Abstract
Aims
Patients often require combination therapies to achieve LDL cholesterol (LDL-C) targets for the primary prevention of atherosclerotic cardiovascular disease. This study investigates the effect of inclisiran, a small interfering ribonucleic acid targeting hepatic proprotein convertase subtilisin/kexin type 9 production, in primary prevention patients with elevated LDL-C despite statins.
Methods and results
This pre-specified analysis of the placebo-controlled, randomized ORION-11 trial included 203 individuals at risk of, but without prior, cardiovascular events and LDL-C ≥2.6 mmol/L, despite maximally tolerated statins. Inclisiran 284 mg or placebo was administered on Days 1, 90, and thereafter every 6 months up to 540 days. Co-primary endpoints were percentage LDL-C change from baseline to Day 510 and time-adjusted change from baseline after Day 90 and up to Day 540. Key secondary endpoints included percentage and absolute changes in atherogenic lipoproteins. Safety was assessed over 540 days. The mean baseline (SD) LDL-C was 3.6 (1.5) mmol/L. At Day 510, the placebo-corrected LDL-C change with inclisiran was −43.7% [95% confidence interval (CI): −52.8 to −34.6] with a corresponding time-adjusted change of −41.0% (95% CI: −47.8 to −34.2); (P < 0.0001). The placebo-corrected absolute change in LDL-C at Day 510 with inclisiran was −1.5 mmol/L (95% CI: −1.8 to −1.2), with a respective time-adjusted change of −1.3 mmol/L (95% CI: −1.6 to −1.1). Inclisiran significantly lowered non-HDL cholesterol and apolipoprotein B (apoB) at Day 510 vs. placebo (P < 0.0001 for both), with a greater likelihood of attaining lipoprotein and apoB goals, and was well-tolerated except for mainly mild, treatment-emergent adverse events at the injection site.
Conclusion
Inclisiran was generally well-tolerated in primary prevention patients with elevated LDL-C, who derived significant reductions in atherogenic lipoprotein levels with twice-yearly maintenance dosing.
Keywords: ASCVD, Inclisiran, Hypercholesterolaemia, Lipoproteins, LDL cholesterol, Primary prevention
Structured Graphical Abstract
Structured Graphical Abstract.
This pre-specified secondary analysis from the ORION-11 trial showed that inclisiran, an siRNA therapy targeting PCSK9 production, was generally well-tolerated in primary prevention patients with elevated LDL-C and resulted in significant reductions in atherogenic lipoprotein levels with twice-yearly maintenance dosing. ApoB, apolipoprotein B; CV, cardiovascular; DNA, deoxyribonucleic acid; LDL-C, low-density lipoprotein cholesterol; mRNA, messenger ribonucleic acid; non-HDL-C, non-high-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; RNA, ribonucleic acid; siRNA, small interfering ribonucleic acid.
See the editorial comment for this article ‘Inclisiran in primary prevention: reality or fiction?’, by Baris Gencer and François Mach, https://doi.org/10.1093/eurheartj/ehac653.
Permissions information.
The authors do hereby declare that all illustrations and figures in the manuscript are original and do not require reprint permission.
Introduction
Global guidelines generally identify four patient populations with elevated cardiovascular (CV) risk.1,2 The first group comprises patients with clinical atherosclerotic CV disease (ASCVD; secondary prevention). The remaining three groups comprise individuals without clinical ASCVD (primary prevention) and include (i) those with an elevated predicted 10-year risk, (ii) those with high-risk conditions such as diabetes mellitus (DM), and (iii) those with familial hypercholesterolaemia (FH).1,2 In absolute terms, the vast majority of CV events occur in individuals without a prior history of ASCVD.3,4 Therefore, effective primary prevention strategies are integral to reducing the global burden of ASCVD.5 Considering that the causes of ASCVD are multi-factorial, policies directed at behaviour and lifestyle, as well as individualized approaches to control conventional risk factors such as lipid levels and blood pressure, are complementary strategies to promote public health.1,2
After diet and lifestyle, the foundational pharmacological approach to lower atherogenic lipoproteins in primary prevention patients is to reduce low-density lipoprotein cholesterol (LDL-C) with statins.1,2 Other lipid-lowering therapies are added if the lowering of LDL-C with statins is considered insufficient for the level of individual risk. Beyond LDL-C, suboptimal control of other lipid parameters, including non-high-density lipoprotein cholesterol (non-HDL-C) and apolipoprotein B (apoB), may also necessitate additional lipid-lowering therapies beyond statins.
Inclisiran is a small interfering ribonucleic acid (siRNA)-based therapy that targets hepatic proprotein convertase subtilisin/kexin type 9 (PCSK9) production, thus reduces PCSK9-mediated degradation of LDL receptors (LDLRs), consequently leading to higher LDLR expression and hepatic uptake of LDL-C, thereby lowering plasma LDL-C levels.6 Inclisiran resulted in significantly reduced LDL-C levels compared with placebo in patients with heterozygous FH (HeFH; primary and secondary prevention),7 in a secondary prevention population,8 and in a mixed population of primary and secondary prevention patients in the ORION-11 trial.8 Here, we report the efficacy and safety profile of inclisiran in a pre-specified primary prevention cohort from the ORION-11 trial.
Methods
Design overview
The design and overall results of the ORION-11 trial (NCT03400800) have been published previously.8 Briefly, this was a randomized, double-blind, placebo-controlled, Phase 3 trial of subcutaneous inclisiran sodium 300 mg (equivalent to 284 mg inclisiran) vs. placebo in patients on appropriate guideline-based medical therapies for other risk factors, including maximally tolerated statins. This study assessed the efficacy and safety of inclisiran over 540 days in patients with clinical ASCVD or ASCVD risk equivalent (high-risk primary prevention patients), in whom LDL-C levels were elevated despite the use of maximally tolerated doses of statins with or without additional lipid-lowering therapy. Patients not receiving statins had documented evidence of intolerance to at least two different statins, one at the lowest approved dose. Statin intolerance requires documentation of historical adverse events (AEs) attributable to any dose of any statin with recorded evidence in source documents and the study’s case report form.
The trial protocol was approved by an institutional review board or independent ethics committee at each participating institution, with all participants providing written informed consent.
