SUMMARY
The microbiome of the female reproductive tract defies the convention that high biodiversity is a hallmark of an optimal ecosystem. Although not universally true, a homogeneous vaginal microbiome composed of species of Lactobacillus is generally associated with health, whereas vaginal microbiomes consisting of other taxa are generally associated with dysbiosis and a higher risk of disease. The past decade has seen a rapid advancement in our understanding of these unique biosystems. Of particular interest, substantial effort has been devoted to deciphering how members of the microbiome of the female reproductive tract impact pregnancy, with a focus on adverse outcomes, including but not limited to preterm birth. Herein, we review recent research efforts that are revealing the mechanisms by which these microorganisms of the female reproductive tract influence gynecologic and reproductive health of the female reproductive tract.
KEYWORDS: vaginal microbiome, cervical microbiome, uterine microbiome, female reproductive tract, upper genital tract, Lactobacillus, bacterial vaginosis, vaginitis, pregnancy, preterm birth
INTRODUCTION
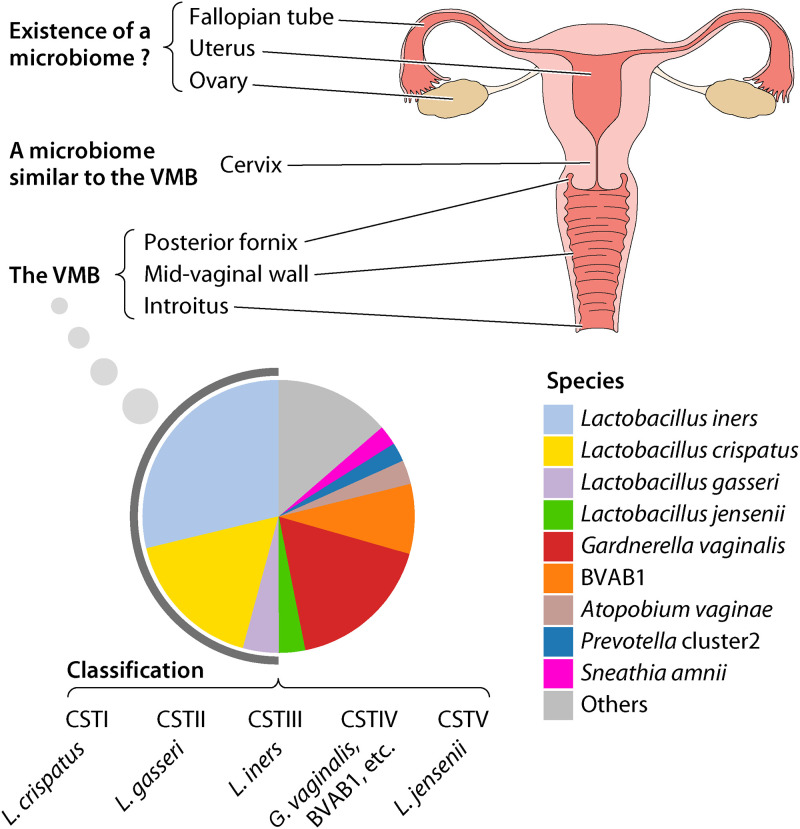
The female reproductive tract is composed of the vagina, the cervix, the uterus, the fallopian tubes, and the ovaries, and the cervix connects the upper reproductive tract to the vagina (Fig. 1) (1). The existence and invasion of microbes in the female reproductive tract have long been known to impact genital and reproductive health. Species of Lactobacillus, generally the most abundant taxa in the vaginal microbiome (VMB), produce lactic acid (2) and probably bacteriocins (3) that inhibit dysbiosis-associated microbes and work to maintain homeostasis and reduce risks of disease. A Lactobacillus-dominated VMB has been the hallmark of female reproductive health. The VMB is less complex than the microbiomes of other body sites, e.g., the oral cavity and the gastrointestinal tract, and the compositions of the microbiomes of the posterior fornix and introitus generally reflect that of the vaginal wall (4). Compared to that in early pregnancy, the VMB in late pregnancy trends toward an even more stable and Lactobacillus-dominated state (5–8), possibly an evolutionarily selected mechanism to ensure a successful pregnancy. VMBs dominated by other taxa, e.g., those associated with bacterial vaginosis (BV), are generally considered suboptimal and have been associated with a higher risk of adverse health (9, 10), including an increased risk of bacterial, viral, and parasitic sexually transmitted infections (STIs) (11), and adverse pregnancy outcomes, including but not restricted to preterm birth (PTB) (5, 12–15). Inflammation caused by microorganisms invading the upper genital tract can lead to adverse pregnancy outcomes, but the source of such microbes remains unclear, and the existence of a natural microbiome in the upper genital tracts of healthy females remains controversial (16, 17).
FIG 1.
Microbiome of the female reproductive tract. In the pie chart showing the composition of the VMB, the taxa are color coded and assigned to the species level. Species belonging to the genus Lactobacillus are highlighted by the gray stripe at the periphery of the pie chart. Data in the pie chart are from a previous study of 2,582 asymptomatic reproductive-age women, 27.2% of whom were pregnant and who had a racial distribution of 52% Black, 20% white, and 28% non-Black Hispanic (8). The VMB can be classified to five community state types (CSTs), and the predominant species in these CSTs are listed. The composition of the cervical microbiome is similar to that of the VMB. The existence of a microbiome in the upper female reproductive tract is still controversial.
Recent studies have provided a deeper understanding of the mechanisms by which the microbiota of the female reproductive tract affects gynecologic and reproductive health (5, 15, 18–23). Herein, we review the microbes in multiple niches of the female reproductive tract and their apparent impacts on human health. Mechanisms by which microbes contribute to maintain overall vaginal health or increase risk for adverse reproductive health are discussed. A particularly impactful target of recent research has been the contributions of microorganisms in the female reproductive tract to adverse pregnancy outcomes. Our review invokes research from the past decade, during which advanced multi-omics technologies, including high-throughput genomic and transcriptomic analyses, have led to rapid advancement of the field. Strategies for the prediction, prevention, and possible intervention to prevent adverse reproductive health outcomes are discussed.
THE VAGINAL MICROBIOME
Composition of the Human Vaginal Microbiome
The initial objective of the National Institutes of Health Human Microbiome Project (HMP; www.hmpdacc.org/hmp), launched in 2008, was to define the human microbiome in health. These early studies confirmed that, compared to the microbiomes of other body habitats, such as the oral cavity and the gastrointestinal tract, the VMB of asymptomatic white reproductive-age females exhibits the lowest community richness and diversity, that it is usually dominated by members of the genus Lactobacillus (4, 24) (Fig. 1), and that the microbiotas of the vaginal introitus, midvaginal wall, and posterior fornix show little distinction (4). Studies of more racioethnically diverse cohorts showed that Lactobacillus spp., i.e., Lactobacillus crispatus, L. gasseri, L. jensenii, and L. iners, and several other taxa, e.g., BV-associated bacterium 1 (BVAB1; “Candidatus Lachnocurva vaginae”), Gardnerella vaginalis, Sneathia amnii, and others (Fig. 1), are the most abundant species in the VMB (5, 8, 25). A landmark study employing high-throughput 16S rRNA taxonomic profiling classified VMBs of nonpregnant reproductive-age women with diverse racioethnicity into four community state types (CSTs) dominated by Lactobacillus spp., i.e., CST I (L. crispatus), CST II (L. gasseri), CST III (L. iners), and CST V (L. jensenii), and a fifth, CST IV, that is more complex and dominated by several anaerobic species (25) (Fig. 1). A more recent study subdivided CST IV into 7 subtypes dominated by different non-Lactobacillus species (26). An alternate but similar approach to classification of VMBs places them into “vagitypes” based on the dominant taxon in the sample (5, 8, 27). Recently, the VIRGO database, which permits classification of the vaginal bacteria at subspecies levels using metagenomic and metatranscriptomic data, was established (28). These classification approaches are based on bioinformatic analyses of taxonomic or gene profiles of the VMB as revealed by high-throughput nucleic acid sequencing. In diagnostic settings, clinical and semiquantitative microscopic observations are generally used to classify the vaginal microbiome (29, 30); i.e., Amsel’s criteria or the semiquantitative Nugent score is used to diagnose BV, and Donder’s score is used for diagnosis of aerobic vaginitis (see below).
Host Factors Affecting the Composition of the Vaginal Microbiome
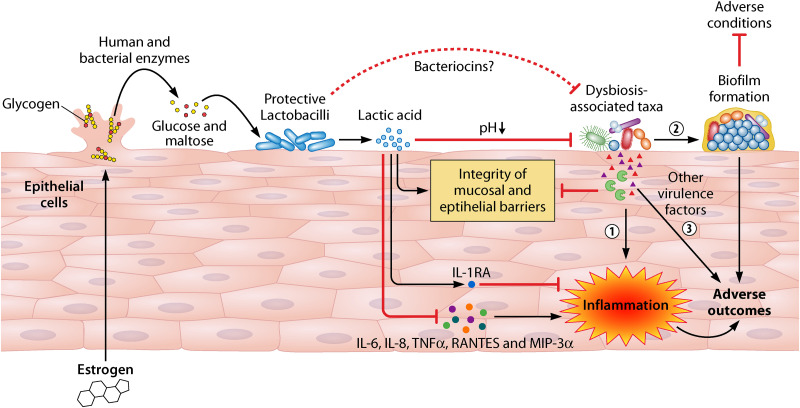
Estrogen is a key host factor in maintaining the vaginal microbiome. It promotes thickening of the vaginal epithelium and generation of intracellular glycogen (9). Host glycogen can be hydrolyzed to glucose and maltose by host alpha amylase, and lactobacilli ferment glucose and maltose into lactic acid, lowering the local pH (2) (Fig. 2). In addition, both in vitro and in silico studies illustrate that multiple vaginal bacteria encode amylase-like enzymes that can metabolize glycogen into glucose and maltose (31–33). Glycogen is probably released from lysed epithelial cells that are induced by high concentrations of lactic acid and cytolysins excreted by Lactobacillus species, and the release of glycogen could be associated with hyaluronidase-1 and matrix metalloproteinase-8 (2). As estrogen levels increase at puberty, the pH of the female genital tract becomes acidic, and meta-analyses on multiple cohorts indicate that the VMB becomes more stable and more likely to be dominated by acidophilic Lactobacillus spp. (6, 34, 35). Likewise, the stability of the VMB and the concentration of estrogen fluctuate during a menstrual cycle and pregnancy, but a positive correlation between these two subjects is consistently observed; i.e., a higher level of estrogen is associated with an increased stability of the VMB, apparently promoting vaginal health (Fig. 3). The opposite occurs after pregnancy and during menopause as estrogen levels decrease, and estrogen therapy of postmenopausal women maintains a more Lactobacillus-dominated state (36, 37). Not surprisingly, use of estrogen-containing contraceptives tends to increase Lactobacillus prevalence (38) while reducing the incidence of BV (39) in reproductive-age women. These data further support the causal relationship between estrogen and the modulation of the VMB. Unlike estrogen, the impact of progesterone on the VMB is not consistent in different studies, as shown in a recent review (40), while an increased level of testosterone seems to be associated with a more complex VMB of women with polycystic ovary syndrome (41).
FIG 2.
Role of the vaginal microbiome in gynecologic and obstetric health. Maternal estrogen promotes the production of glycogen in vaginal epithelial cells. Glycogen released into the female reproductive tract by detachment from or lysing epithelial cells is metabolized to glucose and maltose by human and bacterial α-amylases. Glucose and maltose can be further fermented to lactic acid by Lactobacillus, consequently supporting proliferation of members of this genus. Lactic acid decreases the vaginal pH and, as a result, inhibits the colonization and proliferation of dysbiosis-associated species. Bacteriocins may play roles in controlling colonization and proliferation of microbes generally linked to BV and other adverse conditions. However, lactic acid seems to inhibit host inflammation potentially induced by infections with opportunistic microbes by increasing the anti-inflammatory cytokine IL-1RA and inhibiting proinflammatory cytokines and chemokines, including IL-6, IL-8, TNF-α, RANTES, and MIP-3α. Furthermore, Lactobacillus taxa also seem to promote the integrity of the mucosal and epithelial barriers, thus helping to prevent establishment of these deleterious microbes. In contrast, less favorable taxa induce adverse outcomes by inducing inflammation (circle 1), undergoing biofilm formation (circle 2), and producing other virulence factors, including but not limited to toxins (e.g., vaginolysin and inerolysin), proteases, mucinases, or sialidases (circle 3). See Table 2 for additional BV-associated virulence factors.
FIG 3.
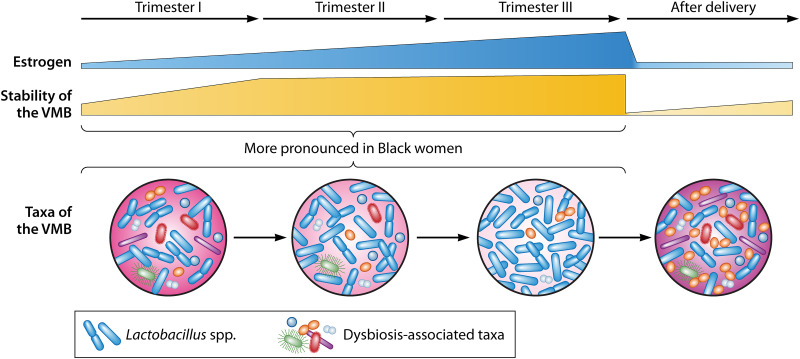
Shift of the vaginal microbiome in pregnancy. Estrogen is one of the key factors that modulate the stability of the VMB. The concentration of estrogen and the stability of the VMB increase during pregnancy. The abundance of lactobacilli increases at the expense of species often related to vaginal dysbiosis, e.g., G. vaginalis, A. vaginae, and P. bivia. This shift in the VMB occurs early in pregnancy and is more pronounced in Black women. After delivery, estrogen levels, along with the stability of the VMB, decrease greatly, and the VMB converts from an optimal state to a state of dysbiosis, which often takes over 40 weeks to recover.
Racioethnicity is another important factor that discriminates the composition of the VMB of reproductive-age women (8, 42, 43). The most dominant taxon in white women is generally L. crispatus, whereas L. iners is prevalent in Asian, Hispanic, and Black women (8, 25). The average vaginal pH of white and Asian women is lower than that of Hispanic and Black women, consistent with a higher abundance of Lactobacillus (25). The VMBs of Black women are less stable, consistent with their more complex microbial communities (8, 43), and exhibit a much higher prevalence of BVAB1, G. vaginalis, S. amnii, and several other anaerobes associated with BV. Black women also suffer a higher risk for STIs and adverse pregnancy outcomes (5, 8, 43, 44). Both genetic differences (45, 46), e.g., known sequence variants among individuals of different races, and environmental differences (47–49), e.g., socioeconomic status and stress, among races likely contribute to these racioethnic differences in VMB composition, and several studies have identified a genetic link between a woman’s genetic background and specific bacterial taxa (45, 46, 50). Moreover, these studies have suggested a genetic association between racial background and certain bacterial taxa in the VMB (45, 50). Hence, the impact of racioethnicity on the VMB is likely mediated by multiple factors, including both the individual’s genetic background and environmental conditions. Since structural racism is key in influencing environmental conditions (51), the difference of the VMB associated with racioethnicity could be potentially caused by structural racism (52). However, current studies have yet to quantify the contribution of these factors to the composition of the VMB, and the mechanisms by which these factors impact the VMB remain unclear.
Lactobacilli and Vaginal Health
Lactobacillus spp. are usually the dominant taxa in the VMB, and these taxa play important roles in antagonizing dysbiosis-associated microorganisms. As described above, lactobacilli lower the vaginal pH (2). Low pH inhibits proliferation of anaerobes commonly associated with BV (53, 54) (see below) and is considered partially responsible for reduced susceptibility to human immunodeficiency virus (HIV) infection (22, 55, 56), other viral infections (57) and other STIs (11) (Fig. 2). Lactic acid also permeabilizes the outer membranes of Gram-negative taxa, possibly potentiating the impact of other factors on these bacteria in vitro (58). The ability of many species of Lactobacillus to produce H2O2 could provide a selective advantage over other potential vaginal colonizers under aerobic conditions, but the largely anaerobic environment in the female reproductive tract would likely preclude in vivo production of H2O2 (59). Lactobacillus spp. also produce bacteriocins, which target other bacterial taxa, permitting the former to proliferate. Multiple putative bacteriocin genes have been identified in the genomes of L. crispatus isolates recovered from cultures of vaginal specimens (60). Gassericin E, identified in a L. gasseri strain, was shown to inhibit other species, including G. vaginalis, in vitro (3). Although the antimicrobial spectrum of these bacteriocins may influence the composition of the VMB, there remains a lack of in vivo evidence that Lactobacillus bacteriocins function to attenuate growth or colonization of other taxa. Finally, in vitro studies showed that various adhesins produced by Lactobacillus spp. promote their colonization of epithelial surfaces (61–64) and inhibit colonization of dysbiosis-associated microorganisms, e.g., G. vaginalis (61) and Escherichia coli (62). L. crispatus also reduces Candida adhesion by producing a biosurfactant in vitro (65) and inhibits Candida albicans infection by promoting epithelial cell defenses through modulation of the production of Toll-like receptors (2 and 4), interleukin 8 (IL-8), and β-defensins 2 and 3 in a HeLa cell model (66).
