Abstract
Our objective is to develop a prophylactic vaccine strategy that can be evaluated for surgical and other high-risk hospitalized patients. In this paper, we describe the preparation and preclinical evaluation of a liposomal complete-core lipopolysaccharide (LPS) vaccine that is nontoxic and broadly antigenic. Complete-core (Ra-chemotype) LPSs were isolated from four gram-negative bacterial strains (Escherichia coli K-12, E. coli R1, Pseudomonas aeruginosa PAC608, and Bacteroides fragilis), mixed together to form a cocktail of complete-core LPSs, and then incorporated into multilamellar liposomes consisting of dimyristoyl phosphatidyl choline, dimyristoyl phosphatidylglycerol, and cholesterol in a 4:1:4 molar ratio. The endotoxic activities of these LPS-containing liposomes were less than 0.1% of the endotoxicities of the original free LPSs as measured by the Limulus amoebocyte lysate assay. In vivo administration of liposomal complete-core LPS mixed with Al(OH)3 to rabbits resulted in no pyrogenicity or overt toxicity over a 7-day period. In immunoblots, sera from rabbits following active immunization elicited cross-reactive antibodies to a large panel of rough and smooth LPSs from numerous clinically relevant gram-negative bacteria, including E. coli (serotypes O1, O4, O6, O8, O12, O15, O18, O75, O86, O157, and O111), P. aeruginosa (Fisher-Devlin serotypes 1, 2, and 3, which correspond to International Antigenic Typing Scheme types 6, 11, and 2, respectively), Klebsiella pneumoniae (serotypes O1, O2ab, and O3), B. fragilis, and Bacteroides vulgatus. Active immunization of mice with liposomal complete-core LPS provided protection against a lethal challenge with E. coli O18 LPS. The vaccine tested was nontoxic, nonpyrogenic, and immunogenic against a wide variety of pathogens found in clinical settings.
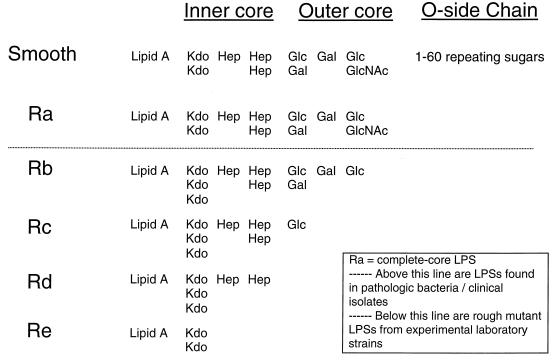
The primary lipid on the surfaces of gram-negative bacteria is lipopolysaccharide (LPS; endotoxin). LPS can be generally characterized as consisting of three structural regions: (i) the lipid A backbone, (ii) an oligosaccharide core, and (iii) the O-polysaccharide outer region (Fig. 1). The lipid region of lipid A is embedded in the outer monolayer of the outer bacterial membrane, and the oligosaccharide core region is positioned between lipid A and the O-polysaccharide outer region. Lipid A has the same basic structure in practically all gram-negative bacteria and is the main endotoxic determinant. LPS oligosaccharide core regions show a high degree of similarity among bacterial genera and consist of a limited number of sugars. The O-polysaccharide outer region (also called O-specific antigen or O-specific side chain) is highly variable and is composed of one or more oligosaccharide repeating units characteristic of the serotype.
FIG. 1.
LPS structures. Ra refers to complete-core LPS. Above the dotted line are LPSs found in pathologic bacteria and clinical isolates. Below the dotted line are rough mutant LPSs from experimental laboratory strains. Kdo, 3-deoxy-d-manno-octulosonic acid; Hep, heptose; Glc, glucose; Gal, galactose; GlcNAc, N-acetylglucosamine.
The different core structures, i.e., chemotypes, of LPS are conventionally designated by the terms Ra, Rb, Rc, Rd, and Re (Fig. 1). It is important to note that in many pathogenic gram-negative bacteria, LPS expressed on a cell's outer membrane is a mixture of rough complete-core LPS (i.e., Ra chemotype without an O-polysaccharide side chain) and smooth complete-core LPS (i.e., Ra chemotype with from 1 to >60 O-polysaccharide side chains). With the exception of those of Chlamydia species, incomplete-core LPS chemotypes (e.g., Escherichia coli J5 Rc and Salmonella minnesota 595 Re) are generally not found in pathogenic bacteria; they are generally found only in laboratory strains of gram-negative bacteria. Hence, given the differences in chemotype structures, it is not surprising that there can be marked variability in the three-dimensional conformations and biological properties (i.e., endotoxicities and antigenicities) of different LPSs.
Several clinical studies have demonstrated an association between low preoperative serum antiendotoxin antibody levels and adverse outcome following surgery (6, 18, 22, 24). For example, it has been found in a study of 301 cardiac surgical patients that low preoperative serum anticore antibody levels predicted adverse postoperative outcome independent of known risk factors and overall humoral immunity (6). An active immunization strategy enhancing natural immunity to LPSs found in clinical settings should reduce complications in and the duration of hospitalization for surgical and high-risk hospitalized patients.
Our objective is to develop a prophylactic vaccine strategy that can be evaluated for surgical and other high-risk hospitalized patients. The first phase of this work has involved the development of a vaccine that is nontoxic and nonpyrogenic and yet elicits antibodies to a large panel of clinically relevant endotoxins. In this paper we describe the preparation and preclinical evaluation of a liposomal complete-core LPS vaccine.