Settings and patients
This trial was conducted in six European countries and South Africa and enrolled adults (aged ≥18 years), including high-risk, primary prevention patients (referred to as ‘risk equivalent’ in the study protocol) or those with ASCVD (secondary prevention). Patients with a 10-year risk of ≥20% of a CV event assessed by the Framingham risk score, or equivalent, type 2 DM, or FH, were included in the primary prevention group. FH was recorded by the physician using the patient’s medical history. Patients with evidence of coronary heart disease, cerebrovascular disease, and peripheral arterial disease were included in the secondary prevention group (see Supplementary material online, Appendix S2). The eligibility criteria stipulated that primary prevention patients had LDL-C levels ≥2.6 mmol/L (≥100 mg/dL) and secondary prevention patients had LDL-C levels ≥1.8 mmol/L (≥70 mg/dL) at the qualifying study visit (screening). Patients were required to be receiving maximally tolerated stable doses of background lipid-lowering therapies for at least 30 days before screening; however, patients were excluded if treated with a monoclonal antibody against PCSK9 within 90 days of screening. Detailed inclusion and exclusion criteria are available in Supplementary material online, Appendix S2. The present report presents a previously unpublished, detailed analysis of the primary prevention cohort. Where relevant, data on the secondary prevention cohort are presented in the Supplementary material for completeness.
Randomization and interventions
Randomization was stratified by background use of statins and by country, and patients were allocated (1:1) to receive either 300 mg of inclisiran sodium or a matching placebo under blinded conditions. Inclisiran or placebo were administered as 1.5 mL subcutaneous injections on Days 1, 90, 270, and 450 (see Supplementary material online, Figure S1), with additional clinic visits on Days 30, 150, 330, and 510 for follow-up and laboratory assessments. The end-of-study visit was conducted on Day 540.
Endpoints
The pre-specified co-primary endpoints were the percentage change in LDL-C from baseline to Day 510 and the time-adjusted percentage change in LDL-C from baseline after Day 90 and up to Day 540. The latter was calculated as the average percentage change in LDL-C from baseline over the period after Day 90 and up to Day 540, which considered measurements taken on Days 150, 270, 330, 450, 510, and 540 (peak and trough measurements). This latter was included as a co-primary endpoint to investigate whether the less frequent administration of inclisiran (twice-yearly) would sustain its lipid-lowering effectiveness over the 6-month duration. There were six key secondary endpoints: absolute change in LDL-C from baseline to Day 510; time-adjusted absolute change in LDL-C from baseline after Day 90 and up to Day 540; percentage change in PCSK9, total cholesterol, apoB, and non-HDL-C from baseline to Day 510.
Other pre-specified secondary endpoints included both absolute and percentage changes in very low-density lipoprotein cholesterol (VLDL-C) from baseline to Day 510, and lipoprotein(a) [Lp(a)] from baseline to Day 540. The proportion of primary and secondary prevention patients achieving, respectively, levels of: LDL-C <2.6 mmol/L (100 mg/dL) and <1.8 mmol/L (70 mg/dL) was a pre-specified other secondary endpoint. Other non pre-specified exploratory endpoints included the proportion of primary and secondary prevention patients achieving respectively, levels of: non-HDL-C <3.4 mmol/L (130 mg/dL) and <2.6 mmol/L (100 mg/dL); apoB <100 mg/dL and <80 mg/dL. The proportion of patients achieving a ≥50% LDL-C reduction was another pre-specified secondary endpoint. AEs and clinical laboratory values were recorded at all visits through to the end-of-trial visit, with AEs classified by investigators according to organ class and as mild, moderate, or severe using standard Medical Dictionary for Regulatory Activities (MedDRA) nomenclature.
Statistical analysis
The statistical assumptions for the overall ORION-11 trial are reported in Supplementary material online, Appendix S3. In this pre-specified subgroup analysis, the first of the co-primary endpoints was analysed using an analysis of covariance (ANCOVA) with a multiple imputation washout model for missing data that assumes missing data are missing at random (MAR), and the second was analysed using mixed models for repeated measures (MMRM), with a control-based pattern-mixture model (CB-PMM) for missing data imputation. The co-primary endpoints and key secondary endpoints used multiple imputation to account for missing data. Other secondary endpoints, absolute and percentage changes in VLDL-C and Lp(a), were analysed using MMRM analysis that assumes missing data are MAR. Supplementary material online, Tables S1 and Table S2 present the missing LDL-C and PCSK9 measurements at specific time points, and Supplementary material online, Table S3 shows the missing lipid measurements at Day 510. The proportion of individuals attaining pre-specified lipid ‘goals’ is reported as a percentage and reflects the population with available data (the denominator), rather than the whole intention-to-treat (ITT) population and thus uses observed values. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated from data with observed values using a logistic regression model and reflect the likelihood of goal attainment with inclisiran vs. placebo.
Results
Patient characteristics
Overall, 2381 patients were screened, and 1617 were randomized. Of those randomized, 203 (12.6%) were categorized as primary prevention patients, with 98 assigned to inclisiran and 105 to placebo. The trial was completed through Day 540 by 94.1% of the primary prevention patients (see Supplementary material online, Figure S2).
The patient characteristics are shown in Table 1. Patients in the inclisiran and placebo groups were well matched by randomization. Within the primary prevention cohort, 132 (65.0%) had DM, 30 (14.8%) had FH, and 114 (56.2%) patients had a 10-year predicted CV risk of ≥20% (non-mutually exclusive groups).
Table 1.
