ABSTRACT
Secretory immunoglobulin A (IgA) plays a crucial role in mucosal immunity for preventing the invasion of exogenous antigens; however, little is understood about the neutralizing activity of serum IgA. Here, to examine the role of IgA antibodies against COVID-19 illnesses, we determined the neutralizing activity of serum/plasma IgG and IgA purified from previously SARS-CoV-2-infected and COVID-19 mRNA vaccine-receiving individuals. We found that serum/plasma IgA possesses substantial but rather modest neutralizing activity against SARS-CoV-2 compared to IgG with no significant correlation with the disease severity. Neutralizing IgA and IgG antibodies achieved the greatest activity at approximately 25 and 35 days after symptom onset, respectively. However, neutralizing IgA activity quickly diminished to below the detection limit approximately 70 days after onset, while substantial IgG activity was observed until 200 days after onset. The total neutralizing activity in sera/plasmas of those with COVID-19 largely correlated with those in purified IgG and purified IgA and levels of anti-SARS-CoV-2-S1-binding IgG and anti-SARS-CoV-2-S1-binding IgA. In individuals who were previously infected with SARS-CoV-2 but had no detectable neutralizing IgA activity, a single dose of BNT162b2 or mRNA-1273 elicited potent serum/plasma-neutralizing IgA activity, but the second dose did not further strengthen the neutralization antibody response. The present data show that the systemic immune stimulation with natural infection and COVID-19 mRNA-vaccines elicits both SARS-CoV-2-specific neutralizing IgG and IgA responses in serum, but the IgA response is modest and diminishes faster than the IgG response.
IMPORTANCE Secretory dimeric immunoglobulin A (IgA) plays an important role in preventing the invasion of foreign objects by its neutralizing activity on mucosal surfaces, while monomeric serum IgA is thought to relate to the phagocytic immune system activation. Here, we report that individuals with the novel coronavirus disease (COVID-19) developed both systemic neutralizing IgG (nIgG) and IgA (nIgA) active against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although the nIgA response was quick and reached the highest activity earlier than the nIgG response, nIgA activity was modest and diminished faster than nIgG activity. In individuals who recovered from COVID-19 but had no detectable nIgA activity, a single dose of COVID-19 mRNA vaccine elicited potent nIgA activity, but the second dose did not further strengthen the antibody response. Our study provides novel insights into the role and the kinetics of serum nIgA against the pathogen in both naturally infected and COVID-19 mRNA vaccine-receiving COVID-19-convalescent individuals.
KEYWORDS: COVID-19, SARS-CoV-2, humoral immunity, neutralizing antibodies, immunoglobulin A, immunoglobulin G, anti-SARS-CoV-2 immunoglobulin, COVID-19 mRNA vaccine
INTRODUCTION
Immunoglobulin A (IgA) is the most abundant type of antibody in the body (1), comprising most of the immunoglobulin in secretions primarily in the gut, milk, and bronchial secretions as a noninflammatory antibody against microbes (2). Such secretory-IgA plays a crucial role in neutralizing the viruses, toxins, and inflammatory microbial molecules invading the mucosal epithelial cells (3) and exerts greater efficacy in preventing infections than serum IgG (4). Thus, selective IgA deficiency, the most common immunologic defect in humans (5), causes recurrent sinopulmonary infections, autoimmune disorders, and allergic disorders. However, most individuals with selective IgA deficiency are asymptomatic, and serum IgA levels in patients do not necessarily correlate with the occurrence or severity of these disorders (6). Serum IgA is the second most abundant isotype following IgG (7), and the functions of serum IgA appear to be related to the phagocytic system activation mediated through the Fc-alpha-RI (CD89) (8), although it is not fully understood. In this regard, it had been recognized that immunization via mucosal routes can elicit robust mucosal immune responses, while the systemic vaccination approach (e.g., administered intramuscularly or intradermally) mainly induces IgG and apparently induces, in part, protective mucosal IgA responses (9).
In terms of the novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), we previously reported that highly neutralizing activity-confirmed COVID-19 convalescent plasma and purified-IgG block the Syrian hamster disease progression with limited viral antigen-positive cells in terminal bronchioles and alveolar regions (10). Sterlin et al. reported that mucosal IgA produced shortly after symptom onset plays a crucial role in the early stage of the disease (11). It has also been reported that COVID-19 mRNA vaccines elicit high titers of anti-SARS-CoV-2-S1-binding IgG (S1-binding IgG) and IgA (S1-binding IgA) antibodies in serum (12–14). In this regard, while systemic neutralizing IgG (nIgG) antibodies induced by COVID-19 and mRNA vaccines are thought to be responsible for the protection against symptomatic infection, further evaluation of the role of IgA in COVID-19 infection and COVID-19 vaccines, especially the evaluation of the neutralizing activity of such natural infection- or vaccine-induced IgA, are needed.
Here, we report that individuals with COVID-19 developed both systemic nIgG and nIgA irrespective of the severity of the disease; however, even though the nIgA response was quick, the activity was modest and diminished faster than that of nIgG. We also report that the COVID-19 mRNA vaccines elicit highly neutralizing serum IgA in COVID-19-experienced individuals.
RESULTS
Clinical characteristics of the participants.
A total of 14 individuals who were confirmed to have SARS-CoV-2 infection with positive RNA-quantitative-PCR (RNA-qPCR) results and were admitted to the Center Hospital of the National Center for Global Health and Medicine in Tokyo, Japan, from February to April 2020 (COVID-19 group) (Table 1) and 8 individuals who received a COVID-19 mRNA vaccine (either BNT162b2 or mRNA-1273) from April to July 2021 after recovery from COVID-19 (convalescent-vaccine group) (Table 2) were enrolled. These individuals agreed to participate in the present clinical studies. All the individuals were Japanese, and 2 out of 14 (14.3%) in the COVID-19 group and 2 out of 8 (25.0%) in the convalescent-vaccine group were female (Tables 1 and 2). The median (range) ages were 53 (37 to 68) and 53 (35 to 61) years in the COVID-19 group and convalescent-vaccine group, respectively (Tables 1 and 2). In the COVID-19 group, seven individuals (50%) had moderate symptoms of lower respiratory disease or imaging with no oxygen requirement, while seven individuals (50%) had severe symptoms and required oxygen treatment during the clinical course without any sequential organ failure. There were no significant differences in the age, sex, or sample collection dates between the moderate and severe symptom groups (Table 1). Individuals in the COVID-19 group received experimental therapeutic agents, which are now mostly considered to be ineffective (see Table S1 in the supplemental material) (15). The convalescent-vaccine group received the primary series of COVID-19 mRNA vaccine 70 to 458 (median, 306) days after disease onset (Table 2).
TABLE 1.
Characteristics of COVID-19-experienced individuals (COVID-19 group) in the study
| Characteristic | All patients (n = 14) | Moderate (n = 7) | Severe (n = 7) |
|---|---|---|---|
| Age, yrs (median) | 37–68 (53) | 47–62 (53) | 37–68 (63) |
| Sex [n (%)] | |||
| Male | 12 (85.7) | 6 (85.7) | 6 (85.7) |
| Female | 2 (14.3) | 1 (14.3) | 1 (14.3) |
| Oxygen requirement | |||
| Days [median (%)] | NAa | 2–15 (6.5) | |
| None | 7 (50.0) | 7 (100) | 0 (0) |
| Nasal canula | 6 (42.9) | 0 (0) | 6 (85.7) |
| High-flow nasal canula | 1 (7.1) | 0 (0) | 1 (14.3) |
| Sample collection, days (median) | |||
| Acute phase | 3–15 (9) | 3–13 (8) | 4–15 (9) |
| Convalescent phase | 17–201 (86) | 19–201 (88) | 17–196 (74) |
NA, not applicable.