MATERIALS AND METHODS
Bacterial strains, LPS, and lipid A.
E. coli K-12 (NCTC 13116), E. coli R1 (NCTC 13114), Pseudomonas aeruginosa PAC608 (NCTC 13124), and Bacteroides fragilis (NCTC 9343) were obtained from the National Collection of Type Cultures, Central Public Health Laboratory, London, England. Klebsiella pneumoniae O1, O2ab, and O3 were kindly provided by M. Trautman (Ulm, Germany). All smooth E. coli strains; P. aeruginosa Fisher types 1, 2, and 3, corresponding to International Antigenic Typing Scheme types 6, 11, and 2; and Bacteroides vulgatus MPRL 10582 were from the strain collection of the Microbial Pathogenicity Research Laboratory, Department of Medical Microbiology, University of Edinburgh, Edinburgh, Scotland.
Bacterial strains were fermented in 15 liters of nutrient broth (Oxoid code CM1) until an optical density at 600 nm of 1.4 (path length, 1 cm) was achieved, harvested, and then lyophilized to dryness. LPSs from E. coli serotypes O1, O4, O6, O8, O12, O15, O18, O75, O86, O157, and O111; K. pneumoniae serotypes O1, O2ab, and O3; and P. aeruginosa Fisher types 1, 2, and 3 were isolated from dried cells by phenol-water extraction (25). LPSs from E. coli K-12 (NCTC 13116), E. coli R1 (NCTC 13114), P. aeruginosa PAC608 (NCTC 13124), and B. fragilis (NCTC 9343) were obtained from dried cells by both the phenol-chloroform-petroleum spirits (boiling point, 40 to 60°C) (PCP) (25) and Triton X-100–MgCl2 (triton) extraction procedures (37). The PCP purification method includes solvents and routinely resulted in LPS that was relatively free (<1%) of protein. These PCP-extracted LPSs routinely appeared as a single band of LPS on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE) with a molecular weight of approximately 5,000. In contrast, the triton method does not involve solvents and resulted in a higher degree (5 to 10%) of residual protein. Initial immunizations were carried out using PCP-extracted LPS, and subsequent experiments used triton-extracted LPS. LPS from E. coli J5 (Rc chemotype) was purchased from List Biological Laboratories, Inc. (Campbell, Calif.).
Purified LPS and lipid A were tested for endotoxic activity using the Limulus amoebocyte lysate (LAL) assay (Pyrotell and Pyrochrome from Associates of Cape Cod, Cape Cod, Mass.). LPS was tested for residual protein using the Lowry assay. LPS was also analyzed by SDS-PAGE to assess potential heterogeneity in the sizes of the purified LPSs as well as to detect residual protein (30).
Incorporation of LPS and lipid A into liposomes.
All liposomes were prepared using depyrogenated glassware and reagents. Multilamellar vesicles (MLVs) were prepared according to the method of Dijkstra et al. (16) with some modifications. Phospholipids were obtained from Avanti Polar Lipids (Alabastar, Ala.) and consisted of dimyristoyl phosphatidyl choline (DMPC), dimyristoyl phosphatidylglycerol (DMPG), and cholesterol in a 4:1:4 molar ratio. Briefly, a phospholipid-cholesterol stock solution in chloroform was rotovapped to dryness at 50°C. LPS suspended in an aqueous phase containing 0.1% triethylamine was added to the dried lipid film in lipid LPS ratios of 1,000:1, 100:1, 10:1, or 1:1 (wt/wt). The LPS-lipid suspensions were then vigorously vortexed and sonicated using a bath sonicator for not less than 5 min. The preparations were then rotovapped (or lyophilized) to dryness and resuspended with vigorous vortexing in a phosphate-buffered saline buffer. MLVs were then spun down with a bench centrifuge. MLV-type liposomes were stored at −20 or at 4°C. For some experiments, small unilamellar vesicles (SUVs) were made from MLV-type liposomes by sonication with a bath sonicator designed to form SUVs (Laboratory Supplies Company, Hicksville, N.Y.). All liposomes were tested for endotoxic activity using the LAL assay.
Lipid/LPS (wt/wt) ratios were 10:1 for all formulations used in immunizations and pyrogenicity testing because this ratio provided the maximum amount of LPS that we could incorporate into the liposomes (see Results). Formulations involving a cocktail of complete-core LPS antigens used LPS component antigens in equal amounts. Immediately prior to administration to animals, 0.5 ml of Al(OH)3 (1 mg of Al per ml suspended in phosphate-buffered saline–2% Alhydrogel [E. M. Sereant Pulp & Chemical Co., Clifton, N.J.]) was added to and completely mixed with 0.5 ml of liposomal formulation.
In vitro liposomal LPS testing and stability.
To determine whether LPS was incorporated into liposomes, the endotoxic activity of a given liposomal LPS formulation was measured by the LAL assay and compared with the LAL activity of the same LPS not subjected to incorporation into liposomes (free LPS).
Presumed leakage of LPS from a given liposomal LPS formulation was tested in the following way. The LAL activity of the formulation was tested at baseline (day 0) and on days 119, 140, and 175 after storage at 4°C. A given formulation was also LAL tested at an accelerated condition (37°C) on days 0, 23, and 60. The amount of presumed leakage was expressed as LAL activity as a percentage of the baseline activity (day 0).
Liposomal LPS pyrogenicity and toxicity.
