Abstract
Aims
Sodium-glucose co-transporter 2 (SGLT2) inhibitors improve cardiovascular outcomes in diverse patient populations, but their mechanism of action requires further study. The aim is to explore the effect of empagliflozin on the circulating levels of intracellular proteins in patients with heart failure, using large-scale proteomics.
Methods and results
Over 1250 circulating proteins were measured at baseline, Week 12, and Week 52 in 1134 patients from EMPEROR-Reduced and EMPEROR-Preserved, using the Olink® Explore 1536 platform. Statistical and bioinformatical analyses identified differentially expressed proteins (empagliflozin vs. placebo), which were then linked to demonstrated biological actions in the heart and kidneys. At Week 12, 32 of 1283 proteins fulfilled our threshold for being differentially expressed, i.e. their levels were changed by ≥10% with a false discovery rate <1% (empagliflozin vs. placebo). Among these, nine proteins demonstrated the largest treatment effect of empagliflozin: insulin-like growth factor-binding protein 1, transferrin receptor protein 1, carbonic anhydrase 2, erythropoietin, protein-glutamine gamma-glutamyltransferase 2, thymosin beta-10, U-type mitochondrial creatine kinase, insulin-like growth factor-binding protein 4, and adipocyte fatty acid-binding protein 4. The changes of the proteins from baseline to Week 52 were generally concordant with the changes from the baseline to Week 12, except empagliflozin reduced levels of kidney injury molecule-1 by ≥10% at Week 52, but not at Week 12. The most common biological action of differentially expressed proteins appeared to be the promotion of autophagic flux in the heart, kidney or endothelium, a feature of 6 proteins. Other effects of differentially expressed proteins on the heart included the reduction of oxidative stress, inhibition of inflammation and fibrosis, and the enhancement of mitochondrial health and energy, repair, and regenerative capacity. The actions of differentially expressed proteins in the kidney involved promotion of autophagy, integrity and regeneration, suppression of renal inflammation and fibrosis, and modulation of renal tubular sodium reabsorption.
Conclusions
Changes in circulating protein levels in patients with heart failure are consistent with the findings of experimental studies that have shown that the effects of SGLT2 inhibitors are likely related to actions on the heart and kidney to promote autophagic flux, nutrient deprivation signalling and transmembrane sodium transport.
Keywords: Heart failure, Proteomics, SGLT2 inhibitors, Differentially expressed proteins
Structured Graphical Abstract
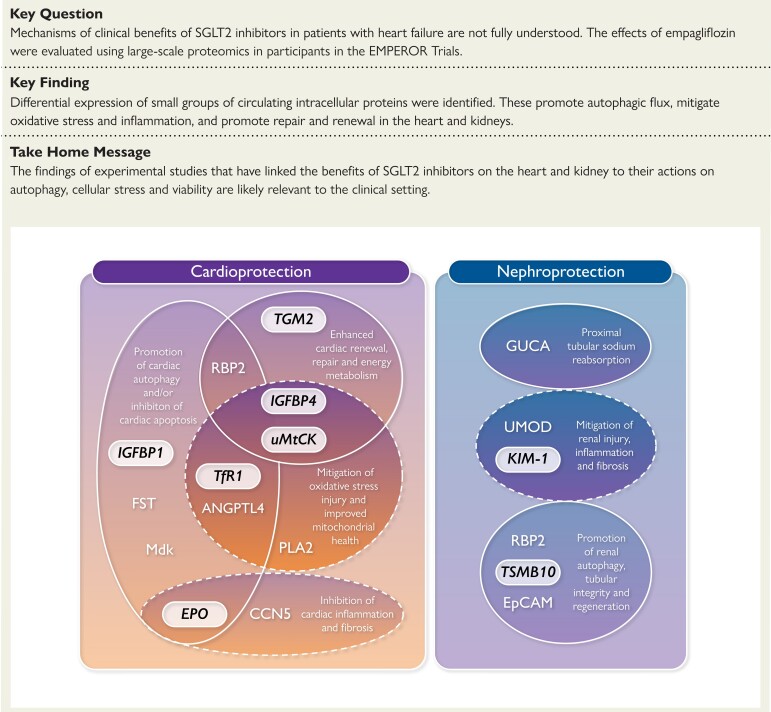
Structured Graphical Abstract.
Favourable biological and cellular actions of differentially expressed proteins in the heart and kidney. IGFBP1, insulin-like growth factor-binding protein 1; TfR1, transferrin receptor protein 1; EPO, erythropoietin; TGM2, protein-glutamine gamma-glutamyltransferase 2; TMSB10, thymosin beta-10; uMtCK, mitochondrial creatine kinase U-type; IGFBP4, insulin-like growth factor-binding protein 4; EpCAM, epithelial cell adhesion molecule; PLA2, phospholipase A2; ANGPTL4, angiopoietin-related protein 4; RBP2, retinol-binding protein 2; CCN5, CCN family member 5; FST, follistatin; Mdk, midkine; GUCA, guanylin.
See the editorial comment for this article ‘SGLT2 inhibitors in heart failure: insights from plasma proteomics’, by Clemens Gutmann et al., https://doi.org/10.1093/eurheartj/ehac624.
Introduction
Sodium-glucose co-transporter 2 (SGLT2) inhibitors improve cardiovascular outcomes across a wide range of high-risk patients, including those with Type 2 diabetes, chronic kidney disease, and heart failure with a reduced or preserved ejection fraction.1 These benefits have been characterized by a decrease in the risk of cardiovascular mortality and heart failure hospitalizations and a reduction in major adverse renal events.2
The underlying mechanisms by which SGLT2 inhibitors improve heart failure and renal outcomes are incompletely understood. Recent evidence suggests that SGLT2 inhibitors may induce a state of starvation mimicry, characterized by enhanced nutrient deprivation signalling and autophagic flux, which leads to an improvement in mitochondrial function, a decrease in oxidative stress, and suppression of proinflammatory and profibrotic pathways.3 It has also been hypothesized that SGLT2 inhibitors may decrease the activity of sodium–hydrogen exchangers in the heart and kidneys, which may reduce intracellular sodium in cardiomyocytes and promote urinary sodium excretion.4 Mediation analyses have identified increases in haemoglobin and decreases in uric acid as statistical intermediaries of the benefits of SGLT2 inhibitors on heart failure and renal outcomes.5,6 Changes in haemoglobin and uric acid may reflect the effects of enhanced nutrient deprivation signalling on erythropoietin production and the effects of augmented autophagic flux on oxidative stress.3,7
We performed large-scale proteomic analyses of biosamples collected in the EMPEROR-Reduced and EMPEROR-Preserved trials before and following short- and long-term treatment with empagliflozin to gain insights into the potential mechanisms of action of SGLT2 inhibitors in patients with chronic heart failure.
Methods
Study populations and biosampling
A total of 9718 patients were randomized into the EMPEROR-Reduced (n = 3730) and EMPEROR-Preserved (n = 5988) trials. Participation in sampling for biobanking of plasma, serum, DNA, and urine was voluntary and not a prerequisite for participation in the trials. Biosamples were collected only if a separate informed consent had been signed, in accordance with local ethical and regulatory requirements. The countries that participated in the biobanking substudy and the methodology of sample collection are described in Supplementary material online, Methods S1.
In EMPEROR-Reduced, 3831 patients gave consent for the collection of biosamples, and of these, 1963 patients had samples available at baseline (i.e. before randomization) and at one or both on-treatment sampling visits (at Week 12 or Week 52). In EMPEROR-Preserved, of the 3067 patients who gave consent for the collection of biosamples, 1463 patients had samples available at baseline and at one or both on-treatment sampling visits (at Week 12 or Week 52). The plasma samples of 600 patients (out of 1963) in EMPEROR-Reduced and of 539 (out of 1463) in EMPEROR-Preserved were randomly selected, and patients were stratified by treatment assignment and data availability. This sample size was determined by budgetary constraints. A total of 599 patients (out of the 600) in EMPEROR-Reduced and 535 (out of 539) in EMPEROR-Preserved had good quality samples with available data both at baseline and at one or both post-randomization visits; thus, a total of 1134 patients were included in the pooled analysis (EMPEROR-Pooled), see Supplementary material online, Methods S2.