TABLE 2.
Characteristics of COVID-19 mRNA-vaccinees after recovery from the disease (convalescent-vaccine group) in the study
| Characteristic | All participants (n = 8) | mRNA-1273 (n = 3) | BNT162b2 (n = 5) |
|---|---|---|---|
| Age, yrs (median) | 35–61 (53) | 55–61 (55) | 35–56 (44) |
| Sex [n (%)] | |||
| Male | 6 (75.0) | 2 (66.6) | 4 (80.0) |
| Female | 2 (25.0) | 1 (33.3) | 1 (20.0) |
| Severity [n (%)] | |||
| Mild | 5 (62.5) | 2 (66.6) | 3 (60.0) |
| Moderate | 2 (25.0) | 0 (0) | 2 (40.0) |
| Severe | 1 (12.5) | 1 (33.3) | 0 (0) |
| Days to vaccination after onset (median) | 70–458 (306) | 173–458 (436) | 70–335 (286) |
| Sample collection, day (median) | |||
| Before 1st vaccination (median) | 17–298 (209) | 97–249 (209) | 17–298 (219) |
| After 1st vaccination (median) | 4–103 (56) | 4–91 (57) | 8–103 (55) |
SARS-CoV-2-neutralizing serum/plasma IgA response occurs earlier and diminishes faster than the IgG response.
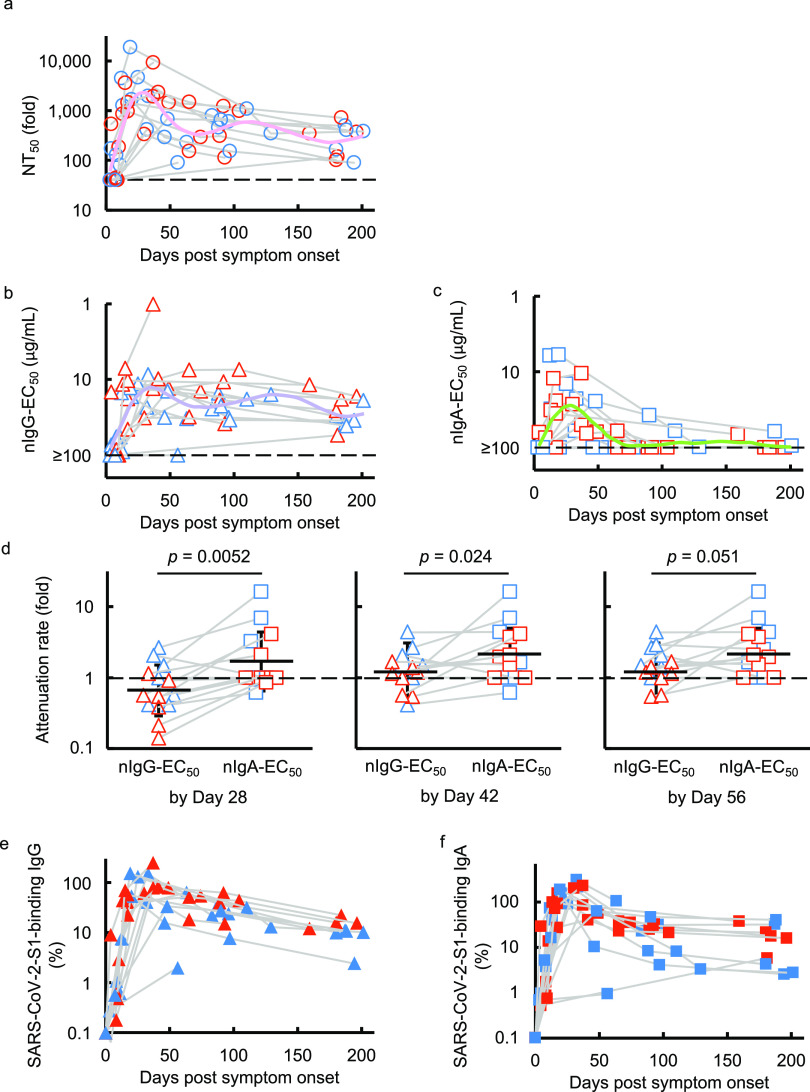
We previously described the kinetics of neutralizing activity of immunoglobulin G (IgG) fractions purified from plasmas of 43 SARS- CoV-2-infected individuals using cell-based assays (16). In the current study, we chose 14 individuals with moderate to severe COVID-19 symptoms and evaluated the neutralizing activity of whole sera/plasmas and purified-IgG and -IgA fractions against wild-type SARS-CoV-205-2N (PANGO lineage B). As shown in Fig. 1a, whole sera/plasmas from all 14 SARS-CoV-2-infected individuals had significantly high titers of SARS-CoV-2-neutralizing activity by 30 days after symptom onset, and thereafter their neutralizing activity gradually decreased, but the decay became slower after around 50 days (Fig. 1a). Significant levels of neutralizing activity persisted in sera/plasmas in all participants as examined on up to day 200 (Fig. 1a). Purified-IgG from sera/plasmas also exerted neutralizing activity expressed as a 50% effective concentration (EC50) of up to 1.0 μg/mL (Fig. 1b) and showed substantial neutralizing activity by around 200 days postonset. Substantial amounts of S1-binding IgG antibodies were also seen by around 200 days after the onset (Fig. 1e). We also identified good immune response to produce nIgA following the emergence of COVID-19 symptoms; however, the decay of the IgA neutralizing activity occurred much earlier than that of nIgG, and by 129 days after onset, 12 of the 14 individuals (85.7%) had an EC50 value of ~100 μg/mL or undetectable (>100 μg/mL) neutralizing activity (Fig. 1c). Of the 14 individuals, 3 (21.4%) showed no detectable nIgA activity throughout the study. In contrast to the early decay in nIgA activity, substantial amounts of S1-binding IgA persisted for up to 200 days after the onset (Fig. 1f).
FIG 1.
Kinetics of SARS-CoV-2-neutralizing activity and S1-binding antibodies. (a to c) VeroE6TMPRSS2 cells were exposed to wild-type SARS-CoV-205-2N with or without various concentrations of diluted sera/plasmas (a), purified IgG (b), or purified IgA (c), and the neutralizing activity and the amounts of S1-binding antibodies were determined. The dashed line denotes the assay limit values (≤40-fold for the panel a and ≥100 μg/mL for panels b and c). Note that the highest viral neutralizing activity of purified IgG and IgA was seen around 35 and 25 days, respectively, after onset. Furthermore, the neutralizing activity of serum IgA diminished much quicker than that of IgG. The colored lines (pink, NT50 for pink; purple, nIgG-EC50; light green, nIgA-EC50) denote the fitted curve. (d) Attenuation rate of the nIgG-EC50 and nIgA-EC50 between the highest neutralizing activity of purified IgG and IgA by day 28, 42, and 56 postonset and neutralizing activity determined latest in the study. (e and f) The kinetics of the amount of S1-binding IgG (e) and IgA (f) are also shown. The amount of S1-binding IgG and IgA increased by approximately day 21 post-symptom onset, followed by a gradual decrease. Note that in contrast, substantial amounts of S1-binding IgG and IgA persisted around 200 days after onset, while the decay occurred more rapidly in IgA. Blue symbols denote the samples collected from individuals with moderate symptoms, while red symbols are those from individuals with severe symptoms.