An established rabbit pyrogenicity model at the Biological Test Center (Irvine, Calif.) was used to assess the relative levels of pyrogenicity in vivo of complete-core LPSs in both free and liposomal forms. Briefly, mature female New Zealand White rabbits between 1.8 and 3.0 kg were sham tested twice in order to ensure their suitability for pyrogenicity testing. Rectal temperatures of the rabbits were measured to ensure a steady baseline, and following administration of the test sample, each rabbit was monitored at 5-min intervals for 3 h using an automatic recording system. A material was considered nonpyrogenic if no rabbit showed an individual rise in temperature of >0.5°C above baseline. Experimental groups included three control groups that were administered increasing doses of purified LPS and three groups that were administered increasing doses of liposomal complete-core LPS. All animal experiments were performed in accordance with institutional guidelines.
Rabbit immunizations.
Initial experiments focused on evaluating the immunogenicities of different complete-core LPS structures as single antigens (e.g., E. coli K-12) and in various antigen cocktails (e.g., E. coli K-12, E. coli R1, B. fragilis, and P. aeruginosa PAC608). The immunogenicity of complete-core (Ra-chemotype) LPS was compared with the immunogenicity of J5 Rc-chemotype LPS incorporated into liposomes.
For all experiments, groups of three mature Dutch female rabbits were immunized intramuscularly with 1.0 ml of a liposome-LPS-Al(OH)3 mixture. Equimolar amounts of antigens were calculated and administered since the molecular mass of Ra-chemotype LPS (≈5,000 Da) is greater than that of Rc-chemotype LPS. All animal experiments were performed in accordance with institutional guidelines.
Gel electrophoresis and blotting.
The cross-reactivities of sera from immunized rabbits were determined by the standard method of binding of sera to purified LPS in Western blots (25). LPSs extracted from smooth bacteria are separated by electrophoresis into bands corresponding to LPS molecules having different molecular weights, depending on the size of the O-specific side chain. PAGE analysis was performed on 14% (wt/vol) acrylamide gels with the buffer system of Laemmli (27), except SDS was omitted from the stacking and separating gel buffers. Samples of LPS (1 to 10 μg, mixed with Laemmli's sample buffer) were loaded onto the gel and electrophoresed at 50 V until the sample had entered the separating gel and then at 150 V until the dye front had migrated 10 cm through the gel. The separated LPS was transferred to a 0.2-μm-pore size nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) at 10 to 12 V for 16 h at 4°C with the Tris-glycine-methanol buffer of Towbin et al. (34). The transferred LPS was immunostained as described in the work of Hancock and Poxton (25).
Mouse lethality: galactosamine (d-GalN) model.
In initial experiments, C57BL 12-week-old male mice were administered 12 mg of galactosamine via the intraperitoneal route followed by various amounts of E. coli O18 LPS. E. coli O18 is a common clinical isolate that expresses the prevalent R1 core type (20) and is a smooth strain bearing both rough (unsubstituted) and smooth (substituted) forms of LPS. Mice were observed for 48 h, and the 100% lethal dose (LD100) was calculated. The LD100 for E. coli O18 LPS was determined to be 12.5 ng. Subsequently, groups of C57BL 12-week-old male mice (at least eight mice per group) were actively immunized via the intraperitoneal route with 10 μg of complete-core liposomal LPS on days 0 and 14. Animals in the control group received no injections. On day 21 all mice (both vaccinated and controls) were administered 12 mg of galactosamine and then challenged with twice the LD100 of E. coli O18 LPS (25 ng). Animals were observed for 48 h, and the percent survival was calculated for each group. All animal experiments were performed in accordance with institutional guidelines.
Statistical procedures.
Statistical significance (P) was set at 0.05. Parametric analyses (two-tailed t test) were used to test for differences between continuous variables. Analysis of variance and Fisher extract analyses were used to test for differences between categorical variables.
RESULTS
Liposomal LPS: in vitro testing and stability.
We measured the endotoxic activity of the LPS in liposomes (before centrifugation and after centrifugation) by LAL assay and compared it with the endotoxic activity of free LPS. Our results routinely suggested that we could incorporate 96 to 99.8% of the LPS into MLVs prior to a centrifugation and washing step. That is, the LAL activity of the liposomal LPS could be reduced to <0.2% of its original value, presumably by incorporation into the MLVs. Moreover, centrifugation of the MLVs and resuspension in buffer further lowered the LAL readings to less than 0.1% of their initial values. Various concentrations of LPS in the bilayers, ranging from 1,000:1 to 1:1 (wt/wt) phospholipid-LPS, were prepared and evaluated. By LAL testing we found that the maximum amount of LPS that could be incorporated into the bilayer was a 10:1 lipid/LPS ratio, consistent with the result of Petrov et al. (32).
A nonspecific inhibitory effect of lipids or MLVs on the LAL test was ruled out since there was no reduction in LAL activity when blank MLVs and LPS were mixed together and then tested. The reduction in LAL activity may have been due to the LPS being either incorporated into the lipid bilayer or entrapped in the aqueous spaces between bilayers in the MLVs. However, when the MLVs were sonicated to form SUVs, the LAL activity was still extremely low compared to that of purified LPS, indicating that greater than 95% of the LPS was located within the lipid bilayer matrix.
In stability experiments there was no change in the LAL activity of any of these samples at any of the time points measured over the >6-month period, i.e., there was no increase in the LAL activity of any of the samples under both routine (4°C) and accelerated (37°C) conditions. These results suggest that there was no appreciable leakage of LPS from these liposomes over an extended time period at suitable storage temperatures.