In these 1134 patients, measurements of circulating proteins were performed using the Olink® Explore 1536 platform, which utilizes proximity extension assay (PEA) technology with a dual-recognition DNA-coupled readout, in which oligonucleotide-labelled antibody probe pairs bind to their respective targets.8 The Olink® Explore 1536 platform consists of 1472 protein assays and 64 internal controls, and divided into four 384-plex panels, and in each panel, overlapping assays of interleukin-6, interleukin-8, and tumour necrosis factor-α are included as an additional quality control. The platform provides log2 normalized protein expression values with relative quantification. The performance of the assays was blinded to the treatment allocation. Samples were allocated in a random order to avoid any systematic influence of study or timepoint. Protein expression values were missing when samples failed quality control (as specified by the manufacturer), and missing values were not imputed. Proteins that were below the limit of detection in >33% of baseline samples were excluded from the analysis. A total of 1283 proteins were evaluated.
Statistical and bioinformatical analysis
Differences in the level of proteins were assessed using mixed models for repeated measurements, in which we calculated the between-group difference (empagliflozin vs. placebo) in the change in plasma concentrations for each protein from baseline to Week 12 and Week 52, while adjusting for the baseline covariates that had been pre-specified in the statistical plan for each trial [i.e. age, sex, geographical region, diabetes, left ventricular ejection fraction, estimated glomerular filtration rate (eGFR)]. We also pre-specified an adjustment for the baseline protein expression and trial assignment (EMPEROR-Reduced and EMPEROR-Preserved). The change from baseline in eGFR was included as a covariate in a post hoc sensitivity analysis.
We identified differentially expressed proteins as those fulfilling two independent criteria: (i) proteins with |log2 fold change| >log2(1.1), corresponding to a ≥10% increase from baseline (log2 fold change >0.1375) or a symmetric >9.09% decrease from baseline and (ii) a false discovery rate q-value (FDRq) <1%, which applies a Benjamini–Hochberg correction to the raw P-value, in order to minimize inflation of a false-positive error rate due to multiplicity of comparisons.9 Significance of differences between treatment effects at Weeks 12 and 52 were investigated by a treatment-by-visit interaction term in the model.
All proteins measured in the Olink® platform are known to exert diverse function across many biological domains. We initially grouped differentially expressed proteins according to their reported actions in oncology, but these groupings had little meaning in cardiology and nephrology. Therefore, we performed an extensive biomedical literature search to identify the biological effects of differentially expressed proteins in the heart and kidneys.
Results
Patient characteristics
The 1134 patients had a mean age of 70 years, 32% were women, 85% were White, and 57% were recruited in Europe. In addition, 48% had Type 2 diabetes, the mean left ventricular ejection fraction was 40%, and the mean eGFR was 61 mL/min/1.73 m2. When the 1134 patients whose biosamples were analysed were compared with the 8584 patients who were randomized in the EMPEROR trial but were not represented in the current analyses, patients not in the current analysis were more likely to be women (37 vs. 32%), less likely to be White (72 vs. 85%), or from Europe (40 vs. 57%), and had a higher ejection fraction (45 vs. 40%), refer Supplementary material online, Table S1.
Differentially expressed proteins at Week 12
Based on our pre-specified criteria and covariate adjustment, 32 of 1283 proteins fulfilled our threshold for being differentially expressed at Week 12; all were increased by ≥10% (log2 fold change >0.1375) and had a false discovery rate <1% (corresponding generally to a raw P-value <0.0007 for the difference between empagliflozin and placebo), Table 1.
Table 1.
Differential expressed proteins (empagliflozin vs. placebo) at Week 12
| Protein | UniprotID | Before adjustment for change in eGFR | After adjustment for change in eGFR | ||
|---|---|---|---|---|---|
| Empagliflozin vs. placebo, log2 fold change (95% CI) | False discovery rate (%) | Empagliflozin vs. placebo, log2 fold change (95% CI) | False discovery rate (%) | ||
| IGFBP1 | P08833 | 0.27 (0.14–0.40) | 0.16 | 0.25 (0.13–0.38) | 0.55 |
| TfR1 | P02786 | 0.26 (0.19–0.32) | <0.0001 | 0.25 (0.18–0.32) | <0.001 |
| CA2 | P00918 | 0.25 (0.11–0.39) | 0.81 | 0.26 (0.12–0.40) | 1.02 |
| EPO | P01588 | 0.25 (0.11–0.38) | 0.65 | 0.24 (0.10–0.37) | 1.53 |
| TGM2 | P21980 | 0.25 (0.11–0.38) | 0.60 | 0.25 (0.12–0.38) | 1.02 |
| TMSB10 | P63313 | 0.22 (0.11–0.34) | 0.43 | 0.19 (0.08–0.31) | 2.18 |
| uMtCK | P12532 | 0.21 (0.10–0.32) | 0.43 | 0.22 (0.11–0.33) | 0.69 |
| IGFBP4 | P22692 | 0.21 (0.13–0.30) | 0.01 | 0.17 (0.09–0.25) | 0.31 |
| AFABP4 | P15090 | 0.21 (0.12–0.29) | 0.02 | 0.17 (0.09–0.25) | 0.31 |
| CCL18 | P55774 | 0.20 (0.11–0.29) | 0.08 | 0.19 (0.10–0.28) | 0.31 |
| Follistatin | P19883 | 0.20 (0.13–0.26) | <0.0001 | 0.19 (0.12–0.26) | <0.0001 |
| Midkine | P21741 | 0.20 (0.11–0.29) | 0.14 | 0.18 (0.09–0.27) | 0.55 |
| Guanylin | Q02747 | 0.20 (0.13–0.26) | <0.0001 | 0.17 (0.11–0.23) | <0.001 |
| SPINK1 | P00995 | 0.20 (0.13–0.26) | <0.01 | 0.16 (0.10–0.23) | 0.02 |
| Rarres2 | Q99969 | 0.19 (0.10–0.29) | 0.18 | 0.17 (0.08–0.27) | 0.96 |
| CNDP1 | Q96KN2 | 0.19 (0.11–0.28) | 0.07 | 0.19 (0.11–0.27) | 0.19 |
| EpCAM | P16422 | 0.19 (0.10–0.28) | 0.17 | 0.20 (0.10–0.29) | 0.29 |
| CCL27 | Q9Y4X3 | 0.19 (0.09–0.29) | 0.61 | 0.17 (0.07–0.27) | 2.16 |
| SRCR | Q8WTU2 | 0.18 (0.09–0.27) | 0.21 | 0.18 (0.09–0.27) | 0.48 |
| PLA2 | P14555 | 0.18 (0.08–0.27) | 0.54 | 0.16 (0.07–0.26) | 2.07 |
| Promotilin | P12872 | 0.18 (0.09–0.26) | 0.26 | 0.16 (0.08–0.25) | 1.02 |
| ANGPTL4 | Q9BY76 | 0.17 (0.11–0.23) | 0.00 | 0.16 (0.09–0.22) | 0.01 |
| RBP2 | P50120 | 0.17 (0.07–0.26) | 0.98 | 0.15 (0.05–0.24) | 4.02 |
| Uromodulin | P07911 | 0.16 (0.10–0.22) | <0.0001 | 0.18 (0.12–0.23) | <0.0001 |
| Cystatin-F | O76096 | 0.16 (0.07–0.25) | 0.63 | 0.15 (0.06–0.24) | 1.89 |
| CCN5 | O76076 | 0.15 (0.08–0.22) | 0.14 | 0.14 (0.07–0.21) | 0.69 |
| CCL16 | O15467 | 0.15 (0.08–0.22) | 0.19 | 0.13 (0.06–0.21) | 1.02 |
| Osteopontin | P10451 | 0.14 (0.08–0.21) | 0.18 | 0.13 (0.06–0.20) | 1.02 |
| ANGPTL2 | Q9UKU9 | 0.14 (0.07–0.22) | 0.54 | 0.14 (0.06–0.21) | 1.16 |
| NPDC1 | Q9NQX5 | 0.14 (0.08–0.21) | 0.07 | 0.11 (0.06–0.17) | 1.02 |
| Elafin | P19957 | 0.14 (0.07–0.21) | 0.44 | 0.11 (0.04–0.18) | 3.96 |
| Cystatin-M | Q15828 | 0.14 (0.08–0.20) | 0.10 | 0.11 (0.05–0.17) | 1.02 |
IGFBP1, insulin-like growth factor-binding protein 1; TfR1, transferrin receptor protein 1; CA2, carbonic anhydrase 2; EPO, erythropoietin; TGM2, protein-glutamine gamma-glutamyltransferase 2; TMSB10, thymosin beta-10; uMtCK, creatine kinase U-type, mitochondrial; IGFBP4, insulin-like growth factor-binding protein 4; AFABP, adipocyte fatty acid-binding protein 4; CCL18, C–C motif chemokine 18; SPINK1, serine protease inhibitor Kazal-type 1; Rarres2, retinoic acid receptor responder protein 2; CNDP1, beta-Ala-His dipeptidase; EpCAM, epithelial cell adhesion molecule; CCL27, C–C motif chemokine 27; SRCR, scavenger receptor cysteine-rich domain-containing group B protein; PLA2, phospholipase A2; ANGPTL4, angiopoietin-related protein 4; RBP2, retinol-binding protein 2; CCN5, CCN family member 5; CCL16, C–C motif chemokine 16; NPDC1, neural proliferation differentiation and control protein 1; ANGPTL2, angiopoietin-related protein 2.