Lactobacillus spp. interact with the host to impact overall vaginal health. Multiple vaginal epithelial cell models show that d- and l-lactic acid and a mixture of VMB-associated metabolites induce the anti-inflammatory cytokine IL-1RA while inhibiting proinflammatory cytokines, including IL-6, IL-8, TNF-α, RANTES, and macrophage inflammatory proteins 3α (MIP-3α) (67, 68) (Fig. 2). IL-1RA induction and IL-8 inhibition by l-lactic acid was confirmed in an organotypic tissue model of female reproductive tract epithelium (67). Although other in vitro studies suggested that several Lactobacillus strains stimulated proinflammatory responses (69, 70), none of these strains are abundant in the VMB. Both in vivo and cervical epithelial cell model studies show that lactic acid produced by lactobacilli and the resulting acidic vaginal pH also promote integrity of the vaginal epithelial barrier inhibiting colonization by other anaerobes and pathogens (Fig. 2) (22, 71). Thus, it is clear that the predominance of protective lactobacilli is associated with lower risks of suboptimal health states. However, overgrowth of lactobacilli can be associated with cytolytic vaginosis, defined by epithelial cell damage, lysis, and scaling due to overproduction of lactic acid (20, 72–79). Cytolytic vaginosis is less common than BV but emphasizes the importance of quantitative assessment of the VMB in clinical settings.
L. iners and L. crispatus are generally the most abundant Lactobacillus spp. in the VMB, followed by L. gasseri and L. jensenii (Fig. 1). L. crispatus, L. jensenii, and L. gasseri produce H2O2, and L. crispatus is associated with lower vaginal pH (25, 80) and is heritable in white women (45). Furthermore, the L. crispatus-dominated VMB yields higher levels of d- but not l-lactic acid than the L. iners-dominated VMB, and decreased d-lactic acid has been associated with PTB (81). As described above, a lower pH both inhibits dysbiosis and generally promotes anti-inflammatory and antibacterial effects (67). The L. crispatus-dominated VMB is more stable during pregnancy (8, 14, 24) and is often reduced in prevalence in pregnant women who go on to experience PTB (5, 12, 14, 82). Supernatants of L. crispatus attenuate disruption of the cervical epithelial barrier mediated by LPS or G. vaginalis and reverse the G. vaginalis-induced inflammation-associated microRNA (miRNA) expression in cervical epithelial cell models (71). L. iners often predominates in Black women (8). It does not produce H2O2 (80), is less efficient than other lactobacilli in production of d-lactic acid (81, 83), produces a cholesterol-dependent cytolysin (inerolysin) (84), and does not inhibit disruption of the cervical epithelium by lipopolysaccharide (LPS) or G. vaginalis (71). Moreover, in contrast to L. crispatus, which is largely exclusionary to other bacterial taxa, L. iners often coexists with G. vaginalis in vivo (12) and has been associated with PTB, miscarriage, and instances of an insufficient cervix during pregnancy (14, 82). Although lactobacilli, especially L. crispatus, seem beneficial for overall vaginal health, the role of L. iners in maintaining vaginal health is less clear (85). Recent strain-level studies illustrate that L. iners genomes are quite conserved and similar with regard to single nucleotide polymorphisms (SNPs) and the presence of characterized genes (24, 86) but differ in the presence of phages, plasmids, and some uncharacterized genes (24), and the impact of these genes on the VMB and women’s health requires further exploration. It remains unclear if these apparent strain differences are associated with the differential impact of L. iners on women’s reproductive health. Interestingly, expression of several L. iners genes, including those encoding inerolysin, mucin, glycerol transport and related metabolic enzymes, and proteins belonging to a CRISPR system, are upregulated in the VMB of women with BV (87), suggesting that the modulation of virulence gene expression in L. iners is associated with dysbiosis of the VMB, but the mechanism remains unclear.
A recent large-scale metabolomics study illustrates that vaginal cytokine profiles and the prevalence of Lactobacillus in the VMB can be predicted by metabolite profiles of the VMB using machine learning models (88). These models can further distinguish between L. crispatus-dominated and L. iners-dominated VMBs using metabolite profiles. Thus, this study demonstrates the interaction among the composition of the VMB, microbial metabolites, and host immune responses.
The Vagina Microbiome in Adverse Health Conditions
Bacterial vaginosis.
Vaginitis is a term to describe various conditions of infection or inflammation of the vagina. The most common kinds of vaginitis are BV, vulvovaginal candidiasis or “yeast” vaginitis, and trichomoniasis vaginitis. BV, with a global prevalence ranging from 23% to 29% (89), is characterized by displacement of lactobacilli in the VMB by anaerobic Gram-negative bacteria (44). Although many women with BV-like vaginal microbiomes lack clinical complaints, a recent meta-analysis estimated that ~34.9% are actually symptomatic (89). These women suffer from increased vaginal discharge, odor, and itching, generally without significant local inflammation (90). Diagnosis by Amsel’s criteria requires the presence of three of the following: vaginal discharge, a pH of ≥4.5, presence of sloughed “clue” cells coated with bacteria, and an amine odor with application of potassium hydroxide to the discharge (91). Nugent scoring (30) involves enumerating large Gram-positive rods (lactobacilli), small Gram-variable rods (G. vaginalis), small Gram-negative rods (Bacteroides spp.), and curved Gram-variable rods (other taxa). Several taxa, including Gardnerella, Atopobium, Prevotella, Porphyromonas, Sneathia, Mobiluncus, Mycoplasma, BVAB1, BVAB2, Mageeibacillus indolicus (BVAB3), and Peptostreptococcus, are often enriched in VMBs of BV patients (44) (Table 1). Although Mobiluncus spp. have been considered a primary contributor to the curved rods observed in BV, recent molecular studies suggest that these curved rods are mostly BVAB1 (44). Treatment of BV with metronidazole is often initially successful, but recurrence is common at a rate approaching 50% within as little as 12 weeks (92) to 12 months (93, 94).
TABLE 1.
Composition of the vaginal microbiome in vaginitisa
| Condition | Vaginal microbiome features |
|---|---|
| Cytolytic vaginosis | Overgrowth of Lactobacillus spp. (20) |
| Bacterial vaginosis | Enrichment of Atopobium vaginae,b BVAB1, BVAB2, BVAB3, Gardnerella vaginalis, Mobiluncus spp., Mycoplasma spp., Porphyromonas spp., Prevotella spp., Sneathia spp., and Ureaplasma spp. (44); depletion of Lactobacillus spp. (44) |
| Vulvovaginal candidiasis | Normal to BV-like vaginal microbiome (19, 120, 122); colonization by Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, or Candida krusei (19, 120, 122) |
| Trichomoniasis | BV-like vaginal microbiome (133, 134); invasion by Trichomonas vaginalis (133, 134) |
| Aerobic vaginitis or desquamative inflammatory vaginitis | Depletion of Lactobacillus spp. (29, 139); frequent detection of aerobic, enteric bacteria, e.g., E. coli, Enterococcus spp., Staphylococcus spp., and Streptococcus spp. (29, 139) |
BVAB1, “Candidatus Lachnocurva vaginae”; BVAB2, Lachnospiraceae BV-associated bacterium 2; BVAB3, Mageeibacillus indolicus.
A. vaginae was reclassified to a new genus and renamed Fannyhessea vaginae in 2018 (266).
G. vaginalis is present in at least 95% of clinically diagnosed BV (95), but its role remains unproven. G. vaginalis isolates have recently been reclassified into four clades and 13 putative genomic species, including “true” G. vaginalis, G. leopoldii, G. piotii, and G. swidsinskii (96, 97). A recent study reported that clades 1 and 2, which include G. vaginalis and genomic species 2 and 3, are associated with BV (97). Another study reported that clades 1 (G. vaginalis and genomic species 2) and 3 (genomic species 8, 9, and 10) are associated with BV, while clade 2 (genomic species 3) is associated with intermediate microbiotas (98). Many, but not all, isolates of G. vaginalis produce vaginolysin, a cholesterol-dependent pore-forming cytolysin that kills mammalian cells by punching holes in their membranes. Species-specific variations found in the vaginolysin amino acid sequence could contribute to differential pathogenicity associated with these Gardnerella strains (99). Members of all four clades are found, albeit in low abundance, in women with no symptoms, and most women with Gardnerella in their VMBs harbor multiple Gardnerella species (98). Some strains of Gardnerella are more proficient than others at biofilm formation (100) or in competition with Lactobacillus species (101, 102) in vitro. Much remains to be learned about the various strains of Gardnerella, its diverse subgroups, and their association with gynecologic and obstetric health.
BV-associated bacteria produce biofilms, disrupt the vaginal mucosal and epithelial barriers by sialidase, cytolysins, and other enzymes, increase the vaginal pH, and produce enzymes that enhance their ability to colonize (Table 2). Gene expression in the VMB has been shown to be impacted by the composition of the VMB (103). Since most BV-associated bacteria have higher relative abundances in CST IV (44), genes responsible for sialidase production have the highest expression in the VMB with CST IV, and higher expression of genes encoding cholesterol-dependent cytolysins in L. iners and G. vaginalis is associated with the depletion of Lactobacillus (103). A cervicovaginal epithelial cell model shows that a mixture of BV-associated vaginal metabolites can promote vaginal inflammation by increasing proinflammatory cytokines, i.e., tumor necrosis factor alpha (TNF-α) and IL-8, but inhibit the production of chemokines, i.e., RANTES and IP-10, which could be a mechanism of immune evasion (68). Other epithelial cell models show that pore-forming toxins, such as vaginolysin produced by G. vaginalis (104) and perhaps inerolysin from L. iners (84), bind to tethered lipid rafts embedded in the plasma membranes of vaginal epithelial cells and mediate cytolysis, a plausible pathogenic mechanism in BV. Interestingly, women taking statins to reduce systemic cholesterol generally exhibit reduced prevalence of Gardnerella, and statins protect cultured vaginal epithelial cells from lysis by vaginolysin (105). Other BV-associated taxa, e.g., Sneathia amnii (106), also express toxins that have yet to be clearly implicated in pathogenesis.
TABLE 2.
Bacterial vaginosis-associated virulence factorsa
| Mechanism | Bacterial product(s) | Species |
|---|---|---|
| Disruption of mucosal barrier | Sialidase | Gardnerella vaginalis, Prevotella bivia, Mycoplasma hominis, Bacteroides fragilis (267, 268) and Prevotella timonensis (269) |
| Glycosulfatase | Prevotella spp. (270) | |
| Disruption of epithelial barrier | Vaginolysin | G. vaginalis (268, 271) |
| Inerolysin | Lactobacillus iners (84) | |
| Phospholipase C | G. vaginalis (268), Ureaplasma urealyticum (272) | |
| Hemolysin | G. vaginalis (273), Sneathia amnii (106) | |
| Urease | U. urealyticum (274) | |
| Increase of the vaginal pH | Amine production | P. bivia (275), BVAB1, Dialister micraerophilus (276) |
| Antibiotic resistance | Resistance gene: 5-nitroimidazole, macrolides, tetracycline, β-lactam and aminoglycoside antibiotics | Widely distributed in the vaginal microbiome (277) |
| Immune evasion | IgA protease | U. urealyticum (278) |
| Organic acids (succinic acid, acetic acid, etc.) | Prevotella spp., Mobiluncus spp. (68, 279) | |
| Growth of diseases-associated bacteria | Amino acids | P. bivia (280) |
| Ammonia | P. bivia (275) | |
| Proinflammatory responses | Organic acids (53, 68) | G. vaginalis (71), Atopobium vaginae (264), P. timonensis (269, 281), Megasphaera elsdenii (281) |
| Biofilm formation | G. vaginalis, Atopobium vaginae, Mobiluncus spp., Fusobacterium nucleatum (107) | |
BVAB1, “Candidatus Lachnocurva vaginae.”
Vaginal biofilms protect BV-associated taxa from clearance by lower pH and lactic acid, antibacterials produced by lactobacilli, the host immune system, and antibiotics (44, 107) (Fig. 2). Although still poorly understood, these biofilms are thought to impair the integrity of the epithelial barrier (44, 107) and have been cited as important contributors to establishment of BV and its predilection for recurrence (107–109). Transcription of genes related to growth and vaginolysin production in G. vaginalis are downregulated in biofilms relative to planktonic cultures in vitro, which could be beneficial for long-term survival of G. vaginalis in the vagina (110). However, coculture of G. vaginalis with Enterococcus faecalis and Actinomyces neuii seems to promote biofilm formation and virulence of G. vaginalis at the transcriptional level (111). Known biofilm matrices contain polysaccharide, extracellular proteins, and extracellular DNA. DNase treatment reduces biofilm formation of G. vaginalis in vitro, suggesting that extracellular DNA is an important component in the biofilm matrix of G. vaginalis (112). However, studies on genes related to the production of polysaccharide and extracellular proteins in G. vaginalis are limited by the lack of genetic tools for this taxon. Interspecies interaction of G. vaginalis with BV-associated bacteria apparently promotes biofilm formation. In vitro studies illustrate that initial attachment of G. vaginalis may be mediated by L. iners or Peptoniphilus spp. and biofilm formation is enhanced by additional taxa, e.g., Atopobium vaginae, Prevotella bivia, Fusobacterium nucleatum, and Mobiluncus spp. (107, 111). A better understanding of vaginal biofilms is required to better support female reproductive health.
Several biogenic amines and short- and long-chain fatty acids, e.g., tyramine, N-acetylputrescine, cadaverine, deoxycarnitine, etc., associated with increased vaginal pH, abnormal vaginal odor or discharge, or the presence of clue cells have higher concentrations in BV patients or in VMBs with CST IV (113–116) and are associated with activation of proinflammatory responses (88, 114, 117). Consistent with these results, genes responsible for biogenic amine production have been discovered in the genomes of many BV-associated bacteria (114). In contrast, the concentrations of metabolites associated with health, e.g., lactate, phenylalanine, tyrosine, glutathione, and others, are higher in asymptomatic participants and in VMBs predominated by protective Lactobacillus (113–116).
Vulvovaginal candidiasis.
Vulvovaginal candidiasis often occurs when the vaginal environment is altered by antibiotic treatment, hormonal changes, metabolic disease, immunological incompetence, sexual activity, or other conditions that permit yeast, mainly Candida spp., to colonize the female reproductive tract in hyphal form (118, 119). Candida albicans and other species of Candida, which bind to mannose-binding lectin on the epithelial cell membranes, are the primary etiological agents (120). In response to altered host environmental conditions, Candida undergoes global transcriptional changes while transitioning into hyphal forms. Hyphae from some C. albicans strains form vaginal biofilms and secrete candidalysin, a cytolytic peptide toxin that damages epithelial cell membranes, permitting penetration of epithelial cell layers. Epidermal growth factor receptor is activated, inducing mitogen-activated protein kinase (MAPK) signaling, MKP1 activation and promotion of proinflammatory mediators, neutrophil recruitment, and type 17 immunity (121). The VMB of reproductive-age women with vulvovaginal candidiasis is generally more complex than that of asymptomatic women but is statistically less complex than that of women with BV (122, 123) (Table 1). In vivo studies suggest that colonization by Lactobacillus may not reduce the risk of vulvovaginal candidiasis (124), and colonization by L. crispatus is even associated with increased C. albicans colonization (125, 126). However, an L. crispatus-dominated VMB is associated with lower risks of C. albicans colonization and vulvovaginal candidiasis than an L. iners-dominated VMB in vivo (19, 123), and L. crispatus inhibits hypha formation of C. albicans (127) as well as the innate immune responses induced by yeast in vitro (66). Thus, it is clear that vulvovaginal candidiasis is associated with the VMB, but it is unclear if it is associated with a specific Lactobacillus species, e.g., L. crispatus, or the predominance rather than the presence of lactobacilli.
Sexually transmitted infections.
Vaginal dysbioses are associated with increased risk for acquisition of STIs, including HIV (55), herpes simplex virus (HSV) (128), human papillomavirus (HPV) (129), gonorrhea, chlamydia, and trichomoniasis, as well as an increased persistence of HPV infection (11). HSV infection has also been shown to promote vaginal dysbiosis (11). Dysbiosis-associated taxa in the VMB were reported to be associated with an increase in CD4-positive T cells in cervicovaginal lavage fluid from young South African women and in a murine model (55). This increased level of CD4-positive T cells, the target of HIV, likely favors HIV transmission in vaginal dysbioses. Evidence that BV-associated bacterial taxa enhance HIV RNA expression has also been documented both in vitro and in vivo (130, 131). BV-associated virulence factors, e.g., vaginolysin, inerolysin, mucinase, sialidase, and others, likely impact the integrity of the vaginal epithelium and mucosal barrier, also possibly facilitating these infections (44, 107). In contrast, protective Lactobacillus spp. inhibit the growth of BV-associated species, as outlined above (10), as well as the proinflammatory responses of epithelial cells and protects the integrity of epithelial barrier (22, 67), which may explain the attenuation of HIV infections by these taxa (22, 55).