Liposomal LPS pyrogenicity and toxicity.
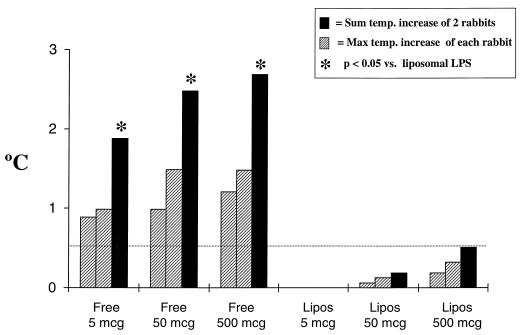
As shown in Fig. 2, free (unincorporated) LPS was pyrogenic in a dose-dependent fashion. In contrast, identical amounts of LPS incorporated into liposomes were not pyrogenic even at the highest dose tested. In experiments involving over 15 rabbits, no animal exhibited signs of demonstrable toxicity (e.g., weight loss, lethargy, and altered breathing) over a 7-day period after administration of 500 μg of LPS incorporated into liposomes.
FIG. 2.
Pyrogenicity in rabbits following administration of free versus liposomal complete-core LPS (Lipos). The dotted line represents the threshold for pyrogenicity (0.5°C). Max, maximum; temp. temperature.
Antigenicity following rabbit immunizations.
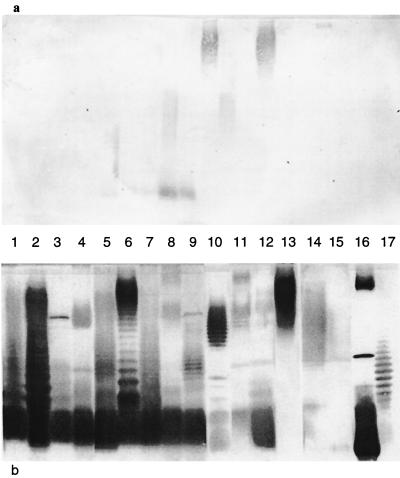
For all experiments, sera on day 0 (prevaccination) showed uniformly low or no detectable antibodies to the LPSs tested by enzyme-linked immunosorbent assay (ELISA), dot blotting, or immunoblotting. Preliminary experiments using ELISA and dot blotting (data not shown) demonstrated that liposomal complete-core LPS antigens elicited antibodies to a broad panel of smooth and rough LPSs. Based on these experiments, the following cocktail of four complete-core LPS antigens was chosen for subsequent experiments: E. coli K-12, E. coli R1, P. aeruginosa PAC608, and B. fragilis. By immunoblotting (Fig. 3b), this liposomal complete-core LPS cocktail elicited various amounts of antibodies to all LPSs tested, i.e., E. coli serotypes O1, O4, O6, O8, O15, O18, O75, O86, and O111; P. aeruginosa Fisher types 1, 2, and 3; K. pneumoniae serotypes O1, O2ab, and O3; and Bacteroides spp. (B. fragilis and B. vulgatus). Sera from rabbits prior to vaccination demonstrated low or undetectable levels of antibodies to these same LPSs by immunoblotting (Fig. 3a).
FIG. 3.
Reactivities by immunoblotting of rabbit sera following active immunization with a liposomal cocktail of complete-core LPS. Groups of three rabbits were immunized on days 0, 14, and 28 with a liposomal cocktail of four complete-core LPS antigens (500-μg total LPS dose) and then bled to obtain sera on day 38. The reactivities of day 0 (a) and day 38 (b) rabbit sera were tested against a panel of LPSs. Lanes 1 to 17, E. coli serotypes O1, O4, O6, O8, O15, O18, O75, O86, and O111; P. aeruginosa Fisher-Devlin types 1, 2, and 3; K. pneumoniae serotypes O1, O2ab, and O3; B. fragilis; and B. vulgatus, respectively.
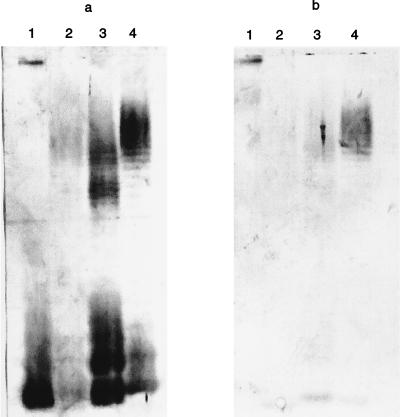
Figure 4 shows a comparison of antibody specificities elicited by liposomes containing equal amounts of either complete-core LPS or the extensively studied incomplete-core J5 Rc. Liposomal E. coli K-12 Ra-chemotype LPS elicited antibodies to E. coli LPSs O18, O12, and O15 as well as to LPS from wild-type Salmonella enterica serovar Typhimurium (Fig. 4a). In contrast, liposomal E. coli J5 Rc-chemotype LPS elicited fewer or no antibodies to these same LPSs (Fig. 4b). This finding was not related to reduced immunogenicity of the J5 Rc LPS since immunization with this LPS elicited very significant binding to the homologous structure.
FIG. 4.