Among these, nine proteins demonstrated the largest treatment effect of empagliflozin (log2 fold change >0.201, corresponding to a >15% increase) with a false discovery rate of <1%. These were insulin-like growth factor-binding protein 1 (IGFBP1), transferrin receptor protein 1 (TfR1), carbonic anhydrase 2 (CA2), erythropoietin (EPO), protein-glutamine gamma-glutamyltransferase 2 (TGM2), thymosin beta-10 (TMSB10), mitochondrial creatine kinase U-type (uMtCK), insulin-like growth factor-binding protein 4 (IGFBP4), and adipocyte fatty acid-binding protein 4 (AFABP4). Among these, the effect of empagliflozin on TfR1 was particularly noteworthy with a nearly 20% increase and an exceptionally low false discovery rate (2.5 × 10−10) with a raw P-value of 1.9 × 10−13.
The effects of empagliflozin (corrected for placebo) on all measured circulating proteins (corrected for placebo) from the baseline to Week 12 are shown in Supplementary material online, Table S2 and displayed in Supplementary material online, Figure S1. No differentially expressed protein showed a statistically significant interaction between EMPEROR-Reduced and EMPEROR-Preserved at Week 12, Supplementary material online, Table S3. No differentially expressed protein met the criteria for a meaningful decrease in level at Week 12. Of note, N-terminal prohormone B-type natriuretic peptide declined at 12 weeks, but the change was not statistically significant.
In a post hoc sensitivity analysis, we examined the effects of empagliflozin vs. placebo on the changes in protein levels from the baseline for all 1283 proteins after the inclusion of the change in eGFR from baseline to Week 12 as an additional covariate. No new proteins were found to be differentially expressed. Of the 32 differentially expressed proteins in our original model, adjustment for the change in eGFR did not meaningfully influence the effect size for proteins with the largest treatment effect, but five of the six proteins with the smallest changes in our original model now fell meaningfully below the threshold of log2 fold change of 0.1375 following adjustment (Table 1). For the remaining 27 proteins, the false discovery rate generally increased modestly, but remained <5% for all proteins, and was <2% for all but 4 proteins.
Differentially expressed proteins at Week 52
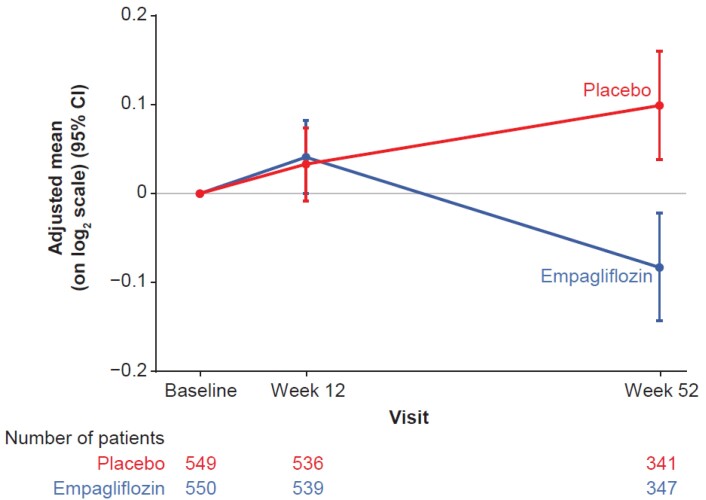
The changes of the proteins from baseline to Week 52 were generally concordant with the changes from baseline to Week 12 (see Supplementary material online, Figure S1), as demonstrated by a lack of significant treatment-by-time interactions for all proteins, with one exception. When compared with placebo, empagliflozin did not have a meaningful effect on kidney injury molecule-1 (KIM-1) at Week 12, but it decreased KIM-1 at Week 52 (Figure 1), log2 fold change −0.18 (95% CI −0.10 to −0.27), a 12% decrease with a false discovery rate of 0.006%. The effect of empagliflozin on KIM-1 at 52 weeks was significantly different from that at 12 weeks, visit-by-treatment interaction false discovery rate of 0.008%.
Figure 1.
Kidney injury molecule-1 (KIM-1). Changes from baseline in the empagliflozin and placebo groups at Week 12 and Week 52.
Biological actions of differential expressed proteins on the heart and kidney
As noted above, after adjustment for changes in eGFR, the circulating levels of 27 proteins were increased by ≈10% or more by empagliflozin at 12 weeks, and one protein was decreased by at least 10% by the drug at 52 weeks. The known biological effects of these 28 proteins are shown in Table 2.10–62
Table 2.
Biological effect of differentially expressed proteins on heart and kidney
| Protein | Cellular action | Effects on heart, kidney, and other Sites |
|---|---|---|
| Proteins with effects on the heart | ||
| Insulin-like growth factor-binding protein 1 (IGFBP1) | Promotion of cardiac autophagy | IGF1 promotes heart failure by inhibiting cardiac autophagy; increases in IGFBP1 interfere with actions of IGF1, thus promoting autophagy. 10 IGFBP1 also promotes HIF-1α stability and inhibits cardiomyocyte apoptosis.11Expression of IGFBP1 is upregulated by sirtuin-1.12 |
| Transferrin receptor protein 1 (TfRI) | Promotion of cardiac iron metabolism and cardiac autophagy; improved mitochondrial health | Required for iron transport into cardiomyocytes. TfR1 knockout leads to mitochondrial dysfunction, down-regulation of cardiac autophagy proteins and cardiomyopathy, which can be prevented by sirtuin-1 activation. 13 TfR1-mediated changes in transmembrane electron transport provides NAD + required for sirtuin activation. 14 |
| Erythropoietin (EPO) | Promotion of cardiac autophagy and inhibition of cardiac fibrosis | Prevents cardiac remodelling, resulting from promotion of autophagy and mitigates apoptosis and inflammation and fibrosis in the heart. 15–17 Expression and actions are AMPK- and sirtuin-dependent. 15,17,18 |
| Follistatin (FST) | Promotion of cardiac autophagy | Follistatin and follistatin-like proteins interact with the same receptors. Follistatin-like protein 1 reduces myocardial injury, apoptosis, remodelling, hypertrophy, and fibrosis by promoting autophagy through effects on AMPK. 19,20 |
| Retinol-binding protein 2 (RBP2) | Promotion of renal autophagy; inhibition of cardiac apoptosis and enhanced cardiac regenerative capacity | Retinoic acid levels decline in heart failure. Facilitates dietary retinol uptake, thereby mitigating cardiomyocyte apoptosis and promoting cardiac regenerative capacity. 21–23 Prevents renal injury by promoting autophagy in the kidney. 24 |
| Midkine (Mdk) | Inhibition of cardiac apoptosis | Reduces cardiac injury, mitigation of cardiac apoptosis and remodelling; promotes angiogenesis. 25,26 |
| Phospholipase A2, membrane-associated (PLA2) | Promotion of cardiac repair following oxidative stress | Calcium-independent PLA2 in ventricular cardiomyocytes localizes to mitochondria and peroxisomes and acts as a phospholipid repair enzyme following oxidative damage. Inhibition of phospholipase A2 is a mechanism underlying anthracycline cardiotoxicity. 27,28 |
| Angiopoietin-related protein 4 (ANGPTL4) | Reduction of oxidative stress and promotion of endothelial cell autophagy | Inhibits lipoprotein lipase in cardiomyocytes. Prevents fatty acid-induced oxidative stress. 29 Preserves endothelial integrity by promotion of autophagy, thereby supporting myocardial function. 30 |
| Insulin-like growth factor-binding protein 4 (IGFBP4) | Reduction of oxidative stress and promotion of cardiac regenerative capacity | Produces cardioprotection by minimizing the DNA injury produced by oxidative stress and promotes angiogenesis. 31 Enhances induction of cardiomyocytes from pluripotential stem cells by inhibition of the Wnt/β-catenin signalling, independent of its binding to IGF. 32 |