To quantify and compare the time-dependent kinetics of serum/plasma, nIgG, and nIgA activity, we generated fitted curves by using the generalized additive model (17, 18), which showed that the nIgA response occurred significantly earlier than the nIgG response; it took 25 days postonset for the nIgA response to reach its peak but 35 days for the nIgG response to reach its peak (Fig. 1b and c). It was also noted that the nIgA response diminished faster than the nIgG response; the average nIgA-EC50 value virtually reached the detection limit (≥100 μg/mL) approximately 70 days postonset (Fig. 1c), while substantial nIgG activity persisted until ~200 days postonset (Fig. 1b). We also attempted to quantify the time-dependent reduction of the nIgG-EC50 and nIgA-EC50 by calculating the attenuation rate between the highest neutralizing activity of purified IgG and IgA by days 28 (range, 3 to 25), 42 (range, 3 to 41), and 56 (range, 3 to 56) postonset and neutralizing activity determined in the latest in the study (Fig. 1d). As shown in Fig. 1d, the attenuation rates of nIgA-EC50 were significantly greater than those of nIgG-EC50 by 28 days (4 weeks; Fig. 1d, left panel) and 42 days (6 weeks; Fig. 1d, middle panel) postonset, with P values of 0.0052 and 0.024, respectively. The same trend was seen when the attenuation rates of nIgG-EC50 and nIgA-EC50 were determined by 56 days (8 weeks; Fig. 1d, right panel; P = 0.051).
Sterlin and his colleagues previously reported that IgA dominates the early neutralizing antibody response to SARS-CoV-2 in patients with COVID-19 (11). Thus, we attempted to examine whether the amounts of S1-binding IgA produced predominated timewise over those of S1-binding IgG by using the slope indexes determined with the initial (first) value determined and the following (second) value determined for S1-binding IgA and IgG amounts in each individual. Then, the slope indexes of S1-binding IgA and IgG amounts were compared using the Wilcoxon signed-rank test. We found that the slope indexes made with the first and second S1-binding IgA were significantly greater than those made with S1-binding IgG, suggesting that the amount of S1-binding IgA produced significantly predominated over the amount of S1-binding IgG at the early phase of the antibody response after symptom onset (P = 0.009) (Fig. 1e and f).
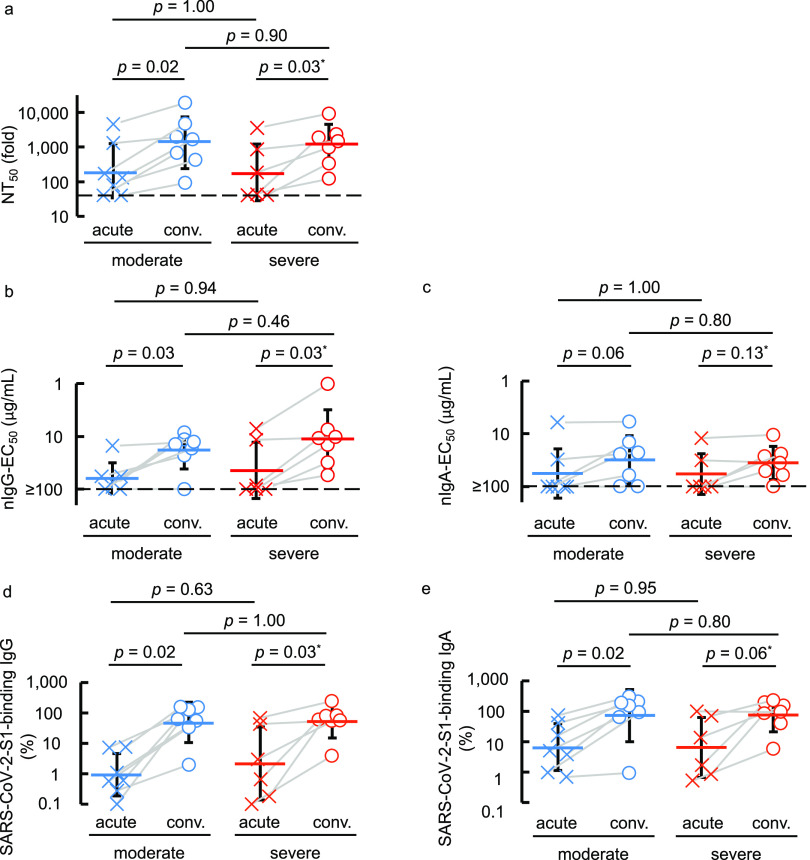
Neutralizing IgG activity is greater in the COVID-19-convalescent phase than in the acute phase regardless disease severity.
We next asked if higher neutralization activity is seen in the acute (less than 14 days postonset or when oxygen treatment was required) or convalescent (14 days postonset and when no oxygen was required) phase or in patients with moderate or severe COVID-19. A significant increase was seen in 50% neutralizing titers (NT50) of sera/plasmas in the convalescent phase compared to the acute phase in both moderate and severe symptom groups (P = 0.02 and 0.03, respectively) (Fig. 2a). There was also a significant increase in nIgG activity in the convalescent phase in both moderate and severe symptom groups (P = 0.03 and 0.03, respectively) (Fig. 2b). The same pattern was seen in S1-binding IgG amounts (Fig. 2d). In contrast, there was no significant difference in nIgA activity between acute and convalescent phases in either the moderate or severe symptom group (Fig. 2c). The amounts of S1-binding IgA were higher in the convalescent than in the acute phase in the moderate symptom group, although the difference in the severe symptom group was not significant (Fig. 2e).
FIG 2.
COVID-19-convalescent individuals possess greater neutralizing activity and SARS-CoV-2-S1-binding antibody levels than those at the acute phase. (a to e) The neutralizing activity of sera/plasmas, purified IgG, and purified IgA (a, b, and c, respectively) and the amounts of S1-binding IgG and IgA (d and e, respectively) were compared between the acute phase (less than 14 days post-symptom onset or when the individual required oxygen treatment) and the convalescent phase (14 days post-symptom onset and beyond which no oxygen was required). Blue symbols denote the samples collected from individuals with moderate symptoms, while red symbols are those from individuals with severe symptoms.
The contribution of neutralizing IgG antibody to serum/plasma neutralizing activity is greater than that of neutralizing IgA.
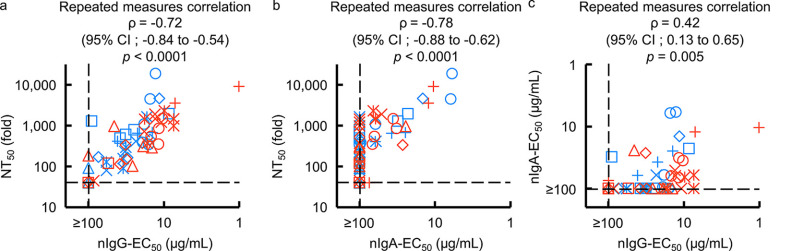
The amounts of S1-binding IgG antibodies in sera from patients with COVID-19 correlate highly with SARS-CoV-2-specific neutralizing activity levels in serum IgG fraction (16, 19). However, the role of SARS-CoV-2-specific humoral IgA antibodies in protecting against SARS-CoV-2 infection remains to be clarified. Thus, we asked whether S1-binding IgA antibody amounts correlate with nIgA activity. The NT50 values of sera/plasmas from patients with COVID-19 proved to correlate well with nIgG activity (nIgG-EC50 values) with the rho (ρ) value of −0.72 (95% confidence interval [CI], −0.84 to −0.54) (Fig. 3a), in line with our previous observations (16). In the case of purified-IgA from patients with COVID-19, a high correlation was also observed with nIgA activity (nIgA-EC50 values), with a ρ value of –0.78 (95% CI, –0.88 to –0.62), although 31 of 56 IgA samples had very low or undetectable (≥100 μg/mL) neutralization activity (Fig. 3b). Between nIgG-EC50 and nIgA-EC50 values, however, a moderate correlation was seen, with a ρ value of 0.42 (95% CI, 0.13 to 0.65) (Fig. 3c). The NT50 values of sera/plasmas and nIgG-EC50 values also had a high correlation with S1-binding IgG amounts (Fig. S2a and b). There was also a high correlation between the NT50 values of sera/plasmas and nIgA-EC50 values with S1-binding IgA amounts (Fig. S2c and d). S1-binding IgA in nasopharyngeal swab samples collected at the earliest point of the infection (fewer than 20 days post-symptom onset) tend to have a higher amount as time progresses (Fig. S3a). Further, the nasal S1-binding IgA was highly correlated with serum S1-binding IgA, with a Spearman’s ρ value of 0.73 (95% CI, 0.40 to 0.89) (Fig. S3b). On the other hand, the serum total human IgG and IgA were consistent during the study period (Fig. S3c and d), with a low correlation (Fig. S3e).