Trichomoniasis, the most common nonviral STI (132), causes severe damage to vaginal tissue and disruption of the vaginal ecology by eliciting host inflammatory responses (132). Trichomonas vaginalis is closely associated with some bacterial taxa, including Mycoplasma hominis, “Candidatus Mycoplasma girerdii,” Veillonella montpellierensis, Prevotella amnii, Sneathia sanguinegens, Anaerococcus, and Parvimonas spp. in the VMB (133–135) (Table 1). M. hominis is considered obligately intracellular, and “Ca. Mycoplasma girerdii” may be a facultative endosymbiont. Moreover, there is some evidence to suggest that these taxa coexist with T. vaginalis (136, 137) and that at least M. hominis potentiates the pathogenicity of T. vaginalis (138).
Desquamative inflammatory vaginitis and aerobic vaginitis.
Desquamative inflammatory vaginitis (139, 140), first described over 6 decades ago, is an inflammatory disorder of questionable etiology associated with purulent discharge, vaginal itching, dyspareunia, and an inflamed vaginal wall (29). A similar condition associated with vaginal colonization by aerobic, enteric bacteria, including, among others, Escherichia, Streptococcus, Staphylococcus, and Enterococcus species, has been termed aerobic vaginitis (29, 141) (Table 1). Both conditions are associated with a paucity of vaginal lactobacilli and have an epidemiologic prevalence similar to that of BV (29). Their bacterial etiology is supported by positive responses to antibiotic treatment, but steroids that reduce inflammation similarly reduce the symptoms (29, 139). Thus, the role of the microbiome in aerobic and desquamative inflammatory vaginitis remains controversial.
The bacteria most frequently encountered in aerobic vaginitis, e.g., group B Streptococcus (GBS, or Streptococcus agalactiae), E. coli, and Staphylococcus aureus, usually have low abundances in the VMB (5, 7, 25, 26). However, in utero infections of these bacteria can cause serious reproductive outcomes, including but not limited to stillbirth and neonatal sepsis (142–144). GBS colonization seems not to affect the composition of the VMB of reproductive-age nonpregnant women (145), but G. vaginalis in the VMB has been reported to enhance GBS colonization and infection in a mouse model (146).
The Vaginal Microbiome and Pregnancy
As discussed above, increased estrogen levels during pregnancy promote generation of glycogen and lead to production of lactic acid by Lactobacillus (Fig. 2). Consistent with these observations, the VMB in late pregnancy seems to exhibit greater stability and Lactobacillus dominance than that in early pregnancy (5–8), and the transitions of the VMB during pregnancy occur earlier in gestation (8) (Fig. 3). The higher abundance of lactobacilli in late pregnancy is coupled with a commensurate reduced abundance of taxa associated with vaginal dysbiosis, including but not limited to G. vaginalis, A. vaginae, and P. bivia (5).
Vaginal communities dominated by Lactobacillus species are quite stable or tend to convert to vaginal communities dominated by other Lactobacillus species during pregnancy (5, 8, 147). Vaginal communities associated with dysbiosis tend to shift toward communities dominated by lactobacilli by the second trimester. The G. vaginalis-dominated VMB is less stable, often converting to an L. iners-dominated VMB (5, 8). These shifts are consistent with an evolution toward a more favorable vaginal environment in pregnancy.
Likely because Lactobacillus spp. are already more dominant in white than Black women before pregnancy (8), the transition to Lactobacillus-dominated profiles and the increase of the stability in the VMB is more evident in Black than white women during pregnancy (5, 8) (Fig. 3). Similarly, metagenomics data illustrate a simplification of metabolic activity only in Black women, not in non-Black women (8, 24). Consequently, pregnancy seems to have a greater apparent impact on the VMB of Black women.
Estrogen levels drop rapidly after delivery. Likely as a consequence, the stability of the VMB also drops (147–149), and recovery of a more stable VMB may take several months in asymptomatic women (147, 148) (Fig. 3). This postpartum disturbance of the VMB occurs irrespective of both the community structure during pregnancy and racioethnic background and manifests in reduced prevalence of Lactobacillus and increased prevalence of anaerobes, including Peptoniphilus, Prevotella, and Anaerococcus, that are generally associated with less optimal health outcomes (147, 148) (Fig. 3). Consistent with this observation, an interpregnancy interval shorter than 1 year is associated with increased risks for pregnancy complications, including PTB (150–152).
THE CERVICAL MICROBIOME
The cervix connects the uterus to the vagina (Fig. 1). Cervical brushes or swabs are used to collect cervical samples (18, 153), and vaginal contamination is minimized during this collection. The composition of the cervical microbiome is generally similar to that of the vaginal microbiome in reproductive-age women (18, 153). Most recent studies of the cervical microbiome focus on its association with cervical cancer. Cervical cancer is one of the most prevalent infectious cancers and is tightly associated with infection by high-risk HPV (154). Persistent high-risk HPV infection increases the risk of cervical intraepithelial neoplasia (CIN). In CIN, HPV DNA integrates into chromosomes of cervical epithelial cells, inducing changes that lead to cervical malignancy (155). The cervical microbiome is more complex in patients with CIN (23) and cervical cancer (18, 156, 157). The loop electrosurgical excision procedure for removing intraepithelial lesions was shown to decrease the complexity of the cervical microbiome in a cohort of Asian women (158). The composition of the cervical microbiome in HPV-negative participants is significantly different from that in participants with intraepithelial lesions or cervical cancer (157, 158). Several taxa, e.g., Gardnerella (18, 159), L. iners (160), A. vaginae (160), Mycoplasma (23), Sneathia (157), and Fusobacterium (157), most of which are associated with dysbiosis of the VMB (Table 1) and produce virulence factors in BV (Table 2), have been reported as risk factors for CIN and cervical cancer. Damage to the integrity of the epithelial (71) and mucosal (161) barriers due to overgrowth of dysbiosis-associated microorganisms has been hypothesized to permit HPV attack on cervical epithelial cells, but the mechanism is unproven. Since chronic inflammation seems to favor malignancy (162), local inflammation associated with dysbiosis of the cervical microbiome may also be involved in the progression of cervical and other gynecologic cancers. In contrast, the genus Lactobacillus is associated with a higher clearance of incident high-risk HPV infection (18), and L. crispatus is associated with lower risk of CIN in the cervical microbiome (160), possibly due to Lactobacillus spp. inhibiting growth of dysbiosis-associated microorganisms and host inflammatory responses.
Similar to the cervical microbiome, the VMB is more complex (163, 164) and contains a higher abundance of dysbiosis-associated taxa, e.g., Sneathia (165) and G. vaginalis (164), in individuals with HPV infection and CIN. Since the vagina and the cervix are in close proximity, it is not surprising that their respective microbiomes are similar in composition and exhibit similar changes in the progression of cervical cancer.
THE MICROBIOTA OF THE UPPER GENITAL TRACT
Is There a Microbiome in the Upper Genital Tract?
The concept that the uninfected upper genital tract has an intrinsic microbiome remains controversial. Multiple studies have supported the existence of the microbiome in the upper genital tract (166–168). However, because of low microbial biomass in these sites, potential contamination in the process of sample collection and processing or the presence of even low levels of bacterial DNA in the so-called “kitome” confounds results based on DNA sequencing. Recent reviews have argued that the uterus lacks a native microbiome (169, 170), whereas others find evidence of a microbiome in the upper genital tract (171). Several carefully controlled studies did not detect a normal microbiome in placental samples (16, 17, 172), and another did not detect a microbiota in fetal meconium before birth (173). Nevertheless, it is clear that pathogens can cause infections in the placenta (16, 174), uterus, fallopian tubes, and ovaries (175–178), and additional study is required to confirm the existence of and characterize a native microbiome in the upper female reproductive tract.
Origin of the Microbiota in the Upper Genital Tract
The correlation between dysbiosis of the VMB and PTB (5, 13, 15, 179, 180) and the similarity of taxa found in the amniotic fluid (174, 181) and membranes (182) to the VMB suggests that pathogens ascend from the lower reproductive tract into the uterine cavity causing uterine infections and problems in pregnancy, including PTB. Consistent with this hypothesis, a study in pregnant mice observed the ascension of bioluminescent E. coli from the vagina into the uterine cavity, resulting in premature delivery (183). However, the mouse and human female reproductive tracts are quite divergent in both anatomy and physiology, rendering these observations difficult to generalize. It has also been proposed that bacteria spread hematogenously from the oral cavity, as taxa similar to those found in the mouth have been found in the uterus (174) and periodontitis has been identified as a risk factor of PTB (184). Taxa common to those in both vaginal and oral microbiomes have also been reported in chorioamnionitis (185), and oral bacteria have been reported in the amniotic fluid in women and mice that experience premature rupture of membranes due to intrauterine infections (186–188). However, human oral taxa are also common in the VMB (25, 148), and their presence in the uterus does not preclude contiguous spread from the lower reproductive tract. Other hypotheses on the origin of microbes in the upper genital tract include migration from the infected fallopian tubes (189) and accidental transmission in invasive procedures (190). Metagenomic studies that provide strain-level resolution will be useful to confirm or refute the association between the VMB, the oral microbiome, intrauterine infections, and adverse pregnancy outcomes.
Ascension of Microbes to the Uterine Cavity
Ascension of microbes to the uterine cavity is generally prevented by the cervical mucus plug (191). However, this plug could be compromised by bacterial products (192–194). GBS produces hyaluronidases that digest hyaluronan, a protective polysaccharide (195), and can promote ascending infection (193, 196). Surprisingly, sloughing (also known as exfoliation or shedding) of infected epithelial cells does not reduce GBS vaginal colonization but increases GBS dissemination and ascension (197). Sialidases and mucinases produced by BV-associated bacteria, e.g., G. vaginalis, P. bivia, M. hominis, and Bacteroides fragilis, compromise mucosal membranes and the cervical plug, are conducive to invasion of the upper reproductive tract in a murine model (198), and increase the risk of very early PTB in vivo (199). However, it remains uncertain whether these sialidases promote ascending infections of other microbes, e.g., GBS or E. coli, in humans, although a short cervical length is thought to be beneficial for microbe ascension and has been shown as a risk factor of PTB (190, 200, 201). Additionally, cervical-fundal uterine peristaltic contractions have been shown to promote the transport of albumin macrospheres of the size of spermatozoa through the cervix (202), which could also promote ascension of pathogens.
THE MICROBIOTA OF THE FEMALE REPRODUCTIVE TRACT AND PRETERM BIRTH
The Microbiota and Preterm Birth
PTB is defined as childbirth after less than 37 weeks of gestation due to multiples causes, e.g., cesarian section or labor induction for medical reasons, preterm premature rupture of the membranes (PPROM), spontaneous labor with intact membranes, etc. (190). PTB is the leading cause (17.7%) of deaths among children under 5 years of age worldwide (203). Intrauterine infections caused by microorganisms have been hypothesized to activate the innate immune system and consequently enhance the risk for spontaneous PTB (190). It has been estimated that up to 25 to 40% of spontaneous PTBs have microbial etiology.
Bacterial species, e.g., Chlamydia trachomatis (204–206), Klebsiella pneumoniae (207, 208), E. coli (208, 209), Fusobacterium nucleatum (210), GBS (16, 208, 211), Mycoplasma hominis (204), Neisseria gonorrhoeae (206), Staphylococcus aureus (194, 209), and Streptococcus mitis (209), have been detected in intrauterine infections and induce PTB (Table 3). Although it is uncertain whether ascension and intrauterine infections are required for vaginal microbes to induce spontaneous PTB, several microorganisms frequently detected in the vagina have increased relative abundances in the VMB of women who later experience PTB, e.g., Ureaplasma, GBS, and several BV-associated taxa. Other microbes, e.g., HIV (212, 213) and HPV (214), have been reported to be associated with increased risk of PTB, but the PTB induced by HIV seems to be independent from the VMB (212).
TABLE 3.
Risk factors for preterm birth associated with the microbiome or microbial infections of the female reproductive tracta
| Classification | Risk factor(s) |
|---|---|
| Factors in the VMB | |
| Vaginal taxa increased in relative abundance in PTB | Aerococcus spp. (5, 15), Atopobium spp. (15, 226, 282), BVAB1 (5), BVAB2 (5), Chlamydia spp. (208), Clostridium sensu stricto (15), Coriobacteriaceae species (5), Dialister spp. (5, 15), Escherichia coli (208), Fusobacterium nucleatum (282), Gardnerella spp. (12, 147), GBS (208, 211), Klebsiella pneumoniae (208), BVAB3 (282), Megasphaera spp. (15, 226), Mobiluncus curtisii/Mobiluncus mulieris (226), Mycoplasma hominis (282), Olsenella sp. (15), Parvimonas sp. (5), Porphyromonas asaccharolytica (226), Prevotella spp. (5, 15, 148), Sneathia amnii (5), Sneathia sanguinegens (5, 226), TM7-H1 (5), Trichomonas vaginalis (208, 283), Ureaplasma spp. (81, 147, 209, 283) |
| Vaginal taxa reduced in relative abundance in PTB | Lactobacillus crispatus (5, 12, 14, 228, 229), Lactobacillus spp. (5, 12, 15, 147, 226, 227, 229) |
| Bacterial virulence factors | BV-associated bacteria: sialidase (199, 284); GBS: β-hemolysin/cytolysin (192) and hyaluronidase (193); unidentified taxa: lipopolysaccharide (285); unidentified taxa: volatile organic compound (286) |
| Bacterial load | A higher vaginal bacterial load is a risk for PTB recurrence (245) |
| Host factors associated with the VMB | |
| Host cytokines/chemokines | Amniotic fluid: IL-1β (287), IL-6 (184, 217, 287), IL-8 (184), IL-10 (218), and IL-18 (219); cervicovaginal fluid: IL-1β (5, 88), IL-6 (5, 216, 217), eotaxin (5, 225), and MIP-1β (5); plasma: GM-CSF (220) |
| Host antimicrobial peptide | Reduced β-defensin-2 (226) |
| Other factors | Short cervix (14, 234), cervical cerclage with braided suture (compared to monofilament suture) (236) |
| Microbial invasion | |
| Bacterial intrauterine infectionsb | Chlamydia trachomatis (204, 205), Escherichia coli (208, 209), Fusobacterium nucleatum (210), GBS (16, 208, 211), Klebsiella pneumoniae (207, 208), Mycoplasma hominis (204), Staphylococcus aureus (194, 209), Streptococcus mitis (209) |
| Viral infections | HIV (213, 288, 289), HPV (214) |
| Other sexually transmitted infections | Neisseria gonorrhoeae (289–291), Treponema pallidum (290), Chlamydia trachomatis (289, 291) |
Unless stated otherwise, risk factors show elevated concentrations/abundances in women who later experience PTB. BVAB1, “Candidatus Lachnocurva vaginae”; BVAB2, Lachnospiraceae BV-associated bacterium 2; BVAB3, Mageeibacillus indolicus; TM7-H1, “Candidatus Saccharibacteria” genomospecies TM7-H1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Most of the vaginal taxa whose abundance increased in PTB were also reported as risk factors for intrauterine infections (174).
The Microbiota and Immune Responses
GBS generates a β-hemolysin/cytolysin that induces inflammation and the disruption of maternal-fetal barriers, leading to higher risk for PTB in a mouse model (192). Although commonly present in the VMB with relatively high abundance, several BV-related bacteria stimulate proinflammatory responses by producing short-chain fatty acid in ectocervical, endocervical, and dendritic cell models (53) (Table 2) and the increased proinflammatory responses caused by non-Lactobacillus taxa in the VMB are associated with PTB (88) (Table 3). Lipopolysaccharide is widely used to promote inflammation to induce PTB in mouse models (183, 215), but the function of lipopolysaccharide in the female reproductive tract is not clear. Several proinflammatory cytokines and chemokines, i.e., IL-1β (5), IL-6 (5, 184, 216–218), IL-8 (184), IL-10 (218), IL-18 (219), granulocyte-macrophage colony-stimulating factor (GM-CSF) (218, 220), MIP-1β (5), and eotaxin (5), have been reported to have elevated concentrations in the amniotic fluid, cervicovaginal fluid, and plasma of pregnant individuals who later experience spontaneous PTB, and cervical levels of IL-1β, IL-6, IL-8, IL-10, eotaxin, MIP-1β, and GM-CSF have been associated with bacteria in the VMB (221–225) (Table 3). β-Defensin-2, a peptide with broad antimicrobial activity, has been reported to be associated with the lower risk of spontaneous PTB previously linked to cervicovaginal microorganisms, but this phenomenon has been observed only in Black women (226).