Baseline demographic and clinical characteristics (intention-to-treat population)
| Inclisiran (n = 98) | Placebo (n = 105) | Total (n = 203) | |
|---|---|---|---|
| Age, years, mean (SD) | 62.7 (10.6) | 63.6 (9.2) | 63.2 (9.9) |
| Male, n (%) | 45 (45.9) | 50 (47.6) | 95 (46.8) |
| White race, n (%) | 94 (95.9) | 101 (96.2) | 195 (96.1) |
| BMI, kg/m2, mean (SD) | 31.1 (5.2) | 32.3 (6.8) | 31.7 (6.1) |
| Cardiovascular risk factors, n (%) | |||
| Smoking (current)a | 19 (19.4) | 16 (15.2) | 35 (17.2) |
| Hypertension | 67 (68.4) | 79 (75.2) | 146 (71.9) |
| DMb | 66 (67.3) | 66 (62.9) | 132 (65.0) |
| FHb | 17 (17.3) | 13 (12.4) | 30 (14.8) |
| 10-year predicted CV risk ≥20%b | 54 (55.1) | 60 (57.1) | 114 (56.2) |
| Concomitant lipid-modifying therapies, n (%) | |||
| Yes | 81 (82.7) | 91 (86.7) | 172 (84.7) |
| Statin use | 79 (80.6) | 89 (84.8) | 168 (82.8) |
| High-intensity statins | 60 (61.2) | 65 (61.9) | 125 (61.6) |
| Ezetimibec | 4 (4.1) | 7 (6.7) | 11 (5.4) |
| Lipid measures, mmol/L, mean (SD) | |||
| LDL-C | 3.7 (1.7) | 3.5 (1.2) | 3.6 (1.5) |
| Total cholesterol | 6.0 (1.8) | 5.8 (1.5) | 5.9 (1.7) |
| Non-HDL-C | 4.6 (1.8) | 4.4 (1.4) | 4.5 (1.6) |
| HDL-C | 1.3 (0.4) | 1.3 (0.4) | 1.3 (0.4) |
| VLDL-Cd | 0.9 (0.4) | 0.9 (0.4) | 0.9 (0.4) |
| Apolipoprotein B, mg/dL | 122.2 (40.5) | 117.4 (29.8) | 119.7 (35.4) |
| Lipoprotein(a), nmol/L | |||
| Median | 40 | 27 | 34 |
| IQR | 17, 148 | 14, 138 | 14, 142 |
| Triglycerides, mmol/L | |||
| Median | 1.8 | 1.8 | 1.8 |
| IQR | 1.3, 2.3 | 1.4, 2.3 | 1.3, 2.3 |
| Other laboratory measures | |||
| PCSK9, µg/L, mean (SD) | 362.3 (99.3) | 356.1 (100.6) | 359.1 (99.8) |
Current smoking status included patients with history of smoking for past the 30 days.
Some patients had more than one high-risk factor (DM, FH, or a 10-year risk of a cardiovascular event of 20% or greater as assessed by the Framingham Risk Score for Cardiovascular Disease or equivalent) and thus, the total number does not add up to 100%.
Ezetimibe use was obtained from concomitant medication records.
VLDL-C calculated.
BMI, body mass index; DM, diabetes mellitus; FH, familial hypercholestrolaemia; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; SD, standard deviation; VLDL-C, very low-density lipoprotein cholesterol.
At the time of randomization, statins were used by 82.8% of patients with 61.6% receiving high-intensity statins, and 5.4% using ezetimibe in combination with statins or other lipid-lowering therapies. As expected from the protocol inclusion criteria, mean (SD) LDL-C levels at baseline were high in the primary prevention cohort at 3.6 (1.5) mmol/L [139.4 (58.0) mg/dL]. In addition, baseline levels of other atherogenic lipoproteins, notably non-HDL-C and apoB, were also elevated, but triglyceride and Lp(a) levels were not markedly deranged (Table 1).
Efficacy
Percentage changes in atherogenic lipoproteins
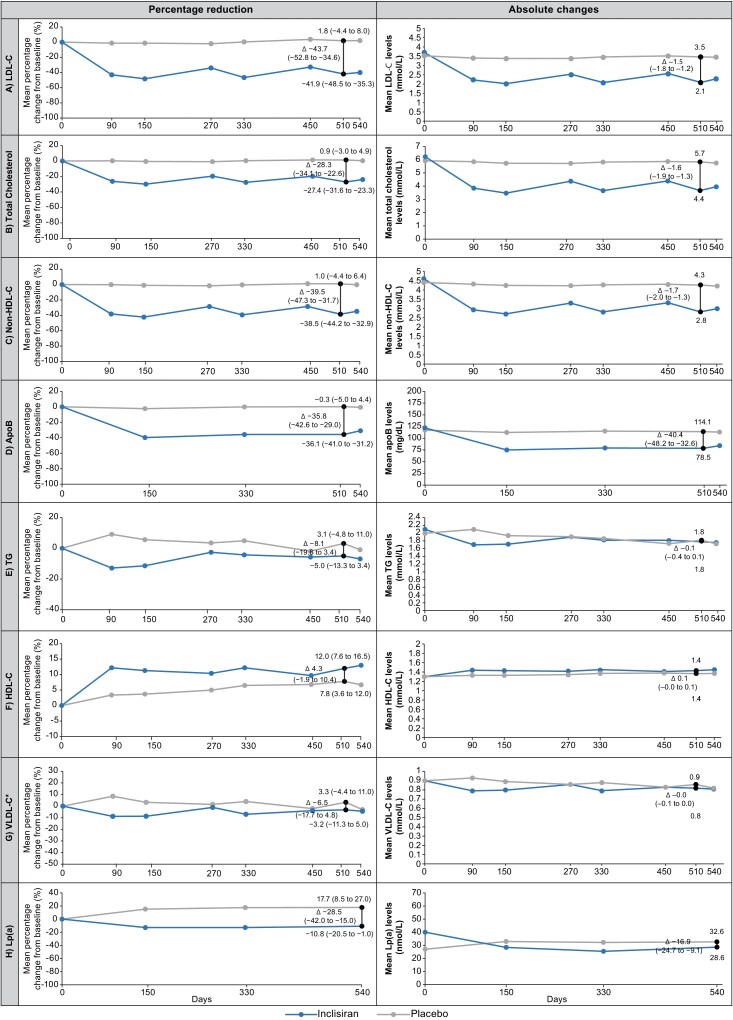
The effects of inclisiran or placebo on the mean percentage changes in lipid levels from the baseline to Day 510 are shown in Figure 1, and the corresponding time-adjusted changes from the baseline after Day 90 and up to Day 540 are shown in Table 2. The mean percentage change in LDL-C from baseline to Day 510 (co-primary endpoint) was −41.9% with inclisiran and +1.8% with placebo, resulting in a −43.7% difference between groups (95% CI, −52.8 to −34.6; P < 0.0001). The mean time-adjusted percentage change in LDL-C from baseline after Day 90 and up to Day 540 (co-primary endpoint) was −40.4% for inclisiran and +0.6% for placebo, reflecting a −41.0% difference between groups (95% CI, −47.8 to −34.2; P < 0.0001).
