fig. S1). A total of 38 genomes carried at least one of the 19 acquired ARGs available in the ABRicate databases. Most of these genes were harbored by F. ulcerans, F. varium, F. mortiferum, F. periodonticum, F. nucleatum, F. perfoetens, F. equinum, F. naviforme, and F. necrogenes. However, F. necrophorum subsp. funduliforme B35 was the only F. necrophorum strain that carried ARGs.
We found the lsaE gene, which confers resistance to pleuromutilin, lincosamide, and streptogramin A, in 13 genomes of F. ulcerans and F. varium except for one genome (Fusobacterium sp. strain HMSC073F01). Also, we identified the presence of vatB, which confers streptogramin resistance, in F. ulcerans. Furthermore, F. necrophorum subspecies funduliforme B35 harbored both macrolide [msr(D)] and tetracycline [tet(T)] resistance genes.
Recombination events in F. necrophorum core genes.
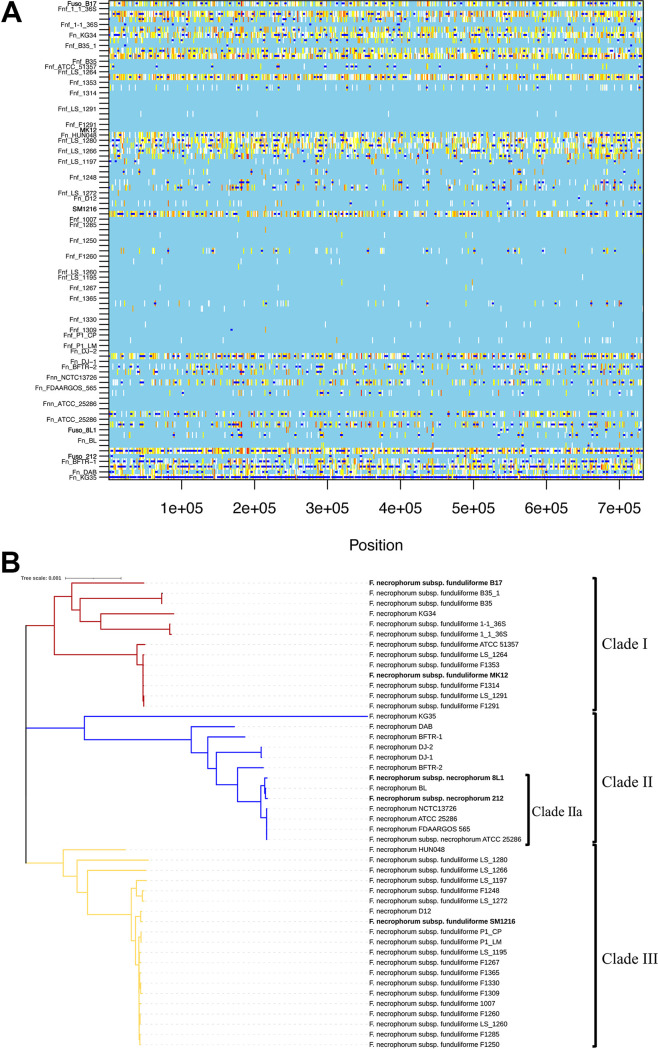
Recombination events can occur from one lineage to another within a single species and can lead to subspeciation. To investigate whether F. necrophorum subspeciation results from recombination events, we applied the ClonalFrameML model. Genome-wide recombination events in F. necrophorum, based on 46 genomes representing carriage and reference isolates assigned to F. necrophorum assembly genomes, are represented in Fig. 4A and B. Figure 4A is the graphical presentation of the recombination events, and Fig. 4B shows a phylogenetic tree constructed based on the recombination events. The dark blue line segments represent recombination sites. For a given branch, sites with no substitutions are shown in light blue, and any color ranging from white to red indicates substitution via mutation. Overall, we detected 1,615 recombination events.
FIG 4.
(A) ClonalFrameML analysis of recombination in F. necrophorum based on 46 genomes mapped to F. necrophorum assembly genomes. In the recombination plot, white vertical bars indicate reconstructed substitutions and dark blue horizontal bars indicate recombination events for each branch of the maximum-likelihood tree. The strain names are on the left, and the sizes and gene positions of the recombination events are shown across the alignment. (B) Phylogenetic tree constructed based on ClonalFrameML analysis of recombination in 46 F. necrophorum genomes, five tests and 41 reference strains. Three distinct clades are formed, with the red and yellow branches representing clade I and III, specific to F. necrophorum subsp. funduliforme, and the blue branch representing clade II, specific to F. necrophorum subsp. necrophorum (clade IIa), with F. necrophorum strains not identified to the subspecies level. Strain KG35 had the longest branch, which indicates significant recombination events. Test strains are in bold. The scale bar represents the length of the branch corresponding to the number of changes per site.