FIG 3.
Correlations of purified IgG and IgA neutralizing activities with serum/plasma neutralizing titers. (a and b) The neutralizing activity of purified-IgG (a) (nIgG-EC50) and -IgA (b) (nIgA-EC50) against serum/plasma neutralizing activity (NT50) values are plotted. (c) The nIgA-EC50 values are plotted against the nIgG-EC50 values. Note that a high correlation is observed between NT50 values and nIgG-EC50 values (repeated measures correlation ρ = −0.72 [95% CI, –0.84 to –0.54]) (a) and between the NT50 values and NIgA-EC50 values (ρ = –0.78 [95% CI, –0.88 to –0.62]) (b), while moderate correlation was observed between nIgA-EC50 and nIgG-EC50 (repeated measures correlation ρ = 0.42 [95% CI, 0.13 to 0.65]) (c). Each symbol denotes the sample from one individual. Blue symbols denote the samples collected from individuals with moderate symptoms, while red symbols denote those from individuals with severe symptoms.
The present data suggest that the neutralizing activity seen in sera/plasmas of patients with COVID-19 is largely composed of the neutralizing activity of serum IgG (Fig. 3a) but also of that of IgA (Fig. 3b). Moreover, as has been seen in the case of neutralizing activity of sera/plasmas that are in large part correlated with the amount of S1-binding IgG (16, 20), the neutralizing activity of serum IgA is correlated with the amounts of S1-binding IgA (Fig. S2d), while the neutralizing activity of IgA was modest compared to that of IgG (Fig. 1b and c, and Fig. 3c).
mRNA-COVID-19 vaccine induces high-level neutralizing activity in COVID-19 convalescent individuals.
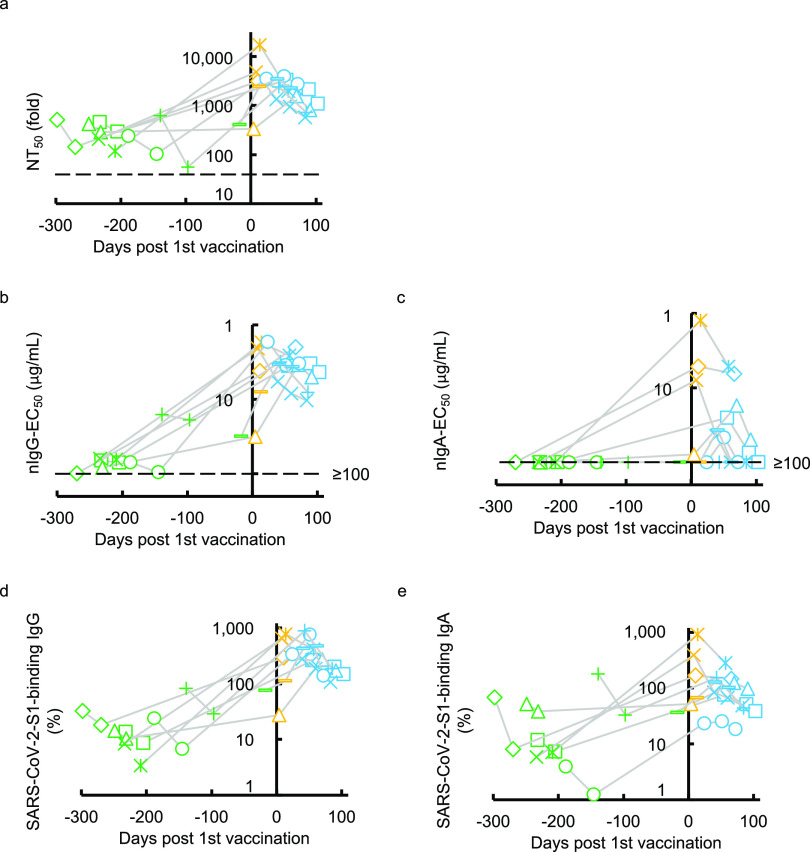
We next examined the SARS-CoV-2-specific IgG and IgA neutralizing activity elicited with the primary series of mRNA vaccine administration (BNT162b2 or mRNA-1273) in eight individuals who had experienced quantitative PCR (qPCR)-confirmed symptomatic COVID-19 70 to 458 days before the first immunization (Table 2). All eight individuals had low but detectable to moderate levels of neutralizing activity in sera/plasmas before vaccination (Fig. 4a). Most of these individuals had significantly high titers of neutralizing activity within 28 days after the first vaccination. The high NT50 titers were not further boosted following the second dose, which is a quite different pattern of NT50 values from the patterns seen in those who were SARS-CoV-2-naive and received the first and second doses of vaccine (20–22). A similar pattern was seen when nIgG-EC50 values were determined in the same participants (Fig. 4b). In the case of nIgA-EC50 values, none of the participants had detectable neutralizing activity (≥100 μg/mL) before the COVID-19 mRNA-vaccination, but 3 of the 8 participants had a substantial increase in the nIgA-EC50 values before the second dose of mRNA vaccine (Fig. 4c). In contrast, these COVID-19-experienced individuals had moderate to high levels of S1-binding IgG and IgA before the first dose, and greater levels of S1-binding IgG and IgA were documented following the first dose, although no further increase was seen after the second dose (Fig. 4d and e). It was noted, however, that the nIgA activity rapidly decreased (Fig. 4c) compared to nIgG activity (Fig. 4b), although such rapid decay was not seen in the amounts of S1-binding IgA antibodies (Fig. 4e).
FIG 4.
Kinetics of neutralizing activity and S1-binding antibody levels before and after COVID-19 mRNA vaccination. (a to e) The kinetics of neutralizing activity (a, b, and c) and S1-binding antibody levels (d and e) in eight previously COVID-experienced individuals who received the COVID-19 mRNA vaccine are shown. Note that all the values significantly rose after the first dose of the vaccine. Also note that none of the participants had detectable nIgA-EC50 values (≥100 μg/mL) before vaccination (c). On the other hand, all of them had low to high levels of nIgA-EC50 after a single dose of the vaccine, and such levels quickly decreased (c), and their S1-binding IgA levels persisted after the two doses of vaccine in the study period (e). The dashed line denotes the assay detection limit (≤40-fold dilution for NT50 and ≥100 μg/mL for nIgG-EC50 and nIgA-EC50). Green symbols denote the samples collected before COVID-19 mRNA vaccination, while yellow and light-blue symbols denote samples collected after the first and second doses, respectively. Each symbol denotes the sample from one individual.