Figure 1.
Mean percentage and absolute changes from baseline over time in (A) low-density lipoprotein cholesterol, (B) total cholesterol, (C) non-high-density lipoprotein cholesterol, (D) apolipoproteinB, (E) triglycerides, (F) high-density lipoprotein cholesterol, (G) very low-density lipoprotein cholesterol, and (H) lipoprotein(a) (intention-to-treat population). * VLDL-C calculated. Mean percentage change from baseline and absolute change for all lipoproteins were measured at baseline and on Days 90, 150, 270, 330, 450, 510, and 540; except for apoB, which was measured on Days 150, 330, 510, and 540. VLDL-C was measured on Day 510 but otherwise calculated. Lp(a) was measured on Days 150, 330, and 540. Graphs show data analysed by mixed models for repeated measures, except for the mean percentage and absolute changes in LDL-C from baseline at Day 510, which were analysed by analysis of covariance and control-based pattern-mixture model, respectively. The black vertical line represents least squares mean (95% CI) percentage or absolute change from baseline to Day 510 for all lipoproteins and Day 540 for lp(a). The P-values for placebo-corrected mean percentage change from baseline to Day 510 were P < 0.0001 for all except triglycerides (P = 0.169), HDL-C (P = 0.170) and VLDL-C (P = 0.257). The P-values for the placebo-corrected absolute change from baseline to Day 510 were P < 0.0001 for all except triglycerides (P = 0.381), HDL-C (P = 0.112) and VLDL-C (P = 0.343). Inclisiran, n = 98; placebo, n = 105. ApoB, apolipoprotein B; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); TG, triglycerides; VLDL-C, very low-density lipoprotein cholesterol.
Table 2.
Time-adjusted percentage and absolute change in atherogenic lipoproteins from baseline after Day 90 and up to Day 540 (intention-to-treat population)
| Inclisiran (n = 98) | Placebo (n = 105) | Least squares mean difference (95% CI) between groups | |
|---|---|---|---|
| Time-adjusted percentage change in lipid measurements, LS mean % (95% CI) a | |||
| LDL-Cb,c | −40.4 (−45.3, −35.5) | 0.6 (−4.1, 5.3) | −41.0 (−47.8, −34.2) (P < 0.0001) |
| Total cholesterol | −24.7 (−28.2, −21.3) | 0.3 (−3.0, 3.6) | −25.1 (−29.8, −20.3) (P < 0.0001) |
| Non-HDL-C | −35.5 (−40.0, −30.9) | −0.2 (−4.5, 4.2) | −35.3 (−41.6, −29.0) (P < 0.0001) |
| ApoB | −35.5 (−39.5, −31.6) | −0.7 (−4.5, 3.0) | −34.8 (−40.3, −29.3) (P < 0.0001) |
| Triglycerides | −5.9 (−11.9, 0.0) | 2.5 (−3.2, 8.2) | −8.4 (−16.6, −0.2) (P = 0.045) |
| HDL-C | 11.0 (8.0, 14.1) | 6.0 (3.1, 8.9) | 5.0 (0.8, 9.3) (P = 0.020) |
| VLDL-Cd | −4.8 (−10.2, 0.6) | 1.1 (−4.0, 6.3) | −5.9 (−13.4, 1.5) (P = 0.116) |
| Lipoprotein(a) | −12.1 (−20.8, −3.4) | 16.8 (8.5, 25.1) | −28.9 (−40.9, −16.9) (P < 0.0001) |
| Time-adjusted absolute change in lipid measurements, LS mean (95% CI) a | |||
| LDL-Cb | −1.4 (−1.6, −1.3) | −0.1 (−0.3, 0.1) | −1.3 (−1.6, −1.1) (P < 0.0001) |
| Total cholesterol, mmol/L | −1.5 (−1.7, −1.3) | −0.1 (−0.3, 0.1) | −1.4 (−1.7, −1.1) (P < 0.0001) |
| Non-HDL-C, mmol/L | −1.6 (−1.8, −1.4) | −0.1 (−0.3, 0.0) | −1.5 (−1.7, −1.2) (P < 0.0001) |
| ApoB, mg/dL | −43.0 (−47.5, −38.4) | −3.6 (−8.0, 0.7) | −39.3 (−45.6, −33.1) (P < 0.0001) |
| Triglycerides, mmol/L | −0.3 (−0.4, −0.1) | −0.2 (−0.3, −0.0) | −0.1 (−0.3,0.1) (P = 0.245) |
| HDL-C, mmol/L | 0.1 (0.1, 0.2) | 0.1 (0.0, 0.1) | 0.1 (0.0,0.1) (P = 0.012) |
| VLDL-C, mmol/Ld | −0.1 (−0.1, −0.0) | −0.0 (−0.1, −0.0) | −0.0 (−0.1,0.0) (P = 0.216) |
| Lipoprotein(a), nmol/L | −12.5 (−17.1, −8.0) | 5.5 (1.2, 9.9) | −18.1 (−24.3, −11.8) (P < 0.0001) |
Mixed-effects model for repeated measures was used for the analysis.
A control-based pattern-mixture model was used.
Co-primary endpoint.
VLDL-C calculated.
ApoB, apolipoprotein B; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LS, least squares; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
The mean placebo-corrected percentage change in non-HDL-C and apoB at Day 510 was −39.5% and −35.8%, respectively (P < 0.0001 for both), and the placebo-corrected time-adjusted percentage change after Day 90 and up to Day 540 was −35.3% and −34.8%, respectively (P < 0.0001 for both).
The placebo-corrected percentage change and the time-adjusted percentage change for other atherogenic lipoproteins [total cholesterol, HDL-C, VLDL-C, and Lp(a)] analysed by MMRM can be found in Figure 1 and Table 2. In addition, the percentage change in total cholesterol, non-HDL-C, and apoB from baseline to Day 510, calculated using CB-PMM, is shown in Supplementary material online, Table S4.