| Protein-glutamine gamma-glutamyltransferase 2 (TGM2) | Enhanced cardiac ATP synthesis, cardiac repair and regenerative capacity | Enhanced fatty acid utilization and ATP synthesis. 33 Promotes cardiac repair and regenerative capacity. 34 |
| Mitochondrial creatine kinase, U-type (uMtCK) | Support of cardiac energy metabolism | Mediates the transfer of high energy phosphate from mitochondria to cytosol. Compensatory increase following oxidative stress has cardioprotective effects. 35–37 |
| Connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed family member 5 (CCN5) | Inhibition of cardiac fibrosis | Inhibits cardiac hypertrophy. Interferes with endothelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation, thereby reducing cardiac fibrosis. Prevents structural and electrical remodelling. Knockout leads to cardiomyopathy. 38,39 |
| Adipocyte fatty acid-binding protein 4 (AFABP4) | Suppression of cardiac contractility | Lipid binding protein that reduces fatty acid uptake by myocardium, thus suppresses cardiac contractility. 40,41 May act as indicator of increased sirtuin-1 signalling and autophagic flux.42 |
| Retinoic acid receptor responder protein 2 (Rarres2, chemerin) | Promotion of cardiac hypertrophy, apoptosis, and angiogenesis | Adipokine that induces cardiomyocyte hypertrophy, apoptosis, and angiogenesis. 43,44 May act as biomarker of renal function, being inversely related to glomerular filtration rate. 45 |
| Proteins with effects on the kidneys | ||
| Carbonic anhydrase 2 (CA2) | Renal tubular sodium, bicarbonate, and water homeostasis | Binds to and enhances the activity of NHE3 in the proximal renal tubule. 46 May offset increases in urinary bicarbonate and water resulting from SGLT2 inhibition. |
| Guanylin (GUCA) | Renal tubular sodium transport | After the dietary salt load, guanylin and uroguanylin promote natriuresis by inhibiting NHE3 in proximal tubule 47,48 |
| Uromodulin (UMOD) | Renal tubular sodium transport and muting of renal inflammation | Promotes sodium reabsorption in the thick ascending limb of the loop of Henle. 49,50 Acts as a trap for proinflammatory renal cytokines; loss-of-function mutations lead to chronic kidney disease. 51 |
| Kidney injury molecule-1 (KIM-1) | Promotion of renal proximal tubular injury, inflammation, and fibrosis | Mediates renal tubular cell injury and apoptosis and promotes tubulointerstitial inflammation and fibrosis 52–54 |
| Epithelial cell adhesion molecule (EpCAM) | Promotion of renal tubular integrity and regeneration | Promotes adhesion and polarity of renal tubular cells. EpCAM is suppressed by nephrotoxic agents and is enhanced during renal tubular regeneration 55,56 |
| Thymosin beta-10 (TMSB10) | Promotion of renal tubular integrity and regeneration | Plays important role in organization of cytoskeleton, thus promoting adhesion and polarity of renal parenchymal cells 57,58 |
| Beta-Ala-His dipeptidase (carnosinase 1, CNDP1) | Degradation of nephroprotective carnosine | Carnosine protects against the development of nephropathy, and gain-of-function mutations of carnosinase-1 cause carnosine depletion and increase risk of chronic kidney disease. 59 |
| Angiopoietin-like protein 2 (ANGPTL2) | Promotes renal inflammation and fibrosis | Expressed in endothelial cells. Promotes oxidative stress, inflammation and fibrosis in the kidney and heart. 60,61 May act as a marker of increased HIF-1α activity.62 |
| Proteins With Effects Other Than on the Heart and Kidneys | ||
| Promotilin (MLN) | No known action in the heart or kidney | Prohormone for motilin, which promotes gastrointestinal motility. Produces relaxation of vascular smooth muscle, leading to systemic vasodilatation |
| Serine proteases inhibitor Kazal-type 1 (SPINK1) | No known action in the heart or kidney | Inhibits trypsin with proposed role in the pathogenesis of pancreatitis. May be biomarker of renal function, since it increases in proportion to decreases in glomerular filtration rate |
| C–C motif chemokine 18 (CCL18) | No known action in the heart or kidney |
Expressed in lung, antigen-presenting dendritic cells and M2 macrophages and is chemoattractant for
T cells and lymphocytes |
| C–C motif chemokine 27 (CCL 27) | No known action in the heart or kidney | Expressed in skin and mediates homing of memory T lymphocytes to cutaneous sites |
| Cystatin-F (CYTF, CST6) | No known action in the heart or kidney | Inhibits cathepsin C-directed protease. Expressed in immune cells and causes down-regulation of killing efficiency of cytotoxic T lymphocytes |
| Scavenger receptor cysteine-rich domain-containing group B protein (SRCR) | No known action in the heart or kidney | Expressed in epithelial cells and plays a role in mucosal immunity and innate defence |
RBP2 has effects on the heart and kidneys, but to avoid duplication, t is described only in the section on the heart.
AMPK, adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; IGF1, insulin-like growth factor 1; NAD+, nicotinamide adenine dinucleotide; NHE3, sodium–hydrogen exchanger isoform 3; Wnt, Wingless-related integration site glycoprotein; HIF-1α, hypoxia-inducible factor-1α.
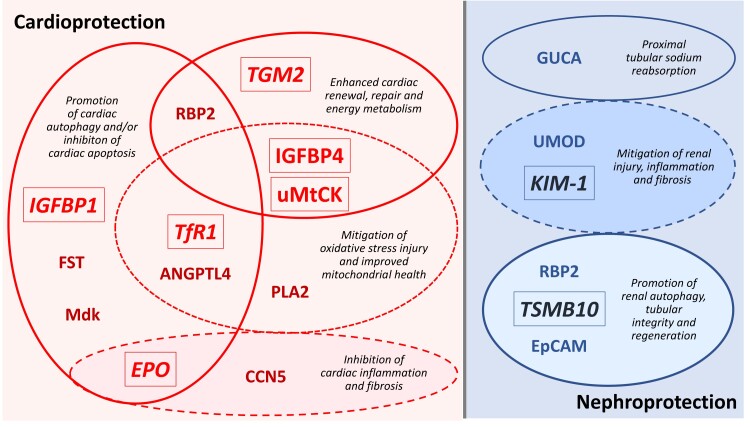
Fourteen proteins have established effects in the heart, and of these, the most common effect was the promotion of autophagic flux (five proteins), which was particularly characteristic of the three cardiac-acting proteins that had the largest effect size (IGFBP1, TfR1, and EPO), Table 1.10,13,15 Most of the other 11 proteins have been shown to reduce oxidative stress or its consequences, inhibit apoptosis, inflammation and fibrosis, and enhance the energy, repair and regenerative capacity of the heart (Table 2 and Figure 2).
Figure 2.
Favourable biological and cellular actions of differentially expressed proteins in the heart and kidney. Empagliflozin had the largest treatment effect on proteins shown in a larger font and in italics. RBP2 has both cardioprotective and nephroprotective effects. IGFBP1, insulin-like growth factor-binding protein 1; TfR1, transferrin receptor protein 1; EPO, erythropoietin; TGM2, protein-glutamine gamma-glutamyltransferase 2; TMSB10, thymosin beta-10; uMtCK, mitochondrial creatine kinase U-type; IGFBP4, insulin-like growth factor-binding protein 4; EpCAM, epithelial cell adhesion molecule; PLA2, phospholipase A2; ANGPTL4, angiopoietin-related protein 4; RBP2, retinol-binding protein 2; CCN5, CCN family member 5; CCL16, C–C motif chemokine 16; NPDC1, neural proliferation differentiation and control protein 1; FST, follistatin; Mdk, midkine; GUCA, guanylin.