Based on the ClonalFrameML analysis, we used the following metrics to study the relative effect of recombination and mutation on the F. necrophorum core genome (Data Set S8). This analysis yielded the following metrics: the relative rate of recombination with mutation (R/θ) was 0.30455, the mean length of the recombined DNA (δ) was 286, and the mean divergence of imported DNA (ν) was 0.0371. Each recombination event generated approximately 10.61 substitutions (i.e., the product of ν and δ). The ratio of changes introduced by recombination to changes introduced by mutation (r/m) was 3.226 (i.e., the product of R/θ, δ, and ν) (19). These findings suggest that recombination caused three times more substitutions than mutation. Consistent with this, our pangenome analysis revealed minimal core gene similarity between F. necrophorum species, supporting our finding that there has been extensive genomic-level recombination. This is likely the case because core functions are less susceptible to recombination, which would confer a fitness disadvantage and result in the loss of bacteria from the population. This output suggests that recombination occurred in accessory genes associated with pathogenesis and other accessory functions. These accessory genes can undergo recombination without causing loss of function and may result in a divergent group within species. Extensive recombination events occurred in strain KG35 (Fig. 4A), and the recombination events commonly occurred among F. necrophorum subsp. necrophorum strains, giving rise to multiple evolutionary distances within the species.
Because recombination events are usually constructed to correct branch length and identify true phylogeny, we used recombination events analysis to determine whether the recombining genes and events support putative virulence character evolution in Fusobacterium. Our phylogenetic analysis (Fig. 4B) revealed that F. necrophorum subsp. funduliforme possibly underwent several recombination events to evolve into three distinct F. necrophorum subsp. funduliforme clades (clades I and III) and a group of uncharacterized F. necrophorum strains (clade II). Later, clade II evolved to form a distinct clade of F. necrophorum subsp. necrophorum (clade IIa), which includes test strains 8L1 and 212 and reference strains BL and FDAARGOS 565. When comparing the genomic size with respect to recombination, we found that the genomic size of the strains in clade II is greater than that of F. necrophorum subsp. funduliforme (Fig. 4B). These findings suggest that widespread recombination in clade II may give rise to subspecies different from F. necrophorum subsp. funduliforme. However, we did not identify a genomic size correlation with respect to strain KG35, which shows proximity in phylogeny-based recombination within clade II. Similarly, test strains MK12 and B17 appeared to have undergone fewer recombination events and lie closer to their ancestor strains, but each fell in a different branch, while SM1216 was in a different clade of F. necrophorum subsp. funduliforme descendants.
DISCUSSION
In the present study, we provided genomic sequence analysis of the genus Fusobacterium and the species F. necrophorum to understand genomic variability and species relatedness and to characterize five clinical isolates. To date, very few studies have investigated the population structure of Fusobacterium. We included 167 Fusobacterium strains for which the whole genome has been sequenced (the 162 latest [July 2019] RefSeq strains of Fusobacterium, including F. necrophorum RefSeq strains, plus five test strains) for our analyses. The Fusobacterium phylogenic topology published by McGuire et al. is comparable to the phylogeny in our study (20), although F. necrophorum strain D12 was shown to be in closer relation to F. gonidiaformans ATCC 25563. However, the data comparison of this study was based on only 26 genomes. In our analysis, which consisted of 167 Fusobacterium genomes, we observed a similar output in our UBCG-based cladogram (Fig. 1A) and noted a close relationship of F. necrophorum with F. gonidiaformans. Furthermore, D12 is far inside with proximity to the test strain SM1216 in the cladogram of our study. Our analyses showed that F. necrophorum KG35 was the outermost genome, as this strain appeared to be an outlier in our subsequent analyses. This finding suggests that strain KG35 may be the most ancestral genome available for F. necrophorum, which could have descended from the species F. gonidiaformans during evolution. On the other hand, KG35 could belong to a different entity than F. necrophorum. This hypothesis could be corroborated by evaluating the ANI output for ANI values ranging below <95%, because 95% ANI-based demarcation is considered acceptable for intraspecies classification.
We used conserved genes or core genes to understand Fusobacterium species’ phylogeny. Our analysis revealed a wide range of genomic variability, with only 29 core genes shared in the genus Fusobacterium, including those that encode ribosomal proteins and some essential catalyzing enzymes (Fig. 2A). The accessory genes contributed to >88% of the pangenome, thus indicating substantial heterogeneity among Fusobacterium species. All bacteria have developed ways to transfer or acquire genes through various mechanisms with high recombination rates (21). The variability we observed within this genus indicates that these bacterial species had been through a series of evolutionary events that gave rise to 28 species in the genus Fusobacterium (22), suggesting that these core genes may be essential for the survival of the bacteria.