Absolute changes in atherogenic lipoproteins
The absolute change in lipid parameters over time is shown in Figure 1, with corresponding time-adjusted changes shown in Table 2. The absolute change in LDL-C from baseline to Day 510 was −1.6 mmol/L (−60.7 mg/dL) in the inclisiran group vs. −0.06 mmol/L (−2.3 mg/dL) in the placebo group, with a difference of −1.5 mmol/L (−58.4 mg/dL) between groups [95% CI, −1.8 to −1.2 (95% CI in mg/dL −70.4 to −46.4), P < 0.0001]. The time-adjusted absolute change in LDL-C from baseline after Day 90 and up to Day 540 was −1.4 mmol/L (−55.3 mg/dL) with inclisiran and −0.1 mmol/L (−3.4 mg/dL) with placebo, meaning a difference of −1.3 mmol/L (−51.8 mg/dL) and 95% CI, −1.6 to −1.1 (95% CI in mg/dL, −61.1 to −42.6), P < 0.0001 between groups.
The placebo-corrected absolute changes in non-HDL-C and apoB from baseline to Day 510 were −1.7 mmol/L (−63.7 mg/dL) and −40.4 mg/dL, respectively (P < 0.0001 for both). The placebo-corrected time-adjusted absolute changes in non-HDL-C and apoB from baseline after Day 90 and up to Day 540 were −1.5 mmol/L (−56.5 mg/dL) and −39.3 mg/dL, respectively (P < 0.0001 for both).
Other efficacy endpoints
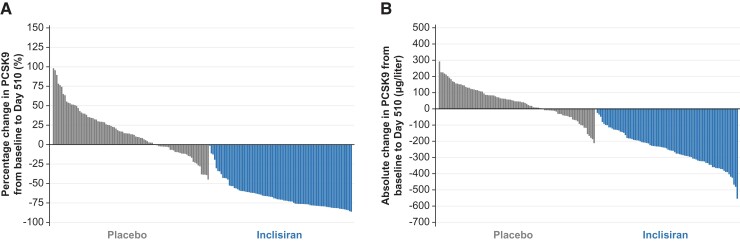
The effects of inclisiran vs. placebo on the percentage and absolute change in PCSK9 levels from baseline are shown in Figure 2. PCSK9 levels decreased by 65.2% with inclisiran and increased by 15.7% with placebo from the baseline to Day 510, representing a between-group difference of −80.8% (95% CI, −87.4 to −74.3; P < 0.0001). At Day 510, a greater proportion of patients receiving inclisiran was likely to achieve at least a 50% reduction in LDL-C (see Supplementary material online, Table S5). Similarly, the proportions of patients achieving LDL-C levels <2.6 mmol/L (100 mg/dL) or <1.8 mmol/L (70 mg/dL) or <1.3 mmol/L (<50 mg/dL) were greater with inclisiran vs. placebo (see Supplementary material online, Figure S3) with 77.6%, 49.4%, and 34.1% patients treated with inclisiran achieving these LDL-C levels, respectively. Importantly, 66.0% of inclisiran-treated patients achieved at least a 50% reduction in LDL-C levels from baseline at any time during the study, compared with 8.7% of placebo-treated patients. Similarly, the proportions of patients achieving non-HDL-C levels <3.4 mmol/L (130 mg/dL) and <2.6 mmol/L (100 mg/dL) or apoB levels <100 mg/dL and <80 mg/dL at Day 510, respectively, were higher with inclisiran (see Supplementary material online, Table S5) vs. placebo.
Figure 2.
Inter-individual variation in proprotein convertase subtilisin/kexin type 9 from baseline to Day 510 (intention-to-treat population). Panel (A) and (B) show the percentage and absolute change in PCSK9, respectively, from baseline to Day 510. The waterfall plot shown represents the change in PCSK9between two specified time points in individuals with measurements at both baseline and Day 510. Data are presented in the following order: patients with the greatest increase to patients with the greatest decrease for each parameter. Inclisiran, n = 98; placebo, n = 105. ITT, intention-to-treat; PCSK9, proprotein convertase subtilisin/kexin type 9.
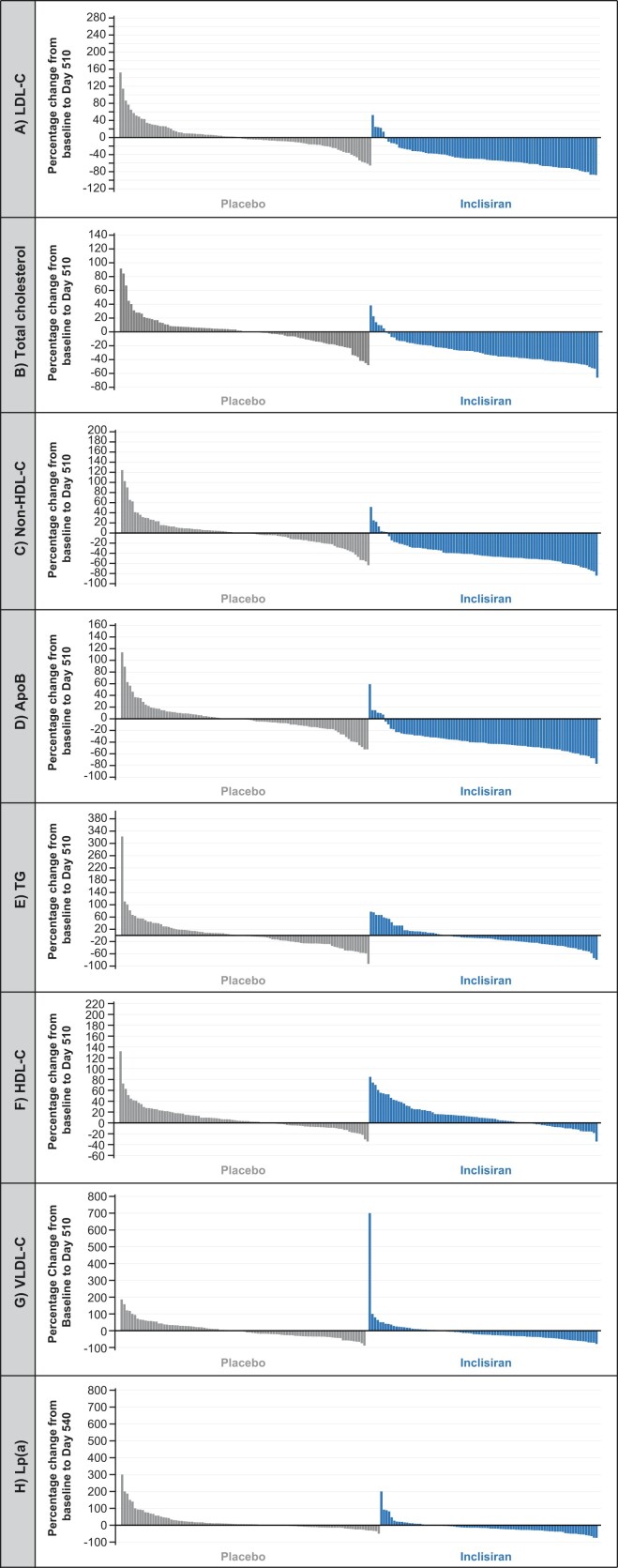
Waterfall plots depicting inter-individual variation in the percentage change in different lipid parameters at Day 510 [Lp(a) at Day 540] show that the majority of inclisiran-treated patients had reductions in atherogenic lipoproteins, including Lp(a) and increases in HDL-C levels (Figure 3). In contrast, among placebo-treated patients, there was considerable variation, with some patients demonstrating marked increases or decreases between baseline and Day 510 (Figure 3), potentially indicative of inconsistent adherence to background statin therapy, although this was not specifically assessed.