Nine proteins have established effects in the kidney (Table 2), and of these, three proteins (CA2, guanylin, and uromodulin) have actions on sodium transport in the renal tubules.46,47,50 Two proteins have been shown to inhibit renal injury, inflammation, and fibrosis (uromodulin and KIM-1),51,54 and three proteins play a role in promoting renal autophagy, tubular integrity and regeneration [retinol-binding protein 2 (RBP2), TSMB10 and epithelial cell adhesion molecule (EpCAM)], Figure 2 and Table 2.24,56,57
Discussion
Our proteomics analysis of blood samples taken from participants pooled from the EMPEROR-Reduced and EMPEROR-Preserved trials indicate that circulating levels of approximately 30 proteins changed meaningfully during SGLT2 inhibition with empagliflozin (Structured Graphical Abstract). Concentrations of these proteins changed by at least 10%, and they did so with substantial consistency, yielding results that were highly statistically significant, even after major adjustment for multiplicity of testing. Our thresholds for identifying differentially expressed proteins were similar to or more stringent than those used in other cardiovascular proteomics analyses; we used a false discovery rate of <1%, rather than the <5% that has been commonly used in other studies.63 Importantly, the changes in the circulating levels of most differentially expressed proteins were not related to changes in renal clearance than may have been produced by empagliflozin as a result of its action to reduce intraglomerular filtration pressures, since our results did not meaningfully change following adjustment for changes in glomerular filtration rate. It should be understood that most of the proteins that we assessed act intracellularly, and it is plausible that changes in circulating levels parallel changes in the intracellular concentration of these proteins with subsequent release from the cytosol. However, the magnitude of this release does not necessarily reflect the intracellular expression or activation of these proteins, whose stochiometric relationships may not be linear. Furthermore, our proteomics analyses cannot identify the organ sources of this protein release.
In experimental studies, SGLT2 inhibitors have been shown to exert cardioprotective and nephroprotective effects by promoting autophagic flux and mitigating apoptosis, muting oxidative and other cellular stresses, inhibiting proinflammatory and profibrotic pathways, and enhancing cellular energy stores and metabolism.3,64–68 It is therefore noteworthy that most of the 33 differentially expressed proteins identified in our proteomics analyses have been shown to exert favourable effects on the heart and kidney in hypothesis-driven experimental studies performed in animal models of cardiac and renal injury (Table 2). Of particular note, six proteins have been shown to promote autophagic flux in the heart, kidney or endothelium, i.e. IGFBP1, TfR1, EPO, follistatin, angiopoietin-related protein 4 (ANGPTL4), and RBP2.10,11,13,15,19,24,30
Five proteins have been shown to reduce the levels or consequences of oxidative stress and improve mitochondrial health in the myocardium (TfR1, phospholipase A2 (PLA2), ANGLPTL4, IGFBP4, and uMtCK).13,28,29,31,36 Four proteins have been reported to enhance energy metabolism, cardiac repair or the renewal of cardiomyocytes (RBP2, IGFBP4, TGM2, and uMtCK).23,32–35 As a result of these and other actions, many of the differentially expressed proteins have favourable effects to retard maladaptive ventricular hypertrophy or remodelling, Table 2. In addition, two proteins have demonstrated effects to mitigate renal injury and inflammation (uromodulin and KIM-1),49,52 three proteins have been shown to promote renal autophagy or enhance the integrity or regeneration of renal tubular cells (RBP2, TSMB10, and EpCAM),55,57 and three proteins (CA2, guanylin, and uromodulin) play a role in renal tubular sodium transport.47,49 Therefore, our proteomics analyses suggest that the pathways that are influenced by SGLT2 inhibitors in patients with heart failure are similar to those influenced by these drugs in animal models of cardiac or renal stress.64–69
Importantly, our conclusions remained unaltered even when we focused on proteins with the most marked changes, Figure 1. The protein that showed the most striking increase was IGFBP1, which binds to insulin-like growth factor 1 (IGF1) to limit its activity. IGF1 has demonstrable deleterious effects on the heart to suppress autophagy, and its upregulation promotes the development of cardiomyopathy.10 The protein that showed the second largest treatment effect in the current study was TfR1, which is required for iron transport into cardiomyocytes to prevent contractile dysfunction,70 but it also enhances nutrient deprivation signalling.14 Knockout of TfR1 leads to mitochondrial derangements, suppression of autophagy and the development of cardiomyopathy.13 The third most differentially expressed protein related to heart function was EPO, which is classically known as the principal stimulus to red blood cell production. However, EPO also exerts direct effects on the heart (independent of its actions on erythrocytosis) to promote autophagy, prevent adverse ventricular remodelling, and mute proinflammatory and profibrotic signalling in the myocardium.15–17 Interestingly, all three proteins are functionally related to the increases in haemoglobin seen in clinical trials with SGLT2 inhibitors, a physiological change that is the most important statistical mediator of the benefit of SGLT2 inhibitors to reduce heart failure and major adverse renal events.5,6 Specifically, IGFBP1 promotes the stability of hypoxic inducible factor 1-α,11 an important endogenous stimulus to the production or erythropoietin, while TfR1 ensures the adequacy of intracellular levels of iron in erythrocytes.71 Interestingly, increased levels of ANGPTL2 may also serve as an indicator of increased hypoxic inducible factor 1-α signalling.62
Three prior studies have reported the results of proteomics analyses before and after SGLT2 inhibition, but the three studies each evaluated fewer than 80 patients, <10% of the patients in the current EMPEROR proteomics substudy.63,72,73 Of the three, the study most comparable to our analyses was carried out by Ferrannini et al.,63 although that study differed from ours in several important respects. First, Ferrannini et al. studied the effects of empagliflozin in patients with Type 2 diabetes, whereas we studied the effects of empagliflozin in patients with chronic heart failure. Ferrannini et al measured proteins using an aptamer-based assay, whereas we performed measurements using a PEA method. Ferrannini et al. performed measurements after only 4 weeks of treatment, whereas we carried out our assessments after 12 and 52 weeks of therapy. Despite these differences, it is noteworthy that Ferrannini et al. also observed that the proteins most prominently affected by SGLT2 inhibition were IGFBP1, TfR1, EPO and AFABP4, findings that are strikingly concordant with the major observations of the current proteomics analyses from the EMPEROR trials. Interestingly, IGFBP1, TfR1, EPO, and AFABP4 are all linked to increased activity of sirtuin-1, a nutrient deprivation sensor that promotes autophagy and is known to be increased by SGLT2 inhibitors in experimental studies and that appears to be essential to mediating their benefits.74 Experimentally, the effects of SGLT2 inhibitors to reduce cellular stress and attenuate apoptosis in cardiomyocytes are abolished when the actions of sirtuin-1 are inhibited.75 It is therefore noteworthy that sirtuin-1 directly stimulates the expression of both IGFBP1 and AFABP4,12,42 TfR1 is required for sirtuin-1 synthesis,14 and sirtuin-1 can stimulate the production of EPO by an action on hypoxia-inducible factor 2-α.18
Our proteomics findings are also consistent with the upregulation of proteins that have favourable effects on renal structure and function. Upregulation of RBP2 may enhance the ability of retinoic acid to prevent renal injury by promoting autophagic flux in renal parenchymal cells.24 Increases in circulating levels of TSAMB10 and EpCAM may be critical to the establishment of the adhesion and polarity of renal tubular cells, a critical step in their regeneration following injury.55–58 During long-term treatment, SGLT2 inhibition produced meaningful decreases in KIM-1, which mediates renal tubular cell injury and apoptosis and promotes tubulointerstitial inflammation and fibrosis.52–54 The concerted actions of these differentially increased proteins closely mimic the known effects of SGLT2 inhibitors to prevent renal injury, inflammation, and fibrosis in experimental studies of renal stress in animal models.64
Interestingly, several of our differentially expressed proteins may represent a mechanism by which the kidney may compensate for the renal tubular actions of SGLT2 inhibitors. SGLT2 inhibitors interfere with sodium reabsorption in the proximal renal tubule through an action to inhibit both SGLT2 and sodium–hydrogen exchanger isoform 3 (NHE3).69,76 Guanylin also acts in the proximal tubule to inhibit NHE3,47 but it is secreted following a dietary salt load;77 its action reinforces the effect of SGLT2 inhibitors to promote the urinary excretion of sodium and bicarbonate.76,78 Yet, the observed increase in levels of CA2 may represent a compensatory mechanism to these effects. CA2 binds to NHE3 to increase its activity,46 and CA2 enhances the renal tubular adaptation to the increase in urinary bicarbonate that follows the action of SGLT2 inhibitors and guanylin to inhibit NHE3.79 In addition, CA2 acts to counter the increase in free water clearance that is seen when glycosuria is induced by SGLT2 inhibitors.80 Uromodulin can further counteract the natriuretic effect of SGLT2 inhibitors by enhancing sodium reabsorption more distally in the nephron.50 The activation of these counterregulatory mechanisms may explain why the initial natriuretic and osmotic diuretic response to SGLT2 inhibitors is not sustained.81–83 Accordingly, we observed no meaningful decrease in NT-proBNP in the current study, a finding consistent with our earlier observations based on a conventional ELISA assay.84
Our findings should be considered in light of certain strengths and limitations. Our proteomic analyses examined >1200 proteins in blood collected from >1100 patients who participated in two placebo-controlled randomized trials. Therefore, our study represents the largest proteomic database to date on the effects of SGLT2 inhibitors. Despite this advantage, we had an insufficient number of patients in each trial to adequately examine potential differences in the effects of SGLT2 inhibitors between patients with a reduced and preserved ejection fraction, and we needed to pool our data across both trials to maximize statistical power. Another strength of the study is that we assessed changes at both 12 and 52 weeks, enabling us to show that the effects seen early in treatment were generally not meaningfully different during long-term therapy. Although we adjusted for changes in renal function that occurred during the first 12 weeks, we did not adjust for changes in concomitant medications, which have the potential to influence protein expression independent of the study medication; however, changes in background therapy during the first 12 weeks were modest.