The results of this study also explain the evolutionary changes that led to the genetic divergence of the species F. necrophorum into F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme. Critically, there were some F. necrophorum RefSeq strains used in this study that were not identified to the subspecies level. We were able to predict the subspecies of these unclassified RefSeq strains based on the constructed phylogenetic tree and how these strains clustered (Fig. 1B and Table 1). Therefore, our analyses predicted that strain BL and FDAARGOS 566 are F. necrophorum subsp. necrophorum. Additionally, our analyses indicated that the uncharacterized F. necrophorum strains DAB, BFTR-1, BFTR-2, DJ-1, and DJ-2 could belong to F. necrophorum subsp. necrophorum. Although the orthologous-group-based (pangenome) analysis confirmed genetic dissimilarity among subspecies, this analysis did not provide any definite correlation between GC content and source (23). Despite this, the genome was larger in F. necrophorum subsp. necrophorum than in F. necrophorum subsp. funduliforme.
To specify the genes unique to the subspecies, further analysis using pangenome output and the Scoary program identified 14 and 26 genes unique to the F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme, respectively. Among the unique genes specific to subspecies, no direct virulence gene was observed in any subspecies. However, different transferases, including those involved in vitamin biosynthesis that have a specific role in biochemical pathways and are integral to bacterial survival, were identified. Some vitamins are known to promote virulence, for example, vitamin B2 and vitamin B6 (24–26). Similarly, biosynthetic transferases for the vitamins cobalamin and thiamine identified in our analyses are also known to be involved in virulence and influence the ability of the pathogen to infect a host studied in some bacteria (27, 28). Some of the other transferases, such as 4-diphosphocytidyl-2-C-methyl-D erythritol kinase, which is involved in isoprene biosynthesis, could be involved in survival and competition in a mixed-culture environment and be specific to F. necrophorum subsp. funduliforme (29). Similarly, the iron transporter gene hmu is known to be involved in hemin utilization and is involved in iron acquisition in Yersinia pestis (30). The acquisition of iron is essential for bacterial growth and promotion, as well as for pathogenicity and virulence. This feature is specific to F. necrophorum subsp. necrophorum.
To evaluate genetic relatedness and characterize the five clinical isolates, we evaluated the ANI of orthologous fragment pairs shared between the two genomes (31). Our analysis revealed that the calculated ANI values between the F. necrophorum strains and the five test strains were ≥95% identical, a recommended threshold for demarcation for bacterial species (32). These results further confirmed that all test strains (8L1, 212, B17, MK12, and SM1216) belonged to Fusobacterium necrophorum. Kook et al. used the same approach to calculate ANI values in F. nucleatum subspecies and found that all four examined subspecies (F. nucleatum subsp. nucleatum, F. nucleatum subsp. polymorphum, F. nucleatum subsp. vincentii, and F. nucleatum subsp. animalis) had a pairwise ANI of >96% (33). The study by Kook et al. led to a result similar to that in Fig. 1A, where the phylogenetic tree based on the 16S rRNA gene was constructed and revealed that F. hwasookii clustered with F. nucleatum subsp. polymorphum. However, it has been argued that the above-mentioned subspecies of F. nucleatum should be considered separate species, as they all differ significantly at the DNA level (34).
With an increase in Fusobacterium pathogenesis, it is necessary to identify virulence genes that can be exploited to treat related diseases. The rapid rise of sequencing technologies and genomic analysis has provided an important platform for exploring causes of pathogenicity, as well as determining virulence genes and antimicrobial resistance patterns. However, the lack of reference genomes for more virulent subspecies, such as F. necrophorum subsp. necrophorum, for which few sequenced genomes are publicly available, has made phylogenetic analysis and identification of potential virulence factors a challenge. To overcome this challenge, we performed a custom-based virulence gene search using all 46 F. necrophorum strains, which enabled us to broaden the search probability. Based on this search, we identified 13 different virulence genes distributed among F. necrophorum strains. Some of these virulence genes were explicitly found in only one subspecies. For example, virulence genes encoding the type IV secretion system (T4SS), hemin receptor, and FadA adhesin were found only in the F. necrophorum subspecies necrophorum. On the other hand, YadA-like family proteins, hemagglutinin, OmpA, and Fic toxin, were only found in F. necrophorum subsp. funduliforme. Also, a set of genes, including ompH and those encoding virulence-associated protein (vapD), hemolysin III family protein, cell envelope integrity protein (creD), and leukotoxin, were found in both subspecies. However, there were notable exceptions for creD and leukotoxin A genes, as creD was absent in F. necrophorum subsp. funduliforme F1365 and leukotoxin A was absent in F. necrophorum DJ1.
Our study found the fadA gene in F. necrophorum subsp. necrophorum. Because fadA is a virulence gene involved in host cell attachment and encodes a vital invasion factor for active invaders in F. nucleatum infections (35), our findings suggest that F. necrophorum subsp. necrophorum could be classified as an active invader (20, 36). The absence of the invasion factor (FadA) in F. necrophorum subsp. funduliforme could explain why F. necrophorum subsp. funduliforme often occur in association with mixed bacterial infection (37–39). However, more studies are needed to confirm whether F. necrophorum subsp. necrophorum is an active invader.