Figure 3.
Inter-individual variation from baseline to Day 510 in (A) low-density lipoprotein cholesterol, (B) total cholesterol, (C) non-high-density lipoprotein cholesterol, (D) apolipoproteinB, (E) triglycerides, (F) high-density lipoprotein cholesterol, (G) very low-density lipoprotein cholesterol, and (H) lipoprotein(a) (intention-to-treat population). The waterfall plots shown represent the changes in LDL-C, total cholesterol, non-HDL-C, apoB, triglycerides, HDL-C, and VLDL-C between two specified time points in individuals with observed data both at baseline and Day 510 and for lp(a) at baseline and Day 540. Data are presented in the following order: patients with the greatest increase to patients with the greatest decrease for each parameter. Inclisiran, n = 98; placebo, n = 105. ApoB, apolipoproteinB; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); TG, triglycerides; VLDL-C, very low-density lipoprotein cholesterol.
Safety
The primary prevention safety population comprised 203 patients (98 exposed to inclisiran and 105 to placebo). The proportion of patients with treatment-emergent adverse events (TEAEs) is shown in Table 3.
Table 3.
Adverse events and key laboratory measurements (safety population)a
| Inclisiran (n = 98) | Placebo (n = 105) | |
|---|---|---|
| Adverse events, n (%) | ||
| Patients with at least one adverse event | 91 (92.9) | 88 (83.8) |
| Patients with at least one event leading to drug discontinuation | 5 (5.1) | 3 (2.9) |
| Serious adverse events, n (%) | ||
| Patients with at least one serious adverse event | 20 (20.4) | 13 (12.4) |
| Death | 1 (1.0) | 1 (1.0) |
| Cancer | 1 (1.0) | 1 (1.0) |
| New worsening or recurrent malignancy | 5 (5.1) | 2 (1.9) |
| Clinically relevant adverse events at the injection site, n (%) b | ||
| Any event | 4 (4.1) | 0 (0.0) |
| Mild | 3 (3.1) | 0 (0.0) |
| Moderate | 1 (1.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) |
| Persistentc | 0 (0.0) | 0 (0.0) |
| Clinically relevant laboratory measurements, n (%) | ||
| Liver function | ||
| ALT >3× ULN | 1 (1.0) | 1 (1.0) |
| AST >3× ULN | 0 (0.0) | 1 (1.0) |
| ALP >3× ULN | 0 (0.0) | 0 (0.0) |
| Bilirubin 2×ULN | 1 (1.0) | 1 (1.0) |
| Kidney function | ||
| Creatinine >2 mg/dL | 0 (0.0) | 2 (1.9) |
| Muscle | ||
| CK >5×ULN | 3 (3.1) | 1 (1.0) |
| Haematology | ||
| Platelet count <75 × 109/L | 0 (0.0) | 0 (0.0) |
The safety population included all the patients who received at least one dose of inclisiran or placebo. Adverse events were recorded over the trial period of 540 days.
Adverse events at the injection site included the preferred terms injection site erythema, injection site hypersensitivity, injection site pruritus, injection site rash, and injection site reaction.
Events with a duration <6 months were not persistent.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; ULN, upper limit of normal.
AEs that occurred during the study period, regardless of causality, were reported in 91/98 (92.9%) of inclisiran-treated and 88/105 (83.8%) of placebo-treated patients. The majority of AEs in each cohort were reported as mild-to-moderate, with the most common AEs occurring at a similar frequency between treatment groups within each cohort. Compared with placebo, the proportion of patients with at least one reported AE [91/98 (92.9%) vs. 88/105 (83.8%)] or a serious AE [SAE; 20/98 (20.4%) vs. 13/105 (12.4%)] was greater with inclisiran (Table 3). Clinically relevant TEAEs at the injection site were greater with inclisiran vs. placebo [4/98 (4.1%) vs. 0], with the majority graded as mild and none as severe or persistent. Clinically relevant laboratory measures of safety were similar between treatment groups (Table 3).