Comparison of reactions of complete-core and J5 Rc incomplete-core LPS antigens by immunoblotting. Groups of three rabbits were immunized on days 0, 14, and 56 with equimolar doses of K-12 Ra-chemotype LPS (500 μg) (a) or J5 Rc-chemotype LPS (358 μg) (b) and then bled to obtain sera on day 63. The reactivities of day 56 rabbit sera were tested against a panel of LPSs. Lanes 1 to 4, wild-type S. enterica serovar Typhimurium and E. coli serotypes O12, O15, and O18, respectively.
Mouse lethality: galactosamine (d-GalN) model.
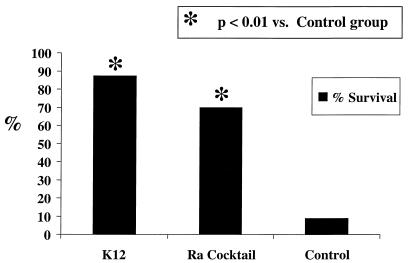
Vaccination with liposomal complete-core LPS antigens significantly protected mice from a lethal challenge with E. coli O18 LPS 7 days following vaccination. As demonstrated in Fig. 5, only 9% (1 of 11) of mice (95% confidence interval [CI], 0 to 18%) in the control group survived. In contrast, 70% (7 of 10) (95% CI, 56 to 84%) of mice vaccinated with a cocktail of Ra-chemotype LPS survived challenge with twice the LD100 of LPS (P < 0.01 compared with results for control animals). Also, 88% (seven of eight) (95% CI, 76 to 98%) of mice vaccinated with liposomal E. coli K-12 complete-core LPS survived challenge with twice the LD100 of LPS (P < 0.01 compared with results for control animals). The protection from the LPS challenge demonstrates that the antibodies elicited by these complete-core LPS antigens, shown in immunoblots, have biological activities in vivo.
FIG. 5.
Protection from challenge with twice the LD100 of E. coli O18 LPS in the mouse lethality (d-GalN) model. Percentages of survival following lethal challenge are shown for three groups: mice given liposomal E. coli K-12 complete-core LPS, mice given liposomal cocktail of Ra-chemotype LPS, and control mice.
DISCUSSION
Gram-negative-LPS-induced systemic inflammation is an important and should be a preventable cause of morbidity in surgical and other high-risk hospitalized patients (5). Several clinical studies have demonstrated an association between low preoperative serum antiendotoxin core antibody levels and adverse outcome following a wide variety of surgical procedures (6, 18, 22, 24). Enhancement of natural immunity to LPS should reduce complications in and the duration of hospitalization for surgical and high-risk patients, and an LPS-based vaccine can have applicability for a broad group of surgical and medical patients. Such a vaccine must be nontoxic, nonpyrogenic, and immunogenic against a wide variety of pathogens found in clinical settings.
Several potential vaccine strategies have been explored. One strategy involves polyvalent vaccines (10, 12) which are intended to elicit antibodies to the O-specific side chain from each of the LPS serotypes included in the particular vaccine. They are not intended to generate antibodies that can bind to LPS from more than one serotype or species of gram-negative bacteria. For example, a polyvalent vaccine comprising 24 Pseudomonas and Klebsiella antigens has been described previously (10) but was not reported to elicit antibodies to other potentially clinically relevant pathogens such as E. coli and Bacteroides. This vaccine strategy has several limitations. The clinical and commercial viability of these vaccines may be limited due to the cost and complexity involved in manufacturing the large number of antigens required. Furthermore, the phenomenon of antigen competition may prevent these vaccines from eliciting antibodies to the large number of antigens.
In another potential vaccine strategy, incomplete core E. coli J5 (Rc-chemotype) and S. minnesota 595 (Re-chemotype) heat-killed bacterial cells have been used extensively as active immunogens and to generate hyperimmune anti-LPS serum for passive immunization of patients (4, 9, 15, 29, 40). Studies using these incomplete-core LPS antigens had inconsistent results, and these antigens have not been reported to elicit antibodies to LPSs from a large panel of clinically relevant gram-negative bacteria (e.g., E. coli, Pseudomonas, Klebsiella, and Bacteroides). McCabe and colleagues concluded that Ra-chemotype structures were not good antigens based on experiments performed over 2 decades ago (28). In those experiments, active immunization with Re-chemotype-killed cells was effective whereas immunization with Ra-chemotype-killed cells was not. In contrast, experiments performed by our group using complete-core LPS chemotypes incorporated into liposomes indicate that a cocktail of Ra-chemotype antigens can elicit cross-reactive antibodies.
The liposomal complete-core LPS vaccine described in this paper was not pyrogenic (Fig. 2) or toxic, yet was broadly antigenic in several models of vaccine safety and efficacy (Fig. 3 to 5). Liposomes have previously been shown to mask the toxic lipid A moieties of certain lipid A-containing structures (16, 19, 32).
The lipids used in our liposomal LPS preparations were chosen from classes of naturally occurring membrane lipids to provide a suitable, stable lipid matrix for the LPS. Saturated phospholipids were used because of their resistance to oxidation and their long shelf life. To maximize mixing of LPS and the lipid matrix, we chose phospholipids (DMPC and DMPG) containing hydrocarbon chains with the same hydrocarbon length (14 carbons) as that of the saturated chains in LPS. Indeed, our results indicated a high degree of incorporation of the complete-core LPS antigens used into liposomes. Relatively high concentrations of cholesterol (44 mol%) were used to maintain the lipids in a liquid-crystalline phase over a wide range of temperatures and to provide mechanical stability to the liposomes, as demonstrated by low LAL assay readings after the liposomes had been stored for extended time periods at 4 and 37°C. Moreover, all of these lipids are quite inexpensive, a relevant issue since the vaccine is intended to be administered prophylactically and hence needs to be relatively inexpensive. We note that these same lipids (i.e., DMPC, DMPG, and cholesterol) were previously used in a lipid A-containing liposomal vaccine tested in a phase 1 clinical trial (19). This malaria vaccine was stable over a long period of time and did not cause systemic toxicity even at the highest dose tested (2.2 mg of lipid A) (19).