Previous reports have documented the role of T4SS in horizontal gene transfer of antimicrobial resistance and virulence genes (40, 41). Since F. necrophorum appeared to have evolved from F. nucleatum, the presence of T4SS in F. necrophorum subsp. necrophorum indicates the possibility of horizontal acquisition of fadA from F. nucleatum (42). Furthermore, the hemin receptor (hemin_rec), which was found in F. necrophorum subsp. necrophorum (43), plays an essential role in the colonization and proliferation of many pathogenic bacteria. Therefore, the hemin receptor may be a potential target for the development of vaccines and therapies for F. necrophorum infections.
Fusobacterial infections are commonly treated with antibiotics such as penicillin, amoxicillin, clindamycin, and imipenem. Although antimicrobial resistance is not commonly found in this genus, in contrast to other pathogens, the emergence of resistance is inevitable. Our genome sequence analysis showed few ARGs distributed among all Fusobacterium species other than F. necrophorum. Only two ARGs were found in F. necrophorum (1/46 strains tested), indicating the chances of antimicrobial resistance emergence in Fusobacterium species. Therefore, it is necessary to develop novel and effective therapeutic and prevention strategies, such as vaccination, before the development of resistance in this bacterial species. It is of the utmost importance to search for potential virulence targets that could be used to develop therapeutic and prevention strategies against fusobacterial infection.
Pathogenic and pandemic fusobacterial strains are often the result of recombination events. We focused this study on F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme, as their biological activities differ. We investigated recombination events that might have influenced evolutionary diversification of the F. necrophorum subspecies. The results revealed that F. necrophorum subsp. funduliforme may have undergone a genomic expansion during evolution, leading to a clade of a virulent form of F. necrophorum subsp. funduliforme and another unclassified clade of F. necrophorum. Later, this unclassified F. necrophorum subsp. necrophorum clade could have evolved to more virulent forms of F. necrophorum subsp. necrophorum. Similarly, some of the virulence genes found only in F. necrophorum subsp. necrophorum reflect evidence of recombination during evolution in the F. necrophorum subsp. necrophorum genome. Our data demonstrate that the difference observed is evidence for the effect of recombination events on the evolutionary relationship with other Fusobacterium necrophorum strains (Fig. 4A) (44). Taken together, the results of this study suggest that recombination events played a significant defining role in F. necrophorum, resulting in F. necrophorum subsp. necrophorum being more virulent than F. necrophorum subsp. funduliforme.
MATERIALS AND METHODS
Strains.
Five strains of F. necrophorum, including four bovine isolates (8L1, B17, 212, and SM1216) and one human isolate (MK12), were used in this study (Table 1). These strains were isolated and identified from previously described clinical cases (37). For all strains, DNA extraction was performed as previously mentioned (45). Briefly, the strains were grown in prereduced anaerobically sterilized brain heart infusion (PRAS-BHI) broth. After overnight growth, cells were pelleted by centrifugation (6,500 × g) and resuspended in TES buffer (50 mM Tris-HCl [pH 7.5], 1 mM EDTA, and 25% sucrose). The cells were then pretreated with lysozyme (room temperature, 30 min) and lysed using Sarkosyl NL (Sigma-Aldrich, St. Louis, MO, USA) with proteinase K (Sigma-Aldrich) (60°C, for 1 h). The crude lysate was resuspended in Tris-buffer-saturated phenol-chloroform. DNA was extracted by precipitation in 2.5 mL ice-cold ethanol, and the pellet was resuspended in TE buffer (10 mM Tris-HCl and 1 mM EDTA; pH 8). Subsequently, the DNA was purified with a cesium chloride density gradient followed by dialysis against double-distilled water. The purity and concentration were checked spectrophotometrically. Purified DNA was used for paired-end sequencing at HiSeq using the Illumina platform.
Furthermore, 162 Fusobacterium sequence files and 41 F. necrophorum RefSeq assemblies were downloaded from the NCBI database (Data Sets S1 and S2). The sequences downloaded for this study were selected to generate a diverse data set for comparative genome analysis. Furthermore, genomes of strains belonging to two different genera, Leptotrichia buccalis DSM 1135 and Cetobacterium somerae ATCC BAA-474, were included in the data as an outgroup.
Bioinformatic analysis.
(i) Data preprocessing. All raw read preprocessing was performed on the Galaxy platform (https://usegalaxy.org/). For quality control, raw sequences were preprocessed using FastQC software v0.11.7. Based on the FastQC report, quality trimming was performed using Trimmomatic to filter and trim low-quality reads (46). The processed paired-end reads were used for subsequent analysis.
(ii) Genome assembly, quality check, and annotation. The quality trimmed reads were assembled on the Galaxy platform (https://usegalaxy.org/) using SPAdes with default parameters (47), and contigs shorter than 200 bp were discarded. The quality of the final assemblies was checked using QUAST, a quality assessment tool (48). Using the Rapid Prokaryotic Genome Annotation (PROKKA) annotation, curated assemblies were annotated (49). Subsequently, the annotation files (.gff) produced by PROKKA analysis were used to create the pangenome.