Discussion
Previous publications report the effect of inclisiran on reducing LDL-C and other atherogenic lipoproteins in the combined primary and secondary prevention population from the ORION-11 trial and the pooled ORION-9, ORION-10, and ORION-11 trials.8,9 Here we present the primary and secondary endpoints of lipid changes specifically in the primary prevention cohort. Data relevant to the secondary prevention population are presented in Supplementary online material (see Supplementary material online, Tables S4–S8, and Supplementary material online, Figures S4–S7). In a primary prevention population with a mean baseline LDL-C level of 3.6 mmol/L (139.4 mg/dL), treatment with inclisiran administered on Day 1, 90, and every 6 months thereafter, resulted in a sustained lowering of circulating PCSK9 levels by 80.8% (placebo-corrected). This translated into significant peak reductions in a range of circulating atherogenic lipoproteins, including LDL-C (43.7%), non-HDL-C (39.5%), and apoB (35.8%) at Day 510 (Structured Graphical Abstract). Additionally, the placebo-corrected percentage reduction in Lp(a) levels from baseline to Day 540 was 28.5%. Perhaps more importantly, the infrequent dosing regimen (twice-yearly) did not result in attenuation of efficacy, providing clinically significant time-adjusted reductions in levels of LDL-C by 41.0%, non-HDL-C by 35.3%, and apoB by 34.8%, indicating a substantial and sustained lipid-lowering response among those treated with inclisiran. Among the study population, four-fifths of whom were receiving statin therapy (including two-thirds on high-intensity statins), these time-adjusted percentage reductions corresponded to time-adjusted mean absolute reductions in levels of LDL-C by 1.3 mmol/L (−51.8 mg/dL), non-HDL-C by 1.5 mmol/L (−56.5 mg/dL) and apoB by 39.3 mg/dL, over ∼15 months (from baseline after Day 90 and up to Day 540) of treatment. Taken together, these findings demonstrate the efficiency and feasibility of this siRNA-based therapy as an adjunctive lipid-lowering treatment in the primary prevention setting. The overall safety profile observed in this smaller primary prevention cohort was mostly consistent with what was observed in the secondary prevention cohort (see Supplementary material online, Table S8), showing that inclisiran was generally well-tolerated in the primary prevention population. The higher proportion of AEs, SAEs, and TEAEs at the injection site (mainly mild; none were severe or persistent) reported with inclisiran in the primary prevention cohort may be due to the small number of patients in this cohort, and as such, the clinical relevance is uncertain. There were no TEAEs at the injection site reported among placebo-treated patients in the primary prevention cohort.
CV disease remains the leading cause of death and disability worldwide.10 More than 50% of these deaths occur in individuals without a prior history of CV disease;11 hence, population health strategies that improve approaches to primary prevention at a global level are important. The multi-factorial nature of CV disease necessitates a comprehensive approach to prevention, starting first with diet and lifestyle modifications before considering pharmacotherapy to improve all modifiable risk factors, including LDL-C, if appropriate, in the setting of shared decision-making. Most prescriptions for statins (approximately two-thirds of all use) are for the primary prevention of CV disease.12 This includes individuals with a very high 10-year predicted risk of fatal and non-fatal CV events (as estimated through risk calculators), those with high-risk conditions such as DM, which increases the lifetime risk of CV disease, and those with inherited dyslipidaemias, such as FH, which results in lifetime cumulative exposure to elevated LDL-C levels.1,2 Many of these high-risk primary prevention patients have LDL-C levels that exceed guideline recommendations for their level of risk. Some reasons for this include the inability to tolerate high-intensity statins,13 poor adherence to prescribed therapeutic regimens,13 pharmacogenomic variability in response,14 or high LDL-C levels at treatment initiation. In the present study, the high baseline LDL-C level of 3.6 mmol/L (139.4 mg/dL) represents a significant but potentially modifiable residual risk15 as lowering LDL-C further by 1.0 mmol/L would be expected to reduce CV risk in relative terms by approximately one-fifth.16,17 However, the sample size of the present study is too small and the follow-up duration too short to demonstrate CV risk reduction, which will require a formal prospective evaluation.
In the present study, 77.6%, 49.4%, and 34.1% of primary prevention patients achieved LDL-C levels <2.6 mmoL/L (100 mg/dL), < 1.8 mmol/L (70 mg/dL), and <1.3 mmol/L (50 mg/dL), respectively, with inclisiran at Day 510. Of course, these percentages reflect the threshold level of LDL-C for inclusion in the study and may not apply to a broader primary prevention population. The use of other lipid markers such as non-HDL-C and apoB as secondary measures of therapeutic efficacy and residual lipid-related risk is increasingly relevant among individuals with DM, obesity, or elevated triglycerides, where LDL-C levels alone may underestimate risk.1,2 With similar caveats as with LDL-C goal achievement, 76.5% of inclisiran-treated primary prevention patients in this study achieved non-HDL-C levels <3.4 mmol/L (130 mg/dL) and 81.2% achieved apoB levels <100 mg/dL.
The ORION-11 trial also included 1414 secondary prevention patients (see Supplementary material online, Figure S4), and the modestly lower placebo-corrected difference in LDL-C with inclisiran (43.7% vs. 50.6%) in the primary prevention cohort merits a brief discussion. Firstly, the primary prevention cohort is significantly smaller (203 patients), contributing to greater uncertainty around the precision of the point estimate for reduction in the primary endpoint (−52.8 to −34.6% for primary prevention and −54.0 to −47.2% for secondary prevention). Compared with the secondary prevention cohort, the primary prevention cohort was characterized by ∼10% lower use of statins and 20% lower use of high-intensity statins, a much greater proportion of women and patients with FH, and, by study inclusion criteria, a higher mean baseline LDL-C level of 3.6 mmol/L. Although baseline PCSK9 levels were similar between cohorts, by study design, the change in LDL-C assumes that background adherence to lipid-lowering therapy (mostly statins) is consistent between the two-time points when LDL-C is measured. Examination of the placebo group waterfall plots shows wide within-person variation in LDL-C, likely attributable to background lipid-lowering therapy adherence. The impact of changes in this adherence, together with much smaller sample size, higher baseline LDL-C, and lower use of high-intensity statins, may have contributed to observed differences rather than any true biological effect. This could, in time, be explored further in larger studies with a wider set of demographic characteristics in the setting of primary prevention. That said, the magnitude of the absolute reduction in LDL-C in the primary prevention cohort was clinically meaningful and resulted in a greater proportion of patients achieving lower LDL-C goals when added to statins as compared with placebo.