All of the NT50, nIgG-EC50, nIgA-EC50, percent S1-binding IgG, and percent S1-binding IgA values proved to have significantly increased following primary series administration (Fig. S4a to e). These data demonstrate that COVID-19 mRNA vaccines induce high titers of nIgA in previously COVID-19-experienced individuals after a single dose of vaccine. However, such nIgA activity apparently diminished faster than the nIgG activity, while the difference was not statistically significant (P = 0.069) (Fig. 4b and c). Such an early decay of nIgA activity had been seen in those with symptomatic infection with SARS-CoV-2 (Fig. 1c). The second-dose vaccination did not significantly slow the speed of decay (Fig. 4c). We also examined whether there are correlations among NT50, nIgG-EC50, nIgA-EC50, and S1-binding IgG and IgA values. As we have seen that nIgG activity greatly contributes to serum/plasma SARS-CoV-2-neutralizing activity compared to that of nIgA activity in individuals with COVID-19 (Fig. 3a to c and Fig. S2a to d), similar profiles were identified in previously COVID-19-contracted individuals following COVID-19 mRNA vaccination (Fig. S5a to g).
DISCUSSION
In respiratory tract infections such as influenza virus infection, natural infection induces systemic IgG responses (23, 24) and mucosal secretory-IgA responses (25). In terms of COVID-19, Sterlin et al. reported that IgA-expressing-circulating plasmablasts detected shortly after symptom onset have a consistent phenotype with that found in lung and eventually produced mucosal IgA (11). Our present data also showed that the amounts of nasal S1-binding IgA antibody and the amounts of serum S1-binding IgA are highly correlated (Pearson’s ρ = 0.73, Fig. S3b), suggesting the serum S1-binding IgA and mucosal S1-binding IgA share similar, albeit not the same, antigenic determinants or immunological repertoire in response to SARS-CoV-2-S1. In this regard, it is of note that Wang et al. have suggested that serum IgA monomers are produced by the same cells that produce secretory dimers (19). Of note, Sterlin et al. reported that serum IgA specific to the receptor-binding domain (RBD), which represents a critical target for neutralization, was detected earlier than anti-RBD IgG as assessed with a photonic ring immunoassay (11). In the present study, we also showed that the nIgA response occurred significantly earlier than the nIgG response; it took 25 days postonset for the nIgA response to reach its peak and 35 days for the nIgG response to reach its peak (Fig. 1b and c). Moreover, S1-binding IgA production significantly predominated over S1-binding IgG production (Fig. 1d and e). These data are in line with the observations by Sterlin et al. (11).
Although the neutralization of pathogens is attributed to the neutralizing activity of IgG, providing long-term immunity for as long as decades in cases such as mumps, varicella-zoster virus (VZV), and Epstein-Barr virus (EBV) (26), protective immunity to seasonal coronaviruses (27), SARS-CoV, and Middle East respiratory syndrome (MERS)-CoV (28) is known to be short-lived. Vanshylla et al. reported that the neutralizing activity in serum waned quickly (half-life, 3.6 months) compared to the neutralizing activity of purified-IgG (half-life, 7.8 months), and such a short half-life of activity of serum is thought to be partially attributed to the presence of S-binding IgA and IgM in serum (29). Moreover, Iyer et al. reported that RBD-binding IgA antibodies are short-lived compared to RBD-binding IgG antibodies (30). In the present study, we extended the observations by Vanshylla et al. and Iyer et al. and demonstrated that SARS-CoV-2-neutralizing activity of serum/plasma IgA was identified earlier and diminished faster than that of IgG as assessed in 14 individuals with COVID-19 (Fig. 1b and c). Moreover, when such activity was determined following mRNA vaccination in eight COVID-19-experienced individuals, SARS-CoV-2-neutralizing activity in serum/plasma IgA also quickly diminished compared to that in serum/plasma IgG (Fig. 4b and c). However, there were no significant differences in the decay rate of SARS-CoV-2-S1-binding IgG and IgA levels (Fig. 1d and e and Fig. 4d and e). The faster decay in the nIgA activity compared to that in nIgG may derive from the difference in half-lives of serum IgA and IgG (i.e., 3 to 5 and 21 days, respectively). Also, it is possible that since the total amount of serum IgG in the body is greater than that of IgA, the consumption and absorption of nIgA by the viral antigens could be more apparent than in the case of IgG.
While the 1st-dose vaccination in the COVID-19-experineced individuals elicited a good response comparable to the response seen in COVID-19-unexperineced individuals following the 2nd dose (20, 31), the response following the 2nd dose was comparable to or even less than the response after the 1st dose in those COVID-19-experienced individuals (Fig. 4a to e). In this respect, it is noteworthy that the intervals following the 1st dose until the 2nd dose was administered were 3 or 4 weeks. These intervals were probably too short for eliciting the otherwise boosted immune response. In fact, there are several published articles that describe the antibody responses after COVID-19 vaccination in previously COVID-19-expereineced individuals (21, 22, 32–34). In such articles, a single dose of COVID-19 mRNA vaccine (whether BNT162b2 or mRNA1273) or adenoviral vector-based vaccine (AZD1222) induced substantial neutralizing antibodies and T cells responses against the virus that are compatible with the responses seen after two doses of vaccine in individuals without prior SARS-CoV-2 infection. While the mechanism of such robust immunogenicity seen with a single dose of vaccine in COVID-19-experienced individuals remains to be clarified, it is presumed that the prior COVID-19 infection served as the primary immunization.
It has been reported that the neutralizing activity of IgM and IgA are dramatically greater than that of IgG when the activity of recombinant monoclonal antibodies, which share the same anti-SARS-CoV-2 spike protein Fab region, was examined using a pseudotyped lentivirus coated with the SARS-CoV-2 spike protein and angiotensin-converting enzyme 2 (ACE2)-transfected Crandell-Rees feline kidney cells as the host cell line (35). In the current study, unlike their findings, we observed that the neutralizing activity of purified-IgA is modest compared to that of purified-IgG (Fig. 1b and c, and Fig. 3c). In this regard, we used a cell-based neutralization assay using IgA fractions purified from sera/plasmas, which are of polyclonal nature. Thus, our data should possibly represent the more comprehensive protective effect of serum-derived IgA, although more studies are needed.
There are reports that individuals with selective IgA deficiency tend to have higher risks of severe COVID-19 (36, 37). Thus, we initially hypothesized that individuals with moderate symptoms would possess greater neutralizing activity of serum IgA than those with severe COVID-19. However, there were no significant differences in nIgA-EC50 values between those with moderate and severe disease (Fig. 2d and e). In this regard, we have lately shown that patients with severe COVID-19 had greater nIgG levels in serum than those with mild COVID-19 (16), and we reasoned that the exposure to larger amounts of SARS-CoV-2 over the long term in those with severe COVID-19 resulted in greater nIgG activity (29).
The kinetics of S-binding IgG, IgA, and IgM upon natural SARS-CoV-2 infection has been well described during the 2 years of the COVID-19 pandemic (11, 30, 38, 39). There is a good body of literature regarding SARS-CoV-2-neutralizing IgG antibody responses, yet there are only a few reports on the nIgA antibody. However, those reports describe the neutralizing activity of monoclonal IgA antibodies produced by peripheral (35) or mucosal (40) memory B cells. There are also a number of reports describing immune responses elicited by various types of vaccines, such as mRNA (BNT162b2 or mRNA1273), nonreplicating adenoviral vector (AZD1222, Sputnik V, or Ad26.COV2.S) and inactivated (BBIBP-CorV) vaccines (41, 42); however, such articles have only described S-binding IgA antibodies, and no neutralizing activity of such IgA antibodies has been evaluated. Thus, our present report, which describes the nIgA activity in detail together with the nIgG activity in individuals with COVID-19, should shed light on the understanding of the immune response upon SARS-CoV-2 infection.