Phylogenetic analysis by core gene alignment and average nucleotide identity.
Phylogenetic trees were constructed based on the core gene alignment (UBCG) and the average nucleotide identity (ANI) values.
(i) Up-to-date bacterial core gene. To predict genomic relationships at the genus and species levels, a UBCG approach with default parameters was used (50). These trees were constructed using the concatenated alignment of the core genes of bacteria. A manually annotated GenBank file with 162 NCBI reference Fusobacterium sequences and the latest NCBI RefSeq assembly of 41 F. necrophorum species were downloaded (https://www.ncbi.nlm.nih.gov/assembly; last accessed July 2019). The sets of 167 Fusobacterium genus genomes (162 NCBI RefSeq sequences plus five test strains) were used for the analysis of evolutionary relationships at the genus level between different species. A similar phylogenetic analysis was performed for 46 (41 NCBI RefSeq sequences plus five test strains) F. necrophorum genomes. For each UBCG, the core gene sequences were aligned with multiple-sequence alignment. The resulting alignments were concatenated, and phylogeny was inferred using the maximum-likelihood (ML) algorithm in the RAxML v 7.8.6 tool using the GTR+I+G4 model of nucleotide substitution (51). All trees were visualized using iTOL, a web tool for phylogenetic tree display (https://itol.embl.de/) (52) (Data Set S3).
(ii) Average nucleotide identity analysis. The DNA-DNA relatedness and orthologous genes shared between species of F. necrophorum were analyzed using the FastANI algorithm by constructing an ANI heat map (53). Genetic relatedness for pairwise comparisons of 46 genome sequences, including five test strains, was measured by the ANI of all conserved orthologous genes calculated using the FastANI method (version 1.3). ANI values were calculated as previously described (53). Items in the FastANI matches that showed ≥95% nucleotide sequence identity and ≥70% sequence coverage were selected to calculate ANI. The fragment length was set at 1,000 bases and a k-mer size of 16, with the minimum fraction set to 0.5. The output was used to generate a heat map and identify relatedness.
Pangenome analysis.
Pangenome analyses are composed of core genome detection (genes common to all strains) and detection of accessory or ancillary genes (genes common to only a subset of the strains and strain-specific genes). Comparative genomic analysis was performed for the five curated genome assemblies using the published NCBI RefSeq database for Fusobacterium and F. necrophorum at both the genus and species levels. Genome sequences annotated by the PROKKA (49) pipeline using the Roary program (54) were used to create the pangenome. The core gene alignment file obtained from Roary was taken for model testing using RAxML. Based on RAxML, the GTR+I+G4 model was the best-scoring model to generate the ML estimate. Later, this model was used to generate the phylogenetic trees; ML and iTOL were used for phylogenetic tree construction.
A pairwise comparison was performed to identify subspecies specific gene differences between F. necrophorum subspecies. The comparison was performed in the Scoary program to identify the uniqueness of the gene via evaluation of the highest-scoring nonintersecting gene pairs, sorted by P value (55). The analysis was done using the gene presence-absence output of Roary and UBCG subspecies classification as phenotypic traits.
Identification of virulence genes.
We methodically searched the literature to identify potential virulence genes in Fusobacterium and related species for virulence genes. The literature search results were used to create a list of potential virulence genes; then, a local database was created through a manual search with the FASTA sequences of these genes (Data Set S5). These genes were checked against the strains used for comparative analysis at the genus and species levels.
Identification of antimicrobial resistance genes.
ARGs were investigated in the latest NCBI RefSeq sequences of the genus Fusobacterium and the contigs of the five test strains. The ABRicate v0.8.10 search engine (https://github.com/tseemann/ABRicate) was used to screen ARGs with the following databases: ResFinder (56), NCBI AMR FinderPlus (57), and CARD (v2.0.3) (58). Positive-hit cutoffs of 50% sequence identity and 30% sequence coverage were used for analysis.
Recombination analysis.
Recombination events and evidence of positive selection were analyzed using ClonalFrameML, an interactive application using default parameters (59). Briefly, multiple-sequence alignments of RefSeq F. necrophorum genomes with test strains were inferred using RAxML and analyzed using ClonalFrameML. An ML tree was constructed based on the recombination events, and the clonal genealogy was reiterated until the best tree was obtained. ClonalFrameML uses Baum-Welch expectation maximization and Viterbi algorithms to obtain an ML estimate of the recombination parameters, branch lengths of clonal genealogy, and ML importation status.
Data availability.