Vaccines with endotoxin, e.g., typhoid vaccine (17), have been administered to millions of subjects over several decades and shown to be safe except for transient side effects (e.g., fever, malaise) attributable to free LPS (17). A vaccine intended for prophylactic use should be virtually free of toxicity, i.e., not cause fever or flu-like symptoms. Rabbits respond to LPS in a manner similar to that of humans at clinically relevant doses of LPS. The lack of pyrogenicity or overt toxicity observed in rabbits administered our vaccine (Fig. 2) strongly suggests that this formulation will not cause pyrogenicity or flu-like symptoms in humans. Vaccine lots used for clinical trials will be manufactured according to stringent good manufacturing practices, which may result in a safety profile superior to the one described herein (3).
A vaccine intended for use in surgical and medical patients should also be broadly antigenic. In recent studies assessing the association between natural (i.e., endogenous) immunity to core endotoxin and postoperative outcome, two- and threefold differences in preoperative levels were associated with differences in the incidences of postoperative complications (6, 24). We developed a vaccine with the long-term objective of enhancing natural immunity to LPS and improving outcome in surgical and other high-risk hospitalized patients. This approach focuses on the reduction or prevention of LPS-mediated complications, in contrast to strategies that focus on the treatment of infections and LPS-mediated injury.
In our study, the antibody specificities elicited by the vaccine were predominantly assessed by the standard method of immunoblotting (33). In contrast to very sensitive assays, such as ELISA, that may detect low-affinity antibodies, immunoblotting has been reported to be a relatively insensitive assay, i.e., more antibodies or higher-affinity antibodies must be present in order to obtain a positive result using this assay (33). Our complete-core LPS vaccine elicited cross-reactive antibodies (Fig. 3 and 4) to a broad panel of bacterial genera that, based on previous studies, appear to have a high likelihood of being pathogenic either as viable organisms (i.e., causing infection) or through toxicity of shed LPS, e.g., Escherichia (11, 23, 31), Klebsiella (35, 36), Pseudomonas (38), and Bacteroides (2, 7, 13, 14).
For example, the three serotypes of K. pneumoniae used in this study represented 79.8% of 378 clinical isolates from a series of hospitalized patients (36). The postimmunization serum showed particularly strong binding to K. pneumoniae serotype O1, which represented 39.2% of all isolates (36). Furthermore, the three serotypes of P. aeruginosa used in this study represented 85.7% of 207 clinical isolates from a series of hospitalized patients (38). The postimmunization serum showed particularly strong binding to P. aeruginosa Fisher-Devlin serotype 1, which accounted for 50% of bacteremic isolates in a previous study (38). The E. coli serotypes and Bacteroides strains used to assess the postimmunization sera in our study also represent common clinical isolates for these organisms (11, 23, 31). In one study of 861 E. coli clinical isolates, serotypes O1, O4, O6, O15, O18, and O75 accounted for 92% of cases of fatal E. coli bacteremia (23).
In addition to our finding that elicited antibodies bound LPS by immunoblotting, we also demonstrated that these antibodies have biological activities in vivo. In an established mouse galactosamine lethality model, these antibodies conferred protection (Fig. 5) following challenge with twice the LD100 of LPS from E. coli O18. This clinically relevant serotype of E. coli accounted for 27% of fatal cases of E. coli bacteremia (23).
Despite decades of controversy in this field, there is some evidence that prophylactic strategies using gram-negative-LPS-based vaccines can be efficacious. Approximately 3 million dairy cows are vaccinated annually in the United States with gram-negative-LPS-based vaccines. In one randomized clinical trial, a vaccine administered prophylactically reduced the incidence and severity of mastitis in cattle (21). In another example of vaccine efficacy, cancer and burn patients immunized with antigens derived from Pseudomonas bacteria had fewer infections and complications than the control group in several randomized clinical trials conducted over 2 decades ago (1, 26, 39). In one of these studies involving 361 hospitalized cancer patients (39), active immunization with a Pseudomonas-based vaccine reduced deaths attributable to Pseudomonas infection (7 versus 17%, P < 0.01). These studies support the theory that gram-negative LPS causes clinically relevant hospital complications. These studies are also important because they demonstrate that patients with severe illness can mount an effective humoral response to these vaccines in time to confer clinical benefit. Despite these positive results, Pseudomonas-based vaccines are not routinely used today for several reasons. These vaccines, designed over 2 decades ago, caused toxicity (e.g., fever and malaise) attributable to the crude nature of the LPS. Furthermore, these vaccines were not shown to raise antibodies to other clinically relevant bacterial pathogens, such as Escherichia, Bacteroides, and Klebsiella.
In humans a vaccine approach targeted towards LPS might enhance immunity that is already present. Hence, it is likely that prophylactic administration of a vaccine will result in the rapid synthesis of antiendotoxin antibodies. Surgical and other high-risk hospitalized patients often develop gram-negative infections and LPS-mediated injury several days to a week following admission to the hospital. Thus, vaccination of high-risk individuals prior to surgery or on admission to the hospital or intensive care unit may prevent complications from developing several days later.