It should be noted that the limitation of the present work is that we did not systematically characterize SARS-CoV-2-specific secretory IgA antibodies, which represent the dominating immunoglobulins in exocrine secretions. In the literature, there are currently only a few reports documenting the role of secretory IgA antibodies in protection against SARS-CoV-2 infection. Studies to elucidate the protective effect of secretory IgA upon SARS-CoV-2 infection and anti-COVID-19 vaccination remain to be conducted.
In conclusion, the present data showed that the SARS-CoV-2-neutralizing serum/plasma IgA response is seen earlier than the nIgG response, suggesting that the humoral IgA plays a critical role in the acute phase of the infection, although that nIgA response diminishes faster than the nIgG response, which should in turn play a role in the later phase of infection. Further, in previously SARS-CoV-2-infected individuals, the first (initial) administration of COVID-19 mRNA vaccines induces high titers of nIgG and nIgA; however, the neutralizing activity of IgA also diminishes faster than that of IgG.
MATERIALS AND METHODS
Participants.
A total of 14 individuals who were diagnosed with COVID-19 based on positive RNA-quantitative-PCR (RNA-qPCR) results from February to April 2020 and another 8 individuals who received a COVID-19 mRNA vaccine (either BNT162b2 or mRNA-1273) from April to July 2021 after recovery from COVID-19 and agreed to participate in the clinical studies (Certified Review Board of National Center for Global Health and Medicine approval numbers NCGM-G-003472 and NCGM-G-003536) for specimen collection and convalescent plasma donation (10, 43) were enrolled in the present work. The data were analyzed anonymously. Nasopharyngeal swab samples were collected at early time points after admission and stored at −80°C until use. Sera or plasmas were obtained intermittently and stored at −20°C until use.
Cells, viruses, and immunoglobulin purification.
Transmembrane protease serine 2 (TMPRSS2)-overexpressing VeroE6 (VeroE6TMPRSS2) cells (RRID CVCL_YQ49) were obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank (Osaka, Japan). VeroE6TMPRSS2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL penicillin, 100 μg/mL kanamycin, and 1 mg/mL G418 under a humidified atmosphere containing 5% CO2 at 37°C. A SARS-CoV-2 strain, SARS-CoV-205-2N (PANGO lineage B) was isolated in March 2020 in Tokyo, Japan, as previously described (16). IgG fractions were obtained from SARS-CoV-2-infected individuals’ sera or plasmas using spin column-based antibody purification kit (Protein G) (Cosmo Bio, Tokyo, Japan). IgA fractions were purified from the IgG purification flowthrough using Pierce Jacalin agarose (Thermo Fisher Scientific, Waltham, MA) and eluted in phosphate-buffered saline (PBS) using Zeba spin desalting columns, 40K MWCO (Thermo Fisher Scientific). The total human IgG and IgA concentrations were determined using the human IgG enzyme-linked immunoassay (ELISA) kit and human IgA ELISA kit, respectively (abcam, Cambridge, UK). The purity of the IgG and IgA was determined using the capillary electrophoresis Simple Western Jess apparatus and the total protein detection module (Protein Simple, San Jose, CA), anti-human IgA, alpha-chain specific, horseradish peroxidase (HRP)-linked antibody no. 80403, and anti-human IgG, Fc gamma fragment-specific, HRP-linked antibody no. 32935 (Cell Signaling Technology, Danvers, MA). The purities of the IgG and IgA were approximately 85% (84.0 ± 2.4) and 75% (75.2 ± 1.6), respectively, as four representative IgG and IgA samples were examined.
Antiviral assays.
The SARS-CoV-2 neutralizing activity of donated plasma and purified immunoglobulin against the wild-type SARS-CoV-2 (PANGO lineage B) was determined as previously described (10, 16, 20). In brief, VeroE6TMPRSS2 cells were seeded in 96-well flat-bottom microtiter culture plates at a density of 1 × 104 cells/well. On the following day, the virus (SARS-CoV-205-2N) was mixed with the various concentrations of the serum/plasma or purified immunoglobulin fractions and incubated for 20 min at 37°C. The preincubated mixture was inoculated to the cells at a multiplicity of infection (MOI) of 0.01. The cells were cultured for 3 days, and the number of viable cells in each well was measured using cell counting kit 8 (Dojindo, Kumamoto, Japan). The potency of SARS-CoV-2 inhibition by sera/plasmas or purified immunoglobulin was determined based on its inhibitory effect on virally induced cytopathicity in VeroE6TMPRSS2 cells. The amounts of S1-binding antibodies in each plasma sample were determined using anti-SARS-CoV-2 ELISA (IgG) and (IgA) (Euroimmun, Lübeck, Germany). The serially diluted donor 84 (D84) plasma (10) was used as a reference (100%) for quantification with a four-parameter logistic curve calculated using ImageJ (Fiji) (Fig. S1) (44).
Statistical analysis.
The 50% neutralizing titers of sera/plasmas (NT50), 50% effective concentration of purified IgG and IgA (nIgG-EC50 and nIgA-EC50, respectively), and the amounts of anti-SARS-CoV-2-S1-binding IgG and anti-SARS-CoV-2-S1-binding IgA (S1-binding IgG and S1-binding IgA, respectively) were determined and compared between the acute and convalescent phases of COVID-19 and between the moderate and severe symptoms using a Wilcoxon signed-rank test and Wilcoxon rank sum test, respectively. The attenuation rates of nIgG-EC50 and nIgA-EC50 were calculated by dividing the nIgG-EC50 or nIgA-EC50 values determined to be the latest in the study with the highest neutralizing activity (lowest nIgG-EC50 or nIgA-EC50 values) by days 28, 42, and 56 postonset. To examine which of the nIgG-EC50 and nIgA-EC50 values diminished faster in the convalescent-vaccine group, the values obtained by subtracting the lowest EC50 values from the highest EC50 values post-1st vaccine administration were compared. Then, the attenuation rates of nIgG-EC50 and nIgA-EC50 and the differences after vaccination were compared by the Wilcoxon signed-rank test. To compare the amounts of S1-binding IgG and IgA timewise, the slope indexes were determined with the initial (first) value obtained and the following (second) value obtained for S1-binding IgA and IgG amounts in each individual. Then, the slope indexes of S1-binding IgA and IgG amounts were compared using the Wilcoxon signed-rank test. The correlations and corresponding 95% confidence intervals of NT50, IgG-EC50, IgA-EC50, S1-binding IgG, and S1-binding IgA were determined using the repeated measures correlation method to consider the within-individual association (45) using rmcorr (R package v. 0.4.6) (46). The computed correlation coefficients were considered high if the absolute value was above 0.7, moderate if the absolute value was between 0.4 and 0.7, and low if the absolute value was below 0.4, according to Guilford’s rule of thumb. The nIgG-EC50 and nIgA-EC50 kinetics were fitted with a generalized additive model (17) with mgcv (R package v. 1.8-40). The fitting was implemented for superimposed data of all samples. All the analyses were performed using R statistical software v. 4.1.3 (47). Statistical significance was defined as P < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the Japan Agency for Medical Research and Development (AMED) (grant no. JP20fk0108160 and JP20fk0108502 to K.M. and JP20fk0108502, JP20fk0108257, and JP20fk0108510 to H.M.), by MHLW Research on Emerging and Re-emerging Infectious Diseases and Immunization Program (grant no. JPMH20HA1006 to K.M.), by a grant from National Center for Global Health and Medicine Research Institute (grant no. 20A2003D to K.M.), and by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (H.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Mariko Kato for technical assistance.