Sequencing raw data are available from the latest version of RefSeq: https://www.ncbi.nlm.nih.gov/assembly/?term=Fusobacterium and https://www.ncbi.nlm.nih.gov/assembly/?term=Fusobacterium±necrophorum. Assembly accession numbers (or GenBank Assembly IDs and RefSeq Assembly IDs) for the assemblies analyzed in this manuscript are provided in the supplementary material see Data Set S1.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to the team at South Dakota State University, where all bioinformatic coding was performed for this study. The clinical test strains were isolated at Kansas State University as part of a research study.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Sanjeev K. Narayanan, Email: sanjeev@purdue.edu.
Philip N. Rather, Emory University School of Medicine
REFERENCES
- 1.Finegold SM. 1996. Anaerobic Gram-negative bacilli, 4.11.1–4.11.17. In Baron S (ed), Medical Microbiology, 4th ed. University of Texas Medical Branch at Galveston, Galveston, TX. [PubMed] [Google Scholar]
- 2.Riordan T, Wilson M. 2004. Lemierre’s syndrome: more than a historical curiosa. Postgrad Med J 80:328–334. doi: 10.1136/pgmj.2003.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riordan T. 2007. Human infection with Fusobacterium necrophorum (necrobacillosis), with a focus on Lemierre’s syndrome. Clin Microbiol Rev 20:622–659. doi: 10.1128/CMR.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos-Vara JA, Rook J, Scanlan CM, Mugli F, Yamini B. 1997. Fusobacterium necrophorum septicemia in a lamb: pathologic and microbiologic characterization. J Vet Diagn Invest 9:79–82. doi: 10.1177/104063879700900115. [DOI] [PubMed] [Google Scholar]
- 5.Bennett KW, Eley A. 1993. Fusobacteria: new taxonomy and related diseases. J Med Microbiol 39:246–254. doi: 10.1099/00222615-39-4-246. [DOI] [PubMed] [Google Scholar]
- 6.Narongwanichgarn W, Misawa N, Jin JH, Amoako KK, Kawaguchi E, Shinjo T, Haga T, Goto Y. 2003. Specific detection and differentiation of two subspecies of Fusobacterium necrophorum by PCR. Vet Microbiol 91:183–195. doi: 10.1016/S0378-1135(02)00295-X. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen LH, Prag J. 2000. Human necrobacillosis, with emphasis on Lemierre’s syndrome. Clin Infect Dis 31:524–532. doi: 10.1086/313970. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraja TG, Narayanan SK, Stewart GC, Chengappa MM. 2005. Fusobacterium necrophorum infections in animals: pathogenesis and pathogenic mechanisms. Anaerobe 11:239–246. doi: 10.1016/j.anaerobe.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan SK, Nagaraja TG, Okwumabua O, Staats J, Chengappa MM, Oberst RD. 1997. Ribotyping to compare Fusobacterium necrophorum isolates from bovine liver abscesses, ruminal walls, and ruminal contents. Appl Environ Microbiol 63:4671–4678. doi: 10.1128/aem.63.12.4671-4678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narayanan SK, Nagaraja TG, Chengappa MM, Stewart GC. 2001. Electrophoretic mobility anomalies associated with PCR amplification of the intergenic spacer region between 16S and 23S ribosomal RNA genes of Fusobacterium necrophorum. J Microbiol Methods 46:165–169. doi: 10.1016/s0167-7012(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson LA, Morrow CJ, Corner LA, Hodgson ALM. 1994. Phylogenetic relationship of Fusobacterium necrophorum A, AB, and B biotypes based upon 16S rRNA gene sequence analysis. Int J Syst Bacteriol 44:315–319. doi: 10.1099/00207713-44-2-315. [DOI] [PubMed] [Google Scholar]
- 12.Okwumabua O, Tan Z, Staats J, Oberst RD, Chengappa MM, Nagaraja TG. 1996. Ribotyping to differentiate Fusobacterium necrophorum subsp. necrophorum and F. necrophorum subsp. fundiliforme isolated from bovine ruminal contents and liver abscesses. Appl Environ Microbiol 62:469–472. doi: 10.1128/aem.62.2.469-472.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanan S, Stewart GC, Chengappa MM, Willard L, Shuman W, Wilkerson M, Nagaraja TG. 2002. Fusobacterium necrophorum leukotoxin induces activation and apoptosis of bovine leukocytes. Infect Immun 70:4609–4620. doi: 10.1128/IAI.70.8.4609-4620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marler LM, Siders JA, Wolters LC, Pettigrew Y, Skitt BL, Allen SD. 1991. Evaluation of the new RapID-ANA II system for the identification of clinical anaerobic isolates. J Clin Microbiol 29:874–878. doi: 10.1128/jcm.29.5.874-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read TD, Massey RC. 2014. Characterizing the genetic basis of bacterial phenotypes using genome-wide association studies: a new direction for bacteriology. Genome Med 6:109. doi: 10.1186/s13073-014-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertelli C, Greub G. 2013. Rapid bacterial genome sequencing: methods and applications in clinical microbiology. Clin Microbiol Infect 19:803–813. doi: 10.1111/1469-0691.12217. [DOI] [PubMed] [Google Scholar]