ACKNOWLEDGMENTS
We thank Emily E. Conway for her assistance in preparing liposomal formulations and Robert Brown for assistance with animal immunizations and antibody analyses. This work was paid for by Duke University (Durham, N.C.) and by Medical Defense Technologies, LLC (New York, N.Y.).
REFERENCES
- 1.Alexander J W, Fisher M W, MacMillan B G. Immunological control of Pseudomonas infection in burn patients: a clinical evaluation. Arch Surg. 1971;102:31–35. doi: 10.1001/archsurg.1971.01350010033008. [DOI] [PubMed] [Google Scholar]
- 2.Allan E, Poxton I R, Barclay G R. Anti-Bacteroides lipopolysaccharide IgG levels in healthy adults and sepsis patients. FEMS Immunol Med Microbiol. 1995;11:5–12. doi: 10.1111/j.1574-695X.1995.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Alving C R. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–446. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner J D, Heumann D, Calandra T, Glauser M P. Antibodies to lipopolysaccharides after immunization of humans with the rough mutant Escherichia coli J5. J Infect Dis. 1991;163:769–772. doi: 10.1093/infdis/163.4.769. [DOI] [PubMed] [Google Scholar]
- 5.Bennett-Guerrero E. Systemic inflammation. In: Kaplan J A, Reich D L, Konstadt S N, editors. Cardiac anesthesia. 4th ed. Philadelphia, Pa: W. B. Saunders; 1998. pp. 297–318. [Google Scholar]
- 6.Bennett-Guerrero E, Ayuso L, Hamilton-Davies C, White W D, Barclay G R, Smith P K, King S A, Muhlbaier L H, Newman M F, Mythen M G. Relationship of preoperative serum anti-endotoxin-core antibody levels and adverse outcomes following cardiac surgery. JAMA. 1997;277:646–650. [PubMed] [Google Scholar]
- 7.Bennett-Guerrero, E., M. E. Youssef, G. R. Barclay, S. Hosain, F. Vela-Cantos, L. A. Andres, and I. R. Poxton. Exposure to Bacteroides fragilis endotoxoin during cardiac surgery. Anesth. Analg. 90:819–823. [DOI] [PubMed]
- 8.Boswell G W, Buell D, Bekersky I. AmBisome (liposomal amphotericin B): a comparative review. J Clin Pharmacol. 1998;38:583–592. doi: 10.1002/j.1552-4604.1998.tb04464.x. [DOI] [PubMed] [Google Scholar]
- 9.Calandra T, Glauser M P, Schellekens J, Verhoef J. Treatment of gram-negative septic shock with human IgG antibody to Escherichia coli J5: a prospective, double-blind, randomized trial. J Infect Dis. 1988;158:312–319. doi: 10.1093/infdis/158.2.312. [DOI] [PubMed] [Google Scholar]
- 10.Campbell W N, Hendrix E, Cryz S, Jr, Cross A S. Immunogenicity of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered to victims of acute trauma. Clin Infect Dis. 1996;23:179–181. doi: 10.1093/clinids/23.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Ceddia T, Cialfi R, Mancini A, Marinucci M C. Frequency of Escherichia coli serotypes in urinary infections. Boll Soc Ital Biol Sper. 1979;6:538–543. [PubMed] [Google Scholar]
- 12.Cryz S J, Jr, Que J O, Cross A S, Furer E. Synthesis and characterization of a polyvalent Escherichia coli O-polysaccharide–toxin A conjugate vaccine. Vaccine. 1995;13:449–453. doi: 10.1016/0264-410x(94)00009-c. [DOI] [PubMed] [Google Scholar]
- 13.Delahooke D M, Barclay G R, Poxton I R. A re-appraisal of the biological activity of Bacteroides lipopolysaccharide. J Med Microbiol. 1995;42:102–112. doi: 10.1099/00222615-42-2-102. [DOI] [PubMed] [Google Scholar]
- 14.Delahooke D M, Barclay G R, Poxton I R. Tumor necrosis factor induction by an aqueous phenol-extracted lipopolysaccharide complex from Bacteroides species. Infect Immun. 1995;63:840–846. doi: 10.1128/iai.63.3.840-846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeMaria A, Jr, Johns M A, Berberich H, McCabe W R. Immunization with rough mutants of Salmonella minnesota: initial studies in human subjects. J Infect Dis. 1988;158:301–311. doi: 10.1093/infdis/158.2.301. [DOI] [PubMed] [Google Scholar]
- 16.Dijkstra J, Ryan J L, Szoka F C. A procedure for the efficient incorporation of wild-type lipopolysaccharide into liposomes for use in immunological studies. J Immunol Methods. 1988;114:197–205. doi: 10.1016/0022-1759(88)90174-3. [DOI] [PubMed] [Google Scholar]
- 17.Engels E A, Falagas M E, Lau J, Bennish M L. Typhoid fever vaccines: a meta-analysis of studies on efficacy and toxicity. Br Med J. 1998;316:110–116. doi: 10.1136/bmj.316.7125.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman R, Gould F K. Prevention of fever and gram negative infection after open heart surgery by antiendotoxin. Thorax. 1985;40:846–848. doi: 10.1136/thx.40.11.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fries L F, Gordon D M, Richards R L, Egan J E, Hollingdale M R, Gross M, Silverman C, Alving C R. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci USA. 1992;89:358–362. doi: 10.1073/pnas.89.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibb A P, Barclay G R, Poxton I R, di Padova F. Frequencies of lipopolysaccharide core types among clinical isolates of Escherichia coli defined with monoclonal antibodies. J Infect Dis. 1992;166:1051–1057. doi: 10.1093/infdis/166.5.1051. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez R N, Cullor J S, Jasper D E, Farver T B, Bushnell R B, Oliver M N. Prevention of clinical coliform mastitis in dairy cows by a mutant Escherichia coli vaccine. Can J Vet Res. 1989;53:301–305. [PMC free article] [PubMed] [Google Scholar]