The limitations of the present analysis merit consideration. The primary prevention cohort comprised only 203 patients within the overall ORION-11 trial, warranting large, dedicated studies in this patient population. Additionally, longer-term safety data would provide further assurances, although the tolerability of inclisiran in this distinct group over a treatment period of 18 months appears to be similar to observations in secondary prevention patients and patients with HeFH in the ORION-9 trial, the majority of whom were primary prevention patients.7 Most of these patients have been enrolled in the open-labeled extension ORION-8 trial (NCT03814187), investigating the long-term safety of inclisiran. Although the background use of high-intensity statins was generally high (61.6%) in the primary prevention cohort, the use of ezetimibe was low (5.4%). It is likely that more patients in the placebo group would have reached pre-specified LDL-C goals had the use of other LDL-C lowering therapies been maximized. Notwithstanding this limitation, the background lipid-lowering therapy of the current primary prevention cohort is similar to real-world findings from a patient registry study (comprising 3000 primary prevention patients) conducted in the same geographical region during roughly the same time period.18 The use of ezetimibe in primary prevention patients in the present study was similar to that of the primary prevention cohort in the DA VINCI registry, while the use of high-intensity statins was two-fold higher.18
In conclusion, inclisiran administered twice-yearly (after the initial and 3-month doses) by subcutaneous injection was generally well-tolerated and provides effective and sustained reductions in multiple atherogenic lipoproteins across a broad range of high-risk primary prevention patients with elevated LDL-C despite maximally tolerated statins.
Supplementary Material
Acknowledgements
The authors thank all the investigators, trial site staff, and patient volunteers who participated in the trials. The authors thank Vennila Dharman, MBBS (Novartis Healthcare Pvt. Ltd. India) and Aisling Towell, PhD (Novartis Ireland Ltd.) for providing medical writing support in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3), and Klaus Molle, PhD (Novartis Pharma AG) for critical review of the manuscript and editorial guidance. Prof. Ray receives support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre; his institution (Imperial College London) receives support from the NIHR Applied Research Collaboration Northwest London.
Contributor Information
Kausik K Ray, Imperial Centre for Cardiovascular Disease Prevention, Department of Primary Care and Public Health, Imperial College London, Reynolds Building, St Dunstans Road, London W6 8RP, UK.
David Kallend, DalCor Pharmaceuticals, Montreal, QC, Canada; LIB Therapeutics, Cincinnati, OH, USA.
Lawrence A Leiter, Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Frederick J Raal, Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Wolfgang Koenig, German Heart Centre, Technical University Munich, DZHK (German Centre for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany; Institute of Epidemiology and Medical Biometry, University of Ulm, Ulm, Germany.
Mark J Jaros, Summit Analytical, Denver, CO, USA.
Gregory G Schwartz, Cardiology Division, University of Colorado School of Medicine, Aurora, CO, USA.
Ulf Landmesser, Department of Cardiology, Charité-University Medicine Berlin, Berlin Institute of Health (BIH), DZHK, Partner Site Berlin, Germany.
Lorena Garcia Conde, Novartis Pharma AG, Basel, Switzerland.
R Scott Wright, Mayo Clinic Department of Cardiology, Rochester, MN, USA.
Authors contributions
The principal investigator (K.K.R.) and steering committee in collaboration with the sponsor designed the study protocol. The principal investigator wrote the first draft of the manuscript and all authors participated in its revision and concurred with the decision to submit the final manuscript for publication. The authors disclose that Summit Analytical, Denver, Colorado, United States of America carried out all statistical analysis, with funding from Novartis Pharma AG, Basel, Switzerland. All authors confirm that they approve of the data presented in the manuscript.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Novartis Pharma AG, Basel, Switzerland
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
- 1. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol 2019;73:e285–e350. 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 3. Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA 2011;306:2120–2127. 10.1001/jama.2011.1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, et al. Relations between implementation of new treatments and improved outcomes in patients with non-ST-elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J 2018;39:3766–3776. 10.1093/eurheartj/ehy554 [DOI] [PubMed] [Google Scholar]
- 5. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khvorova A. Oligonucleotide therapeutics — a new class of cholesterol-lowering drugs. N Engl J Med 2017;376:4–7. 10.1056/NEJMp1614154 [DOI] [PubMed] [Google Scholar]
- 7. Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med 2020;382:1520–1530. 10.1056/NEJMoa1913805 [DOI] [PubMed] [Google Scholar]
- 8. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–1519. 10.1056/NEJMoa1912387 [DOI] [PubMed] [Google Scholar]
- 9. Wright RS, Ray KK, Raal FJ, Kallend D, Jaros M, Koenig W, et al. Patient-Level pooled analysis of inclisiran efficacy and safety in the ORION phase III program. J Am Coll Cardiol 2021;77:1182–1193. 10.1016/j.jacc.2020.12.058 [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . Cardiovascular diseases (CVDs). Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed 25 August 2022).
- 11. Pender A, Lloyd-Jones DM, Stone NJ, Greenland P. Refining statin prescribing in lower-risk individuals: informing risk/benefit decisions. J Am Coll Cardiol 2016;68:1690–1697. 10.1016/j.jacc.2016.07.753 [DOI] [PubMed] [Google Scholar]
- 12. Byrne P, Cullinan J, Murphy C, Smith SM. Cross-sectional analysis of the prevalence and predictors of statin utilisation in Ireland with a focus on primary prevention of cardiovascular disease. BMJ Open 2018;8:e018524. 10.1136/bmjopen-2017-018524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khunti K, Danese MD, Kutikova L, Catterick D, Sorio-Vilela F, Gleeson M, et al. Association of a combined measure of adherence and treatment intensity with cardiovascular outcomes in patients with atherosclerosis or other cardiovascular risk factors treated with statins and/or ezetimibe. JAMA Netw Open 2018;1:e185554. 10.1001/jamanetworkopen.2018.5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485–494. 10.1016/j.jacc.2014.02.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Byrne P, Cullinan J, Smith A, Smith SM. Statins for the primary prevention of cardiovascular disease: an overview of systematic reviews. BMJ Open 2019;9:e023085. 10.1136/bmjopen-2018-023085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J 2017;38:2459–2472. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the cholesterol treatment trialists collaboration. Eur Heart J 2018;39:2540–2545. 10.1093/eurheartj/ehx450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, et al. EU-Wide Cross-Sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2021;28:1279–1289. 10.1093/eurjpc/zwaa047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.