- 17.Tadepalli S, Narayanan SK, Stewart GC, Chengappa MM, Nagaraja TG. 2009. Fusobacterium necrophorum: a ruminal bacterium that invades liver to cause abscesses in cattle. Anaerobe 15:36–43. doi: 10.1016/j.anaerobe.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Xiong Z, Sun L, Yang J, Jin Q. 2012. VFDB 2012 update: toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Res 40:D641–D645. doi: 10.1093/nar/gkr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Didelot X, Falush D. 2007. Inference of bacterial microevolution using multilocus sequence data. Genetics 175:1251–1266. doi: 10.1534/genetics.106.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuire AM, Cochrane K, Griggs AD, Haas BJ, Abeel T, Zeng Q, Nice JB, Macdonald H, Birren BW, Berger BW, Allen-Vercoe E, Earl AM. 2014. Evolution of invasion in a diverse set of Fusobacterium species. mBio 5:e01864-14. doi: 10.1128/mBio.01864-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francino MP. 2012. The ecology of bacterial genes and the survival of the new. Int J Evol Biol 2012:394026. doi: 10.1155/2012/394026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parte AC. 2018. LPSN—list of prokaryotic names with standing in nomenclature (Bacterio.net), 20 years on. Int J Syst Evol Microbiol 68:1825–1829. doi: 10.1099/ijsem.0.002786. [DOI] [PubMed] [Google Scholar]
- 23.Mira A, Martín-Cuadrado AB, D’Auria G, Rodríguez-Valera F. 2010. The bacterial pan-genome: a new paradigm in microbiology. Int Microbiol 13:45–57. doi: 10.2436/20.1501.01.110. [DOI] [PubMed] [Google Scholar]
- 24.Bonomi HR, Marchesini MI, Klinke S, Ugalde JE, Zylberman V, Ugalde RA, Comerci DJ, Goldbaum FA. 2010. An atypical riboflavin pathway is essential for Brucella abortus virulence. PLoS One 5:e9435. doi: 10.1371/journal.pone.0009435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dick T, Manjunatha U, Kappes B, Gengenbacher M. 2010. Vitamin B6 biosynthesis is essential for survival and virulence of Mycobacterium tuberculosis. Mol Microbiol 78:980–988. doi: 10.1111/j.1365-2958.2010.07381.x. [DOI] [PubMed] [Google Scholar]
- 26.Grubman A, Phillips A, Thibonnier M, Kaparakis-Liaskos M, Johnson C, Thiberge J-M, Radcliff FJ, Ecobichon C, Labigne A, de Reuse H, Mendz GL, Ferrero RL. 2010. Vitamin B6 is required for full motility and virulence in Helicobacter pylori. mBio 1:e00112-10. doi: 10.1128/mBio.00112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowley CA, Kendall MM. 2019. To B12 or not to B12: five questions on the role of cobalamin in host-microbial interactions. PLoS Pathog 15:e1007479. doi: 10.1371/journal.ppat.1007479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schauer K, Stolz J, Scherer S, Fuchs TM. 2009. Both thiamine uptake and biosynthesis of thiamine precursors are required for intracellular replication of Listeria monocytogenes. J Bacteriol 191:2218–2227. doi: 10.1128/JB.01636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eoh H, Narayanasamy P, Brown AC, Parish T, Brennan PJ, Crick DC. 2009. Expression and characterization of soluble 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase from bacterial pathogens. Chem Biol 16:1230–1239. doi: 10.1016/j.chembiol.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson JM, Jones HA, Perry RD. 1999. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect Immun 67:3879–3892. doi: 10.1128/IAI.67.8.3879-3892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu XW, De Meyer S, Trujillo ME. 2018. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol 68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 32.Lee I, Kim YO, Park SC, Chun J. 2016. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol 66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 33.Kook JK, Park SN, Lim YK, Cho E, Jo E, Roh H, Shin Y, Paek J, Kim HS, Kim H, Shin JH, Chang YH. 2017. Genome-based reclassification of Fusobacterium nucleatum subspecies at the species level. Curr Microbiol 74:1137–1147. doi: 10.1007/s00284-017-1296-9. [DOI] [PubMed] [Google Scholar]
- 34.Gupta RS, Sethi M. 2014. Phylogeny and molecular signatures for the phylum Fusobacteria and its distinct subclades. Anaerobe 28:182–198. doi: 10.1016/j.anaerobe.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Yamada M, Li M, Liu H, Chen SG, Han YW. 2007. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem 282:25000–25009. doi: 10.1074/jbc.M611567200. [DOI] [PubMed] [Google Scholar]