We thank all the patients who participated in the clinical trial for the collection of convalescent plasma and staff of the Diseases Control and Prevention Center, Center for Clinical Sciences, Department of Hematology, Clinical Laboratory Department, Department of Clinical Engineering, and Nursing Department at the Central Hospital of the National Center for Global Health and Medicine.
Conceptualization: Y.T. and H.M.; methodology: Y.T., K.M., and H.M.; formal analysis: K.O., Y.S., and Y.U.; investigation: Y.T., K.O., and N.K.-I.; data curation: Y.T., Y.S., N.K.-I., M.T., and T.S.; resources: N.K.-I., M.T., T.S., and S.M.; writing-original draft: Y.T. and H.M.; writing-review and editing: K.O., Y.S., N.K.-I., Y.U., and K.M.; visualization: Y.T.; supervision: N.O., K.M., and H.M.; project administration: H.M.; funding acquisition: K.M. and H.M.
We have declared that no competing interests exist.
Footnotes
Supplemental material is available online only.
Contributor Information
Hiroaki Mitsuya, Email: hmitsuya@hosp.ncgm.go.jp.
Takamasa Ueno, Kumamoto University.
REFERENCES
- 1.Cerutti A, Rescigno M. 2008. The biology of intestinal immunoglobulin A responses. Immunity 28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerutti A, Cols M, Gentile M, Cassis L, Barra CM, He B, Puga I, Chen K. 2011. Regulation of mucosal IgA responses: lessons from primary immunodeficiencies. Ann N Y Acad Sci 1238:132–144. doi: 10.1111/j.1749-6632.2011.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 4.Mazanec MB, Coudret CL, Fletcher DR. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol 69:1339–1343. doi: 10.1128/JVI.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiehm ER. 2008. The four most common pediatric immunodeficiencies. J Immunotoxicol 5:227–234. doi: 10.1080/15476910802129646. [DOI] [PubMed] [Google Scholar]
- 6.Shkalim V, Monselize Y, Segal N, Zan-Bar I, Hoffer V, Garty BZ. 2010. Selective IgA deficiency in children in Israel. J Clin Immunol 30:761–765. doi: 10.1007/s10875-010-9438-x. [DOI] [PubMed] [Google Scholar]
- 7.Woof JM, Kerr MA. 2006. The function of immunoglobulin A in immunity. J Pathol 208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 8.Bakema JE, van Egmond M. 2011. The human immunoglobulin A Fc receptor FcαRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol 4:612–624. doi: 10.1038/mi.2011.36. [DOI] [PubMed] [Google Scholar]
- 9.Su F, Patel GB, Hu S, Chen W. 2016. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum Vaccin Immunother 12:1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takamatsu Y, Imai M, Maeda K, Nakajima N, Higashi-Kuwata N, Iwatsuki-Horimoto K, Ito M, Kiso M, Maemura T, Takeda Y, Omata K, Suzuki T, Kawaoka Y, Mitsuya H. 2022. Highly neutralizing COVID-19 convalescent plasmas potently block SARS-CoV-2 replication and pneumonia in Syrian hamsters. J Virol 96:e0155121. doi: 10.1128/JVI.01551-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt C-E, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, Lacorte J-M, Yssel H, Parizot C, Dorgham K, Charneau P, Amoura Z, Gorochov G. 2021. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisnewski AV, Campillo Luna J, Redlich CA. 2021. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One 16:e0249499. doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zurac S, Nichita L, Mateescu B, Mogodici C, Bastian A, Popp C, Cioplea M, Socoliu C, Constantin C, Neagu M. 2021. COVID-19 vaccination and IgG and IgA antibody dynamics in healthcare workers. Mol Med Rep 24:578. doi: 10.3892/mmr.2021.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzi L, Dalla Gasperina D, Veronesi G, Shallak M, Ietto G, Iovino D, Baj A, Gianfagna F, Maurino V, Focosi D, Maggi F, Ferrario MM, Dentali F, Carcano G, Tagliabue A, Maffioli LS, Accolla RS, Forlani G. 2022. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine 75:103788. doi: 10.1016/j.ebiom.2021.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Solidarity Trial Consortium. 2021. Repurposed antiviral drugs for Covid-19: interim WHO Solidarity Trial results. N Engl J Med 384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda K, Higashi-Kuwata N, Kinoshita N, Kutsuna S, Tsuchiya K, Hattori S-I, Matsuda K, Takamatsu Y, Gatanaga H, Oka S, Sugiyama H, Ohmagari N, Mitsuya H. 2021. Neutralization of SARS-CoV-2 with IgG from COVID-19-convalescent plasma. Sci Rep 11:5563. doi: 10.1038/s41598-021-84733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood SN. 2017. Generalized additive models: an introduction with R, 2nd ed Chapman and Hall/CRC, New York, NY. [Google Scholar]
- 18.van den Hoogen LL, Verheul MK, Vos ERA, van Hagen CCE, van Boven M, Wong D, Wijmenga-Monsuur AJ, Smits G, Kuijer M, van Rooijen D, Bogaard-van Maurik M, Zutt I, van Vliet J, Wolf J, van der Klis FRM, de Melker HE, van Binnendijk RS, den Hartog G. 2022. SARS-CoV-2 Spike S1-specific IgG kinetic profiles following mRNA or vector-based vaccination in the general Dutch population show distinct kinetics. Sci Rep 12:5935. doi: 10.1038/s41598-022-10020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, Cipolla M, Hoffmann H-H, Oliveira TY, Oren DA, Ramos V, Nogueira L, Michailidis E, Robbiani DF, Gazumyan A, Rice CM, Hatziioannou T, Bieniasz PD, Caskey M, Nussenzweig MC. 2021. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med 13:eabf1555. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda K, Amano M, Uemura Y, Tsuchiya K, Matsushima T, Noda K, Shimizu Y, Fujiwara A, Takamatsu Y, Ichikawa Y, Nishimura H, Kinoshita M, Matsumoto S, Gatanaga H, Yoshimura K, Oka S-I, Mikami A, Sugiura W, Sato T, Yoshida T, Shimada S, Mitsuya H. 2021. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci Rep 11:22848. doi: 10.1038/s41598-021-01930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzoni A, Di Lauria N, Maggi L, Salvati L, Vanni A, Capone M, Lamacchia G, Mantengoli E, Spinicci M, Zammarchi L, Kiros SK, Rocca A, Lagi F, Colao MG, Parronchi P, Scaletti C, Turco L, Liotta F, Rossolini GM, Cosmi L, Bartoloni A, Annunziato F, COVID-19 Research Group . 2021. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest 131:e149150. doi: 10.1172/JCI149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson M, Stec M, Rewane A, Landay A, Cloherty G, Moy J. 2021. SARS-CoV-2 antibody responses in infection-naive or previously infected individuals After 1 and 2 doses of the BNT162b2 vaccine. JAMA Netw Open 4:e2119741. doi: 10.1001/jamanetworkopen.2021.19741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds HY. 1988. Immunoglobulin G and its function in the human respiratory tract. Mayo Clin Proc 63:161–174. doi: 10.1016/S0025-6196(12)64949-0. [DOI] [PubMed] [Google Scholar]
- 24.Krammer F. 2019. The human antibody response to influenza A virus infection and vaccination. Nat Rev Immunol 19:383–397. doi: 10.1038/s41577-019-0143-6. [DOI] [PubMed] [Google Scholar]