Most importantly, although our proximal extension assay has certain advantages over aptamer-based methods (which measure protein fragments), our Olink platform measured only a small fraction of the intracellular proteins that have been implicated in the action of SGLT2 inhibitors in experimental studies. Furthermore, we did not measure many of the proteins that Ferrannini et al.63 noted to be meaningfully changed by empagliflozin using aptamer-based methods (including growth differentiation factor 15 and ferritin). Most importantly, we did not directly measure any of the proteins that are involved in enhanced nutrient deprivation signalling and have been implicated in mediating the actions of SGLT2 inhibitors on cardiomyocytes and renal tubular cells (e.g. sirtuin-1, proliferator-activated receptor gamma coactivator 1-α, phosphorylated mammalian target of rapamycin, and phosphorylated AMP-activated protein kinase). These should be the focus of further investigations.
Conclusion
Proteomics is rapidly emerging as an innovative approach to gaining potential insights into the mechanisms of disease and drug action. Our proteomics analysis of blood samples taken from participants pooled from two trials of empagliflozin in heart failure identified differential expression of a small select group of circulating proteins following SGLT2 inhibition. The biological effects of differentially expressed proteins on the heart were primarily focused on the promotion of autophagic flux, but they also included favourable effects to reduce oxidative stress, inhibit inflammation and fibrosis, and enhance the energy stores and the repair and regenerative capacity of the heart. The effects of differentially expressed proteins in the kidney involved the enhancement of renal autophagy, suppression of renal inflammation and fibrosis, and promotion of renal tubular integrity and regeneration as well as compensatory mechanisms that can limit the ability of SGLT2 inhibitors to produce sustained increases in sodium and water excretion. The actions of differentially expressed proteins identified in patients with heart failure are consistent with the findings of experimental studies that have linked the benefits of SGLT2 inhibitors on the heart and kidney to their actions on autophagy, inflammation and fibrosis, and cellular stress and viability. Our findings suggest that the results of these experimental studies are likely to be highly relevant to the clinical setting.
Supplementary Material
Acknowledgements
Graphical assistance was provided by 7.4 Limited and supported financially by Boehringer Ingelheim.
Contributor Information
Faiez Zannad, Université de Lorraine, Inserm, Centre d'Investigations Cliniques Plurithématique 1433, and Inserm U1116, CHRU, F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists), 5, rue du Morvan, 54500 Vandoeuvre-Les-Nancy, France.
João Pedro Ferreira, Université de Lorraine, Inserm, Centre d'Investigations Cliniques Plurithématique 1433, and Inserm U1116, CHRU, F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists), 5, rue du Morvan, 54500 Vandoeuvre-Les-Nancy, France; Cardiovascular R&D Centre-UnIC@RISE, Cardiovascular Research and Development Center, Department of Surgery and Physiology, Faculty of Medicine of the University of Porto, Alameda Professor Hernâni Monteiro 4200-319 Porto, Portugal; Internal Medicine Department, Centro Hospitalar de Vila Nova de Gaia/Espinho, R. Conceição Fernandes S/N, 4434-502 Vila Nova de Gaia, Portugal.
Javed Butler, Heart and Vascular Research, Baylor Scott and White Research Institute, 34 Live Oak St Ste 501, Dallas, TX 75204, USA; University of Mississippi Medical Center, 2500 North State Street Jackson, MS 39216, USA.
Gerasimos Filippatos, Heart Failure Unit, National and Kapodistrian University of Athens School of Medicine, Mikras Asias 75, Athina 115 27 Athens, Greece.
James L Januzzi, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St, Boston, MA 02114, USA; The Baim Institute for Clinical Research, 930 Commonwealth Ave #3, Boston, MA 02215, USA.
Mikhail Sumin, Boehringer Ingelheim International GmbH, Binger Str. 173, 55218 Ingelheim am Rhein, Germany.
Matthias Zwick, Boehringer Ingelheim Pharma GmbH & Co. KG, Birkendorfer Str. 65, 88400 Biberach an der Riss, Germany.
Maral Saadati, Elderbrook Solutions GmbH on behalf of Boehringer Ingelheim Pharma GmbH & Co. KG, Birkendorfer Str. 65, 88400 Biberach an der Riss, Germany.
Stuart J Pocock, London School of Hygiene and Tropical Medicine, Keppel St, London WC1E 7HT, UK.
Naveed Sattar, BHF, UK School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Stefan D Anker, Department of Cardiology (CVK) Berlin Institute of Health Center for Regenerative Therapies (BCRT) German Centre for Cardiovascular Research (DZHK) partner site Berlin, Charité Universitätsmedizin Berlin, Charité, Campus Virchow-Klinikum, Augustenburger Platz 1, D-13353 Berlin, Germany; Institute of Heart Diseases, Wroclaw Medical University, Borowska Street 213, 50-556 Warsaw, Poland.
Milton Packer, Baylor Heart and Vascular Hospital, Baylor University Medical Center, 621 N Hall St, Dallas, TX 75226, USA; Imperial College, London, Exhibition Rd, South Kensington, London SW7 2BX, UK.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The EMPEROR-Reduced and Preserved trials were funded by Boehringer Ingelheim and Eli Lilly (EMPEROR-Reduced ClinicalTrials.gov number, NCT03057977 and EMPEROR-Preserved ClinicalTrials.gov number, NCT03057951).
Data availability
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on Vivli—Center for Global Clinical Research Data, and earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit Medical & Clinical Trials | Clinical Research | MyStudyWindow for further information. https://www.mystudywindow.com/msw/datasharing.
References
- 1. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 Inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 2. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 Inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 3. Packer M. Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation 2020;141:2095–2105. [DOI] [PubMed] [Google Scholar]
- 4. Packer M. Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 2017;136:1548–1559. [DOI] [PubMed] [Google Scholar]
- 5. Fitchett D, Inzucchi SE, Zinman B, Wanner C, Schumacher M, Schmoor C, et al. Mediators of the improvement in heart failure outcomes with empagliflozin in the EMPA-REG OUTCOME trial. ESC Heart Fail 2021;8:4517–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li JW, Neal B, Perkovic V, de Zeeuw D, Neuen BL, Arnott C, et al. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int 2020;98:769–777. [DOI] [PubMed] [Google Scholar]
- 7. Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. J Card Fail 2020;26:977–984. [DOI] [PubMed] [Google Scholar]
- 8. PEA—a high-multiplex immunoassay technology with qPCR or NGS readout. In: https://www.olink.com/content/uploads/2021/09/olink-white-paper-pea-a-high-multiplex-immunoassay-technology-with-qpcr-or-ngs-readout-v1.0.pdf; 2020.