The Vaginal Microbiome in Preterm Birth
16S rRNA taxonomic profiles of the VMBs in pregnancies that deliver prematurely are generally more complex than the VMBs in pregnancies that go to term (5, 13, 15). Because of the protective effects of Lactobacillus spp. on overall vaginal health (Fig. 2), it is not surprising that a decrease in prevalence of lactobacilli, primarily L. crispatus, in pregnancies that end in PTB has been observed (5, 12, 15, 147, 226–229) (Table 3). Moreover, several taxa have been identified as putative risk factors for PTB (Table 3). These taxa are generally more readily observed earlier in pregnancy (5, 13, 15), likely due to the general homogenization effect of pregnancy toward a more Lactobacillus-dominated VMB (8). Similarly, the vaginal pH and Gram staining in early pregnancy can better predict PTB in asymptomatic women (230). Many of the taxa associated with PTB, e.g., Gardnerella, Atopobium, Dialister, Megasphaera, Prevotella, and Sneathia, and others, are components of CST IV (25) (Table 3). Thus, not surprisingly, CST IV has a positive association with PTB (82, 147, 229, 231). As described above, virulence factors that compromise mucosal membranes and the cervical plug and promote proinflammatory responses, e.g., sialidase, β-hemolysin/cytolysin, and hyaluronidase, increase ascension of microbes to the uterine cavity. It is reasonable that bacteria that produce these virulence factors (192, 193, 199) and increase proinflammatory responses (92, 221, 222, 232), e.g., Gardnerella spp., Mycoplasma hominis, Prevotella spp., and GBS, increase the risk for PTB (Table 3). However, PTB-associated virulence factors have yet to be identified in other PTB-associated taxa, e.g., BVAB1, TM7-H1, Dialister spp., etc. Continued study is required to define which taxa are keys in the enhanced risk of PTB and explore more PTB-associated virulence factors and the mechanisms by which they induce PTB. Other studies have failed to identify components of the microbiome associated with risk of PTB (7, 13, 180). These studies may have missed signals due to the racioethnic composition of their cohorts, gestational age of sampling, low sample size, or treatments (e.g., antibiotics and estrogen) received by the participants. There were also significant technical differences in these studies, including the definition of spontaneous PTB, the varied sequencing technologies employed, and the differential analysis pipelines and databases applied.
Metagenomic and metatranscriptomic data of the VMB, not surprisingly, have identified essentially the same panel of bacteria as risk factors for PTB in 16S rRNA-based studies (5). Recent metabolomics studies on the VMB and PTB have been inconsistent in study design, sample sources, metabolomic techniques, and statistical methods, leading to inconsistent conclusions (233). A recent comprehensive review of metabolomic publications listed 163 metabolites that were potentially associated with PTB, only four of which, i.e., myoinositol, creatinine, histidine, and 5-oxoproline, were associated with PTB across multiple studies (233). Thus, more in-depth metabolomic studies are warranted.
Clinical Issues Associated with the Vaginal Microbiome and Preterm Birth
As introduced above, a short cervix is a risk factor of spontaneous PTB, likely by promoting microbe ascension (190, 200, 201) (Table 3). Furthermore, a short cervical length in reproductive-age women is associated with suboptimal VMBs dominated by L. iners or anaerobes rather than L. crispatus (14, 234), which could further increase the risk of PTB. Cervical cerclage is widely used to prevent PTB in pregnancies at risk for PTB (235). Cerclage with a braided suture is associated with greater dysbiosis in the VMB and higher risk for PTB than cerclage with a monofilament suture, likely due to its irregular structure of the former providing more readily colonized niches (236).
Cervical remodeling is a term to describe the changes of the cervix in extracellular matrix structure and mechanical properties after pregnancy (237). Premature cervical remodeling leads to 12.5% of PTB (238) and can be induced by lipopolysaccharide in a mouse infection model (239). A study with a cohort dominated by white women shows that extreme cervical shortening is associated with a nonoptimal VMB (240). According to these observations, proinflammatory responses caused by microbes are thought to be a risk factor stimulating premature cervical remodeling and subsequent PTB (238, 241).
Similar to the association between the VMB and PTB, a more complex VMB with reduced Lactobacillus spp. is associated with PPROM (227, 242–244). The association between PTB and PPROM was recently reviewed (242).
It is worth noting that not only the composition of the VMB but also a higher vaginal bacterial load detected in the second trimester is associated with recurrent PTB (245), which again emphasizes the importance of quantitative assessment of the VMB in clinical settings.
Bacterial Vaginosis and Preterm Birth
Interestingly, G. vaginalis, which has long been the hallmark of a VMB of women with BV and which produces known toxins, e.g., vaginolysin and sialidase, has not been universally associated with PTB (5, 227). However, as discussed above, G. vaginalis has recently been redefined into four clades and up to 13 putative genomic species (8, 96, 97), and although at least one subgroup was tentatively associated with PTB (12), additional work is required to verify the possible contributions of each of these taxa to reproductive health and risk of PTB. Although BV and some BV-associated taxa are identified as risk factors for PTB (179, 246) (Tables 1 and 3) and an effective BV treatment seems to attenuate the PTB-associated proinflammatory responses (221, 222), current treatment strategies, usually with metronidazole or clindamycin, do not seem to reduce the risk for PTB (208, 247). Although this observation might suggest that the BV-associated VMB is not contributory to PTB risk, other interpretations are plausible. Thus, it is also possible that resistance to metronidazole by the relevant bacteria may be responsible for the persistence or rapid regrowth of these bacteria or that treatment fails to reverse the negative impacts; e.g., inflammatory responses, of these taxa (92, 221, 248, 249). Alternatively, sequestration of metronidazole by L. iners could reduce efficacy of the antibiotic, permitting BV recurrence (250) and a consequent failure to reduce PTB risk. It seems possible that a more effective elimination of the BV-associated VMB, e.g., with multiple antibiotics, followed by reestablishment of a Lactobacillus-dominant microbiome, perhaps with prebiotics or probiotics, would both improve treatment of BV and reduce risk of PTB.
Prediction of Preterm Birth
A variety of parameters; e.g., cervical length (200, 201), the composition of the VMB (5, 12, 15, 147, 226, 227), and the local expression of inflammatory cytokines (5, 184, 217, 220), have been reported to be predictive of PTB. Several reports suggest that some of these biomarkers are more relevant in earlier stages of pregnancy, likely due to the tendency of the VMB to become more homogeneous and Lactobacillus dominated as pregnancy progresses (5, 13, 15) (Fig. 3). A model for PTB prediction using the abundance of four taxa, i.e., S. amnii, BVAB1, Prevotella cluster 2, and TM7-H1, in the VMB early in pregnancy showed high sensitivity and specificity (5). Other recent models for prediction of risk for PTB invoked integrated proteomics and transcriptomics data from plasma and metabolomics from urine samples (251) or a combination of cervical length, gestational age, amniotic fluid glucose, and IL-6 (200). Honing of such models to include vaginal taxa abundance, human gene expression, metabolomics (e.g., d-lactic acid) (81) and cytokine profiling (e.g., IL-6) (217) as well as demographic and clinical parameters (e.g., cervical length, preterm-birth history, racioethnicity, and socioeconomic status) has promise for accurate early prediction of risk for preterm birth and other adverse pregnancy outcomes.
THE VAGINAL VIROME AND VAGINAL HEALTH
Still largely missing in studies of the microbiomes of the female reproductive tract is the virome component. Bacteriophages have been shown to help maintain stability in the gut microbiome (252) and are also abundant in the VMB (253). Recent studies suggest that the vaginal virome is associated with the composition of the VMB and BV status, and vaginal viruses targeting BV-associated taxa have been identified (254, 255). Also, CRISPR genes, which function as an antibacteriophage defense system, have been reported to be upregulated in the VMBs of women with BV (87, 255), implying that bacteriophages may play a role in modulating bacterial components of the VMB. Additionally, one early study implied that higher viral diversity and richness of the eukaryotic virome in the first trimester of pregnancy were associated with higher risk for PTB (256), thus suggesting that the virome could play a significant role in obstetric health. Thus, the virome of the reproductive tract should be further investigated.
ADDRESSING DYSBIOSES OF THE VAGINAL MICROBIOME
Approximately 50% of women with BV experience a recurrence within as few as 12 weeks (92) to 12 months (93, 94) after treatment with metronidazole. Treatment with other antibiotics more effective against Gram-positive bacteria, e.g., erythromycin, seems to alter the balance of the microbiome, leading to vaginal dysbiosis (227). As outlined above, estrogen enriches the vaginal epithelium in glycogen and favors proliferation of lactobacilli. Estrogen is used to treat atrophic vaginitis associated with estrogen deficiency, e.g., menopause and postpartum dysbiosis (257). However, certain clinical criteria, such as gynecological, breast, or other malignancy, or risk thereof, could be a contraindication to the use of estrogen. Thus, low-dose transvaginal topical estrogen has been shown to be effective at engendering a more optimal Lactobacillus-rich VMB while not increasing the risk of estrogen-sensitive cancers (257, 258). Additionally, more attention should be focused on nonestrogen therapy, e.g., probiotics, prebiotics, VMB transplantation, or possibly statins. Women taking statins are more likely to have a VMB dominated by lactobacilli, possibly due to inhibition of toxicity of the vaginolysin toxin from G. vaginalis (105). A recent report showed that introduction of L. crispatus into the reproductive tract after metronidazole treatment decreases BV recurrence by 15% at week 12 (259). Similarly, Lactobacillus-rich VMB transplants from healthy donors to five reproductive-age women with recurrent BV resulted in long-term remission in at least four of the recipients (21). Although such transplants introduce risks and other challenges, these results are promising for future development of noninvasive probiotic treatment of dysbioses of the VMB.
PERSPECTIVES
Our understanding of the microbiome of the female reproductive tract has advanced rapidly in the past decade. Although not universally true, an optimal VMB is usually dominated by species of Lactobacillus (25), particularly L. crispatus, L. jensenii or L. gasseri, and VMBs dominated by other taxa, e.g., G. vaginalis, A. vaginae, BVAB1, Mycoplasma spp., Sneathia spp., Ureaplasma spp., E. coli, and GBS, are associated with adverse conditions, including BV, aerobic vaginitis, vulvovaginal candidiasis, STIs, viral infections, cervical cancer, and adverse pregnancy outcomes (e.g., PTB). However, this dogma may be overstated, as VMB profiles that might normally be considered adverse are common in the absence of any clinical symptoms, complaints, or complications. Although much progress has been made, it is clear that there is still much lacking in our understanding of the contribution of the microbiomes of the female reproductive tracts to human health and disease.
Dysbioses of the female reproductive tract are generally treated with antibiotics or antifungals with considerable initial response. Although BV usually responds initially to antibiotic therapy, its high recurrence rate remains an enigma (92–94). Several virulence factors, e.g., biofilm formation and antibiotic resistance, are relevant to BV recurrence (260), and in-depth strain-level taxonomic and gene-centric studies are required to dissect the mechanisms by which the relevant taxa persist. Application of probiotics in combination with antibiotics and estrogen may be an option that aids in improving the homeostasis of the VMB and thereby vaginal health. Vaginal protective lactobacilli, in contrast to other Lactobacillus strains, have attracted significant attention as possible probiotics (259). Other combinations of pharmaceuticals, e.g., statins (105) and vitamin D (261, 262), may be helpful as well. Future study will undoubtedly clarify these possibilities. However, it is abundantly clear that much work remains to be done to find a combination of therapies that leads to a stable health-promoting VMB.
Although mice and epithelial cell models have been developed, study of the VMB is hampered by the lack of better models, as even nonhuman primates have vaginal physiology that differs greatly from that of the human female reproductive tract. In the absence of good animal models, establishment of three-dimensional (3D) organoid models would advance the field significantly (263). Most of the abundant taxa in the VMB are culturable, e.g., G. vaginalis (110), S. amnii (106), A. vaginae (264), and P. bivia (264), but other taxa, e.g., BVAB1 and “Ca. Mycoplasma girerdii,” remain difficult to manipulate in vitro. Molecular methods such as single gene deletion, genome-wide mutagenesis, and spatial genomics and transcriptomics are also important in clarifying the mechanism by which these bacteria cause disease. Although metagenomic and metatranscriptomic results have largely confirmed that the compositions of the VMB (4, 5, 24, 265) and bacterial risk factors for PTB are those identified in 16S rRNA-based studies (5), more in-depth longitudinal metagenomic and metatranscriptomic studies would be helpful to identify clades and genes relevant to BV, particularly in G. vaginalis (98) and L. iners (87).
In sum, recent studies have modified and greatly improved our understanding of the impact of the microbiome of the female reproductive tract. Continued study is required to elucidate the specific taxa that are relevant and the mechanisms by which they exert their effects. The contribution of host genetics and the virome are yet to be explored. Overall, a better understanding of the microbiome of the reproductive tract holds great promise to improve human health and well-being.
ACKNOWLEDGMENTS
We gratefully acknowledge the thousands of participants who contributed specimens and data to these studies. We also acknowledge the contributions of all members of the Vaginal Microbiome Consortium at VCU, whose contributions made this report possible. We thank Kimberly K. Jefferson and Jeffrey Donowitz for careful review of the manuscript.
We were funded by grants UH3AI083263, U54HD080784 and R01HD092415 from the National Institutes of Health and support from the GAPPS BMGF PPB grant from the Global Alliance to Prevent Prematurity and Stillbirth.
B.Z. and Z.T. were the primary authors of this review. M.G.S., G.A.B., and L.E. assisted in the organization, writing, and proofreading of the manuscript.