- 22.Gould E K, Harvey J A, Dytrych J K. Antibody to endotoxin is associated with decreased frequency of postoperative infection. Am J Obstet Gynecol. 1989;160:317–319. doi: 10.1016/0002-9378(89)90433-x. [DOI] [PubMed] [Google Scholar]
- 23.Gransden W R, Eykyn S J, Phillips I, Rowe B. Bacteremia due to Escherichia coli: a study of 861 episodes. Rev Infect Dis. 1990;6:1008–1018. doi: 10.1093/clinids/12.6.1008. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton-Davies C, Barclay G R, Cardigan R A, McDonald S J, Purdy G, Machin S J, Webb A R. Relationship between preoperative endotoxin immune status, gut perfusion, and outcome from cardiac valve replacement surgery. Chest. 1997;112:1189–1196. doi: 10.1378/chest.112.5.1189. [DOI] [PubMed] [Google Scholar]
- 25.Hancock I C, Poxton I R. Bacterial cell surface techniques. Chichester, United Kingdom: John Wiley & Sons; 1988. [Google Scholar]
- 26.Jones R J, Roe E A, Gupta J L. Controlled trial of Pseudomonas immunoglobulin and vaccine in burn patients. Lancet. 1989;ii:1263–1265. doi: 10.1016/s0140-6736(80)92334-x. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1990;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.McCabe R, Bruins S C, Craven D E, Johns M. Cross-reactive antigens: their potential for immunization-induced immunity to gram-negative bacteria. J Infect Dis. 1977;136:S161–S166. doi: 10.1093/infdis/136.supplement.s161. [DOI] [PubMed] [Google Scholar]
- 29.McCutchan J A, Wolf J L, Ziegler E J, Braude A I. Ineffectiveness of single-dose human antiserum to core glycolipid (E. coli J5) for prophylaxis of bacteremic, gram-negative infections in patients with prolonged neutropenia. Schweiz Med Wochenschr Suppl. 1983;14:40–45. [PubMed] [Google Scholar]
- 30.Nelson D, Neill W, Poxton I R. A comparison of immunoblotting, flow cytometry and ELISA to monitor the binding of anti-lipopolysaccharide monoclonal antibodies. J Immunol Methods. 1990;133:227–233. doi: 10.1016/0022-1759(90)90363-z. [DOI] [PubMed] [Google Scholar]
- 31.Olesen B, Kolmos H J, Orskov F, Orskov I. A comparative study of nosocomial and community-acquired strains of Escherichia coli causing bacteraemia in a Danish University hospital. J Hosp Infect. 1995;4:295–304. doi: 10.1016/0195-6701(95)90208-2. [DOI] [PubMed] [Google Scholar]
- 32.Petrov A B, Semenov B F, Vartanyan Y P, Zakirov M M, Torchilin V P, Trubetskoy V S, Koshkina N V, L'Vov V L, Verner I K, Lopyrev I V, Dmitriev B A. Toxicity and immunogenicity of Neisseria meningitidis lipopolysaccharide incorporated into liposomes. Infect Immun. 1992;60:3897–3903. doi: 10.1128/iai.60.9.3897-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poxton I R. Antibodies to lipopolysaccharide. J Immunol Methods. 1995;186:1–15. doi: 10.1016/0022-1759(95)00123-r. [DOI] [PubMed] [Google Scholar]
- 34.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trautmann M, Held T K, Susa M, Karajan M A, Wulf A, Cross A S, Marre R. Bacterial lipopolysaccharide (LPS)-specific antibodies in commercial human immunoglobulin preparations: superior antibody content of an IgM-enriched product. Clin Exp Immunol. 1998;111:81–90. doi: 10.1046/j.1365-2249.1998.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trautmann M, Ruhnke M, Rukavina T, Held T K, Cross A S, Marre R, Whitfield C. O-antigen seroepidemiology of Klebsiella clinical isolates and implications for immunoprophylaxis of Klebsiella infections. Clin Diagn Lab Immunol. 1997;4:550–555. doi: 10.1128/cdli.4.5.550-555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchida K, Mizushima S. A simple method for isolation of lipopolysaccharides from Pseudomonas aeruginosa and some other bacterial strains. Agric Biol Chem. 1987;51:3107–3114. [Google Scholar]
- 38.Vazquez F, Mendoza M C, Villar M H, Vindel A, Mendez F J. Characteristics of Pseudomonas aeruginosa strains causing septicemia in a Spanish hospital 1981–1990. Eur J Clin Microbiol Infect Dis. 1992;8:698–703. doi: 10.1007/BF01989973. [DOI] [PubMed] [Google Scholar]
- 39.Young L S, Meyer R D, Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med. 1973;79:518–527. doi: 10.7326/0003-4819-79-4-518. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler E J, McCutchan J A, Fierer J, Glauser M P, Sadoff J C, Douglas H, Braude A I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]