- 9. Green GH, Diggle PJ. On the operational characteristics of the Benjamini and hochberg false discovery rate procedure. Stat Appl Genet Mol Biol 2007;6:Article27. 10.2202/1544-6115.1302 [DOI] [PubMed] [Google Scholar]
- 10. Abdellatif M, Trummer-Herbst V, Heberle AM, Humnig A, Pendl T, Durand S, et al. Fine-tuning cardiac insulin-like growth factor 1 receptor signaling to promote health and longevity. Circulation 2022;145:1853–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang X, Jiang H, Lin P, Zhang Z, Chen M, Zhang Y, et al. Insulin-like growth factor binding protein-1 regulates HIF-1α degradation to inhibit apoptosis in hypoxic cardiomyocytes. Cell Death Discov 2021;7:242. 10.1038/s41420-021-00629-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gan L, Han Y, Bastianetto S, Dumont Y, Unterman TG, Quirion R. FoxO-dependent and -independent mechanisms mediate SirT1 effects on IGFBP-1 gene expression. Biochem Biophys Res Commun 2005;337:1092–1096. [DOI] [PubMed] [Google Scholar]
- 13. Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep 2015;13:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crane FL, Navas P, Low H, Sun IL, de Cabo R. Sirtuin activation: a role for plasma membrane in the cell growth puzzle. J Gerontol A Biol Sci Med Sci 2013;68:368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin C, Zhang M, Zhang Y, Yang K, Hu J, Si R, et al. Helix B surface peptide attenuates diabetic cardiomyopathy via AMPK-dependent autophagy. Biochem Biophys Res Commun 2017;482:665–671. [DOI] [PubMed] [Google Scholar]
- 16. Gut N, Piecha G, Aldebssi F, Schaefer S, Bekeredjian R, Schirmacher P, et al. Erythropoietin combined with ACE inhibitor prevents heart remodeling in 5/6 nephrectomized rats independently of blood pressure and kidney function. Am J Nephrol 2013;38:124–135. [DOI] [PubMed] [Google Scholar]
- 17. Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett 2017;275:28–38. [DOI] [PubMed] [Google Scholar]
- 18. Chen R, Xu M, Hogg RT, Li J, Little B, Gerard RD, et al. The acetylase/deacetylase couple CREB-binding protein/sirtuin 1 controls hypoxia-inducible factor 2 signaling. J Biol Chem 2012;287:30800–30811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang W, Duan Q, Zhu X, Tao K, Dong A. Follistatin-like 1 attenuates ischemia/reperfusion injury in cardiomyocytes via regulation of autophagy. Biomed Res Int 2019;2019:9537382. 10.1155/2019/9537382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, et al. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci USA 2011;108:E899–E906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calderon RM, Smith CA, Miedzybrodzka EL, Silvaroli JA, Golczak M, Gribble FM, et al. Intestinal enteroendocrine cell signaling: retinol-binding protein 2 and retinoid actions. Endocrinology 2022;163:bqac064. 10.1210/endocr/bqac064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Da Silva F, Jian Motamedi F, Weerasinghe Arachchige LC, Tison A, Bradford ST, Lefebvre J, et al. Retinoic acid signaling is directly activated in cardiomyocytes and protects mouse hearts from apoptosis after myocardial infarction. Elife 2021;10:e68280. 10.7554/eLife.68280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin SC, Dollé P, Ryckebüsch L, Noseda M, Zaffran S, Schneider MD, et al. Endogenous retinoic acid regulates cardiac progenitor differentiation. Proc Natl Acad Sci U S A 2010;107:9234–9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu J, Zheng C, Wan X, Shi M, McMillan K, Maique J, et al. Retinoic acid alleviates cisplatin-induced acute kidney injury through activation of autophagy. Front Pharmacol 2020;11:987. 10.3389/fphar.2020.00987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sumida A, Horiba M, Ishiguro H, Takenaka H, Ueda N, Ooboshi H, et al. Midkine gene transfer after myocardial infarction in rats prevents remodelling and ameliorates cardiac dysfunction. Cardiovasc Res 2010;86:113–121. [DOI] [PubMed] [Google Scholar]
- 26. Takenaka H, Horiba M, Ishiguro H, Sumida A, Hojo M, Usui A, et al. Midkine prevents ventricular remodeling and improves long-term survival after myocardial infarction. Am J Physiol Heart Circ Physiol 2009;296:H462–H469. [DOI] [PubMed] [Google Scholar]
- 27. Swift L, McHowat J, Sarvazyan N. Anthracycline induced phospholipase A2 inhibition. Cardiovasc Toxicol 2007;7:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McHowat J, Creer MH. Catalytic features, regulation and function of myocardial phospholipase A2. Curr Med Chem Cardiovasc Hematol Agents 2004;2:209–218. [DOI] [PubMed] [Google Scholar]
- 29. Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, et al. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res 2010;106:1712–1721. [DOI] [PubMed] [Google Scholar]
- 30. Zhan W, Tian W, Zhang W, Tian H, Sun T. ANGPTL4 Attenuates palmitic acid-induced endothelial cell injury by increasing autophagy. Cell Signal 2022;98:110410. 10.1016/j.cellsig.2022.110410 [DOI] [PubMed] [Google Scholar]
- 31. Wo D, Peng J, Ren DN, Qiu L, Chen J, Zhu Y, et al. Opposing roles of wnt inhibitors IGFBP-4 and Dkk1 in cardiac ischemia by differential targeting of LRP5/6 and β-catenin. Circulation 2016;134:1991–2007. [DOI] [PubMed] [Google Scholar]
- 32. Xue Y, Yan Y, Gong H, Fang B, Zhou Y, Ding Z, et al. Insulin-like growth factor binding protein 4 enhances cardiomyocytes induction in murine-induced pluripotent stem cells. J Cell Biochem 2014;115:1495–1504. [DOI] [PubMed] [Google Scholar]
- 33. Szondy Z, Mastroberardino PG, Váradi J, Farrace MG, Nagy N, Bak I, et al. Tissue transglutaminase (TG2) protects cardiomyocytes against ischemia/reperfusion injury by regulating ATP synthesis. Cell Death Differ 2006;13:1827–1829. [DOI] [PubMed] [Google Scholar]
- 34. Song H, Chang W, Lim S, Seo HS, Shim CY, Park S, et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells 2007;25:1431–1438. [DOI] [PubMed] [Google Scholar]
- 35. Whittington HJ, Ostrowski PJ, McAndrew DJ, Cao F, Shaw A, Eykyn TR, et al. Over-expression of mitochondrial creatine kinase in the murine heart improves functional recovery and protects against injury following ischaemia-reperfusion. Cardiovasc Res 2018;114:858–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santiago AP, Chaves EA, Oliveira MF, Galina A. Reactive oxygen species generation is modulated by mitochondrial kinases: correlation with mitochondrial antioxidant peroxidases in rat tissues. Biochimie 2008;90:1566–1577. [DOI] [PubMed] [Google Scholar]
- 37. Lygate CA, Hunyor I, Medway D, de Bono JP, Dawson D, Wallis J, et al. Cardiac phenotype of mitochondrial creatine kinase knockout mice is modified on a pure C57BL/6 genetic background. J Mol Cell Cardiol 2009;46:93–99. [DOI] [PubMed] [Google Scholar]
- 38. Jeong D, Lee MA, Li Y, Yang DK, Kho C, Oh JG, et al. Matricellular protein CCN5 reverses established cardiac fibrosis. J Am Coll Cardiol 2016;67:1556–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoon PO, Lee MA, Cha H, Jeong MH, Kim J, Jang SP, et al. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol 2010;49:294–303. [DOI] [PubMed] [Google Scholar]
- 40. Umbarawan Y, Kawakami R, Syamsunarno MRAA, Koitabashi N, Obinata H, Yamaguchi A, et al. Reduced fatty acid uptake aggravates cardiac contractile dysfunction in streptozotocin-induced diabetic cardiomyopathy. Sci Rep 2020;10:20809. 10.1038/s41598-020-77895-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamounier-Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, et al. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res 2009;105:326–334. [DOI] [PubMed] [Google Scholar]
- 42. Josephrajan A, Hertzel AV, Bohm EK, McBurney MW, Imai SI, Mashek DG, et al. Unconventional secretion of adipocyte fatty acid binding protein 4 is mediated by autophagic proteins in a sirtuin-1-dependent manner. Diabetes 2019;68:1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab 2010;95:2476–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodríguez-Penas D, Feijóo-Bandín S, García-Rúa V, Mosquera-Leal A, Durán D, Varela A, et al. The adipokine chemerin induces apoptosis in cardiomyocytes. Cell Physiol Biochem 2015;37:176–192. [DOI] [PubMed] [Google Scholar]
- 45. Blaszak J, Szolkiewicz M, Sucajtys-Szulc E, Konarzewski M, Lizakowski S, Swierczynski J, et al. High serum chemerin level in CKD patients is related to kidney function, but not to its adipose tissue overproduction. Ren Fail 2015;37:1033–1038. [DOI] [PubMed] [Google Scholar]