REFERENCES
- 1.Chumduri C, Turco MY. 2021. Organoids of the female reproductive tract. J Mol Med (Berl) 99:531–553. 10.1007/s00109-020-02028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. 2017. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 168:782–792. 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Maldonado-Barragán A, Caballero-Guerrero B, Martín V, Ruiz-Barba JL, Rodríguez JM. 2016. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol 16:37. 10.1186/s12866-016-0663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, Xu J, Jimenez NR, Vivadelli SC, Fong SS, Sheth NU, Jean S, Lee V, Bokhari YA, Lara AM, Mistry SD, Duckworth RA, III, Bradley SP, Koparde VN, Orenda XV, Milton SH, Rozycki SK, Matveyev AV, Wright ML, Huzurbazar SV, Jackson EM, Smirnova E, Korlach J, Tsai YC, Dickinson MR, Brooks JL, Drake JI, Chaffin DO, Sexton AL, Gravett MG, Rubens CE, Wijesooriya NR, Hendricks-Muñoz KD, Jefferson KK, Strauss JF, III, Buck GA. 2019. The vaginal microbiome and preterm birth. Nat Med 25:1012–1021. 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur H, Merchant M, Haque MM, Mande SS. 2020. Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women's gynecological lifecycle. Front Microbiol 11:551. 10.3389/fmicb.2020.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. 2014. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2:4. 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano MG, Parikh HI, Brooks JP, Edwards DJ, Arodz TJ, Edupuganti L, Huang B, Girerd PH, Bokhari YA, Bradley SP, Brooks JL, Dickinson MR, Drake JI, Duckworth RA, Fong SS, Glascock AL, Jean S, Jimenez NR, Khoury J, Koparde VN, Lara AM, Lee V, Matveyev AV, Milton SH, Mistry SD, Rozycki SK, Sheth NU, Smirnova E, Vivadelli SC, Wijesooriya NR, Xu J, Xu P, Chaffin DO, Sexton AL, Gravett MG, Rubens CE, Hendricks-Muñoz KD, Jefferson KK, Strauss JF, Fettweis JM, Buck GA. 2019. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat Med 25:1001–1011. 10.1038/s41591-019-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. 2012. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 160:267–282. 10.1016/j.trsl.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happel A-U, Kullin B, Gamieldien H, Wentzel N, Zauchenberger CZ, Jaspan HB, Dabee S, Barnabas SL, Jaumdally SZ, Dietrich J, Gray G, Bekker L-G, Froissart R, Passmore J-AS. 2020. Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLoS Pathog 16:e1008559. 10.1371/journal.ppat.1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis FM, Bernstein KT, Aral SO. 2017. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol 129:643–654. 10.1097/AOG.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, Biggio JR, Wong RJ, Druzin ML, Shaw GM, Stevenson DK, Holmes SP, Relman DA. 2017. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci USA 114:9966–9971. 10.1073/pnas.1705899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout MJ, Zhou Y, Wylie KM, Tarr PI, Macones GA, Tuuli MG. 2017. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol 217:356.e1–356.e18. 10.1016/j.ajog.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, Holmes E, Nicholson JK, Teoh TG, MacIntyre DA. 2017. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 5:6. 10.1186/s40168-016-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosti I, Lyalina S, Pollard KS, Butte AJ, Sirota M. 2020. Meta-analysis of vaginal microbiome data provides new insights into preterm birth. Front Microbiol 11:476. 10.3389/fmicb.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS, Smith GCS. 2019. Human placenta has no microbiome but can contain potential pathogens. Nature 572:329–334. 10.1038/s41586-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leiby JS, McCormick K, Sherrill-Mix S, Clarke EL, Kessler LR, Taylor LJ, Hofstaedter CE, Roche AM, Mattei LM, Bittinger K, Elovitz MA, Leite R, Parry S, Bushman FD. 2018. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6:196. 10.1186/s40168-018-0575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, Gonzalez P, Safaeian M, Schiffman M, Burk RD, Costa Rica HPV Vaccine Trial (CVT) Group . 2020. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog 16:e1008376. 10.1371/journal.ppat.1008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tortelli BA, Lewis WG, Allsworth JE, Member-Meneh N, Foster LR, Reno HE, Peipert JF, Fay JC, Lewis AL. 2020. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am J Obstet Gynecol 222:471.e1–e471.e9. 10.1016/j.ajog.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Zhang X, Yao W, Sun Y, Zhang Y. 2019. Characterization of the vaginal microbiome during cytolytic vaginosis using high-throughput sequencing. J Clin Lab Anal 33:e22653. 10.1002/jcla.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, Strahilevitz J, Moses AE, Shapiro H, Yagel S, Elinav E. 2019. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med 25:1500–1504. 10.1038/s41591-019-0600-6. [DOI] [PubMed] [Google Scholar]
- 22.Dizzell S, Nazli A, Reid G, Kaushic C. 2019. Protective effect of probiotic bacteria and estrogen in preventing HIV-1-mediated impairment of epithelial barrier integrity in female genital tract. Cells 8:1120. 10.3390/cells8101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein C, Gonzalez D, Samwel K, Kahesa C, Mwaiselage J, Aluthge N, Fernando S, West JT, Wood C, Angeletti PC. 2019. Relationship between the cervical microbiome, HIV status, and precancerous lesions. mBio 10:e02785-18. 10.1128/mBio.02785-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goltsman DSA, Sun CL, Proctor DM, DiGiulio DB, Robaczewska A, Thomas BC, Shaw GM, Stevenson DK, Holmes SP, Banfield JF, Relman DA. 2018. Metagenomic analysis with strain-level resolution reveals fine-scale variation in the human pregnancy microbiome. Genome Res 28:1467–1480. 10.1101/gr.236000.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108:4680–4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.France MT, Ma B, Gajer P, Brown S, Humphrys MS, Holm JB, Waetjen LE, Brotman RM, Ravel J. 2020. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8:166. 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks JP, Buck GA, Chen G, Diao L, Edwards DJ, Fettweis JM, Huzurbazar S, Rakitin A, Satten GA, Smirnova E, Waks Z, Wright ML, Yanover C, Zhou YH. 2017. Changes in vaginal community state types reflect major shifts in the microbiome. Microb Ecol Health Dis 28:1303265. 10.1080/16512235.2017.1303265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma B, France MT, Crabtree J, Holm JB, Humphrys MS, Brotman RM, Ravel J. 2020. A comprehensive non-redundant gene catalog reveals extensive within-community intraspecies diversity in the human vagina. Nat Commun 11:940. 10.1038/s41467-020-14677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donders GGG, Bellen G, Grinceviciene S, Ruban K, Vieira-Baptista P. 2017. Aerobic vaginitis: no longer a stranger. Res Microbiol 168:845–858. 10.1016/j.resmic.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29:297–301. 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhandari P, Tingley JP, Palmer DRJ, Abbott DW, Hill JE. 2021. Characterization of an α-glucosidase enzyme conserved in Gardnerella spp. isolated from the human vaginal microbiome. J Bacteriol 203:e00213-21. 10.1128/JB.00213-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith RW, Brittingham A, Wilson WA. 2016. Purification and identification of amylases released by the human pathogen Trichomonas vaginalis that are active towards glycogen. Mol Biochem Parasitol 210:22–31. 10.1016/j.molbiopara.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 33.van der Veer C, Hertzberger RY, Bruisten SM, Tytgat HLP, Swanenburg J, de Kat Angelino-Bart A, Schuren F, Molenaar D, Reid G, de Vries H, Kort R. 2019. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: implications for in vivo dominance of the vaginal microbiota. Microbiome 7:49. 10.1186/s40168-019-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farage M, Maibach H. 2006. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet 273:195–202. 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 35.Farage MA, Miller KW, Sobel JD. 2010. Dynamics of the vaginal ecosystem—hormonal influences. Infect Dis (Auckl) 3:IDRT.S3903. 10.4137/IDRT.S3903. [DOI] [Google Scholar]
- 36.Shen J, Song N, Williams CJ, Brown CJ, Yan Z, Xu C, Forney LJ. 2016. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep 6:34119. 10.1038/srep34119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cauci S, Driussi S, De Santo D, Penacchioni P, Iannicelli T, Lanzafame P, De Seta F, Quadrifoglio F, de Aloysio D, Guaschino S. 2002. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol 40:2147–2152. 10.1128/JCM.40.6.2147-2152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks JP, Edwards DJ, Blithe DL, Fettweis JM, Serrano MG, Sheth NU, Strauss JF, III, Buck GA, Jefferson KK. 2017. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception 95:405–413. 10.1016/j.contraception.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, De Guingand D, Morton AN, Fairley CK. 2013. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis 56:777–786. 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 40.Ratten LK, Plummer EL, Bradshaw CS, Fairley CK, Murray GL, Garland SM, Bateson D, Tachedjian G, Masson L, Vodstrcil LA. 2021. The effect of exogenous sex steroids on the vaginal microbiota: a systematic review. Front Cell Infect Microbiol 11:732423. 10.3389/fcimb.2021.732423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong X, Qin P, Yin J, Shi Y, Xuan Y, Chen Z, Zhou X, Yu H, Peng D, Wang B. 2021. Clinical manifestations of polycystic ovary syndrome and associations with the vaginal microbiome: a cross-sectional based exploratory study. Front Endocrinol (Lausanne) 12:662725. 10.3389/fendo.2021.662725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fortenberry JD. 2013. The uses of race and ethnicity in human microbiome research. Trends Microbiol 21:165–166. 10.1016/j.tim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, Strauss JF, The Vaginal Microbiome C, Jefferson KK, Buck GA. 2014. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading) 160:2272–2282. 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onderdonk AB, Delaney ML, Fichorova RN. 2016. The human microbiome during bacterial vaginosis. Clin Microbiol Rev 29:223–238. 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright ML, Fettweis JM, Eaves LJ, Silberg JL, Neale MC, Serrano MG, Jimenez NR, Prom-Wormley E, Girerd PH, Borzelleca JF, Jr, Jefferson KK, Strauss JF, III, York TP, Buck GA. 2021. Vaginal microbiome Lactobacillus crispatus is heritable among European American women. Commun Biol 4:872. 10.1038/s42003-021-02394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta SD, Nannini DR, Otieno F, Green SJ, Agingu W, Landay A, Zheng Y, Hou L. 2020. Host genetic factors associated with vaginal microbiome composition in Kenyan women. mSystems 5:e00502-20. 10.1128/mSystems.00502-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. 2005. Socioeconomic status in health research: one size does not fit all. JAMA 294:2879–2888. 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 48.Evans GW, Schamberg MA. 2009. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci USA 106:6545–6549. 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jašarević E, Howerton CL, Howard CD, Bale TL. 2015. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 156:3265–3276. 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Si J, You HJ, Yu J, Sung J, Ko G. 2017. Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 21:97–105. 10.1016/j.chom.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. 2017. Structural racism and health inequities in the USA: evidence and interventions. Lancet 389:1453–1463. 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 52.Benezra A. 2020. Race in the microbiome. Sci Technol Human Values 45:877–902. 10.1177/0162243920911998. [DOI] [Google Scholar]
- 53.Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, Cone RA, Tachedjian G. 2015. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 6:164. 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Seta F, Campisciano G, Zanotta N, Ricci G, Comar M. 2019. The vaginal community state types microbiome-immune network as key factor for bacterial vaginosis and aerobic vaginitis. Front Microbiol 10:2451. 10.3389/fmicb.2019.02451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, Padavattan N, Desai C, Droit L, Moodley A, Dong M, Chen Y, Ismail N, Ndung'u T, Ghebremichael MS, Wesemann DR, Mitchell C, Dong KL, Huttenhower C, Walker BD, Virgin HW, Kwon DS. 2017. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46:29–37. 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyssen D, Wang YY, Hayward JA, Agius PA, DeLong K, Aldunate M, Ravel J, Moench TR, Cone RA, Tachedjian G. 2018. Anti-HIV-1 activity of lactic acid in human cervicovaginal fluid. mSphere 3:e00055-18. 10.1128/mSphere.00055-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conti C, Malacrino C, Mastromarino P. 2009. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol 60:19–26. [PubMed] [Google Scholar]
- 58.Alakomi HL, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol 66:2001–2005. 10.1128/AEM.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tachedjian G, O'Hanlon DE, Ravel J. 2018. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 6:29. 10.1186/s40168-018-0418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdelmaksoud AA, Koparde VN, Sheth NU, Serrano MG, Glascock AL, Fettweis JM, Strauss JF, Buck GA, Jefferson KK. 2016. Comparison of Lactobacillus crispatus isolates from Lactobacillus-dominated vaginal microbiomes with isolates from microbiomes containing bacterial vaginosis-associated bacteria. Microbiology (Reading) 162:466–475. 10.1099/mic.0.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ojala T, Kankainen M, Castro J, Cerca N, Edelman S, Westerlund-Wikström B, Paulin L, Holm L, Auvinen P. 2014. Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis. BMC Genomics 15:1070. 10.1186/1471-2164-15-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Xu J, Shuai J, Chen J, Zhang Z, Fang W. 2007. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int J Food Microbiol 115:307–312. 10.1016/j.ijfoodmicro.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Nishiyama K, Nakazato A, Ueno S, Seto Y, Kakuda T, Takai S, Yamamoto Y, Mukai T. 2015. Cell surface-associated aggregation-promoting factor from Lactobacillus gasseri SBT2055 facilitates host colonization and competitive exclusion of Campylobacter jejuni. Mol Microbiol 98:712–726. 10.1111/mmi.13153. [DOI] [PubMed] [Google Scholar]
- 64.Abramov VM, Kosarev IV, Priputnevich TV, Machulin AV, Khlebnikov VS, Pchelintsev SY, Vasilenko RN, Sakulin VK, Suzina NE, Chikileva IO, Derysheva EI, Melnikov VG, Nikonov IN, Samoilenko VA, Svetoch EE, Sukhikh GT, Uversky VN, Karlyshev AV. 2020. S-layer protein 2 of Lactobacillus crispatus 2029, its structural and immunomodulatory characteristics and roles in protective potential of the whole bacteria against foodborne pathogens. Int J Biol Macromol 150:400–412. 10.1016/j.ijbiomac.2020.02.065. [DOI] [PubMed] [Google Scholar]
- 65.De Gregorio PR, Parolin C, Abruzzo A, Luppi B, Protti M, Mercolini L, Silva JA, Giordani B, Marangoni A, Nader-Macías MEF, Vitali B. 2020. Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb Cell Fact 19:133. 10.1186/s12934-020-01390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzo A, Losacco A, Carratelli CR. 2013. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human β-defensins 2 and 3. Immunol Lett 156:102–109. 10.1016/j.imlet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 67.Hearps AC, Tyssen D, Srbinovski D, Bayigga L, Diaz DJD, Aldunate M, Cone RA, Gugasyan R, Anderson DJ, Tachedjian G. 2017. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol 10:1480–1490. 10.1038/mi.2017.27. [DOI] [PubMed] [Google Scholar]
- 68.Delgado-Diaz DJ, Tyssen D, Hayward JA, Gugasyan R, Hearps AC, Tachedjian G. 2019. Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal vaginal microbiota. Front Cell Infect Microbiol 9:446. 10.3389/fcimb.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galdeano CM, Perdigón G. 2006. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol 13:219–226. 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rocha-Ramírez LM, Pérez-Solano RA, Castañón-Alonso SL, Moreno Guerrero SS, Ramírez Pacheco A, García Garibay M, Eslava C. 2017. Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res 2017:4607491. 10.1155/2017/4607491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anton L, Sierra LJ, DeVine A, Barila G, Heiser L, Brown AG, Elovitz MA. 2018. Common cervicovaginal microbial supernatants alter cervical epithelial function: mechanisms by which Lactobacillus crispatus contributes to cervical health. Front Microbiol 9:2181. 10.3389/fmicb.2018.02181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cibley LJ, Cibley LJ. 1991. Cytolytic vaginosis. Am J Obstet Gynecol 165:1245–1249. 10.1016/s0002-9378(12)90736-x. [DOI] [PubMed] [Google Scholar]
- 73.Cerikcioglu N, Beksac MS. 2004. Cytolytic vaginosis: misdiagnosed as candidal vaginitis. Infect Dis Obstet Gynecol 12:13–16. 10.1080/10647440410001672139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puri S. 2020. Cytolytic vaginosis: a common yet underdiagnosed entity. Ann Trop Pathol 11:29. 10.4103/atp.atp_18_19. [DOI] [Google Scholar]
- 75.Hacisalihoglu U, Acet F. 2021. A clinicopathological diagnostic and therapeutic approach to cytolytic vaginosis: an extremely rare entity that may mimic vulvovaginal candidiasis. J Cytol 38:88–93. 10.4103/JOC.JOC_169_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Z, Zhou W, Mu L, Kuang L, Su M, Jiang Y. 2015. Identification of cytolytic vaginosis versus vulvovaginal candidiasis. J Low Genit Tract Dis 19:152–155. 10.1097/LGT.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 77.Yang S, Zhang Y, Liu Y, Wang J, Chen S, Li S. 2017. Clinical significance and characteristic clinical differences of cytolytic vaginosis in recurrent vulvovaginitis. Gynecol Obstet Invest 82:137–143. 10.1159/000446945. [DOI] [PubMed] [Google Scholar]