- 36.Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK, Deng CX. 2005. Identification and characterization of a novel adhesin unique to oral fusobacteria. J Bacteriol 187:5330–5340. doi: 10.1128/JB.187.15.5330-5340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechtenberg KF, Nagaraja TG, Chengappa MM. 1998. Antibiotic susceptibility of Fusobacterium necrophorum isolated from liver abscesses. Am J Vet Res 59:44–747. [PubMed] [Google Scholar]
- 38.Langworth BF. 1977. Fusobacterium necrophorum: its characteristics and role as an animal pathogen. Bacteriol Rev 41:373–390. doi: 10.1128/br.41.2.373-390.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scanlan CM, Hathcock TL. 1983. Bovine rumenitis-liver abscess complex: a bacteriological review. Cornell Vet 73:288–297. [PubMed] [Google Scholar]
- 40.Fronzes R, Christie PJ, Waksman G. 2009. The structural biology of type IV secretion systems. Nat Rev Microbiol 7:703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallden K, Rivera-Calzada A, Waksman G. 2010. Type IV secretion systems: versatility and diversity in function. Cell Microbiol 12:1203–1212. doi: 10.1111/j.1462-5822.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascales E, Christie PJ. 2003. The versatile bacterial type IV secretion systems. Nat Rev Microbiol 1:137–149. doi: 10.1038/nrmicro753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagy G, Dobrindt U, Kupfer M, Emödy L, Karch H, Hacker J. 2001. Expression of hemin receptor molecule ChuA is influenced by RfaH in uropathogenic Escherichia coli strain 536. Infect Immun 69:1924–1928. doi: 10.1128/IAI.69.3.1924-1928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stott CM, Bobay LM. 2020. Impact of homologous recombination on core genome phylogenies. BMC Genomics 21:829. doi: 10.1186/s12864-020-07262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narayanan SK, Nagaraja TG, Chengappa MM, Stewart GC. 2001. Cloning, sequencing, and expression of the leukotoxin gene from Fusobacterium necrophorum. Infect Immun 69:5447–5455. doi: 10.1128/IAI.69.9.5447-5455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nurk S, Bankevich A, Antipov D, Gurevich A, Korobeynikov A, Lapidus A, Prjibelsky A, Pyshkin A, Sirotkin A, Sirotkin Y, Stepanauskas R, McLean J, Lasken R, Clingenpeel SR, Woyke T, Tesler G, Alekseyev MA, Pevzner PA. 2013. Assembling genomes and mini-metagenomes from highly chimeric reads, p 158–170. In Deng M, Jiang R, Sun F, Azhang X (ed), Research in computational molecular biology, RECOMB 2013. Lecture notes in computer science, vol 7821. Springer, Berlin, Germany. [Google Scholar]
- 48.Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 50.Na SI, Kim YO, Yoon SH, min Ha S, Baek I, Chun J. 2018. UBCG: up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J Microbiol 56:280–285. doi: 10.1007/s12275-018-8014-6. [DOI] [PubMed] [Google Scholar]
- 51.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brynildsrud O, Bohlin J, Scheffer L, Eldholm V. 2016. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol 17:238. doi: 10.1186/s13059-016-1108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, Tyson GH, Zhao S, Hsu CH, McDermott PF, Tadesse DA, Morales C, Simmons M, Tillman G, Wasilenko J, Folster JP, Klimke W. 2019. Validating the AMRFINder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob Agents Chemother 63:e00483-19. doi: 10.1128/AAC.00483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FSL, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Didelot X, Wilson DJ. 2015. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLoS Comput Biol 11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Set S1. Download spectrum.00297-22-s0001.xlsx, XLSX file, 0.03 MB (30.5KB, xlsx)
Data Set S2. Download spectrum.00297-22-s0002.xlsx, XLSX file, 0.01 MB (12.5KB, xlsx)
Data Set S3. Download spectrum.00297-22-s0003.xlsx, XLSX file, 0.01 MB (13.6KB, xlsx)
Data Set S4. Download spectrum.00297-22-s0004.xlsx, XLSX file, 1.8 MB (1.8MB, xlsx)
Data Set S5. Download spectrum.00297-22-s0005.xlsx, XLSX file, 0.01 MB (10KB, xlsx)
Data Set S6. Download spectrum.00297-22-s0006.xlsx, XLSX file, 0.01 MB (11.6KB, xlsx)
Data Set S7. Download spectrum.00297-22-s0007.xlsx, XLSX file, 0.01 MB (10KB, xlsx)
Data Set S8. Download spectrum.00297-22-s0008.xlsx, XLSX file, 0.05 MB (55.9KB, xlsx)
Fig. S1. Download spectrum.00297-22-s0009.pdf, PDF file, 0.2 MB (165.1KB, pdf)
Data Availability Statement
Sequencing raw data are available from the latest version of RefSeq: https://www.ncbi.nlm.nih.gov/assembly/?term=Fusobacterium and https://www.ncbi.nlm.nih.gov/assembly/?term=Fusobacterium±necrophorum. Assembly accession numbers (or GenBank Assembly IDs and RefSeq Assembly IDs) for the assemblies analyzed in this manuscript are provided in the supplementary material see Data Set S1.