- 46. Krishnan D, Liu L, Wiebe SA, Casey JR, Cordat E, Alexander RT. Carbonic anhydrase II binds to and increases the activity of the epithelial sodium-proton exchanger, NHE3. Am J Physiol Renal Physiol 2015;309:F383–F392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lessa LM, Carraro-Lacroix LR, Crajoinas RO, Bezerra CN, Dariolli R, Girardi AC, et al. Mechanisms underlying the inhibitory effects of uroguanylin on NHE3 transport activity in renal proximal tubule. Am J Physiol Renal Physiol 2012;303:F1399–F1408. [DOI] [PubMed] [Google Scholar]
- 48. Sindic A. Current understanding of guanylin peptides actions. ISRN Nephrol 2013;2013:813648. 10.5402/2013/813648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, et al. Rampoldi L; SKIPOGH team: common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med 2013;19:1655–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, et al. Activation of the bumetanide-sensitive na+, K+,2Cl- cotransporter (NKCC2) is facilitated by tamm-horsfall protein in a chloride-sensitive manner. J Biol Chem 2011;286:30200–30210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Y, El-Achkar TM, Wu XR. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem 2012;287:16365–16378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao X, Chen X, Zhang Y, George J, Cobbs A, Wang G, et al. Kidney injury molecule-1 is upregulated in renal lipotoxicity and mediates palmitate-induced tubular cell injury and inflammatory response. Int J Mol Sci 2019;20:3406. 10.3390/ijms20143406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mori Y, Ajay AK, Chang JH, Mou S, Zhao H, Kishi S, et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab 2021;33:1042–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Humphreys BD, Xu F, Sabbisetti V, Grgic I, Movahedi Naini S, Wang N, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 2013;123:4023–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trzpis M, McLaughlin PM, van Goor H, Brinker MG, van Dam GM, de Leij LM, et al. Expression of EpCAM is up-regulated during regeneration of renal epithelia. J Pathol 2008;216:201–208. [DOI] [PubMed] [Google Scholar]
- 56. Schnell U, Cirulli V, Giepmans BNG. EpCAM: structure and function in health and disease. Biochim Biophys Acta 2013;1828:1989–2001. [DOI] [PubMed] [Google Scholar]
- 57. Gerosa C, Fanni D, Nemolato S, Locci A, Marinelli V, Cabras T, et al. Thymosin beta-10 expression in developing human kidney. J Matern Fetal Neonatal Med 2010;23:125–128. [DOI] [PubMed] [Google Scholar]
- 58. Rosenblum ND, Yager TD. Changing patterns of gene expression in developing mouse kidney, as probed by differential mRNA display combined with cDNA library screening. Kidney Int 1997;51:920–925. [DOI] [PubMed] [Google Scholar]
- 59. Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, et al. Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005;54:2320–2327. [DOI] [PubMed] [Google Scholar]
- 60. Morinaga J, Kadomatsu T, Miyata K, Endo M, Terada K, Tian Z, et al. Angiopoietin-like protein 2 increases renal fibrosis by accelerating transforming growth factor-β signaling in chronic kidney disease. Kidney Int 2016;89:327–341. [DOI] [PubMed] [Google Scholar]
- 61. Xiang H, Xue W, Li Y, Zheng J, Ding C, Dou M, et al. Knockdown of ANGPTL2 protects renal tubular epithelial cells against hypoxia/reoxygenation-induced injury via suppressing TLR4/NF-κB signaling pathway and activating Nrf2/HO-1 signaling pathway. Cell Transplant 2020;29:963689720946663. 10.1177/0963689720946663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen W, Wang J, Wang X, Chang P, Liang M. Knockdown of hypoxia-inducible factor 1-alpha (HIF1α) interferes with angiopoietin-like protein 2 (ANGPTL2) to attenuate high glucose-triggered hypoxia/reoxygenation injury in cardiomyocytes. Bioengineered 2022;13:1476–1490. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63. Ferrannini E, Murthy AC, Lee YH, Muscelli E, Weiss S, Ostroff RM, et al. Mechanisms of sodium-glucose cotransporter 2 inhibition: insights from large-scale proteomics. Diabetes Care 2020;43:2183–2189. [DOI] [PubMed] [Google Scholar]
- 64. Packer M. Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J Am Soc Nephrol 2020;31:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Packer M. Critical examination of mechanisms underlying the reduction in heart failure events with SGLT2 inhibitors: identification of a molecular link between their actions to stimulate erythrocytosis and to alleviate cellular stress. Cardiovasc Res 2021;117:74–84. [DOI] [PubMed] [Google Scholar]
- 66. Li X, Flynn ER, do Carmo JM, Wang Z, da Silva AA, Mouton AJ, et al. Direct cardiac actions of sodium-glucose cotransporter 2 inhibition improve mitochondrial function and attenuate oxidative stress in pressure overload-induced heart failure. Front Cardiovasc Med 2022;9:859253. 10.3389/fcvm.2022.859253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moellmann J, Mann PA, Kappel BA, Kahles F, Klinkhammer BM, Boor P, et al. The sodium-glucose co-transporter-2 inhibitor ertugliflozin modifies the signature of cardiac substrate metabolism and reduces cardiac mTOR signalling, endoplasmic reticulum stress and apoptosis. Diabetes Obes Metab 2022. 10.1111/dom.14814 [DOI] [PubMed] [Google Scholar]
- 68. Yang L, Liang B, Li J, Zhang X, Chen H, Sun J, et al. Dapagliflozin alleviates advanced glycation end product induced podocyte injury through AMPK/mTOR mediated autophagy pathway. Cell Signal 2022;90:110206. 10.1016/j.cellsig.2021.110206 [DOI] [PubMed] [Google Scholar]
- 69. Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 2014;25:2028–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, et al. Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J 2017;38:362–372. [DOI] [PubMed] [Google Scholar]
- 71. Richard C, Verdier F. Transferrin receptors in erythropoiesis. Int J Mol Sci 2020;21:9713. 10.3390/ijms21249713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao YX, Borjigin S, Yan ZL. Functional annotation and enrichment analysis of differentially expressed serum proteins in patients with type 2 diabetes after dapagliflozin. World J Diabetes 2022;13:224–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail 2021;9:578–589. [DOI] [PubMed] [Google Scholar]
- 74. Packer M. Cardioprotective effects of sirtuin-1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (sodium-glucose cotransporter 2) inhibitors. Circ Heart Fail 2020;13:e007197. 10.1161/CIRCHEARTFAILURE.120.007197 [DOI] [PubMed] [Google Scholar]
- 75. Ren FF, Xie ZY, Jiang YN, Guan X, Chen QY, Lai TF, et al. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin 2022;43:1721–1732. 10.1038/s41401-021-00805-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol 2020;319:F712–F728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Michell AR, Debnam ES, Unwin RJ. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu Rev Physiol 2008;70:379–403. [DOI] [PubMed] [Google Scholar]
- 78. da Silva Lima V, Crajoinas RO, Carraro-Lacroix LR, Godinho AN, Dias JL, Dariolli R, et al. Uroguanylin inhibits H-ATPase activity and surface expression in renal distal tubules by a PKG-dependent pathway. Am J Physiol Cell Physiol 2014;307:C532–C541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic na+/HCO3- cotransporter. J Biol Chem 2007;282:13508–13521. [DOI] [PubMed] [Google Scholar]
- 80. Vilas G, Krishnan D, Loganathan SK, Malhotra D, Liu L, Beggs MR, et al. Increased water flux induced by an aquaporin-1/carbonic anhydrase II interaction. Mol Biol Cell 2015;26:1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boorsma EM, Beusekamp JC, Ter Maaten JM, Figarska SM, Danser AHJ, van Veldhuisen DJ, et al. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail 2021;23:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. Circulation 2020;142:1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Scholtes RA, Muskiet MHA, van Baar MJB, Hesp AC, Greasley PJ, Hammarstedt A, et al. The adaptive renal response for volume homeostasis during 2 weeks of dapagliflozin treatment in people with type 2 diabetes and preserved renal function on a sodium-controlled diet. Kidney Int Rep 2022;7:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Januzzi JL Jr, Zannad F, Anker SD, Butler J, Filippatos G, Pocock SJ, et al. Packer M; EMPEROR-reduced trial committees and investigators. Prognostic importance of NT-proBNP and effect of empagliflozin in the EMPEROR-reduced trial. J Am Coll Cardiol 2021;78:1321–1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on Vivli—Center for Global Clinical Research Data, and earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit Medical & Clinical Trials | Clinical Research | MyStudyWindow for further information. https://www.mystudywindow.com/msw/datasharing.