- 78.Sanches JM, Giraldo PC, Amaral R, Eberlin MN, Marques LA, Migliorini I, Nakahira M, Bieleveld MJM, Discacciati MG. 2018. Vaginal lipidomics of women with vulvovaginal candidiasis and cytolytic vaginosis: a non-targeted LC-MS pilot study. PLoS One 13:e0202401. 10.1371/journal.pone.0202401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qi W, Li H, Wang C, Li H, Zhang B, Dong M, Fan A, Han C, Xue F. 2021. Recent advances in presentation, diagnosis and treatment for mixed vaginitis. Front Cell Infect Microbiol 11:759795. 10.3389/fcimb.2021.759795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antonio MA, Hawes SE, Hillier SL. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis 180:1950–1956. 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 81.Witkin SS, Moron AF, Ridenhour BJ, Minis E, Hatanaka A, Sarmento SGP, Franca MS, Carvalho FHC, Hamamoto TK, Mattar R, Sabino E, Linhares IM, Rudge MVC, Forney LJ. 2019. Vaginal biomarkers that predict cervical length and dominant bacteria in the vaginal microbiomes of pregnant women. mBio 10:e02242-19. 10.1128/mBio.02242-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang DH, Shin J, Rhee MS, Park KR, Cho BK, Lee SK, Kim BC. 2020. Vaginal microbiota profiles of native Korean women and associations with high-risk pregnancy. J Microbiol Biotechnol 30:248–258. 10.4014/jmb.1908.08016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. 2013. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio 4:e00460-13. 10.1128/mBio.00460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ragaliauskas T, Plečkaitytė M, Jankunec M, Labanauskas L, Baranauskiene L, Valincius G. 2019. Inerolysin and vaginolysin, the cytolysins implicated in vaginal dysbiosis, differently impair molecular integrity of phospholipid membranes. Sci Rep 9:10606. 10.1038/s41598-019-47043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. 2017. Lactobacillus iners: friend or foe? Trends Microbiol 25:182–191. 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Tortelli BA, Lewis AL, Fay JC. 2021. The structure and diversity of strain-level variation in vaginal bacteria. Microb Genom 7:mgen000543. 10.1099/mgen.0.000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Macklaim JM, Fernandes AD, Di Bella JM, Hammond JA, Reid G, Gloor GB. 2013. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 1:12. 10.1186/2049-2618-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pruski P, Correia GDS, Lewis HV, Capuccini K, Inglese P, Chan D, Brown RG, Kindinger L, Lee YS, Smith A, Marchesi J, McDonald J, Cameron S, Alexander-Hardiman K, David AL, Stock SJ, Norman JE, Terzidou V, Teoh TG, Sykes L, Bennett PR, Takats Z, MacIntyre DA. 2021. Direct on-swab metabolic profiling of vaginal microbiome host interactions during pregnancy and preterm birth. Nat Commun 12:5967. 10.1038/s41467-021-26215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. 2019. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sexual Trans Dis 46:304–311. 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 90.Klebanoff MA, Schwebke JR, Zhang J, Nansel TR, Yu KF, Andrews WW. 2004. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol 104:267–272. 10.1097/01.AOG.0000134783.98382.b0. [DOI] [PubMed] [Google Scholar]
- 91.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22. 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 92.Mtshali A, San JE, Osman F, Garrett N, Balle C, Giandhari J, Onywera H, Mngomezulu K, Mzobe G, de Oliveira T, Rompalo A, Mindel A, Abdool Karim SS, Ravel J, Passmore JS, Abdool Karim Q, Jaspan HB, Liebenberg LJP, Ngcapu S. 2021. Temporal changes in vaginal microbiota and genital tract cytokines among South African women treated for bacterial vaginosis. Front Immunol 12:730986. 10.3389/fimmu.2021.730986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. 2006. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 193:1478–1486. 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 94.Sobel JD, Schmitt C, Meriwether C. 1993. Long-term follow-up of patients with bacterial vaginosis treated with oral metronidazole and topical clindamycin. J Infect Dis 167:783–784. 10.1093/infdis/167.3.783. [DOI] [PubMed] [Google Scholar]
- 95.Muzny CA, Schwebke JR. 2013. Gardnerella vaginalis: still a prime suspect in the pathogenesis of bacterial vaginosis. Curr Infect Dis Rep 15:130–135. 10.1007/s11908-013-0318-4. [DOI] [PubMed] [Google Scholar]
- 96.Vaneechoutte M, Guschin A, Van Simaey L, Gansemans Y, Van Nieuwerburgh F, Cools P. 2019. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int J Syst Evol Microbiol 69:679–687. 10.1099/ijsem.0.003200. [DOI] [PubMed] [Google Scholar]
- 97.Ahmed A, Earl J, Retchless A, Hillier SL, Rabe LK, Cherpes TL, Powell E, Janto B, Eutsey R, Hiller NL, Boissy R, Dahlgren ME, Hall BG, Costerton JW, Post JC, Hu FZ, Ehrlich GD. 2012. Comparative genomic analyses of 17 clinical isolates of Gardnerella vaginalis provide evidence of multiple genetically isolated clades consistent with subspeciation into genovars. J Bacteriol 194:3922–3937. 10.1128/JB.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balashov SV, Mordechai E, Adelson ME, Gygax SE. 2014. Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J Med Microbiol 63:162–175. 10.1099/jmm.0.066407-0. [DOI] [PubMed] [Google Scholar]
- 99.Garcia EM, Serrano MG, Edupuganti L, Edwards DJ, Buck GA, Jefferson KK. 2021. Sequence comparison of vaginolysin from different Gardnerella species. Pathogens 10:86. 10.3390/pathogens10020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan S, Voordouw MJ, Hill JE. 2019. Competition among Gardnerella subgroups from the human vaginal microbiome. Front Cell Infect Microbiol 9:374. 10.3389/fcimb.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teixeira GS, Carvalho FP, Arantes RME, Nunes AC, Moreira JLS, Mendonça M, Almeida RB, Farias LM, Carvalho MAR, Nicoli JR. 2012. Characteristics of Lactobacillus and Gardnerella vaginalis from women with or without bacterial vaginosis and their relationships in gnotobiotic mice. J Med Microbiol 61:1074–1081. 10.1099/jmm.0.041962-0. [DOI] [PubMed] [Google Scholar]
- 102.Castro J, Alves P, Sousa C, Cereija T, França Â, Jefferson KK, Cerca N. 2015. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci Rep 5:11640. 10.1038/srep11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.France MT, Fu L, Rutt L, Yang H, Humphrys MS, Narina S, Gajer PM, Ma B, Forney LJ, Ravel J. 2022. Insight into the ecology of vaginal bacteria through integrative analyses of metagenomic and metatranscriptomic data. Genome Biol 23:66. 10.1186/s13059-022-02635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia EM, Kraskauskiene V, Koblinski JE, Jefferson KK. 2019. Interaction of Gardnerella vaginalis and vaginolysin with the apical versus basolateral face of a three-dimensional model of vaginal epithelium. Infect Immun 87:e00646-18. 10.1128/IAI.00646-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abdelmaksoud AA, Girerd PH, Garcia EM, Brooks JP, Leftwich LM, Sheth NU, Bradley SP, Serrano MG, Fettweis JM, Huang B, Strauss JF, III, Buck GA, Jefferson KK. 2017. Association between statin use, the vaginal microbiome, and Gardnerella vaginalis vaginolysin-mediated cytotoxicity. PLoS One 12:e0183765. 10.1371/journal.pone.0183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harwich MD, Jr, Serrano MG, Fettweis JM, Alves JM, Reimers MA, Buck GA, Jefferson KK, Vaginal Microbiome Consortium . 2012. Genomic sequence analysis and characterization of Sneathia amnii sp. nov. BMC Genomics 13(Suppl 8):S4. 10.1186/1471-2164-13-S8-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jung HS, Ehlers MM, Lombaard H, Redelinghuys MJ, Kock MM. 2017. Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit Rev Microbiol 43:651–667. 10.1080/1040841X.2017.1291579. [DOI] [PubMed] [Google Scholar]
- 108.Swidsinski A, Dörffel Y, Loening-Baucke V, Schilling J, Mendling W. 2011. Response of Gardnerella vaginalis biofilm to 5 days of moxifloxacin treatment. FEMS Immunol Med Microbiol 61:41–46. 10.1111/j.1574-695X.2010.00743.x. [DOI] [PubMed] [Google Scholar]
- 109.Swidsinski A, Mendling W, Loening-Baucke V, Swidsinski S, Dörffel Y, Scholze J, Lochs H, Verstraelen H. 2008. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 198:97.e1-6–97.e6. 10.1016/j.ajog.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 110.Castro J, França A, Bradwell KR, Serrano MG, Jefferson KK, Cerca N. 2017. Comparative transcriptomic analysis of Gardnerella vaginalis biofilms vs. planktonic cultures using RNA-seq. NPJ Biofilms Microbiomes 3:3. 10.1038/s41522-017-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castro J, Machado D, Cerca N. 2019. Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J 13:1306–1317. 10.1038/s41396-018-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hymes SR, Randis TM, Sun TY, Ratner AJ. 2013. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis 207:1491–1497. 10.1093/infdis/jit047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, Marrazzo JM, Fredricks DN. 2015. Metabolic signatures of bacterial vaginosis. mBio 6:e00204-15. 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nelson TM, Borgogna JL, Brotman RM, Ravel J, Walk ST, Yeoman CJ. 2015. Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front Physiol 6:253. 10.3389/fphys.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McMillan A, Rulisa S, Sumarah M, Macklaim JM, Renaud J, Bisanz JE, Gloor GB, Reid G. 2015. A multi-platform metabolomics approach identifies highly specific biomarkers of bacterial diversity in the vagina of pregnant and non-pregnant women. Sci Rep 5:14174. 10.1038/srep14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vitali B, Cruciani F, Picone G, Parolin C, Donders G, Laghi L. 2015. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur J Clin Microbiol Infect Dis 34:2367–2376. 10.1007/s10096-015-2490-y. [DOI] [PubMed] [Google Scholar]
- 117.Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT. 2012. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with Toll-like receptor ligands. Am J Reprod Immunol 67:391–400. 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. 2018. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 18:e339–e347. 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- 119.Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. 2016. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 42:905–927. 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- 120.Ilkit M, Guzel AB. 2011. The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: a mycological perspective. Crit Rev Microbiol 37:250–261. 10.3109/1040841X.2011.576332. [DOI] [PubMed] [Google Scholar]
- 121.Naglik JR, Gaffen SL, Hube B. 2019. Candidalysin: discovery and function in Candida albicans infections. Curr Opin Microbiol 52:100–109. 10.1016/j.mib.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu MB, Xu SR, He Y, Deng GH, Sheng HF, Huang XM, Ouyang CY, Zhou HW. 2013. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS One 8:e79812. 10.1371/journal.pone.0079812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ceccarani C, Foschi C, Parolin C, D'Antuono A, Gaspari V, Consolandi C, Laghi L, Camboni T, Vitali B, Severgnini M, Marangoni A. 2019. Diversity of vaginal microbiome and metabolome during genital infections. Sci Rep 9:14095. 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McClelland RS, Richardson BA, Hassan WM, Graham SM, Kiarie J, Baeten JM, Mandaliya K, Jaoko W, Ndinya-Achola JO, Holmes KK. 2009. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis 199:1883–1890. 10.1086/599213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brown SE, Schwartz JA, Robinson CK, O’Hanlon DE, Bradford LL, He X, Mark KS, Bruno VM, Ravel J, Brotman RM. 2019. The vaginal microbiota and behavioral factors associated with genital Candida albicans detection in reproductive-age women. Sex Transm Dis 46:753–758. 10.1097/OLQ.0000000000001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eastment MC, Balkus JE, Richardson BA, Srinivasan S, Kimani J, Anzala O, Schwebke J, Fiedler TL, Fredricks DN, McClelland RS. 2021. Association between vaginal bacterial microbiota and vaginal yeast colonization. J Infect Dis 223:914–923. 10.1093/infdis/jiaa459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jang SJ, Lee K, Kwon B, You HJ, Ko G. 2019. Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci Rep 9:8121. 10.1038/s41598-019-44579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. 2003. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 37:319–325. 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 129.Watts DH, Fazzari M, Fazarri M, Minkoff H, Hillier SL, Sha B, Glesby M, Levine AM, Burk R, Palefsky JM, Moxley M, Ahdieh-Grant L, Strickler HD. 2005. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis 191:1129–1139. 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 130.Simoes JA, Hashemi FB, Aroutcheva AA, Heimler I, Spear GT, Shott S, Faro S. 2001. Human immunodeficiency virus type 1 stimulatory activity by Gardnerella vaginalis: relationship to biotypes and other pathogenic characteristics. J Infect Dis 184:22–27. 10.1086/321002. [DOI] [PubMed] [Google Scholar]
- 131.Cu-Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CC, HIV Epidemiology Research Study . 2001. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis 33:894–896. 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 132.Mercer F, Johnson PJ. 2018. Trichomonas vaginalis: pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol 34:683–693. 10.1016/j.pt.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jarrett OD, Srinivasan S, Richardson BA, Fiedler T, Wallis JM, Kinuthia J, Jaoko W, Mandaliya K, Fredricks DN, McClelland RS. 2019. Specific vaginal bacteria are associated with an increased risk of Trichomonas vaginalis acquisition in women. J Infect Dis 220:1503–1510. 10.1093/infdis/jiz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Masha SC, Owuor C, Ngoi JM, Cools P, Sanders EJ, Vaneechoutte M, Crucitti T, de Villiers EP. 2019. Comparative analysis of the vaginal microbiome of pregnant women with either Trichomonas vaginalis or Chlamydia trachomatis. PLoS One 14:e0225545. 10.1371/journal.pone.0225545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fettweis JM, Serrano MG, Huang B, Brooks JP, Glascock AL, Sheth NU, Strauss JF, III, Jefferson KK, Buck GA, Vaginal Microbiome Consortium . 2014. An emerging mycoplasma associated with trichomoniasis, vaginal infection and disease. PLoS One 9:e110943. 10.1371/journal.pone.0110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vancini RG, Benchimol M. 2008. Entry and intracellular location of Mycoplasma hominis in Trichomonas vaginalis. Arch Microbiol 189:7–18. 10.1007/s00203-007-0288-8. [DOI] [PubMed] [Google Scholar]
- 137.Costello EK, Sun CL, Carlisle EM, Morowitz MJ, Banfield JF, Relman DA. 2017. Candidatus Mycoplasma girerdii replicates, diversifies, and co-occurs with Trichomonas vaginalis in the oral cavity of a premature infant. Sci Rep 7:3764. 10.1038/s41598-017-03821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dessì D, Margarita V, Cocco AR, Marongiu A, Fiori PL, Rappelli P. 2019. Trichomonas vaginalis and Mycoplasma hominis: new tales of two old friends. Parasitology 146:1150–1155. 10.1017/S0031182018002135. [DOI] [PubMed] [Google Scholar]
- 139.Paavonen J, Brunham RC. 2018. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med 379:2246–2254. 10.1056/NEJMra1808418. [DOI] [PubMed] [Google Scholar]
- 140.Gardner HL. 1968. Desquamative inflammatory vaginitis: a newly defined entity. Am J Obstet Gynecol 102:1102–1105. 10.1016/0002-9378(68)90399-2. [DOI] [PubMed] [Google Scholar]
- 141.Kaambo E, Africa C, Chambuso R, Passmore JS. 2018. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front Public Health 6:78. 10.3389/fpubh.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lawn JE, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, Madrid L, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, Le Doare K, Madhi SA, Rubens CE, Saha SK, Schrag S, Sobanjo-Ter Meulen A, Vekemans J, Seale AC. 2017. Group B streptococcal disease worldwide for pregnant women, stillbirths, and children: why, what, and how to undertake estimates? Clin Infect Dis 65:S89–s99. 10.1093/cid/cix653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, Carlo WA, Cotten CM, D'Angio CT, Kazzi SNJ, Poindexter BB, Van Meurs KP, Hale EC, Collins MV, Das A, Baker CJ, Wyckoff MH, Yoder BA, Watterberg KL, Walsh MC, Devaskar U, Laptook AR, Sokol GM, Schrag SJ, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . 2020. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr 174:e200593. 10.1001/jamapediatrics.2020.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McMullan BJ, Campbell AJ, Blyth CC, McNeil JC, Montgomery CP, Tong SYC, Bowen AC. 2020. Clinical management of Staphylococcus aureus bacteremia in neonates, children, and adolescents. Pediatrics 146:e20200134. 10.1542/peds.2020-0134. [DOI] [PubMed] [Google Scholar]
- 145.Rosen GH, Randis TM, Desai PV, Sapra KJ, Ma B, Gajer P, Humphrys MS, Ravel J, Gelber SE, Ratner AJ. 2017. Group B Streptococcus and the vaginal microbiota. J Infect Dis 216:744–751. 10.1093/infdis/jix395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gilbert NM, Foster LR, Cao B, Yin Y, Mysorekar IU, Lewis AL. 2021. Gardnerella vaginalis promotes group B Streptococcus vaginal colonization, enabling ascending uteroplacental infection in pregnant mice. Am J Obstet Gynecol 224:530.e1–530.e17. 10.1016/j.ajog.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DS, Wong RJ, Shaw G, Stevenson DK, Holmes SP, Relman DA. 2015. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci USA 112:11060–11065. 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, Lehne B, Arulkumaran S, Brown R, Teoh TG, Holmes E, Nicoholson JK, Marchesi JR, Bennett PR. 2015. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 5:8988. 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang X, Zhai Q, Wang J, Ma X, Xing B, Fan H, Gao Z, Zhao F, Liu W. 2022. Variation of the vaginal microbiome during and after pregnancy in Chinese women. Genomics Proteomics Bioinformatics 10.1016/j.gpb.2021.08.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shachar BZ, Mayo JA, Lyell DJ, Baer RJ, Jeliffe-Pawlowski LL, Stevenson DK, Shaw GM. 2016. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. BJOG 123:2009–2017. 10.1111/1471-0528.14165. [DOI] [PubMed] [Google Scholar]