- 25.Pakkanen SH, Kantele JM, Moldoveanu Z, Hedges S, Häkkinen M, Mestecky J, Kantele A. 2010. Expression of homing receptors on IgA1 and IgA2 plasmablasts in blood reflects differential distribution of IgA1 and IgA2 in various body fluids. Clin Vaccine Immunol 17:393–401. doi: 10.1128/CVI.00475-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amanna IJ, Carlson NE, Slifka MK. 2007. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 27.Edridge AWD, Kaczorowska J, Hoste ACR, Bakker M, Klein M, Loens K, Jebbink MF, Matser A, Kinsella CM, Rueda P, Ieven M, Goossens H, Prins M, Sastre P, Deijs M, van der Hoek L. 2020. Seasonal coronavirus protective immunity is short-lasting. Nat Med 26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 28.Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, Borgert BA, Moreno CA, Solomon BD, Trimmer-Smith L, Etienne V, Rodriguez-Barraquer I, Lessler J, Salje H, Burke DS, Wesolowski A, Cummings DAT. 2020. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun 11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanshylla K, Di Cristanziano V, Kleipass F, Dewald F, Schommers P, Gieselmann L, Gruell H, Schlotz M, Ercanoglu MS, Stumpf R, Mayer P, Zehner M, Heger E, Johannis W, Horn C, Suárez I, Jung N, Salomon S, Eberhardt KA, Gathof B, Fätkenheuer G, Pfeifer N, Eggeling R, Augustin M, Lehmann C, Klein F. 2021. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe 29:917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer AS, Jones FK, Nodoushani A, Kelly M, Becker M, Slater D, Mills R, Teng E, Kamruzzaman M, Garcia-Beltran WF, Astudillo M, Yang D, Miller TE, Oliver E, Fischinger S, Atyeo C, Iafrate AJ, Calderwood SB, Lauer SA, Yu J, Li Z, Feldman J, Hauser BM, Caradonna TM, Branda JA, Turbett SE, LaRocque RC, Mellon G, Barouch DH, Schmidt AG, Azman AS, Alter G, Ryan ET, Harris JB, Charles RC. 2020. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol 5:eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi P-Y, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. 2020. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG, Cheng S, Sobhani K. 2021. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havervall S, Marking U, Greilert-Norin N, Ng H, Gordon M, Salomonsson A-C, Hellström C, Pin E, Blom K, Mangsbo S, Phillipson M, Klingström J, Hober S, Nilsson P, Åberg M, Thålin C. 2021. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. EBioMedicine 70:103523. doi: 10.1016/j.ebiom.2021.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angyal A, Longet S, Moore SC, Payne RP, Harding A, Tipton T, Rongkard P, Ali M, Hering LM, Meardon N, Austin J, Brown R, Skelly D, Gillson N, Dobson SL, Cross A, Sandhar G, Kilby JA, Tyerman JK, Nicols AR, Spegarova JS, Mehta H, Hornsby H, Whitham R, Conlon CP, Jeffery K, Goulder P, Frater J, Dold C, Pace M, Ogbe A, Brown H, Ansari MA, Adland E, Brown A, Chand M, Shields A, Matthews PC, Hopkins S, Hall V, James W, Rowland-Jones SL, Klenerman P, Dunachie S, Richter A, Duncan CJA, Barnes E, Carroll M, Turtle L, de Silva TI, et al. 2022. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe 3:e21–e31. doi: 10.1016/S2666-5247(21)00275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisil Y, Yazici Z, Shida H, Miura T. 2021. Is SARS-CoV-2 neutralized more effectively by IgM and IgA than IgG having the same Fab region? Pathogens 10:751. doi: 10.3390/pathogens10060751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinti I, Mortari EP, Fernandez SA, Milito C, Carsetti R. 2021. IgA antibodies and IgA deficiency in SARS-CoV-2 infection. Front Cell Infect Microbiol 11:655896. doi: 10.3389/fcimb.2021.655896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Çölkesen F, Kandemir B, Arslan Ş, Çölkesen F, Yıldız E, Korkmaz C, Vatansev H, Evcen R, Aykan FS, Kılınç M, Aytekin G, Feyzioğlu B, Doğan M, Teke T. 2022. Relationship between selective IgA deficiency and COVID-19 prognosis. Jpn J Infect Dis 75:228–233. doi: 10.7883/yoken.JJID.2021.281. [DOI] [PubMed] [Google Scholar]
- 38.Ma H, Zeng W, He H, Zhao D, Jiang D, Zhou P, Cheng L, Li Y, Ma X, Jin T. 2020. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol 17:773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marot S, Malet I, Leducq V, Zafilaza K, Sterlin D, Planas D, Gothland A, Jary A, Dorgham K, Bruel T, Burrel S, Boutolleau D, Schwartz O, Gorochov G, Calvez V, Marcelin A-G, Sorbonne Université SARS-CoV-2 Neutralizing Antibodies Study Group . 2021. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun 12:844. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Planchais C, Fernández I, Bruel T, de Melo GD, Prot M, Beretta M, Guardado-Calvo P, Dufloo J, Molinos-Albert LM, Backovic M, Chiaravalli J, Giraud E, Vesin B, Conquet L, Grzelak L, Planas D, Staropoli I, Guivel-Benhassine F, Hieu T, Boullé M, Cervantes-Gonzalez M, Ungeheuer M-N, Charneau P, van der Werf S, Agou F, Dimitrov JD, Simon-Lorière E, Bourhy H, Montagutelli X, Rey FA, Schwartz O, Mouquet H, French COVID Cohort Study Group, CORSER Study Group . 2022. Potent human broadly SARS-CoV-2-neutralizing IgA and IgG antibodies effective against Omicron BA.1 and BA.2. J Exp Med 219:e20220638. doi: 10.1084/jem.20220638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafon E, Jäger M, Bauer A, Reindl M, Bellmann-Weiler R, Wilflingseder D, Lass-Flörl C, Posch W. 2022. Comparative analyses of IgG/IgA neutralizing effects induced by three COVID-19 vaccines against variants of concern. J Allergy Clin Immunol 149:1242–1252.e12. doi: 10.1016/j.jaci.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adjobimey T, Meyer J, Sollberg L, Bawolt M, Berens C, Kovačević P, Trudić A, Parcina M, Hoerauf A. 2022. Comparison of IgA, IgG, and neutralizing antibody responses following immunization with Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm’s COVID-19 vaccines. Front Immunol 13:917905. doi: 10.3389/fimmu.2022.917905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terada M, Kutsuna S, Togano T, Saito S, Kinoshita N, Shimanishi Y, Suzuki T, Miyazato Y, Inada M, Nakamoto T, Nomoto H, Ide S, Sato M, Maeda K, Matsunaga A, Satake M, Matsubayashi K, Tsuno H, Kojima M, Kuramistu M, Tezuka K, Ikebe E, Okuma K, Hamaguchi I, Shiratori K, Sato M, Kawakami Y, Inaba K, Igarashi S, Yamauchi R, Matsumura M, Ishimaru K, Zhang B, Kuge C, Ishihara M, Gouda M, Tanaka K, Ishizaka Y, Ohmagari N. 2021. How we secured a COVID-19 convalescent plasma procurement scheme in Japan. Transfusion 61:1998–2007. doi: 10.1111/trf.16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakdash JZ, Marusich LR. 2017. Repeated measures correlation. Front Psychol 8:456. doi: 10.3389/fpsyg.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakdash JZ, Marusich LR. 2022. rmcorr: repeated measures correlation. https://CRAN.R-project.org/package=rmcorr. [DOI] [PMC free article] [PubMed]
- 47.R Core Team. 2022. The R project for statistical computing. https://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5 and Table S1. Download spectrum.02716-22-s0001.pdf, PDF file, 0.3 MB (261.7KB, pdf)