- 151.McKinney D, House M, Chen A, Muglia L, DeFranco E. 2017. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol 216:316.e1–316.e9. 10.1016/j.ajog.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kangatharan C, Labram S, Bhattacharya S. 2017. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update 23:221–231. 10.1093/humupd/dmw043. [DOI] [PubMed] [Google Scholar]
- 153.Jacobson JC, Turok DK, Dermish AI, Nygaard IE, Settles ML. 2014. Vaginal microbiome changes with levonorgestrel intrauterine system placement. Contraception 90:130–135. 10.1016/j.contraception.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 154.Brusselaers N, Shrestha S, van de Wijgert J, Verstraelen H. 2019. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol 221:9–18.E8. 10.1016/j.ajog.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 155.Doorbar J. 2006. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 110:525–541. 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 156.Tango CN, Seo SS, Kwon M, Lee DO, Chang HK, Kim MK. 2020. Taxonomic and functional differences in cervical microbiome associated with cervical cancer development. Sci Rep 10:9720. 10.1038/s41598-020-66607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, Martínez-Barnetche J, Cortina-Ceballos B, López-Estrada G, Delgado-Romero K, Burguete-García AI, Cantú D, García-Carrancá A, Madrid-Marina V. 2016. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS One 11:e0153274. 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang H, Lu J, Lu Y, Cai Q, Liu H, Xu C. 2018. Cervical microbiome is altered in cervical intraepithelial neoplasia after loop electrosurgical excision procedure in china. Sci Rep 8:4923. 10.1038/s41598-018-23389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Curty G, Costa RL, Siqueira JD, Meyrelles AI, Machado ES, Soares EA, Soares MA. 2017. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci Rep 7:17364. 10.1038/s41598-017-17351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seo SS, Oh HY, Lee JK, Kong JS, Lee DO, Kim MK. 2016. Combined effect of diet and cervical microbiome on the risk of cervical intraepithelial neoplasia. Clin Nutr 35:1434–1441. 10.1016/j.clnu.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 161.Valenti P, Rosa L, Capobianco D, Lepanto MS, Schiavi E, Cutone A, Paesano R, Mastromarino P. 2018. Role of Lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front Immunol 9:376. 10.3389/fimmu.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mantovani A, Allavena P, Sica A, Balkwill F. 2008. Cancer-related inflammation. Nature 454:436–444. 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 163.Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, Gravitt PE. 2014. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis 210:1723–1733. 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. 2016. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 4:58. 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee JE, Lee S, Lee H, Song YM, Lee K, Han MJ, Sung J, Ko G. 2013. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8:e63514. 10.1371/journal.pone.0063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, Xie H, Chen X, Zeng C, Wen B, Zeng L, Du H, Tang H, Xu C, Xia Y, Xia H, Yang H, Wang J, Wang J, Madsen L, Brix S, Kristiansen K, Xu X, Li J, Wu R, Jia H. 2017. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 8:875. 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miles SM, Hardy BL, Merrell DS. 2017. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil Steril 107:813–820.E1. 10.1016/j.fertnstert.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 168.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. 2014. The placenta harbors a unique microbiome. Sci Transl Med 6:237ra65. 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baker JM, Chase DM, Herbst-Kralovetz MM. 2018. Uterine microbiota: residents, tourists, or invaders? Front Immunol 9:208. 10.3389/fimmu.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. 2017. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5:48. 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peric A, Weiss J, Vulliemoz N, Baud D, Stojanov M. 2019. Bacterial colonization of the female upper genital tract. Int J Mol Sci 20:3405. 10.3390/ijms20143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Theis KR, Romero R, Winters AD, Greenberg JM, Gomez-Lopez N, Alhousseini A, Bieda J, Maymon E, Pacora P, Fettweis JM, Buck GA, Jefferson KK, Strauss JF, III, Erez O, Hassan SS. 2019. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am J Obstet Gynecol 220:267.e1–267.e39. 10.1016/j.ajog.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kennedy KM, Gerlach MJ, Adam T, Heimesaat MM, Rossi L, Surette MG, Sloboda DM, Braun T. 2021. Fetal meconium does not have a detectable microbiota before birth. Nat Microbiol 6:865–873. 10.1038/s41564-021-00904-0. [DOI] [PubMed] [Google Scholar]
- 174.Payne MS, Bayatibojakhi S. 2014. Exploring preterm birth as a polymicrobial disease: an overview of the uterine microbiome. Front Immunol 5:595. 10.3389/fimmu.2014.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lenz JD, Dillard JP. 2018. Pathogenesis of Neisseria gonorrhoeae and the host defense in ascending infections of human fallopian tube. Front Immunol 9:2710. 10.3389/fimmu.2018.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hafner LM. 2015. Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 92:108–115. 10.1016/j.contraception.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 177.El Khoury J, Stikkelbroeck MM, Goodman A, Rubin RH, Cosimi AB, Fishman JA. 2001. Postmenopausal tubo-ovarian abscess due to Pseudomonas aeruginosa in a renal transplant patient: a case report and review of the literature. Transplantation 72:1241–1244. 10.1097/00007890-200110150-00010. [DOI] [PubMed] [Google Scholar]
- 178.Brunham RC, Gottlieb SL, Paavonen J. 2015. Pelvic inflammatory disease. N Engl J Med 372:2039–2048. 10.1056/NEJMra1411426. [DOI] [PubMed] [Google Scholar]
- 179.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. 2003. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 189:139–147. 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 180.Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, Vo KC, Caughey AB, Hilton JF, Davis RW, Giudice LC. 2014. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci 21:32–40. 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.He Q, Kwok L-Y, Xi X, Zhong Z, Ma T, Xu H, Meng H, Zhao F, Zhang H. 2020. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 12:1794266. 10.1080/19490976.2020.1794266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Parnell LA, Briggs CM, Cao B, Delannoy-Bruno O, Schrieffer AE, Mysorekar IU. 2017. Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci Rep 7:11200. 10.1038/s41598-017-11514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suff N, Karda R, Diaz JA, Ng J, Baruteau J, Perocheau D, Tangney M, Taylor PW, Peebles D, Buckley SMK, Waddington SN. 2018. Ascending vaginal infection using bioluminescent bacteria evokes intrauterine inflammation, preterm birth, and neonatal brain injury in pregnant mice. Am J Pathol 188:2164–2176. 10.1016/j.ajpath.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dörtbudak O, Eberhardt R, Ulm M, Persson GR. 2005. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol 32:45–52. 10.1111/j.1600-051X.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 185.Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, Sweeney EL, Knox CL, Lambers DS, Jobe AH, Chougnet CA, Kallapur SG, Aagaard KM. 2016. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol 214:627.e1–627.e16. 10.1016/j.ajog.2016.01.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rehbinder EM, Lødrup Carlsen KC, Staff AC, Angell IL, Landrø L, Hilde K, Gaustad P, Rudi K. 2018. Is amniotic fluid of women with uncomplicated term pregnancies free of bacteria? Am J Obstet Gynecol 219:289.e1–289.e12. 10.1016/j.ajog.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 187.Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, Shamonki JM, Redline RW. 2010. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol 115:442–445. 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. 2004. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 72:2272–2279. 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goldenberg RL, Hauth JC, Andrews WW. 2000. Intrauterine infection and preterm delivery. N Engl J Med 342:1500–1507. 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 190.Goldenberg RL, Culhane JF, Iams JD, Romero R. 2008. Epidemiology and causes of preterm birth. Lancet 371:75–84. 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. 2002. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol 187:137–144. 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 192.Randis TM, Gelber SE, Hooven TA, Abellar RG, Akabas LH, Lewis EL, Walker LB, Byland LM, Nizet V, Ratner AJ. 2014. Group B Streptococcus β-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis 210:265–273. 10.1093/infdis/jiu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, Adams Waldorf KM, Rajagopal L. 2016. Bacterial hyaluronidase promotes ascending GBS infection and preterm birth. mBio 7:e00781-16. 10.1128/mBio.00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Doster RS, Kirk LA, Tetz LM, Rogers LM, Aronoff DM, Gaddy JA. 2017. Staphylococcus aureus infection of human gestational membranes induces bacterial biofilm formation and host production of cytokines. J Infect Dis 215:653–657. 10.1093/infdis/jiw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Akgul Y, Word RA, Ensign LM, Yamaguchi Y, Lydon J, Hanes J, Mahendroo M. 2014. Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. J Clin Invest 124:5481–5489. 10.1172/JCI78765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Coleman M, Armistead B, Orvis A, Quach P, Brokaw A, Gendrin C, Sharma K, Ogle J, Merillat S, Dacanay M, Wu T-Y, Munson J, Baldessari A, Vornhagen J, Furuta A, Nguyen S, Adams Waldorf KM, Rajagopal L. 2021. Hyaluronidase impairs neutrophil function and promotes group B Streptococcus invasion and preterm labor in nonhuman primates. mBio 12:e03115-20. 10.1128/mBio.03115-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vornhagen J, Armistead B, Santana-Ufret V, Gendrin C, Merillat S, Coleman M, Quach P, Boldenow E, Alishetti V, Leonhard-Melief C, Ngo LY, Whidbey C, Doran KS, Curtis C, Waldorf KMA, Nance E, Rajagopal L. 2018. Group B streptococcus exploits vaginal epithelial exfoliation for ascending infection. J Clin Invest 128:1985–1999. 10.1172/JCI97043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gilbert NM, Lewis WG, Lewis AL. 2013. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One 8:e59539. 10.1371/journal.pone.0059539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cauci S, Hitti J, Noonan C, Agnew K, Quadrifoglio F, Hillier SL, Eschenbach DA. 2002. Vaginal hydrolytic enzymes, immunoglobulin A against Gardnerella vaginalis toxin, and risk of early preterm birth among women in preterm labor with bacterial vaginosis or intermediate flora. Am J Obstet Gynecol 187:877–881. 10.1067/mob.2002.127454. [DOI] [PubMed] [Google Scholar]
- 200.Cobo T, Aldecoa V, Figueras F, Herranz A, Ferrero S, Izquierdo M, Murillo C, Amoedo R, Rueda C, Bosch J, Martínez-Portilla RJ, Gratacós E, Palacio M. 2020. Development and validation of a multivariable prediction model of spontaneous preterm delivery and microbial invasion of the amniotic cavity in women with preterm labor. Am J Obstet Gynecol 223:421.e1–421.e14. 10.1016/j.ajog.2020.02.049. [DOI] [PubMed] [Google Scholar]
- 201.Gudicha DW, Romero R, Kabiri D, Hernandez-Andrade E, Pacora P, Erez O, Pedro Kusanovic J, Jung E, Paredes C, Berry SM, Yeo L, Hassan SS, Chaur-Dong HSU, Tarca AL. 2020. Personalized assessment of cervical length improves prediction of spontaneous preterm birth: a standard and a percentile calculator. Am J Obstet Gynecol 224:288.e1–288.e17. 10.1016/j.ajog.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kunz G, Beil D, Deiniger H, Einspanier A, Mall G, Leyendecker G. 1997. The uterine peristaltic pump. Normal and impeded sperm transport within the female genital tract. Adv Exp Med Biol 424:267–277. 10.1007/978-1-4615-5913-9_49. [DOI] [PubMed] [Google Scholar]
- 203.Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, Prieto-Merino D, Cousens S, Black RE, Liu L. 2022. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health 6:106–115. 10.1016/S2352-4642(21)00311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Anonymous. 1989. Association of Chlamydia trachomatis and Mycoplasma hominis with intrauterine growth retardation and preterm delivery: investigators of the Johns Hopkins Study of Cervicitis and Adverse Pregnancy Outcome. Am J Epidemiol 129:1247–1257. 10.1093/oxfordjournals.aje.a115244. [DOI] [PubMed] [Google Scholar]
- 205.Rours GI, de Krijger RR, Ott A, Willemse HF, de Groot R, Zimmermann LJ, Kornelisse RF, Verbrugh HA, Verkooijen RP. 2011. Chlamydia trachomatis and placental inflammation in early preterm delivery. Eur J Epidemiol 26:421–428. 10.1007/s10654-011-9569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Curry A, Williams T, Penny ML. 2019. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician 100:357–364. [PubMed] [Google Scholar]
- 207.Bonasoni MP, Palicelli A, Dalla Dea G, Comitini G, Nardini P, Vizzini L, Russello G, Bardaro M, Carretto E. 2021. Klebsiella pneumoniae chorioamnionitis: an underrecognized cause of preterm premature rupture of membranes in the second trimester. Microorganisms 9:96. 10.3390/microorganisms9010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Carey JC, Klebanoff MA. 2005. Is a change in the vaginal flora associated with an increased risk of preterm birth? Am J Obstet Gynecol 192:1341–1346. 10.1016/j.ajog.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 209.Kikhney J, von Schöning D, Steding I, Schulze J, Petrich A, Hiergeist A, Reischl U, Moter A, Thomas A. 2017. Is Ureaplasma spp. the leading causative agent of acute chorioamnionitis in women with preterm birth? Clin Microbiol Infect 23:119.e1–119.e7. 10.1016/j.cmi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 210.Chan E, Brundler MA, Zhang K. 2019. Identification of Fusobacterium nucleatum in formalin-fixed, paraffin-embedded placental tissues by 16S rRNA sequencing in a case of extremely preterm birth secondary to amniotic fluid infection. Pathology 51:320–322. 10.1016/j.pathol.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 211.Bianchi-Jassir F, Seale AC, Kohli-Lynch M, Lawn JE, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, Le Doare K, Madhi SA, Saha SK, Schrag S, Sobanjo-Ter Meulen A, Vekemans J, Rubens CE. 2017. Preterm birth associated with group B Streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 65:S133–S142. 10.1093/cid/cix661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Trombetta A, Comar M, Tommasini A, Canton M, Campisciano G, Zanotta N, Cason C, Maso G, Risso FM. 2021. SARS-CoV-2 infection and inflammatory response in a twin pregnancy. Int J Environ Res Public Health 18:3075. 10.3390/ijerph18063075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Anderson K, Kalk E, Madlala HP, Nyemba DC, Jacob N, Slogrove A, Smith M, Kroon M, Harrison MC, Eley BS, Boulle A, Myer L, Davies MA. 2021. Preterm birth and severe morbidity in hospitalized neonates who are HIV exposed and uninfected compared with HIV unexposed. AIDS 35:921–931. 10.1097/QAD.0000000000002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wiik J, Nilsson S, Kärrberg C, Strander B, Jacobsson B, Sengpiel V. 2021. Associations of treated and untreated human papillomavirus infection with preterm delivery and neonatal mortality: a Swedish population-based study. PLoS Med 18:e1003641. 10.1371/journal.pmed.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Giusto K, Patki M, Koya J, Ashby CR, Jr, Munnangi S, Patel K, Reznik SE. 2019. A vaginal nanoformulation of a SphK inhibitor attenuates lipopolysaccharide-induced preterm birth in mice. Nanomedicine (Lond) 14:2835–2851. 10.2217/nnm-2019-0243. [DOI] [PubMed] [Google Scholar]
- 216.Jun JK, Yoon BH, Romero R, Kim M, Moon JB, Ki SH, Park JS. 2000. Interleukin 6 determinations in cervical fluid have diagnostic and prognostic value in preterm premature rupture of membranes. Am J Obstet Gynecol 183:868–873. 10.1067/mob.2000.109034. [DOI] [PubMed] [Google Scholar]
- 217.Wei SQ, Fraser W, Luo ZC. 2010. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 116:393–401. 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 218.Kumar M, Murugesan S, Singh P, Saadaoui M, Elhag DA, Terranegra A, Kabeer BSA, Marr AK, Kino T, Brummaier T, McGready R, Nosten F, Chaussabel D, Al Khodor S. 2021. Vaginal microbiota and cytokine levels predict preterm delivery in asian women. Front Cell Infect Microbiol 11:639665. 10.3389/fcimb.2021.639665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jacobsson B, Holst RM, Mattsby-Baltzer I, Nikolaitchouk N, Wennerholm UB, Hagberg H. 2003. Interleukin-18 in cervical mucus and amniotic fluid: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation and preterm delivery. BJOG 110:598–603. 10.1046/j.1471-0528.2003.02445.x. [DOI] [PubMed] [Google Scholar]
- 220.Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD, Das A, Caritis SN, Roberts JM, Miodovnik M, Menard K, Thurnau G, Dombrowski MP, McNellis D. 2000. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. Am J Obstet Gynecol 182:625–630. 10.1067/mob.2000.104210. [DOI] [PubMed] [Google Scholar]
- 221.Yudin MH, Landers DV, Meyn L, Hillier SL. 2003. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstet Gynecol 102:527–534. 10.1097/00006250-200309000-00019. [DOI] [PubMed] [Google Scholar]
- 222.Armstrong E, Hemmerling A, Miller S, Burke KE, Newmann SJ, Morris SR, Reno H, Huibner S, Kulikova M, Liu R, Crawford ED, Castañeda GR, Nagelkerke N, Coburn B, Cohen CR, Kaul R. 2022. Metronidazole treatment rapidly reduces genital inflammation through effects on bacterial vaginosis-associated bacteria rather than lactobacilli. J Clin Invest 132:e152930. 10.1172/jci152930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gautam R, Borgdorff H, Jespers V, Francis SC, Verhelst R, Mwaura M, Delany-Moretlwe S, Ndayisaba G, Kyongo JK, Hardy L, Menten J, Crucitti T, Tsivtsivadze E, Schuren F, van de Wijgert JH, Vaginal Biomarkers Study Group . 2015. Correlates of the molecular vaginal microbiota composition of African women. BMC Infect Dis 15:86. 10.1186/s12879-015-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung'u T, Dong KL, Walker BD, Fichorova RN, Kwon DS. 2015. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42:965–976. 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Campisciano G, Gheit T, De Seta F, Cason C, Zanotta N, Delbue S, Ricci G, Ferrante P, Tommasino M, Comar M. 2019. Oncogenic virome benefits from the different vaginal microbiome-immune axes. Microorganisms 7:414. 10.3390/microorganisms7100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, Ravel J. 2019. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun 10:1305. 10.1038/s41467-019-09285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brown RG, Marchesi JR, Lee YS, Smith A, Lehne B, Kindinger LM, Terzidou V, Holmes E, Nicholson JK, Bennett PR, MacIntyre DA. 2018. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med 16:9. 10.1186/s12916-017-0999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Feehily C, Crosby D, Walsh CJ, Lawton EM, Higgins S, McAuliffe FM, Cotter PD. 2020. Shotgun sequencing of the vaginal microbiome reveals both a species and functional potential signature of preterm birth. NPJ Biofilms Microbiomes 6:50. 10.1038/s41522-020-00162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tabatabaei N, Eren AM, Barreiro LB, Yotova V, Dumaine A, Allard C, Fraser WD. 2019. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case-control study. BJOG 126:349–358. 10.1111/1471-0528.15299. [DOI] [PubMed] [Google Scholar]
- 230.Hauth JC, Macpherson C, Carey JC, Klebanoff MA, Hillier SL, Ernest JM, Leveno KJ, Wapner R, Varner M, Trout W, Moawad A, Sibai B. 2003. Early pregnancy threshold vaginal pH and Gram stain scores predictive of subsequent preterm birth in asymptomatic women. Am J Obstet Gynecol 188:831–835. 10.1067/mob.2003.184. [DOI] [PubMed] [Google Scholar]
- 231.Dunlop AL, Satten GA, Hu YJ, Knight AK, Hill CC, Wright ML, Smith AK, Read TD, Pearce BD, Corwin EJ. 2021. Vaginal microbiome composition in early pregnancy and risk of spontaneous preterm and early term birth among African American women. Front Cell Infect Microbiol 11:641005. 10.3389/fcimb.2021.641005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Joag V, Obila O, Gajer P, Scott MC, Dizzell S, Humphrys M, Shahabi K, Huibner S, Shannon B, Tharao W, Mureithi M, Oyugi J, Kimani J, Kaushic C, Ravel J, Anzala O, Kaul R. 2019. Impact of standard bacterial vaginosis treatment on the genital microbiota, immune milieu, and ex vivo human immunodeficiency virus susceptibility. Clin Infect Dis 68:1675–1683. 10.1093/cid/ciy762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Carter RA, Pan K, Harville EW, McRitchie S, Sumner S. 2019. Metabolomics to reveal biomarkers and pathways of preterm birth: a systematic review and epidemiologic perspective. Metabolomics 15:124. 10.1007/s11306-019-1587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gerson KD, McCarthy C, Elovitz MA, Ravel J, Sammel MD, Burris HH. 2020. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am J Obstet Gynecol 222:491.e1–491.e8. 10.1016/j.ajog.2019.11.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Conde-Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O'Brien JM, Cetingoz E, da Fonseca E, Creasy G, Soma-Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. 2013. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol 208:42.e1–42.e18. 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kindinger LM, MacIntyre DA, Lee YS, Marchesi JR, Smith A, McDonald JA, Terzidou V, Cook JR, Lees C, Israfil-Bayli F, Faiza Y, Toozs-Hobson P, Slack M, Cacciatore S, Holmes E, Nicholson JK, Teoh TG, Bennett PR. 2016. Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. Sci Transl Med 8:350ra102. 10.1126/scitranslmed.aag1026. [DOI] [PubMed] [Google Scholar]
- 237.Yoshida K, Jayyosi C, Lee N, Mahendroo M, Myers KM. 2019. Mechanics of cervical remodelling: insights from rodent models of pregnancy. Interface Focus 9:20190026. 10.1098/rsfs.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Timmons B, Akins M, Mahendroo M. 2010. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab 21:353–361. 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gonzalez JM, Xu H, Chai J, Ofori E, Elovitz MA. 2009. Preterm and term cervical ripening in CD1 mice (Mus musculus): similar or divergent molecular mechanisms? Biol Reprod 81:1226–1232. 10.1095/biolreprod.108.075309. [DOI] [PubMed] [Google Scholar]
- 240.Di Paola M, Seravalli V, Paccosi S, Linari C, Parenti A, De Filippo C, Tanturli M, Vitali F, Torcia MG, Di Tommaso M. 2020. Identification of vaginal microbial communities associated with extreme cervical shortening in pregnant women. J Clin Med 9:3621. 10.3390/jcm9113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yellon SM. 2017. Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol Reprod 96:13–23. 10.1095/biolreprod.116.142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bennett PR, Brown RG, MacIntyre DA. 2020. Vaginal microbiome in preterm rupture of membranes. Obstet Gynecol Clin North Am 47:503–521. 10.1016/j.ogc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 243.Paramel Jayaprakash T, Wagner EC, van Schalkwyk J, Albert AY, Hill JE, Money DM, PPROM Study Group . 2016. High diversity and variability in the vaginal microbiome in women following preterm premature rupture of membranes (PPROM): a prospective cohort study. PLoS One 11:e0166794. 10.1371/journal.pone.0166794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Brown RG, Al-Memar M, Marchesi JR, Lee YS, Smith A, Chan D, Lewis H, Kindinger L, Terzidou V, Bourne T, Bennett PR, MacIntyre DA. 2019. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl Res 207:30–43. 10.1016/j.trsl.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Goodfellow L, Verwijs MC, Care A, Sharp A, Ivandic J, Poljak B, Roberts D, Bronowski C, Gill AC, Darby AC, Alfirevic A, Muller-Myhsok B, Alfirevic Z, van de Wijgert JHHM. 2021. Vaginal bacterial load in the second trimester is associated with early preterm birth recurrence: a nested case-control study. BJOG 128:2061–2072. 10.1111/1471-0528.16816. [DOI] [PubMed] [Google Scholar]
- 246.Hendler I, Andrews WW, Carey CJ, Klebanoff MA, Noble WD, Sibai BM, Hillier SL, Dudley D, Ernest JM, Leveno KJ, Wapner R, Iams JD, Varner M, Moawad A, Miodovnik M, O’Sullivan MJ, Van Dorsten PJ. 2007. The relationship between resolution of asymptomatic bacterial vaginosis and spontaneous preterm birth in fetal fibronectin-positive women. Am J Obstet Gynecol 197:488.e1–488.e5. 10.1016/j.ajog.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 247.Lewis AL, Laurent LC. 2020. USPSTF 2020 recommendations on screening for asymptomatic bacterial vaginosis in pregnancy. JAMA 323:1253–1255. 10.1001/jama.2019.22311. [DOI] [PubMed] [Google Scholar]
- 248.Vodstrcil LA, Muzny CA, Plummer EL, Sobel JD, Bradshaw CS. 2021. Bacterial vaginosis: drivers of recurrence and challenges and opportunities in partner treatment. BMC Med 19:194. 10.1186/s12916-021-02077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Verwijs MC, Agaba SK, Darby AC, van de Wijgert J. 2020. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am J Obstet Gynecol 222:157.e1–157.e13. 10.1016/j.ajog.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lee CY, Cheu RK, Lemke MM, Gustin AT, France MT, Hampel B, Thurman AR, Doncel GF, Ravel J, Klatt NR, Arnold KB. 2020. Quantitative modeling predicts mechanistic links between pre-treatment microbiome composition and metronidazole efficacy in bacterial vaginosis. Nat Commun 11:6147. 10.1038/s41467-020-19880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jehan F, Sazawal S, Baqui AH, Nisar MI, Dhingra U, Khanam R, Ilyas M, Dutta A, Mitra DK, Mehmood U, Deb S, Mahmud A, Hotwani A, Ali SM, Rahman S, Nizar A, Ame SM, Moin MI, Muhammad S, Chauhan A, Begum N, Khan W, Das S, Ahmed S, Hasan T, Khalid J, Rizvi SJR, Juma MH, Chowdhury NH, Kabir F, Aftab F, Quaiyum A, Manu A, Yoshida S, Bahl R, Rahman A, Pervin J, Winston J, Musonda P, Stringer JSA, Litch JA, Ghaemi MS, Moufarrej MN, Contrepois K, Chen S, Stelzer IA, Stanley N, Chang AL, Hammad GB, Wong RJ, Alliance for Maternal and Newborn Health Improvement, the Global Alliance to Prevent Prematurity and Stillbirth, and the Prematurity Research Center at Stanford University , et al. 2020. Multiomics characterization of preterm birth in low- and middle-income countries. JAMA Netw Open 3:e2029655. 10.1001/jamanetworkopen.2020.29655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Garretto A, Miller-Ensminger T, Wolfe AJ, Putonti C. 2019. Bacteriophages of the lower urinary tract. Nat Rev Urol 16:422–432. 10.1038/s41585-019-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.da Costa AC, Moron AF, Forney LJ, Linhares IM, Sabino E, Costa SF, Mendes-Correa MC, Witkin SS. 2021. Identification of bacteriophages in the vagina of pregnant women: a descriptive study. BJOG 128:976–982. 10.1111/1471-0528.16528. [DOI] [PubMed] [Google Scholar]
- 254.Jakobsen RR, Haahr T, Humaidan P, Jensen JS, Kot WP, Castro-Mejia JL, Deng L, Leser TD, Nielsen DS. 2020. Characterization of the vaginal DNA virome in health and dysbiosis. Viruses 12:1143. 10.3390/v12101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Happel A-U, Balle C, Maust BS, Konstantinus IN, Gill K, Bekker L-G, Froissart R, Passmore J-A, Karaoz U, Varsani A, Jaspan H. 2021. Presence and persistence of putative lytic and temperate bacteriophages in vaginal metagenomes from South African adolescents. Viruses 13:2341. 10.3390/v13122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wylie KM, Wylie TN, Cahill AG, Macones GA, Tuuli MG, Stout MJ. 2018. The vaginal eukaryotic DNA virome and preterm birth. Am J Obstet Gynecol 219:189.e1–189.e12. 10.1016/j.ajog.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bachmann GA, Nevadunsky NS. 2000. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 61:3090–3096. [PubMed] [Google Scholar]
- 258.Donders G, Neven P, Moegele M, Lintermans A, Bellen G, Prasauskas V, Grob P, Ortmann O, Buchholz S. 2014. Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat 145:371–379. 10.1007/s10549-014-2930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, Green L, Miller S, Powell J, Parks T, Hemmerling A. 2020. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med 382:1906–1915. 10.1056/NEJMoa1915254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bradshaw CS, Sobel JD. 2016. Current treatment of bacterial vaginosis-limitations and need for innovation. J Infect Dis 214(Suppl 1):S14–S20. 10.1093/infdis/jiw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kamronrithisorn T, Manonai J, Vallibhakara SA, Sophonsritsuk A, Vallibhakara O. 2020. Effect of vitamin D supplement on vulvovaginal atrophy of the menopause. Nutrients 12:2876. 10.3390/nu12092876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rosen EM, Martin CL, Siega-Riz AM, Dole N, Basta PV, Serrano M, Fettweis J, Wu M, Sun S, Thorp JM, Jr, Buck G, Fodor AA, Engel SM. 2022. Is prenatal diet associated with the composition of the vaginal microbiome? Paediatr Perinat Epidemiol 36:243–253. 10.1111/ppe.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Heremans R, Jan Z, Timmerman D, Vankelecom H. 2021. Organoids of the female reproductive tract: innovative tools to study desired to unwelcome processes. Front Cell Dev Biol 9:661472. 10.3389/fcell.2021.661472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. 2014. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis 209:1989–1999. 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 265.Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, Novak RM, McCorrister S, McKinnon LR, Cohen CR, Mackelprang R, Lingappa J, Lauffenburger DA, Klatt NR, Burgener AD. 2016. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog 12:e1005889. 10.1371/journal.ppat.1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T, Kyrpides NC, Pukall R, Klenk HP, Goodfellow M, Göker M. 2018. Genome-based taxonomic classification of the phylum actinobacteria. Front Microbiol 9:2007. 10.3389/fmicb.2018.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP. 2001. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transm Infect 77:402–408. 10.1136/sti.77.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mohammadzadeh R, Sadeghi Kalani B, Kashanian M, Oshaghi M, Amirmozafari N. 2019. Prevalence of vaginolysin, sialidase and phospholipase genes in Gardnerella vaginalis isolates between bacterial vaginosis and healthy individuals. Med J Islam Repub Iran 33:85. 10.34171/mjiri.33.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ilhan ZE, Łaniewski P, Tonachio A, Herbst-Kralovetz MM. 2020. Members of Prevotella genus distinctively modulate innate immune and barrier functions in a human three-dimensional endometrial epithelial cell model. J Infect Dis 222:2082–2092. 10.1093/infdis/jiaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Roberton AM, Wiggins R, Horner PJ, Greenwood R, Crowley T, Fernandes A, Berry M, Corfield AP. 2005. A novel bacterial mucinase, glycosulfatase, is associated with bacterial vaginosis. J Clin Microbiol 43:5504–5508. 10.1128/JCM.43.11.5504-5508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gelber SE, Aguilar JL, Lewis KL, Ratner AJ. 2008. Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol 190:3896–3903. 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.DeSilva NS, Quinn PA. 1999. Characterization of phospholipase A1, A2, C activity in Ureaplasma urealyticum membranes. Mol Cell Biochem 201:159–167. 10.1023/a:1007082507407. [DOI] [PubMed] [Google Scholar]
- 273.Cauci S, Scrimin F, Driussi S, Ceccone S, Monte R, Fant L, Quadrifoglio F. 1996. Specific immune response against Gardnerella vaginalis hemolysin in patients with bacterial vaginosis. Am J Obstet Gynecol 175:1601–1605. 10.1016/s0002-9378(96)70112-6. [DOI] [PubMed] [Google Scholar]
- 274.Blanchard A, Razin S, Kenny GE, Barile MF. 1988. Characteristics of Ureaplasma urealyticum urease. J Bacteriol 170:2692–2697. 10.1128/jb.170.6.2692-2697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pybus V, Onderdonk AB. 1997. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis 175:406–413. 10.1093/infdis/175.2.406. [DOI] [PubMed] [Google Scholar]
- 276.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R, Marrazzo JM, Fredricks DN. 2012. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 7:e37818. 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bostwick DG, Woody J, Hunt C, Budd W. 2016. Antimicrobial resistance genes and modelling of treatment failure in bacterial vaginosis: clinical study of 289 symptomatic women. J Med Microbiol 65:377–386. 10.1099/jmm.0.000236. [DOI] [PubMed] [Google Scholar]
- 278.Spooner RK, Russell WC, Thirkell D. 1992. Characterization of the immunoglobulin A protease of Ureaplasma urealyticum. Infect Immun 60:2544–2546. 10.1128/iai.60.6.2544-2546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Al-Mushrif S, Eley A, Jones BM. 2000. Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J Med Microbiol 49:1023–1030. 10.1099/0022-1317-49-11-1023. [DOI] [PubMed] [Google Scholar]
- 280.Pybus V, Onderdonk AB. 1998. A commensal symbiosis between Prevotella bivia and Peptostreptococcus anaerobius involves amino acids: potential significance to the pathogenesis of bacterial vaginosis. FEMS Immunol Med Microbiol 22:317–327. 10.1111/j.1574-695X.1998.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 281.van Teijlingen NH, Helgers LC, Zijlstra-Willems EM, van Hamme JL, Ribeiro CMS, Strijbis K, Geijtenbeek TBH. 2020. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J Reprod Immunol 138:103085. 10.1016/j.jri.2020.103085. [DOI] [PubMed] [Google Scholar]
- 282.He YH, Xiao BB, Yang HX. 2021. A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study). Am J Obstet Gynecol 224:634. 10.1016/j.ajog.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 283.Lamont RF. 2015. Advances in the prevention of infection-related preterm birth. Front Immunol 6:566. 10.3389/fimmu.2015.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ng S, Chen M, Kundu S, Wang X, Zhou Z, Zheng Z, Qing W, Sheng H, Wang Y, He Y, Bennett PR, MacIntyre DA, Zhou H. 2021. Large-scale characterisation of the pregnancy vaginal microbiome and sialidase activity in a low-risk Chinese population. NPJ Biofilms Microbiomes 7:89. 10.1038/s41522-021-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nold C, Anton L, Brown A, Elovitz M. 2012. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth. Am J Obstet Gynecol 206:208.e1–208.e7. 10.1016/j.ajog.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 286.Lacey L, Daulton E, Wicaksono A, Covington JA, Quenby S. 2020. Volatile organic compound analysis, a new tool in the quest for preterm birth prediction-an observational cohort study. Sci Rep 10:12153. 10.1038/s41598-020-69142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cox SM, Casey ML, MacDonald PC. 1997. Accumulation of interleukin-1beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update 3:517–527. 10.1093/humupd/3.5.517. [DOI] [PubMed] [Google Scholar]
- 288.Gudza-Mugabe M, Havyarimana E, Jaumdally S, Garson KL, Lennard K, Tarupiwa A, Mugabe F, Marere T, Mavenyengwa RT, Masson L, Jaspan HB. 2020. Human immunodeficiency virus infection is associated with preterm delivery independent of vaginal microbiota in pregnant African women. J Infect Dis 221:1194–1203. 10.1093/infdis/jiz584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ravindran J, Richardson BA, Kinuthia J, Unger JA, Drake AL, Osborn L, Matemo D, Patterson J, McClelland RS, John-Stewart G. 2021. Chlamydia, gonorrhea, and incident HIV infection during pregnancy predict preterm birth despite treatment. J Infect Dis 224:2085–2093. 10.1093/infdis/jiab277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Baer RJ, Chambers CD, Ryckman KK, Oltman SP, Rand L, Jelliffe-Pawlowski LL. 2019. An evaluation of sexually transmitted infection and odds of preterm or early-term birth using propensity score matching. Sexual Trans Dis 46:389–394. 10.1097/OLQ.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 291.Burdette ER, Young MR, Dude CM, Wall KM, Haddad LB. 2021. Association of delayed treatment of chlamydial infection and gonorrhea in pregnancy and preterm birth. Sexual Trans Dis 48:925–931. 10.1097/OLQ.0000000000001490. [DOI] [PubMed] [Google Scholar]